Abstract
The prostate is a principal accessory genital gland that is vital for normal fertility. Epithelial cells lining the prostate acini release in a defined fashion (exocytosis) organellar nanosized structures named prostasomes. They are involved in the protection of sperm cells against immune response in the female reproductive tract by modulating the complement system and by inhibiting monocyte and neutrophil phagocytosis and lymphocyte proliferation. The immunomodulatory function most probably involves small non-coding RNAs present in prostasomes. Prostasomes have also been proposed to regulate the timing of sperm cell capacitation and induction of the acrosome reaction, since they are rich in various transferable bioactive molecules (e.g. receptors and enzymes) that promote the fertilising ability of sperm cells. Antigenicity of sperm cells has been well documented and implicated in involuntary immunological infertility of human couples, and antisperm antibodies (ASA) occur in several body fluids. The propensity of sperm cells to carry attached prostasomes suggests that they are a new category of sperm antigens. Circulating human ASA recognise prostasomes, and among 12 identified prostasomal antigens, prolactin- inducible protein (95 %) and clusterin (85 %) were immunodominant at the expense of the other 10 that were sporadically occurring.
Keywords: Prostasomes, Non-coding RNA, Seminal fluid, Spermatozoa, Antioxidants, Immunosuppression
Introduction
The acinar, epithelial cells of the prostate gland have the capacity for secretion. These cells are not alone (although being in majority) in the epithelial lining of prostatic ducts, since there are also minor elements of basal and endocrine–paracrine (APUD) cells. The secretion is not only the result of the synthesising activity of the epithelial cells but depends also on transudation from blood plasma. Besides soluble components, the human prostate gland secretes a particular fraction organised in well-defined organelles (extracellular vesicles, EVs) termed prostasomes (Ronquist and Brody 1985) that were first reported in the 1970s (Ronquist and Hedstrom 1977). They are surrounded by a bilayered membrane and have a globular appearance (Fig. 9.1) and can be recovered in prostatic and seminal fluids with similar ultrastructure. Subsequent electron microscopy examinations revealed that the EVs corresponded to intracellular vesicles inside another larger vesicle, a so-called storage vesicle, equivalent to a multivesicular body (MVB) of late endosomal origin (Ronquist and Brody 1985). Prostasomes exhibited similar mean diameters of about 150 nm regardless of being intracellular or extracellular (after isolation) (Ronquist and Brody 1985), but the size varied and the diameter of a vast majority of prostasomes was within the range of 30–200 nm (Ronquist 2012). Ultrastructural studies revealed that prostasomes were delivered into the glandular duct by so-called exocytosis (Ronquist and Brody 1985; Sahlen et al. 2002). Functional effects of prostasomes on sperm cells were early registered (Stegmayr and Ronquist 1982).
Fig. 9.1.
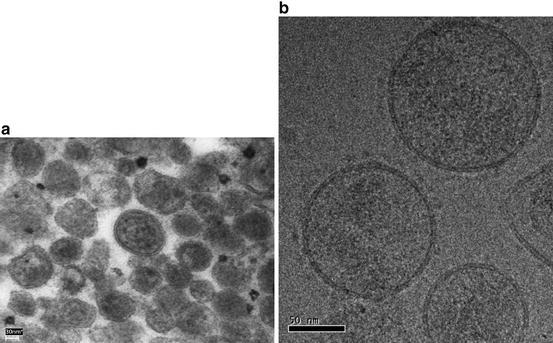
Ultrastructure of prostasomes. (a) Thin-section transmission electron microscopy (EM) image of human prostasomes isolated from seminal plasma. Prostasomes display rounded structures with varied sizes more or less filled with electron-dense material. (b) Ultrastructural appearance of prostasomes by cryo-EM. The samples have been vitrified in liquid ethane to prevent the formation of perturbating ice crystals. The rounded prostasomes are surrounded by classical biological membranes (Brisson A & Ronquist G, unpublished 2013)
Biochemical Characteristics of Prostasomes
Proteins
The protein composition of human prostasomes is varied and has been comprehensively examined (Ronquist et al. 2013). There are almost 1,000 different proteins in prostasomes and many proteins are enzymes. In order to investigate possible contamination from testes and epididymides in our prostasome preparation, we followed the Mg2+ and/or Ca2+ ATPase activity (a marker enzyme of prostasomes) in seminal plasma of 13 men before and after vasectomy. Interestingly, there was no change in ATPase activity meaning that the contribution of sperm cells with regard to ATPase activity and therewith prostasomes was nil. Similar precautions were carried out by Aalberts et al. (2012), and they isolated from vasectomised men two distinct seminal prostasome populations. Both types of prostasomes resembled exosomes in terms of their buoyant density, size and presence of characteristic exosome markers.
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) separation of prostasomal proteins gave rise to a characteristic and consistent banding pattern (Ronquist et al. 2011) (Fig. 9.2) that was distinctly different from that obtained after SDS-PAGE separation of pellets from seminal vesicle secretion subjected to ultracentrifugation, again emphasising the prostate gland as solitary origin of prostasomes. The SDS-PAGE characteristics of prostasomes were three distinct bands identified as aminopeptidase N (CD13; 110 kDa), dipeptidyl peptidase 4 (CD26; 88 kDa) and enkephalinase or neutral endopeptidase, NEP (CD10; 86 kDa) (Fig. 9.2). Mammalian aminopeptidase N (APN) is an important player in many physiological processes, such as sperm motility, cell–cell adhesion, immune cell chemotaxis, tumour angiogenesis and metastasis and coronavirus entry.
Fig. 9.2.
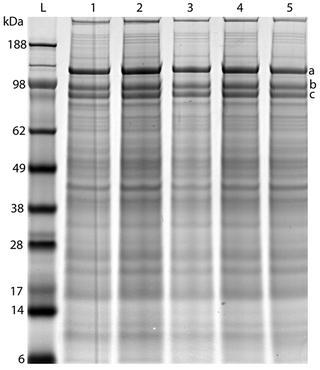
Highly reproducible SDS-PAGE pattern of preparations from different pools of seminal prostasomes (1–5) over a 2-year period. The three marked bands, a–c, in the high molecular weight range have been identified as follows: (a) CD13—aminopeptidase N (approximately 110 kDa), (b) CD26—dipeptidylpeptidase 4 (approximately 88 kDa), (c) CD10—neprilysin (approximately 86 kDa) (Reprinted from Ronquist et al. (2011) with permission of the publisher. Copyright 2014 John Wiley & Sons A/S)
We identified dipeptidyl peptidase 4 (DPP-4) as the antigen of a monoclonal antiprostasome antibody on human prostasomes, and the specific activity of DPP-4 in its prostasomal context is unprecedentedly high (Vanhoof et al. 1992). The DPP-4 antigen (CD26) and enzymatic activity were present in human prostatic fluid but absent from that of the seminal vesicles (Wilson et al. 1998) in harmony with the idea of a solitary prostate gland origin of prostasomes. On the T-cell surface, DPP-4 functions as adenosine deaminase binding protein. A transfer of CD26 from prostasomes to sperm cells was possible, followed by an interaction of prostasomal adenosine deaminase and the transferred CD26 on sperm cells, ultimately leading to fusion between prostasomes and sperm cells (Minelli et al. 1999).
Prostasome-associated enkephalinase/neutral endopeptidase, NEP (CD10), was characterised by Aumüller’s group (Renneberg et al. 2001). Endogenous opioid peptides, among which the naturally occurring opioid pentapeptides (enkephalins) are to be found, participate in the regulation of reproductive physiology at multiple sites.
