Abstract
Oral forms of medications contain “inactive” ingredients to enhance their physical properties. Using data analytics, we characterized the abundance and complexity of inactive ingredients in approved medications. A majority of medications contain ingredients that could cause adverse reactions, underscoring the need to maximize the tolerability and safety of medications and their inactive ingredients.
One Sentence Summary:
Inactive ingredients in oral medications are generally poorly appreciated and many include materials associated with adverse reactions in patients.
Active and inactive ingredients
Oral drug products include both the active pharmaceutical ingredient (API) and a specific mixture of inactive ingredients (excipients). The United States Food and Drug Administration (FDA) defines the API as the compound intended to provide the desired pharmaceutical effect. Conversely, inactive ingredients are broadly defined as “any component of a drug product other than an active ingredient” (1). These components are not intended or expected to have a direct biological or therapeutic effect but instead are added to alter the physical properties of an oral solid dosage form (tablet or capsule) to facilitate absorption or to improve stability, taste, appearance, or to render the therapeutic tamper-resistant (2). Together, the API and the inactive ingredients make up a specific pharmaceutical formulation.
Decades of pharmaceutical development have tailored inactive ingredient components to ensure that the desired properties of the formulation are met. Manufacturers will often design formulations by borrowing from thousands of known inactive ingredients (3) because approval of novel excipients can require extensive toxicological profiling (4). Although established excipients have precedence of showing safety on the population level and can be reviewed to evaluate their toxicities, health effects that are undetectable in current preclinical toxicology screenings could remain obscured. Scattered case reports (5–7) have brought this to the attention of formulation scientists (4), clinicians (5), and legislative agencies (8, 9), but the magnitude and scope of this challenge is currently unknown.
Allergies and intolerances
Increasing numbers of clinical reports have documented adverse reactions triggered by an inactive ingredient in a medication (Fig. 1A) (2, 5–7). These adverse reaction-associated inactive ingredients (ARAIIs) can commonly cause symptoms in the form of an allergy [“an immunologically mediated response to a pharmaceutical and/or formulation (excipient) agent in a sensitized person” (10)] or an intolerance. Many allergic reactions to inactive ingredients are Type I hypersensitivity reactions, mediated by Immunoglobulin E recognition of an antigen and characterized by symptoms associated with histamine release such as urticaria, angioedema, bronchospasm, and