Abstract
In addition to supporting cellular energetic demands and providing building blocks for lipid synthesis, fatty acids (FAs) are precursors of potent signaling molecules. In particular, the presence of conjugated double bonds on the fatty-acyl chain provides a preferential target for nitration generating nitro-FAs (NO2-FAs). The formation of NO2-FAs is a nonenzymatic process that requires reactive nitrogen species and occurs locally at the site of inflammation or during gastric acidification. NO2-FAs are electrophilic and display pleiotropic signaling actions through reversible protein alkylation. This review focuses on the endogenously formed NO2-FAs, mechanism of absorption, systemic distribution, signaling, and preclinical models. Understanding the dynamics of these processes will facilitate targeted dietary interventions and further the current pharmacological development aimed at low-grade inflammatory diseases.
Diet, Fas, and Signaling
It has been almost 90 years since the pioneering discovery that some FAs are essential dietary components [1,2]. These findings demonstrated that both linoleic acid (LA) and α-linolenic acid (αLA) are essential FAs, as animals lack Δ−12 and Δ−15 desaturases required for their synthesis. Deficiency of these FAs in early parenteral formulations led to severe dermatitis [3,4]. Thus, not only do FAs serve as energy sources but are also critical structural components of membranes and signaling molecules, with LA and αLA serving as precursors to arachidonic acid, eicosapentaenoic acid, and docosahexaenoic acid. Bioactive signaling mediators are generated using these FAs as substrates through the oxidative action of cyclooxygenases, lipoxygenases, and cytochrome P450 activity [5]. In addition to providing essential bis-allylic FAs, diets also provide other important FAs that have conjugated double bonds such as conjugated linoleic- and linolenic-acids (CLA and CLnA, respectively; see Glossary) [6]. CLA and CLnA are ruminant-derived FAs present in meat and dairy products [7]. Additionally, other CLnA isomers are acquired from plant sources such as pomegranate and bitter melon [8]. Alternatively, the microbiome participates in the isomerization of LA into CLA, contributing to the systemic CLA levels [9]. While conjugated FAs are not used as substrates during enzymatic conversion to bioactive lipids, the lipoxygenase-driven oxidation of polyunsaturated FAs results in the conjugation of double bonds. Moreover, the sequential oxidation of arachidonic, eicosapentaenoic, and docosahexaenoic acids by lipoxygenases and other oxidases leads to oxidized bioactive FAs that can contain multiple conjugated double bonds (e.g., lipoxins, leukotrienes, maresins, and resolvins) [5]. The presence of conjugated double bonds in the alkyl chain is essential for nitration reactions as it largely increases the efficiency of the reaction and yields of nitrated products [10]. Nonetheless, oxidized FAs containing conjugated double bonds or bis-allylic carbons are not endogenous substrates of nitration reactions. While most pharmacological efforts focus on nitrated oleic acid (nitro-oleic acid, NO2-OA), human data show that endogenous nitration reactions occur almost exclusively on conjugated FAs [11,12]. This review covers FA nitration (focusing predominantly on conjugated FAs), mechanisms of absorption and distribution of nitro-fatty acids (NO2-FA), and the protective effects of endogenously formed NO2-FAs. Additionally, preclinical animal data on pharmacological approaches to treat a variety of diseases (Figure 1) instigated by inflammatory, oxidative stress, and fibrotic processes are discussed.
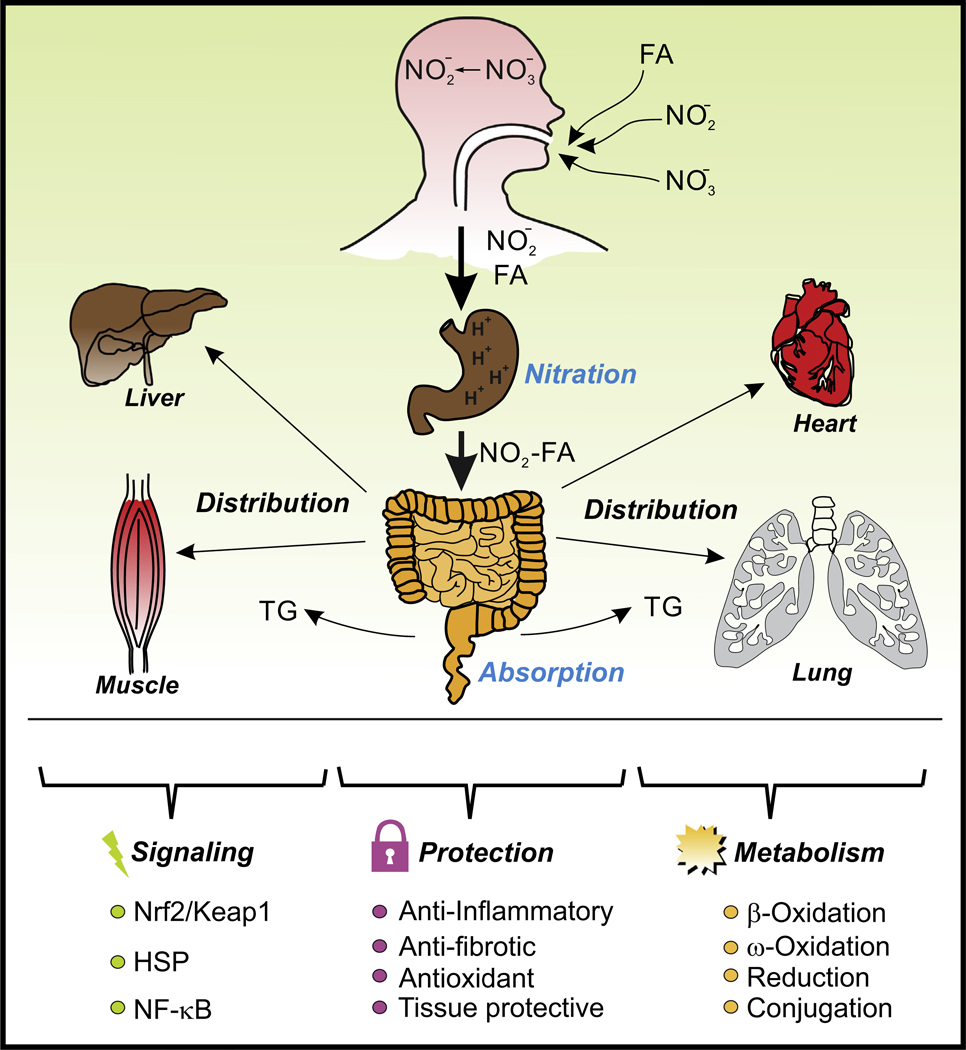
Figure 1. Overview of NO2-FA Formation, Distribution, Metabolism, Signaling, and Protection.
NO2-FAs are mainly formed in the gastric compartment and, upon absorption and distribution, exert protective effects by activating antioxidant, anti-inflammatory, and antifibrotic signaling pathways. NO2-FAs are finally metabolized and eliminated, at least partially, through kidney filtration. Abbreviations: HSP, ; NF-κB, nuclear factor-κB; NO2-FA, nitro-fatty acid; Keap1, ; Nrf2, nuclear factor (erythroid-derived 2)-like 2; TG, triglyceride.
Let the Chemistry Explain the Biology
The discovery of nitrotyrosine as a hallmark of peroxynitrite formation, oxidative stress, and later, nitration induced by the activity of peroxidases (e.g., myeloperoxidase, eosinophil peroxidase, and lactoperoxidase), led to efforts to find additional modifications of biomolecules that could be either pathological biomarkers or drive disease pathogenesis [13]. As a consequence of these efforts, nitration of oligonucleotides, catecholamines, and lipids was described, with the first two groups of biomolecules leading to the formation of 8-nitro-guanidine, 8-nitro-guanosine, 8-nitro-cGMP, 6-nitro-dopamine, and 6-nitro-epinephrine, among others [14,15]. While the nitration of DNA and RNA bases is associated with inflammation, the formation of NO2-cGMP has been studied and shown to exert cellular redox, vascular, and signaling functions [16]. With regards to lipids, initial efforts were hindered by analytical limitations and low instrument sensitivity. OA and LA were initially reported as in vivo lipid nitration targets [17–22]. These findings and interpretations were later revised when Bonacci et al. reported that the yields obtained from the nitration of CLA were >105 times higher than those of nonconjugated dienes, and that nitro-conjugated linoleic acid (NO2-CLA) was the most abundant endogenously formed NO2-FA [10]. The identification of the endogenous substrate permitted the evaluation of the biological conditions under which these were formed, the characterization of the concentrations, metabolism, and signaling properties [23].
The mechanism involved in tyrosine nitration starts with an initial one-electron oxidation [e.g., by hydroxyl-, carbonate-, lipid-peroxyl-, lipid-alkoxyl- and nitrogen dioxide (•NO2)-radicals] [13], generating a tyrosyl radical that is further stabilized by resonance [13]. This is followed by a radical–radical coupling reaction between the tyrosyl radical and •NO2 to form nitrotyrosine (Figure 2A). The product of tyrosine nitration is stable and occurs mainly in proteins, conveying biological activity through the modification of the physicochemical properties of the tyrosine residues that occur when the nitro group is incorporated. This causes a significant decrease in the pKa of the tyrosine hydroxyl group induced by the strong electron withdrawing characteristics of the adjacent nitro group [13].
