Abstract
Membrane fusion is not spontaneous. Therefore, enveloped viruses have evolved membrane-fusion mediating glycoproteins that, once activated, refold, and release energy that fuses viral and cellular membranes. The influenza A virus hemagglutinin (HA) protein is a prototypic structural class I viral fusion glycoprotein that, once primed by proteolytic cleavage, is activated by endosomal low pH to form a fusogenic “leash-in-grooves” hairpin structure. Low-pH induced HA protein refolding is an irreversible process, so acid exposure in the absence of a target membrane leads to virus inactivation. The HA proteins of diverse influenza virus subtypes isolated from a variety of species differ in their acid stabilities, or pH values at which irreversible HA protein conformational changes are triggered. Recently, efficient replication of highly pathogenic avian influenza (HPAI) viruses such as H5N1 in avian species has been associated with a relatively high HA activation pH. In contrast, a decrease in H5N1 HA activation pH has been shown to enhance replication and airborne transmission in mammals. Mutations that alter the acid stabilities of H1 and H3 HA proteins have also been discovered that influence the amantadine susceptibilities, replication rates, and pathogenicities of human influenza viruses. An understanding of the role of HA acid stability in influenza virus biology is expected to aid in identifying emerging viruses with increased pandemic potential and assist in developing live attenuated virus vaccines. Acid-induced HA protein activation, which has provided a paradigm for protein-mediated membrane fusion, is now identified as a novel determinant of influenza virus biology.
Keywords: Influenza Virus, Membrane Fusion, Avian Influenza Virus, Highly Pathogenic Avian Influenza, Severe Acute Respiratory Syndrome
Introduction
The influenza A viruses are negative-strand RNA viruses of the Orthomyxoviridae family. They encode a variety of proteins from eight gene segments (Shaw and Palese 2013): PB2, PB1, PA, HA, NP, NA, M, and NS. The HA and neuraminidase (NA) envelope glycoproteins and the M2 ion channel are embedded in the host cell-derived viral membrane. Internal proteins include the M1 matrix protein, the polymerase proteins (PB1, PB2, and PA), the nucleoprotein (NP), and the NEP/NS2 protein. Nonstructural accessory proteins include NS1 and the more recently discovered proteins PB1-N40 (Wise et al. 2009), PA-X (Jagger et al. 2012), PB1-F2 (Chen et al. 2001), M42 (Wise et al. 2012), NS3 (Mohammed Selman et al. 2012), and PA-N155 and PA-N182 (Muramoto et al. 2013).
Diverse influenza A viruses circulate in a reservoir of wild aquatic birds including 16 HA and 9 NA subtypes (Krauss and Webster 2010). Two additional HA (H17 and H18) and NA (N10 and N11) subtypes have recently been discovered in influenza viruses isolated from bats (Tong et al. 2012, 2013). Avian influenza viruses occasionally infect non-avian species including humans, causing limited outbreaks or global pandemics (Russell and Webster 2005). The replication efficiency, transmissibility, and pathogenicity of a given influenza virus in a particular host depend on multiple viral and host factors (Salomon and Webster 2009; Belser et al. 2010; Fukuyama and Kawaoka 2011; Sorrell et al. 2011). The focus of this review is on the role of the HA protein in viral entry and influenza virus biology with a focus on the acid stability of the HA protein. The HA protein is a prototypic structural class I viral fusion glycoprotein that is triggered by low pH in the endosome to undergo irreversible conformational changes that cause membrane fusion. The HA proteins from circulating viruses differ in the pH required for fusion activation, and recent studies identify HA acid stability as one of several important factors in the interspecies adaptation and evolution of influenza A viruses.
Molecular and Cell Biology of the HA Protein
Hemagglutinin (HA) Protein Synthesis and Maturation
As with the other influenza viral genes, HA mRNA is transcribed in the nucleus and exported to the cytoplasm (Shaw and Palese 2013). The HA0 precursor protein is a type I integral membrane protein. HA0 contains an N-terminal signal sequence (which targets protein synthesis to the ER before being cleaved and released), an ~513-residue ectodomain, an ~27-residue transmembrane (TM) domain, and an ~11-residue cytoplasmic tail (CT) (Fig. 1). In the presence of chaperones in the ER, HA0 monomers are extensively folded and form intramolecular disulfide bonds before forming a noncovalently linked homotrimeric complex (Copeland et al. 1988). N-linked glycosylation contributes to protein folding in the ER (Hebert et al. 1995). As the HA protein trafficks intracellularly to the cell surface, N-linked glycosylation sites undergo maturation and free cysteine residues in the TM domain and CT are acylated with fatty acids (Naeve and Williams 1990; Veit et al. 1991; Steinhauer et al. 1991b).
Fig. 1.

Alignment of HA protein sequences with key features identified. Residues are identified by H3 numbering above the alignment and real numbering (starting with methionine 1) to the right of the sequences. Alpha helical—(cylinders) and beta strand—(arrows) secondary structures are included above the alignments. Both prefusion and postfusion secondary structures are shown in HA2 (they are equivalent in HA1). Fusion (red), esterase (yellow), and receptor-binding (blue) subdomains are color-coded in the secondary structures. Signal peptide, fusion peptide, transmembrane (TM), and cytoplasmic tail (CT) regions are also identified. Residues governing HA acid stability (Table 1) are identified in the primary sequence by yellow boxes. Sequences are from A/Aichi/2/68/X-31 (H3N2), A/Shanghai/02/2013 (H7N9), A/California/04/2009 (H1N1), and A/Vietnam/1203/04 (H5N1)
The HA0 precursor protein is incapable of causing membrane fusion, and proteolytic cleavage into an HA1/HA2 complex is required to prime the protein into a fusion-competent form (Steinhauer 1999). The HA0 proteins from HPAI viruses contain a polybasic (arginine and lysine) cleavage site with an R-X-R/K-R sequence that is recognized intracellularly in the trans-Golgi network by ubiquitously expressed subtilisin-like enzymes such as furin and PC6 (Garten et al. 1981; Webster and Rott 1987; Vey et al. 1992; Stieneke-Gröber et al. 1992). For human and low pathogenic avian influenza ( LPAI) viruses, HA0 is cleaved extracellularly by soluble trypsin-like proteases including tryptase Clara (Kido et al. 1992), mini-plasmin (Murakami et al. 2001), and ectopic anionic trypsin I (Towatari et al. 2002). More recently, cell-associated serine proteases have also been discovered to cleave monobasic HA0 proteins including TMPRSS2, human airway trypsin-like (HAT) protease (Böttcher et al. 2006), and TMPRSS4 (Chaipan et al. 2009). MSPL and TMPRSS13 serine proteases have been found to cleave HA proteins from HPAI isolates (Okumura et al. 2010).
Virus Assembly and Budding
At the plasma membrane, the HA and NA glycoproteins associate with lipid rafts while the M2 ion channel is largely excluded (Takeda et al. 2003; Leser and Lamb 2005). Lipid rafts are variable-sized regions of the plasma membrane enriched with cholesterol and sphingolipid (Lingwood and Simons 2010) that serve as a platform for virus assembly and budding (Suomalainen 2002; Rossman and Lamb 2011) before M2-mediated scission (Rossman et al. 2010). The NA protein desialyates cellular and viral glycoconjugates, enabling efficient release of progeny virions from the host cell and preventing HA-mediated virion–virion aggregation (Compans et al. 1969; Palese et al. 1974). Efficient influenza virus replication appears to require an optimal balance between HA receptor-binding and NA receptor-destroying activities, which can be disturbed by reassortment, interspecies transmission, or NA-inhibitor treatment [reviewed in (Wagner et al. 2002)]. A role for NA enzymatic activity in HA-mediated membrane fusion has also been described (Huang et al. 1980). Inhibition of NA activity decreases the pH required to activate the HA proteins of some H5N1 isolates (Reed et al. 2010), and the absence of the NA protein results in decreased membrane fusion by an H1N1 HA protein (Su et al. 2009). The influence of NA activity on membrane fusion activity may be limited to specific isolates and its mechanism is unknown, although it may include destabilizing the HA protein via modification of HA glycosylation sites.
HA Protein Prefusion Structures
X-ray crystallographic structures of bromelain-released HA ectodomain from A/Aichi/68 (H3N2) in its HA0 uncleaved form (Chen et al. 1998) and its fusion-primed HA1/HA2 form (Wilson et al. 1981) reveal at atomic resolution the prefusion conformation of this prototypic structural class I viral fusion protein (Fig. 2a). Tethered to the viral envelope is the more highly conserved fusion (F) domain (also known as stalk or stem). The F domain is trapped in a high-energy conformation in part by the presence of the membrane-distal globular receptor-binding domain (RBD) head domains. The RBD consists of receptor-binding and vestigial esterase subdomains (identified in Fig. 1) that were most likely inserted into a primordial F protein during the virus’ evolution (Rosenthal et al. 1998). The immunodominant, poorly conserved RBD head is directed toward target cells and contains the receptor-binding pocket (R.B.P.) and most of the major antigenic sites (Wiley et al. 1981). The long, fibrous F domain has at its core a 54-residue triple-stranded coiled coil (helices C and D) that is flanked by spring-loaded B loops, A helices, and membrane-proximal regions (Fig. 2b). The prefusion HA0 and HA1/HA2 structures are superimposable with the exception of five residues upstream of the cleavage site and the first 12 residues of the fusion peptide located near the base of the molecule. In uncleaved HA0, these 17 residues form a prominent loop that is protease-accessible (Chen et al. 1998); whereas, in cleaved HA1/HA2 the fusion peptide tucks into a pocket formed by residues from HA1 and the HA2 D and A helices (Wilson et al. 1981).
