Abstract
Intestinal/multivisceral transplantation has evolved from an experimental procedure to the treatment of choice for patients with irreversible intestinal failure and serious complications related to long-term parenteral nutrition. Children who are likely to suffer permanent intestinal failure and benefit from intestinal transplantation include those with a remaining small bowel length of less than 30–40 cm, absence of the ileocecal valve, colonic resection and malabsorptive syndromes. Indications for transplant include frequent severe bouts of catheter associated sepsis, threatened loss of vascular access and the development of liver cirrhosis from cholestasis. Children who are more likely to experience cholestasis from total parenteral nutrition include those who experience persistent hyperbilirubinemia (greater than 6 mg/dl despite enteral nutrition), those with recurrent sepsis and/or bacterial overgrowth and those with minimal tolerance of any enteral feeds in the first few months post resection. The 1 year survival rate after intestinal transplantation has markedly improved over the last several years but long term survival rates have remained unchanged. The improved short term survival rates have led to an increased prevalence of this patient population in intensive care units. Management of intestinal and multivisceral transplant recipients is uniquely challenging because of complications arising from the high incidence of transplant rejection and its treatment. In the ICU, the complexity of medical care for the transplant recipient requires a multidisciplinary approach with coordination by an intensivist in collaboration with the transplant surgeon, gastroenterologist, and other specialists.
Keywords: Gastroschisis; Necrotizing enterocolitis; Multivisceral transplantation; Acute cellular rejection; Antibody mediated rejection; Chronic rejection; Graft vs. host disease; Calcineurin inhibitors; Tacrolimus; Thymoglobulin; Rituximab; Post-transplant lymphoproliferative disorder; Posterior reversible encephalopathy; Herpes virus infections; Viral infection, reactivation
Introduction
Intestinal/multivisceral transplantation has evolved from an experimental procedure to the treatment of choice for patients with irreversible intestinal failure and serious complications related to long-term parenteral nutrition. Each year nearly 200 individuals undergo intestinal transplantation and approximately one half of recipients are less than 18 years of age [1]. At the end of 2007, there were nearly 400 living pediatric intestinal transplant recipients [1]. Management of intestinal and multivisceral transplant recipients is uniquely challenging because of complications arising from the high incidence of graft rejection and its treatment. When performed in a young, immunologically naive population the likelihood of post-transplant life threatening infections is high. Long-term co-morbidities, such as diabetes, hypertension, chronic kidney failure, and neurological sequelae, also develop in this patient population, especially as short-term survival improves. Thus, the medical care of intestinal transplant recipients may involve the management of insufficiency of every organ system.
In the ICU, the complexity of medical care for the transplant recipient requires a multidisciplinary approach with coordination by an intensivist in collaboration with the transplant surgeon, gastroenterologist, and other specialists [2]. The majority of children are transplanted in a few high volume centers and increasing collective experience has contributed to improved survival [3]. When these patients present to ICUs distant from the home transplant center, early communication with that center is essential and transfer after stabilization may be necessary.
Indications for Transplant
Intestinal transplantation does not offer benefit for all children dependent on total parenteral nutrition (TPN). With new approaches to nutritional support and bowel lengthening procedures, 1 and 5 years survival rates for children with short bowel syndrome are 94 and 80 %, respectively [4]. Eighty percent of children can wean from TPN – most do so between 1 and 2 years of age [4]. Less than 10 % die from TPN related complications [4]. Whereas children were once institutionalized while receiving TPN, today many of these children can live at home while undergoing bowel rehabilitation.
Several reviews have identified the following characteristics of children who are likely to suffer permanent intestinal failure: remaining small bowel length less than 30–40 cm, absence of the ileocecal valve, and colonic resection [4, 5]. Children who are more likely to experience TPN-induced cholestasis include those who experience persistent hyperbilirubinemia (greater than 6 mg/dL despite enteral nutrition), those with recurrent sepsis and/or bacterial overgrowth, and those with minimal tolerance of any enteral feeds in the first few months post resection [4, 5]. Indications for transplant include frequent severe bouts of catheter-associated sepsis and threatened loss of vascular access [4]. Children should be referred for transplant evaluation when they experience liver fibrosis, a sustained bilirubin elevation over 5 mg/dL, signs of portal hypertension, and loss of 50 % of vascular access sites. A ratio of aspartate aminotransferase (AST) to platelet count greater than 1.5 was associated with the early development of fibrosis and may be used to guide the timing of listing for isolated intestinal transplantation [6]. Children with malabsorptive conditions such as micro-villous inclusion disease, tufting enteropathy and mega-cystic microcolon intestinal hypo-peristalsis syndrome should also be referred to a transplantation center as they have no intestinal rehabilitation potential [4, 5]. Waiting times for an isolated intestine allograft are much shorter than waiting times for a liver allograft [3]. Children who are ill enough to require hospitalization at the time of transplantation have a higher mortality than those waiting at home. Over the last several years short term survival rates have improved and fewer children are noted to be in the hospital at the time of transplantation, likely due to earlier referral [1].
Intestinal transplantation can also be used for the treatment of specific neoplastic diseases involving the mesenteric root. In pediatrics, neoplastic disease is an indication for transplant in approximately 1 % of cases. Moon et al. described a series of 15 patients, 12 of whom had desmoid tumors, who underwent multivisceral transplantation. This subset of patients had 1 and 5 years survival rates of 69 and 50 % respectively [7].
Pre-transplant Evaluation
The pre-transplant evaluation process is designed to identify contraindications to transplant and co-morbidities that will complicate the post-transplant course. Contraindications include profound neurologic deficits, non-correctable life-threatening conditions that are not related to liver and intestinal failure, immunodeficiencies, non-resectable malignancies, multisystem autoimmune disorders, and insufficient vascular access [4]. Cancer survivors must be 5 years out from their last treatment and cleared by an oncologist before being considered transplant candidates. Co-morbidities are common in intestinal transplant candidates. At the University of Miami, the most common cause of intestinal failure for pediatric intestinal transplant is gastroschisis (27 %) followed by necrotizing enterocolitis (20 %) [8]. These specific conditions are associated with prematurity, prolonged hospitalization, colonization with resistant pathogens, chronic lung disease, failure to thrive, poor vascular access, limited abdominal capacity, and poor nutrition. In adult transplantation, mesenteric thrombosis is a common primary diagnosis and a coexisting hypercoaguable state may lead to additional complications [8, 9].
A thorough history must include screening of the family history for thrombophilia, hypercholesterolism, and adverse anesthetic incidents. A thorough psychosocial evaluation is necessary to evaluate potential barriers to both emergency care and routine monitoring. Imaging including abdominal, central nervous systems and vascular access must be reviewed. Certainly liver assessment is critical and liver biopsy to evaluate the extent of fibrosis is routine. Renal function should be evaluated to determine if a kidney should be included in the graft. Serologic tests for HIV, hepatitis B and C are required. Every effort is made to bring the child into compliance with vaccination recommendations before transplant. The complete pre-transplant evaluation should be available to the intensivist. Ideally, a pediatric intensivist should participate in a meeting where candidate eligibility is discussed prior to listing.
Surgical Approach
There are several surgical approaches to intestinal transplantation [8]. Figure 30.1 gives an overview of the different types of grafts used in intestinal transplantation. Isolated intestinal transplantation has an advantage over combined transplants in that the intestine can be explanted should severe rejection or other complications such as posttransplant lymphoproliferative disorder (PTLD) arise. With this scenario, the patient becomes TPN dependent once again and immunosuppression is stopped. If the child has significant hepatic fibrosis or liver dysfunction and a liver transplant is required, it is recommended that the transplantation of the liver is performed at the time of intestinal transplantation. A combined liver and intestinal transplant may include the duodenum and pancreas, whole or in part, for technical reasons [2]. The term multivisceral transplantation applies to the en bloc replacement of liver, pancreas, stomach, and small intestine after evisceration of native abdominal organs including the spleen; however, there is great variability in the extent of transplantation of other organs such as the spleen and proximal colon [2, 3, 8, 10]. Colonic transplantation was once thought to be associated with higher infection rates, but this appears to no longer be true and colonic transplantation may reduce fluid losses [8, 11, 12]. A modified multivisceral transplantation refers to en bloc transplantation of multiple abdominal organs excluding the liver [8, 9]. To simplify clinical research, some scientists have recommended that procedures be categorized as intestinal transplant with or without liver transplant and the term multivisceral be abandoned [3]. However, these terms are already recognized and used in comparative analysis of specific transplant type [10].
Fig. 30.1.

Overview of types of grafts used in intestinal and multivisceral transplantation (Reprinted from Hopfner et al. [152]. With permission from SAGE Publications, Inc.)
Postsurgical Care and Surgical Complications
Upon reperfusion the transplanted intestine undergoes significant swelling. The patient requires aggressive fluid resuscitation and the edema may prevent closure of the abdomen. Postoperatively, central venous pressure (CVP) monitoring and close attention to perfusion, abdominal fluid losses, and urine output is crucial. In small children, the preoperative blood pressure may be the best guide for postoperative target blood pressure to prevent excessive fluid overload. Hypotension not responsive to fluid administration is relatively rare, and there is no data to support the use of a specific vasoconstrictor for hypotension at this time [2]. In fact, hypertension is more frequent than hypotension in these patients related to corticosteroids, IV calcineurin inhibitors, and hypervolemia. Hypertension is typically managed by short-acting calcium channel blockers [2]. Frequent assessment of serum lactic acid concentrations can also guide fluid therapy. Oncotic pressure is often low as evidenced by hypoalbuminemia, and albumin supplementation may be beneficial. The large aortic anastomosis reduces the risk of hepatic artery thrombosis seen in isolated liver transplantation. Still, there are concerns about intestinal perfusion. Therefore, the hemoglobin is maintained at around 9–10 mg/dl and mild abnormalities of coagulation are not corrected [2]. Platelet counts are typically as low as 40,000 without evidence of bleeding and are not corrected. The color of the stoma must be frequently observed. When combined with a small abdominal compartment, abdominal distension may compromise diaphragmatic excursion and functional residual capacity. If there is any question of intra-abdominal hypertension, bladder pressure should be measured [2].
Typically capillary leak begins to subside 24 h postoperatively. At this time IV fluids can be gradually decreased. Postoperative ileus generally persists for several days, so continuous gastric suctioning is necessary to reduce intra-luminal pressure. Most centers begin feeding around the fifth postoperative day upon detection of bowel sounds and stoma output but a few wait until 10–14 days postoperatively [13]. Short peptide-based isotonic formulas are infused at small but increasing volumes, while optimal intake is supported by parenteral nutrition [13]. Due to lymphatic disruption, absorption of fat as chylomicrons is impaired until collateral channels reform at around 180 days postoperatively [13]. Some centers supplement with intralipids or medium chain triglycerides and fat soluble vitamins [13]. It is not unusual to have high ostomy output. This can be controlled with anti-peristaltic agents such as loperamide and diphenoxylate in high doses.
