Abstract
The expression of sialic acids (Sia) is highly conserved in deuterostomes, i.e., from echinoderms to humans. They constitute components of cell surface glycoproteins and gangliosides, where they occupy mainly the terminal position as individual monosaccharides and, more rarely, as oligo- or polymers. They are frequently found in secreted glycoconjugates and in oligosaccharides, mainly of blood serum, milk, and mucus secretions [1, 2].
Keywords: Sialic acid O-acetylation, Sialate-O-acetylesterases, Immunoregulation, Lymphocytes, Virulence, Ganglioside O-acetylated GD3
Introduction
The expression of sialic acids (Sia) is highly conserved in deuterostomes, i.e., from echinoderms to humans. They constitute components of cell surface glycoproteins and gangliosides, where they occupy mainly the terminal position as individual monosaccharides and, more rarely, as oligo- or polymers. They are frequently found in secreted glycoconjugates and in oligosaccharides, mainly of blood serum, milk, and mucus secretions [1, 2].
The term “sialic acids” defines a group of various N- and O-derivatives of the basic neuraminic acid molecule. After N-acetylneuraminic acid (Neu5Ac), the most frequent species are N-glycolylneuraminic acid (Neu5Gc) and O-acetylated derivatives. Evidence is accumulating that esterified Sia are more ubiquitous than Neu5Gc. In contrast to Neu5Gc, they also occur in microorganisms. Figure 28.1 shows the Sia family. One Sia molecule may carry one or several, up to three, O-acetyl groups. N-Acetyl-9-O-acetylneuraminic acid (Neu5,9Ac2) is most common. Acetyl ester groups are often a combined neuraminic acid with other residues such as N-glycolyl, O-lactyl, or O-methyl groups, and they may also be linked to 2-keto-3-deoxynononic acid (KDN) [1, 2].
Fig. 28.1.

The family of naturally occurring Sia. The nomenclature, abbreviations, combinations of substituents, and distribution in tissues and cells are described in [1, 2]
The first insight into the biosynthesis of O-acetylated Sia was obtained in live tissue slices from bovine submandibular gland [3]. An O-acetyltransferase involved in Sia modification was solubilized from Golgi membranes and partially purified [4]. Whereas the enzymatic and molecular genetic backgrounds of Neu5Gc have been well elucidated, knowledge of Sia O-acetylation is scanty. Sialate-O-acetyltransferases (SOATs) have been cloned from a few bacterial species [5–9], but so far this has not been possible for eukaryotic SOAT genes, in spite of many attempts [10].
O-Acetylated Sia seem to be involved in many biological phenomena. Their predominance and variability of expression during development and malignancy, also as part of so-called “oncofetal antigens,” suggests their participation in physiological and pathological processes. Its role as a potent regulator of cellular interactions [1, 11, 12] classifies Sia O-acetylation with other biochemical regulations of cell function such as protein acetylation, phosphorylation, or modification with N-acetylglucosamine (GlcNAc) and methylation.
In the following methods for analyzing structure, the occurrence and metabolism as well as the pathophysiological functions of O-acetylated Sia will be reviewed. Special emphasis will be given to their role in immune defense mechanisms, both of innate and adaptive immunity, as components of mucins and other glycoconjugates expressed on tumor cells and lymphocytes. Interestingly, new observations speak in favor of an intracellular regulatory function of O-acetylated GD3 during apoptosis.
Analysis and Occurrence of O-Acetylated Sia
When studying the roles of O-acetylated Sia, exact analysis of their nature in biological materials is indispensable. The fundaments of these topics were described in this book’s previous edition and elsewhere [2–14]. Here, only recent developments or considerations relevant to the following will be presented. Acquiring a better understanding of O-acetylated Sia was hampered since the ester groups are labile and easily hydrolyzed, in particular under acidic or alkaline conditions, possibly during isolation of glycoconjugates or by liberation of Sia from their glycosidic linkages. In addition, esterases, de-O-acetylating Sia, are abundant in tissues [15] and may also reduce the quantity of these Sia in biological materials when released during the tissue extraction process. In spite of these problems, the nature of Sia and the position and number of O-acetyl groups can best be identified in a free state after their liberation from glycosidic linkage and possibly by purification through ion-exchange chromatography. Hydrolysis of glycosidic bonds is carried out by sialidase or mild acids. The latter method has the advantage that all Sia linkages are opened at similar rates, in contrast to enzymatic hydrolysis. The use of propionic acid (2 M, 4 h, 80°C) gives an excellent yield of O-acetylated Sia without significant isomerization of the ester groups. During concentration of the hydrolyzate, propionic acid evaporates azeotropically, i.e., without concentration, which preserves ester groups; this is in contrast to the hydrochloric or sulfuric acid used earlier, which can damage the Sia.
Now, better and more handy analytical techniques are available: direct application, i.e., without prior purification by ion-exchange chromatography, of Sia hydrolytically released; and chromatography on cellulose or silica gel thin layers, unless the sample contains too many impurities. Recently, it could be shown [16] that ion-exchange purification on strong alkaline resin, as carried out routinely for a long time [14], leads to isomerization of Neu5,7Ac2 to Neu5,9Ac2. Analysis of such a crude Sia sample is also possible by high-performance liquid chromatography (HPLC) of the fluorescence-labeled Sia [14, 16]. The accuracy of the assay can be increased by de-esterification with mild alkali or influenza C virus esterase and sialate–pyruvate lyase treatment of the sample. Mass spectrometric and nuclear magnetic resonance (NMR) spectroscopy analyses of free and glycosidically linked O-acetylated Sia are described in [2, 14, 16].
Histochemistry is a powerful tool for screening native tissues and cells for the occurrence of O-acetylated Sia. Staining is possible by mild periodate oxidation, leaving the other sugar residues intact, followed by diaminobenzaldehyde (Schiff’s reagent) treatment or by direct staining using specific antibodies, influenza C virus hemagglutinin, or other Sia-recognizing lectins [17]. Saponification and sialidase treatment serve as controls and increase the specificity of this method, avoid de-esterification, and may allow direct localization of the O-acetylated Sia within cells.
O-Acetylated Sia can frequently be detected in microorganisms and animals of the deuterostome lineage, from echinoderms to humans. The extent of O-acetylation in a given tissue can be rather variable, due to the developmental state or the stage of malignancy during tumor transformation. The tissues in which the highest concentrations of O-acetylated Sia were found, both by chemical and histochemical methods, are bovine submandibular gland [1, 2, 18, 19] and human colon mucosa [20–22]. The mucins from these tissues are extremely rich in these Sia. For example, in fresh bovine submandibular gland mucin (BSM), most Sia residues are esterified with one or two, and rarely three, acetyls at the Sia glycerol side chain. Remarkably, 50% of the Sia of snake muscle were found to be O-acetylated even after acid hydrolysis (unpublished data).
Metabolism of O-Acetylated Sia Biosynthesis
Three O-acetyltransferases (SOATs) that specifically esterify Sia in different positions have been discovered: sialate-4-O-acetyltransferase, sialate-7-O-acetyltransferase, and sialate-9-O-acetyltransferase (Fig. 28.2, Table 28.1). The SOAT from bovine submandibular gland was solubilized from its subcellular membrane location [32] by detergents and partially purified [4]. The enzyme has a molecular mass of 150–160 kDa, requires an (unknown) activator for full activity, and shows optimum activity at pH 6.5. CMP-Neu5Ac revealed to be the best substrate. It primarily incorporates the O-acetyl group at C-7 of Neu5Ac and can thus be defined as acetyl-CoA:sialate-7-O-acetyltransferase (EC 2.3.1.45). Before, it was not clear whether it could also esterify the hydroxyl residue at C-9. 9-O-Acetylated Sia exist in high amounts in BSM beside the 7-O-acetylated Sia and always coexist with Neu5,7Ac2 in other cells and tissues where Sia O-acetylation takes place at the side chain. This may be the result of one of two hypotheses: either 9-O-acetyltransferase activity is present additionally in these biological sources, or isomerization of Neu5,7Ac2 to Neu5,9Ac2 occurs. We favor the second hypothesis since it was shown that the O-acetyl group can migrate spontaneously to C-9, yielding Neu5,9Ac2 [33]. The frequent occurrence of small amounts of Neu5,8Ac2 may be another product of this isomerization process. Since the migration speed is relatively slow at physiological pH, the existence of an isomerase (migrase) is still under discussion; however, firm evidence is lacking [4]. After the arrival of the acetyl at C-9, another acetylation at C-7 is possible, and after migration of this group to O-8, a third acetyl group may be attached to C-7, leading to a fully esterified side chain of Sia. Such Neu5,7,8,9Ac4 was detected in small quantities in BSM, which have high O-acetylation potential. Furthermore, Neu5Ac is primarily O-acetylated at C-7 in starfish gonads (unpublished data) and in normal human lymphocytes (unpublished data) as well as in lymphoblasts of childhood acute lymphoblastic leukemia (ALL) [25]. The ganglioside GD3 was the best substrate in these experiments. The exact identification of the SOAT specificity was possible by using improved analytical tools, i.e., direct thin-layer chromatography (TLC) or HPLC analysis of the enzyme reaction products by which O-acetyl migration is avoided [23].
Fig. 28.2.

Metabolism of Sia O-acetylation. This figure represents our knowledge of enzymes involved in the transfer and removal of Sia O-acetyl groups. The stars indicate the hydroxyls found to be O-acetylated. The flashed arrows symbolize the position specificity of the SOAT activities discovered: sialate-4-O-acetyltransferase, sialate-7-O-acetyl-transferase, and sialate-9-O-acetyl-transferase. The scissors represent the sialate-9-O-acetylesterase and sialate-4-O-acetylesterase involved in hydrolysis of the ester groups. The O-acetyl group at the Sia glycerol side chain can migrate between C-7 and C-9, whereas the 4-O-acetyl group seems to be immobile. For further details, see the text (from [23] with permission of the publishers)
Table 28.1.
