Abstract
The gene silencing capability of RNA interference (RNAi) is being used to study individual gene's biological function and role in biochemical pathways. However, the efficacy of RNAi depends upon efficient delivery of the intermediates of RNAi, small interfering RNA (siRNA) oligonucleotides. The delivery challenge is even greater when the aim is to inhibit the expression of target genes in disease tissues. In vivo delivery of siRNA is complicated and challenging, and recent works on various animal disease models and early successes in human clinical trials are enlightening the tremendous potential of RNAi therapeutics. In this chapter, the latest developments of in vivo delivery of siRNA and the critical issues related to this effort are addressed.
Keywords: RNA interference, Small interfering RNA, In vivo delivery, RNAi therapeutics, Nanoparticle, Local delivery, Systemic delivery
1 Introduction
RNA interference (RNAi) has been rapidly adopted for the discovery and validation of gene function through cell culture and animal model studies, using sequence-specific small interfering RNA (siRNA) [1, 2]. These siRNA intermediates (21–23-nt oligos) were found to bind to an RNA induced silencing complex (RISC) and then selectively degrade the complementary single-stranded target RNA in a sequence-specific manner. Not only is siRNA being used to characterize gene function in high throughput screens for potential therapeutic targets, the growing success as a research tool has also stirred up tremendous interest in using siRNA as a therapeutic agent. With many reports on the in vivo application of siRNA inhibitors and the success of several early phase clinical trials, a broad therapeutic application of RNAi therapeutics is coming. One of the major hurdles to realize the RNAi therapeutic potential is to overcome the obstacles to the siRNA delivery locally and systemically to the disease tissues. In this review, we address the challenges and problems for in vivo siRNA delivery for potential therapeutic applications.
2 Challenges of In Vivo siRNA Delivery
As a potent and specific inhibitor of gene expression, siRNA is being rapidly adopted as the preferred tool for functional genomics research [3, 4]. siRNA oligos are typically used to inhibit an individual gene, though targeting multiple genes or groups of genes are possible by using a combination of multiple siRNA sequences [5, 6]. The success of using siRNA to knockdown gene expression in vitro has led to a growing interest in applications of siRNA inhibitors in vivo for evaluation of the therapeutic potentials of the gene targets of interest, potency of siRNA inhibitors, the route of administration, and the unwanted adverse effects. These applications should eventually provide validated targets for conventional therapeutic modalities such as small molecule and monoclonal antibody inhibitors, as well as validation of siRNA drug lead itself (Fig. 3.1) [3].
Fig. 3.1.
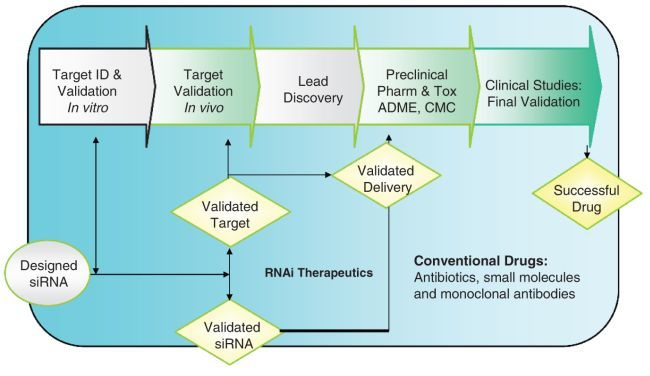
Delivery of siRNA for drug discovery and development. Effective siRNA deliveries in vitro and in vivo are playing very important role for siRNA-based target validation and potential RNAi therapeutics [5]. When targets are validated through in vivo siRNA delivery process, there are already three types of outcomes: validated targets, validated siRNA duplexes, and validated in vivo delivery
One example is using siRNA oligos specifically targeting angiogenesis factors, such as VEGFs, EGF, FGFs, and their receptors, representing the most widely recognized targets that took years to validate. One study using siRNA-mediated downregulation of these proangiogenesis genes, VEGF and VEGF R2, in clinically relevant xenograft tumor models resulted in a significant antitumor efficacy. Thus, the functions of these two targets were further validated rapidly in a matter of weeks [7]. This example demonstrates the power of in vivo target validation with siRNA inhibitors. In this case, not only the roles of the proangiogenic factors were validated, the siRNA inhibitors themselves were also validated as potential anticancer drugs.
Delivering siRNA oligos in vivo to animal tissues and keeping them active in the targeted cells are complicated and involve using a physical, chemical, or biological approach and in some cases their combination [7]. Since the main goal of in vivo delivery is to have active siRNA oligos in the target cells, the stability of siRNA oligos in both the extracellular and intracellular environment after a systemic administration is the most challenging issue (Fig. 3.2). The first hurdle is the size of the 21–23-nt double-stranded siRNA oligos: they are relatively small and thus rapidly excreted through urine when administrated into the blood stream, even if those siRNA molecules remain stable through chemical modifications. Second, the double-stranded siRNA oligos are relatively unstable in the serum environment and are potentially degraded by RNase activity within a short period of time. Third, when siRNA is administered systemically, the nonspecific distribution of the oligos throughout the body will significantly decrease the local concentration at the site of disease. In addition, the siRNA oligos need to overcome the blood vessel endothelial wall and multiple tissue barriers in order to reach the target cells. Finally, when siRNA reaches the target cells, cellular uptake of the oligos and intracellular RNAi activity require efficient endocytosis and intact double-stranded oligos.
