Abstract
To characterize the antigenic epitopes of the hemagglutinin (HA) protein of H1N1 influenza virus, a panel consisting of 84 clones of murine monoclonal antibodies (mAbs) were generated using the HA proteins from the 2009 pandemic H1N1 vaccine lysate and the seasonal influenza H1N1(A1) vaccines. Thirty-three (39%) of the 84 mAbs were found to be strain-specific, and 6 (7%) of the 84 mAbs were subtype-specific. Twenty (24%) of the 84 mAbs recognized the common HA epitopes shared by 2009 pandemic H1N1, seasonal A1 (H1N1), and A3 (H3N2) influenza viruses. Twenty-five of the 84 clones recognized the common HA epitopes shared by the 2009 pandemic H1N1, seasonal A1 (H1N1) and A3 (H3N2) human influenza viruses, and H5N1 and H9N2 avian influenza viruses. We found that of the 16 (19%) clones of the 84 mAbs panel that were cross-reactive with human respiratory pathogens, 15 were made using the HA of the seasonal A1 (H1N1) virus and 1 was made using the HA of the 2009 pandemic H1N1 influenza virus. Immunohistochemical analysis of the tissue microarray (TMA) showed that 4 of the 84 mAb clones cross-reacted with human tissue (brain and pancreas). Our results indicated that the influenza virus HA antigenic epitopes not only induce type-, subtype-, and strain-specific monoclonal antibodies against influenza A virus but also cross-reactive monoclonal antibodies against human tissues. Further investigations of these cross-reactive (heterophilic) epitopes may significantly improve our understanding of viral antigenic variation, epidemics, pathophysiologic mechanisms, and adverse effects of influenza vaccines.
Keywords: Influenza virus; H1N1; HA protein; Human tissues, Heterophilic antigen epitopes
Introduction
Influenza A virus (IAV), which possesses the capacity to infect humans, mammals, birds and poultry, has caused considerable health and economic threats globally (Webster et al., 1992). IAV belongs to the Orthomyxoviridae family and is a single stranded negative sense RNA virus with eight RNA segments encoding at least 11 proteins. Hemagglutinin (HA), encoded by the fourth RNA segment, is the major membrane glycoprotein that mediates viral attachment and fusion and determines pathogenicity and virulence. Neuraminidase (NA), encoded by the sixth RNA fragment, is the second major membrane glycoprotein. Both HA and NA are crucial antigenic components of the virus. Due to immune selection pressures, low fidelity of RNA transcription, and RNA reassortment of the viral genome, a variety of genetic and immunogenic HA and NA variants emerge constantly. Up to now, 18 HA and 9 NA subtypes of type A influenza virus have been identified (Tong et al., 2013).
In the 21st century, the pandemic H1N1 influenza virus emerged in 2009 from Mexico and quickly spread all over the world (WHO, 2012). In the first year of infection alone, H1N1 caused 151,700–575,400 deaths, and there were an estimated 60 million cases of the 2009 H1N1 pandemic strain and a number of severe cases in young adults without underlying disease (cdc.gov). People at higher risk of serious complications include the elderly (>65 years), younger children (<5), and pregnant women. In severe cases, symptoms included pneumonia, respiratory failure, thrombocytopenia and death (Desdouits et al., 2013, Guedj et al., 2012, Mammas et al., 2011). Vaccination is the best preventive strategy against influenza. The safety and efficacy of the vaccine for the 2009 pandemic H1N1 strain was evaluated (Liang et al., 2010), and the adverse events associated with 2009 H1N1 vaccination raised concerns that affected the large-scale administration of the influenza vaccine (CDC, 2009, Williams et al., 2011, Choe et al., 2011). Guillain–Barre syndrome was one of the several adverse events observed after vaccination with the 2009 H1N1 vaccine (Deeks et al., 2011, Greenberg et al., 2009). However, the pathological mechanisms underlying influenza A virus infection and vaccine-induced serious adverse reactions are still poorly understood. Monsalvo et al. (2011) found non-protective antibodies in the sera from patients with severe pandemic 2009 H1N1 influenza disease, and these non-protective-antibodies were found to be associated with immune complexes. We hypothesized that autoimmunity plays a role in the development of severe symptoms after virus infection and vaccination-related adverse events (Ahmed et al., 2014, Perdan-Pirkmajer et al., 2012). However, more evidence is required in support of this hypothesis.
