Abstract
Introduction
We sought to determine whether non-mandated or passive centralization of radical cystectomy (RC) to higher-volume centers leads to enhanced processes of care and outcomes.
Methods
This is a population-based, retrospective, cohort study that used the Ontario Cancer Registry (OCR) to identify all incident patients who underwent RC from 1994–2013. Electronic records of treatment were linked to OCR; pathology records were obtained for all cases and reviewed by a team of trained data abstractors. The primary objective was to describe annual provider RC volumes. Secondary objectives included investigating process and outcome measures.
Results
For the 5574 patients identified, the mean annual surgeon volume and hospital volume of RC from 1994–2008 was 4.5 (95% confidence interval [CI] 4.4–4.7) and 12.2 (95% CI 11.8–12.5), respectively. From 2009–2013, these volumes significantly increased to 6.8 (95% CI 6.5–7. 1) and 16.4 (95% CI 15.8–16.9). Process variables improved over time, including the use of neoadjuvant chemotherapy. Over the study period, there was a substantial improvement in cancer-specific survival (CSS): hazard ratio (HR) 0.60 (95% CI 0.53–0.67) for 2009–2013. During the most recent era, there was still evidence of a provider volume effect on both process measures and CSS.
Conclusions
There has been recent passive centralization of RC to higher-volume providers in the province of Ontario, with measurable improvements in processes of quality care. Although centralization was also associated with improvement in CSS, in the most recent era, there continues to be low-volume providers with a residual volume-outcome effect.
Introduction
Centralization of complex surgical care is now focal in the contemporary global conversation to improve outcomes, particularly in surgical oncology. The rationale has been founded by cumulative evidence that high-volume providers have better outcomes across multiple cancer sites.1,2 Despite concerns with the evidence base supporting this volume effect, strong associations with optimized processes of care in alignment with improved early and late outcomes would seem to satisfy both face and construct validity of the concept.3,4 To capitalize on this knowledge, mandated centralization of care to high-volume centers and surgeons or benchmarking key processes of care for lower-volume providers could be considered. Although there is increasing experience internationally,5,6 criticism of mandated centralization of surgical care remains, particularly regarding the potential to decrease access to care.7,8 Accordingly, widespread adoption across cancer sites has been halting.
Radical cystectomy (RC) for bladder cancer in as an interesting model to explore these concepts in the North American context. First, RC is a relatively common and complex oncological surgical procedure and population studies of routine surgical care have suggested suboptimal results compared to high-volume centers of excellence.2,9,10 Second, ideal outcomes for invasive bladder cancer are dependent on a multidisciplinary approach. Third, several perioperative processes of care have been well-delineated, including use of perioperative chemotherapy, which can lead to a modest but definitive improvement in outcomes.2,11 Despite some experience with mandated centralization in other jurisdictions, RC in North America has not been the subject of such focus from health authorities, notwithstanding several calls from key stakeholders.12 We hypothesized that some degree of passive centralization of RC will have occurred in recent years due to rising awareness of the volume-outcomes phenomenon within the urologic oncology community. Furthermore, we hypothesized that any passive centralization in a large population would be associated with measurable changes in processes of care and improvements in oncological outcomes.
Methods
This is a population-based, retrospective, cohort study that used the Ontario Cancer Registry (OCR) to identify all incident patients in Ontario who underwent RC from 1994–2013.13 The primary objectives of this current study were to identify trends indicating passive centralization of RC in the contemporary era and to evaluate any associations between hospital and surgeon RC volumes and the processes of care that are linked to early and late outcomes. In order to determine if any passive centralization coincided with potential detrimental patient-centered effects, we looked at global wait times and patient distance travelled to surgical care. Finally, we theorized that any degree of centralization of surgical care might dampen well-described provider volume effects on outcomes.
