Abstract
Lungs are the most commonly involved organ by HIV/AIDS related diseases, and pulmonary infections are the main reasons for the increasing death rate from AIDS. Pathogens of HIV related pulmonary infections include parasites, fungi, mycobacteria, viruses, bacteria and toxoplasma gondii. According to international reports, pathogens have different geographical distribution, which is also closely related to the socioeconomic status of the region to produce varied AIDS related diseases spectra. For instance, in the United States, pneumocystis carnii pneumonia (PCP), tuberculosis and recurrent bacterial pneumonia (at least twice within 1 year) occur frequently in HIV infected patients. An international report published 10 years ago indicated that PCP is the most common and serious pulmonary opportunistic infections in HIV infected patients. Now its incidence has dropped with the application of antiretroviral treatment and preventive measures. PCP will continue to occur initially in patients who are aware of their HIV infection. In addition, HIV related viral and parasitic infections have been reported both domestically and internationally. In this section, the clinical manifestations and imaging findings of HIV related pulmonary infections are analyzed and discussed, which provide effective diagnosis basis, so as to reduce the incidence of HIV-related pulmonary infections.
Keywords: HIV/AIDS related pneumocystis carnii pneumonia (PCP), HIV/AIDS related pulmonary bacterial infections, HIV/AIDS related pulmonary fungal infections, HIV/AIDS related pulmonary virus infections, HIV/AIDS related pulmonary parasitic diseases, HIV/AIDS related pulmonary neoplasm
General Overview of HIV/AIDS Related Respiratory Diseases
Lungs are the most commonly involved organ by HIV/AIDS related diseases, and pulmonary infections are the main reasons for the increasing death rate from AIDS. Pathogens of HIV related pulmonary infections include parasites, fungi, mycobacteria, viruses, bacteria and toxoplasma gondii. According to international reports, pathogens have different geographical distribution, which is also closely related to the socioeconomic status of the region to produce varied AIDS related diseases spectra. For instance, in the United States, pneumocystis carnii pneumonia (PCP), tuberculosis and recurrent bacterial pneumonia (at least twice within 1 year) occur frequently in HIV infected patients. An international report published 10 years ago indicated that PCP is the most common and serious pulmonary opportunistic infections in HIV infected patients. Now its incidence has dropped with the application of antiretroviral treatment and preventive measures. PCP will continue to occur initially in patients who are aware of their HIV infection. In addition, HIV related viral and parasitic infections have been reported both domestically and internationally. In this section, the clinical manifestations and imaging findings of HIV related pulmonary infections are analyzed and discussed, which provide effective diagnosis basis, so as to reduce the incidence of HIV-related pulmonary infections.
AIDS Related Pulmonary Infections
Pneumocystis Carnii Pneumonia
Pneumocystis has been believed to be a kind of protozoon. Recently, based on its ultrastructure and ribosomal RNA phylogenetic analysis, pneumocystis is now believed to be a kind of fungus, with high affinity to the lung tissues. Due to the compromised immunity, 95 % AIDS patients sustain different types of pulmonary infections, of which PCP is the most common life-threatening opportunistic infection with an incidence rate of about 60–85 %. About 90–95 % patients suffering from AIDS complicated by PCP are adolescents and adults with their CD4 T cell counts being less than 200/μl. Clinical manifestations of typical PCP are fever, cough (dry cough without phlegm), dyspnea, chest distress and shortness of breath. Dyspnea is shown as progressive difficulty in breathing, which initially occurs after physical activities and develops into difficulty breathing even in resting state. PCP is commonly accompanied by weight loss, fatigue, anemia, general upset and lymphadenectasis. All these symptoms are non-specific, but patients often report subjective feelings of severe symptoms while physical signs are mild. By auscultation, the lungs are normal or with slightly dry, moist rales. These are the clinical findings characteristic to AIDS complicated by PCP. In most patients with PCP, the serum LDH level increases but it is non-specific. In cases of AIDS complicated by PCP, the blood PO2 reduces, commonly being lower than 70 mmHg in patients in the middle and advanced stages. The diagnostic imaging for PCP includes chest X-ray and CT scanning. Due to the low resolution of chest X-ray, its demonstrations are negative for PCP patients in the early stage or only include thickened pulmonary markings and decreased pulmonary transparency. However, CT scanning demonstrates tiny lesions or more detailed changes in lungs. Especially with the application of HRCT, the detection rate of PCP lesions has been greatly improved. It has been internationally reported that nearly 10 % of PCP patients show negative findings by the chest X-ray but with abnormal findings by HRCT. Due to the rapid progression of PCP as well as its complex pathological changes, CT scanning demonstrations are diverse with specificity. According to different pulmonary CT scanning demonstrations in different stages of the illness, PCP is divided into early stage (exudative and infiltrative stage), middle stage (fusion and parenchymal stage) and advanced stage (absorption or fibrosis stage). The early typical manifestations include intrapulmonary multiple miliary nodules, mainly distributed in both middle and lower lung fields. It may be accompanied by enlarged hilar shadow, which should be differentiated from acute miliary tuberculosis.
The middle stage is a period of infiltration. As the disease progresses, miliary and patchy shadows fuse and expand into a dense infiltrative shadow with even density, showing a diffuse ground glass liked change. The typical manifestations include bilaterally symmetric foci with the hilus as the center. The foci infiltrates from the hilus to bilateral pulmonary interstitium, progressing from the both middle lungs to both lower lungs. HRCT can more clearly demonstrate the foci, showing a map liked or gravel road liked appearance, with clearly demonstration of gas containing bronchus penetrating the foci. The pulmonary apex is involved later. The exterior stripe of the lung field has increased transparency, showing typical willow leaf sign or moon bow sign which is the manifestation of compensatory pulmonary emphysema.
During the late compensatory repair period, the intrapulmonary lesions are mainly parenchymal changes and fibrosis, with large flaky high density shadows as well as cords liked and reticular changes. Pneumothorax, mediastinal emphysema, pneumatocele, pleural effusion and other complications may occur, with an incidence of pneumatocele in about 10–20 % patients. The autopsy grossly demonstrates swelling of the lung tissue, and the alveoli are filled with large quantity foamy liquid. The pathological changes mainly manifested as interstitial pneumonia, with early manifestations of increased permeability of the capillary wall basement membrane in the alveolar walls, which leads to fluid exudation. The Pneumocystis carinii proliferate in large quantity and adhere to cause degeneration of the type I alveolar epithelial cells and shedding of the basement membrane. Vascular congestion, edema as well as infiltration of lymphocytes, plasma cells and mononuclear cells can be found in the pulmonary interstitium.
Fungal Infections
Due to the extensive existence of aspergillus in natural world, sputum smear positive often fails to define its invasive infection. In the cases with aspergillus infection, hyphae can be found in the sputum. Fungal infections often occur in patients with CD4 T cell count below 100/μl, of which the most common pulmonary infection is aspergillus infection, followed by penicillium marneffei infection. Pulmonary infections caused by Candida albicans and histoplasma are rarely found. The incidence of cryptococcal pulmonary infection is still in a disagreement, which is increasing recently. There are also some common endemic fungal infections, such as the most commonly found fungal infections of AIDS complicated by penicillium infections in Guangxi Zhuang Autonomous Region and Hong Kong, China. Aspergillus has an extensive existence in the natural world. AIDS complicated by aspergillus infection is related to the application of corticosteroid hormone or broad-spectrum antibiotics, which occurs commonly in the advanced/critical stage of AIDS. The cases of pulmonary fungal infections, with findings of hyphae (aspergillus or candida) or yeast in cytoplasm (Histoplasma capsulatum) in tissue sections and simultaneous findings of histiocytic reactions including the infiltration of neutrophilic granulocyte and the necrosis of histocytes, can be diagnosed as having invasive fungal infection.
Cryptococcal Pneumonia
Cryptococcal infection occurs when CD4 count is below 200/μl. Especially when CD4 count is below 50/μl, the incidence is increasing. Cryptococcal infection often manifests as meningitis or meningoencephalitis. Its pulmonary infection is simple or with accompanying meningitis. The clinical manifestations are the fever, cough, shortness of breath and rarely accompanying chest pain. About 10 % patients with cryptococcal infection have respiratory failure. Cryptococcal pneumonia can occur in any part of the lungs, commonly with multiple foci in both lower lungs. Its imaging demonstrations commonly include increased pulmonary markings, singular or multiple nodules and the fusion of multiple nodules into mass. In 1992, Jones et al. [103] divided the pulmonary lesions into four groups: (1) Primary syndrome composed of subpleural lesions and the involved lymph nodes; (2) Granuloma, which is larger parenchymal granuloma; the granuloma contains large quantity inactive yeast and surrounded by fibrous granulation tissues, which contains large quantity macrophages. (3) Intrapulmonary miliary lesions, the lesions being in miliary size and diffusively distributing; (4) Formation of cavities, with central necrosis of the foci to form cavity; 10–16 % patients may have thin wall cavities. The manifestations of bilateral diffuse interstitial infiltration, patchy fusion, nodules, pleurisy, and hilar lymphadenectasis have also been reported. There are also some individual reports of cryptococcal pneumonia with pulmonary mass, singular pleural effusion and pneumothorax. In addition, some cases of cryptococcal infection may show negative pulmonary manifestations. The pathological changes include mainly exudative or granulomatous reaction, infiltrations of macrophages, lymphocytes and multinuclear macrophages in the foci, and rarely found purulent lesions. Chronic granuloma may be accompanied with extensive fibrosis.
Aspergillus Pneumonia
Aspergillus is a conditional pathogenic fungus. In AIDS patients, aspergillus invades the bronchial wall and the lung tissues after inhaled to cause exudative and necrotic lesions as well as secondary suppurative pneumonia and lung abscess. After the pus fluids and necrotic substances expelled, cavity forms. Sometimes a fungus ball occurs in the cavity. Due to the different types of aspergillus as well as their different targets of invasion and different defense mechanism of the human body, the demonstrations of aspergillus pneumonia are also varying, which can be divided into four types. The first type includes bronchial pneumonia, disseminated aspergillus sepsis, aspergillus ball, and allergic reaction, with clinical manifestations of chills, fever, wheezing, cough, mucous sputum, hemoptysis and chest pain. Hemoptysis is a serious symptom of pulmonary aspergillosis, which can be the main reason of death. By chest X-ray, there are pulmonary infiltration and cavity lesion that is a round shadow with clear boundary, movable with posture. In the cases with bronchial occlusion, the fluid in the cavity shows liquid level, and sometimes in air crescent sign, based on which neoplasm can be excluded. Aspergillus infection secondary to the cavity is the specific demonstration for the imaging diagnosis of aspergillosis.
Penicillium Marneffei Pneumonia
Penicillium marneffei is a new species of penicillium genus, which was discovered in 1956 [104], with a distribution in Southeast Asia and southern China. Rhizomys is its natural host. As an conditional pathogen, people with compromised immunity are susceptible to Penicilliosis marneffei (PSM). Penicillium marneffei can spread through the contaminated soil by faeces of Rhizomys, and infect people via the respiratory tract, gastrointestinal tract and skin defects. PSM is believed to be one of the most common opportunistic infections in AIDS patients of Southeast Asia, and its incidence is still increasing. The clinical manifestations include long-term fever, progressive weight loss, cough and expectoration, skin rash, anemia, and lymphadenectasis. The pathological process of PM pneumonia is that PM yeast phase pigment with a strong hydrophobicity promotes the conidium of the mould phase and the cells of the yeast phase to adhere to the alveolar macrophages and macrophages in other parts, which enlarge the organs with abundant mononuclear macrophages, such as lymphadenectasis. Macrophagic granuloma may occur, with multinucleated cytomegalic responses. Kudeken et al. [13] conducted a study on PM infected but immunocompetent rats, which demonstrated that PM can cause fatal high inflammatory responses after complex CD4 T cells mediation. But in AIDS patients with PM pulmonary infections, due to the serious insufficiency of the CD4 T cells, the phagocytosis of macrophages is obviously weakened, with less exudative changes but commonly proliferative changes to cause only non-reactive necrotic inflammation with cavity formation. Zhang et al. [105] studies a group of cases, with imaging characteristic demonstrations of clustering cavities in the irregularly thick wall, reflecting its pathological features of mainly proliferation and necrotic cavities. The clustering may be related to the spreading of PM along the bronchi. The pulmonary puncture for biopsy demonstrates that microscopically tissue culture at a temperature of 25 °C may find mycelium branches and septa as well as the string of microspores or growth of hyphae in broom liked appearance.
Candida Pneumonia
Candida albicans is yeast liked fungus, which is widespread in the natural world. It can parasitize in the mocous of skin, oral cavity, intestinal tract and vagina of the human being. Candida albicans cannot cause disease in immunocompetent people but is pathogenic in immunocompromised population. After its invasion into the tissues, it turns into mycelia and multiplies in large quantity with great toxicity. It also has the ability to fight against phagocytosis. Clinically, its infection is characterized by a chronic onset and clinical symptoms of low and moderate grade fever but rarely high fever, cough, shortness of breath, cyanosis, irritation or dysphoria. The pulmonary signs include weakened breathing sounds by auscultation and obvious moist rales of lungs. The serious cases may have symptoms of systemic poisoning. The illness is prolonged and repeated during its whole progression. By diagnostic imaging demonstrations, it can be divided into the following types: (1) Bronchitis type, with chest X-ray demonstrations of increased pulmonary markings in lower fields of both lungs; (2) Pneumonia type, commonly with accompanying extrapulmonary lesions. The lesions are mainly located in the middle and lower lung fields and lesions in the lower lung field are more common. The apex is generally not involved. The lesions are recurring one after another. A small number of patients may sustain complications of exudative pleurisy. (3) Disseminated type, with miliary shadows, diffuse nodular shadows or multiple small abscesses. The lesions often involve the middle and lower lungs. Chest X-ray demonstrates thickened pulmonary markings and accompanying spots, small flakes and large flakes of parenchyma shadows, in manifestations of bronchial pneumonia. In some serious cases, the foci may fuse together and enlarge to involve the entire lobe. CT scanning demonstrates pulmonary nodules and few have ground glass liked changes of the lungs. Pathological changes include acute inflammatory lesions in the lungs, alveolar exudation and infiltration of monocytes, lymphocytes and neutrophils. Acute disseminated lesions often cause multiple small abscesses, central caseous necrosis, spores and hyphae in and around the lesions.
Histoplasma Capsulatum Pneumonia
Histoplasma capsulatum belongs to moniliales family, deuteromycetes class and fungal kingdom, whose growth requires organic nitrogen. It is often isolated from the soil with abundant contents of birds or bats faeces and spreads along with chickens, birds, dogs, cats, and mice. When the conidia and mycelial fragments of histoplasmosis are inhaled, most can be expelled by the defense mechanism of the human body. Granulomas may form, but in immunocompromised patients, it may cause disseminated histoplasmosis. When the CD4 T cell count in AIDS patients is less than 150/μl, histoplasma capsulatum infection of lungs may occur. Histoplasma capsulatum pneumonia has a higher incidence in South America, Africa and India. In the slight cases, the clinical manifestations are similar to symptoms of the cold, with low-grade fever, cough, and general upset. In the serious cases, there are symptoms of influenza, including chills, high fever, cough, chest pain, dyspnea, fatigue and poor appetite. In the cases of acute cavity, thin-walled cavity may form within a month. Complications may be pericarditis, arthritis, skin nodules, rash fibrous mediastinitis and mediastinal granuloma. Diagnostic imaging demonstrations are non-specific, with scattering pulmonary acinus exudation, multiple nodules in a diameter of about 3 mm with accompanying thickened septa, and formation of granulomas with accompanying calcification. It should be differentiated from bacterial pneumonia, tuberculosis and other pulmonary fungal infections by laboratory tests to define the diagnosis. The specificity of the glycogen antigen detection of histoplasma capsulatum is up to 98 %.
Mucor Pneumonia
Mucor spreads through the respiratory tract. It commonly invades the blood vessels, especially arteries. It reproduces locally or causes thrombosis and embolism. Clinical manifestations are high fever, cough, sputum, shortness of breath, chest distress, chest pain and hemoptysis (pulmonary artery involvement). The diagnostic imaging demonstrates flakes inflammatory foci, with manifestations of pulmonary cavity and pulmonary infarction. The pathological changes are hemorrhagic infarction of local tissue, pneumonia and exudation of neutrophils. Hemorrhagic infarction of local tissue may be related to hyphae induced minor arteries lesions.
Pulmonary Tuberculosis
It is estimated that one third of the world population was/is infected with tubercle bacillus and 9 % of them are AIDS patients. The WHO reported that there are 88,000 newly infected patients of TB each year and 8.4 % of them are caused by AIDS. It is estimated that each year in 1,000 HIV infected patients, 35–162 sustain active TB, and there is a great risk of active TB progressed from the latent tuberculosis infection. HIV infected patients with tuberculosis are commonly young and middle aged adults, with more male patients than female patients. Tuberculosis can occur at any stage of AIDS and at any level of CD4 T cell counts. It has been internationally reported that HIV infection complicated by TB has no specific imaging demonstrations. It has an acute onset, with an incidence of acute onset 2.5 times as high as that in non-HIV infected patients. The lesions are morphologically diverse, which are different from non-HIV infected patients with TB. HIV infection complicated by TB has commonly an acute onset, while TB in non-HIV infected patients is commonly secondary to other lesions, with cavities, fibrosis, pleural thickening and calcification. A study conducted in China has demonstrated that for AIDS complicated by TB, the acute cases mainly have military and exudative lesions, with an incidence of 33 and 49 % respectively; while the incidence of chronic cases including cavity, fibrosis and calcification is declining, being 11, 11 and 2 respectively. A later occurrence of tuberculosis in HIV infected patients indicates a more seriously immunocompromised immunity, with less typical clinical manifestations and imaging demonstrations. When the CD4 T cell count level is above 350–400/μl, the systemic symptoms are fever, chills, night sweating, fatigue, poor appetite and weight loss. Respiratory symptoms are cough, expectoration, hemoptysis, chest pain and dyspnea. It manifests as primary tuberculosis, with its foci distributing in the middle and lower lungs, involving multiple lobes and segments. When the CD4 T cell count decrease, the impact of TB increase including the occurrence of extrapulmonary tuberculosis and disseminated disease. When the CD4 T cell count drops below 200/μl, pulmonary tuberculosis manifests as acute onset (such as miliary tuberculosis) or extrapulmonary tuberculosis (such as ileocecal tuberculosis) and peripheral lymph nodes tuberculosis. Its difference from the clinical manifestations of non-HIV infected patients is as the following: (1) More common pulmonary infiltration with multiple involvements and rare cavities; (2) Higher incidence of dissemination (87–96 %) commonly along with blood flow and higher incidence of extrapulmonary tuberculosis (60–70 %); (3) More common lymph node tuberculosis, such as hilar, mediastinal and extrapleural lymphadenectasis; (4) Lower positive rate of tuberculin test (PPD); (5) More patients with no expectoration, with sputum smear for acid-fast bacilli staining is negative; (6) Higher incidence of resistant strains, high recurrence rate, and higher mortality (Table 17.1).
Table 17.1.
Clinical manifestations of HIV/AIDS related tuberculosis and Non-HIV/AID related tuberculosis
| HIV/AID tuberculosis | Non-HIV/AID tuberculosis | |
|---|---|---|
| Tuberculin test | Early positive | Accumulation of somatic cells |
| Advanced conversion into negative | Generation of lymphokine (ThI type) | |
| Chest X-ray | Atypical | Infiltration |
| More common in the lower lung | More commonly cavity | |
| Rare cavity | Common occurrence in the apex and posterior segment, with downwards spreading | |
| Extrapulmonary TB | More common | Mainly intrapulmonary, rarely extrapulmonary |
| Spreading to extrapulmonary tissues | ||
| Detection rate of MTB | Low (19 %) | High (30–73 %) |
| Anti-TB therapies | Poor efficacy and more side effects | Favorable efficacy and less side effects |
Foci in the cases with AIDS complicated by pulmonary tuberculosis are change quickly. After anti-TB treatment, the lesions are absorbed quickly. Those receiving no anti-TB therapy, the foci tend to fuse together to form a mass or diffusely distribute.
Bacterial Pneumonia
Bacterial septicemia often occurs in AIDS patients. Many opportunistic pathogens can cause respiratory infections, including bacterial bronchitis, pneumonia and pleuritis. The incidence rate of bacterial pneumonia (BP) is 3–5 %. BP has a larger range of impact on HIV infected patients than on non-HIV infected groups. Repeated episode of BP is considered to be the first manifestations of latent HIV infection. Therefore, for those individuals who have recurrent pneumonia without other risk factors, they should be alert to HIV infection. The common pathogenic bacteria include Streptococcus pneumoniae, Staphylococcus aureus, Rhodococcus equi, Haemophilus and pseudomonas aeruginosa. As non-HIV infected patients, the most common pathogens of pneumonia are Streptococcus pneumoniae and Haemophilus influenzae. Legionella and Klebsiella are also common. Many factors, such as the reduced T lymphocytes in HIV infected patients, manufacturing disorders of neutrophils, mononuclear cells and cytokines, and dysfunctional B lymphocytes, provide chances for opportunistic bacterial infections. In addition, the application of broad-spectrum antibiotics also increases the chance of opportunistic infections. BP can occur in any stage of HIV and at any level of CD4 T cell count. When the CD4 T cell count decreases, the incidence of BP also increases. The clinical manifestations of HIV infected patients are the same as non-HIV infected patients, with acute onset (3–5 days), high fever (39–40 °C), chills, chest pain, dyspnea, cough, purulent sputum (bloody or rusty). Being different from non-HIV infection, pulmonary infection in HIV-infected patients is often recurrent.
The imaging demonstrations of HIV/AIDS related bacterial pneumonia are similar to those in non-HIV infected patients. Most cases of streptococcal pneumonia and haemophilus pneumonia have unilateral, confined and partially fused foci with accompanying pleural effusion. The imaging demonstrations include thickened and deranged pulmonary markings, alveolar filling of inflammatory exudates with the progression of the illness, large flaks inflammatory infiltration shadows or parenchymal shadows, bronchial gas filling phase in the parenchymal shadows. The lesions distribute along the pulmonary segments or lobes, rarely with accompanying pleural effusion. During the absorption period, the density of the parenchymal shadows gradually reduces and the scope narrows down. There may be cavities in some individual cases. But in most cases it is completely absent after 3–4 weeks. Lesions absorption are slow in elderly patients and recurrent patients, which is difficult to be completely absorbed and often develop into organic pneumonia.
Rhodococcus equi was initially discovered in 1923 and nominated as corynebacterium equi. After structure analysis of the cell wall, it was found to be different from Corynebacterium, and therefore it is classified into Rhodococcus. Rhodococcus equi is generally considered as the pathogens of horses, pigs and cattle. Human rhodococcus equi infection is rare. But in recent years, due to an increase in patients with immunodeficiency syndrome, reports of rhodococcus equi induced human respiratory infection and sepsis are increasing. Rhodococcus equi is an intracellular facultative parasitic bacterium. Its optimum temperature for growth is 30 °C, and suitable temperature for its growth is 10–40 °C. The acid-fast staining for rhodococcus equi shows uncertain results. Due to its various morphology, it is commonly mistaken as diphtheroids bacilli, Bacillus or Micrococcus. On sheep blood agar plate, the bacterium can have synergistic hemolysis with staphylococcus aureus, mononuclear Listeria and Corynebacterium pseudotuberculosis. Toxicity mechanisms of Rhodococcus equi has been recently discovered the existence of toxic plasmid, which provides a new idea for the full understanding of its pathogenesis. Clinical symptoms are usually cough, orange red sputum, high fever and other symptoms. E Marchiori et al. in 2005 studied five cases of AIDS complicated by Rhodococcus equi pulmonary infection. All the patients had a case history of cough and fever history for 1–2 months with accompanying shortness of breath and chest pain. Li et al. in 2009 [106] studied a group of 13 cases. All patients had fever, with a body temperature being 38–40 °C, cough, orange red sputum. The typical clinical manifestations of the disease are fever, dyspnea and chest pain. Other symptoms such as weight loss, diarrhea and joint pain are not representative. Based on the course of the disease, the diagnostic imaging demonstrations of Rhodococcus equi pulmonary infection can be divided into early stage, showing round liked flaky blurry shadows surrounding unilateral hilum that has blurry boundary; middle stage (parenchymal change), showing central sphere liked high density shadow surrounding unilateral hilum, in parenchymal changes and with clear boundary; advanced stage (necrosis) showing secondary cavity of the pulmonary mass, possibly with hydropneumothorax and pleurisy. The imaging demonstrations are characteristic, but lack of specificity. And it should be differentiated from pulmonary tumors. The pathological changes include the most commonly chronic suppurative bronchopneumonia and extensive pulmonary abscesses. The histopathology demonstrates massive bleeding in alveolar space, large quantity erythrocytes, intact cellular wall and large quantity epithelial cells. The predominant pathological changes may also be fibroblasts, with parenchymal changes of lung tissue and thickened alveolar septa. Accumulating piles of strip liked purple Rhodococcus equi can be found by PAS staining.
Viral Pneumonia
Common pathogenic viruses of the opportunistic pulmonary infections in HIV infected patients are cytomegalovirus (CMV) and influenza virus. CMV is the most common pathogen of HIV/AIDS related pulmonary infection. By autopsy, 49–82 % patients with HIV/AIDS have CMV infection, only second to Pneumocystis carinii pneumonia. Moskowitz et al. [32] reported that among the direct causes of death in AIDS patients, 19 % is due to pulmonary cytomegalovirus infection. Because of the lack of typical clinical manifestations and sensitive examinations for its early diagnosis, the definitive diagnosis rate of cytomegalovirus pneumonia is only 13–24 % before autopsy. The clinical manifestations of CMV infection are non-specific. The systemic symptoms are fever, soreness of joints and muscles. Respiratory symptoms are paroxysmal dry cough, progressive shortness of breath, difficulty in breathing during activities. Pulmonary CMV infection may develop secondary fungal infection or be complicated by bacterial, fungal, and Pneumocystis carinii infections. The cytomegalovirus can widely spread in the organs and tissues of the infected patients, and the infections can directly lead to the damage of infected host cells. In addition, the virus can also cause pathogenic effects via immune pathological mechanism. Some scholars classified CMV pneumonia into diffuse, miliary necrosis and cytomegalic. Diffuse and cytomegalic CMV pneumonia are often accompanied by diffuse alveolar damage (DAD), which is more common in the diffuse type of CMV pneumonia but less common in cytomegalic type of CMV pneumonia. The pathological basis of diffuse small nodules in lungs is hemorrhagic necrosis. Sometimes CMV infection is concurrent with other infections in the lungs, and even co-infects one cell. Pulmonary parenchymal changes indicate bacterial and fungal infections, such as findings of inclusion bodies in the cells, commonly known as Eagle’s Eye sign. The imaging demonstrations of cytomegalovirus pneumonia are diverse. Some studies summarize that the lungs commonly have manifestations of diffuse interstitial infiltration or alveolar infiltration, with ground glass liked changes, pulmonary parenchymal changes, grid liked changes, thickend bronchial wall, bronchiectasis, pulmonary nodules or masses. The principal changes include the early lesions of ground glass liked changes and advanced lesions of pulmonary masses.
Lymphoid Interstitial Pneumonia
Lymphoid interstitial pneumonia is the abnormal hyperplasia of the pulmonary lymphoid tissue. Its occurrence is related to autoimmune diseases, and is believed to be a direct response of the lungs to HIV. The clinical manifestations are recurrent infections, poor appetite, hepatomegaly and splenomegaly, and arrested development. The diagnostic imaging demonstrates no characteristic changes by CT scanning, with thickened bronchial wall, diffuse central lobular nodules or bronchiectasis, grid liked and cords liked shadows in uneven thickness. The pathological changes include accumulating lymphocytes and plasma cells that are mixed to infiltrate the pulmonary interstitium and expand to surrounding areas of the bronchi.
Toxoplasma Pneumonia
Toxoplasma pneumonia is caused by the infection of the intracellular parasite, Toxoplasma gondii. Ludlam et al. in 1963 firstly proposed the concept of pulmonary toxoplasmosis, which was believed to cause atypical pneumonia [107]. The clinical manifestations are cough and expectoration. In the serious cases, dyspnea and cyanosis can occur. In the chronic cases, there are long term low grade fever, cough, weight loss and enlarged lymph nodes. The diagnostic imaging demonstrates bronchopneumonia, interstitial pneumonia and pleurisy. (1) Bronchial pneumonia is also known as lobular pneumonia, with scattered patchy and blurry density shadows. (2) Interstitial pneumonia has typical manifestations of reticular and nodular shadows. (3) Pleurisy is rare, showing pleural effusion, limited diaphragmatic activity. The imaging demonstrations are non-specific, which can be defined in combination with the etiologic examinations. The pathological changes are congestion and edema of the surrounding connective tissue of the alveolar wall and bronchial walls, widened pulmonary interstitium, small quantity serous fibrin exudation from alveoli and pulmonary interstitium, and infiltration of macrophages and lymphocytes. Toxoplasma cysts and tachyzoites may be found in pulmonary interstitium and macrophages as well as alveolar epithelium.
HIV/AIDS Related Pulmonary Tumors
Kaposi’s Sarcoma
Kaposi’s sarcoma, a vascular tumor, was discovered in 1872, and is also known as multiple hemorrhagic sarcomas, multiple vascular sarcomas, or multiple pigmented sarcomas. Kaposi’s sarcoma is believed to be the defining tumor of AIDS. Outbreak of KS occurred in male homosexuals in Europe and the United States. Data show that in about 30 % Caucasian homosexuals, Kaposi’s sarcoma is a major complication of in HIV/AIDS patients. It has been confirmed that, though Kaposi’s sarcoma has strong invasion, the disease itself has little impact on the mortality of AIDS. The cause of death in majority of the patients is still opportunistic infections. The clinical manifestations include face and neck lesions in dark red to purple red plaques. The plaques do not fade away when pressed, with surrounding brown ecchymosis. It commonly involves multiple organs including lungs, spleen, oral cavity, lymph nodes, gastrointestinal tract and liver. The lungs are the major target of invasion. The diagnostic imaging demonstrates hilar lymphadenectasis and its surrounding nodular infiltration, bilateral interstitial changes, and pleural effusion that are its typical X-ray demonstration. Early pathological changes are similar to those of common angioma; with gathering of capillaries into groups containing histocytes engulfed hemosiderin and orderly arranged vascular endothelial cells. It further progression see active proliferation of endothelial cells and fibroblasts, increased nuclear mitosis with anaplasia, and scattered lymphocytes and histocytes between blood vessels. In the advanced stage, occlusion and necrosis of the vascular lumen can be found. Irregular lumen and fissures of the new capillaries can be commonly found in the tumor, filled with blood and common hemorrhage. In China, KS is relatively rare. Its definitive diagnosis can be made by pathological examination.
Other HIV/AIDS Related Malignancies
Other HIV/AIDS related malignancies include Burkitt’s lymphoma, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma and lung cancer. In summary, HIV/AIDS related pulmonary infections are important diseases in the disease spectrum of HIV/AIDS imaging. The diagnostic imaging is irreplaceable examinations for pulmonary infections. Early prevention and correct diagnosis are the keys to improve the quality of life and prolong the lives of patients. The complexity and multiplicity of HIV/AIDS related pulmonary diseases present challenges for the clinicians. Firstly, HIV/AIDS related diseases should be optimally classified. Each type should has a disease spectrum, which can be used for exclusion in combination with immunological indices to make the diagnosis and differential diagnosis. The diagnosis of HIV/AIDS related pulmonary infections should be made in combination with case history and laboratory tests for targeting individualized diagnosis to serve clinical practice.
HIV/AIDS Related Pneumocystis Carnii Pneumonia (PCP)
Pneumocystis carinii (PC) pneumonia is caused by the opportunistic fungus, Pneumocystis carinii. The disease occurs in immunocompromised patients, mostly are HIV infected persons. In these patients, PCP is one of the manifestations of AIDS. The risk factors of PCP include HIV infection, primary immunodeficiency, premature birth, neoplasms, the use of immunosuppressant after organ transplantation and long-term use of high dose corticosteroids. Currently, HIV infection is the cause of the vast majority cases of PCP. The Pneumocystis carinii is re-classified as a fungus by genome, which is a widespread micro-organism. Immunocompromised people are susceptible to its infection. In the early 1980s, due to the limited knowledge about HIV/AIDS complicated by opportunistic infections, Pneumocystis carinii infection is rarely diagnosed. In the recent 15 years, since the worldwide prevalence of AIDS, Pneumocystis carinii infection is the most common and serious opportunistic infection in HIV infected patients. In North America, Pneumocystis carinii infection is listed as the defining disease of AIDS, with more than 85 % HIV infected patients can be infected by Pneumocystis carinii and their CD4 T cell count is usually lower than 100/μl. When PCP is diagnosed, the average CD4 T cell count is approximately 50/μl. The mortality rate of Pneumocystis carinii infection induced acute respiratory failure is higher than 80 %, which can be reduced to 50 % after systematic treatment. Pneumocystis carinii infection has a high recurrence rate, being more than 65 % within 18 months. Therefore, when the CD4 T cell count of HIV infected patients is lower than 100/μl, preventive treatment against Pneumocystis carinii infection should be administered.
Pathogens and Pathogenesis
The pathogen is the trophozoites and cysts produced by Pneumocystis carinii, principally living in the lungs. Pneumocystis carinii was used to being categorized as as protozoon, but recently, it is believed to be belonged to fungus according to its ultrastructure and Pneumocystis ribosomal RNA phylogenetic analysis. The main infection route of PCP is airborne transmission and reactivation of in vivo latent Pneumocystis carinii. Inflammatory and immune responses of the host include phagocytosis of Pneumocystis carinii by the alveolar macrophages, infiltration of lymphocytes in peribronchial and vascular area, proliferation of type II alveolar cells, local and systemic increase of antibody.
Pathophysiological Basis
By naked eyes observation, there are extensive and diffuse invasion of lungs, which is soft like waterlogged sponge and in milky white with black spots. The filled foamy substance in the alveoli and bronchioles is a mixture of necrotic fungus and immunoglobulin. The alveolar septum has infiltration of plasma cells and lymphocytes, resulting in thickened alveolar septa up to 5–20 times as the normal thickness that occupy 3/4 of the entire lung volume. The cysts are firstly located in the macrophage cytoplasm of the alveolar septa. Subsequently, the alveolar cells containing cysts sheds off into the alveolar space. After the rupture of the cystic wall, sporozoite is discharged to turn into free trophozoites, which gains its access into the alveolar space. The alveolar exudates include plasma cells, lymphocytes and histocytes (Fig. 17.1a–c).
Fig. 17.1.
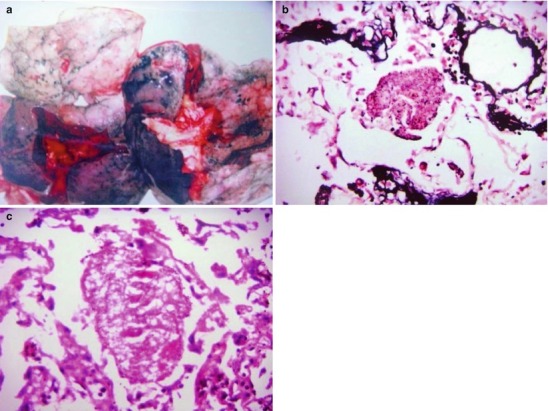
(a) Gross specimens’ observation demonstrates foamy liquid filling in the lung tissues. (b) HE demonstrates pneumocystis in the alveolar exudates, which can be stained black by silver methenamine staining, ×400. (c) HE demonstrates the foamy substance in the alveolar space, ×400
Clinical Symptoms and Signs
The clinical symptoms include dry cough, shortness of breath and an indoor hypoxia. About 95 % AIDS patients have multi-pathogens induced pulmonary infections. The most significant laboratory abnormality in most PCP patients is hypoxemia. Based on correlation between PCP and arterial oxygen partial difference, hypoxemia is divided into three degrees. The slight cases at indoor conditions have their PaO2 being above 70 mmHg, or alveolar-arterial oxygen pressure difference being less than 35 mmHg, or both. The moderate and severe cases have their PaO2 being usually less than 70 mmHg, or alveolar-arterial oxygen pressure difference being above 35 mmHg, or both. The most common manifestations of AIDS complicated by PCP are progressive subacute onset of dyspnea, fever, dry cough and chest distress, the symptoms aggravating in a few days or weeks. Pulmonary examination is usually negative in slight cases. As the disease aggravates, the cases show shortness of breath, cyanosis, tachycardia, and diffuse dry rales. Pneumocystis carinii infection accounts for 60–85 % of AIDS patients, which is one of the major causes of death in AIDS patients.
Examination Methods and Selections
Diagnostic Imaging
The diagnostic imaging examinations include chest X-ray, CT scanning and nuclear medicine examination. (1) Chest X-ray is the conventional examination for screening. Early lesions tend to be missed for the diagnosis due to the limited resolution or atypical lung lesions. (2) CT scanning with high resolution is superior to chest X-ray. (3) Nuclear medicine examination demonstrates increased uptake of the isotope-labeled monoclonal antibodies in the lung tissues of the PCP patients.
Etiological Examination
(1) By tracheal suction or lung tissue biopsy, the detection rate of Pneumocystis carinii is up to 90 %. By tissue section staining, abundant protozoa can be found in intra-alveolar foamy eosinophil substance mass (By methenamine silver nitrate staining, the dark brown round or oval shaped cysts can be found in a diameter of 6–8 μm out of the cells). (2) By ELISA, Pneumocystis IgG antibody can be detected and by latex particle agglutination test, the protozoa antigen can be detected. (3) Molecular biology techniques, such as PCR can be applied for early diagnosis.
Other Examinations
The following examinations are non-specific, but can be used to assess the severity of PCP and its progression. (1) By arterial blood gas analysis, the patients may show reduced blood oxygen saturation and respiratory alkalosis. (2) By serum enzyme spectrum analysis, the patients may show increased LDH. (3) It can be detected to have increased alveolar-arterial oxygen partial pressure difference.
Imaging Demonstrations
Chest X-ray
In the early stage (exudation period), alveolar fluids exudate, with diffuse granular shadows in the bilateral lung fields extend from the hilum to the surrounding. In the middle stage (infiltration and fusion period), the intrapulmonary lesions fuse, with ground glass liked or cloudy shadows that are bilaterally symmetric like butterfly wings. In the middle and advanced stages (parenchymal changes period), the lung tissues show parenchymal changes, with high density shadows and accompanying air bronchogram. The lung periphery shows stripes of transparent shadows. In the advanced stage (pulmonary fibrosis period), the pulmonary interstitium is thickened in dense cords liked appearance, with interval irregular patchy shadows. The pulmonary ventilation improves and the lung periphery shows dense parenchymal shadows with emphysema, pneumomediastinum and pneumothorax.
CT Scanning
In the early stage (exudation period), the lesions radiatus develop from the hilum to lung field. In the early stage, the diffuse exudative lesions distribute as pulmonary acinus, with changes similar to pulmonary interstitial changes. It was believed to be interstitial pneumonia. However, acute PCP is actually exudation of alveoli and spaces containing gas. The parenchymal changes are accompanied by infiltration of small quantity plasma cells, with demonstrations of spots and granular shadows with clear boundaries. In the middle stage (infiltration and fusion period), about 3–4 weeks later, the lesions fuse to show typical alveolar exudative lesions. The fused foci are demonstrated as non-specific infiltration shadows in ground glass liked appearance. In the middle and advanced stages (parenchymal changes period), about 5–6 weeks later, the intrapulmonary parenchymal changes show an obvious bronchus sign. In the advanced stage (pulmonary fibrosis period), about 7–8 weeks later, the lobular septa of both lungs are significantly thickened, with cords liked pulmonary fields, grid liked changes and decreased transparency. It can be complicated by pulmonary psuedocysts, with thin and clear cystic wall and with no liquid gas level.
In the Early Stage (Exudation Period)
Case Study 1
A male patient aged 34 years was confirmatively diagnosed as having AIDS by the CDC. He complained of dyspnea and wheezing for 3 days and his CD4 T cell count was 85/μl.
Fig. 17.2.
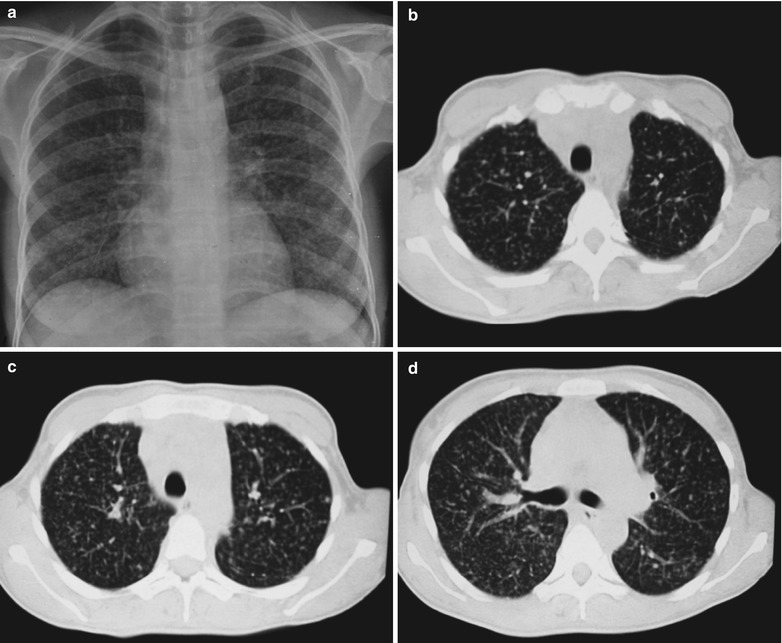
(a–f) HIV/AIDS related Pneumocystis carinii pneumonia. (a) DR demonstrates scattered miliary increased density shadows in both lungs, with even size, density and distribution. The shadows of both hila are dense, with sharp both costophrenic angles. (b–f) CT scanning demonstrates scattered miliary nodular shadows in both lungs, which is more obviously in the middle pulmonary strip and with quite even size and density. Trachea and bronchi are unblocked
Case Study 2
A male patient aged 31 years was confirmatively diagnosed as having AIDS by the CDC. He complained of dyspnea and wheezing for 1 week and his CD4 T cell count was 115/μl.
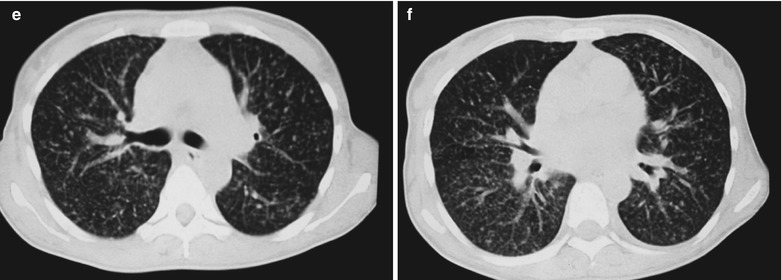
Fig. 17.3.
(a–e) HIV/AIDS related Pneumocystis carinii pneumonia. (a) DR demonstrates cloudy and scattered miliary increased density shadows in both lungs, with enlarged and thickened hilum of both lungs. (b–e) CT scanning demonstrates even miliary increased density shadows in the middle and upper lungs as well as the dorsal segment of the lower lung field, with some fused in thin cloudy shadows
In the Middle Stage (Infiltration and Fusion Period)
Case Study 3
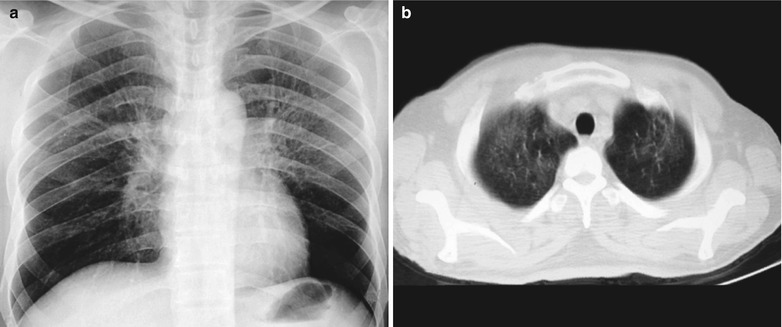
A male patient aged 38 years was confirmatively diagnosed as having AIDS by the CDC. He complained of dyspnea and wheezing for 20 days and his CD4 T cell count was 105/μl.
Fig. 17.4.
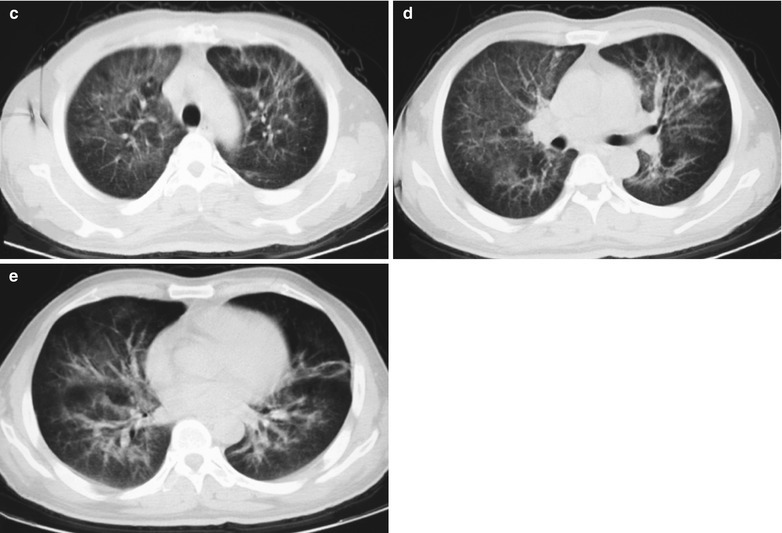
(a–e) HIV/AIDS related Pneumocystis carinii pneumonia. (a) DR demonstrates cloudy or ground glass liked increased density shadows in both lungs, with enlarged and thickened hilum of both lungs. (b–e) CT scanning demonstrates even miliary increased density shadows in the middle and upper lungs as well as the dorsal segment of lower lungs, with some fused into thin cloudy ground glass liked shadows with increased density, with decreased transparency of both lungs and enlarged hilar shadows in both lungs
Case Study 4
A male patient aged 41 years was confirmatively diagnosed as having AIDS by the CDC. He complained of chest distress, dyspnea and wheezing for 1 month and his CD4 T cell count was 104/μl.
Fig. 17.5.
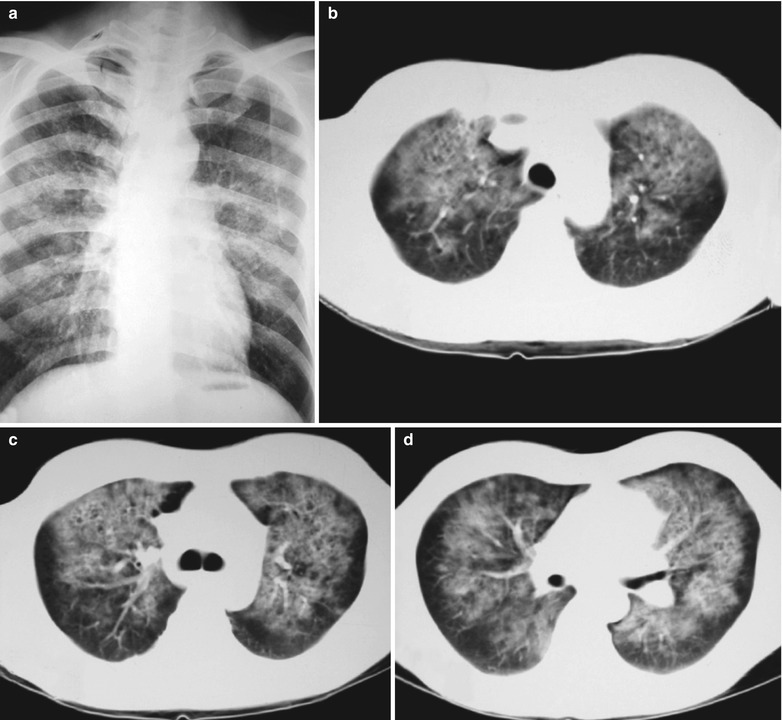
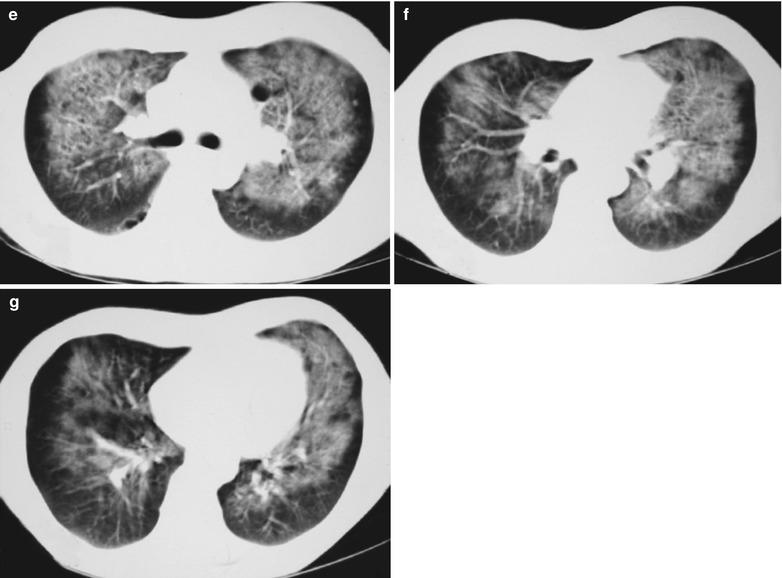
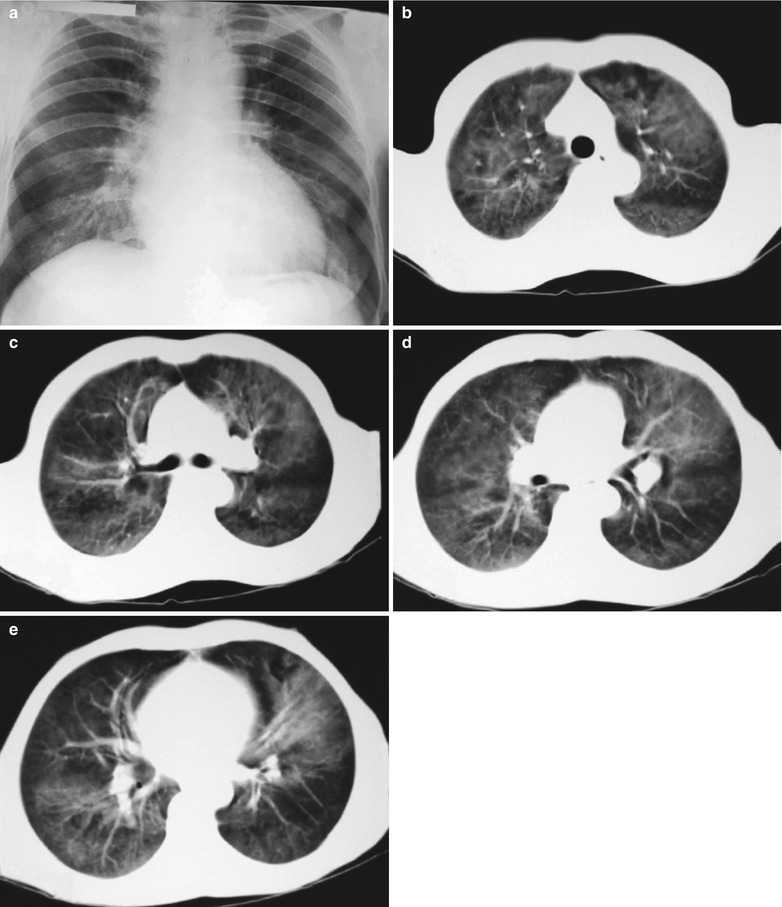
(a–g) HIV/AIDS related Pneumocystis carinii pneumonia. (a) DR demonstrates patchy shadows with increased density in both lungs, with thickened hilar shadows in both lungs. (b–g) CT scanning demonstrates flaky ground glass liked density shadows in upper lungs and dorsal segment of both lungs, which is more obvious in the middle inner strips. There are extrapulmonary stripes transparent shadows, with some bronchial walls thickened and enlarged hilar shadows in both lungs
Case Study 5
A female patient aged 31 years was confirmatively diagnosed as having AIDS by the CDC. She complained of chest distress, dyspnea and wheezing for 1 week and her CD4 T cell count was 115/μl.
Fig. 17.6.
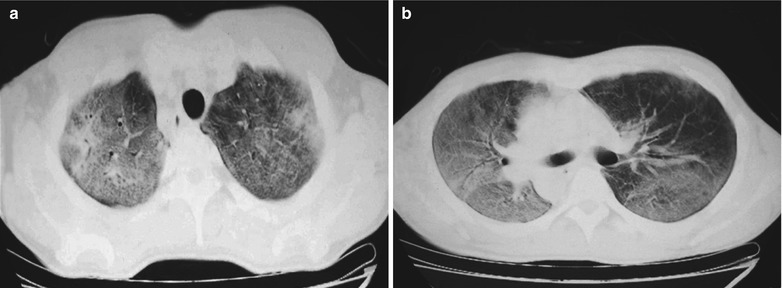
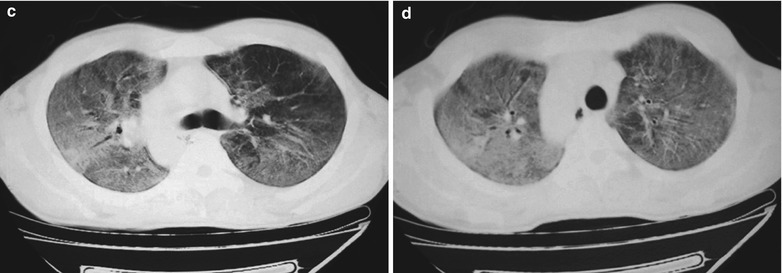
(a-d) HIV/AIDS related Pneumocystis carinii pneumonia. (a–d) CT scanning demonstrates flaky ground glass liked density shadows in upper lobes of both lungs, with bronchial shadows in them; flaky parenchymal shadows in the subpleural apical segment; and thickened bronchial walls in the anterior and posterior segments of the right upper lobe
Case Study 6
A male patient aged 31 years was confirmatively diagnosed as having AIDS by the CDC. He complained of chest pain, chest distress, dyspnea and wheezing for 2 weeks and his CD4 T cell count was 85/μl.
Fig. 17.7.
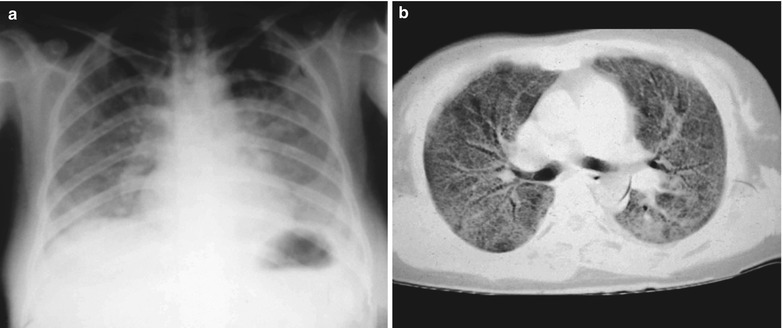
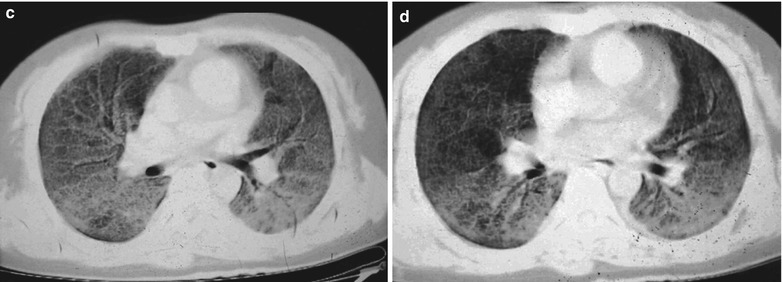
(a–d) HIV/AIDS related Pneumocystis carinii pneumonia. (a) DR demonstrates diffusely distributed shadows with increased density in both lungs that is more obvious in the middle and lower lungs. The hilar shadows in both lungs are enlarged. Both diaphragmatic surfaces and phrenic angles are blurry. (b–d) CT scanning demonstrates flaky shadows with increased density in both lungs, with parenchymal shadows in the lingular segment of left upper lobe and in the dorsal segments of both lower lobes and bronchial shadows in them. There are also thickened bronchial walls and enlarged hilar shadows in both lungs
In the Middle-Advanced Stage (Parenchymal Changes Period)
Case Study 7
A male patient aged 31 years was confirmatively diagnosed as having AIDS by the CDC. He complained of dyspnea, cyanosis and wheezing for 3 weeks, with obviously decreased oxygen saturation. His CD4 T cell count was 45/μl.
Fig. 17.8.
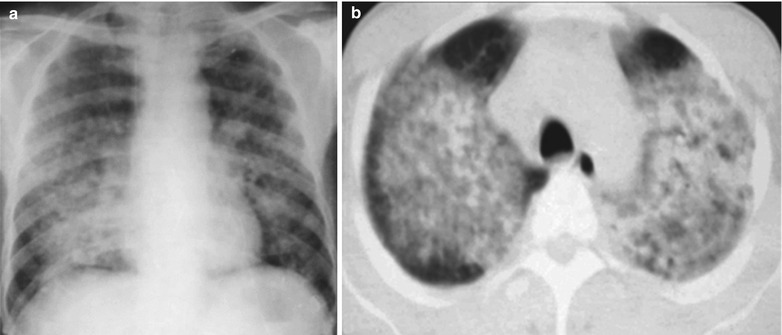
(a–g) HIV/AIDS related Pneumocystis carinii pneumonia. (a) DR demonstrates large flaky parenchyma shadows in both lungs which is more obvious in the middle and lower lobes of both lungs. There are also enlarged hilar shadows in both lungs and sharp both costophrenic angles. (b–g) CT scanning demonstrates large flaky parenchyma shadows in concentric and symmetrical distribution, bronchial shadows in them and thickened bronchial walls
Case Study 8
A male patient aged 31 years was confirmatively diagnosed as having AIDS by the CDC. He complained of dyspnea, cyanosis and wheezing for 4 weeks, with obviously decreased oxygen saturation. His CD4 T cell count was 45/μl.
