Abstract
There are several conditions with mucocutaneous findings that are potentially life-threatening, particularly in certain vulnerable populations. In this chapter, leptospirosis, typhoid fever, dengue, diphtheria, and murine typhus are reviewed. The disease time course of classic and atypical presentations is detailed to assist making the diagnosis in subtle cases. Associated symptoms are discussed as well as a comparison with disease mimics and differential diagnoses. Key diagnostic features are emphasized, and evidence-based management of each condition is detailed in this chapter.
Keywords: Leptospirosis, Typhoid fever, Dengue, Diphtheria, Murine typhus
Leptospirosis
Background
Leptospirosis is a potentially fatal zoonotic infection seen in both temperate and tropical regions exposed to heavy rainfall and flooding. Rodents are the most important reservoir for transmission. Commonly carried by the brown rat (Rattus norvegicus), the virus is excreted through urine of the rat or other infected animal and contracted by contact with mucosal surfaces or skin breaks [1]. It is most commonly seen in tropical areas subject to poverty, large rainfall, and flooding; however, cases do occur within the United States, particularly the South Pacific coastal states and Hawaii [2]. The organism can infect many types of mammals which can be either asymptomatic or fatal. Spontaneous abortion is a common complication in animals and frequently described in cattle, swine, sheep, and goats. Risk factors for acquiring leptospirosis infection include activities with exposure to infected animals or water. Farmers and animal caretakers are at particular risk. Sporadic outbreaks commonly occur and have been described in athletes participating in triathlons who swam in infected water [3–6].
Clinical Presentation
Symptoms are largely nonspecific, and patients often present with flu-like illness including fever, myalgias, and headache. However, there are several distinguishing features that herald a diagnosis of leptospirosis (see Table 23.1). Ocular findings are quite common and may be present in up to 90% of cases. In particular, conjunctival suffusion, a dilatation of conjunctival vessels, is a common presentation in leptospirosis but rarely seen in other infectious diseases (see Fig. 23.1). Other ocular findings may include subconjunctival hemorrhage, icterus (seen in severe disease), and hypopyon [7].
Table 23.1.
Classic features of leptospirosis
| Typical presentation |
| Flu-like illness |
| Conjunctival suffusion a |
| Myalgias (especially calf pain) |
| Headache with retro-orbital pain |
aDistinguishing clinical feature
Fig. 23.1.
Conjunctival suffusion with subconjunctival hemorrhage in a patient with leptospirosis. (From Lin et al. [57]. (Link to image: (may be better quality via the link) https://www.ncbi.nlm.nih.gov/core/lw/2.0/html/tileshop_pmc/tileshop_pmc_inline.html?title=Click%20on%20image%20to%20zoom&p=PMC3&id=3269263_tropmed-86-187-g003.jpg)
Rash is less common and may lead one to consider other diagnoses such as dengue, hantavirus, chikungunya, and others; however, other cutaneous findings such as jaundice with intense pruritus (secondary to liver and renal failure) as well as petechiae or ecchymosis from hemorrhagic complications may be present [7].
Atypical Presentation
There have been documented cases of a pretibial rash with leptospiral infection; however, this is uncommon, as the majority of leptospiral diseases are without cutaneous findings. Other atypical findings include pharyngeal injection, maculopapular skin rashes, and cutaneous hyperesthesias [8, 9].
Associated Symptoms
Systemic symptoms are common, predominantly fever, myalgias, and headache. The headache is frequently described as a throbbing, bitemporal, frontal headache often accompanied by retro-orbital pain and photophobia. Myalgias with back and calf tenderness are commonly described. Cough, nausea, vomiting, diarrhea, and abdominal pain are also not infrequent [9].
Disease Progression
With leptospirosis, the disease course may vary significantly from patient to patient. Following exposure, the average incubation period is 10 days (see Fig. 23.2). In typical cases, patients then proceed to a biphasic illness. In the first phase, patients experience rapid onset of fever, rigors, myalgias, and headache that lasts 4–9 days. Patients then experience an afebrile phase for up to 3 days, followed by a recurrence of fever and possible complications such as meningitis and uveitis [9].
Fig. 23.2.
Leptospirosis disease timeline
Common Mimics and Differential Diagnosis
Differential diagnosis includes malaria, dengue, chikungunya, typhus, rickettsial disease, and hantavirus. See Table 23.2 for distinguishing features of the differential diagnoses.
Table 23.2.
Differential diagnoses for leptospirosis
| Differential diagnoses | |
|---|---|
| Malaria |
Cyclical fevers Severe headache Rash is uncommon |
| Dengue |
High fevers Headache with retro-orbital pain Conjunctival injection Maculopapular rash Palmar desquamation Petechiae/ecchymosis and positive tourniquet test |
| Chikungunya |
High fever Maculopapular rash Myalgias, arthralgias |
| Typhoid fever |
Fever with relative bradycardia Rose spot rash GI symptoms |
| Rocky Mountain spotted fever |
Fever Headache Arthralgias, myalgias Maculopapular rash, petechiae, purpura that moves centripetally. Can affect palms and soles Predominantly eastern United States |
| Murine typhus |
Fever Maculopapular rash that starts centrally and classically spares palms and soles |
| Hantavirus |
Fever Headache GI symptoms Rash is uncommon Non-cardiogenic pulmonary edema |
Complications
Severe leptospiral infections can lead to significant and potentially lethal complications (see Table 23.3). Weil’s syndrome is characterized by liver and renal failure usually in association with altered level of consciousness, hemorrhage, and anemia. Advanced age and jaundice are associated with a higher mortality rate. Severe infections may also be complicated by progression to ARDS and circulatory collapse [9, 10].
Table 23.3.
Complications of leptospirosis
| Complications |
|---|
| Weil’s syndrome: hepatic and renal failure |
| Hemorrhagic complications: epistaxis, GI bleed, pulmonary hemorrhage, hemolytic anemia |
| Myocarditis |
| Neurologic: broad neurologic symptoms, aseptic meningitis, transverse myelitis |
| ARDS |
| Circulatory collapse |
Hemorrhagic complications may also occur, secondary to coagulopathy and thrombocytopenia. Pulmonary hemorrhage is one of the most severe complications with a demonstrated fatality rate over 50%. GI bleeding, severe epistaxis, and hemolytic anemia are other hemorrhagic complications that may be present [1, 11].
Myocarditis, although rare, is another well documented complication of leptospirosis [9].
Patients may also experience a myriad of neurologic complications including aseptic meningitis (relatively common) and transverse myelitis [9].
Management
Diagnosis
Definitive diagnosis is made from PCR, antibody titers, or culture. However, as these diagnostic measures take time, empiric treatment should be initiated if there is a high clinical suspicion of the disease [12].
Treatment
While the majority of leptospirosis cases are mild and will resolve without intervention, early antibiotic therapy may prevent disease progression (see Table 23.4). Outpatient therapy is indicated for mild disease and consists of either doxycycline or azithromycin, with azithromycin being the preferred treatment for pregnant women and children. If rickettsial infection is a possible diagnosis, doxycycline should be given (see Chap. 10.1007/978-3-319-75623-3_16 for further discussion on rickettsial infections). Patients with evidence of complications, hemodynamic instability, or severe dehydration should be admitted for supportive care, targeted treatment of the specific complication and intravenous antibiotics [1, 13]. Inpatient treatment includes IV penicillin, ampicillin, ceftriaxone, or cefotaxime. A Jarisch-Herxheimer reaction may occur following treatment, which should be managed supportively [14].
Table 23.4.
Treatmentof leptospirosis
| Treatment regimens | |
|---|---|
| Outpatient |
Doxycycline 100 mg orally twice daily for 7 days Azithromycin 500 mg orally daily for 3 days Amoxicillin 50 mg/kg in 3 equally divided oral doses for 7 days |
| Inpatient |
IV penicillin 1.5 million units every 6 h for 7 days Doxycycline 100 mg IV twice daily for 7 days Ceftriaxone 1–2 g IV once daily for 7 days Efotaxime 1 g IV every 6 h for 7 days |
Bottom Line: Leptospirosis Clinical Pearls
Leptospirosis can range from mild flu-like illness to multi-organ dysfunction.
Patients typically present with flu-like illness, headache, and myalgias, predominantly in the back and calves.
Ocular findings are a key diagnostic clue to leptospirosis.
Conjunctival suffusion is the most common ocular finding in leptospirosis.
Other ocular findings include subconjunctival hemorrhage, scleral icterus, and scattered petechiae.
Diagnosis should be made clinically and is confirmed with serology, PCR, and culture.
Outpatient treatment includes doxycycline, azithromycin, or amoxicillin.
Inpatient regimens include IV penicillin, doxycycline, ceftriaxone, and cefotaxime.
