Abstract
Here, we review the current knowledge about viral derived membranotropic peptides, and we discuss how they may be used for many therapeutic applications. While they have been initially discovered in viral fusion proteins and have been involved in the mechanism of viral entry, it is now clear that their features and their mode of interaction with membrane bilayers can be exploited to design viral inhibitors as well as to favor delivery of cargos across the cell membrane and across the blood–brain barrier. The peptide gH625 has been extensively used for all these purposes and provides a significant contribution to the field. We describe the roles of this sequence in order to close the gap between the many functions that are now emerging for membranotropic peptides.
Keywords: Membranotropic peptide, Hydrophobicity, Fusion, Delivery, Viral inhibition
Graphical abstract
Highlights
-
•
Membranotropic peptides and their therapeutic applications
-
•
Membrane fusion, viral inhibition, drug delivery
-
•
gH625, a peptide derived from Herpes simplex virus type I: a case study
-
•
gH625 in vitro and in vivo delivery across the blood–brain barrier
1. Introduction
Over the past few decades peptides have progressively achieved increased value in drug design and pharmaceutical delivery. Moreover, great interest has been dedicated to the identification of peptides as drug candidates. The number of peptides in the pharmaceutical industry is continuously growing and about 10% of the entire drug market is represented by peptide based drugs [1], [2]. Bioactive peptides can be derived from natural sources or can be discovered through rational engineering, high-throughput screening, or structure-based design starting from defined protein regions [3]. Among the many peptides playing a relevant role in biology, some show a high propensity for binding to lipid membranes due to their simultaneous hydrophobic and amphipathic nature.
This class of hydrophobic peptides is characterized by the presence of unusual conspicuous amounts of alanine and glycine residues and sometimes also prolines. Such a degree of Ala/Gly content is uncommon for hydrophobic domains such as signal sequences and transmembrane anchors; in fact, their presence may account for the intrinsic conformational flexibility which is a typical feature of membrane interacting peptides.
Also aromatic residues are generally present and dominate the interactions that take place at this unique physical–chemical environment of the water–membrane interface [4]. The favorable interactions of aromatic side chains with phospholipid moieties located at the membrane interface contribute to the insertion of the peptide into the bilayer.
Amphipathicity is a key feature of these peptides. The term amphipathicity generally refers to molecules with both hydrophilic and hydrophobic faces [5]. Peptides can be amphipathic in their primary structure or secondary structure. Primary amphipathic peptides correspond to the sequential assembly of a domain of hydrophobic residues with a domain of hydrophilic residues divided by a spacer domain; while secondary amphipathic peptides are generated by the conformational state which allows positioning of hydrophobic and hydrophilic residues on opposite sides of the same molecule. In particular, amphipathic, hydrophobic peptides present one face with large and aromatic residues and the other with small residues such as Ala/Gly. This distribution of amino acid residues facilitates the membrane interaction and peptide insertion into the bilayer [6].
Conformational polymorphism plays a key role; in fact, the ability to shift from random to α/β conformations as a consequence of membrane composition and peptide concentration has emerged as a common structural pattern for this class of peptides [6].
There are several types of membrane active peptides which can be roughly divided in antimicrobial peptides [7], viral peptides [8] and cell penetrating peptides [9]. Although very different in primary sequence one from the other, it may be hypothesized that their common physical features could result in a shared mechanism of action and essentially determines the many roles that they can play in nature. Among the hydrophobic peptides with a propensity for membrane binding, characterized by a high interfacial hydrophobicity or amphipathicity, the ones derived from enveloped virus glycoproteins are attracting considerable attention. These peptides can interfere with enveloped virus entry by direct physical interaction with the hydrophobic surfaces present on membranes and/or fusion proteins and are, thus, critical for both fusion and entry.
Viral glycoproteins undergo conformational changes as a consequence of either low endosomal pH or receptor binding which leads to the exposure of hydrophobic peptides, loops or patches, which then interact with and destabilize one or both the opposing membranes. Crystallographic data on the post-fusion structures of viral fusion proteins have allowed the identification and characterization of three different classes [10], [11]. Class I fusion proteins are characterized by trimers of hairpins with a central α-helical coiled-coil structure and have been identified in orthomyxoviruses, paramyxoviruses, retroviruses, filoviruses and coronaviruses [12], [13], [14], [15], [16]. Class II fusion proteins are present on viral envelopes as pre-fusion dimers which convert into post-fusion trimers of hairpins composed of β structures and have main representatives in the Flaviviridae and Togaviridae families [17], [18]. Class III fusion proteins are characterized by a central α-helical trimeric core similar to Class I and two fusion loops located at the tip of an elongated β-sheet similar to Class II fusion proteins and members are present in Herpesviridae and Rhabdoviridae families [19].
Despite several differences in the mechanism of entry elicited by the three classes of fusion glycoproteins, they all induce membrane fusion in a similar manner through the formation of an analogous hairpin structure which allows fusion peptides to insert into cell membranes and to drive membrane destabilization. Thus, during the viral entry process, the hydrophobic surfaces that become exposed are characterized by somewhat variable but at the same time detailed physical characteristics which include the size, shape and secondary structure of exposed hydrophobic patches, as well as the nature of the neighboring polar or charged residues.
2. Membranotropic peptides and fusion ability
The Wimley–White interfacial hydrophobicity scale (WWIHS) is an experimentally-determined free energy scale that calculates the propensity of individual amino acids in peptide sequences to partition from water into a phosphatidylcholine interface [20], [21] and has been effectively used to identify fusion peptides in viral glycoproteins. The hydropathy analysis allows calculating a hydrophobicity score along the sequence of a protein, identifying segments with a propensity to interact with membrane interfaces. WWIHS values are calculated assuming random coil peptides partitioned into the bilayer interface; the values are minimum possible values and the ΔG may get more favorable if peptide binding also promotes an increase in secondary structure [22], [23].
The interfacial helical hydrophobic moment (iHHM) is a further physico-chemical factor that is important for membrane interactions and secondary structure formation of peptides bound to membrane interfaces. The iHHM describes the degree to which a peptide sequence would have segregated hydrophobic and hydrophilic faces if it folded into an α-helix [24]. A peptide with a large iHHM can interact strongly with membranes as a helix due to partitioning–folding coupling [22], [23] even with a WWIHS score that is not positive overall.
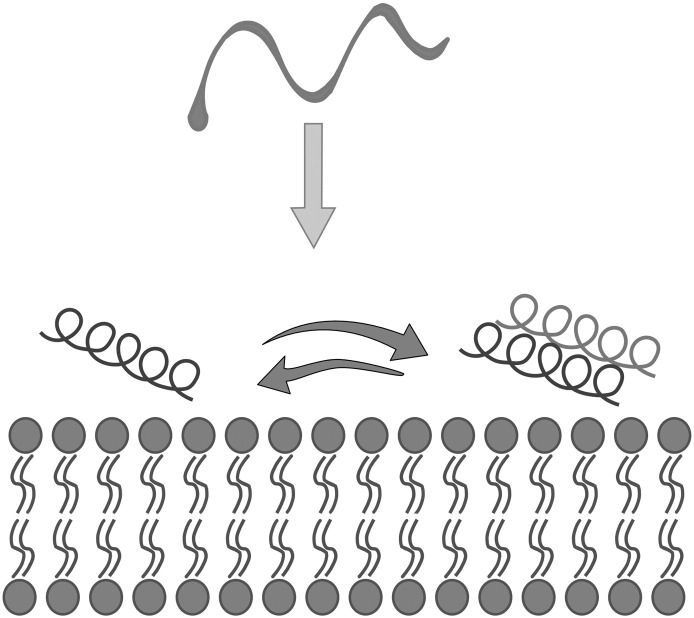
The presence of the fusion peptide within the ectodomain exposed to the aqueous phase is a feature shared by all viral fusion proteins and constitutes an absolute requirement for their fusogenic activity. Fusion peptides are typically 20–30 residues long and potentially fold into amphipathic helices and are rich in glycines and alanines, providing them a high degree of conformational flexibility. Thus, their structure is polymorphic and strongly dependent on the environment. The fusion peptide of influenza virus, for example, has been observed in random coil, α-helical and β-sheet conformations in different environments [25] (Fig. 1 ).
Fig. 1.
The conformational flexibility of membranotropic peptides.
It has been proposed that all three forms have some physiological relevance; the peptide may be unstructured in solution on the way to the target membrane; it may be helical at low concentrations but may self-associate in β-sheets at higher concentrations in the membrane interface. The fusion peptide of HIV also undergoes conformational transitions; it adopts α helical or β sheet structures depending on concentration, lipids and ionic conditions [26].
The helical form of the influenza fusion peptide is probably a key determinant to promote fusion. The structure of the peptide in membrane shows a kink which separates the N terminal and the short C terminal helices which together form a boomerang shaped structure [27]. Both helical arms are amphipathic with bulky hydrophobic residues facing the membrane interior. The conserved N-terminal glycine residue is critical for fusion and for the correct structure of the peptide inside the membrane; in fact, when it is mutated to a valine the N-terminal helix is partially unwound and the fusion peptide is inactive [28].
Actually, there are numerous studies demonstrating that a delicate balance between α and β structures, is essential for membrane fusion and is influenced by environmental conditions such as pH, ionic strength, peptide sequence, presence or absence of divalent cations, cholesterol content and also by the lipid/peptide ratio. For instance, studies performed on the Ebola fusion peptide, show that the conformational transition from an α-helix to a β-sheet is induced by a change in the peptide to lipid ratio in the membrane [29]. At low peptide concentration in lipids, it is essentially an α-helix; while as the local peptide concentration increases in the membrane, the proportion of α-helix drops off in favor of a mainly antiparallel β-sheet structure. This concentration dependent effect on peptide conformation might be of biological relevance [29].
