Abstract
Purpose
To explore the clinical, microbiological and immunological features of patients with fever and thrombocytopenia.
Methods
Patients with unexplained fever and thrombocytopenia were enrolled. Viruses were detected using real-time PCR, and bacteria were measured by culturing methods. Serum cytokines, platelet antibody IgG (PA-IgG) and Helicobacter pylori (HP) were detected using ELISA.
Results
Pathogens were detected in 74.68% of patients, which included single fungal/viral/bacterial infection and multiple infection. The pathogens could not be unidentified in 25.32% of cases. Cytokines including Interleukin (IL)-6, IL-10, interferon-γ(IFN-γ), platelet activating factor (PAF) and PA-IgG were significantly higher in patients as compared to healthy controls (P < .01 or P < .05). Principal component analyses extracted four groups of parameters that have a strong positive predicting value, revealing that disease status evaluation would be more accurate if we combined the platelet parameters and inflammatory biomarkers. While event-free survival (EFS) that indicates the time of platelet elevated after therapy was the highest in patients with single bacterial or fungal infection, EFS was affected by the levels of cytokines and PA-IgG.
Conclusions
Differences in immune function may be the main factors affecting the prognosis of patients with fever and thrombocytopenia, while treatment based on precise etiological diagnosis is important for therapeutic efficacy.
Keywords: Thrombocytopenia, Fever, Cytokines, Platelet antibody IgG
Highlights
-
•
Pathogens were detected in 74.68% of patients with fever and thrombocytopenia.
-
•
The IL-6, IL-10, IFN-γ, PAF and PA-IgG levels were significantly higher in patients.
-
•
The event-free survival was affected by the levels of cytokines and PA-IgG.
-
•
It's better to assess disease status by combining clinical parameters and biomarkers.
1. Introduction
Fever and thrombocytopenia are frequent symptoms in patients with viral infection. Severe fever with thrombocytopenia syndrome (SFTS), caused by a novel bunyavirus, was initially identified in 2009. SFTS produces a broad spectrum of clinical manifestations that range from acute self-limited febrile illness to severe disease, with a reported fatality rate varying between 12% and 30% [1]. Due to its poor prognosis and higher mortality, SFTS has quickly become an emerging infectious disease that has aroused extensive surveillance in public health. Currently, SFTS, together with meningitis syndrome and respiratory syndrome, are the primary three syndromes that are monitored in clinic. Unlike other inflammatory disease, the etiology of fever and thrombocytopenia is quite complicated; thrombocytopenia may arise due to a lack of platelet production, retention of platelets (PLTs) in the spleen, platelet destruction, or increased use and dilution of PLTs. In clinic, most thrombocytopenia patients are diagnosed as primary immune thrombocytopenia (ITP) that is defined as a peripheral blood platelet count <100 × 109/l in the absence of any obvious cause of thrombocytopenia [2]. Because the etiology of ITP is so complicated that physicians seldom relies on its etiology to formulate treatment plans, then, it is difficult to explain why some patients have poor therapeutic efficacy.
Fever is a common feature and consequence for ITP patients with viral infection. Evidence regarding the cross-reactivity of virus-specific antibodies with normal platelet antigens was firstly obtained in patients developing ITP after contracting human immune deficiency virus [3] and varicella-zoster virus infection [4]. Since then, it has been discovered that thrombocytopenia and viral infections occur in succession. Thrombocytopenia as a major clinical sign is found in some viral infectious diseases including Zika virus, HIV-1, hepatitis B/hepatitis C virus, dengue virus, Epstein-Barr virus, B19 virus, and novel bunyavirus [1,[5], [6], [7], [8], [9], [10], [11], [12]]. A previous study showed that patients with influenza virus A/B or parainfluenza virus 1 infections tend to have a lower PLT count than patients who did not have respiratory viral infection, while patients with human metapneumovirus, parainfluenza virus 2, coronavirus 229, or adenovirus infections did not show significant changes in the PLT count [13]. In some patients, thrombocytopenia caused by viral infection was transient without serious bleeding and spontaneously resolved within a few weeks without treatment [14]; however, other patients have a severe bleeding syndrome and worse prognosis. The mechanism remains to be elucidated.
Thrombocytopenia is also a common complication during or after bacterial infections. Direct interaction between platelets and bacteria may contribute to thrombocytopenia [15]. Helicobacter pylori (HP) has proven to be the most important bacterium causing ITP. Additionally, it was observed that salmonella survived and replicated in macrophages that spread to the lymphatic vessels, spleen, and bone marrow, and thrombocytopenia may be associated with hemophagocytic histiocytes in the bone marrow [16]. Fecal microbial transplantation may also lead to thrombocytopenia during inflammation [17]. Staphylococcus aureus activates platelets through several surface-expressed proteins, and binds to adhered platelets to cause platelet activation and aggregation, resulting in thrombocytopenia [18]. Unfortunately, the role of bacterial infection in thrombocytopenia is often clinically ignored.
A viral/bacterial antigen may be recognized as a similar platelet antigen, a process termed molecular mimicry, which then gives rise to cross-reactive anti-platelet autoantibodies [19,20]. Platelets with bound autoantibodies are subsequently recognized by phagocytes bearing Fcγ –receptors (FcγRs), which results in enhanced antibody-mediated platelet phagocytosis and destruction primarily in the spleen [21]. In patients with ITP autoantibodies targeting platelet surface glycoproteins, primarily GPIIb-IIIa and GPIb-IX, lead to Fc-dependent clearance via macrophages [22]. Platelets express FccRIIA, an IgG receptor used to identify immune complexes (ICs). FccRIIA is involved in the initiation of strong effectors functions in leukocytes, including cytokine release, antibody-dependent cell-mediated pathogen killing, and ICs internalization, which is critical for immune and inflammatory responses. Virus or bacteria can activate platelets via FccRIIA that plays an important role in the interaction of platelet and pathogen infection, and the polymorphism of FccRIIA-131 directly affects the susceptibility and severity of infection [23]. However, the relationship among the platelet autoantibodies, cytokines, and other parameters associated infection is still not clear.
Many questions regarding the pathophysiology, mechanism, diagnosis, natural history, and management of ITP remain to be resolved [24,25]. The obscure etiology and mechanisms of thrombocytopenia greatly affect its therapeutic efficacy. First-line therapy for ITP consists of corticosteroids with or without intravenous immunoglobulin [2], and some patients respond to this while others do not. Nowadays, there are no valuable biomarkers to predict the therapeutic efficacy. Etiologic diagnosis of thrombocytopenia may be beneficial for treatment, to some ITP caused by viral infections, accurate etiologic diagnosis can reduce the amount of antibiotics used. Unfortunately, the diagnosis of ITP is still based on exclusion [26]. The relationships among the etiology of fever and thrombocytopenia, the intrinsic immune mechanism, and therapeutic efficacy remain to be elucidated. To clarify the above issues, patients with unexplained fever and thrombocytopenia were enrolled in this study. The associations among the etiology, platelet autoantibody, cytokine expression, and therapeutic efficacy were investigated with the aim of determining valuable parameters that mirror the pathogenesis and predict therapeutic efficacy of thrombocytopenia.