Three of the glycolytic enzymes detected in prostasomes on proteomic examinations (Ronquist et al. 2013) belong to the top ten proteins found in most exosomes. Moreover, not only human prostasomes but also bovine, canine and equine prostasomes demonstrated a capacity for adenosine triphosphate (ATP) production (Ronquist et al. 2013). The effect of extracellular ATP on the activation of sperm cells has revealed a signal transduction mechanism for ATP involving the purinergic receptor-mediated release of second messengers culminating in acrosomal exocytosis (Luria et al. 2002).
Lipids
Prostasomes exhibit a peculiar lipid composition with an exceptionally high cholesterol-to-phospholipid molar ratio around 2.0. Sphingomyelin (at the expense of phosphatidylcholine) was the predominant phospholipid class representing nearly half of the total phospholipid measured. One third of the fatty acids in sphingomyelin were palmitic acid. Remaining fatty acids consisted largely of saturated and monounsaturated fatty acids (Arvidson et al. 1989). This peculiar pattern suggested the lipids in the prostasome membrane to be highly ordered. This conclusion was corroborated by our electron spin-labelling experiments showing that the order parameters for prostasomes and extracted prostasome lipids were very high (Arvidson et al. 1989) rendering the prostasomal membrane robust features withstanding physical violence, e.g. freezing and thawing. The two types of “true” prostasomes mentioned above and described by Aalberts et al. (2012) also harboured the characteristic lipid pattern. In addition, these authors reported the presence of monohexosylceramides. Prostasome/exosome formation may be dependent on hydrolysis of sphingomyelin by neutral sphingomyelinase in the lipid raft membrane domain of endosomes (Trajkovic et al. 2008). The hydrolysis product, ceramide, may serve as a trigger of endocytosis. The lipid raft hypothesis was launched in 1997 alleging that lipids play a regulatory role in which they mediate protein clustering and protein diffusion within the lipid bilayer of the membrane (Simons and Ikonen 1997). In this model, the membrane can undergo phase separation into coexisting liquid-disordered and liquid-ordered phases. The liquid-ordered phase, termed lipid raft (Simons and Ikonen 1997), was envisaged as enriched in cholesterol and saturated sphingolipids and phospholipids and characterised by tight lipid packing and reduced molecular diffusion, as we previously noticed for prostasomes (Arvidson et al. 1989).
Nucleic Acids
Different types of RNA have been detected in EVs from various sources (Raposo and Stoorvogel 2013). Technological developments have allowed for the deep sequencing of RNA involving also prostasomes isolated from the ejaculate of vasectomised men, revealing that the majority is neither mRNA nor miRNA (Aalberts et al. 2014). However, recent data provide evidence that prostasomes carry several small RNA biotypes with immunomodulatory functions when delivered to target cells (Vojtech et al. 2014). Fragments of human chromosomal DNA were identified inside purified prostasomes (Ronquist et al. 2009). A genome-wide DNA copy number analysis revealed that they contained fragments of DNA randomly selected from the entire genome (Ronquist et al. 2011). It has been argued that it cannot be excluded that small apoptotic vesicles, which are known to contain fragmented DNA, were co-isolated with prostasomes (Aalberts et al. 2014). Round bodies agreeing with apoptotic vesicles have indeed been identified in human semen, but their dimensions ranged from those similar to sperm head to much larger (Marchiani et al. 2007) meaning a size range considerably larger than that of prostasomes from which DNA was isolated. We prepared human seminal prostasomes in accordance with the prevailing protocol for exosome preparation including passage through a 0.2 μm filter and centrifugation in a sucrose gradient (Ronquist et al. 2012). Filterable prostasomes contained about half the amount of DNA when compared with nonfilterable prostasomes (Table 9.1). The DNA pattern in both types of prostasomes ranged from 1–2 kbp (kilobase pairs) to 10–14 kbp which was similar to what was found when examining prostasomes not subjected to filtration and sucrose gradient separation (Ronquist et al. 2009, 2011).
Table 9.1.
DNA content in filterable and nonfilterable prostasomes (duplicate assays) obtained from the main band after sucrose gradient centrifugation
| Prostasome type | Prostasome concentration (mg protein)/mL | Volume of prostasomes (mL) | Total prostasome content (mg) | DNA concentration (ng/μL) | Elution volume (μL) | Total DNA (μg) |
|---|---|---|---|---|---|---|
| Filterable | 2 | 1.2 | 2.4 | 57 | 50 | 2.85 |
| Filterable | 2 | 1.2 | 2.4 | 96 | 50 | 4.8 |
| Nonfilterable | 2 | 1.2 | 2.4 | 160 | 50 | 8 |
| Nonfilterable | 2 | 1.2 | 2.4 | 160 | 50 | 8 |
From Ronquist et al. (2012)
Neuroendocrine Components
Prostasomes contain the neuroendocrine markers chromogranin B, neuropeptide Y and vasoactive intestinal polypeptide in about equimolar amount. Synaptophysin and chromogranin A were found in about 10 % and 2 %, respectively, of that amount (Stridsberg et al. 1996). It was reported that prostasomes express a common secretory granule protein, viz. granulophysin (Skibinski et al. 1994). This molecule has a similar structure as the neuroprotein synaptophysin mentioned above.
Human Reproduction and Role of Prostasomes
Prostasome Regulation of Sperm Cell Function
The physiological relevance of prostasomes was brought out by the finding that prostasomes are able to interact with sperm cells, albeit that both prostasomes and sperm cells display a net negative surface charge favouring repulsive forces (Ronquist et al. 1990). This important extracellular reaction between a cell and an organelle was subsequently confirmed in different ways. Accordingly, prostasomes can carry information from prostate cells to sperm cells. Transfer of a message to target cells could occur by three possible mechanisms: (1) by direct contact between the prostasomal membrane and the sperm cell plasma membrane, (2) by fusion of the two membranes and (3) by sperm cell internalisation of the prostasome. The female reproductive tract is equipped with a well-balanced immune system, and prostasomes are able to mediate different abilities to sperm cells which are important for their survival in a hostile environment to reach and penetrate the zona pellucida for fertilisation of the ovum (Park et al. 2011). Achievement of zona penetration by sperm cells means an ability of both lysis (hydrolytic enzymes) and thrust (hyperactivated motility). It seems reasonable that at least a part of the prostasomes (that are in great excess over sperm cells in an ejaculate) are able to deliver their cargo to sperm cells. This is well-founded, since there might be a conflict within the sperm cell between the critical demand of functional abilities and the silence of protein translation. Transcription ceases several days before the end of spermiogenesis, and the time between when expression is shut down and when acquisition of a distinct pattern of motility known as hyperactivation is needed may be weeks.
Sperm Motility, Capacitation and the Acrosome Reaction
Sperm motility is a critical factor in judging semen quality, and the motility pattern influences the fertilising capability of sperm cells. In the lower female reproductive tract, motility is important to penetrate the cervical mucus, while vigorous beating of the sperm tail is necessary for penetration of zona pellucida in the upper tract (Aitken et al. 1985). The motility pattern of sperm cells evoked by prostasomes (Fabiani et al. 1994; Arienti et al. 2004) helps oocyte fertilisation. We and others have suggested that prostasomes may be able to regulate the divalent cation concentrations in the microenvironment of sperm cells to promote motility (Arienti et al. 2004; Ronquist 1987). On the other hand, it has been claimed that the sperm plasma membrane is extremely impermeable to direct calcium entry into the cytoplasm (Vijayaraghavan and Hoskins 1989). In comparison with the surrounding seminal plasma, an unambiguous enrichment of calcium was observed in prostasomes (Stegmayr et al. 1982). Prostasomes may deliver calcium to sperm cells after fusion, and a detectable increase was indeed noted after 2 min of fusion (Palmerini et al. 1999). Progesterone was influential on the process, and the increased calcium accumulation in sperm cells produced by the fusion with prostasomes and by stimulation of progesterone was independent and additive phenomenon (Arienti et al. 2001). New evidence indicates that human sperm cells have a clever way to solve the conflict between the critical demand for Ca2+ signalling tools and the silence of protein translation (Park et al. 2011).