The functional meaning of the conformational polymorphism is unclear, although it is believed to be fundamental to enable backbone reorientation of the fusion protein; therefore, the ability to insert at various levels might be required for the evolving of final stages of the fusion cascade [30]. Independently from the principal conformational organization, the degree of insertion plays a key role for inducing membrane fusion. It has also been hypothesized that [31] fusion domains first assemble as β-sheets on the surface of the membrane and later convert into α-helices to complete fusion.
Aromatic residues are generally present in fusion peptides and may help in overcoming the energy cost of peptide bond partitioning into membranes. The interactions with phospholipid moieties located at the membrane interfaces may also help in stabilizing the insertion into just one leaflet of the bilayer. The initial interaction with the external leaflet is thought to generate elastic stresses which drive to bilayer fusion, helping to overcome the hydration repulsion forces between approaching bilayers by orienting the poorly solvated face toward the external medium [32]. The asymmetric insertion into one membrane monolayer may promote expansion of the polar head region and determine a curvature stress onto the overall lipid bilayer; the created bulges that protrude from the membrane can facilitate the formation of lipid contacts between fusing bilayers [33].
Particular attention has also been devoted to the effect of additional membranotropic sequences on the overall fusogenicity. The presence of additional fusogenic sequences was evidenced in Sendai F1 [34], Measles F1 [35], SARS-CoV S2 [36], Hepatitic C virus E1 and E2 [37], Dengue E [38], [39] and Herpes Virus gB and gH [40], [41], [42]. The idea that a single fusion peptide is the solely responsible for the complete membrane fusion event has been substituted by the assumption that a concerted action of different membranotropic regions is necessary for membrane interacting/perturbing activity. As a matter of fact, also membrane proximal regions (pre-TM) play a key role in fusion [41], [43], [44], [45]. The pre-TM domains are particularly rich in aromatic residues which enable them to insert into the membrane interface.
3. Viral inhibition
Effective therapeutics against enveloped viruses are still scarcely represented. A few drugs have been developed against HIV, influenza virus, hepatitis virus and a few other viruses but they are still not ideal and in some cases have proved to induce resistance [46]. As a consequence, for most enveloped viruses, there are no effective therapies and entry inhibitors represent an interesting and underutilized target.
Peptides with a propensity for membrane binding can also interfere with enveloped virus entry by direct physical interaction with the hydrophobic surfaces present on cell membranes and/or fusion proteins. As recently reviewed by Badani et al. [46], there are many peptide inhibitors that are somewhat hydrophobic and/or amphipathic with a propensity to bind to bilayer membrane interfaces and other hydrophobic surfaces. It is not known whether membrane binding directly affects viral fusion, or whether interaction with the fusion protein itself is an absolute requirement for entry inhibition. It is widely accepted that membrane binding of an inhibitory peptide will greatly increase the effective concentration of the peptide close to the fusion protein, indicating that the interaction with membrane and the interaction with the fusion protein may be effectively coupled [47].
As a matter of fact, the potential of numerous fusion peptides and/or membranotropic peptides derived from proteins of enveloped viruses as entry inhibitors has been widely described in literature [48], [49], [50], [51]. The accepted view is that the inhibition of infectivity may be due to the formation of inactive aggregates between the fusogenic stretches present in both the viral protein and the synthetic peptides. These aggregates are formed as a consequence of their ability to oligomerize or to mimic the modes of binding of their original domains in their partner protein. It has been hypothesized that they stabilize a pre-fusion intermediate and prevent merging of the bilayers.
It is now evident that several domains are essential for membrane fusion and thus peptides involved in the fusion mechanism may all interfere with the intramolecular interactions between the several domains and may represent interesting targets for the design of entry inhibitors. Thus, membrane physical properties could be critically important to the events that drive viral entry and it is conceivable that peptides interfere with the function of viral fusion proteins by changing the physical chemistry of the membrane itself by direct interaction. Many laboratories are working to unravel the mechanism of action of viral membranotropic peptides and several hypotheses have been proposed. Many studies suggest that multiple mechanisms may take place simultaneously. Indirect ways in which interfacial binding peptides can affect viral entry have also been hypothesized. Self-oligomerization of membrane embedded fusion peptides has been proposed to be responsible of inhibition [52], [53]. The most studied case is that of HIV, where the inhibition has been attributed to the formation of structurally defined oligomeric complexes [54], [55]; while mutants with a lower helical content and tendency to self-associate into β-sheets [56] are able to inhibit membrane fusion at various stages.
It is interesting to note that there are clinical studies on a peptide called VIRIP, which is designed as an inhibitor of the HIV fusion peptide [57], [58]. This sequence was able to block HIV-1 infection by targeting gp41 fusion peptide [58] and optimized versions of this sequence proved to be as potent as inhibitors targeting the coiled coil sequences and moreover were devoided of cellular toxicity. A 10-day monotherapy clinical trial enrolling 18 HIV-1 infected patients [57] showed that the drug can be well tolerated by patients and reduces their plasma viral load. Its identification and clinical evaluation represent the first proof of concept that membranotropic sequences could suppress viral replication in infected individuals and have potential clinical effectiveness.
The hypothesis that peptide entry inhibitors act by a physical–chemical interaction with hydrophobic surfaces exposed during the fusion process suggests that this novel approach may be a general rule; moreover, instead of focussing on the structure-based design, it would be possible to design novel hydrophobic/amphipathic inhibitors which could be easily made protease resistant by the introduction of non-natural or d-amino acids.
4. Delivery tools
The membrane bilayer represents a semi-permeable barrier, defining the interior of an individual cell; its existence confers cells their potential to survive and function properly. Nevertheless, crossing of the cellular membranes remains one of the major obstacles for the proper delivery of therapeutics [59], [60]. The lipophilic nature of biological membranes restricts the direct intracellular delivery of most compounds; whereas small molecules and ions can diffuse across the bilayer, larger molecules are generally excluded from simple diffusion into the cell. The differing hydrophobicity/hydrophilicity of the lipid membrane renders the transfer across this barrier extremely difficult due to differences in solubility. Notwithstanding the therapeutic potential of a number of novel molecules, their pharmaco-distribution properties hamper the possibility to reach the stage of pharmaceutical preparations and stimulate industrial interest; in fact, these molecules need to be delivered intracellularly to exert their therapeutic action inside the cytoplasm or onto individual organelles. It is, thus, evident that the therapeutic potential of a drug is largely dependent on the development of delivery tools able to selectively and efficiently carry it to target cells with minimal toxicity.
The translocation across the membrane is by far less well understood than the binding step. There is a significant similarity in the physico-chemical parameters between membrane partitioning peptides and membrane translocating peptides [61]. A novel intriguing hypothesis is that hydrophobic peptides that partition into membranes may also be able to cross cell membranes and enter cells. Therefore, these peptides may also cross endothelial layers in vivo, including the blood–brain barrier [62], [63].
Delivery across cellular membranes involves several membrane reorganization processes such as transient permeabilization of the cell membrane, which are similar to the ones involved in the entry of viruses. Membrane fusion and its disruption are related processes, although leakage and fusion capacities of peptides do not always correlate and the features/activities of membranotropic peptides may depend on particular environmental and temporal conditions. Since not all membranotropic peptides are able to cross the membrane bilayer, it is essential to identify structural characteristics of hydrophobic peptides know to enter the cell membrane to highlight any feature that is involved in the penetration which may help in the design of novel delivery tools. Thus, an important feature to consider is the structural requirements for cellular uptake and the ability of membranotropic peptides to interact with the cell surface and lipid moieties of the cell membrane. A very complete review describing the binding and translocation of membrane active peptides has been recently published [64] which highlights the fact that peptide translocation is not coupled with dye flux. Graded dye flux would occur concomitant with peptide translocation, which would explain incomplete dye release; whereas all-or-none flux would occur with peptides unable to translocate, and therefore these peptides accumulate on the membrane until a rupture point is reached, resulting in complete dye release [64].
Cell-penetrating peptides (CPPs) have been widely used due to their capability to transport several kinds of macromolecules across the membrane bilayer in vitro and in vivo [65], [66], [67]. CPPs are short and usually basic amino acid rich peptides originating from proteins that are able to cross biological barriers, such as the viral TAT protein. Although the uptake mechanism of CPPs is still debated, it seems to involve mainly the endocytic pathway, trapping the conjugated cargo in endosomes eventually ending in lysosomes where common enzymatic degradation mechanisms take place, therefore leading to a limited delivery of therapeutic agents to the intracellular target.
Hydrophobic peptides that efficiently traverse biological membranes, promoting lipid-membrane reorganizing processes, such as fusion or pore formation and involving temporary membrane destabilization and subsequent reorganization [8], [68], may be able to circumvent the endosomal entrapment either favoring the escape from the endosome or by translocating a cargo through the plasma membrane directly into the cytosol.
This idea has been exploited to design the drug delivery tool called MPG. MPG is an amphipathic peptide whose primary sequence is composed of the hydrophobic amino acids of the HIV-1 fusion peptide (GALFLGFLGAAGSTMGA) associated to a hydrophilic domain derived from the Nuclear Localization Sequence (NLS) of Simian virus 40 (SV40) large T antigen (PKKKRKV). These hydrophilic and hydrophobic segments are separated by a three amino-acid spacer (WSQ) [69], [70]. This peptide exploits the known properties of the glycine-rich HIV fusion peptide essential for membrane fusion activity and the NLS of the SV40 large T antigen to improve the nuclear addressing of the peptide [71], [72].
