Abstract
Despite 75 years of research into prevention and treatment of influenza, the viruses that cause this disease continue to rank as some of the most important pathogens afflicting humans today. Progress in development of therapeutics for influenza has been slow for much of that time, but has accelerated in pace over the last two decades. Two classes of antiviral medications are used in humans at present, but each has limitations in scope and effectiveness of use. New strategies involving these licensed agents, including alternate forms of delivery and combination therapy with other drugs, are currently being explored. In addition, several novel antiviral compounds are in various clinical phases of development. Together with strategies designed to target the virus itself, new approaches to interrupt host–pathogen interactions or modulate detrimental aspects of the immune response have been proposed. Therapy for influenza will likely undergo substantial changes in the decades to come, evolving with our knowledge of pathogenesis as new approaches become viable and are validated clinically.
Keywords: Influenza Virus, H1N1 Virus, Seasonal Influenza, Influenza Virus Infection, H5N1 Influenza Virus
Introduction
Influenza is a contagious respiratory illness caused by influenza viruses. Every year, influenza epidemics cause numerous deaths and millions of hospitalizations. The most frightening effects, however, are seen when new strains of the virus emerge from different species causing worldwide outbreaks of infection. In April 2009 a novel influenza virus of H1N1 subtype emerged from a swine reservoir, causing the first pandemic in more than 40 years (Perez-Padilla et al. 2009). The clinical attack rate was highest in children, and children and young adults of school age were the main vectors of transmission (Nishiura et al. 2009). Surprisingly, however, much of the severe disease from the new pandemic strain also occurred in school-age children and young adults, groups that are typically spared by the most serious outcomes during seasonal influenza (Reichert et al. 2010). Despite the unexpected emergence of a pandemic H1N1 strain, significant concern remains over the potential of highly pathogenic avian influenza viruses of the H5N1 subtype to emerge and achieve similar worldwide spread (Webby and Webster 2003; McCullers 2008). Although transmission of H5N1 influenza viruses from birds to humans is currently inefficient, the capacity to infect humans and cause severe pneumonia with rapid progression to acute respiratory distress syndrome and multi-organ failure (Beigel et al. 2005; bdel-Ghafar et al. 2008) suggests a pandemic from one of these viruses might be much more severe than that from the pandemic 2009 H1N1 strain.
Several antiviral compounds have been developed against influenza virus to interfere with specific events in the replication cycle (McCullers 2005). Among these, two classes of drugs are currently approved as antiviral agents by the food and drug administration (FDA) of the United States. The adamantanes are inhibitors of viral uncoating (amantadine, rimantadine), while the neuraminidase (NA) inhibitors (zanamivir, oseltamivir) interfere with the viral budding process. While these drugs are effective in reducing symptomatology from influenza, increasing evidence of resistance to these conventional antiviral drugs and the narrow time window during which their administration is effective are driving an increased push for novel therapeutic targets, drug combinations, or optimization of the existing antiviral regimens.
M2 Ion Channel Inhibitors
Pharmacokinetics and Clinical Use
The first clinically useful anti-influenza drugs were the adamantane derivatives, amantadine (1-aminoadamantane hydrochloride; trade name Symmetrel) and its methyl derivate rimantadine (α-methyl-1-adamantane-methylamine-hydrochloride; trade name Flumadine) (Fig. 1). The first report of the antiviral activity of amantadine against influenza A viruses was published in 1964 (Davies et al. 1964). Amantadine was initially approved in the United States in 1966, and rimantadine in 1993. Pharmacokinetic studies carried out with healthy immunocompetent adults demonstrated 85–95% oral bioavailability of amantadine and rimantadine and systemic distribution of these drugs with the ability to cross the placenta and the blood–brain barrier; distribution was seen into breast milk, saliva, tears, nasal secretions, and cerebral spinal fluid (Aoki and Sitar 1988). Amantadine is rapidly and almost completely absorbed from gastrointestinal tract with time to peak plasma concentration 2–4 h and a plasma half-life of 17 h (range 10–25 h) (Endo Pharmaceuticals 2007). More than 90% of amantadine is excreted unchanged in urine by glomerular filtration and renal tubular secretion. Rimantadine is also rapidly absorbed after oral administration, with a time to peak plasma concentration of 5–7 h in healthy adults and a plasma half-life of 25 h (range 13–65 h) (Forest Pharmaceuticals 2010). Unlike amantadine, rimantadine is extensively metabolized in the liver through hydroxylation such that less than 25% is excreted unchanged in the urine. This may account for the lower incidence of central nervous system- related side effects, such as insomnia and difficulty concentrating, that are experienced with rimantadine, compared to amantadine (Dolin et al. 1982). The peak and steady-state concentrations are higher and the half-life of adamantanes is prolonged in patients who are elderly or who have renal impairment (Forest Pharmaceuticals 2010; Endo Pharmaceuticals 2007).
Fig. 1.
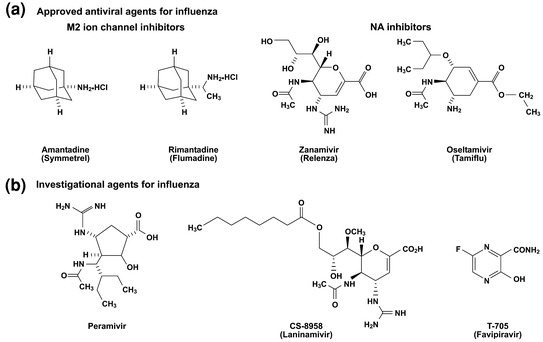
Structures of antiviral agents active against influenza viruses. a Approved antiviral agents for influenza. b Investigational agents for influenza
The adamantanes have been shown to be efficacious in the treatment of influenza A virus infection caused by different subtypes (H1N1, H2N2, and H3N2) but are ineffective against influenza B viruses (Table 1) (Wingfield et al. 1969; Doyle et al. 1998; Van Voris et al. 1981; Hayden and Monto 1986). Defervescence, improvement in symptoms, resolution of symptoms, and return to normal activity all occurred about 1 day earlier in treated subjects than in those receiving placebo. Although no studies of sufficient size have been performed to convincingly address whether adamantanes treatment prevents complications of influenza, animal data (McCullers 2004) and a challenge trial in adult volunteers (Doyle et al. 1998) suggest that there is a lack of effect. The adult therapeutic regimen of amantadine or rimantadine is 200 mg/day either as a single dose or divided twice daily for 7 days, and for best therapeutic effect should be administered within 48 h of onset of symptoms (Harper et al. 2009). Efficacy in populations other than healthy adults or when administration is delayed beyond 48 h has not been studied thoroughly. Prophylaxis of healthy adults during influenza outbreaks showed 71–91% efficacy compared to placebo in preventing laboratory-confirmed influenza virus infection in two trials using amantadine (Monto et al. 1979; Dolin et al. 1982), and 85% efficacy using rimantadine in a single trial (Dolin et al. 1982). Prophylactic administration at a dose of 100 mg/dose once daily can be used for up to 6 weeks or until active immunity can be expected from immunization with inactivated influenza A virus vaccine. A recent meta-analysis of published clinical data concluded that the major effects of amantadine and rimantadine treatment were to shorten the duration of fever by about 1 day in treated, infected individuals, and prevent ~60–70% of influenza cases when used as prophylaxis (Jefferson et al. 2010).
Table 1.
Approved antiviral agents for influenza
| Characteristics | M2 Inhibitors | NA Inhibitors | ||
|---|---|---|---|---|
| Amantadine | Rimantadine | Zanamivir | Oseltamivir | |
| FDA approved |
1966 Symmetrel |
1993 Flumadine |
1999 Relenza |
1999 Tamiflu |
| Efficacy | Influenza A virus infection | Influenza A and B virus infection | ||
|
Treatment regimen (adults) |
100 mg orally bid × 5 days |
100 mg orally bid × 5 days |
2 inhaled doses (10 mg) bid × 5 days |
75 mg orally bid × 5 days |
|
Treatment regimen (children) |
5 mg/kg/d orally (max 150 mg/d) in 2 doses × 5 days | 5 mg/kg/d orally (max 150 mg/d) in 2 doses × 5 days |
>7 years 2 inhalaled doses (10 mg) bid × 5 days |
>1 year 12–75 mg orally bid × 5 days |
| Prophylaxis regimen (adults) |
100 mg orally bid |
100 mg orally bid |
2 inhalaled doses (10 mg) qd × 10 days |
75 mg orally qd × 10 days |
| Prophylaxis regimen (children) |
5 mg/kg/d orally (max 150 mg/d) in 2 doses |
5 mg/kg/d orally (max 150 mg/d) in 2 doses |
>5 years 2 inhalat. (10 mg) qd × 10 days |
>1 year 12–75 mg orally qd × 10 days |
|
Adverse effects |
Nausea, dizziness, insomnia, vomiting, nervousness, light headedness, impaired concentration, seizures | Nausea, dizziness, insomnia, vomiting, light headedness; less pronounced CNS adverse effects | Bronchospasm | Nasal congestion, nausea, vomiting, discomfort |
| Mechanism of action | Inhibit viral replication by blocking the ion channel at the stage of virus entry into cells; prevent virus release by altering the conformation of the HA protein | Block the activity of the NA enzyme and interrupt an established infection at a later stage of virus replication by inhibiting the release of virions from infected cells | ||
| Molecular markers of resistancea | Mutations in M2 protein at positions: L26F; V27A/T/S/G; A30V/T/S; S31N/G; G34E |
Mutations in NA: Q136K (N1and N2 subtypes); E119D/G/A (B virus) |
Mutations in NA: H275Y, N295S (N1 subtype); E119V, R292K (N2 subtype); R152K, D198N (B virus) | |
Note N1 and N2 numbering is used to designated NA mutations in corresponding NA subtypes of influenza A viruses; N2 numbering is used to designated NA mutations for influenza B viruses.aBased on sequence analysis of M gene (M2 inhibitors) or NA gene (NA inhibitors). Q136KNA mutation has been reported in MDCK-propagated clinical isolates of seasonal H1N1 and H3N2 viruses with the reduced susceptibility to zanamivir (Hurt et al. 2009; Dapat et al. 2010)
Mechanism of Action
Adamantanes (amantadine and rimantadine) possess two concentration-dependant mechanisms of antiviral action (Pinto et al. 1992). At micromolar concentrations (0.1–5 μM) adamantanes selectively inhibit two different steps in the replication cycle in a strain-specific manner (Appleyard 1977). Prior to membrane fusion, the low pH of the endosome activates the M2 channel to conduct protons across the viral envelope, which results in the acidification of the viral interior. Adamantanes block the ion channel activity of the M2 protein of influenza A virus, and viral replication is inhibited by the blockade of hydrogen ion flow into the virus particle, principally at the stage of virus entry and uncoating (Wang et al. 1993). Amantadine also acts at a late stage of replication by preventing virus release of certain influenza strains that possess intracellularly cleavable hemagglutinin (HA), in particular the H5 and H7 subtypes. This effect is proposed to result from irreversible conversion of the HA to its low-pH conformation form within the trans-Golgi network in the absence of M2 function (Grambas et al. 1992; Betakova et al. 2005). When cells are incubated in vitro with adamantanes at concentrations >0.1 mM, amantadine and rimantadine are concentrated in lysosomes, and the acid-dependent activation of HA-mediated membrane fusion is inhibited through an increase in the lysosomal pH (Gething et al. 1986; Steinhauer et al. 1996). However, the clinical utility of this second effect is not known as it is not thought to occur at deliverable drug concentrations.
