Abstract
Half a century has passed since the first orthotopic heart transplant took place. Surgical innovations allowed for heart, lung, and heart-lung transplantation to save lives of patients with incurable chronic cardiopulmonary conditions. The complexity of the surgical interventions, chronic host health conditions, and antirejection immunosuppressive medications makes infectious complications common. Infections have remained one of the main barriers for successful transplantation and a source of significant morbidity and mortality. Recognition of infections and its management in this setting require outstanding clinical skills since transplant recipients may not exhibit classic signs or symptoms of disease, and laboratory work has some pitfalls. The prevention, identification, and management of infectious diseases complications in this population are a priority to undertake to improve the medical outcomes of transplantation. Herein, we reviewed the historical aspects, epidemiology, and prophylaxis of infections in heart, lung, and heart-lung transplantation. We also discuss the most prevalent organisms affecting the host and the organ systems involved.
Keywords: Heart transplantation, Lung transplantation, Heart-lung transplantation, Bacterial infections, Virus diseases, Fungal infections, Epidemiology, Prophylaxis, Diagnosis
Historical Perspective
We have conceived the human heart as the main source of our deep emotions and feelings. A place where our very conscious resides as portrayed by Edgar Allan Poe in his famous The Tell-Tale Heart short story: “I felt that I must scream or die! And now—again!—hark! Louder! Louder! Louder! Louder!” Dr. John Gibbon Jr. used for the first time in 1953 a heart-lung respirator to keep a patient alive while performing heart surgery. Dr. Norman Shumway at Stanford developed and perfected the first surgical technique leading to heart transplantation surgery. After Dr. Christian Barnard’s first orthotopic heart transplant in December 1967, and Dr. Shumway first heart transplant in the United States in January 1968, heart transplantation became a standard therapeutic option for life-threatening congestive failure and started to be performed in the hundreds over the next following years at different centers. Heart transplant surgery faced complications due in part to rejection and infection. However, the development of more selective immunosuppressive therapy and improvements in prevention, detection, and treatment of infections allowed for heart transplant surgery to increase rapidly worldwide.
Four thousand and ninety six heart (3529 adults) transplants were reported to the International Society of Heart and Lung Transplant Registry (ISHL) in 2011 [1]. The landscape of infection affecting heart transplant patients has been shaped by different factors: (A) implementation of more selective calcineurin-based immunosuppressive protocols, (B) lessened immunosuppressive induction regimens, (C) the institution of antimicrobial prophylaxis resulting in a significant decrease or delay in the emergence of major infections episodes including P. jirovecii (PCP), Nocardia spp., Listeria spp., Toxoplasma gondii, cytomegalovirus, toxoplasmosis, cytomegalovirus (CMV), herpes simplex virus (CMV), varicella zoster virus (VZV), and invasive fungal infections, (D) introduction of novel diagnostic technology facilitating earlier recognition and treatment of infections, (E) expansion in the criteria to select donors and recipients to include various scenarios dealing with HBV, HCV, and HIV infections [2], and (F) shift toward predominantly Gram-positive bacterial infections and multiresistant bacteria in recent years [3–5].
A Stanford team lead by Dr. Bruce Reitz performed a Lung transplantation as a combined heart-lung transplant procedure in 1981 [6]. Shortly after, thoracic surgeons optimized the single- and double-lung transplant procedures. Improvement of surgical techniques, especially bronchial anastomosis and evolution of flush perfusion lung preservation, decreased the perioperative bronchial complications substantially. Similarly to heart transplantation, improvements in immunosuppressive regimens, antimicrobial prophylaxis, and graft preservation led to enhancement in survival among lung transplant recipients. In contrast to cardiac, lung transplantation has faced the challenge of infections unique to the transplant of this organ. Mold infections of the anastomotic site, host versus graft disease, and serious infections with Mycobacterium abscessus, Chlamydia spp., bronchiolitis, and Burkholderia cepacia complex are among infectious complications rarely observed in other transplant patients [7].
Transplantation of thoracic organs has improved the quality of life and prevented the death of thousands of individuals worldwide. Graft survival and life expectancy have been markedly improved in these patients due to the introduction of more optimal immunosuppression, antimicrobial prophylaxis, and diagnostic technology allowing the earlier diagnosis and treatment of infection and rejection. Finally, further control of infection is likely to result from implementation of new approaches to assess the net state of immunosuppression in these patients.
Epidemiology
Infection was recognized as a major threat to thoracic transplantation from the early inception days [8]. There are several factors predisposing thoracic transplant recipients to infections: (A) factors present before transplantation: age, presence of comorbidities (e.g., chronic kidney disease, diabetes mellitus, cancer, etc.), nutrition status, latent infections, colonization with healthcare-associated organisms, and occult community-acquired infections; (B) factors during the surgery: duration of the transplant procedure, graft injury including ischemic time, colonization or latent infection of the graft, surgical instrumentation (e.g., mechanical ventilation, invasive devices such as catheters, drains, Foley catheters, etc.), ICU stay, and need for re-interventions; and (C) factors present after transplant: degree of immunosuppression, CMV infection, and rejections (Table 2.1).
Table 2.1.
Clinical features modifying infection risk in transplantation
| Before transplantation |
| Age |
| Comorbidities: diabetes mellitus, chronic kidney disease, cancer, etc. |
| Nutrition status |
| Latent infections or occult community-acquired infections |
| Colonization with healthcare-associated organisms |
| During transplantation |
| Duration of the transplant procedure |
| Graft injury |
| Ischemic time |
| Anastomosis site in lung transplant |
| Denervation of allograft (e.g., diminished cough reflex) |
| Lymphatic drainage disruption |
| Colonization or latent infection of the graft |
| Surgical instrumentation (e.g., mechanical ventilation) |
| ICU stay |
| Need for re-interventions |
| After transplantation |
| Immunosuppression |
| CMV infection |
| Rejections |
Heart Transplant Infections
A total of 4096 heart transplants were performed in 2011. Heart transplant recipients have an average age of 54 years and are predominantly man (76%). They have a significant history of smoking (46%) and hypertension (45%) and have cardiomyopathy (54%) followed by coronary artery disease (37%) as the leading causes of transplant [1]. The historical (pediatric and adult transplants between 1982 and 2011) 1-year, 5-year, and 10-year survival rates are 81%, 69%, and 50%, respectively. Overall median survival is 11 years, but it increases up to 13 years for those surviving the first year after transplantation. Although not associated with increased posttransplant mortality, infections before transplant can affect up to 25% of heart transplant candidates. Being bronchitis and soft tissue infections, the more commonly present [9]. Despite no major changes in the distribution of causes of death since 1994, infections remained a predominant factor of mortality during the first 3 years after transplant. It contributes with up to almost 20% of causes of death [3]. The global incidence of infections in heart transplant ranges between 30% and 60% and the associated mortality between 4% and 15% [10]. The incidence of infection measured as major infectious episodes per patient has steadily declined from 2.83 in the early 1970s to 0.81 in the early 2000s [3, 8, 11]. The most frequent type of infection is bacterial (44%), followed by viral (42%), fungal including Pneumocystis jirovecii (14%), and protozoa (0.6%). Unfavorable functional outcomes are observed in patients who developed infections in the first year of transplant, mainly associated with bloodstream, CMV, and lung infections [12]. Pulmonary and central nervous system (CNS) infections are independent predictors of mortality among heart transplant recipients. Reactivation of latent parasitic infections residing in extra-cardiac tissues in the host or transmitted in the transplanted heart is an important consideration. The classic example is the reactivation of Trypanosoma cruzi. Chagas disease is a vector-borne illness transmitted by triatomine bugs, and it is endemic in Latin America. The ethnicity or origin of either the donor or the recipient from these regions should raise the concern for possible reactivation. Chagas reactivation was documented in 38.8% of cases in a cohort of Brazilian heart transplant recipients, where Chagas cardiomyopathy was the second most common indication for transplant (34.9%) [13]. Chagas can also reactivate from the transplanted heart procured from a seropositive donor and transplanted into a seronegative recipient. Although with a substantial decreased on its prevalence in the most recent eras, toxoplasmosis is another important consideration in this setting. Similarly to Chagas, Toxoplasma gondii—also with a predilection to invade the myocardium—can be transmitted by reactivation of quiescent cysts in the recipient or the transplanted heart [14].
Lung and Heart-Lung Transplant Infections
By 2011, 3640 adults received lung transplantation, the highest reported number of procedures up to that date, driven mainly by the increase of double-lung transplants. Double-lung transplant is indicated for septic lung diseases (e.g., cystic fibrosis). Around 66% of recipients were aged 45–65 years old. The most frequent indications for transplant were COPD (34%), followed by interstitial lung disease (ILD) (24%), bronchiectasis associated with cystic fibrosis (CF) (17%), and α1AT deficiency-related COPD (6%) [15]. The overall (from 1994 to 2011) 1-year, 5-year, and 10-year survival rates among lung recipients are 79%, 53%, and 31%, respectively. Overall median survival is 5.6 years. Lung transplants from CMV seronegative donors have better survival rates than from CMV seropositive donor. Thirty-day mortality was led by graft failure (24.7%) and non-CMV infections (19.6%). During the remainder of the year, non-CMV infections were the leading cause of death (35.6%). Infection is still prominent as the cause of death following the first year of transplant after bronchiolitis obliterans syndrome (BOS)/chronic lung rejection or graft failure [15]. Other infections complications historically present among the ten primary causes of death within the first year include sepsis, pneumonia, and fungal infections [16]. High lung allocation score (LAS) at the time of transplantation is associated with a lower 1-year survival and higher rates of infections among lung transplant recipients [17].
Sixty-three adult Heart-Lung transplantations were reported to the ISHL registry in 2011. Sixty-six percent of recipients were in the group range from 18 to 49 years old. Sixty-three percent of the indications were for congenital heart disease and idiopathic pulmonary arterial hypertension. Heart-lung transplant for CF was higher in Europe and other centers compared to North American. When compared to lung only transplants, short-term survival was worse, but long-term survival was better for the heart-lung transplant recipients. Their 1-year, 5-year, and 10-year survival rates were 63%, 44%, and 31%, respectively. The median survival was 3.3 years and 10 years for those surviving the first year. Similarly, they have graft failure (27%), technical complications (21.9%), and non-CMV infections (17.8%) as leading causes of death during the first 30 days posttransplant. Non-CMV infections (35.1%) were the top cause of death after 1 month and within 1 year of transplant. After the first year, BOS/late graft failure and non-CMV infections were the predominant causes of death [15]. Among other risk factors for mortality in lung transplantation are cystic fibrosis, nosocomial infections, and mechanical ventilation before transplant [18].
Infections in lung transplant recipients are predominantly bacterial (48%), viral (35%), fungal (13%), and mycobacterial (4%) [19]. In 60%, the infection site is pulmonary. Risk factors for infection vary by the type of organism. Mechanical ventilation (MV) for >5 days immediately following transplant surgery and isolation of Staphylococcus aureus (SA) from airway cultures in the recipient were considered risk factors for invasive SA infections in a retrospective study of patients with lung and heart-lung transplants [20]. Likewise, risk factors for the development of healthcare-associated infections with Gram-negative organisms, Aspergillus, Legionella, and MRSA (methicillin-resistant Staphylococcus aureus), include prolonging MV, renal failure, use of ATG (antithymocyte globulin), and recurrent rejections episodes [21]. Additionally, α-1-antitrypsin deficiency and repeat transplantation are also risk factors for nosocomial infections. Mycobacterium tuberculosis transmission from lung donors with latent infection has been documented in highly endemic areas [22]. Colonization with MDR organisms (Pseudomonas aeruginosa, Burkholderia, Acinetobacter, nontuberculous mycobacteria (NTM), and Scedosporium) before transplant—especially important in CF patients—can predict the development of challenging infections to treat after transplant [23].
Pretransplant Evaluation of Recipients and Donors
Pretransplant Screening of Recipients
Patients should undergo a comprehensive evaluation of potential infectious complications associated with transplantation. A detailed medical history including previous vaccinations, history of past infections, exposures (geographical, occupational, animal, etc.), travel, and foreign-born status among others should be obtained.
Clinicians shuold perform routine serologies for the detection of pathogen-specific IgG for CMV, HSV, EBV (VCA), VZV, hepatitis B (HBsAg, HBsAb, HBcAb), HIV, hepatitis C, and syphilis. Toxoplasma IgG should also be performed in heart and heart-lung transplant candidates. Additionally, we recommend to obtain UA, urine culture, CXR, and tuberculin skin test (TST), or a Quantiferon assay. In lung and heart-lung transplant candidates, sputum should be cultured for bacterial, fungal, and AFB studies.
Some centers advocate the screening of patients for colonization with MDR (multidrug resistant) bacteria such as MRSA and VRE (vancomycin resistant Enterococci), which it may have an impact on the type of antibacterial prophylaxis used preoperatively or the empirical antibiotics should sepsis develop in the immediate postoperative period. In potential lung recipients, previous respiratory colonization with MDR Pseudomonas, especially in CF patients, should not exclude them from transplant [24]. On the other hand, if colonization with B. cenocepacia (genomovar III) in CF is present transplant is relatively contraindicated [25, 26].
Checking for endemic fungi such as Coccidioides immitis or for the parasites Trypanosoma cruzi, Strongyloides stercoralis, and Leishmania spp. is indicated in the presence of the appropriate risk factors [27–31].
Histoplasma capsulatum has reactivated during immunosuppressive therapy [32]. Infections after solid organ transplantation (SOT) are rare and attributable to transmission from the donor [33]. Furthermore, latent histoplasmosis can be present with negative serologies and treatment after transplant carries a good outcome. Therefore the role of screening for histoplasmosis is of questionable significance [34].
Pretransplant Screening of Donors
The type of evaluation may change if the donor is alive or deceased depending on the available time to collect the samples. Similarly to recipients, donors should undertake a comprehensive assessment including a complete history, assessment of risk factors, exposures, immunizations, and previous or current infections. Donors should be screened for HIV, hepatitis B/C, syphilis, and tuberculosis. Furthermore, we recommend to obtain serologies for CMV, EBV, HSV, VZV, and Toxoplasma gondii, and for HTLV-1/HTLV-2 in endemic areas. In high-risk donors, the use of nucleic acid amplification tests (NAAT) for HBV, HCV, and HIV should be considered. Additionally, blood cultures to document an occult bacteremia are recommended. In lung transplant donors, we recommend obtaining respiratory cultures through bronchoscopy to detect colonizing organisms and target them to prevent invasive infections in the donor. Culturing the media of the allograft during acquisition or processing have been advocated to reduce the risk of mycotic aneurysms among kidney transplant recipients, which may apply to other SOT [35]. Screening of donors for endemic mycosis is not well established. On the other hand, heart transplant donors should be screened for Chagas if the donor was born in Latin America [29]. Finally, it is important to highlight the increase recognition of emerging, unusual viral infections such as West Nile virus, lymphocytic choriomeningitis virus, rabies, and different human coronaviruses [34, 36]. Testing for those organisms should be done based on individual assessments. Table 2.2 describes and summarizes the diagnostic workup recommend among donors and recipients.
Table 2.2.
