Abstract
Picornaviruses, which include the human rhinoviruses (HRVs) and enteroviruses (EVs), are the most frequent cause of acute human illness worldwide. HRVs are the most prevalent cause of acute respiratory tract illnesses (ARIs) which usually commence in the upper respiratory tract (URT). ARIs are the leading cause of morbidity in children under 5 years and occur in all seasons. ARIs linked to HRV infections are associated with excessive and perhaps inappropriate antibiotic prescribing and with significant direct and indirect healthcare expenditure. ARI incidence is highest in the first 2 years of life, with up to thirteen episodes per year including up to six positive for an HRV, and it is not uncommon to average one infection per child-month.
Keywords: Chronic Obstructive Pulmonary Disease, Lower Respiratory Tract, Common Cold, Respiratory Virus, Human Respiratory Syncytial Virus
Introduction
Picornaviruses, which include the human rhinoviruses (HRVs) and enteroviruses (EVs), are the most frequent cause of acute human illness worldwide [1]. HRVs are the most prevalent cause of acute respiratory tract illnesses (ARIs) which usually commence in the upper respiratory tract (URT). ARIs are the leading cause of morbidity in children under 5 years and occur in all seasons [2, 3]. ARIs linked to HRV infections are associated with excessive and perhaps inappropriate antibiotic prescribing [4] and with significant direct and indirect healthcare expenditure [5, 6]. ARI incidence is highest in the first 2 years of life, with up to 13 episodes per year including up to six positive for an HRV, and it is not uncommon to average one infection per child-month [3, 7–9]. In preschool-aged children, nearly 50 % of general practitioner visits are for ARI [10], many of which are self-limiting. ARIs can often be managed in the community with supportive care from parents, but complications can arise that require a medical visit for management of asthma, otitis media, or sinusitis [11]. HRVs replicate in nasal cells, sinus cells, bronchial epithelial cells (BECs) [12, 13], and smooth muscle cells [14] but not in monocytes [15] or dendritic cells (DCs) [16]. The inflammatory immune response they trigger very soon after infection has its greatest impact in the young, the elderly, those with asthma or chronic obstructive pulmonary disease (COPD), and in the immunocompromised. First infections usually elicit a stronger response. Antiviral interventions have been under development for decades; to date most have met with varying degrees of failure or unacceptability. Vaccines have been considered unachievable because of the large number of diverse and distinct viral types.
There are 100 classically defined and recognized HRV serotypes grouped into two species, HRV-A and HRV-B, and a recently defined third species, HRV–C, containing more than 60 genotypes identified and characterized entirely by molecular means. Their cousins, the four enterovirus species (EV-A, EV-B, EV-C, and EV-D), are also found in the airways at times. Most systematic and mechanistic studies of HRV etiology and pathogenesis have been informed by studies in adults, mostly prior to the discovery of HRV-Cs. Adults exhibit reduced symptoms from HRV infections because of prior exposure and the resultant protective immune memory which that imparts (see Sect. 7.3). Furthermore, many modern studies (1) draw conclusions about lower respiratory tract (LRT) disease using URT specimens and (2) infrequently sample, doing so across small cross sections of time. These limitations have hampered attempts to associate virus detection and disease. Current thinking is that HRV-Cs may be key players in asthma exacerbations although our inability to culture them routinely has hindered our progress in understanding their role. The impact of the HRVs has been underestimated for decades, and the concept of the HRVs as a very large assemblage of genetically, immunogenically, antigenically, and temporally distinct and stable viral entities remains rare; they are more commonly considered a single variable virus, a view that science does not support.
Historical Background
The disease most commonly associated with the airways and resulting from HRV infection is the common cold, a self-limiting coryzal illness [17–19]. The term dates back to ancient Greece, but evidence that the syndrome and asthma, another disease most frequently due to HRV infection, has been with us since ancient times can be viewed in writings on the Ebers papyrus, a medical document written in the sixteenth century BC [20, 21]. In 1930 the common cold was considered either to be due to exposure to the elements or to infection by bacteria [22]. It was later understood to be largely due to something in bacteria-free filtrates, and so the search for viral causes began [23, 24].
The Common Cold Unit (CCU) was established in Salisbury, UK, to seek solutions to the mysteries of the common cold, mostly through adult volunteer infection studies and careful systematic science [23]. The CCU functioned for 44 years (1946–1990), and it was here in 1953 that the first in vitro culture of an HRV was achieved using lung tissue from a particular embryo (Fig. 29.1) [25, 26]. Propagation failed once this tissue was expended [22, 33].
Fig. 29.1.
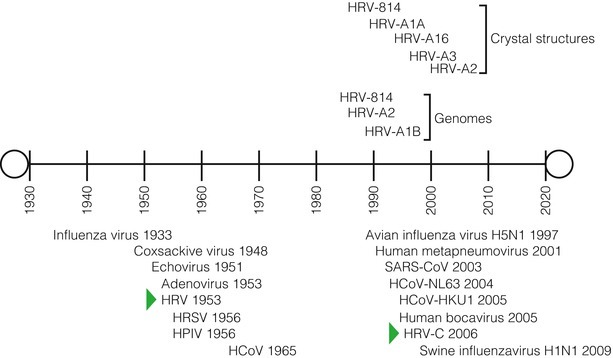
A timeline of virus discovery from the human respiratory tract. The date of each virus’s published description is shown, as are the dates the HRV crystal structures were defined and the first HRV genomes sequenced
Once HRV isolation was possible, viral serotyping developed and culture techniques were further refined. This leads to an international effort to characterize and name the HRVs [27–30].
In 2006 renewed interest in HRV research was triggered by the description of a distinct clade of HRV types [31] found using molecular typing. The resultant flurry of HRV research raised questions about many earlier paradigms of rhinovirology and of the role of established respiratory viruses in ARIs. The novel clade was proposed as a new species, HRV-C, which was taxonomically confirmed in 2009 [32–34]. Prior to the discovery of the HRV-Cs, the genus Rhinovirus had been abolished and the HRV-A and HRV-B species assigned to the genus Enterovirus within the family Picornaviridae [35]. The HRV-Cs have been assigned a new naming scheme based on genetic sequence in the absence of antigenic or serological data. While the sequencing of all serotyped HRV genomes was completed in 2009, few of the HRV-Cs or apparently novel HRV-As or HRV-Bs have been similarly characterized, so the full spectrum of HRV genomes, the rhinovirome, remains incomplete. In this chapter we have described individual serotyped HRVs as the “classical” types, a type being the description for a single, genetically stable, stand-alone HRV.
Methods for Epidemiologic Analysis
The Pre-molecular Era
The original clinical definition of an HRV infection was written using data from cell and tissue culture and adult human infection studies. After 1953 in vitro isolation methods employed a virus interference test to more easily determine successful isolation; cultures suspected of infection with an uncharacterized HRV prevented infection by another, readily titratable virus [36]. Later, Price (1956; the JH strain) and then Pelon and co-workers (1957; 2,060 strain) developed culture systems that permitted HRV replication to be more easily identified [37, 38]. The early HRVs were initially classified as echoviruses (ECHO 28; later HRV-1) [39]. At the same time, propagation of the HGP (HRV-2) strain resulted from using increased acidity, lowered cultivation temperatures, and constant motion (rotation) [40, 41]. Despite the challenges [42], virus isolation was a more sensitive indicator of infection than an antibody rise in paired sera [43].
It was found that several cell lines and methods were required to encompass virus concentrations ranging from 101 to 105 TCID50/mL [44–47] and growth differences among the different virus types. Additionally, cell age after plating (<72 h), inoculum volume (relevant to the culture vessel), medium pH (6.8–7.3), and cell density were important factors for the reproducible appearance of HRV-induced plaques and for higher virus yields [48–51]. The HRVs can grow at temperatures above 35 °C (some prefer that under certain conditions) [52], but rolling at 33 °C, preceded by a 2–4-h stationary incubation period [41], has historically provided the highest yield and fastest in vitro HRV growth [36, 50, 53, 54].
Serodiagnosis grew increasingly impractical as the number of serotypes increased [49, 55]. However, antibody-based methods were essential for type-specific neutralization of infection [56] from which early epidemiology data were derived and around which the HRV nomenclature system evolved in 1967 [28]. The first classical strains were officially named in 1967 [57], the last in 1987 [30].
Today we know that cell culture-based methods are unreliable for accurately representing respiratory virus epidemiology; although enhanced by immunofluorescence, they are still used [58]. The HRV-Cs have not been successfully cultured in any cell lines or primary cell culture, although many attempts have been described [32, 59–62]. In 2011 HRV-C15 and W23 (another HRV-C) were shown to grow using organ culture [63]. Sinus tissue hosted increasing levels of viral RNA, as did adenoid, tonsil, and nasal polyp tissue, but much less effectively, as measured by in situ hybridization [63]. The sinus organ culture system also allowed testing of the first reverse engineered HRV-C (pC15) [63]. Isolation identified HRVs in ~23 % of adults with ARIs, associated with 0.5 illnesses per year [64].
The Molecular Era
Because culture is inefficient and subjective and requires expertise, even for the culturable HRV types, it is becoming an art lost to clinical laboratories the world over. It is unsurprising that PCR-based methods now prevail, providing a much improved understanding of the nature and scope of HRV infections. The virological and immunobiological cost of this improvement is a paucity of low passage “wild” HRV isolates to work with; thus, many research findings from recent years have employed easy to grow highly passaged and adapted HRV isolates. The impact of virus adaptation on the reliability of data from use of such viruses is unknown.
PCR-based assays have dramatically increased the frequency of HRV detection [65–70]. The improved sensitivity and reduced turnaround time have shown that HRVs, as a group, are usually the predominant viruses in ARI cases [71–73]. With reliable detection levels that extend from as few as 102 TCID50/sample to well above clinically relevant loads, PCR can detect virus levels which are commonly shed during all stages of experimental infection studies [74, 75].
The common understanding of the systemic [76–78] or symptomatic [79, 80] context of HRV detections was established during the era of culture detection, and PCR has challenged these paradigms by detecting virus more often than culture. HRVs are sometimes found in “healthy controls”; however, it is likely that with more thoughtful definitions of “healthy,” these detections would reduce. It is not uncommon to experience a feeling that one is “coming down” with something that never develops further. This is likely due to a transient infection or reinfection by an HRV or other respiratory virus that is eliminated quickly by the host response. It is possible to correlate viral nucleic acid load at the sampling site with disease severity; however, this is made difficult by the highly variable sampling efficiency of respiratory tract specimens which only permit the generation of reliable quantitative PCR (qPCR) data if serial specimens are available [81].
The 5′ untranslated region (UTR; Figs. 29.2 and 29.3) is the most common target for diagnostic oligonucleotides since the first HRV RT-PCR in 1988 [82], and the region has retained relevance for virus detection by its adaptation to reverse transcriptase real-time methods (RT-rtPCR) [53, 65, 66, 69, 74–76, 79, 83–103]. The 5′UTR is comprised of a number of conserved sequence “islands” (Fig. 29.2) that permit the robust detection of the majority of HRVs and those “respiratory EVs” which can be regularly detected in the respiratory tract [104, 105]. The detection of respiratory EVs in no way detracts from the importance of supporting clinical decision making using these assays. However, repositioning these primers or changing the method of employing them [106–109] may undermine assay performance, as evidenced by predicted hybridization mismatches, uncommonly low detection frequencies [110], and by comparison of multiple primer sets using the same specimens [111]. The addition of an oligoprobe rtPCR method increases amplicon detection sensitivity and specificity, identifying 100-fold fewer TCID50/mL or 10 fold fewer genome copies than agarose gel detection of amplicon [75, 79, 112].
Fig. 29.2.

A schematic of conserved sequence regions in a generalized HRV 5′ UTR, based on a map described by Andeweg et al. [68]. The PCR primers of broadly reactive conventional RT-PCR [82, 113] and RT-rtPCR [75, 114] assays are shown
Fig. 29.3.
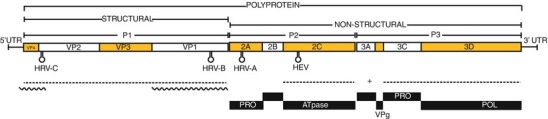
A schematic of the ~7,200 nucleotide ssRNA genome and key regions of a typical HRV member of the genus Enterovirus. The polyprotein and precursory (P1–P3) and 11 matured peptides are named in genome boxes and functionally identified underneath. The RNA is polyadenylated at the 3′ end and covalently bound to the virion protein, genome (VPg encoded by 3B) at the 5′ terminus. Regions essential for genus- and species-level identification are underlined (dashed line) as are those which are more commonly used in the clinical research setting (wavy line). The distinctively located HRV and EV cis-acting replication elements are shown as stem loop structures and protease (PRO) and polymerase (POL) functional regions are labeled (Adapted with permission from McErlean et al. [33])
Other molecular tools, capable of detecting multiple targets, have evolved in recent years [58, 70, 115–121], and some have gone on to be approved for clinical laboratory use [122]. Microarrays can detect thousands of viral targets, but are expensive for routine use (USD30–300 per sample) and not sensitive enough to avoid a pre-hybridization PCR amplification when using clinical specimens. At their most robust, microarrays, like PCR, rely on the existence of conserved regions of sequence to detect unknown viruses allowing them to detect previously unknown HRV types [123]. High-throughput or “deep” sequencing platforms have become less expensive and more readily available, and they have succeeded in finding new diversity within the HRV species [124]. The experiments remain costly so have not yet found a place for regular screening tasks and remain coupled to a need for pre-PCR steps. Rapid protein- or virion-based assays are not (yet) adequately sensitive [125, 126].
Because of the high number of HRVs and the high frequency of infections, genotyping methods have become an essential accompaniment for understanding HRV epidemiology. Nucleotide sequencing of the VP1, 5′UTR+VP4+VP2 (called hereafter VP4/VP2), or 5′UTR region has replaced traditional serological methods, because of its speed and need for fewer specialized reagents compared to serotyping. VP1 yields the most comprehensive subgenomic genotyping information and is essential for the minimal definition of a new HRV type [127]. The VP4/VP2 region (Fig. 29.3) is considered easier to use because it encompasses sufficient genetic diversity to confirm the identity of a clinical HRV type while also providing broad enough sensitivity to amplify the ~160 HRVs from a challenging biological substrate, clinical specimens [128]. Screening of airway specimens for HRVs is not routine [111] due to factors including cost and the perceived low clinical relevance of detection. Genotyping is mostly relegated to research facilities. Because of this, HRV molecular epidemiology studies tend to be smaller and focused on a specific disease or research question.
Biological Characteristics
Most in-depth molecular studies of HRV replication have focused on a single HRV type. Generally, it is presumed that results can be extrapolated to the other HRV types and to the in vivo situation. HRVs replicate in the cytoplasm (Fig. 29.4) [129] with membrane-associated replication structures containing double-stranded RNA (dsRNA) replicative intermediates (RI) which are formed in cells 4 h after infection [52, 130]. Single-stranded infectious RNA forms after RIs start to accumulate [130]. Genomic RNA (plus strand) is the template for complementary minus strand synthesis which in turn is the template for new genomic plus strands that become incorporated into virions [131]. Virions are synthesized from 4 to 7 h after infection and reach maximum release levels at 10–18 h [131].
Fig. 29.4.
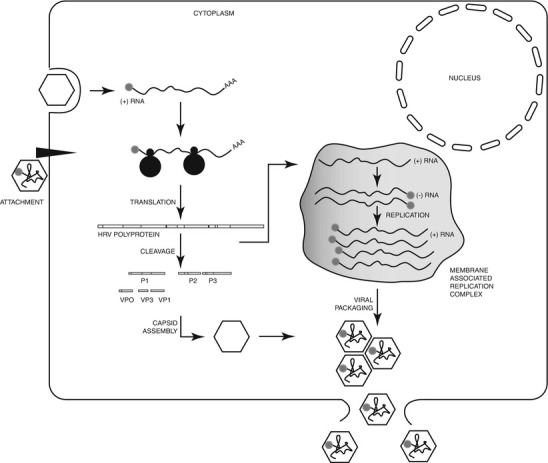
Schematic of a general HRV attachment and entry process. Genome replication in association with membranes produces the viral polyprotein which is co- and posttranslationally processed by 2APRO and 3CPRO into the proteins (P1–P3) and structural peptides (VP1–VP4; VP2 and VP4 derive from the VP0 precursor protein) that assemble into protomers, pentamers, and finally capsids. Nonstructural proteins are also released in these cleavages as well as through autoproteolytic cleavage. Mature HRV virions packaged with an ssRNA genome escape by cell lysis (Adapted with permission from Arden et al. [138])
HRV replication in epithelial cells may shut off host cell transcriptional activity via direct cleavage of transcription factors and nuclear pore complex components. Protease 2A (2APRO) of HRV-B2 may directly cleave eukaryotic initiation factor 4G (eIF4G) when bound to eIF4E [132, 133]. The eIFs have key roles in initiation and rate control of host cell translation [132]. Host cellular protein production is virtually replaced by HRV-B14 proteins after only 6 h of infection [134]. HRV-B14-infected cells also display reduced nuclear importing and degraded nuclear pore complex (NPC) components [135]. This may represent another HRV strategy for limiting the host response by preventing or reducing key signaling pathway molecules (e.g., IRF-3, STAT1, NF-κB) and shutting down host cell protein synthesis. Protease 3C (3CPRO) from HRV-A16 targets the nucleus and can disrupt active and passive nucleocytoplasmic transport [129, 136]. Recombinant 2APRO protein from HRV-A16,HRV-A89, HRV-B4, HRV-B14, HRV-C2, and HRV-C6 exhibited differing specificities and kinetics against eIF4G as well as NPC components demonstrating functional diversity between HRV types [137]. This finding underscores the functional diversity within the HRV species and the risk of extrapolating too greatly from the study of single HRV types. It is apparent from a wealth of immunobiological data that HRVs still efficiently trigger a proinflammatory immune response that has considerable clinical impact among at-risk groups, and that their putative interruption of host cell machinery does little to hinder this.
The Rhinovirus Genome
The virion encapsulates an approximately 7 kb positive sense RNA genome (Fig. 29.3), which tends to be more adenine and uracil (A+U) rich than the EV genome [139]. In particular, A+U more frequently occupies the third or “wobble” codon position. The single RNA “gene” acts as messenger RNA to encode the single multi-domain, proteolytically processed “polyprotein.” The coding region is bracketed by UTRs which perform regulatory functions necessary for genome duplication [140]. These are very similar genomic, transcriptional, and translational features to those of their close cousins, the EVs. Most of the information currently required for virus identification by the International Committee on Taxonomy of Viruses (ICTV) can be found through analysis of the genetic features of HRVs (Fig. 29.3).
There are 158 complete HRV polyproteins on the GenBank database (Fig. 29.5). The first complete HRV genome sequence (HRV-B14) was described in 1984 [141] followed by HRV-A2 in 1985 [142] and HRV-A1b in 1988[143](Fig. 29.3). In 2007 Kistler et al. added 28 genomes [144] and Tapparel et al. 12, including one common to both studies [145]. Sequencing of the VP4/VP2 region was completed for all classical strains in 2002 [146], and the complete set of 1D regions were available in 2004 [147]. Currently there are at least 50 named HRV-C VP1 regions available and 20 complete HRV-C genomes. Many more genomes are appearing as part of the Rhinovirus Consortium’s efforts to complete and study the rhinovirome using high-throughput sequencing technologies to genetically characterize HRVs from their combined clinical specimen stores (http://www.international-rhinovirus-consortium.org/). Many 5′UTR and VP4/VP2 sequences reside on the GenBank database, most of which are labeled using in-house laboratory schemes rather than an approved nomenclature. Analysis of the full-length genomes supports the use of 5′UTR, VP1, and VP4/VP2 subgenomic regions for useful representation of HRV species and types [144, 147].
Fig. 29.5.

The current spectrum of 168 complete HRV complete polyprotein amino acid sequences available on the GenBank database. The alignment was conducted using MAFFT within Geneious Pro v5.6 [148]. The phylogenetic and molecular evolutionary analyses were conducted using MEGA version 5 (Poisson model, 500 bootstraps with consensus support shown at the nodes where space permitted [149]) (Reprinted with permission from Miller and Mackay [150])
Recombination, the process of genetic exchange which results in a chimeric genome [151], can only be detected in mature viruses after the fact, and it must therefore be inferred indirectly through genomic analysis and comparison. Predictions of infrequent recombination among the HRVs [83] have been made based on examination of the available set of HRV coding and noncoding regions [152]. Intensive analyses reported that recombination is not a driving force for the evolution of HRV types [144, 153, 154]. Some discrepancies are likely because of the different number of sequences used, the different origins of the viruses used for sequencing, and the analysis methods employed. HRV-C evolution seems to have been more affected by prior recombination, than is apparent for members of HRV-A or HRV-B. This is similar to the EV species but with far fewer predicted recombination events than for EV evolution [114, 151, 155, 156]. Most of the recombination proposed to have affected the HRVs occurred between HRV-C and HRV-A and is often found within the 5′UTR or at the 5′UTR/VP4 junction [83, 157, 158] but rarely in coding sequence (2A [158] or 3C [83]). The high sequence diversity among the individual HRV polyprotein coding sequences may keep recombination events to a minimum in order to retain viral fitness [158]. The ability of HRVs to recombine in practice awaits empirical evidence; the extent of recombination among all HRV or EV types and the frequency with which viable recombinants arise are entirely unquantified.
The Rhinovirus Capsid
The 28–30 nm HRV virion has been visualized for only a handful of HRV-A and HRV-B types (including HRV-A1a, HRV-A2, HRV-B3, HRV-B14, and HRV-A16), but no HRV-C structures have been empirically determined to date. The first, HRV-B14, was described in 1985 [159] followed by HRV-A1a in 1989 [160], HRV-A16 in 1993 [161], HRV-A3 in 1996 [162], and HRV-A2 in 2000 [163]. HRV-C structure has only been predicted using computer modeling, but their basic structure seems to be that expected of an HRV (Fig. 29.6) [33]. The HRV capsid shell is composed of 60 protomers, each comprising one copy of the viral proteins VP4, VP3, VP2, and VP1. VP1, VP2, and VP3 (each ~30 kDa) are to some extent exposed on the capsid surface, whereas VP4 (~7 kDa) is internalized and associated with viral RNA. Five protomers come together at a point around a fivefold axis, and this cluster is called the pentamer. The fivefold axis is circumscribed by a cleft referred to as the “canyon.” VP1, VP2, and VP3 are each formed by a convoluted set of protein sheets and loops [159]. The loops protrude beyond the external capsid surface and contain discontinuous antigenic sites. Of the HRV types studied, four neutralizing antibody immunogenic (NIm) regions have been identified on HRV-B14 and HRV-A16: NIm-1A (located in VP1), NIm-1B (VP1), NIm-II (VP2 and VP1), and NIm-III (VP3 and VP1) [159]. Antigenic sites identified on HRV-A2 are called A, B, and C [164]. The scope and location of antigenic and immunogenic moieties among the HRV-Cs is unknown. Using known receptor binding sequence as a guide for computer modeling (Fig. 29.6), it has been predicted that when discovered, the receptor for the HRV-Cs will differ from the major and minor receptors defined for the HRV-As and HRV-Bs [33].
Fig. 29.6.

Predicted HRV-C3 pentamers compared to major (HRV-B14) and minor (HRV-A2) group HRV pentamers which have been obtained from X-Ray crystallography. (a) HRV-C3 versus HRV-B14 SimPlot data projected onto a space filling depiction of the predicted HRV-C3 pentamer. Shading represents the amino acid identity (26–69 %). The yellow-dashed triangle represents a single icosahedral asymmetric unit (T = p3 conformation) composed of VP1 and VP2 from the same protomer and VP3 from an adjacent protomer. The major group domains of interest are divided between two asymmetric units for ease of viewing. Receptor (white) and antigenic (red) sites are shown in outline. (b) Bird’s eye view of a major group HRV pentamer in ribbon form (HRV-B14, gray) with labeled antigenic neutralization sites (NImIA-III, green) and combined HRV-A16 and HRV-B14 intercellular adhesion molecule (ICAM)-1 receptor footprints (red) [165, 166]. Magnified areas of interest (boxed) highlight computer-based comparisons to the equivalent HRV-C3 (orange) predicted structures of interest. (c) HRV-C3 versus HRV-A2 SimPlot data projected onto the HRV-C3 pentamer. The domains of interest are mostly shown within a single asymmetric unit. (d) A minor group pentamer (HRV-A2, gray) including antigenic sites (sites a–c, green) and very-low-density-lipoprotein receptor (VLDLR) footprint (red) [167]. Attachment of the VLDL-R involves adjacent VP1 molecules. Magnified VP1 area represents one half of a VLDL-R footprint [168]. Amino acid substitutions (arrowed) contributed to the differences between minor group sites b and c (Adapted with permission from McErlean et al. [33])
Classification of the HRVs
The three HRV species within the genus Enterovirus are a genetically, immunogenically, and antigenically diverse assemblage of >160 viral types (Table 29.1). This accounts for the combination of HRV-A1a and -A1b, exclusion of HRV-87, which is actually EV-D68 despite confusion over acid liability [169–171] and combination of HRV-Hanks which is actually HRV-A21 [147]. Serological studies indicate that some HRV-A and HRV-B types may not be distinct enough to deserve a unique identity [147]. Species within the genus share >70 % amino acid (aa) identity in the polyprotein and in 2C+3CD and >60 % aa identity in P1 (Fig. 29.3) as well as their host cell receptors, a limited natural host range, a genome base composition (G+C) that varies by no more than 2.5 %, and a similar compatibility of proteolytic processing, replication, encapsidation, and genetic recombination [172]. A variant of the same HRV type shares 87–88 % aa identity or more in VP1 [129]. Much of the nongenetic criteria remain undefined for the HRV-Cs. In 2008 the genera Enterovirus and Rhinovirus were officially combined, retaining the former genus name Enterovirus with the Human enterovirus C as the prototype species. A genus in the order Picornavirales, family Picornaviridae, is at least 58 % different in its amino acid identity from any other genus. In 2009 a proposal establishing the species Human rhinovirus C was ratified by the ICTV. Formal HRV-C numbering commenced in 2010, and type numbers were initially assigned based on the date of submission of relevant sequences to GenBank (HRV-C1, formerly NAT001; HRV-C2, f. NAT045; HRV-C3, f. QPM; HRV-C4, f. C024, etc.; Table 29.1) [127]. A clinical detection of an HRV-C can be considered a novel type principally based on its VP1 sequence or provisionally (“C_pat,” Table 29.1) based on VP4/VP2 [146] and could be confirmed as a variant of a previously characterized HRV-C by identity thresholds to either region. The 5′UTR can be and still is used [173, 174] for HRV genotyping, but it is a more problematic region than VP1 or VP4/VP2 because of the recombination activity that affects this region, especially among the HRV-Cs [175]. This is presented as phylogenetic intermingling of some HRV-A and HRV-C types [114]. Nonetheless, careful application of sequence identity thresholds when comparing clinical sequences to the GenBank database (≥96 % identity required before assigning a clinical detection to a particular type) succeeds in characterizing HRV species and types [9]. There are currently 50 types within HRV-C (which includes the types once grouped together under HRV-“A2,” HRV-X, and HRV-NY clades), 78 HRV-A types, and 25 HRV-Bs. The most up-to-date information on current taxonomic trends can be found at the ICTV Picornaviridae study group website (http://www.picornastudygroup.com/).
