Abstract
BACKGROUND:
Enhanced recovery programs have been associated with improved outcomes following gynecologic surgery. There is limited data on the effect of enhanced recovery programs on healthcare costs or healthcare service utilization.
OBJECTIVES:
To evaluate differences in hospital charges for women undergoing surgery for a suspected gynecologic cancer managed in an enhanced recovery program as compared with conventional perioperative care.
STUDY DESIGN:
We performed a retrospective cohort study of women undergoing open abdominal surgery for a suspected gynecologic cancer before and after implementation of an enhanced recovery after surgery [ERAS] program. Consecutive patients from May to October 2014 and from November 2014 to November 2015 comprised the conventional perioperative care [pre-ERAS] and enhanced recovery after surgery [ERAS] cohorts, respectively. Patients were excluded if they underwent surgery with a multidisciplinary surgical team or minimally invasive surgery. All technical and professional charges were ascertained for all healthcare services from the day of surgery until postoperative day 30. Charges for adjuvant treatment were excluded. Charges were classified according to the type of clinical service provided. The primary outcome was the difference in total hospital charges between the pre-ERAS and ERAS groups. Secondary outcomes were between group differences in hospital charges within clinical service categories.
RESULTS:
A total of 271 patients were included in the analysis with 58 and 213 patients in the pre-ERAS and ERAS cohort, respectively. A total of 70,177 technical charges and 6,775 professional charges were identified and classified. The median hospital charge for a patient decreased 15.6% in the pre-ERAS to ERAS groups [95% CI 0–39%; p=0.008]. Patients in the ERAS group also had lower charges for laboratory services [20%; 95% CI 0–39%; p=0.04], pharmacy services [30%; 95% CI 14–41%; p<0.001], room-and-board [25%; 95% CI 20–47%; p=0.005], and material goods [64%; 95% CI 44–81%; p < 0.001]. No differences in charges were observed for perioperative services, diagnostic procedures, emergency department care, transfusion-related services, interventional radiology procedures, physical/occupational therapy, outpatient care, or other services.
CONCLUSIONS:
Hospital charges and healthcare service utilization were lower for enhanced recovery patients compared with patients receiving conventional perioperative care following open surgery for a suspected gynecologic cancer. Enhanced recovery programs may be considered as high value in healthcare as they provide improved outcomes while lowering resource use.
Keywords: Enhanced recovery after surgery, gynecologic oncology, gynecologic surgery, postoperative care
INTRODUCTION
The benefit of enhanced recovery after surgery [ERAS] programs after gynecologic surgery has been documented.1 Such programs apply evidence-based perioperative management strategies with the goal to standardize patient care and mitigate the physiologic dysfunction that can occur as a result of surgery and postoperative recovery.1–3 Implementation of ERAS programs have resulted in shorter postsurgical admissions, fewer postoperative complications, decreased use of opioid pain medication, and improvements in functional recovery.4–8 There has been broad interest in the creation of ERAS programs nationally and globally.2,3,8
Value in healthcare is frequently described as an equation of “health outcomes achieved per dollar spent.” 9 In the context of ERAS programs, considerable interest has focused on the improvement in perioperative outcomes. Less attention has been directed towards the cost required to achieve those outcomes. Ascertaining ‘cost’ in the American healthcare system is difficult given the substantial variability observed in the expense to a provider to offer a service, the amount billed to a patient or payer, and the reimbursement actually provided for that service.
Cost reductions have been observed or projected from the implementation of ERAS programs in other surgical literatures.10–14 A recent systematic review and mea-analysis of patients undergoing major abdominal surgery across a variety of surgical disciplines estimated a mean cost reduction of $5100 for ERAS patients.15 Reports from several ERAS programs in gynecologic surgery have also reported cost savings as secondary outcomes.1,16,17 Cost savings observed with ERAS programs may be attributed to reductions in the length of postoperative hospital admission. Our goal with this investigation was to evaluate differences in hospital costs both in aggregate and within discrete categories of perioperative clinical services for patients undergoing gynecologic surgery in an ERAS program as compared with conventional postoperative care.
