Abstract
Objective:
Our objective is to describe the short-term, health status benefits of ARNI initiation in patients with heart failure and reduced ejection fraction (HFrEF).
Background:
While sacubitril/valsartan, a neprilysin inhibitor (ARNI), improved patients’ health status (compared with enalapril) at 8 months in PARADIGM-HF, the early impact of ARNI on patients’ symptoms, function and quality of life is unknown.
Methods:
Health status was assessed with the 12-item Kansas City Cardiomyopathy Questionnaire (KCCQ-12) in 3,918 outpatients with HFrEF (LVEF ≤ 40%) across 140 US centers in the CHAMP-HF registry. ARNI was initiated in 508 patients, who were matched 1:2 to 1,016 patients not started on ARNI (no-ARNI) using a non-parsimonious time-dependent propensity score (6 sociodemographic factors, 23 clinical characteristics), prior KCCQ Overall Summary Score (KCCQ-OS)), and ACEI/ARB status.
Results:
Multivariable linear regression demonstrated a greater mean improvement in KCCQ-OS in patients initiated on an ARNI (5.3±19 vs. 2.5±17.4, p < 0.001) over a median (IQR) of 57 (32, 104) days. The proportions of ARNI versus no-ARNI groups with a ≥10-point (large) and a ≥20-point (very large) improvement in KCCQ-OS were 32.7% vs. 26.9% and 20.5% vs. 12.1%, consistent with numbers needed to treat of 18 and 12, respectively.
Conclusions:
In routine clinical care, ARNI initiation was associated with early improvements in health status, with 20% experiencing a very large health status benefit, as compared with 12% not started on ARNI. These findings support the use of ARNI to improve patients’ symptoms, function, and quality of life.
Keywords: Heart Failure, Sacubitril/Valsartan, Health Status
Introduction
A primary treatment goal in patients with heart failure and reduced ejection fraction (HFrEF) is to optimize their health status (e.g., symptoms, function, and quality of life).1 Health status is not only important from patients’ and providers’ perspectives,2 but also a strong and independent predictor of cardiovascular morbidity and mortality.3–6 Accordingly, regulatory agencies are increasingly recognizing the significance of systematically quantifying patients’ health status using patient-reported outcomes (PROs), to assess the health status benefits of novel therapies.7–9 However, despite an era of rapidly expanding treatments, few pharmacologic interventions in HFrEF have been shown to improve patients’ quality of life and reduce their symptom burden.1,10
The PARADIGM-HF trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) compared sacubitril/valsartan (angiotensin receptor-neprilysin inhibitor [ARNI]) with enalapril and demonstrated improved survival and lower hospitalization rates with ARNI. It, also, showed significantly less deterioration in patients’ health status with ARNI from baseline to 8 months.11 These data led the 2016 European and North American guideline authors to recommend ARNI for patients with HFrEF, or as a replacement for angiotensin converting-enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) in patients with HFrEF.12,13 However, a limitation of PARADIGM-HF was that patients’ health status was not assessed prior to the run-in phase, precluding an assessment of the early health status benefits of ARNI. Moreover, the effectiveness of ARNI on patients’ health status in routine clinical practice is unknown.
To address these gaps in knowledge, we used data from the Change the Management of Patients with Heart Failure (CHAMP-HF) registry14 to examine the association between ARNI initiation and patient-reported health status. CHAMP-HF is a prospective, multicenter, observational registry of outpatients with HFrEF that captured serial health status outcomes using the 12-item Kansas City Cardiomyopathy Questionnaire (KCCQ-12),15 making it an ideal data source to describe the health status benefits of ARNI in routine clinical practice.
Methods
Study Design
CHAMP-HF is a multicenter, observational registry designed to capture the care and outcomes of patients with HFrEF across heterogeneous outpatient practices in the United States.14 Eligibility criteria included a chronic diagnosis of HFrEF (left ventricular ejection fraction ≤ 40%) and use of ≥1 oral pharmacotherapy for heart failure. Minimum required exposure to ARNI was defined as uninterrupted ARNI therapy, at any dose either at the time of or between the most recent KCCQ assessment and the next closest assessment occurring at least two weeks following ARNI treatment initiation. Those with limited life expectancy, being considered for advanced mechanical support (e.g. left ventricular assist device, heart transplantation), and requiring hemodialysis were excluded. Study coordinators at each practice site were responsible for consecutively identifying and enrolling patients during the course of a routine outpatient HFrEF visit. For this analysis, other exclusion criteria included: (1) ARNI use prior to enrollment, (2) documented to have a contraindication/intolerance to ARNI, and (3) completion of < 2 KCCQ-12 assessments (1 prior to or at ARNI initiation and 1 at least 2 weeks after initiation; Figure 1). CHAMP-HF was funded by Novartis. All participating sites received institutional review board approval and informed consent was signed by each participant prior to enrollment.