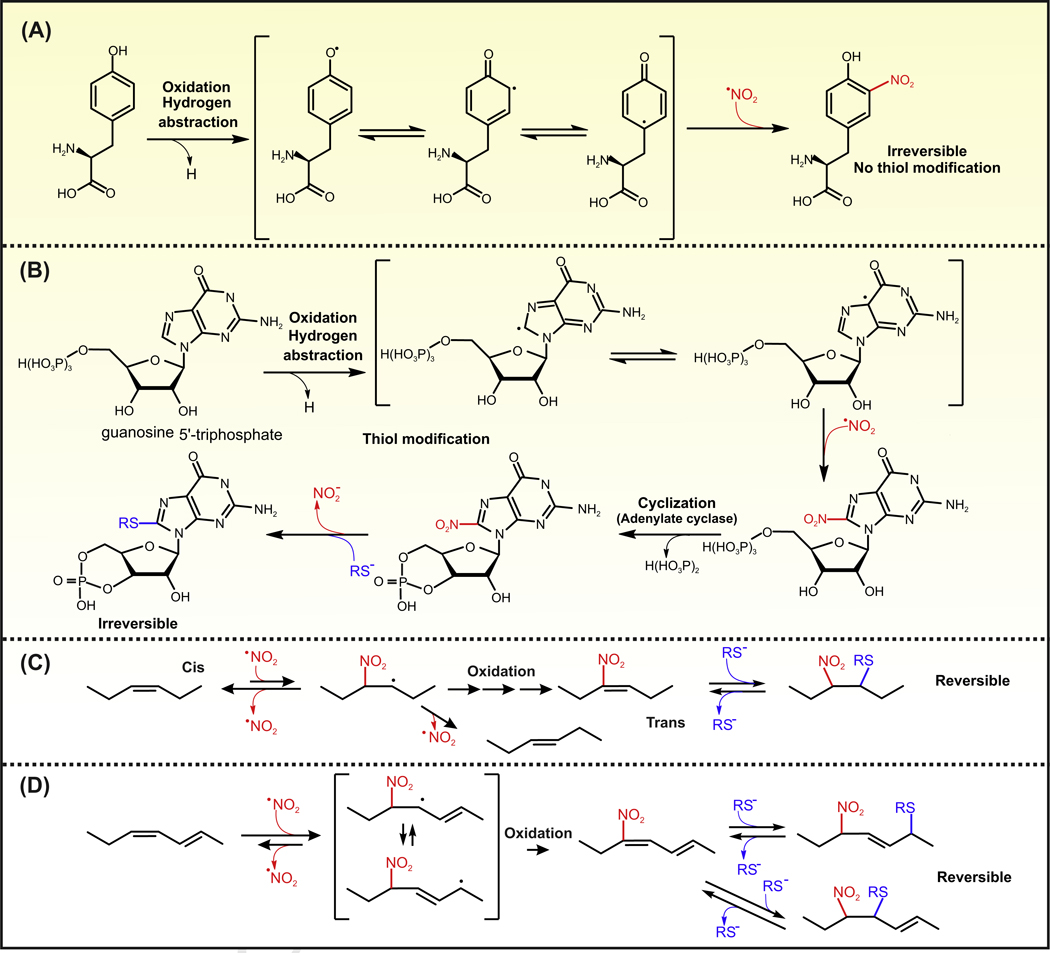
Figure 2. Mechanism of Biologically Relevant Nitration Reactions.
(A) Tyrosine nitration: a one-electron oxidation of tyrosine leads to the formation of a tyrosyl radical, which is stabilized by resonance. A radical–radical reaction between the tyrosyl radical and •NO2 results in the formation of nitrotyrosine. Nitrotyrosine is not reactive and exerts its biological activity by inducing protein conformational changes induced by charge and spatial modifications. (B) Formation of 8-NO2-cGMP: 8-nitro-cGMP is formed upon the activity of guanylate cyclase on NO2-GTP. GTP levels in cells largely exceed the amount of cGMP and as a consequence are the preferred biological substrate for purine nitration. Nitration of GTP is initiated by one-electron oxidation to form a radical that can be stabilized by resonance. As for the tyrosyl radical, a radical-radical reaction with •NO2 forms NO2-GTP. 8-NO2-cGMP reacts slowly and irreversibly with thiols with the concomitant release of nitrite. (C) Nitration of monounsaturated and bis-allylic FAs: this nitration mechanism is inefficient as the initial addition of •NO2 results in an unstable radical. The radical intermediate reverses back to the alkene with the elimination of •NO2, usually resulting in cis–trans isomerization of the double bond. This reaction renders NO2-FAs only in the presence of high concentrations of •NO2. (D) Nitration of conjugated dienes: •NO2 adds to the double bond and the resulting radical is also stabilized by resonance. This radical is then oxidized to form an NO2-FA with conjugated double bonds. The resulting molecule contains two electrophilic carbon, and thiols can add reversibly via Michael addition reaction to the β and δ carbon. Abbreviations: NO2-FA, nitro-fatty acid.
The nitration of GTP also proceeds through an initial one-electron oxidation with hydrogen abstraction that is stabilized by resonance (between C5 and C8 radicals). The radical at C8 reacts with •NO2 to restore the aromatic purine ring leading to the formation of 8-NO2-GTP. Through the enzymatic activity of guanylate cyclase, 8-NO2-GTP is converted into the electrophilic product 8-NO2-cGMP, which reacts with protein thiols through nucleophilic substitution releasing the nitro group (Figure 2B). This reaction forms a stable Cys–cGMP adduct; a process termed protein S-guanylation. When compared to other endogenous electrophiles, the reactivity of 8-NO2-cGMP with glutathione (GSH) is low with a second-order rate constant of 0.03 M/s. This is orders of magnitude slower than the reaction constants determined for other biologically relevant electrophiles such as 4-hydroxynonenal, 15-deoxy-prostaglandin J2, NO2-OA, nitro-linoleic acid (NO2-LA), and NO2-CLA (reaction constants of 1.3, 0.7, 355, 183, and 34 M/s, respectively) [24–26]. Most of the biologically relevant electrophiles are rapidly detoxified through the enzymatic activity of glutathione S-transferases [27]. By contrast, the conjugation reaction between 8-NO2-cGMP and GSH has not been reported to be catalyzed by glutathione S-transferases [28].
Nitration reactions of FAs occur when a NO2 group is added to an alkyl chain. One mechanism involves a direct radical–radical reaction between •NO2 and an alkyl radical (resembling the second step of tyrosine nitration) [29]. This route will not be covered in this review as it lacks biological relevance, nitration yields are very low, and no derivatives of these reactions have been observed in vivo [29]. The biologically relevant nitration reactions typically involve the direct addition of •NO2 to the double bond (Figure 2C). This addition reaction is reversible and occurs with all double bonds yielding a β-nitroalkyl radical. Under most biological conditions, these unstable radicals eliminate •NO2 (reverse reaction) inducing cis–trans isomerizations of the double bond (Figure 2C). When these reactions occur on lipids containing conjugated double bonds, the initial radical is stabilized by resonance, which decreases the rate of the elimination reaction and favors reactions with oxygen and nitrogen oxides (e.g., •NO2 and •NO) producing intermediates that decompose to form electrophilic nitroalkenes (e.g., NO2-CLA; Figure 2D) [10].
Main Sites of NO2-CLA Formation
A strong rationale supported by the nitration chemistry and potential physiological actions of nitrated compounds motivated studies to evaluate the mechanisms and sites of endogenous NO2-FA formation. The first evidence of endogenous NO2-CLA formation in pathological conditions was observed in a mouse model of heart ischemia–reperfusion (I/R). Although initially identified as a trans-NO2-LA given its increased retention time compared to cis-NO2-LA synthetic standards [30], this observation was later corrected, and definitive chemical assignment was provided using isotopically labeled NO2-CLA standards and HPLC-MS/MS analysis [10]. Historically, nitration reactions were strongly linked to inflammation driving initial detection efforts to endogenous sites where proinflammatory and oxidative conditions would prevail. Thus, the role of phagocytic cells, mitochondria, I/R events, and acute and chronic inflammation was investigated [10]. The evaluation of NO2-CLA formation proved difficult; the main reason being the electrophilic nature displayed by NO2-FAs and their reactivity towards thiols. If the concentration of nucleophilic intracellular targets, which range from 2 nM to 17 mM for GSH and from 10 mM to 50 mM for protein thiols [31,32], is considered, >99% of NO2-CLA would be predicted to be covalently bound to cysteine through Michael addition reactions in cells [26]. Advances in the investigation of the local formation of these species have been limited by the inherent difficulty to accurately quantify the adducted fraction [33]. In addition, the local formation has yet to be shown to impact systemic levels measured in circulation.
