Abstract
This is a prospective observational study of a cohort of inpatients exposed to a severe acute respiratory syndrome (SARS) outbreak. Strict infection control policies were instituted. The 70 patients exposed to the SARS outbreak were isolated from the rest of the hospital. They were triaged, quarantined and cohorted in three open plan wards. Selective isolation was carried out immediately when symptoms and signs suspicious of SARS manifested clinically. The patients' ages ranged from 21 to 90 years and 56% had surgery before the quarantine. Sixteen patients with unexplained fever during the period of quarantine were isolated, seven of whom were eventually diagnosed with probable SARS. The crude incidence of SARS in our cohort was 10%. The SARS case fatality was 14%. No secondary transmission of the SARS virus within the cohort was observed. Strict infection control, together with appropriate triaging, cohorting and selective isolation, is an effective and practical model of intervention in cohorts exposed to a SARS outbreak. Such a management strategy eases the logistic constraints imposed by demands for large numbers of isolation facilities in the face of a massive outbreak.
Keywords: Severe acute respiratory syndrome (SARS), Disease outbreak, Emerging communicable diseases, Epidemiology, Singapore
1. Introduction
Severe acute respiratory syndrome (SARS) has spread rapidly since its emergence in Hong Kong and China in early 2003.1., 2., 3. This epidemic is caused by a novel coronavirus4., 5. and information about its infectivity and transmission dynamics are still largely unknown. Such data are, however, crucial for effective public health measures. Singapore was among the first urban centres to report an outbreak of SARS.6 During this outbreak, the disease was largely confined within healthcare institutions.7., 8.
Here, we report the clinical outcome of an entire cohort of patients exposed to the SARS virus after an outbreak in two surgical wards. All these patients were subsequently quarantined in another hospital and observed. Those who developed symptoms or signs suspicious of SARS were isolated and treated. This scenario allowed for a unique opportunity to study the epidemiology of this disease, its clinical infectivity and transmission characteristics in a cohort exposed to the virus. It also provided a framework for future management of a SARS outbreak.
2. Methods
This observational epidemiological study was approved by the internal review board of the Singapore General Hospital ethics committee.
On 5 and 6 April 2003, a cluster of healthcare workers (HCWs) and patients from two surgical wards (wards 57 and 58) in Singapore General Hospital (SGH) developed unexplained fever. In the midst of an outbreak of SARS, this event was ominous in a hospital that had no previous SARS cases and it was classified as a possible SARS outbreak. The source was unknown but we assumed that all patients in both wards had been exposed to the virus. A later epidemiological study using contact tracing pointed to an index case having infected a total of 24 HCWs, patients (excluding those in the study cohort) and visitors in the two wards. In view of the speed of the outbreak and the potential for spread to the rest of the 1400 bed general hospital, the SARS taskforce in the hospital together with the Ministry of Health (MOH) decided to isolate the entire cohort of patients on the two wards.
This was effected on the 6 April (D-day) as follows: patients from both wards were transferred to a dedicated SARS hospital designated by the MOH. A team of 131 doctors, nurses, physiotherapist and ancillary staff from the affected surgical wards was seconded to take care of these patients. The rest of the surgical department was decommissioned.
These patients were subsequently managed in two general wards and a high-dependency ward, and isolated from the rest of the dedicated SARS hospital. The patients were triaged on admission to the wards. Those requiring close hourly monitoring, invasive central venous pressure monitoring and tracheostomy care were admitted to the high-dependency ward (ward C). The less seriously ill were admitted to the general wards. These patients were triaged accordingly: ward A, those who had a current fever or a recent history of fever related to a surgical cause (e.g. surgical infections and postoperative complications); and ward B, those who had no history of fever in their admission before transfer and included patients with chronic surgical problems. The wards were of an open design and patients were subject to cohorting. Limited numbers of isolation rooms were available on the general wards and in the high-dependency ward. No visitors were allowed and there was no interaction of patients between wards. Patients were discouraged from interacting with one another within the ward, but as their condition improved, many, in fact, did so. They shared facilities within the ward such as the toilets and bathing facilities. The HCWs looking after them wore full personal protective equipment (PPE) at all times using precautions for airborne, droplet and contact transmission. The PPE included 3M (1860/1860S) N95 particulate respirator masks, long-sleeved disposable gowns, goggles or visors, hair covers and gloves. The technique of applying and removing the PPE was standardized for all HCWs. The N95 masks were test fitted for all HCWs before patient contact. Gowns and gloves were discarded after each patient contact and strict handwashing was observed. Contaminated goggles and stethoscopes were cleaned with 70% alcohol before re-use as was other equipment necessary for routine physical examination. The use of PAPR (powered airway pressure respirators, 3M Jupiter) was indicated for procedures involving aerosolization such as suctioning and chest physiotherapy. The use of nebulizers in the general ward was prohibited. Surgical procedures were carried out in a specially designated operating theatre.
