Abstract
Study objective
In a pilot study conducted during March 14 to April 2, 2003, 2 severe acute respiratory syndrome (SARS) screening scores were developed for predicting SARS among febrile patients presenting to the emergency department (ED). The objective of this study is to validate these scoring systems with a different set of patients.
Methods
All adult patients with documented fever, measured at home or at the hospital, and presenting to the ED of National Taiwan University Hospital, a 2,400-bed tertiary care teaching hospital in northern Taiwan, were prospectively enrolled. Two previously developed SARS screening scores were applied to all patients. The final diagnosis of SARS was made by the Expert Committee of the Center for Disease Control Taiwan, Republic of China, according to the criteria of Centers for Disease Control and Prevention, Atlanta, GA.
Results
A total of 239 adult patients, including 117 men and 122 women, were enrolled. Eighty-two patients were finally diagnosed with SARS. Compared with the SARS patients in the derivation cohort, those in the validation cohort were older (44.5±15.9 versus 33.9±15.9 years), more likely to acquire the disease locally (76.8% versus 37.5%), and more likely to have cough before or during fever. For the non-SARS patients, cases in the validation cohort presented with less cough and coryza but more diarrhea. For the 4-item symptom score, the sensitivity reached 96.3% (95% confidence interval [CI] 89.7% to 98.7%) and the specificity 51.6% (95% CI 43.8% to 59.3%). For the 6-item clinical score, the sensitivity reached 92.6% (95% CI 84.8% to 96.6%) and the specificity 71.2% (95% CI 63.6% to 77.7%). When the clinical score was applied to patients with a positive symptom score, the combined sensitivity reached 90.2% (95% CI 82.0% to 95.0%), and the combined specificity reached 80.1% (95% CI 73.2% to 85.6%).
Conclusion
This prospective study validated the scoring system previously developed by using a different cohort. The scoring systems could be applied to settings where mass screening of SARS is needed during future outbreaks.
Capsule Summary.
What is already known on this topic
No rapid tests currently exist to distinguish severe acute respiratory syndrome (SARS) from common minor respiratory ailments in the emergency department setting.
What question this study addressed
A clinical scoring system for SARS developed in a prior pilot study was prospectively validated in 239 adults with fever.
What this study adds to our knowledge
A scoring system based on the presence of cough before or concomitant with fever, myalgia, diarrhea, rhinorrhea/sore throat, lymphopenia, and thrombocytopenia identified cases that ultimately met the Centers for Disease Control and Prevention definition of probable SARS with a sensitivity of more than 90% and a specificity of more than 70%.
How this might change clinical practice
This scoring system may be helpful to rapidly identify SARS, but it may not perform as well in other epidemiologic settings and SARS was not confirmed by serology or polymerase chain reaction testing in all cases.
Introduction
Background
Since March 12, 2003, when the World Health Organization (WHO) issued the first global alert about cases of severe respiratory illness that may spread to hospital staff,1 severe acute respiratory syndrome (SARS) has become a global public health concern. According to the WHO's report, a total of 8,437 people in 30 countries were affected by the disease, with 831 deaths.2 Taiwan is an island with a total area of 36,000 km2 and a population of 23 million. Because of the close proximity to China and Hong Kong, frequent travel and business contacts resulted in the importation of SARS. From March to July 2003, a total of 150,628 persons were under home quarantine in Taiwan,3 and 3,032 febrile patients were isolated in hospitals.4 Large-scale quarantine, isolation, and body temperature measurement practices had created huge psychological and economic impacts on the society. There were 665 SARS cases reported in Taiwan; about one eighth of the cases were diagnosed at the emergency department (ED) of National Taiwan University Hospital. Soon after the first 2 SARS cases in Taiwan were diagnosed at National Taiwan University Hospital on March 14, 2003,5 the ED was inundated by febrile patients requesting screening for SARS. The situation worsened after April 24, when intrahospital outbreaks occurred in 2 municipal hospitals.6 Because of the proximity of these 2 hospitals to National Taiwan University Hospital, the ED was crowded with patients being screened for SARS in the study period. A total of 875 patients presented to our ED for SARS screening; 754 of them came during the endemic period from April 23 to May 12, or almost 40 cases daily.
Importance
Despite efforts to develop rapid laboratory assays for SARS, it is not yet practical and economical to use these laboratory tests to screen large numbers of people. Although a low sensitivity of 25.8% was reported by Rainer et al,7 the WHO criteria remain the sole screening tool for many hospitals. Screening tools that have acceptable sensitivity and use easily available symptomatic and laboratory items are highly desirable, especially in mass screening.
