Abstract
Hydroids, one of the dominant components of the zoobenthic communities, share comparable growth patterns with higher plants because of their modular body organization, high potential of asexual reproduction, and phenotypic plasticity. These features, together with the ability to enter dormancy to overcome unfavorable conditions, make hydroids successful organisms adaptable to a wide range of environmental scenarios. Depending on their wide range of shapes and sizes, hydroids form three-dimensional forests at different dimensional scales, establishing both trophic and non-trophic relationships with several other organisms, from virus to vertebrates.
Despite numerous researches conducted to study the hydroid ecology, the putative importance of hydroids in structuring zoobenthic communities is underestimated. Here, information available about hydroid ecology is summarized, in order to emphasize the role of hydroids as forest formers, as well as their function in the bentho-pelagic coupling.
Keywords: Ecological role, Emerging threats, Hydroid forests, Protection, Seasonal and perennial habitat formers
Introduction
Clonal animals, according to Jackson and Coates (1986), have either uniserial or multiserial growth patterns. Uniserial colonies are also called “runners” and do not form large assemblages, whereas multiserial colonies perform lateral and distal growth and tend to persist, with the possibility of forming large assemblages. Multiserial colonies, hence, are able to form “forests” and behave as trees, whereas uniserial colonies are more similar to weeds. The two strategies are also used to distinguish guerrilla from phalanx species (Humphrey and Pyke 1998). Philopatric colonies (Knowlton and Jackson 1993) can produce a high number of genetically identical colonies (each termed a ramet) that, together, form a genet: an assemblage of genetically identical ramets. This tendency is conducive to the formation of animal forests.
The colonies of the Hydrozoa show highly diverse growth forms that cover both categories (Bouillon et al. 2006). Small polyp colonies (usually less than 1 cm high) are reptant and tend either to grow on other organisms or to form “meadows,” growing directly on primary substrates where they can play a certain ecological role in becoming habitats for other species. Small hydroids are mostly overlooked, being considered as mere epizoites. Consequently, very little information is available on the ecological traits of small hydroids and the role they play in both benthic and pelagic communities. Clytia hummelincki (Leloup, 1935) represents a case of meadow-forming hydroid (Gravili et al. 2008).
Large colonies (ranging from 10 cm to 1 m) grow on primary substrates and become substrate for other organisms, forming forests comparable in sizes and abundances to those of either algae or gorgonians, and likely play roles of habitat formers as those documented for anthozoan forests (Cerrano et al. 2010; Ponti et al. 2016; Valisano et al. 2016).
Hydroids are one of the main components of zoobenthic communities. At their relative dimensional scale, hydroids change the features of the geological habitat, becoming habitat formers by affecting water movement and light penetration and providing settling space, shelter, or food to several associated species, so enhancing local biodiversity. Moreover, hydroid forests release a consistent amount of planulae, medusoids, or medusae in the surrounding environment, contributing to bentho-pelagic coupling and affecting biogeochemical cycles (Gili et al. 1998; Rossi et al. 2012).
The modular organization of hydroids gives them a high plasticity and a potentially unlimited growth (Marfenin 1997; Kosevich 2006); hence they can adapt their shape, growth strategies, trophic behavior, and reproductive strategies to a vast array of environmental conditions (Boero 1984; Gili and Hughes 1995; Bouillon et al. 2006).
Even if hydroid ecology is rather well known (Fig. 1), their putative importance in structuring zoobenthic communities is underestimated by benthic ecologists. In spite of several documented cases of hydroids as formers of benthic assemblages comparable to algal forests, in fact, the formal definition of habitats rarely gave them much importance. For the Mediterranean Sea, for example, in the list of habitats assembled by RAC-SPA (2006), there is just a single item comprising all hydroid forests (i.e., facies with large hydrozoa), whereas each type of algal forests is carefully identified with the name of the main species; the same treatment is given to the Bryozoa. Fraschetti et al. (2008) proposed a rationale of benthic habitat classification that allows accommodating hydroid forests into the seasonal or even permanent habitat formers.
Fig. 1.
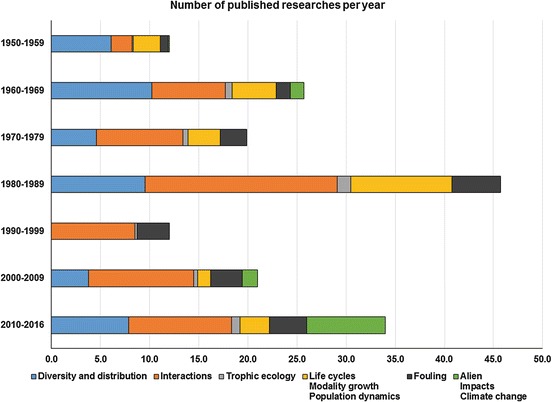
Trend of published papers per year from 1950 to 2016. Over 1,500 papers focusing on hydroid ecologywere considered (Data extracted from BiblioHydro, Gravili et al. 2000)
One of the reasons why hydroids are neglected is that hydroid specialists contributed less than marine botanists to the formal definition of habitats, and this led to underappreciation of the role of these animals, in respect to algae. Furthermore, at least in the Mediterranean Sea, field activities are mostly carried out in the favorable season when algae dominate benthic assemblages and most hydroids are dormant, leading to underappreciation of their importance in structuring benthic communities.
Analyzing papers focusing on hydroid ecologycould help in understanding why hydroid ecological role is overlooked. From 1950 to today (Fig. 1), most of the papers were published during the decade 1980–1989 (over 45 per year); the most explored topic is the study of interactions between hydroids and other organisms (up to about 20 papers per year).
During the last years (2010–2016), there was an increasing interest in studying hydroids belonging to fouling communities, alien species, and effects of climate change on hydroid assemblages.
Among the researches published in the last two decades, very few papers put in relation hydroid distribution and population dynamics with abiotic and/or biotic factors (Azzini et al. 2003; Ronowicz et al. 2008; Orejas et al. 2013; Di Camillo et al. 2012a; Rossi et al. 2012).
Similarly to what highlighted by Becerro (2008) regarding sponges, most of researches on hydroid ecology generally are descriptive and focus on one or few species, interesting a narrow readership. Lack of published quantitative data expressed in terms of biomass prevents estimation of the potential of hydrozoan forests in terms of trophic impact, food source, and reproductive output and does not allow the comparison with other animal forests or within the animal forests.
Deciduous Hydroid Forests
Deciduous hydroids , typical of shallow waters at cold and temperate latitudes, range from less than 1 mm (many Campanulariids, Campanuliniids) to about 20 cm in height (i.e., several Eudendriids, Pennariids, Tubulariids, Aglaopheniids, etc.). In these seasonal species, hydranth resorption or shedding occurs in response to periodic adverse environmental conditions, followed by dormancy of remaining fragments of tissue (coenosarc) enclosed in stems or hydrorhizae acting as resting stages (Bouillon et al. 2006). When environmental conditions become favorable again, the regeneration of the colonies takes place from the dormant tissue.
Seasonality drives the succession of species with different ecological characteristics; consequently, winter and summer species may occupy the same space, but in distinct temporal windows (Boero and Fresi 1986; Coma et al. 2000; Bavestrello et al. 2006; Puce et al. 2009). The duration of dormancy/active phases can change along bathymetric or geographical ranges characteristic of each species. Deciduous forests undergo strong seasonal variations in their biomass in relation to fluctuation of an intricate mixture of abiotic/biotic factors (i.e., temperature, irradiance, salinity, sedimentation, oxygen concentration, food availability, space competition, predation). Further studies are needed to understand physiological processes triggering quiescence or renovation and to test the duration of dormancy in relation to different ranges of environmental constraints since research focused on a limited number of species (Boero 1994 for a review).
Many studies on hydroid seasonality were conducted in the Mediterranean Sea. Boero and Fresi (1986) were the first to generalize a seasonal pattern of community structure in the Mediterranean benthos while giving paramount importance to hydroids, highlighting the importance of seasonal fluctuations in the composition of the sessile benthos, with a dominance of the algal component in the spring and summer and a prevalence of hydroids in the fall and winter. This was studied in detail for the dominant winter species Eudendrium glomeratum Picard, 1951 (Boero et al. 1986) and later better formalized in general studies (e.g., Boero 1994; Coma et al. 2000). Bavestrello et al. (2006) showed that, in the Mediterranean Sea, 50% of the species thrive in the winter, 30% in the summer, and only 20% tend to be always present.
Because of their plasticity and fast growth, deciduous hydroid forests have a prominent role in shaping zoobenthos dynamics. A good example is supplied by the comparison of the different life strategies of (Cavolini, 1785) from different localities. At the Medes Islands (MI), E. racemosum is also constantly present (Rossi et al. 2012), whereas it is sharply seasonal in the Adriatic Sea (AS), where temperatures are significantly lower in the winter (Di Camillo et al. 2012a) (Fig. 2). The longer duration of the fertility period and the highest polyp production in the population from the AS are likely related to the conspicuous food availability of this area. The considerable food amounts ingested by the polyps from the AS (Di Camillo et al. 2012a) suggest that (i) the hydroid plays a role in energy cycling and in removing particles from the water column and that (ii) local trophic levels are a key factor regulating variations in biomass of this suspension feeder. Indeed, in the MI, the summer regression of the population of E. racemosum, as well as the low polyp production, is probably related to the summer food paucity (Coma et al. 2000; Rossi et al. 2012). Moreover, in the MI, the hydroid growth could be limited in summer due to competition with algae, as occurring at other localities of the Western Mediterranean (Boero 1984; Boero and Fresi 1986; Boero et al. 1986; Rossi et al. 2012).
Fig. 2.
