Abstract
Being aware and implementing the latest and best scientific evidence in infection control and health care epidemiology is critical to enhancing patient outcomes. In this review, the latest published scientific data in health care epidemiology and patient safety were reviewed for the period May 2003-May 2004. Medline reviews and reviews of infection control and infectious diseases journals were used for this period. The latest guidelines and publications on antimicrobial resistance, nursing or infection control professional staffing, West Nile virus, and Severe Acute Respiratory Syndrome (SARS) are included. Awareness of these and other important infection control publications is essential if the latest measures are to be implemented to prevent and control health care-associated infections.
Full implementation of the best evidence-based science in health care epidemiology and infection control is essential if patient outcomes are to be optimized and health care costs minimized. With the rapid expansion of the health care epidemiology, infection control, and patient safety literature, it is increasingly difficult to stay up to date with the latest science in the field and to identify promptly and implement lifesaving prevention interventions. Guideline recommendations for prevention of health care-associated infections (HAI) are published periodically, but, often, important prevention measures or other advances in the science occur between guideline updates and may not be brought to the attention of infection control personnel (ICP). The purpose of the State of the Science presentation at the annual meeting of the Association for Professionals in Infection Control and Epidemiology, Inc (APIC) is to review the latest scientific advances in the field each year and to bring these findings to the attention of the infection control community. This paper will summarize the advances in the state of the science of health care epidemiology, infection control, and patient safety during the last year.
Methods
The medical literature published from May 1, 2003 through May 30, 2004 was reviewed for recent advances in infection control and patient safety. A Medline search was conducted using the key terms nosocomial infection, hospital infection, antimicrobial resistance, outbreaks, surveillance, infection control, hospital epidemiology, and patient safety. In addition, the indexes of the 11 periodicals (ie, New England Journal of Medicine, Lancet, Journal of Hospital Infection, Pediatrics, Journal of Pediatrics, Journal of Infectious Diseases, Clinical Infectious Diseases, Annals of Internal Medicine, Infection Control and Hospital Epidemiology, American Journal of Infection Control, and the Centers for Disease Control and Prevention's Morbidity and Mortality Report (MMWR) were reviewed.
Because of the large number of articles meeting the review criteria, only guidelines from national organizations and publications advancing the fields of antimicrobial resistance, nurse or ICP staffing, West Nile virus, and Severe Acute Respiratory Syndrome (SARS) are included.
Results
During this period, 5 guidelines were published.1., 2., 3., 4., 5. These guidelines provide important recommendations for prevention of health care-associated pneumonia, environmental infection control, infection control in dental settings, controlling or preventing antimicrobial-resistant pathogens, and preventing infections associated with endoscopy. ICPs should read these guidelines and implement the most highly supported evidence-based recommendations.
Antimicrobial resistance
Does contact isolation really reduce transmission of antimicrobial-resistant pathogens?
This is the question addressed by Lepelletier et al6 in a 3-year study in a hospital in France. Over this period, they focused their analysis on methicillin-resistant Staphylococcus aureus (MRSA), Enterobacteriacae resistant or intermediately susceptible to third-generation cephalosporins, Pseudomonas aeruginosa, or Acinetobacter spp resistant to ticarcillin, ceftazidime, and/or imipenem. Routine clinical cultures were used to detect these pathogens; active surveillance cultures were not performed. Extensive educational efforts, including (1) meetings and training of staff; (2) meetings to discuss target pathogens and their epidemiology, transmission, and control measures; (3) use of a hygienist to promote infection control and prevent cross transmission; and (4) isolation precaution monitoring for each patient were included in the intervention. Target multidrug-resistant bacteria increased during the study period from 143 isolates in 1999 to 202 in 2001; 44% were MRSA, 20% were P aeruginosa, 22% were Enterobacteriacae, and 4% were Acinetobacter spp. Despite very high compliance with contact isolation precaution recommendations during 1999 and 2001 (ie, 82%-91% for isolation sign, 51%-85% for alcohol hand rub use, 78% for gown use, 85% for glove use, and 80%-85% for private room use), MRSA rates per 100 admissions increased from 0.29 to 0.42: P aeruginosa remained stable at 0.26, and Enterobacteriacae increased from 0.15 to 0.27. The authors concluded that “prevention programs which merely fully implement contact precautions and routine clinical cultures (ie, lack active surveillance cultures) are not sufficient to decrease the incidence of multidrug-resistant bacteria.” Furthermore, they recommended screening for carriage of multidrug-resistant bacteria in high-risk areas and patients (ie, active surveillance cultures), together with rapid isolation of colonized or infected patients for the control of multidrug-resistant bacteria.