MATERIALS AND METHODS
A retrospective cohort study was performed as a secondary analysis of our institutional quality improvement project implementing an ERAS program in gynecologic surgery that was established November 2014 [Table 1].7,18 In brief, the program consists of management strategies that can be categorized into the preoperative [clear fluids until 2 hours before surgery, avoidance of bowel prep, preoperative analgesia], intraoperative [opioid-sparing multimodal anesthesia, goal-directed fluid therapy, minimizing the use of surgical drains], and postoperative phases [limited intravenous fluids, multimodal opioid-sparing analgesia, quick resumption of a general diet, encouraging ambulation, discouraging bedrest]. Before implementation of the ERAS program, perioperative management of patients undergoing open surgery with a gynecologic oncologist was not standardized with regards to the use of bowel preparations, postoperative intravenous fluids, resumption of diet after surgery, pain management, blood product transfusion, bedrest, and postoperative mobilization.
Table 1.
ERAS Program Components.
| Phase of Care | Intervention | Enhanced Recovery Approach |
|---|---|---|
| Pre-operative | ||
| Counseling | Pre-operative teaching and optimization | |
| Diet | Nutritional counseling during preoperative visit No solid food after midnight Clear liquids until 2 hours prior to surgery Carbohydrate loading |
|
| Bowel preparation | None | |
| Pre-medication | Tramadol Pregabalin Celecoxib Acetaminophen Prophylactic Heparin |
|
| Intravenous Fluids | Saline lock IV | |
| Intra-operative | ||
| Antibiotics | Prophylaxis per ACOG guidelines | |
| Anesthesia | Emphasis on total intravenous anesthesia No epidurals Wound infiltration with local anesthetic |
|
| Intravenous Fluids | Goal-directed (non-invasive cardiac monitoring) | |
| NGT/drain placement | Not utilized on a routine basis | |
| Foley catheter | Removal morning of POD1 | |
| Post-operative | ||
| Intravenous Fluids | IV fluids 40mL/hour until morning of POD1 Saline lock IV when tolerating 500mL by mouth |
|
| Multimodal Analgesia | Acetaminophen Ibuprofen Pregabalin Oxycodone as needed Hydromorphone IV as needed |
|
| Diet | Dietitian counseling Regular diet upon arrival to hospital floor Oral hydration |
|
| Ambulation | Ambulate ≥8 per day All meals in chairs Out of bed ≥8 hours daily |
|
| Blood Transfusion | Transfuse for hemoglobin <7g/dl |
ERAS: Enhanced Recovery After Surgery
IV: intravenous
ACOG: American College of Obstetricians and Gynecologists
NGT: nasogastric tube
POD: postoperative day.
Hospital charge data, defined as the amount billed by a healthcare provider for a service, was collected for all consecutive patients for one year [November 2014 to November 2015] following the implementation of the ERAS program [ERAS cohort]. Patients who underwent surgery within 6 months prior to ERAS implementation [May 2014 to October 2014] served as historical controls [pre-ERAS cohort]. Patients were eligible for inclusion in the analysis if they underwent open gynecologic surgery. Patients who had concurrent procedures with any additional surgical services or who underwent minimally invasive surgery were excluded. Demographic and clinical data were ascertained, including: age, race, body mass index, American Society of Anesthesiologists (ASA) Score, Charlson Comorbidity Index, procedure length, tumor site, tumor type, perioperative complications, surgical complexity score, reoperation, and readmission. Perioperative complications were categorized using the Clavien-Dindo classification system.19 Ovarian, fallopian tube and primary peritoneal cancer cases were characterized as low, medium, or high using the surgical complexity score developed by Aletti et al.20 In patients who underwent reoperation, their categorization into either the pre-ERAS or ERAS groups was by their initial surgery. Itemized data for all technical and professional services was collected from the day of surgery until 30-days postoperatively. The technical component of a charge reflects the cost of equipment, supplies, non-physician personnel, facilities, and other elements related to the provision of service. The professional component reflects the fee for the physician’s work or expertise in the delivery of a diagnostic or therapeutic service. For patients who received adjuvant therapy related to their cancer diagnosis, charges for these services were excluded from the analysis. Hospital charges were classified according to clinical service group, including laboratory services, pharmacy services, perioperative care [e.g. PACU services], diagnostic procedures, interventional radiology procedures, emergency department care, transfusion-related services, pathology-related services, physical and occupational care, room and board-related services, charges for material goods, outpatient care, and other. Patients who experienced postoperative complications or hospital readmission that extended their hospital stay beyond postoperative day 30 were not excluded from the analysis.
