Abstract
Background
Respiratory protective devices exposed to pathogenic microorganisms present a potential source of transmission of infection during handling. In this study, the efficacy of 4 antimicrobial respirators to decontaminate MS2, a surrogate for pathogenic viruses, was evaluated and compared with control N95 filtering face piece respirators, which did not contain any known antimicrobial components.
Methods
MS2 containing droplet nuclei were generated using a Collison nebulizer and loaded onto respirator coupons at a face velocity of 13.2 cm/seconds for 30 minutes. The coupons were incubated at 2 different temperature and relative humidity (RH) conditions and analyzed for viable MS2 at different time intervals.
Results
Results showed that log10 reduction of MS2 was not statistically significant (P > .05) between the control and antimicrobial respirator coupons, when stored at 22°C and 30% RH up to 20 hours. Coupons from 1 of the 4 antimicrobial respirators showed an average MS2 log10 reduction of 3.7 at 37°C and 80% RH for 4 hours, which was statistically significant (P ≤ .05) compared with coupons from the control respirators.
Conclusion
Results from this study suggest that MS2 virus decontamination efficacy of antimicrobial respirators is dependent on the antimicrobial agent and storage conditions.
Key Words: Antimicrobial technology, respirator, decontamination efficacy, virus aerosol, survivability
Experts predict that an influenza pandemic is likely to occur, and, as a result, the federal government has instituted a number of initiatives to enhance pandemic influenza planning efforts. To minimize and delay the spread of an influenza pandemic, a number of nonpharmaceutical interventions that would be readily available at the onset of a pandemic are recommended by government agencies and nongovernment organizations. The use of respiratory protective devices is one intervention designed to reduce exposure to infectious influenza aerosols. National Institute for Occupational Safety and Health (NIOSH)-approved respirators are also recommended for protection of health care workers against several other pathogenic microorganisms including Mycobacterium tuberculosis, severe acute respiratory syndrome, Bacillus anthracis, and others.1, 2
Respirators contaminated by influenza or other infectious aerosol can become a potential source of transmission. Fomites are inanimate objects capable of aiding the dissemination of infectious organisms. Viruses can contaminate and survive in the inanimate environment, persist after drying, and become reaerosolized.3, 4, 5 Use of respirators in infected environments can potentially result in the deposition of infectious agents on the surface as well as inner layers of the respirator that may cause its conversion to a fomite, thereby becoming a vehicle for direct or indirect transmission. Prior studies have demonstrated the survival of various infectious microorganisms (eg, bacteria6, 7, 8 and fungi9) on respirators, but little work has been done with respect to contamination of respirators by viruses.10 These prior studies indicate that some bacteria and fungi can survive on respirator filters, but their survivability is dependent on the specific organism, filter material, and storage conditions.
Other studies have focused on handling contaminated devices. For example, a recent study addressed the issue of virus transmission among health care workers handling contaminated personal protective equipment (PPE).11 In that study, respirators and other PPE including goggles, gowns, and gloves donned by volunteers were intentionally contaminated with MS2. Participants performed tasks such as measuring blood pressure on a mannequin and then removed PPE according to standard protocol. One-handed removal of respirator and goggle resulted in approximately 1- to 2-log10 of MS2 (most probable number) transfer to hands. Removal of other PPE including gowns and gloves showed higher levels of contamination. Similarly, cross contamination of viruses by surface to hand12 and hand to hand13 have been reported. Handling rhinovirus-contaminated coffee cups and plastic tiles caused transmission of virus in some test subjects.12 Similarly, hand-to-hand contact was shown to transfer rhinovirus infection more effectively than aerosols produced by cough, sneeze, and loud talk or prolonged exposure.13 In another study, influenza A and B viruses survived for 24 to 48 hours on hard, nonporous surfaces such as stainless steel and plastics but only survived for <8 to 12 hours on cloth, paper, and tissues.14 The study also demonstrated that influenza A could be transferred from the environmental surfaces to the hands and survives long enough for self-inoculation to occur.
Recent reports from the Institute of Medicine related to the reusability of face masks and PPE for health care workers have recommended research on “alternative materials, including bioactive fibers”15 and “chemical treatments to impart biocidal properties to PPE to enhance their protection capability and extend their useful life.”16 It is speculated that a respirator or surgical mask incorporating a proven antimicrobial substance (hereafter referred to as an “antimicrobial respirator”) could potentially kill or prevent the growth of microorganisms such as bacteria, fungi, or viruses that have been deposited on the respirator surface or trapped in the filter media.17 With increasing media reports on influenza pandemic planning issues, there is a growing interest in the area of antimicrobial respirators. Several manufacturers have produced prototype and commercially available filtering face piece respirators (FFRs) and other types of face masks claiming antimicrobial efficacy for a wide range of microorganisms.
However, there is limited knowledge on the efficacy of antimicrobial respirators to reduce fomite potential. Among the studies reported in the literature, many employed iodine-treated filters to investigate microorganism viable removal efficiency using different techniques.18, 19, 20, 21, 22 These studies investigated whether antimicrobial filter media was able to reduce the viable virus particles passing through the filter media by inactivation in addition to particle capture. This was done by comparing the number of viable virus particles that pass through the control and antimicrobial filters. The conclusion from these studies18, 20, 21 was that viruses and bacteria were inactivated by iodine at the filter level. Interestingly, a recent study indicated that iodine released from the filter and dissolved in the virus extraction medium may also contribute to virus inactivation.23 The authors also showed that the filter materials contained many MS2 particles after extraction, indicating the possibility for a fomite. Thus, it is of great scientific interest to focus on the question of whether the potential for the respirators to act as a fomite was reduced or not. Some limited studies have been reported on the survivability of spores, bacteria, and fungi on antimicrobial air filter media,18 electrospun nanofiber webs,24 activated carbon fibers,25 military fabrics,26 and surgical masks17 after storage. These studies found that the storage conditions (time, temperature, humidity) played an important role in the efficacy of the antimicrobial agent. However, it is difficult to make generalizations across studies because they all used different test methods and each study evaluated only a single antimicrobial agent. Furthermore, none of these studies reported data on the viability of viral aerosols applied to the devices. Manufacturers often provide unpublished antimicrobial efficacy data obtained from third-party test laboratories using different test protocols for their products. For example, in some testing, a small volume of the microorganism suspension is applied on to filter swatches, whereas other tests use the whole respirator/mask to load bioaerosol particles. There is a general need for a standardized protocol based on realistic test conditions for assessing the efficacy of antimicrobial respirators.