There are few reports of the surgical complications after transplantation. Intestinal perforation/anastomotic leak appears to be the most common complication, followed by sub-acute bowel obstruction, often related to ostomy prolapse [14]. Intestinal perforation can be difficult to detect as the use of steroids diminishes an inflammatory response and the typical signs and symptoms of rebound tenderness and fever may be lacking [2]. The incidence of abdominal compartment syndrome has been reduced by staged closure of the abdomen [15]. Other complications include portocaval thrombosis, biliary leak, wound dehiscence, intra-abdominal bleeding, failed abdominal closure, and fistula formation [2, 14]. Rarely, mycotic aneurysm at the aortic anastomosis can occur due to a relatively high level of contamination of the surgical field. Aneurysm rupture leads to a sudden catastrophic intra-abdominal bleeding. Frequent monitoring and use of imaging studies such as ultrasound and CT can assist the clinician in identifying fluid collections and thrombotic complications [2].
Complications Related to Immunosuppression
Immunosuppression Protocols
The first attempts at intestinal transplantation over two decades ago often failed due to refractory acute rejection and its common sequelae, bacterial infection [15]. With the introduction of tacrolimus-based immunosuppression in 1990, the short-term survival of intestinal transplant recipients has substantially improved, but long-term patient survival remains challenging [1, 3]. Intestinal and multivisceral transplants differ from other solid organ transplants because of the complex interaction between the intestinal allograft and the native immune system, including the gut-associated lymphoid tissue (GALT) of the bowel mucosa [16]. There is evidence that enterocytes have the capacity to function as antigen presenting cells resulting in stimulation of donor lymphocytes [17]. As a consequence, intestinal and multivisceral transplant recipients need greater immunosuppression than other solid organ recipients and experience higher rates of acute and chronic rejection [1].
The implementation of efficient induction therapy reduces the risk of rejection [8, 9, 11, 16, 18–21]. Induction therapy varies by center but typically consists of anti-lymphocyte preparations such as rabbit anti-thymocyte globulin (rATG, Thymoglobulin®, Genzyme, Cambridge, MA), alemtuzumab (Campath-1H®, Berlex Laboratories, Montville, NJ) or daclizumab (Zenapax®, Roche Pharmaceuticals, Nutley, NJ) [1, 3, 8, 9, 11]. However, the use of Campath-1H in pediatric transplant recipients was associated with significantly higher morbidity and mortality due to infection in children younger than 4 years [21]. Maintenance immunosuppression consists of the calcineurin inhibitor tacrolimus (Prograf®, Fujisawa Pharmaceuticals, Deerfield, IL) and steroids. The initial tacrolimus target whole blood trough concentration measured at 12 h also varies by center but is generally over 10 ng/mL for the first 3 months. Subsequently, concentrations are kept slightly lower, 8–12 ng/mL [1–3, 8, 9]. High dose steroids are given intraoperatively (up to 50 mg/kg) and tapered over the first 4 days. Maintenance steroid management also varies by center, some reduce the steroid dose 2–4 weeks after transplant, and others reduce the steroid dose 6–9 months after transplantation [1]. Whether specific induction therapies will permit reductions in long term immunosuppression and improve long term survival in intestinal and multivisceral transplants recipients is still being evaluated.
Acute Cellular Rejection
Acute cellular rejection (ACR) occurs in approximately 40 % of children after intestinal transplantation, generally within the first 3 months post-transplant [1, 21, 22]. In multivisceral transplantation, the most common site of ACR is the intestine, while other organs appear to be spared [8]. Although non-compliance is a risk factor for transplant rejection, documentation of non-compliance is difficult in intestinal transplantation. Tacrolimus is metabolized by enterocytes and impaired metabolism during rejection results in elevated tacrolimus concentrations at the time of presentation with rejection [23, 24]. Acute cellular rejection is characterized by fever, diarrhea, abdominal pain and/or distension, and is often associated with positive blood cultures due to bacterial translocation of enteric organisms [25]. For this reason, some authors recommend initiation of prophylactic anti-bacterial and anti-viral therapy during any episode of intestinal allograft rejection [26]. The systemic inflammatory response that accompanies rejection can mimic sepsis with fever, tachycardia, tachypnea, hypoxemia, metabolic acidosis and hypotension, and requires careful monitoring and aggressive resuscitation.
Early detection of rejection is crucial as ACR remains the greatest risk factor for permanent graft failure; especially, if there is a severe rejection episode or if rejection is not resolved within 21 days [8, 21]. Surveillance endoscopic biopsies and zoom video endoscopy are currently performed biweekly during the first month, weekly during the next 2 months, then monthly afterward. Intestinal rejection can be localized: therefore, multiple biopsies must be obtained from several locations. Additional endoscopies are required when fever, diarrhea, or bacteremia occur. During suspected or proven episodes of rejection, endoscopies are performed as often as every other day until symptoms and the histopathological features resolve [22].
The endoscopic findings of ACR include blunted villi, edematous and friable mucosa, ulcers, and mucosal exfoliation [27]. Histopathologically, acute allograft rejection is graded mild (grade 1), moderate (grade 2) or severe (grade 3) based on a combination of crypt injury, mucosal infiltration primarily by mononuclear cells, and increased crypt cell apoptosis (Fig. 30.2) [27]. Exfoliative severe rejection is defined by denudation of the intestinal epithelium and is associated with high rates of mortality and graft loss [28].
Fig. 30.2.
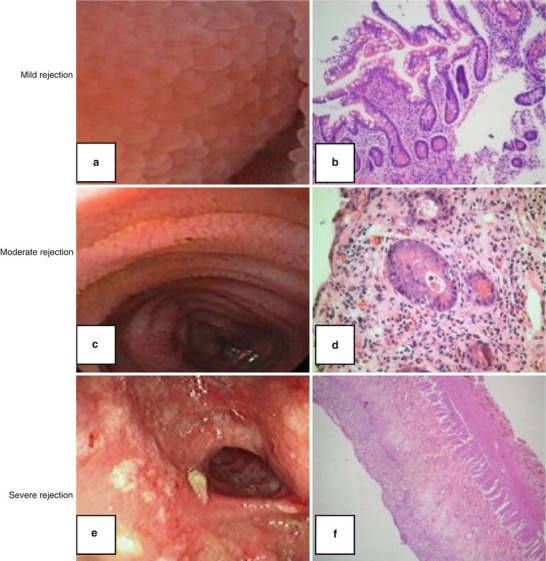
Endoscopic findings and histopathology of acute cellular rejection (ACR): Mild ACR (grade 1): Mild mixed inflammatory infiltrate in lamina propria. Few apoptotic bodies in crypts (a, b). Moderate ACR (grade 2): Increase of mixed inflammatory infiltrate in lamina propria with frequent apoptotic bodies in crypts and confluent apoptosis (c, d). Severe ACR: (grade 3). Marked degree of crypt damage and architectural distortion with loss of crypts. Marked diffuse mixed inflammatory infiltrate with epithelial cell apoptosis in the surviving crypts (e, f) (Reprinted from Hopfner et al. [152]. With permission from SAGE Publications, Inc.)
Acute cellular rejection is treated by modulation of immunosuppression (Table 30.1). Mild acute rejection is treated with pulsed steroids (methylprednisolone 15–30 mg/kg), followed by a weaning cycle along with an increase in targeted tacrolimus concentration to early post-transplant values. Steroid-resistant episodes as well as moderate and severe rejection are generally treated with Muromonab-CD3 (Orthoclone OKT3®) or Thymoglobulin [1, 21]. Treatment can vary from a single dose to 14 days of therapy depending on severity and response [1]. In a recent adult series, four cases of OKT3 resistant and three cases of steroid resistant ACR responded to infliximab, a monoclonal antibody which blocks TNF α (alpha) [29].
Table 30.1.
Overview of treatment of acute cellular rejection dependent on severity
| Severity of rejection | Treatment | Rescue treatment |
|---|---|---|
| Mild rejection | Steroid bolus and cycle | Consider Thymoglobulin |
| Increase in baseline immunosuppression | ||
| Moderate rejection | Muromonab-CD3 (Orthoclone OKT3®) or Thymoglobulin (1–14 days) | |
| Increase in baseline immunosuppression | ||
| Severe rejection | Muromonab-CD3 (Orthoclone OKT3®) or Thymoglobulin (14 days) | Consider removal of graft for treatment failures |
| Increase in baseline immunosuppression |
Since histopathologic changes are a delayed manifestation of rejection and may occur in isolated areas, potential biomarkers for accurate early detection of rejection have been investigated. Plasma citrulline, synthesized exclusively by enterocytes of the small intestine, has been used as a biomarker of intestinal mass and function in patients with intestinal failure [30, 31]. Several recent studies have shown a strong correlation between lower plasma citrulline concentrations and grade of rejection [32–34]. Using a citrulline concentration cutoff point of 13 μmol/L for moderate or severe ACR, the sensitivity was 96 %, but specificity was only 69 %. A plasma citrulline concentration of more than 13 μmol/L excluded moderate and severe ACR with greater than 99 % certainty [33]. Because normal plasma concentrations of citrulline take up to 3 months to achieve after intestinal transplant, this assay is not useful in the first weeks post-transplant [34]. Moreover, plasma citrulline concentrations decrease only after epithelial destruction, which makes it less useful as an early marker of rejection.
Fecal calprotectin, produced by neutrophils and macrophages, is elevated in conditions with mucosal leukocyte infiltrate such as inflammatory bowel disease, and has been evaluated as a screening tool to indicate a need for endoscopy, but does not seem to be able to differentiate between ACR and other intestinal pathologies [35–37]. In addition to biomarkers of rejection, direct measures of immune system activity such as complement-dependent cytotoxicity panel- reactive antibody testing (CDC-PRA), peripheral lymphocyte subsets and the ImmuKnow® (Cylex Inc., Columbia, Maryland, USA) immune cell function assay are under investigation as early indicators of rejection risk or excessive immunosuppression [38, 39].
Antibody Mediated Rejection
Circulating donor specific antibodies have long been known to be associated with hyperacute rejection where organ dysfunction develops in minutes to hours after transplant due to pre-existing antibodies to donor tissue that bind to endothelium and activate complement [40]. Plasmapheresis combined with intensified immunosuppression has shown some success in single organ transplant recipients. High dose immunoglobulin (IVIG) has also been associated with decreased antibody titers and successful reversal of humoral rejection [40]. While plasmapheresis and IVIG reduce circulating antibodies, Rituximab, a monoclonal antibody against CD20 present on B-lymphocytes, has been used to stop antibody production [41, 42]. Acute humoral rejection is not specifically reported in intestinal transplant but may play a role in a few patients with early decompensation.
Antibody–mediated rejection is increasingly considered to play a role in delayed graft rejection, ACR not responsive to immunosuppression, and in chronic graft rejection. Approximately 20 % solid organ transplant recipients develop donor specific antibodies to HLA class I or II donor antigens, non-HLA antibodies to blood group antigens, and endothelial antigens [40]. Circulating antibodies are not always associated with organ dysfunction, but patients with HLA antibodies have significantly higher rates of graft failure [40]. Kato and colleagues showed HLA antibodies developed in 5 of 28 patients post intestinal/multivisceral transplantation [43]. Three of the five patients had strongly positive plasma reactive antibody (PRA) titer; all three had temporally associated significant episodes of acute rejection; whereas only 1 of the 25 patients with a weak or negative PRA titer had a rejection episode around the time of sampling [43]. Other investigators reported correlation between humoral sensitization and vascular changes in the intestinal mucosa [44].