Fig. 2.

Structural changes in the HA protein after low-pH induced activation. a Prefusion HA1/HA2 trimer with HA1 shown in magenta and HA2 secondary structural elements shown in multiple colors. The receptor-binding pocket (R.B.P.) is located in the membrane-distal head domain and the metastable fusion domain is located in the membrane-proximal stalk. b Prefusion HA2 trimer shown without HA1 residues. c Postfusion HA2 trimer showing changes in secondary and tertiary structure after acid-induced irreversible protein refolding. In each panel, two protomers are colored gray. Figure adapted from (Bullough et al. 1994) using A/Aichi/2/68/X-31 (H3N2) protein data bank structures 1HGF and 1QUI
X-ray crystal structures have been determined for bromelain-released and baculovirus-expressed HA1/HA2 and HA0 prefusion structures from numerous influenza A viruses including those isolated from humans (H1N1, pH1N1, H2N2, H3N2, H5N1, H7N7, H7N9), avians (H1, H2, H3, H5, H7, H13, H16), swine (H1, H9), and bat (H17). Despite amino-acid sequence identity of less than 50 %, the structures across subtypes are highly conserved (RMSD values of ~ 1 Å within domains) and can be classified into two structural/antigenic groups (Air 1981; Nobusawa et al. 1991; Gamblin and Skehel 2010): group 1 (H1, H2, H5, H6, H8, H9, H11, H12, H13, H16, and H17) and group 2 (H3, H4, H7, H10, H14, and H15). The main differences between group 1 and 2 HA structures are (a) an ~20° axial rotation and ~4 Å displacement of the globular RBD head atop the central coiled coil in the stalk and (b) the sharpness of the turn of the B loop back toward the base of the C helix (Ha et al. 2002). These differences have been ascribed to differences in HA1 residue 107 (in the 110 helix of the vestigial esterase domain), residues 63 and 75 in the HA2 B loop, and residue 88 in the HA2 C helix (Ha et al. 2002).
H3 Numbering Convention
HA protein structure and function are highly conserved across subtypes despite substantial differences in primary amino-acid sequence and numbering. To facilitate the description of significant residues (e.g., residues in the R.B.P. or those altering HA acid stability), HA residues are conventionally identified using “H3 numbering.” H3 numbering derived from the first HA structure of A/Aichi/68 (H3N2) (Wilson et al. 1981) is described in Fig. 1 and will be used throughout this review.
Influenza Virus Internalization into a New Host Cell
After virus egress and HA priming by cleavage, an infectious progeny virion is poised to invade a new host cell. During influenza A virus entry, the HA protein binds host cell receptors containing sialic acid, or N-acetyl-neuraminic acid (Weis et al. 1988; Chu and Whittaker 2004). It should be noted that A/WSN/33 (H1N1) has been shown to enter cells in the absence of sialyated N-glycans by a dynamin-dependent mechanism (de Vries et al. 2012) and the HA protein from a recently discovered H17N10 bat virus does not bind sialic acid-containing receptors (Tong et al. 2012; Sun et al. 2013). In general, the HA proteins from human-adapted viruses preferentially bind to α(2,6)-linked sialosides while those from avian viruses tend to prefer those with an α(2,3) linkage (Matrosovich et al. 2009). As few as one amino-acid mutation in the R.B.P. can switch receptor-binding specificity (Rogers et al. 1983; Matrosovich et al. 2000) and, in turn, can alter the tropism, host range, and pathogenicity of an influenza virus (Belser et al. 2010; Fukuyama and Kawaoka 2011; Sorrell et al. 2011). Receptor binding triggers internalization of the virion into the host cell (Rust et al. 2004), which can occur via clathrin-mediated or clathrin-independent endocytosis (Sieczkarski and Whittaker 2002; Lakadamyali et al. 2006) or by macropinocytosis (de Vries et al. 2011; De Conto et al. 2011).
Acid-induced Activation of the HA Protein
Once internalized, an influenza virus trafficks through the endosomal network (Lakadamyali et al. 2004; Sun and Whittaker 2013). Within 5 min, endocytosed material is exposed to pH 6.5–6.0 in early endosomes and then is slowly acidified (over 30–40 min) to pH 5.5–5.0 in late endosomes and pH 5.0–4.6 in lysosomes (Mellman et al. 1986; Cain et al. 1989; Grove and Marsh 2011). Exposure to low pH triggers fusion-primed HA1/HA2 to undergo irreversible conformational changes that cause membrane fusion in the presence of target membranes or virion inactivation in the absence of target membranes (Skehel and Wiley 2000). While the biological trigger for HA activation is low pH, the native protein is trapped in a metastable (high-energy) conformation and its activation energy barrier can also be overcome in vitro by other mild destabilizing agents such as heat and the denaturant urea (Scholtissek 1985; Ruigrok et al. 1986; Carr et al. 1997). Human H1, H2, and H3 HA proteins from twentieth century pandemics were found to be activated and cause membrane fusion at pH values of 5.0–5.2 (Hoekstra and Klappe 1993; Galloway et al. 2013), leading to the classical view that HA-mediated membrane fusion takes place in late endosomes (Grove and Marsh 2011; Sun and Whittaker 2013). However, broader surveys that include avian and swine isolates have shown that HA activation pH values range from pH 4.6 to 6.0 (Scholtissek 1985; Galloway et al. 2013). This raises the possibility that isolates with relatively high activation pH values may cause membrane fusion in early endosomes, as has been proposed for HPAI H5N1 viruses (Reed et al. 2010; DuBois et al. 2011b). The pH values of endocytic compartments may also vary between host species, tissues, and individuals.
Low-pH-induced Structural Changes
Several large-scale structural changes occur during HA protein refolding (Fig. 2). Upon reaching a threshold pH, protonation appears to induce deformations in the RBD and relax the B loops at the top of the stalk (Xu and Wilson 2011). Next, the RBD protomers dissociate from each other and the stalk (Bottcher et al. 1999; Huang et al. 2002) with the overall fold within the RBD domains remaining intact (Bizebard et al. 1995; DuBois et al. 2011a). Solvent penetration into the stalk induces conformational changes within the central helices (Xu and Wilson 2011). Meanwhile, dissociation of the RBDs from the stalk enables the B loops to spring out and extend the central coiled coil from helix C through helix A, an energetically favorable process (Carr and Kim 1993) that propels the fusion peptide harpoon toward the target membrane (Bullough et al. 1994; Huang et al. 2003). This prehairpin intermediate is topologically reminiscent of those formed by fellow structural class I viral fusion glycoproteins from HIV gp41 (Chan and Kim 1998) and the paramyxovirus F protein (Russell et al. 2001). Subsequently, HA2 residues 106–112 switch from a coiled coil to a reverse turn (Wilson et al. 1981; Bullough et al. 1994). This allows the membrane-proximal “leash” region to zipper up in the grooves of the central coiled coil in an antiparallel orientation, juxtaposing the TM domains and fusion peptides (Fig. 2c).
Membrane Fusion
Membrane fusion is not spontaneous and requires energy. Energy barriers that must be overcome for membrane fusion include (a) bringing together two membranes containing repulsive charges; (b) inducing lipid curvatures and deformations to form a hemifusion stalk; (c) in some cases, forming a hemifusion diaphragm; and (d) forming and expanding a fusion pore to allow mixing of contents (Chernomordik and Kozlov 2008; Martens and McMahon 2008). The two fusing membranes are brought into proximity first by high-avidity binding of multiple trimeric HA proteins to the host cell. Additionally, HA protein refolding juxtaposes target-membrane interacting fusion peptides with viral-envelope spanning TM domains (Durrer et al. 1996; Chen et al. 1999). Formation of the leash-in-grooves hairpin by the HA protein helps drive lipid mixing and hemifusion (Borrego-Diaz et al. 2003; Park et al. 2003). Similarly, the energy released by six-helix bundle formation in HIV gp41 and paramyxovirus F is coupled to the work of membrane fusion for those other class I fusion proteins (Melikyan et al. 2000; Russell et al. 2001). Lipid packing is also disturbed by penetration into the host cell membrane by the fusion peptide, which adopts a bent “boomerang” structure (Han et al. 2001). The presence of the TM domains helps to break the hemifusion stalk or diaphragm (Melikyan et al. 1995; Armstrong et al. 2000). Finally, continued HA protein refolding outside the initial contact zone is necessary for fusion pore expansion that is sufficient for delivery of the viral RNA and internal structural proteins into the cytosol of the host cell (Leikina et al. 2004).