Complications may affect nearly any organ system and include hemorrhagic complications, liver failure, myocarditis, neurologic manifestations, ARDS, and renal failure.
Typhoid Fever
Background
Typhoid fever is a febrile illness common among travelers endemic to Asia, Africa, Latin America, and the Caribbean. It is caused by Salmonella enterica serotypes (Typhi and Paratyphi) and is commonly spread through urine and feces. Humans are the only reservoir and it is endemic in areas with poor sanitation [15].
Clinical Presentation and Disease Progression
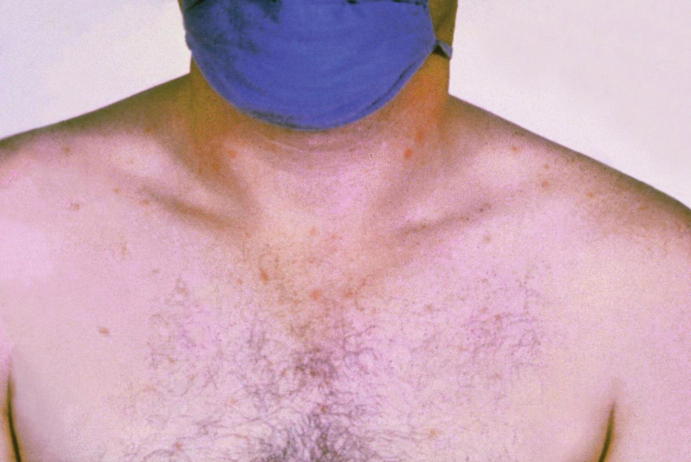
Typhoid fever is a febrile illness that often develops mucocutaneous findings. Classically, typhoid fever is known for its “rose-spot” rash, which is characterized as a blanching salmon-colored maculopapular rash that appears across the trunk in groupings of 5–15 papules (Fig. 23.3). These lesions may extend across the back and to proximal extremities as well [16].
Fig. 23.3.
Patient with a rose-colored rash on chest and shoulders with acute typhoid fever. (Photo courtesy: https://phil.cdc.gov/details.aspx?pid=2215)
Atypical Presentation
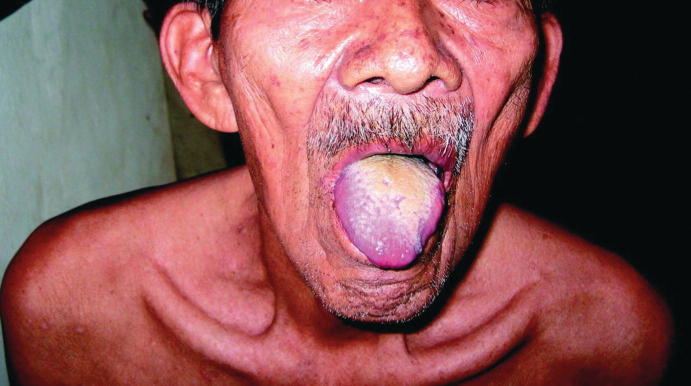
One notable atypical finding that appears to be specific to typhoid fever is “furry tongue” (see Fig. 23.4). There are well-documented cases of white-yellow-coated tongues following infection with a reported specificity up to 94% [17, 18].
Fig. 23.4.
Coated tongue in a patient with typhoid fever. (From Bal and Czarnowski [18]. Open access https://www.ncbi.nlm.nih.gov/pmc/articles/PMC374215/bin/21FF1.jpg)
Associated Symptoms
In addition to fever and rash, patients commonly present with abdominal pain. This abdominal pain may be associated with either constipation or diarrhea which occur with similar frequency. In addition, relative bradycardia (Faget’s sign/sphygmothermic dissociation) is a well-described phenomenon as are headache, sleep disturbance, cough, arthralgias, and myalgias (see Table 23.5) [19].
Table 23.5.
Classic features of typhoid fever
| Typical presentation |
|---|
| Fever with relative bradycardia |
| Maculopapular rash in groupings |
| Abdominal pain and GI symptoms |
| Flu-like illness including headache, cough, arthralgias, myalgias |
Time Course
Classically, typhoid feverpresents in stages (see Fig. 23.5). The incubation period lasts roughly 5–21 days after ingestion of the microorganism. In the first week of clinical symptoms, patients often develop fever which classically rises in a stepwise fashion and, if left untreated, may persist for weeks. In the second week of infection, the classic “rose-spot” rash appears with lesions usually lasting 3–5 days before remitting. Patients commonly complain of abdominal pain at this time and may suffer from a myriad of GI symptoms. In the third week, patients may develop complications of ongoing illness as discussed below [15].
Fig. 23.5.
Typhoid fever disease timeline
Common Mimics and Differential Diagnosis
Findings in typhoid fever are largely nonspecific, and therefore a wide differential needs to be maintained. The differential diagnosis includes malaria, tuberculosis, brucellosis, tularemia, leptospirosis, rickettsial infections, dengue fever, hepatitis, and infectious mononucleosis.
Complications
Left untreated, typhoid fever can lead to a number of significant complications, which commonly present as GI complaints in the later weeks of infection (see Table 23.6). These include small bowel ulceration, intestinal perforation, and subsequent septic shock. Other complications are also possible, including psychosis, neurologic deficits, and “typhoid encephalopathy” which can be described as altered level of consciousness or delirium. The bacteria can also seed nearly every other organ system so there may potentially be cardiac, respiratory, genitourinary, musculoskeletal, and central nervous system abnormalities. Finally, patients if untreated, may become chronic carriers causing autoinoculation and or transmission to other contacts [19–21].
Table 23.6.
Complications of typhoid fever
| Complications |
|---|
| GI: Ulceration, perforation, peritonitis |
| Neurologic: Encephalopathy, psychosis, neurologic deficits |
| Sepsis |
| Nearly any other organ system may be affected, but the above complications are the most common |
Management
Diagnosis
The diagnosis of typhoid fever should largely be clinical with high suspicion in patients exposed to endemic areas. Diagnosis can be confirmed with cultures from blood, stool, urine, rose spots, and bone marrow; however, these diagnostic methods are imperfect. Serology is of limited utility as a positive result may indicate a prior infection rather than an ongoing one. Other developing modalities include ELISA and PCR [22].
Treatment
Historically, chloramphenicol or amoxicillin were the drugs of choice for treatment of typhoid fever; however, drug resistance has become a significant problem (see Table 23.7). Treatment should be directed by local resistance patterns and severity of illness. In severe disease with systemic signs, IV ceftriaxone or fluoroquinolones should be initiated if there is local susceptibility. Glucocorticoid therapy has been shown to reduce severity of illness and mortality in patients with severe disease based on a randomized, double-blind placebo-controlled trial out of Indonesia with a relatively low side effect profile. Therefore, if patients develop delirium, coma, shock, or DIC, glucocorticoid therapy should be considered with dexamethasone loading at 3 mg/kg IV followed by 1 mg/kg IV every 6 h for eight doses. Steroid treatment beyond this is contraindicated as it may increase relapse rate [23–26].
Table 23.7.
Treatment of typhoid fever
| Treatment |
|---|
| Select treatment based on severity of disease and local susceptibilities |
| Inpatient regimen: IV ceftriaxone or fluoroquinolones |
| Outpatient regimen: PO azithromycin or fluoroquinolones |
| In severe cases, add IV dexamethasone 3 mg/kg followed by repeat doses at 1 mg/kg every 6 h |
In uncomplicated disease, oral agent therapy with ciprofloxacin 500–750 mg PO BID for 14 days should be initiated. In quinolone-resistant regions, azithromycin 1 g PO may be taken daily for 5 days [23].
Bottom Line: Typhoid Fever Clinical Pearls
Typhus is endemic to Mexico, Peru, Indonesia, and the Indian subcontinent.
Classic presentation includes high fevers, abdominal discomfort, and a maculopapular rash that appears in clusters across the torso.
Significant complications may occur from untreated typhoid fever particularly throughout the GI tract, but any organ may be affected.
Diagnosis is clinical and treatment initiated empirically in suspected cases, while confirmatory tests are pending.
Treatment choice should be based on severity of disease and local susceptibilities.
Dengue Fever
Background
Dengue fever, caused by several serotypes of Flaviviridae , is one of the most common etiologies of arthropod-borne viral disease in the world. While often asymptomatic and self-limited, dengue fever can vary significantly in severity and is a significant public health concern in developing nations [27].
