Abstract
Accumulating evidence suggests that the gut microbiota plays an important role in the pathogenesis of colitis and that its composition could be modulated by exposure to dietary components. Thus, it may be possible to ameliorate colitis disease severity through administration of dietary components. Herein, we determined the effects of orally administered resveratrol on the gut microbiota composition and resulting inflammatory status of a dextran sodium sulfate (DSS)-induced colitis mouse model. Our results supported our hypothesis that dietary resveratrol altered microbial composition and restored microbial community diversity in DSS-treated mice. Specifically, resveratrol effectively decreased the abundance of genera: Akkermansia, Dorea, Sutterella and Bilophila, and increased the proportion of Bifidobacterium in colitic mice. Resveratrol was also able to prevent mouse body weight loss, reduce the disease activity index, attenuate tissue damage, and down-regulate the expression of pro-inflammatory cytokines such as IL-2, IFN-γ, GM-CSF, IL-1β, IL-6, KC/GRO, and TNF-α in the colon of DSS-treated mice. A Pearson’s correlation analysis indicated significant correlations between the relative levels of these pro-inflammatory cytokines and alterations of gut microbiota. Our results demonstrated that dietary resveratrol attenuated inflammatory status and alleviated gut microbiota dysbiosis in a colitis mouse model.
Keywords: Anti-inflammation, Cytokines, Colitis, Dietary Resveratrol, Gut microbiota
Introduction
Inflammatory bowel disease (IBD), encompassing relapsing-remitting Crohn’s disease and ulcerative colitis, is a chronic inflammatory disease of the gastrointestinal tract.1 Although the precise etiology of IBD remains unknown, the most accepted hypothesis to date is that genetically predisposed hosts experience continual immune and inflammatory responses to their gut microbiota or ingested matieral.1, 2 The possible implication of the gut bacterial community in the pathogenesis of IBD has been highlighted only recently. Existing lines of evidence for such a relationship include: noted microbial markers for the progression of IBD such as reduced community diversity and altered bacterial composition when compared to healthy subjects,3–5 germ-free dextran sodium sulfate (DSS)-treated mice demonstrating resistance against colitis development compared to their germed counterpart,6 as well as IL-10-deficient mice (a murine model of IBD) not developing colitis under germ-free conditions.7 These results suggest that microbial factors might directly contribute to the generation of colonic inflammation and adverse metabolic consequences. Therefore, the gut microbiota has become an important target in the treatment of IBD, and therapy has focused on correcting intestinal microbial imbalances and dysbiosis.
As the sole source of energy input for bacteria residing in the gastrointestinal tract, an individual’s diet has a large influence on the gut microbiota composition. Food components (e.g. fiber, polysaccharides, polyphenols) that are natively non-digestible are subjected to intestinal microbial metabolism.8, 9 Previous studies estimated that 90–95% of ingested polyphenols may accumulate in the large intestine where they interact with the gut microbial community.10 Resveratrol (3,5,4’-trihydroxy-trans-stilbene), a polyphenol found in various berries and peanuts, is highly accumulated in the gastrointestinal tract (65.1%).11 Studies have reported that dietary resveratrol alleviates gut dysbiosis caused by a high-fat diet, namely by increasing the Firmicutes/Bacteroidetes ratio, inhibiting the growth of Enterococcus faecalis, and increasing the prevalence of probiotics Lactobacillus and Bifidobacterium in the gut.12, 13
The protective effects of resveratrol against acute or chronic colitis in different models were demonstrated in previous works.14–17 In several instances, dietary resveratrol down-regulated inflammatory biomarkers, reduced oxidative stress, and attenuated clinical symptoms in experimental murine colitis models.14, 16, 18 A human study revealed that six weeks of dietary resveratrol could alleviate clinical colitis.19 A separate study reported increases in Lactobacillus and Bifidobacterium by resveratrol treatment and a moderating effect on the growth of Enterobacteria in an acute colitis rat model.17 Recently, Haider et al. reported that resveratrol modulated the gut microbiota to prevent colitis in an acute 2,4,6-trinitrobenzenesulfonic acid-induced colitis mouse model.20 In this study, 16S rRNA sequencing was used to identify gut microbiota community shifts by resveratrol in DSS-induced chronic colitis mice. Our study provides insights into the effects of resveratrol on the gut microbiota and their interaction to affect chronic colitis.
Materials and Methods
Animals and experimental design
This study was performed in strict accordance with the NIH guidelines for the care and use of laboratory animals and was approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Amherst. 40 male CD-1 mice (6–8 weeks old) were purchased from Charles River Laboratories (Wilmington, MA, USA). Animals were randomly assigned to four groups and housed at 3–4 mice per cage in a temperature-controlled environment (22 ± 2°C) with 65% relative humidity and fixed 12h light/dark cycle. For the first week of standard diet feeding, cage rotation was performed to minimize individual variation. After the one-week diet acclimation, the four groups, each sized n=10, were designated as follows: the control group (DSS-/RES-) fed with standard chow AIN93G diet (Dyets Inc., Bethlehem, PA, USA); the resveratrol group (DSS-/RES+) fed with standard diet supplemented with 0.025% resveratrol (w/w, >99% purity) (Quality Phytochemicals, East Brunswick, NJ, USA); the DSS group (DSS+/RES-) treated with standard diet and 1.5% DSS water (w/v, salt form) (International Lab, Chicago, IL, USA); and the DSS-resveratrol group (DSS+/RES+) received 0.025% resveratrol (w/w) supplemented diet and 1.5% DSS drinking water (w/v). DSS was added to sterilized drinking water and offered ad libitum for 4 days, followed by 7 days of pure water for recovery. This cycle was repeated four times. Food and water consumption per cage were recorded and averaged to each mouse throughout the entire experimental period. At the end of the fourth cycle of DSS treatment, all mice were sacrificed with CO2 asphyxiation. The spleen was quickly excised and weighted. The colon from individual mouse was dissected, measured in length, cut open longitudinally. Fecal samples were collected from the colon immediately, snap-frozen, and then stored at −80°C for later analysis.
DAI and histological assessment
Disease activity index (DAI) was scored based on the extent of rectal bleeding: 0 (none), e1 (hemoccult positive), 2 (blood), 3 (gross bleeding); stool consistency: 0 (normal), 1 (soft but formed), 2 (loose and formless), 3 (diarrhea); and weight loss: 0 (0%), 1 (1–5%), 2 (5–10%), 3 (10–20%), 4 (>20%).21 The final macroscopic score for each animal is the sum of these three separate scores.