Infectious screening during transplantation
| Diagnostic workup among donors and recipients |
|---|
| Routine tests obtained among donors and recipients: |
| Viral test: HIV Elisa, hepatitis C antibody, HBV (HBsAg, HBcAb total, HBsAb), IgG antibody for CMV, HSV, EBV VCA, VZV |
| Bacterial: Treponemal antibody (e.g., EIAs, FTA-ABS), QFN assay or PPD |
| Parasite: Toxoplasmosis IgG (routinely indicated for heart transplant patients) |
| Other screening to consider among donors or recipients in the presence of specific risk factors: |
| Viral: NAAT for HIV, HCV, HBV in high-risk donors. HTLV-1/HTLV-2 in donors from endemic areas |
| Bacterial: |
| Recipients: UA, urine culture, CXR, and sputum culture. Optional: To consider screen for colonization with MDR organisms (MRSA or VRE) |
| Donors: Blood cultures, allograft media culture, and bronchoscopy with culture from respiratory specimens in lung donors |
| Parasite: Ortho EIA and Abbott Prism Chagas test to screen for Trypanosoma cruzi in donors or recipients from Latin America. Strongyloides stercoralis and Leishmania spp. serologies should be obtained in recipients in the presence of appropriate geographic risk factors |
| Fungal: EIA for coccidioidal antibodies or complement fixing antibodies for cocci |
Abbreviations: NAAT Nucleic acid amplification test, CXR chest X-ray, MDR multidrug resistant, EIA enzyme-linked immunosorbent assay
Prevention of Infections
Immunizations
Immunization should be optimized before transplantation since the recipient will have better chances to mount an adequate immune response [37]. The advisory committee on immunization practices (ACIP) [38] and the guidelines for immunizations in solid organ transplantation [39] recommend inactivated influenza vaccine annually. Tetanus, diphtheria, and acellular pertussis (Tdap) should be administered to all adults who have not previously received Tdap or have an unknown status. Varicella vaccination with two doses in patients without evidence of immunity or a single dose of zoster vaccination, inactivated polio vaccine, hepatitis A/B, HPV (three series through 26 years of age), and meningococcal and pneumococcal vaccines should be administered [38]. It is remarkably important to vaccinate all household members as well. BCG and rabies vaccines can be considered under some extenuating or exposure-related indications. See Table 2.3.
Table 2.3.
Immunizations recommendations during transplantation
| Recommended vaccines among heart, lung, and heart-lung recipients |
|---|
| Annual inactivated influenza vaccine |
| Tdap (should be administered to all adults who have not previously received Tdap or have an unknown status) |
| VZV (two series) in patients without evidence of immunity |
| Zoster vaccine should be given in varicella-positive candidates age ≥60 years and considered in candidates aged 50–59 (>4 weeks before transplant) |
| Inactivated polio |
| Hepatitis A series |
| Hepatitis B series |
| HPV (three series through 26 years of age) |
| Pneumococcal: Pneumococcal conjugate 13-valent (PCV13) followed by pneumococcal polysaccharide 23 (PPSV23) vaccine 8 weeks later (If PPSV23 was received first; PCV13 should be given at least 1 year after) |
| Meningococcal conjugate vaccine |
| Under special circumstances: Rabies and BCG |
Avoidance of Exposures
Education of the patient and the family members is a cornerstone to establishing effective preventive measures. Emphasis should be enforced about hand hygiene and food handling. Additionally, potential sources of bacteria, fungi (e.g., Aspergillus), and toxoplasmosis such as plants and flowers, cleaning pet’s litter or cages, eating uncooked meat, acquiring new pets, construction areas, farming, barnyard activities, and smoking marihuana should be avoided. If those recreational or occupational exposures are unavoidable; appropriate gear, such gloves, must be worn. Education about possible community exposures is also important. Close contacts with persons with fevers or rash potentially infected with VZV, herpes zoster, or influenza should be circumvented as well. Patients should cook all meals thoroughly, wash all fruits and vegetables, and shun all unpasteurized products. Safe sex practices are recommended. If any foreign travel is planned, seeking evaluation in a specialized travel clinic is advisable.
Prophylaxis
Guidelines for the management of surgical antimicrobial prophylaxis list cefazolin (2 g, 3 g for patients with weight >120 Kg every 4 h) as the recommended regimen for heart, lung, and heart-lung transplantation surgery. Clindamycin (900 mg every 6 h) or vancomycin (15 mg/kg) can be substituted as alternative agents in beta-lactam allergic patients [40, 41]. This recommendation can be adjusted individually, based on local hospital surveillance data or previous knowledge of colonizing organisms (e.g., addition of aztreonam, gentamicin, or a single-quinolone dose). However, the widespread use of quinolones may increase the resurgence of antimicrobial resistance. The antibiotic should be administered within 60 min before surgical incision (within 120 min for vancomycin or quinolones) and to be continued for 24–48 h in heart transplants and 48–72 h and no longer than 7 days in lung and heart-lung transplant recipients. Recommendation to continue antibacterial prophylaxis until chest and mediastinal tubes are removed lacks sufficient evidence. Redosing will depend on the procedure duration and associated blood loss.
The recipient does not need treatment if a localized infection was present in the donor, except during meningitis where concomitant bacteremia often coexist. In meningitis and bacteremia, it is prudent to treat the recipient for 2–4 weeks [34].
Indications for antifungal prophylaxis in heart transplant recipients are not clear. A systemic review showed no benefit of antifungal therapy to prevent invasive fungal infections in transplants recipients other than liver [42]. Although a prospective cohort of heart transplant recipients showed targeted prophylaxis—an echinocandin for a median of 30 days with the presence of at least one risk factor for invasive aspergillosis (IA) (reoperation, cytomegalovirus disease, posttransplantation hemodialysis, and another patient with IA in the program 2 months before or after the procedure)—was highly effective and safe in preventing IA episodes [43], no consensus exists for universal antifungal prophylaxis in heart transplant recipients. Most centers have adopted antifungal prophylaxis including inhaled amphotericin B, oral itraconazole, or IV targeted echinocandin prophylaxis.
In lung and lung-heart transplant recipients, fungal prophylaxis should be considered, especially if pretransplantation respiratory cultures either from the donor lung or recipient airways shows Aspergillus or Candida. One approach is to use inhaled amphotericin B (50 or 100 mg in extubated or intubated patients, respectively) daily until 4 days after transplant and then weekly until hospital discharge in patients with no known colonization [44, 45]. If a mold has been isolated, voriconazole is recommended up to 4 months after transplant. Although evidence and efficacy need to be confirmed, combination antifungal prophylaxis therapies is used at some centers [46].
Pneumocystis jiroveci prophylaxis is done with trimethoprim-sulfamethoxazole (TMP-SMX) for 6 months, up to 1 year. Some centers extend the PJP prophylaxis to lifelong. TMP-SMX also confers protection against Toxoplasma, Nocardia, and Listeria species infections. Alternatively, dapsone, inhaled pentamidine, or atovaquone can be used in patients with a history of sulfa allergy. TMP-SMX is recommended at many centers for lifelong in toxoplasmosis seronegative recipients of seropositive cardiac donors (Toxoplasma D+/R−) [11].
CMV prevention is recommended to all D+/R− and R+ patients. There are two common strategies for CMV prevention: antiviral prophylaxis and preemptive therapy. Both approaches possess similar success rate and their advantages and disadvantages [47]. Guidelines recommend valganciclovir or intravenous ganciclovir as the preferred antivirals. Oral ganciclovir is an option in heart transplant patients, although it possesses a low oral bioavailability and therefore the theoretical risk of increased resistance. Often, CMV immune globulin is used as an adjunctive agent. In heart recipients, prophylaxis is recommended for 3–6 months in D+/R− and 3 months in R+. In lung and heart-lung recipients, the duration of prophylaxis is 12 months and 6–12 months in D+/R− and R+ recipients, respectively [48]. In D−/R− patients, otherwise not receiving CMV active agents, antiviral prophylaxis against other herpes viruses, such as HSV and VZV, should be considered. Use of oral CMX001 (oral liposomal formulation of cidofovir) in hematopoietic-cell transplants reduced CMV-related events and may have a potential role in preventing CMV in other transplant settings [49]. Refer to Table 2.4 for a list of prophylaxis recommendations.
Table 2.4.
Antimicrobial prophylaxis
| Prophylaxis in heart, lung, and heart-lung transplant |
| Bacterial a: |
| Preferred: Cefazolin 2 g (3 g for patients with weight >120 Kg). Redose every 4 h for extended procedure time and significant blood loss |
| Alternative: Vancomycin 15 mg/kg or clindamycin, 900 mg IV |
| CMV prophylaxis b: |
| Valganciclovir, 900 mg PO once daily |
| IV ganciclovir, 5 mg/kg IV once daily |
| Oral ganciclovir (heart transplant), 1 gr PO three times a day |
| Consider adjuvant therapy with CMV immune globulin |
| Pneumocystis jiroveci: |
| TMP-SMX, one single tablet a day or one double-strength tablet three to seven times a week for 6–12 months |
| Alternatively, dapsone (100 mg PO daily); inhaled pentamidine (300 mg/dose monthly) or atovaquone (1500 mg PO once daily) can be used |
| Other: In D−/R− patients, otherwise not receiving CMV prophylaxis, consider acyclovir to prevent HSV/VZV reactivation |
| Prophylaxis in heart transplants |
| Parasitic: |
| Consider lifelong TMP-SMX in toxoplasmosis mismatch recipients (D+/R−) |
| CMV prophylaxis: |
| Doses as above. Duration: 3–6 months in D+/R− and 3 months in R+ recipients |
| Fungal (optional): |
| IV echinocandin daily for 30 days in the presence of IA risk factors |
| Prophylaxis in lung and heart-lung transplants |
| Bacterial: |
| Consider the addition of aztreonam, gentamycin, or a single-quinolone dose in the presence of previous respiratory cultures positive for Gram negatives |
| CMV prophylaxis b: |
| Doses as above. Duration: 12 months in D+/R− and 6–12 months in R+ recipients |
| Fungal: |
| Negative pretransplant respiratory cultures: Inhaled amphotericin B, 50 or 100 mg in extubated or intubated patients, respectively, daily until 4 days after transplant and then weekly until hospital discharge |
| Positive pretransplant respiratory cultures for Aspergillus: voriconazole, 6 mg/kg every 12 h for 2 doses; followed by maintenance dose of 4 mg/kg every 12 h, is recommended up to 4 months after transplant. Maintenance dose can be achieved with oral voriconazole 200 mg PO every 12 h |
Abbreviation: IA Invasive aspergillosis
aThe antibiotic should be administered within 60 min before surgical incision (within 120 min for vancomycin or quinolones) and to be continued for 24–48 h in heart transplants and 48–72 h and no longer than 7 days in lung and heart-lung transplant recipients
bDoses of valganciclovir, ganciclovir, and other antibiotics may require adjustment for renal function
Risk of Infection Posttransplantation
<1 Month
This period is characterized more commonly for nosocomial, bacterial infections. Thus, the bacterial organisms present are often MDR (e.g., VRE, MRSA). In heart transplant recipients, skin and soft tissue infections (SSTI), surgical site infection, and mediastinitis are of concern during this period. Likewise, lung and lung-heart transplant recipients may develop infections related to previous respiratory colonization (Pseudomonas, Aspergillus). Other significant infections include aspiration pneumonitis, healthcare- and ventilator-associated pneumonia, catheter-related bloodstream infections (CRBSI), nosocomial UTIs, and Clostridium difficile colitis. Donor-derived infections during this period can be present and will include HSV, lymphocytic choriomeningitis virus (LCMV), rhabdovirus (rabies), West Nile virus (WNV), and HIV. Toxoplasma gondii and Trypanosoma cruzi are also serious donor-derived infections in heart transplant recipients that can develop within the first 6 months posttransplantation [50].
1–6 Months
During this period, reactivation of latent infections usually occurs. Hence, bacterial infections such as those caused by Nocardia asteroides, Listeria monocytogenes, and Mycobacteria tuberculosis typically occur. Additionally, fungal infections by Aspergillus spp., Cryptococcus neoformans, and P. jiroveci and parasitic by Toxoplasma gondii, Leishmania spp., Strongyloides, and Trypanosoma cruzi can also be seen. Viral infections present during this period include herpesviruses (HSV, VZV, CMV, and EBV) and adenovirus.
>6 Months
Development of infections after 6 months are predominantly community-acquired pneumonia and urinary tract infections. Other diseases include Aspergillus and Mucor species, Nocardia, Rhodococcus, and late viral infections including CMV, hepatitis B and C, JC polyomavirus infection, posttransplant lymphoproliferative disorder (PTLD), HSV encephalitis, and viral community-acquired infections (e.g., coronavirus, West Nile virus, influenza).
Monitoring
Infections
It is important to recognize transplant recipients as a patient population with increased susceptibility to infections and have a low threshold to perform diagnostic workup in the presence of any concerning signs or symptoms. Infections monitoring is also done in a structured way when preemptive therapy for CMV is in place (as opposed to universal prophylaxis). Protocols vary by the transplant center but, usually, implies a weekly CMV PCR or pp65 Ag monitoring [51]. Likewise, monitoring of cell-mediated immunity (CMI) using a Quantiferon-CMV assay may be useful predicting late-onset CMV disease once CMV prophylaxis has been stopped [52]. CMI also have been monitored for EBV using an enzyme-linked immunoSpot assay [53].
Immunoglobulin G (IgG), C3, IgG2 levels, and NK cell counts have been proposed as an attempt to identify the risk of infection in heart transplant recipients within the first year [54].
Drug-Drug Interactions
Significant drug-drug interactions exist among antimicrobial and immunosuppressive agents. Patient medication list should be reviewed carefully. CTP3A4 strong inducers such as nafcillin reduce tacrolimus serum concentrations. In contrast, azoles such as fluconazole can result in increased levels of tacrolimus or cyclosporine. For voriconazole, the dose of tacrolimus needs to be reduced by two-thirds [55] and the cyclosporine dose by 50% [56]. Rifamycins can have an opposite drug-drug interaction by decreasing the concentrations of prednisone, cyclosporine, tacrolimus, sirolimus, and mycophenolate mofetil (MMF) [57, 58]. Likewise, tacrolimus administration along with quinolones may cause QT prolongation [59].
Infections in Heart Transplantation
Infecting Microbial Agents
Bacterial
In heart transplant patients, bacterial infections have similar clinical manifestations commonly observed in other patient populations. However, clinical signs may be subtle or absent (e.g., afebrile). They are the most frequent type of infections in this setting, reaching up to 50% of all infections [3]. The most common are pulmonary infections followed by bacteremias, mediastinal, and skin infections. Staphylococcus aureus—predominantly methicillin-resistant—can cause SSTI, ventilator-associated pneumonia, mediastinitis, CRBSI, other forms of bacteremia, and osteomyelitis. In contrast, coagulase-negative Staphylococcus is more commonly associated with CRBSI. Among Gram-negative bacteria, Pseudomonas aeruginosa is common, usually of pulmonary origin. Escherichia coli is the primary causal organism of UTIs. Extended-spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae, Escherichia coli, Klebsiella oxytoca, and Citrobacter freundii are also found in 2.2% of heart transplant recipients [60].