The data collection and management were approved as a component of an institutional quality improvement project [QI-6033]. The retrospective analysis of these data was approved by the institutional review board [PA18–1136]. Numerical values presented represent percent differences in hospital charges both in aggregate and within categorized clinical services. As is the case among many healthcare organizations, numerical cost data is protected and proprietary information at our institution. Absolute numerical values regarding hospital costs are unable to be disclosed in this report to protect institutional cost-to-charge ratios.
The primary objective was to determine if there were any differences in the total median hospital charges for patients who were managed in an ERAS program compared to those receiving conventional postoperative care. The secondary objectives were to determine if there were differences in median hospital charges in categorized clinical services between the pre-ERAS and ERAS groups. Descriptive statistics were used to summarize the demographic and clinical characteristics of the pre- and ERAS cohorts. A Wilcoxon rank-sum test was used to compare distributions among continuous variables and a Fisher’s exact test was used to test associations among categorical variables. Wilcoxon rank-sum tests were used to evaluate for differences in the distributions of charges between the pre-ERAS and ERAS cohorts for both the total hospital charges as well as for the service-related charge categories. The absolute percent change from pre-ERAS to ERAS was calculated between the median charges overall and for each charge category. Bootstrap methods were used to calculate the 95% confidence interval for the absolute percent change.21,22 The bootstrap sample included 1000 unrestricted random samples of k size, where k is the number of observations selected from each group (58 pre-ERAS and 213 ERAS; bootstrap sample size N=271,000). The lower and upper limits of the 95% CI was derived from the 2.5th and 97.5th percentiles of the calculated median percent changes from the bootstrap samples, respectively. A p-value < 0.05 was considered statistically significant. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
There were 271 patients included in the analysis; 58 patients and 213 patients in the pre-ERAS and ERAS cohort, respectively. A total of 70,177 technical charges and 6,775 professional charges were attributed to the entire study population. The pre-ERAS and ERAS groups had similar demographic and clinical characteristics [Table 2]. The median ages for the pre-ERAS and ERAS groups were 58 (range; 32–85) and 57 (range; 18–79) years, respectively (p = 0.808). Median operating times were 221.5 (range; 98–484) and 210 (range; 33–497) minutes for the pre-ERAS and ERAS groups, respectively (p = 0.271). Complications of any grade were observed in 43.1% (25/58) and 38.5% (82/213) of patients in the pre-ERAS and ERAS groups, respectively (p = 0.547). The case mix of the two groups was similar in terms of surgical complexity (p = 0.153). The majority of procedures were performed by gynecologic oncologists [99.6%; 270/271]. Three patients (5.2%; 3/58) in the pre-ERAS group and four patients (1.9%; 4/213) in the ERAS group underwent reoperation. Eight patients (13.8%; 8/58) in the pre-ERAS group and 27 (12.7%; 27/213) in the ERAS group were readmitted after surgery. Gastrointestinal complications [e.g. ileus, nausea/emesis, obstruction, perforation] were the most common reason for readmission in both the pre-ERAS [37.8%; 3/8] and ERAS groups [44.4%; 12/27]. Median length of stay was 1 day shorter for the ERAS group compared with the pre-ERAS group [4 vs. 3 days; p<0.001]. Compliance with the institutional ERAS program was at least 70% or more of the interventions.
Table 2.
