Abstract
Collective knowledge regarding the occurrence of influenza among swine is incomplete due to inconsistent surveillance of swine populations. In this chapter, we review what surveillance activities exist and some of the practical challenges encountered. Furthermore, to support robust surveillance activities, accurate laboratory assays are needed for the detection of the virus and viral nucleic acids within clinical samples, or for antiviral antibodies in serum samples. The most common influenza diagnostic assays used for swine are explained and their use as surveillance tools evaluated.
Keywords: Influenza Virus, Avian Influenza, Surveillance Program, Avian Influenza Virus, Hemagglutination Inhibition
Surveillance for Influenza Viruses in Swine
Influenza A viruses in swine typically cause an acute respiratory disease which, in uncomplicated cases, is mild and self-limiting (Radostits et al. 2000). Infection of swine with influenza A virus is common (Brown 2000) and occurs throughout the year (Vincent et al. 2008). However, seasonal peaks occur in months with moderate temperatures and humidity (Shaman and Kohn 2009) similar to the pattern of disease seen in humans. Because endemic swine influenza is highly prevalent but causes minimal mortality in infected pigs, the World Organization for Animal Health (Office International de Epizooties), and the U.S. Department of Agriculture (USDA) have not classified swine influenza as a notifiable or reportable disease (OIE 2009; USDA/APHIS 2009). Further complicating the coordinated surveillance efforts are the limited resources available for animal disease surveillance in general. For these financial and biological reasons, systematic and rigorous surveillance is focused on diseases of much higher consequence to animal health and international trade, such as brucellosis and foot-and-mouth disease. Animal disease surveillance in general is labor-intensive and costly and hence animal health authorities at the international, national, provincial, and state levels have precluded assigning it a higher priority for funding (Pappaioanou and Gramer 2010). Given these challenges, the efficient and effective surveillance of influenza viruses in swine will require a strategic approach, encompassing all the attributes of a successful surveillance program.
Attributes of Disease Surveillance Systems
When considering an influenza virus surveillance program for swine populations the key attributes of disease surveillance systems developed and used by leading public health authorities for detecting diseases of public health importance in human populations (CDC 2009) must be considered. These attributes are summarized below.
-
A.
Simplicity. This refers to the surveillance system’s structure and ease of operation. As with most successful operations, systems that prove to be the most valuable utilize methods that are as simple as possible while still fulfilling the primary objectives.
-
B.
Flexibility. A system that can adapt to changing needs, such as the addition of new collection methods or employing new and more specific diagnostic assays has built-in flexibility to capture the required information.
-
C.
Acceptability. A surveillance system must appeal to all interested parties and, once found acceptable, it reflects the willingness of individuals and organizations to participate in the surveillance system (e.g., swine farmers, veterinarians, and veterinary diagnostic laboratory personnel who are asked to report cases of disease).
-
D.
Timeliness. After initial diagnosis, how quickly the cases are entered into the surveillance system or the time that elapses between onset of infection, diagnosis, case report, information sharing, and action, is often regarded as key to a surveillance system’s success (Jajosky and Groseclose 2004). While timeliness is of critical importance, it is often very difficult to measure (Jajosky and Groseclose 2004).
-
E.
Completeness. Completeness is the attribute of a surveillance system that is most directly linked to the true discipline of epidemiology. Completeness is reflected by the proportion of all cases of disease in a specified population that are detected by the surveillance system and is affected by the likelihood that: (a) animals with infection or disease are tested; (b) the condition is correctly diagnosed (skill of animal health provider, accuracy of diagnostic tests); and (c) the case is reported to the surveillance system once it has been diagnosed. The factors that may affect completeness of a surveillance system are addressed in more detail in Sect. 1.3.
-
F.
Representativeness. A surveillance system that accurately describes the occurrence of disease over time and its distribution in the study population by location, group, and severity can be referred to as representative. Consideration of this attribute is especially important for large populations with variable prevalence because most systems simply cannot detect every single case of infection or disease. The common idiom “tip of the iceberg” is a popular way of referring to how the documented and described cases of a disease that are evident as the result of a surveillance program truly represent the largely undetected/unseen cases in the vast population.
While there are several challenges that inherently exist when trying to conduct surveillance on animal populations (further discussed in Sect. 1.3), if an organization is forward thinking and keeps these key attributes in mind, a wealth of information can be generated. The information garnered from an influenza surveillance system for pigs is driven in large part by the initial design and rationale for the surveillance.
Rationale Behind Influenza Surveillance Systems for Swine
While the majority of a surveillance effort is designed to answer “how” and “what”, e.g., logistics and population selection, the better part of the planning time should be devoted to determining “why” to invest in the activity in the first place. For influenza virus surveillance in animals, the simple, altruistic reason is that surveillance must be done to protect public health and prevent pandemics (Patriarca and Cox 1997). Philanthropic intentions aside, it is important to find rationale for surveillance that will also benefit or provide information to all stakeholders. A recent Institute of Medicine (IOM) review for the National Academies, “Sustaining Global Surveillance and Response to Emerging Zoonotic Diseases,” hits the nail on the head with its recommendations to improve early detection and response to zoonotic diseases, such as influenza. Specifically, they are of the opinion that comprehensive surveillance would be best achieved in the following manner: “Multidisciplinary teams of professionals that have relevant expertise and field experience would identify populations at risk and causes and risk factors for infection, and then rapidly and widely disseminate this information so that immediate and longer term disease prevention and control interventions can be implemented (IOM/NRC 2009).”
For influenza virus surveillance in swine in the United States, the rationale for a surveillance system includes not only protection of public health, but detection, discovery, and sharing of virus isolates to facilitate updates for vaccines, refine diagnostic assays, and determine the distribution of new influenza strains in swine to inform further policy decisions (USDA/APHIS 2009). In Europe, the Research Programme of the European Commission funded the coordination of the European Surveillance Network for Influenza in Pigs (ESNIP), a group that set out on a coordinated surveillance mission many years earlier, in 2001, with the stated goal of being to first standardize diagnostic techniques used for surveillance and detection of influenza viruses in pigs. Once the initial goals were achieved, the wealth of information from the surveillance efforts was leveraged for a second round of studies (ESNIP 2007) on the epidemiology and evolution of influenza viruses in European pigs and to optimize influenza diagnostic assays for swine (Kyriakis et al. 2010a). Naturally, a listed rationale for ESNIP 2 is, “to obtain insights into the public health risk of influenza in swine by monitoring swine for avian influenza viruses and by comparison of influenza viruses in swine and in human populations.” ESNIP 3 has since been launched to “…increase the knowledge of the epidemiology and evolution of swine influenza virus in European pigs” with significant research investment directed toward detailed antigenic and genetic characterization of influenza virus strains isolated from pigs (European Commission 2010).
The surveillance programs for swine influenza in developed countries such as the United States and those of the European Union are striving to best coordinate efforts not only with multiple disciplines and agencies, but also with swine producers. While the benefits to human and public health are tangible in that understanding influenza virus patterns in swine may lead to more accurate and timely diagnosis of zoonotic influenza events, the reward for swine producers is less definable. The perception that influenza surveillance programs for pigs have fewer advantages for pork producers is likely due to several reasons, including the minimal impact of influenza virus infection on overall swine health and productivity, fear that trade and profits will be negatively affected, and the lack of readily available, consistently reliable, and inexpensive vaccines to control influenza once it is detected. Furthermore, the funding for such surveillance efforts in pigs requires commitment from all sectors, including animal agriculture, food production, human, and public health (AASV 2009). Securing funding and increasing participation in influenza surveillance programs for swine are challenges that need to be addressed.
Challenges to Surveillance in Animal Populations
From the outset, any effort to conduct influenza surveillance in pigs faces several unique challenges. For starters, respiratory disease is relatively common in pigs and the clinical signs and gross lesions associated with influenza virus in pigs are not entirely specific to influenza. While influenza virus is a significant disease in pigs, there is no official disease reporting requirement for influenza because clinical disease seldom leads to dramatic mortality or severe economic losses in a herd. Additionally, influenza virus is a highly mutagenic virus that can be exchanged among multiple species, with most concerning exchanges occurring between animals and humans. Due to the nature of the global economy, both humans and animals are increasingly mobile regionally and internationally, making comprehensive surveillance difficult across species and geographic boundaries. These factors pose challenges to any influenza surveillance effort in pigs and illustrate the importance of a coordinated surveillance approach.
The first challenge to swine influenza surveillance, and to any early warning system for swine infectious disease, is the inability to reliably detect disease through observations of clinical signs. The clinical signs associated with influenza virus in pigs are generally attributed to Porcine Respiratory Disease Complex (PRDC), a polymicrobial pneumonia caused by several common swine respiratory viruses and bacteria (Brockmeier et al. 2002; Straw et al. 2006). While it is true that influenza A viruses are frequently isolated from pigs with PRDC and evidence of exposure via serological assays is common in growing swine (Brockmeier et al. 2002), it would be wrong to ascribe all clinical signs of respiratory disease in a swine population to influenza without more discriminatory diagnostic methods. Therefore, while reliance on clinical signs and gross lesions for disease detection in pigs has proven to improve the sensitivity of disease detection for other swine diseases such as classical swine fever (Elbers et al. 2003), the sole use of clinical signs as a detection method can lack specificity (Engel et al. 2005). Even swine diseases with hallmark clinical signs, e.g. vesicular exanthema of swine, have cases that may be mild enough to fail detection by clinical observation alone (Schnurrenberger et al. 1987). Similarly, many uncomplicated influenza virus infections in pigs are also mild. It has been shown that single virus infections result in transient clinical signs (Van Reeth et al. 1996). Hence, clinical signs as a method of detection for influenza virus would also likely result in numerous missed cases as well as an abundance of false positive cases. Finally, using clinical signs to detect influenza requires observation by a trained veterinary professional or animal caretaker. Thus, it bears mentioning that surveillance methods, like direct clinical observation, requiring close contact with animals infected by with a potentially zoonotic disease can pose health risks to workers, another potential challenge (Myers et al. 2006; Bos et al. 2010).
The challenges associated with tracking “transboundary” viruses in animals, including influenza, have been reviewed previously (Domenech et al. 2006; Lynn et al. 2006; Gubernot et al. 2008) and the impact of human travel on respiratory disease epidemics such as Severe Acute Respiratory Syndrome (SARS) in people has been examined extensively. However, the component of virus transmission from humans to pigs has not been a significant consideration other than in retrospective analysis of the 1918 Spanish Flu pandemic up until 2009 H1N1 pandemic (Hofshagen et al. 2009). Clearly the impact of human travel and the potential for infecting pigs with novel influenza viruses are evident now. Yet few surveillance systems that exist are capable of capturing both the human and animal data needed to shed light on the existing barriers that prevent or gateways that allow transmission to occur between species.