Chronic rejection is one of the most common causes of intestinal graft loss in the second post-transplant year [8]. In a recent retrospective study, chronic rejection was diagnosed in approximately 20 % of isolated intestinal transplants and 5 % of multivisceral transplants [45]. The reason for the lower incidence of rejection in the latter group is unclear. Some investigators hypothesize that it is due to the protective effect of the liver or the preceding evisceration and thus decreased recipient lymphocyte population. Patients typically present with abdominal pain, chronic diarrhea, and weight loss. Major histological features of chronic rejection are nonspecific and include progressive obliterative arteriopathy, apoptosis, mural fibrosis and perivascular inflammation. In multivisceral transplants, the pancreas might be the organ most susceptible to chronic rejection [45]. Currently, re-transplantation is the only definitive treatment for chronic rejection with graft failure [46], but if chronic rejection is an antibody mediated process there are potential new therapeutic approaches [40]. Bortezomib, a proteasome inhibitor used in the treatment of multiple myeloma, causes apoptosis in plasma cells, thereby decreasing alloantibody production. It has been found to be useful in treating antibody mediated rejection in kidney transplant recipients and may become a promising agent in the treatment of rejection after intestinal and multivisceral transplantation [47]. A recent case report showed successful reversal of refractory rejection with bortezomib in a patient following multivisceral transplantation [48]. Similarly, blockade of terminal complement activation by eculizumab, a humanized monoclonal antibody with high affinity for C5, appeared effective in a case report of ACR in a kidney transplant recipient [49].
Graft-Versus-Host Disease
Graft-versus-Host Disease (GvHD), defined as a reaction of donor immunocompetent cells against host tissue, occurs in 4–10 % of intestinal transplant recipients [8, 21, 50]. In classic GvHD seen after hematopoietic stem cell transplantation, inflammatory cytokines and donor derived lymphocytes damage skin, native liver and native intestine. In intestinal/multivisceral organ transplantation, however, the skin is often the only involved organ, although pulmonary GvHD is reported [50]. The diagnosis is based on clinical symptoms, detection of mixed chimerism with circulating donor lymphocytes, and histopathology. In a retrospective analysis of 46 children undergoing intestinal/multivisceral transplantation, five patients developed GvHD at a median time of 47 days after transplantation [50]. All of the patients had cutaneous symptoms with a maculopapular generalized rash, and two patients had native gastrointestinal involvement in the form of diarrhea. In addition, three patients developed respiratory symptoms and four patients had severe hematologic abnormalities (pancytopenia, T-cell lymphoma, myeloid dysplasia, autoimmune hemolytic anemia). Three of the five patients with GvHD died a few months after diagnosis. In another study of 123 children, six developed confirmed GvHD, (0.5 ± 0.1 cases per 100 patient months of follow-up), and four of these six died [8]. The risk of GVHD appears to be greater in multivisceral transplantation than in isolated intestinal transplantation [8].
Although there is no proven standard therapy for GvHD, the initial approach is reduction of the maintenance immunosuppression and high dose steroids. The prognosis worsens dramatically for steroid-nonresponders [51, 52]. Salvage regimens include sirolimus, mycophenolate-mofetil and pentostatin, a nucleoside analog that irreversibly inhibits adenosine deaminase resulting in severe immunosuppression [53]. In addition, various monoclonal antibodies and anti-cytokine therapies such as infliximab/etanercept (anti-TNF α (alpha)) and daclizumab (anti-IL2 receptor) have been evaluated and show success in the treatment of GvHD in patients after bone marrow transplant [53–55]. In steroid resistant multivisceral recipients with a transplanted spleen, splenectomy is a possible last resort treatment option [8]. Extracorporeal photochemotherapy (ECP) is a promising therapeutic procedure that is currently under examination in a prospective multicenter phase 3 trial [56, 57]. Extracorporeal photochemotherapy (ECP) kills T-cells by first exposing them to the chemical methoxsalen and then ultraviolet A light [57].
Other Postoperative Complications
Infectious Complications
The main determinants of infectious complications in transplant recipients are the degree of immunosuppression and exposure to pathogens through the transplanted organ or the environment [58, 59]. A similar pattern of post-transplant infections has been described when institutions use similar tacrolimus or cyclosporine based immunosuppressive regimens following solid organ transplant; however, in the era of induction therapy for pediatric intestinal/multivisceral transplantation viral infections seem to appear earlier than previously reported in other transplant populations [38, 58, 59]. These viruses have a predilection for the graft and therefore may be activated latent infections from donor derived tissues [60]. Cytomegalovirus infection occurs in 30 % of recipients, while adenovirus is reported in 20 % of recipients [61–63]. The use of antiviral prophylactic strategies has made herpes virus infections uncommon, but the use of reverse transcriptase polymerase chain reaction (RT-PCR) assays for the detection and monitoring of viral DNA has identified other pathogens, including polyoma viruses such as BK virus and paramyxoviruses such as parainfluenza and metapneumovirus [38, 64]. Recurrent and chronic viral infections may eventually lead to graft dysfunction [45]. Opportunistic infections such as Pneumocystis jiroveci pneumonia and Toxoplasma spp can be prevented with trimethoprim-sulfamethoxazole (TMP/SMX).
Respiratory Failure
Intestinal/multivisceral transplant recipients observed over a 10 year period at the University of Miami were four times more likely than liver transplant recipients (24 % versus 6 %) to be re-admitted to the ICU with respiratory failure after transplantation [65]. The overall survival rate for intestinal/multivisceral transplant recipients who developed respiratory failure after transplant was 75 %, higher than the 58 % observed in a liver transplant population and higher than survival rates generally reported following bone marrow transplantation [8, 66–68]. This difference may be explained in part by the high prevalence of pulmonary edema from fluid overload and low oncotic pressure complicating acute tacrolimus toxicity in intestinal/multivisceral transplant recipients experiencing rejection. Less common non-infectious causes include transfusion related lung injury, GvHD, diffuse alveolar hemorrhage, and sirolimus toxicity [66–68]. Sirolimus-induced pneumonitis is reported in at least 2 % of liver transplant recipients and should be suspected in patients who develop respiratory symptoms while receiving sirolimus [68]. When intestinal and/or multi-visceral transplant are performed in children with a diminished abdominal cavity, abdominal distension can limit diaphragmatic excursion and compromise respiratory function.
Infectious pulmonary complications in intestinal/multivisceral transplant recipients include bacterial pneumonia, viral pneumonitis and acute respiratory distress syndrome (ARDS) related to sepsis. In one single center, pneumonia related respiratory failure occurred in 18 % of patients and was associated with younger age and higher mortality [8]. Due to a lack of published data on respiratory failure in intestinal transplant recipients, extrapolation of management principles from other immunocompromised populations is required. Optimal outcome depends on identifying the cause of the lung infiltrate through analysis of tracheal fluid, bronchoalveolar lavage fluid or tissue obtained from open lung biopsy and instituting specific therapy at the earliest possible time [67–69]. In a prospective cohort of 200 immunocompromised patients with infiltrates, infectious agents were recovered in over three fourths of all subjects [67]. While some viral infections such as influenza and RSV can be detected via immunoflourescence assay or culture from nasopharyngeal washing samples, the detection of common respiratory viral pathogens has been improved by use of RT-PCR applied to nasopharyngeal and endotracheal samples [64, 70, 71]. Through this technology, community acquired viruses such as influenza virus, parainfluenza virus, adenovirus and rhinovirus have been increasingly recognized as major causes of serious lower respiratory illness in transplanted children (Table 30.2). Due to the diminished inflammatory response in immunocompromised patients typical radiologic appearance for any infectious agent may be absent [72]. In half of adults with neutropenia and in adult kidney transplant recipients computed tomography images revealed lesions not visualized on plain radiographs [71–74]. Studies have shown that in 70–86 % of mechanical ventilated patients, information obtained from open lung biopsy resulted in significant changes in management [75–77]. Because viral killing requires T-cell activation and therefore leads to severe pneumonia, immunosuppression needs to be profoundly reduced and even withheld for several days to allow immunologic recovery while increasing monitoring of the graft [2].
Table 30.2.
Common viral pathogens isolated in transplanted children with lower respiratory tract infection
| Rhinovirus |
| Influenza |
| Parainfluenza |
| Respiratory syncytial virus |
| Adenovirus |
| Metapneumovirus |
Respiratory failure in intestinal/multivisceral transplant recipients should be managed as it is in other critically ill populations with a few caveats. The use of non-invasive ventilation should be limited to a select population. Disrupted innervations of the transplanted stomach and/or proximal intestine impair gastrointestinal motility and increase the risk of aspiration. In addition, a small abdominal compartment may be compromised by intra-abdominal hypertension with even a small increase in intestinal air. Once intubated, sedation can be difficult in this population and higher doses of sedatives are required either because of upregulated drug clearance enzymes or a high liver mass to body weight ratio. Typically, when plateau pressures are greater than 30–35 cmH2O we have implemented high frequency oscillatory ventilation. This requires pharmacologic paralysis in a population with many risk factors for prolonged paralysis and critical illness polyneuropathy so drug holidays and or daily monitoring of train of four are recommended.
Sepsis and Septic Shock
Bacterial sepsis is a major cause of mortality in organ recipients and early-onset bacteremia is associated with greater mortality compared to late-onset bacteremia after solid organ transplant [78]. Blood stream infection occurs more frequently in recipients of intestinal transplantation than in other types of solid organ transplantation [26]. This is mainly due to the presence of bacterial flora along the lumen of the allograft, enterocyte injury during rejection, the higher amount of immunosuppressive therapy, and the development of lymphoproliferative disorder [26, 79, 80]. Enteric bacteremia is an indication for endoscopic evaluation and biopsy of the graft to rule out rejection. As shown in multiple studies, early initiation of antibacterial therapy has had a direct effect on reducing mortality in sepsis and a transplant recipient should receive empiric antibiotics whenever febrile [81–83]. This includes coverage for enteric gram negative and gram positive organisms such as Enterococcus spp. Antifungal coverage may also be appropriate. Early goal-directed therapy requires intravenous access for fluid resuscitation and vasoactive therapy, ongoing patient assessment, and normalization of the physiologic parameters mean arterial pressure and mixed venous oxygen saturation; this approach results in a decrease in both in-hospital and overall mortality [84, 85]. A state of absolute or relative adrenal insufficiency has been well described in sepsis [85]. Transplant recipients are at greater risk due to long-term steroid use. Therefore, stress-doses of hydrocortisone with or without fludrocortisone should be administered. In the presence of active bacterial infections and no evidence of graft rejection, a reduction in immunosuppression is appropriate for transplant recipients but by how much and for how long is uncertain.
