Abstract
The amygdala is a key subcortical region thought to contribute to emotional components of pain. As opioid receptors are found in both the central (CeA) and basolateral (BLA) nuclei of the amygdala, we investigated the effects of morphine microinjection on evoked pain responses, pain motivated behaviors, dopamine release in the nucleus accumbens (NAc), and descending modulation in rats with left side spinal nerve ligation (SNL). Morphine administered into the right or left CeA had no effect on nerve injury induced tactile allodynia or mechanical hyperalgesia. Right, but not left, CeA morphine produced conditioned place preference (CPP) and increased extracellular dopamine in the NAc selectively in SNL rats, suggesting relief of aversive qualities of ongoing pain. In SNL rats, CPP and NAc dopamine release following right CeA morphine was abolished by blocking mu opioid receptor (MOR) signaling in the rostral anterior cingulate cortex (rACC). Right CeA morphine also significantly restored SNL-induced loss of the diffuse noxious inhibitory controls (DNIC), a spino-bulbo-spinal pain modulatory mechanism, termed conditioned pain modulation in humans. Microinjection of morphine into the BLA had no effects on evoked behaviors and did not produce CPP in nerve injured rats. These findings demonstrate that the amygdalar action of morphine is specific to the right CeA contralateral to the side of injury and results in enhancement of net descending inhibition. Additionally, engagement of MORs in the right CeA modulates affective qualities of ongoing pain through endogenous opioid neurotransmission within the rACC, revealing opioid-dependent functional connections from the CeA to the rACC.
Summary
Opioids in the right central amygdala nucleus contralateral to neuropathic injury restore diffuse noxious inhibitory controls in rats with nerve ligation and relieve aversive qualities of ongoing pain.
Introduction
Opioids modulate sensory processing and preferentially alter affective qualities of pain [4]. Human brain imaging and preclinical studies have suggested a role for endogenous opioid peptides and their receptors in the rostral anterior cingulate cortex (rACC) in the modulation of aversive qualities of pain [38; 60]. Opioid receptors are also highly expressed in subcortical areas including the amygdala [11; 43], but the contribution of these circuits to the modulation of emotional/affective dimensions of pain remain unclear. In addition to processing pain affect, central opioid circuits are thought to control the descending pain modulatory pathways that are engaged by placebo analgesia, expectations of pain or pain relief, or through bottom-up mechanisms during a concurrent pain stimulation (i.e., conditioned pain modulation; CPM) [30; 51; 55]. CPM, termed diffuse noxious inhibitory controls (DNIC) in animal studies, is a “pain inhibits pain” mechanism and is characterized by reduction of both subjective pain scores and objective pain measures, including pain-evoked potentials and pain thresholds [42]. Remarkably, amygdala activity has been shown to directly correlate with the extent of pain reduction during CPM in healthy volunteers [30].
The amygdala consists of several cytologically and neurochemically heterogenous sub-nuclei that vastly differ in their afferent and efferent connections with other brain regions. The most prominent clusters of amygdala nuclei include the lateral/basolateral complex and the central amygdala nucleus (CeA) [41]. The lateral (LA) and basolateral (BLA) amygdala nuclei consist of mostly glutamatergic pyramidal neurons, have reciprocal connections with the cerebral cortex and densely project to the CeA [40]. In contrast, the CeA, comprising of the capsular, lateral, and medial subdivisions, contains predominantly inhibitory GABA-ergic neurons that may co-synthetize opioid or non-opioid neuropeptides [6; 16]. The CeA receives nociceptive inputs from the spinal cord via the parabrachial area and processed pain information from the LA/BLA and the cerebral cortex [54]. CeA neurons do not have direct projections to the cortex; however, they innervate forebrain structures, including the bed nuclei of the stria terminalis and substantia innominata, that may provide an indirect connection to the prefrontal cortex and the ACC [44]. Additionally, CeA neurons prominently innervate autonomic and descending pain modulatory regions including the hypothalamus, periaqueductal gray area, and rostral ventromedial medulla (RVM) [49]. Collectively, the organization of the amygdala supports its role in integrating direct nociceptive input with affective, motivational, and cognitive information and also its role of modulating autonomic, affective, and perhaps spinal processing, such as reflexive withdrawal behavioral responses through descending pain pathways.
Here, we investigated the effects of MOR activation in different amygdala subdivisions on sensory and affective aspects of pain by measuring mechanical allodynia and hyperalgesia, strength of the DNIC effect, conditioned place preference (CPP), and dopamine release in the nucleus accumbens (NAc) of rats with neuropathic injury. In addition, we investigated a possible functional interaction between opioid amygdala and rACC circuitry.
Materials and methods
Animals
Male, Sprague-Dawley rats, weighing 250–300 g were obtained from Harlan Laboratories (Indianapolis; IN). Rats were housed on a 12 h/12 h light/dark cycle with food and water available ad libitum. All animal procedures received approval from the Institutional Animal Care and Use Committee (IACUC) of the University of Arizona. Rats were monitored throughout the duration of the study to reduce unnecessary stress and/or pain, and the number of animals used was in accordance with the International Association for the Study of Pain ethical guidelines. Investigators for all behavioral experiments were blinded to the treatment groups.
Surgery
Spinal nerve ligation surgery
Spinal nerve ligation (SNL) surgery was performed as described previously [24]. Rats were maintained under 2% v/v isoflurane anesthesia delivered in a 3:2 ratio of nitrous oxide and oxygen. A paraspinal incision was made and the left tail muscle excised. Part of the L5 transverse process was removed to expose the left L5 and L6 spinal nerves, which were then isolated and ligated with a non-absorbable 6–0 braided silk thread proximal to the formation of the sciatic nerve. The surrounding skin and muscle were closed with absorbable 3–0 sutures. Sham surgery was performed in an identical manner omitting the ligation step. All rats were monitored for normal behaviors (grooming and mobility) and for general health and weight gain post-surgery.
Intracranial CeA, BLA, ACC and NAc cannulation
Stereotaxic cannulation surgeries were performed in rats anesthetized with a ketamine/xylazine combination (80/12 mg/kg, i.p.; Western Medical Supply/Sigma, Arcadia; CA). Bilateral cannulation of the rACC was performed as previously described [23; 39]. A pair of 26-gauge stainless steel guide cannulas cut 4 mm below the pedestal (Plastics One Inc., Roanoke; VA) were directed toward the rACC injection site (anteroposterior (AP): +2.6 mm from bregma; medial-lateral (ML): ± 0.6 mm from midline; dorsoventral (DV): −1.8 mm from skull). Unilateral 26-gauge guide cannulae were implanted into the left or right CeA (AP: bregma −2.0 mm; ML: +/− 4.0 mm; DV: −7.0 mm), or the left or right BLA (AP: bregma −3.3 mm; ML: +/− 5.0 mm; DV: −8.0 mm). For microdialysis, a single guide cannula (AG-8, EICOM Corp., Kyoto, Japan) was implanted into the left NAc (AP: bregma +1.7 mm; ML: −1.0 mm; DV: −6.0 mm). Guide cannulas were cemented in place and secured to the skull by small stainless-steel machine screws. Stainless steel dummy cannulas were inserted into each guide to keep the guide free of debris. Rats then received a subcutaneous gentamycin (1 mg/ml; VetOne, Boise; ID) injection, and were allowed to recover for 7–10 days. Rats used in the microdialysis and CPP experiments were housed individually after cannulation. All rats were monitored and assessed daily for overall health.