Natural fertilisation to occur implies interrelationships between the female and the sperm cells, and fertilising ability is only acquired in the female reproductive tract through a functional maturation process that is capacitation. It means a complex of structural and functional changes in sperm cells throughout their transit through the female reproductive tract and is considered to be complete when the sperm cells are able to respond to ligands in the zona pellucida by undergoing the acrosome reaction. The capacitation concept involves sperm alterations such as loss of cholesterol from the membrane, increased protein phosphorylation, increased intracellular concentrations of Ca2+ and cyclic nucleotides and hyperpolarisation of membrane potential (Visconti 2009). Capacitated sperm cells change their motility characteristics probably in order to facilitate their passage through the latter parts of the female reproductive tract (Ho et al. 2009). Herewith, they are primed to undergo the acrosome reaction in case they should come in contact with the zona pellucida and/or cumulus cells surrounding the ovum (Publicover et al. 2007), and sperm cells that acrosome react before a contact with these structures are invalid to fertilise. Hence, the acrosome reaction must take place after binding to a homologous zona.
Prostasomes bind primarily to the head of live sperm cells, and in vivo, prostasomes may bind to sperm cells in the uterus, to be carried (as “rucksacks”) into oviduct and to fuse with the sperm cell only during the final approach of the ovum (Aalberts et al. 2013). This is in line with the observation that prostasome fusion with the sperm cell was an obligatory prerequisite for the transfer from prostasomes of a range of calcium ion signalling tools (including receptors and enzymes) for regulating sperm flagella (Park et al. 2011) and guaranteeing hyperactivated motility necessary for zona pellucida penetration. Apparently, this might be interpreted as a logical consequence of the limited outfit of the sperm cell. Progesterone released by cumulus cells surrounding the ovum is a potent stimulator of the acrosome reaction (Lishko et al. 2011). Human sperm cells are extremely sensitive to progesterone, demonstrating a chemotactic response to picomolar concentration of the hormone (Teves et al. 2006). Park et al. (2011) found that picomolar concentrations of progesterone induced a well-adapted, high-amplitude, calcium ion signal in sperm cells, provided they had fused with prostasomes. This is concordant with other data corroborating the view that prostasome–sperm cell fusion can stimulate the acrosome reaction making sperm cells more sensitive to the effect of progesterone (Palmerini et al. 2003).
Protective Ability Against Oxidative Damage
Reactive oxygen species (ROS) are a major cause of idiopathic male infertility. An abnormally high production of ROS has been demonstrated in 40 % of semen samples from infertile individuals (Iwasaki and Gagnon 1992). Leukocytes infiltrating the semen, particularly the polymorphonuclear neutrophils, seem to be the major source of ROS generation (Saez et al. 1998). Prostasomes have the ability to interact with neutrophils and reduce their capacity to produce superoxide anion after stimulation (Skibinski et al. 1992). Hence, prostasomes could play a role as an antioxidant factor, and it was demonstrated that prostasomes indeed reduced ROS production by sperm preparations containing polymorphonuclear neutrophils (Saez et al. 1998). Subsequent work suggested that prostasomes inhibit the NADPH (nicotinamide adenine dinucleotide phosphate, reduced form) oxidase activity of polymorphonuclear neutrophils by lipid transfer from prostasomes to the plasma membrane of these cells (Saez et al. 2000), therewith inhibiting the ongoing machinery of ROS production.
Protective Ability Against Bacteria
Prostasomal chromogranin B is in abundance over chromogranin A, which is unusual (Stridsberg et al. 1996). What is more, a C-terminal fragment of chromogranin B possesses a potent antibacterial ability. This peptide (secretolytin) forms a three-dimensional structure similar to the insect-derived proteins, cecropins, that are antibacterial as well. The biological activity of these peptides results from their ability to form channels through the bacterial wall leading to bacterial cell death. Also, other parts of both chromogranin B and chromogranin A were antibacterial (Metz-Boutigue et al. 1998). Prostasomes at low concentrations displayed distinct antibacterial effects associated with membrane cavities and bacterial cell death (Carlsson et al. 2000).
A group of peptide antibiotics is the cathelicidin family. The human cationic antimicrobial protein (hCAP-18) is the only known member of this family of proteins in humans (Larrick et al. 1994). The antimicrobial peptide LL-37 becomes activated when released from the C-terminal end of the hCAP-18 holoprotein. In addition to its antimicrobial activity, LL-37 has chemotactic activity for neutrophils, monocytes and T-cells (Agerberth et al. 2000). Hence, LL-37 may contribute to both innate and adaptive immunity, the latter by recruiting immunocompetent cells to sites of microbial invasion. hCAP-18 is expressed in the male reproductive system and high levels were found in seminal plasma (Malm et al. 2000). It appeared in two distinct fractions after gel chromatography of seminal plasma, and the high molecular fraction (the major part) represented hCAP-18 bound to prostasomes (Andersson et al. 2002). In other words, prostasomes can well serve as a reservoir of this precursor of the antibiotic peptide LL-37. It seems like prostasomes may exert antibacterial activities by more than one route.
Involvement of Prostasomes in Immune Responses
Seminal Plasma Immunosuppressive Activity
The prostate gland is equipped with an active immunological armamentarium and is able to respond to several nonself antigens. It has CD8+ suppressor/cytotoxic T lymphocytes, macrophages and B cells. Prostate epithelium and stromal cells express toll-like receptors and respond to various antigens (Hoover and Naz 2012). This is somewhat contrary to the general belief that the blood–testis barrier forms an immunological barrier excluding the entry of immunoglobulins and lymphocytes into the luminal compartment and preventing germ cell components crossing the barrier to elicit an immunological response in the body. Still, immunosuppressive factors exist and play a pivotal role in successful fertilisation, implantation and fetal growth by protecting female and male reproductive cells from the immunosurveillance system. Since the female genital tract is not an immunologically privileged site, the presence within seminal fluid of powerful immunosuppressive agents is called for. The activity of seminal plasma in this regard has been measured by suppression of the proliferation of lymphocytes that have been activated by mitogen and also by suppression of natural killer (NK) cell activity. The studies of inhibition of NK cell function have all concluded that the prostaglandins in semen are responsible for the inhibition. However, in studies that used the mitogen induced lymphoproliferation assay, activity was detected in high molecular weight fractions (Lee and Ha 1989), and this led to some confusion about the active substances in human semen. Kelly et al. (1991) solved this issue by identifying the high molecular weight immunosuppressive factor as prostasomes. The prostasomes not only inhibited lymphoproliferation but also the ability of macrophages to phagocytose latex beads. As a matter of fact, prostasomes bound rapidly to the leukocyte plasma membrane followed by endocytosis that was mediated by an undefined plasma membrane receptor. Interactions of prostasomes with neutrophils and monocytes inhibited their ability to phagocytose latex particles (Skibinski et al. 1992).
