Abstract
Extraordinary incidents resulting in airborne infectious disease outbreaks could produce patient isolation requirements that exceed most hospitals' capacity. This article investigates expedient methods to establish airborne infection isolation areas using a commercially available portable filtration unit and common hardware supplies. The study was conducted within a conventional, nonisolation hospital room, and researchers evaluated several airborne isolation configurations that did not require building ventilation or structural modifications. A portable high-efficiency particulate air filtration unit and full-length plastic curtains established a “zone-within-zone” protective environment using local capture and directional airflows. The cost of constructing the expedient configurations was less than US$2,300 and required fewer than 3 person-hours to construct. A medical nebulizer aerosolized polystyrene latex microspheres to generate respirable condensation nuclei. Aerosol spectrometers sized and counted respirable particles at the source patient and health care worker positions and in areas outside the inner zone. The best-performing designs showed no measurable source migration out of the inner isolation zone and mean respirable particle counts up to 87% lower at the health care worker position(s) than those observed directly near the source patient location. Investigators conclude that with careful implementation under emergency circumstances in which engineered isolation rooms are unavailable, expedient methods can provide affordable and effective patient isolation while reducing exposure risks and potential disease transmission to health care workers, other patients, and visitors.
Introduction
Background
The health care burden during an infectious disease outbreak, such as the recent experience with severe acute respiratory syndrome (SARS), or a large bioterrorism event will fall disproportionately on health care providers at the local level. Hospital emergency departments (EDs), outpatient clinics, and even physician offices could be required to handle a surge of patients, many potentially infectious, others motivated by fear to seek medical care for their nonspecific “flu-like” or respiratory symptoms. In addition to providing patient care, health care facilities must also protect their patients, staff, and visitors from exposure to potentially infectious patients. Between April 15 and June 9, 2003, 74 SARS cases were reported to Toronto Public Health. Of these, 29 (39%) of 74 were among health care workers, 28 (38%) of 34 occurred as a result of exposure during hospitalization, and 17 (23%) of 74 occurred among hospital visitors.1 Under these scenarios, staffing shortages are probable, appropriate respiratory protection supplies will be in high demand, and the need to isolate potentially infectious patients will exceed the availability of airborne infection isolation rooms.
Importance
Although the US government has been working to address shortcomings in our emergency medical response plan for extraordinary incidents, feasible solutions that are applicable across multiple demographics have been slow to develop and appear costly to implement. Recent governmental reports indicate that the US health care system generally lacks the patient isolation capacity to handle a significant airborne infectious epidemic or bioterrorism event.2., 3. Attempts to identify alternative isolation approaches have had few details and much controversy.4., 5. Draft recommendations prepared by the Centers for Disease Control and Prevention (CDC) for community-level preparedness and response to SARS include a few references to the use of portable filtration units and other engineering controls to address surge-patient isolation requirements; however, more guidance is needed about their selection and effective use.6
Goals of this investigation
The purpose of this work is to evaluate the potential feasibility of expedient, negative-pressure, high-efficiency particulate air (HEPA)–filtered patient enclosures for control of airborne pathogens during emergencies requiring isolation surge capacity. HEPA filters are at least 99.97% efficient in removing particles that are 0.3 μm (the size most difficult to capture) and essentially 100% efficient for particles either larger or smaller than 0.3 μm. The selection of portable filtration technology as an evaluated approach was fueled by its compatibility with existing ventilation systems, its affordability, and its recognition in published literature as an available engineering control to assist in patient isolation.7., 8., 9., 10. In particular, these references cite the use of portable filtration units as a way to increase the effective dilution of airborne contaminants within a designated airborne infection isolation room. By virtue of a “zone-within-zone” approach to configuring patient isolation areas, the researchers sought to improve on the traditional whole-room dilution approach to contaminant control by significantly increasing source containment and capture efficiency within a smaller inner isolation zone occupied by the patient. In this manner, contaminant spread to the remainder of the room (the outer zone) could be minimized, thus reducing health care worker exposure potential.