Goals of this investigation
In a previous pilot study, we developed 2 SARS scores, the 4-item symptom score and the 6-item clinical score, from a cohort of 70 febrile patients.8 This study was conducted to validate both scores by using a second set of 239 febrile patients presenting to the ED during the outbreak.
Materials and methods
Theoretical model of the problem
Definite diagnosis of SARS relies on clinical manifestations, epidemiologic data, and laboratory test results, including virologic and serologic results.9 Early in the course of the disease, however, most of the manifestations of SARS are vague and difficult to differentiate from other airway infections. It takes more than 2 days for the chest radiograph to develop infiltrates, and the initial changes are difficult to differentiate from other forms of pneumonia.10 It also takes 8 to 10 days for the reverse transcriptase–polymerase chain reaction to achieve the maximal sensitivity (92.9%).11 The contact or travel history may not be helpful if community outbreak has already taken place. Therefore, early diagnosis of SARS and isolation decisions would be based mainly on clinical presentations. Although the initial presentation of SARS appears nonspecific, we believe that the clinical finding of SARS was unique among the febrile diseases. A scoring system combining symptoms and laboratory results may help in the diagnosis and decisionmaking when the physician is confronted with febrile patients during SARS outbreaks.
Study design
From March 15 to April 2, 2003, we conducted a prospective pilot study to derive SARS screening scores from a cohort of 70 febrile patients presenting to the ED of National Taiwan University Hospital.5 Two sets of scores were developed: the 4-item symptom score and the 6-item clinical score. This prospective observational study was conducted to validate these scores by using a second cohort of patients.
Setting
This study was conducted at National Taiwan University Hospital, a 2,400-bed tertiary care university teaching hospital in northern Taiwan, from April 3 to May 12, 2003. While the study was conducted, Taiwan became a SARS endemic area. The numbers of SARS patients diagnosed and treated in National Taiwan University Hospital were highest in Taiwan during this outbreak. Although National Taiwan University Hospital is a tertiary care teaching hospital, patients could present to the ED without referral. The ED also received patients referred from other hospitals or from clinics.
Selection of participants
From April 3 to May 12, 2003, all patients older than 15 years who presented to the ED with a documented temperature of 38.0°C (100.3°F) or greater, measured at home or at the hospital, regardless of risk of exposure were enrolled in this prospective validation study. These patients were directed to a separate area for SARS screening. Staff emergency physicians and residents who underwent intensive training for the identification and management of SARS were responsible for screening the febrile patients.
All enrolled patients were assessed by emergency physicians using a structured record form. Initial investigations included detailed medical history, physical examination, essential laboratory tests by WHO recommendation,12 and chest radiography. Symptom score and clinical score were applied to all enrolled patients.8 Decisions for disposition were made by the criteria described as follows.
The scoring system contained 2 scores, the symptom score and the clinical score. The respective items and their corresponding scores are shown in Table 1. With the cutoff value of zero, sensitivity was 100% (95% confidence interval [CI] 67.6% to 100%) and specificity was 75.9% (95% CI 63.9% to 84.8%) for the symptom score in the derivation cohort. With a cutoff value of 1, the sensitivity was 100% (95% CI 67.6% to 100%) and the specificity was 86.3% (95% CI 75.5% to 93.0%) for the clinical score in the derivation cohort.8
Table 1.
The SARS scores.∗
| Items | Initial Symptoms and Laboratory Findings | Score |
|---|---|---|
| A | Myalgia | 1 |
| B | Diarrhea | 1 |
| C | Cough before or during fever | −2 |
| D | Rhinorrhea or sore throat | −1 |
| E | Lymphopenia† | 1 |
| F | Thrombocytopenia‡ | 1 |
Symptom score = A + B + C + D. If the total score is <0, then SARS is less likely. Clinical score = A + B + C + D + E + F. If the total score is >0, then SARS is likely.
Lymphopenia is defined as lymphocyte count below 1.0×109/L.
Thrombocytopenia is defined as platelet count below 150×109/L.
Admission for isolation was indicated in patients with at least 1 of the following conditions: (1) infiltrates on chest radiograph; (2) significantly abnormal laboratory data, such as severe leukopenia; and (3) symptom score of 0 or greater, and clinical score of 1 or greater. Patients not meeting the admission criteria were discharged home for quarantine and followed up by telephone interview or scheduled outpatient clinic visit 3 days later. Admission for isolation was also indicated for fever lasting more than 3 days.