Detection of sialate-O-acetyltransferase activities
| Cells and tissues | References |
|---|---|
| Bovine submandibular glanda | [4, 23] |
| Bovine livera | Unpublished |
| Human colon mucosaa | [21, 22, 24] |
| Human leucocytesa | Unpublished |
| Acute lymphoblastic leukaemia (CEMC 7 cells)a | [25] |
| Human skin melanomab | [26] |
| Rat liverb | [27, 28] |
| Guinea pig liverc | [29] |
| Equine submandibular glandc | Unpublished |
| Starfish Asterias rubens gonadsa | Unpublished |
| Campylobacter jejuni d | [30] |
| Escherichia coli e | [6, 31] |
a O-acetylation primarily at C-7 of Sia
b O-acetylation found at C-7 and C-9, but primary enzymatic insertion site not yet clearly demonstrated
c O-acetylation at C-4
d O-acetylation at C-9
e O-acetylation at Sia side chain (C-7, C-9)
A transferase responsible for Sia O-acetylation directly at C-9 seems to exist in Campylobacter jejuni [30]. This was shown by NMR analysis of the SOAT product using α2,8-linked Neu5Ac as a substrate. Thus, this bacterial enzyme appears to be a distinct SOAT and may be numbered as EC 2.3.1.46 in the Enzyme Nomenclature. The exact position specificities of the other bacterial SOATs cloned from Neisseria meningitides [5], Escherichia coli [6], and group B Streptococcus [8] have not yet been enzymatically investigated in detail, although they also seem to modify the Sia side chain.
Another SOAT, so far found only in mammals, is sialate-4-O-acetyltransferase (4-SOAT; EC 2.3.1.44). This enzyme was intensively studied in Golgi membranes from guinea pig liver [29]. It exhibits a broad substrate specificity ranging from free Sia, sialylated oligosaccharides, glycoproteins, and gangliosides. This 4-SOAT has neither been isolated nor cloned. Corresponding activity was demonstrated also in the microsomal fraction from submandibular gland of horse. The tissues of this animal are rich in 4-O-acetylated Sia (unpublished data).
The firm attachment of eukaryotic SOATs to subcellular membranes [32], the difficulty in solubilizing these, and the failure to isolate the labile SOAT from BSM in pure form indicate that they are part of a protein complex. This possibly has functional significance. A cooperation of various proteins during SOAT reaction using AcCoA was already discussed by Varki’s group, when studying the enzyme in rat liver microsomes [27]. Based on this and as a result of our studies with BSM, a model is shown in Fig. 28.3. Accordingly, relying on in vitro studies, O-acetylation occurs before the transfer of Sia onto nascent mucin glycoproteins, since CMP-Neu5Ac is an excellent substrate for bovine [4] and human colon [24] 7-SOAT.
Fig. 28.3.

Biosynthesis of Sia and their O-acetylation in eukaryotic cells. After synthesis of Neu5Ac in the cytosol and activation in the nucleus, CMP-Neu5Ac enters the Golgi via the transporter and is O-acetylated by the sialate-7-O-acetyltransferase and then transferred to glycoproteins or glycolipids by sialyltransferase. The transporters and the transferases seem to be part of a protein complex. This model is mainly based on studies with the bovine submandibular gland [4]. It may be differrent in, for example, human lymphocytes, where GD3 was found to be the most suitable substrate for the O-acetyltransferase in vitro (unpublished data)
That this may also occur in vivo is demonstrated by the isolation of CMP-glycoside of O-acetylated Sia from BSM [3]. O-Acetyl migration is believed to take place after glycoprotein sialylation. Carriers for CMP-Neu5Ac and AcCoA, as well as sialyltransferase, may be part of this complex. This model may be typical for mucin-producing cells, in contrast to lymphocyte homogenates, for example, where GD3 is O-acetylated at the best rate (unpublished data).
Degradation
Three enzyme groups are directly involved in the catabolism of Sia: esterases, sialidases, and sialate–pyruvate lyases. The first and main players in the degradation of O-acetylated Sia are esterases, which act on free and bound Sia. They participate in regulation of the degree of O-acetylation in glycoconjugates, and thus, together with the O-acetyltransferases, are responsible for the biology of O-acetylated Sia. Such esterases were found in some virus and bacterium species and seem to be abundant in cells of higher animals in various forms [15, 28, 34].
In toroviruses, group 2 coronaviruses [35], and influenza C virus [36], esterases seem to function as receptor-destroying enzymes during the process of cell infection, although the exact mechanism is not yet known. They are specific for either 4-O-acetyl or 9-O-acetyl groups on cell membrane Sia. Toroviruses are described to be an exception because they can hydrolyze both 7- and 9-O-acetyl groups [35]. Influenza C virus esterase is the best characterized enzyme, and it is in wide use as a tool to specifically remove 9-O-acetyl groups from Sia and, in this way, to study the nature of O-acetylated Sia and their cellular localization and function [17, 37].
The Sia-degrading esterases of bacteria are less well characterized, but they are assumed to play a prominent role in colonization of epithelia by bacteria and in their virulence [20]. Esterases may provide new ligands, i.e., non-O-acetylated Sia, for binding of bacteria and also facilitate the action of sialidases and lyases, which show reduced activity with O-acetylated Sia (see below). In this way, the penultimate sugars of epithelia or mucins are unmasked by Sia removal, mostly galactose or N-acetylgalactosamine, which are distinct constituents of ligands for bacterial colonization other than Sia. Thus, O-acetyl groups on Sia hinder degradation of the mucinous defense barrier on epithelia by bacteria and colonization and thus eventual destruction of epithelia. This may be valid for human intestine, where the amount and O-acetylation of Sia increase from small intestine to colon [38].
Mammalian esterases hydrolyzing Sia O-acetyl groups are frequent and were best investigated in rat and horse liver and bovine brain [15, 28, 34]. Interestingly, they can only hydrolyze 4- or 9-O-acetyl groups at different rates, but not 7-O-acetyl residues. The reason for this hydrolytic resistance of 7-O-acetyl may be found in the three-dimensional structure of the O-acetylated glycans, as demonstrated by computer-aided modeling (Fig. 28.4). The statistically most frequent position of the 7-O-acetyl group is adjacent to the terminal Sia, while the 9-O-acetyl group is protruding. Correspondingly, the 7-O-acetyl group cannot bind to the catalytic center of the esterases, which is in contrast to the protruding 9-O-acetyl residue. Before hydrolysis, the 7-O-acetyl group has to migrate to C-9. In horse liver, two esterases were found that can hydrolyze only 4-O-acetyl esters and both 4- and 9-acetyls, respectively [15].
Fig. 28.4.
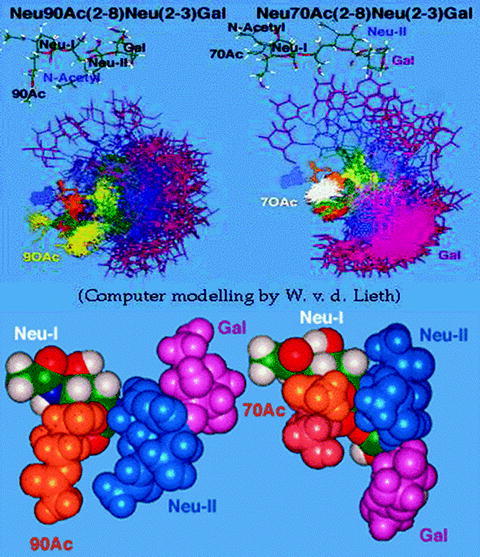
Modeling of the structures of 7-O-acetyl GD3 (right) and 9-O-acetyl GD3 (left). All depictions show that the 9-O-acetyl of the terminal Neu5Ac (Neu-I) protrudes from the ganglioside glycan chain, while the 7-O-acetyl remains closer to Neu-I
Sia esterases mainly occur in lysosomes but were also demonstrated in Golgi regions, plasma membranes, and cytosol [15, 28]. These locations explain the enzymes’ involvement not only in the degradation of glycoconjugates in lysosomes, but also in the regulation of expression of O-acetylated Sia. It was shown in human colon mucosa cells that the degree of O-acetylation depends on the ratio of the activities of both O-acetyltransferase and esterase and not on the activity of O-acetyltransferase alone [24].
Sialidases continue the degradation of sialylated glycoconjugates mainly in lysosomes (Neu1). However, they may also occur in the plasma membrane (Neu3) and in the cytosol (Neu2) and were cloned from these sites [39]. Also, many bacteria and viruses often produce large amounts of this enzyme [1, 2, 40–42]. Bacteria can use secreted sialidase to unmask penultimate sugars for binding to the host cells, or/and they consume liberated Sia for nutritional purposes. Influenza virus sialidases destroy the receptor for hemagglutinin (receptor-destroying enzymes), in particular after propagation and release from host cells, thus facilitating spreading.
Sialidases are mentioned in this chapter because their activities are strongly influenced by Sia O-acetylation. All sialidases studied in this regard are reduced in their activity by 50–80% in the case of O-acetylation of the Sia glycerol side chain [2, 4, 40, 42]. With the exception of influenza virus sialidase, these enzymes do not release 4-O-acetylated Sia from their glycosidic linkage [43]. The ester groups inhibit sialidases sterically, not competitively or in any other way.