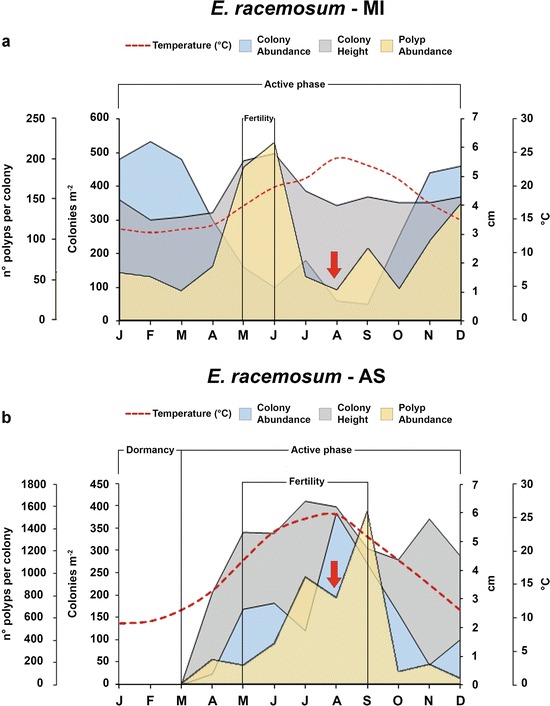
Comparison of different population dynamics of (Cavolini, 1785) from Medes Islands (MI) (a) and Adriatic Sea (AS) (b) Red arrows indicate when the nudibranch predation is most intense
Under different local conditions, the life cycle patterns of Eudendrium racemosum can further differ from those described above: for example, in the Ligurian Sea, E. racemosum living on artificial substrates and in eutrophic conditions was observed all year round, while the population living on a natural rocky cliff was present in summer only (Azzini et al. 2003).
These considerations highlight that studies on fast-growing suspension feeders are indispensable to understand how population dynamics mirror environmental pressures and that the natural variability of these phenomena is great.
In tropical areas, seasonality is due to rainfalls, and biomass fluctuations are probably related to variations in food abundance during the wet and dry season (Boero 1994). In Indonesian waters, the maximal density of hydroids coincides with the period of heavy rainfall and river inputs (Di Camillo et al. 2008). However, most of the projects carried out in tropical waters focused on the census of marine organisms, while long-term monitoring programs are scant. Similar knowledge gaps are present at the poles, where a dark and ice-dominated season alternates with a long period of total darkness (Orejas et al. 2013).
Seasonal forests can regress as a result, for instance, of the impact of global warming on species of cold-water affinity, such as Eudendrium glomeratum in the Ligurian Sea (Boero et al. 1986). The regression of seasonal species is usually given less importance than that of perennial species, such as those of gorgonians in the Ligurian Sea (Cerrano et al. 2000). Seasonal species are preadapted to undergo long periods of adverse conditions with dormant phases but, nevertheless, if negative situations become permanent (e.g., with constantly higher temperatures than previous ones), species might become locally extinct.
Perennial Hydroid Forests
Perennial hydroids are always present in their erect forms (e.g., the calcareous Milleporidae and Stylasteridae) and are more common than seasonal species where variations in environmental conditions are small. Habitat stability allows enduring species to develop large and sturdy colonies and to reach considerable sizes ranging from 20 cm up to 2 m: for example, Plumularia elongata Billard, 1913, Solanderia spp., or Millepora spp. at tropics; Lytocarpia myriophyllum (Linnaeus, 1758) on soft bottoms; Errina spp., and Amphisbetia operculata (Linnaeus, 1758) on hard substrates from temperate regions (Table 1, Figs. 3 and 4). Perennial hydroids can give rise to animal forests comparable to those formed by gorgonians. Those with calcified skeletons are probably slow growing, but their growth rates are poorly estimated (Lewis 2006). During their theoretically unlimited life span, perennial hydroids host several taxa of sessile and vagile organisms, represent a food source for several animals, and, in their turn, likely collect great amounts of feeding particles from the water column. These forests undoubtedly have a significant – but little explored – role in energy transfer from plankton to benthos (Gili et al. 1998; Gili and Coma 1998). Enduring hydroid forests can be indicators of habitat health conditions: for example, the presence of intact Errina gardens on hard substrates (Häussermann and Försterra 2007; Salvati et al. 2010), Plumularia elongata on tropical reefs (Di Camillo et al. 2010), and Lytocarpia miriophyllum on sandbanks (Di Camillo et al. 2013; Cerrano et al. 2015) is linked to the existence of pristine or scarcely disturbed habitats.
Table 1.
Summary of the ecological characteristics of some large forest former hydrozoans (A, Anthomedusae; L Leptomedusae)
| Order | Species | Geographic distribution | Vertical zonation | Substrate | Shape and skeleton | Reproduction | Associated organisms | Threats |
|---|---|---|---|---|---|---|---|---|
| A | Millepora spp. | Circumtropical | From reef crest | Hard | Larger colonies branched; calcified skeleton | Eumedusoids | Zooxanthellae, Wanella elongatum (Hiro, 1931) (barnacle), Hermodice carunculata (Pallas, 1766) (polychaete, predator), several species of crustaceans, echinoderms, mollusks, nemerteans, polychaetes, and sipunculids | Global warming, bomb fishing, pollution |
| A | Solanderia spp. | Tropical and subtropical | Fore reef | Hard | Fan-shaped to bushy; perisarc stiff | Cryptomedusoids or eumedusoids | Medioantenna spp., serpulids (polychaetes), Licnophora spp. (protozoans), Jason mirabilis M.C. Miller, 1974 and Pleurolidia juliae Burn, 1966 (nudibranches, predators), bivalves, gastropods, tunicates, ophiuroids, copepods, barnacles, amphipods, bryozoans | |
| A | Pseudosolanderia spp. | Circumtropical | Fore reef | Hard | Fan-shaped to bushy; chitinous or from partly to quite totally calcified | Eumedusoids | Lagisca zibrowii Hartmann-Schröder, 1992 (polynoid polychaete), Pachyprocerastea hydrozoicola (Hartmann-Schröder, 1992) and Procerastea simpliseta Hartmann-Schröder, 1990 (syllid polychaetes) | |
| A | Stylasterids | Temperate | Deep waters | Hard | Branched colonies; calcified skeleton | Fixed gonophores in ampullae | Algae, other hydrozoans, scleractinians, Epizoanthus sp. (zoanthid), sponges, bryozoans, commensal polychaetes, Pachylasma giganteum (Philippi, 1836) (barnacle), Pedicularia spp. (gastropods) | Destructive fishing techniques |
| L | Amphisbetia operculata (Linnaeus, 1758) | Temperate and subtropical | From shallow waters in cold regions | Hard substrates on soft bottoms | Bushy with pinnate cormoids; perisarc flexible | Cryptomedusoids | Diatoms, macroalgae, ciliates, other hydrozoans, entoprocta, Doto eireana Lemche, 1976 (nudibranch, predator), other mollusks, bryozoans, tunicates | |
| L | Sertularella diaphana (Allman, 1885) | Tropical and subtropical | Fore reef | Hard | Fan-shaped to bushy; perisarc stiff | Cryptomedusoids | Bivalves, amphipods | |
| L | Sertularia argentea Linnaeus, 1758 | Temperate | From shallow waters in cold regions | Hard | Spiralate colonies; pinnate branches; flexible perisarc | Heteromedusoids | Ornamental harvesting | |
| L | Hydrallmania falcata (Linnaeus, 1758) | Temperate | From shallow to mesophotic zone | Hard substrates on soft bottoms | Pinnate cormoids spirally arranged; perisarc flexible | Fixed gonophores | Other hydroids | Ornamental harvesting |
| L | Aglaophenia cupressina Lamouroux, 1816 | Indo-Pacific | Between reef crest and fore reef | Hard | Bushy with pinnate cormoids; perisarc very flexible | Fixed gonophores protected by corbulae | Zooxanthellae, Cuthona diversicolor Baba, 1975 (nudibranch, predator), cyclopoid copepods, Hyastenus bispinosus Buitendijk, 1939 (crab), fish | Global warming |
| L | Lytocarpia myriophyllum (Linnaeus, 1758). | From boreal to subtropical regions | Mesophotic and deep waters | Soft | Bushy with pinnate cormoids; perisarc very flexible | Fixed gonophores |
Forams, other hydroids, Amphianthus dohrnii (Koch, 1878) (actinian), Dondice banyulensis Portmann & Sandmeier, 1960 (nudibranch, predator, egg deposition), bivalves, stalked barnacles, solenogaster, gastropods, caprellids and gammarids, bryozoans |
Destructive fishing techniques |
| L | Macrorhynchia spp. | Tropical and subtropical | Fore reef | Hard | Fan-shaped, pinnate cormoids; moderately flexible | Fixed gonophores or medusoids, protected by phylactocarps | Other hydroids (Hebellidae), decapod palaemonids | |
| L | Streptocaulus dollfusi (Billard, 1924) | Eastern Atlantic, Strait of Gibraltar | Mesophotic and deep waters | Hard | Erect and polysiphonic colonies, pinnate cormoids | Fixed gonophores protected by phylactocarps | ||
| L | Plumularia elongata (Billard, 1913) | Indo-Pacific | Fore reef | Hard | Fan-shaped to bushy; with pinnate cormoids; strongly polysiphonic and stiff | Fixed or swimming gonophores | Hydrozoanthus gracilis (Lwowsky, 1913) and Hydrozoanthus sp.1 (zoanthids), pycnogonids | |
| L | Pseudoplumaria spp. | Temperate and subtropical | From shallow to deep waters | Soft | With pinnate cormoids | Fixed gonophores | ||
| L | Nemertesia spp. | Cosmopolitan | Mesophotic and deep waters | Hard substrates on soft bottoms | Bushy due to very close unbranched cormoids | Fixed gonophores | Sponges, other hydroids, Alcyonium digitatum Linnaeus, 1758 (anthozoan), nemerteans, bivalves, gastropods (prosobranchs and opistobranchs), Ophiothrix fragilis (Abildgaard, in O.F. Müller, 1789) (ophiuroid), Antedon bifida (Pennant, 1777) (comatulid), polychaetes, gammarid and caprellid amphipods, copepods, cirripeds, cumaceans, isopods, decapods, pycnogonids, entoprocts, bryozoans, tunicates | Destructive fishing techniques |
| L | Polyplumaria flabellata G.O. Sars, 1874 | Temperate and subtropical | Deep waters | Hard | Fan-shaped; with pinnate cormoids; stiff perisarc | Fixed gonophores | ||
| L | Halecium spp. | Boreal and temperate | Mesophotic and deep waters | Hard | Fan-shaped to bushy, pinnate cormoids | Fixed gonophores | ||
| L | Hartlaubella gelatinosa (Pallas, 1766) | Temperate | Deep waters | Mixed substrates | Bushy | Fixed gonophores | Amphipods and caprellids |
Fig. 3.