Amino-acid Residues Influencing HA Acid Stability
Over 70 mutations in H1, H2, H3, H5, and H7 HA proteins have been discovered that alter the pH at which the HA protein is triggered to undergo its irreversible conformational changes and cause membrane fusion (Table 1). Residues determining HA acid stability are located throughout the sequence (Fig. 1) and structure (Fig. 3). In general, the mutations are located in regions of the HA molecule that undergo dramatic changes in secondary and tertiary structure during the prefusion to postfusion transition (Wilson et al. 1981; Daniels et al. 1985; Bullough et al. 1994; Thoennes et al. 2008). As activation pH-altering mutations have not altered substantially the backbone structures of human H3N2 (Weis et al. 1990), avian H5N1 (DuBois et al. 2011b; Xiong et al. 2013; Zhang et al. 2013; de Vries et al. 2014), and human H2N2 (Xu and Wilson 2011) HA proteins in the prefusion conformation, acid stability mutations may in general exact their greatest influence on protein folding intermediates.
Table 1.
Mutations altering the activation pH of the influenza A virus HA protein
| H3 #a |
Sub-type | Mutation and change in activation pH | Regionb | Reference |
|---|---|---|---|---|
| 171 | H3N2 | H17R (+0.6), H17A (0.4), H17Q (+0.25), H17Y (-0.3) | 1 | Daniels et al. (1985), Steinhauer et al. (1996), Lin et al. (1997), Thoennes (2008) |
| 171 | H5N1 | Y17H (+0.4) | 1 | Reed et al. (2009), Zaraket et al. (2013a) |
| 181 | H5N1 | H18Q (−0.3) | 1 | Reed et al. (2009), Zaraket et al. (2013a) |
| 321 | H7N1 | R32G (+0.2) | 2 | Daniels et al. (1985) |
| 451 | H5N1 | K45D (−0.1) | 1 | Reed et al. (2009) |
| 701/252 | H1N1 | L701P/Q252H (−0.2) | 6/7 | Koerner et al. (2012) |
| 911 | H7N1 | R91L (+0.3), R91Q (+0.1) | 3 | Daniels et al. (1985) |
| 1041/1151 | H5N1 | D104N/I115T (−0.3) | 3 | Hulse et al. (2004), DuBois et al. (2011b) |
| 1101 | H5N1 | H110Y (−0.4) | 3 | Herfst et al. (2012), Zhang et al. (2013) |
| 1351/42 | H3N2 | G1351E/G42E (+0.4) | 5/1 | Lin et al. (1997) |
| 1371/822 | H3N2 | N137D/K82T (+0.4) | 5/2 | Lin et al. (1997) |
| 1621 | H3N2 | P162S (+0.2) | 5 | Keleta et al. (2008) |
| 1791 | H3N2 | L179P (+0.5) | 5 | Nakowitsch et al. (2011) |
| 2101 | H3N2 | Q210R (+0.15) | 4 | Keleta et al. (2008) |
| 2161 | H5N1 | E216K (−0.4), K216E (+0.4) | 4 | Hulse et al. (2004), DuBois et al. (2011b) |
| 2181 | H3N2 | G218E,W(+0.4) | 4 | Steinhauer et al. (1996), Lin et al. (1997), Narasaraju et al. (2009), Keleta et al. (2008) |
| 2211 | H5N1 | S221P (−0.15) | 4 | Hulse et al. (2004), DuBois et al. (2011b) |
| 2241/2261 | H5N1 | N224K/Q226L (+0.2) | 5 | Imai et al. (2012) |
| 3001 | H7N1 | R300S (+0.3) | 2 | Daniels et al. (1985) |
| 3181 | H5N1 | T318I (−0.2) | 2 | Imai et al. (2012) |
| 12 | H3N2 | G1F,H,I,L (+0.3) | 1 | Steinhauer et al. (1995) |
| 32 | H7N1 | F3L (+0.4) | 1 | Daniels et al. (1985) |
| 42 | H3N2 | G4A (+0.4) | 1 | Steinhauer et al. (1995) |
| 62 | H3N2 | I6M (+0.3) | 1 | Daniels et al. (1985) |
| 82 | H3N2 | G8A (+0.5) | 1 | Steinhauer et al. (1995) |
| 92 | H3N2 | F9L (+0.6) | 1 | Daniels et al. (1985) |
| 232 | H7N7 | G23C (−1.0) | 1 | Ilyushina et al. (2007) |
| 412/852 | H3N2 | T41A/E85D (+0.4) | 1/2 | Lin et al. (1997) |
| 472 | H7N1 | Q47L (+0.45) | 2 | Daniels et al. (1985) |
| 472 | H3N2 | Q47R (+0.35) | 2 | Daniels et al. (1985) |
| 472 | H1N1 | E47K (−0.4) | 2 | Cotter et al. (2014) |
| 512 | H3N2 | K51A,E (+0.2) | 2 | Thoennes et al. (2008) |
| 542 | H3N2 | R54E (+0.2) | 2 | Steinhauer et al. (1996) |
| 542 | H7N1 | R54K (+0.3), R54G,S (+0.1) | 2 | Daniels et al. (1985) |
| 582 | H3N2 | K58I (−0.7) | 2 | Steinhauer et al. (1991a, 1996) |
| 582 | H5N1 | K58I (−0.5) | 2 | Reed et al. (2009, 2010), Zaraket et al. (2013a, b) |
| 752 | H3N2 | G75R (+0.4) | 2 | Nakowitsch et al. (2011) |
| 812 | H3N2 | E81G (+0.3) | 2 | Daniels et al. (1985) |
| 812 | H7N1 | I81S (+0.1) | 2 | Daniels et al. (1985) |
| 1052 | H3N2 | Q105K (+0.3), Q105A (-0.2) | 2 | Daniels et al. (1985), Thoennes (2008) |
| 1052 | H5N1 | E105K (−0.2) | 2 | Reed et al. (2009) |
| 1062 | H3N2 | H106A (+0.1), H106F (-0.1) | 2 | Thoennes et al. (2008) |
| 1062 | H2N2 | R106H (−1.0) | 2 | Xu and Wilson (2011) |
| 1122 | H3N2 | D112A (+0.5), D112G (+0.4), D112N (+0.35) D112E (+0.25) | 1 | Daniels et al. (1985), Steinhauer et al. (1996), Thoennes et al. (2008) |
| 1122 | H7N1 | D112G (+0.4) | 1 | Daniels et al. (1985) |
| 1122 | H5N1 | D112G (+0.3) | 1 | Reed et al. (2009) |
| 1142 | H7N1 | E114K (+0.5) | 1 | Daniels et al. (1985) |
| 1142 | H5N1 | N114K (+0.5) | 1 | Reed et al. (2009, 2010) |
| 1142 | H3N2 | E114K (+0.6) | 1 | Daniels et al. (1985) |
| 1162 | H3N2 | N116D (+0.4) | 1 | Lin et al. (1997) |
| 1172 | H3N2 | K117R (+0.4) | 1 | Lin et al. (1997) |
| 1172 | H1N1 | N117D (+0.4) | 1 | Murakami et al. (2012) |
| 1322 | H3N2 | D132N (+0.2) | 7 | Doms et al. (1986) |
| 1562 | H3N2 | T156N (−0.1) | 7 | Keleta et al. (2008) |
aH3 numbering is described in Fig. 1 and subscripts refer to HA1 and HA2 subunits
bStructural regions are defined as: (1) Fusion peptide and its surrounding pocket, (2) Spring-loaded coiled coil, (3) Esterase-stalk interface, (4) HA1-HA1 protomer interface, (5) Near or in receptor-binding pocket, (6) Esterase-RBD interface, and (7) Membrane proximal region
Fig. 3.
