Abstract
Background and Purpose:
The 2018 AHA guidelines recommend perfusion imaging to select patients with acute large vessel occlusion (LVO) for thrombectomy in the extended window. However, the relationship between noncontrast CT and CT perfusion imaging has not been sufficiently characterized >6h after last known normal (LKN).
Methods:
From a multicenter prospective cohort of consecutive adults who underwent thrombectomy for anterior LVO 0-24 hours after LKN, we correlated baseline core volume (rCBF<30%) and the Alberta Stroke Program Early CT Scale (ASPECTS) score. We compared perfusion findings between patients with an unfavorable ASPECTS (<6) against those with a favorable ASPECTS (≥6), and assessed findings over time.
Results:
Of 485 enrolled patients, 177 met inclusion criteria (median age 69y [IQR 57-81], 49% female, median ASPECTS 8 [IQR 6-9], median core 10cc [IQR 0-30]). ASPECTS and core volume moderately correlated (r=−0.37). A 0cc core was observed in 54 (31%) patients, 70% of whom had ASPECTS <10. Of the 28 patients with ASPECTS <6, 3 (11%) had a 0cc core. After adjustment for age and stroke severity, there was a lower ASPECTS for every 1hr delay from LKN (cOR 0.95, 95%CI 0.91-1.00, p=0.04). There was no difference in core (p=0.51) or penumbra volumes (p=0.87) across patients over time.
Conclusions:
In this multicenter prospective cohort of patients who underwent thrombectomy, one-third of patients had normal CTP core volumes despite nearly three quarters of patients showing ischemic changes on CT. This finding emphasizes the need to carefully assess both non-contrast and perfusion imaging when considering thrombectomy eligibility.
Keywords: CT, CT Perfusion, Stroke
INTRODUCTION
The American Heart Association recommends endovascular thrombectomy for patients with acute ischemic stroke due to large vessel occlusion (LVO) with favorable non-contrast computed tomography (CT) imaging if they present within 6 hours of symptom onset.1 After 6 hours, the recommendations suggest the use of perfusion imaging in addition to the non-contrast CT for the selection of thrombectomy candidates.
The use of automated perfusion imaging may accurately identify patients with a minimal burden of irreversible ischemia and a large volume of salvageable penumbra, who could potentially benefit from endovascular recanalization. Using RAPID automated software (iSchemaView, Redwood, CA), the DAWN and DEFUSE-3 clinical trials have proven a robust clinical response to thrombectomy for patients with proximal LVO (ICA-terminus or M1) who present within 6-24 hours after the time last known normal (LKN).2,3 Patients for either trial were excluded from randomization if non-contrast imaging showed evidence of infarct involving more than one-third of the middle cerebral artery territory (DAWN), ASPECTS <6 (DEFUSE-3), or perfusion imaging showed significant perfusion core infarct volume (≥70cc for DEFUSE-3 and ≥31-to-51cc for DAWN, depending on age and stroke severity).
While perfusion software platforms like RAPID automatically calculate volumes of ischemic and oligemic tissue, interpretation of the non-contrast CT is arguably more subjective and concerns surround the inter-rater reliability.4,5 We aimed to understand 1) the relationship between non-contrast CT ASPECTS score and automated, CTP-based core infarct volume estimation in the extended window; 2) time dependent changes in both these measures; and 3) differences in outcomes of patients based on favorability of the ASPECTS who otherwise had a favorable perfusion profile.
METHODS
Patient selection
A prospective, multi-center, observational cohort of consecutively treated patients over 18 years of age with acute LVO was used in this analysis. The design of this cohort (the Blood Pressure after Endovascular Stroke Treatment study) and patient characteristics have been described previously.6 Briefly, consecutive patients with anterior circulation LVO who underwent endovascular thrombectomy at one of 12 academic centers in the US were prospectively enrolled in this observational study. Patient characteristics, neuroimaging features, and clinical outcomes were collected by local investigators at each site using a centrally monitored, HIPAA-compliant, online platform, as previously described.7 This study was approved by each site’s Institutional Review Board, except one that deemed it exempt. Data included herein may be requested from the corresponding author at reasonable request.
Consecutive patients with acute LVO of the anterior circulation (ICA-terminus, M1, or M2) were screened for inclusion in BEST. Diagnosis of LVO was confirmed by CTA of the head, which was acquired in conjunction with the non-contrast CT and CTP. For this subgroup analysis, patients were excluded if the time last known normal (LKN) was unknown or if CTP was not performed.
Imaging
Non-contrast head CT, CT angiography of the head and neck, and CTP of the head were performed according to each institution’s protocol. CTP studies were postprocessed using RAPID software to generate automated, operator-independent, motion-corrected, deconvolution-based maps of the ischemic core and hypoperfusion, as in recently published clinical trials.2,3 Relative cerebral blood flow (rCBF), and time-to-maximum of the tissue residue function (Tmax) were calculated and the automated values were used in this study, irrespective of potential artifactual findings. RAPID automatically segments and calculates volumes of the ischemic core (rCBF<30%) and of hypoperfusion (Tmax>6s), based on consensus recommendations.8 Alberta Stroke Program Early CT Scale (ASPECTS) score was extracted from the radiology report of the initial non-contrast CT scan or assessed by trained local study personnel. For patients who underwent CTP, the quantitated perfusion core (rCBF <30%) and hypoperfusion (Tmax>6s) volumes were automatically calculated.
