Several different infectious agents may cause acute undifferentiated diarrhea in calves. They include enteropathogenic strains of Eschenchia colt, rotavirus, coronavirus, and Cryptosporidia, and perhaps other infectious agents. There is a reasonably reliable relationship between the age of the calf at onset of diarrhea and the etiologic agent. E. coli, which possesses the K99~antigen, causes diarrhea in calves usually under three days of age and only rarely over five days of age. Rotavirus and coronavirus cause diarrhea at from seven days to several weeks of age. Cryptosporidia causes diarrhea at five to seven days and up to three to five weeks of age. Mixed infections with a combination of all of these pathogens are also common. The predominant clinical sign in all of these infections is diarrhea; therefore, they are difficult to diagnose and differentiate from each other. Because calf diarrhea can be caused by more than one pathogen at the same time, it is difficult to control reliably using any specific technique. Because improper management is one of the most important causes of herd problems, the successful control of the disease will depend on management of the beef herd throughout the winter and especially at calving time.
In western Canada, the management cycle of most beef herds follows a typical pattern. Cows with their spring calves are dispersed onto summer pasture or range areas in April and May for breeding and remain there until calves are weaned, usually in October or November. Pregnant cows are fed winter roughage, usually mixed hay or straw plus some grain, or silage, for a variable length of time from November to May, depending on the amount of snowfall and the availability of feed. Calving begins as early as January in some herds and may continue into May and June; however, peak calving rates occur in March and April.
Many herd problems with calf diarrhea are initiated because cows are crowded or confined during the winter feeding or calving intervals. As the degree or duration of confinement in the winter feeding area increases, the risk of transmission of enteropathogens from carrier cows to other cows and heifers in the herd also increases. Hence, a large portion of cows may be shedding enteropathogens in their feces or have contaminated udders and underbellies when they are moved to the calving area. When this occurs, calving grounds that were relatively “clean” can become heavily contaminated very quickly. A similar pattern of events may occur even when cows are not confined during the winter period but are crowded into a relatively small area for calving. Even worse, winter feeding and calving may be done in the same area.
Newborn calves that are confined to the calving area further increase the population density, which not only increases the transmission of infectious agents but also increases stress and reduces transfer of passive immunity. Calves born into an environment that is more highly contaminated may become infected during or shortly after birth and remain clinically normal but shed enteropathogens, thereby contributing further to environmental contamination. Diarrheic calves become a primary source of infection not only for other calves but also for adult animals and the environment. Hence, management of the beef herd throughout the winter and spring should be aimed at breaking the cycle of transmission of enteropathogens from carrier animals to the environment and eventually to newborn calves. This can be done by increasing resistance and decreasing the risk of infection of both cows and newborn calves.
The management techniques used for prevention and control of epidemics of diarrhea are similar. However, preventive management provides many more options because it involves integrating a variety of procedures into the herd management program throughout the year. In contrast, when faced with trying to control an epidemic that is already underway, producers are restricted to using far fewer management procedures. They must deal with an immediate problem and therefore can use only those procedures that can be adapted and applied quickly. Alteration of management during spring calving is made even more difficult because resources such as labor, feed, and bedding are limited, or their application is restricted by inclement weather or shortage of facilities.
Proper management implies several things. It implies the ability to recognize a potentially dangerous situation and to adjust management procedures to remove the potential hazards. For example, a heavy snowfall creates a potentially dangerous situation for several reasons. Herds are often confined, causing a build-up of contamination in the wintering area and on the cows. When the snow starts to melt, usually after some calves are already on the ground, the ground surface becomes wet and muddy. This places a stress on the calves and helps to spread infection. Under these conditions a potential exists for severe outbreaks. Steps should be taken before calving starts to decrease this potential. Cows should not be wintered and calved on the same ground. The proposed calving area should be kept relatively free of snow and the herd should be moved onto this area shortly before the onset of calving.