2. Material and methods
2.1. Patients and sample collection
During the period of January 2016 to June 2017, patients with unexplained fever (body temperature >37.6 °C) and thrombocytopenia (platelet count<100 × 10 9/l) were enrolled in our study. Patients with solid tumors, leukemia, liver disease, or upper and lower gastrointestinal or other traumatic bleeding were excluded. Ten healthy volunteers were selected as the controls. The study was approved by the Ethics Committee of the First Affiliated Hospital of Shantou University Medical College, and informed consent was obtained from all patients. All samples were collected at the onset of disease and before treatment.
2.2. Real-time PCR detection of viruses
The nucleic acid from the serum samples was extracted using a viral DNA/RNA magnetic bead extraction kit (TIANLONG, China) and NP968 nucleic acid extraction instrument (TIANLONG, China). A single-tube TaqMan® real-time reverse transcription polymerase chain reaction (PCR) strategy (AgPath-ID™ One-Step RT-PCR kit from Applied Biosystems, USA) was used to detect viruses. Bunyavirus was detected using the severe fever with thrombocytopenia syndrome virus (SFTSV) Bunyavirus Real Time RT-PCR Kit (Liferiver, China), and human parvovirus B19 was detected using the Human Parvovirus B19 Nucleic Acid Detection Kit (Daangene, China). PCR amplification was performed using a 7500 Real-Time PCR System (Applied Biosystems, USA), according to the manufacturer's instructions.
2.3. Fungus/bacteria detection
For bacteria identification, the blood and sputum specimens of patients were cultured according to National Standard Protocols of China. Specimens were inoculated in blood agar, eosin methylene blue agar, and chocolate agar and incubated for 24–48 h at 37 °C. Colony identification was undertaken using a VITEK 2 Compact system (Biomérieux, France). Helicobacter pylori was detected using a commercial kit (Human Anti-Helicobacter Pylori Antibody (IgG) ELISA kit (Cusabio, China)).
2.4. Detection of PA-IgG and cytokines in serum
The PA-IgG and six cytokines PAF, interleukin (IL)-6, IL-10, IL-17A, IL-12/23P40, and IFN-γ in serum samples were detected using a Human platelet-associated IgG antibody (PA-IgG) ELISA kit (Shanghai Jingkang, China), Human Platelet Activating Factor ELISA Kit (Dakewe, China) and Human IL-6/IL-10/IL-17A/IL-12/23P40/IFN-γ precoated ELISA Kit (CUSABIO, China), according to the manufacturer's instructions. The absorbance values of six cytokines were measured using an Infinite M200 Pro microplate reader (Tecan, Switzerland) and then converted to concentration values according to the reagent instructions. The sensitivity of PA-IgG, PAF, IL-6, IL-10, IL-17A, IL-12/23P40, and IFN-γ detection was 0.1 μg/l, 1.17 ng/ml, 2 pg/ml, 5 pg/ml, 31.3 pg/ml, 15 pg/ml, and 5 pg/ml, respectively.
2.5. Statistical analyses
Statistical analyses were performed using SPSS 19.0 software. Continuous variables with normal distributions were used mean ± standard deviation (SD) to describe and compare using multiple variance of multiple samples. Medians and interquartile ranges (IQRs) were used to describe non-normal distribution, with comparisons based on the Mann-Whitney U test between any two groups. The correlations between serum cytokine and other parameters were obtained using the non-parametric rank-based Spearman's correlation coefficient. Principal component analysis (PCA) was performed to obtain the significant principal components that most accurately reflect the status of thrombocytopenia. Receiver operating characteristic (ROC) curve analysis was used to determine and distinguish the usefulness of biomarkers. EFS was calculated from the date of complete recovery (platelet count return to normal range) until the first event (any cause of recurrence or death) or until the last follow-up. Survival curves were calculated using the Kaplan–Meier method, and survival was compared using Mantel's log-rank test. Thrombocytopenia with or without improvement were analyzed using binary logistic regression. The level of statistical significance was set at 5%.
3. Results
3.1. The basic clinical characteristics of the patients
In Table 1 , the 79 patients included 48 males and 31 females. Four cases occurred in children with a mean age (years) of 7.75 (1–14 years), and 75 cases occurred in adults with a mean age of 59.87 (17–93 years). Various body temperatures (T) were obtained from the patients, including 11 patients with normal T (37.6–37.9 °C), 44 (55.7%) patients with moderate T (38.0–38.9 °C), and 24 cases with high T (≥39.0 °C). Pathogen detection showed that a single virus infection was found in 7 patients (8.86%), which mainly consisted of three types of viruses: 229E, PIV2, and Flu A. Single fungus infection was found in 5 cases, and single bacterial infection was found in 24 cases of Helicobacter pylori(HP), 3 cases of Acinetobacter baumannii, 3 cases of Acinetobacter baumannii concurrent with HP, 1 case of Haemophilus influenzae and 1 case of Serratia marcescens concurrent with HP. Multiple-infection was found in 15 patients (18.99%) (shown in Table 1). The pathogens could not be measured in 20 cases (25.32%). Of these 79 patients, 52 patients treated with antimicrobials, and most used antibiotics were quinolones, followed by cephalosporins and beta-lactams. Few patients were treated with anti-viral drugs.
Table 1.
Patient's general characteristics and pathogen detection results.
| Cases | Percentage | ||
|---|---|---|---|
| Total | 79 | ||
| Age | |||
| 0–14 | 4 | 5.06% | |
| ≥15 | 75 | 94.94% | |
| Gender | |||
| Male | 48 | 60.76% | |
| Female | 31 | 39.24% | |
| Temperature (°C) | |||
| 37.6–37.9 | 11 | 13.92% | |
| 38.0–38.9 | 44 | 55.70% | |
| 39.0–39.9 | 16 | 20.25% | |
| ≥40.0 | 8 | 10.13% | |
| Pathogen infection | |||
| Single virus | PIV2 | 3 | 3.80% |
| 229E | 2 | 2.53% | |
| Flu A | 2 | 2.53% | |
| Single fungus | 5 | 6.33% | |
| Single bacteria | Helicobacter pylori | 24 | 30.38% |
| Acinetobacter baumannii | 3 | 3.80% | |
| Acinetobacter baumannii + HP | 3 | 3.80% | |
| Haemophilus influenzae | 1 | 1.27% | |
| Serratia marcescens + HP | 1 | 1.27% | |
| Multiple pathogens | 229E+ HP | 6 | 7.59% |
| PIV2+ HP | 3 | 3.80% | |
| PIV2 + Fungus | 1 | 1.27% | |
| 229E + Fungus+ HP | 1 | 1.27% | |
| Flu A + Fungus+ HP | 1 | 1.27% | |
| PIV2 + Klebsiella pneumoniae | 1 | 1.27% | |
| PIV2 + Acinetobacter baumannii + HP | 1 | 1.27% | |
| Flu A+ Escherichia coli + HP | 1 | 1.27% | |
| Unidentified | 20 | 25.32% | |
Abbreviations: 229E, coronaviruses 229E; PIV2, parainfluenza viruses 2; Flu A, influenza viruses types A; HP, Helicobacter pylori.
