Abstract
Geraniaceae family plants are highly reputed aromatic and medicinal perennial branched herbs. The high economic value of these plants is due to their secondary metabolites, especially essential oil of foliage, which is a complex mixture of volatile phytochemicals, such as terpenes, esters, aldehydes, alcohols, ketones and phenols. The main phytoconstituents of the essential oil belong to the terpenoid group of metabolites, such as monoterpenes, sesquiterpenes, diterpenes and their esters. Of these, geraniol, linalool, citronellol and their esters (50–70%) generally constitute a major portion of essential oil, responsible for its fragrance. Essential oil is biosynthesized in specialized tissues known as glandular trichomes present in leaves, green branches and fresh flowers. Geraniaceae family plants have been highly useful in the perfumery, cosmetics, aromatherapy, pharmaceuticals and food industries. Several pharmacological properties such as antifungal, anti-inflammatory, anti-cancerous, anti-depressant, antibacterial, antioxidant, antiseptic, anti-dysentery, and antidiabetic properties are attributed to the presence of geranium oil. Further, it improves blood circulation, treats congestion, cleans the lymphatic system, strengthens the immune system, and is effective in combating nervousness, constipation, insomnia, anxiety and high blood pressure. The chapter discusses the phytochemical composition, pharmacological properties, genomics of essential oil biosynthetic pathway, enhancement of essential oil yield, and several biotechnological approaches to enhance the quantity as well as quality of essential oil in geranium.
Keywords: Rose-scented geranium, Pelargonium, Essential oil, Terpene, Geraniol
Introduction
Geraniaceae family plants (Pelargonium sp.) are perennial medicinal and essential oil-yielding branched herbs, growing in subtropical and temperate climates. Pelargonium genus comprises more than 750 species, and most of them originated from Europe and Africa. They have remarkable commercial applications due to their characteristic essential oil. The essential oil distillate of a high-value Geraniaceae plant, rose-scented geranium (Fig. 12.1), has a very strong, pleasant and rosy fragrance with a minty top; therefore, it is used as a substitution of the expensive rose oil, and is also known as ‘poor man’s rose oil’. The essential oil of rose-scented geranium was extensively used as a flavouring agent in the food, cosmetic, perfumery, and pharmaceutical industries. The rose-scented geranium essential oil is also well known for its effectiveness in various health-related treatments such as aromatherapy and for its antimicrobial properties (Narnoliya et al. 2017, 2018a; Jadaun et al. 2017). In India, geranium was introduced in the nineteenth century in the southern climate, and now it grows in different parts of India. They are also used as ornamental plants, for example, Pelargonium x hortorum and Pelargonium graveolens, and grown in gardens and parks to provide a pleasant fragrance (Ravindra and Kulkarni 2015).
Fig. 12.1.

Rose-scented geranium plant
There are four species of geranium which have commercial applications: zonal geranium (Pelargonium x hortorum), scented geranium (Pelargonium sp.), regal pelargonium (Pelargonium x domesticum) and regal ivy geranium (Pelargonium peltatum). The essential oil of geranium is synthesized in specialized cells known as trichomes. The vegetative and reproductive organs of rose-scented geranium are reported to have non-glandular and glandular trichomes. Generally, glandular trichomes are the major reservoirs for essential oil (Boukhris et al. 2013; Narnoliya et al. 2017). More than 200 species of geranium occur naturally, out of which P. graveolens, P. odoratissimum, P. radens, and P. capitatum are more commonly used for harvesting the essential oil. The hydro-distillation method is commonly used for extraction of oil from the aerial part, especially leaves and stem. Essential oil of rose-scented geranium is composed of complex volatile phytochemicals, produced as secondary metabolites, such as terpenes, esters, aldehydes, ketones, alcohols, and phenols. Generally, they play a crucial role in ecological adjustment of the plant and protect it from pathogen and herbivore attacks. Thus, essential oil components are the key substances of rose-scented geranium for its defence system (Babu and Kaul 2005; Jadaun et al. 2017; Ravindra and Kulkarni 2015).
Geranium oil comprises more than 120 phytoconstituents, which include monoterpenes, sesquiterpenes, diterpenes, and low molecular weight aroma compounds. There are three main components, linalool, citronellol and geraniol, and their esters, which constitute more than 60% of total essential oil, and they are responsible for determining its odour. Other components are menthone, nerol, isomenthone, rose oxides, terpineol, pinene and myrcene (Jadaun et al. 2017; Ravindra and Kulkarni 2015). Thus, terpenes are the major contributors in the essential oil of rose-scented geranium, and these terpenes are biosynthesized through the terpenoid pathway. It was less explored in terms of its genomics, transcriptomics, gene expression and enzyme characterization. Recently, the transcriptomic information of rose-scented geranium leaf has been reported, which provides a foundation for the molecular study of primary and secondary metabolism (Narnoliya et al. 2017, 2018a). Recently, a gene, 1-deoxy-d-xylulose-5-phosphate synthase (DXS) was cloned from this plant and its recombinant protein was physico-kinetically characterized and heterologously overexpressed in Withania somnifera to evaluate the effect of DXS on withanolides (Jadaun et al. 2017).
Hence, recent genomics and functionality studies provided a platform to enhance the quantity and quality of essential oil. Using modern biotechnological and synthetic biological approaches, with the aid of available biological information, it could be possible to enhance the productivity of rose-scented geranium’s essential oil. Herein, we discuss the phytochemical composition of geranium essential oil, and its biological effects. Further, different biotechnological approaches have also been explored to enhance the production of high-value essential oil.
Phytochemical Composition of Geranium
Rose-scented geranium is famous for its fragrance produced by its high-value essential oil. Aroma and fragrance of an oil depend on its composition. Essential oil of geranium contains more than 200 types of organic compounds, of which, terpenes, phenylpropanoids, and some other low molecular weight phytoconstituents occur predominantly (Table 12.1). Terpenes constitute the major part of essential oil. Terpene is composed of a five-carbon isoprene unit (CH2-C (CH3)-CH-CH2). The common formula of terpene is (C5H8)n, where ‘n’ is the number of isoprene units. Further, depending on the number of isoprene units, terpenes are classified into different categories such as monoterpenes (2 isoprene units, i.e., 10 carbons), sesquiterpenes (3 isoprene units, i.e., 15 carbons), diterpenes (4 isoprene units, i.e., 20 carbons), triterpenes (6 isoprene units, i.e., 30 carbons) and tetraterpenes (8 isoprene units, i.e., 40 carbons). In essential oil, these terpenes are present either in their simple form or in alcoholic, ketonic, aldehyde, and ester forms, and sometimes as chlorinated or oxygenated derivatives. On the basis of carbon arrangement, these terpenes are present in different structures like acyclic, monocyclic, and bicyclic structures.
Table 12.1.
Different types of chemicals extracted from rose-scented geranium
| Chemical Category | Examples of Chemicals |
|---|---|
| Aliphatic hydrocarbons | Butane; isoprene; 1,3-pentadiene; hexane; isooctane; octadecane; nonadecane; nonadecene; eicosane; henicosane; decosane; tricosane; tetracosane; pentacosane |
| Aromatic hydrocarbons | Toluene; p-cymene |
| Terpene hydrocarbons | α-Pinene; β-pinene; α-phellandrene; β-phellandrene; camphene; myrcene; sabinene; limonene; γ-terpinene; terpinolene; cis-β-ocimene; trans-β-ocimene; dehydro-1,8-cineole; 1,4-cineole; p-menthadiene; perillene; piperitone |
| Sesquiterpene hydrocarbons | α-Copaene; α-cadinene; γ-cadinene; δ-cadinene; guaia-6-9-diene; β-bisabolene; α-calcorene, calamenene, β-selinene, α-muurolene; γ-muurolene; α-bourbonene; β-bourbonene; 11-orbourbonene; β-caryophyllene; γ-caryophyllene; bicyclo-germacrene; germacrene D; longifolene; β-gurjunene; β-farnesene; (E,E)-α-farnesene; α-cubebene; β-cubebene; β-elemene; β-maaline; α-humulene; viridiflorene; zonzrene; α-ylangene; allo-aromadendrene; selina- 4,11-diene; α-guaiene |
| Aliphatic alcohols | Methanol; ethanol; t-butanol; pentanol; 1-penten-3-ol-2-propanol; hexanol; 2-methylpropanol; 2-dimethylpropanol; 2-methylbutanol; 2-methyl-3-buten-2-ol; 3-metylbutanol; 3-methylpentan-1-ol; cis-3-hexenol; trans-2-hexenol; 3-hexen-1-ol; octanol; 1-octen-3-ol; 2-octanol |
| Terpene alcohols | Geraniol; isogeraniol; isopulegol; 7-hydroxy-6, 7-dihydrogeraniol; nerol; epi-photonerol A; linalool; menthol; isomenthol; neoisomenthol; α-terpineol; citronellol; 7-hydroxydyhydrocitronellol; borneol; isoborneol; terpinen-4-ol |
| Aromatic alcohols | 2-Phenylethyl alcohol |
| Sesquiterpene alcohols | 10-Epi-γ-eudesmol; β-eudesmol; 11-selina-4-α-ol; junenol; farnesol; guaiol; spathulenol; T-cadinol; elemol |
| Aliphatic esters | Methyl formate; methyl butyrate; 2-methylbutyl formate; 3-methybutyl formate; 2-methylpropyl formate; 3-methylpentyl formate; ethyl formate; butyl formate; propyl formate; 2-propyl formate; hexyl formate; benzyl tiglate; (Z)-3-hexenyl acetate |
| Aromatic esters | 2-Phenylethyl tiglate; 2-phenylethyl propionate; 2-phenylethyl butyrate; 2-phenylethyl isobutyrate; 2-phenylethyl isovalerate; 2-phenylethyl acetate |
| Terpene esters | 3-Hexenyl acetate; geranyl formate; geranyl butyrate; geranyl isobutyrate; geranyl 2-methyl butyrate; geranyl tiglate; geranyl acetate; geranyl propionate; geranyl valerate; geranyl 3-methylvalerate; geranyl 4-methylvalerate; geranyl hexanoate; geranyl heptanoate; geranyl nonanoate; geranyl isovalerate; methyl geranate; geranyl 3-methyl pentanoate; geranyl octanoate; citronellyl acetate; citronellyl formate; citronellyl butyrate; citronellyl tiglate; citronellyl propionate; citronellyl valerate; citronellyl 4-methylvalerate; citronellyl isovalerate; citronellyl hexanoate; citronellyl isohexanoate; citronellyl heptanoate; citronellyl octanoate; citronellyl nonanoate; furopelargonic acetate; linalyl acetate; bornyl acetate; neryl acetate; neryl formate |
| Aliphatic ketones | Acetone; 2-butanone; 2-pentanone; 3-methyl-2-butanone; 2-methyl-3-pentanone; 4-methyl-2-pentanone; 2-methylcyclopentanone; 3-methylcyclo-pentanone; 3-metylcyclohexanone; 4-methyl-3-penten-2-one; 2-hexanone; methylheptenone; 6-methyl-5-hepten-2-one; methyl-3-methylcyclo-pentenyl ketone |
| Terpene ketones | Menthone; isomenthone |
| Sesquiterpene ketones | 1,7-Dihydrofurapelargone; furapelargone A; furapelargone B; 7,8 dihydrofurapelargone |
| Aliphatic aldehydes | Benzaldehyde; ethanol; decanal; 2-methylpropanal; 3-methyl-2-butanal; 3-methylbutanal; 2-furfuraldehyde; nonanal; (E)-2-hexenal |
| Terpene aldehydes | Geranial; citronellal; neral; photocitrl A; epi-photocitral A; photocitral B; p-menth-1-en-9-al |
| Terpene oxides | Cis-rose oxide; trans-rose oxide; cis-linalool oxide; trans-linalool oxide; anhydrolinalool oxide; bois-de-rose oxide; nerol oxide |
| Sesquiterpene oxides | Caryophyllene oxide |
| Aliphatic acids | Formic acid; propionic acid; acetic acid; caprylic acid |
| Terpene acids | 6-oxo-6-7-dihydrocitronellic acid; geranic acid; citronellic acid |
| Miscellaneous | Dimethyl sulphide; eugenol; methyl eugenol; furan; α-agarofuran; juniper camphor; vetispirans theaspirans; rose furan; epoxy-rose furan |
The other main component of essential oil is phenylpropanoid, a derivative of the aromatic amino acid phenylalanine, synthesized via the shikimic acid pathway. Cinnamic acid and para-hydroxycinnamic acid are the precursors for generation of a variety of phenylpropanoids. Generally, they are present in nonvolatile glycosylated forms, but whenever they are catalysed by enzymatic reactions, the resultant aglyconic moiety produces a characteristic aroma and flavour (Friedrich 1976; Dudareva et al. 2004).