Mechanical allodynia (von Frey test)
On the day of the experiment, rats were placed in suspended chambers with wire mesh floors for 30 minutes to habituate prior to testing. A series of calibrated von Frey filaments (Stoelting, Wood Dale; IL) in logarithmically spaced increments ranging from 0.41 to 15 g (4–150 N) were applied perpendicular to the plantar surface of the ipsilateral hind paw until the filament buckled. Withdrawal threshold was determined by sequentially increasing and decreasing the stimulus strength (“up and down” method), analyzed using a Dixon nonparametric test, and expressed as the mean withdrawal threshold [10].
Mechanical hyperalgesia (Randall–Selitto test)
The Randall–Selitto paw pressure test (Ugo Basile, Varese; Italy) was used to measure changes in static sensory thresholds (mechanical hyperalgesia) and as the test stimulus to quantify the strength of the DNIC effect in sham and SNL rats on day 14 post-surgery. The pressure at which the rat vocalized or withdrew its hind paw was recorded as the paw withdrawal threshold (PWT). PWT was measured three times for each hind paw at each time point and averaged prior to data analysis.
DNIC measurement
Following baseline Randall-Selitto PWT measurement of the right and left hind paws, capsaicin solution (125 μg/50 μl) was interdermally injected into the left forepaw as the conditioning stimulus to induce DNIC as previously described [32; 33]. PWT of the right and left hind paws were measured again at 20, 40, 60, and 90 min post capsaicin administration. Capsaicin (Sigma-Aldrich, St. Louis; MO) was mixed to an initial concentration of 50 μg/μl in a solution containing 1:1 ethanol:tween 80 and then diluted to the final concentration of 2.5 μg/μl using 0.9% saline. Rats were briefly anesthetized with isoflurane for the capsaicin injection.
Conditioned place preference (CPP)
A single trial conditioning protocol was used for CPP as previously described [25; 35]. Rats underwent daily handling by the experimenter before the pre-conditioning phase. On preconditioning day, rats were placed into the CPP boxes with free access to explore all chambers. To verify whether a pre-existing chamber bias existed, automated software (Photobeam Activity System 2.0.7) was used to determine the time spent in each chamber across 15 minutes. Rats spending more than 80% (720 s) or less than 20% (180 s) of the total time in either chamber were eliminated from further testing. On conditioning day, rats with BLA or CeA cannulas received a saline injection into the cannula, and were placed into a conditioning chamber for 30 min. Four hours later, rats received morphine into the BLA or CeA and were placed into the opposite chamber for 30 min. On test day, rats were placed into the middle CPP chamber and were allowed to explore all chambers again for 15 minutes; the time spent in each chamber was automatically recorded using the Photobeam Activity System 2.0.7 to determine chamber preference. To determine the role of endogenous opioids in the rACC in right CeA-mediated CPP, rats with rACC and right CeA (R-CeA) cannulas received β-funaltrexamine (β-FNA) or saline bilaterally into the rACC on the first CPP day immediately after baseline assessment. The next day (conditioning day), they were conditioned as described above with R-CeA saline in the morning and R-CeA morphine 4 h later in the afternoon and were tested for chamber preference on the third day (test day). Difference scores were calculated as the time spent in the morphine-paired chamber on test day minus the time spent in the same chamber on the preconditioning day.
In vivo microdialysis and dopamine quantification
Microdialysis experiments were done in awake, freely moving rats. The microdialysis probe (AI-8–2, EICOM; Japan) was inserted into the NAc with 2 mm semipermeable membrane projecting beyond the guide cannula and perfused at 1.25 μl/min with artificial cerebrospinal fluid (aCSF: 147.0 mM NaCl, 2.8 mM KCl, 1.2 mM MgCl2 and 1.2 mM CaCl2). After a 90 min washout period, one 90 min baseline and one 90 min treatment fraction were collected into 4°C pre-chilled Eppendorf tubes containing 1.0 μl 40x antioxidant solution (6.0 mM L-cysteine, 2.0 mM oxalic acid and 1.3% w/v glacial acetic acid) [19]. All rats were then injected with cocaine (20 mg/kg, i.p.) and dialysates collected for an additional 90 min. Fractions were analyzed using Agilent 1100 HPLC system (Agilent, Santa Clara; CA) with a 5020-guard cell, MD-150 column and Coulochem III 5014B electrochemical detector (ESA; MA). The guard cell was set at 350 mV, Electrode1 at −150 mV and Electrode2 at 250 mV. A standard curve was obtained from seven serial dilutions of dopamine (2.5 – 160 pg in 20 μl aCSF plus antioxidant cocktail). The Limit of Detection (LOD) and Limit of Quantification (LOQ) were calculated according to the formulas: LOD= 3.3 (SDr/S); LOQ= 10 (SDr/S); where the standard deviation of the response SDr (SD of y-intercepts of regression lines) and the slope of the standard curve S were determined from the measurements of 10 independent standard curves. Data from rats that failed to generate dopamine efflux post-cocaine treatment were excluded. Dopamine concentrations were expressed as percent of the corresponding baseline level.
Drug administration (intra-brain injections)
A unilateral injection cannula extending 1 mm beyond the end of the guide cannula was connected to a 2 μl Hamilton syringe and driven by a syringe pump (Stoelting, Quintessential Stereotaxic Injector, Wood Dale; IN). Microinjections of morphine sulfate (National Institute of Drug Abuse Drug Supply Program, Bethesda; MD) or vehicle (saline) were administered unilaterally into either right or left CeA or BLA at a dose of 1 μg/0.2 μl. Bilateral injections of β-funaltrexamine (β-FNA, Tocris, Ellisville; MO) or saline into the rACC were done 20–24 h prior to testing at a dose of 3 μg/μl/side. Post-experiment, rats were euthanized with CO2 in accordance with the ethical standards set forth by the American Association of Veterinary Medicine, and 0.5 μl of Black India Ink was injected into the cannula to verify injection location. Data from rats with misplaced cannulas were removed from subsequent data analysis.
Statistical analyses:
Statistical analyses were calculated using GraphPad Prism 7 (GraphPad Software, La Jolla; CA). Tactile response time-course experiments (von Frey and Randall-Selitto tests) were analyzed using a three-way ANOVA with “time”, “pain” model and “drug” treatment as variable factors. Where significance was observed, a Tukey’s post hoc test was performed. For CPP experiments, data are presented as difference scores (i.e., the difference between the time spent in the drug-paired chamber on test day minus the time spent in that chamber on baseline day). Previous experiments confirmed that the employed CPP procedure is unbiased. Thus, a positive CPP score represents place preference, a negative score indicates aversion, and zero indicates no preference [26; 29]. To evaluate whether the rats show significant preference or aversion, differences from a hypothetical value of 0 (i.e., no preference) were determined for each group’s difference score using a one-sample t-test. Subsequently, to compare between the two treatment groups, an unpaired t-test was used. All results were expressed as mean ± SEM. Significance was set at p<0.05.
Results
Morphine in the right CeA has no effect on tactile allodynia or mechanical hyperalgesia in SNL rats.