In Vivo Nitration
Over the past 15 years, it has become clear that nitrite is an active inorganic anion that mediates vasodilation, induces nitrosation and participates in nitration reactions [34,35]. Nitrite is a product of •NO oxidation that can be acquired through diet or be formed in the oral cavity after reduction of dietary nitrate by commensal bacteria [36]. The low pH gastric compartment of the stomach provides ideal conditions for nitrite protonation, generating different nitrogen oxides including dinitrogen trioxide (N2O3) and •NO2 [36,37]. In the presence of conjugated FAs, these reactions generate NO2-FAs in the gastric compartment [10,12]. Pioneering work by Lima et al. recognized the formation of a nitrated LA in the stomach, with the levels in circulation increasing during the postprandial state [19,20]. In retrospect, although initially identified as NO2-LA, it is likely that those observations would have corresponded to NO2-CLA. The authors compared endogenous NO2-LA with synthetic standards and reported differences in spectra and retention times, recognizing that these could be due to different positional isomers in vivo [20]. Furthermore, the analysis of NO2-FA expanded to include NO2-LA-containing cholesterol esters. The evaluation of these species is more complex and its structural characteristics have yet to be fully elucidated [19].
The relevance of gastric NO2-CLA formation stemmed from studies in mice and rats showing that coadministration of 15N-labeled nitrite and CLA resulted in the appearance of 15NO2-CLA in plasma, tissue, and urine [10]. Moreover, gastric NO2-CLA formation is able to induce expression of the nuclear factor (erythroid-derived 2)-like 2 (Nrf2)-dependent gene heme-oxygenase-(HO)-1 in colon epithelial cells, reinforcing the concept that diet-dependent formation of NO2-FA actively participates in signaling and tissue-protective mechanisms [10]. These studies were later translated into humans with oral coadministration of CLA (3 g) and 20 mg 15N-labeled nitrite, resulting in a rapid increase of 15NO2-CLA and a peak concentration (Cmax) in plasma at 1 h of 25 nM [12]. Basal levels of plasma NO2-CLA in human healthy volunteers are ~1 nM [10,12]. To add context to the levels derived from dietary sources and formed during digestion, animals administered subcutaneously with a pharmacological dose of 2 mg/kg/day using osmotic minipumps had steady-state concentrations of plasma NO2-OA of around 6 nM [38]. Based on area under the curve, similar exposures are obtained between the dietary and pharmacological approaches further highlighting the potential for diet-based interventions that modulate the levels of NO2-CLA.
Besides gastric formation, the characterization and description of endogenous NO2-FA formation have been challenging. The first observation of endogenous NO2-CLA formation in vivo occurred in a murine model of focal cardiac I/R [30]. In this same model, exogenous administration of NO2-OA resulted in significant protection against I/R injury, with a marked preservation of left ventricular function and significant reduction in infarct size. After that observation, efforts focused on the evaluation of NO2-CLA formation during inflammation. In cell culture, macrophage activation in the presence of CLA results in significant NO2-CLA formation [10,23,39]. This formation required the generation of •NO and was abolished by the pharmacological inhibition of •NO synthase. While •NO was required, addition of isotopically labeled nitrite (15) resulted in the incorporation of 15N in NO2-CLA, without modifying the overall yields [39]. This indicated that in cell culture, CLA nitration was mostly dependent on •NO auto-oxidation reactions and not nitrite acidification or reactions forming peroxynitrite. These observations were translated into an animal model of sterile sepsis to show that peritoneal inflammation formed NO2-CLA. Using 18O- and 15N-labeling strategies, this work again revealed that these reactions were not related to acidification in vivo but to the formation and auto-oxidation of •NO. Similar to cell culture experiments, in vivo oxidation of •NO resulted in the formation of symmetrical N2O3 and concomitant formation of •NO2. These reactions were specific to CLA and did not result in nitration of LA or other FAs. This work was extended using intraperitoneal zymosan A from Saccharomyces cerevisiae to induce inflammation [39]. The local formation of NO2-CLA decreased the formation of proinflammatory cytokines and inhibited leukocyte recruitment, suggesting that NO2-CLA acts as an adaptive mediator to control inflammation and protect neighboring tissues through the activation of Nrf2-dependent genes [23]. The therapeutic effects of NO2-FAs have been demonstrated in many preclinical animal models (Table 1), but the question regarding the extent of the protective effect of endogenously produced NO2-CLA at the site of inflammation, injury, or I/R has not been completely addressed yet.
Table 1.
NO2-FA Evaluation in Preclinical Animal Modelsa
| Disease state | Animal | Disease model | Dose | Duration | Route of admin | Outcomes | Refs |
|---|---|---|---|---|---|---|---|
| ALS | Female B6SJL-TgN (SOD1-G93A) 1Gur mice | Onset of disease @ 90 d followed by treatment until 140 d | 16 mg/kg/day | 7.14 wk | SC injection (3×/wk) | NO2-OA crossed the BBB and was neuroprotective in this ALS model | [80] |
| Atherosclerosis | 8-wk-old apoE−/− mice | Western diet | 8 mg/kg/day | 3 wk | SC minipump | NO2-OA reduced atherosclerotic lesion formation via decreasing inflammation, macrophage infiltration and limiting the expression of adhesion molecules | [81] |
| Atrial fibrosis | C57BL/6J mice | AngII infusion via mini pump (1.5 ng/g/min) | 6 mg/kg/day | 2 wk | SC minipump | NO2-OA decreased the Ang-II-induced fibrotic response in heart | [82] |
| Atrial fibrosis and fibrillation | NO2-OA decreased Ang-II-induced vulnerability for atrial fibrillation and inhibited atrial fibrosis | [83] | |||||
| Breast cancer | 6-wk-old female athymic nude mice | MDA-MB-231 xenograft tumor growth; NO2-OA started once tumor size was 50–100 mm3 | 7.5 mg/kg/d | 4 wk | Oral gavage | NO2-OA mediates in vivo growth suppression of MDA-MB-231 cells with no overt toxic effects | [72] |
| Cardiac I/R – MI | 8–12-wk-old C57/Bl6 mice | I/R: 30 min unilateral ischemia, 24 h reperfusion | 6.6 mg/kg | At time of reperfusion | IP | NO2-OA has protective effects in cardiac I/R resulting in decreased infarct size and improved left ventricular function in all treatment regimens | [30] |
| 15 min prior to reperfusion | IP | ||||||
| 3 d prior to ischemia | SC minipump | ||||||
| Diabetes | 8–10-wk-old C57BL/6J or Lepob (ob/ob) male mice | Genetic model of obesity and insulin resistance on normal chow | 8 mg/kg/d | 4 wk | SC minipump | NO2-OA normalized hyperglycemia in diabetic mice. Plasma levels of 30 nM were enough to exert a pharmacological effect | [84] |
| Hypertension | 8–10-wk-old C57BL/6J mice | Ang II infusion | 5 mg/kg/day | 2 wk | SC mini-pump | NO2-OA lowers BP by acting as an antagonist of Ang-II-induced hypertension | [55] |
| Pre-existing hypertension: Ang II injection | 1.25, 2.5, 5, 10, 20 mg/kg | 10 min before or 3 d after Ang II delivery | IV – jugular infusion | Dose-dependent reduction in BP. While PPARγ agonist had no effect on BP reduction by NO2-OA | |||
| C57BL/6 | Ang II infusion via mini pump at 1 mg/kg/d | 5 mg/kg/d | 3 days after Ang II infusion | SC minipump | NO2-OA protects against Ang-II-induced hypertension and is mediated via the inhibition of soluble epoxide hydrolase | [56] | |
| Inflammation (multiorgan sepsis) | 8–10-wk-old male C57BL/6 mice | Escherichia coli-induced septic shock (single IP injection of 10 mg/kg); 18 hr | 0.2 mg/kg/d | 48 h before LPS challenge of 18 h | SC minipump | NO2-OA protects against endotoxin-induced endotoxemia and multiorgan injury in mice | [85] |
| Inflammation (pulmonary and sepsis) | 8-wk-old male C57BL/6/J and 5- LO-deficient mice | LPS (20mg/ kg, IP); 16 h | 6.6 mg/kg | 1, 4 h before and 4 h after LPS | IP | NO2-OA attenuates LPS-induced neutrophil and monocyte mobilization, irreversibly inhibits 5-LO and inhibits lung injury | [86] |
| Inflammation (skin) | 6–12-wk-old female Balb/c mice | CHS-sensitization with 0.5% DNFB, FITC, or oxazolone | 0.84 mg/kg | 18 h prior to skin insult | topical | Topical application of NO2-OA augments CHS response | [75] |
| and male and female FoxP3DTR mice | 18 h prior to skin insult | SC injection | SC injection of NO2-OA inhibits skin inflammation in ACD | [74] | |||
| Inflammation (vascular) | C57BL/6J mice | Tail vein injection of LPS (0.5 mg/kg LPS); 3 h | 5 mg/kg/d | 3 d | SC minipump | NO2-OA decreased vascular inflammation by disrupting TLR4 signaling and inhibiting leukocyte adhesion | [87] |
