Abstract
Geriatric psychiatric syndromes might serve as the starting point for a medical classification of psychiatric disorders, because their medical and neurological comorbidity and their clinical, neuropsychological, and neuroimaging features often reflect specific brain abnormalities. Geriatric syndromes, however, consist of complex behaviors that are unlikely to be caused by single lesions. We propose a model in which aging-related changes in specific brain structures increase the propensity for the development of certain psychiatric syndromes. The predisposing factors are distinct from the mechanisms mediating the expression of a syndromic state, much like hypertension is distinct from stroke, but constitutes a morbid vulnerability. We argue that research seeking to identify both brain abnormalities conferring vulnerability as well as the mediating mechanisms of symptomatology has the potential to lead to a medical classification of psychiatric disorders. In addition, a medical classification can guide the effort to improve treatment and prevention of psychiatric disorders as it can direct therapeutic efforts to the underlying predisposing abnormalities, the syndrome-mediating mechanisms, and to development of behavioral skills needed for coping with adversity and disability.
The principal function of medical diagnosis is to guide treatment. Effective treatment strategies depend on the physician’s ability to identify a clinical picture with distinct underlying biological and nonbiological contributors that can be targeted with the available interventions. Despite the advantages of the medical diagnostic system, psychiatric nosology consists of phenomenologically defined syndromes that are either biologically heterogeneous or have unknown biological contributors. We argue that geriatric psychiatric syndromes, and geriatric depression in particular, can serve as the starting point for a medical classification in psychiatry, because they occur in the context of medical and neurological comorbidity and are accompanied by clinical, neuropsychological, and neuroimaging findings pointing to specific brain abnormalities. This paper reviews relevant literature and proposes a model and a research agenda that might lead to a medical classification.
The DSM-III Revolution
Historically, the DSM-III was created to provide an operational framework for the classification of clinical observations during an era in which diagnoses had previously been made on the basis of clinicians’ individual views and even feelings. Indeed, the DSM-III and its successors revolutionized the field by creating a common language for clinicians to use in characterizing their patients. An equally profound change occurred in psychiatric research, where systematic assessment of psychopathology became standard practice. Like all landmark ideas, the DSM-III soon began to be used for purposes beyond its original raison d’ etre; it became a nosology without a medical basis. One of the reasons for this development has been the absence of definitive knowledge of biological contributors to most of the major DSM syndromes.
A diagnostic system that relies exclusively on phenomenology has significant limitations. Phenomenology strives for syndromic homogeneity, although syndromic homogeneity is neither feasible nor desirable. First, use of the DSM-III or DSM-IV criteria results in nonhomogeneous syndromes, because different symptom permutations might qualify for the same diagnosis. Second, psychiatric disorders need not have homogeneous clinical presentations any more than medical illnesses do. Authoritative textbooks of medicine, like the Harrison’s Principles of Internal Medicine, permit a variety of accompanying features in describing the clinical picture of medical illnesses, (e.g., pneumonia might be accompanied by dyspnea, fever). The typical presentation of a syndrome rarely occurs in all patients with the same medical illness (e.g., myocardial infarction without precordial pain is common among older adults). Some diseases are, in fact, characterized by their clinical heterogeneity. Syphilis has so many diverse clinical presentations that it once was referred to as the great imitator. The diverse clinical presentations of mycoplasma pneumonia led to the early term atypical pneumonia. Insistence on uniform clinical presentation can be counterproductive, because it might classify as “non-syndromes” conditions with common pathogenetic contributors. Nonetheless, improved understanding of specific contributors to the pathogenesis of a larger syndrome might provide information on specific symptoms. For example, subjects with frontrostriatal dysfunction and depression might exhibit executive function deficits, whereas individuals with an early-onset depression with significant genetic contribution might exhibit other clinical features.
Readiness for a Medical Classification
The etiology of psychiatric disorders remains unknown; however, advancements in understanding biological correlates of psychiatric disorders and the context in which they occur place us in a better position to propose a research agenda that would eventually lead to a medical classification of psychiatric disorders.
Medical classification requires that some of the clinical manifestations of a disorder are mediated by biological mechanisms, rather than mandating a unitary biological explanation for all aspects of the disorder. Therefore, medical classification allows nonbiological factors to contribute to the development of a disorder. For example, severe acute respiratory syndrome (SARS) is a viral respiratory infection with diverse clinical presentation, a higher incidence during the cold months of the year, and a higher mortality among elders and medically compromised patients. Therefore, even in an infectious disorder like SARS, environmental facilitation, and noninfectious medical burden (nonspecific to the SARS virus) play a role in the development and course of the disease.
Geriatric psychiatric syndromes and depression, in particular, occur in patients with medical and neurological disorders and offer an opportunity for research leading to a medical classification model. Like disorders complying with the demands of medical classification, biological research on psychiatric syndromes needs to take into consideration the biological and nonbiological context in which geriatric psychiatric disorders occur.