Studies on mechanisms of microbial infection and immunity have revealed the presence of heterophilic antigens (also called molecular mimicry) that are associated with pathogenic microorganism-triggered infections. For example, shared antigens have been identified between the hemolytic streptococcus polysaccharide and protein and the human glomerular basement membrane. The anti-streptococcal antibodies produced upon hemolytic streptococci infection bind to the glomerular basement membrane causing glomerulonephritis (Kraus and Beachey, 1998). Moreover, antigens shared by Escherichia coli O14 type lipopolysaccharide and human colonic mucosa have been related to the pathogenesis of ulcerative colitis caused by E. coli O14 (Laercrantz et al., 1968). Anti-autoantibodies have also been identified in lung tissue from patients with atypical pneumonia infected with coronavirus (Li et al., 2005).
In addition to their role in receptor binding, fusion, and assembly, influenza virus HA is also the major antigenic determinant inducing the adaptive immune response of the host. We speculated that H1N1 influenza virus HA and human tissues/cells have common (heterophilic) epitopes, which could induce a heterophilic antibody response in infected hosts. These heterophilic antibodies may be involved in the pathogenicity of influenza virus infection and the mechanisms of vaccine-induced adverse reactions.
To provide evidence for our hypothesis, 84 clones of monoclonal antibodies were generated using the HA proteins from the influenza A/2009 H1N1 vaccine lysate and the seasonal A1 vaccine. The cross-reactivity of these mAbs against different subtypes of influenza virus, respiratory pathogens, and human tissue was evaluated. Our results suggested that there are shared epitopes between influenza virus HA antigen, certain of respiratory pathogens, and some human tissues. Further studies of these cross-reactive epitopes in the H1N1 HA protein will significantly improve our understanding of epitope evolution, virus epidemic, pathophysiologic mechanisms of infection and vaccine-induced adverse events.
Methods and materials
Immunogens and chemicals
HA protein from 2009 H1N1 vaccine lysate, the vaccine was prepared from A/California/7/2009-NYMC X-179A by Hualan Vaccine Ltd. (Xinxiang, China) and approved by State Food and Drug Administration (SFDA, S20090015). The seasonal influenza A1, A3 (A1 from A/Brisbane/59/2007, A3 from A/Victoria/210/2009 NYMC X-187) vaccines were obtained from Dalian Yalifeng Biotechnology Ltd. (Dalian, China). Respiratory pathogens (respiratory syncytial virus (RSV), 1,2,3-type parainfluenza virus (PIV1,2,3), adenovirus (AdV), Mycoplasma pneumoniae, and Chlamydia pneumoniae were purchased from Bio Co., Ltd. Shenzhen Fei Peng. Mouse myeloma cells (Sp2/0) were purchased from ATCC. BALB/c mice (8 weeks old, female) were purchased from Experimental Animal Center of the Fourth Military Medical University. HRP-labeled goat anti-mouse secondary antibody was purchased from Zhongshan Golden Bridge Company. Polyethylene glycol (PEG) was purchased from Sigma. The mAb subtype identification kit SBA ClonotypingTM System/HRP was obtained from Southern Biotech Inc. HAT and cell culture medium with bovine serum (DMEM) were purchased from Gibco. Human tissue chips were purchased from Shaanxi Chaoying Biotech Co., and tissue immunohistochemical staining kit was purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.
Mice immunization
All experimental mice were fed sterilized water and feed. Mice (6–8 weeks old) were immunized with 25 μg antigen (2009 H1N1 vaccine lysate and seasonal influenza A1) by the intraperitoneal route in 0.1 mL of PBS emulsified with an equal amount of Freund's complete adjuvant. Booster immunizations were administered intraperitoneally at the same dose but emulsified with Freund's incomplete adjuvant.