We must be precise in the terms that we use
An article entitled “Rapid control of an outbreak of Staphylococcus aureus on a neonatal intensive care department using standard infection control practices and nasal mupirocin”7 states that active surveillance cultures, in addition to cohorting of patients and staff, contact isolation precautions, hand hygiene, and nasal mupriocin were used as the intervention. The outbreak was terminated in 4 weeks. However, it is clear that standard precautions alone were not used (as implied by the article title) but rather that active surveillance cultures were used in addition to contact precautions. If we are not precise in the terms that we use (as in the use of “standard infection control practices”in this paper), readers will be misled or confused in interpreting the results.
How often do health care worker hands become contaminated after contacting environmental surfaces?
A study conducted at a Veteran's Administration hospital evaluated how often health care worker's (HCW) hands became contaminated on 8 wards/units randomly selected over 2 weeks.8 At the hospital, stool specimen positive for Clostridium difficile were screened for vancomycin-resistant enterococcus (VRE), but no active surveillance cultures were performed for MRSA. Target pathogens were MRSA, VRE, C difficile, and gram-negative bacteria. HCW hands were cultured immediately after use of an alcohol rub and then after contact with a variety of environmental surfaces in patient's rooms (ie, bed rail, bed table, or nearby surface). Imprints of the hands were performed on blood agar. All control cultures (after alcohol rub and before environmental contact) were negative. In occupied rooms, 34 of 64 (53%) of HCW hands were positive for ≥1 target pathogen. In cleaned rooms, 6 of 25 (24%) of HCW hands were positive for ≥1 target pathogen. The authors concluded that HCWs “commonly acquire nosocomial pathogens when they contact environmental surfaces near patients who are not in contact isolation.” This paper again reemphasizes the importance of hand hygiene during all patient and environmental contacts.
Is fecal incontinence a risk factor for environmental VRE contamination?
In a prospective study at a university hospital, the frequency of VRE contamination in rooms of 15 continent and 15 incontinent patients culture positive for VRE was evaluated by culturing bed rails, bedside tables, and call buttons at baseline and 2 and 5 days after disinfection.9 Regardless of continence or incontinence status (with or without diarrhea or occasional or frequent incontinence), the rate of environmental VRE contamination was common. All baseline, day 2, or day 5 cultures were positive ≥50% of the time, except for day 2 cultures in continent patients without diarrhea, which were positive 44% of the time. The authors concluded “environmental contamination occurs frequently in rooms of patients who are continent or incontinent and VRE-colonized. Similar infection control measures should be implemented for continent and incontinent patients.” This study shows that incontinence is not a risk factor for environmental VRE contamination. Given the data that VRE colony counts in the stool of colonized or infected patients are similar,10 these data show that the infection control precautions implemented for VRE-colonized or infected patients should be the same whether or not they are continent or incontinent.
Current factors that argue for a more aggressive approach to control of health care-associated MRSA and VRE
During the past few years, we have seen the emergence of community-acquired MRSA. Some have argued that, with the emergence of MRSA in the community, efforts to control MRSA in health care settings should not be a priority. However, although the prevalence of community-acquired MRSA varies from region to region, several studies show that the prevalence of community-acquired MRSA is much, much lower than that of health care-associated MRSA. Three recent studies, 2 in pediatric populations and a third in the homeless population, found the prevalence of community-acquired MRSA to be approximately 0.2%.11., 12., 13. In addition, a national prevalence study of the US population found the prevalence of MRSA to be <1%. Thus, the prevalence of community-acquired MRSA pales in comparison with the >50% prevalence of MRSA in health care settings. Furthermore, over the past 2 years, we have witnessed the emergence of true vancomycin resistance in S aureus (VRSA).14 In 2004, the third VRSA-infected patient was identified in a long-term care facility.15 Data from the CDC's National Nosocomial Infections Surveillance (NNIS) system show that the incidence of MRSA and VRE continues to increase in our health care facilities, even at facilities with ICPs committed to infection control, suggesting that the current CDC isolation guideline recommendations have not worked (and will not in the future) in preventing transmission of MRSA or VRE in health care settings. The current incidence of MRSA exceeds 50%, and current incidence of VRE exceeds 25% in intensive care unit (ICU) settings ( Fig 1; NNIS, MRSA, and VRE). In addition, recent data monitoring the emergence of VRE in San Francisco Bay area hospitals illustrate that, if aggressive control measures are not taken, VRE will become endemic in your institution.16 In 33 hospitals in the San Francisco Bay area, from 1993 through 1998, VRE increased from 1 isolate at 1 hospital to 864 isolates in all 33 hospitals (and 100 bloodstream infections). These data again illustrate the importance of identifying and isolating patients who are colonized and those who are infected with VRE.
Fig 1.
