Abstract
Purpose:
Mycosis fungoides (MF) is the most common subtype of cutaneous T-cell lymphoma. Skin-directed treatments often improve but do not cure MF skin lesions. The purpose of this study was to 1) assess whether remission was associated with malignant T cell clone depletion at treated sites using either low dose radiation therapy (LDRT, 8 Gy) or topical steroids and 2) assess whether a clone-ablative therapy, like LDRT, is associated with overall survival in high-risk early-stage CTCL patients.
Experimental design:
Pre- and post-treatment biopsies from 20 lesional skin samples of 18 MF patients who received either 8 Gy LDRT (n=16) or topical steroids (n=4) underwent high-throughput T-cell receptor sequencing (HTS) of the TCRB gene to quantify the malignant T cell clone. For the retrospective chart review, overall survival of 47 high-risk early-stage patients was compared between patients who did or did not receive radiation.
Results:
LDRT eradicated the clone in 5/16 lesions and reduced it >90% in 11/16; there were no recurrences in these lesions. Patients treated with topical steroids appeared to clinically improve but the malignant clone persisted. We found that the number of residual malignant T cells predicted lesion recurrence. A retrospective review showed that early stage high-risk patients who received radiation as part of their treatment regimen had prolonged overall survival compared to patients who did not.
Conclusions:
These findings demonstrate that LDRT can eradicate malignant T cells in MF, provides robust disease control, and is associated with improved survival in high-risk early stage patients.
Keywords: cutaneous T-cell lymphoma (CTCL), radiation, steroids, high-throughput T-cell receptor sequencing, residual disease
Introduction
Cutaneous T-cell lymphomas (CTCL) are non-Hodgkin lymphomas derived from T cells that traffic to the skin. Mycosis fungoides (MF), a clinical subtype of CTCL, typically presents as an inflammatory skin disease without extracutaneous involvement.1 More than 80% of these early stage patients (Stage IA-IIA) will have an indolent course.2,3 However, approximately 20% of early stage patients will develop highly aggressive disease that can be fatal. Recently, the tumor clone frequency (TCF) as determined by high-throughput sequencing (HTS) of the TCRB gene is a reliable biomarker that identifies early stage patients at-risk of disease progression.4 Although skin-directed therapies like topical steroids can suppress the inflammatory skin lesions of MF, none are curative and skin lesions tend to recur.5–7 To accomplish the goal of finding curative therapies in CTCL, it is imperative that we better understand how our skin-directed therapies induce disease remissions and identify molecular markers that predict long-term disease control at the site of treatment.
Radiation therapy is an effective skin-directed therapy for MF.8,9 Electron beam therapy and superficial brachytherapy are the radiation modalities most commonly used to treat MF skin lesions. Delivery of electrons can be controlled to minimize penetration beyond the skin but dose conformity is lost when curved surfaces are treated. Brachytherapy uses custom-made surface molds into which radiation sources are placed using flexible catheters. Brachytherapy provides homogeneous dosing over areas with complex topography, such as the face.
Complete response rates up to 96% after localized radiation therapy are observed at doses >7 Gy.10 However, the likelihood of recurrence is inversely related to dose, with recurrence rates up to 42% seen at doses <10 Gy.11 More recently, low-dose radiotherapy using a total dose of 8 or 16 Gy has shown CR rates >90% without notable toxic effects. Most patients experience months to years of disease-free skin at the site of treatment.12,13
Topical steroids are first-line therapies to treat skin lesions in early-stage CTCL patients. Topical steroids are highly efficacious in inducing remission of the treated lesions but these responses are often short-lived.6,7 The effects of topical steroids and radiation therapy on the malignant T cell burden in the skin have not been studied, in large part because it is difficult to distinguish malignant T cells from activated benign infiltrating T-cells in early-stage lesions.
High-throughput sequencing of the TCRB gene (HTS) provides quantitative analyses of how many distinct T-cell clones are present within a sample, the relative frequency of each clone, and the exact nucleotide sequences of each T-cell clone’s complementarity determining region-3 region (CDR3).14 HTS facilitates the diagnosis of early-stage disease,15 allows tracking of specific T-cell clones over time and in different tissues,16–18 and detects low levels of residual disease after treatment.19 HTS has been used to demonstrate that topical treatment with resiquimod, a TLR8 agonist, can eradicate the malignant T cell clone from skin in a subset of MF patients.20 To date, no other skin-directed therapy has been shown to eliminate malignant T cells from MF skin lesions. In this manuscript, we find that low dose radiation therapy (LDRT, 8 Gy) but not topical steroid therapy can eliminate malignant T cells from treated MF skin lesions and that the risk of lesion recurrence correlates with the number of residual malignant T cells in skin. Moreover, patients treated with radiation therapy in our retrospective high-risk early stage patient cohort was associated with improved overall survival compared to patients with similar disease burden and prior therapies who did not receive radiation. Our study demonstrates that LDRT provides long-term durable remissions for treated lesions and is associated with improved survival in high-risk early stage CTCL patients.
Materials and Methods
Human subjects
All studies were conducted in accordance with the Declaration of Helsinki. This is an experimental laboratory study performed on human tissue samples. Written consent was obtained from all patients before study entry and sample collection. Patients who were studied met the World Health Organization-European Organization for Research and Treatment of Cancer (WHO-EORTC) criteria for MF. Each was assigned a unique study number permitting the lead author and principal investigator to have access to patient historical information. Lesional skin from patients with CTCL was obtained from patients seen at the Dana-Farber/Brigham and Women’s Cancer Center (DF/BWCC) Cutaneous Lymphoma Program. All tissues were collected with previous approval from the Partners and Dana-Farber Institutional Review Boards. J.O., A.M., N.R.L, C.L., T.S.K., P.M.D and R.C. analyzed the data and all authors had access to primary data.
Study procedures and treatment of prospective cohort
Eligibility criteria included a confirmed diagnosis of CTCL after a review of the clinical, molecular, and histological data according to the ISCL/EORTC criteria. Prior to study inclusion, treatments received by the patient were determined by physician choice. In general, every patient was treated with the least aggressive treatment that led to at least a partial response and symptomatic relief. In early-stage patients with no evidence of blood involvement, skin-directed therapies were utilized with systemic therapies implemented if skin-directed therapies alone were unable to control disease. Treatment-resistant lesions were chosen to receive radiation for this study. The patients that received topical steroids were early-stage patients who had not previously attempted a full course of alternating class I and class III pulsed topical corticosteroids, the first-line treatment implemented at our institution. Patients were enrolled only if they were consented to a pre- and post-lesional skin biopsy prior to study enrollment. Patients were excluded if they showed evidence of peripheral blood involvement at the time of study enrollment. Sixteen subjects with stage IA-IIB CTCL underwent low-dose (4 Gy x2 fractions) brachytherapy (n=12) or electron beam therapy (n=4) (Tables S1 and S2). A skin biopsy of the lesion was obtained before treatment and an adjacent skin biopsy was obtained approximately 8 weeks after therapy. During the time after treatment, the only topical therapies used were nonmedicated emollients. A separate 4 patients were treated with biweekly alternating Class I and Class III strength topical corticosteroids (TCS) for a total of 12 weeks with evaluation approximately 4 weeks after stopping steroid application. Clinical endpoints examined were lesional skin clinical response and duration of response. Lesional skin response to treatment was determined by the modified Composite Assessment of Index Lesion Severity (mCAILs).21 Responders were defined as complete (100% clearance), partial (50-99% clearance of skin disease from pre-treatment mCAILs) and stable (<25% increase to <50% clearance in skin disease from baseline) at the post-treatment evaluation period.21 Duration of response was defined as the date when criteria for response was first met until the date response was first lost.21 Two dermatologists who were blinded to treatment (N.R.L and C.L) performed this analysis using patient photographs. The only exception to this was patient 650 who had mCAILS performed at the day of evaluation by J.T.O. The data for lesion follow-up were collected at the reference time point of November 23,2018.