The OCR is a population-based registry that captures diagnostic and demographic information on 98% of all incident cases of cancer diagnosed in Ontario.14 Stage of disease was not routinely available from the existing data source, hence pathology records were obtained for all cases and reviewed by a team of trained data abstractors, with periodic quality audits performed by the study investigators. Hospital care and surgical intervention data was obtained from the Canadian Institute for Health Information15 and chemotherapy utilization information was obtained using treatment records from Ontario regional cancer centers and physician billing records as previously described.13 These datasets were linked using unique encoded identifiers and analyzed at ICES.
Comorbidity was assessed using the Charlson comorbidity index and socioeconomic status information was obtained as previously described.16 Surgical wait times were measured from time of diagnosis documented within OCR to the time of their RC, and distance travelled was aerial distance between residence and hospital location. The number of cystectomies performed at each hospital per year was derived from the overall study population. A volume level for each hospital and surgeon was derived as previously described using the mean annual number of cystectomies over a five-year study period.2 Although there are no validated volume benchmarks for RC, we assigned high-volume providers as >6.2 cases per year for surgeons and >20 cases per year for hospitals based on previous work in a historical Ontario cohort.2
To compare proportions between study groups, the Chi-square test was used. From the OCR, survival data was determined from the date of surgery using the Kaplan-Meier technique, and comparisons within groups were made using the log-rank test. Cox-proportional hazards regression models were used to assess the association between patient-, disease-, and treatment-related factors on overall survival (OS) and cancer-specific survival (CSS). All analyses were performed using SAS, version 9.4 (SAS institute, Cary, NC). The study was approved by the Research Ethics Board of Queen’s University. This study was designed, analyzed, and reported in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement.17
Results
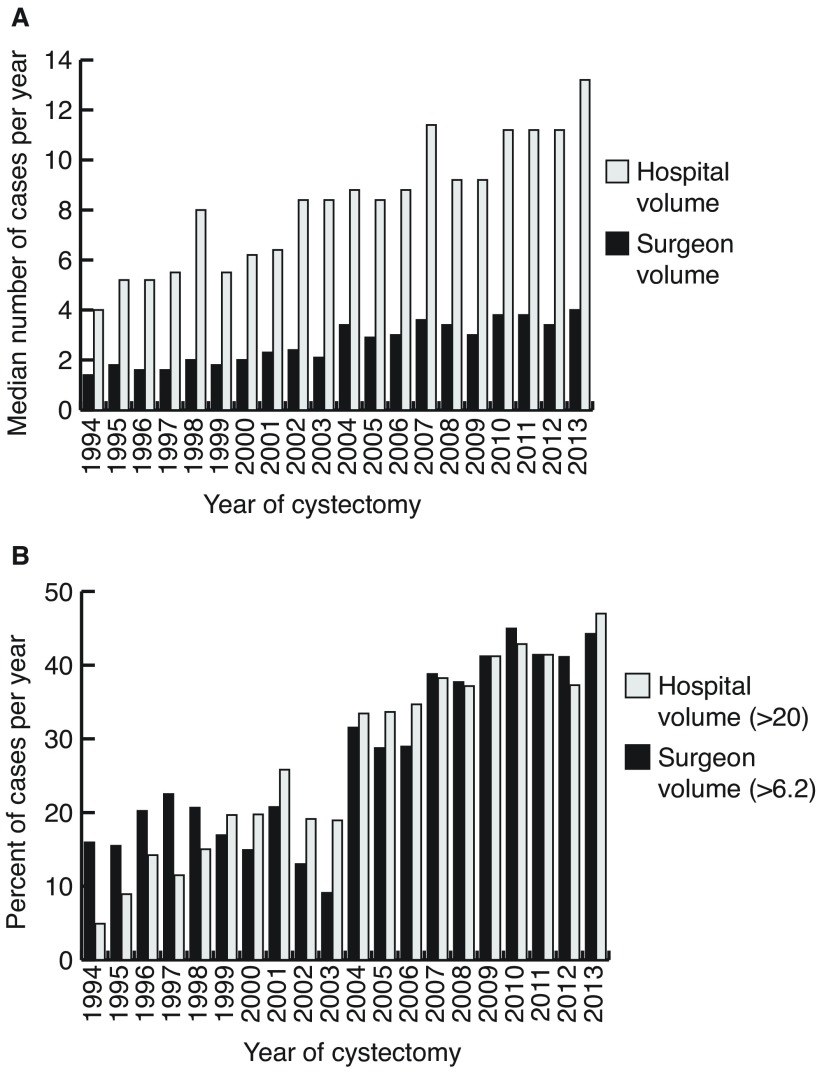
Between 1994 and 2013, 5574 patients were identified having undergone RC for bladder cancer in Ontario. Characteristics of the study population can be found in Table 1. Cases were divided into four eras spanning the 20 years of the study, with a stable number of RC being performed in the two most recent eras (2004–2013). As depicted in Fig. 1A, over the entire study period there has been a marginal increase in the median number of cases done by surgeons but a substantial increase in