Abstract
Enzyme immunoassays (EIAs) to detect and quantify antibodies against respiratory syncytial virus (RSV) and RSV proteins in human plasma or sera are described. The first EIA uses RSV lysate antigens produced in HEp-2 cell line. The second EIA uses RSV F or G gene-expressed antigen in HEp-2 cells. The third EIA uses 30-amino acid synthetic peptides from central conserved region of G protein of RSV A2 or RSV B1 virus and a peptide from the SARS CoV nucleoprotein as a negative control peptide. All three EIAs have been evaluated for detecting and quantifying the respective antibodies in human sera or plasma.
Key words: Respiratory syncytial virus, Enzyme immunoassay, Peptide, RSV F gene, RSV G gene
Introduction
Antibodies against RSV proteins play an important role in preventing disease with RSV by various mechanisms including virus neutralization, antibody-dependent cellular cytotoxicity (ADCC), and complement-mediated neutralization. The RSV attachment (G) and fusion (F) proteins are the major targets of RSV-specific neutralizing antibodies but antibodies against other virus proteins are also induced by infection and can contribute to serological diagnosis of infection. EIA detects both neutralizing and non- neutralizing antibodies and use of RSV lysate antigen in EIA provides a way to detect antibodies against multiple RSV proteins. Use of F or G gene-expressed antigens, e.g., in a HEp-2 cell line, can be used to determine protein-specific antibody response. The 30-amino acid synthetic peptides are from a region of the G protein that is immune active and antibodies to this region can prevent some aspects of RSV disease.
Materials
Production of RSV Virus and HEp-2 Lysate Antigens
Class II biosafety laminar flow cabinet.
175 cm2 Vented cell culture flasks.
HEp-2 cells.
RSV virus stock.
37 °C Incubator with 5 % CO2.
SF-MEM: Serum-free minimum essential medium (MEM).
5 % Fetal bovine serum-minimum essential medium (FBS-MEM): MEM, 5 % v/v FBS, 1× penicillin/streptomycin, 1× glutamine.
−80 °C Freezer.
15 mL Conical tubes.
50 mL Conical tubes.
2 mL Cryovials.
Liquid nitrogen.
Benchtop centrifuge with rotor for 50 mL conical tubes.
Determining the Dilution of RSV Lysate Antigen to be Used in the RSV Lysate EIA
Class II biosafety laminar flow cabinet.
96-Well EIA plates.
200 μL Multichannel pipette.
Micropipettes.
Pipette tips.
Fluid reservoirs.
RSV lysate antigen.
HEp-2 lysate antigen.
10× PBS: 1.37 M NaCl, 27 mM KCL, 100 mM Na2HPO4, 10 mM KH2PO4. Dissolve the reagents in water, adjust pH to 7.4 with HCl, and sterilize by autoclaving.
PBS: To prepare 1× PBS dilute 10× PBS 1:10 in sterile distillated water.
EIA coating buffer (carbonate-bicarbonate buffer 0.05 M, pH 9.6): 1.6 g of Na2CO3, 2.94 g of NaHCO3, 0.2 g of NaN3, distillated water to 1 L. Check for pH 9.4–9.6; usually there will be no need to adjust pH. Store in screw-capped bottle at room temperature.
EIA washing buffer: 200 mL of 10× PBS, 1 mL of Tween-20, and 1800 mL of water. Add 10× PBS, distilled H2O and Tween 20 in a 2 L measuring cylinder and stir for 10 min using a magnetic stirrer at 200 rpm. Store at room temperature.
EIA blocking buffer: 1× PBS, 1 % w/v cold water fish skin gelatin, 1 % w/v skim milk, and 0.05 % Tween-20.
Goat anti-RSV polyclonal serum (AB1128, Millipore Inc., USA).
Anti-goat IgG-HRP-labeled secondary antibody of choice.
0.15 M Citric acid: 7.74 g of C5H7COOH⋅1H2O, 17.93 g Na2HPO4, distillated water to 1 L. Adjust pH 6.0 if needed. Store at room temperature.
