Abstract
The mitochondrion descends from a bacterium that, about two billion years ago, became endosymbiotic. This organelle represents a Pandora’s box whose opening triggers cytochrome-c release and apoptosis of cells from multicellular animals, which evolved much later, about six hundred million years ago. BCL-2 proteins, which are critical apoptosis regulators, were recruited at a certain time point in evolution to either lock or unlock this mitochondrial Pandora’s box. Hence, particularly intriguing is the issue of when and how the “BCL-2 proteins–mitochondria–apoptosis” triptych emerged. This chapter explains what it takes from an evolutionary perspective to evolve a BCL-2-regulated apoptotic pathway, by focusing on the events occurring upstream of mitochondria.
Keywords: Cell death, Apoptosis, BCL-2, BH3, Mitochondria
Introduction
It is in the form of cells that life has continued over generations for billions of years. Most of the time, these building blocks of life are defined as self-replicating elements, overlooking the fact that cells endowed with the ability to self-destruct were described in all branches of the tree of life (Bozhkov and Lam 2011; Dwyer and Winkler 2013; Kerr et al. 1972; Madeo et al. 1997). In multicellular animals (metazoans), organismal success and complexity are built upon the silent destruction and rapid removal of cells through a genetically encoded cell death process called apoptosis. This form of active (or programmed) cell death functions to sculpt shapes, optimize functions and eliminate damaged, superfluous, or harmful cells from the body, thus playing crucial roles in animal development and homeostasis. Adding to its importance as a physiological phenomenon, apoptosis dysregulation is involved in a wide range of diseases such as cancer (Czabotar et al. 2014; Elmore 2007). Studies on vertebrate cells have revealed the existence of two major apoptotic pathways: the extrinsic pathway initiated by the ligation of death receptors by extracellular ligands at the surface of target cells and the intrinsic (mitochondrial or BCL-2-regulated) pathway, which can be stimulated by a plethora of signals (e.g., DNA damage, endoplasmic reticulum stress, hypoxia, growth factor deprivation, and developmental cues) (Czabotar et al. 2014; Tait and Green 2013).
The BCL-2-regulated apoptotic pathway is initiated through transcriptional and/or post-transcriptional activation of so-called BH3-only proteins, which form a disparate group of proteins traditionally considered as sensors of cellular stress and damage (Doerflinger et al. 2015; Shamas-Din et al. 2011). In response to distinct upstream signaling events, some of these death effectors (i.e., BIM, PUMA, tBID) serve as ligands to activate the pro-apoptotic BCL-2 family members BAX and BAK through direct interaction, while all of them can activate BAX/BAK indirectly by binding to and inhibiting the prosurvival BCL-2 homologous proteins. Once activated through a complex multi-step process, BAX and BAK are thought to homo-oligomerize and form (or participate to) pores in the mitochondrial outer membrane (Tait and Green 2013; Volkmann et al. 2014; Westphal et al. 2014). These oligomeric pores cause the release of mitochondrial intermembrane space proteins, including cytochrome-c (cyt-c), in the cytosol (in a process termed MOMP, for mitochondrial outer membrane permeabilization). Leaked cyt-c then triggers the activation of a family of death proteases called caspases through a well-defined post-mitochondrial pathway (which will not be reviewed here). Prosurvival BCL-2 proteins can inhibit BAX-BAK activity through one or more possible mechanisms: sequestration of the “direct activator” BH3-only proteins (Llambi et al. 2011) or local inhibition of BAX (and BAK) at the mitochondrial outer membrane level (via inhibitory complex formation, oligomer disassembly, and/or retrotranslocation to the cytosol) (Billen et al. 2008a; Edlich et al. 2011; Subburaj et al. 2015). The “sensitizer” BH3-only proteins can neutralize the prosurvival BCL-2 proteins through direct binding, thus releasing the direct activators to promote BAX-BAK activation and apoptosis. An important concept that emerges from this mechanistic description is that the BCL-2-regulated pathway appears to be organized in a hierarchical manner, from cellular sentinels (BH3-only proteins) to the BCL-2/BAX apoptotic switch controlling cytosolic release of mitochondrial apoptogenic factors (Fig. 9.1). The next sections will address evolutionary perspectives on all three categories of constituents, with a particular emphasis on BCL-2 homologous proteins [aka BCL-2 family members, see https://bcl2db.ibcp.fr/BCL2DB/BCL2DBNomenclature for an explanation of nomenclature (Aouacheria 2014; Rech de Laval et al. 2014)].
Fig. 9.1.
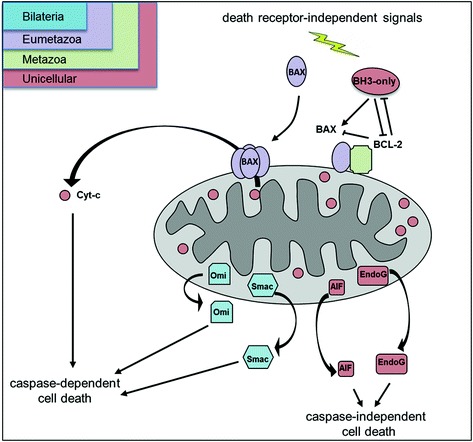
Simplified representation of the mitochondrial apoptotic pathway. Mitochondrial outer membrane permeabilization (MOMP) constitutes the pivotal event in the mitochondrial or BCL-2-regulated intrinsic death pathway and results in the release of cytochrome-c (cyt-c) and other mitochondrial apoptogenic factors from the mitochondrial intermembrane space to the cytosol. Once in the cytoplasm, cyt-c activates a family of death proteases called caspases that leads to the cleavage of a myriad of cellular substrates, causing cell demise. This pathway is initiated by activation of BH3-only proteins which serve as ligands to activate proapoptotic BCL-2 family members (e.g., BAX) through direct interaction or by binding to and inhibiting prosurvival BCL-2 homologous proteins (like BCL-2). Once activated, BAX homo-oligomerizes and forms pores in the mitochondrial outer membrane which cause the release of apoptogenic factors. Among these apoptogenic proteins, cytochrome-c, Omi/HtrA2, and SMAC/Diablo promote caspase-dependent cell death, whereas AIF and endoG induce caspase-independent cell death. Gene products are colored by their phyletic distribution (inset, see text for details)
Mitochondrial Intermembrane Space Proteins
The output of MOMP corresponds to the cytosolic release of mitochondrial apoptogenic proteins normally sequestered within the intermembrane space. The following five death-promoting factors have received significant characterization: cyt-c, apoptosis-inducing factor (AIF), second mitochondrial activator of caspases (Smac)/Diablo, Omi/HtrA2 and endonuclease G (endoG). During apoptosis, cyt-c directly induces caspase activation whereas Smac/Diablo and Omi/HtrA2 neutralize the inhibition of caspase activation (Lorenzo and Susin 2004; Saelens et al. 2004). AIF and endoG translocates to the nucleus to trigger caspase-independent DNA fragmentation (Arnoult et al. 2003; Cregan et al. 2004). All of these mitochondrial factors are encoded in the nucleus. Cyt-c and AIF have the widest phylogenetic distribution as they are found both in prokaryotes (Archaea, bacteria) and eukaryotes including protists, plants, fungi, and animals. Omi/HtrA2 and endoG also display a wide phylogenetic pattern and are present in all kingdoms of life except Archaea. Based on this, it seems reasonable to infer that these four mitochondrial apoptogenic proteins represent endosymbiotic acquisitions from the mitochondrial ancestor. In contrast, Smac/Diablo orthologues are only present in vertebrate species, suggesting a late phylogenetic origin. Interestingly, most of these mitochondrial intermembrane space proteins can act as “pencils–erasers”: cyt-c, for instance, has a vital daily job in respiration (as an essential electron carrier) and becomes cytotoxic only when its gets to the cytosol (Garrido and Kroemer 2004). AIF was also suggested to exert vital normal functions (possibly pertaining to its oxidoreductase activity) (Porter and Urbano 2006; Vahsen et al. 2004; Sorrentino et al. 2015). EndoG may be involved in DNA recombination and repair in addition to proliferation (Buttner et al. 2007; Huang et al. 2006). These examples illustrate an important but often neglected aspect of many apoptotic players: their polyfunctional nature. Pleiotropy is backed by a peculiar subcellular compartmentation, i.e., sequestration of conditionally toxic proteins in a normally non-accessible subcellular compartment (the mitochondrial intermembrane space). The molecular determinants underlying the apoptotic and non-apoptotic functions have been deciphered for cyt-c and AIF (Cheung et al. 2006; Hao et al. 2005) but await characterization for the other mitochondrial factors.
