Abstract
Enthusiasm for cell therapy for myocardial injury has waned due to equivocal benefits in clinical trials. In an attempt to improve efficacy, we investigated repeated cell therapy and adjunct renal denervation (RDN) as strategies for augmenting cardioprotection with cardiosphere-derived cells (CDCs). We hypothesized that combining CDC post-conditioning with repeated CDC doses or delayed RDN therapy would result in superior function and remodeling. Wistar–Kyoto (WKY) rats or spontaneously hypertensive rats (SHR) were subjected to 45 min of coronary artery ligation followed by reperfusion for 12–14 weeks. In the first study arm, SHR were treated with CDCs (0.5 × 106 i.c.) or PBS 20 min following reperfusion, or additionally treated with CDCs (1.0 × 106 i.v.) at 2, 4, and 8 weeks. In the second arm, at 4 weeks following myocardial infarction (MI), SHR received CDCs (0.5 × 106 i.c.) or CDCs + RDN. In the third arm, WKY rats were treated with i.c. CDCs administered 20 min following reperfusion and RDN or a sham at 4 weeks. Early i.c. + multiple i.v. dosing, but not single i.c. dosing, of CDCs improved long-term left ventricular (LV) function, but not remodeling. Delayed CDC + RDN therapy was not superior to single-dose delayed CDC therapy. Early CDC + delayed RDN therapy improved LV ejection fraction and remodeling compared to both CDCs alone and RDN alone. Given that both RDN and CDCs are currently in the clinic, our findings motivate further translation targeting a heart failure indication with combined approaches.
Keywords: Cellular post-conditioning, Sympathetic nervous system, Fibrosis, Stem cells, Ischemia–reperfusion injury, Cell therapy, Left ventricular function
Introduction
The current paradigm for treating myocardial infarction (MI) is timely reperfusion. Unless reperfusion is prompt, ischemic and reperfusion-induced injury lead to scar formation, wall thinning, and adverse cardiac remodeling. Many strategies to reduce infarct size and provide long-term structural and functional benefit have failed [19]. Specifically, treatment with stem and progenitor cells has been optimistically pursued with the ultimate goal of cardiac regeneration. However, enthusiasm for cell-based therapies for cardiac injury has been stifled by inconsistent or undetectable benefits in single-dose clinical trials [8, 11, 28]. The lack of efficacy of cell therapy has been suggested to be due to poor engraftment of stem/progenitor cells and the limitations of single-dose administration. Several lines of evidence suggest that repeated doses of transplanted cells result in enhanced myocardial protection or recovery [4, 10, 26]. Protection is likely due to the paracrine effects of the transplanted cells, which produce a host of protective factors [9, 13, 17]. Cell engraftment is limited and transient, at levels < 3% by 35 days, and regenerative capacity by canonical stem cell mechanisms is likely below levels that reach clinical significance [14].
Both cell therapy and renal denervation (RDN) have shown some promise, particularly in preclinical settings, but both have met setbacks in clinical testing [3, 15, 16, 23, 27, 30]. Cell therapy has been proposed as a means to offset loss of functional muscle, while RDN blocks secondary maladaptive autonomic pathways that perpetuate and exacerbate post-MI heart failure. Here, we investigated potential modalities for improving cell therapy following MI. First, we investigated the efficacy of repeated cell administrations. Secondly, because cell therapy and RDN target different steps in the pathophysiology of heart failure, we hypothesized that therapy with cardiosphere-derived cells (CDCs) during early reperfusion, coupled with delayed RDN, would provide more robust and durable effects on cardiac structure and function than either therapy alone. The advantage for combining cell therapy with RDN, as opposed to pharmacologic agents is that neither therapy requires pretreatment or dependence on patient adherence, increasing the clinical viability of the combination.
Here, we demonstrate, in a rodent model of MI, that treatment with CDCs soon after reperfusion has short-term benefits in systolic function, which attenuate over time. This improvement in function, however, is sustained with follow-up doses of i.v. infusions of CDCs. Moreover, while single-dose CDC administration soon after reperfusion only improves short-term function and has no impact on long-term remodeling, RDN prevents late-stage cardiac dilation and systolic dysfunction. When combined, we find that the two approaches lead to enhanced lasting improvements of systolic function, as well as salutary changes in structural remodeling.
Methods
Experimental animals
Male Wistar–Kyoto (WKY) rats or spontaneously hypertensive rats (SHR) 19–20 weeks of age (Charles River Laboratories) were used in the study. All animals were housed in a temperature-controlled animal facility with a 12-h light/dark cycle, with water and rodent chow provided ad libitum. All animals received humane care in compliance with the Principles of Laboratory Animal Care formulated by the National Society of Medical Research and the Guide for the Care and Use of Laboratory Animals published by the NIH (8th Edition, Revised 2011). The LSUHSC New Orleans IACUC approved all animal procedures.
Myocardial ischemia/reperfusion
Myocardial ischemia–reperfusion was performed as described previously by our group [24]. Following 45 min of ischemia, rats underwent coronary reperfusion and were maintained on study for 12 or 14 weeks. During this time, cardiac structure and function were assessed using two-dimensional echocardiography. Animals were excluded from the study protocol if there was < 15% reduction in LVEF at 2 weeks in the SHR studies and < 10% reduction in LVEF at 2 weeks in WKY studies.
Cardiosphere-derived cell (CDC) administration
CDCs from WKY rats were derived, expanded, and prepared as previously described [7]. At 20 min of reperfusion, the aorta was cross-clamped for 15 s during which time 0.5 × 106 cells or PBS were injected with a 30-gauge needle into the LV lumen to achieve effective intracoronary delivery [12]. In the multiple dosing study, 1.0 × 106 cells or PBS were also injected with a 30-gauge needle into the tail vein at the 2, 4, and 8-week time points.
Radiofrequency renal denervation procedure
SHR and WKY were randomly divided into either a RF-RDN or sham-RDN group 4 weeks after ischemia–reperfusion. RF-RDN was performed as previously described [24].
Echocardiography
Prior to MI, baseline parasternal long-axis, two-dimensional and M-mode echocardiography was performed using MS250 13–24-MHz probe on a Vevo 2100 (Visualsonics) under anesthesia with isoflurane (1%) supplemented with 100% O2. Serial echocardiography was also performed in the same manner. LV end-diastolic dimension (LVEDD) and LV end-systolic dimension (LVESD) were analyzed from EKV™ (ECG-Gated Kilohertz Visualization)-generated M-mode long-axis images. LV ejection fraction (LVEF) was determined using LV Trace from an EKV™-generated long-axis B-mode image.
Biofluorescence imaging
CDCs (1 × 106) were labeled with the liposomal dye DiD (ThermoScientific) and then delivered intravenously (tail vein infusion) following myocardial ischemia–reperfusion. Two hours later, organs (heart, lungs, liver, kidneys, spleen) were excised, rinsed in PBS, and then imaged for biofluorescence (Xenogen IVIS Lumina, PerkinElmer). CDC biofluorescence was normalized against organ-specific auto-fluorescence by acquiring CDC-treated and control organs within the same field.
Left ventricular and arterial blood pressure measurements
At 14 weeks, LV end-diastolic pressure and carotid pressures were determined using a pressure catheter (Transonic Scisense Pressure Measurement system) under 1.0% isoflurane supplemented with 100% O2. Analysis was performed using LabChart Pro software.
Assessment of cardiac fibrosis
At the 12 or 14-week end points, hearts were arrested in diastole (potassium chloride), fixed in zinc formalin, embedded in paraffin, sectioned at one level, cut twice and stained for Masson’s trichrome to detect fibrosis. In a blinded fashion, the infarct area of LV was calculated as total fibrotic area/myocardium area × 100. Infarct expansion scores were determined in a blinded fashion (Alizee Pathology LLC, Thurmont, Maryland, USA) as previously described [25]. 0 = the solid fibrous scar has a well-defined external border without evidence of interstitial fibrosis extending into the surrounding myocardium. 1 = the solid fibrous scar has a well-defined external border with evidence of interstitial fibrosis extending slightly (up to ~ 300 μm) into the surrounding myocardium in few areas with most areas having 100 microns or less of border zone expansion. 2 = the solid fibrous scar has poorly defined external borders with interstitial fibrosis extending greater than 300 μm, but less than 500 μm in numerous areas surrounding the solid scar. 3 = the solid fibrous scar has poorly defined external borders with widespread extension of interstitial fibrosis (greater than 500 μm) in multiple areas surrounding the solid scar.