At the moment this is the only viral fusion peptide that has been widely exploited for applications in drug delivery.
5. A case study: gH625, a membranotropic peptide derived from Herpes simplex virus type I
A milestone in understanding the role of hydrophobic viral peptides is represented by the sequence “gH625” derived from glycoprotein H of Herpes simplex virus type I. Herpes simplex virus (HSV) is an important human pathogen, responsible for significant morbidity and mortality worldwide and is characterized by a complex multi-component entry machinery. HSV enters host cells by fusion of the viral envelope with either the plasma membrane or an endosomal membrane, and the entry pathway is likely determined by both virus and host cell factors and involves multiple viral glycoproteins and cellular receptors in a cascade of molecular interactions [73], [74], [75], [76]. The envelope glycoproteins gH/gL, gB and gD are all essential for the entry process and their expression is able to induce the fusion of cellular membranes in a virus-free system [77], [78]. Both gH/gL and gB constitute the core fusion machinery and cooperate to induce the initial lipid destabilization that ends in fusion [79] and both gB and gH contain several membranotropic sequences [40], [41], [42], [49], [50], [80], [81], [82]. Although it has recently become available the crystal structure of the gH–gL complex [83], it is still debated whether gH is merely a fusion regulator or it plays a more direct role in the fusion process and many studies suggest that the gH–gL complex may undergo dynamic rearrangements [77], [84]. In particular, some peptides derived from the gH ectodomain block virus entry, while others have the ability to bind and disrupt model membranes. gB is considered a canonical Class III fusion protein and has been demonstrated to be involved in virus attachment, penetration and cell-to-cell spread. The crystal structure of gB is a trimer in which multiple contacts between protomers throughout the molecule contribute to its stability [85]. It has been hypothesized [86] that gB refolds similarly to Class I fusion proteins and that the packing of the C-terminal arm against the coiled-coil provides the driving force for gB refolding from the prefusion to the post-fusion conformation. The gB structure corresponds to a post fusion conformation and it is now widely accepted that gB undergoes conformational changes upon variations of pH in order to bring about fusion [87], [88], [89], [90]. Several synthetic gB peptides induced the fusion of large unilamellar vesicles and inhibited herpes virus infection [42], [91].
When the crystal structures of gB and of gH/gL were not yet available, we reported the identification of several sequences in gH and gB with the ability to interact with the membrane and among these sequences there was also the canonical fusion peptide of gB [40], [42], [80], [92]. Although it is not yet well understood the role played by the other membranotropic sequences and in particular by the glycoprotein gH in the whole fusion process, it is now widely accepted that several regions in the fusion glycoproteins are involved in the local destabilization of the membrane bilayer which ends in the fusion of the viral envelope with the host cell membrane.
gH625 was first selected and characterized by our group in 2005 [40] and still is the HSV-1 peptide with the highest fusion capability and the most widely studied. It was initially identified using the WWIH scale and subsequent works allowed determining the many applications of this sequence from membrane fusion, to viral inhibition and drug delivery [63], [93], [94].
5.1. General features and membrane interactions
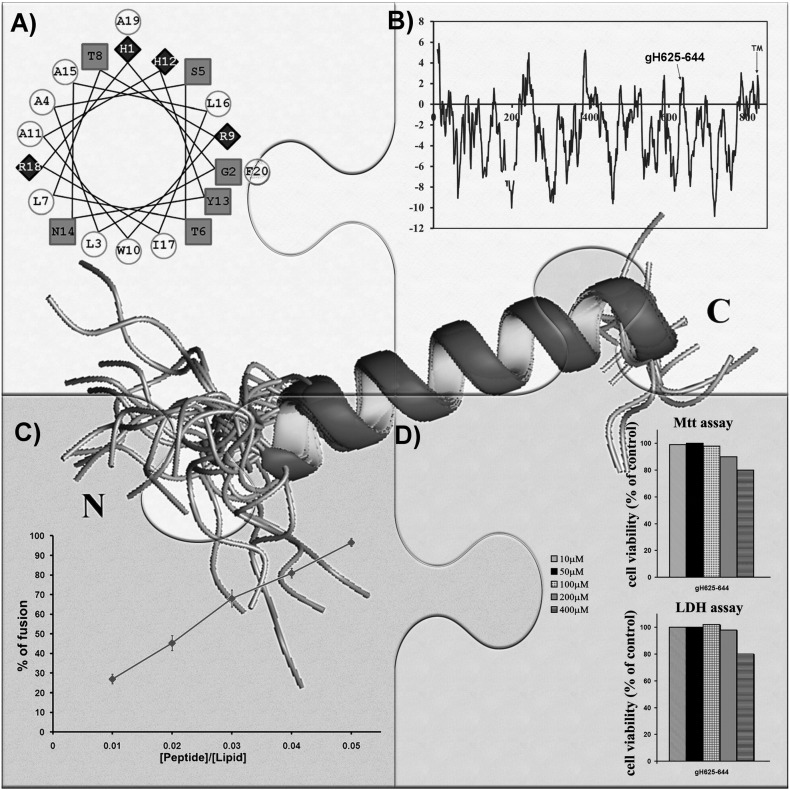
The twenty residue peptide gH625 (from aa 625 to aa 644) is a membrane-perturbing domain, (Fig. 2 ) which interacts with biological membranes and is implicated in the merging of the viral envelope and the cellular membrane [42], [50]. The peptide contains residues crucial for its capacity to interact and destabilize target lipid membranes. It is rich in hydrophobic residues including glycines, leucines, alanines, and aromatic residues such as tryptophan and tyrosines, which are known to be located preferentially at the membrane interface. The peptide–lipid interactions are initiated by the arginine residue located at the C-terminus; in fact, when the arginine is mutated, the fusogenic activity of the peptide is strongly impaired. The hydrophobic domain is also crucial for its insertion into the membrane and further supports the view that hydrophobic interactions between fusion proteins and cell-membrane phospholipids initiate membrane perturbation in the early stages of viral fusion. The many biophysical experiments performed on gH625 have shown that the peptide interacts with model membranes, penetrates the bilayer from its N-terminal side, has a tryptophan residue buried inside the bilayer, and adopts a helical conformation with its hydrophobic residues on one face of the helix and polar or charged residues on the opposite face [40], [49], [50].
Fig. 2.
The many features of gH625: A) helical wheel representation of gH625; B) WWIHS hydrophobicity plot of the glycoprotein gH showing the peak corresponding to gH625; C) peptide-promoted membrane fusion of PC/Chol (1:1) LUVs as determined by lipid mixing; D) toxicity assays performed on gH625 up to a concentration of 400 μM.
The analysis of peptides with longer and shorter sequences derived from this region and of their interactions with membranes clearly demonstrated that the activity of this region depends on the amino acid sequence and on its length. The presence of a histidine residue at the N-terminus of the native sequence strongly increases the fusion activity [50]. The importance of a single histidine residue as a switch for triggering viral fusion was also reported for other viruses [95] such as paramyxoviruses, therefore supporting the importance and specificity of the histidine moiety in activating fusion. Furthermore, a conserved histidine in one of the fusion loops of Semliki Forest virus E1 protein was found to be fundamental [96]. The histidine in gH625 both helps the initial interactions with the membrane and the oligomerization process [50]. This hypothesis is further supported by the fact that the histidine is located at the N-terminus and correct configuration of the N-terminal fusion peptide appears to be crucial for the fusogenic function of several fusion proteins as well as its location in the membrane core after peptide-bilayer interaction. The addition of one histidine at the N-terminus of gH625 is sufficient to make the peptide approximatively 8-fold more active. In particular, the addition of any other residue at the N-terminus impaired the fusion ability of the sequence (data not published).
gH625 strongly interacts and spontaneously penetrates the lipid-phase and inserts into membranes with a α-helical structure [50], [81], [82]. Both the tryptophan and tyrosine are on the same side of the helix in the three-dimensional structure, forming an amphiphilic helix in which one side is constituted by aromatic and hydrophobic residues, whereas the other side is formed by hydrophilic or small residues. The interaction between the aromatic ring of tryptophan and the side chain of tyrosine is important for maintenance of structural stability during the interaction with the membrane. An amphipathic α-helix is believed to be an important feature of membranotropic peptides playing a crucial role for mediating lipid–protein interactions during the binding of proteins to membranes and once bound, the hydrophobic face of the amphipathic peptide would allow the peptide to enter the membrane interior, thereby triggering local fusion of the membrane leaflets, transient pore formation, cracks and membrane fusion.
gH625 has the ability to penetrate deep into the bilayer as a helix without causing significant bilayer perturbations which may help explaining its ability to perform several different roles.
5.2. Viral inhibition
gH625 showed a significant inhibitory effect and this effect appears conditioned by its ability to partition into membranes and aggregate within them. Since the peptide self-associates in aqueous and lipid solutions, it is possible that it binds to its counterpart in the gH protein. gH625 may also interact with the host cell membrane, therefore its ability to moderately inhibit viral entry when cells are treated first, is dependent on the possibility that the virus will find a modified cell membrane still exhibiting on its surface the peptide [48]. Moreover, gH625 does not have any activity in virus preincubation experiments, indicating that an eventual binding partner site on the pre-fusion gH protein is probably hidden and not available to interactions with free peptides, as also demonstrated by the analysis of the gH crystallographic structure.
While the N-terminal histidine residue was proven to be fundamental for the interaction with the membrane bilayer and for translocation across the membrane, the absence of this residue induced similar levels of viral inhibition when compared with the full length peptide. The substitution of Leu627 with a valine residue does not alter the hydrophobicity of the peptide, and does not influence its infectivity inhibition properties while its substitution with a polar residue (serine) substantially reduces its inhibitory activity [49].
