Abstract
Proteases are enzymes that have the capacity to hydrolyze peptide bonds and degrade other proteins. Proteases can promote inflammation by regulating expression and activity of different pro-inflammatory cytokines, chemokines and other immune components in the lung compartment. They are categorized in three major subcategories: serine proteases, metalloproteases and cysteine proteases especially in case of lung diseases. Neutrophil-derived serine proteases (NSPs), metalloproteases and some mast cell-derived proteases are mainly focused here. Their modes of actions are different in different diseases for e.g. NE induces the release of IL-8 from lung epithelial cells through a MyD88/IRAK/TRAF-6-dependent pathway and also through EGFR MAPK pathway. NSPs contribute to immune regulation during inflammation through the cleavage and activation of specific cellular receptors. MMPs can also influence the progression of various inflammatory processes and there are many non-matrix substrates for MMPs, such as chemokines, growth factors and receptors. During lung inflammation interplay between NE and MMP is an important significant phenomenon. They have been evaluated as therapeutic targets in several inflammatory lung diseases. Here we review the role of proteases in various lung inflammatory diseases with emphasis on their mode of action and contribution to immune regulation during inflammation.
Keywords: Serine Protease, Metalloprotease, Inflammation, Inflammatory lung diseases, Pro-inflammatory cytokines, Chemokines, Neutrophil
Introduction
Numerous environmental pathogens, particulate matters, allergens and harmless antigens are present in the air we breathe. Although airways are the main port of entry for pathogens and allergens during inhalation of the inspired air (10,000 L per day in humans) [1], the lung is one of the most challenged organs of the body. For this reason, air-breathing animals have developed several defense mechanisms in this compartment [2, 3].
Inflammation, a host defense mechanism, is an immediate response of the body to tissue injury caused by harmful stimuli, such as pathogens, damaged cells or chemicals [4]. Lung inflammation is a broad term which covers various acute and chronic inflammatory diseases such as Acute Lung Injury (ALI), Acute Respiratory Distress Syndrome (ARDS), Emphysema, Airway hyper-reactivity (AHR) or Asthma, Allergic Asthma or Chronic eosinophilic inflammation, Chronic Obstructive Pulmonary Disease (COPD), Fibrotic lung disease, Pulmonary edema, Tuberculosis, Pneumococcal infection etc. This complex and dynamic process is characterized by an innate immune response, which involves a coordinated expression of inflammatory cytokines and implication of various cell types particularly immune cells aimed at clearing the pathogenic agent, damaged cells etc. [5]. Modulation of expression and activity of these inflammatory cytokines and other immune components are regulated by various proteases in the lung compartment. Here we present the information relative to some relevant proteases which have roles in different inflammatory lung diseases.
Proteases are enzymes that have the ability to hydrolyze peptide bonds and degrade other proteins. According to the active groups of their catalytic center they are categorized and in case of lung disease, basically three major protease group serine proteases, cysteine proteases, and the matrix metalloproteases (MMPs) are studied [6]. There is another group the ADAM (a disintegrin and metalloprotease) family of proteases, which has an emerging role in mucin production and cytokine processing [7]. A number of studies have elucidated the role of proteases in human diseases such as cancer, thrombotic and inflammatory disorders [8, 9]. Various inflammatory cells, such as neutrophils, mast cells, macrophages, and lymphocytes are the major source of proteases within the lung [10–12]. Other cells, including epithelial, endothelial, and fibroblasts, also synthesize proteases [13, 14]. Serine proteases, including Neutrophil Elastase (NE), Cathepsin G (CG), and Proteinase 3 (PR 3), are packaged in primary granules within neutrophils [15]. Some of the metalloproteases, MMP-8 and MMP-9, are also packaged into specific and gelatinase granules, respectively, in the neutrophil [16].
Proteases in Lung and Their Mode of Action
In the lung, proteases function either intracellularly or extracellularly after cellular activation. Proteases that may be present in the respiratory tract and activate PARs include the endogenous enzymes mast cell tryptase (activates PAR2), trypsin (PAR1, PAR2 and PAR4), chymase (PAR1) and cathepsin G (PAR4), as well as exogenous enzymes such as Der p1 (PAR2) that are inhaled. However, these and other enzymes within the respiratory tract may also inactivate or disarm various PARs by cleaving them at other sites that remove the tethered ligand sequence [17]. Proteases have crucial role in chemotaxis of all of the various inflammatory cell types to the lung. MMP-9 and serine proteases increase eosinophil chemotaxis, and MMP-12 is responsible for eosinophil and macrophage accumulation [18, 19]. However, maximum work has focused on neutrophil chemoattraction to the lung by interleukin 8 (IL-8) and leukotriene B4 to study the effect of proteases on cell migration in inflammatory lung diseases.
Serine Proteases
Neutrophil-Derived Serine Proteases (NSPs)
Neutrophils are essential for host defense against invading pathogens. They are the first inflammatory cell lines to enter tissue during inflammation [20]. They engulf and degrade microorganisms using an array of weapons that include reactive oxygen species, antimicrobial peptides, and proteases such as Cathepsin G, Neutrophil Elastase and Proteinase 3. After release, these proteases also contribute to the extracellular killing of microorganisms, and regulate non-infectious inflammatory processes by activating specific receptors and modulating the levels of cytokines [21]. In addition to their involvement in pathogen destruction and the regulation of proinflammatory processes, NSPs are also involved in a variety of inflammatory human conditions, including chronic lung diseases (chronic obstructive pulmonary disease, cystic fibrosis, acute lung injury, and acute respiratory distress syndrome) [22–25]. In these disorders, accumulation and activation of neutrophils in the airways result in excessive secretion of active NSPs, thus causing lung matrix destruction and inflammation.
Neutrophil Elastase (NE)
NE is a serine-protease of the chymotrypsin family stored in primary (azurophilic) granules of PMNs along with proteinase-3 and cathepsin G, two other neutrophil serine-proteases. Intracellular stored NE comes in action when azurophilic granules are incorporated to the phagosome [26]. However, in vitro stimulation of PMNs with physiological relevant pro-inflammatory stimuli induces either transfer of NE to the plasma membrane (membrane-bound NE associated to by proteoglycans) [27, 28], or a secretion in the extracellular space specially in case of pulmonary chronic (CF and COPD) [29, 30] or acute lung injury [22], where high efflux of PMNs in the alveolar space increase the release of NE from necrotic PMNs. Functions of NE are not only concerned with degrading bacteria [31–33] and extracellular matrix molecules, they also operate on various bioactive molecules including chemokines, cytokines, growth factors and cell surface receptors [34–36], thus the “deleterious” concept of NE has changed towards a multifunctional molecule able to regulate inflammatory process and immune responses. Indeed, extracellular NE (free, chromatin-bound or membrane-bound) participates in: (1) direct killing of bacteria [31–33]; (2) processing and release of chemokines, cytokines and growth factors [34, 35], (3) modulation of immune cell activity through interaction with cell surface receptors [36, 37], (4) mucus secretion [38].
Proteases can also modulate cytokine activity and release from immune cells through mechanisms independent of cytokine receptors. For example, it has been shown that NE induces the release of IL-8 from lung epithelial cells [39–42] through a MyD88/IRAK/TRAF-6-dependent pathway [42] that also involves TLR4 [39] and also through EGFR MAPK pathway (Fig. 1) [43]. How NE activates TLR4 is unknown but liberation of proteolytic fragments from host targets able to recognize PRR could be possible as described for TLR2 [44]. Serine proteases such as NE can induce IL-8 expression by bronchial epithelial cells (Fig. 1) and leukotriene B4 expression by macrophages [42, 45]. NE appears to be the most important regulatory factor present in the cystic fibrosis (CF) lung responsible for IL-8 expression because inhibition of NE activity in CF bronchoalveolar lavage fluid (BALF) almost completely blocks IL-8 message in bronchial epithelium [40]. It has been shown that NE generally act at least in part via an IL-1 receptor-associated kinase-1/myeloid differentiation factor-88/nuclear factor-κB-dependent pathway in bronchial epithelial cells; this can be inhibited by a dominant negative variant of myeloid differentiation factor-88 [42]. This gives new therapeutic approaches targeted at inhibiting the NE-activated intracellular pathways rather than NE itself. It is quite clear that expression of IL-8 and leukotriene B4 are responsible for neutrophil migration to the lung, and given the high neutrophil and NE burden present in the CF lung this has lead to the “vicious cycle” hypothesis whereby NE is the main player behind IL-8 production and neutrophil influx into the CF lung. From further experiments it has been found that an initial inflammatory event can stimulate further inflammation i.e., epithelial cell injury in mice leads to secretion of the murine homolog of IL-8, which in turn binds to an adhesive component of the extracellular matrix, syndecan-1 [46]. MMP-7 cleaves this syndecan-1-murine IL-8 complex and this is crucial for attracting neutrophils to the damaged epithelial surface (Fig. 2).
Fig. 1.
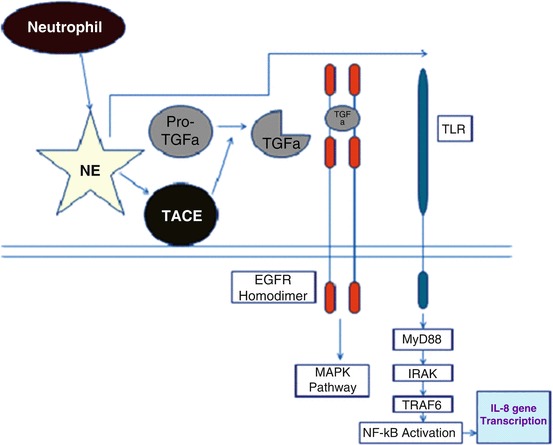
Mechanism of neutrophil elastase (NE) induced-release of IL-8 from lung epithelial cells. Following its release from the azurophilic granules in response to pathogenic/pathologic insult, NE activates TNFa converting enzyme (TACE), which in turn cleaves proTGFa (pro-transforming growth factor a) to generate soluble TGFa as a ligand for the epidermal growth factor receptor (EGFR). EGFR co-localizes with toll-like receptor-4 (TLR4) and a signal transduction cascade is initiated via myeloid differentiation factor 88 (MyD88 or Mal), IL-1 receptor-associated kinases (IRAKs), tumor necrosis factor receptor-associated factor 6 (TRAF6), transforming growth factor-beta-activated kinase 1 (TAK1) and the IkB kinases (IKKs), leading to a degradation of inhibitor of NF-kB (IkB) proteins, activation of nuclear factor-kB (NF-kB) and increased IL-8 gene transcription
Fig. 2.

