Abstract
Acute disseminated encephalomyelitis (ADEM) is an autoimmune demyelinating disease of central nervous system (CNS). ADEM is most commonly seen in children, although adults can also be affected. The disease typically starts with an abrupt onset within day to weeks after a viral infection or immunization. Presenting features include an acute encephalopathy with multifocal neurologic signs and fever. ADEM generally has a monophasic course, although recurrent ADEM has also been described and is defined as multiphasic ADEM. MRI shows widespread lesions located in both brain and spinal cord. An involvement of basal ganglia and thalami has also been described. Analysis of cerebrospinal fluid (CSF) may reveal mild lymphocytic pleocytosis and increased proteins, whereas oligoclonal bands are usually negative. In the absence of specific biologic markers, ADEM remains a diagnosis of exclusion and it is still based on clinical manifestations, imaging, and laboratory features. Therapy is based on steroid administration and the prognosis is usually favorable.
Introduction
This chapter reviews the current knowledge on epidemiology, etiopathogenesis, clinical features, diagnostic procedures, and treatment approaches of acute disseminated encephalomyelitis (ADEM). ADEM is considered a rare autoimmune demyelinating disorder of the central nervous system (CNS), traditionally characterized by a monophasic course. The term ADEM encompasses postinfectious, postvaccination, and idiopathic encephalomyelitis [1]. The majority of subjects report recent infections (generally nonspecific upper respiratory tract and flu-like syndromes) or active immunization several weeks before disease onset. Typically, ADEM manifests an acute onset, with nonspecific symptoms, such as headache, fever, nausea, and vomiting with or without altered mental status. In some cases, the presentation may be severe and dramatic, requiring intensive care and mechanical ventilation. Despite the clinical severity, the long-term prognosis is generally favorable and with complete recovery in the majority of patients.
Epidemiology
The average annual incidence of ADEM, depending on population-based studies, is included between 0.07 and 0.6 per 100,000 individuals per year [2–4].
Geographic Distribution
Population-based studies of pediatric patients with ADEM performed in the United States and in the United Kingdom revealed a slightly lower incidence compared to Asian Countries, in particular: the incidence of pediatric ADEM was estimated between 0.47/100,000 and 0.64/100,000 children in Asian countries [2, 4], whereas in Europe and San Diego the annual incidence ranged between 0.07/100,000 and 0.30/100,000 [3, 5, 6]. In addition, similarly to multiple sclerosis (MS), ADEM incidence seems to be influenced by latitude, as in the United States, where it is higher in the North than in the South, increasing with the distance from the equator [7]. Epidemiological data of adult ADEM have been reported only in a few studies, due to the variability of age at presentation.
Demographic Features
Although ADEM may occur at any age, it is more frequent during childhood. In child population the median age of onset ranges between 5 and 8 years, with a slight male predominance (male to female ratio 1.8:1) [8]. In adult cases, the age of presentation is between 33 and 41 years, without gender predominance. Despite the difference of incidence among various populations, no specific ethnic distribution has been described.
Risk Factors
In 50–85% of ADEM cases, disease onset is preceded by a viral/bacterial infection or a vaccination [5, 8, 9]. The pathogens causing the infection associated with ADEM are mostly unknown [10]. Prodromal manifestations include a flu-like syndrome (56–61%) and nonspecific upper respiratory or gastrointestinal manifestations (7–17%). In pediatric ADEM, the onset is frequently preceded by exanthematous diseases [11]. Common viruses (Epstein-Barr, measles, mumps, rubella, and coxsackie B) are the most common pathogens associated with postinfectious ADEM [11]. Bacterial infections, such as Borrelia burgdorferi, Mycoplasma pneumoniae, and Legionella pneumophila, are rarely reported [12, 13]. The list of pathogens involved in ADEM is reported in Table 5.1 [14]. The time interval between prodromal manifestations and ADEM onset varies from 4 to 42 days [11]. The disease has a seasonal peak in winter and spring, according to its relative infectious etiologies.
Table 5.1.
Causes of postinfectious and postvaccinal acute disseminated encephalomyelitis
| Causes of ADEM |
| Viral infections |
| Measles |
| Varicella zoster |
| Rubella |
| Mumps |
| Influenza A and B |
| Hepatitis A |
| Hepatitis C |
| Epstein-Barr virus |
| HIV |
| Nonspecific upper respiratory tract infections |
| Human herpesvirus 6a |
| Herpes simplex virusa |
| Dengue virusa |
| Coxsackie Ba |
| Coronavirusa |
| Nonviral infections |
| Group A b-hemolytic streptococci |
| Legionella pneumophila |
| Salmonella typhi |
| Leptospirosis |
| Plasmodium falciparum |
| Mycoplasma pneumoniae |
| Rickettsia rickettsii |
| Borrelia burgdorferi |
| Postvaccinal ADEM |
| Rabies vaccine made in brain or spinal cord preparations |
| Measles |
| Japanese encephalitis virus |
| Oral poliovirus |
| Tetanus toxoid |
| Influenza |
| Hepatitis B recombinant vaccine |
| Tick-borne encephalitisa |
The risk of developing post-immunization ADEM is significantly lower than the risk of the postinfectious one, as less than 5% of ADEM cases follow immunization [13]. Currently, vaccinations most often associated with postvaccination ADEM are measles, mumps, and rubella. The mean latency between immunization and the ADEM onset ranges from 2 to 30 days [15].
In the literature, only a few studies on genetic susceptibility in ADEM patients are reported. One study has recently demonstrated an association between ADEM and HLA-DR genes, suggesting that ADEM patients may have a genetic predisposition [15]. Association with other systemic diseases is still unknown. However, a tendency to develop encephalopathy with white matter (WM) lesions has been reported in patients with congenital adrenal hyperplasia or acquired adrenal insufficiency [15].