Kidney norepinephrine measurement
At the 14-week end point, kidney cortex was homogenized and norepinephrine levels were measured using ELISA technique according to the manufacturer’s recommendations (Abnova Co.)
Plasma angiotensin II and aldosterone measurements
At the 14-week end point, plasma concentrations of angiotensin II and aldosterone were determined using ELISA technique according to the manufacturer’s recommendations (Enzo Life Sciences).
Statistical analyses
All pooled data are expressed as the mean ± SEM. A repeated measure two-way ANOVA with matched values and Bonferroni post-test were used for echocardiography analyses. Multiple Mann–Whitney tests were used for ranked histological analysis. p values of < 0.05 were considered statistically significant. Unmarked p values indicate no statistical difference between groups.
Results
Biodistribution of CDCs following i.v. dosing
Following MI/R, rats were infused intravenously with either PBS or CDCs (DiD-labeled, 1 × 106). Organs were harvested 2 h later to examine CDC distribution in various organs following i.v. delivery. CDCs were primarily concentrated in the lungs and liver (Fig. 1b) with ~ 3500-fold higher concentration of CDCs in the lungs than the heart. CDCs were undetectable in the kidneys at this time point.
Fig. 1.
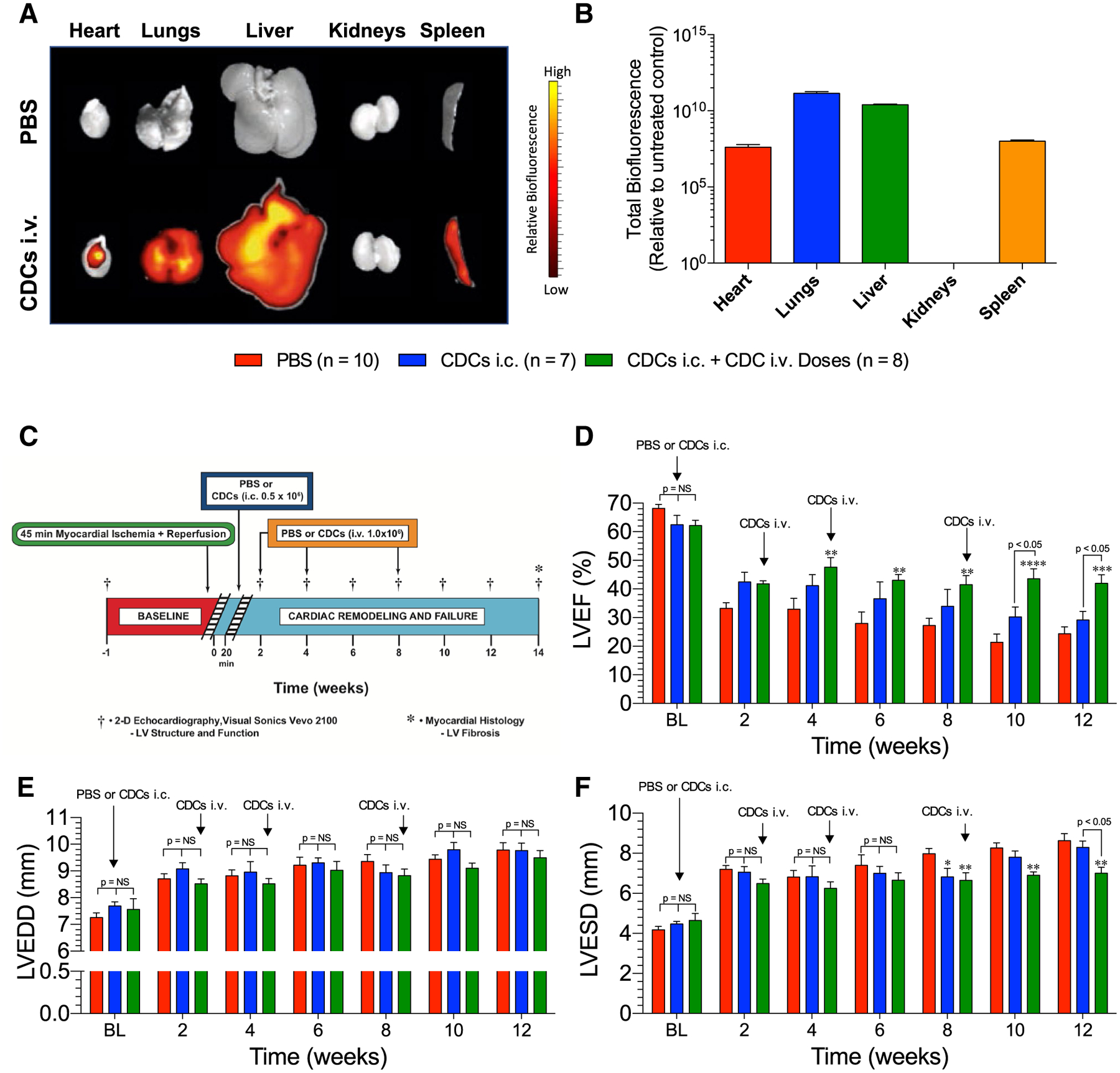
a Cardiosphere-derived cell (CDC) biodistribution following MI/R. Representative biofluorescence images of organs harvested from rats treated intravenously with PBS or CDCs (DiD-labeled; 1.0 × 106) 2 h into reperfusion. b Quantification of total CDC biofluorescence relative to control tissue (n = 4). c Ischemic heart failure protocol. Myocardial ischemic reperfusion injury protocol in SHR. Rats were subjected to 45 min of coronary artery ligation followed by 12 weeks of reperfusion. SHR were treated with either PBS or 0.5 × 106 CDCs via intracardiac delivery with aortic cross-clamp 20 min into reperfusion. SHR were then treated with either PBS or 1.0 × 106 CDCs via tail vein injection at 2, 4, and 8 weeks. d LV ejection fraction, e LV end-diastolic dimensions, and f LV end-systolic dimensions. Mean is represented as ± SEM. *p < 0.05 compared to PBS, **p < 0.01 compared to PBS, ***p < 0.001 compared to PBS, and ****p < 0.001 compared to PBS
Early i.c. CDCs + repeated i.v. CDC therapy has superior sustaining improvements on LV ejection fraction compared to single-dose i.c. CDCs
CDCs were administered at 20 min after reperfusion into the LV lumen under aortic cross-clamp, or additionally by i.v. infusion at 2, 4, and 8 weeks following myocardial ischemia–reperfusion injury in SHR (Fig. 1c). Single-dose injection of CDCs soon after MI yielded trending transient improvements in EF compared to control (Fig. 1d). In contrast, multiple-dose treatment resulted in improvements in EF beginning as early as 4 weeks, and these benefits were maintained throughout the study (Fig. 1d). Delta EF relative to baseline values are shown in Supplemental Fig. 1a. Neither single- nor multiple-dose treatments of CDCs improved cardiac dilation; and all improvements in ejection fraction were due to a reduction in end-systolic dimensions compared to control (Fig. 1e, f).
Cardiac fibrosis following CDC therapy for myocardial infarction
Analysis of cardiac fibrosis at the study end point revealed that single i.c. injection of CDCs at reperfusion did not reduce cardiac fibrosis compared to PBS when quantified as total LV fibrosis, infarct zone fibrosis (LV free wall), or remote zone fibrosis (septal wall) in SHR (Fig. 2). However, multiple-dose treatment reduced total LV fibrosis compared to the PBS and single-dose treatment groups, reduced infarct zone fibrosis compared to the single-dose group, and lowered remote zone fibrosis compared to the PBS and single-dose groups (Fig. 2). The remote protection distant from the infarct injury shows that repeated CDC therapy has cardioprotective actions which extend beyond tissue repair of ischemic myocardial cells.
Fig. 2.