Resistance
Rapid development of fully pathogenic and transmissible resistant variants after amantadine or rimantadine treatment and their ineffectiveness against influenza B virus infection are the main drawbacks of M2 blockers (Hayden 1996). The markers of resistance to adamantanes are well established and include substitution of one of five amino acids (positions 26, 27, 30, 31, and 34) within the transmembrane domain of M2 protein (Table 1); each change confers resistance to both amantadine and rimantadine (Hay et al. 1986; Belshe et al. 1988; Pinto et al. 1992). Amantadine-resistant influenza A viruses are found among 30–80% of isolates after only a few days of drug therapy in both immunocompetent and immunocompromised patients (Shiraishi et al. 2003).
The incidence of naturally occurring amantadine-resistant variants has increased dramatically since 2003, and these resistant influenza A (H1N1) and A (H3N2) viruses spread widely and reached nearly 100% even in countries without substantial amantadine use (Bright et al. 2006; Centers for Disease Control 2006). However, it is important to note that the percentage of resistant variants varies in different countries and among different influenza A virus subtypes. With the decrease in the use of adamantanes over the last several years driven by these trends in susceptibility, resistance to adamantanes in seasonal influenza A (H1N1) viruses from the United States has returned to relatively low levels, 10.7 and 0.7% for the 2007–2008 and 2008–2009 seasons, respectively (Centers for Disease Control 2010). Conversely, adamantane resistance in South East Asia has remained elevated (33–100%) since 2007 (Barr et al. 2008). Phylogenetically, the M genes of amantadine-sensitive and amantadine-resistant influenza A (H1N1) viruses form two distinct clades: 2B and 2C, respectively (Deyde et al. 2007). Among recent seasonal influenza A (H1N1) viruses, clade 2C viruses carry the S31N mutation in the M2 protein, the most commonly detected amantadine resistance marker, while clade 2B viruses are primarily amantadine sensitive. If amantadine resistance is detected in the primarily amantadine-sensitive clade 2B viruses, it is usually linked with the development of resistance during amantadine treatment (Hayden 2006). The S31N mutation is also present in current seasonal A (H3N2) viruses. Widespread circulation of amantadine resistance has been prevalent in influenza A (H3N2) viruses during recent seasons (33 and 100% for the 2007–2008 and 2008–2009 seasons, respectively) despite the decrease in usage of the drug (Bright et al. 2005; Barr et al. 2007, 2008). However, amantadine resistance in A (H3N2) viruses was acquired independently and may be related to the collective effects of drug pressure, spontaneous mutations, or reassortments in the viral genome (Deyde et al. 2009; Nelson et al. 2009). For this reason, the United States Centers for Disease Control and Prevention (CDC) has discouraged use of M2 inhibitors until the frequency of this phenotype has subsided (Centers for Disease Control 2006).
NA Inhibitors
Pharmacokinetics and Clinical Use
Development of the NA inhibitors was a significant milestone in antiviral development as this was the first example of synthesis of such a drug based on the crystal structure of a target enzyme (von Itzstein et al. 1996; Kim et al. 1997; Babu et al. 2000). The NA inhibitor zanamivir (4-guanidino-Neu5Ac2en, GG167, trade name Relenza) was designed to be a competitive inhibitor of sialidases (Fig. 1). A second NA inhibitor developed shortly after zanamivir, the prodrug oseltamivir phosphate (oseltamivir) (ethyl[3R,4R,5S]-4-acetamido-5-amino-3-[1-ethylpropoxy]-1-cyclohexene-1-carboxylate, trade name Tamiflu), is rapidly cleaved into the active oseltamivir carboxylate ([3R,4R,5S]-4-acetamido-5-amino-3-[1-ethylpropoxy]-1-cyclohexene-1-carboxylic acid) by esterases in the gastrointestinal tract, liver, or blood (Gubareva et al. 2000; McClellan and Perry 2001). The NA inhibitors were approved by FDA for the treatment and prevention of influenza in 1999. The oral bioavailability of zanamivir is <5%, which led to development of a dry powder formulation for inhalation (5 mg zanamivir per 20 mg lactose) (Cass et al. 1999). Systemic absorption was improved by this route, with about 15% total bioavailability, a time to peak plasma concentration of 1–2 h, and a plasma half-life of 3–5 h. More than 90% of absorbed zanamivir is excreted unchanged in the urine (Cass et al. 1999). Oseltamivir carboxylate has oral bioavailability ~80%, peak plasma concentration is achieved 3–4 h after administration, and the plasma half-life is 6–10 h (McClellan and Perry 2001). Oseltamivir is not thought to distribute into the brain (Straumanis et al. 2002), although central nervous system toxicity in juvenile rats with an immature blood-brain barrier has led to caution in the use of this agent in children under 1 year of age (Kimberlin et al. 2010). Oseltamivir carboxylate is eliminated primarily by renal excretion through a combination of glomerular filtration and anionic renal tubular secretion (He et al. 1999). In general, adverse events after oral administration of oseltamivir are considered to be mild and include nausea and vomiting. Inhalation of zanamivir is generally well tolerated but may cause bronchospasm in some patients with underlying lung disease (Gubareva et al. 2000).
Both oseltamivir and zanamivir are effective in early treatment of influenza A viruses in experimentally infected volunteers (Hayden et al. 1999b; Calfee et al. 1999), and were effective and well tolerated in adults treated for natural influenza infection (Hayden et al. 1997; Treanor et al. 2000; Nicholson et al. 2000). Reduced effectiveness for influenza B viruses as compared to influenza A viruses has been reported for oseltamivir (Kawai et al. 2008), and, in general, only sparse data are available from randomized trials of NA inhibitor effectiveness against influenza B viruses. The therapeutic benefits of NA inhibitors have been reported to include reductions of about 24 h in the time to alleviation of illness, resumption of usual activities, and duration of fever, as well as decreases in illness severity, ancillary medication use, viral titers, and the frequency of antibiotic prescriptions for lower respiratory complications (Hayden et al. 1997; Nicholson et al. 2000; Treanor et al. 2000). Oseltamivir both decreases the incidence of secondary bacterial pneumonia and reduces the severity of complications in an animal model (McCullers 2004). Similar data are not available from a single, well-powered trial in humans, although a meta-analysis of data from multiple trials including unpublished data suggests these results can be extrapolated at least to healthy adults (Kaiser et al. 2003). In children, however, oseltamivir was shown to reduce the occurrence of otitis media by 44% compared to placebo (Whitley et al. 2001). Retrospective reviews of insurance claims databases suggest that NA inhibitors reduce the risk of otitis media, pneumonia, respiratory illnesses other than pneumonia, and hospitalization in both adults and children (Gums et al. 2008; Piedra et al. 2009). Limited data are also available on reduction of risk in adult diabetics, where fewer respiratory illnesses and hospitalizations were noted using this methodology (Orzeck et al. 2007).
Both zanamivir and oseltamivir have been shown to be efficacious in preventing laboratory-confirmed influenza in healthy adults during an outbreak of influenza (Monto et al. 1999; Hayden et al. 1999a) and have demonstrated the ability to interrupt household transmission (Welliver et al. 2001; Hayden et al. 2000, 2004). Zanamivir is approved for the treatment of acute influenza in adults and in children 7 years and older with a recommended dosage of 10 mg twice daily for 5 days by inhalation (Harper et al. 2009). Oseltamivir is indicated for the treatment of acute influenza in patients aged >1 year, and is administered orally to adults at 75 mg twice daily for 5 days starting within 2 days of symptom onset (Table 1). The oseltamivir treatment dosage for children of age 1–12 is based on weight. Early administration of oseltamivir increases the benefit seen in healthy adults relative to treatment at 48 h (Aoki et al. 2003), but no randomized, controlled trials have been conducted studying treatment outside of the first 48 h , so no data are available to examine the effects of late treatment on prevention of complications. Indeed, since persons with chronic illness, who might be more likely to benefit from late treatment as viral control might be established later in such individuals, have typically been excluded from antiviral studies this question is currently unanswered.
Zanamivir is approved for prophylaxis in adults and in children 5 years and older, using a single daily 10 mg dose for 10 days for household prophylaxis and for 28 days for seasonal prophylaxis (Harper et al. 2009). Oseltamivir is also approved for prophylaxis of influenza at a dosage of 75 mg per day for up to 6 weeks (Harper et al. 2009). Higher doses and longer durations of therapy with oseltamivir (150 mg twice daily for 10 days) have been attempted anecdotally for severe infections with H5N1 subtype viruses or in immunocompromised subjects (Beigel et al. 2005; Le et al. 2005; Memoli et al. 2010), but no data from randomized trials are available to assess the effectiveness of these measures. For seasonal influenza, the usage of high dosages of oseltamivir (up to 450 mg twice daily) have been addressed in a pilot manufacturer-sponsored study, showing dose-linear pharmacokinetics and good tolerability (Dutkowski et al. 2010).
Mechanism of Action
The NA is second to HA as the most abundant protein on the viral surface, with 50–100 molecules per virion. Although the NA and HA surface glycoproteins of influenza virus evolve and change continuously, conserved residues were identified through all influenza subtypes at the NA active site (Colman 1994). The NA contains 19 residues at the active site that are conserved in all NA subtypes of influenza viruses, including eight catalytic residues (R118, D151, R152, R224, E276, R292, R371, and Y406; N2 numbering) that directly interact with the substrate (sialic acid) and 11 framework residues for functional binding and catalysis (E119, R156, W178, S179, D/N198, I222, E227, H274, E277, N294, and E425) that support the catalytic residues (Colman et al. 1993). The NA inhibitors were designed based on the knowledge of the three-dimensional structure of the NA complex with sialic acid (von Itzstein et al. 1996).
The primary function of the NA enzyme in the life cycle of influenza viruses is to cleave the α-ketosidic bond linking the terminal sialic acid residue from the glycoconjugate, destroying the receptor association with the HA (Gubareva et al. 2000). In this manner the influenza viral NA removes sialic acid residues from the surface of the infected cell and from mucins in the respiratory tract, facilitating the release of newly synthesized virus particles and allowing the virus to spread (Matrosovich et al. 2004). The cleavage of HA receptors by NA is also essential to prevent attachment of new viruses to one another and to glycopeptides present on the cell membrane (Colman 1994).