BCL-2 Homologous Proteins
BCL-2 family proteins control apoptosis upstream of the release of mitochondrial apoptogenic proteins and subsequent activation of caspases. This family of proteins comprises anti-apoptotic BCL-2 and pro-apoptotic BAX and their respective homologs. Our previous phylogenomic studies have revealed that BCL-2 homologous genes were restricted to metazoan species (and some animal viruses) and absent in fully sequenced genomes from Archaea, Eubacteria, Viridiplantae, Fungi, and other unicellular Eukaryota (Aouacheria et al. 2005), suggesting that this family arose in the metazoan stem. Logically, BCL-2 homologs are not found in the fully sequenced genomes of the choanoflagellate Monosiga brevicollis and the filasterean Capsaspora owczarzaki (King et al. 2008; Suga et al. 2013). Therefore, BCL-2 homologs most probably evolved only about 600 or 700 MYA and do not trace their origin back to the mitochondrial ancestor, their appearance being concomitant to the emergence of metazoan multicellularity.
Previous analysis indicated that gene duplication (for instance in marine invertebrates and fishes) and loss (e.g., in nematodes) played a prominent role in the evolution of the BCL-2 family and contributed to the generation of lineage-specific diversity. Six representatives are present in the demosponge Amphimedon queenslandica (Srivastava et al. 2010), four in the placozoan Trichoplax adhaerens (Srivastava et al. 2008), nine in the cnidarian Hydra vulgaris (Lasi et al. 2010), ten in the zebrafish Danio rerio (Kratz et al. 2006), and fourteen in the humans (Aouacheria et al. 2005), whereas the worm Caenorhabditis elegans has a unique BCL-2-like gene (called CED-9) (Hengartner and Horvitz 1994) and the fruit fly (Drosophila melanogaster) only a pair of homologs (known as Buffy and Debcl) (Clavier et al. 2015). Thus, the BCL-2 gene complement of extant metazoans is not a mere function of organismal complexity but include differential gene expansion and loss across lineages. As a result, BCL-2 homologous genes are present in multiple paralogs showing substantial sequence divergence and BH (BCL-2 Homology) motif arrangements (Aouacheria et al. 2005; Aouacheria et al. 2013; Guillemin et al. 2009) (see Fig. 9.2). Phylogenetic reconstruction indicates that, in vertebrates, BCL-2 homologs segregate into three major clades: BCL-2-like, BAX-like, and BID-like members (Aouacheria et al. 2013). BCL-2-like and BAX-like members correspond to prosurvival and pro-apoptotic proteins, respectively, while BID-like members form a divergent group of proteins with diverse activities toward apoptosis. Within this last group, BPR/BCL2L12 is an anti-apoptotic protein (shown to reside in the nucleocytoplasmic compartment rather than mitochondria) (Stegh and DePinho 2011), BFK/BCL2L15 is poorly characterized but may constitute a pro-apoptotic protein (Coultas et al. 2003), and BCL-G/BCL2L14 appears to be neutral against apoptosis (Tischner and Villunger 2012). BCL-2 family members have been reported in multiple invertebrate species, including sponges, cnidarians, echinoderms, and mollusks (see Table 9.1), and many more are predicted [e.g., in Trichoplax adhaerens (Srivastava et al. 2008), Ciona intestinalis (Terajima et al. 2003), Bombyx mori (Zhang et al. 2010), Apis mellifera (Dallacqua and Bitondi 2014), and Octopus vulgaris (Castellanos-Martinez et al. 2014)]. Unfortunately, only a small proportion of these proteins have been fully characterized in an experimental way. Although there have been numerous published phylogenetic studies, none of them has addressed the full diversity of BCL-2 family members in invertebrates and a robust, comprehensive phylogenetic analysis is not yet available.
Fig. 9.2.
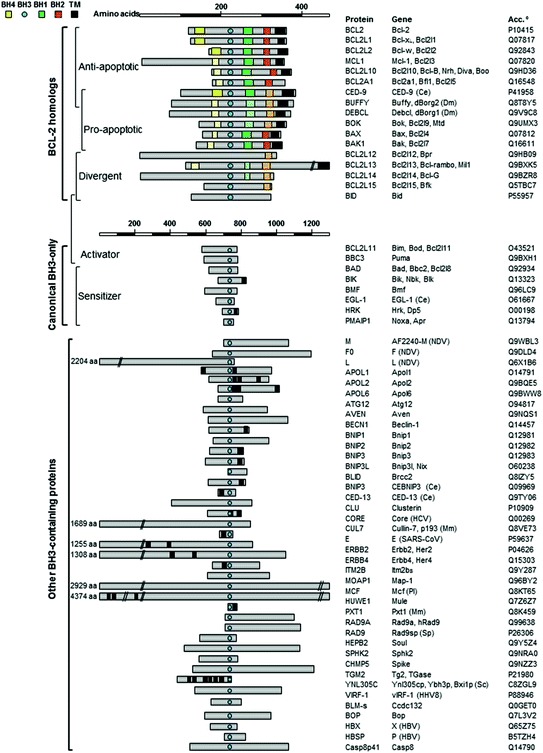
BH motif composition in BCL-2 homologous proteins and BH3-containing proteins. Schematic representation and BH motif composition of BCL-2 homologous proteins (including BCL-2-like, BAX-like and BID-like subgroups), canonical BH3-only proteins and other reported BH3-containing proteins (with UniProtKB accession numbers). Light shades depicted BH motif is uncertain. Total amino acid (aa) number is indicated for proteins that were not drawn to scale. Abbreviations for non-human proteins: Ce Caenorhabditis elegans; Dm Drosophila melanogaster; NDV Newcastle disease virus; Mm Mus musculus; HCV hepatitis C virus; Pl Photorhabdus luminescens; Sp Schizosaccharomyces pombe; SARS-CoV human SARS coronavirus; Sc Saccharomyces cerevisiae; HHV8 Human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus)
Table 9.1.
