Abstract
Case series
Patients: Female, 68-year-old • Female, 60-year-old • Male, 36-year-old • Male, 49-year-old • Male, 3-year-old
Final Diagnosis: Fungal endophthalmitis
Symptoms: Blurred vision • fatty keratic precipitates • floater cells • hypopyon
Medication: —
Clinical Procedure: An intravitreal injection of 10 μg of amphotericin B
Specialty: Infectious Diseases
Objective:
Challenging differential diagnosis
Background:
In clinical practice, the presentation of fungal endophthalmitis is often occult and confusing, so it is difficult to make an early diagnosis. The aim of this study was to evaluate the utility of β-d-glucan (BDG) testing in diagnosis, management, and prognosis of fungal endophthalmitis.
Case Reports:
We present a retrospective, observational case series of 5 fungal endophthalmitis cases, 3 of which were endogenous and 2 exogenous. There were significantly elevated BDG levels in all cases, which was consistent with the pathological diagnosis. Four cases were diagnosed as fungal endophthalmitis through smear or culture and gene chip analysis of intraocular fluid.
Conclusions:
Fungal endophthalmitis is rare, and its diagnosis is difficult because of its occult nature. Therefore, BDG testing may be required as an auxiliary examination for the early diagnosis of fungal endophthalmitis. Compared to cultures and smears, intraocular fluid BDG testing has a higher sensitivity for detecting fungal endophthalmitis.
MeSH Keywords: Aqueous Humor, Endophthalmitis, Intravitreal Injections
Background
Fungal endophthalmitis is a relatively rare, sight-threatening disease, which is divided into exogenous and endogenous infections. Exogenous endophthalmitis is often the result of direct inoculation of a pathogen during intraocular surgery or from a penetrating trauma [1]. Generally, the risk factors for endogenous fungal endophthalmitis (EFE) include systemic diseases (e.g., diabetes, liver cirrhosis, neutropenia, malignant tumors, and acquired immunodeficiency syndrome), solid-organ or stem cell transplantations, catheter-related factors (e.g., intravenous hyperalimentation), and/or the presence of an infectious lesion (e.g., liver abscesses, meningitis, and lung abscesses) [2].
Although blood cultures, tissue cultures, and histological testing are the criterion standards for diagnosing systemic invasive fungal infections, up to 13% of all cases of ocular candidiasis may be missed during the initial evaluation [3]. Moreover, fungal organisms do not always grow in blood cultures, and this tissue is difficult to obtain safely for pathological testing and culturing. Therefore, there are quite a few challenges in the diagnosis of fungal endophthalmitis.
β-D-glucan (BDG) is a polysaccharide cell wall component found in many fungal species, and it is released into the bloodstream during an invasive fungal infection [4]. Many researchers have reported that serum BDG testing has a higher sensitivity for detecting an invasive fungal infection compared to a blood culture [5], and it can identify such an infection prior to the appearance of clinical symptoms and prior to obtaining radiological findings and positive culture results [6]. Although serum BDG testing has been used primarily in immunocom-promised individuals who are at a high risk for invasive fungal infections, it can help monitor clinical responses to systemic antifungal therapy [7]. However, BDG testing has seldom been mentioned in the ophthalmological literature [8–11], and it may be clinically underused in suspected fungal endophthalmitis cases. In this series of case reports, we discuss the utility of BDG testing and its potential future applications in the field of ophthalmology. We performed β-D-glucan quantification using a fungal (1–3)-β-D-Glucan assay kit (Zhanjiang A&C Biological, China) and LKM Kinetic Tube Reader equipment (Labkinetics LLC, USA) by spectrophotometry.
Case Report
Case 1
A 68-year-old woman presented with photophobia and blurred vision 4 months after undergoing cataract surgery in her right eye. She appeared to be healthy, without a history of immuno-suppression, autoimmune disease, or animal exposure. Upon presentation, her visual acuities and intraocular pressures (IOPs) were finger counting/20 cm and 18 mmHg for the right eye and 100/200 and 15 mmHg for the left eye. Fatty keratic precipitates (KPs), floater cells in the anterior chamber (Figure 1A, 1B), and severe vitreous opacity were observed in the right eye. She was initially treated using a subconjunctival injection of 4 mg of triamcinolone acetonide and prednisolone acetate eye drops 6 times daily. After 1 week, the clinical symptoms had worsened, so a pars plana vitrectomy (PPV) was performed and a vitreous specimen was obtained. The results of the vitreous examination showed that the inflammatory cytokine concentrations had increased remarkably, as follows: vascular endothelial growth factor (VEGF) <1 pg/ml (normal range: <40.0 pg/ml), transforming growth factor (TGF) 561 pg/ml (normal range: <1.0 pg/ml), interleukin-6 (IL-6) 1745.71 pg/ml (normal range: <50.0 pg/ml), IL-10 14.21 pg/ml (normal range: <5.0 pg/ml), vascular cell adhesion protein (VCAM) was 11 316.31 pg/ml (normal range: 200∼1000 pg/ml), and IL-8 was 4676.81 pg/ml (normal range: <20.0 pg/ml).
