Abstract
Saponins are one of the most numerous and diverse groups of plant natural products. They serve a range of ecological roles including plant defence against disease and herbivores and possibly as allelopathic agents in competitive interactions between plants. Some saponins are also important pharmaceuticals, and the underexplored biodiversity of plant saponins is likely to prove to be a vital resource for future drug discovery. The biological activity of saponins is normally attributed to the amphipathic properties of these molecules, which consist of a hydrophobic triterpene or sterol backbone and a hydrophilic carbohydrate chain, although some saponins are known to have potent biological activities that are dependent on other aspects of their structure. This chapter will focus on the biological activity and the synthesis of some of the best-studied examples of plant saponins and on recent developments in the identification of the genes and enzymes responsible for saponin synthesis.
Keywords: Triterpenes, Natural products, Plant defence, Pharmaceuticals, Biosynthesis, Oxidosqualene cyclase, Cytochrome p450, Acyltransferase, Glycosyltransferase
Introduction
Saponins are glycosides of triterpenes and steroids (Fig. 28.1). Steroidal glycoalkaloids are sometimes also referred to as saponins. The triterpene and steroid backbones are both derived from the mevalonic acid pathway, the common precursor being 2,3-oxidosqualene (Fig. 28.2). The name “saponin” derives from the soap-like properties of these compounds. The highly polar sugar moieties together with the non-polar triterpene or sterol backbones result in a highly amphipathic compound. Hence, these compounds produce stable foams, a feature often associated with aqueous extracts from saponin-accumulating plants (Hostettmann and Marston 1995). Indeed, the names of some plants originate from this property, such as soapwort (Saponaria officinalis), which was historically used as a source of detergent.
Fig. 28.1.
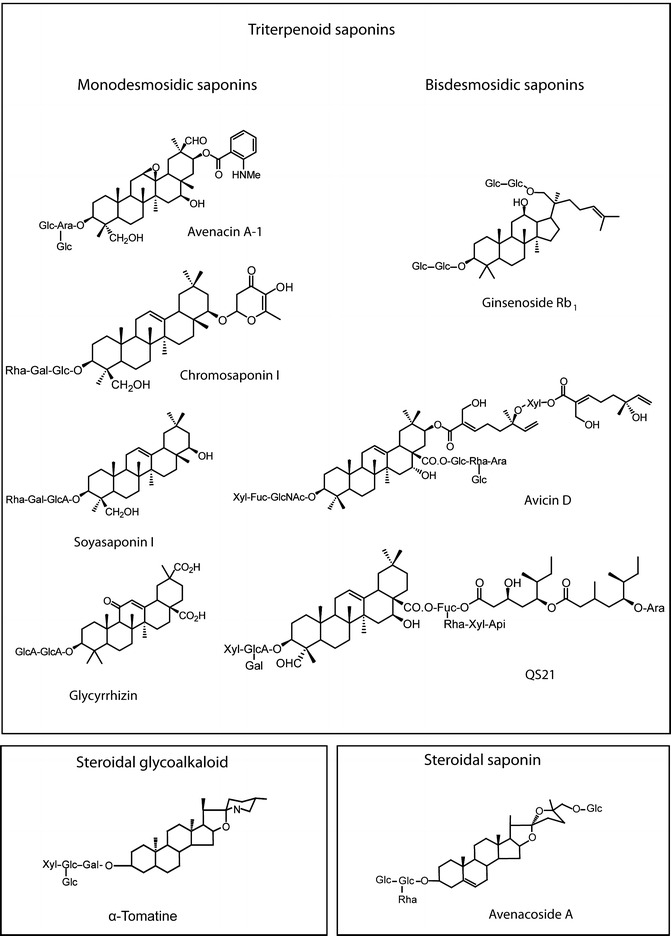
Structures of plant saponins. Triterpenoid saponins (top panel ): avenacin A-1 from oat roots (Avena spp.), chromosaponin I from pea seed (Pisum sativum), ginsenoside Rg1 from ginseng roots (Panax spp.), soyasaponin I (also known as soyasaponin Bb) from soya (Glycine max), avicin D from Acacia victoriae seed pods, glycyrrhizin from liquorice roots (Glycyrrhiza spp.) and QS21 from Quillaja saponaria bark. Examples of both monodesmosidic (one sugar chain) and bisdesmosidic (two sugar chains) saponins are shown. Bottom panels: Steroidal glycoalkaloid α-tomatine from tomato leaves (Solanum lycopersicum); the steroidal saponin avenacoside A from oat leaves (Avena spp.)
Fig. 28.2.
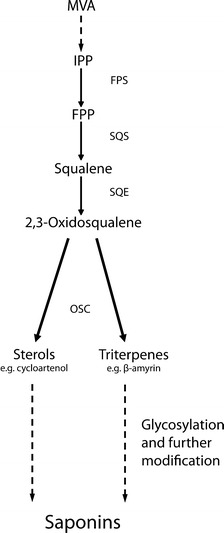
Saponin biosynthesis in plants. Farnesyl diphosphate (FPP) is synthesised from isopentyl diphosphate (IPP) by farnesyl diphosphate synthase (FPS). Squalene synthase (SQS) converts FPP to squalene, and squalene epoxidase (SQE) then oxidises squalene to produce 2,3-oxidosqualene. 2,3-Oxidosqualene serves as the substrate for a range of oxidosqualene cyclase (OSC) enzymes, including cycloartenol synthase for primary sterol synthesis and β-amyrin synthase. These enzymes are responsible for the synthesis of the major sterol and triterpene precursors of saponin biosynthesis, respectively. Triterpenes and sterols derived from 2,3 oxidosqualene are further elaborated by oxidative and other modifications, and by glycosylation, leading to the synthesis of saponins
Saponins represent a sizable proportion of the number of known plant natural products, which is in excess of 200,000 (Dixon 2001; Hartmann 2007; Osbourn et al. 2011). While plant natural products used to be regarded as waste products of a “luxurious metabolism”, they are now accepted as the products of natural selection with diverse biological activities and important ecological roles (Dixon 2001; Hartmann 2007). The structural diversity of saponins is reflected in the array of different biological activities associated with these compounds, and these diverse compounds provide a significant resource for drug and agrochemical discovery. Indeed, many plant-derived saponins are currently used as important pharmaceuticals in the treatment of a range of diseases in conventional and traditional medicine (Arase et al. 1997; Cinatl et al. 2003; Jayatilake et al. 2003; Germonprez et al. 2004; Harada 2005). Research into the functions and synthesis of saponins has provided a wealth of information on the properties of this important group of compounds, both for human use and in plants.
The basis of the diversity of saponins lies in several aspects of their structure (Fig. 28.1) (Hostettmann and Marston 1995). Firstly, the aglycone triterpenes and sterols themselves encompass a wide range of structures, with variation in the degree and nature of cyclization and oxidation of the backbone. Secondly, the nature of glycosylation is widely variable with respect to the number and type of sugar molecules, the types of inter-sugar linkages and the presence of one or more sugar chains. Monodesmosidic saponins have a single sugar chain attached at the C-3 position, while bidesmosidic saponins have an additional sugar chain at the C-28 (for triterpenoid saponins) or C-26 (steroid saponins) position. Further modifications of the saponin backbone give rise to even greater structural diversity, such as the addition of acyl- or ether-linked groups derived from organic acids (e.g. avenacin A-1, chromosaponin I, avicin D and QS21; Fig. 28.1) (Begley et al. 1986; Kudou et al. 1993; Yoshikawa et al. 1994, 1997, 2000, 2005; Germonprez et al. 2004; Zou et al. 2005).
The function and synthesis of saponins in plants will be discussed in this chapter, with particular focus on triterpenoid saponins and on the oat root triterpenoid saponins known as avenacins.
Function
Biological Activity of Saponins
As might be expected from their chemical diversity, saponins collectively have a wide range of biological activities. Many of these compounds have antimicrobial and/or anti-herbivore activity and so may have roles in plant defence (Osbourn 1996; Morrissey and Osbourn 1999; Francis et al. 2002; Friedman 2002, 2006; Sparg et al. 2004). Saponins also have a range of important pharmaceutical properties, for example, anti-inflammatory, antifungal, antibacterial, anti-parasitic, anti-cancer and antiviral activities (reviewed by Sparg et al. 2004; Podolak et al. 2010). Saponins have further applications in a range of industries extending beyond pharmaceuticals. Their surfactant properties are important in the beverage and cosmetics industries, and saponins are used as foaming agents for a variety of purposes including in fire extinguishers (Hostettmann and Marston 1995). In addition, some saponins are used as flavourings due to their intense sweetness or bitterness (Price et al. 1987; Grenby 1991; Kitagawa 2002; Heng et al. 2006). For example, the sweetness of liquorice root is attributable to the presence of the triterpenoid saponin glycyrrhizin (Kitagawa 2002).
Saponins generally act by permeabilising plasma membranes. Their amphipathic properties enable them to penetrate membranes, where they complex with sterols and cause pore formation (Roddick 1979; Roddick and Drysdale 1984; Steel and Drysdale 1988; Fenwick et al. 1992; Armah et al. 1999). While membrane permeabilisation is a common feature of saponins, these compounds are also likely to have further effects on cells, for example, by interfering with cellular processes, such as enzyme activities, transport, organelle integrity, redox-related functions and other signal transduction processes and through triggering apoptosis (e.g. McManus et al. 1993; Ohana et al. 1998; Sparg et al. 2004; Haridas et al. 2001a; Lemeshko et al. 2006). For some saponins, it has been shown that biological activity does not depend on amphipathicity, making it unlikely that their mode of action is through membrane permeabilisation (Oda et al. 2003; Simons et al. 2006).
The biological properties of saponins in the context of their ecological functions and commercial applications are discussed below.
Ecological Roles
Oat Saponins
Avenacins are triterpenoid saponins that are found in the tips of oat roots (Crombie et al. 1984; Crombie and Crombie 1986; Hostettmann and Marston 1995). Oats appear to be unique amongst the cereals in being able to synthesise saponins (Ohmoto and Ikuse 1970; Osbourn et al. 2003). There are four forms of avenacin. The major form (avenacin A-1) is shown in Fig. 28.1. Avenacins are oleane-type triterpenoids derived from β-amyrin (Begley et al. 1986; Haralampidis et al. 2002). β-Amyrin is elaborated by addition of various functional groups including hydroxyls and an epoxide and by addition of a branched trisaccharide chain consisting of one l-arabinose and two d-glucose molecules. In addition, avenacins are acylated at the C-21 carbon of the triterpene with either N-methyl anthranilate (avenacins A-1 and B-1) or benzoate (avenacins A-2 and B-2). The N-methylanthraniloyl acyl group confers bright blue fluorescence under UV illumination, and this fluorescence can be readily seen in the root tip. Avenacins are potent antifungal compounds and are effective against the fungal pathogen Gaeumannomyces graminis var. tritici, which is the casual agent of take-all disease (Papadopoulou et al. 1999). Take-all causes major yield losses in wheat crops throughout the world, and there is currently no effective means of control. In contrast, oats are highly resistant to infection by G. graminis. Around half a century ago, it was suggested that the resistance of oats to this disease might be associated with the blue fluorescent material in the root tips of oat plants, which was shown to be antifungal (Goodwin and Pollock 1954; Turner 1960). Isolation of the antifungal components of oat roots then led to the purification and structural identification of the avenacins (Burkhardt et al. 1964; Maizel et al. 1964). Osbourn et al. (1994) provided further evidence to highlight the importance of avenacins in resistance to take-all. Avenacins are only found in the Avena genus, and most oat species synthesise the compounds, suggesting that avenacins confer a selective advantage. One oat species, Avena longiglumis, was found to lack avenacins in its roots and was also shown to be susceptible to G. graminis var. tritici, while all other oat accessions investigated produced avenacins and were resistant to this pathogen. Further compelling evidence for a role for avenacins in plant defence came from the mutagenesis of a diploid avenacin-producing oat species (Avena strigosa), and the demonstration that avenacin-deficient mutants (isolated by screening for reduced root fluorescence) have enhanced susceptibility to a range of soil-borne fungal pathogens including G. graminis var. tritici (Papadopoulou et al. 1999).
