Abstract
Many conditions of pediatric patients require fluid therapy. Depending on the veterinarian's assessment of hydration and perfusion status, fluids can be administered orally, subcutaneously, intraperitoneally, intravenously, or by the intraosseous route. Pediatric patients are prone to hypothermia, hypovolemia, hypoglycemia, and hypokalemia, which must be addressed during fluid therapy in pediatric patients. Typical parameters used to assess hydration status in adult animals do not always apply to pediatric patients. Veterinarians should be aware of differences between pediatric patients and adult animals in terms of physical assessment, common presentations, and fluid requirements for resuscitation and maintenance needs.
In dogs and cats, the term “pediatric” generally refers to the first 12 weeks of life [1]. This period can be further divided into the neonatal stage (0–2 weeks), the infant stage (2–6 weeks), and the juvenile stage (6–12 weeks) [2]. Fluid therapy often is required in sick pediatric patients, but the methods and specific conditions that require fluid support vary according to the stage of life and severity of the underlying condition. Pediatric patients differ from adult patients in many ways [3]. This article addresses specific considerations and indications for fluid therapy in each of the stages of life in pediatric patients.
Fluid Therapy in Neonates
Indications for fluid therapy in neonates include postpartum resuscitation, shock, trauma, dehydration, hypoglycemia, hypothermia, separation from the dam, sepsis, malnutrition, and inability to nurse [4]. Compared with adult animals, neonates are much more prone to dehydration for several reasons [3]. They have a greater surface area-to-volume ratio and increased skin permeability, which make them susceptible to rapid fluid losses. Neonates also have higher total body water content (80% of body weight compared with 60% in adult animals) and have immature renal function. Neonates are unable to concentrate their urine until 8 weeks of age in kittens and 12 weeks of age in puppies. Because of rapid water turnover, dehydration can occur much more acutely in neonates than in older animals [3].
In addition to being prone to dehydration, neonates also are at much higher risk for hypoglycemia than adult animals. Neonates have inefficient hepatic gluconeogenesis and limited glycogen stores, and they are unable to maintain glucose homeostasis if deprived of food for more than a few hours. The need for glucose always must be taken into consideration during rehydration and resuscitation of sick neonates.
Neonates also are much more predisposed to hypothermia than adult animals because of their increased surface area and lack of shivering response (the shivering response does not develop until day 6 of life) [3]. The normal body temperature of neonates also ranges from 95° to 97°F during the first week of life, and does not reach normal “adult” body temperature until after 2 weeks of age [1]. It is important to always administer warmed fluids to treat or prevent hypothermia. Hypothermic neonates develop bradycardia and gastrointestinal ileus. Oral feeding is not effective until normothermia is restored.
Initial Assessment of the Sick Neonate
Typical methods for assessing hydration in adult animals may be unreliable in neonates [3]. Whereas tenting of the skin is used to detect dehydration in adult animals, skin turgor is less reliable in neonates because of increased water content and decreased fat content of the skin. The thin skin on the ventral abdomen is the best place to evaluate hydration status in neonates. In adult animals, severe dehydration causes tachycardia and concentrated urine. These variables cannot be relied on in neonates because the heart rate normally is rapid and urine is not concentrated in neonates. Mucous membranes often remain moist in neonates until dehydration is severe. Mucous membrane color and capillary refill time are good indicators of perfusion in neonates and adult animals. Neonates normally have hyperemic mucous membranes for the first week of life [4]. Pale mucous membranes and slow capillary refill time (> 1.5 sec) are seen when dehydration is severe enough to cause hypovolemic shock. Puppies with poor perfusion still may have normal skin turgor even when mucous membrane color and capillary refill time indicate shock. Clinical signs of sepis, hypovolemia, or shock in neonates include pale mucous membranes, decreased urine output, cold extremities, limp body tone, constant crying, and reluctance to suckle [5]. The veterinarian should determine whether the animal has simple dehydration or actual perfusion deficits. The sicker the animal, the more aggressive the route chosen for fluid therapy. Neonates with sepsis or severe hypovolemia need rapid and aggressive fluid resuscitation by either the intravenous or intraosseous route [2]. Neonates with mild to moderate dehydration can receive fluids orally (by stomach tube), subcutaneously, or intraperitoneally.
