Abstract
Design, controlled synthesis, physico-chemical and biological characteristics of novel well-defined biodegradable star-shaped copolymers intended for advanced drug delivery is described. These new biocompatible star copolymers were synthesised by grafting monodispersed semitelechelic linear (sL) N-(2-hydroxypropyl)methacrylamide copolymers onto a 2,2-bis(hydroxymethyl)propionic acid (bisMPA)-based polyester dendritic core of various structures. The hydrodynamic diameter of the star copolymer biomaterials can be tuned from 13 to 31 nm and could be adjusted to a given purpose by proper selection of the bisMPA dendritic core type and generation and by considering the sL copolymer molecular weight and polymer-to-core molar ratio. The hydrolytic degradation was proved for both the star copolymers containing either dendron or dendrimer core, showing the spontaneous hydrolysis in duration of few weeks. Finally, it was shown that the therapy with the biodegradable star conjugate with attached doxorubicin strongly suppresses the tumour growth in mice and is fully curative in most of the treated animals at dose corresponding approximately to one fourth of maximum tolerated dose (MTD) value. Both new biodegradable systems show superior efficacy and tumour accumulation over the first generation of star copolymers containing non-degradable PAMAM core.
Keywords: HPMA, star-like polymers, drug delivery, doxorubicin, bisMPA, cancer
1. Introduction
Polymer materials have been increasingly studied in recent decades as potential biomaterials for various applications in medicine. Moreover, a considerable number of water-soluble polymer materials with bound anti-cancer therapeutics suitable for drug delivery to tumour tissue have been developed using macromolecular chemistry, given that macromolecules, including polymer drug conjugates, polymer micelles and polymer particles, can passively accumulate in solid tumours due to enhanced permeability and retention (EPR) effect.[1–3] The conjugation of low-molecular-weight drugs with water-soluble polymers confers several advantages, such as prolonged circulation in blood and higher accumulation in tumour tissue, enabling highly effective anti-tumour therapy with minimal drug side effects.[4, 5] The bioactive molecules are typically covalently attached to the polymer carrier via biodegradable spacers, thus enabling the safe transport of inactive drug into the blood stream and controlled drug release in the target tissue.[6–8]
Based on their excellent water-solubility, biocompatibility and non-immunogenic properties, N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer biomaterials [5, 6, 9] are among the most studied water-soluble synthetic polymer carriers. In recent decades, conjugates based on HPMA copolymers containing the cytotoxic drugs doxorubicin (DOX), pirarubicin (THP), or docetaxel [10, 11], bound via a pH- sensitive hydrazone bond, have been developed as highly potent drug-delivery systems for cancer treatment in vivo.[12] The accumulation of these polymer carriers in tumour tissue is molecular-weight dependent.[4] In addition, the size and shape of polymer carriers is critical to their biological behaviour, and various structures of HPMA copolymers with different molecular weights have thus been designed.[13, 14]
Such biomaterials as macromolecules or particles with a higher hydrodynamic diameter (in the range of 10–150 nm) accumulate in tumour tissue to a higher extent than small molecules.[15] HPMA polymer carriers with various structures, such as grafted, block, branched and star polymer conjugates have been designed to fulfil the requirements for an effective drug delivery system (DDS), as their higher hydrodynamic diameter predetermines a higher accumulation in tumour tissue than that of linear polymers with a molecular weight below the limit of the renal threshold.[5, 7] Despite similarities in the “grafting to” procedure used in the synthesis of grafted and star polymers, biodegradable star-like polymer precursors represent the most effective way to increase molar mass and hydrodynamic size in a defined manner with low molecular-weight dispersity above the limit of renal threshold.[16]
Dendrimers are frequently used as cores for star-like polymeric materials due to their well-defined structure and availability in terms of diversity of molecular mass and functional groups. We previously showed that high-molecular-weight star-like polymer DOX conjugates with a polyamidoamine (PAMAM) dendrimer core and HPMA copolymer arms can accumulate in solid tumours in mice to a considerably greater extent than corresponding linear polymer conjugates with DOX.[17] However, the safe elimination of these polymers via the kidney has not yet been verified. The renal threshold for linear polymer carriers based on HPMA is approximately 50–70 kg mol−1.[17] The PAMAM dendrimer core is non-biodegradable, as well as corresponding star-like polymers, meaning that star-like polymer precursor with either an enzymatically or a reductively cleavable spacer between the core and the polymers arms have been designed in order to ensure their renal excretion.[4, 18] A disadvantage of this approach is the multistep synthetic procedure required for star polymers and the slow biodegradation rate of the enzymatically cleavable spacer. Moreover, both enzymatically and reductively degradable spacers are designed to be degradable only within the target cell, meaning that the star-like polymer is transported in the bloodstream and subsequently into tumour tissue as a high-molecular-weight structure of bulky hydrodynamic size. Indeed, the considerable hydrodynamic size can restrict extravasation and penetration within the tumour tissue, decrease eliminability from the organism and decrease the maximum tolerated dose (MTD). [19] These limitations may exclude these in principle non-degradable materials from translation into clinical studies. The use of biodegradable dendrimer cores may facilitate the design of high-molecular star-like polymer carriers with enhanced tumour accumulation without the need to incorporate a degradable spacer. Polyester cores have been shown to hydrolytically degrade within the organism, and thus represent as a degradable core of high-molecular-weight polymer materials that can be degraded even extracellularly in tumour tissue, thereby facilitating pervasion into tumours. [12, 20]
In the present study, we focused on the design and novel advanced and controllable synthesis of high-molecular-weight star-like polymeric materials based on HPMA copolymers using 2,2-bis(hydroxymethyl)propionic acid (bisMPA) dendrons and dendrimers as the biodegradable cores.[21] The bisMPA cores are biocompatible dendritic cores, rendering them valuable candidates as a multifunctional core for a new generation of star-like carriers. To obtain high-molecular-weight polymer materials with a well-defined structure, the use of monodispersed semitelechelic linear copolymers with reactive end group functionality close to one is necessary. Therefore, sL copolymers were synthesised using a controlled polymerisation technique termed RAFT (Reversible Addition–Fragmentation chain Transfer) polymerisation using chain transfer agent with suitable reactive groups. The physico-chemical characteristics, degradation kinetics, in vitro cytotoxicity and in vivo anti-tumour activity as well as the biodistribution in tumour-bearing mice are described in detail here.
2. Materials and methods
2.1. Chemicals
Tert-butyl alcohol, methanol, ethyl acetate, dimethyl sulfoxide (DMSO), 2,4,6-trinitrobenzene-1-sulfonic acid (TNBSA), N,N-dimethylacetamide (DMA), CuBr, 4-quinolinol, and N,N-diisopropylmethylamine (DIPEA) were purchased from Merck-Sigma Aldrich (Germany). Doxorubicin hydrochloride (DOX) was purchased from Meiji Seika, Japan. Dendritic cores; PFD-G4-TMP-azide (48 end groups), PFD-G3-TMP-azide (24 end groups), and PFD-G2-TMP-azide (12 end groups) dendrimers; PFD-G5-acetylene-ammonium (32 end groups); PFD-G4-acetylene-ammonium (16 end groups) and PFD-G3-acetylene-ammonium (8 end groups) dendrons were purchased from Polymer Factory (Sweden). PAMAM dendrimers were purchased from Dendritech (USA). Azo initiator V-70 was purchased from Wako Chemicals (Japan). p-SCN-Bn-Deferoxamine was purchased from Macrocyclics™ (USA). All solvents were of gradient grade or high-performance liquid chromatography (HPLC) grade.
2.2. Synthesis of monomers
N-(2-hydroxypropyl)methacrylamide (HPMA) and N-(tert-butoxycarbonyl)-N’-(6-(methacryloylamino)hexanoyl)hydrazine (Ma-Ah-NHNH-Boc) were synthesized as described previously.[6],[10] 1H NMR spectra of prepared monomers are plotted as Figures SI7 and Figure SI8.
2.3. Synthesis of chain transfer agent for RAFT polymerisation
Synthesis of thiazolidine-2-thione functional chain transfer agent (CTA) 2-cyano-5-oxo-5-(2-thioxo-1,3-thiazolidin-3-yl)pentan-2-yl ethyl carbontrithioate (TTc-TT)
4-Cyano-4-(ethylthiocarbonothioylthio)pentanoic acid (1.51 g, 5.72 mmol) and 2-thiazoline-2-thiol (0.71 g, 6.0 mmol) were dissolved in 15 mL of dichloromethane (DCM) and N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride (1.50 g, 7.80 mmol) was added to the solution followed by a catalytic amount of 4-dimethylaminopyridine. The reaction mixture was stirred at laboratory temperature for 2 h, and extracted with distilled water (2 × 20 mL), before the organic layer was dried with Na2SO4 and the DCM evaporated. The remaining oily residue was diluted with a mixture of hexane:ethyl acetate (1:1; 30 mL) and purified by silica gel chromatography using hexane:ethyl acetate (1:1). The eluent was evaporated and the resulting yellow oily product was crystallised in a refrigerator. The final yield was 1.33 g (64 %).
TT-TTc was characterised using a HPLC Shimadzu system with reverse-phase column Chromolith® HighResolution RP-18e, 100 × 4.6 mm (Merck, Germany) equipped with a UV/VIS photodiode array detector. Gradient elution was performed with 5% - 95% of acetonitrile with 0.1% of TFA for 15 min at a flow rate of 1.0 mL min−1. HPLC yielded a single peak at 305 nm with a retention time of 11.7 min.
ESI−MS: m/z = 365.08 (M-H)+; 1H NMR (600 MHz, CDCl3, δ) 4.59 (t, 2H, NCH2CH2), 3.60–3.66 (m, 2H, CH3CH2S), 3.34 (t, 2H, CH2CH2S), 3.34 (t, 2H, CH2CH2CO), 2.49 (t, 2H, C(CH3)CH2CH2), 1.89 (s, 3H, (CH3)C(CN)), 1.36 (t, 3H, CH3CH2).
