Abstract
The neck region contains around 300 lymph nodes (LNs) out of 800 LNs in the whole body. The detailed study of LNs by Rouviere in 1932 [1] and the later illustration of metastatic predilection of head and neck malignancies to certain LN regions by Lindberg et al. [2] paved the road to a clinically sound classification. The American Academy of Otolaryngology and Head and Neck Surgery (AAO-HNS) and the American Joint Committee on Cancer (AJCC) developed the currently widely accepted levels classification of the cervical LNs (Table 8.1, Figs. 8.1 and 8.2).
Keywords: Kawasaki Disease, Neck Dissection, Cervical Lymphadenopathy, Radical Neck Dissection, Selective Neck Dissection
Anatomical Considerations
The neck region contains around 300 lymph nodes (LNs) out of 800 LNs in the whole body. The detailed study of LNs by Rouviere in 1932 [1] and the later illustration of metastatic predilection of head and neck malignancies to certain LN regions by Lindberg et al. [2] paved the road to a clinically sound classification. The American Academy of Otolaryngology and Head and Neck Surgery (AAO-HNS) and the American Joint Committee on Cancer (AJCC) developed the currently widely accepted levels classification of the cervical LNs (Table 8.1, Figs. 8.1 and 8.2).
Table 8.1.
Cervical LN level classification according to the AAO-HNS
| Level | Area | Sublevels | Areas drained |
|---|---|---|---|
| I |
Submental LNs Submandibular LNs |
IA IB |
Floor of mouth, anterior oral tongue, anterior mandibular alveolar ridge, and lower lip Oral cavity, anterior nasal cavity, midface soft tissue structures, submandibular gland |
| II | Upper internal jugular nodes; lying between the skull base and the hyoid bone | IIA, IIB | Oral cavity, nasal cavity, nasopharynx, oropharynx, hypopharynx, larynx, and parotid gland |
| III | Middle internal jugular nodes; lying between the hyoid bone and cricoid cartilage | Oral cavity, nasopharynx, oropharynx, hypopharynx, and larynx | |
| IV | Lower internal jugular nodes; extending between cricoid cartilage and the clavicle | The hypopharynx, cervical esophagus, and larynx | |
| V | Posterior triangle (spinal accessory chain) LNs |
VA VB |
VA: nasopharynx and oropharynx VB: thyroid gland |
| VI | Pretracheal + prelaryngeal + paratracheal LNs | Thyroid gland, glottic and subglottic larynx, apex of piriform sinus, cervical esophagus | |
| VII | Upper mediastinal LNs |
Fig. 8.1.
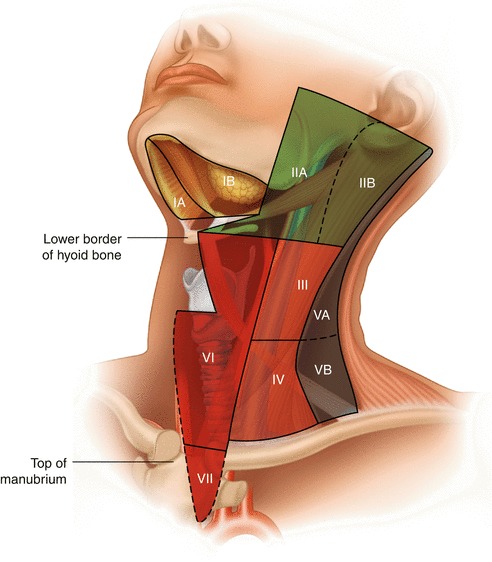
Regional nomenclature of cervical nodes
Fig. 8.2.
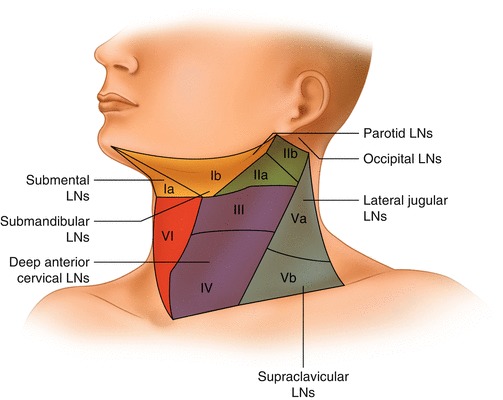
Head and neck regions draining into each nodal level
Delineation of the Levels of Cervical Lymph Nodes [3] (Fig. 8.1)
Level IA: Submental Region
These LNs lie within the triangle bounded by the anterior belly of the digastric muscles (both sides) and the hyoid bone inferiorly.
Level IB: Submandibular Region
These LNs lie within the triangle bounded by the anterior and posterior bellies of digastric muscle inferiorly and the body of the mandible superiorly. It should be noted that the submandibular gland should be included in the removed specimen if the LNs within this level are excised.
Level IIA and IIB: Upper Jugular Group
Those are the LNs surrounding the upper third of the internal jugular vein (IJV). They extend from the skull base superiorly to the level of the inferior border of the hyoid bone inferiorly. Anteriorly, it is bounded by the lateral border of the sternohyoid muscle and the stylohyoid muscle. Posteriorly, it is bounded by the posterior border of the sternocleidomastoid (SCM) muscle. Level IIA LNs lie anterior to the vertical plane of the spinal accessory nerve, while IIB LNs lie posterior to this plane. Radiologically, level IB and level IIA LNs are separated by the vertical plane at the posterior aspect of the submandibular gland.
Level III: Middle Jugular Group
This level extends from the inferior border of the hyoid bone superiorly to the inferior border of the cricoid cartilage inferiorly and is located around the middle third of the IJV. Again, the anterior boundary is represented by the lateral border of sternohyoid muscle, and the posterior boundary is the posterior border of the SCM. The jugulo-omohyoid LN belongs to this group.
Level IV: Lower Jugular Group
These LNs extend from the lower border of the cricoid cartilage to the clavicle, and they are located around the lower third of the IJV. Anteriorly, this group is bounded by the lateral border of sternohyoid muscle and posteriorly bounded by the posterior border of SCM. It should be noted that the “Virchow” LN belongs to this group.
Levels VA and VB: Posterior Triangle Group
The upper limit of this group is the convergence of the trapezius and SCM muscles, while the lower boundary is formed by the clavicle. Anteriorly, it is bounded by the posterior border of SCM, and the posterior boundary is formed by the anterior border of the trapezius muscle. Level VA LNs and VB LNs are separated by an imaginary horizontal plane marking the lower border of the cricoid cartilage. Level VA LNs include the spinal accessory LNs, while VB includes LNs located around the transverse cervical vessels and the supraclavicular LNs.
Level VI: Central Compartment Group
This level includes pretracheal, paratracheal, pre-cricoid (Delphian), and peri-thyroidal LNs (including LNs located along the recurrent laryngeal nerves [RLN]). This region extends from the hyoid bone superiorly to the suprasternal notch inferiorly. Laterally, it is bounded by the common carotid arteries (CCAs).
Neck Dissection
Historical Overview
The term neck dissection refers to the removal of the fibrofatty tissue of the neck with the intention of treating lymphatic metastasis in the neck region. This surgical procedure was first described by George Crile in 1906 although reports of similar procedures of en bloc resections go back to 1888 when Franciszek Jawdynski – a Polish surgeon – reported four cases. The work of Crile was further popularized by several surgeons including the famous American surgeon, Hayes Martin. However, the morbidity accompanying the radical approach of the original procedure stimulated the development of a more conservative approach. Based on anatomical understanding of the lymphatic anatomy of the neck, Suarez introduced the principles of functional neck dissection in 1963. Later, Ettore Bocca published a description of an operative technique in the English literature aiming at the preservation of the non-lymphatic structures of the neck resulting in a better functional outcome and less morbidity, at the same time obtaining equivalent oncologic results [4, 5].
The ongoing experimentation and clinical studies resulted in a more precise understanding of the metastatic nodal patterns in head and neck malignancies [2, 6]. The resulting data allowed for a more conservative approach depending on the negligible risk of involvement of certain nodal regions in various oncologic scenarios ending in a wide range of surgical procedures. This variation called for an updated classification and nomenclature of operations involving surgical excision of the cervical lymphatics. In order to achieve this, AAO-HNS sponsored the work of the Committee of Head and Neck Surgery and Oncology to develop a unified classification of neck dissection operations. The first classification was established in 1991; this was later modified in 2002 and 2008 to become the currently accepted classification [7–11].
Classification of Neck Dissection (AAO-HNS and American Head and Neck Society) [3]
Radical Neck Dissection (RND)
This is the original standard operation of cervical lymphadenectomy to which all the other modifications have been applied. It entails removal of ipsilateral nodal groups extending from the lower border of the mandible superiorly to the clavicle inferiorly and from the lateral border of sternohyoid muscle, hyoid bone, and contralateral anterior belly of digastric muscle medially to the anterior border of trapezius muscle laterally. Thus, nodal groups of level I–V are removed, while the anterior compartment (central, level VI), suboccipital, peri-parotid (except for the infra-parotid LNs lying in the submandibular triangle), retropharyngeal, and buccinators LNs are not. It also includes the removal of spinal accessory nerve (SAN), IJV, and SCM.
Modified Radical Neck Dissection (MRND) (Figs. 8.3 and 8.4)
Fig. 8.3.
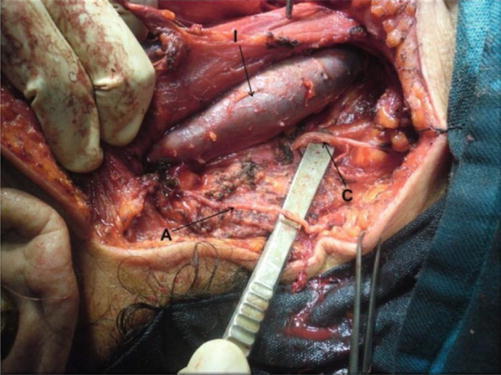
Level V dissected, showing accessory nerve (A), branch from cervical plexus (C), and IJV (I)
Fig. 8.4.
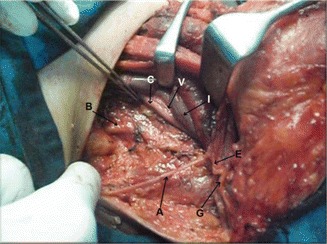
Levels III, IV and V dissected, showing accessory nerve (A), great auricular nerve (G), Erb’s point (E), IJV retracted (I), common carotid artery (C), vagus nerve (V), and trunks of brachial plexus (B)
This entails the removal of the same nodal groups as described in RND while preserving one or more of the non-lymphatic structures (SAN, IJV, and SCM). According to the current terminology, the preserved non-lymphatic structure should be named in the operation title, e.g., modified RND with preservation of IJV and SAN.
Selective Neck Dissection (SND)
This refers to the cervical lymphadenectomy with preservation of one or more nodal groups included in the original RND. This approach is based on the predictable patterns of nodal metastasis according to the site of the primary malignancy. As a result, different variations of lymphadenectomy have been described including:
Supraomohyoid neck dissection: removal of levels I–III.
Lateral neck dissection: removal of levels II–IV.
Posterolateral neck dissection: removal of levels II–V, suboccipital, postauricular, and the external jugular LNs.
Anterior neck dissection: removal of level VI LNs.
To obtain a unified terminology as regards to the variations in the extent of excision, it is recommended to mention the levels and sublevels excised following the term SND, e.g., SND [level VI].
Extended Radical Neck Dissection (ERND)
This entails removal of additional LN groups (including retropharyngeal, superior mediastinal, paratracheal, or buccinator LNs) or non-lymphatic structures or both, which are not included in the standard RND operation. Examples of non-lymphatic structures that can be removed include the vagus nerve, hypoglossal nerve, carotid artery, paraspinal muscles, or the overlying skin. The additional removed structures should be mentioned in the operation title.