Oral Fluids
Stomach tube feeding can be used to give fluids and nutrition to neonates that are not hypothermic, hypoglycemic, or dehydrated. It is an effective method of providing hydration and nutrition to neonates that cannot suckle from the dam. A 5- to 8-Fr feeding tube is used to measure from the tip of the nose to the last rib ( Fig. 1). The tube is marked, lubricated, and passed down the left side of the mouth. It should pass easily, but it is important to remember that a gag reflex is not present until approximately 10 days of age [1]. Proper placement can be assured by instilling a small amount of sterile saline first and making sure that it does not come out of the nose. The stomach capacity of the neonate is approximately 50 mL/kg [1], although filling the stomach to capacity is not recommended because the risk of aspiration increases with a full stomach. Commercial milk replacer can be used, and the amount fed can be determined from the label on the can. Initially, puppies can be fed 10 mL every 2 to 4 hours, increasing the amount by 1 mL per feeding. Kittens can be started at 5 mL every 2 to 4 hours, increasing by 1 mL per day. Food should be warmed to approximately 100°F. If diarrhea occurs, the formula should be diluted 1:2 with balanced electrolyte solution until diarrhea resolves. When removing the stomach tube after feeding, it is important to kink it to prevent aspiration of liquid that otherwise could drain from the tube as it is being pulled out.
Fig. 1.
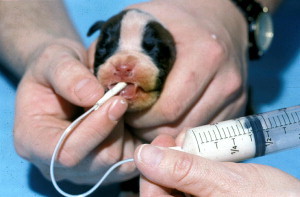
Oral feeding through a stomach tube can be an effective way of maintaining nutrition and fluid requirements in neonates that are unable to nurse.
Neonates also can be fed with a syringe, eye dropper, or bottle. If using a syringe or eye dropper, care must be taken not to administer the liquid too fast because aspiration pneumonia is a common complication. If using a bottle or pet nurser, the hole in the nipple may need to be enlarged with a hot needle so that a drop of milk forms easily when the bottle is turned upside down.
Subcutaneous Fluids
Neonates with mild to moderate dehydration but normal perfusion can be treated by subcutaneous administration of fluids. Maintenance requirements are two to three times higher than for adult animals (120–180 mL/kg/d) [3]. This amount of fluid plus the dehydration deficit (% dehydration × body weight in kg = liters of crystalloid fluid) can be divided into several equal portions and administered subcutaneously at the intrascapular space. Dextrose should not be added to isotonic fluids because the resultant fluid becomes hypertonic and actually draws fluid into the subcutaneous space. The best fluid to correct mild dehydration is a balanced electrolyte solution, such as lactated Ringer's solution or Normosol-R. If dextrose supplementation is desired, 0.45% NaCl with 2.5% dextrose can be administered safely by the subcutaneous route. If hypokalemia is present, up to 30 mEq/L of KCl can be added to fluids for subcutaneous administration.
Neonates that are unable to nurse for the first 24 hours are deprived of colostrum and develop failure of passive transfer, which puts them at high risk for developing infections [6]. Subcutaneous administration of serum from the dam or some other well-vaccinated adult dog can supply protective antibodies. The recommended dose to supply adequate immunity is 16 mL for puppies and 15 mL for kittens. This volume can be divided into two to three equal portions and administered subcutaneously every 6 to 8 hours [6], [7].
Intraperitoneal Fluids
Colostrum, whole blood, or crystalloid fluids can be administered intraperitoneally. Hypertonic dextrose solutions should not be given intraperitoneally, especially to dehydrated neonates, because fluid is pulled from the intravascular space and interstitium into the abdominal cavity. Blood given intraperitoneally is not absorbed for 48 to 72 hours, so it is not an effective method for treating animals with life-threatening anemia [2]. Instillation of warm fluids into the peritoneal cavity can be a useful method of treating hypothermia and increasing core body temperature.