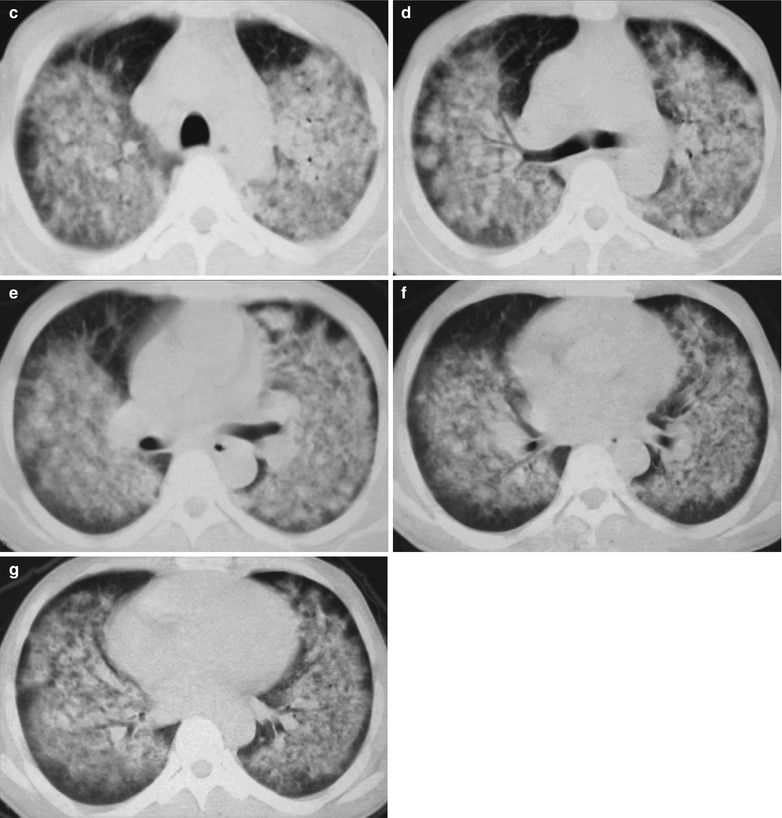
Fig. 17.9.
(a–d) HIV/AIDS related Pneumocystis carinii pneumonia. (a–d) CT scanning demonstrates large flaky parenchyma shadows in both lungs, with transparent areas in some foci. The trachea and bronchi are unblocked, with thickened bronchial walls in the middle and lower lobes
Case Study 9
A male patient aged 43 years was confirmatively diagnosed as having AIDS by the CDC. He complained of dyspnea, cyanosis and wheezing for 4 weeks, with obviously decreased oxygen saturation. His CD4 T cell count was 45/μl.
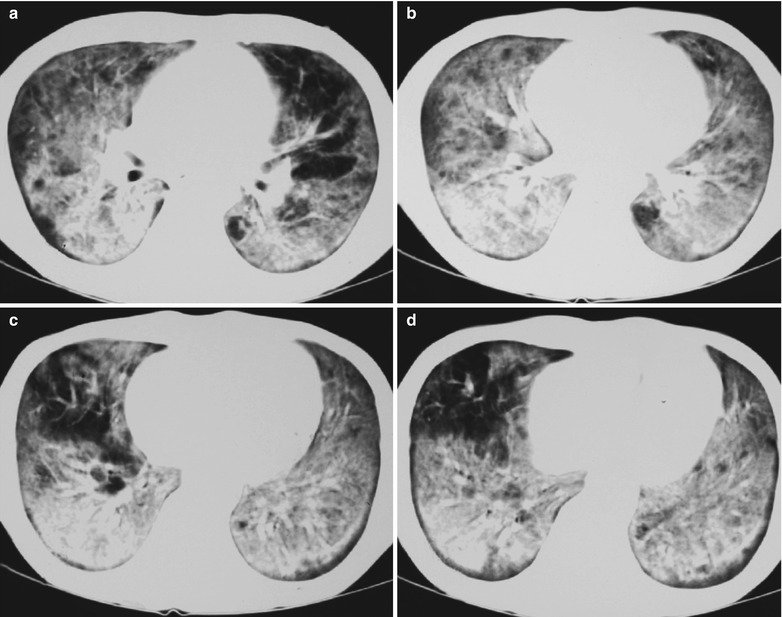
Fig. 17.10.
(a–d) HIV/AIDS related Pneumocystis carinii pneumonia. (a–d) CT scanning demonstrates large flaky and mass liked parenchyma shadows in both lungs which is more obvious in the right lung, bronchial shadows in them, and thickened bronchial walls in the middle lobe of the right lung
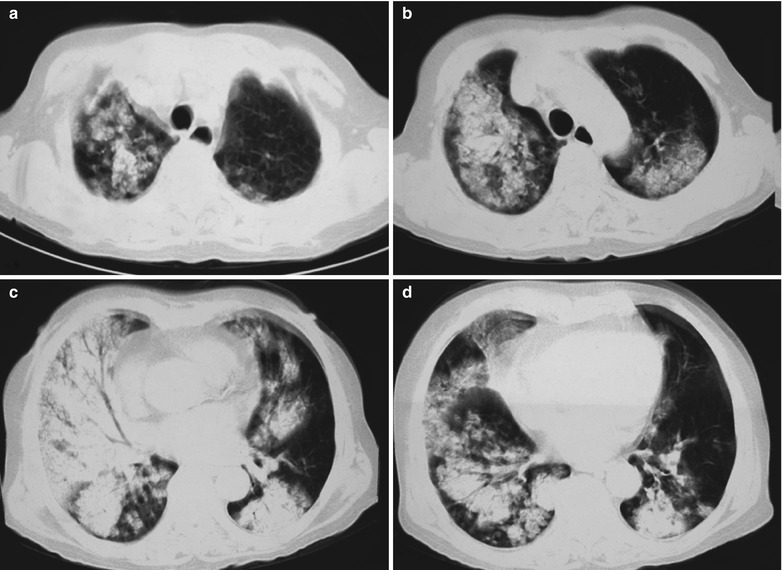
In the Absorption Period
Case Study 10
A male patient aged 31 years was confirmatively diagnosed as having AIDS by the CDC. He complained of dyspnea, cyanosis and wheezing for 7 weeks, with obviously decreased oxygen saturation. His CD4 T cell count was 75/μl.
Fig. 17.11.
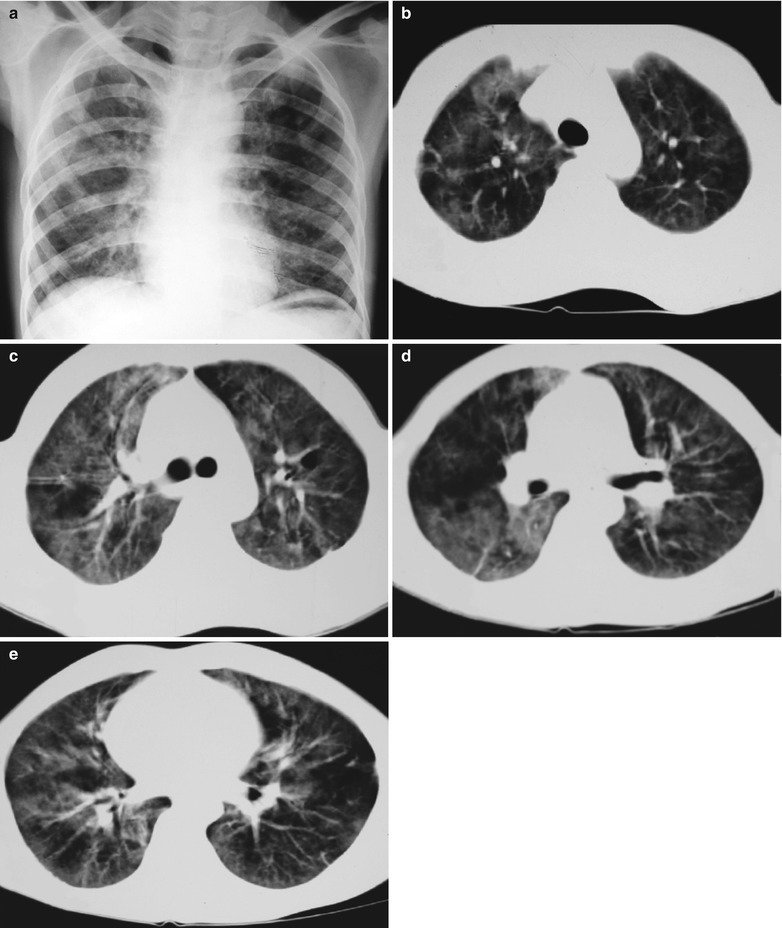
(a–e) HIV/AIDS related Pneumocystis carinii pneumonia. (a) DR demonstrates scattered patchy shadows with increased density in both lungs and a few cords liked shadows which are more obvious in the right lung. There are thickened both hilar shadows and sharp both costophrenic angles. (b–e) CT scanning demonstrates flaky and mass liked ground glass density shadows in both lungs and a few cords liked shadows which are more obvious in the right lung. The trachea and bronchi are unblocked
Case Study 11
A female patient aged 51 years was confirmatively diagnosed as having AIDS by the CDC. She complained of dyspnea, cyanosis and wheezing for 8 weeks, with obviously decreased oxygen saturation. Her CD4 T cell count was 65/ μl.
Fig. 17.12.
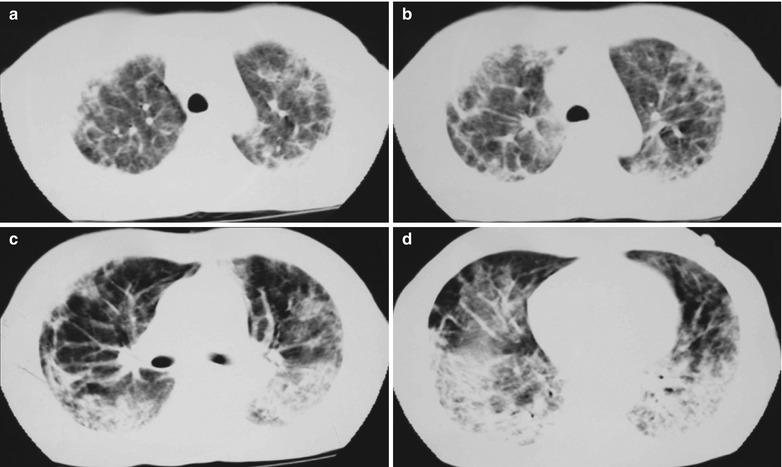
(a–d) HIV/AIDS related Pneumocystis carinii pneumonia. (a–d) CT scanning demonstrates multiple patchy parenchyma shadows and fibrous cords liked shadows in both lungs which are more obvious in the dorsal segment of both lower lungs, bronchial shadows in them, and thickened bronchial walls in the middle lobe. The hilar shadows in both lungs are enlarged
Case Study 12
A female patient aged 37 years was confirmatively diagnosed as having AIDS by the CDC. She complained of dyspnea, cyanosis and wheezing for 8 weeks, with obviously decreased oxygen saturation. Her CD4 T cell count was 45/ μl.
Fig. 17.13.
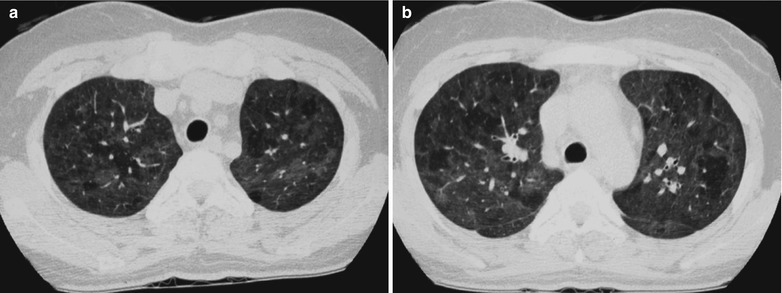
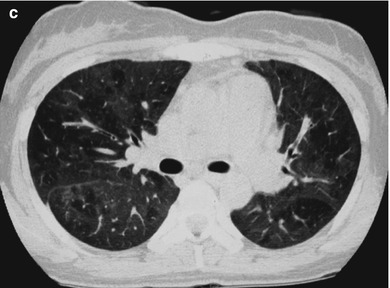
(a–c) HIV/AIDS related Pneumocystis carinii pneumonia. (a–c) CT scanning demonstrates multiple ground glass liked density shadows in both lungs, transparent areas in them and unblocked trachea and bronchi
Case Study 13
A male patient aged 41 years was confirmatively diagnosed as having AIDS by the CDC. He complained of dyspnea, cyanosis and wheezing for 8 weeks. His CD4 T cell count was 45/μl.
Fig. 17.14.
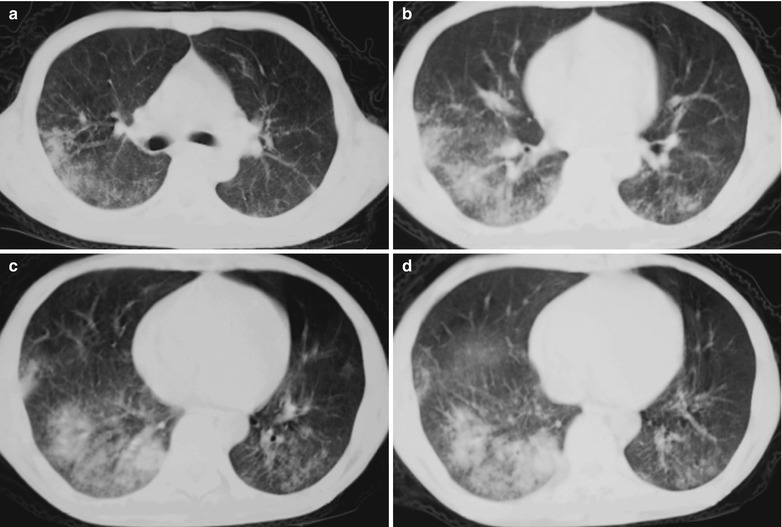
(a–d) HIV/AIDS related Pneumocystis carinii pneumonia. (a–d) CT scanning demonstrates multiple ground glass liked density shadows in both lungs, mass and flakes of parenchymal shadows in the posterior segment of the right upper lobe and in the dorsal segment of both lower lobes which is more obvious in the right lung, and bronchial shadows in them
In the Advanced Stage (Pulmonary Fibrosis Period)
Case Study 14
A female patient aged 31 years was confirmatively diagnosed as having AIDS by the CDC. She complained of dyspnea, cyanosis and wheezing for 5 weeks, with obviously decreased oxygen saturation. Her CD4 T cell count was 45/ μl.
Fig. 17.15.
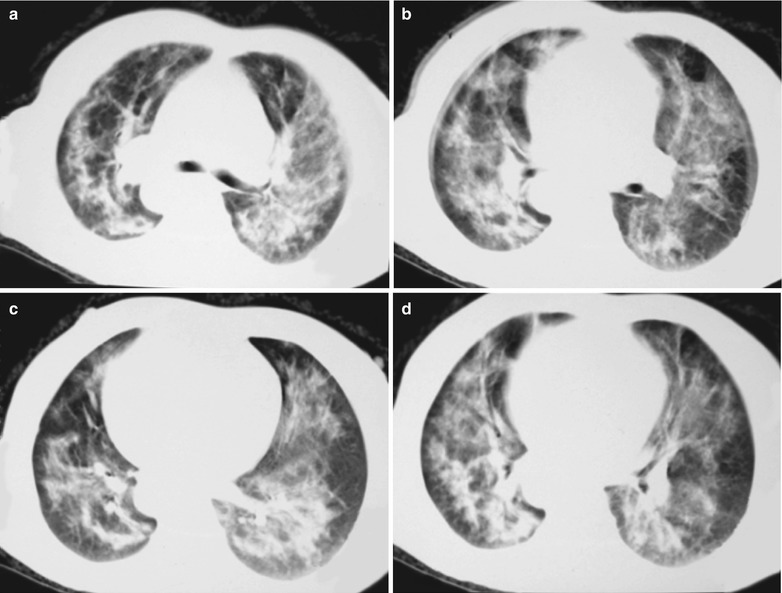
(a–d) HIV/AIDS related Pneumocystis carinii pneumonia. (a–d) CT scanning demonstrates multiple patchy blurry shadows and fibrous cords liked shadows in both lungs which are more obvious in the middle inner parts of both lungs, with transparent areas in them. The bronchial walls are thickened
Case Study 15
An AIDS patient was confirmatively diagnosed by the CDC. He sustained Pneumocystis carinii pneumonia.
Fig. 17.16.
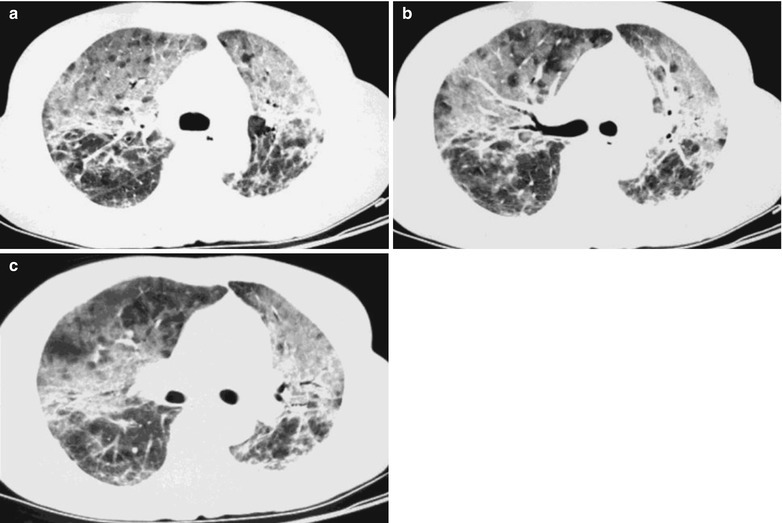
(a–c) HIV/AIDS related Pneumocystis carinii pneumonia. (a–c) CT scanning demonstrates multiple fibrous cords liked shadows in lungs, multiple patchy parenchyma shadows and ground glass liked density shadows in both upper lobes, with multiple transparent areas in them. The bronchial walls are thickened in the anterior and posterior segments of the right upper lobe as well as in the lingual segment of the left lung
Case Study 16
A female patient aged 30 years was confirmatively diagnosed as having AIDS by the CDC. She complained of dyspnea, cyanosis and wheezing, with obviously decreased oxygen saturation. Her CD4 T cell count was 3/μl.
Fig. 17.17.
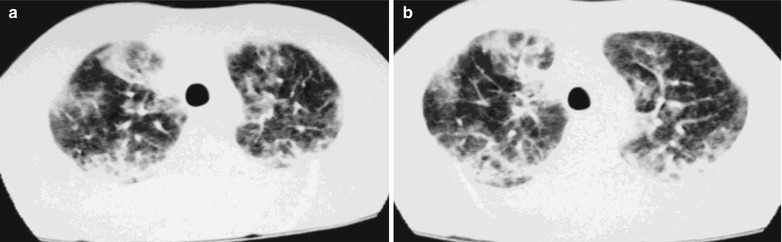
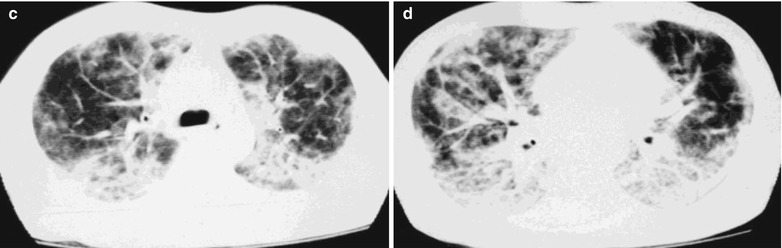
(a–d) HIV/AIDS related Pneumocystis carinii pneumonia. (a–d) CT scanning demonstrates multiple patchy parenchymal shadows and fibrous cords liked shadows in both lungs which are more obvious in both lower lungs. The trachea and bronchi are unblocked, with enlarged hilar shadows in both lungs
Case Study 17
A female patient aged 38 years was confirmatively diagnosed as having AIDS by the CDC. She complained of dyspnea, cyanosis and wheezing, with obviously decreased oxygen saturation. Her CD4 T cell count was 5/μl.
Fig. 17.18.
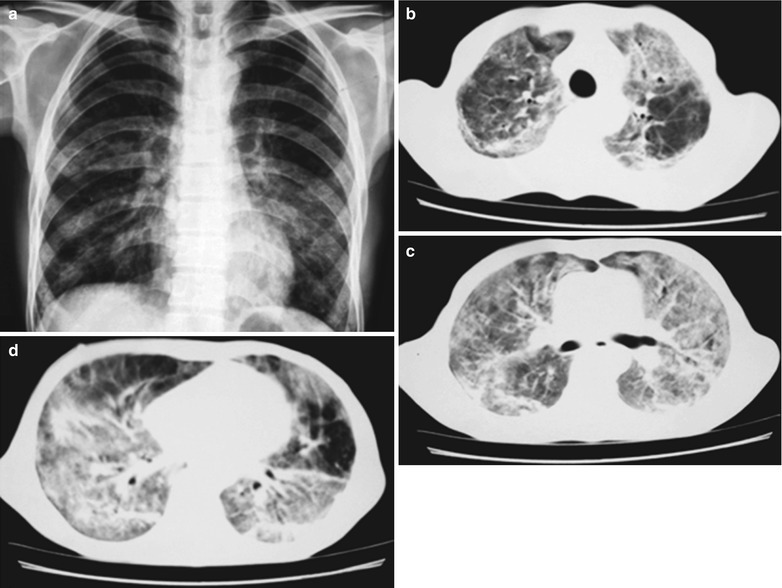
(a–d) HIV/AIDS related Pneumocystis carinii pneumonia. (a) DR demonstrates multiple patchy shadows with increased density in both lungs which are more obvious in both middle and lower lungs. The hilar shadows in both lungs are enlarged, with sharp both costophrenic angles. (b–d) CT scanning demonstrates multiple patchy and mass liked parenchyma shadows in both lungs, ground glass density shadows in the apical segment of both upper lobes, transparent areas in the medial segment of the right middle lobe as well as in the lingual segment of the left upper lobe, and unobstructed trachea and bronchi
Diagnostic Basis
Case History
Patients with acquired immunodeficiency.
Clinical Symptoms
The early symptoms include fever, dry cough and shortness of breath. The advanced symptoms are serious dyspnea, cyanosis, progressive hypoxemia and respiratory failure. By pulmonary examinations, scattered dry and moist rales can be heard.
Bronchoalveolar Lavage (BAL)
Trophozoites of Pneumocystis cysts can be found by liquid Giemsa staining.
Biopsy or Autopsy for Pathological Examination
Slight and moderate interstitial inflammation responses mainly involve lymphocytes and alveolar macrophages. The detection of cysts containing sporozoites is the basis to define the diagnosis.
Imaging Demonstrations
Chest X-ray
Chest X-ray demonstrations of PCP can be classified into four types. (1) Early pulmonary interstitial infiltration and diffuse miliary alveolar exudation; (2) In the middle stage, there are alveolar exudates, with fusion and parenchymal changes; (3) In the middle-advanced stage, diffuse parenchymal changes; (4) Pulmonary interstitial fibrosis and lung cavity or lung bulla, as well as pneumothorax and emphysema.
CT Scanning with High Resolution
For the cases with negative or atypical findings by chest X-ray, CT scanning with high resolution should be performed. CT scanning demonstrates early lesions of multiple symmetric diffuse miliary nodal shadows, which have clear boundaries. In the middle stage, there are thin cloudy shadows or ground glass liked density shadows. In the middle-advanced stage, the lung tissues show parenchymal shadows, with trachea-bronchial sign. In the outer strip of the lung, a transparent area in shape of willow leaf can be demonstrated. In the advanced stage, fibrous cords liked shadows are demonstrated some lung tissues with compensatory emphysema and even pulmonary pseudocysts.
Nuclear Medicine Examinations
The intake of the isotope-labeled monoclonal antibody by lung tissues of PCP patients increases.
Differential Diagnosis
HIV/AIDS related PCP should be differentiated from bacterial pneumonia, pulmonary tuberculosis, viral pneumonia, fungal pneumonia, ARDS, and lymphocytic interstitial pneumonia (LIP).
Bacterial Pneumonia
Bacterial pneumonia has more focal lesions but less diffuse lesions.
Pulmonary Mycobacterium Tuberculosis Infection
Pulmonary mycobacterium tuberculosis infection has manifestations of military pulmonary tuberculosis by chest X-ray, which is difficult to be differentiated from early PCP. HIV/AIDS related PCP shows miliary nodules, which further fuse into cloudy or ground glass liked shadows or parenchymal changes. The lesions are commonly symmetrical, with the hilus as the center. The clinical manifestations include fever, dry cough or accompanying difficulty breathing, and even cyanosis. But in the cases of pulmonary Mycobacterium tuberculosis infections, most show miliary nodules, which further fuse into large nodules or mass. After about 2 weeks treatment in the early stage, the military nodules in both lungs can be absent, with common clinical symptom of high fever. Correlation studies of miliary tuberculosis and peripheral blood CD4 T cell count have demonstrated that the general incidence of miliary tuberculosis is low, only 6–9 %, but it is the main manifestation of HIV/AIDS related pulmonary miliary tuberculosis. Generally, when CD4 T cell count is below 200/μl, the incidence of cavity lesions is 29 %, non-cavity lesions 58 %, complicated by pleural effusion 11 % and lymphadenectasis 20 %. When CD4 T cell count is between 200 and 390/μl, the incidence of cavity lesions and non-cavity lesions each accounts for 44 %, complicated pleural effusion 11 % and lymphadenectasis 14 %. When CD4 T cell count above 400/μl, the manifestation is commonly pneumonia type, in flaky shadows or parenchymal shadows in just one pulmonary segment. The incidence of cavity lesions is 63 %, non-cavity lesions 33 %, complicated by pleural effusion 3 % and no lymphadenectasis.
Cytomegalovirus Pneumonia
Chest X-ray demonstrates cytomegalovirus pneumonia negative in 1/3 patients. The foci are commonly bilateral, with reticular particles in 33 % patients, alveolar foci in 22 % patients, nodular foci in 11 % patients, complicated by cavity in 11 % patients, cysts in 6 % patients, pleural effusion in 33 % patients and lymphadenectasis in 11 % patients.
Cryptococcus Neoformans Pneumonia
The incidence of diffuse foci in the cases of cryptococcus neoformans pneumonia is 76 %, interstitial foci or mixed foci 76 %, alveolar foci 19 %, nodular foci 5 %, lymphadenectasis 11 % and pleural effusion 5 %.
Lymphoid Interstitial Pneumonia
HIV/AIDS related PCP is more likely to occur in children with AIDS, which presents difficulty for its differentiation form lymphoid interstitial pneumonia. However, lymphoid interstitial pneumonia commonly has a chronic onset, with commonly manifestations of cough and dry rales. Systemic lymphadenectasis and enlargement of salivary glands can also be found. By lung tissues biopsy, EBV-DNA1 can be detected, which provides basis for their differentiation.
Discussion
Pneumocystis, a unicellular organism, is the pathogen of Pneumocystis carinii pneumonia. Pneumocystis carinii pneumonia is one of common opportunistic infections in AIDS patients, which is also the leading cause of death in AIDS patients. In the initial episode of PCP, most patients have a CD4 T cell count of less than 100/μl. Diagnostic imaging demonstrates bilaterally symmetrical ground glass liked shadows, which can be diffusely distributed and tend to mainly involve the periphery of the hilus or the middle and lower lung fields. HRCT scanning is commonly applied to assess early PCP that is demonstrated negative by chest X-ray. HRCT scanning demonstrates bilaterally symmetric patchy or fused ground glass liked shadows. The pathological basis of ground glass liked shadows and parenchymal areas reflect that the acinus is filled by the foamy exudates, which are composed of surface active substances, cellulose and cell debris. All of the ground glass liked shadows, overlapping septa and the intralobular linear shadows are in gravel road liked manifestation. The septa and intralobular linear shadows demonstrate pulmonary interstitial edema or cellular infiltration.
In the middle-advanced stage of PCP, there are manifestations of small pulmonary nodules, pulmonary parenchymal changes, thickened interlobular septa, intralobular linear shadows, mass like lesions, pleural effusion, and lymphadenectasis. The cysts tend to mainly involve the upper lobes, which can be unilateral or bilateral pulmonary cysts, pneumothorax, mild or severe interstitial fibrosis and traction bronchiectasis. HRCT scanning demonstrations of PCP are non-specific. Its diagnosis should be in combination with HIVPH13 and etiological examinations.
HIV/AIDS Related Pulmonary Bacterial Infections
HIV/AIDS Related Tuberculosis
Pathogen and Pathogenesis
Mycobacterium tuberculosis is still an important pathogen for pulmonary infection in HIV positive patients. Since the mid-1980s, the main cause of the increasing incidence of tuberculosis is the prevalence of HIV infection. The incidence of tuberculosis in AIDS patients is 200–500 times higher than the general population. HIV infection is the most dangerous factor for progression of latent tuberculosis into active tuberculosis. Tubercle bacillus belongs to Mycobacterium family of Mycobacterium genus, which is divided into types of human, bovine and murine. The main cause of human tuberculosis is human Mycobacterium tuberculosis, which is known as acid-fast bacilli. Tubercle bacillus wall is the complex containing high molecular weight fatty acids, lipids, proteins and polysaccharides, which are related to its pathogenicity and immune responses. Lipid can cause the infiltration of human monocytes, epithelial cells and lymphocytes to form tuberculous nodules. Its protein contents can cause allergic reactions, and infiltration of neutrophils and mononuclear cells. Polysaccharides participate in certain immune responses (such as agglutination). These pathogenic factors lay the foundation for the occurrence of tuberculosis in AIDS patients.
Pathophysiological Basis
Human immunity, allergic responses as well as the number and pathogenicity of tubercle bacilli are closely related to the quality, range, spreading rate and the progression of tuberculosis. Its pathological changes are characterized by exudation, infiltration, proliferation and hyperplasia, degenerative necrosis (caseous necrosis) and cavity formation.
Exudation Based Lesions
The manifestations include congestion, edema and infiltration of leukocytes. The exudative lesions occur in early stage of tuberculosis inflammation or when the lesions deteriorate. It can also be found in the serosa tuberculosis. There is neutrophilic granulocytes in the exudative lesions, which are gradually substituted by monocytes (phagocytes). The engulfed tubercle bacilli can be found in the large mononuclear cells. The exudative lesions are absorbed and dissipated through the phagocytosis of the mononuclear-phagocyte system, even with no scar.
Proliferation Based Lesions
When large mononuclear cells engulf and digest tubercle bacilli, the phospholipid of the bacteria render the large mononuclear cells to enlarge and be flat, similar to epithelial cells, which is known as epithelioid cells. These epithelioid cells gather into groups, with central Langhans giant cells that pass the messages of the bacteria antigens to lymphocytes. Surrounding the Langhans giant cells, there are often many lymphocytes to form typical tuberculous nodules, which are characteristic lesions of tuberculosis. This is why it is called Tuberculosis. In the tuberculous nodules, tubercule bacilli are usually undetectable. Proliferation based lesions often occur in the cases with less bacteria invasion and when human cells mediated immunity is predominant.
Degeneration Based Lesions (Caseous Necrosis)
Degeneration often occurs on the basis of the exudative or proliferative lesions. Tubercle bacilli overcome macrophages and then continually proliferate in large quantity. After the cells become cloudy and swelling, the foci show fatty degeneration, dissolved into fragments, until the occurrence of necrosis. After the death of inflammatory cells, proteolytic enzymes are released to dissolve the tissues that results in necrosis, which is coagulative necrosis. By naked eyes observation, they are yellowish gray, with loose and brittle quality like caseous. Therefore it is known as caseous necrosis. Microscopic examination demonstrates an area of solid and Eosin staining red necrotic tissues with no tuberculosis.
Results of Tuberculosis
Tubercle bacilli in the foci of caseous necrosis proliferate in large quantity to cause liquefaction, which is related to infiltration of neutrophile granulocytes and large monocytes. Part of liquefied caseous necrotic substances can be absorbed and part can be discharged by the bronchus to form cavities. Otherwise, it may cause intrapulmonary spreading along with bronchi. The small caseous necrosis or proliferative lesions can be shrunk and absorbed after treatment, with only residues of slight fibrous scars. Due to the compromised immunity in AIDS patients, the lesions rarely show fiber tissues proliferation, but form cords liked scar. Calcification rarely occurs.
Spread and Deterioration of Tuberculosis Lesions
If the necrotic lesions erode the blood vessels, tubercle bacilli can cause systemic miliary tuberculosis along with blood flow, including brain, bones and kidneys. Large quantity sputum containing tubercle bacilli gains its access into the gastrointestinal tract. It can also cause intestinal tuberculosis and peritoneal tuberculosis. Pulmonary tuberculosis can cause tuberculosis pleurisy via direct spreading to the pleura (Fig. 17.19a–c).
Fig. 17.19.
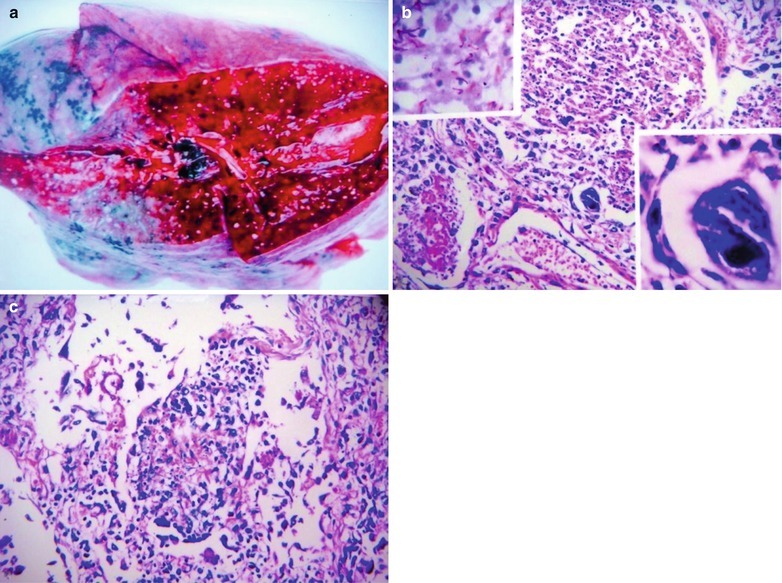
(a) Gross observation in autopsy demonstrates disseminated pulmonary tuberculosis, with grayish white military nodules in diffuse distribution in the lung tissues section. (b) It is demonstrated that mycobacteriumavium-intracellularcomplex infection in the lung tissue, with atypical tuberculosis nodules and acid-fast staining positive (left top). There are a subnodular giant cell, eosinophilic inclusion bodies in the nucleus and bradyzoites in cytoplasm of T. gondii. HE × 100. (c) HE demonstrates mycobacteriumavium-intracellularcomplex infection, with atypical tuberculosis nodular changes, HE × 200
Clinical Symptoms and Signs
Clinically, it is a chronic progression, with rare acute onset. The clinical symptoms are commonly systemic, with fever and fatigue. The respiratory symptoms include cough and hemoptysis. Pulmonary TB can be divided into primary and secondary, with the initial episode commonly being primary (type I). The residual bacteria after primary infection can cause secondary infection (type II-IV) when the immunity is compromised via spreading along blood flow or direct spreading.
Primary Tuberculosis (Type I)
It is common in HIV positive children. Most cases are asymptomatic, sometimes with symptoms of low grade fever, mild cough, sweating, rapid heartbeat, and poor appetite.
Hematogenous Disseminated Pulmonary Tuberculosis (Type II)
HIV/AIDS related miliary tuberculosis is one of the major manifestations of pulmonary tuberculosis, which is more common. The onset of acute miliary tuberculosis is rapid, with symptoms of chills and high fever with a body temperature up to 40 °C, mostly remittent fever or continuous fever. There may be decreased leukocytes count and accelerated sedimentation rate. The progression of subacute and chronic hematogenous disseminated pulmonary tuberculosis is relatively slow.
Infiltrative Pulmonary Tuberculosis (Type III)
Infiltrative pulmonary tuberculosis in AIDS patients commonly occurs in both middle and lower lung fields, with flaky and flocculent foci or parenchymal changes in lobes or segments. Caseous lesions are rare. The early stage of infiltrative pulmonary tuberculosis is commonly asymptomatic, with later occurrence of fever, cough, night sweating, chest pain, weight loss, expectoration and hemoptysis.
Chronic Fibrous Cavity Pulmonary Tuberculosis (Type IV)
This type of pulmonary TB rarely occurs in AIDS patients. In non-AIDS patients, chest X-ray demonstrates three major changes, namely cavity, fibrosis, and bronchial dissemination. In the AIDS patients, the pulmonary manifestations include single or multiple nodular shadows with clear boundaries.
Tuberculous Pleuritis
Tuberculous pleuritis is an exudative inflammation caused by the direct invasion of tubercle bacillus from the primary lesion near the pleura into the pleura, or hematogenous dissemination via the lymphatic vessels to the pleura. The routes for occurrence of tuberculous pleurisy include: (1) The bacteria in the hilar lymph tuberculosis counterflow to the pleura along lymph vessels. (2) TB lesions adjacent to pleura rupture to cause direct access of the tubercle bacilli or products of tuberculosis infection into the pleural cavity. (3) Acute or subacute hematogenous disseminated tuberculosis causes pleuritis. (4) Due to the increased allergic responses, the pleura highly respond to tuberculosis toxins to cause exudation. (5) Thoracic tuberculosis and rib tuberculosis rupture into the pleural cavity. Clinically, pleuritis can be divided into three types, dry pleuritis, exudative pleuritis and tuberculous empyema (rare). The common clinical manifestations are fever, cough with accompanying chest pain of the affected side and shortness of breath.
Examinations and Their Selection
Sputum Tuberculin Test
(1) Sputum smear examination is simple to manipulate, with high accuracy rate. The findings of the tubercle bacilli can define the diagnosis. It still is the golden criteria for the diagnosis of pulmonary tuberculosis. (2) Sputum tubercle bacilli culture has high reliability. Tubercle bacilli drug sensitivity test can be performed but requires 6–8 weeks to obtain the results. Therefore, its application is limited.
Immunological Diagnosis of Pulmonary TB
(1) Tuberculin purified protein derivative (PPD) test is commonly used. Its positive result is one of the evidence confirming a past history of TB infection. (2) BACTEC test can be performed to detect the metabolites of mycobacterium tuberculosis. Generally, mycobacterium can be detected in 2 weeks. The quantity of mycobacteria can affect the period required for test results. (3) PCR has poor specificity but high sensitivity of up to 98–100 %.
Thoracoscopy and Mediastinoscopy
Both can be applied to observe the enlarged lymph nodes in the chest and mediastinum. In addition, they can be applied to obtain specimens for biopsy, which facilitates the diagnosis and differential diagnosis.
Diagnostic Imaging
Diagnostic imaging examinations include chest X-ray and CT scanning. Chest X-ray can demonstrate the location, quality and range of the lesions. It can also help to assess the therapeutic efficacy. CT scanning can demonstrate small or hidden lesions, with a high resolution.
Imaging Demonstrations
Primary Tuberculosis
Primary pulmonary tuberculosis, also known as primary syndrome, is rare in adult AIDS patients. Chest X-ray demonstrates intrapulmonary patchy or large flaky parenchymal changes, hilar and mediastinal lymphadenectasis in connection to irregular cords liked shadows (located between intrapulmonary lesion and the hilum). Lymph node tuberculosis is demonstrated to have mediastinal lymphadenectasis that sometimes fuse into mass. In AIDS patients, simple mediastinal lymph node tuberculosis is more common than primary syndrome.
Hematogenous Disseminated Pulmonary Tuberculosis
(1) The acute cases are demonstrated to have diffused miliary nodules in both lungs with even distribution, even size and even density. (2) The subacute and chronic cases are demonstrated to have nodules in both lungs, with uneven distribution, uneven size and uneven density. Sometimes calcification occurs in the nodules, with fibrous cords and thickened pleura.
Secondary Pulmonary Tuberculosis
Infiltrative pulmonary tuberculosis are demonstrated to have patchy parenchymal changes in the middle and lower lung fields as well as parenchymal changes, cavities and fibrous cords liked foci in the segments and lobes. It can also occur in the upper lung fields, commonly with accompanying mediastinal and hilar lymph node tuberculosis.
Chronic Fibrous Cavity Pulmonary Tuberculosis
It commonly occurs in the advanced stage of AIDS,, with manifestations of pulmonary interstitial fibrosis and formation of cavities. This type of pulmonary tuberculosis is less common.
Tuberculous Pleuritis
It rarely occurs, mostly in the early stage of AIDS. It is rare in the middle and advanced stages of AIDS. Dry pleuritis has manifestations of blunt costophrenic angle and limited diaphragm mobility. Exudative pleuritis is manifested as small quantity pleural effusion and thickened pleura, commonly with encapsulated effusion of the lateral pleura. Calcification is rare.
HIV/AIDS Related Lymph Node Tuberculosis
Case Study 1
A male patient aged 28 years was confirmatively diagnosed as having AIDS by the CDC. He complained of dull chest pain, dyspnea, fever, night sweating, fatigue and anorexia. His CD4 T cell count was 65/μl.
Fig. 17.20.
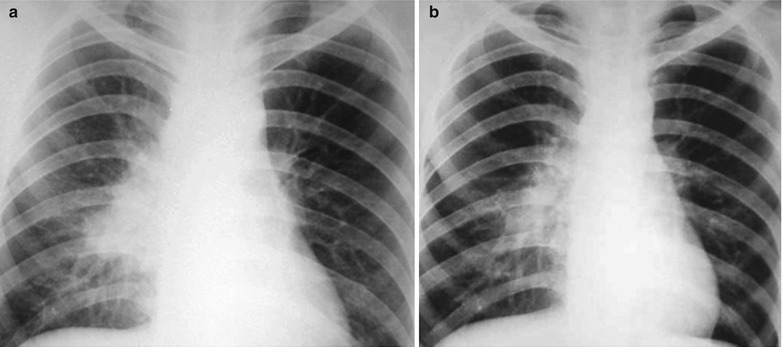
(a, b) HIV/AIDS related lymph node tuberculosis. (a) DR demonstrates enlarged right hilum in nodular dense shadows with peripheral thickened and blurry pulmonary markings, and no obvious abnormalities of the left hilum. (b) DR demonstrates smaller right hilum after treatment for 1 month
Case Study 2
A male patient aged 37 years was confirmatively diagnosed as having AIDS by the CDC. He complained of dull chest pain, dyspnea, fever, night sweating and fatigue. His CD4 T cell count was 65/μl.
Fig. 17.21.
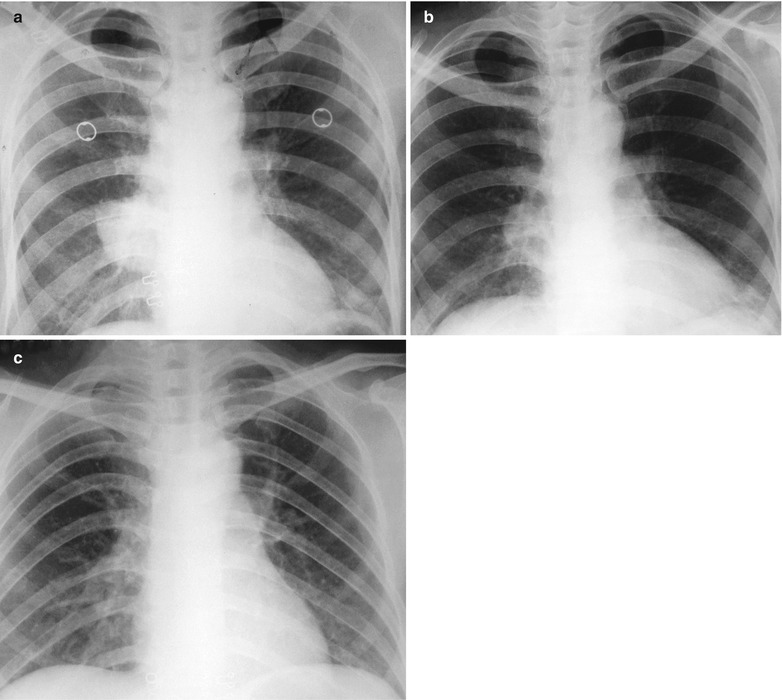
(a–c) HIV/AIDS related lymph node tuberculosis. (a) DR demonstrates enlarged right hilum in mass liked dense shadow, with peripheral thickened and blurry pulmonary markings, and no obvious abnormalities of the left hilum. (b) DR demonstrates smaller right hilum after anti-tuberculosis therapy for 1 month. (c) DR demonstrates absent tumor in the right hilum and normal left hilum
Case Study 3
A male patient aged 48 years was confirmatively diagnosed as having AIDS by the CDC. He complained of dull chest pain, dyspnea, fever, night sweating, fatigue and anorexia. His CD4 T cell count was 45/μl.
Fig. 17.22.
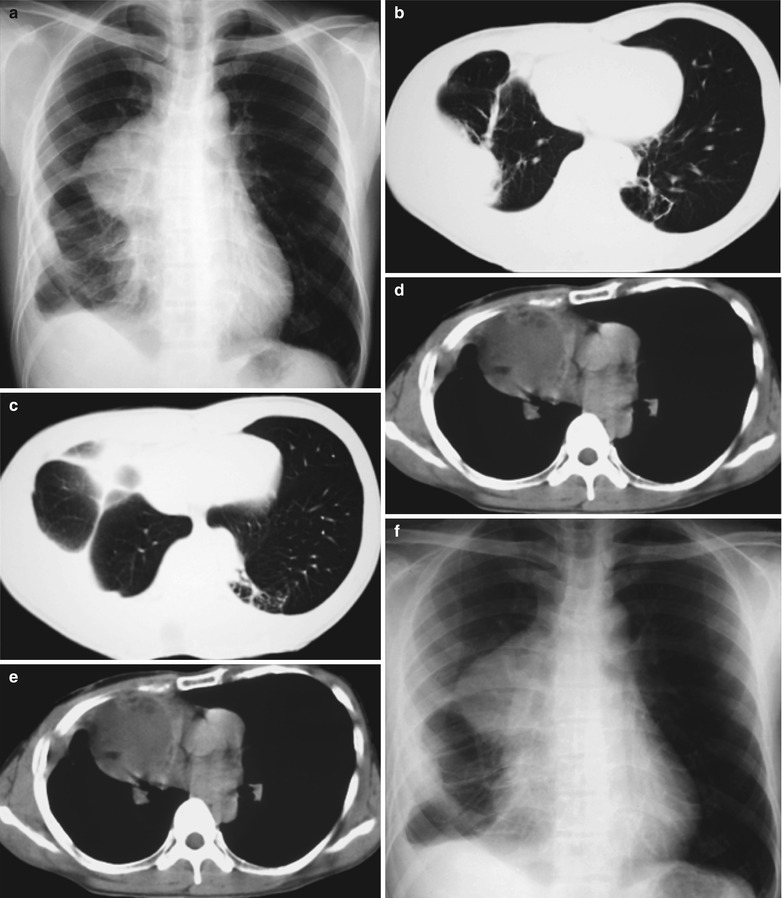
(a–f) HIV/AIDS related lymph node tuberculosis. (a) DR demonstrates semicircular mass liked dense shadow in the right hilum that protrudes to the lung field with peripheral thickened and blurry pulmonary markings, thickened pleura of lateral chest wall, and blunt costophrenic angle in Jan. 2008. (b–e) CT scanning demonstrates narrowed right thorax, thickend pleura of lateral chest wall with encapsulated effusion, uneven density mass in the right hilum, thinner right bronchus due to compression and no obvious abnormalities in the left hilum. (f) DR in Aug. 2008 demonstrates no obvious changes of the lesions after anti-tuberculosis treatment for 1 month
Case Study 4
A female patient aged 36 years was confirmatively diagnosed as having AIDS by the CDC. She complained of dull chest pain, dyspnea, fever, night sweating, fatigue and anorexia. Her CD4 T cell count was 55/μl.
Fig. 17.23.
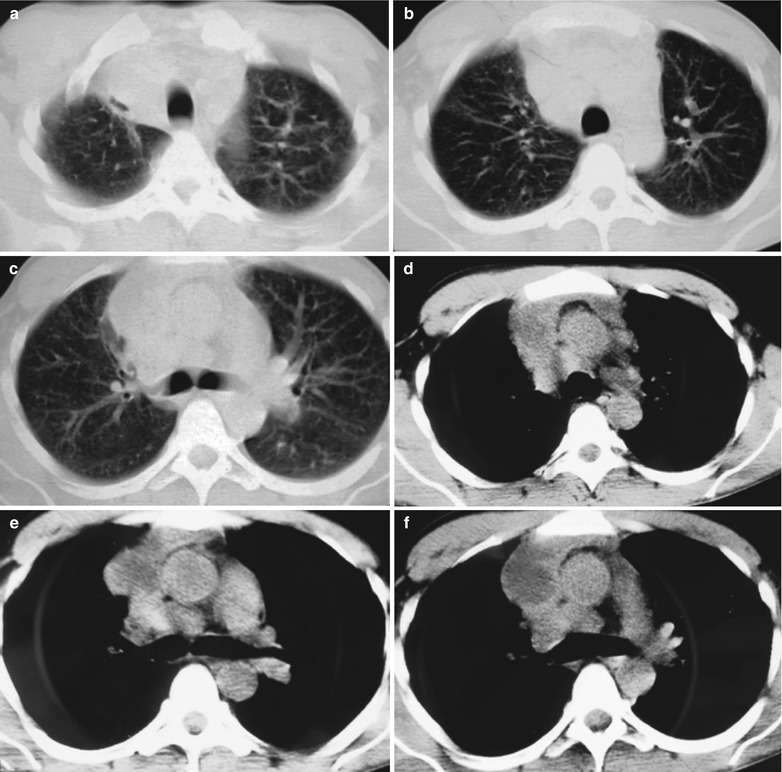
(a–f) HIV/AIDS related lymph node tuberculosis. (a–c) CT scanning of the pulmonary window demonstrates dense mass shadow beside the right aortic arch, and thinner right bronchus due to compression. (d–f) CT scanning of the mediastinal window demonstrates low density mass shadow besied the right aortic arch with clear boundary
Case Study 5
A female patient aged 46 years was confirmatively diagnosed as having AIDS by the CDC. She complained of dull chest pain, dyspnea, fever and fatigue. Her CD4 T cell count was 35/μl.
Fig. 17.24.
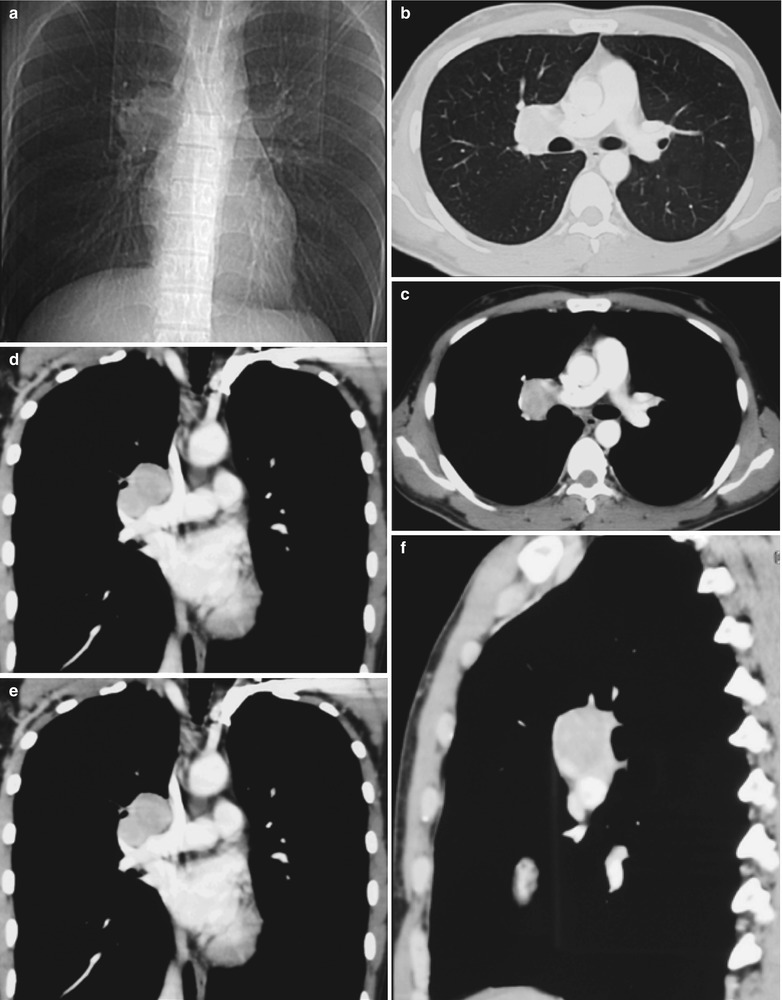
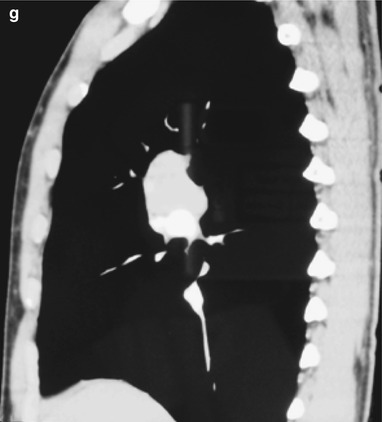
(a–g) HIV/AIDS related lymph node tuberculosis. (a) DR demonstrates circular mass dense shadow in the right hilum that protrudes to the lung field with peripheral thickened and blurry pulmonary markings, thickened pleura in the lateral chest cavity and blunt costophrenic angle in Jan. 2008. (b–e) CT scanning demonstrates multiple uneven mass density shadows in right hilum in a size of about 3 × 3.5 × 3.8 cm. (f–g) Enhanced CT scanning demonstrates slight uneven enhancement of the lesion and no obvious abnormalties in the left hilum
Case Study 6
A female patient aged 36 years was confirmatively diagnosed as having AIDS by the CDC. She complained of dull chest pain, dyspnea, fever, night sweating and fatigue. Her CD4 T cell count was 85/μl.
Fig. 17.25.
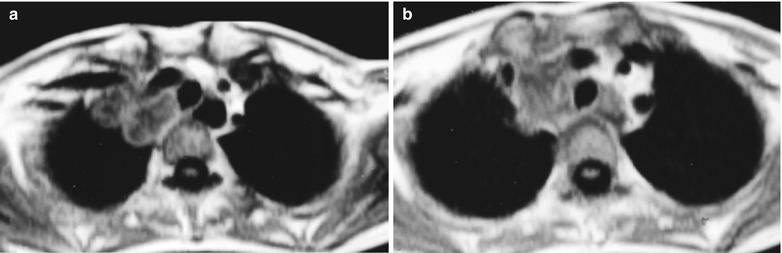
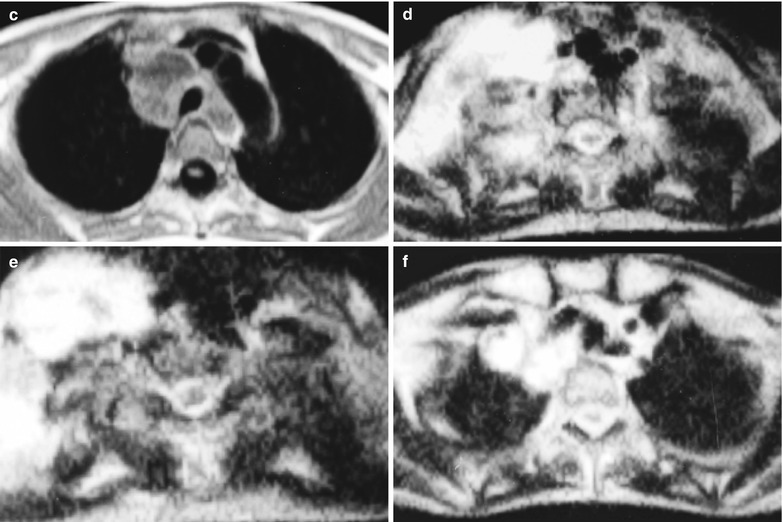
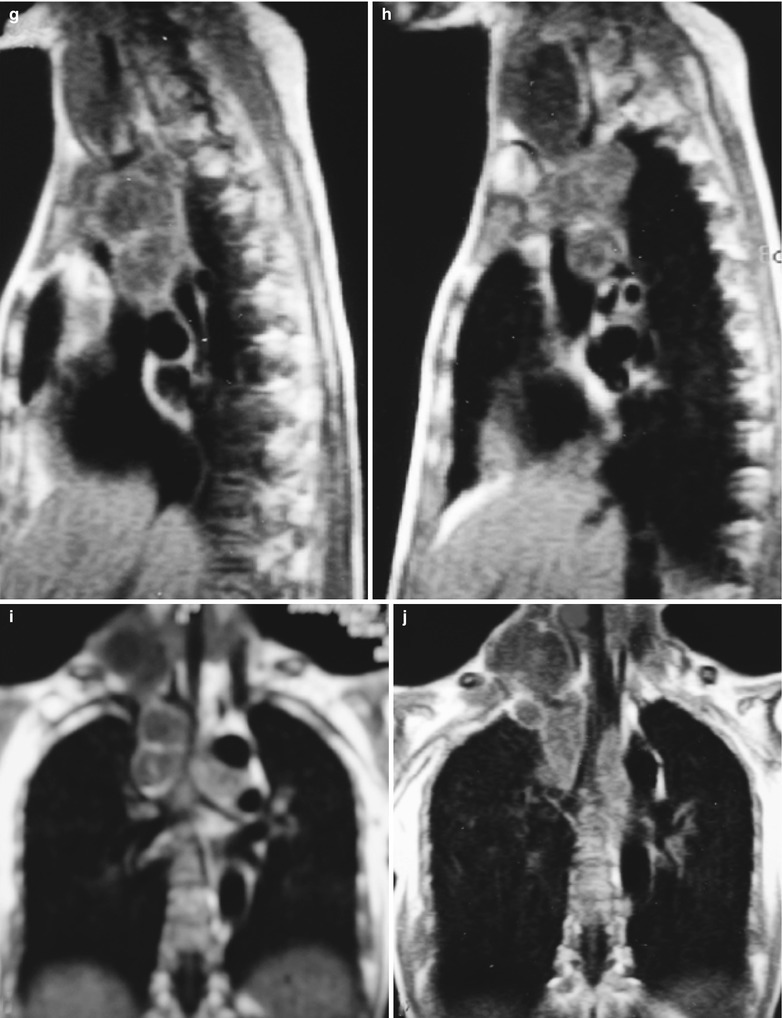
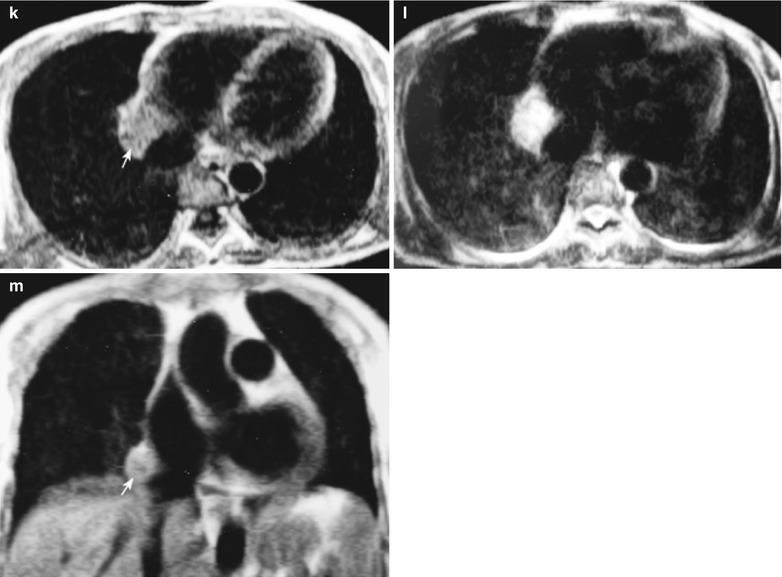
(a–m) HIV/AIDS related lymph node tuberculosis. (a–f) MR imaging demonstrates multiple round liked long T1WI long T2WI signal adjacent to the right sternocleidomastoid as well as in the supraclavicular fossa and the right upper mediastinum. (g–j) Sagittal MR imaging demonstrates multiple round liked sugar-coated haws liked masses in the entrance of the thorax and right upper mediastinum, with central long T1WI signal and unclear boundary. (k, l) Axial MR imaging demonstrates round liked long T1WI long T2WI signal in the right cardiophrenic angle (arrow). (m) Coronal MR imaging demonstrates round liked equal signal shadow
Miliary Tuberculosis
Case Study 1
A male patient aged 41 years was confirmatively diagnosed as having AIDS by the CDC. He was infected HIV via blood transfusion, with complaints of cervical lymph node tuberculosis, ascites and abdominal infection, fungal stomatitis, biliary stones with infection and severe anemia. His CD4 T cell count was 33/μl.