In this study, coupons from antimicrobial respirators from 4 manufacturers representing 4 different antimicrobial agents were loaded with aerosolized MS2 virus containing droplet nuclei, incubated for different time periods, and, then, viable virus particles were recovered and enumerated. The Bio-Aerosol Respirator Test System used in this study was designed to deliver a representative challenge mimicking the airborne transmission of virus containing droplet nuclei.27 After coupon loading, the antimicrobial effect of the test samples was evaluated at 2 different storage conditions, namely 22°C and 30% relative humidity (RH) and 37°C and 80% RH and, then, assaying the viable viruses trapped on respirators at different time points. It was hypothesized that relatively fewer viable MS2 would be recovered from the coupons obtained from the antimicrobial respirators compared with the control N95 FFR coupons containing no known antimicrobial components. Furthermore, it was hypothesized that storage conditions would affect the ability of the antimicrobial respirator to render the virus inactive.
Materials and methods
Respirators
Antimicrobial FFRs (respirators) from 4 manufacturers were obtained (Table 1 ). One of them was a NIOSH-approved P95 particulate respirator. The other 3 were not NIOSH-approved respirators. All manufacturers claimed incorporating antimicrobial treatments in their respirators, but none of them were approved by any US regulatory agencies for their antimicrobial efficacy. One manufacturer embedded silver-copper based antimicrobial technology throughout the fiber on the outer layer of the mask. Similarly, EnvizO3-Shield technology (oxygen species) was incorporated on the outer layer of the mask by another manufacturer. In other cases, TiO2 coated filtering layers were placed beneath the outer layer of the mask or iodine-activated resin incorporated filtering layer was positioned within the respirator. The presence of antimicrobial agents on the straps and sealing surfaces is not known. NIOSH-approved FFRs, containing no known antimicrobial components, from a single manufacturer were used as the control.
Table 1.
Test antimicrobial respirators
| Respirator manufacturer | Antimicrobial component | Incorporation | Availability |
|---|---|---|---|
| A | Silver-copper based technology | Embedded into filter fiber throughout and placed on the outer layer | Prototype |
| B | EnvizO3-Shield technology | Incorporated on outer layer of respirator | Prototype |
| C | Iodinated resin | Incorporated on filtering layer and positioned within respirator | Commercially available |
| D | TiO2 | Coated on filtering layers and placed beneath the outer layer | Prototype |
Test respirator coupons
Circular coupons (5 cm2) were cut from NIOSH-approved N95 FFRs (control) and 4 antimicrobial respirators as described previously.27 The coupons were not sterilized prior to loading but were handled with sterile forceps to minimize chemical and biologic contamination. Two N95 FFR and 4 antimicrobial FFR coupons (excised from at least 2 different FFRs) were placed into separate test specimen holders and attached to the sample ports of the Bio-Aerosol Respirator Test System (BARTS) for MS2 loading.27 For controls, N95 FFR coupons were used instead of soluble gelatin filters used in some studies to enumerate viable microorganisms.
Selection of virus: MS2
Coliphage MS2 virus was selected as a surrogate virus for this study because it is nonpathogenic and easy to handle in a biosafety level 1 laboratory. MS2 is a nonenveloped virus and less sensitive to decontamination agents.28 Many researchers have used MS2 as a stringent test case to assess the antimicrobial efficacy of respiratory materials.21, 22 Previous studies also showed that MS2 survival was better at low RH conditions than at high RH.29, 30
Escherichia coli and MS2 cultures
The bacterium Escherichia coli was used as a host for the virus MS2. E coli 15597 and MS2 virus (ATCC 15597-B1) were obtained from American Type Culture Collection (ATCC). E coli was replicated in ATCC medium 271 (www.atcc.org) as described previously.27 Briefly, 20 μL E coli stock was inoculated in 10 mL medium 271 and incubated overnight at 37°C in an incubator. An aliquot (1 mL) of overnight culture of E coli was added to 100 mL medium 271 and incubated at 37°C with shaking at 100 rpm for 5 hours.
MS2 virus was replicated using E coli (ATCC 15597) as the host bacterium. An aliquot (1 mL) of the 5-hour culture of E coli was used to inoculate 30 mL of ATCC medium 271 in a conical flask. After 2.5 hours, 1.5 mL of MS2 stock was added to the conical flask containing E coli and incubated overnight at 37°C. The medium 271 containing replicated MS2 was centrifuged at 7100g for 30 minutes at 4°C (IEC Multi RF, Thermo Electron Corporation, Waltham, MA). The supernatant containing MS2 was filtered through a 0.22-micron millipore filter (Fisherbrand, Fisher Scientific Company, Pittsburgh, PA). The virus suspension (approximately 1011 plaque-forming units (pfu)/mL) was stored in a 50-mL plastic conical tube (Falcon; Becton Dickinson. Franklin Lakes, NJ) at 4°C.