2.4. Synthesis of polymer precursors
Semitelechelic HPMA copolymers with the 2-thiazolidine-2-thione (TT) end groups poly(HPMA-co-Ma-Ah-NHNH-Boc)-TT (P1-TT and P2-TT) were prepared by RAFT polymerisation of HPMA and Ma-Ah-NHNH-Boc using 2,2’-azobis(4-methoxy-2,4-dimethylvaleronitrile) (V-70) as an initiator and TTc-TT as a CTA in molar ratios of monomer:CTA:initiator 280:2:1 for P1-TT and 580:2:1 for P2-TT. The molar ratio of HPMA to Ma-Ah-NHNH-Boc in the reaction mixture was 92:8. An example of the synthesis for P1-TT is as follows: HPMA (3.0 g, 20.95 mmol) was dissolved in 25.6 mL of tert-butyl alcohol and mixed in a polymerisation ampule with Ma-Ah-NHNH-Boc (0.571 g, 1.82 mmol), TTc-TT (61.9 mg, 162.7 μmol) and initiator V-70 (25.1 mg, 81.3 μmol) dissolved in 2.8 mL of anhydrous DMA. The polymerisation mixture was bubbled with argon for 10 min and sealed. Copolymerisation was carried out at 30°C for 72 h. The copolymer was isolated by precipitation in a mixture of acetone and diethyl ether (2:1), filtered off and dried under a vacuum. The yield was 2.7 g (76 %). The content of TT groups was determined spectrophotometrically in methanol using the extinction coefficient, ε305=10 500 L mol−1 cm−1.
TTc groups from precursors P1-TT and P2-TT were removed by the reaction with excess of AIBN in DMA (80°C, 3 h) (modified from Perrier et al.[22]).
Semitelechelic copolymers with propargyl-end groups poly(HPMA-co-Ma-Ah-NHNH-Boc)-propargyl (P1-Prg, P2-Prg) were prepared by aminolytic reaction of TT end groups of appropriate polymer precursors with propargylamine. An example of the synthesis of P1-Prg is as follows: P1-TT (1 g, 0.02 mmol) and propargylamine (2.5 mg, 0.04 mmol) were dissolved in methanol and stirred for 1h at laboratory temperature. Unreacted propargylamine was removed by column chromatography (Sephadex LH-20, methanol). The copolymer was isolated by precipitation into a mixture of acetone and diethyl ether (2:1), filtered off, and dried under a vacuum. The course of the reaction was monitored spectrophotometrically by disappearing the spectrum characteristic of TT reactive groups. 1H NMR spectrum of representative linear precursor is shown in Figure SI9.
The synthesis of sL copolymer precursor P3-TT by free radical polymerisation (FRP) was performed as described previously by Etrych et al. [23]
2.5. Synthesis of star-like precursors and drug conjugates
Star-like precursor S1 was prepared using the “click” reaction of bisMPA dendrimer core PFD-G3-TMP-azide terminating with azide groups and sL copolymer precursor P1-Prg with propargyl end group in the molar ratio 1:16 in the presence of CuBr as a catalyst. PFD-G3-TMP-azide core (2.2 mg, 0.37 mmol) and sL copolymer P1-Prg (111.6 mg, 5.9 mmol) were dissolved in 2 mL DMA and bubbled with argon. After 30 min, CuBr (7 mg, 49 mmol) was added and the mixture was bubbled with argon for 3h. The remaining CuBr was chelated with 4-quinolinol and removed using column chromatography (Sephadex LH-20, methanol).
Star-like precursor S2 was prepared by the reaction of amino groups of bisMPA dendron PFD-G5-acetylene-ammonium with the TT-end group of sL copolymer precursor (P2-TT) in the molar ratio 1:10 in the presence of DIPEA. PFD-G5-acetylene-ammonium (8.5 mg, 1.4 mmol) and DIPEA (5.9 mg, 45.7 mmol) were dissolved in 400 μL DMA. After 40 min, P2-TT (600 mg, 14 mmol) in 11 mL DMA was added. The reaction mixture was stirred for 3h at laboratory temperature. The crude product was precipitated to ethyl acetate and dried under vacuum.
Star-like precursors S3 or S4 were prepared by the reaction of PAMAM dendrimer with 32 amino groups (G3) in the presence of DIPEA with sL copolymer precursor with TT-end group, P2-TT (RAFT polymerisation) or P3-TT (FRP polymerisation) in the molar ratio 1:12 or 1:10. An example of the synthesis of S3 is as follows: PAMAM (16.45 mg, 2.4 mmol) and DIPEA (29.0 mg, 228.5 mmol) were dissolved in 1 mL DMSO. After 40 min, P2-TT (1200 mg, 28.8 mmol) in 20 mL DMSO was added.
The star precursors S1, S2 and S3 were purified from unreacted sL copolymers using size exclusion chromatography (SEC) (Sephacryl S-300, 0.15M NaCl). Chromatograms of the reaction mixture and product after purification are provided in the supplementary data – (Figure SI2). The purified product was desalted using SEC (Sephadex G-25, milli-Q water) and freeze dried to obtain the final product. 1H NMR spectrum of representative star precursor is shown in Figure SI10.
The Boc-groups protecting hydrazides of all of the above described star-like polymers (S1 to S4) were removed thermally in distilled water as described previously.[24] 100 mg of star-like polymer was dissolved in 5 mL of milli-Q water, bubbled with argon for 10 min in a polymerisation vial, and sealed. A deprotection reaction was carried out at 100°C for 30 min. The final product was obtained by freeze-drying, and the content of free hydrazide groups was determined using a modified TNBSA assay as described previously. [25]
Star-like polymer conjugates containing DOX bound via a pH-sensitive hydrazone bond were prepared by the reaction of deprotected S1–S4 precursors (bearing free hydrazide groups) with DOX in methanol (MeOH) in the presence of acetic acid. An example of this reaction is as follows: the polymer S1 (100 mg) was dissolved in MeOH (800 μL) and the solution was added to solid DOX (10.7 mg). Next, 80 μL of concentrated acetic acid was added and the reaction mixture was stirred in the dark at laboratory temperature for 20 h. The reaction mixture was then diluted with MeOH and the crude product was purified by column chromatography (Sephadex LH-20, methanol) where the unbound drug was removed. The polymer fraction was concentrated under vacuum, precipitated to ethyl acetate, and dried to a constant weight. The content of bound DOX was determined spectroscopically (ε488=11 200 L mol−1 cm−1, methanol).
For whole-body excretion studies of polymer biomaterials, star-like conjugates with deferoxamine (DFO) S2-DFO and S3-DFO were prepared. Precursors were synthesised in the same way as S2 and S3 in a molar ratio of polymer arm to core of 6:1. The resulting star-like polymer precursors (100 mg) with free amino groups on the cores and p-SCN-Bn-Deferoxamine (10 mg) were dissolved in DMSO (1.9 mL) and stirred overnight. Unreacted DFO was removed using SEC column chromatography (Sephacryl S-300, 0.15M NaCl) in one step together with unreacted sL copolymer. The DFO content was determined using 1H NMR and UV-Vis. S2-DFO (Mw = 312 000 g mol−1, 0.35 mol% DFO) and S3-DFO (Mw = 224 000 g mol−1, 0.58 mol% DFO) were utilised in a biodistribution study with free hydrazide groups on the polymer arms.
2.6. Characterisation of polymer conjugates
The weight-average molecular weight Mw, number-average molecular weight Mn and dispersity Ð of polymer precursors and conjugates were measured using SEC on a HPLC Shimadzu system equipped with a SPD-M20A photodiode array detector (Shimadzu, Japan), differential refractometer (Optilab®rEX), and multiangle light scattering (DAWN HELLEOS II) detectors (both from Wyatt Technology Co., USA). The mobile phase for the TSKgel SuperSW3000, TSKgel3000AW+TSKgel4000AW combination and TSKgel4000SWxl columns was a mixture of methanol:sodium acetate buffer (CH3COONa:CH3COOH; pH = 6.5) (80 : 20; v:v); the flow rate was 0.3 mL min−1 for TSKgel SuperSW3000, 0.6 mL min−1 for TSKgel3000AW+TSKgel4000AW and 0.5 mL min−1 for TSKgel4000SWxl.
The hydrodynamic diameter (Dh) of the conjugates was measured by dynamic light scattering (DLS) (Nano-ZS, Malvern) in a 0.15M NaCl solution at polymer concentration 5 mg mL−1. Nanoparticle tracking analysis (NTA) was performed using NS 300 instrument. NTA is an image analysis method based on Brownian motion of nanomaterial, therefore one can see the uniformity as well as the shape pattern of nanomaterials.
2.7. In vitro release of drug
DOX release rates from conjugates were measured in phosphate buffers at pH 5.0 or 7.4 (0.15 M) at 37°C using a conjugate concentration of 3 mg mL−1. The amount of released DOX was determined by HPLC with an SEC column as described above. The relative area of the peaks (PDA detection at 488 nm) corresponding to the released drug and the polymer-bound drug were used into calculation.
2.8. In vitro degradation of star-like polymers
The degradation rates of star-like copolymers S1 and S2 were studied in 0.15M phosphate buffers at pH 5.0, pH 6.5 or 7.4 at 37°C using a S1 and S2 concentration of 3 mg mL−1. The amount of released linear polymer was determined by HPLC with an SEC column TSKgel4000SWxl (instrument and detectors are as described in section 2.6). The degradation rate was evaluated as the ratio of peaks corresponding to star-like polymer and linear polymer obtained from differential refractive index detector.
2.9. In vitro cytotoxicity
The murine EL4 T cell lymphoma (ATCC TIB-39), 4T1 mammary carcinoma (ATCC CRL-2539), and CT26 colon carcinoma (ATCC CRL-2638) cell lines were purchased from ATCC (USA) and maintained as recommended by the provider. For in vitro cytotoxicity assays, the cells were harvested, washed, and dispensed in to 96 well flat bottom plates (Thermo Fisher Scientific) at 5,000/well (EL4, CT26), or 2,500/well (4T1). Next, the cells were cultivated with serial dilutions of the star DOX conjugates C1–C4 for 3 days and their metabolic activity or proliferation was determined by MTT (EL4 cell line), or 3H-Thymidine incorporation assay (4T1, CT26). The inhibition of cell growth was calculated as the IC50, the concentration of cytotoxic drug which inhibited metabolic activity or proliferation by 50 %. At least four parallel samples were used for each experimental condition.
2.10. In vivo therapeutic activity
A murine EL4 T cell lymphoma model was established in in vivo experiments as follows. Female C57BL/6 mice (H-2b) were obtained from the Institute of Physiology CAS (Prague, Czech Republic). For tumour growth, the mice were subcutaneously (s.c.) transplanted at day zero with 1.105 EL4 cells. Next, the mice were treated intravenously (i.v.) with the star DOX conjugates C1–C4 at day 8, when the diameter of the tumours was 5–8 mm, at a dose of 7.5 mg DOX/kg i.v. or at days 8 and 12 at a dose of 5 mg DOX/kg i.v. Tumour growth and survival time were monitored. The tumour volume was calculated as V=a.b2/2, where a = longer diameter, and b = shorter diameter. Next, mice with fully regressed tumours (long-term survivors, LTS) were re-transplanted with the same number of EL4 lymphoma cells and left untreated.