Etiopathogenesis
The etiopathogenesis of ADEM is not fully understood. However, the prevailing theory suggests that it is an autoimmune disorder of the CNS, triggered by an environmental stimulus in genetically susceptible individuals. Molecular sequencing has revealed that viral or bacterial microorganisms may share antigenic determinants with myelin autoantigens such as myelin basic protein (MBP), myelin-associated oligodendrocyte basic protein (MOBP), oligodendrocyte-specific protein (OSP), myelin oligodendrocyte glycoprotein (MOG), and proteolipid protein (PLP). The hypothesis of molecular mimicry is supported by studies showing the presence of anti-MOG antibodies in the serum and CSF during the acute phase of the disease and their progressive decline with disease resolution. However, the pathogenetic role of these antibodies is still debated. Moreover, also IgG autoantibodies targeting epitopes derived from MBP, PLP, and MOBP, have been described.
These short stretches of homologies have the capacity to activate T-cell clones via T-cell molecular mimicry, provoking a CNS autoimmune T-cell-mediated response and inflammation with subsequent demyelination [16, 17]. In addition to this mechanism, the infection may lead to a nonspecific self-sensitization of T cells against myelin autoantigens, by activation of costimulatory pathways and cytokine release [18]. This phenomenon, called “bystander activation,” may provoke the breakdown of T-cell tolerance. Hence, both cell-mediated and humoral effector mechanisms may play a role in the immune-mediated damage to the CNS in ADEM.
Pathologically, the hallmarks of ADEM include perivenular sleeves of demyelination, edema and perivenous inflammation with foamy macrophages containing myelin products, but also T and B lymphocytes, neutrophils, plasma cells, microglial cells, and eosinophilic granulocytes may be present [13, 19]. Sometimes these perivenous lesions may coalesce, forming confluent areas. Typically, axons in the demyelinating lesions are relatively spared, but, in fatal cases of ADEM, extensive axonal damage has been observed. Axonal damage is demonstrated by an increased level of phosphorylated microtubule-associated protein (TAU), which is primarily located in neuronal axons, in the cerebrospinal fluid (CSF). Atypical findings include glial nodules in the gray matter (GM), perivascular necrosis, infiltration of the meninges and vasculitic-like lesions [20].
Clinical Manifestations
Classic ADEM is a monophasic disease following a preceding illness, or, less often, a vaccination. Latency period ranges from several days to few months and typical presentation includes the acute onset of multifocal neurologic symptoms, often with rapid deterioration of the state of consciousness.
Typical Features
Prodromal symptoms: are similar in adults and children and include headache, malaise, irritability, fever, nausea, and vomiting. Generally, neurological symptoms occur within 2–5 days from these manifestations [19].
Altered mental status: encephalopathy comprises changes in behavior and/or consciousness, ranging in severity from lethargy to coma. Impairment of consciousness is present in 46–73% of pediatric patients and in 20–56% of adult cases [21] and may require hospitalization in intensive care (43%) or use of mechanical ventilation (16%) [8]. In children, the presence of encephalopathy is included as a diagnostic criterion of ADEM, and helps to distinguish ADEM from MS [22]. Patients with GM involvement may experience more profound states of encephalopathy, as the cerebral cortex is responsible for higher levels of sensory processing. Some patients may also show irritability, confusion, and psychosis.
Focal neurological symptoms: patients may present different symptoms, depending on the area of the brain involved. A damage in the contest of occipital lobes may result in homonymous visual field defects and, if severe and bilateral, also cortical blindness. Patients may also show higher cortical function deficits, such as agraphia, alexia, aphasia, and acalculia. In the case of involvement of the motor cortex, patients may have pyramidal signs (i.e., Babinski sign, spastic hypertonus, and hyperreflexia). Sensory symptoms include astereognosis and agraphesthesia, or loss of proprioception, as well as reduction of pain and temperature perception. Patients with brainstem involvement may present palsy of the cranial nerves. The most common symptoms are: diplopia (both cranial nerve palsies and/or inflammation of the gaze-control centers), dysphagia, dysarthria, vertigo, nystagmus, hearing loss, reduced taste and smell sensitivity, and respiratory failure [19]. Brainstem involvement is usually associated with poorer prognosis and with a higher risk of fulminant disease course [23].
Meningism: is present in 26–31% of cases and is caused by the infiltration of the meninges by lymphocytes.
Atypical Symptoms
Persistent headache, stroke-like events, recurrent seizures (predominant in pediatric cases), dystonia or parkinsonism, neuropsychiatric symptoms and progressive onset are atypical findings in ADEM and, when present, other conditions should be carefully excluded [19].
Natural History of ADEM
The classic form of ADEM, which accounts for the 70–90% of all cases, is characterized by a monophasic disease course with an excellent functional prognosis (90% of cases) and low mortality risk (≤5%) [5, 8]. However, variable proportions of patients (10–30% of all cases) with a multiphasic course (M-ADEM) have been described in the literature [11]. The time interval from onset to first relapse is quite variable, usually ranging from 2 months to 8 years [8]. Recurrence after three decades has been described in a single case [24]. Prognostic risk factors for a relapse include the coexistence of an optic neuritis, the presence of criteria for MS at magnetic resonance imaging (MRI) and a family history of CNS disorders [25]. In most of M-ADEM patients, long-term clinical and imaging follow-up has shown the resolution of lesions with no long-lasting neurologic impairments [22]. Patients with the coexistence of M-ADEM and anti-MOG antibodies typically do not develop new lesions on MRI during the asymptomatic period [26].
Variants
Acute hemorrhagic leukoencephalitis (AHLE): AHLE is considered an extremely severe, hyperacute, variant of ADEM and has been reported in 2% of pediatric cases [8]. Typically, it presents with meningism, headache, seizures, multifocal neurologic signs, asymmetrical neurologic deficits, and rapid progression to coma [27]. Prodromal symptoms, including fever, malaise, and myalgia, are more common than in classic ADEM and are followed by rapidly progressive hemorrhagic demyelination of the WM.