Formalin-fixed colon tissue was processed for paraffin embedding, with a section thickness of 5 μm, and haematoxylin and eosin (H&E) staining as previously described.22–24 Three parameters were graded: surface epithelial loss, crypt destruction, and inflammatory cell infiltration into the mucosa based on the established criteria.25 A score of 0–4 was assigned to each parameter (maximum macroscopic score = 12).
Enzyme-linked immunosorbent assay (ELISA)
30 mg of colonic mucosa was scraped and homogenized in 200 μL of Tris lysis buffer (Meso Scale Discovery, Rockville, MD, USA) containing 1% protease inhibitor cocktail (Boston Bioproducts, Ashland, MA, USA). Supernatant was collected by centrifugation at 16 000 g for 20m at 4°C, then loaded in sandwich ELISA kits (Meso Scale Discovery, Rockville, MD, USA) to determine the concentrations of cytokines: granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon gamma (IFN-γ), Interleukin 10 (IL-10), Interleukin 2 (IL-2), Interleukin 1 beta (IL-1β), Interleukin 6 (IL-6), keratinocyte chemoattractant/human growth-regulated oncogene (KC/GRO), and tumor necrosis factor alpha (TNF-α) according to the manufacturer’s instructions.
Fecal DNA extraction, 16S rRNA analysis and Illumina Mi-Seq sequencing
Fecal bacterial DNA was extracted using the PowerFecal DNA isolation kit (MoBio Laboratories, Inc., Carlsbad, CA, USA) following the supplier’s protocol. The DNA was quantified, and quality checked by NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA, USA) and samples were normalized to 20ng/μL. The V3-V4 regions of the 16S rRNA gene was targeted for amplification by PCR using forward primer 5’TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG and reverse primer 5’GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC as described by Klindworth et al.26 Successful amplification was verified by gel electrophoresis. PCR products were then purified with AMPure XP beads (Beckman Coulter, Danvers, MA, USA). Dual indices and Illumina sequencing adapters were attached by PCR using the Nextera XT Index Kit (Illumina, San Diego, CA, US). The quantity and size of PCR products were determined and verified using Qubit dsDNA BR Assay kit (Life Technologies Corporation, Carlsbad, CA, USA) and ScreenTape Assay on Tape Station 2200 (Agilent Technologies, Santa Clara, CA, USA). Final PCR products were pooled in equimolar volumes and diluted to 4 nM. After combining the amplicon library and PhiX control, the samples were loaded onto the 600-cycle MiSeq Reagent kit v3 cartridge and sequenced by the Illumina MiSeq platform (Illumina Inc, San Diego, CA, USA).
Statistical analysis
Automatically demultiplexed Illumina sequence reads were processed using Quantitative Insights Into Microbial Ecology (QIIME) software pipeline v1.9.1.27 QIIME-generated operational taxonomic units (OTUs) clustered at ≥97% sequence similarity were further subjected to alpha- and beta-diversity and principal coordinate analysis (PCoA). Cladogram construction and Linear discriminant analysis (LDA) were performed using the LEfSe tool from The Huttenhower Lab.28 Correlation analyses were performed with SPSS 17.0 software (Chicago, IL, USA). Data were presented as the mean ± standard deviation (SD). The statistical significance of differences among groups was performed using one-way ANOVA followed by Tukey’s post hoc test or one-way nonparametric ANOVA Kruskal-Wallis test.
Results and discussion
Dietary resveratrol attenuated colitis symptoms
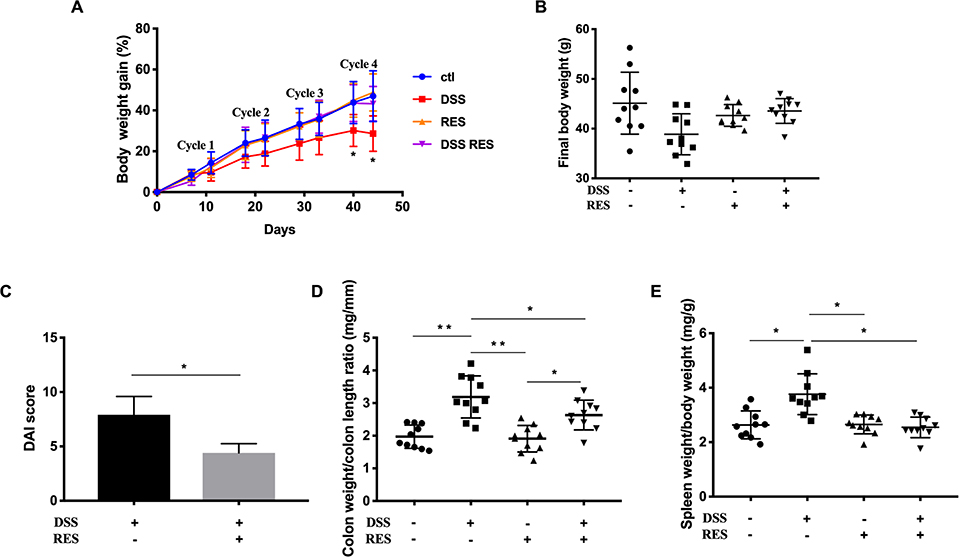
There were no significant differences of drink and food consumptions among cages and groups. The resveratrol dose applied is equivalent to ca. 2.3 mg/kg/day in humans, which is achievable through dietary supplementation. Administration of DSS water in four phases successfully induced chronic colitis and the development of colorectal dysplasia in mice.29 Body weight was monitored at the end of every DSS and recovery cycle as an indicator of colitis severity. As DSS-treatment cycle number increased, body weight increased correspondingly but to a lesser extent in DSS+/RES- mice compared to the control group, with the difference reaching statistical significance at and following the fourth DSS cycle (P < 0.05). Dietary resveratrol prevented this trend in DSS+/RES+ mice (Fig. 1A). DSS+/RES+ mice experienced average final body weight gain from 36.92 ± 3.05g to 44.78 ± 1.61g (P < 0.01) (Fig. 1B).
Figure 1.
Dietary resveratrol attenuated colitis symptoms. (A) Percentage of body weight gain during the entire experiment; (B) Final body weight; (C) Disease activity index (DAI); (D) Colon weight to colon length ratio; (E) Spleen weight to body weight ratio. Data presented as mean ± standard deviation (SD) (n= 10). Significant differences are indicated: *, P < 0.05; * *, P < 0.01.