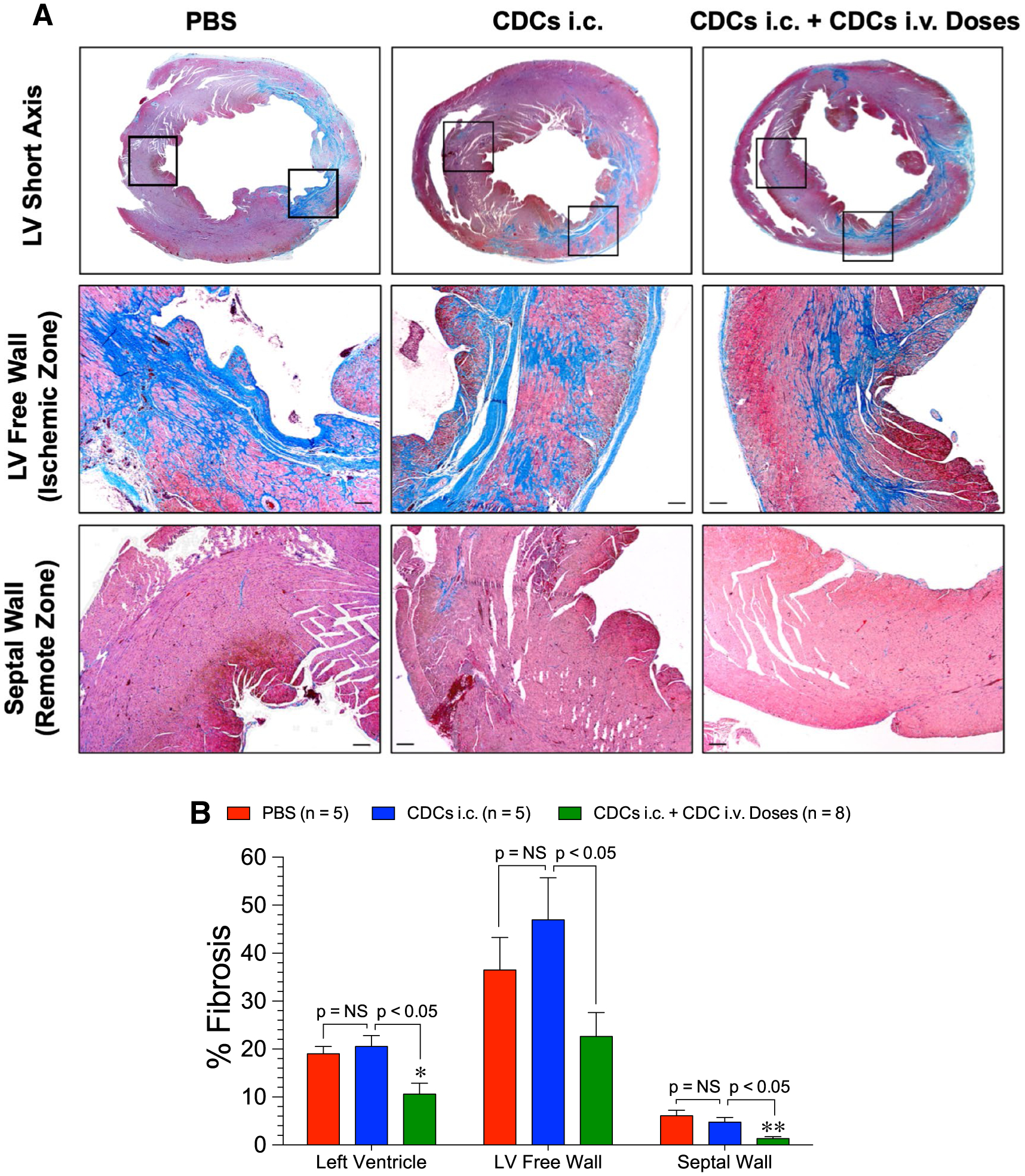
Cardiac fibrosis at 12 weeks following ischemic injury in SHR. a Representative images stained with Masson’s trichrome of the mid-ventricular short axis LV, free wall (ischemic zone) and the remote zone of the septum. b Total LV, LV free wall, and septal fibrosis in the PBS, CDCs i.c., and CDCs i.c. + CDC i.v. Doses groups. Mean is represented as ± SEM. *p < 0.05 compared to PBS and **p < 0.01 compared to PBS
Delayed CDC + RDN therapy improves ventricular function, but is not superior to delayed CDC treatment
CDC or CDC + RDN therapies were given at 4 weeks following myocardial ischemia–reperfusion injury in SHR (Fig. 3a). Delayed single-dose i.c. injection of CDCs post-MI yielded lasting improvements in EF compared to control (Fig. 3b). Additionally, delayed CDC + RDN treatment resulted in lasting improvements in EF compared to control, but not beyond those of independent CDC therapy. LVEF improvements were observed as early as 2–4 weeks after treatments. Delta EF relative to baseline values is shown in Supplemental Fig. 1b. Both CDC and CDC + RDN similarly improved LV end-systolic diameter compared to control (Fig. 3d). Neither CDCs alone nor CDC + RDN significantly mitigated cardiac dilation as measured by LV end-diastolic diameter, although there was a trending improvement in both treatment groups (Fig. 3c).
Fig. 3.
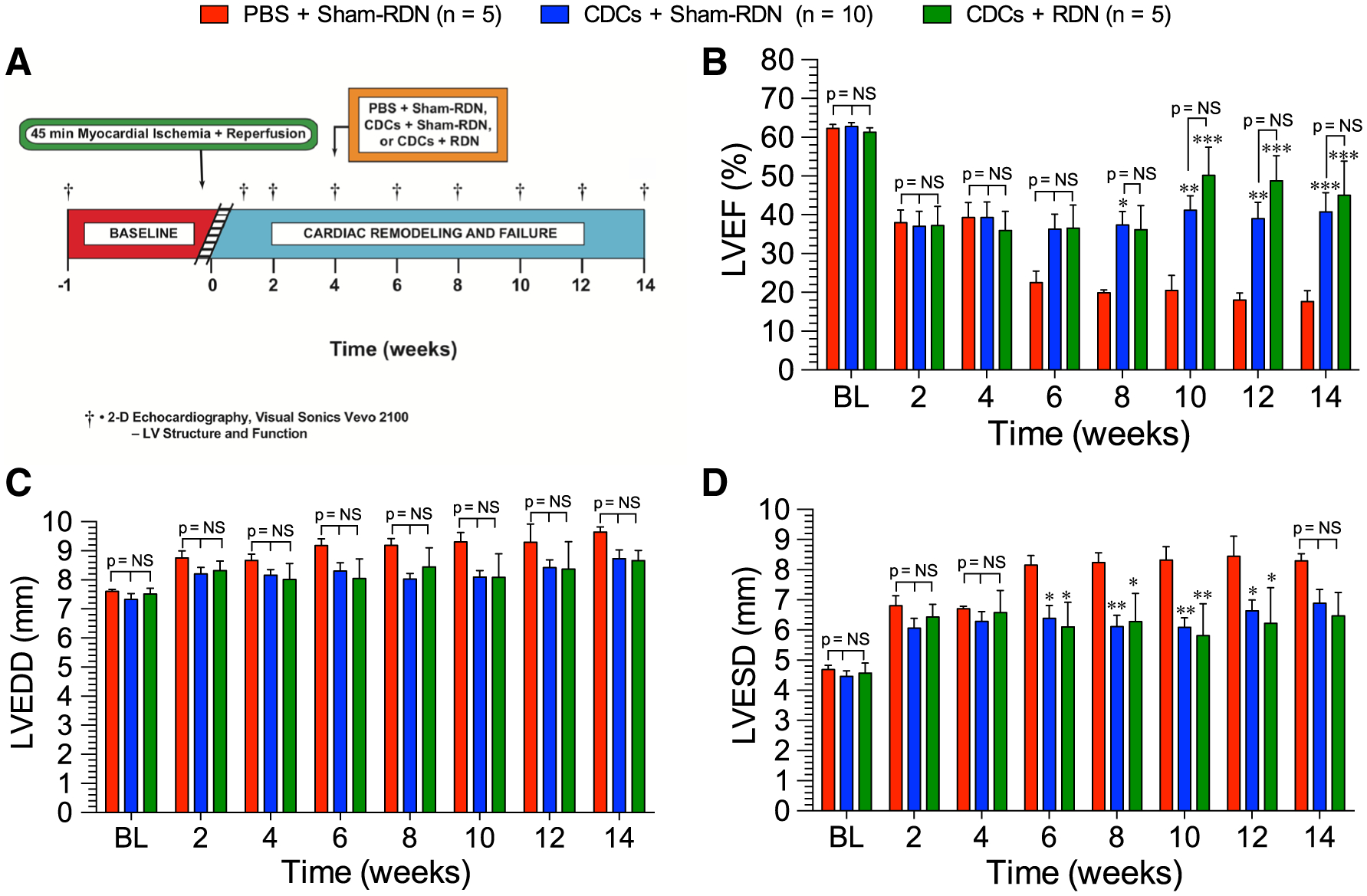
a Ischemic heart failure protocol. Myocardial ischemic reperfusion injury protocol in SHR. Rats were subjected to 45 min of coronary artery ligation followed by 14 weeks of reperfusion. SHR were treated with either PBS or 0.5 × 106 CDCs via intracardiac delivery with aortic cross-clamp 4 weeks into reperfusion. SHR were also treated with either Sham-RDN or bilateral radiofrequency RDN at 4 weeks into reperfusion. b LV ejection fraction, c LV end-diastolic dimensions, and d LV end-systolic dimensions. Mean is represented as ± SEM. *p < 0.05 compared to PBS, **p < 0.01 compared to PBS, and ***p < 0.001 compared to PBS
CDCs improve short-term ejection fraction, while RDN preserves long-term function
Given the lack of additional benefit of combining CDCs + RDN therapy at 4 weeks of reperfusion compared to single therapy, we modified the treatment protocol to administer CDCs early into reperfusion followed by RDN at 4 weeks post-reperfusion treatment time point in normotensive WKY rats (Fig. 4). CDC therapy alone, given 20 min into reperfusion, preserved LVEF through the 2-, 4-, and 8-week time points (Fig. 5a). However, by 14 weeks, the systolic function improvements in CDC-treated WKY diminished such that LVEF was no longer significantly superior to PBS + Sham-RDN. Improvements in LVEF in the RDN-treated rats were observed as early as 4 weeks after denervation and were maintained above the PBS + Sham-RDN-treated rats throughout the 14-week experiment. At 8 weeks, LVEF in CDC + RDN-treated rats trended to be superior to either CDCs or RDN alone. These differences were statistically significant by 14 weeks, when LVEF with combined CDC + RDN treatment was 22% and 30% greater than RDN and CDCs alone, respectively. Delta EF relative to baseline values is shown in Supplemental Fig. 1c.