Histopathologically, the hallmarks of AHLE include edema, vessel fibrinoid necrosis, hemorrhages, perivascular exudation, and granulocyte infiltration, with perivascular demyelination and reactive astrocytosis. Perivascular astrocytes demonstrate dystrophic and swollen processes, suggesting astrocytic damage, which may appear at early stages of the disease [19]. On MRI, AHLE lesions tend to be larger than classic ADEM, are surrounded by conspicuous edema and are frequently associated with hemorrhagic foci (Fig. 5.1). Although cases of recovery after high-dose steroid therapy or neurosurgical decompression have been reported, most patients die due to massive brain edema and intracranial hypertension within the first weeks after the onset of neurologic symptoms [28].
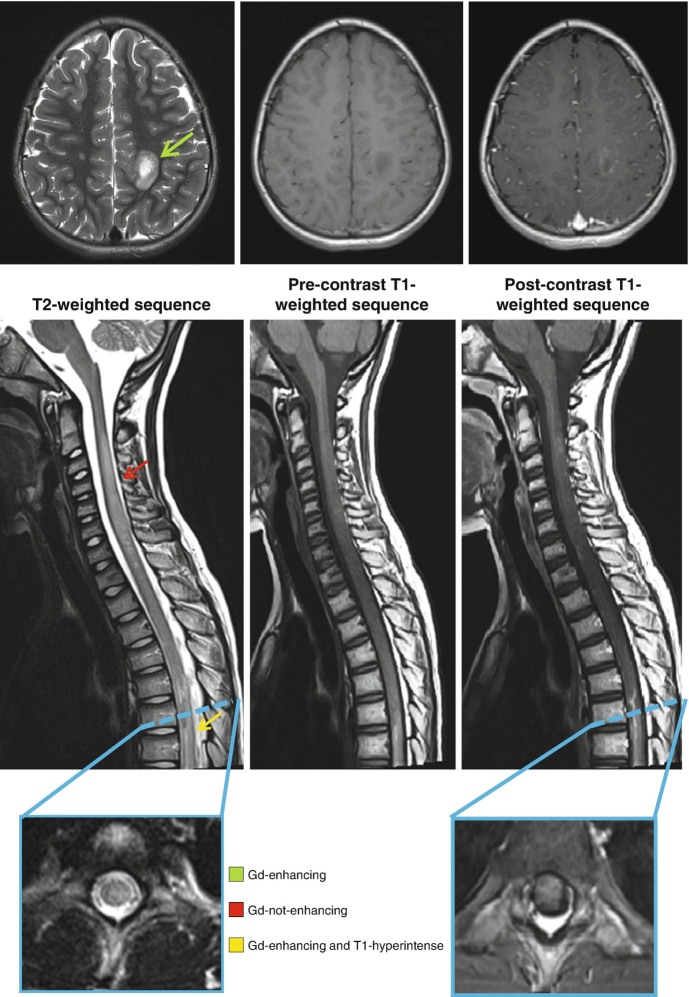
Fig. 5.1.
MRI acquired from a 10-year-old boy affected by acute hemorrhagic leukoencephalitis (AHLE) with major expression at spinal cord level. At the level of the brain (green arrow), a T2-hyperintense, T1-hypointense and gadolinium-enhancing lesion is shown. At the level of the spinal cord, a cervical (red arrow) T2-hyperintense, T1-hypointense and gadolinuim not-enhancing lesion and a thoracic (yellow arrow), T2-hyperitense, T1-hyperintense and gadolinium-enhancing lesion are visible. The spontaneous T1-hyperintensity suggests the presence of deposits of hemosiderin, typical of the hemorrhagic phenotypes of ADEM. On the axial plane (blue boxes), lesions are centrally located and involve more than half of the cross-sectional area of the spinal cord
Recurrent optic neuritis (ADEM-ON): unilateral or bilateral optic neuritis (ON), with retro-orbital pain exacerbated with eye movement, dyschromatopsia, and subacute visual loss can occur. When recurrent ON is present in the contest of ADEM, the disease is called ADEM-ON [29]. ADEM-ON is rare, the incidence is unknown, and the data about this entity are insufficient. Further studies are needed to allow recommendations on treatment or prognosis.
Acute transverse myelitis (ATM): ATM is a common manifestation of postinfectious or postvaccinal ADEM (indeed in 30–60% of idiopathic cases there is a history of preceding respiratory, gastrointestinal, or systemic illness). Like cerebral ADEM, ATM has also been reported following measles, rubella, influenza, and hepatitis-B vaccination. However, the trigger event leading to such an autoimmune response against the spinal cord is still unclear [30]. In one-third of patients the onset of myelitis is preceded by pain located in the distribution area of the involved segments of the spinal cord. During the acute phase, about half patients with ATM are paraplegic. Other common symptoms are paresthesia, dysesthesia, numbness, impaired breathing, bowel and bladder dysfunction, paralysis, muscle weakness, or nerve pain. Initial severity of weakness and evidence of denervation on electromyography have been considered poor prognostic indicators for subsequent recovery [31]. Disability in these patients is mainly related to motor impairment and to a lesser extent to sensory symptoms. Approximately one-third of patients with ATM recover completely, one-third show partial recovery with moderate disabilities, and the rest experience permanent severe disabilities.
Combined central and peripheral involvement of the nervous system: patients with postinfectious neurologic syndromes, like ADEM, may have clinical and/or electrodiagnostic evidence of peripheral nervous system involvement [32]. Clinical symptoms of peripheral nerve injury include distal limb paresthesia, perineal anesthesia, and muscle atrophy. Patients with peripheral nerve involvement are generally significantly older, have a worse prognosis, and a higher risk of relapses [33, 34].