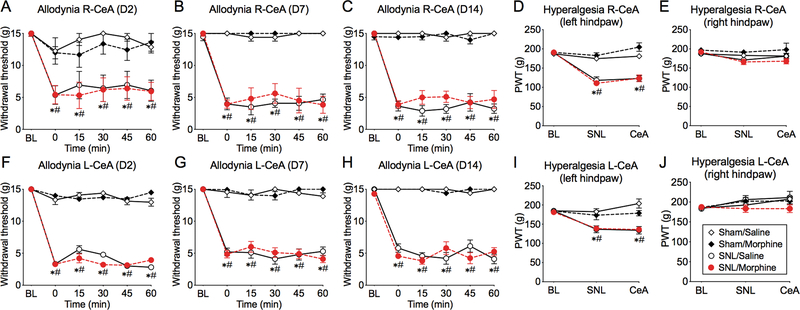
Rats were implanted with a unilateral cannula into the right CeA and SNL or sham surgery was performed on the left side. At 2–14 days following SNL surgery, rats demonstrated significantly reduced paw withdrawal thresholds (PWT) to von Frey filaments in the left hind paw (i.e., ipsilateral to the ligation side) compared to pre-surgery baselines (Figure 1A-C; three-way ANOVA with Tukey’s multiple comparison test, see Table 1 for the results of statistical analyses), confirming the development of mechanical allodynia. SNL rats also developed mechanical hyperalgesia in the ipsilateral hind paw, demonstrated by decreased PWT with the Randall-Selitto test at 14 days post-surgery (Figure 1D,E; three-way ANOVA, Table 1). Morphine microinjection (1 μg/0.2 μL) into the right CeA had no effect on hind paw withdrawal thresholds to von Frey filaments or Randall-Selitto testing at any time after the surgery in either sham or SNL rats (Figure 1A-D). These data indicate that MOR signaling in the right CeA does not influence subthreshold (allodynia) or suprathreshold (hyperalgesia) mechanical withdrawal responses.
Figure 1. Morphine microinjection into the right or left CeA has no effect on mechanical allodynia and hyperalgesia in SNL.
(A-C; F-H) SNL, but not sham-operated, rats developed tactile allodynia 2–14 days following surgery, demonstrated as significantly reduced ipsilateral (left) hind paw withdrawal thresholds from pre-surgery baselines (BL). Time courses of hind paw withdrawal thresholds following morphine microinjection into the right (A, B, C) or left (F, G, H) CeA show no effect of morphine at any time point after the surgery. In SNL rats, PWT remain significantly lower than BL and significantly lower than in sham rats at corresponding times. (D, I) Compared to baseline (BL), SNL, but not sham, rats developed mechanical hyperalgesia in the left hind paw, shown by significantly decreased paw withdrawal thresholds (PWT) with the Randall-Selitto test at 14 days post-surgery (SNL). (E, J) No hyperalgesia was observed in the right hind paw. Administration of morphine into the right (D, E) or left (I, J) CeA did not affect withdrawal thresholds in any group (CeA). Data display means ± SEM, n= 5–8 rats/group, (three-way ANOVA with Tukey’s post-hoc test; # represents a significant difference from BL, * represents a significant difference from sham rats at corresponding times).
Table 1. Summary of statistical analyses.
| Figure | Analysis | Time | Pain | Drug | Time × Pain | Time × Drug | Pain × Drug | T × P × D |
|---|---|---|---|---|---|---|---|---|
| 1A | 3-way ANOVA | P<0.0001 F (5, 128) = 16.23 | P<0.0001 F (1, 128) = 111.2 | P=0.2395 F (1, 128) = 1.396 | P<0.0001 F (5, 128) = 6.899 | P=0.8346 F (5, 128) = 0.4193 | P=0.6454 F (1, 128) = 0.2128 | P=0.9920 F (5, 128) = 0.0997 |
| 1B | 3-way ANOVA | P<0.0001 F (5, 114) = 26.17 | P<0.0001 F (1, 114) = 660.0 | P=0.3215 F (1, 114) = 0.9915 | P<0.0001 F (5, 114) = 25.12 | P=0.8115 F (5, 114) = 0.4514 | P=0.6814 F (1, 114) = 0.1694 | P=0.9832 F (5, 114) = 0.1378 |
| 1C | 3-way ANOVA | P<0.0001 F (5, 150) = 32.47 | P<0.0001 F (1, 150) = 760.9 | P=0.3430 F (1, 150) = 0.9049 | P<0.0001 F (5, 150) = 32.30 | P=0.5427 F (5, 150) = 0.8123 | P=0.0482 F (1, 150) = 3.969 | P=0.9567 F (5, 150) = 0.2128 |
| 1D | 3-way ANOVA | P<0.0001 F (2, 63) = 47.89 | P<0.0001 F (1, 63) = 141.7 | P=0.1877 F (1, 63) = 1.774 | P<0.0001 F (2, 63) = 37.59 | P=0.3863 F (2, 63) = 0.9657 | P=0.0685 F (1, 63) = 3.435 | P=0.5132 F (2, 63) = 0.6742 |
| 1E | 3-way ANOVA | P=0.0230 F (2, 63) = 4.006 | P=0.0032 F (1, 63) = 9.391 | P=0.3755 F (1, 63) = 0.7965 | P=0.1823 F (2, 63) = 1.749 | P=0.8358 F (2, 63) = 0.1799 | P=0.0400 F (1, 63) = 4.396 | P=0.4385 F (2, 63) = 0.8353 |
| 1F | 3-way ANOVA | P<0.0001 F (5, 102) = 204.5 | P<0.0001 F (1, 102) = 3150 | P=0.7577 F (1, 102) = 0.0957 | P<0.0001 F (5, 102) = 128.4 | P<0.0001 F (5, 102) = 6.342 | P=0.0951 F (1, 102) = 2.839 | P=0.9487 F (5, 102) = 0.2298 |
| 1G | 3-way ANOVA | P<0.0001 F (5, 120) = 51.45 | P<0.0001 F (1, 120) = 1047 | P=0.6073 F (1, 120) = 0.2655 | P<0.0001 F (5, 120) = 42.59 | P=0.9831 F (5, 120) = 0.1382 | P=0.8181 F (1, 120) = 0.05313 | P=0.2042 F (5, 120) = 1.471 |
| 1H | 3-way ANOVA | P<0.0001 F (5, 114) = 76.32 | P<0.0001 F (1, 114) = 1968 | P=0.4119 F (1, 114) = 0.6782 | P<0.0001 F (5, 114) = 73.17 | P=0.2347 F (5, 114) = 1.386 | P=0.4199 F (1, 114) = 0.6553 | P=0.0072 F (5, 114) = 3.364 |
| 1I | 3-way ANOVA | P<0.0001 F (2, 54) = 12.10 | P<0.0001 F (1, 54) = 51.47 | P=0.2140 F (1, 54) = 1.581 | P<0.0001 F (2, 54) = 12.84 | P=0.6565 F (2, 54) = 0.4241 | P=0.1970 F (1, 54) = 1.707 | P=0.4155 F (2, 54) = 0.8928 |
| 1J | 3-way ANOVA | P=0.0278 F (2, 54) = 3.832 | P=0.4521 F (1, 54) = 0.5737 | P=0.1621 F (1, 54) = 2.009 | P=0.8039 F (2, 54) = 0.2192 | P=0.2517 F (2, 54) = 1.415 | P=0.0653 F (1, 54) = 3.541 | P=0.3131 F (2, 54) = 1.187 |
| 2B | 3-way ANOVA | P<0.0001 F (4, 125) = 61.65 | P<0.0001 F (1, 125) = 120.6 | P<0.0001 F (1, 125) = 24.90 | P<0.0001 F (4, 125) = 8.641 | P=0.2807 F (4, 125) = 1.282 | P=0.0015 F (1, 125) = 10.51 | P=0.2616 F (4, 125) = 1.332 |
| 2C | 3-way ANOVA | P<0.0001 F (4, 125) = 54.07 | P=0.2528 F (1, 125) = 1.320 | P=0.7617 F (1, 125) = 0.09236 | P=0.7111 F (4, 125) = 0.5338 | P=0.9183 F (4, 125) = 0.2347 | P=0.4385 F (1, 125) = 0.6040 | P=0.7480 F (4, 125) = 0.4832 |
| 5A | 3-way ANOVA | P<0.0001 F (5, 108) = 286.1 | P<0.0001 F (1, 108) = 6869 | P<0.0001 F (1, 108) = 17.66 | P<0.0001 F (5, 108) = 277.2 | P=0.4474 F (5, 108) = 0.9573 | P=0.0013 F (1, 108) = 10.83 | P=0.0985 F (5, 108) = 1.910 |
| 5B | 3-way ANOVA | P<0.0001 F (5, 120) = 416.1 | P<0.0001 F (1, 120) = 10217 | P=0.0077 F (1, 120) = 7.352 | P<0.0001 F (5, 120) = 431.7 | P=0.1520 F (5, 120) = 1.650 | P=0.7880 F (1, 120) = 0.07264 | P=0.0001 F (5, 120) = 5.501 |
| Figure | Analysis | Test side | Pain | Drug | Side × Pain | Side × Drug | Pain × Drug | S × P × D |
| 2D | 3-way ANOVA | P=0.0401 F (1, 25) = 4.688 | P=0.4481 F (1, 25) = 0.5940 | P=0.2142 F (1, 25) = 1.624 | P=0.5761 F (1, 25) = 0.3209 | P=0.0267 F (1, 25) = 5.540 | P=0.0111 F (1, 25) = 7.520 | P=0.1953 F (1, 25) = 1.771 |
Morphine in the left CeA has no effect on tactile allodynia or mechanical hyperalgesia in SNL rats.