Statistical analysis
In the primary analysis, patients were divided into two groups based on the timing of recanalization (≤6h from time LKN [“early window”] vs. >6h after LKN [“extended window”]). Time was recorded as a continuous variable from LKN to time recanalization was achieved for angiography/thrombectomy—or if recanalization was unsuccessful, the time when angiography was aborted. The time of CT/CTP acquisition was not collected. The two primary dependent variables of interest were the (1) ASPECTS score and (2) ischemic core volume. A sensitivity analysis was also performed to estimate the association between ASPECTS and time from LKN to recanalization, after excluding one outlier who underwent thrombectomy at 52 hours. The Spearman’s rank-order correlation was used to estimate the association of ASPECTS score and perfusion core volume. Secondary pre-specified dependent variables included the absolute mismatch ratio (defined as the volume of hypoperfused tissue divided by the volume of ischemic core), hypoperfusion volume using RAPID software, hemorrhagic transformation within 72 hours of thrombectomy, symptomatic ICH (sICH, defined by a worsening of the NIHSS by 4 or more points within 24 hours of admission with associated ICH detected within 72 hours), “good functional outcome” (defined as a 90-day modified Rankin Scale [mRS] score of 0-2), and 90-day mRS.
Subgroup analysis according to DAWN/DEFUSE-3 perfusion criteria
In a sensitivity analysis, only patients who underwent CTP and met DAWN3 or DEFUSE-32 perfusion imaging criteria were analyzed. To be included in this subgroup analysis, patients were not required to have met the non-contrast imaging criteria in each of these trials. A minimum, core volume-varying NIHSS was required per DAWN.3 However, we did not require a minimum NIHSS of 7 to apply DEFUSE-32 perfusion imaging criteria to this subgroup of patients.
In this subgroup analysis, patients were categorized as having unfavorable non-contrast CT imaging (ASPECTS <6) or favorable ASPECTS (≥6). Patients had to have met at least one trial’s perfusion imaging criteria as summarized: For DEFUSE-3,2 a perfusion core volume <70cc, mismatch ratio >1.8, and mismatch volume >15 ml; or for DAWN,3 the patient must have met core volume criteria according to age and stroke severity. The primary outcome of this analysis was a “good functional outcome” at 90 days.
Normality of continuous variables was evaluated histographically and with the Shapiro-Wilk test. Categorical data were presented as proportions, and continuous data were reported as medians with interquartile range. Between-group comparisons were made using Chi-square or Fisher’s exact test for categorical variables, or the Wilcoxon Rank-Sum test, where appropriate. Crude and adjusted logistic and linear regression analyses were performed to correlate independent variables with outcomes of interest. Ordered logistic regression was used to generate a proportional odds model for the change in ASPECTS score over time (grouped by scores 1–5, 6, 7, 8, 9, and 10, due to the small number of patients with ASPECTS <6), with a lower odds indicating a lower probability for having a higher ASPECTS category, and was adjusted for age and baseline stroke severity. The proportional odds assumption of the ordered logit model was confirmed using the Brant test (p=0.24). Age, baseline NIHSS, time to recanalization, treatment with IV tPA, and successful recanalization (TICI 2b/3) were incorporated into multivariable models assessing odds of hemorrhagic transformation and functional outcome measures. All tests were two-sided and performed using STATA 15.0 (STATA Corp, College Station, TX). P-values are reported for conventional purposes only, and should be interpreted as hypothesis generating. Missing data was not imputed. No adjustments were made for multiple comparisons as all analyses were exploratory.
RESULTS
Of 485 enrolled patients in the overall BEST study, 177 underwent CTP and were included in the analyses. The median age was 69 years (IQR 57-81), 87 were female (49%), with a median baseline NIHSS of 17 (IQR 11-20). Seventy-two patients (41%) presented in the early window. Compared to patients who were treated beyond 6 hours, those treated in the early window more frequently received intravenous tissue plasminogen activator (65% versus 10%, p<0.01), but there were no other meaningful differences in baseline patient characteristics (Table 1). No patients received intra-arterial thrombolysis. When patients were dichotomized by treatment within the early window (<6h) or extended window (6-24h), there was no significant difference in ASPECTS score, perfusion core volume, or hypoperfusion volume (Table 2).
Table 1.
Patient demographics according to timing of recanalization.
| EVT 0-6 hours (n=72) | EVT >6 hours (n=105) | p-value | |
|---|---|---|---|
| Age, median years (IQR) | 68 (58-80) | 69 (57-81) | 0.62 |
| Sex, n female (%) | 37 (52%) | 50 (48%) | 0.62 |
| Ethnicity, n (%) | 0.03 | ||
| Black | 12 (17%) | 21 (20%) | |
| Caucasian | 35 (49%) | 50 (48%) | |
| Asian | 0 (0%) | 2 (2%) | |
| Other | 20 (28%) | 15 (14%) | |
| Unknown | 5 (7%) | 19 (18%) | |
| Baseline NIHSS, median (IQR) | 18 (13-21) | 16 (10-20) | 0.17 |
| Occlusion location, n (%) | 0.89 | ||
| Internal carotid artery terminus occlusion | 21 (29%) | 30 (29%) | |
| M1 occlusion | 38 (53%) | 53 (50%) | |
| M2 occlusion | 13 (18%) | 22 (21%) | |
| Treatment with intravenous thrombolysis, n (%) | 47 (65%) | 10 (10%) | <0.01 |
| Time from last seen normal to recanalization or angiography completion, median minutes (IQR) | 232 (182-274) | 720 (540-969) | <0.01 |
| TICI 2b/3, n (%) | 69 (96%) | 85 (81%) | <0.01 |
EVT: endovascular thrombectomy, n: number of patients, IQR: interquartile range, NIHSS: National Institutes of Health Stroke Scale, M1: the proximal middle cerebral artery segment, M2: the second division of the middle cerebral artery, and TICI: thrombolysis in cerebral infarction, with a score of 2b/3 indicating >50% recanalization of the previously occluded vessel.
Table 2.
