Abstract
The capsids of most plant viruses are simple and robust structures consisting of multiple copies of one or a few types of protein subunit arranged with either icosahedral or helical symmetry. In many cases, capsids can be produced in large quantities either by the infection of plants or by the expression of the subunit(s) in a variety of heterologous systems. In view of their relative simplicity, stability and ease of production, plant virus particles or virus-like particles (VLPs) have attracted attention as potential reagents for applications in bionanotechnology. As a result, plant virus particles have been subjected to both genetic and chemical modification, have been used to encapsulate foreign material and have, themselves, been incorporated into supramolecular structures.
Keywords: Coat Protein, Virus Particle, Plant Virus, Virus Surface, Brome Mosaic Virus
Introduction
The majority of plant viruses have particles that are nonenveloped and consist of only one or a few types of subunit arranged either with icosahedral or helical symmetry around a single-stranded RNA genome. In many cases plant virus particles can be produced in large quantities in plants and are generally very stable. Virus-like particles (VLPs) also can be produced by expressing the coat protein in a variety of heterologous systems, such as Escherichia coli, yeast or insect cells. The advent of techniques for the manipulation of RNA genomes coupled with a detailed knowledge of their virion structure and the use of heterologous expression systems has meant that plant viruses have been at the forefront of the exploitation of virus particles for applications in both bio- and nano-technology. As plant viruses are clearly biological entities which can also be considered as nanoparticles, it is perhaps best to consider plant virus particles as being used in bionanotechnology.
Plant viruses to date have been exploited in three different ways in bionanotechnology: modification of the outer capsid surface (either genetically, chemically or by a combination of both), filling of the inner cavity, and the incorporation of particles into supramolecular structures. This chapter is therefore divided into sections dealing separately with the modification of the outer surface, the incorporation of materials within particles and the incorporation of particles within higher order structures. However, it should be noted that particles with modifications to their outer capsid surface can be used to incorporate foreign material and can, themselves, be incorporated into higher order structures.
Modification of the Outer Surface of Plant Virus Particles
The first examples of the modificationof the outer surface of virus particles involved the genetic modification of the coat protein (Lomonossoff and Johnson 1996). The original motivation for this work was to modify particles to express antigenic peptides; such modified particles (chimaeras) could potentially serve as novel subunit vaccines. Subsequently, the alternative approach of chemically modifying particles was explored. Several of the amino acids within viral coat protein subunits have side chains which are suitable for chemical modification. Prominent among these are the ε-amino group of lysine, the carboxyl groups of aspartic and glutamic acid, the thiol group of cysteine and the hydroxyl group of tyrosine. When such side chains are exposed on the outer surface of the virus particle, they are addressable by a number of chemical reactants allowing the virus particles to be modified in vitro. This allows the introduction of a greater range of moieties than it is possible to introduce genetically. It is also possible to combine genetic and chemical modification of the virus surface by the introduction or elimination of defined amino acids, thereby modulating the reactivity of the virus particles. It is also possible to genetically insert peptides which catalyse certain reactions, such as the specific deposition of minerals. The capsids of a number of plant viruses with varying morphologies have been modified, both genetically and chemically, on their exterior surfaces. The main prerequisites for genetic modification are that the presence of the foreign sequence does not interfere with the ability of the modified coat protein to assemble into virions or VLPs and that the inserted sequence is, indeed, displayed on the outer surface of assembled particles. For chemical modification, it is important the reaction conditions are not so harsh that they disrupt the virus structure and that some information is available about the numbers and types of addressable groups. For these reasons, attention to date has focussed on those viruses which are known to be robust and for which there is at least some information available about the topology of the coat protein in the assembled virions.
Icosahedral Viruses
Cowpea Mosaic Virus
Cowpea mosaic virus (CPMV) was the first plant virus to be developed as a system for the display of foreign peptides (Usha et al. 1993; Porta et al. 1994, 1996) and has subsequently been used extensively for chemical modification (Steinmetz et al. 2009a). CPMV is a bipartite RNA virus, with the particles consisting of 60 copies each of a Large (L) and a Small (S) coat protein (CP) arranged with icosahedral symmetry in a particle of approximately 28 nm diameter (Fig. 1). Both coat proteins are produced by proteolytic cleavage of a precursor (VP60) by the virus-encoded 24K proteinase (Franssen et al. 1982; Saunders et al. 2009). The virus particles are attractive candidates for both genetic and chemical modification for a number of reasons: they can be purified in large quantities from infected tissue, they are very robust, surviving at 60°C for at least one hour, across the range of pH 4–10, and in some organic solvent–water mixtures, and their 3-dimensional structure is known to atomic resolution (Lin and Johnson 2003). Furthermore infectious cDNA clones of both RNAs have been available for the past 20 years enabling precise genetic changes to be introduced. The availability of a detailed 3-dimensional structure of the virus particles initially enabled a rational choice to be made regarding potential sites for the insertion of heterologous peptides into the viral coat proteins such that they would be surface-exposed and would not adversely affect particle assembly (Lomonossoff and Johnson 1995; Johnson et al. 1997) and has subsequently proved invaluable for making precise chemical modifications (Steinmetz et al. 2009a).
Fig. 1.
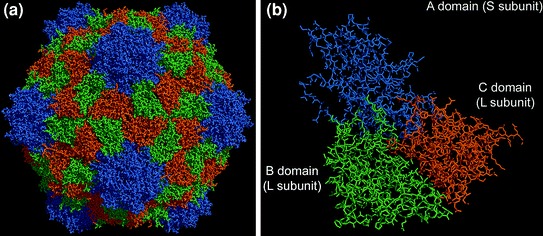
Structure of a the Cowpea mosaic virus (CPMV) capsid and b the asymmetric unit. The capsid is comprised of the small(S) and large(L) subunit. (Steinmetz and Evans 2007)
The initial studies on the genetic alteration of the outer surface of CPMV particles were aimed at creating antigenic chimaeras for vaccine purposes. A number of sites were identified as suitable for the insertion of foreign peptides. In most cases, the foreign peptide has been inserted into the most exposed loop of the virus surface, the βB-βC loop of the S protein. However, other sites, such as the βE-αB loop of the L protein and the βC’-βC” loop of the S protein, have also been used successfully (Brennan et al. 1999; Taylor et al. 2000; Chatterji et al. 2002; Porta et al. 2003). Generally, provided the inserted peptide was less than 40 amino acids and had a pI below 9.0 (Porta et al. 2003) the yields of modified particles were similar to those obtained with wild-type CPMV (up to 1mg of particles per gram of infected leaf tissue). In each case, the chimaeric virus particles present 60 copies of the inserted peptide on the virus surface, though preliminary experiments indicate that it will be possible to utilise more than one insertion site simultaneously. Work on the production of chimaeras for vaccine purposes culminated in the demonstration of protective immunity in target animals (Dalsgaard et al. 1997; Langeveld et al. 2001) and the ability to correlate the structure that a peptide adopts with its immunological properties (Lin et al. 1996; Taylor et al. 2000). Work on the immunological properties of antigenic chimaeras is outside the scope of the current chapter and for further information the reader is referred to specialist reviews on the subject (Lomonossoff and Hamilton 1999; Cañizares et al. 2005; Lomonossoff, 2005; Sainsbury et al. 2010).
More recently, the ability to express peptides on the CPMV surface has been exploited to introduce peptides which are capable of, or promote, subsequent chemical modification. Medintz et al. (2005) introduced a hexa-histidine at various points on the virus surface and the modified particles were bound via the introduced histidines to glass slides coated with nickel-nitrilotriacetic acid (Ni–NTA). The exposed histidine sequences on the upper virus surface were then used to bind a bifunctional Ni–NTA-biotin reagent which allowed the subsequent attachment of quantum dots via the biotin-avidin interaction. In addition, it has been possible to exploit the observation that short peptides can promote specific mineralisation of virus particles. CPMV chimaeras have been produced in which peptides designed to promote the deposition of silica or an iron-platinum (FePt) alloy on the virion surface were expressed in the βB-βC loop of the S protein (Steinmetz et al. 2009b; Shah et al. 2009). In the case of the CPMV-silica chimaera, silication was achieved via a sol–gel process. The silicated CPMV particles could be easily visualised in unstained electron microscopy images. The diameter of silicated particles was estimated to be ∼32 nm, consistent with an average silica coating on each particle of 2 nm. In a similar approach, Shah et al. (2009) created a CPMV chimaera that promotes the formation of an FePt alloy on the capsid surface to give monodisperse, hollow, FePt nanoparticles of about 30 nm diameter (Fig. 2). The mineralised particles produced in this way are highly monodisperse and are not readily prepared by other synthetic methods. Further, the process is environmentally benign; as it proceeds at room temperature and pressure, requires no organic solvents, and produces little waste.
Fig. 2.