Thus, essential oil is the key component of rose-scented geranium, and its content in geranium ranges from 0.06% to 0.16%. Approximately 20–170 kg/ha of essential oil was produced by the growers in different locations (Rao 2009). On the basis of available reports in the literature, it is confirmed that citronellol, geraniol, linalool, and citronellyl formate are the major phytoconstituents of geranium essential oil, but their ratio is variable with cultivation zone and season (Džamić et al. 2014). Geranium, grown in Tajikistan, showed that 79 compounds acquire 95.1% of the total essential oil. In this essential oil, 37.5% citronellol, 6% geraniol, 3.7% caryophyllene oxide, 3.1% menthone, 3% linalool, 2.7% β-bourbonene, 2.1% iso-menthone, 2.0% geranyl formate, and 3.1% menthone were reported to be present (Sharopov et al. 2014). Citronellol (36.4%), and citronellyl formate (12.1%) were also found as main components of oil isolated from aerial parts of geranium, grown in Isfahan Province and Central Iran (Ghannadi et al. 2012). Essential oil of aerial parts of geranium grown in Dr. Josif Pančić, an institute of medicinal plants, Belgrade, was reported to contain 55 compounds, constituting 99.32% in weight of total oil in which 59.74% and 0.49% comprises oxygenated monoterpenes and monoterpene hydrocarbons, respectively. Citronellol (24.54%), geraniol (15.33%), citronellyl formate (10.66%) and linalool (9.80%) are present predominantly (Džamić et al. 2014).
There are several factors, such as environmental conditions (climate, soil, humidity, fertilizer and seasonal variation), genotypic and physiological conditions of plants and distillation method, which affect the essential oil yield and composition of phytoconstituents in oil (Rao 2009; Sangwan et al. 2001; Verma et al. 2013; Cannon et al. 2013). There is also a clear ageing effect on the composition of oil in geranium, as shown in a report by Rajeswara Rao et al. (1993) that essential oil yield (1.56%) and geraniol content (34.6%) were the highest in the youngest leaf. Essential oil yield not only varies with different cultivars, but even the same cultivar may produce essential oil of altered composition in different seasons. Different cultivars of geranium, such as Bourbon, CIM-Pawan and Kelkar, showed variations in essential oil production from 0.05% to 0.12%, depending on the season of its cultivation (Verma et al. 2014). Oil composition fluctuates with climatic conditions, as citronellol-, nerol-, geraniol- and menthone-rich oil was obtained from plants grown in temperate climates of high-altitude regions; on the other side, isomenthone-, linalool-, citronellyl formate-rich oil was found in plants grown in lower altitudes (Rajeswara Rao et al. 1990).
Apart from climatic conditions, soil conditions also have a significant impact on the essential oil yield in geranium. An experiment was performed on growing geranium plants under water-stress conditions, and it was observed that essential oil yield was indirectly proportional to the duration of interval periods of irrigation (Putievsky et al. 1990). The nearby vegetation in cultivation area also affects oil yield and composition, as reported earlier that presence and absence of weeds affect the ratio of phytoconstituents in oil. However, some experiments showed that growing another crop at a particular distance from geranium crop does not affect the oil composition up to a significant level, and hence, income can be doubled from the same land area (Rajeswara Rao and Bhattacharya 1997; Singh et al. 2013). The phytochemical composition of rose-scented geranium oil is presented in Table 12.1.
Biosynthesis of Essential Oil
There are two types of phytoconstituents present in rose-scented geranium, that is, terpene (major) and phenylpropanoids (minor). Terpenes are synthesized through the terpenoid/isoprenoid pathway and phenylpropanoids through the shikimate pathway.
Terpene Biosynthetic Pathway
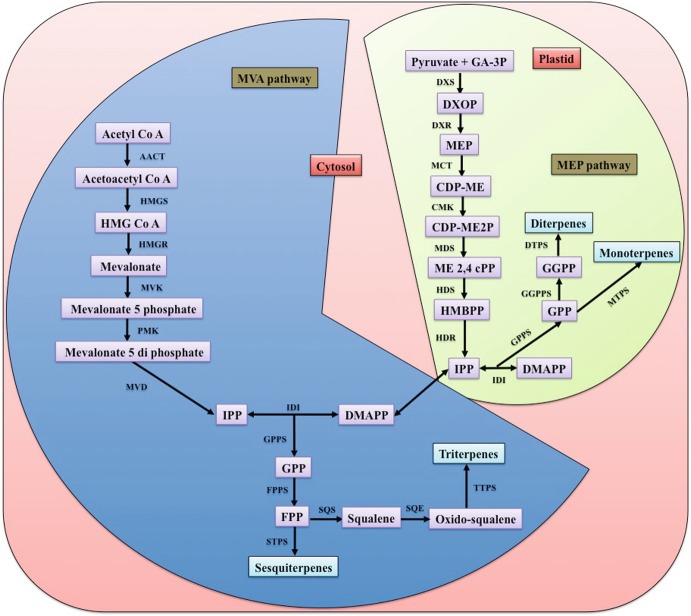
Terpenes are synthesized by the participation of two pathways, one is the cytosolic mevalonate (MVA) or classical acetate pathway, and another is the plastidial non-mevalonate or 2-C-methyl-D-erythritol 4-phosphate (MEP) or glyceraldehyde phosphate/pyruvate or 1-deoxy-D-xylulose 5-phosphate (DXP) pathway (Fig. 12.2). There are several evidences to prove that the MVA pathway is operated in cytosol, and endoplasmic reticulum plays a major role in the biosynthesis of sesquiterpenes (C15), triterpenes (C30), and polyterpenes, while the MEP pathway is dedicated for synthesis of isoprenes (C5), monoterpenes (C10), diterpenes (C20), and tetraterpenes (C40). The names of the pathways indicate the name of their first products such as mevalonic acid and 1-deoxy-D-xylulose 5-phosphate in the MVA and MEP pathways, respectively. Isopentenyl pyrophosphate (IPP), and dimethylallyl diphosphate (DMAPP), generated from both the pathways, function as precursors for manufacturing a plethora of terpenes. Terpenes are biosynthesized as a result of several enzymatic reactions catalyzed by terpene synthase enzymes. For many years, MVA was thought to be solely responsible for the synthesis of terpenes, but radioactive tracer experiments revealed the discovery of the MEP pathway for terpene biosynthesis (Lichtenthaler 1999; Eisenreich et al. 2004). Although both pathways contribute to the biosynthesis of geranium essential oil, the MEP pathway is predominantly involved, as evident by the higher concentration of monoterpenes than sesquiterpenes in geranium oil (Jadaun et al. 2017).
Fig. 12.2.
The terpene biosynthetic pathway in rose-scented geranium. AACT acetoacetyl-CoA thiolase/acetyl-CoA acetyltransferase, HMGS hydroxymethylglutaryl-CoA synthase, HMGR hydroxymethylglutaryl-CoA reductase, MVK mevalonate kinase, PMK phosphomevalonate kinase, MVD mevalonate diphosphate decarboxylase, DXS 1-deoxy-D-xylulose 5-phosphate synthase, DXR 1-deoxy-D-xylulose 5-phosphate reductoisomerase, MCT 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase, CMK 4-(cytidine 5′-diphospho)-2-C-methyl-D-erythritol kinase, MDS 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase, HDS (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase, HDR (E)-4-hydroxy-3-methylbut 2-enyl diphosphate reductase, IDI isopentenyl-diphosphate delta isomerase, GPPS geranyl diphosphate synthase, FPPS farnesyl pyrophosphate synthase, GGPPS geranylgeranyl diphosphate synthase, MTPS: monoterpene synthase, STPS sesquiterpene synthase, DTPS diterpene synthase, HMG CoA hydroxymethylglutaryl-CoA, IPP isopentenyl pyrophosphate, DMAPP dimethylallyl pyrophosphate GA-3P: glyceraldehyde 3-phosphate, DXOP 1-deoxy-D-xylulose-5-phosphate, MEP 2-C-methyl-d-erythritol-phosphate, CDP-ME 4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol, CDP-ME2P 2-phospho 4-(cytidine 5′-diphospho)2-c-methyl-d-erythritol, ME 2,4 cPP C-methyl-D-erythritol 2,4-cyclodiphosphate, HMBPP 1-hydroxy-2-methyl-2-butenyl 4-diphosphate, GPP geranyl pyrophosphate, FPP farnesyl pyrophosphate, GGPP geranylgeranyl pyrophosphate, MVA mevalonic acid
The MVA pathway starts with condensation reaction of two molecules of acetyl CoA, which produces acetoacetyl CoA that further undergoes condensation reaction with another acetyl CoA, and forms 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) as the product. This HMG-CoA is reduced into mevalonic acid (MVA) by the action of the enzyme HMG-CoA reductase (HMGR). Mevalonic acid kinase (MVK) and phosphomevalonate kinase (PMK) further convert MVA into mevalonate diphosphate through the phosphorylation process. Mevalonate-5-diphosphate decarboxylase (MPD) catalyses an ATP-coupled decarboxylation reaction for the production of isopentenyl diphosphate (IPP). IPP can be converted into dimethylallyl diphosphate (DMAPP) by an enzyme, IPP/DMAPP isomerase (IDI) (Fig. 12.2). Recently, a modified MVA pathway was proposed in which mevalonate-5-phosphate underwent decarboxylation forming isopentenylphosphate, which can be transformed into isopentenyl diphosphate (IPP) by the enzyme isopentenyl phosphate kinase (IPK) (Chen and Poulter 2010; Hayakawa et al. 2017).