Some large warm-affinity hydrozoans. (a, b) The subtropical Solanderia ericopsis (Carter, 1873) (a) from New Zealand predated by the nudibranch Jason mirabilis M. C. Miller, 1974 (photo curtesy of Ian Skip, http://www.ianskipworth.com and Solanderia secunda (b) from the North Sulawesi. The insets show the polynoid Medioantenna variopinta and the nudibranch Pleurolidia juliae. (c–d) Aglaophenia cupressina from the North Sulawesi; the inset shows a colony explored by razor fish. (e) Plumularia elongata from Bali: a large colony completely covered with the zoanthid Hydrozoanthus sp. 1 (inset). (f–g) Macrorhynchia spectabilis (f) and Sertularella diaphana (g) from the North Sulawesi. The insets in Fig. (g) show a pteriod bivalve and numerous amphipods respectively on the main axis and on hydrorhiza of S. diaphana
Fig. 4.
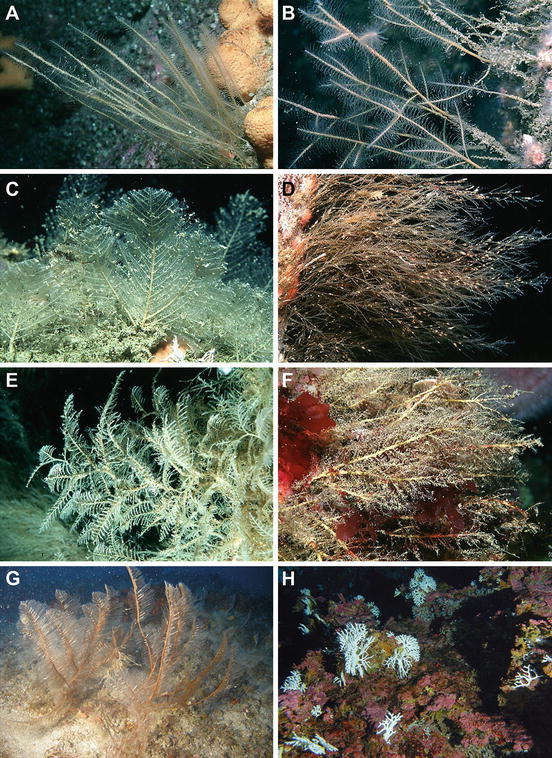
Some large cold-affinity hydrozoans. (a–f). Hydrozoans from British Isles, photo curtesy of Dr. Bernard Pictet http://www.habitas.org.uk/marinelife/. (a) Nemertesia antennina. (b) Nemertesia ramosa. (c) Polyplumaria flabellata. (d) Amphibestia operculata. (e) Hydrallmania falcata. (f) Halecium muricatum. (g) Lytocarpia myriophyllum from the Western Mediterranean. (h) The deep stylasterid Errina aspera from the Strait of Messina
Loss of their habitats would lead to decline or disappearance of the enduring hydroid forests and their ecosystem services, as observed for L. myriophyllum in Northern Ireland because of intense bottom trawling (Goodwin et al. 2011). In Ireland and Great Britain, the hydroid is considered a priority species (Goodwin et al. 2011), while, in the Mediterranean, surveys of L. myriophyllum should be performed to collect much information about its distribution and abundance and to develop a proper conservation strategy of this species (Di Camillo et al. 2013).
Indeed, the extension of either perennial or seasonal hydroid forests has never been mapped as carefully as other benthic features, such as coralligenous formations or seagrass meadows.
Phenology and distribution of enduring species should be known to evaluate their importance for ecosystem functioning and to plan conservation programs.
Interactions with Other Organisms
Hydroids can establish different kinds of symbiotic relationships with several organisms from viruses to vertebrates, and, due to their wide size range, they can be both hosts and epibionts.
Hydroids increase habitat complexity and enhance biodiversity as demonstrated through the study of temporal variations in composition and biomass of the organisms associated to Tubularia indivisa Linnaeus, 1758 from the North Sea (Zintzen et al. 2008). The biomass of most of the symbionts was positively correlated to that of the host, and the maximal epibiont density reached very high values (about 450,000 ind. m−2).
Even if several papers describe the entire assemblage of fauna and flora associated to hydroids (e.g., Hughes 1975; Lagardère and Tardy 1980; Bavestrello et al. 1996; Genzano 2001; Zintzen et al. 2008; Meretta and Genzano 2015), the majority of the available literature studies associations involving few taxa, such as endosymbiotic algae (29% of published papers, Fig. 5) and mollusks (19%). There are over 600 papers focusing on interactions between hydroids and other taxa, highlighting that hydroid forests represent a mosaic of microhabitats, exploitable by numerous and diversified organisms. The perisarc composition and the production of secondary metabolites probably affect the attractiveness of hydroid basibionts; moreover, the organic matter trapped by hydroid colonies may support detritus-feeder communities (Bavestrello et al. 2008), whereas other organisms such as amphipods can use the inorganic fraction imprisoned under the hydrorhiza to build their burrows (Genzano 2001). Even the smallest hydroids can host a dense microbiome, composed both of prokaryotic and eukaryotic symbionts (Fig. 6). Stabili et al. (2006) found prokaryotic communities on the perisarcal exoskeleton of Aglaophenia octodonta Heller, 1868, while Östman (2000) and Di Camillo et al. (2012b) reported bacteria on the bare epidermis of Pennaria disticha Goldfuss, 1820, and Ectopleura crocea (L. Agassiz, 1862). Schuett and Doepke (2009) recorded microbes inside the tissues of Tubularia indivisa Linnaeus, 1758. Among eukaryotes, the microbiome includes diatoms, sessile ciliata such as Vorticella and suctorians (Tazioli and Di Camillo 2013), foraminifera, and macroalgae (Bavestrello et al. 2008).
Fig. 5.
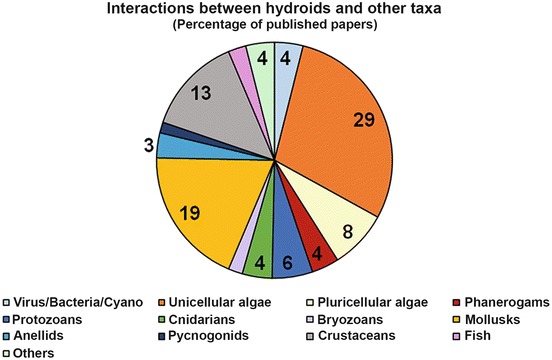
Percentages of published papers concerning interactions between hydroids and other organisms
Fig. 6.
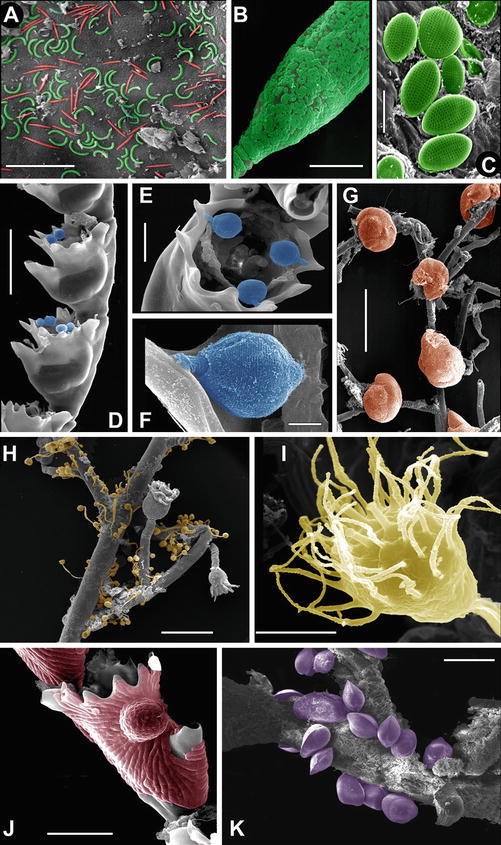
Microforests living on hydrozoans at scanning electron microscope; recolored pictures. (a) Two microbial morphotypes, one horseshoe shaped (green) and another one fusiform (red), living on the bared epidermis of Ectopleura crocea. (b–c) Portion of the hydrotheca of Clytia linearis (b) almost completely covered by the diatom Cocconeis pseudonotata (c). (d–f) Sessile ciliated of the genus Vorticella settled on the thecal margin of Aglaophenia kirkenpaueri. (g) Foraminifers living on cauli of Eudendrium armatum. (h–i) A dense assemblage of suctorians settled on a colony of Eudendrium racemosum. (j) Hydrotheca of Aglaophenia tubiformis covered by the coralline alga Hydrolithon cf. farinosum. (k) Juvenile of Mytilus galloprovincialis on Eudendrium racemosum. Scale bars: (a, c, f) 10 μm; (b, d) 200 μm; (e) 50 μm; (g–h) 1 mm; (i) 20 μm; (j) 100 μm; (k) 500 μm
Millepora spp. and Aglaophenia cupressina Lamouroux, 1816, are the only large forest-forming hydroids with conspicuous presences in scleractinian-dominated shallow reefs. The fact that both species host zooxanthellae (Symbiodinium spp.) suggests that the success of these hydroids on the upper part of the reef could be linked to their ability to obtain part of their nutrition from autotrophic sources (Lewis 2006). On the other hand, many zooxanthellate hydroids (e.g., Hydra spp., Myrionema amboinense Pictet, 1893, Eudendrium moulouyensis Marques, Peña Cantero, Vervoort, 2000, Halecium nanum Alder, 1859) do have zooxanthellae but do not form forests.