Clinical Presentation
Dengue fever classically presents with high fever, headache, abdominal pain, myalgias, and arthralgias as well as mucocutaneous findings (see Tables 23.8 and 23.9). During the febrile portion of the illness, conjunctival injection and oropharynx hyperemia are commonly present (see Fig. 23.6). Facial flushing or erythematous mottling may occur at the beginning of fever or just before, usually resolving within 2 days after onset of symptoms (Fig. 23.7). Rash can occur in up to 50% of patients, occurring either early or late. The rash is typically described as maculopapular and can occur diffusely across face, throat, abdomen, and extremities. Patients may endorse pruritus as well. With defervescence, a late cutaneous eruption develops, characterized by confluent and erythematous islands, which are often pruritic in nature (Figs. 23.8 and 23.9). The rash often resolves within 2–3 days but may last up to as many as 5 days. In addition, as the disease resolves, pruritic desquamation of the palms and soles may occur. Other cutaneous manifestations occur secondarily to hemorrhagic complications, as discussed below, and include petechiae, purpura, and ecchymosis (see Fig. 23.10) [28].
Table 23.8.
Mucocutaneous findings in dengue
| Mucocutaneous findings |
|---|
| Conjunctival injection |
| Oropharynx hyperemia |
| Facial flushing |
| Maculopapular rash across face, throat, abdomen, and extremities |
| Confluent erythematous islands |
| Desquamation of palms and soles |
| Petechiae, purpura, ecchymosis |
Table 23.9.
Typical presentation of dengue
| Typical presentation |
|---|
| Flu-like illness including fevers, myalgias, arthralgias |
| Mucocutaneous findings as above |
| Headache with retro-orbital pain |
| Nausea and vomiting |
| Relative bradycardia |
| Less common findings: anorexia, altered taste sensation, sore throat |
Fig. 23.6.
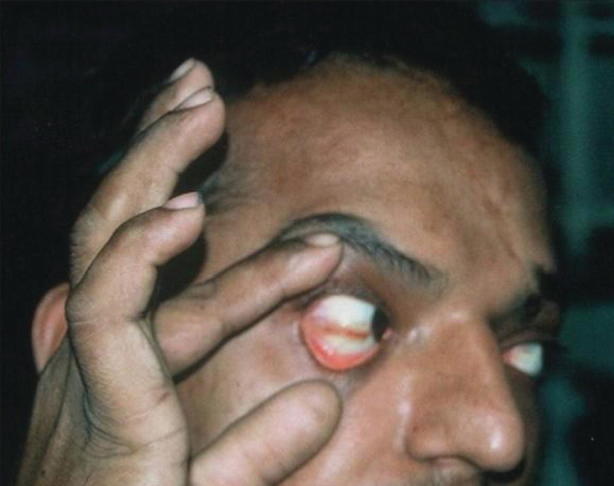
Scleral injection in a patient with dengue fever. (From Thomas et al. [58] (open access))
Fig. 23.7.
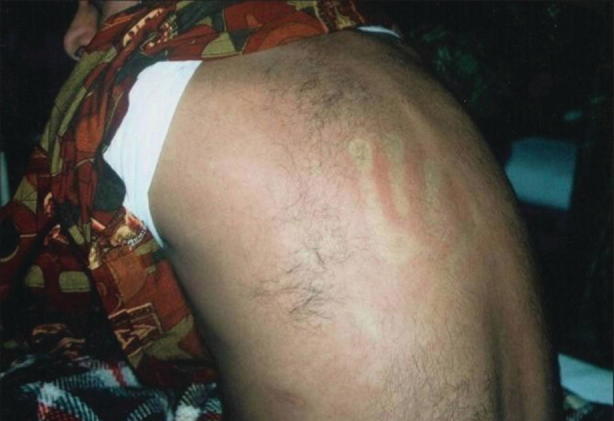
Erythematous blanching rash of a patient with dengue infection. (From Thomas et al. [58] (open access))
Fig. 23.8.
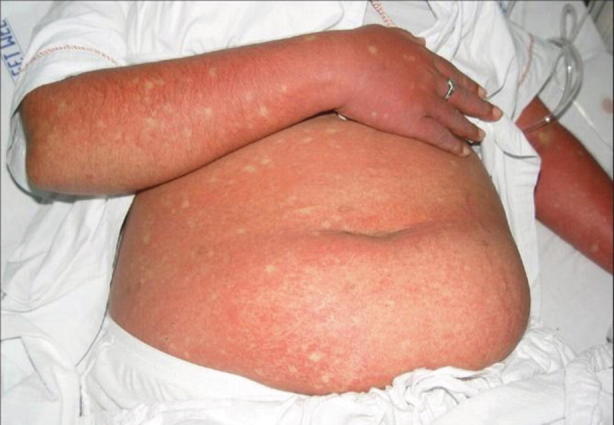
Confluent erythematous rash with islands of sparing in a patient with dengue fever. (From Thomas et al. [58] (open access))
Fig. 23.9.
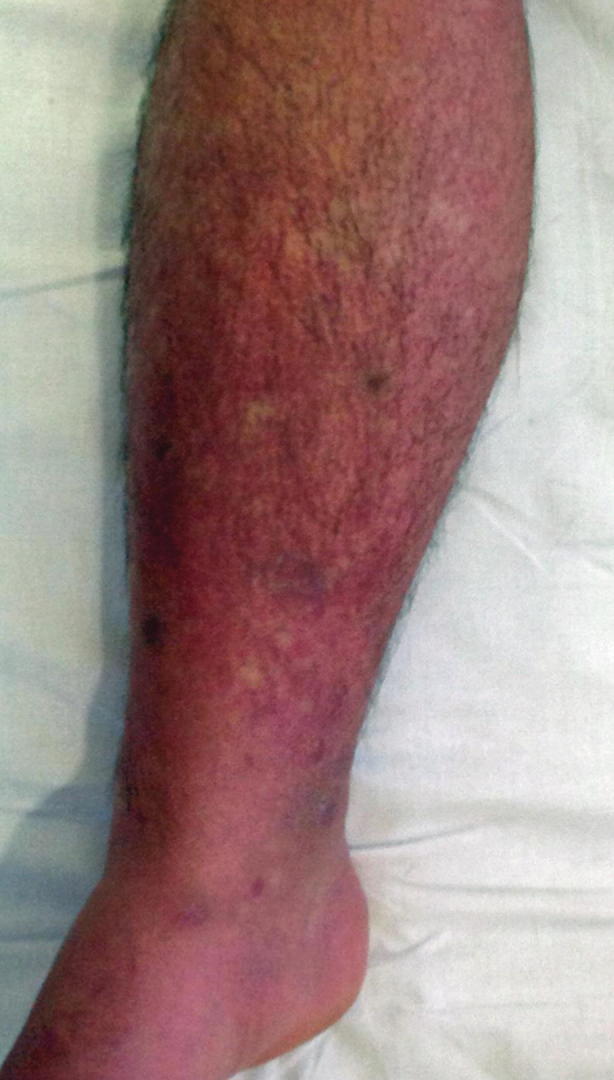
Early erythematous mottling and secondary maculopapular rash. (Used with permission from Kenzaka and Kumabe [59])
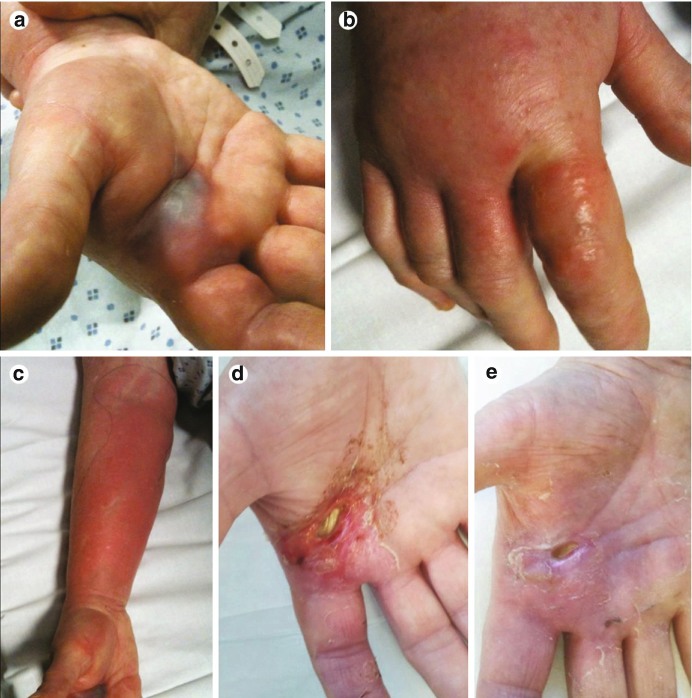
Fig. 23.10.
