Abstract
Since thermostable Taq DNA polymerase was discovered in 1987, nucleic acid amplification techniques have made great strides and contributed greatly to progress in the life sciences. These techniques were introduced into the clinical laboratory and have produced great changes in diagnostic tools and tests. In particular, there have been many innovative molecular testing developments in the field of diagnostic microbiology.
Keywords: Extraction Method, Diatomaceous Earth, Clinical Specimen, Nucleic Acid Extraction, Automate Extraction
Introduction
Since thermostable Taq DNA polymerase was discovered in 1987, nucleic acid amplification techniques have made great strides and contributed greatly to progress in the life sciences. These techniques were introduced into the clinical laboratory and have produced great changes in diagnostic tools and tests. In particular, there have been many innovative molecular testing developments in the field of diagnostic microbiology.
Culture methods of bacterial identification are labor-intensive and time-consuming. However, they are simple, cheap, and remain the gold standard. It also is possible to perform antimicrobial susceptibility testing on cultured isolates, so conventional culture methods with biochemical phenotyping are still the most common procedures performed in clinical microbiology laboratories [1]. To further assist in microbial identification, nucleic acid amplification has been introduced in the clinical microbiology laboratory. Such testing was initially done for viruses, allowing detection of small amounts of viral nucleic acid quickly. Similar tests also have been applied to bacteria, especially those that require cell cultures, are difficult to grow on routine culture media, or are slow growing such as Chlamydia, Neisseria gonorrhoeae, and Mycobacterium. In addition, there are ongoing attempts to apply these new techniques for routine clinical microbiology testing, including the diagnosis of sepsis [1].
The development of nucleic acid amplification has proceeded at an unprecedented pace and achieved higher sensitivity and specificity [2]. However, in order to obtain satisfying results with this new technique, the testing must go through several important steps. Preanalytical testing variables comprise sample collection and preparation, specimen transport and storage, stability of the nucleic acid in the samples, and nucleic acid extraction [3, 4].
Nucleic acid extraction is the first step of any amplification experiment no matter what kind of amplification is used to detect a specific pathogen [1, 5]. It is a crucial preanalytic step in the development and performance of any successful molecular diagnostic method and ensures a reliable result [3, 4]. We must pay attention to the technical progress of the nucleic acid extraction as well as to the method for amplification and detection of nucleic acids in order to obtain satisfactory results. Nucleic acid extraction consists of three major processes: isolation, purification, and concentration. Commercial extraction kits are commonly used in the clinical microbiology laboratory [2]. These kits provide the essential requirements for nucleic acid extraction. These essential requirements have been well described by Boom et al. [6, 7] as follows: Extraction should be simple, rapid, and should show high sensitivity and specificity. It is preferred that there be no requirements for specialized equipment or special knowledge and skills. The final nucleic acid should be pure and easy to modify for various amplification techniques. The reagents and their product should be harmless, and the process of preparation should resist contamination with other specimens. If the final volume of eluate is small, detection limits are maximized. When we deal with clinical specimens, we also should consider the elimination of potential inhibitors of the DNA polymerase and the removal of pathogenicity from hazardous pathogens as well as good target recovery and establishment of the integrity of nucleic acid targets [2]. Ideally, the final target is pure nucleic acid without amplification inhibitors or contaminants such as protein, carbohydrate, and other nucleic acids [8].
There are a few points to be specially considered when we consider the use of nucleic acid extraction in the field of clinical microbiology. Usually, we extract DNA or RNA, and some times, we may extract both, depending on the circumstances. The targets for nucleic acid extraction are diverse. They can be the cultured bacterial isolates themselves. Alternatively, we can use culture media, including blood culture bottles or various clinical specimens such as sputum, stool, urine, tissue, or cerebrospinal fluid [1]. In terms of nucleic acid extraction, they may target the same nucleic acid, but they have different implications for the extraction procedure itself. Nucleic acid extraction from cultured bacteria is relatively simple because they are pure colonies and they contain large numbers of organisms. However, we need to recognize that gram-positive bacteria have thick walls, such that nucleic acid extraction is more difficult than it is from gram-negative bacteria, which have thinner walls [5]. For clinical specimens, the details of the method depend on the characteristics of each specimen. It is important to remember that the subject is nucleic acid, not of humans, but of bacteria, virus, or fungus. If we extract from clinical specimens containing human cells, we cannot help mixing human DNA and sometimes, recovery of microbial DNA can be diminished by the presence of human DNA.
Nucleic Acid Extraction Techniques
Cesium Chloride/Ethidium Bromide Density Gradient Centrifugation
Since 1950, density gradient centrifugation using cesium chloride (CsCl)/ethidium bromide (EtBr) has been used as for DNA extraction method and has become standard in research laboratories [9, 10]. The basic principle of this method is to use the difference in density between the cesium ion and water and intercalation of EtBr, which shows good results for separation of various DNAs and the procurement of high-yield DNA [11]. For example, each DNA can be separated as independent bands as a result of the differences in each DNA’s density in the gradient by the intercalation of EtBr [7]. However, it has important limitations in that it requires an expensive ultracentrifuge and considerable time, it is difficult to perform, and EtBr is harmful [7, 8]. Consequently, this method is not suitable for clinical microbiology and has not been used in the clinical laboratory.
Phenol–Chloroform Extraction
Phenol–chloroform extraction is widely used. The process consists of vigorous mixing of phenol–chloroform solution and sample followed by centrifugation [7]. Phenol does not completely inhibit RNase activity, and this characteristic enables isolation of nucleic acid by combination with chloroform and alcohol [12]. After centrifugation, the upper (aqueous) phase containing the DNA can be separated from the lower (organic) phase containing denatured proteins, and DNA can be precipitated by adding ethanol or isopropanol with a high concentration of salt [8]. After washing with 70% ethanol to remove any remaining ethanol or isopropanol, the final target DNA is collected by dissolving it in TE buffer or sterile distilled water [13]. This method is also used for RNA extraction by concomitant use of guanidinium isothiocyanate. This combination can overcome the limitation of RNA extraction using the guanidinium isothiocyanate itself, so RNA could be isolated conveniently using a single-step technique by Chomczynski et al. [12, 14]. Total RNA is recovered by precipitation with isopropanol after separation of the upper phase containing the total RNA from the lower phase containing DNA and proteins [12, 14]. Although the phenol–chloroform method is relatively easy compared with CsCl/EtBr and is very useful for the extraction of nucleic acids, it is problematic for the clinical microbiology laboratory because phenol has important limitations due to it being toxic, caustic, and flammable [5, 15, 16].