Nocardia species are well recognized as an opportunistic pathogen in this setting. Although relatively rare in heart transplant recipients (frequency <1%), Nocardia is only second in frequency in heart transplant after lung transplant recipients [61–63]. Pertinent-independent risk factors associated with the development of this infection in SOT include high-dose steroids, history of CMV disease, and high levels of calcineurin inhibitors [62]. With the almost universal prophylaxis with TMP-SMX, Nocardia infection is less common and often present late, usually after 1 year posttransplant [63]. When they occurred, they affect the lung predominantly, which is the port of entry for disseminated infections and CNS invasion. Also, it can cause skin nodules and abscesses. Listeria monocytogenes can also be seen in heart transplant recipients and can count for a significant proportion of the bacterial meningitis cases in this setting [64]. Additionally, myocarditis and myocardial abscesses with this organism have also been documented [65]. Mycobacterium tuberculosis and nontuberculous mycobacteria (NTM), although, documented to occur in heart transplantation, are rare in the United States [66, 67]. However, it is important to recognize that the development of tuberculosis (TB) can be more prevalent in some endemic regions and often present with extrapulmonary involvement [68, 69]. Legionellosis and Rhodococcus equi with mainly pulmonary manifestations (pneumonia, pulmonary infiltrates, or cavitation) are another significant infections among heart transplant recipients [70].
Fungal
Fungal infections excluding PCP represent around 4.0% of all the infections. From them, invasive mold infections (IMI) are a significant contribution to morbidity and mortality among heart transplant recipients. The incidence in this population can reach 10 per 1000 person-years, and its associated mortality is approximately 17% [71]. Aspergillus represents up to 65% of all IMI. Its median time of onset is about 46 days, although late presentation (>90 days) has been more recently recognized associated with receipt of sirolimus in conjunction with tacrolimus for refractory rejection or cardiac allograft vasculopathy [72]. The most common clinical presentation for aspergillosis includes fever, cough, and single or multiple pulmonary nodules [73]. Extrapulmonary manifestations include spondylodiscitis, infective endocarditis, mediastinitis, endophthalmitis, and brain and cutaneous abscesses [74–78]. Dissemination tends to affect the CNS in a good proportion of the cases. Mucormycosis is the second most frequent mold affecting heart transplant recipients. Mucor, along with other non-Aspergillus molds (e.g., Scedosporium, Ochroconis gallopava), are associated with disseminated infections, CNS involvement, and poorer outcomes [79, 80]. Pneumocystis jiroveci (PCP)—although with a marked reduction in incidence with the introduction of universal prophylaxis—is still a significant pathogen and cases may occur late after heart transplant. Cryptococcosis, although infrequent among SOT patients, has its higher incidence in heart transplant recipients [81]. Usually, its manifestations present late and affect the lungs and the CNS predominantly. Histoplasmosis and coccidioidomycosis occurred typically in the first year after transplant. Antigenuria was the most sensitive diagnostic test in SOT for histoplasmosis [82]. Finally, Candida infections are an important cause of morbidity and mortality as well. Rate of colonization is higher than in the general population [83]. Candida most commonly causes an oral mucosa infection. Although there has been a decline of invasive infections over time, these do occur and typically in the form of bloodstream infections secondary to catheter-related infections, tracheobronchitis, or disseminated disease [84]. Additionally, other confined end-organ injuries such as endophthalmitis and esophagitis can also be seen.
Viral
CMV infection is of critical importance among SOT. In heart transplant recipients, CMV has been inconsistently associated with cardiac allograft vasculopathy [85]. Furthermore, CMV leads to upregulation of pro-inflammatory cytokines, increase procoagulant response, left ventricular dysfunction, allograft rejection, and an increase of opportunistic infections [86]. The greatest risk for developing CMV disease is CMV-negative recipients of CMV-positive organs (D+/R−), followed by D+/R+ and D−/R+. A clinical report estimated that the rate of infections in heart transplant ranges between 9% and 35%, and disease is present in around 25% of patients [87]. The clinical manifestations are not unique to heart transplant recipients and include a CMV syndrome (fevers, myalgias, arthralgias, malaise, leukopenia, and thrombocytopenia). CMV-associated end-organ injury in this setting includes most frequently pneumonitis and gastrointestinal disease [10]. Other manifestations comprise myelosuppression, hepatitis, and pancreatitis. In contrast to the high frequency observed in AIDS patients, chorioretinitis in heart transplant patients is relatively rare [87]. Guidelines on CMV diagnosis and managements are discussed in more detail in Chap. 55 and also have been published elsewhere [88]. Other herpes viruses are of important consideration as well. EBV-associated T-cell PTLDs are more frequent in heart transplant recipients (0.4%) than in other SOT patients [89]. PTLD is a significant contributor to morbidity and mortality in the pediatric heart transplant population [90]. Human T-lymphotropic virus type I (HTLV1), human herpes virus (HHV)-6, HHV-7, and HHV-8 might play a role in EBV(−) T-cell PTLDs as well. Herpes viruses can manifest, as in other hosts, as mucocutaneous lesions for HSV, herpes zoster for VZV, infectious mononucleosis in the case of EBV, Kaposi sarcoma for HHV-8, and encephalitis for HHV-6/7. Hepatitis, colitis, pneumonitis, and gastrointestinal disease have also been attributed to dissemination with certain herpes viruses. Herpes viruses can present with disseminated skin lesions (with or without vesicle formation) and fever of unknown origin.
Adenovirus has been associated with rejection, ventricular dysfunction, coronary vasculopathy, and the need for re-transplantation. The current standard treatment for adenovirus is cidofovir, but outcomes are not optimal [91].
Chronic hepatitis without an identifiable cause should prompt testing for hepatitis E virus (HEV). Chronic HEV infection leads to the rapid development of fibrosis. HEV testing should be done with RNA PCR due to a delay in the antibody response. We recommend decreased immunosuppression and ribavirin therapy for 3 months [92, 93]. Other less common manifestation that should be considered under the correct epidemiologic risk factors include HTLV-1/HTLV-2-associated myelopathy, rabies, lymphocytic choriomeningitis virus, subacute measles encephalitis, mumps (associated parotitis, orchitis, vestibular neuritis, and allograft involvement), dengue virus, orf virus, human coronavirus, and influenza [36].
Parasitic
Cardiac transplant itself is one the predictors for development of toxoplasmosis [94]. Other associated risk factors include negative serum status before transplant, diagnosis of cytomegalovirus (CMV) infection, and high-dose prednisone. Toxoplasmosis can be transmitted by the donor heart (D+/R−, especially during the first 3 months) or can reactivate from the recipient (>3 months). Most of the infections developed during the first 6 months posttransplant and are predominantly primary infections. About 22% of infected patients had a disseminated infection carrying an estimated 17% mortality. Toxoplasmosis can manifest otherwise with myocarditis, encephalitis, pneumonitis, or chorioretinitis. Diagnosis requires identification of tissue cysts surrounded by an abnormal inflammatory response, detection of Toxoplasma DNA in body fluids by PCR, or positive Toxoplasma-specific immunohistochemistry in affected organs. Posttransplant serological tests are not helpful for diagnosis and may be misleading since results may change or not regardless of the presence of toxoplasmosis [95]. The preferred treatment regimen is a combination of pyrimethamine with sulfadiazine [96].
Advanced Chagasic cardiomyopathy is a primary indication for heart transplantation in some centers [13]. Trypanosoma cruzi, the causal organism of Chagas disease, can be transmitted up to 75% of the time from infected heart donors (D+/R−) [97]. Additionally, Chagas disease can reactivate from the donor once immunosuppression is in place (R+). The reactivation rate can range between 22% and 90% in recipients with chronic chagasic cardiomyopathy undergoing heart transplant [98–100]. Additional risk factors for reactivation include rejection episodes, neoplasms, and use of MMF [98]. The mean onset of symptoms is approximately 112 days [101]. Once manifested, Chagas can present with nonspecific symptoms such as fever, malaise, anorexia, hepatosplenomegaly, and lymphadenopathy. Myocarditis, pericarditis, and encephalitis are also seen. Reactivation can mimic rejection and exhibits congestive heart failure, AV block and skin manifestations such as nodules and panniculitis. Increased eosinophil count and anemia can be indirect indicators of reactivation [102]. Diagnosis is made with the visualization of circulating trypomastigotes in peripheral blood. Additionally, blood and tissue PCR can be used. Tissue amastigotes can be seen in biopsy H&E preparations (Fig. 2.1). Finally, serologies are a crucial aspect in the diagnosis especially if seroconversion have been documented. In asymptomatic individuals, when the diagnosis of Chagas has been established in the donor, monitoring should be instituted with weekly blood T. cruzi PCR and microscopy [29]. Preferred antitrypanosomal therapy consists on benznidazole. Nifurtimox is an alternative treatment option. Posaconazole has anti-parasitic activity but carries high failure rates [103, 104]. GI disease with Isospora (Cystoisospora) belli, Cryptosporidium, Cyclospora, and Microsporidia has been reported to affect SOT recipients. Microsporidiosis can manifest with disseminated disease: fever, keratoconjunctivitis, CNS involvement, cholangitis, cough, and thoracic/abdominal pain [94]. Other rare parasitic infections affecting heart transplants include leishmaniasis, strongyloidiasis, and free-living amoebas [94, 105].
Fig. 2.1.
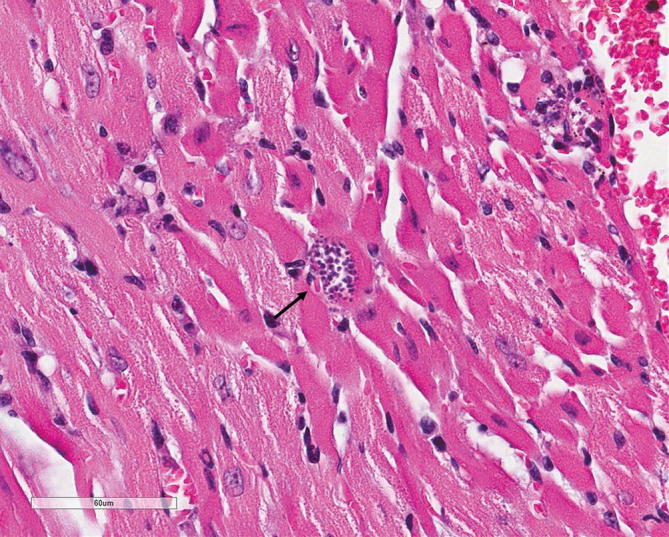
Trypanosoma cruzi amastigote in heart tissue (H&E stain, 400×)
Sites and Types of Infection
Skin, Soft Tissue, and Bone
The rate of surgical site infections (SSI)—sternal wound infections—in patients receiving antimicrobial prophylaxis ranged from 5.8% to 8.8% following heart transplant procedures [41]. Heart transplantation itself is an independent risk factor for SSIs. Other risk factors include age, prophylaxis with ciprofloxacin alone, positive wire cultures, female gender, previous left ventricular assist device (VAD) placement, BMI >30 kg/m2, previous cardiac procedures, and inotropic support for hemodynamic instability [41, 106]. Similarly to other hosts, Staphylococcus species are the predominant organism causing SSTIs. MRSA can reach up to 21% of the cases. Gram-positive organisms: VRE (E. faecalis), coagulase-negative staphylococci, and other Enterococcus species are other etiologic agents. Candida and selected gram negatives such as Enterobacteriaceae, P. aeruginosa, and Stenotrophomonas maltophilia can cause SSIs as well [107]. Sternal osteomyelitis often complicates deep SSI. Additionally, sternal wound infections by NTM and fungi such as Aspergillus and Scedosporium have been documented [108, 109]. Herpes zoster is also an important consideration and source of morbidity. Herpes zoster (HZ) is found as a complication in 19–22% of the patients with a median time of presentation ranging from 0.73 to 2.10 years [64, 110]. Close to half may develop postherpetic neuralgia. Multi-dermatome involvement, zoster ophthalmicus, and meningoencephalitis are also described. Exposure to MMF is an independent risk factor. Conversely, CMV prophylaxis reduces the risk for HZ.
Bloodstream
Bloodstream infections (BSIs) are a risk factor for mortality among heart transplant recipients. Likewise, SOT recipient status is an independent risk factor for developing bacteremia [111]. In heart transplant recipients; the rate of BSI ranged between 16% and 24%. The median onset is about 51–191 days, and the sources are in order of frequency: lower respiratory tract, urinary tract, and CRBSI. Gram-negative bacteria were more commonly isolated. They are in order of appearance E. coli, P. aeruginosa, and K. pneumoniae. More common Gram-positive bacteria were S. aureus, S. epidermidis, E. faecalis, and L. monocytogenes. Directly attributable mortality is 12.2%. Among the identifiable independent risk factors to develop BSI are hemodialysis, prolonged intensive care unit stay, and viral infections [112, 113]. Infective endocarditis (IE) is seen more frequently among heart transplant recipients than in the general population. With IE occurred, it most commonly involves the mitral and tricuspid valves and Staphylococcus aureus and Aspergillus are the main etiologic organisms. The main predisposing factors in this setting are believed to be the frequent use of vascular indwelling catheters and the frequency of endomyocardial biopsies [114]. Staphylococcus aureus bacteremia in heart transplant recipients ranges from 10% to 38% [11, 115]. The sources of SA bacteremia in SOT are CRBSI (30%), pneumonia (24%), wound (14%), endocarditis (10%), intra-abdominal infections (9%), bone and joint (7%), cardiac devices (3%), UTI (1%), and SSTI (1%) [115].
Chest
Immediately following heart transplant and during the 1st month, patients are more susceptible to develop pneumonia, most of which are healthcare or ventilator associated and therefore caused by nosocomial organisms such as MRSA, Pseudomonas aeruginosa, and other Gram negatives including Acinetobacter and ESBL-Enterobacteriaceas. Pneumonia is one the major contributors to mortality in the early postoperative period. Pneumonia-related mortality approaches 15% [116]. After the 1st month, interstitial pneumonia and pneumonitis can develop, and the differential includes herpesviruses (HSV, CMV, VZV) and respiratory syncytial virus (RSV), Toxoplasma gondii and Pneumocystis jiroveci. Pulmonary nodules with or without cavitation can be caused by fungi such as coccidioidomycosis, aspergillosis, mucormycosis, cryptococcosis; bacterial including actinomycosis, tuberculosis, atypical mycobacterial infections, Nocardia, Rhodococcus equi, and Gram-negative bacilli; and noninfectious causes like pulmonary infarction or lymphoproliferative disorders [117, 118]. Pulmonary nodules are seen in about 10% of the patients, and the median detection time is about 66 days. The associated symptoms are fever and cough. The most frequent etiology is Aspergillus followed by Nocardia, and Rhodococcus. CMV is an exceedingly rare cause of pulmonary nodules. The diagnostic approach with the higher yield is transthoracic fine needle aspiration followed by bronchoalveolar lavage and transtracheal aspiration [118]. Community-acquired pneumonia caused by Streptococcus pneumonia, Legionella spp., mycoplasma, and influenza is another source of morbidity [10].
Mediastinitis is a common complication in this setting. In patients receiving antimicrobial prophylaxis, mediastinitis develops in 3–7% of the patients [107, 119]. A CT scan is usually necessary to determine the extension of the infection. MRSA Staphylococcus epidermidis, Gram-negative bacteria, and Aspergillus fumigatus are frequently found as the causal organisms [120]. Antimicrobial therapy should be accompanied by aggressive surgical debridement [121].