Outcome measures
All admitted patients were followed up by contact with the treating physicians and medical record review. Initially discharged patients who exhibited defervescence within 3 days were followed up by telephone interview 10 days later, if possible. The final diagnoses of SARS were made by the consensus of the SARS Expert Committee of the Center for Disease Control Taiwan, Republic of China, a team of respiratory and infectious diseases experts, according to criteria from the Centers for Disease Control and Prevention (CDC), Atlanta, GA.9 The decision was made by review of all relevant clinical, epidemiologic, radiographic, and laboratory data, including SARS-associated coronavirus (SARS-coronavirus) antibodies and reverse transcriptase–polymerase chain reaction when available. All patients who exhibited defervescence within 3 days or whose alternative diagnosis could fully explain the clinical findings were classified as non-SARS.
First, individual components of both scores (4 symptom-related and 2 laboratory-related items) were evaluated for their predictive ability. Subsequently, SARS screening scores were validated as an independent predictor of SARS in febrile patients presenting to the ED by calculation of the sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio. Receiver operator characteristic (ROC) curves of both scores were plotted, and the areas under the curves were calculated.
Primary data analysis and data presentation
Data were entered, processed, and analyzed with SPSS for Windows (Release 10.0; SPSS, Inc., Chicago, IL). Data are reported as mean±SD unless otherwise specified; 95% CIs were also computed. Binomial variables were analyzed with the χ2 test. The unpaired Student's t test was used for comparisons of continuous variables. Logistic regression modeling was adopted to obtain the adjusted odds ratios (ORs) of individual components in predicting SARS.
Results
From April 3 to May 12, 2003, 875 patients presented to the ED for SARS screening. Among them, 273 patients had a documented temperature greater than 38°C (>100.3°F), measured at home or at the hospital. Thirty-four patients younger than 15 years were excluded. All the remaining 239 patients were enrolled.
Characteristics of study subjects
There were 117 men and 122 women. The mean age was 40.3±16.0 years (range 15 to 87 years). One hundred eighty-seven (78.2%) patients had a risk of exposure to SARS. For the remaining 52 patients, no definite contact history could be traced.
Ninety-seven (40.6%) patients were admitted after initial ED evaluation. Only 65 patients had abnormal chest radiograph results on initial presentation at the ED. Of the 32 admitted patients with initial normal chest radiograph results, 13 patients developed pneumonia after admission and were diagnosed with SARS. Eight of the 138 discharged patients had persistent fever at home. They were admitted later and finally diagnosed with SARS. Sixty-one patients were tested for SARS-coronavirus reverse transcriptase–polymerase chain reaction at initial presentation; among 55 SARS patients, 21 had positive test results (sensitivity 38.2%). Follow-up indirect fluorescent-antibodies assay results for SARS-coronavirus were available for 32 SARS patients admitted to our hospital, and 31 of these patients showed seroconversion (96.9%). Forty-four patients tested positive for SARS by either polymerase chain reaction or serology in our study group.
The demographic data, initial clinical presentations, and laboratory results of patients in both cohorts are listed in Table 2. The patients in the validation group are older than those in the derivation group (40.3±16.0 versus 35.0±15.8 years), mainly because the SARS group in the validation cohort were older (44.5±15.9 versus 33.9±15.9 years). The reasons for contracting the SARS virus changed from travel-related (62.5%) in the derivation cohort to contact-related (76.8%) in the validation cohort. No significant difference in the symptoms and laboratory results was noted in SARS patients between the 2 cohorts, except for the increased cough before or during fever in the validation cohort (19.5% versus 0%). Compared with the non-SARS patients in the derivation cohort, patients in the validation cohort had significantly less cough and coryza but more diarrhea. No significant difference in laboratory results was noted in non-SARS patients between these 2 cohorts.
Table 2.