Transmission electron micrograph of unstained FePt-coated CPMV showing monodisperse mineralised particles
Wild-type CPMV particles have five exposed lysines and eight or nine exposed carboxylates (from aspartic and glutamic acid residues) per asymmetric unit, (the asymmetric unit consists of one copy each of the L and S protein) (Fig. 1). This means that each particle should have 300 addressable amine and 480–540 addressable carboxyl groups per virus particle. There are also exposed tyrosines, but no cysteines. All the naturally occurring addressable groups have been exploited to produce chemically modified particles. Probably the most frequently utilised group has been the ε-amino group of surface-exposed lysines. Initial studies revealed that approximately 240 dye molecules could be attached per particle under forcing conditions (Wang et al. 2002a, b) suggesting that four of the five exposed lysines can be modified. The ability to modify lysine residues has since been extensively exploited for a number of purposes. The first example of this was reported by Raja et al. (2003) who derivatised the lysine residues of CPMV with polyethylene glycol (PEG). PEG is known to moderate the immune response to a particle, a phenomenon which may be of considerable use if plant viruses are to be used as novel vaccines. The reaction was shown to give conjugates with altered densities and immunogenicities, consistent with the known chemical and biological properties of PEG. These studies were subsequently refined by Steinmetz and Manchester (2009) who showed that only longer PEG chains effectively shield particles from interacting with cells or tissues. CPMV particles which have been fluorescently labelled at exposed lysines have been successfully used for high-resolution vascular imaging (Lewis et al. 2006). This application makes use of the observations that CPMV particles are nontoxic and rapidly cleared when administered to mammals (Singh et al. 2007) and the propensity of CPMV particles to bind to endothelium surface-expressed vimentin (Koudelka et al. 2009). The exposed lysines have also been extensively used to couple biotin to the virus surface to enable the particles to bind to avidin (Medintz et al. 2005; Steinmetz et al. 2006a) and this introduced binding ability has been exploited for the creation of supramolecular structures (see Sect. 4.1.2). Lysines have also been modified with ferrocenecarboxylate to produce redox-active nanoparticles bearing approximately 240 ferrocene moieties per particle (Steinmetz et al. 2006b). Electrochemical measurements showed that the multiple, redox-active, organometallic centres behave as electronically isolated units. These studies have subsequently been refined and the effect of the length of the linker between the virus surface and the ferrocene on the properties of the resulting particles assessed (Aljabali et al. 2010a) (Fig. 3); the ability to vary this length will be of importance in the design of CPMV-based bioelectronic components and devices. The redox-active particles resulting from these studies may lead to the development of electron-transfer mediators in redox catalysis, amperometric biosensors, and eventually to nanoelectronic devices such as molecular batteries.
Fig. 3.

Redox-active ferrocene-CPMV nanoparticles prepared with various linker lengths and coupling strategies. (Aljabali et al. 2010a)
A further refinement of the use of addressable lysines has been the combination of their modification with advanced conjugation chemistries such as Cu(I)-catalysed azide-alkyne1,3-dipolar cycloaddition (CuCAAC) reactions (“click” chemistry). Click reactions are a particularly useful strategy for bioconjugation because of their specificity (Strable and Finn, 2009). This approach has been used to attach a variety of molecules, including fluorescent dyes (Meunier et al. 2004; Sen Gupta et al. 2005a), metal complexes (Prasuhn et al. 2007), sugars and polymers (Sen Gupta et al. 2005a, b; Kaltgrad et al. 2007; Astronomo et al. 2010), fullerenes (Steinmetz et al. 2009c) and chemically sensitive RNA fragments (Hong et al. 2009), to the surface of CPMV. The use of this bioorthogonal ligation chemistry with polyvalent display of carbohydrates on the virus surface is an efficient method for the production of anti-glycan polyclonal antibodies with the potential for applications in diagnostics and immunotherapeutics (Kaltgrad et al. 2007).
Though for many purposes modification of the majority of the lysine residues on the CPMV surface is appropriate, others require a greater degree of selectivity. To investigate the reactivity of the various lysines and to examine the possibility of selectively removing some of them, Chatterji et al. (2004) created a series of mutants in which the exposed lysines were substituted with arginines. The results showed that all the lysine residues identified as being exposed are, indeed, addressable and contribute to the overall reactivity of the virus particles. The ability of particles in which all but one of the exposed lysines had been eliminated to allow highly specific attachment of a foreign entity was demonstrated by reacting two mutants, each with a single lysine per asymmetric unit, with monosulfo-NHS-Nanogold followed by cryo-electron microscopy of the resulting conjugates. The electron density of the gold particles corresponded only to the position of single remaining lysine residue which differed in the two mutants (Chatterji et al. 2004).
As an alternative to addressing lysines, Steinmetz et al. (2006c) demonstrated that it is possible to couple the redox-active compound viologen via the surface-exposed carboxyl groups of aspartic and glutamic acids. Though examination of the virus surface indicated that each particle should have 480–540 addressable carboxyl groups only 180 viologen moieties per particle were added. Carboxylates have also been used to introduce ferrocenes (Aljabali et al. 2010a) (Fig. 3). The aromatic side-chain of surface-exposed tyrosine residues has also been investigated as an alternative to the more traditional sites for modification. Using the tripeptide NH2-Gly–Gly-His-COOH (GGH) in the presence of nickel acetate and magnesium monoperoxyphthalate, dityrosine crosslinks were introduced into the virus capsids and the procedure could also be used to make covalent attachments to the virion by trapping with a functionalised disulfide (Meunier et al. 2004). It is also possible to simultaneously address different functionalities of the CPMV surface. Recently, Brunel et al. (2010) have developed a hydrazone ligation strategy to produce targeted CPMV particles which display 133 copies of the VEGFR-1 ligand, 55 copies of a PEGylated peptide, and a total of 188 fluorescent dyes on the virus surface.
In addition to utilising naturally occurring amino acids on the CPMV surface, additional reactive sites can be introduced by mutagenesis. Thiol-addressable CPMV mutants with cysteine residues on the exterior surface were generated by insertion of cysteine residues at specific points on the virus surface (Wang et al. 2002c). The mutant particles reacted with monomaleimido-Nanogold and the derivatised particles imaged by cryo-electron microscopy. The gold particles could clearly be seen at the positions of the inserted cysteine residues, demonstrating that the CPMV particle can function as a convenient and programmable platform for chemical reactions (Wang et al. 2002c) (Fig. 4). Gold nano-particles attached to the surface of cysteine-substituted CPMV particles have been interconnected using molecular wires to create a 3-D conducting network (Blum et al. 2005). The cysteine-substituted mutants have also proved useful for the conjugation of a number of other moieties (Strable and Finn 2009). The presence of genetically introduced cysteine residues has, however, been found to promote aggregation of the virus, making preparations difficult to store and handle. To overcome this problem, a method of chemically introducing thiol groups has been developed by coupling the versatile, thiol-protected, N-succinimidyl-S-acetylthiopropionate to surface lysines. On deprotection, the thiol groups are reactive but the particles do not aggregate over several weeks (Steinmetz et al. 2007). A more complete description of the modifications which have been chemically introduced on CPMV particles can be found in Steinmetz and Evans (2007) and Strable and Finn (2009).
Fig. 4.

Cryoelectron microscopy of derivatised CPMV-Cys mutant. a Three-dimensional reconstruction of CPMV particles with 1.4 nm nanogold clusters. b Difference electron density map showing the nucleic acid (green) and the gold particles. c A pentameric section of the difference electron density map around the five-fold symmetry axis superimposed on the atomic model of CPMV showing that the gold is attached at the site of the Cys mutation. (Wang et al. 2002c, with permission, Copyright 2002 Wiley–VCH Verlag GmbH & Co. KGaA.)
All the studies described above have been conducted using particles, either wild-type or genetically modified, produced by the infection of plants. Approximately 90% of the particles produced in this way contain either RNA-1 or RNA-2 and the preparations retain their ability to infect plants and spread in the environment. In addition, while CPMV RNAs have not been shown to be capable of replication in mammalian cells, uptake of particles does occur both in vitro and in vivo (Steinmetz et al. 2009a), raising biosafety concerns. To address these issues, there have been attempts to inactivate or eliminate the viral RNAs within CPMV preparations either by irradiation with ultraviolet light (Langeveld et al. 2001; Rae et al. 2008) or chemically (Ochoa et al. 2006; Phelps et al. 2007). However, these processes have to be carefully monitored as they risk altering the structural properties of the particles and require that the virus retains its functionality to infect and spread in plants. The recent observation that co-inoculation of plants with the VP60 precursor to the L and S coat protein and the 24K viral proteinase results in the production of empty (RNA-free) CPMV capsids (Saunders et al. 2009) suggests a way around this problem. Thus many future studies involving the modification of the outer surface may well be conducted using particles produced in this manner rather than by infection. Furthermore, the empty particles produced in this manner could potentially be loaded with foreign “cargo” (see Sect. 3.1.5).
Cowpea Chlorotic Mottle Virus
Cowpea chlorotic mottle virus (CCMV) is a tripartite virus which has a capsid that consists of 180 identical coat protein subunits which form a spherical particle 28 nm in diameter. The CCMV capsid displays addressable lysines and carboxylates derived from aspartic and glutamic acid. Amine and carboxy-selective chemistry has been used to selectively attach fluorescent dyes to the virus surface, with approximately 540 lysine residues and 560 carboxylates being addressable (Gillitzer et al. 2002). It also proved possible to genetically introduce two solvent-exposed cysteines per CP into the virus capsid. Probing the resultant particles with thiol-selective dyes showed that approximately a third of the introduced thiols could be addressed. Subsequently a large diversity of ligands including fluorescent dyes, an organometallic photosensitiser, biotin, small peptides and even intact IgG antibodies can be effectively chemically linked to the exterior surface of CCMV, clearly illustrating that chemical modification is a generic approach to surface modification of the virus capsid (Gillitzer et al. 2002; Suci et al. 2007a, b). Further details of the chemical attachment of molecules to the surface of CCMV can be found in Steinmetz and Evans (2007) and Young et al. (2008).
A particular advantage of CCMV is the ability of the coat proteins subunits, isolated either from virus particles or through the expression of the CCMV coat protein in heterologous expression systems, to assemble into VLPs in vitro. Using this ability, a method has been developed that allows hybrid CCMV particles containing differentially modified subunits to be produced through mixed self-assembly (Gillitzer et al. 2006). To achieve this, CCMV particles were independently decorated with two different types of ligands to generate two populations of labelled virions. The two types of particles were disassembled in vitro and the resulting subunits separately purified. Reassembly was performed using varying ratios of the two types of subunits, thereby controlling the stoichiometries of the two ligands on the final assembled virions (Fig. 5).
Fig. 5.
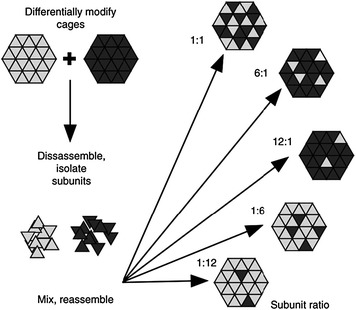
Schematic for the assembly of multifunctionalised Cowpea chlorotic mottle virus particles. (Gillitzer et al. 2006, with permission, Copyright 2006 Wiley–VCH Verlag GmbH & Co. KGaA.)