mAb production
Hybridoma cell fusion, screening and cloning was carried out according to a previously published protocol (Naundorf et al., 2002). Briefly, the spleen cells of immunized mice were fused with myeloma SP2/0 cells at a ratio of 10:1 (spleen:SP 2/0). The fused cell mix was allowed to proliferate in hypoxanthine aminopterin thymidine (HAT) supplemented medium in 96-well plates incubated in 5% CO2 for 7–10 days in a 37 °C water bath. After adding 1 mL fusogen to the stirred cells, the cells were allowed to stand at 37 °Cfor 45 s and then 1 mL of serum-free DMEM was added. A total of 3 mL of serum-free DMEM was finally added to terminate the integration effect of the fusing agent. After centrifuging the cells, the supernatant was discarded and 60 mL HAT medium was slowly added to the cells. Subsequently, the cells were resuspended gently and plated in a pre-prepared 96-well plate at 100 μL/well. After fusion, the cell fusion medium was changed once every 3 days. Between day 3 and day 6, half the medium was replaced with HAT selection medium. On day 9 and day 12 the medium was replaced with HT culture medium. When the fused cells proliferated and settled at the bottom of the wells, 10–50% positive antibody-secreting cells were screened via indirect ELISA using the H1N1 HA protein. The positive cells were subcloned using the limited dilution method and purified by subcloning 3 times to establish stable hybridoma cell lines. Stable lines were cultured and frozen at −20 °C for future use.
Characterization of the mAbs
Isotyping of the mAbs was performed with the Isotyping kit (SBA Clonotyping™ System/HRP) according to the instruction manual.
Specificity testing of purified mAbs
Indirect ELISA was conducted for specificity testing of the 84 mAbs. Reactivity of the mAbs against the H1N1 influenza virus HA vaccine, the seasonal influenza vaccine (A1, A3), avian influenza (H5N1 and H9N2), RSV, PIV1,2,3, AdV, M. pneumoniae, and C. pneumoniae was evaluated. The allantoic fluid and SP2/0 cells were used as the negative controls.
Hemagglutinin (HA) and hemagglutination inhibition (HI) test
HA and HI tests were performed according to a previously published protocol (Yin and Liu, 1997). A 2-fold serial dilution of the 2009 H1N1 virus and seasonal influenza virus A1 was plated in 96-well U-bottomed microtiter plates (100 μl/well) with an equal volume of 0.5% chicken erythrocytes. After incubating the cells at 37 °C for 1 h, the HA titer of each virus was measured. For the HI test, 50 μl of 4 HA units of different subtypes of the influenza virus and 50 μl of a 2-fold serially diluted mAbs mixture were added to each well of the 96-well U-bottomed microtiter plate, mixed well, and incubated at 37 °C for 30 min. In the next step, 50 μl of 0.5% chicken erythrocytes were added to each well, mixed well, and incubated again at 37 °C or 30 min. HI titer was determined as the highest dilution of the virus that prevented hemagglutination.
Immunohistochemistry
Cross-reaction of H1N1 HA mAbs with human tissues was examined via immunohistochemical staining of tissue histological sections. Briefly, paraffin sections of tissue were deparaffinized, hydrated, blocked with endogenous peroxidase at room temperature for 20 min with 3% H2O2, and then blocked with buffer containing goat serum for 30 min. The samples were stained with primary antibody (mAbs) at 4 °C overnight, kept at room temperature for 60 min, washed 3 times with PBS, and incubated with HRP-labeled sheep anti-mouse secondary antibody at 37 °C for 40 min. After 3 washes with PBS, diaminobenzidine (DAB) and hematoxylin were added for color development and color enhancement, respectively, according to the manufacturer's instructions.
Western blot
A total of 20 μg of each HA sample (1.392 mg/ml obtained from H1N1 vaccine lysate) was mixed with SDS electrophoresis sample buffer and resolved on a 12% SDS-PAGE gel, and transferred onto a nitrocellulose membrane. After blocking with 5% skimmed milk, the membranes were treated with H1N1 mAb supernatants. Membranes were probed with HRP-linked anti-mouse polyclonal secondary antibody and bands visualized via chemiluminescence using the ECL reagent.