| Inflammatory bowel disease | 7–8-wk-old female BALB/c mice | DSS-induced (2% in drinking water for 7 d) | 0.5 or 5 mg/kg/d | 7 d | SC minipump | NO2-OA reduced disease index, prevented colon shortening and the increase in p65 expression by increasing PPARγ expression | [88] |
| Kidney – diabetic nephropathy | 12-wk-old Leprdb/db(db/db) and Leprdb/m (db/m) | Genetic model; coupled NO2-OA with losartan | 5 mg/kg/d | 2 wk | SC minipump | NO2-OA or losartan alone mildly decreased kidney injury. However, when treatments were combined, the diabetic renal injury was reversed | [89] |
| Kidney – nephropathy | Male BALB/c mice | ADR-induced nephropathy; mice sacrificed 7 d after 10 mg/kg ADR injection | 5 mg/kg/day | 2 d before ADR single injection | SC minipump | NO2-OA protects against ADR nephropathy by decreasing inflammation and reactive species generation | [90] |
| Kidney – Renal I/R | 3-mo-old male B6129SF2/J mice | I/R: 30 min warm, bilateral ischemia, 24 h reperfusion | 0.5 mg/kg | Starting 1 h after ischemia, every 6 h for 24 h | IP | Delayed administration of NO2-OA attenuates renal I/R injury in the mouse likely via inhibition of the inflammatory response | [91] |
| NAFLD/NASH | 8-wk-old male C57BL/6J and apoE−/− mice | WD (42% fat, TD.88137) NASH (40% fat, D17010103) | 5 or 8 mg/kg/d | 12 wk | SC minipump | NO2-OA protects against NASH-diet-induced liver damage, hepatomegaly and steatohepatitis | [70] |
| 6–8-wk-old C57BL/6J mice | HFD (60% fat; D12492) | 8 mg/kg/d | 6 wk | SC minipump | NO2-OA protects against obesity-induced insulin resistance and steatosis | [92] | |
| Obesity | 4-mo-old obese Zucker rats – fa/fa mutation in leptin receptor | Genetic model on normal chow | 0.0075 mg/kg/d | 2 wk | SC minipump | NO2-OA decreased plasma triglyceride levels, normalizes plasma nonesterified free FAs and increases plasma high-density lipoproteins in obese Zucker rats. NO2-FA may be a safe and effective therapeutic for obesity | [93] |
| Pulmonary arterial hypertension | 8–10-wk-old C57BL/6J mice | Hypoxia (10% O2) for 28 d on normal chow | 8 mg/kg/d | 2 and 4 wk | SC mini-pump | NO2-OA attenuated hypoxia-induced pulmonary hypertension | [94] |
| 6–8-wk-old C57BL/6J mice | HFD (60% fat; D12492) | 8 mg/kg/d | 6.5 wk | SC minipump | NO2-OA protects against obesity-induced insulin resistance and PAH | [95] | |
| Vascular injury | 6–8-wk-old C57BL/6 mice | Wire injury of femoral artery | 2 mg/kg/d | 3 wk | SC minipump | NO2-OA inhibited neointimal hyperplasia after wire-induced femoral artery injury via HO-1-dependent mechanisms | [38] |
| Ventral hernia | 10–12-wk-old female Sprague–Dawley rats | Microparticle delivery of NO2-OA in a ventral hernia rat model | ~1200 pmol/scaffold (in vitro assessment) | 8 wk | Scaffold implantation | NO2-OA repaired the abdominal wall and increased angiogenesis | [96] |
Abbreviations: ACD, ; ADR, adriamycin; ALS, amyotrophic lateral sclerosis; BBB, blood–brain barrier; CHS, contact hypersensitivity; DNFB, 1-fluoro-2,4-dinitrobenzene; DSS, dextran sodium sulfate; HFD, high-fat diet; IP, intraperitoneal; IV, intravenous; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; LO, lipoxygenase; LPS, lipopolysaccharide; MI, myocardial infarction; SC, subcutaneous; WD, Western diet.
NO2-FA Distribution and Metabolism
Most of the studies characterizing NO2-FAs measured the concentrations of the free acid form in plasma [17,18,21,22,38]. It has become clear that most of the NO2-FAs are absorbed at the enterocyte level and packaged into chylomicrons that reach the systemic circulation via the thoracic lymphatic duct and left subclavian vein [40]. In animals, bolus oral administration of NO2-OA results in esterified levels that are 40 times greater than the levels of the free acid form [40]. This is relevant as plasma levels of free acid NO2-CLA concentrations in healthy human volunteers remain stable around 1–3 nM [12]. Because of technical challenges related to the stability of NO2-CLA under hydrolysis conditions, the level of esterified NO2-CLA has yet to be determined in humans.
The distinction and the consequences of the NO2-FAs being distributed through lipoproteins (mainly chylomicrons and very low-density lipoproteins) are far reaching, as tissue delivery depends on the local activity of lipoprotein lipases, as is the case for other FAs. The activity of lipoprotein lipases (LPLs) is tightly regulated by angiopoietin-like 4 (Angptl4) and is highest in tissues that rely on FAs as energy sources like heart, kidney, and muscle, or to store FAs as triglycerides and provide heat (white and brown adipose tissue, respectively) [41]. Importantly, the reported expression and activity of LPL correlate with the distribution of tissue radioactivity in rats orally administered with [14C]-NO2-OA [42]. LPL is synthesized by parenchymal tissue and transported to the lumen of capillaries. Hydrolysis of FAs from triglycerides is catalyzed when LPL is bound to glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPIHBP1) [43]. The accumulation of [14C]-NO2-OA in adipose tissue indicates that adipocytes act as reservoirs as well as a buffering system that maintains NO2-FA levels in plasma at constant levels [42,44].
Adipose tissue releases NO2-FAs in the free FA form into the blood, which are then transported back to the liver tightly bound to albumin. Considering that cultured adipocytes actively reduce NO2-FAs into their corresponding nonelectrophilic nitroalkanes [42,44], the extent of fat release of active nitroalkenes has yet to be established. While albumin represents the most abundant thiol in plasma (~600 μM, 75% Cys 34 in the reduced state), representing a potential sink for NO2-FA in circulation, it has been shown that the reaction with Cys34 is minimal [26]. Instead, NO2-FAs actively bind to the hydrophobic pockets present in albumin and are thereby stabilized and transported [26,45]. Once in the liver, these bioactive lipids exert their signaling actions, are recycled and packed into VLDL, metabolized through β and ω oxidation, or inactivated through the activity of prostaglandin reductase 1 [46]. It remains to be established whether the metabolites produced by the liver are excreted through bile or returned to the circulation for renal elimination. While 35% of orally administered [14C]-NO2-OA is disposed of via urinary excretion, it is unclear whether the remaining 65% is not absorbed or actively excreted in the feces through the biliary system [11,42].
Endothelium and Lipid Metabolism
The endothelium provides a physical barrier between blood and tissues. This single layer of endothelial cells essentially acts as an overall gatekeeper that communicates signals between blood and tissue by actively or passively transporting vasoactive biomolecules into and out of the circulation. Although it has not been studied in detail, data suggests that NO2-FAs are transported through the same mechanisms as FAs via FA-binding proteins (FABPs) and translocases. The delivery of lipids to tissues and their consequent metabolism is tightly regulated by endothelial cells [47]. The docking protein GPIHBP1 anchors LPL to the endothelial lumen, to temporarily immo-bilize and hydrolyze FAs off triglycerides carried by transporting lipoproteins (chylomicrons and VLDL) [48,49]. This activity is tissue specific and differentially modulated during fed/fasting states [50]. During the fed state, higher LPL activity and GPIHBP1 expression in adipose tissue facilitate directed transport to storage areas, while fasting promotes a higher delivery to muscle, heart, and kidney (figure 3). This mechanism of distribution also applies to NO2-FAs, where [14C]-labeling studies have shown preferential localization to adipose tissue, heart, liver, and kidney [42]. Once released from lipoproteins, FAs are actively transported across the endothelial Cells by the fatty acid transporter CD36 in coordination with FABPs [51]. NO2-FAs exert signaling actions not only at target tissues, but also endothelial cells and vascular smooth muscle cells found in larger arteries. Distinctly, after reaching adipose tissue, NO2-FAs become esterified and part of a larger pool of releasable FAs. The extent to which fat tissue contributes to buffering systemic NO2-FAs through adipocyte-controlled release has yet to be established but systemic levels are maintained even after days of discontinuing administration suggesting that adipose tissue the main reservoir. NO2-FAs are mobilized from adipocytes through the activity of adipose triglyceride lipase (ATGL) and transported back to the liver bound to albumin (Figure 3).