the volume at the hospital level. The mean annual surgeon volume and hospital volume of RC during 1994–2008 was 4.5 (95% confidence interval [CI] 4.4–4.7) and 12.2 (95% CI 11.8–12.5), respectively. In the more contemporary era, these volumes significantly increased to 6.8 (95% CI 6.5–7.1) (p<0.01) and 16.4 (95% CI 15.8–16.9) (p<0.01). Furthermore, there has been an increasing trend in the proportion of RC completed by higher-volume surgeons (>6.2 cases) and at higher volume centers (>20 cases) over the entire study period (Fig. 1B) (p<0.001 for both surgeon and center volume). In the most recent years, >40% of RC were done by higher-volume providers.
Table 1.
Characteristics of patients with bladder cancer treated with cystectomy in Ontario from 1994–2013
| (n=5574) n (%) | |
|---|---|
| Patient-related | |
| Year of surgery | |
| 1994–1998 | 927 (17%) |
| 1999–2003 | 1231 (22%) |
| 2004–2008 | 1721 (31%) |
| 2009–2013 | 1695 (30%) |
| Age (years)1 | |
| 20–49 | 213 (4%) |
| 50–59 | 699 (13%) |
| 60–69 | 1469 (26%) |
| 70–79 | 2134 (38%) |
| 80+ | 1059 (19%) |
| Sex | |
| Male | 4182 (75%) |
| Female | 1392 (25%) |
| SES by quintile2 | |
| 1 | 1098 (20%) |
| 2 | 1244 (22%) |
| 3 | 1206 (22%) |
| 4 | 1042 (19%) |
| 5 | 931 (17%) |
| Unknown | 53 (1%) |
| Comorbidity score | |
| 0 | 3856 (69%) |
| 1+ | 1718 (31%) |
| Disease-related | |
| Pathologic T stage | |
| <T2 | 1058 (19%) |
| T2 | 1272 (23%) |
| T3 | 2107 (38%) |
| T4 | 1121 (20%) |
| TX | 16 (0%) |
| Pathologic N stage | |
| N negative | 3102 (56%) |
| N positive | 1377 (25%) |
| NX | 1095 (20%) |
| LVI | |
| No | 1509 (27%) |
| Yes | 2186 (39%) |
| Unstated | 1879 (34%) |
Age is based on date of cystectomy.
Socioeconomic status, quintile 1 represents communities where the poorest 20% of the Ontario population resided.
LVI: lymphovascular invasion; SES: socioeconomic status.
Fig. 1.
(A) Median annual surgeon and hospital volume across the study years. (B) Proportion of cases per year high-volume surgeon (>6.2) and for high-volume hospital (>20).
After excluding cases with <pT2, a total of 4246 patients from the cohort would have been eligible to receive perioperative chemotherapy. Over the study period, there was a significant increase in use of perioperative chemotherapy from 19.0% in the earliest era to 35.2% in the most recent (p<0.001). Patient referral to medical oncology preoperatively increased from 12.1% to 31.5% (p<0.001), with similar increasing trends for postoperative referrals (Supplementary Fig. 1; available at cuaj.ca). In alignment with modern guidelines, the use of neoadjuvant chemotherapy (NACT) increased substantially from 4.4% in 1994–2008 to 18.7% in 2009–2013 (p<0.001). Patients in the contemporary period were more likely to have had a preoperative referral to radiation oncology (data not shown).