OPD substrate: 25 mL of 0.15 M citric acid, 15 mg O-phenylenediamine (OPD), 15 μL H2O2. Prepare in a 50 mL tube wrapped in aluminum foil, screw cap the tube, and mix by vigorous shaking for 45 s. Prepare freshly, store at room temperature in the dark, and use within 10–20 min.
4 N H2SO4: 444.5 mL distillated water, 55.5 mL of 18 M H2SO4. In 1 L glass bottle, add H2SO4 to water in chemical full exhaust hood, allow to cool, then screw cap the bottle, and store in screw-capped bottle at room temperature.
ELISA plate reader.
Detection of Anti-RSV Antibodies in Human Plasma or Serum Using RSV Lysate EIA
-
20.
96-Well EIA plates.
-
21.
50 mL Conical tubes.
-
22.
200 μL Multichannel pipette.
-
23.
Micropipettes.
-
24.
Pipettes tips.
-
25.
Fluid reservoirs.
-
26.
EIA coating buffer.
-
27.
EIA washing buffer.
-
28.
EIA blocking buffer.
-
29.
Human reference antiserum to RSV (available from NIH BEI Resources, USA, Cat # NR-4020).
-
30.
Goat-anti-human IgG—HRP-labeled antibody of choice.
-
31.
OPD substrate.
-
32.
4 N H2SO4.
-
33.
ELISA plate reader.
Detection of Anti-RSV G or F Protein Antibodies in Human Plasma or Serum
96-Well culture plates.
HEp-2 cells.
Mammalian expression plasmid containing the RSV F or G gene (pCDNA3.1+ or similar).
DNA transfection reagent of choice.
Complete medium: MEM, 10 % v/v FBS, 1× glutamine.
Opti-MEM® I Reduced serum medium or regular high-glucose DMEM without serum.
Vortex.
15 mL Conical tubes.
96-Well EIA plates.
200 μL Multichannel pipette.
Micropipettes.
Pipettes tips.
Fluid reservoirs.
PBS.
4 % PFA: 4 g of paraformaldehyde, distillated water to 100 mL, pH 7.4 (see Note 1).
EIA coating buffer.
EIA washing buffer.
EIA blocking buffer.
Goat-anti-human IgG—HRP-labeled antibody.
OPD substrate.
4 N H2SO4.
ELISA plate reader.
Detection of Anti-RSV Antibodies in Human Plasma or Serum Using RSV Lysate EIA
96- Well EIA plates.
200 μL Multichannel pipette.
200 μL pipette tips.
EIA coating buffer.
50 mL Conical tubes.
RSV G protein and SARS CoV N protein peptides (see Table 1).
Fluid reservoirs.
Table 1.
Peptides used for quantification of human anti-RSV group A and B antibodies to the central conserved region of the G protein
| Peptide ID | Amino acid location | Amino acid sequence |
|---|---|---|
| RSV A2-glycoprotein | 161–190 | NDFHFEVFNF VPCSICSNNP TCWIACKRIP |
| RSV B1-glycoprotein | 161–190 | DDYHFEVFNF VPCSICGNNQ LCKSICKTIP |
| SARS CoV-nucleoproteina | 1–30 | MDLFMRFFTL GSITAQPVKI DNASPASTVH |
aThe SARS CoV nucleoprotein peptide 1–30 amino acid (30 mer) is used as a negative control peptide
Methods
The RSV lysate antigen-based enzyme immunoassay (EIA) detects antibodies against RSV proteins present in preparation including anti-F, anti-G, and anti-N protein antibodies. TheEIA can be performed using just an RSV group A or RSV group B strain or by combining lysates from each group. TheEIA’s sensitivity for detecting past infection can be improved, e.g., in young children, by using both RSV A and RSV B group antigens.
All the procedures involving handling and production of infectious RSV virus in cell culture must be performed in a Class II biosafety laminar flow cabinet adhering to the biosafety guidelines.