Interaction of NE and MMPs during inflammatory responses in the respiratory tract. Neutrophil Elastase (NE) induces interleukin-8 (IL-8) synthesis, resulting in chemoattraction of neutrophils to the respiratory tract. MT1-matrix metalloprotease (MMP) 14 processes IL-8 and monocyte chemoattractant protein 1 (MCP-1), and MMP-7 cleaves the syndecan-1-IL-8 complex to generate active IL-8, which in turn acts as a neutrophil chemoattractant. MMP-12 released from macrophages also helps in chemoattraction of different inflammatory cell
Proteinase-3 (PR-3)
Proteinase-3 (PR-3) is a serine protease that cleaves TNF into membrane-associated TNF and soluble TNF form [47]. It has been hypothesized that PR-3-mediated TNF processing may be an important mechanism in inflammatory lung diseases [48]. PR-3 is able to degrade extracellular matrix, and its potential involvement in pulmonary inflammatory disease has been demonstrated by the induction of emphysema in hamsters following intratracheal instillation [49]. PR-3 is also enhanced in the sputum of cystic fibrosis patients, and correlates with disease severity [50]. The main sources of PR-3 are neutrophils and it has been hypothesized that there would be an increased contribution of PR-3 to TNF processing in diseases with abundant alveolar neutrophils such as usual interstitial pneumonia (UIP) [48].
NSPs contribute to immune regulation also through the cleavage and activation of specific cellular receptors. NE, PR3, and CG can process the N-terminal extracellular domains of protease-activated receptors (PARs), which are a subfamily of related G-protein-coupled receptors [51, 52]. These receptors are ubiquitously expressed in various tissues and cells and, more especially, in platelets and endothelial cells. Processing of PAR extracellular domains occurs through exposure of a tethered ligand that allows the auto-activation of the receptor and subsequent activation of an intracellular signaling cascade via phospholipase C [51, 52]. Four PARs have been identified so far; three of them, PAR-1, PAR-3, and PAR-4, can be activated by thrombin. Apart from thrombin, CG released from activated neutrophils can also activate PAR-4 at the surface of platelets and initiate their aggregation [53]. All three NSPs cleave PAR-1, which impairs their activation by thrombin [54]. Serine proteases cleave the amino acids at a specific site of the extracellular N-terminus of the molecule to expose a new N-terminal ligand domain that binds to another site on the same molecule, thereby activating the receptor. The amino acid sequence of each cleavage site is specific for the particular PAR, and mAb(monoclonal Antibody) assays for the protein and PCR assays for mRNA are available [55]. Activation of PAR2 results in the production and secretion of IL-8 and chemokine (C-Cmotif) ligand 2 [56, 57].
Human Airway Trypsin-Like Protease (HAT)
HAT belongs to the family of Type II Transmembrane Serine Proteases (TTSP) [58]. Members of this family present a short intracellular domain connected to a single-pass transmembrane domain followed by a large extracellular domain containing a highly variable stem region and a C-terminal serine-protease domain of the chymotrypsin (S1) fold [58]. It is preferentially expressed in human bronchial and tracheal respiratory tract [59, 60], particularly in ciliated cells [61]. A soluble form after proteolytic cleavage is also found in the sputum of patients with chronic airways diseases [59]. Concerning the lung compartment, in vitro experiments have revealed various HAT activity such as: (1) fibrinogenolytic activity in lung airway anticoagulation processes [62]; (2) proteolytic inactivation of urokinase-type plasminogen activator receptor (uPAR) in lung epithelial cells [63]; (3) proteolytic activation of the hemagglutinin antigen of influenza virus leading to multicycle replication and propagation of influenza virus in vitro [64, 65]; (4) stimulation of lung fibroblast proliferation through a PAR-2-dependent MEK-MAPK mediated pathway [66]; (5) increase of intracellular Ca2+ concentration in bronchial cells through PAR-2-dependent mechanisms [67].
Metalloproteases
Matrix Metalloproteinases (MMPs)
Matrix metalloproteinases (MMPs) are zinc-dependent neutral endopeptidases that form a family of extracellular matrix proteolytic enzymes. They are primarily responsible for the degradation of extracellular matrix components during the remodeling processes essential for normal tissue growth and repair. In the lung, inappropriate expression and excessive activity of several MMPs, including MMP-12, have been implicated in the tissue-destructive processes associated with chronic lung diseases, including COPD and asthma [68–73]. It is well established that MMP-12 has definite role in the pathogenesis of COPD [74]. Patients suffering from COPD have increased secretion and activity of MMPs, especially MMP-2 and MMP-9, been identified in inflammatory cells and tissues isolated from those patients [75]. In our previous study, we found increased expression of MMP-2 in case of cadmium induced lung inflammation in mice model [76]. Kundu et al. (2009) performed SDS-PAGE and gelatine zymography to find out the expression of MMP-2 from lung cell extracts at different time points after the induction of cadmium chloride and found there was increased expression of MMP-2 even after use of ibuprofen, a non-steroidal anti-inflammatory drug (Fig. 3). Expression and activities of MMP-2 and MMP-9 are increased in case of Influenza virus infection both in vivo and in vitro. It has been demonstrated that H3N2 virus infection induces expression of MMP-2 and MMP-9 in murine lungs in vivo and alveolar epithelial cells in vitro [77]. Gelatinases (including MMP-2 and MMP-9) are zinc-dependent endopeptidases, degrade major components of the basement membrane such as gelatin and collagen IV, and exert deleterious effects on the epithelium and endothelium in the thin alveolar-capillary barrier [78].
Fig. 3.
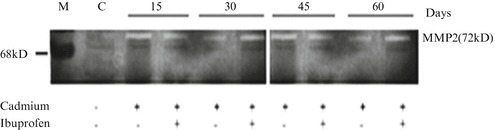
Increased expression of MMP-2 in cadmium induced lung inflammation by SDS-PAGE and Gelatin zymography. Detection of matrix metalloproteinase-2 (MMP-2) expression at different time points after the induction of Cadmium chloride (5 mg/kg body weight). Lung cell extracts were prepared at days of 15, 30, 45 and 60 from normal (N), Cadmium treated and Cadmium plus Ibuprofen treated (three individual animals per dose) mice. The zymography was developed and stained. The picture shows increased expression of MMP-2(72 kDa), which was not inhibited by Ibuprofen. Gel is representative of three comparable experiments indicate p < 0.05 with respect to the control
A number of studies reveal that MMPs can also influence the progression of various inflammatory processes and there are many non-matrix substrates for MMPs, such as chemokines, growth factors and receptors [79]. MMP-14 is a protease who does the processing of IL-8 by removing a pentapeptide from the N-terminus of the protein, resulting in a more biologically active form. MMP-9 also process IL-8 to a form 20-fold more active as a chemoattractant [80, 81]. They process monocyte chemoattractant proteins (MCPs) to produce antagonists of chemokine receptors, indicating a role for MMP-14 in dampening inflammation [82]. Another potent pro-inflammatory cytokine IL-1β that requires proteolytic processing before activation not only by caspase-1 but also several MMPs, including MMP-2, MMP-3 and MMP-9. Interestingly, MMP-3 can degrade the mature IL-1beta cytokine, suggesting potentially dual roles for MMPs in either stimulating or inhibiting IL-1beta effects [83]. The mechanism by which MMPs control inflammation is the regulation of chemokine gradients and that includes both the immobilization of chemokines to the components of extracellular matrix and the generation of chemotactic concentration gradients which provide indications for leukocyte migration. Thus, MMPs can indirectly control influx of inflammatory cells by cleaving proteins in the pericellular environment that bind chemokines. One well established example of this mechanism is MMP7-dependent shedding of syndecan-1 in acute lung injury [46]. In response to lung injury, both CXCL1 (KC) and MMP7 are induced, and MMP7 sheds syndecan-1, a ubiquitous heparan sulfate proteoglycan, that releases the CXCL1-syndecan-1 complex to generate a chemokine gradient. MMP7-null mice that lack this shedding are unable to create a CXCL1 gradient, and thus, neutrophils fail to efflux into the alveolar space and instead remain in the perivascular space [84].
There is also interplay between NE and MMPs in inflammation. NE activates MMP-9 directly and indirectly by inactivating TIMP-1, the naturally occurring inhibitor of MMP-9 [85, 86]. Furthermore, NE may activate MMP-2 through a mechanism that requires MMP-14 expression [87]. Therefore, a variety of proteases liberated from neutrophils or actively expressed on epithelial cells may interact with each other, thereby perpetuating a cycle of inflammation. If we consider CF lung, there is high concentration of NE due to the increased neutrophil burden present in the CF lung and surplus the levels of MMP-14-processed monocyte chemoattractant protein antagonists. This is supported by evidence showing significant IL-8 levels present in CF bronchoalveolar lavage fluid [40].
ADAM (A Disintegrin and Metalloprotease)
ADAMs are a family of type I transmembrane proteins belonging to the adamalysin subfamily of metalloproteinases [88]. Members of this family present a metalloprotease domain and a domain of interaction with integrin (disintegrin domain) [89], indicating that ADAMs are both proteases and adhesion molecules. At least 40 ADAMs has been described so far, 25 of which are expressed in humans [90, 91]. ADAMs family has been implicated in the control of membrane fusion, cytokine and growth factor shedding, and cell migration, as well as processes such as muscle development, fertilization, and cell fate determination.