Diagnostic Criteria
In 2007, the International Pediatric Multiple Sclerosis Study Group (IPMSSG) proposed diagnostic criteria for pediatric acquired demyelinating disorders of the CNS, including ADEM. In 2013, the original definitions were updated, but ADEM remains a diagnosis of exclusion.
All the following criteria are required for a diagnosis of ADEM [22], once other possible neurological conditions have been reasonably excluded:
a first multifocal clinical CNS event of presumed inflammatory demyelinating cause;
encephalopathy: defined as stupor and/or lethargy or behavioral changes unexplained by fever, systemic illness, or postictal symptoms;
brain MRI is abnormal during the acute (3 months) phase; and
no new clinical or MRI findings 3 months or more after the clinical onset.
Typical brain MRI findings include the presence of diffuse, poorly demarcated, and large (>1–2 cm) T2-hyperintense lesions involving predominantly the brain WM. Deep GM lesions (e.g., thalamus or basal ganglia) can be present. T1-hypointense lesions in the WM are rare.
In addition to these criteria, the IPMSSG also specified that clinical symptoms and radiologic findings of ADEM can fluctuate in severity and evolve in the first 3 months after the clinical onset. A second event is defined as the development of new symptoms at least 3 months after the start of incident illness. However, data supporting the biological rationale for the 3-month requirement are still needed [22].
Diagnostic Procedures
ADEM diagnosis is challenging, as no specific biomarkers are available and clinical manifestations are heterogeneous. No definitive laboratory biomarkers or tests other than postmortem investigation allow a definitive diagnosis of ADEM to be formulated, but various tests may offer supportive evidence.
Detailed clinical history and neurologic examination, which often reveals multifocal symptoms, are the first step of evaluation. The presence of encephalopathy is supportive of the diagnosis. In patients with clinical manifestations highly suspicious of ADEM, physicians should request: complete blood count, c-reactive protein, erythrocyte sedimentation rate, anti-nuclear antibodies, serology for herpes simplex virus (HSV), enterovirus, Epstein-Barr virus (EBV), mycoplasma, anti-Acquaporin4 (AQP4), and anti- MOG antibodies [19].
MRI
MRI is the best method to evaluate a patient with a suspicion of ADEM. MRI of the brain with gadolinium administration is indicated when the patient is stabilized. MRI of the cervical and dorsal spinal cord may also be performed to confirm the extent of inflammation and when there are symptoms or signs suggestive of myelopathy [35].
In patients with ADEM at presentation, MRI findings may be similar to MS, but several findings are helpful in their differentiation.
Lesion features: T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences typically demonstrate multifocal hyperintense lesions in the brain, which vary from small round/ovoid foci to flocculent “cotton ball” lesions with very hyperintense areas and “fuzzy” margins [36]. WM involvement is typically bilateral and asymmetric. ADEM lesions can involve both the WM and GM, affecting the subcortical and central WM, cortical GM-WM junction and deep GM of brainstem, cerebellum thalami, and basal ganglia (Fig. 5.2) [37].
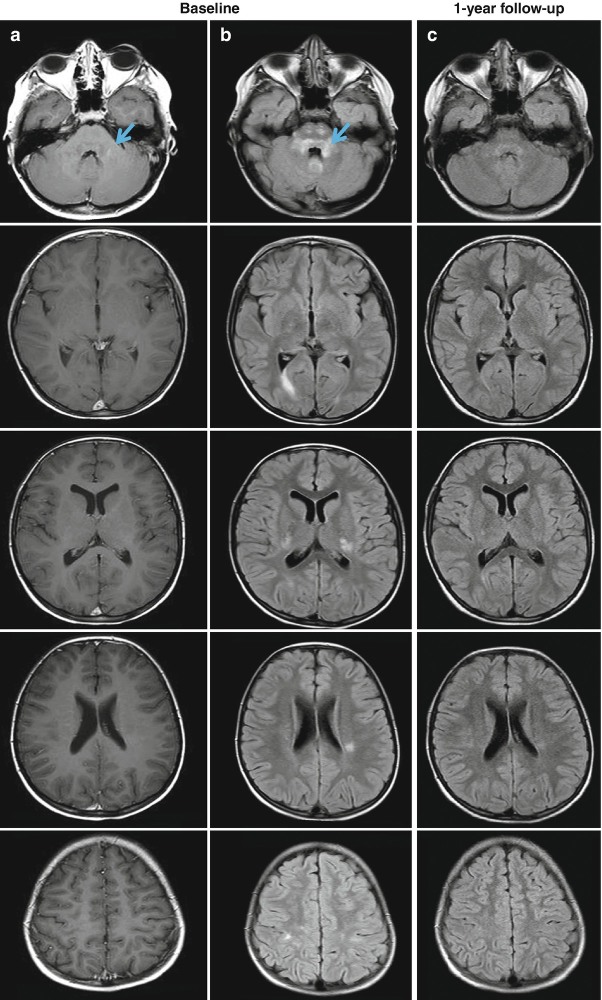
Fig. 5.2.
Brain axial post-contrast T1-weighted spin-echo (a) and fluid-attenuated inversion recovery (FLAIR) magnetic resonance images (b) from an 8-year-old patient with acute disseminated encephalomyelitis (ADEM). In (b), multiple hyperintense lesions involving both the white and gray matter are visible, whereas only one infratentorial lesion shows a mild enhancement (a, blue arrow). The one-year follow-up of the patient (c) shows a significant reduction of the size of hyperintense lesions on the FLAIR scan, without the formation of any new lesions
In patients with ADEM, pre-contrast T1-weighted sequences are usually inconspicuous, unless lesions are large, in which case a weak hypointensity has been reported [38].
Post-contrast T1-weighted sequences demonstrate enhancing lesions in 30–100% of patients [39] with a quite variable pattern of enhancement (incomplete ring or arch enhancement along the edge of inflammation, nodular, gyral, or spotty enhancement).