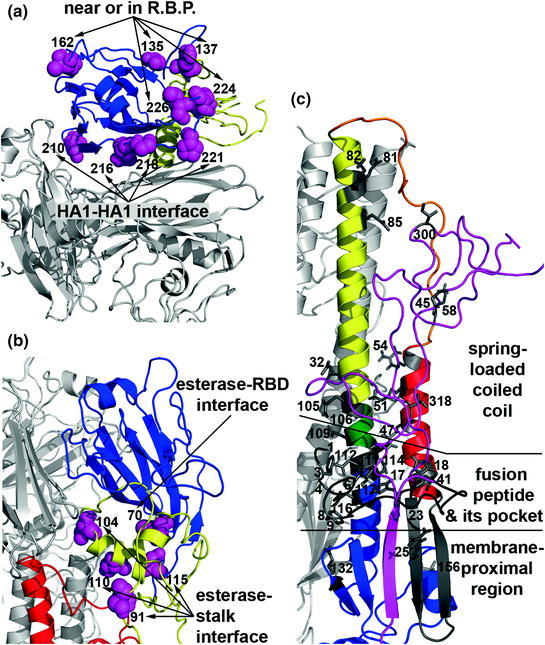
Locations of HA acid stability mutations in the prefusion conformation. a RBD residues near or in the receptor-binding pocket (R.B.P.) or at the interface of HA1-HA1 protomers. b Esterase sub-domain residues at the interface of the receptor-binding subdomain and the stalk region. c HA1 and HA2 residues in the spring-loaded coiled coil region, in and around the fusion peptide pocket, and in the membrane-proximal region. In panels A and B, the receptor-binding subdomain is colored blue and the esterase subdomain is colored yellow. In panel C, HA1 residues in the stalk are colored magenta and HA2 secondary structural elements are colored as in Fig. 2. Residues governing HA acid stability are identified using H3 numbering on the A/Aichi/2/68/X-31 (H3N2) protein data bank structure 1HGF. Acid stability mutations are described in detail in Table 1
Pioneering work to identify HA acid stability mutants involved the selection of amantadine-resistant viruses that had elevated activation pH values (Daniels et al. 1985). These mutations were later mapped to four structural regions (Bullough et al. 1994): (1) the fusion peptide and its pocket; (2) helix A and loop B packing against the spring-loaded long CD helix; (3) the HA2-HA1 interface between the RBD esterase subdomain, helix C, and loop B; and (4) the HA1-HA1 interface between RBD protomers. Mapping the locations of the expanded list of acid stability mutations adds three more structural regions (Fig. 3): (5) near and in the R.B.P.; (6) at the interface of RBD and esterase subdomains; and (7) in the membrane-proximal region. Furthermore, it is likely that mutations in the TM domain (Zhou et al. 2013) and cytoplasmic tails may also influence the energy required for HA protein activation, just as has been reported for other structural class I viral fusion proteins from retroviruses and paramyxoviruses (Li et al. 2001; Tong et al. 2002; Waning et al. 2004). Some combinations of acid stability mutations are additive while others or not (Steinhauer et al. 1996), consistent with multiple structural transitions occurring during HA protein refolding. A great variety of acid stability mutations have been shown to be functionally equivalent with respect to amantadine resistance (Daniels et al. 1985). It has been hypothesized, but has not yet been demonstrated, that acid stability mutations are also functionally equivalent with respect to other biological properties (Zaraket et al. 2013a, b).
Impact of HA Acid Stability on Influenza Virus Biology
Adaptation of HA Acid Stability In Vitro
The acid stability of the HA protein has been shown to play a role in amantadine susceptibility and interspecies adaptation in vitro. Mutations that increase the pH of activation of HA proteins from H3N2 and H7N1 viruses have been shown to enhance viral fitness in the presence of ammonium chloride and high concentrations of amantadine, compounds that raise endosomal pH (Daniels et al. 1985; Doms et al. 1986; Steinhauer et al. 1995, 1996). Alternatively, HPAI H7N1 and H7N7 viruses containing mutations that decrease the HA activation pH are resistant to lower concentrations of amantadine that prevent M2-mediated neutralization of the secretory pathway (Steinhauer et al. 1991a; Ilyushina et al. 2007). The adaptation of egg-adapted A/Aichi/2/68 (H3N2) to mammalian MDCK and MDBK cells (Lin et al. 1997) and A/PR/8/34 (H1N1) to Vero cells (Murakami et al. 2012) resulted in increases in HA activation pH from pH 5.2 to 5.6 for the H3N2 virus and from pH 5.2 to 5.4 for the H1N1 virus.
Decrease in HA Activation pH Promotes HPAI Virus Adaptation to Mammals
Mutations that alter HA acid stability have also been implicated in the adaptation of avian influenza viruses from wild aquatic birds, the reservoir of influenza A viruses (Kim et al. 2009), to land-based poultry and mammals. A comparison of LPAI H7N3 viruses found that a virus isolated from a turkey had a lower HA activation pH than a related virus previously isolated from a duck, suggesting the adaptation of avian H7 viruses from wild aquatic birds to terrestrial poultry may be associated with enhanced HA acid stability (Giannecchini et al. 2006). The HPAI virus A/chicken/Vietnam/C58/2004 ( H5N1), with an HA activation pH of 5.9 (Reed et al. 2009), was rendered unfit for growth and transmission in mallards by mutations that substantially raised (HA1-Y17H, activation pH 6.3) or lowered (HA2-K58I, activation pH 5.4) the HA activation pH. While the acid-stabilizing K58I mutation attenuated H5N1 replication in ducks, this same mutation increased replication and pathogenesis in mice (Zaraket et al. 2013b) and enhanced early growth of A/Vietnam/1203/04 (H5N1) in the upper respiratory tracts of ferrets (Zaraket et al. 2013a). Thus, a decrease in the HA activation pH has been shown to assist in the adaptation of H5N1 to mammals. On the other hand, an increase in HA activation pH (in the range of pH 5.2–6.0) has been associated with increased H5N1 virus growth and virulence in chickens (Hulse et al. 2004; DuBois et al. 2011b). This agrees with observations that the HA proteins from HPAI influenza viruses tend to have relatively high (pH 5.6–6.0) activation pH values while those from human-adapted seasonal influenza viruses are lower (pH 5.0–5.2) (Scholtissek 1985; Reed et al. 2010; DuBois et al. 2011b; Galloway et al. 2013).
Two recent studies provide compelling evidence for a role of HA acid stability in the adaptation of avian H5-containing viruses to mammals (Imai et al. 2012; Herfst et al. 2012). Two H5 viruses were adapted to transmit in ferrets, either A/Indonesia/5/2005 (H5N1) (Herfst et al. 2012) or a reassortant virus containing the HA gene from A/Vietnam/1203/04 (H5N1) and the internal genes of A/California/04/09 (H1N1) (Imai et al. 2012). In both studies, three analogous rounds of mutations occurred. First, two mutations were introduced into the R.B.P. that switched receptor-binding specificity from a preference of α(2,3) to α(2,6). Second, a mutation atop the RBD head removed a glycosylation site, increasing the accessibility of the R.B.P. The final mutation necessary for airborne transmissibility in ferrets in both cases was an HA1 mutation adjacent to (H110Y) or in (T318I) the fusion-mediating stalk domain that decreased the HA activation pH to ~5.6. While an acid-stabilizing mutation was required for airborne transmissibility in ferrets, such a mutation was not sufficient in the absence of α(2,6) receptor-binding specificity and/or glycosylation site deletion (Shelton et al. 2013; Zaraket et al. 2013a).
Adaptation of Human Influenza Viruses to Murine Lungs
The adaptation of human influenza viruses for replication in the murine lung has been associated with increases in HA activation pH (Narasaraju et al. 2009). An increase in HA activation pH from 5.2 to 5.6 resulted in increased growth and virulence of A/Hong Kong/1/68 (H3N2) in mice (Keleta et al. 2008). Similarly, an increase in HA activation pH from 5.3 to 5.8 enhanced A/PR/8/34 (H1N1) growth and virulence in mice (Koerner et al. 2012). Mouse-adapted A/Philippines/82 (H3N2) was also found to have increases in HA activation pH and virulence in mice (Hartley et al. 1997). The effects of activation-pH altering mutations on influenza A virus growth in the murine lung may not extend to growth in ferrets, as the acid-stabilized HA2-K58I mutant of A/Vietnam/1203/04 (H5N1) had increased growth in the lungs of mice but reduced growth in the lungs of ferrets (Zaraket et al. 2013a).
HA Acid Stability in Live Attenuated Vaccine Development
Just as knowledge of optimal HA activation pH values in various species may enhance risk assessment of emerging influenza viruses with increased pandemic potential, such an understanding may also enhance the development of live attenuated vaccines. Acid-destabilizing mutations that increase the HA activation pH from 5.8 to 6.2–6.3 have been shown to impair the immunogenicity of live attenuated, NS1-deleted H3N2 vaccine candidates in ferrets (Nakowitsch et al. 2011). In contrast, the acid-stabilizing HA2-K58I mutation that enhances upper respiratory tract growth of A/Vietnam/1203/04 in ferrets (Zaraket et al. 2013a) has been shown also to increase the infectivity and immunogenicity of a live attenuated, NS1-deleted H5N1 vaccine candidate in mice (Krenn et al. 2011). Similarly, a decrease in the pH of activation of the HA protein from 5.4 to 5.0 has been shown to enhance the stability and infectivity of p2009 H1N1 vaccine candidates (Cotter et al. 2014). Overall, HA acid-stabilizing mutations may enhance live attenuated influenza virus vaccines.