Proper management also implies the ability to recognize and correct those conditions that are causing a problem. No one can predict and avoid all of the potential dangers that will arise, and epidemics will still occur in spite of apparent good management in some herds. .However, the duration and severity of many outbreaks can be limited by altering or modifying management programs as soon as problems arise. For example, a farmer may have calved his herd of 100 cows in a 12-acre field for the past two years and not had any diarrheic calves. However, this year he has increased the number of calving cows to 120 and early spring storms have made the ground surface much wetter than it was during the past two years. About 50 per cent of the calves have diarrhea. He should recognize that the transmission distance between animals is reduced since there are fewer square feet per cow-calf pair this year because more cows are calving within the same total area. Also, if the ground surface is wet, the level of contamination with infectious agents is likely to be higher. He should allow each cow-calf pair more space, perhaps by dividing the herd into two groups of 60 or by moving to a larger area. He should also supply more clean, dry bedding than in previous years.
Prevention of Epidemics of Neonatal Calf Diarrhea
It is not possible to outline one management system that is suitable for all herds under all circumstances. There are at least five basic management principles for the prevention and control of calf diarrhea, each of which can be adapted in individual herds to a greater or lesser degree. An epidemiologic survey of the disease in beef herds in western Canada from 1973 to 1976 provided most of the data used to illustrate these principles. The five principles are as follows: (1) Remove the source of the infection from the calf’s environment. (2) Remove the calf from the contaminated environment. (3) Increase the nonspecific resistance of the calf. (4) Increase the specific immunity of the calf. (5) Reduce stress.
Remove the Source of the Infection from the Calf’s Environment
It is not possible to completely remove the infectious agents that cause diarrhea from the calf’s environment. Some enteropathogens are carried by the cow and are transmitted to the calf during or shortly after calving. It is also likely that some bacterial and viral pathogens can survive for long periods in manure, contaminated bedding, and perhaps soil. Excess surface water, which is often a problem in calving grounds during the spring thaw, may be another source of contamination. Calving inside during the winter and spring can be particularly dangerous because contamination builds up quickly in poorly ventilated, damp areas that are not exposed to sunlight. After treating or handling diarrheic calves, humans also become a primary source of contamination.
Prevention
The major objective is to keep the level of environmental contamination low so that the calf’s natural defense mechanisms are not overwhelmed, particularly before it ingests colostrum. The following recommendations will assist in reducing and controlling the level of contamination:
-
1.
Avoid confining the herd as much as possible during the winter feeding period. Rotate feeding and bedding areas so that cows and heifers are not forced to remain in a contaminated environment. This will help to reduce the number of infected “carrier” cows, which shed enteropathogens in their manure. Udders and underbellies will also be less contaminated, thus reducing the risk that cows and heifers will be a primary source of contamination when they are moved into the calving area.
-
2.
Do not calve cows and heifers in the same location in which they were held during the winter months.
-
3.
Move cows and heifers into the calving area one to two weeks prior to the onset of calving. This will prevent the excessive accumulation of manure and contaminated bedding in the calving area.
-
4.
Do not allow cows and heifers to become “overconfined” during calving. The herd, particularly the heifers, must be observed during calving. However, they should not be restricted to small areas, particularly muddy corrals or small paddocks. Even animals calved on large areas can become confined by excessive snow or by restricting the placement of feed or bedding to the same area.
-
5.
Allow animals plenty of space at calving. If the ground surface is muddy or wet, allow more square feet per cow-calf pair. If the calving herd cannot be turned out because of excessive snow, plow, or bulldozed strips on sidehills and hilltops so they have room to disperse. This technique was used successfully to control outbreaks in several herds when record high snowfalls occurred in western Canada in 1974. Change the location of feeding and bedding grounds every few days to encourage the herd to migrate within the calving area.
-
6.
Do not calve in the same location year after year, particularly if diarrhea has been a problem in that area previously. Attempt to rotate calving grounds from year to year.
-
7.
Locate calving grounds to take advantage of natural shelter and where the type of soil and the contour of the land allow natural drainage away of surface water.
-
8.
Calving areas should be cleaned up and left vacant through the hot, dry summer months. All manure and excess bedding should be removed so fresh, underlying soil is exposed.