Imaging and outcome variables according to timing of recanalization.
| EVT 0-6 hours (n=72) | EVT >6 hours (n=105) | p-value | |
|---|---|---|---|
| ASPECTS, median (IQR) | 8 (6-9) | 8 (6-9) | 0.51 |
| Volume of ischemic core, median rCBF <30% mL (IQR) | 14 (1-36) | 7 (0-26) | 0.13 |
| Volume of perfusion deficit, median Tmax >6s mL (IQR) | 118 (81-167) [n=71] | 112 (64-159) [n=105] | 0.21 |
| Mismatch ratio, median (IQR) | 4.9 (3.1-10.2) [n=55] | 6.4 (2.9-17.1) [n=68] | 0.37 |
| Any ICH within 72 hours, n (%) | 24 (31%) | 32 (33%) | 0.83 |
| Symptomatic ICH† within 72 hours, n (%) | 3 (4%) | 9 (9%) | 0.37 |
| Good functional outcome*, n (%) | 28/64 (44%) | 31/99 (31%) | 0.11 |
| 90-day mRS, median (IQR) | 3 (1-4) [n=64] | 3 (2-5) [n=99] | 0.09 |
Good functional outcome indicates mRS of 0-2 90 days after infarct.
Symptomatic ICH indicates worsening of the NIHSS by 4 or more points within 24 hours of admission with associated intracranial hemorrhage identified on computed tomography detected within 72 hours.
EVT: endovascular thrombectomy, n: number of patients, ASPECTS: Alberta Stroke Program Early CT Scale, IQR: interquartile range, rCBF: regional cerebral blood flow, Tmax >6s: time to tissue maximum residue function greater than 6 seconds, ICH: intracerebral hemorrhage, and mRS: modified Rankin Scale score.
Relationship between ASPECTS and rCBF
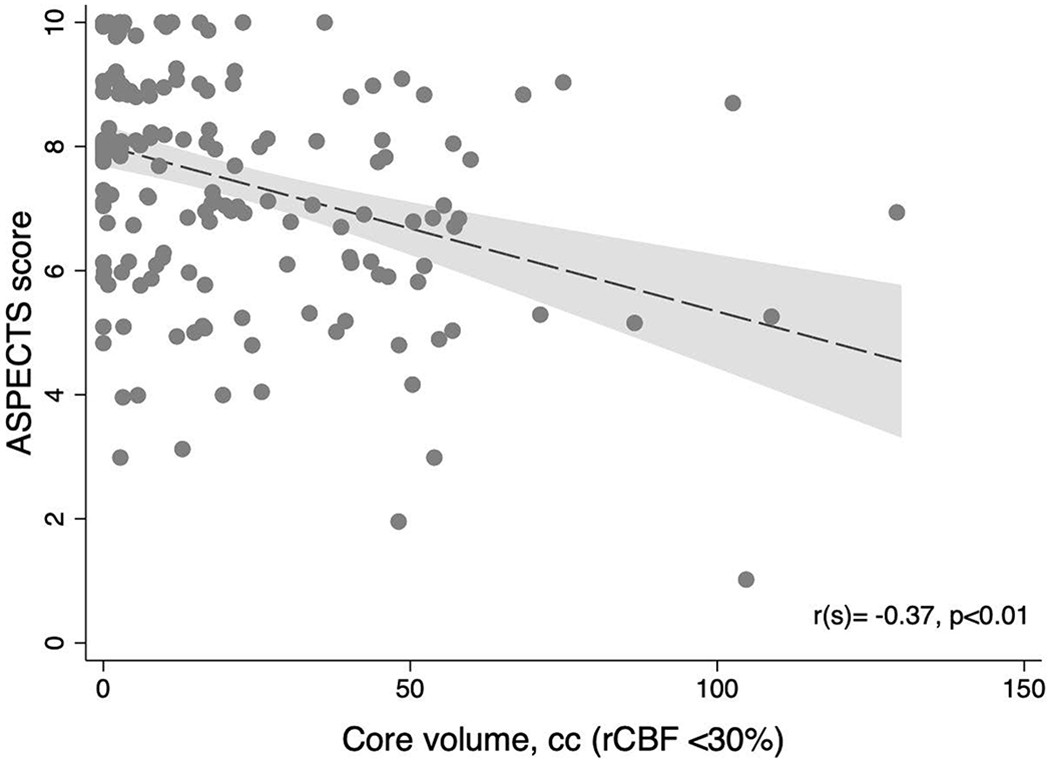
Among all included patients, the median non-contrast CT ASPECTS score was 8 (IQR 6-9) with a median perfusion core volume of 10cc (IQR 0-30). ASPECTS and core volume moderately correlated (rs=−0.37, p<0.01; Figure 3). This degree of correlation persisted among patients who underwent early recanalization (rs=−0.40, p<0.01) or recanalization in the extended window (6-24h, rs=−0.38, p<0.01). Of all included patients, a core volume of 0cc was observed in 54 (31%), 38 of whom (70%) had an ASPECTS <10. Among patients who presented beyond 6 hours (n=105), a core volume of 0cc was observed in 37 patients (35%)—29 of whom (78%) had an ASPECTS <10. Twenty-eight patients had an ASPECTS score <6, including 14 who presented in the extended window (13% of all extended window patients), and 3 who had a core volume of 0cc.
Figure 3.
Relationship between ASPECTS and core volume on CTP.
ASPECTS: Alberta Stroke Program Early Computed Tomography Scale, rCBF: Relative cerebral blood flow, cc: Cubic centimeters.