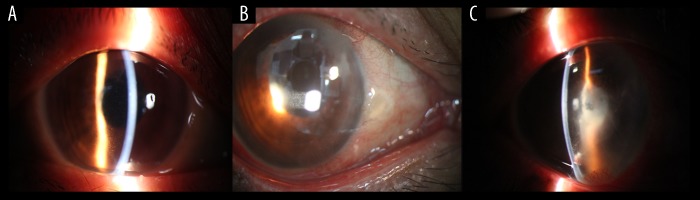
Figure 1.
Case 1: Preoperative anterior chamber findings, including KP, flare, and fibrinoid exudation. (A, B) Keratic precipitates and cells in the anterior chamber. (C) After 4 months, the symptoms recurred, and the visual acuity decreased to light perception. There were 3+ cells and a flare in the anterior chamber, as well as fibrinous exudation without hypopyon.
One month later, the patient’s vision had increased to 25/200. However, after 4 months, the symptoms recurred and her visual acuity decreased to light perception. There were 3+ cells and a flare in the anterior chamber, along with excessive fibrinous exudation without hypopyon in the right eye (Figure 1C). Another 23-gauge PPV was performed, and the IOL was taken out. In addition, a vitreous specimen was sent for a microculture, and the aqueous humor underwent BDG testing. The fungal smear was positive, and the BDG level was high (859 pg/ml, normal range: <151.5 pg/ml), which was strongly suggestive of fungal endophthalmitis. She was promptly given an intravit-real injection of 10 μg of amphotericin B. Unfortunately, after 1 year of treatment, her right eye underwent an enucleation due to uncontrollable intraocular inflammation and retinal detachment, which resulted in eyeball atrophy.
Case 2
A 60-year-old woman presented with a 2-month history of blurred vision in her left eye, and she had an unknown history of uveitis for 3 months. Previous blood tests showed negative results for HIV, syphilis, rheumatism, tuberculosis, and C-reactive protein. One month previously, she had a fever due to urinary stones. One week ago her aqueous examination revealed negative results for tuberculous bacillus DNA, cytomegalovirus (CMV), EB virus, herpes simplex virus (HSV), and varicella-zoster virus (VZV). In addition, her IL-6 level was 13 409.0 pg/ml and IL-10 level was 17.7 pg/ml.
An ocular examination of her left eye showed a best corrected visual acuity (BCVA) of 20/200 and an IOP of 9 mmHg. There were 3+ cells, a flare, and 2+ KPs in the anterior chamber and numerous floater cells and white filaments in the vitreous body (Figure 2A). At the local hospital, she was started on empirical treatment via subconjunctival injection of 8 mg of triamcinolone acetonide and oral administration of 10 mg of prednisone for 1 week, followed by a reduction to 5 mg for 1 week.
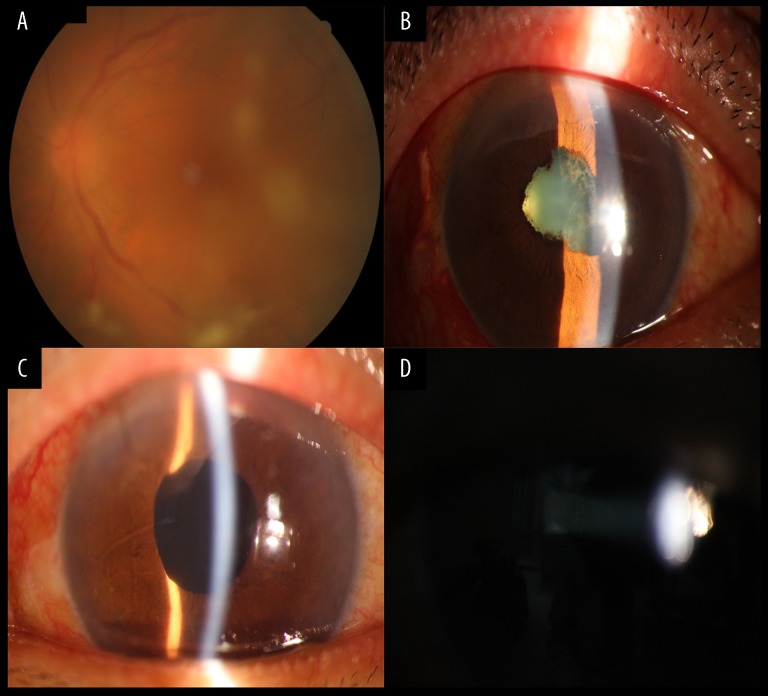
Figure 2.