anaphylaxis (10). Such rare effects can lead to drastic adverse events in small patient subpopulations (5, 11). Conversely, intolerances to an inactive ingredient can cause symptoms through mechanisms such as malabsorption, which causes gastrointestinal symptoms via direct osmotic effects or as a result of their fermentation in the digestive system. These potentially affect a much larger population with more benign symptoms compared to allergic reactions (12, 13). These pathways might lead to adverse drug effects that affect patients’ well-being and adherence to drug regimens if the inactive ingredients are present in sufficient quantities to trigger a reaction.
Fig. 1. Active versus inactive ingredients and complexity of formulations.
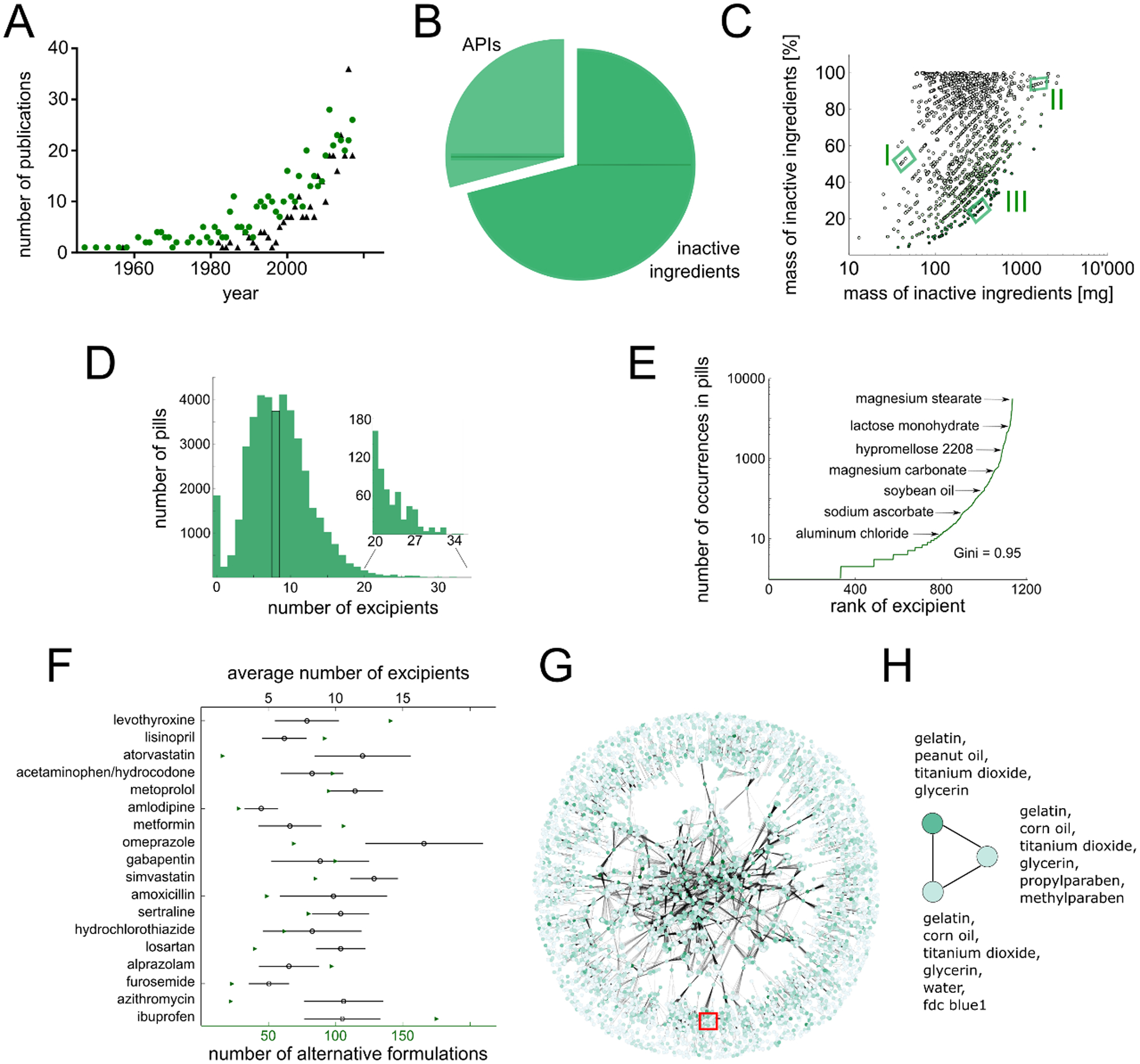
(A) Number of publications in PubMed containing the search terms “excipient allergy” (green circles) or “excipient irritation” (black triangles) per year. (B) Percentage of the mass of a medication corresponding to inactive (dark green) versus active (light green) ingredients. (C) Correlation analysis between the mass and percentage of inactive ingredients in a given medication. Green shading denotes dose. Different formulations for the same API and dose are grouped together [e.g. valsartan 40 mg (I), cyclosporine 100 mg (II), and amoxicillin 1 g (III)]. (D) Distribution of inactive ingredients in oral solid dosage forms. The median (eight) is highlighted in black. Insert shows the distribution of 596 pills/capsules with 20 inactive ingredients or more. (E) Frequency of inactive ingredients. Gini coefficient = 0.95. (F) Formulation heterogeneity for the 18 most-prescribed single-agent oral medications during 2016 (14). Green triangles denote the number of different available formulations; the mean and standard deviation of the distribution of the number of inactive ingredients contained in these formulations are depicted by black circles and lines, respectively. (G) Formulation network highlighting complexity of formulation space. Each node corresponds to a specific combination of inactive ingredients; two nodes are connected when at least one API has been commercially formulated with each of these separate combinations of inactive ingredients. Node color corresponds to frequency of formulation usage, edge thickness corresponds to number of APIs that have been formulated with either of the two inactive ingredient combinations. Few clusters of inactive ingredients are exclusively applied to certain drugs (periphery), whereas other formulations are heavily applied to many different APIs and form a complex relationship (central region). The red box highlights region of valproic acid formulations. (H) Enlarged valproic acid region from (G). Network for three different combinations of inactive ingredients currently used to formulate valproic acid. Darker green indicates more frequent use.