Recently, poly(amide)-based dendrimers functionalized at their termini with gH625 were shown to inhibit both HSV-1 and HSV-2 at a very early stage of the entry process, most likely through an interaction with the viral envelope glycoproteins; thus, preventing the virus from coming into close contact with cellular membranes, a prerequisite for viral internalization [97]. The 50% inhibitory concentration was 100 and 300 nM against HSV-1 and HSV-2 respectively, with no evidence of cell toxicity at these concentrations, indicating that the functionalization of a dendrimer with a membranotropic peptide represents a promising strategy for inhibition of viruses of the Herpesviridae family. The multivalent display of gH625 on the dendrimer scaffold results in an almost six fold increase of antiviral activity for HSV-1 and two fold for HSV-2 in comparison to the activity of the dendrimer itself, and more than 100-fold increase in the activity of the unsupported peptide. The cytotoxicity profile measured by the MTT assay showed that the peptidodendrimer is not toxic to Vero cells up to the highest concentration investigated in antiviral testing, while some toxicity was observed for the unfunctionalized dendrimer, especially at higher concentrations, demonstrating another advantage of the peptide functionalization [97]. Any inhibitory activity was excluded when the compounds were added at a post-entry step and also when cells were pre-treated with the dendrimer derivatives, indicating that both the peptidodendrimer and the dendrimer are not able to interfere with viral replication once the virus has gained access to the cellular milieu. The peptidodendrimer might sterically hinder the gH relative domain, either in a pre-fusogenic or in an intermediate conformation, preventing a complete and functional interaction between gH and the membrane to fuse. The mechanism of inhibition may involve binding to gH itself through oligomerization of the gH625 domain present on the glycoprotein or interaction with other glycoproteins present on the virion envelope, such as gB or gD.
The modification of a dendrimer scaffold with membranotropic peptides represents an attractive strategy for the design of a new class of antiviral drugs that exert their effect, coupling the intrinsic anti-viral properties of the dendrimer with the activity of membranotropic peptides and have the potential of being developed as multifunctionalized scaffolds to provide a therapeutic molecule to directly deliver to its target [97].
The inhibition of membrane fusion represents an attractive target for drug design and although further studies are needed to better define the exact mechanism of inhibition by hydrophobic peptides and the specific nature or location of their interactions with viral targets, the data obtained for gH625 suggest that hydrophobic domains play a significant role in membrane fusion and provide an alternative approach to the development of viral peptide inhibitors outside of the classical inhibitory heptad repeat regions.
5.3. Applications to drug delivery
gH625 cellular uptake is associated with its hydrophobic and amphipathic characters which provide the necessary ability to interact with membrane lipids and to form a transient helical structure that temporarily affects membrane organization, thereby facilitating insertion into the membrane and translocation [98].
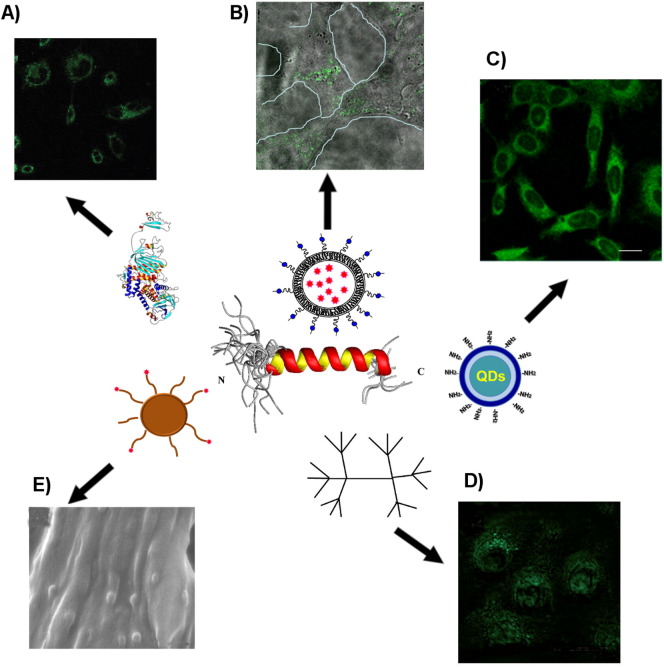
Compared to TAT peptide (a positively charged CPP) which mainly exploits the endocytic pathway, gH625 crosses membrane bilayers mainly through a translocation mechanism. A one amino acid shorter version of this fusogenic peptide was also found to improve the endosomal release of DNA/Lipofectamine lipoplexes and transgene expression up to 30-fold in human cell lines [99]. It has been recently demonstrated that gH625 is able to traverse the membrane bilayer and to transport into the cytosol several compounds, such as QDs [98], liposomes [100], NPs [62], dendrimers [101], and proteins [102]. Examples of using gH625 as an intracellular delivery enhancer are provided in the remaining part of the paragraph (Fig. 3 ).
Fig. 3.
The many applications of gH625 to drug delivery. Confocal microscopy images showing the internalization of gH625 functionalized: A) proteins [102]; B) liposomes [100]; C) Qdots [98]; D) dendrimers [101]. E) Scanning electron microscopy images of functionalized polystyrene nanoparticles [62].
QDs are fluorescent probes under intense research and development for broad applications in molecular, cellular and in vivo imaging [103]. Although considerable success has been achieved in using QDs for labeling fixed cells and for imaging cell membrane proteins, only limited progress has been made for molecular imaging inside living cells because of their insufficient ability to traverse cell membranes. Several authors have recently reported on the functionalization of QDs with positively charged CPPs and established that the main route of entrance is via endosomal uptake, therefore, escape from the endosomal system is of paramount importance [104], [105], [106]. gH625-QD internalization was demonstrated to be highly successful and to involve the endocytic pathway only to a minor extent [98].
Liposomal aggregates have also attracted great attention due to their success as in vivo carriers of drugs [107]. To enhance the antitumor efficacy of liposomal drugs, the efforts of many research groups are directed toward the improvement of cellular internalization of liposomes through the addition of surface ligands and cell penetrating peptides. Liposomes decorated with gH625 and loaded with doxorubicin (Dox) [100], were able to penetrate inside living HeLa cells. The results obtained suggest that the functionalization of liposomes with gH625 could affect the uptake mechanism of liposomes and their intracellular distribution and Dox release. This evidence could be useful in the design of carriers for a controlled delivery and release of Dox in order to avoid side effects associated to Dox itself.
Dendrimers [108], [109] also represent a very promising tool for drug delivery, combining the advantageous features of nanoparticles (ideal size as in vivo carriers, multivalency), of polymeric materials (low cost, tunable properties, biocompatibility) and of small molecules (monodispersity and detailed control of their properties) [109], [110]. Their surface modification by means of conjugation or adsorption of a biospecific ligand, may allow their delivery to specific sites and modulation of drug release minimizing toxic effects and increasing intracellular bioavailability [111]. Thus, the dendrimeric scaffolds may be a promising tool for an efficient drug delivery engine. Little information is available on the mechanism of dendrimer uptake and intracellular trafficking [112]. Studies performed on PAMAM dendrimers [113] and PAMAM dendrimers functionalized with the TAT [114] indicate that endocytosis mechanisms contribute to the internalization and intracellular trafficking and that adding the TAT failed to enhance delivery efficiency. The attachment of gH625 to the termini of a poly(amide)-based dendrimer allows the conjugate to penetrate into the cellular matrix, whereas the unfunctionalized dendrimer is excluded from translocation. The peptide-functionalized dendrimer is rapidly taken into the cells mainly through a non-active translocation mechanism [101]. The combination of the benefits of dendrimers and peptides chemistry could be useful for the development of a selective carrier which could cross the membrane and be efficiently internalized into the cellular targets.
5.4. In vitro and in vivo delivery across the blood–brain barrier
Many therapeutic drugs are excluded from entering the brain, due to their lack of transport through the blood–brain-barrier (BBB) [115]. The development of new strategies for enhancing drug delivery to the brain is fundamental in diagnostics and therapeutics of central nervous diseases (CNS). Most strategies to transport drugs inside the CNS cause disruption of the anatomical texture of the BBB, therefore impairing its natural function; as a consequence, effective delivery approaches should be cautiously assessed considering their impact on the overall protective function of the BBB [116]. Targeted delivery of a therapeutic cargo to the intended site of action in the brain appears to be one of the most promising non-invasive approach to overcome the BBB, combining the advantages of brain targeting, high incorporation capacity, reduction of side effects and circumvention of the multidrug efflux system [117], [118], [119], [120].
Polystyrene nanoparticles (NPs) decorated on their surface by gH625 showed that the uptake of NPs with gH625 by brain endothelial cells was greater than that of the NPs without the peptide and functionalized NPs were free to move intracellularly [62]. Most importantly, gH625 decreased NP intracellular accumulation as large aggregates and enhanced the NP BBB crossing. The surface functionalization with gH625 may change NPs fate and provides a good strategy for the design of promising carriers to deliver drugs across the BBB for the treatment of brain diseases.