The 18 patients in the prospective study were a part of the DFCI-02016 longitudinal study at Dana-Farber and these cases occurred between 2010 and 2016. Eligible patients were inquired to their interest in the tissue study and all consenting patients who had a pre- and post-treatment biopsy were included. Stratification and matching were not used in case selection. Data were last collected on November 23,2018 and the median follow-up time was 3 years.
DNA and RNA isolation from skin
DNA was isolated from frozen, optimum cutting temperature compound (OCT)-embedded or FFPE skin samples as previously described4. For each DNA sample, third complementarity-determining regions (CDR3) of the TCRB gene were amplified and sequenced using ImmunoSEQ (Adaptive Biotechnologies, Seattle, WA).22–24
High throughput sequencing of the TCRB gene and determining normalized numbers of malignant T cells in the skin
The putative malignant clone was identified and malignant clone frequency was calculated as previously described.4 Total nucleated cells were estimated as previously described.15 To normalize the samples based on total input DNA, we divided the number of T cells/100 ng input DNA to get malignant T cells/100ng DNA.
All samples were anonymous and assays were performed blinded to the patient’s outcome.
Retrospective chart review of early stage patients who received radiation therapy
The initial cohort comprised 210 patients with early-stage CTCL prospectively included in study DFCI-02-016 at the DFCI Cutaneous Lymphoma Clinic from 1998 to 2016 who had high-throughput sequencing of the TCRB gene performed4 (Table S4). All patients gave informed consent, and had a confirmed diagnosis of CTCL after review of the clinical, molecular and histological data.25 CTCL staging and disease progression were evaluated according to the ISCL/EORTC criteria.21,26 High-risk patients from this 210-patient cohort were defined by the initial skin biopsy specimen having a tumor clone frequency >25% and the clinical characteristics of this cohort, including other treatments received, can be found in Table S6. Therapies received by the patients were recorded and overall survival was compared based on the use of radiation therapy or phototherapy in the early stage of the disease. Excluded from the cohort were patients who received their first radiation treatment after they had progressed to Stage IIB or greater. Cases were well-balanced with respect to age and stage in this cohort (Table S6). The end of the follow-up period was December 23,2016 and the median follow-up time was 3.9 years (range 4 months-189 months).
Clinical endpoints examined were overall survival as defined according to the ISCL/EORTC criteria.26 Overall survival is the time interval between the date of initial biopsy confirming mycosis fungoides and date of death from any cause. The data were collected at the reference time point of December 23, 2016. Analyses were performed using R version 3.4.3 and SAS 9.4.
Statistics
Primary methods of data analysis included descriptive statistics and are described in the figure legends.
For the prospective cohort, the distribution of response duration was summarized using the Kaplan-Meier method with significance determined by the log-rank test and defined as the time between the pre-treatment biopsy and either worsening of treated lesion or censoring at last follow-up. Age, gender, serum LDH, folliculotropism, large-cell transformation, the tumor clone frequency, number of skin-directed therapies received, number of systemic therapies received, BSA affected and clone reduction as determined by number of malignant cells/100 ng DNA were assessed for their association with disease recurrence using univariable logistic regression. Since there were only 20 lesions and 6 recurrences, multivariable models were not constructed. Each model had recurrence/no recurrence as the binary outcome. Odds ratios were based on marginal inference using generalized estimated equations to account for repeated measurements. The issue of sparse/zero data was addressed using Firth’s penalized likelihood.
Given the rarity of CTCL and the willingness of patients to agree to a post-treatment biopsy, this study was not powered to detect a specific effect size.
For the retrospective cohort, high-risk patients were defined by the initial skin biopsy specimen having a tumor clone frequency >25%.4 Patients were then stratified based on whether they had received radiation therapy or not (Fig. 6) or whether they had phototherapy or not (Fig. S3). Age, gender, stage, LDH, folliculotropism, large cell transformation, number of skin-directed therapies received, number of systemic therapies received, BSA affected, presence of plaques, involvement of lymph nodes21, and whether the patient received radiation therapy or phototherapy were assessed for their association with overall survival using univariable Cox proportional hazards models. Hazard ratios are presented with 95% confidence intervals estimated using log(-log) transformation; inference was based on Wald p-values. Survival probabilities were estimated using the method of Kaplan-Meier.
Figure 6. Treatment of high-risk early stage MF patients with radiation therapy is associated with increased overall survival.
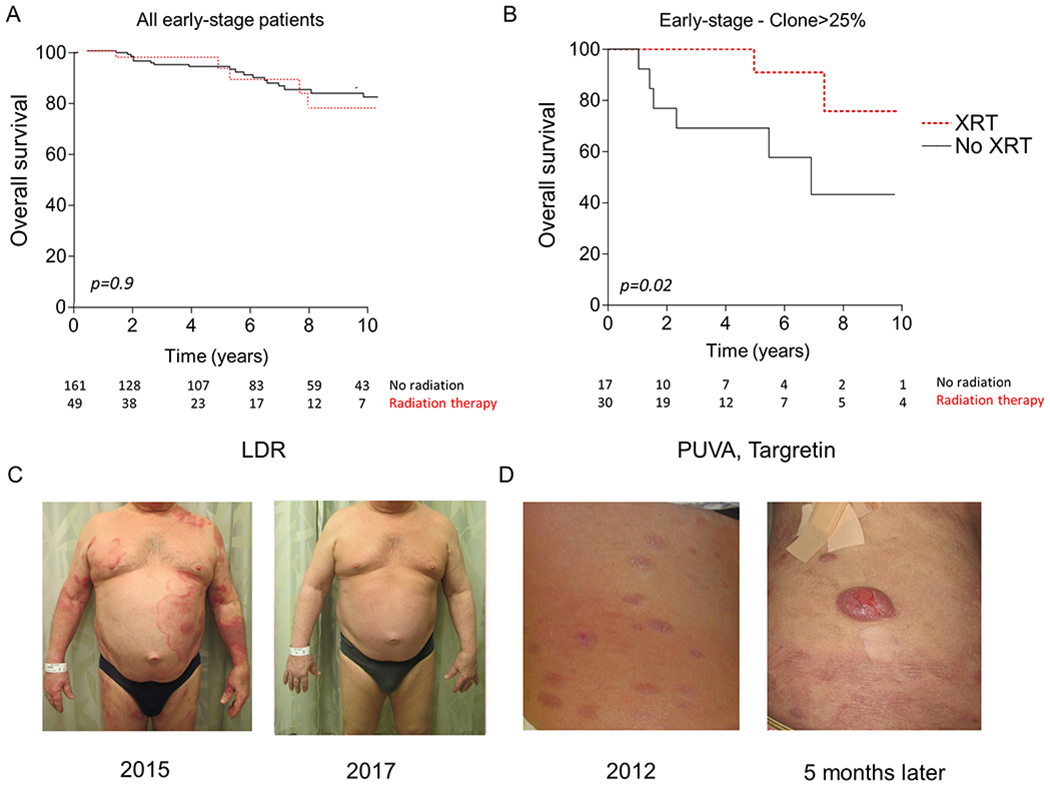
(A) Kaplan-Meier estimates of overall survival in 210 patients with early-stage mycosis fungoides, according to the use of radiation therapy (brachytherapy or electron-beam therapy) in early stage patients. (B) Kaplan-Meier estimates of overall survival in 47 high-risk patients (defined as skin lesions with >25% TCF) with early-stage mycosis fungoides, according to the use of radiation therapy. Numbers at-risk are indicated at the bottom and curves were compared using the log-rank test, p<0.05 considered significant. (C) Clinical photographs of a Stage IB patient who received subsequent low-dose total skin electron beam with resultant complete remission that has remained stable over the past 3 years managed conservatively with NBUVB. (D) Clinical photographs of Stage IB patient treated with PUVA and bexarotene who then progressed to tumoral nodules and peripheral blood involvement 5 months later. This patient ultimately succumbed to her lymphoma.