1. Patient Exclusion Flowsheet.
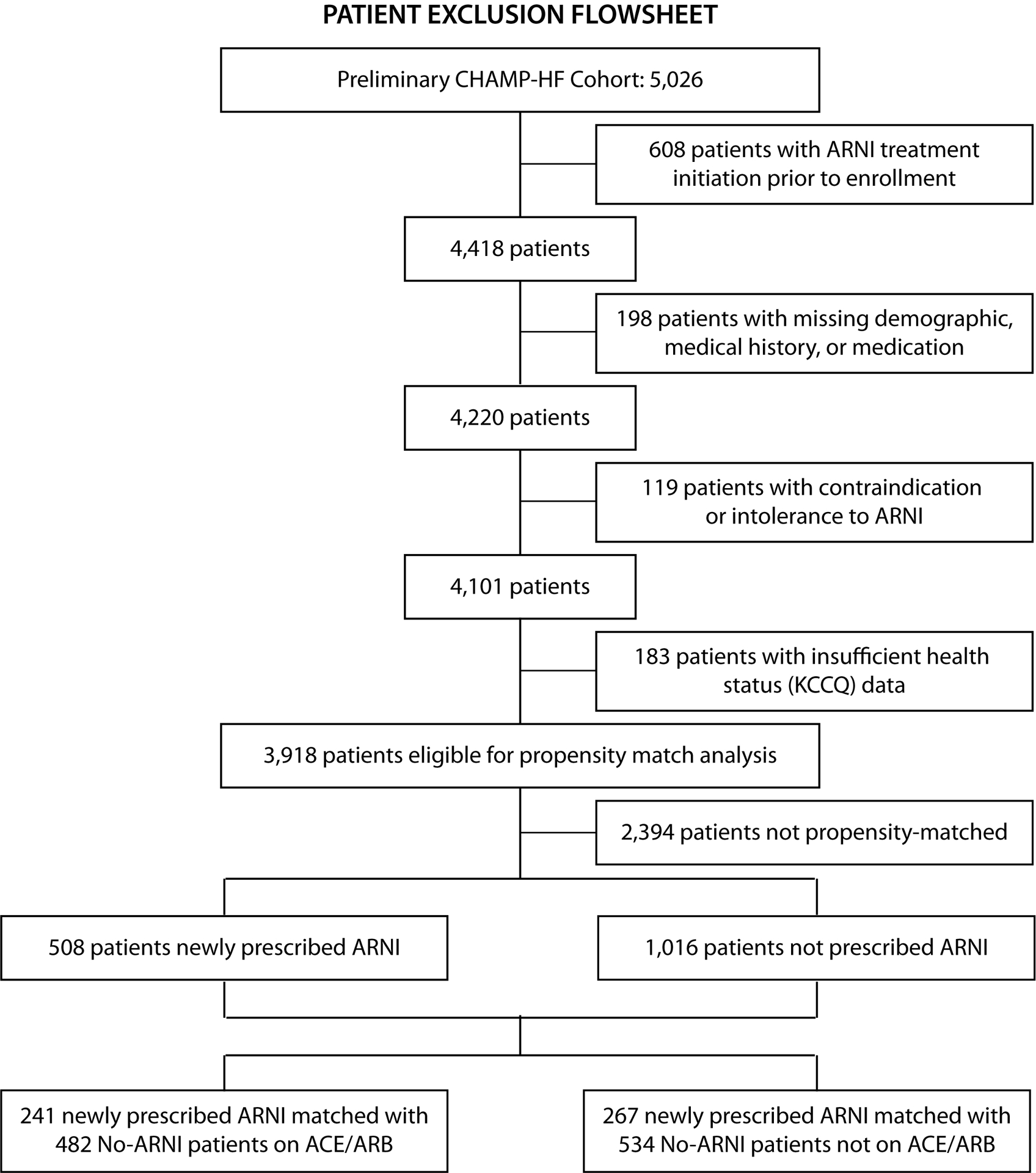
Patients with ARNI initiation prior to enrollment, contraindications to ARNI treatment, and missing covariate and KCCQ data were excluded.
Data Collection and Primary Analysis Outcome
At the time of patient enrollment, study coordinators interviewed patients to collect their baseline sociodemographic and health status information and performed chart abstraction to establish a comprehensive clinical history and medication profile. On all subsequent visits (1, 3, 6, and 12 months), patient-reported data were collected either during in-person or telephone interviews. The study did not dictate or recommend any changes in therapy, nor mandatory laboratory measurements.
The primary outcome for this analysis was change in KCCQ-12, a well-validated disease-specific patient-reported outcome measure that measures patients’ health status over the preceding two weeks that preserves the psychometric properties of the KCCQ-23 used in the PARADIGM-HF trial.15 The KCCQ Overall Summary score (KCCQ-OS) is comprised of four, equally-weighted domains – physical limitation (KCCQ-PL), symptom frequency (KCCQ-SF), quality of life (KCCQ-QoL), and social limitation (KCCQ-SL) – which were secondary outcomes of the study. All domains and the KCCQ-OS score range from 0 to 100, where higher scores indicate better health status.15 Prior and extensive work has defined the clinical significance of both group mean and individual patient-level changes. Patient-level changes of <5, 5 to <10, 10 to <20, and ≥20 points represent worse to small, moderate, large, and very large improvements, respectively.3,6,16,17
Study Cohorts and Defining ARNI Use
Participants were allocated into ARNI versus no-ARNI treatment groups, contingent on whether they began ARNI at any time after enrollment. Because ARNI may have been a preferred, initial treatment, or patients may have switched from an ACEI/ARB to an ARNI, we directly matched patients on their pre-ARNI ACEI/ARB status (use of an ACEI/ARB within the preceding 2 weeks; Online Figure 1). Due to the time-dependent nature of this analysis, if a no-ARNI patient completed ≥ 2 KCCQ assessments after initial propensity matching and prior to ARNI initiation, this patient was then potentially eligible to be included twice in the analysis – initially as a no-ARNI patient using the pre-ARNI KCCQ data and then as an ARNI patient using the post-ARNI KCCQ data.