Large hydroids are basibionts for a conspicuous number of taxa due to (i) the large surface offered by their colonies; (ii) the high variety of microhabitats represented by the hydrorhiza, the proximal polysiphonic portions, and the thinner distal branches; (iii) the elevation from the substrate, allowing the settlement of acrophilous species; and (iv) in some case the stability over time of at least the elder and larger basal portions, where organisms are particularly abundant (Garcia et al. 2009; Hughes 1975, 1977).
Some large hydroids such as Nemertesia antennina and Lytocarpia myriophyllum form a complex rootlike apparatus, which may be composed by several generations of hydrorhizae (Hughes 1977; Di Camillo et al. 2013). In proximity of the substrate, the anchoring system accumulates sediments due to the reduced current speed (Hughes 1978). This three-dimensional holdfast represents a perennial habitat for several organisms, such as those of meiofauna (Cerrano et al. 2015), sponges, pycnogonids, bryozoans (Hughes 1978), or amphipods. The biomass of associated organisms may reduce in distal portions due to the possible presence of defensive/cleaning zooids (Gili and Hughes 1995; Hughes 1978) or because of a higher turnover of the apical branches.
Hydroid’s colonies can be the primary substrate for the recruitment of other invertebrates, such as mussel’s larvae (Standing 1976; Di Camillo et al. 2012a; Genzano et al. 2003). Big colonies of Amphisbetia operculata (Linnaeus, 1758) and Plumularia setacea (Linnaeus, 1758) represent the unique filamentous structure in sublittoral outcrops from temperate southwestern Atlantic (Buenos Aires coast), and blue mussel spats use these colonies as primary substrata (Genzano et al. 2003); colonies of Symplectoscyphus subdichotomus (Kirchenpauer, 1884) are the main primary settlement substrate of scallop spats in fishing grounds in Argentine sea (Bremec et al. 2008).
Some examples of relationships between large hydroids and associated organisms are summarized in Table 1. Millepora spp. form functional biogenic reefs positively influencing fish abundance and species richness, especially in scleractinian-poor Southwestern Atlantic (Coni et al. 2013; Lewis 2006; Pereira et al. 2012; Rogers et al. 2014). Besides fish, Millepora forests were found in association with a rich epi- and endofauna represented by crustaceans, echinoderms, mollusks, nemerteans, polychaetes, tunicates, and sipunculids (Cook et al. 1991; Garcia et al. 2009, 2010; Pérez and Gomes 2012).
In temperate waters from southwestern Atlantic Ocean, intertidal colonies of the Anthomedusan Ectopleura crocea (Agassiz, 1862) can form dense clumps that support many epizoical microcrustaceans. Colonies also trap sediments under their hydrorhizae where other sediment-associated fauna lives (Genzano 2001). In sublittoral outcrops, two large Leptomedusan hydroid, P. setacea and A. operculata, represent the most abundant available substrata for numerous vagile and sessile organisms (Meretta and Genzano 2015).
Stylasterid corals enhance habitat complexity offering refuge, food, and a hard substrate to several vagile and sessile invertebrates (Braga-Henriques et al. 2011; Salvati et al. 2010; Pica et al. 2015).
Trophic Ecology, Feeding Behavior, and Reproductive Strategies
Hydroid habitat formers do not give rise to large colonies and forests everywhere, suggesting that formation of forests occurs only where food availability can support their development.
The experimental studies performed to define the trophic role of hydroids in marine communities (Table 2) show that hydroids can exploit large quantities of seston, contributing to control the secondary production in coastal waters (Gili and Coma 1998; Gili et al. 1998). Zooplanktonic preys, eggs, merobenthic larvae, phytoplankton, bacteria, and detritus represent the preferential food items of hydroids. The prey composition and predation rates vary in relation to hydroid species, sizes of preys and hydranths (feeding polyps), characteristics of the study areas, temporal fluctuations of environmental parameters (such as temperature, salinity, current intensity), and biotic factors (i.e., predation and competition). Captures are related to the food availability, which can vary daily or seasonally (Table 2). Predation rate is inversely correlated to digestion time (Coma et al. 1994) which, in its turn, is related to hydroid characteristics but also to environmental conditions (i.e., temperature and salinity, Kinne and Paffenhöfer 1965; Gili and Hughes 1995). Besides the size, other features of the ingested preys can condition digestion times, such as the presence of stiff exoskeletons (Orejas et al. 2013). Times required for digestion of some hydrozoans are compared in Table 3.
Table 2.
Predation rates of some hydroids from different localities
| Species | Locality | Dominant preys | Predation rate | Carbon intake (mg C m−2 day−1) | References |
|---|---|---|---|---|---|
| Campanularia everta Clark, 1876 | Western Mediterranean | POM (88), copepod eggs (7%) | From 4,000 (summer) to 800,000 (winter) preys m−2 day−1 | 6.4 | Coma et al. 1995; Gili et al. 1998 |
| Ectopleura crocea (Agassiz, 1862) | Mar del Plata | Diatoms (100% in winter), crustaceans (summer) | 115·(summer), 93 (autumn), 77 (winter), 200 (spring) preys ind.−1 day−1 | – | Genzano 2005 |
| Ectopleura larynx (Ellis & Solander, 1786) | Cumbrae (SW Scotland) | Crustacean eggs, nauplii, copepodites | From 36 (day) to 360 (night) preys ind.−1 day−1 | 89.9 | Gili et al. 1996, 1998 |
| Eudendrium racemosum (Cavolini, 1785) | Medes Islands (Mediterranean) | – | 372,000 preys m−2 day−1 in June | 30.7 | Rossi et al. 2012 |
| Eudendrium racemosum (Cavolini, 1785) | North Adriatic Sea (Mediterranean) | Bivalve larvae (43%), tintinnids (32%), invertebrate eggs (7%), invertebrate larvae (6%), amphipods (4%) | Over 6,000 preys m−2 day−1 in summer (without considering POM) | 13 | Di Camillo et al. 2012 |
| Eudendrium racemosum (Cavolini, 1785) | Western Mediterranean | Copepod eggs (28%), Copepods (22%), invertebrate larvae (10%) | 120,000 preys m−2 day−1 | 12 | Gili et al. 1998 |
| Eudendrium racemosum (Cavolini, 1785) | Western Mediterranean | Above all zooplanktonic preys (crustacean fragments, copepods, larvae) | 100,000 preys m−2 day−1 | – | Barangé and Gili 1998 |
| Lytocarpia myriophyllum (Linnaeus, 1758) | Ligurian Sea | – | – | 13,000a | Cerrano et al. 2015 |
| Millepora complanata Lamarck, 1816 | Barbados | Copepods (63%) | 480,000 preys m−2 of the corallum’s surface day−1 | 360,000 mg C m−2 of the corallum’s surface day−1 | Lewis 1992 |
| Nemalecium lighti (Hargitt, 1924) | San Blas Islands (Panamá) | Diatoms (28%), POM (23%); invertebrate larvae (19%); bivalve larvae (11%) | 400,000 preys m−2 day−1 | 6 | Gili et al. 1998; Coma et al. 1999 |
| Obelia dichotoma (Linnaeus, 1758) | Kongsfjorden (Spitsbergen, Arctic) | Fecal pellets (up to 57% of the total ingested food), phytoplankton (up to 50%), and organic matter (up to 35%) | 261,182 preys m−2 day−1 | 5.5–8.9 | Orejas et al. 2013 |
| Obelia geniculata (Linnaeus, 1758) | Chile | Fecal pellets (48%), Copepod eggs (29%), diatoms (17%) | 3,200,000 preys m−2 day−1 | 48 | Gili et al. 1998 |
| Silicularia rosea Meyen, 1834 | King George Island (Antarctica) | Benthic diatoms (95%), eggs (2%) | 4,000,000 preys m−2 day−1 | 66 | Gili et al. 1996, 1998 |
aEstimated applying the lowest capture rate calculated for cnidarians (0.01 mg C ind.−1 day−1, from Gili and Coma 1998)
Table 3.
Digestion time of some hydroidsestimated in experimental conditions
| Species | Sampling site | Rearing conditions | Preys | Digestion time (h) | References |
|---|---|---|---|---|---|
| Ectopleura larynx (Ellis & Solander, 1786) |
Cumbrae (SW Scotland) |
15 °C | Small | 2–3 | Gili et al. 1996 |
| Large | 5 | ||||
| Ectopleura crocea (Agassiz, 1862) | Mar del Plata | 4–5 | |||
| Obelia dichotoma (Linnaeus, 1758) | Arctic waters | 6 °C | A single diatom | 20 | Orejas et al. 2013 |
| Hydractinia echinata (Fleming, 1828) |
Knähaken Reef (Øresund, NE Atlantic) |
4 °C | 40 | Chistensen 1967 | |
| 16 °C | 5 | ||||
| Silicularia rosea Meyen, 1834 | King George Island (Antarctica) | 0–2 °C | Diatoms | 12 | Gili et al. 1996 |
| Campanularia everta (Clark, 1876) | Medes Islands (NW Mediterranean) | 18 °C | 2 | Coma et al. 1995 | |
| Clava multicornis (Forsskål, 1775) | Helgoland (North Sea) |
12 °C (32‰ S) |
Artemia larvae | 8 | Kinne and Paffenhöfer 1965 |
|
17 °C (32‰ S) |
Artemia larvae | 6 | |||
|
22 °C (32‰ S) |
Artemia larvae | 4 | |||
| Garveia franciscana (Torrey, 1902) | Russia | 23–26 °C | 3–4 | Simkina 1980 | |
| Eudendrium racemosum (Cavolini, 1785) | Medes Islands (NW Mediterranean) | 18 °C | 5 | Barangé and Gili 1988 |
Different preys have diverse carbon contents; however, the total carbon intake (mg C m−2 day−1) of the considered hydroid species is comparable to that of other suspension feeders (Gili and Coma 1998, Table 1), suggesting that hydroids play a key role in energy transfer from the pelagic to the benthic realms (Coma et al. 1995, 1998). In particular, hydroids forming large, multibranched colonies could have a great impact on seston since they have a number of feeding polyps much higher than small hydroid species. Large colonies of Lytocarpia myriophyllum from the Ligurian Sea have about 1,300,000 feeding polyps m−2 (Cerrano et al. 2015), that, applying the lowest feeding rate reported for a cnidarian (0.01 mg C polyp−1 day−1, Gili and Coma 1998), could remove up to 13,000 mg C m−2 day−1.