Abdominal/Genitourinary
There are not distinctive abdominal-pelvic complications among heart transplant recipients. Clostridium difficile is a common hospital-related cause of diarrhea associated with the use of antimicrobials. Other etiology for diarrhea secondary to acute gastroenteritis can present in a protracted way in this setting. Listeria infection can present as a febrile gastroenteritis illness as well. Nontyphoid Salmonella infection has been described to complicate the early postoperative period in a center in Taiwan [122]. Acute cholecystitis can affect heart transplant recipients advocating to have a low threshold to use ultrasound as a screening method [123]. Acute pancreatitis with abscess formation has also been described [124]. As pointed above, hepatitis E can present with persistently abnormal liver tests.
Although less frequent than in kidney transplant recipients, urinary tract infections are an important cause of morbidity. UTIs are predisposed by Foley catheters. The organisms most commonly involved are Gram-negative bacteria, Enterococcus, and Candida. Polyomavirus nephropathy by BK virus has been described in heart transplant recipients and might be a contributor to chronic kidney disease [125].
Central Nervous System
The need for urgent transplantation and multiple transfusions are independently associated with infectious, neurologic complications. Its overall mortality can reach 12% [64]. Donor-derived meningoencephalitides affecting heart transplant recipients usually manifest within the first 30 days. These infections include West Nile virus, arenaviruses (e.g., LCMV), and rabies. WNV can manifest with a Guillain-Barré-like axonopathy with cerebrospinal fluid (CSF) pleocytosis. In addition to meningitis or encephalitis, ataxia, myelitis, optic neuritis, polyradiculitis, and seizures can also be observed [126]. WNV can be also acquired by the recipient in the community or through blood transfusions and present at a later time [127]. Other infectious forms of meningitis and encephalitis that can present after the 1st month include listeriosis, Streptococcus pneumoniae, Trypanosoma cruzi, Toxoplasma, HHV-6, and disseminated herpes virus infections (CMV, VZV, HSV, and EBV) [128–130]. The absence of appropriate primary prophylaxis or monitoring increases their risk. Aspergillus causes the majority of brain abscess. Additionally Toxoplasma, tuberculosis, Listeria spp., Cryptococcus neoformans, Scedosporium spp., and Nocardia can also be causative agents [129]. Concomitant pulmonary involvement is common, particularly for those whose portal of entry is the respiratory tract.
Progressive multifocal leukoencephalopathy (PML), a demyelinating disease caused by the reactivation of JC virus, has a usual median onset of 27 months. It carries a marked high case fatality rate and a median survival of 6.4 months in SOT [131]. The use of rituximab as an antirejection treatment seems to confer an increased risk for PML [132]. HTLV-1-associated myelopathy (HAM) has been described as well in SOT.
Infections in Lung and Heart-Lung Transplantation
Infecting Microbial Agents
Bacterial
Bacterial infections are the most common type of infections among lung and lung-heart transplant recipients. The anatomic site most frequently affected is the respiratory tract, usually manifested with pneumonia, sinusitis, or tracheobronchitis. Previous colonization, healthcare associated, and procedures related are the primary sources. For patients with cystic fibrosis (CF), knowledge of previous colonization results may provide some diagnostic and therapeutic advantages. Pseudomonas aeruginosa is a predominant colonizing pathogen in CF. However, Acinetobacter baumannii, Burkholderia species, Stenotrophomonas maltophilia, Achromobacter xylosoxidans, NTM, Pandorea, and Ralstonia are also observed [23]. Furthermore, pathogens that are known to cause nosocomial pneumonia during the 1st month include Staphylococcus aureus, Pseudomonas aeruginosa, other Gram negatives (Klebsiella pneumoniae, Enterobacter cloacae, Serratia marcescens, Escherichia coli, Acinetobacter species), and anaerobes.
Gram-positive bacteria are a common source of infections making up to 40% of them [133]. The most common sites affected were the respiratory tract, followed by bacteremia, skin, wound, and catheter related. The pathogens more frequently identified are Staphylococcus species (77%), Enterococcus species (12%), Streptococcus species (6%), Pneumococcus (4%), and Eubacterium lentum (1%). Staphylococcus aureus infection can develop up to 20% of lung recipients. SA commonly causes pneumonia, followed by tracheobronchitis, bacteremia, intrathoracic infections, and SSTIs [20]. Streptococcus pneumoniae is community acquired and present with pneumonia, usually after 6 months posttransplant. Pseudomonas aeruginosa has high rates of colonization (up to 40%) and disease (30%) [134]. Other significant bacterial infections that may present after the 1st month are Mycobacterium tuberculosis, NTM, Nocardia, Rhodococcus, and Legionella. Isolation of NTM in lung transplant recipients without evidence of disease is not associated with increased mortality [135]. Nocardiosis can occur in about 2% of the lung transplant recipients. The median time of onset ranges from 14.3 to 34.1 months [136, 137]. Nocardia asteroides, N. farcinica, N. nova, and N. brasiliensis have been reported. N. farcinica appears to carry worse outcomes. This infection can present as a breakthrough in the presence of trimethoprim-sulfamethoxazole for P. jiroveci prophylaxis, although the isolates may remain susceptible. Mortality has been reported to range between 18% and 40%. The native lung is more frequently affected in single-lung transplant recipients. Nodules are the more prevalent radiographic finding. Extrapulmonary involvement affecting the skin and brain can be seen. Hypogammaglobulinemia and neutropenia seem to confer additional risk factors for nocardiosis in this setting [137].
Fungal
Fungal infections are frequent complications in lung and lung-heart transplant. They present in about 15–35% and carry an overall mortality close to 60% [138]. Aspergillus and Candida are the most frequent causative agents. Other important fungi include Cryptococcus spp., mucormycosis, endemic fungi (Histoplasma, Coccidioides, and Blastomyces spp.), Scedosporium spp., Fusarium spp., and dematiaceous molds. Candida infections are prominent during the 1st month after transplantation. It can be one of the most common causes of BSI in this setting [139]. Although colonization of the upper airways and gastrointestinal tract is common, Candida additionally can cause mucocutaneous disease, tracheobronchitis, anastomosis site infections, CRBSI, and disseminated disease. Aspergillus spp. lead as the cause of invasive fungal infections. Its attack rate of infection is almost ten times compared to that in other SOT patients (estimated incidence of 6% among lung transplant recipients) [140, 141]. A. fumigatus is the most common species, but A. terreus, A. flavus, and A. niger have been described as well. The main predisposing risk factors in this setting are intense immunosuppression, previous colonization with Aspergillus spp., airway ischemia, and BOS. Single-lung transplant possesses the greatest risk to developing an invasive Aspergillus infection carrying a higher mortality than double-lung and heart-lung transplant recipients. Single-lung recipients are usually older and more likely to have COPD as the indication for transplantation [140]. Aspergillus infections can present as tracheobronchitis, pneumonia, or disseminated disease. Extrapulmonary involvement includes sinusitis, CNS or orbits infections, and vertebral osteomyelitis. Aids in the diagnosis can include surveillance bronchoscopies (bronchoalveolar lavage stain and culture; biopsy), chest CT and serum/BAL galactomannan, beta-D-glucan, and PCR. The presence of pulmonary nodular lesions in invasive infections can carry better outcomes [142]. Voriconazole is the treatment of choice. It is important to note that immune reconstitution inflammatory syndrome (IRIS) can develop at a median of 56 days in 7% of treated lung transplant recipients [143]. In Aspergillus tracheobronchitis, nebulized amphotericin B and debridement of the bronchial anastomosis are important adjuvant measures to systemic antifungal therapy [144, 145]. Pneumocystis jirovecii pneumonia manifests from 1 to 6 months. Its incidence has been reduced dramatically with universal TMP/SMX prophylaxis. Cryptococcosis with a rate of 2% in lung transplant recipients presents with pulmonary involvement, but dissemination with meningitis can occur. Furthermore, Cryptococcus skin manifestations like cellulitis and Cryptococcus-associated IRIS have been documented [146, 147].
Viral
Viral infections are a common cause of morbidity among lung transplant recipients. The most common viruses are (1) CMV among the herpes viruses and (2) community-acquired respiratory viruses. As in other SOT recipients, the higher risk to develop CMV infection is among D+/R−, followed by D+/R+, D−/R+, and D−/R−. This last scenario carries less than 5% of risk [48, 148]. Lung transplant recipients possess higher risk for CMV than other SOT with an estimated incidence of 30–86% [87]. The lung is considered a primary reservoir for CMV latency, and abundant lymphocytic tissue surrounds the transplanted organ. Additionally, the use of antilymphocyte antibodies to treat rejection or for immunosuppression and other herpesviruses infections are additional risk factors for CMV disease [149]. Interferon (IFN)-γ (+874T/T) polymorphism increases IFN levels and may be a predisposition for CMV disease [150]. CMV is significantly associated with BOS, which reduces survival after the first year posttransplant [151]. CMV disease is most commonly manifested by pneumonitis or viral syndrome and less frequently with gastrointestinal disease. Among lung transplant recipients, ganciclovir-resistant CMV carries an increased morbidity and mortality [152].
Infections with community-acquired respiratory viruses ranged from 7.7% to 64%. These infections are associated with increased risk to develop pneumonia, graft dysfunction manifested by lung function loss, BOS, high calcineurin inhibitor blood levels, and increase mortality [153–155]. These viruses include influenza, parainfluenza, respiratory syncytial virus (RSV), coronaviruses, human rhinovirus, adenovirus, human metapneumoviruses, and bocaviruses. The hospitalization rates are higher for influenza and parainfluenza (50% and 17%, respectively) [154]. Symptoms are usually nonspecific. Diagnosis often requires detection of viral nucleoprotein antigens in nasopharyngeal swabs or bronchoalveolar lavage (BAL) by enzyme immunoassay or fluorescent antibody or the amplification of nucleic acid by PCR. Ribavirin may possess activity against Paramyxoviruses (RSV, Metapneumovirus, and parainfluenza). Ribavirin is administered inhaled, orally, or intravenously. Oseltamivir or zanamivir is the treatment choice of influenza A or B [156]. Adamantanes (amantadine and rimantadine) are not active against influenza B, and there is a marked increase resistance among influenza A strains [156]. Similarly to other SOT recipients, DNA viruses like non-CMV herpesviruses (HSV-1,-2), VZV, HHV-6,-7,-8, and EBV are a source of significant morbidity including but not limited to CMV-negative viral syndrome, rash, pneumonitis, hepatitis, and encephalitis [157]. Lastly, polyomavirus such as BK virus (BKV), JC virus (JCV), and simian virus 40 (SV40)—although frequently encountered in lung transplant recipients with an unclear causality—may cause worsening renal function or survival [158]. PTLD is also a well-recognized complication. A trend toward late PTLD presentation (>1 year) has been documented where B symptoms are more predominant as well as extra-graft involvement [159].
Parasitic
As other immunosuppressive states , certain parasitic infections can complicate lung and heart-lung transplants recipients. It is critical to elicit a detailed history and geographic risk factors to determine the risk of acquisition and the potential etiologic agent. Toxoplasmosis can result from primary infection or reactivation of previous latent infections. Toxoplasmosis can develop in patients with negative epidemiological history for cat ownership or consumption of undercooked meat. In patients with primary toxoplasmosis, nonspecific symptoms such as fever, lymphadenopathy, or organ injury may be present. Reactivation can cause encephalitis with or without space-occupying brain lesions, seizures, chorioretinitis, fever of unknown origin, pneumonitis, myocarditis, and rash. Although cases of the lung fluke, Paragonimus westermani have not been reported in lung transplantation, it can be a potential threat in endemic areas where this organism is endemic. Other parasites that can target the lung in immunosuppressive states include Echinococcus, Schistosoma , and Strongyloides stercoralis [160]. Strongyloidiasis can present as hyperinfection syndrome [161]. Leishmania, although infrequently seen, has been reported among lung and lung-heart recipients [30]. Free-living amoebas can affect this population as well. Amoebic granulomatous dermatitis and disseminated infection presenting with ulcerative skin lesions, respiratory failure, and seizures have been described in lung transplant recipients [162, 163]. Finally, alimentary protozoa , including Cryptosporidium, which present with diarrhea and may elevate tacrolimus levels [164], and microsporidia, which present with unusual manifestations like myositis or granulomatous interstitial nephritis, affects lung transplant recipients [165, 166].
Sites and Types of Infection
Skin, Soft Tissue, and Bone
The overall rate of SSIs is about 13% with a significant proportion of infections being organ or space occupying (72%), deep incisional (17%), and superficial (10%) [18, 41]. Independent risk factors to develop SSI are diabetes, female donor, prolonged ischemic time, and the number of red blood cells transfusion during the perioperative period [167]. SSIs are associated with a 35% mortality within the first year of transplantation. The most common organisms found to cause SSI or mediastinitis are P. aeruginosa, Candida species, S. aureus (including MRSA), Enterococcus, coagulase-negative Staphylococci, Burkholderia cepacia, E. coli, Proteus mirabilis, Serratia marcescens, Acinetobacter baumannii, Enterobacter cloacae, and Klebsiella species. There is a correlation in up to 33% of the patients’ SSI causative organisms with previous pathogens colonizing recipients’ native lungs at the time of the transplant [167]. The median onset is 25 days after lung transplant [167]. Although rare, NTM can cause SSI infections among lung transplant recipients. The most frequently encountered are Mycobacterium avium complex followed by Mycobacterium abscessus and Mycobacterium gordonae. NTM SSI infections can be complicated by progressive disseminated disease or requirement of lifelong suppressive therapy [135]. Other organisms such as Mycoplasma hominis and Lactobacillus spp. have also been described. Deep infections can affect up to 5% of the patients. Sternal osteomyelitis can reach up to 6% of these deep infections. Causative organisms for sternal osteomyelitis include Pseudomonas aeruginosa, Serratia marcescens, and Scedosporium. Non-sternal osteomyelitis affecting the calcaneus bone has complicated a disseminated infection with Aspergillus fumigatus [168].
Bloodstream
Bloodstream infections (BSIs) occur with an estimated rate of 25% among lung transplant recipients. A major proportion of BSIs occur in the early posttransplant period. BSIs infections are significantly associated with worse survival [139, 169]. The most common organisms encountered are Staphylococcus aureus, Pseudomonas aeruginosa, and Candida [139]. Pseudomonas aeruginosa BSI—predominantly present during the transplant hospitalization period and more commonly affecting CF patients—is followed in frequency by Burkholderia cepacia and Candida albicans. Conversely, Staphylococcus aureus was the predominant organism after transplantation discharge. In an estimated 70% of BSI, the source was pulmonary, followed in frequency by CRBSI , gastrointestinal infection, peritonitis, and UTI. A pulmonary source of bacteremia in SOT often develops into septic shock [170]. Although unusual, cases of Aspergillus fumigatus endocarditis have been described following lung transplantation [171]. Often patients had CF as the underlying lung disease and a median of 8 ± 6 months presentation. This complication carries a high mortality and often requires a combination of antifungal therapy with valvular replacement surgery.