D-Glyceraldehyde 3-phosphate and pyruvate are the precursor molecules in the MEP pathway. They condense together and form the first intermediate 1-deoxy-D-xylulose 5-phosphate (DXP). In the next step, DXP is reductively isomerized by reducto-isomerase (DXR/IspC) into MEP and subsequently couples with cytidine 5′-triphosphate (CTP), generating methyl erythritol cytidyl diphosphate (CDP-ME) by CDP-ME synthetase (IspD). Then, CDP-ME is phosphorylated to produce 4-diphosphocytidyl-2-C methyl-D-erythritol-2-phosphate (CDP-MEP). An ATP-dependent enzyme IspE catalyses this reaction. At the next level, cyclization of CDP-MEP is performed by IspF, which leads to the generation of 2-C-methyl-D erythritol-2,4- cyclodiphosphate (MEcPP). Further, IspG catalysed transformation of MEcPP into 4-hydroxy-3-methylbutenyl 1-diphosphate (HMBPP). The final step of this pathway is performed by the IspH protein, which generates IPP and DMAPP.
For the synthesis of diverse terpenes, isoprene units are joined together in a head-to-tail pattern. Geranyl pyrophosphate (GPP), a monoterpene precursor molecule, is synthesized by the action of the geranyl pyrophosphate synthase (GPPS) enzyme. Further, addition of one more isoprene unit with GPP by farnesyl pyrophosphate synthase (FPPS) leads to generation of a sesquiterpene precursor, farnesyl pyrophosphate (FPP). Head-to-tail condensation of FPP with IPP produces geranylgeranyl pyrophosphate (GGPP), a diterpene. In the next stage, addition of one more IPP with GGPP forms C25 compounds known as sesterterpene. Interestingly, condensation of farnesyl pyrophosphate (FPP) is used for the synthesis of the C30 triterpene compound (squalene), and in a similar fashion, tail-to-tail condensation of geranylgeranyl pyrophosphate (GGPP) results in the synthesis of C40 molecules, tetraterpenes (Narnoliya et al. 2017, 2018b).
The arrangement of carbon molecules in the chain takes place according to the types of terpenes produced. The most common pattern is cyclization of terpenes, which takes place through generation of an intermediate carbenium ion. For example, heterolysis of the carbon oxygen bond of geranyl pyrophosphate produces geranyl carbocation, and when this carbocation reacts with water, it produces geraniol and subsequently its oxidation leads to synthesis of citral. Granyl carbocation undergoes intramolecular electrophilic addition reaction to generate monocyclic carbocation, which produces limonene after a proton elimination reaction. Further, many other different kinds of intra- and intermolecular interactions are required for the production of diversified terpene molecules (Narnoliya et al. 2017, 2018b).
Phenylpropanoid Biosynthesis
Phenylpropanoids are the aromatic compounds which are synthesized by the shikimate acid pathway, and aromatic amino acids phenylalanine and tyrosine are the precursors of this pathway. The shikimic acid pathway starts with the joining of D-erythrose 4-phosphate with phosphoenol pyruvic acid, and shikimic acid is produced as an intermediate, followed by the generation of chorismate. This chorismate is further utilized in the generation of phenylalanine, which is converted into cinnamic acid, and further, it leads to the generation of multiple types of phenylpropanoids.
Pharmacological Properties of Geranium
Pelargonium contains a number of pharmacological properties. However, there are very limited reports available on the pharmacological properties of rose-scented geranium. Herein, we discuss the pharmacological properties related to Pelargonium sp. including rose-scented geranium.
Antibacterial Properties
Geranium essential oil possesses significant antibacterial properties. Bigos et al. (2012) stated that geranium oil possesses a compelling antibacterial property against clinical isolates of Staphylococcus aureus strain ATCC 433000, which contains multidrug-resistant capacity. Due to its antibacterial effect and almost zero toxicity, geranium oil can be used in food processing. During quiche filling, addition of geranium essential oil at different concentrations (250 ppm, 500 ppm and 1000 ppm) showed significant antibacterial property (Lis-Balchin et al. 1998). Essential oil can be applied in combination with other oils or with standard drugs, and results of such combinatorial experiments exhibit unexpectedly higher inhibitory rates. For example, when citricidal™ and geranium oil were applied together, this combination showed a highly inhibitory influence against MRSA (methicillin-resistant S. aureus) and geranium oil methicillin-sensitive S. aureus (Edwards-Jones et al. 2004). Besides, S. aureus is also effective against other gram-positive strains like Bacillus cereus, and Bacillus subtilis (Silva and Fernandes 2010). Carmen and Hancu (2014) also found that geranium essential oil is able to inhibit the growth of gram-negative bacteria (E. coli, Pseudomonas aeruginosa, and Proteus mirabilis) as well as gram-positive bacteria (S. aureus, and Enterococcus faecalis). Surprisingly, geranium oil, either in free form or in capsulated form, acts as an inhibitory factor against Mycobacterium sp., the most targeted pathogen of current drug professionals. Geranium oil is effective against M. abscessus, M. massiliense, M. smegmatis, and M. avium, even at minimal inhibitory concentrations (MICs) [17.9–35.9 μg/ml] (Giongo et al. 2015). Along with Mycobacterium sp., geranium oil has potent antimicrobial activities against other screened pathogenic bacteria such as S. aureus, Streptococcus, Staphylococcus, Listeria monocytogenes, Pseudomonas aeruginosa, and Salmonella enteritidis (Giongo et al. 2015).
Antifungal Properties
In addition to the antimicrobial properties against many gram-positive and gram-negative bacteria, geranium oil is also found to be effective against Candida albicans and Cryptococcus neoformans fungi, causing severe diseases in humans. Further, the effect of the individual component of geranium oil was estimated, and results suggested that citronellol exhibits the most effective fungicidal property, followed by geraniol, isomenthone, geranyl formate and citronellyl formate (Rath et al. 2005). In another study, geranium oil also exhibited significant antifungal property against C. albicans (Carmen and Hancu 2014). When essential oil was supplemented in nanocapsule, it exhibited significant inhibitory effects on the growth of more than one Candida species, C. albicans, C. glabrata, C. parapsilosis, C. krusei, C. geochares, C. magnoliae, C. kefyr, C. guilliermondii, C. catenulata, C. membranefaciens, C. lusitaniae, and C. dubliniensis (Giongo et al. 2015).
Antifungal properties of Geranium herbarium was tested against Saprolegnia parasitica, an oomycete pathogen which causes diseases in freshwater fishes, affecting the fish market. In a study, rainbow trout (Oncorhynchus mykiss) eggs were infected with S. parasitica and then treated with geranium oil at different concentrations (1 ppm, 5 ppm, 10 ppm, 25 ppm, 50 ppm, and 100 ppm). The geranium oil concentration of 100 ppm was noted as minimum inhibitory concentration (MIC) that makes a significant difference from the control (untreated) sample (Khosravi et al. 2012). The essential oil harvested from Pelargonium graveolens exhibited remarkable antifungal properties against Rhizoctonia solani, a plant pathogenic fungus, and Malassezia, a fungus causing skin diseases in animals (Bouzenna and Krichen 2013; Naeini et al. 2011).
Anti-inflammatory and Antioxidant Properties
Essential oil of geranium displayed significant anti-inflammatory properties against mice in which ear oedema was induced by croton oil. Almost 73–88% reduction was obtained at doses of 5 and 10 ml of oil/ear, respectively. Inhibition of inflammation was also confirmed by histological analysis (Nadjib Boukhatem et al. 2013). According to Džamić et al., geranium oil exhibited potent antioxidant properties, and it successfully reduced 2,2-diphenyl-1-picrylhydrazyl (DDPH) radicals in a dose-dependent manner (Džamić et al. 2014).
Insecticidal Properties
The insecticidal/antifeedant property of geranium oil was well known for the past many years against various types of insects (Lis 1996). L-Quisqualic acid (C5H7N3O5), an excitatory amino acid, isolated from the petals of Pelargonium x hortorum, displayed a paralytic effect on Japanese beetles (Range et al. 2011). Essential oil of geranium also exhibits insecticidal properties against the insect Rhyzopertha dominica at a dose of 50 μl/petri dish, 8.5 cm in diameter (Bouzenna and Krichen 2013).
Anti-neuroinflammatory Properties
Neurodegenerative disorders like Alzheimer’s disease are the consequences of neuroinflammation and neural cell death, which are caused by the activation of microglial cells, leading to the production of pro-inflammatory factors like nitric oxide (NO). Application of geranium oil showed an inhibitory effect on NO production with a reduced expression of cyclooxygenase-2 (COX-2) and nitric oxide synthase (iNOS) enzymes. Interestingly, when individual components were used in the experiment, they were unable to show significant reduction in inflammation; therefore, the inhibitory effect of essential oil is a kind of synergistic effect (Elmann et al. 2010).
