Abstract
Context
Atypical femur fractures (AFFs) are serious adverse events associated with bisphosphonates and often show poor healing.
Evidence acquisition
We performed a systematic review to evaluate effects of teriparatide, raloxifene, and denosumab on healing and occurrence of AFF.
Evidence synthesis
We retrieved 910 references and reviewed 67 papers, including 31 case reports, 9 retrospective and 3 prospective studies on teriparatide. There were no RCTs. We pooled data on fracture union (n = 98 AFFs on teriparatide) and found that radiological healing occurred within 6 months of teriparatide in 13 of 30 (43%) conservatively managed incomplete AFFs, 9 of 10 (90%) incomplete AFFs with surgical intervention, and 44 of 58 (75%) complete AFFs. In 9 of 30 (30%) nonoperated incomplete AFFs, no union was achieved after 12 months and 4 (13%) fractures became complete on teriparatide. Eight patients had new AFFs during or after teriparatide. AFF on denosumab was reported in 22 patients, including 11 patients treated for bone metastases and 8 without bisphosphonate exposure. Denosumab after AFF was associated with recurrent incomplete AFFs in 1 patient and 2 patients of contralateral complete AFF. Eight patients had used raloxifene before AFF occurred, including 1 bisphosphonate-naïve patient.
Conclusions
There is no evidence-based indication in patients with AFF for teriparatide apart from reducing the risk of typical fragility fractures, although observational data suggest that teriparatide might result in faster healing of surgically treated AFFs. Awaiting further evidence, we formulate recommendations for treatment after an AFF based on expert opinion.
Keywords: osteoporosis, antiresorptives, anabolics
Antiresorptive drugs such as bisphosphonates are widely used for the treatment of osteoporosis. Although effective for prevention of osteoporotic fractures, use of bisphosphonates is associated with rare but serious adverse events such as osteonecrosis of the jaw and atypical femur fractures (AFFs). An AFF is a spontaneous or low-trauma, subtrochanteric or femur shaft fracture often complicated by delayed or nonunion (26%–39%) and bilateral occurrence (2, 3).
The age-adjusted incidence rate of AFF has been estimated to be 1.8 per 100 0000 person-years in patients on bisphosphonate use under 2 years, increasing to 113 per 100 000 person-years with more than 8 years’ duration (4). It is thought that decreased bone resorption in bisphosphonate users results in suppressed bone turnover with accumulation of microcracks and homogeneously mineralized bone, making the bone more brittle and allowing the development of a spontaneous femur fracture. However, it is uncertain if bisphosphonates are causally related to AFF, and, incidentally, AFFs do occur in bisphosphonate-naïve individuals (5). Usually, bisphosphonates are discontinued after AFF is diagnosed. It has been shown that the risk of AFF decreases 70% per year from the last use of antiresorptive drugs (6), although it is not certain that this risk reduction is also seen in patients who have already sustained an AFF.
It is unclear if alternative osteoporosis drugs, particularly anabolic drugs, can promote AFF healing. Moreover, there is no guideline on how patients should be treated after an AFF where the risk of causing new atypical fractures should be weighed against the risk of fragility fractures when not treating osteoporosis. It has been proposed that teriparatide, an analog of parathyroid hormone, is a safe option for treatment of osteoporosis in patients with AFF, especially since it may also have a beneficial effect on the healing of AFF itself (7). Teriparatide is the only anabolic osteoporosis drug that is currently globally available. It directly stimulates osteoblasts that might enable the formation of new, heterogeneously mineralized bone at the fracture site of AFF. Besides teriparatide, antiresorptive drugs, other than bisphosphonates, such as raloxifene and denosumab may be considered for osteoporosis treatment in patients with AFF. Denosumab is a human monoclonal antibody to RANKL and a potent inhibitor of bone resorption. Although AFFs have been reported in patients exposed to denosumab in case reports, it has not been clearly established in epidemiological studies how often denosumab, with or without preceding bisphosphonate use, is associated with AFF. The radiological healing or deterioration of AFF while on denosumab treatment is also not known. Raloxifene is a selective estrogen receptor modulator (SERM) that acts as an estrogen agonist in bone, with an antiresorptive effect that is milder than that of bisphosphonates and denosumab. The relationship between raloxifene and the occurrence of AFF has not been investigated. To our knowledge, this is the first review that explored denosumab and raloxifene in addition to teriparatide for medical management of osteoporosis in patients with AFF. Further, we investigated whether AFF occurs as an adverse event in clinical trials with 2 novel drugs for osteoporosis, romosozumab and abaloparatide. Romosozumab, an antibody to sclerostin with both anabolic and antiresorptive effects, was recently approved in Europe, Japan, and the United States for the treatment of (severe) osteoporosis. Abaloparatide is a synthetic analog of parathyroid hormone-related protein. Strontium ranelate was not included in this review since this drug is no longer available in most countries.
We performed a systematic literature review to assess both the occurrence and the radiological healing of AFFs in patients who had used or were using teriparatide, denosumab, or raloxifene. We formulate recommendations for healing of the AFF itself and for osteoporosis management in patients who have sustained an AFF and are at high risk of fragility fractures.
Methods
We performed a search using key words related to AFFs and teriparatide, denosumab, and/or raloxifene in Embase, Medline Epub (Ovid), Web of Science and Cochrane Central on May 28, 2018. We separately searched for AFF as an adverse event in clinical trials with romosozumab or abaloparatide. Reviews and articles written in a language other than English were excluded. Conference abstracts and original research articles were included. Articles were reviewed when AFF was diagnosed during or after the use of teriparatide, denosumab, and raloxifene or when the radiological healing of AFF in a specified amount of time was reported using these drugs.
A complete AFF was defined as a noncomminuted subtrochanteric or femur shaft fracture with a predominantly transverse fracture line that may become oblique as it progresses medially, after no or minimal trauma. An incomplete form of AFF was defined as a localized endosteal or periosteal thickening of the lateral cortex of the subtrochanteric femur with or without the presence of a lucent line. When the authors did not describe whether a fracture line was visible, we assessed medical imaging in the article to review the presence of a fracture line.
We extracted data on sex, median age, ethnicity, use of bisphosphonates, surgical interventions, and clinical or functional outcome after the AFF as far as this information was available.
We assessed the occurrence of newly diagnosed AFF during or after the use of teriparatide, denosumab, or raloxifene. Newly diagnosed AFF could either be the first clinical presentation of AFF, a second AFF of the contralateral femur, or recurrent AFF at the ipsilateral femur.
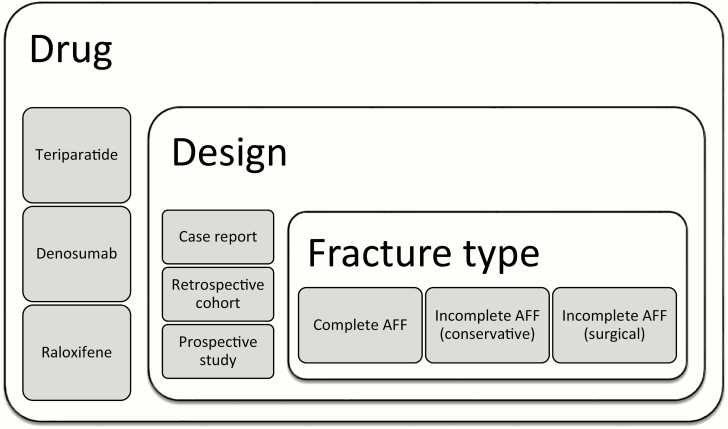
For the assessment of radiological healing, the results were categorized for each type of drug according to study design (case report, retrospective cohort, and prospective studies) and fracture type (complete AFF, incomplete AFF with or without surgical treatment) (Fig. 1).
Figure 1.
The results for each type of drug were categorized according to study design and fracture type. Abbreviation: atypical femur fracture.
We assessed the total number of AFFs described in the literature with complete radiological healing at 6 months and 12 months after medical management. The number of conservatively treated incomplete AFFs that developed a lucent line or progressed to complete AFF was also noted. We pooled these data on healing from all article types to provide better insight into the effectiveness of the drugs for the healing of AFF.
Radiological healing in complete AFFs and surgically treated incomplete AFFs was defined as adequate callus bridging. Radiological healing of an incomplete AFF on conservative management was defined by disappearance of a visible fracture line. Radiological healing of incomplete AFFs without a lucent line included flattening of cortical thickening, disappearance of bone marrow edema on magnetic resonance imaging (MRI) scan, or fading of hotspots on bone scintigraphy. Incomplete AFFs with localized cortical thickening only, without abnormalities on MRI scan or bone scintigraphy, were excluded from assessment of radiological healing because focal cortical thickening can remain unchanged for more than 5 years after diagnosis of incomplete AFF (8). We give our recommendations for teriparatide, denosumab, and raloxifene in the medical treatment of patients with AFF. In order to address the decision-making in individual cases, we have formulated treatment advice for patients with a new diagnosis of AFF and patients with AFF who have completed a 2-year course of teriparatide. These considerations are based on the findings in this review and our expert opinion.
Results: Systematic Review
Our search retrieved 910 references. We selected 2 conference abstracts and 130 articles after screening of title and abstract. We replaced one conference abstract with the article that was published shortly after our search date (9, 10). After full-text reading, 67 articles were included for this review. Sections on teriparatide, denosumab, and raloxifene have overlapping references because some case descriptions report on a combination of these treatments in patients with AFF.