RESULTS
Low dose radiation therapy can eradicate the malignant T cell clone in MF patches and plaques
Patients with patches and plaques of MF often have excellent initial response rates when treated with either palliative low-dose radiation doses >7 Gy or topical corticosteroids (TCS).6,7,11,12,26,27 While both therapies reduce inflammation, it is unclear to what degree these therapies reduce the malignant T cell clone. We studied the lesional skin of patients with MF before and after treatment with either TCS or LDRT using mCAILS to grade clinical inflammation and HTS to quantitate the malignant T cell clone. For the TCS treated group, four stage IA patients were treated for a total of 12 weeks with a wash-out period until the post-treatment biopsy was taken approximately 4 weeks later (range 2-14 weeks) (Supplemental Table 1). For the LDRT group, 12 lesions in 11 patients received 2 fractions of 4 Gy radiation delivered by either electron beam (n=4) or brachytherapy (n=8) and post-treatment biopsies were taken approximately 8 weeks after therapy (range 7-45 weeks, median 9 weeks, Table S2). The pre-treatment mCAILS was similar between all groups of patients. All patients had reduced mCAILS post-treatment ranging from 43-100% (Fig. 1A–B, Fig. S1). There were 6 complete responses (CR), 5 partial responses (PR) and one stable lesion (SD) in patients treated with LDRT. All lesions treated with TCS had a CR. These results suggest that both treatments were effective in reducing clinical inflammation.
Figure 1. HTS sensitively quantifies residual malignant T cells in skin after therapy and reveals that LDRT but not TCS can eradicate the malignant T cell.
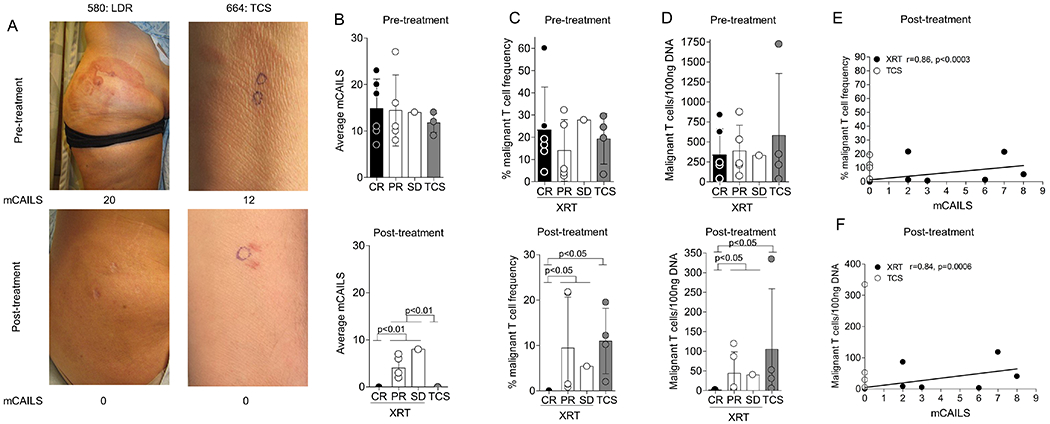
(A) Representative photographs and corresponding mCAILS of complete responders showing before and after therapy (LDRT, left and TCS, right). (B-D) Combined dot plot and bar graphs showing average mCAILS (B), % malignant clone frequency (C), and malignant T cells/100ng DNA (D) between the LDRT treatment response subgroup and the TCS treated group before (upper panels) and after (lower panels) treatment. Significance was determined between post-XRT treated groups by the Kruskal-Wallis Test followed by Dunn’s multiple comparison test. (E-F) Dot plot and Spearman correlation between the % TCF and mCAILS (E) or malignant T cells/100ng DNA and mCAILS (F) in post-treatment biopsies. Spearman’s r and p-value are indicated with p<0.05 considered significant.
To assess the malignant clone burden, we identified the malignant T cell clone as the top productive sequence in the pre-treatment skin biopsy and looked at both the malignant clone frequency as a measure of all infiltrating T cells as well as the normalized numbers of malignant T cells per unit skin (ie malignant T cells/100ng input DNA) as both these measures have clinical significance.4,15 The pre-treatment TCF and malignant T cells/100ng DNA were similar between groups (p=0.9) (Fig. 1C–D). Six lesions receiving LDRT showed a CR with malignant clone eradication in 4 lesions and a residual TCF <0.05% in the other 2 lesions. Lesions that showed a PR or SD, in contrast, had significantly higher residual TCF (range 0.8-22%) and malignant T cells/100ng DNA (range 3-119) compared to lesions that had a CR (p<0.05) (Fig. 1C–D). The number of malignant T cells in skin and mCAILs were positively correlated (Fig. 1E–F). In contrast, the TCS treated lesions did not show a significant reduction in malignant T cells despite complete resolution of clinical inflammation (p=0.2) (Fig. 1D–F). These results suggested that normal appearing skin may still harbor residual malignant T cells.
LDRT can induce long-term remissions in heavily pre-treated MF tumors
The efficacy of LDRT in heavily pre-treated tumoral MF lesions of the syringotropic variant is not well-characterized.27–29 We report a 69 year-old male with a history of CLL and MF who developed debilitating tumors over a 3 month period on the plantar surface of the left foot (Figure 2A). Prior therapies, including phototherapy, acitretin and methotrexate were unsuccessful. A biopsy showed syringotropic CTCL. At the time of presentation to our clinic, the patient had been unable to walk for approximately 2 years secondary to pain. A decision was made to treat the affected area with palliative LDRT. The patient went into complete remission with residual hyperkeratosis over the biopsy site scar (Figure 2B).
Figure 2. Syringotropic tumoral nodule resistant to topical, light and systemic therapies completely responds to 4 Gray (Gy) x2 radiation with long-term durable response with >99% reduction of malignant T cell burden.