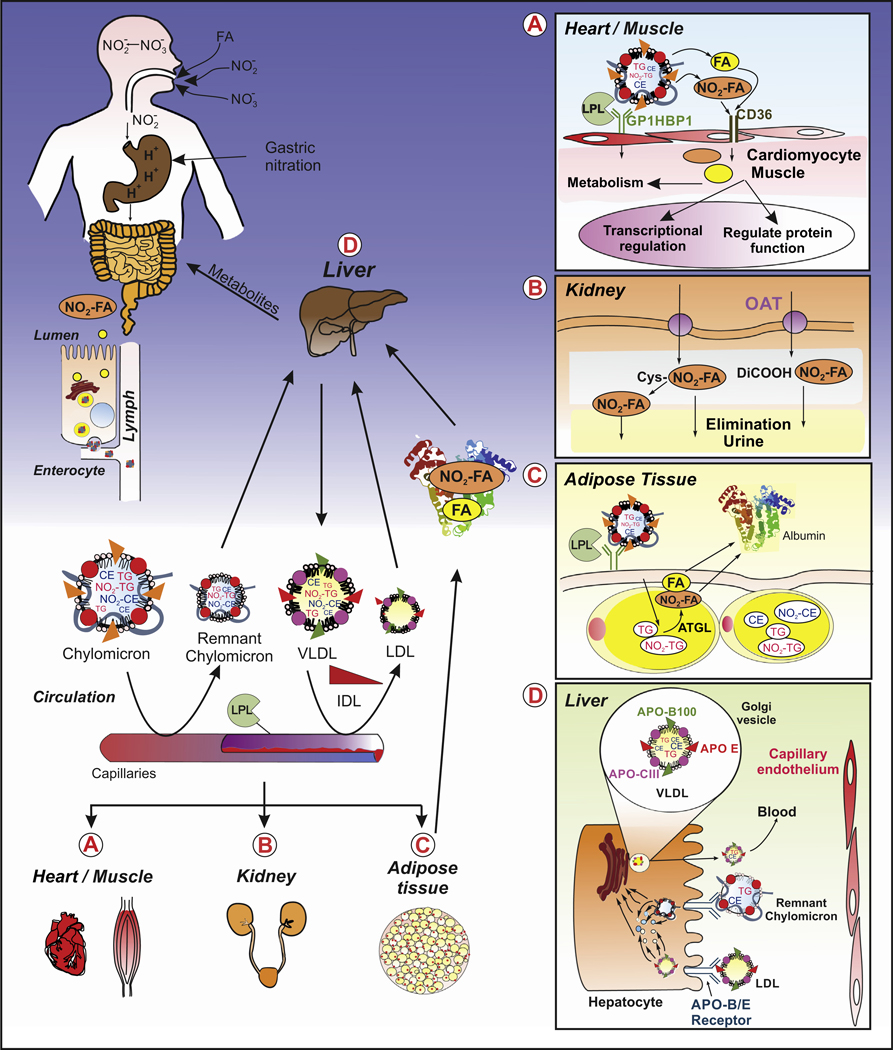
Figure 3. Formation and Biodistribution of NO2-FA.
NO2-CLA formation is promoted under the acidic conditions found in the stomach through the reaction of dietary nitrite-derived radicals and CLA (usually found esterified in triglycerides). For therapeutic purposes, NO2-FAs can also be administered orally as a drug as is the case for CXA-10 (Complexa Inc. – currently undergoing Phase II clinical trials). Through the activity of lipases, triglycerides are hydrolyzed and NO2-FAs are released and absorbed together with other FAs by enterocytes, packed into chylomicrons as triglycerides and moved into the circulation via the lymphatic system. (A) Once the chylomicrons reach the capillaries, the NO2-FAs are cleaved off the triglycerides by the activity of lipoprotein lipases in a process that requires the presence of docking protein GPIHBP1. The released NO2-FAs can bind to fatty acid transporters (e.g., CD36) or diffuse into the endothelial cells and reach the parenchymal cells, such as cardiomyocytes. Heart, kidney, muscle, liver, and adipose tissue are among the main targets. Once the NO2-FAs reach the target cells, they participate in signaling pathways via post-translational modifications of proteins, are metabolized, and then enzymatically inactivated. (B) Hydrophilic metabolites of NO2-Fas, including dicarboxylic acid derivatives, β-oxidation products, mercapturic acids, and cysteine adducts, are filtered in the kidney and eliminated in the urine. (C) NO2-FAs that reach the adipose tissue are re-esterified into triglycerides for storage. Degradation products formed during tissue metabolism and NO2-FAs released from adipocytes through ATGL activity reach the circulation where they bind to albumin and are transported and delivered to the liver for excretion or filtered into urine by the kidneys. (D) Alternatively, NO2-FAs that reach the liver can be reincorporated into triglycerides, assembled in the endoplasmic reticulum and Golgi compartments into VLDL particles, and mature VLDL particles containing Apo B, E, andCIII released to circulation and distributed systemically to target tissues. This initiates a new cycle of delivery, signaling, inactivation, metabolism, and elimination. Finally, the liver clears remaining LDL particles and remnant chylomicrons through selective uptake and breakdown. Abbreviations: Apo, apolipoprotein; ATGL, adipose triglyceride lipase; GPIHBP1, glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1; IDL, intermediate density lipoprotein; LDL, low density lipoprotein; LPL, lipoprotein lipase; NO2-CLA, nitro-conjugated linoleic acid; NO2-FA, nitro-fatty acid; OAT, ; TG, triglyceride; VLDL, very low density lipoprotein.
NO2-FAs Regulate Vascular Tone
The regulatory actions exerted by NO2-FAs on endothelial cells have not been explored as extensively as other cell types and tissues. Among these, NO2-FAs regulate vascular tone by phosphorylating endothelial NO synthase (eNOS) and increasing eNOS protein expression in endothelial cells, which ultimately enhances •NO bioavailability [52]. Additionally, NO2-FAs significantly enhance Nrf2-dependent HO-1 expression and activity in endothelial and vascular smooth muscle cells [38,52,53], and thereby increases CO, which also is a potent endogenous vascular modulator via guanylyl cyclase activation. Endothelin (ET)-1 is a vasoconstrictor and antagonizes the vasodilating effects of •NO. NO2-FAs induce ET-1 clearance through the Nrf2-dependent increase of endothelin receptor B expression in vascular ECs [54]. These protective effects in cell culture translate to hypertension models in mice. NO2-FAs decrease angiotensin (Ang) II-induced blood pressure in mice [55,56]. These protective effects have been attributed to NO2-FA covalently adducting Ang II type 1 receptor which interferes with G-protein-coupled receptor signaling and limits calcium flux in vascular smooth muscle cells [55]. Subsequent studies also revealed that NO2-FA inhibits soluble epoxide hydrolase (sEH), resulting in the accumulation of endogenous vasodilators [56]. By increasing endogenous vasodilation mediators and suppressing the vasoconstrictive effects of ET-1, NO2-FAs are able to regulate blood pressure. Most of these findings can be attributed to a regulation of EC function or a consequence of the EC exposition to NO2-FAs during their transport across the endothelial barrier to reach parenchymal or smooth muscle cells.
Reactivity Explains Signaling
The nitroalkene group present in NO2-CLA and NO2-OA is electrophilic and actively participates in reversible Michael addition reactions [24,26,57]. Main targets are soft nucleophiles such as cysteine present in proteins and/or GSH, but proteomic approaches have also shown the formation of histidine adducts [58]. Glutathione, a tripeptide, is the most abundant cysteine-containing molecule in cells. While many endogenous electrophiles react covalently with these targets, these reactions are either irreversible or display small Koff reaction constants resulting in very slow elimination reactions [58]. By contrast, NO2-FAs display higher on and off reaction rates resulting in a highly movable pool of intracellular NO2-FAs characterized by rapid addition and elimination kinetics. Ultimately, the reactions with cysteines are the ones that have been proven to activate or deactivate signaling pathways and enzymatic activities [59].
Rationale for Using NO2-OA as a Potential Therapeutic
NO2-OA is a minor endogenous NO2-FA and often remains undetectable in tissues and body fluids. Yet, most of the signaling and pharmacology has been performed with it. Why?
This is closely related to the findings and evolution of the field throughout the years and the historic use of OA and LA as nitration substrates. The first evidence of the nitration of lipids came from toxicological work performed by Pryor and Lightsey to understand the impact of •NO2 exposure to the lungs [60]. These studies focused on low and high •NO2 levels and demonstrated that nitration occurred only at a high concentration of •NO2 in both single and methylene interrupted double bonds [60–62]. Studies on the mechanisms and nitration products were later expanded using both OA and LA as substrates [60,63,64]. Initially, nitration of LA was examined, which was then followed by reports of NO2-OA detection [17,21,22]. While •NO2 also adds to nonconjugated double bonds, as is the case for OA and LA, the resulting intermediate radical products are predicted to be highly unstable. Under biological conditions, most of these formed radical intermediates will undergo •NO2 elimination and induce cis–trans isomerization before they can be stabilized (Figure 2C). This is the main difference with CLA, where additional stability of the NO2-CLA intermediate radical is achieved through resonance structures, greatly increasing the nitration yields when compared to OA and LA.