Table 29.1.
ICTV-approved nomenclature for the members of the HRV species
| Human rhinovirus | ||||||
|---|---|---|---|---|---|---|
| A | B | C | ||||
| 1 M,B | 34 B | 64 B | 3 H,A | C3 (f. QPM) | C26 | C_pat14 (f. SA365412) |
| 2 M,B | 36 B | 65 B | 4 A | C10 (f. QCE) | C27 | C_pat15 (f. HRV-CO-1368) |
| 7 H,B | 38 B | 66 B | 5 A | C1 (f. NAT001) | C28 | C_pat16 (f. RV1250) |
| 8 H,A | 39 B | 67 B | 6 H,A | C2 (f. NAT045) | C29 | C_pat17 (f. RV1039) |
| 9 H,B | 40 B | 68 B | 14 H,A | C4 (f. C024) | C30 | C_pat18 (f. RV546) |
| 10 H,B | 41 B | 71 B | 17 H,A | C5 (f. C025) | C31 | C_pat19 (f. China/GDYY100/2008) |
| 11 H,B | 43 A | 73 B | 26 H,A | C6 (f. C026) | C32 | C_pat20 (f. 202511) |
| 12 H,B | 44 B | 74 B | 27 H,B | C7 (f. NY074) | C33 | C_pat21 (f. 202092) |
| 13 H,A | 45 A | 75 B | 35 A | C8 (f. N4) | C34 | C_pat22 (f. 20264) |
| 15 H,A | 46 B | 76 B | 37 A | C9 (f. N10) | C35 | C_pat24 (KR1868) |
| 16 H,B | 47 B | 77 B | 42 A | C11 (f. CL-170085) | C36 (f. NAT069) | C_pat27 (f. PV68) |
| 18 H,A | 49 B | 78 B | 48 A | C12 | C37 (f. NAT059) | C_pat28 (f. Cd08-1009-U) |
| 19 H,B | 50 B | 80 B | 52 A | C13 | C38 (f. tu34) | |
| 20 H,B | 51 B | 81 B | 69 A | C14 | C39 (f. g2-11) | |
| 21 H,B | 53 B | 82 B | 70 A | C15 | C40 (f. g2-25) | |
| 22 H,B | 54 A | 85 B | 72 A | C16 | C41 (f. g2-23) | |
| 23 H,B | 55 B | 88 B | 79 A | C17 | C42 (f. g2-28) | |
| 24 H,B | 56 B | 89 B | 83 A | C18 | C43 (f. 06-230) | |
| 25 H,B | 57 B | 90 B | 84 A | C19 | C44 (f. PNC40168) | |
| 28 H,B | 58 B | 94 B | 86 A | C20 | C45 (f. PNC40449) | |
| 29 M,B | 59 B | 95 A | 91 A | C21 | C46 (f. PNC40449) | |
| 30 M,B | 60 B | 96 B | 92 A | C22 | C47 (f. K1091_301104 | |
| 31 M,B | 61 B | 98 B | 93 A | C23 | C48 (f. PNG7293-3193) | |
| 32 A | 62 B | 100 B | 97 A | C24 | C49 (f. IN-36) | |
| 33 B | 63 B | N13 | 99 A | C25 | C50 (f. SG1,SO5986) | |
M and H indicate early cell tropism-based classification (monkey, human) abandoned in favor of a sequential numbering system [177]. HRV types were later divided into the major and minor groups defined by receptor tropism [184, 185]. Receptor-designated minor group HRV types are underlined, and major group types are shown in bold. Antiviral groups (A and B) are labeled [165, 194]. HRV-A8 and HRV-A95 are also likely the same serotype [147]. A full list of genetically close serotype pairings was presented by Ledford et al. [147] HRV-C nomenclature was defined in 2010 and currently includes a number of provisionally assigned types (pat) which are confirmed once preliminary VP4/VP2 data can be confirmed with VP1 sequence and the provisional number removed (e.g., C_pat1 to C_pat13 have already been reassigned)
Historically a key feature distinguishing the HRVs from the EVs was the instability of the HRV capsid in the presence of acid and their lower preferred laboratory propagation temperature (33–34 °C versus 37 °C for EVs). Over time HRVs have been subclassified in different ways. The first was based on tissue tropism and host range. HRVs that preferred growth using monkey cells were called “M” strains and those (the majority) that grew only in human cell cultures, “H” strains [56, 176–180]. These two groups correlate with receptor usage [131] (Table 29.1) and possibly with the titer of the inoculum employed [181]. In 1962 it was proposed to abandon this terminology in favor of a sequential numbering system [177].
Picornaviruses recognize a variety of cellular receptors [169, 182, 183]. HRV types are also subdivided into major and minor groups defined by use of one of the two main receptor molecules [184, 185]. The capsid of the majority of classical HRVs (n = 89) [184] interacts with the amino-terminal domain of the 90 kDa intercellular adhesion molecule (ICAM-1; CD54) [186–189]. Receptor binding destabilizes the HRV capsid, probably by dislodging the “pocket factor,” and initiates uncoating [164, 182, 190]. ICAM-1 interacts with its receptor, leukocyte function antigen-1 (LFA-1), and plays a role in recruitment and migration of immune effector cells [191]. The minor group [184] of classical viruses employ members of the low-density lipoprotein receptor (LDLR) family to attach to cells [167]. Binding of VLDL-R occurs outside of the canyon employing a different destabilizing and uncoating mechanism. Heparan sulfate may act as a receptor under specific conditions [183, 192, 193].
In 1990 Andries et al. defined, and Laine et al. refined, two “antiviral groups” (A and B) based on their susceptibility to a panel of antiviral molecules [165, 194]. These groupings reflected the nature of the amino acid (and hence nucleotide) sequence of the region interacting with the antiviral molecules. These antiviral groups can also be visualized using phylogeny [194]. When sequences from other subgenomic regions, including P1, 2C, and 3CD, were examined by phylogeny, the species were found, in most cases, to inversely correlate with antiviral grouping labels (Table 29.1).
Today, sequencing and phylogeny play a central role in species classification within the genus, and together, they are surrogates for the important biological classification criteria [146, 147, 165, 195–197]. For the HRV-Cs, first described as the “HRV-A2” clade (not to be confused with the single virus, HRV-A2, this naming scheme appeared after the HRV-C clade’s name was proposed) of viruses in 2006 [31], sequencing of 5′UTR and VP4/VP2 has provided the bulk of HRV information from clinical studies. While culture in primary sinus tissue has been reported [63], no receptor is yet defined.
Descriptive Epidemiology
HRVs are the most numerous and frequently detected of all the “respiratory viruses,” so-called because of their predominant detection in and tropism for the human URT or LRT (Fig. 29.7). The circulation of HRVs varies with population age, underlying disease, immunocompromise, over time, and across distance. Circulation is influenced by the nature, strength, distinctiveness, and memory of the immune response HRVs trigger and by the nature and prevalence of other concurrently circulating respiratory, and perhaps nonrespiratory, viruses. With the recent discovery of the unculturable HRV-Cs came the realization that previous HRV epidemiology was only reliable if conducted by one or more suitably broad-spectrum HRV PCR assays [111]; hence, prior to 1988, detection of the full spectrum of ≥160 HRVs did not occur. After 1988, the ability to detect all types very much depended on the nature of the PCR primers and detection methods used. The great number of distinct HRV types has burdened the search for answers to epidemiology-related questions. However, as for other important respiratory viruses including human respiratory syncytial virus (HRSV) and the influenza viruses (IFVs), the virus types within a species show evidence of being both distinct and discrete viruses that are independently recognized by their host and consequently independently infect their hosts. Each HRV type is also genetically stable [144].
Fig. 29.7.
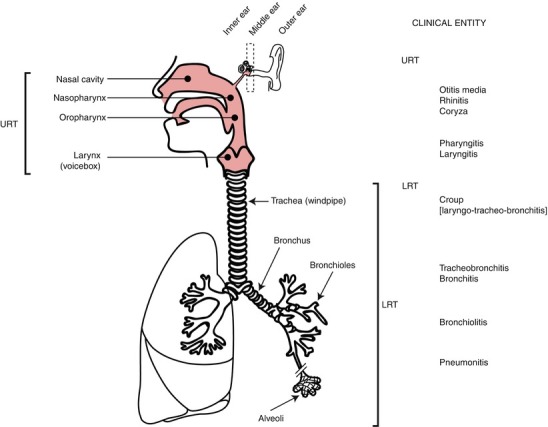
A schematic representation of the human respiratory tract. The upper (shaded pink) and lower respiratory tract (URT/LRT) and the components of the ear are indicated as are the approximate locations of URT and LRT diseases associated with respiratory virus infection (Adapted from Mackay et al. [200] with permission from Caster Academic Press)
The HRV species circulate variably from year to year with evidence of epidemics of distinct types. A prospective longitudinal cohort study over 6 months examined HRV frequency and diversity in 272 specimens from 18 healthy children (0–7 years of age) [198]. A median of three HRVs and a maximum of six were detected per child. A similar outcome resulted from an Australian cohort study [9].
Genotyping reveals more of the HRV diversity at a single site than culture ever could with molecular studies finding between 34 and 70 distinct HRVs at a single location [9, 128, 199]. The number of additional HRV cases that occur in children outside of specifically defined symptomatic periods remain to be defined, with current studies indicating that a much higher number of HRV infections may occur. More comprehensive investigation of HRV type and illness will be undertaken during analysis of data from the Australian-based Observational Research in Childhood Infectious Diseases (ORChID) study (http://clinicaltrials.gov/show/NCT01304914).
Interestingly, the HRV-Bs are often underrepresented, even when accounting for the smaller number of known HRV-B types [128]. A number of studies have not found any robust patterns between the circulating HRV types or species and clinical outcome, but the majority of studies seeking this information are short and sample infrequently, limiting their ability to find the patterns they seek [128].
Specimen Collection
Studies into the relative sensitivities of nasopharyngeal aspirates (NPA) and swab sampling methods produce differing results, but generally, if seeking the best diagnostic yield for as many respiratory viruses as possible (i.e., seeking a laboratory diagnosis to support clinical decision making), NPAs are the sample of optimal choice. One study reported similar clinical sensitivities between swabs and NPAs for human coronaviruses (HCoVs), IFVs, and HRSV, but reduced sensitivities using swabs for HRVs, human adenoviruses (HAdVs), human metapneumovirus (HMPV), or parainfluenza viruses (HPIVs) [201]. A second study reported no difference in sensitivities for HRVs, HAdVs, and HPIVs but a reduced sensitivity for HRSV and IFVs when using swabs [202]. Nasopharyngeal washes also yield more viral culture success than either nasal or pharyngeal swabs. Nonetheless, many studies use nasal swabs as the sample of choice because they allow self-collection and involve much less discomfort than NPAs, and PCR has meant that infectious virus is not required, only viral nucleic acid which relaxes some limitations imposed by the need for rapid, careful, temperature-controlled, and expensive transport requirements [64, 203, 204]. Bronchoalveolar lavage samples are best for seeking LRT etiologies, especially in adults where nasal wash viral loads can be low compared to those in children, but this is an invasive method with some risk attached [205].
Host Population Distribution
HRVs infect all people, all around the globe. Spread of HRVs is most obvious and frequent from child to child and from child to parent [206]. In populations of mixed age, the majority of HRV detections occur in children [128]. Among 272 specimens from 18 healthy children, over a third (37 %) were HRV positive. Children less than 5 years of age (44 % of whom were HRV positive) were shown to have more HRV infections and a wider diversity of HRV types than children more than 5 years old (28 % HRV positive) [198]. Healthy adults in the military [54, 207], at university [208], at home [209–212], and in the workplace [209] have also featured prominently in historical, culture-based, and volunteer infection studies and heavily influenced our view of HRV infection outcomes [64, 206]. Although studies of children in hospital-based populations usually report more significant clinical outcomes (relating to the LRT) [213] than community-based studies, these data are still broadly applicable. Hospital populations originate from the community and reflect the more serious and perhaps first exposures to the virus. Hospital-based populations define the potential of a virus to cause severe clinical outcomes. Disease at this end of the spectrum has the strongest influence on future prioritization of therapeutic research and developments [214].
Modern air travel contributes to the rapid spread of respiratory viruses as seen in their often frequent detection among travelers [215] including those with febrile illnesses [216]. Apart from children, HRVs are found with the great clinical impact in the elderly (described as 60–90 years of age) with 50 % of ARIs positive for an HRV, sometimes with a greater burden of disease than IFVs [217]. Those with asthma or COPD are also affected by the ARI triggering exacerbations of wheezing illness (see Sect. 8.2). It is thought that this is not a different type of infection but rather a different response to infection by the host. Wheezing can also result from infection in atopic people who do not have underlying asthma or COPD. HRVs cause significant impact in the immunocompromised, and this group is the only population to date that has been found to host truly persistent HRV infections (see Sect. 5.7). Because the HRVs are the largest group of viruses to infect humans, it is not surprising that they confuse differential diagnoses during pandemics and have key roles in co-detections and asymptomatic disease. The study of HRVs is the study of all respiratory viruses; while each can be considered in isolation, this will likely be detrimental to a greater understanding of respiratory virus pathogenesis.
Seasonality
HRVs circulate throughout the year but usually with a bimodal peak in temperate locations in both hemispheres. The highest peaks, mostly defined using adult populations, are in the autumn (fall) and spring [64, 66, 211] (and, peculiarly, on a Monday [218]). The major winter dip in HRV prevalence closely coincides with the peaks of other respiratory viruses, particularly IFVs [219] and HRSV [66]. One hypothesis states that a miasma exists in the school classroom, of particular relevance to those who suffer asthma exacerbations, and this miasma maintains immune stimulation, which subsequently wanes among school children during holidays, to be challenged anew upon return to school [220]. It is clear that an interplay or interference takes place between viruses at the population level, particularly evident among RNA viruses.
There is a correlation between spiking spring and autumnal HRV case numbers and an asthma exacerbation “season” 10–24 days after return to school from holidays, in a range of climates [220–223]. This was particularly obvious among asthma hospitalizations of children (5–15 years of age) in Ontario, Canada, which peaked at weeks 37–39 across a decade [223]. Upon investigation, HRVs were the most prevalent of the viruses found in a 1-year analysis of emergency room presentations in Ontario [223]. HRVs also predominate during “hay fever season” [172]. Although a defined seasonality is not always found in the tropics [224], this may sometimes be due to testing that does not include HRVs [222, 225] or only some HRVs [226].
Recurrence
All the HRV types continue to circulate today, including those named in the earliest of the nomenclature assignments. At a single site during 12–24 months, 70 or more types can co-circulate [8, 9] [174], dropping [198, 227] if the study time frame at the site is shortened. A recurring HRV type, defined using molecular tools, accounted for 1.6 % of any virus detected in a birth cohort followed for 12 months [8] and, in another cohort, occurred twice in two children, within a 6-month period [198].
Within a given year and across different years, it is apparent that HRV species exchange predominance [9, 36, 60, 227–229]. No evidence exists to satisfactorily explain this; however, herd immunity may be a factor.
Coinfection
The use of cell and tissue culture underestimated the frequency of multiple infections in patients, most likely because the dominant virus out-replicated any others, or due to viral load differences, specimen quality issues, differing cell tropisms, or the triggering of an antiviral state by the first virus. When the majority of respiratory viruses are sought using PCR techniques, multiple virus-positive specimens can comprise a third of those tested [230], dropping to around a fifth of ARI episodes when fewer viruses are sought [217]. There is sometimes an emphasis on the high number of HRV cases that are identified in the presence of another virus, and including HRV testing does raise the frequency of pathogen detection above one per sample [231]. Coinfections, or, more correctly for PCR-based studies, co-detections (since PCR cannot determine infectivity), have been found to either increase [71, 232–236] or have no impact on the clinical outcome in their host [237–241], and so the issue of clinical relevance of co-detections is still uncertain. In extreme cases, half of all HRV detections can be found concurrently with another virus. On the surface, this is a significant fraction, and yet 80 % or more of HRSV, HMPV, EV, and IFV detections and 71 % of HCoV-NL63 detections can be found in the company of another virus [242]. Other studies find different, but still higher proportions of co-detections involving non-HRVs [217]. Whether co-detections represent a particular synergism between the involved viruses, a differential capability to manipulate the host immune response, a sign of innocuousness for the most frequently involved virus [243], or a chance due to overlapping seasons remains unclear. It is clear, however, that co-detections are not an anomaly or an error due to “overly sensitive” PCR tests; they are evidence of further biological complexity that, until recently, remained hidden from us. Recent studies have shown that the initial impression of HRVs being overrepresented in these cases was incorrect. Closer analysis of viral co-detections has revealed patterns [231, 244]. These became clear when co-detections were examined bidirectionally, not just how many HRVs were positive for virus X but also how many of virus X cases were positive for an HRV. Whether in a hospital or a community setting, HRVs more often occur as the sole virus detected in ARIs [9, 244]. Considering their ubiquity, it is interesting that relatively low numbers of concurrent detections occur [245, 246], supporting the concept that HRVs have a direct role in the clinical outcome of their infection [247]. The HRV partnership with host immunity may be a mutualistic one, inadvertently imparting an advantage to the host by protecting against more cytopathic respiratory viral pathogens, while the host provides a vessel for HRV replication and transmission. Studies of single respiratory viruses without being in the context of the respiratory virome are of limited value in drawing conclusions about clinical impact.
Virus Interference and the HRVs
Much of the longitudinal epidemiology data previously relied upon to form assessments of HRV significance was acquired using culture-based techniques. With improved and more comprehensive testing, patterns can be seen among the interactions of HRVs and other respiratory viruses.
Virus interference is a type of virus-virus interaction (VVI) that has been known for decades. VVI has recently been categorized into types [248]. At the population level, it has been noted that during trials of live attenuated IFV (LAIV) vaccines, an interferon (IFN) response was triggered that protected vaccinees against off-target viruses for 7 days postvaccination [249]. This 1970 study went so far as to suggest such effects could be maintained for a prolonged period using a regime of consecutive schedule vaccinations, each separated by 7 days or more, during times of a prolonged epidemic [249]. A similar effect was produced using live EV vaccines (LEV) to replace pathogenic EV types and interrupt outbreaks [250]. Orally administered LEVs succeeded in their principal task but also reduced the incidence of ARIs during epidemics by 50 % overall [250]. This shows that immune activation in the gastrointestinal system generates an anatomically distinct protective effect and there may be a similar effect on the gut’s inflammatory status after respiratory virus infection. In contrast to the LAIV results, the off-target protective effect was reversed in a study using a trivalent inactivated IFV vaccine [251]. The mechanism underneath these opposing outcomes is unclear.
During the heyday (1960s) of tissue culture for virus studies, a common biological assay for infection with HRV involved attempted infection of the culture with an enterovirus (EV) or HPIV-1 [252, 253]. Failure of the superinfecting virus to grow heralded the likely presence of a non-cytopathogenic HRV. Virus interference has been used to measure IFN in specimens through its inhibition of HRV growth [254]. More recently HRV-HAdV dual PCR-positive cases were found less often than expected and harbored lower viral loads of HRV than did specimens from cases of sole HRV infections [255]. Significantly, the majority of these instances of VVI involve RNA viruses [244]. It has been shown that dual infections of peripheral blood mononuclear cells (PBMCs) with viruses other than HRSV (including HRVs) induced immune responses similar to those of single infections, but coinfections including an HRSV resulted in reduced IFN-γ responses [71]. VVIs are affected by the ability of each to moderate the host response against them.
Virus interference has also been identified in virus positives as a series of patterns among respiratory specimens tested for up to 17 respiratory viruses (Fig. 29.8) [9, 244]. Statistical analyses supported that many of the co-detections occurred in patterns, in particular that fewer co-detections involved an HRV than would have been expected by chance alone (p ≤ 0.05). For some period, RNA virus infection, especially the HRV group, may render the host less likely to be infected by other viruses and, by extrapolating to the community level, help constrict the epidemic periods of other viruses by reducing the number of fully susceptible hosts.
Fig. 29.8.
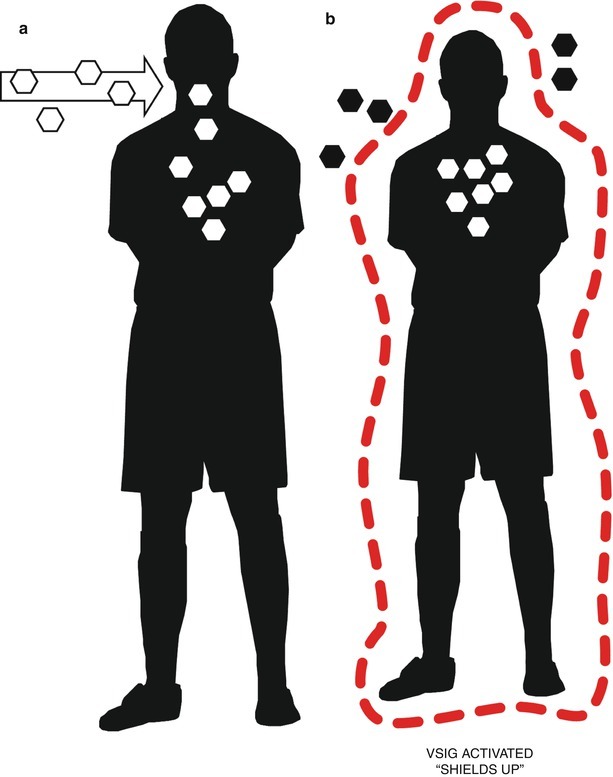
A simplified representation of the impact of a first respiratory virus infection on subsequent respiratory virus superinfections. Very shortly after the host is infected, (a) the local early innate immune response creates an antiviral state in neighboring cells (see Sect. 7.1), perhaps also in distant epithelia, mediated by circulating immune cells. The resultant inflammatory response (b) creates a shield of sorts, reducing the likelihood of infection by a superinfecting virus mediated by viral stress-inducible gene (VSIG) products
Virus interference as a feature of respiratory virus epidemiology can also be seen in results of other studies [256]. During an 8-week period that spanned peak 2009 H1N1 pandemic influenza season in Wisconsin, it was influenza A virus (IFAV) that seemed to dominate HRV in children with asthma who were sampled weekly [236]. Whether this reflects all IFV-HRV interactions or just those involving a novel IFV such as 2009 H1N is unclear. It was found that PBMCs from these children exhibited normal immune responses [236].
HRV Shedding and Persistence
Reports of subjects with continuous and extended (greater than 2–3 weeks) periods of HRV positivity [3, 257] increased as PCR methods replaced cell culture for HRV detection. This had only rarely been recorded using culture [54]. HRV RNA has been detected days prior to symptoms commencing and for as long as 5 or more weeks after they cease [3, 258–261]. Studies that only define the period between ARIs in children as that time when specimens are RT-PCR negative [3] will not detect overlapping serial infections (Fig. 29.9). Epidemiology that incorporates HRV typing generally does not find chronic shedding [204]. HRV shedding normally ceases within 11–21 days, after signs and symptoms have stopped [3, 9, 44, 75, 85, 260]. Thus, the perception of persistence is probably due to serial or overlapping infections by multiple untyped strains [8, 54, 210, 262]. Few studies [263] have suitably addressed persistence in HRV infections involving healthy subjects since pre- and post-sampling clinical data are rarely described [80, 264].
Fig. 29.9.

The impact of HRV typing and of sampling based only on symptoms. The example provided here diagrammatically represents a single, hypothetical monitoring period, starting at time = 0, for a single individual. The period of potentially detectable HRV is indicated by an open box. If sampling occurred at each time point (0–6) and HRV positives were genotyped, it would be apparent that three different strains infected the individual, although discerning HRV-X from HRV-Z at time point 3 would require a molecular cloning approach. Illness, in different forms, may have continued over the entire period depending on the symptoms required/recorded and the period of time represented by the monitoring period. In this case a clinical diagnosis may record only a single symptomatic episode. Genotyping may not be performed, and sampling may be intermittent, and so association between viral type or species and disease is impossible. In the study examples indicated by (a) start and finish sampling or (b) symptomatic sampling, (asterisks mark sampling times in filled bars), the laboratory data would have made only one or two identifications, respectively. In the third example, (c) frequent sampling of this type has previously led to conclusions of HRV persistence or chronic shedding; when combined with genotyping, it becomes apparent that different HRV types are present
To date, true persistence—an ongoing detection of a single confirmed HRV type—has been limited to individuals with underlying immunosuppression or immune dysfunction [260]. HRV-Cs were detected more than three times longer in immunocompromised young patients than in immunocompetent children, with a mean of 16 versus 53 days [265]. Multiple detection of the same HRV type (100 % identical HRV-1a sequence in each patient over time) extended to 4 months in hematopoietic stem cell transplant recipients.
Asymptomatic Infections
The proof of causality is as difficult to achieve as the proof of innocuousness when it comes to respiratory viruses and ARIs. The definition of “well” subjects prior to or at the time of sampling or inoculation is sometimes not clear, especially for young children who cannot reliably report symptoms [3, 96, 204]. Often parents notice a symptomatic illness before an infection is detected in the laboratory [3], supporting the importance of diaries in longitudinal home-based community studies. Nonetheless, even with the support of telephone interviews and home visits, milder cold symptoms may be missed. It is not uncommon for an asymptomatic control to subsequently become symptomatic or have been symptomatic before sampling [8, 266]. Some studies employ sensitive symptom scoring systems [267], but the criteria for being symptomatic are usually designed to describe and clearly discriminate overt or more “severe” illnesses, those with obvious and measurable signs. Strict definitions help improve patient management and the commencement or better direction of treatment or cohorting. However, in research studies the arbitrary degree of severity required for reporting a symptomatic event often overlooks very simple changes in host biology due to a virus’s replication. These changes to the norm are mild but nonetheless represent disease (a disorder of structure or function that produces specific symptoms or that affects a specific location and is not simply a direct result of physical injury) in the literal sense. Such minor or short-lived, often unrecorded [3], indications of infection include sinus pain, headache, sore throat, earache, watery eyes, fatigue, muscle aches and pains, and mood changes. Within families, HRVs are frequently transmitted from children who are usually symptomatic [204]. Infants frequently exposed to other children have more asymptomatic viral infections [8]. Among infected adult family members, asymptomatic infections are more likely [204]. Among older parents, whether their children live at home or not, asymptomatic infections are more frequent following HRV challenge than among adults without children or in younger parents [268]. In a study of viral species in age-stratified cases and controls, significantly lower viral loads were found in those without the required symptoms [269]. QPCR may prove useful to determine viral load cutoffs to address this issue in the future, although the respiratory tract is a difficult tissue for qPCR [200].