Solid-Phase Extraction
McCormick et al. introduced a new DNA extraction method involving solid-phase extraction in 1989 [17]. They used an insoluble siliceous core particle rather than liquid phenol. The function of this siliceous core particle is similar to that of phenol, but it has a few advantages in that it is safer, and cross-contamination can be reduced. It is well known that the precipitation in the phenol/chloroform method causes DNA loss, and Meijer et al. could reduce it by replacing the precipitating step with silica particles [18]. Solid-phase nucleic acid extraction was incorporated into many commercial kits, and it still is the basis of many extraction methods, although siliceous core particles have been replaced by other materials such as silica matrices, glass particles, diatomaceous earth, and anion-exchange carriers (Fig. 11.1) [7].
Fig. 11.1.
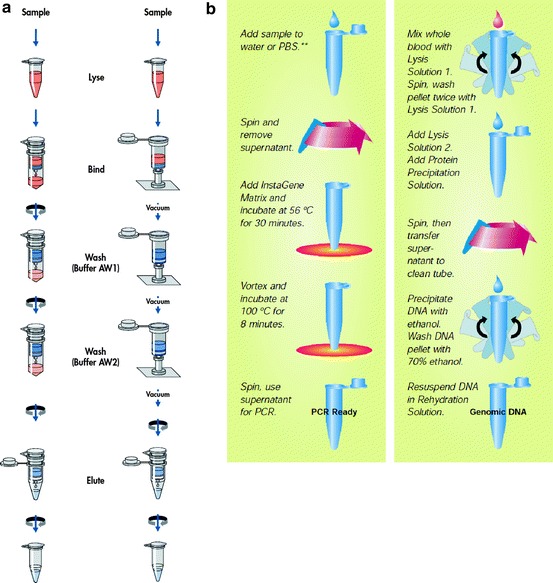
Schematic diagrams of (a) solid-phase, spin-column nucleic acid extraction method (QIAamp DNA Mini kit from Qiagen, http://www.qiagen.com) and (b) liquid-based nucleic acid extraction method (InstaGene Matrix and Genomic DNA kit from Bio-Rad, http://www.bio-rad.com)
Solid-phase extraction uses a spin column operated by centrifugal force allowing DNA to be purified rapidly and efficiently without the limitations of liquid extraction, including incomplete phase separation [8]. Solid-phase extraction using silica now is one of the most common methods for nucleic acid extraction. Silica that possesses a positive charge combines strongly with DNA, which possesses a negative charge, so it can enable rapid, pure, and quantitative purification [7]. In 1990, Boom et al. [6] used an innovative approach in which diatomaceous earth served as a matrix for solid-phase extraction. The principle of this method is that it immobilizes DNA onto its particles in the presence of a chaotropic agent. The technique can purify rRNA as well as single-stranded and double-stranded DNA. It takes only a short time and can be applied to clinical specimens as well as to DNA and bacteria. The process of solid-phase extraction involves cell lysis, nucleic acid adsorption, washing, and elution [7, 8]. Column conditioning is obtained using a buffer at a particular pH [19]. The nucleic acid will be released after cell lysis and decanting of lysis buffer into the column. Nucleic acid adsorption is completed in a chaotropic salt solution [19]. Washing buffer contains a competitive agent and can remove contaminants such as proteins and salts. In elution, TE buffer is applied to the column so that purified nucleic acid will be released [19].
Magnetic Bead Method
There is another important modification of solid-phase extraction, that is, the magnetic bead method. The beads have a negative surface charge and bind proteins and cellular debris selectively [7]. So, DNA can be isolated easily from specimens by removing proteins and cellular debris on the beads. This has the potential advantages of removing the need for repeated centrifugation, vacuum filtration, and column separation for washing and elution as well as organic solvents [7, 8]. The magnetic bead method is very simple and convenient; so many commercial kits are available for this method [8]. Some manufacturers combined the techniques of solid-phase extraction using silica and magnetic beads, which satisfies the customers’ requests for time- and labor-effectiveness and efficiency (Fig. 11.2). This method is commonly used in automated extraction methods such as miniMag (bioMerieux) and MagNA Pure (Roche). In terms of new technology, additional commercial kits using this new technique are being launched into the market. The enzymatic method is an example of these new extraction methods [20]. These new methods help investigators by giving advantages of more convenience, requirement for only small volumes of specimen, and enhancement of DNA recovery.
Fig. 11.2.
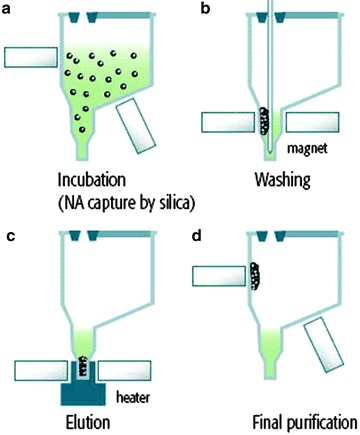
Extraction principles using magnetic silica particles. (a) During incubation of the lysed samples, all the target nucleic acid is captured by magnetic silica particles. (b) The NucliSENS easy MAG magnetic device attracts all the magnetic silica, enabling the system to purify the nucleic acids through several washing steps. (c) The heating step releases the nucleic acids from the silica. (d) In the final step, the magnetic silica particles are separated from the eluate by the magnetic device (NucliSENS easyMAG from biomerieux, http://www.biomerieux-diagnostics.com)
Applications to Clinical Specimens
Nucleic acid extraction from clinical specimens is quite different from that from cultured isolates of bacteria or fungi. This extraction step can influence the subsequent performance of the diagnostic tests; the efficiency of nucleic acid extraction is related directly to the sensitivity of the final test results [21]. Each clinical specimen has diverse characteristics. Blood and stool are composed of many substances, and among these, heme and bile act as inhibitors of amplification and should be removed [5]. We can find the comparison results for nucleic acid extraction; however, this cannot ensure that we can adapt this result to different specimens and pathogens. In previous reports, herpes simplex virus DNA was isolated relatively easily from genital swabs [5, 22, 23], but obtaining bacterial DNA from stool samples was more complex [5, 24].
To overcome these limitations, the extraction method must be evaluated before routine testing of specific pathogens from specific specimens. For detection of clinically important viruses, extraction efficiency was evaluated in various specimens, including serum, urine, and cerebrospinal fluid, and good performance was confirmed [2, 25, 26]. However, we should not extrapolate these specific results to all types of virus and specimens.