Teriparatide
We found 31 case reports, 9 retrospective cohort studies, and 3 prospective studies that have reported the effect of teriparatide on the radiological healing of AFF or occurrence of AFF. There were no published randomized controlled trials (RCTs). Detailed study descriptions of case reports, retrospective cohorts, and prospective studies on teriparatide use in patients with AFF can be found in Supplement 1(1). The demographic characteristics of the patients with AFF on teriparatide in case reports are stated in Table 1. Clinical variables and main findings from retrospective cohorts and prospective studies are summarized in Table 2 and Table 3, respectively. The pooled data on radiological healing of AFF with teriparatide treatment are shown in Table 4.
Table 1.
Demographic Characteristics of Case Reports on Teriparatide Use in AFF Patients
| Reference | n = Patientsa | n = Incomplete AFFb | Fracture Line Visible | n = Complete AFFb | Sex | Mean Age (years) | Backgroundc | Antiresorptivesd | Condition | Mean Duration Treatment in Years (range)e |
|---|---|---|---|---|---|---|---|---|---|---|
| Al Azzani, 2015 | 1 | 1 | yes | 0 | M | 54 | Caucasian | alendronate (5), ibandronate (5) | cystic fibrosis | 10 |
| Carvalho, 2011 | 1 | 0 | - | 1 | F | 77 | Caucasian | alendronate | postmenopausal osteoporosis | 4 |
| Cerveró, 2015 | 1 | 1 | yes | 1 | F | 71 | (Spain) | alendronate | postmenopausal osteoporosis | 5 |
| Chiu, 2013 | 1 | 2 | yes | 0 | F | 63 | (Taiwan) | alendronate | postmenopausal osteoporosis | 7 |
| Chew, 2013 | 1 | 1 | yes | 0 | F | 70 | (Malaysia) | alendronate | back pain | 6 |
| Etxebarria-Foronda, 2015 | 1 | 0 | - | 1 | M | 21 | (Spain) | pamidronate iv (3), alendronate (5) | osteogenesis imperfecta | 8 |
| Fukuda, 2014 | 1 | 1 | yes | 1 | F | 74 | (Japan) | alendronate | postmenopausal osteoporosis | 6 |
| Giannotti, 2013 | 1 | 1 | no | 1 | F | 65 | Caucasian | “bisphosphonates” | NS | 6 |
| Gomberg, 2011 | 1 | 2 | no (2) | 0 | F | 63 | Caucasian | alendronate | glucocorticoid-induced osteoporosis | 13 |
| Holm, 2014f | 1 | 0 | - | 1 | NS | NS | (Norway) | “bisphosphonates” | osteogenesis imperfecta | 9 |
| Huang, 2012 | 1 | 1 | yes | 0 | F | 63 | Asian | alendronate | vertebral fractures | 3 |
| Iwata, 2014 | 1 | 0 | - | 2 | F | 56 | Asian | incadronic iv 10 mg two-weekly (3), pamidronate iv 90 mg monthly (1), zoledronate iv 4 mg monthly (5) | metastatic bone disease | 9 |
| Jain, 2011 | 1 | 1 | NS | 1 | F | 75 | (India) | alendronate | osteopenia | 6 |
| Kaur, 2016 | 1 | 1 | no | 0 | F | 70 | Guyanese | alendronate | postmenopausal osteoporosis | 10 |
| Lampropoulou-Adamidou, 2013 | 1 | 0 | - | 1 | F | 84 | Caucasian | alendronate (12), ibandronate (1) | postmenopausal osteoporosis | 13 |
| Mastaglia, 2016 | 1 | 1 | no | 1 | F | 57 | Caucasian | alendronate | osteopenia | 7 |
| Nguyen, 2017 | 1 | 0 | - | 1 | F | 65 | (Australia) | alendronate | postmenopausal osteoporosis | 11 |
| Ramchand, 2016 | 1 | 2 | no (2) | 0 | F | 82 | (Australia) | alendronate (6), risedronate (1) | rib fracture osteoporosis | 7 |
| Reddy, 2012 | 1 | 0 | - | 1 | M | 70 | Asian | zoledronate iv 4 mg monthly | androgen deprivation therapy | 2 |
| Righetti, 2018 | 1 | 2 | yes (1) | 0 | F | 67 | Armenian | alendronate | hypophosphatasia | 10 |
| Román, 2015 | 1 | 0 | - | 2 | M | 72 | (Spain) | alendronate | glucocorticoid-induced osteoporosis | 11 |
| Schilcher, 2015 | 1 | 0 | - | 1 | F | 84 | (Sweden) | “bisphosphonates” | rheumatoid arthritis/Wegener granulomatosis | 16 |
| Selga, 2016 | 1 | 0 | - | 2 | F | 62 | (Spain) | alendronate (10), risedronate (2), ibandronate (3), denosumab (2) | osteoporosis | 17 |
| Spyridonidis, 2014 | 1 | 1 | yes | 1 | F | 78 | (Greece) | alendronate | osteoporosis | 8 |
| Stathopoulos, 2011 | 1 | 0 | - | 1 | F | 76 | Caucasian | zoledronate iv 4 mg yearly | osteoporosis | 6 |
| Tan, 2017 | 1 | 1 | yes | 0 | M | 63 | (Singapore) | alendronate (7), etidronate (2) | osteogenesis imperfecta | 9 |
| Tarazona-Santabalbina, 2013 | 1 | 1 | yes | 1 | F | 73 | (Spain) | alendronate | osteoporosis | 13 |
| Tsuchie, 2015 | 2 | 3 | yes (3) | 0 | F | 78 | (Japan) | alendronate | osteoporosis | 5 (4–6) |
| Uppin, 2016 | 1 | 0 | - | 2 | F | 56 | (India) | alendronate | rheumatoid arthritis | 4 |
| Vaishya, 2013 | 1 | 2 | yes (2) | 0 | F | 63 | (India) | alendronate | osteoporosis | 3 |
| Visekruna, 2008 | 2 | 0 | - | 3 | F | 69 | Caucasian | alendronate, raloxifene | steroid-dependent rheumatoid arthritis | 13 (10–16) |
Abbreviations: AFF, atypical femur fracture; F, female; M, male; iv, intravenous; NS, not stated
aFrom case series, only patients in whom the effect of teriparatide could be assessed on healing or occurrence of AFFs were included in this table.
bThe number of AFFs included (contralateral) AFFs that had already healed by the time teriparatide was started. This means that the total number of AFFs in this table is higher than the total number of AFFs that were treated with teriparatide.
cThe country of the affiliation is given when ethnicity of the AFF case was not specified in the article.
dThe types of bisphosphonates prior to the occurrence of the first AFF. When a patient had used several antiresorptive drugs, the total number of years the patient had used this specific drug is indicated in parentheses. In some cases, type of bisphosphonates was unknown (“bisphosphonates”). For intravenous bisphosphonates, the dosage and treatment interval are given in the table. Alendronate dosages included 70 mg weekly or 10 mg daily. Etidronate was given 400 mg every 2 weeks, ibandronate 150 mg monthly, risedronate 35 mg weekly, and raloxifene 60 mg daily.
eThe total duration of antiresorptive drugs use prior to the first diagnosis of AFF is given in years, not including drug holidays.
fNo access to full-text article.
Table 2.
Summary of Retrospective Cohorts of AFF Patients and Use of Teriparatide
| Reference | Total Cohort, n | Patients on TPT, n | Controls Without TPT, n | Fracture Type of TPT Usersa | Female, n (%) | Mean Age (years) | Country | AR Use | Mean Duration AR, (years; range) | Main Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Cheung, 2013 | 22 | 22 | 0 | Incomplete: Surgical = 3 AFFs Conservative = 19 AFFs | 22 (100%) | 66 | Canada | Yes (100%) | 12 (3.4–28.7) | Of 19 incomplete AFFs without surgery, 2 healed, 5 were healing, 12 were stable after 2 years of TPT, but 4 patients developed new incomplete AFFs |
| Lee, 2013 | 51 | 19 | 32 | Incomplete: Surgical = 12 AFFs Conservative = 7 AFFs | 50 (98%) | 70.4 | South Korea | Yes (77%) | 4.5 | 7 patients on TPT and 19 patients without TPT required surgery; use of teriparatide did not significantly reduce the need for surgery (P = 0.210) |
| Lee, 2017 | 44 | 14 | 30 | Complete: n = 14 AFFs | 44 (100%) | 70.1 | South Korea | Yes (100%) | 5.1 | Time to healing was 4.9 months in TPT group, 6.6 months in non-TPT group, and 7.1 months in those continued on bisphosphonates |
| Miyakoshi, 2015 | 34 | NS (21 AFFs) | NS (24 AFFs) | Incomplete: Surgical = 5 AFFs Conservative = 5 AFFs Complete: n = 11 AFFs | 34 (100%) | 78.5 | Japan | Alendronate or risedronate (100%) | 4.4 (1–11.7) | Time to healing was significantly shorter for all surgically treated AFF in TPT group (5.4 vs 8.6 months) |
| Petraszko, 2016 | 7 | 6 | 1 | Incomplete: Conservative = 8 AFFs Surgical = 1 AFF | 7 (100%) | 70.7 | USA | Yes (100%) | 10.6 (7–15) | Fracture line disappeared in 2 of 6 AFFs with a visible line within 1 year of TPT |
| Saleh, 2012 | 10 | 9 | 1 | Incomplete: conservative = 13 AFFs | 10 (100%) | 66.8 | USA | Alendronate or risedronate (100%) | 10 (4–17) | 5 AFFs without line all healed, 7 of 8 AFFs with fracture line had surgery after 3 months of TPT |
| Sato, 2017 | 12 | 6 | 6 | Incomplete: conservative = 6 AFFs | 12 (100%) | 55.6 | Japan | Alendronate (100%) | 5.9 (3.1–9.3) | All AFFs on continued bisphosphonates deteriorated; 1 AFF progressed to complete fracture after 8 months of TPT |
| Takakubo, 2017 | 8 | 4 | 4 | NS surgical = 5 AFFs | 8 (100%) | 54.9 | Japan | Alendronate, risedronate, minodronate (100%) | 4.3 (2–10) | Time to healing was 11.5 months in 5 AFFs on TPT and 13.3 months in 6 AFFs without TPT, but 1 AFF was not healed after 1 year and lost to follow- up in the TPT group |
| Yeh, 2017 | 13 | NS (8 AFFs) | NS (8 AFFs) | Complete: n = 8 AFFs | 13 (100%) | 70.2 | Taiwan | Alendronate (100%) | 4.0 (2.5–6) | Time to healing was 4.4 months in the TPT group vs 6.2 months in the non- TPT group |
Percentage of women, mean age, antiresorptive use and mean duration of antiresorptive treatment were based on the whole cohort, including controls.