Since Sia are involved in so many biological processes including immunological ones (see the following sections), and since desialylation by autochthonous or microbial sialidases from infections can destroy the manifold functions of glycoconjugates and cells and can lead to premature or early degradation of molecules and cells [44], O-acetylation seems to hinder such destructive processes. This defense mechanism therefore can be considered as part of the organism’s innate immune system. It can be “misused” by over-O-acetylation of tumor cells (see below) or possibly can be beneficially used for the extension of the lifespan of recombinant glycoproteins of pharmaceutical interest. It was shown, for example, that O-acetylation of Sia in rabbit erythrocyte membranes protects the red blood cell from phagocytosis [45]. Esterases counteract this trend and can facilitate the breakdown of sialoglycoconjugates.
O-Acetylation of Sia seems to provide protection from trypanosomal diseases, too. Trypanosoma cruzi in South America and Trypanosoma brucei in Africa, the latter causing sleeping sickness transmitted by tsetse flies, express trans-sialidase, which has both sialidase and transferase activity [46]. Trypanosomes are coated with Sia by direct transfer of Sia from host glycoconjugates to Gal or GalNAc residues forming only α2,3-linkages, which protect the parasites from being well recognized by the host’s immune system. O-Acetylation appreciably hinders this enzymatic transfer [47], thus representing a defense system against trypanosomiasis. Indeed, N’dama cattle expressing O-acetylated Sia on erythrocytes are less prone to trypanosomal infection than Zebu cattle [48].
Sia liberated by sialidase action are transported from the lysosomes to the cytosol and further degraded by Sia-specific lyases yielding pyruvate and acylmannosamine (acetyl- and glycolylmannosamine) [2, 14, 42]. Both Sia and acylmannosamines can be recycled and reused for sialoglycoconjugate biosynthesis in the Golgi. Here again, O-acetyl groups hinder the cleavage of Sia, those at position C-4 of Sia completely and at C-7 or C-9 partially. The physiological significance of this is unknown, and it may be discussed that it promotes the reuse of these Sia, unless cytosolic esterase facilitates their degradation. O-Acetylated Sia can be activated with CMP and transferred by sialyltransferases in the Golgi [1, 2, 49]. In bacteria, the lyase is necessary to use Sia released by sialidase for nutritive purposes [50].
General Biological Roles of O-Acetylated Sia in Mammalian Cells
Interest in O-acetylated Sia is increasing due to the variety of their biological and especially pathophysiological effects. These were summarized, and ample literature was supplied, in the last edition of this series [13]. Accordingly, Sia ester groups increase the hydrophobic properties of glycoconjugates, which may influence the structural and rheological properties of cell membranes and mucins. The influence on the activity of the catabolic enzymes (trans-)sialidases and lyases and their biological implications was discussed above.
A most important effect of Sia O-acetylation is modulation of the ligand function of terminal Sia residues on glycans. Sia become ligands, well investigated with viruses. These either recognize 9-O- or 4-O-acetylated Sia [1, 35, 36, 51], or their binding to Sia is prevented by O-acetylation. The attachment of influenza A and B viruses was reported to be inhibited by 9-O-acetylation of the Sia ligands [52]. Humans are susceptible to all three influenza virus types (A, B, and C) because we can synthesize both 9-O-acetylated and unsubstituted Neu5Ac. In an investigation of one person’s nasal mucin, about 20% Neu5,9Ac2 was found (unpublished data). It appears theoretically possible that a person with a high grade of Sia O-acetylation of the mucin from the respiratory tract is less susceptible to influenza A or B infection. O-Acetylation also inhibits the activity of viral sialidase, which may influence spreading of the virus and thus represents a protective effect against influenza.
Further examples for the specific recognition of O-acetylated Sia are some crustacean proteins and achatinin-H from the snail Achatina fulica [1, 12, 25]. The latter lectin is successfully applied for the study of lymphoblast Sia O-acetylation in ALL [25]. Also, many antibodies are known, the specificity and binding of which require O-acetylated Sia in ganglioside or glycoprotein antigens (see below).
There is evidence that Sia O-acetylation blocks the ligand function of Sia in other systems, not only during binding of influenza A and B viruses. An early observation in this respect was the negative influence of Sia O-acetylation on activation of the complement pathway [53]. Although nothing is known about the effect of esterification on the binding of bacteria to mucins or host cells, O-acetylation of mouse erythrocytes was found to inhibit attachment of the malaria parasite Plasmodium falciparum [54]. Sia recognition in vitro by siglecs 1, 2, and 4 (sialoadhesin, CD22, and myelin-associated glycoprotein) was also found to be impaired by O-acetylation [55]. It appears rewarding to study the biological effects of this Sia modification, for example, whether and how it influences the function of siglecs [1, 56, 57].
Besides the hydrophobicity effect, O-acetyl groups influence the biology of Sia in three predominant ways (1) by delaying the rate of degradation of Sia residues of physiologically relevant glycoconjugate molecules; (2) by creating new Sia ligands; and (3) by masking existing ligand functions.
Thus, Sia-O-acetylation is an important and versatile postglycosylation modification of biomolecules [58], frequently used in cell biology as judged from the astonishingly wide occurrence of esterified Sia in animals and microorganisms. These three effects may be sufficient to explain most of the phenomena, such as the stimulatory effect of O-acetylated Sia on cell growth (summarized in [13]), the increased or decreased antigenicity of glycoconjugates and cells, and their nature as differentiation- and tumor-associated antigens. Inhibition of apoptosis by O-acetylation, a new and very important observation in sialobiology and discussed below, may also belong to this category of effects of Sia modification.
Increasing evidence points to an antioxidative effect of free and bound Sia. While in the case of oxidation of free Neu5Ac with H2O2, a loss of the carboxylic group and the formation of an octonic acid derivative were reported [59], the site of oxidation of mucin-bound Sia is unknown [60]. Tanaka et al. [61] reported desialylation of human low-density lipoproteins by radical reactions, although the mechanism was not explained. Since O-acetylation at C-9 strongly hinders oxidation with mild periodate concentrations of the Sia side chain when compared with the unsubstituted monosaccharide in vitro [18], O-acetylation of this part of the Sia molecule may hinder premature destruction of the cell-protecting Sia layer by reactive oxygen species (ROS) in vivo and thus the development of atherosclerosis. Sia lining the lumen of blood vessels were shown by histological means to be O-acetylated in rats [17, 37, 62] and thus support this assumption. The O-acetyl groups on endothelial cells could also influence receptor interactions. An unusually high concentration of Sia was observed on the surface of vascular endothelia of human, rabbit, and guinea pig, which was decided to be required for the repulsion of blood components [63]. On the other hand, unsubstituted or 4- or 7-O-acetylated Sia may serve as a scavenger of ROS.
Bacteria
Sia O-acetylation was described earlier [64] to increase the virulence of bacteria. In E. coli K1, the polysialic acid capsule (K1 antigen) functions by inhibiting innate immunity [9] and infectiosity. O-Acetylation of this antigen, which is governed by the O-acetyltransferase gene neuO, is expected to strongly influence the host–microbe association and to modulate pathogenicity. In these processes, the relative resistance of colominic acid towards degradation by exosialidases by esterification – as well as reduced binding of cationic antimicrobial peptides to the acidic capsular polysaccharide – may play a role [65]. The binding of bacteria to mucous layers, host cell surfaces, and polysialic acid antibodies could be influenced by the hydrophobic substituents, too.
Special Immunological Implications
Glycobiology has profited from modern technologies making structural and functional analysis of glycoconjugates easier. For example, by means of monoclonal antibodies, carbohydrate structures can now be analyzed in the steric context of intact cell surfaces. Some of these antibodies recognize even subtle differences in carbohydrate structures, e.g., O-acetylation at various positions. Since it became obvious that carbohydrate-specific monoclonal antibodies are not only useful for basic cell biological research but also for clinical use, some of them have been integrated into the cluster of differentiation (CD) system of monoclonal antibodies detecting cell surface molecules of leukocytes and cells involved in immune reactions [66]. Most of these CD antibody-defined carbohydrates can be subsumed under the term “histo-blood group antigens.” Monoclonal antibodies that are able to distinguish between GD3 and its 7- and 9-O-acetylated variants have been designated as CD60a (GD3), CD60b (9-O-acetyl GD3), and CD60c (7-O-acetyl-GD3) [67]. These antibodies became valuable tools for investigating their expression on lymphocytes. Interestingly, some of these structures are also often expressed on tumor cells.
These findings and the recognition that carbohydrate–lectin interactions play a decisive role in many immune reactions have recently led to a closer collaboration between immunologists and glycobiologists, finally giving rise to the field of glycoimmunology, which is gradually finding its way into modern textbooks and Web-based communication platforms (e.g., Consortium for Functional Glycomics: http://www.functionalglycomics.org).