Cellular Component
Tissue is an important clinical specimen for diagnosing localized cytomegalovirus infections in a transplanted organ as biopsy is a common method used to evaluate potential CMV infection [27]. However, tissue specimens have problems because the large amount of human tissue contains cellular DNA, proteins, and other materials [28]. So, a more complex step to extract the nucleic acid of the microbial pathogen is needed. Most commercial kits for tissue specimens extract human nucleic acid also. In recent years, we have been able to extract the viral nucleic acid from clinical specimens having cellular components, and there have been a few trials of these kits to detect various clinically important viruses [29–31]. There is one other report concerning the extraction of six viruses from clinical cellular specimens, and the investigators compared four commercial extraction methods [28]. The viruses included in this study are BK virus, cytomegalovirus, Epstein–Barr virus, human herpesvirus 8, herpes simplex virus, and varicella-zoster virus. All four kits could extract DNA from all six viruses.
Stool is an important clinical specimen for the detection of viruses causing diarrheal illnesses. The results can be affected by the efficiency of nucleic acid extraction from stool, because stool is a mixture of many unrecognized materials, including bacteria, protein, and other cellular materials. So, stool specimens are considered one of the most difficult specimens for nucleic acid extraction in the clinical laboratory. In one report by Peiris et al. [32], the positive rate was 97% for severe acute respiratory syndrome coronavirus in stool samples. However, the detection rates were quite different in another report: only 26.2 and 68.0% between 1 and 2 weeks after disease onset [33]. It is suggested that the difference in positive rates is a consequence of variations in the RNA extraction method [34].
Serum, Plasma, and Whole Blood
Clinicians place great emphasis on the detection of bacteria and fungi in blood. Therefore, nucleic acid extraction from blood has become very important. Many researchers have found that there are numerous PCR inhibitors in blood culture bottles such as sodium polyanetholesulfonate (SPS) and hemins [35]. Millar and collaborators compared several commercial and in-house extraction methods used to detect bacteria and fungi in BacT/Alert blood culture bottles [36]. To reduce the detection time, the serum, plasma, or whole blood is used as a main specimen for detection of bacteria and fungi. Serum or plasma is more efficient and convenient than whole blood because whole blood includes many PCR inhibitors [37]. Most commercial kits showed a high recovery rate of pathogen DNA, but only those methods that used heat lysis with an alkali wash could remove PCR inhibitors. Detection of brucellosis was highly sensitive even though Brucellae are facultative intracellular pathogens [38]. Similarly, kits containing proteinase K showed better yield of Brucella in serum specimens [39]. However, to enhance the sensitivity of PCR amplification, whole blood is considered as a final target because it contains more pathogens than serum or plasma [40]. Although the nucleic acid kits were not developed to extract microbial DNA from whole blood, all commercial kits are able to do so [21]. In recent years, we have been able to use several automated systems to extract bacterial or fungal DNA from whole blood [30, 41, 42], although they are expensive. They are suitable for high-throughput detection [43].
It also is important to consider the concentrations of pathogens. The recovery of Toxoplasma gondii was similar for two DNA extraction techniques when using various PCR methods [44]. However, the results were different when there were low concentrations of tachyzoites in blood [45] vs. amniotic fluid [44]. We can see the similar result in Chlamydia pneumoniae detection from stools [34]. The positive rates were lowered when the RNA concentrations dropped and this confirms the clinical importance of the extraction methods used for stool samples.
The use of dried blood spots (DBSs) is an alternative to whole blood and is an important and common specimen used for the diagnosis of congenital infections [46]. It is different from whole blood in that the tiny specimen may contain very few causative pathogens. At present, many kinds of commercial or noncommercial DBSs are in use. Because the amount of blood is small, inadequate DNA extraction can be a problem and result in a low sensitivity. Several investigators attempted to detect CMV DNA in DBS using several extraction methods and found that the recovery of CMV DNA differed according to the extraction method used [46–50]. The lysis buffer used also can affect the yield of RNA from DBS, and column extraction methods revealed significant loss in RNA recovery [51].
Influence of Specific Pathogen
Even when we use clinical specimens to extract nucleic acid, we should recognize that recovery is influenced by the physical properties of the pathogen [44]. The effect will be greater if the method does not include proteinase K in the lysis step. The Apicomplexa phylum including Toxoplasma is well known to be resistant to detergent lysis [52].
Fungi are problematic when attempting to extract nucleic acid because it is difficult to break their cell walls in order to release the DNA [15, 42]. Moreover, the detection rates in certain clinical specimens such as whole blood are low because of the very low loads of fungal cells [53–55]. So the extraction once again is a crucial step and can determine the sensitivity of a particular PCR assay [56, 57]. To recover small amounts of fungal DNA from clinical specimens, a protocol should be established for an optimal extraction method. The QIAamp DNA blood kit was successful in extracting Candida DNA and was suitable to use with a TaqMan-based PCR assay, whereas all other kits tested failed to detect low amounts of Candida DNA [15]. In another study, the investigators were successful with all of the extraction methods used in their study, even though those kits were not specifically designed for the extraction of fungal DNA from whole blood [58]. We encounter a similar difficulty in extracting nucleic acid from Mycobacteria. Many researchers have tried to find optimal extraction methods for most clinically important specimens; the most appropriate method for each laboratory’s situation should be applied [59].
PCR Inhibitors
There are many factors that affect the efficiency of nucleic acid extraction, an important one being the presence of many kinds of inhibitors. It is well known that bile salts, hemoglobin, and polysaccharides can inhibit PCR [60, 61]. There also may be contaminating bacterial or fungal DNA in the reagents [39, 58, 62]. Most PCR assays can be influenced by reaction inhibitors and other contaminants, especially when the various clinical specimens containing these inhibitors are used as samples, and nucleic acid extraction thus becomes the crucial step that determines their influence [63]. In previous reports [29, 64], the presence of inhibitors was confirmed when the MagNA Pure Compact system was used for a principal nucleic acid extraction. In one other report [45], the authors compared the MagNA Pure Compact system and QIAamp DNA minikit for the detection of Toxoplasma DNA from blood. The sensitivity of PCR using the MagNA Pure Compact system was lower than that of the QIAamp DNA minikit, so the presence of inhibitors may have been responsible for the difference of sensitivity between the two methods. Moreover, the combination of the extraction kit and the master mix can make a difference in PCR performance in terms of inhibition [44]. We should also consider the fact that many human DNAs are mixed with relatively rare pathogen DNAs in clinical specimens, meaning abundant human DNA will be obtained during extraction for pathogen detection [65].