Materials and methods
Room description and isolation zone configurations
The performance of a free-standing, portable, HEPA filtration unit ( Figure 1) as an aid to establish expedient airborne infection isolation areas was evaluated within a hospital room of roughly 2,500-cubic-foot volume (71 m3). The room's floor space measured approximately 20×16 ft (6×5 m). The original room design accommodated 3 patient beds, with no provisions for airborne infection isolation. A restroom measuring approximately 5×10 ft (1.5×3.0 m) was connected to the patient room. The door to the restroom remained closed throughout the testing. For the patient isolation research, there were 2 evaluated configurations: a 2-patient configuration with independent isolation areas for each patient and a single-patient isolation configuration. Of the 7 trial scenarios evaluated during the study, 3 scenarios were variations of the 2-patient configuration, and 4 scenarios were variations of the single-patient configuration.
Figure 1.

Photographs of the NuAire portable HEPA filtration unit used in this work. A, The assembled unit viewed from the inlet side (cellular telephone shown for scale); B, inlet grille and one 2×2 ft (0.61×0.61 m) prefilter removed to show fan unit; C, rear view with exhaust grille removed to show the 4.5×2 ft (1.22×0.61 m) HEPA filter.
The same HEPA filtration unit (Model NU-114, NuAire Inc., Plymouth, MN; US$2,195.00) provided the air-cleaning capacity for each configuration. The existing cotton privacy curtains were removed from their tracks and replaced by floor-to-ceiling plastic curtains. In actual practice, the cotton privacy curtains could remain in place, and the plastic curtains could be mounted inside of the cotton. The plastic curtains were constructed from 4-mil plastic sheeting sold as painting drop cloth in home improvement and painting supply stores. The plastic curtains were mounted to within approximately 0.5 inch (1.3 cm) of the ceiling curtain track using the existing curtain hooks, thus retaining the curtain's ability to be completely opened and closed. The curtain extended 8 feet (2.4 m) downward to approximately 0.5 inch (1.3 cm) above the floor.
For the 2-patient configuration, the HEPA unit was placed equidistant between the footboards of the 2 patient beds and diagonally across from the entrance into the individual patient areas ( Figure 2). The inlet perimeter of the HEPA unit was tightly secured to the 2 curtains using plastic sheeting and strips of sheathing tape. In this configuration, the HEPA unit pulled “contaminated” air from the 2 patient isolation areas (inner zones) and discharged clean air into the remainder of the room (the common outer zone). After initial tests revealed a transfer of source aerosol between the 2 patient isolation zones, additional 4-mil plastic sheeting was used to construct a floor-to-ceiling vertical partition at the inlet-side of the HEPA unit to prevent such migration. A photograph of the inlet side of the HEPA unit showing the vertical partition, as well as the plastic curtains taped into place, is shown in Figure 3. To facilitate controlled airflow into the inner isolation zones, each curtain was retracted to create a gap approximately 10 inches (0.25 m) wide near the head of the respective patient beds where the patient care provider might frequently stand. The gap width selection was based on qualitative smoke tests using a handheld smoke generator (Cumulus Air Flow Indicator, Draeger Safety Inc., Pittsburgh, PA). This gap provided a strategic path of least resistance for air to enter the inner isolation zone and flow past the health care worker, over the patient, and toward the HEPA unit with minimal recirculation inside the enclosure.
Figure 2.
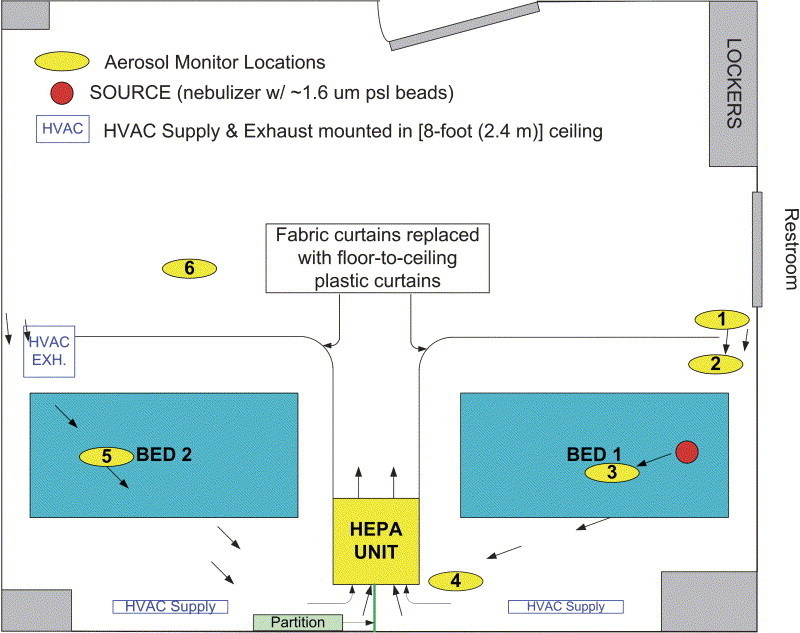
Schematic showing a 20×16 ft (6×5 m), 3-bed nonisolation hospital room expediently converted to contain isolation zones for 2 potentially infectious patients. In this configuration, a single HEPA filtration unit provides the air cleaning capacity for both isolation zones.