MS2 virus aerosol generation and loading on to test respirator coupons
Droplet nuclei containing MS2 virus were generated using a Collison nebulizer (BGI Inc., Waltham, MA) and loaded onto 6 respirator coupons using the Bio-Aerosol Respirator Test System.27 The count median diameter of the particles loaded onto coupons was measured using a scanning mobility particle sizer (SMPS, Model 3080; TSI, Inc., Shoreview, MN) and found to be 32 nm with a corresponding mass median diameter of 141 nm.27 Briefly, 23 mL of MS2 (approximately 105-108 pfu/mL) suspension in 1% ATCC medium 271 was added to a Collison nebulizer glass jar. Compressed filtered air was passed into the nebulizer (20 psi, 10 L/min) to aerosolize MS2 along with the other constituents of the media. The proteins and other components of the aerosolization fluid serve as a protective factor, which has been shown to neutralize or serve a physical barrier to reduce the effectiveness of some antimicrobial agents.27 The aerosol was diluted with high-efficiency particulate air filtered air (13 psi, 50 L/min) downstream of the nebulizer outlet. The diluted aerosol entered the aerosol chamber and was allowed to equilibrate. After 5 minutes, MS2 aerosol was passed in parallel through 6 specimen holders, each containing a test coupon fixed in the sample port at 4 L/min flow rate (face velocity: 13.2 cm/seconds [s]) regulated by vacuum lines. MS2 droplet nuclei were allowed to deposit throughout the 6 respirator coupons for 30 minutes. Coupons from the controls and the antimicrobial respirator being tested were randomly assigned to a particular sample port (holder) to avoid any bias caused by port location within the Bio-Aerosol Respirator Test System chamber.
In each experiment, 4 coupons from the N95 FFR control and 2 coupons from one of the antimicrobial respirators were loaded with MS2. Of the 4 control coupons, 2 were used to determine MS2 loading levels. The remaining 2 control and 2 antimicrobial coupons were stored under controlled environmental conditions.
Storage conditions for MS2 loaded respirator coupons
MS2 loaded respirator coupons were stored at 2 different environmental conditions in a Caron 6010 Environmental Test Chamber (Caron Products & Services Inc; Marietta, OH) for different time intervals. In the first set of experiments, MS2 loaded coupons were stored at 22°C and 30% RH, and virus particles recovered at 0, 8, and 20 hours were enumerated. A second set of coupons were loaded with MS2 and stored at 37°C and 80% RH for 0, 2, and 4 hours, and viable MS2 from coupons were enumerated. The experiment was repeated 3 times for each storage time point for each environmental condition. MS2 recovery from coupons and enumeration of viable virus particles were accomplished by the methods described below.
Virus recovery
Following storage or in the case of the loading level control coupons immediately following loading, each respirator coupon was resuspended in 10 mL ATCC medium 271 in a 50 mL conical tube and vortexed at the highest setting for 2 minutes. The coupons were removed and discarded. The supernatant containing virus was enumerated for viable MS2 by a plaque assay as described below.
Plaque assay
MS2 virus was enumerated using a single agar layer method as described previously.26 Each MS2 virus suspension was serially diluted. Briefly, 8 mL of medium 271 with 0.5% agar was placed into each glass culture tube and incubated at 47°C in a water bath. E coli (0.5 mL) at log phase, and 100 μL or 1 mL of the MS2 suspension was added to the culture tubes. The soft agar containing E coli and MS2 was poured into an empty Petri plate and mixed by swirling. The plates were allowed to harden at room temperature and placed in an incubator at 37°C and 30% RH overnight. MS2 plaques were counted manually, and those containing 30 to 300 pfu were recorded.
Toxicity assay
Experiments were conducted to determine the toxicity of iodine species released in to the recovery medium. Three coupons from both the N95 control and antimicrobial respirator C were stored at 37°C and 80% RH. After 4 hours, each coupon was placed in a separate 50-mL conical tube containing 10 mL of 271B medium and approximately 106 MS2 pfu. Each sample containing a coupon, 271B medium, and virus was vortexed for 1 minute at the highest setting. The coupon was removed, and the recovery medium was allowed to stand for 3 hours at room temperature before a plaque assay was performed.
Data analysis
Log reduction of MS2 (ie, decontamination efficacy) for each antimicrobial respirator coupon and the control N95 FFR respirator coupon was calculated by dividing the average number of viable MS2 virus recovered from the loading level coupons by the number of viable MS2 virus recovered from each control N95 FFR respirator or each antimicrobial respirator coupon. These values were then converted to log10 units, and the average and the standard deviations of the 6 replicates reported. Statistical analysis comparing the average log10 values for the coupons from 4 antimicrobial respirators and the coupons from the control N95 FFR respirator was done with a series of t tests for each of the 4 antimicrobial respirator models, time points, and storage conditions using Sigma Stat (Jandel Corporation, Systat Software Inc., San Jose, CA).
Results
Table 2 shows that MS2 loading levels for the coupons from the N95 FFR controls and the 4 antimicrobial respirators were consistent. The relative standard deviations for the loading levels of the N95 control and antimicrobial respirators were within reasonable limits.
Table 2.
MS2 loading on to N95 filtering face piece respirators and antimicrobial respirators
| Antimicrobial respirator |
|||||
|---|---|---|---|---|---|
| MS2 loading | N95 FFR | A | B | C | D |
| Set 1: loading (log10) | 5.96 ± 0.18 (3%) | 5.93 ± 0.2 (3%) | 5.67 ± 0.07 (1%) | 6.19 ± 0.27 (4%) | 6.38 ± 0.07 (1%) |
| Set 2: loading (log10) | 5.71 ± 0.31 (5%) | 6.50 ± 0.15 (2%) | 5.13 ± 0.33 (6%) | 5.81 ± 0.36 (6%) | ND |
NOTE. Numbers in parentheses are relative standard deviations of the loading levels for N95 FFRs and antimicrobial respirators.
