Abstract
Terpenes, also known as terpenoids are the largest and most diverse group of naturally occurring compounds. Based on the number of isoprene units they have, they are classified as mono, di, tri, tetra, and sesquiterpenes. They are mostly found in plants and form the major constituent of essential oils from plants. Among the natural products that provide medical benefits for an organism, terpenes play a major and variety of roles. The common plant sources of terpenes are tea, thyme, cannabis, Spanish sage, and citrus fruits (e.g., lemon, orange, mandarin). Terpenes have a wide range of medicinal uses among which antiplasmodial activity is notable as its mechanism of action is similar to the popular antimalarial drug in use—chloroquine. Monoterpenes specifically are widely studied for their antiviral property. With growing incidents of cancer and diabetes in modern world, terpenes also have the potential to serve as anticancer and antidiabetic reagents. Along with these properties, terpenes also allow for flexibility in route of administration and suppression of side effects. Certain terpenes were widely used in natural folk medicine. One such terpene is curcumin which holds anti-inflammatory, antioxidant, anticancer, antiseptic, antiplasmodial, astringent, digestive, diuretic, and many other properties. Curcumin has also become a recent trend in healthy foods and open doors for several medical researches. This chapter summarizes the various terpenes, their sources, medicinal properties, mechanism of action, and the recent studies that are underway for designing terpenes as a lead molecule in the modern medicine.
Keywords: Terpenes, Essential oils, Antiplasmodial, Curcumin, Anti-cancer, Anti-diabetic
Introduction
What Are Terpenes?
Terpenes, also known as isoprenoids are the largest and most diverse group of naturally occurring compounds that are mostly found in plants but larger classes of terpenes such as sterols and squalene can be found in animals. They are responsible for the fragrance, taste, and pigment of plants.1 Terpenes are classified on the basis of organization and number of isoprene units it contains (see footnote 1). An isoprene unit is a building block of terpenes that is a gaseous hydrocarbon that contains the molecular formula C5H8 (see footnote 1). Terpenes and terpenoids are terms that are often used interchangeably but the two terms have slight differences; terpenes are an arrangement of isoprene units that are naturally occurring, volatile, unsaturated 5-carbon cyclic compounds that give off a scent or a taste to defend itself from organisms that feed off of certain types of plants (see footnote 1). Terpenes have many functions in plants such as a thermoprotectant, signaling functions, and not limited to, pigments, flavoring, and solvents but also have various medicinal uses (Yang et al. 2012). Table 15.1 shows the different types of terpenes discussed in this chapter along with an example of that terpene.
Table 15.1.
Different types of terpenes and their properties
| Classification | Carbon atoms | Species produced from | Medicinal uses | References |
|---|---|---|---|---|
| Monoterpenes | C10 | Quercus ilex | Fragrances, repellent | Loreto et al. (2002) |
| Sesquiterpenes | C15 | Helianthus annuus | Treat malaria, treat bacterial infections, and migraines | Chadwick et al. (2013) |
| Diterpenes | C20 | Euphorbia, salvia miltiorrhiza | Anti-inflammatory, cardiovascular diseases | Vasas and Hohmann (2014), Zhang et al. (2012) |
| Triterpenes | C30 | Centella asiatica | Wound healing, increases circulation | James and Dubery (2009) |
Plants that Carry Medicinal Terpene
Terpene is a natural compound with various medical properties and found in both plants and animals (Gershenzon 2007). Among natural products that mediate antagonistic and beneficial interactions within the organism, terpene play a variety of roles (Gershenzon 2007). Terpene protects many living organisms like microorganisms, animals and plants from abiotic and biotic stresses (Gershenzon 2007). Terpene can ward off pathogens, predators, and competitors. Living organisms use terpene for multiple reasons like medicinal purposes and communications about food, mates, or enemies (Gershenzon 2007). It is impressive how different organisms use terpene for common purposes even though terpene contain many forms and varieties (Gershenzon 2007).
So far only a small percentage of terpene is investigated (Franklin et al. 2001). Cannabis is one of the most common sources for the medicinal terpene (Franklin et al. 2001). This plant contains many medicinal properties like anticancer, antimicrobial, antifungal, antiviral, antihyperglycemic, analgesic, anti-inflammatory, and antiparasitic (Franklin et al. 2001). Terpene is also used to enhance skin penetration, prevent inflammatory diseases (Franklin et al. 2001). Nowadays modern medication use large scales of terpene for various treatment drugs (Franklin et al. 2001).
There are commonly used plants like tea (Melaleuca alternifolia), thyme, Cannabis, Salvia lavandulifolia (Spanish sage), citrus fruits (lemon, orange, mandarin) etc. that provide wide range of medicinal values (Perry et al. 2000). Tea tree oil has increased in popularity in recent years when it comes to alternative medicine (Perry et al. 2000). Tea tree oil is a volatile essential oil and is famous for its antimicrobial properties, and acts as the active ingredient that is used to treat cutaneous infections (Carson et al. 2006) Apart from the flavor that gives to food, essential oil contain antimicrobial properties (Bound et al. 2015). Thyme is one of plants that synthesize terpene alcohols and phenols which contain powerful antibacterial and antifungal properties (Bound et al. 2015). Terpene synthesized from cannabis also long served as medicines (Perry et al. 2000). They also contain psychoactive properties and used against many infectious diseases (Perry et al. 2000). is famous for anti-dementia (current memory-enhancing) drugs by enhancing cholinergic activity via inhibition of cholinesterase (Perry et al. 2000). In vitro examination method was used to study the effects of constituent terpenes on human erythrocyte acetylcholinesterase (Perry et al. 2000). Some of the medicinal properties of terpenes are listed in Table 15.2.
Table 15.2.
Medicinal Properties of terpenes from different sources
| Terpene | Medicinal properties | References |
|---|---|---|
| Tea tree | Contains the active ingredient to treat cutaneous infections | Carson et al. (2006) |
| Thyme | Possesses powerful antibacterial and antifungal properties | Bound et al. (2015) |
| Cannabis | Possesses psychoactive properties and used against many infectious diseases | Friedman et al. (2006) |
| Spanish sage | Enhances memory and is used in anti-dementia drugs | Lopresti (2016) |
| Citrus fruits | Drugs against pediculosis | Mehlhorn et al. (2011) |
| Citral | Antibacterial and antifungal effects | Silva et al. (2008) |
| Lemongrass | Insect repellent | Silva et al. (2008) |
Properties Associated with Terpene
Important properties associated with terpene are difficult to overstress (Franklin et al. 2001). There are many important uses with terpene and these include anti-insect properties, antimicrobial properties and anti-herbivore properties (Franklin et al. 2001). Terpene can be extracted through plants and thorough some insects (Franklin et al. 2001).
Anti-insect
Without using harsh chemicals that could potentially contain side effects, terpene is a healthy alternative to ward off insects (Franklin et al. 2001). There have been many pesticides made for killing domestic pests like lice, or mites (Franklin et al. 2001). In these cases, it is very important to make sure that these pesticides do not affect humans in harmful ways (Franklin et al. 2001). There are many options like shampoo, sprays, lotions that were manufactured against pests that include one or more terpenes that are employed in the instant invention (Franklin et al. 2001). These naturally occurring terpenes are generally not modified they were used in their raw form and the environment protection agency in the USA classified as “GRAS” which mean Generally Regards as Safe (Franklin et al. 2001).
Certain terpene is highly effective against both lice and lice eggs and there is a less than significant chance of resistance developing against this terpene based pesticides; reason for this is their observed modes of action (Franklin et al. 2001). Unlike other types of pediculosis medication this terpene based instant inventions are not neurotoxins (Franklin et al. 2001) Terpenes are also used combined with terpene aldehyde called citral. Citral derives from an essential oil that is extracted from lemongrass ( ) (Franklin et al. 2001). Citral possesses antibacterial and antifungal properties, while lemongrass possesses anti-insect properties (Franklin et al. 2001).
A series of anti-insect formulation contain many terpenes (Franklin et al. 2001) Most of these pesticides are a mix of terpene and citral (Franklin et al. 2001). Table 15.3 consists of what these terpenes include.
Table 15.3.
Terpenes added in anti-insect formulations
| Terpene type | Function | Features | References |
|---|---|---|---|
| Limonene | This is strongly preferred. Limonene enhances the properties of other terpenes | Redistilled limonene has less odor, more stable than d-limonene | Franklin et al. (2001) |
| Beta-ionone | Antibacterial and antifungal properties | Beta-ionone has prophylactic value. | Mikhlin et al. (1983) |
| Geraniol | Similar level activity like beta-ionone. Geraniol possesses antibacterial and antifungal properties. | Geraniol gives a pleasant fragrance. | Chen and Viljoen (2010) |
| Eugenol | This is also the active terpene in clove oil. This possesses anesthetic properties which help with the itching that comes with bug bites. Also contain antibacterial and antifungal properties | Contain a distinct fragrance which is like geraniol | Franklin et al. (2001) |
| Myrcene | Possesses antifungal, antibacterial properties | Famous for its fragrance properties | Filipowicz et al. (2003) |
Antimicrobial
Antimicrobial properties or the ability to kill or stop growth of a microorganism in terpenes are commonly used in traditional and modern medicine (Himejima et al. 1992). There are many terpenes with antimicrobial activities (Himejima et al. 1992). The following plants produce terpenes which have antimicrobial properties: Pinus ponderosa (Pinaceae), spices (sage, rosemary, caraway, cumin, clove, and thyme), Cretan propolis, Helichrysum italicum, Rosmarinus officinalis, and so on (Himejima et al. 1992). These antimicrobial terpenes can also be used against food borne pathogen like , , and (Himejima et al. 1992).
cell extract contain wide-ranging antimicrobial activities (Himejima et al. 1992). After steaming and distillation from Pinus ponderosa cell extract, a distillate and a residue are obtained (Himejima et al. 1992). The distillate consists of monoterpenes and some sesquiterpenes while the residue consists of four diterpene acids (Himejima et al. 1992). It was also reported that when a physical damage is caused to the pine tree or any other terpene containing tree from insect attacks, resin which contains terpene secret to protect the tree from further damage (Himejima et al. 1992).