Demographic and initial clinical presentations of the SARS and non-SARS patients in validation and derivation groups.
| Validation Group |
Derivation Group |
|||
|---|---|---|---|---|
| Characteristics | Non-SARS (N=157) | SARS (N=82) | Non-SARS (N=62) | SARS (N=8) |
| Age, y±SD | 38.3±15.8 | 44.5±15.9 | 44.0±9.8 | 33.9±15.9 |
| Sex, men/women (%) | 80/69 (53.7) | 29/40 (42.0) | 40/22 (64.5) | 4/4 (50) |
| Risk of exposure, No. (%) | ||||
| Contact history | 77 (49.0) | 63 (76.8) | 16 (25.8) | 3 (37.5) |
| Travel history | 44 (28.0) | 3 (3.7) | 46 (74.2) | 5 (62.5) |
| No risk of exposure | 36 (23.0) | 16 (19.5) | 0 | 0 |
| Symptoms | ||||
| Fever (at presentation to ED), No. (%) | 35 (23.0) | 50 (61.0) | 19 (30.7) | 3 (37.5) |
| Period from fever to ED, days±SD | 2.11±1.70 | 3.96±1.95 | 2.14±3.4 | 6.36±3.25 |
| Cough, No. (%) | 77 (49.0) | 31 (37.8) | 65 (79.3) | 7 (87.5) |
| Cough before or during fever, No. (%) | 65 (41.4) | 16 (19.5) | 40 (64.5) | 0 |
| Rhinorrhea (coryza), No. (%) | 26 (16.7) | 3 (3.7) | 19 (30.6) | 0 |
| Sore throat, No. (%) | 61 (38.9) | 5 (6.1) | 26 (41.9) | 1 (12.5) |
| Myalgias, No. (%) | 39 (24.8) | 56 (68.3) | 17 (27.4) | 6 (75) |
| Headache, No. (%) | 12 (7.6) | 17 (20.7) | 6 (9.68) | 3 (37.5) |
| Diarrhea, No. (%) | 35 (22.3) | 33 (40.2) | 6 (9.68) | 4 (50) |
| Signs, mean±SD | ||||
| Body temperature, °C | 37.4±0.88 | 38.2±0.91 | 37.3±0.9 | 37.7±1.0 |
| Mean blood pressure, mm Hg | 100.9±15.7 | 94.5±15.7 | 100.2±16.2 | 97±10 |
| Pulse rate, beats/min | 101.5±18.3 | 99.1±15.0 | 100±19.8 | 103±13 |
| Oxygen saturation on room air, % | 97.8±2.5 | 97.4±3.0 | 98.0±1.6 | 97.8±1.5 |
| Laboratory data | ||||
| WBC count, ×109/L, mean±SD | 8.3±4.0 | 5.5±2.4 | 8.6±3.7 (N=59) | 6.1±5.1 (N=8) |
| Hemoglobulin, g/dL, mean±SD | 13.1±1.6 | 12.6±1.6 | 13.4±2.6 (N=59) | 13.7±1.6 (N=8) |
| Platelet count, ×109/L, mean±SD | 223.5±79.7 | 160.3±58.4 | 211.6±78.8 (N=59) | 144.1±36.3 (N=8) |
| Lymphocyte count, ×109/L, mean±SD | 1.4±0.7 | 0.9±0.4 | 1.5±1.1 (N=59) | 0.9±0.3 (N=8) |
| Absolute neutrophil count, ×109/L, mean±SD | 6.2±3.8 | 4.2±2.1 | 6.2±3.3 (N=59) | 4.8±5.2 (N=8) |
| Serum aspartate transaminase, U/L, mean±SD | 29.2±28.7 | 53.2±72.7 | 28.5±13.6 (N=34) | 47.1±25.1 (N=8) |
| Creatinine phosphokinase, U/L, mean±SD | 116.3±124.5 | 434.6±1676.2 | 152.5±195.1 (N=17) | 292.2±209.5 (N=5) |
| C-reactive protein, mg/dL, mean±SD | 1.9±2.5 | 4.0±2.9 | 2.2±2.7 (N=35) | 3.4±2.1 (N=7) |
| Infiltrates on initial chest radiograph, No. (%) | 16 (10.2) | 65 (79.3) | 3/62 (4.8) | 6/8 (75) |
| SARS-CoV RT-PCR, No. (%) | 0/6 (0) | 21/55 (38.2) | NA | 2/6 (33.3) |
| Seroconversion for SARS-CoV IFA, No. (%) | NA | 31/32 (96.9) | NA | 6/6 (100) |
IFA, Indirect fluorescent antibody assay; NA, not applicable; SARS-CoV RT-PCR, SARS coronavirus reverse transcriptase–polymerase chain reaction.