Production of RSV Virus and HEp-2 Lysate Antigens
Seed 175 cm2 vented cell culture flasks with 3 × 106 HEp-2 cells in 30 mL of 5 % FBS-MEM. Seed six flasks per every RSV virus (e.g., RSV A2, RSV B1) and HEp-2 lysate antigen control. Incubate the flask at 37 °C in 5 % CO2 for 24–36 h (see Note 2).
Remove the medium and wash cells with 5 mL of SF-MEM three times.
Thaw the RSV virus stock to use on ice. Once thawed, dilute virus in SF-MEM to achieve 1 × 105 pfu/mL (MOI = 0.1).
Infect the HEp-2 cells with 3 mL of diluted virus and incubate at 37 °C in 5 % CO2 for 2 h by rocking every 15 min to spread the virus inoculum on the cell monolayer. For the flasks that will be used to produce HEp- 2 lysate antigen control use 3 mL of SF-MEM instead (mock infection).
Remove the inoculums and wash cells thrice with 5 mL of SF-MEM medium.
Add 40 mL 5 % FBS-MEM and incubate flasks at 37 °C in 5 % CO2 for 72 h.
On day 3 (72 h) post-infection, when viral cytopathic effects (CPE) are first becoming evident, remove culture medium and wash the flask thrice with 5 mL of SF-MEM (see Note 3).
Add 30 mL fresh SF-MEM and incubate the flask at 37 °C/5 % CO2 for further 24–48 h.
On days 4–5 post-infection, when 90 % viral ) CPE is observed, freeze the 175 cm2 flasks at −80 °C.
Thaw the flasks, remove 2 mL of the virus fluid from each flask, and pool in a 15 mL tube. Subdivide into 0.5 mL aliquots in cryovials, snap freeze using liquid nitrogen, and then store at −80 °C for 24 h. Refreeze the 175 cm2 flasks at −80 °C until determination of virus titer.
Take out one cryovial that has been frozen at −80 °C for 24 h and perform a plaque assay (see Chapter 10.1007/978-1-4939-3687-8_3) to determine virus titer.
Thaw, freeze, and thaw the 175 cm2 flask to lyse the cells, and then immediately after the third thaw transfer the cell lysate to 50 mL conical tubes. Centrifuge the cell lysate at 1880 × g for 20 min at 4 °C. Aliquot the supernatant in 1.5 mL into cryovials and store at −80 °C.
Determining the Dilution of RSV Virus Lysate Antigen to be Used in the RSV Lysate EIA
Perform serial twofold dilutions (1:2 to 1:256) of the RSV lysateEIA and HEp-2 cell lysate inEIA coating buffer.
Dispense 100 μL of diluted antigen solution in each column of 96- wellEIA plate using multichannel pipette. Seal the plate with adhesive plastic sheet and incubate 37 °C for 2 h and then at 4 °C for 16 h.
Remove the lysate antigen and wash five times with 300 μL ofEIA washing buffer using an automated plate washer or by hand pipetting up and down.
Dry the plates by blotting on paper towel at the end of washing but not between washes.
Dispense 200 μL ofEIA blocking buffer per well, seal the plates with adhesive plastic sheet, and incubate at 37 °C for 1 h.
Wash five times with 300 μL ofEIA washing buffer.
Dilute goat anti-RSV polyclonal serum inEIA blocking buffer, add 100 μL per well, and incubate for 1 h at 37 °C.
Wash five times with 300 μL ofEIA washing buffer.
Dilute anti-goat IgG-HRP-labeled secondary antibody inEIA blocking buffer. Add 100 μL per well and incubate at 37 °C for 1 h.
Wash five times with 300 μL ofEIA washing buffer and blot the plates on paper towel at the end of washing.
Add 100 μL of OPD substrate per well and incubate the plate for 30 min in the dark at room temperature.
Stop the reaction by adding 50 μL of 4 N H2SO4 and gently rock the plate to mix it.
Read absorbance at 490 nm within 10–15 min of stopping theEIA reaction.
Data analysis can be performed on Microsoft Excel or similar software.
Report the highest dilution of RSV lysate antigen that gives corrected (RSV antigen—HEp- 2 control antigen) absorbance >1.5 at 490 nm to be used in the subsequent assays.