Hypothesis for Role of HA Acid Stability in Influenza Virus Biology
While further studies are needed to define the mechanism by which the pH of activation of the HA protein influences the interspecies adaptation of influenza A viruses, a working hypothesis is emerging. Intracellular pH is determined by the presence and activity of cellular vacuolar H+-ATPases (V-ATPases) (Mellman et al. 1986; Jefferies et al. 2008), which may differ between different cell types, tissues, hosts, and metabolic states. For example, increased glucose exposure in MDCK cells leads to increased vATPase activity and decreased endosomal pH, thereby enhancing the replication of A/PR/8/34 (H1N1) (Kohio and Adamson 2013), which has a relatively low HA activation pH of 5.0–5.1 (Galloway et al. 2013). For avian H5N1 viruses, a relatively high HA activation pH enhances replication in the enteric and respiratory tracts of ducks and chickens (Reed et al. 2010; DuBois et al. 2011b), presumably because a destabilized HA protein is more readily triggered for membrane fusion during viral entry. In contrast, a high HA activation pH appears to be a liability for growth in the mammalian upper respiratory tract (Imai et al. 2012; Herfst et al. 2012; Shelton et al. 2013; Zaraket et al. 2013a), most likely because it enhances the susceptibility of pre-cleaved H5 HA trimers to irreversible inactivation in airway tissues. Mammalian airway tissue is acidic (pH 5.5–6.9) (Washington et al. 2000; Fischer and Widdicombe 2006) and acid secretion into the airway is enhanced upon infection by influenza viruses, lowering the nasal pH to 5.2 (Jacoby et al. 1988; Fischer et al. 2002). Human-adapted influenza viruses with monobasic cleavage sites may also benefit by having a relatively low HA activation pH value, which may help prevent premature inactivation in the mammalian respiratory tract. Human-adapted H1N1, H2N2, and H3N2 viruses from the twentieth century pandemics have HA activation pH values of pH 5.0–5.2 (Scholtissek 1985; Galloway et al. 2013), and a naturally occurring mutation in circulating p2009 H1N1 viruses has been shown to decrease the HA activation pH from 5.4 to 5.0 (Cotter et al. 2014).
Conclusions and Future Perspectives
Membrane fusion is not a spontaneous process but instead is driven by the formation of hairpin structures that bring together the viral TM domain (embedded in the viral envelope) and the viral fusion peptide (inserted into the host cell membrane) so as to juxtapose the viral and cellular membranes. HA-mediated membrane fusion has been a paradigm for understanding viral membrane fusion and is topographically similar to intracellular membrane fusion driven by SNARE proteins (Söllner 2004; Wickner and Schekman 2008). Recent studies add HA protein acid stability, or the pH at which the HA is triggered to undergo irreversible structural changes to cause membrane, to the list of molecular properties that contribute to the interspecies adaptation, pathogenesis, and transmissibility of influenza A viruses. Influenza viruses are internalized by endocytosis, and the HA protein is activated for membrane fusion by low pH. Many other enveloped viruses invade host cells similarly including hepatitis C virus, Epstein-Barr virus, vesicular stomatitis virus, avian leukemia virus, human rhinovirus, dengue virus, and severe acute respiratory syndrome (SARS) coronavirus (Grove and Marsh 2011). These other viruses may also regulate their host range and tropism by adaptive mutations that alter the acid stabilities of their fusion glycoproteins.
HA acid stability is just one of multiple simultaneous factors that dictate influenza virus biology, and many questions remain with respect to its mechanism and breadth of impact. It is not yet clear the extent to which this one molecular property interacts with others long known for influencing the replication, interspecies adaptation, pathogenicity, and transmissibility of influenza viruses (Salomon and Webster 2009; Belser et al. 2010; Fukuyama and Kawaoka 2011; Sorrell et al. 2011). For example, the mechanism by which NA activity influences HA acid stability is unknown (Huang et al. 1980; Su et al. 2009; Reed et al. 2010). It is also unknown whether mutations that alter the acid stabilities of monobasic HA proteins exert as strong of effects as those altering intracellularly cleaved (and more acid-neutralization sensitive) polybasic HA proteins. Compared to HPAI viruses, LPAI viruses are reported to have a broader and lower range of HA activation pH values (5.3–6.0 in ducks and 5.1–5.5 in chickens and turkeys) (Galloway et al. 2013). Avian influenza viruses disseminate throughout the enteric tracts of birds, including environments of extreme low pH, yet it is not clear how these viruses avoid inactivation. The pH values in endocytic vesicles have been measured in vitro in cell monolayers, but it is unknown how the pH values of early and late endosomes vary in vivo in the tissues of various living species and individuals with altered metabolic states. Perhaps a knowledge of species-specific and tissue-specific endosomal pH values and extracellular pH may better explain why a relatively low HA activation pH is disfavored by HPAI viruses in avian species yet favored in mammals. The preferred range of HA activation pH in swine and whether swine may serve as a mixing vessel during the acquisition of human-preferred HA acid stability is not yet known. Finally, the long-term viability of HA stalk-binding antiviral drugs and antibodies, in addition to the development of stalk-based universal vaccines, will also depend on the extent to which such treatments and prophylactics are prone to altering HA acid stability and the concomitant effects of such changes on influenza virus replication and the host response.
Abbreviations
- CT
Cytoplasmic tail
- ER
Endoplasmic reticulum
- HA
Hemagglutinin
- HAT
Human airway trypsin-like
- HPAI
Highly pathogenic avian influenza
- LPAI
Low pathogenic avian influenza
- MSPL
Mosaic serine protease large-form
- NA
Neuraminidase
- NP
Nucleoprotein
- RBD
Receptor-binding domain
- R.B.P.
Receptor-binding pocket
- RMSD
Root-mean-square deviation
- SARS
Severe acute respiratory syndrome
- TM
Transmembrane
- TMPRSS
Transmembrane protease, serine
Contributor Information
Richard W. Compans, Phone: +1(404)727-2015, Email: rcompan@emory.edu
Michael B. A. Oldstone, Phone: +1858-784-8054, Email: mbaobo@scripps.edu
Charles J. Russell, Email: charles.russell@stjude.org
References
- Air GM. Sequence relationships among the hemagglutinin genes of 12 subtypes of influenza A virus. Proc Natl Acad Sci USA. 1981;78:7639–7643. doi: 10.1073/pnas.78.12.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RT, Kushnir AS, White JM. The transmembrane domain of influenza hemagglutinin exhibits a stringent length requirement to support the hemifusion to fusion transition. J Cell Biol. 2000;151:425–437. doi: 10.1083/jcb.151.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Maines TR, Tumpey TM, Katz JM. Influenza A virus transmission: contributing factors and clinical implications. Expert Rev Mol Med. 2010;12:e39. doi: 10.1017/S1462399410001705. [DOI] [PubMed] [Google Scholar]
- Bizebard T, Gigant B, Rigolet P, et al. Structure of influenza virus haemagglutinin complexed with a neutralizing antibody. Nature. 1995;376:92–94. doi: 10.1038/376092a0. [DOI] [PubMed] [Google Scholar]
- Borrego-Diaz E, Peeples ME, Markosyan RM, et al. Completion of trimeric hairpin formation of influenza virus hemagglutinin promotes fusion pore opening and enlargement. Virology. 2003;316:234–244. doi: 10.1016/j.virol.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Bottcher C, Ludwig K, Herrmann A, et al. Structure of influenza haemagglutinin at neutral and at fusogenic pH by electron cryo-microscopy. FEBS Lett. 1999;463:255–259. doi: 10.1016/s0014-5793(99)01475-1. [DOI] [PubMed] [Google Scholar]
- Böttcher E, Matrosovich T, Beyerle M, et al. Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J Virol. 2006;80:9896–9898. doi: 10.1128/JVI.01118-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullough PA, Hughson FM, Skehel JJ, Wiley DC. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- Cain CC, Sipe DM, Murphy RF. Regulation of endocytic pH by the Na+, K+-ATPase in living cells. Proc Natl Acad Sci USA. 1989;86:544–548. doi: 10.1073/pnas.86.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CM, Chaudhry C, Kim PS. Influenza hemagglutinin is spring-loaded by a metastable native conformation. Proc Natl Acad Sci USA. 1997;94:14306–14313. doi: 10.1073/pnas.94.26.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CM, Kim PS. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993;73:823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- Chaipan C, Kobasa D, Bertram S, et al. Proteolytic activation of the 1918 influenza virus hemagglutinin. J Virol. 2009;83:3200–3211. doi: 10.1128/JVI.02205-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- Chen J, Lee KH, Steinhauer DA, et al. Structure of the hemagglutinin precursor cleavage site, a determinant of influenza pathogenicity and the origin of the labile conformation. Cell. 1998;95:409–417. doi: 10.1016/s0092-8674(00)81771-7. [DOI] [PubMed] [Google Scholar]