Reported invertebrate BCL-2 proteins
| Species | Reference | Gene/protein (Acc. °) | Motif composition (as published) | Function |
|---|---|---|---|---|
| Echinodermata | ||||
| Strongylocentrotus purpuratus | Robertson et al. (2006) |
SPU_024469 SPU_006124 SPU_021416 SPU_001916 SPU_016028 SPU_014028 SPU_010641 SPU_010786 SPU_017154 |
BH4 TM TM BH3, TM BH3, TM BH3, TM |
|
| Arthropoda | ||||
| Apis mellifera | Dallacqua and Bitondi (2014) | Ambuffy (A0A088ACI8) | BH1-4, TM | |
| Bombyx mori | Pan et al. (2014) | Bmbuffy (E9JEG2) | BH1-3, TM | Anti |
| Drosophila melanogaster |
Quinn et al. (2003) Colussi et al. (2000) |
Buffy (Q8T8Y5) Debcl (Q9V9C8) |
BH1-3, TM BH1-3, TM |
Anti Pro |
| Mollusca | ||||
| Ruditapes philippinarum | Lee et al. (2013) |
RpBCL-2A (KC506418) RpBCL-2B (KC506419) |
BH1-4, TM BH1-3, no TM |
|
| Mytilus galloprovincialis | Estevez-Calvar et al. (2013) |
Bcl2 (KC545829) Bax (KC545830) |
BH1-4, TM BH1-3, TM |
|
| Chlamys farreri | Qi et al. (2015) |
CfBcl-2 (KJ611244) CfBax (KJ620057) |
CfBcl-2: BH4, BH3, BH1, BH2, no TM CfBax: BH3, BH1, BH2, no TM |
|
| Crassostrea hongkongensis | Xiang et al. (2015) |
ChBax (KM262836) ChBak (KM262837) |
BH3, BH1, BH2, TM | |
| Nematoda | ||||
| Caenorhabditis elegans | Hengartner and Horvitz (1994) | P41958 | BH1-4, TM | Anti |
| Platyhelminthes | ||||
| Schmidtea mediterranea | Bender et al. (2012) |
Smed-Bak-1 (JN621808) Smed-Bak-2 (JN621809) Smed-bak-3 (JN621810) Smed-bok-2 (JN621814) Smed-bcl2-1 (FJ807655) Smed-bcl2-3 (JN621816) |
Pro | |
| Schistosoma mansoni | Lee et al. (2011) |
sjA/smA sjB /smB sjBcl2/2 smBcl2/2 sjBcl2/1 smBcl2/1 sjC /smC sjD |
BH1-4, TM BH1-4, TM BH1-4, TM BH1-4, no TM BH1 BH1 BH3 BH3 |
Anti Pro Pro |
| Cnidaria | ||||
| Aiptasia pallida | Dunn et al. (2006) | ABHP (DQ211980) | BH1, BH2, no TM | |
| Stylophora pistillata | Kvitt et al. (2011) | StyBcl-2-like (EU715319) | BH1-4, TM | |
| Hydra magnipapillata | Lasi et al. (2010) |
HyBak-like 1 (EF104645) HyBak-like 2 (EU035760) HyBcl-2-like 1 (EF104646) HyBcl-2-like 2 (EF104647) HyBcl-2-like 3 (EU035765) HyBcl-2-like 4 (EU035764) HyBcl-2-like 5 (EU035763) HyBcl-2-like -6 (EU035762) HyBcl-2-like 7 (EU035761) HyBH3-only 1 (hma2.230679) HyBH3-only 2 (hma2.221399) HyBH3-only 3 (hma2.218794) HyBH3-only 4 (hma2.224514) |
BH1-3, TM BH1-3, TM BH1-4, TM BH1-4, TM BH1-4, TM BH1-4, TM BH1-4, TM BH1-4, TM BH1-4, TM BH3 BH3 BH3 BH3 |
Pro Pro Anti Anti Anti Anti Slightly pro Anti Anti ND Pro Neutral Neutral |
| Porifera | ||||
| Geodia cydonium | Wiens et al. (2001) | BHP2-GC (AJ293508) | BH1, BH2, TM | Anti |
|
Geodia cydonium Suberites domuncula |
Wiens et al. (2000) |
BHP1_GC (CAB97129) BHP1_SD (CAB97205) |
BH1, BH2, TM | |
| Lubomirskia baicalensis | Wiens et al. (2006) |
BAK-2_LUBAI (CAJ12144) BCL-2a_LUBAI (CAJ12145) |
BH3, BH2, TM BH1-4, TM |
|
Since most bcl-2 family genes and proteins share commonalities in structure (e.g., an intron dividing the BH2 motif, and a similar “helical bundle” tridimensional fold—see Fig. 9.3), it appears likely that the diversity of metazoan BCL-2 genes was generated from a single precursor. The origin and functions of this ancestral BCL-2 protein are unknown. The early discovery that BCL-2 homologous proteins bear structural resemblance (analogy) to microbial toxins like colicins or the translocation domain of diphtheria toxin (Muchmore et al. 1996) has led to the speculation that they might have been acquired by horizontal gene transfer from the bacterial world. However, a set of viral proteins structurally related to BCL-2 (but functionally divergent) were recently characterized (Graham et al. 2008; Neidel et al. 2015), suggesting that the hypothesis of a viral origin for the founder gene should also be considered (Fig. 9.3). Whatever their origins, it seems reasonable to infer that the appearance of BCL-2 proteins might have been instrumental to the emergence of metazoan multicellularity, through the recruitment of mitochondria to a cell death program enabling tissue differentiation and homeostasis. However, the opposite assertion could also be true, namely recruitment of BCL-2 family proteins into a mitochondrial death program as a consequence of animal multicellularity. This issue is particularly interesting because in addition to their daily job in apoptosis, BCL-2 proteins have been shown to exert physiological, non-apoptotic functions such as regulation of mitochondrial dynamics, metabolism, DNA damage response, calcium homeostasis, general autophagy, and mitophagy (Pattingre et al. 2005; Alavian et al. 2011; Autret and Martin 2009; Chen et al. 2011; Chen and Pervaiz 2007; Gross 2006; Hardwick and Soane 2013; Hollville et al. 2014; Karbowski et al. 2006; Laulier and Lopez 2012; Murakawa et al. 2015; Perciavalle et al. 2012; Pinton and Rizzuto 2006; Wang et al. 2013). These findings suggesting that the function of BCL-2 proteins is pleiotropically linked to prosurvival traits in extant metazoan species raise the possibility that the ancestral function of BCL-2 proteins was unrelated to apoptosis regulation and that these proteins were exapted from an ancestor with an originally different function.
Fig. 9.3.
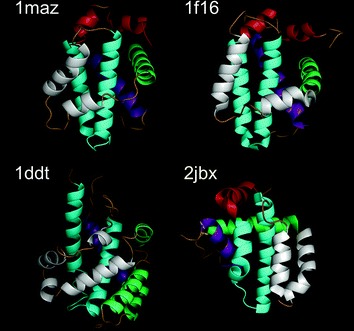
Structural similarity between BCL-2 homologs and microbial proteins. Ribbon diagrams of anti-apoptotic protein BCL-xL (PDB code: 1maz), proapoptotic protein BAX (1f16), diphtheria toxin translocation domain (1ddt), and myxoma virus antiapoptotic protein M11L (2jbx). These proteins form a compact α-helical bundle with a pair of central helices (in cyan) surrounded by other (mainly amphipathic) helices. The figure was made with PyMol
Evolutionary information is scarce about the beginnings of paralog divergence in the family and about how the repertoire of BCL-2 family genes evolved in the different metazoan lineages. Evidence of conserved colinearity (i.e., relict linkage) was gathered for the divergent BCL-2 homologs BID and BCL2L13 in vertebrate genomes (Aouacheria et al. 2005), providing information about the time when the cross talk between the intrinsic and extrinsic apoptosis pathways—which is mediated by BID—evolved. An early duplication involving these genes at the invertebrate-to-vertebrate transition, followed by two further duplications gave rise to the BID-like clade characterized by the absence of the C-terminal transmembrane segment (TM) (Aouacheria et al. 2013), illustrating the fact that many gene (sub) family expansions probably originally occur as tandem or proximal duplications (Charon et al. 2012; Fan et al. 2008; Srivastava et al. 2008). In the case of BCL2A1/BFL-1, a prosurvival BCL-2 homolog which appears to be found only in mammals, the exon encoding this TM segment has been replaced by a heterologous sequence, possibly as a result of duplication and shuffling events (Ko et al. 2007). BID, BCL2L13, and BCL2A1 correspond to phylogenetically recent innovations in metazoans, but other BCL-2 family genes are of more ancient origin such as proapoptotic BAK and prosurvival BCL2L1 (BCL-xL), for which close or divergent homologs, respectively, can be found in early-branching metazoans (Srivastava et al. 2010; Wiens et al. 2001; Wiens et al. 2000).