Detection of Anti-RSV Antibodies in Human Plasma or Serum Using RSV Lysate EIA
Using sterile 50 mL conical tubes, dilute the HEp-2 lysate antigen and RSV lysate antigen (according to dilution determined in Subheading 3.2) inEIA coating buffer.
Dispense 100 μL of diluted antigen solution in each column of 96- wellEIA plate using multichannel pipette as required in the plate layout. Use new tips to dispense RSV and HEp-2 lysate antigens.
Seal the plate with adhesive plastic sheet and incubate 37 °C for 2 h and then at 4 °C for 16 h.
Remove RSV lysate antigen or HEp-2 lysate antigen liquid from the wells and add 200 μL ofEIA washing buffer. Make sure that the micropipette tips do not touch the surface of the wells and cross contaminate the wells.
Wash five times with 300 μL ofEIA washing buffer using an automated plate washer or by hand pipetting up and down.
Dry the plates by blotting on paper towels at the end of washing but not between washes.
Dispense 200 μL ofEIA blocking buffer per well, seal the plates with adhesive plastic sheet, and incubate at 37 °C for 1 h.
Wash five times with 300 μL ofEIA washing buffer and dry the plates by blotting on paper towels at the end of washing but not between washes.
Dilute human plasma samples inEIA blocking buffer to 1:200 dilution in triplicate, dilute the human reference antiserum to 1:200, and perform serial fourfold dilutions (Fig. 1).
Add 100 μL of diluted samples per well and incubate for 1 h at 37 °C.
Wash five times with 300 μL ofEIA washing buffer and dry the plates by blotting on paper towel at the end of washing but not between washes.
Dilute the goat-anti-human IgG-HRP-labeled secondary antibody inEIA blocking buffer. Add 100 μL per well and incubate at 37 °C for 1 h (see Note 4).
Wash five times with 300 μL ofEIA washing buffer and dry the plates by blotting on paper towels.
Add 100 μL of OPD substrate per well and incubate the plate for 30 min in the dark at room temperature.
Stop the reaction by adding 50 μL of 4 N H2SO4 and gently rock the plate to mix it.
Read optical density/absorbance at 490 nm within 10–15 min of stopping theEIA reaction.
Data analysis can be performed on Microsoft Excel or similar software (see Note 5).
Fig. 1.
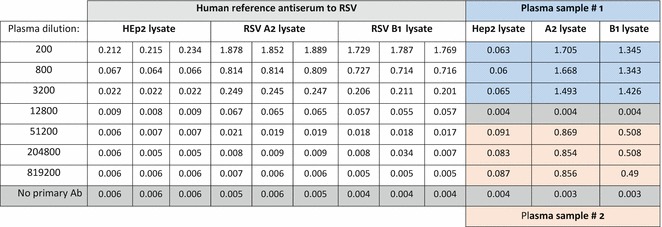
Example result of an RSV lysate EIA assay for quantification of antibodies in human plasma
Detection of Anti-RSV G or F Protein Antibodies in Human Plasma or Serum
In a 96-well culture plate seed 2 × 104 HEp-2 cells/well in 100 μL of complete medium and incubate at 37 °C in 5 % CO2 for 18–24 h.
Prepare the transfection complexes by mixing the mammalian expression plasmid containing the RSV F or G gene and the transfection reagent of choice. Avalanche®-Omni transfection reagent will be used as an example for this protocol.
In a 15 mL conical tube add 11 μg of DNA into 1250 μL of Opti-MEM® Reduced-Serum medium or regular high-glucose DMEM without serum (see Note 6). Mix by vortexing.
Briefly vortex Avalanche®-Omni and add 11 μL into the 15 mL conical tube containing the DNA. Mix by inversion 8–10 times and incubate for 15 min at room temperature.
Add 9.75 mL of complete medium to the 15 mL conical tube containing the transfection complexes and mix by inversion 8–10 times.
Add 100 μL per well of the plasmid-Avalanche®-Omni lipid polymer complexes to the 96-well culture plate. Incubate at 37 °C in 5 % CO2 for 36–48 h.