Although medical/neurological comorbidity offers a research window to the brain, it is unreasonable to expect a direct lesion-syndrome relationship. There are at least five reasons for the absence of such a one-to-one relationship. First, the most likely brain abnormalities associated with behavioral disorders affect high-level neural systems. These systems are redundant and interactive. Unlike peripheral nerve lesions and, to a lesser extent, lesions of the internal capsule and some brain stem areas, lesions of high-level cortical and subcortical structures do not result in identical behavioral abnormalities. Neural system redundancy and plasticity might explain the heterogeneity in symptom expression, because other centers assume some of the function of the lesioned circuitry. In addition, the complex interactions among neural systems (lateral prefrontal cortex, anterior thalamus, anterior cingulate, subgenual cingulate, orbitofrontal cortex, hippocampus, and medial frontal cortex) might explain why different brain lesions or pathological processes can lead to similar syndromes. Moreover, there is evidence that differences in limbic-cortical activation are associated with differential response to antidepressant treatments; more limited limbic-cortical and cortical-cortical connections differentiate responders to cognitive behavioral therapy from responders to pharmacotherapy (Seminowicz et al 2004). A second reason for a lack of a direct central nervous system (CNS) lesion-syndrome relationship is the contribution of non-CNS medical burden that, either directly or through the resultant disability, facilitates the development of psychopathology. Third, the premorbid competencies that each person possesses at the time of the CNS insult might influence the character and course of psychopathology. These can be behavioral (e.g., skills relevant to problem solving and dealing with stress) or cognitive (e.g., ability of a person to assess risks and benefits of specific situations and decisions and act accordingly). A fourth reason is that environmental adversity might lead to adaptation problems and enhance abnormal behavioral patterns. Environmental adversity needs to be viewed in the context of the individual’s physical, behavioral, and cognitive skills, because a mild environmental change might overwhelm a person with underdeveloped skills. Finally, genetic make-up is an important contributor in early-onset depression, but also increases the risk of depression in patients with late-onset depression occurring after a brain insult (e.g., personal or family history of a depressive disorder has been shown to increase the incidence of post-stroke depression as much as, if not more than, the site of the vascular lesion; Morris et al 1992).
Accordingly, research aiming to generate the grounds for a medical classification of psychiatric disorders needs to focus on CNS abnormalities that confer a morbid vulnerability to psychopathology development, rather than on those leading to a distinct behavioral syndrome (Figure 1). The affected neural systems might determine, to some extent, the clinical presentation and course of the ensuing behavioral disorder, however, medical burden, cognitive and behavioral competence and their relationship to the individual’s environment, and genetic make-up might also influence the neurobiology as well as the clinical presentation and outcomes of the behavioral disorder. Additional studies might focus on the mechanisms mediating the expression of syndromic states in predisposed individuals.
Figure 1.
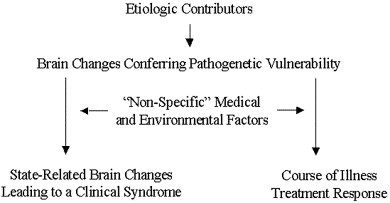
A Model for Late-life Psychiatric Disorders.
Geriatric Depression
Geriatric depression might serve as a model entity for the work that is needed toward a medical classification of psychiatric disorders. First, geriatric depression resembles medical diseases. Along with changes in mood, ideation, and behavior, geriatric depression is associated with peripheral body changes. These include the vegetative syndrome, but also hypercortisolemia, increased abdominal fat, decreased bone density, and increased risk for type-II diabetes and hypertension (Brown et al 2004). Depression of older adults often is accompanied by cognitive impairments resulting from abnormally functioning neural systems; dysfunction of frontostriatal pathways, the amygdala, and the hippocampus have been implicated. In addition to CNS pathology, geriatric patients have multiple medical illnesses requiring treatment with agents crossing the blood-brain barrier, experience disability, and often are presented with difficulties in negotiating their environment. Each of these factors might contribute to the heterogeneity of the clinical presentation and the course of geriatric depression.
Although geriatric depressed patients vary in their clinical presentation and course, most of them have aging-related CNS changes. Thus, an appropriate first step for research relevant to medical classification is the search for CNS pathology predisposing to depressive symptoms and influencing their course (Figure 1). This work needs to be followed by studies examining the relationships of CNS pathology to the context in which geriatric depression occurs (e.g., nonneurological medical burden, disability, poor matching of the depressed person to his/her habitat, psychiatric history).
Abnormalities in more that one brain region might predispose to depression. There are at least two reasons for this assumption. First, abnormalities in one region might influence other functionally connected regions. Second, elderly patients might have abnormalities in more than one brain region with complex interactions, leading to vulnerability to depression. In the following sections are three examples of brain structure abnormalities that, available findings suggest, increase vulnerability to depression. It should be emphasized, however, that these might not be the only brain structures whose compromise predisposes to depression.
Frontostriatal Dysfunction
Two sets of clinical findings suggest a relationship between frontostriatal dysfunction and geriatric depression. First, executive dysfunction, a disturbance resulting from compromised integrity of frontal structures and their subcortical connections, is common in geriatric depression (Lockwood et al 2002, Nebes et al 2001) and persists after improvement of mood-related symptoms (Murphy and Alexopoulos 2004, Nebes et al 2003). Second, disorders compromising frontostriatal pathways are often complicated by depression. Subcortical dementing disorders, including vascular dementia, Parkinson’s disease, and Huntington’s disease are more likely to result in depression than cortical dementias (Sobin and Sackeim 1997). Moreover, development of depression is more likely to occur in Alzheimer’s patients with subcortical atrophy (Starkstein et al 1995).