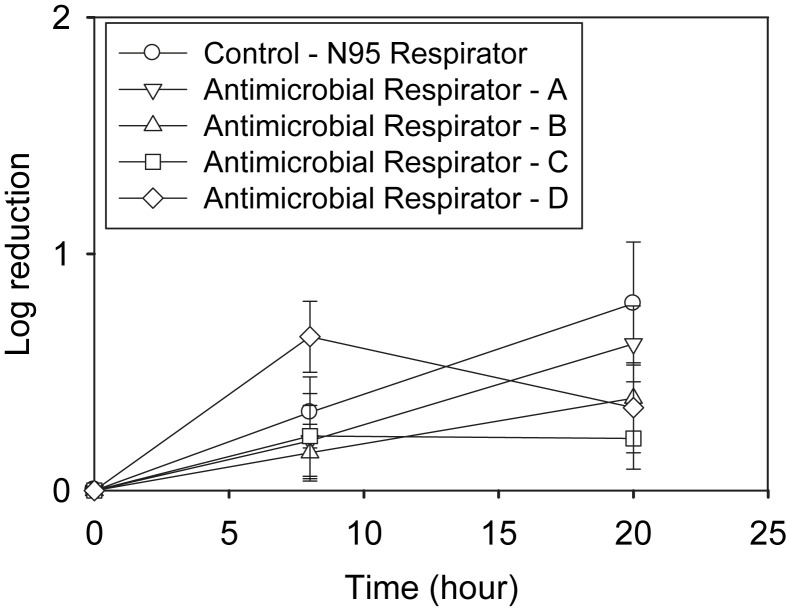
The first set of MS2 loaded coupons was stored at 22°C and 30% RH. MS2 survival was measured at 0, 8, and 20 hours. Figure 1 shows that the log10 reduction of MS2 increased with time up to 20 hours for all respirator coupons except antimicrobial respirator D. All respirator coupons showed <1-log10 reduction even at 20 hours storage time. There was no significant difference (P > .05) in the log10 reduction of MS2 between the control N95 FFR and the antimicrobial respirator coupons (A, B, C, and D) at 8 or at 20 hours.
Fig 1.
MS2 loaded respirator coupons were incubated at 22°C and 30% relative humidity (RH), and viable MS2 were enumerated at 8 and 20 hours.
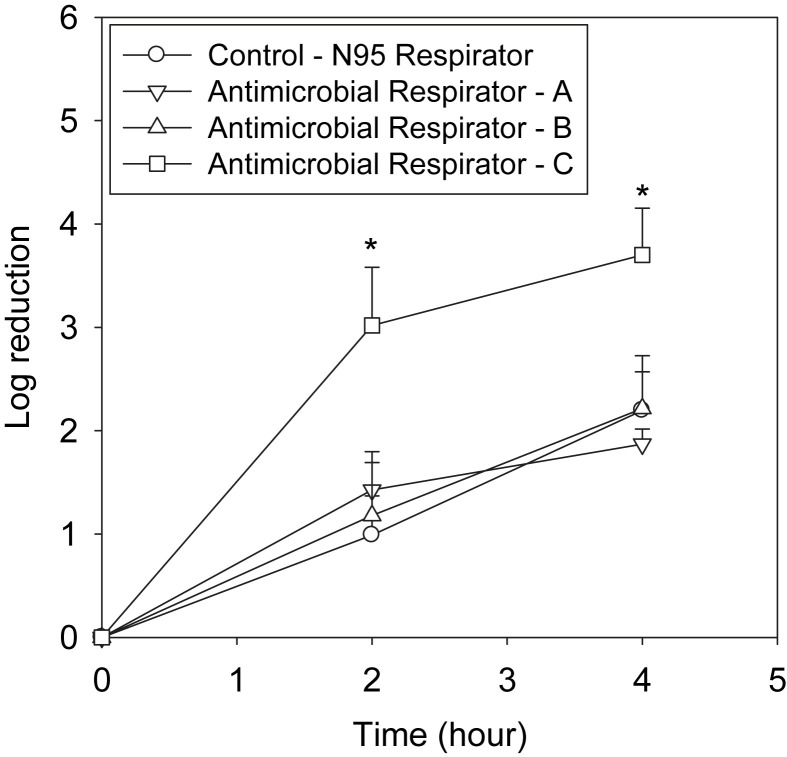
In the second set of experiments, MS2 loaded coupons were stored at 37°C and 80% RH, and the log10 reduction was measured at 0, 2, and 4 hours. Figure 2 shows that the log10 reduction of MS2 increased with time for all the antimicrobial respirators and N95 FFR controls tested. MS2 reductions for coupons from antimicrobial respirators A and B were approximately 2-log10 units and were not significantly (P > .05) different from the control N95 FFR. At the same time, coupons from antimicrobial respirator C showed 3.7-log10 reduction of MS2 at 4 hours of storage, which was significantly (P ≤ .05) different from the N95 FFR control coupons. Coupons from antimicrobial respirator D were unable to be tested at the high RH conditions.
Fig 2.
MS2 loaded respirator coupons were incubated at 37°C and 80% relative humidity (RH), and viable MS2 were enumerated at 2 and 4 hours. ∗Significantly (P < .05) different from the control N95 respirator.
Coupons from antimicrobial respirator C were compared with N95 control respirators for MS2 reduction at the 2 different storage conditions (Fig 1, Fig 2). MS2 reduction was approximately 2-log10 and 3.7-log10 units (4 hours) at 37°C and 80% RH for coupons from the control and antimicrobial respirator C, respectively. However, coupons from both the N95 FFR control and the antimicrobial respirators showed approximately <1-log10 MS2 reduction (8 hours) at 22°C and 30% RH. To determine whether MS2 inactivation of respirator C was due to iodine released from respirator coupon, a control toxicity assay was performed. MS2 survival was found to be 6.27 ± 0.03 and 6.23 ± 0.10 log10 units for the N95 control and respirator C, respectively, with no significant difference between them.
Discussion
The respirators employed in the study were manufactured with different antimicrobial technologies and tested at 2 different storage conditions. Other studies on the effectiveness of antimicrobial devices18, 26 have used similar storage conditions. Furthermore, the low temperature, low RH setting (22°C and 30% RH) can be considered based loosely on room temperature conditions found in health care facilities. Some anecdotal evidence suggests that health care facilities in North America maintain RH below 40%.31 The high temperature, high RH setting (37°C and 80%) can be considered similar to the warm and moist conditions found in the breathing zone of a respirator user.