Measurement of DNA Quality
The classical method to check DNA purity is to measure the adsorption of UV light at 260 and 280 nm. The DNA content is proportional to the adsorption of UV light at 260, and adsorption at 280 nm reflects protein contamination. So, we can easily calculate the DNA purity using the OD260/OD280 ratio. In recent years, newly developed methods such as PicoGreen have been introduced and are becoming more popular in clinical laboratories, although the spectrophotometric method does have many advantages [66]. PicroGreen is based on the use of fluorescence and needs only a minute volume of sample.
Comparison of Nucleic Acid Extraction Methods
The method used for nucleic acid extraction differs greatly in clinical microbiology laboratories. When we deal with cultured bacteria to get genomic DNA, it is common to use simple heating, but this has many limitations and is not appropriate for use directly on clinical specimens. There are many reports comparing various extraction methods, including commercial kits, from various specimens for bacteria, virus, and fungi [21, 25, 26, 29, 30, 39, 42, 44, 67–71]. The methods can be divided into solution or column based according to differences of their principles, and most commercial extraction kits we use can be divided the same way. DNA recovery was better when a spin column method was used for extraction of C. pneumoniae DNA from vascular tissue [72]. However, DNA recovery ability can differ among kits even though all use the spin-column method as the principal tool [73]. So the method itself does not give assurance, and we should keep in mind that the DNA recovery can be different among various kits that use the similar principles.
Regardless of specific kits, specific companies, and their protocols, they have common steps in their procedures for optimal extraction [8]. Cell lysis must be the first step. After nucleoprotein complexes have been denatured, nucleases are inactivated. The contaminants are removed, and nucleic acid is purified. Even though these basic steps are not changed, there has been a vast alteration in nucleic acid extraction, namely, development of automated instrumentation. The method for the nucleic acid extraction can be divided into manual or automated, and this is an important point in the classification of nucleic acid extraction methods.
Manual Method
Many commercial kits have been developed for nucleic acid extraction. These kits are composed of a few reagents and are designed primarily for manual extraction. These kits are suitable for use in clinical laboratories and have replaced older in-house methods (Table 11.1). There are many publications that have evaluated the performance of these commercial nucleic acid extraction methods and compared them with conventional methods such as phenol–chloroform and the alkali wash/heat lysis [15, 36, 39, 58]. These manual commercial extraction kits show good performance for nucleic acid extraction compared with in-house methods.
Table 11.1.
Manual methods of nucleic acid extraction and purifications for rapid real-time PCR assays
| Kit/manufacturer/homepage | Technologic principle | Specimen throughput | Specimen type |
|---|---|---|---|
|
High Pure Roche Applied Science |
Nucleic acid capture by glass fiber fleece immobilized in a special plastic filter tube and subjected to centrifugation | 24 samples in 1 h | Serum, whole blood, plasma, urine, stool, sterile body fluids, respiratory tract specimens, swabs (genital, dermal) |
|
QIAamp Qiagen |
Nucleic acid capture by silica gel membrane placed in tube column and subjected to centrifugation or vacuum conditions |
24 samples in 1 h for DNA 24 samples in 1.5 h for RNA |
Respiratory tract specimens, plasma, stool, serum, whole blood, urine, sterile body fluids, swabs (nasal, fecal) |
|
IsoQuick Orca Research |
Nucleic acid is partitioned into an aqueous phase and then precipitated with ethanol and resuspended in water or buffer |
24 samples in 1 h for DNA 24 samples in 2 h for RNA |
Plasma, whole blood, stool, respiratory tract specimens, sterile body fluids, swabs (dermal, fecal, genital) |
|
IsoCode Stix Schleichar & Schuell |
DNA bound to matrix and released by simple water and heat elution | Processed individually | Whole blood |
Reprinted with permission from Espy MJ et al. (2006) Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin Microbiol Rev 19(1):165–256
Their ability to recover pure DNA and to remove the contaminants, including proteins, is of great importance, but there are also important differences in cost, time demands, labor intensity, and principles of each method. Given these differences, there are numerous choices available; the most appropriate method for a particular laboratory should be selected. Both liquid- and column-based methods are commonly used at present.
Most of these kits use noncorrosive agents, so they are safe and easy to deal with. However, there still are some pitfalls. Although the entire extraction procedure is standardized by the manufacturer’s manual, the process is still complicated and is performed manually. Therefore, problems with reproducibility by different persons can be seen. To minimize such reproducibility problems, it is necessary to provide continuous training and quality control [5]. Ethanol is used for precipitation of the nucleic acid in some manual kits, and the inhibition of PCR can occur when this ethanol is not completely removed [74]. The manual extraction method has been designated as a high-complexity test according to Clinical Laboratory improvement Amendments of 1988 (CLIA ’88) regulations (http://www.cms.hhs.gov/clia/), so only trained laboratory personnel can perform it. Moreover, the number of specimens for molecular testing is increasing, which places more stress on the technologists who are processing these specimens. This can affect the accuracy of tests as a result of a processing mistake or by contamination attributable to the complex processing procedure.
Automatic Methods
The introduction of commercial manual extraction kits was a valuable adjunct for molecular testing in the clinical microbiology laboratory. However, the manual extraction method is still labor-intensive and time-consuming and requires a well-trained technologist. There were also reports of outbreaks of cross contamination when the samples were treated at the same time [39, 75]. In recent years, many manufacturers developed and launched various automated extraction instruments; these instruments vary in principle, procedure time, cost, and size (Table 11.2). The automated extraction instruments are easily divided by their workload capacity; the most appropriate instrument thus can be selected according to each laboratory’s workload situation from low-throughput instruments such as MagNA Pure compact, NucliSens miniMAG, and BioRobot EZ1 systems to medium- to high-throughput instruments such as the MagNA Pure LC and BioRobot M48/9604 [76].
Table 11.2.