Whether multifunctional nanosystems, designed and tested in vitro, are able to properly work in vivo into mammalian hosts, is not fully granted. To address this issue, in vivo studies are necessary, thus validating design strategies and facilitating optimization and further functionalization. Although numerous studies showed that gH625 is an efficient carrier for bioactive cargoes in vitro [121], these results did not guarantee that it can be developed into a useful pharmaceutical delivery platform. The ability of gH625 to cross the BBB in vivo was also recently evaluated [122]. gH625 was administered in vivo to rats and its presence in the liver and in the brain was detected. Within 3.5 h from its i.v. administration, gH625 can be found beyond the BBB in proximity of cell neurites. gH625 has no toxic effect in vivo, since it does not affect brain maximal oxidative capacity and mitochondrial respiration rate. The data suggest that gH625, for its ability to cross the BBB, represents a novel nano-carrier system for drug delivery to the central nervous system. These results open new possibilities for direct delivery of drugs into patients in the context of theranostics and might address the treatment of several human diseases.
Other peptides have been proposed as a drug delivery system; it was demonstrated that TAT was able to enter tissues in vivo in mice [123]; ANTP was able to activate endogenous T cells in mice [124]. These peptides are highly positively charged, and absorptive-mediated transcytosis has been proposed for their transport across the BBB. A bradykinin analogue has also been reported to increase the penetration of small molecules by transitory opening of the BBB [125]. gH625 is the first viral membranotropic peptide which was shown to be a potential delivery system for macromolecules in vivo; these results coupled with previous in vitro data support the view that gH625 enters the BBB without involving endocytic processes. Hence, the eventual cargo may be immediately and completely available [122].
The presence of multiple metabolic barriers may restrict the application of such peptide-based ligand for targeted drug delivery in vivo. Peptides alone or conjugated on the surface of nanocarriers are subject to proteolysis in the blood after systemic administration. In addition, the BBB is also a metabolic barrier due to the presence of various enzymes in brain capillary endothelial cells. gH625 starts to be degraded after 1 h of incubation but the intact peptide is still present after 3.5 h of incubation and thus holds the potential for extending brain targeting efficiency due to its resistance to proteolysis for 3.5 h [122].
6. Conclusions
It is still under-recognized that some amino acid sequences in virtue of their specific features can play many different roles in nature. Membranotropic viral peptides derived from fusion glycoproteins are widely studied especially for their ability to fuse membranes but there are many literature data also describing other roles besides membrane fusion. gH625 is an example of how these sequences can be employed for completely different purposes: fusion of membranes, viral inhibition and drug delivery. Till now, gH625 is the only membranotropic peptide that has been extensively used for many applications and among them as a drug delivery system for the brain. In the development of new therapies to treat brain pathologies, the BBB represents a major obstacle against the use of potential drugs for treating disorders of the CNS due to the impermeable nature of the cell membranes of this compartment to several molecules [115], [126]. The data reported on the in vivo application of gH625 for brain delivery, support the novel view that synthetic peptides derived from viral membranotropic sequences can be used successfully to deliver biologically active substances inside the BBB.
The exact molecular mechanism of gH625 entry remains to be established but it appears to be a general feature of membranotropic peptides, which may be used for mediating delivery to virtually any tissue and in particular across the BBB, conveying a wide variety of cargoes with intact bioactivity into virtually any tissue or organ. Other sequences have been found to be useful for drug delivery which happens to have the same features of the viral fusion peptide, indicating that it may be possible to design novel sequences with pre-determined characteristics which can be useful to treat many diseases.
However, still much work has to be done on this type of peptides; we should not forget that their mechanism of perturbation of membrane bilayers may also allow the design of new membranotropic peptides with the ability to denature the membrane bilayer of bacteria and thus we may add to their many roles also the antibacterial activity which may represent an alternative to classical antibiotics in order to combat the antibiotic resistance problem. Much of the vast literature on membranotropic peptides is devoted to single activities of these sequences; yet compelling structure–function relationship studies bridging the gap among all these activities are necessary.
Acknowledgement
The authors thank Luca De Luca for excellent technical assistance.
References
- 1.Craik D.J., Fairlie D.P., Liras S., Price D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013;81:136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- 2.Vlieghe P., Lisowski V., Martinez J., Khrestchatisky M. Synthetic therapeutic peptides: science and market. Drug Discov. Today. 2010;15:40–56. doi: 10.1016/j.drudis.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Sato A.K., Viswanathan M., Kent R.B., Wood C.R. Therapeutic peptides: technological advances driving peptides into development. Curr. Opin. Biotechnol. 2006;17:638–642. doi: 10.1016/j.copbio.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Yau W.M., Wimley W.C., Gawrisch K., White S.H. The preference of tryptophan for membrane interfaces. Biochemistry. 1998;37:14713–14718. doi: 10.1021/bi980809c. [DOI] [PubMed] [Google Scholar]
- 5.Harris F., Wallace J., Phoenix D.A. Use of hydrophobic moment plot methodology to aid the identification of oblique orientated α-helices. Mol. Membr. Biol. 2000;17:201–207. doi: 10.1080/09687680010018826. [DOI] [PubMed] [Google Scholar]
- 6.Joanne P., Nicolas P., El Amri C. Antimicrobial peptides and viral fusion peptides: how different they are? Protein Pept. Lett. 2009;16:743–750. doi: 10.2174/092986609788681814. [DOI] [PubMed] [Google Scholar]
- 7.Cruz J., Ortiz C., Guzman F., Fernandez-Lafuente R., Torres R. Antimicrobial peptides: promising compounds against pathogenic microorganisms. Curr. Med. Chem. 2014;21:2299–2321. doi: 10.2174/0929867321666140217110155. [DOI] [PubMed] [Google Scholar]
- 8.Falanga A., Cantisani M., Pedone C., Galdiero S. Membrane fusion and fission: enveloped viruses. Protein Pept. Lett. 2009;16:751–759. doi: 10.2174/092986609788681760. [DOI] [PubMed] [Google Scholar]
- 9.Wang F., Wang Y., Zhang X., Zhang W., Guo S., Jin F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J. Control. Release. 2014;174:126–136. doi: 10.1016/j.jconrel.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Harrison S.C. Mechanism of membrane fusion by viral envelope proteins. Adv. Virus Res. 2005;64:231–261. doi: 10.1016/S0065-3527(05)64007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison S.C. Viral membrane fusion. Nat. Struct. Mol. Biol. 2008;15:690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson I.A., Skehel J.J., Wiley D.C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981;289:366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- 13.Fass D., Harrison S.C., Kim P.S. Retrovirus envelope domain at 1.7 angstrom resolution. Nat. Struct. Biol. 1996;3:465–469. doi: 10.1038/nsb0596-465. [DOI] [PubMed] [Google Scholar]
- 14.Weissenhorn W., Carfi A., Lee K.H., Skehel J.J., Wiley D.C. Crystal structure of the Ebola virus membrane fusion subunit, GP2, from the envelope glycoprotein ectodomain. Mol. Cell. 1998;2:605–616. doi: 10.1016/s1097-2765(00)80159-8. [DOI] [PubMed] [Google Scholar]
- 15.Yin H.S., Wen X., Paterson R.G., Lamb R.A., Jardetzky T.S. Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation. Nature. 2006;439:38–44. doi: 10.1038/nature04322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y., Liu Y., Lou Z., Qin L., Li X., Bai Z., Pang H., Tien P., Gao G.F., Rao Z. Structural basis for coronavirus-mediated membrane fusion. Crystal structure of mouse hepatitis virus spike protein fusion core. J. Biol. Chem. 2004;279:30514–30522. doi: 10.1074/jbc.M403760200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rey F.A., Heinz F.X., Mandl C., Kunz C., Harrison S.C. The envelope glycoprotein from tick-borne encephalitis virus at 2 Å resolution. Nature. 1995;375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 18.Lescar J., Roussel A., Wien M.W., Navaza J., Fuller S.D., Wengler G., Rey F.A. The fusion glycoprotein shell of Semliki Forest virus: an icosahedral assembly primed for fusogenic activation at endosomal pH. Cell. 2001;105:137–148. doi: 10.1016/s0092-8674(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 19.Backovic M., Jardetzky T. Class III viral membrane fusion proteins. In: Dittmar T., Zänker K.S., editors. Cell Fusion in Health and Disease. Springer; Netherlands: 2011. pp. 91–101. [Google Scholar]