Remove the medium from the 96-well culture plate by aspiration and wash wells four times with 250 μL of PBS.
Fix the cells with 100 μL of 4 % PFA for 20 min.
RemovePFA and wash the wells four times with PBS (see Note 7).
Dispense 200 μL ofEIA blocking solution per well, seal the plate with adhesive plastic sheet, and incubate at 37 °C 1 h.
Wash five times with 300 μL ofEIA washing buffer using an automated plate washer or by hand pipetting up and down. Blot the plates on paper towel at the end of washing.
In triplicate wells, add 100 μL of test plasma or serum diluted inEIA blocking buffer at a final dilution of 1:200, and incubate the plate at 37 °C for 1 h.
Wash five times with 300 μL ofEIA washing buffer.
Dilute the goat-anti-human IgG-HRP-labeled secondary antibody inEIA blocking buffer, dispense 100 μL per well, and incubate at 37° for 1 h.
Wash five times with 300 μL ofEIA washing buffer.
Add 100 μL of OPD substrate to each well and incubate the plate for 20 min in the dark at room temperature.
Stop reaction by adding 50 μL of 4 N H2SO4 and gently rock the plate to mix it.
Read optical density/absorbance at 490 nm within 10–15 min of stopping theEIA reaction.
Data analysis can be performed on Microsoft Excel or similar software (see Fig. 2 and Note 8).
Fig. 2.
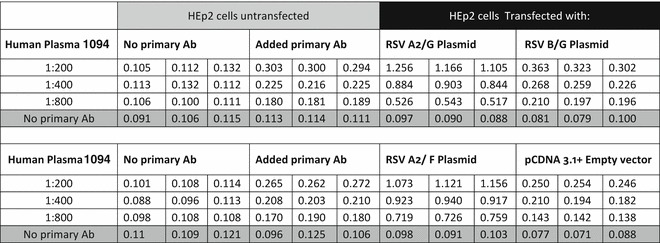
Example result of an RSV F and G protein EIA assay for quantification of antibodies in human plasma
Detection of Antibodies to Central Conserved Region of the RSV G Protein (seeNote9)
Using sterile 50 mL conical tubes dilute RSV A2, RSV B1, and SARS peptide to 5000 ng/mL inEIA coating buffer. Mix five times by vortexing with 5-min intervals in between each vortex until completely dissolved.
Transfer peptides to separate fluid reservoirs.
Dispense 100 μL of diluted peptide solution in each column of 96- wellEIA plate using a multichannel pipette as per the assay design plate layout (see Fig. 3 and Note 10)
After adding peptides, seal the plate with adhesive plastic sheet and incubate at 37 °C for 1 h or at 4 °C overnight.
Aspirate the liquid from the wells and wash five times with PBS + 0.05 % Tween 20.
Dispense 200 μL ofEIA blocking solution per well, seal the plate with adhesive plastic sheet, and incubate at 37 °C for 1 h.
Wash five times with 300 μL ofEIA washing buffer using an automated plate washer or by hand pipetting up and down. Blot the plates on paper towel at the end of washing.
In triplicate wells, add 100 μL of test plasma or serum diluted inEIA blocking buffer at a final dilution of 1:200 and incubate the plate at 37 °C for 1 h.
Wash five times with 300 μL ofEIA washing buffer using an automated plate washer or by hand pipetting up and down. Blot the plates on paper towel at the end of washing.
Dilute the goat-anti-human IgG-HRP-labeled secondary antibody inEIA blocking buffer, dispense 100 μL per well, and incubate at 37 °C for 1 h.
Wash five times with 300 μL ofEIA washing buffer. Blot the plates on paper towel at the end of washing.
Add 100 μL of OPD substrate to each well and incubate the plate for 20 min in the dark at room temperature.
Stop reaction by adding 50 μL of 4 N H2SO4 and gently rock the plate to mix it.
Read optical density/absorbance at 490 nm within 10–15 min of stopping theEIA reaction.
Data analysis can be performed on Microsoft Excel or similar software (see Note 11).
Fig. 3.