Particularly, intriguing is the issue of how opposite activities evolved in proteins that share a similar 3D structure, as do BCL-2-type and BAX-type proteins. The precise molecular determinants that underpin the extreme functional divergence between structural homologs of the BCL-2 family are not completely understood. Distinct regions of the BCL-2 domain were shown to be involved in the functional dichotomy between pro- and anti-apoptotic members: the BH4 region (which is often located in the first α-helix) (Borner et al. 1994; Lee et al. 1996), the BH3 motif (Lee et al. 2014), and the α5-α6 helical hairpin motif (often referred to as a “pore-forming” domain) (Bleicken et al. 2013; Guillemin et al. 2010). However, subtle differences are scattered along the entire protein domain of pro- and anti-apoptotic BCL-2 proteins and it is expected that various sites may contribute to their antagonist actions on cell survival. A prime difference between BAX-type and BCL-2-type proteins might be related to the ability of proapoptotic homologs to self-assemble by forming dimers and higher order oligomers (Subburaj et al. 2015; Westphal et al. 2014), and of the prosurvival ones to inhibit these association processes in the context of the mitochondrial membrane by behaving like chain terminators, i.e., dominant negative forms (Reed 2006; Westphal et al. 2014). If this scenario is correct, then what distinguishes both types of proteins should be looked for in contact interfaces (with partners and/or lipids) in addition to isolated regions, bearing in mind that those interactions can be “cloudy” and involve distant residues. An alternative view may be that the separation between BCL-2-like and BAX-like family members has been overstated and that some kind of dualistic thinking is at work that somehow hides the partly artificial nature of the pro- versus anti-apoptotic dichotomy. This alternative scenario is not without support from a variety of experimental data, including the demonstration that (i) most prosurvival BCL-2 family proteins can be converted into death factors following proteolytic cleavage (Cheng et al. 1997; Clem et al. 1998; Kucharczak et al. 2005; Michels et al. 2004; Xue and Horvitz 1997); (ii) proapoptotic isoforms can be produced by alternative splicing of prosurvival bcl-2-like genes (Bae et al. 2000; Boise et al. 1993); (iii) pro-apoptotic BAK or BAK proteins can behave as prosurvival factors in specific cellular contexts or cell types (Kiefer et al. 1995; Lewis et al. 1999); and (iv) a number of BCL-2 family members were characterized both as pro- and anti-apoptotic factors (e.g., BCL2L10, BOK, and Bcl-rambo/BCL2L13) (Lee et al. 2001; Song et al. 1999; Aouacheria et al. 2001; Inohara et al. 1998; Ke et al. 2001; Zhang et al. 2001; Jensen et al. 2014). Hence, the scission between prosurvival and proapoptotic BCL-2 family members might be less definitive and clear than currently assumed.
BH3-Only Proteins
BCL2 homologous proteins act as receptors for BH3-only proteins, which are structurally unrelated proapoptotic molecules. In response to death signals, BH3-only proteins either inhibit the BCL-2-like apoptosis inhibitors or activate the BAX-like death activators. These proteins therefore form a signal-processing layer that connects onto the BCL-2/BAX core machinery the various inputs telling the cell either to survive or to commit suicide. Synthetic peptides encompassing the BH3 motif of various BH3-only proteins were shown to bind with high affinities to a hydrophobic groove at the surface of prosurvival BCL-2 homologous proteins (Petros et al. 2000). Following this finding, BH3-mimetic drugs were developed that rapidly entered clinical trials as anticancer agents (Davids and Letai 2012). Given their key roles, the discovery of novel BH3-only proteins has represented and continues to represent a critical endeavor in the cell death field. Historically, this protein group contained nine non-homologous proteins discovered in the “1990s and early 2000s” (BIM, BMF, PUMA, NOXA, BAD, HRK, BIK, EGL1, and BID, herein termed “canonical” BH3-only proteins), which were sometimes erroneously appended to the protein family of BCL-2 homologs. In fact, only BID qualifies both as a BH3-only protein, as it contains a single BH3 motif, and as a BCL-2 homologous protein, because it shares a similar 3D structure with BCL-2 and BAX (Billen et al. 2008b; Chou et al. 1999; McDonnell et al. 1999). Current models of apoptosis regulation and a majority of review articles exclusively focus on the proapoptotic activity of these nine BH3-only proteins, ignoring the fact that the number of claimed BH3-only proteins has dramatically increased to reach a total of ~40 (Aouacheria et al. 2015; Aouacheria et al. 2013) (Fig. 9.2). Contrary to the other BH motifs that were only detected in BCL-2 homologous proteins, BH3 motifs are now found in a gamut of folded (e.g., BCL-2) and unstructured protein domains [note that, except BID, all BH3-only proteins are intrinsically disordered proteins (Barrera-Vilarmau et al. 2011; Craxton et al. 2012; Hinds et al. 2007; Rogers et al. 2013; Yan et al. 2004)], bringing the grand total number of reported BH3 sequences to more than 60 unique instances. As a result, the evolutionary histories of BH3 motifs are singular, inherently coupled to the evolution of the proteins that harbor them, and therefore difficult to disentangle collectively. Depending on the case, evolution of BH3 motifs can be attributed to homologous processes (e.g., duplication divergence of BCL-2 family genes) or homoplastic mechanisms (e.g., random coincidence or convergence, as in the case of the E3 ubiquitin ligase MULE and the insecticidal toxin Mcf1, among many other putative instances). Interestingly, inspection of gene structures suggests that transfer events (e.g., exon shuffling) could also be involved, as illustrated by the relatively high similarity of the BCL-2 homolog BAK and the BH3-only gene BIK in their BH3 regions (Aouacheria et al. 2015).
The reason that explains this complicated situation has its root in the very nature of the BH3 motif, whose sequence signatures are diverse and of low complexity (i.e., very predictable) (Aouacheria et al. 2013). Following on this observation, we recently advanced the argument that the BH3 motif meets the criteria for classification as a short linear motif (SLiM) or a molecular recognition element/feature (MoRE/MoRF) involved in protein–protein interactions between structured domains (e.g., globular domains of the BCL-2 type) and between structured domains and intrinsically disordered proteins (as exemplified by the interaction between canonical BH3-only proteins and BCL-2-like or BAX-like proteins). Rather than considering the BH3 as an apoptotic motif per se, this novel conceptual framework poses this motif as a versatile and evolutionary plastic module associated with binding events in various branches of the tree of life, within metazoans but also probably outside the animal kingdom as well. Future experiments will have to (i) assess the prevalence of BH3 motifs in proteins from non-metazoan species, (ii) unravel the identity of their putative receptors, and (iii) determine their possible roles in the biology of the cognate organisms.
Conclusion
To sum up, the BCL-2-regulated apoptotic pathway (a metazoan synapomorphy) emerged as the result of the interplay between an eukaryotic organelle (the mitochondrion) sequestrating proteins which have both vital and proapoptotic roles, a membranotropic structural domain (the BCL-2 globular fold) able to convey opposite activities toward cell survival and cell death, and a short and evolutionary plastic module (BH3) mediating protein–protein interactions. It is likely that acquisition of a proto-bcl-2 gene occurred only once during the evolution of the first multi-celled animals, followed by vertical evolutionary descent, lineage-specific diversification, and gene losses, contributing to the numerous morphological and lifestyle features of animals. Although sequences are “documents of evolutionary history” [in reference to Zuckerkandl and Pauling (1965)], it is hard to figure out in any real way whether the repertoire of molecules involved in the control of active cell death was “simple” or “complex” in the last common ancestor of modern-day animal species. Yet, as they are descendants of lineages that diverged early in the history of multicellular animals, the study of basal metazoan species can offer useful clues, e.g., about the presence of a BH3-dependent mitochondrial apoptotic pathway in their ancestors, or about the possible non-apoptotic function(s) of the BCL-2 ancestral protein. Whether metazoan BCL-2 homologous proteins emerged as stress-signaling molecules, or as switches connecting and controlling the execution of the various pathways involved in cell survival and death (including apoptosis, autophagy and programmed necrosis), or as key players serving biochemical functions distinct from cell death regulation remains an open question.
Acknowledgements
The authors thank P. Pontarotti for the invitation to write this chapter. We are grateful to Dr. Valentine Rech De Laval for help with illustrations.
Contributor Information
Pierre Pontarotti, Email: pierre.pontarotti@univ-amu.fr.
Abdel Aouacheria, Email: abdelouahab.aouacheria@umontpellier.fr, Email: aouacheria.abdel@gmail.com.