The DAI score is commonly used to evaluate the inflammatory severity in mice with colitis, which encompasses the fecal consistency, hematochezia, and body weight loss.21 The DAI score rated as high as 7.9 ± 1.9 in the DSS+/RES- group, whereas it was markedly attenuated 1.8-fold with resveratrol treatment (P < 0.05) (Fig. 1C). Colon weight/length ratio is another metric correlated with the progression of colorectal inflammation. As shown in Fig.1D, the difference between DSS+/RES- and DSS-/RES- colon weight/length ratio was highly significant (P < 0.05) while the administration of resveratrol nullified this effect in DSS-treated mice. Furthermore, remarkable spleen enlargement was observed in DSS-treated mice compared to control mice. The consumption of dietary resveratrol was demonstrated to restrict the spleen to final body weight ratio to that of the control level (P < 0.05) (Fig. 1E). These results were in agreement with previous research,14, 15, 17, 18 that demonstrated that dietary resveratrol prevented colitis progression indicators.
Dietary resveratrol ameliorated colonic tissue damage
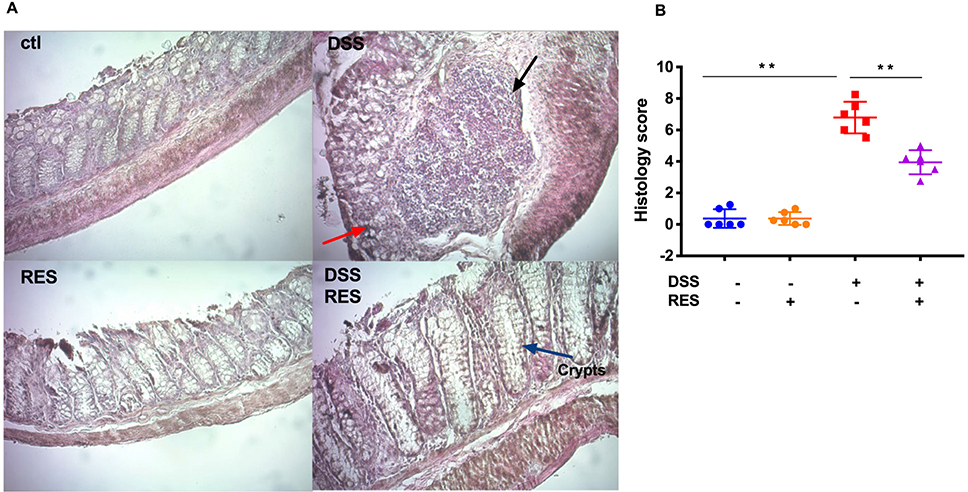
H&E staining of the colonic segments from DSS+/RES- mice showed severe inflammatory damage, characterized by significant distortion of crypt structure, edema of submucosa, and instances of immune cell dysplasia (Fig. 2A). Resveratrol supplementation significantly mitigated morphological alterations observed in the DSS+/RES- group, protecting colonic tissue integrity. The majority of colonic epithelium and mucosal architecture remained intact (Fig. 2A), which was consistent with previous studies.12, 14 Damage of colonic mucosa rated by overall histological score was 1.7-fold higher (P < 0.01) in the DSS+/RES- compared to the DSS+/RES+ group (Fig. 2B).
Figure 2.
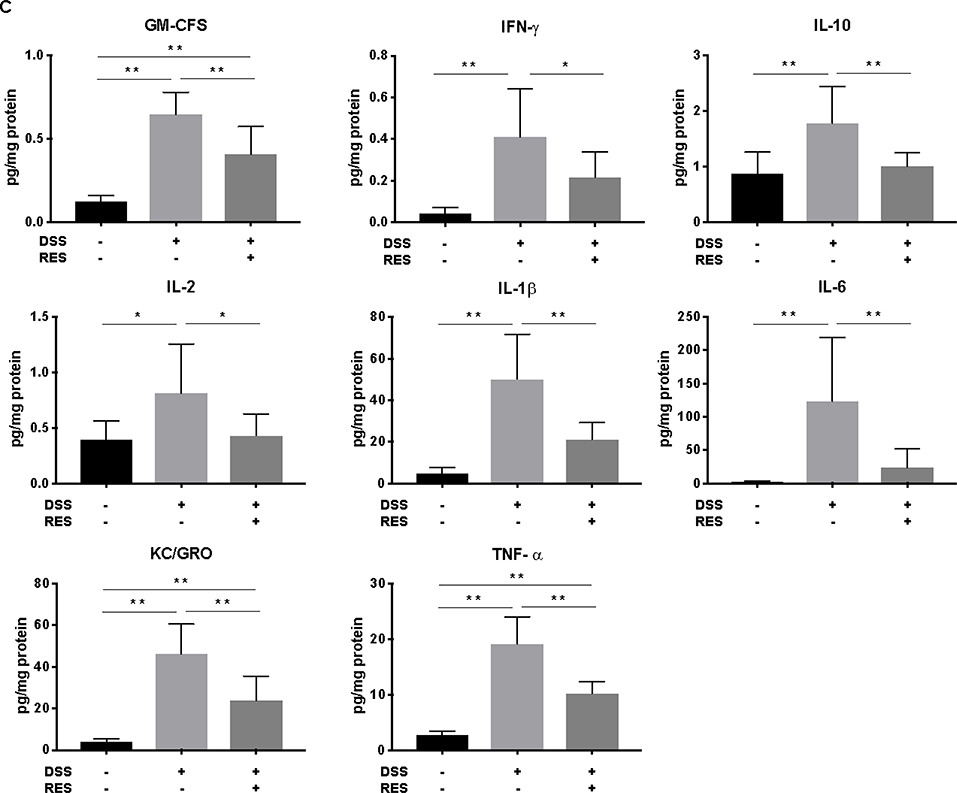
Dietary resveratrol improved colonic tissue damage and suppressed the overexpression of inflammatory cytokines. (A) Representative colon tissue images of H&E-stained colorectum sections (300X magnification). Crypts destruction associated with epithelial damage (red arrow) and inflammatory cell infiltrations (black arrow) were evident in DSS group; (B) Histological score of colonic abnormality (n=6); (C) Quantification of inflammatory cytokines (GM-CSF, IFN-γ, IL-10, IL-2, IL-1β, IL-6, KC/GRO, and TNF-α) in the colonic mucosa (n=8). Data are mean ± SD). Significant differences are indicated as: *, P < 0.05; * *, P < 0.01.
