Primary aldosteronism is caused by the autonomous and excessive secretion of aldosterone and is the most common cause of secondary hypertension with a prevalence of 5–10% 1. Aldosterone secretion from the zona glomerulosa of the adrenal is stimulated primarily by the renin-angiotensin system, which in turn is responsive to sodium balance. Renin, angiotensin II and aldosterone increase during sodium depletion and are suppressed during sodium overload. The diagnosis of autonomous and excessive secretion of aldosterone in patients with hypertension is based on demonstration of the lack of suppression with administration of excessive sodium by oral or intravenous route, sometimes in combination with a mineralocorticoid agonist 1. Until 2011 research on primary aldosteronism concentrated on defining the prevalence of the disorder, on determining sporadic versus familial origin, and on classification of the disease into aldosterone-producing adenoma, bilateral idiopathic hyperaldosteronism, or unilateral hyperaldosteronism. The discovery of somatic mutations of the inward rectifying potassium channel KCNJ5 in about one third of adenomas represented a major breakthrough in 2011 2. This was followed by the identification of somatic mutations of multiple genes including calcium channels CACNA1D and CACNA1H, sodium potassium ATPase ATP1A1, calcium ATPase ATP2B3, chloride channel CLCN2 and activating mutations of β-catenin in approximately two-thirds of patients 3. The CYP11B2 is the terminal enzyme in the synthesis of aldosterone and serves as a marker where aldosterone is synthesized. A study by De Sousa et al used CYP11B2 immunohistochemistry as a tool to guide the search for mutations in conjunction with new generation sequencing and revealed that 93.75% of adenomas had an aldosterone-driving mutation 4. Other studies using similar techniques have demonstrated similar prevalence of different mutations which varied according to sex and ethnic origin 5.
CYP11B2 expression in the adrenal of young humans is like rodents in that it is expressed in scattered cells throughout the zona glomerulosa underneath the capsule. With ageing, however, the expression groups into aldosterone-producing cell clusters (APCC) that are widely separated from each other beneath the capsule. Adrenals from reportedly normotensive kidney donors frequently have multiple APCCs and 35% of them have aldosterone-driving mutations. The mutations were mostly of the calcium channel CACNA1D with a lesser number of the ATP1A1; none of the somatic mutations found were of the potassium channel KCNJ5. It is unknown if these APCCs with somatic mutations in normotensive individuals result in autonomous production of aldosterone, and their significance remains unknown 6. It is possible that these somatic mutations are inconsequential unless there is an unknown second hit which leads to excessive production of aldosterone and stimulation of cell proliferation resulting in an aldosterone-producing adenoma 7. Conclusions are limited by the small sample size of normal human adrenals, but one can speculate that if KCNJ5 mutations occur, then the cells might be more susceptible to a second hit leading to the formation of aldosterone-producing adenoma 7. KCNJ5 is a good marker of zona glomerulosa and its presence in adjacent cells to the adenoma is likely to define latent zona glomerulosa cells that their CYP11B2 is suppressed by the high levels of aldosterone and salt that people consume in the western world. Adenomas with KCNJ5 mutations tend to be larger with more pronounced hyperaldosteronism than with other mutations, however, overexpression of KCNJ5 mutants alone in cells actually has an anti-proliferative effect which suggests there must be a second hit phenomenon 7. As shown by De Sousa et al 4 and others, the expression of KCNJ5 in these tumors is significantly less than in adjacent normal zona glomerulosa which might limit its anti-proliferative effect.
The adrenals from patients with an aldosterone-producing adenoma frequently also have APCCs or micronodules and in the study of De Sousa et al 4 had a high frequency of aldosterone-driving mutations. Interestingly, many of them had a mutation different from the adenoma. ‘Furthermore, there is wide variability between adenomas with some expressing the CYP11B2 in a homogenous pattern and others in a heterogeneous pattern; the activating mutation can be present in cells expressing CYP11B2 or in cells not expressing CYP11B2. The reason for this heterogeneity is unknown. In the normal human adrenal there is a clear separation of enzyme expression into zones, with the CYP11B2 being expressed only in the zona glomerulosa and the CYP11B1 and CYP17A1 expressed only in the zona fasciculata, but in some adenomas there is not only mingling of cells expressing the different enzymes, but some cells can co-express CYP11B2 with either CYP11B1 or CYP17A1. This finding seems to be more often present in cells that bear mutations of the KCNJ5. There has been a lot of interest in trying to correlate a steroidogenic pattern with a given mutation and, as shown in the paper by De Sousa et al 4, the secretion of hybrid steroids 18-oxocortisol and 18-hydroxycortisol correlates with mutations of the KCNJ5. This could be explained by the abnormal proximity of cells with CYP11B1 and CYP17A1 providing the substrate cortisol (or 11-deoxycortisol) for transformation by adjacent cells bearing the CYP11B2 enzyme. However, it seems more likely that these hybrid steroids are formed within the cells that co-express at least the CYP17A1 and CYP11B2. This assumption stems from the fact that the adrenal has one of the highest levels of expression of the P-glycoprotein that pumps more polar steroids out of the cell, such that an extracellular substrate would be unlikely to accumulate from the adjacent cells.