Abbreviations: AFF, atypical femur fracture; AR, antiresorptive; NS, not stated; TPT, teriparatide.
aWhen the number of AFFs is not stated in the article, the number of patients is given.
Table 3.
Summary of Prospective Studies on AFF Patients and Use of Teriparatide
| Reference | Total Cohort, n | Patients on TPT, n | Controls Without TPT, n | Fracture Type of TPT Users a | Female, (n, %) | Mean Age, (years) | Country | AR Use | Mean Duration AR, (years, range) | Main Outcome |
| Chiang, 2013 | 14 | 5 | 9 | Incomplete: n = 4 Complete: n = 1 | 13 (93%) | 76 | Australia | 100%(Alendronate,risedronate,pamidronate,zoledronate) | 7 (4–10) | TPT users: 2 healed, 3 had partial healing Controls: 3 prophylactic surgery, 1 contralateral AFF, 6 with nonunion |
| Greenspan, 2018 | 13 | 13b | 0 | Incomplete: Surgical = 1 Complete n = 12 | 13 (100%) | 74 | USA | 100%(Risedronate,ibandronate,alendronate) | NS | Higher bone healing scores in immediate TPT group, but not statistically significant |
| Watts, 2017 | 14 | 14 | 0 | Complete: n = 9 Incomplete: Surgical = 1 Conservative = 4 | 14 (100%) | 68 | USA | 100%(Alendronate,ibandronate,zoledronate,risedronate) | 8.8 (3–14.5) | Complete AFFs: 4 healed, 5 partial healing, 1 nonunion;Incomplete AFF: 4 partial healing, 3 unchanged; 2 contralateral complete AFFs |
Percentage of women, mean age, antiresorptive use and mean duration of antiresorptive treatment were based on the whole cohort, including controls.
AFF, atypical femur fracture; AR, antiresorptive; NS, not stated; TPT, teriparatide.
aThe number of patients is given.
b7 immediate postsurgery, 6 on teriparatide 6 months postoperatively.
Table 4.
Radiological Healing of AFF After Teriparatide: Pooled Data
| Incomplete AFF (conservative) | Incomplete AFF (surgical) | Complete AFF | ||
|---|---|---|---|---|
| Fracture Healing and Teriparatide Use; n = 140 Patients | TPT | TPT | TPT | No TPT |
| Number of AFFs (total 165) | 30 | 10 | 58 | 67 |
| Healing ≤ 6 months of TPT | 13 (43%) | 9 (90%) | 44 (76%) | 34 (51%) |
| Healing 6–12 months of TPT | 4 (13%) | 1 (10%) | 9 (16%) | 29 (43%) |
| No union achieved at 12 months | 9 (30%) | - | 5 (9%) | 4 (6%) |
| Progression to complete AFF | 4 (13%) | NA | NA | NA |
Abbreviations: AFF, atypical femur fracture; NA, not applicable; TPT, teriparatide.
Five AFFs that underwent surgical procedures from Takakubo et al were categorized as complete fractures. In the study by Miyakoshi et al, 1 nonoperated incomplete AFF and 1 surgically treated incomplete AFF on teriparatide and 8 complete AFFs without teriparatide were labeled as healed by the authors between 6 and 24 months. These fractures were categorized as “healing at 12 months.” From the study by Sato et al, only progression to complete AFF in 1 patient on teriparatide and 1 without teriparatide could be established, while for the other 19 incomplete AFFs, the fracture healing was not specified.
Excluded: Patients (n = 7) without fracture consolidation after ≤ 6 months of teriparatide use (18, 50, 51) (n = 3), (20) (n = 3 with surgery after 3 months), (48) (n = 1, case no. 3), fracture healing could not be assessed with certainty (52, 53), duration of fracture healing or fracture type were not reported (14, 19, 27, 28).
Teriparatide use and occurrence of AFF
. New AFF cases during or after teriparatide use were reported in 8 patients and always occurred in patients with previous bisphosphonate exposure. The new AFFs occurred after 4, 11, 18, and 24 months of teriparatide treatment in 4 patients (11–14). The remaining 4 patients were described in a conference abstract that did not report the duration of teriparatide at time of diagnosis, but all developed new incomplete AFFs during teriparatide therapy in the same femur in which the first incomplete AFF was diagnosed (15).
Six of the 8 patients had been diagnosed with another AFF before, but in 2 patients, the AFFs during teriparatide were the first AFFs (12, 13). One patient was diagnosed with a complete and contralateral incomplete AFF 2 years after stopping teriparatide without any antiresorptive use in the meantime, but the patient had been treated for 8 years with antiresorptives in the past (12).
Teriparatide use after AFF
Descriptive data of case reports, retrospective and prospective studies.
In 33 patients, a total of 24 incomplete AFFs and 27 complete AFFs were reported at the time of starting teriparatide treatment in 31 case reports. In 13 (54%) incomplete AFFs, a fracture line was described or visible on the images in the publication, while the other cases of incomplete AFFs only showed focal cortical thickening on x-ray. The majority of cases were women (n = 27, 82%). The mean age of all patients with AFF was 67 years, ranging from 21 to 84 years. Only a minority of studies (39%) reported ethnicity in 13 patients, of whom 9 were Caucasian. All cases of AFF were associated with the use of bisphosphonates. A total of 27 (82%) patients were previously exposed to alendronate therapy. The mean treatment duration with antiresorptive drugs was 8.3 years, with a minimum duration of 2 years and a maximum exposure of 17 years. Three patients were diagnosed before the AFF with osteogenesis imperfecta (16–18), and 1 patient was genetically tested after the occurrence of bilateral incomplete AFFs that revealed hypophosphatasia (19).
Nine retrospective cohorts that comprised a total of 201 patients with AFF reported the effect of teriparatide use on radiological healing. Five cohorts involved incomplete forms only (15, 20–23), 3 cohorts described complete fractures only (24–26), and 1 cohort was mixed (27). Six cohorts consisted of entirely Asian populations. In 8 cohorts, all AFF cases were exposed to antiresorptive therapy and 1 cohort had 23% bisphosphonate-naïve patients.
Three prospective studies comprised a total of 31 women and 1 man, with a mean age of 73 years, who were treated for bisphosphonate-associated AFFs with teriparatide. Only 1 of these studies had controls (n = 9 patients) without teriparatide treatment (28). All 3 studies had a mix of complete and incomplete AFFs. Teriparatide was started immediately after surgery in 1 study and compared with delayed commencement of teriparatide 6 months postoperatively (29), while in the other 2 studies, teriparatide was started between 7 weeks to just over 1 year after the diagnosis of AFF (28, 30). The study by Greenspan et al included 4 individuals with periprosthetic fractures (29), which strictly does not adhere to the diagnostic criteria for AFF as formulated by the American Society for Bone and Mineral Research (3).
Radiological healing of AFF after teriparatide: pooled data.
We pooled findings on fracture union and teriparatide use in case reports and retrospective studies. Apart from deterioration of incomplete AFFs to complete fractures in 2 patients (30), no data on radiological healing from the 3 prospective studies could be used for this analysis because either the fracture type (28) or time to healing (29, 30) could not be established from these publications.
Data on fracture healing of 165 AFFs in 140 patients were pooled in Table 4, of which 96% were women (11, 14, 16–18, 21–27, 30–49). Teriparatide treatment was given for 98 (59%) AFFs while 67 AFFs from control groups in the cohort studies (all complete AFFs) did not receive teriparatide. The number of incomplete nonoperated AFFs without teriparatide was too small for comparison (n = 4), and there were no controls for surgically managed incomplete AFF. Healing of the fracture was achieved within 6 months of starting teriparatide in 13 (43%) incomplete nonoperated AFFs, 9 (90%) surgically treated incomplete AFFs, and 44 (76%) complete AFFs. In the non-teriparatide-treated group, 34 (51%) complete AFFs healed within 6 months. Complete AFFs appeared to heal faster with teriparatide compared with controls without teriparatide, but in both groups, nonhealing occurred at 12 months postoperatively in a small portion of patients: 5 (9%) AFFs in the teriparatide users; and 4 (6%) AFFs in those without teriparatide. Teriparatide was started in 11 patients because of signs of delayed healing or nonunion, ranging from 2 months to 2 years after the initial diagnosis of AFF (n = 2 incomplete, conservatively managed AFFs; n = 9 complete AFFs) (14, 17, 18, 26, 31, 34, 36, 39, 41, 42, 44). Sixteen patients with 18 fractures had not discontinued bisphosphonates immediately after the diagnosis of AFF, ranging from 3 weeks up to 1 year, including 4 AFFs in 4 patients in the teriparatide-treated group (n = 2 incomplete, conservatively managed AFFs; n = 2 complete AFFs) and 12 controls with 14 complete AFFs (24, 25, 30, 31, 45). Progression from incomplete to complete AFFs occurred in 4 patients after initiation of teriparatide at varying intervals: 9 days, 2 months, 8 months, and 21 months (23, 30, 48).
Denosumab
Denosumab use and occurrence of AFF.