Figure 3.

Photograph showing inlet side of the HEPA filtration unit located between 2 patient isolation zones. The vertical partition at the inlet grill successfully prevented source aerosol from transferring between the 2 isolation zones.
In the single-patient configuration, the HEPA unit was placed near the wall at the foot of the patient bed and incorporated into the curtain boundary as shown in Figure 4. Additional plastic sheeting was taped around the HEPA inlet to form a tight seal between the plastic curtain, the wall, and the inlet perimeter of the HEPA unit. Because of the increased airflow within the single isolation zone, the entrance curtain gap was increased to 12 inches (30 cm) on the basis of results from the qualitative smoke tests.
Figure 4.
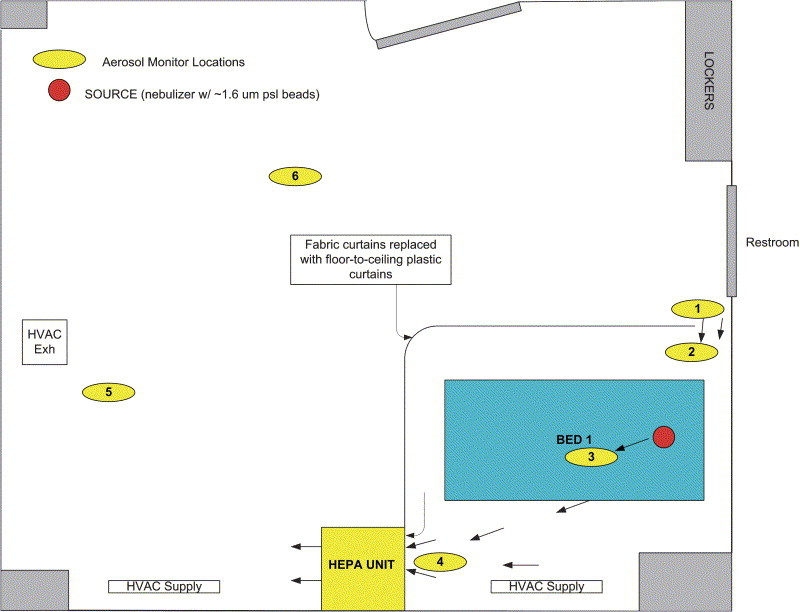
Schematic showing a 20×16 ft (6×5 m), 3-bed nonisolation hospital room expediently converted to contain a single zone for a potentially infectious patient. In this configuration, the HEPA filtration unit is reoriented to serve the single isolation zone.
Both configurations were designed to pull clean air into the inner isolation zone and into the space occupied by a bedside health care worker. The air path continued past the worker and across the patient position where the air became potentially “contaminated.” The contaminated air from the inner isolation zone was pulled toward the HEPA unit, cleaned, and then discharged into the room's outer zone, thus maintaining the inner zone at a negative pressure relative to the outer zone and its adjacent areas. Smoke tests were conducted along the top and bottom edge of the plastic curtains to verify a consistent inward airflow.
Airflow measurements
Volumetric flow rates through heating, ventilating, and air conditioning (HVAC) supply air diffusers, exhaust grilles, and the HEPA unit were measured using an Alnor Electronic Balometer (Model APM 150, TSI Incorporated, Shoreview, MN). The balometer's 1×4-ft (0.3×1.2 m) extension hood was used to measure airflow rates through the HVAC supply diffusers and exhaust grille. The 2×4-ft (0.6×1.2 m) extension hood was used to measure the airflow rate on the discharge side of the HEPA unit.