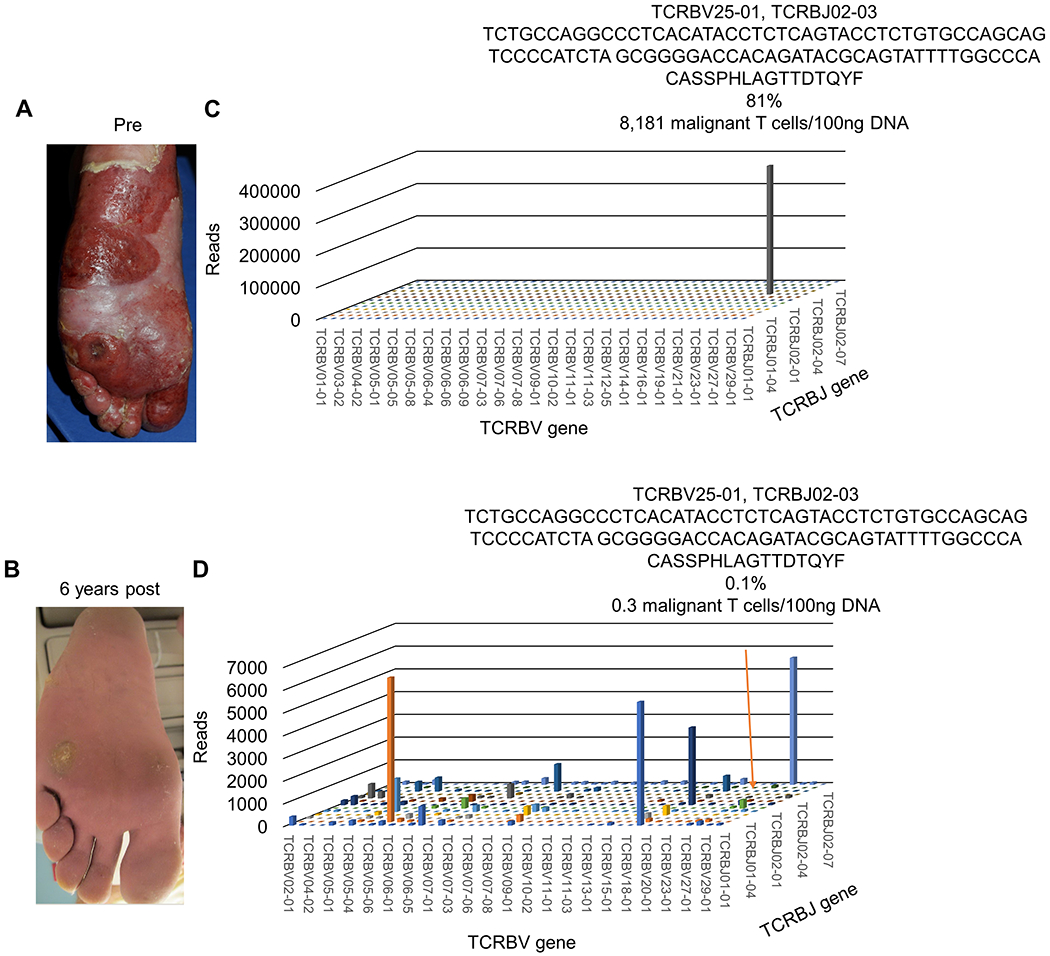
(A) Photo of tumoral nodule and plaques on left plantar foot prior to LDRT and (B) 6 years after LDRT showing residual biopsy scar but continued remission at site of therapy. (C) 3D histogram of the TCRB sequencing data shows V and J gene expression of hyperexpanded malignant T cell clone with resultant nucleotide and translated amino acid (AA) sequence of the CDR3 region. Based on productive templates, HTS revealed a malignant clone frequency of 81% and 8,181 malignant T cells/100ng DNA. (D) 3D histogram of the TCRB sequencing data shows a polyclonal population of benign T cells with a drastic reduction of the original tumor clone frequency to 0.14% and after normalization of sequencing data, the equivalent of 0.3 residual malignant T cells/100ng DNA (orange arrow).
Over six years the patient has developed additional patches and plaques at other body sites while the treated area has remained clear of disease. To determine the malignant clone burden at this quiescent site, HTS was performed on the DNA from the original diagnostic biopsy and on a second adjacent biopsy taken six years later. The malignant clone comprised 81% of the total T cells in the skin at initial biopsy at 8,181 malignant T cells/100ng DNA. The TCF was reduced to 0.1% with a corresponding 0.3 malignant T cells/100ng DNA (Fig 2C–D). This suggested that LDRT can drastically reduce the malignant clone burden and induce a long-term durable remission in heavily pre-treated MF tumors.
LDRT can eradicate the clone in folliculotropic MF
Folliculotropic CTCL can be difficult to treat, often occurs in cosmetically sensitive areas like the scalp and face, and definitive doses of radiation are associated with permanent alopecia.30 A 40-year-old woman with folliculotropic CTCL developed thick alopecic plaques of her scalp that were unresponsive to TCS, carmustine, and nitrogen mustard with a PET-CT showing enlarged dermatopathic nodes and HTS showing a TCF of 19% (Figure 3A–C). The patient underwent LDRT and experienced a complete clinical clearance and resolution of inflamed lymph nodes 11 weeks after therapy and hair regrowth 6 months later (Fig. 3D–E). Interestingly, the patient had lighter, curlier hair upon regrowth, which has been reported in conjunction with electron beam radiation and chemotherapeutics.31 HTS revealed eradication of the malignant T cell clone (Fig. 3F). Thus, LDRT can be an effective therapy for folliculotropic MF that spares permanent hair loss.
Figure 3. LDRT spares hair follicle destruction and can eradicate the malignant T cell clone in folliculotropic CTCL.
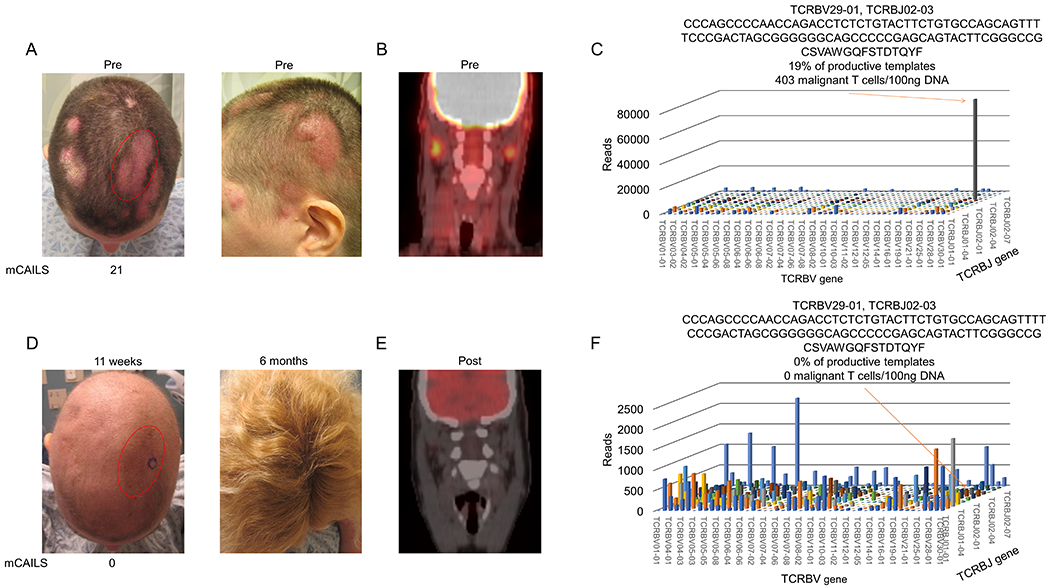
(A) Clinical photographs showing numerous erythematous plaques with overlying scale (left) with index lesion scored by mCAILS highlighted in red. Photo on right demonstrates thickness of plaques. (B) PET-CT scan showing PET-avid dermatopathic skin-draining lymph nodes prior to therapy. (C) 11 weeks after LDRT, clinical photograph showing anagen effluvium and clearance of original plaque stage disease (left) with 6-month follow-up picture (right) showing complete hair regrowth. (D) Repeat PET-CT scan after therapy showing resolution of PET-avid lymph nodes. (E) 3D histogram of TCRB sequencing showing V and J gene expression of hyperexpanded malignant T cell clone with resultant nucleotide and translated amino acid (AA) sequence of the CDR3 region. Based on productive templates, HTS revealed a malignant clone frequency of 19% and 403 malignant T cells/100ng DNA. (F) 3D histogram of TCRB sequencing shows eradication of the malignant T cell clone with recovery of benign T cell populations.