Five different kinds of terpene can be isolated from , they are, the diterpenes, 14,15-dinor-13-oxo-8(17)-labden-19-oic acid and a mixture of labda-8(17),13E-dien-19-carboxy-15-yl oleate, palmitate and triterpene (Popova et al. 2009). Spectroscopic analysis and chemical evidence has been used to establish the structures of the different compounds (Popova et al. 2009). These compounds that were isolated from terpene was tested for its antimicrobial activity against bacteria like gram positive and gram negative (Popova et al. 2009). It was all tested for human pathogenic fungi which has broad-spectrum antimicrobial activity (Popova et al. 2009).
essential oil was analyzed using gas chromatography and mass spectrometry to fraction into terpene and terpenoid. Fifty two compounds, including hydrocarbons of the oil; α-pinene (10.2%), α-cedrene (9.6%) aromadendrene (4.4%), β-caryophyllene (4.2%), and limonene (3.8%), neryl acetate (11.5%), 2-methylcyclohexyl pentanoate (8.3%), 2-methylcyclohexyl octanoate (4.8%), and geranyl acetate (4.7%) were identified (Mastelic et al. 2017).
Monoterpenes
The smallest of terpenes are monoterpenes . They contain the compound C10H16, come from different flowers, fruits and leaves and are known as the main component of essential oils, fragrances and many structural isomers (see footnote 1). Monoterpenes are also the most fragrant of all the classes of terpenes (see footnote 1). Examples for the types of monoterpenes found in natural scents are α-pinene, which imparts scent to pine trees, and limonene from citrus plants (see footnote 1).
What is thought to be one of the main purposes of monoterpenes is to attract pollinators or to serve the purpose of repelling other organisms from feeding off of plants. They also may be related to the flowering process of the plants (Loreto et al. 2002). They are isolated from their plant sources by distillation with steam and have a boiling points in the range of 150 °C to 185 °C (see footnote 1). Monoterpenes are purified using fractional distillation at pressures that are reduced or use another process in order to form a crystalline derivative (see footnote 1).
Monoterpene Emission Under Heat Stress
Many studies test the hypothesis of high emissions of monoterpenes under high temperatures using the leaves of Quercus ilex, also known as evergreen oak (Table 15.1). The evergreen tree is native to the Mediterranean area where it has to survive under hot and dry conditions and synthesis of these monoterpenes may have been an adaptive mechanism for the plants to survive under heat stress.2 This tree does not emit isoprenes but it emits monoterpenes and is able to handle different environmental stresses such as drought, salt, and heat (see footnote 2). A particular study done by Loreto et al. (2002) were conducted to visualize monoterpene production in response to high temperatures and to see if thermotolerance is increased with monoterpenes (Loreto et al. 2002). In this study, the leaves were exposed in 5 °C intervals ranging from the temperatures 30 °C to 55 °C and leaves were kept under conditions in which inhibited or allowed monoterpenes to synthesize (Loreto et al. 2002). The results that were found in this experience was a discovery of seven most abundant monoterpenes which was emitted at the maximum temperature of 35 °C and decreased its abundance over time as the temperatures increased and α-pinene had the greatest abundance of emittance at 35 °C as well as other terpenes but greatly reduced over higher temperatures (Loreto et al. 2002). At 55 °C the monoterpenes, myrcene and limonene had higher emission rates compared to temperatures around 35 °C (Loreto et al. 2002). Photosynthesis was also decreased when the leaves were exposed to any temperature that was higher than 30 °C and at 55 °C showed a loss of CO2 and recovery occurred around 30 °C (Loreto et al. 2002). Overall, the monoterpenes showed that their optimal temperature for emission was around 30–35 °C (Loreto et al. 2002). Researchers prove that the emission of monoterpenes is under enzymatic control due to their optimal temperatures (Loreto et al. 2002).
Sesquiterpenes
Sesquiterpenes , containing the chemical formula C15H24, are much larger compounds than monoterpenes and are much more stable in comparison.3 They are isolated by distillation with steam or by extraction and purified by methods such as vacuum fractional distillation or gas chromatography (see footnote 1). Oxidation or rearrangement of isoprene units that are made to sesquiterpenes produce the corresponding sesquiterpenoids (see footnote 1). Sesquiterpenes are naturally occurring and found in plants , fungi, and insects and act as a defensive mechanism or attract mates with pheromones in insects (see footnote 1). Acyclic compounds of sesquiterpenes such as farnesans can be used as a natural pesticide for insects and also as pheromones for some insects and mammals such as elephants, to attract mates or to mark their territory (see footnote 1).
Sesquiterpenes have a vital role in plant growth hormones and signaling properties in response to its environment (Giraudat 1995). Abscisic acid has a role in plants such as development, germination, cell division, and synthesis of protein storage and signalling (Giraudat 1995). It also plays a role in plants in response to various environmental stresses. It regulates the closure of the stoma by regulating ion channels and exchange of water across the plasma membrane (Giraudat 1995). Cyclic ADP-ribose signals abscisic acid in response to drought-stressing conditions from the environment (Giraudat 1995). Abscisic acid is not unique to plants, it has shown to be present in the central nervous system of other organisms such as pigs and may play a role in humans as a pro-inflammatory cytokine and stimulator of insulin release in the human pancreas (Chadwick et al. 2013). Gossypol is a sesquiterpene that is found in cotton plants. It has anticancer properties and can potentially inhibit fertility in male humans which is why it must be removed from essential oils and various other products before human use or consumption. Avarol, a sesquiterpenoid that has shown to have antimicrobial and antifungal uses, is effective against the AIDS virus in humans (see footnote 3).4
The medicinal properties of sesquiterpenes typically come from flowering plants that are included in the Asteraceae family, which include, but not limited to sunflowers, marigolds, and daisies. This family of flowers is a significant resource for potent sesquiterpene lactones, which are usually found in the leaves and the flower portion of plants and are constantly being produced at high levels (Chadwick et al. 2013). The role of sesquiterpenes in these flowering plants are not solely made for human use but for the purpose of protecting the plant from predators and are produced de novo in response to microbial attack and ultraviolet ray protection (Chadwick et al. 2013). Their bitter taste is a defense mechanism against herbivores from feeding on them but some have sweet tastes or tastes that are pleasant to certain organism for the purpose of spreading their seeds and being fertilized in different areas (Chadwick et al. 2013). Sesquiterpenes have many uses in traditional, western medicine because they contain so many anticancer, antiplasmodial, and anti-inflammatory activities (Chadwick et al. 2013). Sesquiterpenes lactones are able to reduce stomach ulcers in some people and are also present in powerful antimalarial drugs (Chadwick et al. 2013). Artemisinin, a metabolite produced from Artemisia annua, which contains sesquiterpene lactone produced in the roots and shoots of the plants, is used in drugs to treat malaria (Chadwick et al. 2013). Other uses of this family of flowers is for treatment of bacterial infections, migraines, and to improve skin (Chadwick et al. 2013). Lettuce opium has been used for many years as a painkiller (Chadwick et al. 2013).
Diterpenes
Diterpenes are naturally occurring chemical compounds that contain the molecular formula, C20H32. Diterpenes have physiologically active groups such as vitamin A activity well as plant growth hormones that regulate germination, flowering and switch reproductive cycles (from asexual to sexual reproduction) of plants (Lee et al. 2015). They can also be classified as a phytol, which is an oxygenated acyclic diterpene. Over 650 diterpenoids have been isolated from Euphorbia plants, which is a very diverse genus of flowering plants (Popova et al. 2009). Diterpenes have many therapeutic benefits such as antitumor, cytotoxic, and anti-inflammatory (Vasas and Hohmann 2014). They are present in anticancer drugs such as taxol, and the tumor promoter, phorbol (Vasas and Hohmann 2014).
Tanshinones are a class of diterpenes that are isolated from dried roots or rhizomes of an herb in traditional Chinese medicine called also known as Danshen or Tanshen (Zhang et al. 2012). Tanshinones were first isolated in the 1930s, and since then, more than 90 chemicals have been identified and split up into two groups: 40 lipophilic and 50 hydrophilic compounds (Zhang et al. 2012). Tanshinones have recently been extensively researched for their anticancer properties in vitro and in vivo (Zhang et al. 2012). Their potential use as an anticancer drug comes from their broad range of activities such as anti-proliferation and inhibiting adhesion, migration, and invasion (Zhang et al. 2012). Analogues of tanshinone have been synthesized in many clinical trials because they have many anticancer attributes (Lee et al. 2015). This herb has been used in many Asian countries for preventative and therapeutic solutions to many diseases such as heart disease, vascular diseases, and arthritis (Zhang et al. 2012). Tanshinones may also reduce inflammation and increase immune responses (Zhang et al. 2012).
Cafestol and kahweol are diterpene alcohols that are found in the oil derived from coffee beans. These chemical structures are very similar but only differ by an extra double bond that is present in kahweol’s chemical structure.5 Researchers have reported that coffee lowers the risk of depression in women, prostate cancer in men, stroke, diabetes, and some cancers (see footnote 5). It is thought that the anti-inflammatory and antioxidant properties of these particular diterpenes are responsible for such events (see footnote 5). Coffee benefits the liver as well by lowering liver enzymes that are in response to inflammation and damage and may offer some protection against liver cancer as well (see footnote 5). The adverse result of these diterpenes is that they raise cholesterol level, but it seems to be limited to coffee that has been unfiltered and has oily droplets of cafestol and kahweol (see footnote 5). Filtered coffee may not have much impact on cholesterol levels (see footnote 5).
Triterpenes
Triterpenes are composed of three or six isoprene units and have the chemical formula C30H48 which includes steroids and sterols with squalene being the biological precursor of all triterpenes (see footnote 1). Triterpenes are produced by animals, plants, and fungi. They play a role as precursors to steroids in animal and plant organisms, and are derived from mevalonic acid (see footnote 1). Saponins come from the skins of many plants and have emulsion like properties that make them excellent detergents in the human digestive system (see footnote 1). Chemical structures of steroid saponins are similar to hormones that are produced in the human body (see footnote 1).
The medicinal uses of triterpenes are not quite as recognized as other different types of terpenes but their uses are being continuously investigated by researchers. Their properties have been studied for anticancer, antioxidant, antiviral, and anti-atherosclerotic activities (Nazaruk and Borzym-Kluczyk 2015). Some studies have shown that there is promising potential for the use of triterpenes for people with diabetes by aiming to reduce glucose levels and also by reducing sweetness inhibitors in sweet and high calorie foods (Nazaruk and Borzym-Kluczyk 2015). Saponins have detoxification properties and act as a diuretic for the kidneys and wound healing properties (Nazaruk and Borzym-Kluczyk 2015).