Fig. 17.26.
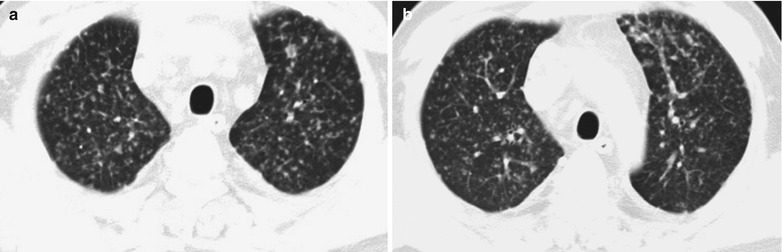
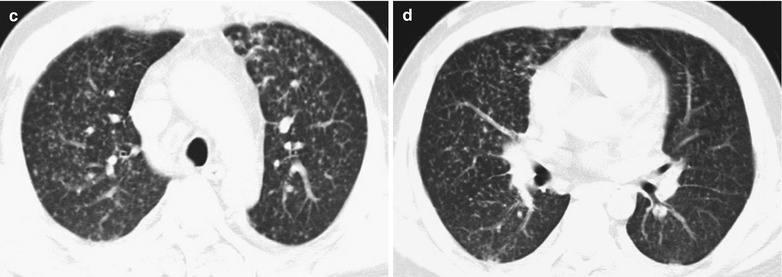
(a–d) HIV/AIDS related miliary tuberculosis. (a–d) CT demonstrates diffuse scattering miliary shadows with increased density in both lungs, which are bilaterally symmetric as well as in even size and distribution
Case Study 2
A female patient aged 39 years was confirmatively diagnosed as having AIDS by the CDC. She complained of fever and night sweating. Her CD4 T cell count was 73/μl.
Fig. 17.27.
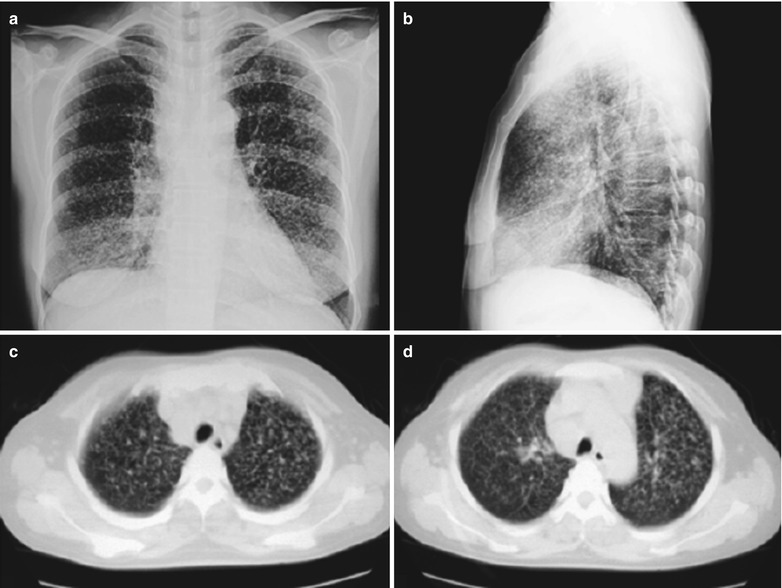
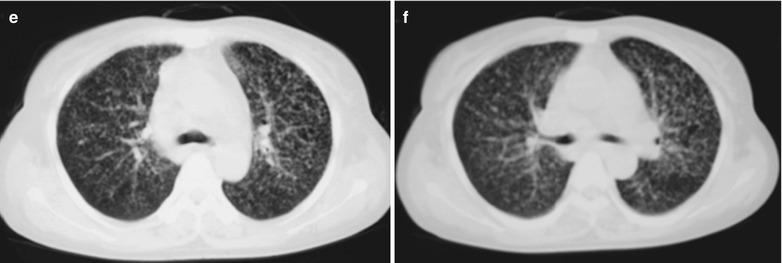
(a–f) HIV/AIDS related miliary tuberculosis. (a–b) Anteroposterior and lateral DR demonstrates diffuse miliary shadows in both lungs, which are bilaterally symmetric and in even size and distribution. (c–f) CT demonstrates diffuse scattering miliary shadows with increased density in both lungs, which are bilaterally symmetric as well as in even size and distribution
Case Study 3
A female patient aged 42 years was confirmatively diagnosed as having AIDS by the CDC. In the recent 1 month, she sustained chills, fever, aggravating night sweating and diarrhea with watery stools for five to six times daily and no bloody mucopurulent stool as well as obvious weight loss. By physical examinations on admission, T 35.6 °C, P 80/min, R 20/min, BP 90/60 mmHg; by laboratory tests, AST 83 u/l, ALB 26.7 g/L, GLO 36.4 g/L, GGT 233u/L, ALP 438 u/L; electrolytes normal; WBC 1.4/L, LYM% 12.1 %, NEuT% 84.4 %; blood sedimentation 20 mm/h; RPR-Ab Weak positive. Her CD4 T cell count was 53/μl.
Fig. 17.28.
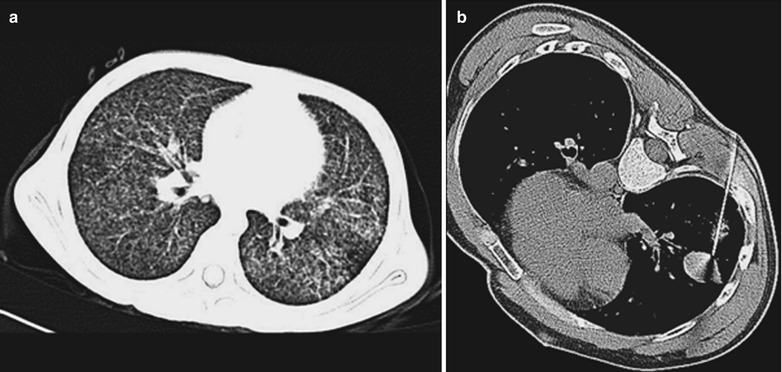
(a–b) HIV/AIDS related miliary tuberculosis. (a) CT scanning demonstrates diffuse scattering miliary shadows with increased density in both lungs, which are bilaterally symmetric and in even size and distribution. (b) CT scanning of the mediastinal window demonstrates round liked nodular shadows in the right lower lung. CT guided puncture for biopsy is performed to define the diagnosis
Case Study 4
A female patient aged 46 years was confirmatively diagnosed as having AIDS by the CDC. She complained of chills, fever and night sweating for 1 week. Her CD4 T cell count was 23/μl.
Fig. 17.29.
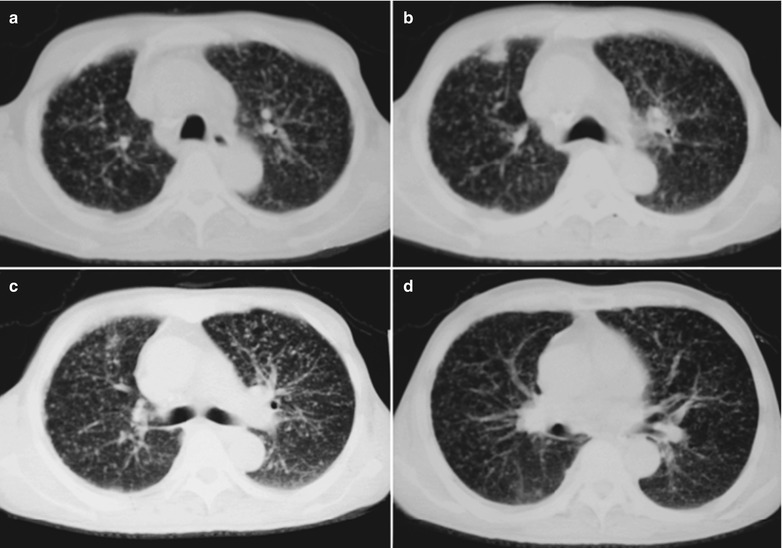
(a–d) HIV/AIDS related miliary tuberculosis. (a–d) CT scanning demonstrates diffuse scattering miliary shadows with increased density in both lungs, which are bilaterally symmetric and in even size and distribution. There are nodular shadows in the anterior segment of the right middle lung lobe with clear boundary
Case Study 5
A female patient aged 32 years was confirmatively diagnosed as having AIDS by the CDC. She complained of chill and fever for 2 weeks. Her CD4 T cell count was 63/μl.
Fig. 17.30.
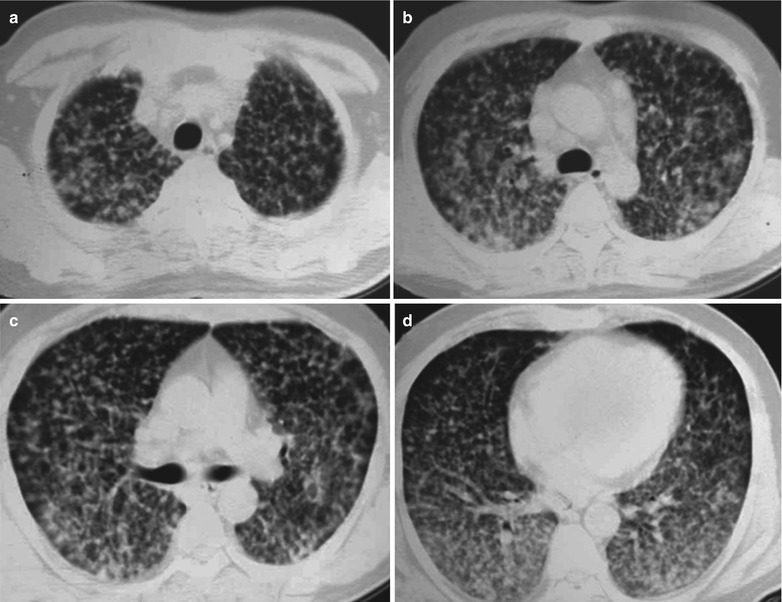
(a–d) HIV/AIDS related miliary tuberculosis. (a–d) CT scanning demonstrates diffuse scattering miliary shadows with increased density in both lungs and fusion of some military shadows, which are bilaterally symmetric and in even size and distribution
Case Study 6
A male patient aged 37 years was confirmatively diagnosed as having AIDS by the CDC. He had a history of blood transfusion, with complaints of finding HIV infected for 4 years, intermittent cough and fever for 6 months. His CD4 T cell count was 50/μl.
Fig. 17.31.
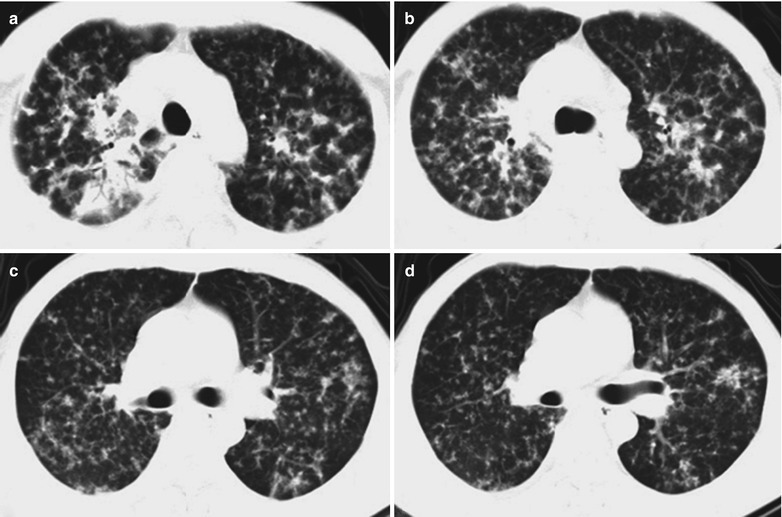
(a–d) HIV/AIDS related miliary tuberculosis. (a–d) CT scanning demonstrates diffuse scattering miliary shadows with increased density in both lungs, fusion of some military shadows into patchy or mass liked shadow, diffusely distributed lung lesions
Fusion Period of Miliary Pulmonary TB
Case Study 7
A male patient aged 47 years was confirmatively diagnosed as having AIDS by the CDC. He had a history of blood transfusion, with complaints of finding HIV infected for 6 years, intermittent cough and fever for 6 months. His CD4 T cell count was 10/μl.
Fig. 17.32.
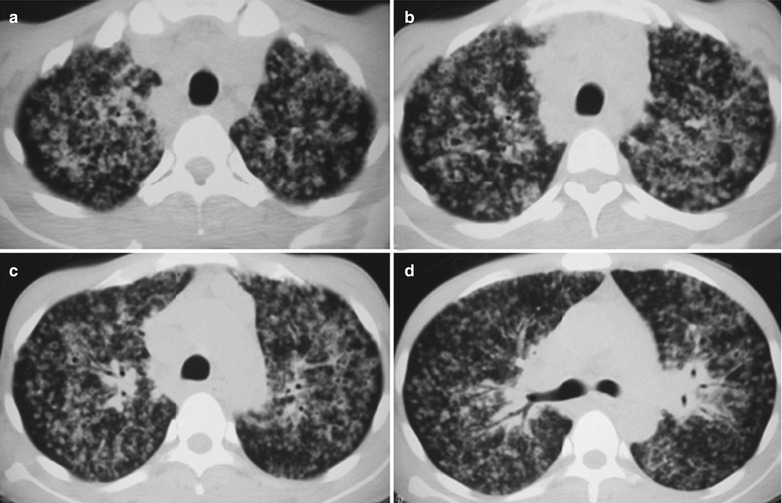
(a–d) HIV/AIDS related miliary tuberculosis. (a–d) CT demonstratesdiffuse scattering miliary shadows with increased density in both lungs, fusion of some military shadows into patchy or mass liked shadows and diffusely distributed lung lesions
Infiltrative Pulmonary Tuberculosis
Case Study 1
A male patient aged 41 years was confirmatively diagnosed as having AIDS by the CDC. He sustained HIV infection for 7 years, with chief complaints of cough and dyspnea. His CD4 T cell count was 14/μl.
Fig. 17.33.
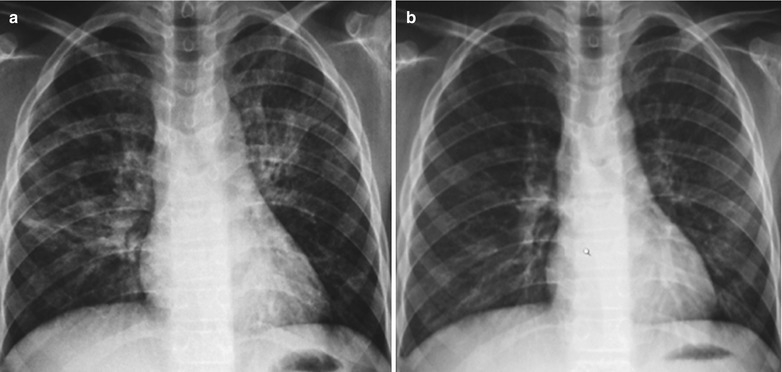
(a, b) HIV/AIDS related infiltrative pulmonary tuberculosis. (a) DR demonstrates scattering patchy and cords liked blurry density shadows in the right middle and upper lung fields as well as enlarged and thickened hilum. (b) DR demonstrates that the lungs lesions are almost absent compared to (a), after anti-TB treatment for 5 months
Case Study 2
A female patient aged 31 years was confirmatively diagnosed as having AIDS by the CDC. She sustained HIV infection for 8 years, with chief complaints of cough and dyspnea. Her CD4 T cell count was 34/μl.
Fig. 17.34.
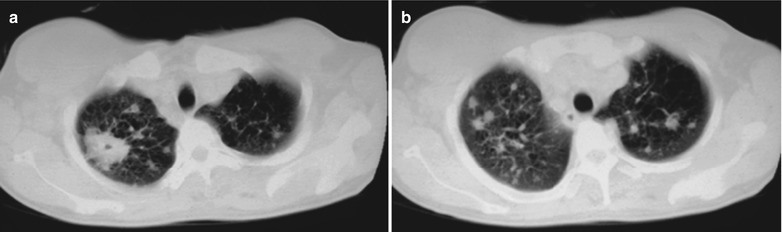
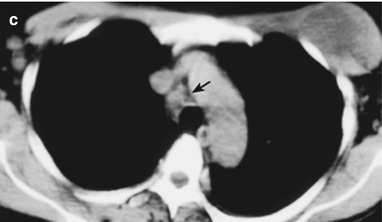
(a–c) HIV/AIDS related infiltrative pulmonary tuberculosis. (a, b) CT scanning of the pulmonary window demonstrates patchy shadows with increased density in the right upper lung and multiple satellite lesions scattering around. (c) CT scanning of the mediastinal window demonstrates lymphadenectasis of aortic window, subcutaneous soft tissue mass shadow in the left anterior chest wall with central low density shadow as well as right axilliary lymphadenectasis
Case Study 3
A male patient aged 41 years was confirmatively diagnosed as having AIDS by the CDC. He sustained HIV infection for 7 years, with chief complaints of cough and dyspnea. His CD4 T cell count was 14/μl.
Fig. 17.35.
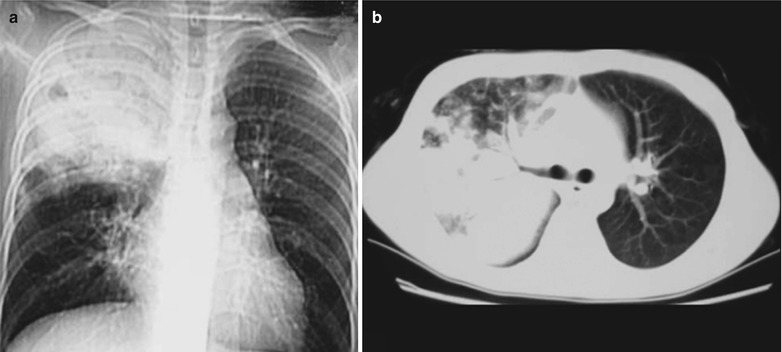
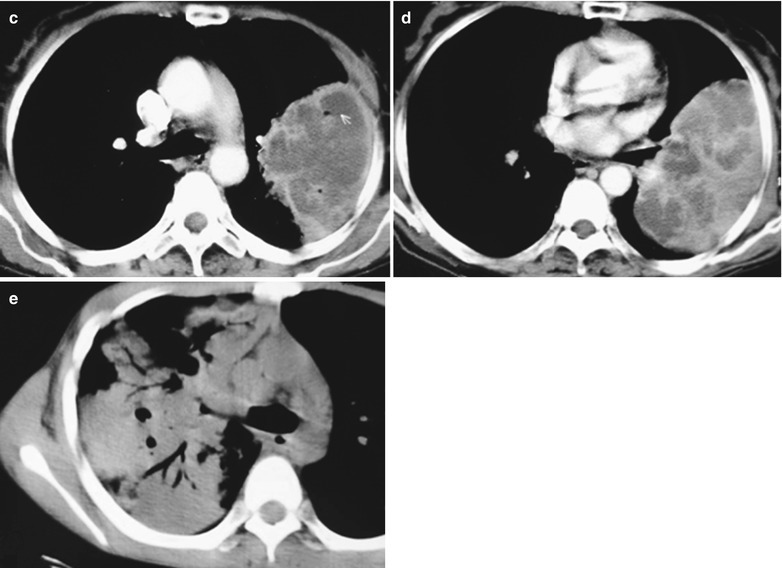
(a–e) HIV/AIDS related infiltrative pulmonary tuberculosis. (a) DR demonstrates diffuse large flaky dense shadows in the right upper lung with transparent areas in them. (b–c) CT scanning of the pulmonary window demonstrates large flaky shadows with increased density in the right upper lung, with multiple satellite lesions scattering around. (d, e) CT scanning of the mediastinal window demonstrates large flaky parenchymal shadows in the right upper lung with air bronchogram sign as well as mediastinal lymphadenectasis
Case Study 4
A male patient aged 36 years was confirmatively diagnosed as having AIDS by the CDC. He sustained HIV infection for 6 years, with chief complaints of fever and left chest pain for more than 1 month. Examinations demonstrated HIV positive, acid-fast bacilli positive in sputum culture, PPD strong positive; CD4 T cell count 168/μl, CD4/CD8 0.27. Pathology demonstrated caseous and granulation tissue. The clinical diagnosis was type III tuberculosis with accompanying left pleurisitis.
Fig. 17.36.
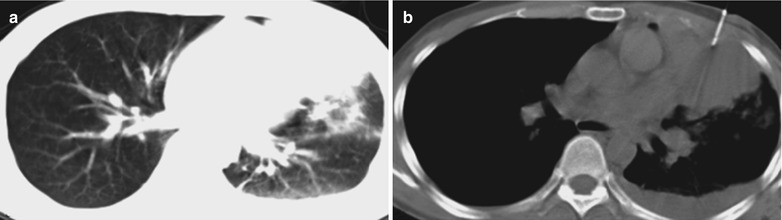
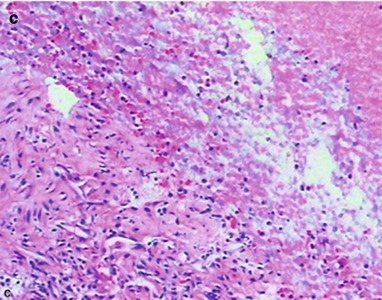
(a–c) HIV/AIDS related infiltrative pulmonary tuberculosis. (a) Pulmonary CT scanning of the pulmonary window demonstrates parenchymal shadows in the left lingual lobe with surrounding pulmonary acinar nodular shadows. (b) CT guided pucture biopsy of left lingula. (c) The pathology demonstrates granulation tissue and caseous necrosis, being in consistency with tuberculosis changes. HE × 100
Chronic Fibrous Cavity Pulmonary Tuberculosis (with Rare Occurrence)
Case Study 1
A male patient aged 38 years was confirmatively diagnosed as having AIDS by the CDC. He complained of fever and left chest pain for 1 month. By examinations, HIV positive, acid-fast bacilli positive in the sputum culture and a CD4 T cell count 201/μl.
Fig. 17.37.
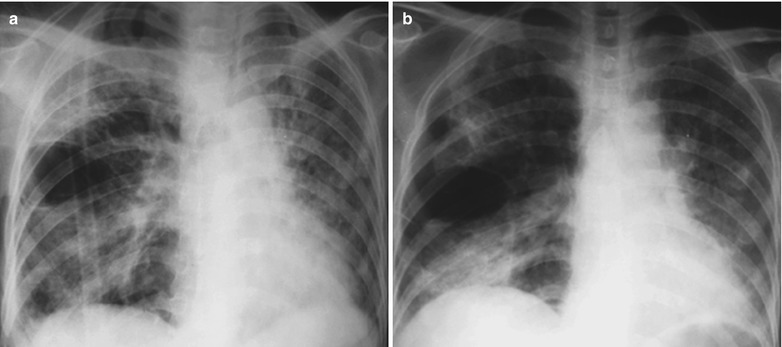
(a, b) HIV/AIDS related chronic fibrous cavity pulmonary tuberculosis. (a, b) DR demonstrates diffuse scattering patchy shadows with increased density, and oval thin wall cavity shadows in the right middle lung field
Case Study 2
A male patient aged 43 years was confirmatively diagnosed as having AIDS by the CDC. He complained of finding HIV positive for 6 years, as well as fever, cough and left chest pain for 1 month. The acid-fast bacillus was positive in sputum culture and his CD4 T cell count was 261/μl.
Fig. 17.38.
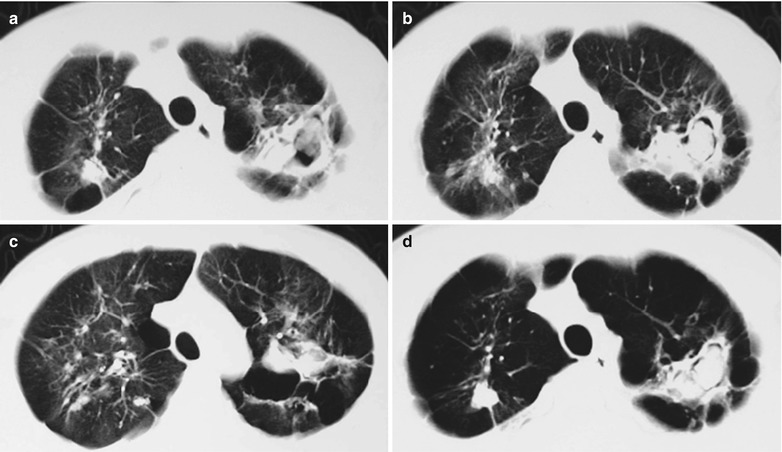
(a–d) HIV/AIDS related chronic fibrous cavity pulmonary tuberculosis. (a) CT scanning of the pulmonary window demonstrates patchy shadows with increased density in both upper lungs, peripheral crab feet liked or cords liked shadows due to pleural traction with cavity shadows in them and periperal satellite lesions. (b–d) CT scanning of the mediastinal window demonstrates round liked mass shadows with high density in the cavities of the left upper lung, surrounding transparent shadows, no nodules in the walls and surrounding nodular satellite lesions. By pathological examination, the diagnosis is defined as chronic fibrous cavity pulmonary tuberculosis complicated by Aspergillus infection
Case Study 3
A female patient aged 41 years was confirmatively diagnosed as having AIDS by the CDC. She had a case history of cavity pulmonary tuberculosis for several years, with complaints of fever, cough and chest pain for 2 months. Acid-fast bacilli were positive in the sputum culture. Her CD4 T cell count was 151/μl.
Fig. 17.39.
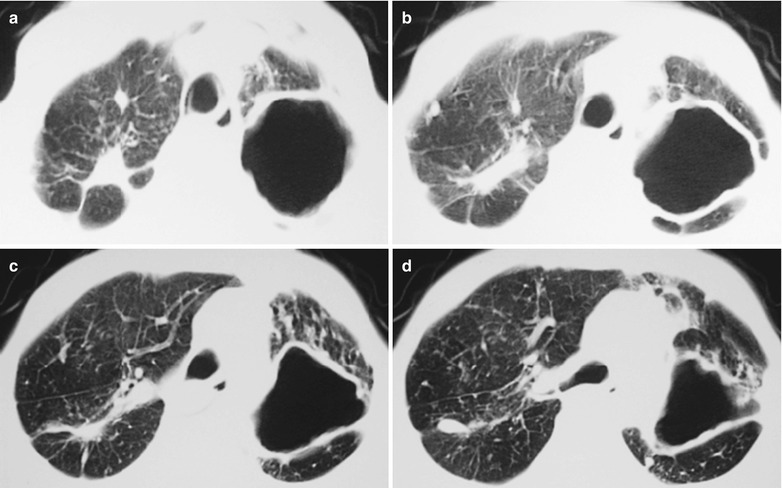
(a–d) HIV/AIDS related chronic fibrous cavity pulmonary tuberculosis. (a, b) CT scanning of the pulmonary window demonstrates patchy shadows with increased density in the right upper lung, periphery crab feet liked or cords liked shadows due to pleural traction and satellite lesions. (c, d) CT scanning of the mediastinal window demonstrates round liked or triangle shaped thick wall cavities in the left upper lung, no nodules in the wall and peripheral nodular satellite lesions
Case Study 4
A female patient aged 35 years was confirmatively diagnosed as having AIDS by the CDC. She was hospitalized due to complaints of chest distress, cough and expectoration for 2 months, with after noon low grade fever and weight loss. On admission, she was confirmed HIV positive and a CD4 T cell count of 120/μl.
Fig. 17.40.
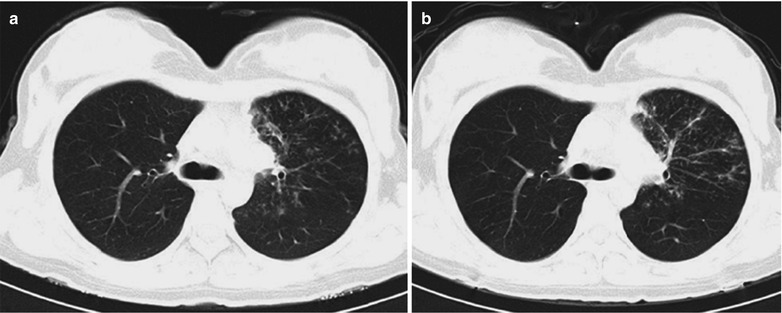
(a, b) HIV/AIDS related endobronchial tuberculosis. (a, b) Chest CT scanning demonstrates narrowed left major bronchus, irregular thickening of the brounchial wall, multiple irregular flaky, patchy and military shadows in the posterior apical, anterior and lingual segments of the left upper lung. Bronchobierscopy demonstrates narrowed left major bronchus, which is possibly caused by endobronchial tuberculosis
Tuberculous Pleuritis
Case Study 1
A female patient aged 37 years was confirmatively diagnosed as having AIDS by the CDC. She complained of fever and chest pain for 2 months, with acid-fast bacilli positive in the pleural fluid culture. Her CD4 T cell count was 71/μl.
Fig. 17.41.
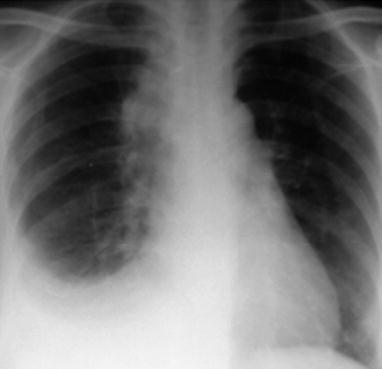
HIV/AIDS related tuberculous pleuritis. DR demonstrates arch shaped dense shadows with higher exterior density and lower interior density in the right lower lung field and covered right edge of the heart
Case Study 2
A female patient aged 40 years was confirmatively diagnosed as having AIDS by the CDC. She complained of fever and chest pain for 2 months, with acid-fast bacilli positive in the pleural fluid culture. Her CD4 T cell count was 891/μl.
Fig. 17.42.
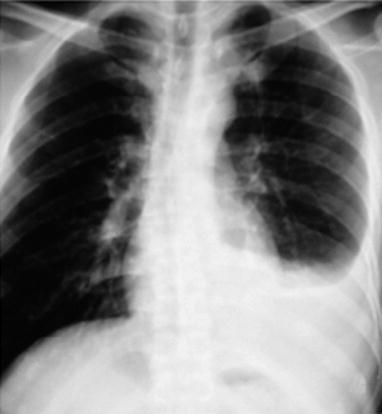
HIV/AIDS related tuberculous pleuritis. DR demonstrates arch shaped dense shadows with higher exterior density and lower interior density in the left lower lung field and covered right edge of the heart
Case Study 3
A female patient aged 34 years was confirmatively diagnosed as having AIDS by the CDC. She complained of fever and chest pain for 2 months, with acid-fast bacilli positive in the pleural fluid culture. Her CD4 T cell count was 51/μl.
Fig. 17.43.
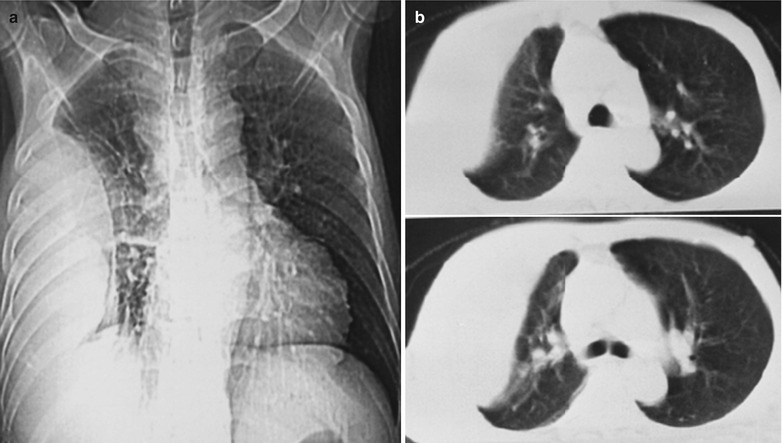
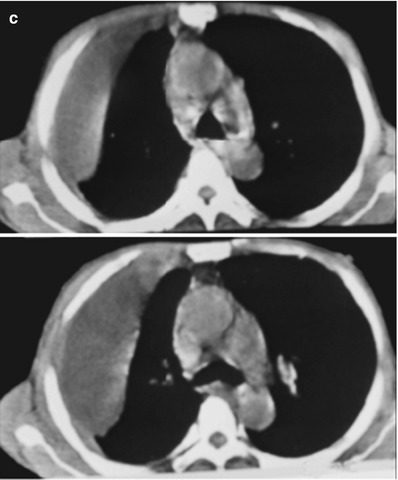
(a–c) HIV/AIDS related tuberculous pleuritis. (a) DR demonstrates thickened pleura in the right lateral chest wall in spindle liked dense shadows. (b) CT scanning of the pulmonary window demonstrates thickened pleura in the right lateral chest wall in spindle liked dense shadows. (c) CT scanning of the mediastinal window demonstrates encapsulated fluid density shadow in the pleura of the right lateral chest wall
Diagnostic Basis
Clinical Symptoms and Signs
Cough expectoration, chest pain, dyspnea, fever, night sweating, fatigue, anorexia, lymphadenectasis and rapid progression of the conditions.
Tuberculin Test
PPD skin test with a resulted diameter of more than 5 mm should be considered as tuberculosis infection. But its positive rate remains low.
Bacterial Culture
The culture of sputum and bronchoalveolar lavage fluid can detect the pathogens.
Molecular Biology Examination
Nucleic acid analysis or DNA probe technique, PCR and chromatography can be applied to detect tubercle bacilli.
Diagnostic Imaging
The commonly used diagnostic imaging examinations include chest X-ray and CT scanning. The main demonstrations include (1) intrapulmonary and extrapulmonary lymphadenectasis; (2) miliary tuberculosis manifestations; (3) infiltrative (pneumonia type) pulmonary tuberculosis; (4) Pulmonary interstitial fibrosis, cavity, pulmonary emphysema, nodules, emphysema, and bronchiectasis with accompanying infections. In the cases with their CD4 T cell count being above 400/μl, the imaging demonstrations are similar to those of non-HIV/AIDS patients with pulmonary tuberculosis. In the cases with their CD4 T cell count being above 400/μl, the manifestations are intrapulmonary large flaky parenchymal changes, surrounding satellite lesions as well as mediastinal and hilar lymphadenectasis. Sometimes the manifestations may be only mediastinal lymphadenectasis and their fusion into mass. In the cases with CD4 T cell count being above 200/μl, there may be accompanying extrapulmonary tuberculosis, such as tuberculous peritonitis, bone tuberculosis, brain tuberculosis and splenic tuberculosis. In the cases with their CD4 T cell count being lower than 100/μl, the manifestations are mostly miliary tuberculosis.
Differential Diagnosis
HIV/AIDS related tuberculosis should be principally differentiated from pneumocystis carinii pneumonia, fungal infections, other pneumonia and lung cancer.
Pneumocystis Carinii Pneumonia
HIV/AIDS related tuberculosis should be differentiated from PCP. PCP is mainly manifested as multiple lesions with hilum as the center to extending symmetrically to outside of the lungs. In the advanced stage, PCP has main lesions of pulmonary interstitial fibrosis, with less accompanying mediastinal and hilar lymphadenectasis. The laboratory tests can facilitate to define the diagnose.
Fungal Infections
HIV/AIDS related tuberculosis should be differentiated from fungal infections. Fungal infections are relatively less common than tuberculosis. The imaging findings of HIV/AIDS related pulmonary fungal infections are diverse, with manifestations of miliary, flaky flocculent liked, parenchymal, mass, and interstitial changes. In general, the diffuse lesions are mainly interstitial changes, while confirmed lesions, compared to TB lesions, are more likely to have thick walled cavities. Satellite lesions of fungal infections are less than those of tuberculosis, with less accompanying mediastinal and hilar lymphadenectasis. Sometimes laboratory tests are necessary to define the diagnosis.
Non-tuberculosis Mycobacteria Pneumonia
HIV/AIDS related tuberculosis should be differentiated from non-tuberculosis mycobacteria pneumonia. Their imaging findings are similar to each other, which presents challenges for their differential diagnosis. Molecular biology examinations play an important role in the differentiation.
Other Pneumonia
HIV/AIDS related tuberculosis should be differentiated from other pneumonia. Non-bacterial pneumonia (mycoplasma, viral and allergic) often shows patchy shadows, which are similar to the manifestations of early infiltrative pulmonary tuberculosis. When bacterial pneumonia shows lobar lesions, it may be confused with tuberculous caseous pneumonia, which should also be differentiated for the diagnosis. Symptoms of mycoplasma pneumonia are mild, with imaging findings being always inconsistency with the clinical symptoms, which usually subside within 2–3 weeks. In the cases of allergic pneumonia, eosinophils in the blood increase, with intrapulmonary mobile shadows, which are the basis for their differentiation. Bacterial pneumonia can have acute onset, with chills, high fever, rust colored sputum, and streptococcus pneumoniae positive. Recovery is rapid after antibiotic treatment and all these symptoms can subside within 1 month.
Pulmonary Abscesses
HIV/AIDS related tuberculosis should be differentiated from pulmonary abscesses. In the cases of infiltrative pulmonary tuberculosis with cavities, it should be differentiated from pulmonary abscess. Especially, tuberculosis with cavities in the apical segment of inferior lobe should be differentiated from acute pulmonary abscess. Chronic fibrous cavity tuberculosis should be differentiated from chronic pulmonary abscess. The key points for the differentiation are tubercle bacilli positive by sputum culture in the cases of TB, while tubercle bacilli negative by sputum culture in the cases of abscesses. Pulmonary abscess has an acute onset, with increased leukocytes and neutrophils as well as favorable therapeutic efficacy of antibiotics. But sometimes tuberculosis with cavity may develop into bacterial infection, with undetectable tubercle bacillus by sputum culture.
Lung Cancer
HIV/AIDS related tuberculosis should be differentiated from lung cancer. The central type of lung cancer has nodular shadow in the hilum or hilar and mediastinal lymph node metastasis, which should be differentiated from lymphatic tuberculosis. The peripheral type of lung cancer has small flaky infiltration and nodules in the periphery of the lungs, which should be differentiated from tuberculoma or tuberculosis infiltrative lesions. Lung cancers occur commonly in people aged above 40 years. The central type mainly is squamous carcinoma and the cases often have a history of long term smoking, with symptoms of no fever but difficulty breathing or chest distress as well as gradually increasing chest pain. There are also symptoms of irritated cough with blood phlegm and progressive weight loss. The cases with supraclavicular metastasis have palpable harden lymph nodes. The intrapulmonary nodules can lobulated with fine spikes, no satellite lesions, generally no calcification and possible vacuole sign. The peripheral type of lung cancer shows pleura invagination sign. Tuberculin test often shows negative in the cases of lung, positive or weakly positive in the cases with TB, and negative or weak positive in AIDS patients.
Discussions
HIV infection is known to be the main factor for the development of latent tuberculosis into active tuberculosis. It has been estimated that there are globally about 42 million HIV infected patients, of which more than 25 % sustains active tuberculosis. Most of these patients are in Africa and some developing countries of Asia, with insufficient health care resources. The clinical manifestations of tuberculosis in AIDS patients are affected by the degree of immunosuppression. The imaging findings in the cases with slight immunosuppression are similar to those of secondary tuberculosis in healthy hosts. The disease mainly involves the posterior apical segment of the upper lobe to show focal parenchymal areas and nodular shadows. About 20 % patients may have cavities and 10 % patients may have lymphadenectasis. The imaging demonstrations in the cases with serious immunosuppression are similar to those in the cases with primary tuberculosis, with characteristic abnormal manifestations of hilar and/or mediastinal lymphadenectasis and parenchymal changes of the air chambers. CT scanning demonstrates enlarged nodules with low density. Enhanced scanning demonstrates marginal enhancement of the lymph nodes. The incidence of military tuberculosis in AIDS patients is increasing due to reduced thymic T lymphocytes in AIDS patients and the defects of delayed allergic responses, which result in the formation of granulomas and impaired functions to kill bacilli and confine the lesions.
HIV/AIDS Related Pulmonary Nontuberculous Mycobacterial Infections
Nontuberculous mycobacteria (NTM) refer to the mycobacteria except for mycobacterium tuberculosis complex (human, cattle, African and vole) and mycobacterium leprae. The most commonly known nomination is nontuberculous mycobacteria (NTM). More than 100 kinds of NTM have been found so far. According to Berger Manual of Systematic Bacteriology, NTM is divided into two categories, rapid growth type and slow growth type.
Pathogens and Pathogenesis
NTM are widely spread in nature, such as soil, dust, flowing water and raw milk. Under a microscope, NTM is morphologically similar to tubercle bacilli, with red stained findings by acid-fast staining. According to the growth of NTM in solid medium, the Runyon classification divides NTM into the following four groups, light chromogenic bacteria; dark chromogenic bacteria that can cause cervical lymphnoditis in children, intrapulmonary or extrapulmonary infections and abrasive abscess; non-chromogenic bacteria including mycobacterium avium complex, intracellular mycobacteria that can cause pulmonary infections, lymphnoditis, arthritis and meningitis; rapid growth bacteria including mycobacterium fortuitum, mycobacterium, mycobacterium abscessus that can cause pulmonary diseases and skin infections.
Pathophysiological Basis
Immunocompromised populations, such as HIV infected patients, patients with neoplasms, patients with long-term use adrenocortical hormone or immunosuppressive agents, are more susceptible to disseminated NTM infection. Immunocompetent people may have mycobacterium kansasii and mycobacterium avium infections. It was reported in the United States that the occurrence of mycobacterium avium complex infection in HIV positive patients is up to more than 95 %. The pathological changes of NTM infections are similar to those of tuberculosis. NTM lymphnoditis is pathologically characterized by granulomatous inflammation. Tuberculous nodules formed by epithelioid cells and Langhans giant cells are rare, with no accompanying central caseous necrosis. Due to the weak pathogenicity of NTM, the pathological changes are slight, but there is difference in the pathological changes of NTM infections in terms of location, type and host. Cavities are common in the cases with pulmonary NTM infection, commonly being multiple or multilocular thin wall cavities. The pleuron is rarely involved, with non-specific pathological changes of inflammation but with large quantity pathogens of NTM.
Clinical Symptoms and Signs
Patients often have a history of chronic obstructive pulmonary disease, tuberculosis, silicosis, pulmonary abscess, bronchiectasis, cystic fibrosis, diabetes, ulcer as well as use of hormone or immunosuppressive agents. Its occurrence is more common in males than in females. The symptoms include cough, expectoration, hemoptysis, chest pain, difficulty breathing, low grade fever, weight loss and fatigue. The symptoms are non-specific and the conditions progress slowly.
Examinations and Their Selection
Bacteriologic Examinations
For patients with suspected diagnosis of pulmonary NTM infection, sputum smear for acid-fast staining, sputum culture and bronchial lavage specimen culture can be performed. The positive findings should be identified with two to three times repeated culture. The same finding of NTM can define the diagnosis.
Pathological Examinations
Pathological biopsy can be performed for the diagnosis of NTM lymphnoditis.
Molecular Biological Examinations
Using 16S-23 SrDNA gene spacer sequence (IGS) of NTM for PCR-restriction fragment length polymorphism analysis (PCR-RFLP), NTM species can be identified, which is more accurate, faster and simpler than the conventional morphological and biochemical examinations.
Mantoux Skin Test
Mycobacterium tuberculosis and NTM have common antigen. PPD skin test produces cross-reaction, but there are still differences between mycobacterium tuberculosis and NTM. PPD-T of the mycobacterium tuberculosis and PPD-NTM of NTM are simultaneously obtained for Mantoux skin tests. The induration diameter of PPD-T in NTM patients is generally within 15 mm. For the cases with the induration diameter of PPD-NTM skin test being 5 mm larger or over 25 % larger than that of PPD-T skin test, NTM infection can be confirmed.
Chest X-ray and CT Scanning
Both are the most commonly used imaging examinations.
Imaging Demonstrations
Imaging demonstrations include various lesions such as infiltration, cavity, nodules, fibrous caseation and extensive fiber contraction in unilateral or bilateral lungs. The incidence of cavity is up to 80 %, being singular or multiple. Cavities caused by intracellular Mycobacterium are mostly found in the pleura, with thin wall and less surrounding exudates.
Case Study
A female patient aged 26 years was confirmatively diagnosed as having AIDS by the CDC. She complained of cough and chest distress for half a month, fever for 10 days, 1 day after cesarean section and finding of HIV positive for 1 day. Her CD4 T cell count was 54/μl, with Treponema pallidum antibody negative and PPD test negative.
Fig.17.44.
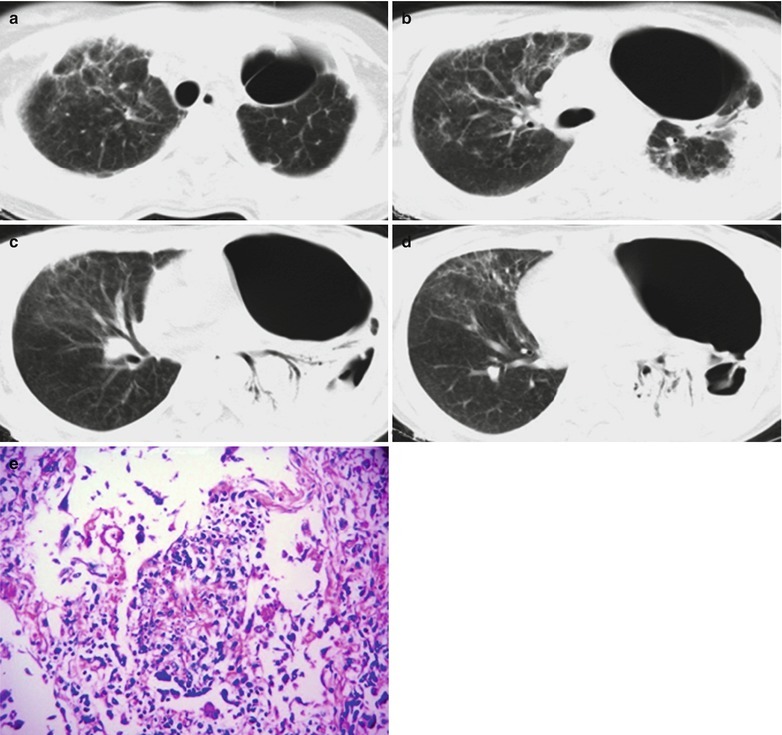
(a–e) HIV/AIDS related pulmonary nontuberculous mycobacterial infection. (a–d) CT scanning demonstrates multiple cavities in the left lung field, bilateral multiple lobular central nodules and extensive branches liked linear shadows in tree buds sign. There are also large flaky parenchymal changes of the lung tissues in the left lower lung field in high density shadows, with accompanying air bronchogram sign. (e) HE staining demonstrates avium intracellular complex mycobacteria infection of lung tissues in atypical tuberculous nodular changes. (HE × 200)
Diagnostic Basis
Respiratory symptoms or accompanying systemic symptoms.
Sputum culture find the same kind of NTM three times.
Bronchial biopsy for NTM culture is positive and lung tissue biopsy demonstrates similar granuloma to NTM lesions.
Chest X-ray demonstrates a variety of lesions, such as infiltration, cavity, nodules, fibrous caseation and extensive fiber contraction in the right upper lung. CT scanning demonstrates multiple lobular central nodules and tree buds sign, which are susceptible to cavities.
Differential Diagnosis
HIV/AIDS related pulmonary nontuberculous mycobacterial infection should be differentiated from tuberculosis, bronchiectasis, mycoplasmal pneumonia, pulmonary cystic fibrosis, Legionnaires disease, pulmonary fungal diseases and Pneumocystis carinii diseases, which depends on the PPD-NTM skin test and etiological examinations. The cases with their induration diameter of PPD-NTM skin test being 5 mm larger or over 25 % larger than that of PPD-T skin test, NTM infection can be defined. In its differential diagnosis from tuberculosis, the lesion tissues specimen collected for mycobacterial culture is all positive. But the colony state and growth conditions of NTM are different from Mycobacterium tuberculosis complex. Disseminated NTM disease should be differentiated from septicemia, typhoid fever, disseminated fungal diseases, and systemic miliary tuberculosis, which mainly depends on the PPD-NTM skin test and etiological examinations. The specific identification procedures are as above.
Discussion
The incidence of non-tuberculous mycobacterial infections in AIDS patients is high. MAC infection is usually caused by the initial exposure rather than the reactivation of latent pathogens. In patients with complications of MAC related lung diseases, most of the imaging findings are normal. The most common manifestation is mediastinal or hilar lymphadenectasis. And the pulmonary symptoms are similar to those of tuberculosis. In the cases with multiple patchy parenchymal changes, cavities can be found, as well as nodules with blurry boundaries, pleural effusion and rarely found miliary nodules. Sputum or bronchoalveolar lavage fluid culture positive, clinical symptoms, imaging findings, and response to treatment can define the diagnosis.
HIV/AIDS Related Staphylococcus Aureus Pneumonia
Pathogens and Pathogenesis
Staphylococcus aureus is a Gram positive coccus and is coagulase positive staphylococcus. The pathogenic substances of staphylococcus aureus mainly are toxins and enzymes, such as hemolytic toxins, leukocidin and enterotoxin, which play a role in hemolysis, necrosis, killing leukocytes and vascular spasm. The staphylococcus aureus coagulase is the main reason for suppurative infection.
Pathophysiological Basis
Pneumonia caused by inhaled staphylococcus aureus through the respiratory tract often shows lesions in the large lobes or extensive fusion of bronchopneumonia lesions. Bronchial and alveolar rupture allows gas to enter the pulmonary interstitium, which is communicated with the bronchi. In the cases of bronchiolar blockage by necrotic tissues or pus, the one-way valve effect is formed to cause tension pulmonary emphysema. In the cases with superficial pulmonary emphysema with excessively high tension, it ruptures to form pneumothorax or pyopneumothorax, as well as bronchooleural fistula (Fig. 17.45a, b).
Fig. 17.45.
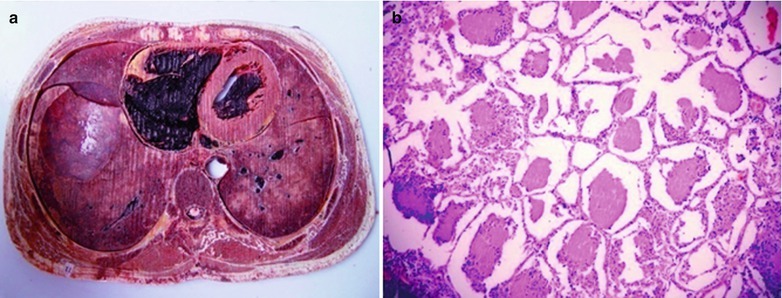
(a, b) HIV/AIDS related Staphylococcus aureus pneumonia. (a) Gross specimen observation demonstrates bilateral purplish brown lesions, patchy white infiltration at the base of the lungs. (b) HE staining demonstrates alveolar diffuse lesions, exudation of the serous fluid and inflammatory cells in the alveolar cavity, and alveolar wall congestion
Clinical Symptoms and Signs
The symptoms include chills, persistent high fever, cough, expectoration, chest pain and other symptoms. There is no sign in the early period. Symptoms are scattered moist rales in both lungs, being in consistency to severe toxic symptoms and respiratory symptoms. Yellow purulent sputum is the typical characteristics of staphylococcus aureus pneumonia. In the cases with larger lesions or fusion of lesions, signs of parenchymal changes, pneumothorax or pyopneumothorax can be found.
Examinations and Their Selection
Bacteriological Examinations
In the sputum or pleural fluid smears examinations, the bacteria with a concentration being no less than 107 cfa/ml is the pathogen, the bacteria with a concentration being 105–107 cfa/ml is the suspected pathogen, and the bacteria with a concentration being less than 105 cfa/ml is the contaminated bacteria.
Blood Tests
There are increased WBC count and neutrophils, leftward migration of the nucleus and possibly no increase of WBC count in AIDS patients.
Immunological Examinations
Immunofluorescence, enzyme-linked immunosorbent assay and counter immunoelectrophoresis can be performed to detect serum antigen or antibody of the pathogenic bacteria, which can define the diagnosis. Polymerase chain reaction has certain significance in pathogen detection.
Bronchofiberoscopy
The protected bronchoscopic specimen (PBS) and bronchoalveolar lavage (BAL) can be applied to collect the specimen, which has reduced chances of specimens contamination by oral bacteria.
Percutaneous Puncture Biopsy
Biphasic TV monitors guided pulmonary puncture and suction for pulmonary tissues examinations can be performed to detect the real pathogenic bacteria.
Chest X-ray and CT Scanning
Both are the most commonly used imaging examinations.
Imaging Demonstrations
The diagnostic imaging demonstrates staphylococcus aureus pneumonia as lesions in the inner zone of both middle lower lungs. There are singular or multiple parenchymal changes in patchy or lobar distribution that may fuse into large flakes. It may be complicated by cavity and pulmonary emphysema, with surrounding compensatory emphysema.
Case Study 1
A male patient aged 37 years was confirmatively diagnosed as having AIDS by the CDC. He complained of high fever with a body temperature of about 39 °C and chest pain for 2 months. His CD4 T cell count was 31/μl.
Fig. 17.46.
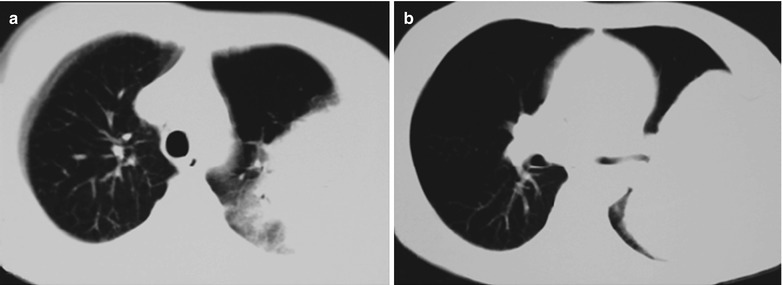
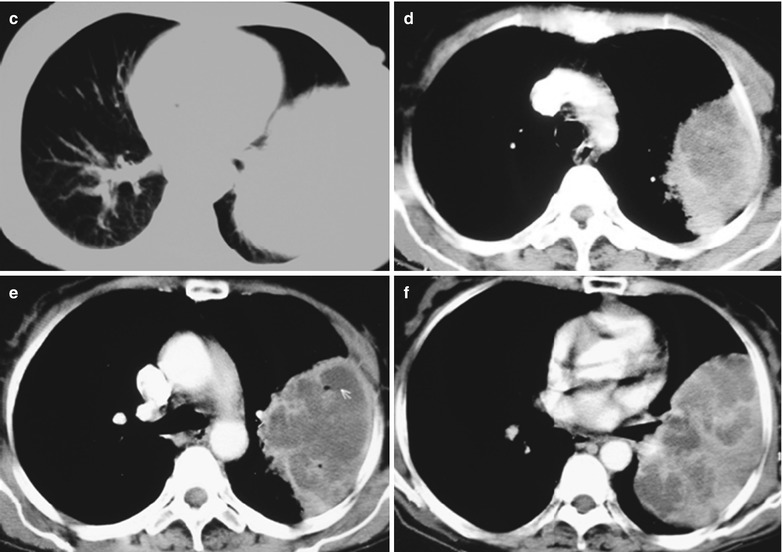
(a–f) HIV/AIDS related staphylococcus aureus pneumonia. (a–c) CT scanning demonstrates large flaky dense shadows in the middle-outer zone of the left middle lung field, and narrowed left bronchus. (d–f) CT scanning demonstrates large flaky shadows with uneven density in the left lateral chest wall and even lower density shadow in them
Case Study 2
A male patient aged 40 years was confirmatively diagnosed as having AIDS by the CDC. He complained of high fever with a body temperature of about 40 °C and chest pain for 2 months. His CD4 T cell count was 85/μl.
Fig. 17.47.
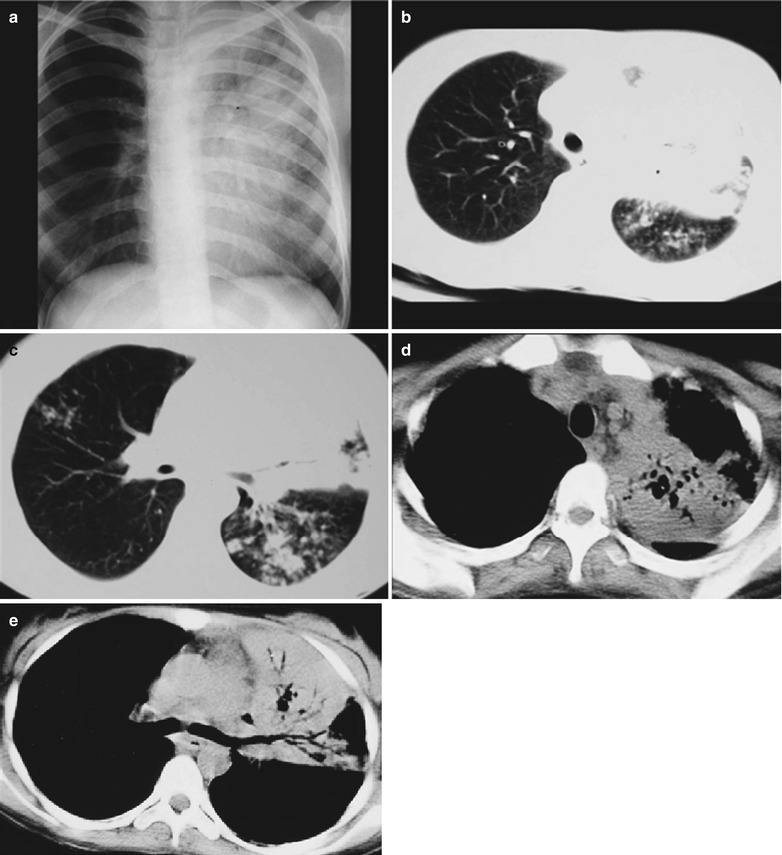
(a–e) HIV/AIDS related staphylococcus aureus pneumonia. (a) DR demonstrates large flaky dense shadows with increased density in the middle-inner zone of the left upper lung field, with blurry boundaries. The lung tissue are atelectatic and the mediastinum migrates leftwards. (b, c) CT scanning demonstrates large flaky dense shadows with increased density in the middle-inner zone of the left upper lung field, with blurry boundaries, with surrounding acinar or particle liked shadows that fuse into flaky shadows. (d, e) CT scanning demonstrates large flaky shadow of parenchymal changes in the middle-inner zone of the left upper lung field, with cyst liked transparent shadows and air bronchogram sign in them, as well as mediastinal lymphadenectasis
Case Study 3
A male patient aged 40 years was confirmatively diagnosed as having AIDS by the CDC. He complained of general fatigue and dizziness for more than 2 months as well as cough and expectoration for 1 month. His CD4 T cell count was 46/μl.