Cervical Lymphadenopathy: Pathology and Clinical Pattern
Definition
Lymphadenopathy (LA) is a pathological process of LNs manifested by abnormally increased size or altered consistency or number [12]. Cervical lymphadenopathy (CLA) refers to cervical nodal tissue measuring more than 1 cm in diameter [13]. However, palpable supraclavicular nodes of any size are considered abnormal [14]. It is not a disease by itself; rather, it is a sign of an underlying pathology that ranges from a trivial infection to a metastatic malignant neoplasm.
Classification
Etiological
Cervical lymphadenopathy may be classified according to its etiology into malignant, infectious, autoimmune, miscellaneous, and iatrogenic (due to medications). These broad categories can be easily remembered using the mnemonic MIAMI [14].
Pathological
In General, CLA may be due to lymphoid hyperplasia or infiltration [15].
Lymphoid hyperplasia is further subclassified, based on the anatomic and histopathology, into the following patterns: (1) follicular hyperplasia, (2) sinus hyperplasia, (3) paracortical hyperplasia, (4) necrotizing granulomatous lymphadenitis, (5) necrotizing non-granulomatous lymphadenitis, and (6) acute lymphadenitis [12].
A lymph node may be infiltrated by malignant cells, in malignant lymphoma/leukemia, or by other cells, like lipid cells and glycogen-laden macrophages [15]. The former category includes a wide spectrum of lymphoid and hematopoietic neoplasms that are classified by the World Health organization (WHO) into (1) precursor B and T cell neoplasm, (2) mature B cell neoplasm, (3) mature T cell and NK cell neoplasm, (4) Hodgkin’s lymphoma, (5) histocytic and dendritic neoplasm, and (6) post-transplantation lymphoproliferative disorders ( LPDs) [16].
An additional pathological entity is spindle cell lesions of LN which include (1) bacillary angiomatosis, (2) Kaposi sarcoma, (3) palisaded myofibroblastoma, and (4) inflammatory pseudotumor of LN [17].
Clinical
Based on distribution: CLA may be localized (only one area is involved), regional (two or more contagious areas are involved), or part of generalized (two or more noncontiguous areas are involved) [18].
Based on the duration: CLA is further classified into acute (2 weeks duration), subacute (2–6 weeks duration), and chronic (does not resolve by 6 weeks).
Etiology
A wide range of causes can result in CLA (Table 8.2). A recent cross-sectional study has demonstrated that the most likely cause of CLA depends on the age group; reactive or nonspecific inflammation was the most common cause in those younger than 14 years; tuberculous lymphadenopathy was the predominant pathology in 14–59-year group, while cancer should be suspected if the patient is 60 years or older [19].
Table 8.2.
Causes of CLA
| I. Infectious | II. Malignant | VI. Medications |
|
Viral Infectious mononucleosis Infectious hepatitis Herpes simplex Rubella Measles Adenovirus HIV Bacterial Streptococcus Staphylococcus Cat-scratch disease Tularemia Tuberculosis Syphilis leprosy Diphtheria Chlamydia Lymphogranuloma venereum Trachoma Rickettsial Scrub typhus Rickettsial pox Fungal Histoplasmosis Coccidiomycosis Parasitic Toxoplasmosis Leishmaniasis |
Hodgkin’s lymphoma Non-Hodgkin’s lymphoma (NHL) Acute lymphoblastic leukemia Chronic lymphoblastic leukemia Hairy cell leukemia T cell lymphoma Multiple myeloma with amyloidosis Metastatic |
Allopurinol Atenolol Captopril Carbamazepine Cephalosporin Gold Hydralazine Penicillin Phenytoin Primidone Pyrimethamine Quinidine |
| III. Immunological disease | VII. Miscellaneous | |
|
Rheumatoid arthritis Systemic lupus erythematosus (SLE) Sjogren’s syndrome Drug hypersensitivity Silicone associated Serum diseases Graft versus host disease |
Sarcoidosis Histiocytosis X Kikuchi’s disease Kawasaki’s disease Castleman’s disease Lymphomatoid granulomatosis |
|
| IV. Endocrine disease | ||
|
Hyperthyroidism Thyroiditis Adrenal insufficiency | ||
| V. Lipid storage disorders | ||
|
Gaucher’s disease Niemann-Pick’s disease |
Infections
Cervical lymphadenopathy may occur when the draining nodes react to a nearby infection in the head and neck region including upper respiratory infection or when the infectious agent localizes in the node itself.
Acute bacterial infections are most commonly caused by Staphylococcus aureus or Streptococcus pyogenes and should be suspected in patients with pustular or vesicular lesions of the face or scalp, history of earache or discharge, and history of sore throat or cough suggesting skin, ear, or upper respiratory infections, respectively. Infection with anaerobes like bacteroides may be suspected in patients with dental abscess or periodontal disease. Pasteurella multocida infection may follow animal bites, while Yersinia pestis infection may follow flea bites [21]. Clinically, adenopathy is often solitary unilateral and usually involving submandibular, upper deep cervical, submental, and occipital area in decreasing order of frequency. Enlarged LNs are tender and may be fluctuant, and the overlying skin is warm and erythematous [15]. The patient is feverish and usually has an evident source of infection.
Acute viral adenitis typically follows an upper respiratory tract infection by rhinovirus, parainfluenza virus, influenza virus, respiratory syncytial virus, coronavirus, and adenovirus. Other less common etiologies are mumps, measles, rubella, varicella, and herpes simplex. Clinically, adenopathy is commonly multiple and bilateral. Lymph nodes are relatively small, typically not tender, and rarely suppurate, and the overlying skin is not warm nor erythematous. Patients usually have low-grade fever and commonly complaining of cough, rhinorrhea, conjunctivitis, or skin rash.
Infectious mononucleosis (IMN) is particularly common in children and adolescents and is caused by Epstein-Barr virus which spreads primarily by saliva and replicates inside B lymphocytes and epithelial cells of the pharynx. Diagnosis is based on Hoagland’s criteria: at least 50 % of lymphocytes and at least 10 % of atypical lymphocytes in the presence of fever, pharyngitis, and adenopathy. Cervical adenopathy usually involves the posterior group and may be associated with axillary adenopathy, inguinal adenopathy, or splenomegaly. Diagnosis should be confirmed serologically through the classic Paul-Bunnell test, which detect heterophil antibodies by agglutination of sheep red cells, or through the more sensitive detection of antibodies to viral capsid antigens [22].
Chronic infectious lymphadenopathy is defined by failure to resolve or improve despite 2–6 weeks of appropriate therapy. Important causes of chronic infectious lymphadenopathy are detailed in the following sections.
Tuberculosis (TB)
Tuberculosis (TB) is an ancient multisystem disease that has been detected in Egyptian mummies dating to 5000 BC. It continues to be one of the most prevalent communicable diseases particularly among third-world countries. The developed countries witness a health challenge caused by TB because of increasing migration from developing countries and rising incidence of HIV in these countries [23]. The causative organism is Mycobacterium tuberculosis, though it can also be caused by other mycobacteria. Though TB can virtually involve any body organ, the lymphatic system is the most common site of extrapulmonary affection, within which cervical LNs are the most commonly involved [24]. The portal of entry usually determines the affected nodal group; involvement of jugulodigastric LNs usually denotes infection entering through tonsils or adenoids, while a pulmonary source is usually manifested by supraclavicular lymphadenopathy [25]. Histopathologically, tuberculous LNs demonstrate epithelioid, macrophages and giant cells, caseation necrosis, and scanty acid fast bacilli appearing as fragmented or beaded rods inside or outside cells [26].
In a study of 102 patients presenting with neck mass (Fig. 8.5) of tuberculous etiology, fever was present in 64 % of patients, weight loss in 42 %, and sweating in 18 % [24]. Locally, Jones and Campbell classified tuberculous lymphadenopathy into five distinct stages (Table 8.3) [27].
Fig. 8.5.
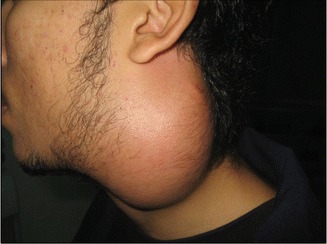
Matted tuberculous cervical lymph nodes in a 23-year-old young gentleman
Table 8.3.
Stages of TB lymphadenopathy
| Stage | Description | Pathogenesis | Clinical features |
|---|---|---|---|
| 1 | Discrete LNs | Nonspecific reactive hyperplasia; | Large, firm, and mobile LNs |
| 2 | Matted LNs | Periadenitis | Large and rubbery LNs, fixed to surrounding tissues |
| 3 | Cold abscess | Central softening and caseation deep to the deep fascia | Soft, smooth, nontender, fluctuant selling without involvement of the skin |
| 4 | Collar stud abscess | As a result of increased pressure caseous material perforates the deep fascia | Abscess is adherent to the overlying skin |
| 5 | Tuberculous sinus/ulcer | When the abscess bursts | Chronic nonhealing sinus or ulcer with thin, bluish, undermined edges and scanty watery discharge |
Syphilis
Syphilis is a sexually transmitted disease, caused by Treponema pallidum, which can invade mucous membranes not only of the genital region but also of the head and neck such as the lips, tongue, and tonsils. Clinically, chancre is usually present at primary site of infection; its absence, however, is reported in some cases in which diagnosis becomes more difficult. Enlarged LNs are multiple, firm, mobile, and not tender. Diagnosis is usually made serologically through rapid plasma reagin (RPR) test, T. pallidum hemagglutination assay (TPHA) test, and Venereal Disease Research Laboratory (VDRL) slide test [30, 31].
Cat-Scratch Disease
Cat-scratch disease is a zoonotic disease transmitted through cats, caused by Bartonella henselae. Diagnosis requires at least 2 of the following three criteria: (1) presence of typical clinical symptoms, (2) serological detection of antibodies against B. henselae, and (3) detection of Bartonella DNA in the extirpated lymph nodes. Clinical symptoms include fever, lymphadenopathy, asthenia, pharyngitis, laryngitis, and skin rash. Lymphadenopathy is the most common clinical manifestation with the cervical nodes being the most frequently involved [32].
Toxoplasmosis
Toxoplasmosis is a zoonotic disease transmitted through ingestion of undercooked meat containing oocytes of Toxoplasma gondii present in cat’s feces. Cervical lymphadenopathy is present in 90 % of cases and may be associated with fever, sore throat, and myalgias. The nodal enlargement is usually solitary, discrete, mobile, and nontender [33].
Malignancy
Although CLA is more commonly caused by a benign etiology, a sinister underlying malignant process is not uncommon, necessitating careful evaluation of such patients. Exclusion of malignancy is in fact the first and the single most important aim sought by the physician when evaluating CLA. The incidence of malignancy in patients with CLA seems to vary considerably with their demographics: age, gender, and race. The rate of malignant etiologies rises with age. A cross-sectional study by Biswas et al. [19] demonstrated that the rate of malignancy among patients with CLA was 12.1 %, 21.7 %, and 100 % in the age groups <14, 15–59, and ≥60 years, respectively. In their study, Shakya et al. [34] observed a malignancy rate as high as 50 % in patients with CLA in 51–60-year age group. Such high incidence of malignancy among the aforementioned age groups led to the belief that any neck mass in an adult should be suspected as malignant until proven otherwise [35]. Gender issue has also been studied with the uniform finding of higher frequency of malignancy among males [36]. Similarly, incidence of malignancy in patients with CLA differs among different racial groups. A malignancy rate as high as 50 % was observed among Iranians presenting with unknown neck masses [37], while it was 28.2 % and 4.8 % among Indians and Nepalese, respectively [19, 34].