In general, the results from this study (Fig 1, Fig 2) showed that the MS2 decontamination efficacy of the antimicrobial respirator coupons was dependent on the storage conditions and the type of antimicrobial agent. MS2 loaded antimicrobial respirator coupons at 37°C and 80% RH showed significantly higher log10 reductions for only 1 antimicrobial respirator (respirator C) compared with control N95 FFR coupons. At the same time, coupons from all 4 antimicrobial respirators tested in the study showed no significant difference in MS2 reduction compared with the coupons from the control N95 FFRs at 22°C and 30% RH for up to 20 hours.
As expected, high temperature and RH were found to decrease MS2 survival in the N95 FFR control coupons. Log10 reduction of MS2 was <1 at 22°C and 30% RH (20 hours) compared with ∼2 or more at 37°C and 80% RH (4 hours) for all the respirators tested in the study. The effect of RH and temperature on viable MS2 loaded onto respirator coupons is consistent with previous studies that showed MS2 aerosol survival was higher at 20% RH than at 80% RH.29, 30 In addition, MS2 is less sensitive to commonly used antimicrobial agents compared with enveloped viruses.30 This is partly because MS2 is a nonenveloped virus, which lacks a lipid bilayer envelope. In general, nonenveloped viruses are hardier and able to survive longer than enveloped viruses32 such as influenza.
Coupons from respirator C containing iodinated fibers tested in this study showed a significant increase in the log10 reduction of MS2 at 37°C and 80% RH, but not at 22°C and 30% RH, suggesting that the storage condition is a critical factor for the survival of MS2 and activity of this antimicrobial agent. MS2 virus reduction of the iodinated respirator coupon increased significantly at 37°C and 80% RH (4 hours, Fig 2) as did the control N95 respirator coupons, consistent with previous studies that showed MS2 inactivation was maximal at 75% RH and less at 20% and 30% RH.29, 30 However, coupons from antimicrobial respirator C were significantly different from the control N95 respirator coupons only for the tests done at 37°C and 80% RH, suggesting that the MS2 decontamination effect of the coupons from the iodinated respirator may be increased due to the high RH and high temperature employed in our study. Previous studies suggested that MS2 remained in the solid particle state at low RH, and inactivation of MS2 in the solid particle state was less compared with the levels in the fluid conditions.29, 30 This may be a reason why many types of viruses including influenza virus are maximally stable at a low RH environment.33, 34 High temperature and RH also increased MS2 log10 reduction compared with the low temperature and RH condition regardless of the respirator type. Taken together, the results suggest that iodine and high temperature and RH synergistically inactivated MS2 because this effect was not found for coupons from any of the antimicrobial respirators. Only coupons from antimicrobial respirator C showed a significant reduction of MS2 compared with the control coupons at 37°C and 80% RH. The results are in agreement with a previous study that investigated the decontamination efficacy of iodine-treated filter media against bacterial spores.18 Furthermore, comparing the results for respirator C to the controls for both storage conditions lends support to inactivation occurring on the respirator filter sample as opposed to in the extraction medium. This conclusion is further supported by the results from the toxicity assay showing that iodine released into the recovery medium was not directly interfering with the survival of MS2. This indicates that MS2 inactivation had occurred prior to the recovery of virus from coupons. However, one recent study reported that MS2 inactivation can occur in the extraction medium after removal of the virus from an iodine-coated filter.23 Further studies in this area are needed. Iodine inactivation of microorganisms appears to involve different mechanisms. In one study, iodine inactivation of poliovirus was mediated by disruption of the capsid protein coat.35 Results from a recent study employing isoelectric focusing of iodinated MS2 proteins suggested that iodine inactivation involved conformational changes to the protein coat.36 Some studies showed that iodine reaction with sulfhydryl groups caused inactivation of enzymes.37 Iodine also appears to cause nucleic acids damage in bacterial species previously.38
One of the antimicrobial respirators (D) employed an ultraviolet (UV)-A (310 nm - 400 nm), lamp (4W; emission maximum, 380 nm) to activate TiO2 on the respirator placed approximately 15 cm distance. Coupons from this antimicrobial respirator showed no ability to render MS2 inactive more than the control at either storage condition. However, bacterial culture on Petri dish coated with TiO2 when exposed to a UV-A light showed a significant increase in inactivation compared with the controls.39, 40 This may be partly explained by the difference in the intensity of the UV-A on the target organism and other exposure conditions. In our study, access for UV-A light to the TiO2 layer as well as free radicals to MS2 deposited in different layers26 are limiting factors unlike the direct UV-A exposure to bacterial cultures in other studies.39, 40 Alternately, the hydroxyl and oxygen radicals produced in the experimental setup were not sufficient enough to inactivate MS2. On the other hand, MS2 was shown to be highly sensitive to direct UV-C light (254 nm) compared with other virus types.41, 42 This may be attributed to the difference in the wavelengths of the UV light used in those experiments. It is well-known that free radicals damage cells through mechanisms involving oxidative stress and damage of DNA40 as well as RNA.43 The absence of a significant inactivation of MS2 by UV-A and TiO2 obtained in our study suggests the production of insufficient levels of free radicals to cause significant RNA damage.
Other reactive oxygen molecules such as ozone are also excellent antimicrobial agents. Results for MS2 loaded antimicrobial respirator test coupons with incorporated EnvizO3-Shield technology (B) did not show any statistically significant inactivation compared with the controls at either storage condition. However, in other studies, ozone gas was found to inactivate aerosolized bacteriophages including MS2, phi 6, and T7.44 Tseng et al introduced bacteriophage aerosols generated by a Collison nebulizer and ozone produced from a generator into a chamber in a parallel manner.44 Samples were removed from the chamber before and after ozone treatment, and viable bacteriophages were enumerated using a 1-stage Anderson sampler. Ozone at concentrations <10 ppm showed a significant inactivation in 15 seconds. In general, higher inactivation levels were obtained for the bacteriophages at 85% RH compared with the levels at 50% RH. The effect of ozone was attributed to its interaction with capsid protein of the virus.44 The difference in the sensitivity of MS2 to ozone between the 2 studies may partly be attributed to the different forms of oxygen species in the experimental procedures. The failure to obtain inactivation of MS2 using EnvizO3-Shield technology engineered respirators in our study may be explained by the different form of oxygen species as well as to the insufficient levels of oxygen species available for MS2 inactivation throughout the layers of the respirator coupon under the exposure conditions.