Demographic & Clinical Characteristics
| Pre-ERAS (n = 58) | ERAS (n = 213) | All Patients (n = 271) | p-value | ||
|---|---|---|---|---|---|
| Age, y | Median (Min, Max) | 58.0 (32.0, 85.0) | 59.0 (18.0, 79.0) | 59.0 (18.0, 85.0) | 0.808 |
| Length of Stay, d | Median (Min, Max) | 4.0 (2.0, 29.0) | 3.0 (1.0, 57.0) | 3.0 (1.0, 57.0) | < 0.001 |
| Surgical Time, min | Median (Min, Max) | 221.5 (98.0, 484.0) | 210.0 (33.0, 497.0) | 211.0 (33.0, 497.0) | 0.271 |
| Estimated Blood Loss, mL | Median (Min, Max) | 300.0 (40.0, 3500.0) | 250.0 (10.0, 2700.0) | 250.0 (10.0, 3500.0) | 0.095 |
| Race | White or Caucasian | 48 (82.8%) | 152 (71.4%) | 200 (73.8%) | 0.151 |
| Black or African American | 2 (3.4%) | 29 (13.6%) | 31 (11.4%) | ||
| Asian | 1 (1.7%) | 7 (3.3%) | 8 (3.0%) | ||
| Other | 5 (8.6%) | 21 (9.9%) | 26 (9.6%) | ||
| Unknown | 2 (3.4%) | 4 (1.9%) | 6 (2.2%) | ||
| Ethnicity | Hispanic or Latino | 12 (20.7%) | 28 (13.1%) | 40 (14.8%) | 0.333 |
| Not Hispanic or Latino | 43 (74.1%) | 174 (81.7%) | 217 (80.1%) | ||
| Unknown | 3 (5.2%) | 11 (5.2%) | 14 (5.2%) | ||
| Body Mass Index (kg/m2) | < 18.5 | 1 (1.7%) | 5 (2.3%) | 6 (2.2%) | 0.957 |
| 18.5 – 24.9 | 15 (25.9%) | 67 (31.5%) | 82 (30.3%) | ||
| 25.0 – 29.9 | 21 (36.2%) | 66 (31.0%) | 87 (32.1%) | ||
| 30.0 – 34.9 | 11 (19.0%) | 42 (19.7%) | 53 (19.6%) | ||
| 35.0 – 39.9 | 6 (10.3%) | 19 (8.9%) | 25 (9.2%) | ||
| ≥ 40 | 4 (6.9%) | 14 (6.6%) | 18 (6.6%) | ||
| ASA Score | I | - | 1 (0.5%) | 1 (0.4%) | 0.435 |
| II | 13 (22.4%) | 28 (13.8%) | 41 (15.7%) | ||
| III | 44 (75.9%) | 169 (83.3%) | 213 (81.6%) | ||
| IV | 1 (1.7%) | 5 (2.5%) | 6 (2.3%) | ||
| Charlson Comorbidity Index | 0 | 6 (10.3%) | 20 (9.4%) | 26 (9.6%) | 0.928 |
| 1–2 | 22 (37.9%) | 79 (37.1%) | 101 (37.3%) | ||
| ≥ 3 | 30 (51.7%) | 114 (53.5%) | 144 (53.1%) | ||
| Tumor Type | Malignant | 52 (89.7%) | 179 (84.0%) | 231 (85.2%) | 0.634 |
| Benign | 6 (10.3%) | 31 (14.6%) | 37 (13.7%) | ||
| Unavailable | - | 3 (1.4%) | 3 (1.1%) | ||
| Tumor Site a | Cervix | 1 (1.9%) | 7 (3.9%) | 8 (3.5%) | 0.550 |
| Ovary | 35 (67.3%) | 110 (61.5%) | 145 (63.7%) | ||
| Uterine | 7 (13.5%) | 38 (21.2%) | 45 (19.5%) | ||
| Other | 1 (1.9%) | 1 (0.5%) | 2 (0.9%) | ||
| Recurrent Disease | 8 (15.4%) | 28 (15.6%) | 36 (15.6%) | ||
| 30-day Complications, Grade I-IV b | 25 (43.1%) | 82 (38.5%) | 107 (39.5%) | 0.547 | |
| Reoperation | 3 (5.2%) | 4 (1.9%) | 7 (2.6%) | 0.170 | |
| Readmission | 8 (13.8%) | 27 (12.7%) | 35 (12.9%) | 0.826 | |
| Surgical Complexity c | Low | 20 (54.1%) | 78 (68.4%) | 98 (64.9%) | 0.153 |
| Intermediate | 15 (40.5%) | 34 (29.8%) | 49 (32.5%) | ||
| High | 2 (5.4%) | 2 (1.8%) | 4 (2.6%) |
: No disease site specified for benign disease.
: Complications were graded using Clavien-Dindo perioperative complication classification.
: Degree of surgical complexity considered as a variable for cases of ovarian, fallopian tube, or primary peritoneal cancer. All column values are numerical count and percentage unless indicated otherwise. The denominator in the calculation of column percentages for tumor site is the number of patients with malignant disease [52 for pre-ERAS; 179 for ERAS; 231 for all patients]. Abbreviations: ASA, American Society of Anesthesiologists; ERAS, Enhanced Recovery After Surgery.