Statistical Analysis
Time-Dependent Propensity Score Matching
Because patients may have been prescribed ARNI at different times throughout the registry, matching was employed to ensure that no-ARNI patients were identified at the same time during follow-up as those newly prescribed ARNI. All eligible participants were propensity-matched 1:2 ARNI: no-ARNI based upon on a time-dependent propensity score, their most recent KCCQ-OS, and ACEI/ARB status. Propensity scores were calculated using Cox proportional hazards models, where “time to ARNI” (days from enrollment to ARNI initiation) was the dependent variable and all patient-level predictors (except sociodemographics) were allowed to vary over time. Variables in the propensity score included 6 sociodemographic (age, sex, race, Hispanic ethnicity, household income, and employment status) and 23 clinical characteristics, comprised of medical history (atrial fibrillation, ventricular tachycardia/fibrillation, cardiac resynchronization therapy, chronic obstructive pulmonary disease, coronary artery disease, diabetes mellitus, depression/anxiety, essential hypertension, ischemic cardiomyopathy, current smoker, prior HF hospitalization, chronic kidney disease), medication use (beta-blocker, mineralocorticoid antagonist, loop diuretic, hydralazine, digoxin, ivabradine), physiologic measures (body mass index, systolic blood pressure, heart rate, left ventricular ejection fraction), and their most recent KCCQ scores. Due to non-mandatory reporting of laboratory data, these were not included in the model due to high missing rates. Left ventricular ejection fraction, health status data, and medications were updated throughout the analysis while physiologic data (e.g. vital signs) were updated every 6 months (the first value within each 6-month period was used for the matching within that period). Continuous variables were assessed for linearity of their relationship with the primary outcome of the propensity analysis using restricted cubic splines. For categorical variables, multi-level variables (e.g. household income) were categorized into binary categories (white vs. nonwhite, income < $25,000 vs ≥ $25,000, and employed full- or part-time vs. not working) to simplify interpretation. The quality of propensity matching was evaluated using standardized differences; the absolute difference in means (or proportions) divided by the average standard deviation. A standardized difference of <0.10 (10%) reflects good covariate balance between groups.18
Propensity models and matching were conducted, separately, for patients who were and were not on ACEI/ARBs, and then the cohorts were combined. To be included in the ACEI/ARB cohort, patients were required to have been treated with ACEI/ARB within two weeks prior to enrollment (otherwise, they were assigned to the no-ACEI/ARB cohort). These steps insured comparability of newly prescribed ARNI patients and switchers from ACEI/ARB to ARNI. Medians (interquartile ranges) of time (days) from pre-match to post-match KCCQ assessments were calculated. The primary analysis included all patients (ARNI vs. no-ARNI), with comparisons of newly prescribed (ARNI vs no-ACEI/ARB) and switching (ARNI vs. ACEI/ARB) reported as secondary analyses.
The majority of patient-level variables had ≤5% missing data, with the exception of household income (21%) and body mass index (8%). For covariates in the propensity model, missing values were imputed using single imputation with full conditional specification. KCCQ scores were not imputed.
Linear Regression and Responder Analysis
Change in KCCQ was calculated as the difference between post-match and pre-match KCCQ scores. After comparing the propensity-matched cohorts, we compared the mean differences in the matched cohorts and further compared the association of ARNI initiation with changes in health status using five linear regression models of change; KCCQ-OS as the primary outcome and change in KCCQ domain scores as secondary outcomes. The regression models used generalized estimating equations (GEE) to adjust for all covariates and clustering of patients within practice sites.
Because mean KCCQ scores represent a population average effect, we also described the distribution of change so that the proportion of patients with clinically important changes in health status could be appreciated. For each KCCQ score, where there was a significant effect in the main model, the proportion of patients (n; %) across categories of KCCQ improvement (worse to small (<5 points), moderate (≥5 to <10 points), large (10 to <20 points), and very large (≥20 points) changes) were calculated.15 When statistically significant, the number needed to treat (NNT) was calculated by first finding the absolute risk reduction (ARR = PA-PN), where PA and PN represent the proportion of ARNI and no-ARNI patients, respectively, with a given level of health status improvement and 95% confidence intervals (CI) were calculated as ARR +/− 1.96*SE(ARR), where SE=Wald standard error. The reciprocals of these estimates were used to calculate the NNTs.
All estimates were reported using 95% confidence intervals and a p-value ≤0.05 was considered statistically significant. All analyses were performed using SAS software (version 14.3 SAS Institute, Cary, NC).
Results
Study Cohort
At the time of this analysis, 3,918 patients across 140 sites were enrolled in CHAMP-HF between 2015 and 2017, after excluding those that were prescribed ARNI prior to enrollment (n = 608), missing demographic, medical history, or medication data (n = 198), documented to have a contraindication or intolerance to ARNI (n = 119), and missing KCCQ data before or after ARNI initiation (n = 183; Figure 1). Of these, 580 were newly prescribed ARNI and 508 (88%) were successfully matched with 1,016 patients who had not initiated ARNI at the same point during follow-up (Table 1; Online Figure 2). For those patients who began treatment with ARNI, 267 (53%) and 241 (47%) were and were not on prior ACEI/ARB therapy within two weeks of ARNI initiation, respectively. The matched groups were well-balanced in sociodemographic and clinical characteristics, with the exception of chronic kidney disease (standardized difference = 11.7%), beta-blocker (14.1%), MRA (15.3%), and loop diuretic (10.2%) treatment. Similar comparability between groups was observed within the ACEI/ARB (267 ARNI and 534 ACEI/ARB) and no-ACEI/ARB (241 ARNI and 482 no-ACEI/ARB) cohorts (Online Tables 1–2; Online Figures 3–4).