Analysis of the statistical significance of tumour growth and survival time was conducted using two-way ANOVA and log-rank (Mantel-Cox) tests with GraphPad Prism software. Values of p < 0.05 were considered statistically significant. For all animal treatment experiments, the protocol was approved by the Institutional Animal Care and Use of the Academy of Sciences of the Czech Republic and conducted in compliance with local and European guidelines.
2.11. Determination of DOX content in tissues
Female C57BL/6 mice were transplanted with EL4 lymphoma cells and treated at day 8 as in the treatment experiments, with three mice per group/time interval. Heparinised blood, liver, and tumour tissue samples were taken at 6, 12, 24, 48, 72, and 144 h after treatment. The tissues were excised, weighed and homogenised in PBS, and the DOX content (i.e., the sum of free and polymer-bound DOX) was determined after quantitative acid hydrolysis in 1M HCl. After incubation for 1 h at 50°C, the aglycon of DOX (doxorubicinone) was extracted with chloroform, the organic phase was evaporated to dryness, and the remaining solid was completely dissolved in methanol and analysed using a gradient-based HPLC Shimadzu system equipped with Chromolith® HighResolution RP-18 endcapped column and fluorescence detector (Shimadzu RF-10Axl) (λexc= 488 nm, λem=560 nm). A calibration curve was obtained by the injection of exact amounts of free DOX· into the tissue samples obtained from untreated animals, and the samples were analysed as described above after homogenisation, hydrolysis and extraction. All experiments were carried out in quadruplicate.
2.12. Whole-body retention of polymer biomaterials
For quantitative measurement of the whole-body retention and observation of the clearance pattern of the polymer conjugates, whole-body positron emission tomography (PET) scans of healthy mice intravenously injected with 89Zr-labeled S2-DFO or S3-DFO were performed. The polymer-chelator conjugates were then radiolabelled with 89Zr as described previously [26]. Briefly, 37–74 MBq of 89Zr-oxalate was added to 300 μL of 0.5 M HEPES buffer. The pH was neutralised with a 2M solution of Na2CO3 followed by the addition of aqueous solutions of S2-DFO or S3-DFO conjugates. Next, 1.0–1.5 mg of DFO-polymer conjugate was used per 37 MBq of 89Zr and the mixture was incubated for 2 h at 37°C with constant shaking and purified on PD-10 columns using PBS as the mobile phase. Radiochemical yields were determined via instant thin layer chromatography using silica backed iTLC plates and 50 mM EDTA as the running buffer.
Small animal PET scans were performed using an Inveon microPET/microCT rodent model scanner (Siemens Medical Solutions USA, Inc.). Four-to-five-week-old female Balb/c mice (Envigo, n=4) bearing 4T1 murine breast tumours were intravenously injected with 5–10 MBq of the radiolabelled polymer conjugate and static PET scans were performed at 0.5, 4, 24, 48, 72, 120 and 168 h post-injection (p.i.). The images were reconstructed using a maximum a posteriori (MAP) algorithm, with no attenuation or scatter correction. Decay-corrected whole-body images were used to calculate total radioactivity present and are presented as the percentage of injected dose (%ID). All whole-body imaging studies were conducted according to a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee.
3. Results and discussion
Polymer conjugates based on HPMA with bound DOX, particular with linear, graft, or star-like polymer structures, have been studied extensively in a number of studies and shown to have enhanced efficacy in comparison with free drugs and other systems.[4, 5, 27–34] Nevertheless, polymer biomaterials that combine the advantages of high-weight star-like conjugates during transport in the bloodstream, EPR-driven tumour accumulation and linear polymers upon extravasation and penetration within the tumour tissue have not been developed to date. This study focused on the novel synthesis and in-depth evaluation of the physicochemical and biological properties of biodegradable polymer biomaterial to carry DOX and with the capacity to fulfil these requirements. An innovative combination of functions resulted in the increased passive accumulation of biomaterial in solid tumour and its degradation to small polymers that could more easily penetrate in tissues and undergo excretion from the organism by renal filtration.
3.1. Design and synthesis of linear polymer precursors
Semitelechelic linear copolymers P1-TT and P2-TT were prepared with high functionality of the TT α-end group by RAFT polymerisation using CTA TTc-TT and the azo initiator V-70. To retain the highest possible functionality of TT groups, polymerisation was carried out at 30°C for 72 h. By changing the ratio monomer:CTA:initiator two linear polymers with different molecular weights and with low dispersity were synthesized. The ω-TTc end groups of P1-TT and P2-TT were removed by a method [35] that used excess AIBN in DMA, thus preserving the content of α-end TT groups in polymers. P3-TT was synthesised as control semitelechelic linear copolymer by free radical polymerisation using the feed ratio and conditions to obtain a polymer with similar molecular weight to P1-TT [19]. The characteristics of the polymers are summarised in Table 1.
Table 1.
Characteristics of semitelechelic linear HPMA copolymer precursors
| Sample | Mw [kg mol−1] a) | Ð | NHNH2 [mol.%] b) | FTT or FPrg c) |
|---|---|---|---|---|
| P1-TT | 19 | 1.05 | 5.8 | 0.89 |
| P1-Prg | 19.5 | 1.06 | 5.8 | 0.88 |
| P2-TT | 42 | 1.06 | 5.6 | 0.90 |
| P2-Prg | 42.5 | 1.06 | 5.6 | 0.89 |
| P3-TT | 26.5 | 1.84 | 5.4 | 1.31 |
Molecular weights and dispersity were determined by SEC using multi-angle light scattering and refractive index detectors.
Deprotected hydrazide content was determined by TNBSA assay.
TT end group content was determined spectrophotometrically at 305 nm. The value of Prg is estimated.
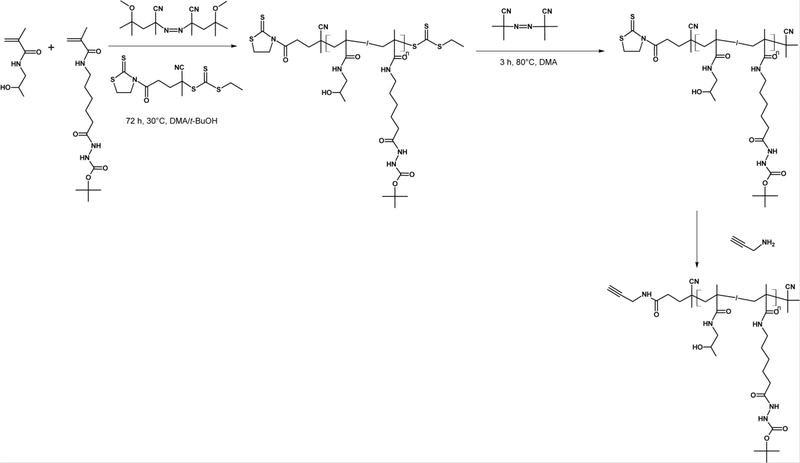
Semitelechelic polymers with α-end propargyl groups were prepared by post-polymerisation modification of the TT end groups of polymers P1-TT or P2-TT using propargylamine. The reaction was quantitative, without a significant alteration in the molecular weight of the polymer precursor. As propargyl groups are difficult to determine precisely, we indirectly determined the content of propargyl groups, by measuring the decrease in absorbance of the TT groups. The reaction scheme of RAFT polymerisation and the post-polymerisation modification of TT end groups as well as the TTc end group removal reaction are shown in Figure 1.
Figure 1.
Reaction scheme of RAFT polymerisation of linear precursors followed by TTc end group removal.
3.2. Design and synthesis of star-like polymer precursors and conjugates
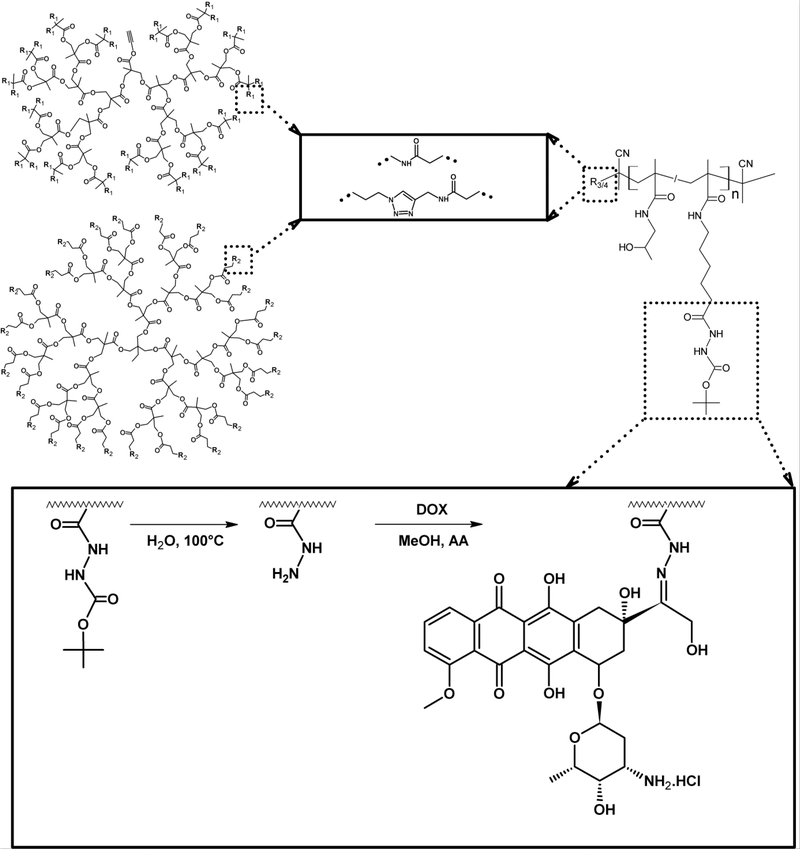
Polyester dendritic cores based on bisMPA were used for the synthesis of biodegradable star-like polymer precursors. Two types of dendritic cores were employed in the synthesis of star polymers: a dendrimer structure with azido groups on the surface or a dendron structure with primary amino groups on the surface. Examples of both cores are detailed in Figure SI1. Star-like polymers were synthetized using the “grafting onto” approach, in which synthetized linear copolymers were grafted onto the dendritic core. Two synthetic methods were employed for the synthesis of star polymer precursors. First, a click reaction between the azido groups of the bisMPA dendrimer core with polymer precursors P1-Prg or P2-Prg catalysed by copper (CuBr) was used for the synthesis of bisMPA dendrimer-based polymer biomaterials. An aminolytic reaction was employed for the second set of star-like polymers synthesised from bisMPA dendron and P1-TT or P2-TT precursors. Both methods are summarised in Figure 2.