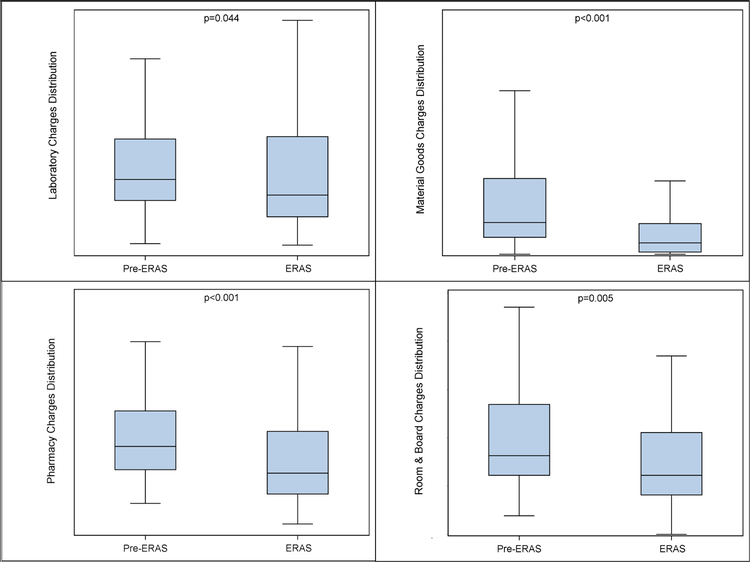
The median 30-day surgical and postoperative hospital charges in the ERAS group were 15.6% [95% CI 5–24.5%] lower than the pre-ERAS group. The underlying distribution of total hospital charges was significantly different with lower median costs in the ERAS group [p=0.008] [Figure 1]. When hospital charges were grouped into clinical service categories, ERAS patients had 20% [95% CI 0–39%] lower median hospital charges for laboratory services [Table 3]. The ERAS patients had 30% [95% CI 14–41%] lower median charges for pharmacy services. The median room-and-board-related hospital charges were decreased in the ERAS group by 25% [95% CI 20–47%]. The median hospital charge for material goods was decreased in the ERAS group by 64% [95% CI 44–81%]. For these clinical service categories, the underlying distribution of hospital charges was significantly different for laboratory services [p=0.044], pharmacy services [p<0.001], room-and-board [p=0.005], and material goods [p<0.001] with lower median hospital charges in the ERAS group [Figure 2]. For the remaining service categories, either no difference in median hospital charges was observed or a difference in median hospital charges for a given service category could not be estimated between the pre-ERAS and ERAS groups. Differences in median hospital charges could not be determined for certain service categories if the median charge in the pre-ERAS and / or ERAS groups was $0 [i.e. no hospital charge in a given service category occurred for a patient] [Table 3].
Figure 1.

Distribution of Hospital Charges in Pre-ERAS and ERAS Cohorts
Observations outlying beyond of the range of the whiskers of the box plots are not shown. ERAS: Enhanced Recovery After Surgery.
Table 3.
Percent Difference in Median Hospital Charges in Pre-ERAS and ERAS Cohorts.
| Percent Difference in Median Hospital Chargesa | p-valuec | |
|---|---|---|
| Laboratory Services | −20% (−39% to 0%) | 0.044 |
| Interventional Radiology | N/Ab | - |
| Emergency Department Care | N/Ab | - |
| Outpatient Care | 3% (−55% to 72%) | 0.364 |
| Perioperative Care | −1% (−9% to 11%) | 0.789 |
| Diagnostic Procedures | N/Ab | - |
| Transfusion-related Services | N/Ab | - |
| Physical / Occupational Therapy | N/Ab | - |
| Pharmacy Services | −30% (−41% to −14%) | < 0.001 |
| Room & Board | −25% (−47% to −20%) | 0.005 |
| Pathology | −12% (−38% to 22%) | 0.285 |
| Material Goods | −64% (−81% to −44%) | < 0.001 |
| Miscellaneous | N/Ab | - |
| Overall | −15.6% (−24.5% to −5%) | 0.008 |
Values within parentheses represent 95% confidence interval.
The absolute percent difference with estimated 95% confidence interval.
Percent change unable to be determined due to median hospital charge in pre-ERAS and/or ERAS group being $0 for given service category.
Wilcoxon rank-sum test among pre- and post-ERAS charges ERAS: Enhanced Recovery After Surgery; N/A: not applicable.
Figure 2.
Distribution of Hospital Charges in Pre-ERAS and ERAS Cohorts in Selected Clinical Service Categories
Observations outlying beyond of the range of the whiskers of the box plots are not shown. Clockwise from top-left: hospital charges for laboratory services; hospital charges for material goods; hospital charges for room & board; and hospital charges for pharmacy services. ERAS: Enhanced Recovery After Surgery.