We then divided all patients into four groups according to the pathogens detected (shown in Table 2 ): single viral group (7 cases), single fungal/bacterial (f/b) group (37 cases), multiple pathogens group (15 cases), and unidentified pathogen group (20 cases). Males were more than females, and average age ranged from 54 to 60 years in each group.
Table 2.
The clinical data among different pathogens infected with fever and thrombocytopenia patients.
| Single virus | Single f/b | Multiple pathogens | Unidentified | |
|---|---|---|---|---|
| Number of cases | 7 | 37 | 15 | 20 |
| Improved case after healing | 3 | 19 | 5 | 8 |
| Deaths cases | 1 | 2 | 2 | 4 |
| Gender (M/F) | 5/2 | 20/17 | 9/6 | 14/6 |
| Age | 57.14 ± 16.24 | 59.22 ± 19.58 | 55.53 ± 20.51 | 54.85 ± 23.10 |
| Temperature (°C) | 38.81 ± 0.78 | 38.64 ± 0.81 | 38.85 ± 0.97 | 38.45 ± 0.51 |
| WBC (×10^9/l) | 7.43(5.99–9.16) | 9.81(5.25–11.29) | 9.58(8.46–13.20) | 7.07(3.79–11.83) |
| LY (%) | 8.64(2.96–19.2) | 9.28(4.10–14.01)⁎ | 13.10(5.55–15.32) | 18.37(6.35–26.33) |
| N (%) | 84.97(71.52–95.22) | 84.28(71.07–92.58) | 82.70(76.21–86.84) | 70.72(59.18–87.19) |
| RBC (×10^12/l) | 3.54 ± 0.98 | 3.54 ± 0.80 | 3.87 ± 0.80 | 3.77 ± 1.06 |
| HB (g/l) | 98.29 ± 23.82 | 105.65 ± 23.16 | 118.00 ± 23.47 | 113.05 ± 29.61 |
| PLT (×10^9/l) | 75.86 ± 14.98▲ | 64.57 ± 21.12 | 52.27 ± 22.78 | 61.85 ± 26.10 |
| MPV (fl) | 9.50 ± 0.86 | 12.44 ± 2.69 | 10.36 ± 1.42 | 9.79 ± 1.69 |
| PDW (fl) | 17.57 ± 0.89 | 17.07 ± 2.20 | 16.19 ± 2.33 | 16.77 ± 1.70 |
| CRP (mg/l) | 181.4(107.00–196.00) | 93.40(28.30–138.50) | 128.44(110.00–140.00) | 75.70(13.25–109.55) |
| PCT (ng/ml) | 1.72(0.54–14.45) | 6.41(2.56–195.46) | 7.75(0.61–61.87) | 1.09(0.23–8.27) |
| ALT (U/l) | 23.0(19.00–62.00) | 29.00(15.50–142.00) | 50.00(24.00–436.00) | 56.50(26.50–118.50) |
| AST (U/l) | 83.00(28.00–129.50) | 66.00(23.00–235.00) | 59.00(34.00–789.00) | 83.50(33.25–133.50) |
Abbreviations: f/b, fungus/bacteria; M, Male; F, Female; WBC, white blood cell; LY, lymphocyte; N, neutrophil; RBC, red blood cell; Hb, hemoglobin; PLT, platelet; MPV, mean platelet volume; PDW, platelet distribution width; CRP, C-reaction protein; PCT, procalcitonin; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
P<0.05 when the single f/b group is compared with unidentified group.
P<0.05 when the single virus group is compared with multiple pathogens group.
In Table 2, the improvement rate for the single f/b infectious group was 51.35% (19/37), the single viral group was 42.86% (3/7), the unidentified pathogen group was 40.00% (8/20), and the multiple infectious group was 33.33% (5/15). In the comparison of laboratory parameters, the white blood cell (WBC) counts in the single f/b group and multiple infectious group were higher than those in the single viral and unidentified pathogen group, and the highest lymphocyte count was found in the unidentified pathogen group (P < .05). The mean PLT count of the four groups was <76.0 × 10^9/l, and the mean PLT count of the multiple pathogens group was the lowest (52.27 × 10^9/l), which was significantly lower than that in the single virus group (P < .05). The mean platelet volume (MPV) count was the highest in the single f/b group (mean = 12.44 fl), which was higher than that in the other three groups. The mean C-reactive protein (CRP) in thrombocytopenia groups was higher than the normal value (CRP <0.05 mg/l), similarly, the median PCT in the four groups were higher than the clinical limit of detection (PCT <0.1 ng/ml), with the median value of the multiple pathogens group being the highest (7.75) (Table 2). The clinical parameters of patients with or without HP infection were also analyzed. The median LY (%) in HP-positive patients were lower than those in negative patients (P < .05). The median PAF concentration was 2.5 times higher in HP-positive patients than that in the negative group.
3.2. The serum PA-IgG and cytokines in different groups
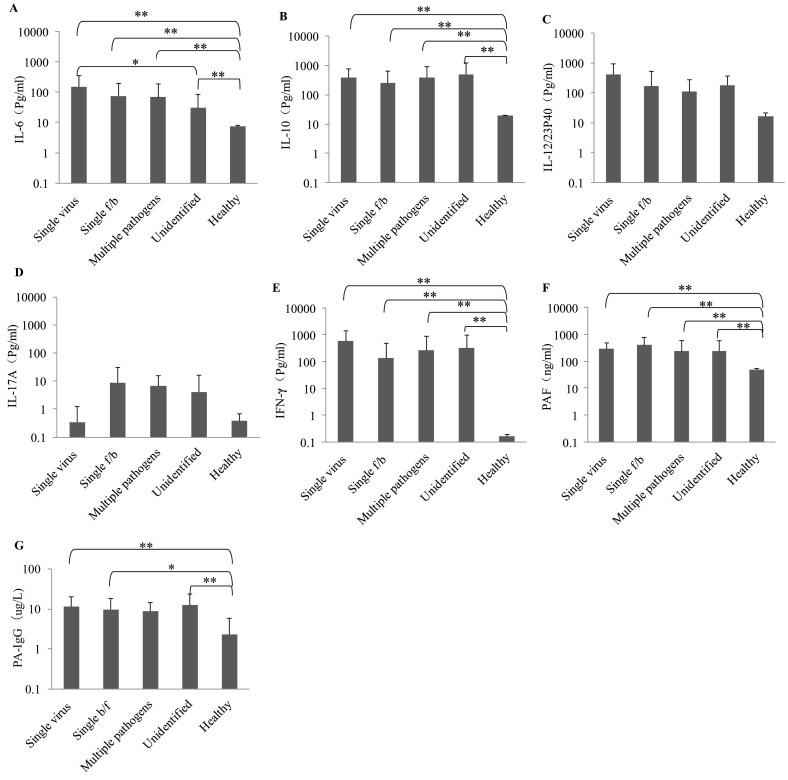
Among the six cytokines measured in this study, there were no significant differences for serum IL-12/23P40 and IL-17A among five groups (P > .05) (Fig. 1 ). However, we found that serum levels of IL-6, IL-10, and IFN-γ, as well as PAF were significantly higher in all patient groups as compared to those in healthy controls (P < .01). Compared to the unidentified pathogen group, IL-6 was significantly higher in the single viral group with a mean concentration of 153.03 pg/ml (P < .05). The highest level of IL-10 was found in the unidentified pathogen group (501.67 pg/ml). The IFN-γ concentration (582.59 pg/ml) was the highest in the single viral group among the five groups. The highest PAF value was found in the single f/b group (400.02 ng/ml). Similarly, the concentration of PA-IgG of healthy people was lower than those of fever and thrombocytopenia patients, and there was a significant difference when compared with three of patients' groups (healthy vs. single virus: P < .01; healthy vs. single f/b: P < .05; healthy vs. unidentified: P < .01) (Fig. 1).