Pathologies such as inflammation and cancer also involve ADAMs family members. ADAMs has been related to lung pathological processes such as cancer [90], asthma [92, 93] and idiopathic pulmonary fibrosis [94]. ADAMs can also modulate cell responses to various signals by acting as cell surface sheddase on membrane associated cytokines, apoptosis ligands, growth factors and receptors. In particular, sheddase activity has important physiological consequences. One of the most documented example is through the production of active tumor necrosis factor-α (TNF-α), a potent inducer of innate inflammatory responses. TNF-α is a homotrimeric transmembrane protein of 26 kDa able to induce inflammatory and cytotoxic effects after cell-to-cell contact [95]. ADAM-17 (also known as TNF-α convertase enzyme or TACE) cleaves transmembrane TNF-α to release the 17 kDa active soluble form of TNF-α [96]. Importantly, ADAMs are also implicated in the shedding of most of the EGFR ligands (EGF, transforming growth factor (TGF)-a, heparin binding (HB)-EGF, betacellulin, epiregulin and amphiregulin (AR)) [89]. It has been reported that Adam17 knock-out mice presented developmental defects resembling those in animals lacking TGF-a, HB-EGF, AR, or the EGFR [97–99]. In addition, sheddase activity on EGFR ligands has important physiological consequences for mucus production. Recently, it has been confirmed that endothelial ADAM10 and ADAM17 are both required for microvascular permeability [100]. To investigate this phenomenon Dreymueller et al. (2012) did LPS treatment on HMVEC-L (Human Microvascular Endothelial Cells) and found there is release of soluble JAM-A by LPS-challenged HMVEC-L. Release of soluble JAM-A was 1.3-fold enhanced by 4 h of LPS stimulation, further increasing to 2.3-fold by stimulation for 24 h and was completely inhibited by GW280264X-treatment, capable of blocking tumor necrosis factor-alpha-converting enzyme (TACE) and the closely related disintegrin-like metalloproteinase 10 (ADAM10). The knockdown of ADAM10 or ADAM17 by shRNA indicated the involvement of ADAM17 and to a lesser extent of ADAM10 in JAM-A release. They further examined whether ADAM10/17 activity might influence transendothelial migration of neutrophils. The inhibitor GW280264X reduced transmigration in response to the neutrophil-attracting cytokine IL-8 by 70 %. Silencing or knock-out of either ADAM10 or ADAM17 alone was sufficient to abrogate transmigration in response to IL-8. Thus, endothelial ADAM10 and ADAM17 are both required for microvascular permeability and for IL-8-mediated transmigration of neutrophils in vitro.
TACE (TNF-α Converting Enzyme)
It has been reported that TACE mediates a critical step in the development of post-transplantation lung injury [101]. Goto et al. (2004) evaluated the role of TACE in acute inflammation using an inhibitor of the enzyme in a rat model of lung transplantation. Inhibition of this protease results decreased neutrophil accumulation in the alveolar space and other histological changes such as intercellular adhesion molecule-1 (ICAM-1) expression. In addition, significantly lower levels of monocyte chemotactic protein-1 (MCP-1), cytokine induced neutrophil chemoattractant-1 (CINC-1), high mobility group box-1 (HMGB1), and soluble epithelial cadherin and decreased neutrophil elastase activity were observed in bronchoalveolar lavage fluid from the rats treated with the inhibitor.
Mast Cell-Derived Proteases
Proteases are the most abundant class of proteins produced by mast cells. Many of these are stored in membrane-enclosed intracellular granules until liberated by degranulating stimuli, which include cross-linking of high affinity IgE receptor F(c)εRI by IgE bound to multivalent allergen [102]. It has been investigated that b-tryptase, a major protease released during mast cell activation, cleaves IgE and this tryptase-mediated IgE cleavage affects IgE binding to allergens [103]. From their study, IgE degradation products were detected in tryptase-containing tissue fluids collected from sites of allergic inflammation [103] and it has been confirmed that tryptase cleaves IgE and abolishes binding of IgE to allergens and F(c)εRI. It is a natural mechanism for controlling allergic reactions is supported by experiments performed with purified proteins, as well as by cellular in vitro and in vivo data [103].
The lung epithelial cells could be activated by airborne proteases from molds, mites, or pollens. Activation of other cells in the airway by various endogenous and exogenous proteases that increase production of IgE antibody and enhance infiltration of eosinophils, basophils, neutrophils, monocytes, and lymphocytes. Apart from that, smooth muscle contraction is enhanced, nerves are made more reactive, and airway responsiveness is increased [104]. Eosinophils and mast cells are degranulated and stimulated to produce inflammatory molecules, such as nitric oxide, major basic protein, leukotrienes, histamine, and mast cell tryptase itself. Mast cell tryptase is likely to be especially important in the late phase of the allergic response [105].
Proteases Involvement in Disease Progression
Chronic Obstructive Pulmonary Disease (COPD) and Pulmonary Emphysema
Chronic obstructive pulmonary disease (COPD) represents a group of diseases, including chronic bronchitis and emphysema, which are characterized by an airflow limitation that is not fully reversible [106]. The pathogenic roles of NSPs in COPD are attributed to their ability to break down connective tissue components and generate proinflammatory peptides from these components [107, 108], to induce mucus secretion by submucosal glandular cells and goblet cells, and to express proinflammatory cytokines from airway epithelial cells [109–111]. Pulmonary emphysema is a destructive lesion of the lung parenchyma. It has been demonstrated that the lesion of the matrix which results in emphysema is the end-result of crosstalk between macrophage metalloelastase and neutrophil elastase [112, 113]. However, the latter protease is responsible for the greater portion of the final proteolytic attack [112].
We have already mentioned before the role of MMP-12 in the pathogenesis of COPD. The most well studied MMPs in human COPD and emphysema are MMP-1, MMP-8, MMP-9, and MMP-12, which all have been implicated in tissue destruction in human COPD and emphysema [114]. The lymphocytes present in emphysematous lungs have a strong Th1 bias, expressing higher levels of CXCL10 (IP-10) and CXCL9 (MIG). This CXCL9 can upregulate the expression of MMP-12 in pulmonary macrophages [115], providing a mechanism for chronic and progressive destruction of lung parenchyma that is seen in emphysema.
Interstitial Lung Disease and Idiopathic Pulmonary Fibrosis (IPF)
Idiopathic pulmonary fibrosis (IPF), one of the most common forms of interstitial lung diseases, is a progressive fibrotic lung condition of unknown etiology [116]. The diagnosis of IPF is made by surgical lung biopsy, and the histopathological features include the presence of patchy inflammatory cells, foci of proliferating fibroblasts and myofibroblasts, and collagen deposition [117, 118]. From a study it has been observed that MMP-2 activity is increased in a dose-dependent manner in A549 cells treated for 48 h by TGF-β stimulation [119] because TGF-β plays a key role in stimulation of fibroblast proliferation and has been implicated in progression of IPF [120].
It has been postulated that NE may be involved in the early stages of lung inflammation during the development of pulmonary fibrosis [121]. Neutrophil elastase acts as a putative link between emphysema and fibrosis and this dual role of NE has been reported from a recent study that has highlighted NE as a common pathogenic mechanism linking pulmonary emphysema and fibrosis [122]. This study was done in two animal models in which emphysema and fibrosis were induced either by bleomycin (BLM) or by chronic exposure to cigarette smoke. In order to study whether BLM-induced lesion is protease-dependent or not, a group of mice was treated with 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (a serine proteinase inhibitor active against neutrophil elastase). In DBA/2 mice that develop both emphysema and fibrosis after chronic cigarette-smoke exposure, the presence of NE in alveolar structures is also associated with a positive immunohistochemical reaction of both TGF-β and TGF-α [123]. These results strongly suggest that neutrophil elastase may be a common pathogenic link between emphysema and fibrosis, acting as a regulatory factor in the generation of soluble cytokines with mitogenic activity for mesenchymal cells resulting either in emphysema or in fibrosis or both [122]. A recent study has provided evidence that different interstitial levels of NE burden in emphysema may be associated with different routes of collagen clearance (intracellular vs. extracellular) and different degrees of remodeling of the ECM in emphysema [124]. This point merits further investigation. The implication of NE in lung destruction and repair and its pathogenic role in emphysema and fibrosis could lead to a novel approach for therapeutic interventions.
Cystic Fibrosis (CF)
Cystic fibrosis (CF) is a common, inheritable genetic disorder that results in malfunctioning of the chloride channels of many exocrine epithelial linings including the airway epithelia [125]. The exact mechanism or pathophysiology of progressive lung inflammation in patients with CF is not clear, but patients with CF have increased levels of MMP-2, MMP-8, and MMP-9 in their BAL [126]. In a study of 23 children with CF, BAL fluid concentrations of MMP8 and MMP9 were higher in untreated children and lower in those who were treated with DNase [127]. In children with stable CF, there is a significant inverse relationship between MMP-9 and lung function, as measured by FEV1. Furthermore, levels of MMP-9 are higher in sputum of asymptomatic children with CF compared with controls, suggesting that MMP-9 and total neutrophil count may be useful markers of airway injury and airflow obstruction in persons with CF [126]. As discussed in Sect. 7, MMPs play a critical role in the host defense against pathogens in the lung.
Neutrophils express three closely related serine proteases, NE, CG and PR3, in a coordinated fashion. It has been reported that IL-6 is susceptible to cleavage by all three proteases [128] but now a days, degradation of SIL-6R in a time and dose dependent manner has also been proved in the context of cystic fibrosis [129]. It has been studied that on the basis of molar concentration, among these proteases, CG is the most potent protease with maximal degradation occurring within 60 min at a concentration of 250 nM. NE also shows potent activity at 250 nM but required up to 4 h for complete degradation of sIL-6R to occur. PR3 was the least active protease with minimal degradation of sIL-6R occurring in the presence of 1,000 nM protease after 1 h and complete degradation being observed after 4 h in the presence of 500 nM of protease. If we consider CF lung, unregulated proteolytic activity is the main contributor to pathology of this disease and is also responsible for reduced expression and activity of a number of important components of the immune response [130]. Targeting of IL-6 by NSPs [128] has previously been suggested as an explanation for the surprisingly low pulmonary expression of IL-6 in CF patients [131] however, this is the first study to show specific proteolysis of this cytokine by serine proteases in CF BALF [129].
Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS)
These are inflammatory disorders of the lung most commonly caused by trauma, sepsis, and pneumonia, the latter two being responsible for approximately 60 % of cases [132]. Early in the initiation of ALI and ARDS, massive number of neutrophils accumulates in the vasculature of the lung. Neutrophils and their cytotoxic products, including oxidants and proteases, have main pathological importance in ALI and ARDS. It has been observed that there is increased elastolytic activity [133] in patients with ARDS [134]. Increased levels of HNE in plasma and in BAL have also been observed with at-risk patients who later developed ALI [135, 136]. Pathologic effects of HNE are associated with microvascular injury, causing endothelial damage, increased capillary permeability, and interstitial edema. HNE may also potentiate the inflammatory response by increasing the expression and release of cytokines [137] and by increasing mucin production [138]. In experimental animal models, intratracheal administration of exogenous HNE induces lung hemorrhage and ALI, whereas administration of pharmacological HNE inhibitors prevents lung injury, which further supports the role of NSPs in lung injury [22, 139, 140]. Owen et al. (2004) propose an interesting role of MMP-8 that it may be anti-inflammatory during acute lung injury, because MMP-8 null mice given intratracheal LPS have significantly greater accumulation of neutrophils in the alveolar space than wild-type mice [141].