NK cells may represent an important component of innate immunity in the female reproductive tract, and the role of prostasomes in the regulation of NK cell activity showed that prostasomes expressed high levels of CD48, the ligand for the activating receptor CD244 (Tarazona et al. 2011). The interactions between NK cells and prostasomes resulted in a decrease of CD244 expression. Furthermore, the decreased NK cell activity observed in NK cells cultured in the presence of prostasomes suggested that prostasomes may immunomodulate the local environment within the female reproductive tract preventing immune-mediated sperm destruction and prolonging their survival rate (Tarazona et al. 2011). Hence, prostasomes play a significant role in neutralising immune defences against sperm cells in the hostile environment that the female reproductive tract constitutes.
The complement system consists of about 30 plasma and cellular proteins (receptors and regulators) with a primary function in host defence, to differentiate between self and nonself, and as a purging system of the body. The complement system destroys invading foreign cells and organisms, either by direct lysis or by recruitment of leukocytes. The main event in the activation of complement is the activation of C3 into C3b and C3a. This is achieved by two enzyme complexes called convertases, and the classic pathway is triggered by the formation of antigen–antibody complexes (Doekes et al. 1984). The complement system is controlled by several soluble and membrane-bound regulators. Most of the regulators are members of the “regulators of complement activation” (RCA) superfamily encoded by a gene cluster on chromosome 1 that mainly regulates the two types of convertases. Decay accelerating factor (DAF, CD55) and membrane cofactor protein (MCP, CD46) belong to this family. CD59 antigen is an 18–20 kDa membrane protein that is a regulator of membrane attack complex (MAC). Seminal plasma is known to have complement-inhibiting activity, (Tarter and Alexander 1984) and the trophoblast/leukocyte common (TLX) antigen occurring on sperm cells (Anderson et al. 1989) is identical with the above-mentioned MCP, an inhibitor of complement activation (Purcell et al. 1990). Rooney et al. described that all detectable CD59 in seminal plasma was associated with prostasomes representing a protection to sperm cells by being a pool of CD59 from which protein lost from sperm cells, perhaps as a result of low level complement attack or of normal membrane turnover, can be replenished (Rooney et al. 1993). CD46 and CD55 were subsequently found to be prostasome-associated as well (Rooney et al. 1996). These findings should be considered in the light of findings suggesting a functionally active complement system in the female reproductive tract (Jin et al. 1991). Therefore, it is reasonable to conclude that sperm cells are at risk within the female reproductive tract. The interaction of prostasomes with the local female immune system including immunomodulatory skills exerted by prostasomal small RNA biotypes (Vojtech et al. 2014) may prevent sperm cells from being phagocytosed, damaged or killed and therewith prolongs their lifespan in an otherwise hostile environment.
Sperm Cell Immunogenicity
Antisperm Antibodies
The addition of carbohydrates (glycosylation) is the most common form of covalent posttranslational modification of newly synthesised proteins. A variety of functions can be ascribed to the carbohydrate modifications of proteins. The common feature of the varied functions is that they either mediate specific recognition events (e.g. cell–cell, cell–matrix, immune responses) or that they modulate protein function (e.g. ligand binding; intra- and intercellular trafficking) (Varki 1993). Important constituents of a biological membrane (including that of the sperm cell) are peripherally located and integral proteins. Therefore, surface coat antigens that are peripherally associated with the sperm plasma membrane should be distinguished from those linked to integral plasma membrane proteins. A salient feature of this reasoning is the maintenance of the strict distinction between on the one hand immunity to sperm surface antigens that could theoretically play a role in gamete interactions leading to fertilisation and, on the other hand, due to, e.g. steric hindrance, hidden antigens of sperm cells that would not (Bronson 1999). The other variable in this complex of problems is that the immunoglobulin class responsible for the reactivity with the sperm cell antigens is not a single one but rather three (IgG, IgA and IgM) that appear in different concentrations, among which antisperm IgM in blood plasma hardly enters the male genital tract secretions due to its ungainly molecular structure (Bronson 1999). Despite the antigenic nature of sperm cells, the vast majority of males do not produce antisperm antibodies (ASA). This would in turn presuppose mechanisms that suppress this reaction, involving genital tract structures and sperm cells themselves. Freshly ejaculated and capacitated human sperm cells have been claimed to react with different types of ASA (Fusi and Bronson 1990). The alterations in antigenicity associated with capacitation may reflect a surface redistribution associated with changes in the functional state of sperm cells. ASA, which are more frequent than oocyte antibodies, may fulfil the criteria of an autoimmune disease in the male (Omu et al. 1999). ASA can inhibit fertility by interfering with motility, penetration of the cervical mucus, capacitation/acrosomal exocytosis, zona pellucida binding and sperm–oocyte fusion (Bohring and Krause 2003; Naz and Butler 2013). ASA are prevalent in the infertile male population, and the presence of ASA in males can reduce fecundity, but the causality is not strictly clear. It should be mentioned that ASA occur in several body fluids like seminal plasma and bound to the sperm cell surface and blood plasma of men and women but also in oviduct fluid, cervical mucus and follicular fluid of women. The presence of ASA has been described in 1.2–19 % of fertile couples, suggesting that not all ASA cause infertility. ASA from infertile patients may be directed to dissimilar sperm antigens and/or clusters of antigens or possess different antigen-binding characteristics differing from those of fertile individuals (Chamley and Clarke 2007). Bohring et al. (2001) investigated seminal plasma samples from 20 infertile patients, and 18 proteins associated with sperm membranes were detected as antigens and 6 proteins were biochemically identified.
Circulating ASA Recognise Prostasomes
A prostasome coat on swim-up sperm cells was found when we immunostained the samples with seven different monoclonal antibodies directed against purified prostasomes and all monoclonal antibodies tested demonstrated a similar staining pattern (Allegrucci et al. 2001). Therefore, the probability of a possible cross-reactivity with some other seminal plasma component was weak and prompted us to suggest that prostasomes may be a candidate antigen for ASA and we raised polyclonal chicken antibodies against purified seminal prostasomes (Allegrucci et al. 2001). Chicken antibodies have the advantage that they eliminate interference caused by the human complement system, rheumatoid factor and cellular Fc receptors and they resemble human autoantibodies in their reactivity. Human sperm cells incubated with increasing concentrations of chicken polyclonal antiprostasome antibodies caused approximately 80 % of the sperm cells to agglutinate. The agglutination displayed several types of sperm formation, mostly tail to tail contacts, but the design of interaction was dependent upon the concentration of the antiprostasome antibody. It should be pointed out that the agglutination of sperm cells by the chicken prostasome antibody was similar to the agglutination caused by sera from patients having ASA. When antiprostasome antibodies were preincubated with high concentrations of prostasomes, no agglutination was observed during the subsequent contact with sperm cells (Allegrucci et al. 2001) herewith underlining the specific role of prostasomes as antigens in this context. IgG antibodies against sperm cells were detected in the 20 sera of ASA-positive patients investigated for infertility (15 men and 5 women). In the majority of cases (90 %), the sera of the patients elicited complement activation, measured by the deposition of C3 on the sperm cells. A significant positive correlation was found between sperm cell-bound C3 and ASA titer and especially between deposition of C3 and IgG (Allegrucci et al. 2001). ASA of the IgG type in serum of infertile men and women do recognise prostasomes as antigens, and prostasomes are strongly immunogenic and they should not be overlooked in immunological infertility assessment.