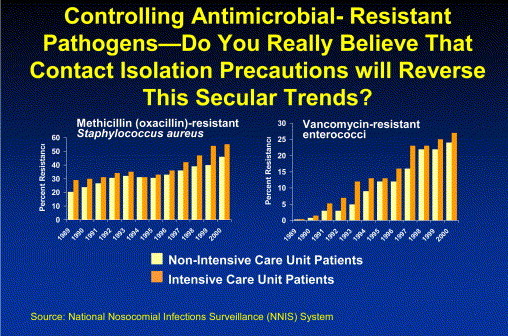
Secular Trend in MRSA- and VRE-infection rates, National Nosocomial Infections Surveillance (NNIS) System, 1989-2000.
The SHEA Guideline
The SHEA Guideline for preventing nosocomial transmission of multidrug-resistant strains of S aureus and enterococcus presents a proven approach for controlling MRSA, VISA, VRSA, and VRE.4 The major recommendations include the following: (1) active surveillance cultures to identify the reservoir (ie, colonized patients) for spread, (2) hand hygiene, (3) barrier precautions (contact isolation or cohorting), (4) antibiotic stewardship, and (5) decolonization of MRSA- or VRE-colonized patients or HCWs (in selected settings). The purpose of the active surveillance cultures is to identify all colonized patients. The guideline does not recommend that all patients admitted to the hospital be cultured. Rather, it recommends that high-risk patients be identified. Examples of such high-risk patients might include those on prolonged antibiotics, those admitted to specific areas of the hospital (eg, dialysis, ICUs, oncology wards), those with underlying diseases (eg, dialysis patients), or those with prolonged hospital stays.
What data are there to show that the SHEA Guideline approach works?
In Table 1, the impact of implementing the SHEA recommended approach in a variety of settings (entire acute care hospitals at university or community hospitals; medical, surgical or neonatal ICUs, surgical or oncology wards) is shown. In each of these interventions presented at the 2004 SHEA annual meeting, the SHEA recommendations significantly reduced MRSA compared with the experience in the previous year. In the one instance in which the intervention did not reduce MRSA, subsequent investigation revealed that compliance with surveillance cultures was only 61%, and, after enhancing compliance in obtaining the active surveillance cultures, the rates of MRSA have been significantly reduced (personnel communication, L. Mermel, MD). It seems extremely unlikely that all of these studies (and those that have been already been published) would all have findings in 1 direction—reduction of MRSA or VRE—if the results were due to chance alone. Arguments that all these studies are flawed rings hollow. Where are similar data showing that hand hygiene alone, standard precautions alone, or the implementation of the current or draft CDC HICPAC Isolation Guideline recommendations have had a similar impact? In fact, CDC NNIS data show that the prevalence of MRSA and VRE have continued to increase despite implementation of CDC-HICPAC recommendations at US hospitals.
Table 1.
Impact of active surveillance cultures, contact precautions, and hand hygiene on methicillin-resistant Staphylococcus aureus (MRSA) infection rates, SHEA annual meeting, 2004
| Abstract No. | Author | ASC | Location | Results | Prior 1–2 y |
|---|---|---|---|---|---|
| 14 | Clancy | Admit, wk | MICU | 9.5-5.3/1000 pd | 100% Increase |
| SICU | 7.8-5.1/1000 pd | ||||
| 52 | Salgado | Admit, wk | HW | 45%-34% | 41% increase |
| 56 | Fonseca | Admit, wk | NICU, Brazi | Outbreak terminated | |
| 287 | West | Admit, wk | 2 hospitals | A: 0.6-.04 | 29% increase |
| HR | B: 0.7-0.4 | ||||
| 297 | Black | Admit, wk | Univ Hosp MICU | 27-5.5 per 10,000 pd | |
| 384 | Tomic | Admit, wk | HW 237 bed Hosp-Slovenia | 50%-15% pt with HA-MRSA | |
| 446 | Ben-David | Admit, wk | HW Univ Hosp | .39%-.55% | 61% ASC compliance |
| 447 | Muder | Admit, DC | Surg ward | 1.5-0.77 infect/1000 pt | Rest hosp NC |
| 455 | Boyce | Admit, wk | SICU Hem-Onc | 1.5-0.65 per 100 admits | Cost: $11,420 |
| 313 | Karchmer | Admit, wk | HR Adult/Ped ICU & IM | 19.5%-10.4% | 15% increase |
MICU, Medical intensive care unit; pd, patient-days; SICU, surgical intensive care unit; HW, hospital wide; HR, high risk; HA, hospital-acquired; ASC, active surveillance cultures; NC, no change; hem-onc, hematology-oncology ward; IM, intermediate care unit.
What do mathematical models tell us is the best approach to control VRE?