In patients with acute respiratory distress syndrome, salbutamol (b2-agonist) increases MMP-9 activity in bronchoalveolar lavage fluid, and increases MMP-9 but decreases TIMP-1 and -2 expressions in distal lung epithelial cells [142]. Similar findings are reported for formoterol (b2-adrenoceptor agonist) in a rat model of pulmonary inflammation [143]. From another study of Zhang et al., an interesting data found that formoterol and ipratropium bromide partially protect the lungs against the inflammation by reducing neutrophilic infiltration. This protective effect is associated with reduced MMP-9 activity known to play an important pro-inflammatory role in acute inflammatory process [143].
Asthma
Asthma is characterized by episodic dyspnea, lung inflammation, and in some patients, progressive irreversible airway dysfunction [144]. Expression of several MMPs has been associated with asthma; enhancement of MMP-1, MMP-2, MMP-3, MMP-8, and MMP-9 all have been found in sputum and BAL from patients with asthma [69]. With severe asthmatic conditions, patients have increased MMP9 activity in BAL relative to that from patients with mild asthma or control patients. Wenzel et al. [145] found an increase in MMP-9 activity in the subepithelial basement membrane that is accompanied by higher TGF-β. These studies suggest that in patients with severe asthma, neutrophils play a key role in lung remodeling because they express both MMP-9 and TGF-β, which are involved in breakdown and repair of tissue, respectively. In asthma, MMP-9 is expressed in bronchial epithelium and submucosa, where its abundance correlates with tissue eosinophil number [146] because MMP-9 is also produced by eosinophils, macrophages, and neutrophils [147, 148]. In sputum, concentration of MMP-9 is positively correlated with neutrophil number [149] and also with the cumulative macrophage, neutrophil, and eosinophil count [150] during asthma. BAL taken from asthmatic patients after allergen challenge increases the mitogenic indices even more than a normal patient’s BAL [151]. This phenomenon could contribute to the airway remodeling observed in patients with chronic asthma; however, the pathway underlying the proliferation remains unclear. EGFR, TGF-β, or platelet-derived growth factors are all candidates based on their presence in lung during disease and their relationship to various MMPs [152].
Asthmatic patients express an increased amount of PAR-2 on respiratory epithelial cells but not on smooth muscle or alveolar macrophages [153, 154]. Schmidlin and colleagues [155] have studied the effect of PAR-2 on ovalbumin challenge of immunized mice. Compared with wild-type animals, eosinophil infiltration was inhibited by 73 % in mice lacking PAR-2 and increased by 88 % in mice over expressing PAR-2. Similarly, compared with wild-type animals, airway hyperreactivity to inhaled methacholine was diminished by 38 % in mice lacking PAR-2 and increased by 52 % in mice over expressing PAR-2. PAR-2 deletion also reduced IgE levels to ovalbumin sensitization 4-fold compared with levels seen in wild-type animals. Mast cell chymase induces eosinophil infiltration, presumably by activating PAR-1 [156]. Secretary leukocyte protease inhibitor administered intratracheally before allergen challenge prevented bronchoconstriction, airway hyperresponsiveness, and leukocyte influx [157]. The actions of PARs and their activating proteinases in the airways have also been studied extensively to determine their role in various lung diseases [158].
Pseudomonas aeruginosa Infection in Lung
Pseudomonas aeruginosa (Pa) is an opportunistic pathogen that infects over 80 % of CF adult lungs [159] and is a major cause of ventilator-associated pneumonia (VAP) in hospitalized patients [160]. A broad spectrum of Pa virulence factors has been identified including proteases secreted by various mechanisms [161]. Proteases such as elastase (LasB), alkaline protease (AprA), staphylolysin (LasA) and Protease IV (a serine-endoprotease) have been identified in CF lung [162, 163]. There are other virulence factors (proteases) of Pa which are capable to degrade host proteins, including matrix components and components of the immune system such as immunoglobulins and serum alpha proteins [164]. The most studied Pa protease is the elastolytic metalloproteinase LasB, a type II secretion system enzyme. There are several functions of Pa elastase has been reported such as it can alters epithelial barrier function [165], disables PAR-2 receptor in lung epithelial cells [166], cleaves uPAR leading to disruptions of uPAR-dependent cellular interaction [167], inactivates complement and immunoglobulins, and inhibits cell chemotaxis, phagocytosis and microbicidal activities in human leukocytes [168, 169].
The present findings reveal a protective role for extracellular NE against the pathogen. It has been demonstrated that NE, an endogenous effecter, could also participate in the orchestration of lung inflammatory response against P. aeruginosa infection by modulating the expression of cytokines (e.g., induction of the expression of the pro-inflammatory TNF-α, MIP-2, and IL-6) [5].
Allergic bronchopulmonary Aspergillosis
Allergic Bronchopulmonary Aspergillosis (ABPA) occurs in nonimmunocompromised patients and belongs to the hypersensitivity disorders induced by Aspergillus sp. Genetic factors and activation of bronchial epithelial cells in asthma or cystic fibrosis are responsible for the development of a CD4 + Th2 lymphocyte activation and IgE, IgG and IgA-AF antibodies production [170]. It appears that total serum IgE levels are extremely high, and not all of it is specific antibody to Aspergillus species antigens. The pathological changes such as pulmonary infiltrates of eosinophilic pneumonitis, granulomatous central bronchiectasis, and segmental pulmonary fibrosis are seen [171]. Aspergillus species produces proteases [172–176] which can desquamate epithelial cells and stimulate IL-6 and IL-8 production [172]. Aspergillus species proteases also initiate growth factor release from epithelial cells and are possibly responsible for the central bronchiectasis [177]. It still remains unclear what is abnormal about the response in the few patients in whom this disease develops and why it is especially common in individuals with cystic fibrosis.
Conclusions
As we have mentioned before that generally three major classes of proteases—serine proteases, MMPs, and cysteinyl proteases—have been identified in the lung. They are associated traditionally with various inflammatory lung diseases and airway extracellular matrix destruction. From ample evidences it has been proved that each protease family has a multitude of regulatory functions, which makes them of pivotal importance in inflammation, innate immunity, and infection. For example, if we consider role of NE in lung inflammation, recent observations suggest that it’s role is more complex than the simple degradation of ECM components. Several lines of evidence propose that NE aims specifically at a variety of regulatory functions in local inflammatory processes but their relevance under various pathophysiological conditions still remains poorly understood and need further investigation. NE, acting as a link between pulmonary emphysema and fibrosis that could lead to a novel strategy for therapeutic interventions.
Among various lung inflammatory diseases, in case of chronic infective lung diseases, the normal physiological processes become deregulated because of extracellular protease activity, which leads ultimately to upregulation of proinflammatory mediators, increased recruitment of inflammatory cells to the lung, impaired phagocytosis, enhanced mucin production, and inactivation of important innate and antimicrobial proteins. This results in sustained inflammation and predisposition to infection. One way to treat such protease-mediated events in chronic infective lung disease is with antiprotease therapy, which neutralizes excessive extracellular protease activity without compromising the normal physiologic role of proteases. Antiprotease trials have been performed using α1-antitrypsin and secretary leukoprotease inhibitor, both of which have successfully inhibited NE activity in vivo in CF and α1-antitrypsin deficiency [178–180]. Other inhibitors of serine proteases and MMPs are being developed, including synthetic inhibitors to combat protease-induced lung destruction [181].
As these NSPs participate in a variety of pathophysiological processes, they appear as potential therapeutic targets for drugs that inhibit their active site or impair activation from their precursor. Overall, the available preclinical and clinical data suggest that inhibition of NSPs using therapeutic inhibitors would suppress or attenuate deleterious effects of inflammatory diseases, including lung diseases [182].
MMPs compromise a structurally and functionally related group of proteolytic enzymes, which play a key role in the tissue remodeling and repair associated with inflammation [183]. Among various MMPs, MMP-12 has massive importance for lung remodeling in patients suffering from COPD, and its importance was confirmed in MMP knockout mice that were protected against smoke-induced emphysema [184]. Presently it has been found that MMP-12 activity was higher in ex-smokers with COPD compared with that seen in smokers with COPD, and this suggests that the smoking effect keep increasing MMP-12 activity, is irreversible and more severe disease can be associated with deregulated MMP-12 function [74]. Other MMP such as MMP-2 would be the target for treatment of cadmium induced lung inflammation [76]. Animal models of human lung diseases and clinical studies have provided ample evidences for the involvement of MMPs in the development or progression of a number of common lung diseases such as COPD, emphysema, and asthma. However, merely detecting increases in MMPs expression in various lung inflammatory conditions may not provide sufficient information to understand the contribution of MMPs in human lung diseases [152].
Metalloproteinases of the ADAM family have been recognized as potential therapeutic targets in several diseases. However, due to their broad activity, systemic application of inhibitors may not represent an appropriate treatment strategy. ADAM17 mediates a number of shedding events that influence several components of acute lung inflammation including vascular leakage, leukocyte recruitment and cytokine release. Particularly in endothelial cells, ADAM17 appears to act as a central regulator in pulmonary inflammation. So, in near future inhibition of ADAM17, possibly locally to reduce systemic side effects, may be a promising approach for the treatment of Acute Lung Injury [100].
Given that they are involved in the pathogenesis of various diseases, they can be good therapeutic targets along with their specific protease inhibitors [185]. There could be a further option of combining protease modulators with monoclonal antibodies against cytokines to treat such inflammatory lung diseases. From clinical point of view whether anti-protease treatment or use of these proteases along with their specific inhibitors for curing these inflammatory lung diseases is beneficial, needs further investigation and research.
Acknowledgements
Authors want to thank Mr. Subhadip Kundu (Postdoctoral Fellow, Department of Biochemistry, Uniformed Services University of Health and Science) for his guide in SDS-PAGE and Gelatine Zymography data analysis. Author also likes to thank University Grant Commission for providing fellowship. This work was supported by grants from Department of Science and Technology, Govt. of India FIST Program in Department of Zoology, University of Calcutta for instrument support.
Contributor Information
Sajal Chakraborti, Email: s_chakraborti@hotmail.com.
Naranjan S. Dhalla, Phone: +12042353421, FAX: +1204233-6723, Email: nsdhalla@sbrc.ca
Arindam Bhattacharyya, Email: arindam19@yahoo.com.