Panel A shows a typical petechial rash in an infant with dengue. Panel B shows minor bleeding around injection sites, a very common feature in dengue. Panel C shows a hematoma in a patient with severe dengue. Panel D shows characteristic diffuse macular rash that appears after recovery from the acute illness in an adult patient with dengue (typically 3-6 days after fever onset). Note the “islands of white” of normal skin surround by an erythematous rash Simmons et al. [60]
Associated Symptoms
As above, patients may experience a myriad of symptoms consistent with viral illness. Patients commonly complain of frontal headache with retro-orbital pain, myalgias, arthralgias, nausea, and vomiting. During the febrile phase of the illness, patients commonly have a relative bradycardia to the degree of fever. Less common associated symptoms include anorexia, altered taste sensation, and mild sore throat. Other symptoms may occur secondarily to complications and will be discussed below [28].
Physical Exam
Patients may have conjunctival injection and pharyngeal erythema. Given propensity for hemorrhagic complications, patients commonly develop petechiae, purpura, and ecchymosis. Additionally, lymphadenopathy, hepatomegaly, facial plethora, or signs of overload secondarily to vascular leak may be present [28].
Time Course of Disease
Dengue virus is typically transmitted by the Aedes mosquito and is typically found in densely forested areas. The viral incubation period is 3–14 days after inoculation. Dengue fever is divided into three phases: the febrile phase, the critical phase, and the convalescent phase [28].
In the febrile phase, patients experience fever that waxes and wanes, lasting from 2 to 7 days, as well as many of the symptoms above [28].
The critical phase occurs around the time of defervescence, often 3–7 days after fever onset. This phase usually lasts 24–48 h and may be complicated by systemic vascular leak syndrome. This syndrome is characterized by plasma leak, bleeding, shock, and organ dysfunction [29].
The convalescent phase, often occurring 1–2 days after defervescence, is characterized by resolution of plasma leakage and hemorrhage. A pruritic confluent erythematous rash often is present during this phase as seen above [28].
Common Mimics and Differential Diagnosis
Differential diagnosis should include malaria, dengue, chikungunya, typhus, rickettsial disease, and hantavirus. See Table 23.2 for distinguishing features of the differential diagnoses.
Complications
The complications of dengue are many and involve multiple organ systems with potential organ failure. Table 23.10 outlines many of the common complications [28–33].
Table 23.10.
Complicationsof dengue
| Complications | |
|---|---|
| Systemic vascular leak syndrome |
Third spacing Liver injury and failure Acute kidney injury |
| Hemorrhagic |
Easy bleeding Petechiae and purpura DIC |
| Neurologic |
Encephalopathy Seizures Neuropathies (including pure motor weakness) Guillain-Barre Transverse myelitis |
| Cardiovascular |
Myocarditis Arrhythmia Heart failure |
Management
Diagnosis
Early diagnosis ofdengue fever is usually clinical and should be suspected in patients with the signs and symptoms above and exposure to endemic regions. Although neither sensitive nor specific, providers may perform a tourniquet test to help in diagnosis (Figs. 23.11 and 23.12). In this test, a blood pressure cuff is inflated midway between systolic and diastolic pressures and left for 5 min. A positive test is characterized by ten or more new petechiae in 1 square inch. Diagnosis may be confirmed by detection of viral components in serum or PCR, although these methods are labor intensive and costly. Serology is also available but is of lower specificity. Viral culture is also available but of limited utility given time needed for test to result [34–36].
Fig. 23.11.
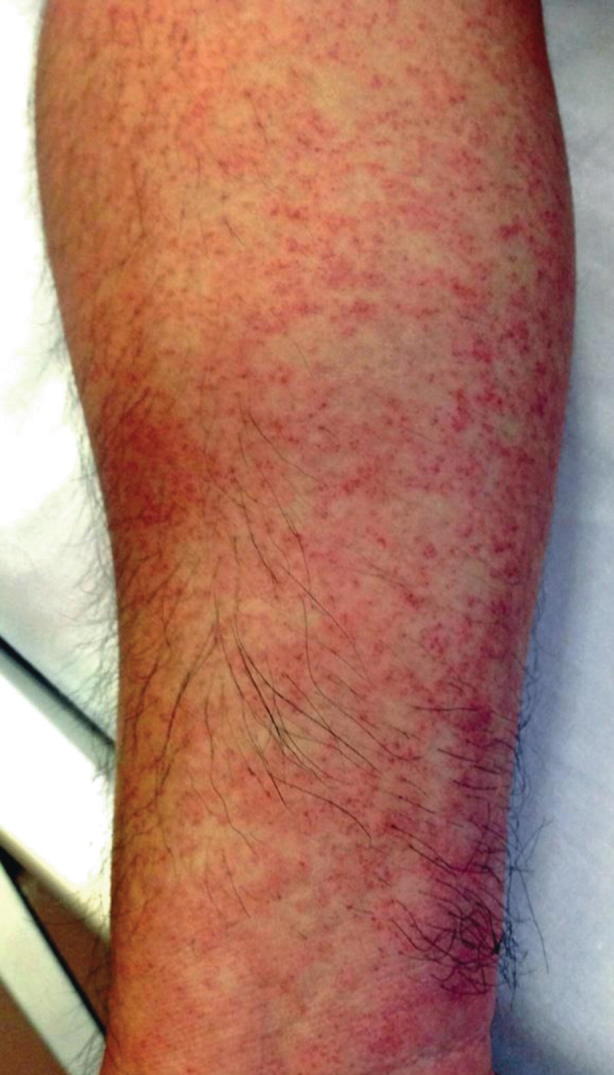
Positive tourniquet test in dengue. (Used with permission from Kenzaka and Kumabe [59])
Fig. 23.12.
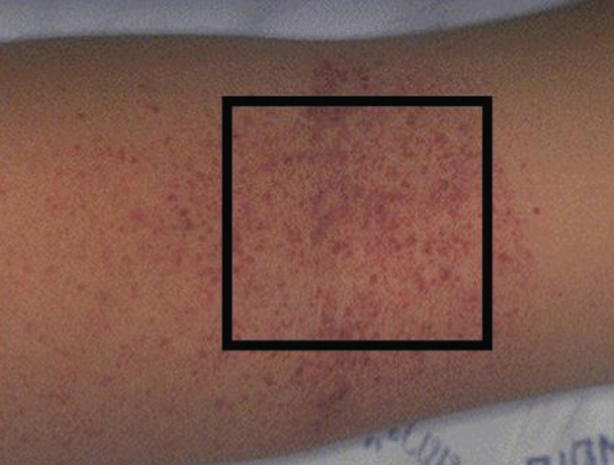
Positive tourniquet test in dengue. (Source: https://www.cdc.gov/dengue/training/cme/ccm/images/tourniquet_test1.png)
Management
Managementis largely supportive because there is no antiviral therapy for dengue fever. Outpatient management may be appropriate in patients without systemic complications or comorbid conditions such as pregnancy, infancy, advanced age, renal failure, underlying hemolytic disease, or poor social support/access to follow-up care [37].
Inpatient management should be considered for any patient with comorbid conditions or for those with signs of severe infection. Signs of severe infection include abdominal pain, persistent nausea and vomiting, fluid accumulation (ascites, pleural effusion), mucosal bleeding, lethargy or altered mental status, hepatomegaly, increased hematocrit with rapid decrease in platelet count, and signs of shock or end-organ dysfunction. Aggressive IV fluid supplementation is warranted in those with signs of dehydration. Fevers, myalgias, and arthralgias can be managed with acetaminophen. NSAIDs and aspirin should be avoided, given the potential hemorrhagic complications. During the disease course, hemoglobin and platelets should be monitored. Transfusion with packed red blood cells is indicated for worsening anemia and suspected bleeding. Platelet transfusion is indicated for severe thrombocytopenia (<10,000/mm3) but should not be pursued prophylactically. Vitamin K may be indicated if the prothrombin time is prolonged which may occur as a result of liver dysfunction or DIC [37–39].
Bottom Line: Dengue Fever Clinical Pearls
Dengue is endemic to tropical regions and transmitted by the Aedes mosquito.
The disease presents in three phases: The febrile phase, the critical phase, and the convalescent phase.
Symptoms may vary significantly; patients commonly present with fever, headache, retro-orbital pain, GI symptoms, and rash.
The initial rash is maculopapular and may last several days.
A secondary rash may present in the convalescent phase, which is erythematous, occurs in clusters, and is often pruritic.
There are a wide range of complications that can occur from dengue fever, including vascular leak, hemorrhagic sequelae, and multi-organ dysfunction.
Diagnosis is largely clinical and confirmed with titers and PCR.
Treatment is largely supportive with IV fluids and antipyretics, particularly acetaminophen.
Aspirin and NSAIDs should be avoided due to potential hemorrhagic complications.
Transfusion and vitamin K may be required for hemorrhagic complications.
Diphtheria
Background
Caused by the gram-positive rod , diphtheria is a disease that can cause a myriad of symptoms, ranging from asymptomatic infection to respiratory distress. It is often associated with mucocutaneous symptoms. Diphtheria was a significant cause of morbidity and mortality in the pre-vaccine era; however, since the advent of the diphtheria vaccine, the disease has largely been eliminated in developed countries but may sporadically occur [40].
