Abstract
There is compelling evidence of a global problem of poor vitamin D status in expecting mothers and postnatal life; and even more critical, is the evidence showing the association of vitamin D deficiency with increased morbidity and mortality risks from respiratory infections. Viral and bacterial pneumonia kills more children than any other illness, accounting for 19 % of all deaths in children less than five years of age worldwide; and under-nutrition, which includes vitamin D insufficiency/deficiency, has been implicated in 53 % of all these deaths. Poor vitamin D status is a result of insufficient sunlight exposure and/or poor dietary intake. Greater understanding of the role of vitamin D deficiency in precipitating lung infections grew from the use of rodent models and observational and intervention studies in infants and toddlers. Vitamin D adequacy is important to maintaining the key protective mechanism of developing lungs since it mediates the synthesis of antimicrobial peptides, the lungs strongest defense against viral and bacterial pathogens. If vitamin D intervention currently under study in several clinical trials is proven successful, then implementation of new fortification practices, revised guidelines for healthy sun exposure and public health programs for vitamin D supplementation of pregnant/lactating women and their infants may be effective strategies to aide in preventing neonates and children under five from developing pneumonia. Globally, there is potential to save more than a million young lives with preventive treatment, a compelling reason why the efficacy of optimizing vitamin D mediated defense against respiratory pathogens in infants and children merits further study.
Keywords: vitamin D deficiency, infants, vitamin D supplementation, wheezing, skeletal health, respiratory infections, breast fed infants
Introduction
Vitamin D is a secosteroid hormone that is synthesized in humans upon solar UVB (290–315 nm) mediated conversion of endogenous skin 7-dehydrocholesterol to cholecalciferol (vitamin D3) (MacLaughlin et al., 1982), and is also derived from food and dietary supplements. During the winter when adequate sunlight is limited for synthesis, there is greater dependency on dietary intake and thus the term ‘vitamin’ meaning essential nutrient was applied to this sunshine-derived hormone. Since vitamin D occurs naturally in a limited number of foods, fortified foods such as milk and milk products, margarines, and breakfast cereals constitute the major dietary sources of the two forms of vitamin D, cholecalciferol or ergocalciferol (vitamin D2) in the US and Canada (Calvo et al., 2004). After absorption or synthesis, vitamin D2 or D3 is transported by vitamin D binding protein to the liver where the enzyme 25-hydroxylase converts it to 25(OH)D. This intermediary metabolite circulates and delivers the precursor to the active or hormonal form of vitamin D to different tissues. In this capacity, plasma 25(OH)D serves as the main status indicator for vitamin D. The mitochondrial enzyme, 1α-hydroxylase converts 25(OH)D to the active form of vitamin D, 1,25(OH)2D (Holick, 2007). Renal tissues are thought to be the major source of circulating levels of 1,25(OH)2D, but most extra-renal tissues including the immune and airway epithelial cells constitutively or upon activation express 1α-hydroxylase and can produce 1,25(OH)2D (Hansdottir and Monick, 2011).
Vitamin D has pleiotropic functions, and is involved in the regulation of approximately 1000 human genes (Tavera-Mendoza and White, 2007), including genes associated with immune responses and lung development. While its classical role in bone mineralization is well documented, the interplay of non-rachitic vitamin D status to in utero and post-natal health outcomes such as susceptibility to early-life respiratory infections continues to evolve. In this chapter, we review the current public health concern about vitamin D status in infants and toddlers in North America, the dietary guidelines and Federal regulations governing the level of vitamin D in foods and supplements during pregnancy and early childhood, and examine the evidence for association between poor vitamin D status and risk of respiratory infections in infants and toddlers.
Vitamin D status and dietary guidelines during pregnancy and early childhood
Plasma 25(OH)D is considered the best biomarker for vitamin D status as it is relatively stable and reflects contributions from all sources of vitamin D (diet and endogenous synthesis). The IOM, an independent non-governmental body, recently defined adequate vitamin D status as having serum 25(OH)D concentrations greater than 50 nmol/l (or 20 ng/ml; 1 ng/ml = 2.5 nmol 25(OH)D/l) in both the general population and pregnant women, and concentrations between 30–49 nmol/l (or 12–20 ng/ml) or <30 nmol/l (or <12 ng/ml) were considered to be inadequate or deficient respectively (IOM, 2011). The IOM cutoff levels defining adequate, insufficient and deficient plasma vitamin D status are specific to bone health and do not consider other systems that are affected by circulating levels of 25(OH)D such as the immune system and respiratory health (IOM, 2011). Although serum 25(OH)D levels of at least 25 nmol/l prevents rickets, it has been proposed by some investigators that concentrations around 80 nmol/l (32 ng/ml) are optimal, since these levels lead to the greatest calcium absorption and the highest bone mass (Bischoff-Ferrari et al., 2006; Dawson-Hughes, 2008) and are associated with better health outcomes involving non-skeletal tissue (Hollis, 2011).
An accurate determination of the overall dietary requirements for maintaining vitamin D adequacy is impossible because of substantial variation in skin synthesis due to season, location, age, duration of exposure, pollution, skin pigmentation and exposed area, and use of sun screen. Consequently, assuming minimal sun exposure and after thorough review of literature on skeletal health, the IOM established a RDA of 600 IU (15 μg; 1 μg = 40 IU vitamin D2 or D3) vitamin D for pregnant and lactating women and toddlers. With insufficient evidence to develop an RDA in infants, an adequate intake of 400 IU (10 μg) was proposed to ensure vitamin D nutritional adequacy in this population. The IOM derives the RDA from an EAR, which was determined for vitamin D for the first time in 2011. From a public health perspective the EAR (400 IU or 10 μg/d for individuals >1 year) is used to evaluate the nutrient intake adequacy of a population at the 50th percentile (median) level of intake for all age groups (Whiting and Calvo, 2011). There appears to be global variation in recommended vitamin D intake guidelines for pregnant women, infants and toddlers (Table 16.1). This variation could be a result of differences due to season, demographics of light versus dark skinned individuals, national supplementation and fortification policies, and more importantly skepticism associated with non-skeletal health benefits from basic science experiments and observational studies as compared to randomized clinical trials.
Table 16.1.
Examples of global recommendations for dietary vitamin D intake (IU/d) (FAO/WHO, 2001; Holick et al., 2011; IOM, 1997, 2011; Vidailhet et al., 2012).