Recently, Perencevich et al17 published a study using mathematical modeling of their ICU data evaluating a variety of VRE control strategies in ICU settings. In this model, they compared standard precautions with no active surveillance cultures and no isolation of previously known VRE-positive patients on readmission, passive surveillance and isolation of those known to be VRE-culture positive on admission or when clinical cultures detect the VRE-infected patient (ie, the CDC HICPAC approach), or active surveillance cultures with either isolation of the VRE-colonized patient and removal from isolation when the culture is negative or only placing the patient in isolation when VRE culture positivity is detected. When these approaches were compared, passive surveillance only reduced VRE by 4.2% compared with standard precautions alone. In contrast, active surveillance cultures with isolation of the patient after the culture is positive reduced VRE by 39% and active surveillance cultures with immediate isolation and removal if culture negative reduced VRE by 65% compared with no surveillance. The authors concluded that “active surveillance are projected to be effective in reducing VRE transmission in ICU settings.”
Staffing and patient safety
A variety of studies have been published in the past year on infection control staffing and the impact of infection control efforts (especially isolation) and patient safety.
What level of infection control professional staffing is necessary?
In a study by O'Boyle et al, the Delphi method was used to survey ICPs to determine staffing requirements in infection control.18 There were 45 respondents (30 at acute care and 15 at nonacute care settings) to 10 surveys. The consensus was that 1 ICP per 100 beds would be required to perform the expanded functions required of this position (eg, bioterrorism preparedness, SARS preparedness, occupational health, HIV/AIDS prevention, antimicrobial-resistance and antimicrobial use, and others).
What is the state of infection control programs in Canada?
In a survey of infection control programs in Canada, 238 ICPs were surveyed and 172 (72%) responded.19 The median staffing at these hospitals in Canada was 1 ICP per 250 beds; however, 42% of respondents had <1 ICP per 250 beds. In addition, 21% had no doctorial degree level person involved in the infection control program. Of the respondents, 62% reported surgical site infection (SSI) rates to the chief of surgery, and 37% reported SSI rates to individual surgeons. These data are very discouraging considering the low level of implementation of measures identified in the Study of the Efficacy of Infection Control (SENIC) project as significantly associated with effective infection control programs.20 As Zoutman et al concluded, “there were deficits in the identified components of effective infection control programs. Greater investment in resources is needed.” Somehow, in the United States, Canada, and around the world, a greater appreciation for the impact and benefits of vigorous infection control programs need to be recognized by our governments and health care facility administrators.
Contact isolation: does it impair patient care?
One of the increasingly voiced arguments against attempts to control aggressively the antimicrobial-resistant pathogens is that isolation precautions negatively impact on patient care. Several studies were published in the past 12 months addressing this issue. In the first, Saint et al conducted a prospective cohort study at 2 university hospitals from October 1999 to March 2000 in which they observed senior medical residents and their attending physicians caring for patients.21 Of 139 patients observed, 31 (22%) were in contact precautions. Senior medical residents were equally likely to enter the room and examine the patient whether the patient was in isolation or not (26/31 [84%) vs 94/108 [87%]. In contrast, attending physicians were less likely to enter a room and examine a patient if they were in isolation (11/31 (35%) vs 79/108 [73%], P < .001). In the second paper, Stelfox et al examined the quality of medical care received by patients isolated for at least 2 days and compared the care with (1) patients in the same room either before or after the MRSA patient in the hospital in Canada or (2) with a disease-specific cohort (congestive hear failure) of patients admitted to Brigham and Women' Hospital in Boston, Massachusetts.22 The study period was January 1, 1999 to January 1, 2000 (Canada) or January 1, 1999 to July 1, 2002 (Boston). Processes of care examined included vital signs, clinician notes, left ventricular function, stress test or angiogram, daily weights, heart failure education within 4 weeks of discharge, admission, and discharge cardiovascular medications. Outcomes of care measured included injuries that prolonged hospitalization or produced disability or transient laboratory value abnormality. Patient satisfaction measures included medical record documentation of complaints, leaving against medical advice, attempted suicide, and altercations and files from public relations for unsolicited complaints, access to staff, communication, humaneness, cleanliness, or billing. Patient medical records were abstracted onto a 1-page sheet by medical record analysts; this page then was reviewed by 2 physicians. Process of care measures showed that isolated patients were significantly more likely than nonisolated patients to have fewer than expected number of vital signs, more incompletely recorded vital signs, days with no vital signs (range, 1-6 days), days with vital signs not recorded as ordered, days with no nursing narrative, and days with no physician progress notes. General nature and severity of adverse events evaluation showed that isolated patients had significantly greater length of stay, preventable adverse events, supportive care failures–falls, pressure ulcers, and fluid or electrolyte disorders. There was no difference in overall mortality in isolated or nonisolated patients. Measures of patient dissatisfaction were more common in isolated (12 [8%]) than nonisolated (3 [1%]) patients; these included any complaint, informal complaint, or formal complaint. More controls complained about the lack of humaneness of staff and billing. For each category of measurement, the range of complaints was from 0 to 5. The authors conclude that there is a large body of studies “that support the effectiveness of isolation policies in hospitals in preventing nosocomial infections.” They point out that, although their study was quasiexperimental and raises questions of whether the group differences are simply a function of severity of illness, it is essential that the most essential components of isolation policy be identified. These studies also illustrate that ICPs must educate patient caretakers of the importance of infection control practices and that compliance with these practices is essential. We should not tolerate a physician not examining a patient because of the inconvenience of donning appropriate gowns and gloves. Would we tolerate a surgeon who did not want to scrub or wear a gown and gloves in surgery?