Acute Kidney Injury
Recipients of intestinal/multivisceral transplants have multiple risk factors for development of acute or chronic kidney failure, both pre- and post-transplant. Predictors of renal dysfunction include low pretransplant glomerular filtration rate (GFR), critical illness prior to transplant, and high-dose tacrolimus therapy [86]. At the time of transplant many recipients have already has a decline in GFR, on average to 83 % of the norm [86]. Although this renal dysfunction appears relatively mild, it predisposes to further decline of the kidney function after transplantation due to lower renal reserve. Post-transplant, recipients receive prolonged therapy with the nephrotoxic calcineurin inhibitors, tacrolimus or cyclosporine [87–89]. A higher incidence of infections mandates exposure to nephrotoxic antimicrobials. Other common complications post-transplantation also contribute to an increased risk of renal dysfunction, e.g. hypoperfusion, dehydration, chronic hypertension, diabetes mellitus, infection with BK virus and dyslipidemia.
The overall incidence of acute kidney injury (AKI) after adult solid organ transplantation is approximately 25 % with 8 % requiring renal replacement therapy [90]. In the intestinal/multivisceral transplant patients, acute tacrolimus toxicity is a common cause of AKI. This is in part due to an erratic intestinal absorption of tacrolimus and its metabolism by enterocytes. In the face of low renal reserve, whole blood tacrolimus concentrations over 20 ng/mL are associated with elevated serum creatinine, blood urea nitrogen (BUN), and oliguria [90]. Various vasoregulatory molecules, including endothelin and adenosine, are involved in calcineurin inhibitor related renal artery vasoconstriction [90]. Hence, acute tacrolimus toxicity frequently does not respond to diuretic therapy. Fenoldapam, a selective dopamine-1 receptor agonist that decreases vascular resistance while increasing renal blood flow, preserves renal function by counter-balancing the renal vasoconstrictive effects of cyclosporine in kidney and liver transplant patients [91, 92]. Aminophylline, a nonselective adenosine receptor antagonist, enhanced the response to loop diuretic in pediatric abdominal organ transplant recipients [93].
The indications for renal replacement therapy in multiple organ transplantation are the same as in other patient populations [94]. Peritoneal dialysis is not an option in the child after extensive abdominal surgery with removal of the peritoneum. Continuous venovenous hemodiafiltration is preferred to intermittent hemodialysis in many centers because of the latter’s potential for large fluid shifts leading to hypotension and reduced organ perfusion [94]. Limited vascular access is a common finding in patients with intestinal failure, and functional vascular access is the most important technical component determining successful provision of continuous renal replacement therapy. In children less than 10 kg, circuit survival is significantly lower with smaller catheter size [95]. Furthermore, extracorporeal circuit volumes comprising more than 10–15 % of patient blood volume must be primed with whole blood to avoid hypotension and anemia, yet the bradykinin release syndrome (BRS) with acute hypotension is often associated with blood priming of the circuit [96]. Because multivisceral transplant patients are often poor candidates for hemodialysis, AKI must be prevented by close monitoring of tacrolimus concentrations and renal function, and avoidance of nephrotoxic drugs and hypoperfusion.
Chronic Kidney Disease
The incidence of chronic kidney disease (CKD) in adult intestinal and multivisceral transplant recipients 5 years after transplantation is 21.3 % and therefore higher than in other solid organ transplant populations [86]. Long-term use of calcineurin inhibitors is a major risk factor for the development of kidney dysfunction and the cumulative tacrolimus concentration has been described as predictor of decreased GFR, both in children and adults [8, 87, 97]. In a study of 44 pediatric intestinal transplant patients, without acute tacrolimus toxicity, the mean GFR decreased significantly from 138 mL/min/1.73 m [2] pre-transplantation to 102 mL/min/1.73 m [2] 18–24 months post-transplantation [97]. In rapid and persistent deterioration of the kidney function, sirolimus, a macrolide with potent immunosuppressive properties, can be used as maintenance therapy instead of tacrolimus. However, in intestinal organ transplants experience with sirolimus is limited. Several intestinal/multivisceral transplant recipients have undergone subsequent kidney transplants [1].
Hematologic Complications
After organ transplantation derangement of all hematopoietic cell lines has been described, resulting in anemia, leukocytopenia and thrombocytopenia. Generally cytopenia arises from decreased production or increased destruction of cell lines due to infection, drug toxicity, and formation of auto-antibodies. Hematologic complications are best described after kidney, heart and liver transplantation. Several drugs used for immunomodulation, antiviral and antibacterial treatment or as ancillary therapy can cause hematologic side effects; in intestinal multivisceral transplantation, most importantly tacrolimus, mycophenolate mofetil, sirolimus, ganciclovir and TMP/SMX [98–100].
Post-transplant anemia is frequent after pediatric solid organ transplantation, affecting 85, 68 and 82 % of patients at 1 month, 12 months and 5 years, respectively [101]. The most common reason for anemia in transplant patients is iron deficiency secondary to poor nutritional iron supplementation and depletion of iron stores. In addition, in pediatric patients frequent phlebotomies after major surgery can play a significant role in the development of anemia [102]. Pure red cell aplasia (PRCA) or selective suppression of red cells is much less frequent than pancytopenia [101]. In vitro studies have shown no direct suppression of the bone marrow by tacrolimus, but reversible pure red cell aplasia has been reported [101, 103].
Neutropenia occurs in more than 10 % of transplanted patients related to drugs and infections [104]. Ganciclovir and valganciclovir, routinely used in intestinal and multivisceral transplant recipients for prophylaxis and treatment of CMV infection, are associated with neutropenia, as is TMP/SMX, used for prophylaxis of Pneumocystis jiroveci [105]. Bone marrow suppression associated with medication is usually reversible with dose reduction or discontinuation [106, 107]. Alternative options for prophylaxis in case of TMP/SMX toxicity are pentamidine and dapsone. In addition, treatment with hematopoietic growth factors (G-CSF, GM-CSF) can shorten the duration of drug-induced severe neutropenia and agranulocytosis with a reduction in the number of infections and fatal complications [105]. Table 30.3 shows a summary of drugs commonly used after transplantation associated with severe neutropenia and agranulocytosis.
Table 30.3.
Drugs commonly used in transplant recipients incriminated in the occurrence of severe neutropenia and agranulocytosis
| Drug category | Frequently used drugs in transplant recipients incriminated in neutropenia |
|---|---|
| Antimicrobials | Aciclovir, cephalosporins, ciprofloxacin, clindamycin, dapsone, fluconazole, ganciclovir, gentamicin, imipenem, linezolid, macrolides, metronidazole, penicillins, rifampicin, trimethoprim–sulfamethoxazole, valganciclovir, vancomycin |
| Anticonvulsants | Phenytoin, valproic acid |
| Cardiovascular drugs and diuretics | Acetazolamide, coumarins, hydralazine, metolazone, nifedipine, propranolol, spironolactone, thiazides, ticlopidine |
| Gastrointestinal drugs | Cimetidine, famotidine, metoclopramide, omeprazole, ranitidine |
| Immunomodulators | Alemtuzumab, infliximab, glucocorticoids, mycophenolate-mofetil, OKT3, rituximab, sirolimus, tacrolimus, thymoglobulin |
In transplant recipients, viral infections may lead to bone marrow suppression. Active CMV infection should be ruled out prior to stopping ganciclovir or valganciclovir in response to marrow suppression as CMV disease often presents with neutropenia and/or thrombocytopenia. Parvovirus B19, a small single-stranded DNA-virus, infects erythroid progenitor cells by binding to their cellular receptor, the P-antigen [108]. Severe and persistent aplastic anemia due to B19 infection has been reported in adult and pediatric organ transplant recipients [109]. The prevalence of symptomatic B19 infection in studies of adult solid organ recipients ranged between 1 and 2 % [110]. The prevalence in children after solid organ transplantation is unknown. The median time of presentation with B19 induced anemia after pediatric solid organ transplant is 8 months [110]. Associated thrombocytopenia and leukocytopenia are reported but infrequent. Detection of parvovirus B19 DNA in serum or bone marrow by PCR is the diagnostic gold standard, as serology is confounded by the impaired humoral response to infections. Although there is currently no specific treatment for B19 infection, IVIG has been successfully used in cases of severe anemia in immunocompromised patients [111–113]. Reduction of immunosuppression should be considered in therapy resistant cases [114].
In transplant recipients, presence of donor lymphocytes and a deregulated immune system can lead to antibody-mediated destruction of hematologic cell lines. Alloantibody-mediated hemolytic anemia usually occurs in the first weeks after solid organ transplantation [115]. This is caused by donor lymphocytes, in what is called the passenger lymphocyte syndrome whereby the concurrently transplanted B-cells produce antibodies that react against recipient red blood cell antigens [116]. Donor antibody-induced hemolysis in ABO-mismatched solid organ transplantation is usually mild and self-limiting, [117] whereas alloimmune hemolytic anemia due to antibodies with anti-D (Rhesus) specificity can be severe needing multiple transfusions [116]. Passenger lymphocyte syndrome can also affect the platelet count. Severe and resistant alloimmune thrombocytopenia with alloantibodies from passenger donor lymphocytes directed against the HPA1a-platelet antigen was demonstrated in three patients receiving kidney or liver transplantation [118]. Lacaille and coworkers described six children who developed severe antibody-mediated anemia or thrombocytopenia while treated with tacrolimus after liver or intestinal transplantation [119]. All patients had positive anti-platelet antibodies or Coombs’ positive anemia. The authors suspected interference of tacrolimus with T-cell function or with endogenous control mechanisms of T-cell activation and down-regulation as the underlying mechanism. Therapy was successful with steroids or anti-CD20 monoclonal antibody [119]. In a more recent case series, four children who had undergone combined liver and bowel transplantation developed autoimmune-hemolytic anemia. Three of the four children also developed thrombi in major vessels suggesting that anticoagulation should be used when this condition presents [120].
Thrombotic microangiopathy (TMA) is a rare but potentially lethal complication encountered in solid organ transplantation. This condition encompasses the clinical diagnoses of thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS) and is associated with tacrolimus and cyclosporine in transplant recipients [121–123]. In severe cases, microvessel occlusion leads to tissue ischemia and organ dysfunction, which may manifest in renal failure, pancreatitis, intestinal ischemia and stroke. Besides discontinuation of the drug, plasmapheresis appears to be the most effective treatment of TMA [122, 123].
Epstein-Barr Virus and Posttransplant Lymphoproliferative Disorders
Epstein-Barr virus (EBV) is a herpes virus that can infect several cell lines including lymphocytes and has been associated with different malignancies, including posttransplant lymphoproliferative disorders (PTLD), a group of heterogeneous lymphoproliferative diseases arising in the pharmacologically immunosuppressed population after organ transplant [124, 125]. Seronegative EBV status at time of transplant is a risk factor for PTLD, hence children are at increased risk compared to adults [126]. Generally, the risk of PTLD corresponds with the duration and intensity of the immunosuppressive therapy [126]. Specifically, the use of anti-T-cell antibodies for induction and rejection exposes intestinal recipients to a higher risk of PTLD compared to other solid organ graft recipients. The incidence is reportedly 5–10 % for intestinal recipients and appears to be higher for multivisceral recipients [21, 38, 127].