Automated nucleic acid extraction methods and their characteristics
| Manufacturer | Instrument | Technologic principle | Specimens/run (batch size) |
|---|---|---|---|
| BioMerieux | Easy MAG | Magnetic bead extraction | 24 |
| BioMerieux | MiniMAG | Magnetic bead extraction | 12 |
| Roche | MagNA Pure LC | Magnetic bead extraction | 32 (1–32) |
| Roche | MagNA Pure Compact | Magnetic bead extraction | 8 (1–8) |
| QIAGEN | BioRobot Universal/BioRobot MDx |
Vacuum-based and/or magnetic bead extraction |
96 (8) |
| QIAGEN | QIAsymphony SP | Magnetic bead extraction | 96 (1–24) |
| QIAGEN | QIAxtractor | Vacuum-based extraction | 96 (8–96) |
| bioneer | ExiPrep 16 Plus, ExiPrep 16 Pro | Magnetic bead extraction | 16 (1–16) |
| Abbott Molecular | M2000sp | Magnetic bead extraction | 96 (24) |
| Eppendorf | epMotion 5070/epMotion 5075 | Vacuum-based and/or magnetic bead extraction | 384 (1–384) |
| Fisher Scientific | Thermo KingFisher Flex | Magnetic bead extraction | 1–96 (1–96) |
| Beckman Coulter | Biomek NXp Span 8/Biomek NXp Multichannel 96 | Magnetic bead extraction | 96 (8) |
The automated extraction methods have many advantages compared with manual methods, and these instruments have proven to be very useful adjuncts for PCR testing. The steps proceed automatically with fast turnaround. This reduces the working time and avoids mistakes such as pipetting error. Many specimens can be analyzed at the same time [43]. It provides constant reproducibility for recovery of nucleic acid, avoiding person-to-person variations seen with manual extraction methods. It can also diminish cross contamination by reducing unnecessary handling steps and avoiding mistakes by personnel [5]. It has an additional advantage for quality control monitoring, whereas the manual method needs intensive work for quality control monitoring [77].
Since many automated extraction instruments and kits have been developed, numerous evaluations have been reported [67, 78–81]. These studies included various kinds of extraction kits, clinical specimens, and pathogens. Even though some reports showed high detection rates with manual extraction method, the results of automated extraction methods were similar to or better than those of manual methods in most of these studies [28, 29, 45, 64, 67, 78–81].
For example, Cook et al. [28] evaluated the performance of four commercial automated extraction kits for the detection of viruses using clinical specimens. They compared viral yield using cultured cells containing CMV, EBV, HSV, BK, VZV, or HHV-8. The procedures of the kits were similar, and DNA extraction was successful with all kits. There were some variations of viral yields, which were only 50% compared with those of the manual kits. The differences of yields are not of great significance if we consider the biologic range of viral loads in clinical practice as the manufacturer’s recommendation. In the study of multiplex molecular detection of infection in septic patients using automated extraction, the recovery of DNA was similar to that of the conventional manual method at the point of maximal binding surface of MagNA pure nanoparticles [76]. Many leukocytes are present in the blood sample, so the final DNA amounts recovered by the manual method can be about three times those obtained by MagNA pure extraction [82]. We can face different results for the evaluation of automated extraction instrument. The efficiency of the NucliSens easyMAG was low for CMV [49] and respiratory viruses [29]; however, in another study, the NucliSens miniMAG showed the best results for the isolation of polyomavirus BK virus and the human beta-actin gene from urine specimens [2] and severe respiratory syndrome coronavirus RNA in stool samples [34].
The most important drawback we must consider is the economic aspect of the automated methods. To use such a system, an expensive instrument and extraction reagents, including disposables, are needed. Sometimes, this increased cost for the automated system precludes its use. However, the influenza A (H1N1) pandemic in 2009 demonstrated the value of an automated extraction system. At that time, requests for influenza A (H1N1) identification were increasing rapidly, and many laboratories could not perform all the requested tests because of limited personnel. This made clear the usefulness of the automated extraction system. Although the detection rates and yield recovery are the most important factors in selecting commercial extraction methods, other factors, including ease of use and cost per extraction, also must be considered [28].
Conclusion
In recent years, advanced molecular tests have come to occupy an important position in the diagnosis of infectious diseases because of their high sensitivity and specificity [83, 84]. Efficient nucleic acid extraction is essential. The optimal extraction method should fulfill the following conditions: speed, short working time, cost-effectiveness, high sensitivity and specificity, good reproducibility, and safety [1]. It will be ideal for use with all kinds of specimens and pathogens. However, at present, there is no one extraction method that satisfies all these conditions. On the contrary, there are significant differences between extraction kits because nucleic acids can be different in specific clinical specimens. So, it is important to carefully evaluate the performance of any extraction method used in the clinical microbiology laboratory.
Contributor Information
Yi-Wei Tang, Phone: 212-639-8181, FAX: 615343-6160, Email: tangy@mskcc.org.
Charles W. Stratton, Email: charles.stratton@Vanderbilt.Edu
Jeong Hwan Shin, Email: jhsmile@inje.ac.kr.
References
- 1.Mancini N, Carletti S, Ghidoli N, Cichero P, Burioni R, Clementi M. The era of molecular and other non-culture-based methods in diagnosis of sepsis. Clin Microbiol Rev. 2010;23:235–251. doi: 10.1128/CMR.00043-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang YW, Sefers SE, Li H, Kohn DJ, Procop GW. Comparative evaluation of three commercial systems for nucleic acid extraction from urine specimens. J Clin Microbiol. 2005;43:4830–4833. doi: 10.1128/JCM.43.9.4830-4833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anwar A, Wan G, Chua KB, August JT, Too HP. Evaluation of pre-analytical variables in the quantification of dengue virus by real-time polymerase chain reaction. J Mol Diagn. 2009;11:537–542. doi: 10.2353/jmoldx.2009.080164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippi G, Guidi GC, Mattiuzzi C, Plebani M. Preanalytical variability: the dark side of the moon in laboratory testing. Clin Chem Lab Med. 2006;44:358–365. doi: 10.1515/CCLM.2006.073. [DOI] [PubMed] [Google Scholar]