Similarly, separate cohorts of rats with unilateral cannulas in the left CeA developed mechanical allodynia at 2–14 days following left spinal nerve ligation (Figure 1F-H; three-way ANOVA, Table 1), as well as mechanical hyperalgesia observed in the injured (left) hind paw at day 14 (Figure 1I; three-way ANOVA, Table 1). Morphine microinjection into the left CeA had no effect on hind paw withdrawal thresholds in either the von Frey or Randall-Selitto tests (Figure 1F-J). Thus, MOR signaling in the left CeA does not directly impact mechanical pain withdrawal responses.
SNL attenuates the DNIC response in the ipsilateral hind paw.
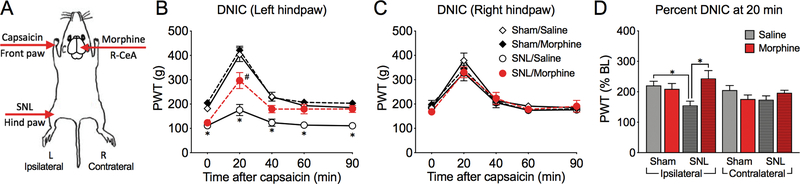
Prior work in our laboratory has shown ipsilateral-specific loss of the DNIC response in SNL rats [47] demonstrating dysfunction of dynamic descending pain modulation during application of a remote conditioning pain stimulus. We therefore investigated whether CeA morphine may restore functional engagement of the descending pain pathways during the DNIC response. Randall-Selitto paw pressure threshold measurements on the right or left hind paws were used as the test stimuli and intradermal application of capsaicin into the left forepaw was used as the second (conditioning) stimulus. The schematics of the experimental design is depicted in Figure 2A. First, we evaluated the effect of nerve injury on the effectiveness of the DNIC response in rats receiving saline in the right CeA. In sham rats, capsaicin significantly increased withdrawal thresholds of both the left and right hind paws, demonstrating an effective DNIC analgesic response at 20 min post-capsaicin (Figure 2B,C; three-way ANOVA; Tukey’s post-test, Table 1). However, compared to shams, the DNIC response in the left (injured) hind paw was significantly diminished in SNL rats (Figure 2B; Table 1). No significant reduction in the right (uninjured) hind paw DNIC response was observed in SNL, compared to sham rats receiving R-CeA saline (Figure 2C; Table 1). These results confirm predominantly site-specific (ipsilateral) attenuation of the DNIC effect in the injured hind paw of R-CeA-saline treated SNL rats.
Figure 2. Loss of the DNIC response observed selectively in the ipsilateral hind paw is restored by morphine microinjection into the right CeA.
(A) A schematic depicting the sides of SNL surgery, conditioning stimulus with capsaicin and the side of CeA injections. (B) Time-course of DNIC experiment in the ipsilateral hind paw. A significant loss of DNIC was seen at 20 minutes post-capsaicin in SNL rats pretreated with saline in the right CeA, but not in SNL/Morphine treated rats. (C) Time-course of DNIC in the contralateral hind paw of right CeA-pretreated rats. (D) DNIC responses at 20 min post capsaicin from Figs B and C are plotted as percent increase from BL. Data display means ± SEM, n= 4–10 rats/group, *p<0.05 (sham/saline vs. SNL/saline, three-way ANOVA with Tukey’s post-test); #p<0.05 (SNL/saline vs. SNL/morphine, three-way ANOVA with Tukey’s post-test).
Morphine microinjection into the right CeA restores the DNIC response in SNL rats.
Next, we evaluated the effects of morphine microinjection in the right CeA on the DNIC response. There was no effect of R-CeA morphine on the DNIC response in either paw in sham-operated rats. In SNL rats, R-CeA morphine had no significant effect on DNIC measured in the right hind paw (Figure 2C). However, in the left (injured) hind paw, SNL rats pre-treated with morphine into the right CeA showed a significantly larger DNIC response at 20 min post capsaicin than the saline-pretreated group (Figure 2B, Table 1). To further compare the effects of pain and drug treatment on ipsilateral (left) and contralateral (right) DNIC amongst all groups, we plotted the percent PWT increase between baseline and 20 min post capsaicin [(PWT at 20)/(PWT at 0)*100%] for all experimental groups in a bar graph (Figure 2D). Three-way ANOVA confirmed a significant effect of the paw side tested, as well as a significant effect of the interaction between the drug and pain condition and the drug and the ipsilateral or contralateral paw (Table 1). Tukey’s post-test shows significant loss of DNIC in the ipsilateral paw of R-CeA saline-treated SNL rats and DNIC restoration in R-CeA morphine treated SNL rats (Figure 2D). These results suggest that right CeA morphine reinstates normal function of descending pain modulation during DNIC in SNL rats.
Morphine microinjection into the right, but not left, CeA produces CPP in SNL rats.
