Summary
Investigations into the suspected airborne transmission of pathogens in healthcare environments have posed a challenge to researchers for more than a century. With each pathogen demonstrating a unique response to environmental conditions and the mechanical stresses it experiences, the choice of sampling device is not obvious. Our aim was to review bioaerosol sampling, sampling equipment, and methodology. A comprehensive literature search was performed, using electronic databases to retrieve English language papers on bioaerosol sampling. The review describes the mechanisms of popular bioaerosol sampling devices such as impingers, cyclones, impactors, and filters, explaining both their strengths and weaknesses, and the consequences for microbial bioefficiency. Numerous successful studies are described that point to best practice in bioaerosol sampling, from the use of small personal samplers to monitor workers' pathogen exposure through to large static samplers collecting airborne microbes in various healthcare settings. Of primary importance is the requirement that studies should commence by determining the bioefficiency of the chosen sampler and the pathogen under investigation within laboratory conditions. From such foundations, sampling for bioaerosol material in the complexity of the field holds greater certainty of successful capture of low-concentration airborne pathogens. From the laboratory to use in the field, this review enables the investigator to make informed decisions about the choice of bioaerosol sampler and its application.
Keywords: Airborne pathogens, Healthcare, Bioaerosol sampling, Bioefficiency
Introduction
Recent outbreaks such as severe acute respiratory syndrome (SARS), H1N1 influenza, and the H5N1 avian influenza pandemic have raised concerns among infection control teams about the importance of the aerosol transmission of pathogens and have been an impetus to investigate the transmission dynamics of bioaerosols including influenza.1, 2, 3 Studies of suspected airborne transmission routes of various pathogens have been undertaken with differing degrees of success.4
Bioaerosol material is derived from biological origins, including aerial suspensions of bacteria, viruses, fungi, enzymes, and pollen. The size range varies from submicron-sized viral particles to fungal spores and pollen grains up to 1 mm in diameter. If carried by a favourable air flow, bioaerosol material may be distributed over large distances with potentially fatal results. For example, a community-wide outbreak of Legionnaires' disease, which resulted in 18 fatalities, had an outbreak source in industrial cooling towers 6 km from the affected community.5 However, bioaerosols may be relatively delicate structures susceptible to damage due to environmental conditions, such as desiccation.6
There are several different types of bioaerosol sampler available to investigators, which broadly fall into four categories including impingers, cyclones, impactors, and filters (Figure 1 ). Impingers and cyclones collect airborne particles into a liquid collection medium, whereas impactors collect particles on to solid/semi-solid mediums and filters trap bioaerosol material on fine fibres or porous membrane surfaces. The mechanisms of each collection method and their associated benefits/drawbacks are discussed in detail in the subsequent section. This paper constitutes a review of bioaerosol sampling mechanisms and seeks to address practical issues such as choosing a bioaerosol sampling device, bioefficiency, and operational considerations.
Figure 1.
(a) SKC BioSampler (impinger); (b) Coriolis sampler (cyclone); (c) SKC BioStage Impactor; (d) SKC Button Sampler (filter).
Methods
A comprehensive literature search was performed, using electronic databases to retrieve English language papers on bioaerosol sampling within hospitals and the wider environment. The databases searched were Science Direct, Medline (Web of Science), ProQuest, and Taylor & Francis Online. The primary search criterion was bioaerosol with secondary search criteria being sampling, hospital, pathogen, infection, environment, cyclone, impactor, impinger, and aerosol. The final search was on January 16th, 2016. Initially papers were included from 1994 to present day; however, having received expert advice, the search was revised to included papers from 1940 onwards. Patents and foreign language papers were excluded from the literature review. The search yielded 314 publications, of which 131 were included in the review. The software programme EndNote was used for reference management.
Principles of bioaerosol collection
Bioaerosols may be collected using passive or active sampling systems as described in the following sections, with active sampling devices involving a mechanical component.7, 8
Passive sampling
Passive sampling is arguably the most readily available, economic, and unobtrusive method of bioaerosol sampling and relies on particles settling by means of gravity, on a collection substrate housed in a settle plate. The collected particles are usually quantified in terms of the number of colony-forming units (cfu) within the area of the settling plates for the duration of a specified time-period (for example in units of cfu/m2/h). As no mechanical aids, such as a pump, are required, passive sampling has the benefit of not disturbing the surrounding air. The settling velocity of a particle describes the speed of the particle as it descends in still air and is dependent on particle size and density.9 Smaller, lighter particles will remain airborne for longer than larger, denser particles; and if the air speed exceeds the settling velocity the particle will remain suspended indefinitely. In addition, as airflow, even within an enclosed room, will be driven by subtle variations in temperature, the source volume of air for the passively collected sample will be unknown. The combination of these factors has allowed passive sampling to be regarded as both quantitatively and qualitatively inaccurate, and as a subordinate collection method to active sampling. However, this is an oversimplification to the invariably complex nature of bioaerosol sampling. If the area of interest is the dust contamination of surfaces, for example wounds or surgical instruments, then the assessment of the microbial fallout, as opposed to particles remaining suspended in the air, is the imperative.10 Substantial effort has been made to standardize the use of settle plates in the investigation of microbial surface contamination, with consideration given to plate size, position, and length of exposure.11 The 1/1/1 scheme refers to the positioning of 90 mm diameter Petri dishes at a height of 1 m above floor level, 1 m from a wall, and with an exposure time of 1 h. This standardized method also allows for description of the microbial contamination of the surrounding atmosphere through the use of an index of microbial air contamination (IMA). More recently an investigation into contamination in modern operating theatres (OTs) with turbulent airflows suggested that the IMA value could lead to an underestimation of the risk.12
In a study which compared air and surface sampling for Aspergillus sp. using contact plates within a hospital ward, a significant difference between the collection of airborne and surface spores was noted, with aspergillus accounting for >25% of the fungi isolated in the air but <2% of fungi isolated from surfaces.13 This study underlined the need to be aware of the fact that pathogens which have settled on to a surface (subsequently collected on a contact plate) or settle plate may not give an accurate reflection of the suspended airborne concentrations of that pathogen. Another study concluded that although settle plates could demonstrate a close correlation with bioaerosol collection undertaken by active samplers, there were exceptions, with settle plates shown to be less sensitive to the collection of fungal spores.14 To countermand this deficiency an increase of the exposure time of settle plates from 1 to 4 h was proposed. By contrast, using a 30 min settle plate exposure time, a study in post-flood central Thailand demonstrated that settle plates can be used as an alternative to active sampling systems.15 The study did, however, acknowledge that the higher than average fungal bioaerosol presence may have limited the generalizability of their findings.
Surface sampling in intensive care units has been undertaken using tryptic soy agar contact plates accompanied by air sampling using a Sampl'air lite (Aes Chemunex, Bruz, France).16 The total viable count of collected microbes varied between the surface and air sampling methods, which suggested that the source of the contamination may be different. Surface contamination is especially influenced by human activity such as touch. Surface and air sampling both concluded that the bed areas were consistently highly contaminated. The effectiveness of nitrocellulose membranes as an alternative to replication detection and organism counting (RODAC) plates for surface sampling has been demonstrated.17 The membranes have the advantage of being more effective at removing microbes, while also enabling samples to be taken from curved surfaces.
Passive sampling can also be used to describe more unusual methods of collection of bioaerosol material such as solid phase microextraction (SPME).18 Microbial volatile organic compounds (MVOC) have been successfully collected using an 85 μm stableflex carboxen/polydimethylsiloxane fibre contained in a commercial housing.
Active sampling
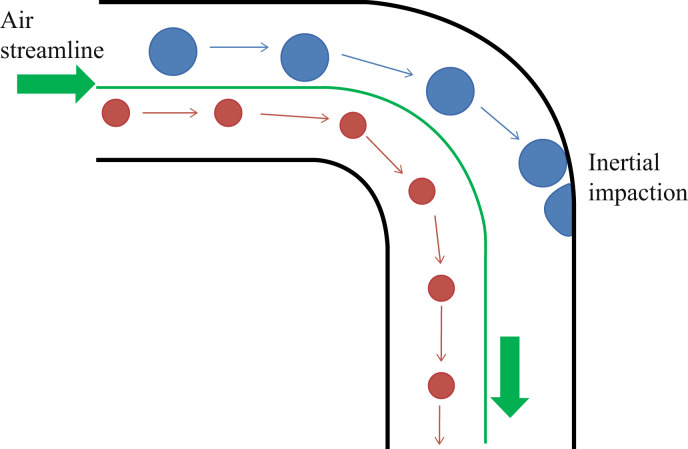
Active sampling cannot be discussed without reference to a particle's mass and inertia. The mass of a particle is equal to its volume multiplied by its density, so it is possible for two particles to have the same mass but differing volumes and densities. If, however, two particles have the same density but differ in volume, then the larger particle will have a greater mass than the smaller particle. The inertia of a particle can be described as follows: consider two particles with differing masses being carried by an air flow inside a pipe. On reaching a bend in the pipe, the particle with the smaller mass will be able to travel along the airflow streamline and continue beyond the bend, whereas the particle with the larger mass will be unable to turn as quickly and will hit the wall and potentially become attached (Figure 2 ). When a particle collides with a wall because its mass is too great to allow it to travel with the airflow, the collision is known as inertial impaction. If particles of varying size all have the same density then the larger particles will succumb more readily than the smaller particles to inertial impaction, hitting the wall at a bend in the pipe. Hence the smaller particles will be collected in the sampling devices but the larger ones will not.
Figure 2.
Inertial impaction in a pipe.
There are several active sampling devices including impingers, cyclones, and impactors, which will be discussed in detail later. However, all active bioaerosol sampling systems consist of five fundamental elements which are necessary to undertake accurate sampling:19
-
1.
inlet to the sampling device
-
2.
transport of the air sample through the device
-
3.
particle size selection (not always present)
-
4.
collecting medium
-
5.
pump and calibrated flow monitoring.