Relationship of ASPECTS and rCBF with time
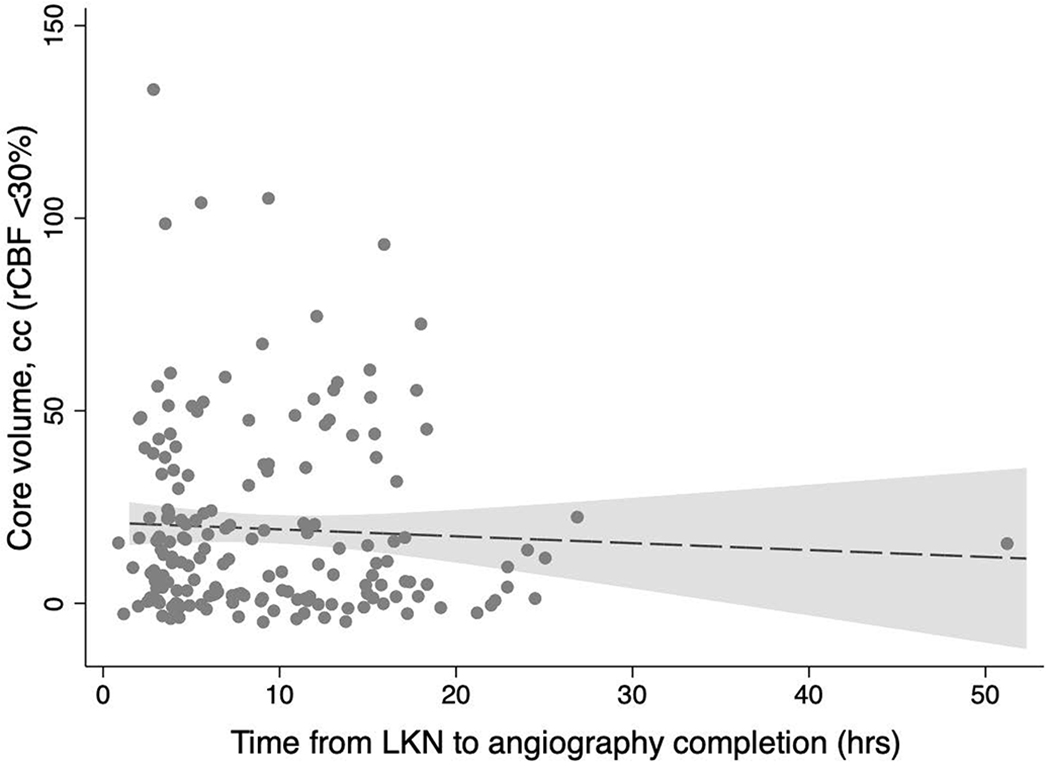
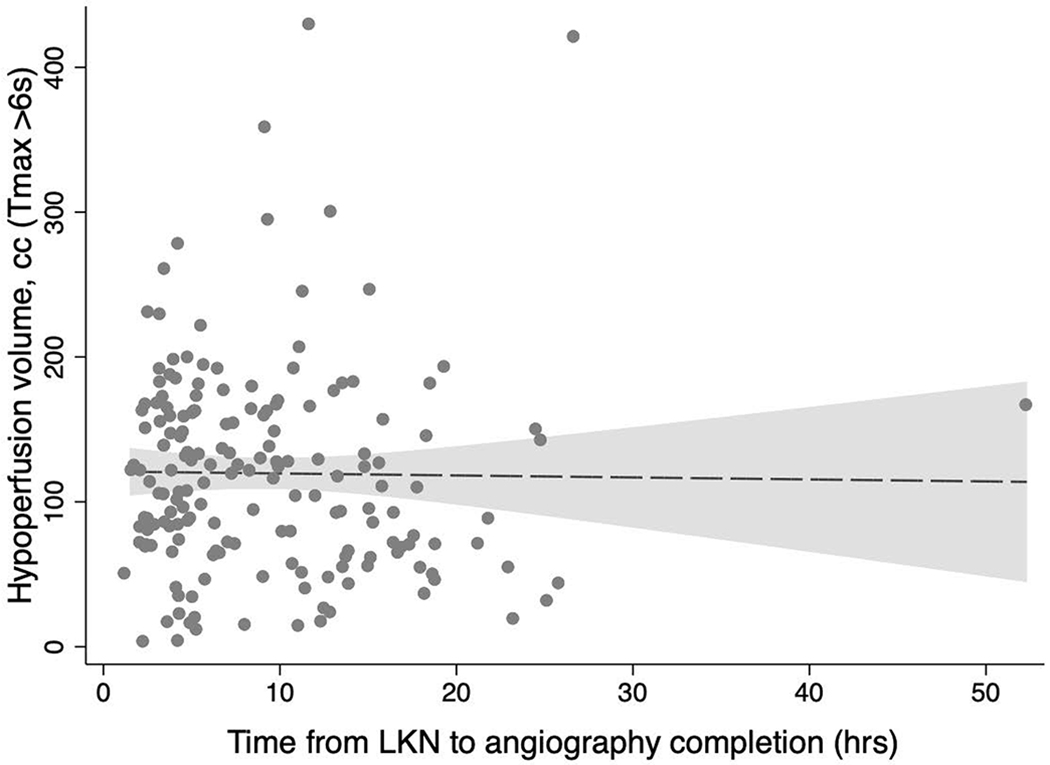
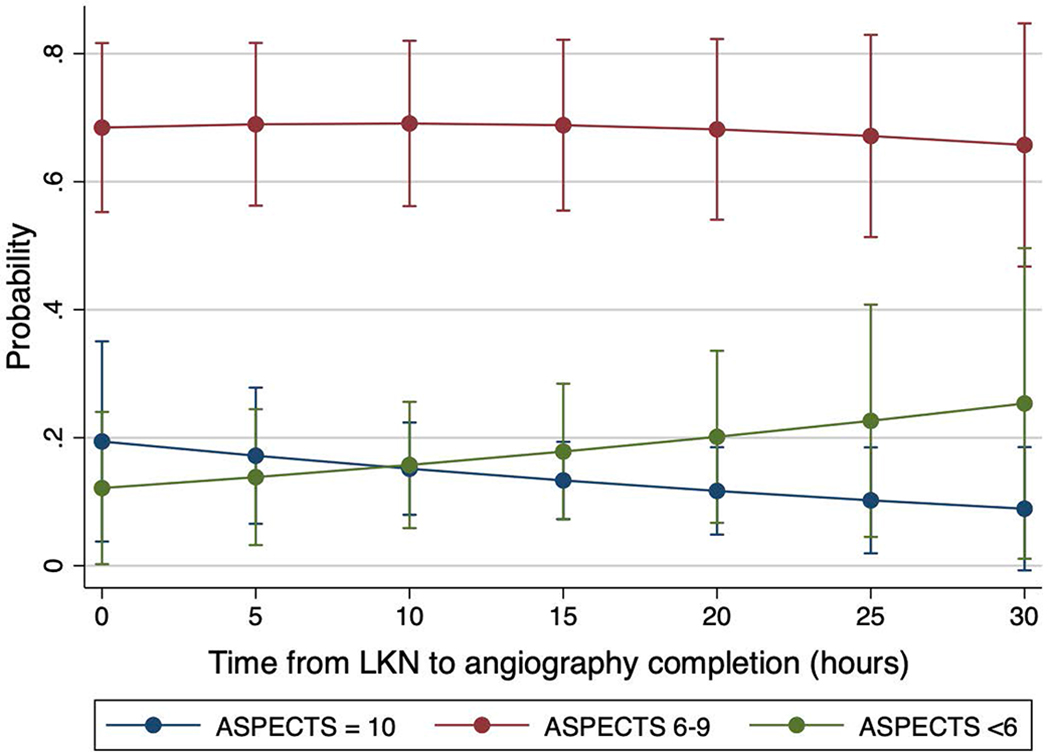
ASPECTS scores were non-significantly lower for every passing hour from LKN (common OR 0.96, 95%CI 0.91-1.00, p=0.06) whereas the core (β= −0.18, 95%CI −0.72 – 0.36 p=0.51) and penumbra volumes (β= −0.14, 95%CI −1.72 – 1.45, p=0.87) remained unchanged. After adjustment for age and baseline NIHSS, the odds of a higher ASPECTS for every hour after LKN dwindled (cOR 0.95, 95%CI 0.91-1.00, p=0.04; Figure 1), while the core (β= −0.15, 95%CI −0.67 – 0.38, p=0.58) and penumbra volumes (β= −0.07, 95%CI −1.63 – 1.49, p=0.93) remained unchanged (Figure 2). After excluding one outlier who underwent thrombectomy at 52 hours after LKN, ASPECTS scores were lower for every hour after LKN, however this change was not statistically significant (adjusted cOR 0.96, 95%CI 0.91-1.01, p=0.12).
Figure 1.
Probability of a given ASPECTS score according to time from last known normal.
The probability of a higher ASPECTS score (10) lessens with time, while the probability of a lower ASPECTS score (1-5) grows with time. Of note, one outlier patient was evaluated at 52 hours after last known normal and was excluded from this plot. They presented with severe deficits (National Institutes of Health Stroke Scale of 18) and an internal carotid artery occlusion, ASPECTS score of 5, perfusion core volume of 15cc and a hypoperfusion volume of 167cc. They underwent thrombectomy with 100% recanalization, which was complicated by an asymptomatic hemorrhage and mild clinical improvement. By 90 days, their modified Rankin Scale was 4. LKN: Last known normal, ASPECTS: Alberta Stroke Program Early CT Scale.
Figure 2.
Change in perfusion core and hypoperfusion volumes over time.
Scatter plots for core volume (A) and hypoperfusion volume (B) versus time shown with fitted lines and 95% confidence intervals. LKN: Last known normal, hrs: Hours, cc: Cubic centimeters, rCBF: Relative cerebral blood flow, and Tmax >6s: Time to tissue maximum residue function greater than 6 seconds.
ASPECTS-based outcomes among patients meeting trial perfusion criteria