A significant increase over time in the performance of pelvic lymph node dissection (PLND) (p<0.001) was observed (Supplementary Fig. 2; available at cuaj.ca). An explicit node count was available in 3544 of the 4246 PLND cases and of these cases, 1135 had positive nodes. Median node counts significantly increased (p<0.001) over time, with a subsequent decrease in median node density per year (p<0.001). The margin positive rate was variable over the years, from 6.6% to 19.6%, without any significant trends over the study period. The use of continent urinary diversions appears to have significantly increased over the study period, from 10.9% in the first era to 13.5% in 2009–2013 (p<0.001).
There did not appear to be any substantive change in distance travelled to surgical care with the mean (± standard deviation) in the earliest era 41 km (±84) compared to 46 (±92) (p=0.250) in the contemporary era. As centralization of care could potentially translate to longer wait times, both for intervening diagnostic tests and resulting greater workloads of higher-volume providers, we assessed the wait times over the study period and, indeed, there appeared to be an associated increase over the four study periods; the proportion of patients receiving RC within three months of initial diagnosis decreased from 57% in the first era to 47% in the most recent (p<0.001) (Supplementary Table 1; available at cuaj.ca).
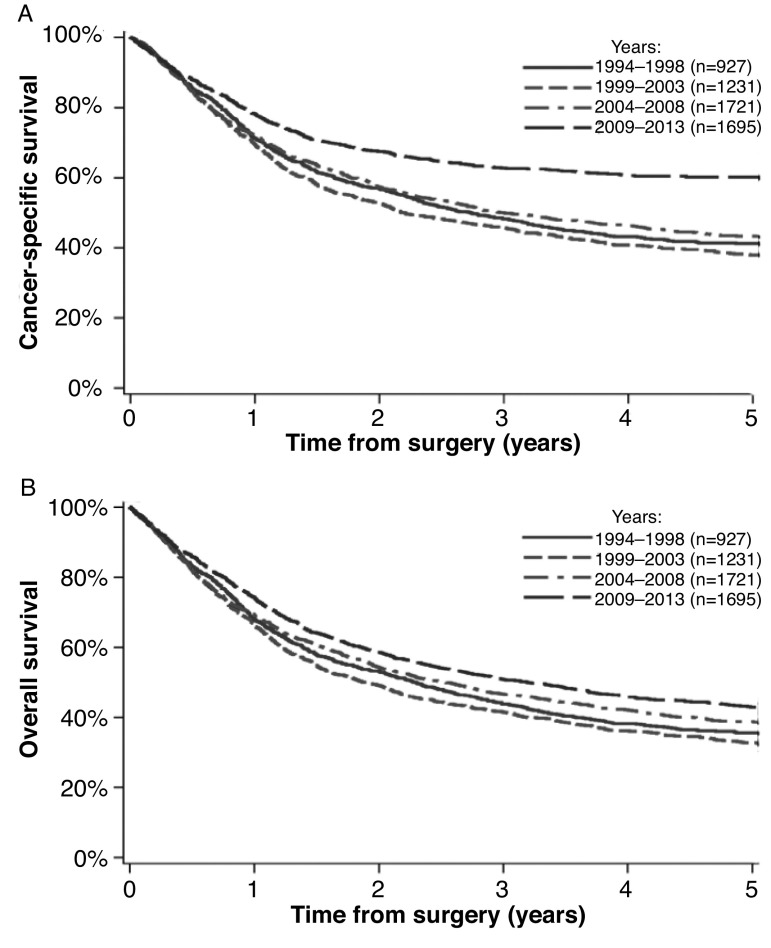
Centralization of RC to higher-volume providers was associated with a trend to an effect on re-admission rates at 30 (p=0.003) and 90 days (p=0.087). In adjusted analyses, there was no evidence of any change on 30- or 90-day mortality. There was, however, a significant improvement in CSS and OS over the study period (Fig. 2) (Adjusted CSS hazard ratio [HR] 0.60 [95% CI 0.53–0.67], OS HR 0.80 [95% CI 0.73–0.89] in 2009–2013). When assessing survival adjusting for age, comorbidity, lymphovascular invasion (LVI), T stage, N stage, and adjuvant chemotherapy (excluding NACT cases, as stage would be confounded), we still demonstrated an effect on CSS over time, with a HR of 0.73 (95% CI 0.64–0.84) in 2009–2013.