Tetraterpenes
Tetraterpenes are also known as carotenoids that have the molecular formula C40H56 and can be in the category of terpenes because they are made from isoprene units.6 Most carotenoids are highly unsaturated and for this reason, they are extremely difficult to isolate and purify (see footnote 1). They are found in all different types of fungi, bacteria, and plants and mainly responsible for red, yellow, or orange fat-soluble plant and animal pigments (see footnote 6). One of the most crucial and common tetraterpene is beta-carotene that contributes to the yellow pigment in carrots. It is important to mammals especially because it is a precursor in producing vitamin A and other important terpenoids for vision (see footnote 1).
Higher order terpenes have been shown to increase thermotolerance (Singsaas 2001). The permeability of the thylakoid membranes increase at higher temperatures and this happens by an increase in cyclic photophosphorylation around photosystem II (Singsaas 2001). When the temperature of the atmosphere continues to rise, the photophosphorylation system is not able to keep up with protons leaking, which causes the transmembrane gradient to drop and a reduction in ATP synthesis occurs (Singsaas 2001). All these events can potentially cause lowering in the Rubisco activation state due to an inhibition of RuBP regeneration (Singsaas 2001).
MEP Pathway
The MEP pathway , also known as the non-mevalonate pathway or methylerythritol phosphate pathway, is a metabolic pathway for isoprenoid biosynthesis that creates the products isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). This pathway occurs in the chloroplasts and produce monoterpenes, specific sesquiterpenes, diterpenes, and carotenoids (Zhang et al. 2012). The vital application of this pathway is to develop antimicrobial agents to target diseases such as malaria and sexually transmitted diseases (Hunter 2007). Since this pathway does not occur in humans, it is a valuable resource to develop antibacterial and antiparasitic drugs (Seemann et al. 2009).
The first steps of this pathway involve pyruvate and d-glyceraldehyde 3-phosphate to produce DOXP which is catalyzed by 1-deoxy-d-xylulose-5-phosphate (DXS) (Hunter 2007). 1-Deoxy-d-xylulose-5-phosphate reductoisomerase, otherwise known as IspC, coverts DOXP to MEP. From MEP, it reacts with CTP to create 4-diphosphocytidyl-2C-methyl-d-erythritol (Hunter 2007). A phosphate is released in this reaction and then reacts with ATP-dependent IspE to make 4-diphosphocytidyl-2C-methyl-d-erythritol 2-phosphate and ADP and then reacts with the enzyme IspF to create 2C-methyl-d-erythritol 2,4-cyclodophosphate (Hunter 2007). The enzyme requires metal cations. Then finally, in the least understood step of the reaction, the two enzymes, IspG and IspH make the two products, IPP and DMAPP by using a two-electron reduction (Hunter 2007). The pathway is regulated by control of repression or activation of gene expression via feedback loops within the pathway or by effector molecules which target an enzyme or downstream activities (Hunter 2007).
MVA Pathway
The MVA pathway or mevalonic acid pathway occurs in the cytosol. It is responsible for the synthesis of sterols, specific sesquiterpenes, and also may play a role in the synthesis of transhinones (Zhang et al. 2012). In gram-positive bacteria, the genes in the metabolic pathways such as MVA are organized into operons and are thought to be regulated by transcription (Hunter 2007).
Cannabis
The use of cannabis is increasing for medicinal uses that commonly treat pain, the side effects of chemotherapy in cancer patients such as nausea, anxiety and depression, and its uses and benefits are continuously being researched by scientists (Cathcart et al. 2015). There are at least 80 compounds that come from the cannabis plant that are regarded as cannabinoids that cause psychotropic effects in the human brain due to CB1 receptors (Klein et al. 2011). The main active ingredient, delta-9-tetrahydrocannabinol, otherwise known as THC, is a psychoactive agent and is a focus for controversy in society because it binds to the human endocannabinoid receptors in areas of the brain such as the hippocampus and the frontal cortex, which are responsible for memory, cognition and attention.7 How THC works is by taking the place of endocannabinoids, naturally occurring chemicals in the human body (see footnote 7). One of the most common and well known molecules that THC replaces in the human body is called anadamide (see footnote 7). To this day, scientists are researching to discover the exact role of this molecule in the human body.
Cannabidiol, or CBD is also a common ingredient in cannabis but compared to THC, it is a non-psychoactive and it can potentially reduce the effects of THC (Klein et al. 2011). CBD does not bind to the same receptors as THC does in the human body and it works by inhibiting FAAH or the enzyme fatty acid amide hydroxyls (see footnote 7). This enzyme is responsible for degrading anadamide in the body and by inhibiting FAAH, CBD increases natural endocannabinoids already in the human system (Klein et al. 2011). CBD is thus an agent that works for depression, anxiety and neuroprotective effects (Klein et al. 2011).
What are major components in cannabis are the monoterpenes that are responsible for many different medicinal properties. One of the main uses for THC is the potential for cancer treatment and can play a role in reducing size of tumors (see footnote 7). THC can also reduce inflammation caused by certain diseases in patients. Other conditions that THC can help but are not limited to are ADHD, Arthritis, migraines, and glaucoma (see footnote 7).8 It can also improve the symptoms in individuals that suffer from HIV by helping their appetite and thus causing weight again, improving their depression symptoms and their quality of life (Lutge et al. 2013).
Antiplasmodial Activity
Terpenes have been shown to have a favorable antiplasmodial activity. With the rising malarial infections and drug resistance, terpenes have gained more attention towards it through antiplasmodial activity (Nogueira and Lopes 2011). The interesting mechanism behind the terpene activity is that it binds to the hemin part of infected erythrocytes and kills the parasite just like the famous antimalarial drug chloroquine (Orjih et al. 1981; Kayembe et al. 2012). Hemin is made of iron which is necessary for the plasmodium development in the erythrocytes. Though hemin breaking enzymes are not yet found in plasmodium, it could be one reason why hemin binding accounts for parasite lysis (Ginsburg and Demel 1984). Another study suggests that drug-hemin complex binds to phospholipid layers thereby disrupting the respective membrane structure and causing cell lysis (Ginsburg and Demel 1984). Moreover, it is also known that hemin can affect the carbohydrate metabolism of the parasites, which could lead to lysis of parasites (Rodriguez and Jungery 1986). Thus, terpenes can be designed to be promising drugs for malaria.
Different kinds of terpenes show different effects on the parasites. For instance, beta-myrcene the most common terpenes, is proven to have in vitro antiplasmodial activity (Kpoviessi et al. 2014). Beta-myrcene from , the plant which is high in terpenes, does not show an anti plasmodial effect but extracts from stem, leaves, and seeds of clove basil showed a good antiplasmodial activity (Small 2017; Kpoviessi et al. 2014). Additionally, it was also reported to have antitrypanosomal activity when tested against (Habila et al. 2010). This data leads to the fact that terpenes are effective against pathogenic Protista.
Limonene regarded as the second most commonly found terpene, also possesses antiplasmodial activity against . Limonene achieves its goal by targeting the intermediates of the active isoprenoid pathway of the parasite. Isoprenoid pathway plays a major role in parasite survival by mediating cell signaling, protein translation and several other biological processes (Jordão et al. 2011). Specifically, the isoprenic products that are inhibited from being synthesized are dolichol and ubiquinone (Goulart et al. 2004). The isoprenoid pathway of parasites is distinct from that found in mammals, which makes limonene a reliable constituent of antimalarial drug (Goulart et al. 2004). Thus, the host cell pathway will not be affected by the administration of the drug.
Pinene, commonly found monoterpene in pine trees is composed of two classes—alpha-pinene and beta-pinene. Both the classes of pinene were reported to be effective against the W2 strain of , which is resistant to chloroquine (Boyom et al. 2010). Of particular interest is the increase in antiplasmodial activity of pinene in cumin seed oil with increase in the distillation time. The study concluded that the optimal distillation time for increased antimalarial activity is 0–5 and 5–7.5 min (Zheljazkov et al. 2015). Further investigation is needed to ascertain if distillation time is just increasing the yield of pinenes in the oil or improving the bioactivity of pinenes.
The next most abundant terpene, caryophyllene has the ability to both prevent and cure malaria. Caryophyllene is an active component of insect repellents especially for mosquitoes and other blood-feeding Diptera (Maia and Sarah 2011). Recent studies ensured that silver nanoparticles synthesized from caryophyllene are highly effective against (Kamaraj et al. 2017). Thus, terpenes could be a safer and a cost effective alternative for malarial treatment.
Antiviral Activity
The emerging viral diseases have necessitated the research for new effective antiviral agents such as terpenes . As a result, scientists evaluated various terpenes for their properties, among which monoterpenes showed a good result. Monoterpenes are terpene classes that possess two isoprene units. They form a major constituent of essential oils in plants which indicates monoterpenes play a major role in defense for plants (Grabmann 2005). A 2005 study evaluated the in vitro antiviral activity of several essential oils extracted from South American plants (Duschatzky et al. 2005). The oil extracts were tested against three major human viruses—herpes simplex virus-1 (HSV1), dengue virus type 2, and Junin virus. The oils that were proved to be virucidal were mainly composed of monoterpenes, namely, carvone, carveol limonene, alpha- and beta-pinene, caryophylene, camphor, beta-ocimene, and one sesquiterpene which is germacrene (Duschatzky et al. 2005). A similar study in 2008 analyzed the essential oils of seven plants from Lebanon for in vitro antiviral activity (Loizzo et al. 2008). The viruses under investigation were HSV1 and severe acute respiratory syndrome corona virus (SARS CoV). The results were positive for antiviral effects, and the major constituents were alpha- and beta-pinene, beta-ocimene, and 1,8-cineole (Loizzo et al. 2008). Following this, a 2009 study on also had similar results which suggested 1,8-cineole, α-pinene, caryophyllene oxide, and sabinene to be the major components of virucidal oils (Alim et al. 2009). Functional data from these studies reveal that a few monoterpenes are shared by various plants for antiviral properties (Alim et al. 2009). These shared monoterpenes could be of importance as they are present universally.