Clinical Presentation
Patients infected with C. diphtheria usually present in one of two fashions: respiratory diphtheria or cutaneous diphtheria. Respiratory diphtheria is often caused by toxigenic strains, whereas cutaneous diphtheria may be caused by both toxigenic and non-toxigenic strains.
Respiratory diphtheria often presents with sore throat, malaise, cervical lymphadenopathy, and low-grade fever. Early in the disease course, only pharyngeal erythema may be present, which then progresses to isolated areas of gray and white exudate (see Figs. 23.13 and 23.14; Table 23.11). Although known as the hallmark of this disease, pseudomembranes form in only one third of cases. Pseudomembranes are easily friable gray tissue adhering to underlying tissue, composed of necrotic fibrin, leukocytes, epithelial cells, and organisms. These membranes can spread anywhere along the respiratory tract; however, the majority of cases are found along tonsils and oropharynx. Extensive spreading of these membranes can lead to respiratory compromise which will be discussed below [41].
Fig. 23.13.
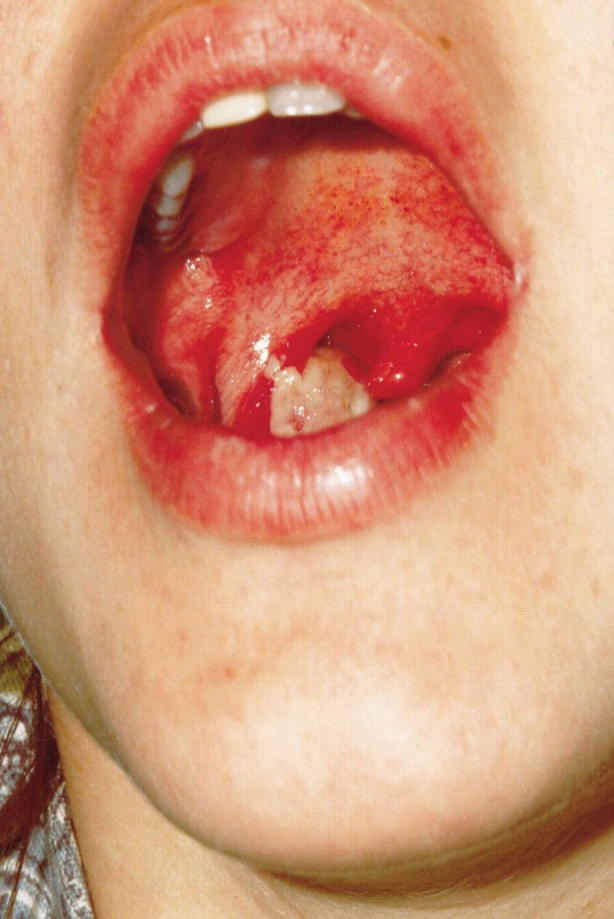
Early diphtheria in a 26-year-old female. Note the membrane on the right tonsil. (Used with permission from Kadirova et al. [61])
Fig. 23.14.
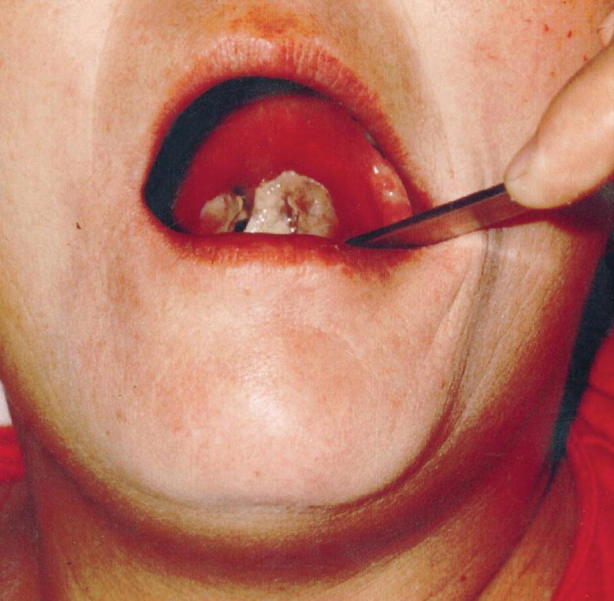
Diphtheritic membrane extending from the uvula to the pharyngeal wall with associated neck edema in a 47-year-old female. (Used with permission from Kadirova et al. [61])
Table 23.11.
Mucocutaneous findings in diphtheria
| Classic presentations | |
|---|---|
| Respiratory diphtheria |
Pharyngeal erythema Gray-white exudate Pseudomembrane note that these findings can occur anywhere along respiratory tract |
| Cutaneous diphtheria |
Blisters or pustules that progress to shallow ulcers Gray membranes Hemorrhagic or violaceous base Painful lesion that becomes anesthetic Heals poorly |
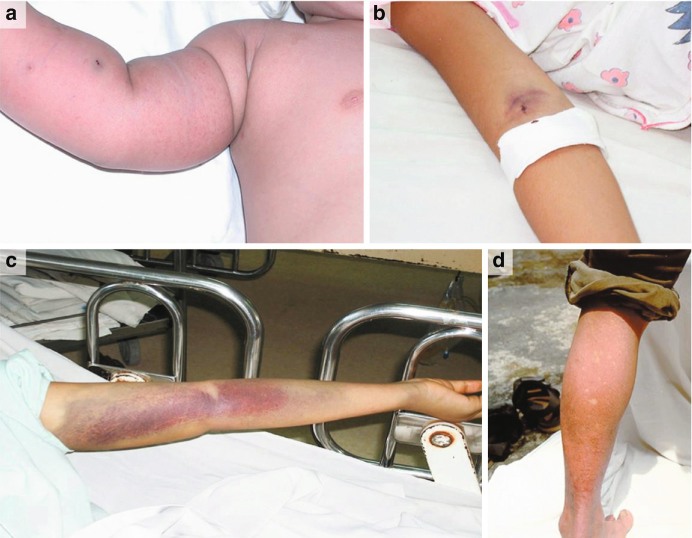
Cutaneous diphtheria is often the more benign of the two conditions as systemic toxicity is rare. Early, patients may experience a small blister or pustule, often over a site of minor skin trauma, with straw-colored fluid that ruptures early forming a punched-out ulcer. These shallow ulcers usually become chronic, poorly healing lesions (taking from 6 weeks to up to a year to heal) that become covered by gray membrane. While initially painful during the first 2 weeks, these lesions often become anesthetic and develop a hemorrhagic or purple base (see Fig. 23.15). In addition, this variant of the disease tends to predominately affect the impoverished and intravenous drug users. Patients with cutaneous diphtheria rarely develop the respiratory variant of the disease but commonly serve as a reservoir of infection for others [42–44].
Fig. 23.15.
Clinical presentation and progression of Corynebacterium ulcerans cutaneous diphtheria. (a) Palmar aspect of the hand at time of presentation. (b) Dorsal aspect of the hand at the time of presentation. (c) Ipsilateral forearm with spreading inflammatory response at time of presentation. (d) Palmar aspect of hand after surgical debridement of synovial sheath necrotic tissue at 7 days after presentation. (e) Palmar aspect of hand at 28 days after presentation and debridement. (Used with permission from Moore et al. [42])
Cutaneous Diphtheria
Associated Symptoms
Respiratory diphtheria often presents similar to streptococcal pharyngitis with sore throat, malaise, lymphadenopathy, and low-grade fever; however, patients may also experience cough as areas of the respiratory tract become involved. Symptoms may become significantly more severe in cases of systemic toxicity as discussed below. Symptoms are not solely limited to cutaneous and respiratory systems, however, and further manifestations will be discussed below [45, 46].
Physical Exam
As above, findings consistent with diphtheria are posterior oropharynx erythema and pseudomembrane formation. In cases of nasal diphtheria, the clinician may find serosanguineous to purulent nasal discharge. In laryngeal diphtheria, hoarseness and cough may be present. In severe cases, the pseudomembranes may be extensive causing massive swelling of the tonsils, uvula, cervical lymph nodes, submandibular region, and neck. This presentation is colloquially known as the “bull neck” of diphtheria, and such swelling may cause stridor and respiratory distress (see Fig. 23.16) [41].
Fig. 23.16.