COMMENT
Principal Findings & Meaning
In this study, we found that the median total hospital charges for a woman undergoing open gynecologic surgery was reduced by more than 15% following implementation of an ERAS program. Although hospital costs and charges vary across organizations, the percent difference observed allows for generalizability and estimation of the magnitude of cost savings that may be expected from implementation of an ERAS program. The percent difference in hospital charges observed may also be interpreted in the context of national cost estimates for healthcare spending on the surgical and postoperative care of women with gynecologic cancer. For example, a recent national estimate of the mean reimbursement for surgical and postoperative care of a woman with newly-diagnosed advanced stage epithelial ovarian cancer is $30,501.23 With this considered as a baseline estimate and assuming the percent difference in hospital charges observed in this study is a reasonable approximation for reduction in healthcare spending, implementation of an ERAS program would be estimated to reduce the amount spent by a healthcare payer on the surgical and postoperative care of each patient by approximately $4,760. Although changes in hospital charges do not correspond perfectly with payer reimbursement, these estimates would suggest that nationwide application of ERAS programs to the care of women with ovarian cancer could save more than $100 million per year in healthcare spending. Similar to the results of this analysis, previous reports of ERAS programs in gynecologic surgery have described reductions in healthcare costs as secondary outcomes.1,16,17 Variable or unclear definitions of healthcare costs can make interpretation of these findings difficult. Our investigation adds to the existing literature by reiterating the cost savings seen from an ERAS program while also providing a clear definition of how we defined the monetary values in this analysis. Further, these results add more insight into how ERAS programs provide monetary savings by examining the differences in charges across categorized hospital services.
Ascertainment of ‘cost’ in our healthcare system is difficult with variable definitions depending on the perspective of the individual or organization defining it. From the standpoint of a healthcare provider, hospital charges indirectly reflect the cost [i.e. the resources used to provide a service] to care for patients. To a third-party payer, hospital charges may more directly reflect a ‘cost’ as the reimbursement made for the services provided. However, it is very uncommon for third-party payers to reimburse the full charge amount as they often engage in complex contract negotiations with healthcare providers that affect the payment provided for the healthcare service rendered to their beneficiaries. In this study, we are unable to determine any impact on the out-of-pocket costs to patients based on the implementation of an ERAS program. However, the differences in charges observed in this analysis do reflect both the costs to provide care to patients as well as the reimbursement costs that accrue to third-party payers, albeit indirectly. In doing so, we were able to show evidence that an enhanced recovery program adds value by reducing charges for the surgical and 30-day postoperative care of patients undergoing open gynecologic surgery.
Prior reports regarding ERAS programs have examined healthcare costs in aggregate for the entire surgical and postoperative care episode. By categorizing hospital charges into categorical clinical services provided, we found that the median charges were lower in the ERAS group for laboratory services, pharmacy services, room-and-board, and material goods as compared with the pre-ERAS cohort. The decrease in median charges for these services correlates with the decrease in total length of stay. However, we did not observe charge differences for services that would be unaffected by length of stay. For example, charges for perioperative care were unchanged in the pre-ERAS and ERAS groups while charges for laboratory and pharmacy services were decreased by 20% and 30%, respectively. This observation validates the intuitive assumption that ERAS provides cost-savings through reduced healthcare service utilization by shortening overall length of postsurgical admission. Simply put, patients who stay in the hospital less use fewer hospital services and accrue fewer costs for these services.