Inactive ingredients: A major component of drug mass
Oral solid dosage formulations of the most frequently prescribed medications in the United States (14), as supplied from the pharmacy at Brigham and Women’s Hospital, consist of 75% ± 26% inactive ingredients (table S1). The lipid-lowering agent atorvastatin calcium 80 mg (Major Pharma) is indicated for the prevention of various cardiovascular diseases and contains the largest amount of inactive ingredient among these pills, with an inactive ingredient mass of 770 mg. Simvastatin 5 mg (McKesson), belonging to the same medication class as atorvastatin, contained the lowest amount of inactive ingredients (50 mg), which nevertheless accounted for 90% of its total mass. The German database “Gelbe Liste” (www.gelbe-liste.de) captures the piece weights for a large set of 1,902 different medications, extending the scope of our analysis of the most frequently prescribed medication. We mined these data and observed a similar average value of 71% ± 26% (Fig. 1B), highlighting that inactive ingredients make up the major part of the administered material. In terms of mass, an average tablet or capsule contains 280 mg of inactive ingredient and only 164 mg of API. Close to half (41.3%) of all drug products contain more than 250 mg of inactive ingredients (Fig. 1C). Such doses are further multiplied by polypharmacy (simultaneous usage of multiple medications), which is particularly prevalent in older adults: 39.0% of Americans over the age of 65 take at least five prescription medications daily (15), and 11.7% of a similar cohort of Swedes took more than 10 prescription medications daily (16). A patient taking 10 prescription medications would ingest an average of 2.8 g of inactive ingredients daily. This is a substantial amount of excipient material that is administered to patients every day and merits further consideration.
Complexity of the formulation landscape
The Pillbox database (https://pillbox.nlm.nih.gov) contains information on 42,052 oral solid dosage formulations consisting of a total of 354,597 inactive ingredients. According to this data, an average tablet or capsule contains 8.8 inactive ingredients (Fig. 1D). 596 oral solid dosage forms contain 20 different inactive ingredients or more (Fig. 1D inset). Individual inactive ingredients occur in vastly different numbers (Fig. 1E, table S2): magnesium stearate can be found in 30,263 oral solid dosage forms (72%), whereas a third of all inactive ingredients (333, 30%) only occur once. We calculated the Gini index to measure disparity in inactive ingredient occurrence. The Gini index is a value ranging from zero (perfect equality, all ingredients occur in the same frequency) to one (perfect inequality, only a single ingredient occurs in all medications and other ingredients never occur). A Gini index of 0.95, close to perfect inequality, indicates that the number of occurrences of inactive ingredients is heavily skewed towards the most commonly occurring inactive ingredients (Table 1).
Table 1. List of critical inactive ingredients that can act as allergens.
Percentage occurrence refers to fraction of all formulations of medications (solid oral dosage forms) that contain the critical ingredient.
| Ingredient | Classification | Percentage occurrence in medications |
|---|---|---|
| Lactose | food | 44.82% |
| Corn starch | food | 36.54% |
| PEG | polymer | 36.03% |
| Povidone | polymer | 35.80% |
| Carboxymethylcellulose | other | 21.38% |