Fig. 1.
The serum cytokines and PA-IgG levels in different groups and comparison (Single virus: n = 7; Single f/b: n = 37; Multiple pathogens: n = 15; Unidentified: n = 20; Healthy: n = 10). The bars represent the mean of each group of data, and the vertical line represents the error line and the end is the standard deviation value. * and ** indicate the statistical significant at P < .05 and P < 0. 01, respectively.
3.3. The correlations between cytokines and platelet parameters in patients with fever and thrombocytopenia
Inner associations were also analyzed between cytokines and the platelet parameters. In this study, the PLT quantity was associated with platelet distribution width (PDW) (r = 0.231, P < .05) and IL-10 (r = 0.331, P < .01). A correlation exists between IL-6 with IL-10 (r = 0.458, P < .01), with PAF (r = 0.370, P < .01), with CRP (r = 0.667, P < .01), and with PCT (r = 0.440, P < .01). The correlations were found between IL-10 with IL-12/23P40 (r = 0.298, P < .01), IFN-γ (r = 0.446, P < .01), PAF (r = 0.257, P < .05) and PCT (r = 0.379, P < .01). Additionally, IL-12/23P40 was correlated with IFN-γ (r = 0.447, P < .01), and PCT was correlated with CRP (r = 0.410, P < .01). No correlations were tested between IL-17A and other cytokines (Table 3 ).
Table 3.
The spearman correlation coefficients of six cytokines in patients with fever and thrombocytopenia.
| IL-6 (pg/ml) | IL-10 (pg/ml) | IL-12/23P40 (pg/ml) | IL-17A (pg/ml) | IFN-γ (pg/ml) | PAF (ng/ml) | PLT (×10^9/l) | MPV (fl) | PDW (fl) | CRP (mg/l) | PCT (ng/ml) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | – | ||||||||||
| IL-10 | r = 0.458** | – | |||||||||
| P = .000 | |||||||||||
| IL-12/23P40 | r = 0.009 | r = 0.298** | – | ||||||||
| P = .937 | P = .008 | ||||||||||
| IL-17A | r = −0.018 | r = 0.167 | r = 0.116 | – | |||||||
| P = .875 | P = .142 | P = .310 | |||||||||
| IFN-γ | r = 0.104 | r = 0.446** | r = 0.447** | r = 0.092 | – | ||||||
| P = .360 | P = .000 | P = .000 | P = .419 | ||||||||
| PAF | r = 0.370** | r = 0.257* | r = 0.062 | r = −0.09 | r = −0.059 | – | |||||
| P = .001 | P = .022 | P = .589 | P = .433 | P = .604 | |||||||
| PLT | r = −0.040 | r = −0.331** | r = 0.212 | r = 0.049 | r = −0.065 | r = 0.125 | – | ||||
| P = .726 | P = .003 | P = .061 | P = .668 | P = .571 | P = .271 | ||||||
| MPV | r = −0.129 | r = 0.280 | r = −0.137 | r = 0.012 | r = 0.019 | r = 0.024 | r = −0.179 | – | |||
| P = .258 | P = .807 | P = .229 | P = .915 | P = .869 | P = .831 | P = .115 | |||||
| PDW | r = 0.080 | r = −0.007 | r = −0.077 | r = −0.024 | r = −0.057 | r = 0.156 | r = −0.231* | r = −0.058 | – | ||
| P = .486 | P = .952 | P = .500 | P = .836 | P = .617 | P = .171 | P = .040 | P = .614 | ||||
| CRP | r = 0.667** | r = 0.148 | r = −0.083 | r = 0.028 | r = −0.057 | r = 0.256 | r = −0.031 | r = −0.134 | r = 0.203 | – | |
| P = .000 | P = .193 | P = .465 | P = .807 | P = .620 | P = .023* | P = .787 | P = .239 | P = .072 | |||
| PCT | r = 0.440** | r = 0.379** | r = −0.017 | r = −0.195 | r = 0.104 | r = 0.185 | r = −0.206 | r = −0.118 | r = 0.046 | r = 0.410** | – |
| P = .000 | P = .001 | P = .880 | P = .086 | P = .363 | P = .103 | P = .069 | P = .301 | P = .687 | P = .000 |
The level of statistical significance (P) was set at 5%.
⁎ and ⁎⁎ indicate the statistically significant spearman correlation at the 0.05 level and 0.01 level, respectively.
3.4. Principal component analysis (PCA)
PCA method was exploited to determine whether the parameters profile reflects disease status in patients with fever and thrombocytopenia. PCA extracted four important principal components with eigenvalues >1 that explained 58.71% of the total variance of the data set. It can be seen from component 1 that IL-6, CRP, and PCT have a strong positive loading. Component 2 includes IL-10, IFN-γ, and PLT, where PLT exert a strong negative loading. Similarly, component 3 included IL-12/23P40 and PDW, component 4 included PA-IgG, IL-17A, PAF and MPV, while PDW, PAF and MPV contributed strong negative loadings (Table 4 ). Our results showed that cytokine loading is larger than other parameters in reflecting disease status (component 1: IL-6 = 0.873 > CRP = 0.767/PCT = 0.758; component 2: IL-10 = 0.674/IFN-γ = 0.714 > PLT = −0.615; component 3: IL-12/23P40 = 0.783 > PDW = −0.678; component 4: PA-IgG = 0.811 > IL-17A = 0.659/PAF = −0.458 > MPV = −0.302). Therefore, to patients with fever and thrombocytopenia, the disease status evaluation would be more accurate if we combined the platelet parameters and inflammatory biomarkers.
Table 4.