Fig.17.48.
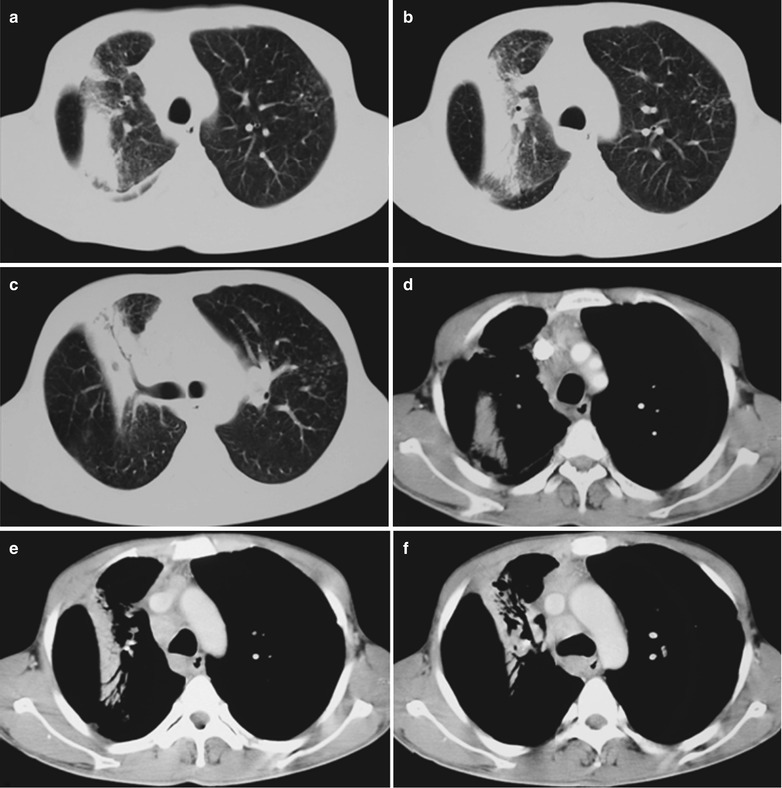
(a–f) HIV/AIDS related Staphylococcus aureus pneumonia. (a–c) CT scanning demonstrates broad band liked high density shadows in the right middle lung field, with air bronchogram sign in them. (d–f) CT scanning of the mediastinal window demonstrates broad band liked uneven parenchymal shadows in the right middle lung field, with uneven thickness of air bronchogram sign
Case Study 4
A male patient aged 40 years was confirmatively diagnosed as having AIDS by the CDC. He complained of general fatigue and dizziness for more than 2 months as well as cough and sore throat for 1 month. His CD4 T cell count was 76/μl and was clinically diagnosed as bacterial pneumonia of the right lung.
Fig.17.49.
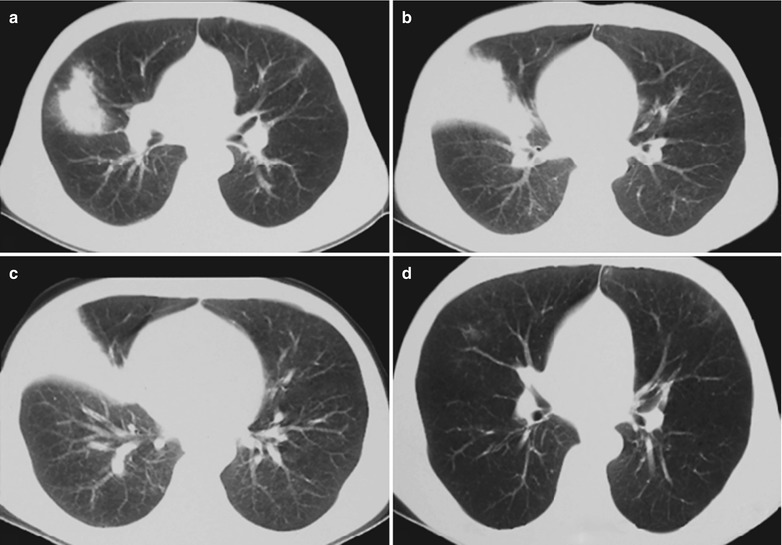
(a–d) HIV/AIDS related staphylococcus aureus pneumonia. (a–c) CT scanning demonstrates fan shaped shadow in the right middle lung field with its apex pointing to the hilar dense shadow, with clear boundaries. (d) CT scanning reexamination demonstrates absence of the lesions in the right lung after anti-bacteria treatment for 2 weeks
Case Study 5
A male patient aged 44 years was confirmatively diagnosed as having AIDS by the CDC. He complained of high fever, cough and expectoration for 1 week. His CD4 T cell count was 116/μl.
Fig.17.50.
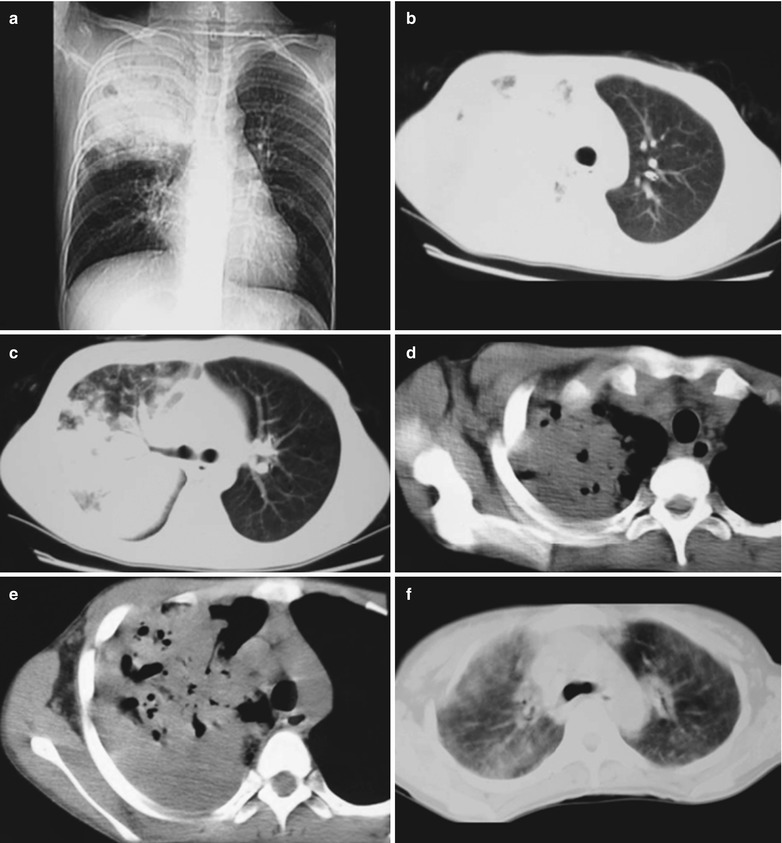
(a–f) HIV/AIDS related Staphylococcus aureus pneumonia. (a) DR demonstrates large flaky dense shadow in the right middle-upper lung field, with blurry boundary. (b, c) CT scanning demonstrates large flaky dense shadow in the right middle upper lung field, with blurry boundary. (d, e) CT scanning demonstrates large flaky dense shadow in the right middle upper lung field, with cystic transparent area in it. (f) Reexamination demonstrates obviously improved pulmonary lesions in the right lung after anti-bacteria treatment for 3 weeks
Case Study 6
A male patient aged 29 years was confirmatively diagnosed as having AIDS by the CDC. He had a history of multiple sexual partners. He was definitively diagnosed in Otc. 2004 and showed symptoms in Dec. 2005. He complained of high fever with the highest body temperature of 39 °C, cough and expectoration of thick sputum, weight loss and fatigue. His CD4 T cell count was 20/μl.
Fig. 17.51.
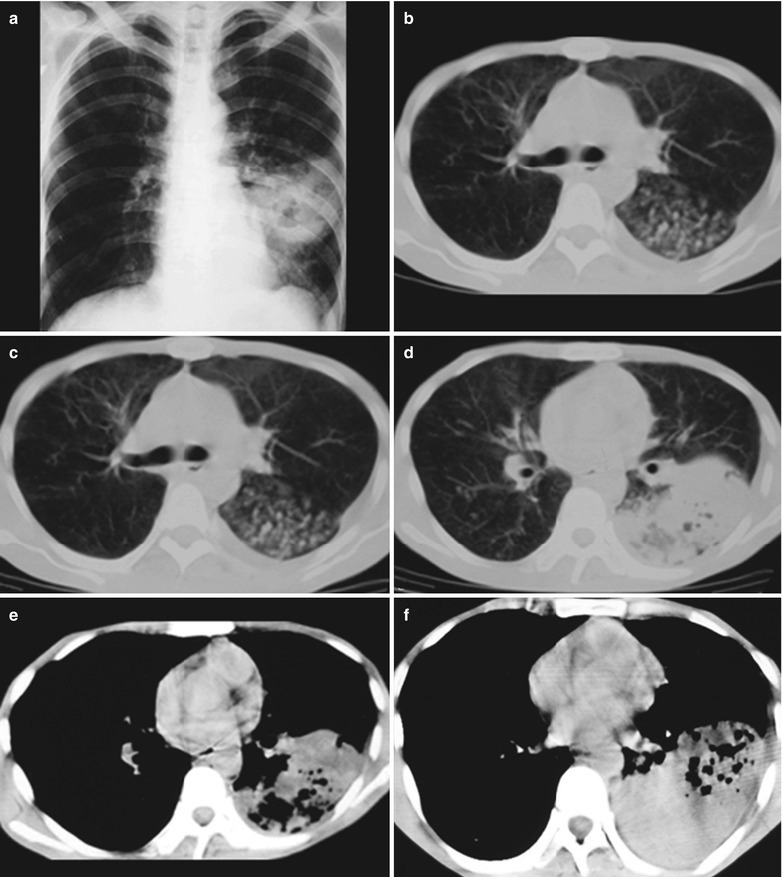
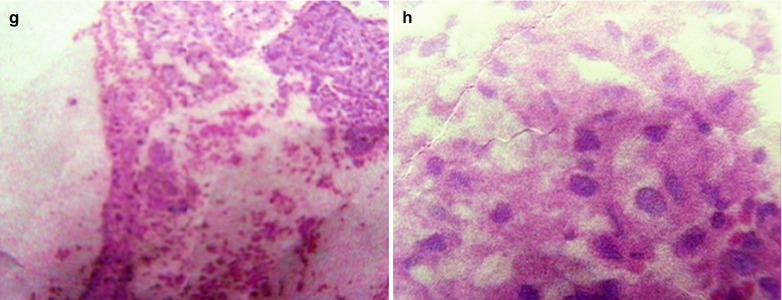
(a–h) HIV/AIDS related staphylococcus aureus pneumonia. (a) Chest X-ray demonstrates large flaky high density shadow in the left middle-lower lung field, with central transparent areas in different sizes and blurry boundaries; parenchyma changes of the left lower lung, predominantly in the posterior and exterior basal segments; unobstructed brounchus and thickened adjacent pleura. (b–d) CT scanning demonstrates large flaky shadows in fan shaped distribution along the bronchus in the left middle-lower lung, with gas containing cavities and high density shadows; and thickened adjacent pleura of the lateral chest wall. (e, f) CT scanning of the mediastinal window demonstrates large flaky fan shaped parenchymal shadows in the left middle-lower lung field, with ventilation shadows in them; and thickened pleura of the lateral chest wall. (g, h) Pathological examination showed staphylococcus aureus
Case Study 7
A male patient aged 38 years was confirmatively diagnosed as having AIDS by the CDC. He had a history of multiple sexual partners. He complained of fever with the highest body temperature of 39 °C, cough and expectoration of thick sputum for 1 week. His CD4 T cell count was 30/μl.
Fig. 17.52.
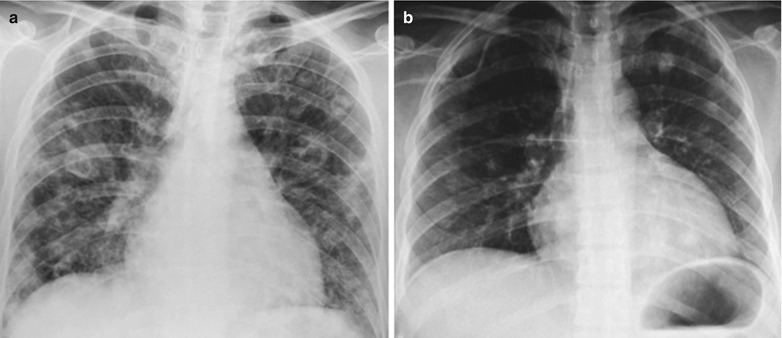
(a, b) HIV/AIDS related staphylococcus aureus pneumonia. (a, b) DR demonstrates diffuse scattered multiple thin-walled transparent areas in both lungs, increased and blurry pulmonary markings, and enlarged heart shadow in flask shape (pericardial effusion)
Diagnostic Basis
Clinical Symptoms
Chills, fever, cough, expectoration, chest pain and other symptoms commonly; hemoptysis and dyspnea rarely
Clinical Signs
The patients may be found with fever appearance, rarely shortness of breath and cyanosis. In the serious cases, the body temperature can be as high as 39–40 °C and blood pressure decreases, with signs of shock. By chest examinations, decreased ipsilateral respiratory motion; increased or decreased fremitus, dull sound in percussion; bronchial breathing sounds or moist rales by auscultation; rarely pleural friction and weakened breathing sounds.
Blood Tests
Increased WBC count and neutrophils, possible the nucleus left shift; no increase or even decrease of WBC count in AIDS patients; Staphylococcus aureus positive by blood culture.
Bacteriological Examinations
Sputum or pleural fluid smears examinations for pathogenic bacteria culture is positive, and antibiotic sensitivity test is positive.
Chest X-ray and CT Scanning
By chest X-ray and CT scanning, the most common demonstrations are lesions of bronchial pneumonia. The findings of pulmonary emphysema and cavities can facilitate the diagnose.
Differential Diagnosis
The lesions should be differentiated from infiltrative parenchymal bronchioloalveolar carcinoma. The smaller lesions should be differentiated from pulmonary infarction. The large lesions should be differentiated from obstructive pneumonia. It is difficult to identify the types of common bacterial pneumonia simply by chest X-ray and CT scanning. In combination to the laboratory tests, the diagnosis can be defined.
Discussion
The incidence of pyogenic bacterial infection is increasing in AIDS patients, which is caused by their weakened cellular and humoral immunity. The manifestations of these most common bacterial infections are similar to those of non-HIV infected patients by chest X-ray. Bacterial pneumonia and purulent bronchitis are the most common causes of pulmonary infections in AIDS patients. Particularly, they are frequently found in patients with a history of intravenous drug abuse and smokers. They are histologically characterized by inflammations of the bronchi and bronchioles as well as inflammatory exudates and mucus in the airway lumens. CT scans facilitates the diagnosis of bronchiolitis and early bronchial pneumonia. The demonstrations are characterized by (1) small centrilobular nodular shadows, which is the cross sectional demonstration of bronchioles filled with inflammatory substances and its surrounding inflammations; (2) Branched linear shadows, which is the long axis demonstration of abnormal bronchioles; (3) Focal parenchymal areas caused by bronchial pneumonia. Bacterial infection usually is the unilateral segmental alveolar infiltration, with manifestations of lobar segmental pneumonia in the exudation period. Early lesions show exudative inflammation in lobar and segmental distribution. The pathological changes are mainly alveolar exudates in a small quantity. The lesions progress into parenchymal changes. According to the case history and the clinical manifestations, the lesions are in lobar and segmental distribution by chest X-ray and CT scanning, with the bronchi in railway track sign for the diagnosis. CT scanning demonstrates fan-shaped or broad band liked distributions of infiltration and parenchymal changes in the lungs.
HIV/AIDS Related Rhodococcus Equi Pneumonia
Pathogens and Pathogenesis
Rhodococcus equi infection is one of the zoonotic diseases, which commonly occurs in the grazing areas. Patients with T lymphocyte immunodeficiency caused by AIDS and other factors are especially susceptible to the infection. Rhodococcus equi was firstly discovered in 1923 and was nominated as corynebacterium equi. After structure analysis of the cell wall, it was found that the bacterium is quite different from Corynebacterium, and therefore classified as Rhodococcus. Rhodococcus equi infection in human is rare. But in recent years, due to an increase of patients with immunodeficiency syndrome, reports on rhodococcus equi caused human respiratory infections and sepsis are increasing. In the past, the toxicity mechanism of Rhodococcus equi was mostly speculated. Until recently, the damage process of toxic plasmid to human tissue is discovered, which presents a new way for the study of the pathogenesis of Rhodococcus equi infections.
Pathophysiological Basis
Rhodococcus equi is one of the facultative parasites in the cells and its optimum growth temperature is 30 °C, with a suitable growth temperature of 10–40 °C. Acid-fast staining of Rhodococcus equi shows uncertain results. Due to its morphological diversity, it is often mistaken as diphtheroid bacillus, bacillus or micrococcus. In sheep blood agar, rhodococcus equi can have synergistic hemolysis with staphylococcus aureus, Listeria monocytogenes and corynebacterium pseudotuberculosis, which is a characteristic manifestation of Rhodococcus equi. The most common pathological changes in Rhodococcus equi infection are chronic purulent bronchitis and extensive lung abscess. Imaging often demonstrates subacute pneumonia, commonly with cavities.
Clinical Symptoms and Signs
The clinical manifestations are poor appetite, drowsiness, fever and shortness of breath. Studies by E Marchiori et al. [30] in 2005 revealed that all the 5 cases of AIDS complicated by rhodococcus equi pulmonary infection have cough and fever lasting for 1–2 months, with accompanying shortness of breath and chest pain. All the 13 cases, studied by Li et al. in 2011 [106], have fever with a body temperature up to 38–40 °C and cough. In addition, there are also expectoration with orange red sputum in 10 cases, hemoptysis in 4 cases, dyspnea in 11 cases, moist rales of lungs in 13 cases, emaciation in 6 cases, poor appetite in 6 cases, diarrhea in 2 cases, joint pain in 1 case, oral candidiasis infections in 13 cases, oral herpes in 4 cases, chest pain in 4 cases, no obvious symptoms in 1 case and hepatitis B in 3 cases. Typical clinical manifestations of this disease are fever, cough, dyspnea and chest pain, while others such as emaciation, diarrhea and joint pain are not representative symptoms.
Examinations and Their Selection
Identification of the Bacteria
Various specimens were inoculated on blood plates at a temperature of 35 °C for 18–24 culture, with bacteria growth of 18 strains. They are biologically characterized by a diameter of about 0.5 mm, non-transparent and slight yellowish colonies. After 48–72 h, the colonies expand to 1–2 mm, which can be emulsified in mucous fluid liked state. Most of the colonies produce orange and orange red pigments, which can be cultured in ordinary agar.
Pathological Examinations
Histopathological findings are typical for Rhodococcus equi infection. H&E staining demonstrates mainly bleeding in the alveolar space, large quantity epithelial cells, possibly predominant fibroblasts, parenchymal changes of lung tissue and thickened alveolar septa. PAS staining demonstrates scattered or clustered rhodococcus equi in pink or purplish red.
Chest X-ray and CT Scanning
Both are the most commonly used imaging examinations.
Percutaneous Lung Puncture for Biopsy
Biphasic TV monitor guided lung puncture can be performed to suck lung tissues for biopsy, based on which the real pathogenic bacteria can be detected.
Imaging Demonstrations
The typical demonstrations include central hilar sphere liked shadow with increased density in unilateral lung, accounted for 70 %. There are also manifestations of exudative infiltration and large flaky or spherical mass shadows in the right or left hilar area. The lesions are in patchy or flaky appearance, radiating from the hilum to the lung field with blurry boundaries.
Case Study 1
A male patient aged 30 years was confirmatively diagnosed as having AIDS by the CDC. He complained of recurrent fever, cough and chest pain for 13 days; and was found HIV positive for 8 days. He was also a carrier of hepatitis C virus, with symptoms of fever with no known causes, cough, chest distress and weak limbs. By examinations, he was found to have complexion of chronic conditions; many moist and dry rales by cardiopulmonary auscultation. He had a past history of HIV positive for 8 years, with drug abuse and extramarital affairs. The history of present illness includes fever, paroxysmal cough with a little whitish yellow thick sputum since May 8, 2008. He also had subjective paroxysmal dull pain in the left chest, and hemoptysis once which was bright red with blood clot in a volume of about 80 ml. His CD4 T cell count was 10/μl. By sputum culture, Rhodococcus equi was found positive. After receiving antibiotic treatment, his conditions improved and he was discharged from the hospital.
Fig.17.53.
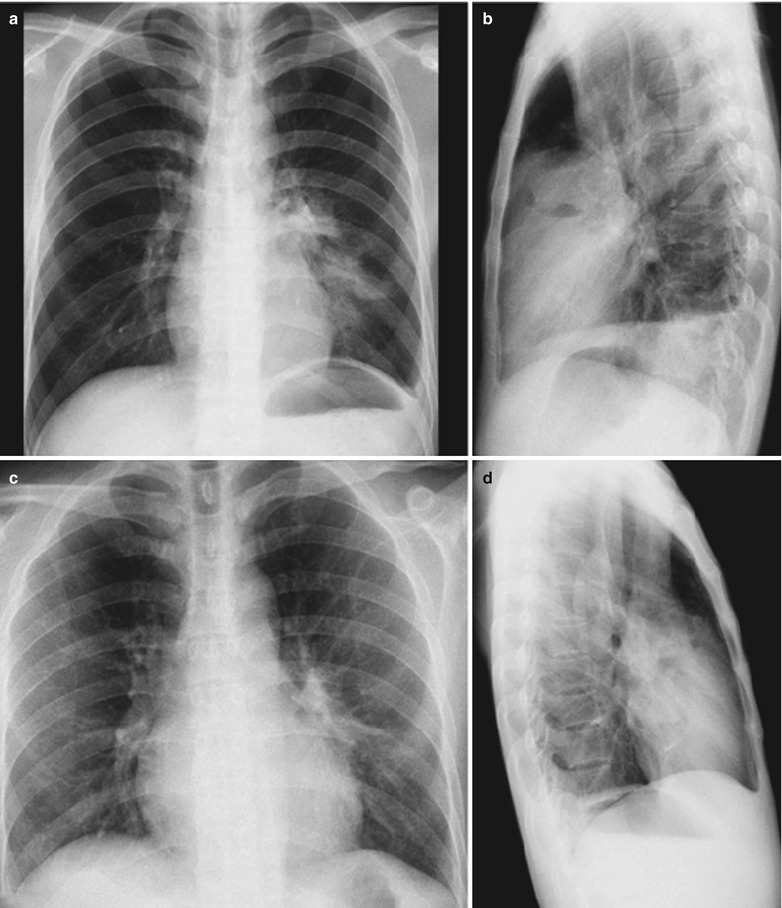
(a–d) HIV/AIDS related Rhodococcus equi pneumonia. (a, b) Anteroposterior and lateral DR demonstrates enlarged and thickened left hilum, and large flaky blurry shadows with increased density in the left lower lung. (c, d) Reexamination demonstrates normal lungs after antibiotic treatment
Case Study 2
A male patient aged 43 years was confirmatively diagnosed as having AIDS by the CDC. He complained of fever with the highest body temperature of 39 °C with no known causes for more than 3 months, with accompanying paroxymal cough and expectoration of small quantity white thick sputum. By blood culture, Rhodococcus equi was found positive. He was confirmatively diagnosed as HIV positive 3 months ago and his CD4 T cell count was 15/μl. By examinations, he had a complexion of chronic illness and many moist and dry rales by cardiopulmonary auscultation.
Fig. 17.54.
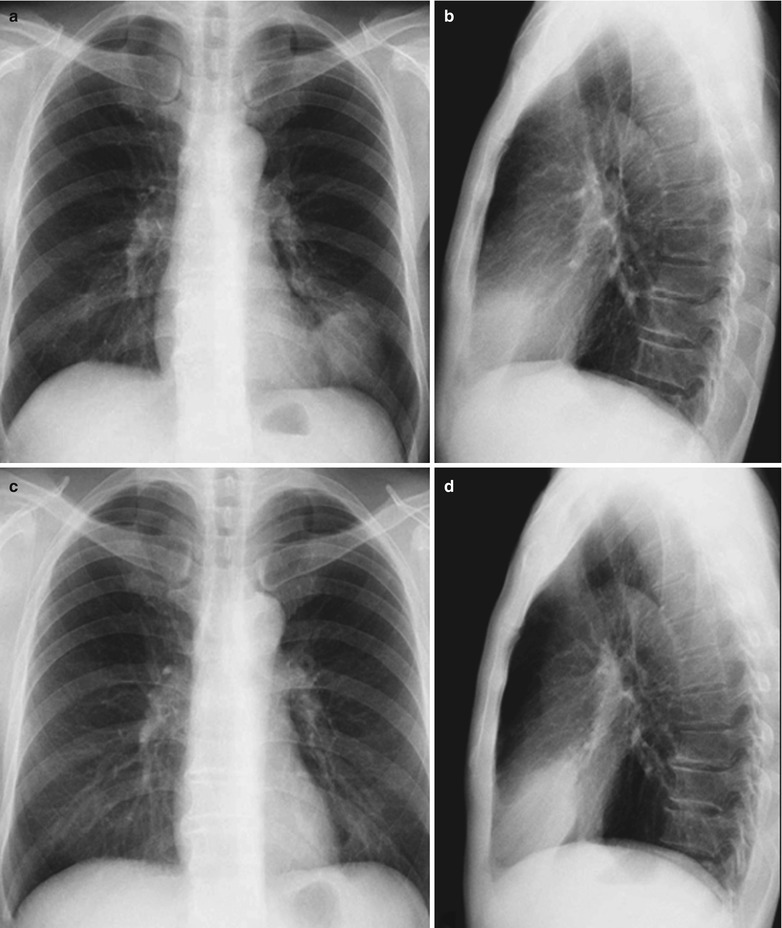
(a–d) HIV/AIDS related Rhodococcus equi pneumonia. (a, b) DR demonstrates round liked large flaky shadows with increased density in the left lower lung, enlarged and thickened hilus. (c, d) DR reexamination after treatment demonstrates flocculent shadows in the left lower lung, with improved conditions than previous findings before treatment (a, b)
Case Study 3
A male patient aged 49 years was confirmatively diagnosed as having AIDS by the CDC. He complained of paroxymal cough and expectoration of white diluted bubble sputum with no known causes since Dec. 2007, as well as other symptoms of after noons fever with the highest body temperature of 40 °C and obvious night sweating. He also complained of chest distress and weak limbs for 5 months, finding of HIV positive for 14 days. By examinations, he had a complexion of chronic illness and his CD4 T cell count was 53/μl. After anti-infection treatment, his conditions were not improved, but deteriorated to cause death.
Fig. 17.55.
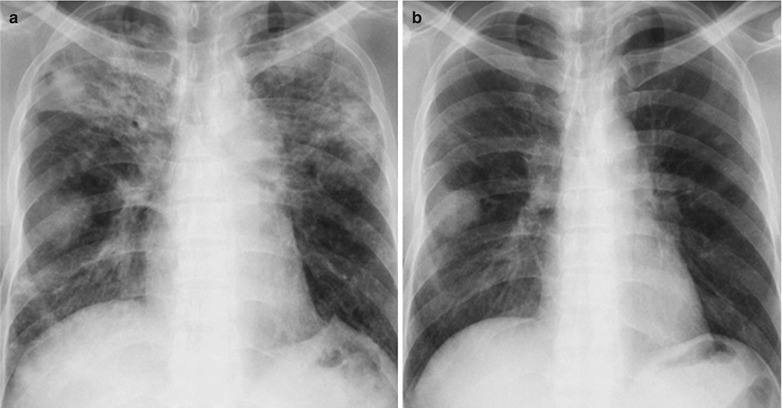
(a, b) HIV/AIDS related Rhodococcus equi pneumonia. (a, b) DR demonstrates round liked large flaky shadows with increased density in the right lower lung, cords liked and flocculent liked blurry shadows in both middle-upper lung fields and in the right lower lung field, and enlarged and thickened hilus
Case Study 4
A male patient aged 38 years was confirmatively diagnosed as having AIDS by the CDC. He complained of fever, cough, expectoration, chest distress and weak limbs for 5 months. His CD4 T cell count was 63/μl.
Fig.17.56.
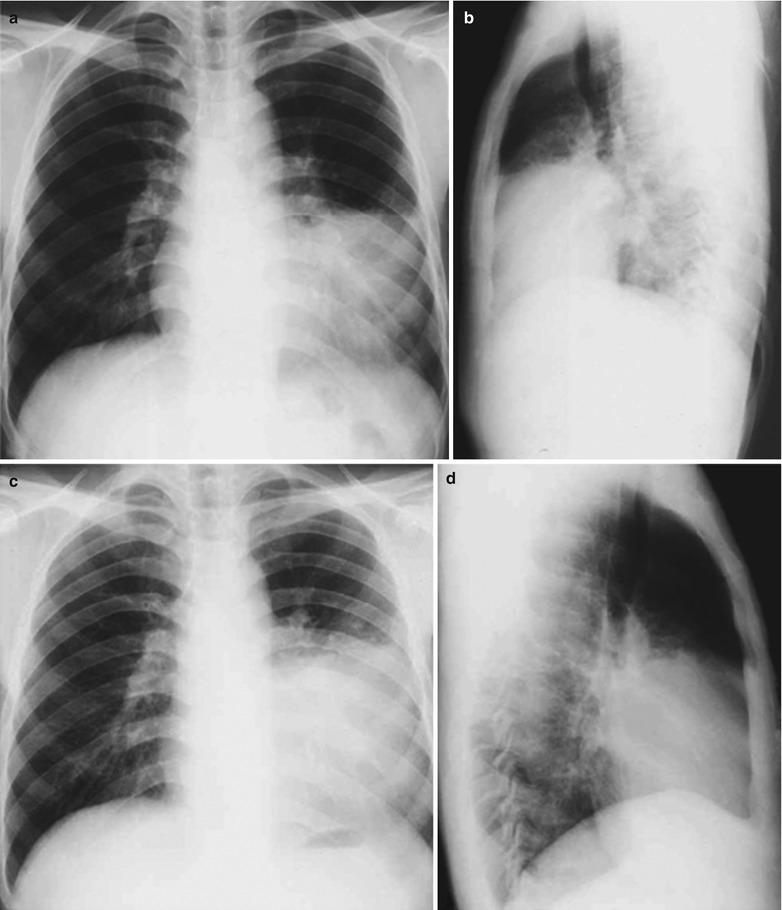
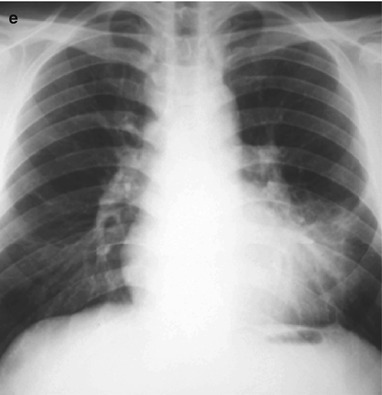
(a–e) HIV/AIDS related Rhodococcus equi pneumonia. (a, b) DR demonstrates huge round large flaky shadows with increased density in the left lower lung, enlarged and thickened hilum and covered right heart edge. (c, d) DR reexamination after treatment for 1 week demonstrates flocculent shadow in the left lower lung, improved than those before the treatment (a, b). (e) DR reexamination after treatment demonstrates flocculent liked shadows in the left lower lung, obviously improved than before the treatment (c, d)
Case Study 5
A male patient aged 39 years was confirmatively diagnosed as having AIDS by the CDC. He complained recurrent cough and fever for 3 days. His CD4 T cell count was 50/μl. Multiple times sputum smears demonstrated Gram positive bacilli, Rhodococcus equi, and yeast liked fungus. Multiple times sputum culture demonstrated smooth ball liked Candida glabrata, Rhodococcus equi and fungi. By lung tissues culture, Rhodococcus equi was detected.
Fig. 17.57.
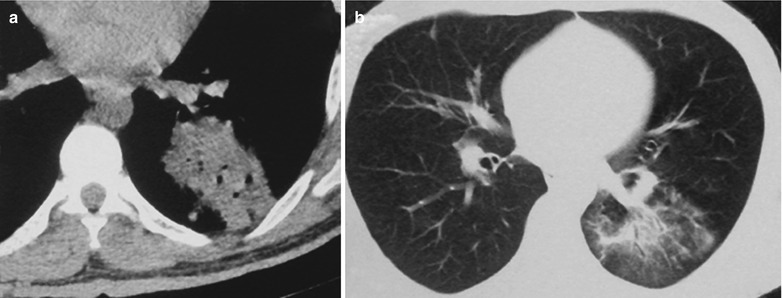
(a, b)HIV/AIDS related Rhodococcus equi pneumonia. (a) CT scanning of the mediastinal window demonstrates soft tissue mass shadows in the dorsal segment of the left lower lung, with ventilation shadows in them. (b) CT scanning of the pulmonary window after the treatment demonstrates absence of the soft tissue mass shadows, and flocculent liked shadow in the left lower lung, obviously improved than previous findings (a)
Case Study 6
A male patient aged 39 years was confirmatively diagnosed as having AIDS by the CDC. He complained of recurrent cough and chest distress for 2 months. His CD4 T cell count was 50/μl.
Fig.17.58.
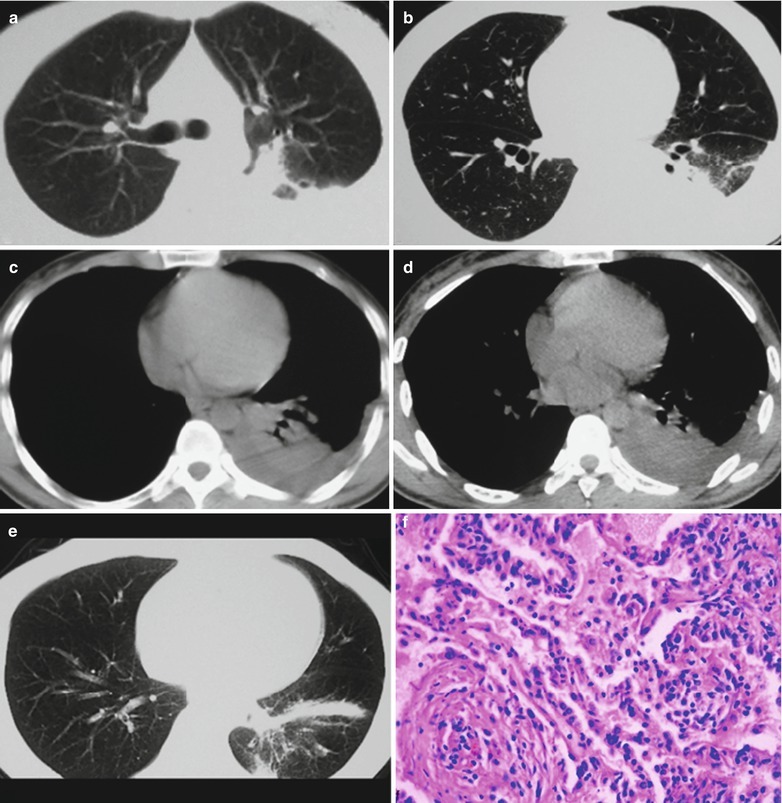
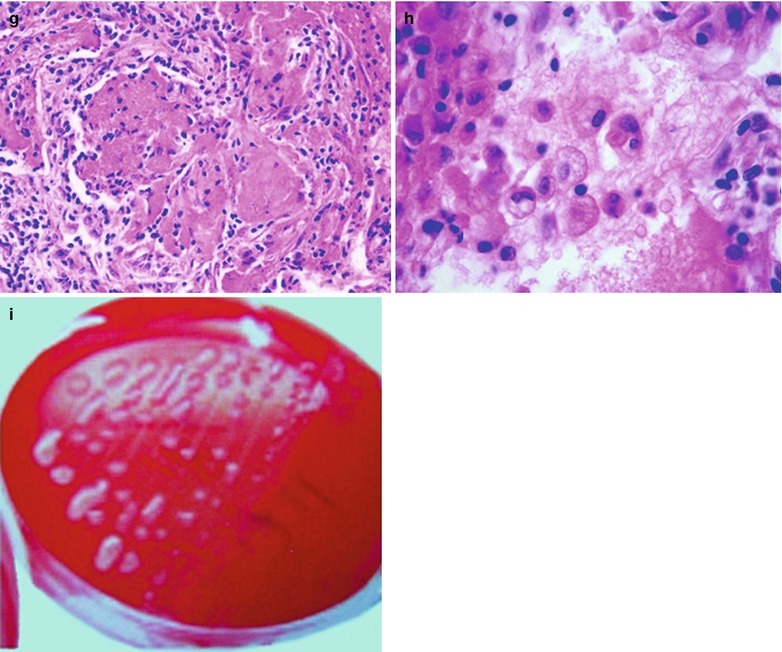
(a–i) HIV/AIDS related Rhodococcus equi pneumonia. (a, b) CT scanning of the pulmonary window before the treatment demonstrates flaky dense shadows in the dorsal segment of the left lower lung, with ventilation shadows in them. (c, d) CT scanning of the mediastinal window before the treatment demonstrates flaky dense shadows in the dorsal segment of the left lower lung, pulmonary atelectasis and pleural effusion, with ventilation shadows in them. (e) CT scanning of the pulmonary window after the treatment demonstrates absence of the mass shadows in the left lower lung with transverse stripes shadows, obviously improved than previous findings (a, b). (f) HE staining demonstrates thickened alveolar septa and exudates from the alveolar cavity. (g) HE staining demonstrates massive bleeding in the alveolar cavity, large quantity erythrocytes and intact cell walls. (h) HE staining demonstrates phagocytized basophilic granules in the leukocytes. (i) HP staining demonstrates purplish red Rhodococcus equi in a shape of crescent in orange red sputum
Case Study 7
A male patient aged 48 years was confirmatively diagnosed as having AIDS by the CDC. He complained of fever, cough, expectoration, chest distress and weak limbs for 3 months. His CD4 T cell count was 53/μl.
Fig. 17.59.
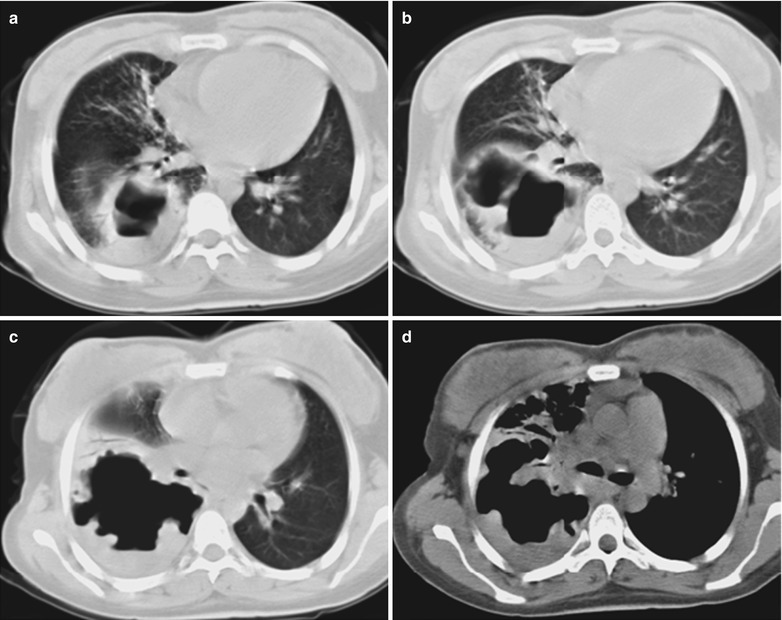
(a–d) HIV/AIDS related Rhodococcus equi pneumonia. (a–c) CT scanning of the pulmonary window demonstrates thick walled cavities shadows in the dorsal segment of the right lower lung, with irregular wall thickness and liquid gas level in them. (d) CT scanning of the mediastinal window demonstrates thick walled cavities shadows in the dorsal segment of the left lower lung, with irregular wall thickness and liquid gas level in them
Case Study 8
A male patient aged 40 years was confirmatively diagnosed as having AIDS by the CDC. He complained of fever, cough, expectoration and chest distress for 1 month. His CD4 T cell count was 70/μl.
Fig. 17.60.
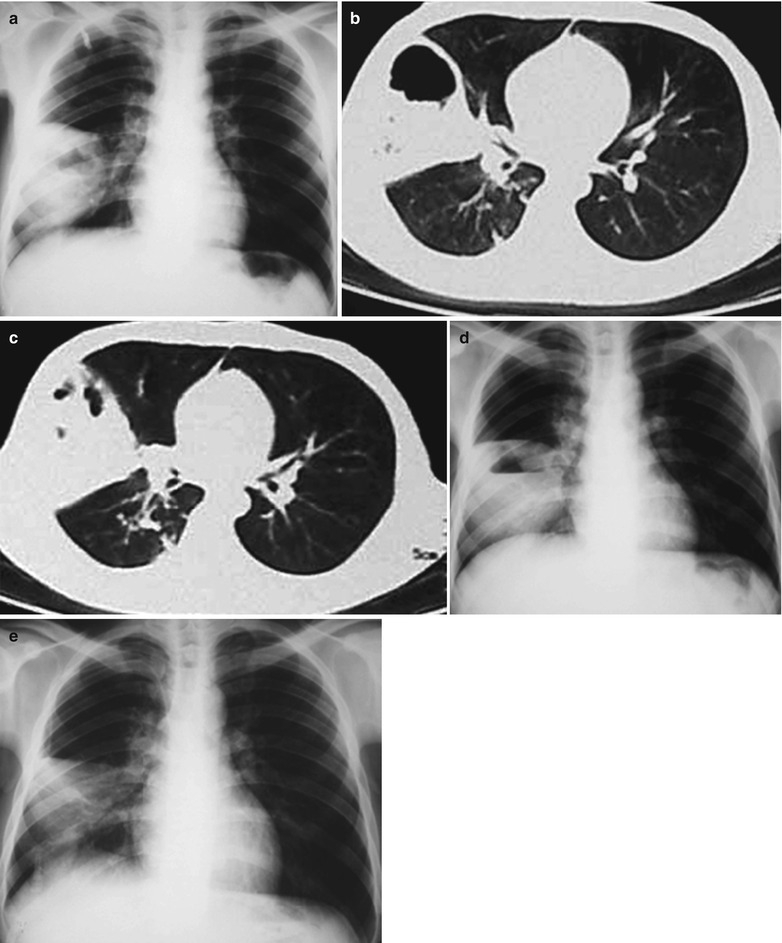
(a–e) HIV/AIDS related Rhodococcus equi pneumonia. (a) DR demonstrates huge sphere liked mass shadow in the middle-outer zone of the right lower lung field, with cavities shdows in them. (b–d) CT scanning demonstrates triangular dense shadow in the middle-outer zone of the right lower lung field, with its apex pointing to the hilum and round liked cavities shadows in them. (e) Reexamination after the treatment demonstrates shrinkage of the huge sphere liked mass shadow in the middle-outer zone of the right lower lung field, with closure of the cavities
Case Study 9
A male patient aged 30 years was confirmatively diagnosed as having AIDS by the CDC. He complained of fever, cough, expectoration and chest distress for 1 week. His CD4 T cell count was 90/μl.
Fig.17.61.
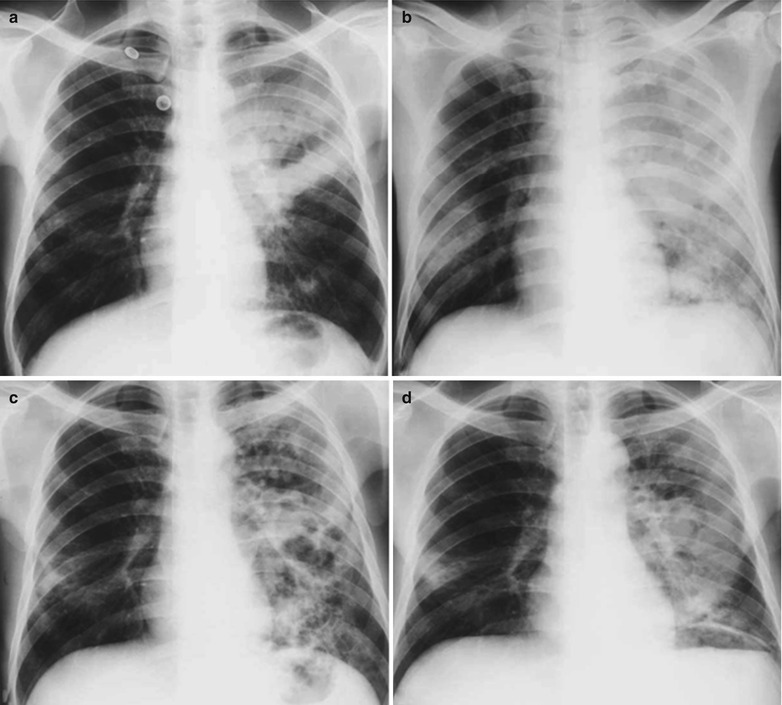
(a–d) HIV/AIDS related Rhodococcus equi pneumonia. (a) DR demonstrates large flaky shadow in the middle-outer zone of the left upper lung field. (b) DR demonstrates diffuse dense shadow in the left lung field, with round liked cavities shadows in them. (c–d) Reexamination after treatment demonstrates multiple cavities shadows in the middle-outer zone of the left upper lung field
Case Study 10
A male patient aged 35 years was confirmatively diagnosed as having AIDS by the CDC. He complained of paroxymal cough, expectoration of white diluted bubble sputum and fever with the highest body temperature of 40 °C. His CD4 T cell count was 93/μl.
Fig. 17.62.
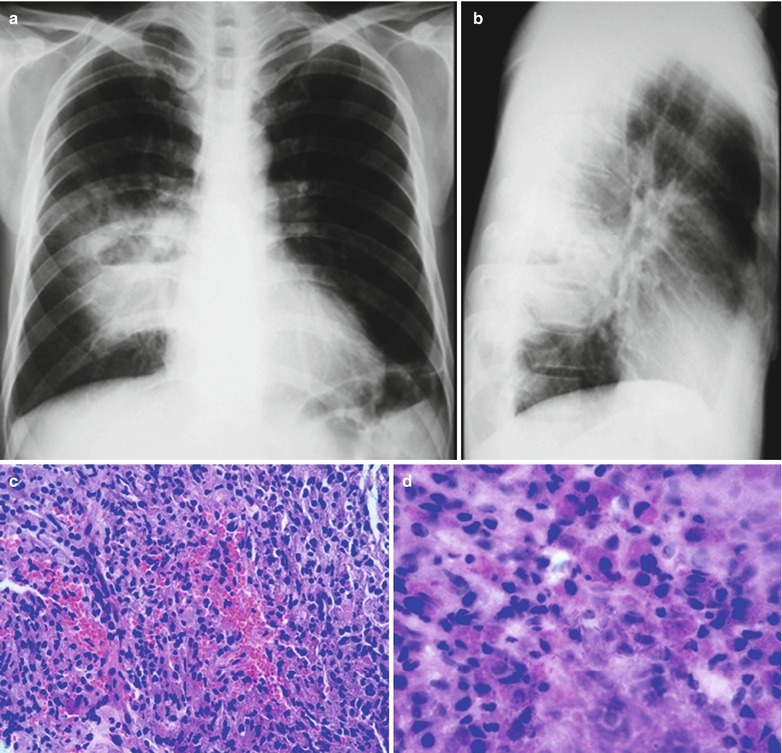
(a–d) HIV/AIDS related Rhodococcus equi pneumonia. (a, b) Anteroposterior and lateral DR demonstrates huge sphere liked mass shadow in the right hilum, with cavities shadows and liquid gas level in it. (c) HE staining demonstrates bleeding in the lung tissues and aggregation of large quantity lymphocytes. (d) Masson staining demonstrates branches liked purplish red Rhodococcus equi
Case Study 11
A male patient aged 34 years was confirmatively diagnosed as having AIDS by the CDC. He complained of right chest pain and hemoptysis for 5 months for admission and was confirmed as HIV positive. His CD4 T cell count was 11/μl.
Fig.17.63.
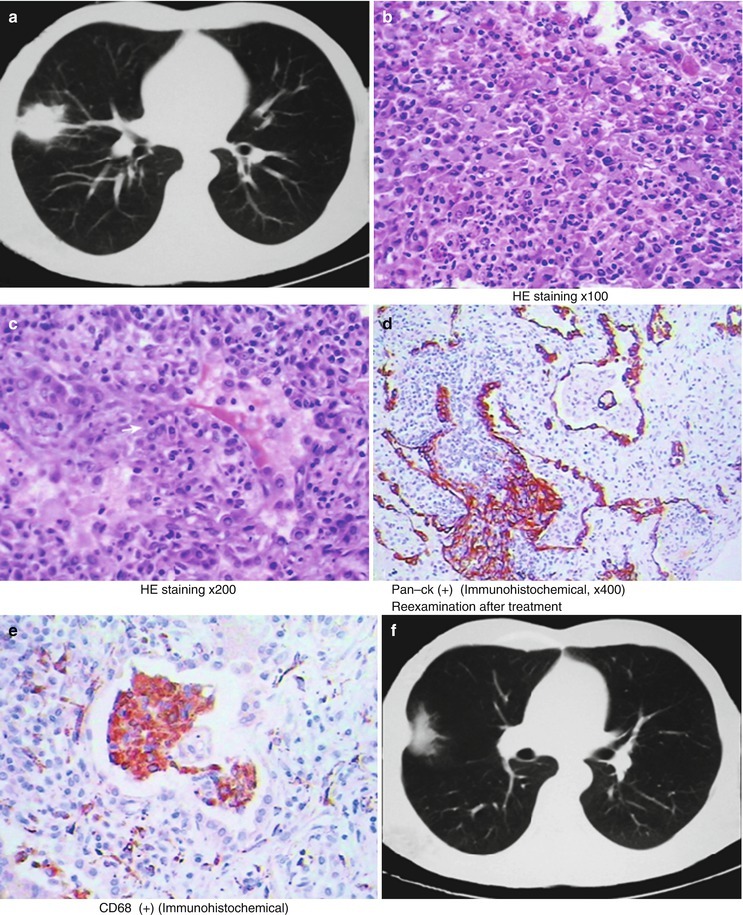
(a–f) HIV/AIDS related Rhodococcus equi pneumonia. (a) Pulmonary CT scanning demonstrates a mass shadow in the lateral segment of the right middle lobe in size of 4.7 × 3.7 × 3.2 cm with uneven density and lace liked boundary, and small bubbles shadows in it. By both sputum culture and lung tissue culture, Rhodococcus equi can be detected. (b, c) Pathological biopsy and HE staining demonstrate inflammatory pseudotumor. (d, e) Immunohistochemistry demonstrates Rhodococcus equi antibody positive. (f) Reexamination after treatment demonstrates obvious shrinkage of the original lung lesions
Diagnostic Basis
Strain Identification
Catalase test of the strain is positive, which is confirmed as Rhodococcus equi by API CORYNE system.
Pulmonary Puncture for Biopsy and Pathological Examinations
H&E staining demonstrates mainly bleeding in the alveolar space, large quantity epithelial cells or mainly fibroblasts, parenchymal changes of lung tissue and thickened alveolar septa. PAS staining and Masson staining demonstrate scattered or clustered distribution of rod rhodococcus equi in pink or purplish red.
Imaging Demonstrations
AIDS complicated by pulmonary rhodococcus equi infection shows subacute inflammation. Firstly, there are exudates in the surrounding area of unilateral hilum as well as sphere shaped mass shadows which is centrally dense and peripherally blurry, with its apex pointing to the hilum. The lesions can be complicated by cavities and fluid level, with thick wall of the cavities. As the disease progresses, the abscess cavities have increased tension, with gradually thinner abscesss cavity walls and uneven wall thickness, even showing pleural effusion. These are its characteristic imaging findings.
Differential Diagnosis
It often needs to be differentiated from Pneumocystis carinii pneumonia, tuberculosis, staphylococcus aureus pneumonia, central type lung cancer and other diseases. Imaging findings of Pneumocystis carinii pneumonia usually are ground glass liked changes in the lung field, with parenchymal changes and centrilobular nodules. Tuberculosis often shows miliary tuberculosis, with lymphadenectasis, large tubercles and parenchymal changes. These characteristic pathological and imaging findings of staphylococcus aureus pneumonia are similar to those of bronchial pneumonia (lobular pneumonia). The lesions are nodules with blurry boundaries in a diameter of 4–10 mm. The disease progresses rapidly, while pulmonary rhodococcus equi infection has a chronic progression. HRCT scanning demonstrates staphylococcus aureus pneumonia as centrilobular nodules and branch linear shadows (tree buds sign), which can be found in 40 % patients, with 15–30 % will develop into commonly singular pulmonary abscess. Chest CT scanning demonstrates round liked abscess cavity with thick wall and liquid level in it. The inner wall of the abscess cavity is often irregular, with various changes within short period. The central type of lung cancer is commonly demonstrated as round liked shadow of unilateral hilum with rough boundary. Lobulation or bronchial stenosis sometimes occurs. However, AIDS complicated by Rhodococcus equi pneumonia is demonstrated as sphere liked mass in the hilum; mostly sphere liked increased density shadow with hilum as the center. The shadow is centrally dense and peripherally blurry, with no bronchial stenosis.
Discussion
Rhodococcus equi was firstly discovered in 1923 and was nominated as corynebacterium equi. After structure analysis of the cell wall, it was found that the bacterium is quite different from Corynebacterium, and therefore classified as Rhodococcus. Rhodococcus equi is generally believed to be the pathogen for horses, pigs and cattle. Human Rhodococcus equi infection is rare. But in recent years, due to an increase of patients with immunodeficiency syndrome, reports on human respiratory tract infection and sepsis caused by Rhodococcus equi are increasing. In sheep blood agar, Rhodococcus equi may have synergistic hemolysis with staphylococcus aureus, Listeria monocytogenes and corynebacterium pseudotuberculosis, which is characteristically rhodococcus equi. Studies by E Marchiori et al. [30] in 2005 revealed that all the 5 cases of AIDS complicated by Rhodococcus equi pulmonary infection have cough and fever lasting for 1–2 months, with accompanying shortness of breath and chest pain. All the 13 cases, studied by Li et al. in 2009 [106], have fever with a body temperature of 38–40 °C and cough. There are symptoms or signs of orange red sputum in 10 cases, hemoptysis in 4 cases, dyspnea in 11 cases, lung moist rales in 13 cases, emaciation in 6 cases, poor appetite in 6 cases, diarrhea in 2 cases, joint pain in 1 case, oral candidal infections in 13 cases, oral herpes in 4 cases, chest pain in 4 cases, no obvious symptoms in 1 case and hepatitis B in 3 cases. In the studies of the horse infected with Rhodococcus equi, Professor CHEN [108] found that it is characterized by chest X-ray demonstrations of abscesses in different sizes in the lungs and significant alveolar fusion in the lesion site. Its basic pathological changes are chronic suppurative bronchopneumonia and extensive pulmonary abscesses. The imaging demonstrations are commonly subacute pneumonia, often with cavities. Among the 13 cases of AIDS patients, studied by Li et al. in 2009 [106], 9 cases have central type sphere liked shadows with increased density distributed in the unilateral hilar region, accounting for 70 %. The largest shadow is in size of 5 × 6 cm, which is centrally dense and peripherally blurry, in a halo sign. The imaging findings are similar to those of lung tumor; with exudative infiltration and large flaky or sphere shaped mass shadow in the right hilar region in 4 cases, infiltration and large patchy or sphere shaped mass shadow in the outer zone of the right lung in 1 case, exudative infiltration and large flaky or sphere shaped mass shadow in left hilar region in 4 cases, diffuse atelectasis in the left lung in 1 cases, partial atelectasis in 5 cases, singular pulmonary abscess cavity in 9 cases, multiple honeycomb liked cavity in 1 case, cavity shadow in the right lung in 6 cases, complicated by fluid level in 4 cases, cavity in the left lung in 3 cases, complicated by fluid level in 1 case, flakey shadows in bilateral hilar regions in 1 case, left pleural effusion in 3 cases and mediastinal lymphadenectasis in 2 cases. Exudative inflammation often occurs around the abscess cavity, possibly with small quantity pleural effusion and/or thickened and adhesive pleura. In the study of 5 cases AIDS complicated by pulmonary Rhodococcus equi infection, conducted by E Marchiori et al. [30] in 2005, CT scanning demonstrates 3 cases with fusion of centrilobular nodules and tree buds sign around the parenchymal changes, among which one case has the lesions in the both lower lobes. Donisi investigated 12 patients with HIV positive complicated by Rhodococcus equi infection and found its mortality rate is 58 % [37]. Harvey reported 11 cases of HIV positive complicated by Rhodococcus equi infection, with 6 cases of death, accounting for 54.5 % [38]. The study of 13 cases of AIDS complicated by Rhodococcus equi infection, conducted by Li et al. [106] found its mortality rate of 7 %. The very different mortality of the two groups may be closely related to the regional economic and medical conditions as well as the administration of antibacterial and HAART therapies. In the study of the AIDS complicated by Rhodococcus equi pulmonary infection, conducted by E Marchiori et al. in 2005 [30], the CD4 T cell count is below 50/μl. In the study of AIDS complicated by Rhodococcus equi pulmonary infection conducted by Donisi et al. in 1996 [37], CD4 T cell count is 47.7/μl. The 13 cases of AIDS complicated by Rhodococcus equi infection studied by Li et al. in 2009 [106] showed a CT4 T cell count of lower than 49/μl. All the results are in consistency. In conclusion, AIDS complicated by pulmonary Rhodococcus equi infection is mainly subacute inflammation. There are exudation around the unilateral hilum as well as centrally dense and peripherally blurry sphere shaped mass shadows, with secondary cavities and parenchyma changes and even pleural effusion. All of these are characteristic imaging demonstrations.