In this section, we focus on the expression and functional impact of O-acetylated carbohydrates on leukocytes and immunity-related cells. Although O-acetylation can occur at various sites of the Sia molecule, from our observations, carbons 7 and 9 are prevalent for this modification in leukocytes. In most studies, it was found that the disialoganglioside GD3 is the major carrier of terminal O-acetylated Sia. Interestingly, GD3 itself has been described as a ganglioside involved in several reactions relevant for the immune system. In earlier in vitro studies, it was shown that gangliosides shed by tumors, such as GD3, inhibit activity of natural killer (NK) cells [68, 69]. In particular, it was speculated that the antitumor cytotoxicity of NK cells was blocked, thereby alleviating undisturbed tumor growth. This idea was later followed up when the new family of Sia-binding lectins, the siglecs, was established. Nicoll et al. [70] described that GD3 expressed on target cells can negatively modulate NK cell cytotoxicity by interacting with siglec-7 on NK cells, which has a preference for binding α2,8-linked disialic acids and seems to be one of the inhibitory NK cell receptors. This observation may have provided a straightforward explanation for a possible protective function of strong GD3 expression in melanoma [71]. However, on the other hand, GD3 expressed on melanoma cells can induce another class of cytotoxic cells, the NKT cells [72]. The fine specificity of GD3-reactive NKT cells is mediated by binding to CD1d, a molecule with a structure like the major histocompatibility antigens on T cells, and with a preference for carbohydrate antigens. This new result has to be compared with the in vivo situation of antimelanoma NK and NKT cell-mediated immunity. Possibly the balance between these two types of cytotoxic cells decides on the outcome of effective immune surveillance in patients with melanoma. Another mechanism to explain the immune escape of melanoma cells may be that GD3 seems able to impair dendritic cell differentiation from monocytes and may induce their apoptosis [73]. Thus, dendritic cells as antigen-presenting cells for efficient T-cell cytotoxicity are missing. Moreover, the GD3-related maturating dendritic cells produced large amounts of the inhibitory cytokine interleukin IL-10 [73]. An open question is how O-acetylation of sialoglycans on tumor cells influences these immune responses. Unfortunately, our knowledge of this aspect is rather scant. Recently, Ravindranath et al. [74] found that, in one patient with melanoma, only metastatic lesions expressed the O-acetylated forms of GD2 and GD3, whereas the primary tumor expressed exclusively the non-O-acetylated gangliosides. In a profound study using a microtiter plate assay with influenza C viruses [71], it was shown that primary melanoma from human skin in most cases expresses elevated levels of 9-O-acetylated GD3, at variable degrees. The same was observed in basalioma, where the expression of this antigen was up to 60-fold higher than in surrounding normal skin [75]. In breast carcinomas, the situation looks somehow more complex. In normal ducts and in benign lesions, 9-O-acetylated sialoglycans were present in Golgi regions and at the plasma membrane as detected by reaction with a CD60b antibody; while in carcinomas of the breast, 9-O-acetylated sialoglycans were distributed in the cytoplasm in a disorderly fashion [76]. Cell surface expression of CD60b structures was only observed in well-differentiated carcinomas, and overall expression decreased with progression of malignancy.
O-Acetylated sialoglycans may also be part of glycoproteins as, for example, in mucins of the colon. Here, sialyl Lewisx (CD15s) moieties of MUC1 and MUC2 were found to be differently O-acetylated [21, 77]. In regards to carcinoma, the O-acetylation decreased from the primary colon carcinomas to their liver metastases, as was observed in mammary carcinomas. Similarly, in colon carcinoma, Sia esterification decreases during increase of malignancy [21]. It may be speculated that O-acetylation of sialyl Lewisx is a negative regulator of the metastasis process, considering that sialyl Lewisx is recognized by selectins of vascular endothelial cells. Thus, the question remains as to whether O-acetylated selectin ligands can inhibit the successful extravasation of tumor cells as a prerequisite for invasion of host tissue.
A function of O-acetylated sialoglycans in the normal intestinal environment may be protection against degradation of mucins by the enteric bacterial flora [78], as described in the metabolism part. Such a protection seems to be most important in human rectum because Sia O-acetylation is highest there [38]. Whether loss of O-acetylation in malignant colon carcinomas is an advantage for tumor progression, with regard to easier accessibility for bacterial sialidases, is still unclear. It has to be considered that, as a rule, primary colon carcinomas are accompanied by severe tissue lesions and inflammation at their borders. It may well be that facilitated degradation of the carbohydrate moiety of colon mucins is one step in this process.
To our knowledge, it has not yet been studied why the submandibular gland of the cow produces such a highly esterified mucin in large amounts, where the ratio of O-acetyl groups to Sia residues is more than one. It is a hypothesis that this phenomenon has to do with the maintenance of bacterial homeostasis in the rumen, where a special microflora is involved in the digestive processes. It is also conceivable that the stability of O-acetylated Sia towards microbial sialidases increases the lifespan and function of the mucin in the bovine digestive tract. That Sia-O-acetylation plays a protective role in bacterial colonization of the rumen can be delineated from the observation that bovine fetal submandibular gland expresses almost no O-acetylated Sia [79]. It should also be considered that high O-acetylation modifies the viscosity, i.e., the rheological properties, of mucus.
The beneficial effects of mucin Sia O-acetylation seem to be a widespread phenomenon. Esterified Sia were also observed in the digestive tract of the striped weakfish Cynoscion guatucupa [80]. Eye mucins also contain such esterified Sia [81].
Human milk, in the colostrum state, has a much higher content of O-acetylated Sia than it does later (unpublished data). Also, the total Sia amount steadily decreases 4–5 times from the beginning until day 100 of lactation. Milk contains a high diversity of glycans that are involved in regulating the physiological colonization of newborns’ intestines with microorganisms [82]. The role of O-acetylated Sia in this process has yet to be elucidated.
Strikingly, chicken erythrocytes are O-acetylated on their surface only after hatching [83]. The function of this is unknown. It could be due to stabilization of the Sia coat on the red blood cells or to a defense mechanism of the chicken during interaction with the microbial environment. Kiehne and Schauer [45] observed a reduced phagocytosis rate in rabbit erythrocytes coated with O-acetylated Sia when compared with de-O-acetylated cells.
It appears rewarding to study all these phenomena, which may represent defense mechanisms and thus can be categorized in a wider sense as part of the innate immune system. Furthermore, studies are necessary to probe O-acetylated Sia for the binding of bacteria, as was already done for viruses to reveal the binding of many virus types to O-acetylated Sia (see above), or to study the effect of de-O-acetylation on binding.
Decrease of O-acetylation may help tumor cells escape from complement-mediated lysis. Recognition of carbohydrates on the cell surface by elements of the alternative complement pathway may be inhibited by O-acetylation [53]. The hindrance of interaction of Sia with siglec O-acetylation was reported above. Whether this possibility is relevant for the in vivo situation needs further clarification. CD22 (siglec-2), for instance, strictly requires α2,6-sialylated lactosamine residues as ligands. To our knowledge, O-acetylation of these carbohydrate compounds has not yet been clearly demonstrated. Generally, siglecs, which are mostly expressed by immune-competent cells, are involved in innate immunity with the potential to trigger apoptosis and to provide inhibitory signals [84]. It is conceivable that these effects can be modulated by Sia modification.
An interesting phenomenon is that patients suffering from certain tumors such as medulloblastoma, a neural tumor disease, carry antibodies of the IgM subtype against O-acetylated GD3 in their serum [85]. Antibodies against O-acetylated sialoglycans were reported for children with ALL [25, 86]. The occurrence of these antibodies may be used for diagnostic purposes. However, it should be noted that in a small percentage of the healthy population, cytotoxic antibodies directed against various carbohydrate structures, preferentially gangliosides, can be found. It is unclear whether these so-called natural autoantibodies are present without prior antigen contact or whether their frequency is increased after contact with microbial structures [87]. It has long been known that after infection with C. jejuni, antiganglioside autoantibodies occur, which may be mediated by molecular mimicry with ganglioside-like oligosaccharides on bacterial lipopolysaccharides [88]. These autoantibodies seem to be involved in Guillain–Barré syndrome, a polyneuropathy of possible autoimmune background.
The existence and frequency of natural antibodies against O-acetylated structures in healthy blood donors need to be determined. Also, the occurrence of antibodies against O-acetylated molecules and autoimmune diseases has not yet been clarified to our knowledge.
Major carriers of surface-expressed 9- and 7-O-acetylated GD3 and related oligosaccharide structures are human T lymphocytes [89, 90]. Originally, 9-O-acetylated sialoglycans on GD3 and possibly also on distinct glycoproteins were described on T lymphocytes of autoimmune lesions [91]. Later, high expression of CD60b (9-O-acetylated GD3) was defined for CD8+ helper T cells [92] after extensively screening expression of both CD60b and CD60c (7-O-acetylated GD3), also on peripheral resting lymphocytes and on mature T lymphocytes in lymph nodes [91; unpublished data]. To a lower extent, tonsillar B lymphocytes also express CD60b and -c [93, 94]. Both CD60b and -c could also be detected on normal and malignant CD34+ hematopoietic precursor cells (unpublished data). Additionally, the occurrence of CD60c was described on monocytes and granulocytes to various extents [90] and on human CD34 hematopoietic progenitor cells derived from bone marrow (unpublished data). The isolation of 7- and 9-O-acetylated GD3 from Molt-4 cells, a T-cell leukemia line, is shown in Fig. 28.5.
Fig. 28.5.

CD60a, -b, and -c antigens in lipid extracts of the T-cell leukemia line Molt-4. The gangliosides were detected by different monoclonal antibodies. Lane A, lipid extract of 5 × 106 Molt-4 cells; lane B, 0.1 μg GD3; lane C, 0.1 μg 7-O-acetyl GD3; lane D, 0.1 μg 9-O-acetyl GD3. The antigens were separated on Si 60 HPTLC plates (Merck) (solvent = chloroform:methanol:water [120:70:17, v/v, including 0.2% CaCl2 (w/v), 40 min]), fixed with polyisobutylmethacrylate, and stained using the antibodies. The mild alkali hydrolysis (0.1 M glycine:NaOH buffer, pH 9.7, 37°C, 30 min) served to induce the migration of the O-acetyl group from the 7 to the 9 position and thus permitted detection by M-T6004
The function of cell surface-expressed CD60b and -c on T and B lymphocytes is not entirely clear. Agonistic effects of CD60b and -c monoclonal antibodies on lymphocytic proliferation are in favor of involvement in activation processes [90, 93, 95]. An interesting observation was that T and B lymphocytes react to stimulation with CD60b and -c antibodies in a different way. In T lymphocytes, CD60c antibodies alone can stimulate proliferation. In B cells, additional signals such as IL-4 and triggering of the B-cell receptor are required. For stimulation with antibodies against CD60b, additional signals are needed in both lymphocyte classes [93]. This differential behavior may have a basis in different surface distribution of the antigens. While CD60b structures are found in dot-like formations, possibly as components of rafts in T and B cells, CD60c on T cells showed a more homogenous distribution on the cell surface [93]. It is most likely that CD60b is a costimulatory signal for raft-concentrated receptors, while CD60c in T cells acts as a mitogen. However, most types of leukocytes express raft-like cell surface structures that contain varying extents of either CD60b or -c or both O-acetylated glycans. When CD34+ precursor cells are pretreated with 9-O-acetyl-specific influenza C esterase, CD60b-specific staining is diminished as expected; however, that of CD60c increases, possibly due to a better accessibility for the CD60c residues after degradation of the CD60b epitope (Table 28.2). This experiment also points to a close neighboring cell surface localization of both O-acetylated CD60 variants.