Loading scores of variables on significant PCA that combined components to predict cytokine profile, PA-IgG and clinical indicators in thrombocytopenia, and receiver operating characteristic curve analysis of components.
| Variables | Components |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| PA-IgG (μg/l) | 0.044 | 0.039 | −0.016 | 0.811 |
| IL-6 (pg/ml) | 0.873 | −0.013 | 0.012 | 0.025 |
| IL-10 (pg/ml) | 0.462 | 0.674 | 0.076 | −0.024 |
| IL-12/23P40 (pg/ml) | −0.010 | 0.250 | 0.783 | 0.011 |
| IL-17A (pg/ml) | −0.143 | −0.041 | −0.009 | 0.659 |
| IFN-γ (pg/ml) | −0.125 | 0.714 | 0.118 | 0.030 |
| PAF (ng/ml) | 0.421 | 0.058 | 0.194 | −0.458 |
| PLT (×10^9/l) | 0.032 | −0.615 | 0.521 | 0.055 |
| MPV (fl) | −0.168 | −0.028 | −0.205 | −0.302 |
| PDW (fl) | 0.158 | 0.111 | −0.678 | 0.028 |
| CRP (mg/l) | 0.767 | −0.147 | −0.124 | −0.053 |
| PCT (ng/ml) | 0.758 | 0.187 | −0.118 | −0.034 |
| Receiver operating characteristic curve analysis | ||||
| AUC | 0.521 | 0.358 | 0.505 | 0.564 |
| P | 0.745 | 0.031 | 0.937 | 0.333 |
| Cut off value | 0.150 | −0.252 | 0.199 | 0.282 |
| Sensitivity (%) | 40 | 65.7 | 97.1 | 60 |
| Specificity (%) | 75 | 9.1 | 22.7 | 68.2 |
Abbreviations: AUC, areas under curve.
3.5. Risk evaluation in patients with/without improvement after treatment
The factors that significantly influenced the prognosis of patients were also analyzed, these were age, PLT quantities, PCT, and IL-17A. Among them, PLT quantities, PCT, and IL-17A were risk factors (OR > 1.0), and age was a protective factor (OR < 1.0) (Table 5 ). We then used Kaplan-Meier survival curves to further assess the suitability of six cytokines and PA-IgG as prognostic factors and to compare the prognosis of different types of pathogen infections. We conducted a follow-up of the patient from hospital admittance to discharge, and survival information was collected. The levels of cytokines and PA-IgG were considered as a stratification variable for survival time analysis. Our data showed that patients with single f/b infection have the highest event-free survival (EFS), and patients with single viral infection had the lowest. EFS in patients with lower levels of IL-6, IL-10, IL-17A, IFN-γ and PA-IgG tended to be longer than in patients with higher levels. However, EFS in patients with lower levels of IL-12/23P40 and PAF tended to be shorter when compared to higher-level patients. We observed no significant difference between the higher and lower groups, and the p-values of the K-M curves were all >0.05, probably due to the small sample size (Fig. 2 ).
Table 5.
Binary logistic regression analysis of risk factors in patients with or without improvement.
| Factors | P value | OR | 95.0% C.I. for OR |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 0.029 | 0.956 | 0.918 | 0.995 |
| T | 0.504 | 1.458 | 0.483 | 4.404 |
| WBC | 0.483 | 1.063 | 0.896 | 1.261 |
| LY | 0.055 | 1.087 | 0.998 | 1.183 |
| N | 0.067 | 1.072 | 0.995 | 1.154 |
| RBC | 0.558 | 2.588 | 0.108 | 62.253 |
| HB | 0.337 | 0.949 | 0.853 | 1.056 |
| PLT | 0.005 | 1.081 | 1.024 | 1.141 |
| MPV | 0.472 | 1.108 | 0.838 | 1.467 |
| PDW | 0.060 | 1.483 | 0.984 | 2.237 |
| CRP | 0.591 | 1.003 | 0.993 | 1.013 |
| PCT | 0.040 | 1.031 | 1.001 | 1.061 |
| ALT | 0.741 | 0.999 | 0.991 | 1.006 |
| IL-6 | 0.180 | 0.992 | 0.981 | 1.004 |
| IL-10 | 0.702 | 1.000 | 0.998 | 1.003 |
| IL-12/23P40 | 0.075 | 0.998 | 0.995 | 1.000 |
| IL-17A | 0.040 | 1.101 | 1.004 | 1.207 |
| IFN-γ | 0.583 | 1.000 | 0.998 | 1.001 |
| PAF | 0.092 | 1.002 | 1.000 | 1.004 |
| PA-IgG | 0.923 | 1.004 | 0.918 | 1.099 |
OR, Odds ratio.
Fig. 2.
Kaplan-Meier survival curves were used to assess the prognosis of different types of pathogen infection groups and the applicability of six serum cytokines and PA-IgG as prognostic factors. (A) The EFS for the single virus group was 7 days shorter than for the multiple pathogens group (EFS = 31 days), followed by the unidentified group for 57 days. The maximum EFS in single f/b group was 113 days; (B) The mean of the IL-6 is 69.90 pg/ml, EFS in patients with IL-6 > 69.90 pg/ml was shorter than the patients with IL-6 < 69.90 pg/ml. (C) The mean of the IL-10 is 353.80 pg/ml, EFS in patients with IL-10 > 353.80 pg/ml was shorter than the patients with IL-10 < 353.80 pg/ml. (D) The mean of the IL-12/23P40 is 182.50 pg/ml, EFS in patients with IL-12/23P40 > 182.50 pg/ml was longer than the patients with IL-12/23P40 < 182.50 pg/ml. (E) The mean of the IL-17A is 6.33 pg/ml, EFS in patients with IL-17A > 6.33 pg/ml was shorter than the patients with IL-17A < 6.33 pg/ml. (F) The mean of the IFN-γ is 246.07 pg/ml EFS in patients with IFN-γ > 246.07 pg/ml was shorter than the patients with IFN-γ < 246.07 pg/ml. (G) The mean of the PAF is 321.80 ng/ml, EFS in patients with PAF > 321.80 ng/ml was longer than the patients with PAF < 321.80 ng/ml. (H) The mean of the PA-IgG is 11.164 μg/l, EFS in patients with PA-IgG > 11.164 μg/l was shorter than the patients with PA-IgG < 11.164 μg/l.