Migratory waterfowl represent another potential transmission source for influenza to pigs, as demonstrated experimentally (Kida et al. 1994) and naturally (Pensaert et al. 1981; Karasin et al. 2000). In the more recent report on natural infection of pigs with H4N6 influenza, waterfowl on a lake near a swine farm in Canada were implicated as the source of infection in pigs (Karasin et al. 2000). Even with confinement rearing of pigs, exposure to water-borne virus is possible in cases where surface water is used untreated as a water supply for the pigs. Pigs raised partially or completely outdoors could face a higher exposure risk. In the case of the H4N6 influenza virus infection, pigs were raised in confinement. The authors provide evidence of pig to pig transmission of the H4N6 influenza virus within the herd.
In contrast, the first widespread detection of H3N2 influenza virus in pigs in the United States in 1998 was followed by widespread dissemination of H3N2 throughout the North American swine population and subsequent reassortment with other influenza viruses (Ma et al. 2006). This is significant in light of the tremendous increase in movement of growing pigs throughout North America over the past 20 years, another significant challenge for surveillance of influenza in pigs. Data from the Minnesota Board of Animal Health on the movement of growing pigs and breeding swine into Minnesota are illustrative of this point (Fig. 1). There has been more than a seven fold increase in the number of feeder pigs imported into Minnesota over a 5-year period (fiscal years 1994–1999) and this number has doubled again in the subsequent 5-year period (fiscal years 1999–2004), with shipments originating from Canada and 31 other U.S. states (source: Minnesota Board of Animal Health). This movement of pigs at young ages (3–11 weeks) provides a source of pigs that are potentially infectious or susceptible (or both) to particular influenza virus strains. This extent of interstate and international movement is an important consideration when designing surveillance methods for influenza in pigs.
Fig. 1.
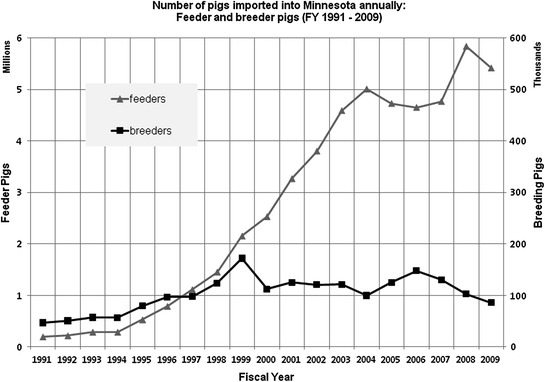
Number of growing and adult pigs imported into Minnesota annually during fiscal years (FY) 1991 and 2009 according to the Minnesota Board of Animal Health. Triangles represent growing (feeder) pigs. Squares represent adult (breeder) pigs
Finally, there is a potential challenge to influenza surveillance in pigs if producers are reluctant to participate in such a program. Diagnostic testing costs can be a barrier to surveillance particularly during protracted periods of unprofitable production such as occurred in 2009 in North America. Producers and veterinarians may also be reluctant to participate in surveillance programs that are perceived to have a potential negative impact on marketability of pigs from a specific site or more generally for the marketing of pork.
Surveillance Design and Logistics
Surveillance design parameters depend on the objectives of the surveillance program as outlined previously. For example, surveillance parameters would be different if the objective is to identify the most prevalent influenza virus subtypes in pigs in a particular region versus whether influenza virus has been eliminated from a specific swine herd (Torremorell et al. 2009). Designing a surveillance program also requires a thorough understanding of the behavior of the virus in pigs, available diagnostic tests, and the production practices used for raising pigs that are to be monitored. Important features of influenza virus infections in pigs are illustrated in Fig. 2 and discussed in detail in other chapters (Clinicopathological Features of Swine Influenza) in this text.
Fig. 2.
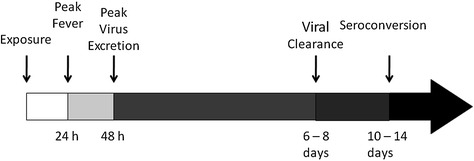
The dynamics of influenza virus infection in swine represented by the simple timeline here are useful for designing surveillance testing protocols
Briefly, it is critical to remember that pigs develop a fever and begin shedding virus rapidly following exposure to influenza virus. Peak virus excretion follows the peak of fever very closely and declines rapidly thereafter. Circulating antibodies are detected within 10–14 days of infection. On an individual pig basis, there is a window of time following infection in which the virus has been cleared, antibodies have not developed, and the pig appears not infected.
Surveillance design is also a function of the tests available for use. Tests intended to detect virus need to be applied during the first week following infection, preferably on samples from pigs that are still febrile. Serological tests such as hemagglutination inhibition can be used to evaluate samples before and after the expected time of seroconversion to specific subtypes of influenza. Serological tests are also available in an ELISA format that detect antibodies against all influenza A subtypes (Ciacci-Zanella et al. 2010) or certain individual subtypes. Influenza diagnostic assays for both antigen and antibody detection are discussed in detail in Sect. 2. Once established diagnostic assays are chosen for the surveillance program, the next critical component is a proper specimen selection and sampling strategy.
Specimen selection and sampling strategies. The specimen of choice within a surveillance program again relates to the objectives of the surveillance as well as the availability of appropriate samples for collection and testing. A variety of specimens are suitable for SIV detection in pigs, including nasal swabs, tracheal swabs, tracheal fluid, lung lavage fluid, and lung sections. For ante-mortem diagnosis of SIV, nasal swabs are one of the more easily obtainable samples. Oral fluids collected from pigs on a group basis represent an alternative to nasal or oropharyngeal swabs. Oral fluids have been used extensively for diagnostic tests in human medicine and are now being applied in swine herds for detecting pathogens and antibodies against the pathogens (Prickett and Zimmerman 2010). Specific applications of oral fluids for influenza virus testing are discussed in Sect. 2.2. Additionally, testing air samples for the presence of swine influenza virus is in the early stages of development (Hermann et al. 2006) and could find application in broader surveillance applications. Postmortem examinations of pigs infected with influenza A viruses have detected the virus (Vincent et al. 2009a; Yazawa et al. 2004) primarily in respiratory tract tissues (nasal turbinates, trachea, and lung), but also in tonsil and bronchial lymph node. The sites for virus replication are similar for historical isolates of “classical” H1N1 swine influenza virus (Yazawa et al. 2004) and 2009 pandemic H1N1 (Vincent et al. 2009a). Postmortem tissues are considered ideal specimens as they can also be examined for gross and microscopic lesions. Thus, complete necropsies with histopathological examinations can further our understanding of the pathogenesis of influenza A viruses in swine. Regardless of the specimen collected, the sample size chosen from the population of concern will affect successful detection of influenza in swine.
Sample size determination for surveillance programs is a function of what test is to be employed and how prevalent the target organism is within the population. In other words, the number of pigs shedding influenza virus at the time of sampling is likely to be different from the number of pigs with serum antibodies depending on when the pigs are sampled. The calculation of an adequate sample size required is fairly straightforward once all the other elements of the sampling frame are established, i.e. sensitivity and specificity of the test, prevalence of the target organism within the population, population size to be sampled, and desired confidence in the end result.
A formula that has been used extensively in swine disease surveillance programs for many years is given below:
where N is the population size, d is the number of positives in the population (expected or threshold prevalence for detection), α is the desired confidence level, and n is the number needed for testing (Cannon and Roe 1982). For example, if one assumes that a diagnostic assay is 100 % sensitive and specific, a sample size of n = 30 from a herd of infinite population N, will provide a 95 % confidence level of detection of disease if the disease prevalence is 10 %. In most situations, the diagnostic assays employed are not 100 % sensitive. As sensitivity decreases, the sample size n must increase.
Databases and sharing of information. Our experience with swine influenza databases indicates that populating the database and sharing the information is most successful when the information is used for a specific and important purpose. Testing for swine influenza virus is a regular activity at veterinary diagnostic laboratories. Serology results are generally used for making decisions on vaccine timing and are not typically collated into common databases. Virus detection by PCR and virus isolation is used to determine the role of influenza virus in clinical disease. Viruses isolated from clinical cases are often used for the production of autogenous vaccines based on the results of virus sequencing information. Virus sequencing information is often assembled into dendrograms to follow virus trends over time and geography. Each piece of the collective diagnostic information has a role in influenza diagnosis and control on a herd or production system basis. By definition, this brings the maintenance of the database close to the end user, who also happen to provide the data inputs.
Financial incentives, such as third party payment for sequencing information, have not appeared to be as important as the direct need for information in terms of motivating producers to participate in surveillance programs up to now. The degree to which producers and veterinarians are interested in sharing disease surveillance information among groups is promising but has not yet been fully determined (Davies et al. 2007).
Examples of Influenza Surveillance in Swine
Comprehensive surveillance programs are needed to detect new influenza strains especially the ones with pandemic potential so we can increase our preparedness to it. Effective surveillance programs should include detection of influenza viruses in humans and animals including pigs. It should also include detection of viruses distributed throughout the world particularly in high risk areas where humans, poultry, and pigs coexist. Surveillance in pigs is considered crucial because pigs have receptors for human, swine, and avian influenza viruses potentially favoring the arising of new viral reassortants. Unfortunately such a global comprehensive surveillance program has not been put in place yet but attempts have been made at the local and regional levels. One limitation of this approach is that the information is not always integrated and shared across species and regions diminishing the effectiveness of comprehensive surveillance efforts.
Detection of influenza viruses in other mammalian species such as cats, dogs, bovine, and equine should also be considered as part of the integrated programs. Although a coordinated global surveillance initiative in pigs does not exist yet, there are examples of programs that over the years have provided a significant but incomplete picture of the circulating influenza viruses in pigs. In addition, the programs that are being planned to actively collect data and specimens for influenza will help to bridge the current gap in influenza surveillance in pigs.
Serosurveillance of pigs in North America. In the US, surveillance studies using serological methods have been based on the sampling of pigs at the point of slaughter and the testing of samples submitted to veterinary diagnostic laboratories. In these studies, pigs originated from various Midwestern States and were representative of pigs owned by multiple enterprises throughout the US. This was the method of choice for many years when other methods of sampling were not available.