Figure 2.
Reaction scheme of star-like polymer formulation. Left: chemical structures of dendron (up) or dendrimer (down). Right: structure of the HPMA-based copolymer. The reaction steps leading to the drug conjugate are shown in the lower frame.
The molecular weight of novel star-like polymer biomaterials and thus the hydrodynamic size in the solution can be easily adjusted to therapeutic demands. To demonstrate this feasibility, an entire library of star-like polymer biomaterials with various generations of dendritic cores was synthetized, as detailed in Tables SI1 – SI4. To further investigate the structure to effectivity relationship, two types of linear copolymers differing in molecular weights were used: a smaller one of 19 kg mol−1 (P1-TT or P1-Prg), corresponding to a polymer easily excretable by glomerular filtration; and a larger one of molecular weight 42 kg mol−1 (P2-TT or P2-Prg), representing a polymer slowly excretable by glomerular filtration.
Using the hydrolytically degradable bisMPA dendron core and aminoreactive polymers P1-TT or P2-TT we synthetized star-like polymers S5–S13 or S28–S36, respectively, with molecular weights ranging from 87 kg mol−1 to 350 kg mol−1 or from 156 to 493 kg mol−1 and low dispersity, respectively. We conclude that the molecular weight can be appropriately adjusted by the generation of bisMPA dendron core, the molecular weight of the semitelechelic copolymer, and the molar ratio of copolymer to core (Tables SI1 and SI3). The hydrodynamic diameters estimated by DLS of the synthetized dendron based star-like polymers in physiological solution (0.15M NaCl) ranged from 13 to 22 nm or from 19 to 28 nm, respectively. Thus, we were able to synthesise the monodispersed star polymer precursors with a broad range of molecular weights starting from slightly less than 100 kg mol−1 up to almost 500 kg mol−1. A representative chromatogram of dendron based star-like polymer after synthesis and after the removing of unreacted semitelechelic copolymer is shown in Figure SI2. Using this novel synthetic route, star-like polymers with tailored sizes could be prepared within a reaction time of 3 h.
The kinetics of this reaction, in which increases in molar mass as well as decreases in the content of TT reactive groups, were monitored, are shown in Figure 3. Moreover, the use of RAFT polymers allowed the formation of the star-like polymers with very high molecular weights and dispersity below 1.1, representing highly suitable candidates for further preclinical evaluation.
Figure 3.
Kinetics of the formation of star-like polymer precursors. ■ : % of remaining TT groups on the polymer chains; ♦ : % of unreacted linear polymer; ⚫ : molar mass of star-like polymer.
Similar to the dendron-based star polymers we successfully synthesised bisMPA dendrimer-based star polymers S14–S27 and S37–S50, as shown in Tables SI2 and SI4. In this scenario, a click reaction using propargyl-azide couples was employed in the preparation of highly defined star polymer biomaterials. The resulting bisMPA dendrimer based star polymers corresponded adequately with the aminolytically prepared bisMPA dendron based star polymers in terms of molecular weight and size: S8 or S11 were comparable with S15 or S18, which were synthetised with the same molar ratio between the dendritic core and linear polymer. For the bisMPA dendrimer-based star polymer biomaterials we were able to control the molecular weight and size in the range of 114 – 720 kg mol−1 and 16–31 nm. The slightly higher molecular weight and diameter found in the bisMPA dendrimer-based star polymers resulted from two factors: i) a greater increase in the ratio of polymer to core (dendrimer of generation 4 had a higher amount of reactive groups); and ii) the click reaction was not deactivated compared with the slow hydrolytic deactivation of TT groups in solution. Representative chromatograms from SEC of bisMPA dendrimer star-like polymers S45, S47, and S50 after the removal of unreacted linear polymers are shown in Figure SI3. The shift in retention time corresponded accurately with increasing molecular weights of the synthetised star-like polymers. In summary, we synthesised various star polymer biomaterials with a broad range of molecular weights and sizes in solution. The molecular weights of these polymer biomaterials were readily adjustable with the proper selection of bisMPA core, sL polymer type, and sL polymer to core ratio.
To demonstrate the dependence of molar mass or size of the star polymers on sL polymer precursor size and type of the core, the characteristics of the selected star polymers are summarised in Table 2. The same ratio of linear polymer to dendritic core (8:1) was employed, and the star-like polymers differed only in the linear precursor used for synthesis, P1-Prg/TT (Dh= 6.9±0.2 nm) or P2-Prg/TT (Dh= 9.8±0.2 nm). Interestingly, the size and molecular weight of star polymers was virtually independent of the type of dendritic core (see S8 versus S18 or S31 versus S41), and therefore also of the type of conjugation reaction. The use of linear polymers with high molecular weight and hydrodynamic size led to the synthesis of star polymers with lower amounts of polymer chains connected to the core but with significantly higher molecular weight. We attribute the lower number of linear precursors linked to the dendritic core of P2-Prg/TT (5.0 or 5.3) compared with the star polymers from P1-Prg/TT (7.2 or 7.6) to steric hindrances on the core.
Table 2.
Characteristics of selected star-like polymers with the same molar ratio of polymer to dendritic core
| Sample | Core/Pol a) | Mw [kg mol−1] b) | Ð | Generation of core | Molar ratio Pol : core | No. of chains on core | Dh (int.) c) [nm] |
|---|---|---|---|---|---|---|---|
| S8 | Dendron/P1-TT | 137 | 1.01 | G4 | 8 : 1 | 7.2 | 16.1±0.6 |
| S31 | Dendron/P2-TT | 222 | 1.06 | G4 | 8 : 1 | 5.3 | 19.5±0.5 |
| S18 | Dendrimer/P1-Prg | 149 | 1.06 | G3 | 8 : 1 | 7.6 | 16.7±0.3 |
| S41 | Dendrimer/P2-Prg | 213 | 1.13 | G3 | 8 : 1 | 5.0 | 20.4±1.1 |
Type of dendritic core used (Dendron = bisMPA dendron, dendrimer = bisMPA dendrimer,), Pol = semitelechelic polymer precursor used for star-like polymer formulation
Molecular weight Mw and dispersity Ð were determined by SEC using multi-angle light scattering and refractive index detectors.
Hydrodynamic diameter was measured by DLS in 0.15M NaCl
Additionally, the highly precise synthetic route permitted the tailored synthesis of star-like biomaterial on demand for the application. The described synthetic procedure is robust, as documented by the data summarised in Table 3. These results unambiguously show that star polymer biomaterials with a molar mass of approximately 150 kg mol−1 can be synthesised using several feed settings with both linear polymers varying in molecular weight, ratio of polymer to dendritic core, and core generation. Moreover, both bisMPA based dendritic cores (dendron or dendrimer) could be used. Thus, this flexibility of the synthetic method confirmed the robustness of the described synthetic strategy of star polymer biomaterials. Characteristics of selected star-like polymers with comparable molar mass are summarized in Table 3 and corresponding GPC chromatograms are plotted in Figure SI11.
Table 3.
Characteristics of selected star-like polymers with comparable molar masses
| Sample | Core/Pola) | [kg mol−1] b) | Ð | Generation of core | Molar ratio Pol : core | No. of chains on core | Dh (int.) c) [nm] |
|---|---|---|---|---|---|---|---|
| S10 | Dendron/P1-TT | 149 | 1.01 | G4 | 16 : 1 | 7.8 | 16.3±0.2 |
| S28 | Dendron/P2-TT | 156 | 1.06 | G3 | 4 : 1 | 3.7 | 18.5 ±0.4 |
| S22 | Dendrimer/P1-Prg | 150 | 1.06 | G4 | 8 : 1 | 7.7 | 17.1±1.0 |
| S37 | Dendrimer/P2-Prg | 166 | 1.12 | G2 | 4 : 1 | 3.9 | 19.8±0.6 |
Type of dendritic core used (Dendron = bisMPA dendron, dendrimer = bisMPA dendrimer,), Pol = polymer precursor used for star-like polymer formulation
Molecular weights and dispersity were determined by SEC using multi-angle light scattering and refractive index detectors.
Hydrodynamic diameter was measured by DLS in 0.15M NaCl
To evaluate the biological behaviour of our star polymer biomaterials, we synthesised four star polymers S1–S4 that differed in structure (Table 4). Star polymers S1 and S2 were prepared from biodegradable bisMPA dendritic cores, while the previously developed non-degradable S3 and S4 were based on non-degradable PAMAM dendrimers and served as control star-like polymers. The star-like polymer S4 was synthesised from linear precursor P3-TT prepared by FRP, and is therefore characterised by higher dispersity.
Table 4.
Characteristics of HPMA star-like polymer precursors employed for the polymer-drug conjugate synthesis
| Sample | Core/Pol a) | Mw [kg mol−1] b) | Ð | No. of chains on core Teor. / real c) | Dh (int.) d) [nm] | ζ-potential [mV] e) |
|---|---|---|---|---|---|---|
| S1 | Dendrimer/P1-Prg | 268 | 1.1 | 16 / 13.7 | 20.5±0.8 | −1,65±0.98 |
| S2 | Dendron/P2-TT | 354 | 1.2 | 10 / 8.4 | 23.9±0.7 | −0,25±0.97 |
| S3 | PAMAM/P2-TT | 456 | 1.3 | 12 / 10.8 | 27.1±0.4 | −1,99±1.28 |
| S4 | PAMAM/P3-TT | 204 | 1.8 | 10 / 8.2 | 22.6±0.9 | −0.3±0.2 |
Type of dendritic core used (Dendron = bisMPA dendron, dendrimer = bisMPA dendrimer, PAMAM = PAMAM dendrimer). Pol = polymer precursor used for star-like polymer formulation
Molecular weights and dispersity were determined by SEC using multi-angle light scattering and refractive index detectors.
Ratio between HPMA copolymer chains and dendrimer core, theoretically calculated from feed and real calculated by dividing star-like Mw with precursor Mw.