- Chen J, Skehel JJ, Wiley DC. N- and C-terminal residues combine in the fusion-pH influenza hemagglutinin HA(2) subunit to form an N cap that terminates the triple-stranded coiled coil. Proc Natl Acad Sci USA. 1999;96:8967–8972. doi: 10.1073/pnas.96.16.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Calvo PA, Malide D, et al. A novel influenza A virus mitochondrial protein that induces cell death. Nat Med. 2001;7:1306–1312. doi: 10.1038/nm1201-1306. [DOI] [PubMed] [Google Scholar]
- Chernomordik LV, Kozlov MM. Mechanics of membrane fusion. Nat Struct Mol Biol. 2008;15:675–683. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu VC, Whittaker GR. Influenza virus entry and infection require host cell N-linked glycoprotein. Proc Natl Acad Sci USA. 2004;101:18153–18158. doi: 10.1073/pnas.0405172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans RW, Dimmock NJ, Meier-Ewert H. Effect of antibody to neuraminidase on the maturation and hemagglutinating activity of an influenza A2 virus. J Virol. 1969;4:528–534. doi: 10.1128/jvi.4.4.528-534.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland CS, Zimmer KP, Wagner KR, et al. Folding, trimerization, and transport are sequential events in the biogenesis of influenza virus hemagglutinin. Cell. 1988;53:197–209. doi: 10.1016/0092-8674(88)90381-9. [DOI] [PubMed] [Google Scholar]
- Cotter CR, Jin H, Chen Z. A single amino acid in the stalk region of the H1N1pdm influenza virus HA protein affects viral fusion, stability and infectivity. PLoS Pathog. 2014;10:e1003831. doi: 10.1371/journal.ppat.1003831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RS, Downie JC, Hay AJ, et al. Fusion mutants of the influenza virus hemagglutinin glycoprotein. Cell. 1985;40:431–439. doi: 10.1016/0092-8674(85)90157-6. [DOI] [PubMed] [Google Scholar]
- De Conto F, Covan S, Arcangeletti MC, et al. Differential infectious entry of human influenza A/NWS/33 virus (H1N1) in mammalian kidney cells. Virus Res. 2011;155:221–230. doi: 10.1016/j.virusres.2010.10.008. [DOI] [PubMed] [Google Scholar]
- de Vries E, de Vries RP, Wienholts MJ, et al. Influenza A virus entry into cells lacking sialylated N-glycans. Proc Natl Acad Sci USA. 2012;109:7457–7462. doi: 10.1073/pnas.1200987109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries E, Tscherne DM, Wienholts MJ, et al. Dissection of the influenza a virus endocytic routes reveals macropinocytosis as an alternative entry pathway. PLoS Pathog. 2011;7:e1001329. doi: 10.1371/journal.ppat.1001329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries RP, Zhu X, McBride R, et al. Hemagglutinin receptor specificity and structural analyses of respiratory droplet-transmissible H5N1 viruses. J Virol. 2014;88:768–773. doi: 10.1128/JVI.02690-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms RW, Gething MJ, Henneberry J, et al. Variant influenza virus hemagglutinin that induces fusion at elevated pH. J Virol. 1986;57:603–613. doi: 10.1128/jvi.57.2.603-613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois RM, Aguilar-Yañez JM, Mendoza-Ochoa GI, et al. The receptor-binding domain of influenza virus hemagglutinin produced in Escherichia coli folds into its native, immunogenic structure. J Virol. 2011;85:865–872. doi: 10.1128/JVI.01412-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois RM, Zaraket H, Reddivari M, et al. Acid stability of the hemagglutinin protein regulates H5N1 influenza virus pathogenicity. PLoS Pathog. 2011;7:e1002398. doi: 10.1371/journal.ppat.1002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrer P, Galli C, Hoenke S, et al. H+-induced membrane insertion of influenza virus hemagglutinin involves the HA2 amino-terminal fusion peptide but not the coiled coil region. J Biol Chem. 1996;271:13417–13421. doi: 10.1074/jbc.271.23.13417. [DOI] [PubMed] [Google Scholar]
- Fischer H, Widdicombe JH. Mechanisms of acid and base secretion by the airway epithelium. J Membr Biol. 2006;211:139–150. doi: 10.1007/s00232-006-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Widdicombe JH, Illek B. Acid secretion and proton conductance in human airway epithelium. Am J Physiol Cell Physiol. 2002;282:C736–C743. doi: 10.1152/ajpcell.00369.2001. [DOI] [PubMed] [Google Scholar]
- Fukuyama S, Kawaoka Y. The pathogenesis of influenza virus infections: the contributions of virus and host factors. Curr Opin Immunol. 2011;23:481–486. doi: 10.1016/j.coi.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway SE, Reed ML, Russell CJ, Steinhauer DA. Influenza HA subtypes demonstrate divergent phenotypes for cleavage activation and pH of fusion: implications for host range and adaptation. PLoS Pathog. 2013;9:e1003151. doi: 10.1371/journal.ppat.1003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin SJ, Skehel JJ. Influenza hemagglutinin and neuraminidase membrane glycoproteins. J Biol Chem. 2010;285:28403–28409. doi: 10.1074/jbc.R110.129809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten W, Bosch FX, Linder D, et al. Proteolytic activation of the influenza virus hemagglutinin: the structure of the cleavage site and the enzymes involved in cleavage. Virology. 1981;115:361–374. doi: 10.1016/0042-6822(81)90117-3. [DOI] [PubMed] [Google Scholar]
- Giannecchini S, Campitelli L, Calzoletti L, et al. Comparison of in vitro replication features of H7N3 influenza viruses from wild ducks and turkeys: potential implications for interspecies transmission. J Gen Virol. 2006;87:171–175. doi: 10.1099/vir.0.81187-0. [DOI] [PubMed] [Google Scholar]
- Grove J, Marsh M. Host-pathogen interactions: the cell biology of receptor-mediated virus entry. J Cell Biol. 2011;195:1071–1082. doi: 10.1083/jcb.201108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha Y, Stevens DJ, Skehel JJ, Wiley DC. H5 avian and H9 swine influenza virus haemagglutinin structures: possible origin of influenza subtypes. EMBO J. 2002;21:865–875. doi: 10.1093/emboj/21.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Bushweller JH, Cafiso DS, Tamm LK. Membrane structure and fusion-triggering conformational change of the fusion domain from influenza hemagglutinin. Nat Struct Biol. 2001;8:715–720. doi: 10.1038/90434. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Reading PC, Ward AC, Anders EM. Changes in the hemagglutinin molecule of influenza type A (H3N2) virus associated with increased virulence for mice. Arch Virol. 1997;142:75–88. doi: 10.1007/s007050050060. [DOI] [PubMed] [Google Scholar]
- Hebert DN, Foellmer B, Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 1995;81:425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- Herfst S, Schrauwen EJA, Linster M, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012;336:1534–1541. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D, Klappe K. Fluorescence assays to monitor fusion of enveloped viruses. Meth Enzymol. 1993;220:261–276. doi: 10.1016/0076-6879(93)20088-k. [DOI] [PubMed] [Google Scholar]
- Huang Q, Opitz R, Knapp EW, Herrmann A. Protonation and stability of the globular domain of influenza virus hemagglutinin. Biophys J. 2002;82:1050–1058. doi: 10.1016/S0006-3495(02)75464-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Sivaramakrishna RP, Ludwig K, et al. Early steps of the conformational change of influenza virus hemagglutinin to a fusion active state: stability and energetics of the hemagglutinin. Biochim Biophys Acta. 2003;1614:3–13. doi: 10.1016/s0005-2736(03)00158-5. [DOI] [PubMed] [Google Scholar]
- Huang RT, Rott R, Wahn K, et al. The function of the neuraminidase in membrane fusion induced by myxoviruses. Virology. 1980;107:313–319. doi: 10.1016/0042-6822(80)90299-8. [DOI] [PubMed] [Google Scholar]
- Hulse DJ, Webster RG, Russell RJ, Perez DR. Molecular determinants within the surface proteins involved in the pathogenicity of H5N1 influenza viruses in chickens. J Virol. 2004;78:9954–9964. doi: 10.1128/JVI.78.18.9954-9964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyushina NA, Govorkova EA, Russell CJ, et al. Contribution of H7 haemagglutinin to amantadine resistance and infectivity of influenza virus. J Gen Virol. 2007;88:1266–1274. doi: 10.1099/vir.0.82256-0. [DOI] [PubMed] [Google Scholar]
- Imai M, Watanabe T, Hatta M, et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature. 2012 doi: 10.1038/nature10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby DB, Tamaoki J, Borson DB, Nadel JA. Influenza infection causes airway hyperresponsiveness by decreasing enkephalinase. J Appl Physiol. 1988;64:2653–2658. doi: 10.1152/jappl.1988.64.6.2653. [DOI] [PubMed] [Google Scholar]