| Year | Country | 3 months1 | 9 months1 | 5 years | Pregnancy2 |
|---|---|---|---|---|---|
| 1991 | United Kingdom | 340 | 280 | −3 | −3 |
| 1996 | Italy | 400 | 700 | 200 | 200 |
| 1997 | IOM (United States/Canada) | 200 | 200 | 200 | 200 |
| 2001 | France | 800-1000 | 800-1000 | 200 | 200 |
| 2001 | World Health Organization (WHO) | 200 | 200 | 200 | 200 |
| 2004 | Nordic countries | 400 | 400 | 300 | 300 |
| 2004 | Germany/Austria/Switzerland | 400 | 400 | 200 | 200 |
| 2005 | Australia and New Zealand | 200 | 200 | 200 | 200 |
| 2011 | IOM (United States/Canada) | 400 | 400 | 600 | 600 |
| 2011 | The Endocrine Society – United States | 400-1000 | 400-1000 | 600-1000 | 1,500-2,000 |
| 2012 | France | 1000-1,200 | 1000-1,200 | 2 doses4 of 80,000- or 100,000-IU in winter – Nov & Feb | 1 dose4 of 80,000- or 100,000-IU @ start of 3rd trimester |
|
1 Breastfed infants. 2 Below 50 yrs of age. 3 400 IU/d in cases of insufficient UVB exposure. 4 Bolus dose. | |||||
Maternal status of vitamin D levels
Maternal vitamin D deficiency during pregnancy is global and widely prevalent, and can be attributed to inadequate vitamin D intake and restricted sunlight exposure during winter months compared to summer months, northern latitudes, and higher melanin levels in dark-skinned or veiled women. Based on a National Human Activity Pattern Survey it has been estimated from the outdoor-sunlight data that US women including those of child-bearing age (22–40 years) get lower annual UVB doses than males because they spend less time outdoors (Godar, 2001; Godar et al., 2001). In the US, 29 % of African-American pregnant women and 5 % of Caucasian pregnant women residing in the northern latitudes are vitamin D deficient (defined as serum 25(OH)D less than 37.5 nmol/l); whereas 54 % of African-American participants and 47 % of Caucasian participants are vitamin D insufficient (defined as serum 25(OH)D 37.5-80 nmol/l) (Bodnar et al., 2007). Moreover, 18 % of pregnant women in the United Kingdom, 25 % in the United Arab Emirates, 80 % in Iran, 42 % in northern India, 61 % in New Zealand and 60-84 % of pregnant non-Western women in the Netherlands have been shown to have serum 25(OH)D concentrations <25 nmol/l (Dawodu and Wagner, 2007). Thus, there is concern that in utero vitamin D deficits could lead to developmental re-programming of body functions and long-term morbidity in infants, children and adults.
Cumulative intake data from the nationally representative NHANES survey conducted from 2007–2008 show that US women of childbearing age have significantly lower (P < 0.01) vitamin D intakes than their age-matched male counterparts (Figure 16.1) (USDA, 2010). A comprehensive assessment of 25(OH)D serum data from participants in NHANES 2001–2006, one year of age and over, after adjusting for age and season, showed that males are less likely to be at risk of deficiency (<30 nmol/l) than females (Figure 16.2) and the prevalence of those at risk of inadequacy (30–49 nmol/l) did not differ by sex. However, in the 2001–2006 NHANES cohort, among women of childbearing age, those who were pregnant or lactating were less likely to be at risk of deficiency compared to women who were not pregnant or lactating (Figure 16.2) and no differences in prevalence of vitamin D inadequacy were observed between the two groups (Looker et al., 2011). These prevalence data reflect 24 hr recall levels of vitamin D intake, which suggests that pregnant and lactating women in general in the US are in compliance with the current dietary guidelines for maintaining vitamin D status or have adequate sun exposure. A rigorous analysis of current NHANES data is needed to determine the association of vitamin D status with sun exposure and total ‘usual’ dietary intake for individual race/ethnicities, since smaller cross-sectional studies have shown association of maternal deficiency, race (African-American), winter birth, and a BMI of ≥35 as risk factors for newborn vitamin D deficiency.
Figure 16.1.
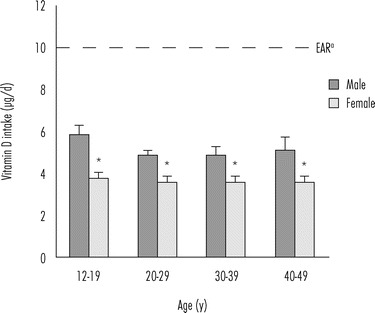
Vitamin D intake in the United States (NHANES, 2007–2008). Mean vitamin D intake (±SE) from food per individual, by gender and age of participants in the 2007–2008 NHANES survey of US population.
a Estimated average requirement (EAR) for vitamin D intake established in 2011 (IOM, 2011). The differences in vitamin D intake between males and females were significant across all age groups (P < 0.01) (USDA, 2010).
Figure 16.2.
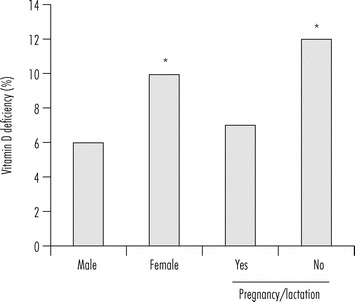
Prevalence of vitamin D deficiency in the United States (NHANES, 2001–2006).
Age- and season-adjusted prevalence of risk for vitamin D deficiency (<30 nmol/l) among men and women, and among women of childbearing age who were pregnant or lactating (Yes) or not (No) among participants of the 2001–2006 NHANES survey. * Indicates significant difference between males and females and between women’s pregnancy/lactation status at P < 0.05. Figure modified from Looker et al. (2011).
Vitamin D status in infants
During pregnancy the fetus is wholly dependent on the mother for vitamin D, and 25(OH)D readily crosses the placenta such that cord blood 25(OH)D levels are between 80 % and 100 % of maternal concentrations (Fleischman et al., 1980; Gertner et al., 1980; Hillman and Haddad, 1974). There is a strong correlation between maternal and newborn 25(OH)D circulating levels as the half-life of 25(OH)D is approximately 2 to 3 weeks, and a low maternal status often results in the infant being deficient in vitamin D during the first weeks post-partum (Hollis and Wagner, 2004a; Yu et al., 2009). Because vitamin D secretion in breast milk is limited (Kovacs, 2008), lactating women require robust serum 25(OH)D levels to support vitamin D status in nursing infants (Hollis and Wagner, 2004b). The World Health Organization recommends supplementation with 400-IU daily vitamin D to all pregnant women; however, because of lack of awareness and poor compliance, 46 % of newborns in industrialized countries are born with insufficient serum levels of 25(OH)D (Belderbos et al., 2011).
More recently Balk and the Council on Environmental Health and Section on Dermatology recommended that children younger than 1-year of age avoid direct sunlight and also use sunscreen (Balk, 2011). Furthermore, for reasons that are not entirely clear, data suggest that the relationship between vitamin D intake and serum 25(OH)D levels is non-linear (Hypponen et al., 2009), and current guideline levels for vitamin D supplementation (200–600 IU/d) are unlikely to achieve optimal serum 25(OH)D levels (IOM, 2011). In a study of 40 mother-infant pairs, 76 % of mothers and 81 % of newborns had a 25(OH)D level below 50 nmol/l at the time of birth, despite the fact that during pregnancy the mothers ingested about 600 IU/d of vitamin D from a prenatal supplement and consumed two glasses of milk (Lee et al., 2007). Thus, it is understandable that infants who are fed only human breast milk are prone to developing vitamin D deficiency leading to a Federal mandate both in the United States and Canada to fortify infant formula with vitamin D at 40–100 IU/kcal and 40–80 IU/kcal, respectively. The effectiveness of infant formula fortification was evident in a cross-sectional study of 247 healthy infants in a primary care setting in Boston as breastfeeding without supplementation markedly increased the odds of vitamin D deficiency compared with infants who were exclusively formula (bottle)-fed (Gordon et al., 2008). Interestingly, although maternal supplementation of 200-IU/d vitamin D was available to all nursing mothers, there may be barriers to obtaining the supplement or lack of awareness of the need to supplement, which resulted in poor compliance. Thus, with emerging data on insufficiency in vitamin D levels with the IOM 1997 recommendation of 200 IU/d, the revised recommendation is now set at 600 IU/d for infants in the United States and Canada (IOM, 2011).