Fig. 4.
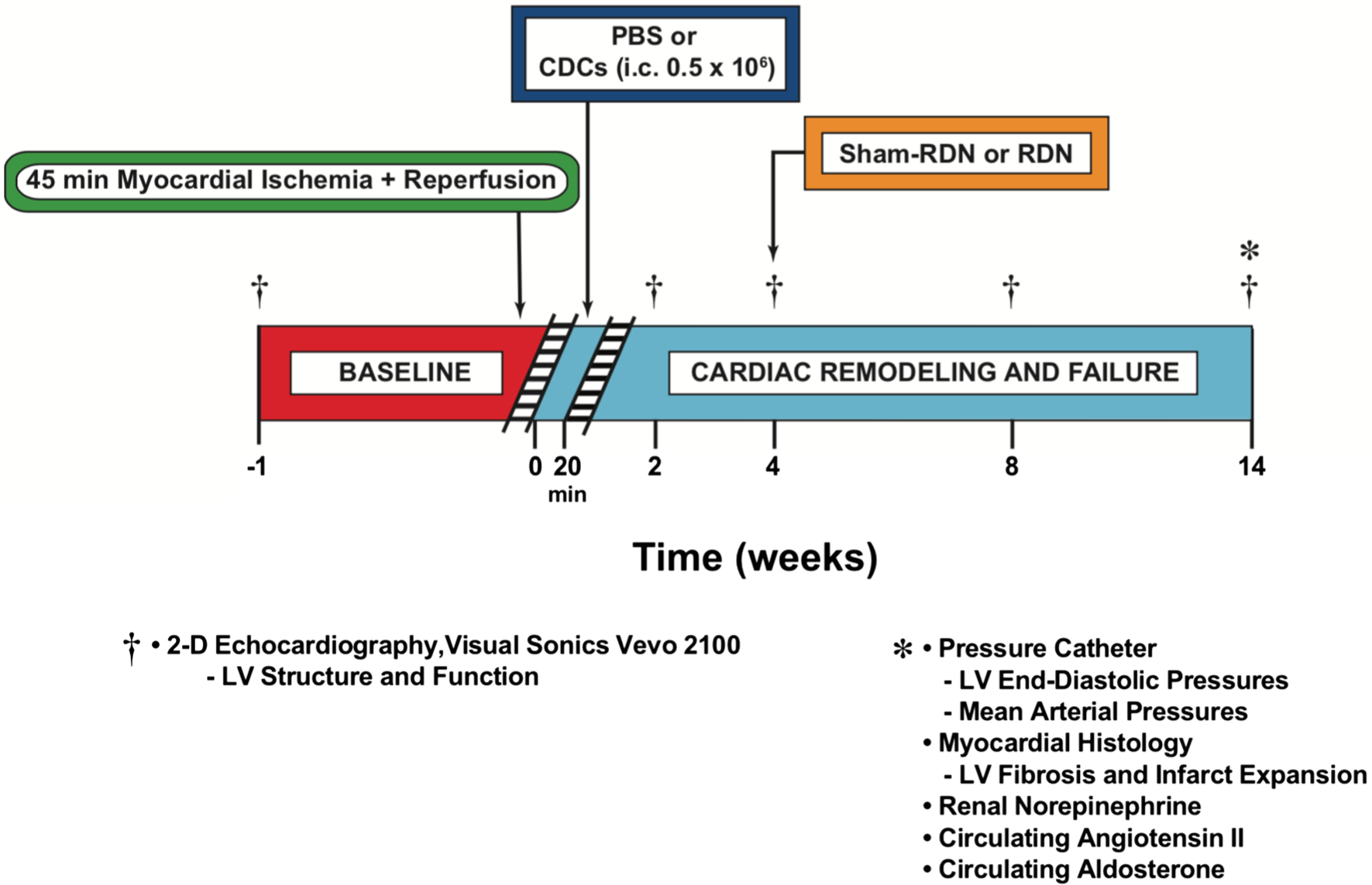
Ischemic heart failure protocol. Myocardial ischemic–reperfusion injury protocol in WKY rats. Rats were subjected to 45 min of coronary artery ligation followed by 14 weeks of reperfusion. WKY rats were treated with either PBS or 0.5 × 106 CDCs via intracardiac delivery with aortic cross-clamp 20 min into reperfusion. WKY rats were then treated with either Sham-RDN or bilateral radiofrequency RDN at 4 weeks into reperfusion
Fig. 5.