Malignant cervical LNs may be caused by secondary metastasis from another primary or less commonly by lymphoma [19, 34]. However, in their audit of 140 patients with CLA, Magsi et al. [38] found that lymphoma was more common than metastasis. Among the latter group, Naeimi et al. [37] found that SCC was the most common pathology regardless of age with the larynx and hypopharynx being the most common sites of the primary tumor. Similar findings were reported by Biswas et al. [19] with adenocarcinoma coming second in frequency. Lymphoma, as a cause of malignant CLA, ranks the second in frequency with NHL being more common in some series [19], while others reported that Hodgkin’s lymphoma was more common [37].
Histologically, the presence of Reed-Stenberg cells (large cells with abundant basophilic cytoplasm and multiple nuclei) in a background of inflammatory cells is characteristic of Hodgkin’s disease (HD) (Fig. 8.6). The WHO classified HD into five subtypes according to the predominant cell types: (1) nodular sclerosis, (2) lymphocyte rich, (3) mixed cellularity, (4) lymphocyte depleted, and (5) nodular lymphocyte predominant [12]. In NHL, parafollicular or marginal zone distribution of B cells occurs in nodal marginal zone b cell lymphoma, while complete, partial, sinus, or interfollicular involvement with variable cytology occurs in diffuse large B cell lymphoma, not otherwise specified [17].
Fig. 8.6.
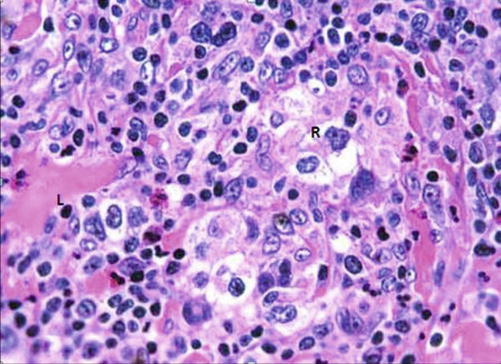
H&E-stained section demonstrating classical pathology in Hodgkin’s lymphoma (L lymphocyte, R Reed-Sternberg cells)
Clinical features suggestive of malignant etiology of CLA include:
Generally: NHL usually presents with generalized lymphadenopathy with or without hepatosplenomegaly, in contrast to Hodgkin’s lymphoma in which the lymphadenopathy is initially localized and subsequently spreads orderly to contiguous nodal regions. Systemic manifestations like fever, weight loss, night sweats, and pruritus are called B symptoms and are suggestive of lymphoma: being less common in non-Hodgkin’s type [18].
Regionally: a pigmented skin lesion or mass that is mobile with deglutition may suggest metastatic deposits from nearby melanoma or papillary thyroid cancer [12].
Locally: enlarged supraclavicular LN carries the highest risk of malignancy and should always be viewed with suspicion [39, 40]. Hard, fixed, painless lymph nodes are highly suggestive of metastatic deposits (Fig. 8.7), while rubbery or firm mobile nodes are more suggestive of lymphoma, though amalgamation (Fig. 8.8) and limitation of mobility may occur later [14].
Fig. 8.7.
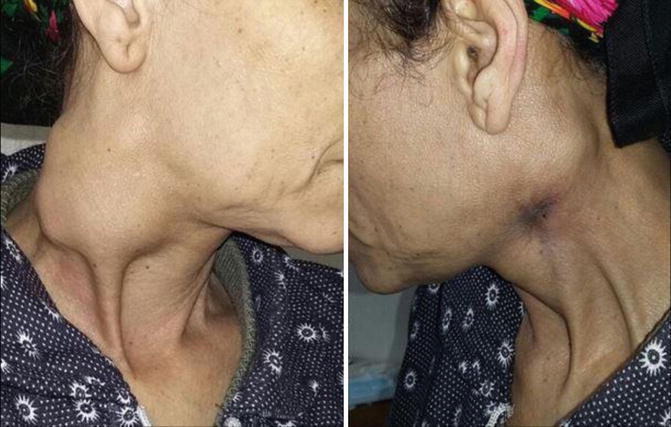
Bilateral cervical lymphadenopathy, metastatic from unknown primary, in an old female patient
Fig. 8.8.
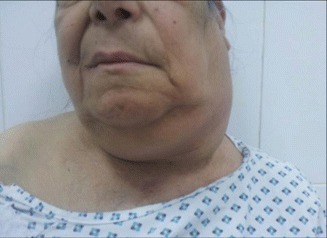
Left-sided neck mass, turned out to be amalgamated LNs, in an old female with lymphoma
Autoimmune Disease
Lymphadenopathy is a detectable physical finding in up to 82 % and 69 % of rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) cases, respectively, but it could also be seen in almost any autoimmune disorder. In these disorders, adenopathy of cervical nodes ranks second in frequency after axillary lymphadenopathy [41]. Histopathologically, inflamed nodes show reactive follicular hyperplasia, polyclonal plasma cell infiltration with occasional mitosis in the interfollicular area, moderate vascular proliferation, and no compression of reticulin fibers; this is to differentiate it from lymphoma in which interfollicular area shows many mitosis, scarce plasma cells, and compressed reticulin fibers [42].
When present, cervical lymphadenopathy is usually associated with axillary and/or inguinal lymphadenopathy. Lymph nodes are usually multiple, relatively small, soft, mobile, and not tender [43]. Diagnosis should be made on the ground of relevant clinical and laboratory criteria. Polyarthritis of small joints, lasting more than 6 weeks together with elevation of serum rheumatoid factor (RF), anti-citrullinated protein antibody (ACPA), and acute phase reactants (APR) is diagnostic for RA [44]. Presence of malar rash, discoid rash, nonerosive arthritis, photosensitivity, oral ulcers, and renal, neurologic, and hematologic disorders and elevated antinuclear antibodies and anti-DNA antibodies are diagnostic of SLE [45]. Dryness of the mouth and eyes is suggestive of Sjogren’s syndrome [46].
Endocrine Disease
Sahlmann et al. [47] reported that cervical lymphadenopathy (levels II–IV, VI) occurred in more than 80 % of patients with autoimmune thyroiditis (AIT). Similarly, cervical lymphadenopathy was detected in 23 % of cases of Hashimoto’s thyroiditis (HT) [48]. Graves’ disease has also been reported to be associated with cervical lymphadenopathy [49, 50]. Pathology is reactive lymphoid hyperplasia. Therefore, AIT including HT should be included in differential diagnosis of patients with thyroid nodes and cervical LNs. Presence of cervical lymphadenopathy in a patient with clinical features of chronic adrenal insufficiency should alert the physician to the possibility of secondary adrenal involvement or to the very rare entity of primary adrenal lymphoma [51, 52].
Drug-Induced Lymphadenopathy
Certain medications can cause diffuse lymphadenopathy; of which phenytoin-induced lymphadenopathy is the most widely described in the literature. The reaction tends to occur few months after initiation of therapy and usually disappear within weeks after stopping drug administration. Pathophysiology is thought to be due to medication-induced immunologic disturbances, including reduced humoral and cellular immunity. Pathology usually reveals partial or complete effacement of the lymph node architecture by polymorphous infiltration of lymphocytes, plasma cells, and eosinophils [53, 54].
Phenytoin, in particular, has also been associated with a characteristic nodal pathology, termed pseudolymphoma. Differentiation from lymphoma is based on the absence of clonal proliferation of T cells. Clinically, cervical adenopathy tends to be bilateral and usually part of generalized lymphadenopathy. Condition is often associated with fever, rash, and eosinophilia. A high index of suspicion should always be kept since the condition can be easily confused with lymphoma. History of phenytoin exposure, improvement on phenytoin discontinuation, and pathological examination of a surgically excised lymph node are the clues to correct diagnosis [55].
Miscellaneous Causes of Cervical Lymphadenopathy
Kawasaki Disease (KD)
It is a type of systemic vasculitis of unknown etiology, also known as lymphocutaneous disease. It commonly occurs in children under 5 years of age. Cervical lymphadenopathy is present in approximately 42–65 % of KD patients [56]. Histopathologically, adenopathy is caused by sinus expansion and paracortical zone enlargement. Lymph nodes may also show ischemic changes in the form of necrotic foci, developing below the capsule and accompanied by fibrin thrombi in the surrounding small vessels [57]. Diagnosis should be clinically suspected in the presence of: (1) fever more than 5 days; (2) cervical lymphadenopathy, usually unilateral; (3) edema, erythema, and/or desquamation of the palms and soles; (4) non-purulent bilateral conjunctivitis; and (5) strawberry tongue. A high index of suspicion should be kept when evaluating these children as the condition may be complicated by coronary artery abnormalities (aneurysm, thrombosis, infarctions) in 25 % of untreated children. Helpful laboratory data are elevated white cell count with neutrophilia and lymphopenia; elevated acute phase reactant, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR); and elevated liver enzyme, aspartate transaminase (AST) and alanine transaminase (ALT) [58].
Sarcoidosis
It is a multisystem chronic inflammatory condition of unknown etiology. Cervical lymphadenopathy is the most common head and neck manifestation of the condition, though it accounts only for 1.7 % of all head and neck lymphadenopathy. Histopathologically, it is characterized by the presence of noncaseating epithelioid cell granulomas (Fig. 8.9).
Fig. 8.9.
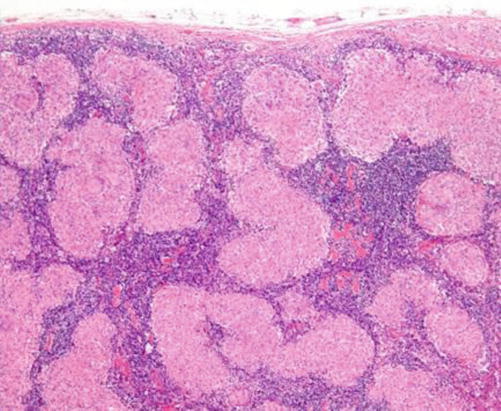
H&E-stained section in an LN showing multiple sarcoid granulomas
Diagnosis is usually established on the basis of compatible clinical and radiologic findings of hilar lymphadenopathy, pulmonary infiltration, and skin and ocular lesions. However, a diagnostic challenge arises when isolated cervical lymphadenopathy is the sole clinical feature. The latter condition usually necessitates exclusion of other granulomatous disease particularly tuberculosis, histopathological examination of surgically excised lymph node, and immunohistochemical detection of high expression of tumor necrosis factor-α (TNF-α) in epithelioid cells [59, 60].
Histiocytosis X
It is a rare disease predominantly affecting children. A controversy exists regarding its etiology: reactive versus neoplastic. Nodal involvement may be an isolated lesion or more commonly a part of systemic disease [61]. The pathological hallmark is presence of clonal proliferation of antigen-presenting dendritic cells called Langerhans cells (LCs) (Fig. 8.10): so-called Langerhans cells histiocytosis [59].
Fig. 8.10.
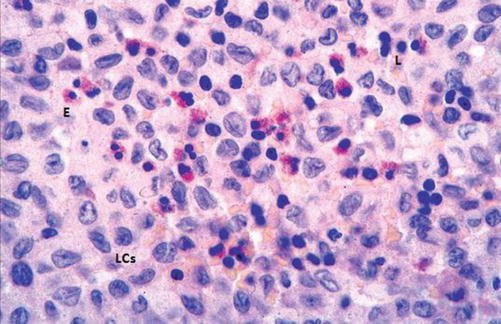
H&E-stained section in a histiocytosis lymph node showing proliferation of Langerhans cell (LCs) and scattered eosinophils (E) and lymphocytes (L)
Clinically, the disease has a broad spectrum of severity. The mildest form is called eosinophilic granuloma and is characterized by solitary bone, skin, lung, or stomach lesions. The moderate form of the disease is called Hands-Schuller-Christian disease and is characterized by a triad of diabetes insipidus, exophthalmos, and lytic bone lesions. The most severe form is Letterer-Siwe disease and is a life-threatening multisystem disorder.