Heavy metals including copper, silver, and mercury have been also used for antimicrobial applications. Results from this study showed that coupons from respirators engineered with silver-copper based antimicrobial technology (A) did not increase the log10 reduction of MS2 virus significantly at either storage condition. Other studies on antimicrobial surgical masks coated with a mixture of silver nitrate and titanium dioxide nanoparticles17 and silver particle coated activated carbon fibers24 demonstrated good antimicrobial properties against E coli and other bacteria, with complete inactivation occurring after 24 hours and 10 minutes of storage, respectively. In a previous report, MS2 virus resuspended in phosphate-buffered saline showed a slight (0.28 log10) reduction upon exposure to silver (0.1 mg/L) for 130 minutes.45 Silver also enhanced the effect of UV light-induced inactivation in a synergistic manner. Silver ion generated reactive oxygen species was found to inactivate microorganisms.46 Similarly, silver nitrate inactivation of herpes simplex virus types 1 and 2 at concentrations 30 μmol/L was reported.47 Another mechanism of heavy metals including silver is believed to be mediated by their ability to bind sulfhydryl groups of capsid proteins of viruses.47 In this study, coupons from the respirator containing silver-copper based antimicrobial technology failed to show a statistically significant decontamination of MS2 compared with the controls. This may be partly explained by the difference in the test methodologies. In our study, MS2 was resuspended in 1% ATCC medium 271 for aerosolization and then in 100% medium 271 for subsequent procedures. The aerosol particles undergo desiccation to become droplet nuclei. The protein contained in the aerosol medium is concentrated through a reduction in volume of the aerosolized particles.27 The high concentration of protein in the droplet nuclei can diminish the decontamination effect of silver-copper based antimicrobial technology on MS2 by competing/interfering with potential binding to sites including the capsid protein sulfhydryl groups of the virus. High concentration of many proteins is also known to inhibit the effect of antimicrobial agents including bleach27 and other agents.30
Contaminated respirators may act as fomites during handling (eg, donning or doffing the respirator) or reuse. In this study, a viral challenge was loaded by passing MS2 containing droplet nuclei with a mass median diameter of 141 nm through the respirator coupons. Under the conditions used in this study and a previous study, varying levels of MS2 virus may be deposited in different layers of the respirator.27 However, significant amounts of virus containing droplet nuclei remain on the surface for cross contamination. MS2 virus loaded as droplet nuclei were found to survive for an extended period of time (>20 hours)—even for the antimicrobial respirators—at room temperature and low RH, consistent with other studies investigating the survivability of viruses on other porous substrates.5 In practice, other studies have shown that MS2 can readily transfer from respirator to hand.11 Because viruses can generally survive for extended periods of time and the time between respirator donning/doffing events can be short, the antimicrobial agent needs to be able to reduce viability within hours and preferably within minutes. If an effective antimicrobial respirator could be developed and validated, it may be possible to reduce the risks of handling of contaminated respirators because the likelihood of transferring active virus particles to the hand would be greatly diminished.
This study has some limitations such as the selection of surrogate virus, procedure for loading respirator coupons with the aerosolized MS2 containing droplet nuclei, and storage conditions. The antimicrobial respirators tested in the study were all previously evaluated by their respective manufacturers using different microorganisms and test protocols. MS2 virus selected for this study is a nonenveloped virus resistant to many antimicrobial agents. Thus, it is not surprising that only 1 antimicrobial respirator was somewhat effective at reducing MS2 viability at 1 storage condition. The decontamination efficacy of antimicrobial respirators is likely to be different for enveloped viruses such as influenza, which are more likely to be rendered inactive by many antimicrobial agents. In this study, MS2 resuspended in 1% ATCC medium 271 was aerosolized and loaded onto coupons. Different results could be obtained using a medium containing different concentration of proteins, leading to higher or lower levels of protective factor. In this study, respirator coupons with MS2 were stored at only 2 different storage conditions. The antimicrobial effect of respirator coupons at additional storage conditions may be necessary to confirm the results.
Conclusion
Coupons from the 4 antimicrobial respirators tested in the study showed consistent MS2 loading levels similar to coupons from the N95 FFR controls. Coupons from all 4 antimicrobial respirators showed <1-log reduction of MS2 at 22°C and 30% RH storage condition up to 20 hours, which was not significantly different from the N95 control FFR coupons. At 37°C and 80% RH, the iodinated antimicrobial respirator coupons showed 3.7-log10 reduction of MS2 at 4 hours, which was significantly higher than the control. The viability of MS2 from the coupons from the other antimicrobial respirators was not significantly different from the controls. In general, high temperature and RH decreased MS2 log10 survivability of control and antimicrobial respirators. The decontamination effect seen with the coupons excised from the iodinated respirators suggests that iodine and high temperature and RH synergistically inactivated MS2. The results suggest that the ability for antimicrobial respirators to reduce the amount of viable MS2 is dependent on the storage conditions and the antimicrobial agent used. Further improvements in antimicrobial respirators are necessary to assure that they reduce the risks of handling after contamination.
Acknowledgments
The authors thank their NIOSH colleagues, Jay Parker and Lisa Delaney, and the antimicrobial respirator manufacturers that participated in the study for their critical review of the manuscript and suggestions.
Footnotes
Supported by NIOSH funding CAN No. 921 Z6PT.
Mention of commercial product or trade name does not constitute endorsement by the National Institute for Occupational Safety and Health. The findings and conclusions of this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Conflicts of interest: None to report.