Particle control measurements
Uniformly sized polystyrene latex microspheres of 1.65-μm diameter (Catalog No. 4016A, Duke Scientific, Palo Alto, CA) were aerosolized as condensation nuclei originating from the patient 1 head position using a standard medical air-jet nebulizer operating at a pressure of 20 psi (138 kPa) (PARI Star nebulizer with ProNeb Ultra compressor Model 85B 0000, PARI Innovative Manufacturing Inc., Midlothian, VA). This particle size was chosen as being representative of the 1- to 3-μm size range of tuberculosis bacteria, spores (including anthrax spores), and other infectious bioaerosols that remain airborne for long periods, are readily inhaled, and penetrate deep into the lung.11 The airborne particles were sized and counted at 3 locations inside of the source patient's (patient 1) isolation zone and 3 locations outside of this zone using real-time light-scattering aerosol spectrometers (Grimm Dust Monitors, Models 1.105, 1.106, and 1.108 [2 each], Labortechnik GmbH & CoKG, Ainring, Germany). These monitors measure the aerosol size distribution in 8 size ranges. Results were logged in the form of particle counts per liter of air at a 1-minute sampling interval. For the purposes of this research, attention was paid to the size range between 1 μm and 2 μm, which corresponds to the 1.65-μm source aerosol. The aerosol monitors were located as shown in Figure 2, Figure 4 and placed at bed height (approximately 36 inches [91 cm] above the floor) except the provider position monitor inside the enclosure, which was placed at a height (60 inches [150 cm] above the floor) representing the breathing zone of a standing health care provider. Monitors outside the enclosure were placed at bed height to indicate the potential for exposure of other patients to particles escaping the enclosure.
Before the polystyrene latex microspheres were aerosolized, the HEPA unit was operated for 45 minutes with the restroom and entry doors closed to minimize background aerosol concentrations. Background concentrations were consistently reduced to less than 20 cpm for 1- to 2-μm particles. This concentration was generally 3% to 5% of the concentration produced near the aerosol generator at the patient head position during experiments. For each trial, the nebulizer cup was prepared by adding 3 drops of the suspended polystyrene latex microspheres into 8 mL of water purified by reverse osmosis (reverse osmosis water). After collection of at least 5 minutes of background readings, the nebulizer was activated and the airborne particle counts were logged throughout a 30-minute nebulization period. The collected particle count data were downloaded to a personal computer for archiving and analysis within an Excel spreadsheet (Microsoft Corporation, Redmond, WA).
In the 2-patient and single-patient configurations, multiple tests were performed incorporating several design and operational combinations associated with the HVAC supply diffusers and exhaust grille. When sealed, the linear HVAC supply diffusers were covered with tape. Similarly, the square exhaust grille was sealed with tape and plastic for all 2-patient configuration trials, where its location fell within patient 2's inner isolation zone. The exhaust was also sealed for 1 of the 4 single-patient configuration trials, even though it fell outside the inner isolation zone, in recognition that despite the containment of the inner zone, some hospitals or jurisdictions may still not allow air recirculation from the overall room back into the general HVAC system.
Discussion
Airflow
The HEPA unit's flow rate was measured at 550 cubic feet per minute (cfm) (0.26 m3/s [cms]). Expressed in terms of air changes per hour, the unit provided more than 13 air changes per hour of filtration for the entire patient room (inner and outer zones) while providing more than 32 air changes per hour for the isolated inner zones of the 2-patient configuration and more than 65 air changes per hour for the isolated inner zone of the single-patient configuration. All of these values compare favorably with design recommendations provided by the CDC, the American Institute of Architects, and the American Society of Heating, Refrigeration, and Air-Conditioning Engineers, which prescribe at least 12 air changes per hour for engineered airborne infection isolation rooms, although for engineered rooms, they also prescribe at least 2 air changes per hour of outdoor air.7., 8., 9., 12. When open, the HVAC supply diffuser near patient bed 1 delivered 185 cfm (0.09 cms), the diffuser near patient bed 2 delivered 130 cfm (0.06 cms), and the exhaust grille removed 155 cfm (0.07 cms) from the room. The bathroom flow rates were 90 cfm (0.04 cms) of supply air and 125 cfm (0.06 cms) of exhaust air directed to the outdoors, resulting in more than 18 air changes per hour within the restroom while maintaining it under negative pressure. Thus, even within the restroom, the airflow values maintained consistency with the prescribed design recommendations for engineered isolation rooms.