Diagnosis can be made from fine-needle aspiration cytology which demonstrates many LCs through its characteristic nuclear features, namely, nuclear grooves and pseudoinclusions, as well as immunohistochemical detection of positivity for S-100, peanut agglutinin (PNA), MHC class II, CD1a, and langerin (CD207) [61].
Kikuchi-Fujimoto Disease (KFD)
It is a benign and self-limited disease, mainly affecting young Japanese women. Etiology is unknown; however, an infectious cause has been proposed. Histopathologically, the LN shows single or multiple areas of necrosis and histiocytic cellular infiltrate (Fig. 8.11), so-called histiocytic necrotizing lymphadenitis. Clinically, patients usually present with localized lymphadenopathy, fever, and leucopenia. The disease may be associated with autoimmune phenomena and may occur in the setting of SLE [62].
Fig. 8.11.
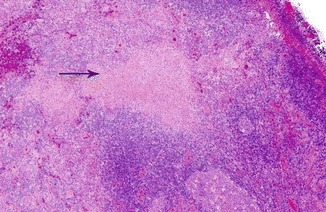
H&E-stained section of an LN in Kikuchi-Fujimoto’s disease showing area of necrosis (arrow)
Castleman’s Disease
It is a lymphoproliferative disorder (LPD) of unknown etiology. Histopathologically, LNs show follicular hyperplasia and marked capillary proliferation with endothelial hyperplasia. Two pathological types have been identified: hyaline vascular type and plasma cell type (Fig. 8.12) [63]. Clinically, there are two variants: unicentric and multicentric. In unicentric variant, the patient is often asymptomatic, and the disease is usually discovered accidentally on imaging which usually detects hilar or mediastinal lymphadenopathy. The multicentric type is a systemic disease, usually presenting with significant peripheral lymphadenopathy and hepatosplenomegaly as well as frequent fevers, night sweats, and fatigue [54]. Diagnosis is based on clinical suspicion, histological examination, and immunohistochemical detection of increased expression of vascular endothelial growth factor [64].
Fig. 8.12.
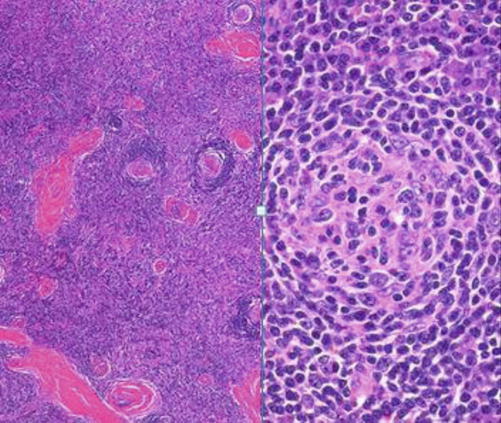
H&E-stained section in lymph nodes with Castleman’s disease (hyaline cell type on the left; plasma cell type on the right)
Neck Staging Under the “TNM Staging System” for Head and Neck Tumors
(AJCC head and neck tumor staging by site [3])
This staging system excludes the nasopharynx and thyroid.
Regional Lymph Nodes (N)
Nx: Regional LNs cannot be assessed
N0: No regional LNs metastasis
N1: Metastasis in a single ipsilateral LN, 3 cm or less in greatest dimension
- N2: Metastasis in a single ipsilateral LN, >3 cm but not >6 cm in greatest dimension; in multiple ipsilateral LNs, none >6 cm in greatest dimension; or in bilateral or contralateral LNs, none >6 cm in greatest dimension
- N2a: Metastasis in a single ipsilateral LN, >3 cm but not >6 cm in greatest dimension
- N2b: Metastasis in multiple ipsilateral LNs, none >6 cm in greatest dimension
- N2c: Metastasis in bilateral or contralateral LNs, none >6 cm in greatest dimension
N3: Metastasis in LNs more than 6 cm in greatest dimension
*Note: A designation of “U” or “L” may be used for any N stage to indicate metastasis above the lower border of the cricoid cartilage (U) or below the lower border of the cricoid cartilage (L). Similarly, clinical/radiological extracapsular spread (ECS) should be recorded as E− or E+.
Nasopharynx
Nx: Regional LNs cannot be assessed
N0: No regional LN metastasis
N1: Unilateral metastasis in cervical LN(s), 6 cm or less in greatest dimension, above the supraclavicular fossa, and/or unilateral or bilateral retropharyngeal LNs, 6 cm or less in greatest dimension
N2: Bilateral metastasis in cervical LN(s), 6 cm or less in greatest dimension, above the supraclavicular fossa
N3: Metastasis in LN >6 cm and/or to supraclavicular fossa
N3a: >6 cm in dimension
N3b: Extension to the supraclavicular fossa
*Note: Midline nodes are considered ipsilateral nodes.
Thyroid Gland
Regional LNs are the central compartment, lateral cervical, and upper mediastinal LNs.
Nx: Regional LNs cannot be assessed
N0: No regional LN metastasis
N1: Regional LN metastasis
N1a: Metastasis to level VI (pretracheal, paratracheal, and prelaryngeal/Delphian LNs)
N1b: Metastasis to unilateral, bilateral, or contralateral cervical (level I, II, III, IV, or V) or superior mediastinal LNs (level VII)
Mucosal Melanoma
Regional LNs are the central compartment, lateral cervical, and upper mediastinal LNs.
Nx: Regional LNs cannot be assessed
N0: No regional LN metastases
N1: Regional LN metastases present
Carcinoma of Unknown Primary (CUP)
Carcinoma of unknown primary site (CUP) represents around 5–10 % of all tumors; those are malignancies presenting with LN metastasis, while efforts for identification of the primary site of malignancy fail [65, 66].
A fair fraction of CUP presents with cervical nodal metastasis, SCC being the most common histological type, followed by adenocarcinoma, undifferentiated malignancy, and others including melanoma and lymphoma. Such affection is commonly unilateral with level II LNs being the most commonly affected group followed by level III. Generally speaking, upper and middle neck LN affections originate from the head and neck region, while isolated lower neck LN affections are usually associated with malignancies below the clavicles [67–69].
Nearly 25 % of malignancies in the pediatric population occur in the head and neck region, where the cervical nodes represent the most common site. Neuroblastoma and leukemia are the most common malignancies associated with cervical nodes during the first 6 years of life, followed by rhabdomyosarcoma and NHL. After 6 years, Hodgkin’s lymphoma (HL) has the highest prevalence, followed by both NHL and rhabdomyosarcoma [70].
Diagnostic Approach
The diagnosis of the etiology of CLA should proceed in a step-by-step fashion, starting with careful history taking, examination, and investigative workup if necessary.
The value of patient history does vary widely. It can point directly to the cause and suggest the possible etiology or it can be nondiagnostic [71, 72].
The first target during the evaluation of CLA is to determine whether it is of the localized or generalized variety. Factors related to the size, consistency, and mobility of the LNs should also be considered as they may point clearly to a malignant nature.
When dealing with localized cervical lymphadenopathy, the draining sites of the affected levels should be thoroughly examined for sources of infection or possible malignant lesion. If the history and examination reveal a source of infection, then no further workup is required and treatment can be initiated. However, follow-up for adequate response is mandatory.
History Taking
The following factors should be considered during history taking:
Age: The probability of malignant nature is higher in older age populations. Data from one study performed in a referral center showed that 79 % of biopsies performed in young patients (<30 years) were of benign nature, while 60 % of biopsies from older patients (>50 years) showed malignant etiology [73].
Symptoms of infection: Fever, conjunctivitis, sore throat, dental pain, ulcers, or discharge. Night sweats and shivers can suggest tuberculosis (TB).
Localized symptoms of malignancy: Hoarseness of voice, dysphagia, stridor, ulcers, and pain should be sought, especially when a high index of suspicion is available.
Generalized symptoms of malignancy: Weight loss: an unexplained weight loss of more than 10 % during a period of 6 months should raise suspicion of lymphoma [74].
Symptoms of collagenic disease: Arthralgia, joint deformities, or myalgia.
Medical history: Allergies to certain drugs are common causes for lymphadenopathy (e.g., phenytoin).
Occupational and epidemiological exposures: History of a high-risk behavior, recent traveling to high-risk regions, or exposure to pets or specific occupational hazards can all suggest a specific underlying etiology, sometimes of a rare incidence.
Duration and response to previous medications: A short history of lymphadenopathy may favor acute infectious etiologies (viral, bacterial, etc.), while persistent nodal enlargement (more than 4 weeks) accompanies chronic infections, collagenic diseases, and malignancies. Also, response to previous medications (e.g., antimicrobials) may help validate the underlying pathology.
Physical Examination
The key features that should be sought are:
Distribution: It is crucial to determine whether the cervical lymphadenopathy is isolated (localized, enlargement of LNs in one region), regional (enlargement of LNs in 2 or more contiguous regions), or part of generalized lymphadenopathy (enlargement of LNs of 2 or more noncontiguous regions). Generalized lymphadenopathy accompanies systemic diseases specially if associated with splenomegaly. In the neck, supraclavicular LNs drain the gastrointestinal tract, genitourinary tract, and lungs. Enlarged supraclavicular LNs should raise a strong suspicion of malignancy as an underlying malignancy occurs in 54–85 % of cases [73, 75–77].
Size: The LNs of subcentimeter diameter are usually of no clinical significance, while LNs more than 2 cm in size of persistent nature mandate thorough diagnostic workup [72].
Mobility: Fixed LNs suggest metastatic causes, while freely movable nodes have a wide range of underlying etiologies including primary malignancies (e.g., lymphoma). Evaluation of supraclavicular LN mobility can be aided by performance of Valsalva maneuver.
Consistency: Hard LNs usually suggest a malignant nature. However, other forms of consistency can still accompany malignant nodes, and therefore consistency should not be relied upon for differentiation of the nodal nature. A tender LN can point out an underlying inflammatory process [78].
Investigations
If history and physical examination are diagnostic of infectious cause (bacterial), treatment should be initiated without further workup. If they are suggestive of viral causes, follow-up and/or specific serological tests can be done.
In case of low estimated risk of malignancy, patients with localized LNs and nondiagnostic initial workup can be followed up for 4 weeks. Empirical antimicrobials and steroids should be avoided for their lympholytic effect thus affecting the results of biopsy.
Imaging Modalities
Ultrasonography (US)
Ultrasonography (US) is the primary diagnostic technique that can be used when the clinical examination is not directly diagnostic of the nature of the LNs. The main target of ultrasonographic examination is to distinguish reactive, tuberculous, lymphomatous, and metastatic LN etiologies. This can be achieved by analysis of various nodal parameters.
Reactive LNs
Typical features include a low short-axis-to-long-axis ratio (S/L < 0.5) except for parotid and submandibular regions where they usually attain a more rounded contour (S/L = or > 0.5), together with the absence of suspicious criteria including irregular margins, peripheral halo, internal echoes, and tendency for fusion. Doppler US cannot distinguish inflammatory and neoplastic LNs on the basis of their flow patterns [61, 79].
Reactive LNs are usually found in the upper part of the neck (submandibular, parotid, and upper cervical) and the posterior triangle. Another important feature is the preservation of the echogenic hilum representing hilar vascularity, which can be further confirmed by color Doppler [79]. Spectral Doppler US shows low vascular resistance in reactive LNs (resistive index, RI, and pulsatility index, PI) [80].
Regarding the size, 9 mm is the upper limit for the minimal axial diameter in both the submandibular and the subdigastric nodes, while 8 mm is the upper limit for the other cervical LNs (Fig. 8.13) [81].
Fig. 8.13.
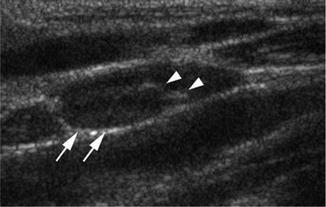
Ultrasound showing reactive LN (white arrows) maintaining its echogenic hilum (arrow heads)
Tuberculous LNs
Features include the presence of fusion tendency (nodal matting), hypoechoic core, posterior enhancement, multiple LNs, adjacent soft tissue edema (displays as a peripheral halo), and the presence of strong internal echoes denoting calcifications, caseation, and granuloma formation [61, 82, 83].