Along with clinical observations, structural neuroimaging studies have documented that neurologically unimpaired depressed patients have low volumes of structures of frontostriatal networks, including the subgenual anterior cingulate (Drevets et al 1997), caudate head (Krishnan et al 1992), and the putamen (Husain et al 1991). Moreover, low volumes of the anterior cingulate, the orbitofrontal cortex, and the rectus gyrus have been reported in geriatric depression (Ballmaier et al 2004), and hyperintensities in subcortical structures and their frontal connections are prevalent in geriatric depression (Coffey et al 1990, Krishnan et al 1997, Steffens et al 1999). Subcortical hyperintensities have been associated with executive dysfunction (Aizenstein et al 2002, Boone et al 1992), the clinical expression of frontostriatal dysfunction. Reduction in glia of the subgenual anterior cingulate gyrus (Rajkowska et al 1999) as well as abnormalities in neurons of the dorsolateral prefrontal cortex have been observed in unipolar depressed patients (Ongur et al 1998b).
Cognitive neuroscience studies suggest that dysfunction in certain parts of frontostriatal circuitry (i.e., the prefrontal cortices and the anterior cingulate) is relevant to the development of depressive symptoms. A model of the prefrontal cortex function proposes that this brain structure maintains the representation of an individual’s goals and sends signals to other brain areas to facilitate the expression of task-appropriate response (Davidson et al 2002). Such signals facilitate the expression of the most adaptive response to a stimulus at the expense of an immediately rewarding response. Theoretically, abnormalities in prefrontal circuitry responsible for positive affect-guided anticipation might predispose to some types of depression and result in failure to anticipate positive incentives. The left medial orbitofrontal cortex is activated in response to reward, and the right orbitofrontal cortex in response to punishment (O’Doherty et al 2001); both of these functions are relevant to depression. The anterior cingulate gyrus has connections to brain structures relevant to the expression of depressive symptoms and is responsible for functions that are often impaired in depression. Accordingly, the affective cingulate subdivision is connected to the orbitofrontal cortex, the amygdala, the lateral hypothalamus, the anterior insula, the periaqueductal grey, and some autonomic stem centers (Bush et al 2000, Ongur et al 1998a). The affective cingulate assesses conflicts between current function and information with motivational consequences and integrates affective and cognitive information. The cognitive cingulate subdivision has connections with the dorsolateral prefrontal cortex, the posterior cingulate, the parietal cortex, and the supplementary motor cortex. Its principal function is to monitor competing responses and modulate attention and executive functions in collaboration with the dorsolateral cortex (Bush et al 2000, Whalen et al 1998).
Frontostriatal dysfunction might influence the clinical presentation of geriatric depression. Among elderly depressed patients, those with executive dysfunction have more pronounced psychomotor retardation and reduced interest in activities than depressed patients without executive dysfunction (Alexopoulos et al 1997b, Alexopoulos et al 2002, Krishnan et al 1997), a clinical presentation resembling medial frontal lobe syndromes. Nonetheless, depressed patients with executive dysfunction have sad mood, hopelessness, helplessness, and worthlessness of similar severity to that of depressed patients without executive dysfunction (Alexopoulos et al 2002) and meet criteria for major depression.
Clinical and laboratory studies suggest that frontostriatal abnormalities influence the course of depression. Executive dysfunction, a clinical expression of frontostriatal abnormalities predicts slow, poor response (Alexopoulos et al 2004, Dunkin et al 2000, Kalayam and Alexopoulos 1999, Potter et al 2004, Simpson et al 1998), and unstable response (Alexopoulos et al 1999) to antidepressants, although some disagreement exists (Butters et al 2004). White matter abnormalities have been associated both with executive dysfunction (Aizenstein et al 2002, Boone et al 1992) and with poor outcomes of geriatric depression (Alexopoulos 2002, Hickie et al 1995, Hickie et al 1997, O’Brien et al 1998, Simpson et al 1998, Steffens et al 2001). Hypometabolism of the rostral anterior cingulate was reported in treatment-resistant depression, whereas cingulate hypermetabolism was associated with favorable response (Mayberg 2001). Anterior cingulate activity is a predictor of the extent of treatment response in depressed young adults (Pizzagalli et al 2001). Finally, increased left frontal error negative wave amplitude after a response inhibition task (mediated by the anterior cingulate) predicts limited or slow change in depressive symptoms in elders receiving citalopram treatment (Kalayam and Alexopoulos 2003).
Amygdalar Dysfunction
Patients experiencing their first depressive episode have larger amygdala volumes than patients with recurrent depression and healthy control subjects (Frodl et al 2003). In contrast, decline in amygdalar volume has been documented in recurrent depression (Sheline et al 1998). Along these lines, a hypermetabolic state of the amygdala has been associated with greater depressive symptoms and negative emotions (Drevets 1999, Drevets 2003). It has been hypothesized that increased activation of the amygdala is a result of inadequate inhibition of this structure by prefrontal centers (Drevets 1999). Thus, the combination of increased amygdalar metabolism with failure of cortical modulation of the emotional input might confer vulnerability to depression.
The association of amygdala to depression is further supported by its role in the processing of stimuli. A series of studies suggest that the amygdala is integral to the modulation of mood states, because it mediates the perception of emotion and particularly the processing of aversive stimuli (Davidson 2001). The amygdala integrates negative emotions and signals to centers responsible for coping behavior and autonomic activity (LeDoux 1993).