Glycosylation of saponins is generally critical for antifungal activity (Sandrock and Van Etten 1998; Morrissey and Osbourn 1999). The loss of a single sugar from the oligosaccharide chain does not greatly reduce the amphipathicity of saponins but can impair the ability to complex with sterols (Arneson and Durbin 1967). Many fungi can hydrolyse sugars from saponins, thereby reducing antifungal activity (Sandrock and Van Etten 1998; Morrissey and Osbourn 1999). For example, the ability of an oat-attacking variant of the take-all fungus (G. graminis var. avenae) to infect oats is dependent on its ability to produce a saponin glycosyl hydrolase known as avenacinase (Bowyer et al. 1995). The various deglucosylated forms of avenacin have significantly reduced antifungal activity and reduced ability to complex membrane sterols. Examples of saponin glycosyl hydrolases have been reported from various other plant pathogenic fungi, including pathogens of oat leaves (which encounter the steroidal avenacosides) and of tomato (which encounter the steroidal glycoalkaloid α-tomatine) (Sandrock and Van Etten 1998; Morrissey and Osbourn 1999) (Fig. 28.1).
Like many plant secondary metabolites, avenacins are localised in the plant vacuole. An interesting insight into the role of saponin glycosylation in self-protection in plants was made recently during investigation of oat mutants that fail to fully glycosylate avenacins (Mylona et al. 2008). These mutants have stunted roots, a root hair-deficiency phenotype and membrane-trafficking defects. These defects were shown to be due to accumulation of the incompletely glucosylated avenacin intermediate. Thus, although this intermediate is less toxic to fungi (Turner 1961; Bowyer et al. 1995) and has a reduced capacity to cause permeabilisation of fungal membranes, it is toxic to plants cells. Glucosylation may be important for transport of avenacins to the vacuole. Consistent with this, the incompletely glucosylated avenacin intermediate has an atypical subcellular distribution and is not appropriately targeted to the vacuole. This suggests that vacuolar sequestration is an important self-protection mechanism (Mylona et al. 2008).
Avenacins are synthesised and accumulate in the epidermal cells of the root tip (Haralampidis et al. 2001). They are also released into the soil (Carter et al. 1999), although it is not clear whether this is an active process or a consequence of sloughing of the root epidermis. Carter et al. (1999) analysed fungi isolated from the roots of field-grown oat plants and found that many of these fungi were resistant to the toxic effects of avenacins and most were able to degrade these saponins. Thus, avenacins are likely to influence the growth of microorganisms in and around oat roots. The release of avenacins into the soil also has implications for competitive interactions between plants. Saponins from other plant species have been shown to have phytotoxic properties and as a consequence have been implicated in allelopathy (Oleszek and Jurzysta 1987; Waller et al. 1993; Hiradate et al. 1999; Li et al. 2004). Avenacins are phytotoxic and may therefore also have functions in suppression of the growth of neighbouring plants (Field et al. 2006). Oats are an important weed of other cereals, and understanding the basis of this competitive ability could lead to benefits for agriculture.
Triterpenoid Saponins from Legumes and Brassicaceae
Ecological roles for saponins have been identified in a range of other plant species. Soyasaponins, like avenacins, have a pentacyclic oleane triterpene skeleton. Members of the soyasaponin group of saponins are found in a variety of agriculturally important legumes (Hostettmann and Marston 1995; Yoshiki et al. 1998; Suzuki et al. 2002, 2005; Agrell et al. 2003), and several of these compounds have important pharmacological properties (Konoshima et al. 1992; Milgate and Roberts 1995; Dixon and Sumner 2003; Gurfinkel and Rao 2003). Soyasaponins are a diverse group of compounds that exist as mono- and bisdesmosidic forms (Hostettmann and Marston 1995). An example of a monodesmosidic soyasaponin is soyasaponin I (Fig. 28.1). In some bisdesmosidic legume saponins, the terminal monosaccharide of the C-22 sugar chain is modified by the addition of a γ-pyranoyl group (an ether-linked 2,3-dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP) group: Yoshiki et al. 1998; Tsurumi et al. 1992). One such γ-pyronyl saponin, chromosaponin I (Fig. 28.1), sometimes also referred to as soyasaponin VI (Hostettmann and Marston 1995), has been shown to have a growth-promoting effect on other plants (Tsurumi and Wada 1995; Tsurumi and Ishizawa 1997; Tsurumi et al. 2000) and is believed to exert its effects through regulation of auxin influx (Rahman et al. 2001). While chromosaponin I promotes plant growth, other legume saponins have been shown to suppress the growth of other plant species, an observation that is of particular relevance to organic farming methods in which these species are used as green fertiliser crops and that may explain why this practice can have a negative impact on subsequent crop yields (Oleszek and Jurzysta 1987; Waller et al. 1993; Hiradate et al. 1999; Li et al. 2004).
Accumulation of a variety of triterpenes including soyasaponin I (Fig. 28.1) (also known as soyasaponin Bb) and the related bidesmosidic saponin, medicagenic acid, occurs in response to fungal elicitors (Suzuki et al. 2002, 2005), wounding and herbivory in Medicago sativa (Agrell et al. 2003) and has been linked with plant defence. Similarly, soyasaponins have been shown to be major insecticidal and antifeedant components of pea seeds (Taylor et al. 2004) and are likely to protect these plants from herbivory by insects. Resistance to insect herbivores in the Brassicaceae is known to be mediated by glucosinolates (Bones and Rossiter 1996; Halkier and Gershenzon 2006). Some specialist herbivores are not affected by glucosinolate toxicity and use the compounds as a signal to stimulate oviposition on the plant (Huang et al. 1994). However, the ability of one brassicaceous species, Barbarea vulgaris, to resist attack by the specialist diamondback moth (Plutella xylostella) was found to be associated with the accumulation of a triterpenoid saponin in the plant (Shinoda et al. 2002). The saponin is associated with antifeedant effects towards the larva of P. xylostella and causes toxicity at high concentrations. Antifeedant activity and/or toxicity to insects have also been suggested for a number of other saponins (Hlywka et al. 1994; Agrell et al. 2003; Taylor et al. 2004) and may be an important component of plant defence. Saponins also impact on palatability for other animals including humans. For example, some saponins are used as sweeteners (Hayashi et al. 1999, 2001a). Conversely, other saponins have anti-sweet properties and are able to suppress the sweet taste of glucose (Yoshikawa et al. 1994, 1997, 2000). The flavour properties of soyasaponins have been investigated through the stimulation of the glossopharyngeal nerve of frogs by direct application of pure saponins (Yoshiki et al. 1998). Anti-herbivore activity and bitterness can also have detrimental consequences for agriculture (Francis et al. 2002), and saponins from legumes can reduce the ability of ruminant mammals to digest plant material (Milgate and Roberts 1995; Dixon and Sumner 2003).
Steroidal Glycoalkaloids from the Solanaceae
Many solanaceous species produce antifungal steroidal glycoalkaloid saponins. Tomato plants produce α-tomatine, a monodesmosidic steroidal glycoalkaloid with a tetrasaccharide side chain that accumulates in the leaves and immature fruit (Friedman 2002) (Fig. 28.1). α-Tomatine is fungitoxic and has been implicated in protection against fungal infection (Sandrock and Van Etten 1998; Morrissey and Osbourn 1999; Friedman 2002). As with other saponins, α-tomatine is toxic to a wide range of fungal species. Toxicity is generally ascribed to the ability of the saponin to complex with sterols and permeabilise fungal plasma membranes (Arneson and Durbin 1968a; Roddick 1979; Keukens et al. 1992, 1995). α-Tomatine has recently also been shown to induce reactive oxygen-mediated programmed cell death in fungi (Ito et al. 2007). Specialist pathogens of tomato generally have a higher level of resistance to α-tomatine when compared with fungi that do not infect tomato (Arneson and Durbin 1968b; Steel and Drysdale 1988; Suleman et al. 1996; Sandrock and Van Etten 1998; Morrissey and Osbourn 1999). The tomato leaf spot fungus, Septoria lycopersici, provides an interesting example of this plant-pathogen relationship. S. lycopersici is resistant to α-tomatine and is able to infect tomato plants. This fungus produces an α-tomatine-hydrolysing enzyme, tomatinase, which deglucosylates α-tomatine to the less toxic product β2-tomatine (Arneson and Durbin 1967). Tomatinase-deficient mutants of S. lycopersici (generated by insertional inactivation of the tomatinase gene) are unable to degrade α-tomatine and have enhanced sensitivity to this saponin. Such mutants are not compromised in their ability to cause disease on tomato leaves. However, they do trigger enhanced cell death and elevated expression of defence genes during early infection (Martin-Hernandez et al. 2000). Heterologous expression of S. lycopersici tomatinase in the phytopathogenic fungi Cladosporium fulvum and Nectria haematococca, both of which are normally unable to degrade α-tomatine, resulted in enhanced sporulation on tomato plants (Melton et al. 1998; Sandrock and Van Etten 2001), providing further evidence for a role for tomatinase in virulence. Another fungal pathogen of tomato, Fusarium oxysporum f. sp. lycopersici, produces a tomatinase enzyme that has a different mode of action to that of S. lycopersici and that hydrolyses α-tomatine to give the tetrasaccharide lycotetraose and the aglycone tomatidine. Gene silencing and targeted gene disruption experiments indicate that F. oxysporum f. sp. lycopersici tomatinase is required for full virulence on tomato (Ito et al. 2002; Pareja-Jaime et al. 2008). Importantly, the α-tomatine hydrolysis products β2-tomatine, tomatidine and lycotetraose have all been shown to suppress induced defence responses in tomato (Bourab et al. 2002; Ito et al. 2004, 2007), suggesting that hydrolysis of α-tomatine may serve a dual function during infection of tomato plants by fungi, namely, detoxification of a preformed toxin and subversion of the hydrolysis products for suppression of induced defences. Significantly, the α-tomatine aglycone, tomatidine, has been shown to inhibit sterol biosynthesis in yeast (Simons et al. 2006), although the mechanism by which tomatidine and other α-tomatine hydrolysis products interfere with induced plant defence responses is as yet unknown.
Roles in Human Health
Saponins are exploited as important pharmaceuticals and for a variety of other industrial uses. The triterpenoid ginsenoside saponins (e.g. ginsenoside Rb1; Fig. 28.1) are the major bioactive components of ginseng, the roots of which are widely used in traditional Chinese medicine. Ginsenosides have multiple pharmacological properties, including anti-tumour, immunomodulatory and neurological activity (Attele et al. 1999). The triterpenoid saponin from liquorice, glycyrrhizin (Fig. 28.1), also has wide-ranging medical uses. This compound has antiviral activity and is used in the treatment of hepatitis (Arase et al. 1997). Glycyrrhizin is also active against the HIV and SARS viruses (Cinatl et al. 2003; Harada 2005) and in addition has anti-inflammatory (Matsui et al. 2004), immunomodulatory (Takahara et al. 1994) and anti-ulcer activity (He et al. 2001). The main use of glycyrrhizin globally is, however, as a sweetener in the food industry (Kitagawa 2002). Avicins (e.g. avicin D; Fig. 28.1) are triterpenoid saponins from the Australian desert tree Acacia victoriae that have anti-tumour activity (Jayatilake et al. 2003) and are used in the treatment of cancer. Avicins have a range of physiological effects in mammalian cells including induction of apoptosis (Haridas et al. 2001a), suppression of inflammatory responses (Haridas et al. 2001b), inhibition of cell proliferation (Mujoo et al. 2001) and prevention of mutagenesis caused by environmental toxins (Hanausek et al. 2001), all of which may contribute to the anti-cancer properties of this compound. Two modes of action have been identified. Avicins permeabilise the mitochondrial outer membrane (Lemeshko et al. 2006; Haridas et al. 2007); they also covalently modify a transcription factor leading to modulation of responses to oxidative stress (Haridas et al. 2005). The main forms of avicin, such as avicin D, are acylated at the C-21 carbon with a group derived from two monoterpenes joined via a xylose (Fig. 28.1) (Jayatilake et al. 2003), and this group is essential for both modes of action (Haridas et al. 2005, 2007).
An important adjuvant used to improve the effectiveness of vaccines is a saponin derived from the bark of the South American tree Quillaja saponaria, known as QS21 (Kensil et al. 1991) (Fig. 28.1). A number of saponins have been shown to act as adjuvants (Barr et al. 1998; Oda et al. 2003). Interestingly, the most effective adjuvant saponins are bidesmosidic (Oda et al. 2003). This contrasts with other biological activities of saponins, which often depend on the amphipathic properties associated with monodesmosidic saponins. A comprehensive review of the pharmacological effects of saponins can be found in Sparg et al. (2004).