In the US, several serological surveys have been conducted. It was demonstrated during 1976/1977 (Hinshaw et al. 1978) and 1988/1989 (Chambers et al. 1991) that influenza virus infections were common among pigs. The percentage of pigs seropositive against classical swine H1N1 viruses ranged from 20 to 47 % in 1976/1977 and 51 % in 1988/1989. In contrast serologic evidence of H3 virus exposure was remarkably lower in both studies (1.4 % in 1976/1977 and 1.1 % in 1988/1989).
In a subsequent study conducted in 1997/1998 (Olsen et al. 2000), 27.7 % of pigs were seropositive to swine H1 virus, 8 % to an H3 human virus, and 7.6 % to an H1 avian virus. These results indicated that pigs were exposed to human H3 and avian viruses to a greater extent than in the past. The finding that the study population tested positive to human H3 influenza virus was of particular significance. Up to that point, detection of H3-subtype influenza viruses in US pigs was rare although it was detected regularly among pigs in Asia and Europe. The findings in 1998 indicated a dramatic pattern change for influenza epidemiology in North America. A Canadian study indicated that seroprevalence to H3N2 viruses in 2002 was negligible although seroprevalence to H1N1 remained high (24.3–61.1 %) (Poliak et al. 2008).
Therefore, influenza surveillance using serological methods has provided useful information in the past but its use has become less reliable due to the broader use of influenza vaccines in pigs and the inability to differentiate antibodies induced by vaccine strains from field strains. In addition, serological methods may not always be able to differentiate infection by strains within a subtype or even between subtypes. The limitations of serological assays are discussed in further detail in Sect. 2.3. For these reasons, virus molecular characterization methods have become widely used and are better able to detect genetic differences among viruses.
Surveillance provided by U.S. Veterinary Diagnostic Laboratories. State and private diagnostic laboratories in the US constitute a rich resource of samples and data for influenza virus surveillance in pigs. Thousands of cases are submitted to the diagnostic laboratories by practitioners and producers to investigate respiratory disease. Most of the cases originate from US herds but may include samples from Canadian herds and a few countries located in Central and Latin America. In many of the cases submitted, influenza virus is detected and diagnosed. As an example, more than 4862 influenza A viruses have been isolated from swine respiratory specimens at the University of Minnesota Veterinary Diagnostic Laboratory (UMVDL) between January 1, 2001 and June 1, 2010 (Gramer and Torrison 2010). In addition 200–700 influenza A virus nucleic acid detection tests (RT-PCR) are conducted monthly on swine respiratory specimens submitted to the UMVDL. The detection of influenza A virus by PCR is followed by subtyping and even partial hemagglutinin gene sequencing when funding is available. Because of confidentiality issues, data derived from these diagnostics is reported solely to the submitting veterinarian and animal owner. While some of the data is shared with the influenza research community, the majority is not automatically released to any publically accessible surveillance databases. Rectifying this situation is not straightforward, but would likely involve discontinuing the institutional practice of considering animal influenza virus isolates as the intellectual property of the owner or researcher and assuring anonymity and prevention of penalties to clients submitting specimens. Nevertheless, diagnostic laboratory data do constitute a valuable resource. In the US, the data generated represent the types of influenza viruses circulating in domestic swine and has resulted in vaccine strain updates and diagnostic reagent revision.
It can be argued that surveillance conducted through routine submissions to diagnostic laboratories is passive, syndromic, and retrospective producing only partial analysis of viruses. Whole genome sequencing of influenza A virus isolates from pigs is needed to detect virus changes and reassortment events that may result in new strains of pandemic potential (Vijaykrishna et al. 2010). Efforts, such as that of the USDA, are designed to integrate the US veterinary diagnostic laboratory network influenza detection and characterization into a more integrated and comprehensive surveillance plan (USDA/APHIS 2009).
Passive/Syndromic surveillance programs. During the last few years discussions have taken place in the US to have an active surveillance influenza program in pigs similar to those for people and poultry for detecting high pathogenic avian influenza or detecting strains of clinical importance. Such a program has not yet been fully possible in pigs although tremendous advances have been made. As a result of the 2009 pandemic, the USDA in cooperation with the CDC and industry allies initiated a voluntary influenza surveillance program in pigs (USDA/APHIS 2009). Although participation in the program has been limited, pork producer, and veterinarian involvement is slowly increasing and contributions of specimens for virus isolation to the surveillance efforts are on the rise.
In addition, influenza is proposed to be part of a comprehensive and integrated surveillance program being designed to protect the US food supply from the impact of diseases considered exotic in the US (AASV 2010). This program has many goals including actively testing for foot-and-mouth disease, classical swine fever, Brucella suis, Aujeszky’s disease, Trichinella spiralis, Toxoplasma gondii, and influenza A virus. Many stakeholders are participating in the design of this program, including the USDA, HHS/CDC, National Pork Board, National Pork Producers Council, American Association of Swine Veterinarians, Veterinary Diagnostic Laboratories, State Animal Health Officials, and State Pork Producer Associations. In regards to influenza, the program aims to determine the prevalence and variety of influenza viruses in US swine, facilitate influenza strain selection for vaccine production, provide continuous improvement of diagnostic testing capabilities, and warrant anonymity to the submitting systems to facilitate cooperation. Such a system should facilitate the cooperation and sharing of information and specimens among stakeholders.
Hong Kong surveillance program for influenza in slaughtered swine. For over a decade, researchers at the University of Hong Kong have participated in an internationally funded, systematic, virological, surveillance program for influenza A viruses in swine slaughtered at one abbatoir in Hong Kong (Vijaykrishna et al. 2010). A majority of the swine slaughtered at this abbatoir are said to originate from mainland China. Routine visits are made to the abbatoir wherein nasal or tracheal swabs from slaughtered pigs are collected, subjected to virus isolation via inoculation in eggs or MDCK cells, and then characterized by hemagglutination inhibition (HI) and sequencing. This slaughter surveillance program has yielded interesting information regarding the genetic constellation of viruses present in China and Hong Kong (Smith et al. 2009; Peiris et al. 2001).
Research-based surveillance. In an effort to bridge the gap on influenza surveillance in pigs, the United States National Institutes of Health funded Centers of Excellence for Influenza Research and Surveillance (CEIRS) have directed some of their research efforts toward active influenza surveillance in swine-dense areas in the Midwestern United States (NIAID 2010). The information from an active surveillance program such as this is sorely needed as growing swine are more representative of the population of pigs most likely to be infected with influenza A virus (Brown 2000), and, because the epidemiology of the virus in swine farms is not well understood (Olsen et al. 2006), an active surveillance program can shed key information on the epidemiology of influenza in swine. In the NIAID sponsored program on active influenza surveillance in swine, thirty nasal swabs are collected every month for 12 consecutive months from growing pigs in 34 separate farms. Swabs are tested for influenza virus by PCR and virus isolation. During collection, the age of the pigs, group clinical signs, and influenza vaccination history are recorded. Farm characteristics, such as herd size, building design, proximity to other farms, biosecurity practices, are also recorded in an attempt to determine possible risk factors associated influenza virus infection. Data on pig age, clinical status, meteorological, and environmental conditions are collected to obtain information on current influenza isolates, their distribution, and disease characteristics.
Summary of international surveillance programs. In Europe, the Research Programme of the European Commission funded the coordination of the European Surveillance Network for Influenza in Pigs (ESNIP). This group became active in 2001 and continues the efforts to increase the knowledge of the epidemiology and evolution of swine influenza virus in European pigs.
In Hong Kong, the surveillance program consists of the isolation of influenza virus at the point of slaughter. Throughout this program a limited but significant number of viral isolates has become available representing the only active systematic influenza surveillance program in the world.
In South and Central America, formal surveillance efforts are nonexistent and are complicated by the fact that some countries consider influenza in pigs an exotic disease limiting the ability to even conduct routine influenza diagnostics.
Diagnostics for Swine Influenza
Diagnosis of swine influenza in the twenty-first century has become more complicated due to the presence of multiple strains of influenza viruses cocirculating in pigs (Webby et al. 2004). Due to the introduction of these multiple strains, the diagnosis and characterization, it is important to understand the many tests that are being used to better characterize influenza virus infections in swine.
Clinicopathology
Clinical signs and characteristic macroscopic and microscopic lesions are useful in making a presumptive, but not definitive, diagnosis of swine influenza infection (see the chapter regarding Clinicopathological Features of Swine Influenza in this text and also Sect. 1.3 and Fig. 2). Laboratory detection of the whole virus, viral antigen, viral nucleic acids or anti-viral antibodies within tissues, serum or other clinical samples is needed for definitive diagnosis.
Direct Detection Methods
Detection of Influenza Virus Antigen
Immunohistochemistry (IHC) and immunofluorescence (IFA) are used to detect influenza virus antigen in frozen or formalin-fixed tissues using different antibodies (Guarner et al. 2000; Haines et al. 1993; Larochelle et al. 1994; Onno et al. 1990; Vincent et al. 1997). The nucleoprotein (NP) is well-conserved among influenza A viruses; therefore, anti-NP antibodies can be used to detect all subtypes of influenza A viruses. However, the hemagglutinin (HA) protein is subtype-specific and hence is used to detect specific subtypes of influenza virus. The NP antigen is located in the nucleus and cytoplasm of infected cells (Guarner et al. 2000; Haines et al. 1993; Larochelle et al. 1994; Vincent et al. 1997) while the HA is located in the cytoplasm and along the cell surface (Guarner et al. 2000).
Direct immunostaining methods use antibodies that are labeled with biotin, fluorophore, enzyme, or colloidal gold (Buchwalow et al. 2010). Although technically difficult and time-consuming, indirect immunostaining methods have higher sensitivity and are more commonly used for diagnostic tests (Buchwalow et al. 2010). These methods use an unlabeled primary antibody followed by a labeled secondary antibody. The application of the substrate then results in amplification of the colorimetric signal produced by the enzyme attached to the secondary antibody (Buchwalow et al. 2010). Of the indirect methods, the standard avidin–biotin complex (ABC) method of IHC has been widely used for SIV detection (Haines et al. 1993; Vincent et al. 1997). However, with this method there can be background staining due to endogenous biotin in the tissues (Vosse et al. 2007). Therefore, these methods have been adapted to polymer-based IHC method (Richt et al. 2006) that uses a polymer backbone on the secondary antibody to attach to the enzyme instead of avidin–biotin complex (Sabattini et al. 1998).