Table 28.2.
Expression of CD60 epitopes on native CD34+ hematopoietic precursor cells derived from bone marrow with and without enzymatic pretreatment
| CD60 subtype | Antibody | Untreated | VCN treateda | SOAE treatedb | OSGE treatedc |
|---|---|---|---|---|---|
| CD60a | R24 | (+)d | − | (+) | (+) |
| CD60b | UM4D4 | ++ | − | − | ++ |
| CD60c | U5 | ++ | − | +++ | ++ |
Bone marrow cells were obtained from bone marrow aspirates and were isolated as described [93]. Biopsies were taken after having received patients’ informed consent. The study was approved by the ethical board of the University of Heidelberg, Germany. CD34+ cells were identified by a CD34-specific monoclonal antibody in flow cytometry, and analysis of CD60 expression on CD34+ cells was further performed in multicolor flow cytometric analysis. Prior to cell surface staining, live cells were pretreated with enzymes as described below to define structural characteristics and possible protein carriers of the respective CD60 epitopes on cell surface-expressed glycoconjugates
The results shown here are representative for several independent measurements on CD34+ cells of healthy donors as well as of patients suffering from CD34+ acute myeloblastic leukemia (Schwartz-Albiez; unpublished data)
aPretreatment of cells with sialidase from Vibrio cholerae (VCN) as described earlier [93]
bPretreatment of cells with recombinant sialate-9-O-acetylesterase (SOAE) of influenza C virus [96]; this enzyme does not attack 7-O-acetylated Neu5Ac
cPretreatment of cells with O-sialoglycoprotein endopeptidase (OSGE), which degrades sialoglycoproteins of the mucin type, as positive control; successful degradation of surface-expressed CD45 on CD34+ cells was shown in flow cytometry
dFor evaluation of expression, both percentage of positive cells as well as fluorescent intensity per cell were considered
Since gangliosides do not have a transmembrane or intracellular domain, direct signal transduction by gangliosides seems rather unlikely. We rather prefer the “tug boat” hypothesis, namely, distinct glycosphingolipids upon cross-linking by antibodies (or any other molecule capable of binding more than one molecule) contribute to raft formation by pulling together a certain array of receptors.
It has been known for some time that intracellular GD3 is involved in CD95- and ceramide-mediated apoptosis. Upon receptor triggering de novo, increased activity of GD3 synthase by synthesized GD3 accumulates intracellularly. GD3 normally restricted in its presence to the Golgi network and plasma membrane is transferred to mitochondria via endosomal transport [97]. Here, it is found in raft-like mitochondrial membrane domains [98]. The mechanisms of GD3-induced apoptosis can be traced back to a GD3-mediated increase of the production of ROS and a change in the mitochondrial membrane potential [99, 100]. O-Acetylation of GD3 suppresses this proapoptotic function of nonacetylated GD3 and does not have the deleterious effects on mitochondria membranes [101]. Cells that are resistant to overexpression of GD3 convert existing GD3 more readily to 9-O-acetylated GD3 [101]. Also, exogenous O-acetylated GD3 given to cells in vitro is internalized and can prevent apoptosis [102]. It was also observed that an apoptosis-resistant variant of the T-cell leukemia cell line Jurkat transferred GD3 into 9-O-acetyl GD3 [102]. Thus, it is most likely that high intracellular expression of 9-O-acetyl GD3 protects tumor cells from apoptosis. While data have been gathered on the antiapoptotic effect of 9-O-acetyl GD3, no data are available on possible effects of 7-O-acetyl GD3. We have observed that in T- and B-cell lines, 7-O-acetyl followed by 9-O-acetyl GD3 is present in an intracellular pool (unpublished data). Given that intracellular 9-O-acetyl GD3 confers antiapoptotic potential, can this protective effect possibly be accelerated by rapid conversion of 7-O-acetyl into 9-O-acetyl GD3? (It was described above that the primary insertion site of O-acetyls catalyzed by mammalian, including lymphocyte, Sia O-acetyltransferases is at C-7, from where it can isomerize to C-9.) We also have no knowledge of the mechanisms for transporting both acetylated forms of GD3 to the cell surface or of those with regard to the balance between the intracellular pool and cell surface expression of CD60b and -c, and what is decisive for regulation of either antiapoptotic or proliferative effects. First experiments showed that human tonsillar B lymphocytes undergoing either spontaneous or staurosporine-induced apoptosis, in vitro, are characterized by expression of CD60b on their surface, but not expression of CD60c [93]. It may be that O-acetylated gangliosides fulfill different tasks at the cell surface and in intracellular compartments.
Conclusions and Outlook
As discussed, our knowledge concerning the functions and metabolism of O-acetylated sialoglycans is still scant; indeed, there are more open questions than sound data, especially with regard to biology and pathology. Many mechanisms behind the regulation of immune reactions in general, and tumor growth in particular, wait to be unraveled.
Open questions concerning the functional impacts of O-acetylation are listed below:
What are the mechanisms for regulating the intracellular pools of 7- and 9-O-acetylated GD3 and their differential transport to the cell surface, and what determines the balance between the intracellular pool and surface expression of O-acetylated glycans?
Do 7- and 9-O-acetylated gangliosides have different tasks in membranous raft formation? In particular, is the composition of O-acetylated gangliosides in rafts at the lymphocytic cell surface functionally linked to distinct activation stages of lymphocytes?
Can O-acetylated gangliosides regulate NK or NKT activity, especially with regard to lectin receptor binding?
What roles do 7- and 9-O-acetylated gangliosides play on the tumor cell surface, especially in melanoma cells, with regard to tumor defense?
Can O-acetylated gangliosides bind to CD1d or related surface molecules?
Which functions do 7-O-acetylated gangliosides have in apoptosis? Does 7-O-acetylated GD3 have an antiapoptotic effect like 9-O-acetyl GD3, or can it reverse this effect?
How does ligand O-acetylation influence interaction with siglecs and what are the biological consequences?
How strong is the effect of O-acetylation on the lifespan of sialylated molecules, e.g., recombinant glycoproteins?
In what ways does O-acetylation influence cell and tissue growth and differentiation?
What are the gene and protein structures of mammalian SOATs, and how are they organized in subcellular compartments?
How is their expression regulated?
Which substrate specificities do these enzymes from different origins exhibit, and is there an isomerase (“migrase”) involved?
How do O-acetyltransferases and esterases cooperate in various tissues at development states?
In which way and to what extent can Sia O-acetylation influence colonization and pathogenicity of bacteria?
In this regard, what is the role of O-acetyl groups on mammalian cells or secreted mucins?
O-Acetylated Sia have always appeared as enigmatic molecules in glycobiology. They were treated as Cinderellas because of their lability and because of the difficulties of purifying enzyme protein and elucidating molecular biology. As these obstacles are being overcome through the use of better techniques, it will become easier and more rewarding to study this kind of Sia modification to benefit our understanding of cell biology and pathology.
Contributor Information
Albert M. Wu, Phone: 3211-8966, FAX: 3211-8456, Email: amwu@mail.cgu.edu.tw
Roland Schauer, Email: schauer@biochem.uni-kiel.de.