The malignant clone persists after LDRT in MF granulomatous slack skin lesions
The granulomatous slack skin (GSS) variant of CTCL is often refractory to therapy.32 The effectiveness of LDRT in GSS is not well-characterized. We used HTS to measure the malignant T cell clone post-LDRT in 2 GSS patients. Both patients had a >10-year history of GSS and had failed at least 5 therapies, including prior treatments with radiation therapy (Supplemental Table 1). They both agreed to undergo another round of LDRT for palliation. Patient 322 received LDRT to a GSS buttock lesion (Figure 4A). At the 11-week follow-up appointment, mCAILS was similar and HTS revealed an increase in both the TCF (43% to 71%) and residual malignant T cells/100ng DNA (1124 to 1419 T cells/100ng DNA) (Figure 4B–D).
Figure 4. Two patients with the granulomatous slack skin (GSS) variant of CTCL had persistent clinical disease and malignant T cell burden after LDRT.
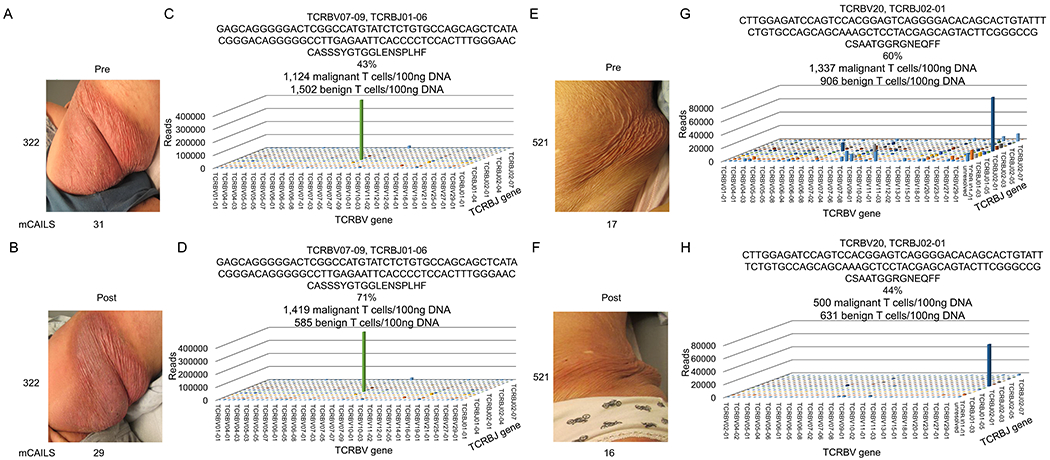
Clinical photographs of patient 322 with corresponding mCAILS scores (A) before and (B) after treatment with LDRT. 3D TCR histogram shows a greatly expanded clone (C) before therapy that increases in both frequency and normalized numbers of malignant T cells (D) after therapy with concomitant decrease in benign T cells. Clinical photographs of patient 521 with corresponding mCAILS score (E) before and (F) after LDRT. (G) 3D TCR histograms show a highly expanded clone before therapy. (H) After therapy, the clone remained highly expanded both in relative frequency and number of malignant T cells/100ng DNA after therapy with a similar number of benign T cells.
Patient 521 received LDRT to a GSS left flank lesion (Fig. 4E). Similar to patient 322, there was minimal improvement in mCAILS (Fig. 4F). While HTS of her pre- and post-treatment lesional skin showed a modest reduction of her TCF (60% to 44%) and malignant T cells/100ng DNA (1,337 to 500 T cells/100ng DNA), the clone persisted at higher levels than the other MF variants studied (Fig. 4H–K). These observations suggest that the malignant T cells present in GSS skin lesions may not be as responsive to LDRT compared to other MF skin lesions.
HTS monitoring of malignant T cells reveal dynamic changes in the skin after treatment
In aggregate, a total of 18 patients and 20 MF lesions were examined. The mCAILS improved to varying degrees in all treated lesions with a range of 6-100% (Fig. 5A). The lesions that had a CR to LDRT had a significantly lower residual TCF and malignant T cells/100 ng DNA compared to those lesions that only showed a PR or SD (p<0.01) (Fig. 5B–D). All skin lesions that underwent a CR demonstrated a >99% reduction of malignant T cells/100ng DNA with 5 lesions showing clone eradication while the malignant T cells in steroid-treated lesions persisted at significantly higher numbers (p<0.04) (Fig. 5D–E).
Figure 5. Low residual malignant clone burden predicts long-term durable responses.
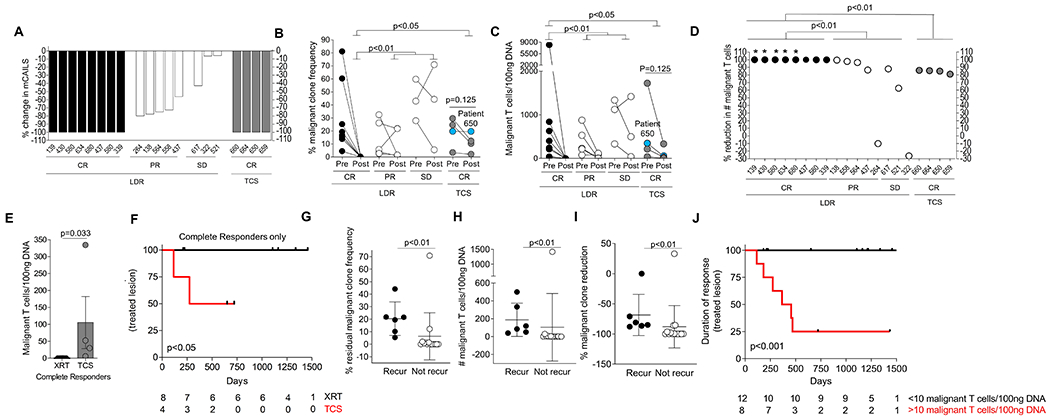
(A) Waterfall plot showing the % reduction in mCAILS scores after therapy between LDRT and TCS treated lesions with complete responders (CR) showing 100% reduction, partial responders (PR) showing 50-99% reduction and stable disease (SD) lesions showing <50% reduction. (B-C) Aggregate data showing the (B) % malignant T cell clone frequency or (C) Malignant T cells/100ng DNA before and after treatment with connecting lines representing individual patients. Patient 650 (blue dot) had a similar TCF in pre- and post-treatment biopsy but reduced normalized numbers of malignant T cells. Significance was determined between groups by the Kruskal-Wallis Test followed by Dunn’s multiple comparison test. P<0.05 was considered significant. (D) Dot plot showing the % reduction in the number of malignant T cells/100ng DNA after therapy with radiaiton (black circles=CR, white circles=PR and SD) or TCS (gray circles, all CR) groups. *=no clone detectable. (E) Dot plot and bar graph of the residual malignant T cell/100ng DNA in the post-treatment biopsies in the complete responders treated with either radiation or TCS. Significance determined by Mann-Whitney U test. (F) Kaplan-Meier estimates of treated lesion recurrence comparing 8 treated lesions with LDRT (black) and 4 TCS treated lesions (red). Numbers at-risk are indicated at the bottom. (G-I) Dot plot comparing lesions in both treatment groups that either recurred or remained in remission looking at (G) malignant T cells/100ng DNA, (H) % residual TCF and (I) % malignant clone reduction of malignant T cells/100ng DNA. Significance determined by Mann-Whitney U test with p-value <0.05 considered significant. (J) Kaplan-Meier estimates of treated lesion recurrence comparing 20 treated lesions with LDRT or TCS based on residual malignant T cells/100ng DNA at a binary cutoff of 10. Numbers at-risk are indicated at the bottom. p-values determined by log-rank test.