A number of rapid immunoassays, most being enzyme-linked immunosorbent assay (ELISA)-based tests kits are commercially available that can detect influenza virus antigen in clinical samples. Most of these tests have been developed specifically for human and avian applications and the viral proteins that are detected by these kits are HA, neuraminidase (NA), or NP. Five of the kits licensed for human application were found to have sensitivity of 67–71 % and specificity of 99–100 % for Influenza A (Hurt et al. 2007). The sensitivity was higher for specimens containing more than 105 copies/ml of influenza virus RNA as determined by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) (Cheng et al. 2009) or 103–105 TCID50/ml of virus as determined by virus titration in cell cultures (Chan et al. 2009; Hurt et al. 2009). For avian samples, in which sensitivity of RT-PCR is known to be lower than that of virus isolation in embryonated chicken eggs, the sensitivity of antigen detection kits was comparable to that of RT-PCR (Cattoli et al. 2004); the minimum amount of virus needed was 5 × 104 TCID50/ml (Fedorko et al. 2006).
Detection of Nucleic Acids
First described in 1985 (Saiki et al. 1985), the polymerase chain reaction (PCR) has been used to clone DNA, sequence, and analyze genes, identify people by their unique genetic fingerprint and diagnose infectious and genetic diseases. The production of complementary DNA (cDNA) from RNA was made possible by the development of RT-PCR. In 1992, PCR was made even more powerful with the innovation of real-time PCR (RRT-PCR) (Higuchi et al. 1992). Although semiquantitative in nature (Kubista et al. 2006), several RRT-PCR testing protocols have been developed for the detection and quantitation of influenza A viruses including SIVs (Spackman et al. 2002; Spackman and Suarez 2008).
The use of RNA extraction and purification methods varies by the type of sample being tested. For example, RNA can be extracted directly from infected amnioallantoic fluids, cell culture supernatants, bronchoalveolar lavage fluids (BALF), and oral fluids. However, for certain clinical diagnostic samples, prior processing is necessary. Tissue samples, such as lungs, are first made into a 10 % w/v homogenate using a balanced salt solution or a viral culture medium while nasal swabs are usually suspended and vortexed in a test tube with 2 ml of the above media. Although labor-intensive, standard organic extraction procedures produce high purity RNA from most any sample, including tissue homogenates, paraffin-embedded tissues, and body fluids (Sun 2010). However, commercial kits that use magnetic beads or solid-phase adsorption are more sensitive and easy to use with consistent results (Sun 2010). Commercial kits, such as RNeasy and QIAamp RNA kits (Qiagen, Valencia, CA) and PureLink™ RNA kit (Invitrogen, Carlsbad, CA) are based on solid-phase adsorption using silica-membrane spin columns. Commercial kits for magnetic bead extraction, such as MagMAX™ (Applied Biosystems, Foster City, CA) and EZ1 (Qiagen, Valencia, CA) are useful for liquid samples that have low virus concentration or contain PCR inhibitors, such as oral fluids, semen, urine, feces, and blood (Chan and McNally 2008; Das et al. 2009).
To detect a broad range of influenza A subtypes, primers for RRT-PCR are designed to target the conserved matrix (M) or nucleoprotein (NP) genes. The USDA-validated avian influenza RRT-PCR for the M gene (Spackman et al. 2002; Spackman and Suarez 2008) has been adapted for the detection of SIV in swine samples. The minimum detectable concentration of the virus for this procedure ranges from 10−1 to 101 TCID50/ml depending on the virus strain (Landolt et al. 2005; Richt et al. 2004). While virus isolation is still the gold standard test for influenza viruses, RT-PCR is an accurate, rapid, and sensitive technique that can be used to screen a large number of samples in a short period of time. The main disadvantage of RT-PCR is that it detects only the viral RNA and does not determine whether virus is viable or not. Since virus isolation depends on sample inoculation in a live culture system and detects the presence of live virus, it is often used in conjunction with RT-PCR to verify the presence of viable virus.
Detection of Whole Virus
Egg inoculation (EI) using nine to eleven-day-old embryonated chicken eggs is considered the gold standard for isolation and propagation of avian influenza viruses and certain egg-adapted SIVs (Clavijo et al. 2002; Swenson et al. 2001). However, it has been demonstrated that human influenza viruses propagated in chicken embryos acquired amino acid changes in their HA gene resulting in antigenic variation of the virus (Katz et al. 1987; Katz and Webster 1992; Meyer et al. 1993; Robertson et al. 1995). Comparatively, there was little to no genetic or antigenic variation in the same viruses when propagated in mammalian cell lines (Katz et al. 1987, 1990; Katz and Webster 1992; Meyer et al. 1993; Robertson et al. 1995), including Vero, MRC-5, BHK-21, and fetal porcine kidney cells. Of these, the Madin-Darby canine kidney (MDCK) cells have the highest sensitivity and are most commonly used in research and diagnostic applications (Meguro et al. 1979). For maximum sensitivity, inoculation of chicken embryos and/or another cell line is recommended in addition to MDCK cells.
Sample preparation for virus culture is the same as described for RT-PCR (Meguro et al. 1979). Influenza A viruses may replicate in cell cultures within 24–48 h or may take up to 5–6 days if the initial virus concentration in the sample is low. Growth of virus in cell cultures induces the production of cell lysis or cytopathic effects (CPE). Often a second blind passage is necessary for certain strains to show CPE. Once the virus has grown in cell cultures, tests can be performed on the culture supernatant to confirm viral identity. Although not a definitive assay, hemagglutination (HA) of chicken erythrocytes can be taken as a presumptive diagnosis of the virus and for approximation of the amount of virus present in the cell culture supernatant (1 HA unit approximates 5–6 log10 of virus). A more accurate method of quantifying virus is virus titration by inoculation of a set of serial dilutions in cell cultures (Villegas and Alvarado 2008). For definitive virus identification, the culture supernatant can be tested by RT-PCR or commercial influenza antigen test kits based on NP or M antigen. Since virus culture usually contains higher concentrations of virus than the original sample, sensitivity issue seen with clinical samples is usually not a problem when using antigen test kits.
Although virus isolation requires specialized equipment and maintenance of cell cultures and/or embryonated eggs, it is a standardized procedure that is available in most diagnostic laboratories. The virus isolated in cell culture can be cryogenically preserved for years and used for further characterization and vaccine production.
Indirect Detection
Although the clinical signs of influenza infection coincide with the presence of virus in nasal secretions, the isolation of virus by the gold standard method of virus culture or its detection by RT-PCR can be difficult when the period of virus shedding is brief. It has been found in vaccine challenge studies that shedding can be as transient as 24–72 h (Heinen et al. 2001, 2002; Van Reeth et al. 2001, 2003, 2006).
In situations when influenza virus is suspected but no longer detectable at the time of testing, detection of specific immunoglobulins may be undertaken. Immunoglobulins (predominantly IgG) are formed in swine at detectable levels within 1–2 weeks post infection and peak at 4–7 weeks (Olsen et al. 2006). For this reason, it has been recommended that serum samples be collected from pigs at the time of infection and at 3–4 weeks after the onset of clinical signs to compare the acute versus convalescent response (Rossow et al. 2003). Since influenza antibodies can be formed in response to both vaccination and exposure status, the interpretation of serologic assays will depend on both the vaccination and exposure status of the animals being tested. The serologic tests used to detect and measure influenza antibodies include: hemagglutination inhibition, serum neutralization, and enzyme-linked immunosorbent assays.
Hemagglutination inhibition (HI). The agglutination of red blood cells (RBCs) is a natural reaction that occurs in the presence of HA protein on the surface of the virus. HA can be specifically inhibited by influenza antibody, which can be measured in an HI assay. Optimum HA and HI reactions in SIVs occur with turkey or chicken RBCs, which are used in standardized tests (OIE 2008). Before conducting HI tests, it is imperative to remove non-specific inhibitors of viral hemagglutination and naturally occurring agglutinins from the serum samples to be tested. Inhibitors can be removed by treatment with receptor destroying enzyme (RDE) from Vibrio cholerae, heat inactivation, kaolin, or potassium periodate. Similarly, non-specific agglutinins can be removed by pretreatment of serum samples with chicken or turkey RBCs (Boliar et al. 2006; Pedersen 2008a; Regula et al. 2000; Ryan-Poirier and Kawaoka 1991; Springer and Ansell 1958; Subbarao et al. 1992). RDE and heat inactivation at 56 °C are the methods currently recommended to remove inhibitors (OIE 2008).
For the HI test, serial two fold dilutions of the test serum (starting at 1:10 and ending at 1:640 or 1:1280) are prepared in 96-well microtiter plates followed by the addition of 4–8 HA units of a single subtype of influenza virus in all wells containing serum dilutions. Following incubation for an hour at room temperature, 0.5 % suspension of RBCs is added to each well. In the absence of specific antibody, the virus is uninhibited (unbound) and is free to bind to the RBCs resulting in hemagglutination. However, if antihemagglutinin antibodies are present in the serum, such as after exposure or vaccination, the antibodies will bind to the hemagglutinin protein on the surface of the influenza virus, thus inhibiting the virus’ ability to agglutinate the RBCs. The reciprocal of the highest serum dilution that inhibits HA is considered to be the HI titer of that serum (Fig. 2). HI titers greater than or equal to 1:40 are usually considered to be protective (Hancock et al. 2009).
The HI test is considered a standard test for the detection of SIV antibody (Villegas and Alvarado 2008) but is somewhat subjective in nature and the results may vary because of operator subjectivity and also upon repeating the test. Also, since there is broad cross-reactivity among the α, β, and γ clusters of the H1 subtype of SIVs, a positive HI titer may indicate a virus related to the virus of exposure, but does not definitively identify it. However, homologous virus reactions are typically stronger than heterologous virus reactions, resulting in higher HI titers. The advantages of this test are that it is a standardized procedure that is inexpensive and easy to perform and the results are comparable to more complicated tests, such as serum neutralization (Leuwerke et al. 2008; Vincent et al. 2006, 2009a).
Serum neutralization (SN) or virus neutralization (VN). The SN test detects virus-specific neutralizing antibody present in a serum sample. Serial two fold dilutions of the serum and a known amount of SIV are preincubated and then added to MDCK cells to determine the highest dilution of serum that can neutralize virus infection of cells and production of CPE (Fig. 3). Neutralizing antibodies in serum sample block viral infection of cell culture and the virus is not available to produce CPE. However, if antibodies are not present, the virus is not blocked and is free to cause CPE in inoculated cell cultures. Reciprocal of the highest serum dilution that can neutralize virus infection is considered to be the SN titer of the serum. Since the test uses very small volumes of serum in cell monolayers contained in 96-well microtiter plates, it is often called micro neutralization. One of the advantages of SN over HI and enzyme-linked immunosorbent assays (ELISA) is that it demonstrates the biologic (neutralizing) activity of the antibodies present in the serum. Some of the disadvantages of this test are that it requires equipment and supplies used for virus cultures and the results can take up to 72 h to obtain. Also, the SN titers may vary when the test is repeated.