Resistance
Two mechanisms of resistance to NA inhibitors have been described. The first is mutations within the NA enzyme catalytic site that disrupt a direct interaction with the drug. The second is mutations in the HA that reduce affinity for its receptor, thus compensating for the effect of the drug on NA activity (Gubareva et al. 2000). Thus, the molecular determinants of NA inhibitor resistance have been mapped not only to NA but also to HA (Gubareva et al. 2000; McKimm-Breschkin 2000). However, mutations at conserved NA residues are reported to be more clinically relevant (McKimm-Breschkin 2002). NA mutations that confer NA inhibitor resistance reduce sialidase activity and/or stability (Staschke et al. 1995; Tai et al. 1998), but the in vitro replication kinetics of these variants do not always reflect the defective NA enzymatic activity. In some cases the replication efficiency of such mutants may be comparable to that of the wild-type virus (Gubareva et al. 1997) or may be compromised (Ives et al. 2002; Tai et al. 1998) in cell culture. The presence of HA mutations that mask the NA defect and the lack of an optimal cell line may limit characterization of the NA inhibitor-resistant variants in vitro (Matrosovich et al. 2003). Sequence analysis of clinically derived drug-resistant viruses revealed that these NA mutations are NA subtype specific and differ with the NA inhibitor used (Ferraris and Lina 2008). The most frequently observed mutations for NA inhibitor-resistant variants for influenza A viruses of N1 NA subtype are H275Y and N295S (N1 numbering); for influenza A viruses of N2 NA subtype are R292K and E119V (N2 numbering), and for influenza B viruses are R152 and D198 N. Initial studies found that NA inhibitor-resistant influenza viruses were severely compromised in vitro and in animal models (Carr et al. 2002; Ives et al. 2002; Herlocher et al. 2002), and thus led to the idea that resistant viruses were unlikely to have an impact on epidemic and pandemic influenza. However, further studies showed that clinically derived H1N1 virus with H275Y NA mutation (Herlocher et al. 2004) and reverse genetically derived H3N2 virus with E119V NA mutation (Yen et al. 2005) possess similar biological fitness and transmissibility as their drug-sensitive counterparts.
Prior to the 2007–2008 influenza season oseltamivir-resistant variants were found in only a small proportion of patients (approximately 4–8% of children and <1% of adults) after treatment with the NA inhibitor (Stilianakis et al. 2002). However, rigorous detection techniques identified resistant mutants in 9 of 50 (18%) Japanese children during treatment with oseltamivir (Kiso et al. 2004). High level of oseltamivir resistance among influenza H1N1 viruses was reported in many European countries starting in the 2007–2008 influenza season. The emergence and widespread of naturally occurring oseltamivir-resistant variants with H275Y NA amino acid substitution among seasonal H1N1 influenza viruses of A/Solomon Islands/3/06-lineage emphasized that drug-resistant viruses can be highly fit and transmissible in humans (Lackenby et al. 2008; Dharan et al. 2009).
During the 2009 H1N1 influenza pandemic, almost all tested viruses remained susceptible to oseltamivir and zanamivir. Oseltamivir-resistant variants with H275Y NA mutation were isolated from individuals receiving prophylaxis (Baz et al. 2009) and from immunocompromised patients (Centers for Disease Control 2009) under drug selection pressure. Oseltamivir-resistant variants also have been isolated from untreated patients (Leung et al. 2009; Zonis et al. 2010) and from a few community clusters (Le et al. 2010). Two cases of suspected nosocomial transmission between immunocompromised patients have been reported, although it is uncertain whether the mutants came from secondary transmission or arose spontaneously (Gulland 2009). The reasons for the relative paucity of resistant strains and the lack of widespread transmission are not yet clear. However, experimental evidence suggests that the oseltamivir-resistant H275Y mutant of the pandemic 2009 H1N1 virus retained efficient transmission through direct contact in a ferret model, but respiratory droplet transmission was decreased as compared to an oseltamivir-sensitive virus (Duan et al. 2010). This suggests that transmission efficiency of the mutants may be decreased, limiting spread between humans.
Changes in Antiviral Policy During 2009 H1N1 Pandemic
The emergence of a novel pandemic strain in 2009 presented several dilemmas regarding the use of antiviral medications for influenza. First, the two licensed classes of drugs, the M2 ion channel inhibitors and NA inhibitors, were only approved for use with acute, uncomplicated influenza within the first 48 h of illness. The major effects of traditional antiviral therapy are symptom reduction (Hayden et al. 1997; Treanor et al. 2000), and earlier treatment is more likely to have beneficial effects (Aoki et al. 2003). During the pandemic, it became apparent that clinical use extended beyond acute, uncomplicated influenza to include severely ill patients with complex, prolonged infections, with treatment often starting beyond the 48 h window (Memoli et al. 2010; Harter et al. 2010). As discussed above, there are limited data on the use of antiviral drugs in hospitalized patients or on the effectiveness of such compounds at preventing complications of influenza. Treatment of such critically ill patients highlighted a second issue that no approved drugs could be given by the intravenous route, which is required in severely ill persons. And finally, resistance complicated management. The pandemic 2009 H1N1 strain was resistant to the adamantanes, but susceptible to the NA inhibitors. Seasonal H1N1 viruses circulating during the 2008–2009 season showed the opposite pattern, susceptibility to adamantanes but resistance to NA inhibitors. Thus, without the ability to not only diagnose but also serotype viruses in the community, the choice of antiviral for empiric therapy was unclear. Furthermore, resistance to NA inhibitors was noted to develop in select circumstances such as prolonged treatment of immunocompromised patients (Memoli et al. 2010), threatening to make all approved agents useless.
In response to these concerns, public health authorities made a number of changes to antiviral policy during the 2009–2010 influenza season. In April 2009, the Department of Health and Human Services (DHHS) issued an Emergency Use Authorization (EUA) for oseltamivir, allowing it to be used in children under 1 year of age, and in patients who were symptomatic for more than 48 h, and hospitalized or severely ill patients. A similar EUA was issued for zanamivir, allowing its use in hospitalized patients and in those after 48 h of onset of symptoms. In addition, an intravenous formulation of zanamivir was made available by the company that produces it on a compassionate use basis for patients whose medical conditions did not allow to use oral or inhaled drugs (Harter et al. 2010). In November 2009, another EUA was issued for a third, unlicensed NA inhibitor, peramivir (Birnkrant and Cox 2009). Based on an expedited review at FDA, peramivir was thought likely to be effective for treatment of influenza, and was authorized for intravenous administration in hospitalized patients with pandemic H1N1 infection. Since oseltamivir-resistant viruses are typically also resistant to peramivir (Moscona 2009; Memoli et al. 2010), only zanamivir could be used for pandemic H1N1 strains which developed oseltamivir resistance during treatment. In June 2010, the EUAs for oseltamivir, zanamivir, and peramivir were terminated by DHHS upon expiration of the declared emergency related to the 2009 pandemic.
Since patients will continue to have severe disease and complications from influenza, further research is necessary to justify permanent approval for these indications using either existing or novel antiviral compounds. In particular, research is needed on prevention of complications such as bacterial superinfections, viral pneumonia, and cardiac events. Patient populations at highest risk of developing complications and requiring hospitalization, including asthmatics, persons with heart disease, infants, and the elderly, should be targeted in these trials. Hopefully data from use outside of the normal indications will become available from the experience during the 2009 H1N1 influenza pandemic.
Combination Therapy
The segmented genome of influenza viruses, allowing reassortment between viruses, and a high mutation rate based on infidelity of the viral polymerase are factors in the emergence of resistance to any single antiviral drug therapy. Resistance may be less of a problem when combination treatment regimens are employed against an infectious agent. This strategy has already proven effective in the management of human immunodeficiency virus (HIV)-infected individuals, where multiple drug combinations of highly active anti-retroviral therapy (HAART) have revolutionized treatment (Raboud et al. 2002; Kuritzkes and Walker 2007). The combined application of antiviral drugs that target multiple distinct functions of the virus possess different modes of action, pharmacokinetics, tolerance profiles, and resistance patterns and make it a logical therapeutic option. The existence of effective antiviral agents for influenza combination therapy may not only potentiate antiviral activity and result in synergistic or additive effects, but may also enhance clinical outcomes by allowing reductions of the doses of individual drugs. The major benefit of dose reduction in this scenario is a reduction in dose-related drug toxicity and side effects.
A sufficient body of information is now available on the advantages of double and triple drug combinations on influenza virus infection in cell culture and mouse models. Initial studies included evaluation of adamantanes (amantadine and rimantadine) and the synthetic nucleoside ribavirin. Ribavirin, a broad-spectrum antiviral agent, inhibits influenza A and B virus infection in vitro and in animal models (De Clerq 2006; Smee et al. 2006; Sidwell et al. 2005). In MDCK cells, rimantadine combined with ribavirin showed additive and synergistic effects against the replication of influenza A viruses (Hayden et al. 1984; Stein et al. 1987). Combinations that paired rimantadine with an NA inhibitor (zanamivir, oseltamivir carboxylate, or peramivir) reduced extracellular H1N1 and H3N2 influenza virus yields in MDCK cells more efficiently than any of the drugs alone (Govorkova et al. 2004). Recent studies have shown highly synergistic activity of a triple combination antiviral drug (TCAD) regimen (oseltamivir carboxylate, amantadine, and ribavirin) against both seasonal viruses and the novel H1N1 pandemic strain in vitro (Nguyen et al. 2009, 2010). The synergy of the TCAD regimen was significantly greater than that of any double combination tested, including a combination comprising two NA inhibitors at concentrations achievable in human plasma (Nguyen et al. 2009). Ribavirin is clinically available in many countries because of its therapeutic activity against hepatitis C virus (Hu et al. 2010). However, it has not been officially approved for use against influenza in the United States, and its approved use is limited in the countries where it is licensed due to a relatively small therapeutic index, induction of hemolytic anemia at high doses, high toxicity, and potential teratogenic effects (De Clerq 2006).
Further, preclinical data from animal models have confirmed the benefits of combination chemotherapy for influenza virus infection (Govorkova and Webster 2010). Combinations of the NA inhibitor peramivir and ribavirin significantly increased survival of mice lethally challenged with influenza A/NWS/33 (H1N1) virus (Smee et al. 2002). A synergistic interaction was reported when rimantadine and oseltamivir were given to mice infected with lethal dose of the 1968 H3N2 pandemic strain (Galabov et al. 2006). Oseltamivir-ribavirin combinations were synergistic against an influenza B virus infection in mice (Smee et al. 2006). Importantly, drug combinations were demonstrated to be efficacious against highly pathogenic H5N1 influenza viruses in vivo (Leneva et al. 2000; Ilyushina et al. 2007, 2008). In a mouse model, oseltamivir combined with amantadine or rimantadine was more effective than monotherapy with oseltamivir in preventing the death of mice infected with H5N1 or H9N2 viruses (Leneva et al. 2000). Combinations of amantadine and oseltamivir produced an additive benefit: survival was 30% with oseltamivir alone, 60% with amantadine alone, and 90% with combination treatment as tested against recombinant amantadine-sensitive A/Vietnam/1203/04 (H5N1) influenza virus (Ilyushina et al. 2007). However, combination therapy was no better than oseltamivir alone against the recombinant amantadine-resistant A/Vietnam/1203/04 (H5N1) influenza virus in this study. An oseltamivir and ribavirin combination therapy showed principally additive efficacy against both clade 1 and clade 2 highly pathogenic H5N1 influenza viruses in a mouse model, although the results were dependent on the H5N1 influenza strain. Higher doses were required to protect mice against A/Turkey/15/06 virus than against A/Vietnam/1203/04 virus (Ilyushina et al. 2008).