A total of 31 AFFs in 22 patients were reported after the use of denosumab in 14 case reports and 2 clinical trials. The characteristics of these patients are summarized in Table 5. Ethnicity was stated only in 3 reports, with subjects of a Caucasian (n = 1) or Japanese (n = 4) background (50–52). Eleven patients with 15 AFFs were treated for osteoporosis with denosumab 60 mg half-yearly (43, 52–60), while 16 AFFs in 11 patients have been reported after denosumab treatment with a high dose of 120 mg monthly for metastatic bone disease (50, 51, 61–64).
Table 5.
Occurrence of AFF During or After the Use of Denosumab
| Osteoporosis (n = 11) | Bone Metastases (n = 11) | Overall (n = 22) | |
|---|---|---|---|
| Number of AFFs | 15 | 16 | 31 |
| Mean age (min-max; years) | 70.7 (59–81) | 54.7 (50–86) | 62.7 (50–86) |
| Female (%) | 10 (91%) | 10 (91%) | 20 (91%) |
| Complete AFFs (%) | 11 (73%) | 6 (38%) | 17 (77%) |
| Incomplete AFFs (%) | 4 (27%) | 10 (62%) | 14 (64%) |
| BP use | 7 (64%) | 7 (64%) | 14 (64%) |
| BP-naïve | 4 (36%) | 4 (36%) | 8 (36%) |
| Mean duration of BP, years (range) | 9.0 (5 wks–15 yrs) | 7.8 (6–11.3) | 8.4 (5 wks—15 yrs) |
| Number of denosumab doses, mean (range) | 3.2 of 60 mg half-yearly (1–14) | 30 of 120 mg monthly (18–48) | - |
| Accumulative dose, mg/year | 120 | 1440 | - |
| Number of denosumab doses in BP-naïve patients, mean (range) | 5.8 (1–14) | 29 (21–42) | - |
Abbreviations: AFF, atypical femur fracture; BP, bisphosphonate.
Parameters are based on the time of the first AFF. Mean duration of bisphosphonates was calculated in bisphosphonate users only. Incomplete fractures with progression to complete fractures were excluded from the number of incomplete AFFs. Denosumab was dosed 120 mg monthly in oncological patients and 60 mg every 6 months in osteoporosis patients. Missing data: age (n = 2) (63), mean duration of bisphosphonates (n = 3) (55), median number of denosumab doses (n = 3) (55). Included articles: (8, 42, 54–68)
AFF occurred in 8 patients without prior bisphosphonate use (9, 52, 59–61, 63, 64), of which 4 were in patients treated in an oncology setting (61, 63, 64), meaning that only 4 cases were documented of AFF after use of denosumab for management of osteoporosis (9, 52, 59, 60). Two bisphosphonate-naïve individuals developed an AFF following the sixth and the fourteenth dose of denosumab in the FREEDOM-trial, a phase 3 clinical trial with denosumab in 4550 women with osteoporosis (59, 60). The first patient stopped denosumab and achieved fracture healing within 6 months, while the latter continued denosumab, but no data on the healing of AFF are available in this case (personal communication by Amgen, October 2018). One 60-year-old male who had been on glucocorticoids for asthma for more than 30 years developed an AFF without any previous bisphosphonate use 2 months after the second dose of denosumab, which was given in a RCT of denosumab in patients with glucocorticoid-induced osteoporosis (9). The fourth case without bisphosphonate exposure concerns an incomplete, medially located AFF after only 1 injection of denosumab and without abnormalities on x-ray but with periosteal reaction on the MRI scan (52). Although stress fractures resembling AFF located on the medial instead of the lateral cortex have been described (65), this case does not meet the diagnostic criteria of AFF according to the American Society for Bone and Mineral Research Task Force (3). The 4 bisphosphonate-naïve AFF cases treated for metastatic bone disease occurred after 21, 24, or 42 doses of 120 mg denosumab monthly (61, 63, 64). In 2 other cases, the influence of bisphosphonates on the risk of AFF cannot be excluded, but AFF was preceded by very short bisphosphonate treatment before starting denosumab (53, 55). These 2 cases are very similar, since both patients had used alendronate for just a few weeks before switching to strontium ranelate because of side effects, which was subsequently replaced by denosumab, again because of intolerance to the drug. Both patients developed an AFF after 3 doses of denosumab (53, 55). These reports of AFF after denosumab with minimal or no previous bisphosphonate use are suggestive of a role for denosumab in the development of AFF, but the numbers are small, and AFFs have also been reported rarely in patients never treated for osteoporosis (5, 66, 67). In another report, the AFF appeared to be triggered by 1 dose of denosumab in December 2012 after 5years of alendronate use between 1994 and 1999 (57), followed by a subsequent drug holiday for 13 years.
Denosumab use after AFF
We found 7 papers that reported on the use of denosumab after an AFF in 10 patients (18, 45, 58, 68–71).
Bisphosphonates switched to denosumab treatment.
Seven patients switched from bisphosphonates to denosumab just before or after the first AFF. One patient with an incomplete AFF after 4 years of risedronate who underwent preventive placement of an intramedullary gamma nail was switched to denosumab and had delayed healing after 6 and 12 months (68). In a case series of complete AFFs associated with alendronate use (69), 4 patients started denosumab after the first AFF. There were 4 different outcomes. One patient had delayed fracture healing at 12 months but with minimal pain and almost the same activity level. One patient had a second complete AFF on the contralateral side 1 year after switching to denosumab; this contralateral AFF showed bridging callus formation at 9 months’ follow-up. One patient had bridging callus formation at 12 months and was pain-free. One patient had resumed normal daily activities at 18 months of follow-up, and radiographs showed bone healing (69). In a case report, 1 patient, who sustained a first complete AFF after 1 dose of denosumab and 8 years of alendronate (58), continued denosumab treatment but sustained a second complete AFF after 3 more doses of denosumab. The authors describe healing of both AFFs within 5 months postoperatively. Another case is described of denosumab started postoperatively for complete AFF, with full weight-bearing after 3 months and no adverse events at 18 months of follow-up; complete bony union was achieved at 1 year postoperatively (70).
Teriparatide switched to denosumab treatment.
Three cases are reported of denosumab therapy following teriparatide. One case involved bilateral incomplete AFFs without visible fracture lines after 7 years of oral bisphosphonates and who was treated with teriparatide for 18 months and a subsequent drug holiday of 12 months (71). The cortical thickening had almost completely flattened on x-rays when denosumab was prescribed as treatment for low bone mineral density (BMD). The authors reported that the patient had increasing thigh pain in both upper legs 6 months after the first dose of denosumab and that x-rays and bone scintigraphy showed recurrent, incomplete bilateral AFFs with presence of a lucent line after which the surgeon decided to perform bilateral internal fixation (71). Two case reports (1 with incomplete AFF and 1 with complete AFF) mention that the initiation of denosumab therapy had a good outcome in the short term (< 1 year) (18, 45).
Raloxifene
Raloxifene use and occurrence of AFF
Six papers (29, 49, 72–75) stated the use of raloxifene prior to the diagnosis of AFF in 8 patients, although in 4 patients, it was unclear whether this was preceded by bisphosphonate treatment (74, 75). Two patients had simultaneous use of raloxifene and bisphosphonates during 6 months and 6 years, respectively (49, 72). One had had prior bisphosphonate use (29). In a case series of surgically treated AFFs from Japan (73), a patient treated with raloxifene only was reported. This concerned a 77-year-old woman who had taken raloxifene and vitamin K2 for only 1 year when she sustained an AFF after a fall from standing height. Because delayed union was suspected, she received low-intensity pulsed ultrasonography 3 months postoperatively, and partial fracture healing was seen 9 months after the surgery (73).
Raloxifene use after AFF
We found reports of 2 patients treated with raloxifene after AFF, in both cases after teriparatide treatment (37, 46). One 63-year-old Asian woman received 10 months of teriparatide after incomplete AFF with a visible fracture line, which was subsequently replaced by raloxifene. The fracture line had already diminished after 3 months of teriparatide and was invisible 15 months after the diagnosis, which was 5 months after starting raloxifene (37). One 78-year-old woman with incomplete AFF with a lucent line received teriparatide; the fracture line had almost disappeared 3 months postoperatively. After 12 months of teriparatide, she switched to a SERM, most likely raloxifene, and had an event-free follow-up 3 years after the diagnosis (46).
Romosozumab
Twelve studies have been performed with romosozumab. Two studies reported 3 cases of AFF. One case of AFF occurred 3.5 months after the first monthly dose in a phase 3 clinical trial (76), but the association between romosozumab and the AFF is questionable, given that the participant had complained of prodromal pain prior to the first romosozumab administration. Two cases of AFFs occurred during open-label alendronate treatment after 1 year of monthly romosozumab in another trial (77).
Abaloparatide
A total of 9 clinical trials with abaloparatide were published. No cases of AFF were reported in patients who used or had used abaloparatide.