Fig. 3.
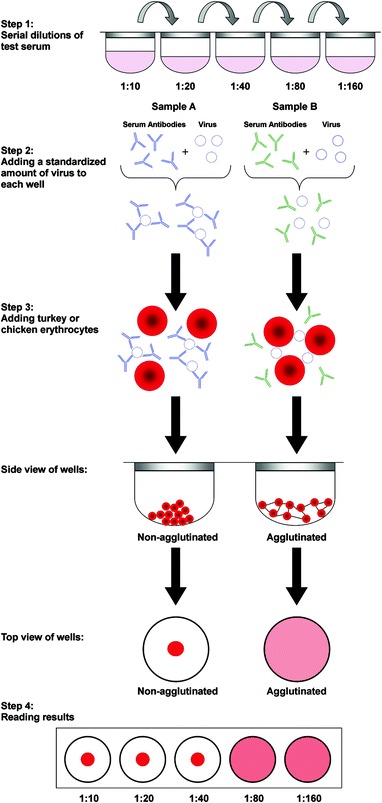
Steps in a hemagglutination inhibition reaction. The antibodies on the left in sample A prevent the virus from agglutinating the erythrocytes. Whereas the antibodies on the right in sample B do not bind to the virus in step 2, which agglutinate the erythrocytes in step 3. The antibody titer shown in step 4 is read out as 1:40
Enzyme-linked immunosorbent assay (ELISA). The ELISA test uses a 96-well plate that has been coated with influenza viral antigen. The serum sample is incubated in the coated wells for antibody attachment. After the unbound material is washed away, an anti-influenza monoclonal antibody that is conjugated to an enzyme is bound to the antigen. The unbound conjugate is washed away and the enzyme substrate (that produces a color change in the presence of the enzyme) is added to the wells. The color-changing reaction is stopped after 15 min and the amount of color produced is read as an optical density (O.D.) in a spectrophotometer (Fig. 4). The O.D. is inversely proportional to the amount of anti-influenza antibodies present in the test sample. Commercially available ELISA test kits include separate ELISA tests for H1N1 and H3N2 subtypes of SIV. Another ELISA that detects antibodies to a range of influenza A viruses is available and has been adapted for use in detecting anti-SIV antibodies (Ciacci-Zanella et al. 2010).
Fig. 4.
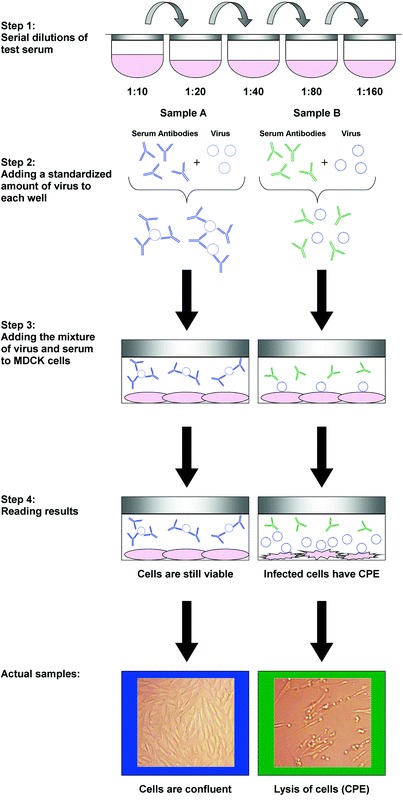
Steps in a serum neutralization reaction. The antibodies in sample A on the left neutralized the virus in step 2. This resulted in no cytopathic effects (CPE) in step 4. Whereas the antibodies in sample B on the right did not neutralize the virus in step 2, resulting in infection of the MDCK cells and CPE in step 4
The commercial H1N1 ELISA uses an antigen prepared from a classical H1N1 SIV and, thus has a limited detection range of swine H1 subtypes. Although the H1N1 test is not designed to detect other influenza subtypes, it may sometimes cross-react with H3N2 because of some common epitopes between H1N1 and H3N2 viruses. In addition, the H1N1 test has been found to miss recently infected animals (Yoon et al. 2004). The H3N2 ELISA test was developed from a cluster I virus leading to lower reactivity with class IV viruses (Yoon et al. 2004). The MultiS-Screen ELISA (FlockChek™, Idexx, Westbrook, ME) uses a highly conserved epitope of influenza A nucleoprotein (NP) (Ciacci-Zanella et al. 2010). Preliminary studies indicate that this kit, while originally designed for use in avian species, also detects antibodies against subtypes common to swine (Ciacci-Zanella et al. 2010) (Fig. 5).
Fig. 5.
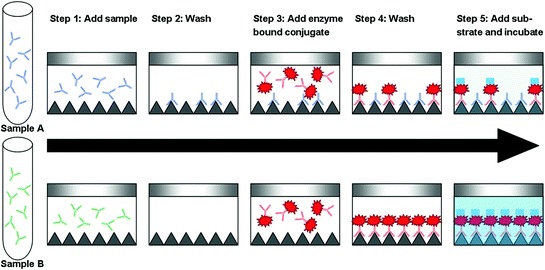
Steps in a blocking ELISA test. The optical density of sample A is lower than sample B because the influenza A antibody in sample A is bound to the antigen coated on the bottom of the well, partially blocking the binding of the enzyme bound conjugate. The antibodies in sample B did not bind to the antigen and were therefore washed out in step 2. Figure adapted from http://www.idexx.com/pubwebresources/pdf/en_us/livestock-poultry/0965846.pdf
Virus Subtyping and Sequencing
Important for host range, antigenicity, and pathogenesis, the 16 HA and 9 NA genes are antigenically and genetically divergent and these variations are used for subtyping the influenza viruses. The cultured viruses were traditionally subtyped using HI and NA inhibition (NI) assays (Pedersen 2008a, b). The NI assay uses a dilution of the cultured virus between 1:4 and 1:32, depending on the virus concentration. There are several steps that include standardized NA antisera (N1–N9), fetuin, periodate, sodium arsenite, and thiobarbituric acid which result in a dark color if there is no inhibition and a light color if there is inhibition; the NA subtype has the light color result. Both of these assays are time-consuming and require standardized NA and HA antisera, which are often difficult to acquire. Therefore, RT-PCR is now regularly used for subtyping. Currently, HA and NA specific primers can be used for both detection and subtyping of influenza A viruses. Additionally, a number of multiplex and nested RT-PCR have been developed for subtyping with and without simultaneous detection of influenza A virus (Chander et al. 2010; Fereidouni et al. 2009; He et al. 2009; Lam et al. 2007; Li et al. 2001; Stockton et al. 1998; Yang et al. 2010).
In addition to subtyping, RT-PCR can also be used for sequencing all eight gene segments of influenza virus (Chander et al. 2010; Jindal et al. 2009). The sequences can be examined and compared to other sequences with molecular analysis tools; uncovering the evolutionary; and geographic relationships of influenza viruses. However, the amount of RNA in clinical samples is usually low compared to the other cellular materials and contaminating bacteria (Spackman and Suarez 2008). Therefore, cell culture supernatants and amnio-allantoic fluid containing a large concentration of whole virus, are recommended for sequencing and other molecular analyses (Spackman and Suarez 2008).
Limitations of Diagnostic Assays
The rapid evolution of influenza A viruses over the last decade has led to genetic and antigenic variation of the virus in North American swine. This has led to limitations in cross-reactivity for the serologic assays. These changes need to be kept in mind when interpreting the results of these tests. Although there is some antigenic cross-reactivity among the classical and reassorted α, β, and γ clusters of the swine H1 subtype, there is little to no cross-reactivity between these three clusters and the human-like δ cluster (Vincent et al. 2006, 2009b). This variability in the antigenic cross-reactivity was demonstrated in 2009 pandemic H1N1 virus for both North American and European swine H1 subtypes using sera from experimentally infected and vaccinated pigs (Kyriakis et al. 2010b; Vincent et al. 2010). The human-like viruses in the SwH1δ cluster were recently found to have two distinct antigenically divergent groups, which could result in additional limitations for serologic assays (Vincent et al. 2009a). Similarly among the swine H3 viruses, there is little to no cross-reactivity between groups I and IV. There is also limited to no cross-reactivity between swine subtypes, which means that multiple viruses from each subtype need to be tested to determine the subtype of the virus that produced the antibodies. To overcome the limitations of cross-reactivity and broaden influenza surveillance, the samples may first be screened by the MultiS-Screen ELISA followed by more specific tests, such as SN and HI assays to determine the subtype of the virus of exposure.
As the influenza virus continues to evolve, the primers for RT-PCR for detection and subtyping need to be continually validated and updated. Current testing stratagems rely on conserved nucleotide sequences for the primers. However, the variability in the HA and NA genes in avian influenzas have resulted in the design of multiple wobble primers to detect one subtype of influenza A without cross-reactivity with other HA and NA subtypes (Sidoti et al. 2010; Starick et al. 2000; Suarez et al. 2007). The avian influenza primers can be used for subtyping influenza viruses from swine or new subtyping primers can be designed using published sequences (He et al. 2009; Huang et al. 2009; Lee et al. 2008; Nagarajan et al. 2010). New technologies, such as enzyme hybridization and microarray, are being used for subtyping of influenza viruses across species (avian, human and swine) and detection of specific influenza viruses like 2009 pandemic H1N1 (He et al. 2009; Huang et al. 2009).
Contributor Information
Jürgen A. Richt, Phone: 785-532 4408, Email: jricht@vet.k-state.edu
Richard J. Webby, Email: richard.webby@stjude.org
Marie Gramer, Email: grame003@umn.edu.