Qualitative smoke tests
In addition to verifying airflow directions and assisting in the selection of the desired curtain gap, the qualitative smoke tests were instrumental in constructing and fine tuning the evaluated configurations. After the initial trial run in the 2-patient configuration revealed low-level source migration into the outer zone, qualitative smoke tests revealed that a stream of contaminated air, induced by the discharge side of the HEPA unit, was escaping under the HEPA unit in the gap created by the wheels. A simple addition of tape and plastic eliminated this path, and no further migration of source aerosol was measured or observed. Smoke tests, such as the one shown in Figure 5, were also helpful in conducting qualitative evaluations to visualize a configuration's ability to quickly capture and remove airborne contaminants.
Figure 5.

Photograph showing a qualitative smoke test used to verify the airflow path across patient head position and toward the HEPA filtration unit's inlet.
Particle control results
There were 3 key items of interest when the feasibility of the zone-within-zone isolation configurations was assessed: (1) ability to contain the source aerosol within the inner isolation zone(s); (2) ability to prevent source contaminant crossover between patient isolation zones in the 2-patient configuration; and (3) ability to maintain a lower concentration of source aerosol at the health care worker position relative to that surrounding the immediate patient position.
A graph from one of the trial configurations is shown in Figure 6. The disparity in particle counts observed at patient bed 1, the provider position, and the remaining measurement locations produces poor resolution at the lower particle counts. Figure 7 is a reduced-scale version of the same graph to allow better resolution of the particle counts measured outside patient 1's inner isolation zone. In evaluation of the ability of a tested scenario to contain the source aerosol within the inner isolation zone, the particle counts logged by the outer zone aerosol monitors were examined for concentration increases within the 1- to 2-μm band corresponding to the released source aerosol. If all of the outer zone monitors reported steady or decreasing concentrations, the inner zone was considered to have contained the generated source aerosol. In the 2-bed configuration trials, the potential migration of source aerosol between patient zones was evaluated by observing concentration trends within the patient 2 isolation zone. Preventing such contaminant crossover could be extremely important if the 2 patients have only a suspect diagnosis, such as with patients recently admitted into a hospital ED. Last, the metric used to determine the relative ability of a tested scenario to reduce the risk of airborne exposure to the health care worker was the mean particle count (within 1- to 2-μm range) observed at the health care worker position divided by that observed near the source patient (monitoring position at center of bed). This value is identified as the “worker/patient exposure ratio.” Although the metric is useful in evaluating the different scenarios and in making comparisons between them, there is insufficient evidence to extrapolate this metric to a quantitative exposure reduction in an actual environment.
Figure 6.
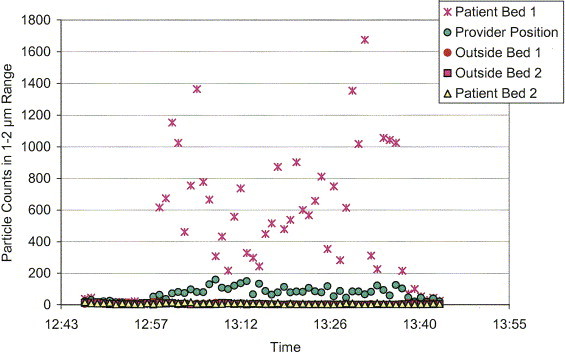
Graph of data results for trial 3, a 2-patient configuration (patient 1 was the source patient) with the HEPA unit divided vertically at the inlet to prevent crossover between patient areas. Note: the red circles are hidden behind the yellow trianges.
Figure 7.
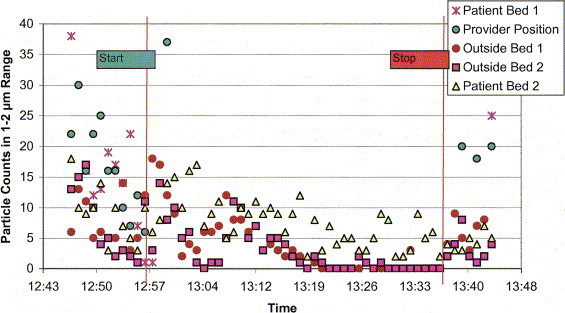
Reduced-scale graph of trial 3 data in Figure 6 allows better resolution of particle counts recorded at patient bed 2 (the nonsource patient) and locations outside the inner isolation zones. The start line represents the starting point of aerosol generation; the stop line represents the deactivation point of the aerosol generator.