The high sensitivity of PCR-based methods has raised concerns over the clinical relevance of a virus-positive result [269]. It is clear that a proportion, around five to 28 % of study-defined asymptomatic control populations [90, 91, 269], are virus positive using sensitive PCR-based methods. This may vary up to nearly 50 % of cases when stratified by age, virus, and season or when including high-risk populations [8, 269]. Every respiratory virus, even IFVs and HRSV, can be found in cases without symptoms at the time of specimen collection even after specific inoculation of adults [137, 269, 270]. This is a complex and incomplete story in need of more research, and so it is frustrating that positivity in asymptomatic people is often used to rank viral importance. Better data are required from asymptomatic controls for any conclusion to be drawn about causality [266], but this requirement often disregards the memory of a normal functioning protective host immunity. It is the host response that defines the degree of clinical severity for the inflammatory disease that is the hallmark of an ARI [271]. It is well known that previous exposure to a virus affords protection from the full clinical spectrum of disease upon repeat exposure to that virus. It should come as no surprise then that HRVs, which usually cause brief infection anyway, could well produce only minor signs and symptoms upon reinfection. The unique and extremely personal infection history of each member of a control group cannot be determined unless they are part of a longitudinal cohort. So, what do cohort studies, supported by comprehensive PCR-based testing, tell us about asymptomatic virus infections?
Some cohort studies do not look in asymptomatic children, seeking samples only at times of symptomatic illness [66, 246, 272]. A birth cohort of children enrolled and sampled when ill and every 6 months for 24 months identified HRVs 14–28 % of infants and toddlers who had no nasal symptoms (defined solely by the presence of rhinorrhea) [273]. The Childhood Origins of ASThma (COAST) birth cohort followed 285 infants at high risk for allergies and asthma for 12 months and identified HRV infections as preceding (mean age of first detection, 4 months) those of HRSV (mean age at least 6 months), and HRVs were found in 35 % of asymptomatic versus 61 % of moderately to severely ill patients; the most frequently symptomatic children also had the greatest proportion of asymptomatic infections [8]. In a study of 58 children with asthma sampled weekly for 5 weeks during each of two peak HRV seasons, nearly two-thirds who were virus positive but not sensitized to at least one allergen showed no asthma symptoms, and nearly half showed no ARI symptoms; in the children who were sensitized, less than one-third showed no asthma symptoms, and only a fifth had no ARI symptoms [227]. A convenience population of 15 healthy children (1–9 years old) without asthma were followed during at least three seasons, and picornaviruses were detected in 5 % of 740 specimens (21 % of infections) not associated with symptoms, although 9 of the 25 infections came from households with an infected sibling [3]. In summary, there is clear evidence for the presence of HRVs in asymptomatic controls. A precise proportion cannot yet be defined. Some study controls show signs of a “lead-in” period where RNA positivity precedes an ARI defined on follow-up, while others may have been defined as symptomatic if more symptoms had been accounted for.
Mechanisms and Routes of Transmission
Source of Infectious Virus
HRVs have been found at extra-respiratory sites. Viremia was determined in the blood of children with LRT infection or pericarditis [274, 275], and HRV-C was more commonly associated with viremia than was HRV-A, supporting possible increased pathogenicity [274]. Blood was also positive for HRV RNA and infectious virus from infants at necropsy [276, 277], and HRV RNA was detected in the plasma of children with asthma, bronchiolitis, or common cold [76]. An HRV was once isolated from feces [203], and more recently higher than expected loads of HRVs were detected in fecal specimens from children with suspected meningitis and fever of unknown origin [77], with gastroenteritis [278], and in a child with pericarditis [275]. Nonetheless, the nasopharynx is still considered the main site of focal virus production [279], regardless of inoculation route [280], and most studies of transmission routes have centered on the URT. In contrast to IFV and HRSV, HRV infection involves less destruction of tissue. Ciliated epithelial cells are sloughed off in proportion to the severity of an HRV ARI, but this damage is minimal and does not occur during the viral incubation period or with subclinical infections [137, 281]. The incubation period between infection and onset of virus shedding into nasal secretions is 1–4 days with shed viral titers peaking in adults between days 2 and 10 [44, 282]. The time until successful HRV transmission among adults in a childless family setting is usually 5–8 days and requires the donor to be shedding at least 103 TCID50 at some stage, to have recoverable virus on the hands and in the nares, enough shared time, and a moderate to severe ARI [283].
The lungs have been shown to host replicating HRV [260], and the reader of such reports may be left with the perception that detection of HRV replication in the LRT explains all LRT symptoms. However, relatively few studies seek or identify true HRV replication in the LRT. While the overwhelming majority of LRT cases detect HRV from the URT, a correlation between URT positivity and LRT disease does exist [284].
Self-Inoculation and Virus Survival
It is well known from experimental inoculation studies that HRV infection can result from inoculation of the conjunctival sac after virus is moved through the nasolacrimal duct [280]. In these studies virus was commonly delivered by aerosol or intranasal instillation of 0.25 mL to 5 mL of suspension [43–46, 280, 285–287]. In the laboratory, HRVs can retain infectivity for hours to days on suitable, nonporous solid surfaces, especially if the inoculum remains damp [47, 287], which supports direct self-inoculation especially in the family setting and indirect inoculation via fomites [288]. In a trial to define the movement of virus from a contaminated donor to a recipient via multiple surfaces or by hand-to-hand contact, 13 % (donor to objects to recipient) and 6 % (donor to recipient fingers) of the virus recoverable from the donor’s fingertips were recoverable from the recipients’ [289]. Even under observation, eye rubbing (0.37 h−1–2.5 h−1) and nose-picking (0.33 h−1–5.3 h−1) occur frequently [47, 290], suggesting self-inoculation could outpace personal hygiene, particularly in the young.
Airborne and Intimate Contact Transmission
It was once thought strange that ARIs were so common, but isolation rates for the expected viruses were so low [36, 291]. With a better understanding of the importance of preexisting antibody (something common among the predominantly adult volunteers used by many studies), the discovery of a third, unculturable species of HRV (still causing ARIs but impossible to isolate or detect using antibody-based systems for which no reagents existed), and a vastly improved diagnostic sensitivity, this is much less confounding. In the past, household cross infection, determined by ARI, was low, about five exposures to infected members required for infection [17] despite viral loads in nasal washings peaking at 1.6 × 105 TCID50/mL [44]. Experimental transmission was also reportedly inefficient [45]. In contrast, “naturally” close-quartered military populations, interacting over 1–4 weeks, experienced rapid spread of HRVs to >50 % of the group [54]. The use of PCR recently clarified this discrepancy, confirming that frequent transmission in families is more common than culture-based studies had identified, often resulting in asymptomatic infection among older siblings and parents [204]. PCR has helped define the scope of viral RNA, if not actual infectious virus, survival, and spread.
Transmission studies require infectious HRV, and so the HRV-Cs do not contribute to the historical data. Under crowded or intimate conditions and with more severe colds, transmission reaches 38–100 % [283, 292]. In some studies, both large- and small-particle aerosols proved inefficient, supported by a low isolation rate from saliva (39 % compared to 65 % of hand washes and 50 % of nasal swabs)[44, 47, 293] and from only 8.3 % of participants exposed to large-particle aerosols [293]. In other human donor-recipient model studies however, aerosol proved to be the main transmission route among antibody-free adults [46, 282]. The discrepancy may have been due to insufficiently long or intense exposure in the earlier aerosol experiments [45, 267]. Apart from particle size, spread of virus by aerosol is affected by existing nasal obstruction which can divert secretions from the nares to contaminate saliva, the presumptive source of virus in coughs and sneezes [44]. When exposed to 10 liters of a small-particle aerosol, 101 TCID50 of HRV-15 was associated with fever and prominent tracheobronchitis in antibody-free (<1:2) adult volunteers but not when delivered via nasal drops or a coarse aerosol [46]. It has also been found that simple breathing releases HRV RNA (the same type was also identified from nasal mucous) from at least a third of adults and children with symptomatic ARIs and infectious HRV could be isolated from a fifth [294, 295].
It is apparent that HRVs accumulate at sites with heavy human traffic, potentially forming a secondary source of infection. HRV RNA can be detected from 32 % of ~47-hour-old filters placed to sample air in office buildings [296]. In aircraft, high efficiency particulate air (HEPA) filters have been found to harbor HRV RNA more than 10 days after they were removed for servicing [297].
Immunity
HRV infections trigger a vigorous proinflammatory immune response that is thought to drive the symptoms experienced as illness [271, 298, 299], but they do not seem to actively prevent or interfere with the host’s immune response the way most other viruses have evolved to do. There may be a role for repeated challenge by HRVs and other respiratory viruses leading to inflammation and tissue remodeling. The host response to HRV infection can be broadly broken into the innate (very fast, encoded in the germ line, nonadaptive) and adaptive (slower to develop, reliant on T cells, B cells, and the generation of antibody) responses. While the innate system is “always watching,” it is significantly amplified by virus infection. The adaptive response is initiated by the host’s first infection with a particular virus and then functions to limit subsequent infections through the production of neutralizing antibodies and amplification of existing cell-mediated immunity.
Innate Immunity and Interferon
After virus-receptor binding and internalization, the earliest host cell immune response to an HRV infection is elicited by the innate immune system (Fig. 29.10). Epithelial cells represent the front line against HRV invasion although alveolar macrophages and DCs are better equipped to respond [300] and do so despite not hosting HRV replication directly [16]. Virus detection is mediated by pattern recognition receptors (PRRs) that have evolved to recognize conserved molecular structures shared among diverse pathogens. Internal- or surface-mounted PRRs include sentinels that specifically recognize picornavirus RNA and protein and, in doing so, trigger an immune circuit that results in the production of IFNs and subsequently hundreds of IFN-stimulated gene products. The innate response to viral infection hinges on inducing two type I IFNs (initially IFN-ß then IFN-α), secreted cytokines that produce antiviral, antiproliferative, and immunomodulatory outcomes [301]. The type III IFNs (IFN-λ1 or IL-29, IFN-λ2 or IL-28A, and IFN-λ3 or IL-28B) are also produced in response to viral infection in a range of cells, although their receptor is not as widespread [302]. The type II IFN, IFN-γ, is produced by activated T cells and natural killer cells rather than in direct response to virus [303]. Detection of viral components triggers protein signaling cascades that regulate IFN synthesis through the activation of viral stress-inducible genes (VSIGs) [301, 304]. These are sometimes expressed constitutively but upregulated after IFN induction following HRV infection [305]. Released IFN-ß binds to the IFN-α/IFN-ß receptor in an autocrine (the same cell) and paracrine (neighboring cells) manner, starting a positive feedback loop for type I IFN production, the “second wave.” VSIGs include the antiviral proteins protein kinase R (PKR), 2′5′OAS/RNaseL, and the Mx proteins [306]. IFN-α upregulates expression of MxA, 2′4′-OAS, and PKR [307]. The Mx pathway is also induced after virus infection but is not constitutively expressed [307]. Depending on the sentinel system stimulated, there are different pathways to VSIG activation. Those VSIGs with antiviral properties (e.g., MxA, PKR, 2′5′OAS/RNaseL) inhibit different stages of virus replication and strengthen an antiviral state in the host. While this state is well known, the nature of its induction by different respiratory viruses and the impact of induction upon the replication of other respiratory viruses are topics for considerable ongoing research.
Fig. 29.10.
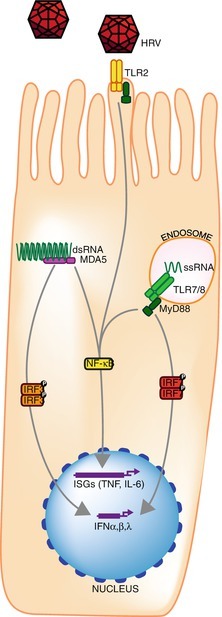
A simplified representation of molecules involved in or that respond to the recognition and response to HRV infection of airway epithelial cells [302, 312–317]. IFN interferon, IRF IFN regulatory factor, ISG IFN-stimulated gene, TLR Toll-like receptor, MDA5 Melanoma Differentiation-Associated protein 6; NF- κB Nuclear factor kappa-light-chain-enhancer of activated B cells, MyD88 myeloid differentiation primary response 8
One pathway to IFN induction relies on the IFN-upregulated cytosolic sentinels retinoic acid inducible gene RIG-I-like receptors (RLRs) RIG-I (specific for IFAV and others) and melanoma differentiation-associated gene 5 (MDA5, specific for picornaviruses and others) [306, 308]. These RNA helicases recognize either RNA with a 5′-triphosphate or distinct dsRNAs, which results in activation of NF-κB leading to “classical” type I IFN induction [301, 306]. Studies into the innate response to HRV infection have been limited to the use of a very few easily cultured types. It is presumed that the result can be extrapolated to most if not all types. This is yet to be tested. RIG-I is degraded by HRV-A16 [309], IFN regulatory factor (IRF)-3 homodimerization is interfered with HRV-B14 which limits IFN-β induction [310, 311], and MDA5 is degraded by HRV-A1a but not HRV-A16 [312].
Another pathway for recognizing HRV infection involves the Toll-like receptors (TLRs), transmembrane PRRs that terminate in an intracellular signaling region. The endosomally localized TLR3, TLR7, TLR8, and TLR9 recognize nucleic acids and are also involved in innate antiviral responses. TLR7 and TLR8 identify G/U-rich ssRNA from endocytosed viruses, while TLR9 recognizes unmethylated CpG DNA present in DNA viruses [301, 318]. TLR2 and TLR4 are found on the cell surface and recognize HRV or HRSV proteins, respectively [318, 319], and TLR3 recognizes dsRNA. TLRs operate mainly, but not exclusively, in plasmacytoid DC [301]. The particular TLR that notifies of an HRV incursion may depend on the method of virus approach [319]. TLR7 activation can reduce 2′5′OAS and MxA mRNA expression and IP10 protein in adolescents with asthma compared to healthy controls [320]. TLR3 activation did not result in a similar disparity [320].
It has been suggested that HRVs may have evolved with humans to such an extent that their symbiotic relationship serves to help train the human immune system [321]. Intriguingly, within the HRV species, there are differences in the type and level of host response induced [322] which may reflect receptor usage, route of entry and cell type infected, HRV species, or the degree of laboratory-adapted virus used during in vitro studies.
Cellular Immunity and Inflammation
After initial HRV infection, the innate response results in production of proinflammatory cytokines, vasoactive peptides, and chemokines that attract leukocytes, granulocytes, DCs, and monocytes (Table 29.2) [321, 323, 324]. The T-lymphocyte response to viral intrusion can be broadly categorized as TH-1-like and TH-2-like. Other T-cell subsets exist, but most work in relation to HRV has been conducted on the earliest defined subsets. The TH-1 cellular response is important in managing cellular immunity and producing interleukin (IL)-2 and IFN-γ. The TH-2 cellular response manages humoral immunity and stimulates B cells via IL4 (initiating production of IgE), IL5 (influencing eosinophils), and IL13 (crucial component of allergen-induced asthma). These two T-cell responses act in concert with epithelial-derived chemokines (e.g., eotaxin) to promote the recruitment and activation of eosinophils and mast cells, contributing to chronic airway inflammation and the hyperresponsiveness of airways to a variety of nonspecific stimuli [325]. TH-2 lymphocytes, opposing TH-1 lymphocytes, contribute to an allergic inflammatory cascade, akin to what occurs to rid humans of parasites [326]. The TH-1 response can also be repressed by binding of microRNA, which leads to an altered balance favoring a TH-2 state in mice and probably in humans [327]. Regulatory T cells (Treg) suppress allergic inflammatory pathways and are therefore fundamental in protecting the airway from allergen sensitization [326].
Table 29.2.
Some important molecules involved in the response to HRV infection
| Molecule | Role |
|---|---|
| IFN-α/IFN-β (type I IFN) | Produced by leukocytes and BECs; numerous subtypes; immunomodulator |
| IFN-γ (type II IFN) | Produced by many cell types after viral infection, especially BECs, PBMC, and DCs; a key TH1 cytokine in intracellular defense through stimulation of antiviral molecules; macrophage and NK cell activation and B-cell proliferation |
| IFN-λ (type III IFN) | Participates in creation of an antiviral state; produced by and influences the maturation of DCs |
| IL-1β | Proinflammatory properties; enhances adhesion molecule expression including ICAM-1; induces IL-2 receptor |
| GM-CSF | A granulocyte and monocyte growth factor |
| IL-2 | Stimulates growth and differentiation of T and B lymphocytes and cytotoxic activity of NK cells and monocytes |
| IL-4 | TH2 differentiation, promotes IgE synthesis |
| IL-6 | Activation, differentiation, and proliferation effects on T and B lymphocytes; induces C-reactive protein stimulating pyrexia |
| IL-8/CXCL-8 | Neutrophil chemoattractant resulting in neutrophilic, monocytic, and lymphocytic recruitment and degranulation activity |
| IL-10 | Anti-inflammatory factor produced by monocytes that acts by inhibiting proinflammatory cytokines IL-1, IL-6, and TNF-α |
| IRF7 | A master hub, regulating antiviral immunity |
| IP10/CXCL10 | Chemoattractant for activated TH1 and NK cells |
| TNF-α | Proinflammatory activity similar to IL-1 β; activates neutrophils; induces vascular permeability |
| MPC-1 | A monocyte attractant |
| Bradykinin | Potent inflammatory mediator, increases vascular permeability |
| TSLP3 | An IL-7-like cytokine that activates myeloid DCs to induce naive T cells into TH2 cells producing IL-4, IL-13, and TNF-α; induced by HRV in the presence of IL-4 |
BEC bronchial epithelial cells, DC dendritic cell, IRF interferon regulatory factor, IFN-γ inducible cytokine protein, NK natural killer, PBMC peripheral blood mononuclear cells, IL interleukin, TNF tissue necrosis factor, TSLP thymic stromal lymphopoietin
Considerable immunobiological research has focused on asthma exacerbation, with which HRVs are intimately involved. Although upregulated by HRV infection, the TH-1 response is comparatively deficient in people with asthma [328, 329]. This is problematic as an increased TH-1-like cytokine response, deduced from higher sputum mRNA IFN-γ/IL5 values, speeds clearance of HRV and symptom amelioration [85]. One possible cause of the TH-1 deficiency in people with asthma is inadequate maturation of type I and III IFN responses due to reduced exposure to infections early in life [330]. The “hygiene hypothesis” [331, 332] posits a pathway for an asthma etiology described [325] in terms of the young, unchallenged immune system, dependent on infections to stimulate the development of its TH-1-like functions. One theory suggests that HRVs play a central role in developing that efficacious antiviral immunity, particularly in infancy, via their frequent, usually mild self-limiting infections [333].
Genome-wide expression analysis of BECs from healthy and asthmatic adult subjects after HRV-A1a infection revealed some significant differences that were found between cell types and response to infection [61]. These included immune response genes (IL1B, IL6, IL8, IL1F9, IL24) and airway remodeling genes (LOXL2, MMP10, FN1) and an overall proinflammatory response and metabolic slowdown consistent with proteolytic cleavage of transcription factors by some HRVs [133, 334–336] in the infected cells. This study further noted some similarities to gene expression changes observed in brushings from people with mild asthma after allergen exposure and in BAL cells from subjects with corticosteroid-resistant asthma [61]. Overall, HRV replication and the host transcriptional response to it were similar in normal or asthmatic BEC cells [61]. This indicated, at least in adults, that something beyond the epithelial cell is an important contributor to more severe clinical outcomes in asthma.
The application of inactivated HRV-B14 was found to promote release of IL10 from monocytes (an immunosuppressive cytokine) and to inhibit the stimulation of IL12 (drives TH-1 development) [337]. However, neither IL10 nor IL12 was significantly induced in asthmatic adult volunteers in response to HRV-A16 compared to healthy subjects [338]. While IFN-α was detected after transfection of DCs with HRV-B14 ssRNA, low TNF-α and IL12 levels were also noted [16]. It was posited that the reduced IL12 could indicate negatively affected local immunity possibly predisposing to secondary infections [337]. Infection of stromal lung cells by HRV-B14 triggered exaggerated levels of the pleiotropic IL11 (an IL6-type cytokine), akin to those triggered by HRSV, which were also detected in nasal secretions from children with wheezing [339].
Other cytokine changes have been identified in atopic adult volunteers challenged with HRV-A16. G-CSF and IL8 (chemo-attractant for neutrophils) levels rose in the URT (as examined by protein detection in nasal lavage) and LRT (mRNA detection in sputum) with concomitant rises in blood and nasal neutrophil numbers [85, 203, 340]. The nasal epithelial cells of atopic individuals, especially in season, express more ICAM-1 than those of nonatopic adults [341] as do normal subjects infected by the major group HRV-B14 [341]. By contrast, IFN-γ and IL8, which appear later postinfection, downregulate ICAM-1 expression in infected cells [191] and encourage infiltration of neutrophils [342], respectively. Changes in ICAM-1 levels may modify T-lymphocyte-mediated cytotoxic or TH interactions with HRV-infected cells, upregulating receptor expression and encouraging eosinophil and T-cell infiltration into the lower airways of asthmatic individuals [187, 343].
Antibodies
Before an HRV can enter a cell, it must pass through a defensive barrier of secreted anti-HRV antibody, mostly IgA. The ease with which this passage occurs is proportional to the progression of clinical disease. Healthy adult volunteers were found to develop IgA by at least 3 days to 2 weeks after inoculation—about the same time as serum antibody—and retain peak levels for at least 8 weeks [178, 344–346], falling faster than serum levels [347, 348]. There is also some evidence for a degree of nasal immune memory [347]. Volunteers with pre-study serum antibody could still be infected in some studies [46, 210, 292], but not in others [349]. Infection is more clear in volunteers without preexisting nasal antibody to experimental challenge virus; they become infected, exhibit more severe ARI, and shed more virus for longer [292, 347]. IgA does not seem to modify illness severity or virus shedding, but high levels prevent reinfection by the initiating virus type. Low levels or absence of IgA does not prevent reinfection by the same HRV type, which may manifest as symptomatic or asymptomatic disease [349].
Older children, adolescents, and adults have greater amounts of HRV-neutralizing antibody than young children [42], accompanying a trend toward decreasing numbers of symptomatic ARIs with increasing age [218, 350]. This feature raises an issue: did the use of older subjects in many common cold studies underplay the pathogenic potential of the HRVs because protective or partially cross-protective antibodies moderated the impact of infection? Consequently, quantifying levels of type-specific serum antibody became routine practice prior to some studies. Adult volunteer studies determined that no infections resulted if preexisting neutralizing antibody titers ≥1:16 existed; as levels grew from 0, so did levels of resistance to infection [43, 210]. Adults were protected by serum titers of 1:3–1:8 [209, 210]. The trend was interrupted by adults in the 20–39 year age group, presumably because they had begun families and their young children acquired and amplified currently circulating types from the community and transmitted them into a household that was either immune naïve or lacking sufficient antibody or cell-mediated memory for protection [351].
Traditional vaccine strategies were quickly ruled out as a prophylactic intervention for HRV illness because of the extensive antigenic variability that is a hallmark of the genus Enterovirus [178, 325]. However, if it were possible to identify “master” strains [352] that exhibit sufficient antigenic cross-reactivity to induce broad heterotypic responses against many other HRV strains, then an effective vaccine could still be possible. In fact, boosting host immunity to an HRV type by repeat infection does heighten immunity to one or more other types [44, 353]. The highest of these heterotypic antibody titers develop against those types with the highest preexisting antibody levels [352]. The first description of a unifying HRV numbering system recounted the appearance of minor serological cross-reactions, which were removed by modification of the technique [57]. Subsequently, cross-reactions were better defined during experimental inoculation when multiple HRV immunogens and antigens were used to deduce the extent of heterotypic responses [56, 210, 352, 354].
Less promising for HRV vaccinology was the description of antigenic variation within HRV types which suggested that immunity to one variant of the type might not protect against infection by other variants [355, 356]. The “prime strain” is a specific antigenic variant of a prototype HRV type that is neutralized to a lesser extent by antisera from the prototype, while yielding antisera that effectively neutralize both itself and the prototype [357]. Another form of this cross-neutralization is ascribed to the “intertypes,” which are HRV isolates that share a lower-level serological relationship with a pair of HRV strains, which themselves share neutralizing reactivity, e.g., HRV-A36 and HRV-A58 [358]. The low-level reciprocal neutralizing activity was not equivalent in both directions; anti-HRV-A36 sera had a higher titer for HRV-A58 than anti-HRV-A58 sera did for HRV-A36 [358]. Over 40 strains were linked directly by such one- or two-way cross-reactions or indirectly through two or more strains. HRV-A67 and HRV-A28 are linked via HRV-A11, HRV-A13, and HRV-A32 (anti-A11 serum neutralizes HRV-A28, anti-A13 neutralizes HRV-A11, and anti-A32 neutralizes both HRV-A13 and HRVa-A67[358, 359]). A surrogate molecular method which provided insight into these interrelationships, perhaps expanding upon them to identify useful patterns for vaccine immunology purposes, would be most welcome.
In summary, heterotypic immunity and HRV intertypes might be exploitable features of HRV immunobiology that could confer maximum protection upon the host from the minimum number of HRV types [358].
Pathogenesis and Host Response
HRVs circulate in great numbers, and any specific roles for distinct HRV types in initiating disease remain to be defined. The relatively inconsequential common cold is the most frequent manifestation of viral infection in humans, with 30 to >80 % of colds positive for an HRV [69, 360, 361]. Furthermore, ARIs due to HRV infection can exacerbate or result in a much greater burden of disease in those with asthma, COPD, or cystic fibrosis. Other complications include otitis media, pharyngitis, and wheeze in atopic people without asthma. The role of viruses in the origin of some of these diseases or their exacerbation is still unresolved. The LRT disease may mask the URT nature of the infection, favoring clinical diagnosis of an LRT illness. Interestingly, during the 2009 H1N1 pandemic, much of the parent-initiated healthcare visits from a birth cohort in the United States were not due to pandemic virus but HRV and HRSV [362]. There is no known natural murine rhinovirus on which to base a small animal model of HRV infection, and mice are not natural hosts for HRVs. A recently developed model of airway disease using mengovirus (a Picornavirus infecting rodents) may yield valuable in vivo airway infection and inflammation data [363].
HRVs are often detected in neonates and infants with LRT signs and symptoms because the very young have narrow, immature airways and are more significantly affected by airway swelling, excessive secretions, and smooth muscle contraction [364]. This may also be due to the relatively naive immunity of very young children. Much of the more severe disease in HRV-positive children occurs in the youngest of them. Some key examples are addressed below.