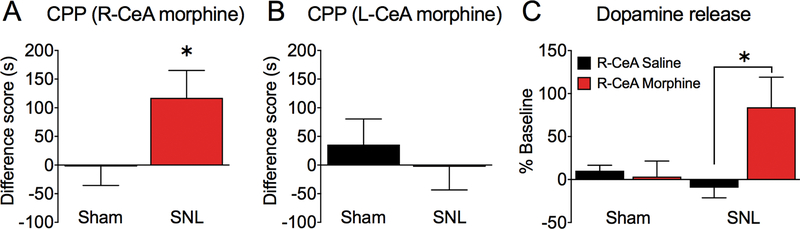
We used the conditioned place preference (CPP) procedure to assess whether activation of opioid receptors in the CeA can relieve the aversive aspects of ongoing neuropathic pain. In sham rats, morphine administration into either the right or left CeA did not produce CPP (Figure 3A,B), demonstrating that in uninjured rats these sites do not mediate the rewarding effects of opioids. However, SNL rats showed significant place preference for the chamber paired with microinjection of morphine in the right CeA (Figure 3A; one sample t-test; Table 2). SNL rats did not develop CPP to morphine administration in the left CeA (Figure 3B; one sample t-test; Table 2).
Figure 3. Morphine microinjection into the right CeA produces CPP and dopamine release in the NAc of SNL rats.
(A) SNL, but not sham-operated, rats showed conditioned place preference for the chamber paired with microinjection of morphine into the right CeA (n= 14–19; *p=0.0248; one sample t-test). (B) Neither sham, nor SNL rats developed CPP to morphine administration in the left CeA (n= 14). (C) Microdialysis evaluation of dopamine levels in the NAc revealed increased DA concentration compared to baseline in SNL, but not sham, rats following morphine administration into the right CeA (n= 6–11; *p=0.0418; one-way ANOVA with Tukey’s multiple comparison test). Data display means ± SEM.
Table 2. Summary of statistical analyses.
P values, t and F ratios and degrees of freedom (df) for statistical data analyses used in Figures 3–5.
| Figure | Analysis | Sham | SNL | Sham × SNL |
|---|---|---|---|---|
| 3A | t-test | P=0.9406 t=0.07593 df=13 | P=0.0248 t=2.448 df=18 | P=0.0656 t=1.909 df=31 |
| 3B | t-test | P=0.4394 t=0.7977 df=13 | P=0.9411 t=0.07537 df=13 | P=0.5261 t=0.6426, df=26 |
| 3C | 1-way ANOVA | P=0.0367 F (3, 29) = 3.232 | ||
| 5C | t-test | P=0.8044 t=0.256 df=8 | P=0.6947 t=0.4007 df=14 | P=0.6613 t=0.4442, df=22 |
| Figure | Analysis | Saline | bFNA | Saline × bFNA |
| 4A | t-test | P=0.0267 t=2.557 df=11 | P=0.4102 t=0.8595 df=10 | P=0.0212 t=2.49 df=21 |
| 4B | t-test | P=0.0129 t=2.685 df=24 |
Morphine microinjection into the right CeA elicits NAc dopamine release in SNL rats.
Motivated behavior to pain relief has been shown to depend on dopamine (DA) release in the nucleus accumbens [36]. To investigate if CPP observed in SNL rats following morphine administration in the right CeA involves activation of dopaminergic neurons, we measured dopamine levels in the NAc using in vivo microdialysis and high-performance liquid chromatography (HPLC) quantification in separate cohorts of SNL and sham rats. Compared to baseline, DA concentrations increased in SNL rats following morphine administration into the right CeA. In contrast, no change was observed after CeA morphine administration in sham rats or after administration of saline in either group (Figure 3C; one-way ANOVA, Table 2). Tukey’s multiple comparison test shows significant difference between SNL saline and SNL morphine groups (p = 0.0418).
Anti-aversive effects mediated by right CeA morphine are dependent on endogenous opioid activity in the rACC.
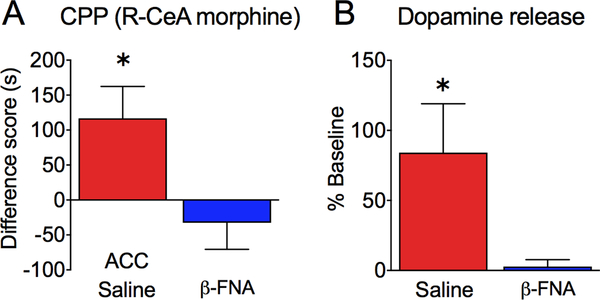
Since pain relief-motivated behavior also depends on endogenous opioid activity in the rACC, we investigated if the effects of morphine administration in the right CeA on CPP and NAc dopamine release would be prevented by blocking endogenous opioid signaling in the ACC with a long-lasting mu opioid antagonist, β-FNA. We implanted SNL rats with bilateral cannula in the rACC and a single cannula in the right CeA. Rats were pre-treated with either saline or β-FNA bilaterally into the rACC and the next day all rats were conditioned in the CPP boxes with R-CeA saline injection in one chamber and R-CeA morphine injection in the opposite chamber, as in the previous experiment. In SNL rats that were pre-treated with saline into the rACC, we observed significant CPP to R-CeA morphine, similar to rats without any ACC manipulations (Figure 4A; one sample t-test; Table 2). However, no CPP was observed following R-CeA morphine in SNL rats pretreated with ACC β-FNA. The difference between saline-pretreated and β-FNA-pretreated rats was statistically significant (Figure 4A; unpaired t-test; Table 2). Consistent with the CPP results, in vivo microdialysis revealed increased NAc dopamine levels following R-CeA morphine administration in rACC saline-pretreated, but not β-FNA-pretreated, SNL rats. The difference between the two groups was statistically significant (Figure 4B; unpaired t-test; Table 2). Thus, in SNL rats, R-CeA morphine-induced CPP and NAc dopamine release were both prevented by rACC β-FNA.
Figure 4. The effects of right CeA morphine on CPP and dopamine release are blocked by rACC β-funaltrexamine.
(A) In SNL rats, CPP to morphine infused into the right CeA is observed only in rats that were pre-treated with saline, but not β-FNA, in the rACC (n= 12; *p=0.0267; one sample t-test). (B) Microdialysis shows that NAc dopamine levels are increased following morphine administration into the right CeA in SNL rats pre-treated with saline, but not β-FNA, in the rACC (n= 11–15; *p=0.0129; unpaired t-test). Data are shown as means ± SEM.
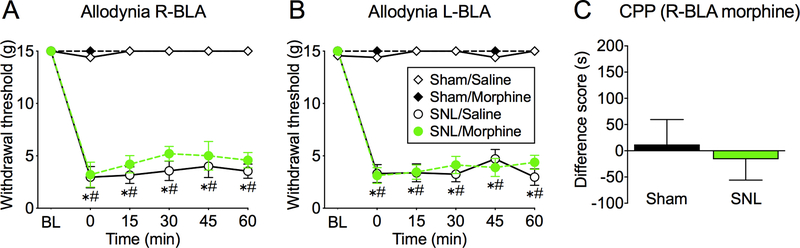
Morphine in the right BLA is not anti-allodynic and does not elicit CPP in SNL rats.
To determine if the observed anti-aversive effects of right CeA morphine are specific to this amygdala sub-region, we investigated if microinjection of morphine into the neighboring basolateral amygdala would modulate sensory or affective pain responses. Administration of morphine into the right or left BLA 14 days post SNL or sham surgery did not affect mechanical withdrawal thresholds (Figure 5A,B; three-way ANOVA, Table 1). Right BLA morphine administration also did not elicit CPP in sham or SNL rats (Figure 5C). Thus, in the amygdala, the anti-aversive effects of morphine appear to be localized to the right (contralateral) CeA.