Table 1.
Patient Characteristics for Combined Matched Cohort
| Post-Match | |||
|---|---|---|---|
| Patient Characteristic (most updated prior to matching) | ARNI N = 508 | no-ARNIa 1016 | Standardized Differences (%)a |
| Sociodemographic | |||
| Age | 64.0 (12.9) | 65.2 (12.7) | 9.0 |
| Female | 30% (154) | 29% (291) | 3.7 |
| White Race | 75% (381) | 74% (750) | 2.7 |
| Hispanic | 9% (47) | 12% (120) | 8.3 |
| Household Income < $25,000 | 34% (175) | 37% (380) | 6.2 |
| Employed Full or Part-Time | 26% (131) | 22% (221) | 9.5 |
| Clinical Measures | |||
| Body Mass Index | 31.2 (7.3) | 30.5 (7.5) | 9.8 |
| Systolic BP | 120.3 (17.3) | 118.8 (17.4) | 8.9 |
| Heart Rate | 74.2 (13.2) | 74.4 (13.4) | 1.8 |
| LVEF (%) | 28.8 (7.1) | 29.5 (8.5) | 9.2 |
| Medical History | |||
| Atrial Fibrillation/Flutter | 38% (195) | 40% (406) | 3.2 |
| History of VT/VF | 23% (119) | 23% (231) | 1.6 |
| CRT therapy | 10% (49) | 9% (94) | 1.3 |
| COPD | 27% (139) | 29% (291) | 2.8 |
| Coronary Artery Disease | 62% (314) | 66% (672) | 9.0 |
| Diabetes | 43% (219) | 43% (439) | 0.2 |
| Depression or Anxiety | 34% (171) | 36% (363) | 4.3 |
| Hypertension | 83% (424) | 83% (841) | 1.8 |
| Ischemic HF Etiology | 41% (209) | 42% (426) | 1.6 |
| Current smoker | 20% (101) | 20% (207) | 1.2 |
| Prior HF Hospitalization | 40% (205) | 41% (419) | 1.8 |
| Chronic Kidney Disease | 18% (92) | 23% (232) | 11.7 |
| Medication | |||
| ACEI/ARB | 53% (267) | 53% (534) | 0.0 |
| Beta-Blocker | 97% (492) | 94% (954) | 14.1 |
| MRA | 49% (250) | 42% (423) | 15.3 |
| Loop Diuretic | 73% (373) | 69% (699) | 10.2 |
| Hydralazine | 4% (22) | 5% (46) | 1.0 |
| Digoxin | 17% (88) | 14% (143) | 8.9 |
| Ivabradine | 2% (8) | 2% (17) | 0.8 |
| Prescribed ≥ 2 HF Medications | 93% (474) | 88% (899) | 16.8 |
| Prescribed ≥ 3 HF Medications | 67% (341) | 57% (580) | 20.8 |
| KCCQ Score | |||
| Overall Score | 64.1 (23.5) | 63.3 (23.9) | 3.3 |
| SF Score | 70.9 (24.5) | 71.1 (24.5) | 0.8 |
| QoL Score | 58.8 (28.3) | 59.3 (28.1) | 1.9 |
| SL Score | 69.3 (27.9) | 68.3 (29.0) | 3.3 |
| PL Score | 67.6 (26.2) | 65.1 (27.2) | 9.6 |
55 ARNI patients were used as no-ARNI matches before their ARNI start date.
Standardized difference is calculated as the differences in means or proportions divided by the standard deviation. A standardized difference >10% is considered imbalance.
Characteristics of the analytic cohort are presented in Online Table 3. Of the total sample, 42.0% were 40–64 and 44.4% were 65–80 years of age, 29.2% were women, and 74.2% were white. Cardiac and non-cardiac comorbidities were common, with 64.6% having coronary artery disease, 27.9% with chronic obstructive lung disease/asthma, 33.7% with depression/anxiety, 42.7% with diabetes, and 38.4% with atrial fibrillation/flutter. Most patients (62.0%) had no HF hospitalizations in the year prior to enrollment, reflecting a relatively stable HF cohort. Mean systolic blood pressure and left ventricular ejection fraction in the cohort was 120 ± 18 mmHg and 28 ± 8%, respectively. Use of HF therapies in the matched cohorts was high, including beta-blockers in 94.3%, mineralocorticoid antagonists (MRA) in 41.4% and loop diuretics in 69.5%, with a minority prescribed digoxin (14.6%) and ivabradine (1.6%). The average KCCQ-OS score at enrollment in CHAMP-HF was 63.6 ± 23.7, corresponding to NYHA Class II.
Association of ARNI Use with Changes in Health Status
Improvements in KCCQ score, from last pre-match to first post-match health status assessment, were observed over a median (IQR) of 57 (32–104) days. Overall, ARNI patients experienced an average 5.3±18.6-point improvement in the KCCQ-OS compared with 2.5±17.4 points for their no-ARNI counterparts (Difference in mean change of 3.2 (95% CI 1.5, 4.9); adjusted group-level difference with regression modeling of 2.9 points (95% CI 1.14, 4.6; p < 0.001); Table 2). This was largely driven by patients’ improvements in the KCCQ-PL (4.8±24.8 points versus 2.0±22.2 points) and KCCQ-QoL (6.4±23.9 points versus 2.7±24.1 points) domains. Similar findings were observed in both the de novo ARNI initiations (mean difference of 2.9 points (95% CI 0.3, 5.5; p = 0.028)) and in those who switched between ACEI/ARB and ARNI (mean difference of 2.7 points (95% CI 0.4, 5.0; p = 0.024)) (Online Tables 4a–4b and 5a–5b).