Common Cold
For the common cold, as for any illness, accurate epidemiology and burden of disease data underpin the prioritization of preventing, treating, and further researching the etiological agent. To assign funds for researching the agent, health policy makers also need to understand how efficacious and cost-effective the development of an intervention will be [365]. The host immune response to HRV replication is the main cause of the signs (quantifiable fever, rhinorrhea) and symptoms (feeling of fever, myalgia, headache, fatigue, and mood change) of a cold that the host experiences [321, 366, 367]. A feature of common colds is increased vascular permeability which, enhanced by kinins, results in increased plasma protein (albumin and immunoglobulin [Ig] G) levels in mucus, approaching the levels in serum [368]. Histamine levels do not rise in nasal secretions of otherwise healthy cold sufferers [368]. During the resolving phase of the ARI, glandular proteins (lysozyme, sIgA) predominate [346].
The common cold syndrome is also described as rhinosinusitis (the agglomeration of rhinitis and sinusitis since they frequently clinically coexist) [369, 370]. This consists of nasal discharge or rhinorrhea, nasal obstruction, sore throat, sinus pain, headache, sneezing, watery eyes, cough, fever, fatigue, muscle aches and pains, and mood changes [367, 371]. These are caused directly or indirectly by viral infection; cough is the result of vagus nerve irritation by mucus; sneeze results from trigeminal nerve irritation; sore throat is likely due to the action of prostaglandins and bradykinins; and fever, psychological effects, fatigue, and myalgia are mediated by cytokines [367]. Hypertrophic adenoids have also been found to have a high proportion of viral, especially HRV, occupation regardless of host symptomatic state [372]. Observation of natural culture-confirmed HRV colds in adults noted that cough usually started by day 1 and was more persistent up to 9 days later [264, 373]. Rhinorrhea, sneezing, and sore throat were reported by half or more of patients and headache by at least a quarter of cases [207, 373]. As neutrophils accumulate at the site of primary URT infection, the myeloperoxidase in their azurophilic granules creates the yellow-green coloration of nasal mucus that was once considered a sign of bacterial superinfection [271, 367]. A common cold caused by an HRV cannot be clinically distinguished from one that caused by any of the other respiratory viruses [207, 371]. As is likely for a single HRV type, once the host has been infected by an HMPV, HPIV, IFV, etc., a secondary exposure to that same virus type will produce less severe clinical outcomes due to pre-primed host immunity.
Asthma and Atopy
Asthma is a clinical diagnosis made on the basis of patient history, physical examination, assessment of airway obstruction or reversibility, and response to bronchodilators [374]. It is a complex chronic respiratory disease involving airway inflammation, airflow obstruction, and airway hyperresponsiveness, which manifests as recurrent reversible attacks with deteriorating asthma control that are generated by interactions between infectious agents and other environmental and genetic factors that remain incompletely characterized [375]. The mechanistic role for HRVs in asthma inception and exacerbation is not yet defined [364, 376] but is being revealed as the extremely complex interplay between inflammation due to virus versus that due to atopy is explored [315]. Possible virus-host interactions include (i) severe HRV infection of healthy infants which may result in subsequent development of asthma; (ii) HRVs may trigger asthma in children with a genetic predisposition toward atopy; (iii) repeated mild infections may protect against more asthmogenic/cytopathic viruses or the overdevelopment of the TH2-type response; and (iv) HRVs may simply exacerbate that which already exists [377]. It is unclear if the risk of atopic asthma during infancy is increased by ARIs which affect the development of the immune system, or whether ARIs lead to asthma development in children with a genetic predisposition to more severe responses to infection [325, 343, 378], or a mix of both. In children with asthma, viruses have been detected in at least 77 % of exacerbations (65 % picornaviruses, probably HRVs [379]) and in 50 % of adults [380].
Acute wheezing episodes (including bronchiolitis and acute asthma) are a frequent, epidemic, and seasonal LRT manifestation of URT respiratory virus infection of children from all ages, especially during the first year of life [214, 381–385]. Bacteria are not major factors in wheezing exacerbations [386]. Wheezing is blamed for high socioeconomic and healthcare costs, overuse of antibiotics, being the primary cause of hospitalization among children, and, rarely, for death [109, 387, 388].
Traditionally HRSV infection has most often been the virus causally associated with expiratory wheezing, wheezy bronchitis, or asthma exacerbations because of the virus’s well-known ability to infect the LRT, its more frequent detection in some studies [386], and the low perceived likelihood of URT viruses such as HRVs replicating in the warmer LRT. Nonetheless, periods of epidemic wheezing in the absence of high rates of HRSV detection are common [383, 389]. HRVs even predominated in some culture-based studies of wheeze [384, 390]. The COAST study used sampling criteria that were intentionally designed to investigate the role of HRSV in illness, but instead indicated that HRVs were the most important predictor of subsequent wheezing in early childhood, and this is supported worldwide [224, 391, 392].
The asthmatic airway is characterized by an infiltration of eosinophils and Th2-type T cells (Th2 cells) [393]. In those with an atopic background, eosinophilia was more common, and the virus isolation rate was higher than in the nonatopic group [394]. The cytokine and eosinophil activation profiles for HRSV-induced wheezing differ from those induced by HRV in which IL5 is significantly higher in serum and nasal aspirates than for HRSV [118]. IP10 was the only cytokine significantly elevated in all symptomatic wheezing groups [118]. Significantly higher rates of HRV detection with more obvious LRT symptoms are more common in children with asthma than in non-asthmatic populations [380, 388, 395, 396]. Exacerbations of asthma are often preceded by a symptomatic rather than asymptomatic HRV infection [17, 379, 388, 397–399] although, in some instances, an exacerbation is the only sign of infection [400]. Reduced peak expiratory volume in children is especially associated with detection of respiratory picornaviruses [379]. Severe “wheezy bronchitis,” a historical term describing an acute illness with preceding ARI and characterized by cough, wheezing, breathlessness, and mucous production, was more often positive for a virus than mild disease [394]. Even the use of culture found that HRVs predominated in both URT and LRT (sputum containing BECs) or combined respiratory tract samples [394]. Bacteria were often present with IFV, but not with HRVs [394].
The airway epithelial cells form a physical barrier in addition to their roles in immune surveillance and regulatory control [393]. However, the asthmatic bronchial epithelium is compromised by incomplete tight junctions that are more sensitive to airborne pollutants [401] and most likely to allergens and respiratory viral infections. This is further specifically disturbed by HRV infection which reduces expression levels of tight and adherens junction proteins [402]. In those with asthma, the presence of an HRV can induce illness that, while often more severe than in non-asthmatics, has been associated with significantly different HRV load or duration of HRV RNA detection in people with asthma compared to those without [403]. HRV-C types are often detected in more serious clinical outcomes than HRV-A or -B [265] although hospitalizations may be fewer for HRV-Cs than the other species [404].
Acute Otitis Media
AOM is diagnosed by middle ear effusion (otorrhea) with simultaneous signs and symptoms of ARI including fever, earache, rhinitis, cough, sore throat, chest wheeze, nocturnal restlessness, irritability, poor appetite, diarrhea, and vomiting. Transient abnormal (negative) ear pressure upon tympanometry occurs in two-thirds to three quarters of uncomplicated colds among healthy children [405, 406]. AOM is a frequent reason for outpatient antibiotic therapy which can reduce the time to resolution of symptoms in infants and has been attributed to reducing the overall hospital burden of AOM [407–412]. Since a longitudinal day-care study in 1982, the association between AOM and viral URT infection has been coalescing, and it is now clear that AOM often occurs with or shortly after a viral ARI, most frequently in the young and occurring more often during winter than summer [413, 414]. The use of influenza vaccines reduced AOM occurrence by a third during an epidemic period [415], but the use of pneumococcal vaccine did not reduce the occurrence of AOM overall, just that relatively small fraction (6 %) due to the target bacteria [416]. The isolation by culture and PCR detection of viruses from middle ear fluids and the refractory nature of some AOM cases to antibiotic therapies confirmed that viruses play an important role in this illness [409, 414, 417]. Studies relying on underperforming culture-based techniques underestimated the role for viral ARIs [414, 418], but other studies using PCR techniques and including HRVs found them to be the most frequently detected virus in middle ear fluids and nasopharyngeal secretions [409, 417].
The use of PCR has identified respiratory viruses, most often HRVs, in nasal secretions of 50–70 % of children with AOM [66, 413]. Because virus is often detected in the nasopharynx at the same time as the middle ear fluid, the question of the relevance of a PCR positive is a valid one [419]. Picornaviruses have been detected in 30 % of nasopharyngeal swabs taken during cold season from AOM-prone infants and young children, and large quantities of HRV RNA have been detected by in situ hybridization of adenoid tissues from 65 % of children with recurrent AOM and/or adenoid hyperplasia [259, 420]. In a cohort of children followed from 2 to 24 months and using culture-RT-PCR, HRVs in the URT were the second most frequent pathogens associated with AOM, after HRSV [66]. Viruses, most often HRVs (30.8 % of AOM with ARI), were also detected concurrently with non-ARI periods associated with AOM episodes (14 % of AOM without ARI)[418] suggesting that AOM may be the only manifestation of some HRV ARIs, just as wheezing sometimes is. In the United States, 180 subjects were enrolled and followed in a birth cohort until the first AOM episode or between 6 and 12 months of age 362]. HRVs accounted for 55 % of viruses detected and 69 % of specimens with a single virus detected. This dominance was maintained even through the 2009 H1N1 influenza pandemic [362].
In the day-care AOM study mentioned above, primary acquisition of Streptococcus pneumoniae or Haemophilus influenzae had minimal importance as an initiation factor for AOM with effusion, but nasopharyngeal colonization was important [413]. Animal studies have shown that virus-bacteria interactions have a role in nasopharyngeal colonization and AOM development [414]. Positive correlation has been made between HRV detection in AOM-prone children and Moraxella catarrhalis infection as well as a tendency toward the copresence of Streptococcus pneumoniae [259]. The presence of HRV-B14 was shown to increase adherence of S. pneumoniae in human tracheal epithelial cell cultures [421]. It is believed that these three bacterial pathogens can colonize without symptoms until a viral ARI shifts the balance toward a cytokine-mediated inflammatory state [419].
Other Diseases in Which HRVs Are Often Detected
Chronic Obstructive Pulmonary Disease
This disorder of older patients encompasses emphysema (alveolar destruction) and chronic bronchitis (large airway inflammation with chronic mucous production) and describes a long-term obstruction to airflow in the lung (compared to asthma which is a reversible obstruction with normal flow between exacerbations). While bacteria are found in half of all exacerbations, antibiotic therapies have often yielded poor outcomes [422]. HRV infections result in more COPD exacerbations (~66 % of cases [92]) than any other virus identified to date [422, 423]. An experimental human model of HRV infection in COPD provided preliminary evidence that HRVs cause exacerbations [286]. Viral culture associated symptomatic HRV infections with exacerbations among chronic bronchitics, including cases of isolation from sputum (LRT sample) in the absence of HRV in the URT [424]. Adding the measurement of an inflammatory marker in the serum, like IL-6, further improves the speed of predicting an infectious etiology for exacerbations of COPD [425].
Pneumonia
Pneumonia is a disease that often occurs early in life, is responsible for millions of deaths each year [426], and is caused by viral and/or bacterial infections. A diagnosis of pneumonia requires a radiologically confirmed inflammatory infiltration of the lung tissue. Childhood community-acquired pneumonia (CAP) is common in developing countries [427]. CAP also complicates existing chronic medical conditions and takes advantage of immunosenesence [428].
The role of HRVs in contributing to the development of bacterial pneumonia is likely underestimated [426, 429]. Determining an etiology is confounded by the rarity of obtaining LRT specimens, by short-term studies, and by the complex milieu of viruses and bacteria involved. Less invasive sampling of the URT permits more routine sampling and screening, and so convenience and reduced risk have led to the detection of putative pathogens in the URT with the general assumption that they account for LRT disease, especially in children under the age of 5 years [430]. Pneumonia studies are complicated by the lack of a suitable control group; sputum is not produced from the healthy lower airway and needle aspiration, while a gold standard is also a hospital procedure with some risk [431].
Studies that are comprehensive and use sensitive molecular testing are also rare for the study of CAP etiology. When used for CAP investigations, PCR methods almost double the microbiological diagnoses over conventional culture and serology techniques, especially improving the identification of mixed infections and fastidious viruses [432]. Rapid diagnosis aids management and helps make decisions about treatment, while prolonged searching for an etiological agent leads to further invasive testing [256, 433]. At least a quarter of clinical CAP cases remain unsupported by microbiological findings [241, 432].
Infections causing pneumonia vary with age and vaccination status [433]. Viruses can be detected in up to 90 % of infants (1–12 months of age) with pneumonia, and these cases follow a seasonal pattern [427, 433]. Bacteria can also be detected in over 90 % of infants and older children, the elderly, and those with severe CAP [432, 434]. Studies that predated the use of PCR pronounced HRSV, followed by HRVs, the major viral contributors to CAP, with viruses comprising 27–72 % of childhood pneumonia cases [429, 434]. In the PCR age, the role of HRVs has received increasing attention, and they are increasingly the major viral group detected from both URT and LRT (sputum) specimens of children with CAP. This holds true even when studies extend across 1 or more years, which presumably would account for seasonal variation in virus prevalence [241, 256, 426, 434]. It is suspected that viruses such as HRV prepare the way for subsequent bacterial infection in some direct or indirect fashion [65, 259, 420, 435]. There are laboratory data which support this [436] as well as observational data showing a high proportion of HRV-bacterial co-detections [256, 437].
Mixed infections including viruses are a possible cause of antibacterial treatment failure and sometimes a puzzle for physicians. Mixed infections occur frequently in LRT diseases such as pneumonia, which is not surprising since new techniques make it clear that the lungs are not the sterile environments we once thought [427, 434, 438, 439]. Viral-bacterial coinfections can comprise 15 % of patients, while viral-viral (2–30 %) and bacterial-bacterial (1–7 %) are much less common [230, 241, 256, 434, 437]. HRSV or HRV is often co-detected with S. pneumoniae in URT samples [256, 437, 440]. HRV detections dominate in younger children with pneumonia during peak HRV seasons, although frequently in co-detections with other viruses [230].
Acute Bronchitis
Acute bronchitis (less than 4-week duration in children) is defined as a sudden cough that often results from large airway infection and frequently involves viruses. Croup or laryngotracheobronchitis (viral or recurrent [441]) is a common LRT illness in children that includes the trachea and larynx as well as the larger airways, resulting in a barking cough. Patients with croup most often have a viral infection with some role for HRVs, although the extent of this is unclear [441, 442]. Despite testing, a third of cases remain without a viral etiology [441]. Tracheobronchitis resulted from some HRV-A15 infection of volunteers [46]. Chest pain and cough have been reported in half or more of adults with HRV infection [207] as well as in children and adults with HRVs detected during exacerbations of bronchitis, with or without an associated ARI [443, 444].
Bronchiolitis
Bronchiolitis occurs seasonally, especially in winter, in infants (1–12 months of age), affecting the small peripheral bronchioles. Winter is the peak season for HRSV circulation, but not usually for HRV. Bronchiolitis is a clinical diagnosis encompassing various disease entities and is most often reported in association with detection of HRSV, a winter virus [445, 446]. However, HRVs make up the majority of HRSV-negative bronchiolitis cases [128], and HRVs are co-detected with HRSV for which hospitalization is prolonged compared to cases positive for either virus alone [447]. Those children positive for an HRV during a clinically diagnosed bout of bronchiolitis have a significantly higher risk of recurrent wheezing in the subsequent year than those in whom another virus is detected [446]. HRVs were reported in over fivefold more cases of bronchiolitis than HRSV among patients in a 2-year prospective cohort of very low birth weight infants in Buenos Aires, Argentina [448].
Sinusitis
After a viral ARI, some proportion of infections may be complicated by sinusitis (inflammation of the sinus mucosa), the extent of which may be underestimated in children if the ARI is mild and unattended by parents [11]. Symptoms may include sinus pain, headache, facial pain, discolored nasal discharge, postnasal drip, cough, sore throat, malaise, and sometimes fever (more so in children) [367, 449]. The precise role for viruses and bacteria in sinusitis is still unclear [450]. Sinusitis is a common comorbidity in those with asthma [451]. The in situ presence of HRV-B14 RNA in maxillary sinus epithelium was reported in seven of 14 adults with acute sinusitis [452]. HRVs were also detected by PCR in half of adults with acute maxillary sinusitis; half of the HRV positives were negative for any bacteria [65]. The common cold is often associated with computed tomographically confirmed sinus cavity occlusion or abnormality in adults with self-diagnosed ARIs [367, 453]. Magnetic resonance imaging identified reversible abnormalities of the paranasal sinuses in a third of healthy adult volunteers following challenge with HRV-A39 [454]. Further evidence of the tropism of HRVs for sinus tissue comes from it being, so far, the only successful host for in vitro HRV-C replication [63].
Cystic Fibrosis
Culture- and serology-based testing has shown that virus infections in cystic fibrosis (CF) patients occur with the same prevalence as the general community, but the consequences of infection are more obvious or severe. These include deterioration of lung function, cough, increased expectoration and weight loss, and a synergistic increase in bacterial growth or acquisition of new bacterial infections [107, 455–458]. The mechanism behind the acquisition of new bacteria is still unknown and not always observed [459], but may involve a reduction in the host’s immune response or viral damage to the respiratory epithelium. There is circumstantial evidence that HRV infections have been associated with respiratory exacerbations in cystic fibrosis patients [459, 460], albeit in very low numbers by nonmolecular studies [461] and without a significantly different clinical outcome from non-HRV ARIs in these patients [107]. Molecular methods have not yet been applied regularly, thoroughly, and systematically, but they generally find HRVs to be prominent among CF children with ARI-associated respiratory exacerbation and involved in mixed viral-bacterial infections [459].
Control and Prevention
Hand washing and disinfectant wipes have been shown to be effective methods of interrupting transfer from fomites to the nose or to conjunctivae [87, 288, 293]. However, with eye rubbing, face touching, and nose-picking occurring frequently [47, 290], self-inoculation often outpaces personal hygiene, particularly in the young.
Hand disinfection is frequently recommended for prevention of HRV infection but has not been supported by controlled clinical trials in a natural setting [462] despite good results in experimental tests [463]. Ethanol-containing disinfectants were more effective than simple hand washing with soap and water for removal of HRV-A39 inoculum, as assessed by culture, and the inclusion of organic acids afforded a residual antiviral effect [463–465]. However, continual hand washing with extra ingredients resulted in skin irritation [462]. The experimental testing [463, 464] may have been biased by short study periods, the absence of a mucus carrier to mimic natural surface deposition and overly stringent control over virus application/hand disinfection compared with the natural study. Additionally, the natural setting study used PCR [462] which detects HRVs more often than culture. The disparity between outcomes may also reflect the contribution of airborne HRV transmission.
Because of the absence of a vaccine or specific antiviral, the most popular method of intervention in uncomplicated HRV ARIs is treatment of the symptoms. This is achieved using analgesics, decongestants, antihistamines, and antitussives. Due to a lack of studies, data are limited on the effectiveness of over-the-counter common cold medications for children [466]. Anticholinergic agents have proven useful to reduce rhinorrhea [467]. For controlling symptoms in those with exacerbated asthma, most of which do not require hospitalization, bronchodilators and oral corticosteroids are the main treatments [468]. The interruption of proinflammatory immune responses or specific signaling pathways using steroids, or other novel therapeutics, may prove to be a more robust approach for treating HRV infections; they have not been successful for HRSV [325].
When initiated early in the illness, a combination of antiviral (IFN-α2b) and anti-inflammatory (chlorpheniramine) components showed promise for interrupting nasal viral replication and symptoms [469].
Antiviral agents (Table 29.3) require early application to effectively precede the pathogenic immune response to HRV infection [325], but they often fail to reproduce their in vitro successes in vivo. Most antirhinoviral drugs are based on capsid-binding agents (Fig. 29.11). Additionally, oral delivery can complicate drug safety because this route increases the risk of systemic side effects compared to a nasal or topical route, but these risks must be considered alongside the disease to be treated; drug side effects are disproportionately severe compared to a common cold than to a severe asthma exacerbation. A systemic route is beneficial if an effect is sought on HRV replication sites that are otherwise inaccessible, such as those not associated with respiratory tract illness [470].
Table 29.3.
Preventive and therapeutic compounds affecting HRV infections
| Therapeutic agent | Primary role | Effect | Evaluation | Reference |
|---|---|---|---|---|
| IFN-α | Elicit cellular antiviral effects | Decreased shedding if administered within 24 h | Toxicity | [471–475] |
| Pirodavir (R77975) | Capsid binder | Intranasal formulation useful against both HRV antiviral groups; three to six doses per day | Variable efficacy, irritation, and mucosal bleeding | [471, 476, 477] |
| WIN54954 | Capsid binder | Broadly active in mice | Reduced efficacy in humans | [471, 478] |
| WIN56291 | Capsid binder | Active against HRV-C15 | Effect only in organ culture so far | [161, 479] [63] |
| Pleconaril (WIN63843) | Capsid binder | Resolved symptoms 1–2 days earlier than placebo. Some types are resistant | FDA issued “not approvable” letter because of side effects | [471, 477] |
| Vapendavir (BTA798) | Capsid binder | Potent binding in animal models | Good bioavailability and safety profile in animals. Phase IIa trial complete | [477, 480] |
| Rupintrivir (AG-7088) | 3C protease inhibitor | Insignificant impact | Discontinued | [470, 471, 481, 482] |
| Enviroxime | Replication inhibitor | Potent anti-replicative activity in vitro | Side effects in vivo | [406, 472] |
| Tremacamra | Soluble ICAM-1 molecule | Could reduce experimental cold symptoms and the quantity of virus shed if administration occurs before or after inoculation but prior to the development of symptoms | [483] | |
| Tiotropium | Anticholinergic agent (bronchodilator) | Reduced HRV-B14 viral load and RNA levels, decreased susceptibility of cells, reduced ICAM-1 mRNA levels and IL-1β, IL-6, and IL-8 protein levels in culture | No obvious change to cell viability in culture | [484] |
| Levofloxacin | Quinolone antibiotic | Reduced HRV-B14 and HRV-A15 viral load (major group HRVs; not the minor group virus, HRV-A2) and RNA levels, decreased susceptibility of cells, reduced ICAM-1 mRNA levels and IL1β, IL6, and IL8 protein levels in culture | No obvious cytotoxicity in culture | [485] |
| Pellino-1 | Regulates IRAK-1 | Controlled primary epithelial cell non-IFN response to HRV-A1; knockdown by siRNA reduced CXCL8 in primary BECs | Did not cause unwanted shutdown of an antiviral response. Target unknown | [486] |
| Azithromycin | Macrolide antibiotic | Significantly increased IFNs and ISG mRNA and proteins resulting from HRV-A1 and HRV-A16 infection in primary BECs. Reduced HRV replication and release | Modest effect in cell culture at relatively high concentration. Mechanism unknown | [487] |
FDA US Food and Drug Administration, ICAM-1 intercellular adhesion molecule 1, IFN interferon, IRAK-1 interleukin-1 receptor-associated kinase-1, BEC bronchial epithelial cells
Fig. 29.11.
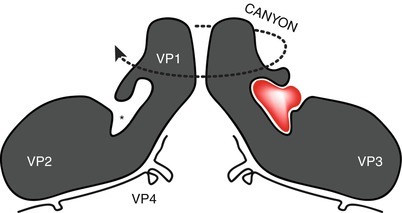
A simplified depiction of two protomers in opposition on a cross section of a pentamer. The positions of the structural peptides are indicated as is the canyon that circumscribes the central axis of the pentamer. The pocket (asterisked) at the base of the canyon is shown without the pocket factor or occupied by a stylized capsid-binding molecule (red). VP1-VP4 Viral protein (Adapted from McErlean et al. [61])
Unresolved Questions and Problems
The recent discovery of the new species, HRV-C, has shone a bright light on how little was known about the HRVs. The HRV-Cs and also the newly discovered HRV-As and HRV-Bs are fastidious in culture, with a single report of HRV-C growth in primary sinus tissue, and the identity of a cellular receptor still unknown. Thus, it is difficult to proceed in many areas, including basic virology, seroepidemiology, immunobiology, and antiviral testing. Determination of the receptors for these new HRVs would aid the search for a more accessible culture system. There would be great interest in a vaccine for some or all of the HRVs, but with increasing evidence of the interactions between HRVs, their hosts, and other respiratory viruses, it may not be wise to interfere before we fully understand what the impact of losing a constantly circulating HRV challenge would be. Antivirals specifically targeting the HRVs may be a better bet, but routine HRV testing and genotyping will first need to be more widespread as surveillance for antiviral resistance will be an important component of monitoring the success of any intervention.
Studies to determine whether there are differences in clinical and immunobiological impact between the many different types are lacking but would greatly improve our ability to plan future routine testing, understand all the clinical responses to the diverse HRVs and to outbreaks of ARI, and improve HRV epidemiology. It is interesting to note that the HRV-Bs are significantly underrepresented in HRV detections. We do not yet know their niche or clinical impact. It may be possible that HRV-Bs are the most well adapted of the HRVs, causing little to no detectable clinical impact, or they may create a different impact than that which we expect, or they may be a species in decline.
The jury remains out on whether HRVs cause or are involved in the development of asthma or merely trigger exacerbations once asthma is established. With a very high healthcare impact from asthma around the world and atopic conditions that may be exacerbated by HRVs on the rise, this is an important area for further investigations.
Acknowledgments
We wish to sincerely thank the following for valuable discussions: John Upham, Anne Chang, Danielle Wurzel, Michael Nissen, Ron Turner James Gern, and Stephen B. Liggett. We are grateful for the extreme patience of Corin and Ronan Mackay, slightly less so for their frequent provision of our firsthand experience in HRV clinical symptoms.
Contributor Information
Richard A. Kaslow, Phone: +1205975-8698, FAX: +1205934-8665, Email: Rkaslow@ms.soph.uab.edu
Lawrence R. Stanberry, Phone: +1212-305-2934, Email: lrs2155@columbia.edu
James W. Le Duc, Email: jwleduc@utmb.edu
Ian M. Mackay, Email: ian.mackay@uq.edu.au.