Discussion
In clinical practice, there is great uncertainty of how to treat patients after they have sustained an AFF. This relates both to potential (positive or negative) effects of bone agents on the healing of the fracture and to the safety of osteoporosis drugs in those patients, who are still at high risk of fragility fracture after an AFF. Bisphosphonates are usually stopped because patients are considered at risk of an AFF of the other femur since bilaterality is commonly reported, varying from 28% up to 44% (2, 7). In this systematic literature review, we aimed to assess the effects of teriparatide, denosumab, raloxifene, romosozumab, and abaloparatide on both the occurrence and healing of AFF in order to give recommendations for medical management. It is difficult to draw firm conclusions because there are no reported RCTs of treatment in patients with AFF with any of these drugs. Based on descriptions of 165 AFFs treated with teriparatide in observational studies, we made a crude estimate of effects of teriparatide on radiological healing of AFF after 6 and 12 months. The majority of surgically treated incomplete (n = 9, 90%) and complete AFFs (n = 44, 76%) healed within 6 months of teriparatide treatment, in contrast with nonoperated incomplete fractures treated with teriparatide (n = 13, 43%) and complete AFFs that were not treated with teriparatide (n = 34, 51%). The reported data are insufficient for an evidence-based recommendation of the use of teriparatide to accelerate healing of AFF. Yet, keeping in mind the flawed study designs and heterogeneity between studies, the observational data might suggest that teriparatide could have a beneficial effect on the healing time of surgically treated AFF, although nonunion after 1 year can still occur. There is no evidence of improved fracture healing for conservatively managed incomplete AFFs based on these observational data. Our findings clearly show that even during and after teriparatide treatment, a new AFF can occur, either as a first presentation of AFF or as a second AFF of the contralateral femur, but only in patients previously treated with bisphosphonates. The role of teriparatide for healing of any type of fracture is debated. One meta-analysis of 5 RCTs in patients with osteoporotic fractures found a significantly shorter healing time in the teriparatide-treated group (78), while another analysis including also nonosteoporotic fractures did not demonstrate any effectiveness for teriparatide with regard to faster union (79). Two RCTs involved subjects with femoral fractures. In one trial with postmenopausal women and low-trauma femoral neck fractures, teriparatide did not improve radiological fracture healing, but the sample size was too small to detect any differences (80). The other RCT involved premenopausal women with acute stress fractures of the lower extremities and who showed a tendency toward improved healing on MRI in the teriparatide group (83.3%) in comparison with the control group (57.1%), but this was not statistically significant (P = 0.18) (81).
There are no documented cases of AFF with the use of abaloparatide. This drug might have equivalent effects on AFF as teriparatide, given the biological similarity. The results from the literature search were insufficient to assess the effects on AFF healing by denosumab and raloxifene. Despite the lack of epidemiological studies, our analysis of the literature suggests that the absolute risk of AFF when using denosumab or raloxifene for osteoporosis is very low given the limited reports of AFF cases using these drugs, 11 and 8 patients respectively, and they also mostly occurred after previous use of bisphosphonates. However, this risk may be increased in patients who have already had an AFF, suggested by the reports of 2 patients with a second complete AFF (58, 69) on denosumab and in another patient with bilateral recurrent incomplete AFFs on denosumab even after use of teriparatide (71). These cases suggest curtailing use of denosumab treatment after an initial unilateral AFF. Romosozumab is linked to 3 AFF cases in clinical trials, but it remains to be seen if more cases of AFF will develop in patients treated with romosozumab with or without bisphosphonate exposure. Based on our findings, we conclude that there is a clear need for RCTs to evaluate whether teriparatide and/or abaloparatide enhances fracture union of AFF (of any type), since this is the only drug that is not associated with the development of AFF without prior use of bisphosphonates. The observational studies in this review are biased and lack information on confounding factors such as time between diagnosis and starting medical treatment, surgical fixation techniques, smoking, body mass index, fracture localization, use of concomitant medication, and postoperative weight-bearing protocols. Currently, 1 clinical trial is ongoing for patients with incomplete AFF who are randomized to receive either placebo injections or teriparatide. Changes in pain score and physical function using the Western Ontario and McMaster Universities Osteoarthritis scale and the proportion of participants requiring surgery after 12 months serve as primary outcomes (82). There are no trials registered investigating teriparatide for complete AFF, nonhealing AFF, or electively operated incomplete AFF. Also, no trials are currently evaluating the risks and benefits of antiresorptive therapy compared with placebo in patients with AFF after stopping teriparatide or in patients with AFF managed conservatively or surgically. It is difficult to set up an adequately powered study because of the low incidence of AFF. Therefore, an international registry of AFF cases could be very useful to gain insight into the safety and efficacy of osteoporosis drugs in relation to fracture healing, bone mineral density, bone turnover, and development of new AFFs in these patients, but this is only possible when patients with AFF are referred to specialized centers.
Recommendations for clinical practice based on expert opinion
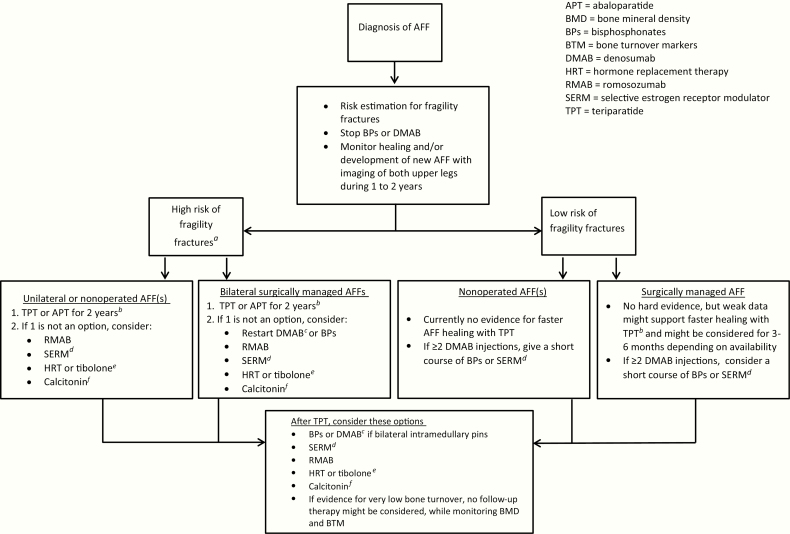
Based on the results in this review and our expert opinion, we advise on medical treatment for patients with AFF. Our recommendations for medical treatment are summarized in a decision tree (Fig. 2), encompassing the occurrence of AFF when using bisphosphonates or denosumab and what to do after a patient with AFF has completed a 2-year course of teriparatide. In any case, extensive monitoring with imaging of both upper legs is advised during the first 1 or 2 years after the diagnosis of AFF because nonhealing of AFF and contralateral AFF may still occur, even on teriparatide.
Figure 2.
Decision tree with considerations for medical management after atypical femur fracture (AFF). aDefinition may vary across countries, eg, a hip BMD T-score ≤ –2.5 SD, older age (70–75 years), a recent fragility fracture, other strong risk factors for fracture, or a FRAX fracture risk score that is above country-specific thresholds (95). dRaloxifene or bazedoxifene are preferably prescribed in relatively young postmenopausal women who are at low risk of hip fractures and deep vein thrombosis (94), or in women in whom the use of teriparatide is contraindicated. eIn case of intolerance to SERMs, hormone replacement therapy or tibolone could be considered in women with a low risk of deep vein thrombosis and breast cancer, without a history of myocardial infarction or stroke (94). bSwitching denosumab to teriparatide may result in progressive BMD loss. cBe aware that antiresorptive therapy may be needed after stopping denosumab. fCalcitonin can be prescribed in patients who are not eligible for bisphosphonates, SERMs, hormone replacement therapy, tibolone, abaloparatide, or teriparatide.
When AFF is diagnosed during the use of bisphosphonates or denosumab, it is recommended to stop this treatment since continuation may lead to worsening of the AFF or a new contralateral AFF. To prevent a rebound effect, discontinuation of denosumab could be followed by a short course of bisphosphonates or SERMs in patients with surgically treated AFFs. In patients at low fracture risk without prevalent vertebral fractures who have only had 1 or 2 half-yearly injections, consider stopping denosumab treatment without subsequent therapy. After healing of bilateral, surgically managed AFFs, bisphosphonates or denosumab may be continued. It should be kept in mind that discontinuation after 3 or more years of bisphosphonate treatment may result in increased risk of hip fractures and clinical vertebral fractures as shown by some studies (83, 84), although this was not found in another recent retrospective analysis of a population-based cohort (85). Continuation of bisphosphonates might lead to a risk of atypical fractures at skeletal sites other than the femur. Anecdotally, spontaneous fractures of other long bones (eg, ulna, forearm, and tibia) have been reported in relation to bisphosphonate use (86–93), but no association has been established, and the potential risk of such atypical fractures does not appear to weigh against the risk of typical osteoporotic fractures. Teriparatide might be started for surgically treated AFFs, although strong evidence for improved fracture union is lacking. Further, teriparatide, SERMs, romosozumab, or abaloparatide may alternatively be considered in patients at high risk of fragility fractures. SERMs are preferably prescribed in relatively young, postmenopausal women who are at low risk of hip fractures and deep vein thrombosis (94). Hormone replacement therapy or tibolone might be considered when SERMs are not tolerated, preferably in younger women (aged < 65 years) who do not have an increased risk of venous thromboembolism and are without a history of myocardial infarction or stroke, also keeping in mind the increased breast cancer risk (94). If the patient is not eligible for any of the aforementioned drugs, calcitonin can be prescribed as in accordance with the recent guideline of the Endocrine Society on pharmacological management of osteoporosis (94). The definition of high risk of fragility fractures varies across countries, but is often defined by a hip BMD T score ≤ –2.5 SD, older age (70–75 years), a recent fragility fracture, other strong risk factors for fracture, or a FRAX (https://www.sheffield.ac.uk/FRAX/) fracture risk score that is above country-specific thresholds (95).
After 2 years of teriparatide, subsequent therapy may be given with raloxifene (or hormone replacement therapy) in women and—in those with bilateral surgical fixation of AFF—denosumab or bisphosphonates. In patients at the end of a (short) course of teriparatide who have low bone turnover markers after teriparatide or who are deemed to be at low risk of osteoporotic fractures, teriparatide may be discontinued without further antiresorptive treatment, but close monitoring of BMD and bone turnover markers is recommended.
The considerations for each individual drug are given in more detail below.