In a retrospective study, presence of strong echoes or a thin echogenic layer had a sensitivity and specificity of 100 % in distinguishing tubercular from malignant LNs [83]. Cystic necrosis is also common in tuberculous LNs resulting in displaced hilar vascularity on Doppler studies (Fig. 8.14) [79, 84].
Fig. 8.14.
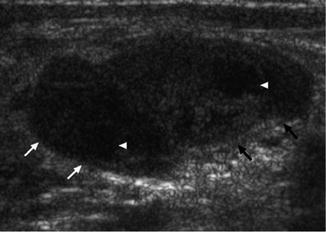
Ultrasound showing tuberculous LN (arrows) with internal caseation (arrow heads)
Lymphomatous LNs
They usually attain a rounded contour, are hypoechoic with absent echogenic hilum, and may show intra-nodal reticulation [85]. On Doppler studies, they show mixed or peripheral vascularity with increased vascular resistance on spectral Doppler US. These features are similar in both Hodgkin’s and NHL [79]. In one study, the main distinguishing feature of such LNs was the homogenous pattern (no echogenic foci) [61]. However, another study demonstrated a heterogeneous micronodular pattern in most of the examined lymphomatous nodes (Fig. 8.15) [85].
Fig. 8.15.
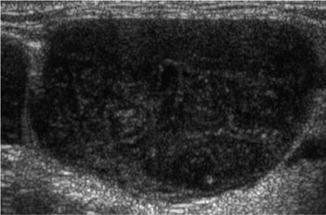
Ultrasound showing LN with NHL
Metastatic LNs
Metastatic malignant LNs usually display a rounded contour with hypoechoic nature with absence of an echogenic hilum. Echogenic foci may also be evident as a sign of coagulation necrosis. Evidence of intra-nodal cystic necrosis should suggest a metastatic nature, a pattern common in metastases from SCC [79]. Metastatic LNs from papillary thyroid carcinoma may display hyper-echogenicity, and punctate calcifications may also be present [80]. Extracapsular spread of the tumor should be suspected when the nodes acquire an ill-defined outline [79]. On color and power Doppler, metastatic LNs show mixed or peripheral vascularity [80].
Ultrasonography is being increasingly used as a great noninvasive modality to differentiate normal and abnormal nodes, where grayscale US has a sensitivity and specificity of 95 and 83 % in categorizing LNs into reactive and metastatic nodes [80]. However, distinguishing lymphomatous, metastatic, and tuberculous LNs can be a more complicated task due to overlapping parameters and relies on the common sonographic features in each category [61]. Doppler studies are essential in the setting of equivocal grayscale US results. One study demonstrated that power Doppler US helped in the correct identification of 17 % of patients with reactive LNs and 5 % of patients with metastatic malignant LNs [86].
Computed Tomography (CT) and Magnetic Resonance Imaging (MRI)
The main application of CT for cervical nodal assessment is in the setting of evaluation of metastatic LNs in the head and neck region in different clinical scenarios:
-
I.
Documented head and neck malignancy by biopsy or other imaging technique with no clinically palpable LNs, for confirmation of the N0 state as any evidence of nodal metastasis will alter both the staging and management of the tumor. It should be noted that cervical LNs metastasis represents the most important prognostic factor for squamous cell carcinoma (SCC) as it decreased the overall survival by 50 %; another 50 % worse prognosis would be expected on evidence of extracapsular extension [87–89]. At the same time, the level, number, and dimensions of the affected LNs have their clinical implications and are proved to correlate with distant metastases [87, 90].
-
II.
Documented head and neck malignancy with palpable LNs on one side of the neck, for evaluation of the contralateral neck side. During evaluation, nodal necrosis should be considered the most important criterion for detection of metastatic LNs in the setting of head and neck cancer with specificity around 95 to 100 %; this appears as focal hypoattenuation of CT and T2 hyperintensity on MRI with peripheral nodal enhancement [91]. Detection of necrosis and cystic changes in LNs not qualifying to be considered metastatic on size basis is crucial during the evaluation process.
Size of the cervical LNs should not be a definitive criterion to exclude malignancy, as LNs less than 1 cm in the largest diameter can still contain malignant tissue and expanding the cutoff size to 1.5 cm in some centers can result in higher rates of false-negative findings.
For proper evaluation, the radiologist should be aware of both the simplified level classification of cervical LNs for easier communication with surgeons and pathologist and the drainage patterns of different head and neck malignancies, meticulous examination of the commonly affected levels is crucial before declaring the neck as N0. A finding of a less commonly affected level in the context of evaluating a certain malignancy should warrant reexamination of the more commonly affected nodes.
The ability of CT to detect micrometastases is restricted. Thus, tumors with high tendency for sending micrometastases are usually managed by neck dissection procedures even if no positive LNs are detected.
Extracapsular extension of tumor tissue has its significant impact in both the prognosis and probability of distant metastasis. However, this does not affect the staging of head and neck malignancies. It should be noted though that CT is not precise in detection of such extension.
-
III.
For evaluation of carotid artery invasion by metastatic nodal tissue.
In many centers and in various clinical scenarios, carotid artery invasion by tumor tissue renders the tumor irresectable (although different surgical techniques may be pursued). Thus, detection of such invasion has a great influence on the planned management. Unfortunately, CT is not precise in detection of direct carotid artery invasion. The reliance on the contact surface alone between tumor tissue and carotid artery can be hugely misleading as a single focal contact can be accompanied by evident intraoperative invasion. Studies show no superiority in CT for detection of carotid artery invasion compared to Doppler studies.
-
IV.
For postoperative follow-up for evidence of recurrent primary and nodal disease.
The CT is a valuable tool in postoperative survey of recurrence at the primary site or at the nodal levels, where radiotherapy and postoperative fibrosis result in difficult LN palpation.
-
V.
In case of metastatic LN proven by biopsy without a clinically proven source of primary (CUP) for the detection of the primary site of malignancy.
Computed tomography of the head and neck, chest, and abdomen can help localize the site of clinically non-evident malignancy. Careful examination of aerodigestive mucosa is mandatory as a finding of focal thickening may save the patient from more aggressive and invasive diagnostic approaches.
It should be noted that a finding of cystic LN in young patients can point out occult metastasis of thyroid origin; this finding can be mistaken with developmental cystic lesions of the neck in this age group.
Other Imaging Modalities
Other imaging modalities such as FDG-PET scan has a high sensitivity for detection of affected LNs. Also, it has a great role in the diagnostic approach for carcinoma of unknown primary (CUP). It has been shown to allow the detection of primary malignancy in 25 % of these cases (PET-CT). However, it can give false-negative results in cases of metastasis from papillary or medullary thyroid cancers.
Another modality is the MRI diffusion, which is being widely tested for better precision in detection of affected LNs.
Biopsy
Biopsy is required for generalized lymphadenopathy when the initial workup is nondiagnostic and for persistent CLA with a high estimated risk of malignancy.
Excisional Biopsy
Excisional biopsy is required to diagnose and grade lymphoma into Hodgkin’s and non-Hodgkin’s varieties. It should be taken from the most abnormal or the largest LN site. However, inguinal LN biopsy should be avoided for its low diagnostic yield. In the setting of high estimated risk of malignancy, an unrevealing biopsy should be considered nondiagnostic rather than negative biopsy, and further workup is required. If results of biopsy show atypical lymphoid hyperplasia, this again should be considered nondiagnostic and further workup including another biopsy should be considered. Tissue biopsy of LNs remains a standard requirement whenever a reactive nature of LNs due to a bacterial or viral cause cannot be confirmed by imaging or serological tests.
Core Needle Biopsy
An US-guided core needle biopsy using automated needles allows for a larger yield of tissue sample and obtaining a specimen with preserved histological architecture allowing for more precise diagnosis and allows for the use of various histological and immunohistochemical techniques. Also, a biopsy obtained by core needle technique may suffice for typing of lymphoma without further need of excisional biopsy.
In a study evaluating 247 patients with cervicofacial lymphadenopathy, US-guided core needle biopsy was shown to have a specificity of 100 %, sensitivity of 98.1 %, and accuracy of 98.7 % in differentiating benign from malignant LNs. In the same study, 80 % of cases of lymphoma could proceed to treatment without the need of excisional biopsy [92].
Traditionally, disadvantages include the probable injury to neural or vascular elements (this can be improved by using imaging guided biopsy) and tumor cell spillage (needle-track metastasis). However, Southam et al. [93] found no cases of track metastasis during a period of 7 years follow-up after applying cutting needle biopsies in head and neck lesions in a large series.
It would be wise to utilize core needle biopsy when results of FNAC are equivocal and a high index of suspicion is present, especially when excisional biopsy carries a higher risk for the patient considering his general medical condition or impossible due to fixation of the nodal tissue to surrounding structures. Also, core needle biopsy can be a time-saving replacement for typing of lymphoma if a sufficient yield can be obtained instead of excisional biopsy, as this usually requires hospitalization and general anesthesia. Still, an equivocal result with considerable suspicion requires repeating the core needle biopsy or open excisional biopsy.
Technique of Modified Radical Neck Dissection (MRND) [94, 95]
Different neck incisions are described for MRND. Classically, the Kocher transverse collar incision can be extended laterally providing adequate exposure in most cases. This incision is known as half apron incision, which carries favorable cosmetic results (Figs. 8.16 and 8.17). The bilateral extension of Kocher incision is called “apron incision.” Good exposure can also be achieved by a vertical extension toward the angle of the jaw. However, cosmetic results are less favorable. A horizontal incision in the upper part of the neck in parallel to the initial incision results in better cosmesis.
Fig. 8.16.
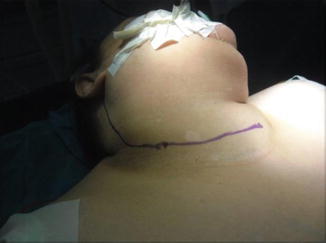
The marked site of skin incision (half apron incision)
Fig. 8.17.
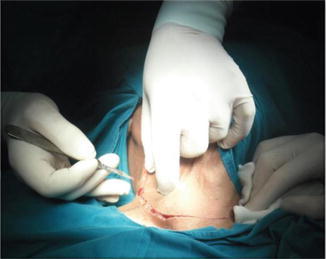
Skin incision (half apron incision)
Dissection then proceeds in the sub-platysmal plane and anterior to the external jugular vein (EJV) for proper elevation of the upper flap (Fig. 8.18).
Fig. 8.18.
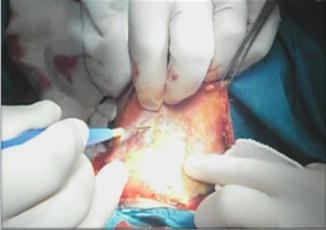
Upper flap elevation
Care should be taken during the advancement of the cranial flap, as vigorous retraction may result in injury of the marginal mandibular branch (MMB) of the facial nerve as it runs in a level just below the mandible. Such injury will result in dribbling from the angle of the mouth and deviation of this angle toward the sound (healthy) side.
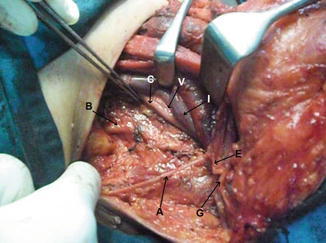
The SCM muscle can usually be preserved and retracted medially or laterally. Fascia over the SCM is then incised longitudinally over its length and gently dissected. The great auricular nerve (GAN) and EJV should be preserved whenever possible and retracted in a posterior direction (Fig. 8.19).
Fig. 8.19.