Control
Once an outbreak has begun, it is very difficult to remove the source of contamination from the calving area. Even if diarrheic calves are isolated, infectious agents may survive for a period of weeks or months in contaminated bams or sheds, in bedding, soil, and ground surface water. Livestockmen who handle and treat sick calves also become a common and potent source of transfer of enteropathogens among calves. If movement away from a contaminated calving area is not possible, provide as much clean, dry bedding as possible and attempt to disperse animals by spreading out bedding and loafing areas. If possible, the person treating sick calves should not have any direct contact with newborn healthy calves.
Remove Calves from the Contaminated Environment
Calves born on open range do not develop severe diarrhea as commonly as calves born and raised in confinement. This is true because as the number of square feet available per cow-calf pair in the calving area decreases, several things happen:
-
1.
The calving area becomes more contaminated.
-
2.
The transmission distance between animals decreases, thereby increasing the rate of passage of infectious agents from animal to animal.
-
3.
The transfer of passive immunity from cow to calf may be impaired.10
-
4.
Stress may increase.
Therefore, the potential for an outbreak of calf scours is higher when the entire pregnant herd is allowed to calve out in the same area and when the calves are allowed to accumulate in the same location until all the calves are born.
Prevention
Avoid Calving in Areas in Which the Level of Contamination with Infectious Agents is Likely to he High. These areas include the following:
-
1.
Bams and sheds: The level of contamination builds up rapidly during the winter because effective cleaning and disinfection are difficult. In addition, ventilation and sunlight are restricted so that infectious agents survive for long periods. Therefore, avoid using barns or sheds that have been used to house or treat affected calves.
-
2.
Corrals and paddocks in which there is an excess of mud and manure or in which the animal population density is high.
-
3.
Local areas within the calving grounds such as restricted feeding, watering, and bedding areas and low-lying areas with poor drainage.
-
4.
Areas that have been contaminated by diarrheic calves.
Attempt to calve in a clean, dry area where animals are not restricted in their movements. If calving must be done in a confined area, try to remove the cow and calf to a new area as soon after birth as possible as described below.
Prevent Crowding in the Calving Area
A management system based on the dual principles of dividing the calving herd into smaller subgroups and dispersing newborn calves soon after birth will help prevent crowding and also increase the ease of providing surveillance and assistance to those cows that need attention. The system has been used successfully for calving heifers on a large commercial ranch that calves approximately 1000 two- year-old heifers annually.5
The principles of two such systems that have been used in western Canada are outlined in Figure 1. The basic design was described by Dr. John Bradley* and used at the Canada Department of Agriculture, Lacombe Research Station, and similar systems are now used by many cow-calf producers.
Figure 1.
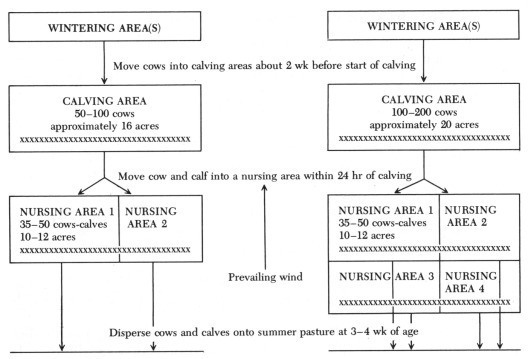
Lacombe-type management system: left, for groups of 50 to 100 cows; right, for groups of 100 to 200 cows. Natural windbreak or fencing of 20 per cent porosity is represented by xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx.
In the Lacombe system, the main herd of several hundred cows is divided into smaller subgroups that are calved in separate areas. In addition, the cow and the calf are moved out of the main calving area into a nursery area within one day of birth of each calf. This system helps to overcome the problems of crowding, mismothering, failure of calves to suck colostrum early enough, and calf diarrhea. By moving the cow-calf pairs out of the main calving area as the calves are born, the job of observing the remaining calving cows is also made much easier.
The following recommendations are suggested for use of the divided calving areas:
-
1.