Saponins also have important dietary properties, and their presence in food crops has implications for human health. Saponins ingested as part of the human diet have been linked with a variety of effects on health, including reducing blood cholesterol levels (Milgate and Roberts 1995; Friedman 2002). The major steroidal glycoalkaloids found in potato are α-chaconine and α-solanine, which are monodesmosides of the steroidal alkaloid aglycone solanidine that differ only in the nature of the carbohydrate chain (Friedman 2006). Glycoalkaloids accumulate in potato tubers in response to insect damage (Hlywka et al. 1994) and also during post-harvest deterioration after exposure to light or as a result of physical damage (Mondy et al. 1987; Dao and Friedman 1994). α-Solanine and α-chaconine are inhibitors of acetylcholine esterase (Abbott et al. 1960; Roddick 1989), which is also the mode of action of many insecticides and can result in neurological symptoms in animals. Consumption of potatoes containing elevated levels of these glycoalkaloids can result in vomiting, diarrhoea, disorientation and death (Hansen 1925; McMillan and Thompson 1979; Korpan et al. 2004), and these symptoms are associated with reduced serum cholinesterase activity (McMillan and Thompson 1979).
The tomato steroidal glycoalkaloid α-tomatine accumulates to high levels in immature tomato fruits (up to 500 mg/kg of fresh fruit weight) (Friedman 2002). However, the consumption of immature tomatoes does not appear to cause symptoms, and likewise, tomato varieties that accumulate high levels of α-tomatine in the mature fruit do not appear to cause ill effects amongst the Peruvians who eat them (Rick et al. 1994), indicating that α-tomatine is not so toxic to humans as the potato glycoalkaloids. In fact, the steroidal glycoalkaloids from these solanaceous species have been found to have health-promoting effects. Both tomato and potato steroidal glycoalkaloids have antiproliferative effects against human cancer cell lines in vitro (Lee et al. 2004) and have also been shown to act as chemosensitisers, increasing the effectiveness of chemotherapeutic drugs by blocking their export through multi-drug resistance-type transport proteins (Lavie et al. 2001). In addition, α-tomatine has recently been shown to protect fish against tumours induced by an environmental toxin (Friedman et al. 2007).
Synthesis
Oxidosqualene Cyclization
Triterpenes and sterols are derived from a common precursor 2,3-oxidosqualene, which is synthesised from acetyl-CoA via mevalonic acid (MVA) and isopentyl diphosphate (IPP) (Haralampidis et al. 2002). Figure 28.2 shows an outline of saponin biosynthesis. The enzymes that convert 2,3-oxidosqualene into the precursors of more elaborate sterols and triterpenes belong to the oxidosqualene cyclase (OSC) family. The products of OSC enzymes are diverse, varying principally in the degree of cyclization (Haralampidis et al. 2002; Phillips et al. 2006; Lodeiro et al. 2007; Vincken et al. 2007; Abe 2007). Collectively, OSCs are capable of cyclising 2,3-oxidosqualene into a diverse range of different products, highlighting the importance of this single enzymatic step. Triterpenoid skeletons alone account for more than 200 different structures that have been described (Segura et al. 2003; Connolly and Hill 2007). The genes encoding the cycloartenol synthase enzyme (CAS) are widely conserved across plant lineages, consistent with the role of this enzyme in the synthesis of essential membrane sterols (Phillips et al. 2006). However, the OSC gene family has expanded and diversified in many plants, providing a molecular basis for triterpene diversity (Suzuki et al. 2002; Ebizuka et al. 2003; Phillips et al. 2006; Field and Osbourn 2008).
Some OSC enzymes produce single cyclization products, while others are multifunctional and generate a variety of different products (Kushiro et al. 2000a; Segura et al. 2000; Basyuni et al. 2006; Phillips et al. 2006; Lodeiro et al. 2007.; Shibuya et al. 2007; Abe 2007). Indeed, a single OSC enzyme from Arabidopsis thaliana was found to be responsible for the synthesis of at least nine distinct triterpenes when heterologously expressed in yeast (Kushiro et al. 2000a). Amongst the best-characterised members of the plant OSC family are the sterol synthase, cycloartenol synthase and the triterpene synthase, β-amyrin synthase (Haralampidis et al. 2002; Abe 2007). Synthesis of β-amyrin is the first committed step in the triterpene pathways leading to avenacins in oat, glycyrrhizin in liquorice and soyasaponins in soy (Fig. 28.1), and β-amyrin synthases have been cloned and characterised from these plant species (Chung et al. 1994; Hayashi et al. 2001a; Haralampidis et al. 2001; Shibuya et al. 2006). Numerous other plant OSC enzymes have also been characterised by heterologous expression in yeast (e.g. Kushiro et al. 2000a; Hayashi et al. 2001a, b; Kawano et al. 2002; Ebizuka et al. 2003; Zhang et al. 2003; Suzuki et al. 2006; Tansakul et al. 2006; Xiang et al. 2006; Shinozaki et al. 2008a, b; Abe 2007). Although there is as yet no crystal structure for plant OSCs, the structures of the related bacterial enzyme, squalene cyclase (Lenhart et al. 2002) and the human OSC lanosterol synthase (Thoma et al. 2004) have been determined empirically. Extensive work has also been carried out on investigating the mode of action of OSCs through site-directed mutagenesis and directed evolution (Dang and Prestwich 2000; Kushiro et al. 2000b; Sato and Hoshino 2001; Meyer et al. 2002; Wu and Griffin 2002; Segura et al. 2002; 2003; Wu and Chang 2004; Wu et al. 2005, 2006). In some cases, this knowledge has enabled the rational engineering of OSC enzymes to give altered product profiles (e.g. Kushiro et al. 2000b; Meyer et al. 2002; Lodeiro et al. 2004; Wu and Chang 2004).
Oxidative Modification of the Saponin Backbone
The diversity of triterpene and sterol saponin skeletons is not solely due to the variety of cyclization reactions but also to the further elaboration of the structure by oxidative modifications. All triterpenes and sterols have in common a C-3 hydroxyl group originating from the epoxide of 2,3 oxidosqualene, although some OSC enzymes can utilise the unusual dioxidosqualene substrate resulting in the synthesis of a product bearing two hydroxyl groups (Shan et al. 2005) (e.g. arabidiol, which is produced by the Arabidopsis thaliana arabidiol synthase enzyme (Xiang et al. 2006)). Also, some OSC enzymes can accept a range of artificial substrates leading to the synthesis of unusual compounds bearing multiple functional groups (Noma et al. 2004; Abe, this volume). Many saponin skeletons include multiple oxidative modifications that are introduced after cyclization, including further hydroxylation, desaturation and epoxidation (Begley et al. 1986; Yoshiki et al. 1998; Jayatilake 2003; Qi et al. 2006; Seki et al. 2008). Oxidation of sterols and triterpenes can influence their biological activity (Ji et al. 1990), although the functional significance of these types of modification for the action of saponins is apparent in only a few examples. The relevance of saponin skeleton modifications has been studied through the chemical modification of these compounds. Chemical modification of glycyrrhizin to alter the number of hydroxyl groups and positions of desaturations had significant impact on the inhibition of interleukins in human cell culture (Matsui et al. 2004). Also, in some cases, the comparison of the biological activities of naturally occurring compounds that differ specifically in these types of modification can reveal the importance of the modifications for activity. For example, the legume saponins avicins D and G differ only by one hydroxyl group, but this difference is sufficient to affect their respective abilities to inactivate caspase (Haridas et al. 2001b), although the hydroxyl group in question is associated with the acyl group and not with the triterpene skeleton. In some instances, this type of approach may also reveal that certain modifications have little impact on biological activity (Yoshikawa et al. 2005).
The avenacin pathway downstream of β-amyrin synthase involves a number of steps, including extensive oxidative modification of the triterpene ring structure. While plant OCS enzymes are relatively well characterised, the enzymes that catalyse the subsequent functionalisation of the triterpene backbone are only poorly understood (Haralampidis et al. 2002). A cytochrome P450 from oat (SAD2) has been shown to be required for avenacin synthesis and is likely to mediate oxygenation of β-amyrin at an as yet undetermined position (Qi et al. 2006). SAD2 belongs to the ancient and highly conserved CYP51 family of cytochrome P450s. Prior to the characterisation of SAD2, CYP51 enzymes were only known to function in the sterol pathway as sterol demethylases. SAD2 is the first CYP51 enzyme to be identified that has a different function – in the synthesis of defence-related triterpene glycosides (avenacins). This unusual CYP450 is the founder member of a monocot-specific divergent subfamily of CYP51 enzymes (defined as the CYP51H subfamily; Nelson et al. 2004). The functions of CYP51H enzymes in other cereals and grasses await investigation. It is possible that these enzymes may also have roles in plant defence.
Two other CYP450s that mediate modification of the triterpene backbones of saponins in other plant species have been identified through bioinformatics-based approaches. The soya CYP93E1 enzyme was identified using a combination of large-scale expressed sequence tag (EST) analysis and gene expression analysis to identify candidate genes involved in soyasaponin biosynthesis. This approach was facilitated by the fact that synthesis of soyasaponins can be induced by elicitor treatment (Shibuya et al. 2006). Candidate CYP450 enzymes identified in these experiments were functionally characterised by heterologous expression in yeast. The CYP93E1 enzyme was shown to catalyse the 24-hydroxylation of β-amyrin and also the formation of the 21-hydroxylated derivative, sophoradiol (Shibuya et al. 2006). A similar approach was used to characterise the triterpene-modifying CYP450 CYP88D6 from liquorice. CYP88D6 catalyses the oxidation of β-amyrin to 11-oxo-β-amyrin when expressed in yeast (Seki et al. 2008).
Glycosyltransferases
Glycosylation of saponins is generally important for the biological activity of these compounds, as discussed above. To date, few of the enzymes responsible for saponin glycosylation have been identified (Bowles et al. 2006; Townsend et al. 2006). Activity-based protein purification studies have been successfully applied to some saponin glycosyltransferases. For example, a UDP-galactose:tomatidine galactosyltransferase has been purified from tomato leaves (Zimowski 1994), and a solanidine glycosyltransferase (SGT) from potato has also been purified (Stapleton et al. 1991). These enzymes both catalyse the transfer of sugar moieties onto steroidal glycoalkaloid aglycones. Unlike triterpene aglycones, the steroidal alkaloid aglycones solanidine and tomatidine have potent antifungal activity (Moehs et al. 1997; Simons et al. 2006). This property was exploited to clone a solanidine glycosyltransferase (SGT) gene from potato by expressing a potato cDNA library in yeast, plating the transformants onto medium containing solanidine and looking for colonies that had increased solanidine tolerance (Moehs et al. 1997). This led to the cloning of the potato SGT1 enzyme, which catalysed the transfer of glucose to the solanidine aglycone (the first committed step in the synthesis of the steroidal glycoalkaloid, α-chaconine) in vitro. The role of SGT in steroidal glycoalkaloid biosynthesis in planta was investigated through antisense RNA-mediated gene silencing (McCue et al. 2005). Silencing of SGT1 did not result in the expected reduction in α-chaconine levels. However, the levels of a different steroidal glycoalkaloid, α-solanine, were substantially reduced in SGT1-silenced plants, suggesting that SGT1 functions as a galactosyltransferase in planta and not as a glucosyltransferase. Indeed, subsequent biochemical characterisation showed that the SGT1 enzyme has a preference for UDP-galactose over UDP-glucose in vitro (McCue et al. 2005). A second potato glycosyltransferase SGT2 was subsequently identified as the primary UDP-glucose:solanidine glucosyltransferase (McCue et al. 2006). More recently, a rhamnosyltransferase implicated in the extension of the sugar chains of solanidine-derived steroidal glycoalkaloids has also been reported (McCue et al. 2007). Solanidine glycosyltransferases have been characterised from aubergine (eggplant; Solanum melongena) (Paczkowski et al. 1997; Zimowski 1997). Also, a steroidal saponin glucosyltransferase has been purified from the medicinal herb Withania somnifera, and the corresponding cDNA cloned (Madina et al. 2007).