Case 2: Anterior chamber appearance before surgery, including vitreous opacity and pupil adhesion. Anterior chamber inflammation was significantly reduced after surgery. (A) Numerous floater cells and white filaments assembling in clumps in the vitreous body. (B) At 3-month follow-up the symptoms were aggravated, including hypopyon, lens opacity, and a sticky pupil. (C, D) Results after BDG testing. PPV was performed followed by intravitreal injection of 100 μg of voriconazole once every 3 days and intravenous administration of 200 mg of voriconazole each day for 10 days. During the follow-up, the patient presented clinical improvement: the hypopyon disappeared, inflammation was reduced, and the vitreous transparency was slowly reestablished.
At her 3-month follow-up, the eye exhibited hypopyon, lens opacity, a sticky pupil, and a fundus that could not be seen clearly (Figure 2B). Therefore, a 23-gauge PPV was performed, and the vitreous specimen was examined using gene chip and BDG testing. The results showed fungal Candida species and a BDG level of 845 pg/ml. This patient was treated with an intravitreal injection of 100 μg of voriconazole with a 4-day interval and the intravenous administration of 200 mg of voriconazole every day for 10 days. During the follow-up, she showed clinical improvement; the flare and hypopyon disappeared, inflammation was reduced, and the vitreous transparency was slowly reestablished (Figure 2C, 2D).
Case 3
A 36-year-old man presented with dark shadow and blurred vision in his left eye 4 months after undergoing cataract surgery due to trauma. Approximately 6 months previously, his left eye was injured by a steel wire, and intravitreal ceftazidime (2.25 mg/0.1 cc) and vancomycin (1 mg/0.1 cc) were administered at the local hospital. The bacterial culture and smear were both negative. At the 1-month follow-up, a PPV was performed in his left eye, intravitreal ceftazidime (2.25 mg/0.1 cc) and vancomycin (1 mg/0.1 cc) were administered again, and the bacterial culture and smear of the vitreous fluid remained negative. One month later, he underwent lens phacoemulsification combined with an intraocular lens implantation, and his post-surgery vision was 100/200. At the 4-month follow-up, he complained of dark shadow and blurred vision in his left eye. One week later, he was referred to our hospital.
The medical condition of his left eye began to deteriorate, and his vision decreased to 40/200. There were 3+ cells, a flare, and 2+ KPs in the anterior chamber, as well as vitreous opacity (Figure 3A–3C). Aqueous fluid was extracted for a smear and culture; however, the results were still negative. At this time, his serum BDG level was 28.9 pg/ml. After 2 weeks, aqueous humor was extracted again, and the BDG level was high (2330 pg/ml). He was given repeated intravitreal injections of 10 μg of amphotericin B and 200 mg of intravenous of voriconazole every day for 10 days. He underwent a PPV and repeated vitreous cavity lavage. After these treatments, the flare and KPs in the anterior chamber decreased significantly. However, after 2 weeks, proliferative vitreoretinopathy (PVR) occurred, resulting in retinal detachment. Finally, a PPV, endolaser coagulation, and a silicone oil tamponade were performed.
Figure 3.
Case 3: Vitreous opacity and persistent anterior chamber inflammation after traumatic cataract surgery. (A, B) There were 3+ cells, a flare, and 2+ keratic precipitates in the anterior chamber. (C) There was significant vitreous opacity.