One notable finding is the observation that although lesions can have a similar TCF before and after treatment, the malignant T cells/100ng DNA were reduced in the post-treatment biopsy. (Fig. 5C–D). For example, patient 650 (blue circle) had an MF patch treated with TCS that showed a TCF of 19% in both the pre- and post-treatment skin biopsy. However, there was an 85% reduction in the malignant T cells/100ng DNA post-treatment. This suggested that examining the malignant T cells per unit skin was a more precise measure of malignant clone burden than solely examining TCF.
The degree of residual malignant clone burden is predictive of lesion recurrence
Treatment with topical corticosteroids abrogated clinical inflammation but these lesions maintained high numbers of malignant T cells. We hypothesized that the duration of response is associated with the amount of residual malignant T cells in the skin. To address this, we have prospectively followed all lesions for the LDRT and TCS cohorts for a median time of 3.1 years (range 0.5 years-4.3 years) and 1.3 years (range 0.8-1.8 years), respectively. Among lesions that showed a CR, 50% of the TCS treated lesions recurred but no lesions in the LDRT group have recurred (Fig. 5F). These data suggest that lesion recurrence likely occurs secondary to the presence of residual malignant T cells in the skin.
To determine if the degree of the residual malignant clone burden in the post-treatment biopsy was associated with loss of treatment response, we examined a variety of clinical and molecular parameters (Fig. 5 G–I and Fig. S2). Significant parameters that differentiated lesions that have recurred or worsened versus ones that have not included >10 malignant T cells/100ng DNA, a residual TCF >5%, and a malignant clone reduction <90%. Lesions that contained fewer than 10 malignant T cells/100ng DNA continued to be in remission. Of the 8 lesions that showed >10 malignant T cells/100ng DNA, 6 lesions have recurred or worsened (p<0.009), (Fig. 5G–I). The number of residual benign T cells, therapy received, body site treated and number of failed therapies were not significantly associated with recurrence (Fig. S2).
To assess how the residual malignant clone burden compared to other adverse risk factors such as age, gender, stage, presence of plaques, folliculotropism, and number of treatments received, we performed a univariable analysis including all 20 treated lesions. There were 6 recurrences including one GSS patient that had worsening of the lesion one year after treatment. Residual malignant clonal burden was the only variable examined significantly related to recurrence at a cutoff of 10 malignant clones/100 ng DNA (OR (> 10 vs. ≤ 10) 65.0, 95% CI: (2.3-∞), p<0.01) (Table S3). Duration of response was also significantly associated with the number of residual malignant T cells/100 ng DNA (Fig. 5J). These data strongly suggest that diminution of the malignant clone burden to <10 cells/100ng DNA is associated with long-term disease control of the treated site.
Radiation therapy is associated with an improved overall survival in early-stage CTCL patients at high risk of disease progression
Recently, we found that early stage patients who had skin biopsies containing a TCF >25% were at 5 fold-higher risk of disease progression.4 In addition, there was a positive linear correlation of increased TCF and time to progression or death.4 Earlier studies in CTCL suggested that progression of disease can be slowed using electron beam therapy.33–36 Given that LDRT can eradicate the malignant T cell clone in a subset of treated lesions, we hypothesized that treatment with radiation in early-stage patients at high risk of disease progression may be associated with improved survival compared to patients who did not receive radiation. To examine this, we retrospectively examined 210 early-stage patients (Stage IA-IIA) and the treatments they received with a median follow-up time of 6.1 years (Supplemental Table 4). When we examined all patients without stratifying for patients at-risk, we did not find a difference in overall survival between early stage patients who received radiation and patients who did not (Fig. 6A). However, when we risk-stratified our cohort to only examine high-risk patients (TCF>25%)4, improved overall survival was associated with those early-stage patients receiving radiation (Fig. 6B). In addition, when we examined patients who received radiation at tumor stage but before having blood and lymph node involvement, radiation continued to show an association with improved overall survival (p=0.04) (Fig. S4A). In contrast, the use of phototherapy (UVB or psoralen +UVA) was not associated with improved OS in these high-risk patients (Fig. S3). In this high-risk group, we found patients that received LDRT to lesions early in their disease course often experienced stable control of their disease with further skin-directed therapies (Fig. 6C). We also found a subset of patients that did not receive radiation or only received radiation in the later stages of disease continued to progress (Fig. 6D).
We also performed further subgroup analyses looking at the association of radiation therapy with overall survival looking lymphoma-specific deaths (n=5) and the time of first radiation treatment to death/censoring. There was a trend of improved overall survival when lymphoma-specific deaths were examined (p=.17) but interpretation of this result is limited given the low number of events in this subgroup (Fig. S4B). Lastly, we determined that there was also a trend toward improved survival from the time of initial radiation therapy (Fig. S4C).
A univariable analysis was performed for all standard prognostic variables to determine if there were other associated variables associated with overall survival in this high-risk cohort. Stage (IA vs >IA) and treatment with radiation remained statistically significant in this comparison (Table S5). To assess whether this finding was confounded by patients not receiving radiation due to more extensive and/or aggressive disease, we compared age, stage, BSA affected, types and number of therapies taken by the patients and found no significant difference in these parameters (Supplemental Tables 6 and 7). Therefore, radiation therapy performed in the early stage high-risk patients was associated with improved overall survival.
DISCUSSION
The optimal outcome for any cancer therapy is eradication of the malignant cells. In this report, we find that LDRT but not topical steroids can eradicate the malignant T cell clone in a subset of patients and this eradication was associated with long-term remissions. Using the highly sensitive technique of HTS, we found that all lesions that showed a complete response had a malignant T cell clone reduction >99% with an undetectable clone in 75% of those LDRT treated lesions. In contrast to LDRT, four patients treated with topical steroids had a complete clinical response but a reservoir of skin malignant T cells remained. Prospective follow-up of these 2 treated patient populations demonstrated that >10 residual malignant T cells/100ng DNA was a significant risk factor for localized disease recurrence. In a retrospective review of high-risk early stage patients, we found an association with improved overall survival in those patients who received radiation therapy compared to those patients who did not. Thus, radiation therapy is a highly effective skin-directed therapy for MF patients and its use in high-risk early-stage patients may positively impact overall survival.