Immunomodulatory and Cytoprotective Properties
A drug, EPs® 7630, was formulated using the root extract of Pelargonium sidoides, and in Germany, this drug is approved for the treatment of bronchitis. This drug has immunomodulatory and cytoprotective effects, showing an inhibitory effect on the interaction of bacteria with its host cell. Simultaneously, it stimulates the respiratory cells by increasing the ciliary beat frequency of these cells (Moyo and Van Staden 2014). This drug was also tested against various viruses related to respiratory infections, and interestingly, this drug showed inhibitory effects against many tested viruses such as influenza A virus strains (H1N1, H3N2), parainfluenza virus, coxsackie virus and human coronavirus. However, it was found to be ineffective against adenovirus, pathogenic avian influenza A virus (H5N1) or rhinovirus (Michaelis et al. 2011). The cumulative effect of antibacterial and antiviral properties of this drug makes it an efficient drug against respiratory infections. There are a limited number of anti-HIV 1 therapies; therefore, the development of drugs against human immunodeficiency virus (HIV) is a task of global concern. In this direction, the use of aqueous extract of Pelargonium sidoides root was also examined against HIV 1, and this extract showed significant anti-HIV 1 activity. The mode of action of this extract is different from previously cited mechanisms as this extract poses an inhibitory impact on the attachment of HIV 1 to host cells, and this happens due to action of its phenolic components (Helfer et al. 2014).
Genomic Analysis of Geranium
Taxonomic position of geranium placed Pelargonium sp. in the Geraniaceae family , and almost all the cultivars grown in different parts of the world are the interspecific hybrids of P. capitatum (L.) L’Herit and P. graveolens L’Herit or P. capitatum (L.) L’Herit and P. radens H.E. Moore. Generally these cultivars are diploids with x = 11 and 2n = 77. These cultivars are sterile so only vegetative cutting is the only way of its propagation (Demarne and Van der Walt 1993). In India, the popular cultivars of geranium are Bourbon, Algerian, and Kelkar.
Nowadays, transcriptome and genomics approaches are largely used to map metabolite pathways in organisms of interest. Transcriptomic data from several medicinal and aromatic plants were available, which revealed genomic information about various valuable metabolic pathway enzymes in Withania somnifera, Centella asiatica, Azadirachta indica, Ocimum sp., etc. (Gupta et al. 2013; Sangwan et al. 2013; Krishnan et al. 2012; Narnoliya et al. 2014; Rastogi et al. 2014). Although transcriptomic data are available from the Geraniaceae family, such as Geranium maderense, Pelargonium x hortorum (Zhang et al. 2013), etc., the essential oil pathway (terpenoid pathway) was deeply explored in a transcriptomic study on rose-scented geranium (Narnoliya et al. 2017). In this analysis, a total of 78,943 unique transcripts were reported, out of which 51,802 contigs showed homology-based functional annotation. Further, putative gene(s) representing terpene, ascorbic acid, tartaric acid and anacardic acid (2-hydroxy-6-alkylbenzoic acid), biosynthetic pathways, hormone metabolism and transcription factors were identified. Transcriptomic study also helped in investigating 6040 simple sequence repeats (SSRs) in rose-scented geranium. The genes encoding DXS, 1-deoxy-d-xylulose reductoisomerase (DXR) and 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) were successfully cloned, and their expression analysis was conducted in rose-scented geranium. Further, DXS gene was overexpressed homologously (rose-scented geranium) as well as heterologously (Withania somnifera), resulting in enhanced production of the essential oil (Jadaun et al. 2017).
Tartaric acid, a high-value food ingredient, is biosynthesized through the catabolism of ascorbic acid via two routes, C2/C3 (via threonic acid) and C4/C5 (via iodic acid) (Debolt et al. 2007; Loews 1999). Geranium is also able to produce a significant quantity of tartaric acid, but the plant species specific preference for the alternative cleavage pathway was unclear. Some reports suggest that geranium follows the C2/C3 cleavage route, but there are insufficient evidences to prove the absence of C4/C5 route. In our recent transcriptome analysis, a putative gene encoding the key regulator of the C4/C5 cleavage reaction, L-idonate-5-dehydrogenase, was annotated with a notable expression level, stipulating the possibilities of occurrence in both the C2/C3 and C4/C5 route for tartaric acid biosynthesis (Narnoliya et al. 2017).
Economic Status of Geranium Essential Oil
Rose-scented geranium is one of the most important commercial plants of the perfumery industry. Geranium is cultivated throughout the world for its good-quality essential oil with roselike fragrance. The demand for essential oil is flourishing rapidly due to the increased awareness of its beneficial effects. It is not only useful to the perfumery and cosmetic related activities, but also used in the food and beverage industries because of its application as food additive without any known side effects. In the global market, currently, Europe accounts as the major producer of essential oil, but Asia-Pacific is also emerging as a promising continental leader in the near future (Dhananjay et al. 2010). The estimated demand of geranium oil is around 250 tons per year. The United States, France, Germany, United Kingdom, Japan and other European countries represent a good market for geranium oil. The main producers and exporters of geranium oil are China, Egypt, Algeria, and Morocco. On the differences of the origin of geranium cultivars, they are majorly divided into three main categories—Reunion Island, Egyptian or North African and Chinese. In Reunion Island-type cultivar, the ratio of citronellol and geraniol is almost 1:1, and the other main components are isomenthone, citronellyl formate, and guaia-6,9-diene. Although Egyptian-type oil also contains a 1:1 ratio of citronellol and geraniol, their prominent components are citronellyl formate, isomenthone, and 10-epi-eudesmol. Chinese-type oil is dominated by citronellol and citronellyl formate with low amounts of geraniol. In grading of quality, Reunion type is followed by Egyptian, and then Chinese cultivars. The price of geranium oil ranges from $55 kg−1 to $110 kg−1, although it depends on oil quality, origin country, and market demand, but still, the price is quite high.
Tissue Culture Study of Geranium
The primary mode of propagation in geranium is cutting, and it rarely occurs through seeds. To maintain the cutting of geranium, a proper cultivation area is required, and due to seasonal variations, in some countries like India, specific growth chambers for protecting the plants against unfavourable conditions are also required. The number of seeds produced from some of the genotypes of geranium is low, and their viability is also very less; therefore, plant regeneration through seeds is not advisable. The cost of geranium seeds is approximately US$120/1000 seeds; therefore, propagation through seeds is very expensive. Another drawback of seed propagation in geranium is that for some time, seed-regenerated plants lack specific horticultural attributes like semi-double florets, and non-shattering features (Harney 1982).
Another reason for low cultivation rates in geranium is susceptibility to many diseases, such as bacterial blight (Xanthomonas campestris pv. pelargonii, and Ralstonia solanacearum), Verticillium wilt, Botrytis blight (Botrytis cinerea), root rot (Pythium spp.), bacterial fasciation (Rhodococcus fascians), rust (Puccinia pelargonii-zonalis), Pelargonium flower break virus (PFBV), etc. (Bi et al. 1999; Nameth et al. 1999; Swanson et al. 2005). So there is a need to develop suitable technologies for achieving a variety of geranium with good agricultural traits like large leaves with high essential oil yield and disease resistance. The tissue culture technology can be applied for maintenance, and improvement of higher-yielding varieties of geranium. The tissue culture techniques can facilitate maintenance and genetic engineering of the desired character in plants, as per the growing demand of geranium in perfumery, pharmaceutical, and food industries. So, here we present a detailed study of tissue culture techniques used in the refinement of phenotypic, and genotypic constitutions of geranium.
Meristem Tip Culture
In 1952, Morel and Martin for the first time introduced the meristem tip culture technique in plants during the practice of production of virus-free Dahlia plants. Afterwards, this technology was used for many ornamental and vegetative plants for production of disease-free plants, whether they were viral, bacterial or fungal diseases (Van Zaayen et al. 1992; Smith 2013; Mohapatra and Batra 2017). Geranium is prone to several pathogens, and the most prominent are Xanthomonas campestris pv. pelargonii, Verticillium dahliae, and Botrytis cinerea. It is necessary to maintain disease-free stocks of cultivars for achieving good market prices (Dunbar 1990). Even chemical treatment is not so fruitful to keep plant infection free because mostly these infections are systemic in nature. Therefore, meristem culture is suggested as a useful technology for production of disease-free plants.
For meristem culture, shoot tips (0.1–0.5 mm) are used as explants for regeneration of plantlets. The plants grown from that meristem explant are further screened for obtaining pathogen-free plants, and presence of pathogen is tested at different stages of growth, and it can be done by keeping the regenerating plantlet under conditions favourable for growth of that pathogen. Modern techniques are more sensitive for testing pathogen contamination in plants. These techniques are enzyme-linked immunosorbent assay (ELISA), colorimetric assays, and polymerase chain reactions (PCR). These techniques are able to detect very low levels of infection. Several protocols have been standardized for production of disease-free geranium plants (Debergh and Maene 1977; Reuther 1982; Mithila et al. 2001).
Virus-infected leaves of Pelargonium zonale showed chlorotic rings, and flecks as disease symptoms. Meristem tip culture is a promising method for the production of virus-free plants. Meristem tips are grown on basal media with various combinations of plant growth hormones, and the meristem responds differently in different media. For example, meristem growth in basal media supplemented with α-naphthalene acetic acid (NAA) and coconut milk results only in callus formation, while supplementation of basal medium with low quantities of auxin, indole acetic acid and kinetin results in plantlet formation. Plants regenerated from virus-free meristem as explants are established as stocks of virus-free mother plants for future usage (Hakkaart and Hartel 1979). Xanthomonas pelargonii-free P. x domesticum is produced by this method (Cassells et al. 1987). Due to some drawbacks, such as low survival rate of explants in initial stages and low numbers of shoot production, this technique has limited applications at the industrial level (Desilets et al. 1993).
In Vitro Organogenesis
Mass propagation of geranium can be achieved by direct or indirect organogenesis. There are several procedures reported to produce plantlets from a variety of explants. Details of these methods, explants, and media composition are described here.
Shoot Tip as Explants
Hybrid geranium is largely a male sterile, so cutting or micropropagation is the only way for its propagation. Earlier, Hamdorf (1976) mentioned the experimental details of the regeneration protocol using shoot tips as explants, which are applicable for many varieties of Pelargonium hybrids (P. zonale, P. peltatum, P. zonalex, P. peltatum, P. grandiflorum). By using shoot tip as an explant, shoots were induced by growing the explants on the Murashige and Skoog (MS) medium containing 2.0 mg l−1 of zeatin and 1.9 mg l−1 of indoleacetic acid (IAA) (Dunbar and Stephens 1989). Desilets et al. (1993) also reported rapid multiplication of the shoot of geranium (Pelargonium x hortorum), where axillary meristem was grown on optimized MS media supplemented with 0.11 pM of l-naphthaleneacetic acid (NAA) and 0.89 pM of 6-benzyladenine (BA). Within one month, 40% of explants responded, giving rise to full shoots, and almost a 90% survival rate was observed during the acclimatization process.