First, the design of the inlet combined with the air flow rate is essential in the collection of a representative sample that correctly reflects the concentration and size distribution of the airborne particles.9 In a moving air stream, such as a ventilation duct, this is achieved by isokinetic sampling. This method considers the ratio of duct and sampling probe diameters, air flows, and inlet orientation to the air stream to ensure successful capture of particles regardless of size or inertia, thus providing a representative sample.
Sampling in still air is also affected by particle inertia, with larger particles being susceptible to evading probe collection; as the particle nears the inlet its velocity increases, thus increasing its stopping distance which may permit the particle to bypass the probe, distorting the concentration in the sample collected. The situation in still air is more straightforward but the probe inlet must be positioned horizontally to prevent an over/underestimation sampling bias.
Sample path
Once the air sample is within the device, the sample path should be as straight and direct as possible in order to minimize losses in the conducting tubing.9 Where this is not possible, for example in a cascade impactor, particle loss will occur through inertial impaction on the bends between collection stages. In devices with sampling probes, particle loss may also occur in the probe head or flexible connective tubing, as particles will be lodged on to the side wall and will not reach the sampling medium. Examples of this include liquid-based bioaerosol samplers, which also experience collection losses through evaporation of the collecting liquid and adhesion of the particles to the device walls. When this occurs the particles do not enter the collection liquid and the sampling process may therefore yield lower or false-negative results.20
Regardless of the mechanism of particle loss, predicting the quantity of losses is problematic, as much depends on the interaction of the air/liquid flows, particle inertia and the design of the sampler. An estimation of losses can be achieved through appropriate laboratory validation, which is discussed in more detail below.
Particle size selection
Identification of particle size selection is not always available in a sampling device; however, there are several ways of selecting by particle size, either by using a pre-classifying cyclone or a series of impaction plates.
Collecting medium
Collection of the bioaerosol samples is normally on to agar or a filter or into liquid, with liquid collection placing less stress on the bioaerosol particles as they are not dried out and are more likely to maintain their viability than the other two methods.6
Calibrated flow modelling
Calibrated flow monitoring and the pump are crucial to the collection procedure as they ensure that the sampling device operates with an accurate air flow rate. Each device will have an optimum speed at which the air flow should pass through the inlet and subsequent tubing to ensure that the bioaerosol particles will be collected with maximum efficiency. Bioaerosol samplers vary considerably in size from large static samplers to smaller, portable personal samplers. Large static samplers operate with higher flow rates of about 12.5–800 L/min, allowing them to collect larger volumes of air more rapidly than personal samplers operating at about 2–4 L/min.21, 22, 23, 24
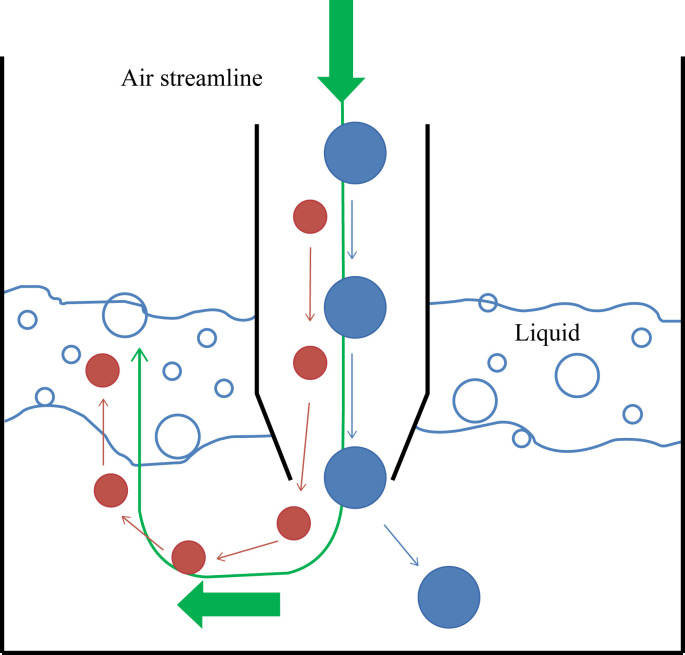
Impingers
Impingers operate by channelling particle-laden air flow through nozzles that exit into a chamber containing liquid (example shown in Figure 1a).6 As the particles exit the nozzles in a jet of air they enter the collection chamber (Figure 3 ). The distance from the nozzle outlet to the surface of the liquid along with the air flow rate influences the diameter of the particles that will be collected. Collection on to liquid prevents desiccation of the collected particles; however, shear forces in the jet in conjunction with the turbulence caused by the air being forced into the chamber may result in loss of viability. This bioefficiency (the ability of the sampling device to maintain the viability of the bioaerosol during and after sampling) may also be reduced through evaporation, re-aerosolization (loss of previously collected particles) and adherence of particles to the internal walls of the collection chamber.20, 25, 26, 27
Figure 3.
Particle-laden airflow in an impinger.
Bioaerosol impingers that are widely used include the All-Glass Impinger (Ace Glass Inc., Vineland, NJ, USA), the BioSampler (SKC Inc., Covington, GA, USA) and the Multistage Liquid Impinger (Burkard Manufacturing Co. Ltd, Rickmansworth, UK) among others.24, 28, 29, 30
Cyclones
In a cyclone sampler (Figure 1b) the particle-laden air is forced by the shape of the collection chamber into a spiral, swirling flow (Figure 4 ). Within this airflow, particles experience a centrifugal force proportional to their diameter, density, and speed. This centrifugal force carries particles with sufficient inertia towards the cyclone wall where they are separated from the air flow into a liquid.31 This generally means that larger particles are more likely to be collected than smaller particles, and, as with all samplers, a calibrated airflow is essential to maintain the correct collection efficiency. On reaching the bottom of the cyclone, the air flow reverses its direction and carries the smaller, uncollected particles out of the cyclone through a vortex finder positioned in the cyclone roof.
Figure 4.
Spiralling airflow pattern in a cyclone.31
To increase the bioefficiency, a film of liquid is injected near to the cyclone's inlet, resulting in wetting of the cyclone walls; the liquid is then collected at the base of the cyclone for analysis.32, 33 Collecting the particles on a liquid film maintains their viability but shear forces may still reduce bioefficiency. Collection losses may again arise from evaporation of the collection liquid, resulting in the re-aerosolization of previously collected material or through liquid carryover where the liquid injected into the cyclone travels over the cyclone roof and vortex finder wall before escaping from the system, carrying with it collected particles.34, 35
Cyclones vary considerably in size and airflow rate, with both the cyclone geometry and the airflow rate affecting the collecting efficiency. Depending on the scale of the cyclone they can be used for collecting large volumes of air while operating at high flow rates or as miniature cyclones that can be worn on a person's clothing in potentially hazardous environments, with the collected material being analysed at the end of each day to assess exposure.36 Cyclones are also frequently used as pre-classifiers, removing larger particles from an airflow before further size classification by other types of sampler.37
Cyclones that are widely used include the Coriolis® μ (Bertin Technologies, Saint Quentin en Yvelines, France), SASS 2300 (Research International, Inc., Monroe, WA, USA), Burkard Cyclone sampler (Burkard Manufacturing Co. Ltd, UK) along with several other cyclones specifically designed for bioaerosol sampling.24, 29, 38, 39, 40, 41, 42, 43
Impactors
In common with cyclones, impactors use the inertia of a particle to facilitate collection. The air sample is passed through an array of nozzles that channel a jet of particle-laden air across a gap towards an agar culture plate, which lies perpendicular to the nozzle outlet. The air flow of the jet will follow a set of curves known as streamlines through the sampler; these curves lie tangentially to the velocity vectors of the flow. The plate deflects the streamlines by 90°, with the air flowing past the plate and through the passageway between the plate and the device walls (Figure 5 ). The particles with sufficiently low inertia will be carried by the streamlines and escape capture. However, particles with higher inertia will be unable to follow the 90° curve of the streamlines, and, under the influence of the centrifugal force, will impact on the agar plate. The collection efficiency of an impactor is therefore primarily dependent on the diameter and density of the particle and the diameter of the nozzle, along with the air velocity of the jet (hence the need to calibrate the air flow through the device). The efficiency of an impactor should have a sharp cut-off curve, with the ideal impactor acting like a sieve, with all particles above a certain size, known as the cut-off size, being captured by the agar plate. This feature makes impactors highly suitable as particle size classifiers, with particles greater than a given size being separated from the air flow, while smaller particles remain airborne. A single-stage impactor has one cut-off size, so only requires one set of nozzles and an agar plate (see example in Figure 1c).
Figure 5.
Particle-laden airflow in a culture plate impactor.