References
- 1.Rotbart HA, Hayden FG. Picornavirus infections: a primer for the practitioner. Arch Fam Med. 2000;9:913–20. doi: 10.1001/archfami.9.9.913. [DOI] [PubMed] [Google Scholar]
- 2.Bryce J, Boschi-Pinto C, Shibuya K, et al. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–52. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 3.Winther B, Hayden FG, Hendley JO. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: association with symptomatic illness and effect of season. J Med Virol. 2006;78:644–50. doi: 10.1002/jmv.20588. [DOI] [PubMed] [Google Scholar]
- 4.Bertino JS. Cost burden of viral respiratory infections: issues for formulary decision makers. Am J Med. 2002;112:42S–9. doi: 10.1016/s0002-9343(01)01063-4. [DOI] [PubMed] [Google Scholar]
- 5.Lambert SB, Allen KM, Carter RC, et al. The cost of community-managed viral respiratory illnesses in a cohort of healthy preschool-aged children. Respir Res. 2008;9:1–11. doi: 10.1186/1465-9921-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackay IM, Arden KE, Lambert SB. Epidemiology. In: Eccles R, Weber O, editors. Common cold. Berlin: Springer; 2009. pp. 77–106. [Google Scholar]
- 7.Monto AS. Epidemiology of viral respiratory infections. Am J Med. 2002;112:4S–12. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 8.Jartti T, Lee W-M, Pappas T, et al. Serial viral infections in infants with recurrent respiratory illnesses. Eur Respir J. 2008;32:314–20. doi: 10.1183/09031936.00161907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackay IM, Lambert SB, Faux CE, et al. Community-wide, contemporaneous circulation of a broad spectrum of human rhinoviruses in healthy Australian preschool-aged children during a 12-month period. J Infect Dis. 2013;207(9):1433–41. doi: 10.1093/infdis/jis476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bridges-Webb C, Britt H, Miles DA, et al. Morbidity and treatment in general practice in Australia. Aust Fam Physician. 1993;22:336–9. [PubMed] [Google Scholar]
- 11.Revai K, Dobbs LA, Nair S. Incidence of acute otitis media and sinusitis complicating upper respiratory tract infection: the effect of age. Pediatrics. 2007;119:e1408–12. doi: 10.1542/peds.2006-2881. [DOI] [PubMed] [Google Scholar]
- 12.Schroth MK, Grimm E, Frindt P, et al. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am J Respir Cell Mol Biol. 1999;20:1220–8. doi: 10.1165/ajrcmb.20.6.3261. [DOI] [PubMed] [Google Scholar]
- 13.Papadopoulos NG, Bates PJ, Bardin PG, et al. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–84. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 14.Hakonarson H, Maskeri N, Carter C, et al. Mechanism of rhinovirus-induced changes in airway smooth muscle responsiveness. J Clin Invest. 1998;102:1732–41. doi: 10.1172/JCI4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gern JE, Dick EC, Lee W-M, et al. Rhinovirus enters but does not replicate inside monocytes or airway macrophages. J Immunol. 1996;156:621–7. [PubMed] [Google Scholar]
- 16.Schrauf C, Kirchberger S, Majdic O, et al. The ssRNA genome of human rhinovirus induces a type I IFN response but fails to induce maturation in human monocyte-derived dendritic cells. J Immunol. 2009;183:4440–8. doi: 10.4049/jimmunol.0804147. [DOI] [PubMed] [Google Scholar]
- 17.Lidwell OM, Sommerville T. Observations on the incidence and distribution of the common cold in a rural community during 1948 and 1949. J Hyg (Lond) 1951;49:365–81. doi: 10.1017/s0022172400066699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson GG, Dowling HF. Transmission of the common cold to volunteers under controlled conditions. IV. Specific immunity to the common cold. J Clin Invest. 1959;38:762–9. doi: 10.1172/JCI103857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner RB. The common cold. Pediatr Ann. 1998;27:790–5. doi: 10.3928/0090-4481-19981201-06. [DOI] [PubMed] [Google Scholar]
- 20.Atzl I, Helms R. A short history of the common cold. In: Eccles R, Weber O, editors. Common cold. Basel: Birkhäuser; 2009. pp. 1–21. [Google Scholar]
- 21.von Klein CH. The medical features of the papyrus Ebers. JAMA. 1905; XLC:1928–1935.
- 22.Hilding A. The common cold. Arch Otolaryngol Head Neck Surg. 1930;12:133–50. [Google Scholar]
- 23.Tyrrell DAJ, Fielder M. Cold wars: the fight against the common cold. New York: Oxford University Press; 2002. [Google Scholar]
- 24.Andrewes C. In pursuit of the common cold. London: William Heinemann Medical Books Limited; 1973. [Google Scholar]
- 25.Andrewes CH, Chaproniere DM, Gompels AEH, et al. Propagation of common-cold virus in tissue cultures. Lancet. 1953;265:546–7. doi: 10.1016/s0140-6736(53)90279-7. [DOI] [PubMed] [Google Scholar]
- 26.Tyrrell DAJ, Buckland FE, Bynoe ML, et al. The cultivation in human-embryo cells of a virus (D.C.) causing colds in man. Lancet. 1962;280:320–2. doi: 10.1016/s0140-6736(62)90107-1. [DOI] [PubMed] [Google Scholar]
- 27.Conant RM, Hamparian VV. Rhinoviruses: basis for a numbering system. I. HeLa cells for propagation and serologic procedures. J Immunol. 1968(100). [PubMed]
- 28.Conant RM, Hamparian VV. Rhinoviruses: basis for a numbering system. II serologic characterization of prototype strains. J Immunol. 1968;100:107–13. [PubMed] [Google Scholar]
- 29.Kapikian AZ, Conant RM, Hamparian VV, et al. A collaborative report: rhinoviruses-extension of the numbering system. Virology. 1971(43). [PubMed]
- 30.Hamparian VV, Colonno RJ, Cooney MK, et al. A collaborative report: rhinoviruses - extension of the numbering system from 89 to 100. Virology. 1987;159:191–2. doi: 10.1016/0042-6822(87)90367-9. [DOI] [PubMed] [Google Scholar]
- 31.Arden KE, McErlean P, Nissen MD, et al. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol. 2006;78:1232–40. doi: 10.1002/jmv.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau SKP, Yip CCY, Tsoi H-W, et al. Clinical features and complete genome characterization of a distinct human rhinovirus genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J Clin Microbiol. 2007;45:3655–64. doi: 10.1128/JCM.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McErlean P, Shackelton LA, Andrewes E, et al. Distinguishing molecular features and clinical characteristics of a putative new rhinovirus species, human rhinovirus C (HRV C) PLoS One. 2008;3:e1847. doi: 10.1371/journal.pone.0001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carstens EB. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2009) Arch Virol. 2010;155:133–46. doi: 10.1007/s00705-009-0547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carstens EB, Ball LA. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2008) Arch Virol. 2009;154:1181–8. doi: 10.1007/s00705-009-0400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrewes CH. Rhinoviruses and common colds. Annu Rev Med. 1966;17:361–70. doi: 10.1146/annurev.me.17.020166.002045. [DOI] [PubMed] [Google Scholar]
- 37.Pelon W, Mogabgab WJ, Phillips LA, et al. A cytopathogenic agent isolated from naval recruits with mild respiratory illnesses. Proc Soc Exp Biol Med. 1957;94:262–7. doi: 10.3181/00379727-94-22915. [DOI] [PubMed] [Google Scholar]
- 38.Price WH. The isolation of a new virus associated with respiratory clinical disease in humans. Proc Natl Acad Sci U S A. 1956;42:892–6. doi: 10.1073/pnas.42.12.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelon W. Classification of the “2060” virus as ECHO 28 and further study of its properties. Am J Hyg. 1961;73:36–54. doi: 10.1093/oxfordjournals.aje.a120164. [DOI] [PubMed] [Google Scholar]
- 40.Tyrrell DAJ, Bynoe ML, Hitchcock G, et al. Some virus isolations from common colds. I. Experiments employing human volunteers. Lancet. 1960;1:235–7. doi: 10.1016/s0140-6736(60)90166-5. [DOI] [PubMed] [Google Scholar]
- 41.Parsons R, Tyrrell DAJ. A plaque method for assaying some viruses isolated from common colds. Nature. 1961;189:640–2. doi: 10.1038/189640a0. [DOI] [PubMed] [Google Scholar]
- 42.Mogabgab WJ, Pelon W. Problems in characterizing and identifying an apparently new virus found in association with mild respiratory disease in recruits. Ann N Y Acad Sci. 1957;67:403–12. doi: 10.1111/j.1749-6632.1957.tb46063.x. [DOI] [PubMed] [Google Scholar]
- 43.Hendley JO, Edmondson WP, Jr, Gwaltney JM., Jr Relation between naturally acquired immunity and infectivity of two rhinoviruses in volunteers. J Infect Dis. 1972;125:243–8. doi: 10.1093/infdis/125.3.243. [DOI] [PubMed] [Google Scholar]
- 44.Douglas RG, Jr, Cate TR, Gerone PJ, et al. Quantitative rhinovirus shedding patterns in volunteers. Am Rev Respir Dis. 1966;94:159–67. doi: 10.1164/arrd.1966.94.2.159. [DOI] [PubMed] [Google Scholar]
- 45.D’Alessio DJ, Meschievitz CK, Peterson JA, et al. Short-duration exposure and the transmission of rhinoviral colds. J Infect Dis. 1984;150:189–94. doi: 10.1093/infdis/150.2.189. [DOI] [PubMed] [Google Scholar]
- 46.Cate TR, Couch RB, Fleet WF, et al. Production of tracheobronchitis in volunteers with rhinovirus in a small-particle aerosol. Am J Epidemiol. 1965;81:95–105. doi: 10.1093/oxfordjournals.aje.a120501. [DOI] [PubMed] [Google Scholar]
- 47.Hendley JO, Wenzel RP, Gwaltney JM., Jr Transmission of rhinovirus colds by self-inoculation. N Engl J Med. 1973;288:1361–4. doi: 10.1056/NEJM197306282882601. [DOI] [PubMed] [Google Scholar]
- 48.Sethi SK. Reproducible plaquing system for rhinovirus serotypes in HeLa cells – agarose suspension. Acta Virol. 1978;22:60–5. [PubMed] [Google Scholar]
- 49.Gwaltney JM., Jr Micro-neutralization test for identification of rhinovirus serotypes. Proc Soc Exp Biol Med. 1966;122:1137–41. doi: 10.3181/00379727-122-31345. [DOI] [PubMed] [Google Scholar]
- 50.Behbehani AM, Lee LH. Growth, plaque production and cationic stabilization of rhinovirus type 1 (Echovirus 28) J Bacteriol. 1964;88:1608–11. doi: 10.1128/jb.88.6.1608-1611.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fiala M, Kenny GE. Enhancement of rhinovirus plaque formation in human heteroploid cell cultures by magnesium and calcium. J Bacteriol. 1966;92:1710–1715. doi: 10.1128/jb.92.6.1710-1715.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jurgeit A, Moese S, Roulin P, et al. An RNA replication-center assay for high content image-based quantifications of human rhinovirus and coxsackievirus infections. Virol J. 2010;7:264. doi: 10.1186/1743-422X-7-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papadopoulos NG, Sanderson G, Hunter J, et al. Rhinoviruses replicate effectively at lower airway temperatures. J Med Virol. 1999;58:100–4. doi: 10.1002/(sici)1096-9071(199905)58:1<100::aid-jmv16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 54.Rosenbaum MJ, De Berry P, Sullivan EJ, et al. Epidemiology of the common cold in military recruits with emphasis on infections by rhinovirus types 1A, 2, and two unclassified rhinoviruses. Am J Epidemiol. 1971;93:183–93. doi: 10.1093/oxfordjournals.aje.a121245. [DOI] [PubMed] [Google Scholar]
- 55.Johnston SL, Bardin PG, Pattemore PK. Viruses as precipitants of asthma symptoms III. Rhinoviruses: molecular biology and prospects for future intervention. Clin Exp Allergy. 1993;23:237–46. doi: 10.1111/j.1365-2222.1993.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 56.Ketler A, Hamparian VV, Hilleman MR. Characterization and classification of ECHO 28-rhinovirus-coryzavirus agents. Proc Soc Exp Biol Med. 1962;110:821–31. doi: 10.3181/00379727-110-27662. [DOI] [PubMed] [Google Scholar]
- 57.Kapikian AZ, Conant RM, Chanock RM, et al. Rhinoviruses: a numbering system. Nature. 1967;213:761–2. doi: 10.1038/213761a0. [DOI] [PubMed] [Google Scholar]
- 58.Schindera C, Kraemer AL, Regamey N, et al. Immunofluorescence versus xTAG multiplex PCR for the detection of respiratory picornavirus infections in children. J Clin Virol. 2010;48:223–5. doi: 10.1016/j.jcv.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McErlean P, Shackelton LA, Lambert SB, et al. Characterisation of a newly identified human rhinovirus, HRV-QPM, discovered in infants with bronchiolitis. J Clin Virol. 2007;39:67–75. doi: 10.1016/j.jcv.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller EK, Edwards KM, Weinberg GA, et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009;123:98–104. doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bochkov YA, Hanson KM, Keles S, et al. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee W-M, Kiesner C, Pappas T, et al. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illness in infants. PLoS One. 2007;2:e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bochkov YA, Palmenberg AC, Lee W-M, et al. Molecular modeling, organ culture and reverse genetics for a newly identified human rhinovirus C. Nat Med. 2011;17:627–32. doi: 10.1038/nm.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gwaltney JM, Jr, Hendley JO, Simon G, et al. Rhinovirus infections in an industrial population I. The occurrence of illness. N Engl J Med. 1966;275:1261–8. doi: 10.1056/NEJM196612082752301. [DOI] [PubMed] [Google Scholar]
- 65.Pitkäranta A, Arruda E, Malmberg H, et al. Detection of rhinovirus in sinus brushings of patients with acute community-acquired sinusitis by reverse transcription-PCR. J Clin Microbiol. 1997;35:1791–3. doi: 10.1128/jcm.35.7.1791-1793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vesa S, Kleemola M, Blomqvist S, et al. Epidemiology of documented viral respiratory infections and acute otitis media in a cohort of children followed from two to twenty-four months of age. Pediatr Infect Dis J. 2001;20:574–81. doi: 10.1097/00006454-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 67.Kämmerer U, Kunkel B, Korn K. Nested PCR for specific detection and rapid identification of human picornaviruses. J Clin Microbiol. 1994;32:285–91. doi: 10.1128/jcm.32.2.285-291.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andeweg AC, Bestebroer TM, Huybreghs M, et al. Improved detection of rhinoviruses in clinical samples by using a newly developed nested reverse transcription-PCR assay. J Clin Microbiol. 1999;37:524–30. doi: 10.1128/jcm.37.3.524-530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arruda E, Pitkäranta A, Witek TJ, et al. Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol. 1997;35:2864–8. doi: 10.1128/jcm.35.11.2864-2868.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Renwick N, Schweiger B, Kapoor V, et al. A recently identified rhinovirus genotype is associated with severe respiratory-tract infection in children in Germany. J Infect Dis. 2007;196:1754–60. doi: 10.1086/524312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aberle JH, Aberle SW, Pracher E, et al. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon-gamma response. Pediatr Infect Dis J. 2005;24:605–10. doi: 10.1097/01.inf.0000168741.59747.2d. [DOI] [PubMed] [Google Scholar]
- 72.Versteegh FGA, Weverling GJ, Peeters MF, et al. Community-acquired pathogens associated with prolonged coughing in children: a prospective cohort study. Clin Microbiol Infect. 2005;11:801–7. doi: 10.1111/j.1469-0691.2005.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hutchinson AF, Ghimire AK, Thompson MA, et al. A community-based, time-matched, case-control study of respiratory viruses and exacerbations of COPD. Respir Med. 2007;101:2472–81. doi: 10.1016/j.rmed.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 74.Arruda E, Hayden FG. Detection of human rhinovirus RNA in nasal washings by PCR. Mol Cell Probes. 1993;7:373–9. doi: 10.1006/mcpr.1993.1055. [DOI] [PubMed] [Google Scholar]
- 75.Lu X, Holloway B, Dare RK, et al. Real-time reverse transcription-PCR assay for comprehensive detection of human rhinoviruses. J Clin Microbiol. 2008;46:533–9. doi: 10.1128/JCM.01739-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xatzipsalti M, Kyrana S, Tsolia M, et al. Rhinovirus viremia in children with respiratory infections. Am J Respir Crit Care Med. 2005;172:1037–40. doi: 10.1164/rccm.200502-315OC. [DOI] [PubMed] [Google Scholar]
- 77.Harvala H, McIntyre CL, McLeish NJ, et al. High detection frequency and viral loads of human rhinovirus species A to C in fecal samples; diagnostic and clinical implications. J Med Virol. 2012;84:536–42. doi: 10.1002/jmv.23203. [DOI] [PubMed] [Google Scholar]
- 78.Victoria JG, Kapoor A, Li L, et al. Metagenomic analyses of viruses in stool samples from children with acute flaccid paralysis. J Virol. 2009;83:4642–51. doi: 10.1128/JVI.02301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnston SL, Sanderson G, Pattemore PK, et al. Use of polymerase chain reaction for diagnosis of picornavirus infection in subjects with and without respiratory symptoms. J Clin Microbiol. 1993;31:111–7. doi: 10.1128/jcm.31.1.111-117.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suvilehto J, Roivainen M, Seppänen M, et al. Rhinovirus/enterovirus RNA in tonsillar tissue of children with tonsillar disease. J Clin Virol. 2006;35:292–7. doi: 10.1016/j.jcv.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 81.Mackay IM, Bustin S, Andrade JM, Nissen MD, Sloots TP. Quantification of microorganisms: not human, not simple, not quick. In: Mackay IM, editor. Real-time PCR in microbiology. Norfolk: Caister Academic Press; 2007. pp. 133–82. [Google Scholar]
- 82.Gama RE, Hughes PJ, Bruce CB, et al. Polymerase chain reaction amplification of rhinovirus nucleic acids from clinical material. Nucleic Acids Res. 1988;16:9346. doi: 10.1093/nar/16.19.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tapparel C, Junier T, Germann D, et al. New respiratory enterovirus and recombinant rhinoviruses among circulating strains. Emerg Infect Dis. 2009;15:719–26. doi: 10.3201/eid1505.081286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Torgersen H, Skern T, Blaas D. Typing of human rhinoviruses based on sequence variations in the 5′ non-coding region. J Gen Virol. 1989;70:3111–6. doi: 10.1099/0022-1317-70-11-3111. [DOI] [PubMed] [Google Scholar]
- 85.Gern JE, Vrtis R, Grindle KA, et al. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–31. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 86.Spence L, Brown WJ, Pyne DB, et al. Incidence, etiology, and symptomatology of upper respiratory illness in elite athletes. Med Sci Sports Exerc. 2007;39:577–86. doi: 10.1249/mss.0b013e31802e851a. [DOI] [PubMed] [Google Scholar]
- 87.Winther B, McCue K, Ashe K, et al. Environmental contamination with rhinovirus and transfer to fingers of healthy individuals by daily life activity. J Med Virol. 2007;79:1606–10. doi: 10.1002/jmv.20956. [DOI] [PubMed] [Google Scholar]
- 88.Khetsuriani N, Kazerouni NN, Erdman DD, et al. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119:314–21. doi: 10.1016/j.jaci.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blomqvist S, Skyttä A, Roivainen M, et al. Rapid detection of human rhinoviruses in nasopharyngeal aspirates by a microwell reverse transcription-PCR-hybridization assay. J Clin Microbiol. 1999;37:2813–6. doi: 10.1128/jcm.37.9.2813-2816.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nokso-Koivisto J, Kinnari TJ, Lindahl P, et al. Human picornavirus and coronavirus RNA in nasopharynx of children without concurrent respiratory symptoms. J Med Virol. 2002;66:417–20. doi: 10.1002/jmv.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kusel MMH, de Klerk NH, Holt PG, et al. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life. Pediatr Infect Dis J. 2006;25:680–6. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 92.Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1618–23. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 93.Papadopoulos NG, Hunter J, Sanderson G, et al. Rhinovirus identification by BglI digestion of picornavirus RT-PCR amplicons. J Virol Methods. 1999;80:179–85. doi: 10.1016/S0166-0934(99)00045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pitkäranta A, Virolainen A, Jero J, et al. Detection of rhinovirus, respiratory syncytial virus and coronavirus infections in acute otitis media by reverse-transcriptase polymerase chain reaction. Pediatrics. 1998;102:291–5. doi: 10.1542/peds.102.2.291. [DOI] [PubMed] [Google Scholar]
- 95.Hayden FG, Herrington DT, Coats TL, et al. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin Infect Dis. 2003;36:1523–32. doi: 10.1086/375069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wright PF, Deatly AM, Karron RA, et al. Comparison of results of detection of rhinovirus by PCR and viral culture in human nasal wash specimens from subjects with and without clinical symptoms of respiratory illness. J Clin Microbiol. 2007;45:2126–9. doi: 10.1128/JCM.02553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Steininger C, Aberle SW, Popow-Kraupp T. Early detection of acute rhinovirus infections by a rapid reverse transcription-PCR assay. J Clin Microbiol. 2001;39:129–33. doi: 10.1128/JCM.39.1.129-133.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Billaud G, Peny S, Legay V, et al. Detection of rhinovirus and enterovirus in upper respiratory tract samples using a multiplex nested PCR. J Virol Methods. 2003;108:223–8. doi: 10.1016/s0166-0934(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 99.Hyypiä T, Auvinen P, Maaronen M. Polymerase chain reaction for human picornaviruses. J Gen Virol. 1989;70:3261–8. doi: 10.1099/0022-1317-70-12-3261. [DOI] [PubMed] [Google Scholar]
- 100.Freymuth F, Vabret A, Galateau-Salle F, et al. Detection of respiratory syncytial virus, parainfluenzavirus 3, adenovirus and rhinovirus sequences in respiratory tract of infants by polymerase chain reaction and hybridization. Clin Diagn Virol. 2000;8:31–40. doi: 10.1016/s0928-0197(97)00060-3. [DOI] [PubMed] [Google Scholar]
- 101.Halonen P, Rocha E, Hierholzer J, et al. Detection of enteroviruses and rhinoviruses in clinical specimens by PCR and liquid-phase hybridization. J Clin Microbiol. 1995;33:648–53. doi: 10.1128/jcm.33.3.648-653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blomqvist S, Roivainen M, Puhakka T, et al. Virological and serological analysis of rhinovirus infections during the first two years of life in a cohort of children. J Med Virol. 2002;66:263–8. doi: 10.1002/jmv.2140. [DOI] [PubMed] [Google Scholar]
- 103.Coiras MT, Aguilar JC, García ML, et al. Simultaneous detection of fourteen respiratory viruses in clinical specimens by two multiplex reverse transcription nested-PCR assays. J Med Virol. 2004;72:484–95. doi: 10.1002/jmv.20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leparc I, Aymard M, Fuchs F. Acute, chronic and persistent enterovirus and poliovirus infections: detection of viral genome by seminested PCR amplification in culture-negative samples. Mol Cell Probes. 1994;8:487–95. doi: 10.1006/mcpr.1994.1070. [DOI] [PubMed] [Google Scholar]
- 105.Lina B, Pozzetto B, Andreoletti L, et al. Multicenter evaluation of a commercially available PCR assay for diagnosing enterovirus infection in a panel of cerebrospinal fluid specimens. J Clin Microbiol. 1996;34:3002–6. doi: 10.1128/jcm.34.12.3002-3006.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ireland DC, Kent J, Nicholson KG. Improved detection of rhinoviruses in nasal and throat swabs by seminested RT-PCR. J Med Virol. 1993;40:96–101. doi: 10.1002/jmv.1890400204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Collinson J, Nicholson KG, Cancio E, et al. Effects of upper respiratory tract infections in patients with cystic fibrosis. Thorax. 1996;51:1115–22. doi: 10.1136/thx.51.11.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Deffernez C, Wunderli W, Thomas Y, et al. Amplicon sequencing and improved detection of human rhinovirus in respiratory samples. J Clin Microbiol. 2004;42:3212–8. doi: 10.1128/JCM.42.7.3212-3218.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jartti T, Lehtinen P, Vuorinen T, et al. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10:1095–101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yoo SJ, Kuak EY, Shin BM. Detection of 12 respiratory viruses with two-set multiplex reverse transcriptase-PCR assay using a dual priming oligonucleotide system. Korean J Lab Med. 2007;27:420–7. doi: 10.3343/kjlm.2007.27.6.420. [DOI] [PubMed] [Google Scholar]
- 111.Faux CE, Arden KE, Lambert SB, et al. Usefulness of published PCR primers in detecting human rhinovirus infection. Emerg Infect Dis. 2011;17:296–8. doi: 10.3201/eid1702.101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Andréoletti L, Lesay M, Deschildre A, et al. Differential detection of rhinoviruses and enteroviruses RNA sequences associated with classical immunofluorescence assay detection of respiratory virus antigens in nasopharyngeal swabs from infants with bronchiolitis. J Med Virol. 2000;61:341–6. doi: 10.1002/1096-9071(200007)61:3<341::AID-JMV10>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gama RE, Horsnell PR, Hughes PJ, et al. Amplification of rhinovirus specific nucleic acids from clinical samples using the polymerase chain reaction. J Med Virol. 1989;28:73–7. doi: 10.1002/jmv.1890280204. [DOI] [PubMed] [Google Scholar]
- 114.Arden KE, Mackay IM. Newly identified human rhinoviruses: molecular methods heat up the cold viruses. Rev Med Virol. 2010;20:156–76. doi: 10.1002/rmv.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nolte FS, Marshall DJ, Rasberry C, et al. MultiCode-PLx system for multiplexed detection of seventeen respiratory viruses. J Clin Microbiol. 2007;45:2779–86. doi: 10.1128/JCM.00669-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Briese T, Palacios G, Kokoris M, et al. Diagnostic system for rapid and sensitive differential detection of pathogens. Emerg Infect Dis. 2005;11:310–3. doi: 10.3201/eid1102.040492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lamson D, Renwick N, Kapoor V, et al. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004–2005. J Infect Dis. 2006;194:1398–402. doi: 10.1086/508551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kato M, Tsukagoshi H, Yoshizumi M, et al. Different cytokine profile and eosinophil activation are involved in rhinovirus- and RS virus-induced acute exacerbation of childhood wheezing. Pediatr Allergy Immunol. 2011;22:e87–94. doi: 10.1111/j.1399-3038.2010.01026.x. [DOI] [PubMed] [Google Scholar]
- 119.Mahony J, Chong S, Merante F, et al. Development of a respiratory virus panel test for detection of twenty human respiratory viruses by use of multiplex PCR and a fluid microbead-based assay. J Clin Microbiol. 2007;45:2965–70. doi: 10.1128/JCM.02436-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dabisch-Ruthe M, Vollmer T, Adams O, et al. Comparison of three multiplex PCR assays for the detection of respiratory viral infections: evaluation of xTAG respiratory virus panel fast assay, RespiFinder 19 assay and RespiFinder SMART 22 assay. BMC Infect Dis. 2012;12:163. doi: 10.1186/1471-2334-12-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Forman MS, Advani S, Newman C, et al. Diagnostic performance of two highly multiplexed respiratory virus assays in a pediatric cohort. J Clin Virol. 2012;55(2):168–72. doi: 10.1016/j.jcv.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mahony JB, Petrich A, Smieja M. Molecular diagnosis of respiratory virus infections. Crit Rev Clin Lab Sci. 2011;48:217–49. doi: 10.3109/10408363.2011.640976. [DOI] [PubMed] [Google Scholar]
- 123.Wang D, Coscoy L, Zylberberg M, et al. Microarray-based detection and genotyping of viral pathogens. Proc Natl Acad Sci U S A. 2002;99:15687–92. doi: 10.1073/pnas.242579699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lysholm F, Wetterbom A, Lindau C, et al. Characterization of the viral microbiome in patients with severe lower respiratory tract infections, using metagenomic sequencing. PLoS One. 2012;7:e30875. doi: 10.1371/journal.pone.0030875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ostroff R, Ettinger A, La H, et al. Rapid multiserotype detection of human rhinoviruses on optically coated silicon surfaces. J Clin Virol. 2001;21:105–17. doi: 10.1016/S1386-6532(01)00150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shanmukh S, Jones L, Driskell J, et al. Rapid and sensitive detection of respiratory virus molecular signatures using a silver nanorod array SERS substrate. Nano Lett. 2006;6:2630–6. doi: 10.1021/nl061666f. [DOI] [PubMed] [Google Scholar]
- 127.Simmonds P, McIntyre CL, Savolainen-Kopra C, et al. Proposals for the classification of human rhinovirus species C into genotypically-assigned types. J Gen Virol. 2010;91:2409–19. doi: 10.1099/vir.0.023994-0. [DOI] [PubMed] [Google Scholar]
- 128.Henquell C, Mirand A, Deusebis AL, et al. Prospective genotyping of human rhinoviruses in children and adults during the winter of 2009–2010. J Clin Virol. 2012;53:280–4. doi: 10.1016/j.jcv.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 129.Watters K, Palmenberg AC. Differential processing of nuclear pore complex proteins by rhinovirus 2A proteases from different species and serotypes. J Virol. 2011;85:10874–83. doi: 10.1128/JVI.00718-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Koliais SI, Dimmock NJ. Replication of rhinovirus RNA. J Gen Virol. 1973;20:1–15. doi: 10.1099/0022-1317-20-1-1. [DOI] [PubMed] [Google Scholar]
- 131.Macnaughton MR. The structure and replication of rhinoviruses. Curr Top Microbiol Immunol. 1982;97:1–26. doi: 10.1007/978-3-642-68318-3_1. [DOI] [PubMed] [Google Scholar]
- 132.Haghighat A, Svitkin Y, Novoa I, et al. The eIF4G-eIF4E complex is the target for direct cleavage by the rhinovirus 2A proteinase. J Virol. 1996;70:8444–50. doi: 10.1128/jvi.70.12.8444-8450.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Svitkin YV, Gradi A, Imataka H, et al. Eukaryotic initiation factor 4GII (eIF4GII), but not eIF4GI, cleavage correlates with inhibition of host cell protein synthesis after human rhinovirus infection. J Virol. 1999;73:3467–72. doi: 10.1128/jvi.73.4.3467-3472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Etchison D, Fout S. Human rhinovirus 14 infection of HeLa cells results in the proteolytic cleavage of the p220 cap-binding complex subunit and inactivates globin mRNA translation in vitro. J Virol. 1985;54:634–8. doi: 10.1128/jvi.54.2.634-638.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gustin KE, Sarnow P. Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J Virol. 2002;76:8787–96. doi: 10.1128/JVI.76.17.8787-8796.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ghildyal R, Jordan B, Li D, et al. Rhinovirus 3C protease can localize in the nucleus and alter active and passive nucleocytoplasmic transport. J Virol. 2009;83:7349–52. doi: 10.1128/JVI.01748-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wilson R, Alton E, Rutman A, et al. Upper respiratory tract viral infection and mucociliary clearance. Eur J Respir Dis. 1987;70:272–9. [PubMed] [Google Scholar]
- 138.Arden KE, Mackay IM. Rhinoviruses. In eLS (John Wiley & Sons, Ltd); 2011. http://www.els.net [doi: 10.1002/9780470015902.a0000431.pub3].