Strengths & Weaknesses
The strengths of our study include that this was an analysis of prospectively-collected data from consecutive patients treated at a high-volume cancer center. There are also several limitations to consider in our study. This was a single institution retrospective review of patients cared for at a large tertiary-level cancer center. This analysis does not incorporate the upfront institutional costs involved in the development and implementation of an ERAS program. Successful implementation of an ERAS program requires substantial initial investment of both human and financial resources. Program development, evaluation, and ongoing improvement requires active participation from a multidisciplinary team that includes surgeons, anesthesiologists, nurses, pharmacists, occupational and physical therapists, research coordinators, and operating room staff 18. We are not able to quantify that investment in this analysis. It is possible that patients may have received services within the first 30-days following surgery outside of our health system. We are unable to ascertain or quantify this using our study design. As discussed above, the use of hospital charges for economic analysis in healthcare is imperfect, especially pertaining to broader perspectives in the accounting of costs. For example, we are unable to ascertain if there was a ‘transfer of costs’ to non-healthcare entities, namely family members or other patient caregivers who may support patients for longer periods of time as they are discharged from the hospital earlier. Institutional policies censoring specific costs [e.g. the financial expenditure required to provide a healthcare service] and total charges impairs our ability to describe the magnitude of difference produced by ERAS program implementation. A recent report from a European tertiary care center partially overcomes this limitation, describing in numerical terms the cost to provide care for patients following gynecologic surgery before and after implementation of an ERAS program.24 Finally, we recognize that one of the limitations of this study is the fact that the number of patients in the pre-ERAS cohort is disproportionately smaller than the post-ERAS cohort. This resulted from a pre-planned strategy by our team to collect, prior to implementation of the ERAS program, all parameters that were going to be measured after the ERAS program was initiated. This was done in order to document baseline data.
Conclusion
In summary, this study offers evidence that ERAS programs reduce the cost of the perioperative care of women undergoing gynecologic surgery. Our analysis suggests that the primary mechanism by which ERAS programs generate cost savings is through reduced utilization of hospital services resulting from shorter postoperative lengths of stay. Value in healthcare is often defined by the equation of outcomes achieved divided by the cost necessary to achieve them.9 Numerous studies have focused on the improvements in perioperative outcomes as the mechanism by which ERAS programs provide value. However, our findings suggest that ERAS programs address both the numerator and denominator of the healthcare value equation, providing improved outcomes as well as reduced costs. Such programs seem to be a highly valuable addition to the surgical care of women with gynecologic cancers.
CONDENSATION:
Lower healthcare costs were seen in the postoperative care of women undergoing surgery for a suspected gynecologic cancer after implementation of an Enhanced Recovery After Surgery program.
AJOG AT A GLANCE:
-
Why was this study conducted?
This investigation was performed to further characterize the value of Enhanced Recovery After Surgery [ERAS] programs in gynecologic cancer surgery. While the clinical benefits of ERAS programs have been described, less focus has been placed on the resources used to achieve improved outcomes. This study evaluates whether implementation of an ERAS program impacts healthcare costs in women undergoing open surgery for a suspected gynecologic cancer.
-
What are the key findings?
Compared to historical controls, women undergoing open gynecologic surgery had lower hospital charges following implementation of an ERAS program. Most of the decrease in healthcare spending seemed to be related to shorter postoperative hospital stay in the ERAS cohort, suggesting that ERAS may be cost-saving by reducing healthcare resource utilization.
-
What does this study add to what is already known?
This investigation offers evidence that ERAS programs in gynecologic surgery provide value through both improved outcomes, such as shorter postoperative hospital stays and reduced opioid consumption, as well as through reduced healthcare costs and healthcare resource use. This analysis also characterizes how ERAS programs provide cost savings – principally by reduction in hospital services uti lization through shorter postoperative length of stay.
Acknowledgements:
Financial support for this research investigation was provided in part by grant funding to the following individuals:
Ross Harrison – Research support from National Institutes of Health T32 grant (#5T32 CA101642).
Larissa Meyer – Research support from National Cancer Institute K award (#K07 CA201013)
University of Texas MD Anderson Cancer Center: National Cancer Institute Cancer Center Support Grant (P30 48CA016672)
ROLE OF FUNDING SOURCES:
The funding sources were not involved in the development of the research hypothesis, study design, data analysis, or manuscript writing. Data access was limited to the authors of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES:
The authors report no conflicts of interest, financial or otherwise, related to the subject matter of the article submitted.
The investigation described in this manuscript was presented in part by Dr. Larissa A. Meyer, as an oral presentation during the 5th ERAS Society World Congress in Lyon, France [May 10–12, 2017].