Cascade impactors can be used to gain information on the particle size distribution of an aerosol, with the particle-laden air flow being passed through successive tiers of nozzles and impaction plates. Each tier, known as a stage, will collect particles of a specific size with the smaller particles remaining airborne and passing on to the next stage. At each subsequent stage, the nozzle diameter will become progressively smaller, hence the jet velocity increases, and the particle cut-off size is reduced. Finally the air flow will pass through a filter to allow remaining small particles to be captured. By weighing the impaction plates from each stage and the filter, before and after sampling, the fraction of the total mass in each particle size range can be established. After cultivation the number of cfu should be enumerated and the counts corrected by positive-hole correction method, which accounts for deposition of multiple bioaerosol particles at the same deposition area.44, 45, 46, 47
In practice, each stage in a cascade impactor will not behave entirely like a sieve, and some particles will be deposited in the passageways between the stages or may bounce off the impaction plates and avoid capture. The airflow over the impaction plates may also be disturbed by the build-up of deposited particles, leading to altered collection efficiencies; however, this can be overcome by the use of multi-jet impactors with >100 nozzles. The bioefficiency of impactors is reduced due to the shear forces on the bioaerosol particles within the jet and on impaction with the agar plates. The microbial species under investigation along with jet velocity and jet-to-plate distance have been found to play an important role in the enumeration of bioaerosols.48 Desiccation of the pathogen will also reduce bioefficiency, which can be overcome by mineral-oil-spread agar plates.49 Despite the drawback of reduced viability, impactors are frequently used in sampling for many airborne pathogens.18, 50, 51
Virtual impactors also use the centrifugal force and inertia to separate particles depending on their diameter. However, virtual impactors do not collect on to an agar plate but instead have a collection probe operating with a minor flow. This works by particles entering the impactor and being carried by the major flow around a bend (Figure 6 ). The smaller particles are able to follow the streamlines around the curve while the particles larger than the cut-off diameter of the apparatus have sufficient inertia to carry them into the collection probe. The minor flow in the collection probe carries these larger particles on to a collection filter. Likewise the smaller particles are collected on a filter in a separate part of the device. Virtual impactors usually have only one or two stages, as each separation stage requires control of both the major and minor flow rates. The use of a collection probe rather than an agar plate avoids issues of particle bounce and deposition build-up; however, virtual impactors suffer collection losses near to the size of the cut-off diameter at the inlet of the probe. A useful feature of these devices is that the airflow in effect concentrates the particles larger than the cut-off size into a smaller volume of air, making virtual impactors useful as particle concentrators.52
Figure 6.
Particle-laden airflow in a virtual impactor.
Slit impactors operate using the same principles of centrifugal force and particle inertia as described with regard to other impactors. Rotating agar plates are especially useful as they provide a record of bioaerosol concentration over a specified time-period to enable certain activities to be monitored. Bioaerosol particles enter the apparatus through a slit, causing the particles to impact on the slowly rotating agar plate below. The smallest particles will escape capture by following the streamlines of the air flows over the plates through the passageways to the outlet.
Impactors may also take the form of sticky plastic rods, such as Rotorods (Ted Brown Associates, Los Altos Hills, CA, USA), or sticky glass plates where the airflow rate through the device can be adjusted in order to vary the collected particle size diameter, for example VersaTrap spore trap cassette (SKC, Inc.).53
Examples of impactors include various single-stage and multi-stage Anderson impactors, Aerotech N-6 impactor (Aerotech Laboratories, Coventry, UK), Air Test Omega (LCB, La Salle, France), Air Samplair Mas-100 (Merck, Lyon, France), and BioImpactor 100-08 (AES), BioStage impactor (SKC, Inc.) among many others.50, 54, 55, 56, 57, 58, 59
Filters
Personal samplers are small, portable devices that are attached to workers' clothing to provide a representative sample of the exposure of the individual to hazardous aerosol. As with larger devices, personal samplers require a pump to draw air through the device, with a sample head, foam, or cyclone being used as pre-selectors for particle size.6 The bioaerosol particles are collected on to filters from where they can be transferred on to plates or dissolved into a liquid solution for culturing, or examined by microscopy (e.g. immunofluorescence). Sampling by filtration is commonplace in aerosol collection but less popular for the collection of bioaerosol particles due to the loss of bioefficiency through desiccation of the pathogen; however, there have been notable successes with filter collection of bioaerosols.60, 61
Fibrous filters consist of layers of fine fibres with relatively substantial gaps between the fibres that allow the filter to be between 70% and 99% air. As particles pass through the filter they are captured by the fibres. Membrane filters have a complex pore-like structure and a porosity of about 50–90% less than fibrous filters. As particle-laden air enters the membrane filter, the particles are deposited on the pore structures, with the benefit that particles much smaller than the pore diameters may be successfully captured.
Personal samplers with various filters include the Inhalable GSP samplers (CIS; BGI, Inc., Waltham, MA, USA) used with Teflon and polycarbonate filters, PAS-6 sampling heads containing polytetrafluoroethylene (PTFE) filters (Millipore, Merck, France) and the Button Aerosol Sampler containing gelatin filters (SKC, Inc.).23, 60, 61
Other bioaerosol sampling techniques
Less widely used bioaerosol sampling techniques include electrostatic precipitation and condensation techniques. On entering the inlet of an electrostatic precipitator, the bioaerosol particles are electrically charged at the inlet before progressing through an electric field, where they are separated from the air flow and deposited on to charged plates. Although there is active research into the natural charge on bioaerosol particles and the efficiency and design of electrostatic precipitators, there is concern that the electric field undermines the viability of microbes and that more extensive investigations are required into this sampling technique.53, 62, 63, 64, 65
Sampling of bioaerosol through condensation techniques involves the air sample being processed through a humidifier. Subsequently the warm, humid air is rapidly cooled with the bioaerosol particles acting as condensation nuclei. Although this method can be used effectively to amplify small microbes, hence improving their chances of detection, the system is complex to use and heat transfer to the microbes may result in a loss of viability.53, 55, 66
Choosing the bioaerosol sampler
Considerations when choosing a bioaerosol sampler include the type and size of micro-organisms under investigation, the environment where the sampling is to be undertaken, and cost. Other factors, more specific to active samplers, should include ease of cleaning/disinfection and precautions that need to be implemented to prevent exhaust air from contaminating the sampling environment.8 Manufacturers' websites usually provide information regarding the suitability and cost of their devices; however, more revealing is the practical use made of bioaerosol samplers by investigators in the field. Sampling for airborne pathogens that may pose a health risk is not limited to healthcare environments. Wastewater treatment plants (WWTPs), farms and slaughterhouses, public and residential buildings, compost facilities and the general outdoor environment have all been the focus for bioaerosol studies and much can be learned from such research.23, 38, 50, 51, 60, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101
If the research is focused on an individual's risk of exposure to harmful airborne microbes, then the obvious choice of device is the personal sampler. These samplers can be worn on the person's clothing and have proven successful in capturing fungi, bacteria, and even viruses.60, 98, 102, 103 One notable study investigated the potential for workers at a Danish WWTP to be exposed to aerosolized noroviruses (NoVs), adenoviruses (AdVs), endotoxins, moulds, and bacteria.60 This is consistent with previous studies reporting increased occurrence of gastrointestinal illness among WWTP workers compared with control groups.104, 105 The study used Inhalable GSP samplers (CIS; BGI, Inc.) to monitor the exposure of sixteen workers. Teflon filters were fitted to the GSP samplers to allow endotoxin capture, whereas polycarbonate filters were successfully used for bacteria, mould, and virus collection. This study was the first to detect viruses, specifically norovirus GI, using GSP samplers.47
The exposure risk experienced by workers in a slaughter house was also undertaken with the wearing of personal samplers.23 The samplers consisted of PAS-6 sampling heads containing 1 μm pore size polytetrafluoroethylene (PTFE) filters (Merck Millipore SAS, Molsheim, France) attached in the breathing zone and were connected to portable sampling pumps (Gilian 3500, Sensidyne, Inc., St Petersburg, FL, USA). The PTFE filters successfully captured WU polyomavirus and human papillomavirus 120 along with other pathogens. One drawback of this study, which was focused on analysing the inhaled breath of the workers, was that it became apparent that the filters were also sampling exhaled breath. This would have been overcome by the use of larger static samplers placed away from workers' immediate environment that were able to sample a bulk background air volume. On occasion, using more than one type of sampler may be necessary to overcome specific sampler limitations.53
Personal samplers have also been used as static samplers in a variety of situations. Gelatin filters (3 mm pore size) fitted to a Button Inhalable Aerosol Sampler (SKC, Inc.) have successfully captured influenza A virus (H5N1) nucleotides, dermatophagoides allergens (Der f 1 and Der p 1), and Bacillus subtilis in a laboratory setting;61 whereas other studies have used gelatin filters with IOM personal samplers (SKC, Inc.) to successfully capture airborne legionellae from a WWTP and shower rooms in nursing homes and Methanobrevibacter species and Saccharopolyspora rectivirgula (causative agents of farmer's lung) in a dairy barn.21, 106 However, gelatin filters were noted to perform poorly in high-humidity environments, as they dissolved when sampling in a shower room for >30 min.21 Gelatin filters fitted to IOM samplers have been shown to have good efficiency in capturing total and viable legionellae but perform very poorly in capturing culturable samples.21 Midget impingers (SKC, Inc.) have been successfully used to assess the effectiveness of a selection of surgical face masks against aerosolized influenza.107
Having undertaken field work, it may be possible to correlate field data to laboratory results to assess the potential risk to workers' health of exposure to other degrees of contamination. A study investigating organic dust toxic syndrome (ODTS) in a seed handling factory used GSP inhalable samplers attached to workers' clothing to collect bioaerosol samples during 8 h shifts.108 In the laboratory it was determined that a rotating drum (HSE Rotating Drum Dustiness Tester, J.S. Holdings, Hertfordshire, Stevenage, UK) containing contaminated dust could successfully aerosolize bacteria and fungi. By comparing results with the samples collected by the personal samplers, it was possible to calculate the concentration of airborne microbes to which the workers would be exposed from the tested dust.