4. Discussion
Platelets participate in the interaction between pathogens and host defense [27], but their function in host defense against infection has received much less attention [28]. Pathogenic microbiome may cause thrombocytopenia by autoimmunity mechanisms, mainly through molecules or epitopes that mimic autoantigens, the immunological recognition of previously unexposed auto-antigens, or via bystanders that activate their own responses to lymphocytes and super-antigen-mediated polyclonal T cell activation with exaggerated cytokine release [29]. Studies have shown that an imbalance of T helper cell (Th) and regulatory T cell (Treg) cytokines may mediate the pathogenesis of thrombocytopenia, which is closely related to the expression of Th1 cytokines (i.e., IFN-γ, IL-2), Th2 cytokines (i.e., IL-4, IL-10), Th17 cytokines (IL-17), and Treg cytokines (TGF-β1) [30]. Consistent with previous studies, up-regulated cytokines were found in our patients with fever and thrombocytopenia, especially IL-6, IL-10, and IFN-γ, indicating that cytokine disorders may be involved in the pathogenesis of newly diagnosed ITPs [31,32]. Notably, IL-6 in thrombocytopenia patients was significantly higher as compared to healthy controls, and IL-6 in the viral group was the highest among all groups. Moreover, our results revealed that IL-6, together with CRP and PCT, has a strong positive loading in mirroring the pathogenesis of thrombocytopenia. PAF is a potent and versatile mediator of pro-inflammation secreted by WBCs [33], which triggers platelet aggregation and activation. Monocytes that encounter dengue virus begin to generate PAF [34], and this leads to enhanced apoptosis of platelets and accelerates platelets clearance in secondary dengue infection [35]. The PAF concentration in our patients with fever and thrombocytopenia was significantly higher as compared to the healthy controls, revealing that PAF may involve in the platelet's apoptosis and clearance in infectious thrombocytopenia. The dose-dependent effect of PAF may induce the release of cytokines and affect the host immune response. A previous study showed that PAF can stimulate mononuclear cells of peripheral blood leading to the release of TNF-α and IL-6 in viral infection [36]. PAF itself or combined with LPS can promote platelet activation and recruitment and worsen endotoxin-induced injury [37,38]. Therefore, PAF and cytokines potentiate each other's effects in vivo to modulate the immune response [39]. The strong associations were found between PAF and IL-6, between PAF and IL-10, between PAF and CRP, revealing the PAF, interacting with IL-6 and IL-10, are jointly responsible for thrombocytopenia [25].
Both antibody-mediated and/or T cell-mediated platelet destruction are key processes of thrombocytopenia [40]. Through antibody-induced immune-mediated phagocytosis and complement activation, alloantibodies destroy platelets and eliminate them from the circulation [41]. In as many as 30% to 40% of the patients, no detectible antibodies can be found, however, studies have shown that antibodies can cause thrombocytopenia even if the platelet-associated antibody levels in humans are lower than the detection limits of conventional methods. Therefore, the detection of the relevant antibodies is the primary condition for diagnosis and treatment [42] as the presence of autoantibodies targeting GPIb-IX is an effective predictor for refractoriness to steroid or IVIG therapy [43]. In this study, only 2 patients were negative in the PA-IgG test in 79 selected patients, indicating that antibody-mediated clearance maybe the main reason for our patients with fever and thrombocytopenia.
Although an informal connection between H. pylori infection and ITP has been suggested by various clinical studies, there are studies that have demonstrated that H. pylori is not associated with thrombocytopenia [44]. The curative effects of anti-H. pylori therapy on improved blood platelet quantity in chronic immune thrombocytopenia is inconsistent [45,46]. In this study, H. pylori has caused the highest morbidity among bacteria inducing thrombocytopenia. There may be a cross-reaction between H. pylori cytotoxin-related gene (CagA) antibodies and platelet antigens, causing platelet-accelerated clearance. In H. pylori-positive patients, significantly higher levels of IL-6, IL-10, and PAF were found when compared with those in the H. pylori-negative group. Because of the involvement of cytokines, the pathogenesis of H. pylori-induced thrombocytopenia is more complex than initially believed. Unfortunately, the mechanism of H. pylori-induced thrombocytopenia is obscure. Revealing the interaction between H. pylori and thrombocytopenia will greatly expand the choices of therapeutic options.
In this study, Kaplan-Meier survival curves were used to further assesses the prognosis of infectious thrombocytopenia. Our data showed that best therapeutic efficacy occurred in the single f/b infectious group, and patients in this group had the highest EFS. The worst therapeutic efficacy was found in the multiple infectious group. Platelet-virus interaction can occur via a variety of receptors expressed on platelets, which are mainly mediated by toll like receptors (TLRs) [47]. Accordingly, different pathogens may interact with its special TLRs on platelets. It is postulated that pathogens recognized by TLRs increase platelet destruction, which is multiplied in the presence of anti-platelet antibodies due to the synergy with TLRs, and then increases R-Fc-mediated phagocytosis [48]. The TLRs activate different signaling pathways and affect cells producing inflammatory cytokines [49]. Therefore, the cytokine production may vary due to the different types of microbiomes. As cytokines involved in immune function adjustment and modulation of the anti-platelet antibody response by B cells [50,51], they may closely related to the platelet destruction. Therefore, we used the levels of cytokines as a stratification variable for EFS, the results showed that cytokine play a significant role in EFS. Patients with lower levels of IL-6, IL-10, IL-17A, and IFN-γ tended to have longer EFS than in patients with higher levels of cytokines. Previous study revealed that IL-22 is elevated in active phases of disease, and decreases in patients responsive to dexamethasone [52]. These results indicate that cytokines could reflect the disease status and the therapeutic effect.
The etiology of thrombocytopenia with a viral/bacterial infection may also be due to non-immunological mechanisms. The virus-induced pro-inflammatory environment itself often leads to further platelet activation in viremic patients [53]. Viruses can trigger a decrease in platelet production by infection of megakaryocytes and hematopoietic stem cells to decrease platelet formation; additionally, virus-infected platelets can expose P-selectin or phosphatidylserine, promoting their phagocytosis by macrophages [35]. Another viable mechanism is the formation of mixed platelet-leukocyte aggregates in adenovirus infection [54] that rapidly reduce platelet counts [55].
5. Conclusions
The etiology, immunologic and clinical features of patients with fever and thrombocytopenia is quite complicated, and multiple factors including type of pathogens, cytokines and autoantibody participate in the platelet's clearance. Finding reliable biomarkers for diagnosis and prognosis prediction are urgently needed for effective therapy.
Acknowledgments
Acknowledgments
This study was supported by the Shantou Science and Technology Project (grant numbers. 180709174010328).
Authors' contributions
Huanzhu Chen and Chun Lin are responsible for the collection of case data, sample collection and testing; Ping He, Zhiqiang Fan, Wenjun Yu and Manxiong Cao are responsible for data collection, collation and statistics; Changwen Ke, Xiaoyang Jiao are responsible for the design of the experiment and the writing of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval and informed consent
This study was approved by the Ethics Committee of Shantou University Medical College and informed consents were obtained from the patients.