Intravenous Fluids
Neonates with perfusion deficits do not absorb subcutaneously administered fluids well because of the peripheral vasoconstriction associated with hypovolemic shock. Aggressive resuscitation and restoration of intravascular volume can be accomplished with intravenously administered fluids. A 24-gauge catheter sometimes can be placed in a cephalic vein, but more commonly the jugular vein is the best site for intravenous catheterization. A 20- to 22-gauge cephalic catheter usually can be placed in the jugular vein ( Fig. 2). If a gram scale is available, an initial bolus of 1 mL per 30 g of body weight (30–45 mL/kg) is administered by slow intravenous push over 5 to 10 minutes. Fluid loading is continued until mucous membrane color and capillary refill time have improved to a bright pink color with 1-second refill time. Warmed crystalloid fluid, such as lactated Ringer's solution or Normosol-R with 5% dextrose added, is the fluid of choice. After the initial bolus, fluid therapy is continued at a rate of 80 to 120 mL/kg/d.
Fig. 2.
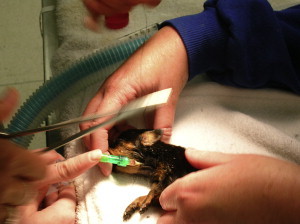
A cephalic catheter can be placed in the jugular vein to provide an effective means for delivering intravenous fluids to critically ill neonates.
Intraosseous Fluids
Sometimes it is impossible to place an intravenous catheter, but the patient has poor perfusion or shock and requires aggressive, rapid resuscitation. Any fluid that can be given intravenously (eg, blood, balanced electrolyte solution, glucose) also can be given by the intraosseous route. A 1- or 2-in spinal needle (18–22 gauge) can be placed easily into the greater trochanter and threaded down the shaft of the femur. The area over the hip must be clipped and surgically prepared before inserting the needle into the femur. The cortical bone is fairly hard compared with the softer medullary cavity. Once the needle is firmly seated in the shaft of the femur, the stylet is removed from the needle and the fluid injected at the same dose and rate as with intravenous resuscitation ( Fig. 3). Once the vascular volume has been restored, it is easier to place an intravenous catheter, and the intraosseous needle can be removed. In some instances, the intraosseous catheter can be bandaged in place for continued fluid therapy support. Once the animal becomes more active, however, the needle is easily dislodged and intraosseous fluid therapy must be discontinued. The intraosseous needle rarely stays in place more than 24 hours.
Fig. 3.
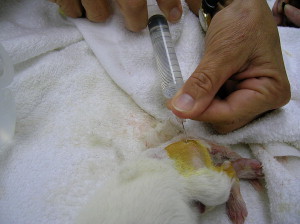
Resuscitation of critically ill neonates can be accomplished by placing a spinal needle in the shaft of the femur and administering warmed fluids by the intraosseous route.
Neonatal Isoerythrolysis
Neonatal isoerythrolysis is a specific condition that occurs when type A or AB kittens are born to type B queens. Type B cats (usually purebred cats of British breed descent) have naturally occurring anti-A antibodies. The kittens are born normally, but when they begin to nurse, they ingest anti-A antibodies in the colostrum, which results in acute life-threatening hemolysis. Clinical signs develop within hours to days and may include sudden death, fading kittens, tail tip necrosis, hemoglobinuria, icterus, and severe anemia. If possible, neonatal isoerythrolysis should be prevented by blood-typing breeding pairs and not breeding type A males to type B females. If the condition does occur, however, the kittens should be removed from the queen for the first 24 hours as soon as a problem is suspected and managed with supportive care as outlined previously. If anemia is severe and a transfusion is required, washed red blood cells from the queen or other type B cat can be administered at a dose of 5 to 10 mL per kitten administered over several hours. This blood is not lysed by the colostral antibodies. The red cells should be suspended in saline rather than plasma to prevent further administration of anti-A antibodies. Subsequent transfusions administered later in life to kittens that survive should consist of type A blood.
The Infant Period
The infant period in pediatric patients ranges from 2 to 6 weeks of age. During this time, the most life-threatening problems are internal and external parasites, juvenile hypoglycemia, dehydration from diarrhea, and trauma [1]. Because renal function does not mature until at least 8 weeks of age, infants remain prone to drug toxicity from decreased renal elimination and dehydration from decreased ability to concentrate their urine.