Case 4
A 49-year-old man presented with pain, redness, and blurred vision in his right eye 1 month after a bone marrow transplantation due to myelodysplastic syndrome. Upon examination, his BCVA was light perception, with an IOP of 45 mmHg. There was mixed congestion in the bulbar conjunctiva, corneal edema, and a large amount of gray-white purulent liver-like material was seen in the anterior chamber. Presumed fungal endophthalmitis was diagnosed, and the BDG testing of the aqueous humor was positive (1329 pg/ml). Subsequently, eight times of intravitreal injections of 100 μg of voriconazole were given with 2-day intervals and 200 mg of intravenous of voriconazole was given every day. Finally, he underwent a lens excision, PPV, endolaser coagulation, and silicone oil tamponade. After the operation, his uncorrected vision acuity was finger counting, BCVA was 0.1, corneal and anterior chambers were transparent, and the flare and cells were obviously decreased (Figure 4).
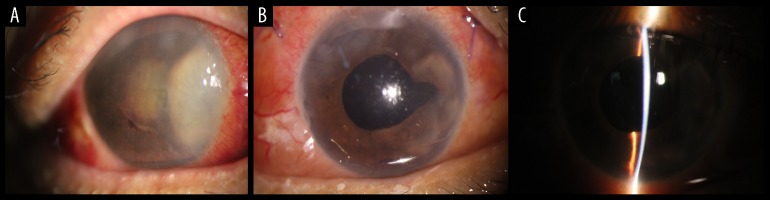
Figure 4.
Case 4: Severe corneal edema and purulent substance before surgery in fungal endophthalmitis; After surgery, corneal edema was reduced, purulent material and inflammation disappeared, and the anterior chamber became clear. (A) Bulbar conjunctival mixed congestion, corneal edema, and a large amount of gray-white purulent material can be seen in the anterior chamber. (B, C) After surgery, the cornea is clear, the purulent-like material disappeared, and the cells and flare were reduced.
Case 5
A 3-year-old boy presented with redness and blurred vision in his right eye 1 month after a bone marrow transplantation. His past medical history was significant for acute myeloid leukemia. Upon examination, there were 3+ cells and a flare in the anterior chamber, the pupillary membrane was closed, there was lens opacity, and the fundus could not be seen. He underwent a lens excision, PPV, endolaser coagulation, and silicone oil tamponade. During the operation, a large fungi mass was seen in the vitreous cavity. The aqueous and vitreous humors underwent BDG testing, and the results were 4019 pg/ml and 1205.2 pg/ml, respectively. The fungal culture results indicated Fusarium. Alternating intravitreal injections of 10 μg of amphotericin B and 100 μg of voriconazole were administrated.
Discussion
A bacterial infection is the main cause of endophthalmitis, and only a small proportion of these cases are believed to be associated with fungal infections [12]. However, fungal endophthalmitis can be a serious threat to vision, and approximately two-thirds of fungal endophthalmitis patients become blind [13]. Chakrabarti et al. reported that the number of fungal endophthalmitis cases has been increasing, mainly in developing countries [14]. Therefore, early diagnosis of fungal endophthalmitis is important for guiding treatment and overall prognosis.
Although blood cultures, tissue cultures, and histological examinations are the criterion standards for diagnosing systemic invasive fungal infections, the diagnosis of fungal endophthalmitis is challenging for clinicians and microbiologists. First, the culture has a long turnaround time, and the clinical presentation and radiographic findings in patients with invasive fungal infections are often nonspecific [15]. Second, fungal organisms often do not grow in blood cultures. Finally, it is difficult to safely obtain a tissue sample for pathological evaluations and cultures. Thus, an ancillary test for the quantification of the BDG levels in the serum, aqueous humor, and vitreous humor is important for the diagnosis of invasive fungal diseases (IFDs) or fungal endophthalmitis.
1,3-BDG is a major polysaccharide cell wall component found in many fungal species, including Candida and Aspergillus species [6]. The mechanism of beta-D-glucan biochemical calorimetric reaction is an enzymatic-based colorimetric assay that takes advantage of a modification of the limulus amebocyte lysate for serum quantification of BDG. Beta-D-glucan binds to the lysate G factor and activates the serine protease zymogen beta subunit, which in turn activates a clotting enzyme that converts coagulogen to coagulin. Then, Boc-Leu-Gly-Argp-nitroanilide is cleaved by the clotting enzyme, which releases paranitroaniline, a chromogenic substance that is measured calorimetrically with absorbance at 405 nm (Figure 5).
Figure 5.