As already mentioned, prostasomes have the ability to adhere to sperm cells albeit that both prostasomes and sperm cells have a net negative surface charge of their membranes favouring repulsive forces. This propensity of sperm cells to carry attached prostasomes, allowed us to regard prostasomes as a new category of sperm antigens (Carlsson et al. 2004a). In a study of the reactivity of ASA-positive sera from 116 suspected immunoinfertile patients, a binding of IgG antibodies to prostasomes was clearly visible in 113 patient sera (97 %) (Carlsson et al. 2004b). Those sera with well-expressed antibody reaction showed 3–10 different prostasomal bands in one-dimensional electrophoresis as antigens. Serum samples from male and female patients agreed with each other, and no difference was observed between sexes (Carlsson et al. 2004b). This reflects a high conformity taking into consideration that the ASA assay was carried out on fresh sperm cells, while the prostasomal antigen represented frozen material that had been thawed before testing. Twenty sera from the 116 suspected immunoinfertile patients with highest titres of antiprostasome autoantibodies were selected for the identification of the proteins corresponding to the prostasomal antigens. Two-dimensional, silver-stained SDS-PAGE gel of separated prostasomal proteins revealed over 200 protein spots in the molecular mass range of 8–180 kDa, and approximately 70 % of the spots displayed isoelectric points (IPs) below 7, i.e. reflecting anionic proteins (Carlsson et al. 2004c). On immunoblotting, most of the ASA-positive sera recognised 3–10 spots in the molecular mass range between 17 and 70 kDa. The size distribution of all protein spots recognised by patient sera after immunoblotting revealed that 19 out of 20 (95 %) of the serum samples discerned protein(s) with a molecular mass range of approximately 17 kDa. Another protein spot with a molecular mass of approximately 40 kDa was found in 17 out of 20 (85 %) of the serum samples. Proteins in 12 spots were identified and are summarised with their theoretical IPs, molecular masses and accession numbers in Table 9.2. The two most frequent antigens are prolactin-inducible protein (PIP), recognised by 19 out of 20 (95 %) patient sera, and corresponding figures for clusterin are 17 out of 20 (85 %) patient sera (Table 9.2). The remaining ten prostasomal antigens were sporadically occurring and identified as given in Table 9.2. A comparison of this study is feasible with that of Bohring et al. (2001) who investigated seminal plasma samples from 20 infertile patients. It should be noted though, that we used blood sera to avoid the problem of antigen excess, due to the huge representation of prostasomes in seminal plasma. The former authors detected 18 proteins associated with sperm membrane antigens, and six proteins were biochemically identified (Bohring et al. 2001), but none of these proteins associated with sperm membranes and recognised by ASA were identical with the 12 proteins associated with prostasomal membranes and yet recognised by ASA. The clearly disproportionate occurrence of only two immunodominant prostasomal antigens (PIP and clusterin) at the expense of the remaining ten sporadically occurring prostasomal proteins deserves careful consideration. It should be mentioned though that Thacker et al. (2011) identified in a pilot study the presence of four major proteins in human seminal plasma that were unique and different in fertile and infertile men. These were PIP, clusterin isoform, prostate-specific antigen (PSA) isoform 1 preprotein and semenogelin II precursor. However, immunoblot analyses of individual serum samples containing ASA from infertile couples generally reveal a certain degree of heterogeneity of antigens recognised by human sperm auto- and isoantibodies (Mathur et al. 1988). Shetty et al. (1999) adopted high-resolution two-dimensional electrophoresis with separation of sperm cell antigens in the first dimension by either isoelectric focusing or nonequilibrium pH gradient electrophoresis to screen a range of proteins with different isoelectric points, followed by SDS-PAGE and a sensitive Western blotting method. They found that a number of antigenic spots among both anionic and cationic proteins were reactive with sera from infertile but not fertile individuals reflecting a considerable diversity in the ASA composition of infertile males and females.
Table 9.2.
Twelve prostasome immunogens recognised by antisperm antisera of 20 patients with suspected immunological infertility in two-dimensional gel electrophoresis. Identification was carried out by MALDI analyses
| No. | pI | Mass (Da) | Accession No. | Protein name |
|---|---|---|---|---|
| 1 | 7.0 | 46,800 | Q13584 | Isocitrate dehydrogenase [NADP] |
| 2 | 5.9 | 86,600 | Q99728 | BRCA1-associated RING domain protein 1 |
| 3 | 6.3 | 36,500 | P10909 | Clusterin |
| 4 | 7.0 | 36,600 | P14550 | Alcohol dehydrogenase [NADP(+)] |
| 5 | 5.7 | 31,100 | O94760 | N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 |
| 6 | 6.8 | 38,700 | P04083 | Annexin A1 |
| 7 | 6.0 | 36,400 | P12429 | Annexin A3 |
| 8 | 7.4 | 32,400 | O00560 | Syntenin-1 |
| 9 | 6.5 | 22,800 | P04792 | Heat shock protein beta-1 (HSP27) |
| 10 | 5.2 | 20,800 | Q04760 | Lactoylglutathione lyase |
| 11 | 5.4 | 21,900 | P32119 | Peroxiredoxin-2 |
| 12 | 5.3 | 16,600 | P12273 | Prolactin-inducible protein |
From Carlsson et al. (2004c)pI = isoelectric point
The two immunodominant prostasomal antigens (PIP and clusterin) are widely expressed proteins. PIP is a 17 kDa glycoprotein (gp17). It exerts multiple important functions in biological systems and is involved in fertility, immunoregulation, antimicrobial activity, apoptosis and tumour progression. PIP has been identified in prostatic secretion and therewith in seminal plasma (Autiero et al. 1995) which is a rich source for its isolation and characterisation (Chiu and Chamley 2003). The binding ability of PIP to the Fc fragment of IgG has improved the understanding of the functional role of PIP in seminal plasma (Witkin et al. 1983). The reduced level of PIP might be associated with infertility, especially in men with ASA (Bronson 1999). Secreted PIP in seminal plasma has been found to have its localisation on the sperm cell surface (Bergamo et al. 1997). PIP is able to bind to CD4+ T-cell receptor and to block CD4-mediated T-cell programmed death which means that PIP may act as a modulator in an immune response reaction (Gaubin et al. 1999). PIP was among some candidate galectin-3 ligands in prostasomes that were identified by tandem mass spectrometry of proteins that co-purified with galectin-3 during lactose affinity chromatography of the membrane fraction containing solubilised human prostasomes (Block et al. 2011). Galectin-3 is a beta-galactoside-binding protein involved in immunomodulation, cell interactions and cancer progression. The intact galectin-3 molecule contains a carbohydrate recognition domain and a non-lectin domain that interacts with protein and lipid moieties. It has a firm association to human prostasomes and more precisely to the prostasome surface (Block et al. 2011), meaning that also PIP may have a surface localisation on human prostasomes. Hence, the multiple functions of galectin-3 are exerted through ligand binding with specific galectin-3 ligands involved (Ochieng et al. 2004). Galectin-3 ligands in human prostasomes were purified, identified and characterised by Kovak et al. (2013), and using a proteomic approach, clusterin as well was among the candidate galectin-3 binding ligands.