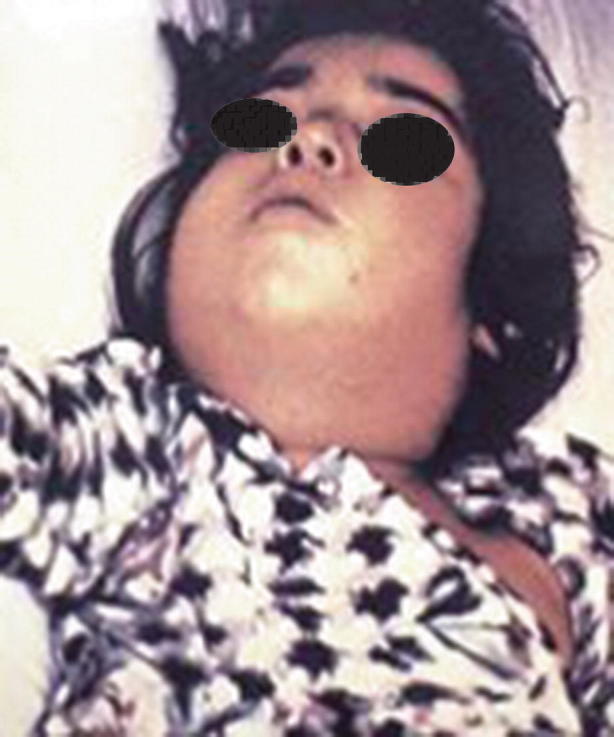
The “bull neck” of diphtheria infection. (Source: https://www.cdc.gov/diphtheria/images/symptoms.jpg)
Time Course of Disease
Humans are the only known reservoir of disease, with spread primarily occurring via respiratory secretions or direct contact with mucocutaneous manifestations. Symptoms often appear 2–5 days postexposure. After symptoms begin, in untreated patients, the disease often lasts up to 2 weeks but can last up to 6 weeks. In appropriately treated patients, the infection is often cleared within 4 days [41, 47].
Common Mimics and Differential Diagnosis
Differential diagnosisshould include infectious mononucleosis, group A streptococcal pharyngitis, epiglottitis, viral pharyngitis, acute necrotizing ulcerative gingivitis, oral candidiasis, and viral pharyngitis.
Complications
See Table 23.12 for a summary of complications associated with diphtheria. The primary and most feared complication is severe membranous pharyngitis which can lead to thickening of the neck, narrowing of the airway, and resultant respiratory distress. Airway management is of the utmost importance in the emergent management of these patients [41].
Table 23.12.
Complications of diphtheria
| Complications | |
|---|---|
| Respiratory |
Airway narrowing Respiratory failure |
| Cardiovascular |
Myocarditis Heart blocks Arrhythmia Heart failure |
| Neurologic |
Cranial nerve palsies Soft palate and posterior pharynx paralysis Peripheral neuropathies |
Another well-described complication of diphtheria is myocarditis, which is more common in severe infections. Cardiac manifestations of diphtheria have been noted in up to 10 to 25 percent of patients with diphtheria, often occurring 1–2 weeks after symptom onset. Patients may experience heart blocks, arrhythmia, heart failure, and, in severe cases, circulatory collapse. These potential complications mandate cardiac monitoring in patients with potential systemic disease [41, 48].
Neurologic toxicity is another well-known complication of severe diphtheria infection. As with cardiac complications, neurologic symptoms are more common with worsening systemic illness. Manifestations of neurologic involvement often include paralysis of the soft palate and posterior pharyngeal wall. These neuropathies can progress to cranial neuropathies and peripheral neuritis ranging from mild weakness to total paralysis [49].
Management
Diagnosis
A diagnosis ofdiphtheria should first be suspected clinically in the unvaccinated or exposed population in the setting of above findings, particularly with friable pseudomembrane formation. Culture from respiratory tract or cutaneous lesions is required for definitive diagnosis; however, a presumptive diagnosis can be made in the setting of gram-positive rods on gram stain with the above findings. PCR and toxin assay are also available to help distinguish whether a toxigenic form of diphtheria is causing thepatient symptoms [50].
Treatment
The most important aspect of treatment of treatment in diphtheria is airway management given risk for obstruction. Patients should also be monitored for dysrhythmias and hypotension secondary to cardiac involvement with supportive management as needed [41].
In cases of early suspected or confirmed respiratory diphtheria, antitoxin should be administered. Of note, antitoxin is only effective before the toxin enters the cell. In addition, there is a 5–20% risk of hypersensitivity or serum sickness; therefore, ideally a scratch test can be performed before IV administration, and epinephrine should be readily available in cases of anaphylaxis [51].
Antibiotic therapy also plays a role in the treatment of diphtheria, with the antibiotics of choice being erythromycin or penicillin. Benefits ofantibiotic therapy are threefold: killing of bacteria preventing further toxin formation, slowing spread of local infection, and reducing of transmission. Treatment with these antibiotic regimens is for 2 weeks followed by a repeat culture to ensure eradication [47, 51]. See Table 23.13 for antibiotic treatment regimens.
Table 23.13.
Antibiotic treatment regimens for diphtheria
| Treatment | |
|---|---|
| Erythromycin | 500 mg four times daily for 2 weeks |
| Penicillin |
PO intolerant Penicillin G IM injections 600,000 units every 12 h PO tolerant: Oral penicillin V 250 mg four times daily for 2 weeks |
Bottom Line: Diphtheria Clinical Pearls
Diphtheria is a potentially life-threatening illness of two variations: Respiratory and cutaneous.
Respiratory diphtheria classically causes pseudomembrane formation which may be diffuse and can lead to airway obstruction.
Cutaneous findings are generally nonspecific, characterized by grayish non-healing ulcers.
Diphtheria may affect multiple organ systems with cardiac and neurologic manifestations being the most common in severe systemic illness.
Diagnosis is initially clinical and confirmed with cultures and PCR.
Treatment includes antibiotics and antitoxin (if early in the disease course).
Murine Typhus
Background
Murine or endemic typhus is a flea-borne infectious disease caused by . The infection is often mild and self-limited and likely frequently remained undiagnosed as a nonspecific febrile illness with rash. As with other rickettsial disease, infection induces a widespread vasculitis [51, 52].
Murine typhus is primarily transmitted via the rat flea, particularly in the developing world and in locations with large rat populations. However, any type of flea may carry and transmit the disease. In suburban United States, cats, opossums, mice, and shrews may host infected fleas. Humans are infected via infected flea bite. Most cases of murine typhus in the United States are reported in people from California, Hawaii, and Texas [51, 52].
Classic Clinical Presentation
Murine typhus is typically a mild illness of nonspecific viral-type symptoms (see Table 23.14). Typically, the onset of illness is abrupt with fever, headache, chills, and myalgias. A rash is present in 20–50 percent of patients. The classic rash is a fine, maculopapular rash that begins on the abdomen and spreads centripetally to the extremities (see Fig. 23.17). Classically, the palms, soles, and face are spared. The rash is frequently faint and less apparent in dark-skinned individuals [51, 53].
Table 23.14.
Classic murine typhus presentation
| Classic presentation |
|---|
| Flu-like illness |
| Fevers |
| Headache |
| Myalgias |
| Fine maculopapular rash |
| Spreads from trunk to extremities |
| No palms or soles involvement |
Fig. 23.17.
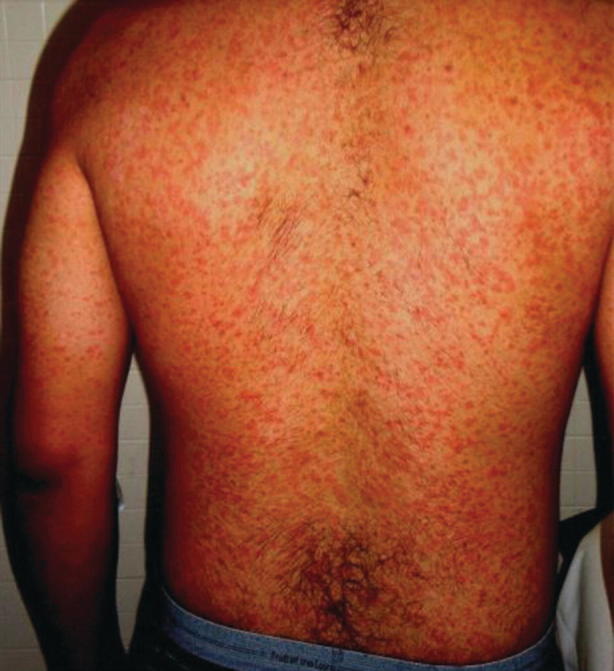
Diffuse murine typhus rashconsisting of multiple erythematous macules. (Source: Gorchynski et al. [53] (open access))
Atypical Presentation
Children are more likely to additionally have abdominal pain, vomiting, and diarrhea [54]. In approximately 10 percent of patients, the rash may be petechial. In contrast to the typical mild disease course, severe complications may potentially occur, particularly in patients with glucose-6-phosphate dehydrogenase deficiency (G6PD) and the elderly (see Complications below).