The study by De Sousa et al 4 raises several issues that might explain some ignored observations since the development of adrenal vein sampling (AVS) 5 decades ago. The diagnosis of unilateral hyperaldosteronism by AVS involves calculation of the lateralization index in order to guide indications for adrenalectomy. Removal of the affected adrenal which has been overproducing aldosterone should result in resolution of the hypertension; however, this only occurs in about a third of patients with improvement in most, but not all, of the others 8. Removal of a dominant aldosterone-producing gland in patients with clear-cut bilateral overproduction of aldosterone also results in “cure” in few patients and improvement in several others. Multiple explanations have been proposed for the lack of cure in a substantial number of patients with no clear answers and has led to considerable controversy as to how AVS should be practiced and interpreted. Some groups propose that AVS should be done using simultaneous catheters to sample both adrenals under basal conditions, while others either propose that it should be done after ACTH stimulation since this improves the success rate of the procedure or to be done under both basal and stimulation with ACTH. In order to assure that the catheter is sampling from an adrenal vein, cortisol is usually measured, and a selectivity index determined by comparison with the peripheral values. The lateralization index is then calculated using a ratio of aldosterone/cortisol from each side, and the contralateral suppression is confidently determined if the ratio of aldosterone/cortisol is less than one. The use of this ratio ignores several factors; first, that cortisol is measured in μMolar concentrations and aldosterone in nMolar concentrations. Secondly, the affected adrenal frequently secretes relatively small but still abnormal amounts of cortisol that can induce a subtle suppression of the zona fasciculata of the contralateral adrenal, thus increasing the aldosterone/cortisol ratio through alteration of cortisol levels. Finally, very few manuscripts report the absolute concentrations secreted by both glands and in many cases the contralateral “unaffected” gland still produces significant amounts of aldosterone in comparison to peripheral values 9. Unilateral adrenalectomy of the dominant adrenal produces a significant reduction of aldosterone in circulation resulting in improvement in the clinical and especially the biochemical abnormalities, but studies support that when the contralateral adrenal is suppressed, post-operative hyperkalemia and cure of the hypertension are more common.
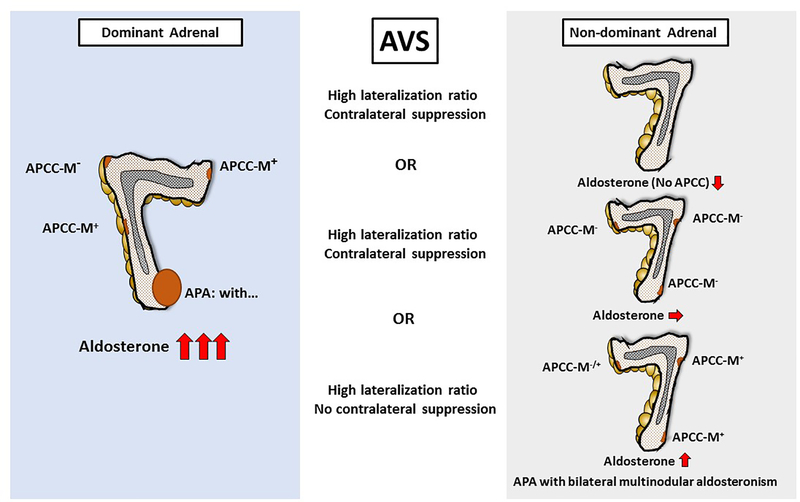
The question of why the contralateral “normal” adrenal may still produce aldosterone in significantly higher concentrations than what is measured in the periphery has not been adequately explored. It is very likely that the contralateral adrenal also has APCCs or micronodules; some of these could retain normal regulation in their secretory properties, and others might have similar mutations to the APCCs that become autonomous (Figure 1). The number of aldosterone over-producing cells in the contralateral adrenal might be sufficiently low that the absolute production of aldosterone is not high enough to produce overt clinical manifestations. This would suggest that many cases of aldosterone-producing adenomas actually represent an asymmetric disorder where initially APCCs bilaterally became autonomous with a second hit in one side that caused significant proliferation of cells leading to a unilateral adenoma. The conditions that resulted in a very high incidence of aldosterone-driving mutations might also exist in other APCCs, but with significantly less proliferation potential. Bilateral hyperaldosteronism has been divided into micronodular and hyperplastic types and may be similar to the proposed aldosterone-producing adenoma with asymmetric hyperplasia 10. It might be time to subclassify aldosterone-producing adenomas as some that are truly unilateral and others that represent bilateral disease with progression of one micronodule or APCC into an adenoma that was subjected to a second hit.