Low dose radiation regimens consisting of low-dose treatments totaling >7 Gy are becoming more commonplace in clinical practice because of the regimens’ high efficacy and improved side effect profile compared to the traditional >24 Gy treatment regimens.12,37 These low-dose regimens are considered palliative but our results suggest that LDRT treated patients who have eradication of malignant T cells may have a localized cure in treated lesions. We acknowledge that longer follow-up over the lifetime of the patient is needed before a localized cure can be definitively determined. Lastly, it is important to note that our HTS studies are representative of the response only in the sampled area and may not reflect responses in other lesions.
Using a low-dose radiation regimen of 4 Gy x2, our study experienced a decreased complete response rate compared to earlier studies (50% vs 92-96%).12,13,37,38 However, these prior studies enrolled patients earlier in their disease course after disease progression on topical steroids, did not use a validated skin assessment such as mCAILS, and had a shorter time to follow-up for first assessment. Our patient cohort progressed on a median of 3 treatments before undergoing LDRT. It is likely that this study population’s skin lesions were more treatment-resistant at baseline and may account for the lower rate of complete responders compared to prior studies.
In other T-cell hematologic malignancies, identification of molecular relapse in the peripheral blood of patients using HTS of the TCRB gene predicts clinical recurrence of disease.14,18,39,40 In CTCL, the identification of the malignant T cell clone in the blood preceded clinical recurrence of disease in those patients who experienced complete clinical remission after undergoing allogeneic stem cell transplant.19 Our data are in line with these prior studies. We find that HTS of the TCRB gene helps to identify patients who will develop long-term control of the treated lesion as well as those lesions most at-risk for recurrence, two critical pieces of information that cannot be attained from the clinical exam.
Strengths of our study include the prospective nature of our topical steroid and LDRT cohorts, the large number of total early stage CTCL patients studied in the retrospective analysis and the high sensitivity and specificity of HTS used to identify the residual malignant clone burden. Limitations of our study include the low number of patients in our prospective cohort and the relative short follow-up time to ascertain lesion recurrence. Because of the generally indolent nature of this lymphoma, recurrence can take many years and patients in our cohort could recur at later time points. Continued observation of these patients and accrual of additional patients is planned.
No therapy thus far in CTCL has been shown to prolong overall survival although early studies suggested that total-skin electron-beam radiation therapy (≥3000 cGy) may be curative in a subset of early-stage patients.35,41,42 Subsequent studies evaluating the impact of other skin-directed therapies in early-stage patients have not shown any survival differences. More recent studies highlight differential effects of PUVA and resiquimod on the malignant T cell clone burden. Both therapies show high rates of clinical remissions at the treated site but skin treated with PUVA continued to harbor residual malignant T cell clones in the skin while resiquimod eradicated the clone in 30% of treated lesions.20,43 With the recent finding that TCF is significantly associated with disease progression in early-stage patients, we now have a method to more specifically assess whether skin-directed treatments impact overall survival in an enriched cohort of at-risk early stage CTCL patients. Indeed, the most significant finding from this study is that high-risk patients who received radiation therapy at an early stage had an improved overall survival compared to patients who did not have radiation as part of their treatment regimen. This association with overall survival was not seen with phototherapy, a skin-directed therapy that is not clone-ablative but with high efficacy in inducing complete responses. While these findings need to be validated prospectively, it supports the idea that certain novel therapies like LDRT may prolong life in patients at high-risk for disease progression. Therapies that have the potential for clone ablation may allow early control of what might otherwise be a progressive lymphoma.
In conclusion, the high clinical response rates and long-term durable control of LDRT is directly associated with the magnitude of malignant T cell reduction in treated lesions. The association with improved overall survival in patients that received radiation treatment early in their disease course suggests that LDRT may be an effective preemptive strike that eliminates high-risk skin lesions. These findings provide a clear rationale for the initiation of future prospective studies examining whether LDRT can improve progression-free and overall survival compared to other first-line skin-directed therapies in high-risk early stage CTCL patients.
Supplementary Material
Translational Relevance:
Mycosis fungoides is a subtype of cutaneous T-cell lymphoma (CTCL) characterized by inflammatory skin lesions that contain malignant T cells. Disease recurrences are common with skin-directed therapies. In this study, we used high-throughput sequencing of the TCRB gene to identify and follow the malignant clone after treatment with either topical steroids or low dose radiation therapy (LDRT). The data support that LDRT, but not topical steroids, can eradicate the malignant T-cells in the treated skin and this is associated with long-term durable remissions. A retrospective analysis of high-risk early stage CTCL patients who received radiation showed an association with improved overall survival. These findings suggest that earlier institution of a clone-eradication therapy like radiation may impact overall survival of early stage CTCL patients.
Acknowledgments
We thank Frank Kuo and the Pathology Specimen Locator Core of the Dana-Farber Harvard Cancer Center.
Financial Support: This study was supported by charitable contributions from E. P. Lawrence and from the Lubin Family Foundation. Support was also obtained from NIH R01 AR063962 (to R.A.C.), NIH P30 AR069625 (to R.A.C.), NIH R01CA203721 (to R.A.C. and T.S.K.), NIH Specialized Program of Research Excellence grant P50 CA9368305 (to T.S.K.) and NIH T32 AR007098 (to T.S.K.). Support also came from a CLARIONS grant from the Cutaneous Lymphoma Foundation (to J.T.O), the Société Française de Dermatologie, Collége des Enseignants de Dermatologie de France, Association pour la Recherche contre le Cancer, Fondation Rene Touraine, and the Philippe Foundation (to A.d.M.). This work was conducted with support from Harvard Catalyst at the Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH award UL1 TR001102 to J.T.O) and financial contributions from Harvard University and its affiliated academic health care centers.
Footnotes
Conflict of Interest Disclosures: T.S.K., R.A.C., J.T.O., and A.d.M. are inventors on patent application US 62/653,854 submitted by The Brigham and Women’s Hospital Inc. that covers the high-throughput screening of TCR genes to predict CTCL progression and prognosis. T.S.K. serves on the Scientific Advisory Board (Hematology) of Adaptive Biotechnologies but does not own stock or receive compensation.