- 139.Hughes PJ, North C, Minor PD, et al. The complete nucleotide sequence of Coxsackievirus A21. J Gen Virol. 1989;70:2943–52. doi: 10.1099/0022-1317-70-11-2943. [DOI] [PubMed] [Google Scholar]
- 140.Rueckert RR, Wimmer E. Systematic nomenclature of picornavirus proteins. J Virol. 1984;50:957–9. doi: 10.1128/jvi.50.3.957-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stanway G, Hughes PJ, Mountford RC, et al. The complete nucleotide sequence of a common cold virus: human rhinovirus 14. Nucleic Acids Res. 1984;12:7859–77. doi: 10.1093/nar/12.20.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Skern T, Sommergruber W, Blaas D, et al. Human rhinovirus 2: complete nucleotide sequence and proteolytic processing signals in the capsid protein region. Nucleic Acids Res. 1985;13:2111–26. doi: 10.1093/nar/13.6.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hughes PJ, North C, Jellis CH, et al. The nucleotide sequence of human rhinovirus 1B: molecular relationships within the rhinovirus genus. J Gen Virol. 1988;69:49–58. doi: 10.1099/0022-1317-69-1-49. [DOI] [PubMed] [Google Scholar]
- 144.Kistler A, Webster DR, Rouskin S, et al. Genome-wide diversity and selective pressure in the human rhinovirus. Virol J. 2007;4:40. doi: 10.1186/1743-422X-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tapparel C, Junier T, Gerlach D, et al. New complete genome sequences of human rhinoviruses shed light on their phylogeny and genomic features. BMC Genomics. 2007;10:224. doi: 10.1186/1471-2164-8-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Savolainen C, Blomqvist S, Mulders MN, et al. Genetic clustering of all 102 human rhinovirus prototype strains: serotype 87 is close to human enterovirus 70. J Gen Virol. 2002;83:333–40. doi: 10.1099/0022-1317-83-2-333. [DOI] [PubMed] [Google Scholar]
- 147.Ledford RM, Patel NR, Demenczuk TM, et al. VP1 sequencing of all human rhinovirus serotypes: insights into genus phylogeny and susceptibility to antiviral capsid-binding compounds. J Virol. 2004;78:3663–74. doi: 10.1128/JVI.78.7.3663-3674.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Drummond AJ, Ashton B, Buxton S, et al. Geneious v5.4. 2011. http://www.geneious.com. 2010. (Ref Type: Computer Program).
- 149.Tamura K, Peterson D, Peterson N, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Miller EK, Mackay IM. From sneeze to wheeze: what we know about rhinovirus Cs. Int J Pediatr. 2013;57(4):291–9. doi: 10.1016/j.jcv.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 151.Simmonds P. Recombination in the evolution of picornaviruses. In: Ehrenfeld E, Domingo E, Roos RP, editors. The Picornaviruses. Washington, DC: ASM Press; 2010. [Google Scholar]
- 152.Blomqvist S, Savolainen-Kopra C, Paananen A, et al. Molecular characterization of human rhinovirus field strains isolated during surveillance of enteroviruses. J Gen Virol. 2009;90:1371–81. doi: 10.1099/vir.0.008508-0. [DOI] [PubMed] [Google Scholar]
- 153.Lewis-Rogers N, Bendall ML, Crandall KA. Phylogenetic relationships and molecular adaptation dynamics of human rhinoviruses. Mol Biol Evol. 2009;26:969–81. doi: 10.1093/molbev/msp009. [DOI] [PubMed] [Google Scholar]
- 154.Simmonds P. Recombination and selection in the evolution of picornaviruses and other mammalian positive-stranded RNA viruses. J Virol. 2006;80:11124–40. doi: 10.1128/JVI.01076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Egger D, Bienz K. Recombination of poliovirus RNA proceeds in mixed replication complexes originating from distinct replication start sites. J Virol. 2002;76:10960–71. doi: 10.1128/JVI.76.21.10960-10971.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kirkegaard K, Baltimore D. The mechanism of RNA recombination in poliovirus. Cell. 1986;47:433–43. doi: 10.1016/0092-8674(86)90600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Palmenberg AC, Spiro D, Kuzmickas R, et al. Sequencing and analyses of all known human rhinovirus genomes reveals structure and evolution. Science. 2009;324:55–9. doi: 10.1126/science.1165557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McIntyre CL, William Leitch EC, Savolainen-Kopra C, et al. Analysis of genetic diversity and sites of recombination in human rhinovirus species C. J Virol. 2010;84:10297–310. doi: 10.1128/JVI.00962-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rossmann MG, Arnold E, Erickson JW, et al. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985;317:145–53. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- 160.Kim SS, Smith TJ, Chapman MS, et al. Crystal structure of human rhinovirus serotype 1A (HRV1A) J Mol Biol. 1989;210:91–111. doi: 10.1016/0022-2836(89)90293-3. [DOI] [PubMed] [Google Scholar]
- 161.Oliveira MA, Zhao R, Lee W-M, et al. The structure of human rhinovirus 16. Structure. 1993;1:51–68. doi: 10.1016/0969-2126(93)90008-5. [DOI] [PubMed] [Google Scholar]
- 162.Zhao R, Pevear DC, Kremer MJ, et al. Human rhinovirus 3 at 3.0 A resolution. Structure. 1996;4:1205–20. doi: 10.1016/s0969-2126(96)00128-1. [DOI] [PubMed] [Google Scholar]
- 163.Verdaguer N, Marlovits TC, Bravo J, et al. Crystallization and preliminary X-ray analysis of human rhinovirus serotype 2 (HRV2) Acta Crystallogr D Biol Crystallogr. 1999;55:1459–61. doi: 10.1107/s0907444999006137. [DOI] [PubMed] [Google Scholar]
- 164.Rossmann MG, He Y, Kuhn RJ. Picornavirus-receptor interactions. Trends Microbiol. 2002;10:324–31. doi: 10.1016/s0966-842x(02)02383-1. [DOI] [PubMed] [Google Scholar]
- 165.Laine P, Blomqvist S, Savolainen C, et al. Alignment of capsid protein VP1 sequences of all human rhinovirus prototype strains: conserved motifs and functional domains. J Gen Virol. 2006;87:129–38. doi: 10.1099/vir.0.81137-0. [DOI] [PubMed] [Google Scholar]
- 166.Lole KS, Bollinger RC, Paranjape RS, et al. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol. 1999;73:152–60. doi: 10.1128/jvi.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hofer F, Gruenberger M, Kowalski H, et al. Members of the low density lipoprotein receptor family mediate cell entry of a minor group common cold virus. Proc Natl Acad Sci U S A. 1994;91:1839–42. doi: 10.1073/pnas.91.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stanway G, et al. Family Picornaviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, et al., editors. Virus taxonomy: eighth report of the international committee in taxonomy of viruses. San Diego: Elsevier Academic Press; 2005. pp. 757–78. [Google Scholar]
- 169.Uncapher CR, DeWitt CM, Colonno RJ. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology. 1991;180:814–7. doi: 10.1016/0042-6822(91)90098-v. [DOI] [PubMed] [Google Scholar]
- 170.Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. Am J Epidemiol. 1967;85:297–310. doi: 10.1093/oxfordjournals.aje.a120693. [DOI] [PubMed] [Google Scholar]
- 171.Ishiko H, Miura R, Shimada Y, et al. Human rhinovirus 87 identified as human enterovirus 68 by VP4-based molecular diagnosis. Intervirology. 2002;45:136–41. doi: 10.1159/000065866. [DOI] [PubMed] [Google Scholar]
- 172.Taxonomy V. Classification and nomenclature of viruses: Ninth Report of the International Committee for the Taxonomy of Viruses. San Diego: Elsevier; 2011. [Google Scholar]
- 173.Landa-Cardena A, Morales-Romero J, Garcia-Roman R, et al. Clinical characteristics and genetic variability of human rhinovirus in Mexico. Viruses. 2012;4:200–10. doi: 10.3390/v4020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Annamalay AA, Khoo SK, Jacoby P, et al. Prevalence of and risk factors for human rhinovirus infection in health Aboriginal and non-Aboriginal Western Australian children. Pediatr Infect Dis J. 2012;31(7):673–9. doi: 10.1097/INF.0b013e318256ffc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Savolainen-Kopra C, Blomqvist S, Smura T, et al. 5′ Noncoding region alone does not unequivocally determine genetic type of human rhinovirus strains. J Clin Microbiol. 2009;47:1278–80. doi: 10.1128/JCM.02130-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tyrrell DAJ, Bynoe ML. Some further virus isolations from common colds. Br Med J. 1961;1:393–7. doi: 10.1136/bmj.1.5223.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Taylor-Robinson D, Tyrrell DAJ. Serotypes of viruses (rhinoviruses) isolated from common colds. Lancet. 1962;1:452–4. doi: 10.1016/s0140-6736(62)91418-6. [DOI] [PubMed] [Google Scholar]
- 178.Cooney MK, Kenny GE. Demonstration of dual rhinovirus infection in humans by isolation of different serotypes in human heteroploid (HeLa) and human diploid fibroblast cell cultures. J Clin Microbiol. 1977;5:202–7. doi: 10.1128/jcm.5.2.202-207.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bloom HH, Forsyth BR, Johnson KM, et al. Relationship of rhinovirus infection to mild upper respiratory disease. JAMA. 1963;186:38–45. doi: 10.1001/jama.1963.63710010001012. [DOI] [PubMed] [Google Scholar]
- 180.Gwaltney JM, Jr, Jordan WS., Jr Rhinoviruses and respiratory disease. Bacteriol Rev. 1964;28:409–22. doi: 10.1128/br.28.4.409-422.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hamre D. Rhinoviruses. Monogr Virol. 1967;1:1–85. [PubMed] [Google Scholar]
- 182.Rossmann MG. Viral cell recognition and entry. Protein Sci. 1994;3:1712–25. doi: 10.1002/pro.5560031010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Khan AG, Pichler J, Rosemann A, et al. Human rhinovirus type 54 infection via heparan sulfate is less efficient and strictly dependent on low endosomal pH. J Virol. 2008;81:4625–32. doi: 10.1128/JVI.02160-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Abraham G, Colonno RJ. Many rhinovirus serotypes share the same cellular receptor. J Virol. 1984;51:340–5. doi: 10.1128/jvi.51.2.340-345.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Colonno RJ, Callahan PL, Long WJ. Isolation of a monoclonal antibody that blocks attachment of the major group of human rhinoviruses. J Virol. 1986;57:7–12. doi: 10.1128/jvi.57.1.7-12.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Greve JM, Davis G, Meyer AM, et al. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839–47. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- 187.Staunton DE, Merluzzi VJ, Rothlein R, et al. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989;56:849–53. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- 188.Rossmann MG, Bella J, Kolatkar PR, et al. Cell recognition and entry by rhino- and enteroviruses. Virology. 2000;269:239–47. doi: 10.1006/viro.2000.0258. [DOI] [PubMed] [Google Scholar]
- 189.Tomassini JE, Graham D, DeWitt CM, et al. CDNA cloning reveals that the major group rhinovirus receptor on HeLa cells is intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1989;86:4907–11. doi: 10.1073/pnas.86.13.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kolatkar PR, Bella J, Olson NH, et al. Structural studies of two rhinovirus serotypes complexed with fragments of their cellular receptor. EMBO J. 1999;18:6249–59. doi: 10.1093/emboj/18.22.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sethi SK, Bianco A, Allen JT, et al. Interferon-gamma (IFN-gamma) down-regulates the rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) on human airway epithelial cells. Clin Exp Immunol. 1997;110:362–9. doi: 10.1046/j.1365-2249.1997.4221440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vlasak M, Goesler I, Blaas D. Human rhinovirus type 89 variants use heparan sulfate proteoglycan for cell attachment. J Virol. 2005;79:5963–70. doi: 10.1128/JVI.79.10.5963-5970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Khan AG, Pickl-Herk A, Gajdzik L, et al. Entry of a heparan sulphate-binding HRV8 variant strictly depends on dynamin but not on clathrin, caveolin, and flotillin. Virology. 2011;412:55–67. doi: 10.1016/j.virol.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 194.Andries K, Dewindt B, Snoeks J, et al. Two groups of rhinoviruses revealed by a panel of antiviral compounds present sequence divergence and differential pathogenicity. J Virol. 1990;64:1117–23. doi: 10.1128/jvi.64.3.1117-1123.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Savolainen C, Laine P, Mulders MN, et al. Sequence analysis of human rhinoviruses in the RNA-dependent RNA polymerase coding region reveals within-species variation. J Gen Virol. 2004;85:2271–7. doi: 10.1099/vir.0.79897-0. [DOI] [PubMed] [Google Scholar]
- 196.Laine P, Savolainen C, Blomqvist S, et al. Phylogenetic analysis of human rhinovirus capsid protein VP1 and 2A protease coding sequences confirms shared genus-like relationships with human enteroviruses. J Gen Virol. 2005;86:697–706. doi: 10.1099/vir.0.80445-0. [DOI] [PubMed] [Google Scholar]
- 197.King AMQ, et al. Virus taxonomy. Seventh Report of the International Committee for the Taxonomy of Viruses. New York: Academic; 2000. [Google Scholar]
- 198.van der Zalm MM, Wilbrink B, van Ewijk BE, et al. Highly frequent infections with human rhinovirus in healthy young children: a longitudinal cohort study. J Clin Virol. 2011;52:317–20. doi: 10.1016/j.jcv.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 199.Bizzintino J, Lee WM, Laing IA, et al. Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J. 2011;37:1037–42. doi: 10.1183/09031936.00092410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mackay IM, Arden KE, Nissen MD, Sloots TP. Real-time PCR in microbiology: from diagnosis to characterization. Norfolk: Caister Academic Press; 2007. Challenges facing real-time PCR characterization of acute respiratory tract infections; pp. 269–318. [Google Scholar]
- 201.Blaschke AJ, Allison MA, Meyers L, et al. Non-invasive sample collection for respiratory virus testing by multiplex PCR. J Clin Virol. 2011;52:210–4. doi: 10.1016/j.jcv.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Heikkinen T, Marttila J, Salmi AA, et al. Nasal swab versus nasopharyngeal aspirate for isolation of respiratory viruses. J Clin Microbiol. 2002;40:4337–9. doi: 10.1128/JCM.40.11.4337-4339.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cate TR, Couch RB, Johnson KM. Studies with rhinoviruses in volunteers: production of illness, effect of naturally acquired antibody, and demonstration of a protective effect not associated with serum antibody. J Clin Invest. 1964;43:56–67. doi: 10.1172/JCI104894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Peltola V, Waris M, Österback R, et al. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis. 2008;197:382–9. doi: 10.1086/525542. [DOI] [PubMed] [Google Scholar]
- 205.Englund JA, Piedra PA, Jewell A, et al. Rapid diagnosis of respiratory syncytial virus infections in immunocompromised adults. J Clin Microbiol. 1996;34:1649–53. doi: 10.1128/jcm.34.7.1649-1653.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gwaltney JM., Jr Epidemiology of the common cold. Ann N Y Acad Sci. 1980;353:54–60. doi: 10.1111/j.1749-6632.1980.tb18905.x. [DOI] [PubMed] [Google Scholar]
- 207.Forsyth BR, Bloom HH, Johnson KM, et al. Patterns of illness in rhinovirus infections of military personnel. N Engl J Med. 1963;269:602–6. doi: 10.1056/NEJM196309192691203. [DOI] [PubMed] [Google Scholar]
- 208.Gwaltney JM, Jr, Jordan WS. Rhinoviruses and respiratory illnesses in University students. Am Rev Respir Dis. 1966;93:362–71. [Google Scholar]
- 209.Hendley JO, Gwaltney JM, Jr, Jordan WS., Jr Rhinovirus infections in an industrial population. IV. Infections within families of employees during two fall peaks of respiratory illness. Am J Epidemiol. 1969;89:184–96. doi: 10.1093/oxfordjournals.aje.a120928. [DOI] [PubMed] [Google Scholar]
- 210.Dick EC, Blumer CR, Evans AS. Epidemiology of infections with rhinovirus types 43 and 55 in a group of university of Wisconsin student families. Am J Epidemiol. 1967;86:386–400. doi: 10.1093/oxfordjournals.aje.a120749. [DOI] [PubMed] [Google Scholar]
- 211.Fox JP, Cooney MK, Hall CE. The Seattle virus watch. V. Epidemiologic observation of rhinovirus infections, 1965–1969 in families with young children. Am J Epidemiol. 1975;101:122–43. doi: 10.1093/oxfordjournals.aje.a112078. [DOI] [PubMed] [Google Scholar]
- 212.Hurrell GD, Sturdy PM, Frood JD, et al. Viruses in families. Lancet. 1971;1:769–74. doi: 10.1016/s0140-6736(71)91214-1. [DOI] [PubMed] [Google Scholar]
- 213.El-Sahly HM, Atmar RL, Glezen WP, et al. Spectrum of clinical illness in hospitalized patients with “Common cold” virus infections. Clin Infect Dis. 2000;31:96–100. doi: 10.1086/313937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Glezen WP, Denny FW. Epidemiology of acute lower respiratory disease in children. N Engl J Med. 1973;288:498–505. doi: 10.1056/NEJM197303082881005. [DOI] [PubMed] [Google Scholar]
- 215.Luna LK, Panning M, Grywna K, et al. Spectrum of viruses and atypical bacteria in intercontinental air travelers with symptoms of acute respiratory infection. J Infect Dis. 2007(195). [DOI] [PMC free article] [PubMed]
- 216.Camps M, Vilella A, Marcos MA, et al. Incidence of respiratory viruses among travelers with a febrile syndrome returning from tropical and subtropical areas. J Med Virol. 2008;80:711–5. doi: 10.1002/jmv.21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nicholson KG, Kent J, Hammersley V, et al. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. Br Med J. 1997;315:1060–4. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Monto AS, Ullman BM. Acute respiratory illness in an American community: the Tecumseh study. JAMA. 1974(227). [PubMed]
- 219.Yu X, Lu R, Wang Z, et al. Etiology and clinical characterization of respiratory virus infections in adult patients attending an emergency department in Beijing. PLoS One. 2012;7:e32174. doi: 10.1371/journal.pone.0032174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tovey ER, Rawlinson WD. A modern miasma hypothesis and back-to-school asthma exacerbations. Med Hypotheses. 2011;76:113–6. doi: 10.1016/j.mehy.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 221.Harju T, Keistinen T, Tuuponen T, et al. Seasonal variation in childhood asthma hospitalisations in Finland, 1972–1992. Eur J Pediatr. 1997;156:436–9. doi: 10.1007/s004310050632. [DOI] [PubMed] [Google Scholar]
- 222.Monteil MA, Juman S, Hassanally R, et al. Descriptive epidemiology of asthma in Trinidad, West Indies. J Asthma. 2000;37:677–84. doi: 10.3109/02770900009087306. [DOI] [PubMed] [Google Scholar]
- 223.Johnston NW, Johnston SL, Norman GR, et al. The September epidemic of asthma hospitalization: school children as disease vectors. J Allergy Clin Immunol. 2006;117:557–62. doi: 10.1016/j.jaci.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 224.Matthew J, Pinto Pereira LM, Pappas TE, et al. Distribution and seasonality of rhinovirus and other respiratory viruses in a cross-section of asthmatic children in Trinidad, West Indies. Ital J Pediatr. 2009;35:16. doi: 10.1186/1824-7288-35-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Monto AS, Johnson KM. Respiratory infections in the American tropics. Am J Hyg. 1968(17). [DOI] [PubMed]
- 226.Do AH, van Doorn HR, Nghiem MN, et al. Viral etiologies of acute respiratory infections among hospitalized Vietnamese children in Ho Chi Minh City, 2004–2008. PLoS One. 2011;6:e18176. doi: 10.1371/journal.pone.0018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Olenec JP, Kim WK, Lee WM, et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125:1001–6. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Miller EK, Williams JV, Gebretsadik T, et al. Host and viral factors associated with severity of human rhinovirus-associated infant respiratory tract illness. J Allergy Clin Immunol. 2011;127:883–91. doi: 10.1016/j.jaci.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gwaltney JM, Jr, Hendley JO. Rhinovirus transmission one if by air, two if by hand. Am J Epidemiol. 1978;107:357–61. doi: 10.1093/oxfordjournals.aje.a112555. [DOI] [PubMed] [Google Scholar]
- 230.Cilla G, Onate E, Perez-Yarza EG, et al. Viruses in community-acquired pneumonia in children aged less than 3 years old: high rate of viral coinfection. J Med Virol. 2008;80:1843–9. doi: 10.1002/jmv.21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Brunstein JD, Cline CL, McKinney S, et al. Evidence from multiplex molecular assays for complex multipathogen interactions in acute respiratory infections. J Clin Microbiol. 2008;46:97–102. doi: 10.1128/JCM.01117-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Greensill J, McNamara PS, Dove W, et al. Human metapneumovirus in severe respiratory syncytial virus bronchiolitis. Emerg Infect Dis. 2003;9:372–5. doi: 10.3201/eid0903.020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Semple MG, Cowell A, Dove W, et al. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191:382–6. doi: 10.1086/426457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Drews AL, Atmar RL, Glezen WP, et al. Dual respiratory virus infections. Clin Infect Dis. 1997;25:1421–9. doi: 10.1086/516137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Foulongne V, Guyon G, Rodiere M, et al. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr Infect Dis J. 2006;25:354–9. doi: 10.1097/01.inf.0000207480.55201.f6. [DOI] [PubMed] [Google Scholar]
- 236.Kloepfer KM, Olenec JP, Lee WM, et al. Increased H1N1 infection rate in children with asthma. Am J Respir Crit Care Med. 2012;185(12):1275–9. doi: 10.1164/rccm.201109-1635OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Choi EH, Lee HJ, Kim SJ, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000–2005. Clin Infect Dis. 2006;43:585–92. doi: 10.1086/506350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wolf DG, Greenberg D, Kalkstein D, et al. Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower respiratory tract infections in hospitalized young children. Pediatr Infect Dis J. 2006;25:320–4. doi: 10.1097/01.inf.0000207395.80657.cf. [DOI] [PubMed] [Google Scholar]
- 239.Garcia-Garcia ML, Calvo C, Perez-Brena P, et al. Prevalence and clinical characteristics of human metapneumovirus infections in hospitalized infants in Spain. Pediatr Pulmonol. 2006;41:863–71. doi: 10.1002/ppul.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Simon A, Wilkesmann A, Muller A, et al. HMPV infections are frequently accompanied by co-infections. Pediatr Pulmonol. 2007;42:98. doi: 10.1002/ppul.20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tsolia MN, Psarras S, Bossios A, et al. Etiology of community-acquired pneumonia in hospitalized school-aged children: evidence for high prevalence of viral infections. Clin Infect Dis. 2004(39). [DOI] [PMC free article] [PubMed]
- 242.Richard N, Komurian-Pradel F, Javouhey E, et al. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatr Infect Dis J. 2008;27:1–5. doi: 10.1097/INF.0b013e31815b4935. [DOI] [PubMed] [Google Scholar]
- 243.Stott EJ, Eadie MB, Grist NR. Rhinovirus infections of children in hospital; isolation of three possibly new rhinovirus serotypes. Am J Epidemiol. 1969;90:45–52. doi: 10.1093/oxfordjournals.aje.a121048. [DOI] [PubMed] [Google Scholar]
- 244.Greer RM, McErlean P, Arden KE, et al. Do rhinoviruses reduce the probability of viral co-detection during acute respiratory tract infections? J Clin Virol. 2009;45:10–5. doi: 10.1016/j.jcv.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mackay IM. Human bocavirus; multisystem detection raises questions about infection. J Infect Dis. 2007;196:968–70. doi: 10.1086/521311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lambert SB, Allen KM, Druce JD, et al. Community epidemiology of human metapneumovirus, human coronavirus NL63, and other respiratory viruses in healthy preschool-aged children using parent-collected specimens. Pediatrics. 2007;120:e929–37. doi: 10.1542/peds.2006-3703. [DOI] [PubMed] [Google Scholar]
- 247.Miller EK, Lu X, Erdman DD, et al. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195:773–81. doi: 10.1086/511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.DaPalma T, Doonan BP, Trager NM, et al. A systematic approach to virus-virus interactions. Virus Res. 2010;149:1–9. doi: 10.1016/j.virusres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Smorodintsev AA, Gvozdilova DA, Romanov YA, et al. Induction of endogenous interferon by use of standard live vaccines for prevention of respiratory viral infections. Ann N Y Acad Sci. 1970;173:811–22. [Google Scholar]
- 250.Voroshilova MK. Potential use of nonpathogenic enteroviruses for control of human disease. Prog Med Virol. 1989;36:191–202. [PubMed] [Google Scholar]
- 251.Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis. 2012;54:1778–83. doi: 10.1093/cid/cis307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hitchcock G, Tyrrell DA. Some virus isolations from common colds. II. Virus interference in tissue cultures. Lancet. 1960;1:237–9. doi: 10.1016/s0140-6736(60)90167-7. [DOI] [PubMed] [Google Scholar]
- 253.Olson LC, Willhight M, Buescher EL. Recovery and characterization of non-cytopathogenic rhinoviruses. J Gen Virol. 1972;17:237–40. doi: 10.1099/0022-1317-17-2-237. [DOI] [PubMed] [Google Scholar]