Clusterin has been found in all body fluids and is a major heterodimeric glycoprotein in mammalian semen. It is synthesised and secreted by a wide variety of cells in different species. Clusterin was named for its ability to elicit clustering among Sertoli cells (Blaschuk et al. 1983). Two forms of clusterin have been identified, viz. the secretory form and the nuclear form. The secretory form is synthesised as a 50–60 kDa protein precursor that is glycosylated and proteolytically cleaved into two subunits (alpha and beta chains) (Leskov et al. 2003). The nuclear form is expressed as a 49 kDa inactive protein precursor that does not undergo a proteolytic cleavage (Reddy et al. 1996). The soluble form of clusterin is present in normal human sperm cells (Thacker et al. 2011), and it has also been observed on the surfaces of immature, low-motile and morphologically aberrant sperm cells (O’Bryan et al. 1994). Clusterin participates in many biological processes such as cell–cell interactions, sperm maturation, agglutination of abnormal sperm cells, membrane recycling, apoptosis and lipid transportation and controls complement-induced sperm cell lysis (Silkensen et al. 1999; Wong et al. 1993). Using a panel of polyclonal and monoclonal antibodies against different parts of the clusterin molecule, Lakins et al. (1998) inferred that in normal rat prostate, clusterin has at least five different glyco/isoforms: fully glycosylated mature pro-protein (76 kDa), cleaved fully glycosylated alpha and beta chains (32 and 48 kDa), two intermediate uncleaved processing forms of pro-protein [presumably the high-mannose (64 kDa) and low-mannose species of clusterin (56 kDa) and full length unglycosylated holoprotein (50 kDa)]. It means that prostate-derived clusterin may act in different immunological shapes herewith including its probable presence at the surface of both sperm cells and prostasomes.
Acknowledgement
The author apologises to those colleagues whose work was not cited due to space limitations.
Contributor Information
Richard Bronson, Phone: +1631-444-2731, Email: Richard.Bronson@stonybrookmedicine.edu.
Gunnar Ronquist, Email: gunnar.ronquist@akademiska.se
References
- Aalberts M, et al. Identification of distinct populations of prostasomes that differentially express prostate stem cell antigen, annexin A1, and GLIPR2 in humans. Biol Reprod. 2012;86(3):82. doi: 10.1095/biolreprod.111.095760. [DOI] [PubMed] [Google Scholar]
- Aalberts M, et al. Spermatozoa recruit prostasomes in response to capacitation induction. Biochim Biophys Acta. 2013;1834(11):2326–2335. doi: 10.1016/j.bbapap.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Aalberts M, Stout TA, Stoorvogel W. Prostasomes: extracellular vesicles from the prostate. Reproduction. 2014;147(1):R1–R14. doi: 10.1530/REP-13-0358. [DOI] [PubMed] [Google Scholar]
- Agerberth B, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96(9):3086–3093. [PubMed] [Google Scholar]
- Aitken RJ, et al. Relationship between the movement characteristics of human spermatozoa and their ability to penetrate cervical mucus and zona-free hamster oocytes. J Reprod Fertil. 1985;73(2):441–449. doi: 10.1530/jrf.0.0730441. [DOI] [PubMed] [Google Scholar]
- Allegrucci C, et al. Circulating human antisperm antibodies recognize prostasomes. Am J Reprod Immunol. 2001;46(3):211–219. doi: 10.1034/j.1600-0897.2001.d01-4.x. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Michaelson JS, Johnson PM. Trophoblast/leukocyte-common antigen is expressed by human testicular germ cells and appears on the surface of acrosome-reacted sperm. Biol Reprod. 1989;41(2):285–293. doi: 10.1095/biolreprod41.2.285. [DOI] [PubMed] [Google Scholar]
- Andersson E, et al. Isolation of human cationic antimicrobial protein-18 from seminal plasma and its association with prostasomes. Hum Reprod. 2002;17(10):2529–2534. doi: 10.1093/humrep/17.10.2529. [DOI] [PubMed] [Google Scholar]
- Arienti G, et al. Progesterone-induced increase of sperm cytosolic calcium is enhanced by previous fusion of spermatozoa to prostasomes. Cell Calcium. 2001;30(3):222–227. doi: 10.1054/ceca.2001.0229. [DOI] [PubMed] [Google Scholar]
- Arienti G, et al. Role of human prostasomes in the activation of spermatozoa. J Cell Mol Med. 2004;8(1):77–84. doi: 10.1111/j.1582-4934.2004.tb00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidson G, et al. Human prostasome membranes exhibit very high cholesterol/phospholipid ratios yielding high molecular ordering. Biochim Biophys Acta. 1989;984(2):167–173. doi: 10.1016/0005-2736(89)90212-5. [DOI] [PubMed] [Google Scholar]
- Autiero M, et al. A 17-kDa CD4-binding glycoprotein present in human seminal plasma and in breast tumor cells. Eur J Immunol. 1995;25(5):1461–1464. doi: 10.1002/eji.1830250550. [DOI] [PubMed] [Google Scholar]
- Bergamo P, et al. CD4-mediated anchoring of the seminal antigen gp17 onto the spermatozoon surface. Hum Immunol. 1997;58(1):30–41. doi: 10.1016/S0198-8859(97)00213-9. [DOI] [PubMed] [Google Scholar]
- Blaschuk O, Burdzy K, Fritz IB. Purification and characterization of a cell-aggregating factor (clusterin), the major glycoprotein in ram rete testis fluid. J Biol Chem. 1983;258(12):7714–7720. [PubMed] [Google Scholar]
- Block AS, et al. Co-purification of Mac-2 binding protein with galectin-3 and association with prostasomes in human semen. Prostate. 2011;71(7):711–721. doi: 10.1002/pros.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohring C, Krause W. Immune infertility: towards a better understanding of sperm (auto)-immunity. The value of proteomic analysis. Hum Reprod. 2003;18(5):915–924. doi: 10.1093/humrep/deg207. [DOI] [PubMed] [Google Scholar]
- Bohring C, et al. Isolation and identification of sperm membrane antigens recognized by antisperm antibodies, and their possible role in immunological infertility disease. Mol Hum Reprod. 2001;7(2):113–118. doi: 10.1093/molehr/7.2.113. [DOI] [PubMed] [Google Scholar]
- Bronson RA. Antisperm antibodies: a critical evaluation and clinical guidelines. J Reprod Immunol. 1999;45(2):159–183. doi: 10.1016/S0165-0378(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Carlsson L, et al. Antibacterial activity of human prostasomes. Prostate. 2000;44(4):279–286. doi: 10.1002/1097-0045(20000901)44:4<279::AID-PROS4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Carlsson L, et al. Prostasome antigens as targets for sperm agglutinating antibodies demonstrated by 1-D gel electrophoresis and immunoblottings. Int J Androl. 2004;27(6):360–367. doi: 10.1111/j.1365-2605.2004.00468.x. [DOI] [PubMed] [Google Scholar]
- Carlsson L, et al. A new test for immunological infertility: an ELISA based on prostasomes. Int J Androl. 2004;27(3):130–133. doi: 10.1111/j.1365-2605.2004.00458.x. [DOI] [PubMed] [Google Scholar]
- Carlsson L, et al. Dominant prostasome immunogens for sperm-agglutinating autoantibodies of infertile men. J Androl. 2004;25(5):699–705. doi: 10.1002/j.1939-4640.2004.tb02844.x. [DOI] [PubMed] [Google Scholar]
- Chamley LW, Clarke GN. Antisperm antibodies and conception. Semin Immunopathol. 2007;29(2):169–184. doi: 10.1007/s00281-007-0075-2. [DOI] [PubMed] [Google Scholar]