References
- 1.Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 Pt 1):319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations--Part I. Gynecol Oncol. 2016;140(2):313–322. [DOI] [PubMed] [Google Scholar]
- 3.Nelson G, Altman AD, Nick A, et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations--Part II. Gynecol Oncol. 2016;140(2):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg. 2017;152(3):292–298. [DOI] [PubMed] [Google Scholar]
- 5.de Groot JJ, Ament SM, Maessen JM, Dejong CH, Kleijnen JM, Slangen BF. Enhanced recovery pathways in abdominal gynecologic surgery: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2016;95(4):382–395. [DOI] [PubMed] [Google Scholar]
- 6.Wijk L, Franzen K, Ljungqvist O, Nilsson K. Implementing a structured Enhanced Recovery After Surgery (ERAS) protocol reduces length of stay after abdominal hysterectomy. Acta Obstet Gynecol Scand. 2014;93(8):749–756. [DOI] [PubMed] [Google Scholar]
- 7.Meyer LA, Lasala J, Iniesta MD, et al. Effect of an Enhanced Recovery After Surgery Program on Opioid Use and Patient-Reported Outcomes. Obstet Gynecol. 2018;132(2):281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey ET, Moulder JK. Perioperative Management and Implementation of Enhanced Recovery Programs in Gynecologic Surgery for Benign Indications. Obstet Gynecol. 2018;132(1):137–146. [DOI] [PubMed] [Google Scholar]
- 9.Porter ME. What is value in health care? The New England journal of medicine. 2010;363(26):2477–2481. [DOI] [PubMed] [Google Scholar]
- 10.Nelson G, Kiyang LN, Crumley ET, et al. Implementation of Enhanced Recovery After Surgery (ERAS) Across a Provincial Healthcare System: The ERAS Alberta Colorectal Surgery Experience. World J Surg. 2016;40(5):1092–1103. [DOI] [PubMed] [Google Scholar]
- 11.Stone AB, Grant MC, Pio Roda C, et al. Implementation Costs of an Enhanced Recovery After Surgery Program in the United States: A Financial Model and Sensitivity Analysis Based on Experiences at a Quaternary Academic Medical Center. J Am Coll Surg. 2016;222(3):219–225. [DOI] [PubMed] [Google Scholar]
- 12.Roulin D, Donadini A, Gander S, et al. Cost-effectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br J Surg. 2013;100(8):1108–1114. [DOI] [PubMed] [Google Scholar]
- 13.Stowers MD, Lemanu DP, Hill AG. Health economics in Enhanced Recovery After Surgery programs. Can J Anaesth. 2015;62(2):219–230. [DOI] [PubMed] [Google Scholar]
- 14.Lee L, Mata J, Ghitulescu GA, et al. Cost-effectiveness of Enhanced Recovery Versus Conventional Perioperative Management for Colorectal Surgery. Ann Surg. 2015;262(6):1026–1033. [DOI] [PubMed] [Google Scholar]
- 15.Visioni A, Shah R, Gabriel E, Attwood K, Kukar M, Nurkin S. Enhanced Recovery After Surgery for Noncolorectal Surgery?: A Systematic Review and Meta-analysis of Major Abdominal Surgery. Ann Surg. 2018;267(1):57–65. [DOI] [PubMed] [Google Scholar]
- 16.Gerardi MA, Santillan A, Meisner B, et al. A clinical pathway for patients undergoing primary cytoreductive surgery with rectosigmoid colectomy for advanced ovarian and primary peritoneal cancers. Gynecol Oncol. 2008;108(2):282–286. [DOI] [PubMed] [Google Scholar]
- 17.Bisch SP, Wells T, Gramlich L, et al. Enhanced Recovery After Surgery (ERAS) in gynecologic oncology: System-wide implementation and audit leads to improved value and patient outcomes. Gynecol Oncol. 2018;151(1):117–123. [DOI] [PubMed] [Google Scholar]
- 18.Miralpeix E, Nick AM, Meyer LA, et al. A call for new standard of care in perioperative gynecologic oncology practice: Impact of enhanced recovery after surgery (ERAS) programs. Gynecol Oncol. 2016;141(2):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am J Obstet Gynecol. 2007;197(6):676 e671–677. [DOI] [PubMed] [Google Scholar]
- 21.Efron B. Bootstrap Methods: Another Look at the Jackknife. The Annals of Statistics. 1979;7(1):1–26. [Google Scholar]
- 22.DiCiccio TJ, Efron B. Bootstrap Confidence Intervals. Statistical Science. 1996;11(3):189–212. [Google Scholar]
- 23.Bercow AS, Chen L, Chatterjee S, et al. Cost of Care for the Initial Management of Ovarian Cancer. Obstet Gynecol. 2017;130(6):1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pache B, Joliat GR, Hubner M, et al. Cost-analysis of Enhanced Recovery After Surgery (ERAS) program in gynecologic surgery. Gynecol Oncol. 2019. [DOI] [PubMed]