Table 2.
Mean Change in Scores and Multivariable Regression-Adjusted Difference in Mean Score Change in Propensity-Matched Patients (Combined Model)
| Mean Change in Scores | Multivariable Regression-Adjusted Difference in Mean Score Change | |||||
|---|---|---|---|---|---|---|
| Change in Score (post minus pre) | P-Value | P-Value | ||||
| KCCQ Score | ARNI (n=508) Mean (SD) | no-ARNI (n=1016) Mean (SD) | ARNI vs. no-ARNI Post KCCQ Score | Difference in Mean Change Estimate (95% CI) | Covariate-Adjusted Difference in Mean Change Estimate (95% CI) | ARNI vs. no-ARNI |
| KCCQ-Overall Summary | 5.3 (18.6) | 2.5 (17.4) | <.001 | 3.2 (1.5, 4.9) | 2.9 (1.1, 4.6) | 0.001 |
| KCCQ-Physical Limitationa | 4.8 (24.8) | 2.0 (22.2) | 0.006 | 3.2 (0.9, 5.5) | 3.3 (0.8, 5.8) | 0.009 |
| KCCQ-Symptom Frequency | 4.3 (20.8) | 1.5 (21.2) | 0.004 | 2.9 (0.9, 4.9) | 2.6 (0.6, 4.6) | 0.010 |
| KCCQ-Social Limitationb | 5.9 (26.0) | 3.6 (23.8) | 0.030 | 2.6 (0.3, 5.0) | 2.7 (0.3, 5.1) | 0.029 |
| KCCQ-Quality of Life | 6.4 (23.9) | 2.7 (24.1) | <.001 | 3.8 (1.6, 6.0) | 3.3 (1.1, 5.4) | 0.003 |
116 sets (348 pts) omitted for missing pre- or post-match Physical Limitation score for one or more members of the set.
76 sets (228 pts) omitted for missing pre- or post-match Social Limitation score for one or more members of the set.
Responder Analysis
The proportion of patients experiencing at least moderate, large and very large health status improvement by change in KCCQ-OS scores were calculated (Table 3). Overall, 43.7% of ARNI patients (versus 39.8% of no-ARNI patients); 32.7% of ARNI patients (versus 26.9% of no-ARNI patients) and 20.5% of ARNI patients (versus 12.1% of no-ARNI patients) experienced at least a moderate (≥5-point increase), large (≥10-point increase) and very large (≥20-point increase) health status benefit, respectively (Online Figures 5–6). Based on these results we found that for every 18 (95% CI= 10, 111) and 12 (95% CI= 9, 24) patients started on an ARNI, one more would be expected to have a large and a very large health status benefit, respectively, as compared with not being started on an ARNI (Tables 3–4 and Central Illustration). Moreover, fewer patients were likely to experience minimal to none improvement or deterioration in their health status (absolute difference of 3.9% between groups), translating to a NNT of 26 to prevent such an outcome.
Table 3.
Patients Categorized by Pre-Post Change in KCCQ in the Combined Matched Cohort
| Pre-Post Change in KCCQ Score | ARNI (n=508) % (n) | no-ARNI (n=1016) % (n) |
|---|---|---|
| Overall Summary | ||
| Very large improvement (≥ 20) | 20.5% (104) | 12.1% (123) |
| Large improvement (10 to <20) | 12.2% (62) | 14.8% (150) |
| Moderate improvement (5 to <10) | 11.0% (56) | 12.9% (131) |
| No or small improvement or worse (< 5) | 56.3% (286) | 60.2% (612) |
| Physical Limitationa | ||
| Very large improvement (≥ 20) | 21.2% (101) | 17.4% (160) |
| Large improvement (10 to <20) | 11.1% (53) | 11.0% (101) |
| Moderate improvement (5 to <10) | 11.6% (55) | 10.4% (96) |
| No or small improvement or worse (< 5) | 56.1% (267) | 61.2% (564) |
| Symptom Frequency | ||
| Very large improvement (≥ 20) | 18.1% (92) | 15.9% (162) |
| Large improvement (10 to <20) | 13.0% (66) | 12.1% (123) |
| Moderate improvement (5 to <10) | 9.8% (50) | 8.1% (82) |
| No or small improvement or worse (< 5) | 59.1% (300) | 63.9% (649) |
| Social Limitationb | ||
| Very large improvement (≥ 20) | 23.9% (116) | 20.1% (192) |
| Large improvement (10 to <20) | 8.5% (41) | 9.1% (87) |
| Moderate improvement (5 to <10) | 9.9% (48) | 12.1% (116) |
| No or small improvement or worse (< 5) | 57.7% (280) | 58.6% (560) |
| Quality of Lifec | ||
| Very large improvement (≥ 20) | 25.6% (130) | 22.3% (227) |
| Large improvement (10 to <20) | 20.7% (105) | 16.9% (172) |
| Moderate improvement (5 to <10) | -- | — |
| No or small improvement or worse (< 5) | 53.7% (273) | 60.7% (617) |
116 sets (348 pts) omitted for missing pre- or post-match Physical Limitation score for one or more members of the set.