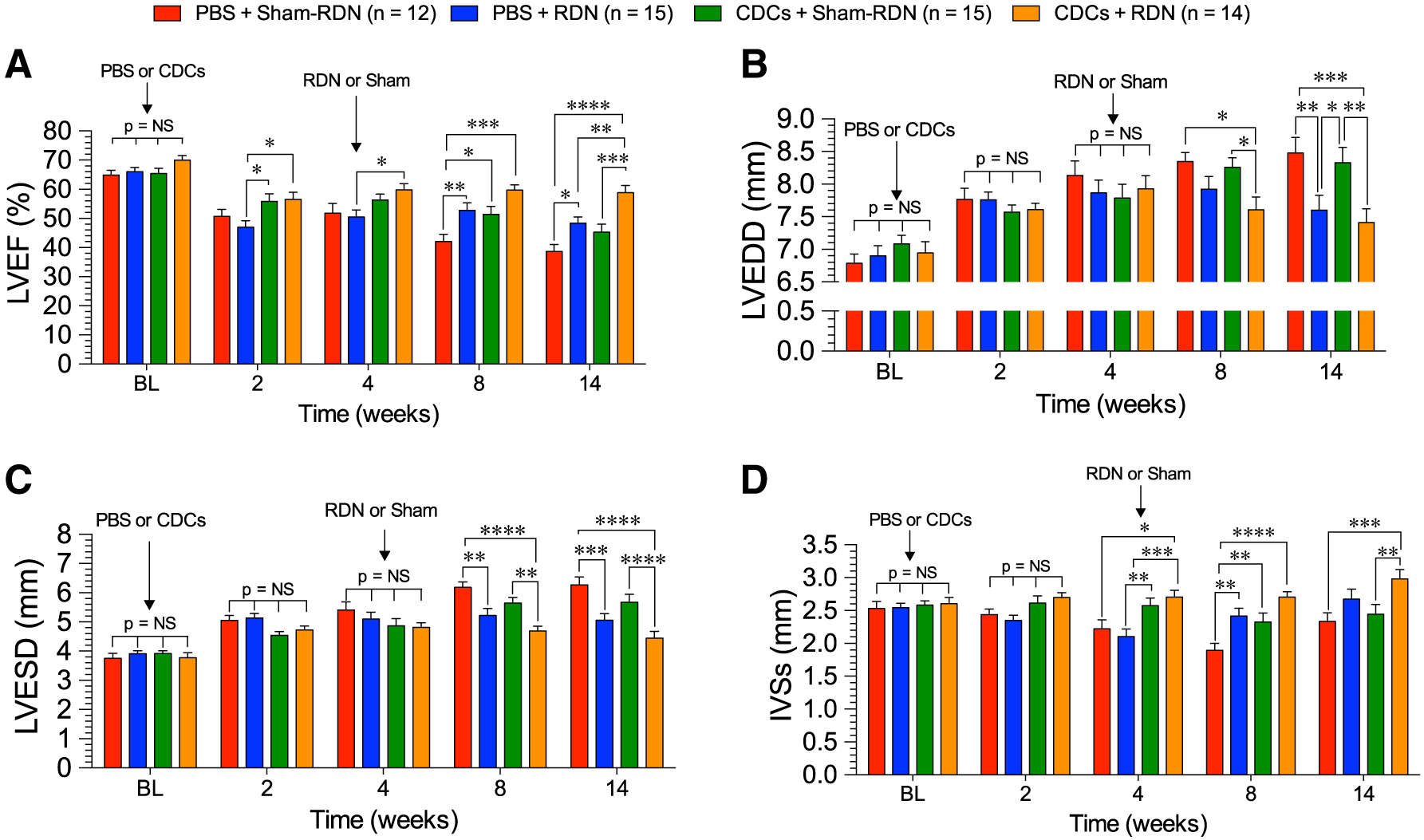
Cardiac structure and function following CDC and RDN therapy in WKY rats. a LV ejection fraction, b LV end-diastolic dimensions, c LV end-systolic dimensions, and d intraventricular septal diameter at systole. Mean is represented as ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 between groups
RDN, but not CDC, therapy improves ventricular dilation following ischemic injury
Left ventricular dimensions at end diastole (Fig. 5b) and end systole (Fig. 5c) revealed that ventricular dilation in the CDC therapy progressed similar to the PBS + Sham-RDN-treated rats by the study end points. Conversely, RDN attenuated remodeling and statistical improvements compared to PBS + Sham-RDN and CDC + Sham-RDN were reached by 14 weeks. There were no greater reductions in dilation in the CDC + RDN group compared to PBS + RDN, indicating that all improvements in remodeling were due to RDN treatment. Wall thickness, as measured by interventricular septal diameter at systole (IVSs), indicated that wall thickness was preserved in the CDC + Sham-RDN group through 8 weeks, but was lost by the 14-week end point (Fig. 5d). However, the CDC + RDN group had a significantly thicker IVSs at 14 weeks compared to CDC + Sham-RDN, indicating that the addition of RDN therapy preserved both structure and function compared to cell therapy alone.
Effects of RDN on the sympathetic nervous system
As we have previously reported, renal denervation exerts sustained effects on the renal sympathetic activation state following ischemia–reperfusion injury. PBS + RDN and CDC + RDN treatments equally reduced kidney norepinephrine levels (NE), which indicates equivalent reductions in renal sympathetic nerve activity (Fig. 6a). CDC + Sham-RDN failed to reduce kidney NE content below PBS + Sham-RDN levels, indicating that CDCs do not alter renal sympathetic nerve activity. In a similar fashion, PBS + RDN and CDC + RDN groups had significantly lower circulating angiotensin II levels compared to PBS + Sham-RDN at 14 weeks (Fig. 6b). There was no difference in plasma angiotensin II concentrations between the PBS + Sham-RDN and CDCs + Sham-RDN groups. The alterations in angiotensin II levels resulting from RDN treatment did no extend to modifications in plasma aldosterone. There were no differences in aldosterone concentrations among study groups at 14 weeks (Fig. 6c).
Fig. 6.
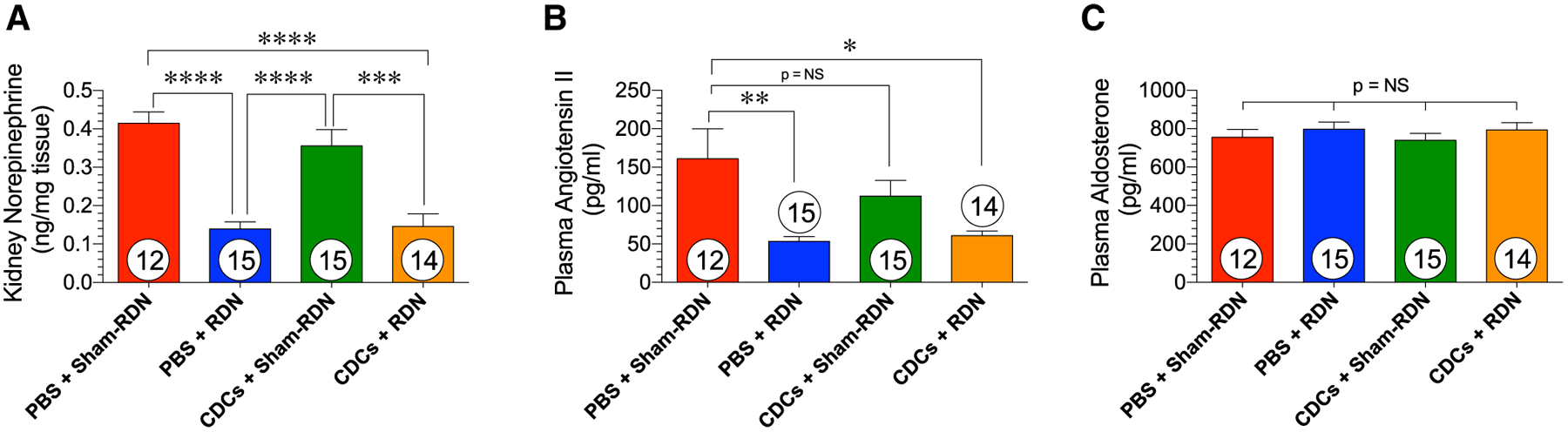
Renal sympathetic tone in Sham-RDN and RDN-treated WKY rats 8 weeks after treatment. a Kidney norepinephrine content, b plasma angiotensin II and c plasma aldosterone levels at the 14-week time point. Mean is represented as ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 between groups
Arterial blood pressure and LV pressure following CDC and RDN
RDN was developed for and is currently used in clinical trials for the treatment of resistant hypertension [3]. Clinical studies have shown that RDN decreases systolic and diastolic blood pressure in both patients on and off antihypertensive medications [2, 16, 30]. However, we have previously demonstrated that in normotensive animals, RDN does not have any blood pressure-lowering effects or heart rate-altering effects [24]. In the present study, we confirmed that CDCs and RDN do not have any significant impact on arterial pressure in the setting of post-ischemic injury in normotensive WKY rats (Fig. 7a). Following ischemic injury, LV end-diastolic pressures rise, which can cause pulmonary congestion. All treatment combinations reduced LVEDP compared to the control PBS + Sham-RDN group, but the combination of CDCs + RDN was not greater than either treatment alone (Fig. 7b).
Fig. 7.