The pathways associated with emotional perception are affected by aging-related changes. Aging-related changes associated with a loss or attenuation of emotional perception might contribute to a depressed or apathetic clinical presentation. Brain diseases (e.g., stroke and subcortical disorders such as Parkinson’s disease) might damage the connections between regions of the amygdala, the medial dorsal thalamic nucleus, and the orbital and medial prefrontal cortex and predispose to depression (Drevets 1999). Finally, hypercortisolemia occurring during chronic medical illnesses is associated with increased amygdalar activity, and conversely, amygdalar activation stimulates cortisol release, representing yet another mechanism by which comorbid illnesses might increase the risk of a depressive syndrome (Erickson et al 2003).
Hippocampal Dysfunction
Growing evidence suggests that hippocampal abnormalities confer vulnerability to depression (Bremner et al 2000, Sheline 2003). Hippocampal volume was found reduced in individuals in their first episode of depression, raising the possibility that hippocampal abnormalities represent a premorbid risk factor (Frodl et al 2002), although some disagreement exits (Campbell et al 2004, Hastings et al 2004). Studies have documented that reduced hippocampal size is correlated with the total length of time spent in a depressed state (Brown et al 2004, Sheline 2000). Moreover, a rapid decline in hippocampal volume has been documented over several years after the first depressive episode, even when treatment was offered (MacQueen 2003).
Abnormalities of the hippocampus are relevant to the aging population, because this structure is particularly vulnerable to aging and aging-related changes. Genetics determine only 40% of the variance in the size of hippocampus, while the rest of the variance is determined by environmental factors (Sullivan 2001). Advanced age is associated with hippocampal atrophy. Moreover, the CA1 hippocampal region and the subiculum are vulnerable to ischemia (MacQueen 2003) and to the effect of hypercortisolemia resulting from stress and chronic medical illness (Miller and O’Callaghan 2003).
Mechanisms of the Depressive Syndrome
The above findings suggest that frontostriatal, amygdalar, and hippocampal dysfunction occur in some patients with geriatric depression; however, depressed elders with clinical or laboratory evidence of dysfunction in these systems have neither a homogeneous clinical presentation nor a homogeneous course of illness. For this reason, frontostriatal, amygdalar, and hippocampal dysfunction are best conceptualized as a morbid vulnerability that predisposes to depression and increases its propensity for chronicity and relapse. This conceptualization allows for other factors to contribute to the development of the syndrome and influence its course, including overall medical burden, disability interfering with adaptation, psychosocial adversity, prior psychiatric history, and so forth.
Predisposition to depression need not be identical to the mechanisms of symptom expression during depressive states. Accordingly, frontostriatal, amygdalar, and hippocampal dysfunction need not be the exact final mechanisms that directly mediate symptom expression during depressive episodes. Functional neuroimaging studies converge to the conclusion that, during depressive states, dorsal neocortical structures are hypometabolic and ventral limbic structures are hypermetabolic (Alexopoulos 2002, Drevets 2000). Similar changes occur in experimentally induced sadness (Mayberg 2001). So the expression of depressive symptoms might be mediated by a common pathway, but the predisposing factor, in this case, frontostriatal, amygdalar, or hippocampal dysfunction, might be unique to a subgroup of depressed patients. In contrast, nondepressed individuals who develop sadness at-will might have a quick normalization of their sadness-associated brain metabolic changes, because they have no predisposing factors to sustain symptoms of depression.
Etiology of Geriatric Depression
Frontostriatal, amygdalar, and hippocampal dysfunction can be caused by various processes. Degenerative, vascular, as well as immune and inflammatory processes might lead to dysfunction of subcortical structures and thus be the etiological factors underlying vulnerability to depression. Depression often occurs in degenerative disorders of subcortical structures with a long preclinical phase (Sobin and Sackeim 1997). Similarly, depression is common in patients with vascular risk factors and disorders resulting in subcortical lesions (Alexopoulos et al 1997a, Krishnan et al 1997). Finally, pro- inflammatory cytokines, including interferon-γ, interleukin-6, tumor necrosis factor-α, and C-reactive protein are elevated during depressed states (Schiepers et al 2005, Thomas et al 2005). Interferon administration often leads to development of depressive symptoms or syndromes, but also to cognitive and electrophysiological changes (Amodio et al 2005).
The preceding paradigm follows the traditional medical conceptualization of disease (i.e., etiologic contributors: brain changes conferring pathogenetic vulnerability: interaction with nonspecific medical and environmental factors: state-related brain changes: clinical syndrome). Moreover, vulnerability-inducing brain changes might influence the course of illness (Figure 1).
Treatment Implications
Identifying neural systems whose abnormalities contribute to depression might guide pharmacological research in selecting candidate antidepressants for specific subgroups of depressed elders. For example, depressed elders with evidence of frontostriatal impairment might be candidates for dopamine enhancing agents, whereas use of such agents in a broader group of depressed patients might dilute the therapeutic signal and lead to the erroneous conclusion that dopaminergic agents have no antidepressant properties for any patients (Alexopoulos 2001). Similarly, research might examine whether adjunct pharmacotherapy with agents enhancing the metabolism of frontostriatal structures (e.g., modafinil; Scammell 2000) can improve the outcomes of depressed patients with evidence of anterior cingulate dysfunction. Although these approaches are sensible, their efficacy must be clinically tested. The reason is that direct lesion-to-syndrome relationships are unlikely in late-life depression. Moreover, even “selectively-acting drugs” have broader effects and change the function of systems other than those on which they exert their immediate action (Millan 2004). Finally, treatment and prevention studies need to investigate the efficacy of behavioral approaches (e.g., problem solving therapy) that remedy behavioral deficits originating from executive dysfunction (Alexopoulos et al 2003).