Fig. 3.2.
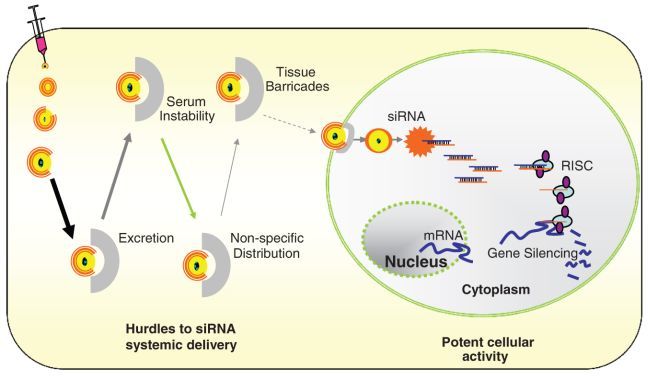
Challenges of systemic in vivo siRNA delivery. The in vivo application, especially systemic delivery of siRNA, is facing challenges from multiple hurdles in the extracellular environment and various barriers for the intracellular uptake. Addressing those issues is critical for efficient in vivo delivery of siRNA in preclinical animal models for drug target validation and potential therapeutics
To increase their stability in both extracellular and intracellular environments, siRNA oligos can be chemically modified by a variety of methods, including change of oligo backbone, replacement of individual nucleotide with nucleotide analogue, and adding conjugates to the oligo. The chemically modified siRNA demonstrated a significant serum resistance and higher stability [8], but it did not solve the problems of excretion through urine and targeted delivery. Therefore, a delivery system capable of protecting siRNA oligos from the urinary excretion and RNase degradation, transporting siRNA oligos through the physical barriers to the target tissue, and enhancing cellular uptake of the siRNA, is the key to the success of in vivo siRNA application.
The accessibility of different tissue types, the presence of various delivery routes, and a variety of pharmacological requirements makes it impossible to have a universal in vivo delivery system suitable to every scenario of siRNA delivery. In terms of in vivo delivery vehicles for siRNA, the “nonviral” carriers are the major type being investigated so far, though some physical and viral delivery approaches are also very effective. The routes of in vivo deliveries are commonly categorized as local or systemic. Some of the delivery vehicles and delivery routes are very effective in animals for target validation but may not be useful for delivery of siRNA therapeutics in humans (Fig. 3.3). Therefore, in vivo siRNA delivery carriers and methods can also be classified as clinically viable and nonclinically viable, according to their suitability for the human use.
Fig. 3.3.
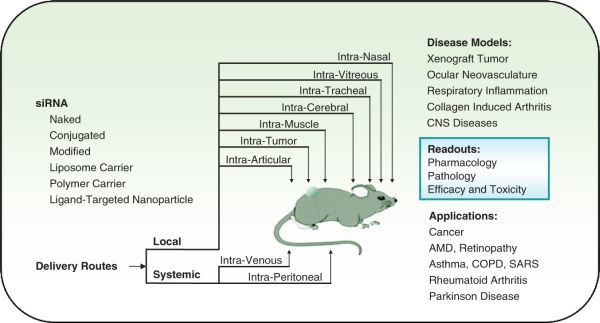
Applications of in vivo siRNA delivery in disease models. Mouse models are widely used for in vivo siRNA delivery studies. siRNA can be delivered by many routes based on the disease types and targeted tissues. The efficacy and toxicity readouts of the siRNA inhibitors from the preclinical models will provide vital information for the in vivo target validation [9]. The clinically viable siRNA delivery provides foundation for designing the administration route and condition of an RNAi therapeutic protocol
3 Delivering siRNA In Vivo Using Nonviral Carriers
Many nonviral carriers are currently used in delivery of either siRNA oligos or DNA-based short hairpin RNA (shRNA) vectors. Because RNAi is a mechanism of action in cytoplasm, achieving effective siRNA delivery is relatively easier than achieving the delivery of DNA-based shRNA systems. Nonviral siRNA delivery to disease tissue usually does not elicit an immune response and it is relatively less toxic to the target cells, which is a great advantage for drug target validation. The possibility of multiple administrations of siRNA makes the therapeutic applications of siRNA very practical.
Cationic lipids and polymers are two major classes of nonviral siRNA delivery carriers and both of them are positively charged and can form complexes with negatively charged siRNA. The siRNA/carrier complex can be condensed into a tiny nanoparticle of size around 100 nm which allows a very efficient cellular uptake of the siRNA agent through the endocytosis process. In a mouse model, the reporter gene silencing and downregulation of TNF-α expression were achieved after intraperitoneal administration of siRNA/lipoplexes [10]. One recent study reported that the use of a cationic derivative of cardiolipin to form lipoplexes with siRNA targeting the c-RAF oncogene led to an inhibition of tumor growth in a sequence-specific manner [11]. In another study, a family of highly branched histidine-lysine (HK) polymer peptides was found to be effective carriers of siRNA [12]. The Raf-1 expression in MDA-MB-435 xenografts was significantly inhibited by intratumoral injection of Raf-1 siRNA complexed with HK polymer (Fig. 3.4) [13]. Another recent study demonstrated a significant inhibition of HER-2 expression and tumor growth through intraperitoneal injection of HER-2 siRNA formulated with polyethylenimine (PEI) [14]. These studies demonstrated that cationic lipids and polymers can enhance siRNA delivery in vivo through systemic routes either intravenously (IV) or intraperitoneally (IP). These carrier-administered siRNA agents were efficiently knocking down the target genes and achieved antitumor efficacies. In contrast, direct intratumoral injection of VEGF siRNA without carrier did not generate any significant antitumor efficacy [15]. In an antiviral study, IV administration of siRNA specific targeting influenza virus RNA genome complexed with PEI was able to inhibit influenza virus production in mice [16].