Chest
Infectious complications related to the chest cavity include mediastinitis, cardiac (pericarditis and myocarditis), lung parenchyma infections (nodular infiltrates, cavitation, or pneumonia), bronchial anastomosis infections, and pleural space infections (bronchopleural fistula and empyema). Empyema followed by mediastinitis and pericarditis, in addition to surgical wound infections and sternal osteomyelitis, is the most frequent deep SSI complications affecting the chest cavity. Empyema presents in around of 3.6% of cases. It occurs during the first 6 months after transplantation (median 46 ± 39 days) carrying an estimated mortality of 28.6% [172]. Most common organisms found are Staphylococcus spp., E. coli, Enterobacter spp., Klebsiella spp., Mycoplasma hominis, VRE, and Candida. Furthermore, Mycobacterium abscessus was isolated as a rare causative agent of empyema as well [173]. The degree of immunosuppression , reduced renal function, previous sternotomy, and re-exploration due to bleeding are listed as potential risk factors for mediastinitis [119]. There is an increased prevalence of mediastinitis caused by Gram negatives and fungi among lung transplant recipients. Causative organisms for mediastinitis are similar to SSI and are listed above. Infectious pericarditis can be present up to 6% of the patients (isolated organisms include MSSA, Mycoplasma hominis, and Scedosporium prolificans) [167, 174, 175]. Due to their high fatal rate, fungal bronchial anastomotic infections are critical to recognize.
Pneumonia is believed to affect around 21% of lung recipients and 40% of heart-lung recipients. Nosocomial organisms cause early pneumonia as in other posttransplant settings. The donor’s lung seems to be the primary source for pneumonic infections, although the recipients’ upper airways or sinuses are also potential sources. Preoperative colonization with Gram-negative rods and colonized infected donor bronchus or perfusate are recognized risk factors for pneumonia. Likewise, pretransplantation colonizing microorganisms from suppurative lung disease are associated with pneumonia development posttransplant [176]. The most common causal organisms are Pseudomonas aeruginosa, Staphylococcus aureus, and Aspergillus spp. Other pathogens include bacteria such as B. cepacia, Enterobacter species, S. maltophilia, Klebsiella species, S. epidermidis, and E. coli, and fungi such as Fusarium spp., Cryptococcus neoformans, and Paracoccidioides brasiliensis [176]. After the 1st month, pneumonia can present as local infiltrates, diffuse interstitial infiltrates, and nodules with or without cavitation. This type of presentation may aid in the possible causative microorganism. The list of potential pathogens is extensive and includes in addition to the already mentioned Nocardia, Chlamydia pneumonia, Legionella, TB, NTM, Pneumocystis jirovecii, Rhodococcus, herpesviruses (CMV, HSV, and VZV), respiratory viruses, endemic fungi (e.g., histoplasmosis), mucormycosis, and Scedosporium spp. [177–179].
Abdominal/Genitourinary
Similarly to other SOT, common infectious complications affecting the gastrointestinal or genitourinary tract include Clostridium difficile colitis and UTIs. Intra-abdominal complication carries an overall increase mortality [180]. Frequent GI symptoms presenting posttransplant are diarrhea which can affect almost 30% of lung transplant recipients and abdominal pain. Abdominal pain should prompt further investigation for potential intra-abdominal causes . In the pediatric population, the possibility of PTLD should be investigated since it carries a high mortality [181]. Other described infectious intra-abdominal complications include digestive perforation (seen in 6%) [182], retroperitoneal abscesses, cholecystitis, perianal abscesses, esophagitis, pancreatitis, pancreatic abscesses, hepatitis, diverticulitis, appendicitis, CMV colitis, megacolon, and colon rupture [180, 183, 184]. In developing countries, persistently abnormal liver enzymes should prompt testing for HEV. HEV RNA should be used for screening. Oral ribavirin seems to be safe and effective in this setting [185].
Central Nervous System (CNS)
CNS symptoms developing during the 1st month following lung or heart-lung transplantation should trigger the concern for donor-derived viral infections. LCMV often is accompanied by CSF normal to low glucose, marked elevated protein, and mild pleocytosis [36]. Although with unclear benefit, ribavirin has been used. Donor-transmitted rabies is an uncommon but neurologic devastating complication that occurs within the first 30 days of transplant. Lung transplantation has been described as a potential causal mechanism [186]. Other organisms known to cause meningitis in lung transplant recipients are Cryptococcus, tuberculosis, WNV, and herpesviruses [187, 188]. Diagnosis of WNV in this setting requires nuclear acid amplification due to the unreliability of serologic testing. Scedosporium apiospermum infections often cause dissemination including CNS abscesses in addition to pulmonary involvement among lung transplant recipients [189]. It is important to differentiate from other molds, since amphotericin B is ineffective against Scedosporium spp. In severe cases or refractory disease without an appropriate surgical debridement, the addition of terbinafine to voriconazole may prove to be useful [190]. Other recognized organisms causing occupying brain lesions are Fusarium, Nocardia, Aspergillus, toxoplasmosis, Cryptococcus neoformans, Listeria, and Cladophialophora bantiana [191–193]. PML, a late manifestation, can be associated with intensified immunosuppression or rituximab. Cidofovir followed by mirtazapine can be considered as a form of therapy for PML.
Conclusions
Infections in heart, lung, and heart-lung transplant recipients are a complex, dynamic, and evolving process. Many factors such as demographics, timing, type of transplant, anatomy, and microbiology, among others, interplay in the development of these fatal complications. Pertinent recognition and treatment of these infections improve transplantation outcomes.
Acknowledgments
No funding agencies had any role in the preparation, review, or approval of this paper. The views expressed in this paper are those of the authors and do not necessarily represent the views of the University of Colorado Denver or Stanford University. No conflict of interests was reported by Andrés F. Henao-Martínez and José G. Montoya.
Contributor Information
Amar Safdar, Email: amarsafdar@gmail.com.
Andrés F. Henao-Martínez, Email: andres.henaomartinez@ucdenver.edu
José G. Montoya, Email: gilberto@stanford.edu
References
- 1.Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, Christie JD, et al. The registry of the international society for heart and lung transplantation: thirtieth official adult heart transplant report—2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):951–964. doi: 10.1016/j.healun.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Durante-Mangoni E, Casillo R, Pinto D, Caianiello C, Albisinni R, Caprioli V, et al. Heart transplantation during active infective endocarditis: case report and review of the literature. Transplant Proc. 2011;43(1):304–306. doi: 10.1016/j.transproceed.2010.09.095. [DOI] [PubMed] [Google Scholar]
- 3.Haddad F, Deuse T, Pham M, Khazanie P, Rosso F, Luikart H, et al. Changing trends in infectious disease in heart transplantation. J Heart Lung Transplant. 2010;29(3):306–315. doi: 10.1016/j.healun.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13(9):785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodford N, Livermore DM. Infections caused by Gram-positive bacteria: a review of the global challenge. J Infect. 2009;59(Suppl 1):S4–16. doi: 10.1016/S0163-4453(09)60003-7. [DOI] [PubMed] [Google Scholar]
- 6.Reitz BA, Wallwork JL, Hunt SA, Pennock JL, Billingham ME, Oyer PE, et al. Heart-lung transplantation. N Engl J Med. 1982;306(10):557–564. doi: 10.1056/NEJM198203113061001. [DOI] [PubMed] [Google Scholar]
- 7.De Soyza A, Meachery G, Hester KL, Nicholson A, Parry G, Tocewicz K, et al. Lung transplantation for patients with cystic fibrosis and Burkholderia cepacia complex infection: a single-center experience. J Heart Lung Transplant. 2010;29(12):1395–1404. doi: 10.1016/j.healun.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Stinson EB, Bieber CP, Griepp RB, Clark DA, Shumway NE, Remington JS. Infectious complications after cardiac transplantation in man. Ann Intern Med. 1971;74(1):22–36. doi: 10.7326/0003-4819-74-1-22. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Padilla M, Caston JJ, Vidal E, Arizon JM, Segura C, Montejo M, et al. Epidemiology and clinical impact of infection in patients awaiting heart transplantation. Int J Infect Dis. 2013;17(9):e681–e685. doi: 10.1016/j.ijid.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Gurgui M, Munoz P. Infection in heart transplantation. Enferm Infecc Microbiol Clin. 2007;25(9):587–597. doi: 10.1157/13111187. [DOI] [PubMed] [Google Scholar]
- 11.Montoya JG, Giraldo LF, Efron B, Stinson EB, Gamberg P, Hunt S, et al. Infectious complications among 620 consecutive heart transplant patients at Stanford University Medical Center. Clin Infect Dis. 2001;33(5):629–640. doi: 10.1086/322733. [DOI] [PubMed] [Google Scholar]
- 12.van de Beek D, Kremers WK, Del Pozo JL, Daly RC, Edwards BS, McGregor CG, et al. Effect of infectious diseases on outcome after heart transplant. Mayo Clin Proc. 2008;83(3):304–308. doi: 10.4065/83.3.304. [DOI] [PubMed] [Google Scholar]
- 13.Godoy HL, Guerra CM, Viegas RF, Dinis RZ, Branco JN, Neto VA, et al. Infections in heart transplant recipients in Brazil: the challenge of Chagas’ disease. J Heart Lung Transplant. 2010;29(3):286–290. doi: 10.1016/j.healun.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Carlos, Gomez SHT, Gould K, Valantine H, Montoya JG. Diagnosis and management of infectious diseases in cardiothoracic transplantation and mechanical circulatory support. ISHLT Monograph Series. 5. Elsevier; 2011. p. 1–10.
- 15.Yusen RD, Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, et al. The registry of the international society for heart and lung transplantation: thirtieth adult lung and heart-lung transplant report—2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):965–978. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Cai J. Thoracic transplantation in the United States: an analysis of UNOS registry data. Clin Transpl. 2006;1:41–56. [PubMed] [Google Scholar]
- 17.Russo MJ, Iribarne A, Hong KN, Davies RR, Xydas S, Takayama H, et al. High lung allocation score is associated with increased morbidity and mortality following transplantation. Chest. 2010;137(3):651–657. doi: 10.1378/chest.09-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattner F, Fischer S, Weissbrodt H, Chaberny IF, Sohr D, Gottlieb J, et al. Post-operative nosocomial infections after lung and heart transplantation. J Heart Lung Transplant. 2007;26(3):241–249. doi: 10.1016/j.healun.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Parada MT, Alba A, Sepulveda C. Early and late infections in lung transplantation patients. Transplant Proc. 2010;42(1):333–335. doi: 10.1016/j.transproceed.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Shields RK, Clancy CJ, Minces LR, Kwak EJ, Silveira FP, Abdel Massih RC, et al. Staphylococcus aureus infections in the early period after lung transplantation: epidemiology, risk factors, and outcomes. J Heart Lung Transplant. 2012;31(11):1199–1206. doi: 10.1016/j.healun.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Speich R, van der Bij W. Epidemiology and management of infections after lung transplantation. Clin Infect Dis. 2001;33(Suppl 1):S58–S65. doi: 10.1086/320906. [DOI] [PubMed] [Google Scholar]
- 22.Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis. 1998;27(5):1266–1277. doi: 10.1086/514993. [DOI] [PubMed] [Google Scholar]
- 23.Shoham S, Shah PD. Impact of multidrug-resistant organisms on patients considered for lung transplantation. Infect Dis Clin N Am. 2013;27(2):343–358. doi: 10.1016/j.idc.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aris RM, Gilligan PH, Neuringer IP, Gott KK, Rea J, Yankaskas JR. The effects of panresistant bacteria in cystic fibrosis patients on lung transplant outcome. Am J Respir Crit Care Med. 1997;155(5):1699–1704. doi: 10.1164/ajrccm.155.5.9154879. [DOI] [PubMed] [Google Scholar]
- 25.Alexander BD, Petzold EW, Reller LB, Palmer SM, Davis RD, Woods CW, et al. Survival after lung transplantation of cystic fibrosis patients infected with Burkholderia cepacia complex. Am J Transplant. 2008;8(5):1025–1030. doi: 10.1111/j.1600-6143.2008.02186.x. [DOI] [PubMed] [Google Scholar]
- 26.Boussaud V, Guillemain R, Grenet D, Coley N, Souilamas R, Bonnette P, et al. Clinical outcome following lung transplantation in patients with cystic fibrosis colonised with Burkholderia cepacia complex: results from two French centres. Thorax. 2008;63(8):732–737. doi: 10.1136/thx.2007.089458. [DOI] [PubMed] [Google Scholar]
- 27.Fitzpatrick MA, Caicedo JC, Stosor V, Ison MG. Expanded infectious diseases screening program for Hispanic transplant candidates. Transpl Infect Dis. 2010;12(4):336–341. doi: 10.1111/j.1399-3062.2010.00517.x. [DOI] [PubMed] [Google Scholar]
- 28.Vikram HR, Dosanjh A, Blair JE. Coccidioidomycosis and lung transplantation. Transplantation. 2011;92(7):717–721. doi: 10.1097/TP.0b013e31822e6e9a. [DOI] [PubMed] [Google Scholar]
- 29.Chin-Hong PV, Schwartz BS, Bern C, Montgomery SP, Kontak S, Kubak B, et al. Screening and treatment of chagas disease in organ transplant recipients in the United States: recommendations from the chagas in transplant working group. Am J Transplant. 2011;11(4):672–680. doi: 10.1111/j.1600-6143.2011.03444.x. [DOI] [PubMed] [Google Scholar]