Tumefactive lesions with horseshoe-shaped enhancement have also been described. Cranial-nerve enhancement is relatively common. Of note, the absence of enhancement does not exclude the diagnosis of ADEM [8].
Diffusion-weighted imaging (DWI) can show a restricted diffusion peripherally, while the center of lesions does not present this abnormality.
In the spinal cord, confluent lesions or lesions extending over multiple segments, associated with cord swelling, have been described in up to one-third of patients.
MRI patterns: four radiologic patterns have been described in ADEM: (1) ADEM with small lesions (<5 mm), (2) ADEM with large confluent WM asymmetric lesions, (3) ADEM with symmetric bithalamic involvement, and (4) ADEM with acute hemorrhagic encephalomyelitis [39].
Lesion evolution: to rule out ongoing disease activity indicating a diagnosis other than ADEM, it has been suggested to reassess patients with at least two additional MRI scans (respectively 3 months and 9/12 months after the clinical onset), as monophasic ADEM is not associated with development of new lesions after 3 months from disease onset. In addition, partial or rarely complete resolution of MRI abnormalities has been described in the majority of patients. Lastly, persistent hypointense lesions in the WM are infrequent in monophasic ADEM and may suggest an alternative diagnosis, such as MS [22].
CSF Examination
Clinical presentation of ADEM may mimic acute encephalitis and CSF examination should be performed to exclude alternative diagnoses. CSF examination should include: biochemistry (in particular protein, glucose, and cell count), gram staining, cytology, culture, and sensitivity for bacteria and virology studies. Polymerase chain reaction (PCR) tests to rule out enteroviruses, HSV and EBV infections and electrophoresis with detection of oligoclonal bands (OCB) and Link index should be performed.
CSF examination may show inflammatory indices, as proteins are usually increased up to 1.1 g/L (range 15–60% of patients) and mild pleocytosis (usually with high percentage of lymphocytes and monocytes) can be seen in 25–65% of patients [5, 40]. In 42–72% of pediatric patients with ADEM, CSF leukocyte count is normal [41].
Intrathecal OCBs are rare in ADEM patients and, when detected, they tend to manifest as mirror pattern in both serum and CSF and would therefore not be considered as true OCBs, but they suggest that the antibody production is not intrathecally restricted [42].
Laboratory Findings
In recent years, different antibodies have been described in patients with ADEM. In particular, anti-MOG antibodies have been recently described, with high titer in the acute phase that tend to decrease and eventually disappear over time [43, 44]. However, their clinical meaning and role in ADEM remains to be clarified. Other IgG autoantibodies targeting myelin autoantigens such as MBP, alpha-B crystalline, and PLP, have been found in several ADEM patients; these may help distinguish ADEM from MS [45]. The presence of serum anti-AQP4-IgG antibodies rules out a diagnosis of ADEM.
Differential Diagnosis
The first priority is to exclude other causes of acquired CNS demyelinating syndromes. The most important differential diagnosis in pediatric ADEM is pediatric MS and this distinction has prognostic and therapeutic implications. The differential diagnosis between ADEM and MS is based on clinical, laboratory, and MRI features:
Clinical manifestations: in most cases of pediatric ADEM, patients present systemic symptoms such as headache, fever, nausea, vomiting, and altered mental status, which are typically uncommon in pediatric MS;
Laboratory findings: the presence of OCBs is a hallmark of MS, and is atypical in ADEM. In challenging cases, anti-MOG antibodies detection may help the differential diagnosis: these antibodies are not generally associated with MS but suggest anti-MOG associated autoimmune demyelination. In patients with ADEM-ON, quantification of anti-AQP4 antibodies should be performed to make the correct diagnosis;
MRI features may help distinguish ADEM from MS [46]. Key brain MRI features found in ADEM and absent in MS include periventricular sparing and the absence of periventricular ovoid lesions perpendicular to the ventricular edge (Dawson fingers) [35, 47, 48]. The discriminatory MRI elements between ADEM and MS are reported in Table 5.2 [19]. According to the current diagnostic criteria, no new clinical or MRI findings must be found 3 months or more after the clinical onset. If radiological evidence of new lesions is present, it is necessary to consider alternative diagnoses, such as MS or neuromyelitis optica spectrum disorders [22].
Table 5.2.
MRI features of ADEM and MS
| MRI features | ADEM: typical | MS: typical |
|---|---|---|
| Deep gray matter/cortical involvement | Yes | No |
| Bilateral diffuse lesions | Yes | No |
| Poorly marginated lesions | Yes | No |
| Large globular lesions | Yes | No |
| Periventricular pattern of lesions | No | Yes |
| Lesions perpendicular to long axis of corpus callosum | No | Yes |
| Ovoid lesions | No | Yes |
| Lesions confined to corpus callosum | No | Yes |
| Sole presence of well-defined lesions | No | Yes |
| Black holes (T1-weighted sequences) | No | Yes |
MRI magnetic resonance imaging, ADEM acute disseminated encephalomyelitis, MS multiple sclerosis
From [19] with permission
Treatment Approaches
ADEM is usually a self-limiting condition that improves spontaneously, making the value of any therapeutic intervention difficult to establish. For this reason, no double-blind placebo-controlled clinical trial on large-scale cohorts exists. As a consequence, in clinical practice, the management of ADEM is based on observational studies.
General Treatment
The maintenance of airway, breathing, and cardiovascular function is essential. Measures to prevent venous thromboembolism are also required in bed-bound patients.
Medical Therapy
High-doses of intravenous (IV) corticosteroids are currently widely accepted as first-line therapy [49]. Adrenocorticotropic hormone and prednisone have been successfully used in the past. Nowadays, recommended treatment consists in IV methylprednisolone at the dose of 20.30 mg/kg/day (up to a maximum of 1000 mg/day) for 5 days [19], followed by an oral tapering of prednisone over 4–6 weeks with a starting dose of 1–2 mg/kg/day [27]. After this treatment, full recovery has been reported in 60–85% of cases [8, 29]. Shorter tapering might increase the risk of relapses. In the first days of disease, IV methylprednisolone might be associated with IV acyclovir, if there is still a suspicion of acute viral encephalitis.