West Nile virus
Since 1999, we have seen the emergence and spread of West Nile virus (WNV) from the East to the West coast of the United States. Although usually associated with community vector-borne transmission, in the past 12 months, we have had several episodes of transmission of WNV associated with health care. In the first, Iwamoto et al describe transmission of WNV from an organ donor to 4 transplant recipients.23 In August 2002, fever and mental status changes developed in recipients of organs from a common donor. An investigation suggested that a blood transfusion was the probably source for the organ donor; the organs from the donor were the source of infection for the 4 recipients. Another study, by Pealer et al, evaluated the risk of transmission of WNV by blood transfusion.24 They examined patients identified with laboratory confirmed evidence of WNV infection within 4 weeks after receipt of a blood component from a donor with viremia. They identified 23 patients with confirmed WNV infection acquired from transfused leukoreduced or nonleukoreduced red cells, platelets, or fresh-frozen plasma. Ten of the 23 (43%) patients were immunocompromised secondary to transplantation or cancer. Eight of 23 (35%) patients were ≥70 years of age. Immunocompromised patients tended to have longer incubation periods. Of the 16 donors linked to the 23 infected recipients, 9 (56%) reported viral symptoms before donation. Fever, new rash, or painful eyes were independently associated with being an implicated donor with viremia. All 16 were WNV-specific IgM antibody negative at the time of donation. These data document that transfused red cells, platelets, or fresh-frozen plasma can transmit WNV. Screening of potential donors with the use of nucleic acid-based assays for WNV may reduce this risk.
Severe Acute Respiratory Syndrome
In the past 12 months, we have witnessed an explosion in the expansion of science relating to SARS. First, we had the identification of the etiologic agent of SARS, a novel coronavirus. The etiologic agent was detected using a combination of virus isolation, electron microscopy, histology, and molecular and serologic assays. Ksaizek et al documented that this new syndrome was caused by a new human coronavirus, SARS-CoV.25 In another study, Isakbaeva et al evaluated potential transmission from 7 laboratory confirmed SARS-CoV-infected persons and their 10 household contacts.26 Performing a longitudinal study, they performed follow-up visits twice a week for the first 3 weeks of illness then once a week for 2 weeks for cases and once a week for contacts. During each visit, they collected demographic, clinical, and epidemiologic, and exposure data and blood, serum, stool, urine, nasopharyngeal (NP), and oraophryngeal specimens. They documented that (1) SARS-CoV was detected in a 14-day sputum in 1 patient and in 5 stool specimens of 2 patients; (2) in 1 case, SARS-CoV persisted in stool for at least 26 days after symptom onset; (3) the highest amounts of virus were in the day 14 sputum and a day 14 stool specimen; and (4) only 1 possible transmission to household contact may have occurred (as the person had traveled to a SARS-affected area). In another study, Peiris et al examined the duration of shedding using polymerase chain reaction (PCR) and found that 90% to 95% of NP aspirates were PCR-positive for SARS-CoV at 10, 13, and 16 days; that 95% to 100% of stools were SARS-CoV PCR-positive at 10, 13, and 16 days; and that approximately 50% of urine is PCR-positive at 10 and 13 days.27 At 21 days after symptom onset, 47% of NP aspirates, 67% of stool, and 21% of urine still are SARS-CoV PCR positive. These data illustrate that persons infected with SARS-CoV can shed the virus (or at least its DNA) for prolonged periods; further studies are needed to evaluate whether the virus is infectious during this long period. Next, Seto et al evaluated what respiratory protection is necessary to prevent SARS-CoV transmission to HCWs.28 At 5 hospitals in Hong Kong, a case-control study was performed, and HCWs were surveyed about their personnel protective equipment use ([PEE], ie, masks, gowns, gloves, and HH [4 measures], as recommended by contact and droplet precautions). Next, they compared 241 noninfected HCWs to 13 SARS-CoV-infected HCWs; all HCWs had documented exposure to 11 patients with SARS. When all 4 PPE measures were implemented, 69 HCWs had exposures, and none became infected. With the omission of ≥1 measure, all 13 HCWs with exposures became SARS-CoV infected. In logistic regression analyses, only masks (of any type, not just N-95 respirators or greater) were associated with protection. The authors concluded that (1) droplet and contact precautions were adequate to significantly reduce the risk of transmission of SARS-CoV, and (2) the protective role of masks suggests that, in hospitals, SARS-CoV is transmitted by droplets. Next, Yu et al analyzed the temporal and spatial distribution of SARS patients from the Amoy Gardens housing complex in Hong Kong.29 The authors examined the date of onset and the location of residents to assess the probability of infection using logistic regression. All