Results
Generation and characterization of the H1N1 mAb panel (hybridoma cell lines)
Using conventional hybridoma technology, a panel of 84 clones of murine monoclonal antibodies were generated using the HA proteins from the 2009 pandemic H1N1 vaccine lysate and the seasonal influenza A1 vaccines. A total of 38 of the 84 mAb clones were against the 2009 pandemic H1N1 vaccine lysate (H1+33–84) and 46 of the 84 mAb clones were against the seasonal influenza A1 vaccines lysate (H1+1–32). After subculturing for three months, the hybridoma cell lines were still found to stably secrete antibodies. Hybridoma cell lines frozen in liquid nitrogen were found to grow well for at least three months after recovery and continued to secrete consistent levels of antibody.
Isotyping and HI assay
The light chains of all the 84 mAb clones obtained were found to be κ chains, whereas IgG1 and IgM accounted for a large proportion of the heavy chains. Sixty three percent (53/84) of the mAb had HI activity (see Table 1 for detailed characteristics).
Table 1.
Ig subclass identification and HI test of the 84 mAb clones.
| Ig typing | Hybridoma cell lines |
HI positive cell lines |
||
|---|---|---|---|---|
| A1 mAb | 2009 H1N1 mAb | A1 mAb | 2009 H1N1 mAb | |
| IgG1 | 23 | 22 | 15 | 12 |
| IgG2a | 1 | 0 | 1 | 0 |
| IgG2b | 5 | 3 | 4 | 2 |
| IgG3 | 0 | 8 | 0 | 5 |
| IgM | 17 | 2 | 10 | 1 |
| IgA | 0 | 3 | 0 | 3 |
| Total | 46 | 38 | 30 | 23 |
Specificity and cross-reactivity of the mAbs
ELISA was performed to determine the specificity and cross-reactivity of the 84 mAbs against the 2009 H1N1 influenza virus vaccine, the seasonal influenza virus A1 and A3 vaccines, and avian flu H5N1 and H9N2 vaccines. Allantoic fluid and SP2/0 ascites were used as negative controls.
Based on their reaction with different subtypes of influenza virus antigen, the mAbs were divided into four categories: (a) 2009 (H1N1) and A1 strain-specific mAbs, (b) 2009 (H1N1) and A1 subtype-specific mAbs, (c) mAbs against 2009 (H1N1) and seasonal influenza viruses with A1 and A3 antigens, and (d) mABs against the common influenza virus antigen shared by 2009 Influenza H1N1 vaccine, seasonal A1 and A3 vaccines, and the avian influenza H5N1 and H9N2 vaccines (Table 2 ).
Table 2.
mAb cross-reactivity with various subtypes of influenza virus.
| mAb category | Number of hybridoma | 2009 H1N1 antigen | Seasonal A1 antigen | HI positive | |
|---|---|---|---|---|---|
| Strain specific | 2009 H1N1 | 11 | 11 | 0 | 8 |
| Seasonal A1 | 22 | 0 | 22 | 16 | |
| H1 subtype | 2009 H1N1 and seasonal A1 | 6 | 4 | 2 | 4 |
| 2009 H1N1 and seasonal A1, A3 common antigen | 2009 H1N1 and seasonal A1/A3 | 20 | 11 | 9 | 8 |
| Common antigen shared by | 2009 H1N1, seasonal A1, A3, avian flu H5N1 and H9N2 | 25 | 12 | 13 | 17 |
| Total | 84 | 38 | 46 | 53 | |
From the results shown in Table 2, it can be concluded that 29% (11/38) of the strain-specific mAb are against the 2009 H1N1 vaccine, of which eight have HI activity, and 48% (22/46) of the strain-specific mAb are against the seasonal A1 vaccine, of which 16 have HI activity. The second category included 6 specific mAbs against the H1 subtype of influenza virus HA protein, accounting for 7% of the panel (6/84) of which four have HI activity. The third category included mAbs reactive against the HA epitopes in the 2009 influenza H1N1 and seasonal influenza virus A1 and A3, accounting for 24% (20/84) of the total mAbs, of which eight mAbs have HI activity. Antibodies in the fourth category were specific to the HA protein epitopes shared by the 2009 H1N1, seasonal A1 and A3, and avian influenza vaccines, accounting for 30% (25/84) of the panel, of which 17 have HI activity.
In the cross-reaction test using ELISA, 16 of the 84 mAbs were found to have different degrees of cross-reactivity with parainfluenza 1–3, respiratory syncytial virus, adenovirus, M. pneumoniae, C. pneumoniae. Of these 16 mAbs, 15 were against the seasonal A1 antigen and only one against the 2009 H1N1 antigen (Table 3 ).