Turnip Yellow Mosaic Virus
Turnip yellow mosaic virus (TYMV) is the type member of the genus Tymovirus. Virions consist of 180 identical coat protein subunits per particle; however only 60 lysines per virus particle (one per 3 subunits) could be readily addressed using standard reagents (Barnhill et al. 2007). In addition, 90–120 carboxyl groups, located in the most exposed region of the coat protein, could also be modified.
Rod-Shaped Viruses
Tobacco Mosaic Virus
Particles of TMV consist of a single molecule of genomic RNA encapsidated by 2130 copies of the 17.5 kDa coat protein arranged with helical symmetry to form rigid particles 300nm in length. The subunits are largely α-helical, with the N- and C-termini being exposed on the outer virus surface. TMV is probably the highest yielding plant virus and thus its particles have attracted considerable interest for both peptide presentation via genetic modification and direct chemical modification. Most attempts to express foreign peptides via genetic fusion have focussed on the C-terminus of the coat protein in view of its exposed location. However, although TMV particles contain a large number of subunits, making the system potentially very attractive for peptide expression, it also creates a problem in that the subunits are very tightly packed, allowing little space on the virus surface for the expression of foreign sequences. A number of strategies have been developed to address this problem. Hamamoto et al. (1993) developed a TMV vector which permitted the synthesis of both native and C-terminally modified versions of the coat protein from the same viral RNA. This was achieved by engineering a leaky termination codon at the C-terminus of the coat protein gene. This system produced particles in plants in which up to 5% of the coat protein subunits were modified at their C-termini and has been used to express epitopes from several animal pathogens (Sugiyama et al. 1995; Turpen et al. 1995). Subsequently, by modifying the site of peptide insertion, TMV-based peptide presentation systems have been developed in which all the coat protein subunits could be modified to express foreign peptides without abolishing virus viability (Turpen et al. 1995; Fitchen et al. 1995). Using a similar approach, Koo et al. (1999) showed that mice immunised with a TMV chimaera expressing a peptide from the spike protein of the coronavirus, Murine hepatitis virus (MHV), were protected from subsequent challenge with the virus.
A problem with using direct fusions of peptides at or near the C-terminus of the TMV coat protein is that the size of inserts which can be tolerated seems to be quite small, the largest reported insert at this site being 23 amino acids (Bendahmane et al. 1999). However, this problem can be alleviated by modifying the C-terminus of the coat protein. Werner et al. (2006) found that a functional fragment of protein A, 133 amino acids in length, could be displayed on the surface of a close relative of TMV, the tobamovirus Turnip vein clearing virus (TVCV), if the sequence was fused to the C-terminus of the coat protein via a 15-amino acid linker. The protein A fragment was able to bind IgG suggesting that the modified virus particles could be used to purify antibodies. Given the success of TMV-based systems for peptide presentation, there has been considerable interest in the commercial development of the technology. For a description of such developments, the reader is referred to recent reviews by McCormick and Palmer (2008) and Smith et al. (2009).
The outer surface of wild-type TMV particles is somewhat devoid of chemically reactive amino acids such as cysteine and lysine. However, Schlick et al. (2005) reported the derivatisation of exterior-exposed tyrosine residues with diazonium salts, resulting in an acetophenone-functionalised conjugate that could react with a wide range of other molecules including PEG monomethyl ethers. To overcome the lack of reactive amino acid chains on the virus surface, several mutants displaying reactive cysteine or lysine residues on the solvent-exposed exterior of the virus have been made, allowing decoration via thiol- or amine-selective chemistry (Demir and Stowell 2002; Yi et al. 2005, 2007). However, in many cases the presence of these added residues adversely affected virus yield. To counteract this, Smith et al. (2006) screened a random collection of TMV mutants which had an additional four amino acids, including a single lysine, inserted near the N-terminus of the coat protein. By selecting those mutants which grew well, the authors were able to identify a particular mutant which could be used for the chemical coupling of a variety of epitopes (McCormick and Palmer 2008).
The external surface of TMV has also been used as a template for deposition reactions including the cocrystallisation of CdS and PbS, oxidative hydrolysis (iron oxides), and sol–gel condensation (SiO2) (Shenton et al. 1999; Fowler et al. 2001; Royston et al. 2009) and for the creation of metal nanoparticles and nanotubes (Dujardin et al. 2003; Knez et al. 2004a; Bromley et al. 2008). Such tubes can be grown from deposited clusters of palladium, platinum and gold on the exterior TMV surface and result in a metallic coat on the virion that serves as a basal layer for electroless deposition of other metals including nickel and cobalt. Royston et al. (2008) reported the deposition of nickel and cobalt on the exterior surface of the virus to create metallic coatings up to 40 nm in thickness. When the nickel-coated virions were incorporated into a nickel–zinc battery system, the electrode capacity of the battery more than doubled. For a more detailed review the reader is referred to Evans (2007).
Potato Virus X
Potato virus X (PVX) has filamentous particles consisting of approximately 1260 coat protein subunits encapsidating a single RNA molecule. Though an atomic resolution structure of the coat protein subunits is not available, the overall architecture of the viral particles is known (Kendall et al. 2008). It has proved possible to genetically fuse peptides to the surface- exposed N-terminus of either a proportion of or all of the subunits. To achieve partial modification, the sequence of the FMDV 2A catalytic peptide was inserted between the peptide and the N-terminus of the coat protein (Santa Cruz et al. 1996) such that both wild-type and N-terminally modified subunits can be produced from the same construct. This approach has the advantage that it potentially permits the expression of longer peptides, including whole proteins, than would be the case if all the subunits are modified. Using the ability of the 2A cleavage strategy to permit the fusion of whole proteins, Smolenska et al. (1998) expressed a single-chain antibody on the particle surface and showed that it retained its binding specificity. Carette et al. (2007) subsequently expressed the enzyme lipase B from Candida antarctica on the surface of the virus. These authors showed that the virus-anchored lipase molecules were catalytically active and suggested that it could act as an anchored biocatalyst.
Using an alternative approach of modifying all the subunits, Marusic et al. (2001) expressed a highly conserved hexapeptide epitope from gp41 of HIV-1 on PVX particles. Mice immunised with the chimaeric particles produced high levels of HIV-1-specific IgG and IgA. To determine the rules which govern the peptides that could be successfully propagated as fusions to the N-terminus of the PVX coat protein, Lico et al. (2006) undertook a detailed analysis of a large number of PVX chimaeras with N-terminal extensions of different lengths and amino acid composition. The results showed that the isoelectric points and tryptophan content of the inserted peptides had a profound influence on the growth of the chimaeras; this knowledge has subsequently been exploited to produce chimaeras in a predictable manner (e.g., Lico et al. 2009). However, there do appear to be occasions when modification of all the subunits is less successful than the partial modification strategy even for short peptides (Uhde-Holzem et al. 2010).
To examine the possibility of chemically modifying PVX, Steinmetz et al. (2010) conducted a detailed study of the reactivity of functional groups present on the surface of the particle. Each of the 1260 PVX CP subunits contains 11 lysine residues, 10 aspartic acid residues and 10 glutamic acid residues, all of which could potentially be modified if they were surface-exposed. In addition, the subunits of some strains of PVX are glycosylated, and the glycan moieties are also potential targets for chemical modification. Preliminary data indicated that none of the carboxylates were addressable under the conditions used and that the coat protein of the particular strain of PVX used for the experiments was not glycosylated. By contrast, lysine residues could be modified with an average of just over one lysine per subunit being modified. The utility of lysine residues for the introduction of PEG molecules of differing sizes and participation in click chemistry was subsequently investigated (Fig. 6).
Fig. 6.
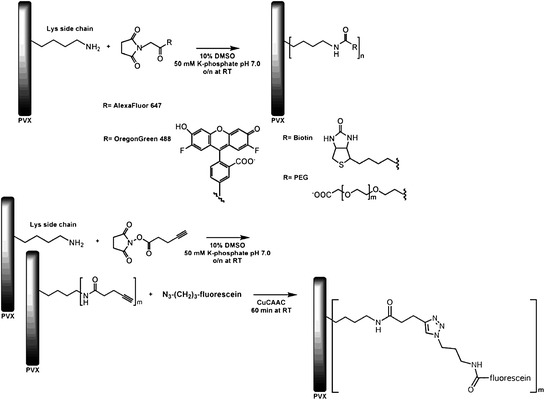
Chemical labelling of Potato virus X with fluorescent dyes and PEG chains using NHS ester-based chemistry or click reactions. (Steinmetz et al. 2010, with permission, Copyright 2010 American Chemical Society)
Utilising the Interior of Plant Virus Particles
The interiors of plant virus capsids potentially provide a nanosize environment for the packaging of foreign materials. There are essentially two approaches that can be taken to encapsulate foreign molecules within the capsid. In the first approach, the foreign molecules are incorporated into the particles during the capsid assembly process. In the second, the foreign molecules are introduced into preassembled particles. Most research has concentrated on utilising the enclosed space of a variety of icosahedral viruses; however the internal channel of TMV, which is open at both ends, has also be used for some specific purposes. In yet another type of application, the ability of the virus coat protein to package specific RNA molecules has been exploited.