Hydrodynamic diameter was measured by DLS in 0.15M NaCl
ζ-potential values were obtained from Malvern Zetasizer Nano ZSP
The polymer precursors S1–S4 were used for the synthesis of star-like polymer conjugates with DOX bound covalently via a hydrazone bond. The resulting conjugates C1–C4, (Table 5) were subsequently used for in vitro as well as in vivo biological evaluation. The conjugation of DOX did not significantly influence the molecular weight, size, and dispersity of the prepared star-like polymer-DOX conjugates. All conjugates contained approximately 10 wt% of DOX. All conjugates were checked for molecular mass and size (Table 5) by SEC, DLS and NTA, an image analysis method based on Brownian motion of nanomaterials. Picture and video captured from this measurement is attached as Figure SI12. Calculated size from NTA measurement was in a good agreement with values obtained from DLS experiments. NTA measurements proved the spherical morphology of the studied star polymers. Curves from DLS for the whole history of conjugate C2, as a representative example, are shown in Figure SI13.
Table 5.
Characteristics of HPMA star-like polymer conjugates with DOX
| Sample | Core/Pol a) | Mw [kg mol−1] b) | Ð | Dh [nm] c) | DOX [wt%] | ζ-potential [mV] e) |
|---|---|---|---|---|---|---|
| C1 | Dendrimer/P1-Prg | 280 | 1.3 | 28.5±0.8 | 9.0 | −3.3±0.9 |
| C2 | Dendron/P2-TT | 360 | 1.2 | 35.4±2.3 | 10.0 | −2.6±0.2 |
| C3 | PAMAM/P2-TT | 511 | 1.3 | 40.4±5.2 | 10.6 | −0.7±0.3 |
| C4 | PAMAM/P3-TT | 220 | 1.7 | 26.0±3.3 | 10.1 | +5.5±0.8 |
Type of dendritic core used (Dendron = bisMPA dendron, dendrimer = bisMPA dendrimer, PAMAM = PAMAM dendrimer), Pol = polymeric precursor used for star-like polymer formulation
Molecular weights and dispersity were determined by SEC using multi-angle light scattering and refractive index detectors.
Hydrodynamic diameter was measured by DLS in 0.15M NaCl
ζ-potential values were obtained from Malvern Zetasizer Nano ZSP
3.3. Degradation study and in vitro drug release
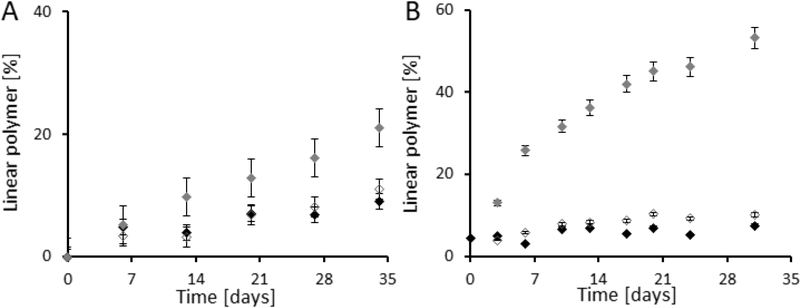
The hydrolytic stability/degradability of the prepared star polymer biomaterials was verified in phosphate buffer at pH 7.4, pH 6.5 and pH 5.0. The results of the incubation experiment demonstrated the influence of the linker structure between the linear polymer and core on the degradation rate of the star biomaterial. The dendron based star-like polymer S2 with an amidic linker had lower hydrolytic stability than dendrimer based polymer S1 with triazine linkage (Figure 4A versus Figure 4B). The degradation rate at pH 5.0 and 6.5 was very slow for all of the star polymers, independent of the type of linker. However, the degradation rate at pH 7.4 was much faster, representing the classical behaviour of ester bond degradation. The course of degradation for polymer S2 is depicted in Figure SI4 (pH 7.4) and Figure SI5 (pH 5.0) on different days as SEC chromatogram overlay. The degradation half-life was calculated for both polymers from the slope of the degradation rate curves. For the dendrimer based star polymer biomaterial S1, the half-life at pH 7.4 was 79 days, while 22.4 days was determined for dendron based star polymer S2. The difference in degradation rate was in agreement with the described slightly rapid hydrolytic degradation of bisMPA dendrons compared with bisMPA dendrimers, which is based on the easier penetration of water molecules into the dendron structure. Moreover, we hypothesise that the triazine linker containing the aliphatic chain partly stabilises the terminal ester bond in the polymer S1. We presuppose that these polymers are even degraded following enzymatic attack in the tumour microenvironment and that degradation proceeds more rapidly, leading to the small polymer degradation product of size < 10 nm.
Figure 4.
Degradation study of star-like polymer S1 with azido groups on dendrimer core (A) and star-like polymer S2 with amino groups on dendron core (B). Percent of linear polymer was estimated from SEC from the RI detector as the ratio between the area under peaks corresponding to star-like and linear polymer. ♦ pH 7.4 ◊ pH 6.5 ♦ pH 5.0
We clearly show that the type of dendritic structure, together with the functional groups on the surface, does not affect the synthesis itself as similar polymer biomaterials can be synthesized (with the same Mw and Rh) using both cores. Nevertheless, the type of core together with the linker structure strongly influences the degradation rate as the hydrolytic half-life of developed biomaterial was adjustable from 22 days up to 80 days.
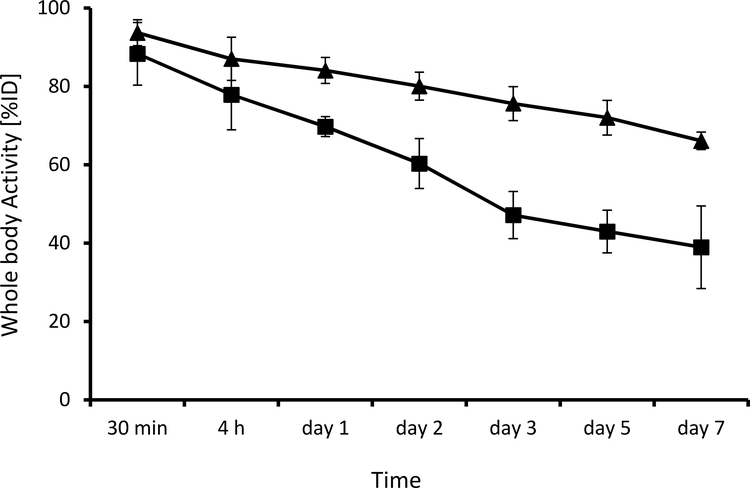
To verify the biodegradability of our materials, bisMPA dendron-based and PAMAM-based star polymer biomaterials, S2-DFO and S3-DFO, were radiolabelled with 89Zr. PET images of up to 7 days were used to investigate the whole-body retention of radioactivity upon intravenous injection of radiolabelled polymers in 4T1 tumour-bearing mice. Both S2-DFO and S3-DFO demonstrated very high radiolabelled yields of above 95%. The results shown in Figure 5 clearly demonstrate that biodegradable star biomaterial based on a dendron core (S2-DFO) was excreted significantly faster than non-degradable material (S3-DFO), which was excreted slowly via the hepatobiliary route. At 24 h p.i., 84.1 ± 3.3 and 69.7 % ± 2.6 %ID of S3-DFO and S2-DFO, respectively, were still present. After one week, more than 60 % of the degradable star biomaterial had already been cleared from the body (38.9 ± 10.5 %ID retained) in comparison with approximately 34% clearance found for the stable PAMAM-based star polymer (66.1 ± 2.2 %ID still present). These data are in accordance with the findings of previous degradation studies. To summarise, biodegradable star polymer biomaterials are degraded in the body at a rate approximately the same as that determined during the previous degradation experiment. Thus, the results indicate that spontaneous hydrolytic degradation is the main cause of biomaterial degradation.
Figure 5.
Whole-body retention of radioactivity, upon intravenous injection of star-like conjugates into 4T1 tumour-bearing mice (n = 4); ■ S3-DFO; ▲ S2-DFO.
Finally, to verify the drug-delivery capacity of the star polymer biomaterials, the anti-cancer drug DOX was conjugated covalently to the star copolymers via pH-sensitive hydrazone bond. As expected, DOX was released more rapidly at acidic pH simulating intracellular pH (pH 5.0), with almost 80 % released in 5 h, or extracellular tumour pH (pH 6.5), with more than 30% released in 36 h, then at pH 7.4 mimicking the pH in the blood stream, where up to 10 % was released in 36 h. We observed no significant differences between the release rate of DOX for all polymer biomaterials at pH 5.0, pH6.5 and 7.4 (see representative data for conjugate C1 in Figure SI6), indicating that hydrolytic attack of the hydrazone bond was not influenced by the carrier structure, regardless of the dendritic core and size of polymer used. In summary, the hydrazone bond enables significant drug release following a small decrease in pH either within the cells or even in the extracellular space of tumours, meaning that the released DOX can readily penetrate tumour cells. Both, degradation and release study was also performed in human plasma. Expectably, no significant differences were found for both degradation rate and drug release rate in comparison to that obtained at phosphate buffer pH 7.4.
3.4. In vitro cytotoxicity
As expected, the cytotoxic activity against EL4 T-cell lymphoma cells in vitro of all conjugates carrying DOX attached via a pH-sensitive hydrazone bond did not differ significantly and was about one order of magnitude lower than that of the free drug (Table 6). In all experiments, the in vitro cytotoxicity of star polymer containing bisMPA-based dendrimer (conjugate C1) was slightly lower than that containing the bisMPA-based dendron (conjugate C2). The conjugates with a non-degradable structure of the core, based on the PAMAM dendrimer (conjugates C3 and C4), showed almost identical in vitro cytotoxic activity, irrespective of any differences in molecular weight and synthesis of the polymer carrier. Cytotoxic activity was slightly higher than the activity seen in conjugates based on bisMPA dendrimer or dendron. Nevertheless, the differences between the star polymers were minimal, indicating that these small differences in in vitro cytotoxicity may not significantly influence the in vivo efficacy in real tumour treatment. Moreover, the in vitro cytostatic activity of the conjugates was determined in two other murine cell lines, murine mammary 4T1 (DOX sensitive) and colon CT26 (cell line with inherent chemoresistance) cells. Experiments in both cell lines showed similar cytotoxicity results for all polymer conjugates, verifying the relationship between bisMPA- and PAMAM-based conjugates seen in the EL4 cell line. The cytostatic activity of free DOX in drug-resistant CT26 cells was lower based on a naturally elevated level of P-gp expression.[36] Interestingly, the cytostatic activity of all polymer conjugates against CT26 cells was only 2 to 4.5 times lower, while the difference between DOX and the conjugates was much higher in the two other cell lines. This observation is in agreement with previously published studies in which HPMA-based conjugates could overcome P-gp-mediated drug resistance.[37] The obtained IC50 values are summarised in Table 6.