Bioinformatics-based approaches to the identification of saponin glycosyltransferases have also been adopted. Medicago truncatula accumulates a range of triterpene saponins in response to elicitor treatment (Suzuki et al. 2002, 2005). Transcriptome analysis has been used to identify transcripts for predicted glycosyltransferases on the basis of co-expression with a cloned β-amyrin synthase gene. Subsequent biochemical characterisation revealed that two glycosyltransferases identified in this way were able to catalyse the glucosylation of triterpene aglycones (Suzuki et al. 2002, 2005; Achnine et al. 2005) and a crystal structure has been obtained for one of these enzymes (Shao et al. 2005). However, these glycosyltransferases had very broad substrate specificity in vitro and were able to glycosylate phenolic compounds more effectively than saponins (Suzuki et al. 2005; Shao et al. 2005). This highlights a common concern associated with analysis of the properties of enzymes in vitro – namely, that data gained from in vitro studies may not reflect the true properties of these enzymes in planta (Suzuki et al. 2005; Bowles et al. 2006). Comparison of the kinetic properties of enzymes in vitro with the availability of substrates in planta offers one means of providing further evidence of likely function in plants. However, the presence of other compounds in planta may modulate the substrate specificity of glycosyltransferases. For example, phospholipids of varying types, while not serving as substrates, have been shown to markedly alter the substrate preference of the aubergine solanidine glycosyltransferase (Paczkowski et al. 2001). It is interesting to note that the glycosyltransferases from M. truncatula that have been implicated in saponin glycosylation are phylogenetically distinct, and each shows similarity to different characterised glycosyltransferases from other species that act on quite different groups of compounds (Gachon et al. 2005).
Acylation of Saponins
Avenacins are acylated at the C-21 carbon with N-methyl anthranilate or benzoate (e.g. avenacin A-1; Fig. 28.1). Acylation at the C-21 position in particular has been suggested to be an important factor in the biological activity of saponins (Podolak et al. 2010). Acylation of avenacins is catalysed by a serine carboxypeptidase-like (SCPL) enzyme (AsSCPL1) which is encoded by the Sad7 gene (Mugford et al. 2009; Mugford and Osbourn 2010). SCPL acyltransferases have previously been identified in dicotyledonous species with roles in the acylation of a range of plant natural products (Milkowski and Strack 2004). Acyl groups have been identified in a number of saponins from a range of plant species (Fig. 28.1) (Warashina et al. 1991; Kudou et al. 1993; Yoshikawa et al. 1994, 1997, 2000; Yoshikawa M et al. 2005; Germonprez et al. 2004; Zou et al. 2005). Examples of triterpenoid saponins acylated with aromatic side chains are found in purple salsify (Tragopogon porrifolius) (Warashina et al. 1991), Stephanotis lutchuensis (Yoshikawa et al. 1994, 1997) and the Vietnamese medicinal species Maesa balansae (Germonprez et al. 2004). Warashina et al. (1991) isolated 18 tragopogonsaponins – all glycosides of echinocystic acid acylated with the phenylpropanoids p-coumarate, ferulate, 4-hydroxyphenyl proponoate or 4-hydroxy, 3-methoxyphenyl proponoate. Yoshikawa et al. (1994, 1997) have identified a number of anti-sweet acylated triterpenoid saponins – sitakisosides – including some that, like avenacin A-1, are acylated with N-methyl anthranilate. Germonprez et al. (2004) identified five forms of triterpenoid saponins from Maesa balansae, collectively known as maesabalides, which contain cinnamate and benzoate acyl groups. The maesabalides were found to exhibit anti-leishmanial activity.
Some saponin acyl groups have been ascribed a biological function through the comparison of acylated saponins and their unacylated counterparts. Avenacin-deficient oat mutants that are defective in avenacin acylation have been identified (Papadopoulou et al. 1999; Qi et al. 2004). These mutants have enhanced susceptibility to fungal pathogens, indicating that acylation is important for disease resistance, although the significance of this modification for the stability and antifungal activity of avenacins is not yet known. Biological activity of theasaponins from tea (Camellia sinensis) has been shown to be dependent upon acylation. Theasaponins are acylated at both the C-21 and C-22 positions by angelate or tiglate ((Z)-or (E)-2-methylbut-2-enoate, respectively) groups (Yoshikawa et al. 2005). Yoshikawa et al. (2005) showed that the gastro-protective effect offered by these compounds against ethanol toxicity was dependent on the presence of these acyl groups. The α-pyranosyl triterpenoid saponin chromosaponin I from pea is conjugated with a 2,3- dihydro-2,5-dihydroxy-6-methyl-4 H-pyran-4-one (DDMP) group by an ether linkage (Fig. 28.1). In addition to its effects on plant growth and development (Tsurumi and Ishizawa 1997, 2000), chromosaponin I also has strong antioxidative capacity (Tsujino et al. 1994). Soya also produces triterpenoid saponins conjugated with DDMP, and the antioxidative capacity of these compounds has been shown to be largely due to the presence of the DDMP group (Yoshiki et al. 1998), suggesting an important contribution of DDMP modification to saponin activity.
The different steps in the synthesis of saponins are likely to occur in different subcellular locations. The early steps in saponin synthesis (mediated by OSC and CYP450 enzymes) are most probably associated with the endoplasmic reticulum (Ruf et al. 2004; Qi et al. 2006; Seki et al. 2008), while glycosyltransferases are typically found in the cytoplasm (Bowles et al. 2006). However, avenacins are sequestered in the vacuole (Mylona et al. 2008), suggesting that at least one transport step is required for their synthesis. Future work should lead to the identification of transporters that are required for saponin synthesis and accumulation.
Genetics and Evolution
While biological activities have been ascribed for many saponins, the demonstration of the importance of these compounds in planta is a difficult matter to resolve, requiring isogenic (or near isogenic) lines that differ solely in ability/inability to produce saponins. So far, the application of reverse genetics-based approaches for investigation of saponin biosynthesis and function has been limited. Transgenic potato plants that have reduced α-solanine content by antisense-mediated silencing of the solanidine glycosyltransferase gene SGT1 have been generated (McCue et al. 2005). This work identified a different function for the enzyme than had been predicted from biochemical analysis in vitro, highlighting the importance of genetic tests of function in planta. These findings are of commercial relevance since they open up opportunities for reducing steroidal glycoalkaloid levels in plants with ensuing benefits for human health. The impact of this modification for broader environmental interactions between potato plants and other organisms was not investigated.
The strong blue fluorescence of avenacin A-1 in oat root tips under UV illumination enables the direct visualisation of the presence of the compound in planta and has provided a facile screen for isolation of avenacin-deficient oat mutants (Papadopoulou et al. 1999). A total of 92 saponin-deficient (sad) mutants with reduced root fluorescence have been identified to date (Papadopoulou et al. 1999; Qi et al. 2006; Qin et al. 2010). These mutants, which represent at least six independent saponin biosynthesis (sad ) loci, have enhanced susceptibility to disease, consistent with a role for avenacins in plant protection (Papadopoulou et al. 1999). Sad1 encodes β-amyrin synthase, the OSC that catalyses the first committed step in avenacin synthesis (Haralampidis et al. 2001). Remarkably, four of the other loci that have been defined by genetic analysis as being required for avenacin synthesis co-segregate with Sad1, indicating that the avenacin biosynthetic genes are clustered (Qi et al. 2004). Physical clustering of avenacin pathway genes was confirmed by the recent cloning of Sad2, (Qi et al. 2006) and of Sad7 (Mugford et al. 2009). The three genes are adjacent and lie within 140 kb of each other.
The finding that the genes for the avenacin pathway form an operon-like gene cluster was surprising, given our current understanding of eukaryotic genome organisation (Osbourn 2010). Metabolic gene clusters are common amongst the fungi, where there are many examples of gene clusters for natural product pathways (Keller and Hohn 1996; Bok et al. 2006; Keller et al. 2005). However, there are an increasing number of examples of gene clusters for metabolic pathways in plants. Other examples include the cyclic hydroxamic acid (2,4-dihydroxy-1,4-benzoxazin-3-one, DIBOA) pathway in maize (Frey et al. 1995, 1997; Gierl and Frey 2001) and the diterpenoid momilactone cluster in rice (Shimura et al. 2007). Both DIBOA and momilactones are implicated in plant defence. Gene clusters for triterpenoid synthesis have also been recently discovered in the model plant Arabidopsis thaliana (Field and Osbourn 2008; Field et al. 2011). Significantly, the triterpenoid gene clusters in oat and Arabidopsis have evolved recently and independently, suggesting that there is selection for clustering of genes for triterpenoid pathways (Qi et al. 2004; Field and Osbourn 2008; Osbourn 2010). Gene clustering will favour inheritance of the genes for the pathway in its entirety by minimising the chances of recombination occurring within the cluster during meiosis, which will provide a selective advantage if the pathway end products confer broad-spectrum disease resistance. There is also evidence to indicate that interference with the integrity of natural product gene clusters can lead to the formation of toxic intermediates (Mylona et al. 2008), so providing further selection pressure to maintain the cluster as a whole. Similarly, the biosynthetic intermediates of some other plant saponins are known to exhibit toxicity to fungi at least and may also have phytotoxic effects (Moehs et al. 1997; Simons et al. 2006). It has been observed that the early steps in several of the pathways encoded by known gene clusters are closely related to various hormone biosynthetic pathways and that this might be a factor in the evolution of the clusters (Chu et al. 2011). Additionally, the clustering of genes may facilitate tight co-ordinate regulation of gene expression at the level of chromatin (Qi et al. 2006; Shimura et al. 2007; Field and Osbourn 2008). The genes belonging to these metabolic clusters do show highly co-ordinated expression. For example, expression of the rice momilactone genes is co-ordinately induced upon treatment with elicitors or UV light (Shimura et al. 2007). The oat avenacin gene cluster and the A. thaliana thalianol gene cluster are both co-ordinately regulated in specific cell types within the roots and are expressed during normal growth and development (Qi et al. 2004, 2006; Field and Osbourn 2008). The genes within the 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one (DIMBOA) gene cluster in maize are expressed in the shoots of young seedlings (Frey et al. 1995), although some members of this gene cluster are also expressed in other tissues (von Rad et al. 2001). However, one would expect the genes required for metabolic pathways to be co-ordinately regulated regardless of physical clustering, and there are many instances of co-ordinate regulation of genes that are not clustered but that belong to a single metabolic pathway (e.g. Hemm et al. 2004). Thus, gene clustering is not essential for co-ordinate expression.
Research into the chemical diversity of saponins is well documented (Hostettmann and Marston 1995), and the molecular genetics underlying the biosynthesis of these compounds is gaining ground. Forward genetics, bioinformatics-based approaches and genome browsing have led to the characterisation of new saponin biosynthetic genes and enzymes (Papadopoulou et al. 1999; Suzuki et al. 2002, 2005; Qi et al. 2004, 2006; Achnine et al. 2005; Seki et al. 2008). The current acceleration in gene discovery will yield biotechnological toolkits (genes, enzymes, regulators, transporters) that will be invaluable in designing strategies for quantitative and qualitative manipulation of saponin content in plants and for production of high-value compounds for commercial use. Future work will also shed further light on the ecological significance of saponins, on the relationship between structure and biological activity and on the mechanisms through which these compounds exert their effects on living cells.
Contributor Information
Thomas J. Bach, Email: bach@unistra.fr
Michel Rohmer, Email: mirohmer@unistra.fr.
Anne Osbourn, Email: anne.osbourn@bbsrc.ac.uk.