Until recently, clinical trials that address the benefits of drug combinations have been limited. The major reason for this lack of clinical study is the high level of resistance to the amantadine among seasonal influenza strains, which had limited the available options using approved agents. A prospective, controlled, trial of oral rimantadine and nebulized zanamivir therapy in seriously ill adults hospitalized with lower respiratory tract manifestations of influenza was conducted during two influenza seasons (January 1998–April 1999) prior to widespread amantadine resistance (Ison et al. 2003). Patients treated with combinations of the drugs demonstrated a trend toward fewer days of virus shedding and were less likely to have a severe cough. Moreover, no resistant variants were found in the group receiving combination therapy, while 2 of 11 patients in the rimantadine monotherapy group had resistant virus (Ison et al. 2003). In a randomized, crossover study (n = 17) it was shown that pharmacokinetics of amantadine (100 mg orally twice daily) were not affected by co-administration of oseltamivir (75 mg orally twice daily), and there was no evidence for an increase in frequency or severity of adverse events when amantadine and oseltamivir were used in combination (Morrison et al. 2007). Given the paucity of clinical data, it is important to now initiate clinical trials specifically designed to evaluate issues regarding combination chemotherapy for influenza. The planning of such studies should include clinical and virological evaluations with determination of influenza virus loads in the patient, the molecular and biological characterization of viruses for resistance and fitness, and pharmacokinetic data to evaluate safety and toxicity.
Investigational Agents for Influenza
Parenteral NA Inhibitors
In addition to further study of existing antivirals, there is an intense need for new antiviral compounds (Hayden 2009). No new influenza antiviral drugs have been approved since 1999 in the United States and none currently have an indication for treatment of severe disease. New formulations of conventional anti-influenza drugs and novel antiviral agents that target either viral proteins or host defense mechanisms are currently at various stages of development (Table 2). Parenteral administration of the NA inhibitors zanamivir (intravenous, IV) and peramivir (IV and intramuscular) is being evaluated in preclinical studies and clinical trials for the treatment of seasonal influenza A infection and were selectively used during the recent 2009 H1N1 pandemic as described above (Harter et al. 2010; Birnkrant and Cox 2009; Memoli et al. 2010). In Japan, parenteral peramivir was licensed under the trade name Rapiacta® in 2010 (Clinical Trials.gov 2010).
Table 2.
Investigational agents for influenza in clinical development
| Agent | Target | Route of administration | Stage of development | Company |
|---|---|---|---|---|
| Virus-targeted approach | ||||
| Relenza (Zanamivir) | NA inhibitor | Parenteral (IV) | Phase 2/3 clinical trials ongoing | GlaxoSmithKline, UK |
| Tamiflu (Oseltamivir) | NA inhibitor | Parenteral (IV) | Phase 3 trial ongoing | Hoffmann-La Roche, Switzerland |
| Peramivir | NA inhibitor | Parenteral (IM and IV) | Licensed in Japan as Rapiacta® in January, 2010; Phase 2/3 clinical trials in US ongoing | BioCryst Pharmaceuticals, USA |
| Laninamivir (CS-8958) |
NA inhibitor, long-acting |
Inhalation | Phase 3 clinical trial completed | Biota, Australia in partnership with Daiichi-Sankyo, Japan |
| Favipiravir (T-705) | Polymerase inhibitor | Oral | Phase 2 clinical trial in Japan ongoing | Toyama Chemical, Japan |
| Triple combination therapy | Combination chemotherapy | Oral | Phase 1–2 clinical trials ongoing | Adamas Pharmaceuticals, Inc., USA |
| Hyper-immune serum | HA neutralization | Parenteral (IV) | Phase 1–2 clinical trials ongoing | Various |
| Host-targeted approach | ||||
| Fludase®, (DAS181) | Influenza virus receptor inactivator | Inhalation | Phase 1 studies ongoing | NexBio, Inc., USA |
| Corticosteroids | Anti-inflammatory | Parenteral | Phase 3 study (suspended) | University of Versailles, France |
| Rosuvastatin | Cholesterol biosynthesis pathway inhibitor | Oral | Phase 3 study ongoing | Vanderbilt University, USA |
Note Data from www.clinicaltrials.gov (Clinical Trials.gov 2010). Triple combination therapy is conducted with Symmetrel, Tamiflu and Ribavirin
Long-Acting NA Inhibitor
The long-acting inhaled NA inhibitor laninamivir (R-125489 = laninamivir and CS-8958 = laninamivir octanoate or the laninamivir prodrug) is a novel, promising drug for the control of influenza (Fig. 1) (Honda et al. 2009; Koyama et al. 2009). Laninamivir is a multimeric zanamivir compound that potently inhibited the NA activities of various influenza A and B viruses, including subtypes N1 to N9 and oseltamivir-resistant viruses (Yamashita et al. 2009), as well as the pandemic 2009 H1N1 virus (Itoh et al. 2009). An attractive feature of this compound is the prolonged retention in the lungs which allows once weekly administration and has shown efficacy superior to that of zanamivir and oseltamivir in mouse models of infection with influenza viruses, including seasonal, pandemic 2009 H1N1, and highly pathogenic H5N1 viruses (Koyama et al. 2009; Kubo et al. 2010; Kiso et al. 2010a). Phase 1 clinical trials have been completed in Japan, and no adverse events related to laninamivir octanoate were observed. The drug is slowly eliminated from the body, lasting up to 144 h after administration with a half-life of about 3 days, suggesting that a single inhalation of laninamivir octanoate can act as a long-acting NA inhibitor in humans (Ishizuka et al. 2010). A double-blind, randomized controlled trial demonstrated that the drug was effective and well tolerated in children with seasonal oseltamivir-resistant influenza A (H1N1) virus infection and effective for treatment of disease caused by oseltamivir-resistant influenza viruses (Sugaya and Ohashi 2010).
Polymerase Inhibitor
Another promising anti-influenza agent which is at advanced stages of development is a substituted pyrazine compound, T-705 (Fig. 1, 6-fluoro-3-hydroxy-2-pyrazinecarboxamide, favipiravir) (Furuta et al. 2002). T-705 inhibits an early to middle stage of viral replication but not the adsorption or release stage. T-705 is converted to the ribofuranosyltriphosphate derivative by host enzymes, and this metabolite selectively inhibits the influenza viral RNA-dependent RNA polymerase in a dose-dependent manner. Interestingly, this compound did not inhibit host DNA and RNA synthesis and only weekly inhibits inosine 5′-monophosphate dehydrogenase (IMPDH) activity (Furuta et al. 2005). T-705 showed a more favorable therapeutic index than did ribavirin in preclinical tests of toxicity in mammalian cells (Furuta et al. 2005). The potent antiviral activity of T-705 in vitro was demonstrated against seasonal influenza A (H1N1, H2N2, and H3N2), B and C viruses (Furuta et al. 2002), influenza A (H5N1) viruses (Sidwell et al. 2007; Kiso et al. 2010b), as well as an oseltamivir-resistant virus (Furuta et al. 2002; Sleeman et al. 2010). Oral treatment with T-705 at the dose of 30 mg/kg/day or more prevented death, inhibited lung consolidation, and reduced lung virus titers in a BALB/c mouse model under lethal challenge with H5N1 and H3N2 subtype viruses (Furuta et al. 2002; Sidwell et al. 2007; Kiso et al. 2010b). In a comparative experiment with oseltamivir, using mice infected with a high challenge dose of influenza A/PR/8/34 (H1N1) virus, T-705 completely prevented death, and the survival rate was significantly higher than in oseltamivir-treated animals (Takahashi et al. 2003). The results of studies of delayed initiation of treatment using influenza A and B viruses showed a marked reduction in mortality even when treatment with T-705 was initiated from 60 to 96 h post virus inoculation. The benefits of using oseltamivir and T-705 in combination to treat H1N1, H3N2, and H5N1 influenza virus infection were recently demonstrated in a mouse model (Smee et al. 2010). Initial unpublished data on human pharmacology are encouraging with regard to oral absorption and tolerability, and Phase 2 efficacy studies of favipiravir have been conducted in Japan (Furuta et al. 2009).
HA Inhibitor
Another potential anti-influenza agent is Cyanovirin-N (CV-N), a carbohydrate-binding protein that inhibits viral entry into cells by specifically binding to high mannose oligosaccharides on the surface glycoproteins of enveloped viruses (O’Keefe et al. 2003). CV-N is a 101 amino acid protein derived from the cyanobacterium Nostoc ellipsosporum and was originally discovered as an inhibitor of HIV, but was later found to inhibit other enveloped viruses such as influenza and Ebola (Boyd et al. 1997; O’Keefe et al. 2003; Barrientos et al. 2003). CV-N showed antiviral activity against a range of influenza A and B viruses in vitro and in mice and ferrets (Smee et al. 2007, 2008). However, the efficacy was strain-specific and depends on the composition of glycosylation sites on the HA. Loss of these glycosylation sites due to mutation of the HA leads to decreases in CV-N binding and antiviral activity (O’Keefe et al. 2003; Smee et al. 2007). A high mannose oligosaccharide at a conserved residue N94 (H3 numbering, corresponds to position 87 in H1 subtype) of the HA1 subunit of HA is the primary target of CV-N, and substitutions at this position by itself confer CV-N resistance. Mutation(s) that affects the receptor binding site for the HA1 may also reduce efficacy of CV-N against influenza viruses (Smee et al. 2007). Clinical studies in humans have not been reported.
Sialic Acid Receptor Inhibitor
Targeting the host cell components required for viral infection is a novel antiviral approach which can theoretically lead to lack or low rates of emergence of drug-resistant variants. Sialic acid (SA)-containing receptors on the surface of susceptible cells are required for infection by influenza viruses. The interaction between HA glycoprotein of influenza virus and SA receptors is essential for initial stages of virus replication, suggesting that targeting this interaction as an therapeutic approach would have some promise. DAS181 (Fludase®) is a recombinant fusion protein containing a sialidase catalytic domain and a respiratory epithelium-anchoring domain [amphiregulin (AR) tag], which can be mass produced in Escherichia coli (Malakhov et al. 2006). The sialidase activity of DAS181 can cleave SAα2,6- and SAα2,3-linked cellular receptors, which are preferentially recognized by human and avian influenza strains, respectively. Because it is host-directed toward the SA acid receptors on airway epithelium, it can also prevent the binding of other respiratory viruses that also utilize these receptors (e.g. parainfluenza) (Moscona et al. 2010). DAS181 potently inhibits infection by seasonal influenza A and B viruses, pandemic 2009 H1N1 viruses, NA inhibitor-resistant influenza viruses, as well as the potentially pandemic H5N1 influenza viruses in MDCK cells, mice, and ferrets (Belser et al. 2007; Triana-Baltzer et al. 2009a, b). In vitro removal of receptors by DAS181 leads to a prolonged antiviral effect, although it is not clear whether this effect will translate into a less-frequent dosing regimen in the clinic. Long-term DAS181 exposure to numerous cell lines and human primary cells does not cause cytotoxicity. The resistance potential of this compound requires further investigations. Preliminary data indicated that DAS181-resistant variants could be generated and mild resistance was developed in two out of six strains tested following up to 30 passages in MDCK cells. DAS181-resistant viruses exhibited an attenuated phenotype in vitro and in mice, and still could be inhibited by the higher concentrations of compound (Moss et al. 2010). Phase 1 clinical studies of DAS181 have been completed, but data on safety are not yet publically available.