Understanding the role of amygdala pathology as a predisposing condition to depression has treatment implications relevant to a medical model classification approach. Preliminary evidence suggests that a persistently hypermetabolic state of the amygdala is associated with increased relapse risk or inadequate response to antidepressant treatment (Drevets 1999). Further research in this area might document that amygdalar hyperactivity demonstrated through neuroimaging studies or its associated hypercortisolemia predict unstable antidepressant response and dictate a cautious course of clinical action.
As in the case of frontostriatal systems and the amygdala, studies of the role of hippocampus in predisposing to depression can guide the development of focused therapies. For example, a treatment study using cognitive behavior therapy for adults with major depression demonstrated that treatment response was associated with increases in metabolism in the hippocampus and dorsal cingulate (Goldapple et al 2004). Understanding these regional changes might also influence the selection of treatment, because new data suggest that cellular plasticity might be enhanced by antidepressant treatment in a way that intercedes in hippocampal loss by inducing regional neurogenesis (Kempermann and Kronenberg 2003). Specifically, serotonergic antidepressants have been shown to increase neurogenesis in the dentate gyrus of the rat hippocampus (Malberg et al 2000).
Implications for Geriatric Psychiatric Syndromes Other than Depression
In the preceding sections, brain abnormalities predisposing to depression were discussed. Discrete brain abnormalities, however, might predispose to a variety of behavioral syndromes, including psychosis, disinhibition, and delirium. For example, the presence of Lewy bodies in limbic and neocortical regions in patients with Lewy body dementia is thought to account for the neuropsychiatric symptoms of this disorder (e.g., visual hallucinations), whereas the more subcortical distribution of Lewy bodies observed in Parkinson’s disease accounts for the predominance of motor symptoms (Barber et al 2001). Along the same lines, progressive supranuclear palsy (PSP) and Parkinson’s disease have different patterns of regional brain degeneration and present rather distinct behavioral pathology (Aarsland et al 2001). Progressive supranuclear palsy leads to degeneration of many subcortical (e.g., the putamen, globus pallidus, caudate, and subthalamus) as well as orbitofrontal and medial frontal regions. In contrast, Parkinson’s disease primarily leads to degeneration of mesocortical dopaminergic and other monoaminergic regions. Consistent with these regional differences is the greater likelihood of patients with PSP to develop disinhibition and apathy syndromes and of patients with Parkinson’s disease to present hallucinations, delusions, and depression. Differences in behavioral pathology between PSP and Parkinson’s disease persist even after accounting for the use of dopaminergic medications (Aarsland et al 2001). Finally, discrete brain abnormalities might predispose to side effects, as in the case of Parkinson’s disease, where degeneration of the mesocortical dopaminergic system might increase vulnerability to l-dopa and lead to delirium.
Toward a Research Agenda
Psychiatric syndromes consist of complex and heterogeneous behaviors unlikely to follow a simple lesion-syndrome relationship. We are positing that aging-related changes in specific brain structures interacting with personal vulnerability (either genetic, neurodevelopmental, or early trauma), or any other individual, biological, or cultural vulnerabilities (e.g., gender or ethnicity), and environmental factors create a propensity for development of certain psychiatric syndromes. The predisposing factors might be distinct from the actual mechanisms operating during the expression of a syndromic state, much like hypertension is distinct from stroke, but represents a predisposing vulnerability.
We argue that research seeking to identify both morbid vulnerabilities to psychiatric syndromes and the mediating mechanisms of symptomatology has the potential to lead to a medical classification of psychiatric disorders. In turn, a medical classification can guide the effort to improve treatment and prevention of psychiatric disorders by focusing therapeutic efforts to the underlying predisposing abnormalities as well as to the syndrome-mediating mechanisms.
The proposed model parallels the etiopathogenesis of syndromes such as myocardial infarction, where obesity, hypertension, cholesterol, and emotional stress are all multimodal risk factors compromising vascular integrity and predisposing to infarction. Similarly, CNS aging or vascular disease as well as behavioral disability, physical deconditioning, and psychosocial adversity might compromise frontostriatal, amygdalar or hippocampal integrity and confer vulnerability to depression. A similar model has been articulated by McEwen, who describes the cumulative effects of the above risk factors as “allostatic load” (McEwen 2003), resulting from the individual’s inability to accommodate to extrinsic stressors.
The research agenda suggested in the following section, in addition to focusing on mechanisms, can guide treatment research targeting persons with specific vulnerabilities. For example, an intervention might involve medication geared toward discrete neural systems predisposing to psychiatric syndromes, treatments preventing further damage to these neural systems (e.g., treatment of hypertension, hyperlipidemia, hypercortisolemia as well as health-promoting behaviors, including stress-reduction, exercise, and perhaps antioxidants), and behavioral approaches aimed at improving coping with adversity and remedying disability. Thus, we propose:
-
1
Studies aimed to understand the role of specific brain structure abnormalities in predisposing to late-life behavioral pathology. Such studies need to focus on interactions between aging-related changes in brain structures, personal vulnerability (either genetic, neurodevelopmental, or early trauma), other individual, biological, or cultural vulnerabilities (e.g., gender or ethnicity), and environmental factors.