References
- 1.Angata T, Varki A. Chemical diversity in the sialic acids and related α-keto acids: an evolutionary perspective. Chem Rev. 2002;102:439–469. doi: 10.1021/cr000407m. [DOI] [PubMed] [Google Scholar]
- 2.Schauer R, Kamerling JP. Chemistry, biochemistry and biology of sialic acids. In: Montreuil J, Vliegenthart JFG, Schachter H, editors. Glycoproteins II. Amsterdam: Elsevier; 1997. pp. 243–402. [Google Scholar]
- 3.Corfield AP, Ferreira do Amaral C, Wember M, Schauer R. The metabolism of O-acyl-N-acylneuraminic acids. Biosynthesis of O-acylated sialic acids in bovine and equine submandibular glands. Eur J Biochem. 1976;68:597–610. doi: 10.1111/j.1432-1033.1976.tb10848.x. [DOI] [PubMed] [Google Scholar]
- 4.Lrhorfi LA, GSrinivasan GV, Schauer R. Properties and partial purification of sialate-O-acetyltransferase from bovine submandibular glands. Biol Chem. 2007;388:297–306. doi: 10.1515/BC.2007.033. [DOI] [PubMed] [Google Scholar]
- 5.Claus H, Borrow R, Achtmann M, Morelli G, Kantelberg C, Longworth E, Frosch M, Vogel U. Genetics of capsule O-acetylation in serogroup C, W-135 and Y. meningococci. Mol Microbiol. 2004;51:227–239. doi: 10.1046/j.1365-2958.2003.03819.x. [DOI] [PubMed] [Google Scholar]
- 6.Deszo EL, Steenbergen SM, Freedberg DI, Vimr ER. Escherichia coli K1 polysialic acid O-acetyltransferase gene, neuO, and the mechanism of capsule form variation involving a mobile contingency locus. Proc Natl Acad Sci USA. 2005;102:5564–5569. doi: 10.1073/pnas.0407428102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steenbergen SM, Lee YC, Vann WF, Vionett J, Wright LF, Vimr ER. Separate pathways for O acetylation of polymeric and monomeric sialic acids and identification of sialyl O-acetyl esterase in Escherichia coli K1. J Bacteriol. 2006;188:6195–6206. doi: 10.1128/JB.00466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis AL, Hensler ME, Varki A, Nizet V. The group B streptococcal sialic acid O-acetyltransferase is encoded by neuD, a conserved component of bacterial sialic acid biosynthetic gene clusters. J Biol Chem. 2006;281:11186–11192. doi: 10.1074/jbc.M513772200. [DOI] [PubMed] [Google Scholar]
- 9.King MR, Vimr RP, Steenbergen SM, Spanjaard L, Plunkett G, III, Blattner FR, Vimr ER. Escherichia coli K1-specific bacteriophage CUS-3 distribution and function in phase-variable capsular polysialic acid O acetylation. J Bacteriol. 2007;189:6447–6456. doi: 10.1128/JB.00657-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiralongo J, Schauer R. The enigma of enzymatic sialic acid O-acetylation. Trends Glycosci Glycotechnol. 2004;16:1–15. [Google Scholar]
- 11.Schauer R. Victor Ginsburg’s influence on my research of the role of sialic acids in biological recognition. Arch Biochem Biophys. 2004;426:132–141. doi: 10.1016/j.abb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Schauer R. The diversity of sialic acids and their interplay with lectins. In: Sansom C, Markman O, editors. Glycobiology. Bloxham: Scion Publishing Ltd; 2007. pp. 136–149. [Google Scholar]
- 13.Schauer R, Schmid H, Pommerencke J, Iwersen M, Kohla G. Metabolism and role of O-acetylated sialic acids. In: Wu AM, editor. The molecular immunology of complex carbohydrates-2. New York: Kluwer Academic/Plenum; 2001. [DOI] [PubMed] [Google Scholar]
- 14.Reuter G, Schauer R. Determination of sialic acids. Methods Enzymol. 1994;230:168–199. doi: 10.1016/0076-6879(94)30012-7. [DOI] [PubMed] [Google Scholar]
- 15.Schauer R, Shukla AK. Isolation and properties of two sialate-O-acetylesterases from horse liver with 4- and 9-O-acetyl specificities. Glycoconj J. 2008;24:625–632. doi: 10.1007/s10719-008-9109-9. [DOI] [PubMed] [Google Scholar]
- 16.Kamerling JP, Vliegenthart JFG, Versluis C, Schauer R. Identification of O-acetylated N-acylneuraminic acids by mass spectrometry. Carbohydr Res. 1975;41:7–17. doi: 10.1016/S0008-6215(00)87002-0. [DOI] [PubMed] [Google Scholar]
- 17.Varki NM, Varki A. Diversity in cell surface sialic acid presentations: implications for biology and disease. Lab Invest. 2007;87:851–857. doi: 10.1038/labinvest.3700656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schauer R, Faillard H. Zur Wirkungsspezifität der Neuraminidase. Das Verhalten isomerer N, O-Diacetyl-neuraminsäureglykoside im Submaxillarismucin von Pferd und Rind bei Einwirkung bakterieller Neuraminidase. Hoppe-Seyler’s Z Physiol Chem. 1968;349:961–968. doi: 10.1515/bchm2.1968.349.2.961. [DOI] [PubMed] [Google Scholar]
- 19.Reuter G, Pfeil R, Stoll S, Schauer R, Kamerling JP, Versluis C, Vliegenthart JFG. Identi-fication of new sialic acids derived from glycoprotein of bovine submandibular gland. Eur J Biochem. 1983;134:139–143. doi: 10.1111/j.1432-1033.1983.tb07542.x. [DOI] [PubMed] [Google Scholar]
- 20.Corfield T. Bacterial sialidases – roles in pathogenicity and nutrition. Glycobiology. 1992;2:509–521. doi: 10.1093/glycob/2.6.509. [DOI] [PubMed] [Google Scholar]
- 21.Corfield AP, Myerscough N, Warren BF, Durdey P, Paraskeva C, Schauer R. Reduction of sialic acid O-acetylation in human colonic mucins in the adenoma-carcinoma sequence. Glycoconj J. 1999;16:307–317. doi: 10.1023/A:1007026314792. [DOI] [PubMed] [Google Scholar]
- 22.Shen Y, Kohla G, Lrhorfi AL, Sipos B, Kalthoff H, Gerwig GJ, Kamerling JP, Schauer R, Tiralongo J. O-Acetylation and de-O-acetylation of sialic acids in human colorectal carcinoma. Eur J Biochem. 2004;271:281–290. doi: 10.1046/j.1432-1033.2003.03927.x. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan GV, Schauer R. Assays of sialate O-acetyltransferases and sialate esterases. Glycoconj J. 2009;26:935–944. doi: 10.1007/s10719-008-9131-y. [DOI] [PubMed] [Google Scholar]
- 24.Shen Y, Tiralongo J, Iwersen M, Sipos B, Kalthoff H, Schauer R. Characterization of the sialate-7(9)-O-acetyltransferase from the microsomes of human colonic mucosa. Biol Chem. 2002;383:307–317. doi: 10.1515/BC.2002.033. [DOI] [PubMed] [Google Scholar]
- 25.Mandal C, Srinivasan GV, Chowdhury S, Chandra S, Mandal C, Schauer R, Mandal C. High level of sialate-O-acetyltransferase activity in lymphoblasts of childhood acute lymphoblastic leukaemia (ALL): enzyme characterization and correlation with disease status. Glycoconj J. 2009;26:57–73. doi: 10.1007/s10719-008-9163-3. [DOI] [PubMed] [Google Scholar]
- 26.Manzi AE, Sjoberg ER, Diaz S, Varki A. Biosynthesis and turnover of O-acetyl and N-acetyl groups in the gangliosides of human melanoma cells. J Biol Chem. 1990;265:13091–13103. [PubMed] [Google Scholar]
- 27.Higa HH, Butor C, Diaz S, Varki A. O-acetylation and de-O-acetylation of sialic acids. O-acetylation of sialic acids in the rat liver Golgi apparatus involves an acetyl intermediate and essential histidine and lysine residues – a transmembrane reaction? J Biol Chem. 1989;264:19427–19434. [PubMed] [Google Scholar]
- 28.Diaz S, Higa HH, Hayes BK, Varki A. O-Acetylation and de-O-acetylation of sialic acids. J Biol Chem. 1989;264:19416–19426. [PubMed] [Google Scholar]
- 29.Iwersen M, Dora H, Kohla G, Gasa S, Schauer R. Solubilisation and properties of the sialate-4-O-acetyl-transferase from guinea pig liver. Biol Chem. 2003;384:1035–1047. doi: 10.1515/BC.2003.116. [DOI] [PubMed] [Google Scholar]
- 30.Houliston RS, Endtz HP, Yuki N, Li J, Jarrell HC, Koga M, van Belkum A, Karwaski MF, Wakarchuk WW, Gilbert M. Identification of a sialate-O-acetyltransferase from Campylobacter jejuni: demonstration of direct transfer to the C9 position of terminal α-2, 8-linked sialic acid. J Biol Chem. 2006;281:11480–11486. doi: 10.1074/jbc.M512183200. [DOI] [PubMed] [Google Scholar]
- 31.Bergfeld AK, Claus H, Vogel U, Mühlenhoff M. Biochemical characterization of the polysialic acid-specific O-acetyltransferase NeuO of Escherichia coli K1. J Biol Chem. 2007;282:22217–22227. doi: 10.1074/jbc.M703044200. [DOI] [PubMed] [Google Scholar]
- 32.Schauer R, Casals-Stenzel J, Corfield AP, Veh RW. Subcellular site of the biosynthesis of O-acetylated sialic acids in bovine submandibular gland. Glycoconj J. 1988;5:257–270. doi: 10.1007/BF01049086. [DOI] [Google Scholar]
- 33.Kamerling JP, Schauer R, Shukla AK, Stoll S, van Halbeek H, Vliegenthart JFG. Migration of O-acetyl groups in N, O-acetylneuraminic acids. Eur J Biochem. 1987;162:601–607. doi: 10.1111/j.1432-1033.1987.tb10681.x. [DOI] [PubMed] [Google Scholar]
- 34.Schauer R, Reuter G, Stoll S. Sialate O-acetylesterases – key enzymes in sialic acid catabolism. Biochimie. 1988;70:1511–1519. doi: 10.1016/0300-9084(88)90288-X. [DOI] [PubMed] [Google Scholar]