Dietary resveratrol mediated increases of colonic pro-inflammatory cytokines caused by DSS treatment
Cytokines play a crucial role in driving and mediating intestinal inflammation. The severity of IBD is associated with up-regulation of pro-inflammatory cytokines and down-regulation of anti-inflammatory cytokines. Hence, restoring balance of these cytokines is a potential mode to alleviate IBD.30 The expression levels of anti- and pro-inflammatory cytokines: GM-CSF, IFN-γ, IL-10, IL-2, IL-1β, IL-6, KC/GRO, and TNF-α in colon mucosa were increased by 5.2, 8.7, 2.0, 2.1, 10.0, 47.9, 10.9, and 7.0-fold respectively over the control group in DSS+/RES- mice (P < 0.05), while dietary resveratrol in the DSS+/RES+ group markedly reduced their maximal expression by 44.2%, 48.5%, 43.2%, 46.8%, 58.0%, 79.9%, 48.5%, and 46.5%, respectively (P < 0.05) (Fig. 2C). These results suggested that the anti-inflammatory effect of resveratrol was at least partially due to improvements in immune homeostasis.
Although significant mitigation of TNF-α, IFN-γ, IL-1β and IL-6 overexpression by resveratrol in DSS-treated mice (Fig. 2C) is in line with several previous studies,16, 18, 31 the modulating effects of dietary resveratrol on colonic GM-CSF, KC/GRO and IL-2 have not been as widely reported. The production of GM-CSF was related to delayed neutrophil apoptosis, which contributes to the tissue injury in IBD.32 Indeed, an increased secretion of GM-CSF had been found in mucosal lesions of mouse models and IBD patients.32, 33 Our present study reports that dietary resveratrol down-regulated the enhanced GM-CSF production in DSS-induced colitis mice (Fig. 2C) for the first time. KC/GRO is a CXC chemokine also known as chemokine (C-X-C motif) ligand 1 (CXCL1). During inflammation, it is involved in neutrophil activation and recruitment.34 It had been reported that the level of CXCL1 in serum was highly correlated with the severity of disease in IBD patients.34 The regulating effects of resveratrol on GM-CSF and KC/GRO suggest that the polyphenol may play a role in neutrophil apoptosis and infiltration in the colon.35 IL-2 supports the proliferation and differentiation of regulatory T cells. Subsequently, aberrant expression of IL-2 promotes the imbalance of effector and regulatory T cells, which leads to multi-organ autoimmune disease and inflammation.36 Our results supported the regulating effects of dietary resveratrol on T cell-associated immune disorders.20
In our study, the observed differential expression of IL-10 in DSS+/RES- and DSS+/RES+ groups (Fig. 2C) was contradictory to previous studies.37 IL-10 exerts anti-inflammatory properties by inhibiting production of pro-inflammatory cytokines such as IL-1, IL-6, IL-12, and TNF-α as well as chemokines.38 We speculate that the possible cause of increased IL-10 in our findings could be due to a triggered response to severe inflammation and increased expression of pro-inflammatory cytokines.39
Resveratrol partially ameliorated gut microbiota dysbiosis induced by DSS
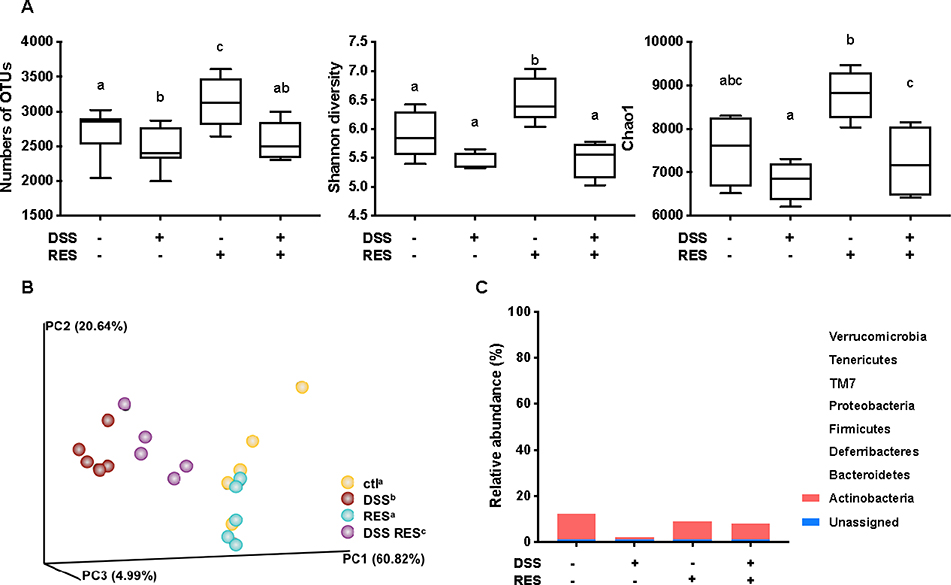
Given the critical role of the gut microbiota in the pathogenesis of colitis, dietary resveratrol was investigated for its ability to counteract the state of chronic gut microbiota dysbiosis in DSS-treated mice. The influences of dietary resveratrol on the richness and evenness of gut microbiota were assessed. OTU number, Shannon diversity and Chao1 indices were measured as they are common measures of α-diversity that indicate the depth of sequence coverage and community diversity within samples.40 As shown in Fig. 3A, dietary resveratrol significantly enriched and diversified the gut microbiota of DSS-/RES+ mice (P < 0.05), while colitic, DSS-treated mice that did not receive resveratrol were measured to have lowered sample species abundance, richness, and diversity (P < 0.05), which supported the prebiotic effects of dietary resveratrol. The relative similarity of the four treatment groups’ microbiota was visualized by PCoA (Fig. 3C) following β-diversity analysis. β-diversity was performed on a rarified sequence set, with a sampling depth equal to the smallest value of the filtered sequences.40 A significant distance between DSS-/RES- and DSS+/RES- groups was observed (P = 0.009), which suggested that DSS treatment dramatically shifted the gut microbiota. Dietary resveratrol shifted this grouping back toward the control group and was clearly distinguishable with the DSS+/RES- group (P = 0.008) (Fig. 3B), which implied that administration of resveratrol partially recovered the dysbiosis caused by DSS and resulting inflammatory status. The presence of overlapping clusters between DSS-/RES- and DSS-/RES+ groups illustrated the relative similarity of microbial composition between these two groups (Fig. 3B). The above results indicated that dietary resveratrol partially recovered the richness, abundance, and evenness of gut microbiota in DSS-treated mice, potentially by protecting numerous specific commensal bacterial clades.
Figure 3.