Consent for publication
We have read and agree to the final draft before submission.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Yu X.J., Liang M.F., Zhang S.Y., Liu Y., Li J.D., Sun Y.L. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011;364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Provan D., Stasi R., Newland A.C., Blanchette V.S., Bolton-Maggs P., Bussel J.B. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–186. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 3.Bettaieb A., Fromont P., Louache F., Oksenhendler E., Vainchenker W., Duedari N. Presence of cross-reactive antibody between human immunodeficiency virus (HIV) and platelet glycoproteins in HIV-related immune thrombocytopenic purpura. Blood. 1992;80:162–169. [PubMed] [Google Scholar]
- 4.Wright J.F., Blanchette V.S., Wang H., Arya N., Petric M., Semple J.W. Characterization of platelet-reactive antibodies in children with varicella-associated acute immune thrombocytopenic purpura (ITP) Br. J. Haematol. 1996;95:145–152. doi: 10.1046/j.1365-2141.1996.d01-1872.x. [DOI] [PubMed] [Google Scholar]
- 5.Karimi O., Goorhuis A., Schinkel J., Codrington J., Vreden S.G.S., Vermaat J.S. Thrombocytopenia and subcutaneous bleedings in a patient with Zika virus infection. Lancet. 2016;387:939–940. doi: 10.1016/S0140-6736(16)00502-X. [DOI] [PubMed] [Google Scholar]
- 6.Aoki A., Moro H., Watanabe T., Asakawa K., Miura S., Moriyama M. A case of severe thrombocytopaenia associated with acute HIV-1 infection. Int. J. STD AIDS. 2015;26:209–211. doi: 10.1177/0956462414531937. [DOI] [PubMed] [Google Scholar]
- 7.Wang X., Jiang W., Li F., Hua F., Zhan Y., Li Y. Abnormal platelet kinetics are detected before the occurrence of thrombocytopaenia in HBV-related liver disease. Liver Int. 2014;34:535–543. doi: 10.1111/liv.12309. [DOI] [PubMed] [Google Scholar]
- 8.Dou J., Lou Y., Wu J., Lu Y., Jin Y. Thrombocytopenia in patients with hepatitis B virus-related chronic hepatitis: evaluation of the immature platelet fraction. Platelets. 2014;25:399–404. doi: 10.3109/09537104.2013.832742. [DOI] [PubMed] [Google Scholar]
- 9.Zucker M.L., Hagedorn C.H., Murphy C.A., Stanley S., Reid K.J., Skikne B.S. Mechanism of thrombocytopenia in chronic hepatitis C as evaluated by the immature platelet fraction. Int. J. Lab. Hematol. 2012;34:525–532. doi: 10.1111/j.1751-553X.2012.01429.x. [DOI] [PubMed] [Google Scholar]
- 10.de Azeredo E.L., Monteiro R.Q., de-Oliveira Pinto L.M. Thrombocytopenia in dengue: interrelationship between virus and the imbalance between coagulation and fibrinolysis and inflammatory mediators. Mediat. Inflamm. 2015;2015 doi: 10.1155/2015/313842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeed M., Dabbagh O., Al-Muhaizae M., Dhalaan H., Chedrawi A. Acute disseminated encephalomyelitis and thrombocytopenia following Epstein-Barr virus infection. J. Coll. Physicians Surg. Pak. 2014;24(Suppl. 3):S216–S218. [PubMed] [Google Scholar]
- 12.de Haan T.R., van den Akker E.S., Porcelijn L., Oepkes D., Kroes A.C., Walther F.J. Thrombocytopenia in hydropic fetuses with parvovirus B19 infection: incidence, treatment and correlation with fetal B19 viral load. BJOG. 2008;115:76–81. doi: 10.1111/j.1471-0528.2007.01555.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim J.K., Jeon J.S., Kim J.W., Kim G.Y. Correlation between abnormal platelet count and respiratory viral infection in patients from Cheonan, Korea. J. Clin. Lab. Anal. 2016;30:185–189. doi: 10.1002/jcla.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labarque V., Van Geet C. Clinical practice: immune thrombocytopenia in paediatrics. Eur. J. Pediatr. 2014;173:163–172. doi: 10.1007/s00431-013-2254-6. [DOI] [PubMed] [Google Scholar]
- 15.Youssefian T., Drouin A., Masse J.M., Guichard J., Cramer E.M. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021–4029. doi: 10.1182/blood-2001-12-0191. [DOI] [PubMed] [Google Scholar]
- 16.Arora S., Gupta N., Kumar A., Kaur I.R. Salmonella enteritidis from a case of fever with thrombocytopenia. Asian Pac J Trop Med. 2011;4:328–329. doi: 10.1016/S1995-7645(11)60097-7. [DOI] [PubMed] [Google Scholar]
- 17.Malnick S.D., Oppenheim A., Melzer E. Immune thrombocytopenia caused by fecal microbial transplantation in a patient with severe recurrent clostridium difficile infection. J. Clin. Gastroenterol. 2015;49:888–889. doi: 10.1097/MCG.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 18.Gafter-Gvili A., Mansur N., Bivas A., Zemer-Wassercug N., Bishara J., Leibovici L. Thrombocytopenia in Staphylococcus aureus bacteremia: risk factors and prognostic importance. Mayo Clin. Proc. 2011;86:389–396. doi: 10.4065/mcp.2010.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W., Nardi M.A., Borkowsky W., Li Z., Karpatkin S. Role of molecular mimicry of hepatitis C virus protein with platelet GPIIIa in hepatitis C-related immunologic thrombocytopenia. Blood. 2009;113:4086–4093. doi: 10.1182/blood-2008-09-181073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi T., Yujiri T., Shinohara K., Inoue Y., Sato Y., Fujii Y. Molecular mimicry by helicobacter pylori CagA protein may be involved in the pathogenesis of H. pylori-associated chronic idiopathic thrombocytopenic purpura. Br. J. Haematol. 2004;124:91–96. doi: 10.1046/j.1365-2141.2003.04735.x. [DOI] [PubMed] [Google Scholar]
- 21.Saleh M.N., Moore D.L., Lee J.Y., LoBuglio A.F. Monocyte-platelet interaction in immune and nonimmune thrombocytopenia. Blood. 1989;74:1328–1331. [PubMed] [Google Scholar]
- 22.Harrington W.J., Minnich V., Hollingsworth J.W., Moore C. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. J. Lab. Clin. Med. 1951;38:1–10. [PubMed] [Google Scholar]
- 23.Arman M., Krauel K. Human platelet IgG Fc receptor FcgammaRIIA in immunity and thrombosis. J. Thromb. Haemost. 2015;13:893–908. doi: 10.1111/jth.12905. [DOI] [PubMed] [Google Scholar]
- 24.Cuker A., Cines D.B., Neunert C.E. Controversies in the treatment of immune thrombocytopenia. Curr. Opin. Hematol. 2016;23:479–485. doi: 10.1097/MOH.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 25.Perera M., Garrido T. Advances in the pathophysiology of primary immune thrombocytopenia. Hematology. 2017;22:41–53. doi: 10.1080/10245332.2016.1219497. [DOI] [PubMed] [Google Scholar]