Of particular interest is the single main monoterpene that is contributing to the virucidal activity. This was studied by Astani et al. (2009) using eucalyptus, tea tree, and thyme essential oil extracts (Astani et al. 2009). They suggested that monoterpene hydrocarbons have a slightly higher virulent activity compared to the monoterpene alcohols against HSV-1. The monoterpenes with the highest virucidal activity were identified to be alpha-pinene and alpha-terpineol (Astani et al. 2009). The mechanism behind the virucidal activity was suggested to be direct inactivation of free viral particles. However, the study concluded that more than isolated single monoterpenes, a mixture of monoterpenes are more effective and possessed lesser toxicity to host cells (Astani et al. 2009). This was further bolstered by another study which evidenced the virucidal property of a combination of monoterpenes obtained from (Zamora et al. 2016). The activity was tested against a human flavivirus West Nile virus. The results were positive both in vivo and in vitro. The underlying mechanism was predicted to be induced cell cycle arrest at G0 or G1 phase. This indicates that a mixture of monoterpenes could act as a better antiviral agent rather than a single monoterpene (Zamora et al. 2016). Recent studies have shown that triketone-terpene adducts also exert antiviral, antimicrobial and antitumor activity (Chen et al. 2017). These adducts are obtained from Myrtaceae as secondary metabolites in the form of sesquiterpenes called myrtucomvalones A, B, and C. The terpene adducts successfully inhibited the respiratory syncytial virus (RSV) (Chen et al. 2017).
The bioactive terpenes present in various plants have shown various results for antiviral property. It would therefore be important to look for various plant source rather than various monoterpenes for therapeutic purposes. Researchers are also focusing on synthesizing terpene hybrid from fungal sources as they are presumed to have antiviral and UV protective properties (Yuan et al. 2017). Terpene synthesis from fungi can lead to cost effective and limited labor methods (Yuan et al. 2017).
Anticancer
The medicinal benefits of terpenes are not limited to pathogenic diseases. Terpenes are widely acclaimed for their anticancer activity too. An early 1997 study concluded that a combination of monoterpenes, diterpenes and sesquiterpenes can be effectively used to treat cancers that occur in colon, brain, prostate gland, and bones.9 It also claimed that administration of terpenes in humans inhibited the growth of prostate cancer cells and sensitized the tumor in such a way it becomes susceptible to radiotherapy (see footnote 9). The major advantage of this treatment was that, the drug can be administered through several routes among which oral and topical were most preferred (see footnote 9).
Among the different kinds of terpenes, limonene is well recognized as an anticancer agent. Limonene is a bioactive food component found in citrus peels, orange peels, and several other citrus fruits (Jirtle et al. 1993). Studies have reported limonene to exhibit strong cancer inhibition activity both in vitro and in vivo. The mechanism behind limonene activity is still under investigation. A study by Jirtle et al. (1993) reported that limonene acts through induction of transforming growth factor B-1 and mannose-6-phosphate/insulin-like growth factor II receptors (Jirtle et al. 1993). In contrast a study by Bishayee and Rabi 2009) suggested that limonene eliminates cancer cells by induction of apoptosis (Bishayee and Rabi 2009). Structural studies on limonene reported that they are lipophilic and have the tendency to be deposited in fatty tissues when administered orally. This indicates that limonene can act as an excellent chemopreventive drug for cancer as it can be deposited in the body (Miller et al. 2010). Another study in 2013 concluded that limonene acts by suppressing the expression of breast tumor cyclin D1 (Miller et al. 2013). This lead to cell cycle arrest and mitigated proliferation of cancer cells in women with early stages of breast cancer (Miller et al. 2013). Recent study showed that limonene from pinecones can kill lung cancer cells in vitro by apoptotic mechanism that is activated through caspase-3 pathway (Lee et al. 2017). These findings indicate a novel application of limonene towards fighting and preventing cancer. Not just limonene, but also its metabolite perillyl alcohol is also said to exhibit antitumor activity in pancreatic cell lines through apoptotic mechanisms (Sobral et al. 2014; Dalessio et al. 2014).
Apart from limonene, the terpene thymoquinone has all been widely studied for its chemoprotective and chemotherapeutic activity. Thymoquinone is found to be an active constituent of the volatile oils of an annual herbaceous plant called (black cumin) (Majdalawieh et al. 2017). The pathways affected by thymoquinone to exert its antitumor properties are p53, PPARγ, MAPK, NF-κB, PI3K/AKT, and STAT3 signaling pathways (Majdalawieh et al. 2017). Thymoquinone has been proved to be anticancerous against several cancers such as breast cancer, skin cancer, non–small cell lung cancer, bile duct cancer, and brain cancer. The basic mechanisms underlying the cancer inhibition is apoptosis and cell cycle arrest (Sobral et al. 2014; Khader and Eckl 2014). Most of the cancer related studies were performed using thermoquinone obtained from the N. sativa extracts. A 2012 study showed that thermoquinone can be obtained in larger amounts from the mint family, namely, Monarda didyma and Monarda media (Taborsky et al. 2012). Thus, thermoquinone from alternative sources has to be tested for its precious potential in cancer therapy.
Other terpenes that have reported cytotoxic effects on cancer cells include alloocimene, camphor, beta-myrcene, pinene, alpha- and gamma-thujaplicin, terpinene, thymohydroquinone, carvone, camphene, and cymene (Sobral et al. 2014). Terpenes being natural compounds are unlikely to affect the healthy cells or create a side effect, which attracts many researchers to exploit its capability in cancer treatment.
Antidiabetic
Diabetes is one of the widely prevalent diseases in the world. It is affecting both children and adults in both developing and developed nations (You and Henneberg 2016; Narayan et al. 2000). The social and economic burden of diabetes continues to grow and it is expected to rise rapidly in developing countries (Sarwar et al. 2010). In USA, diabetes is one of the leading causes for visual impairment, limb amputation, renal diseases, heart diseases and death (Saddinne et al. 1999). Diabetes can be of two types—type 1 (where the immune system of the body acts against the insulin-producing organs) and type 2 (where the insulin produced cannot be used by the body or insulin is produced in low amounts).10 Although there are several medications available, their use is limited due to their adverse effects. Some of the commonly found side-effects include low blood sugar, vomiting, nausea, diarrhea, bloating, and weight gain.11 This led to the research for natural products to be used as effective antidiabetic medication. Phytochemicals from the medicinal plants have been recommended for treating type 2 diabetes, of which terpene forms a major constituent (Jung et al. 2006).
Medicinal plants of Oriental Morocco were studied for their antidiabetic property in rats. The report showed that terpenes, terpene diols, and terpene diol glucosides form major components of the extracts of plants under study (Bnouham et al. 2010). A similar study on medicinal plant and their natural products that were reported from 2001–2005 was conducted by Jung et al. 2006. This study was focused on non–insulin-dependent diabetes mellitus (type 2), and it proved that terpenes along with few other secondary metabolites such as alkaloids and flavonoids exhibit antidiabetic potential (Jung et al. 2006).
The most promising terpene compound for treating diabetes is called andrographolide which is a diterpenoid lactone (Brahmachari 2017). This compound forms the major component of the leaves of the small herbaceous plant . A. paniculata is an Asian plant that has already been reported to be used in traditional medicines for its therapeutic nature (Brahmachari 2017). The terpenoid acts by reducing the plasma glucose and increasing the utilization of glucose by the body in diabetes mellitus rats (Gupta et al. 2008). The actual mechanism by how it does this is it activates the alpha-adrenoreceptors to increase the release of an opioid peptide beta-endomorphin (Brahmachari 2017) which is reported to be secreted in low amounts in diabetic rats (Forman et al. 1985). This increased secretion in turn activates the opioid μ-receptors. These receptors can effectively curb the hepatic gluconeogenesis (glucose synthesis from non-carbohydrate precursors) and elevate the utilization of glucose by muscles. Finally, this results in a reduced plasma glucose concentration (Brahmachari 2017). Andrographolide is also observed to prevent the secondary complications of diabetes such as diabetic retinopathy, a condition that will lead to blindness (Brahmachari 2017). It significantly weakens the retinal angiogenesis and inflammation during the development of the disease (Brahmachari 2017). Moreover, it can also fix the impaired or extended estrous cycle in diabetic rats (Reyes et al. 2006). Andrographolide was orally administered in all the above studies. This indicates its efficiency for being used as a lead molecule in the future drugs designed for treating diabetes mellitus.
Another widely known terpene is curcumin obtained from which commonly called turmeric (Nabavi et al. 2015). It exhibits high antidiabetic property and acts by quashing the oxidative stress and inflammation. By regulating the polyol pathway, it can also reduce the plasma glucose and levels of glycosylated hemoglobin (Nabavi et al. 2015). Moreover, curcumin is also reported to activate the enzymes present in the liver that are essential for glycolysis, gluconeogenesis, and lipid metabolism (Zhang et al. 2013). Alike andrographolide, curcumin is also reported to reduce the complications of diabetes (Nabavi et al. 2015), for example, liver disorder which is a common manifestation of diabetes type 2 (Zhang et al. 2013). Curcumin treats these disorders by reducing the liver weight and lipid peroxidation products. Further, it is also reported to normalize the levels of fetuin-A in serum that contributes to insulin resistance and fatty liver in diabetic rats (Zhang et al. 2013). Other complications that can be attenuated by curcumin include diabetes associated—retinopathy, microangiopathy, neuropathy, and nephropathy (Zhang et al. 2013). These findings confirm that curcumin is likely to be used in the future for diabetes treatment.
Antidepressant
Depression has become a serious health concern by contributing to the emerging mental and emotional disorders throughout the world. It is hitting both the developed and developing countries. Depression can pave way to various health issues from alcoholism to heart diseases (Holden 2000). It is also said to increase the rate of mortality significantly in breast cancer patients (Hjerl et al. 2003). Moreover, depression immobilizes its victims thereby leading to economic loss (Holden 2000). By analyzing the social and economic burdens caused by depression, researchers have stepped out towards finding novel stress-relieving drugs. Synthetic drugs have serious side-effects and unintended interactions with the body that negatively affects the treatment outcome (Jawaid et al. 2011). Hence this necessitated the need for natural drugs. Terpenes serves as one of the most relevant bioactive compound for treating depression and therefore can open doors for designing natural or synthetic antidepressant drugs (Bahramsoltani et al. 2015).
Twenty-five percentage of antidepressant drugs prescribed by doctors are obtained from herbs through various extracts (Saki et al. 2014). To estimate the important compounds contributing to the antidepressant effect, Saki et al. (2014) performed an electronic database based study. The results revealed that terpenes formed a major part of the extracts of medicinal plants that exerted antidepressant effects (Saki et al. 2014). Thus, scientists focused on identifying the active principles of plant extracts contributing to the antistress effects. Different plant had different acting compounds.