References
- Abbott DG, Field K, Johnson EI. Observation on the correlation of anticholinesterase effect with solanine content of potatoes. Analyst. 1960;85:375–377. [Google Scholar]
- Abe I. Enzymatic synthesis of cyclic triterpenes. Nat Prod Rep. 2007;24:1311. doi: 10.1039/b616857b. [DOI] [PubMed] [Google Scholar]
- Achnine L, Huhman DV, Farag MA, Sumner LW, Blount JW, Dixon RA. Genomics-based selection and functional characterization of triterpene glycosyltransferases from the model legume Medicago truncatula. Plant J. 2005;41:875–887. doi: 10.1111/j.1365-313X.2005.02344.x. [DOI] [PubMed] [Google Scholar]
- Agrell J, Oleszek W, Stochmal A, Olsen M, Anderson P. Herbivore-induced responses in alfalfa (Medicago sativa) J Chem Ecol. 2003;29:303–320. doi: 10.1023/a:1022625810395. [DOI] [PubMed] [Google Scholar]
- Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Slaitoh Y, Kobayashi M, Kumada H. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997;79:1494–1500. doi: 10.1002/(sici)1097-0142(19970415)79:8<1494::aid-cncr8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Armah CN, Mackie AR, Roy C, Price K, Osbourn AE, Bowyer P, Ladha S. The membrane-permeabilizing effect of avenacin A-1 involves the reorganization of bilayer cholesterol. Biophys J. 1999;76:281–290. doi: 10.1016/S0006-3495(99)77196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arneson PA, Durbin RD. Hydrolysis of tomatine by Septoria lycopersici: a detoxification mechanism. Phytopathology. 1967;57:1358–1360. [Google Scholar]
- Arneson PA, Durbin RD. Studies on the mode of action of tomatine as a fungitoxic agent. Plant Physiol. 1968;43:683–686. doi: 10.1104/pp.43.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arneson PA, Durbin RD. The sensitivity of fungi to α-tomatine. Phytopathology. 1968;58:536–537. [Google Scholar]
- Attele AS, Wu JA, Yuan C-S. Ginseng pharmacology. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Barr IG, Sjölander A, Cox JC. ISCOMs and other saponin based adjuvants. Adv Drug Deliv Rev. 1998;32:247–271. doi: 10.1016/s0169-409x(98)00013-1. [DOI] [PubMed] [Google Scholar]
- Basyuni M, Oku H, Inafuku M, Baba S, Iwasaki H, Oshiro K, Okabe T, Shibuya M, Ebizuka Y. Molecular cloning and functional expression of a multifunctional triterpene synthase cDNA from a mangrove species Kandelia candel (L.) Druce. Phytochemistry. 2006;67:2517–2524. doi: 10.1016/j.phytochem.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Begley MJ, Crombie L, Crombie WML, Whiting DA. The isolation of avenacin A-1, A-2, B-1 and B-2, chemical defences against cereal “take-all” disease. Structure of their “aglycones”, the avenestergenins, and their anhydro dimers. J Chem Soc Perk Trans. 1986;1:1905–1915. [Google Scholar]
- Bok JW, Noordermeer D, Kale SP, Keller NP. Secondary metabolic gene cluster silencing in Aspergillus nidulans. Mol Microbiol. 2006;61:1636–1645. doi: 10.1111/j.1365-2958.2006.05330.x. [DOI] [PubMed] [Google Scholar]
- Bones AM, Rossiter JT. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol Plantarum. 1996;97:194–208. [Google Scholar]
- Bourab K, Melton R, Peart J, Baulcombe D, Osbourn A. A saponin-detoxifying enzyme mediates suppression of plant defences. Nature. 2002;418:889–892. doi: 10.1038/nature00950. [DOI] [PubMed] [Google Scholar]
- Bowles D, Lim E-K, Poppenberger B, Vaistij FE. Glycosyltransferases of lipophilic small molecules. Annu Rev Plant Biol. 2006;57:567–597. doi: 10.1146/annurev.arplant.57.032905.105429. [DOI] [PubMed] [Google Scholar]
- Bowyer P, Clarke BR, Lunness P, Daniels MJ, Osbourn AE. Host range of a plant pathogenic fungus determined by a saponin detoxifying enzyme. Science. 1995;267:371–384. doi: 10.1126/science.7824933. [DOI] [PubMed] [Google Scholar]
- Burkhardt HJ, Maizel JV, Mitchell HK. Avenacin, an antimicrobial substance isolated from Avena sativa. II. Structure. Biochemistry. 1964;3:427–431. doi: 10.1021/bi00891a021. [DOI] [PubMed] [Google Scholar]
- Carter JP, Spink J, Cannon PF, Daniels MJ, Osbourn AE. Isolation, characterization, and avenacin sensitivity of a diverse collection of cereal-root-colonizing fungi. Appl Environ Microbiol. 1999;65:3364–3372. doi: 10.1128/aem.65.8.3364-3372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu HY, Wegel E, Osbourn AE. From hormones to secondary metabolism: the emergence of metabolic gene clusters in plants. Plant J. 2011;66:66–79. doi: 10.1111/j.1365-313X.2011.04503.x. [DOI] [PubMed] [Google Scholar]
- Chung E, Cho C-W, Kim K-Y, Chung J, Kim J-I, Chung Y-S, Fukui K, Lee J-H. Molecular characterization of the GmAMS1 gene encoding β-amyrin synthase in soybean plants. Russ J Plant Physiol. 1994;54:518–523. [Google Scholar]
- Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of licorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JD, Hill RA. Triterpenoids. Nat Prod Rep. 2007;24:465–486. doi: 10.1039/b507872p. [DOI] [PubMed] [Google Scholar]
- Crombie WML, Crombie L. Distribution of the avenacins A-1, A-2, B-1 and B-2 in oat roots: their fungicidal activity towards take-all fungus. Phytochemistry. 1986;25:2069–2073. [Google Scholar]
- Crombie L, Crombie WML, Whiting DA. Structure of the four avenacins, oat root resistance factors to take-all disease. J Chem Soc Chem Commun. 1984;244:246–248. [Google Scholar]
- Dang TY, Prestwich GD. Site-directed mutagenesis of squalene-hopene cyclase: altered substrate specificity and product distribution. Chem Biol. 2000;7:643–649. doi: 10.1016/s1074-5521(00)00003-x. [DOI] [PubMed] [Google Scholar]
- Dao L, Friedman M. Chlorophyll, chlorogenic acid, glycoalkaloid and protease inhibitor content of fresh and green potatoes. J Agric Food Chem. 1994;42:633–639. [Google Scholar]
- Dixon R. Natural products and disease resistance. Nature. 2001;411:843–847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Sumner LW. Legume natural products: understanding and manipulating complex pathways for human and animal health. Plant Physiol. 2003;131:878–885. doi: 10.1104/pp.102.017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebizuka Y, Katsube Y, Tsutsumi T, Kushiro T, Shibuya M. Functional genomics approach to the study of triterpene biosynthesis. Pure Appl Chem. 2003;75:369–374. [Google Scholar]
- Fenwick GR, Price KR, Tsukamoto C, Okubo K. Saponins. In: D’Mello JPF, Duffus CM, Duffus JH, editors. Toxic substances in crop plants. London: The Royal Society of Chemistry; 1992. pp. 285–327. [Google Scholar]
- Field B, Osbourn AE. Metabolic diversification – independent assembly of operon-like gene clusters in different plants. Science. 2008;320:543–547. doi: 10.1126/science.1154990. [DOI] [PubMed] [Google Scholar]
- Field B, Jordán F, Osbourn A. First encounters – deployment of defence-related natural products by plants. New Phytol. 2006;172:193–207. doi: 10.1111/j.1469-8137.2006.01863.x. [DOI] [PubMed] [Google Scholar]
- Field B, Fiston-Lavier AS, Kemen A, Geisler K, Quesnevillec H, Osbourn AE. Formation of plant metabolic gene clusters within dynamic chromosomal regions. Proc Natl Acad Sci USA. 2011;108:16116–16121. doi: 10.1073/pnas.1109273108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis G, Kerem Z, Makkar HPS, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- Frey M, Kliem R, Saedler H, Gierl A. Expression of a cytochrome P450 gene family in maize. Mol Gen Genet. 1995;246:100–109. doi: 10.1007/BF00290138. [DOI] [PubMed] [Google Scholar]
- Frey M, Chomet P, Glawischnig E, Stettner C, Grün S, Winklmair A, Eisenreich W, Bacher A, Meeley RB, Briggs SP, Simcox K, Gierl A. Analysis of a chemical plant defense mechanism in grasses. Science. 1997;277:696–699. doi: 10.1126/science.277.5326.696. [DOI] [PubMed] [Google Scholar]
- Friedman M. Tomato glycoalkaloids: roles in the plant and in the diet. J Agric Food Chem. 2002;50:5751–5780. doi: 10.1021/jf020560c. [DOI] [PubMed] [Google Scholar]
- Friedman M. Potato glycoalkaloids and metabolites: roles in the plant and in the diet. J Agric Food Chem. 2006;54:8655–8681. doi: 10.1021/jf061471t. [DOI] [PubMed] [Google Scholar]
- Friedman M, McQuistan T, Hendricks JD, Pereira C, Bailey GS. Protective effect of dietary tomatine against dibenzo[a, l]pyrene (DBP)-induced liver and stomach tumors in rainbow trout. Mol Nutr Food Res. 2007;51:1485–1491. doi: 10.1002/mnfr.200700176. [DOI] [PubMed] [Google Scholar]
- Gachon CM, Langlois-Meurinne M, Saindrenan P. Plant secondary metabolism glycosyltransferases: the emerging functional analysis. Trends Plant Sci. 2005;10:542–549. doi: 10.1016/j.tplants.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Germonprez N, Van Puyvelde L, Maes L, Van Tri M, De Kimpe N. New pentacyclic triterpene saponins with strong anti-leishmanial activity from the leaves of Maesa balansae. Tetrahedron. 2004;60:219–228. [Google Scholar]
- Gierl A, Frey M. Evolution of benzoxazinone biosynthesis and indole production in maize. Planta. 2001;213:493–498. doi: 10.1007/s004250100594. [DOI] [PubMed] [Google Scholar]
- Goodwin RH, Pollock BM. Studies on roots. I. Properties and distribution of fluorescent constituents in Avena roots. Am J Bot. 1954;41:516–520. [Google Scholar]
- Grenby TH. Intense sweeteners for the food industry: an overview. Trends Food Sci Technol. 1991;2:2–6. [Google Scholar]
- Gurfinkel DM, Rao AV. Soyasaponins: the relationship between chemical structure and colon anticarcinogenic activity. Nutr Cancer. 2003;47:24–33. doi: 10.1207/s15327914nc4701_3. [DOI] [PubMed] [Google Scholar]
- Halkier BA, Gershenzon J. Biology and biochemistry of glucosinolates. Annu Rev Plant Biol. 2006;57:303–333. doi: 10.1146/annurev.arplant.57.032905.105228. [DOI] [PubMed] [Google Scholar]
- Hanausek M, Gaensh P, Walaszek Z, Arntzen CJ, Slaga TJ, Gutterman JU. Avicins, a family of triterpenoid saponins from Acacia victoriae (Bentham), suppress H-ras mutations and aneuploidy in a murine skin carcinogenesis model. Proc Natl Acad Sci U S A. 2001;98:11551–11556. doi: 10.1073/pnas.191363198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AA. Two fatal cases of potato poisoning. Science. 1925;61:340–341. doi: 10.1126/science.61.1578.340. [DOI] [PubMed] [Google Scholar]
- Harada S. The broad anti-viral agent glycyrrhizin directly modulates the fluidity of plasma membrane and HIV-1 envelope. Biochem J. 2005;329:191–199. doi: 10.1042/BJ20051069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haralampidis K, Bryan G, Qi X, Papadopoulou K, Bakht S, Melton R, Osbourn AE. A new class of oxidosqualene cyclases directs synthesis of antimicrobial phytoprotectants in monocots. Proc Natl Acad Sci U S A. 2001;98:13431–13436. doi: 10.1073/pnas.231324698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haralampidis K, Trojanowska M, Osbourn AE. Biosynthesis of triterpenoid saponins in plants. Adv Biochem Eng Biotechnol. 2002;75:32–47. doi: 10.1007/3-540-44604-4_2. [DOI] [PubMed] [Google Scholar]