- 5.Espy MJ, Uhl JR, Sloan LM, et al. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin Microbiol Rev. 2006;19:165–256. doi: 10.1128/CMR.19.1.165-256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price CW, Leslie DC, Landers JP. Nucleic acid extraction techniques and application to the microchip. Lab Chip. 2009;9:2484–2494. doi: 10.1039/b907652m. [DOI] [PubMed] [Google Scholar]
- 8.Tan SC, Yiap BC. DNA, RNA, and protein extraction: the past and the present. J Biomed Biotechnol. 2009;2009:574398. doi: 10.1155/2009/574398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meselson M, Stahl FW, Vinograd J. Equilibrium sedimentation of macromolecules in density gradients. Proc Natl Acad Sci U S A. 1957;43:581–588. doi: 10.1073/pnas.43.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radloff R, Bauer W, Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967;57:1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carr SM, Griffith OM. Rapid isolation of animal mitochondrial DNA in a small fixed-angle rotor at ultrahigh speed. Biochem Genet. 1987;25:385–390. doi: 10.1007/BF00554547. [DOI] [PubMed] [Google Scholar]
- 12.Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc. 2006;1:581–585. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- 13.Buckingham L, Flaws MI. Molecular diagnostics; fundamentals, methods, & clinical applications. Philadelphia, PA: F.A. Davis; 2007. [Google Scholar]
- 14.Sambrook J, Russel D. Molecular cloning: a laboratory manual, vol. 3. 3. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 15.Maaroufi Y, Ahariz N, Husson M, Crokaert F. Comparison of different methods of isolation of DNA of commonly encountered Candida species and its quantitation by using a real-time PCR-based assay. J Clin Microbiol. 2004;42:3159–3163. doi: 10.1128/JCM.42.7.3159-3163.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randhawa P, Ho A, Shapiro R, et al. Correlates of quantitative measurement of BK polyomavirus (BKV) DNA with clinical course of BKV infection in renal transplant patients. J Clin Microbiol. 2004;42:1176–1180. doi: 10.1128/JCM.42.3.1176-1180.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCormick RM. A solid-phase extraction procedure for DNA purification. Anal Biochem. 1989;181:66–74. doi: 10.1016/0003-2697(89)90394-1. [DOI] [PubMed] [Google Scholar]
- 18.Meijer A, van der Vliet JA, Schouls LM, de Vries A, Roholl PJ, Ossewaarde JM. Detection of microorganisms in vessel wall specimens of the abdominal aorta: development of a PCR assay in the absence of a gold standard. Res Microbiol. 1998;149:577–583. doi: 10.1016/S0923-2508(99)80005-9. [DOI] [PubMed] [Google Scholar]
- 19.Gjerse DT, Hoang L, Hornby D. RNA purification and analysis; sample preparation, extraction, chromatography. 1. Weinheim: Wiley-VCH; 2009. [Google Scholar]
- 20.Coolbear T, Whittaker JM, Daniel RM. The effect of metal ions on the activity and thermostability of the extracellular proteinase from a thermophilic Bacillus, strain EA.1. Biochem J. 1992;287(Pt 2):367–374. doi: 10.1042/bj2870367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith K, Diggle MA, Clarke SC. Comparison of commercial DNA extraction kits for extraction of bacterial genomic DNA from whole-blood samples. J Clin Microbiol. 2003;41:2440–2443. doi: 10.1128/JCM.41.6.2440-2443.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espy MJ, Ross TK, Teo R, et al. Evaluation of LightCycler PCR for implementation of laboratory diagnosis of herpes simplex virus infections. J Clin Microbiol. 2000;38:3116–3118. doi: 10.1128/jcm.38.8.3116-3118.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espy MJ, Uhl JR, Mitchell PS, et al. Diagnosis of herpes simplex virus infections in the clinical laboratory by LightCycler PCR. J Clin Microbiol. 2000;38:795–799. doi: 10.1128/jcm.38.2.795-799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sloan LM, Uhl JR, Vetter EA, et al. Comparison of the Roche LightCycler vanA/vanB detection assay and culture for detection of vancomycin-resistant enterococci from perianal swabs. J Clin Microbiol. 2004;42:2636–2643. doi: 10.1128/JCM.42.6.2636-2643.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beuselinck K, van Ranst M, van Eldere J. Automated extraction of viral-pathogen RNA and DNA for high-throughput quantitative real-time PCR. J Clin Microbiol. 2005;43:5541–5546. doi: 10.1128/JCM.43.11.5541-5546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dundas N, Leos NK, Mitui M, Revell P, Rogers BB. Comparison of automated nucleic acid extraction methods with manual extraction. J Mol Diagn. 2008;10:311–316. doi: 10.2353/jmoldx.2008.070149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerna G, Lilleri D. Monitoring transplant patients for human cytomegalovirus: diagnostic update. Herpes. 2006;13:4–11. [PubMed] [Google Scholar]
- 28.Cook L, Atienza EE, Bagabag A, Obrigewitch RM, Jerome KR. Comparison of methods for extraction of viral DNA from cellular specimens. Diagn Microbiol Infect Dis. 2009;64:37–42. doi: 10.1016/j.diagmicrobio.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Chan KH, Yam WC, Pang CM, et al. Comparison of the NucliSens easyMAG and Qiagen BioRobot 9604 nucleic acid extraction systems for detection of RNA and DNA respiratory viruses in nasopharyngeal aspirate samples. J Clin Microbiol. 2008;46:2195–2199. doi: 10.1128/JCM.00315-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loens K, Bergs K, Ursi D, Goossens H, Ieven M. Evaluation of NucliSens easyMAG for automated nucleic acid extraction from various clinical specimens. J Clin Microbiol. 2007;45:421–425. doi: 10.1128/JCM.00894-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mengelle C, Legrand-Abravanel F, Mansuy JM, Barthe C, Da Silva I, Izopet J. Comparison of two highly automated DNA extraction systems for quantifying Epstein-Barr virus in whole blood. J Clin Virol. 2008;43:272–276. doi: 10.1016/j.jcv.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Peiris JS. Severe acute respiratory syndrome (SARS) J Clin Virol. 2003;28:245–247. doi: 10.1016/j.jcv.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan PK, To WK, Ng KC, et al. Laboratory diagnosis of SARS. Emerg Infect Dis. 2004;10:825–831. doi: 10.3201/eid1005.030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrich A, Mahony J, Chong S, et al. Multicenter comparison of nucleic acid extraction methods for detection of severe acute respiratory syndrome coronavirus RNA in stool specimens. J Clin Microbiol. 2006;44:2681–2688. doi: 10.1128/JCM.02460-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fredricks DN, Relman DA. Improved amplification of microbial DNA from blood cultures by removal of the PCR inhibitor sodium polyanetholesulfonate. J Clin Microbiol. 1998;36:2810–2816. doi: 10.1128/jcm.36.10.2810-2816.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Millar BC, Jiru X, Moore JE, Earle JA. A simple and sensitive method to extract bacterial, yeast and fungal DNA from blood culture material. J Microbiol Methods. 2000;42:139–147. doi: 10.1016/S0167-7012(00)00174-3. [DOI] [PubMed] [Google Scholar]