| Gelatin | food | 16.93% |
| Brilliant blue | dye | 14.47% |
| Sunset Yellow FCF | dye | 12.27% |
| Allura red | dye | 11.20% |
| Propylene glycol | other | 11.14% |
| Indigo carmine | dye | 10.63% |
| Mannitol | sugar | 7.20% |
| Sucrose | sugar | 5.21% |
| Sodium benzoate | other | 1.72% |
| Parabens | other | 1.48% |
| Aspartame | other | 1.46% |
| Erythrosine | dye | 1.03% |
| Tartrazine | dye | 0.95% |
| Saccharin | other | 0.81% |
| Poloxamer | polymer | 0.76% |
| Soybean oil | food | 0.44% |
| Benzyl alcohol | other | 0.43% |
| Vanilla | food | 0.38% |
| Castor oil | food | 0.30% |
| Cetyl alcohol | other | 0.19% |
| Sulfite | other | 0.19% |
| PEG castor oils | food | 0.13% |
| Peanut oil | food | 0.08% |
| Benzoic acid | other | 0.07% |
| Corn syrup | food | 0.05% |
| Sesame Oil | food | 0.05% |
| Starch wheat | food | 0.04% |
| Casein | food | 0.03% |
| Banana essence | food | 0.01% |
| Milk | food | 0.01% |
| Glucosamine | food | 0.00% |
| New coccine | dye | 0.00% |
| Stearyl alcohol | other | 0.00% |
On average, 82.5 alternative formulations are available per API for the 18 most frequently prescribed oral medications in the US (Fig. 1F) (14), highlighting the multiplicity of available versions of the same medication. For example, 140 distinct formulations of the hypothyroidism treatment levothyroxine are produced by 43 different manufactures. Varying numbers of included inactive ingredients in such formulations (Fig. 1F) indicates that different commercially-available versions of medications can contain different excipient mixtures. A “formulation network” can visualize this relationship on a larger scale, depicting available alternatives of all oral solid dosage forms and interchangeabilities of inactive ingredients (Fig. 1G). The network consists of a total of 13,287 nodes, corresponding to the number of unique combinations of inactive ingredients available in Pillbox. The network is populated with a total of 314,866 edges that highlight interchangeability of formulations. Only 1,003 formulations (7.5%) appear unique (isolated nodes on the periphery of the network). Most of these (668, 67%) have been reported only for a single API. A much larger fraction of the network corresponds to inactive ingredient combinations that have been used interchangeably for at least one API (mean value 3.12). These nodes build a convoluted network with distinct relationships between the formulations, highlighting the complexity of available alternatives. A mean degree of 23.7 indicates that, on average, more than 23 alternative combinations of inactive ingredients have been commercialized to deliver the same APIs. These results highlight the multiplicity of available alternatives of medications in terms of their inactive ingredient portion and warrants further study of the differences between those alternatives.
Adverse reactions associated with excipients
A total of 38 inactive ingredients (Table 1) have been described to cause allergic symptoms after oral exposure through direct allergenic potential or through contamination introduced through these ingredients (table S3). These associations are supported by re-challenge with the isolated ARAII or the report of the patient tolerating an alternative formulation. Although these inactive ingredients occur in widely different frequencies (Table 1), a Gini index of 0.75 is lower for ARAIIs compared to all inactive ingredients – indicating a more homogeneous occurrence