Hookworms and fleas can cause severe anemia in infant animals and result in pale mucous membranes, tachycardia, weakness, hematocrit less than 15%, and hypoproteinemia. A blood transfusion may be required to stabilize the animal. Blood is diluted 9:1 with a citrate anticoagulant and given through a millipore blood filter at a dosage of 20 mL/kg over 2 to 4 hours. Intraosseous or intravenous administration is preferred in critical patients, but blood can be administered by the intraperitoneal route as a last resort. Iron supplementation should be given in addition to transfusion in animals with blood-loss anemia [2].
Juvenile hypoglycemia can occur because of immature hepatic enzyme systems, lack of glycogen stores, and increased metabolic requirements for glucose [4]. Clinical signs include weakness, tremors, seizures, stupor, and coma. Treatment involves intravenous or intraosseous administration of glucose (0.5–1 g/kg) diluted to a 5% to 10% solution followed by supportive care.
Dehydration is always a potential problem in juvenile animals that develop diarrhea. Severe consequences can be avoided by administering subcutaneous fluids at one to two times maintenance requirements while attempting to determine the cause of the diarrhea. Common reasons for diarrhea in juvenile animals include overfeeding, lactose intolerance, excess saturated fatty acids, giardiasis, coccidiosis, hookworms, roundworms, coronavirus, rotavirus, parvovirus, campylobacter, salmonella, clostridia, or improper handing of the milk replacement diet [4]. If dehydration is severe, hypovolemia and perfusion deficits should be corrected as outlined previously for neonates.
The Juvenile Period
Juveniles are pediatric patients aged 6 to 12 weeks [1]. They are more similar to adults in terms of renal function and vital signs but still have increased maintenance water requirements (120–200 mL/kg/d) and caloric requirements (180 kcal/kg/d). Because maternal antibody is lost during this time, juvenile patients often are susceptible to infectious diseases unless protected by vaccination. Feline panleukopenia and canine parvovirus are life-threatening diseases that require aggressive fluid therapy support. General concerns regarding prevention of hypoglycemia, dehydration, hypoproteinemia, and anemia need to be addressed during treatment as described for neonates and infants. Young animals that are not eating generally require potassium supplementation in their fluids (20 mEq KCl/L) to prevent hypokalemia [2]. Animals with severe enteritis also often develop hypoproteinemia and need colloid support. Plasma transfusions or hetastarch (20 mL/kg/d) can be administered to maintain colloid osmotic pressure in the range of 16 to 25 mm Hg.
Summary
Pediatric patients have higher maintenance requirements for fluid therapy than adult animals and are more prone to dehydration and hypovolemic shock because of higher total body water content and immature renal function. Aggressive treatment with intravenous or intraosseous fluids or preventive strategies using subcutaneous fluids can be life-saving techniques in these fragile patients.
References
- 1.Hoskins J.D., editor. Veterinary pediatrics. 3rd edition. WB Saunders Co.; Philadelphia: 2001. [Google Scholar]
- 2.Macintire D.K. Pediatric intensive care. Vet Clin North Am Small Anim Pract. 1999;29:971–988. doi: 10.1016/s0195-5616(99)50085-5. [DOI] [PubMed] [Google Scholar]
- 3.Poffenbarger E.M., Ralston A.L., Chandler M.L. Canine neonatology: part 1. Physiologic differences between puppies and adults. Compendium on Continuing Education for the Practicing Veterinarian. 1990;12:601–609. [Google Scholar]
- 4.Lawler D.F. Care and diseases of neonatal puppies and kittens. In: Kirk R.W., editor. Current veterinary therapy x: small animal practice. WB Saunders Co.; Philadelphia: 1989. pp. 1325–1333. [Google Scholar]
- 5.Poffenbarger E.M., Ralston A.L., Chandler M.L. Canine neonatology: part 2. Disorders of the neonate. Compendium on Continuing Education for the Practicing Veterinarian. 1991;13:25–37. [Google Scholar]
- 6.Poffenbarger E.M., Olson P.N., Chandler M.L. Use of adult dog serum as a substitute for colostrum in the neonatal dog. Am J Vet Res. 1992;53:230–233. [PubMed] [Google Scholar]
- 7.Levy J.K., Crawford P.C., Collante W.R. Use of adult cat serum to correct failure of passive transfer in kittens. J Am Vet Med Assoc. 2001;219:1401–1405. doi: 10.2460/javma.2001.219.1401. [DOI] [PubMed] [Google Scholar]