Among the several terpenes, linalool and beta-pinene are commonly found to be active principles (both Guzmán-Gutiérrez et al. 2015; Guzmán-Gutiérrez et al. 2012). They were discovered from the extracts of medicinal plants and and flowers of lavender (Appleton 2012; Guzmán-Gutiérrez et al. 2012; Guadarrama-Cruz et al. 2008). These monoterpenes act by interacting with the 5HT1A receptors of the serotonergic pathway. Serotonins are important in the fact that their release and re-uptake levels can be altered to overcome stress (Chaouloff 2000; Guzmán-Gutiérrez et al. 2012). They also interact with adrenergic receptors of the body that play a major role in stress-induced behavioral changes (Pandey et al. 1995; Guzmán-Gutiérrez et al. 2015). Another interesting finding is the interaction of beta-pinene with dopaminergic receptors namely D1 receptors. This is the mechanism followed by most of the antidepressant drugs available in the market (Guzmán-Gutiérrez et al. 2015). A more interesting study would be to examine the beta-pinene and linalool efficiency through inhalation tests. This is because these monoterpenes are aromatic compounds that generally have an enhanced activity when inhaled as they can directly hit the central nervous system (Guzmán Gutiérrez et al. 2014).
Apart from monoterpenes, sesquiterpenes also exhibit antidepressant effects. One striking example is beta-caryophyllene which was proved to ameliorate the depressive symptoms in mice (Bahi et al. 2014). The underlying mechanism of this compound is binding to a receptor called CB2 and activating it. CB2 is found in the brain and immune cells and plays a major role in regulating depressive-related disorders (Bahi et al. 2014). Thus beta-caryophyllene curbs depression by acting as a CB2 receptor agonist (Bahi et al. 2014).
Other terpenes that have effective antidepressant properties include hyperforin which is present in the extracts of (Subhan et al. 2010). It has been shown that the extracts of H. perforatum differ in their antidepressant potential with the difference in concentration of hyperforin present in the extract (Laakmann et al. 1999). Similar to many other antidepressants hyperforin acts by inhibiting the neuronal uptake of mood regulators such as serotonin, dopamine and norepinephrine. In addition, it also has its own unique mechanism of controlling depression by inhibiting the neurotransmitters GABA and l-glutamate uptake (Müller et al. 2001).
Another fascinating antidepressant plant is , which is a short perennial herb. This plant not only reduces the stress and anxiety levels but also improves the symptoms of depression in humans (Bhattacharyya et al. 2007). The major components of Valeriana extracts are terpenoids called maaliol, patchouli alcohol, and 8-acetoxypatchouli alcohol (Subhan et al. 2010). The terpenoid-less extract of Valeriana was found to be devoid of antidepressant activity which indicates that terpenes are the active components involved in reducing the depression (Subhan et al. 2010).
Uses in Folk Medicine
Folk medicine has always been an eye-opener for designing novel drugs for diseases. To be more specific, almost three-fourths of the plant-based drugs were created based on the knowledge of folk medicine (Table 15.4) (Efferth et al. 2008). Realizing this fact, western worlds are now turning back into old medicines and bioactive plant components to treat modern diseases (Efferth et al. 2007, 2008). This has boosted the export rates of Chinese medicinal products (based on traditional Chinese medicine) from China to other developed nations. Plants used in traditional Chinese medicine (TCM) are being extensively studied for their secondary metabolites and their therapeutic properties (Efferth et al. 2007). One of the active principles of TCM products is terpenes (Liu and Jiang 2012). Due to their large availability and diversity, terpenes contribute the most to industrial and medicinal applications among all the secondary metabolites of plants (Zwenger and Basu 2008).
Table 15.4.
Uses of different terpenes in folk medicine
| No. | Scientific name | Common name | Abundant terpene | Uses in ayurveda | Uses in TCM | References |
|---|---|---|---|---|---|---|
| 1. | Citrus limon | Lemon | Limonene | Oral cavities, digestive problems, abdominal colic pain, and cough | Digestive problems and cleansing the body | a,b |
| 2. | Citrus reticulata | Orange | Limonene | Digestive disorders, abdominal colic pain, and worm infestation | Stomach ache and cough | c,d |
| 3. | Juniperus communis | Juniper | Limonene | Antiseptic, treat cellulite, pain and swelling | Treat cold and urinary problems | e,f |
| 4. | Phyllanthus emblica | Indian gooseberry | Phyllaembicilins |
Boost immunity Strengthen hair follicles Cure acne and pimples Improve circulatory system Cure diarrhea |
Treat diarrhea, jaundice and inflammation |
Zhang et al. (2000), Liu (2016)g |
| 5. | Panax sp. | Ginseng | Humulene |
Boosts energy Used to treat musculoskeletal problems such as rheumatism, arthritis, and so on. |
Memory booster Reduce fatigue Reduce menopause symptoms |
h–j |
| 6. | Cinnamomum verum | Cinnamon | Alpha-pinene, caryophyllene, linalool,alpha-phelandrene, cymene, humulene |
Cold, Diabetes, high cholesterol, digestive problems, bronchitis, sinus congestion |
Cold, diabetes, high cholesterol, digestive problems; control sweating Chest pain |
Ravindran et al. (2004)k |
| 7. | Lycium chinense | Goji berry | Beta-carotene |
Maintains kidney functions Improves eye-sight, fertility, circulation and increases lifetime |
Improves eye-sight, fertility, circulation and increases lifetime | Bungheza et al. (2012)l |
| 8. | Zingiber officinale | Ginger | Zingiberene |
Digestive problems, Joint pain and air sickness |
Cold, cough, wheezing, asthma | m,n |
| 9. | Allium sativum | Garlic | Nerolidol, alpha-pinene, and terpinolene | Treat pimples, tumor, snakebites, wounds, headache, heart disease, gastric problems, ulcer, and measles | Food poisoning and digestive problems | o,p |
| 10. | Ocimum tenuiflorum | Holy basil/tulsi | Eugenol , β-elemene, β-caryophyllene and germacrene |
Restores functions of nervous system Increases fertiltity Used to treat asthma and cold |
Restores functions of the nervous system | Kousik and Baldev (2012)q,r |
ahttp://easyayurveda.com/2012/11/14/health-benefits-of-lemon-ayurveda-details/. Accessed 1 June 2017
bhttp://limoneira.com/lemons-in-traditional-chinese-medicine/. Accessed 7 June 2017
chttp://easyayurveda.com/2011/09/22/benefits-of-orange-fruits-traditional-and-modern-views/. Accessed 5 June 2017
dwww.sacredlotus.com/go/chinese-herbs/substance/chen-pi-orange-peel-citrus-peel-tangerine-peel. Accessed 5 June 2017
ehttp://ayurvedicoils.com/tag/ayurvedic-uses-of-juniper-leaf-oil. Accessed 5 June 2017
fwww.herbs-info.com/juniper.html. Accessed 5 June 2017
ghttp://homeofayurveda.org/the-indian-gooseberry-ayurvedas-wonder-fruit/. Accessed 5 June 2017
hwww.organicfacts.net/health-benefits/herbs-and-spices/health-benefits-of-ashwagandha-or-indian-ginseng.html. Accessed 7 June 2017
ihttp://theleafonline.com/c/science/2014/11/terpene-profile-humulene/. Accessed 5 June 2017
jhttps://en.wikipedia.org/wiki/Ginseng#Uses. Accessed 5 June 2017
khttps://classicalchinesemedicine.org/gpa/guizhi-cinnamon-twig-translations/. Accessed 5 June 2017
lhttps://trueayurveda.wordpress.com/2013/06/25/goji-berries-not-all-that-they-are-cracked-up-to-be/. Accessed 5 June 2017
mwww.mapi.com/ayurvedic-knowledge/food-tips/the-healing-power-of-ginger.html. Accessed 5 June 2017
nhttp://bodymindwellnesscenter.com/ginger-root-in-ayurveda-and-chinese-medicine/. Accessed 5 June 2017
owww.meridian-acupuncture-clinic.com/support-files/garlic-in-tcm.pdf. Accessed 5 June 2017
phttp://ayurveda-foryou.com/health_articles/garlic_benefits.html. Accessed 5 June 2017
qhttps://en.wikipedia.org/wiki/Ocimum_tenuiflorum. Accessed 5 June 2017
rwww.consciouslifestylemag.com/tulsi-holy-basil-sacred-herb/. Accessed 5 June 2017
Paclitaxel is one of the most successful terpenes available in the market today (Efferth et al. 2008). It is made out of yew trees which is a medicinal tree used in TCM.12 Raw material from yew contains taxol (brand name of Paclitaxel) which is used in the treatment of cancers in breast, lung, ovary, pancreas, cervix, and blood (see footnote 12).13,14 Two variations of this drug are used now in chemotherapy —conventional paclitaxel and albumin-bound paclitaxel (see footnote 13). The advantage of the latter is that concentration increases in tumor cells at a rate higher than that of the former (see footnote 13). The mechanism of anticancer activity is described as disruption of microtubules in the mitotic spindle, which will lead to incomplete chromosome separation thereby causing cell death (see footnote 13). In TCM and Ayurveda (herbal medicinal science mainly developed in India), healers used the twigs and barks of the tree to make a special kind of tea that can be given to patients suffering from cancer. However due to the slow growing nature of yew tree, paclitaxel nowadays is produced by coalescing the products of endophytic fungus that grows under the tree and the bark of the tree15,16 (Heinig et al. 2013).
One more common terpene present in the drugs used in TCM is pinene (Wu et al. 2008). Pinene exhibits therapeutic properties such as anti-inflammatory, antiseptic, anticancer, and antibiotic properties.17,18 The source for pinene is Eucalyptus and other related coniferous trees (see footnote 17, Sartorelli et al. 2007) In olden days, the juice from the bark of eucalyptus was collected and mixed in water, milk or wine to be used as a drug (see footnote 17). Currently, they are extracted in the form of oil and sold in the form of syrups and lozenges.19 As eucalyptus oil contains several monoterpenes, a study analyzed the different constituents of eucalyptus oil for its effectiveness against bacteria. Here it was concluded that alpha-pinene is the best monoterpene with the highest inhibitory activity (Sartorelli et al. 2007). Recently scientists are studying another primary terpene in eucalyptus called cineole. Cineole is reported to improve the memory power, cognitive performance and attenuate the symptoms of Alzheimer’s disease in humans (see footnote 19; Moss and Oliver 2012). In addition, studies also showed that cineole is capable of improving the health of bronchitis patients by reducing their cough (Fischer and Dethlefsen 2013). This is in agreement with the fact that eucalyptus oil was used as an expectorant in Ayurvedic medicine.20 It is also known that local Brazilians used the Eucalyptus leaves to treat several human diseases such as cancer (Mathias et al. 2012). Further reports also suggest that eucalyptus oil has been involved in ancient Indian Ayurvedic and Greco-European medicine systems (see footnote 19).