HIV/AIDS Related Pulmonary Fungal Infections
HIV/AIDS Related Pulmonary Aspergillus Infection
Pathogens and Pathogenesis
Most cases of HIV positive complicated by respiratory or pulmonary diseases are caused by Aspergillus fumigatus. Aspergillus fumigatus belongs to filamentous fungi, which is a common opportunistic fungus and has a wide distribution in the nature. As conditional pathogenic bacteria, it can parasitize in the human skin and upper respiratory tract. Human has certain resistance to Aspergillus so it commonly fails to cause diseases. In immunocompromised AIDS patients, the pathogenic bacteria can pass through the defects in the skin and mucous membrane into the blood flow to infect the tissues and organs.
Pathophysiological Basis
Aspergillus commonly violates bronchus and lung, with involvements of rhinal sinuses, external auditory canal, eye and skin. Otherwise, it disseminates to organs of the body along with blood flow. The early lesions are diffuse infiltrative and exudative changes. And advanced lesions are necrosis, pyogenesis or granuloma. Large quantity hyphae can be found in the lesions. The hyphae penetrate the blood vessels to cause vasculitis, perivascular inflammation and thrombosis. And thrombosis can cause ischemia and necrosis of the tissue. According to the pathological changes and imaging findings, it can be divided into three major types: vascular invasion type, bronchopneumonia type and allergic bronchopulmonary aspergillosis type. (1) The vascular invasion type is the result caused by toxins released in the process of aspergillus spreading extensively from the primary focus to the lungs. Vascular infiltration of the pulmonary parenchyma and coagulative necrosis are believed to be the cause of vascular occlusion and pulmonary infarction. (2) Bronchopneumonia type is acute bronchitis caused by inhalation of Aspergillus spores. In the cases of hyphae invasion into the lung tissues, extensive infiltrative pneumonia or focal granuloma are resulted in. It can also cause necrosis, pyogenesis and multiple small abscesses. Spherical pulmonary aspergillosis is often secondary to bronchiectasis, tuberculosis, carcinous cavity and other lung diseases. Mycelia multiply and gather in the cavities of the lungs to form a spherical mass with fibrin and mucosal cells, which are called aspergillar glomera, which do not invade the lung tissue. (3) Allergic bronchopulmonary aspergillosis type is the proliferation and germination of inhaled Aspergillus spore in the airway, often showing obvious related mucosal lesions and eventually resulting in bronchiectasis (Fig. 17.64a–c).
Fig. 17.64.
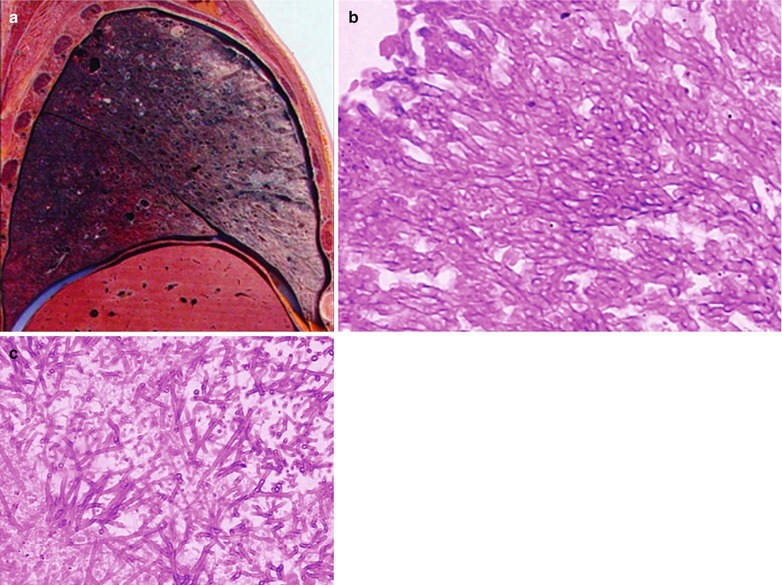
(a) Gross observation demonstrates dark brown lungs in appearance. (b, c) Thology demonstrates hemorrhage and edema of the lung tissue and focal necrosis, with large quantity Aspergillus hyphae and spores in the surrounding area of the necrosis (Combined with pulmonary CMV infection)
Clinical Symptoms and Signs
HIV/AIDS Related Aspergillus Bronchopneumonia
The cases with acute onset have symptoms of high fever or irregular fever, cough, shortness of breath and green purulent sputum. The cases with a chronic onset have symptoms of repeated cough and hemoptysis, which are similar to those of tuberculosis. The pulmonary signs are not obvious, with occasional findings of moist rales.
HIV/AIDS Related Spherical Pulmonary Aspergillosis
Most cases are asymptomatic and sometimes there are fever, cough, shortness of breath, and mucous purulent sputum.
Invasive Pulmonary Aspergillosis
The main symptoms are persistent fever, cough and chest pain. In the serious cases, there is dyspnea.
Examinations and Their Selection
Microscopic Examination of Sputum
By microscopic examination of sputum, Aspergillus hyphae can be found. The culture for aspergillus fumigatus is positive.
Immunologic Assays
Serum IgE is commonly above 2,500 μg/L. Skin test for aspergillus antigen is positive. Serum anti-Aspergillus antigen IgG antibody precipitin is positive.
Fiberobronchoscopy Lavage and Biopsy
Puncture of lungs and pleura for biopsy facilitates the diagnosis of pulmonary fungal infections.
Chest X-rays and CT Scanning
Both are the most commonly used imaging examinations.
Imaging Demonstrations
HIV/AIDS related aspergillus bronchopneumonia is commonly demonstrated to have increased pulmonary markings, diffuse patchy blurry shadows and mass shadows in both lungs. Spherical pulmonary aspergillosis is commonly demonstrated to have sphere liked aspergillar glomera suspending in the cavities to form a crescent shaped transparent area, in characteristic meniscus sign, rolling ball sign and fingertip sign. Meniscus sign is nominated due to a meniscus liked space between the aspergillar glomera growing in the cavity and the cavity wall. Rolling ball sign means that the aspergillar glomera moves along with the changes of posture. Fingertip sign indicates that the substance formed by aspergillar glomera in dilated bronchi is in a finger shape, sometimes in V shape sign and Y shape sign. Invasive lesions refer to lesions invading or destroying lung structures, such as pneumonia, parenchymal changes and necrosis.
Case Study 1
A female patient aged 28 years was confirmatively diagnosed as having AIDS by the CDC. She complained of cough and chest distress, with increased eosinophilic granulocytes. Her CD4 T cell count was 45/μl.
Fig. 17.65.
(a–g) HIV/AIDS related allergic pulmonary Aspergillus infection. (a–f) CT scanning of the pulmonary window demonstrates thickened central pulmonary markings in both lungs, which is turtuous and deranged with fingertip infiltration shadows. (g) DR demonstrates hyperinflation of the right lung, increased and thickened pulmonary markings and ground glass liked shadows with increased density in the right lower lung and left lung lobe
Case Study 2
A female patient aged 30 years was confirmatively diagnosed as having AIDS by the CDC. She complained of high fever or irregular fever, cough and shortness of breath. Her CD4 T cell count was 56/μl.
Fig. 17.66.
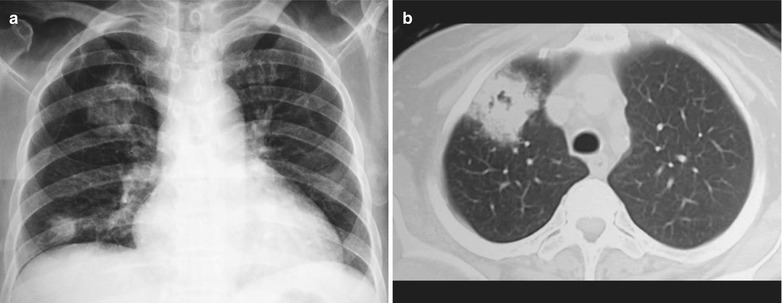
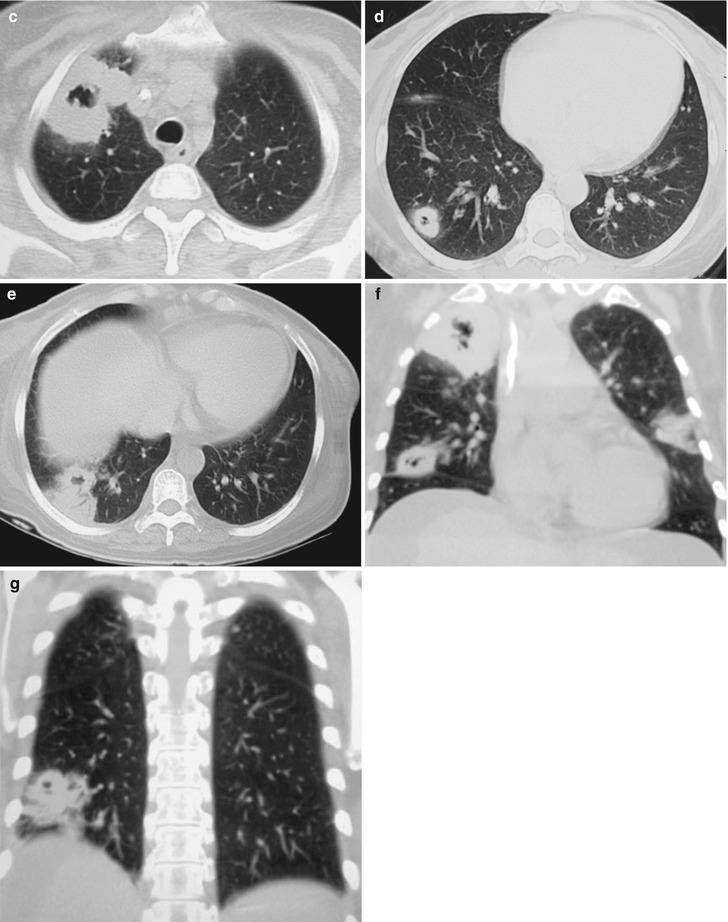
(a–g) HIV/AIDS related pulmonary aspergillosis infection. (a) DR demonstrates large flaky shadows with increased density in the right upper lung, with blurry boundaries; round liked or sphere shaped mass shadows in the right lower lung, with uneven density. (b–e) CT scanning of the pulmonary window demonstrates multiple round liked thick-wall cavities in the dorsal segment of the right lower lung, with small nodular shadow adhering on the cavity wall; and surrounding small nodular shadows and infi ltration shadows. (f, g) Coronal CT scanning reconstruction demonstrates a huge thick-wall cavity in the right upper lung, with irregular thickness of the wall; round liked cavity shadows in the right lower lung, with thick and multilocular walls; and flaky shadows with increased density in the outer zone of the left lower lung
Case Study 3
A male patient aged 36 years was confirmatively diagnosed as having AIDS by the CDC. He complained of high fever or irregular fever, cough and shortness of breath. His CD4 T cell count was 96/μl.
Fig. 17.67.
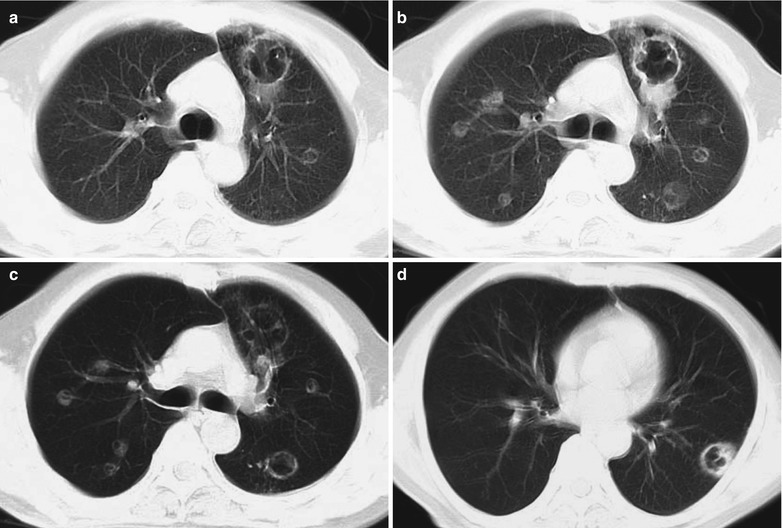
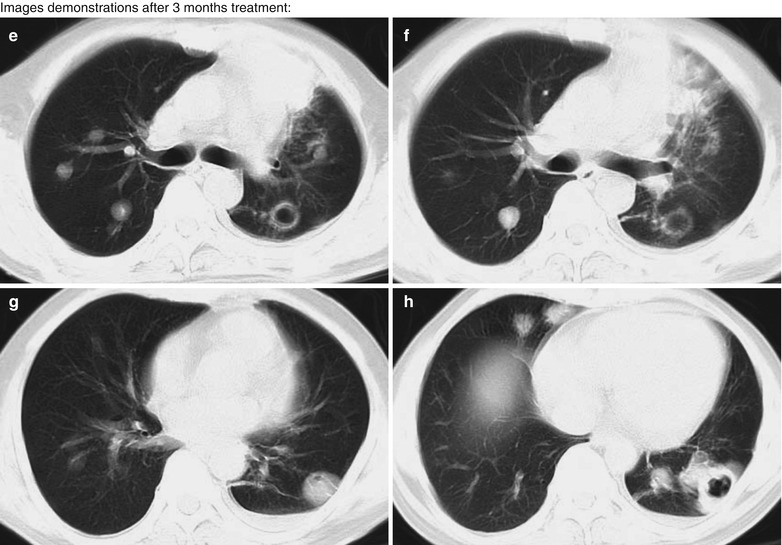
(a–h) HIV/AIDS related pulmonary aspergillosis infection. (a–d) CT scanning of the pulmonary window demonstrates multiple scattered round liked nodular shadows and thick-wall cavity shadows in both lungs, with small nodular shadows in the cavity shadows. (e–h) Reexamination of the pulmonary window after 3 months treatment demonstrates multiple scattered round liked nodular shadows and thick wall cavity shadows in both lungs, with small nodular shadows in the cavity shadows which obviously increase and enlarge compared to previous lesions, with accompanying infiltration shadows around the lesions
Case Study 4
A male patient aged 38 years was confirmatively diagnosed as having AIDS by the CDC. He complained of high fever or irregular fever, cough and shortness of breath. His CD4 T cell count was 76/μl.
Fig. 17.68.
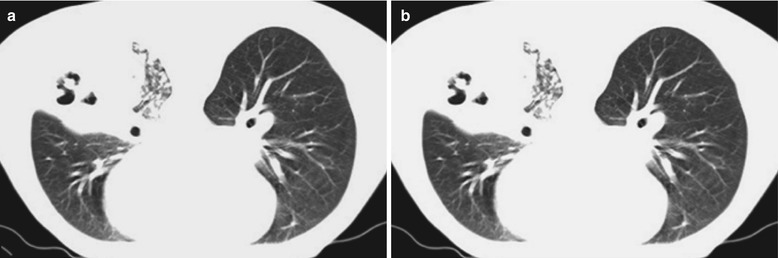
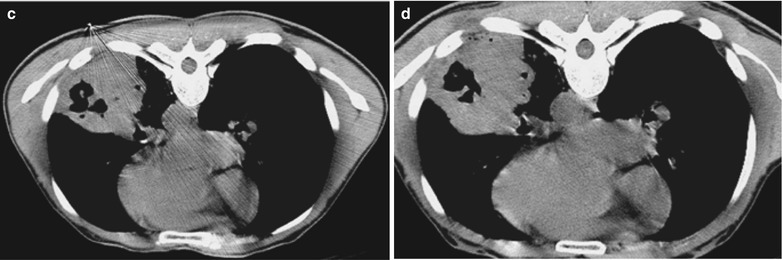
(a–d) HIV/AIDS related pulmonary aspergillosis infection. (a, b) CT scanning of the pulmonary window in prone posture demonstrates mass shadow and thick wall cavity in the dorsal segment of the right lower lung, small nodular shadows in the cavities, surrounding fused miliary infiltration shadows. (c, d) CT scanning of the mediastinal window in the prone posture demonstrates mass shadow and thick wall cavity shadows in the dorsal segment of the right lower lung, small nodular shadows in the cavities, and involved pleura of partial lateral chest wall
Case Study 5
A female patient aged 35 years was confirmatively diagnosed as having AIDS by the CDC. She complained of irregular fever, cough and chest distress. Her CD4 T cell count was 16/μl.
Fig. 17.69.
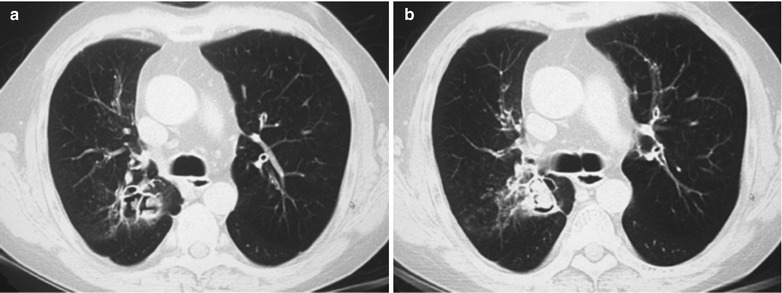
(a, b) HIV/AIDS related pulmonary aspergillosis infection. (a, b) CT scanning of the pulmonary window in the prone posture demonstrates mass shadow and thick wall cavity shadows in the medial basal segment of the right lower lung, irregular nodular shadows in the cavities, and surrounding fused miliary infiltration shadows
Case Study 6
A female patient aged 40 years was confirmatively diagnosed as having AIDS by the CDC. She complained of irregular fever, cough and shortness of breath. Her CD4 T cell count was 36/μl.
Fig. 17.70.
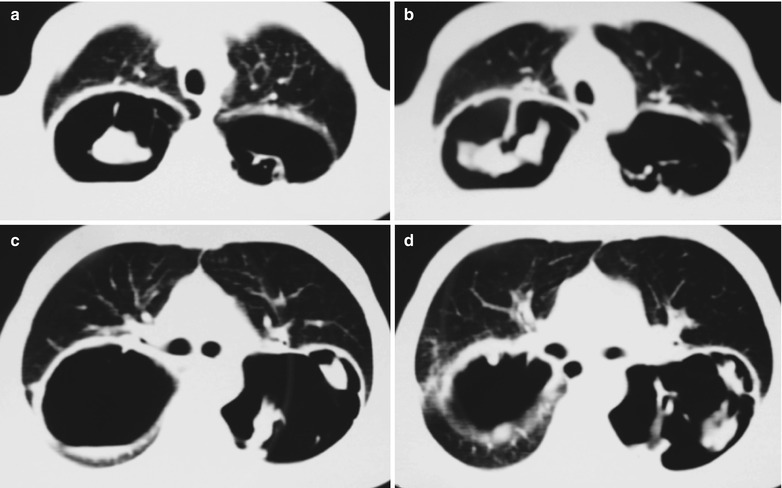
(a–d) HIV/AIDS related pulmonary aspergillosis infection. (a, b) CT scanning of the pulmonary window demonstrates a huge cavity shadow in the dorsal segment of the right lower lung, large nodular shadows in it, liquid gas level in the basal cavity, and the evenly thick wall. (c, d) CT scanning of the mediastinal window in the prone posture demonstrates a huge cavity shadow in the dorsal segment of the left lung, multiple large nodular shadows in it, and involved pleura of the lateral chest wall
Case Study 7
A male patient aged 38 years was confirmatively diagnosed as having AIDS by the CDC. He complained of high fever, cough and chest distress. His CD4 T cell count was 76/μl.
Fig. 17.71.
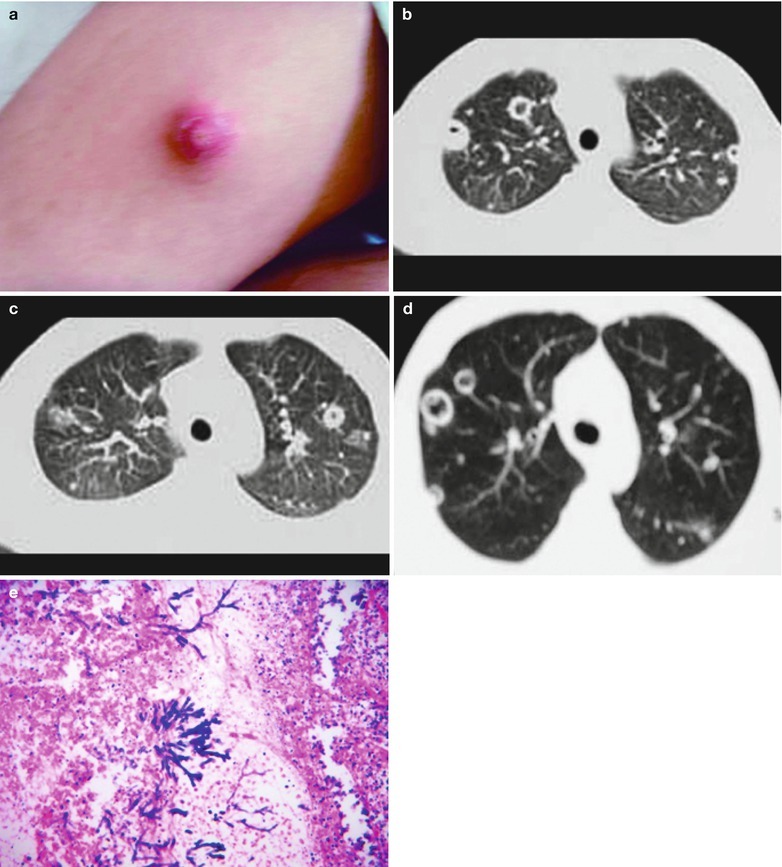
(a–e) HIV/AIDS related pulmonary aspergillosis infection. (a) The gross specimen demonstratesAspergillus abscess in the skin of the forearm. (b–d) CT scanning of the pulmonary window demonstrates multiple round liked nodular shadows and cavity shadows in both lungs, even thickness of cavity wall, and small nodular shadows in some cavities. (e) Pulmonary Aspergillus infection, demonstration purplish blue branches liked or grasses liked growth of hyphae, HE × 400
Case Study 8
A male patient aged 26 years was confirmatively diagnosed as having AIDS by the CDC. He had history of drug abuse and complained of nausea, vomiting gastric contents after meals, abdominal distension, abdominal pain and shortness of breath. By physical examinations, cardiopulmonary auscultation positive, edema of lower limbs. By laboratory tests on admission, WBC 15.8 × 109/L, NEuT 0.534 %, RBC 3.08 × 1012/L, HGB 77 g/L, LYM 0.334 %, GLu 1.1 mmol/L, APTT 64.4 s; liver functions: TBIL 24.6 μmol/L, ALT 88.0 u/L, AST 528 u/L; electrolyte K 5.8 mmol/L; renal functions: HCO3 − 10.1 mmol/L, blood tuberculosis antibody positive, Ascites protein positive, karyocyte 900 × 106/L. Oxygen inhalation was prescribed, with anti-PCP, anti-infection, anti-virus, intravenous dripping, therapies for electrolytes and acid-base balance, diuresis therapies are administered after hospitalized. After 6 o’clock pm, he showed exacerbated shortness of breath, which was diagnosed as episode of PCP. The emergency rescuing was ineffective and death occurred. By B ultrasound, there were hepatosplenomegaly, diffuse lesions in both kidneys, and large quantity ascites.
Fig. 17.72.
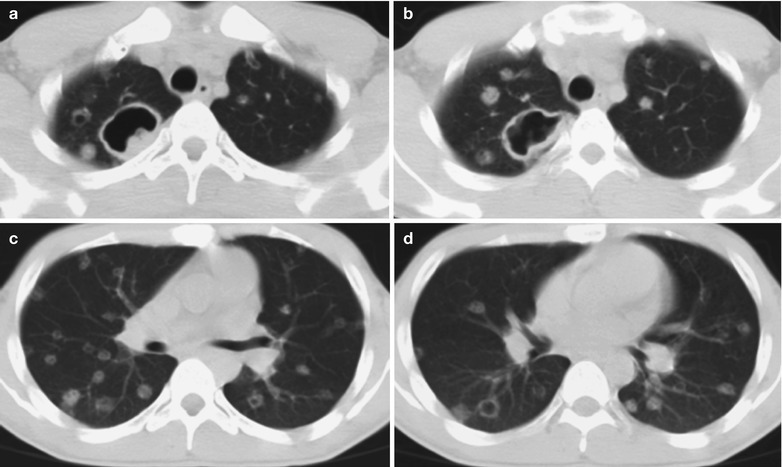
(a–d) HIV/AIDS related pulmonary aspergillosis infection. (a–d) CT scanning of the pulmonary window demonstrates multiple scattered round liked nodular shadows and cavity shadows in both lungs, with even wall thickness and small nodular shadows in some cavities
Case Study 9
A male patient aged 48 years was confirmatively diagnosed as having AIDS by the CDC. He complained of high fever, cough and shortness of breath. His CD4 T cell count was 19/ μl.
Fig. 17.73.
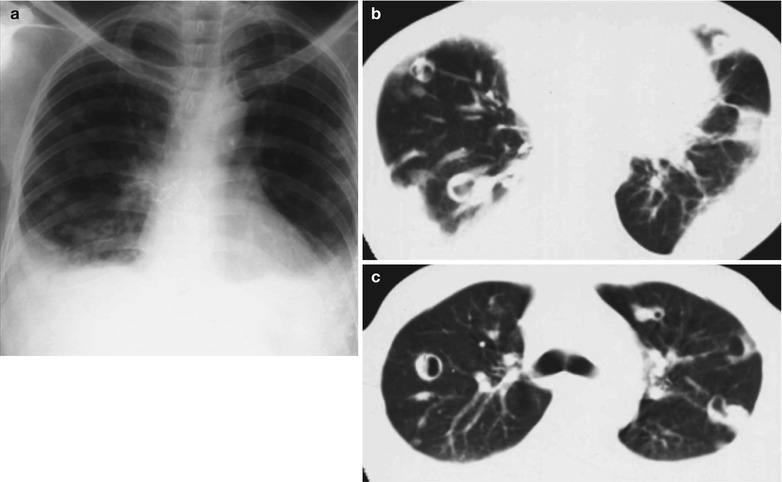
(a–c) HIV/AIDS related pulmonary aspergillosis infection. (a) DR demonstrates diffuse dense shadows in both lower lung fields which is in a arcuate surface with exterior high and interior low (pleural effusion). (b, c) CT scanning of the mediastinal window demonstrates multiple scattered round liked nodular shadows and thick-wall cavity shadows in both lungs, with small nodular shadows in the cavities; thickened pleura of the lateral chest wall, with accompanying encapsulated effusion
Case Study 10
A male patient aged 39 years was confirmatively diagnosed as having AIDS by the CDC. He complained of high fever, cough and chest distress for 1 month. His CD4 T cell count was 29/μl.
Fig. 17.74.
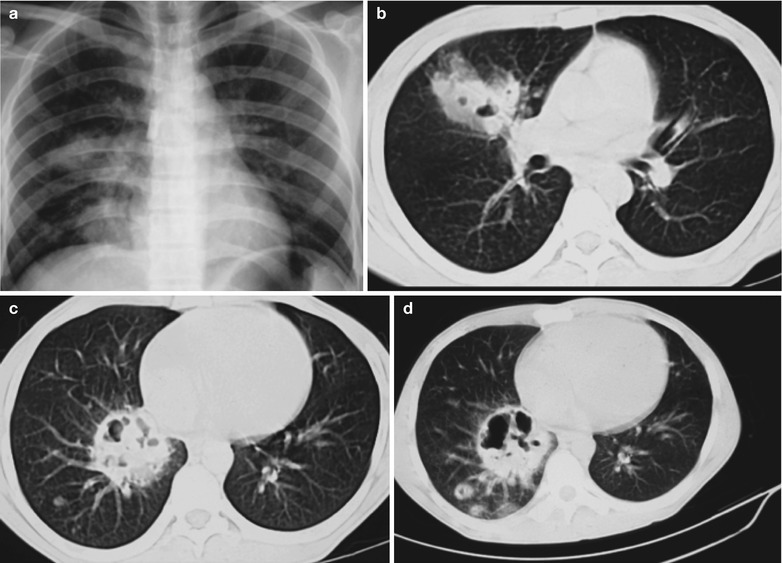
(a–d) HIV/AIDS related pulmonary aspergillosis infection. (a) DR demonstrates round liked uneven density shadows in the medial segments of both middle and lower lungs, multilocular hollow holes in the cavities, surrounding multiple round liked thick-wall small cavity shadows and ground grass liked infiltration shadows. (b–d) CT scanning of the pulmonary window demonstrates scattered round liked uneven density shadows in the right hilum and lower lung, multilocular hollow holes in the cavities; and surrounding multiple round liked thick-wall small cavity shadows and ground grass liked infiltration shadows
Case Study 11
A male patient aged 34 years was confirmatively diagnosed as having AIDS by the CDC. He complained of high fever, cough and chest distress for 3 months. His CD4 T cell count was 49/μl.
Fig. 17.75.
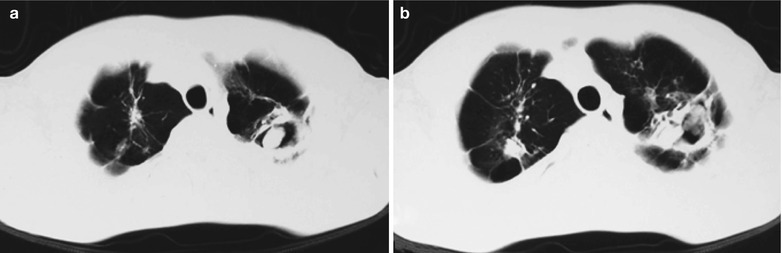
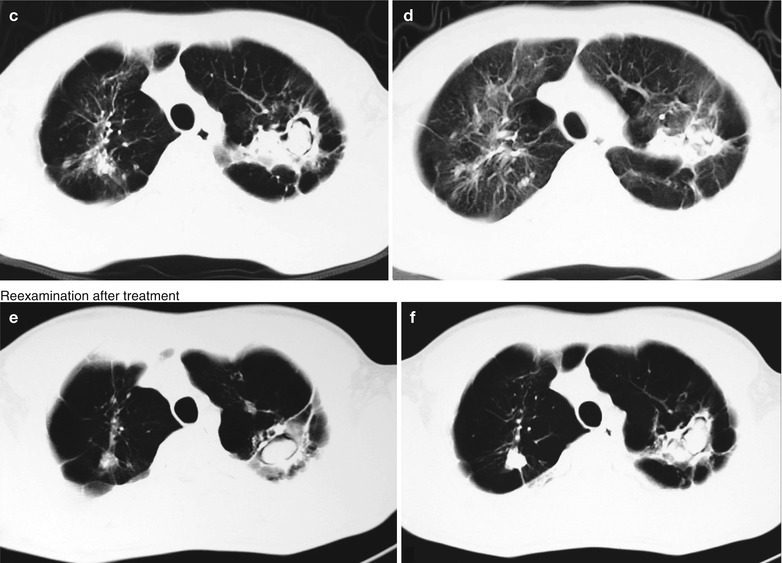
(a–f) HIV/AIDS related pulmonary aspergillosis infection. (a–d) CT scanning of the pulmonary window demonstrates multiple scattered round liked nodular shadows and irregular thick-wall cavity shadows in both lungs, oval or sphere shaped nodular shadows in the cavities with smooth boundaries. (e, f) Reexamination after treatment demonstrates multiple scattered round liked nodular shadows and irregular thick-wall cavity shadows in both lungs, oval nodular shadows in the cavity with smooth boundaries. Compared to the previous imaging findings, the lesions are shrunk, with improved surrounding infiltration
Case Study 12
A male patient aged 35 years was confirmatively diagnosed as having AIDS by the CDC. He was hospitalized due to complaints of fever for 1 week, chest distress and shortness of breath for 1 day. On admission, he was confirmed as HIV positive, with a CD4 T cell count of 9/μl. By physical examinations, he was in poor physical conditions, respiratory rate 27/min, lips cyanotic, coarse breathing sounds of both lungs with small quantity dry rales. His conditions progressed rapidly and death occurred due to respiratory failure after 3 days.
Fig. 17.76.
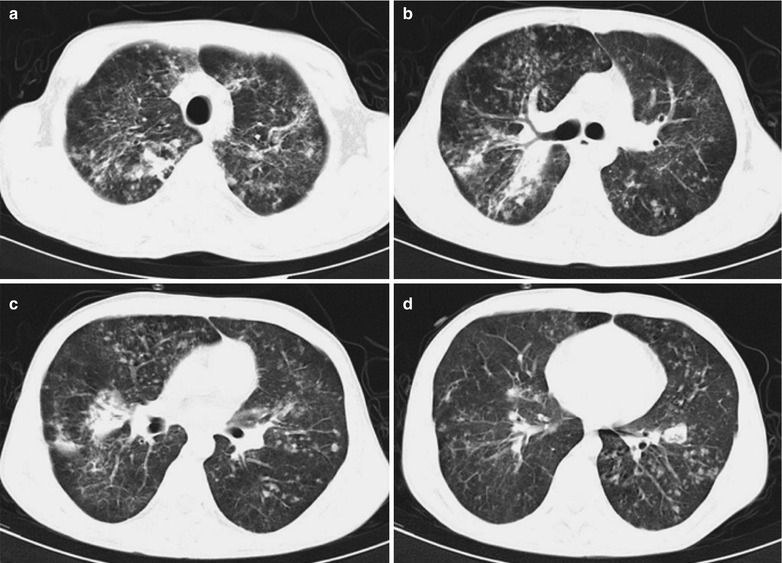
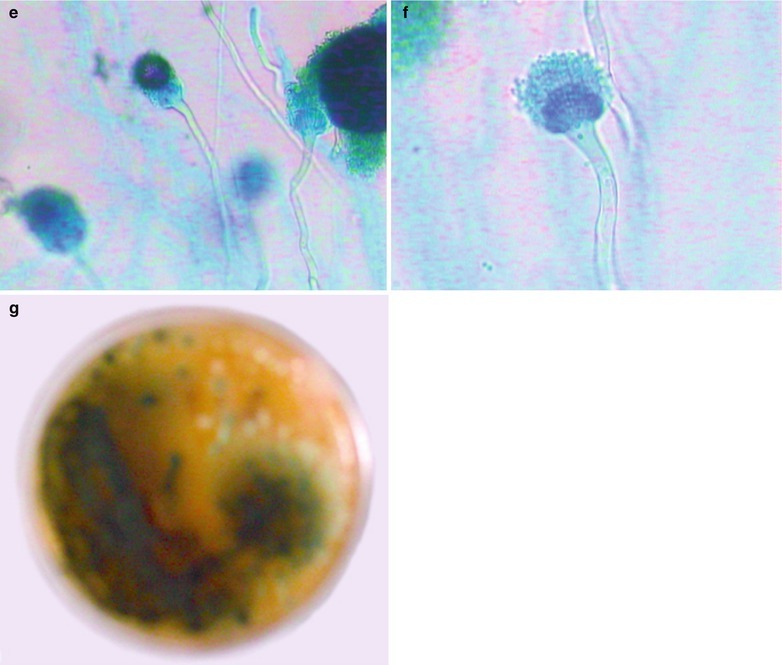
(a–g) HIV/AIDS related pulmonary aspergillosis infection. (a–d) CT scanning demonstrates diffuse scattered thin ground glass liked, patchy, flaky blurry shadows and cords liked shadows in both lungs, with blurry boundaries and uneven density; scattered nodular shadows in different sizes; more lesions in both upper lobes and the right middle lobe; flaky parenchyma shadows in the apical and posterior segments of right upper lobe, with air bronchogram sign in them; unobstructed opening of bronchi as well as lobar and segmental bronchi without stenosis and obstruction; lymphadenectasis in the right hilar region; detected Aspergillus fumigatus by sputum culture. (e, f) Culture for 72 h, lactic acid gossypol blue staining and microscopic observation at ×200 and ×400 demonstrate short column liked conidial head, smooth wall of conidiophores, flask-shaped top capsule and monolayer microconidiophores. (g) Culture in Paul’s medium demonstrates dark green colored colonies
Case Study 2
A female patient aged 30 years was confirmatively diagnosed as having AIDS by the CDC. She complained of high fever or irregular fever, cough and shortness of breath. Her CD4 T cell count was 56/μl.
Diagnostic Basis
Fiberobronchoscopy Lavage and Biopsy
Lung and pleura puncture for biopsy can detect the growth of Aspergillus hyphae.
Sputum Culture
Sputum culture can detect Aspergillus hyphae, with findings of Aspergillus fumigatus positive.
Immunologic Assays
In the cases with allergic bronchopulmonary aspergillosis, the serum IgE is above 2,500 μg/L. Skin test of aspergillus antigen is positive. The serum anti-Aspergillus antigen IgG antibody precipitin is positive.
Diagnostic Imaging
Characteristic CT scanning demonstrations of HIV/AIDS related parasitic aspergillar glomera include pulmonary cavities or cavity lesions with spherical contents, smooth boundaries of the spherical contents with even density, lunate shaped or ring shaped transparent shadows between cavity or cavity walls and the contents, migration of the contents with the body postures. According to the pathological and imaging demonstrations, it can be divided into three major types:
Vascular Invasion Type of Pulmonary Aspergillosis
In the early stage, CT scanning demonstrates soft tissue density nodules or light ground glass liked halo sign around the mass, which is the evidence for the diagnosis of the invasion type pulmonary aspergillosis. Air cresent sign refers to round pulmonary infiltration with accompanying central necrosis and surrounding lunate or ring shaped cavity. Other non-characteristic CT scanning demonstrations include multiple lobular parenchyma lesion shadows or lobular fusion shadows, parenchyma lesion shadows in the lobes, segments and subsegments, nodular or mass shadows and thin/thick wall cavities or low density areas in the mass shadows.
Airway Invasion Type of Pulmonary Aspergillosis
It is demonstrated to have parenchymal lesions around the airway or/and central small nodules in the lower lobes. The parenchymal lesions prove the occurrence of mycotic bronchopneumonia.
Allergic Bronchopulmonary Aspergillosis
The most common imaging finding is the thickened bronchial wall. Central bronchiectasis is its characteristic demonstration. In the cases of dilated bronchi containing sputum bolt or mucus, it shows fingertip shaped or toothpaste shaped shadow, which should be considered as its characteristic demonstration.
Differential Diagnosis
Congenital Bronchial Atresia
HIV/AIDS related pulmonary aspergillosis should be differentiated from congenital bronchial atresia. Most cases of the congenital bronchial atresia are atresia at the proximal pulmonary segment of the bronchi, often with a clearly defined mass. In the typical cases, there are bronchial branches and more branches in fingertip shape, pointing to the pulmonary hilum. Confined pulmonary air retention in the pulmonary lobe and segment of the atresic bronchi is the important evidence for the diagnosis of congenital bronchial atresia.
Allergic Bronchial-Pulmonary Aspergillosis
HIV/AIDS related pulmonary aspergillosis should be differentiated from allergic bronchial-pulmonary aspergillosis. In the cases of allergic bronchial-pulmonary aspergillosis have no clearly defined mass, with demonstrations of V shaped, Y shaped, grapes shaped or fingertip shaped shadows with clearly defined boundaries, which are characteristic in those patients with bronchial asthma or a case history of exposure to dusts containing fungi. There is also increased proportion of eosinophilic granulocytes in the peripheral blood. Detection of aspergillus in phlegm can define the diagnosis.
Central Lung Cancer
HIV/AIDS related pulmonary aspergillosis should be differentiated from central lung cancer. Central lung cancer also can cause mucus impaction of the distal bronchi, with manifestations of bronchial arctia and/or truncation, and the surrounding soft tissue mass shadows.
Pulmonary Cavities and Abscesses Induced by Tuberculoma Dissolved, Secondary Pulmonary TB, Chronic Lung Abscess and Peripheral Lung Cancer as Well as Cystic Bronchiectasis
HIV/AIDS related pulmonary aspergillosis should be differentiated from pulmonary cavities and abscesses induced by dissolved tuberculoma, secondary pulmonary TB, chronic lung abscess and peripheral lung cancer as well as cystic bronchiectasis. Except aspergilloma, spheric morphology caused by other causes is commonly irregular. The cavity contents cannot migrate with body postures, which is the key point for the differential diagnosis.
Discussion
HIV/AIDS related pulmonary aspergillosis can be caused by many pathogenic bacteria and aspergillus fumigatus is the most common one. The infection is often caused by inhaled aspergillus fumigatus in the environment. Vascular invasion type of pulmonary aspergillosis usually has multiple lesions and nodular changes. Generally in pathology, the center of nodule presents typical pale color; commonly with fibrous ring surrounding the nodules resulted from hemorrhage and/or lung parenchymal changes. Histologically, they are characterized by coagulative necrosis of the lung tissues, infiltration of large quantity hyphae in the necrotic tissue, pulmonary vascular infiltration, but usually without responses of vasculitis and thrombosis. The enzymes released by neutrophile granulocytes can cause the separation of necrotic tissue from its adjacent lung tissues to form necrotic mass in the cavities. Airway invasion type of pulmonary aspergillosis, also called aspergillus bronchopneumonia, accounts for 15–30 % of invasive aspergillosis. The most common imaging findings are unilateral/bilateral flaky parenchymal changes, centrilobular small nodules and branches liked linear shadows (tree buds sign). Histologically, it is characterized by necrosis and infiltration of neutrophil granulocytes. The lesions surround the bronchiole and the bronchiole. The invasion of the pulmonary artery can cause bleeding of the adjacent pulmonary parenchyma. Allergic bronchopulmonary aspergillosis rarely has lesions, with no unknown pathogenesis, which is generally believed to be related to type I and type II allergic reactions. It usually shows obvious asthma related mucosal lesions. Hyphae generated by aspergillus fumigatus can induce the production of mucus and additional mucosal lesions, eventually leading to bronchiectasis. Dilatate bronchial lumen is filled with mucus or with absence of epithelium, which is replaced by a granulomatous inflammatory infiltration. The most common imaging manifestations are migratory flocculent, branched Y shaped and V shaped (fingertip sign) shadows in the pulmonary parenchyma, which are related to the infiltration of eosinophils. Pathologically, bronchial cystic dilatation in the pulmonary segment and sub-segment occurs, with large quantity eosinophils in the bronchial mucus and scattered broken aspergillus hyphae. In combination with the case history, the diagnosis can be defined.
HIV/AIDS Related Pulmonary Cryptococcus Infection
Pathogens and Pathogenesis
Compromised immunity is an important cause of cryptococcosis, especially in patients with AIDS or abnormal lymphoproliferative diseases. Cryptococcus neoformans, a single phase mould, exists widely in the natural world. The cryptococcus has a diameter of less than 10 μm, which can be inhaled into the human body via respiratory tract. Under the impact of a high concentration carbon dioxide, it forms a clearly defined protective layer composed of polysaccharide capsule to antagonize the defense mechanisms of the host. Thus, lung infection occurs after its inhalation in immunocompetent people, which is commonly asymptomatic. of cryptococcus, Inhalation of cryptococcus by AIDS patients can lead to hilar lymphadenopathy, as well as singular or multiple subpleural small nodules, being similar to those in the cases of Mycobacterium tuberculosis infection.
Pathophysiological Basis
In the early stage of cryptococcal infection, only a mild inflammatory reaction or diffuse infiltrative exudative changes occur. But in the advanced stage, necrosis, suppuration or granuloma is formed. Large quantity hyphae can be found in the focus. In the cases with hyphae penetrating the blood vessels, vasculitis, perivascular inflammation and thrombosis occur. And thrombosis leads to ischemia and necrosis of the tissue (Fig. 17.77).
Fig. 17.77.
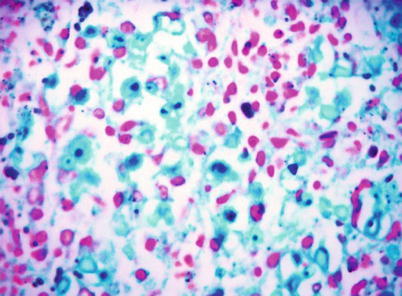
HE staining demonstrates isseminated cryptococci, the Cryptococcus is stained red after mucin carmine staining of cryptococcus neoformans spores in lungs, (HE × 200)
Clinical Symptoms and Signs
Pulmonary cryptococcus infection in AIDS patients often is extensively disseminating, with symptoms of fever, cough, difficulty breathing, expectoration, chest pain caused by pleuritis, and even acute respiratory distress syndrome (ARDS).
Examinations and Their Selection
Chest X-ray and CT scanning are the most commonly used diagnostic imaging examinations.
Laboratory tests include India ink smear or culture of phlegm, chest liquid and CSF; and complement binding reaction test.
Routine blood test can detect slightly and moderately increased WBC count and neutrophils.
Imaging Demonstrations
HIV/AIDS related pulmonary cryptococcus infection has no characteristic imaging demonstrations. Chest X-ray and CT scanning show multiple morphology of the lesions. In the slight cases, there are thickened pulmonary markings in both lower lungs or isolated nodular shadows, and occasionally cavities. In the cases of acute interstitial inflammation, there are diffuse infiltrative or miliary foci, with infiltration, nodules or exudation in any lobe which is more common in bilateral middle and lower lungs, in unilateral lung or confined to one lobe. The foci may be isolated huge spherical or multiple nodular, without obvious surrounding inflammatory responses, similar to those of tubercles or tumors. Otherwise, they are diffuse miliary shadows or flaky infiltrative shadows.
Case Study 1
A male patient aged 38 years was confirmatively diagnosed as having AIDS by the CDC. He had history of paid blood donation for more than 100 times from the year of 1990 to 1994 and was detected HIV positive on Jan. 18, 2004. His CD4 T cell count was 246/μl in the year of 2004, 212/μl in 2005, 120/μl in 2006, 166/μl in 2007, and 27/μl in 2008. He complained of intermittent diarrhea, fever and chest distress for more than 4 months, with alternate occurrence of intermittent diarrhea and fever by watery stool.
Fig. 17.78.
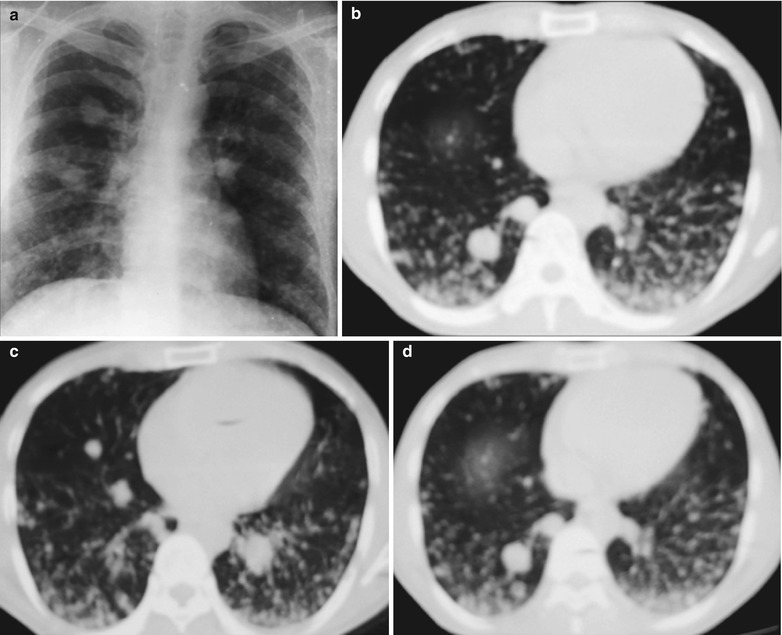
(a–d) HIV/AIDS related pulmonary cryptococcus infection. (a) DR demonstrates multiple scattered nodular and miliary shadows in both lungs. (b–d) Chest CT scanning demonstrates multiple dense nodular shadows with different sizes in both lungs, with clear boundaries. They are intensively distributed in the dorsal segment and the largest one has a diameter of about 2 cm
Case Study 2
An AIDS patient was confirmatively diagnosed by the CDC. He sustained pulmonary Cryptococcus infection.
Fig. 17.79.
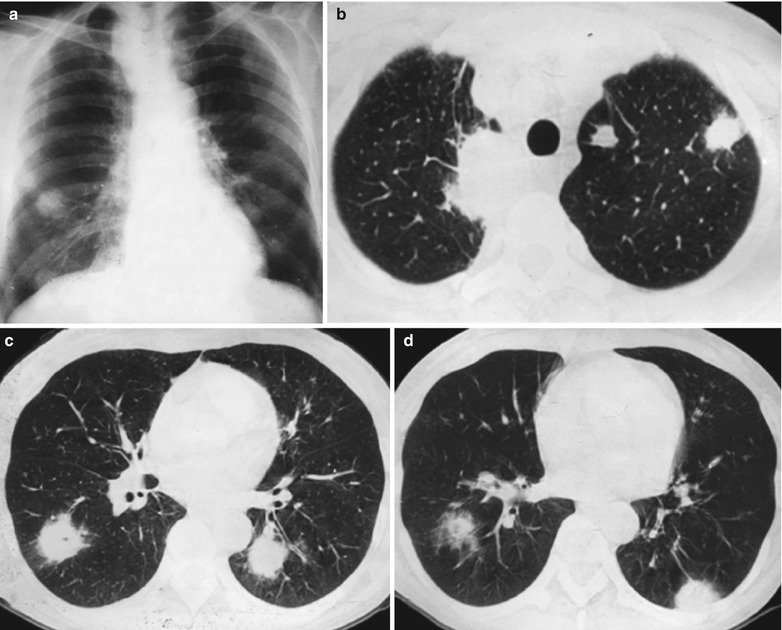
(a–d) HIV/AIDS related pulmonary cryptococcus infection. (a) DR demonstrates multiple scattered nodular shadows in both lower lungs. (b–d) Chest CT scanning demonstrates multiple dense nodular shadows with different sizes and mass shadows in both middle and lower lung fields, with clear boundaries. They are more common in the dorsal segments
Fig. 17.80.
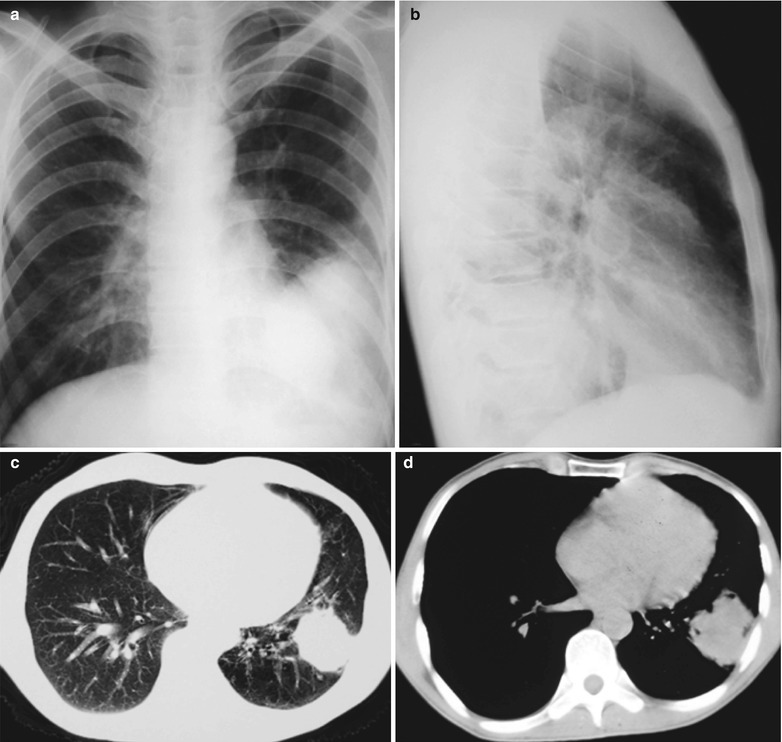
(a–d) HIV/AIDS related pulmonary cryptococcus infection. (a, b) Anteroposterior and lateral DR demonstrate a huge dense mass shadow in the left lower lung, with clear boundary. (c) CT scanning of the pulmonary window demonstrates round liked high density shadow in the left lower lung near left chest wall, with even density. (d) CT scanning of the mediastinal window demonstrates round liked soft tissue density shadows in the left lower lung near left chest wall, with even density, lobulation, and surrounding thick spikes
Case Study 4
Fig. 17.81.
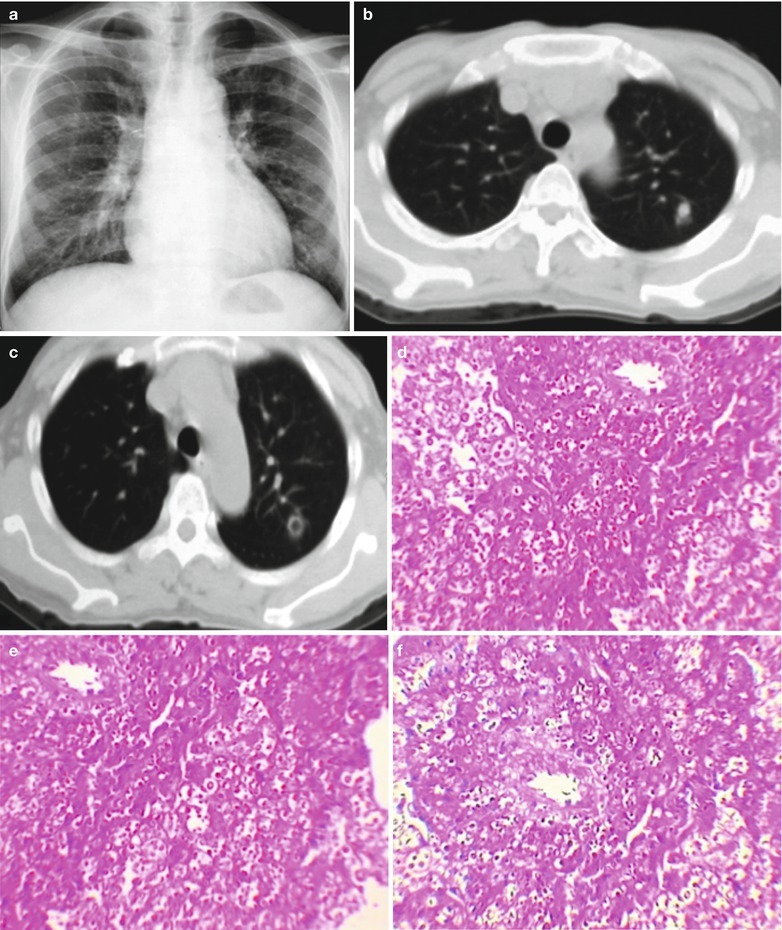
(a–f) HIV/AIDS related pulmonary cryptococcus infection. (a) DR demonstrates thickened lung markings in both lungs and flaky blurry shadows in the left lower lung. (b, c) CT scanning demonstrates round liked nodular and small cavity shadows in the left upper and lower lung, with clear boundaries. (d–f) Pathology demonstrates transparent substrate in lung tissues and many bi-capsular cryptococci in cytoplasm
Case Study 5
An AIDS patient was confirmatively diagnosed by the CDC. He/She was diagnosed as having pulmonary Cryptococcus infection.
Fig. 17.82.
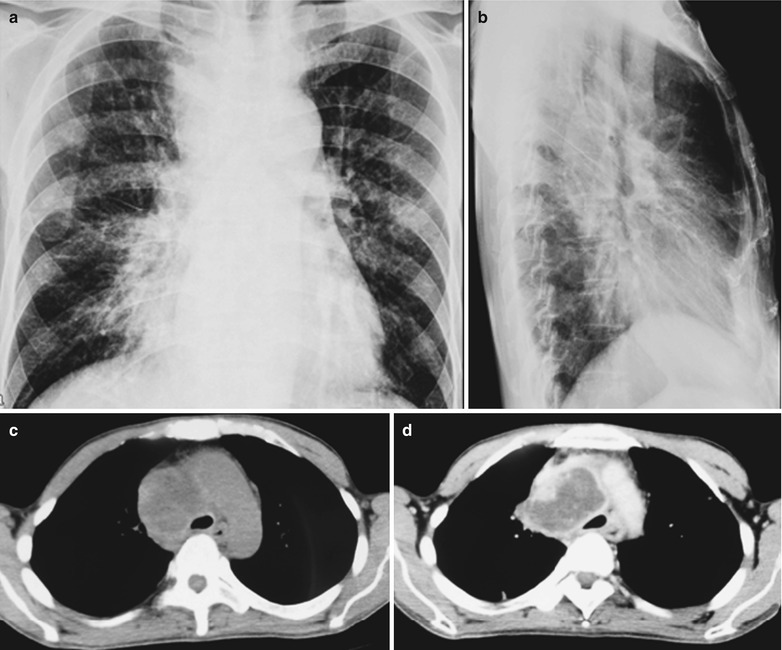
(a–d) HIV/AIDS related pulmonary cryptococcus infection. (a, b) DR demonstrates enlarged blurry hilum in both lungs and thickened pulmonary markings. (c, d) CT scanning demonstrates mediastinal lymphadenectasis, narrowed trachea due to compression with liquefactive necrosis. Enhanced scanning demonstrates marginal enhancement and no central enhancement
Case Study 6
Fig. 17.83.
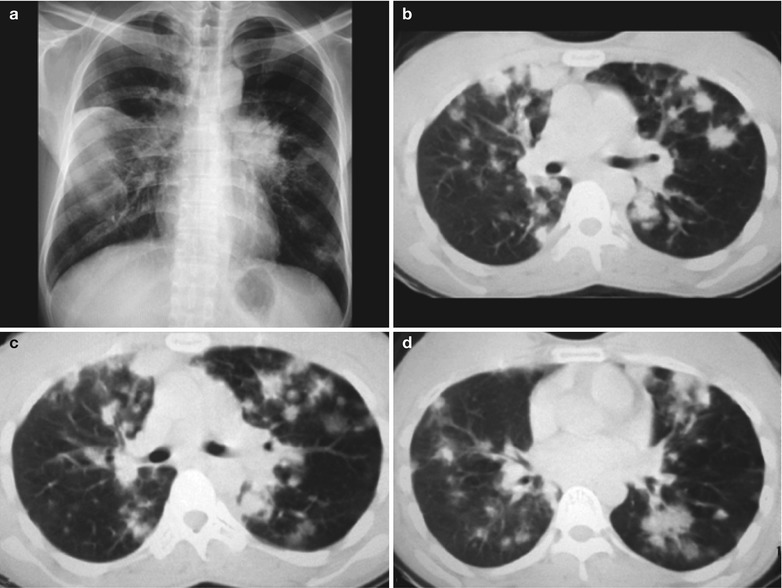
(a–d) HIV/AIDS related pulmonary cryptococcus infection. (a) DR demonstrates enlarged blurry hilum in both lungs and thickened lung markings. (b–d) CT scanning demonstrates multiple scattered nodular or mass dense shadows in both lungs, with lobulation, rough spikes around and fusion of some shadows into mass; surrounding small flaky infiltrative shadows; and mediastinal lymphadenectasis
Case Study 7
A male patient aged 60 years was confirmatively diagnosed as having AIDS by the CDC. He was hospitalized on 2009-2-19 due to headache and vomiting for more than 10 days. In the CSF, Cryptococcus was found. By blood and sputum culture, cryptoccocus was detected. The diagnosis was cryptococcal meningitis, cryptococcal pneumonia and cryptococcal sepsis. After receiving amphotericin B antifungal treatment and dehydration, headache and vomitting were relieved. But chest CT scanning reexamination demonstrated increased pulmonary lesions, which was considered as tuberculosis. On 2009-4-1, he was given HERV anti-tuberculosis treatment. Twenty days ago he sustained weakened lower limbs, which gradually aggravated and completely paralyzed in the recent 1 week, His CD4 T cell count was 34/μl.