- Chiu WW, Chamley LW. Human seminal plasma prolactin-inducible protein is an immunoglobulin G-binding protein. J Reprod Immunol. 2003;60(2):97–111. doi: 10.1016/S0165-0378(03)00084-6. [DOI] [PubMed] [Google Scholar]
- Doekes G, van Es LA, Daha MR. Binding and activation of the first complement component by soluble immune complexes: effect of complex size and composition. Scand J Immunol. 1984;19(2):99–110. doi: 10.1111/j.1365-3083.1984.tb00905.x. [DOI] [PubMed] [Google Scholar]
- Fabiani R, et al. Enhanced recruitment of motile spermatozoa by prostasome inclusion in swim-up medium. Hum Reprod. 1994;9(8):1485–1489. doi: 10.1093/oxfordjournals.humrep.a138735. [DOI] [PubMed] [Google Scholar]
- Fusi F, Bronson RA. Effects of incubation time in serum and capacitation on spermatozoal reactivity with antisperm antibodies. Fertil Steril. 1990;54(5):887–893. doi: 10.1016/s0015-0282(16)53951-x. [DOI] [PubMed] [Google Scholar]
- Gaubin M, et al. Potent inhibition of CD4/TCR-mediated T cell apoptosis by a CD4-binding glycoprotein secreted from breast tumor and seminal vesicle cells. J Immunol. 1999;162(5):2631–2638. [PubMed] [Google Scholar]
- Ho K, Wolff CA, Suarez SS. CatSper-null mutant spermatozoa are unable to ascend beyond the oviductal reservoir. Reprod Fertil Dev. 2009;21(2):345–350. doi: 10.1071/RD08183. [DOI] [PubMed] [Google Scholar]
- Hoover P, Naz RK. Do men with prostate abnormalities (prostatitis/benign prostatic hyperplasia/prostate cancer) develop immunity to spermatozoa or seminal plasma? Int J Androl. 2012;35(4):608–615. doi: 10.1111/j.1365-2605.2011.01246.x. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Gagnon C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil Steril. 1992;57(2):409–416. doi: 10.1016/s0015-0282(16)54855-9. [DOI] [PubMed] [Google Scholar]
- Jin M, Larsson A, Nilsson BO. A functionally active complement system is present in uterine secretion of the mouse prior to implantation. Am J Reprod Immunol. 1991;26(2):53–57. doi: 10.1111/j.1600-0897.1991.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Kelly RW, et al. Extracellular organelles (prostasomes) are immunosuppressive components of human semen. Clin Exp Immunol. 1991;86(3):550–556. doi: 10.1111/j.1365-2249.1991.tb02968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovak MR, et al. Proteomic identification of galectin-3 binding ligands and characterization of galectin-3 proteolytic cleavage in human prostasomes. Andrology. 2013;1(5):682–691. doi: 10.1111/j.2047-2927.2013.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakins J, et al. Clusterin biogenesis is altered during apoptosis in the regressing rat ventral prostate. J Biol Chem. 1998;273(43):27887–27895. doi: 10.1074/jbc.273.43.27887. [DOI] [PubMed] [Google Scholar]
- Larrick JW, et al. A novel granulocyte-derived peptide with lipopolysaccharide-neutralizing activity. J Immunol. 1994;152(1):231–240. [PubMed] [Google Scholar]
- Lee HK, Ha TY. Human seminal plasma suppresses delayed-type hypersensitivity responses to intravaginally deposited sheep red blood cells and sperm: separation of immunosuppressive factors. Int Arch Allergy Appl Immunol. 1989;88(4):412–419. doi: 10.1159/000234726. [DOI] [PubMed] [Google Scholar]
- Leskov KS, et al. Synthesis and functional analyses of nuclear clusterin, a cell death protein. J Biol Chem. 2003;278(13):11590–11600. doi: 10.1074/jbc.M209233200. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011;471(7338):387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- Luria A, et al. Extracellular adenosine triphosphate stimulates acrosomal exocytosis in bovine spermatozoa via P2 purinoceptor. Biol Reprod. 2002;66(2):429–437. doi: 10.1095/biolreprod66.2.429. [DOI] [PubMed] [Google Scholar]
- Malm J, et al. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect Immun. 2000;68(7):4297–4302. doi: 10.1128/IAI.68.7.4297-4302.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiani S, et al. Characterization of M540 bodies in human semen: evidence that they are apoptotic bodies. Mol Hum Reprod. 2007;13(9):621–631. doi: 10.1093/molehr/gam046. [DOI] [PubMed] [Google Scholar]
- Mathur S, et al. Special antigens on sperm from autoimmune infertile men. Am J Reprod Immunol Microbiol. 1988;17(1):5–13. doi: 10.1111/j.1600-0897.1988.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Metz-Boutigue MH, et al. Antibacterial peptides are present in chromaffin cell secretory granules. Cell Mol Neurobiol. 1998;18(2):249–266. doi: 10.1023/A:1022573004910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, et al. CD26 and adenosine deaminase interaction: its role in the fusion between horse membrane vesicles and spermatozoa. Biol Reprod. 1999;61(3):802–808. doi: 10.1095/biolreprod61.3.802. [DOI] [PubMed] [Google Scholar]
- Naz RK, Butler TS. Antibodies to prostate-specific antigen in immunoinfertile women and men. J Reprod Immunol. 2013;97(2):217–222. doi: 10.1016/j.jri.2012.11.005. [DOI] [PubMed] [Google Scholar]
- O’Bryan MK, et al. The use of anticlusterin monoclonal antibodies for the combined assessment of human sperm morphology and acrosome integrity. Hum Reprod. 1994;9(8):1490–1496. doi: 10.1093/oxfordjournals.humrep.a138736. [DOI] [PubMed] [Google Scholar]
- Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2004;19(7–9):527–535. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- Omu AE, et al. Relationship between unexplained infertility and human leukocyte antigens and expression of circulating autogeneic and allogeneic antisperm antibodies. Clin Exp Obstet Gynecol. 1999;26(3–4):199–202. [PubMed] [Google Scholar]
- Palmerini CA, et al. Increase of human spermatozoa intracellular Ca2+ concentration after fusion with prostasomes. Cell Calcium. 1999;25(4):291–296. doi: 10.1054/ceca.1999.0031. [DOI] [PubMed] [Google Scholar]
- Palmerini CA, et al. Fusion of prostasomes to human spermatozoa stimulates the acrosome reaction. Fertil Steril. 2003;80(5):1181–1184. doi: 10.1016/S0015-0282(03)02160-5. [DOI] [PubMed] [Google Scholar]
- Park KH, et al. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci Signal. 2011;4(173):ra31. doi: 10.1126/scisignal.2001595. [DOI] [PubMed] [Google Scholar]
- Publicover S, Harper CV, Barratt C. [Ca2+]i signalling in sperm–making the most of what you’ve got. Nat Cell Biol. 2007;9(3):235–242. doi: 10.1038/ncb0307-235. [DOI] [PubMed] [Google Scholar]
- Purcell DF, et al. The human cell-surface glycoproteins HuLy-m5, membrane co-factor protein (MCP) of the complement system, and trophoblast leucocyte-common (TLX) antigen, are CD46. Immunology. 1990;70(2):155–161. [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KB, et al. Transforming growth factor beta (TGF beta)-induced nuclear localization of apolipoprotein J/clusterin in epithelial cells. Biochemistry. 1996;35(19):6157–6163. doi: 10.1021/bi952981b. [DOI] [PubMed] [Google Scholar]