References
- Alavian KN, Li H, Collis L, Bonanni L, Zeng L, Sacchetti S, Lazrove E, Nabili P, Flaherty B, Graham M, Chen Y, Messerli SM, Mariggio MA, Rahner C, McNay E, Shore GC, Smith PJ, Hardwick JM, Jonas EA. Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat Cell Biol. 2011;13(10):1224–1233. doi: 10.1038/ncb2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouacheria A. The BCL-2 database, Act 2: moving beyond dualism to diversity and pleiotropy. Cell Death Dis. 2014;5:e981. doi: 10.1038/cddis.2013.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouacheria A, Arnaud E, Venet S, Lalle P, Gouy M, Rigal D, Gillet G. Nrh, a human homologue of Nr-13 associates with Bcl-Xs and is an inhibitor of apoptosis. Oncogene. 2001;20(41):5846–5855. doi: 10.1038/sj.onc.1204740. [DOI] [PubMed] [Google Scholar]
- Aouacheria A, Brunet F, Gouy M. Phylogenomics of life-or-death switches in multicellular animals: Bcl-2, BH3-Only, and BNip families of apoptotic regulators. Mol Biol Evol. 2005;22(12):2395–2416. doi: 10.1093/molbev/msi234. [DOI] [PubMed] [Google Scholar]
- Aouacheria A, Combet C, Tompa P, Hardwick JM. Redefining the BH3 death domain as a short linear motif. Trends Biochem Sci. 2015;40(12):736–748. doi: 10.1016/j.tibs.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouacheria A, Rech de Laval V, Combet C, Hardwick JM. Evolution of Bcl-2 homology motifs: homology versus homoplasy. Trends Cell Biol. 2013;23(3):103–111. doi: 10.1016/j.tcb.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoult D, Gaume B, Karbowski M, Sharpe JC, Cecconi F, Youle RJ. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. The EMBO journal. 2003;22(17):4385–4399. doi: 10.1093/emboj/cdg423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autret A, Martin SJ. Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis. Mol Cell. 2009;36(3):355–363. doi: 10.1016/j.molcel.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Bae J, Leo CP, Hsu SY, Hsueh AJ. MCL-1S, a splicing variant of the antiapoptotic BCL-2 family member MCL-1, encodes a proapoptotic protein possessing only the BH3 domain. J Biol Chem. 2000;275(33):25255–25261. doi: 10.1074/jbc.M909826199. [DOI] [PubMed] [Google Scholar]
- Barrera-Vilarmau S, Obregon P, de Alba E. Intrinsic order and disorder in the bcl-2 member harakiri: insights into its proapoptotic activity. PLoS ONE. 2011;6(6):e21413. doi: 10.1371/journal.pone.0021413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender C. E., Fitzgerald P., Tait S. W. G., Llambi F., McStay G. P., Tupper D. O., Pellettieri J., Alvarado A. S., Salvesen G. S., Green D. R. Mitochondrial pathway of apoptosis is ancestral in metazoans. Proceedings of the National Academy of Sciences. 2012;109(13):4904–4909. doi: 10.1073/pnas.1120680109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billen LP, Kokoski CL, Lovell JF, Leber B, Andrews DW. Bcl-XL inhibits membrane permeabilization by competing with Bax. PLoS Biol. 2008;6(6):e147. doi: 10.1371/journal.pbio.0060147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billen LP, Shamas-Din A, Andrews DW. Bid: a Bax-like BH3 protein. Oncogene. 2008;27(Suppl 1):S93–104. doi: 10.1038/onc.2009.47. [DOI] [PubMed] [Google Scholar]
- Bleicken S, Wagner C, Garcia-Saez AJ. Mechanistic differences in the membrane activity of Bax and Bcl-xL correlate with their opposing roles in apoptosis. Biophys J. 2013;104(2):421–431. doi: 10.1016/j.bpj.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74(4):597–608. doi: 10.1016/0092-8674(93)90508-N. [DOI] [PubMed] [Google Scholar]
- Borner C, Martinou I, Mattmann C, Irmler M, Schaerer E, Martinou JC, Tschopp J. The protein bcl-2 alpha does not require membrane attachment, but two conserved domains to suppress apoptosis. J Cell Biol. 1994;126(4):1059–1068. doi: 10.1083/jcb.126.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozhkov PV, Lam E. Green death: revealing programmed cell death in plants. Cell Death Differ. 2011;18(8):1239–1240. doi: 10.1038/cdd.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner S, Eisenberg T, Carmona-Gutierrez D, Ruli D, Knauer H, Ruckenstuhl C, Sigrist C, Wissing S, Kollroser M, Frohlich KU, Sigrist S, Madeo F. Endonuclease G regulates budding yeast life and death. Mol Cell. 2007;25(2):233–246. doi: 10.1016/j.molcel.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Castellanos-Martinez S, Arteta D, Catarino S, Gestal C. De novo transcriptome sequencing of the Octopus vulgaris hemocytes using Illumina RNA-Seq technology: response to the infection by the gastrointestinal parasite Aggregata octopiana. PLoS ONE. 2014;9(10):e107873. doi: 10.1371/journal.pone.0107873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon C, Bruggeman Q, Thareau V, Henry Y. Gene duplication within the Green Lineage: the case of TEL genes. J Exp Bot. 2012;63(14):5061–5077. doi: 10.1093/jxb/ers181. [DOI] [PubMed] [Google Scholar]
- Chen YB, Aon MA, Hsu YT, Soane L, Teng X, McCaffery JM, Cheng WC, Qi B, Li H, Alavian KN, Dayhoff-Brannigan M, Zou S, Pineda FJ, O’Rourke B, Ko YH, Pedersen PL, Kaczmarek LK, Jonas EA, Hardwick JM. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J Cell Biol. 2011;195(2):263–276. doi: 10.1083/jcb.201108059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZX, Pervaiz S. Bcl-2 induces pro-oxidant state by engaging mitochondrial respiration in tumor cells. Cell Death Differ. 2007;14(9):1617–1627. doi: 10.1038/sj.cdd.4402165. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278(5345):1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- Cheung EC, Joza N, Steenaart NA, McClellan KA, Neuspiel M, McNamara S, MacLaurin JG, Rippstein P, Park DS, Shore GC, McBride HM, Penninger JM, Slack RS. Dissociating the dual roles of apoptosis-inducing factor in maintaining mitochondrial structure and apoptosis. EMBO J. 2006;25(17):4061–4073. doi: 10.1038/sj.emboj.7601276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JJ, Li H, Salvesen GS, Yuan J, Wagner G. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell. 1999;96(5):615–624. doi: 10.1016/S0092-8674(00)80572-3. [DOI] [PubMed] [Google Scholar]
- Clavier A, Rincheval-Arnold A, Colin J, Mignotte B, Guenal I. Apoptosis in Drosophila: which role for mitochondria? Apoptosis Int J Program Cell Death. 2015 doi: 10.1007/s10495-015-1209-y. [DOI] [PubMed] [Google Scholar]
- Clem RJ, Cheng EH, Karp CL, Kirsch DG, Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona MA, Hardwick JM. Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci USA. 1998;95(2):554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colussi Paul A., Quinn Leonie M., Huang David C.S., Coombe Michelle, Read Stuart H., Richardson Helena, Kumar Sharad. Debcl, a Proapoptotic Bcl-2 Homologue, Is a Component of theDrosophila melanogasterCell Death Machinery. The Journal of Cell Biology. 2000;148(4):703–714. doi: 10.1083/jcb.148.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L, Pellegrini M, Visvader JE, Lindeman GJ, Chen L, Adams JM, Huang DC, Strasser A. Bfk: a novel weakly proapoptotic member of the Bcl-2 protein family with a BH3 and a BH2 region. Cell Death Differ. 2003;10(2):185–192. doi: 10.1038/sj.cdd.4401204. [DOI] [PubMed] [Google Scholar]