A summary of the results from the 7 evaluated trial scenarios is shown in the Table. The first trial scenario, a 2-patient configuration with the curtain entrance gap closed, was the worst performing trial among both configurations. However, the knowledge gained by this test run aided the success of subsequent trials. After establishing the desired curtain gap and sealing the gap discovered under the HEPA unit, the aerosol count data for trials 2 through 7 revealed continuous reductions in aerosol counts for those monitors located outside the inner zone, despite the activation of the nebulizer and source generation. Counts outside the enclosures were indistinguishable from background levels present at the beginning of the experiments, indicating that the source aerosol was being successfully contained, captured, and filtered within the inner isolation zone. After trials 1 and 2 revealed slight increases in aerosol concentration within the patient 2 isolation zone, the vertical partition was added to the inlet-side of the HEPA unit, thus isolating the 2 inner isolation zones and eliminating this potential path of contaminant crossover.
Table.
Data summary from 7 evaluated trials of 2-patient and single-patient isolation zones created using portable HEPA filtration equipment and common hardware supplies.
| Trial No. | Supply/Exhaust (Open or Sealed) | Source Contained Within Inner Zone? | Source MigrationBetween Patients? | Worker or Patient Exposure Ratio (95% CI)∗ | Comments |
|---|---|---|---|---|---|
| 2-patient configuration | |||||
| 1 | Sealed/sealed | N | Y | 0.59 (0.45–0.76) | Curtain entrance closed, leak found under HEPA unit, no HEPA inlet partition |
| 2 | Sealed/sealed | Y | Y | 0.67 (0.44–1.06) | Curtain entrance gap maintained at 10 in (25 cm), no HEPA inlet partition |
| 3 | Sealed/sealed | Y | N | 0.13 (0.10–0.18) | Curtain entrance gap maintained at 10 in (25 cm), HEPA inlet partition added |
| Single-patient configuration | |||||
| 4 | Sealed/sealed | Y | NA | 0.29 (0.19–0.46) | Increased airflow: curtain entrance gap increased to 12 in (30 cm) |
| 5 | Open/open | Y | NA | 0.55 (0.37–0.86) | HVAC supply reduces incoming air, curtain entrance gap maintained at 10 in (25 cm) |
| 6 | Sealed/open | Y | NA | 0.37 (0.28–0.48) | Room HVAC supply located outside of isolation zone was open |
| 7 | Open/open | Y | NA | 0.70 (0.55–0.92) | Curtain gap maintained at 10 in |
Y, Yes; N, no; CI, confidence interval; NA, not applicable.
This ratio is the mean particle count (within range of 1 to 2 μm) observed at the health care worker position divided by that observed near the source patient. It represents the relative ability of a tested scenario to reduce the risk of airborne exposure to the health care worker.
The worker-patient exposure ratio shown in the Table reveals mean respirable particle counts at the health care worker position ranging from 30% to 87% lower than those observed at the patient position. Even trials 1 and 2, which were operating under less-than-ideal circumstances, provided a protective benefit to the worker position. The most protective trial among all tested configurations was the 2-patient configuration evaluated during trial 3. Trials 4 and 6 among the single-patient configurations also look promising. There were increased variability and diminished protective effect in trials 5 and 7 when the supply air diffusers were opened within the inner isolation zone, which is believed to be caused by the competing air currents increasing turbulent mixing within the inner isolation zone and disrupting the flow path into the HEPA unit. In practice, supply diffusers within the inner isolation zones should probably remain closed, thus relying on tempered transfer air from the outer zone to meet thermal comfort requirements.
The clinical significance of the observed reductions in provider position particle concentration will depend on what pathogen is being controlled and what other protective precautions are used by the provider. In particular, the proper selection, correct fit, and appropriate use of protective devices such as N95, N99, or N100 respirators, as well as provider care to avoid placing oneself in the path of contaminated air movement, will be critical adjuncts to enclosure ventilation in minimizing provider risk. The key characteristic of the observed reductions is the controlled movement of the particles away from the patient and provider and toward the HEPA unit. This controlled movement allows providers to minimize their exposure by positioning themselves away from the airflow path. Standard isolation rooms do not share this feature because room air is mixed to dilute contaminants rather than being directed in a single flow direction for removal. Furthermore, contaminant removal by directed airflow is virtually immediate, whereas removal by dilution takes place during a period determined by the room volume and ventilation rate.