-
2
Studies aimed to understand aging-related processes leading to brain structure damage predisposing to psychiatric disorders.
-
3
Studies aimed to understand the mechanisms mediating psychiatric symptom expression in elderly patients.
-
4
Treatment and prevention studies targeting the predominant neurotransmitter system abnormalities of pathways conferring vulnerability to psychiatric disorders. Such studies should focus on patients with evidence of specific neural network impairment, rather than the whole elderly population with similar symptoms.
-
5
Studies of psychosocial interventions targeting the behavioral deficits produced by abnormalities in neural networks predisposing late-life behavioral pathology.
Acknowledgments
This paper was supported by National Institute of Mental Health grants P30 MH 68638, RO1 MH65652, and the Sanchez Foundation.
Dr. Alexopoulos has received research grants by Forest Pharmaceuticals, Inc. and Cephalon and participated in scientific advisory board meetings of Forest Pharmaceuticals. He has given lectures supported by Forest, Cephalon, Bristol Meyers, Janssen, Pfizer, and Lilly and has received support by Comprehensive Neuroscience, Inc. for the development of treatment guidelines in late-life psychiatric disorders.
References
- Aarsland D., Litvan I., Larsen J.P. Neuropsychiatric symptoms of patients with progressive supranuclear palsy and Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2001;13:42–49. doi: 10.1176/jnp.13.1.42. [DOI] [PubMed] [Google Scholar]
- Aizenstein H.J., Nebes R.D., Meltzer C.C., Fukui M.B., Williams R.L., Saxton J. The relation of white matter hyperintensities to implicit learning in healthy older adults. Int J Geriatr Psychiatry. 2002;17 doi: 10.1002/gps.685. 664–649. [DOI] [PubMed] [Google Scholar]
- Alexopoulos G.S. “The depression-executive dysfunction syndrome of late life”: A specific target for D3 agonists? Am J Geriatr Psychiatry. 2001;9:22–29. [PubMed] [Google Scholar]
- Alexopoulos G.S. Frontostriatal and limbic dysfunction in late-life depression. Am J Geriatr Psychiatry. 2002;10:687–695. [PubMed] [Google Scholar]
- Alexopoulos G.S., Bruce M.L., Hull J., Sirey J.A., Kakuma T. Clinical determinants of suicidal ideation and behavior in geriatric depression. Arch Gen Psychiatry. 1999;56:1048–1053. doi: 10.1001/archpsyc.56.11.1048. [DOI] [PubMed] [Google Scholar]
- Alexopoulos G.S., Kiosses D.N., Klimstra S., Kalayam B., Bruce M.L. Clinical presentation of the “depression-executive dysfunction syndrome” of late life. Am J Geriatr Psychiatry. 2002;10:98–106. [PubMed] [Google Scholar]
- Alexopoulos G.S., Kiosses D.N., Murphy C., Heo M. Executive dysfunction, heart disease burden, and remission of geriatric depression. Neuropsychopharmacology. 2004;29:2278–2284. doi: 10.1038/sj.npp.1300557. [DOI] [PubMed] [Google Scholar]
- Alexopoulos G.S., Meyers B.S., Young R.C., Campbell S., Sibersweig D., Charlson M. The “vascular depression” hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Alexopoulos G.S., Meyers B.S., Young R.C., Kakuma T., Silbersweig D., Charlson M. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562–565. doi: 10.1176/ajp.154.4.562. [DOI] [PubMed] [Google Scholar]
- Alexopoulos G.S., Raue P., Arean P. Problem-solving therapy versus supportive therapy in geriatric major depression with executive dysfunction. Am J Geriatr Psychiatry. 2003;11:46–52. [PubMed] [Google Scholar]
- Amodio P., Toni E.N., Cavaletto L., Mapell D., Bernardinello E., Del Piccolo F. Mood, cognition and EEG changes during interferon alpha (alpha-INF) treatment for chronic hepatitis C. J Affect Disord. 2005;84:93–98. doi: 10.1016/j.jad.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Ballmaier M., Toga A.W., Blanton R.E., Sowell E.R., Lavretsky H., Peterson J. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: An MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004;161:99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- Barber R., Panikkar A., McKeith I.G. Dementia with Lewy bodies: Diagnosis and management. Int J Geriatr Psychiatry. 2001;16:S12–S18. doi: 10.1002/1099-1166(200112)16:1+<::aid-gps562>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Boone K.B., Miller B.L., Lesser I.M., Mehringer C.M., Hill-Gutierrez, Goldberg M.A., Berman N.G. Neuropsychological correlates of white-matter lesions in healthy elderly subjects. A threshold effect. Arch Neurol. 1992;49:549–554. doi: 10.1001/archneur.1992.00530290141024. [DOI] [PubMed] [Google Scholar]
- Bremner J., Narayan M., Anderson E., Staib L., Miller H., Charney D.S. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Brown E.S., Varghese F.P., McEwen B.S. Association of depression with medical illness: Does cortisol play a role? Biol Psychiatry. 2004;55:1–9. doi: 10.1016/s0006-3223(03)00473-6. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Butters M., Whyte E., Nebes R., Begley A.E., Dew M.A., Mulsant B.H. The nature and determinants of neuropsychological functioning in late-life. Arch Gen Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Campbell S., Marriott M., Nahmias C., MacQueen G.M. Lower hippocampal volume in patients suffering from depression: A meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Coffey C.E., Figiel G.S., Djang W.T., Weiner R.D. Subcortical hyperintensity on magnetic resonance imaging: A comparison of normal and depressed elderly subjects. Am J Psychiatry. 1990;147:187–189. doi: 10.1176/ajp.147.2.187. [DOI] [PubMed] [Google Scholar]