Icosahedral Viruses
Cowpea Chlorotic Mottle Virus
The plant virus which has been most extensively used for interior modification is CCMV. Empty (RNA-free) particles of CCMV can be produced in vitro through the assembly of coat protein subunits. CCMV particles have a particular advantage for the encapsulation of foreign molecules as they undergo a pH- and cation-dependent structural transition that can be used to control the loading and release of such material. At pHs above 6.5 and in the absence of divalent cations, the CCMV capsid undergoes a reversible swelling which increases the diameter of the particles by about 10% and leads to the formation of 60 pores (Speir et al. 1995). The interior surface of wild-type particles carries a high positive charge density due to the presence of nine basic residues (arginine and lysine) in the amino-terminal region of each subunit. These positively charged residues normally interact with the negatively charged viral RNA. The positively charged interior surface and the availability of pores have been used to promote mineralisation within the preformed capsid to produce defined inorganic nanoparticles of anionic polyoxometallate salts (Douglas and Young 1998) or titania particles (Klem et al. 2008). The resulting nanoparticles were constrained in both size and shape by the interior dimensions of the CCMV virion. By a photoinitiated stepwise reaction, encapsulated precursor iron complexes within CCMV can be converted to monodisperse Prussian blue nanoparticles (de la Escosura et al. 2008). Further, these CCMV-internalised Prussian blue nanoparticles can be organised on mica and other substrates, assisted by the propensity of CCMV to self-assemble into hexagonal patterns, opening the way for exploitation of their magnetic and optical properties. Using heterologous expression, it has proved possible to produce CCMV particles with an altered interior charge. When the interior was made acidic, it proved possible to catalyse the formation of cationic transition metal oxides inside the particles when they were incubated with the appropriate cations (Douglas et al. 2002). In an alternative approach, the endogenous calcium-binding sites present at the interface of the coat protein subunits in the assembled CCMV capsid were used to bind up to 180 gadolinium (Gd3+) ions (Liepold et al. 2007). The binding of Gd3+ ions to the CCMV capsid results in particles which are paramagnetic and which have potential as MRI contrast agents in vivo.
The CCMV capsid has also shown to be capable of packaging enzymes and polymers. Comellas-Aragonès et al. (2007) demonstrated that one horseradish peroxidase (HRP) molecule, which is about 40 kDa, could be packaged within the CCMV capsid during an in vitro assembly reaction. The packaged HRP remained enzymatically active and substrate accessibility could be modulated by the pH-dependent swelling that CCMV capsids undergo. This result suggests that it may be possible to design nanoreactors based on controlled substrate access to encapsulated enzymes. Subsequently, it was shown (Comellas-Aragonès et al. 2009) that the synthetic polymer, polystyrene sulfonate, can be incorporated within capsids which were modified with PEG on their external surface. Most recently, a strategy has been developed for the self-assembly and loading of CCMV capsids using DNA amphiphiles. The amphiphiles aggregate into micelles with a hydrophobic core and an anionic DNA corona; these negatively charged particles induce capsid formation. By either hybridizing small molecules onto the micelles or incorporating them into the core, co-packaging of various small compounds can be achieved (Kwak et al. 2010). It is anticipated that after further development such nanocarriers may act as high-impact drug delivery systems.
Another potentially useful feature of CCMV is the ability of its coat protein to assemble into structures distinct from the normal virion. For example, it is possible to produce particles containing 60 or 120 subunits, as opposed to 180, by making deletions in the N-terminus of the coat protein (Tang et al. 2006). In an even more dramatic illustration, DNA has also been shown to nucleate the assembly of tube-like structures composed of CCMV coat protein dimers (Mukherjee et al. 2006).
Brome Mosaic Virus
Brome mosaic virus (BMV) is in the same genus as CCMV and BMV particles have similar structure and properties. BMV coat protein has been shown to be able to assemble around preformed gold nanoparticles provided there is a citrate layer between the gold and the protein surface (Dragnea et al. 2003). The encapsulation efficiency of the gold nanoparticles could be improved by coating them with carboxyl terminated polyethylene glycol (Chen et al. 2006); the size of the resultant capsid was found to be influenced by the diameter of the nanogold particle (Sun et al. 2007). Quantum dots have also been encapsulated within BMV particles using in vitro assembly reactions (Dixit et al. 2006). Fluorescence measurements of the material suggested that viral particles containing encapsulated quantum dots could be developed into high-performance luminescent probes. Using a similar approach, Huang et al. (2007) assembled BMV capsids around iron oxide nanotemplates. When the iron oxide core was larger than the inner cavity of native BMV, capsids larger than native BMV particles were obtained. The particles containing the iron oxide were superparamagnetic, suggesting that they could have applications in magnetic imaging and biosensing.
Red Clover Necrotic Mosaic Virus
Red clover necrotic mosaic virus (RCNMV) is a bipartite RNA virus which has particles consisting of 180 identical subunits arranged with icosahedral symmetry and with a diameter of approximately 36 nm. Virus particles are stabilised by an internal protein-RNA cage and their assembly is initiated with the recognition of an origin of assembly site on the viral RNA by the CP (Sit et al. 1998). By attaching an artificial origin of assembly sequence to a gold nanoparticle, it proved possible to achieve the in vitro encapsulation of the gold particle by the viral CP (Loo et al. 2006, 2007). Using this approach, it proved possible to encapsulate a range of gold core sizes. As suggested for BMV, the resultant material could be used for biosensing purposes and RCNMV has been proposed as a targeted particle for cancer treatment (Franzen and Lommel 2009).
Hibiscus Chlorotic Ringspot Virus
Hibiscus chlorotic ringspot virus (HCRSV) is a member of the genus Carmovirus. Virions have 28 nm particles which consist of 180 identical coat protein subunits; empty particles can be produced by the disassembly/reassembly of virions. Anionic polymers, such as polystyrenesulfonic acid and polyacrylic acid, but not neutrally charged dextran molecules, could be successfully loaded into these empty particles (Ren et al. 2006). Ren et al. (2007) made use of this phenomenon to co-encapsulate the anti-cancer drug doxorubicin with polystyrenesulfonic acid into HCRSV particles. To target the particles to cancerous cells, folic acid was conjugated to lysine residues on the outer virus surface. The resultant particles improved the uptake and cytotoxicity of doxorubicin to ovarian cancer cells suggesting that modified plant virus capsids may provide the basis for targeted drug delivery in cancer chemotherapy.
Cowpea Mosaic Virus
Until recently the interior cavity of CPMV particles has not been amenable to loading with foreign molecules as no in vitro assembly system has been available and the production of virions via the infection of plants results in the majority of particles containing the viral RNA. An initial attempt to produce RNA-free, loadable particles involved treating wild-type CPMV at high pH to eliminate the encapsidated virion RNAs (Ochoa et al. 2006). The potential utility of the resultant RNA-free particles was demonstrated by showing that cysteine residues on the inner capsid surface, which are normally occluded by the viral RNA, could now be labelled with a reporter dye. The alternative approach of producing empty particles within plants by the co-expression of VP60 and the 24K proteinase (Saunders et al. 2009) has recently been shown to be able to produce particles which are capable of being loaded with cobalt or iron oxide (Aljabali et al. 2010b). The presence of the metal within the particles allows them to be visualised by electron microscopy in the absence of negative stain. Importantly, the external surface amino acids of the loaded-virus capsid remain amenable to chemical modification (Fig. 7). The ability to both encapsulate materials, such as drugs and nanoparticles, within the capsid and to chemically modify the outer surface, opens up routes for the further development of these systems for the targeted delivery of therapeutic agents.
Fig. 7.
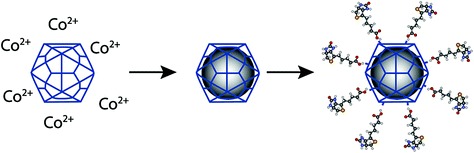
Schematic of the loading of an empty virus-like particle of CPMV and subsequent chemical decoration of the outer surface
The ability of CPMV to encapsidate different lengths of RNA has been exploited to render heterologous RNA highly resistant to degradation. It had previously been observed that RNA-2-containing particles can accommodate RNA-2 molecules with additional lengths of heterologous sequence. These observations have been used to design modified versions of RNA-2 harbouring pathogen-specific sequences that can act as positive controls in highly sensitive real-time PCR-based diagnostic reactions (King et al. 2007).
Rod-Shaped Viruses
Tobacco Mosaic Virus
TMV particles are hollow cylinders with an internal diameter of 4 nm. The interior channel is lined with aspartic and glutamic acid residues and these have been labelled with a variety of small molecules, such as biotin, using carbodiimide coupling reactions (Schlick et al. 2005). Nanowires consisting of bimetallic alloys of CoPt, CoPt3 and FePt3 with lengths up to 100 nm and 4 nm diameter have been synthesised within the TMV capsid channel (Tsukamoto et al. 2007) and the formation of small isolated nanoparticles of silver and nickel within the channel has also been reported (Dujardin et al. 2003). Copper nanowire of 3 nm diameter and up to 150 nm length can be formed within the internal channel by electroless deposition (Balci et al. 2006). Further, judicious choice of reaction conditions enables the channel to be filled with rod-shaped nickel or cobalt nanoparticles or with nickel or cobalt wires of 3 nm width and lengths of several hundred nanometres (Knez et al. 2003) (Fig. 8). Lengths greater than the virion length of 300 nm can be achieved, as the virion has a tendency to aggregate end-to-end, often reaching several micrometres.
Fig. 8.

a Transmission electron micrograph of Tobacco mosaic virus showing two adjacent virion aggregates filled with nickel wires. Inset: a single virion filled with a 200 nm long wire. b TMV containing a 200 nm long cobalt wire (Knez et al. 2003, with permission, Copyright 2003 American Chemical Society)
The encapsidation of TMV RNA by its coat protein is known to proceed from a defined sequence on the viral RNA, the origin of assembly. It has been known for some time that attachment of this sequence to a heterologous RNA will promote encapsidation of the foreign RNA by the coat protein (Gallie et al. 1987). Smith et al. (2007) exploited this phenomenon to deliver RNA encoding the non-structural proteins from Semliki forest virus into mammalian cells. They showed that the encapsidated RNA was uncoated, translated within the cells and stimulated an immune response in mice.
Creating Supramolecular Structures
A major aim of nanotechnology is to incorporate nano-sized components into small-scale devices. In the case of virus-based bionanotechnology, this involves the incorporation of modified viruses or virus-like particles into supramolecular structures, often by binding the particle to surfaces. An early example of the incorporation of a plant virus into a supramolecular structure was reported by Lvov et al. (1994) who incorporated the icosahedral particles of Carnation mottle virus (CarMV), the type member of the genus Carmovirus, into an alternating multilayered thin film. With the advent of methods for the genetic and chemical modification of particles, the range of structures that can be built up has steadily become more sophisticated.