Other Candidates
Advances in understanding the mechanisms of influenza virus replication have revealed a number of potential drug targets (Fig. 2). Small interfering RNAs (siRNAs) can be designed to target viral RNA without engaging host RNA, and therefore can be highly specific, highly effective, have low toxicity, and can be easy to make and formulate (Alvarez et al. 2009). Clinical, proof-of-concept has been shown for an RNA-interference agent targeted against respiratory syncytial virus (DeVincenzo et al. 2010). To this point, only pre-clinical data using RNA inhibitor based therapies are available for influenza (Kumar et al. 2010).
Fig. 2.

A multidrug approach to the management of influenza. HA, hemagglutinin; IFN, interferon; LANI, long-acting neuraminidase inhibitor; NA, neuraminidase; siRNA, small interfering RNA. Reprinted from (White et al. 2009) under the terms of the Creative Commons Attributions License
Antibody therapies are another strategy that has been proposed for treatment or prevention of influenza. This includes intravenous immune globulin (IVIG) preparations, which are used clinically for a variety of purposes, hyperimmune sera from recovered or vaccinated individuals, and specific monoclonal antibody therapies (Bearman et al. 2010; Luke et al. 2010; Martinez et al. 2009). Mouse model data using all three approaches suggest efficacy for primary influenza infections (Marinescu et al. 2009; Ramisse et al. 1998; Krause et al. 2010; Kashyap et al. 2010). Limited clinical data in humans support this approach conceptually, primarily from uncontrolled studies of treatment of pandemic influenza or H5N1 virus-infected patients (Luke et al. 2006; Kong and Zhou 2006; Zhou et al. 2007).
The strategy of immunomodulation to broadly reduce the inflammatory response during severe influenza virus infections (Fig. 2) has also been proposed (Fedson 2009), but is currently not supported by clinical data in humans. Systemic steroids were frequently used as a clinical therapeutic during the 2009 H1N1 pandemic (Falagas et al. 2010). In some published studies more than 50% of severely ill patients were treated with corticosteroids (Kumar et al. 2009). However, no clinical benefit of steroids has thus far been shown for ARDS or specifically for influenza (Steinberg et al. 2006; Napolitano et al. 2010). Alternative candidates targeting specific anti-inflammatory pathways have also been put forward (Fedson 2008), including drugs such as statins, which inhibit a cholesterol biosynthesis pathway enzyme (Liu et al. 2009), agonists of peroxisome proliferator-activated receptors including fibrates and thiozolidinediones (Budd et al. 2007; Aldridge, Jr. et al. 2009), cyclooxygenase pathway inhibitors (Zheng et al. 2008), and antioxidants such as N-acetyl-l-cysteine (Geiler et al. 2010). Each has shown some efficacy in mouse models (Budd et al. 2007; Zheng et al. 2008; Aldridge, Jr. et al. 2009; Geiler et al. 2010). Out of this group of candidates, statins are the only agents that have been studied in humans thus far. To this point, however, cohort studies of persons prescribed statins for their cholesterol-lowering properties have shown no obvious clinical benefit against influenza morbidity (Kwong et al. 2009; Fleming et al. 2010). Further, clinical investigation of these agents and their potential to act in combination with traditional antiviral agents needs to be explored.
Conclusions
Several issues have limited the effectiveness of the currently available antiviral drugs against influenza. First, unlike antibiotics, which can eliminate or greatly reduce pathogen burden, existing influenza antiviral drugs serve only to halt progression of disease by preventing new host cells from being infected. If this intervention is administered early enough in the clinical course, it may alter the tempo of infection, allowing normal immune clearance mechanisms to gain the upper hand. Thus, the major effects of treatment are symptom reduction and a more rapid recovery, not immediate clinical cure. Second, the currently licensed antivirals are all oral medications and, until the recent 2009 pandemic, were authorized only for use in mild to moderate influenza. Critically ill patients with either H5N1 or pandemic 2009 H1N1 infections have been difficult to treat until the recent availability of intravenous peramivir and zanamivir. Third, resistance has been a clinically significant issue for the adamantanes for years, limiting their utility. To combat these issues, more research on existing antivirals and further investigation of novel compounds and strategies are needed. Combination therapy has been explored both with existing, licensed, antivirals as well as with agents not currently approved for use against influenza. Novel agents targeting important viral proteins or host-pathogen interactions are at various stages of development. And finally, novel immunomodulatory strategies targeting the virus-mediated effects or host responses are in development and clinical testing. The future of influenza control likely involves improved means to prevent infection, coupled with combined strategies to both slow the virus as well as mitigate the immunopathologic consequences of infection when it occurs. A coalescence of divergent paths of research to meet these goals is needed if the urgent public health threat of seasonal and pandemic influenza is to be met.
Contributor Information
Jürgen A. Richt, Phone: 785-532 4408, Email: jricht@vet.k-state.edu
Richard J. Webby, Email: richard.webby@stjude.org
Elena A. Govorkova, Phone: +1-901-5952243, FAX: +1-901-5958559, Email: elena.govorkova@stjude.org
References
- Aldridge JR, Jr, Moseley CE, Boltz DA, et al. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci USA. 2009;106:5306–5311. doi: 10.1073/pnas.0900655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez R, Elbashir S, Borland T, et al. RNA interference-mediated silencing of the respiratory syncytial virus nucleocapsid defines a potent antiviral strategy. Antimicrob Agents Chemother. 2009;53:3952–3962. doi: 10.1128/AAC.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki FY, Macleod MD, Paggiaro P, et al. Early administration of oral oseltamivir increases the benefits of influenza treatment. J Antimicrob Chemother. 2003;51:123–129. doi: 10.1093/jac/dkg007. [DOI] [PubMed] [Google Scholar]
- Aoki FY, Sitar DS. Clinical pharmacokinetics of amantadine hydrochloride. Clin Pharmacokinet. 1988;14:35–51. doi: 10.2165/00003088-198814010-00003. [DOI] [PubMed] [Google Scholar]
- Appleyard G. Amantadine-resistance as a genetic marker for influenza viruses. J Gen Virol. 1977;36:249–255. doi: 10.1099/0022-1317-36-2-249. [DOI] [PubMed] [Google Scholar]
- Babu YS, Chand P, Bantia S, et al. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem. 2000;43:3482–3486. doi: 10.1021/jm0002679. [DOI] [PubMed] [Google Scholar]
- Barr IG, Hurt AC, Iannello P, et al. Increased adamantane resistance in influenza A(H3) viruses in Australia and neighbouring countries in 2005. Antiviral Res. 2007;73:112–117. doi: 10.1016/j.antiviral.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Barr IG, Deng YM, Iannello P, et al. Adamantane resistance in influenza A(H1) viruses increased in 2007 in South East Asia but decreased in Australia and some other countries. Antiviral Res. 2008;80:200–205. doi: 10.1016/j.antiviral.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Barrientos LG, O’Keefe BR, Bray M, et al. Cyanovirin-N binds to the viral surface glycoprotein, GP1,2 and inhibits infectivity of Ebola virus. Antiviral Res. 2003;58:47–56. doi: 10.1016/S0166-3542(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Baz M, Abed Y, Papenburg J, et al. Emergence of oseltamivir-resistant pandemic H1N1 virus during prophylaxis. N Engl J Med. 2009;361:2296–2297. doi: 10.1056/NEJMc0910060. [DOI] [PubMed] [Google Scholar]
- bdel-Ghafar AN, Chotpitayasunondh T, Gao Z, et al. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med. 2008;358:261–273. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- Bearman GM, Shankaran S, Elam K. Treatment of severe cases of pandemic (H1N1) 2009 influenza: review of antivirals and adjuvant therapy. Recent Pat Antiinfect Drug Discov. 2010;5:152–156. doi: 10.2174/157489110791233513. [DOI] [PubMed] [Google Scholar]
- Beigel JH, Farrar J, Han AM, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- Belser JA, Lu X, Szretter KJ, et al. DAS181, a novel sialidase fusion protein, protects mice from lethal avian influenza H5N1 virus infection. J Infect Dis. 2007;196:1493–1499. doi: 10.1086/522609. [DOI] [PubMed] [Google Scholar]
- Belshe RB, Smith MH, Hall CB, et al. Genetic basis of resistance to rimantadine emerging during treatment of influenza virus infection. J Virol. 1988;62:1508–1512. doi: 10.1128/jvi.62.5.1508-1512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betakova T, Ciampor F, Hay AJ. Influence of residue 44 on the activity of the M2 proton channel of influenza A virus. J Gen Virol. 2005;86:181–184. doi: 10.1099/vir.0.80358-0. [DOI] [PubMed] [Google Scholar]
- Birnkrant D, Cox E. The emergency use authorization of peramivir for treatment of 2009 H1N1 influenza. N Engl J Med. 2009;361:2204–2207. doi: 10.1056/NEJMp0910479. [DOI] [PubMed] [Google Scholar]
- Boyd MR, Gustafson KR, McMahon JB, et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41:1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright RA, Medina MJ, Xu X, et al. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet. 2005;366:1175–1181. doi: 10.1016/S0140-6736(05)67338-2. [DOI] [PubMed] [Google Scholar]
- Bright RA, Shay DK, Shu B, et al. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. JAMA. 2006;295:891–894. doi: 10.1001/jama.295.8.joc60020. [DOI] [PubMed] [Google Scholar]
- Budd A, Alleva L, Alsharifi M, et al. Increased survival after gemfibrozil treatment of severe mouse influenza. Antimicrob Agents Chemother. 2007;51:2965–2968. doi: 10.1128/AAC.00219-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfee DP, Peng AW, Cass LM, et al. Safety and efficacy of intravenous zanamivir in preventing experimental human influenza A virus infection. Antimicrob Agents Chemother. 1999;43:1616–1620. doi: 10.1128/aac.43.7.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J, Ives J, Kelly L, et al. Influenza virus carrying neuraminidase with reduced sensitivity to oseltamivir carboxylate has altered properties in vitro and is compromised for infectivity and replicative ability in vivo. Antiviral Res. 2002;54:79–88. doi: 10.1016/S0166-3542(01)00215-7. [DOI] [PubMed] [Google Scholar]
- Cass LM, Efthymiopoulos C, Bye A. Pharmacokinetics of zanamivir after intravenous, oral, inhaled or intranasal administration to healthy volunteers. Clin Pharmacokinet. 1999;36(Suppl 1):1–11. doi: 10.2165/00003088-199936001-00001. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control High levels of adamantane resistance among influenza A (H3N2) viruses and interim guidelines for use of antiviral agents—United States, 2005–06 influenza season. MMWR Morb Mortal Wkly Rep. 2006;55:44–46. [PubMed] [Google Scholar]