One major limitation with personal samplers is their relatively low flow rate, which can be as low as 2 L/min.21, 23, 93, 106 Therefore longer sampling times are more appropriate to sample a significant volume of air and this may lead to loss of bioefficiency through desiccation of the pathogen, however the loss of bioefficiency is dependent not only on sampling time, but also the microbial species and relative humidity.6, 109
In comparison with personal samplers larger static samplers, with their associated higher flow rates, enable the capture of larger, more representative air samples over the same time interval. Their associated flows rates vary considerably from about 12.5 L/min for the BioSampler (SKC, Inc.) and the AGI-30 (Ace Glass, Inc.) through 100 L/min for the Coriolis cyclone (Bertin Technologies) and the MAS-100/A (Merck), to 800 L/min for a described impactor.21, 22, 106 Larger samplers have been used extensively in healthcare environments and in other indoor and outdoor environments to successfully capture viruses, bacteria, and spores.50, 110, 111, 112, 113
Investigating the aerial transmission dynamics of influenza gained impetus during the recent H5N1 avian influenza pandemic; however, prior to this and during the H1N1 pandemic, work was undertaken to assess the risk to healthcare workers carrying out aerosol-generating procedures (AGPs) on H1N1-positive patients.1, 2, 3, 4 Using Glass May three-stage impingers (produced at Health Protection Agency, Porton Down, UK), air was sampled 1 m from the head of the H1N1-positive patient while AGPs were being undertaken. The impinger operated at 55 L/min for 40 min intervals and classified the particles into three aerodynamic size ranges (>7.3 μm, 4–7.3 μm and 0.86–4 μm) to assess the respiratory fraction. The air was collected into 15 mL of phosphate-buffered saline and samples analysed using quantitative reverse transcription–polymerase chain reaction. The study showed that the May three-stage impinger proved successful in capturing H1N1 RNA.
Other studies have used larger static samplers to investigate airborne microbial concentration in operating theatres and recovery rooms, the aerial spread of MRSA in hospitals and residential environments, along with investigations undertaken in non-healthcare environments.50, 71, 82, 106, 110, 114, 115
Determining the bioefficiency
The most important aspect of bioaerosol sampling for the user to understand is bioefficiency.7 The bioefficiency of the sampling device is affected by the mechanical stress and desiccation experienced by the pathogen and will vary with the type of sampling device chosen, the sampling time, the type of pathogen under investigation and environmental conditions.48, 109 Many studies have compared the effectiveness of various samplers but unless previous studies have examined the pathogen that you wish to investigate then such studies are of limited use in providing information on bioefficiency.20, 21, 22, 24, 29, 30, 59, 116 It is therefore necessary to test the sampler/pathogen combination in a laboratory, preferably at a similar humidity to that which is expected in the field. This can be undertaken by spiking the sampler with a known concentration of the pathogen and then assessing the concentration collected.60 However, earlier studies used various other methods, such as using two samplers in tandem or parallel, to assess sampling efficiency.117 Surrogate viruses may be used to limit the hazard when investigating high-risk pathogens, but it should be borne in mind that each pathogen responds uniquely to the conditions experienced.118 The time-interval during which the sampler will operate should also be replicated during laboratory testing in order to identify any operational issues or time-related loss of bioefficiency. During such bioefficiency tests, inherent variations in performance of the sampler may also become evident over different particle size ranges.119
With the limitations of different sampling devices being widely acknowledged and variation in collection efficiency between such devices being noted, establishing the bioefficiency of your chosen sampler against the target microbe in itself provides a valuable contribution to the field of bioaerosol sampling.11, 53 If the target microbe is unknown and a general assessment of bioaerosol particles present in an environment is sought, then the use of different types of sampling devices will mitigate the limitations of individual samplers, making a comprehensive study more likely.
Finally this is also a good opportunity to test the storage, enumeration and identification procedure, be that through cultivation and visual enumeration of the cfu, various PCR techniques, metagenomics, mass spectrometry, epifluorescence microscopy, matrix-assisted laser desorption/ionization mass spectrometry or other means.3, 21, 23, 50, 54, 60, 92, 93 These enumeration and identification methods, along with their advantages and limitations, have recently been discussed and are not repeated here; however, it should be noted that quantification of the pathogens captured by active samplers is normally expressed per cubic metre of air, which provides another reason to determine accurately the air flow rate of the device and the sampling time-period.11, 50, 53, 92
Bioaerosol sampling out in the field
The statistical analysis relating to bioaerosol sampling varies considerably depending on the nature of the study, and an investigator would do well to consult a statistician when designing any study.3, 106, 110 Errors arising from bioaerosol sampling are typically threefold: random error of samples containing a finite number of discrete particles; errors due to non-uniformity of the bioaerosol distribution in the atmosphere; and errors due to sampling techniques.117
The sampling period will be influenced by factors that include the operational limitations of the sampling devices, such as the rate of evaporation of the collecting liquid, or the amount of time one has access to a site. However, even with such matters taken into consideration, the sampling time-periods used by investigators varied widely, from as low as 3 min to several hours.60, 110 The longer the sampling period the greater the volume of air being collected, thus the higher probability of capturing airborne pathogens, as long as the bioefficiency of the sampler does not deteriorate with time. When using samplers with differing flow rates concurrently, it may be preferable to calculate the sampling time of each device so that the volume of air captured is the same. If short sampling periods are most suited to the device being used, then repeating the sampling in triplicate should be considered.93 The overall length of the study may span from one day to a couple of years.120 New techniques such as light-induced fluorescence (LIF) methodologies are being implemented in real-time online biological particle sensors, enabling continuous on-site detection of bioaerosol counts.121
The height of the sampling device above floor level within an indoor environment is also important if the investigation is collecting samples from the breathing zone of patients.3, 110 If a more general bioaerosol sampling regime is undertaken, then sampling at different heights within a room and at several spatial locations will provide good sampling coverage.122 Once the samples have been taken, they should be transported and stored in conditions that preserve their efficacy until cultivation and/or identification can be undertaken.
Having previously undertaken a bioefficiency study, the investigator is in a strong position to estimate with reasonable accuracy the concentration of the target bioaerosol in the sampled environment. Combining this information with the genus of the captured microbe, the particle size range (informing on the penetration of the respiratory system), and the health effects on the human or animal population, conclusions can be made regarding bioaerosol concentration and health risk. Presently there is no international consensus on the acceptable exposure limits of bioaerosol concentration, with a recent review drawing attention to this research deficit.53 A lack of bioaerosol studies targeting viruses and archea has also been identified, further limiting our understanding of the impact of airborne microbes on human health.123 Several of the studies discussed in this review were based in bioaerosol-emitting facilities, such as WWTP and compost facilities, where the exposure to harmful microbes is a cause for concern for occupational safety reasons and for risk to health of the population in the surrounding area. In such cases the task for current research is to establish suitable dose–response relationships to enable health-based exposure limits for bioaerosols to be derived.124 Such exposure limits would be designed to protect the general population from the ill effects of long-term exposure to bioaerosols. The situation for healthcare studies is quite different with a wide array of ‘at risk’ groups needing to be considered, making the derivation of health-based exposure limits challenging. The staff, patients or their visitors may be the source of the bioaerosol health risk, such as with SARS virus, respiratory syncytial virus, influenza, measles, mumps, or rubella viruses.53 Their stay in hospital may be brief and may not be contained to one ward, making it difficult to trace the source of an outbreak. The wider environment may also be a source of harmful bioaerosols, such as an increased risk of airborne aspergillus during construction activities or the risk of legionella bacteria in HVAC (heating, ventilation, and air conditioning) or water systems.
Healthcare-specific field studies
To gain a greater understanding of the transmission dynamics of certain airborne diseases and to increase hygiene standards through improved infection control, bioaerosol sampling studies have frequently focused on healthcare environments.
Bioaerosol sampling in operating theatres (OTs) is motivated by the need to reduce the incidence of surgical-site infections. With the inclusion of high-efficiency particulate air (HEPA) filters within OTs, cleanroom technology standards have frequently been applied to these healthcare settings with the airborne particulate count being monitored. With the observations that bioaerosol sampling is time consuming, requires trained personnel and that results are not instantaneous, interest has grown in the correlation between microbiological and dust contamination, to the extent that it has been suggested that microbial sampling should be limited to epidemics, validation of protocols, or changes to the OT environment that may affect microbial content.125
Further investigations have been unable to establish a relationship between dust particles and microbes in OTs, although a relationship between the number of airborne microbes and human activity was confirmed.126 This relationship between increased airborne bacterial concentration and human activity is widely accepted.127, 128 Approximately 5–10% of human skin debris carries bacteria and skin shedding increases with physical activity, with millions of particles being shed per person each day. In addition to measuring the dust count using a light-scattering particle analyser, bioaerosol sampling was undertaken using both passive and active sampling. Settle plates with a 90 mm diameter were placed at strategic locations, 1 m above floor level, throughout the OT and left exposed during 23 surgical operations. The active sampling was undertaken using a single stage slip-type impactor operating at 25 L/min for duration of 20 min, with samples taken during 13 operations. The study did observe an increased concentration of dust particles >5 μm during conventional surgery as opposed to scope procedures. An inverse relationship between dust and bacterial concentration was reported. As the OT door opened into the anaesthetic room, the turbulent airflow resulting from the pressure differential between the two rooms in effect removed dust from the OT. However, the bacterial concentration increased and it was proposed that this may be due to increased movement of the staff. This highlights the need for the investigator to be aware of airflow in and between areas under investigation, in addition to patterns of human activity.
Over a three-year sampling period, a study of surface and airborne microbial contamination was conducted in 29 OTs.14 Both passive and active sampling was conducted during the commissioning of OTs, during major renovations and surgical activities, as well as in adjacent corridors. Passive sampling was undertaken using 90 mm diameter settle plates using the 1/1/1 scheme (explained in the section on ‘Passive sampling’) with tryptic soy agar used for the total aerobic bacterial count, whereas Sabouraud dextrose agar with chloramphenicol was used for fungal isolation. Active sampling of airborne contamination was carried out using a DUOSAS 360 sampler (Pbi International, Milan, Italy) operating at 180 L/min. The study found a moderately strong correlation between the active and passive sampling methods, with the discrepancy between the two techniques being attributed to the relatively short sampling period and limited spatial collection zone of the active sampler compared with the longer exposure time of the settle plates. The investigation also concluded that bioaerosol sampling could be used for the evaluation of the ventilation and air conditioning system within the OT. Comparing the results from sampling during different surgical procedures also had the potential to inform improved surgical hygiene practice.