Of the 177 patients who underwent CTP, 160 (91%) met either DAWN or DEFUSE-3 perfusion imaging criteria and were deemed “perfusion-eligible.” Of the 17 patients who did not meet trial criteria, 4 had a large perfusion core volume (>70cc), 12 had a mismatch ratio <1.8, and 9 had a mismatch volume of <15cc (with overlap between these groups). Twenty-three of the 160 perfusion-eligible patients (14%) had unfavorable baseline non-contrast CT ASPECTS score. Patients with unfavorable baseline ASPECTS (<6) had similar stroke severity, occlusion location, and other baseline measures when compares to those with a favorable ASPECTS score (6-10; Table 3). Patients with unfavorable ASPECTS had larger core and penumbral volumes than those with favorable ASPECTS scores (Table 4).
Table 3.
Demographics for patients meeting trial perfusion imaging criteria.
| Unfavorable ASPECTS (n=23) | Favorable ASPECTS (n=137) | p-value | |
|---|---|---|---|
| Age, median years (IQR) | 66 (61-81) | 70 (56-81) | 0.76 |
| Sex, n female (%) | 13 (56%) | 64 (47%) | 0.38 |
| Ethnicity, n (%) | 0.12 | ||
| Black | 5 (22%) | 28 (20%) | |
| Caucasian | 15 (65%) | 60 (44%) | |
| Asian | 0 (0%) | 2 (1%) | |
| Other | 2 (9%) | 26 (19%) | |
| Unknown | 1 (4%) | 21 (15%) | |
| Baseline NIHSS, median (IQR) | 18 (15-22) | 17 (11-20) | 0.13 |
| Occlusion location, n (%) | 0.68 | ||
| Internal carotid artery terminus occlusion | 10 (44%) | 39 (28%) | |
| M1 occlusion | 11 (48%) | 73 (53%) | |
| M2 occlusion | 2 (9%) | 25 (18%) | |
| Treatment with intravenous thrombolysis, n (%) | 9 (39%) | 45 (33%) | |
| Time from last seen normal to recanalization or angiography completion, median minutes (IQR) | 352 (231-720) | 404 (244-794) | 0.49 |
| TICI 2b/3, n (%) | 21 (91%) | 117 (85%) | 0.45 |
ASPECTS: Alberta Stroke Program Early CT Scale, n: number of patients, IQR: interquartile range, NIHSS: National Institutes of Health Stroke Scale, M1: the proximal middle cerebral artery segment, M2: the second division of the middle cerebral artery, and TICI: thrombolysis in cerebral infarction, with a score of 2b/3 indicating >50% recanalization of the previously occluded vessel.
Table 4.