Fig. 3.4.
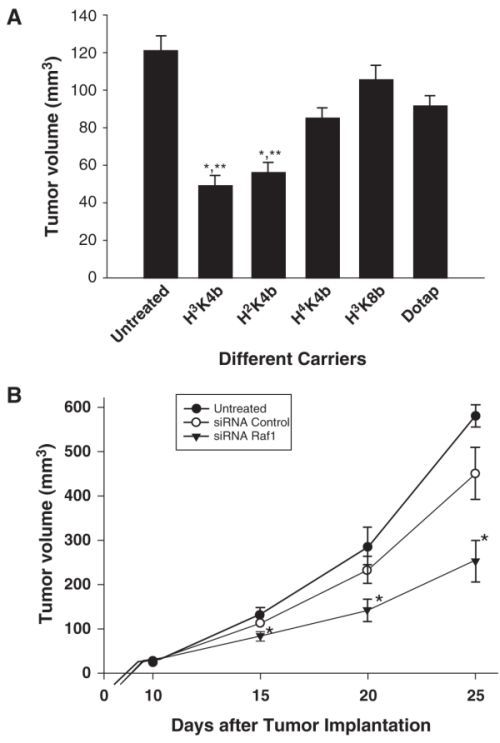
Raf-1 siRNA inhibits tumor growth in vivo. Ten days after the injection of MDA-MB-435 cells into the mammary fat pad, mice with visible tumors were separated into treatment groups. a Mice received 4 μg siRNA/tumor with each intratumoral injection every 5 days to determine the optimal carrier of Raf-1 siRNA. b To confirm the antitumor efficacy of siRNA Raf-1 with the optimal polymer, mice with tumors were divided into these groups: untreated, β-galactosidase (β-gal) siRNA, and Raf-1 siRNA. The β-gal siRNA and Raf-1 siRNA groups were injected with H3K4b complexed with siRNA every 5 days, three times. *P < 0.02, Raf-1 siRNA when compared with untreated; *P < 0.05, Raf-1 siRNA when compared with β-gal siRNA [13]
Some ligand-targeted nucleic acid delivery systems have been developed based on the cationic liposome complex and polymer complex systems. Recent successes of using ligand-targeted complexes to deliver therapeutic siRNA into tumor tissue and liver tissue suggest that targeted systemic delivery for siRNA therapeutics is a possibility [17–19]. We demonstrated an efficient delivery of siRNA to local neovasculature in tumor xenograft or viral infected eye through the systemic administration of a ligand-targeted nanoparticle containing siRNA, targeting VEGF R2, an angiogenesis factor overexpressed in endothelium of new blood vessels. The Arg-Gly-Asp (RGD)-motif peptide ligand is specific to integrin receptor, a marker of the activated endothelial and tumor cells. The ligand-targeted nanoparticle maintains the stability of the siRNA payload, targets to the tumor neovasculature, and enhances the cellular uptake of the siRNA. As a result, VEGF R2 knockdown and antiangiogenesis effects were observed both in xenografts tumor model and in ocular neovascularization disease model [5, 20]. Clearly, this siRNA nanoparticle is a clinically viable delivery system for various applications of siRNA therapeutics.
It has been reported that siRNA can trigger “off-target effects” [21, 22] and activate cellular interferon pathway [23, 24]. These issues raise concerns for the integrity of target validation studies and the safety and selectivity of the potential siRNA therapeutics. However, majority of these alarming results were found from the in vitro studies and many more studies have shown that siRNA inhibitors are highly specific both in vitro and in vivo [25, 26]. One recent study using systemic delivery of unmodified and unformulated siRNA oligos into mice revealed a lack of interferon response [27]. Two recent reports have revealed that siRNA oligos, containing “5-UGUGU-3” motif, were able to induce a Toll-like receptor-mediated interferon response only when they were delivered in vivo with cationic lipid or polymer carrier, through either intravenous or intraperitoneal administration [28, 29]. In contrast, neither the unformulated siRNA oligos containing 5-UGUGU-3 motif nor siRNA containing no 5-UGUGU-3 motif but with cationic carriers were able to induce the interferon response. For this reason, any 5-UGUGU-3 motif and other potential immunostimulatory motifs should be eliminated from the siRNA oligos if cationic lipid or polymer carriers are going to be used.