Correlation between active and passive sampling was also described during a study comparing different ventilation regimes in OTs.12 Using a Surface Air System sampler (SAS, International Pbi, Milan, Italy) operating at 180 L/min and settle plates, both with tryptic soy agar, the study showed that unidirectional airflows within OTs did not guarantee low counts of airborne bacteria. The study also confirmed that an increased number of people and door openings in an OT influenced an increase in bacterial count.
A year-long monitoring of airborne microbial contamination in OTs and surrounding areas has also been studied using mixed effect models to assess the influence of air temperature, relative humidity, number of people in a space and different sampling locations on levels of CO2, suspended particulate matter, and airborne bacteria.110 Bioaerosol sampling was undertaken using an Andersen one-stage viable impactor (N6; Andersen Samplers, Atlanta, GA, USA), with tryptic soy agar. The sampling period was 3 min, with duplicate samples taken at a height of 1.2–1.5 m from floor level to represent the breathing zone of healthcare workers. In concurrence with a previous study, Bacillus spp., Micrococcus spp., and Staphylococcus spp. bacteria were frequently found in the operating theatre area.116 The study found a positive correlation between airborne bacterial concentration and suspended particulate matter (PM10 and PM2.5). A positive correlation was also found between the number of people in a room and CO2 concentrations, but, when temperature, relative humidity and sampling location were accounted for, no significant correlation was found between the number of people and bacterial concentrations. One exception was the postoperative recovery room where there were a greater number of people, higher CO2 levels, and higher concentrations of bacteria. Caution should be exercised when investigating the relationship between the number of human occupants and the concentration of airborne microbes. Although a correlation has been noted within one room of this investigation and in previous studies mentioned in this section, a study carried out in an environmental chamber suggested that outdoor air had a greater influence on the bioaerosol composition.129
Airborne viral and bacterial concentrations were monitored in the outpatient area of a paediatric unit and in the paediatric emergency room twice a week for one year.111 The sampled air was filtered through a closed face, three-piece disposable, plastic cassette containing a 0.2 μm polytetrafluoroethylene filter and operating at 12 L/min. The air within the outpatient area was sampled for 8 h a day whereas the air in the emergency room was monitored during 24 h periods. In both cases, the bioaerosol sampler was positioned in the breathing zone between 1.2 and 1.5 m above floor height. During the course of the study, 186 filter samples were taken and airborne adenovirus and Mycoplasma pneumoniae were detected in both monitored areas, with greatest prevalence found in the outpatient area. The negative control was the use of filters with no air flow passing though the sampling device. No adenovirus and M. pneumoniae was found on the negative controls. The study did, however, notice evidence of seasonal variation in this Taiwanese hospital, with airborne adenovirus peaking in the summer months, whereas M. pneumoniae detection rates increased in the autumn and winter. Identifying peaks in bioaerosol contamination during certain months allows for ventilation rates in affected areas to be increased to reduce the risk to patient health. Effective ventilation and controlled airflow patterns within wards alongside improvements in hygiene and operational procedures are arguably the strongest defence against high concentrations of airborne microbial contamination.4, 112, 130, 131
These hospital-based bioaerosol investigations highlight many of the issues facing the bioaerosol researcher. The methodologies applied differ between research teams. Sampling devices vary with regards to their collection efficiency. Concentrations of airborne pathogens are influenced by airflows within the hospital building and seasonal variation. The influence and correlation between human activity, air quality (humidity, temperature, CO2 concentration) and dust particle count on bioaerosol concentration is uncertain, with contradictory results being presented. The transmission dynamics of some pathogens are not fully understood and an airborne component to transmission should not be overlooked. Even with a good understanding of the concentrations of bioaerosols in an environment, the health-based exposure limits for a diverse group of patients and staff may not be known. Yet such research can inform on appropriate ventilation rates to maintain good air quality, assess the bioaerosol risk to patients and staff, gain a greater understanding of the transmission dynamics of pathogens, and suggest improvements to hygiene procedures. Amid all the uncertainties and difficulties of bioaerosol research, the goal remains to gain a greater understanding of airborne pathogens and to provide safe healthcare environments for our patients and staff.
A summary of the key points in bioaerosol sampling is presented in Box 1 .
Box 1. Key points for bioaerosol sampling.
Passive sampling
-
–Settle plates
-
–Consider using the 1:1:1 scheme with 90 mm plates11
-
–
-
–Surface sampling
-
–Consider using membranes (e.g. nitrocellulose) as an alternative to contact plates on curved surfaces
-
–Surface and aerial contamination may have different sources
-
–
-
–
Results from passive and active samplers should not be assumed comparable
Active sampling
-
–Impactors
-
–Collection on to agar plates
-
–Collection efficiency highly dependent on particle size (should be sieve-like in performance)
-
–Ideal as a particle size classifier
-
–Loss of bioefficiency: shear forces, desiccation, particle bounce, and deposition build-up
-
–
-
–Virtual impactors
-
–Collection into liquid, thus minimizing risk of desiccation
-
–Collection efficiency dependent on particle size
-
–Useful as particle concentrators
-
–
-
–Slit impactors
-
–Collection on to agar plates
-
–Loss of bioefficiency: shear forces, desiccation, particle bounce, and deposition build-up
-
–Records variation in bioaerosol concentration over a specified time-period
-
–
-
–Impingers
-
–Collection into liquid, thus minimizing risk of desiccation
-
–Loss of bioefficiency: shear forces, re-aerosolization, evaporation, adherence to device walls
-
–Collection efficiency dependent on particle size
-
–
-
–Cyclones (wetted)
-
–Collection into liquid, thus minimizing risk of desiccation
-
–Loss of bioefficiency: shear forces, liquid carryover, evaporation, adherence to device walls
-
–May be used as pre-classifiers for particle size
-
–Collection efficiency dependent on particle size
-
–Vary considerably in size and airflow rate
-
–
-
–Filters
-
–Small, portable personal samplers
-
–Loss of bioefficiency: desiccation
-
–Collection efficiency dependent on particle size (sample head, foam, or cyclone being used as pre-selectors)
-
–
In the laboratory
-
–Calibrate the flow rate of the active sampler
-
–Ensures the maximum collection efficiency
-
–Influences the size of particles collected
-
–
-
–Determine the bioefficiency of the sampler against the target pathogen
-
–Test in air conditions expected in the field (relative humidity and temperature)
-
–Spike sampler with known concentration of the target pathogen
-
–Each type of pathogen has a unique response to conditions experienced
-
–Surrogate viruses may be used in place of hazardous pathogens; however, response may differ from target pathogen
-
–Check that bioefficiency is maintained throughout planned sampling time
-
–
-
–
Determine errors in numeration when sampling from a known, repeatable concentration of the target pathogen
-
–
Ensure that the sampler exhaust is not a source of pathogen contamination to the environment
-
–
Test the storage, enumeration, and identification procedure
In the field
-
–Position of the inlet sampler
-
–Avoid strong airflows around the inlet of the sampler
-
–If using an inlet nozzle, position horizontally
-
–Ensure that the sample position is beyond the range of droplet fallout from a source (e.g. coughing/vomiting patient)
-
–
-
–Aerial microbial concentration
-
–Expect non-uniformed concentration in the area studied (expect associated sampling errors)
-
–Consider taking samples at various locations in the area studied
-
–Note human/animal activity and number of humans/animals present, as this may influence concentration of certain microbes
-
–Be aware of airflow patterns due to HVAC (heating ventilation and air conditioning) and natural ventilation
-
–Note air quality: relative humidity, temperature (also consider CO2 and particle dust count)
-
–There may be seasonal variation in concentration of the target pathogen
-
–
-
–Active samplers: quantification of pathogens
-
–Expressed as enumeration per cubic meters of air
-
–Need to know the collection time and flow rate of the sampler
-
–
Conclusion
A wide variety of bioaerosol samplers have been used to investigate airborne pathogens in healthcare facilities and other environments. We have described the underlying principles behind bioaerosol sampling devices along with benefits and disadvantages of various designs. Examples of bioaerosol sampling have been given to point to best practice and to highlight the wide array of devices used and pathogens captured. Due to the unique response of each variety of pathogen to environmental conditions and the stresses experienced in differing sampling devices, the investigator should commence studies by determining the bioefficiency of the chosen sampler and the pathogen under investigation within laboratory conditions. From such foundations, sampling for bioaerosol material in the complexity of the field holds greater certainty of successful capture of low-concentration airborne pathogens.
Conflict of interest statement
None declared.
Funding sources
None.
References
- 1.Aditama T., Samaan G., Kusriastuti R. Avian influenza H5N1 transmission in households, Indonesia. PLoS One. 2012;7:e29971. doi: 10.1371/journal.pone.0029971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herfst S., Schrauwen E., Linster M. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012;336:1534–1541. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson K.-A., Pappachan J., Bennett A. Influenza aerosols in UK hospitals during the H1N1 (2009) pandemic – the risk of aerosol generation during medical procedures. PLoS One. 2013;8(2):e56278. doi: 10.1371/journal.pone.0056278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hobday R.A., Dancer S.J. Roles of sunlight and natural ventilation for controlling infection: historical and current perspectives. J Hosp Infect. 2013;84:271–282. doi: 10.1016/j.jhin.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen T.M., Ilef D., Jarraud S. A community-wide outbreak of legionnaires' disease linked to industrial cooling towers – how far can contaminated aerosols spread? J Infect Dis. 2006;193:102–111. doi: 10.1086/498575. [DOI] [PubMed] [Google Scholar]
- 6.Sykes D. The development of a bioaerosol sampler for the detection of enzymes in industry. University of Teesside, UK; 2005.