- Haridas V, Higuchi M, Jayatilake GS, Bailey D, Mujoo K, Blake ME, Arntzen CJ, Gutterman JU. Avicins: triterpenoid saponins from Acacia victoriae (Benthem) induce apoptosis by mitochondrial perturbation. Proc Natl Acad Sci U S A. 2001;98:5821–5826. doi: 10.1073/pnas.101619098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haridas V, Arntzen CJ, Gutterman JU. Avicins, a family of triterpenoid saponins from Acacia victoriae (Bentham), inhibit activation of nuclear factor-κB by inhibiting both its nuclear localization and ability to bind DNA. Proc Natl Acad Sci U S A. 2001;98:11557–22562. doi: 10.1073/pnas.191363498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haridas V, Kim S-O, Nishimura G, Hausladen A, Stamler JS, Gutterman JU. Avicinylation (thioesterification): a protein modification that can regulate the response to oxidative and nitrosative stress. Proc Natl Acad Sci U S A. 2005;102:10088–10093. doi: 10.1073/pnas.0504430102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haridas V, Li X, Mizumachi T, Higuchi M, Lemeshko VV, Colombini M, Gutterman JU. Avicins, a novel plant-derived metabolite lowers energy metabolism in tumor cells by targeting the outer mitochondrial membrane. Mitochondrion. 2007;7:234–240. doi: 10.1016/j.mito.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Hartmann T. From waste products to ecochemicals: fifty years of research of plant secondary metabolism. Phytochemistry. 2007;68:2831–2846. doi: 10.1016/j.phytochem.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Hirota A, Hiraoka N, Ikeshiro Y. Molecular cloning and characterization of two cDNAs for Glycyrrhiza glabra squalene synthase. Biol Pharm Bull. 1999;22:947–950. doi: 10.1248/bpb.22.947. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Huang P, Kirakosyan A, Inoue K, Hiraoka N, Ikeshiro Y, Kushiro T, Shibuya M, Ebizuka Y. Cloning and characterization of a cDNA encoding β-amyrin synthase involved in glycyrrhizin and soyasaponin biosynthesis in licorice. Biol Pharm Bull. 2001;24:912–916. doi: 10.1248/bpb.24.912. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Huang P, Inoue K, Hiraoka N, Ikeshiro Y, Yazaki K, Tanaka S, Kushiro T, Shibuya M, Ebizuka Y. Molecular cloning and characterization of isomultiflorenol synthase, a new triterpene synthase from Luffa cylindrica, involved in biosynthesis of bryonolic acid. Eur J Biochem. 2001;268:6311–6317. doi: 10.1046/j.0014-2956.2001.02588.x. [DOI] [PubMed] [Google Scholar]
- He J-X, Akao T, Nishino T, Tani T. The influence of commonly prescribed synthetic drugs for peptic ulcer on the pharmacokinetic fate of glycyrrhizin from Shaoyao-Gancao-tang. Biol Pharm Bull. 2001;24:1395–1399. doi: 10.1248/bpb.24.1395. [DOI] [PubMed] [Google Scholar]
- Hemm MR, Rider SD, Ogas J, Murry DJ, Chapple C. Light induces phenylpropanoid metabolism in Arabidopsis roots. Plant J. 2004;38:765–778. doi: 10.1111/j.1365-313X.2004.02089.x. [DOI] [PubMed] [Google Scholar]
- Heng L, Vincken J-P, van Koningsveld GA, Legger A, Gruppen H, van Boekel T, Roozen J, Voragen F. Bitterness of saponins and their content in dry peas. J Sci Food Agric. 2006;86:1225–1231. [Google Scholar]
- Hiradate S, Yada H, Ishii T, Nakajima N, Ohnishi-Kameyama M, Sugie H, Zungsontiporn S, Fujii Y. Three plant growth inhibiting saponins from Duranta repens. Phytochemistry. 1999;52:1223–1228. [Google Scholar]
- Hlywka JJ, Stephenson GR, Sears MK, Yada RY. Effects of insect damage on glycoalkaloid content in potatoes (Solanum tuberosum) J Agric Food Chem. 1994;42:2545–2550. [Google Scholar]
- Hostettmann KA, Marston A. Saponins: chemistry and pharmacology of natural products. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Huang X, Renwick JAA, Sachdev-Gupta K. Oviposition stimulants in Barbarea vulgaris for Pieris rapae and P. napi oleracea: isolation, identification and differential activity. J Chem Ecol. 1994;20:423. doi: 10.1007/BF02064448. [DOI] [PubMed] [Google Scholar]
- Ito S, Takahara H, Kawaguchi T, Tanaka S, Kameya-Iwaki M. Post-transcriptional silencing of the tomatinase gene in Fusarium oxysporum f. sp lycopersici. J Phytopathol. 2002;150:474–480. [Google Scholar]
- Ito S, Eto T, Tanaka S, Yamauchi N, Takahara H, Ikeda T. Tomatidine and lycotetraose, hydrolysis products of α-tomatine by Fusarium oxysporum tomatinase, suppress induced defense responses in tomato cells. FEBS Lett. 2004;571:31–34. doi: 10.1016/j.febslet.2004.06.053. [DOI] [PubMed] [Google Scholar]
- Ito S, Ihara T, Tamura H, Tanaka S, Ikeda T, Kajihara H, Dissanayake C, Abdel-Motaal FF, El-Sayed MA. α-Tomatine, the major saponin in tomato, induces programmed cell death mediated by reactive oxygen species in the fungal pathogen Fusarium oxysporum. FEBS Lett. 2007;581:3217–3222. doi: 10.1016/j.febslet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Jayatilake GS, Freeberg DR, Liu ZJ, Richheimer SL, Blake Nieto ME, Bailey DT, Haridas V, Gutterman JU. Isolation and structures of avicins D and G: in vitro tumor-inhibitory saponins derived from Acacia victoriae. J Nat Prod. 2003;66:779–783. doi: 10.1021/np020400v. [DOI] [PubMed] [Google Scholar]
- Ji Y-H, Moog C, Schmitt G, Luu B. Polyoxygenated sterols and triterpenes: chemical structures and biological activities. J Steroid Biochem. 1990;35:741–744. doi: 10.1016/0022-4731(90)90317-l. [DOI] [PubMed] [Google Scholar]
- Kawano N, Ichinose K, Ebizuka Y. Molecular cloning and functional expression of cDNAs encoding oxidosqualene cyclases from Costus speciosus. Biol Pharm Bull. 2002;25:477–482. doi: 10.1248/bpb.25.477. [DOI] [PubMed] [Google Scholar]
- Keller NP, Hohn TM. Metabolic pathway gene clusters in filamentous fungi. Fungal Genet Biol. 1996;21:17–29. [PubMed] [Google Scholar]
- Keller NP, Turner G, Bennett JW. Fungal secondary metabolism – from biochemistry to genomics. Nat Rev Microbiol. 2005;3:937–947. doi: 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]
- Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterisation of saponins with adjuvant activity from Quillaja saponaria molina cortex. J Immunol. 1991;146:431–437. [PubMed] [Google Scholar]
- Keukens EA, deVrije T, Fabrie CH, Demel RA, Jongen WM, deKruijff B. Dual specificity of sterol-mediated glycoalkaloid induced membrane disruption. Biochim Biophys Acta. 1992;1110:127–136. doi: 10.1016/0005-2736(92)90349-q. [DOI] [PubMed] [Google Scholar]
- Keukens EA, deVrije T, van den Boom C, de Waard P, Plasman HH, Thiel F, Chupin V, Jongen WM, deKruijff B. Molecular basis of glycoalkaloid induced membrane disruption. Biochim Biophys Acta. 1995;1240:216–228. doi: 10.1016/0005-2736(95)00186-7. [DOI] [PubMed] [Google Scholar]
- Kitagawa I. Licorice root. A natural sweetener and an important ingredient in Chinese medicine. Pure Appl Chem. 2002;74:1189–1198. [Google Scholar]
- Konoshima T, Kokumai M, Kozuka M, et al. Anti-tumor-promoting activities of afromosin and soyasaponin I isolated from Wisteria brachybotrys. J Nat Prod. 1992;55:1776–1778. doi: 10.1021/np50090a011. [DOI] [PubMed] [Google Scholar]
- Korpan YI, Nazarenko EA, Skryshevskaya IV, et al. Potato glycoalkaloids: true safety or false sense of security? Trends Biotechnol. 2004;22:147–151. doi: 10.1016/j.tibtech.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Kudou S, Tonomura M, Tsukamoto C, et al. Isolation and structural elucidation of DDMP-conjugated soyasaponins as genuine saponins from soybean seeds. Biosci Biotechnol Biochem. 1993;57:546–550. [Google Scholar]
- Kushiro T, Shibuya M, Masuda K, Ebizuka Y. A novel multifunctional triterpene synthase from Arabidopsis thaliana. Tetrahedron Lett. 2000;41:7705–7710. [Google Scholar]
- Kushiro T, Shibuya M, Masuda K, Ebizuka Y. Mutational studies on triterpene synthases: engineering lupeol synthase into β-amyrin synthase. J Am Chem Soc. 2000;122:6816–6824. [Google Scholar]
- Lavie Y, Harel-Orbital T, Gaffield W, Liscovitch M. Inhibitory effect of steroidal alkaloids on drug transport and multidrug resistance in human cancer cells. Anticancer Res. 2001;21:1189–1194. [PubMed] [Google Scholar]
- Lee K-R, Kozukue N, Han J-S, Park J-H, Chang E-Y, Baek E-J, Chang J-S, Friedman M. Glycoalkaloids and metabolites inhibit the growth of human colon (HT29) and liver (HepG2) cancer cells. J Agric Food Chem. 2004;52:2832–2839. doi: 10.1021/jf030526d. [DOI] [PubMed] [Google Scholar]
- Lemeshko VV, Haridas V, Quijano Pérez JC, Gutterman JU. Avicins, natural anticancer saponins, permeabilize mitochondrial membranes. Arch Biochem Biophys. 2006;454:114–122. doi: 10.1016/j.abb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Lenhart A, Weihofen WA, Pleschke AE, Schulz GE. Crystal structure of a squalene cyclase in complex with the potential anticholesteremic drug Ro48-8071. Chem Biol. 2002;9:639–645. doi: 10.1016/s1074-5521(02)00138-2. [DOI] [PubMed] [Google Scholar]
- Li Y, LiangW ZX, Liu F, Zhu X. Allelopathic activity of root saponins of alfalfa on wheat, corn and barnyardgrass. Allelopathy J. 2004;15:119–123. [Google Scholar]
- Lodeiro S, Segura MJ, Stahl M, Schulz-Gasch T, Matsuda SPT. Oxidosqualene cyclase second-sphere residues profoundly influence the product profile. ChemBioChem. 2004;5:1581–1585. doi: 10.1002/cbic.200400086. [DOI] [PubMed] [Google Scholar]
- Lodeiro S, Xiong QB, Wilson WK, Kolesnikova MD, Onak CS, Matsuda SPT. An oxidosqualene cyclase makes numerous products by diverse mechanisms: a challenge to prevailing concepts of triterpene biosynthesis. J Am Chem Soc. 2007;129:11213–11222. doi: 10.1021/ja073133u. [DOI] [PubMed] [Google Scholar]
- Madina BR, Sharma LK, Chaturvedi P, Sangwan RS, Tuli R. Purification and characterization of a novel glucosyltransferase specific to 27β-hydroxy steroidal lactones from Withania somnifera and its role in plant stress responses. Biochim Biophys Acta. 2007;1774:1199–1207. doi: 10.1016/j.bbapap.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Maizel JV, Burkhardt HJ, Mitchell HK. Avenacin, an antimicrobial substance isolated from Avena sativa. I. Isolation and antimicrobial activity. Biochemistry. 1964;3:424–426. doi: 10.1021/bi00891a020. [DOI] [PubMed] [Google Scholar]
- Martin-Hernandez AM, Dufresne M, Hugouvieux V, Melton R, Osbourn AE. Effects of targeted replacement of the tomatinase gene on the interaction of Septoria lycopersici with tomato plants. Mol Plant Microbe Interact. 2000;13:1301–1311. doi: 10.1094/MPMI.2000.13.12.1301. [DOI] [PubMed] [Google Scholar]
- Matsui S, Matsumoto H, Sonoda Y, Ando K, Aizu-Yokota E, Sato T, Kasahara T. Glycyrrhizin and related compounds down-regulate production of inflammatory chemokines IL-8 and eotaxin 1 in a human lung fibroblast cell line. Int Immunopharmacol. 2004;4:1633–1644. doi: 10.1016/j.intimp.2004.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue KF, Shepherd LVT, Allen PV, Maccree MM, Rockhold DR, Corsini DL, Davies HV, Belknap WR. Metabolic compensation of steroidal glycoalkaloid biosynthesis in transgenic potato tubers: using reverse genetics to confirm the in vivo enzyme function of a steroidal alkaloid galactosyltransferase. Plant Sci. 2005;168:267–273. [Google Scholar]