References
- AASV [American Association of Swine Veterinarians] (2009) American Association of Swine Veterinarians position statement on pandemic (H1N1) 2009 influenza. www.aasv.org/aasv/position-pH1N1.pdf. Accessed 28 May 2010
- AASV [American Association of Swine Veterinarians] (2010) Comprehensive and integrated swine surveillance. http://www.aasv.org/shap/issues/v18n2/v18n2advocacy.htm. Accessed 25 June 2010
- Boliar S, Stanislawek W, Chambers TM. Inability of kaolin treatment to remove nonspecific inhibitors from equine serum for the hemagglutination inhibition test against equine H7N7 influenza virus. J Vet Diagn Invest. 2006;18:264–267. doi: 10.1177/104063870601800305. [DOI] [PubMed] [Google Scholar]
- Bos MEH, te Beest DE, van Boven M, Robert‐Du Ry van Beest Holle M, Meijer A, Bosman A, Mulder YM, Koopmans MPG, Stegeman A (2010) High probability of avian influenza virus (H7N7) transmission from poultry to humans ctive in disease control on infected farms. J Infect Dis 201:1390–1396 [DOI] [PubMed]
- Brockmeier S, Halbur PG, Thacker EL. Porcine respiratory disease complex. In: Brogden K, Guthmiller JM, editors. Polymicrobial diseases. Washington : ASM Press; 2002. pp. 231–257. [Google Scholar]
- Brown IH. The epidemiology and evolution of influenza viruses in pigs. Vet Microbiol. 2000;74:29–46. doi: 10.1016/S0378-1135(00)00164-4. [DOI] [PubMed] [Google Scholar]
- Buchwalow IB, Böcker W, SpringerLink . Immunohistochemistry: basics and methods. Berlin: Springer; 2010. [Google Scholar]
- Cannon RM, Roe RT (1982) Livestock disease surveys: a field manual for veterinarians. Australian Government Publishing Services, Canberra, pp 14
- Cattoli G, Drago A, Maniero S, Toffan A, et al. Comparison of three rapid detection systems for type A influenza virus on tracheal swabs of experimentally and naturally infected birds. Avian Pathol. 2004;33:432–437. doi: 10.1080/03079450410001724058. [DOI] [PubMed] [Google Scholar]
- CDC [Centers for Disease Control and Prevention] (2009) Lesson 5: public health surveillance. In: principles of epidemiology in public health practice, 3rd ed. http://www.cdc.gov/training/products/ss1000/ss1000-ol.pdf Accessed 23 April 2012
- Chambers TM, Hinshaw VS, Kawaoka Y, Easterday BC, Webster RG. Influenza viral infection of swine in the United States 1988–1989. Arch Virol. 1991;116:261–265. doi: 10.1007/BF01319247. [DOI] [PubMed] [Google Scholar]
- Chan DJ, McNally L. Assays for the determination of HIV-1 load in semen: a review of indications, methods and performance in vitro. Current HIV Res. 2008;6:182–188. doi: 10.2174/157016208784324949. [DOI] [PubMed] [Google Scholar]
- Chan KH, Lai ST, Poon LL, Guan Y, Yuen K, Peiris JS. Analytical sensitivity of rapid influenza antigen detection tests for swine-origin influenza virus (H1N1) J Clin Virol. 2009;45:205–207. doi: 10.1016/j.jcv.2009.05.034. [DOI] [PubMed] [Google Scholar]
- Chander Y, Jindal N, Stallknecht D, et al. Full length sequencing of all nine subtypes of the neuraminidase gene of influenza A viruses by primer walking. J Virol Meth. 2010;165:116–120. doi: 10.1016/j.jviromet.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CK, Cowling BJ, Chan KH, Fang VJ, et al. Factors affecting quickvue Influenza A + B rapid test performance in the community setting. Diagn Microbiol Infect Dis. 2009;65:35–41. doi: 10.1016/j.diagmicrobio.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Ciacci-Zanella JR, Vincent AL, Prickett JR, Zimmerman SM, Zimmerman JJ. Detection of anti-influenza A nucleoprotein antibodies in pigs using a commercial influenza epitope-blocking enzyme-linked immunosorbent assay developed for avian species. J Vet Diagn Invest. 2010;22:3–9. doi: 10.1177/104063871002200102. [DOI] [PubMed] [Google Scholar]
- Clavijo A, Tresnan DB, Jolie R, Zhou EM. Comparison of embryonated chicken eggs with MDCK cell culture for the isolation of swine influenza virus. Canadian J Vet Res. 2002;66:117–121. [PMC free article] [PubMed] [Google Scholar]
- Das A, Spackman E, Pantin-Jackwood MJ, Suarez DL. Removal of real-time reverse transcription polymerase chain reaction (RT-PCR) inhibitors associated with cloacal swab samples and tissues for improved diagnosis of avian influenza virus by RT-PCR. J Vet Diagn Invest. 2009;21:771–778. doi: 10.1177/104063870902100603. [DOI] [PubMed] [Google Scholar]
- Davies PR, Wayne SR, Torrison JL, Peele B, de Groot BD, Wray D. Real-time disease surveillance tools for the swine industry in Minnesota. Vet Ital. 2007;43:731–738. [PubMed] [Google Scholar]
- Domenech J, Lubroth J, Eddi C, Martin V, Roger F. Regional and international approaches on prevention and control of animal transboundary and emerging diseases. Ann NY Acad Sci. 2006;1081:90–107. doi: 10.1196/annals.1373.010. [DOI] [PubMed] [Google Scholar]
- Elbers AR, Vos JH, Bouma A, van Exsel AC, Stegeman A. Assessment of the use of gross lesions at post-mortem to detect outbreaks of classical swine fever. Vet Microbiol. 2003;96:345–356. doi: 10.1016/j.vetmic.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Engel B, Bouma A, Stegeman A, Buist W, Elbers A, Kogut J, Döpfer D, de Jong MC. When can a veterinarian be expected to detect classical swine fever virus among breeding sows in a herd during an outbreak? Prev Vet Med. 2005;67(2–3):195–212. doi: 10.1016/j.prevetmed.2004.10.010. [DOI] [PubMed] [Google Scholar]
- ESNIP (2007) The European surveillance network for influenza in pigs. http://www.esnip.ugent.be. Accessed 20 June 2010
- European Commission (2010) European Commission unveils new research projects to fight influenza. http://ec.europa.eu/research/index.cfm?pg=newsalert&lg=en&year=2010&na=na-090310-annexes. Accessed 21 June 2010
- Fedorko DP, Nelson NA, McAuliffe JM, Subbarao K. Performance of rapid tests for detection of avian influenza A virus types H5N1 and H9N2. J Clin Microbiol. 2006;44:1596–1597. doi: 10.1128/JCM.44.4.1596-1597.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fereidouni SR, Starick E, Grund C, Globig A, et al. Rapid molecular subtyping by reverse transcription polymerase chain reaction of the neuraminidase gene of avian influenza A viruses. Vet Microbiol. 2009;135(3–4):253–260. doi: 10.1016/j.vetmic.2008.09.077. [DOI] [PubMed] [Google Scholar]
- Gramer MR, Torrison JL (2010) Happy anniversary to (pandemic 2009 H1N1) flu. National Hog Farmer Weekly Preview. http://nationalhogfarmer.com/weekly-preview/0503-happy-anniversary-pandemic-2009-h1n1/. Accessed 23 June 2010
- Guarner J, Shieh WJ, Dawson J, Subbarao K, et al. Immunohistochemical and in situ hybridization studies of influenza A virus infection in human lungs. Am J Clin Pathol. 2000;114:227–233. doi: 10.1309/HV74-N24T-2K2C-3E8Q. [DOI] [PubMed] [Google Scholar]
- Gubernot DM, Boyer BL, Moses MS. Animals as early detectors of bioevents: veterinary tools and a framework for animal-human integrated zoonotic disease surveillance. Public Health Rep. 2008;123:300–315. doi: 10.1177/003335490812300310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines DM, Waters EH, Clark EG. Immunohistochemical detection of swine influenza A virus in formalin-fixed and paraffin-embedded tissues. Canadian J Vet Res. 1993;57:33–36. [PMC free article] [PubMed] [Google Scholar]
- Hancock K, Veguilla V, Lu X, Zhong W, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- He J, Bose ME, Beck ET, Fan J, et al. Rapid multiplex reverse transcription-PCR typing of influenza A and B virus, and subtyping of influenza A virus into H1, 2, 3, 5, 7, 9, N1 (human), N1 (animal), N2, and N7, including typing of novel swine origin influenza A (H1N1) virus, during the 2009 outbreak in Milwaukee, Wisconsin. J. J Clin Microbiol. 2009;47:2772–2778. doi: 10.1128/JCM.00998-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen PP, van Nieuwstadt AP, de Boer-Luijtze EA, Bianchi AT. Analysis of the quality of protection induced by a porcine influenza A vaccine to challenge with an H3N2 virus. Vet Immunol Immunopathol. 2001;82:39–56. doi: 10.1016/S0165-2427(01)00342-7. [DOI] [PubMed] [Google Scholar]
- Heinen PP, Rijsewijk FA, de Boer-Luijtze EA, Bianchi AT. Vaccination of pigs with a DNA construct expressing an influenza virus M2-nucleoprotein fusion protein exacerbates disease after challenge with influenza A virus. J Gen Virol. 2002;83:1851–1859. doi: 10.1099/0022-1317-83-8-1851. [DOI] [PubMed] [Google Scholar]
- Hermann JR, Hoff SJ, Yoon KJ, Burkhardt AC, Evans RB, Zimmerman JJ. Optimization of a sampling system for recovery and detection of airborne porcine reproductive and respiratory syndrome virus and swine influenza virus. Appl Environ Microbiol. 2006;72:4811–4818. doi: 10.1128/AEM.00472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R, Dollinger G, Walsh PS, Griffith R. Simultaneous amplification and detection of specific DNA sequences. Biotechnology (N.Y) 1992;10:413–417. doi: 10.1038/nbt0492-413. [DOI] [PubMed] [Google Scholar]
- Hinshaw VS, Bean WJ, Webster RG, Easterday BC. The prevalence of influenza viruses in swine and the antigenic and genetic relatedness of influenza viruses from man and swine. Virology. 1978;84:51–62. doi: 10.1016/0042-6822(78)90217-9. [DOI] [PubMed] [Google Scholar]
- Hofshagen M, Gjerset B, Er C, Tarpai A, Brun E, Dannevig B, Bruheim T, Fostad IG, Iversen B, Hungnes O, Lium B. Pandemic influenza A(H1N1)v: human to pig transmission in Norway? Euro Surveill. 2009;14:19406. doi: 10.2807/ese.14.45.19406-en. [DOI] [PubMed] [Google Scholar]
- Huang Y, Tang H, Duffy S, Hong Y, et al. Multiplex assay for simultaneously typing and subtyping influenza viruses by use of an electronic microarray. J Clin Microbiol. 2009;47:390–396. doi: 10.1128/JCM.01807-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt AC, Alexander R, Hibbert J, Deed N, Barr IG. Performance of six influenza rapid tests in detecting human influenza in clinical specimens. J Clin Virol. 2007;39:132–135. doi: 10.1016/j.jcv.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Hurt AC, Baas C, Deng YM, Roberts S, Kelso A, Barr IG. Performance of inflenza rapid point-of-care tests in the detection of swine lineage A(H1N1) influenza viruses. Influenza Other Respir Viruses. 2009;3:171–176. doi: 10.1111/j.1750-2659.2009.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOM/NRC [National Research Council] Sustaining global surveillance and response to emerging zoonotic diseases. Washington: National Academies Press; 2009. [PubMed] [Google Scholar]