All patients had baseline investigations carried out upon transfer. These included a full blood count, serum biochemistry (including liver function tests and lactate dehydrogenase levels) and chest radiograph. Demographic and clinical data of the patient cohort were obtained from the case notes. Data on the clinical course were collected prospectively. The entire cohort was deemed not to have SARS at the onset of quarantine. Subsequently, patients were classified into four groups: (1) non-SARS; (2) observation for unexplained fever (temperature >38 °C); (3) suspected SARS; and (4) probable SARS. Case definition of suspected and probable SARS status was according to prevailing guidelines at the time of the outbreak.9., 10. Patients were followed up daily and clinical parameters monitored at least three times per day. A fever >38 °C or development of symptoms suspicious for SARS prompted a detailed clinical review and a possible upgrading of SARS status. Patients who were upgraded to an observation, suspected or probable SARS status were subsequently removed from the cohort and placed in isolation in a different part of the hospital. Such patients were subsequently co-managed with a team of infectious disease physicians.
Patients remained in quarantine in the wards according to the following guidelines: 10 days must have elapsed after the last case of probable SARS had been isolated from the ward they were in and they had been afebrile and asymptomatic for a minimum of three days and have a normal chest radiograph. They were subsequently discharged home with an official home quarantine order. Patients requiring medical care continued to be managed within the quarantined wards. No transfer to another hospital was allowed. Deaths were managed according to SARS status as defined by the Infectious Diseases Act of Singapore.
3. Results
In total, 70 patients (41 males, 29 females) from the two surgical wards were quarantined. The median age was 59 years (range: 21–90 years). A total of 39 (56%) had a surgical procedure before quarantine and only one patient required an emergency operation during the quarantine period. The patient co-morbidities within the cohort included diabetes mellitus (33%), hypertension (40%), ischaemic heart disease (13%), malignancy (33%), chronic renal failure (6%) and chronic obstructive airway disease (4%). Most patients were ethnic Chinese except for nine Malays, five Indians and five others. A schematic representation of the warding of patients after the transfer is shown in Figure 1 .
Figure 1.
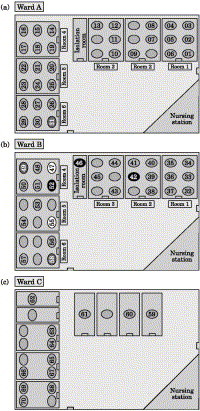
Schematic representation of patients in the two general wards (wards A and B) and in the high-dependency (ward C). The time of conversion to probable SARS is represented by the shading.
3.1. Clinical progress
At the onset of quarantine (D-day), all patients were classified as non-SARS. Their last exposure to the cluster of suspected SARS inpatients and HCWs was the day before quarantine. During the quarantine period, 16 patients were isolated and upgraded from a non-SARS status. Seven of these were eventually reclassified as probable SARS, giving a crude incidence rate of 10%. No patient in ward C (high-dependency ward) developed SARS. In ward A, six patients developed probable SARS. As three patients had developed a fever immediately on day D+1 and were therefore isolated, the incidence in ward A was 12.5%. In ward B, one patient developed probable SARS, i.e. an incidence of 3.2%.
The seven probable SARS patients (one female, six males; median age 55 years, range 37–74 years) developed their infection within 16 days of D-day (one at D+6 days, two at D+7 days, one at D+9 days, one at D+12 days, one at D+15 days and one at D+16 days). All patients remained well except for one who required intubation and intensive care monitoring. Biological confirmation of SARS by polymerase chain reaction assay and serological assay was carried out and all seven patients tested positive. None of the HCWs were infected with SARS by the end of the study.
There were six deaths. Four were not SARS-related. The cause of death was certified as advanced malignancy in three patients and a perforated viscus in the other. The fifth death occurred in a suspected SARS patient who had underlying terminal end-stage renal failure. The patient had multiple intra-abdominal abscesses and an entero-cutaneous fistula. She also had concurrent symptoms of cough and shortness of breath. She had refused further surgical treatment and death was attributed to intra-abdominal abscesses. No post-mortem examination or biological confirmation of SARS infection was carried out. The sixth mortality (patient 46) occurred in a probable SARS patient who was a 74-year-old man admitted for intestinal obstruction secondary to adhesions. He was recovering well on conservative management when he developed fever and progressive breathlessness and was found to have pneumonia. He required ventilation in the intensive care unit and died 17 days after quarantine. The SARS-related case fatality rate was thus 1/7 (14%).