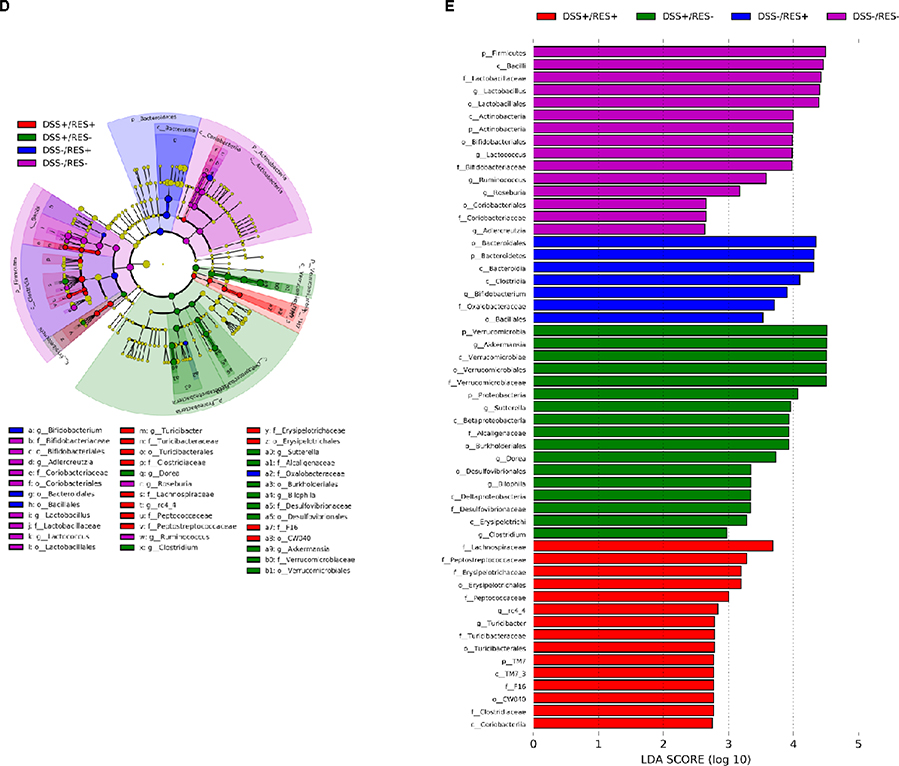
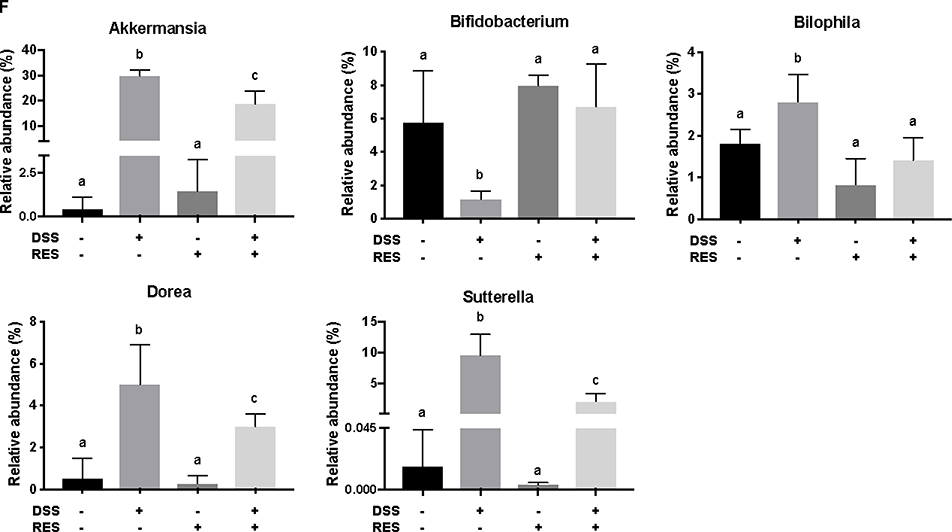
Dietary resveratrol partially relieved gut microbiota dysbiosis in colitic mice. (A) Effects of resveratrol on the α-diversity of the gut microbiota with different indices - numbers of OTUs, Shannon index, and Chao1 (P < 0.05); (B) effects of resveratrol on the β-diversity of the gut microbiota, visualized with PCoA plots (P < 0.01); (C) relative abundance of the gut microbiota at the phylum level; (D) taxonomic cladogram generated from default LEfSe analysis (α < 0.1 for the factorial Kruskal-Wallis test); (E) LDA scores of the differentially abundant taxa (with a LDA score of >2 and a significance of α < 0.1); (F) relative abundance of the gut microbiota at the genus level (P < 0.05). Data are presented as mean ± SD (n = 5). Different letters represent statistically significant differences.
To understand which bacterial clades were affected by dietary resveratrol, we evaluated the relative abundance of the predominant taxa within and among groups (Fig 3C–3F). Linear discriminant analysis effective size (LEfSe) algorithm with significance at p < 0.01 was then used to visualize the differentially representative bacterial clades for each experimental group’s microbiota (Fig 3D–3E). Consistent with previous studies, at the phylum level, we found that Firmicutes, Bacteroidetes, and Actinobacteria accounted for more than 95.0% of the whole gut microbial composition in the DSS-/RES- group.42 Bacteroidetes were significantly more abundant in DSS-/RES+ mice compared with the control group (P < 0.05) (Fig. 3C–3E), which was in agreement with previous studies,43 and were mostly represented in the class Bacteroidia and order Bacteroidales (Fig. 3E–3F). Notably, DSS treatment significantly altered the bacterial structure at the phylum level by contributing to the expansion of Verrucomicrobia and Proteobacteria populations and reduction of Actinobacteria and Firmicutes versus DSS-/RES- mice (P < 0.05) (Fig. 3C–3E), which was also in accordance with previous studies.44 Firmicutes, Bacteroidetes, and Actinobacteria only occupied 56.5% of the total gut microbial community in the DSS-treated group, which is 38.5% lower in relative abundance terms than the DSS-/RES- group. Dietary resveratrol partially reversed this phylum-level shift by limiting the abundance of Verrucomicrobia from 29.7% to 20.8% and Proteobacteria from 11.7% to 3.6% and increasing the abundance of Actinobacteria from 1.2% to 6.9% (P < 0.05) (Fig. 3C–3E).