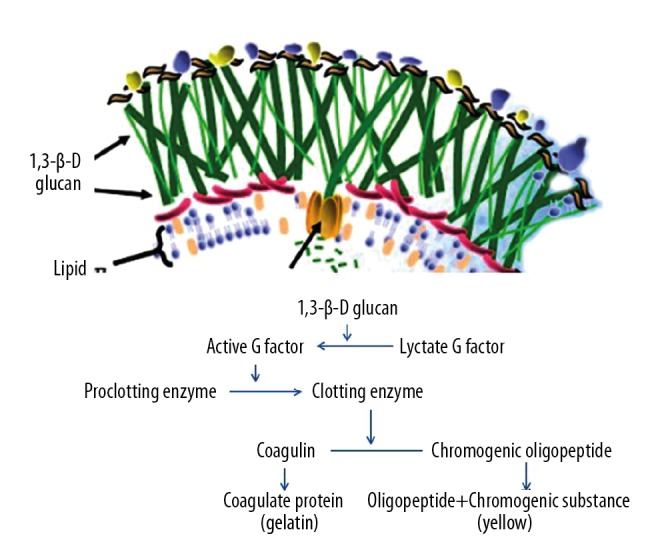
Mechanism of beta-D-glucan biochemical calorimetric reaction.
1,3-BDG can release into the bloodstream in IFD cases. Serum BDG levels have been shown to be significantly higher in some IFD patients than in non-infected individuals. In addition, the trend of an increasing serum BDG level can indicate disease progression and prognosis. Many researchers have reported that detectable BDG antigenemia presents in many critically ill patients with proven or probable IFDs prior to the appearance of clinical symptoms, positive radiological findings, and positive culture results [5]. Therefore, when compared with a traditional pathological diagnosis and tissue culture, BDG testing is less time-consuming, less invasive, and more feasible [16].
Serum BDG testing is being used more routinely in immuno-compromised populations as a means of early surveillance and diagnosis of IFDs in conjunction with clinical and radio-graphic data, and it may be useful for monitoring the clinical response to systemic antifungal therapy [7, 17]. However, BDG testing is seldom mentioned in the ophthalmological literature, and it may be clinically underused in suspected fungal endophthalmitis cases. Recently, with the development and clinical use of BDG testing in ophthalmology, several studies have found elevated serum BDG levels to be one of the most commonly seen clinical characteristics associated with EFE. Shimbo et al. [18] quantified the BDG levels in the vitreous fluid of 26 patients, and they reported high vitreous BDG concentrations in 2 patients with fungal endophthalmitis and below-threshold (10.0 pg/ml) levels in the other patients without fungal endophthalmitis. Thus, they suggested that testing the BDG values in the vitreous fluid are more sensitive than the culture methods, and it may be useful for the diagnosis of fungal endophthalmitis. Tanaka et al. [19] analyzed 79 eyes of 46 patients with EFE treated over a 12-year period, and they concluded that the risk factors for fungal endophthalmitis were as follows: serum BDG level ≥20 pg/ml (90%), intravenous hyperalimentation (87%), fever >38°C (76%), major surgery (76%), male sex (74%), and the presence of cancer (72%).
Patients who develop EFE are often immunosuppressed or have significant systemic risk factors for IFDs. Acute myeloid leukemia and the immunocompromised state of the patient are undoubtedly factors that allow the infection to spread. In such immunocompromised hosts, an extensive evaluation, including computed tomography scans and blood cultures, should be performed to look for possible occult seeding. Fusarium is an emergent opportunistic filamentous fungus. Although the prevalence of fusariosis is less than 1% in patients with hematological malignancies, the rate is increasing [20]. In our study, Cases 4 and 5 were both immunocompromised patients who underwent bone marrow transplantations. Fusarium was eventually identified in the Case 5 culture and Candida albicans was identified in the Case 4 culture. BDG testing was also performed in these 2 cases. With the exception of the systemic risk factors, these cases exhibited typical clinical presentations and fungal cultures. The BDG level of the aqueous humor was 1329 pg/ml in Case 4 and 4109 pg/ml in Case 5, which were both higher than the vitreous humor levels (Table 1). Based on these data, BDG testing may be a useful adjunct to a vitreous culture in the diagnosis of fungal endophthalmitis. However, it is difficult to explain why the BDG levels in the aqueous humors were higher than those in the vitreous humors.
Table 1.