- 254.Smorodintsev AA, Beare AS, Bynoe ML, et al. The formation of interferon during acute respiratory virus infection of volunteers. Arch Gesamte Virusforsch. 1971;33:9–16. doi: 10.1007/BF01254158. [DOI] [PubMed] [Google Scholar]
- 255.Wang Z, Malanoski AP, Lin B, et al. Broad spectrum respiratory pathogen analysis of throat swabs from military recruits reveals interference between rhinoviruses and adenoviruses. Microb Ecol. 2010;59:623–34. doi: 10.1007/s00248-010-9636-3. [DOI] [PubMed] [Google Scholar]
- 256.Hamano-Hasegawa K, Morozumi M, Nakayama E, et al. Comprehensive detection of causative pathogens using real-time PCR to diagnose pediatric community-acquired pneumonia. J Infect Chemother. 2008;14:424–32. doi: 10.1007/s10156-008-0648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Jartti T, Lehtinen P, Vuorinen P, et al. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72:695–9. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]
- 258.Zlateva KT, de Vries JJ, Coenjaerts FE, et al. Prolonged shedding of rhinovirus and re-infection in adults with respiratory tract illness. Eur Respir J. 2014;44(1):169–77. doi: 10.1183/09031936.00172113. [DOI] [PubMed] [Google Scholar]
- 259.Pitkäranta A, Roivainen M, Blomgren K, et al. Presence of viral and bacterial pathogens in the nasopharynx of otitis-prone children. A prospective study. Int J Pediatr Otorhinolaryngol. 2005;70:647–54. doi: 10.1016/j.ijporl.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 260.Kaiser L, Aubert J-D, Pache J-C, et al. Chronic rhinoviral infection in lung transplant recipients. Am J Respir Crit Care Med. 2006;174:1392–9. doi: 10.1164/rccm.200604-489OC. [DOI] [PubMed] [Google Scholar]
- 261.Kling S, Donninger H, Williams Z, et al. Persistence of rhinovirus RNA after asthma exacerbation in children. Clin Exp Allergy. 2005;35:672–8. doi: 10.1111/j.1365-2222.2005.02244.x. [DOI] [PubMed] [Google Scholar]
- 262.Arden KE, Mackay IM. Human rhinoviruses: coming in from the cold. Genome Med. 2009;1:44. doi: 10.1186/gm44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Piotrowska Z, Vázquez M, Shapiro ED, et al. Rhinoviruses are a major cause of wheezing and hospitalization in children less than 2 years of age. Pediatr Infect Dis J. 2009;28:25–9. doi: 10.1097/INF.0b013e3181861da0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sato M, Li H, Ikizler MR, et al. Detection of viruses in human adenoid tissues by use of multiplex PCR. J Clin Microbiol. 2009;47:771–3. doi: 10.1128/JCM.02331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Piralla A, Rovida F, Campanini G, et al. Clinical severity and molecular typing of human rhinovirus C strains during a fall outbreak affecting hospitalized patients. J Clin Virol. 2009;45:311–7. doi: 10.1016/j.jcv.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 266.Garcia-Garcia ML, Calvo C, Pozo F, et al. Human bocavirus detection in nasopharyngeal aspirates of children without clinical symptoms of respiratory infection. Pediatr Infect Dis J. 2008;27:358–60. doi: 10.1097/INF.0b013e3181626d2a. [DOI] [PubMed] [Google Scholar]
- 267.Meschievitz CK, Schultz SB, Dick EC. A model for obtaining predictable natural transmission of rhinoviruses in human volunteers. J Infect Dis. 1984;150:195–201. doi: 10.1093/infdis/150.2.195. [DOI] [PubMed] [Google Scholar]
- 268.Sneed RS, Cohen S, Turner RB, et al. Parenthood and host resistance to the common cold. Psychosom Med. 2012;74:567–73. doi: 10.1097/PSY.0b013e31825941ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Jansen RR, Wieringa J, Koekkoek SM, et al. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol. 2011;49:2631–6. doi: 10.1128/JCM.02094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Carrat F, Vergu E, Ferguson NM, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167:775–85. doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- 271.Hendley JO. The host response, not the virus, causes the symptoms of the common cold. Clin Infect Dis. 1998;26:847–8. doi: 10.1086/513921. [DOI] [PubMed] [Google Scholar]
- 272.Lambert SB, Allen KM, Druce JD. Respiratory illness during winter: a cohort study of urban children from temperate Australia. J Paediatr Child Health. 2005;41:125–9. doi: 10.1111/j.1440-1754.2005.00561.x. [DOI] [PubMed] [Google Scholar]
- 273.van Benten I, Koopman L, Niesters B, et al. Predominance of rhinovirus in the nose of symptomatic and asymptomatic infants. Pediatr Allergy Immunol. 2003;14:363–70. doi: 10.1034/j.1399-3038.2003.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Fuji N, Suzuki A, Lupisan S, et al. Detection of human rhinovirus C viral genome in blood among children with severe respiratory infections in the Philippines. PLoS One. 2011;6:e27247. doi: 10.1371/journal.pone.0027247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Tapparel C, L’Huillier AG, Rougemont AL, et al. Pneumonia and pericarditis in a child with HRV-C infection: a case report. J Clin Virol. 2009;45:157–60. doi: 10.1016/j.jcv.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Urquhart GED, Grist NR. Virological studies of sudden, unexplained infant deaths in Glasgow 1967–1970. J Clin Pathol. 1972;25:443–6. doi: 10.1136/jcp.25.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Urquhart GED, Stott EJ. Rhinoviraemia. Br Med J. 1970;4:28–30. doi: 10.1136/bmj.4.5726.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lau SK, Yip CC, Lung DC, et al. Detection of human rhinovirus C in fecal samples of children with gastroenteritis. J Clin Virol. 2012;53(4):290–6. doi: 10.1016/j.jcv.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Arruda E, Boyle TR, Winther B, et al. Localization of human rhinovirus replication in the upper respiratory tract by in situ hybridization. J Infect Dis. 1995;171:1329–33. doi: 10.1093/infdis/171.5.1329. [DOI] [PubMed] [Google Scholar]
- 280.Winther B, Gwaltney JM, Jr, Mygind N, et al. Sites of rhinovirus recovery after point inoculation of the upper airway. JAMA. 1986;256:1763–7. [PubMed] [Google Scholar]
- 281.Turner RB, Hendley JO, Gwaltney JM., Jr Shedding of infected ciliated epithelial cells in rhinovirus colds. J Infect Dis. 1982;145:849–53. doi: 10.1093/infdis/145.6.849. [DOI] [PubMed] [Google Scholar]
- 282.Dick EC, Jennings LC, Mink KA, et al. Aerosol transmission of rhinovirus colds. J Infect Dis. 1987;156:442–8. doi: 10.1093/infdis/156.3.442. [DOI] [PubMed] [Google Scholar]
- 283.D’Alessio DJ, Peterson JA, Dick CR, et al. Transmission of experimental rhinovirus colds in volunteer married couples. J Infect Dis. 1976;133:28–36. doi: 10.1093/infdis/133.1.28. [DOI] [PubMed] [Google Scholar]
- 284.Gerna G, Piralla A, Rovida F, et al. Correlation of rhinovirus load in the respiratory tract and clinical symptoms in hospitalized immunocompetent and immunocompromised patients. J Med Virol. 2009;81:1498–507. doi: 10.1002/jmv.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Turner RB, Weingand KW, Yeh C-H, et al. Association between interleukin-8 concentration in nasal secretions and severity of experimental rhinovirus colds. Clin Infect Dis. 1998;26:840–6. doi: 10.1086/513922. [DOI] [PubMed] [Google Scholar]
- 286.Mallia P, Message SD, Kebadze T, et al. An experimental model of rhinovirus induced chronic obstructive pulmonary disease exacerbations: a pilot study. Respir Res. 2006;7:116. doi: 10.1186/1465-9921-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Reed SE. An investigation of the possible transmission of rhinovirus colds through indirect contact. J Hyg (Lond) 1975;75:249–58. doi: 10.1017/s0022172400047288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Hendley JO, Gwaltney JM., Jr Mechanisms of transmission of rhinovirus infections. Epidemiol Rev. 1988;10:242–58. [PubMed] [Google Scholar]
- 289.Pancic F, Carpentier DC, Came PE. Role of infectious secretions in the transmission of rhinovirus. J Clin Microbiol. 1980;12:567–71. doi: 10.1128/jcm.12.4.567-571.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347–52. doi: 10.1080/15459620802003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Andrewes CH. The complex epidemiology of respiratory virus infections. Science. 1964;146:1274–7. doi: 10.1126/science.146.3649.1274. [DOI] [PubMed] [Google Scholar]
- 292.Holmes MJ, Reed SE, Stott EJ, et al. Studies of experimental rhinovirus type 2 infections in polar isolation and in England. J Hyg (Lond). 1976;76(379–393). [DOI] [PMC free article] [PubMed]
- 293.Gwaltney JM, Moskalski PB, Hendley JO. Hand-to-hand transmission of rhinovirus colds. Ann Intern Med. 1978;88:463–7. doi: 10.7326/0003-4819-88-4-463. [DOI] [PubMed] [Google Scholar]
- 294.Stelzer-Braid S, Oliver BG, Blazey AJ, et al. Exhalation of respiratory viruses by breathing, coughing, and talking. J Med Virol. 2009;81:1674–9. doi: 10.1002/jmv.21556. [DOI] [PubMed] [Google Scholar]
- 295.Huynh KN, Oliver BG, Stelzer S, et al. A new method for sampling and detection of exhaled respiratory virus aerosols. Clin Infect Dis. 2008;46:93–5. doi: 10.1086/523000. [DOI] [PubMed] [Google Scholar]
- 296.Myatt TA, Johnston SL, Zuo Z, et al. Detection of airborne rhinovirus and its relation to outdoor air supply in office environments. Am J Respir Crit Care Med. 2004;169:1187–90. doi: 10.1164/rccm.200306-760OC. [DOI] [PubMed] [Google Scholar]
- 297.Korves TM, Johnson D, Jones BW, et al. Detection of respiratory viruses on air filters from aircraft. Lett Appl Microbiol. 2011;53:306–12. doi: 10.1111/j.1472-765X.2011.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Dreschers S, Dumitru CA, Adams C, et al. The cold case: Are rhinoviruses perfectly adapted pathogens? Cell Mol Life Sci. 2007;64:181–91. doi: 10.1007/s00018-006-6266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Wark PAB, Bucchieri F, Johnston SL, et al. IFN-g-induced protein 10 is a novel biomarker of rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol. 2007;120:586–93. doi: 10.1016/j.jaci.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Gaajetaan GR, Bruggeman CA, Stassen FR. The type I interferon response during viral infections: a “SWOT” analysis. Rev Med Virol. 2012;22:122–37. doi: 10.1002/rmv.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Seth RB, Sun L, Chen ZJ. Antiviral innate immunity pathways. Cell Res. 2006;16:141–7. doi: 10.1038/sj.cr.7310019. [DOI] [PubMed] [Google Scholar]
- 302.Zhou Z, Hamming OJ, Ank N, et al. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J Virol. 2007;81:7749–58. doi: 10.1128/JVI.02438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 304.Sen GC, Peters GA. Viral stress-inducible genes. Adv Virus Res. 2007;70:233–63. doi: 10.1016/S0065-3527(07)70006-4. [DOI] [PubMed] [Google Scholar]
- 305.Chen Y, Hamati E, Lee PK, et al. Rhinovirus induces airway epithelial gene expression through double-stranded RNA and IFN-dependent pathways. Am J Respir Cell Mol Biol. 2006;34:192–203. doi: 10.1165/rcmb.2004-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Haller O, Kochs G, Weber F. The interferon response circuit: induction and suppression by pathogenic viruses. Virology. 2006;344:119–30. doi: 10.1016/j.virol.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Chai Y, Huang HL, Hu DJ, et al. IL-29 and IFN-alpha regulate the expression of MxA, 2′,5′-OAS and PKR genes in association with the activation of Raf-MEK-ERK and PI3K-AKT signal pathways in HepG2.2.15 Cells. Mol Biol Rep. 2011;38:139–43. doi: 10.1007/s11033-010-0087-1. [DOI] [PubMed] [Google Scholar]
- 308.Berghall H, Siren J, Sarkar D, et al. The interferon-inducible RNA helicase, mda-5, is involved in measles virus-induced expression of antiviral cytokines. Microbes Infect. 2006;8:2138–44. doi: 10.1016/j.micinf.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 309.Barral PM, Sarkar D, Fisher PB, et al. RIG-I is cleaved during picornavirus infection. Virology. 2009;391:171–6. doi: 10.1016/j.virol.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Peng T, Kotla S, Bumgarner RE, et al. Human rhinovirus attenuates the type I interferon response by disrupting activation of interferon regulatory factor 3. J Virol. 2006;80:5021–31. doi: 10.1128/JVI.80.10.5021-5031.2006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 311.Kotla S, Peng T, Bumgarner RE, et al. Attenuation of the type I interferon response in cells infected with human rhinovirus. Virology. 2008;374:399–410. doi: 10.1016/j.virol.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 312.Barral PM, Morrison JM, Drahos J, et al. MDA-5 is cleaved in poliovirus-infected cells. J Virol. 2007;81:3677–84. doi: 10.1128/JVI.01360-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Bonjardim CA, Ferreira PC, Kroon EG. Interferons: signaling, antiviral and viral evasion. Immunol Lett. 2009;122:1–11. doi: 10.1016/j.imlet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Jensen S, Thomsen AR. Sensing of RNA viruses: a review of innate immune receptors involved in recognizing RNA virus invasion. J Virol. 2012;86:2900–10. doi: 10.1128/JVI.05738-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Holt PG, Sly PD. Viral infections and atopy in asthma pathogenesis: new rationales for asthma prevention and treatment. Nat Med. 2012;18:726–35. doi: 10.1038/nm.2768. [DOI] [PubMed] [Google Scholar]
- 316.Aoshi T, Koyama S, Kobiyama K, et al. Innate and adaptive immune responses to viral infection and vaccination. Curr Opin Virol. 2011;1:226–32. doi: 10.1016/j.coviro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 317.Saito T, Gale M., Jr Principles of intracellular viral recognition. Curr Opin Immunol. 2007;19:17–23. doi: 10.1016/j.coi.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 318.Finberg RW, Kurt-Jones EA. Viruses and toll-like receptors. Microbes Infect. 2004;6:1356–60. doi: 10.1016/j.micinf.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 319.Triantafilou K, Vakakis E, Richer EA, et al. Human rhinovirus recognition in non-immune cells is mediated by Toll-like receptors and MDA-5, which trigger a synergetic pro-inflammatory immune response. Virulence. 2011;2:22–9. doi: 10.4161/viru.2.1.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Roponen M, Yerkovich ST, Hollams E, et al. Toll-like receptor 7 function is reduced in adolescents with asthma. Eur Respir J. 2010;35:64–71. doi: 10.1183/09031936.00172008. [DOI] [PubMed] [Google Scholar]
- 321.Kirchberger S, Majdic O, Stöckl J. Modulation of the immune system by human rhinoviruses. Int Arch Allergy Immunol. 2007;142:1–10. doi: 10.1159/000095993. [DOI] [PubMed] [Google Scholar]
- 322.Wark PA, Grissell T, Davies B, et al. Diversity in the bronchial epithelial cell response to infection with different rhinovirus strains. Respirology. 2009;14:180–6. doi: 10.1111/j.1440-1843.2009.01480.x. [DOI] [PubMed] [Google Scholar]
- 323.Message SD, Johnston SL. Host defense function of the airway epithelium in health and disease: clinical background. J Leukoc Biol. 2004;75:5–17. doi: 10.1189/jlb.0703315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Van Cauwenberge PB, van Kempen MJ, Bachert C. The common cold at the turn of the millennium. Am J Rhinol. 2000;14:339–43. doi: 10.2500/105065800781329555. [DOI] [PubMed] [Google Scholar]
- 325.Gern JE, Busse WW. Relationship of viral infections to wheezing illnesses and asthma. Nat Rev Immunol. 2002;2:132–8. doi: 10.1038/nri725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18:673–83. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 327.Lu TX, Hartner J, Lim EJ, et al. MicroRNA-21 limits in vivo immune response-mediated activation of the IL-12/IFN-gamma pathway, Th1 polarization, and the severity of delayed-type hypersensitivity. J Immunol. 2011;187:3362–73. doi: 10.4049/jimmunol.1101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Papadopoulos NG, Stanciu LA, Papi A, et al. A defective type 1 response to rhinovirus in atopic asthma. Thorax. 2002;57:328–32. doi: 10.1136/thorax.57.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.McFadden ER., Jr Acute severe asthma. Am J Respir Crit Care Med. 2003;168:740–59. doi: 10.1164/rccm.200208-902SO. [DOI] [PubMed] [Google Scholar]
- 330.Johnston SL. Innate immunity in the pathogenesis of virus-induced asthma exacerbations. Proc Am Thorac Soc. 2007;4:267–70. doi: 10.1513/pats.200701-030AW. [DOI] [PubMed] [Google Scholar]
- 331.Strachan DP. Hay fever, hygiene, and household size. Br Med J. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax. 2000;55:S2–10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Yoo J, Tcheurekdjian H, Lynch SV, et al. Microbial manipulation of immune function for asthma prevention. Inferences from clinical trials. Proc Am Thorac Soc. 2007;4:277–82. doi: 10.1513/pats.200702-033AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Amineva SP, Aminev AG, Palmenberg AC, et al. Rhinovirus 3C protease precursors 3CD and 3CD’ localize to the nuclei of infected cells. J Gen Virol. 2004;85:2969–79. doi: 10.1099/vir.0.80164-0. [DOI] [PubMed] [Google Scholar]
- 335.Gradi A, Svitkin YV, Sommergruber W, et al. Human rhinovirus 2A proteinase cleavage sites in eukaryotic initiation factors (eIF) 4GI and eIF4GII are different. J Virol. 2003;77:5026–9. doi: 10.1128/JVI.77.8.5026-5029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Hunt SL, Skern T, Liebig HD, et al. Rhinovirus 2A proteinase mediated stimulation of rhinovirus RNA translation is additive to the stimulation effected by cellular RNA binding proteins. Virus Res. 1999;62:119–28. doi: 10.1016/s0168-1702(99)00039-8. [DOI] [PubMed] [Google Scholar]
- 337.Stockl J, Vetr H, Majdic O, et al. Human major group rhinoviruses downmodulate the accessory function of monocytes by inducing IL-10. J Clin Invest. 1999;104:957–65. doi: 10.1172/JCI7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Message SD, Laza-Stanca V, Mallia P, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105:13562–7. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Einarsson O, Geba GP, Zhu Z, et al. Interleukin-11: stimulation in vivo and in vitro by respiratory viruses and induction of airways hyperresponsiveness. J Clin Invest. 1996;97:915–24. doi: 10.1172/JCI118514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Levandowski RA, Weaver CW, Jackson GG. Nasal-secretion leukocyte populations determined by flow cytometry during acute rhinovirus infection. J Med Virol. 1988;25:423–32. doi: 10.1002/jmv.1890250406. [DOI] [PubMed] [Google Scholar]
- 341.Bianco A, Whiteman SC, Sethi SK, et al. Expression of intercellular adhesion molecule-1 (ICAM-1) in nasal epithelial cells of atopic subjects: a mechanism for increased rhinovirus infection? Clin Exp Immunol. 2000;121:339–45. doi: 10.1046/j.1365-2249.2000.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Turner RB. Rhinovirus infection of human embryonic lung fibroblasts induces the production of a chemoattractant for polymorphonuclear leukocytes. J Infect Dis. 1988;157:346–50. doi: 10.1093/infdis/157.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Martin JG, Siddiqui S, Hassan M. Immune responses to viral infections: relevance for asthma. Paediatr Respir Rev. 2006;7S:S125–7. doi: 10.1016/j.prrv.2006.04.225. [DOI] [PubMed] [Google Scholar]
- 344.Cate TR, Rossen RD, Douglas RG, Jr, et al. The role of nasal secretion and serum antibody in the rhinovirus common cold. Am J Epidemiol. 1966;84:352–63. doi: 10.1093/oxfordjournals.aje.a120648. [DOI] [PubMed] [Google Scholar]
- 345.Barclay WS, Al-Nakib W. An ELISA for the detection of rhinovirus specific antibody in serum and nasal secretion. J Virol Methods. 1987(15). [DOI] [PMC free article] [PubMed]
- 346.Igarashi Y, Skoner DP, Doyle WJ, et al. Analysis of nasal secretions during experimental rhinovirus upper respiratory infections. J Allergy Clin Immunol. 1993;92:722–31. doi: 10.1016/0091-6749(93)90016-9. [DOI] [PubMed] [Google Scholar]
- 347.Buscho RF, Perkins JC, Knopf HLS, et al. Further characterization of the local respiratory tract antibody response induced by intranasal instillation of inactivated rhinovirus 13 vaccine. J Immunol. 1972;108:169–77. [PubMed] [Google Scholar]
- 348.Barclay WS, Al-Nakib W, Higgins PG, et al. The time course of the humoral immune response to rhinovirus infection. Epidemiol Infect. 1989;103:659–69. doi: 10.1017/s095026880003106x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Barclay WS, Callow KA, Sergeant M, et al. Evaluation of an enzyme-linked immunosorbent assay that measures rhinovirus-specific antibodies in human sera and nasal secretions. J Med Virol. 1988;25:475–82. doi: 10.1002/jmv.1890250411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Skoner DP, Whiteside TL, Wilson JW, et al. Effect of rhinovirus 39 infection on cellular immune parameters in allergic and nonallergic subjects. J Allergy Clin Immunol. 1993;92:732–43. doi: 10.1016/0091-6749(93)90017-a. [DOI] [PubMed] [Google Scholar]
- 351.Monto AS, Bryan ER, Ohmit S. Rhinovirus infections in Tecumseh, Michigan: frequency of illness and number of serotypes. J Infect Dis. 1987;156:43–9. doi: 10.1093/infdis/156.1.43. [DOI] [PubMed] [Google Scholar]
- 352.Fleet WF, Douglas RG, Cate TR, et al. Antibody to rhinovirus in human sera. II. Heterotypic responses. Proc Soc Exp Biol Med. 1968;127:503–9. doi: 10.3181/00379727-127-32725. [DOI] [PubMed] [Google Scholar]
- 353.Fleet WF, Couch RB, Cate TR, et al. Homologous and heterologous resistance to rhinovirus common cold. Am J Epidemiol. 1965;82:185–96. doi: 10.1093/oxfordjournals.aje.a120543. [DOI] [PubMed] [Google Scholar]
- 354.Cooney MK, Fox JP, Kenny GE. Antigenic groupings of 90 rhinovirus serotypes. Infect Immun. 1982;37:642–7. doi: 10.1128/iai.37.2.642-647.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Stott EJ, Walker M. Antigenic variation among strains of rhinovirus type 51. Nature. 1969;224:1311–2. doi: 10.1038/2241311a0. [DOI] [PubMed] [Google Scholar]
- 356.Patterson LJ, Hamparian VV. Hyper-antigenic variation occurs with human rhinovirus type 17. J Virol. 1997;71:1370–4. doi: 10.1128/jvi.71.2.1370-1374.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Schieble JH, Lennette EH, Fox VL. Antigenic variation of rhinovirus type 22. Proc Soc Exp Biol Med. 1970;133:329–33. doi: 10.3181/00379727-133-34468. [DOI] [PubMed] [Google Scholar]
- 358.Halfpap LM, Cooney MK. Isolation of rhinovirus intertypes related to either rhinoviruses 12 and 78 or 36 and 58. Infect Immun. 1983;40:213–8. doi: 10.1128/iai.40.1.213-218.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Fox JP. Is a rhinovirus vaccine possible? Am J Epidemiol. 1976;103:345–54. doi: 10.1093/oxfordjournals.aje.a112233. [DOI] [PubMed] [Google Scholar]
- 360.Murray CS, Simpson A, Custovic A. Allergens, viruses, and asthma exacerbations. Proc Am Thorac Soc. 2004;1:99–104. doi: 10.1513/pats.2306027. [DOI] [PubMed] [Google Scholar]
- 361.Mäkelä MJ, Puhakka T, Ruuskanen O, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–42. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Ede LC, Loeffelholz MJ, Varez-Fernandez P, et al. Effect of the 2009 influenza A/H1N1 pandemic on viral respiratory infections in the first year of life. Pediatr Infect Dis J. 2012;31(11):1107–12. doi: 10.1097/INF.0b013e31825f29db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Rosenthal LA, Szakaly RJ, Amineva SP, et al. Lower respiratory tract infection induced by a genetically modified picornavirus in its natural murine host. PLoS One. 2012;7:e32061. doi: 10.1371/journal.pone.0032061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Bardin PG, Johnston SL, Pattemore PK. Viruses as precipitants of asthma symptoms II. Physiology and mechanisms. Clin Exp Allergy. 1992;22:809–22. doi: 10.1111/j.1365-2222.1992.tb02825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Fedson DS, Nichol KL. Influenza vaccination: policy versus evidence: no gap between policy and evidence. BMJ. 2006;333:1020. doi: 10.1136/bmj.39024.451389.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Eccles R. Mechanisms of symptoms of the common cold and influenza. Br J Hosp Med. 2007;68:578–82. doi: 10.12968/hmed.2007.68.2.22824. [DOI] [PubMed] [Google Scholar]
- 367.Eccles R. Mechanisms of the symptoms of rhinosinusitis. Rhinology. 2011;49:131–8. [PubMed] [Google Scholar]
- 368.Proud D, Naclerio RM, Gwaltney JM, et al. Kinins are generated in nasal secretions during natural rhinovirus colds. J Infect Dis. 1990;161:120–3. doi: 10.1093/infdis/161.1.120. [DOI] [PubMed] [Google Scholar]
- 369.Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps 2007. Rhinol Suppl. 2007;20:1–136. [PubMed]
- 370.Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 371.Monto AS, Bramley TJ, Sarnes M. Development of a predictive index for picornavirus infections. Clin Infect Dis. 2003;36:253–8. doi: 10.1086/346036. [DOI] [PubMed] [Google Scholar]