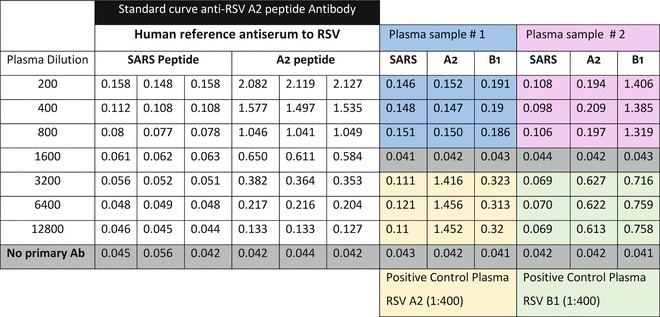
Example result of a G protein central conserved region peptide EIA assay
Notes
To dissolve paraformaldehyde in water add 1 M NaOH and stir gently on a heating block at 60 °C until thePFA is dissolved. Add 10 mL of 10× PBS and allow the mixture to cool to room temperature. Adjust the pH to 7.4 with 1 M HCl (~1 mL), and then adjust the final volume to 100 mL with H2O. Filter the solution through a 0.45 μm membrane filter to remove any particulate matter
The HEp-2 cell culture is grown in 175 cm2 vented cell culture flask and should be processed in a separate hood for mock infection. The HEp-2 cell lysate should be prepared as described for RSV lysate antigen production and 5 % FBS-MEM replaced by SF-MEM at comparable times.
The RSV virus CPE is characterized by formation of syncytia, formation of giant cells, and rounding and sloughing of HEp-2 cells. TheCPE can be observed using an inverted microscope at 40–100× magnification. The uninfected HEp-2 cells that will be used for HEp-2 lysate antigen control should not present anyCPE.
Follow the manufacturer’s recommendation to determine the dilution of secondary antibody. Approximately 10 mL of diluted conjugate is used for one plate. Scale up or down according to the number of plates being tested.
Data analysis can be performed using a standard curve generated for serial twofold dilutions of a human reference antiserum to RSV (available from NIH BEI Resources, USA, Cat # NR-4020) and predict the end point antibody titers in test plasma/serum samples.
As a negative control we recommend to use the empty vector lacking of the RSV F and G gene.
After this step the plates can be stored for up to 2 weeks in zip-lock bags at 4 °C for further use. Before storage, the individual plates should be sealed with adhesive plastic sheet.
Final data can be presented as positive or negative for RSV antibodies (absorbance against F- or G-transfected cells (P) significantly above those for plasmid control (N)) and antibody titer for positive specimens. The titer of RSV antibody can be estimated by comparing corrected absorbance values (P-N) for the specimen to those for serial twofold dilutions of the human reference antiserum to RSV (available from NIH BEI Resources Cat # NR-4020, USA) or an RSV antibody high positive plasma from a donor.
This assay is developed to detect human anti-RSV group A and B antibodies to the central conserved region of glycoprotein. Peptides of 30 amino acid length are custom synthesized at 75 % or higher purity (see Table 1). A standard high-titer serum specimen with reactivity to RSV G peptides should be used to monitor consistency and quality of results. Seven plasma specimens can be tested at one dilution (dilution 1:200) against RSV G peptide and control peptide in one ELISA plate. The SARS CoV nucleoprotein peptide 1–30 amino acid (30 mer) is used as a negative control peptide.
Two plate layouts are used, one that includes serial twofold dilutions of the serum standard to generate a standard curve and the other plates that allows testing seven plasma/serum specimens at a 1:200 dilution. Each dilution or specimen is tested in triplicate wells for RSV peptide and SARS control peptide.
Data analysis can be performed by plotting standard curve for the twofold dilution of standard reference positive plasma or serum sample using Microsoft Excel. The prediction of the antibody titers in test plasma/serum samples can be determined using the standard curve.
Contributor Information
Ralph A. Tripp, Phone: +11706-542-1557, Email: ratripp@uga.edu
Patricia A. Jorquera, Email: jorquera@uga.edu
Larry J. Anderson, Email: larry.anderson@emory.edu