- 7.Breeding D.C. Bioaerosol evaluation in indoor environments. Occup Health Safety. 2003;72:58–66. [PubMed] [Google Scholar]
- 8.ISO 14698-1:2003 . ISO; Geneva: 2003. Cleanrooms and associated controlled environments – biocontamination control – Part 1: General principles and methods. [Google Scholar]
- 9.Hinds W.C. 2nd ed. Wiley Interscience; New York: 1998. Aerosol technology. [Google Scholar]
- 10.French M.L.V., Eitzen H.E., Ritter M.A., Leland D. Environmental control of microbial contamination in the operating room. In: Hunt T.K., editor. Wound healing and wound infection. Appleton-Century-Crofts; New York: 1980. pp. 254–261. [Google Scholar]
- 11.Pasquarella C., Pitzurra O., Savino A. The index of microbial air contamination. J Hosp Infect. 2000;46:241–256. doi: 10.1053/jhin.2000.0820. [DOI] [PubMed] [Google Scholar]
- 12.Agodi A., Auxilia F., Barchitta M. Operating theatre ventilation systems and microbial air contamination in total joint replacement surgery: results of the GISIO-ISChIA study. J Hosp Infect. 2015;90:213–219. doi: 10.1016/j.jhin.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Sautour M., Sixt N., Dalle F. Prospective survey of indoor fungal contamination in hospital during a period of building construction. J Hosp Infect. 2007;67:367–373. doi: 10.1016/j.jhin.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Pasquarella C., Vitali P., Saccani E. Microbial air monitoring in operating theatres: experience at the University Hospital of Parma. J Hosp Infect. 2012;81:50–57. doi: 10.1016/j.jhin.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Khawcharoenporn T., Apisarnthanarak A., Thongphubeth K. Post-flood measurement of fungal bio-aerosol in a resource-limited hospital: can the settle plate method be used? J Hosp Infect. 2013;83:150–152. doi: 10.1016/j.jhin.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Gaudart J., Cloutman-Green E., Guillas S. Healthcare environments and spatial variability of healthcare associated infection risk: cross-sectional surveys. PLoS One. 2013;8(9):e76249. doi: 10.1371/journal.pone.0076249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poletti L., Pasquarella C., Pitzurra M., Savino A. Comparative efficiency of nitrocellulose membranes versus RODAC plates in microbial sampling on surfaces. J Hosp Infect. 1999;41:195–201. doi: 10.1016/s0195-6701(99)90016-6. [DOI] [PubMed] [Google Scholar]
- 18.Lavine B.K., Mirjankar N., LeBouf R., Rossner A. Prediction of mold contamination from microbial volatile organic compound profiles using solid phase microextraction and gas chromatography/mass spectrometry. Microchem J. 2012;103:37–41. [Google Scholar]
- 19.Colbeck I. Blackie Academic & Professional; London: 1998. Physical and chemical properties of aerosols. [Google Scholar]
- 20.Han T., Mainelis G. Investigation of inherent and latent internal losses in liquid-based bioaerosol samplers. J Aerosol Sci. 2012;45:58–68. [Google Scholar]
- 21.Chang C.-W., Hung P.-Y. Evaluation of sampling techniques for detection and quantification of airborne legionellae at biological aeration basins and shower rooms. J Aerosol Sci. 2012;48:63–74. [Google Scholar]
- 22.King M.D., McFarland A.R. Bioaerosol sampling with a wetted wall cyclone: cell culturability and DNA integrity of Escherichia coli bacteria. Aerosol Sci Technol. 2012;46:82–93. [Google Scholar]
- 23.Hall R.J., Leblanc-Maridor M., Wang J. Metagenomic detection of viruses in aerosol samples from workers in animal slaughterhouses. PLoS One. 2013;8(8):e72226. doi: 10.1371/journal.pone.0072226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoisington A., Maestre J.P., King M.D., Siegel J.A., Kinney K.A. The impact of sampler selection on characterizing the indoor microbiome. Building Environ. 2014;80:274–282. [Google Scholar]
- 25.Lin X., Willeke K., Ulevicius V., Grinshpun S.A. Effect of sampling time on the collection efficiency of all-glass impingers. Am Indust Hyg Assoc J. 1997;58:480–488. [Google Scholar]
- 26.Lin X., Reponen A.T., Willeke K., Grinshpun S.A., Foarde K.K., Ensor D.S. Long-term sampling of airborne bacteria and fungi into a non-evaporating liquid. Atmos Environ. 1999;33:4291–4298. [Google Scholar]
- 27.Agranovski I.E., Agranovski V., Grinshpun S.A., Reponen T., Willeke K. Collection of airborne microorganism into liquid by bubbling through porous medium. Aerosol Sci Technol. 2002;36:502–509. [Google Scholar]
- 28.Wen-Hai L., Chih-Shan L. Influence of storage on the fungal concentration determination of impinger and filter samples. Am Indust Hyg Assoc J. 2003;64:102–107. doi: 10.1080/15428110308984798. [DOI] [PubMed] [Google Scholar]
- 29.Langer V., Hartmann G., Niessner R., Seidel M. Rapid quantification of bioaerosols containing L. pneumophila by Coriolis® μ air sampler and chemiluminescence antibody microarrays. J Aerosol Sci. 2012;48:46–55. [Google Scholar]
- 30.Saini D., Hopkins G.W., Chen C.-J. Sampling port for real-time analysis of bioaerosol in whole body exposure system for animal aerosol model development. J Pharmacol Toxicol Methods. 2011;63:143–149. doi: 10.1016/j.vascn.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng W., Hoffmann A.C. Reverse-flow centrifugal separators in parallel: performance and flow pattern. Am Inst Chem Engrs J. 2007;53:589–597. [Google Scholar]
- 32.Krames J., Buttner H. The cyclone scrubber – a high efficiency wet separator. Chem Engng Technol. 1994;17:73–80. [Google Scholar]
- 33.Griffiths W.D., Stewart I.W., Futteer S.J., Upton S.L., Mark D. The development of sampling methods for the assessment of indoor bioaerosols. J Aerosol Sci. 1997;28:437–457. [Google Scholar]
- 34.Nitescu IR. Development of a biosensor for airborne proteases. University of Teesside, UK; 1996.
- 35.Phull MS. An improved wetted wall bioaerosol sampling cyclone. Texas A&M University; 2005.
- 36.Kenny L.C., Gussman R.A. A direct approach to the design of cyclones for aerosol-monitoring applications. J Aerosol Sci. 2000;31:1407–1420. [Google Scholar]
- 37.Griffiths W.D., Boysan F. Computational fluid dynamics (CFD) and empirical modelling of the performance of a number of cyclone samplers. J Aerosol Sci. 1996;27:281–304. [Google Scholar]
- 38.Dungan R.S. Use of a culture-independent approach to characterize aerosolized bacteria near an open-freestall dairy operation. Environ Int. 2012;41:8–14. doi: 10.1016/j.envint.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Moreno-Grau S., Elvira-Rendueles B., Moreno J. Correlation between Olea europaea and Parietaria judaica pollen counts and quantification of their major allergens Ole e 1 and Par j 1-Par j 2. Ann Allergy Asthma Immunol. 2006;96:858–864. doi: 10.1016/S1081-1206(10)61350-6. [DOI] [PubMed] [Google Scholar]
- 40.Macher J., Chen B., Rao C. Field evaluation of a personal, bioaerosol cyclone sampler. J Occup Environ Hygiene. 2008;5:724. doi: 10.1080/15459620802400159. [DOI] [PubMed] [Google Scholar]
- 41.McFarland A.R., Haglund J.S., King M.D. Wetted wall cyclones for bioaerosol sampling. Aerosol Sci Technol. 2010;44:241–252. [Google Scholar]
- 42.Macher J., Chen B., Rao C. Chamber evaluation of a personal, bioaerosol cyclone sampler. J Occup Environ Hygiene. 2008;5:702. doi: 10.1080/15459620802380351. [DOI] [PubMed] [Google Scholar]
- 43.Tolchinsky A.D., Sigaev V.I., Sigaev G.I. Development of a personal bioaerosol sampler based on a conical cyclone with recirculating liquid film. J Occup Environ Hygiene. 2010;7:156. doi: 10.1080/15459620903486768. [DOI] [PubMed] [Google Scholar]
- 44.Andersen A.A. New sampler for the collection, sizing, and enumeration of viable airborne particles. J Bacteriol. 1958;76:471–484. doi: 10.1128/jb.76.5.471-484.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macher J.M. Positive-hole correction of multiple-jet impactors for collecting viable microorganisms. Am Indust Hyg Assoc J. 1989;50:561–568. doi: 10.1080/15298668991375164. [DOI] [PubMed] [Google Scholar]
- 46.Crook B. Inertial samplers: biological perspectives. In: Cox C., Wathes C., editors. Bioaerosols handbook. Lewis Publishers; New York: 1995. pp. 247–257. [Google Scholar]
- 47.Somerville M.C., Rivers J.C. An alternative approach for the correction of bioaerosol data collected with multiple jet impactors. Am Indust Hygiene Assoc J. 1994;55:127. [Google Scholar]
- 48.Yao M., Mainelis G. Effect of physical and biological parameters on enumeration of bioaerosols by portable microbial impactors. J Aerosol Sci. 2006;37:1467–1483. [Google Scholar]
- 49.Xu Z., Wei K., Wu Y. Enhancing bioaerosol sampling by Andersen impactors using mineral-oil-spread agar plate. PLoS One. 2013;8:e56896. doi: 10.1371/journal.pone.0056896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo X., Wu P., Ding W., Zhang W., Li L. Reduction and characterization of bioaerosols in a wastewater treatment station via ventilation. J Environ Sci (China) 2014;26:1575–1583. doi: 10.1016/j.jes.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Persoons R., Parat S., Stoklov M., Perdrix A., Maitre A. Critical working tasks and determinants of exposure to bioaerosols and MVOC at composting facilities. Int J Hygiene Environ Health. 2010;213:338–347. doi: 10.1016/j.ijheh.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 52.Willeke K., Baron P.A. John Wiley & Sons; New York: 1993. Aerosol measurement: principle, techniques and applications. [Google Scholar]