Fig. 17.84.
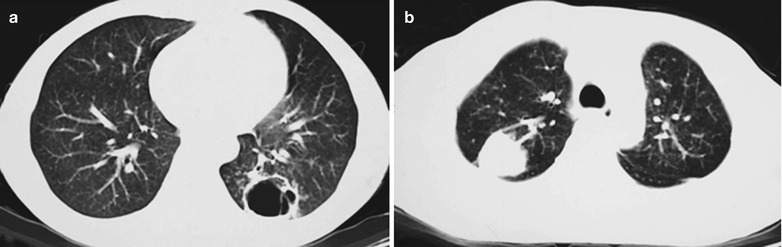
(a, b) HIV/AIDS related pulmonary cryptococcus infection. (a) CT scanning demonstrates round liked cavity shadows in the left upper and lower lung, with uneven thickness of the cavity wall and surrounding infiltrative shadows. (b) CT scanning demonstrates round liked dense mass shadows in the right upper lung, with clear boundaries and bulky drainage vessel shadows
Case Study 8
A male patient aged 50 years was confirmatively diagnosed as having AIDS by the CDC.He complained of cough and chest distress for 3 months. His CD4 T cell count was 35/μl.
Fig. 17.85.
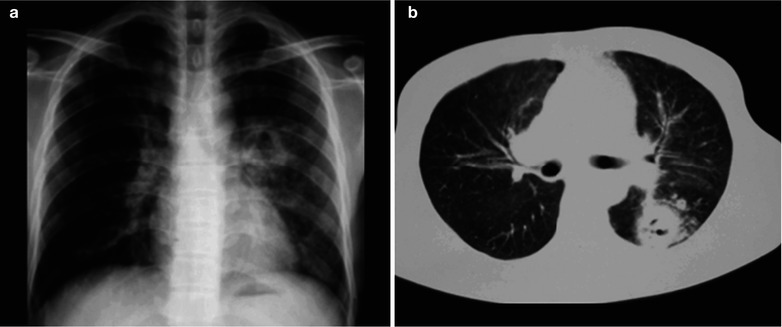
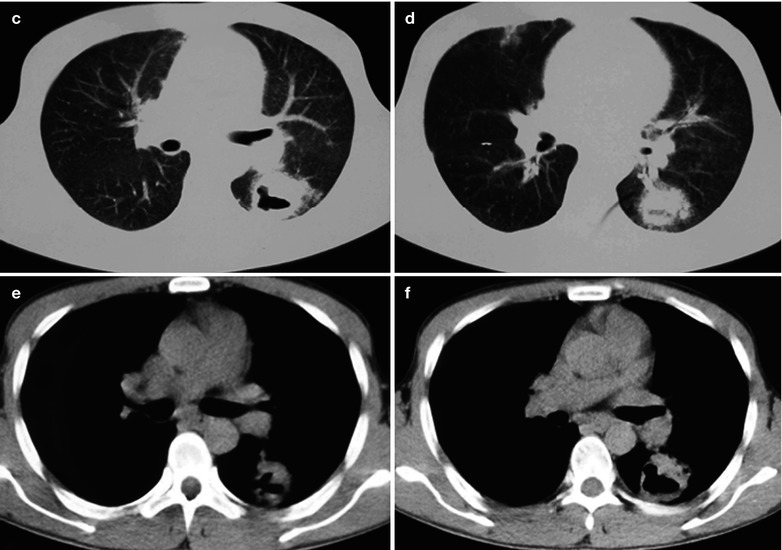
(a–f) HIV/AIDS related pulmonary cryptococcus infection. (a) DR demonstrates round liked thick-wall cavity in the left hilum, with blurred boundary; ground-glass liked shadows with increased density in the left middle and lower lung. (b–d) CT scanning of the pulmonary window demonstrates thick-wall cavity in the dorsal segment of the left lower lung, with uneven thickness of cavity wall. (e, f) CT scanning of the mediastinal window demonstrates thick-wall cavity in the dorsal segment of the left lower lung, with uneven thickness of the cavity wall and surrounding thick spikes
Case Study 3
A female patient aged 54 years was confirmatively diagnosed as having AIDS by the CDC. She complained of repeated fever, headache and vomiting for more than 10 days. More than 10 days ago, she was confirmatively diagnosed as having cryptococcus infection by lumbar puncture for the second time. On May 25, 2009, pressure at lumbar puncture 100 mmH2O; Biochemistry findings: mononuclear cells 74 %, lobulated neutrocytes 26 %, WBC 0.1 × 109/L, PanDi’s test ++, ALT 20 u/L and AST 24 u/L. On Jun. 6, 2009, pressure at lumbar puncture 330 mmH2O; Biochemistry findings: mononuclear cells 74 %, lobulated neutrocytes 26 %, WBC 0.1 × 109/L, PanDi’s test +, cryptococcus (+). Her CD4 T cell count was 153/μl.
Diagnosis Basis
Most AIDS patients are asymptomatic, but they may have low-grade fever and slight cough, usually without positive signs.
Findings of cryptococcus by sputum smear and India ink staining or culture can define the diagnosis.
Blood related antibodies positive by indirect immunofluorescence test can assist the diagnosis.
Findings of Cryptococcus by percutaneous lung biopsy, PAS staining or Alcian blue staining.
Imaging demonstrations of thickened lung markings, scattered nodular or isolated spherical mass under the pleura and surrounding infiltrative lesions commonly in the dorsal segment of the lower lungs, and rarely cavities and mediastinal lymphadenectasis.
Differential Diagnosis
HIV/AIDS related pulmonary cryptococcus infections should be differentiated from tuberculosis, primary or metastatic lung cancer. Tuberculosis mostly is secondary tuberculosis, which is caused by repeated infections of tubercule bacillus. Lesions show flaky or flocculent shadows in the two upper lungs, with blurry boundaries. The wrapped necrotic foci by fibers develop into nodules. It can also show miliary shadows, mostly with mediastinal lymphadenectasis. It should also be differentiated from primary or metastatic lung cancer.
Discussion
Cryptococcus is a relatively common pathogen of pulmonary fungal infection, and mostly develops in AIDS patients. Usually, it is a disseminated disease, with common involvement of the central nervous system and the lungs. In immunocompetent patients, the nodular granuloma caused by the pathogen is similar to those of other pulmonary fungal infections. In patients with serious immunosuppression, wide tissue infiltration of the pathogens may occur in the lungs. A series of imaging demonstrations of pulmonary cryptococcus infection includes reticular or reticulated nodular interstitium. Other less common demonstrations are ground-glass liked shadows, parenchymal changes of air cavity and miliary nodules. The pathogenic fungi can be found mainly in the pulmonary interstitium. Imaging findings include singular or multiple nodules or masses, parenchymal changes of lung lobes and lung segments with clear or unclear boundaries in size of 1–10 cm. There may be also miliary lesions, lymphadenectasis and cavity shadows.
HIV/AIDS Related Pulmonary Candida Infection
Pathogens and Pathogenesis
Candida is an opportunistic pathogen, which widely exists in nature. Candida albicans parasitize in the oral cavity, laryngopharynx, upper respiratory tracts, vaginal and intestinal mucosa of human being. Pulmonary and bronchial moniliasis is commonly caused by candida albicans which has the strongest pathogenicity. After its invasion into the tissues, candida transforms into hyphae and multiplies in a large quantity, with strong toxicity and ability to fight against phagocytosis. AIDS patients may have disseminated pathological changes. Only when the immunity is compromised, the pathogen invades into the bronchus or lungs to cause diseases. Therefore, pulmonary candida infection is commonly secondary.
Pathophysiological Basis
Candidosis can cause acute inflammation in bronchus and lungs, mainly exudation of neutrophils, which can be divided into two types: bronchitis type and pneumonia type. The pathological changes in the early stage are acute suppurative inflammation, accompanied with the formation of abscesses. By the naked eyes observation, they are large flaky parenchymal changes, with central grayish white coagulative necrosis. Under a microscope, the lesions are large flaky caseous necrosis, accompanied with the formation of abscesses, and surrounding infiltration of hyphae and phagocytes. In the advanced, there are caseous necrosis, formation of cavities, fibrosis and granuloma.
Clinical symptoms and signs
Bronchitis Type
Symptoms in AIDS patients are mild, with frequent cough, with a small amount of white mucous phlegm or thick phlegm, no fever or low grade fever; scattered spots of white membranes in the mucosa of oral cavity, throat and bronchus. Dry rales can be heard occasionally in both lungs.
Pneumonia Type
In AIDS patients, the manifestations are mostly acute pneumonia or sepsis, with chills, fever, cough, expectoration of white mucous jelly liked phlegm or thick phlegm often with blood or necrotic tissue. The thick sputum, candidal hyphae and shedding cell debris can be condensed into small colloid clumps, with yeast smell. Other symptoms include even haemoptysis and difficulty breathing. Dry and moist rales can be heard in lungs.
Allergy Type
Symptoms may be difficulty breathing, rhinocnesmus, runny nose and sneezing. Wheezing rales can be heard in both lungs.
Examinations and Their Selection
Chest X-ray and CT scanning are the most commonly used diagnostic imaging examinations.
Sputum smear for direct microscopy can show candida. By fungus culture, the fungus strain can be identified based on colonies growth and microscopy.
Histopathologically, fiberobronchoscopy lavage for smear and biopsy can be performed.
By fungus skin test, candida skin test can provide information for the diagnosis of candida infection. Generally, the results are positive. In the serious cases, the results may be negative.
Fluorescent antibody test for direct smear specimens, colonies by fungus culture and pathohistological tissue section has certain value in the detection of the pathogens.
Molecular biology can be applied to detect the pathogen.
Candida metabolites test and PCR can be applied to determine the gene sequence of candida for the early diagnosis.
Imaging Demonstrations
Chest X-ray
Chest X-ray demonstrates nodular shadows and flaky parenchyma changes in unilateral or bilateral lungs. Sometimes there is miliary infection.
CT Scanning
CT scanning demonstrates most lesions in the middle and lower lung fields, with rare involvement of the apex. There are thickened lung markings or diffuse small flaky/patchy shadows, some of which can fuse into large flaky dense shadows, with blurry boundaries. Nodules, due to bleeding around it, may be surrounded by ground glass liked shadows, which is necrotic bronchopneumonia, usually accompanied with a large quantity neutrophils.
Case Study
A male patient aged 40 years was confirmatively diagnosed as having AIDS by the CDC. He complained of high fever, cough, expectoration, chest pain and shortness of breath, with pulmonary parenchymal changes sign and moist rales. His CD4 T cell count was 18/μl.
Fig. 17.86.
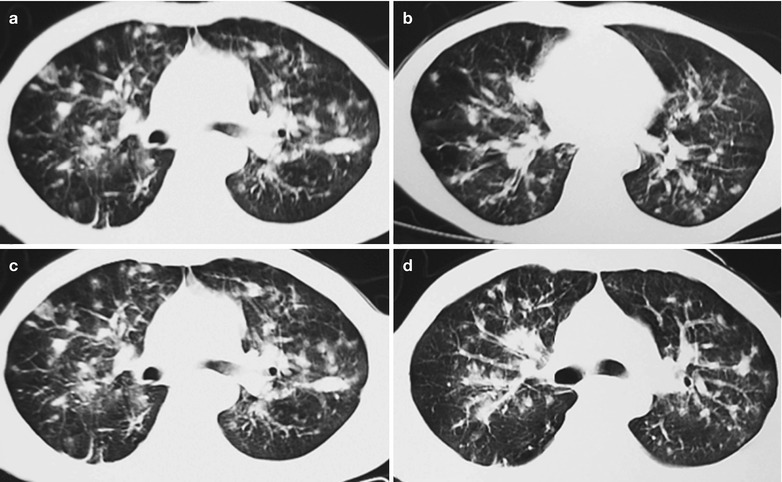
(a–d) HIV/AIDS related pulmonary candida infection. (a–d) CT scanning demonstrates thickened and deranged lung markings in both lungs, diffuse small flaky or patchy shadows, fusion of some small shadows into large flaky dense shadows, with blurry boundaries, enlarged hilum and blurry structures
Diagnostic Basis
Clinical Symptoms
Cough expectoration with white mucous phlegm or thick phlegm, hemoptysis and shortness of breath.
Signs
Examinations of the oral cavity and the throat demonstrate spots liked white membrane covering the surface, and dry and moist rales in the lungs.
Cultures
Successive cultures of phlegm, lung tissue, pleural fluid or cerebrospinal fluid repeatedly demonstrate the same strain of candida, or direct microscopic findings of large quantity pseudohyphae or hyphae and groups of spores can define the diagnosis.
Chest X-ray and CT Scanning
It is demonstrated to have thickened and deranged lung markings in double lung, diffuse small flaky/patchy shadows, fusion of some small shadows into large flaky dense shadows, with blurry boundaries, enlarged hilum and blurry structures. The conditions progress rapidly, with repeated lesions occurrence.
Differential Diagnosis
Bacterial Pneumonia
HIV/AIDS related pulmonary candida infection should be differentiated from bacterial pneumonia. Bacterial pneumonia often has symptoms such as high fever, cough, expectoration, chest pain and shortness of breath. CT scanning demonstrates flocculent infiltrative shadows or parenchyma changes and cavities. The pathogen can be detected in the sputum or chest liquid.
Virus Pneumonia
HIV/AIDS related pulmonary candida infection should be differentiated from virus pneumonia. Viral pneumonia firstly causes upper respiratory tract infection, which spread downward to cause pulmonary inflammation. The demonstrations include ground glass liked changes in the lung fields or mass shadows. The definitive diagnosis should be based on throat swabs, virus isolation from the sputum and serum specific antibodies test.
Pulmonary Tuberculosis
HIV/AIDS related pulmonary candida infection should be differentiated from pulmonary tuberculosis. In the early stage, the symptoms and signs include irritative dry cough, expectoration, hemoptysis and cavities in lungs. (Detailed manifestations of tuberculosis see the section about tuberculosis in this chapter) Its diagnosis mainly should be based on chest X-ray and findings of tubercule bacillus in sputum or other specimens, or tuberculosis specific pathological changes.
Discussion
HIV/AIDS related pulmonary candida albicans is a widespread dimorphism bacteria. The oval shaped budding yeasts and hyphae both can be found in the tissues. Candidiasis is a common disease in AIDS patients. Chest X-ray demonstrates unilateral or bilateral patchy parenchymal changes of the air cavity and nodules with unclear boundaries. Miliary lesions are common. HRCT demonstrates multiple nodular shadows in both lungs, often accompanied with parenchymal changes. Its definitive diagnosis should be based on the findings of candida albicans in the tissues.
HIV/AIDS Related Penicillium Marneffei Infection
Penicillium marneffei (PM) is a newly found penicillium in 1956, which is a special strain with a distribution in South East Asia and Southern China. Rhizomys is its natural host. In 1973, Disalvo et al. [12] reported the first case of natural human PM infection. In 1984, the first case of human PM infection in China was reported in Guangxi Zhuang Autonomous Zone. PM is an opportunistic pathogen and immunocompromised people are susceptible to its infection. Its spreading is along with soil contaminated by Rhizomys feces to invade human body via the respiratory tract, the digestive tract and skin defects. PM infection is believed to be one of the most common opportunistic infections in AIDS patients in Southeast Asia, which has an increasing incidence.
Pathogens and Pathogenesis
PM is the only dimorphic fungus in hyphomycetes penicillium, which is a special strain of penicillium. At different culture temperatures, it shows conversion of biphasic forms: fungal phase at 25 °C and yeast phase at 37 °C. The fungal phase is the hyphae of many cells, with certain biological morphology, such as penicillus, conidiophore, chain liked conidiospore and chains between spores. The yeast phase shows unicellular or bicellular form. In the growth process of PM, large quantity bright rosy or dark rosy pigments are produced, which is characteristically PM. The pigment of yeast phase is secondary metabolites of cells with strong hydrophobicity, which can promote the adhesion of conidiums in fungal phase and cells in yeast phase to the alveolar macrophages and other cells surface in the human body. The pigment monoclonal antibody (MAb) can interrupt the pathological process of adhesion. In addition, this pigment can determine the expression of cluster-encoding genes Mbr through diffusion and penetration of drugs to the cell membrane, thus preventing the penetration of hydrophilic antifungal drugs, such as fluconazole. That is to say, it improves the natural antifungal resistance level of PM. The soluble components of the pigment in fungal phase can trigger the generation of anti-conidium antibody (only IgG) in animals to prevent its spreading in the body. The phenomenon proves that, in terms of tissue invasion, fungal phase is less powerful than yeast phase. Conidium in fungal phase is the carrier of pathogen while the cells in yeast phase are the real pathogenic factors.
Pathophysiological Basis
When PM spreads to the target organ along with the blood flow, it is engulfed by mononuclear phagocytes. In the cases of replication itself and further spreading, reactive proliferation of phagocytes is caused. Mononuclear phagocyte system has strong defense ability. In the cytoplasm of proliferated mononuclear phagocytes, various amounts of PM can be found. PM mainly invades into the body via the respiratory tract, digestive tract and skin defects. In immunocompetent people, local abscesses form in the invasion site, which is characterized by the thick mucus fluid, with mainly necrosis and liquefaction. Vascular reactions and exudation of neutrophil leukocytes and body fluids is less than abscesses induced by common purulent bacteria. The clinical manifestation is confined suppurative inflammation. When the immunity is compromised, due to the insufficiency of immunologic factors, it is difficult for the immune cells to restrict and digest the engulfed pathogens, which leads to confined suppurative reaction. Therefore, it often presents with diffuse lesions. The pulmonary lesions are principally interstitial exudative inflammation.
Fig. 17.87.
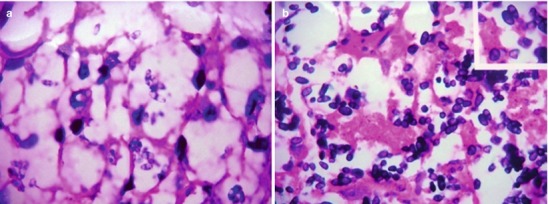
(a) HE staining demonstrates lymph nodes, PM in the yeast phase, with sausage liked shape and obvious septa (HE × 1000). (b) GMS staining demonstrates clearly defined PMs (GMS × 1,000)
Clinical Symptoms and Signs
The typical penicillium marneffei disease has acute or subacute onset, along with fever, chills and shivers, cough and expectoration, hemoptysis, shortness of breath, abdominal pain, diarrhea, bloody stool, fatigue, central necrotic papula mainly in the head and face and scattering in the trunk and extremities as well as hepatosplenomegaly.
Examinations and Their Selection
Pathogen Examinations
Bone marrow smear and PAS staining can be performed to detect the pathogen. Blood, bone marrow, pleuroperitoneal fluid, phlegm and skin defect tissue are collected for the culture at double temperatures with Sabouraud’Broth medium. At the temperature of 25 °C, the colony is in dark red with villous surface, with surrounding red wine liked pigments to gradually spreading into the medium.
Pathological Examinations
Biopsy of lymph nodes and skin defects with PAS staining and Wright & Gimsa staining can be performed.
Laboratory Tests
WBC count, hemochrome, platelets, AST and CD4 T cell count.
Imaging Demonstrations
CT scanning and routine chest X-ray are the diagnostic imaging examinations of choice, which can facilitate to understand the size, morphology, location, quantity and density of the lesions.
Imaging Demonstrations
CT Scanning
It demonstrates multiple small nodular shadows in the lungs, multiple honeycomb liked cavities in both lungs and mediastinal lymphadenectasis. Abdominal scanning demonstrates different degrees of hepatic, splenic and retroperitoneal lymphadenectasis, which can fuse into a huge mass.
Routine Chest X-ray
It demonstrates thickened, deranged and blurry pulmonary markings, small cavities, military nodular shadows, mass liked shadows, spots and patchy shadows, ground glass liked changes, pleuritis and pleural effusion.
Case Study 1
A female patient aged 35 years was confirmatively diagnosed as having AIDS by the CDC. Her husband had a history of drug abuse and she complained of abdominal pain and fever for more than 2 months, with accompanying face rash and diarrhea. Her CD4 T cell count was 5/μl. By examinations, she sustained skin palpula, abdominal tenderness, central concave skin rashes on the face, neck, and upper limbs. There was a palpable mass in the upper left abdomen, hard and tenderness, in a size of 12 × 12 cm. More than 2 months before her admission, she had persistent dull abdominal pain that is commonly in the upper left abdomen, with accompanying fever and face skin rashes that is gradually increasing and spreads to the neck and upper limbs. She also had hepatosplenomegaly, abdominal aortic lymphadenectasis and ascites.
Fig. 17.88.
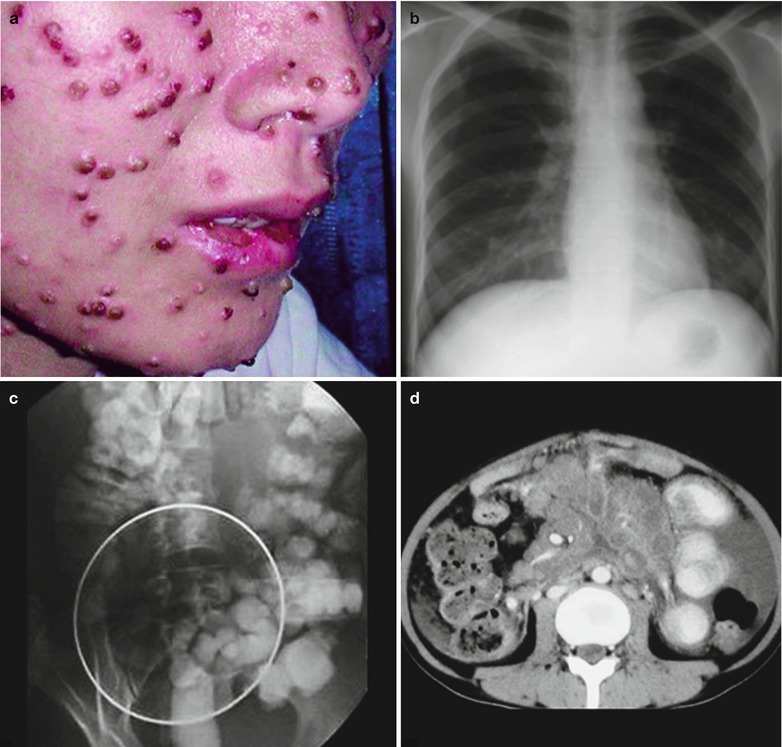
(a–d) HIV/AIDS related penicillium marneffei infection. (a) The gross specimen demonstrates central necrotic pimples on the face. (b) Chest X-ray DR demonstrates cavities in the left upper lung, thickened lung markings in both lower lungs with accompanying multiple spots and flakes shadows. (c) Gastrointestinal barium meal radiology demonstrates left and downwards migration of the intestine due to compression. (d) Enhanced abdominal CT scanning demonstrates retroperitoneal enlarged lymph nodes that fuse into a huge mass, with ring shaped enhancement of the lymph nodes
Case Study 2
A female patient aged 41 years was confirmatively diagnosed as having AIDS by the CDC. She had an unhealthy sexual life, with complaints of fever and cough for more than 1 month, with accompanying concave liked skin rashes in the face and limbs. Her CD4 T cell count was 7/μl.
Fig. 17.89.
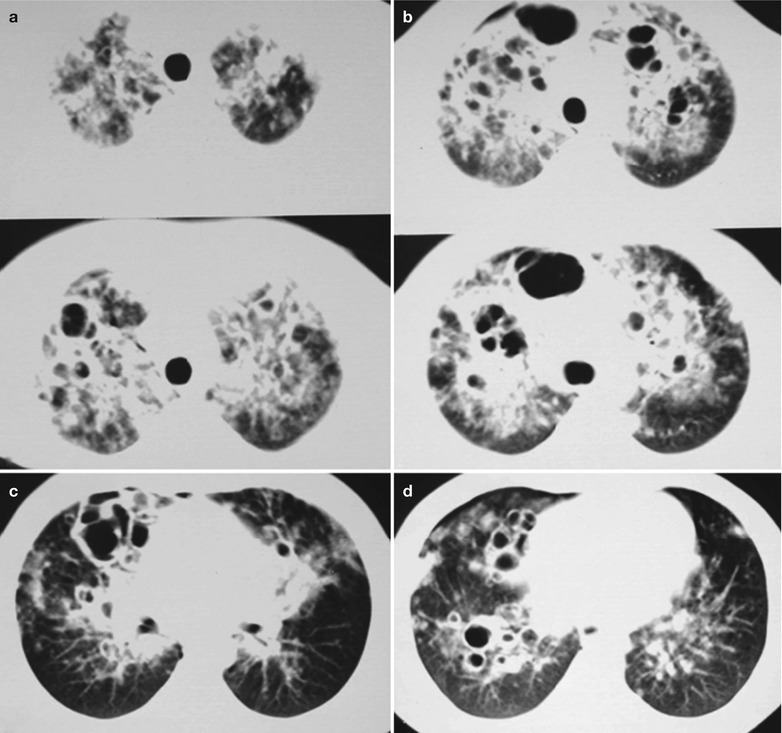
(a–d) HIV/AIDS related penicillium marneffei infection. (a–d) CT scanning demonstrates multiple small nodular shadows in the lungs that fuse into mass with parenchymal shadows, with the hilum as the center and distributing bilaterally symmetric like butterfly wings. There are also multiple clustering cavities or singular large cavity in both lungs
Case Study 3
A female patient aged 29 years was confirmatively diagnosed as having AIDS by the CDC. She had a history of drug abuse, with complaints of fever and cough for more than 1 month and accompanying concave liked skin rashes in the face and limbs. Her CD4 T cell count was 17/μl.
Fig. 17.90.
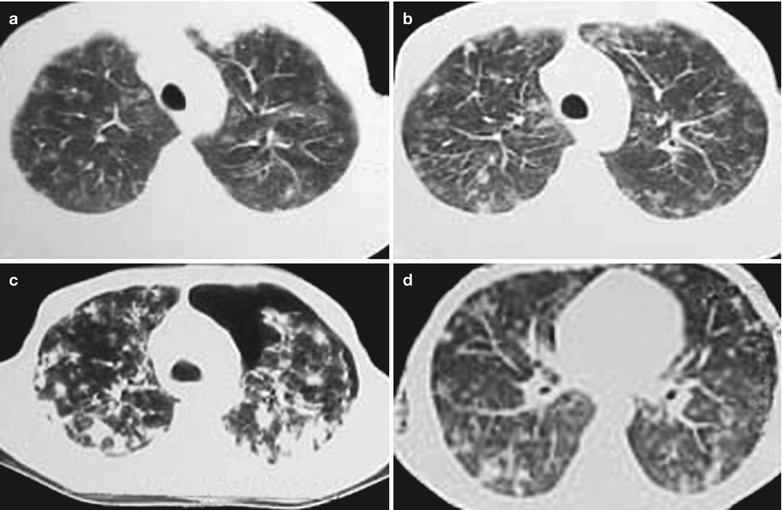
(a–d) HIV/AIDS related penicillium marneffei infection. (a–d) CT scanning demonstrates multiple small nodular shadows in the lungs that fuse into mass in parenchymal shadows, with the hilum as the center and distributing bilaterally symmetric like butterfly wings. There are also multiple clustering cavities or singular large cavity in both lungs
Case Study 4
A female patient aged 51 years was confirmatively diagnosed as having AIDS by the CDC. She had an unhealthy sexual life, with complaints of fever and cough for more than 1 month. Her CD4 T cell count was 7/μl.
Fig. 17.91.
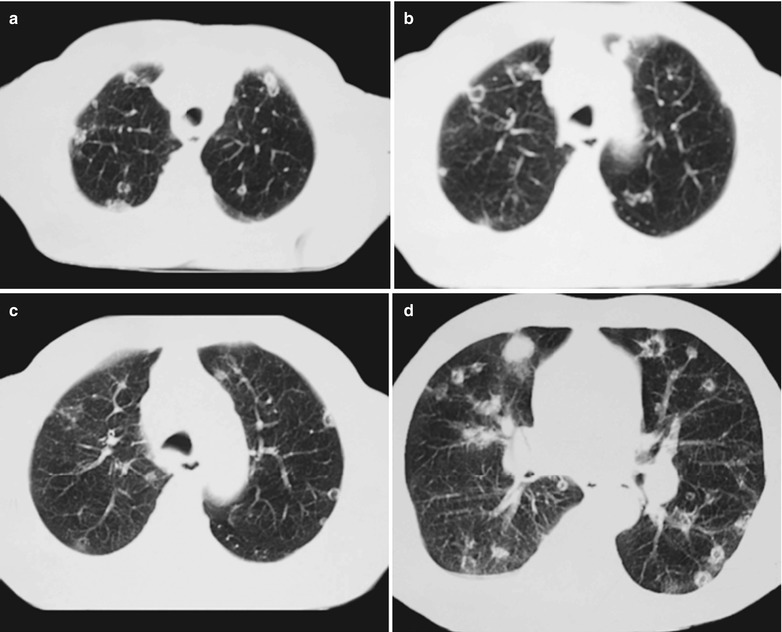
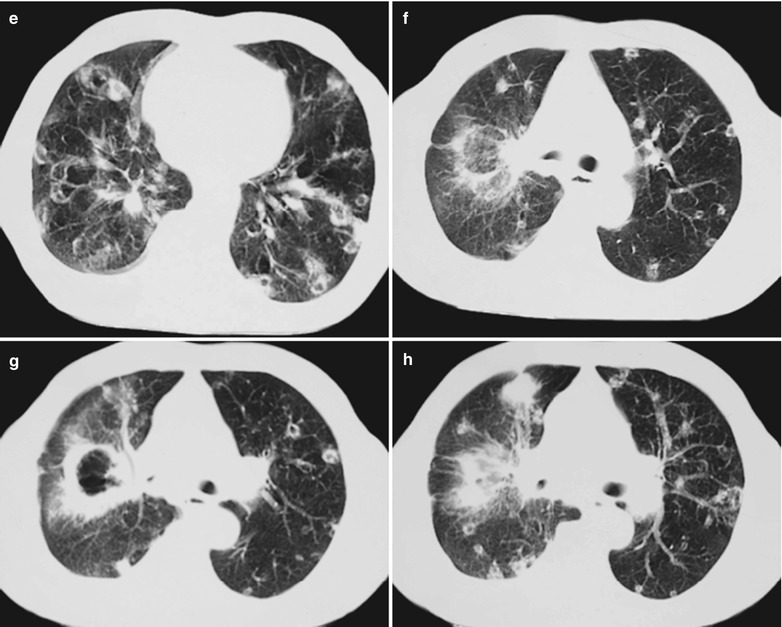
(a–h) HIV/AIDS related penicillium marneffei infection. (a–e) CT scanning demonstrates multiple small nodular shadows and small cavity shadows in the lungs, fusion of some lesions into honeycomb likes cavity shadows or plaque liked dense shadows. (f–h) CT scanning demonstrates large thick-wall cavity shadows in the right lung, surrounding plaque and round liked small cavity shadows and inflammatory infiltrative shadows
Case Study 5
A male patient aged 43 years was confirmatively diagnosed as having AIDS by the CDC. He had a history of drug abuse, with complaints of fever and cough for more than 1 month. His CD4 T cell count was 33/μl.
Fig. 17.92.
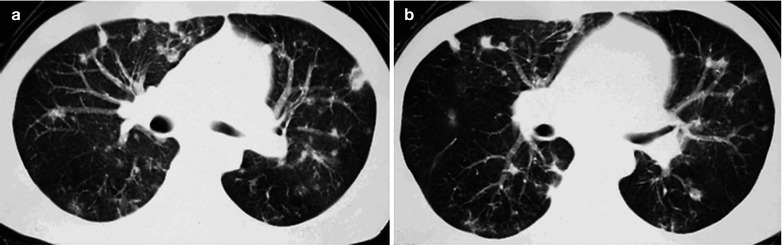
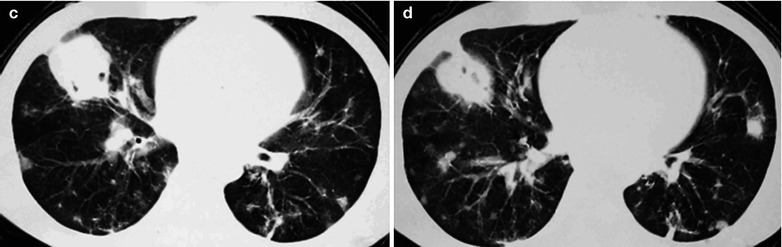
(a–d) HIV/AIDS related penicillium marneffei infection. (a–d) CT scanning demonstrates multiple small nodular shadows and small cavity shadows in the lungs, fusion of the lesions in the right lung into plaque liked shadows with clear boundaries, and multiple round liked small cavity shadows and inflammatory infiltrative shadows in both lungs
Case Study 6
A female patient aged 35 years was confirmatively diagnosed as having AIDS by the CDC. She had a history of extramarital affair, with complaints of fever and cough for more than 1 month. Her CD4 T cell count was 53/μl.
Fig. 17.93.
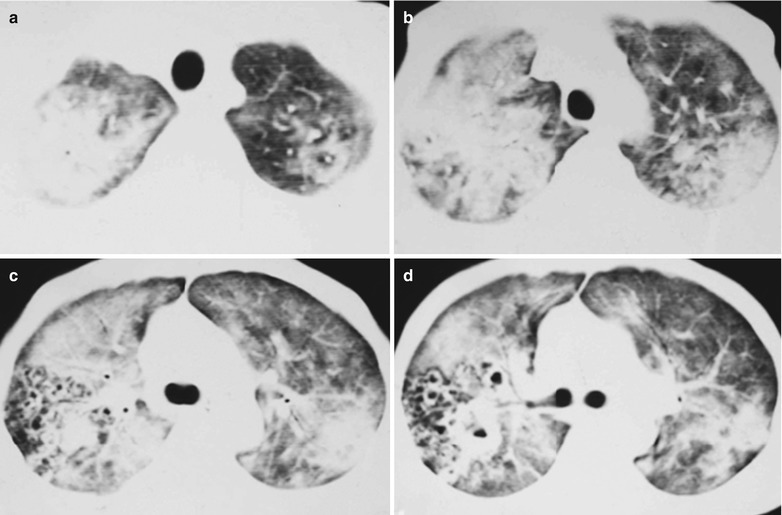
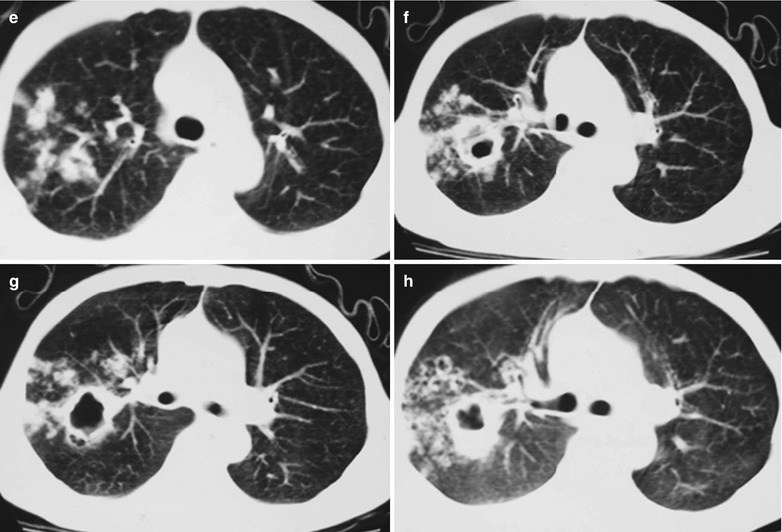
(a–h) HIV/AIDS related penicillium marneffei infection. (a–d) CT scanning demonstrates diffuse small cavity in honeycomb liked and infiltrative parenchyma shadows, with the hilum as the center to distribute bilaterally symmetric like butterfly wings; multiple honeycomb liked cavity shadows in both lungs. (e–h) CT scanning reexamination demonstrates large irregular thick-wall cavities in the right lung, surrounding scattering nodular, honeycomba liked and infiltrative shadows after anti-PM infection treatment for 3 months
Case Study 7
A male patient aged 38 years was confirmatively diagnosed as having AIDS by the CDC. He had a history of extramarital affair, with complaints of fever and cough for more than 1 month. His CD4 T cell count was 93/μl.
Fig. 17.94.
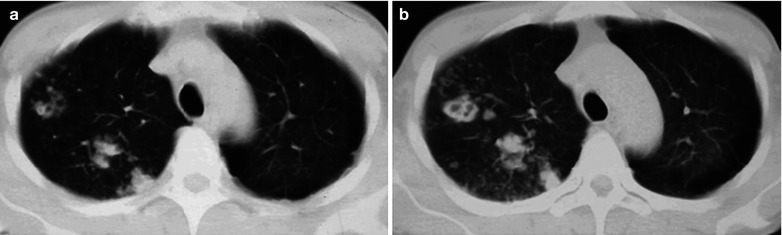
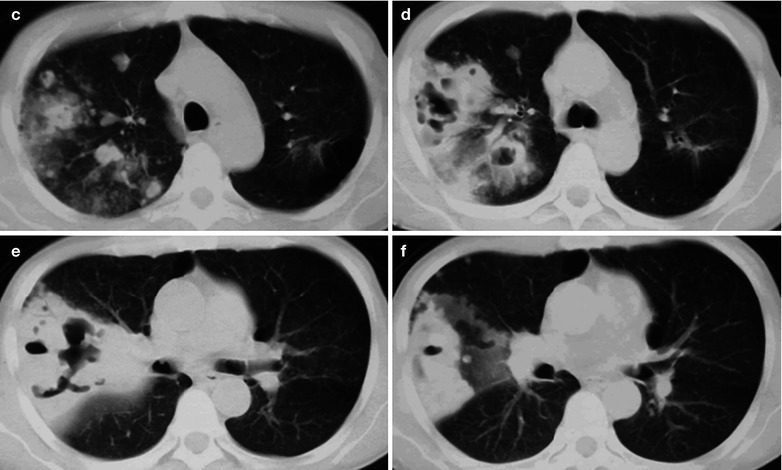
(a–f) HIV/AIDS related penicillium marneffei infection. (a–f) CT scanning demonstrates multiple nodules and small cavities in honeycomb liked shadows and infiltrative parenchyma shadows in the right lung; flaky transparent areas (bullae of lung) in the right anterior margin of the heart and in the outer zone of the left lung near lateral chest wall
Case Study 8
A male patient aged 35 years was confirmatively diagnosed as having AIDS by the CDC. He had been found to be HIV positive for 5 years, with complaints of irregular fever, cough, fatigue and dizziness for 10 days to be hospitalized. By examinations, his CD4 T cell count was 40/μl, HIV positive, Subcultivation of strains demonstrated typical biphasic penicillium. By fungus culture, typical penicillus was found. By bone marrow smear, the round corpuscles mainly located in the macrophages.
Fig. 17.95.
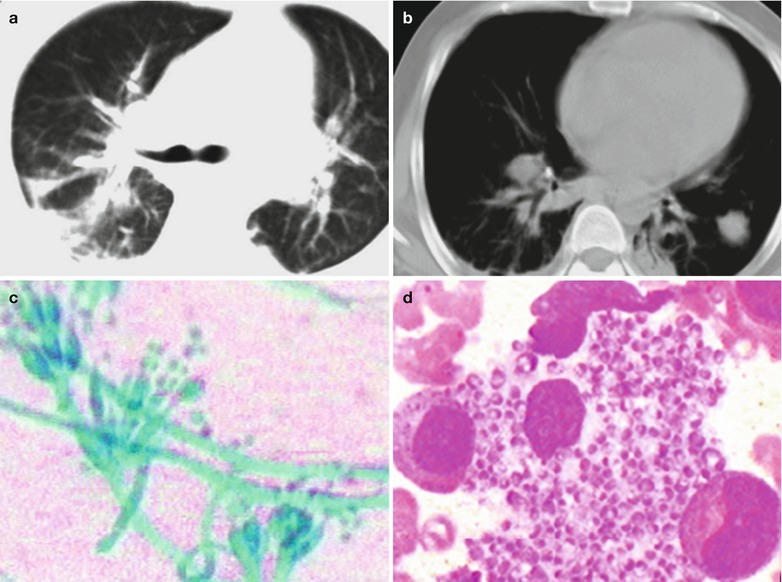
(a–d) HIV/AIDS related penicillium marneffei infection. (a) CT scanning of the pulmonary window demonstrates irregular large flaky shadows with increased density in the dorsal segment of both lower lobes; enlarged hilum in both lungs, cords liked thickening of the vascular vessels. (b) CT scanning of the mediastinal window demonstrates flaky parenchyma shadows in the left lower lung, thickening of both pleura, enlarged hilum shadows in both lungs, thickened right lower bronchial wall. (c) Microscopy after culture at 25 °C demonstrates branches and separated hyphae and its string of small spores, with typical penicillus but no sporangium (Medan staining, ×400). (d) Bone marrow smear demonstrates round or oval cells like the yeast phase within the macrophages; longer cells like the yeast phase outside the macrophages. The two kinds of cells have slightly curved ends in sausages liked appearance (HE staining, ×400)
Case Study 9
A male patient aged 32 years was confirmatively diagnosed as having AIDS by the CDC. He had a history of extramarital affair, with complaints of fever, cough, chest distress, shortness of breath, fatigue, poor appetite, poor sleep, weight loss and shortness of breath after activities for more than 3 months. His CD4 T cell count was 23/μl. AST 156 μ/L. By B ultrasound, multiple low echo masses adjacent the abdominal aorta, in different sizes. The largest one in size of 13 × 8 mm with clear boundary and even distribution of the inner light spots. All the findings indicated lymphadenectasis adjacent to the abdominal aorta. The signs include a complexion of chronic illness and stool culture with findings of PM.
Fig. 17.96.
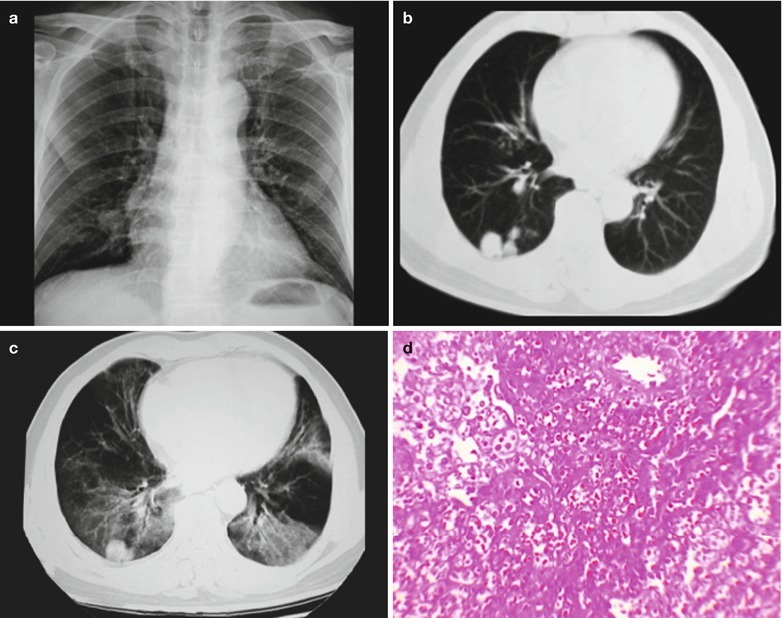
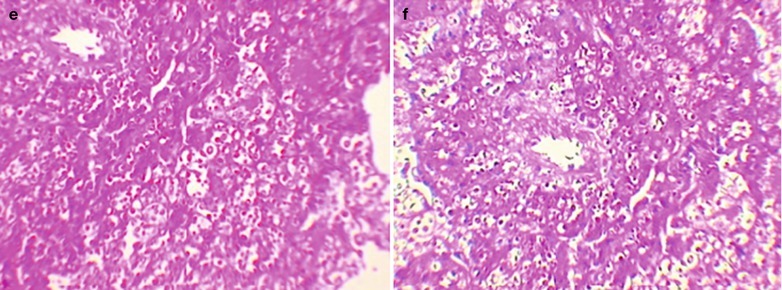
(a–f) HIV/AIDS related penicillium marneffei infection. (a) DR demonstrates flaky blurry dense shadows in the right anterior margin of the heart, with surrounding infiltrative shadows. (b, c) CT scanning of the pulmonary window demonstrates nodular dense shadows in the dorsal segment of the right lower lung, with smooth sharp boundaries; large flaky ground glass liked dense shadows in the dorsal segment of both lungs. (d, e) HE staining demonstrates spores and round liked, long circular or sausage shaped corpuscles in red, in different sizes. (f) HE staining demonstrates spores and round liked, long circular or sausage shaped corpuscles in purplish red, in different sizes
Diagnostic Basis
Chest X-ray and CT Scanning
Both demonstrate multiple nodules in different sizes and cavity shadows. The cavities cluster into honeycomb liked changes with uneven thickness of the walls, clear boundaries and surrounding inflammatory exudates. Some of the nodules infuse into mass dense shadows. Lesions in both lungs have a symmetrical or asymmetric distribution, with no characteristic leions. Mediastinal lymph nodes are obviously enlarged.
Pathogenic Examinations
Sputum smear for direct microscopy demonstrates candida.
Histopathological Examinations
PMs are found by fibrobronchoscopy lavage smear and biopsy.
Immunological Assays
(1) Candida skin test shows positive. (2) Fluorescent antibody test for PMs is performed in procedures of direct smear, fungal colony culture and histopathological examination of the tissue sections.
Metabolites Test and PCR
Metabolites test of PM and PCR can be performed to determine the gene sequences of PM for the early diagnosis.
Differential Diagnosis
HIV/AIDS related penicillium marneffei pneumonia should be differentiated from bronchiectasis and blood borne staphylococcus aureus pulmonary abscess.
Bronchiectasis
Although the cases of bronchiectasis are demonstrated to have clustering round shadows on the cross sections, the wall is thinner and even, accompanying to the spots liked vascular shadows. Some lesions have typical railway liked bronchial dilation signs.
Blood Borne Staphylococcus Aureus Pulmonary Abscess
It is demonstrated to have multiple small cavity lesions in both lungs in line with the evenly distributing blood borne lesions. The lesions are rarely clustering. The wall of the cavities is thick and even with surrounding marginal exudates and blurry boundaries.
Discussion
With the steady increasing of HIV infections, reports on the complication of PM disease have also been increasing recently. The disease can be localized but mostly disseminated, with the involvement of lungs, liver, skin, lymph nodes and other tissues and organs. Therefore, it is known as penicilliosis marneffei or disseminated penicillium marneffei infection in literature reports. Due to the insufficient knowledge about the disease, diagnosis is delayed or missed. In Thailand, penicilliosis marneffei has been the indicator disease of AIDS. About 20 % AIDS patients are infected by PM and 70 % have necrotic papula, which is characteristically disseminated penicillium marneffei infection. CT scanning demonstrates HIV/AIDS complicated by disseminated penicillium marneffei infection as flaky parenchyma shadows in the lungs, clustering of cavities and nodular shadows, mediastinal lymphadenectasis and pleural inflammation responses, which can facilitate its clinical diagnosis.
HIV/AIDS Related Mucor Infection
Pathogens and Pathogenesis
Mucormycosis is a rare kind of conditional fungal disease, with its pathogen, mucor, distributing widely in the natural world. It generally fails to cause diseases, but can cause systematic infections in immunocompromised people. Mucor often invades into the human body via the nose to cause paranasal sinuses and orbital infections, which can further invade into the brain to cause meningitis and frontal abscesses. Pulmonary mucormycosis is only second to the nasal-cerebral infection. It can spread via the respiratory tract to cause pulmonary infections. In addition, there are also skin and gastrointestinal mucormycosis as well as disseminated mucormycosis.
Pathophysiological Basis
The pathological changes of HIV/AIDS related pulmonary mucormycosis are mainly hemorrhagic necrotic inflammation. The defense mechanism of immunocompetent people is to kill the fungal spores with the phagocytosis of macrophages and oxidation killing mechanism. In immunocompromised or immunodeficient patients, the macrophages are often too weak to restrain the engulfed spores from germinating. Therefore, the disease occurs. Vascular vessels are susceptible to the invasion of mucor, especially the arteries. Mucor can locally multiply to cause the formation of blood clots and embolization, and disseminate to other organs along with the blood flow. The main lesions are hemorrhage and necrosis of local tissues and exudation of neutrophils. The lesions of hemorrhage and necrosis are possibly related to the arteriole lesions caused by hyphae.
Clinical Symptoms and Signs
The clinical manifestation of HIV/AIDS related pulmonary mucormycosis is a nonspecific pneumonia. The most common symptoms reported in literatures are persistent high fever, cough, hemoptysis, chest pain and difficulty breathing. It has a rapid progression, with a high mortality rate of 65–96 %. Lung lesions are hemorrhagic infarction or pneumonia, which can cause high fever, cough, expectoration, shortness of breath, chest distress, chest pain, hemoptysis (pulmonary artery involved) and other symptoms. Moist rales can be heard in both lungs and pleural rubs can be heard in the cases of pleura involvement.
Examinations and Their Selection
Assisted by bronchofibroscopy, lung biopsy can be performed.
Histological examinations include bronchial lavage fluid examination, exploratory thoracotomy and puncture of lung tissues for biopsy.
Chest X-ray and CT scanning are conventional effective examinations.
Imaging Demonstrations
The lesions are frequently found in the dorsal and medial segments of both lungs. Early exudation shows miliary shadows, cavity shadows with no wall or with thin wall and small bronchiectasis shadows. Their further progression may cause fusion infiltration, parenchymal changes, nodules, masses, thick-walled cavities and pleural effusion, with accompanying mediastinal lymphadenectasis.
Diagnostic Basis
The findings of mucor or their hyphae by sputum and bronchofibroscopic biopsy.
The diagnostic imaging demonstrates lesions commonly in the dorsal and medial segments of both lungs. There are diffuse scattering miliary shadows, cavity shadows with no wall or with thin walls and small bronchiectasis shadows. They further progress into fusion infiltration, parenchymal changes, nodules, masses, thick-walled cavities and pleural effusion.
Often with accompanying mediastinal lymphadenectasis.
Differential Diagnosis
Chest X-ray of pulmonary mucormycosis demonstrates progressive infiltrated parenchymal changes, or masses, nodules, cavities and pleural effusion. It needs to be differentiated from miliary tuberculosis. HIV/AIDS related miliary tuberculosis are commonly demonstrated as chronic blood borne disseminated tuberculosis, with lesions distributing symmetrically in both lungs. Its long term progression causes fusion into masses. Otherwise, it can be cured by anti-TB therapies. The early lesions are no-wall cavities or thin wall cavities and small bronchioectasis shadows, based on which the differential diagnosis can be made.
Discussion
Almost all cases of HIV/AIDS related pulmonary mucormycosis are found in immunocompromised patients. Chest X-ray demonstrates parenchyma changes and isolated or multiple nodules or masses. The parenchyma changes are patchy or fuse, with unilateral or bilateral distribution. About 20 % patients have pleural effusion and less than 10 % patients have hilar or mediastinal lymphadenosis. CT scanning demonstrates singular or multiple nodules or masses, commonly with clustering or honeycomb liked cavities.
HIV/AIDS Related Pulmonary Virus Infection
HIV/AIDS Related Cytomegalovirus Pneumonia
Pathogen and Pathogenesis
AIDS patients often sustain pulmonary viral infections, and cytomegalovirus infection is the most common one. Generally, its clinical symptoms are not obvious. Viral pneumonia can be simple, or a part of systemic disseminated infections. Cytomegalovirus pneumonia is caused by cytomegalovirus, which belongs to herpes virus B and is a double-chain DNA virus with exterior capsule and spherical nucleus. Cytomegalovirus has two antigens, complement fixing antigen and neutralization antigen. Its pathogenesis is the transmission of cytomegalovirus via various routes, with infants commonly via contacts, and adults commonly via sexual contacts. The onset after infection depends on the amount of the invaded viruses and the immunity status of the human body. Due to the important role the cellular immunity plays in anti-cytomegalovirus infection, patients with deficient cellular immunity have more serious conditions after cytomegalovirus infection. In patients with compromised or deficient immunity, the latent CMV infection can be activated to cause diseases. Therefore, patients with AIDS are susceptible to CMV infection.
Pathophysiological Basis
Cytomegalovirus pneumonia has extensive pathological changes in the lungs. Pathologically, it shows interstitial pneumonia, with the lesions randomly blood borne distributing in the lungs. The distribution can be diffuse, panlobular or focal. The target cells of pathological changes include alveolar cells and macrophages. Diffused pulmonary interstitial edema and fibrosis as well as alveolar swelling, focal necrosis, bleeding and hyperplasia occur after CMV infections to cause hypoxemia. Gross observation of fresh specimens demonstrates pulmonary surface edema and flaky blooding spots. Fixed specimens demonstrate brown hard lung tissues. Under a microscope, pulmonary interstitial congestion as well as infiltration of lymphocytes and mononuclear cells can be found, with the involved epithelial cells enlarged. In the pulmonary interstitium and alveoli, there are intranuclear inclusions, cytoplasmic inclusions and fluid containing abundant proteins. The classical intranuclear inclusions can be found in the cells, purplish red or purplish blue, round or oval, with surrounding halos in eagle eyes sign. Atypical cytomegalic inclusions in cells are slender, long and round liked with abundant cytoplasm and accentric nucleolus, which are blurry, unclear and atypical (Fig. 17.97a–e). Immunohistochemitry demonstrates HIV P24 antigen positive.
Fig. 17.97.
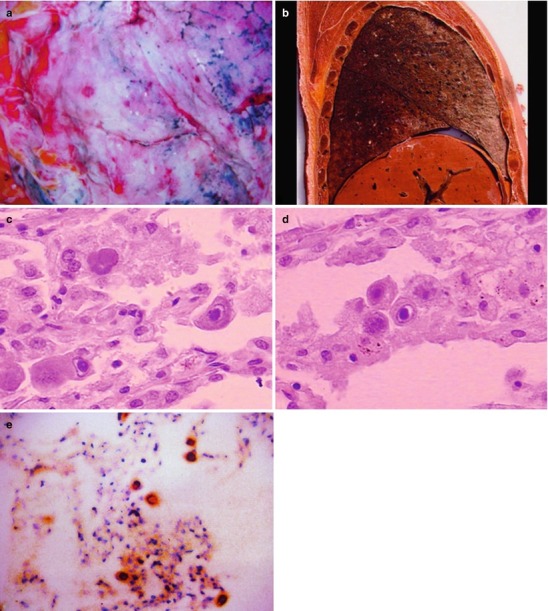
(a) Gross observation of the fresh specimen in autopsy demonstrates pulmonary edema and congestion of cytomegalovirus pneumonia. (b) Gross observation of the formalin fixed specimen in autopsy demonstrates dark brown hard pulmonary tissues. (c, d) HE staining demonstrates large quantity cytomegalovirus inclusions in eagle eyes sign. (e) Immunohistochemical demonstrates HIV P24 antigen positive in macrophages of lung tissues (×400)
Clinical Symptoms and Signs
The systemic symptoms of CMV infection include fever, joint and muscle soreness and pain, abdominal distension and orthostatic hypotension. The respiratory symptoms include paroxysmal dry cough, difficulty breathing, cyanosis and three depressions sign. According to the imaging findings of CMVP, CMV pneumonia can be classified as diffuse, miliary and mass types, among which the diffuse type is the most common.
Examinations and Their Selection
Immunological Assays
Cytomegalovirus can be separated from respiratory secretions culture and urine culture by using human embryonic fibroblasts. By urine sediment smear, giant cell with inclusions can be found. By using immunofluorescence, indirect hemagglutination inhibition and complement fixing test, the antibody titer can be found increased. Indirect immunofluorescence test and immunoenzymic staining test can be applied to detect the anti-CMV-IgG and IgM antibody. In addition, enzyme-linked immuno sorbent assay (ELISA) can also be performed to detect the anti-CMV-IgG and IgM antibody. CMV-IgM antibody positive indicates a recent infection, which has diagnostic value. A single serum CMV-IgG antibody positive indicates a previous infection. And during the acute and recovery phases, double serum CMV-IgG antibody titer being no less than four times increase has diagnostic value, indicating a recent infection.
PCR
PCR can be applied to quantitatively determine the viral load in the whole blood, blood plasma, leukocytes, urine, bronchoalveolar lavage fluid (BALF), cerebrospinal fluid and the tissue specimens, which is believed to be the best way for the diagnosis of invasive CMV infection.
Diagnostic Imaging
Chest X-ray is the most commonly used examination. Chest CT scanning is superior to chest X-ray in terms of resolution and detection rate of the lesions.
Imaging Demonstrations
Pulmonary demonstrations by CMVP include diffuse interstitial infiltration and alveolar infiltration to form reticular shadows, nodules and parenchymal changes. The pathological changes of the airway include thickened bronchial wall and bronchiectasis. Necrotic bronchiolitis can cause obstruction of minor airways. CT scanning demonstrates intrapulmonary multiple small nodule shadows and ground glass liked changes. The pathological basis of nodules or masses is hemorrhage and necrosis. The parenchyma changes indicate complications of bacteria infection or fungal infection.
Case Study 1
A baby boy aged 6 months was confirmatively diagnosed as having AIDS by the CDC. He was infected via vertical transmission from mother to child, with recurrent cough after being born and the most recent cough for 4 days as well as wheezing cough in throat for 1 day before he was hospitalized. He had a past history of premature birth, with primary apnea and bronchial pneumonia and was hospitalized for treatment. Later, he was admitted for three times due to cough, which was diagnosed as interstitial pneumonia. By examinations, WBC 16.3 × 109/L, LYM lymphocyte count 11.7 × 109/L, CMV-Ab weak positive, blood sedimentation 11 mm/h, and tuberculosis antibody negative. After treated by broad-spectrum antibiotic therapy, the therapeutic efficacy is unfavorable.
Fig. 17.98.
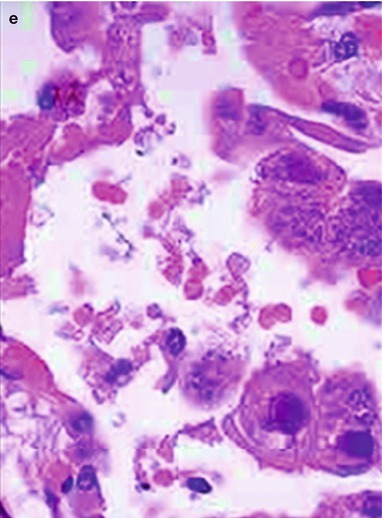
(a–e) HIV/AIDS related cytomegalovirus pneumonia. (a) DR demonstrates thickened lung markings in both lungs, which extend to the outer zone of the lungs. (b) DR demonstrates thickened and deranged lung markings in both lungs with nodular blurry shadows; and cloudy shadows in lung fields. (c, d) CT scanning demonstrates thickened lung markings in both lungs, with diffuse nodular shadows; and cloudy changes in lung fields. (e) HE staining demonstrates cytomegalovirus inclusions
Case Study 2
A female patient aged 45 years was confirmatively diagnosed as having AIDS by the CDC. She had a history of paid blood donation in 1995 and was confirmatively diagnosed in Oct. 2003. In Jul. 2005, she sustained symptoms of irregular fever, cough and emaciation for months. Her CD4 T cell count was 56/μl.