- Craxton A, Butterworth M, Harper N, Fairall L, Schwabe J, Ciechanover A, Cohen GM. NOXA, a sensor of proteasome integrity, is degraded by 26S proteasomes by an ubiquitin-independent pathway that is blocked by MCL-1. Cell Death Differ. 2012;19(9):1424–1434. doi: 10.1038/cdd.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregan SP, Dawson VL, Slack RS. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene. 2004;23(16):2785–2796. doi: 10.1038/sj.onc.1207517. [DOI] [PubMed] [Google Scholar]
- Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15(1):49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- Dallacqua RP, Bitondi MM. Dimorphic ovary differentiation in honeybee (Apis mellifera) larvae involves caste-specific expression of homologs of ark and buffy cell death genes. PLoS ONE. 2014;9(5):e98088. doi: 10.1371/journal.pone.0098088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids MS, Letai A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J Clin Oncol: Official J Ame Soc Clin Oncol. 2012;30(25):3127–3135. doi: 10.1200/JCO.2011.37.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger M, Glab JA, Puthalakath H. BH3-only proteins: a 20-year stock-take. FEBS J. 2015;282(6):1006–1016. doi: 10.1111/febs.13190. [DOI] [PubMed] [Google Scholar]
- Dunn Simon R., Phillips Wendy S., Spatafora Joseph W., Green Douglas R., Weis Virginia M. Highly Conserved Caspase and Bcl-2 Homologues from the Sea Anemone Aiptasia pallida: Lower Metazoans as Models for the Study of Apoptosis Evolution. Journal of Molecular Evolution. 2006;63(1):95–107. doi: 10.1007/s00239-005-0236-7. [DOI] [PubMed] [Google Scholar]
- Dwyer DJ, Winkler JA. Identification and characterization of programmed cell death markers in bacterial models. Methods Mol Biol. 2013;1004:145–159. doi: 10.1007/978-1-62703-383-1_11. [DOI] [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang C, Neutzner A, Tjandra N, Youle RJ. Bcl-x(L) retrotranslocates bax from the mitochondria into the cytosol. Cell. 2011;145(1):104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez-Calvar Noelia, Romero Alejandro, Figueras Antonio, Novoa Beatriz. Genes of the Mitochondrial Apoptotic Pathway in Mytilus galloprovincialis. PLoS ONE. 2013;8(4):e61502. doi: 10.1371/journal.pone.0061502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Chen Y, Long M. Recurrent tandem gene duplication gave rise to functionally divergent genes in drosophila. Mol Biol Evol. 2008;25(7):1451–1458. doi: 10.1093/molbev/msn089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido C, Kroemer G. Life’s smile, death’s grin: vital functions of apoptosis-executing proteins. Curr Opin Cell Biol. 2004;16(6):639–646. doi: 10.1016/j.ceb.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Graham SC, Bahar MW, Cooray S, Chen RA, Whalen DM, Abrescia NG, Alderton D, Owens RJ, Stuart DI, Smith GL, Grimes JM. Vaccinia virus proteins A52 and B14 Share a Bcl-2-like fold but have evolved to inhibit NF-kappaB rather than apoptosis. PLoS Pathog. 2008;4(8):e1000128. doi: 10.1371/journal.ppat.1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A. BID as a double agent in cell life and death. Cell Cycle. 2006;5(6):582–584. doi: 10.4161/cc.5.6.2575. [DOI] [PubMed] [Google Scholar]
- Guillemin Y, Lalle P, Gillet G, Guerin JF, Hamamah S, Aouacheria A. Oocytes and early embryos selectively express the survival factor BCL2L10. J Mol Med (Berl) 2009;87(9):923–940. doi: 10.1007/s00109-009-0495-7. [DOI] [PubMed] [Google Scholar]
- Guillemin Y, Lopez J, Gimenez D, Fuertes G, Valero JG, Blum L, Gonzalo P, Salgado J, Girard-Egrot A, Aouacheria A. Active fragments from pro- and antiapoptotic BCL-2 proteins have distinct membrane behavior reflecting their functional divergence. PLoS ONE. 2010;5(2):e9066. doi: 10.1371/journal.pone.0009066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Duncan GS, Chang CC, Elia A, Fang M, Wakeham A, Okada H, Calzascia T, Jang Y, You-Ten A, Yeh WC, Ohashi P, Wang X, Mak TW. Specific ablation of the apoptotic functions of cytochrome C reveals a differential requirement for cytochrome C and Apaf-1 in apoptosis. Cell. 2005;121(4):579–591. doi: 10.1016/j.cell.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Hardwick J. M., Soane L. Multiple Functions of BCL-2 Family Proteins. Cold Spring Harbor Perspectives in Biology. 2013;5(2):a008722–a008722. doi: 10.1101/cshperspect.a008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner MO, Horvitz HR. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell. 1994;76(4):665–676. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- Hinds MG, Smits C, Fredericks-Short R, Risk JM, Bailey M, Huang DC, Day CL. Bim, Bad and Bmf: intrinsically unstructured BH3-only proteins that undergo a localized conformational change upon binding to prosurvival Bcl-2 targets. Cell Death Differ. 2007;14(1):128–136. doi: 10.1038/sj.cdd.4401934. [DOI] [PubMed] [Google Scholar]
- Hollville E, Carroll RG, Cullen SP, Martin SJ. Bcl-2 family proteins participate in mitochondrial quality control by regulating Parkin/PINK1-dependent mitophagy. Mol Cell. 2014;55(3):451–466. doi: 10.1016/j.molcel.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Huang KJ, Ku CC, Lehman IR. Endonuclease G: a role for the enzyme in recombination and cellular proliferation. Proc Natl Acad Sci USA. 2006;103(24):8995–9000. doi: 10.1073/pnas.0603445103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara N, Gourley TS, Carrio R, Muniz M, Merino J, Garcia I, Koseki T, Hu Y, Chen S, Nunez G. Diva, a Bcl-2 homologue that binds directly to Apaf-1 and induces BH3-independent cell death. J Biol Chem. 1998;273(49):32479–32486. doi: 10.1074/jbc.273.49.32479. [DOI] [PubMed] [Google Scholar]
- Jensen SA, Calvert AE, Volpert G, Kouri FM, Hurley LA, Luciano JP, Wu Y, Chalastanis A, Futerman AH, Stegh AH. Bcl2L13 is a ceramide synthase inhibitor in glioblastoma. Proc Natl Acad Sci USA. 2014;111(15):5682–5687. doi: 10.1073/pnas.1316700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443(7112):658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- Ke N, Godzik A, Reed JC. Bcl-B, a novel Bcl-2 family member that differentially binds and regulates Bax and Bak. J Biol Chem. 2001;276(16):12481–12484. doi: 10.1074/jbc.C000871200. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer MC, Brauer MJ, Powers VC, Wu JJ, Umansky SR, Tomei LD, Barr PJ. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature. 1995;374(6524):736–739. doi: 10.1038/374736a0. [DOI] [PubMed] [Google Scholar]
- King N, Westbrook MJ, Young SL, Kuo A, Abedin M, Chapman J, Fairclough S, Hellsten U, Isogai Y, Letunic I, Marr M, Pincus D, Putnam N, Rokas A, Wright KJ, Zuzow R, Dirks W, Good M, Goodstein D, Lemons D, Li W, Lyons JB, Morris A, Nichols S, Richter DJ, Salamov A, Sequencing JG, Bork P, Lim WA, Manning G, Miller WT, McGinnis W, Shapiro H, Tjian R, Grigoriev IV, Rokhsar D. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 2008;451(7180):783–788. doi: 10.1038/nature06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JK, Choi KH, Pan Z, Lin P, Weisleder N, Kim CW, Ma J. The tail-anchoring domain of Bfl1 and HCCS1 targets mitochondrial membrane permeability to induce apoptosis. J Cell Sci. 2007;120(Pt 16):2912–2923. doi: 10.1242/jcs.006197. [DOI] [PubMed] [Google Scholar]
- Kratz E, Eimon PM, Mukhyala K, Stern H, Zha J, Strasser A, Hart R, Ashkenazi A. Functional characterization of the Bcl-2 gene family in the zebrafish. Cell Death Differ. 2006;13(10):1631–1640. doi: 10.1038/sj.cdd.4402016. [DOI] [PubMed] [Google Scholar]