Akkermansia, Bifidobacterium, Bilophila, Dorea, and Sutterella were the most affected genera among treatment groups (Fig. 3D–3F). It was observed that there was atypical dominance of Akkermansia in the DSS+/RES- group, accounting for 29.7% of the mean population, while it only accounted for less than 1.5% of the microbial composition in DSS-/RES- and DSS-/RES+ groups. Importantly, dietary resveratrol significantly inhibited the excessive abundance of Akkermansia (18.8%) in DSS+/RES+ mice (P < 0.05) (Fig. 3D–3F). Akkermansia is a Gram-negative, mucin-degrading, bacteria of the Verrucomicrobia phylum with only one species, Akkermansia muciniphila, identified to date.45 Its potential role in the development of colitis remain controversial. A. muciniphila was reported as a potent anti-inflammatory intestinal bacterium that adhered to intestinal enterocytes, strengthened the integrity of the epithelial cell layer, and was inversely correlated with inflammation.46, 47 However, A. muciniphila has also been reported to be increased in mice with DSS-induced colitis and patients suffering from colorectal cancer.48, 49 Seregin et al. concluded that A. muciniphila acted as a pathobiont to promote colitis in a genetically susceptible host under the regulation of nod-like receptor 6 (NLRP6).50 Ganesh et al. demonstrated that the presence of A. muciniphila exacerbated Salmonella-induced colitis in gnotobiotic mice.51 The mechanism by which A. muciniphila promoted colitis will clearly need to be elucidated in future works. Given its mucin-degrading properties, we speculated that A. muciniphila invaded and degraded the colon mucosa, which allowed for higher permeability and access to other microbes which drive inflammation.9, 50
Dietary resveratrol also significantly inhibited the abnormal bloom of Bilophila, Sutterella, and Dorea observed in DSS-fed mice (P < 0.05) (Fig. 3D–3F). Bilophila, belonging to the phylum of Proteobacteria, is a sulfate-reducing bacteria associated with IBD and appendicitis.9, 52 Sutterella wadsworthensis, also belonging to the phylum Proteobacteria, has been suggested to induce inflammation cascades by occupying the mucus layer and provoking host immune responses.53 Limited research on Dorea, belonging to the phylum of Firmicutes, has been published. A clinical study indicated that Dorea was lower in Crohn’s disease sufferers compared to healthy subjects.54 However, its potential role in the pathogenesis of IBD has not been discussed yet. In this work, we observed that dietary resveratrol significantly suppressed the relative abundance of Dorea from 5.0% to 3.8% in DSS-treated mice (Fig. 3F) and their abundance was positively correlated with higher expression of pro-inflammatory cytokines (shown below). From these contradictory results forms a need for future investigation to clarify the role of Dorea in the gut and its potential relationship with colon dysbiosis and inflammation. The above results indicated that dietary resveratrol partially suppressed the abnormal growth of potential pathobionts associated with colitis, including Akkermansia, Bilophila, Sutterella and Dorea (Fig. 3F).
Meanwhile, dietary resveratrol in DSS+ mice experienced significantly increased counts of Bifidobacterium, with a 5-fold gain in its relative abundance compared to DSS+/RES- mice (P < 0.05). From the DSS-/RES- mean level of Bifidobacterium at 6.7%, its relative abundance dropped to 1.2% in DSS+/RES- mice and recovered to a similar level as the control upon resveratrol administration (P < 0.05) (Fig. 3D–3F). The protective effects of Bifidobacterium against DSS-induced colitis, such as reduced pro-inflammatory cytokine expression and oxidative damage, has been demonstrated in other research.55 These results support the trend that dietary resveratrol alleviates microbial community perturbations by selectively restoring the abundance of probiotics and suppressing the growth or otherwise resisting out-competition of potential pathogens in a DSS-induced colitis mouse model.
Gut microbiota alterations are strongly correlated with the aberrant expression of cytokines
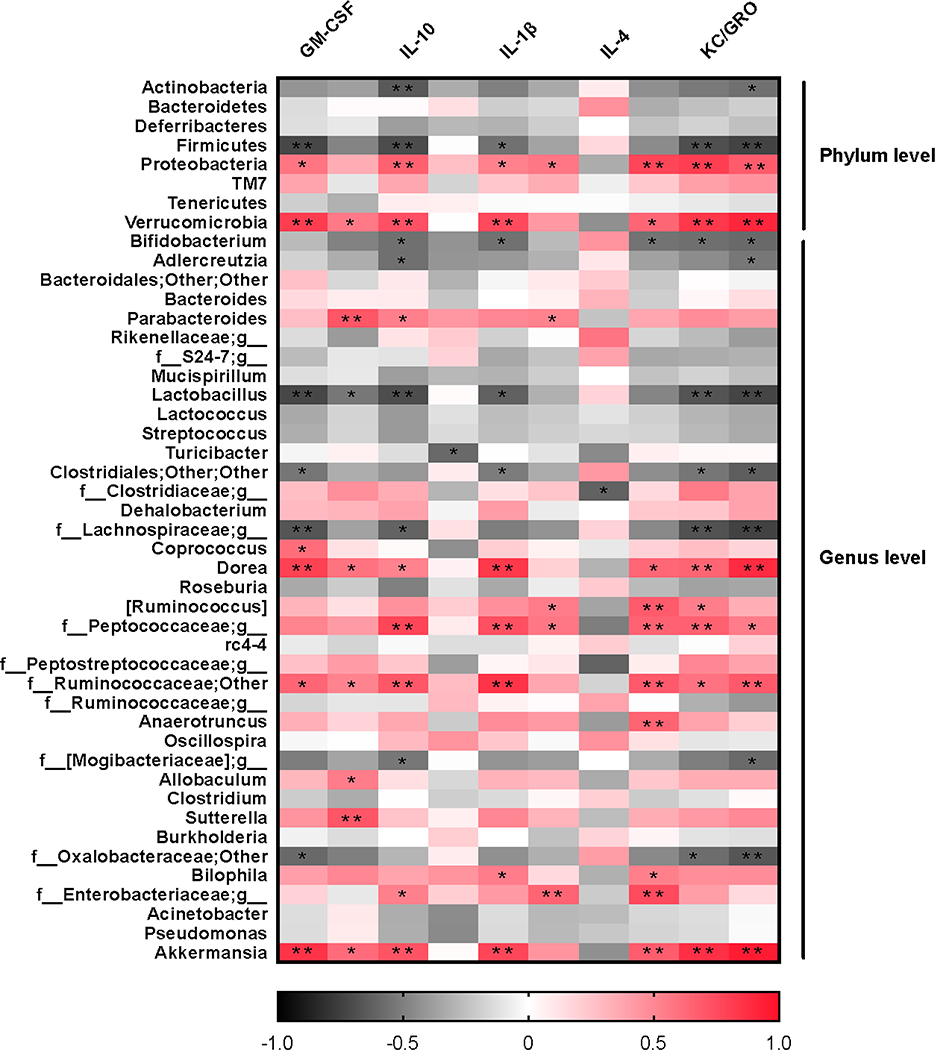
From these findings that dietary resveratrol partially resolved the gut microbiota dysbiosis in colitic mice, we then examined whether these changes were associated with the anti-colitis effects of dietary resveratrol. Correlations between the alterations of gut microbiota and expressions of inflammatory cytokines were analyzed with Pearson’s correlation analysis and visualized in the heatmap as shown in Fig. 4.