4 Delivering siRNA In Vivo Using Local Administration
The choice between local and systemic delivery depends on what tissues and cell types are targeted. For example, skin and muscle can be better accessed using local siRNA delivery, while lung and tumor can be reached efficiently by both local and systemic siRNA deliveries. There is increasing evidence supporting that siRNA can be efficiently delivered to various tissue types, using different approaches (Fig. 3.2).
4.1 Intranasal siRNA Delivery
Airway delivery of siRNA is a very useful method for both target validation and therapeutic development, because of the relevance of respiratory system in various diseases. In a recent study, intranasal delivery of GAPDH-specific siRNA mixed with pulmonary surface active material (InfaSurf) and elastase resulted in lowered GAPDH protein levels in lung, heart, and kidney by ~50–70% at 1 and 7 days after administration, when compared with scrambled siRNA control [30]. Direct delivery of unformulated siRNA into mouse airway led to knockdown of heme oxygenase-1 expression in the lung [31]. Intranasal administration of cationic liposome formulated siRNA specifically targeting the influenza virus RNA genome into mouse lung infected with the influenza virus resulted in a significantly reduced lung virus titer in infected mice and protected animals from lethal challenge [32]. However, in vivo delivery of siRNA with cationic polymer carrier, such as PEI, is often associated with severe toxicity in the host and may induce nonspecific interferon response through the Toll-like receptor pathway, as discussed earlier. Therefore, pulmonary siRNA delivery may require formulations without cationic carriers. Of late, we have successfully delivered siRNA with D5W (5% d-glucose in water) solution into mouse and monkey lungs, achieving effective knockdown of SARS coronavirus RNA (Fig. 3.5) [33].
Fig. 3.5.
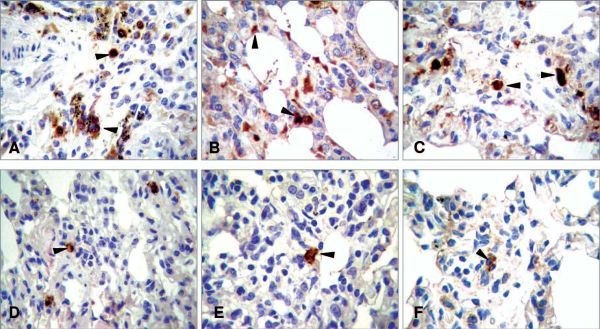
Anti-SARS activity of intranasally delivered siRNA in macaques. The SARS coronavirus (SCV)-specific antigen was detected in alveoli deep into the lungs, including various cell types (original magnification, ×200), confirmed by the specific staining with monoclonal antibodies. a The upper arrow indicates an SCV-infected type II pneumocyte and the lower arrow indicates an infected alveolar macrophage. b Arrows indicate SCV-infected epithelium-originated type I pneumocytes. c Arrows indicate SCV-antigen-positive alveolar macrophages. d–f Arrows indicate SCV-infected cells scattered within the siRNA-treated lungs [33]
4.2 Intraocular siRNA Delivery
An increasing number of clinical protocols have been approved for treating eye diseases with nucleic acid drugs such as antisense oligonucleotides or RNA aptamers. Delivery of nonformulated siRNA specific to VEGF to the subretinal space in a mouse model of retinal neovascularization resulted in a significant reduction of angiogenesis in the eye. Importantly, this study indicated that chemical protection of the siRNA was not essential, at least in the intravitreous compartment of the eye, in contrast to antisense oligonucleotides or RNA aptamers, which need protection by chemical modification for applications in eye [34]. Using a murine model of herpetic stromal ketatitis that develops from herpes simplex virus corneal infection, we found that subconjunctival administration of siRNA targeting several genes in the VEGF pathway significantly inhibited the corneal angiogenesis and disease symptoms [5]. Subconjunctival delivery of siRNA specific to TGF-β significantly reduced the inflammatory response and matrix deposition in a wound-induced mouse model of ocular inflammation [35]. The evidence also provided clinically viable means for the local delivery of siRNA for gene function validation in various eye disease models. Local delivery of siRNA to the front of the eye subconjunctivally or to the back of the eye intravitreously is highly efficient in silencing target gene expression, and therefore are effective administration routes for target validation for eye disease. However, the frequency and time intervals between repeated deliveries may be the limiting factors of these delivery routes, especially for clinical application of siRNA therapeutics.
4.3 Intracerebral siRNA Delivery
The brain tissue is the foundation of the central nervous system (CNS), obviously a very important biological system and one representing considerable interest for both functional genomics research and therapeutic development [36]. A recent study showed that infusion of chemically protected siRNA oligonucleotides in an aqueous solution directly into the brain was able to selectively inhibit gene expression [37]. Treatment of rats with aqueous siRNA against α(2A)-ARs on days 2–4 after birth resulted in an acute decrease in the levels of α(2A)-AR mRNA in the brainstem into which siRNA was injected [38]. Nonviral infusion of siRNA in brain provided a unique approach to accelerate target validation for neuropsychiatric disorders that involve a complex interplay of gene(s) from various brain regions. For example, infusion of siRNA specific to an endogenous dopamine transporter (DAT) gene in regions (ventral midbrain) far distal to the infusion site resulted in a significant downregulation of DAT mRNA and protein in the brain, and elicited a temporal hyperlocomotor response similar to that obtained upon infusion of GBR-12909, a pharmacologically selective DAT inhibitor [39]. However, the difficulty of performing surgical implantation of an infusion pump delivering high dose of siRNA limits its usefulness as a tool for functional genomics. Another recent study on the use of cationic formulations for siRNA delivery to the brain revealed that delivery was more efficient using a lipid carrier than using a polymer carrier [40]. Electroporation is a physical approach which has been used to introduce DNA into the cells. During the process of electroporation, an electric field pulse induces pores (electropores) in cell membrane that allow DNA molecules to enter the cell. Recently, electroporation procedures have been adopted for local delivery of siRNA. In one study, siRNA introduced into hippocampus region by local electroporation led to a marked reduction in the expression of both the mRNA and protein of the target genes, such as GluR2 and Cox-1, without affecting the expression of other proteins [41].