Table 3.
mAb cross-reaction with other respiratory pathogens.
| mAb category cross-reaction | Respiratory pathogens |
Mycoplasma pneumoniae | Chlamydia pneumoniae | ||||
|---|---|---|---|---|---|---|---|
| PIV1 | PIV2 | PIV3 | RSV | ADV | |||
| Seasonal A1 mAb (15/46) | 2 | 1 | 4 | 2 | 15 | 1 | 0 |
| 2009 H1N1 mAb (1/38) | 0 | 0 | 1 | 0 | 0 | 1 | 1 |
| 16/84 | 2/84 | 1/84 | 5/84 | 2/84 | 15/84 | 2/84 | 1/84 |
Immunohistochemistry (reaction of mAbs with human tissue chips)
Cross-reaction of the 84 mAbs with human tissue was screened via microarray immunohistochemical staining. Our results showed that four mAb clones were cross-reactive with human tissue, of which 2 (A1-10 and H1-84) reacted with human brain tissue and 2 (H1-55 and H1-17) with pancreatic tissue (see Table 4 ). The A1-10 monoclonal antibody reacted with tissues of the cerebral cortex and the nucleus of horny, whereas H1-17 and H1-55 reacted with islet α cells (Fig. 1 ).
Table 4.
mAbs cross-reactive with human tissue.
| Tissues | Monoclonal antibodies | |
|---|---|---|
| Brain | A1-10 | H1-84 |
| Pancreatic | H1-17 | H1-55 |
Fig. 1.
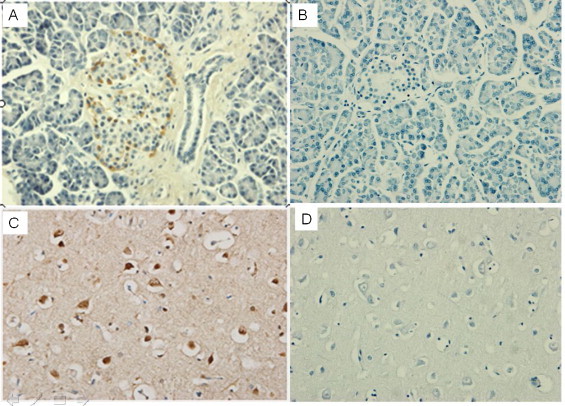
Screening of cross-reactivity of the mAbs against human tissues. (A) Monoclonal antibody H1-17 cross-reaction with human pancreatic tissue. The surrounding islet cells are visible in brown. (B) PBS and human pancreatic tissue cells without staining. (C) Monoclonal antibody A1-10 cross-reaction with human brain tissue. Visible part of the cerebral cortex star horny nuclei is stained brown. (D) PBS and brain tissue.
Western blotting analysis
Four mAbs with cross-reactivity against human tissue were further tested using Western blotting analysis. Fig. 2 shows that the 4 mAbs cross-reactive against human tissues react with the HA protein of influenza virus H1N1.
Fig. 2.

Western blot identification of the specificity of the 4 clones of mAbs with cross-reactivity against human tissues. Lane 1: monoclonal antibody A1-10; lane 2: monoclonal antibody H1-84; lane 3: monoclonal antibody H1-17; lane 4: monoclonal antibodyH1-55.
Discussion
The application of monoclonal antibodies has made it possible to study individual antigen and antibody molecules in depth. Till date the application of monoclonal antibodies against influenza virus has mainly been concentrated on diagnosis, immunotherapy and prophylaxis. The pathological mechanisms underlying the severe symptoms of the 2009 pandemic H1N1 influenza virus infection and the severe adverse events after vaccination are still poorly understood. A panel of 84 clones of murine monoclonal antibodies (mAbs) were successfully generated using the HA proteins from the 2009 pandemic H1N1 vaccine lysate and the seasonal influenza A1 vaccines. Among the 84 clones of mAbs obtained, 38 (H1N1) were made from the 2009 H1N1 influenza vaccine (for example, H1N1 A/South Carolina/1918 (A/SC/1918)), 46 from seasonal influenza A1 vaccine (add information same as before). Forty six of the mABs were found to belong to the IgM subclass1, and because polysaccharide antigens are known to easily elicit IgM antibodies, it was suggested that some of the antibodies in the panel might be specific to polysaccharide epitopes on the H1 glycoprotein.