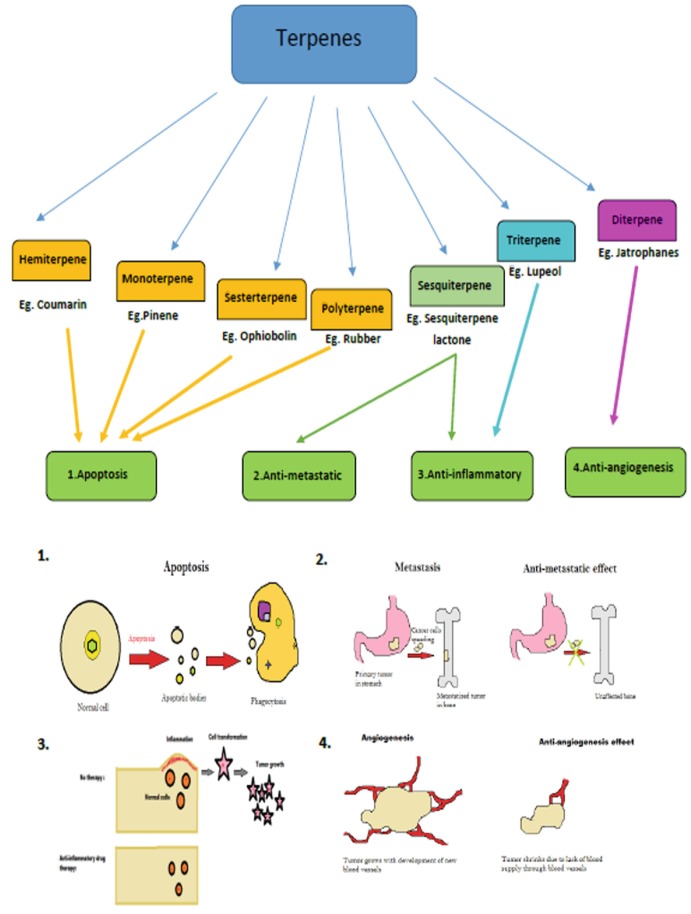
Ayurveda is a popular medicine system which originated about 3000 years ago in India. The ayurvedic medicines are based on medicinal herbs, minerals, and metals (see footnote 16) along with diet regimes such as vegetarianism (Caldecott 2006). This system of medicine has proven to cure chronic disorders that could not be treated by western medicine (Sharma et al. 2007). Interestingly a lot of medicinal plants used by Ayurvedic practitioners owe their therapeutic property to their terpene contents. One good example is turmeric, a family of ginger which is regarded as “Golden Goddess” by medical practitioners (see footnote 18).21 It has numerous therapeutic properties that includes anti-inflammatory, antioxidant, anticancer, antiseptic, antiplasmodial, astringent, digestive, diuretic, and many more (see footnote 18).22 Recently, scientists discovered that most of the turmeric’s properties are laid out by the yellow-colored terpene—curcumin (Kocaadam and Şanlier 2017). Studies are now trying to create curcumin analogues to improve the effects and activity of natural curcumin (Kocaadam and Şanlier 2017). Another popular example is clove which was used by both Ayurveda and TCM as a painkiller in dental cases. It was applied topically on cavities to relieve toothache and abdomen to treat digestive problems (Alqareer et al. 2006). The essential oil of clove is mostly composed of eugenol, a bioactive terpene that is responsible for clove’s aroma (Alqareer et al. 2006). Eugenol by itself is said to enhance the blood circulation in the body and improve metabolism (see footnote 22). Thus, based on the above data we can conclude that various terpenes have been in use even before their discoveries by modern science, due to their amazing medicinal properties. A schematic summary of different terpenes and their medicinal uses, that we discussed, is provided below in Fig. 15.1.
Fig. 15.1.
A brief representation of terpenes and their medicinal uses. Based on (http://www.wisegeek.com/what-are-phagocytes.htm. Accessed 22 Jan 2018) (Zetter 2008; Keklikoglou and Palma 2014)
Footnotes
www.britannica.com/science/isoprenoid. Accessed 22 Jan 2018
www.arborday.org/trees/treeguide/TreeDetail.cfm?ItemID=1094. Accessed 22 Jan 2018
www.cyberlipid.org/simple/simp00042.htm. Accessed 25 Jan 2018
www.cyberlipid.org/simple/simp00041.htm. Accessed 22 Jan 2018
www.health.harvard.edu/staying-healthy/what-is-it-about-coffee. Accessed 22 Jan 2018
www.cyberlipid.org/simple/simp0002.htm#carotene. Accessed 25 Jan 2018
www.herb.co/2016/07/24/what-is-thc/. Accessed 22 Jan 2018
www.chem.libretexts.org/Core/Organic_Chemistry/Lipids/Properties_and_Classification_of_Lipids/Terpenes. Accessed 22 Jan 2018
www.google.com/patents/US5602184. Accessed 23 Jan 2018
www.diabetes.ca/about-diabetes/types-of-diabetes. Accessed 23 Jan 2018
www.diabetes.co.uk/features/diabetes-medication-side-effects.html. Accessed 23 Jan 2018
www.yewbiopharm.com/about-us/. Accessed 29 May 2017
https://www.drugs.com/monograph/paclitaxel.html. Accessed 21 June 2017
www.dailymail.co.uk/health/article-3823690/Could-Chinese-medicine-cure-leukaemia-Taking-herb-alongside-treatment-helps-85-patients-enter-remission.html. Accessed 29 May 2017
www.thepracticalherbalist.com/holistic-medicine-library/pacific-yew-pocket-herbal/. Accessed 29 May 2017
https://nccih.nih.gov/health/ayurveda/introduction.html. Accessed 30 May 2017
http://ayurvedicoils.com/tag/health-benefits-of-a-pinene. Accessed 30 May 2017
www.medicaljane.com/category/cannabis-classroom/terpenes/#terpenes-in-cannabis. Accessed 30 May 2017
https://aromaticstudies.com/about-eucalyptus-globulus-and-18-cineole/. Accessed 30 May 2017
www.eastwesthealingacademy.com/herbs/eucalyptus/. Accessed 30 May 2017
www.ayurvedacollege.com/articles/students/turmeric. Accessed 6 June 2017
http://articles.mercola.com/herbal-oils/clove-bud-oil.aspx. Accessed 6 June 2017
Destinney Cox-Georgian and Niveditha Ramadoss contributed equally to this work.
Contributor Information
Nirmal Joshee, Email: josheen@fvsu.edu.
Sadanand A. Dhekney, Email: sdhekney@uwyo.edu
Prahlad Parajuli, Email: pparajuli@med.wayne.edu.
Chhandak Basu, Email: chhandak.basu@csun.edu.
References
- Alim A, et al. In vitro antimicrobial and antiviral activities of the essential oil and various extracts of Salvia cedronella Boiss. J Med Plant Res. 2009;3(5):413–419. [Google Scholar]
- Alqareer A, et al. The effect of clove and benzocaine versus placebo as topical anesthetics. J Dent. 2006;34(10):747–750. doi: 10.1016/j.jdent.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Appleton, J. “Lavender oil for anxiety and depression.” Nat Med J, vol.4, no. 2, 2012
- Astani A, et al. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother Res. 2009;24(5):673–679. doi: 10.1002/ptr.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi A, et al. β-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol Behav. 2014;135:119–124. doi: 10.1016/j.physbeh.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Bahramsoltani R, et al. Phytochemical constituents as future antidepressants: a comprehensive review. Rev Neurosci. 2015;26(6):699–719. doi: 10.1515/revneuro-2015-0009. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya D, et al. Initial exploratory observational pharmacology of Valeriana wallichii on stress management: a clinical report. Nepal Med Coll J. 2007;9(1):36–39. [PubMed] [Google Scholar]
- Bishayee A, Rabi T. d-Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: generation of reactive oxygen species and induction of apoptosis. J Carcinogen. 2009;8(1):9. doi: 10.4103/1477-3163.51368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bnouham M, et al. Antidiabetic effect of some medicinal plants of oriental Morocco in neonatal non-insulin-dependent diabetes mellitus rats. Hum Exp Toxicol. 2010;29(10):865–871. doi: 10.1177/0960327110362704. [DOI] [PubMed] [Google Scholar]
- Bound J, et al. Synthesis and antibacterial properties of 2,3-dideoxyglucosides of terpene alcohols and phenols. Food Chem. 2015;185:192–199. doi: 10.1016/j.foodchem.2015.03.078. [DOI] [PubMed] [Google Scholar]
- Boyom FF, et al. Antiplasmodial volatile extracts from Cleistopholis patens Engler & Diels and Uvariastrum pierreanum Engl. (Engl. & Diels) (Annonaceae) growing in Cameroon. Parasitol Res. 2010;108(5):1211–1217. doi: 10.1007/s00436-010-2165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari G. Discovery and development of antidiabetic agents from natural products natural product drug discovery. Amsterdam: Elsevier; 2017. [Google Scholar]
- Bungheza IR, et al. Obtaining of carotenoid extract from Lycium Chinense and characterization using spectometrical analysis. Dig J Nanomat Biostruct. 2012;7(2):523–528. [Google Scholar]
- Caldecott T. Ayurveda: the divine science of life. Maryland Heights: Mosby; 2006. [Google Scholar]
- Carson CF, et al. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19(1):50–62. doi: 10.1128/CMR.19.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart P, et al. Cannabis and cancer: reality or pipe dream? Lancet Oncol. 2015;16(13):1291–1292. doi: 10.1016/S1470-2045(15)00302-2. [DOI] [PubMed] [Google Scholar]
- Chadwick M, et al. Sesquiterpenoids lactones: benefits to plants and people. Int J Mol Sci. 2013;14(6):12780–12805. doi: 10.3390/ijms140612780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouloff F. Serotonin, stress and corticoids. J Psychopharmacol. 2000;14(2):139–151. doi: 10.1177/026988110001400203. [DOI] [PubMed] [Google Scholar]
- Chen W, Viljoen AM. Geraniol—a review of a commercially important fragrance material. South African J Bot. 2010;76(4):643–651. doi: 10.1016/j.sajb.2010.05.008. [DOI] [Google Scholar]
- Chen M, et al. Myrtucomvalones A–C, three unusual triketone–sesquiterpene adducts from the leaves of Myrtus communis ‘Variegata’. RSC Adv. 2017;7(37):22735–22740. doi: 10.1039/C7RA02260C. [DOI] [Google Scholar]
- Dalessio P, et al. Skin repair properties of d-limonene and Perillyl alcohol in murine models. Anti-Inflammat Anti-Allerg Agents Med Chem. 2014;13(1):29–35. doi: 10.2174/18715230113126660021. [DOI] [PubMed] [Google Scholar]
- Duschatzky CB, et al. Evaluation of chemical and antiviral properties of essential oils from south American plants. Antivir Chem Chemother. 2005;16(4):247–251. doi: 10.1177/095632020501600404. [DOI] [PubMed] [Google Scholar]
- Efferth T, et al. Molecular target-guided tumor therapy with natural products derived from traditional Chinese medicine. Curr Med Chem. 2007;14(19):2024–2032. doi: 10.2174/092986707781368441. [DOI] [PubMed] [Google Scholar]
- Efferth T, et al. Phytochemistry and pharmacogenomics of natural products derived from traditional Chinese medicine and Chinese Materia Medica with activity against tumor cells. Mol Cancer Ther. 2008;7(1):152–161. doi: 10.1158/1535-7163.MCT-07-0073. [DOI] [PubMed] [Google Scholar]