4.4 Intramuscle siRNA Delivery
The skeletal-muscle tissue is accessible for local siRNA administration. Direct injection of siRNA formulated with cationic lipids or polymers can be considered for local delivery, although inflammation caused by the injection is a common problem. A recent study with nonformulated siRNA delivered by direct injection into mouse muscle, followed by electroporation, demonstrated a significant gene silencing that lasted for 11 days [42]. The electroporation method was also applied in a different study targeting several reporter genes in murine skeletal muscle [43]. A local hydrodynamic approach, in which siRNA in a sufficient volume was rapidly injected into a distal vein of a limb that is transiently isolated by a tourniquet or blood pressure cuff, was tested for siRNA delivery in muscles of animal models and demonstrated a knockdown of both reporter and endogenous gene [44].
4.5 Intratumoral siRNA Delivery
Intratumoral delivery of siRNA is a very attractive approach for functional validation of the tumorigenic genes. We observed inhibition of tumor growth in two human breast cancer xenograft models using intratumoral delivery of VEGF-specific siRNA [45]. It was reported that atelocollagen, a collagen solubilized by protease, can protect siRNA from being digested by RNase when it forms a complex with siRNA. In addition, the siRNA can be slowly released from atelocollagen to efficiently transduce into cells, allowing a long-term target gene silencing [46, 47]. In a mouse xenograft tumor study, after administration of atelocollagen/luc-siRNA complex intratumorally, a reduced luciferase expression was observed. Furthermore, intratumoral injection of atelocollagen/VEGF-siRNA showed an efficient inhibition of tumor growth in an orthotopic xenograft model of a human nonseminomatous germ cell tumor [46]. A similar result was observed in a PC-3 human prostate xenograft tumor model, using the same siRNA delivery approach [47]. Therefore, the atelocollagen-based siRNA delivery method could be a reliable approach to achieve maximal inhibition of gene function in vivo. On the basis of the experience in the successful validation of a group of novel genes for their roles in tumorgenesis using intratumoral delivery of formulated siRNA [2, 4], we believe that intratumoral delivery of siRNA into xenograft tumor models is a very useful platform for in vivo target validation.
5 Delivery of siRNA In Vivo Using Systemic Administration
5.1 Liver-Targeted Systemic Delivery
Some of the first published results showed activity of siRNA in mammals by delivering into mouse liver using the hydrodynamic delivery, a rapid injection of a large volume of aqueous solution into the mouse tail vein creating a high pressure in the vascular circulation that leads to an extensive delivery of siRNA into hepatocytes [48–52]. This procedure allows high efficiency of siRNA uptake and potent siRNA activity in hepatocytes, and thus is a useful tool for functional genomic studies in liver. On the other hand, this procedure is not a clinically viable procedure because of potential damage of liver and other organs, and is limited only to research on liver function and metabolism or liver infectious diseases such as hepatitis [53, 54]. Hepatocyte-specific targeting carriers for siRNA delivery into liver are very attractive approaches for development of siRNA therapeutics for hepatic diseases and are currently under investigation. As one step toward the liver targeting delivery, liver delivery of chemically modified oligonucleotide with cholesterol conjugates was tested, as described in recent publications [8, 55]. However, the data suggested that at least three challenges must be addressed: adequate protection of the siRNA oligonucleotide from serum degradation en route to the liver, protection of the siRNA oligonucleotide from rapid glomerulofiltration by the kidney into the urine, and selective uptake by the target hepatocytes. In addition, the high dose used for intravenous cholesterol-conjugated siRNA delivery indicated a widespread distribution rather than targeting the liver.
5.2 Tumor-Targeted Systemic Delivery
Malignant tumors grow fast and spread throughout the body via blood or the lymphatic system. Metastatic tumors established at distant locations are usually not encapsulated and thus more amenable for systemic delivery. Local siRNA administration methods discussed earlier can meet the requirements for most functional genomics studies by acting on primary tumors or xenograft models which form the basis of most cancer biology research. On the other hand, systemic delivery of siRNA is needed for development of siRNA-based cancer therapeutics.