Standing (1976) and Sutherland and Karlson (1977) showed that hydroids resist overgrowth when present in dense aggregations (forests) being able to prevent the settlement of competitors by feeding on their approaching larvae. This behavior contributed to the proposal of the inhibition model as one of the modes of community development (Connell and Slatyer 1977) and can explain the processes that lead to the establishment of hydroid forests: once established, the forests inhibit the settlement of other species by eating the larvae of potential competitors.
The trophic role of hydroids with medusae is double since they predate both in the benthos and in the plankton. To our knowledge, the complete trophic role of a species with both polyps and medusae has never been studied in detail, accounting for both domains.
Concerning trophic behavior, Miglietta et al. (2000) reviewed a vast array of strategies in the various hydroid species, ranging from passive filter feeding, with the outstretched tentacles used as a filter, to active feeding achieved by movement of the whole polyp in a “searching” pattern, to rhythmic tentacle contraction causing microcurrents that convey small prey toward the mouth. As it might have been expected, specific studies (Gili et al. 1996; Miglietta et al. 2000) showed that small polyps mostly eat small food items, usually protists, whereas large polyps catch larger prey, ranging from crustaceans to other planktonic and benthic prey. Miglietta et al. (2000) distinguish active filter feeders that produce microcurrents that draw food particles toward the mouth (e.g., Aglaophenia) and passive filter feeders that extend their tentacles and wait for prey (e.g., Tubularia). The subantarctic hydroid Silicularia rosea Meyen, 1834, from the intertidal communities of King George Island ingests above all benthic diatoms resuspended by tidal current. The hydroid rhythmically expands and contracts its tentacles in order to increase the flow of particles toward the mouth (Gili et al. 1996). Some species with small hydranths such as Lytocarpia myriophyllum and Aglaophenia cupressina produce conspicuous amount of mucus probably acting as a trap of organic matter (Puce et al. 2002).
Hydroids hosting zooxanthellae (i.e., Myrionema spp., Eudendrium molouyensis, Millepora spp., Aglaophenia cupressina) probably adopt a mixotrophic strategy and exploit products of the photosynthesis in oligotrophic waters.
Trophic strategies are finalized to optimize the hydroid reproductive effort: since the reproductive period overlaps, at least partially, with the higher food intake and the maximal colony size, it is hypothesizable that hydroids store the energy necessary to produce gametes or medusae (Rossi et al. 2012 and references therein). Thanks to their plasticity, hydroids can adapt the shape of their colonies to increase the feeding surface and to give rise to an efficient three-dimensional predation system (Gili and Hughes 1995; Rossi et al. 2012).
The reproductive patterns do have a major role in the persistence of perennial hydroid forests. Large polyp colonies with fixed gonophores such as the Eudendrium studied by Wasserthal and Wasserthal (1973) produce planulae that settle directly in the vicinity of the mother colony, so contributing to the persistence of the forest. Hughes (1977) observed similar patterns for Nemertesia. Furthermore, these large colonies do spend the adverse season as resting hydrorhizae and continue to occupy the substrate. Boero et al. (1986) showed that the forest-forming Eudendrium glomeratum has a mixed strategy, with new colonies deriving from planulae and old colonies that regenerate from the resting stolons. The life histories of most species, however, are unstudied, and further research is needed to substantiate these patterns. The presence of medusae, due to the high vagility of this life form, should have more opportunistic polyps, even though the medusa stage is present in most of the very specialized forms that live in strict symbiosis with other animals (Puce et al. 2008), whereas those associated with plants do not follow this rule.
Emerging Threats for Hydroid Forests
As other “animal forests,” hydroid assemblages represent fragile and diverse systems that could suffer severe threats from direct and indirect impacts and for the lack of a clear responsibility for some human activities in coastal and offshore benthic systems (Rossi 2013).
As shown in Fig. 1, researches on hydroids and environmental/anthropogenic stresses have been increasing. These papers highlight that the three-dimensional habitats formed by hydroids could be threatened from direct (bottom trawling, pollution, urban expansion, tourism, harvesting of precious corals, aquaculture, mining, introduction of alien species) and indirect (ocean acidification and global warming) anthropic impacts (Rossi 2013).
Bottom trawling, among fishing activities, is the most devastating practice for animal forests (Rossi 2013), due to the destruction of the fragile species and the sediment compactness, leading to the simplification of the benthic ecosystems (Althaus et al. 2009; Clark et al. 2010; Rossi 2013). Hydroids living on dredgeable soft substrates, such as Lytocarpia myriophyllum and Nemertesia spp., are vulnerable to the effects of mechanical disturbances. Up to now, the role of soft-bottom habitat formers received little attention despite they create secondary hard substrata (Hughes 1975, 1977, 1978; Ammons and Daly 2008; Cerrano et al. 2015; Di Camillo et al. 2013).
Global warming is leading to changing in the benthic domain (Boero and Bonsdorff 2007; Lejeusne et al. 2010) favoring the establishment of tropical nonindigenous species (Coll et al. 2010; Lejeusne et al. 2010) and leading to the regression or disappearance of autochthonous, cold-affinity species (Boero et al. 2008; Rivetti et al. 2014; McCauley et al. 2015). Analysis of records over time can provide “early warning signals of species” that may encounter higher probabilities of local extinction involving several mechanisms at different spatial and temporal scales (Gravili et al. 2013, 2015). According to Puce et al. (2009) and Gravili and Boero (2014), in the Mediterraneran Sea, larger Halecium species (e.g., Halecium beanii, H. halecinum, H. labrosum) are regressing compared to smaller ones (e.g., Halecium petrosum, H. pusillum, and H. tenellum). Large-size Halecium species, in fact, can be considered as habitat modifiers that create wide marine forests maintaining and modifying habitats (Fraschetti et al. 2008; Piraino and Fanelli 1999; Piraino et al. 2002). Ocean acidification could affect hydroid producing calcified skeletons, such as stylasterids and milleporids, or the partly calcified such as the Alaskan hydractiniid Schuchertinia antonii (Miglietta 2006).
Moreover, intensive aquaculture is responsible for composition changes of the animal forest by water eutrophication, pollution, introduction of invasive species with evident changes in the trophic chain, simplification of the ecosystems, and accumulation of sediments (Borja et al. 2009; Rossi 2013).
Some hydroid species are harvested for ornamental purposes, such as Sertularia argentea and Hydrallmania (Hancock et al. 1956); S. cupressina Linnaeus, 1758 (Wagler et al. 2009); or stylasterids (Cairns 2011), with presumably negative consequences on their conservation status. Moreover, this state is worsened by the deficiency of clear legal rules in the management of marine communities (Rossi 2013).
Other hydroids take advantage from anthropic impacts, such as those able to proliferate on artificial or modified substrates (e.g., among the genera Clytia, Obelia, Ectopleura, and Pennaria) (Morri and Boero 1986).
Deciduous and enduring hydroid forests probably respond in different ways to the effects of the abovementioned threats. Seasonal hydroids, due to their fast growth, plasticity, and above all their ability to enter dormancy, could overcome temporal unfavorable environmental conditions (Di Camillo and Cerrano 2015). Enduring forests formed by large, slow-growing hydroids could be more vulnerable to environmental changes (Di Camillo et al. 2013), especially considering species producing calcified or strongly polysiphonic skeletons or those associated with zooxanthellae (Banaszak 2003).
Conclusions
The scientific literature on hydroids here reported clearly highlights a gap in supplying quantitative data, limiting the possibility to compare information on hydroids with those available for other suspension feeders, to evaluate the impact of hydroids on seston, to calculate energy budgets, and therefore to define their actual role in benthic-pelagic coupling.
Moreover, most of studies focused above all on species easy to rear (i.e., Hydra spp.) or on shallow-water hydroids easy to study in situ. Since several species live in environments difficult and/or expensive to be explored, there are very few data available on cryptic, tropical, polar, and deep hydroids, suggesting that we have only a partial knowledge on hydroid ecology.
Data on enduring species – forming the largest hydroid forests – are still insufficient to understand their function in benthic communities and to assess their vulnerability to climate changes.
Knowledge on ephemeral hydroids allows using them as indicators of several environmental conditions (Mergner 1987). Long-term studies on hydroid diversity and life histories may allow to detect early signs of the effects of water warming and other environmental stresses or to pinpoint nonindigenous species, identifying variations in composition, abundance, and reproductive periods (Puce et al. 2009; Gravili 2016).
The importance given to algal canopies in the description of habitats and in the determination of conservation policies must be extended also to animals that, in the past, where named “zoophytes,” since they have a similar structural role to that of algae as habitat formers, extending also at depths where algal growth is not optimal. Hydroids do have a higher trophic level than algae and represent an important link between the benthic and the planktonic realms, due to the production of medusae and to predation on both benthic and planktonic organisms.