Vitamin D status in toddlers
Most American children may not be going outside enough to meet their vitamin D3 needs from sun exposure (Godar et al., 2012). Recent estimates from everyday outdoor exposure suggest that children (≤5 years) in the northern (45°N) (Figure 16.3a) and southern (35°N) (Figure 16.3b) United States with Fitzpatrick Type II skin (Caucasian) (Fitzpatrick, 1988) can have adequate skin synthesis of vitamin D3 during the summer and children living in the south during the spring as well but only if they do not wear sunscreen at all except during beach vacations. Children with Fitzpatrick Type III and IV skin (olive tone-Hispanic/Asian or brown tone-Indian, respectively) can only synthesize the suggested minimum daily recommendation during the summer months, while those with the darkest pigmentation (skin type V and higher, usually African-Americans) never make the suggested minimum amount of vitamin D3 from sun exposure (Godar et al., 2012). These vitamin D3 estimates from solar exposures assume certain skin types, liberal clothing scenarios during each season and that sunscreens are not worn except during beach vacations. These could be overestimates for infants and toddlers since mothers tend to be overly protective of their children to avoid skin cancer later in life or could be a result of children spending more time indoors watching television. Despite the fact that younger children are spending more time indoor, children in the younger age group (≤5 years) have more cutaneous vitamin D synthesis than those in the older age groups (Godar, 2001).
Figure 16.3.
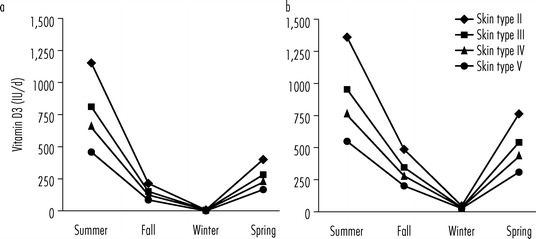
Estimated cutaneous synthesis of vitamin D3 in toddlers according to skin type and season. Average estimated vitamin D3 produced in toddlers (≤5 years of age) from everyday outdoor UV exposures without the use of sunscreens in (a) the northern (45°N) or (b) southern (35°N) United States according to skin type and season. Skin types represent Fitzpatrick skin types II (Caucasian), III (olive skin tone, Hispanic or Asian), IV (brown skin tone, e.g. Indian) and V (light to moderate-skinned African American) (Fitzpatrick, 1988).
With children falling short of the daily needed levels of vitamin D3 from sunshine exposure especially in the winter months, fortified foods are key to maintain adequate vitamin D status in toddlers, and increasingly, the manufacturers of foods targeted to toddlers are recognizing the need to fortify with vitamin D. Levels of vitamin D fortification range from 1 μg (40 IU) per regulatory serving for ready-to-eat cereals to 2.5 μg for fluid milk servings (Calvo and Whiting, 2013). It is evident from reviewing the vitamin D content of the ‘baby foods’ listed in the recent USDA database, Standard Release-24 for nutrient composition of foods that there is a need for newer fortified baby foods. A review of 223 foods showed vitamin D contents ranged from 0 to 0.8 μg vitamin D per 100 g, and approximately 90 % had no listed vitamin D content. With the availability of limited fortified baby foods, in a population study of 750 healthy children aged 6–36 months, the mean overall vitamin D intake from food plus supplements was 290 ± 124 IU/d in 6–12 month age group and 240 ± 128 IU/d in 1–3 years age group (Figure 16.4), and group corresponding serum 25(OH)D levels decreased with age. A quite concerning trend in this cohort was that children discontinued breastfeeding by 5 months of age and most children (84 %) were no longer formula-fed at 5 months (Carpenter et al., 2012). Thus, with the IOM recommended adequate intake of 400 IU/d for infants and 600 IU/d for toddlers approximately 82 % of infants and 2 % of toddlers met the age-specific dietary intake recommendation. These findings suggest that vitamin D-fortified infant formulas provide a positive effect on vitamin D levels and similar supplementary practices are likely to be effective in increasing 25(OH)D status in young children (Carpenter et al., 2012). The mean intake data for toddlers (2–5 years age) from the NHANES 2007–2008 cohort shows a significant difference in vitamin D intake among the racial and ethnic groups with Non-Hispanic blacks consuming less than Non-Hispanic whites, who consume less than Mexican Americans (Figure 16.5) (USDA, 2010). Correspondingly, the prevalence of vitamin D insufficiency and deficiency in children (1–5 years) during the 2001–2004 NHANES was highest among Non-Hispanic blacks followed by Mexican Americans and Non-Hispanic whites (Figure 16.6) (Kumar et al., 2009).
Figure 16.4.
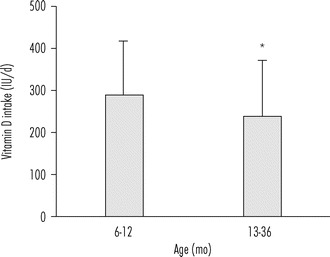
Effect of lack of vitamin D-fortified foods on the daily dietary intake in a cohort of healthy infants and toddlers. Vitamin D daily dietary intake (±SD) in 6–12 months (n = 131) and 13–36 months old (n = 645) healthy children. Significant age differences in vitamin D intake, P < 0.001. Figure drawn from Carpenter et al. (2012).
Figure 16.5.

Vitamin D intake among toddlers in the United States, NHANES, 2007–2008. Mean intake (±SE) of vitamin D (D2 + D3) from NHANES 2007–2008 for toddlers 2–5 years, presented by race and ethnic group. a EAR for vitamin D intake established in 2011 (IOM, 2011). NH: Non-Hispanic. * Indicates significant difference in vitamin D intake between NH Black and Mexican Americans, P < 0.01. Figure drawn with data from USDA (2010).
Figure 16.6.

Prevalence of vitamin D deficiency among toddlers in the United States, NHANES, 2001–2004. Prevalence of vitamin D insufficiency (defined as serum 25(OH)D levels 37–72.5 nmol/l) and deficiency (defined as serum 25(OH)D levels <37 nmol/l) among toddlers and children (1–6 years of age) in NHANES 2001–2004. NH: Non-Hispanic. a Values differ from IOM (2011). Figure redrawn from Kumar et al. (2009).
There is clear evidence for global and widespread vitamin D deficiency or insufficiency due to various risk factors including lack of sunlight exposure, seasonal variation, suboptimal dietary intake, and dark skin pigmentation. More importantly, we have shown the greatest barriers to adequate vitamin D from sun exposure or diet occur in individuals with the poorest vitamin D status. How this widespread deficiency relates to the risk of respiratory tract infections in infants and toddlers is less clear and merits further study.
Fetal and postnatal outcomes associated with vitamin D status
The fetal origins hypothesis, first articulated by David Barker, postulates that in utero epigenetic fetal programming, as a result of environmental events during pregnancy, induces specific genes and genomic pathways that control fetal development and subsequent disease risk (Barker et al., 2002). The biological effects of active vitamin D are achieved through the regulation of gene expression in a cell and tissue specific manner. Briefly, active vitamin D binds to the vitamin D nuclear receptor, and initiates dimerization with the retinoic X receptor. This active complex binds to the nuclear vitamin D responsive elements within the promoter regions of vitamin D-specific responsive genes and initiates gene expression (MacDonald et al., 1993). The developmental periods of in utero and infancy represent critical periods of dynamic development and maturation of key processes. Therefore, in the following sections, we focus on the role of vitamin D in modulating lung structure and innate immune functions, a primer for susceptibility to viral respiratory tract infections during infancy and early childhood (Figure 16.7).