The calving area is sheltered by trees or windbreak fence (8 ft high, 20 per cent porosity). A shelter and handling facility may be included for difficult calvings. Nursery pastures each contain two calf shelters (calf-huts) with windbreak fence at each end. The calf-huts at Lacombe are 24 ft long, 10 ft deep, and 8 ft high in front and are easily movable on skids. They are used primarily as creep areas and are bedded for use of calves only. Calves may spend several hours each day sleeping in them.
-
2.
The calf-huts are moved as necessary when the build-up of manure becomes excessive.
-
3.
The water supply to the calving and nursing areas is provided by a winter water line and tank with an electric heater. If necessary, water troughs may be kept open by using propane or coal heaters, or water may be hauled by truck for short periods.
-
4.
It is preferable to have an extra area (corral, bam) for chronically sick animals, weak calves that are sure to be a problem, and cows with no milk.
-
5.
Move pregnant cows to the calving area approximately two weeks before calving. The bedding should be as clean and dry as possible, especially when calving starts.
-
6.
Within one day after calving, when the bond between the dam and calf is established, move the cow and calf to one of the nursing areas. Do not leave the dam and the calf in the calving area for more than 24 hours unless necessary.
-
7.
Fill the nursing areas to a maximum density of 35 to 40 cows and their calves for each 10- to 12-acre area. Then start in a new nursing area.
-
8.
Do not leave cow and calf in smaller nursing pastures longer than four weeks. If weather permits, move each group out into larger summer pastures when the youngest is three weeks old. Otherwise, problems with parasites and coccidiosis may occur.
-
9.
In small herds it may be possible to calve in a corral, but it is not recommended. Again, cows and calves should be dispersed as soon as possible.
-
10.
For herds larger than 150 cows the system can be duplicated. Alternatively, older cows may be allowed to calve on the range and heifers and other cows of particular concern (exotics, purebreds, known problem-calvers) managed as described.
-
11.
Clean and harrow pastures thoroughly two or three times after use, and leave vacant until grass is reestablished. Graze over summer as required, harrow again in fall, and leave vacant for as long as possible over winter.
It is difficult to define the maximum allowable population density in each area because it will vary depending on weather and ground surface conditions, the level of environmental contamination, and herd immunity. As a general rule, provide as much space per cow as possible. The Lacombe system was originally designed so that 70 cows would calve in 16-acre pastures; however, the entire area was not always available to the cows because of heavy snowfall. Following calving, 35 cow-calf pairs were dispersed onto 12-acre nursing areas. At the University of Saskatchewan Termeunde farm near Lanigan, Saskatchewan, use of a similar system successfully minimized scours when cows were allowed 1000 sq ft (50 cows in 50,000 sq ft), but not when allowed 250 sq ft (50 cows in 12,500 sq ft) in the calving area. Newborn calves were moved to 8-acre nursing areas within two to three hours of birth.
The advantages of a Lacombe-type system are as follows:
-
1.
At calving time it is easier to provide surveillance of the pregnant cows and heifers as one group and the cows that have already calved as another group.
-
2.
Cows and calves are together with their own kind and find one another more readily. Cows that are close to calving will not be claiming another cow’s calf. In large herds this will help to minimize the problem of mismothering, particularly in first-calf heifers.
-
3.
There is a more relaxed environment for the calf, less movement in the herd, and less likelihood of being trampled.
-
4.
Calves of approximately the same age are grouped together, thereby facilitating movement to large pastures according to age, and scheduling for branding and castration.
-
5.
The herd is already divided if an outbreak of diarrhea develops.
Among the disadvantages are the following:
-
1.
The initial cost of construction. This should be more than compensated for by reduced expenditures on medication and calf losses.
-
2.
Increased time and labor for snow removal, feeding, bedding, and observing multiple groups. Reduced time spent on treating sick calves should compensate for these added inputs.
As mentioned above, there are additional risks associated with calving indoors because environmental contamination builds up rapidly. When indoor calving is practiced, a Lacombe-type system is still recommended for postcalving management.