- Centers for Disease Control Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients—Seattle. MMWR Morb Mortal Wkly Rep. 2009;58:893–896. [PubMed] [Google Scholar]
- Centers for Disease Control Update: influenza activity—United States, August 30, 2009–March 27, 2010, and composition of the 2010–11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:423–430. [PubMed] [Google Scholar]
- Clinical Trials.gov (2010). http://www.ClinicalTrials.gov. Accessed 8/4/10
- Colman PM. Influenza virus neuraminidase: structure, antibodies, and inhibitors. Protein Sci. 1994;3:1687–1696. doi: 10.1002/pro.5560031007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman PM, Hoyne PA, Lawrence MC. Sequence and structure alignment of paramyxovirus hemagglutinin-neuraminidase with influenza virus neuraminidase. J Virol. 1993;67:2972–2980. doi: 10.1128/jvi.67.6.2972-2980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapat C, Suzuki Y, Saito R, et al. Rare influenza A (H3N2) variants with reduced sensitivity to antiviral drugs. Emerg Infect Dis. 2010;16:493–496. doi: 10.3201/eid1603.091321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies WL, Grunert RR, Haff RF, et al. Antiviral activity of 1-adamantanamine (amantadine) Science. 1964;144:862–863. doi: 10.1126/science.144.3620.862. [DOI] [PubMed] [Google Scholar]
- De Clerq E. Antiviral agents active against influenza A viruses. Nat Rev Drug Discov. 2006;5:1015–1025. doi: 10.1038/nrd2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVincenzo J, Lambkin-Williams R, Wilkinson T, et al. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci USA. 2010;107:8800–8805. doi: 10.1073/pnas.0912186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyde V, Garten R, Sheu T, et al. Genomic events underlying the changes in adamantane resistance among influenza A(H3N2) viruses during 2006–2008. Influenza Other Respi Viruses. 2009;3:297–314. doi: 10.1111/j.1750-2659.2009.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyde VM, Xu X, Bright RA, et al. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J Infect Dis. 2007;196:249–257. doi: 10.1086/518936. [DOI] [PubMed] [Google Scholar]
- Dharan NJ, Gubareva LV, Meyer JJ, et al. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA. 2009;301:1034–1041. doi: 10.1001/jama.2009.294. [DOI] [PubMed] [Google Scholar]
- Dolin R, Reichman RC, Madore HP, et al. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A infection. N Engl J Med. 1982;307:580–584. doi: 10.1056/NEJM198209023071002. [DOI] [PubMed] [Google Scholar]
- Doyle WJ, Skoner DP, Alper CM, et al. Effect of rimantadine treatment on clinical manifestations and otologic complications in adults experimentally infected with influenza A (H1N1) virus. J Infect Dis. 1998;177:1260–1265. doi: 10.1086/515294. [DOI] [PubMed] [Google Scholar]
- Duan S, Boltz DA, Seiler P, et al. Oseltamivir-resistant pandemic H1N1/2009 influenza virus possesses lower transmissibility and fitness in ferrets. PLoS Pathog. 2010;6:e1001022. doi: 10.1371/journal.ppat.1001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutkowski R, Smith JR, Davies BE. Safety and pharmacokinetics of oseltamivir at standard and high dosages. Int J Antimicrob Agents. 2010;35:461–467. doi: 10.1016/j.ijantimicag.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Endo Pharmaceuticals Inc (2007) Amantadine Package Insert. http://www.endo.com/PDF/symmetrel_pack_insert.pdf
- Falagas ME, Vouloumanou EK, Baskouta E, et al. Treatment options for 2009 H1N1 influenza: evaluation of the published evidence. Int J Antimicrob Agents. 2010;35:421–430. doi: 10.1016/j.ijantimicag.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Fedson DS. Confronting an imminent pandemic with inexpensive generic agents: can it be done? Lancet Infect Dis. 2008;8:571–576. doi: 10.1016/S1473-3099(08)70070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedson DS. Confronting the next influenza pandemic with anti-inflammatory and immunomodulatory agents: why they are needed and how they might work. Influenza Other Respi Viruses. 2009;3:129–142. doi: 10.1111/j.1750-2659.2009.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris O, Lina B. Mutations of neuraminidase implicated in neuraminidase inhibitors resistance. J Clin Virol. 2008;41:13–19. doi: 10.1016/j.jcv.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Fleming DM, Verlander NQ, Elliot AJ, et al. An assessment of the effect of statin use on the incidence of acute respiratory infections in England during winters 1998–1999 to 2005–2006. Epidemiol Infect. 2010;138:1281–1288. doi: 10.1017/S0950268810000105. [DOI] [PubMed] [Google Scholar]
- Forest Pharmaceuticals Inc (2010) Rimantadine Package Insert. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/019649s015lbl.pdf
- Furuta Y, Takahashi K, Fukuda Y, et al. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother. 2002;46:977–981. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Takahashi K, Kuno-Maekawa M, et al. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother. 2005;49:981–986. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Takahashi K, Shiraki K, et al. T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res. 2009;82:95–102. doi: 10.1016/j.antiviral.2009.02.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galabov AS, Simeonova L, Gegova G. Rimantadine and oseltamivir demonstrate synergistic combination effect in an experimental infection with type A (H3N2) influenza virus in mice. Antivir Chem Chemother. 2006;17:251–258. doi: 10.1177/095632020601700502. [DOI] [PubMed] [Google Scholar]
- Geiler J, Michaelis M, Naczk P, et al. N-acetyl-l-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochem Pharmacol. 2010;79:413–420. doi: 10.1016/j.bcp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Gething MJ, Doms RW, York D, et al. Studies on the mechanism of membrane fusion: site-specific mutagenesis of the hemagglutinin of influenza virus. J Cell Biol. 1986;102:11–23. doi: 10.1083/jcb.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorkova EA, Fang HB, Tan M, et al. Neuraminidase inhibitor-rimantadine combinations exert additive and synergistic anti-influenza virus effects in MDCK cells. Antimicrob Agents Chemother. 2004;48:4855–4863. doi: 10.1128/AAC.48.12.4855-4863.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorkova EA, Webster RG. Combination chemotherapy for influenza. Viruses. 2010;2:1510–1529. doi: 10.3390/v2081510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grambas S, Bennett MS, Hay AJ. Influence of amantadine resistance mutations on the pH regulatory function of the M2 protein of influenza A viruses. Virology. 1992;191:541–549. doi: 10.1016/0042-6822(92)90229-I. [DOI] [PubMed] [Google Scholar]
- Gubareva LV, Robinson MJ, Bethell RC, et al. Catalytic and framework mutations in the neuraminidase active site of influenza viruses that are resistant to 4-guanidino-Neu5Ac2en. J Virol. 1997;71:3385–3390. doi: 10.1128/jvi.71.5.3385-3390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubareva LV, Kaiser L, Hayden FG. Influenza virus neuraminidase inhibitors. Lancet. 2000;355:827–835. doi: 10.1016/S0140-6736(99)11433-8. [DOI] [PubMed] [Google Scholar]
- Gulland A. First cases of spread of oseltamivir resistant swine flu between patients are reported in Wales. BMJ. 2009;339:b4975. doi: 10.1136/bmj.b4975. [DOI] [PubMed] [Google Scholar]
- Gums JG, Pelletier EM, Blumentals WA. Oseltamivir and influenza-related complications, hospitalization and healthcare expenditure in healthy adults and children. Expert Opin Pharmacother. 2008;9:151–161. doi: 10.1517/14656566.9.2.151. [DOI] [PubMed] [Google Scholar]
- Harper SA, Bradley JS, Englund JA, et al. Seasonal influenza in adults and children–diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1003–1032. doi: 10.1086/598513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter G, Zimmermann O, Maier L, et al. Intravenous zanamivir for patients with pneumonitis due to pandemic (H1N1) 2009 influenza virus. Clin Infect Dis. 2010;50:1249–1251. doi: 10.1086/651604. [DOI] [PubMed] [Google Scholar]
- Hay AJ, Zambon MC, Wolstenholme AJ, et al. Molecular basis of resistance of influenza A viruses to amantadine. J Antimicrob Chemother. 1986;18(Suppl B):19–29. doi: 10.1093/jac/18.supplement_b.19. [DOI] [PubMed] [Google Scholar]
- Hayden FG, Schlepushkin AN, Pushkarskaya NL. Combined interferon-alpha 2, rimantadine hydrochloride, and ribavirin inhibition of influenza virus replication in vitro. Antimicrob Agents Chemother. 1984;25:53–57. doi: 10.1128/AAC.25.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden FG, Monto AS. Oral rimantadine hydrochloride therapy of influenza A virus H3N2 subtype infection in adults. Antimicrob Agents Chemother. 1986;29:339–341. doi: 10.1128/AAC.29.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden FG (1996) Amantidine and rimantidine-clinical aspects. In: Richman DD (ed) Antiviral Drug Resistance, Wiley, pp. 59–77
- Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- Hayden FG, Atmar RL, Schilling M, et al. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med. 1999;341:1336–1343. doi: 10.1056/NEJM199910283411802. [DOI] [PubMed] [Google Scholar]
- Hayden FG, Treanor JJ, Fritz RS, et al. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA. 1999;282:1240–1246. doi: 10.1001/jama.282.13.1240. [DOI] [PubMed] [Google Scholar]
- Hayden FG, Gubareva LV, Monto AS, et al. Inhaled zanamivir for the prevention of influenza in families. Zanamivir Family Study Group. N Engl J Med. 2000;343:1282–1289. doi: 10.1056/NEJM200011023431801. [DOI] [PubMed] [Google Scholar]
- Hayden FG, Belshe R, Villanueva C, et al. Management of influenza in households: a prospective, randomized comparison of oseltamivir treatment with or without postexposure prophylaxis. J Infect Dis. 2004;189:440–449. doi: 10.1086/381128. [DOI] [PubMed] [Google Scholar]
- Hayden FG. Antiviral resistance in influenza viruses—implications for management and pandemic response. N Engl J Med. 2006;354:785–788. doi: 10.1056/NEJMp068030. [DOI] [PubMed] [Google Scholar]
- Hayden F. Developing new antiviral agents for influenza treatment: what does the future hold? Clin Infect Dis. 2009;48(Suppl 1):S3–S13. doi: 10.1086/591851. [DOI] [PubMed] [Google Scholar]
- He G, Massarella J, Ward P. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64–0802. Clin Pharmacokinet. 1999;37:471–484. doi: 10.2165/00003088-199937060-00003. [DOI] [PubMed] [Google Scholar]
- Herlocher ML, Carr J, Ives J, et al. Influenza virus carrying an R292K mutation in the neuraminidase gene is not transmitted in ferrets. Antiviral Res. 2002;54:99–111. doi: 10.1016/S0166-3542(01)00214-5. [DOI] [PubMed] [Google Scholar]
- Herlocher ML, Truscon R, Elias S, et al. Influenza viruses resistant to the antiviral drug oseltamivir: transmission studies in ferrets. J Infect Dis. 2004;190:1627–1630. doi: 10.1086/424572. [DOI] [PubMed] [Google Scholar]