- McCue KF, Allen PV, Shepherd LVT, Blake A, Whitworth J, Maccree MM, Rockhold DR, Stewart D, Davies HV, Belknap WR. The primary in vivo steroidal alkaloid glucosyltransferase from potato. Phytochemistry. 2006;67:1590–1597. doi: 10.1016/j.phytochem.2005.09.037. [DOI] [PubMed] [Google Scholar]
- McCue KF, Allen PV, Shepherd LVT, Blake A, Maccree MM, Rockhold DR, Novy RG, Stewart D, Davies HV, Belknap WR. Potato glycosterol rhamnosyltransferase, the terminal step in triose side-chain biosynthesis. Phytochemistry. 2007;68:327–334. doi: 10.1016/j.phytochem.2006.10.025. [DOI] [PubMed] [Google Scholar]
- McManus OB, Harris GH, Giangiacomo KM, et al. An activator of calcium-dependent potassium channels isolated from a medicinal herb. Biochemistry. 1993;32:6128–6133. doi: 10.1021/bi00075a002. [DOI] [PubMed] [Google Scholar]
- McMillan M, Thompson JC. An outbreak of suspected solanine poisoning in schoolboys: examinations of criteria of solanine poisoning. Q J Med. 1979;48:227–243. [PubMed] [Google Scholar]
- Melton RE, Flegg LM, Brown JKM, Oliver RP, Daniels MJ, Osbourn AE. Heterologous expression of Septoria lycopersici tomatinase in Cladosporium fulvum: effects on compatible and incompatible interactions with tomato seedlings. Mol Plant Microbe Interact. 1998;11:228–236. doi: 10.1094/MPMI.1998.11.3.228. [DOI] [PubMed] [Google Scholar]
- Meyer MM, Xu R, Matsuda SPT. Directed evolution to generate cycloartenol synthase mutants that produce lanosterol. Org Lett. 2002;4:1395–1398. doi: 10.1021/ol0257225. [DOI] [PubMed] [Google Scholar]
- Milgate J, Roberts DCK. The nutritional and biological significance of saponins. Nutr Res. 1995;15:1233–1249. [Google Scholar]
- Milkowski C, Strack D. Serine carboxypeptidase-like acyltransferases. Phytochemistry. 2004;66:517–524. doi: 10.1016/j.phytochem.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Moehs CP, Allen PV, Friedman M, Belknap WR. Cloning and expression of solanidine UDP-glucose glucosyltransferase from potato. Plant J. 1997;11:227–236. doi: 10.1046/j.1365-313x.1997.11020227.x. [DOI] [PubMed] [Google Scholar]
- Mondy NI, Leja M, Gosselin B. Changes in total phenolic, total glycoalkaloid, and ascorbic acid as a result of bruising. J Food Sci. 1987;52:631–633. [Google Scholar]
- Morrissey JP, Osbourn AE. Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol Mol Biol Rev. 1999;63:708–724. doi: 10.1128/mmbr.63.3.708-724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugford ST, Osbourn A. Evolution of serine carboxypeptidase-like acyltransferases in the monocots. Plant Signal Behav. 2010;5:193–195. doi: 10.4161/psb.5.2.11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugford ST, Qi X, Bakht S, et al. A serine carboxypeptidase-like acyltransferase is required for synthesis of antimicrobial compounds and disease resistance in oats. Plant Cell. 2009;21:2473–2484. doi: 10.1105/tpc.109.065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujoo K, Haridas V, Hoffmann JJ, et al. Triterpenoid saponins from Acacia victoriae (Bentham) decrease tumor cell proliferation and induce apoptosis. Cancer Res. 2001;61:5486–5490. [PubMed] [Google Scholar]
- Mylona P, Owatworakit A, Papadopoulou K, et al. Sad3 and Sad4 are required for saponin biosynthesis and root development in oat. Plant Cell. 2008;20:201–212. doi: 10.1105/tpc.107.056531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DR, Schuler MA, Paquette SM, et al. Comparative genomics of rice and Arabidopsis. Analysis of 727 cytochrome P450 genes and pseudogenes from a monocot and a dicot. Plant Physiol. 2004;135:756–772. doi: 10.1104/pp.104.039826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma H, Tanaka H, Noguchi H, et al. Enzymatic formation of an unnatural novel tetracyclic sesterterpene by β-amyrin synthase. Tetrahedron Lett. 2004;45:8299–8301. [Google Scholar]
- Oda K, Matsuda H, Murakami T, et al. Relationship between adjuvant activity and amphipathic structure of soyasaponins. Vaccine. 2003;21:2145–2151. doi: 10.1016/s0264-410x(02)00739-9. [DOI] [PubMed] [Google Scholar]
- Ohana P, Delmer DP, Carlson RW, et al. Identification of a novel triterpenoid saponin from Pisum sativum as a specific inhibitor of the diguanylate cyclase of Acetobacter xylinum. Plant Cell Physiol. 1998;39:144–152. doi: 10.1093/oxfordjournals.pcp.a029351. [DOI] [PubMed] [Google Scholar]
- Ohmoto T, Ikuse M. Triterpenoids of the gramineae. Phytochemistry. 1970;9:2137–2148. [Google Scholar]
- Oleszek W, Jurzysta M. The allelopathic potential of alfalfa root medicagenic acid glycosides and their fate in soil environments. Plant Soil. 1987;98:67–80. [Google Scholar]
- Osbourn AE. Saponins and plant defence- a soap story. Trends Plant Sci. 1996;1:4–8. [Google Scholar]
- Osbourn AE. Secondary metabolic gene clusters: evolutionary toolkits for chemical innovation. Trends Genet. 2010;26:449–457. doi: 10.1016/j.tig.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Osbourn AE, Clarke BR, Lunness P, Scott PR, Daniels MJ. An oat species lacking avenacin is susceptible to infection by Gaeumannomyces graminis var. tritici. Physiol Mol Plant Pathol. 1994;45:457–467. [Google Scholar]
- Osbourn AE, Qi X, Townsend B, Qin B. Dissecting plant secondary metabolism- constitutive chemical defences in cereals. New Phytol. 2003;159:101–108. doi: 10.1046/j.1469-8137.2003.00759.x. [DOI] [PubMed] [Google Scholar]
- Osbourn AE, Goss RJM, Field RA. The saponins- polar isoprenoids with important and diverse biological activities. Nat Prod Rep. 2011;28:1261–1268. doi: 10.1039/c1np00015b. [DOI] [PubMed] [Google Scholar]
- Paczkowski C, Kalinowska M, Wojciechowski ZA. UDP-glucose:solasodine glucosyltransferase from eggplant (Solanum melongena L.) leaves: partial purification and characterization. Acta Biochim Pol. 1997;44:43–53. [PubMed] [Google Scholar]
- Paczkowski C, Kalinowska M, Wojciechowski ZA. Phospholipids modulate the substrate specificity of soluble UDP-glucose:steroid glucosyltransferase from eggplant leaves. Phytochemistry. 2001;58:663–669. doi: 10.1016/s0031-9422(01)00292-8. [DOI] [PubMed] [Google Scholar]
- Papadopoulou K, Melton RE, Leggett M, Daniels MJ, Osbourn AE. Compromised disease resistance in saponin-deficient plants. Proc Natl Acad Sci U S A. 1999;96:12923–12928. doi: 10.1073/pnas.96.22.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareja-Jaime Y, Roncero MIG, Ruiz-Roldán MC. Tomatinase from Fusarium oxysporum f. sp lycopersici is required for full virulence on tomato plants. Mol Plant Microbe Interact. 2008;21:728–736. doi: 10.1094/MPMI-21-6-0728. [DOI] [PubMed] [Google Scholar]
- Phillips DR, Rasbery JM, Bartel B, Matsuda SPT. Biosynthetic diversity in plant triterpene cyclization. Curr Opin Plant Biol. 2006;9:305–314. doi: 10.1016/j.pbi.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Podolak I, Galanty A, Sobolewska D. Saponins as cytotoxic agents: a review. Phytochem Rev. 2010;6:425–474. doi: 10.1007/s11101-010-9183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KR, Johnson IT, Fenwick GR. The chemistry and biological significance of saponins in foods and feedstuffs. Crit Rev Food Sci Nutr. 1987;26:27–135. doi: 10.1080/10408398709527461. [DOI] [PubMed] [Google Scholar]
- Qi X, Bakht S, Leggett M, Maxwell C, Melton R, Osbourn A. A gene cluster for secondary metabolism in oat – implications for the evolution of metabolic diversity in plants. Proc Natl Acad Sci U S A. 2004;101:8233–8238. doi: 10.1073/pnas.0401301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Bakht S, Qin B, Leggett M, Hemmings A, Mellon F, Eagles J, Werck-Reichhart D, Schaller H, Lesot A, Melton R, Osbourn A. A different function for a member of an ancient and highly conserved cytochrome P450 family: from essential sterols to plant defence. Proc Natl Acad Sci U S A. 2006;103:18848–18853. doi: 10.1073/pnas.0607849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin B, Eagles J, Mellon FA, Mylona P, Peña-Rodríguez L, Osbourn AE. High throughput screening of mutants of oat that are defective in triterpene synthesis. Phytochemistry. 2010;71:1245–1252. doi: 10.1016/j.phytochem.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Rahman A, Ahamed A, Amakawa T, Goto N, Tsurumi S. Chromosaponin I specifically interacts with AUX1 protein in regulating the gravitropic response of Arabidopsis roots. Plant Physiol. 2001;125:990–1000. doi: 10.1104/pp.125.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick CM, Uhlig JW, Jones AD. High α-tomatine content in ripe fruit of Andean Lycopersicon esculentum var. cerasiforme: developmental and genetic aspects. Proc Natl Acad Sci U S A. 1994;91:12877–12881. doi: 10.1073/pnas.91.26.12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddick JG. Complex formation between solanaceous steroidal glycoalkaloids and free sterols in vitro. Phytochemistry. 1979;18:1467–1470. [Google Scholar]
- Roddick JG. The acetylcholinesterase-inhibitory activity of steroidal glycoalkaloids and their aglycons. Phytochemistry. 1989;28:2631–2634. [Google Scholar]
- Roddick JG, Drysdale RB. Destabilization of liposome membranes by the steroidal glycoalkaloid α-tomatine. Phytochemistry. 1984;23:9–25. [Google Scholar]
- Ruf A, Müller F, D’Arcy B, et al. The monotopic membrane protein human oxidosqualene cyclase is active as monomer. Biochem Biophys Res Commun. 2004;315:247–254. doi: 10.1016/j.bbrc.2004.01.052. [DOI] [PubMed] [Google Scholar]
- Ruf A, Müller F, D’Arcy B, et al. The monotopic membrane protein human oxidosqualene cyclase is active as monomer. Biochem Biophys Res Commun. 2004;315:247–254. doi: 10.1016/j.bbrc.2004.01.052. [DOI] [PubMed] [Google Scholar]
- Sandrock RW, Van Etten HD. The relevance of tomatinase activity in pathogens of tomato: disruption of the β2-tomatinase gene in Colletotrichum coccodes and Septoria lycopersici and heterologous expression of the Septoria lycopersici β2-tomatinase in Nectria haematococca, a pathogen of tomato fruit. Physiol Mol Plant Pathol. 2001;58:159–171. [Google Scholar]
- Sato T, Hoshino T. Catalytic function of the residues of phenylalanine and tyrosine conserved in squalene-hopene cyclases. Biosci Biotechnol Biochem. 2001;65:2233–2242. doi: 10.1271/bbb.65.2233. [DOI] [PubMed] [Google Scholar]
- Segura MJ, Meyer MM, Matsuda SPT. Arabidopsis thaliana LUP1 converts oxidosqualene to multiple triterpene alcohols and a triterpene diol. Org Lett. 2000;2:2257–9225. doi: 10.1021/ol006016b. [DOI] [PubMed] [Google Scholar]
- Segura MJR, Lodeiro S, Meyer MM, Patel AJ, Matsuda SPT. Directed evolution experiments reveal mutations at cycloartenol synthase residue His477 that dramatically alter catalysis. Org Lett. 2002;4:4459–4462. doi: 10.1021/ol0269897. [DOI] [PubMed] [Google Scholar]