The final diagnosis of SARS was made for 82 (34.3%) patients and of non-SARS for 157 patients. The demographic data, initial clinical presentations, and laboratory findings of both groups are summarized in Table 2. There was no sex difference between these 2 groups (42.0% men in the SARS group versus 53.7% men in the non-SARS group). The SARS group was significantly older (mean age 44.5±15.9 years versus 38.3±15.8 years).
Compared with the non-SARS group, the SARS group had a longer febrile period before presentation to the ED; a higher percentage of myalgia, headache, and diarrhea; and a lower percentage of cough before or during fever, sore throat, and rhinorrhea.
For laboratory tests, the SARS group had significantly lower levels of leukocyte count, absolute neutrophil count, absolute lymphocyte count, and platelet count and higher levels of serum aspartate aminotransferase and C-reactive protein.
These 2 scores were applied to all patients, and the number of missed patients at different cut points are listed in Table 3. For the 4-item symptom score, with a cutoff value of zero, the sensitivity reached 96.3% (95% CI 89.7% to 98.7%) and the specificity reached 51.6% (95% CI 43.8% to 59.3%). The positive likelihood ratio was 1.99 (95% CI 1.68 to 2.35), and the negative likelihood ratio was 0.07 (95% CI 0.02 to 0.22). The ROC curve of the symptom score is shown in the Figure. The area under the ROC curve is 0.85 (95% CI 0.80 to 0.90). For the 6-item clinical score, with a cutoff value of 1, the sensitivity was 92.6% (95% CI 84.8% to 96.6%) and the specificity was 71.2% (95% CI 63.6% to 77.7%). The positive likelihood ratio was 3.21 (95% CI 2.49 to 4.14), and the negative likelihood ratio was 0.10 (95% CI 0.05 to 0.23). The ROC curve of the clinical score is shown in the Figure. The area under the ROC curve is 0.89 (95% CI 0.85 to 0.93). In clinical practices at the ED, these 2 scores were applied to patients sequentially: febrile patients were assessed for the 4-item symptom scores, and patients with positive results (with a cutoff value of zero) underwent laboratory tests, and the 6-item clinical scores were calculated. Only patients with both scores above the cutoff values were admitted and isolated. In such circumstances, the combined sensitivity was 90.2% (95% CI 82.0% to 95.0%) and the combined specificity was 80.1% (95% CI 73.2% to 85.6%). The combined positive likelihood ratio was 4.54 (95% CI 3.29 to 6.27), and the combined negative likelihood ratio was 0.12 (95% CI 0.06 to 0.24).
Table 3.
Missed SARS patients at different cut points.
| Cutoff Point | Symptom Score, No. (%) | Clinical Score, No. (%) |
|---|---|---|
| ≥–3 | 0 | 0 |
| ≥–2 | 0 | 0 |
| ≥–1 | 1 (1.2) | 0 |
| ≥0 | 3 (3.6) | 1 (1.2) |
| ≥1 | 20 (24.4) | 6 (7.3) |
| ≥2 | 55 (67.1) | 19 (23.2) |
| ≥3 | 42 (51.2) | |
| ≥4 | 69 (84.1) |
Figure.
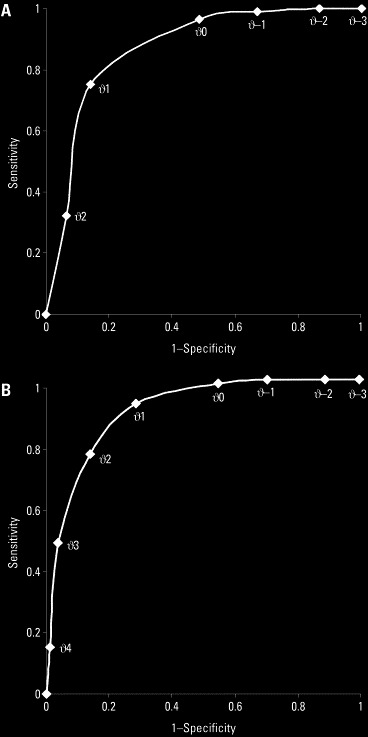
A, ROC curve of the symptom score. B, ROC curve of the clinical score.
In the validation cohort, there were 52 cases without any risk of exposure. SARS was diagnosed in 16 cases, and the SARS-coronavirus reverse transcriptase–polymerase chain reaction result was positive in 4 cases. For the 187 patients with risk of exposure, the combined sensitivity was 90.9% (95% CI 81.6% to 95.8%) and the combined specificity was 71.1% (95% CI 62.5% to 78.4%). For the 52 cases without risk of exposure, the combined sensitivity was 87.5% (95% CI 64.0% to 96.5%) and the combined specificity was 75.0% (95% CI 58.9% to 86.3%).