Figure 5. Morphine microinjection into the right BLA has no effect on tactile allodynia and does not produce CPP in SNL rats.
(A, B) At 14 days after surgery, SNL, but not sham-operated, rats developed tactile allodynia, demonstrated by significantly reduced hind paw withdrawal thresholds from pre-surgery baselines (BL). Time courses of hind paw withdrawal thresholds following morphine microinjection into the right (A) or left (B) BLA show no effect of morphine (n= 5–6 rats/group; *p<0.05; three-way ANOVA with Tukey’s post-hoc test; * represents a significant difference from BL and # represents a significant difference from sham rats at corresponding times). (C) Sham and SNL rats show no preference for right BLA morphine paired chamber (n= 9–15). Data display means ± SEM.
Discussion
Opioids preferentially modulate aversive qualities of pain within corticolimbic circuits, with preservation of physiological nociceptive inputs allowing safety from self-harm in patients [1; 18; 52]. We have shown that engagement of MORs within the rat rACC modulated affective qualities of ongoing pain without altering thresholds to evoked stimuli [14; 38]. Supraspinal MOR-rich regions are also implicated in the modulation of sensory thresholds during CPM [30; 31]. Engagement of top-down circuits to modulate nociception is important for action selection in pain-related behavioral responses [50]. CPM/DNIC is a bottom-up phenomenon involving a spino-bulbo-spinal pathway that is influenced by poorly understood inputs from cortical and subcortical areas including the ACC and the amygdala [30; 31]. CPM/DNIC allows prioritization of some nociceptive inputs that may be critical for learning while suppressing others [42].
We determined if MOR activation in two amygdala subnuclei would (a) preferentially modulate pain aversiveness; (b) require functional relationships with the rACC for the modulation of ongoing pain, and (c) influence the DNIC response. Using the rat SNL model of neuropathic pain, we demonstrate that 1) morphine in the left or right CeA does not modulate mechanical allodynia or hyperalgesia; 2) right (contralateral to injury) CeA morphine restores the loss of DNIC on the injured (left) side; 3) morphine in the right CeA selectively modulates affective components of pain; 4) the anti-aversive effects of right CeA morphine depend on endogenous opioid signaling in the rACC; and 5) BLA morphine did not alter affective measures of pain or reflexive thresholds.
The CeA has been reported to modulate the affective qualities of acute or chronic pain in several pain models [21; 22; 46; 58]. How the amygdala participates in reflexive responses and in descending pain circuits is less clear, with reports of both inhibition and lack of effects on reflexive behaviors [13; 45; 53; 58]. While electrophysiological in vivo recordings demonstrate that CeA neurons exhibit an increased response with increasing intensity of the noxious stimulation, the receptive fields of these neurons are large and often bilateral, suggesting that the CeA does not play a major role in the sensory-discriminative aspects of pain [15; 20].
Opioid receptors are expressed in the amygdala and electrophysiological studies suggest multiple possible sites of action of opioid agonists within the CeA. MOR agonists can regulate glutamate release from presynaptic terminals in the CeA [59], or hyperpolarize intercalated cells (ITCs) located between the BLA and the CeA thereby attenuating GABA-ergic transmission to CeA cells [5]. Furthermore, MOR agonists were found to directly inhibit neurons in the medial CeA, including neurons projecting to the parabrachial area [8]. However, bilateral opioid microinjection into the CeA had no effect on tail flick latency or on the discharge of pain modulatory cells in the rostral ventromedial medulla (RVM) [28]. Consistent with these observations, we did not observe effects on tactile allodynia in awake neuropathic rats following right or left CeA morphine at three different post-injury timepoints. Additionally, right or left CeA morphine at 14 days post-injury had no effect on suprathreshold paw pressure withdrawal responses in sham-operated or SNL rats. These results are consistent with the lack of effect of a MOR agonist on thermal responses in inflammatory pain in rats [58]. These results suggest that activation of MORs in the CeA does not directly modulate mechanical withdrawal responses.
Previous studies have demonstrated that bilateral microinjection of morphine into the BLA caused pain inhibitory “OFF” cells in the RVM to become tonically active while inhibiting tail-flick responses and corresponding “ON” cell discharges in anesthetized uninjured rats [27]. However, our results in awake rats showed that right or left BLA morphine at 14 days post SNL did not alter responses to stimulation with von Frey filaments. The reasons for these differences are not clear but could include time-dependent plasticity within the amygdala circuits that result from ongoing pain and possible influence of anesthesia [15]. BLA morphine failed to reduce mechanical hypersensitivity in an inflammatory pain model, but reversed anxiety-like behaviors and cognitive impairments [17]. The effects of MOR activation in the BLA on modulation of sensory aspects of ongoing pain may therefore be state dependent.
However, amygdala activity has been shown to correlate with nociceptive thresholds during the CPM response. In a recent electroencephalogram (EEG)-based standardized low-resolution brain electromagnetic tomography (sLORETA) study in healthy subjects, increased amygdala activation corresponded to increased heat thresholds during the CPM effect [30]. Many pain conditions are characterized by ineffective CPM responses and this has been replicated in SNL rats as a loss of DNIC [2; 47]. Here, we demonstrate that loss of DNIC in rats with left sided nerve injury is restored by MOR activation in the right CeA. Thus, right (in this case contralateral) CeA opioid signaling may be involved in descending pain modulation under conditions when a concurrent noxious stimulus demands prioritization over a noxious test stimulus.
We used the conditioned place preference procedure, in which animals learn to associate a context with a treatment producing effective pain relief [37], to determine the effects of MOR activation in the CeA on the affective qualities of ongoing pain. A previous study has shown that CeA DAMGO inhibited conditioned place aversion (CPA) in a rat model of inflammatory pain without altering heat-evoked paw withdrawal latencies [58]. We found that right CeA morphine produced CPP in SNL, but not sham, rats, suggesting modulation of aversive qualities of neuropathic pain by opioid circuits in the right CeA. Morphine in the left CeA did not produce CPP in either injured or uninjured rats. Additionally, right BLA morphine did not induce CPP in either sham or SNL rats. We have previously demonstrated that relief of ongoing pain produces negative reinforcement, assessed by CPP, and is associated with the activation of mesolimbic dopamine reward circuits [36]. Complementary to our current CPP observations, R-CeA morphine increased NAc dopamine levels in SNL, but not sham, rats, while R-CeA saline had no effect. These data demonstrate that the anti-aversive effects of amygdalar morphine can be elicited and are lateralized to the right CeA, at least with contralateral injury.
MOR activation within the ACC is known to modulate the aversive aspects of pain [60]. The critical role of opioids in this region is supported by human neuroimaging and by preclinical studies. Notably, functional connectivity between the amygdala and the rACC correlates with placebo analgesia [3]. To investigate this functional interaction and possible interdependency of MOR circuits in the CeA and the rACC, we blocked endogenous opioid signaling in the rACC with bilateral injections of β-FNA, a selective MOR antagonist, and investigated the effects on right CeA morphine-induced CPP and NAc dopamine release. We found that while SNL rats pretreated with rACC saline demonstrated CPP and increased NAc dopamine levels following right CeA morphine, rACC β-FNA, administered prior to right CeA morphine, prevented both the development of CPP and increased NAc dopamine release. These findings demonstrate that the anti-aversive effects mediated by MOR activation in the right CeA are dependent on endogenous opioid release in the rACC, and are consistent with our previous observations that rACC opioid circuits are necessary for pain relief elicited by systemic administration of opioid or non-opioid analgesics [38]. Therefore, activation of subcortical opioid receptors in the CeA promotes pain relief through the release of endogenous opioids within the rACC.