Acknowledgments
The Authors are grateful to Ian Skip and Dr. Bernard Pictet for providing some of the pictures.
The research has been funded by PRIN project (Progetti di Ricerca di Interesse Nazionale 2008) 2008YBEANX_002 – http://prin.miur.it/, Flagship Project RITMARE – The Italian Research for the Sea – coordinated by the Italian National Research Council and funded by the Italian Ministry of Education, University and Research within the National Research Program 2011–2013, the EC Seventh Framework Programme (FP7/2007–2013) under Grant Agreement No. 287844 for the project “Towards COast to COast NETworks of marine protected areas (from the shore to the high and deep sea), coupled with sea-based wind energy potential” (COCONET), the project 689518 – (MERCES Marine Ecosystem Restoration in Changing European Seas).
Contributor Information
Sergio Rossi, Phone: +3434+34935814219, Email: sergio.rossi@uab.cat.
Lorenzo Bramanti, Phone: +3333+33468887319, Email: lorenzo.bramanti@obs-banyuls.fr.
Andrea Gori, FAX: +3434+34932309555, Email: gori@icm.csic.es.
Covadonga Orejas , FAX: +3434+34971404945, Email: cova.orejas@ba.ieo.es.
Cristina Gioia Di Camillo, Email: c.dicamillo@univpm.it.
Giorgio Bavestrello, Email: giorgio.bavestrello@unige.it.
Carlo Cerrano, Email: c.cerrano@univpm.it.
Cinzia Gravili, Email: cinzia.gravili@unisalento.it.
Stefano Piraino, Email: stefano.piraino@unisalento.it.
Stefania Puce, Email: s.puce@univpm.it.
Ferdinando Boero, Email: ferdinando.boero@unisalento.it.
References
- Althaus F, Williams A, Schlacher TA, Kloser RJ, Green MA, Barker BA, Bax NJ, Brodie P, Schlacher-Hoenlinger MA. Impacts of bottom trawling on deep-coral ecosystems of seamounts are long-lasting. Mar Ecol Prog Ser. 2009;397:279e294. doi: 10.3354/meps08248. [DOI] [Google Scholar]
- Ammons A, Daly M. Distribution, habitat use and ecology of deepwater anemones (Actiniaria) in the Gulf of Mexico. Deep-Sea Res II. 2008;55:2657–2666. doi: 10.1016/j.dsr2.2008.07.015. [DOI] [Google Scholar]
- Azzini F, Cerrano C, Puce S, Bavestrello G. Environmental influence on the life history of Eudendrium racemosum (Gmelin, 1791) (Cnidaria, Hydrozoa) in the Ligurian Sea. Biol Mar Mediterr. 2003;10:146–151. [Google Scholar]
- Banaszak AT. Photoprotective physiological and biochemical responses of aquatic organisms. In: Helbling EW, Zagarese HE, editors. UV effects in aquatic organisms and ecosystems. Cambridge: Cambridge University Press; 2003. pp. 329–356. [Google Scholar]
- Barangé M, Gili JM. Feeding cycles and prey capture in Eudendrium racemosum (Cavolini, 1785) J Exp Mar Biol Ecol. 1988;115:281–293. doi: 10.1016/0022-0981(88)90160-8. [DOI] [Google Scholar]
- Bavestrello G, Puce S, Cerrano C, Zocchi E, Boero N. The problem of seasonality of benthic hydroids in temperate waters. Chem Ecol. 2006;22:S197–S205. doi: 10.1080/02757540600670810. [DOI] [Google Scholar]
- Bavestrello G, Cerrano C, Di Camillo CG, Puce S, Romagnoli T, Tazioli S, Totti C. The ecology of protists epibiontic of marine hydroids. J Mar Biol Assoc UK. 2008;88:1611–1617. doi: 10.1017/S0025315408001665. [DOI] [Google Scholar]
- Becerro MA. Quantitative trends in sponge ecology research. Mar Ecol. 2008;29:167–177. doi: 10.1111/j.1439-0485.2008.00234.x. [DOI] [Google Scholar]
- Boero F. The ecology of marine hydroids and effects of environmental factors: a review. PSZNI: Mar Ecol. 1984;5:93–118. doi: 10.1111/j.1439-0485.1984.tb00310.x. [DOI] [Google Scholar]
- Boero F. Fluctuations and variations in coastal marine environments. PSZNI: Mar Ecol. 1994;15:3–25. doi: 10.1111/j.1439-0485.1994.tb00038.x. [DOI] [Google Scholar]
- Boero F, Bonsdorff E. A conceptual framework for marine biodiversity and ecosystem functioning. Mar Ecol. 2007;28:134–145. doi: 10.1111/j.1439-0485.2007.00171.x. [DOI] [Google Scholar]
- Boero F, Fresi E. Zonation and evolution of a rocky bottom hydroid community. Mar Ecol. 1986;7:123–150. doi: 10.1111/j.1439-0485.1986.tb00152.x. [DOI] [Google Scholar]
- Boero F, Balduzzi A, Bavestrello G, Caffa B, Cattaneo-Vietti R. Population dynamics of Eudendrium glomeratum (Cnidaria: Anthomedusae) on the Portofino Promontory (Ligurian Sea) Mar Biol. 1986;92:81–85. doi: 10.1007/BF00392749. [DOI] [Google Scholar]
- Boero F, Bouillon J, Gravili C, Miglietta MP, Parsons T, Piraino S. Gelatinous plankton: irregularities rule the world (sometimes) Mar Ecol Prog Ser. 2008;356:299–310. doi: 10.3354/meps07368. [DOI] [Google Scholar]
- Borja A, Rodríguez JG, Black K, Bodoy A, Emblow C, Fernandes TF, Forte J, Karakassis I, Muxika I, Nickell TD, Papageorgiou N, Pranovi F, Sevastou K, Tomassetti P, Angel D. Assessing the suitability of a range of benthic indices in the evaluation of environmental impact of fin and shellfish aquaculture located in sites across Europe. Aquaculture. 2009;293:231e240. doi: 10.1016/j.aquaculture.2009.04.037. [DOI] [Google Scholar]
- Bouillon J, Gravili C, Gili JM, Boero F. An introduction to Hydrozoa. Mémoires du Muséum National d’Histoire Naturelle, 194. Paris: Publications Scientifiques du Muséum; 2006. p. 1–593.
- Braga-Henriques A, Carreiro-Silva M, Porteiro FM, de Matos V, Sampaio Í, Ocaña O, Ávila SP. The association between a deep-sea gastropod Pedicularia sicula (Caenogastropoda: Pediculariidae) and its coral host Errina dabneyi (Hydrozoa: Stylasteridae) in the Azores. ICES J Mar Sci. 2011;68:399–407.
- Bremec C, Escolar M, Schejter L, Genzano G. Primary settlement substrate of scallop, Zygochlamys patagonica (King and Broderip, 1832) (Mollusca: Pectinidae) in fishing grounds in the Argentine Sea. J Shellfish Res. 2008;27:273–80.
- Cairns SD. Global diversity of the Stylasteridae (Cnidaria: Hydrozoa: Athecatae) PLoS One. 2011;6:e21670. doi: 10.1371/journal.pone.0021670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerrano C, Bavestrello G, Bianchi CN, Cattaneo-Vietti R, Bava S, Morganti C, Morri C, Picco P, Sara G, Schiaparelli S, Siccardi A, Sponga F. A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (Northwestern Mediterranean), summer 1999. Ecol Lett. 2000;3:284–293. doi: 10.1046/j.1461-0248.2000.00152.x. [DOI] [Google Scholar]
- Cerrano C, Danovaro R, Gambi C, Pusceddu A, Riva A, Schiaparelli S. Gold coral (Savalia savaglia) and gorgonian forests enhance benthic biodiversity and ecosystem functioning in the mesophotic zone. Biodivers Conserv. 2010;19:153–167. doi: 10.1007/s10531-009-9712-5. [DOI] [Google Scholar]
- Cerrano C, Bianchelli S, Di Camillo CG, Torsani F, Pusceddu A. Do colonies of Lytocarpia myriophyllum L. 1758 (Cnidaria, Hydrozoa) affect the biochemical composition and the meiofaunal diversity of surrounding sediments? Chem Ecol. 2015;31:1–21. doi: 10.1080/02757540.2014.966699. [DOI] [Google Scholar]
- Christensen H. Feeding and reproduction in Precuthona peachi (Mollusca Nudibranchia). Ophelia. 1977;16:131–42.
- Clark MR, Rowden AA, Schlacher T, Williams A, Consalvey M, Stocks KI, Rogers AD, O’Hara TD, White M, Shank TM, Hall-Spencer J. The ecology of seamounts: structure, function, and human impacts. Annu Rev Mar Sci. 2010;2:253e278. doi: 10.1146/annurev-marine-120308-081109. [DOI] [PubMed] [Google Scholar]
- Coll M, Piroddi C, Steenbeek J, Kaschner K, Ben Rais Lasram F, et al. The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS One. 2010;5:e11842. doi: 10.1371/journal.pone.0011842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coma R, Gili JM, Zabala M, Riera T. Feeding and prey capture cycles in the aposymbiontic gorgonian Paramuricea clavata. Mar Ecol Prog Ser. 1994;115:257–70.
- Coma R, Gili JM, Zabala M. Trophic ecology of a benthic marine hydroid, Campanularia everta. Oceanogr Lit Rev. 1995;119:211–220. [Google Scholar]
- Coma R, Ribes M, Gili JM, Zabala M. An energetic approach to the study of life-history traits of two modular colonial benthic invertebrates. Mar Ecol Prog Ser. 1998;162:89–103. doi: 10.3354/meps162089. [DOI] [Google Scholar]
- Coma R, Ribes M, Orejas C, Gili JM. Prey capture by a benthic coral reef hydrozoan. Coral Reefs. 1999;18:141–5.