Imaging variables for patients who met DAWN or DEFUSE-3 perfusion imaging criteria.
| Unfavorable ASPECTS (n=23) | Favorable ASPECTS (n=137) | p-value | |
|---|---|---|---|
| ASPECTS, median (IQR) | 5 (4-5) | 8 (7-9) | <0.01 |
| Volume of ischemic core, median rCBF<30% mL (IQR) | 19 (8-49) | 6 (0-20) | <0.01 |
| Volume of perfusion deficit, median Tmax >6s mL (IQR) | 163 (94-181) [n=23] | 116 (72-160) [n=136] | 0.04 |
| Mismatch ratio, median (IQR) | 5.2 (3.0-11.4) [n=20] | 6.9 (3.7-17.1) [n=88] | 0.27 |
ASPECTS: Alberta Stroke Program Early CT Scale, n: number of patients, IQR: interquartile range, rCBF: regional cerebral blood flow, Tmax >6s: time to tissue maximum residue function > 6 seconds.
Among patients who met DAWN or DEFUSE-3 perfusion imaging criteria, when compared to patients with favorable ASPECTS, those with unfavorable ASPECTS had poorer clinical outcomes when compared to those with a favorable ASPECTS, including a greater risk of sICH (adjusted OR 16.4 [95%CI 3.7-72.7], p<0.01), poorer mRS at 90 days (adjusted cOR 0.2 [95%CI 0.1-0.5, p<0.01]) and a trend toward a lower odds of a good functional outcome at 90 days mRS 0-2, adjusted OR 0.2 [95%CI 0.04-1.6], p=0.14; Table 5).
Table 5.
Clinical outcomes among patients who met DAWN or DEFUSE-3 perfusion criteria.
| Unfavorable ASPECTS (n=23) | Favorable ASPECTS (n=137) | Unadjusted OR | 95%CI | p-value | Adjusted OR* | 95%CI | p-value | |
|---|---|---|---|---|---|---|---|---|
| Any ICH within 72 hours | 12 (52%) | 38 (28%) | 2.8 | 1.1-7.1 | 0.03 | 2.6 | 1.1-6.3 | 0.04 |
| Symptomatic ICH within 72 hours† | 5 (22%) | 4 (3%) | 9.2 | 2.9-29.4 | <0.01 | 16.4 | 3.7-72.7 | <0.01 |
| Good functional outcome‡ | 3/21 (14%) | 52/126 (41%) | 0.2 | 0.04-1.6 | 0.14 | 0.2 | 0.04-1.6 | 0.14 |
| 90-day mRS | 5 (IQR 4-6) | 3 (IQR 1-5) | 0.2# | 0.1-0.5 | <0.01 | 0.2# | 0.1-0.5 | <0.01 |
Odds ratios adjusted for age, baseline NIHSS, intravenous thrombolysis, TICI 2b/3 recanalization, and time to recanalization, and clustered by hospital site.
Symptomatic ICH indicates worsening of the NIHSS by 4 or more points within 24 hours of admission with associated intracranial hemorrhage identified on computed tomography detected within 72 hours.
Good functional outcome indicates mRS of 0-2 90 days after infarct.
Common odds ratio for achieving a better (lower) mRS score at 90 days.
ASPECTS: Alberta Stroke Program Early CT Scale, n: number of patients, OR: odds ratio, CI: confidence interval, ICH: intracerebral hemorrhage, mRS: modified Rankin Scale, NIHSS: National Institutes of Health Stroke Scale, and TICI: thrombolysis in cerebral infarction, with a score of 2b/3 indicating >50% recanalization of the previously occluded vessel.
DISCUSSION
In this prospective observational cohort study, representing the routine clinical practice at 12 comprehensive stroke centers across the United States, we found that there was a discrepancy between ASPECTS-based and automated CT perfusion-based estimation of irreversible ischemia, and this discrepancy was not dependent on the early versus late window of presentation. Interestingly, there was a time dependent decay in the ASPECTS, but perfusion-based estimation of core did not change over time. While the estimation of the ASPECTS decay lost statistical significance after excluding one outlier who presented at 52 hours after LKN, the effect size remained. Further, patients with unfavorable ASPECTS who otherwise met DAWN and DEFUSE-3 perfusion imaging criteria had worse functional and hemorrhagic outcomes compared to those with favorable ASPECTS.
A small proportion of patients in this study underwent thrombectomy in the extended window (as part of clinical practice) despite ASPECTS scores which would have disqualified them from inclusion in the extended window trials,2,3 although they had acceptable perfusion imaging findings. The non-contrast CT plays a vital role in patient selection and prognostication, since poorer ASPECTS have been associated with higher rates of sICH. Unsurprisingly, ASPECTS scores were lower in patients who presented at a later time from LKN, although there was no significant relationship between time-to-imaging and change in perfusion imaging parameters. These data emphasize the role of the non-contrast CT in identifying early and potentially irreversible cerebral infarction when perfusion imaging may show minimal or no areas of “ischemic core.” It is possible that subcritical oligemia (rCBF >30%, for example) may still result in neuronal death—especially in the extended time window9—but this degree of hypoperfusion could be missed using thresholds prespecified by RAPID and other automated software platforms. Therefore, it remains critical that perfusion imaging be interpreted in conjunction with the simultaneously acquired non-contrast CT, in accordance with the clinical trials which demonstrated efficacy using these tools.
In patients with LVO, perfusion core volumes have been shown to moderately correlate with early ischemic changes on non-contrast CT in the hyperacute (<3h)10 and acute (<6h)11 settings. However, the relationship between early non-contrast CT changes and perfusion core volumes has not been well characterized in the extended window (6 to 24 hours after LKN). We found that among all patients who underwent attempted recanalization within 24 hours of LKN, there was a moderate correlation between the non-contrast ASPECTS score and the rCBF <30% volume. However, as time progressed from LKN, patients were observed to have a lower ASPECTS score while there was no appreciable difference in rCBF, despite a similar pattern of occlusion sites. This suggests that in the extended window (and perhaps beyond) the correlation between ASPECTS and perfusion core estimates may dwindle, and it should remind us that the rCBF estimates tissue perfusion rather than viability. Additionally, this and other estimates of perfusion are highly linked to collateral vessel status, which should be explored in future, dedicated studies.