Table 6.
In vitro cytotoxic activity of star-like conjugates C1-C4 in murine tumour cell lines. IC50 ± SD values are expressed as ng of DOX eq. mL−1. Each IC50 value is the mean of data obtained from at least three independent experiments.
| Sample | Core a) | EL4 b) | CT26 c) | 4T1 c) |
|---|---|---|---|---|
| C1 | Dendrimer | 160.1 ± 51.2 | 86.6 ± 31.3 | 44.1 ± 19.8 |
| C2 | Dendron | 125.8 ± 25.5 | 58.9 ± 19.2 | 41.3 ± 20.3 |
| C3 | PAMAM | 80.4 ± 19.0 | 44.8 ± 1.2 | 28.0 ± 0.2 |
| C4 | PAMAM | 79.2 ± 20.2 | 50.9 ± 17.8 | 36.5 ± 14.5 |
| DOX | 9.5 ± 1.8 | 20.9 ± 3.5 | 7.0 ± 0.9 |
Type of dendritic core used (Dendron = bisMPA dendron, dendrimer = bisMPA dendrimer, PAMAM = PAMAM dendrimer)
Cytotoxic activity detected by MTT assay.
Cytostatic activity detected by 3H-thymidine incorporation.
3.5. Anti-tumour activity in murine tumour model
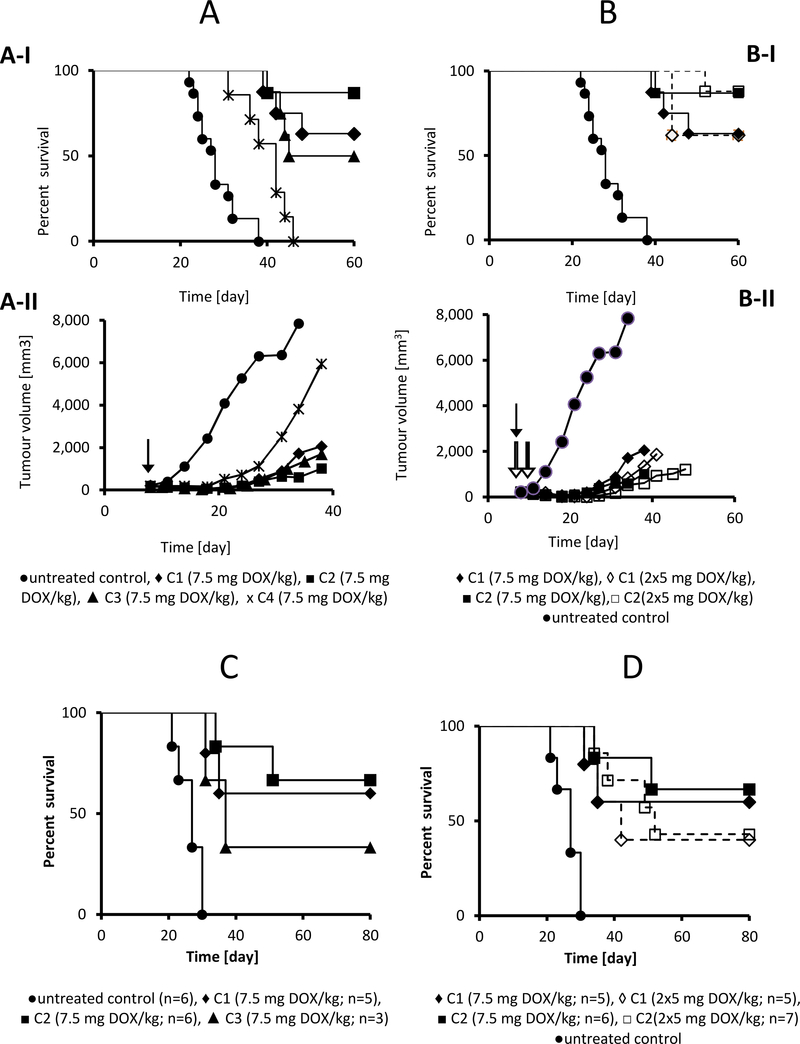
The biodegradable star polymer biomaterials containing DOX (C1 and C2) showed significant anti-tumour activity in the murine EL4 T-cell lymphoma model, even at the low dose of 7.5 mg DOX eq./kg. This dose was equal to one third of MTD of PAMAM-based C4 biomaterial, determined previously as 22.5 mg DOX eq./kg injected i.v. as a single treatment.[19] Moreover, the MTD of biodegradable polymers C1 and C2 was even higher, achieving 30 mg DOX eq./kg; the used dosage was therefore approximately one fourth of the MTD of the novel biomaterials. Both PAMAM-based star polymer biomaterials C3 and C4 significantly reduced the tumour growth (Figure 6A–II), but only C3 was able to cure almost half of the treated mice (Figure 6A–I). The superior effect of C3 compared with C4 may be attributable to its well-defined C3 structure, showing a narrow dispersity of molecular weight. Thus, the C3 biomaterial does not contain, in contrast to C4, a fraction of polymers with low molecular weight, which are much more easily excreted from the organism, and a polymer fraction with very high molecular weight, which cannot extravasate into the tumour. Based on our previous results [38], the changes in Mw and hydrodynamic diameter in the range of 200– 600 kg·mol−1 and 25–50 nm, respectively, do not play a significant role in the efficacy of the polymer nanotherapeutics.
Figure 6.
Overall survival and tumor growth of EL-4 lymphoma-bearing mice treated with polymer conjugates C1-C4. C57BL/6 mice were transplanted s.c. with 1× 105 EL4 cells and treated with 7.5 mg DOX eq./kg of conjugates C1-C4 (A) or with a single dose of 7.5 mg DOX eq./kg or two doses of 5 mg DOX eq./kg of conjugates C1 and C2 (B). Tumour growth and survival were monitored. The figure summarises the results of two independent experiments with eight mice in each experimental group. The difference between the untreated and all treated groups (A-I, B-I) was highly significant (p<0.0001). The difference between the C1, C2 and C3 treated groups (A-I) was not significant. The difference between well-defined conjugates C1-C3 and C4 was significant (p<0.001). Arrows indicate the dosing points. Black arrows indicate the injection of the single dose, white arrows indicate the injections in the two-dosing scheme. Figure 6 - C and D: Survival of mice with fully regressed EL4 T cell lymphomas following treatment with bisMPA-based DOX conjugates (as described in Fig. 6A, 6B). At 170 days after first tumour transplantation, mice were injected s.c. with 1×105 EL4 cells and left untreated. Control = naive mice injected with EL4 cells. Tumour growth (not shown) and survival were monitored.
Interestingly, treatment with the biodegradable star polymer C1 and C2 biomaterials strongly suppressed tumour growth (Figure 6A–II and SI14) and was fully curative in most of the treated animals (Figure 6A–I). The bisMPA dendron-based biomaterial C2 showed a superior effect, inducing complete tumour regression in seven out of eight animals in the experimental group. The enhanced effect of C2 in comparison with C1 is most likely attributable to a more rapid degradation of C2 to smaller polymer fragments that can more easily penetrate tumour tissue. This is in agreement with the hydrolytic stability data of the star polymers S1 and S2. Moreover, we can hypothesise that potential enzymatic degradation in vivo occurs and leads to the increased extravasation of the biodegradable biomaterials. In that event, we would expect the effect to be more pronounced in dendron-based systems, as the enzyme should penetrate the dendron structure of C2 more easily than the more compact dendrimer core of C1.
Two dosing schemes were evaluated for the C1 and C2 conjugates: single dose (7.5 mg DOX eq./kg) injected at day 8 was compared with two consecutive doses (5 mg DOX eq./kg each) administered at days 8 and 12. For both polymer biomaterials, the dosing schemes produced equivalent results (Figure 6B–I, 6B–II, and SI14), pointing to the single dose as the optimal treatment. This findings correlates well with our previous data showing complete cure with single doses of various HPMA-based conjugates of DOX [19, 39], although such a low dose was not previously shown to be effective. Notably, these doses were far below the limit associated with systemic toxicity (MTD 22.5 DOX eq./kg for C4, and 30 mg DOX eq./kg for C1 and C2), inducing no observable signs of systemic toxicity, e.g. weight loss. This significantly points to a remarkably wide therapeutic window. Free parent drug (DOX), even when administered at a greater dose of 10 mg DOX/kg (close to the MTD value) was insufficient in complete tumour regression and only induced prolonged survival as compared with untreated mice (Fig. SI15).
One of the most important features of HPMA copolymer-based drugs containing biomaterials is their capacity to induce tumour-specific, immunologically mediated tumour resistance in the treatment of experimental tumours. [40–42] For the HPMA-copolymer biomaterials carrying DOX bound via a hydrazone bond, the induction of immunogenic cancer cell death has been shown [43] and could contribute to the enhancement of the immune system capability to eliminate tumour cells or metastatic nodules remaining after primary treatment. Here, we re-challenged all animals with tumours that had completely regressed due to treatment with the same (i.e., lethal) dose of EL4 cells, and maintained these animals without additional treatment. Notably, a significant proportion of the mice did not develop a second tumour. Although the number of re-challenged mice was limited, this finding supports the development of effective anti-tumour immune response (Figure 6C, D).
This finding is in agreement with our previously published data.[40–42] We have already proven the anti-tumour immunity in mice cured with various HPMA-based conjugates carrying DOX or taxanes using several assays.[10, 40–43] Again, treatment with a single dose of bisMPA-based star polymers C1 and C2 was associated with a better outcome, as evidenced by the clear trend towards a higher proportion of resistant animals compared with mice cured using two doses of the conjugates (Figure 6D).
3.6. Tumour and liver accumulation
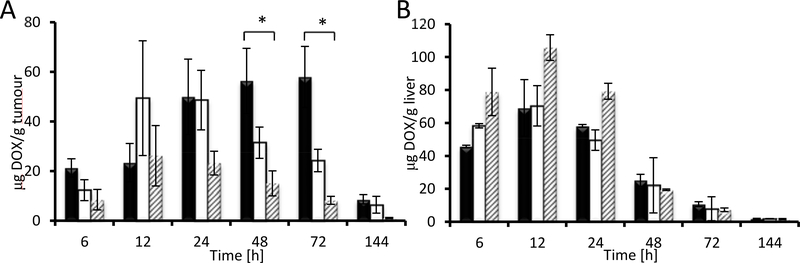
The superior efficacy of conjugates C1 and C2, which had similar Mw and size in aqueous solutions (Table 5), is most likely related to carrier degradation, as the structure is subject to hydrolysis within several days (Figure 4). From these data, it may be inferred that conjugates containing bisMPA cores are relatively stable for several days in the bloodstream and accumulate in tumour tissue at least as effectively as PAMAM star conjugates. [7, 38, 44] Nevertheless, the carrier with bisMPA core disintegrates within several days post-treatment, during which its accumulation is very high. Carrier hydrolysis, either spontaneous or enzymatically driven, produces smaller polymers which can penetrate the interstitial space in the tumour tissue more effectively than non-degradable PAMAM-based conjugates, resulting in higher or more evenly distributed drug in the tumour microenvironment. This also leads to higher accumulation of DOX in the tumour in the case of faster degradable dendron based materials as shown in Figure 7A.