Leaves and Petioles as Explants
Different hormonal combinations and acclimatization conditions were optimized when leaves or foliar segments were used as explants. The combination of 1.3 mg l−1 of benzyl amino purine (BAP) and 0.5 mg l−1 of NAA was found as the most efficient combination for direct regeneration of plantlets, and during acclimatization, the substrate containing coconut powder, Biosafra® (12 g l−1), limestone (1 g l−1) and vermiculite (1:1) was supplemented with MS salts (Arrigoni-Blank et al. 2011). Multiple shoots were obtained from mature leaf explants of Pelargonium rapaceum (L.) L’ Hérit. The best response of explants was observed in the media having a hormonal combination of 0.1 mg l−1 of NAA and 0.1 mg l−1 of BAP (Sukhumpinij et al. 2010). Similarly, using the tissue culture technique, micropropagation of Pelargonium sidoides DC was accomplished successfully, and regenerated plants were hardened in a glasshouse (Theisen and Muller 2012; Moyo et al. 2013). Haploid and diploid cultivars of P. zonale var ver ‘Kleiner Liebling’ plants showed differential patterns in morphological and histological characters during regeneration from stem, leaf and petiole explants. Variations were also seen in response to growth hormones, callusing period and regeneration efficiency (Tuleja et al. 2014).
Cotyledons, Hypocotyls and Root as explants
Different parts of a seedling of Pelargonium x hortorum Bailey were tested as explants to obtain the best material having significant responding efficiency (Chang et al. 1996). The effects of different factors such as seedling age, growth hormone and excision orientation were studied for regeneration efficiency. Among the tested combination, IAA + zeatin-treated cotyledon explants showed the highest rate of regeneration. Substantial shoots were regenerated when the explant was isolated from the basal regions of cotyledons of young (2–4 day old) seedlings. The maximum shoot regeneration was observed when hypocotyls were regenerated on IAA + zeatin or thidiazuron-supplemented media, while root explant regeneration was suggested on zeatin-supplemented media. Croke and Cassells (1997) achieved adventitious shoot regeneration from hypocotyl explants of P. x hortorum. The caulogenic potential of the root of Pelargonium x hortorum FI hybrids was determined by growing root explants on MS media supplemented with tri-iodobenzoic acid (TIBA) and thidiazuron (TDZ), and significant shoot regeneration was obtained (Doyle et al. 1999).
Regeneration Using Mature Seeds
Qureshi and Saxena (1992) reported the production of adventitious shoots and somatic embryos directly by culturing the mature seeds of hybrid geranium (Pelargonium x hortorum Bailey) on MS media supplemented with different plant growth regulators (BAP, BAP + IAA, and thidiazuron).
Somaclonal Variations: A Novel Source for Crop Improvement
Generally, plants regenerated from tissue culture practices are identical to source plants in morphology and genetic constitution, and these plants are termed somaclones because they are generated from the same group of somatic cells. Sometimes, during the tissue culture process, genetic variations are generated in somaclones which distinguish them from the original plant in morphology as well as in other features also; such kinds of variations in somaclones are termed somaclonal variations. These variations are the sources of new traits which may be helpful for crop improvement, and if these variations are genetically stable for many generations, then these lines can be incorporated in plant breeding programmes (Krishna et al. 2016).
In geranium, many authors reported the occurrence of somaclonal variations, which are different in morphology as well as in phytochemical compositions (Dunbar and Stephens 1989; Gupta et al. 2001; Gupta et al. 2002; Kulkarni et al. 2012; Ravindra et al. 2004; Ravindra and Kulkarni 2015). During the screening of calliclones of geranium, Saxena et al. (2008a) observed two morphotypes that differed in the dentation patterns of leaves: one morphotype was with high dentated leaves (HDLs), and another was with low dentated round leaves (LDLs). HDL and LDL were different in many agronomically important traits, such as flowering time, plant height, canopy size and the number of branches and, the most important one, essential oil composition. After greenhouse trials these morphotypes were grown and observed for genetic stability (Saxena et al. 2008a, b). Two isomenthone-rich (64.4% and 67.7%) somaclones were obtained during the propagation practice of Pelargonium sp. (Kulkarni et al. 1998). Although, these variations in tissue culture may be spontaneous, sometimes, they can be incorporated through some agents like mutagens. In geranium, explants of ‘Bourbon’ and ‘Narmada’ cultivars were treated with N-nitroso-N-methylurea (NMU) to obtain somaclonal variations showing better oil yields (Kulkarni et al. 2014). By using somaclonal variation strategies, disease-resistance callus culture of Pelargonium graveolens cv. Hemanti was obtained, which further led to the generation of plants resistant against Alternaria alternata (Saxena et al. 2008a, b).
Somatic Embryogenesis
Somatic embryogenesis is an alternative process of generation of embryo without fusion of gametes. In this process, the somatic cell or group of somatic cell is developed into an embryo or plants after passing through specific embryological stages without undergoing callus formation (Finer 1995). Somatic embryogenesis helps in raising identical plants as parental with fewer chances of variations. Slimmon et al. (1991) optimized somatic embryogenesis in geranium (Pelargonium x hortorum Bailey cv. Scarlet Orbit Improved) and they reported that along with indole acetic acid, other non-indolic compounds like phenylacetic acid (PAA) were capable of inducing somatic embryogenesis (Slimmon et al. 1991). For production of somatic embryo of zonal geranium, petioles and hypocotyls and, for regal geranium, petioles were used as explants (Marsolais et al. 1991). TDZ plays an important role in somatic embryogenesis of geranium, and it was further proved by its application on different explants such as intact seedlings, etiolated hypocotyls and cotyledons (Qureshi and Saxena 1992; Visser et al. 1992; Hutchinson et al. 1997; Haensch 2004).
Croke and Cassells (1997) reported the formation of putative somatic embryos from Pelargonium x hortorum Baily using petioles as explants in TDZ-supplemented MS media. Different factors affecting somatic embryogenesis were also studied such as hormonal doses, pH, basal media composition and genotype. In the case of Pelargonium × hederifolium ‘Bonete’, when petioles were used as explants, adventitious shoot formation occurred through a mixed role of organogenesis and somatic embryogenesis mode of regeneration. Presence of TDZ along with IBA during the induction phase enhances the rate of formation of adventitious structures, and subsequently, improvement was observed in development of shoots (Wojtania et al. 2004). Somatic embryogenesis was also induced in Pelargonium sidoides DC, using somatic cells of inflorescence, shoots and petioles in the presence of TDZ (Duchow et al. 2015). Presence of a symbiotic bacterium positively influenced the somatic embryogenesis phenomenon in P. x hortorum (cv. Ringo Rose) (Visser-Tenyenhuis et al. 1994). Later, identification of this bacterium revealed that it has homology with Bacillus circulans, and inoculation of this bacterium with explants enhances the regeneration process in Pelargonium x hortorum (Murthy et al. 1999).
Genetic Transformation Studies
Genetic transformation technology is a successful tool for improvement of a crop, and till date there are many successful stories in front of us. In geranium, several efforts were put in to achieve model cultivars with desired attributes. The first report of geranium transformation was presented by Pellegrineschi et al. (1994); they infected the stem and leaves with Agrobacterium rhizogenes, and the plants that regenerated from these transformed roots exhibited some major changes that differed from the original plant, such as higher leaf and branch number, altered root morphology, reduced height and increased concentration of geraniol and other aromatic components. In 1995, Robichon and his colleagues first showed the Agrobacterium tumefaciens (strain EHA101, binary plasmid pKHG3)-mediated transformation of Pelargonium x hortorum using cotyledons and hypocotyls as explants (Robichon et al. 1995). All the regenerated plants contained normal morphology and all were fertile. In another study, different strains of Agrobacterium tumefaciens (strain LBA4404 and strain LBG66) were used for raising stable transgenic lines of geranium (KrishnaRaj et al. 1997).
Further, an improved method of Agrobacterium tumefaciens (strain LBA4404 and binary plasmid pLN54)-mediated genetic transformation of regal pelargonium (Pelargonium x domesticum Dubonnet) was established (Boase et al. 1998). In this study, they optimized different factors responsible for enhancement of transformation efficiency such as nature and source of explants, type and concentration of phytohormone and, most importantly, addition of a phenolic substance acetosyringone (100 μM) in the pre-culture and co-cultivation period. Transgenic geranium (Pelargonium sp. ‘Frensham’) plants were raised through Agrobacterium-mediated genetic transformation, and the binary vector had a gene encoding an antimicrobial protein, Ace-AMP1. The transgenic plants showed resistance against Botrytis cinerea, a pathogen causing leaf infection (Bi et al. 1999). Transgenic plants of Pelargonium x hortorum and P. capitatum were generated using a modified transformation protocol with two strains of Agrobacterium tumefaciens, EHA101 and LBA440 (Hassanein et al. 2005). Many more attempts were made to improve the agronomic traits of geranium such as improvement in oil quality (Saxena et al. 2007). Winkelmann et al. (2005) reported an efficient regeneration system and genetic transformation protocol for Pelargonium zonale and Pelargonium peltatum hybrids. Agrobacterium rhizogenes-mediated genetic transformation of Pelargonium sidoides was established for enhancing the content of essential metabolites (Colling et al. 2010). Recently, Singh et al. (2017) showed a modified Agrobacterium tumefaciens (LBA4404)-mediated transformation protocol for generation of transgenic plants of Pelargonium graveolens (cv. CIM-BIO171).
Future Aspects
Geranium is an ornamental and medicinally important crop. Traditionally, it is used for treatment of several diseases due to its unique pharmacological properties. Significant work has been undertaken for improvements in its agronomic traits; however, the functional characterization of genes related to its primary as well as secondary metabolite biosynthesis pathways, especially essential oil biosynthesis pathway, is required at a more vigorous pace. Further, their regulatory elements should be identified and isolated so that the pathway machinery could be manipulated in a desired direction. The protocols for in vitro regeneration and plant transformation were well optimized, which are helpful in incorporation of favourable traits in geranium crop. However, the limitation in geranium is that it has so many species and cultivars, which differ at a high level in their morphology and ploidy level, so any technique optimized for one cultivar may or may not work for another cultivar. Therefore, extensive research is required to enhance the market value of a wide range of cultivars. The industrial importance of geranium depends on the quality of essential oil, so efforts are needed to produce such varieties, which can sustain challenges in the marketing route. Therefore, there is a need to produce improved genotypes, either through classical breeding or through genetic engineering approaches, so that plants with improved essential oil profiling and yield, which exhibit resistance to cold seasons and drought, good aroma, large flower size, altered flower colour and disease-resistant properties can be developed. Interspecific hybridization for the pyramiding of desirable genes can be beneficial in obtaining high oil-yielding varieties. Recent biotechnological and synthetic biology avenues have remarkable potential to further improve geranium for its societal importance.