The results of mAb characterization (Table 2) suggested the following: (I) there are different strain-specific HA epitopes in the 2009 pandemic H1N1 and seasonal A1 influenza viruses, (II) 2009 pandemic H1N1 and the seasonal A1 influenza viruses share common H1 subtype epitopes, (III) 2009 pandemic H1N1 and the seasonal A1, and A3 influenza viruses share common epitopes, and (IV) there are shared HA epitopes between the 2009 pandemic H1N1, seasonal A1 and A3, and avian influenza H5N1 and H9N2 viruses.
Of the 25 mAbs that showed cross-reactivity with the 2009 pandemic H1N1, seasonal A1 and A3, and avian influenza H5N1 and H9N2 viruses, 16 had HI activity, suggesting that this category of Abs are endowed with heterosubtypic neutralizing activity and have potential application in the design of ‘universal’ prophylactic or therapeutic tools (Corti et al., 2011).
More interestingly, immunohistochemical staining (Table 4) revealed 4 mAb clones with different degrees of binding affinity to brain, pancreas or other human tissues. These results suggested that antigenic mimics or heterophilic antigenic epitopes are shared between influenza virus HA protein and human tissues (brain and pancreas). Because such heterophilic antigenic epitopes are capable of inducing autoimmunity in the host, they may be involved in the pathological mechanisms underlying 2009 H1N1 influenza virus infection and vaccine-triggered adverse events. Based on previous studies on some heterophilic antigenic epitopes shared by pathogenic microorganisms and human tissue (Kraus and Beachey, 1998, Laercrantz et al., 1968), we proposed that autoimmunity induced by the influenza HA protein might play a role in the pathogenesis associated with influenza virus infection and vaccination. Carlo Garzelli et al. found that the monoclonal antibody secreted by Epstein–Barr virus-transformed lymphocytes could react with multiple organs including the thyroid, pancreas, stomach, muscles and nerves, indicating that Epstein–Barr virus can induce autoantibodies (Naundorf et al., 2002). Srinivasappa et al. (1968) reported that 600 mAb clones prepared using 11 viral antigens were able to react with 14 different organs from normal uninfected mice. Approximately 3.5% of these mAbs (21/600) reacted with specific types of cells from these organs. Therefore, viral infections might stimulate autoimmune reactions.
Widespread implementation of the influenza vaccination program is the most effective prevention strategy to help high-risk populations establish an immune barrier from influenza infection. However, rare severe adverse events (Guillain–Barre syndrome) after large-scale vaccination raised some concerns (Vellozzi et al., 2010, Evans et al., 2009). Two of the 84 mAbs generated in our study were capable of binding to human brain tissue. These two clones may be useful tools to further understand whether the development of Guillain–Barre syndrome is related to influenza vaccination or not. In addition, 2 more clones were found to react with human islet α cells (data to be published). Our study suggests that autoimmunity might be induced by influenza infection and vaccination. Further investigation of the pathological mechanisms of influenza infection and vaccine-induced adverse events using these 4 cross-reactive mAbs is currently underway in our laboratory.
Conclusion
In summary, a panel of 84 mAbs were generated using the HA proteins from the pandemic H1N1 2009 and the seasonal influenza A1 virus. One group of mAbs that have cross-reactive activity against the 2009 pandemic H1N1, seasonal A1/A3 (what A1, A3), and avian influenza H5N1 and H9N2 viruses, have potential application in the design of ‘universal’ prophylactic or therapeutic strategies. More interestingly, we generated four mAbs that cross-react with human tissues, and could provide a practical tool to further elucidate the mechanisms associated with influenza infection and vaccine-induced adverse events.
Conflict of interest
No any conflict of interest exits in the submission of this manuscript, and all authors approved the manuscript for publication.
Acknowledgement
This research was supported by National Science and Technology Major Projects (No. 2014ZX10004002-002-005).