- 30.Morales P, Torres JJ, Salavert M, Peman J, Lacruz J, Sole A. Visceral leishmaniasis in lung transplantation. Transplant Proc. 2003;35(5):2001–2003. doi: 10.1016/S0041-1345(03)00664-X. [DOI] [PubMed] [Google Scholar]
- 31.Roxby AC, Gottlieb GS, Limaye AP. Strongyloidiasis in transplant patients. Clin Infect Dis. 2009;49(9):1411–1423. doi: 10.1086/630201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain VV, Evans T, Peterson MW. Reactivation histoplasmosis after treatment with anti-tumor necrosis factor alpha in a patient from a nonendemic area. Respir Med. 2006;100(7):1291–1293. doi: 10.1016/j.rmed.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 33.Luong ML, Morrissey O, Husain S. Assessment of infection risks prior to lung transplantation. Curr Opin Infect Dis. 2010;23(6):578–583. doi: 10.1097/QCO.0b013e32833f9f93. [DOI] [PubMed] [Google Scholar]
- 34.Fischer SA, Avery RK. Screening of donor and recipient prior to solid organ transplantation. Am J Transplant. 2009;9(Suppl 4):S7–18. doi: 10.1111/j.1600-6143.2009.02888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matignon M, Botterel F, Audard V, Dunogue B, Dahan K, Lang P, et al. Outcome of renal transplantation in eight patients with Candida sp. contamination of preservation fluid. Am J Transplant. 2008;8(3):697–700. doi: 10.1111/j.1600-6143.2007.02112.x. [DOI] [PubMed] [Google Scholar]
- 36.Waggoner JJ, Soda EA, Deresinski S. Rare and emerging viral infections in transplant recipients. Clin Infect Dis. 2013;57(8):1182–1188. doi: 10.1093/cid/cit456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gangappa S, Kokko KE, Carlson LM, Gourley T, Newell KA, Pearson TC, et al. Immune responsiveness and protective immunity after transplantation. Transpl Int. 2008;21(4):293–303. doi: 10.1111/j.1432-2277.2007.00631.x. [DOI] [PubMed] [Google Scholar]
- 38.Bridges CB, Woods L, Coyne-Beasley T. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedule for adults aged 19 years and older – United States, 2013. MMWR Surveill Summ. 2013;62(Suppl 1):9–19. [PubMed] [Google Scholar]
- 39.Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):309–318. doi: 10.1093/cid/cit816. [DOI] [PubMed] [Google Scholar]
- 40.Soave R. Prophylaxis strategies for solid-organ transplantation. Clin Infect Dis. 2001;33(Suppl 1):S26–S31. doi: 10.1086/320901. [DOI] [PubMed] [Google Scholar]
- 41.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283. doi: 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 42.Playford EG, Webster AC, Sorell TC, Craig JC. Antifungal agents for preventing fungal infections in solid organ transplant recipients. Cochrane Database Syst Rev. 2004;(3):CD004291. [DOI] [PMC free article] [PubMed]
- 43.Munoz P, Valerio M, Palomo J, Giannella M, Yanez JF, Desco M, et al. Targeted antifungal prophylaxis in heart transplant recipients. Transplantation. 2013;96(7):664–669. doi: 10.1097/TP.0b013e31829e6d7b. [DOI] [PubMed] [Google Scholar]
- 44.Drew RH, Dodds Ashley E, Benjamin DK, Jr, Duane Davis R, Palmer SM, Perfect JR. Comparative safety of amphotericin B lipid complex and amphotericin B deoxycholate as aerosolized antifungal prophylaxis in lung-transplant recipients. Transplantation. 2004;77(2):232–237. doi: 10.1097/01.TP.0000101516.08327.A9. [DOI] [PubMed] [Google Scholar]
- 45.Palmer SM, Drew RH, Whitehouse JD, Tapson VF, Davis RD, McConnell RR, et al. Safety of aerosolized amphotericin B lipid complex in lung transplant recipients. Transplantation. 2001;72(3):545–548. doi: 10.1097/00007890-200108150-00036. [DOI] [PubMed] [Google Scholar]
- 46.Avery RK. Antifungal prophylaxis in lung transplantation. Semin Respir Crit Care Med. 2011;32(6):717–726. doi: 10.1055/s-0031-1295719. [DOI] [PubMed] [Google Scholar]
- 47.Beam E, Razonable RR. Cytomegalovirus in solid organ transplantation: epidemiology, prevention, and treatment. Curr Infect Dis Rep. 2012;14(6):633–641. doi: 10.1007/s11908-012-0292-2. [DOI] [PubMed] [Google Scholar]
- 48.Razonable RR, Humar A. Cytomegalovirus in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):93–106. doi: 10.1111/ajt.12103. [DOI] [PubMed] [Google Scholar]
- 49.Marty FM, Winston DJ, Rowley SD, Vance E, Papanicolaou GA, Mullane KM, et al. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med. 2013;369(13):1227–1236. doi: 10.1056/NEJMoa1303688. [DOI] [PubMed] [Google Scholar]
- 50.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 51.Humar A, Snydman D. Cytomegalovirus in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S78–S86. doi: 10.1111/j.1600-6143.2009.02897.x. [DOI] [PubMed] [Google Scholar]
- 52.Kumar D, Chernenko S, Moussa G, Cobos I, Manuel O, Preiksaitis J, et al. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant. 2009;9(5):1214–1222. doi: 10.1111/j.1600-6143.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- 53.Ritta M, Costa C, Sinesi F, Sidoti F, Di Nauta A, Mantovani S, et al. Evaluation of Epstein-Barr virus-specific immunologic response in solid organ transplant recipients with an enzyme-linked ImmunoSpot assay. Transplant Proc. 2013;45(7):2754–2757. doi: 10.1016/j.transproceed.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 54.Sarmiento E, del Pozo N, Gallego A, Fernandez-Yanez J, Palomo J, Villa A, et al. Decreased levels of serum complement C3 and natural killer cells add to the predictive value of total immunoglobulin G for severe infection in heart transplant recipients. Transpl Infect Dis. 2012;14(5):526–539. doi: 10.1111/j.1399-3062.2012.00757.x. [DOI] [PubMed] [Google Scholar]
- 55.Mori T, Aisa Y, Kato J, Nakamura Y, Ikeda Y, Okamoto S. Drug interaction between voriconazole and calcineurin inhibitors in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2009;44(6):371–374. doi: 10.1038/bmt.2009.38. [DOI] [PubMed] [Google Scholar]
- 56.Romero AJ, Le Pogamp P, Nilsson LG, Wood N. Effect of voriconazole on the pharmacokinetics of cyclosporine in renal transplant patients. Clin Pharmacol Ther. 2002;71(4):226–234. doi: 10.1067/mcp.2002.121911. [DOI] [PubMed] [Google Scholar]
- 57.Kuypers DR, Verleden G, Naesens M, Vanrenterghem Y. Drug interaction between mycophenolate mofetil and rifampin: possible induction of uridine diphosphate-glucuronosyltransferase. Clin Pharmacol Ther. 2005;78(1):81–88. doi: 10.1016/j.clpt.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 58.Buffington GA, Dominguez JH, Piering WF, Hebert LA, Kauffman HM, Jr, Lemann J., Jr Interaction of rifampin and glucocorticoids. Adverse effect on renal allograft function. JAMA. 1976;236(17):1958–1960. doi: 10.1001/jama.1976.03270180034016. [DOI] [PubMed] [Google Scholar]
- 59.Kannankeril P, Roden DM, Darbar D. Drug-induced long QT syndrome. Pharmacol Rev. 2010;62(4):760–781. doi: 10.1124/pr.110.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bui Kevin T., Mehta Seema, Khuu Tam H., Ross David, Carlson Margrit, Leibowitz Matthew R., Schaenman Joanna M., Saggar Rajan, Lynch Joseph P., Ardehali Abbas, Kubak Bernard M. Extended Spectrum β-Lactamase–Producing Enterobacteriaceae Infection in Heart and Lung Transplant Recipients and in Mechanical Circulatory Support Recipients. Transplantation. 2014;97(5):590–594. doi: 10.1097/01.TP.0000436928.15650.59. [DOI] [PubMed] [Google Scholar]
- 61.Raso-Raso R, Martinez-Dolz LV, Almenar-Bonet L, Navarro-Manchon J, Blanes-Julia M, Salvador-Sanz A. Nocardia infection in heart transplant recipients. Rev Clin Esp. 2010;210(8):389–393. doi: 10.1016/j.rce.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 62.Peleg AY, Husain S, Qureshi ZA, Silveira FP, Sarumi M, Shutt KA, et al. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: a matched case-control study. Clin Infect Dis. 2007;44(10):1307–1314. doi: 10.1086/514340. [DOI] [PubMed] [Google Scholar]
- 63.Peraira JR, Segovia J, Fuentes R, Jimenez-Mazuecos J, Arroyo R, Fuertes B, et al. Pulmonary nocardiosis in heart transplant recipients: treatment and outcome. Transplant Proc. 2003;35(5):2006–2008. doi: 10.1016/S0041-1345(03)00651-1. [DOI] [PubMed] [Google Scholar]
- 64.Munoz P, Valerio M, Palomo J, Fernandez-Yanez J, Fernandez-Cruz A, Guinea J, et al. Infectious and non-infectious neurologic complications in heart transplant recipients. Medicine (Baltimore) 2010;89(3):166–175. doi: 10.1097/MD.0b013e3181dfa59c. [DOI] [PubMed] [Google Scholar]
- 65.Stamm AM, Smith SH, Kirklin JK, McGiffin DC. Listerial myocarditis in cardiac transplantation. Rev Infect Dis. 1990;12(5):820–823. doi: 10.1093/clinids/12.5.820. [DOI] [PubMed] [Google Scholar]
- 66.Munoz P, Palomo J, Munoz R, Rodriguez-Creixems M, Pelaez T, Bouza E. Tuberculosis in heart transplant recipients. Clin Infect Dis. 1995;21(2):398–402. doi: 10.1093/clinids/21.2.398. [DOI] [PubMed] [Google Scholar]
- 67.Patel R, Roberts GD, Keating MR, Paya CV. Infections due to nontuberculous mycobacteria in kidney, heart, and liver transplant recipients. Clin Infect Dis. 1994;19(2):263–273. doi: 10.1093/clinids/19.2.263. [DOI] [PubMed] [Google Scholar]
- 68.Bodro M, Sabe N, Santin M, Cruzado JM, Llado L, Gonzalez-Costello J, et al. Clinical features and outcomes of tuberculosis in solid organ transplant recipients. Transplant Proc. 2012;44(9):2686–2689. doi: 10.1016/j.transproceed.2012.09.060. [DOI] [PubMed] [Google Scholar]
- 69.Chou NK, Wang JL, Chi NH, Wu IH, Huang SC, Chen YS, et al. Tuberculosis after heart transplantation: twenty years of experience in a single center in Taiwan. Transplant Proc. 2008;40(8):2631–2633. doi: 10.1016/j.transproceed.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 70.Horbach I, Fehrenbach FJ. Legionellosis in heart transplant recipients. Infection. 1990;18(6):361–363. doi: 10.1007/BF01646407. [DOI] [PubMed] [Google Scholar]
- 71.Neofytos D, Treadway S, Ostrander D, Alonso CD, Dierberg KL, Nussenblatt V, et al. Epidemiology, outcomes, and mortality predictors of invasive mold infections among transplant recipients: a 10-year, single-center experience. Transpl Infect Dis. 2013;15(3):233–242. doi: 10.1111/tid.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh N, Limaye AP, Forrest G, Safdar N, Muñoz P, Pursell K, et al. Late-onset invasive aspergillosis in organ transplant recipients in the current era. Med Mycol. 2006;44(5):445–449. doi: 10.1080/13693780600684494. [DOI] [PubMed] [Google Scholar]
- 73.Montoya JG, Chaparro SV, Celis D, Cortes JA, Leung AN, Robbins RC, et al. Invasive aspergillosis in the setting of cardiac transplantation. Clin Infect Dis. 2003;37(Suppl 3):S281–S292. doi: 10.1086/376527. [DOI] [PubMed] [Google Scholar]
- 74.Galimberti R, Kowalczuk A, Hidalgo Parra I, Gonzalez Ramos M, Flores V. Cutaneous aspergillosis: a report of six cases. Br J Dermatol. 1998;139(3):522–526. doi: 10.1046/j.1365-2133.1998.02424.x. [DOI] [PubMed] [Google Scholar]
- 75.Gregory ME, Weir CR, Roberts F, Browne BH. Aspergillus endophthalmitis following orthotopic heart transplant. Can J Ophthalmol. 2009;44(5):607–608. doi: 10.3129/i09-121. [DOI] [PubMed] [Google Scholar]
- 76.Morio F, Treilhaud M, Lepelletier D, Le Pape P, Rigal JC, Delile L, et al. Aspergillus fumigatus endocarditis of the mitral valve in a heart transplant recipient: a case report. Diagn Microbiol Infect Dis. 2008;62(4):453–456. doi: 10.1016/j.diagmicrobio.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 77.Byl B, Jacobs F, Antoine M, Depierreux M, Serruys E, Primo G, et al. Mediastinitis caused by Aspergillus fumigatus with ruptured aortic pseudoaneurysm in a heart transplant recipient: case study. Heart Lung. 1993;22(2):145–147. [PubMed] [Google Scholar]
- 78.Cortet B, Richard R, Deprez X, Lucet L, Flipo RM, Le Loet X, et al. Aspergillus spondylodiscitis: successful conservative treatment in 9 cases. J Rheumatol. 1994;21(7):1287–1291. [PubMed] [Google Scholar]
- 79.Husain S, Alexander BD, Munoz P, Avery RK, Houston S, Pruett T, et al. Opportunistic mycelial fungal infections in organ transplant recipients: emerging importance of non-Aspergillus mycelial fungi. Clin Infect Dis. 2003;37(2):221–229. doi: 10.1086/375822. [DOI] [PubMed] [Google Scholar]
- 80.Cardeau-Desangles I, Fabre A, Cointault O, Guitard J, Esposito L, Iriart X, et al. Disseminated Ochroconis gallopava infection in a heart transplant patient. Transpl Infect Dis. 2013;15(3):E115–E118. doi: 10.1111/tid.12084. [DOI] [PubMed] [Google Scholar]
- 81.Vilchez RA, Fung J, Kusne S. Cryptococcosis in organ transplant recipients: an overview. Am J Transplant. 2002;2(7):575–580. doi: 10.1034/j.1600-6143.2002.20701.x. [DOI] [PubMed] [Google Scholar]
- 82.Assi M, Martin S, Wheat LJ, Hage C, Freifeld A, Avery R, et al. Histoplasmosis after solid organ transplant. Clin Infect Dis. 2013;57(11):1542–1549. doi: 10.1093/cid/cit593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ribeiro PM, Bacal F, Koga-Ito CY, Junqueira JC, Jorge AO. Presence of Candida spp. in the oral cavity of heart transplantation patients. J Appl Oral Sci. 2011;19(1):6–10. doi: 10.1590/S1678-77572011000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schaenman JM, Rosso F, Austin JM, Baron EJ, Gamberg P, Miller J, et al. Trends in invasive disease due to Candida species following heart and lung transplantation. Transpl Infect Dis. 2009;11(2):112–121. doi: 10.1111/j.1399-3062.2009.00364.x. [DOI] [PubMed] [Google Scholar]
- 85.Colvin-Adams M, Agnihotri A. Cardiac allograft vasculopathy: current knowledge and future direction. Clin Transplant. 2011;25(2):175–184. doi: 10.1111/j.1399-0012.2010.01307.x. [DOI] [PubMed] [Google Scholar]
- 86.Potena L, Valantine HA. Cytomegalovirus-associated allograft rejection in heart transplant patients. Curr Opin Infect Dis. 2007;20(4):425–431. doi: 10.1097/QCO.0b013e328259c33b. [DOI] [PubMed] [Google Scholar]
- 87.Snydman DR, Limaye AP, Potena L, Zamora MR. Update and review: state-of-the-art management of cytomegalovirus infection and disease following thoracic organ transplantation. Transplant Proc. 2011;43(3 Suppl):S1–S17. doi: 10.1016/j.transproceed.2011.02.069. [DOI] [PubMed] [Google Scholar]