- Filipowicz N, et al. Phytotherapy research. Hoboken: Wiley; 2003. Antibacterial and antifungal activity of Juniper berry oil and its selected components. [DOI] [PubMed] [Google Scholar]
- Fischer J, Dethlefsen U. Efficacy of cineole in patients suffering from acute bronchitis: a placebo-controlled double-blind trial. Cough. 2013;9(1):25. doi: 10.1186/1745-9974-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman LJ, et al. Diabetes induced by streptozocin results in a decrease in immunoreactive beta-endorphin levels in the pituitary and hypothalamus of female rats. Diabetes. 1985;34(11):1104–1107. doi: 10.2337/diab.34.11.1104. [DOI] [PubMed] [Google Scholar]
- Franklin L et al (2001) Terpene based pesticide treatments for killing terrestrial arthropods including, amongst others, lice, lice eggs, mites and ants
- Friedman H, et al. Addictive drugs and their relationship with infectious diseases. Immunol Med Microbiol. 2006;47(3):330–342. doi: 10.1111/j.1574-695X.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- Gershenzon J. The function of terpene natural products in the natural world. Nat Chem Biol. 2007;3(7):408–414. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- Ginsburg H, Demel RA. Interactions of hemin, antimalarial drugs and hemin-antimalarial complexes with phospholipid monolayers. Chem Phys Lipids. 1984;35(4):331–347. doi: 10.1016/0009-3084(84)90076-8. [DOI] [PubMed] [Google Scholar]
- Giraudat J. Abscisic acid signaling. Curr Opin Cell Biol. 1995;7(2):232–238. doi: 10.1016/0955-0674(95)80033-6. [DOI] [PubMed] [Google Scholar]
- Goulart HR, et al. Terpenes arrest parasite development and inhibit biosynthesis of isoprenoids in Plasmodium falciparum. Antimicrob Agents Chemother. 2004;48(7):2502–2509. doi: 10.1128/AAC.48.7.2502-2509.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabmann J. Terpenoids as Plant Antioxidants. Vitamin Hormon: Plant Hormon; 2005. pp. 505–535. [DOI] [PubMed] [Google Scholar]
- Guadarrama-Cruz G, et al. Antidepressant-like effects of Tagetes lucida Cav. in the forced swimming test. J Ethnopharmacol. 2008;120(2):277–281. doi: 10.1016/j.jep.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Gupta R, et al. An overview of Indian novel traditional medicinal plants with anti-diabetic potentials. African J Tradition Complement Alternat Med. 2008;51:1–17. [PMC free article] [PubMed] [Google Scholar]
- Guzmán Gutiérrez SL, et al. Medicinal plants for the treatment of “nervios”, anxiety, and depression in Mexican traditional medicine. Rev Bras. 2014;24(5):591–608. [Google Scholar]
- Guzmán-Gutiérrez SL, et al. Antidepressant activity of Litsea glaucescens essential oil: identification of β-pinene and linalool as active principles. J Ethnopharmacol. 2012;143(2):673–679. doi: 10.1016/j.jep.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Guzmán-Gutiérrez SL, et al. Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015;128:24–29. doi: 10.1016/j.lfs.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Habila N, et al. Evaluation of in vitro activity of essential oils against Trypanosoma brucei brucei and Trypanosoma evansi. J Parasitol Res. 2010;2010:534601. doi: 10.1155/2010/534601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinig U, Scholz S, Jennewein S. Getting to the bottom of Taxol biosynthesis by fungi. Fungal Divers. 2013;60(1):161. doi: 10.1007/s13225-013-0228-7. [DOI] [Google Scholar]
- Himejima M, et al. Antimicrobial terpenes from oleoresin of ponderosa pine tree Pinus Ponderosa: a defense mechanism against microbial invasion. J Chem Ecol. 1992;18(10):1809–1818. doi: 10.1007/BF02751105. [DOI] [PubMed] [Google Scholar]
- Hjerl K, et al. Depression as a prognostic factor for breast cancer mortality. Psychosomatics. 2003;44(1):24–30. doi: 10.1176/appi.psy.44.1.24. [DOI] [PubMed] [Google Scholar]
- Holden C. Mental health: global survey examines impact of depression. Science. 2000;288(5463):39–40. doi: 10.1126/science.288.5463.39. [DOI] [PubMed] [Google Scholar]
- Hunter WN. The non-mevalonate pathway of isoprenoid precursor biosynthesis. J Biol Chem. 2007;282(30):21573–21577. doi: 10.1074/jbc.R700005200. [DOI] [PubMed] [Google Scholar]
- James JT, Dubery IA. Pentacyclic triterpenoids from the medicinal herb, Centella Asiatica (L.) urban. Molecules. 2009;14(10):3922–3941. doi: 10.3390/molecules14103922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawaid T, Gupta R, Siddiqui ZA. A review on herbal plants showing antidepressant activity. Int J Pharm Sci Res. 2011;90(24):3051–3060. [Google Scholar]
- Jirtle RL, et al. Increased mannose 6-phosphate/insulin-like growth factor II receptor and transforming growth factor beta 1 levels during monoterpene induced regression of mammary tumors. Cancer Res. 1993;53(17):3849–3852. [PubMed] [Google Scholar]
- Jordão FM, et al. Isoprenoid biosynthesis in the erythrocytic stages of Plasmodium falciparum. Mem Inst Oswaldo Cruz. 2011;106(1):134–141. doi: 10.1590/S0074-02762011000900018. [DOI] [PubMed] [Google Scholar]
- Jung M, et al. Antidiabetic agents from medicinal plants. Curr Med Chem. 2006;13(10):1203–1218. doi: 10.2174/092986706776360860. [DOI] [PubMed] [Google Scholar]
- Kamaraj C, et al. Ag nanoparticles synthesized using β-caryophyllene isolated from Murraya koenigii: antimalarial (Plasmodium falciparum 3D7) and anticancer activity (A549 and HeLa cell lines) J Clust Sci. 2017;28(3):1667–1684. doi: 10.1007/s10876-017-1180-6. [DOI] [Google Scholar]
- Kayembe JS et al (2012) In vitro antimalarial activity of 11 terpenes isolated from Ocimum gratissimum and Cassia alata leaves. Screening of their binding affinity with haemin. J Plant Stud 1(2)
- Keklikoglou I, Palma MD. Metastasis risk after anti-macrophage therapy. Nature. 2014;515(7525):46–47. doi: 10.1038/nature13931. [DOI] [PubMed] [Google Scholar]
- Khader M, Eckl PM. Thymoquinone: an emerging natural drug with a wide range of medical applications. Iran J Basic Med Sci. 2014;17(12):950–957. [PMC free article] [PubMed] [Google Scholar]
- Klein C, et al. Cannabidiol potentiates Δ 9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology. 2011;218(2):443–457. doi: 10.1007/s00213-011-2342-0. [DOI] [PubMed] [Google Scholar]
- Kocaadam B, Şanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr. 2017;57(13):2889–2895. doi: 10.1080/10408398.2015.1077195. [DOI] [PubMed] [Google Scholar]
- Kousik DM, Baldev K. A review on therapeutic uses of Ocimum Sanctum Linn (Tulsi) with its pharmacological actions. Int J Res Ayurved Pharm. 2012;3(5):645–647. doi: 10.7897/2277-4343.03512. [DOI] [Google Scholar]
- Kpoviessi BGHK, et al. In vitro antitrypanosomal and antiplasmodial activities of crude extracts and essential oils of Ocimum gratissimum Linn from Benin and influence of vegetative stage. J Ethnopharmacol. 2014;155(3):1417–1423. doi: 10.1016/j.jep.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Laakmann G, et al. St. Johns wort in mild to moderate depression: the relevance of hyperforin for the clinical efficacy. Complement Ther Med. 1999;7(4):265. doi: 10.1055/s-2007-979346. [DOI] [PubMed] [Google Scholar]
- Lee SH, et al. Identification of plant compounds that disrupt the insect juvenile hormone receptor complex. Proc Natl Acad Sci U S A. 2015;112(6):1733–1738. doi: 10.1073/pnas.1424386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, et al. Pinecone of Pinus koraiensis inducing apoptosis in human lung cancer cells by activating Caspase-3 and its chemical constituents. Chem Biodivers. 2017;14(4):1612–1880. doi: 10.1002/cbdv.201600412. [DOI] [PubMed] [Google Scholar]
- Liu Y. Dietary Chinese Herbs: chemistry, pharmacology and clinical evidence. New York: Springer; 2016. Phyllanthus emblica L. 余甘子 (Yuganzi, Indian Gooseberry) [Google Scholar]
- Liu QM, Jiang JG. Antioxidative activities of medicinal plants from TCM. Mini-Rev Med Chem. 2012;12(11):1154–1172. doi: 10.2174/138955712802762239. [DOI] [PubMed] [Google Scholar]
- Loizzo MR, et al. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem Biodivers. 2008;5(3):461–470. doi: 10.1002/cbdv.200890045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopresti L. Salvia (sage): a review of its potential cognitive-enhancing and protective effects. Drugs R&D. 2016;17(1):53–64. doi: 10.1007/s40268-016-0157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, et al. On the monoterpene emission under heat stress and on the increased thermotolerance of leaves of Quercus Ilex L. fumigated with selected monoterpenes. Plant Cell Environ. 2002;21(1):101–107. doi: 10.1046/j.1365-3040.1998.00268.x. [DOI] [Google Scholar]
- Lutge EE, et al. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;4:CD005175. doi: 10.1002/14651858.CD005175.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia M, Sarah JM (2011) Plant-based insect repellents: a review of their efficacy, development and testing. Malar J 10(1) [DOI] [PMC free article] [PubMed]