Costs
The cost of the HEPA filtration unit used in this work was US$2,195, largely because of its heavy-gauge steel construction. However, HEPA units of equivalent filtration capacity are available at significantly lower (and higher) cost, depending on their construction and any additional features such as activated carbon sorption beds for volatile organic chemical removal and ultraviolet biocidal lamps for airstream or filter surface disinfection. The cost of expendable materials used in constructing the enclosures, which included plastic sheeting and packaging tape available in any home improvement store, was well under US$100; however, expendable materials could cost somewhat more in applications requiring larger-scale or more complex construction.
Conclusions
The current research sought to assess the feasibility of using affordable, off-the-shelf equipment and supplies in combination with a zone-within-zone isolation approach to quickly establish airborne infection isolation for 1 or more patients. As a feasibility study, there were multiple scenarios evaluated, with each scenario receiving insufficient scrutiny to develop rigorous performance predictions. Future research will seek to identify uniform implementation recommendations capable of generating optimum predictable results in a variety of physical environments. In the absence of an emergency scenario, the investigated isolation approach should not be considered an acceptable replacement for engineered airborne infection isolation rooms. However, under extraordinary circumstances where the quantity of engineered airborne infection isolation rooms is insufficient to meet surge demand for patient isolation, hospital facilities could quickly deploy portable filtration equipment in combination with zone-within-zone patient isolation configurations.
Hospitals seeking to use these techniques should preferably obtain the necessary equipment and supplies well in advance of their potential implementation. Identifying rooms for potential conversion, preconstructing the isolation zone boundaries (curtains), and qualitatively testing the configurations with visible smoke will all increase the readiness level of the facility. After testing and disassembly, reassembly instructions should be generated and stored with the labeled components for future emergency use. During an actual emergency implementation, an initial quantitative leakage and filter performance test of the HEPA filter unit should be conducted using methodologies consistent with those in the CDC Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Facilities.7 In addition, initial and periodic qualitative testing using visible smoke or equivalent should be conducted to verify capture and containment within the inner isolation zone.
The expedient isolation alternative appears to offer fast, affordable, and effective patient isolation during unique circumstances in which engineered isolation rooms are unavailable. The best-performing designs showed no measurable source migration out of the inner isolation zone and mean respirable particle counts up to 87% lower at the health care worker position(s) than those observed directly near the source patient location. The zone-within-zone patient isolation approach also offers a greater protective benefit to nonprovider hospital workers, visitors, and other patients over approaches that rely solely on isolating entire wards, floors, or designated facilities. Such “big-area” approaches invite a wide contaminant distribution that increases the potential for airborne and surface contact exposures to all who enter. In contrast, the inner isolation zones in the zone-within-zone approach use a much higher ventilation rate while containing contaminant distribution within a much smaller area, thereby reducing the potential for airborne or surface contact. The evaluated expedient designs took fewer than 3 person-hours to construct at a total cost (including US$2,195 for the HEPA unit) of less than US$2,300. Because many hospitals already own high-flow-rate, HEPA-filtered, “negative-air” exhaust systems that they use for dust control during renovation work, the expedient isolation cost could be reduced to perhaps US$50 to US$100 per isolation area because only the plastic sheeting and other expendable materials would be needed. The 3-hour assembly time for the prototype was likely much longer than would be required to erect a preplanned enclosure for which the design was identified and evaluated ahead of time and with which the assemblers were familiar. To minimize construction time, the assembly training and practice could be incorporated into the facility's emergency operations preparation activities.