4. Discussion
When a SARS outbreak was first reported in Singapore, drastic measures including the closure of schools and hospitals were introduced as part of attempts to stop the spread of the virus.6 Such measures were eventually adopted in other urban centres with SARS outbreaks. These were deemed necessary as the transmission dynamics and infectivity rates of the SARS virus are largely unknown. The number of patients that may potentially become affected could overwhelm the capacity of existing medical facilities. Initial reports of SARS suggested a highly contagious virus, causing a disease that had a potentially fatal outcome.11., 12.
This was an observational study of a cohort of surgical patients that had been exposed to the virus after a sudden outbreak of SARS. The quarantine and relocation measures undertaken allowed us to triage, cohort and isolate the patients in a controlled setting for observation and management. Such a study was made possible by the sweeping measures taken to contain the spread of a potentially lethal virus about whose transmission dynamics we had little knowledge. It presented a unique opportunity to study the outcome of such an intervention for control of this new disease and its implications for future public health control efforts.
In the study, patients were subjected to both triaging and cohorting at the point of admission. In the wards, they were isolated immediately after they manifested elevated temperature or symptoms suspicious for SARS. Strict infection control measures were undertaken for contacts between the HCWs and the patients. None of the HCWs assigned to the care of these patients had been diagnosed with SARS by the end of the study. This is a testament to the good protection afforded by these infection control measures against the SARS virus.
Ideally, contacts of clinical SARS patients should be monitored in isolation. The current practice is for such contacts to be on home quarantine until they develop fever. In this study, those exposed were, however, not members of the public but surgical inpatients who continued to require hospital care. These inpatients would normally have required single-room isolation. The role of triaging and cohorting as a public health measure in the management of a large number of exposed institutionalized individuals in a SARS outbreak have not been investigated or reported previously. With such measures, the clinical incidence rate was 10% (7/70) with one SARS-related mortality. There was no transmission within the cohort. These clinical data are useful as health authorities faced with a similar outbreak and a shortage of isolation facilities may take comfort from the observed clinical incidence and case fatality rate.
In the cohort, the majority of patients isolated had an unexplained fever of over 38 °C as the initial suspicious symptom. This was followed by the onset of respiratory symptoms; radiological findings were only evident later on. This experience differs from some previous reports where up to 78% had chest radiographic changes at initial presentation.11 This difference is because temperature and symptom monitoring allowed us to identify SARS early before manifestation of radiological changes. The predominant symptom of unexplained fever, or fever followed by the onset of respiratory symptoms, should serve to heighten the suspicion of a possible SARS infection, especially in the light of contact with SARS patients.
Early isolation of patients with SARS at the onset of fever or respiratory symptoms can affect the clinical transmission of SARS. With this policy, we saw no transmission in the high-dependency ward, and a possible transmission rate of 12.5% in ward A as three probable SARS patients had already been isolated at day 1. These three were presumably infected before quarantine. In ward B, only one patient developed SARS and no further transmission occurred.
The ability to identify SARS-related symptoms is difficult in a cohort of surgical inpatients. Unlike in the community where exposed individuals are usually clinically well, the cohort had underlying co-morbidities and surgical diseases that may mask the symptoms of SARS. More than 50% had also recently undergone a surgical procedure with postoperative complications in the chest or wound complications that produce symptoms and signs that mimic SARS. In the high-dependency ward, no cases of SARS were seen. These patients were the most ill but they were also the least mobile and the most closely monitored. Suspected cases, if any, could have been easily identified and isolated. In ward A, nine cases were suspected and six were eventually classified as probable SARS. The patients triaged and cohorted here had a history of fever related to an infective surgical condition or post-surgical complication. However, they were also less closely monitored compared with those in the high-dependency ward and more mobile around the ward. Three patients (patients 42, 46 and 52) in this ward were identified and isolated early after the quarantine [Figure 1(b)]. In the other three patients (patient 47, 49 and 55) with probable SARS, the original surgical admission was for infective conditions of the biliary and urinary systems. These patients were subsequently identified when their fever recurred despite adequate treatment of their infective condition. This could have masked and delayed identification of the SARS symptoms. This remains a limiting factor if clinical criteria are used for diagnosis. Long incubation periods of up to 16 days after exposure have also been documented and this accounts for their presentation of symptoms after the first week of quarantine.11., 13., 14. Correspondingly in ward B, only one probable SARS case was identified. Although patients were also mobile and less closely monitored, their principal surgical condition made identification and isolation of the fever and symptoms suspicious for SARS easier.
The chain of outbreaks reported in SARS-affected countries points to a propagated epidemic. However, the transmission of the SARS virus in our experience appears to be lower than previously reported. Initial attack rates of over 50% have been documented.15 In the present cohort, 11.4% of patients were diagnosed with suspect SARS and 10% with probable SARS.