- Majdalawieh AF, et al. Anti-cancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativa. Crit Rev Food Sci Nutr. 2017;57(18):3911–3928. doi: 10.1080/10408398.2016.1277971. [DOI] [PubMed] [Google Scholar]
- Mastelic J, et al. Composition and antimicrobial activity of Helichrysum Italicum essential oil and its terpene and terpenoid fractions. Chem Nat Compound. 2017;41(1):35–40. doi: 10.1007/s10600-005-0069-z. [DOI] [Google Scholar]
- Mathias P, et al. In vitro cytotoxic potential of essential oils of Eucalyptus benthamii and its related terpenes on tumor cell lines. Evid Based Complement Alternat Med. 2012;2012:8. doi: 10.1155/2012/342652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn H et al (2011) Compositions comprising citrus flavonoids and quaternary ammonium salts for treating head lice
- Mikhlin ED, et al. Antifungal and antimicrobial activity of beta-ionone and vitamin A derivative. Prikl Biokhim Mikrobiol. 1983;19(6):795–803. [PubMed] [Google Scholar]
- Miller JA, et al. -Limonene: a bioactive food component from citrus and evidence for a potential role in breast cancer prevention and treatment. Oncol Rev. 2010;5(1):31–42. doi: 10.4081/oncol.2011.31. [DOI] [Google Scholar]
- Miller JA, et al. Human breast tissue disposition and bioactivity of limonene in women with early-stage breast cancer. Cancer Prev Res. 2013;6(6):577–584. doi: 10.1158/1940-6207.CAPR-12-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss M, Oliver L. Plasma 1,8-cineole correlates with cognitive performance following exposure to rosemary essential oil aroma. Therap Adv Psychopharmacol. 2012;2(3):103–113. doi: 10.1177/2045125312436573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller WE, et al. Hyperforin—antidepressant activity by a novel mechanism of action. Pharmacopsychiatry. 2001;34(1):98–102. doi: 10.1055/s-2001-15512. [DOI] [PubMed] [Google Scholar]
- Nabavi S, et al. Curcumin: a natural product for diabetes and its complications. Curr Top Med Chem. 2015;15(23):2445–2455. doi: 10.2174/1568026615666150619142519. [DOI] [PubMed] [Google Scholar]
- Narayan V, et al. Diabetes—a common, growing, serious, costly, and potentially preventable public health problem. Diabetes Res Clin Pract. 2000;50(2):77–84. doi: 10.1016/S0168-8227(00)00183-2. [DOI] [PubMed] [Google Scholar]
- Nazaruk J, Borzym-Kluczyk M. The role of triterpenes in the management of diabetes mellitus and its complications. Phytochem Rev. 2015;14(4):675–690. doi: 10.1007/s11101-014-9369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira CR, Lopes LMX. Antiplasmodial Natural Products. Molecules. 2011;16(12):2146–2190. doi: 10.3390/molecules16032146. [DOI] [Google Scholar]
- Orjih A, et al. Hemin lyses malaria parasites. Science. 1981;214(4521):667–669. doi: 10.1126/science.7027441. [DOI] [PubMed] [Google Scholar]
- Pandey SC, et al. β-Adrenergic receptor subtypes in stress-induced behavioral depression. Pharmacol Biochem Behav. 1995;51(2–3):339–344. doi: 10.1016/0091-3057(94)00392-V. [DOI] [PubMed] [Google Scholar]
- Perry NSL, et al. In-vitro inhibition of human erythrocyte acetylcholinesterase by salvia Lavandulaefolia essential oil and constituent terpenes. J Pharm Pharmacol. 2000;52(7):895–902. doi: 10.1211/0022357001774598. [DOI] [PubMed] [Google Scholar]
- Popova M, et al. Terpenes with antimicrobial activity from Cretan Propolis. Phytochemistry. 2009;70(10):1262–1271. doi: 10.1016/j.phytochem.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Ravindran PN, et al. Cinnamon and cassia: the genus Cinnamomum. Boca Raton: CRC Press; 2004. [Google Scholar]
- Reyes BAS, et al. Anti-diabetic potentials of Momordica charantia and Andrographis paniculata and their effects on estrous cyclicity of alloxan-induced diabetic rats. J Ethnopharmacol. 2006;105(1–2):196–200. doi: 10.1016/j.jep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Rodriguez MH, Jungery M. A protein on Plasmodium falciparum-infected erythrocytes functions as a transferrin receptor. Nature. 1986;324(6095):388–391. doi: 10.1038/324388a0. [DOI] [PubMed] [Google Scholar]
- Saddinne JB, et al. Prevalence of self-rated visual impairment among adults with diabetes—United States. Am J Public Health. 1999;89(8):1200–1205. doi: 10.2105/AJPH.89.8.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saki K, et al. The effect of most important medicinal plants on two importnt psychiatric disorders (anxiety and depression)-a review. Asian Pac J Trop Med. 2014;7:S34–S42. doi: 10.1016/S1995-7645(14)60201-7. [DOI] [PubMed] [Google Scholar]
- Sartorelli P, et al. Chemical composition and antimicrobial activity of the essential oils from two species of Eucalyptus. Phytother Res. 2007;21:231–233. doi: 10.1002/ptr.2051. [DOI] [PubMed] [Google Scholar]
- Sarwar N, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Emerging Risk Factors Collaboration. Lancet. 2010;26(375):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann M, et al. Isoprenoid biosynthesis via the MEP pathway: isoprenoid biosynthesis via the MEP pathway in-vivo Moessbauer spectroscopy identifies a [4Fe-4S]2+ Center with unusual coordination sphere in the LytB protein. J Am Chem Soc. 2009;131(37):13184–13185. doi: 10.1021/ja9012408. [DOI] [PubMed] [Google Scholar]
- Sharma H, et al. Utilization of Ayurveda in health care: an approach for prevention, health promotion, and treatment of disease. Part 2—Ayurveda in primary health care. J Altern Complement Med. 2007;13(10):1135–1150. doi: 10.1089/acm.2007.7017-B. [DOI] [PubMed] [Google Scholar]
- Silva C et al (2008) Antifungal activity of the lemongrass oil and Citral against Candida Spp. Braz J Infect Dis 12(1) [DOI] [PubMed]
- Singsaas EL. New Phytologist. Cambridge: Cambridge University Press; 2001. Terpenes and the thermotolerance of photosynthesis. [Google Scholar]
- Small E. Cannabis: a complete guide. Boca Raton: CRC Press; 2017. [Google Scholar]
- Sobral MV, et al. Antitumor activity of monoterpenes found in essential oils. Sci World J. 2014;2014:953451. doi: 10.1155/2014/953451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subhan F, et al. Terpenoid content of Valeriana wallichii extracts and antidepressant-like response profiles. Phytother Res. 2010;24:686–691. doi: 10.1002/ptr.2980. [DOI] [PubMed] [Google Scholar]
- Taborsky J et al (2012) Identification of potential sources of thymoquinone and related compounds in Asteraceae, Cupressaceae, Lamiaceae, and Ranunculaceae families. Open Chem 10(6)
- Vasas A, Hohmann J. Euphorbia Diterpenes: isolation, structure, biological activity, and synthesis (2008–2012) Washington: ACS Publications; 2014. [DOI] [PubMed] [Google Scholar]
- Wu YW, et al. Volatility-dependent 2D IR correlation analysis of traditional Chinese medicine ‘red flower oil’ preparation from different manufacturers. J Mol Struct. 2008;882(1–3):107–115. doi: 10.1016/j.molstruc.2007.09.018. [DOI] [Google Scholar]
- Yang J, et al. Enhancing production of bio-isoprene using hybrid MVA pathway and isoprene synthase in E. Coli. PLoS One. 2012;7(4):e33509. doi: 10.1371/journal.pone.0033509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You W, Henneberg M. Type 1 diabetes prevalence increasing globally and regionally: the role of natural selection and life expectancy at birth. BMJ Open Diabetes Res Care. 2016;4(1):e000161. doi: 10.1136/bmjdrc-2015-000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, et al. Polyketide-terpene hybrid metabolites from an endolichenic fungus pestalotiopsis sp. Biomed Res Int. 2017;2017:1–10. doi: 10.1155/2017/6961928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora AP, et al. The in vitro and in vivo antiviral properties of combined monoterpene alcohols against West Nile virus infection. Virology. 2016;495:18–32. doi: 10.1016/j.virol.2016.04.021. [DOI] [PubMed] [Google Scholar]
- Zetter BR. The scientific contributions of M. Judah Folkman to cancer research. Nat Rev Cancer. 2008;8(8):647–654. doi: 10.1038/nrc2458. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, et al. Novel Norsesquiterpenoids from the roots of Phyllanthus emblica. J Nat Prod. 2000;63(11):1507–1510. doi: 10.1021/np000135i. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. Tanshinones: sources, pharmacokinetics and anti-cancer activities. Int J Mol Sci. 2012;13(10):13621–13666. doi: 10.3390/ijms131013621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DW, et al. Curcumin and diabetes: a systematic review. Evid Based Complement Alternat Med. 2013;2013:636053. doi: 10.1155/2013/636053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheljazkov Valtcho D., Gawde Archana, Cantrell Charles L., Astatkie Tess, Schlegel Vicki. Distillation Time as Tool for Improved Antimalarial Activity and Differential Oil Composition of Cumin Seed Oil. PLOS ONE. 2015;10(12):e0144120. doi: 10.1371/journal.pone.0144120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwenger S, Basu C. Plant terpenoids: applications and potentials. Biotechnol Mol Biol Rev. 2008;3:1–7. [Google Scholar]