76 sets (228 pts) omitted for missing pre- or post-match Social Limitation score for one or more members of the set.
Because of the granularity of the QOL score, a “small improvement” is not possible.
Table 4.
Number Needed to Treat for Moderate and Large Thresholds of Health Status Change (Combined, ACEI/ARB, and no-ACEI/ARB Cohort)
| Cohort | NNT for ≥ Large KCCQ-OS Improvement NNT (95% CI) | NNT for ≥ Very Large KCCQ-OS Improvement NNT (95% CI) |
|---|---|---|
| Combined | 18 (10–111) | 12 (9–24) |
| ACEI/ARBa | -- | 12 (8–31) |
| no-ACEI/ARBa | -- | 13 (8–50) |
In the ACEI/ARB and No-ACEI/ARB cohorts, NNT and 95% CI for ≥moderate KCCQ-OS improvement were not calculated due to lack of statistical significance
Central Illustration.
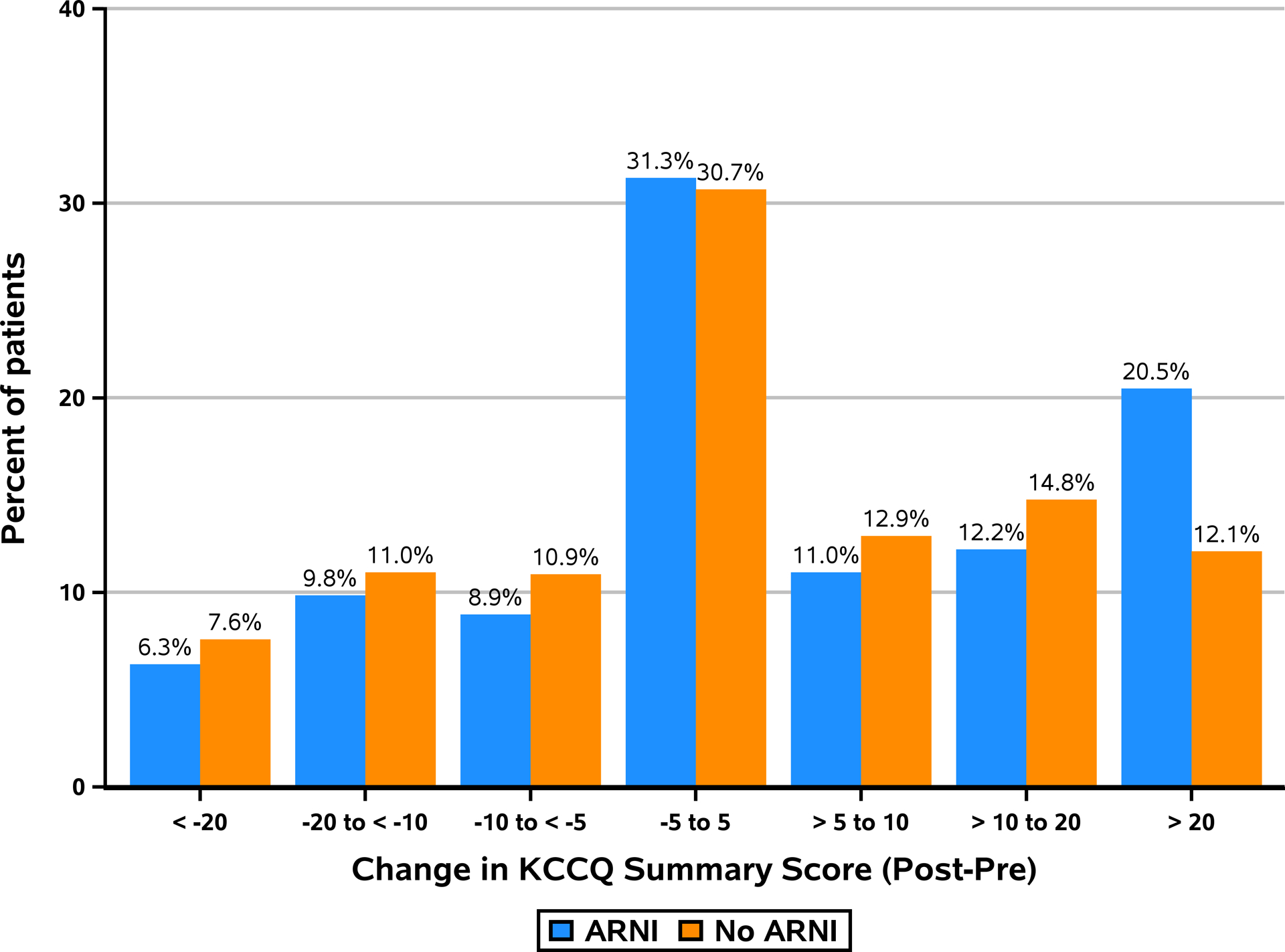
Distribution of Change in KCCQ Overall Summary Score (Combined Cohort)
Consistent findings were observed among ARNI patients who switched from an ACEI/ARB and among patients not previously on an ACEI/ARB (Online Figures 7–8). The proportions experiencing very large clinical improvements in ACEI/ARB cohort were 19.1% vs. 10.5%, respectively suggesting that switching to an ARNI results in an NNT of 12 (95% CI= 8, 31) for a patient to experience a very large improvement in their health status. Among patients not previously on an ACEI/ARB, initiation of an ARNI was associated with a 22% (vs. 13.9%) chance of a very large improvement, corresponding to an NNT of 13 (95% CI= 8, 50; Table 4).