- Jagger BW, Wise HM, Kash JC, et al. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science. 2012;337:199–204. doi: 10.1126/science.1222213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies KC, Cipriano DJ, Forgac M. Function, structure and regulation of the vacuolar (H+)-ATPases. Arch Biochem Biophys. 2008;476:33–42. doi: 10.1016/j.abb.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleta L, Ibricevic A, Bovin NV, et al. Experimental evolution of human influenza virus H3 hemagglutinin in the mouse lung identifies adaptive regions in HA1 and HA2. J Virol. 2008;82:11599–11608. doi: 10.1128/JVI.01393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido H, Yokogoshi Y, Sakai K, et al. Isolation and characterization of a novel trypsin-like protease found in rat bronchiolar epithelial Clara cells. A possible activator of the viral fusion glycoprotein. J Biochem. 1992;267:13573–13579. [PubMed] [Google Scholar]
- Kim JK, Negovetich NJ, Forrest HL, Webster RG. Ducks: the “Trojan horses” of H5N1 influenza. Influenza Other Respir Viruses. 2009;3(IRV084):121–128. doi: 10.1111/j.1750-2659.2009.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner I, Matrosovich MN, Haller O, et al. Altered receptor specificity and fusion activity of the haemagglutinin contribute to high virulence of a mouse-adapted influenza A virus. J Gen Virol. 2012;93:970–979. doi: 10.1099/vir.0.035782-0. [DOI] [PubMed] [Google Scholar]
- Kohio HP, Adamson AL. Glycolytic control of vacuolar-type ATPase activity: a mechanism to regulate influenza viral infection. Virology. 2013;444:301–309. doi: 10.1016/j.virol.2013.06.026. [DOI] [PubMed] [Google Scholar]
- Krauss S, Webster RG. Avian influenza virus surveillance and wild birds: past and present. Avian Dis. 2010;54:394–398. doi: 10.1637/8703-031609-Review.1. [DOI] [PubMed] [Google Scholar]
- Krenn BM, Egorov A, Romanovskaya-Romanko E, et al. Single HA2 mutation increases the infectivity and immunogenicity of a live attenuated H5N1 intranasal influenza vaccine candidate lacking NS1. PLoS One. 2011;6:e18577. doi: 10.1371/journal.pone.0018577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakadamyali M, Rust MJ, Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;124:997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakadamyali M, Rust MJ, Zhuang X. Endocytosis of influenza viruses. Microbes Infect. 2004;6:929–936. doi: 10.1016/j.micinf.2004.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikina E, Mittal A, Cho M-S, et al. Influenza hemagglutinins outside of the contact zone are necessary for fusion pore expansion. J Biochem. 2004;279:26526–26532. doi: 10.1074/jbc.M401883200. [DOI] [PubMed] [Google Scholar]
- Leser GP, Lamb RA. Influenza virus assembly and budding in raft-derived microdomains: a quantitative analysis of the surface distribution of HA, NA and M2 proteins. Virology. 2005;342:215–227. doi: 10.1016/j.virol.2005.09.049. [DOI] [PubMed] [Google Scholar]
- Li M, Yang C, Compans RW. Mutations in the cytoplasmic tail of murine leukemia virus envelope protein suppress fusion inhibition by R peptide. J Virol. 2001;75:2337–2344. doi: 10.1128/JVI.75.5.2337-2344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YP, Wharton SA, Martin J, et al. Adaptation of egg-grown and transfectant influenza viruses for growth in mammalian cells: selection of hemagglutinin mutants with elevated pH of membrane fusion. Virology. 1997;233:402–410. doi: 10.1006/viro.1997.8626. [DOI] [PubMed] [Google Scholar]
- Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- Matrosovich M, Stech J, Klenk HD. Influenza receptors, polymerase and host range. Rev Off Int Epizoot. 2009;28:203–217. doi: 10.20506/rst.28.1.1870. [DOI] [PubMed] [Google Scholar]
- Matrosovich M, Tuzikov A, Bovin N, et al. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J Virol. 2000;74:8502–8512. doi: 10.1128/jvi.74.18.8502-8512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikyan GB, Markosyan RM, Hemmati H, et al. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J Cell Biol. 2000;151:413–423. doi: 10.1083/jcb.151.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikyan GB, White JM, Cohen FS. GPI-anchored influenza hemagglutinin induces hemifusion to both red blood cell and planar bilayer membranes. J Cell Biol. 1995;131:679–691. doi: 10.1083/jcb.131.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Selman M, Dankar SK, Forbes NE et al (2012) Adaptive mutation in influenza A virus non-structural gene is linked to host switching and induces a novel protein by alternative splicing. Emerg Microbes Infect 1:e42. doi:10.1038/emi.2012.38 [DOI] [PMC free article] [PubMed]
- Murakami M, Towatari T, Ohuchi M, et al. Mini-plasmin found in the epithelial cells of bronchioles triggers infection by broad-spectrum influenza A viruses and Sendai virus. Eur J Biochem. 2001;268:2847–2855. doi: 10.1046/j.1432-1327.2001.02166.x. [DOI] [PubMed] [Google Scholar]
- Murakami S, Horimoto T, Ito M, et al. Enhanced growth of influenza vaccine seed viruses in vero cells mediated by broadening the optimal pH range for virus membrane fusion. J Virol. 2012;86:1405–1410. doi: 10.1128/JVI.06009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto Y, Noda T, Kawakami E, et al. Identification of novel influenza A virus proteins translated from PA mRNA. J Virol. 2013;87:2455–2462. doi: 10.1128/JVI.02656-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeve CW, Williams D. Fatty acids on the A/Japan/305/57 influenza virus hemagglutinin have a role in membrane fusion. EMBO J. 1990;9:3857–3866. doi: 10.1002/j.1460-2075.1990.tb07604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakowitsch S, Wolschek M, Morokutti A, Ruthsatz T. Mutations affecting the stability of the haemagglutinin molecule impair the immunogenicity of live attenuated H3N2 intranasal influenza vaccine candidates lacking NS1. Vaccine. 2011;29:3517–3524. doi: 10.1016/j.vaccine.2011.02.100. [DOI] [PubMed] [Google Scholar]
- Narasaraju T, Sim MK, Ng HH, et al. Adaptation of human influenza H3N2 virus in a mouse pneumonitis model: insights into viral virulence, tissue tropism and host pathogenesis. Microbes Infect. 2009;11:2–11. doi: 10.1016/j.micinf.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Nobusawa E, Aoyama T, Kato H, et al. Comparison of complete amino acid sequences and receptor-binding properties among 13 serotypes of hemagglutinins of influenza A viruses. Virology. 1991;182:475–485. doi: 10.1016/0042-6822(91)90588-3. [DOI] [PubMed] [Google Scholar]
- Okumura Y, Takahashi E, Yano M, et al. Novel type II transmembrane serine proteases, MSPL and TMPRSS13, proteolytically activate membrane fusion activity of the hemagglutinin of highly pathogenic avian influenza viruses and induce their multicycle replication. J Virol. 2010;84:5089–5096. doi: 10.1128/JVI.02605-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P, Tobita K, Ueda M, Compans RW. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974;61:397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- Park HE, Gruenke JA, White JM. Leash in the groove mechanism of membrane fusion. Nat Struct Biol. 2003;10:1048–1053. doi: 10.1038/nsb1012. [DOI] [PubMed] [Google Scholar]
- Reed ML, Bridges OA, Seiler P, et al. The pH of activation of the hemagglutinin protein regulates H5N1 influenza virus pathogenicity and transmissibility in ducks. J Virol. 2010;84:1527–1535. doi: 10.1128/JVI.02069-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed ML, Yen H-L, DuBois RM, et al. Amino acid residues in the fusion peptide pocket regulate the pH of activation of the H5N1 influenza virus hemagglutinin protein. J Virol. 2009;83:3568–3580. doi: 10.1128/JVI.02238-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GN, Paulson JC, Daniels RS, et al. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983;304:76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- Rosenthal PB, Zhang X, Formanowski F, et al. Structure of the haemagglutinin-esterase-fusion glycoprotein of influenza C virus. Nature. 1998;396:92–96. doi: 10.1038/23974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman JS, Jing X, Leser GP, Lamb RA. Influenza virus M2 protein mediates ESCRT-independent membrane scission. Cell. 2010;142:902–913. doi: 10.1016/j.cell.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman JS, Lamb RA. Influenza virus assembly and budding. Virology. 2011;411:229–236. doi: 10.1016/j.virol.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok RW, Martin SR, Wharton SA, et al. Conformational changes in the hemagglutinin of influenza virus which accompany heat-induced fusion of virus with liposomes. Virology. 1986;155:484–497. doi: 10.1016/0042-6822(86)90210-2. [DOI] [PubMed] [Google Scholar]
- Russell CJ, Jardetzky TS, Lamb RA. Membrane fusion machines of paramyxoviruses: capture of intermediates of fusion. EMBO J. 2001;20:4024–4034. doi: 10.1093/emboj/20.15.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell CJ, Webster RG. The genesis of a pandemic influenza virus. Cell. 2005;123:368–371. doi: 10.1016/j.cell.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Rust MJ, Lakadamyali M, Zhang F, Zhuang X. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat Struct Mol Biol. 2004;11:567–573. doi: 10.1038/nsmb769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R, Webster RG (2009) The influenza virus enigma. Cell 136:402–410. doi:10.1016/j.cell.S0092-8674(09)00077-4, 10.1016/j.cell.2009.01.029 [DOI] [PMC free article] [PubMed]