Quantitative PCR improves detection and serial monitoring of EBV viral load in peripheral blood [120, 127–129]. Since the rise of EBV viral loads often precedes the development of PTLD, have EBV DNA PCR is routinely monitored [128]. Post-transplant lymphoproliferative disorders can occur at any time, with the highest incidence occurring in the first year after transplantation [38, 124]. A high index of suspicion is needed since the clinical presentation is often nonspecific including symptoms of fever, fatigue and weight loss. Total body imaging studies with computed tomography (CT), magnetic resonance imaging (MRI) or positron emission tomography (PET) scan are necessary for staging of PTLD [130]. Biopsy of the accessible nodal tissues is essential, since histopathologic examination remains the gold standard for diagnosing PTLD [130]. Gastrointestinal tissue sampling may be useful in the absence of a biopsy from lymph nodes, but a negative result does not rule out the disease.
The immediate treatment of PTLD is a reduction in immunosuppression whenever feasible [124, 131]. Even temporary discontinuation of all immunosuppression with close monitoring for rejection does not lead to an increased loss of graft function in liver transplant recipients; [131] however, this aggressive approach is avoided with bowel and multivisceral transplant due to the risk of rejection. Localized disease is often successfully treated with surgery or radiotherapy along with reduction in immunotherapy [132, 133]. Subsequent treatment plans require expertise and collaborative efforts of the transplant, infectious diseases and oncology team [125]. For patients with CD20+ PTLD and who do not respond to immunotherapy reduction, rituximab provides an effective treatment [133–135]. A chemotherapy regimen of cyclosphosphamide and prednisone has been effective for refractory PTLD in children [136]. Survival after PTLD varies across centers but it is worse in disseminated disease and cases that are not controlled with reduction of immunotherapy [137, 138].
Neurological Complications
Pediatric intestinal and multivisceral transplant recipients often have significant delay in cognitive and motor function due to prematurity, malnutrition, hospitalization and severe illness in early childhood. Intraoperatively and post-transplant, they are exposed to multiple drugs with neurotoxic potential, e.g. anesthetics, tacrolimus, and corticosteroids. As a consequence, many pediatric patients display developmental delay in the first 6 months post transplant [139, 140].
After solid organ transplantation, central nervous system (CNS) complications are primarily attributable to the immunosuppressive therapy [141–145]. Cyclosporine and tacrolimus can cause a wide variety of symptoms including encephalopathy and seizures [141–145]. The diagnosis of immunosuppressant-related neurotoxicity is based on exclusion of other causes in addition to a possibly elevated serum drug concentration, but neurotoxicity can occur even without elevated serum concentrations. Treatment consists of lowering the dose or using an alternative immunosuppression. Posterior reversible encephalopathy syndrome (PRES) is associated with calcineurin inhibitors and occurs in 0.5 % of all solid organ transplants. Characterized by headache, confusion, seizure and visual loss with a specific pattern in the MRI of the brain (Fig. 30.3), PRES is frequently associated with hypertension [145]. Unrecognized, PRES may become irreversible with permanent sequelae [145]. The treatment again involves lowering the dose of the drug and treating the frequently associated hypertension. Only one adult study has examined the incidence and spectrum of neurologic complications after intestinal and multivisceral transplantation [146]. This retrospective analysis showed a high incidence of headaches (68 %), encephalopathy (43 %), and seizures (17 %) [146]. Less common were CNS infections, neuromuscular disorders, and ischemic stroke [146]. Use of ganciclovir for EBV and CMV prophylaxis appears to reduce the incidence of previously reported CNS infections. Table 30.4 provides a summary of the pathogens specifically involved in CNS infection in transplant recipients [147–151]. Neurologic complications in pediatric intestinal transplant have not been comprehensively reviewed but given the prolonged use of high dose immunosuppression are likely more common and complex than in other solid organ transplant populations.
Fig. 30.3.
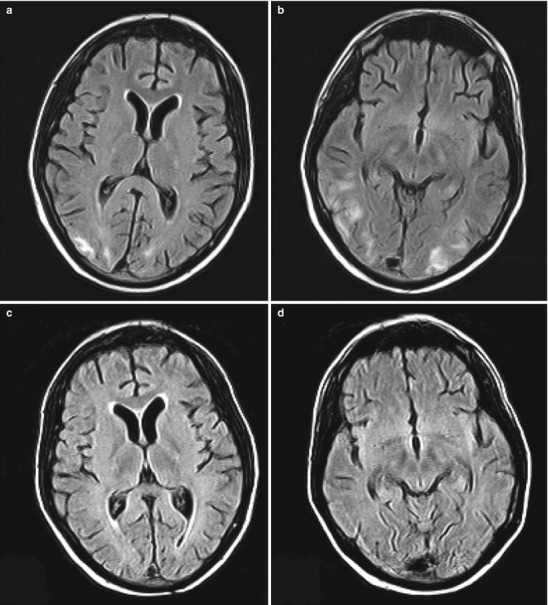
17-year-old patient with PRES: FLAIR sequence of MRI with characteristic subcortical white matter areas of increased signal intensity in the bilateral occipital lobes (a, b). In the follow-up 16 days later the signal abnormalities are no longer visualized (c, d) (Reprinted from Hopfner et al. [152]. With permission from SAGE Publications, Inc.)
Table 30.4.
Pathogens associated with CNS infections specifically in solid organ transplant recipients
| Bacterial pathogens | Fungal pathogens | Viral pathogens |
|---|---|---|
| Nocardia | Aspergillus species | Human herpes virus 6 |
| Listeria monocytogenes | Candida species | Cytomegalovirus |
| Mycobacterium tuberculosis | Cryptococcus neoformans | Epstein Barr virus |
| BK virus | ||
| JC virus |
Conclusion
Improved intermediate survival after intestinal and multivisceral transplant has led to increased application of transplantation to patients with irreversible intestinal failure and an increasing number of surviving transplant recipients. The long term survival rate has remained unchanged for many years because the risk of complications remains high. Bacterial infection and ACR remain the main complications limiting life expectancy, but long-term organ dysfunctions such as chronic kidney failure impact on morbidity and mortality as short term survival improves. Only ongoing collaboration between intensivists and transplant specialists will impact both short-term and long-term complications and improve quality of life.
Contributor Information
Derek S. Wheeler, Phone: +1513 636-4259, Email: Derek.Wheeler@cchmc.org
Hector R. Wong, Phone: +1(513)636-4259, Email: hector.wong@cchmc.org
Thomas P. Shanley, Phone: +1734-998-7606, Email: tshanley@med.umich.edu
Gwenn E. McLaughlin, Email: gmclaugh@med.miami.edu.
Tomoaki Kato, Email: tlc2388@columbia.edu.
References
- 1.2004 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1994–2003. Department of Health and Human Services, Health Resources and Service Administration, Healthcare System Bureau, Division of Transplantation, Rockville; United Network for Organ Sharing, Richmond; University Renal Research and Education Association, Ann Arbor.
- 2.Hauser GJ, Kaufman SS, Matsumoto CS, et al. Pediatric intestinal and multivisceral transplantation: a new challenge for the pediatric intensivist. Intensive Care Med. 2008;34:1141–5. doi: 10.1007/s00134-008-1141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazariegos G, Steffick DE, Horslen S, et al. Intestine transplantation in the United States, 1999–2008. Am J Transplant. 2010;10(Part2):1020–34. doi: 10.1111/j.1600-6143.2010.03044.x. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman SS, Atkinson JB, Bianchi A, et al. Indications for pediatric intestinal transplantation: a position paper of the American Society of Transplantation. Pediatr Transplant. 2001;5:80–7. doi: 10.1034/j.1399-3046.2001.005002080.x. [DOI] [PubMed] [Google Scholar]
- 5.Beath S, Pironi L, Gabe S, et al. Collaborative strategies to reduce mortality and morbidity in patients with chronic intestinal failure including those who are referred for small bowel transplantation. Transplantation. 2008;85:1378–84. doi: 10.1097/TP.0b013e31816dd513. [DOI] [PubMed] [Google Scholar]
- 6.Mangus RS, O’Connor MG, Tector AJ, et al. Use of the aspartate aminotransferase to platelet ration index to follow liver fibrosis progression in infants with short gut. J Pediatr Surg. 2010;45:1266–73. doi: 10.1016/j.jpedsurg.2010.02.098. [DOI] [PubMed] [Google Scholar]
- 7.Moon JI, Selvaggi G, Nishida S, et al. Intestinal transplantation for the treatment of neoplastic disease. J Surg Oncol. 2005;92:284–91. doi: 10.1002/jso.20416. [DOI] [PubMed] [Google Scholar]
- 8.Kato T, Tzakis AG, Selvaggi G, et al. Intestinal and multivisceral transplantation in children. Ann Surg. 2006;243(6):756–64. doi: 10.1097/01.sla.0000219696.11261.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vianna RM, Mangus RS, Tector AJ. Current status of small bowel and multivisceral transplantation. Adv Surg. 2008;42:129–50. doi: 10.1016/j.yasu.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Abu-Elmadg K. The small bowel bontained allografts: existing and proposed nomenclature. Am J Transplant. 2011;11:184–5. doi: 10.1111/j.1600-6143.2010.03354.x. [DOI] [PubMed] [Google Scholar]
- 11.Mazariegos GV. Intestinal transplantation: current outcomes and opportunities. Curr Opin Organ Transplant. 2011;14(5):515–21. doi: 10.1097/MOT.0b013e328330680d. [DOI] [PubMed] [Google Scholar]
- 12.Kato T, Selvaggi G, Gaynor JJ, et al. Inclusion of donor colon and ileocecal valve in intestinal transplantation. Transplantation. 2008;86(2):293–7. doi: 10.1097/TP.0b013e31817ef01c. [DOI] [PubMed] [Google Scholar]
- 13.Gupte GL, Beath SV. Update on intestinal rehabilitation after intestinal transplantation. Curr Opin Organ Transplant. 2009;14:267–73. doi: 10.1097/MOT.0b013e32832ac0f5. [DOI] [PubMed] [Google Scholar]
- 14.Gupte GL, Haghighi KS, Sharif K, et al. Surgical complications after intestinal transplantation in infants and children – UK experience. J Pediatr Surg. 2010;45:1473–8. doi: 10.1016/j.jpedsurg.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Grevious MA, Iqbal R, Raofi V, et al. Stage approach for abdominal wound closure following combined liver and intestinal transplantation from living donors in pediatric patients. Pediatr Transplant. 2009;13:177–81. doi: 10.1111/j.1399-3046.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 16.Abu-Elmadg KM, Cost G, Bond GJ, et al. Evolution of the immunosuppressive strategies for the intestinal and multivisceral recipients with special reference to allograft immunity and achievement of partial tolerance. Eur Soc Organ Transplant. 2009;22:96–109. doi: 10.1111/j.1432-2277.2008.00785.x. [DOI] [PubMed] [Google Scholar]