- Jajosky R, Groseclose S. Evaluation of reporting timeliness of public health surveillance systems for infectious diseases. BMC Public Health. 2004;4:29. doi: 10.1186/1471-2458-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal N, Chander Y, Sreevatsan S, et al. Amplification of four different genes of influenza A viruses using a degenerate primer set in a one step RT-PCR method. J Virol Methods. 2009;160:163–166. doi: 10.1016/j.jviromet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasin AI, Olsen CW, Brown IH, Carman S, Stalker M. H4N6 influenza virus isolated from pigs in Ontario. Can Vet J. 2000;41:938–939. [PMC free article] [PubMed] [Google Scholar]
- Katz JM, Webster RG. Amino acid sequence identity between the HA1 of influenza A (H3N2) viruses grown in mammalian and primary chick kidney cells. J Gen Virol. 1992;73:1159–1165. doi: 10.1099/0022-1317-73-5-1159. [DOI] [PubMed] [Google Scholar]
- Katz JM, Naeve CW, Webster RG. Host cell-mediated variation in H3N2 influenza viruses. Virology. 1987;156:386–395. doi: 10.1016/0042-6822(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Katz JM, Wang M, Webster RG. Direct sequencing of the HA gene of influenza (H3N2) virus in original clinical samples reveals sequence identity with mammalian cell-grown virus. J Virol. 1990;64:1808–1811. doi: 10.1128/jvi.64.4.1808-1811.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida H, Ito T, Yasuda J, Shimizu Y, Itakura C, Shortridge KF, Kawaoka Y, Webster RG. Potential for transmission of avian influenza viruses to pigs. J Gen Virol. 1994;75:2183–2188. doi: 10.1099/0022-1317-75-9-2183. [DOI] [PubMed] [Google Scholar]
- Kubista M, Andrade JM, Bengtsson M, Forootan A, et al. The real-time polymerase chain reaction. Mol Aspects Med. 2006;27:95–125. doi: 10.1016/j.mam.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Kyriakis CS, Brown IH, Foni E, Kuntz-Simon G, Maldonado J, Madec F, Essen SC, Chiapponi C, Van Reeth K. Virological surveillance and preliminary antigenic characterization of influenza viruses in pigs in five European countries from 2006 to 2008. Zoonoses Pub H . 2010 doi: 10.1111/j.1863-2378.2009.01301.x. [DOI] [PubMed] [Google Scholar]
- Kyriakis CS, Olsen CW, Carman S, Brown IH, et al. Serologic cross-reactivity with pandemic (H1N1) 2009 virus in pigs, Europe. Emerg Infect Dis. 2010;16:96–99. doi: 10.3201/eid1601.091190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam WY, Yeung AC, Tang JW, Ip M, et al. Rapid multiplex nested PCR for detection of respiratory viruses. J Clin Microbiol. 2007;45:3631–3640. doi: 10.1128/JCM.00280-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt GA, Karasin AI, Hofer C, Mahaney J, Svaren J, Olsen CW. Use of real-time reverse transcriptase polymerase chain reaction assay and cell culture methods for detection of swine influenza A viruses. Am J Vet Res. 2005;66:119–124. doi: 10.2460/ajvr.2005.66.119. [DOI] [PubMed] [Google Scholar]
- Larochelle R, Sauvageau R, Magar R. Immunohistochemical detection of swine influenza virus and porcine reproductive and respiratory syndrome virus in porcine proliferative and necrotizing pneumonia cases from Quebec. Canadian Vet J. 1994;35:513–515. [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Kang BK, Lee DH, Lyou SH, et al. One-step multiplex RT-PCR for detection and subtyping of swine influenza H1, H3, N1, N2 viruses in clinical samples using a dual priming oligonucleotide (DPO) system. J Virol Methods. 2008;151:30–34. doi: 10.1016/j.jviromet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Leuwerke B, Kitikoon P, Evans R, Thacker E. Comparison of three serological assays to determine the cross-reactivity of antibodies from eight genetically diverse U.S. swine influenza viruses. J Vet Diagn Invest. 2008;20:426–432. doi: 10.1177/104063870802000403. [DOI] [PubMed] [Google Scholar]
- Li J, Chen S, Evans DH. Typing and subtyping influenza virus using DNA microarrays and multiplex reverse transcriptase PCR. J Clin Microbiol. 2001;39:696–704. doi: 10.1128/JCM.39.2.696-704.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn T, Marano N, Treadwell T, Bokma B. Linking human and animal health surveillance for emerging diseases in the United States achievements and challenges. Ann NY Acad Sci. 2006;1081:108–111. doi: 10.1196/annals.1373.011. [DOI] [PubMed] [Google Scholar]
- Ma W, Gramer M, Rossow K, Yoon KJ. Isolation and genetic characterization of new reassortant H3N1 swine influenza virus from pigs in the midwestern United States. J Virol. 2006;80:5092–5096. doi: 10.1128/JVI.80.10.5092-5096.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguro H, Bryant JD, Torrence AE, Wright PF. Canine kidney cell line for isolation of respiratory viruses. J Clin Microbiol. 1979;9:175–179. doi: 10.1128/jcm.9.2.175-179.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer WJ, Wood JM, Major D, Robertson JS, Webster RG, Katz JM. Influence of host cell-mediated variation on the international surveillance of influenza A (H3N2) viruses. Virology. 1993;196:130–137. doi: 10.1006/viro.1993.1461. [DOI] [PubMed] [Google Scholar]
- Myers KP, Olsen C, Setterquist SF, Capuano AW, Donham KJ, Thacker EL, Merchant JA, Gray GC. Are swine workers in the United States at increased risk of infection with zoonotic influenza virus? Clin Infect Dis. 2006;42:14–20. doi: 10.1086/498977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan MM, Simard G, Longtin D, Simard C. Single-step multiplex conventional and real-time reverse transcription polymerase chain reaction assays for simultaneous detection and subtype differentiation of Influenza A virus in swine. J Vet Diagn Invest. 2010;22:402–408. doi: 10.1177/104063871002200309. [DOI] [PubMed] [Google Scholar]
- NIAID [National Institute of Allergy and Infectious Diseases] (2010) Centers of Excellence for Influenza Research and Surveillance (CEIRS). http://www.niaid.nih.gov/LabsAndResources/resources/ceirs/Pages/introduction.aspx. Accessed 25 June 2010
- OIE [Office International des Epizooties] (2009) Pandemic H1N1: questions and answers. www.oie.int/eng/press/h1n1/en_h1_n1_faq.asp. Accessed 29 May 2010
- OIE [World Organization for Animal Health] (2008) Manual of diagnostic tests and vaccines for terrestrial animals. Chapter 2.8.8: Swine Influenza, http://www.oie.int/fr/normes/mmanual/2008/pdf/2.08.08_SWINE_INFLUENZA.pdf. Accessed 22 June 2010
- Olsen CW, Carey S, Hinshaw L, Karasin AI. Virologic and serologic surveillance for human, swine and avian influenza virus infections among pigs in the north-central United States. Arch Virol. 2000;145:1399–1419. doi: 10.1007/s007050070098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CW, Brown IH, Easterday BC, Van Reeth K, et al. Swine Influenza. In: Straw BE, et al., editors. Diseases of swine. 9. Ames: Blackwell; 2006. pp. 469–482. [Google Scholar]
- Onno M, Jestin A, Vannier P, Kaiser C. Diagnosis of swine influenza with an immunofluorescence technique using monoclonal antibodies. Vet Q. 1990;12:251–254. doi: 10.1080/01652176.1990.9694274. [DOI] [PubMed] [Google Scholar]
- Pappaioanou M, Gramer M. Lessons from pandemic H1N1 2009 to improve prevention, detection, and response to influenza pandemics from a one Health perspective. ILAR J. 2010;51:268–280. doi: 10.1093/ilar.51.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarca PA, Cox NJ. Influenza pandemic preparedness plan for the United States. J Infect Dis. 1997;176:S4–S7. doi: 10.1086/514174. [DOI] [PubMed] [Google Scholar]
- Pedersen JC. Hemagglutination-inhibition test for avian influenza virus subtype identification and the detection and quantitation of serum antibodies to the avian influenza virus. In: Spackman E, editor. Methods in Molecular biology, Avian influenza virus. Totowa: Humana Press; 2008. [DOI] [PubMed] [Google Scholar]
- Pedersen JC. Neuraminidase-inhibition assay for the identification of influenza A virus neuraminidase subtype or neuraminidase antibody specificity. In: Spackman E, editor. Methods in Molecular biology, Avian influenza virus. Totowa: Humana Press; 2008. [Google Scholar]
- Peiris JSM, Guan Y, et al. Cocirculation of avian H9N2 and contemporary “human” H3N2 influenza A viruses in pigs in southeastern China: Potential for genetic reassortment? J Virol. 2001;75:9679–9686. doi: 10.1128/JVI.75.20.9679-9686.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert M, Ortis K, Vandeputte J, Kaplan MM, Bachmann PA. Evidence for the natural transmission of influenza A virus from wild ducks to swine and its potential importance for man. Bull World Hlth Org. 1981;59:75–78. [PMC free article] [PubMed] [Google Scholar]
- Poliak Z, Dewey CE, Martin SW, Christensen J, Carman S, Friendship RM. Prevalence of and risk factors for influenza in southern Ontario swine herds in 2001 and 2003. Can J Vet Res. 2008;72:7–17. [PMC free article] [PubMed] [Google Scholar]
- Prickett JR, Zimmerman JJ. The development of oral fluid-based diagnostics and applications in veterinary medicine. Anim Hlth Res Rev. 2010;5:1–10. doi: 10.1017/S1466252310000010. [DOI] [PubMed] [Google Scholar]
- Radostits OM, Gay CC, Blood DC, Hinchcliff KW. Veterinary medicine: a textbook of the diseases of cattle, sheep, pigs, goats, and horses. 9. New York: WB Saunders Co; 2000. pp. 1157–1159. [Google Scholar]
- Regula G, Lichtensteiger CA, Mateus-Pinilla NE, Scherba G, Miller GY, Weigel RM. Comparison of serologic testing and slaughter evaluation for assessing the effects of subclinical infection on growth in pigs. J Am Vet Med Assoc. 2000;217:888–895. doi: 10.2460/javma.2000.217.888. [DOI] [PubMed] [Google Scholar]