Discussion
While a principal goal of HF management is to alleviate patient suffering and improve health status, few pharmacological therapies have been shown to reliably reduce symptoms, improve function and enhance quality of life. In this study, we examined the early health status benefits of instituting an ARNI in a multi-center observational registry of outpatients with chronic HFrEF. We found that, independent of prior treatment with ACEI/ARB, patients prescribed ARNI experienced early and robust improvements in disease-specific health status as measured by the KCCQ. These findings represent the first, real-world evidence describing the potential health status benefits of ARNI in patients with HFrEF.
These findings extend the clinical trial data from PARADIGM-HF,11,19,20 which described significant reductions in cardiovascular mortality and HFrEF hospitalization as well as greater preservation of health status over 8 months with ARNI. A notable limitation of PARADIGM-HF arose from the use of a run-in phase – during which all patients underwent dosing of enalapril, followed by dose-escalation of ARNI – prior to baseline KCCQ assessment. As such, early improvements in health status associated with ARNI use could not be calculated. To address this gap, the CHAMP-HF registry offers a unique perspective by capturing patients’ true baseline health status prior to ARNI treatment initiation, thereby providing a more accurate assessment of early health status changes over time after ARNI initiation. Finding a clinically significant mean increase in KCCQ-OS scores of 5.3 points in the patients treated with ARNI suggests that the early benefits of treatment may have been missed in PARADIGM-HF, but given the minimal further decrease in scores over time in PARADIGM-HF, that these benefits are likely sustained over time. Confirmation of these sustained benefits from CHAMP-HF should be explored as more follow-up in this registry accrues.
When comparing mean differences between groups, it can often be difficult to interpret the clinical significance of changes. Accordingly, we also examined the distribution of change against well-established thresholds of clinically important changes in the KCCQ.15,17 By comparing the proportions of patients by their magnitude of change, we were able to conduct ‘responder’ analyses and estimate the number of patients that would need to be treated for one to have a clinically important change in their health status. The distribution of changes in KCCQ scores for those started on ARNI was shifted, such that a substantial proportion of patients experienced very large clinical improvements in their health status. We found an NNT of 12, meaning that for every 12 patients treated with ARNI, as compared with similar patients not treated, 1 would experience a very large improvement in their health status; regardless of whether ARNI was used as a substitute for ACEI/ARB treatment, or as a de novo therapy. This >20-point change is comparable to the mean health status benefits after transcatheter aortic valve replacement or insertion of a left ventricular assist device.21,22 Understanding whether or not there are particular patient profiles associated with such large health status benefits from ARNI is an important area for future investigation.
Our findings should be interpreted in the context of several potential limitations. First, CHAMP-HF is an observational registry and is thereby susceptible to bias as a result of unmeasured confounding. In particular, we cannot exclude the role of placebo effect due to open-label ARNI use. Second, we were unable to match patients’ laboratory values due to missingness of laboratory data. Third, while a primary aim of CHAMP-HF was to recruit participating sites with diverse treatment backgrounds (e.g. physician specialty, patient population), our findings may not be generalizable throughout the United States or to other countries. In particular, while rates of heart failure medical therapy were (at least) similar to that observed in prior HFrEF registries and HFrEF clinical trials,23–25 rates of MRA, digoxin, and diuretic were lower than those in PARADIGM-HF.25,26 Finally, these results only apply to outpatients with HFrEF and the benefits of ARNI for other outcomes and in other heart failure populations requires further investigation.
Conclusion
In an observational registry of outpatients with HFrEF across the US, we found an association between ARNI initiation and early improvements in patient-reported health status. These improvements were largely driven by a substantially larger proportion of patients treated with ARNI experiencing a very large health status benefit shortly after ARNI initiation. These data supplement the benefits of ARNI therapy in reducing mortality and hospitalizations and underscore the need for future research to better identify patients who are most likely to benefit from ARNI.
Supplementary Material
Clinical Perspectives:
Initiation of ARNI is associated with early, clinically meaningful health status (symptom, function and quality of life) benefits in outpatients with heart failure and reduced ejection fraction.
Translational Outlook:
This work extends prior insights on the clinical benefits of ARNI initiation and demonstrates its early health status benefits in a real-world HFrEF population.
Acknowledgments/Disclosures
Drs. Y. Khariton and M. Nassif are supported by the National Heart, Lung, and Blood Institutes of Health Under Award Number T32HL110837; the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Khariton had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
Dr. John A. Spertus discloses grant funding from NIH, ACCF, Bayer, Novartis, Abbott Vascular. He serves on a Scientific Advisory Board for United Healthcare and as a consultant for Novartis, V-Wave, AstraZeneca, Jansssen, Corvia and Bayer. He has intellectual property rights for the Kansas City Cardiomyopathy Questionnaire and an equity interest in Health Outcomes Sciences
Dr. Laine Thomas reports research funding from Novartis Pharmaceuticals Corporation
Dr. Gregg C. Fonarow reports research support from the National Institute of Health, consulting for Abbott, Amgen, Bayer, Janssen, Medtronic, and Novartis, and serving on the Get With The Guidelines Steering Committee
Dr. Adam D. DeVore receives research Support from the American Heart Association, Amgen, NIH, and Novartis; he provides consulting services for Novartis
Dr. Javed Butler has received research support from the National Institutes of Health and the European Union and serves as a consultant for Amgen, Bayer, Boehringer Ingelheim, Cardiocell, CVRx, Gilead, Janssen, Medtronic, Merck, Novartis, Relypsa, and ZS Pharma.