- 37.Queipo-Ortuno MI, Garcia-Ordonez MA, Colmenero JD, Morata P. Hydrogen peroxide improves the efficiency of a peripheral blood PCR assay for diagnosis of human brucellosis. Biotechniques. 1999;27:248–250, 252. doi: 10.2144/99272bm06. [DOI] [PubMed] [Google Scholar]
- 38.Zerva L, Bourantas K, Mitka S, Kansouzidou A, Legakis NJ. Serum is the preferred clinical specimen for diagnosis of human brucellosis by PCR. J Clin Microbiol. 2001;39:1661–1664. doi: 10.1128/JCM.39.4.1661-1664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Queipo-Ortuno MI, Tena F, Colmenero JD, Morata P. Comparison of seven commercial DNA extraction kits for the recovery of Brucella DNA from spiked human serum samples using real-time PCR. Eur J Clin Microbiol Infect Dis. 2008;27:109–114. doi: 10.1007/s10096-007-0409-y. [DOI] [PubMed] [Google Scholar]
- 40.Ohlin A, Backman A, Bjorkqvist M, Molling P, Jurstrand M, Schollin J. Real-time PCR of the 16S-rRNA gene in the diagnosis of neonatal bacteraemia. Acta Paediatr. 2008;97:1376–1380. doi: 10.1111/j.1651-2227.2008.00924.x. [DOI] [PubMed] [Google Scholar]
- 41.Klingspor L, Jalal S. Molecular detection and identification of Candida and Aspergillus spp. from clinical samples using real-time PCR. Clin Microbiol Infect. 2006;12:745–753. doi: 10.1111/j.1469-0691.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- 42.Loeffler J, Schmidt K, Hebart H, Schumacher U, Einsele H. Automated extraction of genomic DNA from medically important yeast species and filamentous fungi by using the MagNA Pure LC system. J Clin Microbiol. 2002;40:2240–2243. doi: 10.1128/JCM.40.6.2240-2243.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stormer M, Kleesiek K, Dreier J. High-volume extraction of nucleic acids by magnetic bead technology for ultrasensitive detection of bacteria in blood components. Clin Chem. 2007;53:104–110. doi: 10.1373/clinchem.2006.070987. [DOI] [PubMed] [Google Scholar]
- 44.Yera H, Filisetti D, Bastien P, Ancelle T, Thulliez P, Delhaes L. Multicenter comparative evaluation of five commercial methods for toxoplasma DNA extraction from amniotic fluid. J Clin Microbiol. 2009;47:3881–3886. doi: 10.1128/JCM.01164-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edvinsson B, Jalal S, Nord CE, Pedersen BS, Evengard B. DNA extraction and PCR assays for detection of Toxoplasma gondii. APMIS. 2004;112:342–348. doi: 10.1111/j.1600-0463.2004.apm1120604.x. [DOI] [PubMed] [Google Scholar]
- 46.de Vries JJ, Claas EC, Kroes AC, Vossen AC. Evaluation of DNA extraction methods for dried blood spots in the diagnosis of congenital cytomegalovirus infection. J Clin Virol. 2009;46(Suppl 4):S37–S42. doi: 10.1016/j.jcv.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Barbi M, Binda S, Primache V, et al. Cytomegalovirus DNA detection in Guthrie cards: a powerful tool for diagnosing congenital infection. J Clin Virol. 2000;17:159–165. doi: 10.1016/S1386-6532(00)00089-5. [DOI] [PubMed] [Google Scholar]
- 48.Scanga L, Chaing S, Powell C, et al. Diagnosis of human congenital cytomegalovirus infection by amplification of viral DNA from dried blood spots on perinatal cards. J Mol Diagn. 2006;8:240–245. doi: 10.2353/jmoldx.2006.050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soetens O, Vauloup-Fellous C, Foulon I, et al. Evaluation of different cytomegalovirus (CMV) DNA PCR protocols for analysis of dried blood spots from consecutive cases of neonates with congenital CMV infections. J Clin Microbiol. 2008;46:943–946. doi: 10.1128/JCM.01391-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vauloup-Fellous C, Ducroux A, Couloigner V, et al. Evaluation of cytomegalovirus (CMV) DNA quantification in dried blood spots: retrospective study of CMV congenital infection. J Clin Microbiol. 2007;45:3804–3806. doi: 10.1128/JCM.01654-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monleau M, Montavon C, Laurent C, et al. Evaluation of different RNA extraction methods and storage conditions of dried plasma or blood spots for human immunodeficiency virus type 1 RNA quantification and PCR amplification for drug resistance testing. J Clin Microbiol. 2009;47:1107–1118. doi: 10.1128/JCM.02255-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mann T, Gaskins E, Beckers C. Proteolytic processing of TgIMC1 during maturation of the membrane skeleton of Toxoplasma gondii. J Biol Chem. 2002;277:41240–41246. doi: 10.1074/jbc.M205056200. [DOI] [PubMed] [Google Scholar]
- 53.Ahmad S, Khan Z, Mustafa AS, Khan ZU. Seminested PCR for diagnosis of candidemia: comparison with culture, antigen detection, and biochemical methods for species identification. J Clin Microbiol. 2002;40:2483–2489. doi: 10.1128/JCM.40.7.2483-2489.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Challier S, Boyer S, Abachin E, Berche P. Development of a serum-based Taqman real-time PCR assay for diagnosis of invasive aspergillosis. J Clin Microbiol. 2004;42:844–846. doi: 10.1128/JCM.42.2.844-846.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White PL, Shetty A, Barnes RA. Detection of seven Candida species using the Light-Cycler system. J Med Microbiol. 2003;52:229–238. doi: 10.1099/jmm.0.05049-0. [DOI] [PubMed] [Google Scholar]
- 56.Bretagne S, Costa JM. Towards a molecular diagnosis of invasive aspergillosis and disseminated candidosis. FEMS Immunol Med Microbiol. 2005;45:361–368. doi: 10.1016/j.femsim.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Fredricks DN, Smith C, Meier A. Comparison of six DNA extraction methods for recovery of fungal DNA as assessed by quantitative PCR. J Clin Microbiol. 2005;43:5122–5128. doi: 10.1128/JCM.43.10.5122-5128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Metwally L, Fairley DJ, Coyle PV, et al. Improving molecular detection of Candida DNA in whole blood: comparison of seven fungal DNA extraction protocols using real-time PCR. J Med Microbiol. 2008;57:296–303. doi: 10.1099/jmm.0.47617-0. [DOI] [PubMed] [Google Scholar]
- 59.Hosek J, Svastova P, Moravkova M, Vavlik I, Bartos M. Methods of mycobacterial DNA isolation from different biological material: a review. Vet Med. 2006;51:180–192. [Google Scholar]