Control
These recommendations can also be applied during an outbreak. Cows that have not yet calved should be removed from the contaminated calving area to a “clean” location, and one of the systems described above for reducing population density should be started. Most cases of E. coli diarrhea are initiated during the first 24 hours of life, so handling of calves during the first day should be avoided if possible. In order to break the cycle of infection, it may be necessary for livestockmen to take special precautions to avoid contaminating their hands, clothing, and boots. In some cases it may be beneficial to wear disposable rubber gloves and clean coveralls if it is necessary to handle newborn calves when moving them from the calving grounds to holding areas or postcalving areas. Footbaths may also be used by workers moving from one pen to another. A fenceline is normally sufficient to control spread.
Increase the Nonspecific Resistance of the Calf
The newborn calf must ingest a sufficient amount of colostrum within a few hours after birth. The antibodies in the colostrum are absorbed into the blood circulation with maximum efficiency during the first six hours after birth; thereafter the efficiency of absorption is reduced quickly and almost no absorption occurs after 24 hours.15
If calves ingest liberal quantities of colostrum within 12 hours after birth, they will usually attain serum gammaglobulin levels high enough to prevent bacteremia, septicemia, and death from diarrheal dehydration.6., 7. The minimum amount of colostrum that calves should ingest during the first 12 hours to attain satisfactory serum gammaglobulin levels is 50 ml per kg of body weight (5 per cent body weight).8 Therefore, a 40-kg calf should get about 2 liters as soon as possible after birth.
The amount of colostrum ingested by the calf in the first six hours is dependent upon three factors:
-
1.
The amount of colostrum available from the dam. The amount of colostrum available will depend on the maturity of the cow and adequacy of the nutrition throughout the winter. Based on a survey on one Hereford ranch, 50 per cent of the two-year-old heifers had only 500 ml or less of colostrum immediately after calving, 75 per cent had only 750 ml or less, and only 6 per cent had between 1000 and 2000 ml. This suggests that under some conditions heifers may not have sufficient colostrum. This shortage of colostrum is due to a combination of inheritance, lack of maturity, and inadequate nutrition.
Calves that are born weak and cannot or do not want to suck should be offered one to two pints of warm colostrum by nipple bottle or should be force-fed the colostrum with a stomach tube. There is more than adequate absorption of immunoglobulins when colostrum is given by tube within minutes after birth.12
-
2.
The maternal behavior of the dam and whether or not she lets the calf suck. Mismothering occurs commonly in two-year-old heifers (they make no effort to establish a dam-calf relationship), and they abandon their calves or do not allow them to suck. Some of these heifers can be encouraged to accept their calves. The heifer is restrained in a head gate and her hindlimbs are hobbled so that she cannot kick. The calf is held up to the teats and encouraged to suck. Confinement of the heifer with the calf in a small pen for a few days will often result in acceptance of the calf.
When the dam-calf relationship has been established (when the calf is sucking and is encouraged by the mother to suck), the cow and calf should be moved out of the main calving area to a nursery area or pasture. This will prevent overcrowding, which is a common predisposing cause of calf diarrhea.
-
3.
The vigor of the calf and its ability to suck the cow. The vigor of the calf and its desire and ability to stand, “seek the teat,” and suck the necessary amount of colostrum will depend on the health of the calf and the environment. Newborn calves may be weak at birth because of congenital defects and infection or because of a difficult and prolonged birth. The cause of the “weak calf syndrome” is still uncertain, but in utero infection of the fetus late in gestation is a possibility. Prolonged difficult dystocia may cause intrapartum hypoxia, edema of the soft tissue of the head including the tongue, and inability of the calf to suck early enough. All of these calves must be given colostrum as soon as possible either by nipple bottle or force-feeding with a stomach tube. Calves that are born in deep snow or are exposed to very cold and windy weather may become hypothermic, weak, and unable to stand or suck within one hour. These calves must be detected early and fed adequate quantities of colostrum and placed in a dry, weather-protected area until they have regained their strength.
Increase the Specific Immunity of the Calf
The degree of immunity to specific enteropathogens in each animal (individual immunity) and within herds (herd immunity) varies depending on previous exposure to the infectious agent.