Icosahedral Viruses
Cowpea Chlorotic Mottle Virus
CCMV particles have been immobilised on to surfaces with a view to constructing arrays. Immobilisation was achieved by either adsorption of cysteine-containing CCMV particles on to a gold surface (Klem et al. 2003) or via electrostatic interactions of the negatively charged capsids on to positively charged surfaces (Suci et al. 2005, 2006). Furthermore, multilayers consisting of CCMV particles immobilised on a solid support can be constructed using electrostatic interactions or the biotin–streptavidin interaction. The ability to construct thin films of CCMV could be coupled with the multivalent display of various molecules on the capsid surface and with the ability of the virus to encapsulate and release materials from the capsids in a pH-dependent manner. This could potentially lead to the development of semi-permeable functionalised membranes or controlled release coatings.
Cowpea Mosaic Virus
The first approach to the incorporation of modified CPMV particles into a larger structure involved immobilising particles expressing histidine residues (Medintz et al. 2005). In addition to producing continuous layers, single virus particle arrays have also been constructed (Cheung et al. 2003, 2006, 2010; Smith et al. 2003). Techniques such as microcontact patterning or scanning- and dip-pen nanolithography have been used to generate patterns of functional groups on solid supports that can subsequently be used for binding of the viral particles (Smith et al. 2003).
CPMV can be crystallised leading to the formation of 3-D arrays with ordering to near atomic precision. These crystals contain large solvent channels which can potentially be exploited to allow the diffusion of materials into the crystal interior. The channels have been utilised for confined and regular growth of metals such as palladium and platinum (Falkner et al. 2005). After stabilising the crystals by glutaraldehyde cross-linking, they were exposed to solutions that led to the formation of Pd and Pt within the crystal. Transmission electron microscopy studies confirmed that the formation of the metals was confined to the channels.
An alternative method of creating 3-D structures is to build up successive 2-D structures using a layer-by-layer (LbL) approach. To test the feasibility of this with CPMV, particles were covalently labelled with two different ligands: biotin to allow self-assembly via interaction with streptavidin, and fluorescent labels to enable the particles to be imaged (Steinmetz et al. 2006a). Attachment of the different functionalities was achieved via the modification of lysine side chains. The immobilisation of the CPMV particles on a solid support was achieved using either direct binding of cysteine-added mutants to a gold surface or indirectly by binding biotinylated particles mediated via streptavidin. To follow the LbL assembly, biotinylated particles were labelled with different dye molecules. Fluorescence microscopy imaging of the CPMV arrays was consistent with successful binding of successive layers of virus particles (Fig. 9). To study the properties of the multilayers, CPMV LbL assembly was followed in real time and in situ using quartz crystal microbalance with dissipation monitoring (QCMD; Steinmetz et al. 2008a). In particular, the effect of modifying the density of biotin molecules attached via longer and shorter linkers to the virus surface was investigated. These studies revealed that a more regular and densely packed array was produced when CPMV particles displaying a high number of biotin labels attached via the longer linker were assembled. It has also proved possible to build up multilayers of CPMV particles utilising electrostatic interactions (Steinmetz et al. 2008b). Ultrathin films of alternating layers of anionic and cationic polyelectrolytes were built up from a solid surface, by a LbL approach, finishing with a cationic layer. Negatively charged sphere-like CPMV particles were then immobilised on the polyelectrolyte surface. Additional layers of polyelectrolyte-CPMV-polyelectrolyte can readily be introduced (Fig. 10). The incorporation of functionalised virus nanoparticles and polyelectrolytes should lead to new routes for the construction of nano-structured tunable films.
Fig. 9.

Triple layer of CPMV particles on a gold surface. Fluorescence microscopy images (left) and diagrammatic representation of layer structures (right). The red and green flags show AlexaFluor dyes AF488 and AF568, respectively, and the black cross depicts streptavidin (Steinmetz et al. 2006a, with permission, Copyright 2006 American Chemical Society)
Fig. 10.

Scanning electron micrographs showing the sequential build-up of polyelectrolytes and CPMV particles. a Precursor polyelectrolyte thin film. b Initial polyelectrolyte layer coated with a layer of CPMV particles. c Coating of polyelectrolyte-CPMV layer with further polyelectrolyte layers. d Subsequent addition of another layer of CPMV particles (Evans 2007, Copyright 2007 with permission from Elsevier)
Rod-Shaped Viruses
Tobacco Mosaic Virus
The adsorption properties of TMV on various surfaces such as gold, mica, glass and silicon wafers have been investigated (Knez et al. 2004b) and a technique for rapid and large scale assembly of thin film coatings and ordered fibres consisting of aligned TMV particles has also been reported (Kuncicky et al. 2006). Yi et al. (2005, 2007) partially disassembled the coat protein from TMV particles to expose the RNA at the 5′ end of the rods. Oriented assembly of TMV on solid supports was then achieved in a controlled manner via nucleic acid hybridisation using complementary oligonucleotides and the immobilisation of fluorescently labelled TMV onto electrodes was also demonstrated. Furthermore, by using differentially labelled TMV particles and a micropatterned substrate, a patterned TMV microarray has been constructed. Attempts to incorporate TMV particles into multilayers using electrostatic interactions revealed that, unlike the spherical CPMV particles, the rods floated on top of the structures (Steinmetz et al. 2008b).
Conclusions
The past two decades have seen tremendous advances in the manipulation of plant virus particles, both genetically and chemically, and the investigations into the potential uses to which such modified particles can be put. However, at present all studies have been conducted at an academic level. Thus, a major challenge in the future will be the deployment of the technical advances in both the biotechnology and nanotechnology fields. For example, although it has been demonstrated that chimaeric plant virus particles can stimulate protective immunity in experimental animals, this technology has not been approved for use outside the laboratory. The same is true for the potential imaging agents based on the incorporation of foreign materials within particles. In a similar way, a major step in the adoption of plant virus particles in nanotechnology will require the demonstration that some type of device with unusual or highly desirable properties can be produced cost-effectively.
Contributor Information
Kenneth Palmer, Email: kenneth.palmer@louisville.edu.
Yuri Gleba, Phone: 491714903959, Email: gleba@nomadbioscience.com.
George P. Lomonossoff, Phone: +44-1603-450000, FAX: +44-1603-450018, Email: george.lomonossoff@jic.ac.uk
David J. Evans, Email: dave.evans@jic.ac.uk
References
- Aljabali AAA, Barclay JE, Butt JN, et al. Redox-active ferrocene-modified cowpea mosaic virus nanoparticles. Dalton Trans. 2010;39:7569–7574. doi: 10.1039/c0dt00495b. [DOI] [PubMed] [Google Scholar]
- Aljabali AAA, Sainsbury F, Lomonossoff GP, et al. Cowpea mosaic virus unmodified virus-like particles can be loaded with metal and metal oxide. Small. 2010;6:818–821. doi: 10.1002/smll.200902135. [DOI] [PubMed] [Google Scholar]
- Astronomo RD, Kaltgrad E, Udit AK, et al. Defining criteria for oligomannose immunogens for HIV using icosahedral virus capsid scaffolds. Chem Biol. 2010;17:357–370. doi: 10.1016/j.chembiol.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balci S, Bittner AM, Hahn K, et al. Copper nanowires within the central channel of tobacco mosaic virus. Electrochim Acta. 2006;51:6251–6357. [Google Scholar]
- Barnhill NH, Reuther R, Ferguson PL, et al. Turnip yellow mosaic virus as a chemoaddressable bionanoparticle. Bioconjugate Chem. 2007;18:852–859. doi: 10.1021/bc060391s. [DOI] [PubMed] [Google Scholar]
- Bendahmane M, Koo M, Karrer E, et al. Display of epitopes on the surface of tobacco mosaic virus: impact of charge and isoelectric point of the epitope on virus-host interactions. J Mol Biol. 1999;290:9–20. doi: 10.1006/jmbi.1999.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum AS, Soto CM, Wilson CD, et al. An engineered virus as a scaffold for three-dimensional self-assembly on the nanoscale. Small. 2005;1:702–706. doi: 10.1002/smll.200500021. [DOI] [PubMed] [Google Scholar]
- Brennan FR, Jones TD, Gilleland LB, et al. Pseudomonas aeruginosa outer-membrane protein F epitopes are highly immunogenic in mice when expressed on a plant virus. Microbiology. 1999;145:211–220. doi: 10.1099/13500872-145-1-211. [DOI] [PubMed] [Google Scholar]