but 5 patients were in 7 buildings; the index and >50% of cases were in building E. The analysis determined that residents of middle and upper floors were at greater risk than those on lower floors, suggesting the possibility of a rising plume of contaminated ward air. The distribution of risk in buildings B, C, and D corresponded well with the 3-dimensional spread of virus-laden aerosols predicted with computational fluid dynamics modeling. An accompanying editorial raised the possibility that we need to think of airborne transmission of infectious agents as obligate, preferential, or opportunistic; the later being what happened at the Amoy Gardens.30 Last, 3 studies evaluated the risk of transmission of SARS-CoV to household members or HCWs.31., 32., 33. In Pennsylvania, a person with SARS exposed 26 household members, and none became infected.31 In Taiwan, 5 patients with SARS exposed 223 HCWs (many with unprotected exposures); only 1 physician seroconverted, and he had used a surgical mask for respiratory protection.32 Park et al evaluated 110 HCWs with exposure to laboratory-confirmed SARS-CoV patients who had exposures within 3 feet of 6 patients.33 Of those exposed, 45 HCWs used no mask, 72 HCWS used no eye protection, and 40 HCWs had skin contact. No HCWs became infected.
Is influenza the real respiratory pathogen that we should be focusing public health efforts on?
Two recent papers highlight the critical public health importance of influenza A (H5N1).34., 35. In the first, Hien et al describe patients with influenza A (H5N1) admitted to hospitals in Ho Chi Minh City or Hanoi from December 2003 through January 2004.34 During this period, 10 patients were diagnosed (mean age, 13.7 years). None had preexisting conditions. Nine of 10 (90%) patients had a history of direct contact with poultry (median, 3 days before onset). At the same time, there was an epizootic of a highly pathogenic avian influenza in poultry and birds. Clinically, all patients had fever, respiratory symptoms, lymphopenia, thrombocytopenia, and abnormal chest radiographs. Seven patients had diarrhea. There was no evidence of human-to-human transmission. Eight of the 10 patients died. During the same period, a similar epizootic was occurring in Thailand, and there were 23 laboratory-confirmed human cases in Thailand and Vietnam (4/5 in Thailand were boys 6-7 years of age).35 Eighteen of the 23 (76%) died. Antigenically, the isolated influenza A virus was the same as seen in February 2003. Many of the infected persons had chicken or bird exposures. In the last study, Mutsch et al36 describe the association between an intranasal influenza vaccine and Bell's Palsy. The authors conducted a matched case-control study and case series (during October 1, 2000 to April 30, 2001) after introduction of an inactivated intranasal influenza vaccine only used in Switzerland. All primary care; ear, eye, nose, and throat physicians; and neurologists in German-speaking Switzerland were invited to participate. During the study period, 412 of 773 (53%) patients with Bell's Palsy were identified and evaluated. Of these patients, 250 patients were enrolled and matched with 722 controls. In the case-control study, 68 (27%) patients with Bell's Palsy and 8 (1.1%) controls had received intranasal vaccine (Nasalflu; Berna Niotech; P < .001; relative risk = 19); this was 13 excessive cases per 10,000 vaccinees within 1 to 91 days of vaccination. The period of highest risk was 31 to 60 days after vaccination. This study suggests a strong association between the inactivated intranasal vaccine used in Switzerland and Bell's Palsy (this vaccine in no longer in use in Switzerland) and highlights the importance of postmarketing surveillance for adverse events.
Conclusion
During the past 12 months, there have been numerous important advances in infection control and health care epidemiology. In this article, I attempted to highlight the most important. With the large number of infection control and patient safety publications in the medical literature, it is becoming more and more difficult to keep current with the latest literature. Highlights of this year's advances have included new national and international guidelines, further data illustrating the importance of antimicrobial-resistant pathogens, and the critical need to apply proven aggressive control measures to reduce these infections and slow the advance of further antimicrobial-resistant pathogens, such as VRSA. In addition, there have been additional data illustrating the importance of infection control programs and the lack of full implementation of proven infection control measures. In the last year, it has been documented that WNV can be transmitted via donated organs or blood (an arthropod-borne disease can be converted to a hospital pathogen). Last, an enormous amount of scientific data has been provided on the etiology of SARS and the modes and risks of transmission of SARS-CoV. Incorporating the latest information on infection control and health care epidemiology and implementing the most effective and proven methods for prevention should be a high priority in all health care facilities.