Fig. 2.
Survival curves for all bladder cancer cases. (A) Cancer-specific survival; (B) overall survival.
Despite demonstrating some degree of centralization in the later years of the study, there were still a significant number of lower-volume providers. The maximum number of annual cases for the lowest quartile (Q1) surgeons was 1.8 cases compared to 19.2 for the highest quartile (Q4). Similar discrepancies were seen for hospital volume with 6.4 cases for Q1 and 32.2 for Q4. During this most recent era, there was still evidence of a surgeon volume effect on RC process measures, including quality of PLND and use of NACT (Table 2). In adjusted analysis, there was a demonstrable association with surgeon volume and postoperative mortality at 90 days (OR 0.60 [95% CI 0.36–0.99]), although no effect on readmission rates was identified (Supplementary Table 2A; available at cuaj.ca). Survival analysis by surgeon volume, adjusted for age and comorbidity, demonstrated an association with CSS (HR for Q4 0.82 [95% CI 0.65–1.02]), although the trend for OS was not significant (Supplementary Table 2B; available at cuaj.ca). Adjusting for pathological variables (excluding those that received NACT), the significant volume effect on CSS was maintained (HR for Q4 0.83 [95% CI 0.65–1.06]). Similar trends were seen for hospital provider volumes.
Table 2.
Process variables of care around radical cystectomy by surgeon volume
| Surgeon volume | p | ||||
|---|---|---|---|---|---|
|
| |||||
| Q1 (low-volume) | Q2 | Q3 | Q4 (high-volume) | ||
| Preoperative MO referral^ | 102 (23%) | 122 (30%) | 121 (27%) | 116 (29%) | 0.141 |
| Preoperative MO referral* | 95 (28%) | 112 (34%) | 104 (31%) | 99 (35%) | 0.224 |
| Preoperative RO referral^ | 44 (10%) | 50 (12%) | 93 (21%) | 46 (11%) | <0.001 |
| PLND yes^ | 396 (90%) | 364 (89%) | 430 (97%) | 398 (99%) | <0.001 |
| Median node count# (IQR) | 9 (5–15) | 10 (6–15) | 11 (7–18) | 15 (9–21) | <0.001 |
| Mean node density | 0.34±0.27 | 0.31±0.25 | 0.28±0.26 | 0.26±0.25 | 0.096 |
| Margin status | 0.685 | ||||
| Any positive | 83 (19%) | 71 (17%) | 89 (20%) | 71 (18%) | |
| All negative | 349 (79%) | 331 (81%) | 351 (79%) | 330 (82%) | |
| Unstated | 7 (2%) | 6 (1%) | ≤5 (1%) | ≤5 (1%) | |
| NACT rate* | 56 (16%) | 63 (19%) | 52 (15%) | 72 (25%) | 0.009 |
| ACT rate* | 60 (17%) | 77 (23%) | 63 (19%) | 55 (19%) | 0.283 |
Among all surgical cases 2009–2013.
Among those with PLND yes.
Among MIBC cohort 2009–2013.
ACT: adjuvant chemotherapy; IQR: interquartile range; MO: medical oncologist; NACT: neoadjuvant chemotherapy; RO: radiation oncologist; PLND: pelvic lymph node dissection.