Figure 7.
DOX accumulation in tumour (A) and liver (B) after administration of star polymer-DOX biomaterials. Dark bars: bisMPA-dendron based C2; light bars: bisMPA-dendrimer based C1; grey striped bars: PAMAM-based C3.
Following dendron-based C2 administration, DOX accumulation in tumour is initially higher, and then continuously rises and peaks between 24 and 72 h. However, tumour accumulation after dendrimer-based C1 administration is initially lower but rises steeply between 6 and 12 h, reaching a plateau and slowly decreasing after 24 hours. We hypothesize that different pattern of drug accumulation in tumour upon treatment with C1 and C2 is caused by different distribution of star polymer systems within the tumour mass. C2 conjugate is more rapidly degraded to small polymer chain which could penetrate to the tumour mass effectively and increase the overall amount of drug in tumour over time. On the contrary, C1 is degraded very slowly; thus, its large size could be limiting for the enhanced penetration of the conjugate across the interstitium. Thus, the biodegradable C2 conjugate is supposed to ensure regular and fast delivery of the polymer-bound drug to the tumour mass and directly to tumour cells better than the non-degradable C1 conjugate. Notably, both biodegradable star polymer biomaterials were able to deliver significantly higher amounts of DOX to the tumour tissue when compared with the PAMAM-based C3 system.
However, DOX accumulation in the liver over 6–24 h was significantly suppressed for both bisMPA-based biomaterials compared with the PAMAM-based system, showing a more favourable biodistribution profile for the novel biomaterials (see Figure 7B). Moreover, none of the studied biomaterials induced long-term accumulation of drug in liver tissue. Drug accumulation in the liver decreased after 48 h and was negligible at longer-term intervals, indicating that short-time accumulation is likely driven by high perfusion of blood in the liver rather than by persistent interaction-based accumulation.
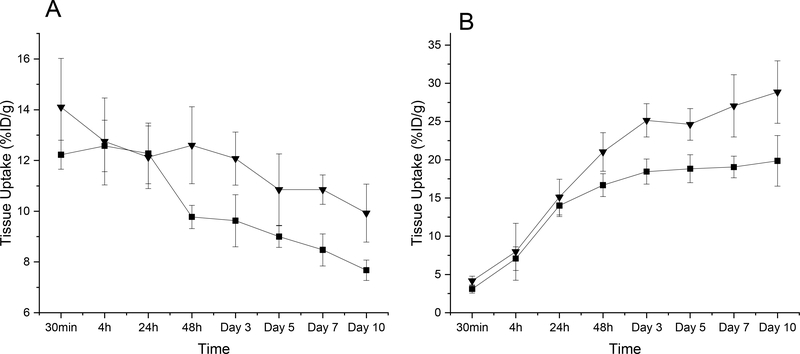
The liver and tumour accumulation of star polymer systems was determined by PET imaging using 89Zr labelled star polymers. In the liver, the initial higher signal of polymers, similarly as that observed in the case of DOX, gradually decreased within the time of experiment (Figure 8A). The liver accumulation of the biodegradable S2-DFO was lower than that of S3-DFO; thus revealing effect of the degradability even in the healthy tissue. Nevertheless, whilst the amount of DOX in the liver tissue decreased after 6 days almost to zero, still 9 to 11% of the injected polymer dose was detected in the liver tissue. We assume that DOX accumulated in the liver is released and degraded and/or removed by urine within 6 days; thus leading to zero concentration detected after this time (Figure 7B). On the contrary, polymer is entrapped in the liver for longer period time as its degradation and removal by urine or hepatobiliary pathway is much slower process.
Figure 8.
Liver (8A) and tumour (8B) retention of radioactivity upon intravenous injection of star-like conjugates into 4T1 tumour-bearing mice (n = 4); ■ S2-DFO; ▼ S3-DFO.
Polymer accumulation in tumour mass was precisely determined using the PET imaging and compared to that of DOX tumour accumulation, see Table SI5. Contrary to DOX, the polymer signal in tumour determined by PET imaging gradually increased in time being the highest for the S3-DFO after 10 days. After two days, the accumulation of nondegradable S3-DFO started to be higher thus proving the influence of biodegradability of S2-DFO on the removal of the polymer system from tumour tissue similarly as in the case of liver. The S2-DFO, on the contrary to S3-DFO, should be degraded to small polymer fragments more easily removable from the tumour tissue and finally from the organism by urine. Comparison of accumulated injected dose (ID) in tumour is enclosed in Table SI5. Significant difference was found between the behaviour of polymer and DOX accumulation for both star polymers which is the basis for the treatment potential of the DOX-containing nanomaterials C2 and C3. After 3 days of treatment using C2 or C3 the tumours started to decrease their size as the destroyed part of the tumour is gradually removed. In parallel, the amount of DOX retained in the regressing tumours also significantly decreases. Indeed, 89Zr labelled S2-DFO and S3-DFO were accumulating during longer time interval and to a higher extent as the tumours expanded during the whole period due to the absence of cytotoxic drug. We speculate that degradation of dendron-based star polymer, S2-DFO or C2, to smaller polymers may lead to the deeper penetration in tumour tissue and released DOX is afterward entrapped and remains accumulated in the tumour mass for longer time. On the other hand, the nondegradable S3-DFO or C3 is stable and stays large during all the time of experiment, what could be limiting for the penetration of the conjugate and the drug across the interstitium. The data clearly demonstrate the beneficial accumulation of DOX carried by degradable star systems, what exactly corresponds with the treatment efficacy.
Based on the results from the in vivo experiments, we can conclude that biodegradable star polymers with a biodegradable bisMPA core grafted with sL HPMA copolymers represent excellent candidates for further extensive pre-clinical evaluation.
4. Conclusion
Here, we describe the design, synthesis, and biological evaluation of novel biodegradable star polymer biomaterials based on a biodegradable bisMPA dendrimer or dendron core grafted with biocompatible sL HPMA copolymers. The novel synthetic strategy allows for the adjustment of the molecular weight Mw and Dh of the biomaterials from 87 to 720 kg mol−1 and from 13 to 31 nm, respectively, based on the selection of dendritic core, sL HPMA copolymer, and ratio of polymer to dendritic core. The degradability of the system was verified in aqueous buffers and plasma in vitro and in vivo using PET imaging. Excellent clearance from the body was shown in vivo for the dendron-based material, with more than 60% eliminated after 7 days. The novel bisMPA star polymer biomaterials represent excellent drug carriers with optimised in vivo biological properties. Treatment with the biodegradable star polymer biomaterials strongly suppressed tumour growth and was fully curative in most of treated animals at low doses of anticancer drug, showing better results for rapidly degradable dendron-containing star polymers. Both biodegradable systems demonstrated superior efficacy over non-degradable star polymers containing a non-degradable PAMAM core. Interestingly, the biodegradable materials showed superior tumour accumulation of carried drug, significantly better than that observed for non-degradable biomaterials with similar physico-chemical properties. Moreover, the excellent anti-tumour efficacy of the novel biodegradable star polymers renders them suitable candidates for further preclinical development as a highly potent biodegradable stimuli-sensitive drug delivery system.