Patient information of 5 cases with fungal endophthalmitis.
| No. | Sex | Age | System condition | Risk factor | Time | Vision | Specimen | BDG (pg/ml) | Culture or smear | Gene chip | Fungus species |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 58 | Health | Cataract surgery | 4 m | FC (right) | Aqueous humor | 859 | Smear (+) | / | / |
| 2 | Female | 60 | Health | Fever | 3 m | 0.1 (left) | Vitreous humor | 845.3 | / | (+) | Candida albicans |
| 3 | Male | 36 | Health | Ocular trauma | 4 m | 0.5 (left) | Aqueous humor, serum | 2230.4 28.9 |
(–) | / | / |
| 4 | Male | 49 | Myelodysplastic syndromes | Bone marrow transplantation | 1 m | LP (right) | Aqueous/Vitreous humor | 1329 985 |
(+) | / | Candida albicans |
| 5 | Male | 3 | Acute myelogenous leukemia | Bone marrow transplantation | 3 m | LP (left) | Aqueous/Vitreous humor | 4109 1205 |
(+) | / | Fusarium |
Exogenous endophthalmitis is often the result of direct inoculation of a pathogen during eye surgery or due to a penetrating trauma [1]. Moreover, the incidence of fungal endophthalmitis after a cataract surgery is correlated with poor medical and health environments during surgery [14]. Several studies have reported that the time to seeking medical treatment for patients with fungal endophthalmitis varies from 1 to 5 months, with an average time of 22 days after surgery, which may be even later in remote areas [12,21,22]. Xu et al. described a case of delayed fungal endophthalmitis secondary to Curvularia 3 years after a cataract surgery [23]. The researchers did not use BDG testing, but, based on the vitreous biopsy, the specimen was positive for Curvularia spp. In our study, the onset times for Cases 1 and 3 were consistent with the literature. Case 1 developed blurred vision 4 months after her cataract surgery. After undergoing various examinations and treatments, fungal endophthalmitis was finally suspected based on the BDG testing of the aqueous humor, which showed a high level (859 pg/ml). The patient received systemic and regional antifungal treatments. Case 3 exhibited blurred vision 4 months after a trauma, but initially had only KPs and floater cells. At first, the serum BDG level was only 28.9 pg/ml, but when the aqueous humor BDG level was assessed 2 weeks later, it had increased to 2230.4 pg/ml. Finally, at that point, fungal endophthalmitis was the suspected diagnosis. As in these 2 cases, the diagnosis of fungal endophthalmitis can be challenging for patients who had a previous cataract surgery or trauma. This may be related to the delayed onset or physician lack of knowledge about the typical clinical presentations of this condition due to its rarity. BDG testing is an effective supplementary examination that is of great importance in the diagnosis of fungal endophthalmitis.
Based on the literature, we know that serum BDG testing is being used more routinely in immunocompromised populations for early surveillance and diagnosis of IFDs, and it may be useful for monitoring the clinical response to systemic antifungal therapy. BDG levels have been quantified in the pleural fluid, cerebrospinal fluid, bronchoalveolar lavage samples, and joint fluid [17,24]. Sims et al. defined successful systemic treatment as a negative slope of the best-fit curve for the serological quantification of BDG levels. If the levels are stable or increasing (i.e., a positive slope), this indicates a suboptimal treatment response or urgent need for further work-up to identify an additional source of infection [25]. However, in intraocular fluid testing, the BDG concentrations in healthy individuals and patients with fungal endophthalmitis have not been established, which makes it difficult to establish a clinical diagnosis and to provide proper treatment based on the BDG levels.
In our study, to compare it with the accuracy of the BDG experiment, we also tested the intraocular fluid BDG levels in 8 patients with non-fungal endophthalmitis, but who were definitively diagnosed with bacterial endophthalmitis by gene chip testing. The results showed that the BDG levels of these patients were all lower than 100 pg/ml, which was significantly different from the fungal endophthalmitis cases (p=0.001) (Table 2). However, the BDG levels were higher (up to 800 pg/ml) in all of the fungal endophthalmitis cases. In a previous report, Shimbo et al. quantified the BDG levels in the vitreous fluid, and reported below-threshold (10.0 pg/ml) levels as being negative for fungal endophthalmitis [18]. However, there is no high-threshold level; therefore, a quantitative study of the BDG levels in the intraocular fluid may be of great clinical value for furthering the diagnostic utility of this testing modality. According to our testing and clinical experience, the criteria that we quantified were as follows: a BDG level below 100.5 pg/ml was negative and a BDG level above 151.5 pg/ml was positive. Of course, these data also depend on the various kits being used. In clinical testing, we found that the BDG levels from the kit produced by Zhanjiang AC Biological were highly consistent with the positive gene chip results.
Table 2.