- 372.Herberhold S, Eis-Hubinger AM, Panning M. Frequent detection of respiratory viruses by real-time PCR in adenoid samples from asymptomatic children. J Clin Microbiol. 2009;47:2682–3. doi: 10.1128/JCM.00899-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Gwaltney JM, Jr, Hendley JO, Simon G, et al. Rhinovirus infections in an industrial population. II. Characteristics of illness and antibody response. JAMA. 1967;202:494–500. [PubMed] [Google Scholar]
- 374.Tai A, Volkmer R, Burton A. Prevalence of asthma symptoms and atopic disorders in preschool children and the trend over a decade. J Asthma. 2009;46:343–6. doi: 10.1080/02770900802660998. [DOI] [PubMed] [Google Scholar]
- 375.Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy. 2009;39:193–202. doi: 10.1111/j.1365-2222.2008.03157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Martinez FD. Gene-environment interactions in asthma. Proc Am Thorac Soc. 2007;4:26–31. doi: 10.1513/pats.200607-144JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Lemanske RF. Viral infections and asthma inception. J Allergy Clin Immunol. 2004;114:1023–6. doi: 10.1016/j.jaci.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 378.Hershenson MB, Johnston SL. Rhinovirus infections more than a common cold. Am J Respir Crit Care Med. 2006;174:1284–5. doi: 10.1164/rccm.200609-1387ED. [DOI] [PubMed] [Google Scholar]
- 379.Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. Br Med J. 1995;310:1225–9. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. Br Med J. 1993;307:982–6. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Rakes GP, Arruda E, Ingram JM, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. Am J Respir Crit Care Med. 1999;159:785–90. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 382.Davoine F, Cao M, Wu Y, et al. Virus-induced eosinophil mediator release requires antigen-presenting and CD4+ T cells. J Allergy Clin Immunol. 2008;122(1):69–77. doi: 10.1016/j.jaci.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 383.Henderson FW, Clyde WA, Collier AM, et al. The etiologic and epidemiologic spectrum of bronchiolitis in pediatric practice. J Pediatr. 1979;95:183–90. doi: 10.1016/s0022-3476(79)80647-2. [DOI] [PubMed] [Google Scholar]
- 384.Horn ME, Gregg I. Role of viral infection and host factors in acute episodes of asthma and chronic bronchitis. Chest. 1973;63:44S–8. doi: 10.1378/chest.63.4_supplement.44s-a. [DOI] [PubMed] [Google Scholar]
- 385.Johnston NW, Sears MR. Asthma exacerbations. 1: epidemiology. Thorax. 2006;61:722–8. doi: 10.1136/thx.2005.045161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.McIntosh K, Ellis EF, Hoffman LS, et al. The association of viral and bacterial respiratory infections with exacerbations of wheezing in young asthmatic children. J Pediatr. 1973;82:578–90. doi: 10.1016/S0022-3476(73)80582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Mallia P, Johnston SL. How viral infections cause exacerbation of airway diseases. Chest. 2006;130:1203–10. doi: 10.1378/chest.130.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Pattemore PK, Johnston SL, Bardin PG. Viruses as precipitants of asthma symptoms. I. Epidemiology. Clin Exp Allergy. 1992;22:325–36. doi: 10.1111/j.1365-2222.1992.tb03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Lemanske RF. The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;15:1–6. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 390.Mitchell IH, Inglis H, Simpson H. Viral infection in wheezy bronchitis and asthma in children. Arch Dis Child. 1976;51:707–11. doi: 10.1136/adc.51.9.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.van der Zalm MM, Uiterwaal CSPM, de Jong BM, et al. Viral specimen collection by parents increases response rate in population-based virus studies. J Allergy Clin Immunol. 2006;117:955–7. doi: 10.1016/j.jaci.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 392.Lemanske RF, Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–7. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 393.Kato A, Favoreto S, Jr, Avila PC, et al. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–7. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Horn ME, Reed SE, Taylor P. Role of viruses and bacteria in acute wheezy bronchitis in childhood: a study of sputum. Arch Dis Child. 1979;54:587–92. doi: 10.1136/adc.54.8.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Minor TE, Baker JW, Dick EC, et al. Greater frequency of viral respiratory infections in asthmatic children as compared with their nonasthmatic siblings. J Pediatr. 1974;85:472–7. doi: 10.1016/S0022-3476(74)80447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Rawlinson WD, Waliuzzaman Z, Carter IW, et al. Asthma exacerbations in children are associated with rhinovirus but not human metapneumovirus infection. J Infect Dis. 2003;187:1314–8. doi: 10.1086/368411. [DOI] [PubMed] [Google Scholar]
- 397.Heymann PW, Platts-Mills TAE, Johnston SL. Role of viral infections, atopy and antiviral immunity in the etiology of wheezing exacerbations among children and young adults. Pediatr Infect Dis J. 2005;24:S217–22. doi: 10.1097/01.inf.0000188164.33856.f9. [DOI] [PubMed] [Google Scholar]
- 398.Green RM, Custovic A, Sanderson G, et al. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. Br Med J. 2007;324:1–5. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Minor TE, Dick EC, DeMeo AN, et al. Viruses as precipitants of asthmatic attacks in children. JAMA. 1974;227:292–8. [PubMed] [Google Scholar]
- 400.Roldaan AC, Masural N. Viral respiratory infections in asthmatic children staying in a mountain resort. Eur J Respir Dis. 1982;63:140–50. [PubMed] [Google Scholar]
- 401.Xiao C, Puddicombe SM, Field S, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011;128:549–56. doi: 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 402.Yeo NK, Jang YJ. Rhinovirus infection-induced alteration of tight junction and adherens junction components in human nasal epithelial cells. Laryngoscope. 2010;120:346–52. doi: 10.1002/lary.20764. [DOI] [PubMed] [Google Scholar]
- 403.van Elden LJ, Sachs AP, van Loon AM, et al. Enhanced severity of virus associated lower respiratory tract disease in asthma patients may not be associated with delayed viral clearance and increased viral load in the upper respiratory tract. J Clin Virol. 2008;41:116–21. doi: 10.1016/j.jcv.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Jin Y, Yuan X-H, Xie Z-P, et al. Prevalence and clinical characterization of a newly identified human rhinovirus C species in children with acute respiratory tract infection. J Clin Microbiol. 2009;47(9):2895–900. doi: 10.1128/JCM.00745-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Sanyal MA, Henderson FW, Stempel EC, et al. Effect of upper respiratory tract infection on eustachian tube ventilatory function in the preschool child. J Pediatr. 1980;97:11–5. doi: 10.1016/s0022-3476(80)80121-1. [DOI] [PubMed] [Google Scholar]
- 406.Heinz BA, Vance LM. The antiviral compound enviroxime targets the 3A coding region of rhinovirus and poliovirus. J Virol. 1995;69:4189–97. doi: 10.1128/jvi.69.7.4189-4197.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Heikkinen T, Chonmaitree T. Importance of respiratory viruses in acute otitis media. Clin Microbiol Rev. 2003;16:230–41. doi: 10.1128/CMR.16.2.230-241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Heikkinen T, Thint M, Chonmaitree T. Prevalence of various respiratory viruses in the middle ear during acute otitis media. N Engl J Med. 1999(340). [DOI] [PubMed]
- 409.Arola M, Ziegler T, Ruuskanen O. Respiratory virus infection as a cause of prolonged symptoms in acute otitis media. J Pediatr. 1990;116:697–701. doi: 10.1016/s0022-3476(05)82650-2. [DOI] [PubMed] [Google Scholar]
- 410.Klein JO. Is acute otitis media a treatable disease? N Engl J Med. 2011;364:168–9. doi: 10.1056/NEJMe1009121. [DOI] [PubMed] [Google Scholar]
- 411.Froom J, Culpepper L, DeMelker RA, et al. Antimicrobials for acute otitis media? A review from the International Primary Care Network. BMJ. 1997;315:98–102. doi: 10.1136/bmj.315.7100.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Tahtinen PA, Laine MK, Huovinen P, et al. A placebo-controlled trial of antimicrobial treatment for acute otitis media. N Engl J Med. 2011;364:116–26. doi: 10.1056/NEJMoa1007174. [DOI] [PubMed] [Google Scholar]
- 413.Henderson FW, Collier AM, Sanyal MA, et al. A longitudinal study of respiratory viruses and bacteria in the etiology of acute otitis media with effusion. N Engl J Med. 1982;306:1377–83. doi: 10.1056/NEJM198206103062301. [DOI] [PubMed] [Google Scholar]
- 414.Chonmaitree T, Heikkinen T. Role of viruses in middle-ear disease. Ann N Y Acad Sci. 1997;830:143–57. doi: 10.1111/j.1749-6632.1997.tb51886.x. [DOI] [PubMed] [Google Scholar]
- 415.Heikkinen T, Ruuskanen O, Waris M, et al. Influenza vaccination in the prevention of acute otitis media in children. Am J Dis Child. 1991;145:445–8. doi: 10.1001/archpedi.1991.02160040103017. [DOI] [PubMed] [Google Scholar]
- 416.Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403–9. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 417.Arola M, Ziegler T, Ruuskanen O, et al. Rhinovirus in acute otitis media. J Pediatr. 1988;113:693–5. doi: 10.1016/S0022-3476(88)80380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Winther B, Alper CM, Mandel EM, et al. Temporal relationships between colds, upper respiratory viruses detected by polymerase chain reaction, and otitis media in young children followed through a typical cold season. Pediatrics. 2007;119:1069–75. doi: 10.1542/peds.2006-3294. [DOI] [PubMed] [Google Scholar]
- 419.Chonmaitree T, Ruohola A, Hendley JO. Presence of viral nucleic acids in the middle ear: acute otitis media pathogen or bystander? Pediatr Infect Dis J. 2012;31:325–30. doi: 10.1097/INF.0b013e318241afe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Rihkanen H, Carpen O, Roivainen M, et al. Rhinovirus in adenoid tissue. Int J Pediatr Otorhinolaryngol. 2004;68:903–8. doi: 10.1016/j.ijporl.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 421.Ishizuka S, Yamaya M, Suzuki T, et al. Effects of rhinovirus infection on the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells. J Infect Dis. 2003;188:1928–39. doi: 10.1086/379833. [DOI] [PubMed] [Google Scholar]
- 422.Sykes A, Mallia P, Johnston SL. Diagnosis of pathogens in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4:642–6. doi: 10.1513/pats.200707-101TH. [DOI] [PubMed] [Google Scholar]
- 423.Johnston SL. Overview of virus-induced airway disease. Proc Am Thorac Soc. 2005;2:150–6. doi: 10.1513/pats.200502-018AW. [DOI] [PubMed] [Google Scholar]
- 424.Eadie MB, Stott EJ, Grist NR. Virological studies in chronic bronchitis. Br Med J. 1966;2:671–3. doi: 10.1136/bmj.2.5515.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Hutchinson AF, Black J, Thompson MA, et al. Identifying viral infections in vaccinated Chronic Obstructive Pulmonary Disease (COPD) patients using clinical features and inflammatory markers. Influenza Other Respi Viruses. 2010;4:33–9. doi: 10.1111/j.1750-2659.2009.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Ruuskanen O, Lahti E, Jennings LC, et al. Viral pneumonia. Lancet. 2011;377:1264–75. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Sinaniotis CA. Viral pneumoniae in children: incidence and aetiology. Paediatr Respir Rev. 2004;5 Suppl A:S197–200. doi: 10.1016/s1526-0542(04)90037-1. [DOI] [PubMed] [Google Scholar]
- 428.Falsey AR. Community-acquired viral pneumonia. Clin Geriatr Med. 2007;23:535–52. doi: 10.1016/j.cger.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Ruuskanen O, Mertsola J. Childhood community-acquired pneumonia. Semin Respir Infect. 1999;14:163–72. [PubMed] [Google Scholar]
- 430.Sinaniotis CA, Sinaniotis AC. Community-acquired pneumonia in children. Curr Opin Pulm Med. 2005;11:218–25. doi: 10.1097/01.mcp.0000159831.82529.85. [DOI] [PubMed] [Google Scholar]
- 431.Vuori-Holopainen E, Salo E, Saxen H, et al. Etiological diagnosis of childhood pneumonia by use of transthoracic needle aspiration and modern microbiological methods. Clin Infect Dis. 2002;34:583–90. doi: 10.1086/338642. [DOI] [PubMed] [Google Scholar]
- 432.Templeton KE, Scheltinga SA, van den Eeden WCJFM, et al. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41:345–51. doi: 10.1086/431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.McCracken GH., Jr Diagnosis and management of pneumonia in children. Pediatr Infect Dis J. 2000;19:924–8. doi: 10.1097/00006454-200009000-00036. [DOI] [PubMed] [Google Scholar]
- 434.Honkinen M, Lahti E, Osterback R, et al. Viruses and bacteria in sputum samples of children with community-acquired pneumonia. Clin Microbiol Infect. 2012;18:300–7. doi: 10.1111/j.1469-0691.2011.03603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Smillie WG, Caldwell EL. A study of pneumonia in a rural area in Southern Alabama. J Exp Med. 1929;50:233–44. doi: 10.1084/jem.50.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Avadhanula V, Rodriguez CA, DeVincenzo JP, et al. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol. 2006;80:1629–36. doi: 10.1128/JVI.80.4.1629-1636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Juvén T, Mertsola J, Waris M, et al. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000(19). [DOI] [PubMed]
- 438.Dagan R, Hall CB, Menegus MA. Atypical bacterial infections explained by a concomitant virus infection. Pediatrics. 1985;76:411–4. [PubMed] [Google Scholar]
- 439.Willner D, Furlan M, Haynes M, et al. Metagenomic analysis of respiratory tract DNA viral communities in cystic fibrosis and non-cystic fibrosis individuals. PLoS One. 2009;4:e7370. doi: 10.1371/journal.pone.0007370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Guggino SE. Evolution of the dF508 CFTR mutation. Trends Microbiol. 1999(7). [DOI] [PubMed]
- 441.Wall SR, Wat D, Spiller OB, et al. The viral aetiology of croup and recurrent croup. Arch Dis Child. 2009;94:359–60. doi: 10.1136/adc.2008.142984. [DOI] [PubMed] [Google Scholar]
- 442.Denny FW, Murphy TF, Clyde WA, Jr, et al. Croup: an 11-year study in a pediatric practice. Pediatrics. 1983;71:871–6. [PubMed] [Google Scholar]
- 443.Taylor-Robinson D, Johnson KM, Bloom HH, et al. Rhinovirus neutralizing antibody responses and their measurement. Am J Hyg. 1963;78:285–92. doi: 10.1093/oxfordjournals.aje.a120347. [DOI] [PubMed] [Google Scholar]
- 444.Stenhouse AC. Rhinovirus infection in acute exacerbations of chronic bronchitis: a controlled prospective study. Br Med J. 1967;3:461–3. doi: 10.1136/bmj.3.5563.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Midulla F, Scagnolari C, Bonci E, et al. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Child. 2010;95:35–41. doi: 10.1136/adc.2008.153361. [DOI] [PubMed] [Google Scholar]
- 446.Midulla F, Pierangeli A, Cangiano G, et al. Rhinovirus bronchiolitis and recurrent wheezing: 1-year follow-up. Eur Respir J. 2012;39:396–402. doi: 10.1183/09031936.00188210. [DOI] [PubMed] [Google Scholar]
- 447.Mansbach JM, Piedra PA, Teach SJ, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med. 2012;166(8):700–6. doi: 10.1001/archpediatrics.2011.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Miller EK, Bugna J, Libster R, et al. Human rhinoviruses in severe respiratory disease in very low birth weight infants. Pediatrics. 2012;129:e60–7. doi: 10.1542/peds.2011-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Meltzer EO, Orgel HA, Backhaus JW, et al. Intranasal flunisolide spray as an adjunct to oral antibiotic therapy for sinusitis. J Allergy Clin Immunol. 1993;92:812–23. doi: 10.1016/0091-6749(93)90058-n. [DOI] [PubMed] [Google Scholar]
- 450.Monto AS, Fendrick AM, Sarnes MW. Respiratory illness caused by picornavirus infection: a review of clinical outcomes. Clin Ther. 2001;23:1615–27. doi: 10.1016/S0149-2918(01)80133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Dotson A, Incaudo GA. Rhinitis, sinusitis, and asthma. In: Gershwin ME, Albertson TE, editors. Bronchial asthma. New York: Springer; 2012. pp. 319–44. [Google Scholar]
- 452.Pitkaranta A, Starck M, Savolainen S, et al. Rhinovirus RNA in the maxillary sinus epithelium of adult patients with acute sinusitis. Clin Infect Dis. 2001;33:909–11. doi: 10.1086/322678. [DOI] [PubMed] [Google Scholar]
- 453.Gwaltney JM, Jr, Phillips CD, Miller RD, et al. Computed tomographic study of the common cold. N Engl J Med. 1994;330:25–30. doi: 10.1056/NEJM199401063300105. [DOI] [PubMed] [Google Scholar]
- 454.Turner BW, Cail WS, Hendley JO, et al. Physiologic abnormalities in the paranasal sinuses during experimental rhinovirus colds. J Allergy Clin Immunol. 1992;90:474–8. doi: 10.1016/0091-6749(92)90172-x. [DOI] [PubMed] [Google Scholar]
- 455.Przyklenk B, Bauernfeind A, Bertele RM, et al. Viral infections of the respiratory tract in patients with cystic fibrosis. Serodiagnosis Immunother Infect Dis. 1988;2:217–25. [Google Scholar]
- 456.Petersen NT, Hoiby N, Mordhorst CH, et al. Respiratory infections in cystic fibrosis patients caused by virus, chlamydia and mycoplasma – possible synergism with Pseudomonas aeruginosa. Acta Paediatr Scand. 1981;70:623–8. doi: 10.1111/j.1651-2227.1981.tb05757.x. [DOI] [PubMed] [Google Scholar]
- 457.Pribble CG, Black PG, Bosso JA, et al. Clinical manifestations of exacerbations of cystic fibrosis associated with nonbacterial infections. J Pediatr. 1990;117:200–4. doi: 10.1016/S0022-3476(05)80530-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Wang EE, Prober CG, Manson B, et al. Association of respiratory viral infections with pulmonary deterioration in patients with cystic fibrosis. N Engl J Med. 1984;311:1653–8. doi: 10.1056/NEJM198412273112602. [DOI] [PubMed] [Google Scholar]
- 459.Wat D, Gelder C, Hibbitts S, et al. The role of respiratory viruses in cystic fibrosis. J Cyst Fibros. 2008;7:320–8. doi: 10.1016/j.jcf.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.de Almeida MB, Zerbinati RM, Tateno AF, et al. Rhinovirus C and respiratory exacerbations in children with cystic fibrosis. Emerg Infect Dis. 2010;16:996–9. doi: 10.3201/eid1606.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Ong EL, Ellis ME, Webb AK, et al. Infective respiratory exacerbations in young adults with cystic fibrosis: role of viruses and atypical microorganisms. Thorax. 1989;44:739–42. doi: 10.1136/thx.44.9.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Turner RB, Fuls JL, Rodgers ND, et al. A randomized trial of the efficacy of hand disinfection for prevention of rhinovirus infection. Clin Infect Dis. 2012;54:1–5. doi: 10.1093/cid/cis201. [DOI] [PubMed] [Google Scholar]
- 463.Turner RB, Fuls JL, Rodgers ND. Effectiveness of hand sanitizers with and without organic acids for removal of rhinovirus from hands. Antimicrob Agents Chemother. 2010;54:1363–4. doi: 10.1128/AAC.01498-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Turner RB, Biedermann KA, Morgan JM, et al. Efficacy of organic acids in hand cleansers for prevention of rhinovirus infections. Antimicrob Agents Chemother. 2004;48:2595–8. doi: 10.1128/AAC.48.7.2595-2598.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Turner RB, Hendley JO. Virucidal hand treatments for prevention of rhinovirus infection. J Antimicrob Chemother. 2005;56:805–7. doi: 10.1093/jac/dki329. [DOI] [PubMed] [Google Scholar]
- 466.Smith MBH, Feldman W. Over-the-counter cold medications: a critical review of clinical trials between 1950 and 1991. JAMA. 1993;269:2258–63. doi: 10.1001/jama.269.17.2258. [DOI] [PubMed] [Google Scholar]
- 467.Borum P, Olsen L, Winther B, et al. Ipratropium nasal spray: a new treatment for rhinorrhea in the common cold. Am Rev Respir Dis. 1981;123:418–20. doi: 10.1164/arrd.1981.123.4.418. [DOI] [PubMed] [Google Scholar]
- 468.Chang AB, Clark R, Sloots TP, et al. A 5- versus 3-day course of oral corticosteroids for children with asthma exacerbations who are not hospitalised: a randomised controlled trial. Med J Aust. 2008;189:306–10. doi: 10.5694/j.1326-5377.2008.tb02046.x. [DOI] [PubMed] [Google Scholar]
- 469.Gwaltney JM, Jr, Winther B, Patrie JT, et al. Combined antiviral-antimediator treatment for the common cold. J Infect Dis. 2002;186:147–54. doi: 10.1086/341455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Patick AK, Brothers MA, Maldonado F, et al. In vitro antiviral activity and single-dose pharmacokinetics in humans of a novel, orally bioavailable inhibitor of human rhinovirus 3C protease. Antimicrob Agents Chemother. 2005;49:2267–75. doi: 10.1128/AAC.49.6.2267-2275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Rotbart HA. Treatment of picornavirus infections. Antiviral Res. 2002;53:83–98. doi: 10.1016/s0166-3542(01)00206-6. [DOI] [PubMed] [Google Scholar]
- 472.Couch RB. The common cold: control? J Infect Dis. 1984;150:167–73. doi: 10.1093/infdis/150.2.167. [DOI] [PubMed] [Google Scholar]
- 473.Atmar RL. Uncommon(ly considered) manifestations of infection with rhinovirus, agent of the common cold. Clin Infect Dis. 2005;41:266–7. doi: 10.1086/430927. [DOI] [PubMed] [Google Scholar]
- 474.Hayden FG, Gwaltney JM., Jr Intranasal interferon-a treatment of experimental rhinoviral colds. J Infect Dis. 1984;150:174–80. doi: 10.1093/infdis/150.2.174. [DOI] [PubMed] [Google Scholar]
- 475.Hayden FG, Mills SE, Johns ME. Human tolerance and histopathologic effects of long-term administration of intranasal interferon-a2. J Infect Dis. 1983;148:914–21. doi: 10.1093/infdis/148.5.914. [DOI] [PubMed] [Google Scholar]
- 476.Hayden FG, Andries K, Janssen PAJ. Safety and efficacy of intranasal Pirodavir (R77975) in experimental rhinovirus infection. Antimicrob Agents Chemother. 1992;36:727–32. doi: 10.1128/aac.36.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Thibaut HJ, De Palma AM, Neyts J. Combating enterovirus replication: state-of-the-art on antiviral research. Biochem Pharmacol. 2012;83:185–92. doi: 10.1016/j.bcp.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 478.Patick AK. Rhinovirus chemotherapy. Antiviral Res. 2006;71:391–6. doi: 10.1016/j.antiviral.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Kim KH, Willingmann P, Gong ZX, et al. A comparison of the anti-rhinoviral drug binding pocket in HRV14 and HRV1A. J Mol Biol. 1993;230:206–27. doi: 10.1006/jmbi.1993.1137. [DOI] [PubMed] [Google Scholar]
- 480.Ryan J, Tucker SP, Luttick A, et al. A new oral rhinovirus inhibitor BTA798. In: 18th international conference on antiviral research. 2005. Ref Type: Conference Proceeding.
- 481.Wang QM, Chen SH. Human rhinovirus 3C protease as a potential target for the development of antiviral agents. Curr Protein Pept Sci. 2007;8:19–27. doi: 10.2174/138920307779941523. [DOI] [PubMed] [Google Scholar]
- 482.Binford S, Weady PT, Maldonado F, et al. In vitro resistance studies of Rupintrivir, a novel inhibitor of human rhinovirus 3C protease. Antimicrob Agents Chemother. 2007;51:4366–73. doi: 10.1128/AAC.00905-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Turner RB, Wecker MT, Pohl G, et al. Efficacy of Tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection. JAMA. 2007;281:1797–804. doi: 10.1001/jama.281.19.1797. [DOI] [PubMed] [Google Scholar]
- 484.Yamaya M, Nishimura H, Hatachi Y, et al. Inhibitory effects of tiotropium on rhinovirus infection in human airway epithelial cells. Eur Respir J. 2012;40:122–32. doi: 10.1183/09031936.00065111. [DOI] [PubMed] [Google Scholar]
- 485.Yamaya M, Nishimura H, Hatachi Y, et al. Levofloxacin inhibits rhinovirus infection in primary cultures of human tracheal epithelial cells. Antimicrob Agents Chemother. 2012;56(8):4052–61. doi: 10.1128/AAC.00259-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Bennett JA, Prince LR, Parker LC, et al. Pellino-1 selectively regulates epithelial cell responses to rhinovirus. J Virol. 2012;86:6595–604. doi: 10.1128/JVI.06755-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Gielen V, Johnston SL, Edwards MR. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur Respir J. 2010;36:646–54. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]
Suggested Reading
- Arden KE, Mackay IM. Newly identified human rhinoviruses: molecular methods heat up the cold viruses. Rev Med Virol. 2010;20:156–76. doi: 10.1002/rmv.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer RM, et al. Do rhinoviruses reduce the probability of viral co-detection during acute respiratory tract infections? J Clin Virol. 2009;45:10–5. doi: 10.1016/j.jcv.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay IM. Human rhinoviruses: the cold wars resume. J Clin Virol. 2008;42:297–320. doi: 10.1016/j.jcv.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P, et al. Proposals for the classification of human rhinovirus species C into genotypically-assigned types. J Gen Virol. 2010;91:2409–19. doi: 10.1099/vir.0.023994-0. [DOI] [PubMed] [Google Scholar]
- Tyrrell DAJ, Fielder M. Cold wars: the fight against the common cold. New York: Oxford University Press; 2002. [Google Scholar]