Supplementary Material
Acknowledgments
This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic within the Inter-excellence program (project MSMT 39022/2018-1) and within the National Sustainability Program II (Project BIOCEV-FAR LQ1604) and by the project “BIOCEV” (CZ.1.05/1.1.00/02.0109), Czech Science Foundation (project 17-13283S and 19-05649S), Ministry of Health of the Czech Republic (project 16-28600A), and the National Institutes of Health (P30CA014520).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer-Chemotherapy - Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Research. 1986;46:6387–92. [PubMed] [Google Scholar]
- [2].Maeda H Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. Journal of controlled release : official journal of the Controlled Release Society. 2012;164:138–44. [DOI] [PubMed] [Google Scholar]
- [3].Zheng X, Pan D, Chen M, Dai X, Cai H, Zhang H, et al. Tunable Hydrophile–Lipophile Balance for Manipulating Structural Stability and Tumor Retention of Amphiphilic Nanoparticles. Advanced Materials. 2019;31:1901586. [DOI] [PubMed] [Google Scholar]
- [4].Etrych T, Subr V, Strohalm J, Sirova M, Rihova B, Ulbrich K. HPMA copolymer-doxorubicin conjugates: The effects of molecular weight and architecture on biodistribution and in vivo activity. Journal of Controlled Release. 2012;164:346–54. [DOI] [PubMed] [Google Scholar]
- [5].Ulbrich K, Hola K, Subr V, Bakandritsos A, Tucek J, Zbořil R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chemical Reviews. 2016;116:5338–431. [DOI] [PubMed] [Google Scholar]
- [6].Kopecek J, Kopeckova P, Minko T, Lu ZR, Peterson CM. Water soluble polymers in tumor targeted delivery. Journal of Controlled Release. 2001;74:147–58. [DOI] [PubMed] [Google Scholar]
- [7].Etrych T, Kovar L, Strohalm J, Chytil P, Rihova B, Ulbrich K. Biodegradable star HPMA polymer-drug conjugates: Biodegradability, distribution and anti-tumor efficacy. Journal of Controlled Release. 2011;154:241–8. [DOI] [PubMed] [Google Scholar]
- [8].Chen K, Cai H, Zhang H, Zhu H, Gu Z, Gong Q, et al. Stimuli-responsive polymer-doxorubicin conjugate: Antitumor mechanism and potential as nano-prodrug. Acta Biomaterialia. 2019;84:339–55. [DOI] [PubMed] [Google Scholar]
- [9].Luo Q, Xiao X, Dai X, Duan Z, Pan D, Zhu H, et al. Cross-Linked and Biodegradable Polymeric System as a Safe Magnetic Resonance Imaging Contrast Agent. ACS Applied Materials & Interfaces. 2018;10:1575–88. [DOI] [PubMed] [Google Scholar]
- [10].Etrych T, Strohalm J, Sirova M, Tomalova B, Rossmann P, Rihova B, et al. High-molecular weight star conjugates containing docetaxel with high anti-tumor activity and low systemic toxicity in vivo. Polymer Chemistry. 2015;6:160–70. [Google Scholar]
- [11].Etrych T, Sirova M, Starovoytova L, Rihova B, Ulbrich K. HPMA copolymer conjugates of paclitaxel and docetaxel with pH-controlled drug release. Molecular pharmaceutics. 2010;7:1015–26. [DOI] [PubMed] [Google Scholar]
- [12].Dozono H, Yanazume S, Nakamura H, Etrych T, Chytil P, Ulbrich K, et al. HPMA Copolymer-Conjugated Pirarubicin in Multimodal Treatment of a Patient with Stage IV Prostate Cancer and Extensive Lung and Bone Metastases. Targeted Oncology. 2016;11:101–6. [DOI] [PubMed] [Google Scholar]
- [13].Etrych T, Subr V, Strohalm J, Sirova M, Rihova B, Ulbrich K. HPMA copolymer-doxorubicin conjugates: The effects of molecular weight and architecture on biodistribution and in vivo activity. Journal of controlled release : official journal of the Controlled Release Society. 2012;164:346–54. [DOI] [PubMed] [Google Scholar]
- [14].Jin X, Sun P, Tong G, Zhu X. Star polymer-based unimolecular micelles and their application in bio-imaging and diagnosis. Biomaterials. 2018;178:738–50. [DOI] [PubMed] [Google Scholar]
- [15].Kostka L, Etrych T. High-Molecular-Weight HPMA-Based Polymer Drug Carriers for Delivery to Tumor. Physiological Research. 2016;65:S179–S90. [DOI] [PubMed] [Google Scholar]
- [16].Yang DP, MNNL Oo, Deen GR, Li Z, Loh XJ. Nano-Star-Shaped Polymers for Drug Delivery Applications. Macromolecular Rapid Communications. 2017;38:1700410. [DOI] [PubMed] [Google Scholar]
- [17].Chytil P, Hoffmann S, Schindler L, Kostka L, Ulbrich K, Caysa H, et al. Dual fluorescent HPMA copolymers for passive tumor targeting with pH-sensitive drug release II: Impact of release rate on biodistribution. Journal of Controlled Release. 2013;172:504–12. [DOI] [PubMed] [Google Scholar]
- [18].Sirova M, Strohalm J, Chytil P, Lidicky O, Tomala J, Rihova B, et al. The structure of polymer carriers controls the efficacy of the experimental combination treatment of tumors with HPMA copolymer conjugates carrying doxorubicin and docetaxel. Journal of Controlled Release. 2017;246:1–11. [DOI] [PubMed] [Google Scholar]
- [19].Tomalova B, Sirova M, Rossmann P, Pola R, Strohalm J, Chytil P, et al. The structure-dependent toxicity, pharmacokinetics and anti-tumour activity of HPMA copolymer conjugates in the treatment of solid tumours and leukaemia. Journal of controlled release : official journal of the Controlled Release Society. 2016;223:1–10. [DOI] [PubMed] [Google Scholar]
- [20].Kostkova H, Schindler L, Kotrchova L, Kovar M, Sirova M, Kostka L, et al. Star Polymer-Drug Conjugates with pH-Controlled Drug Release and Carrier Degradation. Journal of Nanomaterials. 2017. [Google Scholar]
- [21].Feliu N, Walter MV, Montanez MI, Kunzmann A, Hult A, Nystrom A, et al. Stability and biocompatibility of a library of polyester dendrimers in comparison to polyamidoamine dendrimers. Biomaterials. 2012;33:1970–81. [DOI] [PubMed] [Google Scholar]
- [22].Perrier S, Takolpuckdee P, Mars CA. Reversible Addition−FragmentaRon Chain Transfer Polymerization: End Group Modification for Functionalized Polymers and Chain Transfer Agent Recovery. Macromolecules. 2005;38:2033–6. [Google Scholar]
- [23].Etrych T, Mrkvan T, Rihova B, Ulbrich K. Star-shaped immunoglobulin-containing HPMA-based conjugates with doxorubicin for cancer therapy. Journal of Controlled Release. 2007;122:31–8. [DOI] [PubMed] [Google Scholar]
- [24].Koziolova E, Kostka L, Kotrchova L, Subr V, Konefal R, Nottelet B, et al. N-(2-Hydroxypropyl)methacrylamide-Based Linear, Diblock, and Starlike Polymer Drug Carriers: Advanced Process for Their Simple Production. Biomacromolecules. 2018;19:4003–13. [DOI] [PubMed] [Google Scholar]
- [25].Etrych T, Jelinkova M, Rihova B, Ulbrich K. New HPMA copolymers containing doxorubicin bound via pH-sensitive linkage: synthesis and preliminary in vitro and in vivo biological properties. Journal of Controlled Release. 2001;73:89–102. [DOI] [PubMed] [Google Scholar]
- [26].Koziolova E, Goel S, Chytil P, Janouskova O, Barnhart TE, Cai W, et al. A tumor-targeted polymer theranostics platform for positron emission tomography and fluorescence imaging. Nanoscale. 2017;9:10906–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rihova B, Etrych T, Pechar M, Jelinkova M, Stastny M, Hovorka O, et al. Doxorubicin bound to a HPMA copolymer carrier through hydrazone bond is effective also in a cancer cell line with a limited content of lysosomes. Journal of Controlled Release. 2001;74:225–32. [DOI] [PubMed] [Google Scholar]
- [28].Kovar M, Strohalm J, Etrych T, Ulbrich K, Rihova B. Star structure of antibody-targeted HPMA copolymer-bound doxorubicin: A novel type of polymeric conjugate for targeted drug delivery with potent antitumor effect. Bioconjugate Chem. 2002;13:206–15. [DOI] [PubMed] [Google Scholar]
- [29].Jelinkova M, Strohalm J, Etrych T, Ulbrich K, Rihova B. Starlike vs. Classic Macromolecular Prodrugs: Two Different Antibody-Targeted HPMA Copolymers of Doxorubicin Studied in Vitro and in Vivo as Potential Anticancer Drugs. Pharmaceutical Research. 2003;20:1558–64. [DOI] [PubMed] [Google Scholar]
- [30].Kovar L, Etrych T, Kabesova M, Subr V, Vetvicka D, Hovorka O, et al. Doxorubicin attached to HPMA copolymer via amide bond modifies the glycosylation pattern of EL4 cells. Tumor Biol. 2010;31:233–42. [DOI] [PubMed] [Google Scholar]
- [31].Talelli M, Iman M, Varkouhi AK, Rijcken CJF, Schiffelers RM, Etrych T, et al. Core-crosslinked polymeric micelles with controlled release of covalently entrapped doxorubicin. Biomaterials. 2010;31:7797–804. [DOI] [PubMed] [Google Scholar]
- [32].Peng Z-H, Kopecek J. Enhancing Accumulation and Penetration of HPMA Copolymer–Doxorubicin Conjugates in 2D and 3D Prostate Cancer Cells via iRGD Conjugation with an MMP-2 Cleavable Spacer. Journal of the American Chemical Society. 2015;137:6726–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kopecek J, Kopeckova P. HPMA copolymers: Origins, early developments, present, and future. Advanced Drug Delivery Reviews. 2010;62:122–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vicent MJ, Ringsdorf H, Duncan R. Polymer therapeutics: Clinical applications and challenges for development Preface. Advanced Drug delivery Reviews. 2009;61:1117–20. [DOI] [PubMed] [Google Scholar]
- [35].Perrier S, Takolpuckdee P, Mars CA. Reversible addition-fragmentation chain transfer polymerization: End group modification for functionalized polymers and chain transfer agent recovery. Macromolecules. 2005;38:2033–6. [Google Scholar]
- [36].Sivak L, Subr V, Tomala J, Rihova B, Strohalm J, Etrych T, et al. Overcoming multidrug resistance via simultaneous delivery of cytostatic drug and P-glycoprotein inhibitor to cancer cells by HPMA copolymer conjugate. Biomaterials. 2017;115:65–80. [DOI] [PubMed] [Google Scholar]
- [37].Minko T HPMA copolymers for modulating cellular signaling and overcoming multidrug resistance. Advanced Drug Delivery Reviews. 2010;62:192–202. [DOI] [PubMed] [Google Scholar]
- [38].Etrych T, Strohalm J, Chytil P, Rihova B, Ulbrich K. Novel star HPMA-based polymer conjugates for passive targeting to solid tumors. J Drug Target. 2011;19:874–89. [DOI] [PubMed] [Google Scholar]
- [39].Sirova M, Mrkvan T, Etrych T, Chytil P, Rossmann P, Ibrahimova M, et al. Preclinical Evaluation of Linear HPMA-Doxorubicin Conjugates with pH-Sensitive Drug Release: Efficacy, Safety, and Immunomodulating Activity in Murine Model. Pharmaceutical Research. 2009;27:200. [DOI] [PubMed] [Google Scholar]
- [40].Mrkvan T, Sirova M, Etrych T, Chytil P, Strohalm J, Plocova D, et al. Chemotherapy based on HPMA copolymer conjugates with pH-controlled release of doxorubicin triggers anti-tumor immunity. Journal of Controlled Release. 2005;110:119–29. [DOI] [PubMed] [Google Scholar]
- [41].Sirova M, Strohalm J, Subr V, Plocova D, Rossmann P, Mrkvan T, et al. Treatment with HPMA copolymer-based doxorubicin conjugate containing human immunoglobulin induces long-lasting systemic anti-tumour immunity in mice. Cancer Immunology, Immunotherapy. 2007;56:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rihova B, Kovar L, Kovar M, Hovorka O. Cytotoxicity and immunostimulation: double attack on cancer cells with polymeric therapeutics. Trends in Biotechnology. 2009;27:11–7. [DOI] [PubMed] [Google Scholar]
- [43].Sirova M, Kabesova M, Kovar L, Etrych T, Strohalm J, Ulbrich K, et al. HPMA Copolymer-Bound Doxorubicin Induces Immunogenic Tumor Cell Death. Current Medicinal Chemistry. 2013;20:4815–26. [DOI] [PubMed] [Google Scholar]
- [44].Etrych T, Strohalm J, Chytil P, Cernoch P, Starovoytova L, Pechar M, et al. Biodegradable star HPMA polymer conjugates of doxorubicin for passive tumor targeting. European Journal of Pharmaceutical Sciences. 2011;42:527–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.