- 17.Li XC, Almawi W, Jevnikar A, Tucker J, Zhong R, Grant D. Allogeneic lymphocyte proliferation stimulated by small intestine-derived epithelial cells. Transplantation. 1995;60(1):82–9. doi: 10.1097/00007890-199507150-00016. [DOI] [PubMed] [Google Scholar]
- 18.Pirenne J, Kawai M. Intestinal transplantation: evolution in immunosuppression protocols. Curr Opin Organ Transplant. 2009;14(3):250–5. doi: 10.1097/MOT.0b013e32832b2eb7. [DOI] [PubMed] [Google Scholar]
- 19.Pascher A, Kohler S, Neuhaus P, Pratschke J. Present status and future perspectives of intestinal transplantation. Transpl Int. 2008;21(5):401–14. doi: 10.1111/j.1432-2277.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 20.Pirenne J, Kawai M. Tolerogenic protocol for intestinal transplantation. Transplant Proc. 2006;38(6):1664–7. doi: 10.1016/j.transproceed.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 21.Kato T, Gaynor JJ, Selvaggi G, et al. Intestinal transplantation in children: a summary of clinical outcomes and prognostic factors in 108 patients from a single center. J Gastrointest Surg. 2005;9(1):75–89. doi: 10.1016/j.gassur.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Selvaggi G, Gaynor JJ, Moon J, et al. Analysis of acute cellular rejection episodes in recipients of primary intestinal transplantation: a single center, 11-year experience. Am J Transplant. 2007;7(5):1249–57. doi: 10.1111/j.1600-6143.2007.01755.x. [DOI] [PubMed] [Google Scholar]
- 23.Nayyar N, Mazariegos G, Ranganathan S, et al. Pediatric small bowel transplantation. Semin Pediatr Surg. 2010;19(1):68–77. doi: 10.1053/j.sempedsurg.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Novelli M, Muiesan P, Miele-Vergani G, Dhawan A, Rela M, Heaton ND. Oral absorption of tacrolimus in children with intestinal failure due to short or absent small bowel. Transpl Int. 1999;12:463–5. doi: 10.1007/s001470050258. [DOI] [PubMed] [Google Scholar]
- 25.Sano N, Nio M, Shimaoka S, et al. High trough levels of oral FK506 induced by loss of small intestine. Pediatr Transplant. 2001;5:434–8. [PubMed] [Google Scholar]
- 26.Sigurdsson L. Bacteremia after intestinal transplantation in children correlates temporally with rejection or gastrointestinal lymphoproliferative disease. Transplantation. 2000;70:302–5. doi: 10.1097/00007890-200007270-00011. [DOI] [PubMed] [Google Scholar]
- 27.Lee RG, Nakamura K, Tsamandas AC, et al. Pathology of human intestinal transplantation. Gastroenterology. 1996;110(6):1820–34. doi: 10.1053/gast.1996.v110.pm8964408. [DOI] [PubMed] [Google Scholar]
- 28.Sigurdsson L, Kocoshis S, Todo S, Putnam P, Reyes J. Severe exfoliative rejection after intestinal transplantation in children. Transplant Proc. 1996;28(5):2783–4. [PubMed] [Google Scholar]
- 29.Gerlach UA, Koch A, Muller HP, et al. Tumor necrosis factor alpha inhibitors as immunomodulatory antirejection agents after intestinal transplantation. Am J Transplant. 2011;11:1041–50. doi: 10.1111/j.1600-6143.2011.03497.x. [DOI] [PubMed] [Google Scholar]
- 30.Wu G, Knabe DA, Flynn NE. Synthesis of citrulline from glutamine in pig enterocytes. Biochem J. 1994;299(1):115–21. doi: 10.1042/bj2990115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008;27(3):328–39. doi: 10.1016/j.clnu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Gondolesi G, Ghirardo S, Raymond K, et al. The value of plasma citrulline to predict mucosal injury in intestinal allografts. Am J Transplant. 2006;6(11):2786–90. doi: 10.1111/j.1600-6143.2006.01513.x. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz P, Tryphonopoulos P, Island E, et al. Citrulline evaluation in bowel transplantation. Transplant Proc. 2010;42(1):54–6. doi: 10.1016/j.transproceed.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 34.David AI, Selvaggi G, Ruiz P, et al. Blood citrulline level is an exclusionary marker for significant acute rejection after intestinal transplantation. Transplantation. 2007;84(9):1077–81. doi: 10.1097/01.tp.0000287186.04342.82. [DOI] [PubMed] [Google Scholar]
- 35.Mercer DF, Vargas L, Sun Y, et al. Stool calprotectin monitoring after small intestine. Transplantation. 2011;91:1166–71. doi: 10.1097/TP.0b013e318215e709. [DOI] [PubMed] [Google Scholar]
- 36.Akpinar E, Vargas J, Kato T, et al. Fecal calprotectin level measurements in small bowel allograft monitoring: a pilot study. Transplantation. 2008;85(9):1281–6. doi: 10.1097/TP.0b013e31816dcea2. [DOI] [PubMed] [Google Scholar]
- 37.Foell D, Wittkowski H, Roth J. Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut. 2009;58(6):859–68. doi: 10.1136/gut.2008.170019. [DOI] [PubMed] [Google Scholar]
- 38.Avitzur Y, Grant D. Intestine transplantation in children: update 2010. Pediatr Clin North Am. 2010;57(2):415–31. doi: 10.1016/j.pcl.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 39.Zeevi A, Britz JA, Bentklejewski CA, et al. Monitoring immune function during tacrolimus tapering in small bowel transplant recipients. Transpl Immunol. 2005;15:17–24. doi: 10.1016/j.trim.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 40.Colvin RB, Smith RN. Antibody mediated organ allograft rejection. Natures Rev: Immunol. 2005;5:807–17. doi: 10.1038/nri1702. [DOI] [PubMed] [Google Scholar]
- 41.Lefaucheur C, Nochy D, Andrade J, Verine J, et al. Comparison of combination plasmapheresis/IVIG/anti-CD20 versus high-dose IVIG in the treatment of antibody-mediated rejection. Am J Transplant. 2009;9(5):1099–107. doi: 10.1111/j.1600-6143.2009.02591.x. [DOI] [PubMed] [Google Scholar]
- 42.Kaposztas Z, Podder H, Mauiyyedi S, et al. Impact of rituximab therapy for treatment of acute humoral rejection. Clin Transplant. 2009;23(1):63–73. doi: 10.1111/j.1399-0012.2008.00902.x. [DOI] [PubMed] [Google Scholar]
- 43.Kato T, Mizutani K, Terasaki P, et al. Association of emergence of HLA antibody and acute rejection in intestinal transplant recipients: a possible evidence of acute humoral sensitization. Transplant Proc. 2006;38(6):1735–7. doi: 10.1016/j.transproceed.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz P, Garcia M, Pappas P, et al. Mucosal vascular alterations in isolated small-bowel allografts: relationship to humoral sensitization. Am J Transplant. 2003;3(1):43–9. doi: 10.1034/j.1600-6143.2003.30108.x. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi H, Kato T, Delacruz V, et al. Analysis of acute and chronic rejection in multiple organ allografts from retransplantation and autopsy cases of multivisceral transplantation. Transplantation. 2008;85(11):1610–6. doi: 10.1097/TP.0b013e318174d857. [DOI] [PubMed] [Google Scholar]
- 46.Mazariegos GV, Soltys K, Bond G, et al. Pediatric intestinal retransplantation: techniques, management, and outcomes. Transplantation. 2008;86(12):1777–82. doi: 10.1097/TP.0b013e3181910f51. [DOI] [PubMed] [Google Scholar]
- 47.Everly MJ, Everly JJ, Susskind B, et al. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation. 2008;86(12):1754–61. doi: 10.1097/TP.0b013e318190af83. [DOI] [PubMed] [Google Scholar]
- 48.Island ER, Gonzalez-Pinto IM, Tsai HL, et al. Successful treatment with bortezomib of a refractory humoral rejection of the intestine after multivisceral transplantation. Clin Transpl. 2009;465–9. [PubMed]
- 49.Stegall MD. Clinical management of renal transplant patients with donor-specific alloantibody: the state of the art. Clin Transpl. 2010;307–15. [PubMed]
- 50.Andres AM, Santamaría ML, Ramos E, et al. Graft-vs-host disease after small bowel transplantation in children. J Pediatr Surg. 2010;45(2):330–6. doi: 10.1016/j.jpedsurg.2009.10.071. [DOI] [PubMed] [Google Scholar]
- 51.Willenbacher W, Basara N, Blau IW, Fauser AA, Kiehl MG. Treatment of steroid refractory acute and chronic graft-versus-host disease with daclizumab. Br J Haematol. 2001;112(3):820–3. doi: 10.1046/j.1365-2141.2001.02582.x. [DOI] [PubMed] [Google Scholar]
- 52.Baird K, Cooke K, Schultz KR. Chronic graft-versus-host disease (GVHD) in children. Pediatr Clin North Am. 2010;57(1):297–322. doi: 10.1016/j.pcl.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Srinivasan R, Chakrabarti S, Walsh T, et al. Improved survival in steroid-refractory acute graft versus host disease after non-myeloablative allogeneic transplantation using a daclizumab-based strategy with comprehensive infection prophylaxis. Br J Haematol. 2004;124:777–86. doi: 10.1111/j.1365-2141.2004.04856.x. [DOI] [PubMed] [Google Scholar]
- 54.Yanik GA, Uberti JP, Ferrara JLM, et al. Etanercept for sub-acute lung injury following allogeneic stem cell transplantation. Blood. 2003;102:471a. [Google Scholar]
- 55.Busca A, Locatelli F, Marmont F, et al. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft- versus-host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2007;82:45–52. doi: 10.1002/ajh.20752. [DOI] [PubMed] [Google Scholar]
- 56.Messina C, Locatelli F, Lanino E, et al. Extracorporeal photochemotherapy for paediatric patients with graft-versus-host disease after haematopoietic stem cell transplantation. Br J Haematol. 2003;122:118–27. doi: 10.1046/j.1365-2141.2003.04401.x. [DOI] [PubMed] [Google Scholar]
- 57.Baird K, Wayne AS. Extracorporeal photo-apheresis for the treatment of steroid-resistant graft versus host disease. Transfus Apher Sci. 2009;41(3):209–16. doi: 10.1016/j.transci.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Green M, Michaels MG. Infections in solid organ transplant recipients. In: Long SS, Prober CG, Pickering LK, editors. Principles & practice of pediatric infectious diseases. 3. New York: Churchill Livingstone; 2008. pp. 551–7. [Google Scholar]
- 59.Fishman JA. Infection in solid organ transplant recipients. N Engl J Med. 2007;357:2601. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 60.Keough WL, Michaels MG. Infectious complications in pediatric solid organ transplantation. Pediatr Clin North Am. 2003;50:1451–69. doi: 10.1016/s0031-3955(03)00126-3. [DOI] [PubMed] [Google Scholar]