References
- Ahmed S.S., Schur P.H., Macdonald N.E., Steinman L. Narcolepsy, 2009 A (H1N1) pandemic influenza, and pandemic influenza vaccinations: what is known and unknown about the neurological disorder, the role for autoimmunity, and vaccine adjuvants. J. Autoimmun. 2014;50:1–11. doi: 10.1016/j.jaut.2014.01.033. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Safety of influenza A (H1N1) 2009 monovalent vaccines – United States, October 1–November 24, 2009. Morb. Mortal. Wkly. Rep. 2009;58:1351–1356. [PubMed] [Google Scholar]
- Choe Y.J., Cho H., Kim S.N., Bae G.R., Lee J.K. Serious adverse events following receipt of trivalent inactivated influenza vaccine in Korea, 2003–2010. Vaccine. 2011;29:7727–7732. doi: 10.1016/j.vaccine.2011.07.129. [DOI] [PubMed] [Google Scholar]
- Corti D., Voss J., Gamblin S.J., Codoni G., Macagno A., Jarrossay D., Vachieri S.G., Pinna D., Minola A., Vanzetta F., Silacci C., Fernandez-Rodriguez B.M., Agatic G., Bianchi S., Giacchetto-Sasselli I., Calder L., Sallusto F., Collins P., Haire L.F., Temperton N., Langedijk J.P., Skehel J.J., Lanzavecchia A. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- Deeks S.L., Lim G.H., Simpson M.A., Rosella L., Mackie C.O., Achonu C., Crowcroft N.S. Estimating background rates of Guillain–Barré syndrome in Ontario in order to respond to safety concerns during pandemic H1N1/09 immunization campaign. BMC Public Health. 2011;11:329. doi: 10.1186/1471-2458-11-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desdouits M., Munier S., Prevost M.C., Jeannin P., Butler-Browne G., Ozden S., Gessain A., Van Der Werf S., Naffakh N., Ceccaldi P.E. Productive infection of human skeletal muscle cells by pandemic and seasonal influenza A (H1N1) viruses. PLoS ONE. 2013;8:e79628. doi: 10.1371/journal.pone.0079628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D., Cauchemez S., Hayden F.G. Prepandemic immunization for novel influenza viruses, “Swine Flu” vaccine, Guillain–Barre Syndrome, and the detection of rare severe adverse events. J. Infect. Dis. 2009;200:321–328. doi: 10.1086/603560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M.E., Lai M.H., Hartel G.F., Wichems C.H., Gittleson C., Bennet J., Dawson G., Hu W., Leggio C., Washington D., Basser R.L. Response to a monovalent 2009 influenza A (H1N1) vaccine. N. Engl. J. Med. 2009;361:2405–2413. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- Guedj R., Desguerre I., Brassier A., Boddaert N., Hubert P., Oualha M. Unusual muscular injury in an infant with severe H1N1 infection. Pediatr. Neurol. 2012;47:51–54. doi: 10.1016/j.pediatrneurol.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Kraus W., Beachey E.H. Renal autoimmune epitope of group A streptococci specified by M protein tetrapeptide: Ile-Arg-Leu-Arg. Proc. Natl. Acad. Sci. U. S. A. 1998;85:4516–4520. doi: 10.1073/pnas.85.12.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laercrantz R., Hammarström S., Perlmann P., Gustafsson B.E. Immunological studies in ulcerative colitis. IV. Origin of autoantibodies. J. Exp. Med. 1968;128:1339–1352. doi: 10.1084/jem.128.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BA1., He W.P., Liu Y., Shu C.L., Li J., Gao R., Hou J., Li J., Cheng Y. Detection of autoimmune parameter of SARS patients. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2005;19:121–123. [PubMed] [Google Scholar]
- Liang X.F., Wang H.Q., Wang J.Z., Fang H.H., Wu J., Zhu F.C., Li R.C., Xia S.L., Zhao Y.L., Li F.J., Yan S.H., Yin W.D., An K., Feng D.J., Cui X.L., Qi F.C., Ju C.J., Zhang Y.H., Guo Z.J., Chen P.Y., Chen Z., Yan K.M., Wang Y. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2010;375:56–66. doi: 10.1016/S0140-6736(09)62003-1. [DOI] [PubMed] [Google Scholar]