Figure 16.7.
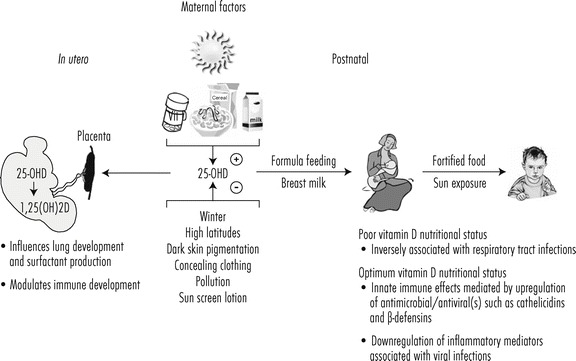
Role of nutrition and sunshine in maintaining vitamin D adequacy and reducing incidence of respiratory tract infections in postnatal life.
Lung development
Lung development occurs predominantly before birth (Stick, 2000) with extensive interactions between epithelial and mesenchymal tissue beginning by the fourth week of gestation and continuing for years after birth, and factors that impair fetal and early childhood lung development have the potential to exert major effects on lung function during childhood and adulthood. Despite differences in the epithelial growth and differentiation during lung development in rodents and humans, rodent models are widely used for lung developmental studies. Rodent models act as a bridge between studies in the laboratory and studies in humans, and have been used to study pulmonary infections (Balan et al., 2011). In rodents, it has been demonstrated that vitamin D modulates key alveolar epithelial-mesenchymal interactions, such as type II pneumocyte and lipofibroblast proliferation and differentiation which are critical for alveolar development and septal thinning during perinatal pulmonary maturation (Sakurai et al., 2009). Vitamin D-deficient rodent models provide mechanistic evidence for a causal link between vitamin D deficiency and deficits in lung function and altered lung structure. Specifically, offspring of vitamin D-deficient mice showed no effect on the overall somatic growth, had marginal reduced numbers of alveoli, and exhibited physiologically significant decreases in lung volume and altered lung mechanics when compared with offspring of vitamin D-replete mice (Zosky et al., 2011). This mouse study complements data showing decreased lung compliance in 50-d-old rats born to vitamin D-deficient mothers (Gaultier et al., 1984), confirming the relationship between vitamin D deficiency and lung function. In addition, as vitamin D deficiency has the potential for premature births in humans (Dawodu and Nath, 2011), studies have shown that prematurity leads to diminished lung function and may predispose premature infants to severe viral lower respiratory tract infections in infancy (Drysdale et al., 2011).
Active vitamin D synthesis has been shown in rodent fetal lung fibroblasts, whereas adjacent alveolar type II pneumocytes express vitamin D receptors and respond to the hormone by undergoing differentiation and maturation resulting in decreased cell glycogen content, and increased surfactant synthesis and secretion (Nguyen et al., 1996, 2004). Surfactant proteins were initially identified as a lipoprotein complex that reduced surface tension at the air-liquid interface of the lung, but recent studies have identified surfactant proteins as components of the lung innate and adaptive immune system with novel roles in the direct killing of inhaled microorganisms and viruses, and control of pulmonary inflammation (Wright, 2005). The vitamin D-mediated rodent type II pneumocyte maturation data looks intriguing but the relevance to humans is uncertain. Regulation of surfactant protein gene expression in human fetal type II pneumocytes by active vitamin D is not coordinated as it is in rodents, but vitamin D receptor immuno-staining is observed in human fetal fibroblasts and type II pneumocytes (Phokela et al., 2005; Stio et al., 1997).
Thus, there is evidence for in utero vitamin D deficiency and association with altered epithelial-mesenchymal maturation, lung mechanics, and immunoregulatory surfactant production. While the association between surfactants in providing innate immunity against respiratory viral infections is conceivable, the role of altered pulmonary function with vitamin D deficiency could also indicate an association with asthma, a chronic inflammatory condition that is described in detail in a separate chapter.
Vitamin D and innate immune protection
The fetus and neonate face a complex set of immunologic demands, and vitamin D has been shown to have an important role in the innate immune system, which helps to prevent infection without the need for immunologic memory from previous exposure to the pathogen (Adams and Hewison, 2008). Innate immunity includes the production of antimicrobial peptides such as Beta-defensins and CAMP by epithelial cells and circulating leukocytes, which are capable of killing a variety of respiratory pathogens including viruses, bacteria, and fungi (Hiemstra, 2007). In early gestation, human decidual cells have been shown to synthesize active vitamin D, which may exert autocrine or paracrine effects on the developing fetal immune system. Vitamin D mediates expression of mRNA for CAMP in decidual cells (Evans et al., 2006), and has been linked to intrauterine immunity (Singh et al., 2005). Human monocytes in an in vitro model, when supplemented with 25(OH)D-deficient cord blood plasma showed a significant decrease in CAMP expression, thus correlating with increased susceptibility to newborn infections (Walker et al., 2011).
In postnatal life, respiratory epithelial cells provide a barrier between the outside environment and internal parenchyma, and are primary targets of respiratory pathogens. The respiratory epithelial cells constitutively activate vitamin D, and are capable of creating a microenvironment that has high levels of active form of the vitamin, resulting in the activation of downstream genes such as those for CAMP. Moreover, viral RNA increases the expression of 1α-hydroxylase, leading to increased activation of vitamin D and further increases in CAMP mRNA (Hansdottir et al., 2008). Hansdottir and colleagues (2010) have shown in an in vitro human tracheo-bronchial epithelial cell model for respiratory syncytial virus infection, that vitamin D attenuates inflammatory cytokine and chemokine response, while maintaining the antiviral activity. This local vitamin D-mediated anti-viral, anti-inflammatory immune response could result in decreased disease severity and morbidity from this common infection (Hansdottir et al., 2010). Thus, vitamin insufficiency and notably, a seasonal decrease of vitamin D-dependent epithelial and leukocyte innate host defense could contribute to increased susceptibility to respiratory infections during winter. A prospective descriptive study of outcomes associated with vitamin D deficiency and pneumonia reported 25(OH)D deficiency associated with increased mortality but not associated with levels of cathelicidin or Beta-defensin (Leow et al., 2011). Clinical trials are underway to assess CAMP expression as a biomarker for fetal-neonatal immune function following antenatal vitamin D supplementation (ClinicalTrials.gov identifier: NCT01126528) and in a separate trial for Intensive Care Unit-associated lung failure, CAMP and Beta-defensin concentrations will be evaluated as a secondary outcome following high-dose vitamin D regimen (ClinicalTrials.gov identifier: NCT01372995).
While we limit our review to the role of innate immune antiviral functions to respiratory infections, vitamin D status has been associated with other prenatal and postnatal immunomodulatory effects, such as promoting peripheral tolerance by rendering antigen presenting dendritic cells tolerogenic and development of T-regulatory cells. Dendritic and T-regulatory cells serve as pivotal links between innate and adaptive immunity, and play a key role in the protection against the inflammatory sequela of airway infections and in the protection against induction and expression of atopic disease (Chambers and Hawrylowicz, 2011; Holt et al., 2008).