References
- 1.CDC Interim domestic guidance on the use of respirators to prevent the transmission of SARS. Centers for Disease Control and Prevention, Department of Health and Human Services, Public Health Services, Tuberculosis Control Division, Atlanta, Georgia, 2003 [Google Scholar]
- 2.CDC Interim recommendations for the selection and use of protective clothing and respirators against biological agents. Centers for Disease Control and Prevention, Department of Health and Human Services, Tuberculosis Control Division, Atlanta, Georgia, 2004 [Google Scholar]
- 3.Hota B. Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis. 2004;39:1182–1189. doi: 10.1086/424667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boone S.A., Gerba C.P. Significance of fomites in the spread of respiratory and enteric viral disease. Appl Environ Microbiol. 2007;73:1687–1696. doi: 10.1128/AEM.02051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker J., Stevens D., Bloomfield S.F. Spread and prevention of some common viral infections in community facilities and domestic homes. J Appl Microbiol. 2001;91:7–21. doi: 10.1046/j.1365-2672.2001.01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brosseau L.M., McCullough N.V., Vesley D. Bacterial survival on respirator and filters and surgical masks. J Am Biol Safety Assoc. 1997;2:32–43. [Google Scholar]
- 7.Johnson B., Winters D.R., Shreeve T.R., Coffey C.C. Respirator filter reuse test using the laboratory simulant Mycobacterium tuberculosis (H37RA strain) J Am Biol Safety Assoc. 1998;3:105–116. [Google Scholar]
- 8.Reponen T.A., Wang Z., Willeke K., Grinshpun S.A. Survival of mycobacteria on N95 personal respirators. Infect Cont Hosp Epidemiol. 1999;20:237–241. doi: 10.1086/501618. [DOI] [PubMed] [Google Scholar]
- 9.Pasanen A.L., Keinanen J., Kalliokoski P., Martikainen P.I., Ruuskanen J. Microbial growth on respirator filters from improper storage. Scand J Work Environ Health. 1993;19:421–425. doi: 10.5271/sjweh.1452. [DOI] [PubMed] [Google Scholar]
- 10.Rengasamy A., Zhuang Z. Berry Ann R. Respiratory protection against bioaerosols: literature review and research needs. Am J Infect Control. 2004;32:345–354. doi: 10.1016/j.ajic.2004.04.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casanova L., Alfano-Sobsey E., Rutala W.A., Weber D.J., Sobsey M. Virus transfer from personal protective equipment to healthcare employees' skin and clothing. Emerg Infect Dis. 2008;14:1291–1293. doi: 10.3201/eid1408.080085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwaltney J.M., Jr., Hendley J.O. Transmission of experimental rhinovirus infection by contaminated surfaces. Am J Epidemiol. 1982;116:828–833. doi: 10.1093/oxfordjournals.aje.a113473. [DOI] [PubMed] [Google Scholar]
- 13.Gwaltney J.M., Jr., Moskalski P.B., Hendley J.O. Hand-to-hand transmission of rhinovirus colds. Ann Intern Med. 1978;88:463–467. doi: 10.7326/0003-4819-88-4-463. [DOI] [PubMed] [Google Scholar]
- 14.Bean B., Moore B.M., Sterner B., Peterson L.R., Gerding D.N., Balfour H.H.J. Survival of influenza viruses on environmental surfaces. J Infect Dis. 1982;146:47–51. doi: 10.1093/infdis/146.1.47. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine . Preparing for an influenza pandemic: personal protective equipment for healthcare workers. In: Goldfrank L.R., Liverman C.T., editors. National Academies Press (U.S.); Washington, DC: 2007. [Google Scholar]
- 16.Institute of Medicine . National Academies Press (U.S.); Washington, DC: 2006. Reusability of facemasks during an influenza pandemic: facing the flu. Committee on the Development of Reusable Facemasks for Use During an Influenza Pandemic, Institute of Medicine (U.S.). Board on Health Sciences Policy. [Google Scholar]
- 17.Li Y., Leung P., Yao L., Song Q.W., Newton E. Antimicrobial effect of surgical masks coated with nanoparticles. J Hosp Infect. 2006;62:58–63. doi: 10.1016/j.jhin.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Lee J.H., Wu C.Y., Wysocki K.M., Farrah S., Wander J. Efficacy of iodine-treated biocidal filter media against bacterial spore aerosols. J Appl Microbiol. 2008;105:1318–1326. doi: 10.1111/j.1365-2672.2008.03855.x. [DOI] [PubMed] [Google Scholar]
- 19.Ratnesar-Shumate S., Wu C.-Y., Wander J., Lundgren D., Farrah S., Lee J.-H. Evaluation of physical capture efficiency and disinfection capability of an iodinated biocidal filter medium. Aerosol Air Qual Res. 2008;8:1–18. [Google Scholar]
- 20.Heimbuch BK, Laventure G, McDonald R, Burr E, Proudfoot E, Wander J: Antimicrobial efficiency of iodinated individual protection filters. Report for U.S. Air Force Research Laboratory Tyndall Air Force Base Contract No. AFRL-ML-TY-TP-2004-4561, Panama City, Florida. U.S. Air Force Research Laboratory; 2004.
- 21.Heimbuch BK, Wander J: Bioaerosol challenges to antimicrobial surface treatments: Enhanced filter efficacy against MS2 coli phage of air filter media coated with polystyrene-4-methyltrimethylammonium triiodide. Report for U.S. Air Force Research Laboratory Tyndall Air Force Base Contract No. AFRL-ML-TY-TP-2006-4527, Panama City, Florida. U.S. Air Force Research Laboratory; 2006.