Fig. 17.99.
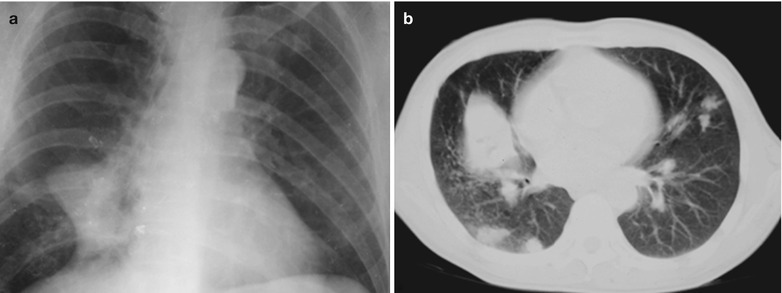
(a, b) HIV/AIDS related cytomegalovirus pneumonia. (a) DR demonstrates enlarged right hilus, thickened and deranged lung markings. (b) Plain CT scanning demonstrates mass shadows in the right hilus, spots shadows in the right middle and lower lobes, and patchy shadows in the lingual segment of the left lung
Diagnostic Basis
CMV antibody positive. When the double serum CMV-IgG antibody titer shows an at least four times increase, reactivation of CMV infection can be considered.
PCR and in situ hybridation can be performed to detect CMV-DNA in the lesions tissues.
Detection of cytomegalovirus inclusion in the lesion tissues is an important evidence for the diagnosis.
Bronchoalveolar lavage fluid, sputum and biopsy tissue can be inoculated into human embryonic fibroblasts for culture; the detection of CMV has more diagnostic value.
Imaging examinations demonstrate diffuse interstitial infiltration and alveolar infiltration to form reticular shadows, nodules and parenchymal changes.
Differential Diagnosis
Mononucleosis caused by pulmonary cytomegalovirus infection is difficult to be differentiated from infectious mononucleosis caused by EBV.
Cytomegalovirus pneumonia should be differentiated from pulmonary infections casued by herpes simplex virus, adenovirus and influenza virus.
Discussion
Cytomegalovirus (CMV) infection is an important cause of pneumonia in patients with compromised immunity. Imaging findings include nodular shadows with blurry boundaries and bilateral flaky parenchymal changes of the lungs. The nodules tend to be bilaterally symmetric or asymmetric, with centrilobular distribution. Histopathological manifestations are nodular alveolar hemorrhage, necrosis and inflammatory lesions, and diffused alveolar lesions. The nodules tend to have a centrilobular distribution, indicating occurrence of bronchiolitis. In AIDS patients, if the diameter of nodules is under 10 cm, it is most likely to be viral infection. The size of the nodules can facilitate the differential diagnosis of pulmonary infections.
HIV/AIDS Related Herpes Simplex Viral Pneumonia
Herpes simplex viral pneumonia (HSVP) often occurs in the upper respiratory tract, and rarely in the lower respiratory tract. Human herpes simplex virus can be divided into two types, namely herpes simplex virus type I (HSV-I) and herpes simplex virus type II (HSV-II). Herpes simplex viral pneumonia mostly occurs in patients with immunodeficiency.
Pathogen and Pathogenesis
Herpes simplex viral pneumonia can be caused by HSV-I and HSV-II, both of which have a nucleocapsid with 20 surfaces. The thickness of the nucleocapsid is about 100 nm, which is composed of 162 capsomeres. The nucleocapsid contains the core of the virus DNA. The virion gains the phospholipid rich viral envelope when it passes through the nuclear envelope. The nucleocapsid gemmates after it passes through the nuclear membrane and is released to the cell surface. The nucleocapsid can also be released outside the cells or gains its access into the neighbour cells for further reproduction. Herpes simplex virus replicates itself in the cell nucleus to produce histopathologic changes of herpes virus replication, with visible Cowdry A type intranuclear inclusions. The pathogenesis process of herpes simplex virus infection in the body can be divided into five stages: initial skin mucosa infection, acute ganglia infection, latent infections, re-activation, and recurrent infections in susceptible hosts. Patients infected by herpes simplex virus can produce IgM, IgG and IgA antibodies to fight directly against virus protein, which may play a role in changing the severity of the infection. Interferon also participates in the control of herpes simplex infection by inhibiting the virus or regulating the defense mechanism. Genetic factors may be also related to the herpes virus infection. Cellular immunity can confine the infection. Herpes virus cannot reproduce in the alveolar macrophages of human body, which is also the reason why herpes virus is less than cytomegalovirus in lungs. Currently, it is believed that herpes simplex virus is an important pathogen of respiratory infections, especially in immunocompromised patients. Localized herpes simplex viral pneumonia occurs due to the direct spreading of virus in the upper and lower respiratory tract. Diffuse herpes simplex viral pneumonia is caused by the virus spread from the reproductive organs lesions or oral lesions (most possibly blood borne). Viremia caused by HSV-I or HSV-II has been reported, and both are related to diffused infections. But in patients without herpes simplex viral infection in skin mucosa, herpes simplex viral pneumonia can also occur.
Fig. 17.100.
The structure of herpes simplex virus
Pathophysiological Basis
Herpes simplex viral pneumonia is caused by the direct spreading of the virus from the upper and lower respiratory tract. Diffuse herpes simplex viral pneumonia is cause by the spreading of the virus from the reproductive organs lesions or oral lesions (most possibly blood borne). Viremia cause by either HSV-I and HSV-II have been reported, both of which are related to diffuse infections. In such cases, the lung tissues may have inflammatory infiltration, lung parenchyma necrosis, bleeding, cellular swelling and round, diffuse interstitial pneumonia. And in most cases, there are accompanying cellular changes of herpes virus infection such as the intranuclear eosinophilic inclusions, necrotic herpes simplex viral trachitis. Herpes simplex viral bronchitis has demonstrations of mucosa erythema, edema, exudation and ulcer, with coverage of the surface by fibrous purulent membranous secretions.
Clinical Symptoms and Signs
The common initial clinical symptoms of herpes simplex viral pneumonia are shortness of breath, cough, and fever with a body temperature being higher than 38.5 °C, decreased WBC count, hypoxemia, respiratory dysfunctioning and azotemia. HSV pneumonia may be accompanied by mucocutaneous lesions by HSV, which show earlier than those of pneumonia. There may be concurrent fungus, cytomegalovirus or bacteria infection. Herpes simplex viral tracheobronchitis may show tracheal or bronchial spasm or stenosis.
Examinations and Their Selection
Etiological Examinations
HSV can be detected in tracheobronchial secretions, bronchoalveolar lavage fluid and lung tissues. Early sampling should be performed under the guidance of a bronchofibroscope.
Cell Culture
Tissue culture is the most sensitive and specific method for the diagnosis, which can also be used for the classification of the virus.
Virus Detection
Papanicolaou (Pap) or Tzank test is a fast and cheap method for cellular diagnosis.
ELISA
ELISA can be used to detect herpes simplex virus, with a sensitivity of up to 95 % and a high specificity.
Diagnostic Imaging
Chest X-ray demonstrations are less valuable for the differential diagnosis. Pulmonary CT scanning can be applied for the differential diagnosis.
Imaging Demonstrations
Herpes simplex viral pneumonia includes three types, namely local, multiple or diffuse interstitial infiltration. In the early stage, typical hilar or diffuse interstitial shadows with increased density can be found, with thickened bronchial wall. As the disease progresses, cloudy or patchy alveolar tamponade and fusion can be found. Chest X-ray may demonstrate negative for herpes simplex viral trachitis and bronchitis.
Case Study
A male patient aged 28 years was confirmatively diagnosed as having AIDS by the CDC. He complained of fever, shortness of breath and cough for 1 week. His CD4 T cell count was 35/μl.
Fig. 17.101.
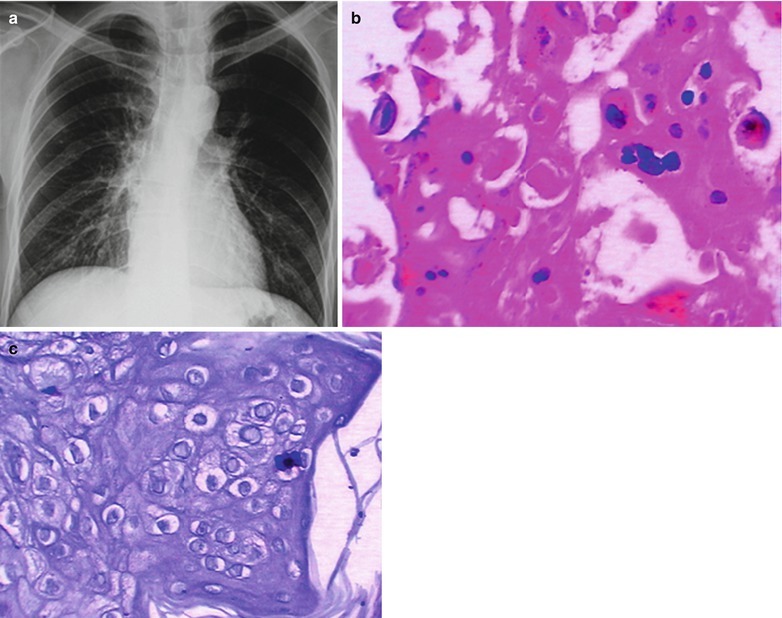
(a–c) HIV/AIDS related Herpes Simplex Viral Pneumonia. (a) DR demonstrates thickened and deranged lung markings in both lungs with accompanying blurry nodular shadows, and cloudy shadows in the lung fields. (b) PAS staining (400×) demonstrates eosinophilic inclusion. (c) Silver methenamine staining demonstrates herpes viral inclusion
Diagnostic Basis
Clinical and radiological manifestations of HSV pneumonia are non-specific. The diagnosis of herpes simplex viral pneumonia, in addition to the clinical manifestations of pneumonia, should also be based on the histological evidence of pulmonary HSV infection and isolation of virus from the lung tissues.
Direct isolation of the virus from the lung tissues can define the diagnosis.
Tracheoscopy in combination to the cytologic examination and virus culture has diagnostic value. Bronchofibroscopy demonstrates tracheobronchial mucosa ulcer and(or) coverage by pseudomembrane, which can also be applied to guide the sampling of bronchial lavage fluid or lung tissues for biopsy. Cytological and histological examinations can provide specific evidence for HSV infection: multinucleated giant cells and intranuclear eosinophilic inclusion. In addition, biopsy demonstrates inflammatory infiltration, parenchyma necrosis and bleeding.
Immunofluorescence can be applied for the histological examination of herpes simplex virus antigen.
The imaging examinations can demonstrate herpes simplex viral pneumonia as localized, multiple or diffuse interstitial infiltration. As the disease progresses, cloudy or patchy alveolar tamponade and fusion can be found.
Differential Diagnosis
Herpes simplex viral pneumonia should be differentiated from bacterial pneumonia, CMV pneumonia, and influenza pneumonia.
Discussion
HIV/AIDS related herpes simplex viral pneumonia is mostly demonstrated by multiple signs, including small nodules, ground glass liked shadows and patchy parenchymal changes. The nodules are in centrilobular distribution, mostly with accompanying branches liked shadows (tree buds sign). Chest X-ray demonstrates diffuse lung parenchymal changes. Imaging finding are parenchymal change areas with bilaterally blurry boundaries. Generally, the nodules have a diameter of 2–10 mm. CT scanning with high resolution demonstrates nodules with surrouding ground glass liked density lesions.
HIV/AIDS Related Lymphoid Interstitial Pneumonia
Lymphoid/Lymphocytic interstitial pneumonia (LIP) is more common in children with AIDS. The CDC in the United States has defined LIP in children under the age of 13 years as the diagnostic indicator of AIDS. The predictive diagnostic criteria include chest X-ray demonstration reticular nodular changes in pulmonary interstitium of both lungs for no less than 2 months, undetectable pathogens and no responses to the antibiotic therapy.
Pathogen and Pathogenesis
Currently, HIV/AIDS related lymphocytic interstitial pneumonia is considered to be related to HIV and Epstein-Barr virus, human T cell leukemia-lymphoma type I virus (HTLV-I) and HIV-I. The infection of the above viruses causes pulmonary lymphatic hyperplasia and other systemic diseases.
Pathophysiological Basis
About 22–75 % children with HIV infection sustain LIP, and 3 % in adults. Most cases of non-HIV infected patients with lymphoid interstitial pneumonia are women, at average age of 56 years old, more commonly in the age group of 40–50 years old and above 70 years old. The pathological manifestations are infiltration of small and mature lymphocytes as well as plasma cells in alveolar septum and the alveolus, extensive interstitial fibrosis and non-caseous granuloma. It is characterized by diffuse infiltration of lymphocytes, plasmocytes and histocytes in the pulmonary interstitium. The lymph follicle with germinal center is more common. Hyperplasia occurs in type II alveolar epithelium, and the macrophage increases in the alveolar cavity. There are rare or mild intraalveoli organization and macrophage aggregation. Staining of the immune globulin light chain demonstrates poly-clone B cells.
Clinical Symptoms and Signs
The clinical symptoms are in progressive development, with cough and suffocation, rare hemoptysis and Sjogren syndrome commonly in mouth and eyes. By examinations, the signs have slight difference between adults and children. In children, there are lymphadenectasis, hepatosplenomegaly, enlargement of parotid gland, clubbing fingers and wheezing sound. In adults, there are lymphadenectasis, slight fine bubbling rales, as well as hepatosplenomegaly and enlargement of parotid gland in 1/3 patients.
Examinations and Their Selection
Peripheral hemogram demonstrates increased lymphocytes and eosinophilic granulocytes.
Myelogram demonstrates increased lymphocytes, plasmocytes and eosinophils.
Blood biochemical examination demonstrates increased immune globulin, predominantly lgM.
Blood gas analysis demonstrates hyoxemia.
Pathogenic examinations by bronchofibroscopy, bronchial alveolar lavage and biopsy can define the diagnosis.
Pulmonary function examinations demonstrate restrictive ventilatory disorder, lower lung compliance and impaired diffusion function. Impaired diffusion function is a more sensitive indicator in monitoring the progress of the disease.
Chest X-ray is the most commonly used imaging examination, while chest CT scanning is commonly applied for the differential diagnosis.
Imaging Demonstrations
Chest X-ray demonstrates HIV/AIDS related lymphoid interstitial pneumonia as reticular or reticular nodular shadows of lung markings in both lungs. HRCT demonstrates bilateral diffuse ground glass liked density shadows. Perivascular thin-walled pneumatocele is common. Pneumatocele induced by LIP is commonly found in the middle lung field, which probably is due to the valve effects caused by infiltration of cells around bronchioles. Manifestations of pneumatocele, together with ground glass liked density shadows, highly indicate LIP. Centrilobular and subpleural small nodules and thickened intralobular septa can be occasionally found.
Case Study 1
A female patient aged 35 years was confirmatively diagnosed as having AIDS by the CDC. She complained of cough, fever and no sputum. Her CD4 T cell count was 45/ μl.
Fig. 17.102.
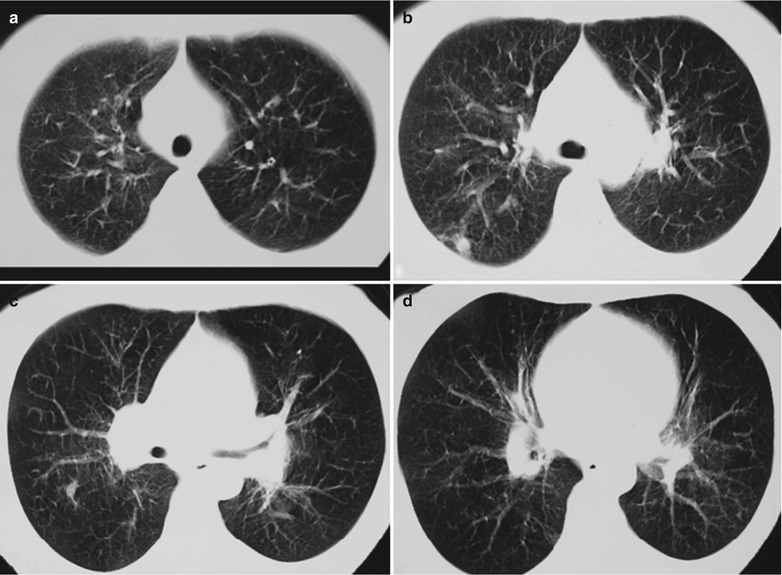
(a–d) HIV/AIDS related lymphoid interstitial pneumonia. (a–d) CT scanning demonstrates thickened and deranged pulmonary markings in both lungs, in reticular appearance; with accompanying multiple small nodular shadows
Case Study 2
A male patient aged 39 years was confirmatively diagnosed as having AIDS by the CDC. He complained of cough and no sputum. His CD4 T cell count was 45/μl.
Fig. 17.103.
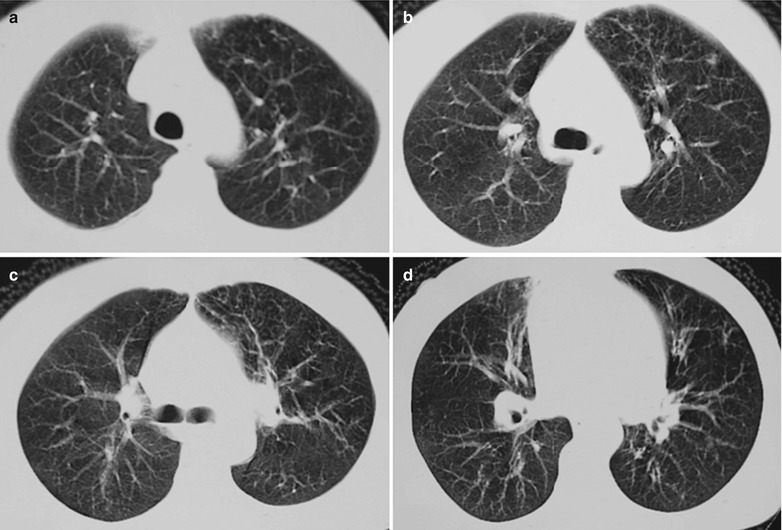
(a–d) HIV/AIDS related lymphoid interstitial pneumonia. (a–d) CT scanning demonstrates thickened and deranged pulmonary markings in both lungs, in reticular appearance; with accompanying multiple small nodular shadows
Case Study 3
A female patient aged 49 years was confirmatively diagnosed as having AIDS by the CDC. She complained of cough and no sputum. Her CD4 T cell count was 7/μl.
Fig. 17.104.
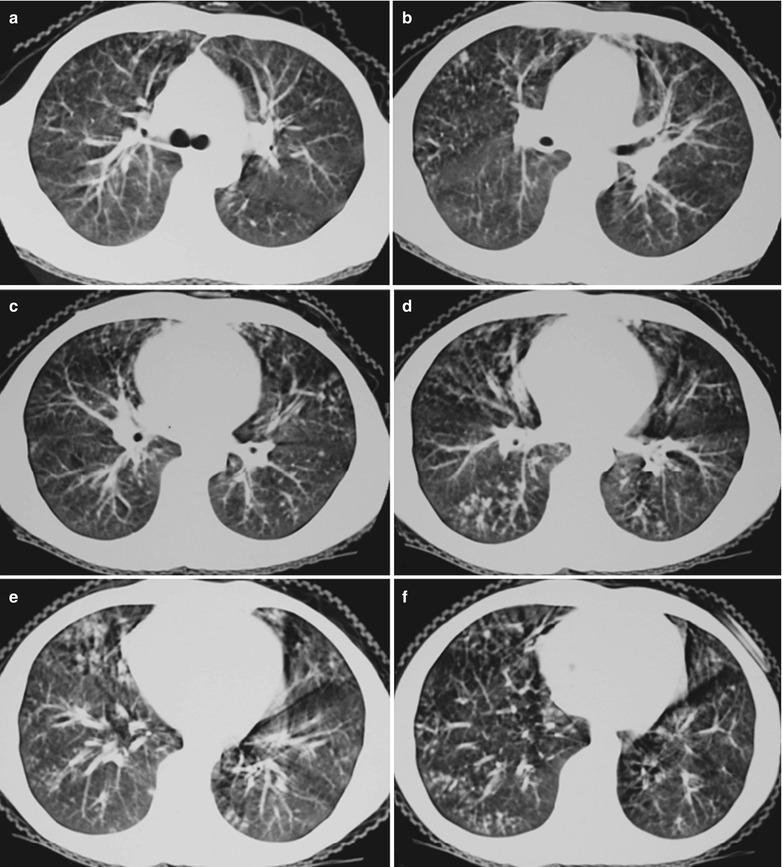
(a–f) HIV/AIDS related lymphoid interstitial pneumonia. (a–f) CT scanning demonstrates thickened and deranged pulmonary markings in both lungs in reticular appearance, with accompanying multiple small nodular shadows, fusion of some nodules into flaky shadows, and ground glass liked changes in the lung fields
Diagnostic Basis
Peripheral hemogram demonstrates increased lymphocytes and eosinophilic granulocytes.
Myelogram demonstrates increased lymphocytes, plasmocytes and eosinophils.
Increased immune globulin, predominantly IgM.
Hyoxemia.
Pathogenic examination of bronchoalveolar lavage fluids fails to find the pathogen.
Pulmonary function examination demonstrates restrictive ventilatory disorder, lower lung compliance and impaired diffusion function.
In terms of the diagnostic imaging, chest X-ray demonstrates thickened pulmonary markings or reticular spots shadows, feathery infiltration shadows and nodular shadows in the basal lung and no hilar lymphadenectasis. In the advanced stage, it develops into pulmonary interstitial fibrosis, showing honeycomb liked lung. CT scanning demonstrates small nodular and ground glass liked shadows. Pulmonary function examinations demonstrate reduced lung volume, restrictive ventilatory disorder, lower lung compliance and dysfunctional diffusion. Dysfunctional diffusion is a more sensitive indicator in monitoring the progress of the disease.
Differential Diagnosis
HIV/AIDS related lymphoid interstitial pneumonia should be differentiated from allergic pneumonia, carcinomatous lymphangitis and pneumocystis carinii cysts.
Discussion
CT scanning with high resolution demonstrates characteristic lesions of HIV/AIDS related lymphoid interstitial pneumonia, including intralobular linear shadows and honeycomb liked changes, with common involvement of the subpleural area and the basal lung. Its characteristic manifestations are clustering gas containing thin-walled cyst in a diameter of 2–10 mm, with clear cyst wall. Shared wall between cysts is its characteristic demonstration. The surrounding ground glass liked density indicates inflammation. Intralobular linear shadows indicate interstitial fibrosis.
HIV/AIDS Related Pulmonary Parasitic Diseases
HIV/AIDS Related Pulmonary Toxoplasmosis
Pulmonary toxoplasmosis (PT) is caused by the toxoplasma parasitizing in cells. Ludlam et al. firstly proposed the concept of pulmonary toxoplasmosis in 1963 [107], arguing that toxoplasma can cause atypical pneumonia. Later, there are some pathological reports about pulmonary toxoplasmosis or disseminated toxoplasmosis with lung involvement. In recent years, due to the global prevalence of AIDS, the incidence of pulmonary toxoplasmosis is increasing, with most cases being disseminated toxoplasmosis with lung involvement. PT has been one of the important opportunistic infections in patients with immunosuppression, especially AIDS patients.
Pathogen and Pathogenesis
HIV/AIDS related pulmonary toxoplasmosis is a zoonotic disease, with cats as its main transmission source, followed by pigs and sheep. People are infected by intake the water or food contaminated by cats’ feces or without cooked meat. Immunocompromised AIDS patients are susceptible to this disease, and its occurrence is rare in immunocompetent people. After its invasion into the human body, the sporozoite in the cystozygote and intracystic cystozoite overflows to penetrate the intestinal wall mucosa and spread to the whole body tissues along with blood or lymph flow. The brain, heart, lymph nodes, and lung are the most vulnerable tissues and organs for the infection. Any abnormality in the process of defense mechanism can cause impaired immune functions to eliminate the toxoplasma, which ultimately causes systemic and pulmonary infections. Pulmonary toxoplasma infection may also be caused by the blood borne spreading of reactivated toxoplasma infection in other body parts, with no exclusion of reactivated pulmonary infection or primary pulmonary infection.
Pathophysiological Basis
Ludlam et al. generally nominated toxoplasmosis as atypical pneumonia [107]. Catterall et al. divided toxoplasmosis into three types: necrosis, inflammatory infiltration and toxoplasma invasion [109]. It can also be classified as type A: subclinical or occult infection; type B: interstitial and atypical pneumonia; type C: necrotic pneumonia; type D: lobar pneumonia; and type E: granulomas pneumonia (toxoplasmoma). By naked eyes observation, the involved lungs are solid, with congestion and red brown section. The pleura have bleeding spots, with moderate peribronchial lymphadenectasis. Under a light microscope, there is exudation of serous fluids in alveolar cavities, occasional formation of transparent membrane or fibrin purulent exudation, infiltration of small quantity neutrophils, proliferation and shedding of alveolar wall cells, and trophozoite and/or cysts of toxoplasma in epithelial cells and macrophages. The pulmonary interstitium may have infiltration of lymphocytes and plasmocytes as well as visible fibroblasts and macrophages. The granuloma changes are also found in the lung tissues, with central stripes or localized necrosis and surrounding lymphocytes and small quantity multinucleated giant cells. It is difficult to find toxoplasma in granuloma, but it can be found in the normal tissues around or near the granuloma.
Clinical Symptoms and Signs
Almost all cases of AIDS complicated by pulmonary toxoplasmosis are caused by disseminated toxoplasmosis with pulmonary involvement. It is commonly diffuse pulmonary inflammation with serious symptoms, including high fever, cough, cyanosis, breathing difficulty, possible occurrence of skin rashes, lymphadenectasis and meningitis. The chronic cases may have long-term low grade fever, cough, and weight loss.
Examinations and Their Selection
Pathogen Examinations
Direct light microscopy of the specimen smear and enprint such as blood, cerebrospinal fluid, bone marrow, anterior aqueous humor, phlegm, urine, saliva, and other osmotic solutions, as well as lymph nodes, muscle tissue or other living tissues can be performed for pathogen examinations.
Immunological Assays
Sabin proposed that the staining test have high sensitivity and specificity according to the findings that mixture of fresh toxoplasma with normal serum can be stained deep by alkaline methylene blue staining, while its mixture with immune serum can be stained light or blank by the same staining. Other assays including indirect fluorescent antibody, indirect blood coagulation, and complement fixation test can provide valuable reference for the diagnosis.
Pathological Examinations
Toxoplasm is tested in pathological eaminations that can provide valuable reference for the diagnosis.
Diagnostic Imaging
Chest X-ray is the most commonly used diagnostic imaging examination. And chest CT scanning can be applied for the differential diagnosis.
Imaging Demonstrations
Imaging findings of HIV/AIDS related pulmonary toxoplasmosis can be divided into four types: bronchial pneumonia, interstitial pneumonia, pleuritis and complication of cardiovascular disease. The type of bronchial pneumonia is also known as lobular pneumonia, with thickened pulmonary markings that distribute along with the bronchi in the middle and lower lung fields, scattered patchy shadows with uneven density and blurry boundaries, fusion of some shadows into large flaky shadow and widened hilar shadow. The type of interstitial pneumonia is demonstrated as reticular and nodular shadows. The interstitial lesions widen the space between the bronchiole and the alveolar wall, with stripes and flocculent shadows. The type of pleuritis is rare, with signs of pleural effusion. The type of complication of cardiovascular disease is demonstrated as heart failure (acute pulmonary edema), with signs of pericardial effusion.
Case Study
A male patient aged 39 years was confirmatively diagnosed as having AIDS by the CDC. He complained of cough and fever. His CD4 T cell count was 29/μl.
Fig. 17.105.
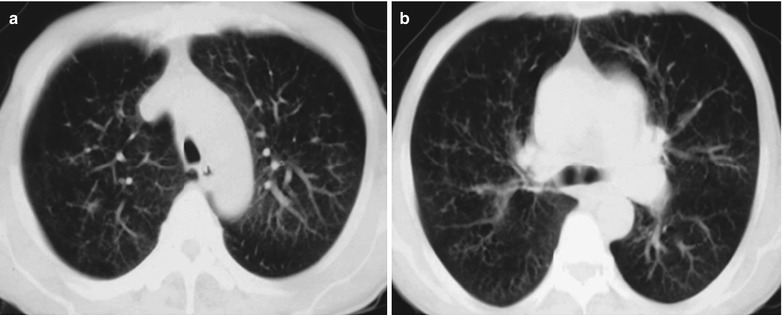
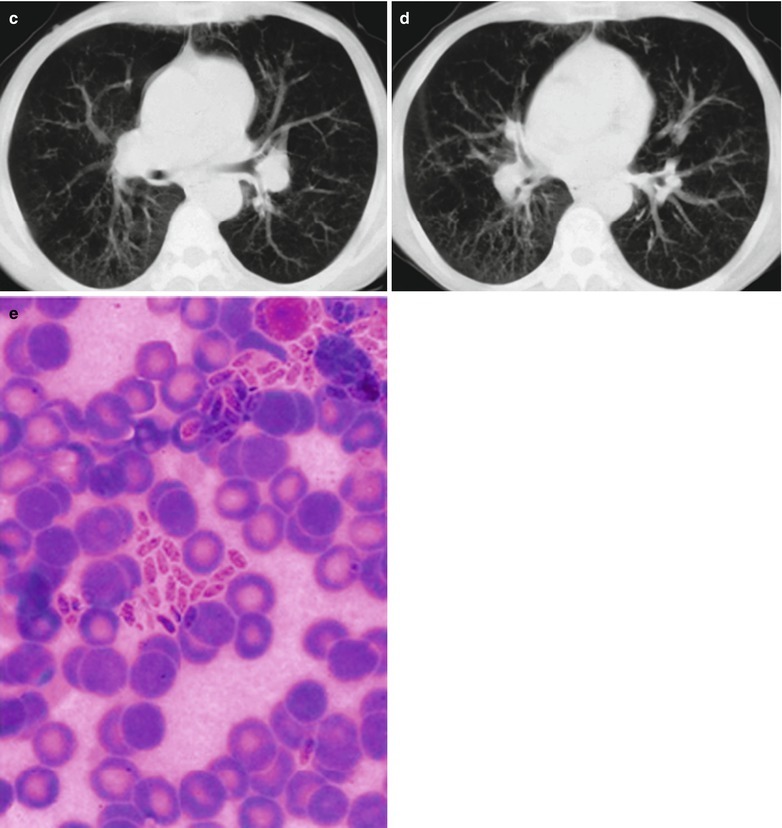
(a–e) HIV/AIDS related pulmonary toxoplasmosis. (a–d) CT scanning demonstrates thickened pulmonary markings in both lungs, which can be enhanced to extend into the middle and outer zones of lungs, in grid liked appearance that is more obvious in the dorsal segment of the lungs. (e) It is demonstrated to have clustering toxoplasma tachyzoites
Diagnostic Basis
Pathogen Examinations
By direct light microscopy, toxoplasma tachyzoites can be found in the specimens such as blood, cerebrospinal fluid, bone marrow, anterior aqueous humor, phlegm, urine, saliva, and other osmotic solutions, as well as lymph nodes, muscle tissues or other living tissues.
Immunological Assays
The fluorescent antibody and complement fixation test are positive.
Pathological Examinations
The biopsy tissue culture and inoculation test are positive. In the lesions and their surrounding tissues of interstitial pneumonia, necrotic bronchitis or granuloma, Toxoplasma can be found.
Diagnostic Imaging
The diagnostic imaging demonstrates any one type of pulmonary toxoplasmosis, including bronchial pneumonia, interstitial pneumonia, pleuritis and cardiovascular disease, can be used as the evidence for the diagnosis of pulmonary toxoplasmosis. The type of bronchial pneumonia is also known as lobular pneumonia, with thickened pulmonary markings with a distribution in both middle and lower lung fields along with the bronchi, scattered patchy shadows with uneven density and blurry boundaries, fusion of some patchy shadows into large flaky shadow and widened hilum. The type of interstitial pneumonia has typical demonstrations of reticular and nodular shadows. The interstitial lesions widen the space between the bronchiole and the alveolar wall, with strip and flocculent shadows. The type of pleuritis is rare, with signs of pleural effusion. The type of cardiovascular disease may have signs of heart failure (acute pulmonary edema), and signs of pericardial effusion.
Differential Diagnosis
HIV/AIDS related pulmonary toxoplasmosis should be clinically differentiated from infectious mononucleosis and mycoplasma pneumonia.
HIV/AIDS Related Pulmonary Strongyloidiasis
Pulmonary infections caused by nematodes in AIDS patients are occasionally reported.
Pathogen and Pathogenesis
Filariform larvae of strongyloides stercoralis invade into skin or mucosa and reach the lungs through lymphatic vessels or venous system and the right heart. They develop into schistosomula in 3–30 days. A few schistosomula develop mature in the lungs or bronchi. Most schistosomula penetrate the pulmonary capillaries into alveoli to cause a series of respiratory symptoms. In the cases of serious infection and in patients with compromised immunity, disseminated lesions occur in lungs and other organs.
Clinical Symptoms and Signs
HIV/AIDS related pulmonary strongyloidiasis can have manifestations of local small bleeding spots, pimples, migratory linear or strips urticaria on skin, as well as manifestations of allergic bronchitis, lobular pneumonia or asthma. AIDS patient may have severe diffuse infection and systemic infection, with symptoms of fever, severe cough, expectoration, hemoptysis, shortness of breath, breathing difficulty, and asthma.
Examinations and Their Selection
Laboratory tests
The pathological examinations include biopsy tissue culture and inoculation test.
Chest X-ray is the most commonly used examination.
Diagnostic Imaging
There are demonstrations of small spots and flaky shadows, thickened hilar shadow and thickened pulmonary markings. Mitchell reported in 1992 that interstitial or alveolar infiltration in both lungs accounts for 62 %, nodular shadows in both lungs 15 %, hilar or mediastinal lymphadenectasis 26 %, pleural fluids 42 %, septal line 25 %, mediastinal lymphadenectasis and ascites are the important clues for the diagnosis.
Diagnostic Basis
The clinical manifestations of HIV/AIDS related pulmonary strongyloidiasis are non-specific. Its diagnosis depends on the etiological examinations. The findings of Strongyloides sterocralis in the patient’s sputum and feces can define the diagnosis.
By laboratory tests, WBC count in peripheral blood increases to 20 × 109∕L; eosinophils granulocytes 25–30 %, or even as high as 70–80 %; the serum total lgE level increases by 50 %; 90 % cases with blood serum lgG and lgE of filariform larvae antigen positive. In the cases with femal strongyloides stercoralis parasitizing in the bronchial epithelium, rhabditiform larva, filariform larva, schistosomula, adult strongyloides stercoralis and the eggs can be found in fresh phlegm, which can define the diagnosis.
Pathological examinations including biopsy tissue culture and inoculation test are positive.
By chest X-ray, the lungs have small spots and flakes of shadows, thickened hilar shadow and thickened pulmonary markings.
Differential Diagnosis
HIV/AIDS related pulmonary strongyloidiasis should be differentiated from HIV/AIDS related pulmonary toxoplasmosis, infectious mononucleosis and mycoplasma pneumonia.
HIV/AIDS Related Pulmonary Neoplasms
HIV/AIDS Related Lymphoma
Pulmonary infiltration of HIV/AIDS related malignant lymphoma commonly has three types: Primary pulmonary lymphoma, which is rare and accounts for 0.5 % of primary pulmonary neoplasms, and 3 % of extranodal lymphomas. Cadranel et al. summarized the characteristics of three typical clonal proliferative diseases in lymphatic system [90], with primary pulmonary lesions. Pulmonary low malignant B cell lymphoma is the most common primary pulmonary lymphoma which is derived from mucosa related lymphoid tissue. The manifestations include slowly decreased alveolar transparency. Pulmonary high malignant B cell lymphoma is extremely rare, which often occurs with singular lesion and primary disease such as immunodeficiency.
Pathogen and Pathogenesis
HIV/AIDS related malignant lymphoma is mostly caused by compromised immunity. HIV/AIDS related Hodgkin’s lymphoma is relatively rare. There are also reports about HIV/AIDS related T cell lymphoma with pulmonary involvement.
Pathophysiological Basis
HIV/AIDS related malignant lymphomas are mostly highly malignant large cells lymphoma. Cerebral lymphoma is one of the defining diseases of AIDS. It has been reported that the clinical incidence of pulmonary infiltration by malignant lymphoma is 10–20 %, but 29–50 % by autopsy.
Clinical Symptoms and Signs
In the early stage, it is commonly asymptomatic. With its progression, symptoms of dry cough, suffocation, and small quantity clear phlegm occur. Mediastinal lymphadenosis includes lymphadenectasis to compress the trachea by, blood vessels and nerves and lead to breathing difficulty, superior vena caval obstruction syndrome, and hoarse voice. The pulmonary parenchyma lesions include reticular structure in the lungs. The clinical symptoms are cough, expectoration, suffocation and breathing difficulty.
Examinations and Their Selection
Laboratory tests
Pathological biopsy, tissue culture and inoculation test.
The diagnostic imaging examinations are the most commonly used examinations for the diagnosis.
Diagnostic Imaging
The imaging demonstrations of HIV/AIDS related malignant lymphomas include:
Mediastinal lymphadenectasis is the most commonly found pulmonary manifestations of malignant lymphoma. The lesions are mainly located in anterior and middle mediastinum, in asymmetric wave liked or lobulated mass. It occurs unilaterally or bilaterally, isolated or fusion.
The incidence of pulmonary parenchymal lesions is 20–30 %. Chest X-ray demonstrates mediastinal lymph nodes extending directly into the lungs, which is susceptible to confusion with pneumonia. They are demonstrated as round shadows in the lung fields or distribute in the whole lung fields. Chest X-ray demonstrates the lymphatic spread of lesions as military nodules in different sizes or isolated intrapulmonary nodules or cavities, commonly accompanying with mediastinal hilar lymphadenectasis. In the cases with its occurrence secondary to endobronchial membrance, obstructive pneumonia or atelectasis can be caused. Some patients may have diffuse pulmonary interstitial changes. Pulmonary infiltration by non-Hodgkin’s lymphoma can also be divided into four types: (1) Nodular type; (2) Pneumonia-alveolar type; (3) bronchial-vascular-lymphatic type, which can be further divided into the central bronchial- vascular type, and diffuse lymphatic type; (4) diffuse lymphatic type can have lesions of reticular or reticular nodular infiltration and its progression into patchy changes.
Miliary-blood borne spreading type is rare.
The pleural lesions is mainly pleural effusion, with bloody or serous pleural fluid.
Case Study 1
A male patient aged 43 years was confirmatively diagnosed as having AIDS by the CDC. He complained of chest distress and cough for more than 1 month. His CD4 T cell count was 56/μl.
Fig. 17.106.
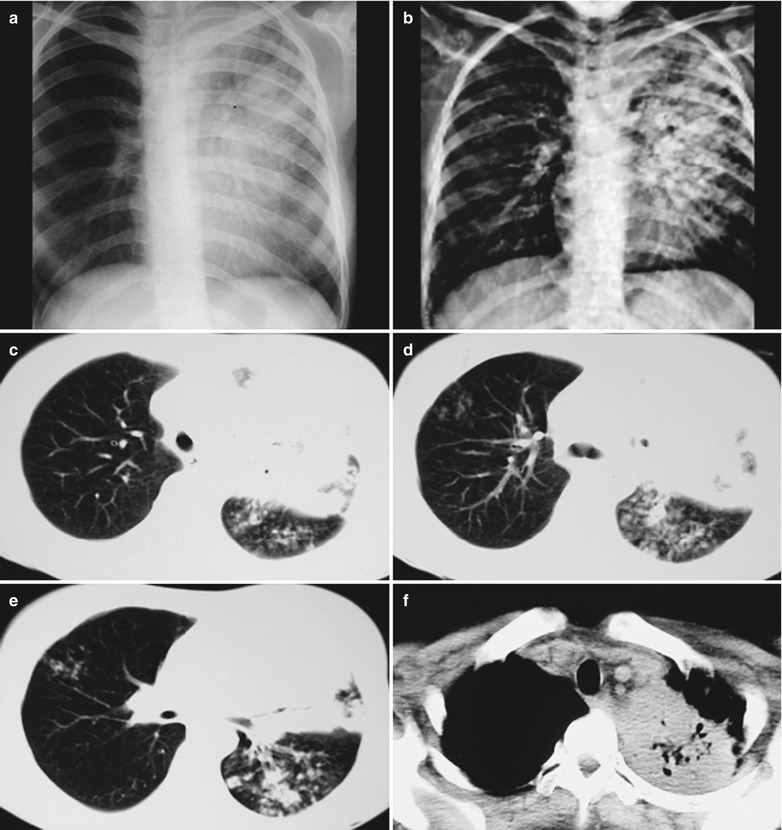
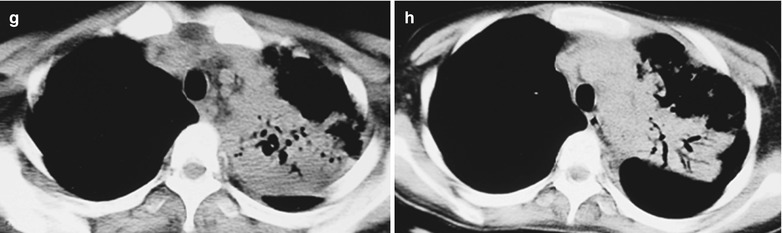
(a–h) HIV/AIDS related lymphoma. (a–b) DR demonstrates enlarged and thickened left hilum in a huge mass shadow. High KV demonstrates a huge mass shadow in the hilum. (c–h) CT scanning of the pulmonary window demonstrates a huge high density mass shadow in the left hilum, surrounding nodular fusion shadows in the lung tissues. CT scanning of the mediastinal window demonstrates a huge high density mass shadow in the left hilum, with air bronchogram sign in the shadow
Case Study 2
A male patient aged 41 years was confirmatively diagnosed as having AIDS by the CDC. He complained of chest distress and chest pain for more than 2 months. His CD4 T cell count was 36/μl.
Fig. 17.107.
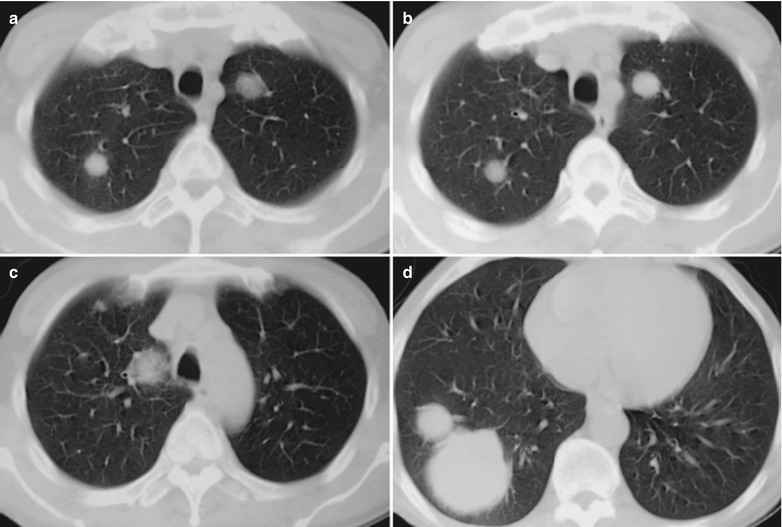
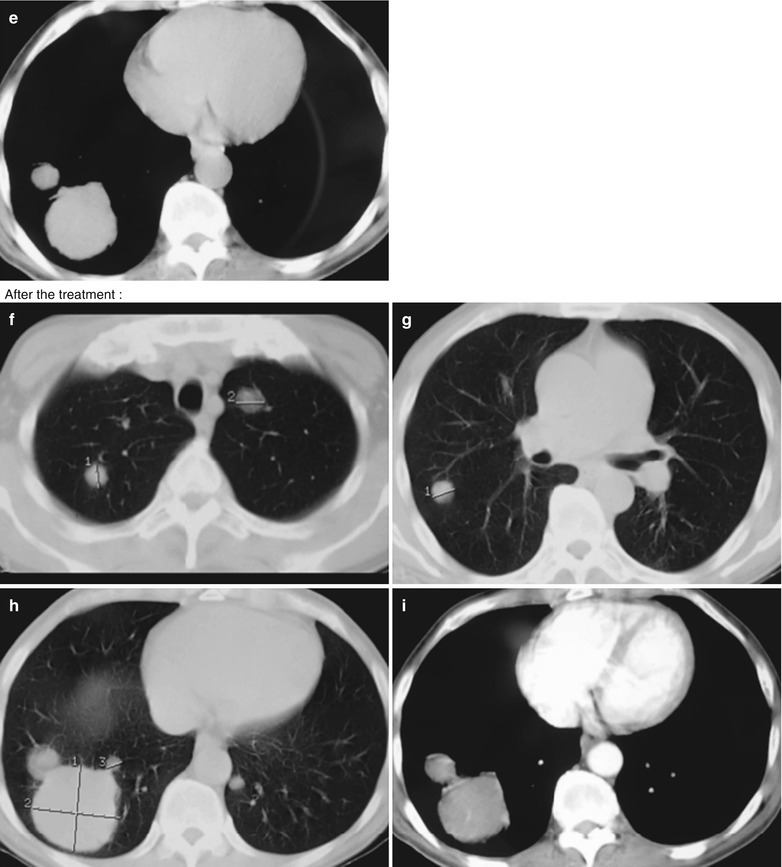
(a–e) HIV/AIDS related lymphoma. (a–e) CT scanning of the pulmonary and mediastinal windows demonstrates multiple round liked nodular shadows with increased density in both lung fields, with clear boundaries; large soft tissue mass shadows in the right lower lung, with slightly lobulated boundaries and spikes. (f–i) CT scanning of the pulmonary and mediastinal windows for reexamination after radiation therapy demonstrates shrinkage of intrapulmonary nodules and masses
Case Study 3
A male patient aged 35 years was confirmatively diagnosed as having AIDS by the CDC. He complained of chest distress and breathing difficulty for 15 days. His CD4 T cell count was 66/μl.
Fig. 17.108.
HIV/AIDS related lymphoma. DR demonstrates widened upper middle mediastinum in a dense shadow, enlarged and thickened hilum, thickened and blurry pulmonary markings with diffuse ground glass liked changes
Case Study 4
A male patient aged 26 years was confirmatively diagnosed as having AIDS by the CDC. He had a history of homosexual behaviors and was hospitalized due to a progressively enlarged subaxillary mass for 3 months. HIV antibody was confirmed positive and his CD4 T cell count was 363/μl.
Fig. 17.109.
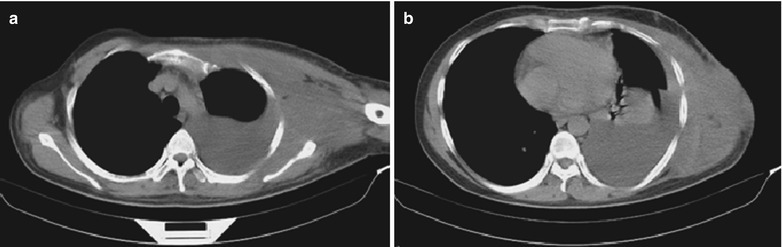
(a, b) HIV/AIDS related lymphoma. (a, b) CT scanning demonstrates a huge soft tissue mass shadow in the left lateral chest wall, with a maximal size of about 7.7 × 13.0 cm and occupying 30 sections with 8 mm in thickness of each section and with the upmost to the supraclavicular area and the bottom in the level of thoracic 12th vertebral body in the lower chest wall. There are also large quantity left pleural effusion and parenchymal changes of the left lower lobe with atelectasis. In the left chest cavity, large quantity liquid density shadows can be found, with compressed lung tissues to the hilum. By puncture and biopsy of the subaxillary mass, the diagnosis is defined as diffuse large B cell lymphoma
Diagnostic Basis
The clinical symptom of difficulty breathing.
By pathological examination of lung tissue biopsy, lymphoma cells are found.
The imaging demonstrations include interstitial infiltration with mediastinal lymphadenectasis or intrapulmonary mass shadows, being singular or multiple with clear boundaries. The masses distribute in the lungs or in the mediastinum and chest wall. The incidence of lymphadenectasis is about 25 %. In the cases with pleural involvement, tumor occurs in the chest wall. The incidence of pleural effusion is 30–50 %. There are also pulmonary demonstrations of reticular nodular shadows, thickened bronchial walls and lobular septum linear shadows.
Differential Diagnosis
HIV/AIDS related pulmonary lymphoma should be differentiated from lung cancer, metastatic tumor and tuberculoma.
Discussion
HIV/AIDS related pulmonary lymphoma can be classified into large cells type (Immunoblastic type) and Burkitt liked type. The vast majority of them have the morphology of the B cells, and most are related to Epstein-Barr viral genome. Its occurrence is at a CD4 T cell count being lower than 100/μl. Hodgkin’s lymphoma tend to occur in the early stage of AIDS, with a CD4 T cell count being above 200/μl. The most common imaging demonstrations include singular or multiple nodules. The singular lesions have a scattering distribution, with clear boundaries. The smooth nodules should be diagnosed as HIV/AIDS related lymphoma. The larger mass sometimes may have cavities in it. Interstitial infiltration with no masses or nodules is rare. Another rare manifestation of HIV/AIDS related B cell lymphoma is pleural, pericardial or abdominal cavity effusion without scattered masses, which is known as coelom-derived or primary exudative lymphoma.
HIV/AIDS Related Kaposi’s Sarcoma
Pathogen and Pathogenesis
In the year of 1994, Moore and Chang identified gamma herpes virus in tissues of Kaposi’s sarcoma and proved their relationship. Kaposi’s sarcoma can be found in any stage of HIV infection, which may occur at even normal level of CD4 T cell count. However, it mostly occurs at a CD4 T cell count being lower than 2,200 × 106/L. The results of epidemiological studies and the fact of HHV-8 being isolated from Kaposi’s sarcoma tissues have demonstrated that Kaposi’s sarcoma is closely related to HHV-8 infection. As a result, HHV-8 is known as Kaposi’s sarcoma related herpes virus. In patients with HIV/AIDS related Kaposi’s sarcoma, its serologic positive rate is 100 %. Kaposi’s sarcoma cells can produce IL-6, during which IL-6 plays a role as an autocrine factor to maintain the cell growth, paracrine cytokines, stimulate proliferation of other interstitial cells and induct the vascular growth. Therefore, Kaposi’s sarcoma is a kind of tumor with abundant blood vessels. Before the application of HARRT, the incidence of Kaposi’s sarcoma in male homosexuals is 21 %. After the clinical application of HARRT treatment, the incidence is decreasing. In addition to HHV-8, some studies indicated that most patients with Kaposi’s sarcoma have HIA-DR5 alleles, suggesting a possible relationship between Kaposi’s sarcoma and the heredity.
Pathophysiological Basis
There is no obvious difference between HIV/AIDS related Kaposi’s sarcoma and classic Kaposi’s sarcoma in pathological changes. Early pathological manifestations are chronic inflammation or granulomatous inflammation, with formation of new vascular and lymphatic vessels and accompanying edema and bleeding. The findings of large and protruding endothelial cells in granuloma tissue with accompanying erythrocytic exudation and hemosiderin particles have great significance for the early diagnosis. The pathological changes in the advanced stage are significant proliferation of the endothelial cells, and proliferation of fibroblasts around capillaries. In the advanced stage, the lesions are often accompanied by extensive connective tissue hyperplasia, which presents difficulty for its differentiation from common sarcoma. When it is difficult to define the diagnosis by light microscopy, immunohistochemical examinations can be used to define the diagnosis. The pathological changes are characterized by lesions confined to the epithelial lamina propria, gathering of spindle cells with mild heteromorphism around many lacuna vasorum with irregular lumen, erythrocytic exudation and hemosiderin sedimentation. The atypical lacuna vasorum can be compressed by proliferative spindle cells to be absent. Vascular endothelial cells and peripheral spindle cells may have mitotic phase in the advanced stage, with increased heteromorphism cells. The inflammatory cells are mainly plasma cells, with acidophilic corpuscles and PAS staining positive, which can assist the pathological diagnosis.
Clinical Symptoms and Signs
Pulmonary Kaposi’s sarcoma in AIDS patients rarely has symptoms. It is commonly concurrent with pulmonary opportunistic infections, with symptoms of cough, difficulty breathing and fever. Other symptoms are related with the location of the tumors. The involvement of trachea or bronchi can cause luminal stenosis. The mediastinal tumor can compress and obstruct lymph vessels to cause pulmonary edema or a large quantity pleural fluids, which result in respiratory difficulty, and even respiratory failure.
Examinations and Their Selection
Sampling by bronchoscopy or endoscopy to prepare pathological section.
Chest X-ray demonstrates its typical manifestation of pleural effusion.
Imaging Demonstrations
DR demonstrates enlarged and deranged hilum in both lungs in bird nest liked appearance. There is light density flaky shadows in the both lower lungs. CT scanning demonstrates multiple rounds liked nodular shadows in the middle and lower lung fields of both lungs with clear boundaries. There are also mediastinal and hilar lymphadenectasis, with common involvement of the pleura and bilateral pleural effusion in a small quantity.
Case Study 1
A male patient aged 33 years was confirmatively diagnosed as having AIDS by the CDC. He had been detected as HIV positive for 5 months, with complaints of recurrent cough and nausea for 10 days and was hospitalized on Jan. 7, 2004. The transmission route was unknown because he denied histories of intraveneous drug abuse, paid blood donation, blood transfusion and unhealthy sexual behaviors. Five months ago, he was diagnosed as AIDS in the stage of AIDS in our hospital, and hospitalized to treat PCP, with a CD4 T cell count of 17/μl. His symptoms were quickly relieved after PCP treatment and he continued the antiviral therapy for almost 5 months after being discharged. By physical examinations, he was in poor spiritual condition, a light blue nodule in size of 0.5 × 0.5 cm in the left upper chest wall with medium hardness, palpable lymph nodes in size of 1.0 × 2.0 in the opisthotic area and inguen, no tenderness and being movable. By the digital rectal examination, a palpable prominent nodule with wide base at 4 cm 7 points away from the anus, with flexible texture and smooth surface. By the auxiliary examinations, WBC 3.9 × 109/L, NEμT 48.3 %, LYM 34.9 %, MON 7.4 %, EOS 9.0 %, RBC 3.27 × 1012/L, HGB 126 g/L, PLT 210 × 109/L, routine urine test normal, blood sedimentation 16 mm/h. By hepatitis B examinations, HBsAg, Anti-HBe and Anti-HBe positive. His CD4 T cell count was 91/μl. By abdominal B ultrasound, multiple low echo nodules in the abdominal cavity, the largest in size of 1.2 × 1.0 cm, which are suspected to be enlarged lymph nodes. On Jan. 14, he received inguinal lymph node biopsy, with pathological report of Kaposi’s sarcoma. During the treatment and following up, the involvement of lungs, digestive tract, lymph nodes and skin is suspected, with the diagnosis of phase II Kaposi’s sarcoma and chemotherapy was recommended. Reexamination by chest X-ray demonstrated normal cardiopulmonary phrenic. Abdominal B ultrasound failed to find enlarged lymph nodes. CT scanning demonstrated shrinkage of lesions in both lungs and mediastinal lymph nodes, with only palpable soybean sized submandibular lymph node. By examinations after chemotherapy, CD4 T cell count 67/μl, viral load 63,000 copies/ml. The patients had multimorphological erythema drug eruptions, which was suspected as drug allergies of chemotherapy, which were absent after symptomatic treatment. The following ups so far show no recurrence of Kaposi’s sarcoma, with his CD4 T cell count fluctuating around 400/μl. He can work as usual.
Fig. 17.110.
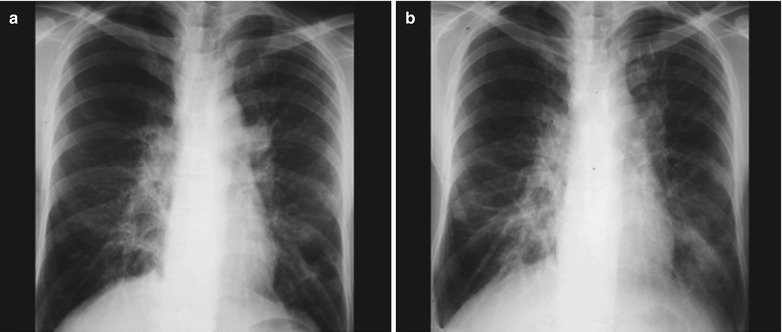
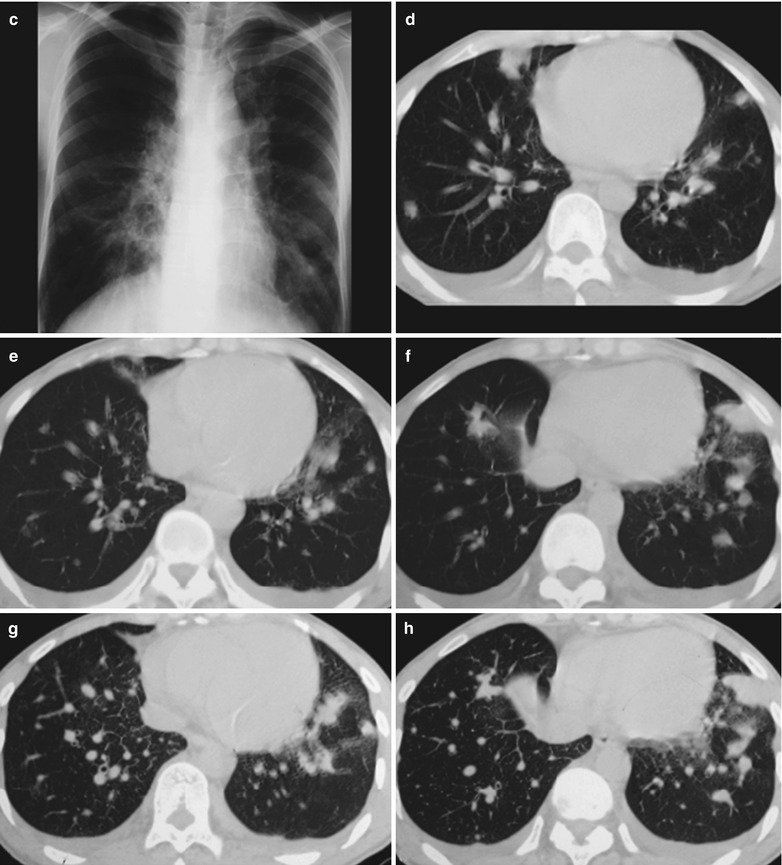
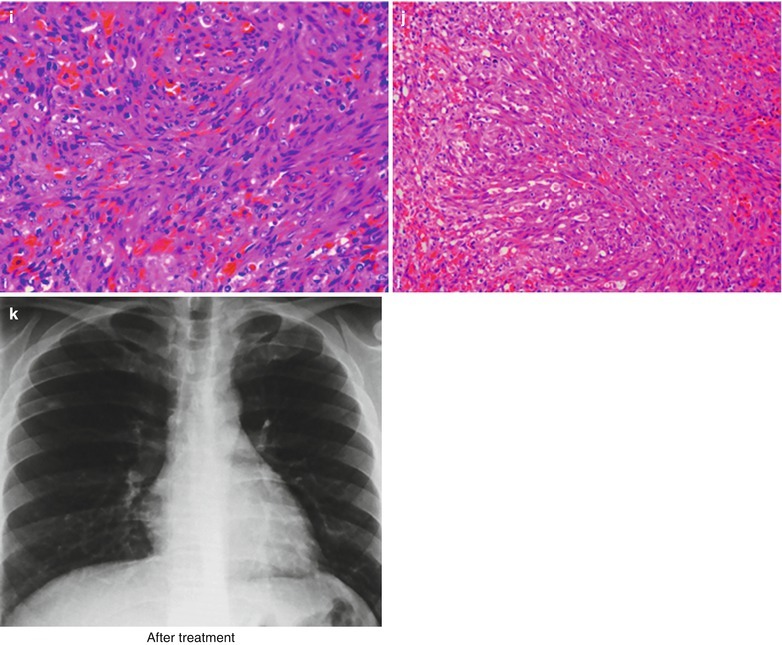
(a–k) HIV/AIDS related Kaposi’s sarcoma. (a–c) DR demonstrates enlarged hilum in both lungs with deranged structure in bird nest liked appearance; light density flaky shadows in the lower lung fields of both lungs and small quantity pleural effusion. (d–h) CT scanning demonstrates multiple round liked nodular shadows in the middle and lower lung fields of both lungs with clear boundaries, multiple mediastinal and hilar enlarged lymph nodes. (i, j) HE staining demonstrates large quantity spindle cells or fusiform cells as well as thick stained nucleoli also in spindle shape. (k) Reexamination after treatment demonstrates no abnormalities in both lungs
Case Study 2
A female patient aged 38 years was confirmatively diagnosed as having AIDS by the CDC. She complained of recurrent cough for 10 days and had a history of unhealthy sexual behaviors. Her CD4 T cell count was 35/μl.