- Coma R, Ribes M, Gili JM, Zabala M. Seasonality in coastal benthic ecosystems. Trends Ecol Evol. 2000;15:448–453. doi: 10.1016/S0169-5347(00)01970-4. [DOI] [PubMed] [Google Scholar]
- Connell JH, Slatyer RO. Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat. 1977;111:1119–44.
- Coni EOC, Ferreira CM, de Moura RL, Meirelles PM, Kaufman L, Francini-Filho RB. An evaluation of the use of branching fire-corals (Millepora spp.) as refuge by reef fish in the Abrolhos Bank, eastern Brazil. Environ Biol Fish. 2013;96:45–55. doi: 10.1007/s10641-012-0021-6. [DOI] [Google Scholar]
- Cook PA, Stewart BA, Achituv Y. The symbiotic relationship between the hydrocoral Millepora dichotoma and the barnacle Savignium milleporum. In: Williams RB, Cornelius PFS, Hughes RG, Robson EA, editors. Coelenterate biology: recent research on cnidaria and ctenophora. Netherlands: Springer; 1991. p. 285–90.
- Di Camillo CG, Bavestrello G, Valisano L, Puce S. Spatial and temporal distribution in a tropical hydroid assemblage. J Mar Biol Assoc UK. 2008;88:1589–1599. doi: 10.1017/S0025315408002981. [DOI] [Google Scholar]
- Di Camillo CG, Bo M, Puce S, Bavestrello G. Association between Dentitheca habereri (Cnidaria: Hydrozoa) and two zoanthids. Ital J Zool. 2010;77:81–91. doi: 10.1080/11250000902740962. [DOI] [Google Scholar]
- Di Camillo CG, Betti F, Bo M, Martinelli M, Puce S, Vasapollo C, Bavestrello G. Population dynamics of Eudendrium racemosum (Cnidaria, Hydrozoa) from the North Adriatic Sea. Mar Biol. 2012;159:1593–1609. doi: 10.1007/s00227-012-1948-z. [DOI] [Google Scholar]
- Di Camillo CG, Luna GM, Bo M, Giordano G, Corinaldesi C, Bavestrello G. Biodiversity of prokaryotic communities associated with the ectoderm of Ectopleura crocea (Cnidaria, Hydrozoa) PLoS One. 2012;7:e39926. doi: 10.1371/journal.pone.0039926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Camillo CG, Boero F, Gravili C, Previati M, Torsani F, Cerrano C. Distribution, ecology and morphology of Lytocarpia myriophyllum (Cnidaria: Hydrozoa), a Mediterranean Sea habitat former to protect. Biodivers Conserv. 2013;22:773–787. doi: 10.1007/s10531-013-0449-9. [DOI] [Google Scholar]
- Di Camillo CG, Cerrano C. Mass mortality events in the NW Adriatic Sea: phase shift from slow-to fast-growing organisms. PloS One. 2015;10:e0126689. doi: 10.1371/journal.pone.0126689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraschetti S, Terlizzi A, Boero F. How many habitats are there in the sea (and where)? J Exp Mar Biol Ecol. 2008;366:109–115. doi: 10.1016/j.jembe.2008.07.015. [DOI] [Google Scholar]
- Garcia TM, Matthews-Cascon H, Franklin-Junior W. Millepora alcicornis (Cnidaria: Hydrozoa) as substrate for benthic fauna. Braz J Oceanogr. 2009;57:153–5.
- Garcia TM, Matthews-Cascon H, Franklin-Junior W. Sipuncula associated with branching fire coral (Millepora alcicornis) in a marine protected area in Northeastern Brazil. Thalassas Int J Mar Sci. 2010;26:9–12. [Google Scholar]
- Genzano GN. Associated fauna and sediment trapped by colonies of Tubularia crocea (Cnidaria, Hydrozoa) from the rocky intertidal of Mar del Plata, Argentina. Biociencias. 2001;9:105–119. [Google Scholar]
- Genzano GN, Excoffon AC, Acuña FH, Zamponi MO. Hydroid colonies as primary substrata for recruits of the mussel Mytilus edulis platensis front off Mar del Plata, Argentina. Ophelia. 2003;57:53–61. doi: 10.1080/00785236.2003.10409505. [DOI] [Google Scholar]
- Gili JM, Coma R. Benthic suspension feeders: their paramount role in littoral marine food webs. Trends Ecol Evol. 1998;13:316–321. doi: 10.1016/S0169-5347(98)01365-2. [DOI] [PubMed] [Google Scholar]
- Gili JM, Hughes RG. The ecology of marine benthic hydroids. Oceanogr Mar Biol Annu Rev. 1995;33:351–426. [Google Scholar]
- Gili JM, Alvà V, Pagès F, Klöser H, Arntz WE. Benthic diatoms as the major food source in the sub-Antarctic marine hydroid Silicularia rosea. Polar Biol. 1996;16:507–512. doi: 10.1007/BF02329070. [DOI] [Google Scholar]
- Gili JM, Alvà V, Coma R, Orejas C, Pagès F, Ribes M, et al. The impact of small benthic passive suspension feeders in shallow marine ecosystems: the hydroids as an example. Zoologische verhandelingen. 1998;323:99–105. [Google Scholar]
- Goodwin C, Edwards H, Breen J, Picton B. Rathlin Island – a survey report from the nationally important marine features project 2009–2011. Northern Ireland Environment Agency Research and Development Series No. 11/03. 2011.
- Gravili C. Zoogeography of Hydrozoa: past, present and a look to the future. In: Goffredo S, Dubinsky Z. (eds). The cnidaria, past, present and future. Springer International Publishing; Switzerland. 2016. p. 95–107.
- Gravili C, Bevilacqua S, Terlizzi A, Boero F. Missing species among Mediterranean non-Siphonophoran Hydrozoa. Biodivers Conserv. 2015;24:1329–57. doi: 10.1007/s10531-015-0859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravili C, Boero F. A bioregionalization of the genus Halecium (Hydrozoa: Haleciidae): sentinel taxon of the global warming? Thalassia Salentina – Proceedings of the 75th National Conference of the Unione Zoologica Italiana. 2014;36(Supplemento):128.
- Gravili C, D’Ambrosio P, Di Camillo C, Renna G, Bouillon J, Boero F. Clytia hummelincki (Hydroidomedusae: Leptomedusae) in the Mediterranean Sea. J Mar Biol Assoc UK. 2008;88:1547–1553. doi: 10.1017/S0025315408001975. [DOI] [Google Scholar]
- Gravili C, Pagliara R, Vervoort W, Bouillon J, Boero F. Trends in hydromedusan research from 1911 to 1997. Scientia Marina. 2000;64:23–29. doi: 10.3989/scimar.2000.64s123. [DOI] [Google Scholar]
- Gravili C, Di Camillo CG, Piraino S, Boero F. Hydrozoan species richness in the Mediterranean Sea: past and present. Mar Ecol. 2013;34(Supplemento 1):41–62. doi: 10.1111/maec.12023. [DOI] [Google Scholar]
- Hancock DA, Drinnan RE, Harris WN. Notes on the biology of Sertularia argentea L. J Mar Biol Assoc UK. 1956;35:307–325. doi: 10.1017/S0025315400010158. [DOI] [Google Scholar]
- Häussermann V, Försterra G. Extraordinary abundance of hydrocorals (Cnidaria, Hydrozoa, Stylasteridae) in shallow water of the Patagonian fjord region. Polar Biol. 2007;30:487–492. doi: 10.1007/s00300-006-0207-5. [DOI] [Google Scholar]
- Hughes RG. The distribution of epizoites on the hydroid Nemertesia antennina (L.) J Mar Biol Assoc UK. 1975;55:275–294. doi: 10.1017/S0025315400015940. [DOI] [Google Scholar]
- Hughes RG. Aspects of the biology and life-history of Nemertesia antennina (L.) (Hydrozoa: Plumulariidae) J Mar Biol Assoc UK. 1977;57:641–657. doi: 10.1017/S0025315400025091. [DOI] [Google Scholar]
- Hughes RG. Life-histories and abundance of epizoites of the hydroid Nemertesia antennina (L.) J Mar Biol Assoc UK. 1978;58:313–332. doi: 10.1017/S0025315400028009. [DOI] [Google Scholar]
- Humphrey LD, Pyke DA. Demographic and growth responses of a guerrilla and a phalanx perennial grass in competitive mixtures. J Ecol. 1998;6: 854, 865.
- Jackson JBC, Coates AG. Life cycles and evolution of clonal (modular) organisms. Philos Trans R Soc Lond. 1986;B313:7–22. doi: 10.1098/rstb.1986.0022. [DOI] [Google Scholar]
- Kinne O, Paffenhöfer GA. Hydranth structure and digestion rate as a function of temperature and salinity in Clava multicornis (Cnidaria, Hydrozoa) Helgoländer Meeresun. 1965;12:329–341. doi: 10.1007/BF01612558. [DOI] [Google Scholar]
- Knowlton N, Jackson JBC. Inbreeding and outbreeding in marine invertebrates. In: Thornhill NW, editor. The natural history of inbreeding and outbreeding. Chicago: University of Chicago Press; 1993. pp. 200–249. [Google Scholar]
- Kosevich IA. Changes in the patterning of a hydroid colony. Zoology. 2006;109:244–259. doi: 10.1016/j.zool.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Lagardère F, Tardy J. Un faciès d’épifaune nouveau: le faciès à Ectopleura dumortieri (van Beneden) et Electra pilosa (Linné) faune associée, cartographie et évolution saissoniére. Cah Biol Mar. 1980;21:265–278. [Google Scholar]
- Lejeusne C, Chevaldonné P, Pergent-Martini C, Boudouresque CF, Perez T. Climate change effects on a miniature ocean: the highly diverse, highly impacted Mediterranean Sea. Trends Ecol Evol. 2010;4:250–260. doi: 10.1016/j.tree.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Lewis JB. Heterotrophy in corals: zooplankton predation by the hydrocoral Millepora complanata. Mar Ecol Prog Ser. 1992;90:251–256. doi: 10.3354/meps090251. [DOI] [Google Scholar]
- Lewis JB. Biology and ecology of the hydrocoral Millepora on coral reefs. Adv Mar Biol. 2006;50:1–55. doi: 10.1016/S0065-2881(05)50001-4. [DOI] [PubMed] [Google Scholar]
- Marfenin NN. Adaptation capabilities of marine modular organisms. In: Naumov AD, Hummel H, Sukhotin AA, Ryland JS (eds). Interactions and adaptation strategies of marine organisms. Dordrecht, the Netherlands; 1997. p. 153–158.