While, initially, formation of both NO2-OA and NO2-LA was reported, there was a preference of NO2-OA over NO2-LA due to less complicated organic synthesis methods and higher stability while maintaining similar signaling actions and reactivity towards thiols [24,26,65]. Thus, NO2-OA was selected as the prototypical NO2-FA and used in most of the preclinical studies (Table 1). Based on these animal models and specific signaling activity, the regio-specific isomer 10-NO2-OA was selected to be developed as a drug candidate to treat inflammatory and fibrotic diseases [66]. It was only years later and supported by more advanced MS techniques that the NO2-CLA was discovered as the main endogenous species [10]. Despite eliciting similar in vitro signaling responses, NO2-CLA has characteristics acteristics that differentiate it from NO2-OA and NO2-LA. In particular, NO2-CLA presents two electrophilic trophilic sites with different Kon and Koff, which are currently being explored [26].
NO2-FAs Signaling
The signaling actions of NO2-FAs go beyond regulating vascular tone, modulating responses in endothelial, parenchymal, and stromal cells. Since the signaling of NO2-FAs is largely driven by its reaction with target cysteines, numerous signaling events and enzymatic activities are modulated. While common pathways include activation of Nrf2, induction of heat shock responses (HSR), and inhibition of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), other more subtle signaling events are cell and tissue specific. Hierarchical transcriptome clustering analysis of cultured endothelial cells treated with NO2-OA revealed that 363 genes were increased and 103 decreased. Of the top 10 mRNA transcripts, eight originate from the heat shock protein family, which had the greatest induction. These findings were validated by qPCR, which showed a maximum induction occurring between 4 h and 8 h in cultured endothelial cells [53]. In response to stress, HSR upregulates transcriptional activity to protect proteins, including expression of folding chaperones and the induction of programs that remove misfolded proteins [67–69]. These microarray data reflect the magnitude of pathway engagement obtained in just cultured endothelial cells. More recently, RNA-sequencing analysis was performed on a model of steatosis and fibrosis using liver from control diet and NASH diet with vehicle, parent nonelectrophilic OA, and electrophilic NO2-OA. The unbiased analysis of liver gene expression revealed that NO2-OA treatment for 12 weeks, once steatosis was established, reversed NASH diet-induced atherosclerotic, fibrotic, inflammatory, lipid metabolism, and stellate cell activation pathways that are involved in the progression to steatohepatitis. More specifically, NO2-OA suppressed hepatic fibrosis genes such as transforming growth factor (TGF)β−1–3, TGFβ receptor 1, tissue inhibitor of metalloproteinase 1 and 2 compared to the nonelectrophilic OA-treated NASH group. The treatment of NO2-OA significantly decreased proinflammatory genes that regulate chemokines, inflammasomes, NF-κB, and Toll-like receptor (TLR)4 signaling pathways. Lipid metabolism is significantly modulated by NO2-OA with the suppression of lipogenic genes (monoacylglycerol o-acyltransferase 2, stearyl-CoA desaturase-2, and sterol regulatory element-binding transcription factor 1) and induction of lipolytic pathways including carnitine palmitoyltransferase (CPT)-1α, CPT-2 and peroxisome proliferator-activated receptor gamma coactivator 1-α [70].
The actions of NO2-FA are protective in an array of preclinical animal models. The observed effects in the preclinical animal models (summarized in Table 1) contrast the technical challenges to identify the mechanisms of actions in all of these models. The pleiotropic effects displayed by the NO2-FAs are closely related with their reactivity. While the activation of Nrf2 and HSR pathways as well as the inhibition of NF-κB are commonly observed actions in the different disease animal models, the relevance of alternative mechanisms of action should always be considered. Inhibition of xanthine oxidase, sEH, Ang II receptor, Rad51, stimulator of interferon genes (STING), and activation of peroxisome proliferator-activated receptor (PPAR)-γ, among others, have been described as subcellular targets of NO2-FAs [55,56,58,71–73]. The engagement of these pathways has gained relevance under certain pathophysiological conditions and need to be addressed individually. This is perhaps the most challenging aspect related to NO2-FAs. As summarized in Table 1, in most cases, the therapeutic dose for NO2-FAs ranges between 2 and 8 mg/kg/day. NO2-FAs have been mostly administered using oral and subcutaneous routes, while topical and intravenous administration has also been tested [55,74,75]. Considering that systemic distribution largely relies on lipoprotein transport, the route of administration is an important variable to be considered in every study design. An important caveat to the routes of NO2-FA administration is the topical application that can result in unwanted dysregulation of immune responses. Similar effects are observed with the therapeutic drug dimethyl fumarate used for the treatment of multiple sclerosis [76]. Skin contact with dimethyl fumarate results in severe dermatitis [77]. While this review does not focus on specific disease states and the potential mechanisms behind the protective effects [58,78,79], Table 1 shows a summary of dose and route of administration, disease condition, and therapeutic outcome.
Concluding Remarks and Future Perspectives
In both transcriptome data sets, in vitro endothelial cells and in vivo liver NASH model, only a small fraction of the pathways, described above, have been closely examined to determine the mech-anistic actions of NO2-FAs. The NO2-FA field is constantly evolving as new pathways involved in the metabolism, absorption, trafficking and signaling are being discovered. These big transcriptome data, coupled with ongoing lipidomic, proteomics- and MS-based approaches, will help prioritize and guide which pathways to pursue. As previously discussed, the data is likely to be cell- and tissue-type specific. The simplistic view of ‘one drug–one target’ strategy will not work for lipid electrophiles given its pleiotropic characteristics. The ‘one drug–multiple targets’ approach with NO2-FAs might provide responses to the unmet clinical needs, particularly for diseases that display multi-factorial pathogenesis.
Highlights.
Nitro-fatty acids (NO2-FAs) are endogenously formed through nonenzymatic mechanisms that require conjugated fatty acids (FAs) and nitrogen dioxide (NO2), a reactive oxidation product of nitrite and nitric oxide (NO). NO2-FAs are formed in the gastric compartment, at sites of inflammation and during ischemia-reperfusion.
Nitro-conjugated linoleic acid (NO2-CLA) is the most abundant endogenously formed NO2-FA and nitro oleic acid (NO2-OA) is a synthetic NO2-FA being developed as a drug.
NO2-FAs exert cell signaling responses through their electrophilic reactivity by reversibly modifying cysteine residues. NO2-FAs regulate signaling activities, mainly through the activation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), heat shock response, and inhibition of NF-κB signaling.
NO2-FAs are distributed to tissues and esterified to triglycerides.
Endothelium is both protected by NO2-FAs and provides the necessary mechanism and regulation to transport NO2-FAs to surrounding tissues.
NO2-FAs have been shown to be effective in a large range of preclinical animal models.
Outstanding Questions.
Does the endogenously generated NO2-CLA in the gastric compartment induce protective signaling actions? Do these preferentially target certain tissues given its biodistribution mechanism?
Could gastric NO2-CLA formation be a viable dietary alternative to treat chronic inflammatory diseases?
What is the impact of NO2-CLA stored in adipocytes on signaling and systemic levels?
What are other signaling pathways engaged by NO2-FAs? Are there clear differences between NO2-OA and NO2-CLA in vivo?
So far, CLA has been identified as the preferred endogenous substrate for FA nitration. The other major source of conjugated FAs that can be obtained from the diet is CLnA. Is CLnA nitrated? Is CLnA a better substrate than CLA?
In vitro treatment concentrations are usually 1–3 μM and in vivo levels are 1–3 nM. While in vivo levels are in a pseudo-steady state with endogenous cysteines, exogenously added NO2-FAs rapidly decrease in concentration given metabolism and thiol addition reactions. How should the concentrations between in vitro experiments and in vivo levels be reconciled to provide bridging between the different settings?
Acknowledgments
We would like to thank Ms. Sonia Salvatore for input and graphic design. This work was supported by R01-GM125944 and R01-DK112854 (F.J.S), AHA 17GRN33660955 (F.J.S), P01-HL103455 (N.K.H.K.), and University of Pittsburgh Medical Center Competitive Medical Research Fund Award (N.K.H.K.).
Glossary
- Conjugated linoleic acid (CLA)
FA characterized by having an 18 carbon alkyl chain containing two conjugated double bonds. Derived from ruminants and mainly found in meat and dairy products. The preferred endogenous substrate for gastric, metabolic and inflammatory-mediaed FA nitration.
- Conjugated linolenic acid (CLnA)
FA characterized by having 18 carbon and two or three conjugated double bonds. The cis–trans configuration and position of the double bonds greatly depend on its biological source. CLnA containing three conjugated double bonds is mainly found in plant-derived oils. Bitter melon and pomegranate are the most common sources of plant-derived dietary CLnA. CLnA containing two conjugated double bonds is most commonly found in dairy products.
- Nitration
reaction that encompasses the addition of a nitro group (−NO2) t oa n organic compound or macromolecule.