- Renneberg H, et al. Identification and characterization of neutral endopeptidase (EC 3. 4. 24. 11) from human prostasomes–localization in prostatic tissue and cell lines. Prostate. 2001;46(3):173–183. doi: 10.1002/1097-0045(20010215)46:3<173::AID-PROS1021>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Ronquist G. Effect of modulators on prostasome membrane-bound ATPase in human seminal plasma. Eur J Clin Invest. 1987;17(3):231–236. doi: 10.1111/j.1365-2362.1987.tb01241.x. [DOI] [PubMed] [Google Scholar]
- Ronquist G. Prostasomes are mediators of intercellular communication: from basic research to clinical implications. J Intern Med. 2012;271(4):400–413. doi: 10.1111/j.1365-2796.2011.02487.x. [DOI] [PubMed] [Google Scholar]
- Ronquist G, Brody I. The prostasome: its secretion and function in man. Biochim Biophys Acta. 1985;822(2):203–218. doi: 10.1016/0304-4157(85)90008-5. [DOI] [PubMed] [Google Scholar]
- Ronquist G, Hedstrom M. Restoration of detergent-inactivated adenosine triphosphatase activity of human prostatic fluid with concanavalin A. Biochim Biophys Acta. 1977;483(2):483–486. doi: 10.1016/0005-2744(77)90078-X. [DOI] [PubMed] [Google Scholar]
- Ronquist G, Nilsson BO, Hjerten S. Interaction between prostasomes and spermatozoa from human semen. Arch Androl. 1990;24(2):147–157. doi: 10.3109/01485019008986874. [DOI] [PubMed] [Google Scholar]
- Ronquist KG, et al. Human prostasomes contain chromosomal DNA. Prostate. 2009;69(7):737–743. doi: 10.1002/pros.20921. [DOI] [PubMed] [Google Scholar]
- Ronquist GK, et al. Prostasomal DNA characterization and transfer into human sperm. Mol Reprod Dev. 2011;78(7):467–476. doi: 10.1002/mrd.21327. [DOI] [PubMed] [Google Scholar]
- Ronquist GK, et al. Prostasomes are heterogeneous regarding size and appearance but affiliated to one DNA-containing exosome family. Prostate. 2012;72(16):1736–1745. doi: 10.1002/pros.22526. [DOI] [PubMed] [Google Scholar]
- Ronquist KG, et al. Prostasomes from four different species are able to produce extracellular adenosine triphosphate (ATP) Biochim Biophys Acta. 2013;1830(10):4604–4610. doi: 10.1016/j.bbagen.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Rooney IA, et al. Physiologic relevance of the membrane attack complex inhibitory protein CD59 in human seminal plasma: CD59 is present on extracellular organelles (prostasomes), binds cell membranes, and inhibits complement-mediated lysis. J Exp Med. 1993;177(5):1409–1420. doi: 10.1084/jem.177.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney IA, Heuser JE, Atkinson JP. GPI-anchored complement regulatory proteins in seminal plasma. An analysis of their physical condition and the mechanisms of their binding to exogenous cells. J Clin Invest. 1996;97(7):1675–1686. doi: 10.1172/JCI118594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez F, et al. Antioxidant capacity of prostasomes in human semen. Mol Hum Reprod. 1998;4(7):667–672. doi: 10.1093/molehr/4.7.667. [DOI] [PubMed] [Google Scholar]
- Saez F, et al. Prostasomes inhibit the NADPH oxidase activity of human neutrophils. Mol Hum Reprod. 2000;6(10):883–891. doi: 10.1093/molehr/6.10.883. [DOI] [PubMed] [Google Scholar]
- Sahlen GE, et al. Ultrastructure of the secretion of prostasomes from benign and malignant epithelial cells in the prostate. Prostate. 2002;53(3):192–199. doi: 10.1002/pros.10126. [DOI] [PubMed] [Google Scholar]
- Shetty J, et al. Human sperm proteome: immunodominant sperm surface antigens identified with sera from infertile men and women. Biol Reprod. 1999;61(1):61–69. doi: 10.1095/biolreprod61.1.61. [DOI] [PubMed] [Google Scholar]
- Silkensen JR, et al. Identification of clusterin sequences mediating renal tubular cell interactions. J Pept Res. 1999;54(5):449–457. doi: 10.1034/j.1399-3011.1999.00145.x. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Skibinski G, et al. Immunosuppression by human seminal plasma–extracellular organelles (prostasomes) modulate activity of phagocytic cells. Am J Reprod Immunol. 1992;28(2):97–103. doi: 10.1111/j.1600-0897.1992.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Skibinski G, Kelly RW, James K. Expression of a common secretory granule specific protein as a marker for the extracellular organelles (prostasomes) in human semen. Fertil Steril. 1994;61(4):755–759. doi: 10.1016/s0015-0282(16)56658-8. [DOI] [PubMed] [Google Scholar]
- Stegmayr B, Ronquist G. Promotive effect on human sperm progressive motility by prostasomes. Urol Res. 1982;10(5):253–257. doi: 10.1007/BF00255932. [DOI] [PubMed] [Google Scholar]
- Stegmayr B, et al. Calcium, magnesium, and zinc contents in organelles of prostatic origin in human seminal plasma. Scand J Urol Nephrol. 1982;16(3):199–203. doi: 10.3109/00365598209179753. [DOI] [PubMed] [Google Scholar]
- Stridsberg M, et al. Prostasomes are neuroendocrine-like vesicles in human semen. Prostate. 1996;29(5):287–295. doi: 10.1002/(SICI)1097-0045(199611)29:5<287::AID-PROS3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Tarazona R, et al. Human prostasomes express CD48 and interfere with NK cell function. Immunobiology. 2011;216(1–2):41–46. doi: 10.1016/j.imbio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Tarter TH, Alexander NJ. Complement-inhibiting activity of seminal plasma. Am J Reprod Immunol. 1984;6(1):28–32. doi: 10.1111/j.1600-0897.1984.tb00105.x. [DOI] [PubMed] [Google Scholar]
- Teves ME, et al. Progesterone at the picomolar range is a chemoattractant for mammalian spermatozoa. Fertil Steril. 2006;86(3):745–749. doi: 10.1016/j.fertnstert.2006.02.080. [DOI] [PubMed] [Google Scholar]
- Thacker S, et al. Evaluation of sperm proteins in infertile men: a proteomic approach. Fertil Steril. 2011;95(8):2745–2748. doi: 10.1016/j.fertnstert.2011.03.112. [DOI] [PubMed] [Google Scholar]
- Trajkovic K, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- Vanhoof G, et al. Distribution of proline-specific aminopeptidases in human tissues and body fluids. Eur J Clin Chem Clin Biochem. 1992;30(6):333–338. doi: 10.1515/cclm.1992.30.6.333. [DOI] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraghavan S, Hoskins D. Quantitation of bovine sperm cytoplasmic calcium with Quin-2 and Fura-2: evidence that external calcium does not have direct access to the sperm cytoplasm. Cell Calcium. 1989;10(4):241–253. doi: 10.1016/0143-4160(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Visconti PE. Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci USA. 2009;106(3):667–668. doi: 10.1073/pnas.0811895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtech L, et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014;42(11):7290–7304. doi: 10.1093/nar/gku347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MJ, et al. Prostate specific origin of dipeptidylpeptidase IV (CD-26) in human seminal plasma. J Urol. 1998;160(5):1905–1909. doi: 10.1016/S0022-5347(01)62441-8. [DOI] [PubMed] [Google Scholar]
- Witkin SS, et al. An IgG-Fc binding protein in seminal fluid. Am J Reprod Immunol. 1983;3(1):23–27. doi: 10.1111/j.1600-0897.1983.tb00207.x. [DOI] [PubMed] [Google Scholar]
- Wong P, et al. Genomic organization and expression of the rat TRPM-2 (clusterin) gene, a gene implicated in apoptosis. J Biol Chem. 1993;268(7):5021–5031. [PubMed] [Google Scholar]