- 88.Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96(4):333–360. doi: 10.1097/TP.0b013e31829df29d. [DOI] [PubMed] [Google Scholar]
- 89.Herreman A, Dierickx D, Morscio J, Camps J, Bittoun E, Verhoef G, et al. Clinicopathological characteristics of posttransplant lymphoproliferative disorders of T-cell origin: single-center series of nine cases and meta-analysis of 147 reported cases. Leuk Lymphoma. 2013;54(10):2190–2199. doi: 10.3109/10428194.2013.775436. [DOI] [PubMed] [Google Scholar]
- 90.Manlhiot C, Pollock-Barziv SM, Holmes C, Weitzman S, Allen U, Clarizia NA, et al. Post-transplant lymphoproliferative disorder in pediatric heart transplant recipients. J Heart Lung Transplant. 2010;29(6):648–657. doi: 10.1016/j.healun.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 91.Florescu DF, Kwon JY, Dumitru I. Adenovirus infections in heart transplantation. Cardiol Rev. 2013;21(4):203–206. doi: 10.1097/CRD.0b013e31828da5b7. [DOI] [PubMed] [Google Scholar]
- 92.Koning L, Pas SD, de Man RA, Balk AH, de Knegt RJ, ten Kate FJ, et al. Clinical implications of chronic hepatitis E virus infection in heart transplant recipients. J Heart Lung Transplant. 2013;32(1):78–85. doi: 10.1016/j.healun.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 93.Kamar N, Izopet J, Tripon S, Bismuth M, Hillaire S, Dumortier J, et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014;370(12):1111–1120. doi: 10.1056/NEJMoa1215246. [DOI] [PubMed] [Google Scholar]
- 94.Coster LO. Parasitic infections in solid organ transplant recipients. Infect Dis Clin N Am. 2013;27(2):395–427. doi: 10.1016/j.idc.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 95.Luft BJ, Naot Y, Araujo FG, Stinson EB, Remington JS. Primary and reactivated toxoplasma infection in patients with cardiac transplants. Clinical spectrum and problems in diagnosis in a defined population. Ann Intern Med. 1983;99(1):27–31. doi: 10.7326/0003-4819-99-1-27. [DOI] [PubMed] [Google Scholar]
- 96.Gallino A, Maggiorini M, Kiowski W, Martin X, Wunderli W, Schneider J, et al. Toxoplasmosis in heart transplant recipients. Eur J Clin Microbiol Infect Dis. 1996;15(5):389–393. doi: 10.1007/BF01690095. [DOI] [PubMed] [Google Scholar]
- 97.Huprikar S, Bosserman E, Patel G, Moore A, Pinney S, Anyanwu A, et al. Donor-derived Trypanosoma cruzi infection in solid organ recipients in the United States, 2001–2011. Am J Transplant. 2013;13(9):2418–2425. doi: 10.1111/ajt.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Campos SV, Strabelli TM, Amato Neto V, Silva CP, Bacal F, Bocchi EA, et al. Risk factors for Chagas’ disease reactivation after heart transplantation. J Heart Lung Transplant. 2008;27(6):597–602. doi: 10.1016/j.healun.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 99.Bestetti RB, Theodoropoulos TA. A systematic review of studies on heart transplantation for patients with end-stage Chagas’ heart disease. J Card Fail. 2009;15(3):249–255. doi: 10.1016/j.cardfail.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 100.Fiorelli AI, Santos RH, Oliveira JL, Jr, Lourenco-Filho DD, Dias RR, Oliveira AS, et al. Heart transplantation in 107 cases of Chagas’ disease. Transplant Proc. 2011;43(1):220–224. doi: 10.1016/j.transproceed.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 101.Bern C. Chagas disease in the immunosuppressed host. Curr Opin Infect Dis. 2012;25(4):450–457. doi: 10.1097/QCO.0b013e328354f179. [DOI] [PubMed] [Google Scholar]
- 102.Theodoropoulos TA, Silva AG, Bestetti RB. Eosinophil blood count and anemia are associated with Trypanosoma cruzi infection reactivation in Chagas’ heart transplant recipients. Int J Cardiol. 2010;145(1):55–56. doi: 10.1016/j.ijcard.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 103.Pinazo MJ, Espinosa G, Cortes-Lletget C, Posada Ede J, Aldasoro E, Oliveira I, et al. Immunosuppression and Chagas disease: a management challenge. PLoS Negl Trop Dis. 2013;7(1):e1965. doi: 10.1371/journal.pntd.0001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Molina I, Gómez i Prat J, Salvador F, Treviño B, Sulleiro E, Serre N, et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med. 2014;370(20):1899–1908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- 105.Golino A, Duncan JM, Zeluff B, DePriest J, McAllister HA, Radovancevic B, et al. Leishmaniasis in a heart transplant patient. J Heart Lung Transplant. 1992;11(4 Pt 1):820–823. [PubMed] [Google Scholar]
- 106.Ramos A, Asensio A, Munez E, Torre-Cisneros J, Blanes M, Carratala J, et al. Incisional surgical infection in heart transplantation. Transpl Infect Dis. 2008;10(4):298–302. doi: 10.1111/j.1399-3062.2008.00316.x. [DOI] [PubMed] [Google Scholar]
- 107.Filsoufi F, Rahmanian PB, Castillo JG, Pinney S, Broumand SR, Adams DH. Incidence, treatment strategies and outcome of deep sternal wound infection after orthotopic heart transplantation. J Heart Lung Transplant. 2007;26(11):1084–1090. doi: 10.1016/j.healun.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 108.Talbot TR, Hatcher J, Davis SF, Pierson RN, 3rd, Barton R, Dummer S. Scedosporium apiospermum pneumonia and sternal wound infection in a heart transplant recipient. Transplantation. 2002;74(11):1645–1647. doi: 10.1097/00007890-200212150-00028. [DOI] [PubMed] [Google Scholar]
- 109.Hummel M, Schuler S, Weber U, Schwertlick G, Hempel S, Theiss D, et al. Aspergillosis with Aspergillus osteomyelitis and diskitis after heart transplantation: surgical and medical management. J Heart Lung Transplant. 1993;12(4):599–603. [PubMed] [Google Scholar]
- 110.Koo S., Gagne L.S., Lee P., Pratibhu P.P., James L.M., Givertz M.M., Marty F.M. Incidence and risk factors for herpes zoster following heart transplantation. Transplant Infectious Disease. 2013;16(1):17–25. doi: 10.1111/tid.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parkins MD, Gregson DB, Pitout JD, Ross T, Laupland KB. Population-based study of the epidemiology and the risk factors for Pseudomonas aeruginosa bloodstream infection. Infection. 2010;38(1):25–32. doi: 10.1007/s15010-009-9145-9. [DOI] [PubMed] [Google Scholar]
- 112.Rodriguez C, Munoz P, Rodriguez-Creixems M, Yanez JF, Palomo J, Bouza E. Bloodstream infections among heart transplant recipients. Transplantation. 2006;81(3):384–391. doi: 10.1097/01.tp.0000188953.86035.2d. [DOI] [PubMed] [Google Scholar]
- 113.Hsu RB, Chang CI, Fang CT, Chang SC, Wang SS, Chu SH. Bloodstream infection in heart transplant recipients: 12-year experience at a university hospital in Taiwan. Eur J Cardiothorac Surg. 2011;40(6):1362–1367. doi: 10.1016/j.ejcts.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 114.Sherman-Weber S, Axelrod P, Suh B, Rubin S, Beltramo D, Manacchio J, et al. Infective endocarditis following orthotopic heart transplantation: 10 cases and a review of the literature. Transpl Infect Dis. 2004;6(4):165–170. doi: 10.1111/j.1399-3062.2004.00074.x. [DOI] [PubMed] [Google Scholar]
- 115.Malinis MF, Mawhorter SD, Jain A, Shrestha NK, Avery RK, van Duin D. Staphylococcus aureus bacteremia in solid organ transplant recipients: evidence for improved survival when compared with nontransplant patients. Transplantation. 2012;93(10):1045–1050. doi: 10.1097/TP.0b013e31824bf219. [DOI] [PubMed] [Google Scholar]
- 116.Atasever A, Bacakoglu F, Uysal FE, Nalbantgil S, Karyagdi T, Guzelant A, et al. Pulmonary complications in heart transplant recipients. Transplant Proc. 2006;38(5):1530–1534. doi: 10.1016/j.transproceed.2006.02.098. [DOI] [PubMed] [Google Scholar]
- 117.Haddad F, Hunt SA, Perlroth M, Valantine H, Doyle R, Montoya J. Pulmonary nocardiosis in a heart transplant patient: case report and review of the literature. J Heart Lung Transplant. 2007;26(1):93–97. doi: 10.1016/j.healun.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 118.Munoz P, Palomo J, Guembe P, Rodriguez-Creixems M, Gijon P, Bouza E. Lung nodular lesions in heart transplant recipients. J Heart Lung Transplant. 2000;19(7):660–667. doi: 10.1016/S1053-2498(00)00119-4. [DOI] [PubMed] [Google Scholar]
- 119.Abid Q, Nkere UU, Hasan A, Gould K, Forty J, Corris P, et al. Mediastinitis in heart and lung transplantation: 15 years experience. Ann Thorac Surg. 2003;75(5):1565–1571. doi: 10.1016/S0003-4975(02)04905-6. [DOI] [PubMed] [Google Scholar]
- 120.Senechal M, LePrince P, Tezenas du Montcel S, Bonnet N, Dubois M, El-Serafi M, et al. Bacterial mediastinitis after heart transplantation: clinical presentation, risk factors and treatment. J Heart Lung Transplant. 2004;23(2):165–170. doi: 10.1016/S1053-2498(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 121.Chou NK, Wang JL, Chi NH, Wu IH, Huang SC, Chen YS, et al. Surgical treatment of mediastinitis after cardiac transplantation. Transplant Proc. 2008;40(8):2629–2630. doi: 10.1016/j.transproceed.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 122.Hsu RB, Lin FY. Nontyphoid Salmonella infection in heart transplant recipients. Am J Med Sci. 2008;336(5):393–396. doi: 10.1097/MAJ.0b013e31816a8973. [DOI] [PubMed] [Google Scholar]
- 123.Menegaux F, Huraux C, Jordi-Galais P, Dorent R, Ghossoub JJ, Pavie A, et al. Cholelithiasis in heart transplant patients. Ann Chir. 2000;125(9):832–837. doi: 10.1016/S0003-3944(00)00004-3. [DOI] [PubMed] [Google Scholar]
- 124.Oaks TE, Pennock JL, Myers JL, Wisman CB. Survival following rupture of a pancreatic abscess in a heart transplant recipient. J Heart Transplant. 1986;5(2):148–153. [PubMed] [Google Scholar]
- 125.Limaye AP, Smith KD, Cook L, Groom DA, Hunt NC, Jerome KR, et al. Polyomavirus nephropathy in native kidneys of non-renal transplant recipients. Am J Transplant. 2005;5(3):614–620. doi: 10.1034/j.1600-6143.2003.00126.x-i1. [DOI] [PubMed] [Google Scholar]
- 126.Murtagh B, Wadia Y, Messner G, Allison P, Harati Y, Delgado R. West Nile virus infection after cardiac transplantation. J Heart Lung Transplant. 2005;24(6):774–776. doi: 10.1016/j.healun.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 127.Kumar D, Prasad GV, Zaltzman J, Levy GA, Humar A. Community-acquired West Nile virus infection in solid-organ transplant recipients. Transplantation. 2004;77(3):399–402. doi: 10.1097/01.TP.0000101435.91619.31. [DOI] [PubMed] [Google Scholar]
- 128.Bestetti RB, Rubio FG, Ferraz Filho JR, Goes MJ, de Santi Neto D, Akio F, et al. Trypanosoma cruzi infection reactivation manifested by encephalitis in a Chagas heart transplant recipient. Int J Cardiol. 2013;163(1):e7–e8. doi: 10.1016/j.ijcard.2012.06.102. [DOI] [PubMed] [Google Scholar]
- 129.van de Beek D, Patel R, Daly RC, McGregor CG, Wijdicks EF. Central nervous system infections in heart transplant recipients. Arch Neurol. 2007;64(12):1715–1720. doi: 10.1001/archneur.64.12.noc70065. [DOI] [PubMed] [Google Scholar]
- 130.Wiesmayr S, Tabarelli W, Stelzmueller I, Nachbaur D, Boesmueller C, Wykypiel H, et al. Listeria meningitis in transplant recipients. Wien Klin Wochenschr. 2005;117(5–6):229–233. doi: 10.1007/s00508-005-0311-5. [DOI] [PubMed] [Google Scholar]
- 131.Mateen FJ, Muralidharan R, Carone M, van de Beek D, Harrison DM, Aksamit AJ, et al. Progressive multifocal leukoencephalopathy in transplant recipients. Ann Neurol. 2011;70(2):305–322. doi: 10.1002/ana.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Loyaga-Rendon RY, Taylor DO, Koval CE. Progressive multifocal leukoencephalopathy in a heart transplant recipient following rituximab therapy for antibody-mediated rejection. Am J Transplant. 2013;13(4):1075–1079. doi: 10.1111/ajt.12153. [DOI] [PubMed] [Google Scholar]
- 133.Gupta MR, Valentine VG, Walker JE, Jr, Lombard GA, LaPlace SG, Seoane L, et al. Clinical spectrum of gram-positive infections in lung transplantation. Transpl Infect Dis. 2009;11(5):424–431. doi: 10.1111/j.1399-3062.2009.00422.x. [DOI] [PubMed] [Google Scholar]
- 134.Zeglen S, Wojarski J, Wozniak-Grygiel E, Siola M, Jastrzebski D, Kucewicz-Czech E, et al. Frequency of Pseudomonas aeruginosa colonizations/infections in lung transplant recipients. Transplant Proc. 2009;41(8):3222–3224. doi: 10.1016/j.transproceed.2009.07.063. [DOI] [PubMed] [Google Scholar]
- 135.Knoll BM, Kappagoda S, Gill RR, Goldberg HJ, Boyle K, Baden LR, et al. Non-tuberculous mycobacterial infection among lung transplant recipients: a 15-year cohort study. Transpl Infect Dis. 2012;14(5):452–460. doi: 10.1111/j.1399-3062.2012.00753.x. [DOI] [PubMed] [Google Scholar]
- 136.Husain S, McCurry K, Dauber J, Singh N, Kusne S. Nocardia infection in lung transplant recipients. J Heart Lung Transplant. 2002;21(3):354–359. doi: 10.1016/S1053-2498(01)00394-1. [DOI] [PubMed] [Google Scholar]
- 137.Poonyagariyagorn HK, Gershman A, Avery R, Minai O, Blazey H, Asamoto K, et al. Challenges in the diagnosis and management of Nocardia infections in lung transplant recipients. Transpl Infect Dis. 2008;10(6):403–408. doi: 10.1111/j.1399-3062.2008.00338.x. [DOI] [PubMed] [Google Scholar]
- 138.Sole A, Salavert M. Fungal infections after lung transplantation. Curr Opin Pulm Med. 2009;15(3):243–253. doi: 10.1097/MCP.0b013e328326f410. [DOI] [PubMed] [Google Scholar]
- 139.Palmer SM, Alexander BD, Sanders LL, Edwards LJ, Reller LB, Davis RD, et al. Significance of blood stream infection after lung transplantation: analysis in 176 consecutive patients. Transplantation. 2000;69(11):2360–2366. doi: 10.1097/00007890-200006150-00025. [DOI] [PubMed] [Google Scholar]
- 140.Singh N, Husain S. Aspergillus infections after lung transplantation: clinical differences in type of transplant and implications for management. J Heart Lung Transplant. 2003;22(3):258–266. doi: 10.1016/S1053-2498(02)00477-1. [DOI] [PubMed] [Google Scholar]