- Bromley KM, Patil AJ, Perriman AW, et al. Preparation of high quality nanowires by tobacco mosaic virus templating of gold nanoparticles. J Mater Chem. 2008;18:4796–4801. [Google Scholar]
- Brunel FM, Lewis JD, Destito G, et al. Hydrazone ligation strategy to assemble multifunctional viral nanoparticles for cell imaging and tumor targeting. Nano Lett. 2010;10:1093–1097. doi: 10.1021/nl1002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañizares MC, Lomonossoff GP, Nicholson L. Development of cowpea mosaic virus-based vectors for the production of vaccines in plants. Expert Rev Vaccines. 2005;4:687–697. doi: 10.1586/14760584.4.5.687. [DOI] [PubMed] [Google Scholar]
- Carette N, Engelkamp H, Akpa E, et al. A virus-based catalyst. Nat Nanotechnol. 2007;2:226–229. doi: 10.1038/nnano.2007.76. [DOI] [PubMed] [Google Scholar]
- Chatterji A, Burns LL, Taylor SS, et al. Cowpea mosaic virus: from the presentation of antigenic peptides to the display of active biomaterials. Intervirology. 2002;45:362–370. doi: 10.1159/000067929. [DOI] [PubMed] [Google Scholar]
- Chatterji A, Ochoa W, Paine M, et al. New addresses on an addressable virus nanoblock: uniquely reactive lys residues on cowpea mosaic virus. Chem. Biol. 2004;11:855–863. doi: 10.1016/j.chembiol.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Chen C, Daniel MC, Quinkert ZT, et al. Nanoparticle-templated assembly of viral protein cages. Nano Lett. 2006;6:611–615. doi: 10.1021/nl0600878. [DOI] [PubMed] [Google Scholar]
- Cheung L, Camarero JA, Woods BW, et al. Fabrication of assembled virus nanostructures on templates of chemoselective linkers formed by scanning probe nanolithography. J Am Chem Soc. 2003;125:6848–6849. doi: 10.1021/ja034479h. [DOI] [PubMed] [Google Scholar]
- Cheung CL, Chung SW, Chatterji A, et al. Physical controls on directed virus assembly at nanoscale chemical templates. J Am Chem Soc. 2006;128:10801–10807. doi: 10.1021/ja0616884. [DOI] [PubMed] [Google Scholar]
- Cheung CL, Rubinstein AI, Peterson EJ, et al. Steric and electrostatic complementarity in the assembly of two-dimensional virus arrays. Langmuir. 2010;26:3498–3505. doi: 10.1021/la903114s. [DOI] [PubMed] [Google Scholar]
- Comellas-Aragonès M, Engelkamp H, Claessen VI, et al. A virus-based single-enzyme nanoreactor. Nat Nanotechnol. 2007;2:635–639. doi: 10.1038/nnano.2007.299. [DOI] [PubMed] [Google Scholar]
- Comellas-Aragonès M, de la Escosura A, Dirks AT, et al. Controlled integration of polymers into viral capsids. Biomacromolecules. 2009;10:3141–3147. doi: 10.1021/bm9007953. [DOI] [PubMed] [Google Scholar]
- Dalsgaard K, Uttenthal Å, Jones TD, et al. Plant-derived vaccine protects target animals against a virus disease. Nat Biotechnol. 1997;15:248–252. doi: 10.1038/nbt0397-248. [DOI] [PubMed] [Google Scholar]
- De la Escosura A, Verwegen M, Sikkema FD et al (2008) Viral capsids as templates for the production of monodisperse Prussian blue nanoparticles. Chem Commun 1542–1544 [DOI] [PubMed]
- Demir M, Stowell MHB. A chemoselective biomolecular template for assembling diverse nanotubular materials. Nanotechnology. 2002;13:541–544. [Google Scholar]
- Dixit SK, Goicochea NL, Daniel MC, et al. Quantum dot encapsulation in viral capsids. Nano Lett. 2006;6:1993–1999. doi: 10.1021/nl061165u. [DOI] [PubMed] [Google Scholar]
- Douglas T, Young M. Host–guest encapsulation of materials by assembled virus protein cages. Nature. 1998;393:152–55. [Google Scholar]
- Douglas T, Strable E, Willits D, et al. Protein engineering of a viral cage for constrained nanomaterials synthesis. Adv Mater. 2002;14:415–418. [Google Scholar]
- Dragnea B, Chen C, Kwak ES, et al. Gold nanoparticles as spectroscopic enhancers for in vitro studies on single viruses. J Am Chem Soc. 2003;125:6374–6375. doi: 10.1021/ja0343609. [DOI] [PubMed] [Google Scholar]
- Dujardin E, Peet C, Stubbs G, et al. Organization of metallic nanoparticles using tobacco mosaic virus templates. Nano Lett. 2003;3:413–417. [Google Scholar]
- Evans DJ. The bionanoscience of plant viruses: templates and synthons for new materials. J Mater Chem. 2007;18:3746–3754. [Google Scholar]
- Falkner JC, Turner ME, Bosworth JK, et al. Virus crystals as nanocomposite scaffolds. J Am Chem Soc. 2005;127:5274–5275. doi: 10.1021/ja044496m. [DOI] [PubMed] [Google Scholar]
- Fitchen J, Beachy RN, Hein MB. Plant virus expressing hybrid coat protein with added murine epitope elicits autoantibody response. Vaccine. 1995;13:1051–1057. doi: 10.1016/0264-410x(95)00075-c. [DOI] [PubMed] [Google Scholar]
- Fowler CE, Shenton W, Stubbs G, et al. Tobacco mosaic virus liquid crystals as templates for the interior design of silica mesophases and nanoparticles. Adv Mater. 2001;13:1266–1269. [Google Scholar]
- Franssen H, Goldbach R, Broekhuijsen M, et al. Expression of middle-component RNA of cowpea mosaic virus: in vitro generation of a precursor to both capsid proteins by a bottom-component RNA-encoded protease from infected cells. J Virol. 1982;41:8–17. doi: 10.1128/jvi.41.1.8-17.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen S, Lommel SA. Targeting cancer with ‘smart bombs’: equipping plant virus nanoparticles for a ‘seek and destroy’ mission. Nanomedicine. 2009;4:575–588. doi: 10.2217/nnm.09.23. [DOI] [PubMed] [Google Scholar]
- Gallie DR, Sleat DE, Watts JW, et al. In vivo uncoating and efficient expression of foreign mRNAs packaged in TMV-like particles. Science. 1987;236:1122–1124. doi: 10.1126/science.3472350. [DOI] [PubMed] [Google Scholar]
- Gillitzer E, Willits D, Young M, et al. Chemical modification of a viral cage for multivalent presentation. Chem Commun. 2002;2002:2390–2391. doi: 10.1039/b207853h. [DOI] [PubMed] [Google Scholar]
- Gillitzer E, Suci P, Young M, et al. Controlled ligand display on a symmetrical protein-cage architecture through mixed assembly. Small. 2006;2:962–966. doi: 10.1002/smll.200500433. [DOI] [PubMed] [Google Scholar]
- Hamamoto H, Sugiyama Y, Nakagawa N, et al. A new tobacco mosaic virus vector and its use for the systemic production of angiotensin-I-converting enzyme inhibitor in transgenic tobacco and tomato. Biotechnology. 1993;11:930–932. doi: 10.1038/nbt0893-930. [DOI] [PubMed] [Google Scholar]
- Hong V, Presolski SI, Ma C, et al. Analysis and optimisation of copper-catalysed azide-alkyne cycloadditions for bioconjugation. Angew Chem Int Ed. 2009;48:9879–9883. doi: 10.1002/anie.200905087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Bronstein LM, Retrum J, et al. Self-assembled virus-like particles with magnetic cores. Nano Lett. 2007;7:2407–2416. doi: 10.1021/nl071083l. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Lin T, Lomonossoff GP. Presentation of heterologous peptides on plant viruses: genetics, structures and function. Annu Rev Phytopathol. 1997;35:67–86. doi: 10.1146/annurev.phyto.35.1.67. [DOI] [PubMed] [Google Scholar]
- Kaltgrad E, Gupta SS, Punna S, et al. Anti-carbohydrate antibodies elicited by polyvalent display on a viral scaffold. ChemBioChem. 2007;8:1455–1462. doi: 10.1002/cbic.200700225. [DOI] [PubMed] [Google Scholar]
- Kendall A, McDonald M, Bian W, et al. Structure of flexible filamentous plant viruses. J Virol. 2008;82:9546–9554. doi: 10.1128/JVI.00895-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DP, Montague N, Ebert K, et al. Development of a novel recombinant encapsidated RNA particle: evaluation as an internal control for diagnostic RT-PCR. J Virol Meth. 2007;146:218–225. doi: 10.1016/j.jviromet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Klem MT, Willits D, Young M, et al. 2-D array formation of genetically engineered viral cages on Au surfaces and imaging by atomic force microscopy. J Am Chem Soc. 2003;125:10806–10807. doi: 10.1021/ja0363718. [DOI] [PubMed] [Google Scholar]
- Klem MT, Young M, Douglas T. Biomimetic synthesis of ß-TiO2 inside a viral capsid. J Mater Chem. 2008;18:3821–3823. [Google Scholar]
- Knez M, Bittner AM, Boes F, et al. Biotemplate synthesis of 3-nm nickel and cobalt nanowires. Nano Lett. 2003;3:1079–1082. [Google Scholar]
- Knez M, Sumser M, Bittner AM, et al. Spatially selective nucleation of metal clusters on the tobacco mosaic virus. Adv Funct Mater. 2004;14:116–124. [Google Scholar]
- Knez M, Sumser MP, Bittner AM, et al. Binding the tobacco mosaic virus to inorganic surfaces. Langmuir. 2004;20:441–447. doi: 10.1021/la035425o. [DOI] [PubMed] [Google Scholar]
- Koo M, Bendahmane M, Lettieri GA, et al. Protective immunity against murine hepatitis virus (MHV) induced by intranasal or subcutaneous administration of hybrids of tobacco mosaic virus that carries an MHV epitope. Proc Natl Acad Sci USA. 1999;96:7774–7779. doi: 10.1073/pnas.96.14.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koudelka KJ, Destito G, Plummer EM, et al. Endothelial targeting of cowpea mosaic virus (CPMV) via surface vimentin. PLoS Pathog. 2009;5:e1000417. doi: 10.1371/journal.ppat.1000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuncicky DM, Naik RR, Velev OD. Rapid deposition and long-range alignment of nanocoatings and arrays of electrically conductive wires from tobacco mosaic virus. Small. 2006;2:1462–1466. doi: 10.1002/smll.200600399. [DOI] [PubMed] [Google Scholar]