among medications. Almost all oral solids (92.8%) contain at least one potential allergen (Fig. 2A). Viewed through the lens of the APIs, only 28% of active ingredients have at least one available formulation that avoids all of these potential allergens, and only 12% of APIs are free of inactive ingredients that have been reported to cause allergic reactions (fig. S1). In many cases, particular APIs will contain a specific ARAII in all available formulations. For example, all available rosuvastatin calcium and diclofenac tablets, among others, contain lactose as an inactive ingredient which might cause allergic reactions through contamination with milk protein (Fig. 2B).
Fig. 2. ARAIIs in drugs.
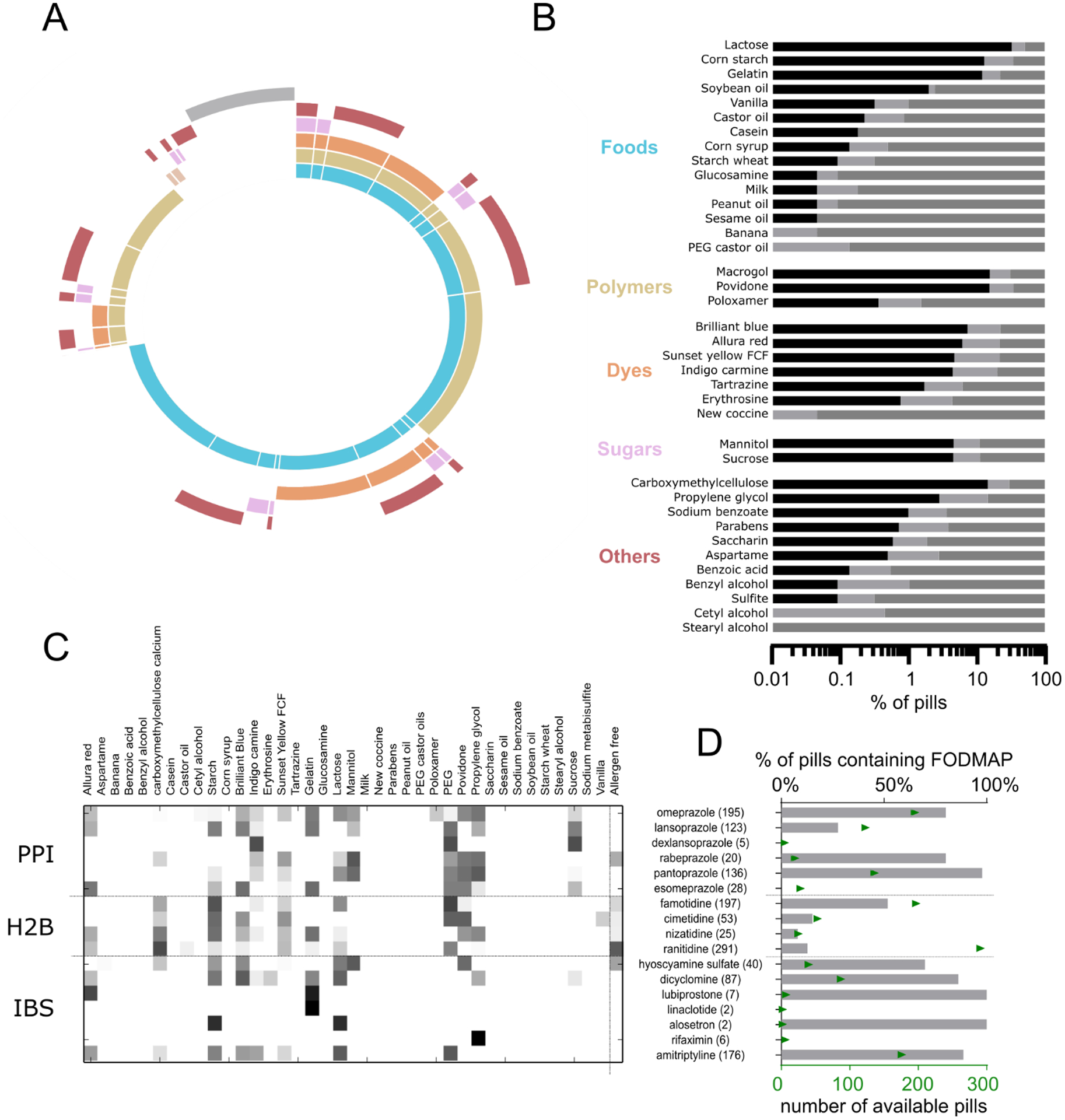
(A) Pie chart depicting percentage of medications containing potential allergen classes (or excipients with the potential to be contaminated with allergens). Gray bar: % of medications without any potential allergens. Colors correspond with classes in (B). (B) Percentage of APIs with potential allergens. Black bar: all formulations of the API contain a specific allergy-associated inactive ingredient; dark gray: all formulations of the API are devoid of the allergen inactive ingredient; light gray: some formulations of the API contain the potential allergen. (C) Heatmap showing the ARAII content of different GI therapeutics, grouped by medication class. Numbers in parentheses indicate number of available formulations; PPI: proton pump inhibitor, H2B: Histamine 2 blockers, IBS: inflammatory bowel syndrome treatments.. (D) Analysis of FODMAP content in gastrointestinal therapeutics
As opposed to the small number of patients who experience severe allergic reactions to inactive ingredients, many more patients are vulnerable to experiencing adverse symptoms caused by the inactive ingredients. For example, the symptoms of irritable bowel syndrome (IBS) are being increasingly managed in part by a diet that is low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) (12). 55% of all oral medications contained at least one FODMAP sugar in their formulation, and 5% contained more than one FODMAP sugar. The most commonly occurring FODMAPs are lactose, mannitol, and polydextrose, found in 45%, 7%, and 4% of all oral solids, respectively. Quantities of these sugars could exceed 500 mg per pill (table S4), contributing to increased FODMAP consumption and potential discomfort.
Allergen ARAII and FODMAP content in oral medications to manage gastrointestinal symptoms is of particular concern because recipients of these medications may experience a worsening of their symptoms due to these ingredients. Certain medication classes are more likely to contain specific ARAIIs, although there were often available medications in the same class that avoided those inactive ingredients (Fig. 2C). For example, polymers such as povidone, polyethylene glycol (PEG), and propylene glycol occur commonly in proton pump inhibitors (PPI), with the exception of dexlansoprazole. Rifaximin tablets (used for treating IBS) contain propylene glycol, which might worsen symptoms. We found that FODMAP sugars were commonly included in formulations across gastrointestinal drug classes, but every investigated class had FODMAP-free alternatives (Fig 2D). These data highlight the need for appropriate selection of not only the API but also the formulation as a whole to help mitigate adverse reactions or improve symptom control in some patients.