Teriparatide
There is no evidence-based indication for teriparatide to enhance healing of AFF, but a tendency toward faster healing with teriparatide for surgically managed AFFs is seen in the limited, observational data. Hence, teriparatide 20 ug daily, when reimbursed, might be considered for surgically treated AFF, both incomplete AFF and complete AFF. Even during the use of teriparatide, nonunions do still occur in surgically managed AFF. The limited data on conservatively managed incomplete forms of AFF and use of teriparatide do not demonstrate improved fracture healing, but should be interpreted with caution, pending the result of an RCT that is awaiting results. When teriparatide is given for the sole purpose to enhance fracture healing of AFF, a short treatment duration of 3 to 6 months may suffice. Teriparatide is a reasonable treatment option for patients who have had an AFF and are still at high risk for fragility fractures. A big clinical dilemma is what to do after a full 2-year course of teriparatide treatment. Normally, antiresorptive therapy is advised after 2 years of teriparatide because the positive effects on bone mass and strength will in time disappear, as with any drug without skeletal retention. Some patients with AFF may have inherent low bone turnover, for example, due to an underlying monogenetic disease (96) or due to previous long-term use of bisphosphonates. It can be speculated that accelerated bone loss after cessation of teriparatide may not occur in these cases. A few studies describe the effect of teriparatide on bone turnover in patients with AFF, but the results are inconclusive. Administration of teriparatide during 6 months has been associated with a significant increase in bone turnover markers in patients with AFF (28, 30) and values returned almost to baseline level after 2 years of teriparatide (30), but pretreatment values varied widely (30, 97) and bone turnover markers did not correlate with histomorphometric findings from bone biopsies before and after teriparatide treatment in patients with AFF (97). We suggest monitoring bone turnover markers on a regular basis in patients with AFF before, during, and after teriparatide treatment and considering antiresorptive drugs when levels start to increase or when BMD starts to decrease in patients at high risk of fractures. In this situation, we suggest either a SERM, romosozumab, calcitonin, tibolone, estrogens, denosumab, or bisphosphonates, based on sex and on bilaterality of surgical intervention (see below).
Denosumab
When a patient sustains an AFF during the use of denosumab, the risk of a rebound effect with rapid loss of BMD and potential risk of multiple vertebral fractures following cessation of denosumab (98) must be weighed against the potentially increased risk of a contralateral AFF when continuing denosumab. Patients who have already had vertebral fractures appear to be at greatest risk of developing multiple vertebral fractures after denosumab discontinuation. In general, a course of bisphosphonates is recommended after stopping denosumab (98). This is not advisable for a conservatively managed incomplete AFF, but a short course of a SERM or bisphosphonates may be considered in patients with bilateral surgically treated AFFs or a unilateral surgically treated AFF without any radiological signs of incomplete AFF of the contralateral femur. Denosumab could be stopped without follow-up therapy in patients at low risk of fragility fractures without prevalent vertebral fractures, especially in those who have only had 1 or 2 half-yearly injections of 60 mg subcutaneously. For patients at high risk of fragility fractures, a switch to teriparatide or a SERM could be considered. However, the rebound effect after stopping denosumab might still occur since teriparatide increases bone turnover. One should also be aware of a decrease in BMD, especially at cortical sites, as was seen in osteoporotic women who transitioned to teriparatide after 2 years of denosumab in the DATA-switch study (99). Alternatively, hormone replacement therapy or tibolone can be considered in women in absence of contraindications such as a high risk of breast cancer or deep vein thrombosis, history of stroke, or myocardial infarction. Calcitonin is an option if the patient does not tolerate any of the aforementioned drugs (94). Denosumab could be continued or initiated when the patient has bilateral surgically treated AFFs and a persistently high risk of fragility fractures, including those who have completed 2 years of teriparatide. Denosumab therapy for up to 10 years has been associated with increasing BMD and low fracture incidence (59). Long-term use of denosumab could especially be considered in elderly patients with a life expectancy of less than 10 years, for whom this may serve as life-long osteoporosis treatment.
Raloxifene
Raloxifene could be considered as follow-up therapy after teriparatide when bone turnover markers are high in postmenopausal women who do not have a history of venous thromboembolic events. Preferably, it is given to women who are relatively young and are at lower risk of hip fractures. As mentioned above, it could also be considered in patients who have to stop denosumab because they are at risk of another AFF and to potentially prevent the rebound in bone turnover and risk of multiple vertebral fractures, especially when they have already had vertebral fractures. However, no studies have been performed using SERMs to prevent rebound after stopping denosumab. Because it has a weaker antiresorptive effect than bisphosphonates or denosumab, and few cases of AFF have been reported on raloxifene, this may be a preferred option after teriparatide (100, 101). Yet it should be kept in mind that raloxifene is not regularly prescribed for osteoporosis, hence a low number of AFFs associated with raloxifene does not guarantee a lower risk of AFF compared with other antiresorptive drugs.
Acknowledgments
The authors acknowledge Gerdien de Jonge, Biomedical Information Specialist of the Medical Library of Erasmus Medical Centre, for her assistance with the systematic literature search.
Author Contributions: Study design: D.M.L and M.C.Z. Systematic review: D.M.L. and M.C.Z. First draft: D.M.L. and M.C.Z. Revision of manuscript: M.C.Z. and M.J.McK., B.A., S.H.R., M.C.S., R.E., B.L., M.N.G.G.
D.M.L. and M.C.Z. take responsibility for the integrity of the data analysis.
Glossary
Abbreviations
- AFF
atypical femur fracture
- BMD
bone mineral density
- MRI
magnetic resonance imaging
- RCT
randomized controlled trial
- SERM
selective estrogen receptor modulator
Additional data have been included in Supplement 1 located in a digital research materials repository (1).
Additional Information
Disclosure Summary: Prof McKenna has received fees for lectures or advice from UCD, Pharma, and Mylan. Prof Langdahl has received research funding to her institution from Amgen and Novo Nordisk and consultancy and lecture fees from Eli Lilly, Amgen, and UCB. Dr Cohen-Solal received fees for lectures from Amgen. Dr Guañabens has in the past received fees for lectures and/or advice from Alexion, Amgen, Eli Lilly, and UCB. Prof Eastell receives consultancy funding from IDS, Roche Diagnostics, GSK Nutrition, FNIH, Mereo, Lilly, Sandoz, Nittobo, Abbvie, Samsung, and Haoma Medica and grant funding from Nittobo, IDS, Roche, Amgen, and Alexion. Prof Zillikens has in the past received fees for lectures and/or advice from Alexion, Amgen, Eli Lilly, Kyowa Kirin, and UCB. All other authors state that they have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Van De Laarschot D, McKenna M, Abrahamsen B, et al. Supplement 1: Detailed study descriptions of case reports, retrospective cohort studies and prospective studies on teriparatide use and AFF. Deposited 27 Nov 2019. 10.5281/zenodo.3555498. [DOI]
- 2. Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: a systematic review of case/case series studies. Bone. 2010;47(2):169–180. [DOI] [PubMed] [Google Scholar]
- 3. Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29(1):1–23. [DOI] [PubMed] [Google Scholar]
- 4. Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27(12):2544–2550. [DOI] [PubMed] [Google Scholar]
- 5. Nguyen HH, van de Laarschot DM, Verkerk AJMH, Milat F, Zillikens MC, Ebeling PR. Genetic risk factors for atypical femoral fractures (AFFs): a systematic review. JBMR Plus. 2018;2(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schilcher J, Koeppen V, Aspenberg P, Michaëlsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop. 2015;86(1):100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shane E, Burr D, Ebeling PR, et al. ; American Society for Bone and Mineral Research . Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25(11):2267–2294. [DOI] [PubMed] [Google Scholar]
- 8. Favinger JL, Hippe D, Ha AS. Long-term radiographic follow-up of bisphosphonate-associated atypical femur fractures. Skeletal Radiol. 2016;45(5):627–633. [DOI] [PubMed] [Google Scholar]
- 9. Saag KG, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol. 2018;6(6):445–454. [DOI] [PubMed] [Google Scholar]
- 10. Saag K, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol. 2018;6(6):445–454. [DOI] [PubMed] [Google Scholar]
- 11. Nguyen HH, Milat F, Ebeling PR. A new contralateral atypical femoral fracture despite sequential therapy with teriparatide and strontium ranelate. Bone Rep. 2017;6:34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spyridonidis TJ, Mousafiris KV, Rapti EK, Apostolopoulos DJ. Bone scintigraphy depicts bilateral atypical femoral stress fractures with metachronous presentation, long before a complete fracture occurs. Hell J Nucl Med. 2014;17(1):54–57. [DOI] [PubMed] [Google Scholar]