Discussion
In this study, we demonstrate passive centralization of RC in Ontario with a substantial shift in practice to higher-volume providers. Despite the lack of mandated consolidation of surgical care for bladder cancer, herein we have demonstrated several resulting associations with the delivery of care for these patients and their outcomes. First, over the study period, there have been substantial improvements in several process-related quality indicators, including consultation with multidisciplinary teams, use of perioperative chemotherapy and enhanced PLND. Second, we have observed some evidence of improved early outcomes over time, including a decrease in re-admission rates. Third, over the study period, we have seen a substantial improvement in CSS. Finally, despite demonstrating some degree of centralization across the province, there was still a significant number of lower-volume providers with a persistent association of the provider volume effect on process-related factors, as well as outcomes.
The relationship between higher provider volumes and improved outcomes has been well-described in many surgical disciplines, including RC for bladder cancer.2,5,9 Survival of patients with muscle-invasive bladder cancer (MIBC) in these higher-volume, cancer-focused centers have consistently been higher18 than those reports from population-level studies of routine clinical care (30–43%).2,18,19 Although some of this discrepancy may be due to case mix or variations in data capture, it is likely that these improved outcomes follow Donebedian’s framework of health quality, where excellent structures lead to optimized processes of care.20 These processes reflect various perioperative factors, such as patient selection and adherence to care pathways, as well as technical proficiencies. Indeed Hollenbeck et al21 demonstrated a number of processes of these factors that may explain some of the volume effect on early mortality in RC. Similarly, we have previously presented models suggesting that the volume effect on CSS was modestly mediated by the quality of PLND and use of perioperative chemotherapy.2
In 2015, leaders from three prominent U.S.-based hospital systems publicly announced a “Take the Volume Pledge” to advocate restraint for certain surgical procedures being done by lower-volume providers and set minimum volume standards for a number of elective surgical procedures.22 There has been some experience of mandated centralization of surgical oncology in Canada and throughout Europe. In urological oncology, the Martini Clinic has centralized radical prostatectomy, performing more than 2000 cases per year with documented superior oncological and functional outcomes when compared to other hospitals.23 A recent report of RC provides evidence that the creation of a centralized high-volume center and concurrent quality program can lead to improvements in outcome within a short period of time.24
Although based on the strong volumes-outcomes literature, reactions to campaigns of regionalization have not been completely positive, with concerns around its scientific underpinnings, including case mix leading to selection bias for higher-volume providers, issues around patient/surgeon autonomy and choice, as well as the logistic issues around moving certain procedures and subsequent personnel to different hospitals. Others worry centralization may create access problems for a substantial proportion of patients in the population and worsen existing disparities between those in rural communities/treated at lower-volume centers.25–27 A recent study by Casey et al describe a complicated relationship between centralization and access in New York State,28 although in this present study, we did not identify any strong signal of access issues during the observed passive centralization in Ontario. We did observe an increase in wait times from diagnosis to RC in the most recent era in Ontario, although these data are limited by the management details prior to the RC and may be confounded by other recent resource constraints. Finally, there is little current literature, specifically in RC for bladder cancer, benchmarking provider volumes and exploring the presence of any ceiling effect of centralization. In the meantime, some patients will continue to require oncological surgery at medium-sized hospitals and care for these patients can only improve if other structures and processes of care can be widely adopted.
Overall, these results do confirm some degree of passive centralization of RC, likely driven by the increasing importance of multidisciplinary care for MIBC, understanding of the volume-outcome paradigm for RC, and perhaps by the marketplace. This centralization was associated with improved processes of care, as well as early and late outcomes. Although unable to confirm a direct cause and effect relationship, our analysis in the most recent years of the study again confirm a strong, ongoing relationship with both surgeon and hospital volume and key processes of care, suggesting that gains made in outcomes of patients after RC are likely secondary to more optimal care by higher-volume providers. However, as this consolidation of care has not been mandated, it remains incomplete, with evidence of ongoing provision of RC by very low-volume surgeons and hospitals. About half of the patients were treated by surgeons performing less than 3.6 cases per year in Ontario from 2009–2013.