- Kwak M, Minten IJ, Anaya D-M. Virus-like particles templated by DNA micelles: a general method for loading virus nanocarriers. J Am Chem Soc. 2010;132:7834–7835. doi: 10.1021/ja101444j. [DOI] [PubMed] [Google Scholar]
- Langeveld JP, Brennan FR, Martinez-Torrecuadrada JL, et al. Inactivated recombinant plant virus protects dogs from a lethal challenge with canine parvovirus. Vaccine. 2001;19:3661–3670. doi: 10.1016/s0264-410x(01)00083-4. [DOI] [PubMed] [Google Scholar]
- Lewis JD, Destito G, Zijlstra A, et al. Viral nanoparticles as tools for intravital vascular imaging. Nat Med. 2006;12:354–360. doi: 10.1038/nm1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lico C, Capuano F, Renzone G, et al. Peptide display on potato virus X: molecular features of the coat protein-fused peptide affecting cell-to-cell and phloem movement of chimeric virus particles. J Gen Virol. 2006;87:3103–3112. doi: 10.1099/vir.0.82097-0. [DOI] [PubMed] [Google Scholar]
- Lico C, Mancinia C, Italiani P, et al. Plant-produced potato virus X chimeric particles displaying an influenza virus-derived peptide activate specific CD8+ T cells in mice. Vaccine. 2009;27:5069–5076. doi: 10.1016/j.vaccine.2009.06.045. [DOI] [PubMed] [Google Scholar]
- Liepold L, Anderson S, Willits D, et al. Viral capsids as MRI contrast agents. Magn Reson Med. 2007;58:871–879. doi: 10.1002/mrm.21307. [DOI] [PubMed] [Google Scholar]
- Lin T, Johnson JE. Structures of picorna-like plant viruses: implications and applications. Adv Virus Res. 2003;62:167–239. doi: 10.1016/s0065-3527(03)62004-x. [DOI] [PubMed] [Google Scholar]
- Lin T, Porta C, Lomonossoff G, et al. Structure-based design of peptide presentation on a viral surface: the crystal structure of a plant/animal virus chimaera at 2.8Å resolution. Folding and Design. 1996;1:179–187. doi: 10.1016/s1359-0278(96)00030-2. [DOI] [PubMed] [Google Scholar]
- Lomonossoff GP (2005) Antigen delivery systems: Use of recombinant plant viruses. In: Mestecky J, Bienenstock J, Lamm ME et al (eds) Mucosal Immunology, 3rd edn. Elsevier, pp 1061–1072
- Lomonossoff GP, Hamilton WDO. Cowpea mosaic virus-based vaccines. Curr Top Microbiol Immunol. 1999;240:177–189. doi: 10.1007/978-3-642-60234-4_9. [DOI] [PubMed] [Google Scholar]
- Lomonossoff GP, Johnson JE. Eukaryotic viral expression systems for polypeptides. Semin Virol. 1995;6:257–267. [Google Scholar]
- Lomonossoff GP, Johnson JE. Use of macromolecular assemblies as expression systems for peptides and synthetic vaccines. Curr Opin Struct Biol. 1996;6:176–182. doi: 10.1016/S0959-440X(96)80072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo L, Guenther RH, Basnayake VR, et al. Controlled encapsidation of gold nanoparticles by a viral protein shell. J Am Chem Soc. 2006;128:4502–4503. doi: 10.1021/ja057332u. [DOI] [PubMed] [Google Scholar]
- Loo L, Guenther RH, Lommel SA, et al. Encapsidation of nanoparticies by red clover necrotic mosaic virus. J Am Chem Soc. 2007;129:11111–11117. doi: 10.1021/ja071896b. [DOI] [PubMed] [Google Scholar]
- Lvov Y, Haas H, Decher G, et al. Successive deposition of alternate layers of polyelectrolytes and a charged virus. Langmuir. 1994;10:4232–4236. [Google Scholar]
- Marusic C, Rizza P, Lattanzi L, et al. Chimeric plant virus particles as immunogens for inducing murine and human immune responses against Human immunodeficiency virus type 1. J Virol. 2001;75:8434–8439. doi: 10.1128/JVI.75.18.8434-8439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick A, Palmer KE. Genetically engineered tobacco mosaic virus as nanoparticle vaccines. Expert Rev Vaccines. 2008;7:33–41. doi: 10.1586/14760584.7.1.33. [DOI] [PubMed] [Google Scholar]
- Medintz IL, Sapsford KE, Konnert JH, et al. Decoration of discretely immobilized cowpea mosaic virus with luminescent quantum dots. Langmuir. 2005;21:5501–5510. doi: 10.1021/la0468287. [DOI] [PubMed] [Google Scholar]
- Meunier S, Strable E, Finn MG. Crosslinking of and coupling to viral capsid proteins by tyrosine oxidation. Chem Biol. 2004;11:319–326. doi: 10.1016/j.chembiol.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Pfeifer CM, Johnson JM, et al. Redirecting the coat protein of a spherical virus to assemble into tubular nanostructures. J Am Chem Soc. 2006;128:2538–2539. doi: 10.1021/ja056656f. [DOI] [PubMed] [Google Scholar]
- Ochoa W, Chatterji A, Lin T, et al. Generation and structural analysis of reactive empty particles derived from an icosahedral virus. Chem Biol. 2006;13:771–778. doi: 10.1016/j.chembiol.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Phelps JP, Dang N, Rasochova L. Inactivation and purification of cowpea mosaic virus-like particles displaying peptide antigens from Bacillus anthracis. J Virol Meth. 2007;141:146–153. doi: 10.1016/j.jviromet.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta C, Spall VE, Loveland J, et al. Development of cowpea mosaic virus as a high-yielding system for the presentation of foreign peptides. Virology. 1994;202:949–955. doi: 10.1006/viro.1994.1417. [DOI] [PubMed] [Google Scholar]
- Porta C, Spall VE, Lin T, et al. The development of cowpea mosaic virus as a potential source of novel vaccines. Intervirol. 1996;39:79–84. doi: 10.1159/000150478. [DOI] [PubMed] [Google Scholar]
- Porta C, Spall VE, Findlay KC, et al. Cowpea mosaic virus-based chimaeras. Effects of inserted peptides on the phenotype, host-range and transmissibility of the modified viruses. Virology. 2003;310:50–63. doi: 10.1016/s0042-6822(03)00140-5. [DOI] [PubMed] [Google Scholar]
- Prasuhn DE, Yeh RM, Obenaus A et al (2007) Viral MRI contrast agents: coordination of Gd by native virions and attachment of Gd complexes by azide-alkyne cycloaddition. Chem Commun, pp 1269–1271 [DOI] [PubMed]
- Rae C, Koudelka KJ, Destito G, et al. Chemical addressability of ultraviolet-inactivated viral nanoparticles (VNPs) PLoS ONE. 2008;3:e3315. doi: 10.1371/journal.pone.0003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja KS, Wang Q, Gonzalez MJ, et al. Hybrid virus-polymer materials. 1. Synthesis and properties of PEG-decorated cowpea mosaic virus. Biomacromolecules. 2003;4:472–476. doi: 10.1021/bm025740+. [DOI] [PubMed] [Google Scholar]
- Wong Ren Y, S-M Lim L-Y. In vitro-reassembled plant virus-like particles for loading of polyacids. J Gen Virol. 2006;87:2749–2754. doi: 10.1099/vir.0.81944-0. [DOI] [PubMed] [Google Scholar]
- Ren Y, Wong S-M, Lim L-Y. Folic acid-conjugated protein cages of a plant virus: a novel delivery platform for doxorubicin. Bioconjug Chem. 2007;18:836–843. doi: 10.1021/bc060361p. [DOI] [PubMed] [Google Scholar]
- Royston ES, Ghosh A, Kofinas P, et al. Self-assembly of virus structured high surface area nanomaterials and their application as battery electrodes. Langmuir. 2008;24:906–912. doi: 10.1021/la7016424. [DOI] [PubMed] [Google Scholar]
- Royston ES, Brown AD, Harris MT, et al. Preparation of silica stabilized tobacco mosaic virus templates for the production of metal and layered nanoparticles. J Colloid Interface Sci. 2009;332:402–407. doi: 10.1016/j.jcis.2008.12.064. [DOI] [PubMed] [Google Scholar]
- Sainsbury F, Cañizares MC, Lomonossoff GP. Cowpea mosaic virus: the plant virus-based biotechnology workhorse. Annu Rev Phytopathol. 2010;48:437–455. doi: 10.1146/annurev-phyto-073009-114242. [DOI] [PubMed] [Google Scholar]
- Santa Cruz S, Chapman S, Roberts AG, et al. Assembly and movement of a plant virus carrying a green fluorescent protein overcoat. Proc Natl Acad Sci USA. 1996;93:6286–6290. doi: 10.1073/pnas.93.13.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders K, Sainsbury F, Lomonossoff GP. Efficient generation of cowpea mosaic virus empty virus-like particles by the proteolytic processing of precursors in insect cells and plants. Virology. 2009;393:329–337. doi: 10.1016/j.virol.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Schlick TL, Ding ZB, Kovacs EW, et al. Dual-surface modification of the tobacco mosaic virus. J Am Chem Soc. 2005;127:3718–3723. doi: 10.1021/ja046239n. [DOI] [PubMed] [Google Scholar]
- Sen Gupta S, Kuzelka J, Singh P, et al. Accelerated bioorthogonal conjugation: a practical method for the ligation of diverse functional molecules to a polyvalent virus scaffold. Bioconjug Chem. 2005;16:1572–1579. doi: 10.1021/bc050147l. [DOI] [PubMed] [Google Scholar]
- Sen Gupta S, Raja KS, Kaltgrad E, et al. Virus-glycopolymer conjugates by copper(I) catalysis of atom transfer radical polymerization and azide-alkyne cycloaddition. Chem Commun. 2005;14:4315–4317. doi: 10.1039/b502444g. [DOI] [PubMed] [Google Scholar]
- Shah SN, Steinmetz NF, Aljabali AAA et al (2009) Environmentally benign synthesis of virus-templated, monodisperse, iron-platinum nanoparticles. Dalton Trans, pp 8479–8480 [DOI] [PubMed]
- Shenton W, Douglas T, Young M, et al. Inorganic–organic nanotube composites from template mineralization of tobacco mosaic virus. Adv Mater. 1999;11:253–256. [Google Scholar]