Evidence for vitamin D status and risk of respiratory infections & childhood wheezing
There is an association of wintertime peaks in respiratory infections especially in the higher latitudes to vitamin D status as the cutaneous synthesis of vitamin D is naturally blunted during that time of the year (Cannell et al., 2006). Respiratory tract infections in the neonatal and pediatric populations are normally viral in origin with accompanying wheezing, pneumonia or bronchiolitis. The infections can be classified into URTIs and LRTIs with the URTI a primary site of contact for inhaled agents, and LRTIs as infections of the intra-thoracic airways and/or lung parenchyma with severe cases leading to bronchiolitis and pneumonia. The main etiological agents include human rhinovirus, RSV, human coronavirus, adenovirus, parainfluenza virus and influenza virus. Cases of bronchiolitis associated with human rhinovirus (46-49 %), RSV (11-27 %), and parainfluenza viruses (5-13 %) account for most of the outpatient sampling in infants with URTIs and LRTIs (Camargo et al., 2011a). Viral infections including rhinovirus and RSV could lead to bronchiolitis and early episodic wheezing in infants (Gern and Busse, 2002), symptoms which are commonly associated with the likelihood of developing reactive airway disease or asthma in early childhood (Asher et al., 2006; Litonjua, 2012; Wu et al., 2008). Large cohort studies, however have demonstrated that many children who wheeze in early childhood during acute respiratory infections do not go on to develop asthma (Camargo et al., 2011b; Martinez et al., 1995; Stein and Martinez, 2004). In addition, accumulating evidence implicates a background of atopy as a leading cause of asthma associated with airway inflammation from respiratory infections (Holt et al., 2012). Nevertheless, asthma is a heterogeneous disease and since clinical diagnosis of asthma in children remains a challenge, epidemiological studies of children often focus on childhood wheezing; and in this chapter, we present selected epidemiological studies on vitamin D deficiency in children and associated respiratory infections and wheezing but not related to asthmatic conditions.
Observational prospective serum 25(OH)D birth cohort studies
Circulating 25(OH)D level provides a distinct advantage to assess vitamin D status than self-reported dietary intake, and cord-blood 25(OH)D concentration is strongly associated with maternal concentration during pregnancy. In a birth cohort study of 922 children cord-blood levels of 25(OH)D had inverse associations with the risk of respiratory infection by three months of age (OR: 1.00 for ≥75 nmol/l, 1.39 for 25–74 nmol/l, and 2.16 for <25 nmol/l). Likewise, cord-blood 25(OH)D levels were inversely associated with risk of wheezing by 15 months, 3 years, and 5 years of age (all P < 0.05) and no association to incident asthma by the age of 5 years. Additional adjustment for potential confounders including seasons of birth did not materially change these results (Camargo et al., 2011b). A recent birth cohort study of 156 healthy neonates showed an association of vitamin D deficiency with increased risk of RSV LRTIs in the first year of life. Neonates born with 25(OH)D concentrations <50 nmol/l had a sixfold (95 % CI = 1.6-24.9; P = 0.01) increased risk of RSV LRTI in the first year of life compared with those with 25(OH)D concentrations ≥75 nmol/l (Belderbos et al., 2011). Similarly, in a population based mother-child cohort study there was a trend of independent association between higher levels of maternal circulating 25(OH)D in pregnancy and decreased odds of LRTIs in offspring (for cohort- and season-specific quartile Q4 vs. Q1, OR: 0.67 (95 % CI = 0.50-0.90); test for trend, P = 0.016). No association was found between 25(OH)D levels in pregnancy and risk of wheezing at age 1 year or 4 years, or asthma at age 4–6 years (Morales et al., 2012).
Case–control studies
Nutritional rickets due in part to vitamin D deficiency is a major health problem in developing countries. It is associated with respiratory muscle weakness and increased risk of respiratory infections. The first association of sub-clinical nutritional rickets to respiratory infections was observed in a case–control trial among Indian children (3–12 years) with multiple episodes of respiratory infections. Administration of oral vitamin D, 60,000 IU/wk and 650 mg of calcium/d for 6 weeks decreased the incidence of respiratory infections in the test population (Rehman, 1994). Similarly in a case–control study, 13-fold higher incidence of nutritional rickets was observed among toddlers (<5 years) with pneumonia than among controls (OR = 13.37; P < 0.001) (Muhe et al., 1997). More recently, significant associations have been identified with non-rachitic vitamin D levels and respiratory tract infections suggesting that vitamin D insufficiency is enough to trigger respiratory infection rather than a secondary manifestation of acute deficiency typically leading to nutritional rickets.
In a hospital-based case–control study of non-rachitic Indian children age 2–60 months, serum 25(OH)D levels of <22 nmol/l had more than 10-fold higher odds of acquiring severe acute LRTIs (OR: 0.09; P < 0.001) (Wayse et al., 2004), and in a similar study design in rural Bangladesh, 25(OH)D levels in children (1–18 months) hospitalized with acute lower respiratory infections (ALRIs) were significantly lower (29.1 nmol/l) than case matched controls (39.1 nmol/l). The unadjusted odds ratio of ALRIs was halved for each 10 nmol/l increase in 25(OH)D (OR = 0.53; 95 % CI = 0.3, 0.96) (Roth et al., 2010). Furthermore, in a neonatal case–control study of ALRIs in Turkey, mean serum level of 25(OH)D was 22.7 ± 22.2 nmol/l compared to age-matched control levels, 40.6 ± 33.6 nmol/l (P = 0.011) and corresponding 25(OH)D levels in mothers of the study group were lower than those in the mothers of the control group (33.4 ± 42 nmol/l and 57 ± 42.3 nmol/l respectively; P = 0.012). These findings suggest that newborns with subclinical vitamin D deficiency may have an increased risk of suffering from acute lower respiratory infection and the strong positive correlation between newborns and mothers 25(OH)D concentrations shows that adequate vitamin D supplementation of mothers should be emphasized during pregnancy (Karatekin et al., 2009).
In contrast, two case–control studies in Canadian children did not reveal an association between vitamin D deficiency and respiratory infections as virtually all children consumed vitamin D fortified infant formula or supplements (Roth et al., 2009). While no difference was observed in vitamin D levels between the entire acute lower respiratory infection group and control group, approximately 50 % of the patients admitted to the pediatric intensive care unit were vitamin D deficient (<50 nmol/l) compared to only 20 % on the general medical floor (OR: 8.23; 95 % CI: 1.4, 48.0; P = 0.02) suggesting that low levels of vitamin D predispose to greater acute LRTI severity (McNally et al., 2009).
Interventional trials
Published data from randomized control clinical trials to evaluate the effects of vitamin D on reducing RTIs in children are limited. Despite mixed results because of study design, lack of measurement of 25(OH)D serum levels and poor compliance, four studies have shown potential for vitamin D intervention to help control respiratory tract infections in the adult population (Aloia and Li-Ng, 2007; Avenell et al., 2007; Laaksi et al., 2007; Li-Ng et al., 2009).