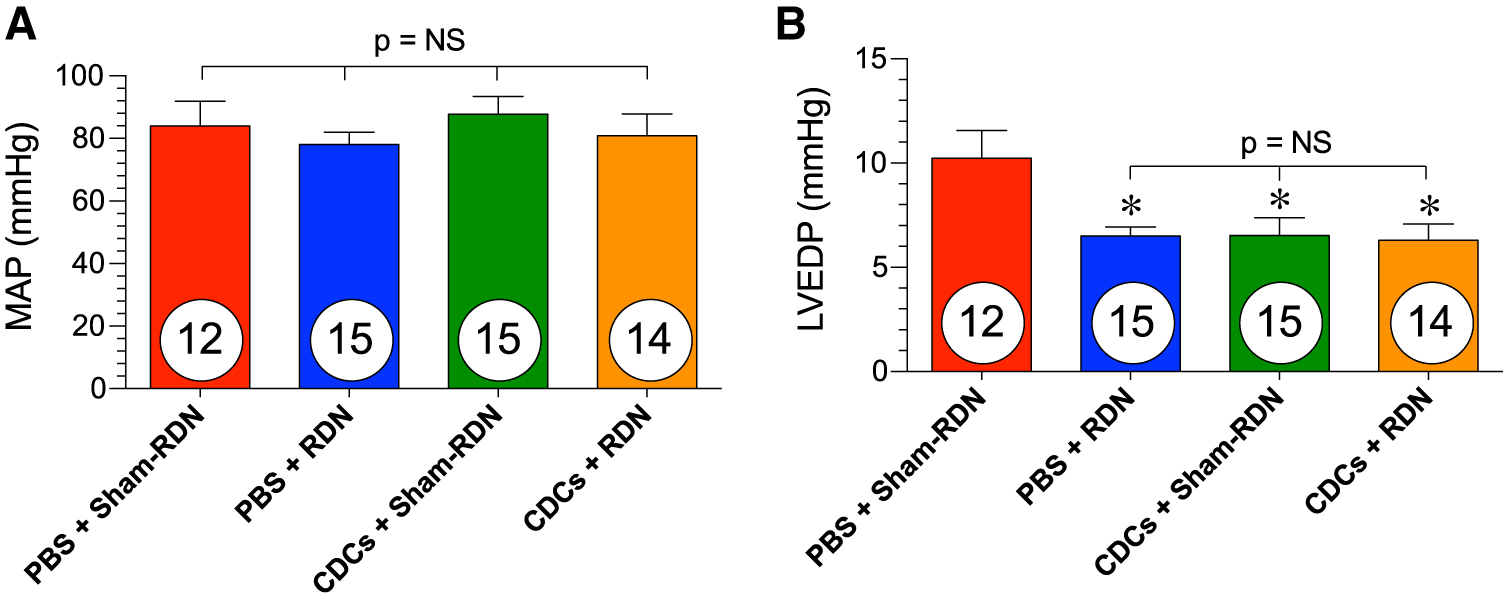
Hemodynamics and ventricular pressures in WKY rats at 14 weeks. a Carotid artery mean arterial pressures and b LV end-diastolic pressures at the 14-week end point. Mean is represented as ± SEM. *p < 0.05 compared to PBS
Cardiac fibrosis following ischemic injury in CDC and RDN-treated rats
CDC and RDN treatments both independently reduced LV fibrosis area compared to the control PBS + Sham-RDN group (Fig. 8c). The combination of these treatments, however, did not further reduce cardiac fibrosis relative to the LV. Similarly, CDC and RDN independently inhibited infarct expansion, and comparable reductions were observed in the CDC + RDN-treated animals following ischemia–reperfusion injury (Fig. 8d).
Fig. 8.
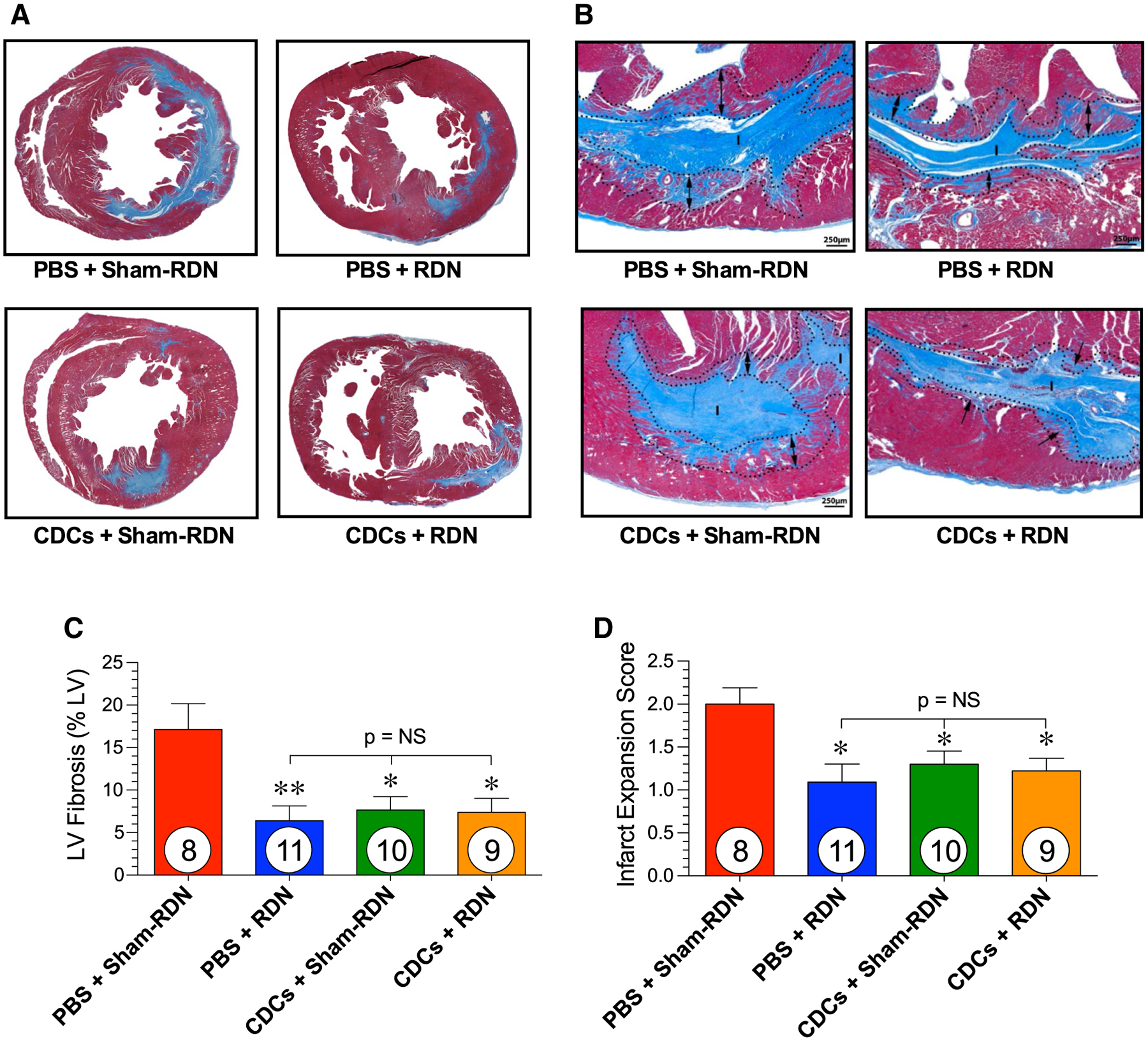
Cardiac fibrosis following ischemic injury in WKY rats. Representative images in LV stained with Masson’s trichrome of the a mid-ventricular short axis and b the solid fibrous infarct (I) and the infarct expansion zone (dotted lines with arrows) at 14 weeks following myocardial infarction in animals treated with PBS + Sham-RDN, PBS + RDN, CDCs + Sham-RDN, and CDCs + RDN. c Total LV fibrosis relative to the LV and d infarct expansion score. Mean is represented as ± SEM. *p < 0.05, **p < 0.01 compared to PBS
Discussion
Cell therapy for cardiac injury, in particular heart failure following MI, has largely failed to meet early hopes and expectations of myocardial regeneration and long-term functional improvements. Although hotly debated, poor clinical efficacy is likely due, in part, to the inability of sufficient cell engraftment, poor cell survival, non-localized cell delivery, and/or improper dosing strategies [22].
We investigated multiple strategies for improving cell therapy effects on post-infarction function and remodeling. In this pursuit, we utilized a thoroughly investigated cell therapy product, CDCs, whose bioactivity and protective mechanisms have been elucidated by > 45 independent laboratories [19]. CDCs secrete vesicles called exosomes, which are packed with signaling RNAs and proteins that modulate macrophages, fibroblasts, endothelial cells, and cadiomyocytes [6, 9, 18]. Modification of resident macrophages dampens the host’s innate immune response to ischemia–reperfusion injury and results in infarct size reduction [7, 19]. Late phase effects of CDCs include alteration in polarization of infiltrating macrophages, increased clearance of necrotic debris, and preservation of structural and functional myocardium [19]. Recently, CDCs have been tested in patients with LV dysfunction and a remote MI (> 1.5 months) in two distinct clinical trials (CADUCEUS [Cardiosphere-Derived Autologous Stem Cells to Reverse Ventricular Dysfunction] [21] and ALLSTAR [Allogeneic Heart Stem Cells to Achieve Myocardial Regeneration] [5]). In both trials, the cells were safe and showed signs of efficacy in improving LV structure and function post-MI, when delivered through the infarct-related coronary artery in chronically injured hearts.
In the first arm of the study, we examined the efficacy of repeated CDC therapy following MI (Figs. 1, 2). As has been consistent with numerous clinical trials, single CDC therapy, when given early into reperfusion, did not have lasting improvements on ventricular function or structure. Conversely, early CDC therapy followed with i.v. CDC doses at 2, 4, and 8 weeks yielded lasting improvements on LV ejection fraction. This is an important finding because not only was a multiple administration strategy efficacious, but also it indicates that i.v. delivery of cells peripherally can lead to a biological response in the myocardium. Consistent with previous work, the major effect of the second injections of CDCs appears to be maintenance of benefit, rather than incremental improvement [26]. Additionally, multiple doses of CDCs reduced cardiac fibrosis in the infarct zone as well as the remote zone of the LV. This attenuation of fibrosis was not observed in the single-treatment group. These findings have far reaching clinical implications for patients who may be able to receive cell therapy efficaciously in a far less invasive i.v. delivery.