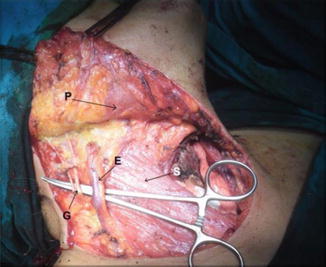
Upper flap elevated, showing SCM muscle (S), platysma (P), external jugular vein (E), and great auricular nerve (G)
The anterior section of the superficial fascia is then dissected from the SCM muscle. It is thus left in continuity with the fascia covering the IJV and its related chain of LNs. Dissection then follows either medially at the junction of the lower part of the IJV and the clavicle or laterally at the junction of the anterior border of the trapezius muscle and the clavicle.
On the left side, the thoracic duct should be identified just above the junction of the innominate vein, IJV, and subclavian veins. Distention of the duct can be achieved by gentle compression over the surrounding areolar tissue making its identification easier (Fig. 8.20). If injured, the thoracic duct should be ligated and divided or else a chyle fistula may result.
Fig. 8.20.
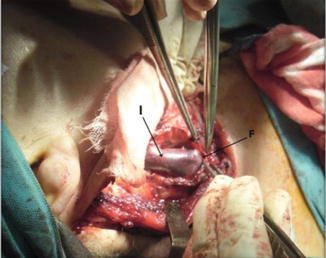
Level III dissection, showing IJV (I) and fascia over it (F)
The IJV is dissected free from its surrounding LN-bearing tissue, which contains the beginning of the MRND. Special attention must be drawn to the lower jugular nodes, which are located behind the vein. The vein should be retracted either medially or laterally to obtain a good view of this area (Fig. 8.21). This retraction should be done gently to avoid tearing the vein, which might cause air embolism.
Fig. 8.21.
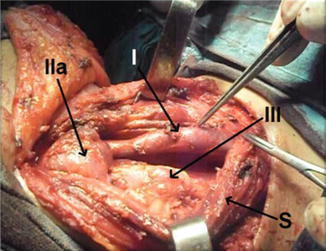
Level III anatomical cervical level of the LNs dissected, showing IJV (I) and SCM muscle retracted (S)
One should then proceed with careful dissection to expose the carotid artery, sympathetic chain, and vagus nerve. The LN-containing fatty tissue is mobilized laterally and superiorly along the clavicle, creating the inferior border of the lateral compartment dissection specimen. At this stage of the operation, care should be taken to avoid injury of the pleura. The phrenic nerve is identified as it runs obliquely on the scalenus anterior muscle. The brachial plexus is identified between the scalenus anterior and medius muscles (Figs. 8.22 and 8.23).
Fig. 8.22.
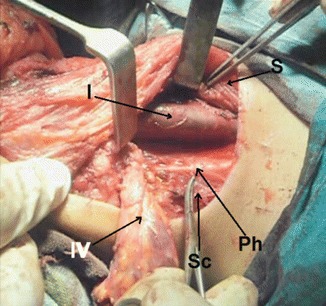
Level IV dissection at right side, showing IJV (I), SCM muscle retracted (S), phrenic nerve (Ph), and scalenus anterior (Sc)
Fig. 8.23.
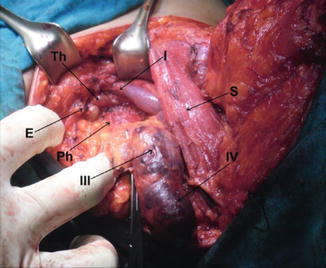
Levels III and IV dissection at left side, showing IJV (I), SCM muscle retracted (S), phrenic nerve (Ph), thoracic duct (Th), and scalenus anterior (Sc)
The anterior border of the trapezius muscle is dissected, and the spinal accessory nerve (SAN) is identified approximately 1 cm anteriorly from the margin of the muscle. The trapezius muscle represents the lateral border of the lateral neck compartment. The SAN runs parallel to the trapezius muscle over the levator muscle of the scapula. The nerve itself is rarely invaded by tumor but is often surrounded by LNs. It should be carefully dissected from the adjacent tissues upward to the cranial part of the SCM muscle (Figs. 8.24 and 8.25).
Fig. 8.24.
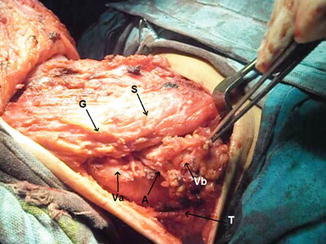
Level V dissection, showing level Va, level Vb, accessory nerve (A), trapezius muscle (T), great auricular nerve (G), and SCM muscle (S)
Fig. 8.25.
Level V dissected, showing accessory nerve (A), great auricular nerve (G), Erb’s point (E), SCM muscle retracted (S), and IJV (I)
A plexus of branches from the cervical sensory nerves (lesser occipital, greater auricular, supraclavicular, and transverse cervical nerves) is located caudal and parallel to the SAN and the phrenic nerve, and these nerves should be preserved when possible (Fig. 8.26). The GAN turns toward the SCM muscle near this point (Fig. 8.27). In this area, too, care must be taken to preserve the branch of the occipital artery, which vascularizes partly the SCM muscle.
Fig. 8.26.
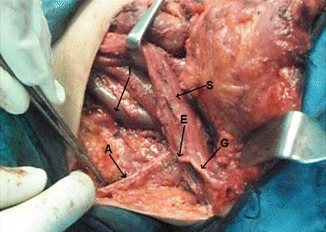
Level V dissected, showing accessory nerve (A), branch from cervical plexus (C), and IJV (I)
Fig. 8.27.
Levels III, IV and V dissected, showing accessory nerve (A), great auricular nerve (G), Erb’s point (E), IJV retracted (I), common carotid artery (C), vagus nerve (V), and trunks of brachial plexus (B)
The occipital artery represents the upper posterior limit of the dissection of the lateral compartment. The dissection continues to the prevertebral fascia. The tissue behind and above the SAN is mobilized from the nerve itself and is dissected upward from the levator muscle of the scapula and splenius muscle of the head (Fig. 8.28).
Fig. 8.28.
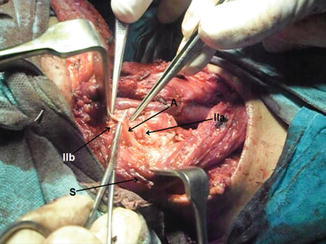
Level II dissection, showing level IIb, level IIa, accessory nerve (A), and SCM muscle retracted (S)
The inferior, lateral, and upper posterior parts of the dissection are completed, and the specimen is passed underneath the SCM muscle, which is now retracted laterally. The anterior part of the specimen is freed from the carotid sheath and jugular vein, and the dissection continues superiorly along the jugular vein, mobilizing the mid- and upper jugular LNs (Fig. 8.29).
Fig. 8.29.
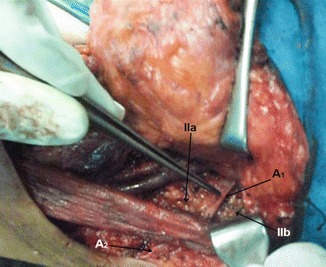
Level II dissected, showing level IIb, level IIa, accessory nerve at level II (A 1), and accessory nerve at level V (A 2)
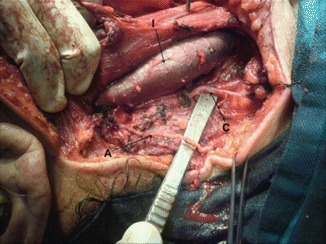
The hypoglossal nerve, which runs behind the facial vein, is identified. Sometimes the facial vein has to be ligated and transected to obtain an adequate exposure to the hypoglossal nerve while removing the upper jugular LNs. The submandibular gland and surrounding nodes are removed en bloc as a level I dissection (Fig. 8.30). The procedure is begun by incising the fascia below the gland, dissecting it up, and identifying the anterior belly of the digastric muscle, clearing the submental fat, and elevating the fascia and LNs from the lateral surface of mylohyoid muscle.
Fig. 8.30.
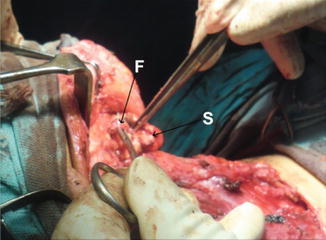
Level I dissection, showing submandibular gland (S), and facial artery (F)
The lateral superior fascia and vessels are divided earlier when the marginal nerve was identified. Care must be taken to include the submental fat pad in the specimen, which is performed by grasping the fat pad just medial to its attachment to the anterior belly of the digastric muscle and dissecting the midline tissue in the submental triangle in an inferior direction.
The mylohyoid muscle is then retracted anteriorly, exposing the lingual nerve. The attachments of the gland to the lingual nerve at the submaxillay ganglion are divided and ligated, and the submandibular duct is divided and ligated (Fig. 8.31).
Fig. 8.31.
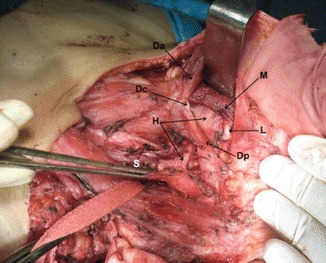
Level I dissected, showing anterior belly (Da), central tendon (Dc), posterior belly (Dp) of digastric muscle, mylohyoid muscle (M), lingual nerve (L), hypoglossal nerve (H), and superior thyroid artery (S)
The gland is retracted inferiorly with the attached pre-vascular nodes on its lateral surface. Leaving the fascia attached to the submandibular gland inferiorly will allow the contents of level I to remain a part of the ND specimen. The specimen can now be removed. Careful hemostasis is performed, and suction drains are often used. The platysma muscle is approximated and the skin is closed (Figs. 8.32, 8.33, 8.34, 8.35, and 8.36).
Fig. 8.32.
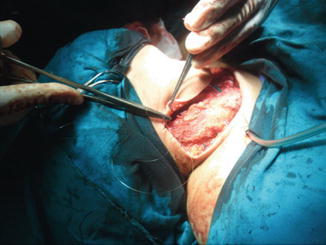
Closure of platysma with drain inserted
Fig. 8.33.
Skin closure with two drains inserted
Fig. 8.34.
Dressing put on the wound
Fig. 8.35.
Level I dissection specimen
Fig. 8.36.
MRND specimen
In the classical radical neck dissection (RND), excision of SCM muscle, SAN, and IJV is performed (Figs. 8.37 and 8.38).
Fig. 8.37.
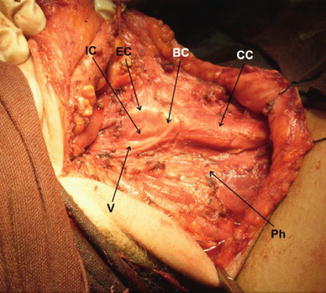
Right neck side after RND, showing common carotid artery (CC), carotid bifurcation (BC), external carotid artery (EC), internal carotid artery (IC), vagus nerve (V), and phrenic nerve (Ph)
Fig. 8.38.
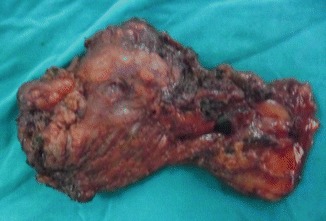
RND specimen
Contributor Information
Mahmoud Sakr, Phone: +2020+2 0100 7834933, FAX: +2020+2 03 4841189, Email: mah_sakr@yahoo.com.
Mahmoud Sakr, Email: mah_sakr@yahoo.com.
References
- 1.Rouvière H. Anatomie des lymphatiques de l’homme. Paris: Masson; 1932. [Google Scholar]
- 2.Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29(6):1446–9. doi: 10.1002/1097-0142(197206)29:6<1446::AID-CNCR2820290604>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 3.Deschler DG, Moore MG, Smith RV. Quick reference guide to TNM staging of head and neck cancer and neck dissection classification, 4th ed. Alexandria: American Academy of Otolaryngology-Head and Neck Surgery Foundation; 2014.