- Scholtissek C. Stability of infectious influenza A viruses at low pH and at elevated temperature. Vaccine. 1985;3:215–218. doi: 10.1016/0264-410x(85)90109-4. [DOI] [PubMed] [Google Scholar]
- Shaw ML, Palese P. Orthomyxoviridae. In: Knipe DM, Howley PM, editors. Fields virology. 6. Philadelphia: Lippincott Williams and Wilkins; 2013. pp. 1151–1185. [Google Scholar]
- Shelton H, Roberts KL, Molesti E, et al. Mutations in hemagglutinin that affect receptor binding and pH stability increase replication of a PR8 influenza virus with H5 HA in the upper respiratory tract of ferrets and may contribute to transmissibility. J Gen Virol. 2013 doi: 10.1099/vir.0.050526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieczkarski SB, Whittaker GR. Influenza virus can enter and infect cells in the absence of clathrin-mediated endocytosis. J Virol. 2002;76:10455–10464. doi: 10.1128/JVI.76.20.10455-10464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- Sorrell EM, Schrauwen EJA, Linster M, et al. Predicting “airborne” influenza viruses: (trans-) mission impossible? Curr Opin Virol. 2011;1:635–642. doi: 10.1016/j.coviro.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner TH. Intracellular and viral membrane fusion: a uniting mechanism. Curr Opin Cell Biol. 2004;16:429–435. doi: 10.1016/j.ceb.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Steinhauer DA. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology. 1999;258:1–20. doi: 10.1006/viro.1999.9716. [DOI] [PubMed] [Google Scholar]
- Steinhauer DA, Martin J, Lin YP, et al. Studies using double mutants of the conformational transitions in influenza hemagglutinin required for its membrane fusion activity. Proc Natl Acad Sci USA. 1996;93:12873–12878. doi: 10.1073/pnas.93.23.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer DA, Wharton SA, Skehel JJ, et al. Amantadine selection of a mutant influenza virus containing an acid-stable hemagglutinin glycoprotein: evidence for virus-specific regulation of the pH of glycoprotein transport vesicles. Proc Natl Acad Sci USA. 1991;88:11525–11529. doi: 10.1073/pnas.88.24.11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer DA, Wharton SA, Skehel JJ, Wiley DC. Studies of the membrane fusion activities of fusion peptide mutants of influenza virus hemagglutinin. J Virol. 1995;69:6643–6651. doi: 10.1128/jvi.69.11.6643-6651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer DA, Wharton SA, Wiley DC, Skehel JJ. Deacylation of the hemagglutinin of influenza A/Aichi/2/68 has no effect on membrane fusion properties. Virology. 1991;184:445–448. doi: 10.1016/0042-6822(91)90867-b. [DOI] [PubMed] [Google Scholar]
- Stieneke-Gröber A, Vey M, Angliker H, et al. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin-like endoprotease. EMBO J. 1992;11:2407–2414. doi: 10.1002/j.1460-2075.1992.tb05305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B, Wurtzer S, Rameix-Welti M-A, et al. Enhancement of the influenza A hemagglutinin (HA)-mediated cell-cell fusion and virus entry by the viral neuraminidase (NA) PLoS One. 2009;4:e8495. doi: 10.1371/journal.pone.0008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Shi Y, Lu X, et al. Bat-derived influenza hemagglutinin h17does not bind canonical avian or human receptors and most likely uses a unique entry mechanism. Cell Rep. 2013;3:769–778. doi: 10.1016/j.celrep.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Sun X, Whittaker GR. Entry of influenza virus. Adv Exp Med Biol. 2013;790:72–82. doi: 10.1007/978-1-4614-7651-1_4. [DOI] [PubMed] [Google Scholar]
- Suomalainen M. Lipid rafts and assembly of enveloped viruses. Traffic. 2002;3:705–709. doi: 10.1034/j.1600-0854.2002.31002.x. [DOI] [PubMed] [Google Scholar]
- Takeda M, Leser GP, Russell CJ, Lamb RA. Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion. Proc Natl Acad Sci USA. 2003;100:14610–14617. doi: 10.1073/pnas.2235620100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoennes S, Li Z-N, Lee B-J, et al. Analysis of residues near the fusion peptide in the influenza hemagglutinin structure for roles in triggering membrane fusion. Virology. 2008;370:403–414. doi: 10.1016/j.virol.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S, Li M, Vincent A, et al. Regulation of fusion activity by the cytoplasmic domain of a paramyxovirus F protein. Virology. 2002;301:322–333. doi: 10.1006/viro.2002.1594. [DOI] [PubMed] [Google Scholar]
- Tong S, Li Y, Rivailler P, et al. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci USA. 2012;109:4269–4274. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S, Zhu X, Li Y, et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013;9:e1003657. doi: 10.1371/journal.ppat.1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towatari T, Ide M, Ohba K, et al. Identification of ectopic anionic trypsin I in rat lungs potentiating pneumotropic virus infectivity and increased enzyme level after virus infection. Eur J Biochem. 2002;269:2613–2621. doi: 10.1046/j.1432-1033.2002.02937.x. [DOI] [PubMed] [Google Scholar]
- Veit M, Kretzschmar E, Kuroda K, et al. Site-specific mutagenesis identifies three cysteine residues in the cytoplasmic tail as acylation sites of influenza virus hemagglutinin. J Virol. 1991;65:2491–2500. doi: 10.1128/jvi.65.5.2491-2500.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vey M, Orlich M, Adler S, et al. Hemagglutinin activation of pathogenic avian influenza viruses of serotype H7 requires the protease recognition motif R-X-K/R-R. Virology. 1992;188:408–413. doi: 10.1016/0042-6822(92)90775-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R, Matrosovich M, Klenk H-D. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev Med Virol. 2002;12:159–166. doi: 10.1002/rmv.352. [DOI] [PubMed] [Google Scholar]
- Waning DL, Russell CJ, Jardetzky TS, Lamb RA. Activation of a paramyxovirus fusion protein is modulated by inside-out signaling from the cytoplasmic tail. Proc Natl Acad Sci USA. 2004;101:9217–9222. doi: 10.1073/pnas.0403339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington N, Steele RJ, Jackson SJ, et al. Determination of baseline human nasal pH and the effect of intranasally administered buffers. Int J Pharm. 2000;198:139–146. doi: 10.1016/s0378-5173(99)00442-1. [DOI] [PubMed] [Google Scholar]
- Webster RG, Rott R. Influenza virus A pathogenicity: the pivotal role of hemagglutinin. Cell. 1987;50:665–666. doi: 10.1016/0092-8674(87)90321-7. [DOI] [PubMed] [Google Scholar]
- Weis W, Brown JH, Cusack S, et al. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988;333:426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- Weis WI, Cusack SC, Brown JH, et al. The structure of a membrane fusion mutant of the influenza virus haemagglutinin. EMBO J. 1990;9:17–24. doi: 10.1002/j.1460-2075.1990.tb08075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W, Schekman R. Membrane fusion. Nat Struct Mol Biol. 2008;15:658–664. doi: 10.1038/nsmb.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley DC, Wilson IA, Skehel JJ. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981;289:373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Skehel JJ, Wiley DC. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981;289:366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Wise HM, Foeglein A, Sun J, et al. A complicated message: Identification of a novel PB1-related protein translated from influenza A virus segment 2 mRNA. J Virol. 2009;83:8021–8031. doi: 10.1128/JVI.00826-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise HM, Hutchinson EC, Jagger BW, et al. Identification of a novel splice variant form of the influenza A virus M2 ion channel with an antigenically distinct ectodomain. PLoS Pathog. 2012;8:e1002998. doi: 10.1371/journal.ppat.1002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Coombs PJ, Martin SR, et al. Receptor binding by a ferret-transmissible H5 avian influenza virus. Nature. 2013;497:392–396. doi: 10.1038/nature12144. [DOI] [PubMed] [Google Scholar]
- Xu R, Wilson IA. Structural characterization of an early fusion intermediate of influenza virus hemagglutinin. J Virol. 2011;85:5172–5182. doi: 10.1128/JVI.02430-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaraket H, Bridges OA, Duan S, et al. Increased acid stability of the hemagglutinin protein enhances H5N1 influenza virus growth in the upper respiratory tract but is insufficient for transmission in ferrets. J Virol. 2013;87:9911–9922. doi: 10.1128/JVI.01175-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaraket H, Bridges OA, Russell CJ. The pH of activation of the hemagglutinin protein regulates H5N1 influenza virus replication and pathogenesis in mice. J Virol. 2013;87:4826–4834. doi: 10.1128/JVI.03110-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Shi Y, Lu X, et al. An airborne transmissible avian influenza H5 hemagglutinin seen at the atomic level. Science. 2013;340:1463–1467. doi: 10.1126/science.1236787. [DOI] [PubMed] [Google Scholar]
- Zhou J, Xu S, Ma J, et al. Recombinant influenza A H3N2 viruses with mutations of HA transmembrane cysteines exhibited altered virological characteristics. Virus Genes. 2013 doi: 10.1007/s11262-013-1011-2. [DOI] [PubMed] [Google Scholar]