References
- 1.Whitsett JA. Intrinsic and innate defenses in the lung: intersection of pathways regulating lung morphogenesis, host defense, and repair. J Clin Investig. 2002;109:565–569. doi: 10.1172/JCI15209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki T, Chow CW, Downey GP. Role of innate immune cells and their products in lung immunopathology. Int J Biochem Cell Biol. 2008;40:1348–1361. doi: 10.1016/j.biocel.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Zaas AK, Schwartz DA. Innate immunity and the lung: defense at the interface between host and environment. Trends Cardiovasc Med. 2005;15:195–202. doi: 10.1016/j.tcm.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Kundu JK, Surh YJ. Emerging avenues linking inflammation and cancer. Free Radic Biol Med. 2012;52:2013–2037. doi: 10.1016/j.freeradbiomed.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 5.Benabid R, Wartelle J, Malleret L, et al. Neutrophil elastase modulates cytokine expression: contribution to host defense against Pseudomonas aeruginosa-induced pneumonia. J Biol Chem. 2012;287:34883–34894. doi: 10.1074/jbc.M112.361352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McElvaney NG, Crystal RG. Proteases and lung injury. In: Crystal RG, West JB, Weibel ER, Barnes PJ, editors. The lung: scientific foundations. Philadelphia, PA: Lippincott-Raven; 1997. pp. 2205–2218. [Google Scholar]
- 7.Nadel JA, Burgel PR. The role of epidermal growth factor in mucus production. Curr Opin Pharmacol. 2001;1:254–258. doi: 10.1016/s1471-4892(01)00045-5. [DOI] [PubMed] [Google Scholar]
- 8.Cheronis JC, Repine JE. Proteases, protease inhibitors, and protease-derived peptides: importance in human pathophysiology and therapeutics. Basel: Birkhauser; 1993. pp. 2–25. [PubMed] [Google Scholar]
- 9.Horl WH, Heidland A. Proteases II: potential role in health and disease. New York, NY: Plenum Press; 1987. pp. 8–12. [Google Scholar]
- 10.Caughey GH. Serine proteinases of mast cell and leukocyte granules: a league of their own. Am J Respir Crit Care Med. 1994;150:S138–S142. doi: 10.1164/ajrccm/150.6_Pt_2.S138. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro SD. Elastolytic metalloproteinases produced by human mononuclear phagocytes: potential roles in destructive lung disease. Am J Respir Crit Care Med. 1994;150:S160–S164. doi: 10.1164/ajrccm/150.6_Pt_2.S160. [DOI] [PubMed] [Google Scholar]
- 12.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapon in the arsenal. Nat Rev Immunol. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 13.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 14.Selman M, Pardo A. The epithelial/fibroblastic pathway in the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:S93–S97. [PubMed] [Google Scholar]
- 15.Jenne DE. Structure of the azurocidin, proteinase 3, and NE genes: implications for inflammation and vasculitis. Am J Respir Crit Care Med. 1994;150:S147–S154. doi: 10.1164/ajrccm/150.6_Pt_2.S147. [DOI] [PubMed] [Google Scholar]
- 16.Kang T, Yi J, Guo A, et al. Subcellular distribution and cytokine- and chemokine-regulated secretion of leukolysin/MT6-MMP/MMP-25 in neutrophils. J Biol Chem. 2001;276:21960–21968. doi: 10.1074/jbc.M007997200. [DOI] [PubMed] [Google Scholar]
- 17.Loew D, Perrault C, Morales M, et al. Proteolysis of the exodomain of recombinant protease-activated receptors: prediction of receptor activation or inactivation by MALDI mass spectrometry. Biochemistry. 2000;39:10812–10822. doi: 10.1021/bi0003341. [DOI] [PubMed] [Google Scholar]
- 18.Okada S, Kita H, George TJ, et al. Migration of eosinophils through basement membrane components in vitro: role of matrix metalloproteinase-9. Am J Respir Cell Mol Biol. 1997;17:519–528. doi: 10.1165/ajrcmb.17.4.2877. [DOI] [PubMed] [Google Scholar]
- 19.Lanone S, Zheng T, Zhu Z, et al. Overlapping and enzyme-specific contributions of matrix metalloproteinases-9 and -12 in IL-13-induced inflammation and remodeling. J Clin Investig. 2002;110:463–474. doi: 10.1172/JCI14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi SD, Voyich JM, Burlak C, DeLeo FR. Neutrophils in the innate immune response. Arch Immunol Ther Exp (Warsz) 2005;53:505–517. [PubMed] [Google Scholar]
- 21.Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 22.Lee WL, Downey GP. Leukocyte elastase: physiological functions and role in acute lung injury. Am J Respir Crit Care Med. 2001;164:896–904. doi: 10.1164/ajrccm.164.5.2103040. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro SD. Proteinases in chronic obstructive pulmonary disease. Biochem Soc Trans. 2002;30:98–102. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 24.Moraes TJ, Chow CW, Downey GP. Proteases and lung injury. Crit Care Med. 2003;31:S189–S194. doi: 10.1097/01.CCM.0000057842.90746.1E. [DOI] [PubMed] [Google Scholar]
- 25.Owen CA. Roles for proteinases in the pathogenesis of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3:253–268. doi: 10.2147/copd.s2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belaaouaj A, McCarthy R, Baumann M, et al. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med. 1998;4:615–618. doi: 10.1038/nm0598-615. [DOI] [PubMed] [Google Scholar]
- 27.Campbell EJ, Owen CA. The sulfate groups of chondroitin sulfate- and heparan sulfate-containing proteoglycans in neutrophil plasma membranes are novel binding sites for human leukocyte elastase and cathepsin G. J Biol Chem. 2007;282:14645–14654. doi: 10.1074/jbc.M608346200. [DOI] [PubMed] [Google Scholar]
- 28.Owen CA, Campbell MA, Boukedes SS, Campbell EJ. Cytokines regulate membrane-bound leukocyte elastase on neutrophils: a novel mechanism for effector activity. Am J Physiol. 1997;272:L385–L393. doi: 10.1152/ajplung.1997.272.3.L385. [DOI] [PubMed] [Google Scholar]
- 29.Snider GL. Chronic obstructive pulmonary disease: risk factors, pathophysiology and pathogenesis. Annu Rev Med. 1989;40:411–429. doi: 10.1146/annurev.me.40.020189.002211. [DOI] [PubMed] [Google Scholar]
- 30.Voynow JA, Fischer BM, Zheng S. Proteases and cystic fibrosis. Int J Biochem Cell Biol. 2008;40:1238–1245. doi: 10.1016/j.biocel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 32.Belaaouaj A, Kim KS, Shapiro SD. Degradation of outer membrane protein A in Escherichia coli killing by neutrophil elastase. Science. 2000;289:1185–1188. doi: 10.1126/science.289.5482.1185. [DOI] [PubMed] [Google Scholar]
- 33.Hirche TO, Benabid R, Deslee G, et al. Neutrophil elastase mediates innate host protection against Pseudomonas aeruginosa. J Immunol. 2008;181:4945–4954. doi: 10.4049/jimmunol.181.7.4945. [DOI] [PubMed] [Google Scholar]
- 34.Lungarella G, Cavarra E, Lucattelli MP, Martorana A. The dual role of neutrophil elastase in lung destruction and repair. Int J Biochem Cell Biol. 2008;40:1287–1296. doi: 10.1016/j.biocel.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Pham CT. Neutrophil serine proteases fine-tune the inflammatory response. Int J Biochem Cell Biol. 2008;40:1317–1333. doi: 10.1016/j.biocel.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roghanian A, Sallenave JM. Neutrophil elastase (NE) and NE inhibitors: canonical and noncanonical functions in lung chronic inflammatory diseases (cystic fibrosis and chronic obstructive pulmonary disease) J Aerosol Med Pulm Drug Deliv. 2008;21:125–144. doi: 10.1089/jamp.2007.0653. [DOI] [PubMed] [Google Scholar]
- 37.Meyer-Hoffert U. Neutrophil-derived serine proteases modulate innate immune responses. Front Biosci. 2009;14:3409–3418. doi: 10.2741/3462. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Verdugo I, Descamps D, Chignard M, et al. Lung protease/anti-protease network and modulation of mucus production and surfactant activity. Biochimie. 2010;92:1608–1617. doi: 10.1016/j.biochi.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Devaney JM, Greene CM, Taggart CC, et al. Neutrophil elastase up-regulates interleukin-8 via toll-like receptor 4. FEBS Lett. 2003;544:129–132. doi: 10.1016/s0014-5793(03)00482-4. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura H, Yoshimura K, McElvaney NG, Crystal RG. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J Clin Investig. 1992;89:1478–1484. doi: 10.1172/JCI115738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sallenave JM, Shulmann J, Crossley J, et al. Regulation of secretory leukocyte proteinase inhibitor (SLPI) and elastase-specific inhibitor (ESI/elafin) in human airway epithelial cells by cytokines and neutrophilic enzymes. Am J Respir Cell Mol Biol. 2010;11:733–741. doi: 10.1165/ajrcmb.11.6.7946401. [DOI] [PubMed] [Google Scholar]
- 42.Walsh DE, Greene CM, Carroll TP, et al. Interleukin-8 up-regulation by neutrophil elastase is mediated by MyD88/IRAK/TRAF-6 in human bronchial epithelium. J Biol Chem. 2001;276:35494–35499. doi: 10.1074/jbc.M103543200. [DOI] [PubMed] [Google Scholar]
- 43.Kuwahara I, Lillehoj EP, Lu W, et al. Neutrophil elastase induces IL-8 gene transcription and protein release through p38/NF-{kappa}B activation via EGFR transactivation in a lung epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2006;291:L407–L416. doi: 10.1152/ajplung.00471.2005. [DOI] [PubMed] [Google Scholar]
- 44.Hartl D, Latzin P, Hordijk P, et al. Cleavage of CXCR1 on neutrophils disables bacterial killing in cystic fibrosis lung disease. Nat Med. 2007;13:1423–1430. doi: 10.1038/nm1690. [DOI] [PubMed] [Google Scholar]