Of the 6 clinical variables, myalgia was the most significant predictor of SARS, with an adjusted OR of 4.34 (95% CI 2.16 to 8.71). The adjusted ORs of individual items are shown in Table 4.
Table 4.
Adjusted ORs of individual items of SARS scores.∗
| Symptom Score |
Clinical Score |
|||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Cough | 0.70 | 0.37–1.32 | 0.75 | 0.38–1.52 |
| URI | 0.17 | 0.07–0.44 | 0.22 | 0.08–0.60 |
| Myalgia | 5.35 | 2.85–10.04 | 4.34 | 2.16–8.71 |
| Diarrhea | 1.71 | 0.87–3.38 | 1.98 | 0.94–4.16 |
| Lymphopenia | 3.48 | 1.71–7.08 | ||
| Thrombocytopenia | 4.05 | 1.89–8.70 | ||
URI, Upper respiratory infection symptoms, including coryza and sore throat.
The reference group is non-SARS patients.
Limitations
There were several limitations in our study: first, these 2 studies (derivation and validation) took place at the same ED with the same physicians. Staff from other settings may not find these scores easy to assess. Second, the interobserver reliability was not assessed because the study was undertaken at a busy ED during the outbreak period. Third, this study was not conducted during influenza season, and the prevalence of other febrile diseases with similar clinical manifestations, such as influenza, may influence the screening ability of this scoring system.
Discussion
Because of its high mortality and infectivity, SARS created a global public health threat during the outbreak period. How to prevent its spreading in the next outbreak, if one ever occurs, is still a major challenge. So far, early interruption of the transmission chain by early detection of patients who have SARS seems to be the only method to contain this disease.13 Overdiagnosis of SARS leads to excessive isolations; on the other hand, underdiagnosis resulted in severe outbreaks in several hospitals. A simple and reliable method for the early detection of SARS patients among all febrile patients has become imperative, especially in settings where mass screening is needed.
This study demonstrated that these 2 scores performed relatively well in the mass screening of SARS at the ED setting, with only 8 of 82 SARS patients initially released from the ED. The proposed screening systems greatly outperformed the WHO criteria, which had a low sensitivity of 25.8% for predicting SARS.7 The symptom score, with its easy application and fairly good sensitivity (96.3%), can serve as an initial triage tool for a larger group of febrile patients, especially in settings in which laboratory tests are unavailable, such as an airport or a triage station. For example, in an ED triage station, patients with negative scores and therefore minimal risks of SARS could be separated immediately from patients at higher risk to reduce the disease transmission at the ED. Patients with positive symptom scores should be directed to further laboratory and radiographic studies and have their clinical score assigned. When a clinical score was applied after a symptom score was positive, the combined sensitivity reached 90.2% and the combined specificity reached 80.1%. With such high sensitivity and specificity, we suggest that only patients with positive symptom and clinical scores undergo isolation. Patients screened as negative by the 2 scores should be discharged to home quarantine and followed up by telephone interview until defervescence. Thus, we could reduce the number of patients needing isolation and improve efficiency in mass screening.
Although all patients in the derivation cohort had a risk of exposure to the SARS virus, patients without a risk of exposure were also enrolled in the validation cohort. This difference in the study design was a natural result of the ever-evolving epidemic conditions in Taiwan during the SARS outbreak. When the virus became endemic and no longer imported, all Taiwan residents fit the epidemiologic criteria of the CDC case definition.9 Although contact history is one of the screening criteria in the CDC and WHO criteria, the history of definite close contact was difficult to clarify at the ED, especially during a community outbreak. A good screening tool must be able to overcome this barrier, namely, it should be able to screen SARS for patients without clear contact history. In a separate analysis excluding patients without contact history, the combined sensitivity (90.9%) and specificity (71.1%) were not significantly different from that of the entire cohort, indicating that the screening systems perform equally well with or without a clear contact history.