The sympathetic nervous system contributes to the pathophysiology of cardiac remodeling and failure in post-MI patients [31]. Because of this, drugs that regulate downstream effects of the sympathetic nervous system (i.e., beta blockers, ACE inhibitors, ARBs) are regularly used, with much success, in this patient population. These drugs have several limitations, including off-target side effects, significant cost burden, and patient non-adherence. Meanwhile, their 5-year mortality rate is at an inadequate rate of 50%. We have previously explored the use of RDN as a means to dampen SNS activity in ischemia-induced heart failure and potentially replace or supplement current HF pharmacotherapies on the market. We found that delayed RDN therapy has lasting improvements on LV function and structure, augments protective circulating natriuretic peptide levels, and reduces cardiac fibrosis [25, 29]. The protective actions of RDN are independent of blood pressure lowering effects. These findings were reaffirmed in the present study that illustrates that RDN has lasting effects on LVEF and cardiac dilation, with sustained reductions in renal sympathetic nerve activity. Because there is no evidence of CDCs, or cell therapy, modulate the pathological actions of the sympathetic nervous system in HF, we proposed combining these complementary treatment strategies that are less susceptible to patient non-adherence.
We first examined the combination of delayed CDC therapy at the same time as delayed RDN following myocardial ischemia–reperfusion injury (Fig. 3). This protocol mimics a treatment regimen for a heart failure patient suffering from chronic heart failure. In this model, single-dose CDCs that were delivered 4 weeks following reperfusion resulted in preserved LV function compared to the declining LVEF in the HF control group. However, the addition of RDN with CDC therapy, when both given at 4 weeks, did not further improve cardiac function beyond CDC treatment levels. Due to the lack of efficacy of the combination therapy, we next explored an alternative strategy.
In the third arm, we delivered CDCs 20 min into reperfusion in an attempt to recruit early cardioprotection (Figs. 4, 5, 6, 7, 8). CDC treatment had early benefits on EF (up to 8 weeks), but the benefit was lost by 14 weeks. Interestingly, CDCs did not prevent LV dilation at any time point. Despite this, CDC treatment alone reduced myocardial fibrosis as effectively as any of the other active treatments, indicating a disconnect between cardiac fibrosis and remodeling. CDC-treated rats had significantly improved LVEDP compared to the PBS + Sham-RDN group indicating long-term improvements in diastolic function, but only short-term improvements in systolic function. Given the efficacy of CDCs to prevent immediate decline in cardiac function following ischemic injury, we investigated combining this post-conditioning treatment with another single-procedure therapy that maintains cardiac function and structure following ischemic injury: RDN [25]. The reduction of cardiac fibrosis by single CDC therapy can be contrasted to the lack of reduction in fibrosis by single CDC therapy in the first arm of the study. In the first arm we utilized a hypertensive SHR, while in the third arm, we utilized a normotensive WKY. It is possible that the pathological effects of long-standing hypertension underline this discrepancy, however, further studies are required to examine the role of hypertension on cell therapy efficacy.
Combining these therapies targets the early inflammatory response as shown in previous CDC MI studies [7, 26] that contributes to infarct development, and also mitigates the later-phase detrimental effects of the SNS on cardiac structure and fibrosis. Previous preclinical studies report that CDCs improve cardiac function and structure, but the tested ischemia–reperfusion protocols do not extend to 14 weeks as was the case in the present study [6, 7, 9]. We found, in fact, that early CDC therapy did not improve long-term ventricular dilation following MI. Moreover, systolic function, as quantified by LVEF, was not significantly superior to the PBS + Sham-RDN control group at this 14-week end point, indicating that CDC therapy is not independently sufficient for improving long-term cardiac performance. Alternatively, RDN mitigated MI-induced LV dilatation and preserved EF above PBS + Sham-RDN at all time points following treatment. The combination of CDCs + RDN did not further reduce dilation compared the PBS + RDN, indicating that improvements in remodeling were independently due to RDN. However, the combination of CDCs and RDN improved LVEF beyond either treatment alone. All treatments equally reduced total cardiac fibrosis and infarct expansion, demonstrating that fibrosis reductions were not the cause of late term improvements in EF and dilation. We propose that the early infarct-reducing, anti-inflammatory actions of CDC therapy coupled with sympathetic modulation of maladaptive changes with RDN (Fig. 5) resulted in lasting effects on systolic function and remodeling that were not seen with either independent therapy.
The present study has several limitations. The first is the sequence and timing of treatments. Given the technical complexity of performing RDN and intracoronary delivery of CDCs in the presence of aortic cross-clamping, we were not able to administer both therapies during myocardial ischemia (i.e., early upon reperfusion). Instead, we either performed both procedures at 4 weeks following reperfusion when the animals were relatively stable or delivered CDCs early upon reperfusion when the chest was open and then performed RDN at 4 weeks following reperfusion. It is possible that treating with CDCs and RDN simultaneously at the time of reperfusion could prove highly effective to attenuate the development of heart failure with reduced ejection fraction. Future studies are required to thoroughly examine and optimize the various combinations and timing; however, it is important to note that, clinically, both treatments can be administered via minimally invasive endovascular techniques and have the potential to be performed during the same percutaneous procedure. Another limitation of the current study is that these experiments were performed in a rodent model of myocardial injury without concomitant cardiovascular diseases or risk factors, aside from hypertension in the studies using SHR. In the first two arms, we utilized the SHR to more closely mimic multifactorial cardiovascular disease state and investigate cell therapy efficacy in hypertension. We then utilized normotensive WKY rats in the third arm to reaffirm our previous work detailing that improvements in LV function and remodeling following RDN therapy is blood pressure independent and not strictly due to ventricular unloading. Hemodynamics and cardiomyocyte signaling, particularly beta-adrenergic signaling, is different in WKY and SHR [1, 20]. Therefore, there may be mechanistic differences associated with remodeling changes in the studies using SHR and WKY rats. Although large animal and clinical results attest to the safety and efficacy of CDCs and RDN therapies individually [9, 19], large animal studies are required the confirm our present findings with combined therapy. This is an essential step for the proper translation of these findings because a clinical, endovascular RDN catheter may perform differently from the extravascular radiofrequency RDN technique used here. The major objective of the current study was to see if there were additive or synergistic effects of the two therapies. Follow-up studies will focus on mechanistic causes of early versus late remodeling differences between the two therapies.
We have introduced an alternative approach for improving cell therapy following MI. Specifically, we report the superiority of early i.c. delivery in combination with a repeated i.v. dosing regimen with cardiac-derived stem cells compared to single-dose i.c. treatment. We also investigated, for the first time, the combination of CDC cell therapy with RDN. Our findings reveal that CDCs improve early systolic function, while RDN maintains late-stage function and remodeling. Interestingly, when both therapies were given, LVEF was maintained at a higher level than either CDCs or RDN alone. Given the numerous limitations of current HF pharmacotherapies, we propose that combining these strategies may have therapeutic potential in treating post-infarction injury to attenuate cardiac remodeling and HF progression.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Heart, Lung, and Blood Institute, Bethesda, MD (National Institutes of Health; 1R01 HL092141 (DJL), 1R01 HL093579 (DJL), 1U24 HL 094373 (DJL), 1P20 HL113452 (DJL), R01 HL133835 (EM), and 18CDA34110445 American Heart Association, Dallas, TX, Career Development Award (GdC). We are also grateful for the generous funding from the LSU Medical School Foundation and the LSU Medical School Alumni Association, New Orleans, LA. We thank Jean Carnal and Dr. Hiroshi Koiwaya for their assistance during these studies.
Footnotes
Conflict of interest E.M. owns founder’s equity in Capricor, Inc. GdC receives consulting fees from Capricor. D.J.P. and D.J.L have a pending patent on the use of RDN and cell therapy to treat cardiovascular diseases. R.K.T., T.E.S., A.S., and T.T.G. have nothing to disclose.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00395-019-0718-1) contains supplementary material, which is available to authorized users.