- 53.Ghosh B., Lal H., Srivastava A. Review of bioaerosols in indoor environment with special reference to sampling, analysis and control mechanisms. Environ Int. 2015;85:254–272. doi: 10.1016/j.envint.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lavine B.K., Mirjankar N., LeBouf R., Rossner A. Prediction of mold contamination from microbial volatile organic compound profiles using head space gas chromatography/mass spectrometry. Microchem J. 2012;103:119–124. [Google Scholar]
- 55.Park C.W., Yoon K.Y., Kim Y.D., Park J.H., Hwang J. Effects of condensational growth on culturability of airborne bacteria: implications for sampling and control of bioaerosols. J Aerosol Sci. 2011;42:213–223. [Google Scholar]
- 56.Zimmerman N.J., Reist P.C., Turner A.G. Comparison of two biological aerosol sampling methods. Appl Environ Microbiol. 1987;53:99–104. doi: 10.1128/aem.53.1.99-104.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spicer R.C., Gangloff H.J. Differences in detection frequency as a bioaerosol data criterion for evaluating suspect fungal contamination. Building Environ. 2010;45:1304–1311. [Google Scholar]
- 58.Nesa D., Lortholary J., Bouakline A. Comparative performance of impactor air samplers for quantification of fungal contamination. J Hosp Infect. 2001;47:149–155. doi: 10.1053/jhin.2000.0883. [DOI] [PubMed] [Google Scholar]
- 59.Zhen S., Li K., Yin L. A comparison of the efficiencies of a portable BioStage impactor and a Reuter centrifugal sampler (RCS) High Flow for measuring airborne bacteria and fungi concentrations. J Aerosol Sci. 2009;40:503–513. [Google Scholar]
- 60.Uhrbrand K., Schultz A.C., Madsen A.M. Exposure to airborne noroviruses and other bioaerosol components at a wastewater treatment plant in Denmark. Food Environ Virol. 2011;3:130–137. [Google Scholar]
- 61.Wu Y., Shen F., Yao M. Use of gelatin filter and BioSampler in detecting airborne H5N1 nucleotides, bacteria and allergens. J Aerosol Sci. 2010;41:869–879. [Google Scholar]
- 62.Wei K., Zou Z., Yao M. Charge levels and Gram (±) fractions of environmental bacterial aerosols. J Aerosol Sci. 2014;74:52–62. [Google Scholar]
- 63.Mainelis G., Adhikari A., Willeke K., Lee S.-A., Reponen T., Grinshpun S.A. Collection of airborne microorganisms by a new electrostatic precipitator. J Aerosol Sci. 2002;33:1417–1432. [Google Scholar]
- 64.Han T., Mainelis G. Design and development of an electrostatic sampler for bioaerosols with high concentration rate. J Aerosol Sci. 2008;39:1066–1078. [Google Scholar]
- 65.Lancereau Q., Roux J.-M., Achard J.-L. Influence of secondary flows on the collection efficiency of a cylindrical electrostatic precipitator. J Aerosol Sci. 2013;63:146–160. [Google Scholar]
- 66.Oh S., Anwar D., Theodore A., Lee J.-H., Wu C.-Y., Wander J. Development and evaluation of a novel bioaerosol amplification unit (BAU) for improved viral aerosol collection. J Aerosol Sci. 2010;41:889–894. doi: 10.1016/j.jaerosci.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hubad B., Lapanje A. The efficient method for simultaneous monitoring of the culturable as well as nonculturable airborne microorganisms. PLoS One. 2013;8(12):e82186. doi: 10.1371/journal.pone.0082186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heinonen-Tanski H., Reponen T., Koivunen J. Airborne enteric coliphages and bacteria in sewage treatment plants. Water Res. 2009;43:2558–2566. doi: 10.1016/j.watres.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Karra S., Katsivela E. Microorganisms in bioaerosol emissions from wastewater treatment plants during summer at a Mediterranean site. Water Res. 2007;41:1355–1365. doi: 10.1016/j.watres.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 70.O'Hara R.E., Rubin R. Reducing bioaerosol dispersion from wastewater treatment and its land application: a review and analysis. J Environ Health. 2005;68:24–29. [PubMed] [Google Scholar]
- 71.Corzo C.A., Romagosa A., Dee S.A., Gramer M.R., Morrison R.B., Torremorell M. Relationship between airborne detection of influenza A virus and the number of infected pigs. Vet J. 2013;196:171–175. doi: 10.1016/j.tvjl.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alvarado C., Gibbs S., Gandara A., Flores C., Hurd W., Green C. The potential for community exposures to pathogens from an urban dairy. J Environ Health. 2012;74:22–28. [PubMed] [Google Scholar]
- 73.Rule A.M., Evans S.L., Silbergeld E.K. Food animal transport: a potential source of community exposures to health hazards from industrial farming (CAFOs) J Infect Public Health. 2008;1:33–39. doi: 10.1016/j.jiph.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 74.Pillai S.D., Ricke S.C. Bioaerosols from municipal and animal wastes: background and contemporary issues. Can J Microbiol. 2002;48:681–696. doi: 10.1139/w02-070. [DOI] [PubMed] [Google Scholar]
- 75.Rahkio T., Korkeala H. Airborne bacteria and carcass contamination in slaughterhouses. J Food Prot. 1997;60:38–42. doi: 10.4315/0362-028x-60.1.38. [DOI] [PubMed] [Google Scholar]
- 76.Brandl H., Fricker-Feer C., Ziegler D., Mandal J., Stephan R., Lehner A. Distribution and identification of culturable airborne microorganisms in a Swiss milk processing facility. J Dairy Sci. 2014;97:240–246. doi: 10.3168/jds.2013-7028. [DOI] [PubMed] [Google Scholar]
- 77.Hospodsky D., Qian J., Nazaroff W.W. Human occupancy as a source of indoor airborne bacteria. PLoS One. 2012;7(4):e34867. doi: 10.1371/journal.pone.0034867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adhikari A., Lewis J.S., Reponen T. Exposure matrices of endotoxin, (1→3)-β-d-glucan, fungi, and dust mite allergens in flood-affected homes of New Orleans. Sci Total Environ. 2010;408:5489–5498. doi: 10.1016/j.scitotenv.2010.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Flores C., Mota L., Green C., Mena K., Balcazar H., Gibbs S. Evaluation of respiratory symptoms and their possible association with residential indoor bioaerosol concentrations and other environmental influences. J Environ Health. 2009;72:8–13. [PubMed] [Google Scholar]
- 80.Adhikari A., Jung J., Reponen T. Aerosolization of fungi, (1→3)-β-d glucan, and endotoxin from flood-affected materials collected in New Orleans homes. Environ Res. 2009;109:215–224. doi: 10.1016/j.envres.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mota L.C., Gibbs S.G., Green C.F., Payan F., Tarwater P.M., Ortiz M. Characterization of seasonal indoor and outdoor bioaerosols in the arid environment of El Paso. Texas. J Environ Health. 2008;70:48–53. [PubMed] [Google Scholar]
- 82.Gandara A., Mota L.C., Flores C. Isolation of Staphylococcus aureus and antibiotic-resistant Staphylococcus aureus from residential indoor bioaerosols. Environ Health Perspect. 2006;114:1859–1864. doi: 10.1289/ehp.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jo W.-K., Seo Y.-J. Indoor and outdoor bioaerosol levels at recreation facilities, elementary schools, and homes. Chemosphere. 2005;61:1570–1579. doi: 10.1016/j.chemosphere.2005.04.103. [DOI] [PubMed] [Google Scholar]
- 84.Portnoy J.M., Barnes C.S., Kennedy K. Sampling for indoor fungi. J Allergy Clin Immunol. 2004;113:189–198. doi: 10.1016/j.jaci.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 85.Luoma M., Batterman S.A. Autocorrelation and variability of indoor air quality measurements. Am Indust Hyg Assoc J. 2000;61:658–668. doi: 10.1080/15298660008984575. [DOI] [PubMed] [Google Scholar]
- 86.Thorne P.S., DeKoster J.A., Subramanian P. Environmental assessment of aerosols, bioaerosols, and airborne endotoxins in a machining plant. Am Indust Hygiene Assoc J. 1996;57:1163–1167. [Google Scholar]
- 87.DeKoster J.A., Thorne P.S. Bioaerosol concentrations in noncompliant, compliant, and intervention homes in the Midwest. Am Indust Hygiene Assoc J. 1995;56:573. [Google Scholar]
- 88.Tsai M.-Y., Liu H.-M. Exposure to culturable airborne bioaerosols during noodle manufacturing in central Taiwan. Sci Total Environ. 2009;407:1536–1546. doi: 10.1016/j.scitotenv.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 89.Jo W.-K., Kang J.-H. Workplace exposure to bioaerosols in pet shops, pet clinics, and flower gardens. Chemosphere. 2006;65:1755–1761. doi: 10.1016/j.chemosphere.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 90.Zhu H., Phelan P., Duan T., Raupp G., Fernando H.J.S. Characterizations and relationships between outdoor and indoor bioaerosols in an office building. China Particuology. 2003;1:119–123. [Google Scholar]
- 91.Douglas P. Bioaerosol emissions from open window composting facilities: emission characterisation and dispersion modelling improvements [PhD thesis]. Cranfield University, UK; 2013.