Hilton Head Island, South Carolina
References
- 1.Guidelines for preventing health-care associated pneumonia, 2003. MMWR. 2004;53:1–36. [PubMed] [Google Scholar]
- 2.Sehulster L., Chinn R., CDC HICPAC Guideline for environmental infection control in healthcare facilities: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) MMWR. 2003;52:1–42. [PubMed] [Google Scholar]
- 3.Kohn W.G., Harte J.A., Malvitz D.M., Collins A.S., Cleveland J.L., Eklund K.J. Centers for Disease Control and Prevention. Guidelines for infection control in dental health care settings, 2003. J Am Dent Assoc. 2004;135:33–47. doi: 10.14219/jada.archive.2004.0019. [DOI] [PubMed] [Google Scholar]
- 4.Muto C.A., Jernigan J.A., Ostrowsky B.E., Richet H.M., Jarvis W.R., Boyce J.M. SHEA Guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol. 2003;24:362–386. doi: 10.1086/502213. [DOI] [PubMed] [Google Scholar]
- 5.Nelson D.B., Jarvis W.R., Rutala W.A., Foxx-Orienstein A.E., Isenberg G., Dash G.R. Multi-society guideline for reprocessing flexible gastrointestinal endoscopes. Infect Control Hosp Epidemiol. 2003;24:532–537. doi: 10.1086/502237. [DOI] [PubMed] [Google Scholar]
- 6.Lepelletier D., Perron S., Huguenin H., Picard M., Bemer P., Caillon J. Which strategies follow from the surveillance of multidrug-resistant bacteria to strengthen the control of their spread? A French experience. Infect Control Hosp Epidemiol. 2004;25:162–164. doi: 10.1086/502368. [DOI] [PubMed] [Google Scholar]
- 7.Lally R.T., Lanz E., Schrock C.G. Rapid control of an outbreak of Staphylococcus aureus on a neonatal intensive care department using standard infection control practices and nasal mupiricin. Am J Infect Control. 2004;32:44–47. doi: 10.1016/s0196-6553(03)00088-9. [DOI] [PubMed] [Google Scholar]
- 8.Bhalia A., Pultz N.J., Gries D.M., Ray A.J., Eckstein E.C., Aron D.C. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol. 2004;25:164–167. doi: 10.1086/502369. [DOI] [PubMed] [Google Scholar]
- 9.Mayer R.A., Geha R.C., Helfand M.S., Hoyen C.K., Salata R.A., Donskey C.J. Role of fecal incontinence in contamination of the environment with vancomycin-resistant enterococci. Am J Infect Control. 2003;31:221–225. doi: 10.1067/mic.2003.45. [DOI] [PubMed] [Google Scholar]
- 10.Montecalvo M.A., Shay D.K., Gerdis C., Petrullo C., Uman J., Rodney K. A semiquantitative analysis of the fecal flora of patients with vancomycin-resistant enterococci colonized patients pose an infection control risk. Clin Infect Dis. 1997;25:929–930. doi: 10.1086/597643. [DOI] [PubMed] [Google Scholar]
- 11.Sa-Leao R., Sanches I.S., Couto I., Alves C.R., de Lensastre H. Low prevalence of methicillin-resistant strains among Staphylococcus aureus colonizing young and healthy members of the community in Portugal. Microb Drug Resist. 2001;7:237–245. doi: 10.1089/10766290152652783. [DOI] [PubMed] [Google Scholar]
- 12.Shopsin B., Mathema B., Martinez J., Ha E., Campo M.L., Fierman A. Prevalence of methicillin-resistant and methicillin-susceptible Staphylococcus aureus in the community. J Infect Dis. 2000;182:359–362. doi: 10.1086/315695. [DOI] [PubMed] [Google Scholar]
- 13.Charlebois E., Perdreau-Remington F., Kreiswirth B., Bangsberg D.R., Ciccarone D., Diep B.A. Origins of community strains of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2004;39:47–54. doi: 10.1086/421090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang S., Sievert D.M., Hageman J.C., Boulton M.L., Tenover F.C., Downes F.P. Infection with vancomycin-resistant Staphylococcus aureus in a home health patient. N Eng J Med. 2003;348:1342–1347. doi: 10.1056/NEJMoa025025. [DOI] [PubMed] [Google Scholar]
- 15.Whitener C.J., Park S.Y., Browne F.A., Parent L.J., Julian K., Bozdogan B. Vancomycin-resistant Staphylococcus aureus in the absence of vancomycin exposure. Clin Infect Dis. 2004;38:1049–1055. doi: 10.1086/382357. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg J., Jarvis W.R., Abbott S.L. Vugia DJ, and the California Emerging Infections Program. Emergence of vancomycin-resistant enterococcus in San Francisco bay area hospitals during 1994-1998. Infect Control Hosp Epidemiol. 2004;25:408–412. doi: 10.1086/502414. [DOI] [PubMed] [Google Scholar]