- 20.White S.H., Wimley W.C. Hydrophobic interactions of peptides with membrane interfaces. Biochim. Biophys. Acta Rev. Biomembr. 1998;1376:339–352. doi: 10.1016/s0304-4157(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 21.Wimley W.C., White S.H. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat. Struct. Biol. 1996;3:842–848. doi: 10.1038/nsb1096-842. [DOI] [PubMed] [Google Scholar]
- 22.Wimley W.C., Hristova K., Ladokhin A.S., Silvestro L., Axelsen P.H., White S.H. Folding of β-sheet membrane proteins: a hydrophobic hexapeptide model. J. Mol. Biol. 1998;277:1091–1110. doi: 10.1006/jmbi.1998.1640. [DOI] [PubMed] [Google Scholar]
- 23.Ladokhin A.S., White S.H. Folding of amphipathic α-helices on membranes: energetics of helix formation by melittin. J. Mol. Biol. 1999;285:1363–1369. doi: 10.1006/jmbi.1998.2346. [DOI] [PubMed] [Google Scholar]
- 24.Segrest J.P., De Loof H., Dohlman J.G., Brouillette C.G., Anantharamaiah G.M. Amphipathic helix motif: classes and properties. Proteins Struct. Funct. Bioinforma. 1990;8:103–117. doi: 10.1002/prot.340080202. [DOI] [PubMed] [Google Scholar]
- 25.Han X., Tamm L.K. pH-dependent self-association of influenza hemagglutinin fusion peptides in lipid bilayers. J. Mol. Biol. 2000;304:953–965. doi: 10.1006/jmbi.2000.4251. [DOI] [PubMed] [Google Scholar]
- 26.Sáez-Cirión A., Nieva J.L. Conformational transitions of membrane-bound HIV-1 fusion peptide. Biochim. Biophys. Acta Biomembr. 2002;1564:57–65. doi: 10.1016/s0005-2736(02)00400-5. [DOI] [PubMed] [Google Scholar]
- 27.Han X., Bushweller J.H., Cafiso D.S., Tamm L.K. Membrane structure and fusion-triggering conformational change of the fusion domain from influenza hemagglutinin. Nat. Struct. Biol. 2001;8:715–720. doi: 10.1038/90434. [DOI] [PubMed] [Google Scholar]
- 28.Tamm L.K., Han X., Li Y., Lai A.L. Structure and function of membrane fusion peptides. Biopolym. Pept. Sci. Sect. 2002;66:249–260. doi: 10.1002/bip.10261. [DOI] [PubMed] [Google Scholar]
- 29.Agopian A., Castano S. Structure and orientation study of Ebola fusion peptide inserted in lipid membrane models. Biochim. Biophys. Acta Biomembr. 2014;1838:117–126. doi: 10.1016/j.bbamem.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Apellaniz B., Huarte N., Largo E., Nieva J.L. The three lives of viral fusion peptides. Chem. Phys. Lipids. 2014;181:40–55. doi: 10.1016/j.chemphyslip.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai A.L., Moorthy A.E., Li Y., Tamm L.K. Fusion activity of HIV gp41 fusion domain is related to its secondary structure and depth of membrane insertion in a cholesterol-dependent fashion. J. Mol. Biol. 2012;418:3–15. doi: 10.1016/j.jmb.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsson P., Kasson P.M. Lipid tail protrusion in simulations predicts fusogenic activity of influenza fusion peptide mutants and conformational models. PLoS Comput. Biol. 2013;9:e1002950. doi: 10.1371/journal.pcbi.1002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozlov M.M., McMahon H.T., Chernomordik L.V. Protein-driven membrane stresses in fusion and fission. Trends Biochem. Sci. 2010;35:699–706. doi: 10.1016/j.tibs.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peisajovich S.G., Samuel O., Shai Y. Paramyxovirus F1 protein has two fusion peptides: implications for the mechanism of membrane fusion. J. Mol. Biol. 2000;296:1353–1365. doi: 10.1006/jmbi.2000.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samuel O., Shai Y. Participation of two fusion peptides in measles virus-induced membrane fusion: emerging similarity with other paramyxoviruses. Biochemistry. 2001;40:1340–1349. doi: 10.1021/bi001533n. [DOI] [PubMed] [Google Scholar]
- 36.Perez-Berna A.J., Guillen J., Moreno M.R., Bernabeu A., Pabst G., Laggner P., Villalain J. Identification of the membrane-active regions of hepatitis C virus p7 protein: biophysical characterization of the loop region. J. Biol. Chem. 2008;283:8089–8101. doi: 10.1074/jbc.M709413200. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Berna A.J., Moreno M.R., Guillen J., Bernabeu A., Villalain J. The membrane-active regions of the hepatitis C virus E1 and E2 envelope glycoproteins. Biochemistry. 2006;45:3755–3768. doi: 10.1021/bi0523963. [DOI] [PubMed] [Google Scholar]
- 38.Nemesio H., Palomares-Jerez F., Villalain J. The membrane-active regions of the dengue virus proteins C and E. Biochim. Biophys. Acta. 2011;1808:2390–2402. doi: 10.1016/j.bbamem.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Nemesio H., Palomares-Jerez M.F., Villalain J. Hydrophobic segment of dengue virus C protein. Interaction with model membranes. Mol. Membr. Biol. 2013;30:273–287. doi: 10.3109/09687688.2013.805835. [DOI] [PubMed] [Google Scholar]
- 40.Galdiero S., Falanga A., Vitiello M., Browne H., Pedone C., Galdiero M. Fusogenic domains in herpes simplex virus type 1 glycoprotein H. J. Biol. Chem. 2005;280:28632–28643. doi: 10.1074/jbc.M505196200. [DOI] [PubMed] [Google Scholar]
- 41.Galdiero S., Falanga A., Vitiello M., D'Isanto M., Collins C., Orrei V., Browne H., Pedone C., Galdiero M. Evidence for a role of the membrane-proximal region of herpes simplex virus type 1 glycoprotein H in membrane fusion and virus inhibition. Chembiochem. 2007;8:885–895. doi: 10.1002/cbic.200700044. [DOI] [PubMed] [Google Scholar]
- 42.Galdiero S., Vitiello M., D'Isanto M., Falanga A., Cantisani M., Browne H., Pedone C., Galdiero M. The identification and characterization of fusogenic domains in herpes virus glycoprotein B molecules. Chembiochem. 2008;9:758–767. doi: 10.1002/cbic.200700457. [DOI] [PubMed] [Google Scholar]
- 43.Lorizate M., Huarte N., Saez-Cirion A., Nieva J.L. Interfacial pre-transmembrane domains in viral proteins promoting membrane fusion and fission. Biochim. Biophys. Acta. 2008;1778:1624–1639. doi: 10.1016/j.bbamem.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peisajovich S.G., Shai Y. Viral fusion proteins: multiple regions contribute to membrane fusion. Biochim. Biophys. Acta. 2003;1614:122–129. doi: 10.1016/s0005-2736(03)00170-6. [DOI] [PubMed] [Google Scholar]
- 45.D'Errico G., D'Ursi A.M., Marsh D. Interaction of a peptide derived from glycoprotein gp36 of feline immunodeficiency virus and its lipoylated analogue with phospholipid membranes. Biochemistry. 2008;47:5317–5327. doi: 10.1021/bi7025062. [DOI] [PubMed] [Google Scholar]
- 46.Badani H., Garry R.F., Wimley W.C. Peptide entry inhibitors of enveloped viruses: The importance of interfacial hydrophobicity. Biochim. Biophys. Acta Biomembr. 2014;1838:2180–2197. doi: 10.1016/j.bbamem.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rapaport D., Ovadia M., Shai Y. A synthetic peptide corresponding to a conserved heptad repeat domain is a potent inhibitor of Sendai virus-cell fusion: an emerging similarity with functional domains of other viruses. EMBO J. 1995;14:5524–5531. doi: 10.1002/j.1460-2075.1995.tb00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galdiero S., Falanga A., Vitiello M., D'Isanto M., Cantisani M., Kampanaraki A., Benedetti E., Browne H., Galdiero M. Peptides containing membrane-interacting motifs inhibit herpes simplex virus type 1 infectivity. Peptides. 2008;29:1461–1471. doi: 10.1016/j.peptides.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galdiero S., Falanga A., Vitiello M., Raiola L., Fattorusso R., Browne H., Pedone C., Isernia C., Galdiero M. Analysis of a membrane interacting region of herpes simplex virus type 1 glycoprotein H. J. Biol. Chem. 2008;283:29993–30009. doi: 10.1074/jbc.M803092200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galdiero S., Falanga A., Vitiello M., Raiola L., Russo L., Pedone C., Isernia C., Galdiero M. The presence of a single N-terminal histidine residue enhances the fusogenic properties of a membranotropic peptide derived from herpes simplex virus type 1 glycoprotein H. J. Biol. Chem. 2010;285:17123–17136. doi: 10.1074/jbc.M110.114819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Y., Rahman N.A., Othman R.B., Hu P., Huang M. Computational identification of self-inhibitory peptides from envelope proteins. Proteins. 2012;80:2154–2168. doi: 10.1002/prot.24105. [DOI] [PubMed] [Google Scholar]
- 52.Chang D.K., Cheng S.F., Lin C.H., Kantchev E.B., Wu C.W. Self-association of glutamic acid-rich fusion peptide analogs of influenza hemagglutinin in the membrane-mimic environments: effects of positional difference of glutamic acids on side chain ionization constant and intra- and inter-peptide interactions deduced from NMR and gel electrophoresis measurements. Biochim. Biophys. Acta. 2005;1712:37–51. doi: 10.1016/j.bbamem.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Lau W.L., Ege D.S., Lear J.D., Hammer D.A., DeGrado W.F. Oligomerization of fusogenic peptides promotes membrane fusion by enhancing membrane destabilization. Biophys. J. 2004;86:272–284. doi: 10.1016/S0006-3495(04)74103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kliger Y., Aharoni A., Rapaport D., Jones P., Blumenthal R., Shai Y. Fusion peptides derived from the HIV type 1 glycoprotein 41 associate within phospholipid membranes and inhibit cell–cell fusion. Structure–function study. J. Biol. Chem. 1997;272:13496–13505. doi: 10.1074/jbc.272.21.13496. [DOI] [PubMed] [Google Scholar]