IV immunoglobulin (IVIG) treatment at the dosage of 2 g/kg administered over 2–5 days is considered as a second-line treatment and may be helpful in steroid-unresponsive ADEM and in patients who have contraindications to corticosteroids [49]. The treatment with IVIG resulted successful in 40–50% of steroid-resistant patients [50]. The mechanism of action of IVIG is not well known, it might have immunomodulatory effects through binding to pathogenic antibodies and inhibiting the activation of myelin-reactive T cells.
Plasma exchange is recommended for therapy-refractory patients with fulminant disease and should be performed immediately after first-line treatment failure, with an estimated efficacy in 40% of cases [51].
Several case reports showing the use of hypothermia or decompressive craniotomy for treatment of fulminant ADEM have been described [52]. In AHLE, in addition to the treatments already described, cyclophosphamide has been used in unresponsive patients [53].
Outcomes
About 65–85% of pediatric ADEM patients typically have a favorable prognosis with a good functional recovery. The improvement of clinical conditions is usually seen within days after treatment start, with a complete recovery in most of cases within few weeks [19]. Residual severe disability is quite rare (7% of pediatric ADEM) [54]. In pediatric series, some patients (20–30% of cases) show residual neurologic deficits, in particular cognitive impairment (especially in the attentive and executive domains) [11] or changes in behavior and personality. These findings are more common in children with diagnosis of ADEM before 5 years of age.
Adult ADEM patients frequently suffer residual focal motor deficits (such as clumsiness, ataxia, and hemiparesis) or epilepsy [8]. In addition, compared to pediatric patients, adults with ADEM have an increased rate of hospitalization, intensive care unit admission, and mortality.
References
- 1.Johnson RT. The pathogenesis of acute viral encephalitis and postinfectious encephalomyelitis. J Infect Dis. 1987;155(3):359–364. doi: 10.1093/infdis/155.3.359. [DOI] [PubMed] [Google Scholar]
- 2.Torisu H, Kira R, Ishizaki Y, Sanefuji M, Yamaguchi Y, Yasumoto S, et al. Clinical study of childhood acute disseminated encephalomyelitis, multiple sclerosis, and acute transverse myelitis in Fukuoka Prefecture, Japan. Brain Dev. 2010;32(6):454–462. doi: 10.1016/j.braindev.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Pohl D, Hennemuth I, von Kries R, Hanefeld F. Paediatric multiple sclerosis and acute disseminated encephalomyelitis in Germany: results of a nationwide survey. Eur J Pediatr. 2007;166(5):405–412. doi: 10.1007/s00431-006-0249-2. [DOI] [PubMed] [Google Scholar]
- 4.Xiong CH, Yan Y, Liao Z, Peng SH, Wen HR, Zhang YX, et al. Epidemiological characteristics of acute disseminated encephalomyelitis in Nanchang, China: a retrospective study. BMC Public Health. 2014;14:111. doi: 10.1186/1471-2458-14-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leake JA, Albani S, Kao AS, Senac MO, Billman GF, Nespeca MP, et al. Acute disseminated encephalomyelitis in childhood: epidemiologic, clinical and laboratory features. Pediatr Infect Dis J. 2004;23(8):756–764. doi: 10.1097/01.inf.0000133048.75452.dd. [DOI] [PubMed] [Google Scholar]
- 6.Gudbjornsson BT, Haraldsson A, Einarsdottir H, Thorarensen O. Nationwide incidence of acquired central nervous system demyelination in Icelandic children. Pediatr Neurol. 2015;53(6):503–507. doi: 10.1016/j.pediatrneurol.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Pellegrino P, Radice S, Clementi E. Geoepidemiology of acute disseminated encephalomyelitis. Epidemiology. 2014;25(6):928–929. doi: 10.1097/EDE.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 8.Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59(8):1224–1231. doi: 10.1212/WNL.59.8.1224. [DOI] [PubMed] [Google Scholar]
- 9.Panicker JN, Nagaraja D, Kovoor JM, Subbakrishna DK. Descriptive study of acute disseminated encephalomyelitis and evaluation of functional outcome predictors. J Postgrad Med. 2010;56(1):12–16. doi: 10.4103/0022-3859.62425. [DOI] [PubMed] [Google Scholar]
- 10.Hung KL, Liao HT, Tsai ML. The spectrum of postinfectious encephalomyelitis. Brain Dev. 2001;23(1):42–45. doi: 10.1016/S0387-7604(00)00197-2. [DOI] [PubMed] [Google Scholar]
- 11.Berzero G, Cortese A, Ravaglia S, Marchioni E. Diagnosis and therapy of acute disseminated encephalomyelitis and its variants. Expert Rev Neurother. 2016;16(1):83–101. doi: 10.1586/14737175.2015.1126510. [DOI] [PubMed] [Google Scholar]
- 12.Menge T, Hemmer B, Nessler S, Wiendl H, Neuhaus O, Hartung HP, et al. Acute disseminated encephalomyelitis: an update. Arch Neurol. 2005;62(11):1673–1680. doi: 10.1001/archneur.62.11.1673. [DOI] [PubMed] [Google Scholar]
- 13.Esposito S, Di Pietro GM, Madini B, Mastrolia MV, Rigante D. A spectrum of inflammation and demyelination in acute disseminated encephalomyelitis (ADEM) of children. Autoimmun Rev. 2015;14(10):923–929. doi: 10.1016/j.autrev.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noorbakhsh F, Johnson RT, Emery D, Power C. Acute disseminated encephalomyelitis: clinical and pathogenesis features. Neurol Clin. 2008;26(3):759–780. doi: 10.1016/j.ncl.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamanouchi H, Moshé SL, Okumura A. Acute encephalopathy and encephalitis in infancy and its related disorders. St. Louis: Elsevier; 2018. [Google Scholar]