- 61.Mañez R, Kusne S, Green M, et al. Incidence and risk factors associated with the development of cytomegalovirus disease after intestinal transplantation. Transplantation. 1995;59:1010–4. doi: 10.1097/00007890-199504150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McLaughlin GE, Delis S, Kashimawo L, Cantwell GP, et al. Adenovirus infection in pediatric liver and intestinal transplant recipients: utility of DNA detection by PCR. Am J Transplant. 2003;3:224–8. doi: 10.1034/j.1600-6143.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 63.Florescu DF, Islam MK, Mercer DF, et al. Adenovirus infections in pediatric small bowel transplant recipients. Transplantation. 2010;90(2):198–204. doi: 10.1097/TP.0b013e3181e0de97. [DOI] [PubMed] [Google Scholar]
- 64.Madan RP, Herold BC. Viral infections in pediatric solid organ transplantation recipients and the impact of molecular diagnostic testing. Curr Opin Organ Transplant. 2010;15:293–300. doi: 10.1097/MOT.0b013e3283398795. [DOI] [PubMed] [Google Scholar]
- 65.McLaughlin GE, Gelman BG, Kuluz JW, et al. Pediatric intensive care unit utilization and outcome in a solid organ transplant population. Pediatr Crit Care Med. 2005;6(1):103. [Google Scholar]
- 66.Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2004;170:22–48. doi: 10.1164/rccm.200309-1322SO. [DOI] [PubMed] [Google Scholar]
- 67.Shorr AF, Sulsa GM, O’Grady NP. Pulmonary infiltrates in the non-HIV infected immunocompromised patient: etiologies, diagnostic strategies and outcomes. Chest. 2004;125:260–71. doi: 10.1378/chest.125.1.260. [DOI] [PubMed] [Google Scholar]
- 68.Duran FG, Piqueras B, Romero M, et al. Pulmonary complications following orthotopic liver transplant. Transpl Int. 1998;11(Suppl 1):S255–9. doi: 10.1007/s001470050473. [DOI] [PubMed] [Google Scholar]
- 69.Rano A, Agusti C, Jimenez P, et al. Pulmonary infiltrates in non-HIV immunocompromised patients: a diagnostic approach using noninvasive and bronchoscopic procedures. Thorax. 2001;56:379–87. doi: 10.1136/thorax.56.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oosterheert JJ, van Loon AM, Schuurman R, et al. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clin Infect Dis. 2005;41:1438–44. doi: 10.1086/497134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Templeton KE, Scheltinga SA, van den Eeden WC, et al. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41:345–51. doi: 10.1086/431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liden PK. Approach to the immunocompromised host with infection in the intensive care unit. Infect Dis Clin North Am. 2009;23:535–56. doi: 10.1016/j.idc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 73.Barloon JT, Galvin JR, Mori M, et al. High-resolution ultrafast chest CT in the management of febrile bone marrow transplant patients with normal or nonspecific chest roentgenograms. Chest. 1991;99:928–33. doi: 10.1378/chest.99.4.928. [DOI] [PubMed] [Google Scholar]
- 74.Heussel CP, Kauczor HU, Heussel G, et al. Early detection of pneumonia in febrile neutropenic patients: use of thin-section CT. Am J Roentgenol. 1997;169:1347–53. doi: 10.2214/ajr.169.5.9353456. [DOI] [PubMed] [Google Scholar]
- 75.Vendryes C, 3rd, McLaughlin GE, Romaguera RL, et al. Open lung biopsy in pediatric patients with respiratory failure after abdominal transplantation. Pediatr Transplant. 2005;9:197–200. doi: 10.1111/j.1399-3046.2004.00283.x. [DOI] [PubMed] [Google Scholar]
- 76.Baumann HJ. Yield and safety of bedside open lung biopsy in mechanically ventilated patients with acute lung injury or acute respiratory distress syndrome. Surgery. 2008;143:426–33. doi: 10.1016/j.surg.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Lim SY, Suh GY, Choi JC, et al. Usefulness of open lung biopsy in mechanically ventilated patients with undiagnosed diffuse pulmonary infiltrates: influence of co-morbidities and organ dysfunction. Crit Care. 2007;11:R93. doi: 10.1186/cc6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wagener MM, Yu VL. Bacteremia in transplant recipients: a prospective study of demographics, etiologic agents, risk factors, and outcomes. Am J Infect Control. 1992;20:239–47. doi: 10.1016/s0196-6553(05)80197-x. [DOI] [PubMed] [Google Scholar]
- 79.Berg RD. Bacterial translocation from the gastrointestinal tract. J Med. 1992;23:217. [PubMed] [Google Scholar]
- 80.Cicalese L, Sileri P, Green M, et al. Bacterial translocation in clinical intestinal transplantation. Transplantation. 2001;71:1414. doi: 10.1097/00007890-200105270-00010. [DOI] [PubMed] [Google Scholar]
- 81.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49(9):3640–5. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kollef MH, Sherman G, Ward S, et al. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115(2):263–74. doi: 10.1378/chest.115.2.462. [DOI] [PubMed] [Google Scholar]
- 83.Ibrahim EH, Sherman G, Ward S, et al. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118(1):146–55. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 84.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 85.Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 86.Watson MJ, Venick RS, Kaldas F, et al. Renal function impacts outcomes after intestinal transplantation. Transplantation. 2008;86(1):117–22. doi: 10.1097/TP.0b013e31817d55ae. [DOI] [PubMed] [Google Scholar]
- 87.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349(10):931–40. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 88.Platz KP, Mueller AR, Blumhardt G, et al. Nephrotoxicity following orthotopic liver transplantation. A comparison between cyclosporine and FK506. Transplantation. 1994;58(2):170–8. [PubMed] [Google Scholar]
- 89.Ueno T, Kato T, Gaynor J, et al. Renal dysfunction following adult intestinal transplant under tacrolimus-based immunosuppression. Transplant Proc. 2006;38(6):1762–4. doi: 10.1016/j.transproceed.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 90.Wyatt CM, Arons RR. The burden of acute renal failure in non-renal solid organ transplantation. Transplantation. 2004;78(9):1351–5. doi: 10.1097/01.tp.0000140848.05002.b8. [DOI] [PubMed] [Google Scholar]
- 91.Biancofiore G, Della Rocca G, Bindi L, et al. Use of fenoldopam to control renal dysfunction early after liver transplantation. Liver Transpl. 2004;10(8):986–92. doi: 10.1002/lt.20145. [DOI] [PubMed] [Google Scholar]
- 92.Jorkasky DK, Audet P, Shusterman N, et al. Fenoldopam reverses cyclosporine-induced renal vasoconstriction in kidney transplant recipients. Am J Kidney Dis. 1992;19(6):567–72. doi: 10.1016/s0272-6386(12)80836-5. [DOI] [PubMed] [Google Scholar]
- 93.McLaughlin GE, Abitbol CL. Reversal of oliguric tacrolimus nephrotoxicity in children. Nephrol Dial Transplant. 2005;20(7):1471–5. doi: 10.1093/ndt/gfh785. [DOI] [PubMed] [Google Scholar]
- 94.Joannidis M. Continuous renal replacement therapy in sepsis and multisystem organ failure. Semin Dial. 2009;22(2):160–4. doi: 10.1111/j.1525-139X.2008.00552.x. [DOI] [PubMed] [Google Scholar]
- 95.Goldstein SL. Overview of pediatric renal replacement therapy in acute kidney injury. Semin Dial. 2009;22(2):180–4. doi: 10.1111/j.1525-139X.2008.00551.x. [DOI] [PubMed] [Google Scholar]
- 96.Brophy PD, Mottes TA, Kudelka TL, et al. AN-69 membrane reactions are pH-dependent and preventable. Am J Kidney Dis. 2001;38:173–8. doi: 10.1053/ajkd.2001.25212. [DOI] [PubMed] [Google Scholar]
- 97.Ueno T, Kato T, Gaynor J, et al. Renal function after pediatric intestinal transplant. Transplant Proc. 2006;38(6):1759–61. doi: 10.1016/j.transproceed.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 98.Andrès E, Maloisel F. Idiosyncratic drug-induced agranulocytosis or acute neutropenia. Curr Opin Hematol. 2008;15(1):15–21. doi: 10.1097/MOH.0b013e3282f15fb9. [DOI] [PubMed] [Google Scholar]
- 99.Andersohn F, Konzen C, Garbe E. Nonchemotherapy drug-induced agranulocytosis: a systematic review of case reports. Ann Intern Med. 2007;46:657–65. doi: 10.7326/0003-4819-146-9-200705010-00009. [DOI] [PubMed] [Google Scholar]
- 100.Caluwe R, Van Laecke S, Emonds MP, et al. Immediate Posttransplantation cotrimoxazole-induced immune thrombocytopenia. Am J Transplant. 2010;10:943–6. doi: 10.1111/j.1600-6143.2010.03028.x. [DOI] [PubMed] [Google Scholar]
- 101.Al-Uzri A, Yorgin PD, Kling PJ. Anemia in children after transplantation: etiology and the effect of immunosuppressive therapy on erythropoiesis. Pediatr Transplant. 2003;7(4):253–64. doi: 10.1034/j.1399-3046.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 102.Sanchez-Giron F, Alvarez-Mora F. Reduction of blood loss from laboratory testing in hospitalized patients using small-volume (pediatric) tubes. Arch Pathol Lab Med. 2008;132:1916–9. doi: 10.5858/132.12.1916. [DOI] [PubMed] [Google Scholar]
- 103.Misra S, Moore TB, Ament ME, Busuttil RW, McDiarmid SV. Red cell aplasia in children on tacrolimus after liver transplantation. Transplantation. 1998;65:575–7. doi: 10.1097/00007890-199802270-00021. [DOI] [PubMed] [Google Scholar]
- 104.Brum S, Nolasco F, Sousa J, et al. Leukopenia in kidney transplant patients with the association of valganciclovir and mycophenolate mofetil. Transplant Proc. 2008;40:752–4. doi: 10.1016/j.transproceed.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 105.Danesi R, Tacca M. Hematologic toxicity of immunosuppressive treatment. Transplant Proc. 2004;36(3):703–4. doi: 10.1016/j.transproceed.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 106.European Mycophenolate Mofetil Cooperative Study Group MMF in renal transplantation: 3-year results from the placebo-controlled trial. Transplantation. 1999;68:391–6. [PubMed] [Google Scholar]
- 107.MacDonald AS, RAPAMUNE Global Study Group A worldwide phase III, randomized controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation. 2001;71:271–80. doi: 10.1097/00007890-200101270-00019. [DOI] [PubMed] [Google Scholar]
- 108.Brown KE, Anderson SM, Young NS. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science. 1993;262:114–7. doi: 10.1126/science.8211117. [DOI] [PubMed] [Google Scholar]
- 109.Broliden K. Parvovirus B 19 infection in pediatric solid organ and bone marrow transplantation. Pediatr Transplant. 2001;5:320–30. doi: 10.1034/j.1399-3046.2001.00035.x. [DOI] [PubMed] [Google Scholar]