References
- 1.Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350(19):1978–1988. [DOI] [PubMed] [Google Scholar]
- 2.Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28(31):4730–4739. [DOI] [PubMed] [Google Scholar]
- 3.Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139(7):857–866. [DOI] [PubMed] [Google Scholar]
- 4.de Masson A, O’Malley JT, Elco CP, et al. High-throughput sequencing of the T cell receptor beta gene identifies aggressive early-stage mycosis fungoides. Sci Transl Med. 2018;10(440). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zackheim HS. Topical carmustine (BCNU) in the treatment of mycosis fungoides. Dermatol Ther. 2003;16(4):299–302. [DOI] [PubMed] [Google Scholar]
- 6.Zackheim HS. Treatment of patch-stage mycosis fungoides with topical corticosteroids. Dermatol Ther. 2003;16(4):283–287. [DOI] [PubMed] [Google Scholar]
- 7.Zackheim HS, Kashani-Sabet M, Amin S. Topical corticosteroids for mycosis fungoides. Experience in 79 patients. Arch Dermatol. 1998;134(8):949–954. [DOI] [PubMed] [Google Scholar]
- 8.Hoppe RT. Mycosis fungoides: radiation therapy. Dermatol Ther. 2003;16(4):347–354. [DOI] [PubMed] [Google Scholar]
- 9.Trowell OA. The sensitivity of lymphocytes to ionising radiation. J Pathol Bacteriol. 1952;64(4):687–704. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Nisce LZ, D’Anglo GJ. Dose-time fractionation study in patients with mycosis fungoides and lymphoma cutis. Radiology. 1976;119(2):439–442. [DOI] [PubMed] [Google Scholar]
- 11.Cotter GW, Baglan RJ, Wasserman TH, Mill W. Palliative radiation treatment of cutaneous mycosis fungoides--a dose response. Int J Radiat Oncol Biol Phys. 1983;9(10):1477–1480. [DOI] [PubMed] [Google Scholar]
- 12.Thomas TO, Agrawal P, Guitart J, et al. Outcome of patients treated with a single-fraction dose of palliative radiation for cutaneous T-cell lymphoma. Int J Radiat Oncol Biol Phys. 2013;85(3):747–753. [DOI] [PubMed] [Google Scholar]
- 13.Neelis KJ, Schimmel EC, Vermeer MH, Senff NJ, Willemze R, Noordijk EM. Low-dose palliative radiotherapy for cutaneous B- and T-cell lymphomas. Int J Radiat Oncol Biol Phys. 2009;74(1):154–158. [DOI] [PubMed] [Google Scholar]
- 14.Robins HS, Campregher PV, Srivastava SK, et al. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114(19):4099–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirsch IR, Watanabe R, O’Malley JT, et al. TCR sequencing facilitates diagnosis and identifies mature T cells as the cell of origin in CTCL. Sci Transl Med. 2015;7(308):308ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaide O, Emerson RO, Jiang X, et al. Common clonal origin of central and resident memory T cells following skin immunization. Nat Med. 2015;21(6):647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu D, Emerson RO, Sherwood A, et al. Detection of minimal residual disease in B lymphoblastic leukemia by high-throughput sequencing of IGH. Clin Cancer Res. 2014;20(17):4540–4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu D, Sherwood A, Fromm JR, et al. High-throughput sequencing detects minimal residual disease in acute T lymphoblastic leukemia. Sci Transl Med. 2012;4(134):134ra163. [DOI] [PubMed] [Google Scholar]
- 19.Weng WK, Armstrong R, Arai S, Desmarais C, Hoppe R, Kim YH. Minimal residual disease monitoring with high-throughput sequencing of T cell receptors in cutaneous T cell lymphoma. Sci Transl Med. 2013;5(214):214ra171. [DOI] [PubMed] [Google Scholar]
- 20.Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126(12):1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29(18):2598–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefranc MP. IMGT, The International ImMunoGeneTics Information System, http://imgt.cines.fr. Methods Mol Biol. 2004;248:27–49. [DOI] [PubMed] [Google Scholar]
- 23.Lefranc MP. IMGT, the International ImMunoGeneTics Information System. Cold Spring Harb Protoc. 2011;2011(6):595–603. [DOI] [PubMed] [Google Scholar]
- 24.Lefranc MP, Giudicelli V, Ginestoux C, et al. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 2009;37(Database issue):D1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):3768–3785. [DOI] [PubMed] [Google Scholar]
- 26.Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110(6):1713–1722. [DOI] [PubMed] [Google Scholar]
- 27.Thein M, Ravat F, Orchard G, Calonje E, Russell-Jones R. Syringotropic cutaneous T-cell lymphoma: an immunophenotypic and genotypic study of five cases. Br J Dermatol. 2004;151(1):216–226. [DOI] [PubMed] [Google Scholar]
- 28.Venturini A, Zane C, Rodella R, Leali C, Calzavara Pinton P, Zorzi F. Syringotropic cutaneous T cell lymphoma treated with PUVA therapy. Eur J Dermatol. 2005;15(4):262–264. [PubMed] [Google Scholar]
- 29.Yost JM, Do TT, Kovalszki K, Su L, Anderson TF, Gudjonsson JE. Two cases of syringotropic cutaneous T-cell lymphoma and review of the literature. J Am Acad Dermatol. 2009;61(1):133–138. [DOI] [PubMed] [Google Scholar]
- 30.Gerami P, Rosen S, Kuzel T, Boone SL, Guitart J. Folliculotropic mycosis fungoides: an aggressive variant of cutaneous T-cell lymphoma. Arch Dermatol. 2008;144(6):738–746. [DOI] [PubMed] [Google Scholar]
- 31.Hinds G, Thomas VD. Malignancy and cancer treatment-related hair and nail changes. Dermatol Clin. 2008;26(1):59–68, viii. [DOI] [PubMed] [Google Scholar]
- 32.Kempf W, Ostheeren-Michaelis S, Paulli M, et al. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the Cutaneous Lymphoma Histopathology Task Force Group of the European Organization For Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144(12):1609–1617. [DOI] [PubMed] [Google Scholar]
- 33.Jones GW, Hoppe RT, Glatstein E. Electron beam treatment for cutaneous T-cell lymphoma. Hematol Oncol Clin North Am. 1995;9(5):1057–1076. [PubMed] [Google Scholar]
- 34.Jones GW, Kacinski BM, Wilson LD, et al. Total skin electron radiation in the management of mycosis fungoides: Consensus of the European Organization for Research and Treatment of Cancer (EORTC) Cutaneous Lymphoma Project Group. J Am Acad Dermatol. 2002;47(3):364–370. [DOI] [PubMed] [Google Scholar]
- 35.Tadros AA, Tepperman BS, Hryniuk WM, et al. Total skin electron irradiation for mycosis fungoides: failure analysis and prognostic factors. Int J Radiat Oncol Biol Phys. 1983;9(9):1279–1287. [DOI] [PubMed] [Google Scholar]
- 36.Micaily B, Miyamoto C, Kantor G, et al. Radiotherapy for unilesional mycosis fungoides. Int J Radiat Oncol Biol Phys. 1998;42(2):361–364. [DOI] [PubMed] [Google Scholar]
- 37.Goddard AL, Vleugels RA, LeBoeuf NR, et al. Palliative Therapy for Recalcitrant Cutaneous T-Cell Lymphoma of the Hands and Feet With Low-Dose, High Dose-Rate Brachytherapy. JAMA Dermatol. 2015;151(12):1354–1357. [DOI] [PubMed] [Google Scholar]
- 38.DeSimone JA, Guenova E, Carter JB, et al. Low-dose high-dose-rate brachytherapy in the treatment of facial lesions of cutaneous T-cell lymphoma. J Am Acad Dermatol. 2013;69(1):61–65. [DOI] [PubMed] [Google Scholar]
- 39.van Dongen JJ, van der Velden VH, Bruggemann M, Orfao A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood. 2015;125(26):3996–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Logan AC, Gao H, Wang C, et al. High-throughput VDJ sequencing for quantification of minimal residual disease in chronic lymphocytic leukemia and immune reconstitution assessment. Proc Natl Acad Sci U S A. 2011;108(52):21194–21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoppe RT, Cox RS, Fuks Z, Price NM, Bagshaw MA, Farber EM. Electron-beam therapy for mycosis fungoides: the Stanford University experience. Cancer Treat Rep. 1979;63(4):691–700. [PubMed] [Google Scholar]
- 42.Kaye FJ, Bunn PA Jr., Steinberg SM, et al. A randomized trial comparing combination electron-beam radiation and chemotherapy with topical therapy in the initial treatment of mycosis fungoides. N Engl J Med. 1989;321(26):1784–1790. [DOI] [PubMed] [Google Scholar]
- 43.Vieyra-Garcia P, Crouch JD, O’Malley JT, et al. Benign T cells drive clinical skin inflammation in cutaneous T cell lymphoma. JCI Insight. 2019;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