Dr. Nancy M. Albert reports consulting for Novartis and Boston Scientific and receiving honoraria from Novartis.
Dr. Puza P. Sharma is an employee of Novartis
Dr. Carol I. Duffy is an employee of Novartis
Mr. Kevin McCague is an employee of Novartis
All other authors have reported no relationships to disclose relevant to the contents of this manuscript.
Funding
CHAMP-HF and this study was funded by the Novartis Pharmaceuticals Corporation
Abbreviations:
- HFrEF
Heart Failure with Reduced Ejection Fraction
- PRO
Patient-Reported Outcomes
- ARNI
Angiotensin-Neprilysin Inhibitor
- KCCQ12
12-Item Kansas City Cardiomyopathy Questionnaire
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. [DOI] [PubMed] [Google Scholar]
- 2.Rumsfeld JS, Alexander KP, Goff DC Jr., et al. Cardiovascular health: the importance of measuring patient-reported health status: a scientific statement from the American Heart Association. Circulation. 2013;127(22):2233–2249. [DOI] [PubMed] [Google Scholar]
- 3.Pokharel Y, Khariton Y, Tang Y, et al. Association of Serial Kansas City Cardiomyopathy Questionnaire Assessments With Death and Hospitalization in Patients With Heart Failure With Preserved and Reduced Ejection Fraction: A Secondary Analysis of 2 Randomized Clinical Trials. JAMA Cardiol. 2017;2(12):1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosiborod M, Soto GE, Jones PG, et al. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation. 2007;115(15):1975–1981. [DOI] [PubMed] [Google Scholar]
- 5.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation. 2004;110(5):546–551. [DOI] [PubMed] [Google Scholar]
- 6.Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150(4):707–715. [DOI] [PubMed] [Google Scholar]
- 7.Committee on Quality of Health Care in America Institute of Medicine: Crossing the quality chasm: a new health system for the 21st century. Washington, D.C: National Academy Press; 2001. [Google Scholar]
- 8.Kansas City Cardiomyopathy Questionnaire (KCCQ) | FDA Voice. [online] Blogs.fda.gov. Available at: https://blogs.fda.gov/fdavoice/index.php/tag/kansas-city-cardiomyopathy-questionnaire-kccq/ [Accessed 7 Jun. 2018].
- 9.National Institutes of Health. The 21st Century Cures Act. [online] Available at: https://www.nih.gov/research-training/medical-research-initiatives/cures [Accessed 14 Jun. 2018].
- 10.Ekman I, Chassany O, Komajda M, et al. Heart rate reduction with ivabradine and health related quality of life in patients with chronic heart failure: results from the SHIFT study. Eur Heart J. 2011;32(19):2395–2404. [DOI] [PubMed] [Google Scholar]
- 11.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. [DOI] [PubMed] [Google Scholar]
- 12.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. [DOI] [PubMed] [Google Scholar]
- 13.Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68(13):1476–1488. [DOI] [PubMed] [Google Scholar]
- 14.DeVore AD, Thomas L, Albert NM, et al. Change the management of patients with heart failure: Rationale and design of the CHAMP-HF registry. American Heart Journal. 2017;189:177–183. [DOI] [PubMed] [Google Scholar]
- 15.Spertus JA, Jones PG. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8(5):469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn KE, Lin L, Moe GW, et al. Relationships between changes in patient-reported health status and functional capacity in outpatients with heart failure. Am Heart J. 2012;163(1):88–94 e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreyer RP, Jones PG, Kutty S, Spertus JA. Quantifying clinical change: discrepancies between patients’ and providers’ perspectives. Qual Life Res. 2016;25(9):2213–2220. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis EF, Claggett BL, McMurray JJV, et al. Health-Related Quality of Life Outcomes in PARADIGM-HF. Circ Heart Fail. 2017;10(8). [DOI] [PubMed] [Google Scholar]
- 20.Chandra A, Lewis EF, Claggett BL, et al. Effects of Sacubitril/Valsartan on Physical and Social Activity Limitations in Patients With Heart Failure: A Secondary Analysis of the PARADIGM-HF Trial. JAMA Cardiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osnabrugge RL, Arnold SV, Reynolds MR, et al. Health status after transcatheter aortic valve replacement in patients at extreme surgical risk: results from the CoreValve U.S. trial. JACC Cardiovasc Interv. 2015;8(2):315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers JG, Aaronson KD, Boyle AJ, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55(17):1826–1834. [DOI] [PubMed] [Google Scholar]
- 23.Fonarow GC, Yancy CW, Heywood JT, et al. Adherence to heart failure quality-of-care indicators in US hospitals: analysis of the ADHERE Registry. Arch Intern Med. 2005;165(13):1469–1477. [DOI] [PubMed] [Google Scholar]
- 24.Patel DB, Shah RM, Bhatt DL, et al. Guideline-Appropriate Care and In-Hospital Outcomes in Patients With Heart Failure in Teaching and Nonteaching Hospitals: Findings From Get With The Guidelines-Heart Failure. Circ Cardiovasc Qual Outcomes. 2016;9(6):757–766. [DOI] [PubMed] [Google Scholar]
- 25.McMurray JJ, Packer M, Desai AS, et al. Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). Eur J Heart Fail. 2014;16(7):817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010;122(6):585–596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