Figure 1;
Schematic representation of an aldosterone-producing adenoma (APA) in the dominant adrenal with corresponding aldosterone levels in the contralateral adrenal. The gland bearing an APA may also have APCCs, some of which may have aldosterone-driving mutations. The contralateral adrenal may have no APCCs, APCCs without mutations and no increased aldosterone production, or APCCs with aldosterone-driving mutations leading to aldosterone concentrations in its adrenal vein several times higher than peripheral values. In the latter case, aldosterone production in the contralateral adrenal is not suppressed, however adrenal vein aldosterone levels are still much lower than from the adrenal with the APA, resulting a high lateralization index.
Acknowledgments
Source of funding:
These studies were supported by National Heart, Lung and Blood Institute grant R01 HL144847 (C.E.GS.), and the National Institute of General Medical Sciences under Award Number 1U54GM115428 (C.E.GS.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: No authors have anything to disclose.
References
- 1.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr., The management of primary aldosteronism: Case detection, diagnosis, and treatment: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–1916 [DOI] [PubMed] [Google Scholar]
- 2.Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Akerstrom G, Wang W, Carling T, Lifton RP. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seccia TM, Caroccia B, Gomez-Sanchez EP, Gomez-Sanchez CE, Rossi GP. The biology of normal zona glomerulosa and aldosterone-producing adenoma: Pathological implications. Endocrine reviews. 2018;39:1029–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Sousa K, Boulkroun S, Baron S, Nanba K, Wack M, Rainey WE, Rocha A, Giscos-Douriez I, Meatchi T, Amar L, Travers S, Fernandes-Rosa FL, Zennaro MC. Genetic, cellular and molecular heterogeneity in adrenals with aldosterone producing adenoma. Hypertension. 2020;in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nanba K, Omata K, Gomez-Sanchez CE, Stratakis CA, Demidowich AP, Suzuki M, Thompson LDR, Cohen DL, Luther JM, Gellert L, Vaidya A, Barletta JA, Else T, Giordano TJ, Tomlins SA, Rainey WE. Genetic characteristics of aldosterone-producing adenomas in blacks. Hypertension. 2019;73:885–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, Liu CJ, Sanjanwala AR, Edwards MA, Gomez-Sanchez CE, Nanba K, Rainey WE. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A. 2015;112:E4591–4599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Sanchez CE, Gomez-Sanchez EP. Mutations of the potassium channel kcnj5 causing aldosterone-producing adenomas: One or two hits? Hypertension. 2012;59:196–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J, Beuschlein F, Kitamoto KK, Pham U, Morimoto R, Umakoshi H, Prejbisz A, Kocjan T, Naruse M, Stowasser M, Nishikawa T, Young WF, Jr. Gomez-Sanchez CE, Funder JW, Reincke M. Outcomes after adrenalectomy for unilateral primary aldosteronism: An international consensus on outcome measures and analysis of remission rates in an international cohort. The lancet. Diabetes & endocrinology. 2017;5:689–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr CE, Cope C, Cohen DL, Fraker DL, Trerotola SO. Comparison of sequential versus simultaneous methods of adrenal venous sampling. J Vasc Interv Radiol. 2004;15:1245–1250 [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki Y, Omata K, Tezuka Y, Gao X, Ogata H, Pieroni J, Ono Y, Morimoto R, Nakamura Y, Gomez-Sanchez CE, Satoh F, Sasano H. Non-neoplastic/hyperplastic primary aldosteronism - its histopathology and genotype. Current Opinion in Endocrine and Metabolic Research. 2019;8:122–131 [Google Scholar]