- Kucharczak JF, Simmons MJ, Duckett CS, Gelinas C. Constitutive proteasome-mediated turnover of Bfl-1/A1 and its processing in response to TNF receptor activation in FL5.12 pro-B cells convert it into a prodeath factor. Cell Death Differ. 2005;12(9):1225–1239. doi: 10.1038/sj.cdd.4401684. [DOI] [PubMed] [Google Scholar]
- Kvitt Hagit, Rosenfeld Hanna, Zandbank Keren, Tchernov Dan. Regulation of Apoptotic Pathways by Stylophora pistillata (Anthozoa, Pocilloporidae) to Survive Thermal Stress and Bleaching. PLoS ONE. 2011;6(12):e28665. doi: 10.1371/journal.pone.0028665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasi M, Pauly B, Schmidt N, Cikala M, Stiening B, Kasbauer T, Zenner G, Popp T, Wagner A, Knapp RT, Huber AH, Grunert M, Soding J, David CN, Bottger A. The molecular cell death machinery in the simple cnidarian hydra includes an expanded caspase family and pro- and anti-apoptotic Bcl-2 proteins. Cell Res. 2010;20(7):812–825. doi: 10.1038/cr.2010.66. [DOI] [PubMed] [Google Scholar]
- Laulier C, Lopez BS. The secret life of Bcl-2: apoptosis-independent inhibition of DNA repair by Bcl-2 family members. Mutat Res. 2012;751(2):247–257. doi: 10.1016/j.mrrev.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Lee E. F., Clarke O. B., Evangelista M., Feng Z., Speed T. P., Tchoubrieva E. B., Strasser A., Kalinna B. H., Colman P. M., Fairlie W. D. Discovery and molecular characterization of a Bcl-2-regulated cell death pathway in schistosomes. Proceedings of the National Academy of Sciences. 2011;108(17):6999–7003. doi: 10.1073/pnas.1100652108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EF, Dewson G, Evangelista M, Pettikiriarachchi A, Gold GJ, Zhu H, Colman PM, Fairlie WD. The functional differences between pro-survival and pro-apoptotic B cell lymphoma 2 (Bcl-2) proteins depend on structural differences in their Bcl-2 homology 3 (BH3) domains. J Biol Chem. 2014;289(52):36001–36017. doi: 10.1074/jbc.M114.610758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LC, Hunter JJ, Mujeeb A, Turck C, Parslow TG. Evidence for alpha-helical conformation of an essential N-terminal region in the human Bcl2 protein. J Biol Chem. 1996;271(38):23284–23288. doi: 10.1074/jbc.271.38.23284. [DOI] [PubMed] [Google Scholar]
- Lee R, Chen J, Matthews CP, McDougall JK, Neiman PE. Characterization of NR13-related human cell death regulator, Boo/Diva, in normal and cancer tissues. Biochim Biophys Acta. 2001;1520(3):187–194. doi: 10.1016/S0167-4781(01)00268-8. [DOI] [PubMed] [Google Scholar]
- Lee Youngdeuk, Whang Ilson, Lee Sukkyoung, Menike Udeni, Oh Chulhong, Kang Do-Hyung, Heo Gang-Joon, Lee Jehee, De Zoysa Mahanama. Two molluscan BCL-2 family members from Manila clam, Ruditapes philippinarum: Molecular characterization and immune responses. Fish & Shellfish Immunology. 2013;34(6):1628–1634. doi: 10.1016/j.fsi.2013.03.366. [DOI] [PubMed] [Google Scholar]
- Lewis J, Oyler GA, Ueno K, Fannjiang YR, Chau BN, Vornov J, Korsmeyer SJ, Zou S, Hardwick JM. Inhibition of virus-induced neuronal apoptosis by bax. Nat Med. 1999;5(7):832–835. doi: 10.1038/10556. [DOI] [PubMed] [Google Scholar]
- Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP, Green DR. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44(4):517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo HK, Susin SA. Mitochondrial effectors in caspase-independent cell death. FEBS Lett. 2004;557(1–3):14–20. doi: 10.1016/S0014-5793(03)01464-9. [DOI] [PubMed] [Google Scholar]
- Madeo F, Frohlich E, Frohlich KU. A yeast mutant showing diagnostic markers of early and late apoptosis. J Cell Biol. 1997;139(3):729–734. doi: 10.1083/jcb.139.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell JM, Fushman D, Milliman CL, Korsmeyer SJ, Cowburn D. Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell. 1999;96(5):625–634. doi: 10.1016/S0092-8674(00)80573-5. [DOI] [PubMed] [Google Scholar]
- Michels J, O’Neill JW, Dallman CL, Mouzakiti A, Habens F, Brimmell M, Zhang KY, Craig RW, Marcusson EG, Johnson PW, Packham G. Mcl-1 is required for Akata6 B-lymphoma cell survival and is converted to a cell death molecule by efficient caspase-mediated cleavage. Oncogene. 2004;23(28):4818–4827. doi: 10.1038/sj.onc.1207648. [DOI] [PubMed] [Google Scholar]
- Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, Ng SL, Fesik SW. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381(6580):335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- Murakawa T, Yamaguchi O, Hashimoto A, Hikoso S, Takeda T, Oka T, Yasui H, Ueda H, Akazawa Y, Nakayama H, Taneike M, Misaka T, Omiya S, Shah AM, Yamamoto A, Nishida K, Ohsumi Y, Okamoto K, Sakata Y, Otsu K. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat commun. 2015;6:7527. doi: 10.1038/ncomms8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidel S, Maluquer de Motes C, Mansur DS, Strnadova P, Smith GL, Graham SC. Vaccinia virus protein A49 is an unexpected member of the B-cell Lymphoma (Bcl)-2 protein family. J Biol Chem. 2015;290(10):5991–6002. doi: 10.1074/jbc.M114.624650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Chun, Hu Yan-Fen, Yi Hua-Shan, Song Juan, Wang La, Pan Min-Hui, Lu Cheng. Role of Bmbuffy in hydroxycamptothecine-induced apoptosis in BmN-SWU1 cells of the silkworm, Bombyx mori. Biochemical and Biophysical Research Communications. 2014;447(2):237–243. doi: 10.1016/j.bbrc.2014.03.093. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Perciavalle RM, Stewart DP, Koss B, Lynch J, Milasta S, Bathina M, Temirov J, Cleland MM, Pelletier S, Schuetz JD, Youle RJ, Green DR, Opferman JT. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat Cell Biol. 2012;14(6):575–583. doi: 10.1038/ncb2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros AM, Nettesheim DG, Wang Y, Olejniczak ET, Meadows RP, Mack J, Swift K, Matayoshi ED, Zhang H, Thompson CB, Fesik SW. Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci: Publ Protein Soc. 2000;9(12):2528–2534. doi: 10.1017/S096183680000331X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Rizzuto R. Bcl-2 and Ca2 + homeostasis in the endoplasmic reticulum. Cell Death Differ. 2006;13(8):1409–1418. doi: 10.1038/sj.cdd.4401960. [DOI] [PubMed] [Google Scholar]
- Porter AG, Urbano AG. Does apoptosis-inducing factor (AIF) have both life and death functions in cells? BioEssays: News Rev Mol Cell Dev Biol. 2006;28(8):834–843. doi: 10.1002/bies.20444. [DOI] [PubMed] [Google Scholar]
- Qi Haigang, Miao Guoying, Li Li, Que Huayong, Zhang Guofan. Identification and functional characterization of two Bcl-2 family protein genes in Zhikong scallop Chlamys farreri. Fish & Shellfish Immunology. 2015;44(1):147–155. doi: 10.1016/j.fsi.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Quinn L. Buffy, a Drosophila Bcl-2 protein, has anti-apoptotic and cell cycle inhibitory functions. The EMBO Journal. 2003;22(14):3568–3579. doi: 10.1093/emboj/cdg355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rech de Laval V., Deleage G., Aouacheria A., Combet C. BCL2DB: database of BCL-2 family members and BH3-only proteins. Database. 2014;2014(0):bau013–bau013. doi: 10.1093/database/bau013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JC. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006;13(8):1378–1386. doi: 10.1038/sj.cdd.4401975. [DOI] [PubMed] [Google Scholar]