Lactose, peanut oil, gluten and chemical dyes
In addition to lactose’s role as an allergen (through potential contamination with milk protein) (5) and FODMAP sugar (12), lactose intolerance is present in 75% of the world population (17). Nevertheless, lactose is commonly used in 45% of all oral solid dosage forms (Table 1), with lactose content reaching close to 600 mg per pill (table S4). Lactose intake from medications has been associated with adverse reactions in multiple published case reports (18, 19), although whether low quantities of lactose elicit reactions remains debated (20, 21). It appears lactose content in medications is too small to cause symptoms for many patients, but individuals with severe cases of lactose intolerance could be affected by less than 200 mg of lactose (7, 13), an amount possibly exceeded by a single medication (table S4). Furthermore, patients with multiple comorbidities could be more susceptible given their exposure to multiple medications each day: for instance, a patient with hypertension and high cholesterol could be on a regimen of amlodipine, simvastatin, and losartan with a combined daily load of lactose close to 1 g (table S4). Under-recognition of the lactose content in medications could be an avoidable cause of medication non-compliance or discontinuation that could be mistakenly attributed to the API.
Conversely, allergens can cause severe reactions even at a very low exposure, with lowest-observed-adverse-effect levels (LOAEL) in the sub milligram range (22), which might trigger reactions after administering only a single agent. For many such ingredients, manufacturers include warning labels emphasizing the physiological relevance of this association. For example, according to the Pillbox data, 100% of progesterone and 62.5% of valproic acid capsules contain peanut oil as a solubilizer (Fig 2B). APIs with such formulations cannot be taken by patients with peanut allergies, limiting therapeutic opportunities (23). Estimates on the prevalence of peanut allergy reach up to 4% of the US population with a growing incidence in children (24). Some formulations of valproic acid replace peanut oil with corn oil, supporting the potential to confer safer adverse effect profiles by substituting critical ingredients with possibly more benign alternatives (Fig 1G and 1H) (25).
Gluten can cause severe reactions in patients suffering from celiac disease (26) at doses as low as 1.5 mg daily when exposed chronically (27). Inactive ingredients produced from wheat starch can result in gluten content in medications. In a survey (28), 18% of manufacturers indicated that their medications contain gluten. Although 69% claimed to produce gluten-free products, only 17% tested their products and could provide documentation on the performed tests. The FDA has recently recommended adding gluten content to product labels (8), indicating an increasing awareness of the potential risk for patients.
Chemical dyes, such as tartrazine, have been suspected to cause severe atopic reactions (6), specifically in patients with existing allergic or asthmatic conditions (29, 30). However, 33% of all medications contain at least one chemical dye associated with allergic reactions in patients (Fig. 2A and 2B, Table 1). Researchers have conducted trials to investigate allergic reactions in patients receiving tartrazine-containing medications versus the same patients receiving tartrazine-free alternatives (11, 31). These trials observed adverse symptoms associated with tartrazine content in about 4% of all patients and higher incidence in sensitive subgroups. This data supports the potential of inactive ingredients as the cause of adverse events in patients.
Conclusions
The future of inactive ingredient research will flourish with more detailed datasets. The mass content of individual inactive ingredients in pills or capsules is largely not reported by manufacturers and therefore is not easily accessible to patients and health care providers. Conversely, for many of the reported allergens and irritants, the distribution of sensitivities among relevant patient populations is sparsely understood. With increasing data availability, future studies can carefully align the precise mass of critical ingredients with the maximum dose tolerated by different patients to characterize affected patient populations and culprit formulations.
It is known that a few select excipients have the potential to alter the pharmacokinetic properties of an API, for example via physicochemical interactions (32) or by modulating metabolic and transport enzymes (33). Appropriate tailoring of a specific formulation for a specific patient could thereby not only avoid adverse reactions but also enable to achieve fine-tuned pharmacokinetic and metabolic profiles.
Accounting for effects of excipients will enable advanced formulations for difficult-to-deliver medications (1) and could lead to personalized medicine for vulnerable subpopulations (9, 15, 25). Such analysis will empower clinicians to make conscious selections of formulations focusing on their patients’ well-being. Recognizing that the inactive portion of a medication, which corresponds on average to two-thirds of the administered material, may be more ‘active’ than previously anticipated, we foresee potential implications for medical protocols, regulatory sciences, and pharmaceutical development of oral medications.
Supplementary Material
Fig. S1. Summary statistics for different allergen classes of potential allergens.
Fig. S2. Flowchart of data curation strategy for Pillbox extraction.