A supplementation of vitamin D (1,200 IU/d) for four winter months among 334 schoolchildren in Japan showed a reduction in influenza A (10.8 %) children in the vitamin D group compared with (18.6 %) children in the placebo group [relative risk (RR), 0.58; 95 % CI: 0.34, 0.99; P = 0.04]. In a sub-group analysis significant reductions of influenza A were more prominent in children taking additional vitamin D supplements (RR 0.36; 95 % CI: 0.17-0.78). The study, however, lacked measurements of serum 25(OH)D and serum antibody concentrations to influenza A (Urashima et al., 2010). A current search of the ClinicalTrials.gov database lists the Vitamin D Outcomes and Interventions In Toddlers (DO IT Trial: NCT01419262, Primary Outcome: Respiratory Infection) and Maternal Vitamin D Supplementation to Prevent Childhood Asthma (VDAART Trial: NCT00920621, Secondary Outcome: LRTIs in the first 3 years of life) as ongoing trials to evaluate the effects of vitamin D on reducing RTIs in infants and toddlers. The gestational supplementation of 4,000 IU/d in the VDAART trial is noted as a recent trial on evaluating clinical safety and effectiveness of vitamin D supplementation during pregnancy concluded that supplementation of 4,000 IU/d is safe and most effective in achieving sufficiency in all women and their neonates regardless of race (Hollis et al., 2011).
Summary and conclusion
We have presented what we believe is compelling evidence of a global problem of poor vitamin D status experienced in utero and perinatally; and even more critical, is the evidence showing the association of vitamin D deficiency during this dynamic period of development with increased morbidity and mortality risks from respiratory infections. Worldwide recognition of this problem is growing and this awareness is stimulating changes in dietary guidelines (Table 16.1), as well as advice on safe sun exposure (Godar et al., 2012) and strategies for national fortification policies (Babu and Calvo, 2010). Viral and bacterial pneumonia kills more children than any other illness, accounting for 19 per cent of all under five deaths worldwide (UNICEF, 2006). According to the 2006 UNICEF/WHO report, an estimated 26 % of neonatal deaths are caused by severe infections during the neonatal period, the majority of which are caused by pneumonia/sepsis. Undernutriton, which includes vitamin D insufficiency/deficiency, has been implicated in 53 % of all deaths among children under five (UNICEF, 2006). If vitamin D intervention currently under study in the clinical trials described in this chapter is proven successful, then implementation of new fortification practices, revised guidelines for healthy sun exposure and public health programs for vitamin D supplementation of pregnant/lactating women and their infants may be effective strategies to aid in preventing neonates and children under five from developing respiratory infections. Globally, there is potential to save more than a million young lives with preventive treatment, a compelling reason why the efficacy of optimizing vitamin D-mediated defense against respiratory pathogens in infants and children merits further study.
Disclaimer
The findings and conclusions presented in this review are those of the authors and do not necessarily represent the views, opinions or policies of the US Food and Drug Administration.
Key facts
Vitamin D deficiency is a result of inadequate sun exposure and/or poor dietary intake.
This chapter addresses the global high prevalence of vitamin D deficiency among women of childbearing age and infants and toddlers.
Vitamin D status (nutritional and sun exposure) is assessed by measuring the transport form of vitamin D, 25-hydroxyvitamin D (25(OH)D).
Active hormonal form is synthesized in most tissues from 25(OH)D.
Vitamin D status is associated with immune development and lung function.
Clinical trials are needed to establish the link between vitamin D deficiency and susceptibility to respiratory tract infections.
Summary points
Vitamin D status of mothers and infants is poorest in darker skinned individuals, particularly those living in Northern latitudes.
The poorest vitamin D status occurs in exclusively breast fed infants who are not supplemented with vitamin D. In contrast, formula fed infants have lower prevalence of vitamin D deficiency; however, older toddlers have limited access to vitamin D-rich foods.
Children with Fitzpatrick skin types II to IV, in the northern and southern United States can only achieve a minimum amount of vitamin D from sun exposure during the summer months if they do not wear sunscreen at all except during beach vacations. Dark-skinned (skin types ≥ V), children fall short of achieving their needs for vitamin D from sun exposure year round.
In vivo animal and in vitro models provide evidence that vitamin D deficiency in utero is associated with altered lung development and immune functions, and increasing postnatal susceptibility to respiratory infections.
Adequate vitamin D is critical for the production of anti-microbial peptides such as cathelicidin and Beta-defensins that are key protective mechanisms in preventing respiratory infections in children under five years of age.
Clinical trials are currently underway to determine if vitamin D supplementation reduces respiratory tract infections in infants and toddlers and during gestation in supplemented pregnant or lactating mothers.
References
- Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nature Clinical Practice: Endocrinology & Metabolism. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia J.F., Li-Ng M. Re: epidemic influenza and vitamin D. Epidemiology and Infection. 2007;135:1095–1096. doi: 10.1017/S0950268807008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous . Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. USA: The National Academies Press (US), Washington (DC); 2011. [PubMed] [Google Scholar]
- Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, Williams H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- Avenell A, Cook JA, Maclennan GS, Macpherson GC. Vitamin D supplementation to prevent infections: a sub-study of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438) Age and Ageing. 2007;36:574–577. doi: 10.1093/ageing/afm091. [DOI] [PubMed] [Google Scholar]
- Babu US, Calvo MS. Modern India and the vitamin D dilemma: evidence for the need of a national food fortification program. Molecular Nutrition & Food Research. 2010;54:1134–1147. doi: 10.1002/mnfr.200900480. [DOI] [PubMed] [Google Scholar]
- Balan KV, Kc P, Mayer CA, Wilson CG, Belkadi A, Martin RJ. Intrapulmonary lipopolysaccharide exposure upregulates cytokine expression in the neonatal brainstem. Acta Paediatrica. 2011;101(5):466–471. doi: 10.1111/j.1651-2227.2011.02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk SJ. Ultraviolet radiation: a hazard to children and adolescents. Pediatrics. 2011;127:e791–817. doi: 10.1542/peds.2010-3502. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. International Journal of Epidemiology. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Belderbos ME, Houben ML, Wilbrink B, Lentjes E, Bloemen EM, Kimpen JL, Rovers M, Bont L. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127:e1513–1520. doi: 10.1542/peds.2010-3054. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. American Journal of Clinical Nutrition. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. Journal of Nutrition. 2007;137:447–452. doi: 10.1093/jn/137.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo, M.S. and Whiting, S.J., 2013. Vitamin D fortification in North America: current status and future considerations. In: V. Preedy (ed.), Handbook of food fortification and health: from concepts to public health applications. King’s College London, London, UK (in press).