This study’s strength includes the large number of cases, removing selection bias by capturing all cases of bladder cancer treated with RC and, therefore, reflecting the Ontario population. However, our data has some limitations, most importantly those inherent in retrospective, observational studies. Although this dataset does include general information regarding disease state, treatments obtained, and related outcomes, it does lack patient-specific details such as specific individual preoperative risk, imaging data, and patient preferences.
Conclusions
This study documents passive centralization of RC to higher-volume providers in the province of Ontario. Associated with this centralization, there have been measurable improvements in processes-related indicators of quality surgical care, including use of the multidisciplinary team. Although there was also an associated improvement in CSS, in the most recent era, there was continued evidence of low-volume providers in Ontario with a remaining volume effect on processes of care and outcomes. Continuing to promote centralization of RC would appear to be appropriate, along with advocacy of quality program implementation and benchmarking of both structure- and process-related indicators of care.
Supplementary Information
Acknowledgements
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). Parts of this material are based on data and information compiled and provided by: MOHLTC, CCO, CIHI. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Footnotes
Supplementary data available at cuaj.ca
See related commentary on page 97
Competing interests: Dr. Siemens has participated in educational talks supported by Ferring; and has participated in clinical trials supported by Astellas, Janssen, and Pfizer. The remaining authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed
References
- 1.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–37. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siemens DR, Mackillop WJ, Peng Y, et al. Processes of care and the impact of surgical volumes on cancer-specific survival: A population-based study in bladder cancer. Urology. 2014;84:1049–57. doi: 10.1016/j.urology.2014.06.070. [DOI] [PubMed] [Google Scholar]
- 3.Christian CK, Gustafson ML, Betensky RA, et al. The volume outcome relationship: Don’t believe everything you see. World J Surg. 2005;29:1241–4. doi: 10.1007/s00268-005-7993-8. [DOI] [PubMed] [Google Scholar]
- 4.National Research Council. Interpreting the volume-outcome relationship in the context of cancer care. National Academies Press; 2001. Aug 11, [PubMed] [Google Scholar]
- 5.Afshar M, Goodfellow H, Jackson-Spence F, et al. Centralization of radical cystectomies for bladder cancer in England, a decade on from the ‘Improving Outcomes Guidance’: The case for super centralization. BJU Int. 2018;121:217–24. doi: 10.1111/bju.13929. [DOI] [PubMed] [Google Scholar]
- 6.Finley CJ, Bendzsak A, Tomlinson G, et al. The effect of regionalization on outcome in pulmonary lobectomy: A Canadian national study. J Thorac Cardiovasc Surg. 2010;140:757–63. doi: 10.1016/j.jtcvs.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stitzenberg KB, Sigurdson ER, Egleston BL, et al. Centralization of cancer surgery: Implications for patient access to optimal care. J Clin Oncol. 2009;27:4671–8. doi: 10.1200/JCO.2008.20.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong ZV, Loehrer AP, Fernández-del Castillo C, et al. Potential impact of a volume pledge on spatial access: A population-level analysis of patients undergoing pancreatectomy. Surgery. 2017;162:203–10. doi: 10.1016/j.surg.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konety BR, Dhawan V, Allareddy V, et al. Impact of hospital and surgeon volume on in-hospital mortality from radical cystectomy: Data from the health care utilization project. J Urol. 2005;173:1695–700. doi: 10.1097/01.ju.0000154638.61621.03. [DOI] [PubMed] [Google Scholar]
- 10.Herr H, Lee C, Chang SA, et al. Bladder Cancer Collaborative Group. Standardization of radical cystectomy and pelvic lymph node dissection for bladder cancer: A collaborative group report. J Urol. 2004;171:1823–8. doi: 10.1097/01.ju.0000120289.78049.0e. [DOI] [PubMed] [Google Scholar]