- Singh P, Prasuhn D, Yeh RM, et al. Bio-distribution, toxicity and pathology of cowpea mosaic virus nanoparticles in vivo. J Control Release. 2007;120:41–50. doi: 10.1016/j.jconrel.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit TL, Vaewhongs AA, Lommel SA. RNA-mediated trans-activation of transcription from a viral RNA. Science. 1998;281:829–832. doi: 10.1126/science.281.5378.829. [DOI] [PubMed] [Google Scholar]
- Smith JC, Lee KB, Wang Q, et al. Nanopatterning the chemospecific immobilization of cowpea mosaic virus capsid. Nano Lett. 2003;3:883–886. [Google Scholar]
- Smith ML, Lindbo JA, Dillard-Telm S, et al. Modified tobacco mosaic virus particles as scaffolds for display of protein antigens for vaccine applications. Virology. 2006;348:475–488. doi: 10.1016/j.virol.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Smith ML, Corbo T, Bernales J, et al. Assembly of trans-encapsidated recombinant viral vectors engineered from tobacco mosaic virus and semliki forest virus and their evaluation as immunogens. Virology. 2007;358:321–333. doi: 10.1016/j.virol.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Smith ML, Fitzmaurice WP, Turpen TH, et al. Display of peptides on the surface of tobacco mosaic virus particles. Curr Top Microbiol Immunol. 2009;332:13–31. doi: 10.1007/978-3-540-70868-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolenska L, Roberts IM, Learmonth D, et al. Production of a functional single chain antibody attached to the surface of a plant virus. FEBS Lett. 1998;441:379–382. doi: 10.1016/s0014-5793(98)01586-5. [DOI] [PubMed] [Google Scholar]
- Speir JA, Munshi S, Wang G, et al. Structures of the native and swollen forms of cowpea chlorotic mottle virus determined by X-ray crystallography and cryo-electron microscopy. Structure. 1995;3:63–78. doi: 10.1016/s0969-2126(01)00135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz NF, Evans DJ. Utilisation of plant viruses in bionanotechnology. Org Biomol Chem. 2007;5:2891–2902. doi: 10.1039/b708175h. [DOI] [PubMed] [Google Scholar]
- Steinmetz NF, Manchester M. PEGylated viral nanoparticles for biomedicine: the impact of PEG chain length on VNP cell interactions in vitro and ex vivo. Biomacromolecules. 2009;13:10784–10792. doi: 10.1021/bm8012742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz NF, Calder G, Lomonossoff GP, et al. Plant viral capsids as nanobuilding blocks: construction of arrays on solid supports. Langmuir. 2006;22:10032–10037. doi: 10.1021/la0621362. [DOI] [PubMed] [Google Scholar]
- Steinmetz NF, Lomonossoff GP, Evans DJ. Decoration of cowpea mosaic virus with multiple, redox-active, organometallic complexes. Small. 2006;2:530–533. doi: 10.1002/smll.200500453. [DOI] [PubMed] [Google Scholar]
- Steinmetz NF, Lomonossoff GP, Evans DJ. Cowpea mosaic virus for material fabrication: addressable carboxylate groups on a programmable nanoscaffold. Langmuir. 2006;22:3488–3490. doi: 10.1021/la060078e. [DOI] [PubMed] [Google Scholar]
- Steinmetz NF, Evans DJ, Lomonossoff GP. Chemical introduction of reactive thiols into a viral nanoscaffold: a method which avoids virus aggregation. ChemBioChem. 2007;8:1131–1136. doi: 10.1002/cbic.200700126. [DOI] [PubMed] [Google Scholar]
- Steinmetz NF, Bock E, Richter RP, et al. Assembly of multilayer arrays of viral nanoparticles via biospecific recognition: a quartz crystal microbalance with dissipation monitoring study. Biomacromolecules. 2008;9:456–462. doi: 10.1021/bm700797b. [DOI] [PubMed] [Google Scholar]
- Steinmetz NF, Findlay KC, Noel TR, et al. Layer-by-layer assembly of viral nanoparticles and polyelectrolytes: The film architecture is different for spheres versus rods. ChemBioChem. 2008;9:1662–1670. doi: 10.1002/cbic.200800070. [DOI] [PubMed] [Google Scholar]
- Steinmetz NF, Lin T, Lomonossoff GP, et al. Structure-based engineering of an icosahedral virus for nanomedicine and nanotechnology. Curr Top Microbiol Immunol. 2009;327:23–58. doi: 10.1007/978-3-540-69379-6_2. [DOI] [PubMed] [Google Scholar]
- Steinmetz NF, Shah SN, Barclay JE, et al. Virus-templated silica nanoparticles. Small. 2009;5:813–816. doi: 10.1002/smll.200801348. [DOI] [PubMed] [Google Scholar]
- Steinmetz NF, Hong V, Spoerke ED, et al. Buckyballs meet viral nanoparticles: candidates for biomedicine. J Am Chem Soc. 2009;131:17093–17095. doi: 10.1021/ja902293w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz NF, Mertens ME, Taurog RE, et al. Potato virus X as a novel platform for potential biomedical applications. Nano Lett. 2010;10:305–312. doi: 10.1021/nl9035753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strable E, Finn MG. Chemical modification of viruses and virus-like particles. Curr Top Microbiol Immunol. 2009;332:1–21. doi: 10.1007/978-3-540-69379-6_1. [DOI] [PubMed] [Google Scholar]
- Suci PA, Klem MT, Douglas T, et al. Influence of electrostatic interactions on the surface adsorption of a viral protein cage. Langmuir. 2005;21:8686–8893. doi: 10.1021/la050217c. [DOI] [PubMed] [Google Scholar]
- Suci PA, Klem MT, Arce FT, et al. Assembly of multilayer films incorporating a viral protein cage architecture. Langmuir. 2006;22:8891–8896. doi: 10.1021/la0612062. [DOI] [PubMed] [Google Scholar]
- Suci PA, Berglund DL, Liepold L, et al. High-density targeting of a viral multifunctional nanoplatform to a pathogenic, biofilm-forming bacterium. Chem Biol. 2007;14:387–398. doi: 10.1016/j.chembiol.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Suci PA, Varpness Z, Gillitzer E, et al. Targeting and photodynamic killing of a microbial pathogen using protein cage architectures functionalized with a photosensitizer. Langmuir. 2007;23:12280–12286. doi: 10.1021/la7021424. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Hamamoto H, Takemoto S, et al. Systemic production of foreign peptides on the particle surface of Tobacco mosaic virus. FEBS Lett. 1995;359:47–250. doi: 10.1016/0014-5793(95)00054-d. [DOI] [PubMed] [Google Scholar]
- Sun J, DuFort C, Daniel MC, et al. Core-controlled polymorphism in virus-like particles. Proc Natl Acad Sci USA. 2007;104:1354–1359. doi: 10.1073/pnas.0610542104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Johnson JM, Dryden KA, et al. The role of subunit hinges and molecular “switches” in the control of viral capsid polymorphism. J Struct Biol. 2006;154:59–67. doi: 10.1016/j.jsb.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Taylor KM, Lin T, Porta C, et al. Influence of three-dimensional structure on the immunogenicity of a peptide expressed on the surface of a plant virus. J Mol Recognit. 2000;13:71–82. doi: 10.1002/(SICI)1099-1352(200003/04)13:2<71::AID-JMR489>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Tsukamoto R, Muraoka M, Seki M, et al. Synthesis of CoPt and FePt3 nanowires using the central channel of Tobacco mosaic virus as a biotemplate. Chem Mater. 2007;19:2389–2391. [Google Scholar]
- Turpen TH, Reinl SJ, Charoenvit Y, et al. Malarial epitopes expressed on the surface of recombinant Tobacco mosaic virus. Biotechnology. 1995;13:53–57. doi: 10.1038/nbt0195-53. [DOI] [PubMed] [Google Scholar]
- Uhde-Holzem K, Schlösser V, Viazov S, et al. Immunogenic properties of chimeric Potato virus X particles displaying the Hepatitis C virus hypervariable region I peptide R9. J Virol Meth. 2010;166:12–20. doi: 10.1016/j.jviromet.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Usha R, Rohll JB, Spall VE, et al. Expression of an animal virus antigenic site on the surface of a plant virus particle. Virology. 1993;197:366–374. doi: 10.1006/viro.1993.1598. [DOI] [PubMed] [Google Scholar]
- Wang Q, Kaltgrad E, Lin T, et al. Natural supramolecular building blocks: wild-type Cowpea mosaic virus. Chem Biol. 2002;9:805–811. doi: 10.1016/s1074-5521(02)00165-5. [DOI] [PubMed] [Google Scholar]
- Wang Q, Lin T, Tang L, et al. Icosahedral virus particles as addressable nanoscale building blocks. Angew Chem Int Ed. 2002;41:459–462. doi: 10.1002/1521-3773(20020201)41:3<459::aid-anie459>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Wang Q, Lin T, Johnson JE, et al. Natural supramolecular building blocks: cysteine-added mutants of cowpea mosaic virus. Chem Biol. 2002;9:813–819. doi: 10.1016/s1074-5521(02)00166-7. [DOI] [PubMed] [Google Scholar]
- Werner S, Marillonnet S, Hause G, et al. Immunoabsorbent nanoparticles based on a tobamovirus displaying protein A. Proc Natl Acad Sci USA. 2006;103:17678–17683. doi: 10.1073/pnas.0608869103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi HM, Nisar S, Lee SY, et al. Patterned assembly of genetically modified viral nanotemplates via nucleic acid hybridization. Nano Lett. 2005;5:1931–1936. doi: 10.1021/nl051254r. [DOI] [PubMed] [Google Scholar]
- Yi HM, Rubloff GW, Culver JN. TMV microarrays: hybridization-based assembly of DNA-programmed viral nanotemplates. Langmuir. 2007;23:2663–2667. doi: 10.1021/la062493c. [DOI] [PubMed] [Google Scholar]
- Young M, Willits D, Uchida M, et al. Plant viruses as biotemplates for materials and their use in nanotechnology. Annu Rev Phytopathol. 2008;46:361–384. doi: 10.1146/annurev.phyto.032508.131939. [DOI] [PubMed] [Google Scholar]