- Robertson Anthony J., Croce Jenifer, Carbonneau Seth, Voronina Ekaterina, Miranda Esther, McClay David R., Coffman James A. The genomic underpinnings of apoptosis in Strongylocentrotus purpuratus. Developmental Biology. 2006;300(1):321–334. doi: 10.1016/j.ydbio.2006.08.053. [DOI] [PubMed] [Google Scholar]
- Rogers JM, Steward A, Clarke J. Folding and binding of an intrinsically disordered protein: fast, but not diffusion-limited. J Am Chem Soc. 2013;135(4):1415–1422. doi: 10.1021/ja309527h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P. Toxic proteins released from mitochondria in cell death. Oncogene. 2004;23(16):2861–2874. doi: 10.1038/sj.onc.1207523. [DOI] [PubMed] [Google Scholar]
- Shamas-Din A, Brahmbhatt H, Leber B, Andrews DW. H3-only proteins: orchestrators of apoptosis. Biochim Biophys Acta. 2011;4:508–520. doi: 10.1016/j.bbamcr.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Song Q, Kuang Y, Dixit VM, Vincenz C. Boo, a novel negative regulator of cell death, interacts with Apaf-1. EMBO J. 1999;18(1):167–178. doi: 10.1093/emboj/18.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino L, Calogero AM, Pandini V, Vanoni MA, Sevrioukova IF, Aliverti A. Key role of the adenylate moiety and integrity of the adenylate-binding site for the NAD(+)/H binding to mitochondrial apoptosis-inducing factor. Biochemistry. 2015;54(47):6996–7009. doi: 10.1021/acs.biochem.5b00898. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Begovic E, Chapman J, Putnam NH, Hellsten U, Kawashima T, Kuo A, Mitros T, Salamov A, Carpenter ML, Signorovitch AY, Moreno MA, Kamm K, Grimwood J, Schmutz J, Shapiro H, Grigoriev IV, Buss LW, Schierwater B, Dellaporta SL, Rokhsar DS. The Trichoplax genome and the nature of placozoans. Nature. 2008;454(7207):955–960. doi: 10.1038/nature07191. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Simakov O, Chapman J, Fahey B, Gauthier ME, Mitros T, Richards GS, Conaco C, Dacre M, Hellsten U, Larroux C, Putnam NH, Stanke M, Adamska M, Darling A, Degnan SM, Oakley TH, Plachetzki DC, Zhai Y, Adamski M, Calcino A, Cummins SF, Goodstein DM, Harris C, Jackson DJ, Leys SP, Shu S, Woodcroft BJ, Vervoort M, Kosik KS, Manning G, Degnan BM, Rokhsar DS. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature. 2010;466(7307):720–726. doi: 10.1038/nature09201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegh AH, DePinho RA. Beyond effector caspase inhibition: Bcl2L12 neutralizes p53 signaling in glioblastoma. Cell Cycle. 2011;10(1):33–38. doi: 10.4161/cc.10.1.14365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subburaj Y, Cosentino K, Axmann M, Pedrueza-Villalmanzo E, Hermann E, Bleicken S, Spatz J, Garcia-Saez AJ. Bax monomers form dimer units in the membrane that further self-assemble into multiple oligomeric species. Nature communications. 2015;6:8042. doi: 10.1038/ncomms9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga H, Chen Z, de Mendoza A, Sebe-Pedros A, Brown MW, Kramer E, Carr M, Kerner P, Vervoort M, Sanchez-Pons N, Torruella G, Derelle R, Manning G, Lang BF, Russ C, Haas BJ, Roger AJ, Nusbaum C, Ruiz-Trillo I. The Capsaspora genome reveals a complex unicellular prehistory of animals. Nat commun. 2013;4:2325. doi: 10.1038/ncomms3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait S. W. G., Green D. R. Mitochondrial Regulation of Cell Death. Cold Spring Harbor Perspectives in Biology. 2013;5(9):a008706–a008706. doi: 10.1101/cshperspect.a008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terajima D, Shida K, Takada N, Kasuya A, Rokhsar D, Satoh N, Satake M, Wang HG. Identification of candidate genes encoding the core components of the cell death machinery in the Ciona intestinalis genome. Cell Death Differ. 2003;10(6):749–753. doi: 10.1038/sj.cdd.4401223. [DOI] [PubMed] [Google Scholar]
- Tischner D, Villunger A. Bcl-G acquitted of murder! Cell Death Dis. 2012;3:e405. doi: 10.1038/cddis.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahsen N, Cande C, Briere JJ, Benit P, Joza N, Larochette N, Mastroberardino PG, Pequignot MO, Casares N, Lazar V, Feraud O, Debili N, Wissing S, Engelhardt S, Madeo F, Piacentini M, Penninger JM, Schagger H, Rustin P, Kroemer G. AIF deficiency compromises oxidative phosphorylation. EMBO J. 2004;23(23):4679–4689. doi: 10.1038/sj.emboj.7600461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann N, Marassi FM, Newmeyer DD, Hanein D. The rheostat in the membrane: BCL-2 family proteins and apoptosis. Cell Death Differ. 2014;21(2):206–215. doi: 10.1038/cdd.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bathina M, Lynch J, Koss B, Calabrese C, Frase S, Schuetz JD, Rehg JE, Opferman JT. Deletion of MCL-1 causes lethal cardiac failure and mitochondrial dysfunction. Genes Dev. 2013;27(12):1351–1364. doi: 10.1101/gad.215855.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal D, Kluck RM, Dewson G. Building blocks of the apoptotic pore: how Bax and Bak are activated and oligomerize during apoptosis. Cell Death Differ. 2014;21(2):196–205. doi: 10.1038/cdd.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens Matthias, Belikov Sergey I., Kaluzhnaya Oxana V., Schröder Heinz C., Hamer Bojan, Perovic-Ottstadt Sanja, Borejko Alexandra, Luthringer Bérengère, Müller Isabel M., Müller Werner E.G. Axial (Apical-Basal) Expression of Pro-apoptotic and Pro-survival Genes in the Lake Baikal Demosponge Lubomirskia baicalensis. DNA and Cell Biology. 2006;25(3):152–164. doi: 10.1089/dna.2006.25.152. [DOI] [PubMed] [Google Scholar]
- Wiens M, Diehl-Seifert B, Muller WE. Sponge Bcl-2 homologous protein (BHP2-GC) confers distinct stress resistance to human HEK-293 cells. Cell Death Differ. 2001;8(9):887–898. doi: 10.1038/sj.cdd.4400906. [DOI] [PubMed] [Google Scholar]
- Wiens M, Krasko A, Muller CI, Muller WE. Molecular evolution of apoptotic pathways: cloning of key domains from sponges (Bcl-2 homology domains and death domains) and their phylogenetic relationships. J Mol Evol. 2000;50(6):520–531. doi: 10.1007/s002390010055. [DOI] [PubMed] [Google Scholar]
- Xiang Zhiming, Qu Fufa, Wang Fuxuan, Xiao Shu, Jun Li, Zhang Yang, Yu Ziniu. ChBax/Bak as key regulators of the mitochondrial apoptotic pathway: Cloned and characterized in Crassostrea hongkongensis. Fish & Shellfish Immunology. 2015;42(2):225–232. doi: 10.1016/j.fsi.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Xue D, Horvitz HR. Caenorhabditis elegans CED-9 protein is a bifunctional cell-death inhibitor. Nature. 1997;390(6657):305–308. doi: 10.1038/36889. [DOI] [PubMed] [Google Scholar]
- Yan N, Gu L, Kokel D, Chai J, Li W, Han A, Chen L, Xue D, Shi Y. Structural, biochemical, and functional analyses of CED-9 recognition by the proapoptotic proteins EGL-1 and CED-4. Mol Cell. 2004;15(6):999–1006. doi: 10.1016/j.molcel.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Zhang H, Holzgreve W, De Geyter C. Bcl2-L-10, a novel anti-apoptotic member of the Bcl-2 family, blocks apoptosis in the mitochondria death pathway but not in the death receptor pathway. Hum Mol Genet. 2001;10(21):2329–2339. doi: 10.1093/hmg/10.21.2329. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Pan MH, Sun ZY, Huang SJ, Yu ZS, Liu D, Zhao DH, Lu C. The genomic underpinnings of apoptosis in the silkworm. Bombyx mori BMC genomics. 2010;11:611. doi: 10.1186/1471-2164-11-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerkandl E, Pauling L. Molecules as documents of evolutionary history. J Theor Biol. 1965;8(2):357–366. doi: 10.1016/0022-5193(65)90083-4. [DOI] [PubMed] [Google Scholar]