- Nitro-conjugated linoleic acid (NO2-CLA)
the most abundant endogenously formed NO2-FA. It is biologically formed as two isomers characterized by the NO2 group located in carbons 9 or 12 that display equivalent signaling potency.
- Nitro-fatty acid (NO2-FA)
electrophilic bioactive FAs that contain a nitro group (−NO2) and display anti-inflammatory, antioxidant, and antifibrotic cell signaling properties, mainly through reactions with cysteines.
- Nitro-oleic acid (NO2-OA)
predominant NO2-FA isomer used in preclinical animal models. CXA-10 which corresponds to NO2-OA with the NO2 group located on carbon 10, is currently undergoing Phase II clinical trials.
- Nitrosation
addition of a nitroso group to an organic compound or macromolecule.
Footnotes
Disclaimer Statement
F.J.S has financial interest in Complexa Inc. N.K.H.K. has no conflicts of interest to declare.
References
- 1.Burr GO and Burr MM (1973) Nutrition classics from The Journal of Biological Chemistry 82:345–67, 1929. A new deficiency disease produced by the rigid exclusion of fat from the diet. Nutr. Rev. 31, 248–249 [DOI] [PubMed] [Google Scholar]
- 2.Spector AA and Kim HY (2015) Discovery of essential fatty acids. J. Lipid Res 56, 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holman RT (1998) The slow discovery of the importance of omega 3 essential fatty acids in human health. J. Nutr 128, 427S–433S [DOI] [PubMed] [Google Scholar]
- 4.Barr LH et al. (1981) Essential fatty acid deficiency during total parenteral nutrition. Ann. Surg 193, 304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dennis EA and Norris PC (2015) Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol 15, 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belury MA (2002) Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Annu. Rev. Nutr 22, 505–531 [DOI] [PubMed] [Google Scholar]
- 7.Gómez-Cortés P. et al. (2018) Milk fatty acids and potential health benefits: an updated vision. Trends Food Sci. Technol 81, 1–9 [Google Scholar]
- 8.Yuan G-F et al. (2014) Conjugated linolenic acids and their bioactivities: a review. Food Funct. 5, 1360–1368 [DOI] [PubMed] [Google Scholar]
- 9.Raimondi S. et al. (2016) Conjugated linoleic acid production by bifidobacteria: screening, kinetic, and composition. Biomed. Res. Int 2016, 8654317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonacci G. et al. (2012) Conjugated linoleic acid is a preferential substrate for fatty acid nitration. J. Biol. Chem 287, 44071–44082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salvatore SR et al. (2013) Characterization and quantification of endogenous fatty acid nitroalkene metabolites in human urine. J. Lipid Res 54, 1998–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delmastro-Greenwood M. et al. (2015) Nitrite and nitrate-dependent generation of anti-inflammatory fatty acid nitroalkenes. Free Radic. Biol. Med 89, 333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrer-Sueta G. et al. (2018) Biochemistry of peroxynitrite and protein tyrosine nitration. Chem. Rev 118, 1338–1408 [DOI] [PubMed] [Google Scholar]
- 14.Tsunoda M. et al. (2007) Determination of nitrocatecholamines in rat brain using high-performance liquid chromatography-peroxyoxalate chemiluminescence reaction detection. J. Chromatogr. A 1164, 162–166 [DOI] [PubMed] [Google Scholar]
- 15.Tsunoda M. et al. (2008) Oxidative stress increases 6-nitronorepinephrine and 6-nitroepinephrine concentrations in rat brain. Biomed. Chromatogr 22, 572–574 [DOI] [PubMed] [Google Scholar]
- 16.Fujii S. and Akaike T. (2013) Redox signaling by 8-nitro-cyclic guanosine monophosphate: nitric oxide- and reactive oxygen species-derived electrophilic messenger. Antioxid. Redox Signal 19, 1236–1246 [DOI] [PubMed] [Google Scholar]
- 17.Baker PR et al. (2005) Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J. Biol. Chem 280, 42464–42475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker PR et al. (2004) Red cell membrane and plasma linoleic acid nitration products: synthesis, clinical identification, and quantitation. Proc. Natl. Acad. Sci. U. S. A 101, 11577–11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima ES et al. (2003) Cholesteryl nitrolinoleate, a nitrated lipid present in human blood plasma and lipoproteins. J. Lipid Res 44, 1660–1666 [DOI] [PubMed] [Google Scholar]
- 20.Lima ES et al. (2002) Characterization of linoleic acid nitration in human blood plasma by mass spectrometry. Biochemistry 41, 10717–10722 [DOI] [PubMed] [Google Scholar]
- 21.Tsikas D. et al. (2009) Specific GC-MS/MS stable-isotope dilution methodology for free 9- and 10-nitro-oleic acid in human plasma challenges previous LC-MS/MS reports. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 877, 2895–2908 [DOI] [PubMed] [Google Scholar]
- 22.Tsikas D. et al. (2009) Nitro-fatty acids occur in human plasma in the picomolar range: a targeted nitro-lipidomics GC-MS/MS study. Lipids 44, 855–865 [DOI] [PubMed] [Google Scholar]
- 23.Villacorta L. et al. (2018) In situ generation, metabolism and im-munomodulatory signaling actions of nitro-conjugated linoleic acid in a murine model of inflammation. Redox Biol. 15, 522–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker LM et al. (2007) Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction. J. Biol. Chem 282, 31085–31093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawa T. et al. (2010) Regulation of redox signaling involving chemical conjugation of protein thiols by nitric oxide and electrophiles. Bioconjug. Chem 21, 1121–1129 [DOI] [PubMed] [Google Scholar]
- 26.Turell L. et al. (2017) The chemical basis of thiol addition to nitro-conjugated linoleic acid, a protective cell-signaling lipid. J. Biol. Chem 292, 1145–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coles BF and Kadlubar FF (2003) Detoxification of electrophilic compounds by glutathione S-transferase catalysis: determinants of individual response to chemical carcinogens and chemotherapeutic drugs? Biofactors 17, 115–130 [DOI] [PubMed] [Google Scholar]
- 28.Akaike T. et al. (2012) Regulation of redox signalling by an electrophilic cyclic nucleotide. J. Biochem 153, 131–138 [DOI] [PubMed] [Google Scholar]
- 29.Buchan GJ et al. (2018) Nitro-fatty acid formation and metabolism. Nitric Oxide 79, 38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudolph V. et al. (2010) Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovasc. Res 85, 155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen RE et al. (2009) Quantifying the global cellular thiol-disulfide status. Proc. Natl. Acad. Sci. U. S. A 106, 422–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Requejo R. et al. (2010) Cysteine residues exposed on protein surfaces are the dominant intramitochondrial thiol and may protect against oxidative damage. FEBS J. 277, 1465–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schopfer FJ et al. (2009) Detection and quantification of protein adduction by electrophilic fatty acids: mitochondrial generation of fatty acid nitroalkene derivatives. Free Radic. Biol. Med 46, 1250–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cosby K. et al. (2003) Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med 9, 1498–1505 [DOI] [PubMed] [Google Scholar]
- 35.DeMartino AW et al. (2019) Nitrite and nitrate chemical biology and signalling. Br. J. Pharmacol 176, 228–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundberg JO and Weitzberg E. (2013) Biology of nitrogen oxides in the gastrointestinal tract. Gut 62, 616–629 [DOI] [PubMed] [Google Scholar]
- 37.Rocha BS et al. (2012) Intragastric nitration by dietary nitrite: implications for modulation of protein and lipid signaling. Free Radic. Biol. Med 52, 693–698 [DOI] [PubMed] [Google Scholar]
- 38.Cole MP et al. (2009) Nitro-fatty acid inhibition of neointima formation after endoluminal vessel injury. Circ. Res 105, 965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vitturi DA et al. (2015) Convergence of biological nitration and nitrosation via symmetrical nitrous anhydride. Nat. Chem. Biol 11, 504–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fazzari M. et al. (2019) Electrophilic fatty acid nitroalkenes are systemically transported and distributed upon esterification to complex lipids. J. Lipid Res 60, 388–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dijk W. and Kersten S. (2014) Regulation of lipoprotein lipase by Angptl4. Trends Endocrinol. Metab 25, 146–155 [DOI] [PubMed] [Google Scholar]
- 42.Fazzari M. et al. (2017) Nitro-fatty acid pharmacokinetics in the adipose tissue compartment. J. Lipid Res 58, 375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birrane G. et al. (2019) Structure of the lipoprotein lipase–GPIHBP1 complex that mediates plasma triglyceride hydrolysis. Proc. Natl. Acad. Sci 116, 1723–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fazzari M. et al. (2015) Generation and esterification of electrophilic fatty acid nitroalkenes in triacylglycerides. Free Radic. Biol. Med 87, 113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turell L. et al. (2013) The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Radic. Biol. Med 65, 244–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitturi DA et al. (2013) Modulation of nitro-fatty acid signaling: prostaglandin reductase-1 is a nitroalkene reductase. J. Biol. Chem 288, 25626–25637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fong LG et al. (2016) GPIHBP1 and plasma triglyceride metabolism. Trends Endocrinol. Metab 27, 455–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldberg IJ (1996) Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J. Lipid Res 37, 693–707 [PubMed] [Google Scholar]