- 4.Bocca E, Pignataro O, Oldini C, Cappa C. Functional neck dissection: an evaluation and review of 843 cases. Laryngoscope. 1984;94(7):942–5. doi: 10.1288/00005537-198407000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Gavilan C, Gavilan J. Five-year results of functional neck dissection for cancer of the larynx. Arch Otolaryngol Head Neck Surg. 1989;115(10):1193–6. doi: 10.1001/archotol.1989.01860340047015. [DOI] [PubMed] [Google Scholar]
- 6.Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160(4):405–9. doi: 10.1016/S0002-9610(05)80554-9. [DOI] [PubMed] [Google Scholar]
- 7.Robbins KT. Classification of neck dissection: current concepts and future considerations. Otolaryngol Clin North Am. 1998;31(4):639–55. doi: 10.1016/S0030-6665(05)70077-3. [DOI] [PubMed] [Google Scholar]
- 8.Ferlito A, Rinaldo A, Robbins KT, Silver CE. Neck dissection: past, present and future? J Laryngol Otol. 2006;120(2):87–92. doi: 10.1017/S0022215105004512. [DOI] [PubMed] [Google Scholar]
- 9.Robbins KT, Clayman G, Levine PA, Medina J, Sessions R, Shaha A, et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol Head Neck Surg. 2002;128(7):751–8. doi: 10.1001/archotol.128.7.751. [DOI] [PubMed] [Google Scholar]
- 10.Robbins KT, Medina JE, Wolfe GT, Levine PA, Sessions RB, Pruet CW. Standardizing neck dissection terminology. Official report of the Academy’s Committee for Head and Neck Surgery and Oncology. Arch Otolaryngol Head Neck Surg. 1991;117(6):601–5. doi: 10.1001/archotol.1991.01870180037007. [DOI] [PubMed] [Google Scholar]
- 11.Robbins KT, Shaha AR, Medina JE, Califano JA, Wolf GT, Ferlito A, et al. Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Head Neck Surg. 2008;134(5):536–8. doi: 10.1001/archotol.134.5.536. [DOI] [PubMed] [Google Scholar]
- 12.Mohseni S, Shojaiefard A, Khorgami Z, Alinejad S, Ghorbani A, Ghafouri A. Peripheral lymphadenopathy: approach and diagnostic tools. Iran J Med Sci. 2014;39(2 Suppl):158–70. [PMC free article] [PubMed] [Google Scholar]
- 13.Sambandan T, Christeffe Mabel R. Cervical lymphadenopathy-a review. JIADS. 2011;2(1):31–3. [Google Scholar]
- 14.Bazemore AW, Smucker DR. Lymphadenopathy and malignancy. Am Fam Physician. 2002;66(11):2103–10. [PubMed] [Google Scholar]
- 15.Parisi E, Glick M. Cervical lymphadenopathy in the dental patient: a review of clinical approach. Quintessence Int. 2005;36(6):423–36. [PubMed] [Google Scholar]
- 16.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–32. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McPhail E, Kurtin P. Lymph node. In: Cheng L, Bostwick DG, editors. Essentials of anatomic pathology. New York: Springer; 2011. pp. 681–721. [Google Scholar]
- 18.Karnath BM. Approach to the patient with lymphadenopathy. Hosp Physician. 2005;41(7):29. [Google Scholar]
- 19.Biswas G, Das A, Haldar D, Mukherjee A, Dutta S, Sinha R. Clinico-pathological correlates of cervical lymphadenopathy: a hospital based study. Indian J Otolaryngol Head Neck Surg. 2013;65(1):42–7. doi: 10.1007/s12070-011-0443-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Upadhyay N. Cervical lymphadenopathy. J Dental Sci. 2012;30–33.
- 21.Gosche JR, Vick L. Acute, subacute, and chronic cervical lymphadenitis in children. Semin Pediatr Surg. 2006;15(2):99–106. doi: 10.1053/j.sempedsurg.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebell MH. Epstein-Barr virus infectious mononucleosis. Am Fam Physician. 2004;70(7):1279–87. [PubMed] [Google Scholar]
- 23.Jha B, Dass A, Nagarkar N, Gupta R, Singhal S. Cervical tuberculous lymphadenopathy: changing clinical pattern and concepts in management. Postgrad Med J. 2001;77(905):185–7. doi: 10.1136/pmj.77.905.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahid F, Afridi H, Javaid M, Khan Q, Shahabi I. Tuberculous cervical lymphadenopathy: FNAC based study of 100 cases J Med Sci. 2011;19(3):119–121
- 25.Khan JA, Mehboob M, Wadood E, Qayyum A, Arbab G. Tuberculous cervical lymphadenopathy. J Surg Pak. 2001;6:16–8. [Google Scholar]
- 26.Eshete Abdurehman, Zeyinudin Ahmed, Ali Solomon, Abera Solomon, Mohammed Mona. M. tuberculosisin Lymph Node Biopsy Paraffin-Embedded Sections. Tuberculosis Research and Treatment. 2011;2011:1–5. doi: 10.1155/2011/127817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones P, Campbell P. Tuberculous lymphadenitis in childhood: the significance of anonymous mycobacteria. Br J Surg. 1962;50(221):302–14. doi: 10.1002/bjs.18005022112. [DOI] [PubMed] [Google Scholar]
- 28.Mohapatra PR, Janmeja AK. Tuberculous lymphadenitis. J Assoc Physicians India. 2009;57:585–90. [PubMed] [Google Scholar]
- 29.Thiagarajan B, Punniyakodi K. Collar stud abscess an interesting case report. Otolaryngol Online J. 2012;2(2). ISSN2250-0359
- 30.Sato J, Tsubota H, Himi T. Syphilitic cervical lymphadenopathy. Eur Arch Otorhinolaryngol. 2003;260(5):283–5. English. [DOI] [PubMed]
- 31.Wang X, Li WQ, Liu HM, Yan HZ, Li YM, He J, et al. Isolated syphilitic cervical lymphadenopathy: report of two cases and review of the literature. J Int Med Res. 2012;40(5):1988–2000. doi: 10.1177/030006051204000541. [DOI] [PubMed] [Google Scholar]
- 32.Ridder GJ, Boedeker CC, Technau-Ihling K, Grunow R, Sander A. Role of cat-scratch disease in lymphadenopathy in the head and neck. Clin Infect Dis. 2002;35(6):643–9. doi: 10.1086/342058. [DOI] [PubMed] [Google Scholar]
- 33.Kale US, Carlin J. Toxoplasmosis as a rare cause of symptomatic cervical lymphadenopathy. Indian J Otolaryngol Head Neck Surg. 2000;52(3):261–3. doi: 10.1007/BF03006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shakya G, Malla S, Shakya KN, Shrestha R. A Study of FNAC of Cervical Lymph Nodes. Journal of Nepal Health Research Council. 1970;7(1):1–5. [Google Scholar]
- 35.Sheykholeslami1 K, Alcantara M , Khan A, Laib D. Asymptomatic neck mass as the only presenting symptom of advanced prostate cancer, a case report and literature review. J Otol Rhinol 2013,2:2. 10.4172/2324-8785.1000117 [DOI]
- 36.Alam K, Khan A, Siddiqui F, Jain A, Haider N, Maheshwari V. Fine needle aspiration cytology (FNAC), a handy tool for metastatic lymphadenopathy. Intern J Pathol. 2010;10(2):1528–8307. [Google Scholar]
- 37.Naeimi M, Sharifi A, Erfanian Y, Velayati A, Izadian S, Golparvar S. Differential diagnosis of cervical malignant lymphadenopathy among Iranian patients. Saudi Med J. 2009;30(3):377–81. [PubMed] [Google Scholar]
- 38.Magsi PB, Jamro BU, Shaikh AA, Sangi HA. An audit of 140 cases of cervical lymphadenopathy at tertiary care hospital. Gomal J Med Sci. 2013;11(1): 47–49.
- 39.Lukić S, Marjanović G, Živanović J. Palpable lymphadenopathy in primary care. Acta Facultatis Medicae Naissensis. 2011;28(1):17–23.
- 40.Mogre D. Chronic cervical lymphadenopathy: a clinicopathological profile. Paripex-Indian J Res. 2014;3(12):19–24.