- 26.Cines D.B., Blanchette V.S. Immune thrombocytopenic purpura. N. Engl. J. Med. 2002;346:995–1008. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- 27.Semple J.W., Italiano J.E., Jr., Freedman J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 28.Flaujac C., Boukour S., Cramer-Borde E. Platelets and viruses: an ambivalent relationship. Cell. Mol. Life Sci. 2010;67:545–556. doi: 10.1007/s00018-009-0209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steed A.L., Stappenbeck T.S. Role of viruses and bacteria-virus interactions in autoimmunity. Curr. Opin. Immunol. 2014;31:102–107. doi: 10.1016/j.coi.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma L., Liang Y., Fang M., Guan Y., Si Y., Jiang F. The cytokines (IFN-gamma, IL-2, IL-4, IL-10, IL-17) and Treg cytokine (TGF-beta1) levels in adults with immune thrombocytopenia. Pharmazie. 2014;69:694–697. [PubMed] [Google Scholar]
- 31.Culic S., Salamunic I., Konjevoda P., Dajak S., Pavelic J. Immune thrombocytopenia: serum cytokine levels in children and adults. Med. Sci. Monit. 2013;19:797–801. doi: 10.12659/MSM.884017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouwman J.J., Visseren F.L., Bosch M.C., Bouter K.P., Diepersloot R.J. Procoagulant and inflammatory response of virus-infected monocytes. Eur. J. Clin. Investig. 2002;32:759–766. doi: 10.1046/j.1365-2362.2002.01041.x. [DOI] [PubMed] [Google Scholar]
- 33.Mitsios J.V., Vini M.P., Stengel D., Ninio E., Tselepis A.D. Human platelets secrete the plasma type of platelet-activating factor acetylhydrolase primarily associated with microparticles. Arterioscler. Thromb. Vasc. Biol. 2006;26:1907–1913. doi: 10.1161/01.ATV.0000228821.79588.ef. [DOI] [PubMed] [Google Scholar]
- 34.Zapata J.C., Cox D., Salvato M.S. The role of platelets in the pathogenesis of viral hemorrhagic fevers. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alonzo M.T., Lacuesta T.L., Dimaano E.M., Kurosu T., Suarez L.A., Mapua C.A. Platelet apoptosis and apoptotic platelet clearance by macrophages in secondary dengue virus infections. J. Infect. Dis. 2012;205:1321–1329. doi: 10.1093/infdis/jis180. [DOI] [PubMed] [Google Scholar]
- 36.Buke A.C., Buke M., Altuglu I.E., Ciceklioglu M., Kamcioglu S., Karakartal G. Tumor necrosis factor alpha and interleukin 6 productions in response to platelet-activating factor in chronic hepatitis B virus infection. Med. Princ. Pract. 2004;13:273–276. doi: 10.1159/000079526. [DOI] [PubMed] [Google Scholar]
- 37.Rabinovici R., Esser K.M., Lysko P.G., Yue T.L., Griswold D.E., Hillegass L.M. Priming by platelet-activating factor of endotoxin-induced lung injury and cardiovascular shock. Circ. Res. 1991;69:12–25. doi: 10.1161/01.res.69.1.12. [DOI] [PubMed] [Google Scholar]
- 38.Beijer L., Botting J., Crook P., Oyekan A.O., Page C.P., Rylander R. The involvement of platelet activating factor in endotoxin-induced pulmonary platelet recruitment in the Guinea-pig. Br. J. Pharmacol. 1987;92:803–808. doi: 10.1111/j.1476-5381.1987.tb11384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camussi G., Tetta C., Baglioni C. The role of platelet-activating factor in inflammation. Clin. Immunol. Immunopathol. 1990;57:331–338. doi: 10.1016/0090-1229(90)90108-3. [DOI] [PubMed] [Google Scholar]
- 40.Zufferey A., Kapur R., Semple J.W. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP) J. Clin. Med. 2017;6 [Google Scholar]
- 41.Hayashi T., Hirayama F. Advances in alloimmune thrombocytopenia: perspectives on current concepts of human platelet antigens, antibody detection strategies, and genotyping. Blood Transfus. 2015;13:380–390. doi: 10.2450/2015.0275-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen X.D., Dugrillon A., Beck C., Kerowgan M., Kluter H. A novel method for simultaneous analysis of specific platelet antibodies: SASPA. Br. J. Haematol. 2004;127:552–560. doi: 10.1111/j.1365-2141.2004.05233.x. [DOI] [PubMed] [Google Scholar]
- 43.Peng J., Ma S.H., Liu J., Hou Y., Liu X.M., Niu T. Association of autoantibody specificity and response to intravenous immunoglobulin G therapy in immune thrombocytopenia: a multicenter cohort study. J. Thromb. Haemost. 2014;12:497–504. doi: 10.1111/jth.12524. [DOI] [PubMed] [Google Scholar]
- 44.Mubarak N., Gasim G.I., Khalafalla K.E., Ali N.I., Adam I. Helicobacter pylori, anemia, iron deficiency and thrombocytopenia among pregnant women at Khartoum, Sudan. Trans. R. Soc. Trop. Med. Hyg. 2014;108:380–384. doi: 10.1093/trstmh/tru044. [DOI] [PubMed] [Google Scholar]
- 45.Sheema K., Ikramdin U., Arshi N., Farah N., Imran S. Role of helicobacter pylori eradication therapy on platelet recovery in chronic immune thrombocytopenic Purpura. Gastroenterol. Res. Pract. 2017;2017 doi: 10.1155/2017/9529752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stasi R., Sarpatwari A., Segal J.B., Osborn J., Evangelista M.L., Cooper N. Effects of eradication of helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood. 2009;113:1231–1240. doi: 10.1182/blood-2008-07-167155. [DOI] [PubMed] [Google Scholar]
- 47.Speth C., Loffler J., Krappmann S., Lass-Florl C., Rambach G. Platelets as immune cells in infectious diseases. Future Microbiol. 2013;8:1431–1451. doi: 10.2217/fmb.13.104. [DOI] [PubMed] [Google Scholar]
- 48.Semple J.W., Aslam R., Kim M., Speck E.R., Freedman J. Platelet-bound lipopolysaccharide enhances Fc receptor-mediated phagocytosis of IgG-opsonized platelets. Blood. 2007;109:4803–4805. doi: 10.1182/blood-2006-12-062695. [DOI] [PubMed] [Google Scholar]
- 49.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 50.Semple J.W., Milev Y., Cosgrave D., Mody M., Hornstein A., Blanchette V. Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood. 1996;87:4245–4254. [PubMed] [Google Scholar]
- 51.Ogawara H., Handa H., Morita K., Hayakawa M., Kojima J., Amagai H. High Th1/Th2 ratio in patients with chronic idiopathic thrombocytopenic purpura. Eur. J. Haematol. 2003;71:283–288. doi: 10.1034/j.1600-0609.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- 52.Cao J., Chen C., Zeng L., Li L., Li X., Li Z. Elevated plasma IL-22 levels correlated with Th1 and Th22 cells in patients with immune thrombocytopenia. Clin. Immunol. 2011;141:121–123. doi: 10.1016/j.clim.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Assinger A. Platelets and infection – an emerging role of platelets in viral infection. Front. Immunol. 2014;5:649. doi: 10.3389/fimmu.2014.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Othman M., Labelle A., Mazzetti I., Elbatarny H.S., Lillicrap D. Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood. 2007;109:2832–2839. doi: 10.1182/blood-2006-06-032524. [DOI] [PubMed] [Google Scholar]
- 55.Koupenova M., Vitseva O., MacKay C.R., Beaulieu L.M., Benjamin E.J., Mick E. Platelet-TLR7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood. 2014;124:791–802. doi: 10.1182/blood-2013-11-536003. [DOI] [PMC free article] [PubMed] [Google Scholar]