Time Course of Disease
After inoculation, there is a 7–14-day incubation period prior to symptom onset. Low-grade fever generally occurs within the first 1–2 days and resolves within 2 days. Rash onset classically occurs 5 days after onset of symptoms and usually resolves spontaneously within 4 days. Total resolution of symptoms generally occurs within 14 days (see Fig. 23.18) [55].
Fig. 23.18.
Murine typhusdisease timeline
Common Mimics and Differential Diagnosis
There is a wide differential diagnosis to the nonspecific symptoms of murine typhus. Important diagnoses to consider include viral infections, rubella, measles (see Chap. 10.1007/978-3-319-75623-3_6), mononucleosis, and classically tropical infections such as dengue and Zika.
Key Physical Exam Findings and Diagnostic Features
Diagnosis typically occurs with initial clinical suspicion in a patient with fever, headache, rash, and possible exposure to infected fleas. Similar to other rickettsial infections, there is no reliable diagnostic test early in the disease course. Serologic confirmation may occur with an indirect fluorescent antibody testing which is typically only available at a health department laboratory. Other laboratory findings are nonspecific and also nondiagnostic. Thrombocytopenia occurs in approximately half of all patients with murine typhus [51, 55].
Management
Treatment of murine typhus rapidly improves clinical symptoms and decreases complication rates; therefore, empiric treatment should be initiated in those with a high enough index of suspicion (see Table 23.15). While tetracyclines and chloramphenicol are effective treatments, doxycycline is the antibiotic of choice for rickettsial infections (see Chap. 10.1007/978-3-319-75623-3_16), and adults should receive 100 mg twice daily. In children, doxycycline is still the recommended agent of choice regardless of age. The recommended dosing of doxycycline in children is 4 mg/kg daily divided into two doses to be received every 12 h. Of note, fluoroquinolones and chloramphenicol may also be used but appear to be less efficacious. In addition, fluoroquinolones should not be used in children under the age of 18 years. While duration of therapy is controversial, some sources recommend treatment until 3 days after defervescence, and evidence of clinical improvement is documented. In addition, it should be noted that most individuals will recover from illness within 2 weeks without treatment [51, 52, 56].
Table 23.15.
Treatmentregimens for murine typhus
| Treatment | |
|---|---|
| Adults | Doxycycline 100 mg twice dailya |
| Children | Doxycycline 4 mg/kg/day divided into two doses every 12 hb |
aTreatment duration is controversial and not clearly outlined
bTetracyclines and chloramphenicol can be used as alternatives, but are generally less effective
Complications
While complicationsare rare, nearly any organ system may be affected by murine typhus. Notable complications include renal dysfunction, pulmonary edema, respiratory failure, aseptic meningitis, splenomegaly, and rarely septic shock and multi-organ system failure. Severe disease is more likely to occur in patients with G6PD deficiency and advanced age [52].
Bottom Line: Murine Typhus Clinical Pearls
Murine typhus is a flea-borne illness that causes an abrupt onset of symptoms including fever, headache, and myalgias.
It is often generally a mild, self-limited disease.
A faint maculopapular rash begins on the trunk and spreads peripherally, sparing the palms and soles.
Patients with G6PD deficiency and advanced age are at risk for life-threatening complications.
Treatment is with doxycycline.
Contributor Information
Emily Rose, Phone: +11323-226-6667, Email: emilyros@usc.edu.
Emily Rose, Email: emilyros@usc.edu.
References
- 1.Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65–97. doi: 10.1007/978-3-662-45059-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jesus MS, Silva LA, Lima KM, Fernandes OC. Cases distribution of leptospirosis in City of Manaus, State of Amazonas, Brazil, 2000-2010. Rev Soc Bras Med Trop. 2012;45(6):713–716. doi: 10.1590/S0037-86822012000600011. [DOI] [PubMed] [Google Scholar]
- 3.Morgan J, Bornstein SL, Karpati AM, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34(12):1593–1599. doi: 10.1086/340615. [DOI] [PubMed] [Google Scholar]
- 4.Stone SC, Mcnutt E. Update: outbreak of acute febrile illness among athletes participating in Eco-Challenge-Sabah 2000 – Borneo, Malaysia, 2000. Ann Emerg Med. 2001;38(1):83–84. doi: 10.1067/mem.2001.116399. [DOI] [PubMed] [Google Scholar]
- 5.Radl C, Müller M, Revilla-fernandez S, et al. Outbreak of leptospirosis among triathlon participants in Langau, Austria, 2010. Wien Klin Wochenschr. 2011;123(23–24):751–755. doi: 10.1007/s00508-011-0100-2. [DOI] [PubMed] [Google Scholar]
- 6.Brockmann S, Piechotowski I, Bock-hensley O, et al. Outbreak of leptospirosis among triathlon participants in Germany, 2006. BMC Infect Dis. 2010;10:91. doi: 10.1186/1471-2334-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rathinam SR. Ocular manifestations of leptospirosis. J Postgrad Med. 2005;51(3):189–194. [PubMed] [Google Scholar]
- 8.Gochenour WS, Smadel JE, Jackson EB, Evans LB, Yager RH. Leptospiral etiology of Fort Bragg fever. Public Health Rep. 1952;67(8):811–813. doi: 10.2307/4588208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanford JP. Leptospirosis – time for a booster. N Engl J Med. 1984;310(8):524–525. doi: 10.1056/NEJM198402233100811. [DOI] [PubMed] [Google Scholar]
- 10.Gulati S, Gulati A. Pulmonary manifestations of leptospirosis. Lung India. 2012;29(4):347–353. doi: 10.4103/0970-2113.102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gouveia EL, Metcalfe J, De carvalho AL, et al. Leptospirosis-associated severe pulmonary hemorrhagic syndrome, Salvador, Brazil. Emerging Infect Dis. 2008;14(3):505–508. doi: 10.3201/eid1403.071064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Limmathurotsakul D, Turner EL, Wuthiekanun V, et al. Fool’s gold: why imperfect reference tests are undermining the evaluation of novel diagnostics: a reevaluation of 5 diagnostic tests for leptospirosis. Clin Infect Dis. 2012;55(3):322–331. doi: 10.1093/cid/cis403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suputtamongkol Y, Niwattayakul K, Suttinont C, et al. An open, randomized, controlled trial of penicillin, doxycycline, and cefotaxime for patients with severe leptospirosis. Clin Infect Dis. 2004;39(10):1417–1424. doi: 10.1086/425001. [DOI] [PubMed] [Google Scholar]
- 14.Guerrier G, D’ortenzio E. The Jarisch-Herxheimer reaction in leptospirosis: a systematic review. PLoS One. 2013;8(3):e59266. doi: 10.1371/journal.pone.0059266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med. 2002;347(22):1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman SL, Punjabi NH, Kumala S, et al. Reduction of mortality in chloramphenicol-treated severe typhoid fever by high-dose dexamethasone. N Engl J Med. 1984;310(2):82–88. doi: 10.1056/NEJM198401123100203. [DOI] [PubMed] [Google Scholar]
- 17.Haq SA, Alam MN, Hossain SM, Ahmed T, Tahir M. Value of clinical features in the diagnosis of enteric fever. Bangladesh Med Res Counc Bull. 1997;23(2):42–46. [PubMed] [Google Scholar]
- 18.Bal SK, Czarnowski C. A man with fever, cough, diarrhea and a coated tongue. CMAJ. 2004;170(7):1095. doi: 10.1503/cmaj.1031079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta SP, Gupta MS, Bhardwaj S, Chugh TD. Current clinical patterns of typhoid fever: a prospective study. J Trop Med Hyg. 1985;88(6):377–381. [PubMed] [Google Scholar]
- 20.Ali G, Rashid S, Kamli MA, Shah PA, Allaqaband GQ. Spectrum of neuropsychiatric complications in 791 cases of typhoid fever. Tropical Med Int Health. 1997;2(4):314–318. doi: 10.1111/j.1365-3156.1997.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 21.Huang DB, Dupont HL. Problem pathogens: extra-intestinal complications of Salmonella enterica serotype Typhi infection. Lancet Infect Dis. 2005;5(6):341–348. doi: 10.1016/S1473-3099(05)70138-9. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman SL, Punjabi NH, Rockhill RC, Sutomo A, Rivai AR, Pulungsih SP. Duodenal string-capsule culture compared with bone-marrow, blood, and rectal-swab cultures for diagnosing typhoid and paratyphoid fever. J Infect Dis. 1984;149(2):157–161. doi: 10.1093/infdis/149.2.157. [DOI] [PubMed] [Google Scholar]