Acknowledgements
The authors acknowledge the Department of Biotechnology (DBT), Government of India. LKN and JSJ acknowledge Science and Engineering Research Board (SERB) N-PDF fellowships, PDF/2015/662 and PDF/2016/445, respectively.
Contributor Information
Sonia Malik, Phone: +555511981021169, Email: 777soniamalik@gmail.com.
Sudhir P. Singh, Email: sudhirsingh@ciab.res.in
References
- Arrigoni-Blank MD, Almeida SA, Oliveira AC, Blank AF. Micropropagation and acclimatization of geranium (Pelargonium graveolens L.) Revista Brasileira de Plantas Medicinais. 2011;13(3):271–275. doi: 10.1590/S1516-05722011000300004. [DOI] [Google Scholar]
- Babu KG, Kaul VK. Variation in essential oil composition of rose scented geranium (Pelargonium sp.) distilled by different distillation techniques. Flavour Fragr J. 2005;20(2):222–231. doi: 10.1002/ffj.1414. [DOI] [Google Scholar]
- Bi YM, Cammue BP, Goodwin PH, KrishnaRaj S, Saxena PK. Resistance to Botrytis cinerea in scented geranium transformed with a gene encoding the antimicrobial protein Ace-AMP1. Plant Cell Rep. 1999;18(10):835–840. doi: 10.1007/s002990050670. [DOI] [Google Scholar]
- Bigos M, Wasiela M, Kalemba D, Sienkiewicz M. Antimicrobial activity of geranium oil against clinical strains of Staphylococcus aureus. Molecules. 2012;17(9):10276–10291. doi: 10.3390/molecules170910276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boase MR, Bradley JM, Borst NK. An improved method for transformation of regal pelargonium (Pelargonium X domesticum Dubonnet) by Agrobacterium tumefaciens. Plant Sci. 1998;139(1):59–69. doi: 10.1016/S0168-9452(98)00177-0. [DOI] [Google Scholar]
- Boukhris M, Nasri-Ayachi MB, Mezghani I, Bouaziz M, Boukhris M, Sayadi S. Trichomes morphology, structure and essential oils of Pelargonium graveolens L’Hér.(Geraniaceae) Ind Crop Prod. 2013;50:604–610. doi: 10.1016/j.indcrop.2013.08.029. [DOI] [Google Scholar]
- Bouzenna H, Krichen L. Pelargonium graveolens L’Her. and Artemisia arborescens L. essential oils: chemical composition, antifungal activity against Rhizoctonia solani and insecticidal activity against Rhyzopertha dominica. Nat Prod Res. 2013;27(9):841–846. doi: 10.1080/14786419.2012.711325. [DOI] [PubMed] [Google Scholar]
- Cannon JB, Cantrell CL, Astatkie T, Zheljazkov VD. Modification of yield and composition of essential oils by distillation time. Ind Crop Prod. 2013;41:214–220. doi: 10.1016/j.indcrop.2012.04.021. [DOI] [Google Scholar]
- Carmen G, Hancu G. Antimicrobial and antifungal activity of Pelargonium roseum essential oils. Adv Pharm Bull. 2014;4(2):511. doi: 10.5681/apb.2014.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassells AC, Carney BF, McCarthy E, McHugh A, Harmey MA. Problems posed by cultivable bacterial endophytes in the establishment of axenic cultures of Pelargonium x domesticum the use of Xanthomonas pelargonii specific ELISA, DNA probes and culture indexing in the screening of antibiotic treated and untreated donor plants. Bacterial and bacteria-like contaminants of plant tissue. Cultures. 1987;225:153–162. [Google Scholar]
- Chang C, Moll BA, Evenson KB, Guiltinan MJ. In vitro plantlet regeneration from cotyledon, hypocotyl and root explants of hybrid seed geranium. Plant Cell Tissue Organ Cult. 1996;45(1):61–66. doi: 10.1007/BF00043429. [DOI] [Google Scholar]
- Chen M, Poulter CD. Characterization of thermophilic archaeal isopentenyl phosphate kinases. Biochemistry. 2010;49:207–217. doi: 10.1021/bi9017957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colling J, Groenewald JH, Makunga NP. Genetic alterations for increased coumarin production lead to metabolic changes in the medicinally important Pelargonium sidoides DC (Geraniaceae) Metab Eng. 2010;12(6):561–572. doi: 10.1016/j.ymben.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Croke JT, Cassells AC. Dark induction and genetic stability of somatic embryos of zonal geraniums (Pelargonium x hortorum Baily) Angew Bot. 1997;71(3–4):119–124. [Google Scholar]
- Debergh P, Maene L. Rapid clonal propagation of pathogen-free Pelargonium plants starting from shoot tips and apical meristems. Acta Hortic. 1977;78:449–454. doi: 10.17660/ActaHortic.1977.78.56. [DOI] [Google Scholar]
- Debolt S, Melino V, Ford CM. Ascorbate as a biosynthetic precursor in plants. Ann Bot. 2007;99:3–8. doi: 10.1093/aob/mcl236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarne FE, Van der Walt JJ. Composition of the essential oil of Pelargonium citronellum (Geraniaceae) J Essent Oil Res. 1993;5(3):233–238. doi: 10.1080/10412905.1993.9698214. [DOI] [Google Scholar]
- Desilets H, Desjardins Y, Bélanger RR. Clonal propagation of Pelargonium x hortorum through tissue culture: effects of salt dilution and growth regulator concentration. Can J Nurs Res. 1993;73(3):871–878. [Google Scholar]
- Dhananjay S, Atul K, Mishra BN. Essential oil: economic and herbal importance in aromatherapy. Int J Plant Sci. 2010;5(2):431–435. [Google Scholar]
- Doyle BM, Lawton DK, Cassells AC. Adventitious regeneration in root culture of a selection of Pelargonium x hortorum cultivars: an assessment of caulogenic potential and genetic stability. Acta Hortic. 1999;530:225–230. [Google Scholar]
- Duchow S, Blaschek W, Classen B. Reproduction of the medicinal plant Pelargonium sidoides via somatic embryogenesis. Planta Med. 2015;81(12/13):1169–1174. doi: 10.1055/s-0035-1545947. [DOI] [PubMed] [Google Scholar]
- Dudareva N, Pichersky E, Gershenzon J. Biochemistry of plant volatiles. Plant Physiol. 2004;135:1893–1902. doi: 10.1104/pp.104.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar KB (1990) Geranium tissue culture for the development of bacterial blight resistance. Ph.D. Thesis, Michigan State University
- Dunbar KB, Stephens CT. Shoot regeneration of hybrid seed geranium (Pelargonium x hortorum) and regal geranium (Pelargonium x domesticum) from primary callus cultures. Plant Cell Tissue Organ Cult. 1989;19(1):13–21. doi: 10.1007/BF00037772. [DOI] [Google Scholar]
- Džamić AM, Soković MD, Ristić MS, Grujić SM, Mileski KS, Marin PD. Chemical composition, antifungal and antioxidant activity of Pelargonium graveolens essential oil. J Appl Pharm Sci. 2014;4(03):001–005. [Google Scholar]
- Edwards-Jones V, Buck R, Shawcross SG, Dawson MM, Dunn K. The effect of essential oils on methicillin-resistant Staphylococcus aureus using a dressing model. Burns. 2004;30(8):772–777. doi: 10.1016/j.burns.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Eisenreich W, Bacher A, Arigoni D, Rohdich F. Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell Mol Life Sci. 2004;61(12):1401–1426. doi: 10.1007/s00018-004-3381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmann A, Mordechay S, Rindner M, Ravid U. Anti-neuroinflammatory effects of geranium oil in microglial cells. J Funct Foods. 2010;2(1):17–22. doi: 10.1016/j.jff.2009.12.001. [DOI] [Google Scholar]
- Finer JJ. Plant cell tissue organ cult. Berlin, Heidelberg: Springer; 1995. Direct somatic embryogenesis; pp. 91–102. [Google Scholar]
- Friedrich H. Phenylpropanoid constituents of essential oils. Lloydia. 1976;39(1):1–7. [PubMed] [Google Scholar]
- Ghannadi A, Bagherinejad MR, Abedi D, Jalali M, Absalan B, Sadeghi N. Antibacterial activity and composition of essential oils from Pelargonium graveolens L’Her and Vitex agnus-castus L. Iran J Microbiol. 2012;4(4):171. [PMC free article] [PubMed] [Google Scholar]
- Giongo JL, Vaucher R, Borin DI, Correa MS, Dos Santos VB, Santos RC, Boligon AA, Athayde ML, Bonez PC, Rossi GG, De Campus MM. Antimycobacterial, antimicrobial and antifungal activities of geranium oil loaded nanocapsules. Int J Pharm Sci. 2015;7:414–419. [Google Scholar]
- Gupta P, Goel R, Pathak S, Srivastava A, Singh SP, Sangwan RS, Asif MH, Trivedi PK. De novo assembly, functional annotation and comparative analysis of Withania somnifera leaf and root transcriptomes to identify putative genes involved in the withanolides biosynthesis. PLoS One. 2013;8(5):e62714. doi: 10.1371/journal.pone.0062714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Banerjee S, Mallavarapu GR, Sharma S, Khanuja SP, Shasany AK, Kumar S. Development of a superior somaclone of rose-scented geranium and a protocol for inducing variants. Hort Sci. 2002;37(4):632–636. [Google Scholar]
- Gupta R, Mallavarapu GR, Banerjee S, Kumar S. Characteristics of an isomenthone-rich somaclonal mutant isolated in a geraniol-rich rose-scented geranium accession of Pelargonium graveolens. Flavour Fragr J. 2001;16(5):319–324. doi: 10.1002/ffj.1002. [DOI] [Google Scholar]
- Haensch KT. Morpho-histological study of somatic embryo-like structures in hypocotyl cultures of Pelargonium× hortorum bailey. Plant Cell Rep. 2004;22(6):376–381. doi: 10.1007/s00299-003-0726-2. [DOI] [PubMed] [Google Scholar]
- Hakkaart FA, Hartel G. Virus eradication from some Pelargonium zonale cultivars by meristem-tip culture. Eur J Plant Pathol. 1979;85(2):39–46. [Google Scholar]