- Davidson R.J. Toward a biology of personality and emotion. Ann N Y Acad Sci. 2001;935:191–207. doi: 10.1111/j.1749-6632.2001.tb03481.x. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., Pizzagalli D., Nitschke J.B., Putnam K. Depression: Perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Drevets W.C. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Drevets W.C. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–819. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Drevets W.C. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Drevets W.C., Price J.L., Simpson J.R., Jr, Todd R.D., Reich T., Vannier M., Raichle M.E. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Dunkin J.J., Leuchter A.F., Cook I.A., Kasl-Godley J.E., Abrams M., Rosenberg-Thompson S. Executive dysfunction predicts nonresponse to fluoxetine in major depression. J Affect Disord. 2000;60:13–23. doi: 10.1016/s0165-0327(99)00157-3. [DOI] [PubMed] [Google Scholar]
- Erickson K., Drevets W., Schulkin J. Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neurosci Biobehav Rev. 2003;27:233–246. doi: 10.1016/s0149-7634(03)00033-2. [DOI] [PubMed] [Google Scholar]
- Frodl T., Meisenzahl E., Zetzsche T., Born C., Groll C., Jager M. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry. 2002;159:1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- Frodl T., Meisenzahl E.M., Zetzsche T., Born C., Jager M., Groll C. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- Goldapple K., Segal Z., Garson C., Lau M., Bieling M., Kennedy S., Mayberg H. Modulation of cortical-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Hastings R., Parsey R., Oquendo M., Arango V., Mann J. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29:952–959. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- Hickie I., Scott E., Mitchell P., Wilhelm K., Austin M.P., Bennett B. Subcortical hyperintensities on magnetic resonance imaging: Clinical correlates and prognostic significance in patients with severe depression. Biol Psychiatry. 1995;37:151–160. doi: 10.1016/0006-3223(94)00174-2. [DOI] [PubMed] [Google Scholar]
- Hickie I., Scott E., Wilhelm K., Brodaty H. Subcortical hyperintensities on magnetic resonance imaging in patients with severe depression-a longitudinal evaluation. Biol Psychiatry. 1997;42:367–374. doi: 10.1016/S0006-3223(96)00363-0. [DOI] [PubMed] [Google Scholar]
- Husain M.M., McDonald W.M., Doraiswamy P.M., Figiel G.S., Na C., Escalona P.R. A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry Res. 1991;40:95–99. doi: 10.1016/0925-4927(91)90001-7. [DOI] [PubMed] [Google Scholar]
- Kalayam B., Alexopoulos G.S. Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry. 1999;56:713–718. doi: 10.1001/archpsyc.56.8.713. [DOI] [PubMed] [Google Scholar]
- Kalayam B., Alexopoulos G.S. A preliminary study of left frontal region error negativity and symptom improvement in geriatric depression. Am J Psychiatry. 2003;160:2054–2056. doi: 10.1176/appi.ajp.160.11.2054. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Kronenberg G. Depressed new neurons-adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry. 2003;54:499–503. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- Krishnan K.R., Hays J.C., Blazer D.G. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- Krishnan K.R., McDonald W.M., Escalona P.R., Doraiswamy P.M., Na C., Husain M.M. Magnetic resonance imaging of the caudate nuclei in depression. Preliminary observations. Arch Gen Psychiatry. 1992;49:553–557. doi: 10.1001/archpsyc.1992.01820070047007. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Emotional memory: In search of systems and synapses. Ann N Y Acad Sci. 1993;702:149–157. doi: 10.1111/j.1749-6632.1993.tb17246.x. [DOI] [PubMed] [Google Scholar]
- Lockwood K.A., Alexopoulos G.S., van Gorp W.G. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119–1126. doi: 10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- MacQueen G.M., Campell S., McEwen B.S., Macdonald K., Amano S., Joffe R.T. Course of illness, hippocampal function and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg J., Eisch A., Nestler E., Duman R. Chronic antidepressant treatment increases neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg H. Depression and frontal-subcortical circuits. Focus on prefrontal-limbic interactions. In: Lichter D.C., Cummings J.L., editors. Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders. The Guilford Press; New York: 2001. pp. 177–206. [Google Scholar]
- McEwen B.S. Mood disorders and allostatic load. Biol Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- Millan M.J. The role of monoamines in the actions of established and novel antidepressant agents: A critical review. Europ J Phamacol. 2004;500:371–384. doi: 10.1016/j.ejphar.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Miller D.B., O’Callaghan J.P. Effects of aging and stress on hippocampal structure and function. Metabolism. 2003;52:17–21. doi: 10.1016/s0026-0495(03)00296-8. [DOI] [PubMed] [Google Scholar]