Table S1. Piece weight analysis of different versions of most commonly prescribed medications.
Table S2. Top ten most common inactive ingredients in Pillbox.
Table S3. List of publications analyzed for identification of reports of allergic reactions or gastrointestinal side effects through inactive ingredients in medications.
Table S4. Lactose content of various medications.
Table S5. Corrected and identified misspellings or alternative spellings in the Pillbox database.
Acknowledgments:
We thank “Gelbe Liste” for providing us with a resource of medication weights and Ellen Reifferscheid for granting access. We thank Caterina Foti and Gianfranco Calogiuri for providing access to their previous work. We are grateful to Robert S. Langer and Aaron Kesselheim for invaluable guidance and comments on this work.
Funding: This work was funded in part by: Swiss National Science Foundation Fellowships P2EZP3_168827 and P300P2_177833 (DR), the Department of Medicine Residency Program (SB), the Alexander von Humboldt Foundation Feodor Lynen Fellowship (CS), the NIH grant EB000244 (GT), the Division of Gastroenterology (GT), and the MIT-IBM Watson AI Lab (DR, CS, GT).
Footnotes
Competing interests: Complete details of all relationships for profit and not for profit for G.T. can be found at the following link: https://www.dropbox.com/sh/szi7vnr4a2ajb56/AABs5N5i0q9AfT1IqIJAE-T5a?dl=0. SB has a consulting relationship with Two River Consulting and is an equity holder in Kronos Bio, Inc.. D.R., S.B., G.T. are co-inventors on a provisional patent application 62/811,502 describing systems to quantify the burden of inactive ingredients in medications.
Data and materials availability: All data associated with this study are present in the paper or supplementary materials.
References and Notes:
- 1.Katdare A, Chaubal MV, Eds., Excipient Development for Pharmaceutical, Biotechnology, and Drug Delivery Systems (Informa Healthcare, 2006). [Google Scholar]
- 2.Abrantes CG, Duarte D, Reis CP, An Overview of Pharmaceutical Excipients: Safe or Not Safe?, J. Pharm. Sci 105, 2019–2026 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Jensen MP, Karoly P, Handbook of Pharmaceutical Excipients – 7th Edition (The Pharmaceutical Press, 2013). [Google Scholar]
- 4.Elder DP, Kuentz M, Pharmaceutical excipients — quality, regulatory and biopharmaceutical considerations, Eur. J. Pharm. Sci 87, 88–99 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Kelso JM, Potential food allergens in medications, J. Allergy Clin. Immunol 133, 1509–1520 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Swerlick RA, Campbell CF, Medication dyes as a source of drug allergy., J. drugs dermatology JDD 12, 99–102 (2013). [PubMed] [Google Scholar]
- 7.Brandstetter RD, Conetta R, Glazer B, Lactose Intolerance Associated with Intal Capsules, New Engl. J. Med 315, 1613–1614 (1986). [DOI] [PubMed] [Google Scholar]
- 8.FDA, Gluten in Drug Products and Associated Labeling Recommendations, Draft Guid. (2017). [Google Scholar]
- 9.Salunke S, Liu F, Batchelor H, Walsh J, Turner R, Ju TR, Tuleu C, European Paediatric Formulation Initiative (EuPFI)—Formulating Ideas for Better Medicines for Children, AAPS PharmSciTech 18, 257–262 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Drug allergy: An updated practice parameter.Ann. Allergy, Asthma Immunol 105, 259–273 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Bhatia MS, Allergy to tartrazine in psychotropic drugs, J. Clin. Psychiatr 61, 473–476 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG, A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome, Gastroenterology 146, 67–75.e5 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Mill D, Dawson J, Johnson JL, Managing acute pain in patients who report lactose intolerance: the safety of an old excipient re-examined, Ther. Adv. Drug Saf (2018), doi: 10.1177/2042098617751498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aitken M, Kleinrock M, Medicines Use and Spending in the U.S.: A Review of 2016 and Outlook to 2021., Rep. by QuintilesIMS Institute. (2017). [Google Scholar]