- 45.Hubbard RC, Fells G, Gadek J, et al. Neutrophil accumulation in the lung in alpha 1-antitrypsin deficiency: spontaneous release of leukotriene B4 by alveolar macrophages. J Clin Investig. 1991;88:891–897. doi: 10.1172/JCI115391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 47.Coeshott C, Ohnemus C, Pilyavskaya A, et al. Converting enzyme independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci U S A. 1999;96:6261–6266. doi: 10.1073/pnas.96.11.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Armstrong L, Godinho SIH, Uppington KM, et al. Tumour necrosis factor-a processing in interstitial lung disease: a potential role for exogenous proteinase-3. Clin Exp Immunol. 2009;156:336–343. doi: 10.1111/j.1365-2249.2009.03906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kao RC, Wehner NG, Skubitz KM, et al. Proteinase 3. A distinct human polymorphonuclear leukocyte proteinase that produces emphysema in hamsters. J Clin Investig. 1988;82:1963–1973. doi: 10.1172/JCI113816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Just J, Moog-Lutz C, Houzel-Charavel A, et al. Proteinase 3 mRNA expression is induced in monocytes but not in neutrophils of patients with cystic fibrosis. FEBS Lett. 1999;457:437–440. doi: 10.1016/s0014-5793(99)01098-4. [DOI] [PubMed] [Google Scholar]
- 51.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 52.Vergnolle N. Protease-activated receptors as drug targets in inflammation and pain. Pharmacol Ther. 2009;123:292–309. doi: 10.1016/j.pharmthera.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 53.Sambrano GR, Huang W, Faruqi T, et al. Cathepsin G activates protease-activated receptor-4 in human platelets. J Biol Chem. 2000;275:6819–6823. doi: 10.1074/jbc.275.10.6819. [DOI] [PubMed] [Google Scholar]
- 54.Renesto P, Si-Tahar M, Moniatte M, et al. Specific inhibition of thrombin-induced cell activation by the neutrophil proteinases elastase, cathepsin G, and proteinase 3: evidence for distinct cleavage sites within the aminoterminal domain of the thrombin receptor. Blood. 1997;89:1944–1953. [PubMed] [Google Scholar]
- 55.Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol. 2004;114(5):997–1008. doi: 10.1016/j.jaci.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 56.Uehara A, Muramoto K, Takada H, Sugawara S. Neutrophil serine proteinases activate human nonepithelial cells to produce inflammatory cytokines through protease-activated receptor 2. J Immunol. 2003;170:5690–5696. doi: 10.4049/jimmunol.170.11.5690. [DOI] [PubMed] [Google Scholar]
- 57.Uehara A, Sugawara Y, Sasano T, et al. Proinflammatory cytokines induce proteinase 3 as membrane-bound and secretory forms in human oral epithelial cells and antibodies to proteinase 3 activate the cells through protease-activated receptor-2. J Immunol. 2004;173:4179–4189. doi: 10.4049/jimmunol.173.6.4179. [DOI] [PubMed] [Google Scholar]
- 58.Bugge TH, Antalis TM, Wu Q, et al. Type II transmembrane serine proteases. J Biol Chem. 2009;284:23177–23181. doi: 10.1074/jbc.R109.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamaoka K, Masuda K, Ogawa H, et al. Cloning and characterization of the cDNA for human airway trypsin-like protease. J Biol Chem. 1998;273:11895–11901. doi: 10.1074/jbc.273.19.11895. [DOI] [PubMed] [Google Scholar]
- 60.Yasuoka S, Ohnishi T, Kawano S, et al. Purification, characterization, and localization of a novel trypsin-like protease found in the human airway. Am J Respir Cell Mol Biol. 1997;16:300–308. doi: 10.1165/ajrcmb.16.3.9070615. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi M, Sano T, Yamaoka K, et al. Localization of human airway trypsin-like protease in the airway: an immunohistochemical study. Histochem Cell Biol. 2001;115:181–187. doi: 10.1007/s004180000243. [DOI] [PubMed] [Google Scholar]
- 62.Yoshinaga S, Nakahori Y, Yasuoka S. Fibrinogenolytic activity of a novel trypsin-like enzyme found in human airway. J Med Invest. 1998;45:77–86. [PubMed] [Google Scholar]
- 63.Beaufort N, Leduc D, Eguchi H, et al. The human airway trypsin-like protease modulates the urokinase receptor (uPAR, CD87) structure and functions. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1263–L1272. doi: 10.1152/ajplung.00191.2006. [DOI] [PubMed] [Google Scholar]
- 64.Bottcher E, Freuer C, Steinmetzer T, et al. MDCK cells that express proteases TMPRSS2 and HAT provide a cell system to propagate influenza viruses in the absence of trypsin and to study cleavage of HA and its inhibition. Vaccine. 2009;27:6324–6329. doi: 10.1016/j.vaccine.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 65.Bottcher E, Matrosovich T, Beyerle M, et al. Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J Virol. 2006;80:9896–9898. doi: 10.1128/JVI.01118-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsushima R, Takahashi A, Nakaya Y, et al. Human airway trypsin-like protease stimulates human bronchial fibroblast proliferation in a protease-activated receptor-2-dependent pathway. Am J Physiol Lung Cell Mol Physiol. 2006;290:L385–L395. doi: 10.1152/ajplung.00098.2005. [DOI] [PubMed] [Google Scholar]
- 67.Miki M, Nakamura Y, Takahashi A, et al. Effect of human airway trypsin-like protease on intracellular free Ca2þ concentration in human bronchial epithelial cells. J Med Invest. 2003;50:95–107. [PubMed] [Google Scholar]
- 68.Boschetto P, Quintavalle S, Zeni E, et al. Association between markers of emphysema and more severe chronic obstructive pulmonary disease. Thorax. 2006;61:1037–1042. doi: 10.1136/thx.2006.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Demedts IK, Brusselle GG, Bracke KR, et al. Matrix metalloproteinases in asthma and COPD. Curr Opin Pharmacol. 2005;5:257–263. doi: 10.1016/j.coph.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 70.Elias JA, Kang MJ, Crouthers K, et al. State of the art. Mechanistic heterogeneity in chronic obstructive pulmonary disease: insights from transgenic mice. Proc Am Thorac Soc. 2006;3:494–498. doi: 10.1513/pats.200603-068MS. [DOI] [PubMed] [Google Scholar]
- 71.Elkington PTG, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006;61:259–266. doi: 10.1136/thx.2005.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gueders MM, Foidart J-M, Noel A, Cataldo DD. Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in the respiratory tract: potential implications in asthma and other lung diseases. Eur J Pharmacol. 2006;533:133–144. doi: 10.1016/j.ejphar.2005.12.082. [DOI] [PubMed] [Google Scholar]
- 73.Lavigne MC, Thakker P, Gunn J, et al. Human bronchial epithelial cells express and secrete MMP-12. Biochem Biophys Res Commun. 2004;324:534–546. doi: 10.1016/j.bbrc.2004.09.080. [DOI] [PubMed] [Google Scholar]
- 74.Chaudhuri R, McSharry C, Brady J, et al. Sputum matrix metalloproteinase-12 in patients with chronic obstructive pulmonary disease and asthma: relationship to disease severity. J Allergy Clin Immunol. 2012;129(3):655–663. doi: 10.1016/j.jaci.2011.12.996. [DOI] [PubMed] [Google Scholar]
- 75.Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodelling. Am J Respir Crit Care Med. 2003;28:12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- 76.Kundu S, Sengupta S, Chatterjee S, et al. Cadmium induces lung inflammation independent of lung cell proliferation: a molecular approach. J Inflamm. 2009;6:19. doi: 10.1186/1476-9255-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ng HH, Narasaraju T, Phoon MC, et al. Doxycycline treatment attenuates acute lung injury in mice infected with virulent influenza H3N2 virus: involvement of matrix metalloproteinases. Exp Mol Pathol. 2012;92:287–295. doi: 10.1016/j.yexmp.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 78.O’Connor CM, FitzGerald MX. Matrix metalloproteases and lung disease. Thorax. 1994;49:602–609. doi: 10.1136/thx.49.6.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol. 2007;82:1375–1381. doi: 10.1189/jlb.0607338. [DOI] [PubMed] [Google Scholar]
- 80.Padrines M, Wolf M, Walz A, Baggiolini M. Interleukin-8 processing by neutrophil elastase, cathepsin G and proteinase-3. FEBS Lett. 1994;352:231–235. doi: 10.1016/0014-5793(94)00952-x. [DOI] [PubMed] [Google Scholar]
- 81.Van den Steen PE, Proost P, Wuyts A, et al. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GROalpha and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–2681. [PubMed] [Google Scholar]
- 82.McQuibban GA, Gong JH, Wong JP, et al. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–1167. [PubMed] [Google Scholar]
- 83.Bernfield M, Gotte M, Park PW, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 84.Brew K. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 85.Ferry G, Lonchampt M, Pennel L, et al. Activation of MMP-9 by neutrophil elastase in an in vivo model of acute lung injury. FEBS Lett. 1997;402:111–115. doi: 10.1016/s0014-5793(96)01508-6. [DOI] [PubMed] [Google Scholar]
- 86.Itoh Y, Nagase H. Preferential inactivation of tissue inhibitor of metalloproteinases-1 that is bound to the precursor of matrix metalloproteinase 9 (progelatinase B) by human neutrophil elastase. J Biol Chem. 1995;270:16518–16521. doi: 10.1074/jbc.270.28.16518. [DOI] [PubMed] [Google Scholar]
- 87.Shamamian P, Schwartz JD, Pocock BJ, et al. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol. 2001;189:197–206. doi: 10.1002/jcp.10014. [DOI] [PubMed] [Google Scholar]
- 88.Wolfsberg TG, Primakoff P, Myles DG, White JM. ADAM, a novel family of membrane proteins containing a disintegrin and metalloprotease domain: multipotential functions in cell-cell and cell-matrix interactions. J Cell Biol. 1995;131:275–278. doi: 10.1083/jcb.131.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 90.Rocks N, Paulissen G, El Hour M, et al. Emerging roles of ADAM and ADAMTS metalloproteinases in cancer. Biochimie. 2008;90:369–379. doi: 10.1016/j.biochi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 91.Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- 92.Foley SC, Mogas AK, Olivenstein R, et al. Increased expression of ADAM33 and ADAM8 with disease progression in asthma. J Allergy Clin Immunol. 2007;119:863–871. doi: 10.1016/j.jaci.2006.12.665. [DOI] [PubMed] [Google Scholar]
- 93.Van Eerdewegh P, Little RD, Dupuis J, et al. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature. 2002;418:426–430. doi: 10.1038/nature00878. [DOI] [PubMed] [Google Scholar]