According to unpublished data from one site participating in the BEST cohort, infarct volumes estimated using CT ASPECTS were greater at later time points after LKN, while the rCBF map remained unchanged. That study was unique in that it screened over 400 consecutive patients who underwent CTP for possible LVO, and included 60 patients with anterior LVO who underwent imaging within 24-hours of LKN, regardless of whether thrombectomy was pursued. The investigators reported a trend for lower ASPECTS for every hour after LKN (proportional OR 0.92, 95%CI 0.84-1.00, p=0.06) after adjusting for age, NIHSS, and thrombolysis, but no change in perfusion core volumes (adjusted p=0.37).(unpublished data) The results of the present multi-center cohort corroborate these single-center findings.
Our results also corroborate prior data12,13 that indicate more extensive regions of irreversible tissue injury are at greater risk of hemorrhagic transformation if recanalization is achieved. Regardless of the time from LKN to recanalization—and despite meeting DAWN/DEFUSE-3 perfusion criteria—patients with an unfavorable non-contrast CT (ASPECTS <6) were at a greater odds of any intracerebral hemorrhage and symptomatic hemorrhage by 72 hours in this observational study. The proportion of patients in this cohort with an unfavorable ASPECTS (but acceptable perfusion parameters) who experienced a good functional outcome was similar to what was observed in the DAWN and DEFUSE-3 trial control groups (14% in BEST vs. 13% in DAWN vs. 17% DEFUSE-3; respectively), which—although our populations are different in many respects—suggests unclear equipoise for this treatment in the extended window for patients with ASPECTS <6. In the early window, however, evidence suggests a benefit of endovascular recanalization despite poorer ASPECTS. According to a post-hoc analysis of the MR CLEAN trial, there remained a non-significant, favorable shift in 90-day functional outcome for patients who underwent thrombectomy with a baseline ASPECTS of 5-7 (cOR 1.9, 95%CI 0.9-4.1), without any increased risk of sICH (9% vs. 7% in patients with a baseline ASPECTS 8-10, p=0.715).14 Additionally, according to data from the HERMES collaboration, the benefit of thrombectomy persisted among patients whose ASPECTS score was 6-8 (cOR 2.34, 95%CI 1.68-3.26 for a shift in 90-day mRS) but was absent if the baseline ASPECTS was 0-5 (cOR 1.24, 95%CI 0.62-2.49).15 In a subsequent meta-analysis including 2 additional randomized clinical trials, the benefit of early window thrombectomy over medical therapy persisted despite ASPECTS of 0-4 (cOR 2.15, 95%CI 1.06-4.37).16 While these patients also experienced higher rates of sICH with intervention (OR 5.00, 95%CI 1.30-19.25), there was no significant increase in mortality (OR 0.81, 95%CI 0.36-1.81). Ultimately, these data highlight the importance of the non-contrast CT as it relates to the safety of endovascular recanalization.
Limitations
While our study was advantageous in its prospective design and its reflection of routine clinical practice at a large number of institutions, it is not without limitations. Perhaps most importantly, our determination of time from LKN does not accurately capture the time from symptom onset to imaging acquisition. As part of the BEST prospective observational study, neither the time of symptom onset nor the time of CT or CTP were recorded. Therefore, we used time from LKN to recanalization (or time when angiography was aborted if thrombectomy had been unsuccessful) as a surrogate measure. This length-time bias may have misclassified patients has having presenting in the extended window when the CT and CTP were acquired in the early window. Despite this limitation, we did observe significantly poorer ASPECTS with every hour of delay from LKN to image acquisition. And while the effect of time on evolution of the non-contrast CT findings could have been attenuated by the exclusion of patients who already had extensive changes on head CT, we found no relationship between time and the standard perfusion parameters. Another limitation is our selective inclusion of patients who underwent attempted endovascular thrombectomy. This introduces a selection bias to the cohort which may have led to excess inclusion of patients with favorable non-contrast CT scans (higher ASPECTS scores) and lower perfusion core volumes. Furthermore, while our study included data from 12 US hospitals, the sample size may have been too small to detect significant differences in perfusion changes over time.
Lastly, all imaging was reviewed at each site by local investigators without central adjudication. The historically reported inter-rater reliability of individual patient ASPECTS scores in acute stroke has been poor, but it may improve when the ASPECTS is categorized into 2 or more groups.4,5,17 For this reason, we grouped patients according to the presence of any early infarct signs (ASPECTS <10) and those with unfavorable ASPECTS scores (ASPECTS <6) which should improve the inter-rater reliability in our cohort. Furthermore, the reliability between raters is thought to be poorest in the acute phase of stroke (e.g., <3 or <6 hours),5,18 but may improve when the non-contrast CT scan is acquired later in the evolution of infarction,19,20 as in the present study. Additionally, we found a clear and significant trend in a lower ASPECTS among patients who present later in the course of their infarction, which further serves to validate these independent assessments. While the use of local readers for each CT is also a limitation to our analysis, it reflects routine clinical practice and medical decision making for patients who are considered for endovascular thrombectomy. Due to the not uncommon incidence rate of artifactual findings of automated hypoperfusion changes in patients with LVO—43% from one center’s experience21—expert interpretation of perfusion findings may have increased the accuracy of these volumetric assessments. However, we did not over-read the automated measurements, as these automated measurements are the presently validated tools used in clinical trials2,3 and current practice.
It would have been ideal to have compared MRI FLAIR volumes over time from LKN, however, this was not acquired for a large proportion of patients prior to thrombectomy. Therefore, our findings should be confirmed with more definitive volumetric MRI assessments in future investigations.