- 15.Charlesworth CJ, Smit E, Lee DSH, Alramadhan F, Odden MC, Polypharmacy Among Adults Aged 65 Years and Older in the United States: 1988–2010., J Gerontol A Biol Sci Med Sci 70, 989–95 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morin L, Johnell K, Laroche M-L, Fastbom J, Wastesson JW, The epidemiology of polypharmacy in older adults: register-based prospective cohort study., Clin. Epidemiol 10, 289–298 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suarez FL, Savaiano DA, Levitt MD, A Comparison of Symptoms after the Consumption of Milk or Lactose-Hydrolyzed Milk by People with Self-Reported Severe Lactose Intolerance, New Engl. J. Med 333, 1–4 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Lieb J, Kazienko DJ, Lactose Filler as a Cause of Drug-Induced Diarrhea, New Engl. J. Med 299, 314–314 (1978). [DOI] [PubMed] [Google Scholar]
- 19.Petrini L, Usai P, Caradonna A, Cabula R, Mariotti S, Lactose intolerance following antithyroid drug medications, J. Endocrinol. Investig 20, 569–570 (1997). [DOI] [PubMed] [Google Scholar]
- 20.Guslandi M, Lactose content of gastrointestinal drugs: does it matter?, Aliment. Pharmacol. Ther 29, 1212–1212 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Montalto M, Gallo A, Santoro L, D’Onofrio F, Curigiliano V, Covino M, Cammarota G, Grieco A, Gasbarrini A, Gasbarrini G, Low-dose lactose in drugs neither increases breath hydrogen excretion nor causes gastrointestinal symptoms, Aliment. Pharmacol. Ther 28, 1003–1012 (2008). [DOI] [PubMed] [Google Scholar]
- 22.FDA, Approaches to establish thresholds for major food allergens and for gluten in food.J. Food Prot, 108 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Cohen AL, Neumayer L, Boucher K, Factor RE, Shrestha G, Wade M, Lamb JG, Arbogast K, Piccolo SR, Riegert J, Schabel M, Bild AH, Werner TL, Window-of-Opportunity Study of Valproic Acid in Breast Cancer Testing a Gene Expression Biomarker, JCO Precis. Oncol, 1–11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burks AW, Peanut allergy, Lancet 371, 1538–1546 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Nagel-Edwards KM, Ko JY, Excipient choices for special populations, Int. J. Pharm. Compd 12, 426–430 (2008). [PubMed] [Google Scholar]
- 26.Reid J, Kelly A, McDonald S, Systematic review of safe level of gluten for people with coeliac disease (Cochrane Australia, 2016). [Google Scholar]
- 27.Chartrand LJ, Russo PA, Duhame AG, Seidman EG, Wheat Starch Intolerance in Patients With Celiac Disease, J. Am. Diet. Assoc 97, 612–618 (1997). [DOI] [PubMed] [Google Scholar]
- 28.King AR, Gluten Content of the Top 200 Medications: Follow-Up to the Influence of Gluten on a Patient’s Medication Choices., Hosp. Pharm 48, 736–43 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stenius BSM, Lemola M, Hypersensitivity to acetylsalicylic acid (ASA) and tartrazine in patients with asthma, Clin. Exp. Allergy 6, 119–129 (1976). [DOI] [PubMed] [Google Scholar]
- 30.Neuman I, Elian R, Nahum H, Shaked P, Creter D, The danger of “yellow dyes” (tartrazine) to allergic subjects, Clin. Exp. Allergy 8, 65–68 (1978). [DOI] [PubMed] [Google Scholar]
- 31.MacCara ME, Tartrazine: a potentially hazardous dye in Canadian drugs., Can. Med. Assoc. J 126, 910–4 (1982). [PMC free article] [PubMed] [Google Scholar]
- 32.Amaral Silva D, Löbenberg R, Davies N, Are Excipients Inert? Phenytoin Pharmaceutical Investigations with New Incompatibility Insights., J Pharm Pharm Sci 21, 29745 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, Li Y, Zou P, Wu M, Zhang Z, Zhang T, The Effects of Pharmaceutical Excipients on Gastrointestinal Tract Metabolic Enzymes and Transporters—an Update, APPS J. 18, 830–843 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Summary statistics for different allergen classes of potential allergens.
Fig. S2. Flowchart of data curation strategy for Pillbox extraction.
Table S1. Piece weight analysis of different versions of most commonly prescribed medications.
Table S2. Top ten most common inactive ingredients in Pillbox.
Table S3. List of publications analyzed for identification of reports of allergic reactions or gastrointestinal side effects through inactive ingredients in medications.
Table S4. Lactose content of various medications.
Table S5. Corrected and identified misspellings or alternative spellings in the Pillbox database.