Comparison of BDG level intraocular fluid in non-fungal endophthalmitis and fungal endophthalmitis and specimens diagnosed by gene chip.
| Non-fungal endophthalmitis group | Fungal endophthalmitis group | |||
|---|---|---|---|---|
| Diagnosis by chip | BDG level (pg/ml) (Negative <100.5; Positive >151.5) | Smear/culture | BDG level (pg/ml) (Aqueous humor) (Negative <100.5; Positive >151.5) | |
| No.1 | Varicella-zoster virus (VZV) (+) | <10 | + | 859 |
| No.2 | Staphylococcus epidermidis (+) | 14.2 | / | 845.3 |
| No.3 | Colibacillus (+) | 97.1 | / | 2230.4 |
| No.4 | Enterococcus faecalis (+) | 32.1 | + | 1329 |
| No.5 | Klebsiella pneumonia (+) | 10.2 | + | 4109 |
| No.6 | Klebsiella pneumonia (+) | 25.7 | ||
| No.7 | Colibacillus (+) | 88.2 | ||
| No.8 | Klebsiella pneumonia (+) | 83.3 | ||
| Mean±SE | 45.1±13.34 | 1875±612.7 | ||
P=0.001.
BDG testing does have some limitations. For example, positive results do not confirm a specific organism or species. Therefore, this test should be used in conjunction with blood and tissue cultures and pathological evaluations, as well as other pertinent clinical tests and findings. At present, the main antifungal drugs that are available are amphotericin B, voriconazole, and fluconazole. In accordance with different fungal species, systematic and regional antifungal therapy should be combined with BDG testing, in which decreasing BDG levels suggest an appropriate treatment response.
Conclusions
Intraocular fluid BDG level is closely associated with fungal endophthalmitis. However, the BDG values from intraocular fluid are more sensitive than culture methods, and these are useful for the diagnosis of fungal endophthalmitis.
Acknowledgments
The measurements of BDG level, cytokine level, and virus load in the intraocular fluids of the cases were performed by GiantMed Diagnostic Corporation, Beijing. Fei Hong, XiaoJuan Wang, and Dan Zhu assisted in taking intraocular fluid samples and managing patients.
Footnotes
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interests
None.
References:
- 1.Alves da Costa Pertuiset PA, Logrono JF. Fusarium endophthalmitis following cataract surgery: Successful treatment with intravitreal and systemic voriconazole. Case Rep Ophthalmol Med. 2016;2016:4593042. doi: 10.1155/2016/4593042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolomeyer AM, Murphy KM, Traband A, et al. Beta-D-glucan testing in patients with fungal endophthalmitis. Retina. 2018;38(4):650–59. doi: 10.1097/IAE.0000000000002049. [DOI] [PubMed] [Google Scholar]
- 3.Krishna R, Amuh D, Lowder CY, et al. Should all patients with candidaemia have an ophthalmic examination to rule out ocular candidiasis? Eye (Lond) 2000;14(Pt 1):30–34. doi: 10.1038/eye.2000.7. [DOI] [PubMed] [Google Scholar]
- 4.Obayashi T, Yoshida M, Tamura H, et al. Determination of plasma (1→>3)-beta-D-glucan: A new diagnostic aid to deep mycosis. J Med Vet Mycol. 1992;30(4):275–80. doi: 10.1080/02681219280000361. [DOI] [PubMed] [Google Scholar]
- 5.Obayashi T, Negishi K, Suzuki T, Funata N. Reappraisal of the serum (1→>3)-beta-D-glucan assay for the diagnosis of invasive fungal infections – ca study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46(12):1864–70. doi: 10.1086/588295. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida K, Shoji H, Takuma T, Niki Y. Clinical viability of Fungitell, a new (1→>3)-beta-D: -glucan measurement kit, for diagnosis of invasive fungal infection, and comparison with other kits available in Japan. J Infect Chemother. 2011;17(4):473–77. doi: 10.1007/s10156-010-0198-6. [DOI] [PubMed] [Google Scholar]
- 7.Karageorgopoulos DE, Vouloumanou EK, Ntziora F, et al. beta-D-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis. 2011;52(6):750–70. doi: 10.1093/cid/ciq206. [DOI] [PubMed] [Google Scholar]