Figure 4.
Heatmap of correlations between gut microbiota and inflammatory cytokines. Positive correlation is in red. Negative correlation is in black. Significant differences are indicated as: *, P < 0.05; * *, P < 0.01.
Consistent positive correlations were observed between potential pathogenic organisms and pro-inflammatory cytokine expression levels. Proteobacteria abundance was significantly positively correlated with GM-CSF, IL-1β, IL-2, IL-6, KC/GRO and TNF-α (p < 0.05) expression levels (Fig. 4). Accordingly, at the genus level, the abundance of Bilophila and Sutterella of the same phylum were positively correlated with pro-inflammatory cytokines, including IL-1β, IL-6 and IFN-γ (p < 0.05). General Verrucomicrobia and one of its genera, Akkermansia, were significantly positively correlated with the expression of GM-CSF, IFN-γ, IL-1β, IL-6, KC/GRP, and TNF-α (P < 0.05) (Fig. 4). These results indicated that gut microbial variations may affect mucosal autoimmunity through regulating or inducing inflammatory cytokine expression.
It was also observed that certain bacterial clades negatively correlated with pro-inflammatory cytokines and positively correlated with anti-inflammatory cytokines. Specifically, Bifidobacterium was highly negatively correlated with IL-1β, IL-6, KC/GRO and TNF-α (P < 0.05), and positively correlated with IL-4. The same was true for Lactobacillus; Negative correlations were observed with pro-inflammatory cytokines GM-CSF, IFN-γ, IL-1β, KC/GRO, and TNF-α (P < 0.05). The protective effect of Lactobacillus and Bifidobacterium in DSS-induced colitis against inflammation by reducing TNF-α has been discussed before.55 These data provided additional clues to the causality between these observations and illustrated potential modes for the pathogenesis of IBD.
Conclusion
In summary, our results corroborated the anti-colitis effects of dietary resveratrol ( at a dose of 2.3 mg/kg/day human equivalent) in DSS-induced colitic mice and provided evidence for the modulatory effects of dietary resveratrol on gut microbiota, including the restoration of bacterial community diversity and the rebalance of probiotics and pathobionts. It was revealed that resveratrol administration not only prevented losses in body weight associated with IBD, reduced the degree of disease activity and other markers of colon inflammation, but it also protected colonic tissue from structure deterioration and dysplasia, and reduced the expression of several pro-inflammatory cytokines when compared to the respective traits in colitic mice that did not receive dietary resveratrol. Grounded in these results, we further revealed that the alterations of select gut microbe abundance were significantly correlated with the expression of inflammatory cytokines, leading us to propose that dietary resveratrol might prevent colitis in large part by modulating the gut microbiota.
Acknowledgement
This work was supported in part by USDA/NIFA, Hatch Fund, and National Institutes of Health (R01 AT010229).
Abbreviations
- DSS
dextran sulfate sodium
- RES
resveratrol
- IBD
inflammatory bowel disease
- DAI
disease activity index
- H&E
hematoxylin & eosin
- OTUs
operational taxonomic units
- PCoA
principal coordinates analysis
- TNF-α
tumor necrosis factor alpha
- IL-1β
Interleukin 1 beta
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- IL-2
Interleukin 2
- IL-6
Interleukin 6
- IL-10
Interleukin 10
- IFN-γ
interferon gamma
- KC/GRO
keratinocyte chemoattractant/human growth-regulated oncogene
- CXCL1
chemokine (C-X-C motif) ligand 1
- LDA
linear discriminant analysis
Reference
- 1.Khor B, Gardet A, and Xavier RJ, Genetics and pathogenesis of inflammatory bowel disease. Nature, 2011. 474(7351): p. 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishida A, et al. , Gut microbiota in the pathogenesis of inflammatory bowel disease. Clinical Journal of Gastroenterology, 2017. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, et al. , Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion, 2016. 93(1): p. 59–65. [DOI] [PubMed] [Google Scholar]
- 4.Fujimoto T, et al. , Decreased abundance of Faecalibacterium prausnitzii in the gut microbiota of Crohn’s disease. J Gastroenterol Hepatol, 2013. 28(4): p. 613–9. [DOI] [PubMed] [Google Scholar]
- 5.Manichanh C, et al. , Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut, 2006. 55(2): p. 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu W, et al. , Precision editing of the gut microbiota ameliorates colitis. Nature, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sellon RK, et al. , Resident Enteric Bacteria Are Necessary for Development of Spontaneous Colitis and Immune System Activation in Interleukin-10-Deficient Mice. Infection and Immunity, 1998. 66(11): p. 5224–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laparra JM and Sanz Y, Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol Res, 2010. 61(3): p. 219–25. [DOI] [PubMed] [Google Scholar]
- 9.Han Y, et al. , Dietary Intake of Whole Strawberry Inhibited Colonic Inflammation in Dextran-Sulfate-Sodium-Treated Mice via Restoring Immune Homeostasis and Alleviating Gut Microbiota Dysbiosis. 2019. [DOI] [PubMed]
- 10.Cardona F, et al. , Benefits of polyphenols on gut microbiota and implications in human health. The Journal of Nutritional Biochemistry, 2013. 24(8): p. 1415–1422. [DOI] [PubMed] [Google Scholar]
- 11.Azorin-Ortuno M, et al. , Metabolites and tissue distribution of resveratrol in the pig. Mol Nutr Food Res, 2011. 55(8): p. 1154–68. [DOI] [PubMed] [Google Scholar]
- 12.Qiao Y, et al. , Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food & function, 2014. 5(6): p. 1241–1249. [DOI] [PubMed] [Google Scholar]
- 13.Etxeberria U, et al. , Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. The Journal of nutritional biochemistry, 2015. 26(6): p. 651–660. [DOI] [PubMed] [Google Scholar]
- 14.Yao J, et al. , Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch Med Res, 2010. 41(4): p. 288–94. [DOI] [PubMed] [Google Scholar]
- 15.Cui X, et al. , Resveratrol Suppresses Colitis and Colon Cancer Associated with Colitis. Cancer Prevention Research, 2010. 3(4): p. 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh UP, et al. , Resveratrol (trans-3,5,4’-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. The Journal of pharmacology and experimental therapeutics, 2010. 332(3): p. 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larrosa M, et al. , Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J Agric Food Chem, 2009. 57(6): p. 2211–20. [DOI] [PubMed] [Google Scholar]
- 18.Cui X, et al. , Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer prevention research (Philadelphia, Pa.), 2010. 3(4): p. 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samsami-Kor M, et al. , Anti-Inflammatory Effects of Resveratrol in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch Med Res, 2015. 46(4): p. 280–5. [DOI] [PubMed] [Google Scholar]