- Honda T, Kubo S, Masuda T, et al. Synthesis and in vivo influenza virus-inhibitory effect of ester prodrug of 4-guanidino-7-O-methyl-Neu5Ac2en. Bioorg Med Chem Lett. 2009;19:2938–2940. doi: 10.1016/j.bmcl.2009.04.067. [DOI] [PubMed] [Google Scholar]
- Hu J, Doucette K, Hartling L, et al. Treatment of hepatitis C in children: a systematic review. PLoS ONE. 2010;5:e11542. doi: 10.1371/journal.pone.0011542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt AC, Holien JK, Parker M, et al. Zanamivir-resistant influenza viruses with a novel neuraminidase mutation. J Virol. 2009;83:10366–10373. doi: 10.1128/JVI.01200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyushina NA, Hoffmann E, Salomon R, et al. Amantadine-oseltamivir combination therapy for H5N1 influenza virus infection in mice. Antivir Ther. 2007;12:363–370. [PubMed] [Google Scholar]
- Ilyushina NA, Hay A, Yilmaz N, et al. Oseltamivir-ribavirin combination therapy for highly pathogenic H5N1 influenza virus infection in mice. Antimicrob Agents Chemother. 2008;52:3889–3897. doi: 10.1128/AAC.01579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka H, Yoshiba S, Okabe H, et al. Clinical pharmacokinetics of Laninamivir, a novel long-acting neuraminidase inhibitor, after single and multiple inhaled doses of Its prodrug, CS-8958, in Healthy Male Volunteers. J Clin Pharmacol. 2010;50:1319–1329. doi: 10.1177/0091270009356297. [DOI] [PubMed] [Google Scholar]
- Ison MG, Gnann JW, Jr, Nagy-Agren S, et al. Safety and efficacy of nebulized zanamivir in hospitalized patients with serious influenza. Antivir Ther. 2003;8:183–190. [PubMed] [Google Scholar]
- Itoh Y, Shinya K, Kiso M, et al. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460:1021–1025. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives JA, Carr JA, Mendel DB, et al. The H274Y mutation in the influenza A/H1N1 neuraminidase active site following oseltamivir phosphate treatment leave virus severely compromised both in vitro and in vivo. Antiviral Res. 2002;55:307–317. doi: 10.1016/S0166-3542(02)00053-0. [DOI] [PubMed] [Google Scholar]
- Jefferson T, Jones M, Doshi P et al (2010) Neuraminidase inhibitors for preventing and treating influenza in healthy adults. Cochrane Database Syst Rev 2:CD001265 [DOI] [PMC free article] [PubMed]
- Kaiser L, Wat C, Mills T, et al. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med. 2003;163:1667–1672. doi: 10.1001/archinte.163.14.1667. [DOI] [PubMed] [Google Scholar]
- Kashyap AK, Steel J, Rubrum A, et al. Protection from the 2009 H1N1 pandemic influenza by an antibody from combinatorial survivor-based libraries. PLoS Pathog. 2010;6:e1000990. doi: 10.1371/journal.ppat.1000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai N, Ikematsu H, Iwaki N, et al. A comparison of the effectiveness of zanamivir and oseltamivir for the treatment of influenza A and B. J Infect. 2008;56:51–57. doi: 10.1016/j.jinf.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kim CU, Lew W, Williams MA, et al. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J Am Chem Soc. 1997;119:681–690. doi: 10.1021/ja963036t. [DOI] [PubMed] [Google Scholar]
- Kimberlin DW, Shalabi M, Abzug MJ, et al. Safety of oseltamivir compared with the adamantanes in children less than 12 months of age. Pediatr Infect Dis J. 2010;29:195–198. doi: 10.1097/INF.0b013e3181bbf26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiso M, Mitamura K, Sakai-Tagawa Y, et al. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet. 2004;364:759–765. doi: 10.1016/S0140-6736(04)16934-1. [DOI] [PubMed] [Google Scholar]
- Kiso M, Kubo S, Ozawa M, et al. Efficacy of the new neuraminidase inhibitor CS-8958 against H5N1 influenza viruses. PLoS Pathog. 2010;6:e1000786. doi: 10.1371/journal.ppat.1000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiso M, Takahashi K, Sakai-Tagawa Y, et al. T-705 (favipiravir) activity against lethal H5N1 influenza A viruses. Proc Natl Acad Sci USA. 2010;107:882–887. doi: 10.1073/pnas.0909603107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LK, Zhou BP. Successful treatment of avian influenza with convalescent plasma. Hong Kong Med J. 2006;12:489. [PubMed] [Google Scholar]
- Koyama K, Takahashi M, Oitate M, et al. CS-8958, a prodrug of the novel neuraminidase inhibitor R-125489, demonstrates a favorable long-retention profile in the mouse respiratory tract. Antimicrob Agents Chemother. 2009;53:4845–4851. doi: 10.1128/AAC.00731-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause JC, Tumpey TM, Huffman CJ, et al. Naturally occurring human monoclonal antibodies neutralize both 1918 and 2009 pandemic influenza A (H1N1) viruses. J Virol. 2010;84:3127–3130. doi: 10.1128/JVI.02184-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo S, Tomozawa T, Kakuta M, et al. Laninamivir prodrug CS-8958, a long-acting neuraminidase inhibitor, shows superior anti-influenza virus activity after a single administration. Antimicrob Agents Chemother. 2010;54:1256–1264. doi: 10.1128/AAC.01311-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- Kumar P, Sood V, Vyas R, et al. Potent inhibition of influenza virus replication with novel siRNA-chimeric-ribozyme constructs. Antiviral Res. 2010;87:204–212. doi: 10.1016/j.antiviral.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Kuritzkes DR, Walker BD. HIV-1: pathogenesis, clinical manifestations, and treatment. In: Knipe DM, Howley PM, editors. Fields virology, Fifth. 5. Philadelphia: Lippincott Williams and Wilkins; 2007. pp. 1697–1701. [Google Scholar]
- Kwong JC, Li P, Redelmeier DA. Influenza morbidity and mortality in elderly patients receiving statins: a cohort study. PLoS ONE. 2009;4:e8087. doi: 10.1371/journal.pone.0008087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackenby A, Hungnes O, Dudman SG, et al. Emergence of resistance to oseltamivir among influenza A(H1N1) viruses in Europe. Euro Surveill. 2008;13:8026. doi: 10.2807/ese.13.05.08026-en. [DOI] [PubMed] [Google Scholar]
- Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437:1108. doi: 10.1038/4371108a. [DOI] [PubMed] [Google Scholar]
- Le QM, Wertheim HF, Tran ND, et al. A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N Engl J Med. 2010;362:86–87. doi: 10.1056/NEJMc0910448. [DOI] [PubMed] [Google Scholar]
- Leneva IA, Roberts N, Govorkova EA, et al. The neuraminidase inhibitor GS4104 (oseltamivir phosphate) is efficacious against A/Hong Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza viruses. Antiviral Res. 2000;48:101–115. doi: 10.1016/S0166-3542(00)00123-6. [DOI] [PubMed] [Google Scholar]
- Leung TW, Tai AL, Cheng PK, et al. Detection of an oseltamivir-resistant pandemic influenza A/H1N1 virus in Hong Kong. J Clin Virol. 2009;46:298–299. doi: 10.1016/j.jcv.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Liu Z, Guo Z, Wang G, et al. Evaluation of the efficacy and safety of a statin/caffeine combination against H5N1, H3N2 and H1N1 virus infection in BALB/c mice. Eur J Pharm Sci. 2009;38:215–223. doi: 10.1016/j.ejps.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Luke TC, Casadevall A, Watowich SJ, et al. Hark back: passive immunotherapy for influenza and other serious infections. Crit Care Med. 2010;38:e66–e73. doi: 10.1097/CCM.0b013e3181d44c1e. [DOI] [PubMed] [Google Scholar]
- Luke TC, Kilbane EM, Jackson JL, et al. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- Malakhov MP, Aschenbrenner LM, Smee DF, et al. Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob Agents Chemother. 2006;50:1470–1479. doi: 10.1128/AAC.50.4.1470-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinescu B, Coman C, Iancu AD, et al. Evaluation of the efficacy of a specific hyperimmune serum in experimental influenza infection in mice. Roum Arch Microbiol Immunol. 2009;68:80–82. [PubMed] [Google Scholar]
- Martinez O, Tsibane T, Basler CF. Neutralizing anti-influenza virus monoclonal antibodies: therapeutics and tools for discovery. Int Rev Immunol. 2009;28:69–92. doi: 10.1080/08830180802593540. [DOI] [PubMed] [Google Scholar]
- Matrosovich M, Matrosovich T, Carr J, et al. Overexpression of the alpha-2,6-sialyltransferase in MDCK cells increases influenza virus sensitivity to neuraminidase inhibitors. J Virol. 2003;77:8418–8425. doi: 10.1128/JVI.77.15.8418-8425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrosovich MN, Matrosovich TY, Gray T, et al. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J Virol. 2004;78:12665–12667. doi: 10.1128/JVI.78.22.12665-12667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan K, Perry CM. Oseltamivir: a review of its use in influenza. Drugs. 2001;61:263–283. doi: 10.2165/00003495-200161020-00011. [DOI] [PubMed] [Google Scholar]
- McCullers JA. Effect of antiviral treatment on the outcome of secondary bacterial pneumonia after influenza. J Infect Dis. 2004;190:519–526. doi: 10.1086/421525. [DOI] [PubMed] [Google Scholar]
- McCullers JA. Antiviral therapy of influenza. Expert Opin Investig Drugs. 2005;14:305–312. doi: 10.1517/13543784.14.3.305. [DOI] [PubMed] [Google Scholar]
- McCullers JA. Preparing for the next influenza pandemic. Pediatr Infect Dis J. 2008;27:S57–S59. doi: 10.1097/INF.0b013e3181684d41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKimm-Breschkin JL. Resistance of influenza viruses to neuraminidase inhibitors–a review. Antiviral Res. 2000;47:1–17. doi: 10.1016/S0166-3542(00)00103-0. [DOI] [PubMed] [Google Scholar]
- McKimm-Breschkin JL. Neuraminidase inhibitors for the treatment and prevention of influenza. Expert Opin Pharmacother. 2002;3:103–112. doi: 10.1517/14656566.3.2.103. [DOI] [PubMed] [Google Scholar]
- Memoli MJ, Hrabal RJ, Hassantoufighi A, et al. Rapid selection of oseltamivir- and peramivir-resistant pandemic H1N1 virus during therapy in 2 immunocompromised hosts. Clin Infect Dis. 2010;50:1252–1255. doi: 10.1086/651605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto AS, Gunn RA, Bandyk MG, et al. Prevention of Russian influenza by amantadine. JAMA. 1979;241:1003–1007. doi: 10.1001/jama.1979.03290360019018. [DOI] [PubMed] [Google Scholar]
- Monto AS, Robinson DP, Herlocher ML, et al. Zanamivir in the prevention of influenza among healthy adults: a randomized controlled trial. JAMA. 1999;282:31–35. doi: 10.1001/jama.282.1.31. [DOI] [PubMed] [Google Scholar]
- Morrison D, Roy S, Rayner C, et al. A randomized, crossover study to evaluate the pharmacokinetics of amantadine and oseltamivir administered alone and in combination. PLoS ONE. 2007;2:e1305. doi: 10.1371/journal.pone.0001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med. 2009;360:953–956. doi: 10.1056/NEJMp0900648. [DOI] [PubMed] [Google Scholar]
- Moscona A, Porotto M, Palmer S, et al. A recombinant sialidase fusion protein effectively inhibits human parainfluenza viral infection in vitro and in vivo. J Infect Dis. 2010;202:234–241. doi: 10.1086/653621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss RB, Davey RT, Steigbigel RT, et al. Targeting pandemic influenza: a primer on influenza antivirals and drug resistance. J Antimicrob Chemother. 2010;65:1086–1093. doi: 10.1093/jac/dkq100. [DOI] [PubMed] [Google Scholar]