- 35.Smits SL, Gerwig GJ, van Vliet ALW, Lissenberg A, Briza P, Kamerling JP, Vlasak R, de Groot RJ. Nidovirus sialate-O-acetylesterases. Evolution and substrate specificity of coronaviral and toroviral receptor-destroying enzymes. J Biol Chem. 2005;280:6933–6941. doi: 10.1074/jbc.M409683200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrler G, Rott R, Klenk HD, Müller HP, Shukla AK, Schauer R. The receptor-destroying enzyme of influenza C virus is neuraminate-O-acetylesterase. EMBO J. 1985;4:1503–1506. doi: 10.1002/j.1460-2075.1985.tb03809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harms G, Reuter G, Corfield AP, Schauer R. Binding specificity of influenza C-virus to variably O-acetylated glycoconjugates and its use for histochemical detection of N-acetyl-9-O-acetylneuraminic acid in mammalian tissues. Glycoconj J. 1996;13:621–630. doi: 10.1007/BF00731450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robbe C, Capon C, Maes E, Rousset M, Zweibaum A, Zanetta JP, Michalski JC. Evidence of regio-specific glycosylation in human intestinal mucins. J Biol Chem. 2003;278:46337–46368. doi: 10.1074/jbc.M302529200. [DOI] [PubMed] [Google Scholar]
- 39.Miyagi T, Wada T, Yamaguchi K, Hata K. Sialidase and malignancy: a mini review. Glycoconj J. 2004;20:189–198. doi: 10.1023/B:GLYC.0000024250.48506.bf. [DOI] [PubMed] [Google Scholar]
- 40.Corfield AP, Michalski JC, Schauer R. The substrate specificity of sialidases from micro-organisms and mammals. In: Tettamanti G, Durand P, Di Donato S, editors. Sialidases and sialidoses, perspectives in inherited metabolic diseases. Milan: Edi Ermes; 1981. pp. 3–70. [Google Scholar]
- 41.Roggentin P, Schauer R, Hoyer LL, Vimr ER. The sialidase superfamily and its spread by horizontal gene transfer. Mol Microbiol. 1993;9:915–921. doi: 10.1111/j.1365-2958.1993.tb01221.x. [DOI] [PubMed] [Google Scholar]
- 42.Schauer R. Sialic acids: fascinating sugars in higher animals and man. Zoology. 2004;107:49–64. doi: 10.1016/j.zool.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Kleineidam RG, Furuhata K, Ogura H, Schauer R. 4-Methylumbelliferyl-α-glycosides of partially O-acetylated N-acetylneuraminic acids as substrates of bacterial and viral sialidases. Biol Chem Hoppe-Seyler. 1990;371:715–719. doi: 10.1515/bchm3.1990.371.2.715. [DOI] [PubMed] [Google Scholar]
- 44.Schauer R. Sialic acids and their roles as biological masks. Trends Biochem Sci. 1985;10:357–360. doi: 10.1016/0968-0004(85)90112-4. [DOI] [Google Scholar]
- 45.Kiehne K, Schauer R. The influence of α- and β-galactose residues and sialic acid O-acetyl groups of rat erythrocytes on the interaction with peritoneal macrophages. Biol Chem Hoppe-Seyler. 1992;373:1117–1123. doi: 10.1515/bchm3.1992.373.2.1117. [DOI] [PubMed] [Google Scholar]
- 46.Engstler M, Schauer R, Brun R. Distribution and developmentally regulated trans-sialidases in the Kineto-plastida and characterization of a shed trans-sialidase activity from procyclic Trypanosoma congolense. Acta Trop. 1995;59:117–129. doi: 10.1016/0001-706X(95)00077-R. [DOI] [PubMed] [Google Scholar]
- 47.Engstler M, Reuter G, Schauer R. Purification and characterization of a novel sialidase in procyclic culture forms of Trypanosoma brucei. Mol Biochem Parasitol. 1992;54:21–30. doi: 10.1016/0166-6851(92)90091-W. [DOI] [PubMed] [Google Scholar]
- 48.Shugaba A, Umar I, Omage J, Ibrahim ND, Andrews J, Ukoha AI, Saror DI, Esievo KA. Biochemical differences (O-acetyl and glycolyl groups) in erythrocyte surface sialic acids trypanotolerant N’dama and trypano-susceptible Zebu cattle. J Comp Pathol. 1994;110:91–95. doi: 10.1016/S0021-9975(08)80273-3. [DOI] [PubMed] [Google Scholar]
- 49.Harduin-Lepers A, Mollicone R, Delannoy P, Oriol R. The animal sialyltransferases and sialyltransferase-related genes: a phylogenetic approach. Glycobiology. 2005;15:805–817. doi: 10.1093/glycob/cwi063. [DOI] [PubMed] [Google Scholar]
- 50.Severi E, Hood DW, Thomas GH. Sialic acid utilization by bacterial pathogens. Microbiology. 2007;153:2817–2822. doi: 10.1099/mic.0.2007/009480-0. [DOI] [PubMed] [Google Scholar]
- 51.Rinninger A, Richet C, Pons A, Kohla G, Schauer R, Bauer HC, Zanetta JP, Vlasak R. Localisation and distribution of O-acetylated N-acetylneuraminic acids, the endogenous substrates of the hemagglutinin-esterase of murine coronaviruses, in mouse tissue. Glycoconj J. 2006;23:73–84. doi: 10.1007/s10719-006-5439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Higa HH, Rogers GN, Paulson JC. Influenza virus hemagglutinins differentiate between receptor deter-minants bearing N-acetyl-, N-glycolyl-, and N, O-diacetylneuraminic acids. Virology. 1985;144:279–282. doi: 10.1016/0042-6822(85)90325-3. [DOI] [PubMed] [Google Scholar]
- 53.Shi WX, Chammas R, Varki NM, Powell L, Varki A. Sialic acid 9-O-acetylation on murine erythroleukemia cells affects complement activation, binding to I-type lectins, and tissue homing. J Biol Chem. 1996;271:31526–31532. doi: 10.1074/jbc.271.49.31526. [DOI] [PubMed] [Google Scholar]
- 54.Klotz FW, Orlandi PA, Reuter G, Cohen SJ, Haynes JD, Schauer R, Howard RJ, Palese P, Miller LH. Binding of Plasmodium falciparum 175 kilodalton erythrocyte binding antigen and invasion of murine erythrocytes requires N-acetylneuraminic acid but not its O-acetylated form. Mol Biochem Parasitol. 1992;51:49–54. doi: 10.1016/0166-6851(92)90199-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelm S, Schauer R. Sialic acids in molecular and cellular interactions. Int Rev Cytol. 1997;175:137–240. doi: 10.1016/S0074-7696(08)62127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crocker PR. Siglecs in innate immunity. Curr Opin Pharmacol. 2005;5:1–7. doi: 10.1016/j.coph.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Varki A, Angata T. Siglecs – the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 58.Yu H, Chen X. Carbohydrate post-glycosylational modifications. Org Biomol Chem. 2007;5:865–872. doi: 10.1039/b700034k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iijima R, Takahashi H, Ikegami S, Yamazaki M. Characterization of the reaction between sialic acid (N-acetylneuraminic acid) and hydrogen peroxide. Biol Pharm Bull. 2007;30:580–582. doi: 10.1248/bpb.30.580. [DOI] [PubMed] [Google Scholar]
- 60.Ogasawara Y, Namai T, Yoshino F, Lee MCI, Ishii K. Sialic acid is an essential moiety of mucin as a hydroxyl radical scavenger. FEBS Lett. 2007;581:2473–2477. doi: 10.1016/j.febslet.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 61.Tanaka K, Tokumaru S, Kojo S. Possible involvement of radical reactions in desialylation of LDL. FEBS Lett. 1997;413:202–204. doi: 10.1016/S0014-5793(97)00917-4. [DOI] [PubMed] [Google Scholar]
- 62.Klein A, Krishna M, Varki NM, Varki A. 9-O-Acetyl-sialic acids have widespread but selective expression: analysis using a chimeric dual-function probe derived from influenza C hemagglutinin-esterase. Proc Natl Acad Sci USA. 1994;91:7782–7786. doi: 10.1073/pnas.91.16.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Born GVR, Palinski W. Unusually high concentrations of sialic acids on the surface of vascular endothelia. Br J Exp Pathol. 1985;66:543–549. [PMC free article] [PubMed] [Google Scholar]
- 64.Ørskov F, Ørskov I, Sutton A, Schneerson R, Lin W, Egan W, Hoff GE, Robbins JB. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J Exp Med. 1979;149:669–685. doi: 10.1084/jem.149.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.King MR, Steenbergen SM, Vimr ER. Going for baroque at the Escherichia coli K1 cell surface. Trends Microbiol. 2007;15:196–202. doi: 10.1016/j.tim.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz-Albiez R, et al. Carbohydrates and lectins. In: Mason D, et al., editors. Leucocyte typing VII. Oxford: Oxford University Press; 2002. pp. 149–164. [Google Scholar]
- 67.Schwartz-Albiez R, Claus C, Kniep B, et al. CD60 Workshop report. In: Mason D, et al., editors. Leucocyte typing VII. Oxford: Oxford University Press; 2002. pp. 185–188. [Google Scholar]
- 68.Bergelson LD, Dyatlovitskaya EV, Klyuchareva TE, Kryukova EV, Lemenovskaya AF, Matveeva VA, Sinitsyna EV. The role of glycosphingolipids in natural immunity: gangliosides modulate the cytotoxicity of natural killer cells. Eur J Immunol. 1989;19:1979–1983. doi: 10.1002/eji.1830191102. [DOI] [PubMed] [Google Scholar]
- 69.Grayson G, Ladisch S. Immunosuppression by human gangliosides II. Carbohydrate structure and inhibition of human NK activity. Cell Immunol. 1992;139:18–29. doi: 10.1016/0008-8749(92)90096-8. [DOI] [PubMed] [Google Scholar]
- 70.Nicoll G, Avril T, Lock K, Furukawa K, Bovin N, Crocker PR. Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via siglec-7-dependent and -independent mechanisms. Eur J Immunol. 2003;33:1642–1648. doi: 10.1002/eji.200323693. [DOI] [PubMed] [Google Scholar]