- 22.Eninger R.M., Adhikari A., Reponen T., Grinshpun S.A. Differentiating between physical and viable penetrations when challenging respirator filters with bioaerosols. Clean--Soil, Air, Water. 2008;36:615–621. [Google Scholar]
- 23.Lee J.H., Wu C.Y., Lee C.N., Anwar D., Wysocki K.M., Lundgren D.A., Farrah S., Wander J., Heimbuch B.K. Assessment of iodine-treated filter media for removal and inactivation of MS2 bacteriophage aerosols. J Appl Microbiol. 2009 doi: 10.1111/j.1365-2672.2009.04375.x. DOI: 10.1111/j.1365-2672.2009.04375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lala N.L., Ramaseshan R., Bojun L., Sundarrajan S., Barhate R.S., Ying-Jun L. Fabrication of nanofibers with antimicrobial functionality used as filters: protection against bacterial contaminants. Biotechnol Bioeng. 2007;97:1357–1365. doi: 10.1002/bit.21351. [DOI] [PubMed] [Google Scholar]
- 25.Yoon K.Y., Byeon J.H., Park C.W., Hwang J. Antimicrobial effect of silver particles on bacterial contamination of activated carbon fibers. Environ Sci Technol. 2008;42:1251–1255. doi: 10.1021/es0720199. [DOI] [PubMed] [Google Scholar]
- 26.Prugh A, Calomiris JJ: Inactivation of Bacillus anthracis spores delivered as liquid suspension or aerosol to self-decontaminating fabric. Air Force Research Laboratory Human Effectiveness Directorate Biosciences and Protection Division Counter-Proliferation Branch Contract No. AFRL-HE-WP-TP-2006-0060, Aberdeen Proving Ground, Maryland. U.S. Air Force Research Laboratory; 2006.
- 27.Fisher E., Rengasamy S., Viscusi D.J., Vo E., Shaffer R.E. Development of a test system to apply virus containing particles to air permeable materials for the evaluation of decontamination procedures for filtering facepiece respirators. Appl Environ Microbiol. 2009;75:1500–1507. doi: 10.1128/AEM.01653-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woolwine J.D., Gerberding J.L. Effect of testing method on apparent activities of antiviral disinfectants and antiseptics. Antimicrob Agents Chemother. 1995;39:921–923. doi: 10.1128/aac.39.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubovi E.J., Akers T.G. Airborne stability of tailless bacterial viruses S-13 and MS-2. Appl Microbiol. 1970;19:624–628. doi: 10.1128/am.19.4.624-628.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trouwborst T., De Jong J.C. Interaction of some factors in the mechanism of inactivation of bacteriophage MS2 in aerosols. Appl Microbiol. 1973;26:252–257. doi: 10.1128/am.26.3.252-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiessen R.J. Filtration of respired gases: theoretical aspects. Respir Care Clin. 2006;12:183–201. doi: 10.1016/j.rcc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Rutala W.A., Weber D.J. Registration of disinfectants based on relative microbicidal activity. Am J Infect Control. 2004;25:333–341. doi: 10.1086/502401. [DOI] [PubMed] [Google Scholar]
- 33.Schaffer F.L., Soergel M.E., Straube D.C. Survival of airborne influenza virus: effects of propagating host, relative humidity, and composition of spray fluids. Arch Virol. 1976;51:263–273. doi: 10.1007/BF01317930. [DOI] [PubMed] [Google Scholar]
- 34.Lowen A.C., Mubareka S., Steel J., Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3:1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez M.E., O'Brien R.T. Mechanisms of inactivation of poliovirus by chlorine dioxide and iodine. Appl Environ Microbiol. 1982;44:1064–1071. doi: 10.1128/aem.44.5.1064-1071.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brion G.M., Silverstein J. Iodine disinfection of a model bacteriophage, MS2, demonstrating apparent rebound. Water Res. 1999;33:169–179. [Google Scholar]
- 37.Li C.H., Lyons W.R., Simpson M.E., Evans H.M. Inactivation of pituitary lactogenic hormone by iodine. Science. 1940;91:530–531. doi: 10.1126/science.91.2370.530. [DOI] [PubMed] [Google Scholar]
- 38.Tennen R., Setlow B., Davis K.L., Loshon C.A., Setlow P. Mechanisms of killing of spores of Bacillus subtilis by iodine, glutaraldehyde and nitrous acid. J Appl Microbiol. 2000;89:330–338. doi: 10.1046/j.1365-2672.2000.01114.x. [DOI] [PubMed] [Google Scholar]
- 39.Pal A., Min X., Yu L.E., Pehkonen Ray M.B. Photocatalytic inactivation of bioaerosols by TiO2 coated membrane. Int J Chem Reactor Eng. 2005;3:A45. [Google Scholar]
- 40.Kuhn K.P., Chaberney I.F., Massholder K., Stickler M., Benz V.W., Sonntag H.-G. Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UVA light. Chemosphere. 2003;53:71–77. doi: 10.1016/S0045-6535(03)00362-X. [DOI] [PubMed] [Google Scholar]
- 41.Tseng C.-C., Li C.S. Inactivation of virus-containing aerosols by ultraviolet germicidal irradiation. Aero Sci Tech. 2005;39:1136–1142. [Google Scholar]
- 42.McDevitt J.J., Lai K.M., Rudnick S.N., Houseman E.A., First M.W., Milton D.K. Characterization of UVC light sensitivity of vaccinia virus. Appl Environ Microbiol. 2007;73:5760–5766. doi: 10.1128/AEM.00110-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nunomura A., Honda K., Takeda A., Hirai K., Zhu X., Smith M.A. Oxidative damage to RNA in neurodegenerative diseases. J Biomed Biotech. 2006;2006:1–6. doi: 10.1155/JBB/2006/82323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tseng C.-C., Li C.-S. Ozone for inactivation of aerosolized bacteriophages. J Aero Sci Tech. 2006;40:683–689. [Google Scholar]
- 45.Butkus M.A., Labare M.P., Starke J.A., Moon K., Talbot M. Use of aqueous silver to enhance inactivation of coliphage MS-2 by UV disinfection. Appl Environ Microbiol. 2004;70:2848–2853. doi: 10.1128/AEM.70.5.2848-2853.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park H.-J., Kim Y.Y., Kim J., Lee J.-H., Hahn J.-S., Gu M.B. Silver-ion-mediated reactive oxygen species generation affecting bacterial activity. Water Res. 2009;43:1027–1032. doi: 10.1016/j.watres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Shimizu F., Shimizu Y., Kumagai K. Specific inactivation of herpes simplex virus by silver nitrate at low concentrations and biological activities of the inactivated virus. Antimicrob Agents Chemother. 1976;10:57–63. doi: 10.1128/aac.10.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]