- Napolitano LM, Park PK, Raghavendran K, et al. Nonventilatory strategies for patients with life-threatening 2009 H1N1 influenza and severe respiratory failure. Crit Care Med. 2010;38:e74–e90. doi: 10.1097/CCM.0b013e3181cc5373. [DOI] [PubMed] [Google Scholar]
- Nelson MI, Simonsen L, Viboud C, et al. The origin and global emergence of adamantane resistant A/H3N2 influenza viruses. Virology. 2009;388:270–278. doi: 10.1016/j.virol.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JT, Hoopes JD, Le MH, et al. Triple combination of amantadine, ribavirin, and oseltamivir is highly active and synergistic against drug resistant influenza virus strains in vitro. PLoS ONE. 2010;5:e9332. doi: 10.1371/journal.pone.0009332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JT, Hoopes JD, Smee DF, et al. Triple combination of oseltamivir, amantadine, and ribavirin displays synergistic activity against multiple influenza virus strains in vitro. Antimicrob Agents Chemother. 2009;53:4115–4126. doi: 10.1128/AAC.00476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000;355:1845–1850. doi: 10.1016/S0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- Nishiura H, Castillo-Chavez C, Safan M et al (2009) Transmission potential of the new influenza A(H1N1) virus and its age-specificity in Japan. Euro Surveill 14 [DOI] [PubMed]
- O’Keefe BR, Smee DF, Turpin JA, et al. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob Agents Chemother. 2003;47:2518–2525. doi: 10.1128/AAC.47.8.2518-2525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzeck EA, Shi N, Blumentals WA. Oseltamivir and the risk of influenza-related complications and hospitalizations in patients with diabetes. Clin Ther. 2007;29:2246–2255. doi: 10.1016/j.clinthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Perez-Padilla R, de la Rosa-Zamboni Ponce, de Leon S, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- Piedra PA, Schulman KL, Blumentals WA. Effects of oseltamivir on influenza-related complications in children with chronic medical conditions. Pediatrics. 2009;124:170–178. doi: 10.1542/peds.2008-0977. [DOI] [PubMed] [Google Scholar]
- Pinto LH, Holsinger LJ, Lamb RA. Influenza virus M2 protein has ion channel activity. Cell. 1992;69:517–528. doi: 10.1016/0092-8674(92)90452-I. [DOI] [PubMed] [Google Scholar]
- Raboud JM, Harris M, Rae S, et al. Impact of adherence on duration of virological suppression among patients receiving combination antiretroviral therapy. HIV Med. 2002;3:118–124. doi: 10.1046/j.1468-1293.2002.00109.x. [DOI] [PubMed] [Google Scholar]
- Ramisse F, Deramoudt FX, Szatanik M, et al. Effective prophylaxis of influenza A virus pneumonia in mice by topical passive immunotherapy with polyvalent human immunoglobulins or F(ab’)2 fragments. Clin Exp Immunol. 1998;111:583–587. doi: 10.1046/j.1365-2249.1998.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert T, Chowell G, Nishiura H, et al. Does glycosylation as a modifier of Original Antigenic Sin explain the case age distribution and unusual toxicity in pandemic novel H1N1 influenza? BMC Infect Dis. 2010;10:5. doi: 10.1186/1471-2334-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi K, Mitamura K, Sakai-Tagawa Y, et al. High frequency of resistant viruses harboring different mutations in amantadine-treated children with influenza. J Infect Dis. 2003;188:57–61. doi: 10.1086/375799. [DOI] [PubMed] [Google Scholar]
- Sidwell RW, Bailey KW, Wong MH, et al. In vitro and in vivo influenza virus-inhibitory effects of viramidine. Antiviral Res. 2005;68:10–17. doi: 10.1016/j.antiviral.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Sidwell RW, Barnard DL, Day CW, et al. Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob Agents Chemother. 2007;51:845–851. doi: 10.1128/AAC.01051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman K, Mishin VP, Deyde VM, et al. In Vitro antiviral activity of favipiravir (T-705) against drug-resistant influenza and 2009 A(H1N1) viruses. Antimicrob Agents Chemother. 2010;54:2517–2524. doi: 10.1128/AAC.01739-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Bailey KW, Morrison AC, et al. Combination treatment of influenza A virus infections in cell culture and in mice with the cyclopentane neuraminidase inhibitor RWJ-270201 and ribavirin. Chemotherapy. 2002;48:88–93. doi: 10.1159/000057668. [DOI] [PubMed] [Google Scholar]
- Smee DF, Bailey KW, Wong MH, et al. Treatment of influenza A (H1N1) virus infections in mice and ferrets with cyanovirin-N. Antiviral Res. 2008;80:266–271. doi: 10.1016/j.antiviral.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Hurst BL, Wong MH, et al. Effects of the combination of favipiravir (T-705) and oseltamivir on influenza A virus infections in mice. Antimicrob Agents Chemother. 2010;54:126–133. doi: 10.1128/AAC.00933-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Wandersee MK, Checketts MB, et al. Influenza A (H1N1) virus resistance to cyanovirin-N arises naturally during adaptation to mice and by passage in cell culture in the presence of the inhibitor. Antivir Chem Chemother. 2007;18:317–327. doi: 10.1177/095632020701800604. [DOI] [PubMed] [Google Scholar]
- Smee DF, Wong MH, Bailey KW, et al. Activities of oseltamivir and ribavirin used alone and in combination against infections in mice with recent isolates of influenza A (H1N1) and B viruses. Antivir Chem Chemother. 2006;17:185–192. doi: 10.1177/095632020601700403. [DOI] [PubMed] [Google Scholar]
- Staschke KA, Colacino JM, Baxter AJ, et al. Molecular basis for the resistance of influenza viruses to 4- guanidino-Neu5Ac2en. Virology. 1995;214:642–646. doi: 10.1006/viro.1995.0078. [DOI] [PubMed] [Google Scholar]
- Stein DS, Creticos CM, Jackson GG, et al. Oral ribavirin treatment of influenza A and B. Antimicrob Agents Chemother. 1987;31:1285–1287. doi: 10.1128/AAC.31.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- Steinhauer DA, Martin J, Lin YP, et al. Studies using double mutants of the conformational transitions in influenza hemagglutinin required for its membrane fusion activity. Proc Natl Acad Sci USA. 1996;93:12873–12878. doi: 10.1073/pnas.93.23.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilianakis NI, Perelson AS, Hayden FG. Drug resistance and influenza pandemics. Lancet. 2002;359:1862–1863. doi: 10.1016/S0140-6736(02)08691-9. [DOI] [PubMed] [Google Scholar]
- Straumanis JP, Tapia MD, King JC. Influenza B infection associated with encephalitis: treatment with oseltamivir. Pediatr Infect Dis J. 2002;21:173–175. doi: 10.1097/00006454-200202000-00021. [DOI] [PubMed] [Google Scholar]
- Sugaya N, Ohashi Y. Long-acting neuraminidase inhibitor laninamivir octanoate (CS-8958) versus oseltamivir as treatment for children with influenza virus infection. Antimicrob Agents Chemother. 2010;54:2575–2582. doi: 10.1128/AAC.01755-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai CY, Escarpe PA, Sidwell RW, et al. Characterization of human influenza virus variants selected in vitro in the presence of the neuraminidase inhibitor GS 4071. Antimicrob Agents Chemother. 1998;42:3234–3241. doi: 10.1128/aac.42.12.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Furuta Y, Fukuda Y, et al. In vitro and in vivo activities of T-705 and oseltamivir against influenza virus. Antivir Chem Chemother. 2003;14:235–241. doi: 10.1177/095632020301400502. [DOI] [PubMed] [Google Scholar]
- Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283:1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- Triana-Baltzer GB, Gubareva LV, Klimov AI, et al. Inhibition of neuraminidase inhibitor-resistant influenza virus by DAS181, a novel sialidase fusion protein. PLoS ONE. 2009;4:e7838. doi: 10.1371/journal.pone.0007838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triana-Baltzer GB, Gubareva LV, Nicholls JM, et al. Novel pandemic influenza A(H1N1) viruses are potently inhibited by DAS181, a sialidase fusion protein. PLoS ONE. 2009;4:e7788. doi: 10.1371/journal.pone.0007788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voris LP, Betts RF, Hayden FG, et al. Successful treatment of naturally occurring influenza A/USSR/77 H1N1. JAMA. 1981;245:1128–1131. doi: 10.1001/jama.1981.03310360020016. [DOI] [PubMed] [Google Scholar]
- von Itzstein M, Dyason JC, Oliver SW, et al. A study of the active site of influenza virus sialidase: an approach to the rational design of novel anti-influenza drugs. J Med Chem. 1996;39:388–391. doi: 10.1021/jm950294c. [DOI] [PubMed] [Google Scholar]
- Wang C, Takeuchi K, Pinto LH, et al. Ion channel activity of influenza A virus M2 protein: characterization of the amantadine block. J Virol. 1993;67:5585–5594. doi: 10.1128/jvi.67.9.5585-5594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webby RJ, Webster RG. Are we ready for pandemic influenza? Science. 2003;302:1519–1522. doi: 10.1126/science.1090350. [DOI] [PubMed] [Google Scholar]
- Welliver R, Monto AS, Carewicz O, et al. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. JAMA. 2001;285:748–754. doi: 10.1001/jama.285.6.748. [DOI] [PubMed] [Google Scholar]
- White NJ, Webster RG, Govorkova EA, et al. What is the optimal therapy for patients with H5N1 influenza? PLoS Med. 2009;6:e1000091. doi: 10.1371/journal.pmed.1000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley RJ, Hayden FG, Reisinger KS, et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J. 2001;20:127–133. doi: 10.1097/00006454-200102000-00002. [DOI] [PubMed] [Google Scholar]
- Wingfield WL, Pollack D, Grunert RR. Therapeutic efficacy of amantadine HCl and rimantadine HCl in naturally occurring influenza A2 respiratory illness in man. N Engl J Med. 1969;281:579–584. doi: 10.1056/NEJM196909112811102. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Tomozawa T, Kakuta M, et al. CS-8958, a prodrug of the new neuraminidase inhibitor R-125489, shows long-acting anti-influenza virus activity. Antimicrob Agents Chemother. 2009;53:186–192. doi: 10.1128/AAC.00333-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HL, Herlocher LM, Hoffmann E, et al. Neuraminidase inhibitor-resistant influenza viruses may differ substantially in fitness and transmissibility. Antimicrob Agents Chemother. 2005;49:4075–4084. doi: 10.1128/AAC.49.10.4075-4084.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng BJ, Chan KW, Lin YP, et al. Delayed antiviral plus immunomodulator treatment still reduces mortality in mice infected by high inoculum of influenza A/H5N1 virus. Proc Natl Acad Sci USA. 2008;105:8091–8096. doi: 10.1073/pnas.0711942105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357:1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- Zonis Z, Engelhard D, Hindiyeh M, et al. Community-acquired oseltamivir-resistant pandemic (H1N1) 2009 in child, Israel. Emerg Infect Dis. 2010;16:1045–1046. doi: 10.3201/eid1606.091875. [DOI] [PMC free article] [PubMed] [Google Scholar]