- 49.Cryer A. (1989) The role of the endothelium in myocardial lipoprotein dynamics. Mol. Cell. Biochem 88, 7–15 [DOI] [PubMed] [Google Scholar]
- 50.Cushing EM et al. (2017) Angiopoietin-like 4 directs uptake of dietary fat away from adipose during fasting. Mol. Metab 6, 809–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glatz JFC and Luiken J. (2018) Dynamic role of the trans-membrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J. Lipid Res 59, 1084–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khoo NKH et al. (2010) Activation of vascular endothelial nitric oxide synthase and heme oxygenase-1 expression by electrophilc nitro-fatty acids. Free Radic. Biol. Med 48, 230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kansanen E. et al. (2009) Nrf2-dependent and -independent responses to nitro-fatty acids in human endothelial cells: identification of heat shock response as the major pathway activated by nitro-oleic acid. J. Biol. Chem 284, 33233–33241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kansanen E. et al. (2017) Nitro-oleic acid regulates endothelin signaling in human endothelial cells. Mol. Pharmacol 92, 481. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J. et al. (2010) Nitro-oleic acid inhibits angiotensin II-induced hypertension. Circ. Res 107, 540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Charles RL et al. (2014) Protection from hypertension in mice by the Mediterranean diet is mediated by nitro fatty acid inhibition of soluble epoxide hydrolase. Proc. Natl. Acad. Sci 111, 8167–8172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Batthyany C. et al. (2006) Reversible post-translational modifycation of proteins by nitrated fatty acids in vivo. J. Biol. Chem 281, 20450–20463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schopfer FJ et al. (2011) Formation and signaling actions of electrophilic lipids. Chem. Rev 111, 5997–6021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bates DJ et al. (2009) Noncatalytic interactions between glutathione S-transferases and nitroalkene fatty acids modulate nitroalkene-mediated activation of peroxisomal proliferator-activated receptor gamma. Biochemistry 48, 4159–4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pryor WA and Lightsey JW (1981) Mechanisms of nitrogen dioxide reactions: initiation of lipid peroxidation and the production of nitrous Acid. Science 214, 435–437 [DOI] [PubMed] [Google Scholar]
- 61.Gallon AA and Pryor WA (1994) The reaction of low levels of nitrogen dioxide with methyl linoleate in the presence and absence of oxygen. Lipids 29, 171–176 [DOI] [PubMed] [Google Scholar]
- 62.Gallon AA and Pryor WA (1993) The identification of the allylic nitrite and nitro derivatives of methyl linoleate and methyl linolenate by negative chemical ionization mass spectroscopy. Lipids 28, 125–133 [DOI] [PubMed] [Google Scholar]
- 63.Napolitano A. et al. (2004) Acid-induced structural modifications of unsaturated Fatty acids and phenolic olive oil constituents by nitrite ions: a chemical assessment. Chem. Res. Toxicol 17, 1329–1337 [DOI] [PubMed] [Google Scholar]
- 64.Jain K. et al. (2008) The mechanism of oleic acid nitration by *NO(2). Free Radic. Biol. Med 45, 269–283 [DOI] [PubMed] [Google Scholar]
- 65.Manini P. et al. (2008) Chemistry of nitrated lipids: remarkable instability of 9-nitrolinoleic acid in neutral aqueous medium and a novel nitronitrate ester product by concurrent autoxidation/nitric oxide-release pathways. J. Org. Chem 73, 7517–7525 [DOI] [PubMed] [Google Scholar]
- 66.Schopfer FJ et al. (2018) Nitro-fatty acids: New drug candidates for chronic inflammatory and fibrotic diseases. Nitric Oxide 79, 31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zügel U. and Kaufmann SH (1999) Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin. Microbiol. Rev 12, 19–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anckar J. and Sistonen L. (2007) Heat shock factor 1 as a co-ordinator of stress and developmental pathways In Molecular Aspects of the Stress Response: Chaperones, Membranes and Networks (Csermely P. and Vígh L, eds), pp. 78–88, SpringerNew York [Google Scholar]
- 69.Buchman TG et al. (1993) Induction of heat shock response leads to apoptosis in endothelial cells previously exposed to endotoxin. Am. J. Phys. Heart Circ. Phys 265, H165–H170 [DOI] [PubMed] [Google Scholar]
- 70.Rom O et al. (2019) Nitro-fatty acids protect against steatosis and fibrosis during development of nonalcoholic fatty liver disease in mice. EBioMedicine 41, 62–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelley EE et al. (2008) Nitro-oleic acid, a novel and irreversible inhibitor of xanthine oxidoreductase. J. Biol. Chem 283, 36176–36184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Woodcock C-SC et al. (2018) Nitro-fatty acid inhibition of triple-negative breast cancer cell viability, migration, invasion, and tumor growth. J. Biol. Chem 293, 1120–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hansen AL et al. (2018) Nitro-fatty acids are formed in response to virus infection and are potent inhibitors of STING palmitoylation and signaling. Proc. Natl. Acad. Sci 115, E7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mathers AR et al. (2017) Electrophilic nitro-fatty acids suppress allergic contact dermatitis in mice. Allergy 72, 656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mathers AR et al. (2018) Topical electrophilic nitro-fatty acids potentiate cutaneous inflammation. Free Radic. Biol. Med 115, 31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kappos L et al. (2008) Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 372, 1463–1472 [DOI] [PubMed] [Google Scholar]
- 77.Lammintausta K et al. (2010) An epidemic of furniture-related dermatitis: searching for a cause. Br. J. Dermatol 162, 108–116 [DOI] [PubMed] [Google Scholar]
- 78.Rom O et al. (2018) Inflammatory signaling and metabolic regulation by nitro-fatty acids. Nitric Oxide 78, 140–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deen AJ et al. (2018) Regulation of stress signaling pathways by nitro-fatty acids. Nitric Oxide 78, 170–175 [DOI] [PubMed] [Google Scholar]
- 80.Trostchansky A et al. (2018) Profile of arachidonic acid-derived inflammatory markers and its modulation by nitro-oleic acid in an inherited model of amyotrophic lateral sclerosis. Front. Mol. Neurosci 11, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rudolph TK et al. (2010) Nitro-fatty acids reduce atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol 30, 938–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ambrozova G et al. (2016) Nitro-oleic acid modulates classical and regulatory activation of macrophages and their involvement in pro-fibrotic responses. Free Radic. Biol. Med 90, 252–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rudolph TK et al. (2016) Nitrated fatty acids suppress angiotensin II-mediated fibrotic remodelling and atrial fibrillation. Cardiovasc. Res 109, 174–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schopfer FJ et al. (2010) Covalent peroxisome proliferator-activated receptor γ binding by nitro-fatty acids: Endogenous ligands act as selective modulators. J. Biol. Chem 285, 12321–12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang H et al. (2010) Nitro-oleic acid protects against endotoxin-induced endotoxemia and multiorgan injury in mice. Am. J. Physiol. Ren. Physiol 298, F754–F762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Awwad K et al. (2014) Electrophilic fatty acid species inhibit 5-lipoxygenase and attenuate sepsis-induced pulmonary inflammation. Antioxid. Redox Signal 20, 2667–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Villacorta L et al. (2013) Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts. Cardiovasc. Res 98, 116–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Borniquel S et al. (2010) Nitrated oleic acid up-regulates PPARγ and attenuates experimental inflammatory bowel disease. Free Radic. Biol. Med 48, 499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Y et al. (2013) Combined losartan and nitro-oleic acid remarkably improves diabetic nephropathy in mice. Am. J. Physiol. Ren. Physiol 305, F1555–F1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu S et al. (2013) Nitro-oleic acid protects against adriamycininduced nephropathy in mice. Am. J. Physiol.-Renal Physiol 305, F1533–F1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu H et al. (2008) Nitro-oleic acid protects the mouse kidney from ischemia and reperfusion injury. Am. J. Physiol. Ren. Physiol 295, F942–F949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khoo NKH et al. (2019) Electrophilic nitro-oleic acid reverses obesity-induced hepatic steatosis. Redox Biol. 22, 101132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang H et al. (2010) Effects of endogenous PPAR agonist nitro-oleic acid on metabolic syndrome in obese Zucker rats. PPAR Res. 2010, 601562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klinke A et al. (2014) Protective effects of 10-nitro-oleic acid in a hypoxia-induced murine model of pulmonary hypertension. Am. J. Respir. Cell Mol. Biol 51, 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kelley EE et al. (2014) Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high fat diet-induced obesity. Cardiovasc. Res 101, 352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.D’Amore A et al. (2017) Nitro-oleic acid (NO2-OA) release enhances regional angiogenesis in a rat abdominal wall defect model. Tissue Eng. A 24, 889–904 [DOI] [PMC free article] [PubMed] [Google Scholar]