- 17.Perenevich E.N., Fishman D.N., Lipsitch M., Harris A.D., Morris J.G., Jr., Smith D.L. Projected benefits of active surveillance for vancomycin-resistant enterococci in intensive care units. Clin Infect Dis. 2004;38:1108–1115. doi: 10.1086/382886. [DOI] [PubMed] [Google Scholar]
- 18.O'Boyle C., Jackson M., Henly S.J. Staffing requirements for infection control programs in U.S. health care facilities: Delphi project. Am J Infect Control. 2002;30:321–333. doi: 10.1067/mic.2002.127930. [DOI] [PubMed] [Google Scholar]
- 19.Zoutman D.E., Ford B.D., Bryce E., Gourdeau M., Hebert G., Henderson E. The state of infection surveillance and control in Canadian acute care hospitals. Am J Infect Control. 2003;31:266–272. doi: 10.1067/mic.2003.88. [DOI] [PubMed] [Google Scholar]
- 20.Haley R.W., Culver D.H., White J.W., Morgan W.M., Emori T.G., Munn V.P. The efficacy of infection surveillance and control programs in preventing nosocomial infections in U.S. hospitals. Am J Epidemiol. 1985;121:182–205. doi: 10.1093/oxfordjournals.aje.a113990. [DOI] [PubMed] [Google Scholar]
- 21.Saint S., Higgins L.A., Nallamothu B.K., Chenoweth C. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control. 2003;31:354–356. doi: 10.1016/s0196-6553(02)48250-8. [DOI] [PubMed] [Google Scholar]
- 22.Stelfox H.T., Bates D.W., Redelmeier D.A. Safety of patients isolated for infection control. JAMA. 2003;290:1899–1905. doi: 10.1001/jama.290.14.1899. [DOI] [PubMed] [Google Scholar]
- 23.Iwamoto M., Jernigan D.B., Guasch A., Trepka M.J., Blackmore C.G., Hellinger W.C. Transmission of West Nile virus from an organ donor to four transplant recipients. N Eng J Med. 2003;348:2196–2230. doi: 10.1056/NEJMoa022987. [DOI] [PubMed] [Google Scholar]
- 24.Pealer L.N., Marfin A.A., Peterson L.R., Lanciotti R.S., Page P.L., Stamer S.L. Transmission of West Nile virus through blood transfusion in the United States in 2002. N Eng J Med. 2003;349:1236–1245. doi: 10.1056/NEJMoa030969. [DOI] [PubMed] [Google Scholar]
- 25.Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S. A novel conoravirus associated with severe acute respiratory syndrome. N Eng J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 26.Isakbaeva E.T., Khetsuriani N., Beard R.S., Peck A., Erdman D., Monroe S.S. SARS-associated cornoavirus transmission, United States. Emerg Infect Dis. 2004;10:225–231. doi: 10.3201/eid1002.030734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peiris J.S., Yuen K.Y., Osterhaus A.D., Stohr K. The severe acute respiratory syndrome. N Eng J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 28.Seto W.H., Tsang D., Yung R.W., Ching T.Y., Ng T.K., Ho M. Effectiveness of precautions against droplet and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) N Eng J Med. 2003;361:1519–1520. doi: 10.1016/S0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu I.T.S., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.H.W. Evidence of airborne transmission of severe acute respiratory syndrome virus. N Eng J Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 30.Roy C.J., Milton D.K. Airborne transmission of communicable infection: the elusive pathway. N Eng J Med. 2004;350:1710–1717. doi: 10.1056/NEJMp048051. [DOI] [PubMed] [Google Scholar]
- 31.Peck A.J., Newbern E.C., Feikin D.R., Issakbaeva E.T., Park B.J., Fehr J. Lack of SARS transmission and U.W. SARS case-patients. Emerg Infect Dis. 2004;10:217–224. doi: 10.3201/eid1002.030746. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y.C., Chen P.J., Chang S.C., Kao C.L., Wang S.H., Wang L.H. Infection control and SARS transmission among healthcare workers, Taiwan. Emerg Infect Dis. 2004;10:895–898. doi: 10.3201/eid1005.030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park B.J., Peck A.J., Keuhnert M.J., Newbern C., Smelser C., Comer J.A. Lack of SARS transmission among healthcare workers, United States. Emerg Infect Dis. 2004;10:244–248. doi: 10.3201/eid1002.030793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hein T., Liem N.T., Dung N.T., San L.T., Mai P.P., Chau N.V.V. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med. 2004;350:1179–1188. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- 35.CDC MMWR Cases of influenza A (N5H1)–Thailand, 2004. MMWR. 2004;53:100–103. [PubMed] [Google Scholar]
- 36.Mutsch M., Zhou W., Rhodes P., Bopp M., Chen R.T., Linder T. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med. 2004;35:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]