The infectivity and transmission of the virus is low when appropriate measures are taken.16 The patients were exposed and subsequently cohorted in the ward with isolation only when an unexplained fever or symptoms arose. Only 10% were infected with SARS and there was no secondary transmission. The other patients remained asymptomatic as they had not been infected.
At the time of the study, the diagnosis of SARS was based on clinical and epidemiological criteria. No diagnostic kit was easily available and none had been validated.17 This made the screening and identification of SARS difficult and insufficiently sensitive.18 However, as the virology and molecular biology is better understood, the case definition of SARS now includes a serological or molecular confirmation.19 Such data will give further insight into transmission dynamics and infectivity of the SARS virus. The development of diagnostic tests is of vital importance for further understanding of this deadly new infection.
In conclusion, effective public health measures are urgently needed for management of a SARS virus outbreak. Strict infection control discipline is important in the management of patients exposed to SARS. This report shows that strict infection control, together with appropriate triaging, cohorting and selective isolation, serve as an acceptable and effective model of intervention in cohorts exposed to a SARS outbreak. Such a management strategy will ease the logistic constraints of the large number of isolation facilities faced by health authorities in the face of a massive outbreak, and provide a guide for future infection control efforts.
Acknowledgements
The authors thank all the members of the ‘Tiger Force Team’ who comprised the 131 medical, nursing and paramedical staff involved in the frontline care of the above cohort of patients. The authors are indebted to the management and staff of Tan Tock Seng Hospital, Singapore for assisting in the transfer and management of patients.
Footnotes
The authors dedicate this report to the patients of this cohort, many of whom we got to know well through the period of quarantine.
References
- 1.Tsang K.W., Ho P.L., Ooi G.C. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 2.Update: outbreak of severe acute respiratory syndrome—worldwide, 2003. MMWR Morb Mortal Wkly Rep 2003;52:269–272. [PubMed]
- 3.Acute respiratory syndrome. China, Hong Kong Special Administrative Region of China, and Viet Nam. Wkly Epidemiol Rec 2003;78:73–74. [PubMed]
- 4.Ksiazek T.G., Erdman D., Goldsmith C.S. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 5.Kuiken T., Fouchier R.A., Schutten M. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Severe acute respiratory syndrome—Singapore, 2003. MMWR Morb Mortal Wkly Rep 2003;52:405–411. [PubMed]
- 7.Tan Y.M., Chow P.K., Soo K.C. Severe acute respiratory syndrome: clinical outcome after inpatient outbreak of SARS in Singapore. BMJ. 2003;326:1394. doi: 10.1136/bmj.326.7403.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu L.Y., Lee C.C., Green J.A. Severe acute respiratory syndrome (SARS) in Singapore: clinical features of index patient and initial contacts. Emerg Infect Dis. 2003;9:713–717. doi: 10.3201/eid0906.030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Outbreak of severe acute respiratory syndrome-worldwide, 2003. MMWR Morb Mortal Wkly Rep 2003;52:226–228. [PubMed]
- 10.Centers for Disease Control and Prevention Updated interim surveillance case definition for severe acute respiratory syndrome (SARS)—United States. JAMA. 2003;289:2637–2639. doi: 10.1001/jama.289.20.2637-b. [DOI] [PubMed] [Google Scholar]
- 11.Lee N., Hui D., Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 12.Ho W. Guideline on management of severe acute respiratory syndrome (SARS) Lancet. 2003;361:1313–1315. doi: 10.1016/S0140-6736(03)13085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdullah A.S., Tomlinson B., Cockram C.S., Thomas G.N. Lessons from the severe acute respiratory syndrome outbreak in Hong Kong. Emerg Infect Dis. 2003;9:1042–1045. doi: 10.3201/eid0909.030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu W., Wang J., Liu P. A hospital outbreak of severe acute respiratory syndrome in Guangzhou, China. Chin Med J (Engl) 2003;116:811–818. [PubMed] [Google Scholar]
- 15.Varia M., Wilson S., Sarwal S. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. CMAJ. 2003;169:285–292. [PMC free article] [PubMed] [Google Scholar]
- 16.Lipsitch M., Cohen T., Cooper B. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966–1970. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO Multicentre Collaborative Networks for Severe Acute Respiratory Syndrome (SARS) diagnosis. Wkly Epidemiol Rec. 2003;78:121–122. [PubMed] [Google Scholar]
- 18.Rainer T.H., Cameron P.A., Smit D. Evaluation of WHO criteria for identifying patients with severe acute respiratory syndrome out of hospital: prospective observational study. BMJ. 2003;326:1354–1358. doi: 10.1136/bmj.326.7403.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoey J. Updated SARS case definition using laboratory criteria. CMAJ. 2003;168:1566–1567. [PMC free article] [PubMed] [Google Scholar]