- 41.Calguneri M, Ozturk MA, Ozbalkan Z, Akdogan A, Ureten K, Kiraz S, et al. Frequency of lymphadenopathy in rheumatoid arthritis and systemic lupus erythematosus. J Int Med Res. 2003;31(4):345–9. doi: 10.1177/147323000303100415. [DOI] [PubMed] [Google Scholar]
- 42.Benaglio Francesca, Vitolo Barbara, Scarabelli Martina, Binda Elisa, Bugatti Serena, Caporali Roberto, Montecucco Carlomaurizio, Manzo Antonio. The Draining Lymph Node in Rheumatoid Arthritis: Current Concepts and Research Perspectives. BioMed Research International. 2015;2015:1–10. doi: 10.1155/2015/420251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richner S, Laifer G. Peripheral lymphadenopathy in immunocompetent adults. Swiss Med Wkly. 2010;140(7–8):98–104. doi: 10.4414/smw.2010.12892. [DOI] [PubMed] [Google Scholar]
- 44.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthrit Rheumat. 2010;62(9):2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 45.Feletar M, Ibañez D, Urowitz MB, Gladman DD. The impact of the 1997 update of the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus: what has been changed? Arthrit Rheumat. 2003;48(7):2067–9. doi: 10.1002/art.11167. [DOI] [PubMed] [Google Scholar]
- 46.Shiboski S, Shiboski C, Criswell L, Baer A, Challacombe S, Lanfranchi H, et al. American College of Rheumatology classification criteria for Sjögren’s syndrome: a data‐driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance Cohort. Arthritis Care Res. 2012;64(4):475–87. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sahlmann CO, Meller J, Siggelkow H, Homayounfar K, Ozerden M, Braune I, et al. Patients with autoimmune thyroiditis. Prevalence of benign lymphadenopathy. Nuklearmedizin. 2012;51(6):223–7. doi: 10.3413/Nukmed-0484-12-03. [DOI] [PubMed] [Google Scholar]
- 48.Paksoy N, Yazal K. Cervical lymphadenopathy associated with Hashimoto’s thyroiditis: an analysis of 22 cases by fine needle aspiration cytology. Acta Cytol. 2009;53(5):491–6. doi: 10.1159/000325374. [DOI] [PubMed] [Google Scholar]
- 49.Dutta MK, Gundgurthi A, Garg MK, Kotwal N. Juvenile Graves’ disease with ophthalmopathy, lymphadenopathy, accelerated growth and congestive cardiac failure. Indian J Pediatr. 2012;79(5):670–2. doi: 10.1007/s12098-011-0503-0. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez MC, Rani D, Faas FH. Unusual clinical course of Graves’ thyrotoxicosis and concomitant sarcoidosis: case report and review of literature. Endocr Pract. 2007;13(2):159–63. doi: 10.4158/EP.13.2.159. [DOI] [PubMed] [Google Scholar]
- 51.Kanwar JB, Gupta S, Agarwal A, Gupta A. Adrenal lymphoma: case report and review of literature. World J Endocrine Surg. 2010;2(1):39–43. doi: 10.5005/jp-journals-10002-1020. [DOI] [Google Scholar]
- 52.Rashidi Armin, Fisher Stephen I. Primary adrenal lymphoma: a systematic review. Annals of Hematology. 2013;92(12):1583–1593. doi: 10.1007/s00277-013-1812-3. [DOI] [PubMed] [Google Scholar]
- 53.Danno K, Kume M, Ohta M, Utani A, Ohno S, Kobashi Y. Erythroderma with generalized lymphadenopathy induced by phenytoin. J Dermatol. 1989;16(5):392–6. doi: 10.1111/j.1346-8138.1989.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 54.Brown JR, Skarin AT. Clinical mimics of lymphoma. Oncologist. 2004;9(4):406–16. doi: 10.1634/theoncologist.9-4-406. [DOI] [PubMed] [Google Scholar]
- 55.Johns Mark E., Moscinski Lynn C., Sokol Lubomir. PHENYTOIN-ASSOCIATED LYMPHOADENOPATHY MIMICKING A PERIPHERAL T-CELL LYMPHOMA. Mediterranean Journal of Hematology and Infectious Diseases. 2010;2(2):e2010028. doi: 10.4084/MJHID.2010.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yanagi S, Nomura Y, Masuda K, Koriyama C, Sameshima K, Eguchi T, et al. Early diagnosis of Kawasaki disease in patients with cervical lymphadenopathy. Pediatr Int. 2008;50(2):179–83. doi: 10.1111/j.1442-200X.2008.02547.x. [DOI] [PubMed] [Google Scholar]
- 57.Yokouchi Y, Oharaseki T, Harada M, Ihara F, Naoe S, Takahashi K. Histopathological study of lymph node lesions in the acute phase of Kawasaki disease. Histopathology. 2013;62(3):387–96. doi: 10.1111/his.12007. [DOI] [PubMed] [Google Scholar]
- 58.Lee K-Y, Rhim J-W, Kang J-H. Kawasaki disease: laboratory findings and an immunopathogenesis on the premise of a. Yonsei Med J. 2012;53(2):262–75. doi: 10.3349/ymj.2012.53.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwon YS, Jung HI, Kim HJ, Lee JW, Choi W-I, Kim JY, et al. Isolated cervical lymph node sarcoidosis presenting in an asymptomatic neck mass: a case report. Tuberculos Respirat Dis. 2013;75(3):116–9. doi: 10.4046/trd.2013.75.3.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen HC, Kang BH, Lai CT, Lin YS. Sarcoidal granuloma in cervical lymph nodes. J Chin Med Assoc. 2005;68(7):339–42. doi: 10.1016/S1726-4901(09)70172-8. [DOI] [PubMed] [Google Scholar]
- 61.Khanna R, Sharma AD, Khanna S, Kumar M, Shukla RC. Usefulness of ultrasonography for the evaluation of cervical lymphadenopathy. World J Surg Oncol. 2011;9:29. doi: 10.1186/1477-7819-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahajan T, Merriman RC, Stone MJ. Kikuchi-Fujimoto disease (histiocytic necrotizing lymphadenitis): report of a case with other autoimmune manifestations. Proceedings (Baylor University Medical Center) 2007;20(2):149–51. doi: 10.1080/08998280.2007.11928275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sudha A, Vivekanand N. Cytologic picture of Castleman’s disease: a report of two cases. J Cytol/Indian Acad Cytolog. 2010;27(4):152–4. doi: 10.4103/0970-9371.73306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishi J, Maruyama I. Increased expression of vascular endothelial growth factor (VEGF) in Castleman’s disease: proposed pathomechanism of vascular proliferation in the affected lymph node. Leuk Lymphoma. 2000;38(3–4):387–94. doi: 10.3109/10428190009087030. [DOI] [PubMed] [Google Scholar]
- 65.Briasoulis E, Tolis C, Bergh J, Pavlidis N, Force EGT. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of cancers of unknown primary site (CUP) Ann Oncol. 2005;16(Suppl 1):i75–6. doi: 10.1093/annonc/mdi804. [DOI] [PubMed] [Google Scholar]
- 66.Saghatchian M, Fizazi K, Borel C, Ducreux M, Ruffie P, Le Chevalier T, et al. Carcinoma of an unknown primary site: a chemotherapy strategy based on histological differentiation – results of a prospective study. Ann Oncol. 2001;12(4):535–40. doi: 10.1023/A:1011129429499. [DOI] [PubMed] [Google Scholar]
- 67.Abbruzzese JL, Abbruzzese MC, Hess KR, Raber MN, Lenzi R, Frost P. Unknown primary carcinoma: natural history and prognostic factors in 657 consecutive patients. J Clin Oncol. 1994;12(6):1272–80. doi: 10.1200/JCO.1994.12.6.1272. [DOI] [PubMed] [Google Scholar]
- 68.Strojan P, Anicin A. Combined surgery and postoperative radiotherapy for cervical lymph node metastases from an unknown primary tumour. Radiother Oncol. 1998;49(1):33–40. doi: 10.1016/S0167-8140(98)00082-6. [DOI] [PubMed] [Google Scholar]
- 69.Vaamonde P, Martin Martin C, del Rio Valeiras M, Labella Caballero T. A study of cervical metastases from unknown primary tumor. Acta Otorrinolaringol Esp. 2002;53(8):601–6. doi: 10.1016/S0001-6519(02)78353-2. [DOI] [PubMed] [Google Scholar]
- 70.Leung AK, Kellner JD. Group A beta-hemolytic streptococcal pharyngitis in children. Adv Ther. 2004;21(5):277–87. doi: 10.1007/BF02850032. [DOI] [PubMed] [Google Scholar]
- 71.Ferrer R. Lymphadenopathy: differential diagnosis and evaluation. Am Fam Physician. 1998;58(6):1313–20. [PubMed] [Google Scholar]
- 72.Kozuch P GM. Lymphadenopathy. Clinical hematology and oncology. New York: Churchill Livingstone; 2003. p. 213–20.
- 73.Lee Y, Terry R, Lukes RJ. Lymph node biopsy for diagnosis: a statistical study. J Surg Oncol. 1980;14(1):53–60. doi: 10.1002/jso.2930140108. [DOI] [PubMed] [Google Scholar]
- 74.Lister TA, Crowther D. Staging for Hodgkin’s disease. Semin Oncol. 1990;17(6):696–703. [PubMed] [Google Scholar]
- 75.Chau I, Kelleher MT, Cunningham D, Norman AR, Wotherspoon A, Trott P, et al. Rapid access multidisciplinary lymph node diagnostic clinic: analysis of 550 patients. Br J Cancer. 2003;88(3):354–61. doi: 10.1038/sj.bjc.6600738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ellison E, LaPuerta P, Martin SE. Supraclavicular masses: results of a series of 309 cases biopsied by fine needle aspiration. Head Neck. 1999;21(3):239–46. doi: 10.1002/(SICI)1097-0347(199905)21:3<239::AID-HED9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 77.Steel BL, Schwartz MR, Ramzy I. Fine needle aspiration biopsy in the diagnosis of lymphadenopathy in 1,103 patients. Role, limitations and analysis of diagnostic pitfalls. Acta Cytol. 1995;39(1):76–81. [PubMed] [Google Scholar]
- 78.Habermann TM, Steensma DP. Lymphadenopathy. Mayo Clin Proc. 2000;75(7):723–32. doi: 10.1016/S0025-6196(11)64620-X. [DOI] [PubMed] [Google Scholar]
- 79.Ahuja AT, Ying M. Sonographic evaluation of cervical lymph nodes. Am J Roentgenol. 2005;184(5):1691–9. doi: 10.2214/ajr.184.5.01841691. [DOI] [PubMed] [Google Scholar]
- 80.Ahuja A, Ying M. Sonography of neck lymph nodes. Part II: abnormal lymph nodes. Clin Radiol. 2003;58(5):359–66. doi: 10.1016/S0009-9260(02)00585-8. [DOI] [PubMed] [Google Scholar]
- 81.van den Brekel MW, Castelijns JA, Stel HV, Golding RP, Meyer CJ, Snow GB. Modern imaging techniques and ultrasound-guided aspiration cytology for the assessment of neck node metastases: a prospective comparative study. Eur Arch Otorhinolaryngol. 1993;250(1):11–7. doi: 10.1007/BF00176941. [DOI] [PubMed] [Google Scholar]
- 82.Ying M, Ahuja AT, Evans R, King W, Metreweli C. Cervical lymphadenopathy: sonographic differentiation between tuberculous nodes and nodal metastases from non-head and neck carcinomas. J Clin Ultrasound. 1998;26(8):383–9. doi: 10.1002/(SICI)1097-0096(199810)26:8<383::AID-JCU2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 83.Asai S, Miyachi H, Suzuki K, Shimamura K, Ando Y. Ultrasonographic differentiation between tuberculous lymphadenitis and malignant lymph nodes. J Ultrasound Med. 2001;20(5):533–8. doi: 10.7863/jum.2001.20.5.533. [DOI] [PubMed] [Google Scholar]
- 84.Ahuja A, Ying M, Yuen YH, Metreweli C. Power Doppler sonography to differentiate tuberculous cervical lymphadenopathy from nasopharyngeal carcinoma. AJNR Am J Neuroradiol. 2001;22(4):735–40. [PMC free article] [PubMed] [Google Scholar]
- 85.Ahuja AT, Ying M, Yuen HY, Metreweli C. ‘Pseudocystic’ appearance of non-Hodgkin’s lymphomatous nodes: an infrequent finding with high-resolution transducers. Clin Radiol. 2001;56(2):111–5. doi: 10.1053/crad.2000.0642. [DOI] [PubMed] [Google Scholar]
- 86.Ahuja A, Ying M. Sonographic evaluation of cervical lymphadenopathy: is power Doppler sonography routinely indicated? Ultrasound Med Biol. 2003;29(3):353–9. doi: 10.1016/S0301-5629(02)00759-7. [DOI] [PubMed] [Google Scholar]
- 87.O’Brien CJ, Smith JW, Soong SJ, Urist MM, Maddox WA. Neck dissection with and without radiotherapy: prognostic factors, patterns of recurrence, and survival. Am J Surg. 1986;152(4):456–63. doi: 10.1016/0002-9610(86)90324-7. [DOI] [PubMed] [Google Scholar]
- 88.Johnson JT, Barnes EL, Myers EN, Schramm VL, Jr, Borochovitz D, Sigler BA. The extracapsular spread of tumors in cervical node metastasis. Arch Otolaryngol. 1981;107(12):725–9. doi: 10.1001/archotol.1981.00790480001001. [DOI] [PubMed] [Google Scholar]
- 89.Chong V. Cervical lymphadenopathy: what radiologists need to know. Cancer Imaging. 2004;4(2):116. doi: 10.1102/1470-7330.2004.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer. 1993;71(2):452–6. doi: 10.1002/1097-0142(19930115)71:2<452::AID-CNCR2820710228>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 91.Kaji AV, Mohuchy T, Swartz JD. Imaging of cervical lymphadenopathy. Semin Ultrasound CT MR. 1997;18(3):220–49. doi: 10.1016/S0887-2171(97)90021-4. [DOI] [PubMed] [Google Scholar]
- 92.Screaton NJ, Berman LH, Grant JW. Head and neck lymphadenopathy: evaluation with US-guided cutting-needle biopsy. Radiology. 2002;224(1):75–81. doi: 10.1148/radiol.2241010602. [DOI] [PubMed] [Google Scholar]
- 93.Southam J, Bradley P, Musgrove B. Fine needle cutting biopsy of lesions of the head and neck. Br J Oral Maxillofac Surg. 1991;29(4):219–22. doi: 10.1016/0266-4356(91)90187-A. [DOI] [PubMed] [Google Scholar]
- 94.Ducci M, Appetecchia M, Marzetti M. Neck dissection for surgical treatment of lymph node metastasis in papillary thyroid carcinoma. J Exp Clin Cancer Res. 1997;16(3):333–5. [PubMed] [Google Scholar]
- 95.Grénman R. Principles and Techniques of Neck Dissection. In: Anniko M, Bernal-Sprekelsen M, Bonkowsky V, Bradley P, Iurato S, editors. Otorhinolaryngology, head and neck surgery. European manual of medicine. Berlin/Heidelberg: Springer; 2010. [Google Scholar]