- 55.Pritsker M., Jones P., Blumenthal R., Shai Y. A synthetic all D-amino acid peptide corresponding to the N-terminal sequence of HIV-1 gp41 recognizes the wild-type fusion peptide in the membrane and inhibits HIV-1 envelope glycoprotein-mediated cell fusion. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7287–7292. doi: 10.1073/pnas.95.13.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y., Han X., Tamm L.K. Thermodynamics of fusion peptide–membrane interactions. Biochemistry. 2003;42:7245–7251. doi: 10.1021/bi0341760. [DOI] [PubMed] [Google Scholar]
- 57.Forssmann W.G., Y.H. The, Stoll M., Adermann K., Albrecht U., Tillmann H.C., Barlos K., Busmann A., Canales-Mayordomo A., Gimenez-Gallego G., Hirsch J., Jimenez-Barbero J., Meyer-Olson D., Munch J., Perez-Castells J., Standker L., Kirchhoff F., Schmidt R.E. Short-term monotherapy in HIV-infected patients with a virus entry inhibitor against the gp41 fusion peptide. Sci. Transl. Med. 2010;2:63re63. doi: 10.1126/scitranslmed.3001697. [DOI] [PubMed] [Google Scholar]
- 58.Münch J., Ständker L., Adermann K., Schulz A., Schindler M., Chinnadurai R., Pöhlmann S., Chaipan C., Biet T., Peters T., Meyer B., Wilhelm D., Lu H., Jing W., Jiang S., Forssmann W.G., Kirchhoff F. Discovery and optimization of a natural HIV-1 entry inhibitor targeting the gp41 fusion peptide. Cell. 2007;129:263–275. doi: 10.1016/j.cell.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 59.Torchilin V.P. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2006;58:1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 60.Bareford L.M., Swaan P.W. Endocytic mechanisms for targeted drug delivery. Adv. Drug Deliv. Rev. 2007;59:748–758. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He J., Kauffman W.B., Fuselier T., Naveen S.K., Voss T.G., Hristova K., Wimley W.C. Direct cytosolic delivery of polar cargo to cells by spontaneous membrane-translocating peptides. J. Biol. Chem. 2013;288:29974–29986. doi: 10.1074/jbc.M113.488312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guarnieri D., Falanga A., Muscetti O., Tarallo R., Fusco S., Galdiero M., Galdiero S., Netti P.A. Shuttle-mediated nanoparticle delivery to the blood–brain barrier. Small. 2013;9:853–862. doi: 10.1002/smll.201201870. [DOI] [PubMed] [Google Scholar]
- 63.Galdiero S., Falanga A., Vitiello M., Grieco P., Caraglia M., Morelli G., Galdiero M. Exploitation of viral properties for intracellular delivery. J. Pept. Sci. 2014;20:468–478. doi: 10.1002/psc.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Almeida P.F. Membrane-active peptides: binding, translocation, and flux in lipid vesicles. Biochim. Biophys. Acta. 2014;1838:2216–2227. doi: 10.1016/j.bbamem.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heitz F., Morris M.C., Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br. J. Pharmacol. 2009;157:195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vivès E., Brodin P., Lebleu B. Truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane. J. Biol. Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 67.Angeles-Boza A.M., Erazo-Oliveras A., Lee Y.-J., Pellois J.-P. Generation of endosomolytic reagents by branching of cell-penetrating peptides. Bioconjug. Chem. 2010;21:2164–2167. doi: 10.1021/bc100130r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galdiero S., Vitiello M., Falanga A., Cantisani M., Incoronato N., Galdiero M. Intracellular delivery: exploiting viral membranotropic peptides. Curr. Drug Metab. 2012;13:93–104. doi: 10.2174/138920012798356961. [DOI] [PubMed] [Google Scholar]
- 69.Morris M.C., Chaloin L., Mery J., Heitz F., Divita G. A novel potent strategy for gene delivery using a single peptide vector as a carrier. Nucleic Acids Res. 1999;27:3510–3517. doi: 10.1093/nar/27.17.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morris M.C., Vidal P., Chaloin L., Heitz F., Divita G. A new peptide vector for efficient delivery of oligonucleotides into mammalian cells. Nucleic Acids Res. 1997;25:2730–2736. doi: 10.1093/nar/25.14.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gallaher W.R. Detection of a fusion peptide sequence in the transmembrane protein of human immunodeficiency virus. Cell. 1987;50:327–328. doi: 10.1016/0092-8674(87)90485-5. [DOI] [PubMed] [Google Scholar]
- 72.Kalderon D., Smith A.E. In vitro mutagenesis of a putative DNA binding domain of SV40 large-T. Virology. 1984;139:109–137. doi: 10.1016/0042-6822(84)90334-9. [DOI] [PubMed] [Google Scholar]
- 73.Delboy M.G., Patterson J.L., Hollander A.M., Nicola A.V. Nectin-2-mediated entry of a syncytial strain of herpes simplex virus via pH-independent fusion with the plasma membrane of Chinese hamster ovary cells. Virol. J. 2006;3:105. doi: 10.1186/1743-422X-3-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roller D.G., Dollery S.J., Doyle J.L., Nicola A.V. Structure–function analysis of herpes simplex virus glycoprotein B with fusion-from-without activity. Virology. 2008;382:207–216. doi: 10.1016/j.virol.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 75.Arii J., Uema M., Morimoto T., Sagara H., Akashi H., Ono E., Arase H., Kawaguchi Y. Entry of herpes simplex virus 1 and other alphaherpesviruses via the paired immunoglobulin-like type 2 receptor alpha. J. Virol. 2009;83:4520–4527. doi: 10.1128/JVI.02601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milne R.S., Nicola A.V., Whitbeck J.C., Eisenberg R.J., Cohen G.H. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J. Virol. 2005;79:6655–6663. doi: 10.1128/JVI.79.11.6655-6663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Connolly S.A., Jackson J.O., Jardetzky T.S., Longnecker R. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat. Rev. Microbiol. 2011;9:369–381. doi: 10.1038/nrmicro2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turner A., Bruun B., Minson T., Browne H. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 1998;72:873–875. doi: 10.1128/jvi.72.1.873-875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farnsworth A., Wisner T.W., Webb M., Roller R., Cohen G., Eisenberg R., Johnson D.C. Herpes simplex virus glycoproteins gB and gH function in fusion between the virion envelope and the outer nuclear membrane. Proc. Natl. Acad. Sci. U. S. A. 2007;104:10187–10192. doi: 10.1073/pnas.0703790104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Falanga A., Tarallo R., Vitiello G., Vitiello M., Perillo E., Cantisani M., D'Errico G., Galdiero M., Galdiero S. Biophysical characterization and membrane interaction of the two fusion loops of glycoprotein B from herpes simplex type I virus. PLoS One. 2012;7:e32186. doi: 10.1371/journal.pone.0032186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galdiero S., Falanga A., Vitiello G., Vitiello M., Pedone C., D'Errico G., Galdiero M. Role of membranotropic sequences from herpes simplex virus type I glycoproteins B and H in the fusion process. Biochim. Biophys. Acta. 2010;1798:579–591. doi: 10.1016/j.bbamem.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 82.Galdiero S., Russo L., Falanga A., Cantisani M., Vitiello M., Fattorusso R., Malgieri G., Galdiero M., Isernia C. Structure and orientation of the gH625-644 membrane interacting region of herpes simplex virus type 1 in a membrane mimetic system. Biochemistry. 2012;51:3121–3128. doi: 10.1021/bi201589m. [DOI] [PubMed] [Google Scholar]
- 83.Chowdary T.K., Cairns T.M., Atanasiu D., Cohen G.H., Eisenberg R.J., Heldwein E.E. Crystal structure of the conserved herpesvirus fusion regulator complex gH–gL. Nat. Struct. Mol. Biol. 2010;17:882–888. doi: 10.1038/nsmb.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsuura H., Kirschner A.N., Longnecker R., Jardetzky T.S. Crystal structure of the Epstein–Barr virus (EBV) glycoprotein H/glycoprotein L (gH/gL) complex. Proc. Natl. Acad. Sci. U. S. A. 2010;107:22641–22646. doi: 10.1073/pnas.1011806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heldwein E.E., Lou H., Bender F.C., Cohen G.H., Eisenberg R.J., Harrison S.C. Crystal structure of glycoprotein B from herpes simplex virus 1. Science. 2006;313:217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 86.Connolly S.A., Longnecker R. Residues within the C-terminal arm of the herpes simplex virus 1 glycoprotein B ectodomain contribute to its refolding during the fusion step of virus entry. J. Virol. 2012;86:6386–6393. doi: 10.1128/JVI.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cantisani M., Falanga A., Incoronato N., Russo L., De Simone A., Morelli G., Berisio R., Galdiero M., Galdiero S. Conformational modifications of gB from herpes simplex virus type 1 analyzed by synthetic peptides. J. Med. Chem. 2013;56:8366–8376. doi: 10.1021/jm400771k. [DOI] [PubMed] [Google Scholar]
- 88.Backovic M., Longnecker R., Jardetzky T.S. Structure of a trimeric variant of the Epstein–Barr virus glycoprotein B. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2880–2885. doi: 10.1073/pnas.0810530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roche S., Bressanelli S., Rey F.A., Gaudin Y. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein G. Science. 2006;313:187–191. doi: 10.1126/science.1127683. [DOI] [PubMed] [Google Scholar]
- 90.Kadlec J., Loureiro S., Abrescia N.G., Stuart D.I., Jones I.M. The postfusion structure of baculovirus gp64 supports a unified view of viral fusion machines. Nat. Struct. Mol. Biol. 2008;15:1024–1030. doi: 10.1038/nsmb.1484. [DOI] [PubMed] [Google Scholar]