- 141.Minari A, Husni R, Avery RK, Longworth DL, DeCamp M, Bertin M, et al. The incidence of invasive aspergillosis among solid organ transplant recipients and implications for prophylaxis in lung transplants. Transpl Infect Dis. 2002;4(4):195–200. doi: 10.1034/j.1399-3062.2002.t01-2-02002.x. [DOI] [PubMed] [Google Scholar]
- 142.Singh N, Suarez JF, Avery R, Lass-Florl C, Geltner C, Pasqualotto AC, et al. Risk factors and outcomes in lung transplant recipients with nodular invasive pulmonary aspergillosis. J Inf Secur. 2013;67(1):72–78. doi: 10.1016/j.jinf.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 143.Singh N, Suarez JF, Avery R, Lass-Florl C, Geltner C, Pasqualotto AC, et al. Immune reconstitution syndrome-like entity in lung transplant recipients with invasive aspergillosis. Transpl Immunol. 2013;29(1–4):109–113. doi: 10.1016/j.trim.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 144.Hadjiliadis D, Howell DN, Davis RD, Lawrence CM, Rea JB, Tapson VF, et al. Anastomotic infections in lung transplant recipients. Ann Transplant. 2000;5(3):13–19. [PubMed] [Google Scholar]
- 145.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(3):327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 146.Rakvit A, Meyerrose G, Vidal AM, Kimbrough RC, Sarria JC. Cellulitis caused by Cryptococcus neoformans in a lung transplant recipient. J Heart Lung Transplant. 2005;24(5):642. doi: 10.1016/j.healun.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 147.Singh N, Lortholary O, Alexander BD, Gupta KL, John GT, Pursell K, et al. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005;40(12):1756–1761. doi: 10.1086/430606. [DOI] [PubMed] [Google Scholar]
- 148.Fishman JA, Emery V, Freeman R, Pascual M, Rostaing L, Schlitt HJ, et al. Cytomegalovirus in transplantation – challenging the status quo. Clin Transplant. 2007;21(2):149–158. doi: 10.1111/j.1399-0012.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 149.Zamora MR. Cytomegalovirus and lung transplantation. Am J Transplant. 2004;4(8):1219–1226. doi: 10.1111/j.1600-6143.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- 150.Mitsani D, Nguyen MH, Girnita DM, Spichty K, Kwak EJ, Silveira FP, et al. A polymorphism linked to elevated levels of interferon-gamma is associated with an increased risk of cytomegalovirus disease among Caucasian lung transplant recipients at a single center. J Heart Lung Transplant. 2011;30(5):523–529. doi: 10.1016/j.healun.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 151.Paraskeva M, Bailey M, Levvey BJ, Griffiths AP, Kotsimbos TC, Williams TP, et al. Cytomegalovirus replication within the lung allograft is associated with bronchiolitis obliterans syndrome. Am J Transplant. 2011;11(10):2190–2196. doi: 10.1111/j.1600-6143.2011.03663.x. [DOI] [PubMed] [Google Scholar]
- 152.Minces LR, Nguyen MH, Mitsani D, Shields RK, Kwak EJ, Silveira FP, et al. Ganciclovir-resistant cytomegalovirus (CMV) infections among lung transplant recipients are associated with poor outcomes despite treatment with foscarnet-containing regimens. Antimicrob Agents Chemother. 2014;58(1):128–135. doi: 10.1128/AAC.00561-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shalhoub S, Husain S. Community-acquired respiratory viral infections in lung transplant recipients. Curr Opin Infect Dis. 2013;26(4):302–308. doi: 10.1097/QCO.0b013e3283630e85. [DOI] [PubMed] [Google Scholar]
- 154.Bridevaux PO, Aubert JD, Soccal PM, Mazza-Stalder J, Berutto C, Rochat T, et al. Incidence and outcomes of respiratory viral infections in lung transplant recipients: a prospective study. Thorax. 2014;69(1):32–38. doi: 10.1136/thoraxjnl-2013-203581. [DOI] [PubMed] [Google Scholar]
- 155.Shah PD, McDyer JF. Viral infections in lung transplant recipients. Semin Respir Crit Care Med. 2010;31(2):243–254. doi: 10.1055/s-0030-1249120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM. Antiviral agents for the treatment and chemoprophylaxis of influenza – recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60(1):1–24. [PubMed] [Google Scholar]
- 157.Clark NM, Lynch JP, 3rd, Sayah D, Belperio JA, Fishbein MC, Weigt SS. DNA viral infections complicating lung transplantation. Semin Respir Crit Care Med. 2013;34(3):380–404. doi: 10.1055/s-0033-1348473. [DOI] [PubMed] [Google Scholar]
- 158.Thomas LD, Milstone AP, Vilchez RA, Zanwar P, Butel JS, Dummer JS. Polyomavirus infection and its impact on renal function and long-term outcomes after lung transplantation. Transplantation. 2009;88(3):360–366. doi: 10.1097/TP.0b013e3181ae5ff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Muchtar E, Kramer MR, Vidal L, Ram R, Gurion R, Rosenblat Y, et al. Posttransplantation lymphoproliferative disorder in lung transplant recipients: a 15-year single institution experience. Transplantation. 2013;96(7):657–663. doi: 10.1097/TP.0b013e31829b0718. [DOI] [PubMed] [Google Scholar]
- 160.Vijayan VK. Parasitic lung infections. Curr Opin Pulm Med. 2009;15(3):274–282. doi: 10.1097/MCP.0b013e328326f3f8. [DOI] [PubMed] [Google Scholar]
- 161.Balagopal A, Mills L, Shah A, Subramanian A. Detection and treatment of Strongyloides hyperinfection syndrome following lung transplantation. Transpl Infect Dis. 2009;11(2):149–154. doi: 10.1111/j.1399-3062.2009.00375.x. [DOI] [PubMed] [Google Scholar]
- 162.Walia R, Montoya JG, Visvesvera GS, Booton GC, Doyle RL. A case of successful treatment of cutaneous Acanthamoeba infection in a lung transplant recipient. Transpl Infect Dis. 2007;9(1):51–54. doi: 10.1111/j.1399-3062.2006.00159.x. [DOI] [PubMed] [Google Scholar]
- 163.Duarte AG, Sattar F, Granwehr B, Aronson JF, Wang Z, Lick S. Disseminated acanthamoebiasis after lung transplantation. J Heart Lung Transplant. 2006;25(2):237–240. doi: 10.1016/j.healun.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 164.Bonatti H, Barroso LF, 2nd, Sawyer RG, Kotton CN, Sifri CD. Cryptosporidium enteritis in solid organ transplant recipients: multicenter retrospective evaluation of 10 cases reveals an association with elevated tacrolimus concentrations. Transpl Infect Dis. 2012;14(6):635–648. doi: 10.1111/j.1399-3062.2012.00719.x. [DOI] [PubMed] [Google Scholar]
- 165.Field AS, Paik JY, Stark D, Qiu MR, Morey A, Plit ML, et al. Myositis due to the microsporidian Anncaliia (Brachiola) algerae in a lung transplant recipient. Transpl Infect Dis. 2012;14(2):169–176. doi: 10.1111/j.1399-3062.2012.00724.x. [DOI] [PubMed] [Google Scholar]
- 166.Levine DJ, Riley DJ, Jorgensen JH, McClain WD, Tio F, Visvesvara GS, et al. Key diagnostic features of granulomatous interstitial nephritis due to Encephalitozoon cuniculi in a lung transplant recipient. Am J Surg Pathol. 2013;37(3):447–452. doi: 10.1097/PAS.0b013e31827e1968. [DOI] [PubMed] [Google Scholar]
- 167.Shields RK, Clancy CJ, Minces LR, Shigemura N, Kwak EJ, Silveira FP, et al. Epidemiology and outcomes of deep surgical site infections following lung transplantation. Am J Transplant. 2013;13(8):2137–2145. doi: 10.1111/ajt.12292. [DOI] [PubMed] [Google Scholar]
- 168.Lodge BA, Ashley ED, Steele MP, Perfect JR. Aspergillus fumigatus empyema, arthritis, and calcaneal osteomyelitis in a lung transplant patient successfully treated with posaconazole. J Clin Microbiol. 2004;42(3):1376–1378. doi: 10.1128/JCM.42.3.1376-1378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Danziger-Isakov LA, Sweet S, Delamorena M, Huddleston CB, Mendeloff E, Debaun MR. Epidemiology of bloodstream infections in the first year after pediatric lung transplantation. Pediatr Infect Dis J. 2005;24(4):324–330. doi: 10.1097/01.inf.0000157089.42020.41. [DOI] [PubMed] [Google Scholar]
- 170.Candel FJ, Grima E, Matesanz M, Cervera C, Soto G, Almela M, et al. Bacteremia and septic shock after solid-organ transplantation. Transplant Proc. 2005;37(9):4097–4099. doi: 10.1016/j.transproceed.2005.09.181. [DOI] [PubMed] [Google Scholar]
- 171.Lazaro M, Ramos A, Ussetti P, Asensio A, Laporta R, Munez E, et al. Aspergillus endocarditis in lung transplant recipients: case report and literature review. Transpl Infect Dis. 2011;13(2):186–191. doi: 10.1111/j.1399-3062.2010.00589.x. [DOI] [PubMed] [Google Scholar]
- 172.Nunley DR, Grgurich WF, Keenan RJ, Dauber JH. Empyema complicating successful lung transplantation. Chest. 1999;115(5):1312–1315. doi: 10.1378/chest.115.5.1312. [DOI] [PubMed] [Google Scholar]
- 173.Fairhurst RM, Kubak BM, Shpiner RB, Levine MS, Pegues DA, Ardehali A. Mycobacterium abscessus empyema in a lung transplant recipient. J Heart Lung Transplant. 2002;21(3):391–394. doi: 10.1016/S1053-2498(01)00339-4. [DOI] [PubMed] [Google Scholar]
- 174.Sayah DM, Schwartz BS, Kukreja J, Singer JP, Golden JA, Leard LE. Scedosporium prolificans pericarditis and mycotic aortic aneurysm in a lung transplant recipient receiving voriconazole prophylaxis. Transpl Infect Dis. 2013;15(2):E70–E74. doi: 10.1111/tid.12056. [DOI] [PubMed] [Google Scholar]
- 175.Mitsani D, Nguyen MH, Silveira FP, Bermudez C, Toyoda Y, Pasculle AW, et al. Mycoplasma hominis pericarditis in a lung transplant recipient: review of the literature about an uncommon but important cardiothoracic pathogen. Transpl Infect Dis. 2010;12(2):146–150. doi: 10.1111/j.1399-3062.2009.00457.x. [DOI] [PubMed] [Google Scholar]
- 176.Campos S, Caramori M, Teixeira R, Afonso J, Jr, Carraro R, Strabelli T, et al. Bacterial and fungal pneumonias after lung transplantation. Transplant Proc. 2008;40(3):822–824. doi: 10.1016/j.transproceed.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 177.Bhaskaran A, Hosseini-Moghaddam SM, Rotstein C, Husain S. Mold infections in lung transplant recipients. Semin Respir Crit Care Med. 2013;34(3):371–379. doi: 10.1055/s-0033-1348475. [DOI] [PubMed] [Google Scholar]
- 178.Bangsborg JM, Uldum S, Jensen JS, Bruun BG. Nosocomial legionellosis in three heart-lung transplant patients: case reports and environmental observations. Eur J Clin Microbiol Infect Dis. 1995;14(2):99–104. doi: 10.1007/BF02111866. [DOI] [PubMed] [Google Scholar]
- 179.Glanville AR, Gencay M, Tamm M, Chhajed P, Plit M, Hopkins P, et al. Chlamydia pneumoniae infection after lung transplantation. J Heart Lung Transplant. 2005;24(2):131–136. doi: 10.1016/j.healun.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 180.Miller CB, Malaisrie SC, Patel J, Garrity E, Vigneswaran WT, Gamelli RL. Intraabdominal complications after lung transplantation. J Am Coll Surg. 2006;203(5):653–660. doi: 10.1016/j.jamcollsurg.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 181.Tai CC, Curtis JL, Szmuszkovicz JR, Horn MV, Ford HR, Woo MS, et al. Abdominal involvement in pediatric heart and lung transplant recipients with posttransplant lymphoproliferative disease increases the risk of mortality. J Pediatr Surg. 2008;43(12):2174–2177. doi: 10.1016/j.jpedsurg.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 182.Bravo C, Gispert P, Borro JM, de la Torre M, Cifrian Martinez JM, Fernandez Rozas S, et al. Prevalence and management of gastrointestinal complications in lung transplant patients: MITOS study group. Transplant Proc. 2007;39(7):2409–2412. doi: 10.1016/j.transproceed.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 183.Maurer JR. The spectrum of colonic complications in a lung transplant population. Ann Transplant. 2000;5(3):54–57. [PubMed] [Google Scholar]
- 184.Augustine SM, Yeo CJ, Buchman TG, Achuff SC, Baumgartner WA. Gastrointestinal complications in heart and in heart-lung transplant patients. J Heart Lung Transplant. 1991;10(4):547–555. [PubMed] [Google Scholar]
- 185.Riezebos-Brilman A, Puchhammer-Stockl E, van der Weide HY, Haagsma EB, Jaksch P, Bejvl I, et al. Chronic hepatitis E infection in lung transplant recipients. J Heart Lung Transplant. 2013;32(3):341–346. doi: 10.1016/j.healun.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 186.Maier T, Schwarting A, Mauer D, Ross RS, Martens A, Kliem V, et al. Management and outcomes after multiple corneal and solid organ transplantations from a donor infected with rabies virus. Clin Infect Dis. 2010;50(8):1112–1119. doi: 10.1086/651267. [DOI] [PubMed] [Google Scholar]
- 187.Hiemann NE, Grimmer S, Kemper D, Knosalla C, Hetzer R. Tuberculous meningitis in a lung transplanted patient. Transpl Infect Dis. 2012;14(4):E19–E22. doi: 10.1111/j.1399-3062.2012.00736.x. [DOI] [PubMed] [Google Scholar]
- 188.Levi ME, Quan D, Ho JT, Kleinschmidt-Demasters BK, Tyler KL, Grazia TJ. Impact of rituximab-associated B-cell defects on West Nile virus meningoencephalitis in solid organ transplant recipients. Clin Transplant. 2010;24(2):223–228. doi: 10.1111/j.1399-0012.2009.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sahi H, Avery RK, Minai OA, Hall G, Mehta AC, Raina P, et al. Scedosporium apiospermum (Pseudoallescheria boydii) infection in lung transplant recipients. J Heart Lung Transplant. 2007;26(4):350–356. doi: 10.1016/j.healun.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 190.Henao-Martinez AF, Castillo-Mancilla JR, Barron MA, Nichol AC. Combination antifungal therapy in the treatment of Scedosporium apiospermum central nervous system infections. Case Rep Infect Dis. 2013;2013:589490. doi: 10.1155/2013/589490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Levin TP, Baty DE, Fekete T, Truant AL, Suh B. Cladophialophora bantiana brain abscess in a solid-organ transplant recipient: case report and review of the literature. J Clin Microbiol. 2004;42(9):4374–4378. doi: 10.1128/JCM.42.9.4374-4378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kleinschmidt-Demasters BK. Disseminated Fusarium infection with brain abscesses in a lung transplant recipient. Clin Neuropathol. 2009;28(6):417–421. doi: 10.5414/NPP28417. [DOI] [PubMed] [Google Scholar]
- 193.Hall WA, Martinez AJ, Dummer JS, Griffith BP, Hardesty RL, Bahnson HT, et al. Central nervous system infections in heart and heart-lung transplant recipients. Arch Neurol. 1989;46(2):173–177. doi: 10.1001/archneur.1989.00520380077017. [DOI] [PubMed] [Google Scholar]