Systemic siRNA delivery imposes several requirements and greater hurdles than does local siRNA delivery. It requires stable oligonucleotides in the blood and in the local environment to enter the target cells. In addition, the siRNA needs to pass through multiple tissue barriers to reach the target cell. A recent study in pancreas xenografts used the systemic administration of CEACAM6-specific siRNA without protection and formulation. The study demonstrated a significant suppression of primary tumor growth by 68%, compared with that by control siRNA, associated with a decreased proliferation index of the tumor cells, impaired angiogenesis, and increased apoptosis. Treatment with CEACAM6-specific siRNA completely inhibited metastasis and significantly improved survival, without apparent toxicity [56]. Recent results revealed the tumor-targeting siRNA delivery using an RGD peptide ligand directed nanoparticle and its application in antiangiogenic treatment for cancer (Fig. 3.6) by systemic siRNA delivery [20, 57], as reviewed elsewhere recently [58].
Fig. 3.6.
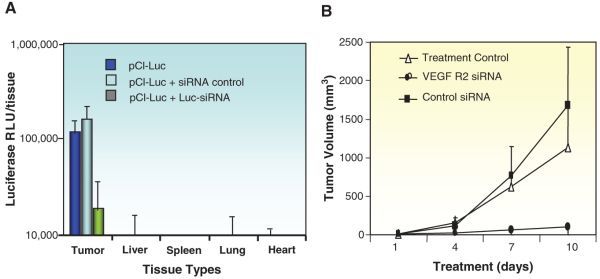
Nanoparticle siRNA delivery for tumor treatment. a N2Atumor-bearing mice received a single intravenous injection of 40 mg pLuc in RPP-nanoplexes only, with control siRNA or with Luc-specific siRNA. 24 h following administration, tissues were assayed for luciferase activity (n = 5). b Mice were inoculated withN2Atumor cells and left untreated (open squares) or treated every 3 days by tail vein injection with RPP-nanoplexes with control siRNA or VEGF R2-specific siRNA at a dose of 40 mg per mouse. Treatment was started when the tumors became palpable (>20 mm3). Only VEGF R2-sequence-specific siRNA inhibited tumor growth, whereas treatment with control siRNA did not affect tumor growth rate when compared with untreated controls (n = 5)
5.3 Other Neovasculature-Targeted Systemic Delivery
In addition to targeting tumor neovasculature, we also studied the RGD ligand targeted nanoparticle for targeting ocular neovasculature tissues [5]. The antiangiogenesis efficacy observed in ocular neovascularization models further demonstrated this approach as a clinically viable method for siRNA therapeutics (Fig. 3.7). Using the HSV DNA induced ocular neovascularization model, we demonstrated that siRNA oligos specific for several genes can be combined in the same nanoparticle as a “cocktail” approach to achieve a stronger antiangiogenesis activity inhibiting the disease pathology [5, 59]. This ligand-directed nanoparticle delivery represents a novel and effective approach for a clinically viable systemic administration of siRNA oligos as the dual-targeted RNAi therapeutics.
Fig. 3.7.
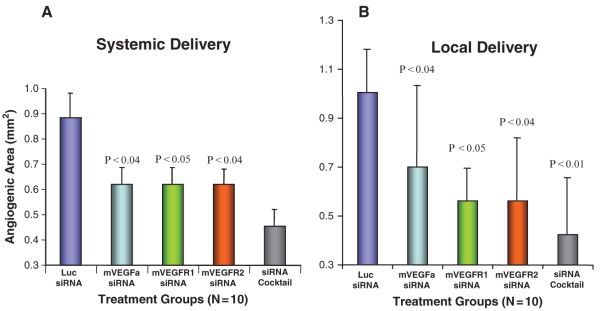
Systemic and local nanoparticle delivery for siRNA therapeutics to treat ocular neovascularization. a Systemic delivery of siRNAs against VEGF pathway genes inhibits the CpG ODN-induced angiogenesis. Individual siRNAs or a mixture of total siRNAs against VEGF pathway genes were delivered with RPP nanoparticles 6 and 24 h after the CpG ODN induction. b Local delivery of siRNAs against VEGF pathway genes inhibits the CpG ODN-induced angiogenesis. Individual siRNAs or a mixture of total siRNAs against VEGF pathway genes were delivered with HKP nanoparticles 6 and 24 h after the induction
6 Conclusion
Currently, delivery of siRNA oligos as a therapeutic agent in vivo, through either local or systemic route, is evolving from the target validation tools to the proof of principle for potential RNAi therapeutics. Therefore, examining the utility of each particular siRNA delivery method in vivo requires confirmation of its robustness during the target validation process with repeated testing in the preclinical models. One significant advantage of siRNA, however, is rapidity with which different siRNA sequences and the matching genes can be studied, which is particularly useful for drug target validation. Moreover, developing and optimizing siRNA delivery in various types of animal disease models will be a challenging but worthy effort to accelerate the novel drug discovery process. Ultimately, this effort will be translated into clinically viable administration method for siRNA-based therapeutics to treat cancer, infectious diseases, and many other critical diseases.