- 23.Thaver D, Zaidi AK, Critchley J, Madni SA, Bhutta ZA. Fluoroquinolones for treating typhoid and paratyphoid fever (enteric fever). Cochrane Database Syst Rev. 2005;(2):CD004530. [DOI] [PubMed]
- 24.Kalra SP, Naithani N, Mehta SR, Swamy AJ. Current trends in the management of typhoid fever. Med J Armed Forces India. 2003;59(2):130–135. doi: 10.1016/S0377-1237(03)80060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler T, Islam A, Kabir I, Jones PK. Patterns of morbidity and mortality in typhoid fever dependent on age and gender: review of 552 hospitalized patients with diarrhea. Rev Infect Dis. 1991;13(1):85–90. doi: 10.1093/clinids/13.1.85. [DOI] [PubMed] [Google Scholar]
- 26.Cooles P. Adjuvant steroids and relapse of typhoid fever. J Trop Med Hyg. 1986;89(5):229–231. [PubMed] [Google Scholar]
- 27.Guzman MG, Harris E. Dengue. Lancet. 2015;385(9966):453–465. doi: 10.1016/S0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- 28.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11(3):480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalayanarooj S, Vaughn DW, Nimmannitya S, et al. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176(2):313–321. doi: 10.1086/514047. [DOI] [PubMed] [Google Scholar]
- 30.Chhour YM, Ruble G, Hong R, et al. Hospital-based diagnosis of hemorrhagic fever, encephalitis, and hepatitis in Cambodian children. Emerging Infect Dis. 2002;8(5):485–489. doi: 10.3201/eid0805.010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomon T, Dung NM, Vaughn DW, et al. Neurological manifestations of dengue infection. Lancet. 2000;355(9209):1053–1059. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- 32.Carod-artal FJ, Wichmann O, Farrar J, Gascón J. Neurological complications of dengue virus infection. Lancet Neurol. 2013;12(9):906–919. doi: 10.1016/S1474-4422(13)70150-9. [DOI] [PubMed] [Google Scholar]
- 33.Neeraja M, Iakshmi V, Teja VD, et al. Unusual and rare manifestations of dengue during a dengue outbreak in a tertiary care hospital in South India. Arch Virol. 2014;159(7):1567–1573. doi: 10.1007/s00705-014-2010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao XT, Ngo TN, Wills B, et al. Evaluation of the World Health Organization standard tourniquet test and a modified tourniquet test in the diagnosis of dengue infection in Viet Nam. Tropical Med Int Health. 2002;7(2):125–132. doi: 10.1046/j.1365-3156.2002.00841.x. [DOI] [PubMed] [Google Scholar]
- 35.Guzman MG, Jaenisch T, Gaczkowski R, et al. Multi-country evaluation of the sensitivity and specificity of two commercially-available NS1 ELISA assays for dengue diagnosis. PLoS Negl Trop Dis. 2010;4(8):e811. doi: 10.1371/journal.pntd.0000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunsperger EA, Muñoz-jordán J, Beltran M, et al. Performance of dengue diagnostic tests in a single-specimen diagnostic algorithm. J Infect Dis. 2016;214(6):836–844. doi: 10.1093/infdis/jiw103. [DOI] [PubMed] [Google Scholar]
- 37.Rajapakse S, Rodrigo C, Rajapakse A. Treatment of dengue fever. Infect Drug Resist. 2012;5:103–112. doi: 10.2147/IDR.S22613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas L, Kaidomar S, Kerob-bauchet B, et al. Prospective observational study of low thresholds for platelet transfusion in adult dengue patients. Transfusion. 2009;49(7):1400–1411. doi: 10.1111/j.1537-2995.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- 39.Khan assir MZ, Kamran U, Ahmad HI, et al. Effectiveness of platelet transfusion in dengue fever: a randomized controlled trial. Transfus Med Hemother. 2013;40(5):362–368. doi: 10.1159/000354837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.English PC. Diphtheria and theories of infectious disease: centennial appreciation of the critical role of diphtheria in the history of medicine. Pediatrics. 1985;76(1):1–9. [PubMed] [Google Scholar]
- 41.Naiditch MJ, Bower AG. Diphtheria; a study of 1,433 cases observed during a ten-year period at the Los Angeles County hospital. Am J Med. 1954;17(2):229–245. doi: 10.1016/0002-9343(54)90261-2. [DOI] [PubMed] [Google Scholar]
- 42.Moore LSP, Leslie A, Meltzer M, Sandison A, Efstratiou A, Sriskandan S. Corynebacterium ulcerans cutaneous diphtheria. Lancet Infect Dis. 2015;15(9):1100–1107. doi: 10.1016/S1473-3099(15)00225-X. [DOI] [PubMed] [Google Scholar]
- 43.Höfler W. Cutaneous diphtheria. Int J Dermatol. 1991;30(12):845–847. doi: 10.1111/j.1365-4362.1991.tb04348.x. [DOI] [PubMed] [Google Scholar]
- 44.Zeegelaar JE, Faber WR. Imported tropical infectious ulcers in travelers. Am J Clin Dermatol. 2008;9(4):219–232. doi: 10.2165/00128071-200809040-00002. [DOI] [PubMed] [Google Scholar]
- 45.Murphy JR. Corynebacterium Diphtheriae. In: Baron S, editor. Medical microbiology. 4. Galveston: University of Texas Medical Branch at Galveston; 1996. [PubMed] [Google Scholar]
- 46.Walters RF. Diphtheria presenting in the accident and emergency department. Arch Emerg Med. 1987;4(1):47–51. doi: 10.1136/emj.4.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kneen R, Pham NG, Solomon T, et al. Penicillin vs. erythromycin in the treatment of diphtheria. Clin Infect Dis. 1998;27(4):845–850. doi: 10.1086/514959. [DOI] [PubMed] [Google Scholar]
- 48.Kneen R, Nguyen MD, Solomon T, et al. Clinical features and predictors of diphtheritic cardiomyopathy in Vietnamese children. Clin Infect Dis. 2004;39(11):1591–1598. doi: 10.1086/425305. [DOI] [PubMed] [Google Scholar]
- 49.Sanghi V. Neurologic manifestations of diphtheria and pertussis. Handb Clin Neurol. 2014;121:1355–1359. doi: 10.1016/B978-0-7020-4088-7.00092-4. [DOI] [PubMed] [Google Scholar]
- 50.Efstratiou A, Engler KH, Mazurova IK, Glushkevich T, Vuopio-varkila J, Popovic T. Current approaches to the laboratory diagnosis of diphtheria. J Infect Dis. 2000;181(Suppl 1):S138–S145. doi: 10.1086/315552. [DOI] [PubMed] [Google Scholar]
- 51.American Academy of Pediatrics . Diphtheria. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Red book: 2015 Report of the Committee on infectious diseases. 30. Elk Grove Village: American Academy of Pediatrics; 2015. pp. 307–311. [Google Scholar]
- 52.Civen R, Ngo V. Murine typhus: an unrecognized suburban vector-borne disease. Clin Infect Dis. 2008;46(6):913–918. doi: 10.1086/527443. [DOI] [PubMed] [Google Scholar]
- 53.Gorchynski JA, Langhorn C, Simmons M, Roberts D. What’s hot, with spots and red all over? Murine typhus. West J Emerg Med. 2009;10(3):207. doi: 10.1111/j.1442-2026.1998.tb00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsioutis C, Zafeiri M, Avramopoulos A, Prousali E, Miligkos M, Karageorgos SA. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16–24. doi: 10.1016/j.actatropica.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 55.Peniche lara G, Dzul-rosado KR, Zavala velázquez JE, Zavala-castro J. Murine typhus: clinical and epidemiological aspects. Colomb Med. 2012;43(2):175–180. [PMC free article] [PubMed] [Google Scholar]
- 56.Fergie JE, Purcell K, Wanat D. Murine typhus in South Texas children. Pediatr Infect Dis J. 2000;19(6):535–538. doi: 10.1097/00006454-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Lin CY, Nan-Chang C, Lee CM. Leptospirosis after Typhoon Americal. J Trop Med Hyg. 2012;86(2):187–188. doi: 10.4269/ajtmh.2012.11-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas EA, Mary J, Kanish B. Mucocutaneous manifestations of dengue fever. Indian J Dermatol. 2010;55(1):79–85. doi: 10.4103/0019-5154.60359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kenzaka T, Kumabe A. Skin rash from dengue fever. BMJ Case Rep. 2013;2013:bcr2013201598. doi: 10.1136/bcr-2013-201598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simmons CP, Farra JJ, van Vinh Chau N, Wills B. Dengue. N Engl J Med. 2012;366:1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 61.Kadirova R, Kartoglu HU, Strebel PM. Clinical characteristics and management of 676 hospitalized cases, Kyrgyz Republic, 1995. J Infect Dis. 2000;181(Suppl 1):S110–S115. doi: 10.1086/315549. [DOI] [PubMed] [Google Scholar]