- 94.Pardo A, Selman M, Kaminski N. Approaching the degradome in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:1141–1155. doi: 10.1016/j.biocel.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 95.Kriegler M, Perez C, DeFay K, et al. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988;53:45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- 96.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 97.Jackson LF, Qiu TH, Sunnarborg SW, et al. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22:2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peschon JJ, Slack JL, Reddy P, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 99.Sternlicht MD, Sunnarborg SW. The ADAM17-amphiregulin-EGFR axis in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2008;13:181–194. doi: 10.1007/s10911-008-9084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dreymueller D, Martin C, Kogel T, et al. Lung endothelial ADAM17 regulates the acute inflammatory response to lipopolysaccharide. EMBO Mol Med. 2012;4:412–423. doi: 10.1002/emmm.201200217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goto T, Ishizaka A, Kobayashi F et al (2004) Importance of tumor necrosis factor-α cleavage process in post-transplantation lung injury in rats. Am J Respir Crit Care Med 170 [DOI] [PubMed]
- 102.Caughey GH. Mast cell proteases as protective and inflammatory mediators. Mast Cell Biology Advances in Experimental Medicine and Biology. 2011;716:212–234. doi: 10.1007/978-1-4419-9533-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rauter I, Krauth M, Westritschnig K, et al. Mast cell–derived proteases control allergic inflammation through cleavage of IgE. J Allergy Clin Immunol. 2008;121:197–202. doi: 10.1016/j.jaci.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 104.Barrios VE, Jarosinski MA, Wright CD. Proteinase-activated receptor-2 mediates hyperresponsiveness in isolated guinea pig bronchi. Biochem Pharmacol. 2003;66:519–525. doi: 10.1016/s0006-2952(03)00292-2. [DOI] [PubMed] [Google Scholar]
- 105.Clark JM, Abraham WM, Fishman CE, et al. Tryptase inhibitors block allergen-induced airway and inflammatory responses in allergic sheep. Am J Respir Crit Care Med. 1995;152:2076–2083. doi: 10.1164/ajrccm.152.6.8520778. [DOI] [PubMed] [Google Scholar]
- 106.Barnes PJ, Stockley RA. COPD: current therapeutic interventions and future approaches. Eur Respir J. 2005;25:1084–1106. doi: 10.1183/09031936.05.00139104. [DOI] [PubMed] [Google Scholar]
- 107.Houghton AM, Quintero PA, Perkins DL, et al. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Investig. 2006;116:753–759. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weathington NM, van Houwelingen AH, Noerager BD, et al. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 109.Sommerhoff CP, Nadel JA, Basbaum CB, Caughey GH. Neutrophil elastase and cathepsin G stimulate secretion from cultured bovine airway gland serous cells. J Clin Investig. 1990;85:682–689. doi: 10.1172/JCI114492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Takeyama K, Agustí C, Ueki I, et al. Neutrophil-dependent goblet cell degranulation: role of membrane-bound elastase and adhesion molecules. Am J Physiol. 1998;275:L294–L302. doi: 10.1152/ajplung.1998.275.2.L294. [DOI] [PubMed] [Google Scholar]
- 111.Witko-Sarsat V, Halbwachs-Mecarelli L, Schuster A, et al. Proteinase 3, a potent secretagogue in airways, is present in cystic fibrosis sputum. Am J Respir Cell Mol Biol. 1999;20:729–736. doi: 10.1165/ajrcmb.20.4.3371. [DOI] [PubMed] [Google Scholar]
- 112.Churg A, Zay K, Shay S, et al. Acute cigarette smoke-induced connective tissue breakdown requires both neutrophils and macrophage metalloelastase in mice. Am J Respir Cell Mol Biol. 2002;27:368–374. doi: 10.1165/rcmb.4791. [DOI] [PubMed] [Google Scholar]
- 113.Shapiro SD, Goldstein NM, Houghton AM, et al. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol. 2003;163:2329–2335. doi: 10.1016/S0002-9440(10)63589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Molet S, Belleguic C, Lena H, et al. Increase in macrophage elastase (MMP-12) in lungs from patients with chronic obstructive pulmonary disease. Inflamm Res. 2005;54:31–36. doi: 10.1007/s00011-004-1319-4. [DOI] [PubMed] [Google Scholar]
- 115.Grumelli S, Corry D, Song L, et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 2004;1:74–83. doi: 10.1371/journal.pmed.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157:199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 117.Schwartz DA, Van Fossen DS, Davis CS, et al. Determinants of progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1994;149:444–449. doi: 10.1164/ajrccm.149.2.8306043. [DOI] [PubMed] [Google Scholar]
- 118.King TEJ, Schwarz MI, Brown K, et al. Idiopathic pulmonary fibrosis. Relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164:1025–1032. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 119.Kasai H, Allen JT, Mason RM, et al. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT) Respir Res. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Willis BC, Liebler JM, Luby-Phelps K, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-ss 1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Taooka Y, Maeda A, Hiyama K, et al. Effects of neutrophil elastase inhibitor on bleomycininduced pulmonary fibrosis in mice. Am J Respir Crit Care Med. 1997;156:260–265. doi: 10.1164/ajrccm.156.1.9612077. [DOI] [PubMed] [Google Scholar]
- 122.Lucattelli M, Bartalesi B, Cavarra E, et al. Is neutrophil elastase the missing link between emphysema and fibrosis? Evidence from two mouse models. Respir Res. 2005;6:83–96. doi: 10.1186/1465-9921-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bartalesi B, Cavarra E, Fineschi S, et al. Different lung responses to cigarette smoke in two strains of mice sensitive to oxidants. Eur Respir J. 2005;25:15–22. doi: 10.1183/09031936.04.00067204. [DOI] [PubMed] [Google Scholar]
- 124.Lucattelli M, Cavarra E, de Santi MM, et al. Collagen phagocytosis by lung alveolar macrophages in animal models of emphysema. Eur Respir J. 2003;22:728–734. doi: 10.1183/09031936.03.00047603. [DOI] [PubMed] [Google Scholar]
- 125.Gadsby DC, Vergani P, Csanady L, et al. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–483. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sagel SD, Kapsner RK, Osberg I, et al. Induced sputum matrix metalloproteinase-9 correlates with lung function and airway inflammation in children with cystic fibrosis. Pediatr Pulmonol. 2005;39:224–232. doi: 10.1002/ppul.20165. [DOI] [PubMed] [Google Scholar]
- 127.Ratjen F, Hartog CM, Paul K, et al. Matrix metalloproteases in BAL fluid of patients with cystic fibrosis and their modulation by treatment with dornase alpha. Thorax. 2002;57:930–934. doi: 10.1136/thorax.57.11.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bank U, Kupper B, Reinhold D, et al. Evidence for a crucial role of neutrophil-derived serine proteases in the inactivation of interleukin-6 at sites of inflammation. FEBS Lett. 1999;461:235–240. doi: 10.1016/s0014-5793(99)01466-0. [DOI] [PubMed] [Google Scholar]
- 129.McGreal EP, Davies PL, Powell W, et al. Inactivation of IL-6 and soluble IL-6 receptor by neutrophil derived serine proteases in cystic fibrosis. Biochim Biophys Acta. 2010;1802:649–658. doi: 10.1016/j.bbadis.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 130.Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 131.Osika E, Cavaillon JM, Chadelat K, et al. Distinct sputum cytokine profiles in cystic fibrosis and other chronic inflammatory airway disease. Eur Respir J. 1999;14:339–346. doi: 10.1034/j.1399-3003.1999.14b17.x. [DOI] [PubMed] [Google Scholar]
- 132.Tsushima K, King LS, Aggarwal NR, et al. Acute lung injury review. Intern Med. 2009;48:621–630. doi: 10.2169/internalmedicine.48.1741. [DOI] [PubMed] [Google Scholar]
- 133.Lee CT, Fein AM, Lippmann M, et al. Elastolytic activity in pulmonary lavage fluid from patients with adult respiratory distress syndrome. N Engl J Med. 1981;304:192–196. doi: 10.1056/NEJM198101223040402. [DOI] [PubMed] [Google Scholar]
- 134.Repine JE. Scientific perspectives on adult respiratory distress syndrome. Lancet. 1992;339:466–469. doi: 10.1016/0140-6736(92)91067-i. [DOI] [PubMed] [Google Scholar]
- 135.Suter PM, Suter S, Girardin E, et al. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am Rev Respir Dis. 1992;145:1016–1022. doi: 10.1164/ajrccm/145.5.1016. [DOI] [PubMed] [Google Scholar]
- 136.Donnelly SC, MacGregor I, Zamani A, et al. Plasma elastase levels and the development of the adult respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:1428–1433. doi: 10.1164/ajrccm.151.5.7735596. [DOI] [PubMed] [Google Scholar]
- 137.Bédard M, McClure CD, Schiller NL, et al. Release of interleukin-8, interleukin-6, and colony-stimulating factors by upper airway epithelial cells: implications for cystic fibrosis. Am J Respir Cell Mol Biol. 1993;9:455–462. doi: 10.1165/ajrcmb/9.4.455. [DOI] [PubMed] [Google Scholar]
- 138.Voynow JA, Young LR, Wang Y, et al. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. Am J Physiol. 1999;276:L835–L843. doi: 10.1152/ajplung.1999.276.5.L835. [DOI] [PubMed] [Google Scholar]
- 139.Kawabata K, Hagio T, Matsuoka S. The role of neutrophil elastase in acute lung injury. Eur J Pharmacol. 2002;451:1–10. doi: 10.1016/s0014-2999(02)02182-9. [DOI] [PubMed] [Google Scholar]
- 140.Zeiher BG, Matsuoka S, Kawabata K, Repine JE. Neutrophil elastase and acute lung injury: prospects for sivelestat and other neutrophil elastase inhibitors as therapeutics. Crit Care Med. 2002;30:S281–S287. doi: 10.1097/00003246-200205001-00018. [DOI] [PubMed] [Google Scholar]
- 141.Owen CA, Hu Z, Lopez-Otin C, Shapiro SD. Membrane-bound matrix metalloproteinase-8 on activated polymorphonuclear cells is a potent, tissue inhibitor of metalloproteinase-resistant collagenase and serpinase. J Immunol. 2004;172:7791–7803. doi: 10.4049/jimmunol.172.12.7791. [DOI] [PubMed] [Google Scholar]