References
- 1.Dykxhoorn D.M, et al. Killing the messenger: short RNAs that silence gene expression. Nat Rev Mol Cell Biol. 2005;4:457–467. doi: 10.1038/nrm1129. [DOI] [PubMed] [Google Scholar]
- 2.Lu P.Y, et al. siRNA-mediated antitumorigenesis for drug target validation and therapeutics. Curr Opin Mol Ther. 2003;5:225–234. [PubMed] [Google Scholar]
- 3.Lu P.Y, et al. In vivo application of RNA interference: from functional genomics to therapeutics. Adv Genet. 2005;54:117–142. doi: 10.1016/S0065-2660(05)54006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie F.Y, et al. Delivering siRNA to animal disease models for validation of novel drug targets in vivo. PharmaGenomics. 2004;July/August:28–38. [Google Scholar]
- 5.Kim B, et al. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis. Am J Pathol. 2004;165:2177–2185. doi: 10.1016/S0002-9440(10)63267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song E, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23(6):709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 7.Lu P.Y., Woodle M.C. Delivering siRNA in vivo for functional genomics and novel therapeutics. In: Appasani K., editor. RNA interference technology. London: Cambridge University Press; 2005. [Google Scholar]
- 8.Soutschek J, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 9.Xie, et al. Harnessing in vivo siRNA delivery for drug discovery and therapeutic development. Drug Discov Today. 2006;11(1/2):67–73. doi: 10.1016/S1359-6446(05)03668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorensen D.R, et al. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol. 2003;327:761–766. doi: 10.1016/S0022-2836(03)00181-5. [DOI] [PubMed] [Google Scholar]
- 11.Chien P.Y, et al. Novel cationic cardiolipin analogue-based liposome for efficient DNA and small interfering RNA delivery in vitro and in vivo. Cancer Gene Ther. 2004;12:321–328. doi: 10.1038/sj.cgt.7700793. [DOI] [PubMed] [Google Scholar]
- 12.Leng Q, et al. Highly branched HK peptides are effective carriers of siRNA. J Gene Med. 2005;7(7):977–986. doi: 10.1002/jgm.748. [DOI] [PubMed] [Google Scholar]
- 13.Leng, Q. et al. (2005). Small interfering RNA targeting Raf-1 inhibits tumor growth in vitro and in vivo. Cancer Gene Ther. Advance online publication, April 1, 2005; doi:10.1038/sj.cgt.7700831 [DOI] [PubMed]
- 14.Urban- Klein B, et al. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005;12:461–466. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- 15.Filleur S, et al. SiRNA-mediated inhibition of vascular endothelial growth factor severely limits tumor resistance to antiangiogenic thrombospondin-1 and slows tumor vascularization and growth. Cancer Res. 2003;63:3919–3922. [PubMed] [Google Scholar]
- 16.Ge Q, et al. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc Natl Acad Sci USA. 2004;101:8676–8681. doi: 10.1073/pnas.0402486101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodle M.C, et al. Sterically stabilized polyplex: ligand-mediated activity. J Control Release. 2001;74:309–311. doi: 10.1016/S0168-3659(01)00339-X. [DOI] [PubMed] [Google Scholar]
- 18.Song E, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23(6):709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 19.Morrissey D.V, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23(8):1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 20.Schiffelers R.M, et al. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32:e149. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal S., Kandimalla E.R. Antisense and siRNA as agonists of toll-like receptors. Nat Biotechnol. 2004;22:1533–1537. doi: 10.1038/nbt1042. [DOI] [PubMed] [Google Scholar]
- 22.Jackson A, et al. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 23.Kariko K, et al. Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through toll-like receptor 3. J Immunol. 2004;172:6545–6549. doi: 10.4049/jimmunol.172.11.6545. [DOI] [PubMed] [Google Scholar]
- 24.Sledz C.A, et al. Activation of the interferon system by short-interfering RNAs. Nat Cell Biol. 2003;5:834–839. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- 25.Chi J.T, et al. Genomewide view of gene silencing by small interfering RNAs. Proc Natl Acad Sci USA. 2003;100:6364–6369. doi: 10.1073/pnas.1131966100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semizarov D, et al. Specificity of short interfering RNA determined through gene expression signatures. Proc Natl Acad Sci USA. 2003;100:6347–6352. doi: 10.1073/pnas.1131959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heidel J.D, et al. Lack of interferon response in animals to naked siRNAs. Nat Biotech. 2004;22:1579–1582. doi: 10.1038/nbt1038. [DOI] [PubMed] [Google Scholar]
- 28.Hornung V, et al. Sequence-specific potent induction of IFN-a by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11:263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 29.Judge A.D, et al. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotech. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 30.Massaro D, et al. Noninvasive delivery of small inhibitory RNA and other reagents to pulmonary alveoli in mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1066–L1070. doi: 10.1152/ajplung.00067.2004. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, et al. Small interfering RNA targeting heme oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. J Biol Chem. 2003;279:10677–10684. doi: 10.1074/jbc.M312941200. [DOI] [PubMed] [Google Scholar]