- Richt JA, Lager KM, Clouser DF, Spackman E, Suarez DL, Yoon KJ. Real-time reverse transcription-polymerase chain reaction assays for the detection and differentiation of North American swine influenza viruses. J Vet Diagn Invest. 2004;16:367–373. doi: 10.1177/104063870401600501. [DOI] [PubMed] [Google Scholar]
- Richt JA, Lekcharoensuk P, Lager KM, Vincent AL, et al. Vaccination of pigs against swine influenza viruses by using an NS1-truncated modified live-virus vaccine. J Virol. 2006;80:11009–11018. doi: 10.1128/JVI.00787-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JS, Cook P, Attwell A, Williams SP. Replicative advantage in tissue culture of egg-adapted influenza virus over tissue-culture derived virus: implications for vaccine manufacture. Vaccine. 1995;13:1583–1588. doi: 10.1016/0264-410X(95)00085-F. [DOI] [PubMed] [Google Scholar]
- Rossow KD, Yeske P, Goyal SM, Webby RJ, Collins JE (2003) Diagnostic investigation of unexpected serology results for swine influenza virus (SIV) and porcine reproductive and respiratory syndrome virus (PRRSV). J Swine Hlth Prod 1133–1135
- Ryan-Poirier KA, Kawaoka Y. Distinct glycoprotein inhibitors of influenza A virus in different animal sera. JVirol. 1991;65:389–395. doi: 10.1128/jvi.65.1.389-395.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabattini E, Bisgaard K, Ascani S, Poggi S, et al. The EnVision++ system: a new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, ChemMate, CSA, LABC, and SABC techniques. J Clin Pathol. 1998;51:506–511. doi: 10.1136/jcp.51.7.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki RK, Scharf S, Faloona F, Mullis KB, et al. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Schnurrenberger P, Sharman RS, Wise GH. Attacking animal diseases: concepts and strategies for control and eradication. Ames: Iowa State University Press; 1987. p. 64. [Google Scholar]
- Shaman J, Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natn Acad Sci. 2009;106:3243–3248. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidoti F, Rizzo F, Costa C, Astegiano S, et al. Development of real time RT-PCR assays for detection of type A influenza virus and for subtyping of avian H5 and H7 hemagglutinin subtypes. Mol Biotechnol. 2010;44:41–50. doi: 10.1007/s12033-009-9211-7. [DOI] [PubMed] [Google Scholar]
- Smith GJD, Vijaykrishna D, et al. Origins and evolutionary genomics of the 2009 swine origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- Spackman E, Suarez DL. Type A influenza virus detection and quantitation by real-time RT-PCR. In: Spackman E, editor. Methods in molecular biology, Avian influenza virus. Totowa: Humana Press; 2008. [DOI] [PubMed] [Google Scholar]
- Spackman E, Senne DA, Myers TJ, Bulaga LL, et al. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer GF, Ansell NJ. Inactivation of human erythrocyte agglutinogens M and N by influenza viruses and receptor-destroying enzyme. Proc Natl Acad Sci USA. 1958;44:182–189. doi: 10.1073/pnas.44.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starick E, Romer-Oberdorfer A, Werner O. Type- and subtype-specific RT-PCR assays for avian influenza A viruses (AIV) J Vet Med B Infect Dis Vet Public Health. 2000;47:295–301. doi: 10.1046/j.1439-0450.2000.00386.x. [DOI] [PubMed] [Google Scholar]
- Stockton J, Ellis JS, Saville M, Clewley JP, Zambon MC. Multiplex PCR for typing and subtyping influenza and respiratory syncytial viruses. J Clin Microbiol. 1998;36:2990–2995. doi: 10.1128/jcm.36.10.2990-2995.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straw BE, Dewey CE, Wilson MR, et al. Differential diagnosis of disease. In: Straw BE, et al., editors. Diseases of swine. 9. Ames: Blackwell; 2006. pp. 241–286. [Google Scholar]
- Suarez DL, Das A, Ellis E. Review of rapid molecular diagnostic tools for avian influenza virus. Avian Dis. 2007;51:201–208. doi: 10.1637/7732-101006-REGR.1. [DOI] [PubMed] [Google Scholar]
- Subbarao EK, Kawaoka Y, Ryan-Poirier K, Clements ML, Murphy BR. Comparison of different approaches to measuring influenza a virus-specific hemagglutination inhibition antibodies in the presence of serum inhibitors. J Clin Microbiol. 1992;30:996–999. doi: 10.1128/jcm.30.4.996-999.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, et al. Nucleic extraction and amplification. In: Grody W, et al., editors. Molecular diagnostics: techniques and applications for the clinical laboratory. 1. San Diego, California: Academic Press; 2010. pp. 35–47. [Google Scholar]
- Swenson SL, Vincent LL, Lute BM, Janke BH, et al. A comparison of diagnostic assays for the detection of type A swine influenza virus from nasal swabs and lungs. J Vet Diagn Invest. 2001;13:36–42. doi: 10.1177/104063870101300108. [DOI] [PubMed] [Google Scholar]
- Torremorell M, Juarez A, Chavez E, Yescas J, Doporto JM, Gramer M. Procedures to eliminate H3N2 swine influenza virus from a pig herd. Vet Rec. 2009;165:74–77. doi: 10.1136/vetrec.165.3.74. [DOI] [PubMed] [Google Scholar]
- USDA/APHIS [Animal and Plant Health Inspection Service] (2009) National Surveillance Plan for Swine Influenza Virus: including novel H1N1 2009 virus. www.aphis.usda.gov/newsroom/hot_issues/h1n1/downloads/H1N1_Surveillance_Plan_2009.pdf. Accessed 21 Dec 2009
- Van Reeth K, Nauwynck H, Pensaert M. Dual infections of feeder pigs with porcine reproductive and respiratory syndrome virus followed by porcine respiratory coronavirus or swine influenza virus: a clinical and virological study. Vet Microbiol. 1996;48:325–335. doi: 10.1016/0378-1135(95)00145-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth K, Labarque G, De Clercq S, Pensaert M. Efficacy of vaccination of pigs with different H1N1 swine influenza viruses using a recent challenge strain and different parameters of protection. Vaccine. 2001;19:4479–4486. doi: 10.1016/S0264-410X(01)00206-7. [DOI] [PubMed] [Google Scholar]
- Van Reeth K, Gregory V, Hay A, Pensaert M. Protection against a European H1N2 swine influenza virus in pigs previously infected with H1N1 and/or H3N2 subtypes. Vaccine. 2003;21:1375–1381. doi: 10.1016/S0264-410X(02)00688-6. [DOI] [PubMed] [Google Scholar]
- Van Reeth K, Labarque G, Pensaert M. Serological profiles after consecutive experimental infections of pigs with European H1N1, H3N2, and H1N2 swine influenza viruses. Viral Immunol. 2006;19:373–382. doi: 10.1089/vim.2006.19.373. [DOI] [PubMed] [Google Scholar]
- Vijaykrishna D, Poon LLM, Zhu HC, et al. Reassortment of pandemic H1N1/2009 influenza A virus in swine. Science. 2010;328:1529. doi: 10.1126/science.1189132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas P, Alvarado I (2008) Chapter 46. In: Dufour-Zavala L, et al. (ed) A laboratory manual for the isolation, identification, and characterization of avian pathogens 5th edn. American Association of Avian Pathologists, Jacksonville, Florida, p 218
- Vincent LL, Janke BH, Paul PS, Halbur PG. A monoclonal-antibody-based immunohistochemical method for the detection of swine influenza virus in formalin-fixed, paraffin-embedded tissues. J Vet Diagn Invest. 1997;9:191–195. doi: 10.1177/104063879700900214. [DOI] [PubMed] [Google Scholar]
- Vincent AL, Lager KM, Ma W, Lekcharoensuk P, et al. Evaluation of hemagglutinin subtype 1 swine influenza viruses from the United States. Vet Microbiol. 2006;118:212–222. doi: 10.1016/j.vetmic.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Vincent AL, Ma W, Lager KM, Janke BH, Richt JA. Swine influenza viruses a North American perspective. Adv Virus Res. 2008;72:127–154. doi: 10.1016/S0065-3527(08)00403-X. [DOI] [PubMed] [Google Scholar]
- Vincent AL, Lager KM, Harland M, Lorusso A, Zanella E, et al. Absence of 2009 pandemic H1N1 Influenza A virus in fresh pork. PLoS ONE. 2009;4:e8367. doi: 10.1371/journal.pone.0008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AL, Ma W, Lager KM, Gramer MR, Richt JA, Janke BH. Characterization of a newly emerged genetic cluster of H1N1 and H1N2 swine influenza virus in the United States. Virus Genes. 2009;39:176–185. doi: 10.1007/s11262-009-0386-6. [DOI] [PubMed] [Google Scholar]
- Vincent AL, Lager KM, Faaberg KS, Harland M, et al. Experimental inoculation of pigs with pandemic H1N1 2009 virus and HI cross-reactivity with contemporary swine influenza virus antisera. Influenza other Respiratory Viruses. 2010;4:53–60. doi: 10.1111/j.1750-2659.2009.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosse BA, Seelentag W, Bachmann A, Bosman FT, Yan P. Background staining of visualization systems in immunohistochemistry: comparison of the Avidin-Biotin Complex system and the EnVision+ system. Appl Immunohistochem Mol Morphol. 2007;15:103–107. doi: 10.1097/01.pai.0000213102.33816.13. [DOI] [PubMed] [Google Scholar]
- Webby RJ, Rossow K, Erickson G, Sims Y, Webster R. Multiple lineages of antigenically and genetically diverse influenza A virus co-circulate in the United States swine population. Virus Res. 2004;103:67−73. doi: 10.1016/j.virusres.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gonzalez R, Huang F, Wang W, et al. Simultaneous typing and HA/NA subtyping of influenza A and B viruses including the pandemic influenza A/H1N1 2009 by multiplex real-time RT-PCR. J Virol Methods. 2010;167:37–44. doi: 10.1016/j.jviromet.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa S, Okada M, Ono M, et al. Experimental dual infection of pigs with an H1N1 swine influenza virus (A/Sw/Hok/2/81) and Mycoplasma hyopneumoniae. Vet Microbiol. 2004;98:221–228. doi: 10.1016/j.vetmic.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Yoon KJ, Janke BH, Swalla RW, Erickson G. Comparison of a commercial H1N1 enzyme-linked immunosorbent assay and hemagglutination inhibition test in detecting serum antibody against swine influenza viruses. J Vet Diagn Invest. 2004;16:197–201. doi: 10.1177/104063870401600304. [DOI] [PubMed] [Google Scholar]