There is some difference in the reported symptomatology of SARS. The incidence of sore throat (6.1%) and rhinorrhea (3.7%) in our SARS patients is similar to that in Canada14 but different from that of Hong Kong, where sore throat was reported to be present in 20% to 35% of cases and rhinorrhea was reported to be present in 22.5% to 26% of cases.7, 15, 16, 17 The causes of the differences in the reported incidence of upper respiratory symptoms remained unclear but might be contributed to the different virus strains.16
In this study, we validated the symptom score and the clinical score in a new cohort. The sensitivities of the score systems dropped from 100% to 96.3% and 92.6%, respectively. The specificity declined from 75.9% and 86.3% to 51.6% and 71.2%, respectively. Several factors might have contributed to the changes in the sensitivity and specificity. In a comparison of the adjusted ORs of individual items for both scores in different cohorts (Table 4), the discriminating abilities of cough and diarrhea for predicting SARS decreased in the validation cohort. The proportion of patients with cough before or during fever, a strong negative predictor in the derivation cohort, increased in the SARS patients and decreased in the non-SARS patients in the validation cohort compared with the derivation cohort. The associated increase in false positive and false negative cases might explain the decrease in the sensitivities and specificities of cough for both scores. On the other hand, the frequency of diarrhea, a positive predictor in the derivation cohort, increased in the non-SARS patients of the validation cohort, which resulted in an increase in the false positive cases and a decrease in the specificities of diarrhea for both scores.
Several factors might have affected the clinical manifestations of SARS and non-SARS patients in the validation cohort, which in turn led to changes in the sensitivities and specificities for various symptom items described here. First, in comparison with patients in the derivation cohort, the SARS patients in the validation cohort were older. More SARS patients contracted the virus in the hospital settings in the validation cohort, and they were older and had more underlying diseases. Many underlying diseases in the elderly, such as chronic obstructive pulmonary disease, could increase the occurrence of cough before or during fever when they contract SARS. Second, most patients in the validation cohort contracted the illness locally. Different virus strains among imported versus local infection might have contributed to the different clinical presentations of the syndrome. Third, the non-SARS patients in the validation cohort had less cough before or during fever, less coryza, and more diarrhea than patients in the derivation cohort. These differences in the symptoms might reflect the activities of other viral diseases.
Attempts were made to modify the scores to maximize sensitivities and specificities. To further explore the clinical significance of the occurrence and timing of cough, the score for cough was modified to −1 (cough before fever), 0 (no cough), and +1 (cough after fever). With the modified scoring system in the validation cohort, the combined sensitivity reached 97.6% (95% CI 91.5% to 99.3%), but the combined specificity dropped to 46.2% (95% CI 38.5% to 54.0%). Other modifications of the scores yield similar results. The score systems in their current formats seem to be simple and balanced in terms of sensitivities and specificities. As the ROC curve demonstrated, selecting lower cutoff points for symptom and clinical scores will increase sensitivity by sacrificing specificity. With cutoff points of −1 and 0 for symptom and clinical scores, respectively, the combined sensitivity reaches 97.6% (95% CI 91.5% to 99.3%), but the combined specificity decreases to 44.2% (95% CI 36.7% to 52.1%). The selection of cutoff points and, therefore, the desired levels of sensitivities and specificities should be based on needs and resources. For example, in nonendemic areas with sufficient health care resource and isolation facilities, higher sensitivities may be desirable.
Our screening systems provide several advantages. First, most SARS patients could be detected and isolated early enough to prevent the disease from spreading. Second, the unnecessary isolations could be reduced to a minimum. Third, it could be used without contact history, which was the limitation of WHO or CDC criteria. Since July 5, 2003, when the WHO removed Taiwan from its list of areas with recent local transmission of SARS, the worldwide outbreak of SARS has come to an end.13 Like many other comparatively new and poorly understood viruses, such as Ebola or Marburg, the SARS-coronavirus could periodically surface to cause outbreaks and then fade away into some animal or environmental reservoirs.18 The scoring system we developed could provide an easy and reliable method for large-scale screening when SARS reappears.
In retrospect
The development of the scoring systems was empirically based on a small sample. Because the total number of SARS patients in Taiwan was less than 20 at that time, we designed this validation study with the expectation that only a small number of SARS patients would follow. Because this validation study was undertaken at a busy ED during the outbreak of SARS in Taiwan, we did not have much time to modify our study and scoring system. If the study could be reconducted, we would follow the Speigelhalter-Knill-Jones approach or the logistic regression model to modify our scoring systems.19
Footnotes
Editor's note: This article was first published on Annals' Web site (www.mosby.com/AnnEmergMed) on December 2, 2003. Articles of particular interest are published on the Web site in advance of their appearance in the print journal. In the future, an increasing percentage of our content will be published first on the Web, predating the print publication as a service to our readers.