- 32.Tompkins S.M, et al. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc Natl Acad Sci USA. 2004;101:8682–8686. doi: 10.1073/pnas.0402630101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li B.J, et al. Prophylactic and therapeutic efficacies of siRNA targeting SARS coronavirus in rhesus macaque. Nat Med. 2005;11(9):944–951. doi: 10.1038/nm1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reich S.J, et al. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol Vis. 2003;9:210–216. [PubMed] [Google Scholar]
- 35.Nakamura H, et al. RNA interference targeting transforming growth factor-beta type II receptor suppresses ocular inflammation and fibrosis. Mol Vis. 2004;10:703–711. [PubMed] [Google Scholar]
- 36.Buckingham S.D, et al. RNA interference: from model organisms towards therapy for neural and neuromuscular disorders. Hum Mol Genet Spec. 2004;2:R275–R288. doi: 10.1093/hmg/ddh224. [DOI] [PubMed] [Google Scholar]
- 37.Dorn G, et al. siRNA relieves chronic neuropathic pain. Nucleic Acids Res. 2004;32:e49. doi: 10.1093/nar/gnh044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shishkina G.T, et al. Attenuation of alpha (2A)-adrenergic receptor expression in neonatal rat brain by RNA interference or antisense oligonucleotide reduced anxiety in adulthood. Neuroscience. 2004;129:521–528. doi: 10.1016/j.neuroscience.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Thakker D.R, et al. Neurochemical and behavioral consequences of widespread gene knockdown in the adult mouse brain by using nonviral RNA interference. Proc Natl Acad Sci USA. 2004;101:17270–17275. doi: 10.1073/pnas.0406214101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassani Z, et al. Lipid-mediated siRNA delivery down-regulates exogenous gene expression in the mouse brain at picomolar levels. J Gene Med. 2004;7:198–207. doi: 10.1002/jgm.659. [DOI] [PubMed] [Google Scholar]
- 41.Akaneya Y, et al. RNAi-induced gene silencing by local electroporation in targeting brain region. J Neurophysiol. 2005;93:594–602. doi: 10.1152/jn.00161.2004. [DOI] [PubMed] [Google Scholar]
- 42.Golzio M, et al. Inhibition of gene expression in mice muscle by in vivo electrically mediated siRNA delivery. Gene Ther. 2004;12:246–251. doi: 10.1038/sj.gt.3302405. [DOI] [PubMed] [Google Scholar]
- 43.Kishida T, et al. Sequence-specific gene silencing in murine muscle induced by electroporation-mediated transfer of short interfering RNA. J Gene Med. 2004;6:105–110. doi: 10.1002/jgm.456. [DOI] [PubMed] [Google Scholar]
- 44.Hagstrom J.E, et al. A facile nonviral method for delivering genes and siRNAs to skeletal muscle of mammalian limbs. Mol Ther. 2004;10(2):386–398. doi: 10.1016/j.ymthe.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Lu P.Y, et al. Tumor inhibition by RNAi-mediated VEGF and VEGFR2 down regulation in xenograft models. Cancer Gene Ther. 2002;10(Suppl.):S4. [Google Scholar]
- 46.Minakuchi Y, et al. Atelocollagen-mediated synthetic small interfering RNA delivery for effective gene silencing in vitro and in vivo. Nucleic Acids Res. 2004;32:e109. doi: 10.1093/nar/gnh093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takei Y, et al. A small interfering RNA targeting vascular endothelial growth factor as cancer therapeutics. Cancer Res. 2004;64:3365–3370. doi: 10.1158/0008-5472.CAN-03-2682. [DOI] [PubMed] [Google Scholar]
- 48.Lewis D.L, et al. Efficient delivery of siRNA for inhibition of gene expression in postnatal mice. Nat Genet. 2002;32:107–108. doi: 10.1038/ng944. [DOI] [PubMed] [Google Scholar]
- 49.McCaffrey A.P, et al. RNA interference in adult mice. Nature. 2002;418:38–39. doi: 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- 50.Song E, et al. RNA interference targeting Fas protects mice from fulminant hepatitism. Nat Med. 2003;9:347–351. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- 51.Zender L, et al. Caspase 8 small interfering RNA prevents acute liver failure in mice. Proc Nat Acad Sci USA. 2003;100:7797–7802. doi: 10.1073/pnas.1330920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Layzer J.M, et al. In vivo activity of nuclease-resistant siRNAs. RNA. 2004;10:766–771. doi: 10.1261/rna.5239604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giladi H, et al. Small interfering RNA inhibits hepatitis B virus replication in mice. Mol Ther. 2004;8:769–776. doi: 10.1016/S1525-0016(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 54.Sen A, et al. Inhibition of hepatitis C virus protein expression by RNA interference. Virus Res. 2003;96:27–35. doi: 10.1016/S0168-1702(03)00170-9. [DOI] [PubMed] [Google Scholar]
- 55.Lorenz C, et al. Steroid and lipid conjugates of siRNAs to enhance cellular uptake and gene silencing in liver cells. Bioorg Med Chem Lett. 2004;14:4975–4977. doi: 10.1016/j.bmcl.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 56.Duxbury M.S. Systemic siRNA-mediated gene silencing: a new approach to targeted therapy of cancer. Ann Surg. 2004;240:667–674. doi: 10.1097/01.sla.0000140755.97224.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dubey Liposomes modified with cyclic RGD peptide for tumor targeting. J Drug Target. 2004;12:257–264. doi: 10.1080/10611860410001728040. [DOI] [PubMed] [Google Scholar]
- 58.Lu P.Y, et al. Modulation of angiogenesis with siRNA inhibitors for novel therapeutics. Trend Mol Med. 2005;11:104–113. doi: 10.1016/j.molmed.2005.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woodle M.C., Lu P.Y. Nanoparticles deliver RNAi therapy. Materials Today, 2005;8(suppl 1):34–41. doi: 10.1016/S1369-7021(05)71035-X. [DOI] [Google Scholar]