- McCauley DJ, Pinsky ML, Palumbi SR, Estes JA, Joyce FH, Warner RR. Marine defaunation: animal loss in the global ocean. Science. 2015;347(6219). doi:10.1126/science.1255641. [DOI] [PubMed]
- Meretta PE, Genzano GN. Epibiont community variation on two morphologically different hydroid colonies: Amphisbetia operculata and Plumularia setacea (Cnidaria, Hydrozoa) Mar Biol Res. 2015;11:294–303. doi: 10.1080/17451000.2014.923101. [DOI] [Google Scholar]
- Mergner H. Hydroids as indicator species of environmental factors on coral reefs. In: Bouillon J, Boero F, Cicognia F, Cornelius PFS, editors. Modern trends in the systematics, ecology and evolution of hydroids and hydromedusae. Oxford: Clarendon Press; 1987. pp. 185–195. [Google Scholar]
- Miglietta MP, Della Tommasa L, Denitto F, Gravili C, Pagliara P, Bouillon J, Boero F. Approaches to the ethology of hydroids and medusae (Cnidaria, Hydrozoa) Sci Mar. 2000;64:63–71. doi: 10.3989/scimar.2000.64s163. [DOI] [Google Scholar]
- Miglietta MP. Hydractinia antonii sp. nov.: a new, partially calcified hydractiniid (Cnidaria: Hydrozoa: Hydractiniidae) from Alaska. J Mar Biol Assoce U.K. 2006;86:993–996. doi: 10.1017/S0025315406013968. [DOI] [Google Scholar]
- Morri C, Boero F.. Catalogue of main marine fouling organisms. vol. 7. Hydroids. Bruxelles: Office d’Etudes Marines et Atmosphériques ODEMA; 1986, 91 pp.
- Orejas C, Rossi S, Peralba A, García E, Gili JM, Lippert H. Feeding ecology and trophic impact of the hydroid Obelia dichotoma in the Kongsfjorden (Spitsbergen, Arctic) Polar Biol. 2013;36:61–72. doi: 10.1007/s00300-012-1239-7. [DOI] [Google Scholar]
- Pereira PHC, Leal ICS, de Araújo ME, Souza AT. Feeding association between reef fishes and the fire coral Millepora spp.(Cnidaria: Hydrozoa). Mar Biodivers Rec. 2012;5:e42.
- Pérez CD, Gomes PB. First record of the fireworm Hermodice carunculata (Annelida, Polychaeta) preying on colonies of the fire coral Millepora alcicornis (Cnidaria, Hydrozoa) Biota Neotropica. 2012;12:217–9. doi: 10.1590/S1676-06032012000200022. [DOI] [Google Scholar]
- Pica D, Cairns SD, Puce S, Newman WA. Southern hemisphere deep-water stylasterid corals including a new species, Errina labrosa sp. n. (Cnidaria, Hydrozoa, Stylasteridae), with notes on some symbiotic scalpellids (Cirripedia, Thoracica, Scalpellidae). ZooKeys. 2015;472:1. [DOI] [PMC free article] [PubMed]
- Piraino S, Fanelli G. Keystone species: what are we talking about. Conserv Ecol. 1999;3,1:r4.
- Piraino S, Fanelli G, Boero F. Variability of species’ roles in marine communities: change of paradigms for conservation priorities. Mar Biol. 2002;140:1067–1074. doi: 10.1007/s00227-001-0769-2. [DOI] [Google Scholar]
- Ponti M, Grech D, Mori M, Perlini RA, Ventra V, Panzalis PA, Cerrano C. The role of gorgonians on the diversity of vagile benthic fauna in Mediterranean rocky habitats. Mar Biol. 2016;163:1–14. doi: 10.1007/s00227-016-2897-8. [DOI] [Google Scholar]
- Puce S, Bavestrello G, Arillo A, Azzini F, Cerrano C. Morpho–functional adapatations to suspension feeeding in Eudendrium (Cnidaria, Hydrozoa) Ital J Zool. 2002;69:301–304. doi: 10.1080/11250000209356473. [DOI] [Google Scholar]
- Puce S, Cerrano C, Di Camillo CG, Bavestrello G. Hydroidomedusae (Cnidaria: Hydrozoa) symbiotic radiation. J Mar Biol Assoc UK. 2008;88:1715–1721. doi: 10.1017/S0025315408002233. [DOI] [Google Scholar]
- Puce S, Bavestrello G, Di Camillo CG, Boero F. Long-term changes in hydroid (Cnidaria, Hydrozoa) assemblages: effect of Mediterranean warming? Mar Ecol (An Evolutionary Perspective) 2009;30:313–326. doi: 10.1111/j.1439-0485.2009.00283.x. [DOI] [Google Scholar]
- RAC/SPA. Classifcation of benthic marine habitat types for the Mediterranean region. 2006. Available online at: http://www.rac-spa.org/sites/default/files/doc_fsd/lchm_en.pdf
- Rivetti I, Fraschetti S, Lionello P, Zambianchi E, Boero F. Global warming and mass mortalities of benthic invertebrates in the Mediterranean Sea. PLoS One. 2014;9:e115655. doi: 10.1371/journal.pone.0115655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R, Correal GO, de Oliveira TC, de Carvalho LL, Mazurek P, Barbosa JEF, Chequer L, Domingos TFS, Jandre KA, Leão LSD, de Andrade Moura L, Occhioni GE, de Oliveira VM, Silva ES, Cardoso AM, de Castro e Costa A, Ferreira CEL. Coral health rapid assessment in marginal reef sites. Mar Biol Res. 2014;10:612–24.
- Ronowicz M, Wlodarska-Kowalczuk M, Kuklinski P. Factors influencing hydroids (Cnidaria: Hydrozoa) biodiversity and distribution in Arctic kelp forest. J Mar Biol Assoc UK. 2008;88:1567–1575. doi: 10.1017/S0025315408001495. [DOI] [Google Scholar]
- Rossi S. The destruction of the ‘animal forests’ in the oceans: towards an oversimplification of the benthic ecosystems. Ocean Coast Manag. 2013;84:77–85. doi: 10.1016/j.ocecoaman.2013.07.004. [DOI] [Google Scholar]
- Rossi S, Bramanti L, Broglio E, Gili JM. Trophic impact of long-lived species indicated by population dynamics in the short-lived hydrozoan Eudendrium racemosum. Mar Ecol Prog Ser. 2012;467:97–111. doi: 10.3354/meps09848. [DOI] [Google Scholar]
- Salvati E, Angiolillo M, Bo M, Bavestrello G, Giusti M, Cardinali A, et al. The population of Errina aspera (Hydrozoa: Stylasteridae) of the Messina Strait (Mediterranean Sea) J Mar Biol Assoc UK. 2010;90:1331–1336. doi: 10.1017/S0025315410000950. [DOI] [Google Scholar]
- Simkina RG. A quantitative study of feeding of the colonies of Perigonimus megas (hydroidea, bougainvillidae) Zool Zhurnal. 1980;59:500–506. [Google Scholar]
- Standing JD. Fouling community structure: effects of the hydroid, Obelia dichotoma, on larval recruitment. In: Mackie GO, editor. Coelenterate ecology and behavior. US: Springer; 1976. vol. 17, p. 155–64.
- Sutherland JP, Karlson RH. Development and stability of the fouling community at Beaufort, North Carolina. Ecol Monogr. 1977;47:425–46.
- Tazioli S, Di Camillo CG. Ecological and morphological characteristics of Ephelota gemmipara (Ciliophora, Suctoria), epibiontic on Eudendrium racemosum (Cnidaria, Hydrozoa) from the Adriatic Sea. Eur J Protistol. 2013;49:590–599. doi: 10.1016/j.ejop.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Valisano L, Notari F, Mori M, Cerrano C. Temporal variability of sedimentation rates and mobile fauna inside and outside a gorgonian garden. Mar Ecol. 2016;37:1303–1314. doi: 10.1111/maec.12328. [DOI] [Google Scholar]
- Wagler H, Berghahn R, Vorberg R. The fishery for whiteweed, Sertularia cupressina (Cnidaria, Hydrozoa), in the Wadden Sea, Germany: history and anthropogenic effects. ICES J Mar Sci. 2009;66:2116–2120. doi: 10.1093/icesjms/fsp201. [DOI] [Google Scholar]
- Wasserthal LT, Wasserthal W. Ökologische Bedeuting der Schleimsekretion bei den Planula-Larven der Hydroidengattung Eudendrium. Mar Biol. 1973;22(4):341–345. doi: 10.1007/BF00391391. [DOI] [Google Scholar]
- Zintzen V, Norro A, Massin C, Mallefet J. Temporal variation of Tubularia indivisa (Cnidaria, Tubulariidae) and associated epizoites on artificial habitat communities in the North Sea. Mar Biol. 2008;153:405. doi: 10.1007/s00227-007-0819-5. [DOI] [Google Scholar]