The lateralization of pain modulation to the right, but not left, CeA has been reported in previous studies, including pERK expression following inflammatory injury [7], neuronal activity following knee inflammation [20], increased neuronal discharge after nerve injury [15], and visceromotor responses from urinary bladder distension [48]. The pain-related effects were localized to the right CeA regardless of which hindlimb was injured [7; 15]. A predominant pronociceptive role of the right CeA is also supported by human imaging studies [56]. Other studies, however, have reported that the side of injury and modality of pain evaluated may influence outcomes [9]. Our studies therefore focused on the role of the right CeA in animals with left-sided spinal nerve ligation. Whether right CeA opioids also modulate affective aspects of pain and the DNIC response in animals with right-sided injuries requires further investigation. While the current study focused on the consequences of MOR activation in pain modulation from the amygdala, other opioid receptors may also participate. In this regard, we previously reported lateralized pronociceptive effects of kappa opioid receptor (KOR) signaling in the right, but not left, CeA in models of stress-related functional pain [32; 57] and have recently demonstrated that blockade of KOR in the right CeA inhibited the aversive qualities of SNL-related neuropathic pain [34]. These findings highlight opposing roles of KOR and MOR activation within the right CeA that, respectively, promote and relieve affective pain responses. The functional organization of MOR and KOR-expressing cells in the CeA may therefore resemble the ON and OFF cells within the RVM [12] and may represent a general principle of pain modulation in the brain. These outcomes may guide therapeutic approaches that could utilize KOR antagonist and MOR agonist strategies for treatment of stress-related pain conditions.
Supplementary Material
Acknowledgements:
This work was supported by DA041809 (FP, EN), NS106902 (VN, FP) and NS038261 (VN) from the National Institutes of Health.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- [1].Ballantyne JC, Sullivan MD. Discovery of endogenous opioid systems: what it has meant for the clinician’s understanding of pain and its treatment. Pain 2017;158(12):2290–2300. [DOI] [PubMed] [Google Scholar]
- [2].Bannister K, Patel R, Goncalves L, Townson L, Dickenson AH. Diffuse noxious inhibitory controls and nerve injury: restoring an imbalance between descending monoamine inhibitions and facilitations. Pain 2015;156(9):1803–1811. [DOI] [PubMed] [Google Scholar]
- [3].Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain 2006;120(1–2):8–15. [DOI] [PubMed] [Google Scholar]
- [4].Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Science translational medicine 2011;3(70):70ra14. [DOI] [PubMed] [Google Scholar]
- [5].Blaesse P, Goedecke L, Bazelot M, Capogna M, Pape HC, Jungling K. mu-Opioid Receptor-Mediated Inhibition of Intercalated Neurons and Effect on Synaptic Transmission to the Central Amygdala. J Neurosci 2015;35(19):7317–7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Butler RK, Ehling S, Barbar M, Thomas J, Hughes MA, Smith CE, Pogorelov VM, Aryal DK, Wetsel WC, Lascelles BDX. Distinct neuronal populations in the basolateral and central amygdala are activated with acute pain, conditioned fear, and fear-conditioned analgesia. Neurosci Lett 2017;661:11–17. [DOI] [PubMed] [Google Scholar]
- [7].Carrasquillo Y, Gereau RWt Hemispheric lateralization of a molecular signal for pain modulation in the amygdala. Mol Pain 2008;4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chieng BC, Christie MJ, Osborne PB. Characterization of neurons in the rat central nucleus of the amygdala: cellular physiology, morphology, and opioid sensitivity. J Comp Neurol 2006;497(6):910–927. [DOI] [PubMed] [Google Scholar]
- [9].Cooper AH, Brightwell JJ, Hedden NS, Taylor BK. The left central nucleus of the amygdala contributes to mechanical allodynia and hyperalgesia following right-sided peripheral nerve injury. Neurosci Lett 2018;684:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 1980;20:441–462. [DOI] [PubMed] [Google Scholar]
- [11].Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch JL, Koch M, Kessler P, Hentsch D, Birling MC, Koutsourakis M, Vasseur L, Veinante P, Kieffer BL, Massotte D. A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct 2015;220(2):677–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fields H. State-dependent opioid control of pain. Nature reviews Neuroscience 2004;5(7):565–575. [DOI] [PubMed] [Google Scholar]
- [13].Gao YJ, Ren WH, Zhang YQ, Zhao ZQ. Contributions of the anterior cingulate cortex and amygdala to pain- and fear-conditioned place avoidance in rats. Pain 2004;110(1–2):343–353. [DOI] [PubMed] [Google Scholar]
- [14].Gomtsian L, Bannister K, Eyde N, Robles D, Dickenson AH, Porreca F, Navratilova E. Morphine effects within the rodent anterior cingulate cortex and rostral ventromedial medulla reveal separable modulation of affective and sensory qualities of acute or chronic pain. Pain 2018;159(12):2512–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Goncalves L, Dickenson AH. Asymmetric time-dependent activation of right central amygdala neurones in rats with peripheral neuropathy and pregabalin modulation. Eur J Neurosci 2012;36(9):3204–3213. [DOI] [PubMed] [Google Scholar]
- [16].Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides 1992;13(3):451–460. [DOI] [PubMed] [Google Scholar]
- [17].Gregoire S, Wattiez AS, Etienne M, Marchand F, Ardid D. Monoarthritis-induced emotional and cognitive impairments in rats are sensitive to low systemic doses or intra-amygdala injections of morphine. Eur J Pharmacol 2014;735:1–9. [DOI] [PubMed] [Google Scholar]
- [18].Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. The Journal of neuroscience : the official journal of the Society for Neuroscience 2007;27(37):10000–10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hubbard KE, Wells A, Owens TS, Tagen M, Fraga CH, Stewart CF. Determination of dopamine, serotonin, and their metabolites in pediatric cerebrospinal fluid by isocratic high performance liquid chromatography coupled with electrochemical detection. Biomedical chromatography : BMC 2010;24(6):626–631. [DOI] [PubMed] [Google Scholar]
- [20].Ji G, Neugebauer V. Hemispheric lateralization of pain processing by amygdala neurons. J Neurophysiol 2009;102(4):2253–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ji G, Neugebauer V. CB1 augments mGluR5 function in medial prefrontal cortical neurons to inhibit amygdala hyperactivity in an arthritis pain model. Eur J Neurosci 2014;39(3):455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jiang H, Fang D, Kong LY, Jin ZR, Cai J, Kang XJ, Wan Y, Xing GG. Sensitization of neurons in the central nucleus of the amygdala via the decreased GABAergic inhibition contributes to the development of neuropathic pain-related anxiety-like behaviors in rats. Mol Brain 2014;7:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nature neuroscience 2004;7(4):398–403. [DOI] [PubMed] [Google Scholar]
- [24].Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992;50(3):355–363. [DOI] [PubMed] [Google Scholar]