- 16.Schirmer L, Srivastava R, Hemmer B. To look for a needle in a haystack: the search for autoantibodies in multiple sclerosis. Mult Scler. 2014;20(3):271–279. doi: 10.1177/1352458514522104. [DOI] [PubMed] [Google Scholar]
- 17.Chastain EM, Miller SD. Molecular mimicry as an inducing trigger for CNS autoimmune demyelinating disease. Immunol Rev. 2012;245(1):227–238. doi: 10.1111/j.1600-065X.2011.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kothur K, Wienholt L, Mohammad SS, Tantsis EM, Pillai S, Britton PN, et al. Utility of CSF cytokine/chemokines as markers of active intrathecal inflammation: comparison of demyelinating, anti-NMDAR and enteroviral encephalitis. PLoS One. 2016;11(8):e0161656. doi: 10.1371/journal.pone.0161656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pohl D, Alper G, Van Haren K, Kornberg AJ, Lucchinetti CF, Tenembaum S, et al. Acute disseminated encephalomyelitis: updates on an inflammatory CNS syndrome. Neurology. 2016;87(9 Suppl 2):S38–S45. doi: 10.1212/WNL.0000000000002825. [DOI] [PubMed] [Google Scholar]
- 20.Young NP, Weinshenker BG, Parisi JE, Scheithauer B, Giannini C, Roemer SF, et al. Perivenous demyelination: association with clinically defined acute disseminated encephalomyelitis and comparison with pathologically confirmed multiple sclerosis. Brain. 2010;133(Pt 2):333–348. doi: 10.1093/brain/awp321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marchioni E, Ravaglia S, Montomoli C, Tavazzi E, Minoli L, Baldanti F, et al. Postinfectious neurologic syndromes: a prospective cohort study. Neurology. 2013;80(10):882–889. doi: 10.1212/WNL.0b013e3182840b95. [DOI] [PubMed] [Google Scholar]
- 22.Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC, et al. International pediatric multiple sclerosis study group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19(10):1261–1267. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- 23.Tenembaum SN. Disseminated encephalomyelitis in children. Clin Neurol Neurosurg. 2008;110(9):928–938. doi: 10.1016/j.clineuro.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Numa S, Kasai T, Kondo T, Kushimura Y, Kimura A, Takahashi H, et al. An adult case of anti-myelin oligodendrocyte glycoprotein (MOG) antibody-associated multiphasic acute disseminated encephalomyelitis at 33-year intervals. Intern Med. 2016;55(6):699–702. doi: 10.2169/internalmedicine.55.5727. [DOI] [PubMed] [Google Scholar]
- 25.Mikaeloff Y, Caridade G, Husson B, Suissa S, Tardieu M, KSGotFNS N. Acute disseminated encephalomyelitis cohort study: prognostic factors for relapse. Eur J Paediatr Neurol. 2007;11(2):90–95. doi: 10.1016/j.ejpn.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Baumann M, Hennes EM, Schanda K, Karenfort M, Kornek B, Seidl R, et al. Children with multiphasic disseminated encephalomyelitis and antibodies to the myelin oligodendrocyte glycoprotein (MOG): extending the spectrum of MOG antibody positive diseases. Mult Scler. 2016;22(14):1821–1829. doi: 10.1177/1352458516631038. [DOI] [PubMed] [Google Scholar]
- 27.Tenembaum SN. Acute disseminated encephalomyelitis. Handb Clin Neurol. 2013;112:1253–1262. doi: 10.1016/B978-0-444-52910-7.00048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payne ET, Rutka JT, Ho TK, Halliday WC, Banwell BL. Treatment leading to dramatic recovery in acute hemorrhagic leukoencephalitis. J Child Neurol. 2007;22(1):109–113. doi: 10.1177/0883073807299971. [DOI] [PubMed] [Google Scholar]
- 29.Dale RC, de Sousa C, Chong WK, Cox TC, Harding B, Neville BG. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain. 2000;123(Pt 12):2407–2422. doi: 10.1093/brain/123.12.2407. [DOI] [PubMed] [Google Scholar]
- 30.Wingerchuk DM, Weinshenker BG. Acute disseminated encephalomyelitis, transverse myelitis, and neuromyelitis optica. Continuum (Minneap Minn) 2013;19(4 Multiple Sclerosis):944–967. doi: 10.1212/01.CON.0000433289.38339.a2. [DOI] [PubMed] [Google Scholar]
- 31.DeSena A, Graves D, Morriss MC, Greenberg BM. Transverse myelitis plus syndrome and acute disseminated encephalomyelitis plus syndrome: a case series of 5 children. JAMA Neurol. 2014;71(5):624–629. doi: 10.1001/jamaneurol.2013.5323. [DOI] [PubMed] [Google Scholar]
- 32.Adamovic T, Riou EM, Bernard G, Vanasse M, Decarie JC, Poulin C, et al. Acute combined central and peripheral nervous system demyelination in children. Pediatr Neurol. 2008;39(5):307–316. doi: 10.1016/j.pediatrneurol.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Ravaglia S, Tavazzi E, Moglia A, Ceroni M, Marchioni E. Combined central and peripheral demyelination: comparison of adult and pediatric series. Pediatr Neurol. 2009;41(1):77. doi: 10.1016/j.pediatrneurol.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Wassmer E, Whitehouse WP. Simultaneous peripheral and central demyelination. J Child Neurol. 2008;23(12):1495. doi: 10.1177/0883073808322328. [DOI] [PubMed] [Google Scholar]