Most strains of E. coli known to cause diarrhea in calves possess the K99+ antigen which helps the bacteria to colonize the calf’s small intestine.4., 14. The K99+ antigen is a filamentous structure found on the surface of most enterotoxigenic E. coli, regardless of serotype.9., 13., 14. Colostral antibodies against the K99+ antigen will prevent diarrhea caused by K99+ enterotoxigenic E. coli.3 Under natural conditions, the colostrum of less than 10 per cent of beef cows contains K99+ antibody; therefore, many calves remain susceptible to E. coli diarrhea during the first few days of life even though they ingest colostrum soon after birth. Immunity to the K99+ antigen can be induced by immunizing pregnant cows during the third trimester of gestation. Bacterins that will prevent diarrhea caused by enterotoxigenic E. coli (Vicogen-Connaught Animal Health, Coligen, Fort Dodge Laboratories) are available and should be administered to cows twice prior to parturition. In the face of an outbreak, some beneficial immunity may develop within three weeks following the first injection. Gows that calve up to 45 to 60 days after the second injection have protective colostral antibody titers.
In contrast to the above, individual as well as herd immunity to rotavirus is high in beef herds in western Canada. In a survey of ten cows from each of 20 herds, 146 cows (73 per cent) and 19 of 20 (95 per cent) herds were positive for rotavirus antibody.11 Therefore, over 70 per cent of beef cows appear to have colostral levels of rotavirus antibody high enough to prevent diarrhea during the first five to seven days of life. However, colostral antibody levels decline rapidly after calving and many calves probably become susceptible to rotavirus diarrhea by one week of age because antibody levels in the milk reaching the lumen of the small intestine are not high enough to prevent infection and multiplication of the virus. Also, because of this decline in antibody levels, outbreaks of rotavirus diarrhea can occur year after year in spite of the presence of colostral antibody in most of the cows at calving.2., 16. When herd immunity is low, outbreaks of rotavirus diarrhea can occur in younger calves, but this appears to be the exception rather than the rule. Sporadic cases will also occur in younger calves if they do not ingest colostrum, or when the volume of colostrum ingested is low, as in heifers. Serum antibody to rotavirus does not prevent diarrhea.16 A similar situation applies for the coronavirus.
A modified live virus vaccine (Calf Guard, Norden Laboratories), which contains both rotaviruses and coronaviruses, can be administered either to the cow prior to parturition or to the calf at birth. By vaccinating the dam, the intent is to delay the normal rapid decline in colostral antibody levels so that antibody persists in the milk for longer than normal. However, sufficient evidence is not yet available to allow a critical evaluation of the vaccine when administered to the dam. Alternatively, the same vaccine, previously marketed as Scourvax II, can be administered orally to newborn calves at birth. To be effective, the manufacturer recommends that all newborn calves in the herd be vaccinated. This procedure is supposed to reduce environmental contamination resulting from passage of the viruses through clinically normal calves. In nursing calves, there is a danger that the vaccine virus will be neutralized in the intestine by colostral antibody, thereby making it ineffective. Because of this problem, it could be counterproductive to administer the vaccine to both the dam and the calf. Initiation of oral calf vaccination in the face of an outbreak may not be effective.
There is not, nor is there likely to be in the near future, a vaccine that is effective against all enteropathogens. Multiple-component vaccines that provide protection against several enteropathogens appear to be technically feasible and may become available within the foreseeable future. Although neither manufacturer of the above vaccines has recommended that both be administered together, there does not appear to be any reason at this time why they should not be administered at the same time, but by separate injection. This type of approach should be most beneficial in herds in which infection with K99+ enterotoxigenic E. coli and rotavirus or coronavirus exists. Colostrum from vaccinated cows can also be stored frozen and fed to weak or neglected calves or used for treatment of sick calves.
Apparent failure of the vaccines under field conditions may occur for a variety of reasons. Other enteropathogens, such as Cryptosporidia, Salmonella, and other viruses that are not related to the antigens in the vaccines, may be present. There may be strains of enterotoxigenic E. coli that colonize the small intestine by some mechanism other than K99+ antigen and against which K99+ antibody will not be protective. Such strains have not yet been identified. There is also increasing evidence that there are different strains of bovine rotavirus that could be immunologically distinct. If so, a viral vaccine prepared from one strain of virus might not protect against all other strains of the virus that occur in the field. Also, in herds in which animals are crowded within the calving area or where the level of environmental contamination is high, the protective level of colostrum may be overwhelmed by infection pressure. Individual calves may also fail to ingest colostrum for a variety of reasons.