- [25].King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nature neuroscience 2009;12(11):1364–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kuo CC, Yen CT. Comparison of anterior cingulate and primary somatosensory neuronal responses to noxious laser-heat stimuli in conscious, behaving rats. Journal of neurophysiology 2005;94(3):1825–1836. [DOI] [PubMed] [Google Scholar]
- [27].McGaraughty S, Farr DA, Heinricher MM. Lesions of the periaqueductal gray disrupt input to the rostral ventromedial medulla following microinjections of morphine into the medial or basolateral nuclei of the amygdala. Brain Res 2004;1009(1–2):223–227. [DOI] [PubMed] [Google Scholar]
- [28].McGaraughty S, Heinricher MM. Microinjection of morphine into various amygdaloid nuclei differentially affects nociceptive responsiveness and RVM neuronal activity. Pain 2002;96(1–2):153–162. [DOI] [PubMed] [Google Scholar]
- [29].Mitchell JM, Margolis EB, Coker AR, Allen DC, Fields HL. Intra-VTA deltorphin, but not DPDPE, induces place preference in ethanol-drinking rats: distinct DOR-1 and DOR-2 mechanisms control ethanol consumption and reward. Alcoholism, clinical and experimental research 2014;38(1):195–203. [DOI] [PubMed] [Google Scholar]
- [30].Moont R, Crispel Y, Lev R, Pud D, Yarnitsky D. Temporal changes in cortical activation during conditioned pain modulation (CPM), a LORETA study. Pain 2011;152(7):1469–1477. [DOI] [PubMed] [Google Scholar]
- [31].Moont R, Crispel Y, Lev R, Pud D, Yarnitsky D. Temporal changes in cortical activation during distraction from pain: a comparative LORETA study with conditioned pain modulation. Brain research 2012;1435:105–117. [DOI] [PubMed] [Google Scholar]
- [32].Nation KM, DeFelice M, Hernandez PI, Dodick DW, Neugebauer V, Navratilova E, Porreca F. Lateralized Kappa Opioid Receptor Signaling from the Amygdala Central Nucleus Promotes Stress-Induced Functional Pain. Pain 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nation KM, Dodick DW, Navratilova E, Porreca F. Sustained exposure to acute migraine medications combined with repeated noxious stimulation dysregulates descending pain modulatory circuits: Relevance to medication overuse headache. Cephalalgia 2018:333102418804157. [DOI] [PubMed] [Google Scholar]
- [34].Navratilova E, Ji G, Phelps C, Qu C, Hein M, Yakhnitsa V, Neugebauer V, Porreca F. Kappa opioid signaling in the central nucleus of the amygdala promotes disinhibition and aversiveness of chronic neuropathic pain. Pain 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Navratilova E, Xie J, King T, Porreca F. Evaluation of reward from pain relief. Annals of the New York Academy of Sciences 2013;1282:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Navratilova E, Xie J, Okun A, Qu C, Eyde N, Ci S, Ossipov M, King T, Fields H, Porreca F. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A 2012;109(50):20709–20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Navratilova E, Xie JY, King T, Porreca F. Evaluation of reward from pain relief. Annals of the New York Academy of Sciences 2013;1282:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A, Arakawa N, Ossipov M, Fields HL, Porreca F. Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. J Neurosci 2015;35(18):7264–7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A, Arakawa N, Ossipov M, Fields HL, Porreca F. Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. The Journal of neuroscience : the official journal of the Society for Neuroscience 2015;35(18):7264–7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Neugebauer V. Amygdala pain mechanisms. Handb Exp Pharmacol 2015;227:261–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist 2004;10(3):221–234. [DOI] [PubMed] [Google Scholar]
- [42].Nir RR, Yarnitsky D. Conditioned pain modulation. Current opinion in supportive and palliative care 2015;9(2):131–137. [DOI] [PubMed] [Google Scholar]
- [43].Oertel BG, Preibisch C, Wallenhorst T, Hummel T, Geisslinger G, Lanfermann H, Lotsch J. Differential opioid action on sensory and affective cerebral pain processing. Clinical pharmacology and therapeutics 2008;83(4):577–588. [DOI] [PubMed] [Google Scholar]
- [44].Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 2010;90(2):419–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pavlovic ZW, Cooper ML, Bodnar RJ. Opioid antagonists in the periaqueductal gray inhibit morphine and beta-endorphin analgesia elicited from the amygdala of rats. Brain Res 1996;741(1–2):13–26. [DOI] [PubMed] [Google Scholar]
- [46].Pedersen LH, Scheel-Kruger J, Blackburn-Munro G. Amygdala GABA-A receptor involvement in mediating sensory-discriminative and affective-motivational pain responses in a rat model of peripheral nerve injury. Pain 2007;127(1–2):17–26. [DOI] [PubMed] [Google Scholar]
- [47].Phelps CE, Navratilova E, Dickenson AH, Porreca F, Bannister K. Kappa Opioid Signaling in the Right Central Amygdala Causes Hindpaw Specific Loss of Diffuse Noxious Inhibitory Controls (DNIC) in Experimental Neuropathic Pain. Pain 2019. [DOI] [PubMed] [Google Scholar]
- [48].Sadler KE, McQuaid NA, Cox AC, Behun MN, Trouten AM, Kolber BJ. Divergent functions of the left and right central amygdala in visceral nociception. Pain 2017;158(4):747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev 2003;83(3):803–834. [DOI] [PubMed] [Google Scholar]
- [50].Schwartz N, Miller C, Fields HL. Cortico-Accumbens Regulation of Approach-Avoidance Behavior Is Modified by Experience and Chronic Pain. Cell Rep 2017;19(8):1522–1531. [DOI] [PubMed] [Google Scholar]
- [51].Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron 2007;55(2):325–336. [DOI] [PubMed] [Google Scholar]
- [52].Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Archives of general psychiatry 2008;65(2):220–231. [DOI] [PubMed] [Google Scholar]
- [53].Tanimoto S, Nakagawa T, Yamauchi Y, Minami M, Satoh M. Differential contributions of the basolateral and central nuclei of the amygdala in the negative affective component of chemical somatic and visceral pains in rats. Eur J Neurosci 2003;18(8):2343–2350. [DOI] [PubMed] [Google Scholar]
- [54].Thompson JM, Neugebauer V. Amygdala Plasticity and Pain. Pain Res Manag 2017;2017:8296501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proceedings of the National Academy of Sciences of the United States of America 2007;104(26):11056–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Woo CW, Schmidt L, Krishnan A, Jepma M, Roy M, Lindquist MA, Atlas LY, Wager TD. Quantifying cerebral contributions to pain beyond nociception. Nat Commun 2017;8:14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Xie JY, De Felice M, Kopruszinski CM, Eyde N, LaVigne J, Remeniuk B, Hernandez P, Yue X, Goshima N, Ossipov M, King T, Streicher JM, Navratilova E, Dodick D, Rosen H, Roberts E, Porreca F. Kappa opioid receptor antagonists: A possible new class of therapeutics for migraine prevention. Cephalalgia 2017;37(8):780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhang RX, Zhang M, Li A, Pan L, Berman BM, Ren K, Lao L. DAMGO in the central amygdala alleviates the affective dimension of pain in a rat model of inflammatory hyperalgesia. Neuroscience 2013;252:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhu W, Pan ZZ. Mu-opioid-mediated inhibition of glutamate synaptic transmission in rat central amygdala neurons. Neuroscience 2005;133(1):97–103. [DOI] [PubMed] [Google Scholar]
- [60].Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 2001;293(5528):311–315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.