- 60.Monteiro L, Bonnemaison D, Vekris A, et al. Complex polysaccharides as PCR inhibitors in feces: Helicobacter pylori model. J Clin Microbiol. 1997;35:995–998. doi: 10.1128/jcm.35.4.995-998.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Widjojoatmodjo MN, Fluit AC, Torensma R, Verdonk GP, Verhoef J. The magnetic immuno polymerase chain reaction assay for direct detection of salmonellae in fecal samples. J Clin Microbiol. 1992;30:3195–3199. doi: 10.1128/jcm.30.12.3195-3199.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Radstrom P, Knutsson R, Wolffs P, Lovenklev M, Lofstrom C. Pre-PCR processing: strategies to generate PCR-compatible samples. Mol Biotechnol. 2004;26:133–146. doi: 10.1385/MB:26:2:133. [DOI] [PubMed] [Google Scholar]
- 63.Bastien P, Procop GW, Reischl U. Quantitative real-time PCR is not more sensitive than “conventional” PCR. J Clin Microbiol. 2008;46:1897–1900. doi: 10.1128/JCM.02258-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schuurman T, de Boer R, Patty R, Kooistra-Smid M, van Zwet A. Comparative evaluation of in-house manual, and commercial semi-automated and automated DNA extraction platforms in the sample preparation of human stool specimens for a Salmonella enterica 5′-nuclease assay. J Microbiol Methods. 2007;71:238–245. doi: 10.1016/j.mimet.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 65.Handschur M, Karlic H, Hertel C, Pfeilstocker M, Haslberger AG. Preanalytic removal of human DNA eliminates false signals in general 16S rDNA PCR monitoring of bacterial pathogens in blood. Comp Immunol Microbiol Infect Dis. 2009;32:207–219. doi: 10.1016/j.cimid.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 66.Ahn SJ, Costa J, Emanuel JR. PicoGreen quantitation of DNA: effective evaluation of samples pre- or post-PCR. Nucleic Acids Res. 1996;24:2623–2625. doi: 10.1093/nar/24.13.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knepp JH, Geahr MA, Forman MS, Valsamakis A. Comparison of automated and manual nucleic acid extraction methods for detection of enterovirus RNA. J Clin Microbiol. 2003;41:3532–3536. doi: 10.1128/JCM.41.8.3532-3536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Loffler J, Hebart H, Schumacher U, Reitze H, Einsele H. Comparison of different methods for extraction of DNA of fungal pathogens from cultures and blood. J Clin Microbiol. 1997;35:3311–3312. doi: 10.1128/jcm.35.12.3311-3312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mygind T, Ostergaard L, Birkelund S, Lindholt JS, Christiansen G. Evaluation of five DNA extraction methods for purification of DNA from atherosclerotic tissue and estimation of prevalence of Chlamydia pneumoniae in tissue from a Danish population undergoing vascular repair. BMC Microbiol. 2003;3:19. doi: 10.1186/1471-2180-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verheyden B, Thielemans A, Rombaut B, Kronenberger P. RNA extraction for quantitative enterovirus RT-PCR: comparison of three methods. J Pharm Biomed Anal. 2003;33:819–823. doi: 10.1016/S0731-7085(03)00312-1. [DOI] [PubMed] [Google Scholar]
- 71.Wilson D, Yen-Lieberman B, Reischl U, Warshawsky I, Procop GW. Comparison of five methods for extraction of Legionella pneumophila from respiratory specimens. J Clin Microbiol. 2004;42:5913–5916. doi: 10.1128/JCM.42.12.5913-5916.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berg HF, Maraha B, Bergmans AM, van der Zee A, Kluytmans JA, Peeters MF. Extraction of Chlamydia pneumoniae DNA from vascular tissue for use in PCR: an evaluation of four procedures. Clin Microbiol Infect. 2003;9:135–139. doi: 10.1046/j.1469-0691.2003.00470.x. [DOI] [PubMed] [Google Scholar]
- 73.Read SJ. Recovery efficiencies on nucleic acid extraction kits as measured by quantitative LightCycler PCR. Mol Pathol. 2001;54:86–90. doi: 10.1136/mp.54.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Valentine-Thon E. Quality control in nucleic acid testing—where do we stand? J Clin Virol. 2002;25(Suppl 3):S13–S21. doi: 10.1016/S1386-6532(02)00196-8. [DOI] [PubMed] [Google Scholar]
- 75.Schabereiter-Gurtner C, Nehr M, Apfalter P, Makristathis A, Rotter ML, Hirschl AM. Evaluation of a protocol for molecular broad-range diagnosis of culture-negative bacterial infections in clinical routine diagnosis. J Appl Microbiol. 2008;104:1228–1237. doi: 10.1111/j.1365-2672.2007.03648.x. [DOI] [PubMed] [Google Scholar]
- 76.Regueiro BJ, Varela-Ledo E, Martinez-Lamas L, et al. Automated extraction improves multiplex molecular detection of infection in septic patients. PLoS One. 2010;5:e13387. doi: 10.1371/journal.pone.0013387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garrett PE. Quality control for nucleic acid tests: common ground and special issues. J Clin Virol. 2001;20:15–21. doi: 10.1016/S1386-6532(00)00150-5. [DOI] [PubMed] [Google Scholar]
- 78.Espy MJ, Rys PN, Wold AD, et al. Detection of herpes simplex virus DNA in genital and dermal specimens by LightCycler PCR after extraction using the IsoQuick, MagNA Pure, and BioRobot 9604 methods. J Clin Microbiol. 2001;39:2233–2236. doi: 10.1128/JCM.39.6.2233-2236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Germer JJ, Lins MM, Jensen ME, et al. Evaluation of the MagNA pure LC instrument for extraction of hepatitis C virus RNA for the COBAS AMPLICOR Hepatitis C Virus Test, version 2.0. J Clin Microbiol. 2003;41:3503–3508. doi: 10.1128/JCM.41.8.3503-3508.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gobbers E, Oosterlaken TA, van Bussel MJ, Melsert R, Kroes AC, Claas EC. Efficient extraction of virus DNA by NucliSens Extractor allows sensitive detection of hepatitis B virus by PCR. J Clin Microbiol. 2001;39:4339–4343. doi: 10.1128/JCM.39.12.4339-4343.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi PY, Kauffman EB, Ren P, et al. High-throughput detection of West Nile virus RNA. J Clin Microbiol. 2001;39:1264–1271. doi: 10.1128/JCM.39.4.1264-1271.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller DN, Bryant JE, Madsen EL, Ghiorse WC. Evaluation and optimization of DNA extraction and purification procedures for soil and sediment samples. Appl Environ Microbiol. 1999;65:4715–4724. doi: 10.1128/aem.65.11.4715-4724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bretagne S, Costa JM. Towards a nucleic acid-based diagnosis in clinical parasitology and mycology. Clin Chim Acta. 2006;363:221–228. doi: 10.1016/j.cccn.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 84.Schuurman T, van Breda A, de Boer R, et al. Reduced PCR sensitivity due to impaired DNA recovery with the MagNA Pure LC total nucleic acid isolation kit. J Clin Microbiol. 2005;43:4616–4622. doi: 10.1128/JCM.43.9.4616-4622.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