- 35.Callen DJ, Shroff MM, Branson HM, Li DK, Lotze T, Stephens D, et al. Role of MRI in the differentiation of ADEM from MS in children. Neurology. 2009;72(11):968–973. doi: 10.1212/01.wnl.0000338630.20412.45. [DOI] [PubMed] [Google Scholar]
- 36.Osborn AG. Osborn’s brain: imaging, pathology, and anatomy. 1. Salt Lake City: Amirsys; 2013. [Google Scholar]
- 37.Atzori M, Battistella PA, Perini P, Calabrese M, Fontanin M, Laverda AM, et al. Clinical and diagnostic aspects of multiple sclerosis and acute monophasic encephalomyelitis in pediatric patients: a single centre prospective study. Mult Scler. 2009;15(3):363–370. doi: 10.1177/1352458508098562. [DOI] [PubMed] [Google Scholar]
- 38.Rossi A. Imaging of acute disseminated encephalomyelitis. Neuroimaging Clin N Am. 2008;18(1):149–161. doi: 10.1016/j.nic.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Tenembaum S, Chitnis T, Ness J, Hahn JS, International Pediatric MSSG Acute disseminated encephalomyelitis. Neurology. 2007;68(16 Suppl 2):S23–S36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 40.Hung PC, Wang HS, Chou ML, Lin KL, Hsieh MY, Wong AM. Acute disseminated encephalomyelitis in children: a single institution experience of 28 patients. Neuropediatrics. 2012;43(2):64–71. doi: 10.1055/s-0032-1309309. [DOI] [PubMed] [Google Scholar]
- 41.Hynson JL, Kornberg AJ, Coleman LT, Shield L, Harvey AS, Kean MJ. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology. 2001;56(10):1308–1312. doi: 10.1212/WNL.56.10.1308. [DOI] [PubMed] [Google Scholar]
- 42.Franciotta D, Columba-Cabezas S, Andreoni L, Ravaglia S, Jarius S, Romagnolo S, et al. Oligoclonal IgG band patterns in inflammatory demyelinating human and mouse diseases. J Neuroimmunol. 2008;200(1–2):125–128. doi: 10.1016/j.jneuroim.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Baumann M, Sahin K, Lechner C, Hennes EM, Schanda K, Mader S, et al. Clinical and neuroradiological differences of paediatric acute disseminating encephalomyelitis with and without antibodies to the myelin oligodendrocyte glycoprotein. J Neurol Neurosurg Psychiatry. 2015;86(3):265–272. doi: 10.1136/jnnp-2014-308346. [DOI] [PubMed] [Google Scholar]
- 44.Di Pauli F, Mader S, Rostasy K, Schanda K, Bajer-Kornek B, Ehling R, et al. Temporal dynamics of anti-MOG antibodies in CNS demyelinating diseases. Clin Immunol. 2011;138(3):247–254. doi: 10.1016/j.clim.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Van Haren K, Tomooka BH, Kidd BA, Banwell B, Bar-Or A, Chitnis T, et al. Serum autoantibodies to myelin peptides distinguish acute disseminated encephalomyelitis from relapsing-remitting multiple sclerosis. Mult Scler. 2013;19(13):1726–1733. doi: 10.1177/1352458513485653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hintzen RQ, Dale RC, Neuteboom RF, Mar S, Banwell B. Pediatric acquired CNS demyelinating syndromes: features associated with multiple sclerosis. Neurology. 2016;87(9 Suppl 2):S67–S73. doi: 10.1212/WNL.0000000000002881. [DOI] [PubMed] [Google Scholar]
- 47.Alper G, Heyman R, Wang L. Multiple sclerosis and acute disseminated encephalomyelitis diagnosed in children after long-term follow-up: comparison of presenting features. Dev Med Child Neurol. 2009;51(6):480–486. doi: 10.1111/j.1469-8749.2008.03136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verhey LH, Branson HM, Shroff MM, Callen DJ, Sled JG, Narayanan S, et al. MRI parameters for prediction of multiple sclerosis diagnosis in children with acute CNS demyelination: a prospective national cohort study. Lancet Neurol. 2011;10(12):1065–1073. doi: 10.1016/S1474-4422(11)70250-2. [DOI] [PubMed] [Google Scholar]
- 49.Koelman DL, Mateen FJ. Acute disseminated encephalomyelitis: current controversies in diagnosis and outcome. J Neurol. 2015;262(9):2013–2024. doi: 10.1007/s00415-015-7694-7. [DOI] [PubMed] [Google Scholar]
- 50.Ravaglia S, Piccolo G, Ceroni M, Franciotta D, Pichiecchio A, Bastianello S, et al. Severe steroid-resistant post-infectious encephalomyelitis: general features and effects of IVIg. J Neurol. 2007;254(11):1518–1523. doi: 10.1007/s00415-007-0561-4. [DOI] [PubMed] [Google Scholar]
- 51.Keegan M, Pineda AA, McClelland RL, Darby CH, Rodriguez M, Weinshenker BG. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology. 2002;58(1):143–146. doi: 10.1212/WNL.58.1.143. [DOI] [PubMed] [Google Scholar]
- 52.Granget E, Milh M, Pech-Gourg G, Paut O, Girard N, Lena G, et al. Life-saving decompressive craniectomy for acute disseminated encephalomyelitis in a child: a case report. Childs Nerv Syst. 2012;28(7):1121–1124. doi: 10.1007/s00381-012-1733-9. [DOI] [PubMed] [Google Scholar]
- 53.Pohl D, Tenembaum S. Treatment of acute disseminated encephalomyelitis. Curr Treat Options Neurol. 2012;14(3):264–275. doi: 10.1007/s11940-012-0170-0. [DOI] [PubMed] [Google Scholar]
- 54.Shilo S, Michaeli O, Shahar E, Ravid S. Long-term motor, cognitive and behavioral outcome of acute disseminated encephalomyelitis. Eur J Paediatr Neurol. 2016;20(3):361–367. doi: 10.1016/j.ejpn.2016.01.008. [DOI] [PubMed] [Google Scholar]