Published online April 8, 2003
http://image.thelancet.com/extras/03cmt87web.pdf
“The terror of the unknown is seldom better displayed than by the response of a population to the appearance of an epidemic, particularly when the epidemic strikes without apparent cause”.1 This quote from 1977 by Edward Kass, describing the fears surrounding the newly recognised legionnaires' disease, aptly describes the public response to the recent appearance of an unexplained atypical pneumonia referred to as severe acute respiratory syndrome (SARS).
In today's Lancet, Joseph Peiris and colleagues provide strong evidence that SARS is associated with a novel coronavirus that has not been previously identified in human beings or animals, and begin the process of eliminating the many unknowns from this new syndrome (figure ). The investigators used classic viral culture and serological techniques, as well as modern molecular genetic methods, to characterise and to determine the cause of the disease in 50 patients with SARS in Hong Kong. One of the strengths of their report, and an important means of establishing causality, is their analysis of specimens from control patients. None of 40 respiratory secretions from patients with other respiratory diseases contained coronavirus RNA, and none of 200 serum samples from blood donors had serum antibody to this new coronavirus. These findings significantly strengthen the tentative aetiological association reported by other investigators from the Centers for Disease Control and Prevention (CDCP) in Atlanta and from Toronto, who have also isolated a novel coronavirus from patients with SARS.2, 3 As other pathogens, such as human metapneumovirus and Chlamydia spp, are identified in SARS patients, it will be important to use control groups to determine their role in causality or as cofactors for severe disease.2, 4
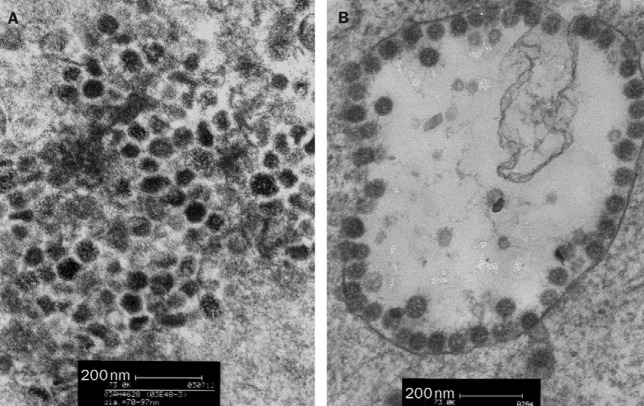
Figure.
Figure 2 from Peiris and colleagues' report on coronavirus as cause of SARS
Thin-section electron micrograph of lung-biopsy sample from patient with SARS and of human pneumonia-associated infected cells.
The clinical features associated with SARS are rapidly becoming available through Peiris' and other reports.2, 5 The Hong Kong investigators identified five clusters of patients by using a modified WHO case definition for SARS, and describe the clinical manifestations of this serious disease. It is notable that nearly 40% of the patients developed respiratory failure that required assisted ventilation. Clinical descriptions will be important in modifying the case definition of this syndrome should it spread, as is likely, beyond the tightly linked clusters that have characterised the epidemiology of SARS thus far. Unfortunately, the early clinical appearance may not allow ready distinction from other common winter-time respiratory viral infections.6, 7 However, certain characteristics of SARS are noteworthy. The constellation of absence of upper respiratory symptoms, the presence of dry cough, and minimal auscultatory findings with consolidation on chest radiographs may alert the clinician to the possible diagnosis of SARS. The presence of lymphopenia, leucopenia, thrombocytopenia, and elevated liver enzymes and creatinine kinase may also raise suspicion.
Clinical diagnosis will become particularly problematic once the association with travel or case contact is lost. Thus, rapid and accurate diagnostic tools will be critical in the management of this epidemic. Given the experience with other respiratory viruses, it is likely that culture and direct antigen-detection from respiratory secretions will not suffice in view of the lethality and contagious nature of this new agent. Rapid diagnosis of SARS, which is important for infection-control measures and potential treatment, will require very sensitive and specific methods. Real-time RT-PCR, currently in use for other respiratory viruses primarily in research settings, may be required as a routine test in clinical diagnostic microbiological laboratories.8, 9
Peiris and colleagues suggest that early therapy with intravenous ribavirin and high-dose glucocorticosteroids may be beneficial. However, the lack of untreated control patients precludes a firm conclusion about benefit. Clinicians often find it difficult to withhold potentially beneficial, yet unproven, therapy in life-threatening situations. Controlled studies may be difficult to do and there are obviously no historical controls for the treatment of SARS. Therefore it will be important for treating physicians to carefully document the dose, timing, and types of therapies used, and the clinical and viral status of patients, so that experiences can be pooled and information productively analysed.
It is truly remarkable and unprecedented that the progress reported by Peiris and colleagues, and elsewhere, on the aetiology and clinical and epidemiological characteristics of SARS has been achieved in less than 2 months. It is fortuitous that this outbreak occurred at a time when viral surveillance-systems headed by WHO in collaboration with CDCP are in place throughout the world. The work of individual laboratories, such as the ones in Hong Kong, Toronto, and CDCP, and cooperation between health authorities in many countries provides protection from the inevitable threat of new epidemic diseases.
References
- 1.Kass EH. Legionnaires' disease. N Engl J Med. 1977;297:1229–1230. doi: 10.1056/NEJM197712012972209. [DOI] [PubMed] [Google Scholar]
- 2.Poutanen SM, Low DE, Henry B. Identification of severe respiratory syndrome in Canada. N Engl J Med. March 31, 2003 doi: 10.1056/NEJMoa030634. http://content.nejm.org/cgi/content/abstract/NEJMoa030634v2 (accessed April 7, 2003). [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention CDC lab analysis suggests new coronavirus may cause SARS. March 24, 2003. http://www.cdc.gov/od/oc/media/pressrel/r030324.htm (accessed April 7, 2003).
- 4.Centers for Disease Control and Prevention CDC telebriefing transcript: CDC update on severe acute respiratory distress syndrome (SARS) April 4, 2003. http://www.cdc.gov/od/oc/media/transcripts/t030404.htm (accessed April 7, 2003).
- 5.Tsang KW, Ho PL, Ooi GC. A cluster of severe acute respiratory syndrome in Hong Kong. N Engl J Med. March 31, 2003 doi: 10.1056/NEJMoa030666. http://content.nejm.org/cgi/content/abstract/NEJMoa030666v2 (accessed April 7, 2003). [DOI] [PubMed] [Google Scholar]
- 6.Falsey AR, Cunningham CK, Barker WH. Respiratory syncytial virus and Influenza A infections in the hospitalized elderly. J Infect Dis. 1995;172:389–394. doi: 10.1093/infdis/172.2.389. [DOI] [PubMed] [Google Scholar]
- 7.Peret TC, Boivin G, Li Y. Characterization of human metapneumoviruses isolated from patients in North America. J Infect Dis. 2002;185:1660–1663. doi: 10.1086/340518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zambon MC, Stockton JD, Clewley JP, Fleming DM. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet. 2001;358:1410–1416. doi: 10.1016/s0140-6736(01)06528-x. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson KG, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community; comparative, prospective, population based study of disease burden. BMJ. 1997;315:1060–1064. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]