The decision to vaccinate the pregnant dams will depend on consideration of the risk factors in the herd. They are as follows:
-
1.
Has the enteropathogen been isolated from diarrheic calves in previous years?
-
2.
Is the disease considered to be economically important in the herd? Vaccination must be cost-effective.
-
3.
What are the characteristics of the calving grounds? Is there sufficient area per calving animal; is the ground surface well drained; is there adequate protection from cold winds, and is it easy to move animals from one place to another?
-
4.
What is the nutritional status of the pregnant animals? Will they have a sufficient amount of colostrum? A major factor in the efficacy of the vaccine is the amount of colostrum ingested by the calf.
-
5.
What is the level of management? Vaccination is not a replacement for inadequate management. The recommendation to use any of the available vaccines for the control of diarrhea in calves in herds that are poorly managed may give the owner a false sense of hope; the results may be poor and the reputation of the vaccine is unjustifiably questioned.
Reduce Stress
Stress is the reaction by which the body of an animal adapts to environmental conditions. The ability of newborn animals to adapt to changes in the environment is limited, and conditions that appear to have no effect on mature animals may be detrimental to the newborn calf. It has been recognized for many years that stress is a contributing factor in many individual cases and in outbreaks of calf diarrhea. However, because many different environmental conditions can cause stress and because stress is difficult to measure, it has not been possible to identify all of the factors that contribute to the problem. Also, some conditions such as “overcrowding” which cause psychological and physical stress also lead to increased levels of contamination and exposure to infectious agents. The results of the epidemiologic survey of beef cattle ranches in Alberta and Saskatchewan have helped to identify some of the factors that contribute to stress.
-
1.
Inclement weather: Many outbreaks occurred within 48 hours following snow storms when the weather was classified as cold and changeable. Later in the spring, rain will also precipitate epidemics.
-
2.
Poor ground surface conditions: Ground surface conditions preceding outbreaks were classified as “wet” by 81 per cent of ranchers. Excess surface water and cold, wet bedding make it difficult for calves to find a comfortable place to sleep and leads to the build-up and spread of contamination. Locate calving areas to take advantage of natural drainage away of surface water. Use clean, dry straw for bedding. Increase the amount and depth of bedding as ground surface conditions become wet or muddy.
-
3.
Crowding: Crowding can cause stress and increase exposure to infectious agents. These two effects are difficult to separate, because as the number of square feet per cow-calf pair decreases, the incidence of diarrhea increases.
During the first two weeks of life calves spend most of their time sleeping or sucking. Under crowded conditions their resting and feeding patterns may be altered. This stress, added to increased exposure to infectious agents may result in a higher incidence of diarrhea. Every effort should be made to ensure that calves have a clean, dry, sheltered area in which nursing and resting are not disturbed.
Summary
A management system designed to control diarrhea in newborn beef calves has been oudined. The calving herd is moved from the wintering grounds to previously prepared, comfortable calving grounds. The herd is divided into small subunits for surveillance of calving. The calving areas should be well-bedded, and a natural or artificial windbreak should be provided. All parts of the calving grounds should be readily accessible.
Every effort is made to ensure that calves receive a liberal supply of colostrum within hours after birth. Obstetrical assistance is provided as necessary to ensure that all calves are born vigorous. At 24 hours of age, the cow-calf pair is moved out of the calving grounds to the nursery pasture. Diarrheic calves are moved to an isolation area and treated accordingly. The system is successful because the newborn calf is moved from contaminated areas of high population density to areas of lower population density that are less contaminated with the common enteropathogens.
Under certain circumstances, the vaccination of the dam in late pregnancy with E. colt K99+ bacterins may be beneficial as an aid to the control of the disease.
Footnotes
Bradley, J.A. : Recommendations for the management of the beef cow herd during calving season, to minimize incidence and severity of acute neonatal diarrhea. Presented at the Calf Scours Seminar, High River, Alberta, 1974.