- Hamdorf G. Propagation of Pelargonium varieties by stem-tip culture. Acta Hortic. 1976;59:143–152. doi: 10.17660/ActaHortic.1976.59.20. [DOI] [Google Scholar]
- Harney PM. Application of plant cell and tissue culture to agriculture and industry. Ontario: University of Guelph; 1982. Tissue culture propagation of some herbaceous horticultural plants; pp. 187–208. [Google Scholar]
- Hassanein A, Chevreau E, Dorion N. Highly efficient transformation of zonal (Pelargonium x hortorum) and scented (P. capitatum) geraniums via Agrobacterium tumefaciens using leaf discs. Plant Sci. 2005;169(3):532–541. doi: 10.1016/j.plantsci.2005.04.014. [DOI] [Google Scholar]
- Hayakawa H, Sobue F, Motoyama K, Yoshimura T, Hemmi H. Identification of enzymes involved in the mevalonate pathway of Flavobacterium johnsoniae. Biochem Biophys Res Commun. 2017;487(3):702–708. doi: 10.1016/j.bbrc.2017.04.120. [DOI] [PubMed] [Google Scholar]
- Helfer M, Koppensteiner H, Schneider M, Rebensburg S, Forcisi S, Müller C, Schmitt-Kopplin P, Schindler M, Brack-Werner R. The root extract of the medicinal plant Pelargonium sidoides is a potent HIV-1 attachment inhibitor. PLoS One. 2014;9(1):e87487. doi: 10.1371/journal.pone.0087487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MJ, KrishnaRaj S, Saxena PK. Inhibitory effect of GA 3 on the development of thidiazuron-induced somatic embryogenesis in geranium (Pelargonium xhortorum Bailey) hypocotyl cultures. Plant Cell Rep. 1997;16(6):435–438. doi: 10.1007/BF01146789. [DOI] [PubMed] [Google Scholar]
- Jadaun JS, Sangwan NS, Narnoliya LK, Singh N, Bansal S, Mishra B, Sangwan RS. Over-expression of DXS gene enhances terpenoidal secondary metabolite accumulation in rose-scented geranium and Withania somnifera: active involvement of plastid isoprenogenic pathway in their biosynthesis. Physiol Plant. 2017;159(4):381–400. doi: 10.1111/ppl.12507. [DOI] [PubMed] [Google Scholar]
- Khosravi AR, Shokri H, Sharifrohani M, Mousavi HE, Moosavi Z. Evaluation of the antifungal activity of Zataria multiflora, Geranium herbarium, and Eucalyptus camaldolensis essential oils on Saprolegnia parasitica infected rainbow trout (Oncorhynchus mykiss) eggs. Foodborne Pathog Dis. 2012;9(7):674–679. doi: 10.1089/fpd.2011.1086. [DOI] [PubMed] [Google Scholar]
- Krishna H, Alizadeh M, Singh D, Singh U, Chauhan N, Eftekhari M, Sadh RK. Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech. 2016;6(1):54. doi: 10.1007/s13205-016-0389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan NM, Pattnaik S, Jain P, Gaur P, Choudhary R, Vaidyanathan S, Deepak S, Hariharan AK, Krishna PB, Nair J, Varghese L. A draft of the genome and four transcriptomes of a medicinal and pesticidal angiosperm Azadirachta indica. BMC Genomics. 2012;13(1):464. doi: 10.1186/1471-2164-13-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KrishnaRaj S, Bi YM, Saxena PK. Somatic embryogenesis and Agrobacterium-mediated transformation system for scented geraniums (Pelargonium sp. ‘Frensham’) Planta. 1997;201(4):434–440. doi: 10.1007/s004250050086. [DOI] [Google Scholar]
- Kulkarni RN, Mallavarapu GR, Baskaran K, Ramesh S, Kumar S. Composition of the essential oils of two isomenthone-rich variants of geranium (Pelargonium sp.) Flavour Fragr J. 1998;13(6):389–392. doi: 10.1002/(SICI)1099-1026(199811/12)13:6<389::AID-FFJ757>3.0.CO;2-2. [DOI] [Google Scholar]
- Kulkarni SS, Ravindra NS, Srinivas KV, Kulkarni RN. A somaclonal variant of rose-scented geranium (Pelargonium spp.) with moderately high content of isomenthone in its essential oil. Nat Prod Commun. 2012;7(9):1223–1224. [PubMed] [Google Scholar]
- Kulkarni SS, Ravindra NS, Srinivas KV, Kulkarni RN. In vitro chemical mutagenesis in exclusively vegetatively-propagated rose-scented geranium (Pelargonium spp.) J Hortic Sci Biotechnol. 2014;89(2):173–178. doi: 10.1080/14620316.2014.11513065. [DOI] [Google Scholar]
- Lichtenthaler HK. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Biol. 1999;50(1):47–65. doi: 10.1146/annurev.arplant.50.1.47. [DOI] [PubMed] [Google Scholar]
- Lis BM. A Chemotaxonomic reappraisal of the Section Ciconium Pelargonium (Geraniaceae) S Afr J Bot. 1996;62:277–279. doi: 10.1016/S0254-6299(15)30657-8. [DOI] [Google Scholar]
- Lis-Balchin M, Buchbauer G, Hirtenlehner T, Resch M. Antimicrobial activity of Pelargonium essential oils added to a quiche-filling as a model food system. Lett Appl Microbiol. 1998;27(4):207–210. doi: 10.1046/j.1472-765X.1998.t01-1-00423.x. [DOI] [PubMed] [Google Scholar]
- Loews FA. Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochemistry. 1999;52:193–210. doi: 10.1016/S0031-9422(99)00145-4. [DOI] [Google Scholar]
- Marsolais AA, Wilson DP, Tsujita MJ, Senaratna T. Somatic embryogenesis and artificial seed production in Zonal (Pelargonium× hortorum) and Regal (Pelargonium× domesticum) geranium. Can J Bot. 1991;69(6):1188–1193. doi: 10.1139/b91-152. [DOI] [Google Scholar]
- Michaelis M, Doerr HW, Cinatl J. Investigation of the influence of EPs® 7630, a herbal drug preparation from Pelargonium sidoides, on replication of a broad panel of respiratory viruses. Phytomedicine. 2011;18(5):384–386. doi: 10.1016/j.phymed.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithila J, Murch SJ, KrishnaRaj S, Saxena PK. Recent advances in Pelargonium in vitro regeneration systems. Plant Cell Tissue Organ Cult. 2001;67(1):1–9. doi: 10.1023/A:1011601517200. [DOI] [Google Scholar]
- Mohapatra PP, Batra VK. Tissue culture of potato (Solanum tuberosum L.): a review. Int J Curr Microbiol App Sci. 2017;6(4):489–495. doi: 10.20546/ijcmas.2017.604.058. [DOI] [Google Scholar]
- Moyo M, Aremu AO, Gruz J, Šubrtová M, Szüčová L, Doležal K, Van Staden J. Conservation strategy for Pelargonium sidoides DC: phenolic profile and pharmacological activity of acclimatized plants derived from tissue culture. J Ethnopharmacol. 2013;149(2):557–561. doi: 10.1016/j.jep.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Moyo M, Van Staden J. Medicinal properties and conservation of Pelargonium sidoides DC. J Ethnopharmacol. 2014;152(2):243–255. doi: 10.1016/j.jep.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy BNS, Vettakkorumakankav N, KrishnaRaj S, Odumeru J, Saxena PK. Characterization of somatic embryogenesis in Pelargonium x hortorum mediated by a bacterium. Plant Cell Rep. 1999;18:607–613. doi: 10.1007/s002990050630. [DOI] [Google Scholar]
- Nadjib Boukhatem M, Kameli A, Amine Ferhat M, Saidi F, Mekarnia M. Rose geranium essential oil as a source of new and safe anti-inflammatory drugs. Libyan J Med. 2013;8(1):22520. doi: 10.3402/ljm.v8i0.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeini AR, Nazeri M, Shokri H. Antifungal activity of Zataria multiflora, Pelargonium graveolens and Cuminum cyminum essential oils towards three species of Malassezia isolated from patients with pityriasis versicolor. J Mycol Med. 2011;21(2):87–91. doi: 10.1016/j.mycmed.2011.01.004. [DOI] [Google Scholar]
- Nameth ST, Daughtrey ML, Moorman GW, Sulzinski MA. Bacterial blight of geranium: a history of diagnostic challenges. Plant Dis. 1999;83(3):204–212. doi: 10.1094/PDIS.1999.83.3.204. [DOI] [PubMed] [Google Scholar]
- Narnoliya LK, Kaushal G, Singh SP, Sangwan RS. De novo transcriptome analysis of rose-scented geranium provides insights into the metabolic specificity of terpene and tartaric acid biosynthesis. BMC Genomics. 2017;18(1):74. doi: 10.1186/s12864-016-3437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narnoliya LK, Rajakani R, Sangwan NS, Gupta V, Sangwan RS. Comparative transcripts profiling of fruit mesocarp and endocarp relevant to secondary metabolism by suppression subtractive hybridization in Azadirachta indica (neem) Mol Biol Rep. 2014;41(5):3147–3162. doi: 10.1007/s11033-014-3174-x. [DOI] [PubMed] [Google Scholar]
- Narnoliya LK, Sangwan RS, Singh SP. Transcriptome mining and in silico structural and functional analysis of ascorbic acid and tartaric acid biosynthesis pathway enzymes in rose-scanted geranium. Mol Biol Rep. 2018;45(3):315–326. doi: 10.1007/s11033-018-4164-1. [DOI] [PubMed] [Google Scholar]
- Narnoliya LK, Jadaun JS, Singh SP. Recent trends and techniques in plant metabolic engineering. Singapore: Springer Nature; 2018. Synthetic biology advances for enrichment of bioactive molecules inplants; pp. 117–145. [Google Scholar]
- Pellegrineschi A, Damon JP, Valtorta N, Paillard N, Tapfer D. Improvement of ornamental characters and fragrance production in lemon-scented geranium through genetic transformation by Agrobacterium rhizogenes. Nature Biotechnol. 1994;12(1):64–68. doi: 10.1038/nbt0194-64. [DOI] [Google Scholar]
- Putievsky E, Ravid U, Dudai N. The effect of water stress on yield components and essential oil of Pelargonium graveolens L. J Essent Oil Res. 1990;2(3):111–114. doi: 10.1080/10412905.1990.9697839. [DOI] [Google Scholar]