- 91.Galdiero S., Vitiello M., D'Isanto M., Falanga A., Collins C., Raieta K., Pedone C., Browne H., Galdiero M. Analysis of synthetic peptides from heptad-repeat domains of herpes simplex virus type 1 glycoproteins H and B. J. Gen. Virol. 2006;87:1085–1097. doi: 10.1099/vir.0.81794-0. [DOI] [PubMed] [Google Scholar]
- 92.Akkarawongsa R., Pocaro N.E., Case G., Kolb A.W., Brandt C.R. Multiple peptides homologous to herpes simplex virus type 1 glycoprotein B inhibit viral infection. Antimicrob. Agents Chemother. 2009;53:987–996. doi: 10.1128/AAC.00793-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Galdiero S., Falanga A., Tarallo R., Russo L., Galdiero E., Cantisani M., Morelli G., Galdiero M. Peptide inhibitors against herpes simplex virus infections. J. Pept. Sci. 2013;19:148–158. doi: 10.1002/psc.2489. [DOI] [PubMed] [Google Scholar]
- 94.Vitiello G., Falanga A., Galdiero M., Marsh D., Galdiero S., D'Errico G. Lipid composition modulates the interaction of peptides deriving from herpes simplex virus type I glycoproteins B and H with biomembranes. Biochim. Biophys. Acta. 2011;1808:2517–2526. doi: 10.1016/j.bbamem.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 95.Krishnan A., Verma S.K., Mani P., Gupta R., Kundu S., Sarkar D.P. A histidine switch in hemagglutinin-neuraminidase triggers paramyxovirus-cell membrane fusion. J. Virol. 2009;83:1727–1741. doi: 10.1128/JVI.02026-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chanel-Vos C., Kielian M. A conserved histidine in the ij loop of the Semliki Forest virus E1 protein plays an important role in membrane fusion. J. Virol. 2004;78:13543–13552. doi: 10.1128/JVI.78.24.13543-13552.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tarallo R., Carberry T.P., Falanga A., Vitiello M., Galdiero S., Galdiero M., Weck M. Dendrimers functionalized with membrane-interacting peptides for viral inhibition. Int. J. Nanomedicine. 2013;8:521–534. doi: 10.2147/IJN.S37739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Falanga A., Vitiello M.T., Cantisani M., Tarallo R., Guarnieri D., Mignogna E., Netti P., Pedone C., Galdiero M., Galdiero S. A peptide derived from herpes simplex virus type 1 glycoprotein H: membrane translocation and applications to the delivery of quantum dots. Nanomedicine. 2011;7:925–934. doi: 10.1016/j.nano.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 99.Tu Y., Kim J.S. A fusogenic segment of glycoprotein H from herpes simplex virus enhances transfection efficiency of cationic liposomes. J. Gene Med. 2008;10:646–654. doi: 10.1002/jgm.1184. [DOI] [PubMed] [Google Scholar]
- 100.Tarallo R., Accardo A., Falanga A., Guarnieri D., Vitiello G., Netti P., D'Errico G., Morelli G., Galdiero S. Clickable functionalization of liposomes with the gH625 peptide from Herpes simplex virus type I for intracellular drug delivery. Chemistry. 2011;17:12659–12668. doi: 10.1002/chem.201101425. [DOI] [PubMed] [Google Scholar]
- 101.Carberry T.P., Tarallo R., Falanga A., Finamore E., Galdiero M., Weck M., Galdiero S. Dendrimer functionalization with a membrane-interacting domain of herpes simplex virus type 1: towards intracellular delivery. Chemistry. 2012;18:13678–13685. doi: 10.1002/chem.201202358. [DOI] [PubMed] [Google Scholar]
- 102.Smaldone G., Falanga A., Capasso D., Guarnieri D., Correale S., Galdiero M., Netti P.A., Zollo M., Galdiero S., Di Gaetano S., Pedone E. gH625 is a viral derived peptide for effective delivery of intrinsically disordered proteins. Int. J. Nanomedicine. 2013;8:2555–2565. doi: 10.2147/IJN.S44186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pinaud F., Clarke S., Sittner A., Dahan M. Probing cellular events, one quantum dot at a time. Nat. Methods. 2010;7:275–285. doi: 10.1038/nmeth.1444. [DOI] [PubMed] [Google Scholar]
- 104.Medintz I.L., Pons T., Delehanty J.B., Susumu K., Brunel F.M., Dawson P.E., Mattoussi H. Intracellular delivery of quantum dot-protein cargos mediated by cell penetrating peptides. Bioconjug. Chem. 2008;19:1785–1795. doi: 10.1021/bc800089r. [DOI] [PubMed] [Google Scholar]
- 105.Lee H., Kim I.K., Park T.G. Intracellular trafficking and unpacking of siRNA/quantum dot-PEI complexes modified with and without cell penetrating peptide: confocal and flow cytometric FRET analysis. Bioconjug. Chem. 2010;21:289–295. doi: 10.1021/bc900342p. [DOI] [PubMed] [Google Scholar]
- 106.Delehanty J.B., Bradburne C.E., Susumu K., Boeneman K., Mei B.C., Farrell D., Blanco-Canosa J.B., Dawson P.E., Mattoussi H., Medintz I.L. Spatiotemporal multicolor labeling of individual cells using peptide-functionalized quantum dots and mixed delivery techniques. J. Am. Chem. Soc. 2011;133:10482–10489. doi: 10.1021/ja200555z. [DOI] [PubMed] [Google Scholar]
- 107.Cukierman E., Khan D.R. The benefits and challenges associated with the use of drug delivery systems in cancer therapy. Biochem. Pharmacol. 2010;80:762–770. doi: 10.1016/j.bcp.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Newkome G.R., Moorefield C.N., Vögtle F. Wiley-VCH; Weinheim: 2001. Dendrimers and Dendrons: Concepts, Synthesis, Applications. [Google Scholar]
- 109.Lee C.C., MacKay J.A., Fréchet J.M.J., Szoka F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 110.Gillies E.R., Frechet J.M. Dendrimers and dendritic polymers in drug delivery. Drug Discov. Today. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 111.Gunaseelan S., Gunaseelan K., Deshmukh M., Zhang X., Sinko P.J. Surface modifications of nanocarriers for effective intracellular delivery of anti-HIV drugs. Adv. Drug Deliv. Rev. 2010;62:518–531. doi: 10.1016/j.addr.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Najlah M., D'Emanuele A. Crossing cellular barriers using dendrimer nanotechnologies. Curr. Opin. Pharmacol. 2006;6:522–527. doi: 10.1016/j.coph.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 113.Seib F.P., Jones A.T., Duncan R. Comparison of the endocytic properties of linear and branched PEIs, and cationic PAMAM dendrimers in B16f10 melanoma cells. J. Control. Release. 2007;117:291–300. doi: 10.1016/j.jconrel.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 114.Kang H., DeLong R., Fisher M.H., Juliano R.L. Tat-conjugated PAMAM dendrimers as delivery agents for antisense and siRNA oligonucleotides. Pharm. Res. 2005;22:2099–2106. doi: 10.1007/s11095-005-8330-5. [DOI] [PubMed] [Google Scholar]
- 115.Lu C.T., Zhao Y.Z., Wong H.L., Cai J., Peng L., Tian X.Q. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int. J. Nanomedicine. 2014;9:2241–2257. doi: 10.2147/IJN.S61288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abbott N.J., Ronnback L., Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 117.Mahajan S.D., Law W.C., Aalinkeel R., Reynolds J., Nair B.B., Yong K.T., Roy I., Prasad P.N., Schwartz S.A. Nanoparticle-mediated targeted delivery of antiretrovirals to the brain. Methods Enzymol. 2012;509:41–60. doi: 10.1016/B978-0-12-391858-1.00003-4. [DOI] [PubMed] [Google Scholar]
- 118.Jain K.K. Nanobiotechnology-based strategies for crossing the blood–brain barrier. Nanomedicine (Lond.) 2012;7:1225–1233. doi: 10.2217/nnm.12.86. [DOI] [PubMed] [Google Scholar]
- 119.Orive G., Ali O.A., Anitua E., Pedraz J.L., Emerich D.F. Biomaterial-based technologies for brain anti-cancer therapeutics and imaging. Biochim. Biophys. Acta. 2010;1806:96–107. doi: 10.1016/j.bbcan.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 120.Qiao R., Jia Q., Huwel S., Xia R., Liu T., Gao F., Galla H.J., Gao M. Receptor-mediated delivery of magnetic nanoparticles across the blood–brain barrier. ACS Nano. 2012;6:3304–3310. doi: 10.1021/nn300240p. [DOI] [PubMed] [Google Scholar]
- 121.Falanga A., Tarallo R., Galdiero E., Cantisani M., Galdiero M., Galdiero S. Review of a viral peptide nanosystem for intracellular delivery. J. Nanophotonics. 2013;7 (071599-071599) [Google Scholar]
- 122.Valiante S., Falanga A., Cigliano L., Iachetta G., Busiello R.A., La Marca V., Galdiero M., Lombardi A., Galdiero S. 2014. The peptide gH625 enters into neuron and astrocyte cell lines and crosses the blood brain barrier in rats. (submitted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fawell S., Seery J., Daikh Y., Moore C., Chen L.L., Pepinsky B., Barsoum J. Tat-mediated delivery of heterologous proteins into cells. Proc. Natl. Acad. Sci. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schutze-Redelmeier M.P., Gournier H., Garcia-Pons F., Moussa M., Joliot A.H., Volovitch M., Prochiantz A., Lemonnier F.A. Introduction of exogenous antigens into the MHC class I processing and presentation pathway by Drosophila antennapedia homeodomain primes cytotoxic T cells in vivo. J. Immunol. 1996;157:650–655. [PubMed] [Google Scholar]
- 125.Borlongan C.V., Emerich D.F. Facilitation of drug entry into the CNS via transient permeation of blood brain barrier: laboratory and preliminary clinical evidence from bradykinin receptor agonist, Cereport. Brain Res. Bull. 2003;60:297–306. doi: 10.1016/s0361-9230(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 126.Patel M.M., Goyal B.R., Bhadada S.V., Bhatt J.S., Amin A.F. Getting into the brain: approaches to enhance brain drug delivery. CNS Drugs. 2009;23:35–58. doi: 10.2165/0023210-200923010-00003. [DOI] [PubMed] [Google Scholar]