Conclusions
Ultimately, our results indicate that the non-contrast CT scan captures vital, early infarct changes that may be missed by perfusion imaging in about one-third of patients. This is especially important in the extended window when perfusion imaging metrics may not change over time, yet the non-contrast CT may show clear and irreversible evolution of cerebral ischemia. The fact that the rCBF captures a low flow state and not a tissue viability state cannot be understated. Further, our results add to the growing literature that even in the presence of favorable perfusion imaging, thrombectomy in patients with significant early ischemic changes carries a low probability of a good functional outcome. This confirms observations made in patients who present in the early window,22,23 and corroborates recently published findings in one cohort treated in the extended window.24 Whether selected patients from this cohort still derive an overall benefit from thrombectomy (versus medical management alone) deserves further investigation and is being reported separately.25 Our findings emphasize the critical importance of reviewing the non-contrast CT before selecting patients for thrombectomy, even in the presence of favorable perfusion imaging profiles.
ACKNOWLEDGEMENTS AND DISCLOSURES:
The authors report no competing financial interests exist. This work was supported by a Society of Vascular and Interventional Neurology (SVIN) pilot grant, University of Cincinnati Gardner Neuroscience Institute pilot grant, National Institutes of Health U01 NS086872, National Institutes of Health U10 NS086512, and National Institutes of Health U10 NS086474.
REFERENCES
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 2.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. New Engl J Med 2018;378:708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. New Engl J Med 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 4.McTaggart RA, Jovin TG, Lansberg MG, et al. Alberta stroke program early computed tomographic scoring performance in a series of patients undergoing computed tomography and mri: Reader agreement, modality agreement, and outcome prediction. Stroke 2015;46:407–12. [DOI] [PubMed] [Google Scholar]
- 5.Mak HK, Yau KK, Khong PL, et al. Hypodensity of >1/3 middle cerebral artery territory versus alberta stroke programme early ct score (ASPECTS): Comparison of two methods of quantitative evaluation of early ct changes in hyperacute ischemic stroke in the community setting. Stroke 2003;34:1194–6. [DOI] [PubMed] [Google Scholar]
- 6.Mistry EA, Sucharew H, Mistry AM, et al. Blood pressure after endovascular therapy for ischemic stroke (BEST): A multi-center prospective cohort study. Stroke 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (redcap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wintermark M, Albers GW, Broderick JP, et al. Acute stroke imaging research roadmap ii. Stroke 2013;44:2628–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bivard A, Kleinig T, Miteff F, et al. Ischemic core thresholds change with time to reperfusion: A case control study. Ann Neurol 2017;82:995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demeestere J, Garcia-Esperon C, Garcia-Bermejo P, et al. Evaluation of hyperacute infarct volume using aspects and brain ct perfusion core volume. Neurology 2017;88:2248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haussen DC, Dehkharghani S, Rangaraju S, et al. Automated ct perfusion ischemic core volume and noncontrast ct aspects (alberta stroke program early ct score): Correlation and clinical outcome prediction in large vessel stroke. Stroke 2016;47:2318–22. [DOI] [PubMed] [Google Scholar]
- 12.Whiteley WN, Slot KB, Fernandes P, et al. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: A systematic review and meta-analysis of 55 studies. Stroke 2012;43:2904–9. [DOI] [PubMed] [Google Scholar]
- 13.Menon BK, Hill MD, Davalos A, et al. Efficacy of endovascular thrombectomy in patients with m2 segment middle cerebral artery occlusions: Meta-analysis of data from the hermes collaboration. J Neurointerv Surg 2019;11:1065–9. [DOI] [PubMed] [Google Scholar]
- 14.Yoo AJ, Berkhemer OA, Fransen PSS, et al. Effect of baseline alberta stroke program early ct score on safety and efficacy of intra-arterial treatment: A subgroup analysis of a randomised phase 3 trial (mr clean). Lancet Neurol 2016;15:685–94. [DOI] [PubMed] [Google Scholar]
- 15.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31. [DOI] [PubMed] [Google Scholar]
- 16.Roman LS, Menon BK, Blasco J, et al. Imaging features and safety and efficacy of endovascular stroke treatment: A meta-analysis of individual patient-level data. Lancet Neurol 2018;17:895–904. [DOI] [PubMed] [Google Scholar]
- 17.Farzin B, Fahed R, Guilbert F, et al. Early ct changes in patients admitted for thrombectomy: Intrarater and interrater agreement. Neurology 2016;87:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Aspects study group. Alberta stroke programme early ct score. Lancet 2000;355:1670–4. [DOI] [PubMed] [Google Scholar]
- 19.Naylor J, Churilov L, Rane N, et al. Reliability and utility of the alberta stroke program early computed tomography score in hyperacute stroke. J Stroke Cerebrovasc Dis 2017;26:2547–52. [DOI] [PubMed] [Google Scholar]
- 20.Naylor J, Churilov L, Chen Z, et al. Reliability, reproducibility and prognostic accuracy of the alberta stroke program early ct score on ct perfusion and non-contrast ct in hyperacute stroke. Cerebrovasc Dis 2017;44:195–202. [DOI] [PubMed] [Google Scholar]
- 21.Siegler JE, Olsen A, Pulst-Korenberg J, et al. Multicenter volumetric assessment of artifactual hypoperfusion patterns using automated ct perfusion imaging. J Neuroimaging 2019;29:573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA 2016;316:1279–88. [DOI] [PubMed] [Google Scholar]
- 23.Manceau PF, Soize S, Gawlitza M, et al. Is there a benefit of mechanical thrombectomy in patients with large stroke (dwi-aspects </= 5)? Eur J Neurol 2018;25:105–10. [DOI] [PubMed] [Google Scholar]
- 24.Bhuva P, Yoo AJ, Jadhav AP, et al. Noncontrast computed tomography alberta stroke program early ct score may modify intra-arterial treatment effect in dawn. Stroke 2019;50:2404–12. [DOI] [PubMed] [Google Scholar]
- 25.Siegler JE, Messe SR, Sucharew H, et al. Thrombectomy in dawn- and defuse-3-ineligible patients: A subgroup analysis from the best prospective cohort study. Neurosurgery 2019; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]