- Calvo MS, Whiting SJ, Barton CN. Vitamin D fortification in the United States and Canada: current status and data needs. American Journal of Clinical Nutrition. 2004;80:1710S–1716S. doi: 10.1093/ajcn/80.6.1710S. [DOI] [PubMed] [Google Scholar]
- Camargo CA, Jr, Ginde AA, Mansbach JM. Vitamin D, Childhood Wheezing, Asthma, and Chronic Obstructive Pulmonary Disease. In: Feldman D, Pike WJ, Adams JS, editors. Vitamin D. Oxford, UK: Academic Press; 2011. pp. 1999–2021. [Google Scholar]
- Camargo CA, Jr, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, Town GI, Pattemore PK, Espinola JA, Crane J. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127:e180–187. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E. Epidemic influenza and vitamin D. Epidemiology and Infection. 2006;134:1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter TO, Herreros F, Zhang JH, Ellis BK, Simpson C, Torrealba-Fox E, Kim GJ, Savoye M, Held NA, Cole DE. Demographic, dietary, and biochemical determinants of vitamin D status in inner-city children. American Journal of Clinical Nutrition. 2012;95:137–146. doi: 10.3945/ajcn.111.018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers ES, Hawrylowicz CM. The impact of vitamin D on regulatory T cells. Current Allergy and Asthma Reports. 2011;11:29–36. doi: 10.1007/s11882-010-0161-8. [DOI] [PubMed] [Google Scholar]
- Dawodu A, Nath R. High prevalence of moderately severe vitamin D deficiency in preterm infants. Pediatrics International. 2011;53:207–210. doi: 10.1111/j.1442-200X.2010.03209.x. [DOI] [PubMed] [Google Scholar]
- Dawodu A, Wagner CL. Mother-child vitamin D deficiency: an international perspective. Archives of Disease in Childhood. 2007;92:737–740. doi: 10.1136/adc.2007.122689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson-Hughes B. Serum 25-hydroxyvitamin D and functional outcomes in the elderly. American Journal of Clinical Nutrition. 2008;88:537S–540S. doi: 10.1093/ajcn/88.2.537S. [DOI] [PubMed] [Google Scholar]
- Drysdale SB, Wilson T, Alcazar M, Broughton S, Zuckerman M, Smith M, Rafferty GF, Johnston SL, Greenough A. Lung function prior to viral lower respiratory tract infections in prematurely born infants. Thorax. 2011;66:468–473. doi: 10.1136/thx.2010.148023. [DOI] [PubMed] [Google Scholar]
- Evans KN, Nguyen L, Chan J, Innes BA, Bulmer JN, Kilby MD, Hewison M. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biology of Reproduction. 2006;75:816–822. doi: 10.1095/biolreprod.106.054056. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Archives of Dermatology. 1988;124:869–871. doi: 10.1001/archderm.1988.01670060015008. [DOI] [PubMed] [Google Scholar]
- Fleischman AR, Rosen JF, Cole J, Smith CM, Deluca HF. Maternal and fetal serum 1,25-dihydroxyvitamin D levels at term. Journal of Pediatrics. 1980;97:640–642. doi: 10.1016/S0022-3476(80)80030-8. [DOI] [PubMed] [Google Scholar]
- Gaultier C, Harf A, Balmain N, Cuisinier-Gleizes P, Mathieu H. Lung mechanics in rachitic rats. American Review of Respiratory Disease. 1984;130:1108–1110. doi: 10.1164/arrd.1984.130.6.1108. [DOI] [PubMed] [Google Scholar]
- Gern JE, Busse WW. Relationship of viral infections to wheezing illnesses and asthma. Nature Reviews: Immunology. 2002;2:132–138. doi: 10.1038/nri725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertner JM, Glassman MS, Coustan DR, Goodman DB. Fetomaternal vitamin D relationships at term. Journal of Pediatrics. 1980;97:637–640. doi: 10.1016/S0022-3476(80)80029-1. [DOI] [PubMed] [Google Scholar]
- Godar DE. UV doses of American children and adolescents. Photochemistry and Photobiology. 2001;74:787–793. doi: 10.1562/0031-8655(2001)074<0787:UDOACA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Godar DE, Pope SJ, Grant WB, Holick MF. Solar UV doses of young Americans and vitamin D3 production. Environmental Health Perspectives. 2012;120:139–143. doi: 10.1289/ehp.1003195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar DE, Wengraitis SP, Shreffler J, Sliney DH. UV doses of Americans. Photochemistry and Photobiology. 2001;73:621–629. doi: 10.1562/0031-8655(2001)073<0621:UDOA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Gordon CM, Feldman HA, Sinclair L, Williams AL, Kleinman PK, Perez-Rossello J, Cox JE. Prevalence of vitamin D deficiency among healthy infants and toddlers. Archives of Pediatrics & Adolescent Medicine. 2008;162:505–512. doi: 10.1001/archpedi.162.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansdottir S, Monick MM. Vitamin D effects on lung immunity and respiratory diseases. Vitamins and Hormones. 2011;86:217–237. doi: 10.1016/B978-0-12-386960-9.00009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. Journal of Immunology. 2008;181:7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansdottir S, Monick MM, Lovan N, Powers L, Gerke A, Hunninghake GW. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. Journal of Immunology. 2010;184:965–974. doi: 10.4049/jimmunol.0902840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemstra PS. The role of epithelial beta-defensins and cathelicidins in host defense of the lung. Experimental Lung Research. 2007;33:537–542. doi: 10.1080/01902140701756687. [DOI] [PubMed] [Google Scholar]
- Hillman LS, Haddad JG. Human perinatal vitamin D metabolism. I. 25-Hydroxyvitamin D in maternal and cord blood. Journal of Pediatrics. 1974;84:742–749. doi: 10.1016/S0022-3476(74)80024-7. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. New England Journal of Medicine. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Hollis BW. Short-term and long-term consequences and concerns regarding valid assessment of vitamin D deficiency: comparison of recent food supplementation and clinical guidance reports. Current Opinion in Clinical Nutrition and Metabolic Care. 2011;14:598–604. doi: 10.1097/MCO.0b013e32834be798. [DOI] [PubMed] [Google Scholar]
- Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. Journal of Bone and Mineral Research. 2011;26:2341–2357. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. American Journal of Clinical Nutrition. 2004;79:717–726. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. American Journal of Clinical Nutrition. 2004;80:1752S–1758S. doi: 10.1093/ajcn/80.6.1752S. [DOI] [PubMed] [Google Scholar]
- Holt PG, Strickland DH, Sly PD. Virus infection and allergy in the development of asthma: what is the connection? Current Opinion in Allergy and Clinical Immunology. 2012;12:151–157. doi: 10.1097/ACI.0b013e3283520166. [DOI] [PubMed] [Google Scholar]
- Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nature Reviews: Immunology. 2008;8:142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- Hypponen E, Berry DJ, Wjst M, Power C. Serum 25-hydroxyvitamin D and IgE – a significant but nonlinear relationship. Allergy. 2009;64:613–620. doi: 10.1111/j.1398-9995.2008.01865.x. [DOI] [PubMed] [Google Scholar]
- Karatekin G, Kaya A, Salihoglu O, Balci H, Nuhoglu A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. European Journal of Clinical Nutrition. 2009;63:473–477. doi: 10.1038/sj.ejcn.1602960. [DOI] [PubMed] [Google Scholar]
- Kovacs CS. Vitamin D in pregnancy and lactation: maternal, fetal, and neonatal outcomes from human and animal studies. American Journal of Clinical Nutrition. 2008;88:520S–528S. doi: 10.1093/ajcn/88.2.520S. [DOI] [PubMed] [Google Scholar]
- Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics. 2009;124:e362–370. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamaki H, Ylikomi T. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. American Journal of Clinical Nutrition. 2007;86:714–717. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clinical Pediatrics. 2007;46:42–44. doi: 10.1177/0009922806289311. [DOI] [PubMed] [Google Scholar]
- Leow L, Simpson T, Cursons R, Karalus N, Hancox RJ. Vitamin D, innate immunity and outcomes in community acquired pneumonia. Respirology. 2011;16:611–616. doi: 10.1111/j.1440-1843.2011.01924.x. [DOI] [PubMed] [Google Scholar]
- Li-Ng M, Aloia JF, Pollack S, Cunha BA, Mikhail M, Yeh J, Berbari N. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiology and Infection. 2009;137:1396–1404. doi: 10.1017/S0950268809002404. [DOI] [PubMed] [Google Scholar]
- Litonjua AA. Vitamin D deficiency as a risk factor for childhood allergic disease and asthma. Current Opinion in Allergy and Clinical Immunology. 2012;12(2):179–185. doi: 10.1097/ACI.0b013e3283507927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker, A.C., Johnson, C.L., Lacher, D.A., Pfeiffer, C.M., Schleicher, R.L. and Sempos, C.T., 2011. Vitamin D status: United States, 2001–2006. NCHS Data Brief, 1–8. [PubMed]
- MacDonald PN, Dowd DR, Nakajima S, Galligan MA, Reeder MC, Haussler CA, Ozato K, Haussler MR. Retinoid X receptors stimulate and 9-cis retinoic acid inhibits 1,25-dihydroxyvitamin D3-activated expression of the rat osteocalcin gene. Molecular and Cellular Biology. 1993;13:5907–5917. doi: 10.1128/mcb.13.9.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaughlin JA, Anderson RR, Holick MF. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science. 1982;216:1001–1003. doi: 10.1126/science.6281884. [DOI] [PubMed] [Google Scholar]
- Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. New England Journal of Medicine. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, Rosenberg AM. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatric Pulmonology. 2009;44:981–988. doi: 10.1002/ppul.21089. [DOI] [PubMed] [Google Scholar]
- Morales E, Romieu I, Guerra S, Ballester F, Rebagliato M, Vioque J, Tardon A, Rodriguez Delhi C, Arranz L, Torrent M, Espada M, Basterrechea M, Sunyer J. Maternal vitamin d status in pregnancy and risk of lower respiratory tract infections, wheezing, and asthma in offspring. Epidemiology. 2012;23:64–71. doi: 10.1097/EDE.0b013e31823a44d3. [DOI] [PubMed] [Google Scholar]
- Muhe L, Lulseged S, Mason KE, Simoes EA. Case–control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997;349:1801–1804. doi: 10.1016/S0140-6736(96)12098-5. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Trubert CL, Rizk-Rabin M, Rehan VK, Besancon F, Cayre YE, Garabedian M. 1,25-Dihydroxyvitamin D3 and fetal lung maturation: immunogold detection of VDR expression in pneumocytes type II cells and effect on fructose 1,6 bisphosphatase. Journal of Steroid Biochemistry and Molecular Biology. 2004;89–90:93–97. doi: 10.1016/j.jsbmb.2004.03.054. [DOI] [PubMed] [Google Scholar]
- Nguyen TM, Guillozo H, Marin L, Tordet C, Koite S, Garabedian M. Evidence for a vitamin D paracrine system regulating maturation of developing rat lung epithelium. American Journal of Physiology. 1996;271:L392–399. doi: 10.1152/ajplung.1996.271.3.L392. [DOI] [PubMed] [Google Scholar]
- Phokela SS, Peleg S, Moya FR, Alcorn JL. Regulation of human pulmonary surfactant protein gene expression by 1alpha,25-dihydroxyvitamin D3. American Journal of Physiology Lung Cellular and Molecular Physiology. 2005;289:L617–626. doi: 10.1152/ajplung.00129.2004. [DOI] [PubMed] [Google Scholar]
- Rehman PK. Sub-clinical rickets and recurrent infection. Journal of Tropical Pediatrics. 1994;40:58. doi: 10.1093/tropej/40.1.58. [DOI] [PubMed] [Google Scholar]
- Roth DE, Jones AB, Prosser C, Robinson JL, Vohra S. Vitamin D status is not associated with the risk of hospitalization for acute bronchiolitis in early childhood. European Journal of Clinical Nutrition. 2009;63:297–299. doi: 10.1038/sj.ejcn.1602946. [DOI] [PubMed] [Google Scholar]
- Roth DE, Shah R, Black RE, Baqui AH. Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatrica. 2010;99:389–393. doi: 10.1111/j.1651-2227.2009.01594.x. [DOI] [PubMed] [Google Scholar]
- Sakurai R, Shin E, Fonseca S, Sakurai T, Litonjua AA, Weiss ST, Torday JS, Rehan VK. 1alpha,25(OH)2D3 and its 3-epimer promote rat lung alveolar epithelial-mesenchymal interactions and inhibit lipofibroblast apoptosis. American Journal of Physiology Lung Cellular and Molecular Physiology. 2009;297:L496–505. doi: 10.1152/ajplung.90539.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh U, Nicholson G, Urban BC, Sargent IL, Kishore U, Bernal AL. Immunological properties of human decidual macrophages – a possible role in intrauterine immunity. Reproduction. 2005;129:631–637. doi: 10.1530/rep.1.00331. [DOI] [PubMed] [Google Scholar]
- Stein RT, Martinez FD. Asthma phenotypes in childhood: lessons from an epidemiological approach. Paediatric Respiratory Reviews. 2004;5:155–161. doi: 10.1016/j.prrv.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Stick S. Pediatric origins of adult lung disease. 1. The contribution of airway development to paediatric and adult lung disease. Thorax. 2000;55:587–594. doi: 10.1136/thorax.55.7.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stio M, Celli A, Lunghi B, Raugei G, Modesti A, Treves C. Vitamin D receptor in IMR-90 human fibroblasts and antiproliferative effect of 1,25-dihydroxyvitamin D3. Biochemistry and Molecular Biology International. 1997;43:1173–1181. doi: 10.1080/15216549700205011. [DOI] [PubMed] [Google Scholar]
- Tavera-Mendoza L.E., White J.H. Cell defenses and the sunshine vitamin. Scientific American. 2007;297:62–65. doi: 10.1038/scientificamerican1107-62. [DOI] [PubMed] [Google Scholar]
- UNICEF, 2006. Pneumonia: The Forgotten Killer of children, UNICEF/WHO.
- United States Department of Agriculture (USDA-ARS), 2010. Nutrient Intakes from Food: Mean Amounts Consumed per Individual, by Race/Ethnicity and Age, What We Eat in America, NHANES 2007–2008. Available at: www.ars.usda.gov/ba/bhnrc/fsrg.
- Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. American Journal of Clinical Nutrition. 2010;91:1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- Walker VP, Zhang X, Rastegar I, Liu PT, Hollis BW, Adams JS, Modlin RL. Cord blood vitamin D status impacts innate immune responses. Journal of Clinical Endocrinology and Metabolism. 2011;96:1835–1843. doi: 10.1210/jc.2010-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. European Journal of Clinical Nutrition. 2004;58:563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- Whiting SJ, Calvo MS. Lifestyle and nutritional determinants of vitamin status. In: Feldman D, Pike WJ, Adams JS, editors. Vitamin D. Oxford, UK: Academic Press; 2011. pp. 979–1007. [Google Scholar]
- Wright JR. Immunoregulatory functions of surfactant proteins. Nature Reviews: Immunology. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- Wu P, Dupont WD, Griffin MR, Carroll KN, Mitchel EF, Gebretsadik T, Hartert TV. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. American Journal of Respiratory and Critical Care Medicine. 2008;178:1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clinical Endocrinology. 2009;70:685–690. doi: 10.1111/j.1365-2265.2008.03403.x. [DOI] [PubMed] [Google Scholar]
- Zosky GR, Berry LJ, Elliot JG, James AL, Gorman S, Hart PH. Vitamin D deficiency causes deficits in lung function and alters lung structure. American Journal of Respiratory and Critical Care Medicine. 2011;183:1336–1343. doi: 10.1164/rccm.201010-1596OC. [DOI] [PubMed] [Google Scholar]