- 11.Herr HW. Extent of surgery and pathology evaluation has an impact on bladder cancer outcomes after radical cystectomy. Urology. 2003;61:105–8. doi: 10.1016/S0090-4295(02)02116-7. [DOI] [PubMed] [Google Scholar]
- 12.Kassouf W, Aprikian A, Black P, et al. Recommendations for the improvement of bladder cancer quality of care in Canada: A consensus document reviewed and endorsed by Bladder Cancer Canada (BCC), Canadian Urologic Oncology Group (CUOG), and Canadian Urological Association (CUA), December 2015. Can Urol Assoc J. 2016;10:E46–80. doi: 10.5489/cuaj.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Booth CM, Siemens DR, Li G, et al. Perioperative chemotherapy for muscle–invasive bladder cancer: A population–based outcomes study. Cancer. 2014;120:1630–8. doi: 10.1002/cncr.28510. [DOI] [PubMed] [Google Scholar]
- 14.Clarke EA, Marrett LD, Kreiger N. Appendix 3 (c) Cancer registration in Ontario: A computer approach Cancer Registration Principles and Methods. Lyon: IARC Publications; 1991. pp. 246–57. [PubMed] [Google Scholar]
- 15.Williams JI, Young W. A summary of studies on the quality of healthcare administrative databases in Canada. Patterns of health care in Ontario: The ICES practice atlas. 2nd ed. Vol. 339. Ottawa: CMA; 1996. p. 45. [Google Scholar]
- 16.Booth CM, Li G, Zhang-Salomons J, et al. The impact of socioeconomic status on stage of cancer at diagnosis and survival: A population–based study in Ontario, Canada. Cancer. 2010;116:4160–7. doi: 10.1002/cncr.25427. [DOI] [PubMed] [Google Scholar]
- 17.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yafi FA, Aprikian AG, Chin JL, et al. Contemporary outcomes of 2287 patients with bladder cancer who were treated with radical cystectomy: A Canadian, multicenter experience. BJU Int. 2011;108:539–45. doi: 10.1111/j.1464-410X.2010.09912.x. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni GS, Urbach DR, Austin PC, et al. Higher surgeon and hospital volume improves long–term survival after radical cystectomy. Cancer. 2013;119:3546–54. doi: 10.1002/cncr.28235. [DOI] [PubMed] [Google Scholar]
- 20.Donabedian A. Evaluating the quality of medical care. Milbank Quarterly. 2005;83:691–729. doi: 10.1111/j.1468-0009.2005.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollenbeck BK, Wei Y, Birkmeyer JD. Volume, process of care, and operative mortality for cystectomy for bladder cancer. Urology. 2007;69:871–5. doi: 10.1016/j.urology.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373:1388–90. doi: 10.1056/NEJMp1508472. [DOI] [PubMed] [Google Scholar]
- 23.Schlomm T, Huland H, Graefen M. Improving outcome of surgical procedures is not possible without adequate quality measurement. Eur Urol. 2014;65:1017–9. doi: 10.1016/j.eururo.2013.11.042. [DOI] [PubMed] [Google Scholar]
- 24.Cathcart P, Sridhara A, Ramachandran N, et al. Achieving quality assurance of prostate cancer surgery during reorganization of cancer services. Eur Urol. 2015;68:22–9. doi: 10.1016/j.eururo.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 25.Liu JH, Zingmond DS, McGory ML, et al. Disparities in the utilization of high-volume hospitals for complex surgery. JAMA. 2006;296:1973–80. doi: 10.1001/jama.296.16.1973. [DOI] [PubMed] [Google Scholar]
- 26.Ward MM, Jaana M, Wakefield DS, et al. What would be the effect of referral to high–volume hospitals in a largely rural state? J Rural Health. 2004;20:344–54. doi: 10.1111/j.1748-0361.2004.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 27.Birkmeyer JD, Siewers AE, Marth NJ, et al. Regionalization of high-risk surgery and implications for patient travel times. JAMA. 2003;290:2703–8. doi: 10.1001/jama.290.20.2703. [DOI] [PubMed] [Google Scholar]
- 28.Casey MF, Wisnivesky J, Le VH, et al. The relationship between centralization of care and geographic barriers to cystectomy for bladder cancer. Bladder Cancer. 2016;2:319–27. doi: 10.3233/BLC-160058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.