- Morris P.L., Robinson R.G., Raphael B., Samuels J., Molloy P. The relationship between risk factors for affective disorder and poststroke depression in hospitalised stroke patients. Aust N Z J Psychiatry. 1992;26:208–217. [PubMed] [Google Scholar]
- Murphy C.F., Alexopoulos G.S. Longitudinal association of initiation/perseveration and severity of geriatric depression. Am J Geriatr Psychiatry. 2004;12:50–56. [PubMed] [Google Scholar]
- Nebes R.D., Butters M.A., Houck P.R., Zmuda M.D., Aizenstein H., Pollock B.G. Dual-task performance in depressed geriatric patients. Psychiatry Res. 2001;102:139–151. doi: 10.1016/s0165-1781(01)00244-x. [DOI] [PubMed] [Google Scholar]
- Nebes R.D., Pollock B.G., Houck P.R., Butters M.A., Mulsant B.H., Zmuda M.D., Reynolds C.F., III Persistence of cognitive impairment in geriatric patients following antidepressant treatment: A randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res. 2003;37:99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- O’Brien J., Ames D., Chiu E., Schweitzer I., Desmond P., Tress B. Severe deep white matter lesions and outcome in elderly patients with major depressive disorder: Follow up study. BMJ. 1998;317:982–984. doi: 10.1136/bmj.317.7164.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J., Kringelbach M.L., Rolls E.T., Hornak J., Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Ongur D., An X., Price J.L. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol. 1998;401:480–505. [PubMed] [Google Scholar]
- Ongur D., Drevets W.C., Price J.L. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D., Pascual-Marqui R.D., Nitschke J.B., Oakes T.R., Larson C.L., Abercrombie H.C. Anterior cingulate activity as a predictor of degree of treatment response in major depression: Evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- Potter G.G., Kittinger J.D., Wagner H.R., Steffens D.C., Krishnan K.R. Prefrontal neuropsychological predictors of treatment remission in late-life depression. Neuropsychopharmacology. 2004;29:2266–2271. doi: 10.1038/sj.npp.1300551. [DOI] [PubMed] [Google Scholar]
- Rajkowska G., Miguel-Hidalgo J.J., Wei J., Dilley G., Pittman S.D., Meltzer H.Y. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Scammell T.E., Estabrooke I.V., McCarthy M.T., Chemelli R.M., Yanagisawa M., Miller M.S., Saper C.B. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20:8620–8628. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiepers O.J., Wichers M.C., Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Bio Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Seminowicz D.A., Mayberg H.S., McIntosh A.R., Goldapple K., Kennedy S., Segal Z., Rafi-Tari S. Limbic-frontal circuitry in major depression: A path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Sheline Y. 3D MRI studies of neuroanatomic changes in unipolar depression: The role of stress and medical comorbidity. Biol Psychiatry. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- Sheline Y. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Sheline Y., Gado M., Price J. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- Simpson S., Baldwin R.C., Jackson A., Burns A.S. Is subcortical disease associated with a poor response to antidepressants? Neurological, neuropsychological and neuroradiological findings in late-life depression. Psychol Med. 1998;28:1015–1026. doi: 10.1017/s003329179800693x. [DOI] [PubMed] [Google Scholar]
- Sobin C., Sackeim H.A. Psychomotor symptoms of depression. Am J Psychiatry. 1997;154:4–17. doi: 10.1176/ajp.154.1.4. [DOI] [PubMed] [Google Scholar]
- Starkstein S.E., Migliorelli R., Teson A., Petracca G., Chemerinsky E., Manes F., Leiguarda A. Prevalence and clinical correlates of pathological affective display in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1995;59:55–60. doi: 10.1136/jnnp.59.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens D.C., Conway C.R., Dombeck C.B., Wagner H.R., Tupler L.A., Weiner R.D. Severity of subcortical gray matter hyperintensity predicts ECT response in geriatric depression. J Ect. 2001;17:45–49. doi: 10.1097/00124509-200103000-00009. [DOI] [PubMed] [Google Scholar]
- Steffens D.C., Helms M.J., Krishnan K.R., Burke G.L. Cerebrovascular disease and depression symptoms in the cardiovascular health study. Stroke. 1999;30:2159–2166. doi: 10.1161/01.str.30.10.2159. [DOI] [PubMed] [Google Scholar]
- Sullivan E.V., Pfefferbaum A., Swan G.E., Carmelli D. Heritability of hippocampal size in elderly twin men: Equivalent influence from genes and environment. Hippocampus. 2001;11:754–762. doi: 10.1002/hipo.1091. [DOI] [PubMed] [Google Scholar]
- Thomas A.J., Davis S., Morris C., Jackson E., Harrison R., O’Brien J.T. Increase in interleukin-1beta in late-life depression. Am J Psychiatry. 2005;162:175–177. doi: 10.1176/appi.ajp.162.1.175. [DOI] [PubMed] [Google Scholar]
- Whalen P.J., Bush G., McNally R.J., Wilhelm S., McInerney S.C., Jenike M.A., Rauch S.L. The emotional counting Stroop paradigm: A functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]