- 110.Gallinella G, Manaresi E, Venturoli S, Grazi GL, Musiani M, Zerbini M. Occurrence and clinical role of active parvovirus B19 infection in transplant recipients. Eur J Clin Microbiol Infect Dis. 1999;18:811–3. doi: 10.1007/s100960050406. [DOI] [PubMed] [Google Scholar]
- 111.Nour B, Green M, Michaels M, et al. Parvovirus B19 infection in pediatric transplant patients. Transplantation. 1993;55:427–30. doi: 10.1097/00007890-199310000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chang FY, Singh N, Gayowski T, Marino IR. Parvovirus B19 infection in a liver transplant recipient: case report and review in organ transplant recipients. Clin Transpl. 1996;10:243–7. [PubMed] [Google Scholar]
- 113.Kurtzman G, Frickhofen N, Kimball J, Jenkins DW, Nienhuis AW, Young NS. Pure red cell aplasia from ten years duration due to persistent parvovirus B19 infection and its cure with immunoglobulin therapy. N Engl J Med. 1989;321:519–23. doi: 10.1056/NEJM198908243210807. [DOI] [PubMed] [Google Scholar]
- 114.Wong TYH, Chan PKS, Leung CB, Szeto CC, Tam JS, Li PKT. Parvovirus B19 infection causing red cell aplasia in renal transplantation on tacrolimus. Am J Kidney Dis. 1999;34:1132–6. doi: 10.1016/S0272-6386(99)70021-1. [DOI] [PubMed] [Google Scholar]
- 115.Frohn C, Jabs WJ, Fricke L, Goerg S. Hemolytic anemia after kidney transplantation: case report and differential diagnosis. Ann Hematol. 2002;81:158–60. doi: 10.1007/s00277-001-0425-4. [DOI] [PubMed] [Google Scholar]
- 116.Ainsworth CD, Crowther MA, Treleaven D, Evanovitch D, Webert KE, Blajchman MA. Severe hemolytic anemia post-renal transplantation produced by donor anti-D passenger lymphocytes: case report and literature review. Transfus Med Rev. 2009;23(2):155–9. doi: 10.1016/j.tmrv.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 117.Ramsey G. Red cell antibodies arising from solid organ transplants. Transfusion. 1991;31:76–86. doi: 10.1046/j.1537-2995.1991.31191096190.x. [DOI] [PubMed] [Google Scholar]
- 118.West KA, Anderson DR, McAlister VC, et al. Alloimmune thrombocytopenia after organ transplantation. N Engl J Med. 1999;341:1504–7. doi: 10.1056/NEJM199911113412004. [DOI] [PubMed] [Google Scholar]
- 119.Lacaille F, Moes N, Hugot JP, Cezard JP, Goulet O, Ruemmele FM. Severe dysimmune cytopenia in children treated with tacrolimus after organ transplantation. Am J Transplant. 2006;6(5 Pt 1):1072–6. doi: 10.1111/j.1600-6143.2006.01304.x. [DOI] [PubMed] [Google Scholar]
- 120.Czubkowski P, Wiliams M, Bagia S, Kelly D, Gupte G. Immune-mediated hemolytic anemia in children after liver and small bowel transplantation. Liver Transpl. 2011;17:921–4. doi: 10.1002/lt.22311. [DOI] [PubMed] [Google Scholar]
- 121.Remuzzi G. HUS and TTP. Variable expression of a single entity. Kidney Int. 1987;32:292–308. doi: 10.1038/ki.1987.206. [DOI] [PubMed] [Google Scholar]
- 122.Ramasubbu K, Mullick T, Koo A, et al. Thrombotic microangiopathy and cytomegalovirus in liver transplant recipients: a case-based review. Transpl Infect Dis. 2003;5:98–103. doi: 10.1034/j.1399-3062.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 123.Trimarchi HM, Truong LD, Brennan S, Gonzalez JM, Suki WN. FK506-associated thrombotic microangiopathy: report of two cases and review of the literature. Transplantation. 1999;67:539–44. doi: 10.1097/00007890-199902270-00009. [DOI] [PubMed] [Google Scholar]
- 124.Allen U, Preiksaitis J. Epstein - Barr virus and posttransplant lymphoproliferative disorder in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S87–96. doi: 10.1111/j.1600-6143.2009.02898.x. [DOI] [PubMed] [Google Scholar]
- 125.Paya CV, Fung JJ, Nalesnik MA, et al. EBV-induced post-transplant lymphoproliferative disorders. ASTS/ASTP EBV PTLD Task Force and the Mayo Clinic Organized International Consensus Development Meeting. Transplantation. 1999;68:1517. doi: 10.1097/00007890-199911270-00015. [DOI] [PubMed] [Google Scholar]
- 126.Opelz G, Daniel V, Naujokat C, et al. Epidemiology of pretransplant EBV and CMV serostatus in relation to posttransplant non-Hodgkin lylmphoma. Transplantation. 2009;88:962–7. doi: 10.1097/TP.0b013e3181b9692d. [DOI] [PubMed] [Google Scholar]
- 127.Gross TG, Savoldo B, Punnet A. Posttransplant lymphoproliferative disorder. Pediatr Clin North Am. 2010;57:481–503. doi: 10.1016/j.pcl.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 128.Stevens SJ, Verschuuren EA, Pronk I, et al. Frequent monitoring of Epstein-Barr virus DNA load in unfractionated whole blood is essential for early detection of posttransplant lymphoproliferative disease in high-risk patients. Blood. 2001;97:1165. doi: 10.1182/blood.v97.5.1165. [DOI] [PubMed] [Google Scholar]
- 129.Fishbein TH. Intestinal transplantation. N Engl J Med. 2009;361(10):1000–10008. doi: 10.1056/NEJMra0804605. [DOI] [PubMed] [Google Scholar]
- 130.Nalesnik MA. The diverse pathology of post-transplant lymphoproliferative disorders: the importance of a standardized approach. Transpl Infect Dis. 2001;3:88–96. doi: 10.1034/j.1399-3062.2001.003002088.x. [DOI] [PubMed] [Google Scholar]
- 131.Hurwitz M, Desai DM, Cox KL, et al. Complete immunosuppressive withdrawal as a uniform approach to post-transplant lymphoproliferative disease in pediatric liver transplantation. Pediatr Transplant. 2004;8:267–72. doi: 10.1111/j.1399-3046.2004.00129.x. [DOI] [PubMed] [Google Scholar]
- 132.Koffman BH, Kennedy AS, Hayman M, et al. Use of radiation therapy in posttransplant lymphoproliferative disorder (PTLD) after liver transplantation. Int J Cancer. 2000;90:104–9. doi: 10.1002/(sici)1097-0215(20000420)90:2<104::aid-ijc6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 133.Frey NV. The management of posttransplant lymphoproliferative disorder. Med Oncol. 2007;24(2):125–36. doi: 10.1007/BF02698031. [DOI] [PubMed] [Google Scholar]
- 134.Milpied N, Vasseur B, Parquet N, et al. Humanized anti-CD20 monoclonal antibody (Rituximab) in post transplant B-lymphoproliferative disorder: a retrospective analysis on 32 patients. Ann Oncol. 2000;11(Suppl 1):113–6. [PubMed] [Google Scholar]
- 135.Oertel SH, Verschuuren E, Reinke P, et al. Effect of anti-CD 20 antibody rituximab in patients with post-transplant lymphoproliferative disorder (PTLD) Am J Transplant. 2005;5:2901–6. doi: 10.1111/j.1600-6143.2005.01098.x. [DOI] [PubMed] [Google Scholar]
- 136.Gross TG, Bucuvalas J, Park J, et al. Low dose chemotherapy for the treatment of refractory post-transplant lymphoproliferative disease in children. J Clin Oncol. 2005;23:6481. doi: 10.1200/JCO.2005.08.074. [DOI] [PubMed] [Google Scholar]
- 137.Leblond V, Dhedin N, Bruneel MM, et al. Identification of prognostic factors in 61 patients with posttransplantation lymphoproliferative disorders. J Clin Oncol. 2001;19(3):772–8. doi: 10.1200/JCO.2001.19.3.772. [DOI] [PubMed] [Google Scholar]
- 138.Trofe J, Buell JF, Beebe TM, et al. Analysis of factors that influence survival with post-transplant lymphoproliferative disorder in renal transplant recipients: the Israel Penn International Transplant Tumor Registry experience. Am J Transplant. 2005;5(4Pt1):775–80. doi: 10.1111/j.1600-6143.2005.00776.x. [DOI] [PubMed] [Google Scholar]
- 139.Thevenin DM, Baker A, Kato T, Tzakis A, Fernandez M, Dowling M. Neurodevelopmental outcomes of infant multivisceral transplant recipients: a longitudinal study. Transplant Proc. 2006;38:1694–5. doi: 10.1016/j.transproceed.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 140.Thevenin DM, Baker A, Kato T, Tzakis A, Fernandez M, Dowling M. Neurodevelopmental outcomes for children transplanted under the age of 3 years. Transplant Proc. 2006;38:1692–3. doi: 10.1016/j.transproceed.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 141.Senzolo M, Ferronato C, Burra P. Neurologic complications after solid organ transplantation. Transpl Int. 2008;22:269–78. doi: 10.1111/j.1432-2277.2008.00780.x. [DOI] [PubMed] [Google Scholar]
- 142.Bechstein WO. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int. 2000;13(5):313–26. doi: 10.1007/s001470050708. [DOI] [PubMed] [Google Scholar]
- 143.Palmer BF, Toto RD. Systemic neurologic toxicity induced by cyclosporine a in three renal transplant patients. Am J Kidney Dis. 1991;18:116–21. doi: 10.1016/s0272-6386(12)80300-3. [DOI] [PubMed] [Google Scholar]
- 144.Gijtenbeek JM, van den Bent MJ, Vecht CJ. Cyclosporine neurotoxicity: a review. J Neurol. 1999;246(5):339–46. doi: 10.1007/s004150050360. [DOI] [PubMed] [Google Scholar]
- 145.Bartynski WS, Tan HP, Boardman JF, et al. Posterior reversible encephalopathy syndrome after solid organ transplantation. Am J Neuroradiol. 2008;29:924–30. doi: 10.3174/ajnr.A0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zivkovic SA, Eidelman BH, Bond GE, Costa G, Abu-Elmagd The clinical spectrum of neurologic disorders after intestinal and multivisceral transplantation. Clin Transplant. 2010;24:164–8. doi: 10.1111/j.1399-0012.2009.01065.x. [DOI] [PubMed] [Google Scholar]
- 147.Czartoski T. Central nervous system infections in transplantation. Curr Treat Options Neurol. 2006;8:193–201. doi: 10.1007/s11940-006-0010-1. [DOI] [PubMed] [Google Scholar]
- 148.Singh N, Husain S. Infections of the central nervous system in transplant recipients. Transpl Infect Dis. 2000;2(3):101–11. doi: 10.1034/j.1399-3062.2000.020302.x. [DOI] [PubMed] [Google Scholar]
- 149.Torre-Cisneros J, Lopez OL, Kusne S, et al. CNS aspergillosis in organ transplantation: a clinicopathological study. J Neurol Neurosurg Psychiatry. 1993;56(2):188–93. doi: 10.1136/jnnp.56.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Van de Beek D, Patel R, Daly RC, McGregor CG, Wijdicks EF. Central nervous system infections in heart transplant recipients. Arch Neurol. 2007;64(12):1715–20. doi: 10.1001/archneur.64.12.noc70065. [DOI] [PubMed] [Google Scholar]
- 151.Takeuchi S, Takasato Y, Masaoka H, et al. Hemorrhagic encephalitis associated with Epstein-Barr virus infection. J Clin Neurosci. 2010;17(1):153–4. doi: 10.1016/j.jocn.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 152.Hopfner R, Tran TT, Island ER, McLaughlin GE. Nonsurgical care of intestinal and multivisceral transplant recipients: a review for the intensivist. J Intensive Care Med. 2013;28(4):215–29. doi: 10.1177/0885066611432425. [DOI] [PubMed] [Google Scholar]