- Mammas I.N., Koutsaftiki C., Papantzimas K., Symeonoglou Z., Koussouri M., Theodoridou M., Myriokefalitakis N. Thrombocytic thrombocytopenic purpura in a child with A/H1N1 influenza infection. J. Clin. Virol. 2011;51:146–147. doi: 10.1016/j.jcv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Monsalvo A.C., Batalle J.P., Lopez M.F., Krause J.C., Klemenc J., Hernandez J.Z., Maskin B., Bugna J., Rubinstein C., Aguilar L., Dalurzo L., Libster R., Savy V., Baumeister E., Aguilar L., Cabral G., Font J., Solari L., Weller K.P., Johnson J., Echavarria M., Edwards K.M., Chappell J.D., Crowe J.E., Jr., Williams J.V., Melendi G.A., Polack F.P. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat. Med. 2011;17:195–199. doi: 10.1038/nm.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naundorf S., Preithner S., Mayer P., Lippold S., Wolf A., Hanakam F., Fichtner I., Kufer P., Raum T., Riethmüller G., Baeuerle P.A., Dreier T. In vitro and in vivo activity of MT201, a fully human monoclonal antibody for pancarcinoma treatment. Int. J. Cancer. 2002;100:101–110. doi: 10.1002/ijc.10443. [DOI] [PubMed] [Google Scholar]
- Perdan-Pirkmajer K., Thallinger G.G., Snoj N., Čučnik S., Žigon P., Kveder T., Logar D., Praprotnik S., Tomšič M., Sodin-Semrl S., Ambrožič A. Autoimmune response following influenza vaccination in patients with autoimmune inflammatory rheumatic disease. Lupus. 2012;21:175–183. doi: 10.1177/0961203311429817. [DOI] [PubMed] [Google Scholar]
- Srinivasappa J., Saegusa J., Prabhakar B.S., Gentry M.K., Buchmeier M.J., Wiktor T.J., Koprowski H., Oldstone M.B., Notkins A.L. Molecular mimicry: frequency of reactivity of monoclonal antiviral antibodies with normal tissues. J. Virol. 1968;57:397–401. doi: 10.1128/jvi.57.1.397-401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S., Zhu X., Li Y., Shi M., Zhang J., Bourgeois M., Yang H., Chen X., Recuenco S., Gomez J., Chen L.M., Johnson A., Tao Y., Dreyfus C., Yu W., McBride R., Carney P.J., Gilbert A.T., Chang J., Guo Z., Davis C.T., Paulson J.C., Stevens J., Rupprecht C.E., Holmes E.C., Wilson I.A., Donis R.O. New world bats harbo diverse influenza A viruses. PLoS Pathog. 2013;9:e1003657. doi: 10.1371/journal.ppat.1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellozzi C., Broder K.R., Haber P., Guh A., Nguyen M., Cano M., Lewis P., McNeil M.M., Bryant M., Singleton J., Martin D., DeStefano F. Adverse events following influenza A (H1N1) 2009 monovalent vaccines reported to the Vaccine Adverse Event Reporting System, United States, October 1, 2009–January 31, 2010. Vaccine. 2010;28:248–7255. doi: 10.1016/j.vaccine.2010.09.021. [DOI] [PubMed] [Google Scholar]
- Webster R.G., Bean W.J., Gorman O.T., Chambers T.M., Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2012. Pandemic (H1N1) 2009. Update 75. Available from: http://www.who.int, http://www.cdc.gov/flu/spotlights/pandemic-global-estimates.htm (accessed 01.01.12) [Google Scholar]
- Williams S.E., Pahud B.A., Vellozzi C., Donofrio P.D., Dekker C.L., Halsey N., Klein N.P., Baxter R.P., Marchant C.D., Larussa P.S., Barnett E.D., Tokars J.I., McGeeney B.E., Sparks R.C., Aukes L.L., Jakob K., Coronel S., Sejvar J.J., Slade B.A., Edwards K.M. Causality assessment of serious neurologic adverse events following 2009 H1N1 vaccination. Vaccine. 2011;29:8302–8308. doi: 10.1016/j.vaccine.2011.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y., Jinghua L. 2nd ed. Beijing Scientific Publisher; 1997. Animal Viruses; pp. 312–318. [Google Scholar]