Fig. 17.111.
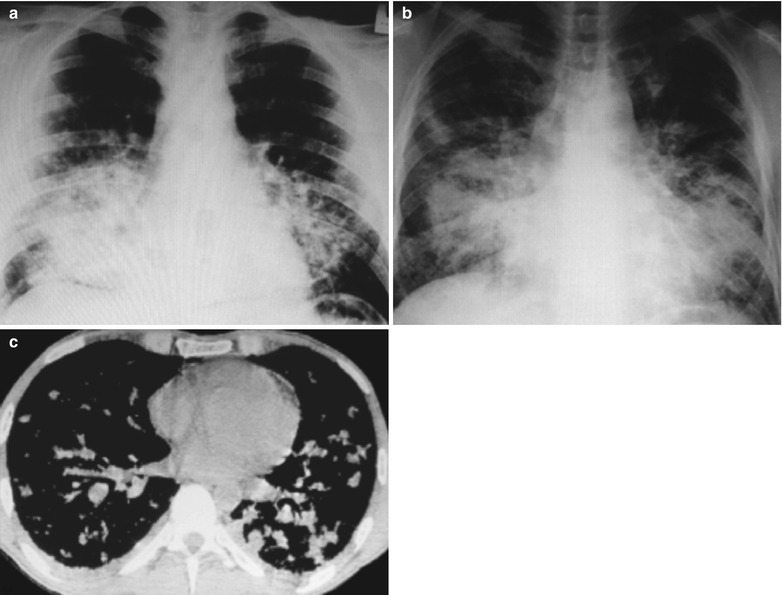
(a–c) HIV/AIDS related Kaposi’s sarcoma. (a, b) DR demonstrates enlarged hilum in both lungs with deranged structure in bird nest liked appearance, light density flaky shadows in lower lung fields of both lungs. (c) CT scanning demonstrates multiple round liked nodular shadows in both middle lower lung fields with clear boundaries, multiple mediastinal and hilar lymphadenectasis, and small quantity pleural effusion in bilateral thoracic cavities
Case Study 3
A male patient aged 39 years was confirmatively diagnosed as having AIDS by the CDC. He complained of recurrent cough for more than 1 month and had a history of unhealthy sexual behaviors. His CD4 T cell count was 55/μl.
Fig. 17.112.
(a, b) HIV/AIDS related Kaposi’s sarcoma. (a, b) DR demonstrates enlarged hilum in both lungs with deranged structure in bird nest liked appearance
Case Study 4
A male patient aged 36 years was confirmatively diagnosed as having AIDS by the CDC. He had a history of homosexual behaviors, with complaints of fever and cough for 2 months as well as chest distress for more than 20 days. Since, July 2010, fever with a body temperature of about 37.5–37.8 °C occurs, with cough, yellowish bloody sputum, and dark purplish patchy skin rashes.
By examinations, his anti-treponema pallidum antibody positive, multiple dark purplish patchy skin rashes on the face, eyelid, lower jaw, hairline, chest and abdomen with skin surface desquamation, palpable bilateral cervical lymphadenectasis and the largest in size of 10 × 19 mm. By laboratory tests, WBC 5.98 × 109/L, N 78.74 %, RBC 2.22 × 1012/L, HGB 71 g/L, PLT 204 × 109/L, CD4 12/μl.
Fig. 17.113.
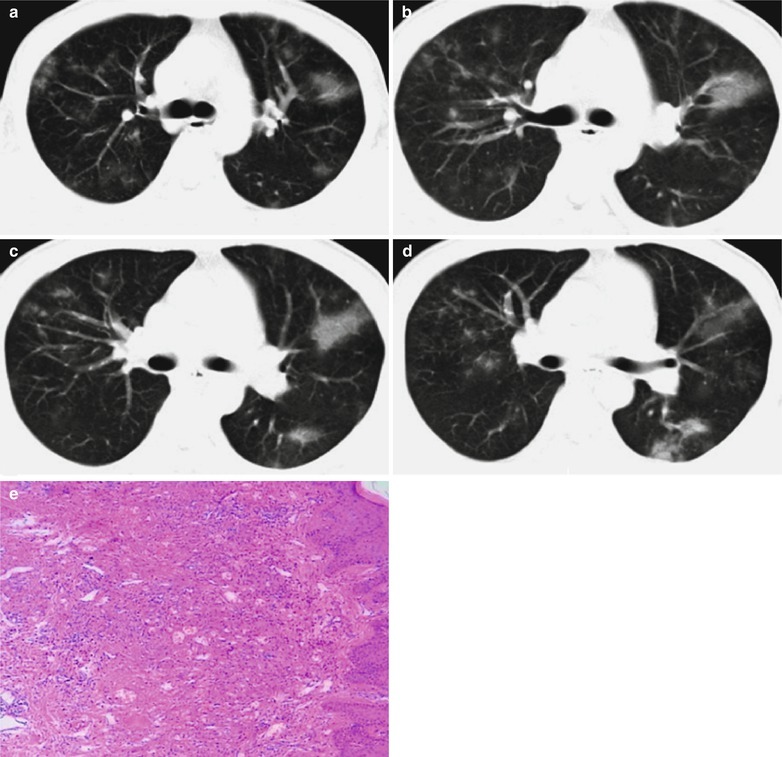
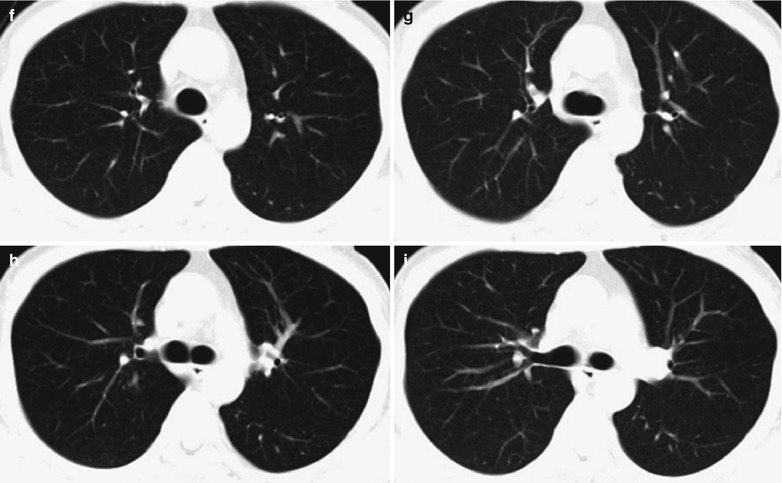
(a–e) HIV/AIDS related Kaposi’s sarcoma. (a–d) Chest CT scanning demonstrates scattered cloudy, mass and flake liked or nodular shadows with increased density. (e) Pathological biopsy demonstrates large quantity heteromorphological spindle cells with large thick stained nucleoli, which are in line with the diagnosis of Kaposi’s sarcoma. (f–i) Cured HIV/AIDS related Kaposi’s sarcoma. (f–i) Reexamination after treatment demonstrates absent lesions in both lungs, with clear lung fields
Case Study 5
A male patient aged 27 years was confirmatively diagnosed as having AIDS by the CDC. He complained of cough for more than 2 months, chest distress for more than 1 month, and bloody sputum for half a month and was hospitalized. He had a history of homosexual behaviors. By examinations on admission, multiple round purplish blue skin rashes nodules on the limbs. His CD4 T cell count was 9/μl.
Fig. 17.114.
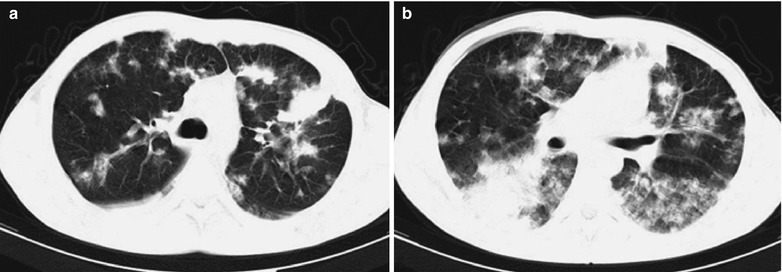
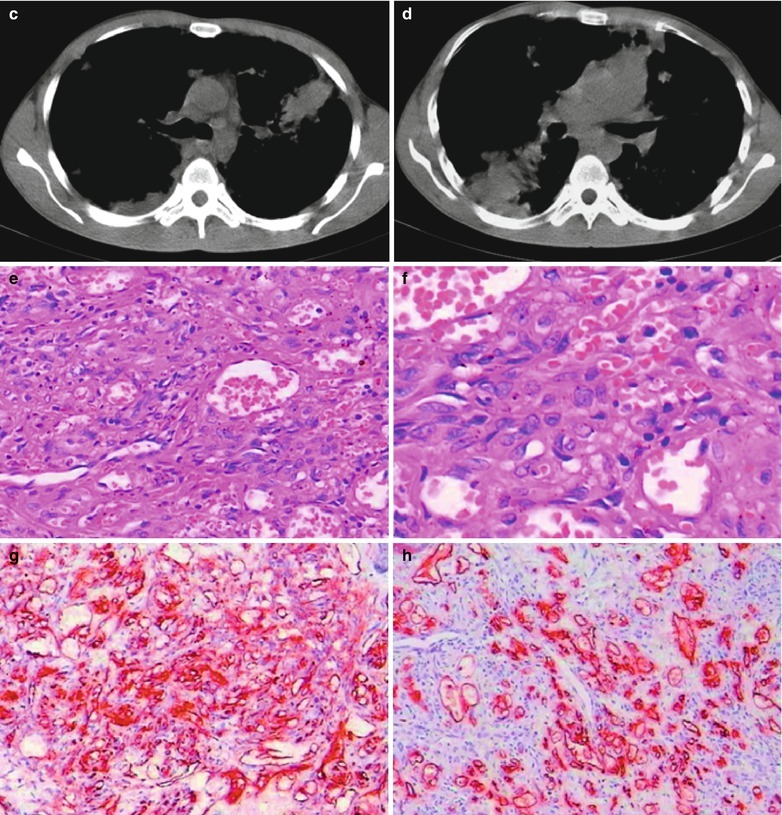
(a–h) HIV/AIDS related Kaposi’s sarcoma. (a–d) CT scanning demonstrates scattered cloudy mass and flakes liked or nodular shadows with increased density in both lungs with uneven density and unclear boundaries, fusion and parenchymal changes of some lesions, more lesions in the lower lobe of both lungs and mostly with parenchymal changes. (e, f) Pathological biopsy demonstrates large quantity heteromorphological spindle cells with large and thick stained nucleoli, which are in line with the manifestations of Kaposi’s sarcoma. (g, h) Immunohistochemical demonstrates positive of C3 and C4
Diagnostic Basis
The appearance of skin lesions of angiosarcoma is gray, grayish black or purplish blue infiltrative nodules or confluent plaques, with possible accompanying bleeding and ulcers. Patients with pulmonary Kaposi’s sarcoma are often accompanied with skin involvement, with the common manifestation of difficulty breathing.
Chest X-ray demonstrates nodular infiltration around the hilum of both lungs with deranged structure in bird nest liked appearance or infiltration of diffuse reticular nodular infiltration and pleural bloody effusion. CT scanning demonstrates enlarged and blurry hilum of both lungs, multiple intrapulmonary round liked nodular shadows with clear boundaries. The CT scanning demonstrations can also be flaky flocculent areas with blurry density or parenchymal density areas along with bronchi.
Lung puncture for histopathological biopsy demonstrates irregular vascular lumen in the dermis, proliferation of endothelial cell with accompanying heteromorphism. In some cases, there are tumor masses composed of spindle cells and epithelial cells.
Differential Diagnosis
Other Sarcoma and Vascular Tumor
HIV/AIDS related Kaposi’s sarcoma should be differentiated from other sarcoma and vascular tumor. KS invasion of the digestive mucosa can cause bleeding and upper gastrointestinal symptoms. The pathological lesions can be diagnosed by upper gastrointestinal endoscopy or biopsy. In the cases of no fever and exclusion of infections, the typical imaging demonstrations and bronchoscopy findings can define the diagnosis of pulmonary KS.
Pneumocystis Carinii Pneumonia
HIV/AIDS related Kaposi’s sarcoma should be differentiated from Pneumocystis carinii pneumonia. The lesions of PCP are mostly symmetric ground glass liked density shadows extending outwards from the hilum in both lungs. In the middle and advanced stages, nodules, fibrosis and cavities occur, rarely with pleural effusion.
HIV/AIDS Related Lung Cancer
Pathogen and Pathogenesis
Lung cancer commonly refers to the cancer in lung parenchyma, usually does not include those mesodermal tumors originating from other pleura, or other malignancies like carcinoid, malignant lymphoma, or metastatic malignancies for other body parts. Therefore, the following lung cancer we are discussing about refers to the malignancies originating from bronchial, or bronchiolar epithelial cells, accounting for 90–95 % of the lung parenchyma malignancies. The cause of lung cancer is still not completely known. Data have indicated that the risk factors of lung cancer include smoking (including second-hand smoke), asbestos, radon, arsenic, ionizing radiation, halogen alkenes, polycyclic aromatic compounds and nickel.
Smoking
Long-term smoking can cause proliferation of the bronchial mucosal epithelial cells and proliferation of squamous epithelium to induce squamous epithelium carcinoma or undifferentiated small cell carcinoma. Non-smokers can also develop lung cancer, but adenocarcinoma is more common among them.
Atmosphere Pollution
Occupational Factors
Long-term exposure to radioactive substances, like uranium and radium, and its derivatives; carcinogenic hydrocarbons, like arsenic, chromium, nickel, copper, tin, ferri, coal tar, bitumen oil, petroleum, asbestos and mustard gas, all can induce lung cancer, which is commonly squamous carcinoma and undifferentiated small cell carcinoma.
Chronic Pulmonary Diseases
Some chronic pulmonary diseases, such as tuberculosis, silicosis and pneumoconiosis, can concurrent with lung cancer. In the cases with these chronic pulmonary diseases, the incidence of cancer is higher than the general population. In addition,bronchopulmonary chronic inflammation and pulmonary fibrous scar lesions may cause metaplasia or hyperplasia of squamous epithelium during their healing processes, based on which some cases can develop into cancer.
Internal Factors of the Human Body
The internal factors of the human body include family heredity, compromised immunity, metabolism and endocrine dysfunction.
Pathophysiological Basis
The lung cancers distribute more in the right lung than in the left lung, more in the upper lobe than in the lower lobe. Its locations range from the major bronchus to the bronchioles. The central type of lung cancer has its origination from the major bronchial lobes and locates adjacent to the pulmonary hilum. The peripheral type of lung cancer has its origination from the lower parts of pulmonary segment bronchi and locates in the peripheral areas in the lungs. In the growth process of the lung cancer, it causes the extension and dilation of the bronchial walls, and penetrates the bronchial walls to invade the adjacent lung tissues and form masses. Meanwhile it intrudes into the bronchi to cause luminal stenosis or obstruction. With its further progression and dissemination, it spreads from the lungs and directly extends into the chest walls, mediastinum, heart, major vessels and other adjacent organs and tissues. Lung cancer can also transfer to other parts of the body along with blood and lymph flows or disseminates to other pulmonary lobes via the respiratory tract. The growth rate and transferring paths of lung cancer depend on its histological types, differentiation degree and other biological characteristics.
Clinical Signs and Symptoms
Early Symptoms and Signs
Lung cancer has no special symptoms in the early period, only has the common symptoms with common respiratory diseases, including cough, bloody sputum, low grade fever, chest pain and chest distress. Therefore, it is often misdiagnosed.
Symptoms in the Advanced Stage
(1) Face and neck edema; (2) Hoarse voice is the most common symptom; (3) Shortness of breath.
Symptoms of Metastatic Lung Cancer
Lung cancer tends to occur distant metastases in the early stage. In the cases with metastatic lesions to the brain, the patients sustain persistent headache and blurry vision. In the cases with metastatic lesions to the bone, bone destruction may occur to cause fracture.
Signs
(1) Restrictive wheezing sound, mostly occurring in the inspiratory phase and recurring after cough; (2) Hoarse voice, caused by lymph nodes transferring to compress and invade the recurrent laryngeal nerve; (3) Superior vena cava syndrome, caused by the compresses or invasion to the superior vena cava by the mass and venous obstruction, with edema in the head, face, neck, and upper limbs, varicose veins and edema in the upper chest, and accompanying dizziness, chest distress, shortness of breath and other symptoms; (4) Horner’s syndrome, with enophthalmos of the affected side, blepharoptosis, shrinkage of the pupils, eye fissure stenosis, increased skin temperature in the upper chest of the affected side and no sweating due to compression or invasion of the apical cancer to the cervical sympathetic ganglia; (5) Should and arm pain, which is radial burning pain in the shoulder and upper limbs of the affected side due to compression or invasion of apical cancer to the brachial plexus nerve; (6) Phrenic nerve paralysis, with symptoms of shortness of breath and chest distress due to invasion to the phrenic nerve; (7) Dysphagia, caused by compressed esophagus by mediastinal lymphadenectasis; and difficulty breathing caused by compressed trachea by mediastinal lymphadenectasis; (8) Pericardial effusion, shortness of breath, arrhythmia and heart dysfunctions due to pericardial invasion; (9) Pleural metastasis, with chest pain and cancerous pleural effusion; (10) Lung cancer metastasis, spreading of lung cancer along with blood flow to the bone, liver, brain, kidney, adrenal gland and subcutaneous tissues. Intrapulmonary metastasis is also common. Metastasis to different locations shows different symptoms and signs. (11) Extrapulmonary signs, commonly including joint pain or joint hypertrophy, clubbing fingers and mental disorders.
Examinations and Their Selection
Imaging Examinations
The diagnostic imaging is the most commonly used and an important examination for the diagnosis of lung cancer. It can facilitate to find some specific manifestations in the lesions, which provide clues for the diagnosis of lung cancer. It is also the main basis for the staging of lung cancer, but fails to define the qualitative diagnosis. Chest X-ray is the main examination for the diagnosis of lung cancer. Anteroposterior and lateral chest X-ray are used for preliminary screening. Chest CT is the diagnostic imaging examination of the choice for the diagnosis of lung cancer. For the central type of lung cancer in the early stage, there are direct signs to define the diagnosis. In the early stage, thin layer scanning with a layer thickness of 1.5–4 mm can be performed to observe the bronchial changes. MR imaging can demonstrate intraluminal nodules, luminal thickness and luminal stenosis of the bronchi from the transverse, coronal, and sagittal perspectives. MR imaging demonstrates favorably cancer in the lesions of obstructive pneumonia, and masses covered by the hilum. PET/CT scanning can be used for the screening of lung cancer metastasis and assessing the therapeutic efficacy after treatment. DSA is used for infusion chemotherapy of bronchial artery in the cases of primary lung cancer.
Bronchoscopy
Bronchoscopy is an important examination for the diagnosis of lung cancer. The pathological changes of the endothelium and the lumen of bronchi can be directly observed by using bronchoscopy. For the cases with caner or cancerous infiltration by bronchoscopy, sampling of the tissues under the guidance of bronchoscopy for biopsy can be performed. Otherwise, bronchial secretions can be suctioned under the guidance of bronchoscopy for cytological examinations to define the diagnosis and the histological classification.
Cytological Examinations
In most cases of primary lung cancer, the shed cancer cells can be found in the sputum, which can also facilitate to define its histological classification.
Exploratory Thoracotomy
After several examinations and short-term exploratory therapies, the qualitative diagnosis cannot be defined and the possibility of lung cancer cannot be excluded. Therefore, exploratory thoracotomy can be performed if the patient’s physical conditions permit.
Imaging Demonstrations
Chest X-ray
Early lesions are confined within the bronchi, causing valve ventilatory disorder and changes of obstructive emphysema. The manifestations include restrictive pulmonary gas increase and sparse lung markings. In the cases with certain degree of bronchostenosis due to unfavorable discharge of the secretions, obstructive pneumonia occurs, showing patchy blurry shadows. In the cases with complete blockage of the bronchi, obstructive atelectasis occurs, showing decreased pulmonary volume, increased density and migration of the mediastinum to the affected side. Obstructive pulmonary bronchiectasis has demonstrations of intrapulmonary cords liked shadows. Lung cancer in the middle and advanced stages are mainly manifested as hilar mass and atelectasis. The mass has a high density with clear boundary. However, the cancer cannot be observed due to its common immersion in the large flaky obstructive pneumonia lesion or large quantity pleural effusion. Atelectasis is commonly manifested as shrinkage of pulmonary segments or shrinkage of unilateral lung, with high density. The shadow of atelectasis widens at the hilum to show prominent mass. In the cases of central type lung cancer in the right upper lobe, a transverse S shape is at the hilum (commonly known as Pancoast cancer). Early diagnosis of central type lung cancer by plain chest X-ray only shows some indirect pulmonary manifestations caused by bronchial obstruction. And these indirect signs are not characteristically lung cancer. In the cases of local obstructive emphysema, these indirect signs can be caused by foreign substances in the bronchi or early inflammation. Obstructive pneumonia is difficult to be differentiated from common pneumonia. Obstructive atelectasis needs to be differentiated from many other conditions.
CT Scanning
(1) Pathological changes in the bronchial lumen including polypoid, nodular or flat papula masses. Benign tumor has smooth boundary and malignant tumor has unsmooth boundary, commonly with wider base and thickened lumen wall. Even the slight bronchial changes caused by the central type of lung cancer can be demonstrated by thin layer CT scanning, including slightly thickened bronchial wall, intraluminal small nodules and lumen stenosis or obstruction. In the middle and advanced stages, the direct signs of the central type lung cancer include thickened bronchial wall, irregular or unsmooth lining of the bronchial lumen. Bronchial obstruction is suddenly truncation or gradual thinning of the lumen to obstruction. (2) Hilar mass locates adjacent or around the bronchi, with smooth or arch shaped boundary. The indirect signs of the central type lung cancer in the middle and advanced stages include secondary changes to bronchial stenosis. Obstructive pneumonia is manifested as patchy blurry shadows or parenchymal changes of the pulmonary segments/lobes, and decreased lung volume.
MR Imaging
MR imaging demonstrates the tumor as long T1 and long T2 signals. In the cases of central type lung cancer with secondary obstructive atelectasis and obstructive pneumonia, enhanced T1 demonstrates the tumor in the lesion of pulmonary atelectasis and obstructive pneumonia. The signal of atelectasis is higher than that of the tumor.
PET/CT Scanning
PET/CT scanning can demonstrates increased and thick stained metabolites of metastatic lesions or residual lesions, which has a diagnostic sensitivity of above 90 %, and a reported specificity of 80–90 %. In addition, it can be applied for the clinical consideration of it hilar, mediastinal lymph node metastasis and extrathoracic distant metastasis, which is an important method to decide clinical stages before lung cancer therapy. But PET has false negative diagnosis in the diagnosis of lung cancer with decreased metabolites, especially the alveolar cell carcinoma. For the diagnosis of pneumonia and pulmonary tuberculosis, it also has false positive results. DSA can demonstrate the blood supply of the tumors.
Case Study
A male patient aged 41 years was confirmatively diagnosed as having AIDS by the CDC. He complained of repeated cough for more than 1 month and reported to have a history of unhealthy sexual behaviors. His CD4 T cell count was 65/ μl.
Fig. 17.115.
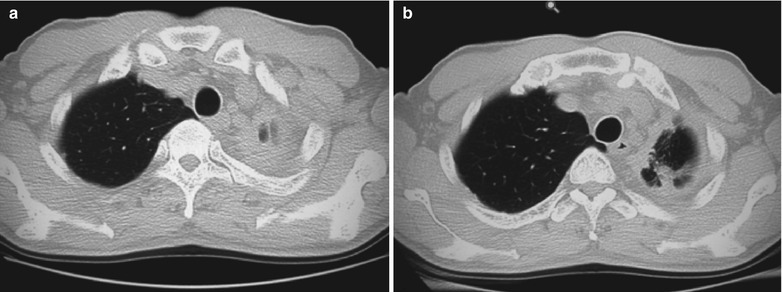
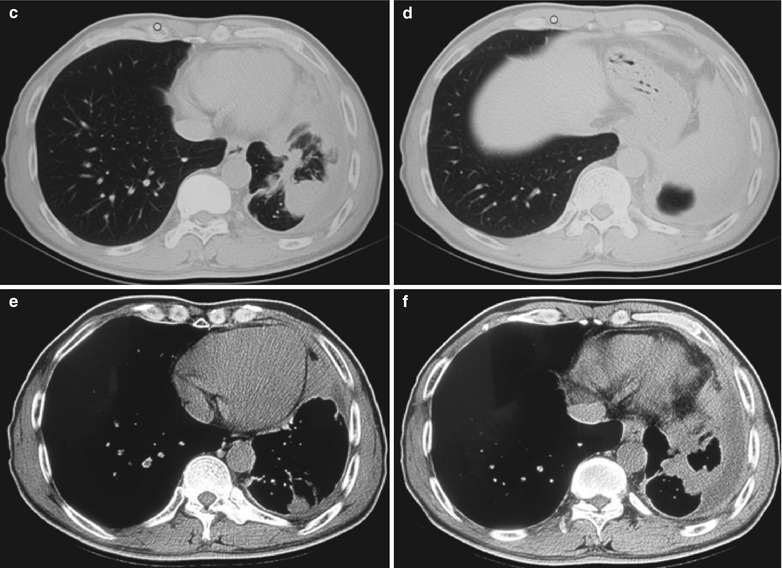
(a–f) HIV/AIDS related lung cancer. (a–f) CT scanning demonstrates diffuse soft tissue density shadows in left upper lung, round liked mass shadows in the middle lung field, thickened pleura in the lateral chest wall with adhesion, and strip liked liquid density shadows
Diagnostic Basis
The case history and related clinical symptoms.
By pathological examination, cancer cells can be found in the tissues.
The examination for the biomarkers of cancer can detect the serum cancer biomarkers such as cancer embryo antigen (CEA), squamous cell carcinoma (SCC) related antigen and cell keratin 19 fragment (CYFRA21-1) positive.
Chest X-ray demonstrations include bronchial obstruction by the cancer to cause obstructive pneumonia or atelectasis, hilar tumor, or widened mediastinal shadow. CT scanning demonstrations include mass shadows intruding into the bronchial lumen, irregular and thickened bronchial wall, and stenosis or obstruction of bronchial lumen. The cases of peripheral type lung cancer have manifestations of nodular or mass shadows around the lung fields, lobulation or incisura with fine and short spikes. The cases of diffuse bronchioloalveolar cell carcinoma have manifestations of infiltrative pathological changes, diffuse scattered nodules and small flake shadows, fusion of them into large flaky shadows, similar to those of pneumonia. CT scanning with high resolution can define early lung cancer. Enhanced scanning can define hilar and mediastinal lymph nodes metastasis. MR imaging is less favorable in demonstrating lesions in the lung parenchyma than CT scanning. Molecular imaging with fluorodeoxyglucose positron emission tomography computed tomography (18FDG-PET-CT) can demonstrate the increased and thick stained metabolites.
Differential Diagnosis
Tuberculosis
(1) Tuberculoma is difficult to be differentiated from the peripheral type of lung cancer. Tuberculoma is more common in young adults, with a long term course of illness. The lesions are commonly found in the apical posterior segment of the upper lobe or dorsal segment of the lower lobe. Chest X-ray demonstrates the lesions with uneven density and satellite lesions. (2) Miliary tuberculosis is difficult to be differentiated from diffuse bronchioloalveolar carcinoma. Miliary tuberculosis is more common in young adults, with obvious symptoms of systemic toxicity. Anti-tuberculosis drug therapy can relieve the symptoms, with gradually absorbed lesions. (3) Chest X-ray demonstrates hilar lymph node tuberculosis as mass shadows in the hilum of lung, which may be misdiagnosed as the central type lung cancer. Hilar lymph node tuberculosis is more common in teenagers, commonly with symptoms of tuberculosis infection but rarely hemoptysis. Lung cancer can be concurrent with pulmonary tuberculosis.
Pulmonary Inflammations
(1) Bronchial pneumonia; Obstructive pneumonia induced by early lung cancer can be misdiagnosed as bronchial pneumonia. Bronchial pneumonia has an acute onset with more obvious symptoms of infection. Chest X-ray demonstrates patchy or spots shadows, with blurry boundaries and uneven density. The lesions are not confined within one segment or one lobe. (2) Pulmonary abscesses; Central necrosis and liquefaction of the lung cancer results in cancerous cavities. By Chest X-ray, the central type lung cancer can be misdiagnosed as pulmonary abscesses. In the acute period, a pulmonary abscess has obvious symptoms of infection, with large quantity purulent sputum. Chest X-ray demonstrates thin cavity wall, smooth inner wall, liquid level and inflammatory changes in the surrounding lung tissues or pleura.
Other Pulmonary Neoplasms
Pulmonary benign tumors including hamartomas, fibroma and chondroma have slow growth. Chest X-ray demonstrates round liked mass shadow, with homogeneous density without lobation.
Extended Readings
- 1.World Health Organization (WHO)/Joint United Nations Programme on HIV/AIDS (uNAIDS). Report on the Global AIDS Epidemic, 2008[EB/OL]. (Accessed 15 Apr 2009). Available from URL: http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp. 2008.
- 2.Jones JL, Hanson DL, Dworkin MS, et al. Surveillance for AIDS-defining opportunistic illnesses, 1992–1997. MMWR CDC Surveill Summ. 1999;48:1–22. [PubMed] [Google Scholar]
- 3.Song WY, Li HJ. Imaging and pathology of HIV related cytomegalovirus pneumonia. Radiol Pract. 2010;25(1):44–6. [Google Scholar]
- 4.Lawrence J, Huang C, George P, et al. Roentgenographic patterns of Pneumocystis Carinii pneumonia in 104 patients with AIDS. Chest. 1987;91(4):323–7. doi: 10.1378/chest.91.3.323. [DOI] [PubMed] [Google Scholar]
- 5.Amorosa JK, Nahass RG, Nosher JL, et al. Radiology distinction of pyogenic pulmonary infection from Pneumocystis Carinii pneumonia in AIDS patients. Radiology. 1990;175(6):721–4. doi: 10.1148/radiology.175.3.2343120. [DOI] [PubMed] [Google Scholar]
- 6.Li H. Atlas of differential diagnosis in HIV/AIDS. Beijing: PMPH; 2009. [Google Scholar]
- 7.The National Institutes of Health (NIH) the Centers for Disease Control and Prevention (CDC), and the HIV Medicine Association of the Infectious Diseases Society of America (HIVMA/IDSA). Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents [EB/OL]. MMWR, 2009. (Accessed 15 Apr 2009). Available from URL: http://aidsinfo.nih.gov/contentfiles/Adult_OI.pdf.
- 8.Visnegarwala F, Graviss EA, Lacke CE, et al. Acute respiratory failure associated with cryptococcosis in patients with AIDS: analysis of predictive factors. Clin Infect Dis. 1998;27:1231–7. doi: 10.1086/514984. [DOI] [PubMed] [Google Scholar]
- 9.Meyohas MC, Roux P, Bollens D, et al. Pulmonary cryptococcosis: localized and disseminated infections in 27 patients with AIDS. Clin Infect Dis. 1995;21:628–33. doi: 10.1093/clinids/21.3.628. [DOI] [PubMed] [Google Scholar]
- 10.Capponi M, Sureau P. Penicillium de Rhizomys Sinensis. Bull Soc Pathol Exot. 1956;49(4):418–21. [PubMed] [Google Scholar]
- 11.Segretain G. Penicillium marneffei agent d’une mycose du system reticuloendothelial. Mycopathol Mycol Appl. 1959;11(4):327–53. doi: 10.1007/BF02089507. [DOI] [PubMed] [Google Scholar]
- 12.Disalvo AF, Fickling AM, Ajello L. Infection caused by Penicillium marneffei description of first natural infection in man. AM J Clin Pathol. 1973;60(2):259–63. doi: 10.1093/ajcp/60.2.259. [DOI] [PubMed] [Google Scholar]
- 13.Kudeken N, Kawakami K, Saito A. CD4+ T cell-mediated fatal hyperinflammatory reactions in mice infected with Penicillium marneffei. Clin Exp Immunol. 1997;107(3):468–73. doi: 10.1046/j.1365-2249.1997.00293.x. [DOI] [PubMed] [Google Scholar]
- 14.Deng Z, Ribas JL, Gibson DW, et al. Infections caused by Penicillium marneffei in China and Southeast Asia: review of eighteen published case and report of our mo re Chinese cases. Rev Infect Dis. 1998;10(3):640–52. doi: 10.1093/clinids/10.3.640. [DOI] [PubMed] [Google Scholar]
- 15.Wheat LJ, Connolly-Stringfield PA, Baker RL, et al. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine (Baltimore) 1990;69:361–74. doi: 10.1097/00005792-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Lacombe C, Lewin M, Monnier-cholley L, et al. Imaging of thoracic pathology in patients with AIDS. J Radiol. 2007;88:1145–54. doi: 10.1016/S0221-0363(07)89926-4. [DOI] [PubMed] [Google Scholar]
- 17.Guilherme FG, Alexandre SM, Cid SF, et al. Clinical and radiographic features of HIV-related pulmonary tuberculosis according to the level of immunosuppression. Rev Soc Bras Med Trop. 2007;40(6):622–6. doi: 10.1590/s0037-86822007000600004. [DOI] [PubMed] [Google Scholar]
- 18.Dolin PJ, Raviglione MC, Kochi A. Global tuberculosis incidence and mortality during 1990-2000. Bull World Health Organ. 1994;72(2):213–20. [PMC free article] [PubMed] [Google Scholar]
- 19.Havlir DV, Barnes PF. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1999;340:367–73. doi: 10.1056/NEJM199902043400507. [DOI] [PubMed] [Google Scholar]
- 20.Batungwanayo J, Taelman H, Dhote R, et al. Pulmonary tuberculosis in Kigali, Rwanda. Impact of human immunodeficiency virus infection on clinical and radiographic presentation. Am Rev Respir Dis. 1992;146:53–6. doi: 10.1164/ajrccm/146.1.53. [DOI] [PubMed] [Google Scholar]
- 21.Jones BE, Young SM, Antoniskis D, Davidson PT, Kramer F, Barnes PF. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148:1292–7. doi: 10.1164/ajrccm/148.5.1292. [DOI] [PubMed] [Google Scholar]
- 22.Awil PO, Bowlin SJ, Daniel TM. Radiology of pulmonary tuberculosis and human immunodeficiency virus infection Gulu, Uganda. Eur Respir J. 1997;10:615–8. [PubMed] [Google Scholar]
- 23.Hirschtick RE, Glassroth J, Jordan MC, et al. Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary Complications of HIV Infection Study Group. N Engl J Med. 1995;333:845–51. doi: 10.1056/NEJM199509283331305. [DOI] [PubMed] [Google Scholar]
- 24.Burack JH, Hahn JA, Saint-Maurice D, et al. Microbiology of community-acquired bacterial pneumonia in persons with and at risk for human immunodeficiency virus type 1 infection. Implications for rational empiric antibiotic therapy. Arch Intern Med. 1994;154:2589–96. doi: 10.1001/archinte.1994.00420220085010. [DOI] [PubMed] [Google Scholar]
- 25.Hondalus MK, Diamond MS, Rosenthal LA, et al. The intracellular bacterium Rhodococcus equi requires Mac21 to mammalian cells. Infect Immun. 1993;61:2919–29. doi: 10.1128/iai.61.7.2919-2929.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh RD, Schoch PE, Cunha BA. Rhodococcus. Infect Control Hosp Epidemiol. 1993;14(5):282–7. doi: 10.1086/646738. [DOI] [PubMed] [Google Scholar]
- 27.Takai S, Sasaki Y, Ikeda T, et al. Virulence of Rhodococcus equi isolates from patients with and without AIDS. J Clin Microbiol. 1994;32(2):457–60. doi: 10.1128/jcm.32.2.457-460.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takai S, Sekizaki T, Ozawa T. Association between large plasmid and 15 to 17 kilo dalton antigens in virulent Rhodococcus equi. Infect Immun. 1991;59:4056–60. doi: 10.1128/iai.59.11.4056-4060.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tkachuk Saad O, Prescott J. Rhodococcus equi plasmids:Isolation and partial characterization. J Clin Microbiol. 1991;29:2696–700. doi: 10.1128/jcm.29.12.2696-2700.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchiori E, Muller NL, de Mendonca RG, Capone D, Souza AS, Jr, Escuissato DL, Gasparetto EL, De Cerqueira EM. Rhodococcus equi pneumonia in AIDS: high-resolution CT findings in five patients. Br J Radiol. 2005;78:783–6. doi: 10.1259/bjr/84768811. [DOI] [PubMed] [Google Scholar]
- 31.Wallace MJ, Hannah J. Cytomegalovirus pneumonitis in patients with AIDS: findings in an autopsy series. Chest. 1987;92:198–203. doi: 10.1378/chest.92.2.198. [DOI] [PubMed] [Google Scholar]
- 32.Moskowitz L, Hensley GT, Chan JC, et al. Immediate causes of death in acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1985;109:735–8. [PubMed] [Google Scholar]
- 33.McKenzie R, Travis WD, Dolan SA, et al. The causes of death in patients with humanimmunodeficiency virus infection: aclinical and pathologic study with emphasis on the role of pμlmonary diseases. Medicine. 1991;70:326–43. doi: 10.1097/00005792-199109000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Geogeann MD, John V, Stuart M, et al. Cytomegalovirus pneumonitis: spectrum of parenchymal CT findings with pathologic correlation in 21 AIDS patients. Radiology. 1994;192:451–9. doi: 10.1148/radiology.192.2.8029414. [DOI] [PubMed] [Google Scholar]
- 35.Scott MA, Graham BS, Verral R, Dixon R, Schaffner W, Tham KT. Rhodococcus equi – an increasingly recognized opportunistic pathogen. Report of 12 cases and review of 65 cases in the literature. Am J Clin Pathol. 1995;103:649–55. doi: 10.1093/ajcp/103.5.649. [DOI] [PubMed] [Google Scholar]
- 36.Kabani M, Boisrame A, Beckerich JM. A highly representative two hybrid genomic library for the yeast Yarrowia lipolvtica. Gene. 2000;241(2):309–15. doi: 10.1016/S0378-1119(99)00476-X. [DOI] [PubMed] [Google Scholar]
- 37.Donisi A, Suardi MG, Caasari S, et al. Rhodococcus equi infection in HIV infected patients. AIDS. 1996;10(4):359–62. doi: 10.1097/00002030-199604000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Harvey RL, Sunstrum JC. Rhodococcus equi infection in patients with and without human immunodeficiency virus infection. Rev Infect Dis. 1991;13(1):139–45. doi: 10.1093/clinids/13.1.139. [DOI] [PubMed] [Google Scholar]
- 39.Wicky S, Cartei F, Mayor B, et al. Radiological findings in nine AIDS patients with Rhodococcus equi pneumonia. Eur Radiol. 1996;6:826–30. doi: 10.1007/BF00240680. [DOI] [PubMed] [Google Scholar]
- 40.Sellon DC, Besser TE, Vivrette SL, et al. Comparison of nucleic acid amplification, serology, and microbiologic culture for diagnosis of Rhodococcus equi pneumonia in foals. J Clin Microbiol. 2001;39:1289–93. doi: 10.1128/JCM.39.4.1289-1293.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanaly ST, Hines SA, Palmer GH. Cytokine modulation alters pulmonary clearance of Rhodococcus equi and development of granulomatous pneumonia. Infect Immun. 1995;63(8):3037–41. doi: 10.1128/iai.63.8.3037-3041.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller NL, Fraser RS, Lee KS, et al. Diseases of the lung: radiologic and pathologic correlations. Philadelphia: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 43.Reittner P, Ward S, Heyneman L, et al. Pneumonia: high-resolution CT findings in 114 patients. Eur Radiol. 2003;13:515–21. doi: 10.1007/s00330-002-1490-3. [DOI] [PubMed] [Google Scholar]
- 44.Amorosa JK, Nahass RG, Nosher JL, Gocke DJ. Radiologic distinction of pyogenic pulmonary infection from Pneumocystis carinii pneumonia in AIDS patients. Radiology. 1990;175:721–4. doi: 10.1148/radiology.175.3.2343120. [DOI] [PubMed] [Google Scholar]
- 45.Gruden JF, Huang L, Turner J, et al. High-resolution CT in the evaluation of clinically suspected Pneumocystis carinii pneumonia in AIDS patients with normal, equivocal, or nonspecific radiographic findings. Am J Roentgenol. 1997;169:967–75. doi: 10.2214/ajr.169.4.9308446. [DOI] [PubMed] [Google Scholar]
- 46.Kuhlman JE, Kavuru M, Fishman EK, Siegelman SS. Pneumocystis carinii pneumonia: spectrum of parenchymal CT findings. Radiology. 1990;175:711–4. doi: 10.1148/radiology.175.3.2343118. [DOI] [PubMed] [Google Scholar]
- 47.Sandhu JS, Goodman PC. Pulmonia in patient with AIDS. Radiology. 1989;173:33–5. doi: 10.1148/radiology.173.1.2789413. [DOI] [PubMed] [Google Scholar]
- 48.Schneider MM, Borleffs JC, Stolk RP, Jaspers CA, Hoepelman AI. Discontinuation of prophylaxis for pneumo-cystis carinii pneumonia in HIV-infected patients treated with highly active antiretroviral therapy. Lancet. 1999;353:201–3. doi: 10.1016/S0140-6736(98)07204-3. [DOI] [PubMed] [Google Scholar]
- 49.White CS, Haramati LB, Elder KH, Karp J, Belani CP. Carcinoma of the lung in HIV-positive patients: findings on chest radiographs and CT scans. Am J Roentgenol. 1995;164:593–7. doi: 10.2214/ajr.164.3.7863877. [DOI] [PubMed] [Google Scholar]
- 50.White DA. Pulmonary complications of HIV-associated malignancies. Clin Chest Med. 1996;17:755–61. doi: 10.1016/S0272-5231(05)70344-0. [DOI] [PubMed] [Google Scholar]
- 51.Frame PT. Pneumocystis carinii infection and AIDS. In: Crowe S, Hoy J, Mills J, editors. Management of the HIV infected patient. London: Cambridge University Press; 1996. pp. 298–308. [Google Scholar]
- 52.Naimey GL, Wuerleer RB. Comparison of histologic stains in the diagnosis of Pnemocystis carinii. Acta Cytol. 1995;39(6):1124–9. [PubMed] [Google Scholar]
- 53.Barnes PF, Bloch AB, Davidson PT, Snider DE., Jr Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991;324:1644–50. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- 54.Di Perri G, Cazzadori A, Vento S, et al. Comparative histopathological study of pulmonary tuberculosis in human immunodeficiency virus-infected and non-infected patients. Tuber Lung Dis. 1996;77:244–9. doi: 10.1016/S0962-8479(96)90008-8. [DOI] [PubMed] [Google Scholar]
- 55.Fishman JE, Saraf-Lavi E, Narita M, Hollender ES, Ramsinghani R, Ashkin D. Pulmonary tuberculosis in AIDS patients: transient chest radiographic worsening after initiation of antiretroviral therapy. Am J Roentgenol. 2000;174:43–9. doi: 10.2214/ajr.174.1.1740043. [DOI] [PubMed] [Google Scholar]
- 56.Greenberg SD, Frager D, Suster B, Walker S, Stavropoulos C, Rothpearl A. Active pulmonary tuberculosis in patients with AIDS: spectrum of radiographic findings (including a normal appearance) Radiology. 1994;193:115–9. doi: 10.1148/radiology.193.1.7916467. [DOI] [PubMed] [Google Scholar]
- 57.Haramati LB, Jenny-Avital ER, Alterman DD. Effect of HIV status on chest radiographic and CT findings in patients with tuberculosis. Clin Radiol. 1997;52:31–5. doi: 10.1016/S0009-9260(97)80302-9. [DOI] [PubMed] [Google Scholar]
- 58.Hocqueloux L, Lesprit P, Herrmann JL, de La BA, Zagdanski AM, Decazes JM, Modaj J. Pulmonary Mycobacterium avium complex disease without dissemination in HIV-infected patients. Chest. 1998;113:542–8. doi: 10.1378/chest.113.2.542. [DOI] [PubMed] [Google Scholar]
- 59.Horsburgh CR., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:1332–8. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 60.Kalayjian RC, Toossi Z, Tomashefski JF, Carey JT, Ross JA, Tomford JW, Blinkhorn RJ., Jr Pulmonary disease due to infection by Mycobacterium avium complex in patients with AIDS. Clin Infect Dis. 1995;20:1186–94. doi: 10.1093/clinids/20.5.1186. [DOI] [PubMed] [Google Scholar]
- 61.Keiper MD, Beumont M, Elshami A, Langlotz CP, Miller WT., Jr CD4 T lymphocyte count and the radiographic presentation of pulmonary tuberculosis. A study of the relationship between these factors in patients with human immunodeficiency virus infection. Chest. 1995;107:74–80. doi: 10.1378/chest.107.1.74. [DOI] [PubMed] [Google Scholar]
- 62.Sekowitz KA, Raffall J, Riley L, et al. Tuberculosis in the AIDS era. Clin Microbiol Rev. 1995;8(2):180–99. doi: 10.1128/cmr.8.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolf DA, Wu CD, Medeiros LJ. Mycobacterial pseudotumors of lymph node. Arch Pathol Lab Med. 1995;119(6):811–4. [PubMed] [Google Scholar]
- 64.Chen KTK. Mycobacterial spindle cell pseudotumor of lymph node. Am J Surg Pathol. 1992;16(3):276–81. doi: 10.1097/00000478-199203000-00008. [DOI] [PubMed] [Google Scholar]
- 65.Hoy J. Managenent of the HIV-infected patient. London: Cambridge University Press; 1996. pp. 285–97. [Google Scholar]
- 66.Sepkowitz KA, Raffall J, Rily L, et al. Tuberculosis in the AIDS era. Clin Microbiol Rev. 1995;8(2):180–99. doi: 10.1128/cmr.8.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uberti-Foppa C, Lillo F, Terreni MR, et al. Cytomegalovirus pneumonia in AIDS patients. Chest. 1998;113(4):919–23. doi: 10.1378/chest.113.4.919. [DOI] [PubMed] [Google Scholar]
- 68.Kida M, Min KW. Atypical cytomegalic cells are diagnostic for cytomegaloviral infection in AIDS. Am J Clin Pathol. 1993;100(3):346–7. [Google Scholar]
- 69.Waxman AB, Goldie SJ, Brett-Suith H, Matthay RA. Cytomegalovirus as a primary pulmonary pathogen in AIDS. Chest. 1987;111:128–34. doi: 10.1378/chest.111.1.128. [DOI] [PubMed] [Google Scholar]
- 70.Chuck SL, Sande MA. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N Engl J Med. 1989;321(12):794–9. doi: 10.1056/NEJM198909213211205. [DOI] [PubMed] [Google Scholar]
- 71.Conces DJ., Jr Endemic fungal pneumonia in immunocompromised patients. J Thorac Imaging. 1999;14:1–8. doi: 10.1097/00005382-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 72.Conces DJ, Jr, Stockberger SM, Tarver RD, Wheat LJ. Disseminated histoplasmosis in ADIS: findings on chest radiographs. Am J Roentgenol. 1993;160:15–9. doi: 10.2214/ajr.160.1.8416614. [DOI] [PubMed] [Google Scholar]
- 73.Connolly JE, Jr, McAdams HP, Erasmus JJ, Rosado-de-Christenson ML. Opportunistic fungal pneumonia. J Thorac Imaging. 1999;14:51–62. doi: 10.1097/00005382-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 74.Denning DW, Follansbess SE, Scolaro M, Norris S, Edelstein H, Stevens DA. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:654–62. doi: 10.1056/NEJM199103073241003. [DOI] [PubMed] [Google Scholar]
- 75.Fish DG, Ampel NM, Galgiani JN, et al. Coccidioidomycosis during human immunodeficiency virus infection. A review of 77 patients. Medicine. 1990;69:384–91. doi: 10.1097/00005792-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 76.Miller WT, Jr, Edelman JM, Miller WT. Cryptococcal pulmonary infection in patients with AIDS: radiographic appearance. Radiology. 1990;175:725–8. doi: 10.1148/radiology.175.3.2343121. [DOI] [PubMed] [Google Scholar]
- 77.Miller WT, Jr, Sais GJ, Frank I, Gefter WB, Aronchick JM, Miller WT. Pulmonary aspergillosis in patients with AIDS. Clinical and radiographic correlations. Chest. 1994;105:37–44. doi: 10.1378/chest.105.1.37. [DOI] [PubMed] [Google Scholar]
- 78.Driver JA, Saunders CA, Heinze-Lacy B, et al. Cryptococcal pneumonia in AIDS: is cryptococcal meningitis preceded by clinically recognizable pneumonia. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9(2):168–71. [PubMed] [Google Scholar]
- 79.Chechani V, Kamholz SL. Pulmonary manifestations of disseminated cryptococcosis in patients with AIDS. Chest. 1990;98(5):1060–6. doi: 10.1378/chest.98.5.1060. [DOI] [PubMed] [Google Scholar]
- 80.Bani-Sadr F, Hamidou M, Raffi F, Chamoux C, Caillon J, Freland C. Clinical and bacteriological aspects of nocardiasis. Presse Med. 1995;24:1062–6. [PubMed] [Google Scholar]
- 81.Furman AC, Jacobs J, Sepkowitz KA. Lung abscess in patients with AIDS. Clin Infect Dis. 1996;22:81–5. doi: 10.1093/clinids/22.1.81. [DOI] [PubMed] [Google Scholar]
- 82.Race EM, Adelson-Mitty J, Kitty J, Kriegel GR, Barlam TF, Reimann KA, Letvin NL, Japour AJ. Focal mycobacterial lym-phadenitis following initiation of protease-inhibitor therapy in patients with advanced HIV-1 disease. Lancet. 1998;351:251–5. doi: 10.1016/S0140-6736(97)04352-3. [DOI] [PubMed] [Google Scholar]
- 83.Shapiro JM, Romney BM, Weiden MD, White CS, O’Toole KM. Rhodococcus equi endobronchial mass with lung abscess in a patient with AIDS. Thorax. 1992;47:62–3. doi: 10.1136/thx.47.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bazot M, Cadranel J, Benayoun S, Tassart M, Bigot JM, Carette MF. Primary pulmonary AIDS-related lymphoma: radiographic and CT findings. Chest. 1999;116:1282–6. doi: 10.1378/chest.116.5.1282. [DOI] [PubMed] [Google Scholar]
- 85.Broder S, Karp JE. The expanding challenge of HIV-associated malignancies. CA Cancer J Clin. 1992;42:69–73. doi: 10.3322/canjclin.42.2.69. [DOI] [PubMed] [Google Scholar]
- 86.Eisner MD, Kaplan LD, Herndier B, Stulbarg MS. The pulmonary manifestations of AIDS-related non-Hodgkin’s lymphoma. Chest. 1996;110:729–36. doi: 10.1378/chest.110.3.729. [DOI] [PubMed] [Google Scholar]
- 87.Sider L, Weiss AJ, Smith MD, VonRoenn JH, Glassroth J. Varied appearance of AIDS-related lymphoma in the chest. Radiology. 1989;171:629–32. doi: 10.1148/radiology.171.3.2717733. [DOI] [PubMed] [Google Scholar]
- 88.Mead JH, Mason TE. Lymphoma versus AIDS. Am J Clin Pathol. 1983;80(4):546–7. doi: 10.1093/ajcp/80.4.546a. [DOI] [PubMed] [Google Scholar]
- 89.Haramati LB, Wong J. Intrathoracic Kaposi’s sarcoma in women with AIDS. Chest. 2000;117:410–4. doi: 10.1378/chest.117.2.410. [DOI] [PubMed] [Google Scholar]
- 90.Khalil AM, Carette MF, Cadranel JL, Mayaud CM, Bigot JM. Intrathoracic Kaposi’s sarcoma. CT findings. Chest. 1995;108:1622–6. doi: 10.1378/chest.108.6.1622. [DOI] [PubMed] [Google Scholar]
- 91.Cathomas G, Stalder A, McGandy CE, et al. Distribution of human herpesvirus 8 DNA in tumorous and nontumorous tissue of patients with acquired immunodeficiency syndrome with and without Kaposi’s sarcoma. Mod Pathol. 1998;11(5):415–20. [PubMed] [Google Scholar]
- 92.Carbone A, Mason TE. Kaposi’s sarcoma in lymphnodes concurrent with Hodgkin’s disease. Am J Clin Pathol. 1983;80(2):228–30. doi: 10.1093/ajcp/80.2.228. [DOI] [PubMed] [Google Scholar]
- 93.Safai B, Johnson KG, Myskowski PT, et al. The natural history of Kaposi’s sarcoma in the acquired immunodeficiency syndrome. Ann Intern Med. 1985;103(5):744–50. doi: 10.7326/0003-4819-103-5-744. [DOI] [PubMed] [Google Scholar]
- 94.Rabaud C, May T, Lucet JC, Leport C, Ambroies-Thomas P, Canton P. Pulmonary toxoplasmosis in patients in-fected with human immunodeficiency virus: a French National Survey. Clin Infect Dis. 1996;23:1249–54. doi: 10.1093/clinids/23.6.1249. [DOI] [PubMed] [Google Scholar]
- 95.Li HJ, Gao YQ, Cheng JL, et al. Diagnostic imaging, preautopsy imaging and autopsy findings of 8 AIDS cases. Chin Med J (Engl) 2009;122(18):2142–8. [PubMed] [Google Scholar]
- 96.Gao JB, Zhang YG, Li HJ, et al. Analysis on the imaging features of AIDS with pulmonary fungal infection. Chin Med J (Engl) 2010;123(24):3583–6. [PubMed] [Google Scholar]
- 97.Li ZC, Li HJ, Dai LL, et al. Liver injury in HIV-1-infected patients receiving non-nucleosides reverse transcriptase inhibitors-based antiretroviral therapy. Chin Med J (Engl) 2010;123(24):3587–90. [PubMed] [Google Scholar]
- 98.Li HJ. MRI demonstrations of AIDS complicated by Toxoplasma gondii infection in cervical spinal cord: with 3 cases reports. Chin Med J (Engl) 2010;123(24):3587–90. [Google Scholar]
- 99.Li HJ. Diagnostic imaging in AIDS in China: current status and clinical application. Chin Med J. 2011;124(7):963–4. [PubMed] [Google Scholar]
- 100.Li HJ, Cheng JL. Imaging and pathological findings of AIDS complicated by pulmonary rhodococcus equi infection. Chin Med J. 2011;124(7):968–72. [PubMed] [Google Scholar]
- 101.Wang L, Shi DP, Han X, Zhao QX, Yan B, Li HJ. Magnetic resonance spectroscopy in the diagnosis of cognitive impairment in AIDS patients. Chin Med J. 2011;124(9):1342–5. [PubMed] [Google Scholar]
- 102.Li H. CT image demonstrations of HIV-seropositive tuberculosis and their relationship with CD4+ T-lymphocyte count. Chin Med J (Engl) 2010;124(4):124–6. [PubMed] [Google Scholar]
- 103.Jones HA, Clark RJ, Schofield JB, et al. Positron emission tomography of 18FDG uptake in localized pulmonary inflammation. Acta Radiol Suppl. 1991;376:148. [PubMed] [Google Scholar]
- 104.Capponi MP, Segretain G, Sureau G. Penicilliosisde Rhizomys sinensis. Bull Soc Pathol Exot Filiales. 1956;49:418–21. [PubMed] [Google Scholar]
- 105.Zhang Y, Wang M, Li H, et al. Accumulation of nuclear mitochondrial DNA in the frontal cortex cells of patients with HIV-associated neurocognitive disorders. Brain Res. 2012;1458:1–11. doi: 10.1016/j.brainres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 106.Li H, Meng Z, Huang K, et al. Imaging findings of AIDS complicated with pulmonary rhodococcus equi infection and correlated with pathology. Radiol Pract. 2009;24(9):943–7. [Google Scholar]
- 107.Ludlam GB, Beattie CP. Pulmonary toxoplasmosis? Lancet. 1963;2(7318):1136–8. doi: 10.1016/S0140-6736(63)90789-X. [DOI] [PubMed] [Google Scholar]
- 108.Chen S, Ma S. Pathogenesis and prevention of rhodococcus equi infection. J Northwest Minorities Univ (Nat Sci) 2001;22(41):44–8. [Google Scholar]
- 109.Catterall JR, Hofflin JM, Remington JS. Pulmonary toxoplasmosis. Am Rev Respir Dis. 1986;133(4):704–5. doi: 10.1164/arrd.1986.133.4.704. [DOI] [PubMed] [Google Scholar]