- Segura MJR, Jackson BE, Matsuda SPT. Mutagenesis approaches to deduce structure-function relationships in terpene synthases. Nat Prod Rep. 2003;30:304–317. doi: 10.1039/b008338k. [DOI] [PubMed] [Google Scholar]
- Seki H, Ohyama K, Sawai S, et al. Licorice β-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proc Natl Acad Sci U S A. 2008;105:14204–14209. doi: 10.1073/pnas.0803876105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan H, Segura MJR, Wilson WK, Lodeiro S, Matsuda SPT. Enzymatic cyclization of dioxidosqualene to heterocyclic triterpenes. J Am Chem Soc. 2005;127:18008–18009. doi: 10.1021/ja055822g. [DOI] [PubMed] [Google Scholar]
- Shao H, He X, Achnine L, Blount JW, Dixon RA, Wang X. Crystal structures of a multifunctional triterpene/flavonoid glycosyltransferase from Medicago truncatula. Plant Cell. 2005;17:3141–3154. doi: 10.1105/tpc.105.035055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M, Hoshino M, Katsube Y, Hayashi H, Kushiro T, Ebizuka Y. Identification of β-amyrin and sophoradiol 24-hydroxylase by expressed sequence tag mining and functional expression assay. FEBS J. 2006;273:948–959. doi: 10.1111/j.1742-4658.2006.05120.x. [DOI] [PubMed] [Google Scholar]
- Shibuya M, Xiang T, Katsube Y, Otsuka M, Zhang H, Ebizuka Y. Origin of structural diversity in natural triterpenes: direct synthesis of seco-triterpene skeletons by oxidosqualene cyclase. J Am Chem Soc. 2007;129:1450–1455. doi: 10.1021/ja066873w. [DOI] [PubMed] [Google Scholar]
- Shimura K, Okada A, Okada K, et al. Identification of a biosynthetic gene cluster in rice for momilactones. J Biol Chem. 2007;282:34013–34018. doi: 10.1074/jbc.M703344200. [DOI] [PubMed] [Google Scholar]
- Shinoda T, Nagao T, Nakayama M, et al. Identification of a triterpenoid saponin from a crucifer, Barbarea vulgaris, as a feeding deterrent to the diamondback moth, Plutella xylostella. J Chem Ecol. 2002;28:587–599. doi: 10.1023/a:1014500330510. [DOI] [PubMed] [Google Scholar]
- Shinozaki J, Shibuya M, Masuda K, Ebizuka Y. Dammaradiene synthase, a squalene cyclase, from Dryopteris crassirhizoma Nakai. Phytochemistry. 2008;69:2559–2564. doi: 10.1016/j.phytochem.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Shinozaki J, Shibuya M, Masuda K, Ebizuka Y. Squalene cyclase and oxidosqualene cyclase from a fern. FEBS Lett. 2008;582:310–318. doi: 10.1016/j.febslet.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Simons V, Morrissey JP, Latijnhouwers M, Csukai M, Cleaver A, Yarrow C, Osbourn A. Dual effects of plant steroidal alkaloids on Saccharomyces cerevisiae. Antimicrob Agents Chemother. 2006;50:2732–2740. doi: 10.1128/AAC.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparg SG, Light ME, van Staden J. Biological activities and distribution of plant saponins. J Ethnopharmacol. 2004;94:219–243. doi: 10.1016/j.jep.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Stapleton A, Allen PV, Friedman M, Belknap WR. Purification and characterization of solanidine glucosyltransferase from the potato. J Agric Food Chem. 1991;39:1187–1193. [Google Scholar]
- Steel CS, Drysdale RB. Electrolytic leakage from plant and fungal tissues and disruption of liposome membranes by α-tomatine. Phytochemistry. 1988;27:1025–1030. [Google Scholar]
- Suleman P, Tohamy AM, Saleh AA, Madkour MA, Straney DC. Variation in sensitivity to tomatine and rishitin among isolates of Fusarium oxysporum f. sp. lycopersici, and strains not pathogenic on tomato. Physiol Mol Plant Pathol. 1996;48:131–144. [Google Scholar]
- Suzuki H, Achnine L, Xu R, Matsuda SPT, Dixon RA. A genomics approach to the early stages of triterpene saponin biosynthesis in Medicago truncatula. Plant J. 2002;32:1033–1048. doi: 10.1046/j.1365-313x.2002.01497.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Reddy MSS, Naoumkina M, et al. Methyl jasmonate and yeast elicitor induce differential transcriptional and metabolic re-programming in cell suspension cultures of the model legume Medicago truncatula. Planta. 2005;220:696–707. doi: 10.1007/s00425-004-1387-2. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Xiang T, Ohyama K, et al. Lanosterol synthase in dicotyledonous plants. Plant Cell Physiol. 2006;47:565–571. doi: 10.1093/pcp/pcj031. [DOI] [PubMed] [Google Scholar]
- Takahara T, Watanabe A, Shiraki K. Effects of glycyrrhizin on hepatitis B surface antigen: a biochemical and morphological study. J Hepatol. 1994;21:601–609. doi: 10.1016/s0168-8278(94)80108-8. [DOI] [PubMed] [Google Scholar]
- Tansakul P, Shibuya M, Kushiro T, Ebizuka Y. Dammarenediol-II synthase, the first dedicated enzyme for ginsenoside biosynthesis, in Panax ginseng. FEBS Lett. 2006;580:5143–5149. doi: 10.1016/j.febslet.2006.08.044. [DOI] [PubMed] [Google Scholar]
- Taylor WG, Fields PG, Sutherland DH. Insecticidal components from field pea extracts: soyasaponins and lysolecithins. J Agric Food Chem. 2004;52:7484–7490. doi: 10.1021/jf0308051. [DOI] [PubMed] [Google Scholar]
- Thoma R, Schulz-Gasch T, D’Arcy B, et al. Insight into steroid scaffold formation from the structure of human oxidosqualene cyclase. Nature. 2004;432:118–122. doi: 10.1038/nature02993. [DOI] [PubMed] [Google Scholar]
- Townsend B, Jenner H, Osbourn A. Saponin glycosylation in cereals. Phytochem Rev. 2006;5:109–114. [Google Scholar]
- Tsujino Y, Tsurumi S, Yoshida Y, Niki E. Antioxidative effects of dihydro-γ-pyronyl-triterpenoid saponin (chromosaponin-I) Biosci Biotechnol Biochem. 1994;58:1731–1732. doi: 10.1271/bbb.58.1731. [DOI] [PubMed] [Google Scholar]
- Tsurumi S, Ishizawa K. Involvement of ethylene in chromosaponin-induced stimulation of growth in lettuce roots. Plant Cell Physiol. 1997;38:668–675. [Google Scholar]
- Tsurumi S, Wada S. Chromosaponin-I stimulates the elongation of cortical-cells in lettuce roots. Plant Cell Physiol. 1995;36:925–929. [Google Scholar]
- Tsurumi S, Takagi T, Hashimoto T. A γ-pyronyl triterpenoid saponin from Pisum sativum. Phytochemistry. 1992;31:2435–2438. doi: 10.1016/0031-9422(92)83294-9. [DOI] [PubMed] [Google Scholar]
- Tsurumi S, Ishizawa K, Rahman A, et al. Effects of chromosaponin I and brassinolide on the growth of roots in etiolated Arabidopsis seedlings. J Plant Physiol. 2000;156:60–67. [Google Scholar]
- Turner EM. The nature of the resistance of oats to the take-all fungus. J Exp Bot. 1960;11:403–412. [Google Scholar]
- Turner EM. An enzymic basis for pathogenic specificity in Ophiobolus graminis. J Exp Bot. 1961;12:169–175. [Google Scholar]
- Vincken J-P, Heng L, de Groot A, Gruppen H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry. 2007;68:275–297. doi: 10.1016/j.phytochem.2006.10.008. [DOI] [PubMed] [Google Scholar]
- von Rad U, Hüttl R, Lottspeich F, Gierl A, Frey M. Two glucosyltransferases are involved in detoxification of benzoxazinoids in maize. Plant J. 2001;28:633–642. doi: 10.1046/j.1365-313x.2001.01161.x. [DOI] [PubMed] [Google Scholar]
- Waller GR, Jurzysta M, Thorne RLZ. Allelopathic activity of root saponins from alfalfa (Medicago sativa L.) on weeds and wheat. Bot Bull Acad Sinica. 1993;34:1–11. [Google Scholar]
- Warashina T, Miyase T, Ueno A. Novel acylated saponins from Tragopogon porrifolius L. Isolation and the structures of tragopogonsaponins A-R. Chem Pharm Bull. 1991;39:388–396. [Google Scholar]
- Wu TK, Chang CH. Enzymatic formation of multiple triterpenes by mutation of tyrosine 510 of the oxidosqualene-lanosterol cyclase from Saccharomyces cerevisiae. Chembiochem. 2004;5:1712–1715. doi: 10.1002/cbic.200400079. [DOI] [PubMed] [Google Scholar]
- Wu TK, Griffin JH. Conversion of a plant oxidosqualene-cycloartenol synthase to an oxidosqualene-lanosterol cyclase by random mutagenesis. Biochemistry. 2002;41:8238–8244. doi: 10.1021/bi0200920. [DOI] [PubMed] [Google Scholar]
- Wu TK, Liu YT, Chang CH. Histidine residue at position 234 of oxidosqualene-lanosterol cyclase from Saccharomyces cerevisiae simultaneously influences cyclization, rearrangement, and deprotonation reactions. Chembiochem. 2005;6:1177–1181. doi: 10.1002/cbic.200500084. [DOI] [PubMed] [Google Scholar]
- Wu TK, Yu MT, Liu YT, Chang CH, Wang HJ, Diau EW. Tryptophan 232 within oxidosqualene-lanosterol cyclase from Saccharomyces cerevisiae influences rearrangement and deprotonation but not cyclization reactions. Org Lett. 2006;8:1319–1322. doi: 10.1021/ol053134w. [DOI] [PubMed] [Google Scholar]
- Xiang T, Shibuya M, Katsube Y, et al. A new triterpene synthase from Arabidopsis thaliana produces a tricyclic triterpene with two hydroxyl groups. Org Lett. 2006;8:2835–2838. doi: 10.1021/ol060973p. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Taninaka H, Kan Y, Arihara S. Antisweet natural products. X: structures of sitakisosides I-V from Stephanotis lutchuensis kodiz. var. japonica. Chem Pharm Bull. 1994;42:2023–2027. doi: 10.1248/cpb.42.2023. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Mizutani A, Kan Y, Arihara S. Antisweet natural products. XII: structures of sitakisosides XI-XX from Stephanotis lutchuensis Kodiz. var. japonica. Chem Pharm Bull. 1997;45:62–67. doi: 10.1248/cpb.45.62. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Hirai H, Tanaka M, Arihara S. Antisweet natural products. XV: structures of jegosaponins A–D from Styrax japonica Sieb. et Zucc. Chem Pharm Bull. 2000;48:1093–1096. doi: 10.1248/cpb.48.1093. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Morikawa T, Li N, Nagatomo A, Li X, Matsuda H. Bioactive saponins and glycosides. XXIII. Triterpene saponins with gastroprotective effect from the seeds of Camellia sinensis -theasaponins E3, E4, E5, E6, and E7. Chem Pharm Bull. 2005;53:1559–1564. doi: 10.1248/cpb.53.1559. [DOI] [PubMed] [Google Scholar]
- Yoshiki Y, Sigemitsu K, Okubu K. Relationship between chemical structures and biological activities of triterpenoid saponins from soybean. Biosci Biotechnol Biochem. 1998;62:2291–2299. doi: 10.1271/bbb.62.2291. [DOI] [PubMed] [Google Scholar]
- Zhang H, Shibuya M, Yokota S, Ebizuka Y. Oxidosqualene cyclases from cell suspension cultures of Betula platyphylla var. japonica: molecular evolution of oxidosqualene cyclases in higher plants. Biol Pharm Bull. 2003;26:642–650. doi: 10.1248/bpb.26.642. [DOI] [PubMed] [Google Scholar]
- Zimowski J. Characterization of UDP-galactose:tomatidine galactosyltransferase from tomato (Lycopersicon esculentum) leaves. Acta Biochim Pol. 1994;41:202–204. [PubMed] [Google Scholar]
- Zimowski J. Synthesis of γ-chaconine and γ-solanine are catalyzed in potato by two separate glycosyltransferases: UDP-glucose:solanidine glucosyltransferase and UDP-galactose:solanidine galactosyltransferase. Acta Biochim Pol. 1997;44:209–214. [PubMed] [Google Scholar]
- Zou K, Tong W-Y, Liang H, Cui J-R, Tu G-Z, Zhao Y-Y, Zhang R-Y. Diastereoisomeric saponins from Albizia julibrissin. Carbohydr Res. 2005;340:1329–1334. doi: 10.1016/j.carres.2004.10.027. [DOI] [PubMed] [Google Scholar]