- 8.Sadiq MA, Hassan M, Agarwal A, et al. Endogenous endophthalmitis: Diagnosis, management, and prognosis. J Ophthalmic Inflamm Infect. 2015;5(1):32. doi: 10.1186/s12348-015-0063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva RA, Sridhar J, Miller D, et al. Exogenous fungal endophthalmitis: An analysis of isolates and susceptibilities to antifungal agents over a 20-year period (1990–2010) Am J Ophthalmol. 2015;159(2):257–64. e251. doi: 10.1016/j.ajo.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Lingappan A, Wykoff CC, Albini TA, et al. Endogenous fungal endophthalmitis: Causative organisms, management strategies, and visual acuity outcomes. Am J Ophthalmol. 2012;153(1):162–166. e161. doi: 10.1016/j.ajo.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Chen KJ, Wu WC, Sun MH, et al. Endogenous fungal endophthalmitis: Causative organisms, management strategies, and visual acuity outcomes. Am J Ophthalmol. 2012;154(1):213–14. doi: 10.1016/j.ajo.2012.03.016. author reply 214. [DOI] [PubMed] [Google Scholar]
- 12.Mithal K, Pathengay A, Bawdekar A, et al. Filamentous fungal endophthalmitis: Results of combination therapy with intravitreal amphotericin B and voriconazole. Clin Ophthalmol. 2015;9:649–55. doi: 10.2147/OPTH.S80387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durand ML. Endophthalmitis. Clin Microbiol Infect. 2013;19(3):227–34. doi: 10.1111/1469-0691.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakrabarti A, Shivaprakash MR, Singh R, et al. Fungal endophthalmitis: Fourteen years’ experience from a center in India. Retina. 2008;28(10):1400–7. doi: 10.1097/iae.0b013e318185e943. [DOI] [PubMed] [Google Scholar]
- 15.Senn L, Robinson JO, Schmidt S, et al. 1,3-Beta-D-glucan antigenemia for early diagnosis of invasive fungal infections in neutropenic patients with acute leukemia. Clin Infect Dis. 2008;46(6):878–85. doi: 10.1086/527382. [DOI] [PubMed] [Google Scholar]
- 16.Alam FF, Mustafa AS, Khan ZU. Comparative evaluation of (1, 3)-beta-D-glucan, mannan and anti-mannan antibodies, and Candida species-specific snPCR in patients with candidemia. BMC Infect Dis. 2007;7:103. doi: 10.1186/1471-2334-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi XY, Liu Y, Gu XM, et al. Diagnostic value of (1→> 3)-beta-D-glucan in bronchoalveolar lavage fluid for invasive fungal disease: A meta-analysis. Respir Med. 2016;117:48–53. doi: 10.1016/j.rmed.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Shimbo M, Ito N, Kadonosono K. [Investigation of beta-D-glucan values in the vitreous] Nippon Ganka Gakkai Zasshi. 2002;106(9):579–82. [in Japansese] [PubMed] [Google Scholar]
- 19.Tanaka M, Kobayashi Y, Takebayashi H, et al. Analysis of predisposing clinical and laboratory findings for the development of endogenous fungal endophthalmitis. A retrospective 12-year study of 79 eyes of 46 patients. Retina. 2001;21(3):203–9. doi: 10.1097/00006982-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Baysal M, Umit E, Boz IB, et al. Fusarium endophthalmitis, Unusual and challenging infection in an acute leukemia patient. Case Rep Hematol. 2018;2018:9531484. doi: 10.1155/2018/9531484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rachitskaya AV, Reddy AK, Miller D, et al. Prolonged Curvularia endophthalmitis due to organism sequestration. JAMA Ophthalmol. 2014;132(9):1123–26. doi: 10.1001/jamaophthalmol.2014.1069. [DOI] [PubMed] [Google Scholar]
- 22.Berbel RF, Casella AM, de Freitas D, Hofling-Lima AL. Curvularia lunata endophthalmitis. J Ocul Pharmacol Ther. 2011;27(5):535–37. doi: 10.1089/jop.2011.0002. [DOI] [PubMed] [Google Scholar]
- 23.Xu K, Almeida DRP, Chin EK, Mahajan VB. Delayed fungal endophthalmitis secondary to Curvularia. Am J Ophthalmol Case Rep. 2016;3:1–4. doi: 10.1016/j.ajoc.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeragh A, Ahmad S, Naseem J, Khan ZU. Candida lusitaniae arthritis in an intravenous drug user. Mycoses. 2007;50(5):430–32. doi: 10.1111/j.1439-0507.2007.01394.x. [DOI] [PubMed] [Google Scholar]
- 25.Sims CR, Jaijakul S, Mohr J, et al. Correlation of clinical outcomes with beta-glucan levels in patients with invasive candidiasis. J Clin Microbiol. 2012;50(6):2104–6. doi: 10.1128/JCM.00773-12. [DOI] [PMC free article] [PubMed] [Google Scholar]