- 142.O’Kane CM, KcKeown SW, Perkins GD, et al. Salbutamol up-regulates matrix metalloproteinase-9 in the alveolar space in the acute respiratory distress syndrome. Crit Care Med. 2009;37:2242–2249. doi: 10.1097/CCM.0b013e3181a5506c. [DOI] [PubMed] [Google Scholar]
- 143.Zhang W, Fievez L, Cheu E, et al. Anti-inflammatory effects of formoterol and ipratropium bromide against acute cadmium-induced pulmonary inflammation in rats. Eur J Pharmacol. 2010;628:171–178. doi: 10.1016/j.ejphar.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 144.Barrios RJ, Kheradmand F, Batts L, Corry DB. Asthma: pathology and pathophysiology. Arch Pathol Lab Med. 2006;130:447–451. doi: 10.5858/2006-130-447-APAP. [DOI] [PubMed] [Google Scholar]
- 145.Wenzel SE, Balzar S, Cundall M, Chu HW. Subepithelial basement membrane immunoreactivity for matrix metalloproteinase 9. Association with asthma severity, neutrophilic inflammation, and wound repair. J Allergy Clin Immunol. 2003;111:1345–1352. doi: 10.1067/mai.2003.1464. [DOI] [PubMed] [Google Scholar]
- 146.Han Z, Jun XU, Zhong N. Expression of matrix metalloproteinases MMP-9 within the airways in asthma. Respir Med. 2003;97:563–567. doi: 10.1053/rmed.2001.1162. [DOI] [PubMed] [Google Scholar]
- 147.Campbell EJ, Cury JD, Shapiro SD, et al. Neutral proteinases of human mononuclear phagocytes: cellular differentiation markedly alters cell phenotype for serine proteinases, metalloproteinases, and tissue inhibitor of metalloproteinases. J Immunol. 1991;146:1286–1293. [PubMed] [Google Scholar]
- 148.Ohno I, Ohtani H, Nitta Y, et al. Eosinophils as a source of matrix metalloproteinase-9 in asthmatic airway inflammation. Am J Respir Cell Mol Biol. 1997;16:212–219. doi: 10.1165/ajrcmb.16.3.9070604. [DOI] [PubMed] [Google Scholar]
- 149.Boulay ME, Prince P, Deschesnes F, et al. Metalloproteinase-9 in induced sputum correlates with the severity of the late allergen-induced asthmatic response. Respiration. 2004;71:216–224. doi: 10.1159/000077418. [DOI] [PubMed] [Google Scholar]
- 150.Lee YC, Lee HB, Rhee YK, Song CH. The involvement of matrix metalloproteinase-9 in airway inflammation of patients with acute asthma. Clin Exp Allergy. 2001;31:1623–1630. doi: 10.1046/j.1365-2222.2001.01211.x. [DOI] [PubMed] [Google Scholar]
- 151.Naureckas ET, Ndukwu IM, Halayko AJ, et al. Bronchoalveolar lavage fluid from asthmatic subjects is mitogenic for human airway smooth muscle. Am J Respir Crit Care Med. 1999;160:2062–2066. doi: 10.1164/ajrccm.160.6.9903131. [DOI] [PubMed] [Google Scholar]
- 152.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87:69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Knight DA, Lim S, Scaffidi AK, et al. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001;108:797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- 154.Roche N, Stirling RG, Lim S, et al. Effect of acute and chronic inflammatory stimuli on expression of protease-activated receptors 1 and 2 in alveolar macrophages. J Allergy Clin Immunol. 2003;111:367–373. doi: 10.1067/mai.2003.6. [DOI] [PubMed] [Google Scholar]
- 155.Schmidlin F, Amadesi S, Dabbagh K, et al. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. 2002;169:5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- 156.Tomimori Y, Muto T, Fukami H, et al. Chymase participates in chronic dermatitis by inducing eosinophil infiltration. Lab Invest. 2002;82:789–794. doi: 10.1097/01.lab.0000018827.78602.f4. [DOI] [PubMed] [Google Scholar]
- 157.Wright CD, Havill AM, Middleton SC, et al. Secretory leukocyte protease inhibitor prevents allergen-induced pulmonary responses in animal models of asthma. J Pharmacol Exp Ther. 1999;289:1007–1014. [PubMed] [Google Scholar]
- 158.Sokolova EE, Reiser GG. A novel therapeutic target in various lung diseases: airway proteases and protease-activated receptors. Pharmacology Therapeutics. 2007;115(1):70–83. doi: 10.1016/j.pharmthera.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 159.FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Pediatr. 1993;122:1–9. doi: 10.1016/s0022-3476(05)83478-x. [DOI] [PubMed] [Google Scholar]
- 160.Hoban DJ, Biedenbach DJ, Mutnick AH, Jones RN. Pathogen of occurrence and susceptibility patterns associated with pneumonia in hospitalized patients in North America: results of the SENTRY antimicrobial surveillance study. Diagn Microbiol Infect Dis. 2003;45:279–285. doi: 10.1016/s0732-8893(02)00540-0. [DOI] [PubMed] [Google Scholar]
- 161.Lazdunski A, Guzzo J, Filloux A, et al. Secretion of extracellular proteins by Pseudomonas aeruginosa. Biochimie. 1990;72:147–156. doi: 10.1016/0300-9084(90)90140-c. [DOI] [PubMed] [Google Scholar]
- 162.Smith L, Rose B, Tingpej P. Protease IV production in Pseudomonas aeruginosa from the lungs of adults with cystic fibrosis. J Med Microbiol. 2006;55:1641–1644. doi: 10.1099/jmm.0.46845-0. [DOI] [PubMed] [Google Scholar]
- 163.Tingpej P, Smith L, Rose B. Phenotypic characterization of clonal and nonclonal Pseudomonas aeruginosa strains isolated from lungs of adults with cystic fibrosis. J Clin Microbiol. 2007;45:1697–1704. doi: 10.1128/JCM.02364-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lederberg J, et al. Pseudomonas. Encyclopedia of Microbiology. 2. San Diego, CA: Academic; 2000. pp. 876–891. [Google Scholar]
- 165.Azghani AO. Pseudomonas aeruginosa and epithelial permeability: role of virulence factors elastase and exotoxin A. Am J Respir Cell Mol Biol. 1996;15:132–140. doi: 10.1165/ajrcmb.15.1.8679217. [DOI] [PubMed] [Google Scholar]
- 166.Dulon S, Leduc D, Cottrell GS. Pseudomonas aeruginosa elastase disables proteinase-activated receptor 2 in respiratory epithelial cells. Am J Respir Cell Mol Biol. 2005;32:411–419. doi: 10.1165/rcmb.2004-0274OC. [DOI] [PubMed] [Google Scholar]
- 167.Leduc D, Beaufort N, de Bentzmann S, et al. The Pseudomonas aeruginosa LasB metalloproteinase regulates the human urokinase-type plasminogen activator receptor through domain specific endoproteolysis. Infect Immun. 2007;75:3848–3858. doi: 10.1128/IAI.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Galloway DR. Pseudomonas aeruginosa elastase and elastolysis revisited: recent developments. Mol Microbiol. 1991;5:2315–2321. doi: 10.1111/j.1365-2958.1991.tb02076.x. [DOI] [PubMed] [Google Scholar]
- 169.Kharazmi A. Mechanisms involved in the evasion of the host defence by Pseudomonas aeruginosa. Immunol Lett. 1991;30:201–205. doi: 10.1016/0165-2478(91)90026-7. [DOI] [PubMed] [Google Scholar]
- 170.Tillie-Leblond I, Tonnel AB. Allergic bronchopulmonary aspergillosis. Allergy. 2005;60:1004–1013. doi: 10.1111/j.1398-9995.2005.00887.x. [DOI] [PubMed] [Google Scholar]
- 171.Greenberger PA. Allergic bronchopulmonary aspergillosis. In: Adkinson NF Jr, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FER, editors. Allergy principles and practice. 6. St Louis, MO: Mosby-Yearbook; 2003. pp. 1353–1371. [Google Scholar]
- 172.Kauffman HF, Tomee JF, van de Riet MA, et al. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J Allergy Clin Immunol. 2000;105:1185–1193. doi: 10.1067/mai.2000.106210. [DOI] [PubMed] [Google Scholar]
- 173.Robinson BW, Venaille TJ, Mendis AH, McAleer R. Allergens as proteases: an Aspergillus fumigatus proteinase directly induces human epithelial cell detachment. J Allergy Clin Immunol. 1990;86:726–731. doi: 10.1016/s0091-6749(05)80176-9. [DOI] [PubMed] [Google Scholar]
- 174.Yu CJ, Chiou SH, Lai WY, et al. Characterization of a novel allergen, a major IgE-binding protein from Aspergillus flavus, as an alkaline serine protease. Biochem Biophys Res Commun. 1999;261:669–675. doi: 10.1006/bbrc.1999.1093. [DOI] [PubMed] [Google Scholar]
- 175.Nigam S, Ghosh PC, Sarma PU. A new glycoprotein allergen/antigen with the protease activity from Aspergillus fumigatus. Int Arch Allergy Immunol. 2003;132:124–131. doi: 10.1159/000073713. [DOI] [PubMed] [Google Scholar]
- 176.Kurup VP, Xia JQ, Shen HD, et al. Alkaline serine proteinase from Aspergillus fumigatus has synergistic effects on Asp-f-2-induced immune response in mice. Int Arch Allergy Immunol. 2002;129:129–137. doi: 10.1159/000065882. [DOI] [PubMed] [Google Scholar]
- 177.Kauffman HF. Immunopathogenesis of allergic bronchopulmonary aspergillosis and airway remodeling. Front Biosci. 2003;8:e190–e196. doi: 10.2741/990. [DOI] [PubMed] [Google Scholar]
- 178.McElvaney NG, Hubbard RC, Birrer P, et al. Aerosol alpha 1-antitrypsin treatment for cystic fibrosis. Lancet. 1991;337:392–394. doi: 10.1016/0140-6736(91)91167-s. [DOI] [PubMed] [Google Scholar]
- 179.McElvaney NG, Nakamura H, Birrer P, et al. Modulation of airway inflammation in cystic fibrosis: in vivo suppression of interleukin-8 levels on the respiratory epithelial surface by aerosolization of recombinant secretory leukoprotease inhibitor. J Clin Investig. 1992;90:1296–1301. doi: 10.1172/JCI115994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hubbard RC, McElvaney NG, Sellers SE, et al. Recombinant DNA-produced alpha 1-antitrypsin administered by aerosol augments lower respiratory tract antineutrophil elastase defenses in individuals with alpha 1-antitrypsin deficiency. J Clin Investig. 1989;84:1349–1354. doi: 10.1172/JCI114305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Taggart CC, Greene CM, Carroll TP, et al. Elastolytic proteases: inflammation resolution and dysregulation in chronic infective lung disease. Am J Respir Crit Care Med. 2005;171:1070–1076. doi: 10.1164/rccm.200407-881PP. [DOI] [PubMed] [Google Scholar]
- 182.Korkmaz B, Horwitz MS, Jenne DE, Gauthier F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol Rev. 2010;62:726–759. doi: 10.1124/pr.110.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 184.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 185.Safavi F, Rostami A. Role of serine proteases in inflammation: Bowman–Birk protease inhibitor (BBI) as a potential therapy for autoimmune diseases. Exp Mol Pathol. 2012;93:428–433. doi: 10.1016/j.yexmp.2012.09.014. [DOI] [PubMed] [Google Scholar]