- Qureshi JA, Saxena PK. Adventitious shoot induction and somatic embryogenesis with intact seedlings of several hybrid seed geranium (Pelargonium x hortorum Bailey) varieties. Plant Cell Rep. 1992;11(9):443–448. doi: 10.1007/BF00232687. [DOI] [PubMed] [Google Scholar]
- Rajeswara Rao BR, Bhattacharya AK. Yield and chemical composition of the essential oil of rose-scented geranium (Pelargonium species) grown in the presence and absence of weeds. Flavour Fragr J. 1997;12(3):201–204. doi: 10.1002/(SICI)1099-1026(199705)12:3<201::AID-FFJ635>3.0.CO;2-X. [DOI] [Google Scholar]
- Rajeswara Rao BR, Bhattacharya AK, Kaul PN, Chand S, Ramesh SI. Changes in profiles of essential oils of rose-scented geranium (Pelargonium sp.) during leaf ontogeny. J Essent Oil Res. 1993;5(3):301–304. doi: 10.1080/10412905.1993.9698224. [DOI] [Google Scholar]
- Rajeswara Rao BR, Sastry KP, Prakasa Rao EV, Ramesh SI. Variation in yields and quality of geranium (Pelargonium graveolens L’Hér. ex Aiton) under varied climatic and fertility conditions. J Essent Oil Res. 1990;2(2):73–79. doi: 10.1080/10412905.1990.9697827. [DOI] [Google Scholar]
- Range CM, Rudolph EW, Ajay PS, Michael ER, Jonathan MF, James C, Charles RK. Rare exitary amino acid from flower of zonal geranium responsible for paralyzing the Japanese beetle. PNAS. 2011;108(4):1217–1221. doi: 10.1073/pnas.1013497108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao BR. Chemical composition and uses of Indian rose-scented Geranium (Pelargonium species) essential oil-A review. J Essent Oil Bear Plant. 2009;12(4):381–394. doi: 10.1080/0972060X.2009.10643735. [DOI] [Google Scholar]
- Rastogi S, Meena S, Bhattacharya A, Ghosh S, Shukla RK, Sangwan NS, Lal RK, Gupta MM, Lavania UC, Gupta V, Nagegowda DA. De novo sequencing and comparative analysis of holy and sweet basil transcriptomes. BMC Genomics. 2014;15(1):588. doi: 10.1186/1471-2164-15-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindra NS, Kulkarni RN. Essential oil yield and quality in rose-scented geranium: variation among clones and plant parts. Sci Hort. 2015;184:31–35. doi: 10.1016/j.scienta.2014.12.023. [DOI] [Google Scholar]
- Reuther G. Propagation of disease-free Pelargonium cultivars by tissue culture. Acta Hortic. 1982;131:311–320. [Google Scholar]
- Robichon MP, Renou JP, Jalouzot R. Genetic transformation of Pelargonium X hortorum. Plant Cell Rep. 1995;15(1):63–67. doi: 10.1007/BF01690255. [DOI] [PubMed] [Google Scholar]
- Rath CC, Dash SK, Rao BR. Antifungal activity of rose-scented geranium (Pelargonium species) essential oil and its six constituents. J Essent Oil Bear Plants. 2005;8(2):218–222. doi: 10.1080/0972060X.2005.10643449. [DOI] [Google Scholar]
- Ravindra NS, Kulkarni RN, Gayathri MC, Ramesh S. Somaclonal variation for some morphological traits, herbyield, essential oil content and essential oil composition in an Indian cultivar of rose-scented geranium. Plant Breed. 2004;123(1):84–86. doi: 10.1046/j.1439-0523.2003.00943.x. [DOI] [Google Scholar]
- Sangwan NS, Farooqi AH, Shabih F, Sangwan RS. Regulation of essential oil production in plants. Plant Growth Regul. 2001;34(1):3–21. doi: 10.1023/A:1013386921596. [DOI] [Google Scholar]
- Sangwan RS, Tripathi S, Singh J, Narnoliya LK, Sangwan NS. De novo sequencing and assembly of Centella asiatica leaf transcriptome for mapping of structural, functional and regulatory genes with special reference to secondary metabolism. Gene. 2013;525(1):58–76. doi: 10.1016/j.gene.2013.04.057. [DOI] [PubMed] [Google Scholar]
- Saxena G, Banerjee S, Verma PC, Mallavarapu GR, Kumar S. Rose-scented geranium (Pelargonium sp.) generated by Agrobacterium rhizogenes mediated Ri-insertion for improved essential oil quality. Plant Cell Tissue Organ Cult. 2007;90(2):215–223. doi: 10.1007/s11240-007-9261-0. [DOI] [Google Scholar]
- Saxena G, Verma PC, Banerjee S, Kumar S. Field performance of somaclones of rose scented geranium (Pelargonium graveolens L’Her Ex Ait.) for evaluation of their essential oil yield and composition. Ind Crop Prod. 2008;27(1):86–90. doi: 10.1016/j.indcrop.2007.08.001. [DOI] [Google Scholar]
- Saxena G, Verma PC, Rahman LU, Banerjee S, Shukla RS, Kumar S. Selection of leaf blight-resistant Pelargonium graveolens plants regenerated from callus resistant to a culture filtrate of Alternaria alternata. Crop Prot. 2008;27(3):558–565. doi: 10.1016/j.cropro.2007.08.013. [DOI] [Google Scholar]
- Sharopov FS, Zhang H, Setzer WN. Composition of geranium (Pelargonium graveolens) essential oil from Tajikistan. Am J Essent Oil Nat Prod. 2014;2:13–16. [Google Scholar]
- Silva NCC, Fernandes AJ. Biological properties of medicinal plants: a review of their antimicrobial activity. J Venom Anim Toxins Incl Trop Dis. 2010;16:402–413. doi: 10.1590/S1678-91992010000300006. [DOI] [Google Scholar]
- Singh M, Singh UB, Ram M, Yadav A, Chanotiya CS. Biomass yield, essential oil yield and quality of geranium (Pelargonium graveolens L. Her.) as influenced by intercropping with garlic (Allium sativum L.) under subtropical and temperate climate of India. Ind Crop Prod. 2013;46:234–237. doi: 10.1016/j.indcrop.2013.01.032. [DOI] [Google Scholar]
- Smith RH. Plant tissue culture: techniques and experiments. Amsterdam: Academic Press; 2013. [Google Scholar]
- Sukhumpinij P, Kakihara F, Kato M. In vitro regeneration from mature leaf explants of Pelargonium rapaceum (L.) L’Hérit. Sci Hortic. 2010;126(3):385–389. doi: 10.1016/j.scienta.2010.07.028. [DOI] [Google Scholar]
- Swanson JK, Yao J, Tans-Kersten J, Allen C. Behavior of Ralstonia solanacearum race 3 biovar 2 during latent and active infection of geranium. Phytopathology. 2005;95(2):136–143. doi: 10.1094/PHYTO-95-0136. [DOI] [PubMed] [Google Scholar]
- Singh P, Khan S, Kumar S, ur Rahman L. Establishment of an efficient Agrobacterium-mediated genetic transformation system in Pelargonium graveolens: an important aromatic plant. Plant Cell Tissue Org Cult (PCTOC) 2017;129(1):35–44. doi: 10.1007/s11240-016-1153-8. [DOI] [Google Scholar]
- Slimmon T, Qureshi JA, Saxena PK. Phenylacetic acid-induced somatic embryogenesis in cultured hypocotylexplants of geranium (Pelargonium x hortorum Bailey) Plant Cell Rep. 1991;10(11):587–589. doi: 10.1007/BF00232517. [DOI] [PubMed] [Google Scholar]
- Theisen LL, Muller CP. EPs® 7630 (Umckaloabo®), an extract from Pelargonium sidoides roots, exerts anti-influenza virus activity in vitro and in vivo. Antivir Res. 2012;94(2):147–156. doi: 10.1016/j.antiviral.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Tuleja M, Krupa A, Góralski G, Płachno BJ. Morphological and histological events in the preliminary tissue culture of haploid and diploid Pelargonium zonale var.‘Kleiner Liebling’. Mod Phytomorphol. 2014;6:39–40. [Google Scholar]
- Van Zaayen A, Van Eijk C, Versluijs JM. Production of high quality, healthy ornamental crops through meristem culture. Acta Bot Neerl. 1992;41(4):425–433. doi: 10.1111/j.1438-8677.1992.tb00512.x. [DOI] [Google Scholar]
- Visser C, Qureshi JA, Gill R, Saxena PK. Morphoregulatory role of thidiazuron substitution of auxin and cytokinin requirement for the induction of somatic embryogenesis in geranium hypocotyl cultures. Plant Physiol. 1992;99:1704–1707. doi: 10.1104/pp.99.4.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser-Tenyenhuis C, Murthy BNS, Odumeru J, Saxena PK. Modulation of somatic embryogenesis in hypocotyl-derived cultures of geranium (Pelargonium x hortorum Bailey) cv. Ringo Rose by a bacterium. In Vitro Cell Dev Biol. 1994;30P:140–143. doi: 10.1007/BF02632203. [DOI] [Google Scholar]
- Verma RK, Chauhan A, Verma RS, Rahman LU, Bisht A. Improving production potential and resources use efficiency of peppermint (Mentha piperita L.) intercropped with geranium (Pelargonium graveolens L. Herit ex Ait) under different plant density. Ind Crop Prod. 2013;44:577–582. doi: 10.1016/j.indcrop.2012.09.019. [DOI] [Google Scholar]
- Verma RK, Verma RS, Rahman LU, Yadav A, Patra DD, Kalra A. Utilization of distillation waste–based vermicompost and other organic and inorganic fertilizers on improving production potential in geranium and soil health. Commun Soil Sci Plant Anal. 2014;45(2):141–152. doi: 10.1080/00103624.2013.854803. [DOI] [Google Scholar]
- Winkelmann T, Kaviani K, Serek M. Development of a shoot regeneration protocol for genetic transformation in Pelargonium zonale and Pelargonium peltatum hybrids. Plant Cell Tissue Organ Cult. 2005;80(1):33–42. doi: 10.1007/s11240-004-5788-5. [DOI] [Google Scholar]
- Wojtania A, Gabryszewska E, Marasek A. Regeneration of Pelargonium× hederifolium ‘Bonete’ from petiole explants. Acta Physiol Plant. 2004;26(3):255–262. doi: 10.1007/s11738-004-0015-x. [DOI] [Google Scholar]
- Zhang J, Ruhlman TA, Mower JP, Jansen RK. Comparative analyses of two Geraniaceae transcriptomes using next-generation sequencing. BMC Plant Biol. 2013;13(1):228. doi: 10.1186/1471-2229-13-228. [DOI] [PMC free article] [PubMed] [Google Scholar]