- 13. Al-Azzani WA, Evans L, Speight L, et al. Hyperpharmacotherapy in ageing cystic fibrosis patients: the first report of an atypical hip fracture. Respir Med Case Rep. 2015;16:148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lampropoulou-Adamidou K, Tournis S, Balanika A, et al. Sequential treatment with teriparatide and strontium ranelate in a postmenopausal woman with atypical femoral fractures after long-term bisphosphonate administration. Hormones. 2013;12(4):591–597. [DOI] [PubMed] [Google Scholar]
- 15. Cheung AM, Adachi J, Khan A, et al. Effect of teriparatide on healing of incomplete atypical femur fractures. J Bone Miner Res. 2013;28. [Google Scholar]
- 16. Etxebarria-Foronda I, Carpintero P. An atypical fracture in male patient with osteogenesis imperfecta. Clin Cases Miner Bone Metab. 2015;12(3):278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holm J, Eiken P, Hyldstrup L, Jensen JE. Atypical femoral fracture in an osteogenesis imperfecta patient successfully treated with teriparatide. Endocr Pract. 2014;20(10):e187–e190. [DOI] [PubMed] [Google Scholar]
- 18. Tan JY, Seow CJ. Management of atypical femoral fracture in a patient with osteogenesis imperfecta. BMJ Case Rep. 2017;2017:bcr-2017-221835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Righetti M, Wach J, Desmarchelier R, Coury F. Teriparatide treatment in an adult patient with hypophosphatasia exposed to bisphosphonate and revealed by bilateral atypical fractures. Joint Bone Spine. 2018;85(3):365–367. [DOI] [PubMed] [Google Scholar]
- 20. Lee YK, Ha YC, Kang BJ, Chang JS, Koo KH. Predicting need for fixation of atypical femoral fracture. J Clin Endocrinol Metab. 2013;98(7):2742–2745. [DOI] [PubMed] [Google Scholar]
- 21. Saleh A, Hegde VV, Potty AG, Schneider R, Cornell CN, Lane JM. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J. 2012;8(2):103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petraszko A, Siegal D, Flynn M, Rao SD, Peterson E, van Holsbeeck M. The advantages of tomosynthesis for evaluating bisphosphonate-related atypical femur fractures compared to radiography. Skeletal Radiol. 2016;45(5):615–623. [DOI] [PubMed] [Google Scholar]
- 23. Sato H, Kondo N, Nakatsue T, et al. High and pointed type of femoral localized reaction frequently extends to complete and incomplete atypical femoral fracture in patients with autoimmune diseases on long-term glucocorticoids and bisphosphonates. Osteoporos Int. 2017;28(8):2367–2376. [DOI] [PubMed] [Google Scholar]
- 24. Takakubo Y, Ohta D, Ishi M, et al. The incidence of atypical femoral fractures in patients with rheumatic disease: Yamagata Prefectural Committee of Atypical Femoral Fractures (YamaCAFe) Study. Tohoku J Exp Med. 2017;242(4):327–334. [DOI] [PubMed] [Google Scholar]
- 25. Lee KJ, Yoo JJ, Oh KJ, et al. Surgical outcome of intramedullary nailing in patients with complete atypical femoral fracture: a multicenter retrospective study. Injury. 2017;48(4):941–945. [DOI] [PubMed] [Google Scholar]
- 26. Yeh WL, Su CY, Chang CW, et al. Surgical outcome of atypical subtrochanteric and femoral fracture related to bisphosphonates use in osteoporotic patients with or without teriparatide treatment. BMC Musculoskelet Disord. 2017;18(1):527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miyakoshi N, Aizawa T, Sasaki S, et al. Healing of bisphosphonate-associated atypical femoral fractures in patients with osteoporosis: a comparison between treatment with and without teriparatide. J Bone Miner Metab. 2015;33(5):553–559. [DOI] [PubMed] [Google Scholar]
- 28. Chiang CY, Zebaze RM, Ghasem-Zadeh A, Iuliano-Burns S, Hardidge A, Seeman E. Teriparatide improves bone quality and healing of atypical femoral fractures associated with bisphosphonate therapy. Bone. 2013;52(1):360–365. [DOI] [PubMed] [Google Scholar]
- 29. Greenspan SL, Vujevich K, Britton C, et al. Teriparatide for treatment of patients with bisphosphonate-associated atypical fracture of the femur. Osteoporos Int. 2018;29(2):501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watts NB, Aggers D, McCarthy EF, et al. Responses to treatment with teriparatide in patients with atypical femur fractures previously treated with bisphosphonates. J Bone Miner Res. 2017;32(5):1027–1033. [DOI] [PubMed] [Google Scholar]
- 31. Carvalho NN, Voss LA, Almeida MO, Salgado CL, Bandeira F. Atypical femoral fractures during prolonged use of bisphosphonates: short-term responses to strontium ranelate and teriparatide. J Clin Endocrinol Metab. 2011;96(9):2675–2680. [DOI] [PubMed] [Google Scholar]
- 32. Cerveró RS, Sastre-Jala B, Heredia-Heredia E, Franco-Ferrando N, Poquet-Jornet J. Atypical femur fractures associated with bisphosphonates: from prodrome to resolution. Rheumatol Rep. 2015;7(1):17–9. [Google Scholar]
- 33. Chew PC, Julaihi B, Ibrahim Z. Spontaneous subtrochanteric femoral stress fracture related to alendronate: a case report. Malays Orthop J. 2013;7(1):70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fukuda F, Kurinomaru N, Hijioka A. Weekly teriparatide for delayed unions of atypical subtrochanteric femur fractures. Biol Ther. 2014;4(1-2):73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Giannotti S, Bottai V, Dell’Osso G, De Paola G, Ghilardi M, Guido G. Pseudoarthrosis in atypical femoral fracture: case report. Osteoporos Int. 2013;24(11):2893–2895. [DOI] [PubMed] [Google Scholar]
- 36. Gomberg SJ, Wustrack RL, Napoli N, Arnaud CD, Black DM. Teriparatide, vitamin D, and calcium healed bilateral subtrochanteric stress fractures in a postmenopausal woman with a 13-year history of continuous alendronate therapy. J Clin Endocrinol Metab. 2011;96(6):1627–1632. [DOI] [PubMed] [Google Scholar]
- 37. Huang HT, Kang L, Huang PJ, et al. Successful teriparatide treatment of atypical fracture after long-term use of alendronate without surgical procedure in a postmenopausal woman: a case report. Menopause. 2012;19(12):1360–1363. [DOI] [PubMed] [Google Scholar]
- 38. Iwata K, Mashiba T, Hitora T, Yamagami Y, Yamamoto T. A large amount of microdamages in the cortical bone around fracture site in a patient of atypical femoral fracture after long-term bisphosphonate therapy. Bone. 2014;64:183–186. [DOI] [PubMed] [Google Scholar]
- 39. Mastaglia SR, Aguilar G, Oliveri B. Teriparatide for the rapid resolution of delayed healing of atypical fractures associated with long-term bisphosphonate use. Eur J Rheumatol. 2016;3(2):87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reddy SV, Gupta SK. Atypical femoral shaft fracture in a patient with non-metastatic prostate cancer on zoledronic acid therapy: effect of therapy or coincidence? Singapore Med J. 2012;53(3):e52–e54. [PubMed] [Google Scholar]
- 41. Román M, de Prado A, Rodríguez de Tembleque F. Bilateral atypical femoral fracture in a man on long-term bisphosphonate and glucocorticoid therapy: a case report. JBJS Case Connect. 2015;5(2):e36–ee5. [DOI] [PubMed] [Google Scholar]
- 42. Schilcher J. High revision rate but good healing capacity of atypical femoral fractures. A comparison with common shaft fractures. Injury. 2015;46(12):2468–2473. [DOI] [PubMed] [Google Scholar]
- 43. Selga J, Nuñez JH, Minguell J, Lalanza M, Garrido M. Simultaneous bilateral atypical femoral fracture in a patient receiving denosumab: case report and literature review. Osteoporos Int. 2016;27(2):827–832. [DOI] [PubMed] [Google Scholar]
- 44. Stathopoulos KD, Kosmidis C, Lyritis GP. Atypical fractures of the femur and ulna and complications of fracture healing in a 76-year-old woman with Sjögren’s syndrome. J Musculoskelet Neuronal Interact. 2011;11(2):208–11; quiz 211. [PubMed] [Google Scholar]
- 45. Tarazona-Santabalbina FJ, Aguilella-Fernández L. Bisphosphonate long-term treatment related bilateral subtrochanteric femoral fracture. Can teriparatide be useful? Aging Clin Exp Res. 2013;25(5):605–609. [DOI] [PubMed] [Google Scholar]
- 46. Tsuchie H, Miyakoshi N, Nishi T, Abe H, Segawa T, Shimada Y. Combined effect of a locking plate and teriparatide for incomplete atypical femoral fracture: two case reports of curved femurs. Case Rep Orthop. 2015;2015:213614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Uppin R, Gupta S, Prakash S. A case report of bisphosphonate-induced bilateral osteoporotic subtrochanteric fracture femurii: review of literature. J Orthop Case Rep. 2016;6(4):31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vaishya R, Vaish A, Nadeem A. Bisphosphonate-induced atypical subtrochanteric femoral fracture. BMJ Case Rep. 2013. :bcr2013201931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Visekruna M, Wilson D, McKiernan FE. Severely suppressed bone turnover and atypical skeletal fragility. J Clin Endocrinol Metab. 2008;93(8):2948–2952. [DOI] [PubMed] [Google Scholar]
- 50. Tateiwa D, Outani H, Iwasa S, et al. Atypical femoral fracture associated with bone-modifying agent for bone metastasis of breast cancer: a report of two cases. J Orthop Surg. 2017;25(3):2309499017727916. [DOI] [PubMed] [Google Scholar]
- 51. Ota S, Inoue R, Shiozaki T, et al. Atypical femoral fracture after receiving antiresorptive drugs in breast cancer patients with bone metastasis. Breast Cancer. 2017;24(4):601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Paparodis R, Buehring B, Pelley EM, Binkley N. A case of an unusual subtrochanteric fracture in a patient receiving denosumab. Endocr Pract. 2013;19(3):e64–e68. [DOI] [PubMed] [Google Scholar]
- 53. Khow KS, Yong TY. Atypical femoral fracture in a patient treated with denosumab. J Bone Miner Metab. 2015;33(3):355–358. [DOI] [PubMed] [Google Scholar]