Recommendations for follow-up work
This study was designed as a preliminary assessment of the utility of expedient isolation enclosures for control of airborne pathogens. As such, it has several limitations that should be considered in interpreting the results. First, the study was limited to a discrete set of enclosure designs evaluated in a single facility, whereas actual work environments will undoubtedly require variations to accommodate specific patient care situations and health care facility engineering characteristics. Additional work is needed to refine design options that integrate the enclosure systems with the facility HVAC systems to ensure pathogen control while maintaining patient comfort, without interfering with HVAC operation in other areas of the facility. Second, the measurements were performed without an actual patient in place and undergoing normal care so that the influence of factors such as patient movement, positioning, breathing, and coughing, as well as care provider movements on system effectiveness, remains to be evaluated. Finally, actual pathogenic bioaerosols were not used in the work; however, this should not be considered a limitation because the determining factor in particle movement and filtration efficiency is particle aerodynamic size. Because the viability and pathogenicity of biologic aerosols are negatively affected by environmental conditions, including temperature, humidity, and oxygen toxicity,13., 14. use of inanimate surrogates actually represents a conservative evaluation of system effectiveness.
Acknowledgments
We thank Phil Comp, MD, Janey Wooley, RN, and Beverly Stiles, RN, of the Oklahoma City VA Medical Center for facilitating the discussed research in their facility; Timothy Cathey, MD, of the Oklahoma State Department of Health, for contributing his medical insights; and Duane Hammond, BSME, National Institute for Occupational Safety and Health, for his assistance in data collection and experimental setup.
Footnotes
The research addressed in this manuscript was presented at the 2004 Public Health Professional Conference, May 16 to 20, Anchorage, AK.
The authors report this study did not receive any outside funding or support.
References
- 1.Health Canada. Update: Severe acute respiratory syndrome: Toronto, 2003 [Health Canada Web site]. Available at: http://www.hc-sc.gc.ca/pphb-dgspsp/publicat/ccdr-rmtc/03vol29/dr2913ea.html. Accessed January 9, 2004.
- 2.Heinrich J. Infectious disease outbreaks: bioterrorism preparedness efforts have improved public health response capacity but gaps remain. Available at: http://www.gao.gov/new.items/d03654t.pdf. Accessed March 12, 2004.
- 3.United States General Accounting Office. Bioterrorism: preparedness varied across state and local jurisdictions. Available at: http://www.gao.gov/new.items/d03373.pdf. Accessed March 12, 2004.
- 4.Yee D. National SARS plan falls short, critics say: medical system called ill-prepared. Cincinnati Enquirer. September 28, 2003; sect A:A3.
- 5.Evans G. Bioterrorism watch: APIC: smallpox plan uses outdated infection control. Ed Management. 2002;14(Suppl):3–4. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Public health guidance for community-level preparedness and response to severe acute respiratory syndrome (SARS). Available at: http://www.cdc.gov/ncidod/sars/guidance/. Accessed March 12, 2004.
- 7.Centers for Disease Control and Prevention Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities. MMWR Morb Mortal Wkly Rep. 1994;43:RR-13. [PubMed] [Google Scholar]
- 8.Sehulster L.M., Chinn R.Y.W., Arduino M.J. American Society for Healthcare Engineering/American Hospital Association; Chicago, IL: 2004. Guidelines for Environmental Infection Control in Health-Care Facilities: Recommendations from CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) [Google Scholar]
- 9.AIA Academy of Architecture for Health and US Department of Health and Human Services . American Institute of Architects Press; Washington, DC: 2001. Guidelines for Design and Construction of Hospital and Health Care Facilities. xv. [Google Scholar]
- 10.Rutala W.A., Jones S.M., Worthington J.M. Efficacy of portable filtration units in reducing aerosolized particles in the size range of Mycobacterium tuberculosis. Infect Control Hosp Epidemiol. 1995;16:391–398. doi: 10.1086/647136. [DOI] [PubMed] [Google Scholar]
- 11.Riley R.L. Airborne infection. Am J Med. 1974;57:466–475. doi: 10.1016/0002-9343(74)90140-5. [DOI] [PubMed] [Google Scholar]
- 12.American Society of Heating Refrigerating and Air-Conditioning Engineers . ASHRAE Handbook: Heating, Ventilating, and Air-Conditioning Applications. American Society of Heating, Refrigerating and Air-Conditioning Engineers; Atlanta, GA: 2003. Health care facilities. [Google Scholar]
- 13.Cox C.S. Stability of airborne microbes and allergens. In: Cox C.S., Wathes C.M., editors. Bioaerosols Handbook. Lewis Publishers; Boca Raton, FL: 1995. pp. 77–99. [Google Scholar]
- 14.Cox C.S. John Wiley & Sons; New York, NY: 1987. The Aerobiological Pathway of Microorganisms. [Google Scholar]