- 92.Conza L., Pagani S.C., Gaia V. Presence of Legionella and free-living amoebae in composts and bioaerosols from composting facilities. PLoS One. 2013;8(7):e68244. doi: 10.1371/journal.pone.0068244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pankhurst L.J., Deacon L.J., Liu J. Spatial variations in airborne microorganism and endotoxin concentrations at green waste composting facilities. Int J Hygiene Environ Health. 2011;214:376–383. doi: 10.1016/j.ijheh.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 94.Drew G.H., Jordinson G.M., Smith M.A., Pollard S.J.T. Evaluating the quality of bioaerosol risk assessments for composting facilities in England and Wales. Resources Conserv Recycling. 2009;53:507–512. [Google Scholar]
- 95.Deacon L.J., Pankhurst L.J., Drew G.H. Particle size distribution of airborne Aspergillus fumigatus spores emitted from compost using membrane filtration. Atmos Environ. 2009;43:5698–5701. [Google Scholar]
- 96.Poulsen O.M., Breum N.O., Ebbehøj N. Sorting and recycling of domestic waste. Review of occupational health problems and their possible causes. Sci Total Environ. 1995;168:33–56. doi: 10.1016/0048-9697(95)04521-2. [DOI] [PubMed] [Google Scholar]
- 97.Chen P.-S., Tsai F.T., Lin C.K. Ambient influenza and avian influenza virus during dust storm days and background days. Environ Health Perspect. 2010;118:1211–1216. doi: 10.1289/ehp.0901782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Borodulin A.I., Desyatkov B.M., Lapteva N.A., Sergeev A.N., Agranovski I.E. Personal sampler for monitoring of viable viruses; modelling of outdoor sampling conditions. Atmos Environ. 2006;40:6687–6695. doi: 10.1016/j.atmosenv.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fang Z., Ouyang Z., Hu L., Wang X., Zheng H., Lin X. Culturable airborne fungi in outdoor environments in Beijing, China. Sci Total Environ. 2005;350:47–58. doi: 10.1016/j.scitotenv.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 100.Cage B.R., Schreiber K., Portnoy J., Barnes C. Evaluation of four bioaerosol samplers in the outdoor environment. Ann Allergy Asthma Immunol. 1996;77:401–406. doi: 10.1016/S1081-1206(10)63339-X. [DOI] [PubMed] [Google Scholar]
- 101.Sercombe J.K., Green B.J., Rimmer J., Burton P.K., Katelaris C.H., Tovey E.R. London Plane Tree bioaerosol exposure and allergic sensitization in Sydney, Australia. Ann Allergy Asthma Immunol. 2011;107:493–500. doi: 10.1016/j.anai.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 102.Kenny L.C., Stancliffe J.D., Crook B. The adaptation of existing personal inhalable aerosol samplers for bioaerosol sampling. Am Indust Hygiene Assoc J. 1998;59:831–841. doi: 10.1080/15428119891011009. [DOI] [PubMed] [Google Scholar]
- 103.Aizenberg V., Grinshpun S.A., Willeke K., Smith J., Baron P.A. Performance characteristics of the button personal inhalable aerosol sampler. Am Indust Hygiene Assoc J. 2000;61:398–404. doi: 10.1080/15298660008984550. [DOI] [PubMed] [Google Scholar]
- 104.Rylander R. Health effects among workers in sewage treatment plants. Occup Environ Med. 1999;56:354–357. doi: 10.1136/oem.56.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thorn J., Beijer L., Rylander R. Work related symptoms among sewage workers: a nationwide survey in Sweden. Occup Environ Med. 2002;59:562–566. doi: 10.1136/oem.59.8.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lecours P.B., Veillette M., Marsolais D., Duchaine C. Characterization of bioaerosols from dairy barns: reconstructing the puzzle of occupational respiratory diseases by using molecular approaches. Appl Environ Microbiol. 2012;78(9):3242–3248. doi: 10.1128/AEM.07661-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Makison Booth C., Clayton M., Crook B., Gawn J.M. Effectiveness of surgical masks against influenza bioaerosols. J Hosp Infect. 2013;84:22–26. doi: 10.1016/j.jhin.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 108.Madsen A.M., Zervas A., Tendal K., Nielsen J.L. Microbial diversity in bioaerosol samples causing ODTS compared to reference bioaerosol samples as measured using Illumina sequencing and MALDI-TOF. Environ Res. 2015;140:255–267. doi: 10.1016/j.envres.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 109.Wang Z., Reponen T., Grinshpun S.A., Górny R.L., Willeke K. Effect of sampling time and air humidity on the bioefficiency of filter samplers for bioaerosol collection. J Aerosol Sci. 2001;32:661–674. [Google Scholar]
- 110.Tang C.-S., Wan G.-H. Air quality monitoring of the post-operative recovery room and locations surrounding operating theatres in a medical centre in Taiwan. PLoS One. 2013;8(4):e61093. doi: 10.1371/journal.pone.0061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wan G.-H., Huang C.-G., Huang Y.-C. Surveillance of airborne adenovirus and Mycoplasma pneumoniae in a hospital paediatric department. PLoS One. 2012;7(3):e33974. doi: 10.1371/journal.pone.0033974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jung C.-C., Wu P.-C., Tseng C.-H., Su H.-J. Indoor air quality varies with ventilation types and working areas in hospitals. Building Environ. 2015;85:190–195. [Google Scholar]
- 113.Tseng C.-C., Li C.-S. Collection efficiencies of aerosol samplers for virus-containing aerosols. J Aerosol Sci. 2005;36:593–607. doi: 10.1016/j.jaerosci.2004.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Creamer E., Shore A.C., Deasy E.C. Air and surface contamination patterns of meticillin-resistant Staphylococcus aureus on eight acute hospital wards. J Hosp Infect. 2014;86:201–208. doi: 10.1016/j.jhin.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 115.Narui K., Noguchi N., Matsunaga N. Change in environmental bacterial flora in a new hospital building. J Hosp Infect. 2009;73:24–33. doi: 10.1016/j.jhin.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 116.Shintani H., Taniai E., Miki A., Kurosu S., Hayashi F. Comparison of the collecting efficiency of microbiological air samplers. J Hosp Infect. 2004;56:42–48. doi: 10.1016/j.jhin.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 117.Bourdillon R.B., Lidwell O.M., Thomas J.C. A slit sampler for collecting and counting air-borne bacteria. J Hyg (Lond) 1941;41:197–224. doi: 10.1017/s0022172400012407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Verreault D., Moineau S., Duchaine C. Methods for sampling of airborne viruses. Microbiol Mol Biol Rev. 2008;72:413–444. doi: 10.1128/MMBR.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Clark S., Lach V., Lidwell O.M. The performance of the Biotest RCS centrifugal air sampler. J Hosp Infect. 1981;2:181–186. doi: 10.1016/0195-6701(81)90028-1. [DOI] [PubMed] [Google Scholar]
- 120.Adhikari A., Sen M.M., Gupta-Bhattacharya S., Chanda S. Airborne viable, non-viable, and allergenic fungi in a rural agricultural area of India: a 2-year study at five outdoor sampling stations. Sci Total Environ. 2004;326:123–141. doi: 10.1016/j.scitotenv.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 121.O'Connor D.J., Daly S.M., Sodeau J.R. On-line monitoring of airborne bioaerosols released from a composting/green waste site. Waste Management. 2015;42:23–30. doi: 10.1016/j.wasman.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 122.Grosskopf K., Mousavi E. Ventilation and transport; bioaerosols in healthcare environments. ASHRAE J. 2014; August:22–31. [Google Scholar]
- 123.Blais-Lecours P., Perrott P., Duchaine C. Non-culturable bioaerosols in indoor settings: impact on health and molecular approaches for detection. Atmos Environ. 2015;110:45–53. doi: 10.1016/j.atmosenv.2015.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Walser S.M., Gerstner D.G., Brenner B. Evaluation of exposure–response relationships for health effects of microbial bioaerosols – a systematic review. Int J Hygiene Environ Health. 2015;218:577–589. doi: 10.1016/j.ijheh.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 125.Dharan S., Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51:79–84. doi: 10.1053/jhin.2002.1217. [DOI] [PubMed] [Google Scholar]
- 126.Scaltriti S., Cencetti S., Rovesti S., Marchesi I., Bargellini A., Borella P. Risk factors for particulate and microbial contamination of air in operating theatres. J Hosp Infect. 2007;66:320–326. doi: 10.1016/j.jhin.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 127.Mitchell N.J., Evans D.S., Kerr A. Reduction of skin bacteria in theatre air with comfortable, non-woven disposable clothing for operating-theatre staff. Br Med J. 1978;1(6114):696–698. doi: 10.1136/bmj.1.6114.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Woodhead K., Taylor E.W., Bannister G., Chesworth T., Hoffman P., Humphreys H. Behaviours and rituals in the operating theatre. J Hosp Infect. 2002;51:241–255. doi: 10.1053/jhin.2002.1220. [DOI] [PubMed] [Google Scholar]
- 129.Adams R.I., Bhangar S., Pasut W. Chamber bioaerosol study: outdoor air and human occupants as sources of indoor airborne microbes. PLoS One. 2015;10:e0128022. doi: 10.1371/journal.pone.0128022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lidwell O.M. The control by ventilation of airborne bacterial transfer between hospital patients, and its assessment by means of a particle tracer: II. Ventilation in subdivided isolation units. J Hygiene. 1972;70:287–297. doi: 10.1017/s0022172400022348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goyal S.M., Anantharaman S., Ramakrishnan M.A. Detection of viruses in used ventilation filters from two large public buildings. Am J Infect Control. 2011;39:e30–e38. doi: 10.1016/j.ajic.2010.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]