- 54. Shabestari M, Eriksen EF, Paschalis EP, et al. Presence of pyrophosphate in bone from an atypical femoral fracture site: a case report. Bone Rep. 2017;6:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Villiers J, Clark DW, Jeswani T, Webster S, Hepburn AL. An atraumatic femoral fracture in a patient with rheumatoid arthritis and osteoporosis treated with denosumab. Case Rep Rheumatol. 2013;2013:249872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schilcher J, Aspenberg P. Atypical fracture of the femur in a patient using denosumab–a case report. Acta Orthop. 2014;85(1):6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thompson RN, Armstrong CL, Heyburn G. Bilateral atypical femoral fractures in a patient prescribed denosumab - a case report. Bone. 2014;61:44–47. [DOI] [PubMed] [Google Scholar]
- 58. Drampalos E, Skarpas G, Barbounakis N, Michos I. Atypical femoral fractures bilaterally in a patient receiving denosumab. Acta Orthop. 2014;85(1):3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised freedom trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513–523. [DOI] [PubMed] [Google Scholar]
- 60. Bone HG, Chapurlat R, Brandi ML, et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the freedom extension. J Clin Endocrinol Metab. 2013;98(11):4483–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Austin DC, Torchia MT, Klare CM, Cantu RV. Atypical femoral fractures mimicking metastatic lesions in 2 patients taking denosumab. Acta Orthop. 2017;88(3):351–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Koizumi M, Gokita T, Toda K. Impending atypical femoral fracture in patients with medullary thyroid cancer with skeletal metastasis treated with long-term bisphosphonate and denosumab. Clin Nucl Med. 2017;42(6):463–464. [DOI] [PubMed] [Google Scholar]
- 63. Yang SP, Kim TW, Boland PJ, Farooki A. Retrospective review of atypical femoral fracture in metastatic bone disease patients receiving denosumab therapy. Oncologist. 2017;22(4):438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sugihara T, Koizumi M, Hayakawa K, Ito Y, Sata N. Impending atypical femoral fracture in a patient of breast cancer with bone metastases receiving long-term denosumab. Clin Nucl Med. 2018;43(5):365–366. [DOI] [PubMed] [Google Scholar]
- 65. van de Laarschot DM, Somford MP, Jager A, Oei EH, Bos PK, Zillikens MC. “Atypical” atypical femur fractures and use of bisphosphonates. Clin Cases Miner Bone Metab. 2016;13(3):204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Szolomayer LK, Ibe IK, Lindskog DM. Bilateral atypical femur fractures without bisphosphonate exposure. Skeletal Radiol. 2017;46(2):241–247. [DOI] [PubMed] [Google Scholar]
- 67. Tan SC, Koh SB, Goh SK, Howe TS. Atypical femoral stress fractures in bisphosphonate-free patients. Osteoporos Int. 2011;22(7):2211–2212. [DOI] [PubMed] [Google Scholar]
- 68. Alfahad A, Thet EM, Radwan F, Sudhakar J, Nini K, Tachtatzis P. Spontaneous incomplete transverse subtrochanteric femoral fracture with cortical thickening possibly secondary to risedronate use: a case report. J Med Case Rep. 2012;6:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ballas EG, Mavrogenis AF, Karamanis E, et al. Low-energy femoral shaft fractures after long-term alendronate therapy: report of seven cases. Eur J Orthop Surg Traumatol. 2015;25(1):181–187. [DOI] [PubMed] [Google Scholar]
- 70. Peake C, Trompeter A. Low-energy atypical femoral shaft and ipsilateral neck fracture: a rare association. BMJ Case Rep. 2017;2017:bcr-2017-222129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ramchand SK, Chiang CY, Zebaze RM, Seeman E. Recurrence of bilateral atypical femoral fractures associated with the sequential use of teriparatide and denosumab: a case report. Osteoporos Int. 2016;27(2):821–825. [DOI] [PubMed] [Google Scholar]
- 72. Osugi K, Miwa S, Marukawa S, Marukawa K, Kawaguchi Y, Nakato S. Diaphyseal femoral fatigue fracture associated with bisphosphonate therapy - 3 more cases. Acta Orthop. 2011;82(1):112–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sasaki S, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab. 2012;30(5):561–567. [DOI] [PubMed] [Google Scholar]
- 74. Funck-Brentano T, Ostertag A, Debiais F, et al. Identification of a p.Arg708Gln variant in COL1A2 in atypical femoral fractures. Joint Bone Spine. 2017;84(6):715–718. [DOI] [PubMed] [Google Scholar]
- 75. Muschitz C, Thaler HW, Dimai HP, et al. Atypical femoral fractures-ongoing and history of bone-specific therapy, concomitant diseases, medications, and survival. J Clin Densitom. 2016;19(3):359–367. [DOI] [PubMed] [Google Scholar]
- 76. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532–1543. [DOI] [PubMed] [Google Scholar]
- 77. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377(15):1417–1427. [DOI] [PubMed] [Google Scholar]
- 78. Lou S, Lv H, Wang G, et al. The effect of teriparatide on fracture healing of osteoporotic patients: a meta-analysis of randomized controlled trials. Biomed Res Int. 2016;2016:6040379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shi Z, Zhou H, Pan B, et al. Effectiveness of teriparatide on fracture healing: a systematic review and meta-analysis. Plos One. 2016;11(12):e0168691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bhandari M, Jin L, See K, et al. Does teriparatide improve femoral neck fracture healing: results from a randomized placebo-controlled trial. Clin Orthop Relat Res. 2016;474(5):1234–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Almirol EA, Chi LY, Khurana B, et al. Short-term effects of teriparatide versus placebo on bone biomarkers, structure, and fracture healing in women with lower-extremity stress fractures: a pilot study. J Clin Transl Endocrinol. 2016;5:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Effect of teriparatide on fracture healing in patients with incomplete atypical femur fractures. NIH U.S. National Library of Medicine. NCT01896011. updated April 18, 2019 Available from: https://clinicaltrials.gov/ct2/show/NCT01896011. Accessed December 1, 2019. [Google Scholar]
- 83. Black DM, Schwartz AV, Ensrud KE, et al. ; FLEX Research Group . Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. Jama. 2006;296(24):2927–2938. [DOI] [PubMed] [Google Scholar]
- 84. Curtis JR, Chen R, Li Z, et al. The impact of the duration of bisphosphonate drug holidays on hip fracture rates. Ann Rheum Dis. 2018;77:58. [Google Scholar]
- 85. Adams AL, Adams JL, Raebel MA, et al. Bisphosphonate drug holiday and fracture risk: a population-based cohort study. J Bone Miner Res. 2018;33(7):1252–1259. [DOI] [PubMed] [Google Scholar]
- 86. Bissonnette L, April PM, Dumais R, Boire G, Roux S. Atypical fracture of the tibial diaphysis associated with bisphosphonate therapy: a case report. Bone. 2013;56(2):406–409. [DOI] [PubMed] [Google Scholar]
- 87. Erdem Y, Atbasi Z, Emre TY, Kavadar G, Demiralp B. Effect of long-term use of bisphosphonates on forearm bone: atypical ulna fractures in elderly woman with osteoporosis. Case Rep Orthop. 2016;2016:4185202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Breglia MD, Carter JD. Atypical insufficiency fracture of the tibia associated with long-term bisphosphonate therapy. J Clin Rheumatol. 2010;16(2):76–78. [DOI] [PubMed] [Google Scholar]
- 89. Lim SY, Rastalsky N, Choy E, Bolster MB. Tibial stress reaction presenting as bilateral shin pain in a man taking denosumab for giant cell tumor of the bone. Bone. 2015;81:31–35. [DOI] [PubMed] [Google Scholar]
- 90. Osada R, Zukawa M, Kimura T. Atypical ulnar fracture associated with long-term bisphosphonate use. J Orthop Sci. 2015;20(6):1132–1135. [DOI] [PubMed] [Google Scholar]
- 91. Ang BF, Koh JS, Ng AC, Howe TS. Bilateral ulna fractures associated with bisphosphonate therapy. Osteoporos Int. 2013;24(4):1523–1525. [DOI] [PubMed] [Google Scholar]
- 92. Moon J, Bither N, Lee T. Atypical forearm fractures associated with long-term use of bisphosphonate. Arch Orthop Trauma Surg. 2013;133(7):889–892. [DOI] [PubMed] [Google Scholar]
- 93. Imbuldeniya AM, Jiwa N, Murphy JP. Bilateral atypical insufficiency fractures of the proximal tibia and a unilateral distal femoral fracture associated with long-term intravenous bisphosphonate therapy: a case report. J Med Case Rep. 2012;6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1595–1622. [DOI] [PubMed] [Google Scholar]
- 95. Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31(10):1910. [DOI] [PubMed] [Google Scholar]
- 96. López-Delgado L, Riancho-Zarrabeitia L, García-Unzueta MT, et al. Abnormal bone turnover in individuals with low serum alkaline phosphatase. Osteoporos Int. 2018;29(9):2147–2150. [DOI] [PubMed] [Google Scholar]
- 97. Miller PD, McCarthy EF. Bisphosphonate-associated atypical sub-trochanteric femur fractures: paired bone biopsy quantitative histomorphometry before and after teriparatide administration. Semin Arthritis Rheum. 2015;44(5):477–482. [DOI] [PubMed] [Google Scholar]
- 98. Tsourdi E, Langdahl B, Cohen-Solal M, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11–17. [DOI] [PubMed] [Google Scholar]
- 99. Leder BZ, Tsai JN, Uihlein AV, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet. 2015;386(9999):1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Adami S, San Martin J, Muñoz-Torres M, et al. Effect of raloxifene after recombinant teriparatide [hPTH(1-34)] treatment in postmenopausal women with osteoporosis. Osteoporos Int. 2008;19(1):87–94. [DOI] [PubMed] [Google Scholar]
- 101. Eastell R, Nickelsen T, Marin F, et al. Sequential treatment of severe postmenopausal osteoporosis after teriparatide: final results of the randomized, controlled European Study of Forsteo (EUROFORS). J Bone Miner Res. 2009;24(4):726–736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.