- 20.Alrafas HR, et al. , Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J Leukoc Biol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JJ, et al. , Investigating Intestinal Inflammation in DSS-induced Model of IBD. Journal of Visualized Experiments: JoVE, 2012(60): p. 3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xian W, et al. , Chemopreventive effects of nobiletin and its colonic metabolites on colon carcinogenesis. Molecular Nutrition and Food Research, 2015. 59(12): p. 2383–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yue S, et al. , Identification of pinostilbene as a major colonic metabolite of pterostilbene and its inhibitory effects on colon cancer cells. Molecular Nutrition and Food Research, 2016. 60(9): p. 1924–1932. [DOI] [PubMed] [Google Scholar]
- 24.Song M, et al. , Dietary 5-demethylnobiletin inhibits cigarette carcinogen NNK-induced lung tumorigenesis in mice. Food Funct, 2017. 8(3): p. 954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Araki Y, et al. , Development of dextran sulphate sodium-induced experimental colitis is suppressed in genetically mast cell-deficient Ws/Ws rats. Clinical and Experimental Immunology, 2000. 119(2): p. 264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klindworth A, et al. , Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Research, 2013. 41(1): p. e1–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caporaso JG, et al. , QIIME allows analysis of high-throughput community sequencing data. Nat Meth, 2010. 7(5): p. 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segata N, et al. , Metagenomic biomarker discovery and explanation. Genome Biology, 2011. 12(6): p. R60–R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clapper ML, Cooper HS, and Chang WC, Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin, 2007. 28(9): p. 1450–9. [DOI] [PubMed] [Google Scholar]
- 30.Guan Q and Zhang J, Recent Advances: The Imbalance of Cytokines in the Pathogenesis of Inflammatory Bowel Disease. Mediators of Inflammation, 2017. 2017: p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao J, et al. , Polydatin ameliorates DSS-induced colitis in mice through inhibition of nuclear factor-kappaB activation. Planta Med, 2011. 77(5): p. 421–7. [DOI] [PubMed] [Google Scholar]
- 32.Noguchi M, et al. , Increased Secretion of Granulocyte-Macrophage Colony-Stimulating Factor in Mucosal Lesions of Inflammatory Bowel Disease. Digestion, 2001. 63(suppl 1)(Suppl. 1): p. 32–36. [DOI] [PubMed] [Google Scholar]
- 33.Dieleman LA, et al. , Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology, 1994. 107(6): p. 1643–1652. [DOI] [PubMed] [Google Scholar]
- 34.Mitsuyama K, et al. , Increased circulating concentrations of growth-related oncogene (GRO)-alpha in patients with inflammatory bowel disease. Dig Dis Sci, 2006. 51(1): p. 173–7. [DOI] [PubMed] [Google Scholar]
- 35.Mayangsari Y and Suzuki T, Resveratrol Ameliorates Intestinal Barrier Defects and Inflammation in Colitic Mice and Intestinal Cells. 2018. 66(48): p. 12666–12674. [DOI] [PubMed] [Google Scholar]
- 36.Klatzmann D and Abbas AK, The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nature Reviews Immunology, 2015. 15: p. 283. [DOI] [PubMed] [Google Scholar]
- 37.Sánchez-Fidalgo S, et al. , Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. European Journal of Pharmacology, 2010. 633(1): p. 78–84. [DOI] [PubMed] [Google Scholar]
- 38.Saraiva M and O’Garra A, The regulation of IL-10 production by immune cells. Nature Reviews Immunology, 2010. 10: p. 170. [DOI] [PubMed] [Google Scholar]
- 39.Peranteau WH, et al. , IL-10 Overexpression Decreases Inflammatory Mediators and Promotes Regenerative Healing in an Adult Model of Scar Formation. Journal of Investigative Dermatology, 2008. 128(7): p. 1852–1860. [DOI] [PubMed] [Google Scholar]
- 40.Odamaki T, et al. , Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiology, 2016. 16: p. 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munyaka PM, et al. , Acute dextran sulfate sodium (DSS)-induced colitis promotes gut microbial dysbiosis in mice. J Basic Microbiol, 2016. 56(9): p. 986–98. [DOI] [PubMed] [Google Scholar]
- 42.Jandhyala SM, et al. , Role of the normal gut microbiota. World Journal of Gastroenterology: WJG, 2015. 21(29): p. 8787–8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen M. l., et al. , Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio, 2016. 7(2): p. e02210–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ritchie LE, et al. , Polyphenol-rich sorghum brans alter colon microbiota and impact species diversity and species richness after multiple bouts of dextran sodium sulfate-induced colitis. FEMS Microbiol Ecol, 2015. 91(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Derrien M, et al. , The Mucin Degrader Akkermansia muciniphila Is an Abundant Resident of the Human Intestinal Tract. Applied and Environmental Microbiology, 2008. 74(5): p. 1646–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reunanen J, et al. , Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of epithelial cell layer. Applied and environmental microbiology, 2015: p. AEM. 04050–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anhe FF, et al. , A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut, 2015. 64(6): p. 872–83. [DOI] [PubMed] [Google Scholar]
- 48.Schwab C, et al. , Longitudinal study of murine microbiota activity and interactions with the host during acute inflammation and recovery. The Isme Journal, 2014. 8: p. 1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weir TL, et al. , Stool Microbiome and Metabolome Differences between Colorectal Cancer Patients and Healthy Adults. PLOS ONE, 2013. 8(8): p. e70803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seregin SS, et al. , NLRP6 Protects Il10(−/−) Mice from Colitis by Limiting Colonization of Akkermansia muciniphila. Cell Rep, 2017. 19(4): p. 733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ganesh BP, et al. , Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic mice. PloS one, 2013. 8(9): p. e74963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loubinoux J, et al. , Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiology Ecology, 2002. 40(2): p. 107–112. [DOI] [PubMed] [Google Scholar]
- 53.Hiippala K, et al. , Mucosal Prevalence and Interactions with the Epithelium Indicate Commensalism of Sutterella spp. Frontiers in Microbiology, 2016. 7(1706). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liguori G, et al. , Fungal Dysbiosis in Mucosa-associated Microbiota of Crohn’s Disease Patients. Journal of Crohn’s and Colitis, 2016. 10(3): p. 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fooks LJ and Gibson GR, Probiotics as modulators of the gut flora. Br J Nutr, 2002. 88 Suppl 1: p. S39–49. [DOI] [PubMed] [Google Scholar]