References
- 1.Anand-Srivastava MB (1992) Enhanced expression of inhibitory guanine nucleotide regulatory protein in spontaneously hypertensive rats. Relationship to adenylate cyclase inhibition. Biochem J 288(Pt 1):79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, Saxena M, Feyz L, Rader F, Lurz P, Sayer J, Sapoval M, Levy T, Sanghvi K, Abraham J, Sharp ASP, Fisher NDL, Bloch MJ, Reeve-Stoffer H, Coleman L, Mullin C, Mauri L (2018) Endovascular ultra-sound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 391:2335–2345. 10.1016/s0140-6736(18)31082-1 [DOI] [PubMed] [Google Scholar]
- 3.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL, Investigators SH- (2014) A controlled trial of renal denervation for resistant hypertension. N Engl J Med 370:1393–1401. 10.1056/NEJMoa1402670 [DOI] [PubMed] [Google Scholar]
- 4.Bolli R (2017) Repeated cell therapy: a paradigm shift whose time has come. Circ Res 120:1072–1074. 10.1161/CIRCRESAHA.117.310710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakravarty T, Makkar RR, Ascheim DD, Traverse JH, Schatz R, DeMaria A, Francis GS, Povsic TJ, Smith RR, Lima JA, Pogoda JM, Marban L, Henry TD (2017) Allogeneic heart stem cells to achieve myocardial regeneration (ALLSTAR) trial: rationale and design. Cell Transpl 26:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Couto G, Gallet R, Cambier L, Jaghatspanyan E, Makkar N, Dawkins JF, Berman BP, Marban E (2017) Exosomal MicroRNA transfer into macrophages mediates cellular postconditioning. Circulation 136:200–214. 10.1161/CIRCULATIONAHA.116.024590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Couto G, Liu W, Tseliou E, Sun B, Makkar N, Kanazawa H, Arditi M, Marban E (2015) Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest 125:3147–3162. 10.1172/JCI81321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher SA, Doree C, Mathur A, Martin-Rendon E (2015) Meta-analysis of cell therapy trials for patients with heart failure. Circ Res 116:1361–1377. 10.1161/CIRCRESAHA.116.304386 [DOI] [PubMed] [Google Scholar]
- 9.Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, Marban L, Ghaleh B, Marban E (2017) Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J 38:201–211. 10.1093/eurheartj/ehw240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo Y, Wysoczynski M, Nong Y, Tomlin A, Zhu X, Gumpert AM, Nasr M, Muthusamy S, Li H, Book M, Khan A, Hong KU, Li Q, Bolli R (2017) Repeated doses of cardiac mesenchymal cells are therapeutically superior to a single dose in mice with old myocardial infarction. Basic Res Cardiol 112:18 10.1007/s00395-017-0606-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gyongyosi M, Wojakowski W, Lemarchand P, Lunde K, Tendera M, Bartunek J, Marban E, Assmus B, Henry TD, Traverse JH, Moye LA, Surder D, Corti R, Huikuri H, Miettinen J, Wohrle J, Obradovic S, Roncalli J, Malliaras K, Pokushalov E, Romanov A, Kastrup J, Bergmann MW, Atsma DE, Diederichsen A, Edes I, Benedek I, Benedek T, Pejkov H, Nyolczas N, Pavo N, Bergler-Klein J, Pavo IJ, Sylven C, Berti S, Navarese EP, Maurer G, Investigators A (2015) Meta-Analysis of Cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res 116:1346–1360. 10.1161/CIRCRESAHA.116.304346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajjar RJ, Schmidt U, Matsui T, Guerrero JL, Lee KH, Gwathmey JK, Dec GW, Semigran MJ, Rosenzweig A (1998) Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci USA 95:5251–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodgkinson CP, Bareja A, Gomez JA, Dzau VJ (2016) Emerging concepts in paracrine mechanisms in regenerative cardiovascular medicine and biology. Circ Res 118:95–107. 10.1161/CIRCRESAHA.115.305373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong KU, Guo Y, Li QH, Cao P, Al-Maqtari T, Vajravelu BN, Du J, Book MJ, Zhu X, Nong Y, Bhatnagar A, Bolli R (2014) c-kit + Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One 9:e96725 10.1371/journal.pone.0096725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F (2006) Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 367:113–121. 10.1016/S0140-6736(05)67861-0 [DOI] [PubMed] [Google Scholar]
- 16.Kandzari DE, Bohm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, Brar S, Cohen SA, Fahy M, Pilcher G, Kario K (2018) Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 391:2346–2355. 10.1016/s0140-6736(18)30951-6 [DOI] [PubMed] [Google Scholar]
- 17.Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ, Kishore R (2015) Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res 117:52–64. 10.1161/circresaha.117.305990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lapchak PA, Boitano PD, de Couto G, Marban E (2018) Intravenous xenogeneic human cardiosphere-derived cell extracellular vesicles (exosomes) improves behavioral function in small-clot embolized rabbits. Exp Neurol 307:109–117. 10.1016/j.expneurol.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 19.Lefer DJ, Marban E (2017) Is cardioprotection dead? Circulation 136:98–109. 10.1161/CIRCULATIONAHA.116.027039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Limas C, Limas CJ (1978) Reduced number of beta-adrenergic receptors in the myocardium of spontaneously hypertensive rats. Biochem Biophys Res Commun 83:710–714 [DOI] [PubMed] [Google Scholar]
- 21.Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marban L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Lardo AC, Marban E (2014) Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol 63:110–122. 10.1016/j.jacc.2013.08.724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marbán E (2018) A mechanistic roadmap for the clinical application of cardiac cell therapies. Nat Biomed Eng 2:353–361. 10.1038/s41551-018-0216-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM (2008) Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J 29:1807–1818. 10.1093/eurheartj/ehn220 [DOI] [PubMed] [Google Scholar]
- 24.Polhemus DJ, Gao J, Scarborough AL, Trivedi R, McDonough KH, Goodchild TT, Smart F, Kapusta DR, Lefer DJ (2016) Radiofrequency Renal Denervation Protects the Ischemic Heart via Inhibition of GRK2 and Increased Nitric Oxide Signaling. Circ Res 119:470–480. 10.1161/circresaha.115.308278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polhemus DJ, Trivedi RK, Gao J, Li Z, Scarborough AL, Goodchild TT, Varner KJ, Xia H, Smart FW, Kapusta DR, Lefer DJ (2017) Renal sympathetic denervation protects the failing heart via inhibition of neprilysin activity in the kidney. J Am Coll Cardiol 70:2139–2153. 10.1016/j.jacc.2017.08.056 [DOI] [PubMed] [Google Scholar]
- 26.Reich H, Tseliou E, de Couto G, Angert D, Valle J, Kubota Y, Luthringer D, Mirocha J, Sun B, Smith RR, Marban L, Marban E (2016) Repeated transplantation of allogeneic cardiosphere-derived cells boosts therapeutic benefits without immune sensitization in a rat model of myocardial infarction. J Heart Lung Transpl 35:1348–1357. 10.1016/j.healun.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 27.Ripa RS, Jorgensen E, Wang Y, Thune JJ, Nilsson JC, Sondergaard L, Johnsen HE, Kober L, Grande P, Kastrup J (2006) Stem cell mobilization induced by subcutaneous granulocyte-colony stimulating factor to improve cardiac regeneration after acute ST-elevation myocardial infarction: result of the double-blind, randomized, placebo-controlled stem cells in myocardial infarction (STEMMI) trial. Circulation 113:1983–1992. 10.1161/CIRCULATIONAHA.105.610469 [DOI] [PubMed] [Google Scholar]
- 28.Sanganalmath SK, Bolli R (2013) Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res 113:810–834. 10.1161/CIRCRESAHA.113.300219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharp TE, Polhemus DJ, Li Z, Spaletra P, Jenkins JS, Reilly JP, White CJ, Kapusta DR, Lefer DJ, Goodchild TT (2018) Renal denervation prevents heart failure progression via inhibition of the renin–angiotensin system. J Am Coll Cardiol 72:2609–2621. 10.1016/j.jacc.2018.08.2186 [DOI] [PubMed] [Google Scholar]
- 30.Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, Watkinson AF, Schmieder RE, Schmid A, Choi JW, East C, Walton A, Hopper I, Cohen DL, Wilensky R, Lee DP, Ma A, Devireddy CM, Lea JP, Lurz PC, Fengler K, Davies J, Chapman N, Cohen SA, DeBruin V, Fahy M, Jones DE, Rothman M, Bohm M (2017) Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet 390:2160–2170. 10.1016/s0140-6736(17)32281-x [DOI] [PubMed] [Google Scholar]
- 31.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J (2009) The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 54:1747–1762. 10.1016/j.jacc.2009.05.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