References
- 1.Acres S.D. University of Saskatchewan; 1976. The epidemiology of acute undifferentiated neonatal diarrhea of beef calves in Western Canada. Ph.D. Thesis. [Google Scholar]
- 2.Acres S.D., Babiuk L.A. Studies on rotaviral antibody in bovine serum and lacteal secretions, using radioimmunoassay. J. Am. Vet. Med. Assoc. 1978;173:555–559. [PubMed] [Google Scholar]
- 3.Acres S.D., Isaacson R.E., Babiuk L.A. Immunization of calves against entero toxigenic colibacillosis by vaccinating dams with purified K99 antigen and whole cell bacterins. Infect. Immun. 1979;25:121–126. doi: 10.1128/iai.25.1.121-126.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellamy J.E.C., Acres S.D. Enterotoxigenic colibacillosis in colostrum-fed calves: Pathologic changes. Am. J. Vet. Res. 1979;40:1391–1397. [PubMed] [Google Scholar]
- 5.Church T.L., Janzen E.D. A system for calving heifers on a large commercial ranch. Bovine Pract. 1978;13:40–44. [Google Scholar]
- 6.Fisher E.W., de la Fuente G.H. Water and electrolyte studies in newborn calves with particular reference to the effects of diarrhea. Res. Vet. Sei. 1972;13:315–322. [PubMed] [Google Scholar]
- 7.Fisher E.W., Martinez A.A., Trainin Z. Studies of neonatal calf diarrhea. II. Serum and fecal immune globulins in enteric colibacillosis. Br. Vet. J. 1974;131:402–414. doi: 10.1016/s0007-1935(17)35236-3. [DOI] [PubMed] [Google Scholar]
- 8.Kruse V. A note on the estimation by simulation technique of the optimal dose and feeding time at first feeding after the calf’s birth. Anim. Prod. 1970;12:661–664. [Google Scholar]
- 9.Lariviere S., Lallier R., Morin M. Evaluation of various methods for the detection of enteropathogenic Escherichia coli in diarrheic calves. Am. J. Vet. Res. 1979;40:130–134. [PubMed] [Google Scholar]
- 10.McBeath D.G., Logan E.F. Influence of neonatal management on serum immunoglobulin levels of suckled calves. Vet. Rec. 1974;1 doi: 10.1136/vr.95.20.466-a. [DOI] [PubMed] [Google Scholar]
- 11.Mohammed K., Babiuk L.A., Saunders J.R. Rotavirus diagnosis: Comparison of various antigens and serological tests. Vet. Microbiol. 1978;3:115–127. [Google Scholar]
- 12.Molla A. Immunoglobulin levels in calves fed colostrum by stomach tube. Vet. Rec. 1978;103:377–380. doi: 10.1136/vr.103.17.377. [DOI] [PubMed] [Google Scholar]
- 13.Moon H.W., Whipp S.C., Skartvedt S.M. Etiologic diagnosis of diarrheal diseases of calves: Frequency and methods of detecting enterotoxin and K99 antigen production by Escherichia coli. Am. J. Vet. Res. 1976;37:1025–1029. [PubMed] [Google Scholar]
- 14.Orskov I., Orskov F., Smith H.W. The establishment of K99, a thermolabile, transmissible Escherichia coli K antigen, previously called “Kco,” possessed by calf and lamb enteropathogenic strains. Acta Pathol. Microbiol. Scand. 1975;83:31–36. doi: 10.1111/j.1699-0463.1975.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 15.Selman I.E., McEwan A.D., Fisher E.W. Serum immune globulin concentrations of calves left with their dams for the first two days of life. J. Comp. Pathol. 1970;80:419–427. doi: 10.1016/0021-9975(70)90074-5. [DOI] [PubMed] [Google Scholar]
- 16.Woode G.N. Epizootiology of bovine rotavirus infection. Vet. Rec. 1978;103:44–46. doi: 10.1136/vr.103.3.44. [DOI] [PubMed] [Google Scholar]