- 71.Hubl U, Ishida H, Kiso M, Hasegawa A, Schauer R. Studies on the specificity and sensitivity of the influenza C virus binding assay for O-acetylated sialic acids and its application to human melanomas. J Biochem. 2000;127:1021–1031. doi: 10.1093/oxfordjournals.jbchem.a022693. [DOI] [PubMed] [Google Scholar]
- 72.Park JE, Wu DY, Prednes M, Lu SX, Ragupathi G, Schrantz N, Chapman PB. Fine specificity of natural killer T cells against GD3 ganglioside and identification of GM3 as an inhibitory natural killer T-cell ligand. Immunology. 2008;123:145–155. doi: 10.1111/j.1365-2567.2007.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Péquet-Navarro J, Portouch M, Popa I, Berthier O, Schmitt D, Portoukalian J. Gangliosides from human melanoma tumors impair dendritic cell differentiation from monocytes and induce their apoptosis. J Immunol. 2003;170:3488–3494. doi: 10.4049/jimmunol.170.7.3488. [DOI] [PubMed] [Google Scholar]
- 74.Ravindranath MH, Muthugounder S, Presser N. Ganglioside signatures of primary and nodal metastatic melanoma cell lines from the same patient. Melanoma Res. 2008;18:47–55. doi: 10.1097/CMR.0b013e3282f43acf. [DOI] [PubMed] [Google Scholar]
- 75.Fahr C, Schauer R. Detection of sialic acids and gangliosides with special reference to 9-O-acetylated species in basaliomas and normal human skin. J Invest Dermatol. 2001;116:254–260. doi: 10.1046/j.1523-1747.2001.01237.x. [DOI] [PubMed] [Google Scholar]
- 76.Gocht A, Rutter G, Kniep B. Changed expression of 9-O-acetyl GD3 (CDw60) in benign and atypical pro-liferative lesions and carcinomas of the human breast. Histochem Cell Biol. 1998;110:217–229. doi: 10.1007/s004180050284. [DOI] [PubMed] [Google Scholar]
- 77.Mann B, Klussmann E, Vandamme-Feldhaus V, Iwersen M, Hanski ML, Riecken EO, Buhr HJ, Schauer R, Kim YS, Hanski C. Low O-acetylation of sialyl-Lex contributes to its overexpression in colon carcinoma metastases. Int J Cancer. 1997;72:258–264. doi: 10.1002/(SICI)1097-0215(19970717)72:2<258::AID-IJC10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 78.Corfield AP, Wagner SA, Clamp JR, Kriaris MS, Hoskins LC. Mucin degradation in the human colon: production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect Immun. 1992;60:3971–3978. doi: 10.1128/iai.60.10.3971-3978.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schauer R, Stoll S, Reuter G. Differences in the amount of N-acetyl- and N-glycoloyl-neuraminic acids, as well as O-acylated sialic acids, of fetal and adult bovine tissues. Carbohydr Res. 1991;213:353–359. doi: 10.1016/S0008-6215(00)90623-2. [DOI] [PubMed] [Google Scholar]
- 80.Díaz AO, García AM, Goldemberg AL. Glycoconjugates in the mucosa of the digestive tract of Cynoscion guatucupa: a histochemical study. Acta Histochem. 2008;110:76–85. doi: 10.1016/j.acthis.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 81.Corfield AP, Donapaty SR, Carrington SD, Hicks SJ, Schauer R, Kohla G. Identification of 9-O-acetyl-N-acetylneuraminic acid in normal canine pre-ocular tear film secreted mucins and its depletion in Keratoconjunctivitis sicca. Glycoconj J. 2005;22:409–416. doi: 10.1007/s10719-005-3698-3. [DOI] [PubMed] [Google Scholar]
- 82.Boehm G, Stahl B. Oligosaccharides from milk. J Nutr. 2007;137:847S–849S. doi: 10.1093/jn/137.3.847S. [DOI] [PubMed] [Google Scholar]
- 83.Herrler G, Reuter G, Rott R, Klenk HD, Schauer R. N-Acetyl-9-O-acetylneuraminic acid, the receptor determinant for influenza C virus, is a differentiation marker on chicken erythrocytes. Biol Chem Hoppe-Seyler. 1987;368:451–454. doi: 10.1515/bchm3.1987.368.1.451. [DOI] [PubMed] [Google Scholar]
- 84.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 85.Ariga T, Suetake K, Nakane M, Kubota M, Usuki S, Kawashima I, Yu RK. Glycosphingolipid antigens in neural tumor cell lines and anti-glycosphingolipid antibodies in sera of patients with neural tumors. Neurosignals. 2008;16:226–234. doi: 10.1159/000111565. [DOI] [PubMed] [Google Scholar]
- 86.Pal S, Chatterjee M, Bhattacharya DK, Bandhyopadhyay S, Mandal C. Identification and purification of cytolytic antibodies directed against O-acetylated sialic acid in childhood acute lymphoblastic leukemia. Glycobiology. 2000;10:539–549. doi: 10.1093/glycob/10.6.539. [DOI] [PubMed] [Google Scholar]
- 87.Schwartz-Albiez R, Laban S, Eichmüller S, Kirschfink M. Cytotoxic natural antibodies against human tumours: an option for anti-cancer immunotherapy? Autoimmun Rev. 2008;7:491–495. doi: 10.1016/j.autrev.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 88.Bowes T, Wagner ER, Boffey J, Nicholl D, Cochrane L, Benboubetra M, Conner J, Furukawa K, Furukawa K, Willison HJ. Tolerance to self gangliosides is the major factor restricting the antibody response to lipopolysaccharide core oligosaccharides in Campylobacter jejuni strains associated with Guillain–Barré syndrome. Infect Immun. 2002;70:5008–5018. doi: 10.1128/IAI.70.9.5008-5018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kniep B, Flegel WA, Northoff H, Rieber EP. CDw60 glycolipid antigens of human leukocytes: structural characterization and cellular distribution. Blood. 1993;82:1776–1786. [PubMed] [Google Scholar]
- 90.Kniep B, Claus C, Peter-Katalinić J, Monner DA, Dippold W, Nimtz M. 7-O-acetyl-GD3 in human T-lymphocytes is detected by a specific T-cell-activating monoclonal antibody. J Biol Chem. 1995;270:30173–30180. doi: 10.1074/jbc.270.50.30173. [DOI] [PubMed] [Google Scholar]
- 91.Fox DA, He X, Abe A, Hollander T, Li LL, Kan L, Friedman AW, Shimizu Y, Shayman JA, Kozarsky K. The T lymphocyte structure CD60 contains a sialylated carbohydrate epitope that is expressed on both gangliosides and glycoproteins. Immunol Invest. 2001;30:67–85. doi: 10.1081/IMM-100104017. [DOI] [PubMed] [Google Scholar]
- 92.Rieber EP, Rank G. CDw60: a marker for human CD8+ T helper cells. J Exp Med. 1994;179:1385–1390. doi: 10.1084/jem.179.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Erdmann M, Wipfler D, Merling A, Cao Y, Claus C, Kniep B, Sadick H, Bergler W, Vlasak R, Schwartz-Albiez R. Differential surface expression and possible function of 9-O- and 7-O-acetylated GD3 (CD60 b and c) during activation and apoptosis of human tonsillar B and T lymphocytes. Glycoconj J. 2006;23:627–638. doi: 10.1007/s10719-006-9000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vater M, Kniep B, Gross HJ, Claus C, Dippold W, Schwartz-Albiez R. The 9-O-acetylated disialosyl carbohydrate sequence of CDw60 is a marker on activated human B lymphocytes. Immunol Lett. 1997;59:151–157. doi: 10.1016/S0165-2478(97)00116-8. [DOI] [PubMed] [Google Scholar]
- 95.Claus C, Schlaak J, Dittmayer M, Meyer zum Buschenfeld K, Dippold W. Inhibition of anti-GD3-ganglioside antibody-induced proliferation of human CD8+ T cells by CD16+ natural killer cells. Eur J Immunol. 1994;24:1208–1212. doi: 10.1002/eji.1830240530. [DOI] [PubMed] [Google Scholar]
- 96.Strasser P, Unger U, Strobl B, Vilas U, Vlasak R. Recombinant viral sialate-O-acetylesterases. Glycoconj J. 2004;20:551–561. doi: 10.1023/B:GLYC.0000043292.64358.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.García-Ruiz C, Colell A, Morales A, Calvo M, Enrich C, Fernández-Checa JC. Trafficking of ganglioside GD3 to mitochondria by tumor necrosis factor-alpha. J Biol Chem. 2002;277:36443–36448. doi: 10.1074/jbc.M206021200. [DOI] [PubMed] [Google Scholar]
- 98.Garofalo T, Tinari A, Matarrese P, Giammarioli AM, Manganelli V, Ciarlo L, Misasi R, Sorice M, Malorni W. Do mitochondria act as “cargo boats” in the journey of GD3 to the nucleus during apoptosis? FEBS Lett. 2007;581:3899–3903. doi: 10.1016/j.febslet.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 99.Rippo MR, Malisan F, Ravagnan L, Tomassini B, Condo I, Costantini P, Susin SA, Rufini A, Todaro M, Kroemer G, Testi R. GD3 ganglioside directly targets mitochondria in a bc1-2-controlled fashion. FASEB J. 2000;14:2047–2054. doi: 10.1096/fj.99-1028com. [DOI] [PubMed] [Google Scholar]
- 100.García-Ruiz C, Colell A, París R, Fernández-Checa JC. Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation. FASEB J. 2000;14:847–858. doi: 10.1096/fasebj.14.7.847. [DOI] [PubMed] [Google Scholar]
- 101.Malisan F, Franchi L, Tomassini B, Ventura N, Condo I, Rippo MR, Rufini A, Liberati L, Nachtigall C, Kniep B, Testi R. Acetylation supresses the proapoptotic activity of GD3 ganglioside. J Exp Med. 2002;196:1535–1541. doi: 10.1084/jem.20020960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kniep B, Kniep E, Ozkucur N, Barz S, Bachmann M, Malisan F, Testi R, Rieber EP. 9-O-acetyl GD3 protects tumor cells from apoptosis. Int J Cancer. 2006;119:67–73. doi: 10.1002/ijc.21788. [DOI] [PubMed] [Google Scholar]