Author contributions: CPS and WCC conceived and designed the study. SYC, CYH, and CMF contributed to acquisition of the data. The manuscript was prepared by CPS and revised by MHMM. PCIK and KCT provided critical revision of the manuscript for important intellectual content. FYS and MHMM were responsible for statistical consultation. The trial was supervised and conducted by SCC, YCC, and WJC. CPS, WCC, and WJC take responsibility for the paper as a whole.
The authors report this study did not receive any outside funding or support.
Contributor Information
Chan-Ping Su, Email: sucp@ha.mc.ntu.edu.tw.
Wen-Jone Chen, Email: jone@ha.mc.ntu.edu.tw.
References
References
- 1.World Health Organization. WHO issues a global alert about cases of atypical pneumonia: cases of severe respiratory illness may spread to hospital staff [World Health Organization Web site]. Available at: http://www.who.int/csr/sars/archive/2003_03_12/en/. Accessed March 12, 2003.
- 2.World Health Organization. Cumulative number of reported probable cases of severe acute respiratory syndrome (SARS) [World Health Organization Web site]. Available at: http://www.who.int/csr/sars/country/2003_07_11/en/. Accessed July 11, 2003.
- 3.Ministry of the Interior, ROC. Status of home compulsory quarantines for class A&B. Available at: http://www.moi.gov.tw/moi/english/addfun_list2.asp?permit=35&name=Status of Home Compulsory Quarantines&id=111. Accessed July 3, 2003.
- 4.Center for Disease Control and Prevention, Taiwan ROC. Taiwan SARS case update. Available at: http://www.cdc.gov.tw/sarsen/. Accessed September 22, 2003.
- 5.Lee ML, Chen CJ, Su IJ. Severe acute respiratory syndrome: Taiwan, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:461–466. [PubMed] [Google Scholar]
- 6.World Health Organization. Update 46: WHO sends team to Taiwan, situation in China [World Health Organization Web site]. Available at: http://www.who.int/csr/sars/archive/2003_05_03/en/. Accessed May 3, 2003.
- 7.Rainer TH, Cameron PA, Smit DV. Evaluation of WHO criteria for identifying patients with severe acute respiratory syndrome out of hospital: prospective observational study. BMJ. 2003;326:1354–1358. doi: 10.1136/bmj.326.7403.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen SY, Su CP, Ma MHM. Predictive model of diagnosing probable cases of severe acute respiratory syndrome in febrile patients with exposure risk. Ann Emerg Med. 2004;43:1–5. doi: 10.1016/S0196-0644(03)00817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Updated interim surveillance case definition for severe acute respiratory syndrome (SARS): United States, April 29, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:1–3. [PubMed] [Google Scholar]
- 10.Wong KT, Antonio GE, Hui DSC. Thin-section CT of severe acute respiratory syndrome: evaluation of 73 patients exposed to or with the disease. Radiology. 2003;228:395–400. doi: 10.1148/radiol.2283030541. [DOI] [PubMed] [Google Scholar]
- 11.Tsang OT-Y, Chau T-N, Choi K-W, et al. Coronavirus-positive nasopharyngeal aspirate as predictor for severe acute respiratory syndrome mortality [Centers for Disease Control and Prevention Web site]. Available at: http://www.cdc.gov/ncidod/EID/vol9no11/03-0400.htm. Accessed September 17, 2003.
- 12.World Health Organization. Management of severe acute respiratory syndrome (SARS). Available at: http://www.who.int/csr/sars/management/en/. Accessed April 11, 2003.
- 13.World Health Organization. Update 96: Taiwan, China: SARS transmission interrupted in last outbreak area [World Health Organization Web site]. Available at: http://www.who.int/csr/don/2003_07_05/en/. Accessed July 5, 2003.
- 14.Booth CM, Matukas LM, Tomlinson GA. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 15.Lee N, Hui D, Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 16.Peiris JS, Lai ST, Poon Ll. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnelly CA, Ghani AC, Leung GM. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet. 2003;361:1761–1766. doi: 10.1016/S0140-6736(03)13410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Update 89: what happens if SARS returns [World Health Organization Web site]? Available at: http://www.who.int/csr/don/2003_06_26/en/. Accessed June 26, 2003.
- 19.Seymour DG, Green M, Vaz FG. Making better decisions: construction of clinical scoring systems by the Spiegelhalter-Knill-Jones approach. [DOI] [PMC free article] [PubMed]


